WO2024240725A1 - Tris[1,2,4]triazolo[1,5-a:1',5'-c:1'',5''-e][1,3,5]triazine derivatives for use in organic electroluminescent devices - Google Patents
Tris[1,2,4]triazolo[1,5-a:1',5'-c:1'',5''-e][1,3,5]triazine derivatives for use in organic electroluminescent devices Download PDFInfo
- Publication number
- WO2024240725A1 WO2024240725A1 PCT/EP2024/063872 EP2024063872W WO2024240725A1 WO 2024240725 A1 WO2024240725 A1 WO 2024240725A1 EP 2024063872 W EP2024063872 W EP 2024063872W WO 2024240725 A1 WO2024240725 A1 WO 2024240725A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- atoms
- aromatic
- radicals
- substituted
- groups
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/22—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains four or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6572—Polycyclic condensed heteroaromatic hydrocarbons comprising only nitrogen in the heteroaromatic polycondensed ring system, e.g. phenanthroline or carbazole
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/18—Carrier blocking layers
Definitions
- the present invention relates to materials for use in electronic devices, in particular in organic electroluminescent devices, and to electronic devices, in particular organic electroluminescent devices, containing these materials.
- Organic-based charge transport materials e.g. triarylamine-based hole transporters
- OLEDs organic light-emitting diodes
- organic photoreceptors in copiers.
- Organic solar cells O-SC
- organic field-effect transistors O-FET
- organic thin-film transistors O-TFT
- organic switching elements O-IC
- organic optical amplifiers and organic laser diodes O-lasers
- Electronic devices in the sense of this invention are understood to be organic electronic devices which contain organic semiconductor materials as functional materials.
- the electronic devices are electroluminescent devices such as OLEDs.
- OLEDs in which organic compounds are used as functional materials are known to those skilled in the art.
- OLEDs are understood to be electronic devices that have one or more layers that comprise organic compounds and emit light when a voltage is applied.
- Electronic devices usually comprise a cathode, an anode and at least one functional, preferably emitting layer.
- they can contain further layers, for example one or more hole injection layers, hole transport layers, hole blocking layers, electron transport layers, electron injection layers, exciton blocking layers, electron blocking layers and/or charge generation layers.
- the object of the present invention is to provide compounds which are suitable for use in an electronic device, in particular an OLED, in particular as material of electron transport layers and/or as host materials, and which lead to good properties there.
- Ar' is on each occurrence, identically or differently, an aromatic or heteroaromatic ring system having 5 to 40 aromatic ring atoms which may be substituted by one or more radicals R 1 , where two or more R 1s may together form an aromatic or heteroaromatic ring system;
- R 2 is, identically or differently at each occurrence, H, D, F, CN or an aliphatic, aromatic or heteroaromatic organic radical having 1 to 20 C atoms, in which one or more H atoms may be replaced by D or F; two or more R 2 substituents may be linked to one another to form a ring.
- An aryl group in the sense of this invention contains 6 to 40 C atoms; a heteroaryl group in the sense of this invention contains 5 to 40 C atoms and at least one heteroatom, with the proviso that the sum of C atoms and heteroatoms at least 5.
- the heteroatoms are preferably selected from N, 0 and/or S.
- An aryl group or heteroaryl group is understood to be either a simple aromatic ring, i.e.
- benzene or a simple heteroaromatic ring, for example pyridine, pyrimidine, thiophene, etc., or a condensed (fused) aryl or heteroaryl group, for example naphthalene, anthracene, phenanthrene, quinoline, isoquinoline, etc.
- Aromatics linked to one another by a single bond, such as biphenyl, are not referred to as aryl or heteroaryl groups, but as aromatic ring systems.
- An aromatic ring system in the sense of this invention contains 6 to 60 C atoms, preferably 6 to 40 C atoms in the ring system.
- a heteroaromatic ring system in the sense of this invention contains 1 to 60 C atoms, preferably 1 to 40 C atoms and at least one heteroatom in the ring system, with the proviso that the sum of C atoms and heteroatoms is at least 5.
- the heteroatoms are preferably selected from N, O and/or S.
- An aromatic or heteroaromatic ring system in the sense of this invention is to be understood as a system which does not necessarily only contain aryl or heteroaryl groups, but in which several aryl or heteroaryl groups can also be connected by a non-aromatic unit (preferably less than 10% of the atoms other than H), such as a C, N or O atom or carbonyl group.
- a non-aromatic unit preferably less than 10% of the atoms other than H
- This also includes systems in which two or more aryl or heteroaryl groups are directly linked to one another, such as biphenyl, terphenyl, bipyridine or phenylpyridine.
- systems such as fluorene, 9,9'-spirobifluorene, 9,9-diarylfluorene, triarylamine, diaryl ether, stilbene, etc. are also to be understood as aromatic ring systems in the sense of this invention, as are systems in which two or more aryl groups are linked, for example, by a linear or cyclic alkyl group or by a silyl group.
- Preferred aromatic or heteroaromatic ring systems are simple aryl or heteroaryl groups and groups in which two or more aryl or heteroaryl groups are directly linked to one another, for example biphenyl, Terphenyl, quaterphenyl or bipyridine, as well as fluorene or spirobifluorene.
- An electron-rich heteroaromatic ring system is characterized by the fact that it is a heteroaromatic ring system that does not contain any electron-poor heteroaryl groups.
- An electron-poor heteroaryl group is a six-membered ring heteroaryl group with at least one nitrogen atom or a five-membered ring heteroaryl group with at least two heteroatoms, one of which is a nitrogen atom and the other is oxygen, sulfur or a substituted nitrogen atom, where further aryl or heteroaryl groups can be condensed onto each of these groups.
- electron-rich heteroaryl groups are five-membered ring heteroaryl groups with exactly one heteroatom selected from oxygen, sulfur or substituted nitrogen, to which further aryl groups and/or further electron-rich five-membered ring heteroaryl groups can be condensed.
- electron-rich heteroaryl groups are pyrrole, furan, thiophene, indole, benzofuran, benzothiophene, carbazole, dibenzofuran, dibenzothiophene or indenocarbazole.
- An electron-rich heteroaryl group is also called an electron-rich heteroaromatic residue.
- An electron-poor heteroaromatic ring system is characterized in that it contains at least one electron-poor heteroaryl group, and particularly preferably no electron-rich heteroaryl groups.
- alkyl group is used as a generic term for both linear or branched alkyl groups and for cyclic alkyl groups.
- alkenyl group and alkynyl group are used as generic terms for both linear or branched alkenyl or alkynyl groups, as well as for cyclic alkenyl or alkynyl groups.
- a cyclic alkyl, alkoxy or thioalkoxy group within the meaning of this invention is understood to mean a monocyclic, a bicyclic or a polycyclic group.
- an aliphatic hydrocarbon radical or an alkyl group or an alkenyl or alkynyl group which can contain 1 to 40 C atoms and in which individual H atoms or CH2 groups can also be substituted by the abovementioned groups, preferably the radicals methyl, ethyl, n-propyl, i-propyl, n-butyl, i-butyl, s-butyl, t-butyl, 2-methylbutyl, n-pentyl, s-pentyl, t-pentyl, 2-pentyl, neo-pentyl, cyclopentyl, n-hexyl, s-hexyl, t-hexyl, 2-hex
- alkoxy group OR 1 with 1 to 40 carbon atoms, preference is given to methoxy, trifluoromethoxy, ethoxy, n-propoxy, i-propoxy, n-butoxy, i-butoxy, s-butoxy, t-butoxy, n-pentoxy, s-pentoxy, 2-methylbutoxy, n-hexoxy, cyclohexyloxy, n-heptoxy, cyclo- heptyloxy, n-octyloxy, cyclooctyloxy, 2-ethylhexyloxy, pentafluoroethoxy and 2,2,2-trifluoroethoxy.
- a thioalkyl group SR 1 with 1 to 40 carbon atoms includes, in particular, methylthio, ethylthio, n-propylthio, i-propylthio, n-butylthio, i-butylthio, s-butylthio, t-butylthio, n-pentylthio, s-pentylthio, n-hexylthio, Cyclohexylthio, n-heptylthio, cycloheptyl-thio, n-octylthio, cyclooctylthio, 2-ethylhexylthio, trifluoromethylthio, pentafluoroethylthio, 2,2,2-trifluoroethylthio, ethenylthio, propenylthio, butenylthio, pentenylthio, cyclopenten
- alkyl, alkoxy or thioalkyl groups according to the present invention can be straight-chain, branched or cyclic, where one or more non-adjacent CH2 groups can be replaced by the abovementioned groups; furthermore, one or more H atoms can also be replaced by D, F, CI, Br, I, CN or NO2, preferably D, F, CI or CN, particularly preferably D, F or CN.
- An aromatic or heteroaromatic ring system with 5 - 60 aromatic ring atoms, preferably 5 - 40 aromatic ring atoms, which can be substituted by the above-mentioned radicals or a hydrocarbon radical and which can be linked to the aromatic or heteroaromatic via any position, is understood to mean in particular groups which are derived from benzene, naphthalene, anthracene, benzanthracene, phenanthrene, pyrene, chrysene, perylene, fluoranthene, naphthacene, pentacene, benzopyrene, biphenyl, biphenylene, terphenyl, triphenylene, fluorene, spirobifluorene, dihydrophenanthrene, dihydropyrene, tetrahydropyrene, cis- or trans- indenofluorene, cis- or trans- indenocarbazole, cis- or trans-
- the nitrogen atom is part of a six-membered ring, as in dibenzo[1,4]oxazine, for example.
- all R and the associated groups if they contain a nitrogen atom with three single bonds, contains, each nitrogen atom with three single bonds is at least part of a five-membered ring.
- the groups R do not contain any substituted or unsubstituted amino groups.
- the group R therefore preferably does not contain any triarylamino groups, but can contain, for example, carbazole groups, i.e. heteroaryl groups that contain nitrogen.
- At least one R comprises an aromatic or heteroaromatic ring system having 9 to 60 aromatic ring atoms, preferably 10 to 40 aromatic ring atoms, very particularly preferably 12 to 30 aromatic ring atoms.
- R and the associated groups do not comprise fused aryl groups.
- At least two substituents R are identical.
- Preferred embodiments of the invention are thus compounds in which two substituents R are identical and the third substituent R is different from the other substituents R, and compounds in which all three substituents R are identical.
- R is selected on each occurrence, identically or differently, from the group consisting of H, D, F, CN, OR 1 , a straight-chain alkyl group having 1 to 10 C atoms or an alkenyl group having 2 to 10 C atoms or a branched or cyclic alkyl group having 3 to 10 C atoms, where the alkyl or alkenyl group may each be substituted by one or more radicals R 1 , but is preferably unsubstituted, and where one or several non-adjacent CH2 groups can be replaced by 0, or an aromatic or heteroaromatic ring system with 6 to 30 aromatic ring atoms, each of which can be substituted by one or more radicals R 1 .
- R is selected on each occurrence, identically or differently, from the group consisting of H, F, CN, a straight-chain alkyl group having 1 to 6 C atoms, in particular having 1, 2, 3 or 4 C atoms, or a branched or cyclic alkyl group having 3 to 6 C atoms, where the alkyl group can be substituted in each case by one or more radicals R 1 , but is preferably unsubstituted, or an aromatic or heteroaromatic ring system having 6 to 24 aromatic ring atoms, which can be substituted in each case by one or more radicals R 1 , preferably non-aromatic radicals R 1 .
- R is selected, identically or differently on each occurrence, from the group consisting of H, D, CN or an aromatic or heteroaromatic ring system having 6 to 24 aromatic ring atoms, each of which may be substituted by one or more radicals R 1 , preferably non-aromatic radicals R 1 .
- all radicals R are selected, identically or differently on each occurrence, from an aromatic or heteroaromatic ring system having 6 to 24 aromatic ring atoms, in particular having 6 to 18 aromatic ring atoms, each of which may be substituted by one or more radicals R 1 , preferably non-aromatic radicals R 1 .
- Suitable aromatic or heteroaromatic ring systems R are selected from phenyl, biphenyl, in particular ortho-, meta- or para-biphenyl, terphenyl, in particular ortho-, meta-, para- or branched terphenyl, quaterphenyl, in particular ortho-, meta-, para- or branched quaterphenyl, fluorene, which can be linked via the 1-, 2-, 3- or 4-position, spirobifluorene, which can be linked via the 1-, 2-, 3- or 4-position, naphthalene, which can be linked via the 1- or 2-position, indole, benzofuran, benzothiophene, which can be linked via the 1-, 2-, 3- or 4-position, dibenzofuran, carbazole, which can be linked via the 1-, 2-, 3- or 4-position, dibenzothiophene, which can be linked via the 1-, 2-, 3- or 4-position, indenocarbazole, indolocarbazole, pyr
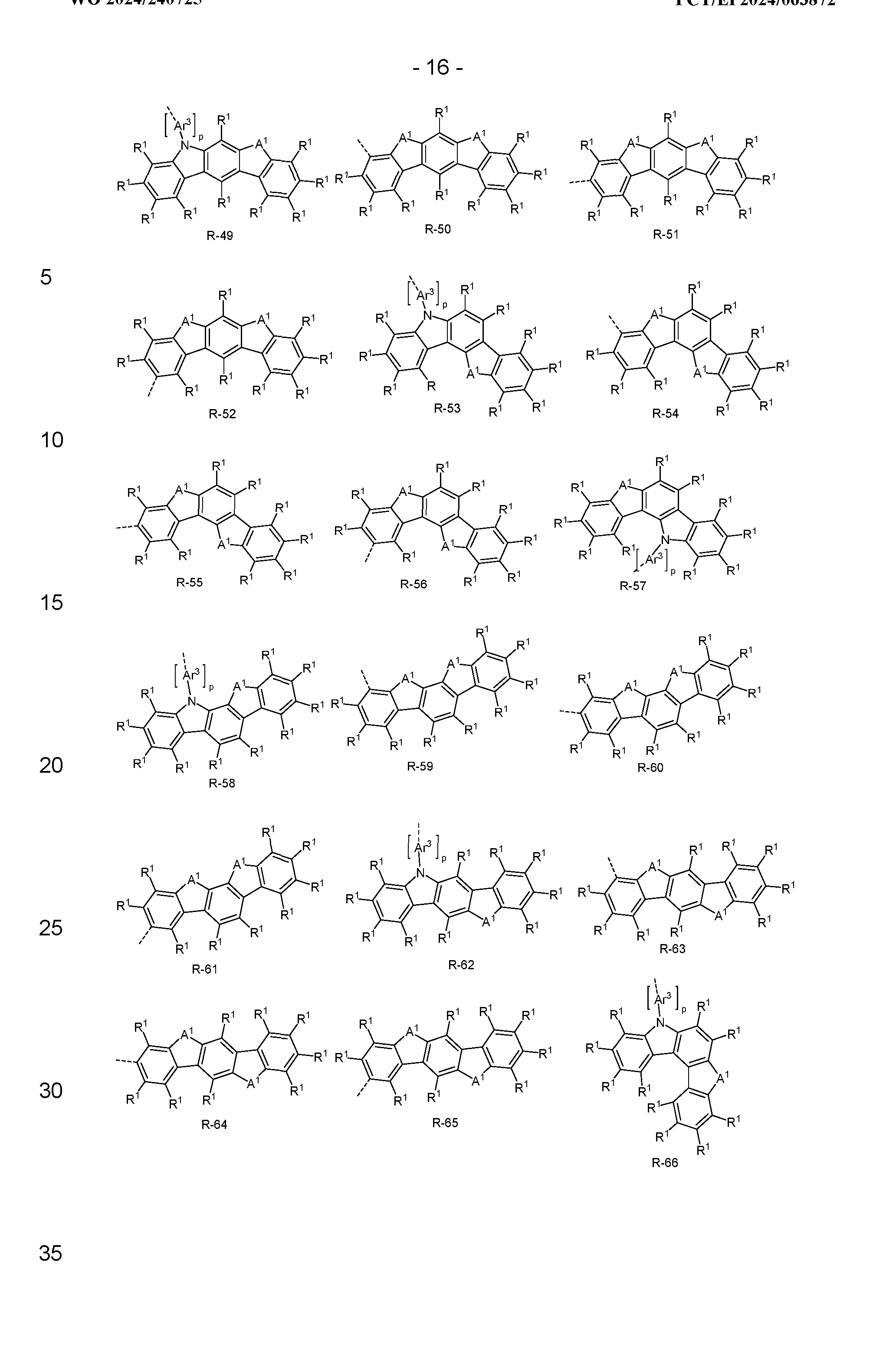
- the groups R when they represent an aromatic or heteroaromatic ring system, are preferably selected from the groups of the following formulas R-1 to R-184,
- Ar 3 is at each occurrence, identically or differently, a bivalent aromatic or heteroaromatic ring system with 6 to 18 aromatic tic ring atoms, each of which may be substituted by one or more radicals R 1 ;
- Ar 3 comprises divalent aromatic or heteroaromatic ring systems based on the groups R-1 to R-184, where p is 0 and the dashed bond and an R 1 represent the bond to the aromatic or heteroaromatic group after R-1 to R-184.
- the substituent R 1 which is bonded to the nitrogen atom preferably represents an aromatic or heteroaromatic ring system having 5 to 24 aromatic ring atoms, which can also be represented by one or more radicals R 2 can be substituted.
- this substituent R 1 is the same or different on each occurrence and represents an aromatic or heteroaromatic ring system having 6 to 24 aromatic ring atoms, preferably having 6 to 12 aromatic ring atoms, and which can each also be substituted by one or more radicals R 2.
- phenyl, biphenyl, terphenyl and quaterphenyl with linkage patterns as listed above for R-1 to R-35 where these structures can be substituted by one or more radicals R 1 , but are preferably unsubstituted.
- a 1 is C(R 1 ) 2
- the substituents R 1 which are bonded to this carbon atom are preferably identical or different on each occurrence and represent a linear alkyl group having 1 to 10 C atoms or a branched or cyclic alkyl group having 3 to 10 C atoms or an aromatic or heteroaromatic ring system having 5 to 24 aromatic ring atoms, which may also be substituted by one or more radicals R 2 .
- R 1 is very particularly preferably a methyl group or a phenyl group.
- the radicals R 1 can also form a ring system with one another, resulting in a spiro system.
- Ar' is the same or different on each occurrence and is an aromatic or heteroaromatic ring system having 6 to 30 aromatic ring atoms, particularly preferably having 6 to 24 aromatic ring atoms and very particularly preferably having 6 to 13 aromatic ring atoms, which may in each case be substituted by one or more radicals R 1 .
- R 1 is the same or different on each occurrence and is selected from the group consisting of H, D, F, CN, a straight-chain alkyl group having 1 to 10 C atoms or an alkenyl group having 2 to 10 C atoms or a branched or cyclic alkyl group having 3 to 10 C atoms, where the alkyl or alkenyl group may each be substituted by one or more radicals R 2 , or an aromatic or heteroaromatic ring system having 6 to 30 aromatic ring atoms, each of which is substituted by one or more radicals R 2 can be substituted; two or more radicals R 1 can form an aliphatic ring system with one another.
- R 1 is the same or different on each occurrence and is selected from the group consisting of H, D, a straight-chain alkyl group having 1 to 6 C atoms, in particular having 1, 2, 3 or 4 C atoms, or a branched or cyclic alkyl group having 3 to 6 C atoms, where the alkyl group can be substituted by one or more radicals R 2 , but is preferably unsubstituted, or an aromatic or heteroaromatic ring system having 6 to 24 aromatic ring atoms, each of which can be substituted by one or more radicals R 2 .
- R 2 is the same or different on each occurrence and is H, D, CN, F, an alkyl group having 1 to 4 C atoms or an aryl group having 6 to 10 C atoms, which may be substituted by an alkyl group having 1 to 4 C atoms, but is preferably unsubstituted.
- all radicals R 1 insofar as they represent an aromatic or heteroaromatic ring system, or R 2 , insofar as they represent aromatic or heteroaromatic groups, are selected from the groups R-1 to R-184, which are then each substituted accordingly with R 2 , or the groups mentioned under R 2 .
- all aromatic or heteroaromatic groups of the radicals R, R 1 or R 2 are selected from the corresponding groups R-1 to R-184.
- the compounds are at least 50%, in particular at least 80%, particularly preferably completely (100%) deuterated. This means that in such a compound the corresponding proportion of the hydrogen atoms contained in the undeuterated compound have been exchanged for D.
- the undeuterated compound is the corresponding compound in which the deuterium has been exchanged for hydrogen and which therefore contains no D. In a fully deuterated compound, all H are exchanged for D.
- the alkyl groups in compounds according to the invention which are processed by vacuum evaporation preferably have no more than five C atoms, particularly preferably no more than 4 C atoms, very particularly preferably no more than 1 C atom.
- compounds which are substituted with alkyl groups, in particular branched alkyl groups, with up to 10 C atoms or which are substituted with oligoarylene groups, for example ortho-, meta-, para- or branched terphenyl or quaterphenyl groups, are also suitable.
- the compounds according to the invention can be prepared by synthesis steps known to the person skilled in the art, such as bromination, Suzuki coupling, Ullmann coupling, Heck reaction, Hartwig-Buchwald coupling, etc.
- the 2,6,10-triaryl/heteroaryl-tris[1,2,4]triazolo[1,5-a:1',5'-c:1",5"-e][1,3,5]triazines according to the invention can be prepared starting from 2,6,10-trichloro-tris[1,2,4]triazolo[1,5-a:1',5'-c:1",5"-e][1,3,5]triazine [879612-44-9] by Suzuki coupling with aryl/heteroaryl-boronic acids or their esters or by SN2Ar reaction with Grignard or organolithium compounds (Scheme 1).
- Typical catalyst systems for the Suzuki coupling are known combinations of palladium compounds and preferably electron-rich phosphines such as SPhos, XPhos, RuPhos, AdaPhos etc., as well as alkali-alkaline earth carbonates, phosphates, hydroxides as typical bases and DMSO, DMF, DMAc, NMP, THF, dioxane as solvents (Lömi) for single-phase reactions or mixtures of water with THF, dioxane, glyme, alcohols, toluene etc.
- Alternative coupling processes such as Negish, Yamamoto, Grignard cross coupling can also be used. are used.
- mixed products can be obtained with respect to the R radical, which can be separated chromatographically.
- the synthesis of mixed compounds can also be carried out by consecutive coupling steps, whereby the dichloroaryl/heteroaryl or the chlorodiaryl/heteroaryl intermediates can be isolated or further reacted in situ.
- the 2,6, 10-tri-N-carbazolyl-tris[1,2,4]triazo lo[1,5- a:1',5'-c:1",5"-e][1,3,5]triazines according to the invention can be prepared starting from 2,6, 10-trichlorotris-[1,2,4]triazolo[1,5-a:1',5'-c:1",5"-e][1,3,5]triazine [879612-44-9] by Buchwald-Hartwig coupling or by SN2Ar reaction with carbazoles (Scheme 2).
- the reactions with indenocarbazoles, indolocarbazoles, etc. can be carried out analogously.
- Typical catalyst systems for the Buchwald-Hartwig coupling are known combinations of palladium compounds and preferably electron-rich phosphines, such as tri-tert-butyl-, tri-cyclohexyl-phosphine, BINAP, SPhos, XPhos, RuPhos, AdaPhos etc.
- typical bases are alcoholates, alkali-alkaline earth carbonates, phosphates and as Solvents THF, dioxane, toluene, DMSO, DMF, DMAc, NMP can be used.
- BuLi, NaH, K2CO3, CS2CO3, K3PO4 in dipolar aprotic solvents such as DMSO, DMF, DMAc, NMP, etc. are used.
- the compounds of the invention can be prepared by the processes described in the literature starting from the corresponding nitriles or carboxamides (e.g. R. Hojo et al., J. Mater. Chem. C, 2022, 10, 13871 or T. Rieth et al., Molecules 2020, 25, 5761).
- a further object of the present invention is therefore a process for preparing the compounds according to the invention, characterized by the following steps: (A) synthesis of the basic skeleton according to formula (1 ) which contains, instead of the radicals R, a reactive leaving group, for example F, CI, Br, I, boronic acid or a boronic acid ester, tosylate or mesylate;
- Another object of the present invention is an oligomer, polymer or dendrimer comprising one or more compounds according to formula (1).
- formulations of the compounds according to the invention are required. These formulations can be, for example, solutions, dispersions or emulsions. It may be preferable to use mixtures of two or more solvents for this purpose.
- Suitable and preferred solvents are, for example, toluene, anisole, o-, m- or p-xylene, methyl benzoate, mesitylene, tetralin, veratrole, THF, methyl-THF, THP, chlorobenzene, dioxane, phenoxytoluene, in particular 3-phenoxytoluene, (-)-fenchone, 1,2,3,5-tetramethylbenzene, 1,2,4,5-tetramethylbenzene, 1-methylnaphthalene, 2-methylbenzothiazole, 2-phenoxyethanol, 2-pyrrolidinone, 3-methylanisole, 4-methylanisole, 3,4-dimethylanisole, 3,5-dimethylanisole, acetophenone, a-terpineol, benzothiazole, butylbenzoate, cumene, cyclohexanol, cyclohexanone, Cyclohexylbenzene, decalin, do
- a further subject of the present invention is therefore a formulation, in particular a solution, dispersion or emulsion, comprising at least one compound according to the invention and at least one further compound.
- the further compound can be, for example, a solvent, in particular one of the above-mentioned solvents or a mixture of these solvents. The preparation of such solutions is known to the person skilled in the art and is described, for example, in WO 2002/072714, WO 2003/019694 and the literature cited therein.
- the further compound can also be at least one further organic or inorganic compound which is also used in the electronic device, for example an emitting compound and/or a matrix material. This further compound can also be polymeric.
- the compounds according to the invention are suitable for use in an electronic device, in particular in an organic electroluminescent device (OLED). Depending on the substitution, the compounds can be used in different functions and layers.
- OLED organic electroluminescent device
- a further object of the present invention is therefore the use of a compound according to the invention in an electronic device.
- Yet another object of the present invention is an electronic device comprising at least one compound according to the invention.
- the compounds according to the invention can be present, in particular when used, as a racemate or as a pure enantiomer.
- the formation of enantiomers is possible, for example, if the radicals R are selected such that rotation around the bond of R to the tristriazolotriazine is hindered and atropisomers are thereby formed.
- An electronic device in the sense of the present invention is a device which contains at least one layer containing at least one organic compound.
- the component can also contain inorganic nic materials or layers that are made entirely of inorganic materials.
- the electronic device is preferably selected from the group consisting of organic electroluminescent devices (OLEDs), organic integrated circuits (O-ICs), organic field-effect transistors (O-FETs), organic thin-film transistors (O-TFTs), organic light-emitting transistors (O-LETs), organic solar cells (O-SCs), dye-sensitized organic solar cells (DSSCs), organic optical detectors, organic photoreceptors, organic photodiodes (OPDs), organic field-quench devices (O-FQDs), light-emitting electrochemical cells (LECs), organic laser diodes (O-lasers) and organic plasmon emitting devices, but preferably organic electroluminescent devices (OLEDs).
- OLEDs organic electroluminescent devices
- O-ICs organic integrated circuits
- O-FETs organic field-effect transistors
- OF-TFTs organic thin-film transistors
- O-LETs organic light-emitting transistors
- O-SCs organic solar cells
- the device is particularly preferably an organic electroluminescent device comprising a cathode, anode and at least one emitting layer, wherein at least one organic layer, which can be an emitting layer, hole transport layer, electron transport layer, hole blocking layer, electron blocking layer or another functional layer, comprises at least one compound according to the invention.
- the layer depends on the substitution of the compound.
- the organic electroluminescent device can contain further layers, for example one or more hole injection layers, hole transport layers, hole blocking layers, electron transport layers, electron injection layers, exciton blocking layers, electron blocking layers, charge generation layers and/or organic or inorganic p/n junctions. Interlayers can also be introduced between two emitting layers, which, for example, have an exciton blocking function. It should be noted, however, that not all of these layers necessarily have to be present.
- the organic electroluminescent device can contain one emitting layer, or it can contain several emitting layers.
- emitting layers are present, these preferably have a total of several emission maxima between 380 nm and 750 nm, so that overall white emission results, ie different emitting compounds that can fluoresce or phosphoresce are used in the emitting layers.
- Systems with three emitting layers are particularly preferred, with the three layers showing blue, green and orange or red emission (the basic structure is described, for example, in WO 2005/011013).
- the organic electroluminescent device according to the invention can also be a tandem OLED, in particular for white-emitting OLEDs.
- the compound according to formula (1) is preferably used in an organic electroluminescent device which comprises one or more phosphorescent emitters.
- the compound according to the invention according to the embodiments listed above can be used in different layers, depending on the precise structure.
- the organic electroluminescent device can contain one emitting layer, or it can contain several emitting layers, with at least one layer containing at least one compound according to the invention. Furthermore, the compound according to the invention can also be used in an electron transport layer and/or in a hole blocking layer and/or in a hole transport layer and/or in an exciton blocking layer.
- phosphorescent compound typically refers to compounds in which the emission of light occurs through a spin-forbidden transition, e.g. a transition from an excited triplet state or a state with a higher spin quantum number, e.g. a quintet state.
- Suitable phosphorescent compounds are in particular compounds that emit light, preferably in the visible range, when suitably excited and also contain at least one atom of atomic number greater than 20, preferably greater than 38 and less than 84, particularly preferably greater than 56 and less than 80.
- All luminescent complexes with transition metals or lanthanides are preferably regarded as phosphorescent compounds, in particular if they contain copper, molybdenum, tungsten, rhenium, ruthenium, osmium, rhodium, indium, palladium, platinum, silver, gold or europium, in particular compounds which contain indium, platinum or copper.
- all luminescent indium, platinum or copper complexes are regarded as phosphorescent emitting compounds.
- Examples of the emitters described above can be found in the applications WO 00/70655, WO 2001/41512, WO 2002/02714, WO 2002/15645, EP 1191613, EP 1191612, EP 1191614, WO 05/033244, WO 05/019373, US 2005/0258742, WO 2009/146770, WO 2010/015307, WO 2010/031485, WO 2010/054731, WO 2010/054728, WO 2010/086089, WO 2010/099852, WO 2010/102709, WO 2011/032626, WO 2011/066898, WO 2011/157339, WO 2012/007086, WO 2014/008982, WO 2014/023377, WO 2014/094961, WO 2014/094960, WO 2015/036074, WO 2015/104045, WO 2015/117718, WO 2016/015815, WO 2016/124304, WO 2017/032439,
- all phosphorescent complexes as used according to the prior art for phosphorescent OLEDs and as known to the person skilled in the field of organic electroluminescence are suitable, and the person skilled in the art can use further phosphorescent complexes without inventive step. It is also possible for the person skilled in the art to use further phosphorescent complexes in combination with the compounds of the formula (1) in organic electroluminescent devices without inventive step. Since the compounds according to the invention can also have a high triplet energy depending on the substitution, it is also possible in particular to use them as matrix material for blue phosphorescent emitters. Further examples are listed in a table below. According to the invention, it is also possible to use the compound of formula (1) in an electronic device containing one or more fluorescent emitting compounds.
- the compounds of the formula (1) are used as electron-transporting material.
- the compounds are preferably contained in an electron-transport layer or a hole-blocking layer or an electron-conducting or bipolar host material. Use in an electron-transport layer is particularly preferred.
- An electron transport layer in the sense of the present application is a layer with an electron-transporting function between the cathode and the emitting layer.
- electron injection layers and hole blocking layers are understood to mean certain embodiments of electron transport layers.
- an electron injection layer is an electron transport layer that directly borders the cathode or is only separated from it by a single coating of the cathode.
- a hole blocking layer is the electron transport layer that directly borders the emitting layer on the cathode side.
- the OLED according to the invention preferably comprises two, three or four electron-transporting layers between the cathode and the emitting layer, of which preferably at least one, particularly preferably exactly one or two, contain a compound of the formula (1).
- the compound of formula (1) is used as an electron transport material in an electron transport layer, an electron injection layer or a hole blocking layer, the compound can be used as a pure material, ie in a proportion of 100% in the electron transport layer, or it can be used in combination with one or more other compounds.
- the compound of formula (1) is used in an emitting layer as a matrix material in combination with one or more emitting compounds, where the emitting compounds can be fluorescent or phosphorescent, preferably phosphorescent.
- the proportion of matrix material in the emitting layer in this case is between 50.0 and 99.9 vol. %, preferably between 80.0 and 99.5 vol. %, particularly preferably between 92.0 and 99.5 vol. % for fluorescent emitting layers and between 85.0 and 97.0 vol. % for phosphorescent emitting layers.
- the proportion of the emitting compound is between 0.1 and 50.0 vol.%, preferably between 0.5 and 20.0 vol.%, particularly preferably between 0.5 and 8.0 vol.% for fluorescent emitting layers and between 3.0 and 15.0 vol.% for phosphorescent emitting layers.
- An emitting layer of an organic electroluminescent device can also comprise systems that contain a large number of matrix materials (mixed matrix systems) and/or a large number of emitting compounds.
- the emitting compounds are generally those that have the smaller proportion in the system and the matrix materials are those that have the larger proportion in the system.
- the proportion of an individual matrix material in the system can be lower than the proportion of an individual emitting compound.
- the compounds of formula (1) are used as a component of mixed matrix systems.
- the mixed matrix systems preferably consist of two or three different matrix materials, particularly preferably of two different matrix materials.
- one of the two materials is a material with hole-transporting properties and the other material is a material with electron-transporting properties.
- the compound of formula (1) is preferably the matrix material with electron-transporting properties.
- the desired electron-transporting and hole-transporting properties of the mixed matrix components can also be predominantly or completely combined in a single mixed matrix component, with the further mixed matrix component(s) fulfilling other functions.
- the two different matrix materials can be present in a ratio of 1:50 to 1:1, preferably 1:20 to 1:1, even more preferably 1:10 to 1:1 and most preferably 1:4 to 1:1.
- Mixed matrix systems are preferably used in phosphorescent organic electroluminescent devices. A source for more detailed information on mixed matrix systems is the application WO 2010/108579.
- the mixed matrix systems can contain one or more emitting compounds, preferably one or more phosphorescent compounds.
- mixed matrix systems are preferably used in phosphorescent organic electroluminescent devices.
- Particularly suitable matrix materials which can be used in combination with the compounds according to the invention as matrix components of a mixed matrix system are selected from the preferred matrix materials for phosphorescent compounds or the preferred matrix materials for fluorescent compounds mentioned below, depending on which type of emitting compound is used in the mixed matrix system.
- Preferred phosphorescent compounds for use in mixed matrix systems are the same as those described above as generally preferred phosphorescent emitter materials.
- Examples of phosphorescent compounds are listed below.
- Preferred fluorescent emitting compounds are selected from the class of arylamines.
- an arylamine or an aromatic amine is understood to mean a compound which contains three substituted or unsubstituted aromatic or heteroaromatic ring systems which are bonded directly to the nitrogen.
- at least one of these aromatic or heteroaromatic ring systems is a condensed ring system, particularly preferably with at least 14 aromatic ring atoms.
- Preferred examples of these are aromatic anthraceneamines, aromatic anthracenediamines, aromatic pyreneamines, aromatic pyrenediamines, aromatic chrysenamines or aromatic chrysenediamines.
- An aromatic anthraceneamine is understood to mean a compound in which a diarylamino group is bonded directly to an anthracene group, preferably in the 9-position.
- An aromatic anthracene diamine is a compound in which two diarylamino groups are bonded directly to an anthracene group, preferably in the 9- and 10-position.
- Aromatic pyrenamines, pyrenediamines, chrysenamines and chrysenediamines are defined analogously, in which the diarylamino groups are bonded to the pyrene preferably in the 1-position or 1,6-position.
- indenofluorenamines or fluorenediamines for example according to WO 2006/108497 or WO 2006/122630
- benzoindenofluorenamines or fluorenediamines for example according to WO 2008/006449
- dibenzoindenofluorenamines or diamines for example according to WO 2007/140847
- the indenofluorene derivatives with condensed aryl groups disclosed in WO 2010/012328 are preferred.
- pyrenearylamines disclosed in WO 2012/048780 and in WO 2013/185871.
- benzoindenofluorenamines disclosed in WO 2014/037077, the benzofluorene- amines, the extended benzoindenofluorenes disclosed in WO 2014/111269 and in WO 2017/036574, the phenoxazines disclosed in WO 2017/028940 and in WO 2017/028941 and the fluorine derivatives bound to furan units or thiophene units disclosed in WO 2016/150544.
- WO 2020/208051 WO 2015102118, WO 2016/152418, WO 2018/095397, WO 2019/004248, WO 2019/132040, US 2020/0161552 and WO 2021/089450 are used.
- Useful matrix materials include materials from different substance classes.
- Preferred matrix materials are selected from the classes of oligoaryls (e.g. 2,2',7,7'-tetraphenylspirobifluorene according to EP 676461 or dinaphthyl-anthracene), in particular oligoaryls with fused aromatic groups, oligoarylenevinylenes (e.g. DPVBi or spiro-DPVBi according to EP 676461), polypodal metal complexes (e.g. according to WO 2004/081017), hole-conducting compounds (e.g.
- electron-conducting compounds in particular ketones, phosphine oxides, sulfoxides etc. (for example according to WO 2005/084081 and WO 2005/084082), atropisomers (for example according to WO 2006/048268), boronic acid derivatives (for example according to WO 2006/117052) or the benzanthracenes (for example according to WO 2008/145239).
- Particularly preferred matrix materials are selected from the classes of oligoarylenes with naphthalene, anthracene, benzanthracene and/or pyrene or atropisomers of these compounds, the oligoarylenevinylenes, the ketones, the phosphine oxides and the sulfoxides.
- Very particularly preferred matrix materials are selected from the classes of oligoarylenes which include anthracene, benzanthracene, benzophenanthrene and/or pyrene or atropisomers of these compounds.
- an oligoarylene is understood to mean a compound in which at least three aryl or arylene groups are connected to one another.
- Preferred matrix materials for phosphorescent compounds are, as well as compounds according to formula (1), aromatic ketones, aromatic phosphine oxides or aromatic sulfoxides or sulfones, e.g. according to WO 2004/013080, WO 2004/093207, WO 2006/005627 or WO 2010/006680, triarylamines, carbazole derivatives, e.g. CBP (N,N-biscarbazolylbiphenyl) or WO 2005/039246, US 2005/0069729, JP 2004/288381, EP 1205527, WO 2008/086851 or WO 2013/041176, indolocarbazole derivatives, e.g. B.
- aromatic ketones aromatic phosphine oxides or aromatic sulfoxides or sulfones
- triarylamines e.g. CBP (N,N-biscarbazolylbiphenyl) or WO 2005/039246, US 2005/
- indenocarbazole derivatives e.g. according to WO 2010/136109, WO 2011/000455, WO 2013/041176 or WO 2013/056776, azacarbazole derivatives, e.g. according to EP 1617710, EP 1617711, EP 1731584, JP 2005/347160, bipolar matrix materials, e.g. according to WO 2007/137725, silanes, e.g. according to WO 2005/111172, azaboroles or boronic esters, e.g. according to WO 2006/117052, triazine derivatives, e.g. B.
- lactams e.g. according to WO 2011/116865 or WO 2011/137951, or dibenzofuran derivatives, e.g. according to WO 2015/169412, WO 2016/015810, WO 2016/023608, WO 2017/148564 or WO 2017/148565.
- another phosphorescent emitter which emits at a shorter wavelength than the actual emitter, can be present in the mixture as a co-host or a compound that does not participate, or does not participate to a significant extent, in the charge transport, as described, for example, in WO 2010/108579.
- US 6,392,250 B1 discloses the use of a mixture consisting of an electron transport material, a hole transport material and a fluorescent emitter in the emission layer of an OLED.
- US 6,803,720 B1 discloses the use of a mixture containing a phosphorescent emitter and a hole and an electron transport material in the emission layer of an OLED.
- composition of the present invention further contains at least one hole-transporting host material in addition to the electron-transporting material.
- the at least one hole-transporting host material is selected from the group of carbazole and triarylamine derivatives, more specifically biscarbazoles, bridged carbazoles, triarylamines, dibenzofuran-carbazole derivatives or dibenzofuran-amine derivatives and carbazolamines.
- the at least one hole-transporting host material is selected from compounds of the formula (h-1) or (h-2): where:
- K is Ar 4 or -L 5 -N(Ar)2;
- Z is CR z or CR A ; or two adjacent Z groups together form a fused ring; RA is -L 3 -AC 5 or -L 4 -N(Ar)2;
- L 4 , L 5 are, identically or differently on each occurrence, a single bond or an aromatic or heteroaromatic ring system having 5 to 30 aromatic ring atoms which may be substituted by one or more radicals R';
- L 3 is a single bond or an aromatic or heteroaromatic ring system having 5 to 30 aromatic ring atoms, which may be substituted by one or more radicals R', where a radical R' on L 3 can form a ring with a radical R z on the carbazole;
- Ar 4 is an aromatic ring system having 6 to 40 aromatic ring atoms or a heteroaromatic ring system having 5 to 40 aromatic ring atoms, which may be substituted by one or more radicals R';
- Ar 5 is, identically or differently at each occurrence, an unsubstituted or substituted heteroaromatic ring system having 5 to 40 aromatic ring atoms, which may be substituted by one or more R';
- E is independently at each occurrence a single bond or a C(R°)2 group
- R° is independently selected at each occurrence from a straight-chain alkyl group having 1 to 10 carbon atoms or a branched or cyclic alkyl group having 3 to 10 carbon atoms, each of which may be substituted by one or more R' radicals;
- x, y are independently selected from 0 or 1, wherein when x or y is 0, the corresponding group E is not present; and
- x + y 1 or 2;
- Ar is, identically or differently at each occurrence, an aromatic or heteroaromatic ring system having 5 to 40 aromatic ring atoms which may be substituted by one or more radicals R", where two or more R" may together form an aromatic or heteroaromatic ring system;
- R"' is on each occurrence, identically or differently, H, D, F, CN or an aliphatic, aromatic or heteroaromatic organic radical having 1 to 20 C atoms, in which one or more H atoms can be replaced by D or F; two or more radicals R"' together can form a ring system.
- the compounds of the formulae (h-1) and (h-2) comprise at least one group Z which stands for R A.
- L 4 , L 5 are, identically or differently on each occurrence, a single bond or an aromatic or heteroaromatic ring system having 5 to 25, more preferably 5 to 20 and even more preferably 6 to 18 aromatic ring atoms, which may be substituted by one or more radicals R'.
- L 3 is a single bond or an aromatic or heteroaromatic ring system having 5 to 25 aromatic ring atoms, more preferably 5 to 20 and even more preferably 6 to 18 aromatic ring atoms, which can be substituted by one or more radicals R', where a radical R' on L 3 can form a ring with a radical R z on the carbazole.
- the group Ar 5 is an unsubstituted or substituted heteroaromatic ring system selected from the groups of formulas (Ar5-1) to (Ar5-6), where the dashed bond indicates the attachment to L 3 or Z;
- V is CR v , with the proviso that V is C when it is bonded to the group of formula (h-1 ) or (h-2); or two adjacent groups
- T is CR T , with the proviso that T is C when bonded to the group of formula (h-1) or (h-2), or two adjacent groups T together form a fused ring;
- M is an aromatic ring system having 6 to 40 aromatic ring atoms or a heteroaromatic ring system having 5 to 40 aromatic ring atoms, which may be substituted by one or more radicals R;
- E 1 is independently at each occurrence a single bond or a group C(R°)2; where R° has the same meaning as above;
- the at least one hole-transporting host material is selected from compounds of the formula (h-1-1) to (h-2-2):
- hole-transporting host materials suitable as a second host material in the composition are shown in the following table:
- the at least one blue phosphorescent metal complex is selected from platinum complexes.
- the at least one blue phosphorescent metal complex has a LUMO of -1.8 eV to -2.2 eV, and the at least one blue phosphorescent metal complex preferably has a HOMO of -5.0 eV to -5.6 eV, as defined by quantum mechanical calculations.
- the energy of the lowest triplet state Ti of the at least one blue phosphorescent metal complex is higher than 2.55 eV, more preferably >2.65 eV, even more preferably >2.75 eV, as defined by quantum mechanical calculations.
- the energy levels of molecular orbitals such as the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), the lowest triplet state Ti or the lowest excited singlet state Si of materials are determined using quantum mechanical calculations.
- HOMO highest occupied molecular orbital
- LUMO lowest unoccupied molecular orbital
- a geometry optimization is first carried out using the "Ground State/Semi-empirical/Default Spin/AM1 /Charge O/Spin Singlet” method.
- An energy calculation is then carried out based on the optimized geometry.
- the "TD-SCF/DFT/Default Spin/B3PW91” method with the "6-31 G(d)" basis set (Charge 0, Spin Singlet) is used.
- the geometry is optimized using the "Ground State/Hartree-Fock/Default Spin/LanL2MB/Charge O/Spin Singlet" method.
- the energy calculation is carried out analogously to the method described above for the organic substances, with the difference that the "LanL2DZ” basis set is used for the metal atom and the "6-31 G(d)" basis set is used for the ligands.
- the energy calculation yields the HOMO energy level HEh or LUMO energy level LEh in Hartree units. From this, the HOMO and LUMO energy levels calibrated using cyclic voltammetry measurements are determined in electron volts as follows:
- the lowest triplet state Ti is defined as the energy of the triplet state with the lowest energy resulting from the quantum chemical calculation described.
- the lowest excited singlet state Si is defined as the energy of the excited singlet state with the lowest energy resulting from the quantum chemical calculation described.
- Ar 50 is, identically or differently on each occurrence, an aromatic or heteroaromatic ring system having 5 to 60 aromatic ring atoms, which may in each case also be substituted by one or more radicals R';
- Ar 51 , Ar 52 , Ar 53 are the same or different and represent a condensed aryl or heteroaryl ring having 5 to 18 aromatic ring atoms, which may each also be substituted by one or more radicals R';
- R co on each occurrence represents a radical selected from H, D, a straight-chain alkyl group having 1 to 40 C atoms, which may be substituted by one or more radicals R', an aryl or heteroaryl group having 6 to 18 aromatic ring atoms, each of which may be substituted by one or more radicals R, where two radicals R c together can form an aliphatic, aromatic or heteroaromatic ring system which is substituted by one or more radicals R';
- R N0 on each occurrence represents a radical selected from H, D, F, a straight-chain alkyl group having 1 to 40 C atoms or a branched or cyclic alkyl group having 3 to 40 C atoms, each of which is substituted by one or more radicals R' and where one or more H atoms may be replaced by D, F or CN, an aromatic or heteroaromatic ring system having 5 to 60 aromatic ring atoms, each of which may be substituted by one or more radicals R';
- R‘ and Ar have the same meaning as above.
- Ar 50 is, identically or differently on each occurrence, an aromatic or heteroaromatic ring system having 5 to 40, more preferably 5 to 30 and even more preferably 6 to 18 aromatic ring atoms, which may in each case also be substituted by one or more radicals R'.
- Ar 51 , Ar 52 , Ar 53 are the same or different and represent a condensed aryl or heteroaryl ring having 6 aromatic ring atoms, which may each also be substituted by one or more radicals R'.
- R co on each occurrence represents a radical selected from H, D, a straight-chain alkyl group having 1 to 10, preferably 1 to 6 and more preferably 1 to 3 C atoms, which may be substituted by one or more radicals R', an aryl or heteroaryl group having 6 to 18 and preferably 6 to 12 aromatic ring atoms, each of which may be substituted by one or more radicals R', where two radicals R co together may form an aliphatic, aromatic or heteroaromatic ring system which is substituted by one or more radicals R'.
- R N0 on each occurrence represents a radical selected from an aromatic or heteroaromatic ring system having 5 to 60, preferably 5 to 40, more preferably 5 to 30 and even more preferably 5 to 18 aromatic ring atoms, which may in each case be substituted by one or more radicals R'.
- the at least one fluorescent emitter in the composition has a peak emission wavelength between 420-550 nm, preferably between 420-470 nm.
- Preferred fluorescent emitters are emitters selected from the emitter classes mentioned above.
- the at least one fluorescent emitter has a full width at half maximum (FWHM) ⁇ 50 nm, preferably FWHM ⁇ 40 nm, more preferably FWHM ⁇ 30 nm.
- FWHM full width at half maximum
- the at least one fluorescent emitter has a LIIMO of -2.1 eV to -2.5 eV, preferably from -2.2 eV to -2.4 eV, as defined by quantum chemical calculations.
- the at least one fluorescent emitter has a HOMO of -4.8 eV to -5.2 eV, preferably from -4.9 eV to -5.1 eV, as defined by quantum chemical calculations.
- the energy of the lowest singlet state Si of the fluorescent emitter is 2.65 eV to 2.9 eV, preferably 2.7 to 2.8 eV, more preferably 2.7 to 2.75 eV, as defined by quantum mechanical calculations.
- Suitable charge transport materials are, in addition to the compounds of formula (1), for example those in Y. Shirota et al., Chem. Rev. 2007, 107(4), 953-1010, or other materials as used in these layers according to the prior art.
- Aromatic amine compounds can be used.
- Other compounds that are preferably used in hole transport layers of the OLEDs according to the invention are in particular indenofluorenamine derivatives (e.g. according to WO 2006/122630 or
- WO 2006/100896 the amine derivatives disclosed in EP 1661888, hexaazatriphenylene derivatives (eg according to WO 01/049806), amine derivatives with fused aromatics (for example according to US 5,061,569), the amine derivatives disclosed in WO 95/09147, monobenzoindenofluorenamines (for example according to WO 08/006449), dibenzoindenofluorenamines (for example according to WO 07/140847), spirobifluorenamines (for example according to WO 2012/034627 or WO 2013/120577), fluorenamines (for example according to WO 2014/015937, WO 2014/015938, WO 2014/015935 and WO 2015/082056), spirodibenzopyranamines (for example according to WO 2013/083216), dihydroacridine derivatives (for example according to WO 2012/150001), Spirodibenzofurans and spirodibenzo
- the OLED according to the invention preferably comprises two or more different electron-transporting layers.
- the compound of formula (1) can be used in none, in one or more or in all electron-transporting layers.
- the compound of formula (1) is used in exactly one or exactly two electron-transporting layers, and other compounds are used in the other electron-transporting layers present.
- Other compounds that can be used in addition to the compounds of formula (1) are all materials that are used according to the state of the art as electron transport materials in the electron transport layer.
- Particularly suitable are aluminum complexes, e.g. Alqs, zirconium complexes, e.g. Zrq4, lithium complexes, e.g.
- Liq Liq, benzimidazole derivatives, triazine derivatives, pyrimidine derivatives, pyridine derivatives, pyrazine derivatives, quinoxaline derivatives, quinoline derivatives, oxadiazole derivatives, aromatic ketones, lactams, boranes, diazaphosphole derivatives and phosphine oxide derivatives.
- Other suitable materials are derivatives of the aforementioned compounds, as disclosed in JP 2000/053957, WO 2003/060956, WO 2004/028217, WO 2004/080975 and WO 2010/072300.
- the device is structured accordingly (depending on the application), contacted and finally sealed to exclude harmful influences from water and air.
- an organic electroluminescent device characterized in that one or more layers are coated using a sublimation process.
- the materials are vapor-deposited in vacuum sublimation systems at an initial pressure of less than 10' 5 mbar, preferably less than 10' 6 mbar. However, it is also possible for the initial pressure to be even lower, for example less than 10' 7 mbar.
- an organic electroluminescent device characterized in that one or more layers are coated using the OVPD (Organic Vapour Phase Deposition) method or with the aid of carrier gas sublimation.
- OVPD Organic Vapour Phase Deposition
- carrier gas sublimation The materials at a pressure between 10' 5 mbar and 1 bar.
- OVJP Organic Vapour Jet Printing
- the materials are applied directly through a nozzle and thus structured.
- an organic electroluminescent device characterized in that one or more layers are produced from solution, such as by spin coating, or using any printing method, such as screen printing, flexographic printing, offset printing, LITI (light induced thermal imaging, thermal transfer printing), ink-jet printing or nozzle printing. Soluble compounds are required for this, which are obtained, for example, by suitable substitution.
- hybrid processes are possible, in which, for example, one or more layers are applied from solution and one or more further layers are vapor-deposited.
- the electronic devices containing one or more compounds of formula (1) can be used in displays, as light sources in lighting applications and as light sources in medical and/or cosmetic applications (e.g. light therapy).
- the compounds according to the invention lead to long service lives. 2.
- the compounds according to the invention lead to high efficiencies, in particular to a high EQE.
- the reactant LS1 is reacted consecutively with two or three equivalents of the Grignard compound, the corresponding di- or triaryl-tris-triazolotriazines can be obtained. If mixtures of aryl bromides are used to synthesize triaryl-tris-triazolotriazines, the resulting product mixture can also be separated into pure components by chromatography. Organolithium compounds can also be used as an alternative to Grignard compounds.
- K3PO4 x H2O is used instead of K2CO3.
- the reaction mixture is allowed to cool and poured into 1500 ml of ice water, filter off the precipitated solid, wash it three times with 100 ml of water each time, three times with 100 ml of ethanol each time and dry in a vacuum.
- the crude product is purified chromatographically (Torrent column machine from A. Semrau). Yield: 26.5 g (61 mmol) 61%; Purity: approx. 97% according to 1 H-NMR.
- the following compounds can be prepared analogously by adjusting the stoichiometry of the reactants.
- Semrau and/or repeated hot extraction crystallization (usual organic solvents or combinations thereof, preferably acetonitrile-DCM, 1:3 to 3:1 vv) and fractional sublimation or annealing under high vacuum. Yield: 30.9 g (68 mmol) 68%; Purity: approx. 99.9% according to HPLC.
- the following compounds can be prepared analogously by adjusting the stoichiometry of the reactants.
- OLEDs according to the invention as well as OLEDs according to the prior art is carried out according to a general process according to WO 2004/058911, which is adapted to the conditions described here (layer thickness variation, materials used).
- the compounds B according to the invention can be used in the hole blocking layer (HBL) and the electron transport layer (ETL). All materials are thermally vapor-deposited in a vacuum chamber.
- the emission layer (EML) always consists of at least one matrix material (host material) SMB (see Table 1) and an emitting dopant (dopant, emitter) D, which is mixed into the matrix material or materials by co-evaporation in a certain volume proportion.
- a specification such as SMB:D (97%:3%) means that the material SMB is present in the layer in a volume proportion of 97% and the dopant D in a proportion of 3%.
- the electron transport layer can also consist of a mixture of two materials, see Table 1. The materials used to produce the OLEDs are shown in Table 5.
- the OLEDs are characterized as standard.
- the electroluminescence spectra, the current efficiency (measured in cd/A), the power efficiency (measured in Im/W) and the external quantum efficiency (EQE, measured in percent) are determined as a function of the luminance, calculated from current-voltage-luminance characteristics (IUL characteristics) assuming a Lambertian radiation characteristic.
- the EQE in (%) and the voltage in (V) are specified at a luminance of 1000 cd/m 2 .
- the OLEDs have the following layer structure: Substrate
- HIL Hole injection layer
- HTM1 Hole injection layer
- HTL Hole transport layer
- EBL Electron blocking layer
- EML Emission layer
- HBL Hole blocking layer
- ETL Electron transport layer
- EIL Electron injection layer
- the compounds B according to the invention can be used in the hole blocking layer (HBL), the electron transport layer (ETL) and in the emission layer (EML) as electron-conducting matrix material (host material) (eTMM).
- HBL hole blocking layer
- ETL electron transport layer
- EML emission layer
- all materials are thermally vapor-deposited in a vacuum chamber.
- the emission layer always consists of at least one or more matrix materials M and a phosphorescent dopant Ir, which is mixed into the matrix material or materials by co-evaporation in a certain volume proportion.
- a specification such as M1:M2:lr (55%:35%:10%) means that the material M1 is in a volume proportion of 55%, M2 in a volume proportion of 35% and Ir in a volume fraction of 10% in the layer.
- the electron transport layer can also consist of a mixture of two materials.
- Table 3 The materials used to manufacture the OLEDs are shown in Table 5.
- the OLEDs are characterized as standard.
- the electroluminescence spectra, the current efficiency (measured in cd/A), the power efficiency (measured in Im/W) and the external quantum efficiency (EQE, measured in percent) are determined as a function of the luminance, calculated from current-voltage-luminance characteristics (IUL characteristics) assuming a Lambertian radiation characteristic.
- the EQE in (%) and the voltage in (V) are specified at a luminance of 1000 cd/m 2 .
- the OLEDs have the following layer structure:
- HIL Hole injection layer made of HTM1 doped with 5% NDP-9 (commercially available from Novaled), 20 nm
- HTL Hole transport layer made of HTM1, 180 nm for blue, 50 nm for green, yellow and red
- Electron blocking layer see Table 3 Emission layer (EML), see Table 3 Hole blocking layer (HBL), see Table 3 Electron transport layer (ETL), see Table 3 Electron injection layer (EIL) made of ETM2, 1 nm Cathode made of aluminum, 100 nm
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Physics & Mathematics (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Optics & Photonics (AREA)
- Electroluminescent Light Sources (AREA)
Abstract
Description
TRIS[1,2,4]TRIAZOLO[1,5-A:r,5,-C:1",5"-E][1,3,5]TRIAZIN-DERIVATE ZUR VERWENDUNG IN ORGANISCHEN ELEKTROLUMINESZENZVORRICHTUNGEN TRIS[1,2,4]TRIAZOLO[1,5-A:r,5 , -C:1",5"-E][1,3,5]TRIAZINE DERIVATIVES FOR USE IN ORGANIC ELECTROLUMINESCENT DEVICES
Die vorliegende Erfindung betrifft Materialien für die Verwendung in elektronischen Vorrichtungen, insbesondere in organischen Elektrolumi- neszenzvorrichtungen, sowie elektronische Vorrichtungen, insbesondere organische Elektrolumineszenzvorrichtungen enthaltend diese Materialien. The present invention relates to materials for use in electronic devices, in particular in organic electroluminescent devices, and to electronic devices, in particular organic electroluminescent devices, containing these materials.
Elektronische Vorrichtungen, welche organische, metallorganische und/oder polymere Halbleiter enthalten, gewinnen zunehmend an Bedeutung, wobei diese aus Kostengründen und aufgrund ihrer Leistungs- fähigkeit in vielen kommerziellen Produkten eingesetzt werden. Als Bei- spiele seien hier Ladungstransportmatenalien auf organischer Basis (z.B. Lochtransporter auf Triarylamin-Basis) in Kopiergeräten, organische Leuchtdioden (OLEDs) in Anzeige- und Displayvorrichtungen oder orga- nische Photorezeptoren in Kopierern genannt. Organische Solarzellen (0- SC), organische Feldeffekt-Transistoren (O-FET), organische Dünnfilm- Transistoren (O-TFT), organische Schaltelemente (O-IC), organische optische Verstärker und organische Laserdioden (O-Laser) sind in einem fortgeschrittenen Entwicklungsstand und können in der Zukunft große Bedeutung erlangen. Electronic devices containing organic, organometallic and/or polymer semiconductors are becoming increasingly important, and are used in many commercial products for reasons of cost and performance. Examples include organic-based charge transport materials (e.g. triarylamine-based hole transporters) in copiers, organic light-emitting diodes (OLEDs) in indicators and display devices, or organic photoreceptors in copiers. Organic solar cells (O-SC), organic field-effect transistors (O-FET), organic thin-film transistors (O-TFT), organic switching elements (O-IC), organic optical amplifiers and organic laser diodes (O-lasers) are at an advanced stage of development and may become very important in the future.
Als elektronische Vorrichtungen im Sinne dieser Erfindung werden organische elektronische Vorrichtungen verstanden, welche organische Halbleitermatenalien als funktionelle Materialien enthalten. Insbesondere stehen die elektronischen Vorrichtungen für Elektrolumineszenzvor- richtungen wie OLEDs. Electronic devices in the sense of this invention are understood to be organic electronic devices which contain organic semiconductor materials as functional materials. In particular, the electronic devices are electroluminescent devices such as OLEDs.
Der Aufbau von OLEDs, in welchen organische Verbindungen als funktionelle Materialien verwendet werden, ist dem Fachmann aus dem Stand der Technik bekannt. Im Allgemeinen werden unter OLEDs elektronische Vorrichtungen verstanden, welche eine oder mehrere Schichten haben, welche organische Verbindungen umfassen, und beim Anlegen einer Spannung Licht emittieren. The structure of OLEDs in which organic compounds are used as functional materials is known to those skilled in the art. In general, OLEDs are understood to be electronic devices that have one or more layers that comprise organic compounds and emit light when a voltage is applied.
In elektronischen Vorrichtungen, insbesondere OLEDs, gibt es einen großen Bedarf, die Leistungsdaten, insbesondere Lebensdauer, Effizienz und Betriebsspannung zu verbessern. Für diese Aspekte konnte bisher keine zufriedenstellende Lösung gefunden werden. In electronic devices, especially OLEDs, there is a great need to improve the performance data, especially lifetime, efficiency and operating voltage. No satisfactory solution has yet been found for these aspects.
Elektronische Vorrichtungen umfassen üblicherweise Kathode, Anode und mindestens eine funktionale, bevorzugt emittierende Schicht. Außer diesen Schichten können sie noch weitere Schichten enthalten, beispiels- weise jeweils eine oder mehrere Lochinjektionsschichten, Lochtransport- schichten, Lochblockierschichten, Elektronentransportschichten, Elek- troneninjektionsschichten, Exzitonenblockierschichten, Elektronenblockier- schichten und/oder Ladungserzeugungsschichten (Charge-Generation Layers). Electronic devices usually comprise a cathode, an anode and at least one functional, preferably emitting layer. In addition to these layers, they can contain further layers, for example one or more hole injection layers, hole transport layers, hole blocking layers, electron transport layers, electron injection layers, exciton blocking layers, electron blocking layers and/or charge generation layers.
Aufgabe der vorliegenden Erfindung ist die Bereitstellung von Verbin- dungen, welche sich für den Einsatz in einer elektronischen Vorrichtung, insbesondere einer OLED, eignen, insbesondere als Material von Elektronentransportschichten und/oder als Hostmaterialien, und dort zu guten Eigenschaften führen. The object of the present invention is to provide compounds which are suitable for use in an electronic device, in particular an OLED, in particular as material of electron transport layers and/or as host materials, and which lead to good properties there.
Überraschend wurde gefunden, dass bestimmte, unten näher beschrie- bene Tristriazolotriazine diese Aufgabe lösen und sich gut für die Verwen- dung in elektronischen Vorrichtungen, insbesondere OLEDs eignen. Dabei weisen die OLEDs insbesondere eine lange Lebensdauer, eine hohe Effizienz und eine geringe Betriebsspannung auf. Diese Verbindungen sowie elektronische Vorrichtungen, insbesondere organische Elektro- lumineszenzvorrichtungen, welche diese Verbindungen enthalten, sind daher der Gegenstand der vorliegenden Erfindung. Surprisingly, it has been found that certain tristriazolotriazines, described in more detail below, solve this problem and are well suited for use in electronic devices, in particular OLEDs. The OLEDs in particular have a long service life, high efficiency and low operating voltage. These compounds and electronic devices, in particular organic electroluminescent devices, which contain these compounds are therefore the subject of the present invention.
Gegenstand der vorliegenden Erfindung ist eine Verbindung gemäß Formel (1 ), Formel (1) wobei für die verwendeten Symbole gilt: R ist bei jedem Auftreten gleich oder verschieden H, D, F, Cl, Br, I, OAr‘, SAr‘, B(OR1)2, CHO, C(=O)R1, CR1=C(R1)2, CN, C(=O)OR1, C(=O)NR1, Si(R1)3, Ge(R1)3, NO2, P(=O)(R1)2, OSO2R1, OR1, S(=O)R1, S(=O)2R1, SR1, eine geradkettige Alkylgruppe mit 1 bis 20 C-Atomen oder eine Alkenyl- oder Alkinylgruppe mit 2 bis 20 C- Atomen oder eine verzweigte oder zyklische Alkylgruppe mit 3 bis 20 C-Atomen, wobei die Alkyl-, Alkenyl- oder Alkinylgruppe jeweils mit einem oder mehreren Resten R1 substituiert sein kann, wobei eine oder mehrere nicht benachbarte CH2-Gruppen durch -R1C=CR1- , -C≡C-, Si(R1)2, CONR1, C=O, C=S, -C(=O)O-, P(=O)(R1), -O-, -S-, SO oder SO2 ersetzt sein können, oder ein aromatisches oder heteroaromatisches Ringsystem mit 5 bis 60 aromatischen Ring- atomen, bevorzugt mit 5 bis 40 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R1 substituiert sein kann; wobei falls R und zugehörige Reste mindestens ein aromatisches Ringsystem, umfassend mindestens ein Stickstoffatom mit drei Einfachbindungen umfassen, für jedes dieser aromatischen Ring- systeme gilt, dass jedes Stickstoffatom mit drei Einfachbindungen Teil mindestens eines Fünfrings ist und/oder Teil eines Sechsrings, umfassend mindestens ein weiteres Heteroatom oder eine C=O- Gruppe, ist; mit der Maßgabe, dass mindestens ein R ein aromatisches oder heteroaromatisches Ringsystem mit 5 bis 60 aromatischen Ring- atomen ist und mit der Maßgabe, dass nicht alle drei R gleichzeitig für eine Phenylgruppe stehen; The present invention relates to a compound according to formula (1), Formula (1) where the following applies to the symbols used: R is, identically or differently on each occurrence, H, D, F, Cl, Br, I, OAr', SAr', B(OR 1 )2, CHO, C(=O)R 1 , CR 1 =C(R 1 )2, CN, C(=O)OR 1 , C(=O)NR 1 , Si(R 1 )3, Ge(R 1 )3, NO2, P(=O)(R 1 )2, OSO2R 1 , OR 1 , S(=O)R 1 , S(=O)2R 1 , SR 1 , a straight-chain alkyl group having 1 to 20 C atoms or an alkenyl or alkynyl group having 2 to 20 C atoms or a branched or cyclic alkyl group having 3 to 20 C atoms, where the Alkyl, alkenyl or alkynyl group can each be substituted by one or more radicals R 1 , where one or more non-adjacent CH2 groups can be replaced by -R 1 C=CR 1 - , -C≡C-, Si(R 1 )2, CONR 1 , C=O, C=S, -C(=O)O-, P(=O)(R 1 ), -O-, -S-, SO or SO2, or an aromatic or heteroaromatic ring system with 5 to 60 aromatic ring atoms, preferably with 5 to 40 aromatic ring atoms, which can each be substituted by one or more radicals R 1 ; where if R and associated radicals comprise at least one aromatic ring system comprising at least one nitrogen atom with three single bonds, for each of these aromatic ring systems, each nitrogen atom with three single bonds is part of at least one five-membered ring and/or part of a six-membered ring comprising at least one further heteroatom or a C=O group; with the proviso that at least one R is an aromatic or heteroaromatic ring system with 5 to 60 aromatic ring atoms and with the proviso that not all three R simultaneously represent a phenyl group;
Ar' ist bei jedem Auftreten gleich oder verschieden ein aromatisches oder heteroaromatisches Ringsystem mit 5 bis 40 aromatischen Ringatomen, das durch einen oder mehrere Reste R1 substituiert sein kann, wobei zwei oder mehr R1 miteinander ein aromatisches oder heteroaromatisches Ringsystem bilden können; Ar' is on each occurrence, identically or differently, an aromatic or heteroaromatic ring system having 5 to 40 aromatic ring atoms which may be substituted by one or more radicals R 1 , where two or more R 1s may together form an aromatic or heteroaromatic ring system;
R1 ist bei jedem Auftreten gleich oder verschieden H, D, F, I, B(OR2)2, CHO, C(=O)R2, CR2=C(R2)2, CN, C(=O)OR2, Si(R2)3, Ge(R2)3, NO2, P(=O)(R2)2, OSO2R2, SR2, S(=O)R2, S(=O)2R2, eine geradkettige Alkylgruppe mit 1 bis 20 C-Atomen oder eine Alkenyl- oder Alkinyl- gruppe mit 2 bis 20 C-Atomen oder eine verzweigte oder zyklische Alkylgruppe mit 3 bis 20 C-Atomen, wobei die Alkyl-, Alkenyl- oder Alkinylgruppe jeweils mit einem oder mehreren Resten R2 substituiert sein kann und wobei eine oder mehrere CH2-Gruppen in den oben genannten Gruppen durch -R2C=CR2-, -C=C-, Si(R2)2, C=O, C=S, -C(=O)O-, CONR2, P(=O)(R2), -S-, SO oder SO2 ersetzt sein können und wobei ein oder mehrere H-Atome in den oben genannten Gruppen durch D, F, CI, Br, I, CN oder NO2 ersetzt sein können, oder ein aromatisches oder heteroaromatisches Ringsystem mit 5 bis 30 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R2 substituiert sein kann, wobei zwei oder mehr Reste R1 miteinander ein aliphatisches, heteroaliphatisches, aroma- tisches oder heteroaromatisches Ringsystem bilden können; R 1 is, identically or differently on each occurrence, H, D, F, I, B(OR 2 )2, CHO, C(=O)R 2 , CR 2 =C(R 2 ) 2 , CN, C(=O)OR 2 , Si(R 2 ) 3 , Ge(R 2 ) 3 , NO 2 , P(=O)(R 2 ) 2 , OSO2R 2 , SR 2 , S(=O)R 2 , S(=O) 2 R 2 , a straight-chain alkyl group having 1 to 20 C atoms or an alkenyl or alkynyl group having 2 to 20 C atoms or a branched or cyclic alkyl group having 3 to 20 C atoms, where the alkyl, alkenyl or alkynyl group is each substituted with one or more radicals R 2 can be substituted and where one or more CH2 groups in the abovementioned groups can be replaced by -R 2 C=CR 2 -, -C=C-, Si(R 2 )2, C=O, C=S, -C(=O)O-, CONR 2 , P(=O)(R 2 ), -S-, SO or SO2 and where one or more H atoms in the abovementioned groups can be replaced by D, F, CI, Br, I, CN or NO2, or an aromatic or heteroaromatic ring system having 5 to 30 aromatic ring atoms, each of which can be substituted by one or more radicals R 2 , where two or more radicals R 1 can form an aliphatic, heteroaliphatic, aromatic or heteroaromatic ring system with one another;
R2 ist bei jedem Auftreten gleich oder verschieden H, D, F, CN oder ein aliphatischer, aromatischer oder heteroaromatischer organischer Rest mit 1 bis 20 C-Atomen, in dem auch ein oder mehrere H-Atome durch D oder F ersetzt sein können; dabei können zwei oder mehr Substituenten R2 miteinander verknüpft sein und einen Ring bilden. R 2 is, identically or differently at each occurrence, H, D, F, CN or an aliphatic, aromatic or heteroaromatic organic radical having 1 to 20 C atoms, in which one or more H atoms may be replaced by D or F; two or more R 2 substituents may be linked to one another to form a ring.
Eine Arylgruppe im Sinne dieser Erfindung enthält 6 bis 40 C-Atome; eine Heteroarylgruppe im Sinne dieser Erfindung enthält 5 bis 40 C-Atome und mindestens ein Heteroatom, mit der Maßgabe, dass die Summe aus C-Atomen und Heteroatomen mindestens 5 ergibt. Die Heteroatome sind bevorzugt ausgewählt aus N, 0 und/oder S. Dabei wird unter einer Aryl- gruppe bzw. Heteroarylgruppe entweder ein einfacher aromatischer Zyklus, also Benzol, bzw. ein einfacher heteroaromatischer Zyklus, beispielsweise Pyridin, Pyrimidin, Thiophen, etc., oder eine kondensierte (anellierte) Aryl- oder Heteroarylgruppe, beispielsweise Naphthalin, Anthracen, Phenanthren, Chinolin, Isochinolin, etc., verstanden. Mitein- ander durch Einfachbindung verknüpfte Aromaten, wie zum Beispiel Biphenyl, werden dagegen nicht als Aryl- oder Heteroarylgruppe, sondern als aromatisches Ringsystem bezeichnet. An aryl group in the sense of this invention contains 6 to 40 C atoms; a heteroaryl group in the sense of this invention contains 5 to 40 C atoms and at least one heteroatom, with the proviso that the sum of C atoms and heteroatoms at least 5. The heteroatoms are preferably selected from N, 0 and/or S. An aryl group or heteroaryl group is understood to be either a simple aromatic ring, i.e. benzene, or a simple heteroaromatic ring, for example pyridine, pyrimidine, thiophene, etc., or a condensed (fused) aryl or heteroaryl group, for example naphthalene, anthracene, phenanthrene, quinoline, isoquinoline, etc. Aromatics linked to one another by a single bond, such as biphenyl, are not referred to as aryl or heteroaryl groups, but as aromatic ring systems.
Ein aromatisches Ringsystem im Sinne dieser Erfindung enthält 6 bis 60 C-Atome, bevorzugt 6 bis 40 C-Atome im Ringsystem. Ein heteroaroma- tisches Ringsystem im Sinne dieser Erfindung enthält 1 bis 60 C-Atome, bevorzugt 1 bis 40 C-Atome und mindestens ein Heteroatom im Ring- system, mit der Maßgabe, dass die Summe aus C-Atomen und Hetero- atomen mindestens 5 ergibt. Die Heteroatome sind bevorzugt ausgewählt aus N, O und/oder S. Unter einem aromatischen oder heteroaromatischen Ringsystem im Sinne dieser Erfindung soll ein System verstanden werden, das nicht notwendigerweise nur Aryl- oder Heteroarylgruppen enthält, sondern in dem auch mehrere Aryl- oder Heteroarylgruppen durch eine nicht-aromatische Einheit (bevorzugt weniger als 10 % der von H verschiedenen Atome), wie z. B. ein C-, N- oder O-Atom oder Carbonyl- gruppe, verbunden sein können. Ebenso sollen hierunter Systeme verstanden werden, in denen zwei oder mehr Aryl- bzw. Heteroaryl- gruppen direkt miteinander verknüpft sind, wie z. B. Biphenyl, Terphenyl, Bipyridin oder Phenylpyridin. So sollen beispielsweise auch Systeme wie Fluoren, 9,9‘-Spirobifluoren, 9,9-Diarylfluoren, Triarylamin, Diarylether, Stilben, etc. als aromatische Ringsysteme im Sinne dieser Erfindung ver- standen werden, und ebenso Systeme, in denen zwei oder mehrere Aryl- gruppen beispielsweise durch eine lineare oder zyklische Alkylgruppe oder durch eine Silylgruppe verbunden sind. Bevorzugte aromatische bzw. heteroaromatische Ringsysteme sind einfache Aryl- bzw. Heteroaryl- gruppen sowie Gruppen, in denen zwei oder mehr Aryl- bzw. Heteroaryl- gruppen direkt miteinander verknüpft sind, beispielsweise Biphenyl, Terphenyl, Quaterphenyl oder Bipyridin, sowie Fluoren oder Spirobi- fluoren. An aromatic ring system in the sense of this invention contains 6 to 60 C atoms, preferably 6 to 40 C atoms in the ring system. A heteroaromatic ring system in the sense of this invention contains 1 to 60 C atoms, preferably 1 to 40 C atoms and at least one heteroatom in the ring system, with the proviso that the sum of C atoms and heteroatoms is at least 5. The heteroatoms are preferably selected from N, O and/or S. An aromatic or heteroaromatic ring system in the sense of this invention is to be understood as a system which does not necessarily only contain aryl or heteroaryl groups, but in which several aryl or heteroaryl groups can also be connected by a non-aromatic unit (preferably less than 10% of the atoms other than H), such as a C, N or O atom or carbonyl group. This also includes systems in which two or more aryl or heteroaryl groups are directly linked to one another, such as biphenyl, terphenyl, bipyridine or phenylpyridine. For example, systems such as fluorene, 9,9'-spirobifluorene, 9,9-diarylfluorene, triarylamine, diaryl ether, stilbene, etc. are also to be understood as aromatic ring systems in the sense of this invention, as are systems in which two or more aryl groups are linked, for example, by a linear or cyclic alkyl group or by a silyl group. Preferred aromatic or heteroaromatic ring systems are simple aryl or heteroaryl groups and groups in which two or more aryl or heteroaryl groups are directly linked to one another, for example biphenyl, Terphenyl, quaterphenyl or bipyridine, as well as fluorene or spirobifluorene.
Ein elektronenreiches heteroaromatisches Ringsystem ist dadurch ge- kennzeichnet, dass es sich dabei um ein heteroaromatisches Ringsystem handelt, das keine elektronenarmen Heteroarylgruppen enthält. Eine elektronenarme Heteroarylgruppe ist eine Sechsring-Heteroarylgruppe mit mindestens einem Stickstoffatom oder eine Fünfring-Heteroarylgruppe mit mindestens zwei Heteroatomen, von denen eines ein Stickstoffatom und das andere Sauerstoff, Schwefel oder ein substituiertes Stickstoffatom ist, wobei an diese Gruppen jeweils noch weitere Aryl- oder Heteroaryl- gruppen ankondensiert sein können. Dagegen sind elektronenreiche Heteroarylgruppen Fünfring-Heteroarylgruppen mit genau einem Hetero- atom, ausgewählt aus Sauerstoff, Schwefel oder substituiertem Stickstoff, an welche noch weitere Arylgruppen und/oder weitere elektronenreiche Fünfring-Heteroarylgruppen ankondensiert sein können. So sind Beispiele für elektronenreiche Heteroarylgruppen Pyrrol, Furan, Thiophen, Indol, Benzofuran, Benzothiophen, Carbazol, Dibenzofuran, Dibenzothiophen oder Indenocarbazol. Eine elektronenreiche Heteroarylgruppe wird auch als elektronenreicher heteroaromatischer Rest bezeichnet. An electron-rich heteroaromatic ring system is characterized by the fact that it is a heteroaromatic ring system that does not contain any electron-poor heteroaryl groups. An electron-poor heteroaryl group is a six-membered ring heteroaryl group with at least one nitrogen atom or a five-membered ring heteroaryl group with at least two heteroatoms, one of which is a nitrogen atom and the other is oxygen, sulfur or a substituted nitrogen atom, where further aryl or heteroaryl groups can be condensed onto each of these groups. In contrast, electron-rich heteroaryl groups are five-membered ring heteroaryl groups with exactly one heteroatom selected from oxygen, sulfur or substituted nitrogen, to which further aryl groups and/or further electron-rich five-membered ring heteroaryl groups can be condensed. Examples of electron-rich heteroaryl groups are pyrrole, furan, thiophene, indole, benzofuran, benzothiophene, carbazole, dibenzofuran, dibenzothiophene or indenocarbazole. An electron-rich heteroaryl group is also called an electron-rich heteroaromatic residue.
Ein elektronenarmes heteroaromatisches Ringsystem ist dadurch gekenn- zeichnet, dass es mindestens eine elektronenarme Heteroarylgruppe enthält, und insbesondere bevorzugt keine elektronenreiche Heteroaryl- gruppen. An electron-poor heteroaromatic ring system is characterized in that it contains at least one electron-poor heteroaryl group, and particularly preferably no electron-rich heteroaryl groups.
Im Rahmen der vorliegenden Erfindung wird der Begriff Alkylgruppe als Oberbegriff sowohl für lineare oder verzweigte Alkylgruppen wie auch für zyklische Alkylgruppen verwendet. Analog werden die Begriffe Alkenyl- gruppe bzw. Alkinylgruppe als Oberbegriffe sowohl für lineare oder ver- zweigte Alkenyl- bzw. Alkinylgruppen, wie auch für zyklische Alkenyl- bzw. Alkinylgruppen verwendet. In the context of the present invention, the term alkyl group is used as a generic term for both linear or branched alkyl groups and for cyclic alkyl groups. Analogously, the terms alkenyl group and alkynyl group are used as generic terms for both linear or branched alkenyl or alkynyl groups, as well as for cyclic alkenyl or alkynyl groups.
Unter einer zyklischen Alkyl-, Alkoxy- oder Thioalkoxygruppe im Sinne dieser Erfindung wird eine monozyklische, eine bizyklische oder eine polyzyklische Gruppe verstanden. Im Rahmen der vorliegenden Erfindung werden unter einem aliphatischen Kohlenwasserstoffrest bzw. einer Alkylgruppe bzw. einer Alkenyl- oder Alkinylgruppe, die 1 bis 40 C-Atome enthalten kann, und in der auch einzelne H-Atome oder CH2-Gruppen durch die oben genannten Gruppen substituiert sein können, bevorzugt die Reste Methyl, Ethyl, n-Propyl, i- Propyl, n-Butyl, i-Butyl, s-Butyl, t-Butyl, 2-Methylbutyl, n-Pentyl, s-Pentyl, t- Pentyl, 2-Pentyl, neo-Pentyl, Cyclopentyl, n-Hexyl, s-Hexyl, t-Hexyl, 2-Hexyl, 3-Hexyl, neo-Hexyl, Cyclohexyl, 1-Methylcyclopentyl, 2-Methyl- pentyl, n-Heptyl, 2-Heptyl, 3-Heptyl, 4-Heptyl, Cycloheptyl, 1-Methylcyclo- hexyl, n-Octyl, Cyclooctyl, 2-Ethylhexyl, 1-Bicyclo[2,2,2]octyl, 2-Bicyclo- [2,2,2]octyl, 2-(2,6-Dimethyl)octyl, 3-(3,7-Dimethyl)octyl, Adamantyl, Trifluormethyl, Pentafluorethyl, 2,2,2-Trifluorethyl, 1,1-Dimethyl-n-hex-1-yl, 1,1-Dimethyl-n-hept-1-yl, 1,1-Dimethyl-n-oct-1-yl, 1,1-Dimethyl-n-dec-1-yl, 1,1-Dimethyl-n-dodec-1-yl, 1,1-Dimethyl-n-tetradec-1-yl, 1,1-Dimethyl-n- hexadec-1-yl, 1,1-Dimethyl-n-octadec-1-yl, 1,1-Diethyl-n-hex-1-yl, 1,1- Diethyl-n-hept-1-yl, 1,1-Diethyl-n-oct-1-yl, 1,1-Diethyl-n-dec-1-yl, 1,1- Diethyl-n-dodec-1-yl, 1,1-Diethyl-n-tetradec-1-yl, 1,1-Diethyl-n-hexadec-1- yl, 1,1-Diethyl-n-octadec-1-yl, 1-(n-Propyl)-cyclohex-1-yl, 1-(n-Butyl)- cyclohex-1-yl, 1-(n-Hexyl)-cyclohex-1-yl, 1-(n-Octyl)-cyclohex-1-yl und 1- (n-Decyl)-cyclohex-1-yl, Ethenyl, Propenyl, Butenyl, Pentenyl, Cyclo- pentenyl, Hexenyl, Cyclohexenyl, Heptenyl, Cycloheptenyl, Octenyl, Cyclooctenyl, Cyclooctadienyl, Ethinyl, Propinyl, Butinyl, Pentinyl, Hexinyl, Heptinyl oder Octinyl verstanden. Unter einer Alkoxygruppe OR1 mit 1 bis 40 C-Atomen werden bevorzugt Methoxy, Trifluormethoxy, Ethoxy, n-Propoxy, i-Propoxy, n-Butoxy, i-Butoxy, s-Butoxy, t-Butoxy, n-Pentoxy, s-Pentoxy, 2-Methylbutoxy, n-Hexoxy, Cyclohexyloxy, n-Heptoxy, Cyclo- heptyloxy, n-Octyloxy, Cyclooctyloxy, 2-Ethylhexyloxy, Pentafluorethoxy und 2,2,2-Trifluorethoxy verstanden. Unter einer Thioalkylgruppe SR1 mit 1 bis 40 C-Atomen werden insbesondere Methylthio, Ethylthio, n-Propyl- thio, i-Propylthio, n-Butylthio, i-Butylthio, s-Butylthio, t-Butylthio, n-Pentyl- thio, s-Pentylthio, n-Hexylthio, Cyclohexylthio, n-Heptylthio, Cycloheptyl- thio, n-Octylthio, Cyclooctylthio, 2-Ethylhexylthio, Trifluormethylthio, Pentafluorethylthio, 2,2,2-Trifluorethylthio, Ethenylthio, Propenylthio, Butenylthio, Pentenylthio, Cyclopentenylthio, Hexenylthio, Cyclohexenyl- thio, Heptenylthio, Cycloheptenylthio, Octenylthio, Cyclooctenylthio, Ethinylthio, Propinylthio, Butinylthio, Pentinylthio, Hexinylthio, Heptinylthio oder Octinylthio verstanden. Allgemein können Alkyl-, Alkoxy- oder Thio- alkylgruppen gemäß der vorliegenden Erfindung geradkettig, verzweigt oder zyklisch sein, wobei eine oder mehrere nicht-benachbarte CH2- Gruppen durch die oben genannten Gruppen ersetzt sein können; weiterhin können auch ein oder mehrere H-Atome durch D, F, CI, Br, I, CN oder NO2, bevorzugt D, F, CI oder CN, besonders bevorzugt D, F oder CN ersetzt sein. A cyclic alkyl, alkoxy or thioalkoxy group within the meaning of this invention is understood to mean a monocyclic, a bicyclic or a polycyclic group. In the context of the present invention, an aliphatic hydrocarbon radical or an alkyl group or an alkenyl or alkynyl group which can contain 1 to 40 C atoms and in which individual H atoms or CH2 groups can also be substituted by the abovementioned groups, preferably the radicals methyl, ethyl, n-propyl, i-propyl, n-butyl, i-butyl, s-butyl, t-butyl, 2-methylbutyl, n-pentyl, s-pentyl, t-pentyl, 2-pentyl, neo-pentyl, cyclopentyl, n-hexyl, s-hexyl, t-hexyl, 2-hexyl, 3-hexyl, neo-hexyl, cyclohexyl, 1-methylcyclopentyl, 2-methylpentyl, n-heptyl, 2-heptyl, 3-heptyl, 4-heptyl, cycloheptyl, 1-methylcyclohexyl, n-octyl, cyclooctyl, 2-ethylhexyl, 1-bicyclo[2,2,2]octyl, 2-bicyclo[2,2,2]octyl, 2-(2,6-dimethyl)octyl, 3-(3,7-Dimethyl)octyl, adamantyl, trifluoromethyl, pentafluoroethyl, 2,2,2-trifluoroethyl, 1,1-dimethyl-n-hex-1-yl, 1,1-dimethyl-n-hept-1-yl, 1,1-dimethyl-n-oct-1-yl, 1,1-dimethyl-n-dec-1-yl, 1,1-Dimethyl-n-dodec-1-yl, 1,1-Dimethyl-n-tetradec-1-yl, 1,1-Dimethyl-n-hexadec-1-yl, 1,1-Dimethyl-n-octadec-1-yl, 1,1-Diethyl-n-hex-1-yl, 1,1-Diethyl-n-hept-1-yl, 1,1-Diethyl-n-oct-1-yl, 1,1-Diethyl-n-dec-1-yl, 1,1- Diethyl-n-dodec-1-yl, 1,1-diethyl-n-tetradec-1-yl, 1,1-diethyl-n-hexadec-1-yl, 1,1-diethyl-n-octadec-1-yl, 1-(n-propyl)-cyclohex-1-yl, 1-(n-butyl)-cyclohex-1-yl, 1-(n-Hexyl)-cyclohex-1-yl, 1-(n-Octyl)-cyclohex-1-yl and 1-(n-Decyl)-cyclohex-1-yl, ethenyl, propenyl, butenyl, pentenyl, cyclopentenyl, hexenyl, cyclohexenyl, heptenyl, cycloheptenyl, octenyl, cyclooctenyl, cyclooctadienyl, ethynyl, propynyl, butynyl, pentinyl, Hexynyl, heptynyl or octynyl understood. Among an alkoxy group OR 1 with 1 to 40 carbon atoms, preference is given to methoxy, trifluoromethoxy, ethoxy, n-propoxy, i-propoxy, n-butoxy, i-butoxy, s-butoxy, t-butoxy, n-pentoxy, s-pentoxy, 2-methylbutoxy, n-hexoxy, cyclohexyloxy, n-heptoxy, cyclo- heptyloxy, n-octyloxy, cyclooctyloxy, 2-ethylhexyloxy, pentafluoroethoxy and 2,2,2-trifluoroethoxy. A thioalkyl group SR 1 with 1 to 40 carbon atoms includes, in particular, methylthio, ethylthio, n-propylthio, i-propylthio, n-butylthio, i-butylthio, s-butylthio, t-butylthio, n-pentylthio, s-pentylthio, n-hexylthio, Cyclohexylthio, n-heptylthio, cycloheptyl-thio, n-octylthio, cyclooctylthio, 2-ethylhexylthio, trifluoromethylthio, pentafluoroethylthio, 2,2,2-trifluoroethylthio, ethenylthio, propenylthio, butenylthio, pentenylthio, cyclopentenylthio, hexenylthio, Cyclohexenylthio, heptenylthio, cycloheptenylthio, octenylthio, cyclooctenylthio, Ethynylthio, propynylthio, butynylthio, pentynylthio, hexynylthio, heptynylthio or octynylthio. In general, alkyl, alkoxy or thioalkyl groups according to the present invention can be straight-chain, branched or cyclic, where one or more non-adjacent CH2 groups can be replaced by the abovementioned groups; furthermore, one or more H atoms can also be replaced by D, F, CI, Br, I, CN or NO2, preferably D, F, CI or CN, particularly preferably D, F or CN.
Unter einem aromatischen oder heteroaromatischen Ringsystem mit 5 - 60 aromatischen Ringatomen, vorzugsweise 5 - 40 aromatischen Ring- atomen, welches noch jeweils mit den oben genannten Resten oder einem Kohlenwasserstoffrest substituiert sein kann und welches über beliebige Positionen am Aromaten bzw. Heteroaromaten verknüpft sein kann, werden insbesondere Gruppen verstanden, die abgeleitet sind von Benzol, Naphthalin, Anthracen, Benzanthracen, Phenanthren, Pyren, Chrysen, Perylen, Fluoranthen, Naphthacen, Pentacen, Benzpyren, Biphenyl, Biphenylen, Terphenyl, Triphenylen, Fluoren, Spirobifluoren, Dihydrophenanthren, Dihydropyren, Tetrahydropyren, cis- oder trans- Indenofluoren, cis- oder trans-lndenocarbazol, cis- oder trans-lndolo- carbazol, cis- oder trans-Monobenzoindenofluoren, cis- oder trans- Dibenzoindenofluoren, Truxen, Isotruxen, Spirotruxen, Spiroisotruxen, Furan, Benzofuran, Isobenzofuran, Dibenzofuran, Thiophen, Benzothio- phen, Isobenzothiophen, Dibenzothiophen, Pyrrol, Indol, Isoindol, Carba- zol, Pyridin, Chinolin, Isochinolin, Acridin, Phenanthridin, Benzo-5,6-chino- lin, Benzo-6,7-chinolin, Benzo-7,8-chinolin, Phenothiazin, Phenoxazin, Pyrazol, Indazol, Imidazol, Benzimidazol, Naphthimidazol, Phenanthrimi- dazol, Pyridimidazol, Pyrazinimidazol, Chinoxalinimidazol, Oxazol, Benz- oxazol, Naphthoxazol, Anthroxazol, Phenanthroxazol, Isoxazol, 1 ,2- Thiazol, 1 ,3-Thiazol, Benzothiazol, Pyridazin, Hexaazatriphenylen, Benzo- pyridazin, Pyrimidin, Benzpyrimidin, Chinoxalin, 1 ,5-Diazaanthracen, 2,7- Diazapyren, 2,3-Diazapyren, 1 ,6-Diazapyren, 1 ,8-Diazapyren, 4,5-Diaza- pyren, 4,5,9, 10-Tetraazaperylen, Pyrazin, Phenazin, Phenoxazin, Pheno- thiazin, Fluorubin, Naphthyridin, Azacarbazol, Benzocarbolin, Phenan- throlin, 1 ,2,3-Triazol, 1 ,2,4-Triazol, Benzotriazol, 1 ,2,3-Oxadiazol, 1 ,2,4- Oxadiazol, 1 ,2,5-Oxadiazol, 1 ,3,4-Oxadiazol, 1 ,2,3-Thiadiazol, 1 ,2,4-Thia- diazol, 1,2,5-Thiadiazol, 1,3,4-Thiadiazol, 1,3,5-Triazin, 1,2,4-Triazin, 1,2,3-Triazin, Tetrazol, 1,2,4,5-Tetrazin, 1,2,3,4-Tetrazin, 1,2,3,5-Tetrazin, Purin, Pteridin, Indolizin und Benzothiadiazol oder Gruppen, die abgeleitet sind von Kombinationen dieser Systeme. Diese Gruppen können auch deuteriert sein. Unter der Formulierung, dass zwei oder mehr Reste miteinander ein Ring- system bilden können, soll im Rahmen der vorliegenden Beschreibung unter anderem verstanden werden, dass die beiden Reste miteinander durch eine chemische Bindung unter formaler Abspaltung von zwei Wasserstoffatomen verknüpft sind. Dies wird durch das folgende Schema verdeutlicht: Weiterhin soll unter der oben genannten Formulierung aber auch ver- standen werden, dass für den Fall, dass einer der beiden Reste Wasser- stoff darstellt, der zweite Rest unter Bildung eines Rings an die Position, an die das Wasserstoffatom gebunden war, bindet. Dies soll durch das folgende Schema verdeutlicht werden: Dabei bedeutet Teil eines Fünfrings für ein Stickstoffatom, dass dieses Stickstoffatom mit vier weiteren Atomen einen Fünfring bildet, wie bei- spielsweise im Carbazol. Im Falle eines Sechsrings ist das Stickstoffatom Teil eines Sechsrings, wie beispielsweise bei Dibenzo[1,4]oxazin. In einer bevorzugten Ausführungsform ist in allen R und den zugehörigen Gruppen, wenn diese ein Stickstoffatom mit drei Einfachbindungen enthält, jedes Stickstoffatom mit drei Einfachbindungen mindestens Teil eines Fünfrings. An aromatic or heteroaromatic ring system with 5 - 60 aromatic ring atoms, preferably 5 - 40 aromatic ring atoms, which can be substituted by the above-mentioned radicals or a hydrocarbon radical and which can be linked to the aromatic or heteroaromatic via any position, is understood to mean in particular groups which are derived from benzene, naphthalene, anthracene, benzanthracene, phenanthrene, pyrene, chrysene, perylene, fluoranthene, naphthacene, pentacene, benzopyrene, biphenyl, biphenylene, terphenyl, triphenylene, fluorene, spirobifluorene, dihydrophenanthrene, dihydropyrene, tetrahydropyrene, cis- or trans- indenofluorene, cis- or trans- indenocarbazole, cis- or trans- indolocarbazole, cis- or trans- monobenzoindenofluorene, cis- or trans- Dibenzoindenofluorene, truxene, isotruxene, spirotruxene, spiroisotruxene, furan, benzofuran, isobenzofuran, dibenzofuran, thiophene, benzothiophene, isobenzothiophene, dibenzothiophene, pyrrole, indole, isoindole, carbazole, pyridine, quinoline, isoquinoline, acridine, Phenanthridine, benzo-5,6-quinoline, benzo-6,7-quinoline, benzo-7,8-quinoline, phenothiazine, phenoxazine, pyrazole, indazole, imidazole, benzimidazole, naphthimidazole, phenanthrimidazole, pyridimidazole, pyrazinimidazole, quinoxalinimidazole, oxazole, benzoxazole, naphthoxazole, Anthroxazole, phenanthroxazole, isoxazole, 1,2-thiazole, 1 ,3-thiazole, benzothiazole, pyridazine, hexaazatriphenylene, benzopyridazine, pyrimidine, benzopyrimidine, quinoxaline, 1,5-diazaanthracene, 2,7-diazapyrene, 2,3-diazapyrene, 1,6-diazapyrene, 1,8-diazapyrene, 4,5-diazapyrene, 4,5,9, 10-tetraazaperylene, pyrazine, phenazine, phenoxazine, phenothiazine, fluorubin, naphthyridine, azacarbazole, benzocarboline, phenanthroline, 1,2,3-triazole, 1,2,4-triazole, benzotriazole, 1 ,2,3-oxadiazole, 1, 2,4-oxadiazole, 1 ,2,5-oxadiazole, 1,3,4-oxadiazole, 1,2,3-thiadiazole, 1,2,4-thiadiazole diazole, 1,2,5-thiadiazole, 1,3,4-thiadiazole, 1,3,5-triazine, 1,2,4-triazine, 1,2,3-triazine, tetrazole, 1,2,4,5-tetrazine, 1,2,3,4-tetrazine, 1,2,3,5-tetrazine, purine, pteridine, indolizine and benzothiadiazole or groups derived from combinations of these systems. These groups can also be deuterated. The phrase that two or more residues can form a ring system with one another is to be understood in the context of the present description to mean, among other things, that the two residues are linked to one another by a chemical bond with the formal elimination of two hydrogen atoms. This is illustrated by the following scheme: Furthermore, the above formulation should also be understood to mean that if one of the two residues represents hydrogen, the second residue binds to the position to which the hydrogen atom was bound, forming a ring. This is illustrated by the following scheme: In this context, part of a five-membered ring for a nitrogen atom means that this nitrogen atom forms a five-membered ring with four other atoms, as in carbazole, for example. In the case of a six-membered ring, the nitrogen atom is part of a six-membered ring, as in dibenzo[1,4]oxazine, for example. In a preferred embodiment, all R and the associated groups, if they contain a nitrogen atom with three single bonds, contains, each nitrogen atom with three single bonds is at least part of a five-membered ring.
In einer bevorzugten Ausführungsform der Erfindung enthalten die Gruppen R keine substituierten oder unsubstituierten Aminogruppen. Bevorzugt enthält die Gruppe R somit keine Triarylaminogruppen, kann aber beispielsweise Carbazolgruppen enthalten, also Heteroarylgruppen, die Stickstoff enthalten. In a preferred embodiment of the invention, the groups R do not contain any substituted or unsubstituted amino groups. The group R therefore preferably does not contain any triarylamino groups, but can contain, for example, carbazole groups, i.e. heteroaryl groups that contain nitrogen.
In einer bevorzugten Ausführungsform umfasst mindestens ein R ein aromatisches oder heteroaromatisches Ringsystem mit 9 bis 60 aroma- tischen Ringatomen, bevorzugt 10 bis 40 aromatischen Ringatomen, ganz besonders bevorzugt 12 bis 30 aromatischen Ringatomen. In a preferred embodiment, at least one R comprises an aromatic or heteroaromatic ring system having 9 to 60 aromatic ring atoms, preferably 10 to 40 aromatic ring atoms, very particularly preferably 12 to 30 aromatic ring atoms.
In einer bevorzugten Ausführungsform umfassen R und die zugehörigen Gruppen keine kondensierten Arylgruppen. In a preferred embodiment, R and the associated groups do not comprise fused aryl groups.
In einer bevorzugten Ausführungsform der Erfindung sind mindestens zwei Substituenten R gleich. Bevorzugte Ausführungsformen der Erfindung sind somit Verbindungen, in denen zwei Substituenten R gleich und der dritte Substituent R von den anderen Substituenten R verschieden ist, und Verbindungen, in denen alle drei Substituenten R gleich sind. In a preferred embodiment of the invention, at least two substituents R are identical. Preferred embodiments of the invention are thus compounds in which two substituents R are identical and the third substituent R is different from the other substituents R, and compounds in which all three substituents R are identical.
Im Folgenden werden bevorzugte Substituenten R, Ar‘, R1 und R2 beschrieben. In einer besonders bevorzugten Ausführungsform der Erfin- dung treten die nachfolgend genannten Bevorzugungen für R, Ar‘, R1 und R2 gleichzeitig auf und gelten für die Strukturen der Formel (1 ) sowie für alle oben aufgeführten bevorzugten Ausführungsformen. Preferred substituents R, Ar', R 1 and R 2 are described below. In a particularly preferred embodiment of the invention, the preferences for R, Ar', R 1 and R 2 mentioned below occur simultaneously and apply to the structures of the formula (1) as well as to all preferred embodiments listed above.
In einer bevorzugten Ausführungsform der Erfindung ist R bei jedem Auf- treten gleich oder verschieden ausgewählt aus der Gruppe bestehend aus H, D, F, CN, OR1, einer geradkettigen Alkylgruppe mit 1 bis 10 C-Atomen oder einer Alkenylgruppe mit 2 bis 10 C-Atomen oder einer verzweigten oder zyklischen Alkylgruppe mit 3 bis 10 C-Atomen, wobei die Alkyl- bzw. Alkenylgruppe jeweils mit einem oder mehreren Resten R1 substituiert sein kann, bevorzugt jedoch unsubstituiert ist, und wobei eine oder mehrere nicht benachbarte CH2-Gruppen durch 0 ersetzt sein können, oder einem aromatischen oder heteroaromatischen Ringsystem mit 6 bis 30 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R1 substituiert sein kann. Besonders bevorzugt ist R bei jedem Auf- treten gleich oder verschieden ausgewählt aus der Gruppe bestehend aus H, F, CN, einer geradkettigen Alkylgruppe mit 1 bis 6 C-Atomen, insbeson- dere mit 1 , 2, 3 oder 4 C-Atomen, oder einer verzweigten oder zyklischen Alkylgruppe mit 3 bis 6 C-Atomen, wobei die Alkylgruppe jeweils mit einem oder mehreren Resten R1 substituiert sein kann, bevorzugt aber unsubsti- tuiert ist, oder einem aromatischen oder heteroaromatischen Ringsystem mit 6 bis 24 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R1, bevorzugt nicht-aromatische Reste R1, substituiert sein kann. Ganz besonders bevorzugt ist R bei jedem Auftreten gleich oder verschieden ausgewählt aus der Gruppe bestehend aus H, D, CN oder einem aromatischen oder heteroaromatischen Ringsystem mit 6 bis 24 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R1, bevorzugt nicht-aromatische Reste R1, substituiert sein kann. Insbe- sondere bevorzugt sind alle Reste R gleich oder verschieden bei jedem Auftreten gewählt aus einem aromatischen oder heteroaromatischen Ring- system mit 6 bis 24 aromatischen Ringatomen, insbesondere mit 6 bis 18 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R1, bevorzugt nicht-aromatische Reste R1 substituiert sein kann. In a preferred embodiment of the invention, R is selected on each occurrence, identically or differently, from the group consisting of H, D, F, CN, OR 1 , a straight-chain alkyl group having 1 to 10 C atoms or an alkenyl group having 2 to 10 C atoms or a branched or cyclic alkyl group having 3 to 10 C atoms, where the alkyl or alkenyl group may each be substituted by one or more radicals R 1 , but is preferably unsubstituted, and where one or several non-adjacent CH2 groups can be replaced by 0, or an aromatic or heteroaromatic ring system with 6 to 30 aromatic ring atoms, each of which can be substituted by one or more radicals R 1 . Particularly preferably, R is selected on each occurrence, identically or differently, from the group consisting of H, F, CN, a straight-chain alkyl group having 1 to 6 C atoms, in particular having 1, 2, 3 or 4 C atoms, or a branched or cyclic alkyl group having 3 to 6 C atoms, where the alkyl group can be substituted in each case by one or more radicals R 1 , but is preferably unsubstituted, or an aromatic or heteroaromatic ring system having 6 to 24 aromatic ring atoms, which can be substituted in each case by one or more radicals R 1 , preferably non-aromatic radicals R 1 . Most preferably, R is selected, identically or differently on each occurrence, from the group consisting of H, D, CN or an aromatic or heteroaromatic ring system having 6 to 24 aromatic ring atoms, each of which may be substituted by one or more radicals R 1 , preferably non-aromatic radicals R 1 . Most preferably, all radicals R are selected, identically or differently on each occurrence, from an aromatic or heteroaromatic ring system having 6 to 24 aromatic ring atoms, in particular having 6 to 18 aromatic ring atoms, each of which may be substituted by one or more radicals R 1 , preferably non-aromatic radicals R 1 .
Geeignete aromatische bzw. heteroaromatische Ringsysteme R sind aus- gewählt aus Phenyl, Biphenyl, insbesondere ortho-, meta- oder para- Biphenyl, Terphenyl, insbesondere ortho-, meta-, para- oder verzweigtem Terphenyl, Quaterphenyl, insbesondere ortho-, meta-, para- oder ver- zweigtem Quaterphenyl, Fluoren, welches über die 1 -, 2-, 3- oder 4- Position verknüpft sein kann, Spirobifluoren, welches über die 1 -, 2-, 3- oder 4-Position verknüpft sein kann, Naphthalin, welches über die 1 - oder 2-Position verknüpft sein kann, Indol, Benzofuran, Benzothiophen, welches über die 1 -, 2-, 3- oder 4-Position verknüpft sein kann, Dibenzo- furan, Carbazol, welches über die 1 -, 2-, 3- oder 4-Position verknüpft sein kann, Dibenzothiophen, welches über die 1 -, 2-, 3- oder 4-Position ver- knüpft sein kann, Indenocarbazol, Indolocarbazol, Pyridin, Pyrimidin, Pyrazin, Pyridazin, Triazin, Chinolin, Chinazolin, Benzimidazol, Phenanthren, Triphenylen oder einer Kombination aus zwei oder drei dieser Gruppen, welche jeweils mit einem oder mehreren Resten R1 sub- stituiert sein können. Wenn R für eine Heteroarylgruppe, insbesondere für Triazin, Pyrimidin oder Chinazolin steht, können auch aromatische oder heteroaromatische Reste R1 an dieser Heteroarylgruppe bevorzugt sein. Suitable aromatic or heteroaromatic ring systems R are selected from phenyl, biphenyl, in particular ortho-, meta- or para-biphenyl, terphenyl, in particular ortho-, meta-, para- or branched terphenyl, quaterphenyl, in particular ortho-, meta-, para- or branched quaterphenyl, fluorene, which can be linked via the 1-, 2-, 3- or 4-position, spirobifluorene, which can be linked via the 1-, 2-, 3- or 4-position, naphthalene, which can be linked via the 1- or 2-position, indole, benzofuran, benzothiophene, which can be linked via the 1-, 2-, 3- or 4-position, dibenzofuran, carbazole, which can be linked via the 1-, 2-, 3- or 4-position, dibenzothiophene, which can be linked via the 1-, 2-, 3- or 4-position, indenocarbazole, indolocarbazole, pyridine, pyrimidine, pyrazine, pyridazine, triazine, quinoline, quinazoline, benzimidazole, Phenanthrene, triphenylene or a combination of two or three of these groups, each of which may be substituted by one or more radicals R 1 . If R is a heteroaryl group, in particular triazine, pyrimidine or quinazoline, aromatic or heteroaromatic radicals R 1 on this heteroaryl group may also be preferred.
Dabei sind die Gruppen R, wenn sie für ein aromatisches bzw. heteroaro- matisches Ringsystem stehen, bevorzugt gewählt aus den Gruppen der folgenden Formeln R-1 bis R-184, The groups R, when they represent an aromatic or heteroaromatic ring system, are preferably selected from the groups of the following formulas R-1 to R-184,
wobei R1 die oben genannten Bedeutungen aufweist, die gestrichelte Bindung die Bindung zum Triazolotriazin in Formel (1 ) darstellt und weiterhin gilt: where R 1 has the meanings given above, the dashed bond represents the bond to the triazolotriazine in formula (1 ) and furthermore:
Ar3 ist bei jedem Auftreten gleich oder verschieden ein bivalentes aroma- tisches oder heteroaromatisches Ringsystem mit 6 bis 18 aroma- tischen Ringatomen, welches jeweils mit einem oder mehreren Resten R1 substituiert sein kann; Ar 3 is at each occurrence, identically or differently, a bivalent aromatic or heteroaromatic ring system with 6 to 18 aromatic tic ring atoms, each of which may be substituted by one or more radicals R 1 ;
A1 ist bei jedem Auftreten gleich oder verschieden BR1, C(R1)2, C=O, NR1, 0 oder S, wobei A1 in den Formeln R-150, R-151 und R-152 für BR1, C=O, NR1, 0 oder S steht; A 1 is, identically or differently at each occurrence, BR 1 , C(R 1 )2, C=O, NR 1 , 0 or S, where A 1 in the formulas R-150, R-151 and R-152 stands for BR 1 , C=O, NR 1 , 0 or S;
A2 ist bei jedem Auftreten gleich oder verschieden C(R1)2, NR1, 0 oder S; p ist 0 oder 1 , wobei p = 0 bedeutet, dass die Gruppe Ar3 nicht vorhan- den ist und dass die entsprechende aromatische bzw. heteroaroma- tische Gruppe direkt an das zugehörige Atom, beispielsweise ein Kohlenstoffatom oder an ein Heteroatom wie ein Stickstoff gebunden ist, wobei, im Fall von Bindung an ein Heteroatom, für die Formeln R- 44, R-49, R-53, R-57, R-58, R-62, R-66, R-70, R-71 , R-112, R-152 bis R-160, R-167, R-172, R-177, R-182 p gleich 1 gilt; r ist 0 oder 1 , wobei r = 0 bedeutet, dass an dieser Position keine Gruppe A1 gebunden ist und an die entsprechenden Kohlenstoffatome stattdessen Reste R1 gebunden sind. A 2 is, identically or differently on each occurrence, C(R 1 ) 2, NR 1 , 0 or S; p is 0 or 1, where p = 0 means that the group Ar 3 is not present and that the corresponding aromatic or heteroaromatic group is bonded directly to the associated atom, for example a carbon atom or to a heteroatom such as a nitrogen, where, in the case of bonding to a heteroatom, for the formulas R- 44, R-49, R-53, R-57, R-58, R-62, R-66, R-70, R-71 , R-112, R-152 to R-160, R-167, R-172, R-177, R-182 p is equal to 1; r is 0 or 1 , where r = 0 means that no group A 1 is bonded at this position and residues R 1 are bonded to the corresponding carbon atoms instead.
In einer bevorzugten Ausführungsform umfasst Ar3 bivalente aromatische oder heteroaromatische Ringsysteme basierend auf den Gruppen der R-1 bis R-184, wobei p gleich 0 gilt und die gestrichelte Bindung und ein R1 für die Bindung zur aromatischen oder heteroaromatischen Gruppe nach R-1 bis R-184 steht. In a preferred embodiment, Ar 3 comprises divalent aromatic or heteroaromatic ring systems based on the groups R-1 to R-184, where p is 0 and the dashed bond and an R 1 represent the bond to the aromatic or heteroaromatic group after R-1 to R-184.
Wenn die oben genannten Gruppen R-1 bis R-184 für R mehrere Gruppen A1 aufweisen, so kommen hierfür alle Kombinationen aus der Definition von A1 in Frage. Bevorzugte Ausführungsformen sind dann solche, in denen eine Gruppe A1 für C(R1)2, NR1, 0 oder S und die andere Gruppe A1 für C(R1)2, NR1, 0 oder S steht. If the abovementioned groups R-1 to R-184 have several groups A 1 for R, all combinations from the definition of A 1 are possible. Preferred embodiments are then those in which one group A 1 stands for C(R 1 ) 2, NR 1 , 0 or S and the other group A 1 stands for C(R 1 ) 2 , NR 1 , 0 or S.
Wenn A1 für NR1 steht, steht der Substituent R1, der an das Stickstoffatom gebunden ist, bevorzugt für ein aromatisches oder heteroaromatisches Ringsystem mit 5 bis 24 aromatischen Ringatomen, welches auch durch einen oder mehrere Reste R2 substituiert sein kann. In einer besonders bevorzugten Ausführungsform steht dieser Substituent R1 gleich oder ver- schieden bei jedem Auftreten für ein aromatisches oder heteroaroma- tisches Ringsystem mit 6 bis 24 aromatischen Ringatomen, bevorzugt mit 6 bis 12 aromatischen Ringatomen, und welches jeweils auch durch einen oder mehrere Reste R2 substituiert sein kann. Besonders bevorzugt sind Phenyl, Biphenyl, Terphenyl und Quaterphenyl mit Verknüpfungsmustern, wie vorne für R-1 bis R-35 aufgeführt, wobei diese Strukturen durch einen oder mehrere Reste R1 substituiert sein können, bevorzugt aber unsubsti- tuiert sind. If A 1 is NR 1 , the substituent R 1 which is bonded to the nitrogen atom preferably represents an aromatic or heteroaromatic ring system having 5 to 24 aromatic ring atoms, which can also be represented by one or more radicals R 2 can be substituted. In a particularly preferred embodiment, this substituent R 1 is the same or different on each occurrence and represents an aromatic or heteroaromatic ring system having 6 to 24 aromatic ring atoms, preferably having 6 to 12 aromatic ring atoms, and which can each also be substituted by one or more radicals R 2. Particularly preferred are phenyl, biphenyl, terphenyl and quaterphenyl with linkage patterns as listed above for R-1 to R-35, where these structures can be substituted by one or more radicals R 1 , but are preferably unsubstituted.
Wenn A1 für C(R1)2 steht, stehen die Substituenten R1, die an dieses Kohlenstoffatom gebunden sind, bevorzugt gleich oder verschieden bei jedem Auftreten für eine lineare Alkylgruppe mit 1 bis 10 C-Atomen oder für eine verzweigte oder zyklische Alkylgruppe mit 3 bis 10 C-Atomen oder für ein aromatisches oder heteroaromatisches Ringsystem mit 5 bis 24 aromatischen Ringatomen, welches auch durch einen oder mehrere Reste R2 substituiert sein kann. Ganz besonders bevorzugt steht R1 für eine Methylgruppe oder für eine Phenylgruppe. Dabei können die Reste R1 auch miteinander ein Ringsystem bilden, was zu einem Spirosystem führt. If A 1 is C(R 1 ) 2 , the substituents R 1 which are bonded to this carbon atom are preferably identical or different on each occurrence and represent a linear alkyl group having 1 to 10 C atoms or a branched or cyclic alkyl group having 3 to 10 C atoms or an aromatic or heteroaromatic ring system having 5 to 24 aromatic ring atoms, which may also be substituted by one or more radicals R 2 . R 1 is very particularly preferably a methyl group or a phenyl group. The radicals R 1 can also form a ring system with one another, resulting in a spiro system.
In einer weiteren bevorzugten Ausführungsform der Erfindung ist Ar' gleich oder verschieden bei jedem Auftreten ein aromatisches oder heteroaroma- tisches Ringsystem mit 6 bis 30 aromatischen Ringatomen, besonders bevorzugt mit 6 bis 24 aromatischen Ringatomen und ganz besonders bevorzugt mit 6 bis 13 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R1 substituiert sein kann. In a further preferred embodiment of the invention, Ar' is the same or different on each occurrence and is an aromatic or heteroaromatic ring system having 6 to 30 aromatic ring atoms, particularly preferably having 6 to 24 aromatic ring atoms and very particularly preferably having 6 to 13 aromatic ring atoms, which may in each case be substituted by one or more radicals R 1 .
In einer weiteren bevorzugten Ausführungsform der Erfindung ist R1 gleich oder verschieden bei jedem Auftreten ausgewählt aus der Gruppe be- stehend aus H, D, F, CN, einer geradkettigen Alkylgruppe mit 1 bis 10 C-Atomen oder einer Alkenylgruppe mit 2 bis 10 C-Atomen oder einer verzweigten oder zyklischen Alkylgruppe mit 3 bis 10 C-Atomen, wobei die Alkyl- bzw. Alkenylgruppe jeweils mit einem oder mehreren Resten R2 substituiert sein kann, oder einem aromatischen oder heteroaromatischen Ringsystem mit 6 bis 30 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R2 substituiert sein kann; dabei können zwei oder mehrere Reste R1 miteinander ein aliphatisches Ringsystem bilden. In einer besonders bevorzugten Ausführungsform der Erfindung ist R1 gleich oder verschieden bei jedem Auftreten ausgewählt aus der Gruppe bestehend aus H, D, einer geradkettigen Alkylgruppe mit 1 bis 6 C-Atomen, insbesondere mit 1 , 2, 3 oder 4 C-Atomen, oder einer ver- zweigten oder zyklischen Alkylgruppe mit 3 bis 6 C-Atomen, wobei die Alkylgruppe mit einem oder mehreren Resten R2 substituiert sein kann, bevorzugt aber unsubstituiert ist, oder einem aromatischen oder hetero- aromatischen Ringsystem mit 6 bis 24 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R2 substituiert sein kann. In a further preferred embodiment of the invention, R 1 is the same or different on each occurrence and is selected from the group consisting of H, D, F, CN, a straight-chain alkyl group having 1 to 10 C atoms or an alkenyl group having 2 to 10 C atoms or a branched or cyclic alkyl group having 3 to 10 C atoms, where the alkyl or alkenyl group may each be substituted by one or more radicals R 2 , or an aromatic or heteroaromatic ring system having 6 to 30 aromatic ring atoms, each of which is substituted by one or more radicals R 2 can be substituted; two or more radicals R 1 can form an aliphatic ring system with one another. In a particularly preferred embodiment of the invention, R 1 is the same or different on each occurrence and is selected from the group consisting of H, D, a straight-chain alkyl group having 1 to 6 C atoms, in particular having 1, 2, 3 or 4 C atoms, or a branched or cyclic alkyl group having 3 to 6 C atoms, where the alkyl group can be substituted by one or more radicals R 2 , but is preferably unsubstituted, or an aromatic or heteroaromatic ring system having 6 to 24 aromatic ring atoms, each of which can be substituted by one or more radicals R 2 .
In einer weiteren bevorzugten Ausführungsform der Erfindung ist R2 gleich oder verschieden bei jedem Auftreten H, D, CN, F, eine Alkylgruppe mit 1 bis 4 C-Atomen oder eine Arylgruppe mit 6 bis 10 C-Atomen, welche mit einer Alkylgruppe mit 1 bis 4 C-Atomen substituiert sein kann, bevorzugt aber unsubstituiert ist. In a further preferred embodiment of the invention, R 2 is the same or different on each occurrence and is H, D, CN, F, an alkyl group having 1 to 4 C atoms or an aryl group having 6 to 10 C atoms, which may be substituted by an alkyl group having 1 to 4 C atoms, but is preferably unsubstituted.
In einer weiteren bevorzugten Ausführungsform der Erfindung sind alle Reste R1, soweit sie für ein aromatisches oder heteroaromatisches Ring- system, bzw. R2 soweit sie für aromatische oder heteroaromatische Gruppen stehen, ausgewählt aus den Gruppen R-1 bis R-184, welche allerdings dann jeweils entsprechend mit R2, bzw. den bei R2 genannten Gruppen substituiert sind. In a further preferred embodiment of the invention, all radicals R 1 , insofar as they represent an aromatic or heteroaromatic ring system, or R 2 , insofar as they represent aromatic or heteroaromatic groups, are selected from the groups R-1 to R-184, which are then each substituted accordingly with R 2 , or the groups mentioned under R 2 .
In einer bevorzugten Ausführungsform der Erfindung sind alle aroma- tischen oder heteroaromatischen Gruppen der Reste R, R1 oder R2 ausgewählt aus den entsprechenden Gruppen R-1 bis R-184. In a preferred embodiment of the invention, all aromatic or heteroaromatic groups of the radicals R, R 1 or R 2 are selected from the corresponding groups R-1 to R-184.
In einer bevorzugten Ausführungsformen sind die Verbindungen mindestens zu 50 %, insbesondere mindestens zu 80 %, besonders bevorzugt vollständig (100 %) deuteriert. Dies bedeutet, dass in einer solchen Verbindungen der entsprechende Anteil der enthaltenen Wasser- stoffatome der undeuterierten Verbindung gegen D ausgetauscht wurden. Die undeuterierte Verbindung ist die entsprechende Verbindung, bei welcher die Deuterium gegen Wasserstoff ausgetauscht sind und welche daher kein D enthält. In einer vollständig deuterierten Verbindung sind alle H gegen D ausgetauscht. In a preferred embodiment, the compounds are at least 50%, in particular at least 80%, particularly preferably completely (100%) deuterated. This means that in such a compound the corresponding proportion of the hydrogen atoms contained in the undeuterated compound have been exchanged for D. The undeuterated compound is the corresponding compound in which the deuterium has been exchanged for hydrogen and which therefore contains no D. In a fully deuterated compound, all H are exchanged for D.
Dabei haben die Alkylgruppen in erfindungsgemäßen Verbindungen, die durch Vakuumverdampfung verarbeitet werden, bevorzugt nicht mehr als fünf C-Atome, besonders bevorzugt nicht mehr als 4 C-Atome, ganz besonders bevorzugt nicht mehr als 1 C-Atom. Für Verbindungen, die aus Lösung verarbeitet werden, eignen sich auch Verbindungen, die mit Alkyl- gruppen, insbesondere verzweigten Alkylgruppen, mit bis zu 10 C-Atomen substituiert sind oder die mit Oligoarylengruppen, beispielsweise ortho-, meta-, para- oder verzweigten Terphenyl- oder Quaterphenylgruppen, substituiert sind. The alkyl groups in compounds according to the invention which are processed by vacuum evaporation preferably have no more than five C atoms, particularly preferably no more than 4 C atoms, very particularly preferably no more than 1 C atom. For compounds which are processed from solution, compounds which are substituted with alkyl groups, in particular branched alkyl groups, with up to 10 C atoms or which are substituted with oligoarylene groups, for example ortho-, meta-, para- or branched terphenyl or quaterphenyl groups, are also suitable.
Die oben genannten bevorzugten Ausführungsformen können beliebig innerhalb der in Anspruch 1 definierten Einschränkungen miteinander kombiniert werden. In einer besonders bevorzugten Ausführungsform der Erfindung treten die oben genannten Bevorzugungen gleichzeitig auf. The above-mentioned preferred embodiments can be combined with one another as desired within the limitations defined in claim 1. In a particularly preferred embodiment of the invention, the above-mentioned advantages occur simultaneously.
Beispiele für bevorzugte Verbindungen gemäß den oben aufgeführten Ausführungsformen sind die in der folgenden Tabelle aufgeführten Verbin- dungen. Examples of preferred compounds according to the embodiments listed above are the compounds listed in the following table.
Die erfindungsgemäßen Verbindungen können nach dem Fachmann bekannten Syntheseschritten, wie z. B. Bromierung, Suzuki-Kupplung, Ullmann-Kupplung, Heck-Reaktion, Hartwig-Buchwald-Kupplung, etc., dargestellt werden. The compounds according to the invention can be prepared by synthesis steps known to the person skilled in the art, such as bromination, Suzuki coupling, Ullmann coupling, Heck reaction, Hartwig-Buchwald coupling, etc.
Die erfindungsgemäßen 2,6, 10-T riaryl-/heteroaryl-tris[1 , 2 , 4]triazolo[ 1 ,5- a: 1 ',5'-c: 1 ",5"-e][1 ,3,5]triazine können ausgehend von 2,6,10-Trichlor- otris[ 1 , 2 , 4]triazolo[ 1 ,5-a: 1 ',5'-c: 1 ", 5"-e][ 1 , 3, 5]triazin [879612-44-9] durch Suzuki-Kupplung mit Aryl-/Heteroaryl-boronsäuren bzw. deren Ester oder durch SN2Ar-Reaktion mit Grignard- oder Organo-Lithium-Verbindungen dargestellt werden (Schema 1 ). Als typische Katalysatorsysteme für die Suzuki-Kupplung können literaturbekannte Kombinationen von Palladium- Verbindungen und bevorzugt elektronenreichen Phosphinen, wie SPhos, XPhos, RuPhos, AdaPhos etc., als typische Basen Alkali-ZErdalkali- carbonate, Phosphate, Hydroxide und als Lösemittel (Lömi) zur ein- phasigen Reaktionsführung DMSO, DMF, DMAc, NMP, THF, Dioxan, bzw. zur zweiphasigen Gemische aus Wasser mit THF, Dioxan, Glyme, Alkoholen, Toluol etc. verwendet werden. Alternative Kupplungsverfahren, wie die Negish-, Yamamoto-, Grignard-Cross-Kupplung können ebenfalls zum Einsatz kommen. Werden Gemische an Aryl-/Heteroarylboronsäuren bzw. deren Ester oder Grignard- oder Organolithium-Verbindungen verwendet, können bezüglich des Restes R gemischte Produkte erhalten werden, die chromatographisch getrennt werden können. Alternativ kann die Synthese von gemischten Verbindungen auch durch konsekutive Kupplungsschritte erfolgen, wobei die Dichlor-Aryl/Heteroaryl- bzw. die Chlordiaryl/Heteroaryl-Intermediate isoliert oder in-situ weiter umgesetzt werden können. The 2,6,10-triaryl/heteroaryl-tris[1,2,4]triazolo[1,5-a:1',5'-c:1",5"-e][1,3,5]triazines according to the invention can be prepared starting from 2,6,10-trichloro-tris[1,2,4]triazolo[1,5-a:1',5'-c:1",5"-e][1,3,5]triazine [879612-44-9] by Suzuki coupling with aryl/heteroaryl-boronic acids or their esters or by SN2Ar reaction with Grignard or organolithium compounds (Scheme 1). Typical catalyst systems for the Suzuki coupling are known combinations of palladium compounds and preferably electron-rich phosphines such as SPhos, XPhos, RuPhos, AdaPhos etc., as well as alkali-alkaline earth carbonates, phosphates, hydroxides as typical bases and DMSO, DMF, DMAc, NMP, THF, dioxane as solvents (Lömi) for single-phase reactions or mixtures of water with THF, dioxane, glyme, alcohols, toluene etc. Alternative coupling processes such as Negish, Yamamoto, Grignard cross coupling can also be used. are used. If mixtures of aryl/heteroarylboronic acids or their esters or Grignard or organolithium compounds are used, mixed products can be obtained with respect to the R radical, which can be separated chromatographically. Alternatively, the synthesis of mixed compounds can also be carried out by consecutive coupling steps, whereby the dichloroaryl/heteroaryl or the chlorodiaryl/heteroaryl intermediates can be isolated or further reacted in situ.
Die erfindungsgemäßen 2,6, 10-T ri-N-carbazolyl-tris[1 , 2 , 4]triazo lo[ 1 ,5- a:1 ',5'-c:1",5"-e][1 ,3,5]triazine können ausgehend von 2,6, 10-Trichlorotris- [1 , 2 ,4]triazolo[ 1 ,5-a: 1 ',5'-c: 1 ", 5"-e][ 1 , 3, 5]triazin [879612-44-9] durch Buchwald-Hartwig-Kupplung oder durch SN2Ar-Reaktion mit Carbazolen dargestellt werden (Schema 2). Analog lassen sich die Reaktionen mit Indenocarbazole, Indolocarbazolen, etc. durchführen. Als typische Kataly- satorsysteme für die Buchwald-Hartwig-Kupplung können literatur- bekannte Kombinationen von Palladium-Verbindungen und bevorzugt elektronenreichen Phosphinen, wie Tri-tert-butyl-, Tri-cyclohexyl-Phosphin, BINAP, SPhos, XPhos, RuPhos, AdaPhos etc., als typische Basen können Alkoholate, Alkali-ZErdalkali-Carbonate, Phosphate und als Lösemittel THF, Dioxan, Toluol, DMSO, DMF, DMAc, NMP verwendet werden. Für die SN2-Ar-Reaktion kommen BuLi, NaH, K2CO3, CS2CO3, K3PO4 in dipolar-aprotischen Lösungsmittel wie DMSO, DMF, DMAc, NMP, etc. zum Einsatz. The 2,6, 10-tri-N-carbazolyl-tris[1,2,4]triazo lo[1,5- a:1',5'-c:1",5"-e][1,3,5]triazines according to the invention can be prepared starting from 2,6, 10-trichlorotris-[1,2,4]triazolo[1,5-a:1',5'-c:1",5"-e][1,3,5]triazine [879612-44-9] by Buchwald-Hartwig coupling or by SN2Ar reaction with carbazoles (Scheme 2). The reactions with indenocarbazoles, indolocarbazoles, etc. can be carried out analogously. Typical catalyst systems for the Buchwald-Hartwig coupling are known combinations of palladium compounds and preferably electron-rich phosphines, such as tri-tert-butyl-, tri-cyclohexyl-phosphine, BINAP, SPhos, XPhos, RuPhos, AdaPhos etc., typical bases are alcoholates, alkali-alkaline earth carbonates, phosphates and as Solvents THF, dioxane, toluene, DMSO, DMF, DMAc, NMP can be used. For the SN2-Ar reaction, BuLi, NaH, K2CO3, CS2CO3, K3PO4 in dipolar aprotic solvents such as DMSO, DMF, DMAc, NMP, etc. are used.
Schema 2: Buchwald-Hartwig-Kupplung bzw. SN2-Ar-Reaktion: Scheme 2: Buchwald-Hartwig coupling or SN2-Ar reaction:
Die Kupplungsreaktionen gemäß Schema 1 und 2 können auch konsekutiv ausgeführt werden, wobei die gemischt Aryl-/Heteroaryl-/N- Carbazolyl-substituierten erfindungsgemäßen Verbindungen erhalten werden. Weiterhin können in der o.g. SN2-Ar-Reaktion auch Alkoholate bzw. Cyanid als Nukleophil eingesetzt und somit die entsprechenden Ether bzw. Nitrile erhalten werden. The coupling reactions according to Schemes 1 and 2 can also be carried out consecutively, whereby the mixed aryl/heteroaryl/N-carbazolyl-substituted compounds according to the invention are obtained. Furthermore, alcoholates or cyanide can also be used as nucleophiles in the above-mentioned SN2-Ar reaction and thus the corresponding ethers or nitriles can be obtained.
Alternativ können die erfindungsgemäßen Verbindungen nach den in der Literatur beschriebenen Verfahren ausgehend von den entsprechenden Nitrilen oder Carbonsäureamiden dargestellt werden (z. B. R. Hojo et al., J. Mater. Chem. C, 2022, 10, 13871 oder T. Rieth et al., Molecules 2020, 25, 5761 ). Alternatively, the compounds of the invention can be prepared by the processes described in the literature starting from the corresponding nitriles or carboxamides (e.g. R. Hojo et al., J. Mater. Chem. C, 2022, 10, 13871 or T. Rieth et al., Molecules 2020, 25, 5761).
Ein weiterer Gegenstand der vorliegenden Erfindung ist daher ein Ver- fahren zur Herstellung der erfindungsgemäßen Verbindungen, gekenn- zeichnet durch die folgenden Schritte: (A) Synthese des Grundgerüsts nach Formel (1 ), das statt der Reste R eine reaktive Abgangsgruppe, beispielsweise F, CI, Br, I, Boronsäure oder ein Boronsäureester, Tosylat oder Mesylat, enthält; A further object of the present invention is therefore a process for preparing the compounds according to the invention, characterized by the following steps: (A) synthesis of the basic skeleton according to formula (1 ) which contains, instead of the radicals R, a reactive leaving group, for example F, CI, Br, I, boronic acid or a boronic acid ester, tosylate or mesylate;
(B) Einführen der Gruppen R durch Kupplungsreaktionen. (B) Introduction of the groups R by coupling reactions.
Ein weiterer Gegenstand der vorliegenden Erfindung ist ein Oligomer, Polymer oder Dendrimer umfassend eine oder mehrere Verbindungen gemäß Formel (1 ). Another object of the present invention is an oligomer, polymer or dendrimer comprising one or more compounds according to formula (1).
Für die Verarbeitung der erfindungsgemäßen Verbindungen aus flüssiger Phase, beispielsweise durch Spin-Coating oder durch Druckverfahren, sind Formulierungen der erfindungsgemäßen Verbindungen erforderlich. Diese Formulierungen können beispielsweise Lösungen, Dispersionen oder Emulsionen sein. Es kann bevorzugt sein, hierfür Mischungen aus zwei oder mehr Lösemitteln zu verwenden. Geeignete und bevorzugte Lösemittel sind beispielsweise Toluol, Anisol, o-, m- oder p-Xylol, Methyl- benzoat, Mesitylen, Tetralin, Veratrol, THF, Methyl-THF, THP, Chlor- benzol, Dioxan, Phenoxytoluol, insbesondere 3-Phenoxytoluol, (-)- Fenchon, 1 ,2,3,5-Tetramethylbenzol, 1 ,2,4,5-Tetramethylbenzol, 1 -Methyl- naphthalin, 2-Methylbenzothiazol, 2-Phenoxyethanol, 2-Pyrrolidinon, 3- Methylanisol, 4-Methylanisol, 3,4-Dimethylanisol, 3,5-Dimethylanisol, Acetophenon, a-Terpineol, Benzothiazol, Butylbenzoat, Cumol, Cyclo- hexanol, Cyclohexanon, Cyclohexylbenzol, Decalin, Dodecylbenzol, Ethyl- benzoat, Indan, NMP, p-Cymol, Phenetol, 1 ,4-Diisopropylbenzol, Di- benzylether, Diethylenglycolbutylmethylether, T riethylenglycolbutylmethyl- ether, Diethylenglycoldibutylether, Triethylenglycoldimethylether, Di- ethylenglycolmonobutylether, Tripropyleneglycoldimethylether, Tetra- ethylenglycoldimethylether, 2-lsopropylnaphthalin, Pentylbenzol, Hexyl- benzol, Heptylbenzol, Octylbenzol, 1 ,1 -Bis(3,4-dimethylphenyl)ethan, 2- Methylbiphenyl, 3-Methylbiphenyl, 1 -Methylnaphthalin, 1 -Ethylnaphthalin, Ethyloctanoat, Sebacinsäure-diethylester, Octyloctanoat, Heptylbenzol, Menthyl-isovalerat, Cyclohexylhexanoat oder Mischungen dieser Löse- mittel. For processing the compounds according to the invention from the liquid phase, for example by spin coating or by printing processes, formulations of the compounds according to the invention are required. These formulations can be, for example, solutions, dispersions or emulsions. It may be preferable to use mixtures of two or more solvents for this purpose. Suitable and preferred solvents are, for example, toluene, anisole, o-, m- or p-xylene, methyl benzoate, mesitylene, tetralin, veratrole, THF, methyl-THF, THP, chlorobenzene, dioxane, phenoxytoluene, in particular 3-phenoxytoluene, (-)-fenchone, 1,2,3,5-tetramethylbenzene, 1,2,4,5-tetramethylbenzene, 1-methylnaphthalene, 2-methylbenzothiazole, 2-phenoxyethanol, 2-pyrrolidinone, 3-methylanisole, 4-methylanisole, 3,4-dimethylanisole, 3,5-dimethylanisole, acetophenone, a-terpineol, benzothiazole, butylbenzoate, cumene, cyclohexanol, cyclohexanone, Cyclohexylbenzene, decalin, dodecylbenzene, ethyl benzoate, indane, NMP, p-cymene, phenetol, 1,4-diisopropylbenzene, di-benzyl ether, diethylene glycol butyl methyl ether, triethylene glycol butyl methyl ether, diethylene glycol dibutyl ether, triethylene glycol dimethyl ether, di- ethylene glycol monobutyl ether, tripropylene glycol dimethyl ether, tetra-ethylene glycol dimethyl ether, 2-isopropylnaphthalene, pentylbenzene, hexylbenzene, heptylbenzene, octylbenzene, 1,1-bis(3,4-dimethylphenyl)ethane, 2-methylbiphenyl, 3-methylbiphenyl, 1-methylnaphthalene, 1 -Ethylnaphthalene, ethyl octanoate, diethyl sebacate, octyloctanoate, heptylbenzene, Menthyl isovalerate, cyclohexylhexanoate or mixtures of these solvents.
Ein weiterer Gegenstand der vorliegenden Erfindung ist daher eine For- mulierung, insbesondere eine Lösung, Dispersion oder Emulsion, umfassend mindestens eine erfindungsgemäße Verbindung und mindes- tens eine weitere Verbindung. Die weitere Verbindung kann beispiels- weise ein Lösemittel sein, insbesondere eines der oben genannten Löse- mittel oder eine Mischung dieser Lösemittel. Die Herstellung solcher Lösungen ist dem Fachmann bekannt und ist beispielsweise beschrieben in WO 2002/072714, WO 2003/019694 und der darin zitierten Literatur. Die weitere Verbindung kann aber auch mindestens eine weitere organische oder anorganische Verbindung sein, die ebenfalls in der elektronischen Vorrichtung eingesetzt wird, beispielsweise eine emittierende Verbindung und/oder ein Matrixmaterial. Diese weitere Verbindung kann auch polymer sein. A further subject of the present invention is therefore a formulation, in particular a solution, dispersion or emulsion, comprising at least one compound according to the invention and at least one further compound. The further compound can be, for example, a solvent, in particular one of the above-mentioned solvents or a mixture of these solvents. The preparation of such solutions is known to the person skilled in the art and is described, for example, in WO 2002/072714, WO 2003/019694 and the literature cited therein. The further compound can also be at least one further organic or inorganic compound which is also used in the electronic device, for example an emitting compound and/or a matrix material. This further compound can also be polymeric.
Die erfindungsgemäßen Verbindungen eignen sich für die Verwendung in einer elektronischen Vorrichtung, insbesondere in einer organischen Elektrolumineszenzvorrichtung (OLED). Abhängig von der Substituierung können die Verbindungen in unterschiedlichen Funktionen und Schichten verwendet werden. The compounds according to the invention are suitable for use in an electronic device, in particular in an organic electroluminescent device (OLED). Depending on the substitution, the compounds can be used in different functions and layers.
Ein weiterer Gegenstand der vorliegenden Erfindung ist daher die Verwen- dung einer erfindungsgemäßen Verbindung in einer elektronischen Vor- richtung. A further object of the present invention is therefore the use of a compound according to the invention in an electronic device.
Ein nochmals weiterer Gegenstand der vorliegenden Erfindung ist eine elektronische Vorrichtung enthaltend mindestens eine erfindungsgemäße Verbindung. Yet another object of the present invention is an electronic device comprising at least one compound according to the invention.
Die erfindungsgemäßen Verbindungen können insbesondere bei ihrer Verwendung als Racemat oder als reines Enantiomer vorliegen. Die Bildung von Enantionmeren ist beispielsweise möglich, wenn die Reste R derart gewählt sind, dass die Rotation um die Bindung von R zum Tris- triazolotriazin gehindert ist und dadurch Atropisomere gebildet werden. The compounds according to the invention can be present, in particular when used, as a racemate or as a pure enantiomer. The formation of enantiomers is possible, for example, if the radicals R are selected such that rotation around the bond of R to the tristriazolotriazine is hindered and atropisomers are thereby formed.
Eine elektronische Vorrichtung im Sinne der vorliegenden Erfindung ist eine Vorrichtung, welche mindestens eine Schicht enthält, die mindestens eine organische Verbindung enthält. Das Bauteil kann dabei auch anorga- nische Materialien enthalten oder auch Schichten, welche vollständig aus anorganischen Materialien aufgebaut sind. An electronic device in the sense of the present invention is a device which contains at least one layer containing at least one organic compound. The component can also contain inorganic nic materials or layers that are made entirely of inorganic materials.
Die elektronische Vorrichtung ist bevorzugt ausgewählt aus der Gruppe bestehend aus organischen Elektrolumineszenzvorrichtungen (OLEDs), organischen integrierten Schaltungen (O-ICs), organischen Feld-Effekt- Transistoren (O-FETs), organischen Dünnfilmtransistoren (O-TFTs), organischen lichtemittierenden Transistoren (O-LETs), organischen Solar- zellen (O-SCs), farbstoffsensibilisierten organischen Solarzellen (DSSCs), organischen optischen Detektoren, organischen Photorezeptoren, orga- nische Photodioden (OPD), organischen Feld-Quench-Devices (O-FQDs), lichtemittierenden elektrochemischen Zellen (LECs), organischen Laser- dioden (O-Laser) und „organic plasmon emitting devices“, bevorzugt aber organischen Elektrolumineszenzvorrichtungen (OLEDs). The electronic device is preferably selected from the group consisting of organic electroluminescent devices (OLEDs), organic integrated circuits (O-ICs), organic field-effect transistors (O-FETs), organic thin-film transistors (O-TFTs), organic light-emitting transistors (O-LETs), organic solar cells (O-SCs), dye-sensitized organic solar cells (DSSCs), organic optical detectors, organic photoreceptors, organic photodiodes (OPDs), organic field-quench devices (O-FQDs), light-emitting electrochemical cells (LECs), organic laser diodes (O-lasers) and organic plasmon emitting devices, but preferably organic electroluminescent devices (OLEDs).
Die Vorrichtung ist besonders bevorzugt eine organische Elektrolumines- zenzvorrichtung umfassend Kathode, Anode und mindestens eine emittierende Schicht, wobei mindestens eine organische Schicht, welche eine emittierende Schicht, Lochtransportschicht, Elektronentransport- schicht, Lochblockierschicht, Elektronenblockierschicht oder eine andere funktionelle Schicht sein kann, mindestens eine erfindungsgemäße Verbindung umfasst. Die Schicht ist abhängig von der Substitution der Verbindung. The device is particularly preferably an organic electroluminescent device comprising a cathode, anode and at least one emitting layer, wherein at least one organic layer, which can be an emitting layer, hole transport layer, electron transport layer, hole blocking layer, electron blocking layer or another functional layer, comprises at least one compound according to the invention. The layer depends on the substitution of the compound.
Außer diesen Schichten kann die organische Elektrolumineszenzvor- richtung noch weitere Schichten enthalten, beispielsweise jeweils eine oder mehrere Lochinjektionsschichten, Lochtransportschichten, Loch- blockierschichten, Elektronentransportschichten, Elektroneninjektions- schichten, Exzitonenblockierschichten, Elektronenblockierschichten, Ladungserzeugungsschichten (Charge-Generation Layers) und/oder organische oder anorganische p/n Übergänge. Ebenso können zwischen zwei emittierende Schichten Interlayer eingebracht sein, welche beispiels- weise eine exzitonenblockierende Funktion aufweisen. Es sei aber darauf hingewiesen, dass nicht notwendigerweise jede dieser Schichten vorhanden sein muss. Dabei kann die organische Elektrolumineszenzvorrichtung eine emittierende Schicht enthalten, oder sie kann mehrere emittierende Schichten enthalten. Wenn mehrere Emissionsschichten vorhanden sind, weisen diese bevorzugt insgesamt mehrere Emissionsmaxima zwischen 380 nm und 750 nm auf, sodass insgesamt weiße Emission resultiert, d. h. in den emittierenden Schichten werden verschiedene emittierende Verbindungen verwendet, die fluoreszieren oder phosphoreszieren können. Insbesondere bevorzugt sind Systeme mit drei emittierenden Schichten, wobei die drei Schichten blaue, grüne und orange oder rote Emission zeigen (Der prinzipielle Aufbau ist beispielsweise in WO 2005/011013 beschrieben). Es kann sich bei der erfindungsgemäßen organischen Elektrolumineszenzvorrichtung auch um eine Tandem-OLED handeln, insbesondere für weiß emittierende OLEDs. In addition to these layers, the organic electroluminescent device can contain further layers, for example one or more hole injection layers, hole transport layers, hole blocking layers, electron transport layers, electron injection layers, exciton blocking layers, electron blocking layers, charge generation layers and/or organic or inorganic p/n junctions. Interlayers can also be introduced between two emitting layers, which, for example, have an exciton blocking function. It should be noted, however, that not all of these layers necessarily have to be present. The organic electroluminescent device can contain one emitting layer, or it can contain several emitting layers. If several emitting layers are present, these preferably have a total of several emission maxima between 380 nm and 750 nm, so that overall white emission results, ie different emitting compounds that can fluoresce or phosphoresce are used in the emitting layers. Systems with three emitting layers are particularly preferred, with the three layers showing blue, green and orange or red emission (the basic structure is described, for example, in WO 2005/011013). The organic electroluminescent device according to the invention can also be a tandem OLED, in particular for white-emitting OLEDs.
Bevorzugt wird die Verbindung gemäß Formel (1 ) in einer organischen Elektrolumineszenzvorrichtung verwendet, welche eine oder mehrere phosphoreszierende Emitter umfasst. Die erfindungsgemäße Verbindung gemäß den oben aufgeführten Ausführungsformen kann dabei in unter- schiedlichen Schichten eingesetzt werden, je nach genauer Struktur. The compound according to formula (1) is preferably used in an organic electroluminescent device which comprises one or more phosphorescent emitters. The compound according to the invention according to the embodiments listed above can be used in different layers, depending on the precise structure.
Dabei kann die organische Elektrolumineszenzvorrichtung eine emittieren- de Schicht enthalten, oder sie kann mehrere emittierende Schichten ent- halten, wobei mindestens eine Schicht mindestens eine erfindungs- gemäße Verbindung enthält. Weiterhin kann die erfindungsgemäße Verbindung auch in einer Elektronentransportschicht und/oder in einer Lochblockierschicht und/oder in einer Lochtransportschicht und/oder in einer Exzitonenblockierschicht eingesetzt werden. The organic electroluminescent device can contain one emitting layer, or it can contain several emitting layers, with at least one layer containing at least one compound according to the invention. Furthermore, the compound according to the invention can also be used in an electron transport layer and/or in a hole blocking layer and/or in a hole transport layer and/or in an exciton blocking layer.
Der Ausdruck „phosphoreszierende Verbindung“ bezeichnet typischer- weise Verbindungen, bei denen die Aussendung von Licht durch einen spin-verbotenen Übergang erfolgt, z. B. einen Übergang von einem ange- regten Triplett-Zustand oder einem Zustand mit einer höheren Spin- Quantenzahl, z. B. einem Quintett-Zustand. Geeignete phosphores- zierende Verbindungen (= Triplett-Emitter) sind insbesondere Verbin- dungen, die bei geeigneter Anregung Licht, vorzugsweise im sichtbaren Bereich, emittieren und außerdem mindestens ein Atom der Ordnungszahl größer als 20, vorzugsweise größer als 38 und kleiner als 84, besonders bevorzugt größer als 56 und kleiner als 80 enthalten. Bevorzugt werden als phosphoreszierende Verbindungen alle lumineszierenden Komplexe mit Übergangsmetallen oder Lanthaniden angesehen, insbesondere wenn sie Kupfer, Molybdän, Wolfram, Rhenium, Ruthenium, Osmium, Rhodium, Indium, Palladium, Platin, Silber, Gold oder Europium enthalten, insbesondere Verbindungen, die Indium, Platin oder Kupfer enthalten. Im Rahmen der vorliegenden Erfindung werden alle lumineszierenden Indium-, Platin- oder Kupferkomplexe als phosphoreszierende emittierende Verbindungen betrachtet. The term “phosphorescent compound” typically refers to compounds in which the emission of light occurs through a spin-forbidden transition, e.g. a transition from an excited triplet state or a state with a higher spin quantum number, e.g. a quintet state. Suitable phosphorescent compounds (= triplet emitters) are in particular compounds that emit light, preferably in the visible range, when suitably excited and also contain at least one atom of atomic number greater than 20, preferably greater than 38 and less than 84, particularly preferably greater than 56 and less than 80. All luminescent complexes with transition metals or lanthanides are preferably regarded as phosphorescent compounds, in particular if they contain copper, molybdenum, tungsten, rhenium, ruthenium, osmium, rhodium, indium, palladium, platinum, silver, gold or europium, in particular compounds which contain indium, platinum or copper. In the context of the present invention, all luminescent indium, platinum or copper complexes are regarded as phosphorescent emitting compounds.
Beispiele der oben beschriebenen Emitter können den Anmeldungen WO 00/70655, WO 2001/41512, WO 2002/02714, WO 2002/15645, EP 1191613, EP 1191612, EP 1191614, WO 05/033244, WO 05/019373, US 2005/0258742, WO 2009/146770, WO 2010/015307, WO 2010/031485, WO 2010/054731 , WO 2010/054728, WO 2010/086089, WO 2010/099852, WO 2010/102709, WO 2011/032626, WO 2011/066898, WO 2011/157339, WO 2012/007086, WO 2014/008982, WO 2014/023377, WO 2014/094961 , WO 2014/094960, WO 2015/036074, WO 2015/104045, WO 2015/117718, WO 2016/015815, WO 2016/124304, WO 2017/032439, WO 2018/011186, WO 2018/041769, WO 2019/020538, WO 2018/178001 , WO 2019/115423 und WO 2019/158453 entnommen werden. Generell eignen sich alle phosphoreszierenden Komplexe, wie sie gemäß dem Stand der Technik für phosphoreszierende OLEDs verwendet werden und wie sie dem Fach- mann auf dem Gebiet der organischen Elektrolumineszenz bekannt sind, und der Fachmann kann ohne erfinderisches Zutun weitere phosphores- zierende Komplexe verwenden. Für den Fachmann ist es auch ohne erfinderische Tätigkeit möglich, weitere phosphoreszierende Komplexe in Kombination mit den Verbindungen der Formel (1 ) in organischen Elektrolumineszenzvorrichtungen zu verwenden. Da die erfindungs- gemäßen Verbindungen je nach Substitution auch eine hohe Triplett- energie aufweisen können, ist es insbesondere auch möglich, diese als Matrixmaterial für blau phosphoreszierende Emitter zu verwenden. Weitere Beispiele sind in einer nachfolgenden Tabelle aufgeführt. Erfindungsgemäß ist es auch möglich, die Verbindung der Formel (1 ) in einer elektronischen Vorrichtung zu verwenden, die eine oder mehrere fluoreszierende emittierende Verbindungen enthält. Examples of the emitters described above can be found in the applications WO 00/70655, WO 2001/41512, WO 2002/02714, WO 2002/15645, EP 1191613, EP 1191612, EP 1191614, WO 05/033244, WO 05/019373, US 2005/0258742, WO 2009/146770, WO 2010/015307, WO 2010/031485, WO 2010/054731, WO 2010/054728, WO 2010/086089, WO 2010/099852, WO 2010/102709, WO 2011/032626, WO 2011/066898, WO 2011/157339, WO 2012/007086, WO 2014/008982, WO 2014/023377, WO 2014/094961, WO 2014/094960, WO 2015/036074, WO 2015/104045, WO 2015/117718, WO 2016/015815, WO 2016/124304, WO 2017/032439, WO 2018/011186, WO 2018/041769, WO 2019/020538, WO 2018/178001, WO 2019/115423 and WO 2019/158453. In general, all phosphorescent complexes as used according to the prior art for phosphorescent OLEDs and as known to the person skilled in the field of organic electroluminescence are suitable, and the person skilled in the art can use further phosphorescent complexes without inventive step. It is also possible for the person skilled in the art to use further phosphorescent complexes in combination with the compounds of the formula (1) in organic electroluminescent devices without inventive step. Since the compounds according to the invention can also have a high triplet energy depending on the substitution, it is also possible in particular to use them as matrix material for blue phosphorescent emitters. Further examples are listed in a table below. According to the invention, it is also possible to use the compound of formula (1) in an electronic device containing one or more fluorescent emitting compounds.
In einer bevorzugten Ausführungsform der Erfindung werden die Verbin- dungen der Formel (1 ) als elektronentransportierendes Material verwen- det. In diesem Fall sind die Verbindungen vorzugsweise in einer Elek- tronentransportschicht oder einer Lochblockierschicht oder einem elek- tronenleitendem oder bipolaren Hostmaterial enthalten. Besonders bevor- zugt ist die Verwendung in einer Elektronentransportschicht. In a preferred embodiment of the invention, the compounds of the formula (1) are used as electron-transporting material. In this case, the compounds are preferably contained in an electron-transport layer or a hole-blocking layer or an electron-conducting or bipolar host material. Use in an electron-transport layer is particularly preferred.
Eine Elektronentransportschicht im Sinne der vorliegenden Anmeldung ist eine Schicht mit elektronentransportierender Funktion zwischen der Kathode und der emittierenden Schicht. An electron transport layer in the sense of the present application is a layer with an electron-transporting function between the cathode and the emitting layer.
Unter Elektroneninjektionsschichten und Lochblockierschichten werden im Rahmen der vorliegenden Anmeldung bestimmte Ausführungsformen von Elektronentransportschichten verstanden. Eine Elektroneninjektions- schicht ist im Falle einer Mehrzahl von Elektronentransportschichten zwischen Kathode und emittierender Schicht eine Elektronentransport- schicht, die direkt an die Kathode angrenzt oder nur durch eine einzige Beschichtung der Kathode von dieser getrennt ist. Eine Lochblockier- schicht ist im Falle mehrerer Elektronentransportschichten zwischen Kathode und emittierender Schicht diejenige Elektronentransportschicht, die kathodenseitig direkt an die emittierende Schicht angrenzt. Vorzugs- weise umfasst die erfindungsgemäße OLED zwischen Kathode und emittierender Schicht zwei, drei oder vier elektronentransportierende Schichten, von denen vorzugsweise mindestens eine, besonders bevorzugt genau eine oder zwei eine Verbindung der Formel (1 ) enthalten. In the context of the present application, electron injection layers and hole blocking layers are understood to mean certain embodiments of electron transport layers. In the case of a plurality of electron transport layers between the cathode and the emitting layer, an electron injection layer is an electron transport layer that directly borders the cathode or is only separated from it by a single coating of the cathode. In the case of several electron transport layers between the cathode and the emitting layer, a hole blocking layer is the electron transport layer that directly borders the emitting layer on the cathode side. The OLED according to the invention preferably comprises two, three or four electron-transporting layers between the cathode and the emitting layer, of which preferably at least one, particularly preferably exactly one or two, contain a compound of the formula (1).
Wird die Verbindung der Formel (1 ) als Elektronentransportmatenal in einer Elektronentransportschicht, einer Elektroneninjektionsschicht oder einer Lochblockierschicht verwendet, so kann die Verbindung als reines Material, d.h. in einem Anteil von 100 %, in der Elektronentransportschicht eingesetzt werden, oder sie kann in Kombination mit einer oder mehreren weiteren Verbindungen verwendet werden. In einer weiteren Ausführungsform der vorliegenden Erfindung wird die Verbindung der Formel (1 ) in einer emittierenden Schicht als Matrix- material in Kombination mit einer oder mehreren emittierenden Verbin- dungen eingesetzt, wobei die emittierenden Verbindungen fluoreszierend oder phosphoreszierend sein können, vorzugsweise phosphoreszierend. If the compound of formula (1) is used as an electron transport material in an electron transport layer, an electron injection layer or a hole blocking layer, the compound can be used as a pure material, ie in a proportion of 100% in the electron transport layer, or it can be used in combination with one or more other compounds. In a further embodiment of the present invention, the compound of formula (1) is used in an emitting layer as a matrix material in combination with one or more emitting compounds, where the emitting compounds can be fluorescent or phosphorescent, preferably phosphorescent.
Der Anteil des Matrixmaterials in der emittierenden Schicht liegt in diesem Fall zwischen 50,0 und 99,9 Vol.-%, bevorzugt zwischen 80,0 und 99,5 Vol.-%, besonders bevorzugt zwischen 92,0 und 99,5 Vol-%. für fluores- zierende emittierende Schichten und zwischen 85,0 und 97,0 Vol.-% für phosphoreszierende emittierende Schichten. The proportion of matrix material in the emitting layer in this case is between 50.0 and 99.9 vol. %, preferably between 80.0 and 99.5 vol. %, particularly preferably between 92.0 and 99.5 vol. % for fluorescent emitting layers and between 85.0 and 97.0 vol. % for phosphorescent emitting layers.
Entsprechend liegt der Anteil der emittierenden Verbindung zwischen 0,1 und 50,0 Vol.-%, bevorzugt zwischen 0,5 und 20,0 Vol.-%, besonders bevorzugt zwischen 0,5 und 8,0 Vol.-% für fluoreszierende emittierende Schichten und zwischen 3,0 und 15,0 Vol.-% für phosphoreszierende emittierende Schichten. Accordingly, the proportion of the emitting compound is between 0.1 and 50.0 vol.%, preferably between 0.5 and 20.0 vol.%, particularly preferably between 0.5 and 8.0 vol.% for fluorescent emitting layers and between 3.0 and 15.0 vol.% for phosphorescent emitting layers.
Eine emittierende Schicht einer organischen Elektrolumineszenzvor- richtung kann auch Systeme umfassen, die eine Vielzahl von Matrix- materialien (Mischmatrixsysteme) und/oder eine Vielzahl von emittierenden Verbindungen enthalten. Auch in diesem Fall sind in der Regel die emittierenden Verbindungen diejenigen, die den kleineren Anteil im System haben und die Matrixmaterialien diejenigen, die den größeren Anteil im System haben. In Einzelfällen kann jedoch der Anteil eines einzelnen Matrixmaterials im System geringer sein als der Anteil einer einzelnen emittierenden Verbindung. An emitting layer of an organic electroluminescent device can also comprise systems that contain a large number of matrix materials (mixed matrix systems) and/or a large number of emitting compounds. In this case too, the emitting compounds are generally those that have the smaller proportion in the system and the matrix materials are those that have the larger proportion in the system. In individual cases, however, the proportion of an individual matrix material in the system can be lower than the proportion of an individual emitting compound.
Vorzugsweise werden die Verbindungen der Formel (1 ) als Bestandteil von Mischmatrixsystemen eingesetzt. Die Mischmatrixsysteme bestehen vorzugsweise aus zwei oder drei verschiedenen Matrixmaterialien, besonders bevorzugt aus zwei verschiedenen Matrixmaterialien. Vorzugs- weise ist in diesem Fall eines der beiden Materialien ein Material mit löchertransportierenden Eigenschaften und das andere Material ist ein Material mit elektronentransportierenden Eigenschaften. Die Verbindung der Formel (1 ) ist vorzugsweise das Matrixmaterial mit elektronentranspor- tierenden Eigenschaften. Die gewünschten elektronentransportierenden und löchertransportierenden Eigenschaften der gemischten Matrix- komponenten können jedoch auch überwiegend oder vollständig in einer einzigen gemischten Matrixkomponente kombiniert sein, wobei die weitere(n) gemischte(n) Matrixkomponente(n) andere Funktionen erfüllt (erfüllen). Die beiden unterschiedlichen Matrixmaterialien können in einem Verhältnis von 1 :50 bis 1 : 1 , bevorzugt 1 :20 bis 1 : 1 , noch bevorzugter 1 :10 bis 1 :1 und am meisten bevorzugt 1 :4 bis 1 :1 vorliegen. Bevorzugt werden Mischmatrixsysteme in phosphoreszierenden organischen Elektrolumines- zenzvorrichtungen eingesetzt. Eine Quelle für detailliertere Informationen über Mischmatrixsysteme ist die Anmeldung WO 2010/108579. Preferably, the compounds of formula (1) are used as a component of mixed matrix systems. The mixed matrix systems preferably consist of two or three different matrix materials, particularly preferably of two different matrix materials. Preferably, in this case, one of the two materials is a material with hole-transporting properties and the other material is a material with electron-transporting properties. The compound of formula (1) is preferably the matrix material with electron-transporting properties. However, the desired electron-transporting and hole-transporting properties of the mixed matrix components can also be predominantly or completely combined in a single mixed matrix component, with the further mixed matrix component(s) fulfilling other functions. The two different matrix materials can be present in a ratio of 1:50 to 1:1, preferably 1:20 to 1:1, even more preferably 1:10 to 1:1 and most preferably 1:4 to 1:1. Mixed matrix systems are preferably used in phosphorescent organic electroluminescent devices. A source for more detailed information on mixed matrix systems is the application WO 2010/108579.
Die Mischmatrixsysteme können eine oder mehrere emittierende Verbin- dungen enthalten, vorzugsweise eine oder mehrere phosphoreszierende Verbindungen. Im Allgemeinen werden Mischmatrixsysteme bevorzugt in phosphoreszierenden organischen Elektrolumineszenzvorrichtungen eingesetzt. The mixed matrix systems can contain one or more emitting compounds, preferably one or more phosphorescent compounds. In general, mixed matrix systems are preferably used in phosphorescent organic electroluminescent devices.
Besonders geeignete Matrixmaterialien, die in Kombination mit den erfin- dungsgemäßen Verbindungen als Matrixbestandteile eines Mischmatrix- systems verwendet werden können, werden aus den unten genannten bevorzugten Matrixmaterialien für phosphoreszierende Verbindungen oder den bevorzugten Matrixmaterialien für fluoreszierende Verbindungen ausgewählt, je nachdem, welche Art von emittierender Verbindung in dem Mischmatrixsystem verwendet wird. Particularly suitable matrix materials which can be used in combination with the compounds according to the invention as matrix components of a mixed matrix system are selected from the preferred matrix materials for phosphorescent compounds or the preferred matrix materials for fluorescent compounds mentioned below, depending on which type of emitting compound is used in the mixed matrix system.
Bevorzugte phosphoreszierende Verbindungen zur Verwendung in gemischten Matrixsystemen sind die gleichen, wie weiter oben als allgemein bevorzugte phosphoreszierende Emittermaterialien beschrieben. Preferred phosphorescent compounds for use in mixed matrix systems are the same as those described above as generally preferred phosphorescent emitter materials.
Beispiele für phosphoreszierende Verbindungen sind nachfolgend aufgeführt. Examples of phosphorescent compounds are listed below.
Bevorzugte fluoreszierende emittierende Verbindungen sind ausgewählt aus der Klasse der Arylamine. Unter einem Arylamin oder einem aroma- tischen Amin wird im Rahmen der vorliegenden Erfindung eine Verbin- dung verstanden, die drei substituierte oder unsubstituierte aromatische oder heteroaromatische Ringsysteme enthält, die direkt an den Stickstoff gebunden sind. Vorzugsweise ist mindestens eines dieser aromatischen oder heteroaromatischen Ringsysteme ein kondensiertes Ringsystem, besonders bevorzugt mit mindestens 14 aromatischen Ringatomen. Bevorzugte Beispiele hierfür sind aromatische Anthracenamine, aroma- tische Anthracendiamine, aromatische Pyrenamine, aromatische Pyren- diamine, aromatische Chrysenamine oder aromatische Chrysendiamine. Unter einem aromatischen Anthracenamin versteht man eine Verbindung, bei der eine Diarylaminogruppe direkt an eine Anthracengruppe, vorzugs- weise in 9-Position, gebunden ist. Unter einem aromatischen Anthracen- diamin ist eine Verbindung zu verstehen, in der zwei Diarylaminogruppen direkt an eine Anthracengruppe gebunden sind, vorzugsweise in der 9- und 10-Position. Analog sind aromatische Pyrenamine, Pyrendiamine, Chrysenamine und Chrysendiamine definiert, bei denen die Diaryl- aminogruppen vorzugsweise in 1 -Position oder 1 ,6-Position an das Pyren gebunden sind. Weitere bevorzugte emittierende Verbindungen sind Indenofluorenamine oder Fluorendiamine, beispielsweise nach WO 2006/108497 oder WO 2006/122630, Benzoindenofluorenamine oder -fluorendiamine, beispielsweise nach WO 2008/006449, und Dibenzoindenofluorenamine oder -diamine, beispielsweise nach WO 2007/140847, sowie die in WO 2010/012328 offenbarten Indenofluoren- derivate mit kondensierten Arylgruppen. Ebenso bevorzugt sind die in WO 2012/048780 und in WO 2013/185871 offenbarten Pyrenearylamine. Ebenfalls bevorzugt sind die in WO 2014/037077 offenbarten Benzo- indenofluorenamine, die in WO 2014/106522 offenbarten Benzofluoren- amine, die in WO 2014/111269 und in WO 2017/036574 offenbarten verlängerten Benzoindenofluorene, die in WO 2017/028940 und in WO 2017/028941 offenbarten Phenoxazine und die in WO 2016/150544 offenbarten an Furaneinheiten oder an Thiopheneinheiten gebundenen Fluorderivate. Weiterhin können Bor-Verbindung gemäß Preferred fluorescent emitting compounds are selected from the class of arylamines. In the context of the present invention, an arylamine or an aromatic amine is understood to mean a compound which contains three substituted or unsubstituted aromatic or heteroaromatic ring systems which are bonded directly to the nitrogen. Preferably, at least one of these aromatic or heteroaromatic ring systems is a condensed ring system, particularly preferably with at least 14 aromatic ring atoms. Preferred examples of these are aromatic anthraceneamines, aromatic anthracenediamines, aromatic pyreneamines, aromatic pyrenediamines, aromatic chrysenamines or aromatic chrysenediamines. An aromatic anthraceneamine is understood to mean a compound in which a diarylamino group is bonded directly to an anthracene group, preferably in the 9-position. An aromatic anthracene diamine is a compound in which two diarylamino groups are bonded directly to an anthracene group, preferably in the 9- and 10-position. Aromatic pyrenamines, pyrenediamines, chrysenamines and chrysenediamines are defined analogously, in which the diarylamino groups are bonded to the pyrene preferably in the 1-position or 1,6-position. Further preferred emitting compounds are indenofluorenamines or fluorenediamines, for example according to WO 2006/108497 or WO 2006/122630, benzoindenofluorenamines or fluorenediamines, for example according to WO 2008/006449, and dibenzoindenofluorenamines or diamines, for example according to WO 2007/140847, and the indenofluorene derivatives with condensed aryl groups disclosed in WO 2010/012328. Likewise preferred are the pyrenearylamines disclosed in WO 2012/048780 and in WO 2013/185871. Also preferred are the benzoindenofluorenamines disclosed in WO 2014/037077, the benzofluorene- amines, the extended benzoindenofluorenes disclosed in WO 2014/111269 and in WO 2017/036574, the phenoxazines disclosed in WO 2017/028940 and in WO 2017/028941 and the fluorine derivatives bound to furan units or thiophene units disclosed in WO 2016/150544. Furthermore, boron compounds according to
WO 2020/208051 , WO 2015102118, WO 2016/152418, WO 2018/095397, WO 2019/004248, WO 2019/132040, US 2020/0161552 und WO 2021/089450 Verwendung finden. WO 2020/208051, WO 2015102118, WO 2016/152418, WO 2018/095397, WO 2019/004248, WO 2019/132040, US 2020/0161552 and WO 2021/089450 are used.
Nützliche Matrixmaterialien, vorzugsweise für fluoreszierende Verbin- dungen, umfassen Materialien verschiedener Substanzklassen. Bevor- zugte Matrixmaterialien sind ausgewählt aus den Klassen der Oligoaryle (z.B. 2,2',7,7'-Tetraphenylspirobifluoren nach EP 676461 oder Dinaphthyl- anthracen), insbesondere der Oligoaryle mit anellierten aromatischen Gruppen, der Oligoarylenvinylene (z.B. DPVBi oder Spiro-DPVBi gemäß EP 676461 ), der polypodalen Metallkomplexe (z.B. gemäß WO 2004/081017), der lochleitenden Verbindungen (z.B. gemäß WO 2004/058911 ), der elektronenleitenden Verbindungen, insbesondere Ketone, Phosphinoxide, Sulfoxide etc. (zum Beispiel nach WO 2005/084081 und WO 2005/084082), die Atropisomere (zum Beispiel nach WO 2006/048268), die Boronsäurederivate (zum Beispiel nach WO 2006/117052) oder die Benzanthracene (zum Beispiel nach WO 2008/145239). Besonders bevorzugte Matrixmaterialien sind ausgewählt aus den Klassen der Oligoarylene mit Naphthalin, Anthracen, Benz- anthracen und/oder Pyren oder Atropisomeren dieser Verbindungen, den Oligoarylenvinylenen, den Ketonen, den Phosphinoxiden und den Sulfoxiden. Ganz besonders bevorzugte Matrixmaterialien sind ausge- wählt aus den Klassen der Oligoarylene, die Anthracen, Benzanthracen, Benzophenanthren und/oder Pyren oder Atropisomere dieser Verbin- dungen umfassen. Unter einem Oligoarylen ist im Rahmen der vorliegen- den Erfindung eine Verbindung zu verstehen, in der mindestens drei Aryl- oder Arylengruppen miteinander verbunden sind. Weiter bevorzugt sind die in WO 2006/097208, WO 2006/131192, WO 2007/065550, WO 2007/110129, WO 2007/065678, WO 2008/145239, WO 2009/100925, WO 2011/054442 und EP 1553154 offenbarten Anthracenderivate, die in EP 1749809, EP 1905754 und US 2012/0187826 offenbarten Pyrenver- bindungen, die in WO 2015/158409 offenbarten Benzanthracenyl- anthracenverbindungen, die in WO 2017/025165 offenbarten Indeno- benzofurane und die in WO 2017/036573 offenbarten Phenanthryl- anthracene. Useful matrix materials, preferably for fluorescent compounds, include materials from different substance classes. Preferred matrix materials are selected from the classes of oligoaryls (e.g. 2,2',7,7'-tetraphenylspirobifluorene according to EP 676461 or dinaphthyl-anthracene), in particular oligoaryls with fused aromatic groups, oligoarylenevinylenes (e.g. DPVBi or spiro-DPVBi according to EP 676461), polypodal metal complexes (e.g. according to WO 2004/081017), hole-conducting compounds (e.g. according to WO 2004/058911), electron-conducting compounds, in particular ketones, phosphine oxides, sulfoxides etc. (for example according to WO 2005/084081 and WO 2005/084082), atropisomers (for example according to WO 2006/048268), boronic acid derivatives (for example according to WO 2006/117052) or the benzanthracenes (for example according to WO 2008/145239). Particularly preferred matrix materials are selected from the classes of oligoarylenes with naphthalene, anthracene, benzanthracene and/or pyrene or atropisomers of these compounds, the oligoarylenevinylenes, the ketones, the phosphine oxides and the sulfoxides. Very particularly preferred matrix materials are selected from the classes of oligoarylenes which include anthracene, benzanthracene, benzophenanthrene and/or pyrene or atropisomers of these compounds. In the context of the present invention, an oligoarylene is understood to mean a compound in which at least three aryl or arylene groups are connected to one another. Further preferred are the anthracene derivatives disclosed in WO 2006/097208, WO 2006/131192, WO 2007/065550, WO 2007/110129, WO 2007/065678, WO 2008/145239, WO 2009/100925, WO 2011/054442 and EP 1553154, the pyrene derivatives disclosed in EP 1749809, EP 1905754 and US 2012/0187826. compounds, the benzanthracenyl-anthracene compounds disclosed in WO 2015/158409, the indeno-benzofurans disclosed in WO 2017/025165 and the phenanthryl-anthracenes disclosed in WO 2017/036573.
Bevorzugte Matrixmaterialien für phosphoreszierende Verbindungen sind, ebenso wie Verbindungen gemäß Formel (1 ), aromatische Ketone, aromatische Phosphinoxide oder aromatische Sulfoxide oder Sulfone, z. B. gemäß WO 2004/013080, WO 2004/093207, WO 2006/005627 oder WO 2010/006680, Triarylamine, Carbazolderivate, z. B. CBP (N,N-Bis- carbazolylbiphenyl) oder WO 2005/039246, US 2005/0069729, JP 2004/288381 , EP 1205527, WO 2008/086851 oder WO 2013/041176, Indolocarbazolderivate, z. B. gemäß WO 2007/063754 oder WO 2008/056746, Indenocarbazolderivate, z. B. gemäß WO 2010/136109, WO 2011/000455, WO 2013/041176 oder WO 2013/056776, Azacarbazol- derivate, z. B. gemäß EP 1617710, EP 1617711 , EP 1731584, JP 2005/347160, bipolare Matrixmaterialien, z. B. gemäß WO 2007/137725, Silane, z. B. gemäß WO 2005/111172, Azaborole oder Boronester, z. B. gemäß WO 2006/117052, Triazinderivate, z. B. gemäß WO 2007/063754, WO 2008/056746, WO 2010/015306, WO 2011/057706, WO 2011/060859 oder WO 2011/060877, Zinkkomplexe, z. B. gemäß EP 652273 oder WO 2009/062578, Diazasilol- bzw. Tetraazasilol-Derivate, z. B. gemäß WO 2010/054729, Diazaphosphol-Derivate, z. B. gemäß WO 2010/054730, verbrückte Carbazol-Derivate, z. B. gemäß WO 2011/042107, WO 2011/060867, WO 2011/088877 und WO 2012/143080, Triphenylen- derivate, z. B. gemäß WO 2012/048781 , Lactame, z. B. gemäß WO 2011/116865 oder WO 2011/137951 , oder Dibenzofuranderivate, z. B. gemäß WO 2015/169412, WO 2016/015810, WO 2016/023608, WO 2017/148564 oder WO 2017/148565. Ebenso kann ein weiterer phos- phoreszierender Emitter, welcher kürzerwellig als der eigentliche Emitter emittiert, als Co-Host in der Mischung vorhanden sein oder eine Verbin- dung, die nicht oder nicht in wesentlichem Umfang am Ladungstransport teilnimmt, wie beispielsweise in WO 2010/108579 beschrieben. Preferred matrix materials for phosphorescent compounds are, as well as compounds according to formula (1), aromatic ketones, aromatic phosphine oxides or aromatic sulfoxides or sulfones, e.g. according to WO 2004/013080, WO 2004/093207, WO 2006/005627 or WO 2010/006680, triarylamines, carbazole derivatives, e.g. CBP (N,N-biscarbazolylbiphenyl) or WO 2005/039246, US 2005/0069729, JP 2004/288381, EP 1205527, WO 2008/086851 or WO 2013/041176, indolocarbazole derivatives, e.g. B. according to WO 2007/063754 or WO 2008/056746, indenocarbazole derivatives, e.g. according to WO 2010/136109, WO 2011/000455, WO 2013/041176 or WO 2013/056776, azacarbazole derivatives, e.g. according to EP 1617710, EP 1617711, EP 1731584, JP 2005/347160, bipolar matrix materials, e.g. according to WO 2007/137725, silanes, e.g. according to WO 2005/111172, azaboroles or boronic esters, e.g. according to WO 2006/117052, triazine derivatives, e.g. B. according to WO 2007/063754, WO 2008/056746, WO 2010/015306, WO 2011/057706, WO 2011/060859 or WO 2011/060877, zinc complexes, e.g. according to EP 652273 or WO 2009/062578, diazasilole or tetraazasilole derivatives, e.g. according to WO 2010/054729, diazaphosphole derivatives, e.g. according to WO 2010/054730, bridged carbazole derivatives, e.g. B. according to WO 2011/042107, WO 2011/060867, WO 2011/088877 and WO 2012/143080, triphenylene derivatives, e.g. according to WO 2012/048781, lactams, e.g. according to WO 2011/116865 or WO 2011/137951, or dibenzofuran derivatives, e.g. according to WO 2015/169412, WO 2016/015810, WO 2016/023608, WO 2017/148564 or WO 2017/148565. Likewise, another phosphorescent emitter, which emits at a shorter wavelength than the actual emitter, can be present in the mixture as a co-host or a compound that does not participate, or does not participate to a significant extent, in the charge transport, as described, for example, in WO 2010/108579.
Eine weitere Möglichkeit, die Leistungsdaten elektronischer Vorrichtungen, insbesondere organischer Elektrolumineszenzvorrichtungen, zu verbessern, besteht darin, Kombinationen aus zwei oder mehr Host- materialien in der Emissionsschicht zu verwenden. In US 6,392,250 B1 wird beispielsweise die Verwendung einer Mischung, bestehend aus einem Elektronentransportmaterial, einem Lochtransportmaterial und einem fluoreszierenden Emitter in der Emissionsschicht einer OLED offenbart. In US 6,803,720 B1 wird die Verwendung einer Mischung, enthaltend einen phosphoreszierenden Emitter sowie ein Loch- und ein Elektronentransportmaterial in der Emissionsschicht einer OLED offenbart. Another possibility to improve the performance of electronic devices, especially organic electroluminescent devices, improve the emission layer is to use combinations of two or more host materials in the emission layer. For example, US 6,392,250 B1 discloses the use of a mixture consisting of an electron transport material, a hole transport material and a fluorescent emitter in the emission layer of an OLED. US 6,803,720 B1 discloses the use of a mixture containing a phosphorescent emitter and a hole and an electron transport material in the emission layer of an OLED.
Daher ist es weiter bevorzugt, dass die Zusammensetzung der vorliegen- den Erfindung zusätzlich zu dem elektronentransportierenden Material ferner mindestens ein lochtransportierendes Hostmaterial enthält. Therefore, it is further preferred that the composition of the present invention further contains at least one hole-transporting host material in addition to the electron-transporting material.
Vorzugsweise ist das mindestens eine lochtransportierende Hostmaterial aus der Gruppe der Carbazol- und Triarylaminderivate, spezieller der Biscarbazole, der verbrückten Carbazole, der Triarylamine, der Dibenzo- furan-Carbazol-Derivate oder der Dibenzofuran-Amin-Derivate und der Carbazolamine ausgewählt. Preferably, the at least one hole-transporting host material is selected from the group of carbazole and triarylamine derivatives, more specifically biscarbazoles, bridged carbazoles, triarylamines, dibenzofuran-carbazole derivatives or dibenzofuran-amine derivatives and carbazolamines.
Weiter bevorzugt ist das mindestens eine lochtransportierende Host- material aus Verbindungen der Formel (h-1 ) oder (h-2) ausgewählt: wobei: More preferably, the at least one hole-transporting host material is selected from compounds of the formula (h-1) or (h-2): where:
K Ar4 oder -L5-N(Ar)2 ist; K is Ar 4 or -L 5 -N(Ar)2;
Z C-Rz oder C-RA ist; oder zwei benachbarte Gruppen Z zusammen einen kondensierten Ring bilden; RA-L3-AC5 oder -L4-N(Ar)2 ist; Z is CR z or CR A ; or two adjacent Z groups together form a fused ring; RA is -L 3 -AC 5 or -L 4 -N(Ar)2;
Rz bei jedem Auftreten gleich oder verschieden ausgewählt ist aus H, D, F, CI, Br, I, N(Ar)2, N(R‘)2, OAr, SAr, CN, NO2, OR‘, SR‘, COOR‘, C(=O)N(R‘)2, Si(R‘)3, B(OR‘)2, C(=O)R‘, P(=O)(R‘)2, S(=O)R‘, S(=O)2R‘, OSChR', einer geradkettigen Alkylgruppe mit 1 bis 20 Kohlenstoffatomen oder einer Alkenyl- oder Alkinylgruppe mit 2 bis 20 Kohlenstoffatomen oder einer verzweigten oder zyklischen Alkylgruppe mit 3 bis 20 Kohlen- stoffatomen, wobei die Alkyl-, Alkenyl- oder Alkinylgruppe jeweils mit einem oder mehreren Resten R substituiert sein kann, wobei eine oder mehrere nicht benachbarte CH2-Gruppen durch Si(R‘)2, C=O, NR‘, O, S oder CONR' ersetzt sein können, oder ein aromatisches oder hetero- aromatisches Ringsystem mit 5 bis 60 aromatischen Ringatomen, bevor- zugt 5 bis 40 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R‘ substituiert sein kann; R z is selected on each occurrence, identically or differently, from H, D, F, CI, Br, I, N(Ar) 2 , N(R') 2 , OAr, SAr, CN, NO 2 , OR', SR', COOR', C(=O)N(R')2, Si(R') 3 , B(OR')2, C(=O)R', P(=O)(R') 2 , S(=O)R', S(=O) 2 R', OSChR', a straight-chain alkyl group having 1 to 20 carbon atoms or an alkenyl or alkynyl group having 2 to 20 carbon atoms or a branched or cyclic alkyl group having 3 to 20 carbon atoms, where the alkyl, alkenyl or alkynyl group can each be substituted by one or more radicals R, where one or more non-adjacent CH2 groups can be replaced by Si(R')2, C=O, NR', O, S or CONR', or an aromatic or heteroaromatic ring system with 5 to 60 aromatic ring atoms, preferably 5 to 40 aromatic ring atoms, each of which can be substituted by one or more radicals R';
L4, L5 bei jedem Auftreten gleich oder verschieden eine Einfachbindung oder ein aromatisches oder heteroaromatisches Ringsystem mit 5 bis 30 aromatischen Ringatomen, das durch einen oder mehrere Reste R‘ substituiert sein kann, sind; L 4 , L 5 are, identically or differently on each occurrence, a single bond or an aromatic or heteroaromatic ring system having 5 to 30 aromatic ring atoms which may be substituted by one or more radicals R';
L3 eine Einfachbindung oder ein aromatisches oder heteroaromatisches Ringsystem mit 5 bis 30 aromatischen Ringatomen, welches durch einen oder mehrere Reste R‘ substituiert sein kann, ist, wobei ein Rest R‘ an L3 mit einem Rest Rz an dem Carbazol einen Ring bilden kann; L 3 is a single bond or an aromatic or heteroaromatic ring system having 5 to 30 aromatic ring atoms, which may be substituted by one or more radicals R', where a radical R' on L 3 can form a ring with a radical R z on the carbazole;
Ar4 ein aromatisches Ringsystem mit 6 bis 40 aromatischen Ring- atomen oder ein heteroaromatisches Ringsystem mit 5 bis 40 aroma- tischen Ringatomen, das durch einen oder mehrere Reste R‘ substituiert sein kann, ist; Ar 4 is an aromatic ring system having 6 to 40 aromatic ring atoms or a heteroaromatic ring system having 5 to 40 aromatic ring atoms, which may be substituted by one or more radicals R';
Ar5 bei jedem Auftreten gleich oder verschieden ein unsubstituiertes oder substituiertes heteroaromatisches Ringsystem mit 5 bis 40 aroma- tischen Ringatomen, das durch ein oder mehrere R‘ substituiert sein kann, ist; Rzbei jedem Auftreten gleich oder verschieden H, D, F, CI, Br, I, N(Ar)2, N(R‘)2, OAr, SAr, CN, NO2, OR‘, SR‘, COOR‘, C(=O)N(R‘)2, Si(R‘)3, B(OR‘)2, C(=O)R‘, P(=O)(R‘)2, S(=O)R‘, S(=O)2R‘, OSO2R‘, eine gerad- kettige Alkylgruppe mit 1 bis 20 Kohlenstoffatomen oder eine Alkenyl- oder Alkinylgruppe mit 2 bis 20 Kohlenstoffatomen oder eine verzweigte oder zyklische Alkylgruppe mit 3 bis 20 Kohlenstoffatomen, wobei die Alkyl-, Alkenyl- oder Alkinylgruppe jeweils mit einem oder mehreren Resten R‘ substituiert sein kann, wobei eine oder mehrere nicht benachbarte CH2- Gruppen durch Si(R‘)2, C=O, NR‘, O, S oder CONR' ersetzt sein können, oder ein aromatisches oder heteroaromatisches Ringsystem mit 5 bis 60 aromatischen Ringatomen, bevorzugt 5 bis 40 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R‘ substituiert sein kann, ist, wobei auch zwei Reste Rz zusammen ein Ringsystem bilden können; Ar 5 is, identically or differently at each occurrence, an unsubstituted or substituted heteroaromatic ring system having 5 to 40 aromatic ring atoms, which may be substituted by one or more R'; R z is, identically or differently on each occurrence, H, D, F, CI, Br, I, N(Ar)2, N(R') 2 , OAr, SAr, CN, NO 2 , OR', SR', COOR', C(=O)N(R') 2 , Si(R') 3 , B(OR')2, C(=O)R', P(=O)(R')2, S(=O)R', S(=O) 2 R', OSO 2 R', a straight-chain alkyl group having 1 to 20 carbon atoms or an alkenyl or alkynyl group having 2 to 20 carbon atoms or a branched or cyclic alkyl group having 3 to 20 carbon atoms, where the alkyl, alkenyl or alkynyl group may each be substituted by one or more radicals R', where one or more non-adjacent CH2 groups are substituted by Si(R')2, C=O, NR', O, S or CONR', or is an aromatic or heteroaromatic ring system having 5 to 60 aromatic ring atoms, preferably 5 to 40 aromatic ring atoms, each of which may be substituted by one or more radicals R', where two radicals R z together may also form a ring system;
E bei jedem Auftreten unabhängig eine Einfachbindung oder eine Gruppe C(R°)2 ist; E is independently at each occurrence a single bond or a C(R°)2 group;
R° bei jedem Auftreten unabhängig ausgewählt ist aus einer gerad- kettigen Alkylgruppe mit 1 bis 10 Kohlenstoffatomen oder einer ver- zweigten oder zyklischen Alkylgruppe mit 3 bis 10 Kohlenstoffatomen, die jeweils durch einen oder mehrere Reste R‘ substituiert sein können; x, y unabhängig aus 0 oder 1 ausgewählt sind, wobei dann, wenn x oder y 0 ist, die entsprechende Gruppe E nicht vorhanden ist; und x + y = 1 oder 2 ist; R° is independently selected at each occurrence from a straight-chain alkyl group having 1 to 10 carbon atoms or a branched or cyclic alkyl group having 3 to 10 carbon atoms, each of which may be substituted by one or more R' radicals; x, y are independently selected from 0 or 1, wherein when x or y is 0, the corresponding group E is not present; and x + y = 1 or 2;
Ar ist bei jedem Auftreten gleich oder verschieden ein aromatisches oder heteroaromatisches Ringsystem mit 5 bis 40 aromatischen Ring- atomen, das durch einen oder mehrere Reste R“ substituiert sein kann, wobei zwei oder mehr R“ miteinander ein aromatisches oder hetero- aromatisches Ringsystem bilden können; Ar is, identically or differently at each occurrence, an aromatic or heteroaromatic ring system having 5 to 40 aromatic ring atoms which may be substituted by one or more radicals R", where two or more R" may together form an aromatic or heteroaromatic ring system;
R‘ bei jedem Auftreten gleich oder verschieden H, D, F, CI, Br, I, N(Ar)2, N(R“)2, OAr, SAr, CN, NO2, OR“, SR“, COOR“, C(=O)N(R“)2, Si(R“)3, B(OR“)2, C(=O)R“, P(=O)(R“)2, S(=O)R“, S(=O)2R“, OSO2R“, eine geradkettige Alkylgruppe mit 1 bis 20 Kohlenstoffatomen oder eine Alkenyl- oder Alkinylgruppe mit 2 bis 20 Kohlenstoffatomen oder eine verzweigte oder zyklische Alkylgruppe mit 3 bis 20 Kohlenstoffatomen, wobei die Alkyl-, Alkenyl- oder Alkinylgruppe jeweils mit einem oder mehreren Resten R“ substituiert sein kann, wobei eine oder mehrere nicht benachbarte CH2-Gruppen durch Si(R“)2, C=O, NR“, O, S oder CONR“ ersetzt sein können, oder ein aromatisches oder heteroaromatisches Ringsystem mit 5 bis 60 aromatischen Ringatomen, bevorzugt 5 bis 40 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R“ substituiert sein kann, ist, wobei auch zwei Reste R‘ zusammen ein Ringsystem bilden können; R' is, identically or differently at each occurrence, H, D, F, CI, Br, I, N(Ar) 2 , N(R“)2, OAr, SAr, CN, NO2, OR“, SR“, COOR“, C(=O)N(R“) 2 , Si(R“) 3 , B(OR“)2, C(=O)R“, P(=O)(R“)2, S(=O)R“, S(=O) 2 R“, OSO2R“, a straight-chain alkyl group having 1 to 20 carbon atoms or a Alkenyl or alkynyl group having 2 to 20 carbon atoms or a branched or cyclic alkyl group having 3 to 20 carbon atoms, where the alkyl, alkenyl or alkynyl group can each be substituted by one or more radicals R", where one or more non-adjacent CH2 groups can be replaced by Si(R")2, C=O, NR", O, S or CONR", or an aromatic or heteroaromatic ring system having 5 to 60 aromatic ring atoms, preferably 5 to 40 aromatic ring atoms, which can each be substituted by one or more radicals R", where two radicals R' together can also form a ring system;
R“ bei jedem Auftreten gleich oder verschieden H, D, F, CI, Br, I, N(R“‘)2, CN, NO2, OR“‘, SR, COOR'“, C(=O)N(R‘“)2, Si(R‘“)3, B(OR‘“)2, C(=O)R“‘, P(=O)(R“‘)2, S(=O)R“‘, S(=O)2R“‘, OSO2R'“, eine geradkettige Alkylgruppe mit 1 bis 20 Kohlenstoffatomen oder eine Alkenyl- oder Alkinylgruppe mit 2 bis 20 Kohlenstoffatomen oder eine verzweigte oder zyklische Alkylgruppe mit 3 bis 20 Kohlenstoffatomen, wobei die Alkyl-, Alkenyl- oder Alkinylgruppe jeweils mit einem oder mehreren Resten R“‘ substituiert sein kann, wobei eine oder mehrere nicht benachbarte CH2- Gruppen durch Si(R“‘)2, C=O, NR“‘, O, S oder CONR'“ ersetzt sein können, oder ein aromatisches oder heteroaromatisches Ringsystem mit 5 bis 60 aromatischen Ringatomen, bevorzugt 5 bis 40 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R“‘ substituiert sein kann, ist, wobei auch zwei Reste R“ zusammen ein Ringsystem bilden können; R“ is, identically or differently on each occurrence, H, D, F, CI, Br, I, N(R“')2, CN, NO2, OR“', SR, COOR'“, C(=O)N(R'“) 2 , Si(R'“) 3 , B(OR'“) 2 , C(=O)R“', P(=O)(R“')2, S(=O)R“', S(=O)2R“', OSO2R'“, a straight-chain alkyl group having 1 to 20 carbon atoms or an alkenyl or alkynyl group having 2 to 20 carbon atoms or a branched or cyclic alkyl group having 3 to 20 carbon atoms, where the alkyl, alkenyl or alkynyl group may each be substituted by one or more radicals R“', where one or more non-adjacent CH2 groups are replaced by Si(R“')2, C=O, NR"', O, S or CONR'", or is an aromatic or heteroaromatic ring system having 5 to 60 aromatic ring atoms, preferably 5 to 40 aromatic ring atoms, each of which may be substituted by one or more radicals R"', where two radicals R" together may also form a ring system;
R“‘ ist bei jedem Auftreten gleich oder verschieden H, D, F, CN oder ein aliphatischer, aromatischer oder heteroaromatischer organischer Rest mit 1 bis 20 C-Atomen, in dem auch ein oder mehrere H-Atome durch D oder F ersetzt sein können; dabei können zwei oder mehr Reste R“‘ zusammen ein Ringsystem bilden. mit der Maßgabe, dass die Verbindungen der Formeln (h-1 ) und (h-2) mindestens eine Gruppe Z umfassen, die für RA steht. Vorzugsweise sind L4, L5 bei jedem Auftreten gleich oder verschieden eine Einfachbindung oder ein aromatisches oder heteroaromatisches Ring- system mit 5 bis 25, weiter bevorzugt 5 bis 20 und noch weiter bevorzugt 6 bis 18 aromatischen Ringatomen, welches durch einen oder mehrere Reste R‘ substituiert sein kann. R"' is on each occurrence, identically or differently, H, D, F, CN or an aliphatic, aromatic or heteroaromatic organic radical having 1 to 20 C atoms, in which one or more H atoms can be replaced by D or F; two or more radicals R"' together can form a ring system. with the proviso that the compounds of the formulae (h-1) and (h-2) comprise at least one group Z which stands for R A. Preferably, L 4 , L 5 are, identically or differently on each occurrence, a single bond or an aromatic or heteroaromatic ring system having 5 to 25, more preferably 5 to 20 and even more preferably 6 to 18 aromatic ring atoms, which may be substituted by one or more radicals R'.
Vorzugsweise ist L3 eine Einfachbindung oder ein aromatisches oder heteroaromatisches Ringsystem mit 5 bis 25 aromatischen Ringatomen, weiter bevorzugt 5 bis 20 und noch weiter bevorzugt 6 bis 18 aroma- tischen Ringatomen, welches durch einen oder mehrere Reste R‘ substi- tuiert sein kann, wobei ein Rest R‘ an L3 mit einem Rest Rz an dem Carbazol einen Ring bilden kann. Preferably, L 3 is a single bond or an aromatic or heteroaromatic ring system having 5 to 25 aromatic ring atoms, more preferably 5 to 20 and even more preferably 6 to 18 aromatic ring atoms, which can be substituted by one or more radicals R', where a radical R' on L 3 can form a ring with a radical R z on the carbazole.
Vorzugsweise ist die Gruppe Ar5 ein unsubstituiertes oder substituiertes heteroaromatisches Ringsystem, das aus den Gruppen der Formeln (Ar5-1 ) bis (Ar5-6) ausgewählt ist, wobei die gestrichelte Bindung die Anbindung an L3 oder Z anzeigt; Preferably, the group Ar 5 is an unsubstituted or substituted heteroaromatic ring system selected from the groups of formulas (Ar5-1) to (Ar5-6), where the dashed bond indicates the attachment to L 3 or Z;
V C-Rv ist, mit der Maßgabe, dass V für C steht, wenn es an die Gruppe der Formel (h-1 ) oder (h-2) gebunden ist; oder zwei benachbarte GruppenV is CR v , with the proviso that V is C when it is bonded to the group of formula (h-1 ) or (h-2); or two adjacent groups
V zusammen einen kondensierten Ring bilden; V together form a condensed ring;
T C-RT ist, mit der Maßgabe, dass T für C steht, wenn es an die Gruppe der Formel (h-1 ) oder (h-2) gebunden ist, oder zwei benachbarte Gruppen T zusammen einen kondensierten Ring bilden; T is CR T , with the proviso that T is C when bonded to the group of formula (h-1) or (h-2), or two adjacent groups T together form a fused ring;
M ein aromatisches Ringsystem mit 6 bis 40 aromatischen Ringatomen oder ein heteroaromatisches Ringsystem mit 5 bis 40 aromatischen Ringatomen, das durch einen oder mehrere Reste R substituiert sein kann, ist; M is an aromatic ring system having 6 to 40 aromatic ring atoms or a heteroaromatic ring system having 5 to 40 aromatic ring atoms, which may be substituted by one or more radicals R;
E1 bei jedem Auftreten unabhängig eine Einfachbindung oder eine Gruppe C(R°)2 ist; wobei R° die gleiche Bedeutung wie oben hat; E 1 is independently at each occurrence a single bond or a group C(R°)2; where R° has the same meaning as above;
RT’ Rv bei jedem Auftreten gleich oder verschieden ausgewählt ist aus H, D, F, CI, Br, I, N(Ar)2, N(R‘)2, OAr, SAr, CN, NO2, OR‘, SR‘, COOR‘, C(=O)N(R‘)2, Si(R‘)3, B(OR‘)2, C(=O)R‘, P(=O)(R‘)2, S(=O)R‘, S(=O)2R‘, OSChR', einer geradkettigen Alkylgruppe mit 1 bis 20 Kohlenstoffatomen oder einer Alkenyl- oder Alkinylgruppe mit 2 bis 20 Kohlenstoffatomen oder einer verzweigten oder zyklischen Alkylgruppe mit 3 bis 20 Kohlenstoffatomen, wobei die Alkyl-, Alkenyl- oder Alkinylgruppe jeweils mit einem oder mehreren Resten R‘ substituiert sein kann, wobei eine oder mehrere nicht benachbarte CH2-Gruppen durch Si(R‘)2, C=O, NR‘, O, S oder CONR' ersetzt sein können, oder ein aromatisches oder hetero- aromatisches Ringsystem mit 5 bis 60 aromatischen Ringatomen, bevor- zugt 5 bis 40 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R‘ substituiert sein kann, wobei auch zwei Reste RT zusammen ein Ringsystem bilden können und zwei Reste Rv zusammen ein Ringsystem bilden können; x1, y1 unabhängig aus 0 oder 1 ausgewählt sind, wobei dann, wenn x1 oder y1 0 ist, die entsprechende Gruppe E1 nicht vorhanden ist; mit der Maßgabe, dass x1 + y1 = 1 oder 2; und wobei R‘ und Ar die gleiche Bedeutung wie oben haben. R T ' R v is selected on each occurrence, identically or differently, from H, D, F, CI, Br, I, N(Ar) 2 , N(R') 2 , OAr, SAr, CN, NO 2 , OR', SR', COOR', C(=O)N(R') 2 , Si(R') 3 , B(OR') 2 , C(=O)R', P(=O)(R') 2 , S(=O)R', S(=O) 2 R', OSChR', a straight-chain alkyl group having 1 to 20 carbon atoms or an alkenyl or alkynyl group having 2 to 20 carbon atoms or a branched or cyclic alkyl group having 3 to 20 carbon atoms, where the alkyl, alkenyl or alkynyl group can each be substituted by one or more radicals R', where one or more non-adjacent CH2 groups can be replaced by Si(R')2, C=O, NR', O, S or CONR', or an aromatic or heteroaromatic ring system with 5 to 60 aromatic ring atoms, preferably 5 to 40 aromatic ring atoms, each of which can be substituted by one or more radicals R', where two radicals R T together can form a ring system and two radicals R v together can form a ring system; x 1 , y 1 are independently selected from 0 or 1, wherein when x 1 or y 1 is 0, the corresponding group E 1 is not present; with the proviso that x 1 + y 1 = 1 or 2; and wherein R' and Ar have the same meaning as above.
Gemäß einer bevorzugten Ausführungsform ist das mindestens eine lochtransportierende Hostmaterial aus Verbindungen der Formel (h-1 -1 ) bis (h-2-2) ausgewählt: According to a preferred embodiment, the at least one hole-transporting host material is selected from compounds of the formula (h-1-1) to (h-2-2):
wobei die Symbole die gleiche Bedeutung wie oben haben und wobei die Indices die folgende Bedeutung haben: x, y, x1,y1 haben die gleiche Bedeutung wie oben; c, f stehen unabhängig für 0, 1 , 2, 3 oder 4; d, e stehen unabhängig für 0, 1 , 2 oder 3; g steht für 0, 1 , 2 oder 3, wenn x1=0; oder für 0, 1 oder 2, wenn x1=1 ; h steht für 0, 1 , 2, 3 oder 4, wenn y1=0; oder für 0, 1 , 2 oder 3, wenn y1=1 ; k steht für 0, 1 , 2, 3 oder 4, wenn x=0; oder für 0, 1 , 2 oder 3, wenn x=1 ; und where the symbols have the same meaning as above and where the indices have the following meaning: x, y, x 1 ,y 1 have the same meaning as above; c, f independently stand for 0, 1 , 2, 3 or 4; d, e independently stand for 0, 1 , 2 or 3; g stands for 0, 1 , 2 or 3 if x 1 =0; or for 0, 1 or 2 if x 1 =1 ; h stands for 0, 1 , 2, 3 or 4 if y 1 =0; or for 0, 1 , 2 or 3 if y 1 =1 ; k stands for 0, 1 , 2, 3 or 4 if x=0; or for 0, 1 , 2 or 3 if x=1 ; and
I steht für 0, 1 , 2 oder 3, wenn y=0; oder für 0, 1 oder 2, wenn y=1 . I stands for 0, 1 , 2 or 3 if y=0; or for 0, 1 or 2 if y=1 .
Beispiele für lochtransportierende Hostmaterialien, die als zweites Hostmaterial in der Zusammensetzung geeignet sind, sind in der folgenden Tabelle abgebildet: Examples of hole-transporting host materials suitable as a second host material in the composition are shown in the following table:
Des Weiteren ist es bevorzugt, dass der mindestens eine blau phospho- reszierende Metallkomplex aus Platinkomplexen ausgewählt ist. Vorzugsweise hat der mindestens eine blau phosphoreszierende Metall- komplex ein LUMO von -1 ,8 eV bis -2,2 eV, und der mindestens eine blau phosphoreszierende Metallkomplex hat vorzugsweise ein HOMO von -5,0 eV bis -5,6 eV, wie durch quantenmechanische Berechnungen definiert. Furthermore, it is preferred that the at least one blue phosphorescent metal complex is selected from platinum complexes. Preferably, the at least one blue phosphorescent metal complex has a LUMO of -1.8 eV to -2.2 eV, and the at least one blue phosphorescent metal complex preferably has a HOMO of -5.0 eV to -5.6 eV, as defined by quantum mechanical calculations.
Vorzugsweise ist die Energie des niedrigsten Triplettzustands Ti des mindestens einen blau phosphoreszierenden Metallkomplexes höher als 2,55 eV, weiter bevorzugt >2,65 eV, noch weiter bevorzugt >2,75 eV, wie durch quantenmechanische Berechnungen definiert. Preferably, the energy of the lowest triplet state Ti of the at least one blue phosphorescent metal complex is higher than 2.55 eV, more preferably >2.65 eV, even more preferably >2.75 eV, as defined by quantum mechanical calculations.
Wie oben erwähnt, werden die Energieniveaus von Molekülorbitalen, wie dem höchsten besetzten Molekülorbital (Highest Occupied Molecular Orbital, HOMO) und dem niedrigsten unbesetzten Molekülorbital (Lowest Unoccupied Molecular Orbital, LUMO), des niedrigsten Triplettzustands Ti oder des niedrigsten angeregten Singulettzustands Si von Materialien über quantenmechanische Rechnungen bestimmt. Zur Berechnung orga- nischer Substanzen ohne Metalle wird zuerst eine Geometrieoptimierung mit der Methode „Ground State/Semi-empirical/Default Spin/AM1 /Charge O/Spin Singlet“ durchgeführt. Im Anschluss erfolgt auf Grundlage der optimierten Geometrie eine Energierechnung. Hierbei wird die Methode „TD-SCF/DFT/Default Spin/B3PW91“ mit dem Basissatz „6-31 G(d)“ (Charge 0, Spin Singlet) verwendet. Für metallhaltige Verbindungen wird die Geometrie über die Methode „Ground State/ Hartree-Fock/Default Spin/LanL2MB/Charge O/Spin Singlet“ optimiert. Die Energierechnung erfolgt analog zu der oben beschriebenen Methode für die organischen Substanzen mit dem Unterschied, dass für das Metallatom der Basissatz „LanL2DZ“ verwendet wird und für die Liganden der Basissatz „6-31 G(d)“ verwendet wird. Aus der Energierechnung erhält man das HOMO-Energie- niveau HEh bzw. LUMO-Energieniveau LEh in Hartree-Einheiten. Daraus werden die anhand von Zyklovoltammetriemessungen kalibrierten HOMO- und LUMO-Energieniveaus in Elektronenvolt wie folgt bestimmt: As mentioned above, the energy levels of molecular orbitals, such as the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), the lowest triplet state Ti or the lowest excited singlet state Si of materials are determined using quantum mechanical calculations. To calculate organic substances without metals, a geometry optimization is first carried out using the "Ground State/Semi-empirical/Default Spin/AM1 /Charge O/Spin Singlet" method. An energy calculation is then carried out based on the optimized geometry. The "TD-SCF/DFT/Default Spin/B3PW91" method with the "6-31 G(d)" basis set (Charge 0, Spin Singlet) is used. For metal-containing compounds, the geometry is optimized using the "Ground State/Hartree-Fock/Default Spin/LanL2MB/Charge O/Spin Singlet" method. The energy calculation is carried out analogously to the method described above for the organic substances, with the difference that the "LanL2DZ" basis set is used for the metal atom and the "6-31 G(d)" basis set is used for the ligands. The energy calculation yields the HOMO energy level HEh or LUMO energy level LEh in Hartree units. From this, the HOMO and LUMO energy levels calibrated using cyclic voltammetry measurements are determined in electron volts as follows:
HOMO(eV) = ((HEh*27,212)-0,9899)/1 ,1206 HOMO(eV) = ((HEh*27.212)-0.9899)/1 .1206
LUMO(eV) = ((LEh*27,212)-2,0041)/1 ,385 Diese Werte sind im Sinne dieser Anmeldung als HOMO- bzw. LUMO- Energieniveaus der Materialien anzusehen. LUMO(eV) = ((LEh*27.212)-2.0041)/1.385 For the purposes of this application, these values are to be regarded as HOMO or LUMO energy levels of the materials.
Der niedrigste Triplettzustand Ti ist definiert als die Energie des Triplett- zustands mit der niedrigsten Energie, der sich aus der beschriebenen quantenchemischen Rechnung ergibt. The lowest triplet state Ti is defined as the energy of the triplet state with the lowest energy resulting from the quantum chemical calculation described.
Der niedrigste angeregte Singulettzustand Si ist definiert als die Energie des angeregten Singulettzustands mit der niedrigsten Energie, der sich aus der beschriebenen quantenchemischen Rechnung ergibt. The lowest excited singlet state Si is defined as the energy of the excited singlet state with the lowest energy resulting from the quantum chemical calculation described.
Die hierin beschriebene Methode ist unabhängig von dem verwendeten Softwarepaket und liefert immer dieselben Ergebnisse. Beispiele für oft benutzte Programme für diesen Zweck sind „GaussianO9W‘ (Gaussian Inc.) und Q-Chem 4.1 (Q-Chem, Inc.). The method described here is independent of the software package used and always gives the same results. Examples of commonly used programs for this purpose are 'GaussianO9W' (Gaussian Inc.) and Q-Chem 4.1 (Q-Chem, Inc.).
Als blau phosphoreszierende Metallkomplexe eignen sich die Verbin- dungen der Formel (Pt-1 ) gemäß nachstehender Definition sehr gut: wobei: The compounds of the formula (Pt-1 ) according to the following definition are very suitable as blue phosphorescent metal complexes: where:
Y1, Y2, Y3, Y4, Y5 bei jedem Auftreten gleich oder verschieden für eine Gruppe CRY oder N stehen; oder Y1-Y2 und/oder Y3-Y4 oder Y4-Y5 einen kondensierten Aryl- oder Heteroarylring mit 5 bis 18 aromatischen Ringatomen bilden können, der jeweils auch durch einen oder mehrere Reste R‘ substituiert sein kann; E50 bei jedem Auftreten gleich oder verschieden für C(RC0)2, NRN0, 0 oder S steht; Y 1 , Y 2 , Y 3 , Y 4 , Y 5 on each occurrence, identically or differently, represent a group CR Y or N; or Y 1 -Y 2 and/or Y 3 -Y 4 or Y 4 -Y 5 can form a condensed aryl or heteroaryl ring having 5 to 18 aromatic ring atoms, which in each case can also be substituted by one or more radicals R'; E 50 stands for C(R C0 )2, NR N0 , 0 or S, identically or differently at each occurrence;
Ar50 bei jedem Auftreten gleich oder verschieden ein aromatisches oder heteroaromatisches Ringsystem mit 5 bis 60 aromatischen Ringatomen, das jeweils auch durch einen oder mehrere Reste R‘ substituiert sein kann, ist; Ar 50 is, identically or differently on each occurrence, an aromatic or heteroaromatic ring system having 5 to 60 aromatic ring atoms, which may in each case also be substituted by one or more radicals R';
Ar51, Ar52, Ar53 gleich oder verschieden für einen kondensierten Aryl- oder Heteroarylring mit 5 bis 18 aromatischen Ringatomen, der jeweils auch durch einen oder mehrere Reste R‘ substituiert sein kann, stehen; Ar 51 , Ar 52 , Ar 53 are the same or different and represent a condensed aryl or heteroaryl ring having 5 to 18 aromatic ring atoms, which may each also be substituted by one or more radicals R';
RY bei jedem Auftreten gleich oder verschieden für einen Rest ausgewählt aus H, D, F, CI, Br, I, CHO, CN, C(=O)Ar, P(=O)(Ar)2, S(=O)Ar, S(=O)2Ar, N(R‘)2, N(Ar)2, NO2, Si(R‘)3, B(OR‘)2, OSO2R', einer geradkettigen Alkyl-, Alkoxy- oder Thioalkylgruppe mit 1 bis 40 C-Atomen oder einer verzweig- ten oder zyklischen Alkyl-, Alkoxy- oder Thioalkylgruppe mit 3 bis 40 C- Atomen, die jeweils durch einen oder mehrere Reste R‘ substituiert sein können, wobei jeweils eine oder mehrere nicht benachbarte CH2-Gruppen durch R‘C=CR‘, C^C, Si(R‘)2, Ge(R‘)2, Sn(R‘)2, C=O, C=S, C=Se, P(=O)(R‘), SO, SO2, O, S oder CONR' ersetzt sein können und wobei ein oder mehrere H-Atome durch D, F, CI, Br, I, CN oder NO2 ersetzt sein können, einem aromatischen oder heteroaromatischen Ringsystem mit 5 bis 60 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R‘ substituiert sein kann, und einer Aryloxygruppe mit 5 bis 60 aromatischen Ringatomen, die durch einen oder mehrere Reste R‘ sub- stituiert sein kann, steht, wobei zwei Reste RY zusammen ein alipha- tisches, aromatisches oder heteroaromatisches Ringsystem, das durch einen oder mehrere Reste R‘ substituiert sein kann, bilden können; R Y on each occurrence, identically or differently, represents a radical selected from H, D, F, CI, Br, I, CHO, CN, C(=O)Ar, P(=O)(Ar) 2 , S(=O)Ar, S(=O) 2 Ar, N(R') 2 , N(Ar) 2 , NO2, Si(R')3, B(OR')2, OSO2R', a straight-chain alkyl, alkoxy or thioalkyl group having 1 to 40 C atoms or a branched or cyclic alkyl, alkoxy or thioalkyl group having 3 to 40 C atoms, each of which may be substituted by one or more radicals R', where in each case one or more non-adjacent CH2 groups are substituted by R'C=CR', C^C, Si(R') 2 , Ge(R') 2 , Sn(R') 2 , C=O, C=S, C=Se, P(=O)(R'), SO, SO2, O, S or CONR' and where one or more H atoms can be replaced by D, F, CI, Br, I, CN or NO2, an aromatic or heteroaromatic ring system having 5 to 60 aromatic ring atoms, each of which can be substituted by one or more R' radicals, and an aryloxy group having 5 to 60 aromatic ring atoms, which can be substituted by one or more R' radicals, where two R Y radicals together can form an aliphatic, aromatic or heteroaromatic ring system, which can be substituted by one or more R'radicals;
Rco bei jedem Auftreten gleich oder verschieden für einen Rest ausge- wählt aus H, D, einer geradkettigen Alkylgruppe mit 1 bis 40 C-Atomen, die durch einen oder mehrere Reste R‘ substituiert sein kann, einer Aryl- oder Heteroarylgruppe mit 6 bis 18 aromatischen Ringatomen, die jeweils durch einen oder mehrere Reste R substituiert sein kann, steht, wobei zwei Reste Rc zusammen ein aliphatisches, aromatisches oder hetero- aromatisches Ringsystem, das durch einen oder mehrere Reste R‘ sub- stituiert ist, bilden können; R co on each occurrence, identically or differently, represents a radical selected from H, D, a straight-chain alkyl group having 1 to 40 C atoms, which may be substituted by one or more radicals R', an aryl or heteroaryl group having 6 to 18 aromatic ring atoms, each of which may be substituted by one or more radicals R, where two radicals R c together can form an aliphatic, aromatic or heteroaromatic ring system which is substituted by one or more radicals R';
RN0 bei jedem Auftreten gleich oder verschieden für einen Rest aus- gewählt aus H, D, F, einer geradkettigen Alkylgruppe mit 1 bis 40 C- Atomen oder einer verzweigten oder zyklischen Alkylgruppe mit 3 bis 40 C-Atomen, die jeweils durch einen oder mehrere Reste R‘ substituiert ist und wobei ein oder mehrere H-Atome durch D, F oder CN ersetzt sein können, einem aromatischen oder heteroaromatischem Ringsystem mit 5 bis 60 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R‘ substituiert sein kann, steht; R N0 on each occurrence, identically or differently, represents a radical selected from H, D, F, a straight-chain alkyl group having 1 to 40 C atoms or a branched or cyclic alkyl group having 3 to 40 C atoms, each of which is substituted by one or more radicals R' and where one or more H atoms may be replaced by D, F or CN, an aromatic or heteroaromatic ring system having 5 to 60 aromatic ring atoms, each of which may be substituted by one or more radicals R';
R‘ und Ar die gleiche Bedeutung wie oben haben. R‘ and Ar have the same meaning as above.
Vorzugsweise ist Ar50 bei jedem Auftreten gleich oder verschieden ein aromatisches oder heteroaromatisches Ringsystem mit 5 bis 40, weiter bevorzugt 5 bis 30 und noch weiter bevorzugt 6 bis 18 aromatischen Ringatomen, das jeweils auch durch einen oder mehrere Reste R‘ substituiert sein kann. Preferably, Ar 50 is, identically or differently on each occurrence, an aromatic or heteroaromatic ring system having 5 to 40, more preferably 5 to 30 and even more preferably 6 to 18 aromatic ring atoms, which may in each case also be substituted by one or more radicals R'.
Vorzugsweise stehen Ar51, Ar52, Ar53 gleich oder verschieden für einen kondensierten Aryl- oder Heteroarylring mit 6 aromatischen Ringatomen, der jeweils auch durch einen oder mehrere Reste R‘ substituiert sein kann. Preferably, Ar 51 , Ar 52 , Ar 53 are the same or different and represent a condensed aryl or heteroaryl ring having 6 aromatic ring atoms, which may each also be substituted by one or more radicals R'.
Vorzugsweise steht RY bei jedem Auftreten gleich oder verschieden für H, D, F, eine geradkettige Alkyl-, Alkoxy- oder Thioalkylgruppe mit 1 bis 40, vorzugsweise 1 bis 20 und weiter bevorzugt 1 bis 10 C-Atomen oder eine verzweigte oder zyklische Alkyl-, Alkoxy- oder Thioalkylgruppe mit 3 bis 40, vorzugsweise 3 bis 20 und weiter bevorzugt 3 bis 10 C-Atomen, die jeweils durch einen oder mehrere Reste R‘ substituiert sein kann, wobei jeweils eine oder mehrere nicht benachbarte CH2-Gruppen durch R‘C=CR‘, C=C, O oder S ersetzt sein können und wobei ein oder mehrere H-Atome durch D oder F ersetzt sein können, ein aromatisches oder heteroaromatisches Ringsystem mit 5 bis 60, vorzugsweise 5 bis 40, weiter bevorzugt 5 bis 30 und besonders bevorzugt 5 bis 18 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R‘ substituiert sein kann. Preferably, R Y on each occurrence is identical or different and represents H, D, F, a straight-chain alkyl, alkoxy or thioalkyl group having 1 to 40, preferably 1 to 20 and more preferably 1 to 10 C atoms or a branched or cyclic alkyl, alkoxy or thioalkyl group having 3 to 40, preferably 3 to 20 and more preferably 3 to 10 C atoms, each of which may be substituted by one or more R' radicals, where one or more non-adjacent CH2 groups may be replaced by R'C=CR', C=C, O or S and where one or more H atoms may be replaced by D or F, an aromatic or heteroaromatic ring system having 5 to 60, preferably 5 to 40, more preferably 5 to 30 and particularly preferably 5 to 18 aromatic ring atoms, each of which may be substituted by one or more R' radicals.
Vorzugsweise steht Rco bei jedem Auftreten gleich oder verschieden für einen Rest ausgewählt aus H, D, einer geradkettigen Alkylgruppe mit 1 bis 10, vorzugsweise 1 bis 6 und weiter bevorzugt 1 bis 3 C-Atomen, die durch einen oder mehrere Reste R‘ substituiert sein kann, einer Aryl- oder Heteroarylgruppe mit 6 bis 18 und vorzugsweise 6 bis 12 aromatischen Ringatomen, die jeweils durch einen oder mehrere Reste R‘ substituiert sein kann, wobei zwei Reste Rco zusammen ein aliphatisches, aroma- tisches oder heteroaromatisches Ringsystem, das durch einen oder mehrere Reste R‘ substituiert ist, bilden können. Preferably, R co on each occurrence, identically or differently, represents a radical selected from H, D, a straight-chain alkyl group having 1 to 10, preferably 1 to 6 and more preferably 1 to 3 C atoms, which may be substituted by one or more radicals R', an aryl or heteroaryl group having 6 to 18 and preferably 6 to 12 aromatic ring atoms, each of which may be substituted by one or more radicals R', where two radicals R co together may form an aliphatic, aromatic or heteroaromatic ring system which is substituted by one or more radicals R'.
Vorzugsweise steht RN0 bei jedem Auftreten gleich oder verschieden für einen Rest ausgewählt aus einem aromatischen oder heteroaromatischen Ringsystem mit 5 bis 60, bevorzugt 5 bis 40, weiter bevorzugt 5 bis 30 und noch weiter bevorzugt 5 bis 18 aromatischen Ringatomen, das jeweils durch einen oder mehrere Reste R‘ substituiert sein kann. Preferably, R N0 on each occurrence, identically or differently, represents a radical selected from an aromatic or heteroaromatic ring system having 5 to 60, preferably 5 to 40, more preferably 5 to 30 and even more preferably 5 to 18 aromatic ring atoms, which may in each case be substituted by one or more radicals R'.
Beispiele für besonders geeignete blau phosphoreszierende Metall- komplexe sind nachstehend abgebildet: ■ Examples of particularly suitable blue phosphorescent metal complexes are shown below: ■
10 c ” 10 c ”
30 30
35 35
Vorzugsweise hat der mindestens eine fluoreszierende Emitter in der Zusammensetzung eine Peakemissionswellenlänge zwischen 420-550 nm, vorzugsweise zwischen 420-470 nm. Preferably, the at least one fluorescent emitter in the composition has a peak emission wavelength between 420-550 nm, preferably between 420-470 nm.
Bevorzugte fluoreszierende Emitter sind Emitter, die aus den oben genannten Emitterklassen ausgewählt sind. Preferred fluorescent emitters are emitters selected from the emitter classes mentioned above.
Vorzugsweise hat der mindestens eine fluoreszierende Emitter eine Halbwertsbreite auf halber Peakhöhe (Full Width at Half Maximum, FWHM) < 50 nm, bevorzugt FWHM < 40 nm, weiter bevorzugt FWHM < 30 nm. Das Verfahren zur Bestimmung der FWHM ist im nachstehenden experimentellen Teil beschrieben. Preferably, the at least one fluorescent emitter has a full width at half maximum (FWHM) < 50 nm, preferably FWHM < 40 nm, more preferably FWHM < 30 nm. The method for determining the FWHM is described in the experimental part below.
Vorzugsweise hat der mindestens eine fluoreszierende Emitter ein LIIMO von -2,1 eV bis -2,5 eV, bevorzugt von -2,2 eV bis -2,4 eV, wie durch quantenchemische Berechnungen definiert. Vorzugsweise hat der mindestens eine fluoreszierende Emitter ein HOMO von -4,8 eV bis -5,2 eV, bevorzugt von -4,9 eV bis -5,1 eV, wie durch quantenchemische Berechnungen definiert. Preferably, the at least one fluorescent emitter has a LIIMO of -2.1 eV to -2.5 eV, preferably from -2.2 eV to -2.4 eV, as defined by quantum chemical calculations. Preferably, the at least one fluorescent emitter has a HOMO of -4.8 eV to -5.2 eV, preferably from -4.9 eV to -5.1 eV, as defined by quantum chemical calculations.
Vorzugsweise liegt die Energie des niedrigsten Singulettzustands Si des fluoreszierenden Emitters bei 2,65 eV bis 2,9 eV, bevorzugt 2,7 bis 2,8 eV, weiter bevorzugt 2,7 bis 2,75 eV, wie durch quantenmechanische Berechnungen definiert. Preferably, the energy of the lowest singlet state Si of the fluorescent emitter is 2.65 eV to 2.9 eV, preferably 2.7 to 2.8 eV, more preferably 2.7 to 2.75 eV, as defined by quantum mechanical calculations.
Beispiele für geeignete fluoreszierende Emitter sind in der nachstehenden Examples of suitable fluorescent emitters are given below.
Geeignete Ladungstransportmaterialien, wie sie in der Lochinjektions- oder Lochtransportschicht oder in der Elektronensperrschicht oder in der Elektronentransportschicht des erfindungsgemäßen elektronischen Bau- elements verwendet werden können, sind neben den Verbindungen der Formel (1 ) zum Beispiel die in Y. Shirota et al., Chem. Rev. 2007, 107(4), 953-1010, oder andere Materialien, wie sie in diesen Schichten gemäß dem Stand der Technik verwendet werden. Suitable charge transport materials, as can be used in the hole injection or hole transport layer or in the electron barrier layer or in the electron transport layer of the electronic component according to the invention, are, in addition to the compounds of formula (1), for example those in Y. Shirota et al., Chem. Rev. 2007, 107(4), 953-1010, or other materials as used in these layers according to the prior art.
Als Materialien für die Löchertransportschicht können alle Materialien verwendet werden, die nach dem Stand der Technik als Lochtransport- materialien in der Lochtransportschicht eingesetzt werden. Es können aromatische Aminverbindungen eingesetzt werden. Weitere Verbin- dungen, die vorzugsweise in löchertransportierenden Schichten der erfindungsgemäßen OLEDs eingesetzt werden, sind insbesondere Indenofluorenamin-Derivate (z.B. nach WO 2006/122630 oderAll materials that are used according to the prior art as hole transport materials in the hole transport layer can be used as materials for the hole transport layer. Aromatic amine compounds can be used. Other compounds that are preferably used in hole transport layers of the OLEDs according to the invention are in particular indenofluorenamine derivatives (e.g. according to WO 2006/122630 or
WO 2006/100896), die in EP 1661888 offenbarten Aminderivate, Hexa- azatriphenylen-Derivate (z.B. nach WO 01/049806), Aminderivate mit anellierten Aromaten (zum Beispiel nach US 5,061 ,569), die in WO 95/09147 offenbarten Aminderivate, Monobenzoindenofluorenamine (zum Beispiel nach WO 08/006449), Dibenzoindenofluorenamine (zum Beispiel nach WO 07/140847), Spirobifluorenamine (zum Beispiel nach WO 2012/034627 oder WO 2013/120577), Fluorenamine (zum Beispiel nach WO 2014/015937, WO 2014/015938, WO 2014/015935 und WO 2015/082056), Spirodibenzopyranamine (zum Beispiel gemäß WO 2013/083216), Dihydroacridin-Derivate (zum Beispiel gemäß WO 2012/150001 ), Spirodibenzofurane und Spirodibenzothiophene (zum Beispiel nach WO 2015/022051 , WO 2016/102048 und WO 2016/131521 ), Phenanthrendiarylamine (zum Beispiel nach WO 2015/131976), Spirotribenzotropolone (zum Beispiel gemäß WO 2016/087017), Spirobifluorene mit meta-Phenyldiamingruppen (zum Beispiel gemäß WO 2016/078738), Spirobisacridine (zum Beispiel gemäß WO 2015/158411 ), Xanthendiarylamine (zum Beispiel gemäß WO 2014/072017), und 9,10-Dihydroanthracen-Spiroverbindungen mit Diarylaminogruppen gemäß WO 2015/086108. WO 2006/100896), the amine derivatives disclosed in EP 1661888, hexaazatriphenylene derivatives (eg according to WO 01/049806), amine derivatives with fused aromatics (for example according to US 5,061,569), the amine derivatives disclosed in WO 95/09147, monobenzoindenofluorenamines (for example according to WO 08/006449), dibenzoindenofluorenamines (for example according to WO 07/140847), spirobifluorenamines (for example according to WO 2012/034627 or WO 2013/120577), fluorenamines (for example according to WO 2014/015937, WO 2014/015938, WO 2014/015935 and WO 2015/082056), spirodibenzopyranamines (for example according to WO 2013/083216), dihydroacridine derivatives (for example according to WO 2012/150001), Spirodibenzofurans and spirodibenzothiophenes (for example according to WO 2015/022051, WO 2016/102048 and WO 2016/131521), phenanthrenediarylamines (for example according to WO 2015/131976), spirotribenzotropolones (for example according to WO 2016/087017), spirobifluorenes with meta-phenyldiamine groups (for example according to WO 2016/078738), spirobisacridines (for example according to WO 2015/158411), xanthenediarylamines (for example according to WO 2014/072017), and 9,10-dihydroanthracene spiro compounds with diarylamino groups according to WO 2015/086108.
Ganz besonders bevorzugt ist die Verwendung von durch Diarylamino- gruppen in 4-Position substituierten Spirobifluorenen als löchertranspor- tierende Verbindungen, insbesondere die Verwendung derjenigen Verbindungen, die in WO 2013/120577 beansprucht und offenbart sind, und die Verwendung von durch Diarylaminogruppen in 2-Position substituierten Spirobifluorenen als löchertransportierende Verbindungen, insbesondere die Verwendung derjenigen Verbindungen, die in WO 2012/034627 beansprucht und offenbart sind. Very particular preference is given to the use of spirobifluorenes substituted by diarylamino groups in the 4-position as hole-transporting compounds, in particular the use of those compounds which are claimed and disclosed in WO 2013/120577, and the use of spirobifluorenes substituted by diarylamino groups in the 2-position as hole-transporting compounds, in particular the use of those compounds which are claimed and disclosed in WO 2012/034627.
Vorzugsweise umfasst die erfindungsgemäße OLED zwei oder mehr verschiedene elektronentransportierende Schichten. Die Verbindung der Formel (1 ) kann dabei in keiner, in einer oder mehreren oder in allen elektronentransportierenden Schichten verwendet werden. In einer bevorzugten Ausführungsform wird die Verbindung der Formel (1 ) in genau einer oder genau zwei elektronentransportierenden Schichten eingesetzt, und in den weiteren vorhandenen elektronentransportierenden Schichten werden andere Verbindungen eingesetzt. Weitere Verbin- dungen, die neben den Verbindungen der Formel (1 ) verwendet werden können, sind alle Materialien, die nach dem Stand der Technik als Elektronentransportmatenalien in der Elektronentransportschicht eingesetzt werden. Besonders geeignet sind Aluminiumkomplexe, z.B. Alqs, Zirkoniumkomplexe, z.B. Zrq4, Lithiumkomplexe, z.B. Liq, Benzimidazol-Derivate, Triazin-Derivate, Pyrimidin-Derivate, Pyridin- Derivate, Pyrazin-Derivate, Chinoxalin-Derivate, Chinolin-Derivate, Oxadiazol-Derivate, aromatische Ketone, Lactame, Borane, Diaza- phosphol-Derivate und Phosphinoxid-Derivate. Weitere geeignete Materialien sind Derivate der vorgenannten Verbindungen, wie sie in JP 2000/053957, WO 2003/060956, WO 2004/028217, WO 2004/080975 und WO 2010/072300 offenbart sind. The OLED according to the invention preferably comprises two or more different electron-transporting layers. The compound of formula (1) can be used in none, in one or more or in all electron-transporting layers. In a preferred embodiment, the compound of formula (1) is used in exactly one or exactly two electron-transporting layers, and other compounds are used in the other electron-transporting layers present. Other compounds that can be used in addition to the compounds of formula (1) are all materials that are used according to the state of the art as electron transport materials in the electron transport layer. Particularly suitable are aluminum complexes, e.g. Alqs, zirconium complexes, e.g. Zrq4, lithium complexes, e.g. Liq, benzimidazole derivatives, triazine derivatives, pyrimidine derivatives, pyridine derivatives, pyrazine derivatives, quinoxaline derivatives, quinoline derivatives, oxadiazole derivatives, aromatic ketones, lactams, boranes, diazaphosphole derivatives and phosphine oxide derivatives. Other suitable materials are derivatives of the aforementioned compounds, as disclosed in JP 2000/053957, WO 2003/060956, WO 2004/028217, WO 2004/080975 and WO 2010/072300.
Die Vorrichtung wird entsprechend (je nach Anwendung) strukturiert, kontaktiert und abschließend versiegelt, um schädliche Einflüsse durch Wasser und Luft auszuschließen. The device is structured accordingly (depending on the application), contacted and finally sealed to exclude harmful influences from water and air.
In den weiteren Schichten der erfindungsgemäßen organischen Elektro- lumineszenzvorrichtung können alle Materialien verwendet werden, wie sie üblicherweise gemäß dem Stand der Technik eingesetzt werden. Der Fachmann kann daher ohne erfinderisches Zutun alle für organische Elektrolumineszenzvorrichtungen bekannten Materialien in Kombination mit den erfindungsgemäßen Verbindungen gemäß Formel (1 ) bzw. den oben ausgeführten bevorzugten Ausführungsformen einsetzen. In the further layers of the organic electroluminescent device according to the invention, all materials can be used as are usually used according to the prior art. The person skilled in the art can therefore, without inventive step, use all materials known for organic electroluminescent devices in combination with the compounds according to the invention according to formula (1) or the preferred embodiments described above.
Weiterhin bevorzugt ist eine organische Elektrolumineszenzvorrichtung, dadurch gekennzeichnet, dass eine oder mehrere Schichten mit einem Sublimationsverfahren beschichtet werden. Dabei werden die Materialien in Vakuum-Sublimationsanlagen bei einem Anfangsdruck kleiner 10’5 mbar, bevorzugt kleiner 10’6 mbar aufgedampft. Es ist aber auch möglich, dass der Anfangsdruck noch geringer ist, beispielsweise kleiner 10’7 mbar. Also preferred is an organic electroluminescent device, characterized in that one or more layers are coated using a sublimation process. The materials are vapor-deposited in vacuum sublimation systems at an initial pressure of less than 10' 5 mbar, preferably less than 10' 6 mbar. However, it is also possible for the initial pressure to be even lower, for example less than 10' 7 mbar.
Bevorzugt ist ebenfalls eine organische Elektrolumineszenzvorrichtung, dadurch gekennzeichnet, dass eine oder mehrere Schichten mit dem OVPD (Organic Vapour Phase Deposition) Verfahren oder mit Hilfe einer Trägergassublimation beschichtet werden. Dabei werden die Materialien bei einem Druck zwischen 10’5 mbar und 1 bar aufgebracht. Ein Spezialfall dieses Verfahrens ist das OVJP (Organic Vapour Jet Printing) Verfahren, bei dem die Materialien direkt durch eine Düse aufgebracht und so strukturiert werden. Also preferred is an organic electroluminescent device, characterized in that one or more layers are coated using the OVPD (Organic Vapour Phase Deposition) method or with the aid of carrier gas sublimation. The materials at a pressure between 10' 5 mbar and 1 bar. A special case of this process is the OVJP (Organic Vapour Jet Printing) process, in which the materials are applied directly through a nozzle and thus structured.
Weiterhin bevorzugt ist eine organische Elektrolumineszenzvorrichtung, dadurch gekennzeichnet, dass eine oder mehrere Schichten aus Lösung, wie z. B. durch Spincoating, oder mit einem beliebigen Druckverfahren, wie z. B. Siebdruck, Flexodruck, Offsetdruck, LITI (Light Induced Thermal Imaging, Thermotransferdruck), Ink-Jet Druck (Tintenstrahldruck) oder Nozzle Printing, hergestellt werden. Hierfür sind lösliche Verbindungen nötig, welche beispielsweise durch geeignete Substitution erhalten werden. Also preferred is an organic electroluminescent device, characterized in that one or more layers are produced from solution, such as by spin coating, or using any printing method, such as screen printing, flexographic printing, offset printing, LITI (light induced thermal imaging, thermal transfer printing), ink-jet printing or nozzle printing. Soluble compounds are required for this, which are obtained, for example, by suitable substitution.
Weiterhin sind Hybridverfahren möglich, bei denen beispielsweise eine oder mehrere Schichten aus Lösung aufgebracht werden und eine oder mehrere weitere Schichten aufgedampft werden. Furthermore, hybrid processes are possible, in which, for example, one or more layers are applied from solution and one or more further layers are vapor-deposited.
Diese Verfahren sind dem Fachmann generell bekannt und können von ihm ohne erfinderisches Zutun auf organische Elektrolumineszenzvor- richtungen enthaltend die erfindungsgemäßen Verbindungen angewandt werden. These methods are generally known to the person skilled in the art and can be applied by him without inventive step to organic electroluminescent devices containing the compounds according to the invention.
Erfindungsgemäß können die elektronischen Vorrichtungen, die eine oder mehrere Verbindungen der Formel (1 ) enthalten, in Displays, als Licht- quellen in Beleuchtungsanwendungen und als Lichtquellen in medizi- nischen und/oder kosmetischen Anwendungen (z.B. Lichttherapie) einge- setzt werden. According to the invention, the electronic devices containing one or more compounds of formula (1) can be used in displays, as light sources in lighting applications and as light sources in medical and/or cosmetic applications (e.g. light therapy).
Die erfindungsgemäßen Verbindungen und die erfindungsgemäßen orga- nischen Elektrolumineszenzvorrichtungen zeichnen sich durch einen oder mehrere der folgenden Eigenschaften aus: The compounds according to the invention and the organic electroluminescent devices according to the invention are characterized by one or more of the following properties:
1 . Die erfindungsgemäßen Verbindungen führen zu langen Lebens- dauern. 2. Die erfindungsgemäßen Verbindungen führen zu hohen Effizienzen, insbesondere zu einer hohen EQE. 1 . The compounds according to the invention lead to long service lives. 2. The compounds according to the invention lead to high efficiencies, in particular to a high EQE.
3. Die erfindungsgemäßen Verbindungen führen zu geringen Betriebs- spannungen. 3. The connections according to the invention lead to low operating voltages.
Die Erfindung wird durch die nachfolgenden Beispiele näher erläutert, ohne sie dadurch einschränken zu wollen. Der Fachmann kann aus den Schilderungen die Erfindung im gesamten offenbarten Bereich ausführen und ohne erfinderisches Zutun weitere erfindungsgemäße Verbindungen herstellen und diese in elektronischen Vorrichtungen verwenden bzw. das erfindungsgemäße Verfahren anwenden. The invention is explained in more detail by the following examples, without intending to restrict it thereby. From the descriptions, the person skilled in the art can carry out the invention in the entire disclosed area and, without inventive step, produce further compounds according to the invention and use them in electronic devices or apply the method according to the invention.
Beispiele: Examples:
Die nachfolgenden Synthesen werden, sofern nicht anders angegeben, unter einer Schutzgasatmosphäre in getrockneten Lösungsmitteln durch- geführt. Die Lösungsmittel und Reagenzien können z. B. von Sigma- ALDRICH bzw. ABCR bezogen werden. Die jeweiligen Angaben in eckigen Klammern bzw. die zu einzelnen Verbindungen angegebenen Nummern beziehen sich auf die CAS-Nummern der literaturbekannten Verbindungen. Unless otherwise stated, the following syntheses are carried out under a protective gas atmosphere in dried solvents. The solvents and reagents can be obtained from Sigma-ALDRICH or ABCR, for example. The respective information in square brackets or the numbers given for individual compounds refer to the CAS numbers of the compounds known from the literature.
B) Synthese von Synthonen S und den Verbindungen B: Beispiel S1 : B) Synthesis of synthons S and compounds B: Example S1 :
Variante 1 : Grignard-Kupplung Variant 1 : Grignard coupling
Durchführung analog D. Bhattacharyya et al., Org. Lett. 2021 , 23, 869, wobei anstelle von 2,4,6-Trichloro-1 ,3,5-triazin LS1 eingesetzt wird. Ansatz: 30.4 g (100 mmol) LS1 , 10.5 ml (100 mmol) Brombenzol, 24.3 g (100 mmol) Magnesium. Reinigung des Rohprodukts erfolgt chromato- graphisch (Torrent Säulenautomat der Fa. A. Semrau). Ausbeute: 26.1 g (76 mmol) 76 %; Reinheit: ca. 97 % ig n. 1H-NMR. Wird das Edukt LS1 konsekutiv mit zwei bzw. drei Äquivalenten der Grignard-Verbindung umgesetzt, können die entsprechenden Di- bzw. Triaryl-tris-triazolotriazine erhalten werden. Werden zur Synthese von Triaryl-tris-triazolotriazinen Gemische an Arylbromiden eingesetzt, kann das entstandene Produkt- gemisch ebenfalls chromatographisch in die Reinkomponenten zerlegt werden. Alternativ zu den Grignard-Verbindungen können auch Organolithium-Verbindungen eingesetzt werden. Carrying out the procedure analogously to D. Bhattacharyya et al., Org. Lett. 2021 , 23, 869, using LS1 instead of 2,4,6-trichloro-1,3,5-triazine. Batch: 30.4 g (100 mmol) LS1, 10.5 ml (100 mmol) bromobenzene, 24.3 g (100 mmol) magnesium. The crude product is purified chromatographically (Torrent column machine from A. Semrau). Yield: 26.1 g (76 mmol) 76%; purity: approx. 97% according to 1 H-NMR. If the reactant LS1 is reacted consecutively with two or three equivalents of the Grignard compound, the corresponding di- or triaryl-tris-triazolotriazines can be obtained. If mixtures of aryl bromides are used to synthesize triaryl-tris-triazolotriazines, the resulting product mixture can also be separated into pure components by chromatography. Organolithium compounds can also be used as an alternative to Grignard compounds.
Variante 2: Suzuki-Kupplung Variant 2: Suzuki clutch
Eine gut gerührte Lösung von 30.4 g (100 mmol) LS1 , 12.2 g (100 mmol) Phenylboronsäure [98-80-6], 27.6 g (200 mmol) Kaliumcarbonat, 423 mg (0.5 mmol) XPhos Pd G3 [564483-18-7], 100 g Glaskugeln (3 mm) und 500 ml DMSO wird ca. 16 h bei 80 °C gerührt (DC-Kontrolle auf voll- ständigen Umsatz). Man entfernt das DMSO weitgehend im Vakuum, nimmt den Rückstand in einem Gemisch aus 500 ml Dichlormethan (DCM) und 200 ml Ethylacetat (EE) auf und filtriert über ein mit DCM vorge- schlämmtes Kieselgel ab. Die weitere Reinigung des Rohprodukts erfolgt chromatographisch (Torrent Säulenautomat der Fa. A. Semrau). A well-stirred solution of 30.4 g (100 mmol) LS1, 12.2 g (100 mmol) phenylboronic acid [98-80-6], 27.6 g (200 mmol) potassium carbonate, 423 mg (0.5 mmol) XPhos Pd G3 [564483-18-7], 100 g glass beads (3 mm) and 500 ml DMSO is stirred for approx. 16 h at 80 °C (TLC control for complete conversion). The DMSO is largely removed in vacuo, the residue is taken up in a mixture of 500 ml dichloromethane (DCM) and 200 ml ethyl acetate (EE) and filtered through a filter paper pre-treated with DCM. elutriated silica gel. The crude product is further purified by chromatography (Torrent column machine from A. Semrau).
Ausbeute: 25.3 g (74 mmol) 76 %; Reinheit: ca. 97 %ig n. 1H-NMR. Yield: 25.3 g (74 mmol) 76%; Purity: about 97% according to 1 H-NMR.
Durchführung analog S1 , Variante 2, Einsatz von 34.6 g (100 mmol) S1 und 19.8 g (100 mmol) 3-Biphenylboronsäure [5122-95-2], Die Reinigung des Rohprodukts erfolgt chromatographisch (Torrent Säulenautomat der Fa. A. Semrau). Ausbeute: 31.0 g (67 mmol) 67 %; Reinheit: ca. 97 %ig n. 1H-NMR. Procedure analogous to S1, variant 2, using 34.6 g (100 mmol) S1 and 19.8 g (100 mmol) 3-biphenylboronic acid [5122-95-2]. The crude product is purified chromatographically (Torrent column machine from A. Semrau). Yield: 31.0 g (67 mmol) 67%; purity: approx. 97% according to 1H-NMR.
Durchführung analog S1 , Variante 2, Einsatz von 46.4 g (100 mmol) S100 und 23.8 g (120 mmol) 2-Biphenylboronsäure [4688-76-0], Die Reinigung des Rohprodukts erfolgt jeweils durch Chromatographie (Torrent Säulen- automat der Fa. A. Semrau) und/oder wiederholte Heißextraktionskristalli- sation (übliche org. Lösungsmittel bzw. deren Kombinationen, bevorzugt Acetonitril-DCM, 1 :3 bis 3:1 vv) sowie fraktionierte Sublimation bzw. Tempern im Hochvakuum. Ausbeute: 35.0 g (60 mmol) 60 %; Reinheit: ca. 99.9 %ig n. HPLC. Carry out analogously to S1, variant 2, using 46.4 g (100 mmol) S100 and 23.8 g (120 mmol) 2-biphenylboronic acid [4688-76-0]. The crude product is purified by chromatography (Torrent column machine from A. Semrau) and/or repeated hot extraction crystallization (usual organic solvents or combinations thereof, preferably acetonitrile-DCM, 1:3 to 3:1 vv) and fractional sublimation or annealing under high vacuum. Yield: 35.0 g (60 mmol) 60%; purity: approx. 99.9% according to HPLC.
Durchführung analog S1 , Variante 2, Einsatz von 9.1 g (30 mmol) LS1 und 20.7 g (100 mmol) ß-([1 ,1 '-Biphenyl]-3-yl-2,2',3',4,4',5,5',6,6'-c/9)- boronsäure [2368221 -45-6], Die Reinigung des Rohprodukts erfolgt jeweils durch Chromatographie (Torrent Säulenautomat der Fa. A. Semrau) und/oder wiederholte Heißextraktionskristallisation (übliche org. Lösungsmittel bzw. deren Kombinationen, bevorzugt Acetonitril-DCM, 1 :3 bis 3:1 vv) sowie fraktionierte Sublimation bzw. Tempern im Hochvakuum. Ausbeute: 13.1 g (19 mmol) 63 %; Reinheit: ca. 99.9 %ig n. HPLC. Procedure analogous to S1, variant 2, use of 9.1 g (30 mmol) LS1 and 20.7 g (100 mmol) ß-([1,1'-biphenyl]-3-yl-2,2',3',4,4',5,5',6,6'-c/9)-boronic acid [2368221-45-6]. The crude product is purified by chromatography (Torrent column machine from A. Semrau) and/or repeated hot extraction crystallization (usual organic solvents or combinations thereof, preferably acetonitrile-DCM, 1:3 to 3:1 vv) and fractional sublimation or annealing under high vacuum. Yield: 13.1 g (19 mmol) 63%; purity: approx. 99.9% according to HPLC.
Werden in den o.g. Reaktionen Boronester eingesetzt, wird K3PO4 x H2O anstelle von K2CO3 verwendet. If boronate esters are used in the above reactions, K3PO4 x H2O is used instead of K2CO3.
Analog können folgende Verbindungen dargestellt werden. Cl The following connections can be represented analogously. Cl
Ein gut gerührtes Gemisch aus 16.7 g (100 mmol) LS1 , Carbazol [86-74- 8], 24.0 g (100 mmol) Natrium hydrid [7646-69-7] und 100 g Glaskugeln (3 mm Durchmesser) in 300 ml Dimethylsulfoxid (DMSO) wird bis zur beendeten Wasserstoffentwicklung bei 40 °C gerührt. Dann lässt man, unter gutem Rühren, eine Lösung von 30.4 g (100 mmol) LS1 in 200 ml warmem DMSO zulaufen, steigert die Temperatur langsam auf 60-100 °C und rührt bis zum vollständigen Umsatz nach (ca. 8 h, DC-Kontrolle). Man lässt die Reaktionsmischung erkalten, gießt, unter gutem Rühren, in 1500 ml Eiswasser ein, saugt vom ausgefallenen Feststoff ab, wäscht diesen dreimal mit je 100 ml Wasser, je dreimal mit je 100 ml Ethanol und trocknet im Vakuum. Die Reinigung des Rohprodukts erfolgt chromato- graphisch (Torrent Säulenautomat der Fa. A. Semrau). Ausbeute: 26.5 g (61 mmol) 61 %; Reinheit: ca. 97 %ig n. 1H-NMR. A well-stirred mixture of 16.7 g (100 mmol) LS1, carbazole [86-74-8], 24.0 g (100 mmol) sodium hydride [7646-69-7] and 100 g glass beads (3 mm diameter) in 300 ml dimethyl sulfoxide (DMSO) is stirred at 40 °C until hydrogen evolution has ceased. Then, with good stirring, a solution of 30.4 g (100 mmol) LS1 in 200 ml warm DMSO is added, the temperature is slowly increased to 60-100 °C and stirring is continued until complete conversion (approx. 8 h, DC control). The reaction mixture is allowed to cool and poured into 1500 ml of ice water, filter off the precipitated solid, wash it three times with 100 ml of water each time, three times with 100 ml of ethanol each time and dry in a vacuum. The crude product is purified chromatographically (Torrent column machine from A. Semrau). Yield: 26.5 g (61 mmol) 61%; Purity: approx. 97% according to 1 H-NMR.
Durchführung analog S200, Einsatz von 43.5 g (100 mmol) S200 und 27.7 g (110 mmol) 3-Phenyl-9H-carbazol [103012-26-6], Die Reinigung des Rohprodukts erfolgt chromatographisch (Torrent Säulenautomat der Fa. A. Semrau). Ausbeute: 41.5 g (65 mmol) 65 %; Reinheit: ca. 97 %ig n. 1H-NMR. Procedure analogous to S200, use of 43.5 g (100 mmol) S200 and 27.7 g (110 mmol) 3-phenyl-9H-carbazole [103012-26-6]. The crude product is purified chromatographically (Torrent column machine from A. Semrau). Yield: 41.5 g (65 mmol) 65%; Purity: approx. 97% according to 1H-NMR.
Durchführung analog S200, Einsatz von 64,2 g (100 mmol) S300 und 24.3 g (100 mmol) 2-Phenyl-9H-carbazol [88590-00-5], Die Reinigung des Rohprodukts erfolgt jeweils durch Chromatographie (Torrent Säulen- automat der Fa. A. Semrau) und/oder wiederholte Heißextraktionskristalli- sation (übliche org. Lösungsmittel bzw. deren Kombinationen, bevorzugt Acetonitril-DCM, 1 :3 bis 3:1 vv) sowie fraktionierte Sublimation bzw. Tempern im Hochvakuum. Ausbeute: 41.5 g (65 mmol) 65 %; Reinheit: ca. Carrying out the procedure analogously to S200, using 64.2 g (100 mmol) of S300 and 24.3 g (100 mmol) of 2-phenyl-9H-carbazole [88590-00-5]. The crude product is purified by chromatography (Torrent column machine from A. Semrau) and/or repeated hot extraction crystallization (usual organic solvents or combinations thereof, preferably acetonitrile-DCM, 1:3 to 3:1 vv) and fractional sublimation or. Annealing in high vacuum. Yield: 41.5 g (65 mmol) 65%; Purity: approx.
99.9 %ig n. HPLC. 99.9% by HPLC.
Analog können folgende Verbindungen, unter Anpassung der Stöchio- metrie der Edukte, dargestellt werden. The following compounds can be prepared analogously by adjusting the stoichiometry of the reactants.
Beispiel B600: Example B600:
Ein gut gerührtes Gemisch aus 46.4 g (100 mmol) S100 und 5.4 g (110 mmol) Natriumcyanid und 100 g Glaskugeln (3 mm Durchmesser) in 300 ml DMSO wird 18 h bei 140 °C gerührt (DC-Kontrolle). Man lässt die Reaktionsmischung erkalten, gießt sie unter gutem Rühren in 1500 ml Eiswasser ein, saugt vom ausgefallenen Feststoff ab, wäscht diesen dreimal mit je 100 ml Wasser, je dreimal mit je 100 ml Ethanol und trocknet im Vakuum. Die Reinigung des Rohprodukts erfolgt jeweils durch Chromatographie (Torrent Säulenautomat der Fa. A. Semrau) und/oder wiederholte Heißextraktionskristallisation (übliche org. Lösungsmittel bzw. deren Kombinationen, bevorzugt Acetonitril-DCM, 1 :3 bis 3:1 vv) sowie fraktionierte Sublimation bzw. Tempern im Hochvakuum. Ausbeute: 30.9 g (68 mmol) 68 %; Reinheit: ca. 99.9 %ig n. HPLC. A well-stirred mixture of 46.4 g (100 mmol) S100 and 5.4 g (110 mmol) sodium cyanide and 100 g glass beads (3 mm diameter) in 300 ml DMSO is stirred for 18 h at 140 °C (TLC control). The reaction mixture is allowed to cool, poured into 1500 ml ice water while stirring well, the precipitated solid is filtered off with suction, washed three times with 100 ml water each time, three times with 100 ml ethanol each time and dried in a vacuum. The crude product is purified by chromatography (Torrent column machine from A. Semrau) and/or repeated hot extraction crystallization (usual organic solvents or combinations thereof, preferably acetonitrile-DCM, 1:3 to 3:1 vv) and fractional sublimation or annealing under high vacuum. Yield: 30.9 g (68 mmol) 68%; Purity: approx. 99.9% according to HPLC.
Analog können folgende Verbindungen, unter Anpassung der Stöchio- metrie der Edukte, dargestellt werden. The following compounds can be prepared analogously by adjusting the stoichiometry of the reactants.
Beispiel: Herstellung der OLEDs Example: Production of OLEDs
Die Herstellung von erfindungsgemäßen OLEDs sowie OLEDs nach dem Stand der Technik erfolgt nach einem allgemeinen Verfahren gemäß WO 2004/058911 , das auf die hier beschriebenen Gegebenheiten (Schichtdickenvariation, verwendete Materialien) angepasst wird. The production of OLEDs according to the invention as well as OLEDs according to the prior art is carried out according to a general process according to WO 2004/058911, which is adapted to the conditions described here (layer thickness variation, materials used).
In den folgenden Beispielen werden die Ergebnisse verschiedener OLEDs vorgestellt. Gereinigte Glasplättchen (Reinigung in Miele Laborspül- maschine, Reiniger Merck Extran), die mit strukturiertem ITO (Indium Zinn Oxid) der Dicke 50 nm beschichtet sind, werden 25 Minuten mit UV-Ozon vorbehandelt (UV-Ozon Generator PR-100, Firma UVP). Diese beschichteten Glasplättchen bilden die Substrate, auf welche die OLEDs aufgebracht werden. a) Blaue Fluoreszenz-OLED- Bauteile - BF: The following examples present the results of various OLEDs. Cleaned glass plates (cleaned in a Miele laboratory dishwasher, Merck Extran cleaner) coated with structured ITO (indium tin oxide) with a thickness of 50 nm are pretreated with UV ozone for 25 minutes (UV ozone generator PR-100, UVP). These coated glass plates form the substrates onto which the OLEDs are applied. a) Blue Fluorescence OLED Devices - BF:
Die erfindungsgemäßen Verbindungen B können in der Lochblockier- schicht (HBL) und der Elektronentransportschicht (ETL) verwendet werden. Alle Materialien werden in einer Vakuumkammer thermisch aufgedampft. Dabei besteht die Emissionsschicht (EML) immer aus mindestens einem Matrixmaterial (Hostmaterial, Wirtsmaterial) SMB (s. Tabelle 1 ) und einem emittierenden Dotierstoff (Dotand, Emitter) D, der dem Matrixmaterial bzw. den Matrixmaterialien durch Co-Verdampfung in einem bestimmten Volumenanteil beigemischt wird. Eine Angabe wie SMB:D (97%:3%) bedeutet hierbei, dass das Material SMB in einem Volumenanteil von 97% und der Dotand D in einem Anteil von 3% in der Schicht vorliegt. Analog kann auch die Elektronentransportschicht aus einer Mischung zweier Materialien bestehen, s. Tabelle 1. Die zur Herstellung der OLEDs verwendeten Materialien sind in Tabelle 5 gezeigt. The compounds B according to the invention can be used in the hole blocking layer (HBL) and the electron transport layer (ETL). All materials are thermally vapor-deposited in a vacuum chamber. The emission layer (EML) always consists of at least one matrix material (host material) SMB (see Table 1) and an emitting dopant (dopant, emitter) D, which is mixed into the matrix material or materials by co-evaporation in a certain volume proportion. A specification such as SMB:D (97%:3%) means that the material SMB is present in the layer in a volume proportion of 97% and the dopant D in a proportion of 3%. Analogously, the electron transport layer can also consist of a mixture of two materials, see Table 1. The materials used to produce the OLEDs are shown in Table 5.
Die OLEDs werden standardmäßig charakterisiert. Hierfür werden die Elektrolumineszenzspektren, die Strom effizienz (gemessen in cd/A), die Leistungseffizienz (gemessen in Im/W) und die externe Quanteneffizienz (EQE, gemessen in Prozent) in Abhängigkeit der Leuchtdichte, berechnet aus Strom-Spannungs-Leuchtdichte-Kennlinien (IUL-Kennlinien) unter Annahme einer lambertschen Abstrahlcharakteristik, bestimmt. Die Angabe der EQE in (%) und der Spannung in (V) erfolgt bei einer Leucht- dichte von 1000 cd/m2. The OLEDs are characterized as standard. For this purpose, the electroluminescence spectra, the current efficiency (measured in cd/A), the power efficiency (measured in Im/W) and the external quantum efficiency (EQE, measured in percent) are determined as a function of the luminance, calculated from current-voltage-luminance characteristics (IUL characteristics) assuming a Lambertian radiation characteristic. The EQE in (%) and the voltage in (V) are specified at a luminance of 1000 cd/m 2 .
Die OLEDs haben den folgenden Schichtaufbau: Substrat The OLEDs have the following layer structure: Substrate
Lochinjektionsschicht (HIL) aus HTM1 dotiert mit 5 % NDP-9 (kommerziell erhältlich von der Fa. Novaled), 20 nm Lochtransportschicht (HTL), aus HTM1 , 180 nm Elektronenblockierschicht (EBL), s. Tabelle 1 Emissionsschicht (EML), s. Tabelle 1 Lochblockierschicht (HBL), s. Tabelle 1 Elektronentransportschicht (ETL), s. Tabelle 1 Elektroneninjektionsschicht (EIL) aus ETM2, 1 nm Kathode aus Aluminium, 100 nm Tabelle 1 : Aufbau blaue Fluoreszenz-OLED-Bautei e Hole injection layer (HIL) made of HTM1 doped with 5% NDP-9 (commercially available from Novaled), 20 nm Hole transport layer (HTL), made of HTM1, 180 nm Electron blocking layer (EBL), see Table 1 Emission layer (EML), see Table 1 Hole blocking layer (HBL), see Table 1 Electron transport layer (ETL), see Table 1 Electron injection layer (EIL) made of ETM2, 1 nm Cathode made of aluminum, 100 nm Table 1: Structure of blue fluorescent OLED components
Tabelle 2: Ergebnisse Blaue Fluoreszenz-OLED- Bauteile b) Phosphoreszenz-OLED-Bauteile: Table 2: Results of blue fluorescence OLED devices b) Phosphorescent OLED components:
Die erfindungsgemäßen Verbindungen B können in der Lochblockier- schicht (HBL), der Elektronentransportschicht (ETL) und in der Emissions- schicht (EML) als elektronenleitendes Matrixmaterial (Hostmaterial, Wirts- material) (eTMM) verwendet werden. Hierfür werden alle Materialien in einer Vakuumkammer thermisch aufgedampft. Dabei besteht die Emissionsschicht immer aus mindestens einem bzw. mehreren Matrix- materialien M und einem phosphoreszierenden Dotierstoff Ir, der dem Matrixmaterial bzw. den Matrixmaterialien durch Co-Verdampfung in einem bestimmten Volumenanteil beigemischt wird. Eine Angabe wie M1 :M2:lr (55%:35%:10%) bedeutet hierbei, dass das Material M1 in einem Volumenanteil von 55%, M2 in einem Volumenanteil von 35% und Ir in einem Volumenanteil von 10% in der Schicht vorliegt. Analog kann auch die Elektronentransportschicht aus einer Mischung zweier Materialien bestehen. Der genaue Aufbau der OLEDs ist Tabelle 3 zu entnehmen. Die zur Herstellung der OLEDs verwendeten Materialien sind in Tabelle 5 gezeigt. The compounds B according to the invention can be used in the hole blocking layer (HBL), the electron transport layer (ETL) and in the emission layer (EML) as electron-conducting matrix material (host material) (eTMM). For this purpose, all materials are thermally vapor-deposited in a vacuum chamber. The emission layer always consists of at least one or more matrix materials M and a phosphorescent dopant Ir, which is mixed into the matrix material or materials by co-evaporation in a certain volume proportion. A specification such as M1:M2:lr (55%:35%:10%) means that the material M1 is in a volume proportion of 55%, M2 in a volume proportion of 35% and Ir in a volume fraction of 10% in the layer. Analogously, the electron transport layer can also consist of a mixture of two materials. The exact structure of the OLEDs can be found in Table 3. The materials used to manufacture the OLEDs are shown in Table 5.
Die OLEDs werden standardmäßig charakterisiert. Hierfür werden die Elektrolumineszenzspektren, die Strom effizienz (gemessen in cd/A), die Leistungseffizienz (gemessen in Im/W) und die externe Quanteneffizienz (EQE, gemessen in Prozent) in Abhängigkeit der Leuchtdichte, berechnet aus Strom-Spannungs-Leuchtdichte-Kennlinien (IUL-Kennlinien) unter Annahme einer lambertschen Abstrahlcharakteristik, bestimmt. Die Angabe der EQE in (%) und der Spannung in (V) erfolgt bei einer Leucht- dichte von 1000 cd/m2. The OLEDs are characterized as standard. For this purpose, the electroluminescence spectra, the current efficiency (measured in cd/A), the power efficiency (measured in Im/W) and the external quantum efficiency (EQE, measured in percent) are determined as a function of the luminance, calculated from current-voltage-luminance characteristics (IUL characteristics) assuming a Lambertian radiation characteristic. The EQE in (%) and the voltage in (V) are specified at a luminance of 1000 cd/m 2 .
Die OLEDs haben den folgenden Schichtaufbau: The OLEDs have the following layer structure:
Substrat substrate
Lochinjektionsschicht (HIL) aus HTM1 dotiert mit 5 % NDP-9 (kommerziell erhältlich von der Fa. Novaled), 20 nm Hole injection layer (HIL) made of HTM1 doped with 5% NDP-9 (commercially available from Novaled), 20 nm
Lochtransportschicht (HTL) aus HTM1 , 180 nm für Blau, 50 nm für Grün, Gelb und Rot Hole transport layer (HTL) made of HTM1, 180 nm for blue, 50 nm for green, yellow and red
Elektronenblockierschicht (EBL), s. Tabelle 3 Emissionsschicht (EML), s. Tabelle 3 Lochblockierschicht (HBL), s. Tabelle 3 Elektronentransportschicht (ETL), s. Tabelle 3 Elektroneninjektionsschicht (EIL) aus ETM2, 1 nm Kathode aus Aluminium, 100 nm Electron blocking layer (EBL), see Table 3 Emission layer (EML), see Table 3 Hole blocking layer (HBL), see Table 3 Electron transport layer (ETL), see Table 3 Electron injection layer (EIL) made of ETM2, 1 nm Cathode made of aluminum, 100 nm
Tabelle 3: Aufbau Phosphoreszenz-OLED-Bauteile Table 3: Structure of phosphorescent OLED components
Tabelle 4: Ergebnisse Phosphoreszenz-OLED-Bauteile Table 4: Results of phosphorescent OLED devices
Tabelle 5: Strukturformeln der verwendeten Materialien Table 5: Structural formulas of the materials used
Claims
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP23175260.1 | 2023-05-25 | ||
| EP23175260 | 2023-05-25 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2024240725A1 true WO2024240725A1 (en) | 2024-11-28 |
Family
ID=86603654
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2024/063872 Pending WO2024240725A1 (en) | 2023-05-25 | 2024-05-21 | Tris[1,2,4]triazolo[1,5-a:1',5'-c:1'',5''-e][1,3,5]triazine derivatives for use in organic electroluminescent devices |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2024240725A1 (en) |
Citations (153)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5061569A (en) | 1990-07-26 | 1991-10-29 | Eastman Kodak Company | Electroluminescent device with organic electroluminescent medium |
| WO1995009147A1 (en) | 1993-09-29 | 1995-04-06 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element and arylenediamine derivative |
| EP0652273A1 (en) | 1993-11-09 | 1995-05-10 | Shinko Electric Industries Co. Ltd. | Organic material for electroluminescent device and electroluminescent device |
| EP0676461A2 (en) | 1994-04-07 | 1995-10-11 | Hoechst Aktiengesellschaft | Spiro compounds and their application as electroluminescence materials |
| JP2000053957A (en) | 1998-06-23 | 2000-02-22 | Koto Gijutsu Kenkyuin Kenkyu Kumiai | Novel organometallic luminescent material and organic electroluminescent device containing the same |
| WO2000070655A2 (en) | 1999-05-13 | 2000-11-23 | The Trustees Of Princeton University | Very high efficiency organic light emitting devices based on electrophosphorescence |
| WO2001041512A1 (en) | 1999-12-01 | 2001-06-07 | The Trustees Of Princeton University | Complexes of form l2mx as phosphorescent dopants for organic leds |
| WO2001049806A1 (en) | 1999-12-31 | 2001-07-12 | Lg Chemical Co., Ltd | Electronic device comprising organic compound having p-type semiconducting characteristics |
| WO2002015645A1 (en) | 2000-08-11 | 2002-02-21 | The Trustees Of Princeton University | Organometallic compounds and emission-shifting organic electrophosphorescence |
| EP1191613A2 (en) | 2000-09-26 | 2002-03-27 | Canon Kabushiki Kaisha | Luminescence device, display apparatus and metal coordination compound |
| EP1191614A2 (en) | 2000-09-26 | 2002-03-27 | Canon Kabushiki Kaisha | Luminescence device and metal coordination compound therefor |
| EP1191612A2 (en) | 2000-09-26 | 2002-03-27 | Canon Kabushiki Kaisha | Luminescence device, display apparatus and metal coordination compound |
| EP1205527A1 (en) | 2000-03-27 | 2002-05-15 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device |
| US6392250B1 (en) | 2000-06-30 | 2002-05-21 | Xerox Corporation | Organic light emitting devices having improved performance |
| WO2002072714A1 (en) | 2001-03-10 | 2002-09-19 | Covion Organic Semiconductors Gmbh | Solutions and dispersions of organic semiconductors |
| WO2002002714A3 (en) | 2000-06-30 | 2002-10-24 | Du Pont | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| WO2003019694A2 (en) | 2001-08-24 | 2003-03-06 | Covion Organic Semiconductors Gmbh | Solutions of polymer semiconductors |
| WO2003060956A2 (en) | 2002-01-18 | 2003-07-24 | Lg Chem, Ltd. | New material for transporting electrons and organic electroluminescent display using the same |
| WO2004013080A1 (en) | 2002-08-01 | 2004-02-12 | Covion Organic Semiconductors Gmbh | Spirobifluorene derivatives, their preparation and uses thereof |
| WO2004028217A1 (en) | 2002-09-20 | 2004-04-01 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element |
| WO2004058911A2 (en) | 2002-12-23 | 2004-07-15 | Covion Organic Semiconductors Gmbh | Organic electroluminescent element |
| WO2004081017A1 (en) | 2003-03-11 | 2004-09-23 | Covion Organic Semiconductors Gmbh | Metal complexes |
| WO2004080975A1 (en) | 2003-03-13 | 2004-09-23 | Idemitsu Kosan Co., Ltd. | Nitrogen-containing heterocycle derivative and organic electroluminescent element using the same |
| US6803720B2 (en) | 2000-12-15 | 2004-10-12 | Universal Display Corporation | Highly stable and efficient OLEDs with a phosphorescent-doped mixed layer architecture |
| JP2004288381A (en) | 2003-03-19 | 2004-10-14 | Konica Minolta Holdings Inc | Organic electroluminescent element |
| WO2004093207A2 (en) | 2003-04-15 | 2004-10-28 | Covion Organic Semiconductors Gmbh | Mixtures of matrix materials and organic semiconductors capable of emission, use of the same and electronic components containing said mixtures |
| WO2005011013A1 (en) | 2003-07-21 | 2005-02-03 | Covion Organic Semiconductors Gmbh | Organic electroluminescent element |
| WO2005019373A2 (en) | 2003-08-19 | 2005-03-03 | Basf Aktiengesellschaft | Transition metal complexes comprising carbene ligands serving as emitters for organic light-emitting diodes (oled's) |
| US20050069729A1 (en) | 2003-09-30 | 2005-03-31 | Konica Minolta Holdings, Inc. | Organic electroluminescent element, illuminator, display and compound |
| WO2005033244A1 (en) | 2003-09-29 | 2005-04-14 | Covion Organic Semiconductors Gmbh | Metal complexes |
| EP1553154A1 (en) | 2002-08-23 | 2005-07-13 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device and anthracene derivative |
| WO2005084081A1 (en) | 2004-02-20 | 2005-09-09 | Merck Patent Gmbh | Organic electronic devices |
| US20050258742A1 (en) | 2004-05-18 | 2005-11-24 | Yui-Yi Tsai | Carbene containing metal complexes as OLEDs |
| WO2005111172A2 (en) | 2004-05-11 | 2005-11-24 | Merck Patent Gmbh | Novel material mixtures for use in electroluminescence |
| JP2005347160A (en) | 2004-06-04 | 2005-12-15 | Konica Minolta Holdings Inc | Organic electroluminescence element, lighting device and display device |
| EP1617711A1 (en) | 2003-04-23 | 2006-01-18 | Konica Minolta Holdings, Inc. | Organic electroluminescent device and display |
| WO2006005627A1 (en) | 2004-07-15 | 2006-01-19 | Merck Patent Gmbh | Oligomeric derivatives of spirobifluorene, their preparation and use |
| WO2006048268A1 (en) | 2004-11-06 | 2006-05-11 | Merck Patent Gmbh | Organic electroluminescent device |
| EP1661888A1 (en) | 2004-11-29 | 2006-05-31 | Samsung SDI Co., Ltd. | Phenylcarbazole-based compound and organic electroluminescent device employing the same |
| WO2006097208A1 (en) | 2005-03-16 | 2006-09-21 | Merck Patent Gmbh | Novel materials for organic electroluminescent devices |
| WO2006100896A1 (en) | 2005-03-18 | 2006-09-28 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative and organic electroluminescence device utilizing the same |
| WO2006108497A1 (en) | 2005-04-14 | 2006-10-19 | Merck Patent Gmbh | Compounds for organic electronic devices |
| WO2006117052A1 (en) | 2005-05-03 | 2006-11-09 | Merck Patent Gmbh | Organic electroluminescent device and boric acid and borinic acid derivatives used therein |
| WO2006122630A1 (en) | 2005-05-20 | 2006-11-23 | Merck Patent Gmbh | Compounds for organic electronic devices |
| EP1731584A1 (en) | 2004-03-31 | 2006-12-13 | Konica Minolta Holdings, Inc. | Organic electroluminescent device material, organic electroluminescent device, display and illuminating device |
| WO2006131192A1 (en) | 2005-06-09 | 2006-12-14 | Merck Patent Gmbh | New materials for organic electroluminescence devices |
| EP1749809A1 (en) | 2004-05-27 | 2007-02-07 | Idemitsu Kosan Co., Ltd. | Asymmetric pyrene derivative and organic electroluminescent device using same |
| WO2007063754A1 (en) | 2005-12-01 | 2007-06-07 | Nippon Steel Chemical Co., Ltd. | Compound for organic electroluminescent element and organic electroluminescent element |
| WO2007065550A1 (en) | 2005-12-08 | 2007-06-14 | Merck Patent Gmbh | Novel materials for organic electroluminescent devices |
| WO2007065678A1 (en) | 2005-12-08 | 2007-06-14 | Merck Patent Gmbh | Novel materials for organic electroluminiescent devices |
| WO2007110129A1 (en) | 2006-03-24 | 2007-10-04 | Merck Patent Gmbh | New materials for organic electroluminescent devices |
| WO2007137725A1 (en) | 2006-05-31 | 2007-12-06 | Merck Patent Gmbh | New materials for organic electroluminescent devices |
| WO2007140847A1 (en) | 2006-06-02 | 2007-12-13 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2008006449A1 (en) | 2006-07-11 | 2008-01-17 | Merck Patent Gmbh | Novel materials for organic electroluminescent devices |
| EP1905754A1 (en) | 2005-07-06 | 2008-04-02 | Idemitsu Kosan Co., Ltd. | Pyrene derivative and organic electroluminescence device making use of the same |
| WO2008056746A1 (en) | 2006-11-09 | 2008-05-15 | Nippon Steel Chemical Co., Ltd. | Compound for organic electroluminescent device and organic electroluminescent device |
| WO2008086851A1 (en) | 2007-01-18 | 2008-07-24 | Merck Patent Gmbh | Carbazole derivatives for organc electroluminescent devices |
| WO2008145239A2 (en) | 2007-05-29 | 2008-12-04 | Merck Patent Gmbh | Benzanthracene derivatives for organic electroluminescent devices |
| WO2009062578A1 (en) | 2007-11-12 | 2009-05-22 | Merck Patent Gmbh | Organic electroluminescent devices comprising azomethine-metal complexes |
| WO2009100925A1 (en) | 2008-02-13 | 2009-08-20 | Merck Patent Gmbh | Novel materials for organic electroluminescent devices |
| WO2009146770A2 (en) | 2008-06-05 | 2009-12-10 | Merck Patent Gmbh | Electronic device comprising metal complexes |
| WO2010006680A1 (en) | 2008-07-18 | 2010-01-21 | Merck Patent Gmbh | Materials for organic electroluminescence devices |
| WO2010012328A1 (en) | 2008-07-29 | 2010-02-04 | Merck Patent Gmbh | Compounds for electronic devices |
| WO2010015306A1 (en) | 2008-08-08 | 2010-02-11 | Merck Patent Gmbh, | Organic electroluminescence device |
| WO2010015307A1 (en) | 2008-08-04 | 2010-02-11 | Merck Patent Gmbh | Electronic devices comprising metal complexes having isonitrile ligands |
| WO2010031485A1 (en) | 2008-09-22 | 2010-03-25 | Merck Patent Gmbh | Materials for organic electroluminescence devices |
| WO2010054731A1 (en) | 2008-11-13 | 2010-05-20 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2010054730A1 (en) | 2008-11-11 | 2010-05-20 | Merck Patent Gmbh | Organic electroluminescent devices |
| WO2010054728A1 (en) | 2008-11-13 | 2010-05-20 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2010054729A2 (en) | 2008-11-11 | 2010-05-20 | Merck Patent Gmbh | Materials for organic electroluminescence devices |
| WO2010072300A1 (en) | 2008-12-22 | 2010-07-01 | Merck Patent Gmbh | Organic electroluminescent device comprising triazine derivatives |
| WO2010086089A1 (en) | 2009-02-02 | 2010-08-05 | Merck Patent Gmbh | Metal complexes |
| WO2010099852A1 (en) | 2009-03-02 | 2010-09-10 | Merck Patent Gmbh | Metal complexes having azaborol ligands and electronic device having the same |
| WO2010102709A1 (en) | 2009-03-13 | 2010-09-16 | Merck Patent Gmbh | Materials for organic electroluminescence devices |
| WO2010108579A1 (en) | 2009-03-23 | 2010-09-30 | Merck Patent Gmbh | Organic electroluminescent device |
| WO2010136109A1 (en) | 2009-05-29 | 2010-12-02 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2011000455A1 (en) | 2009-06-30 | 2011-01-06 | Merck Patent Gmbh | Materials for organic electroluminescence devices |
| WO2011032626A1 (en) | 2009-09-16 | 2011-03-24 | Merck Patent Gmbh | Metal complexes |
| WO2011042107A2 (en) | 2009-10-08 | 2011-04-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2011054442A2 (en) | 2009-11-06 | 2011-05-12 | Merck Patent Gmbh | Materials for electronic devices |
| WO2011057706A2 (en) | 2009-11-14 | 2011-05-19 | Merck Patent Gmbh | Materials for electronic devices |
| WO2011060877A2 (en) | 2009-11-17 | 2011-05-26 | Merck Patent Gmbh | Materials for organic light emitting devices |
| WO2011060859A1 (en) | 2009-11-17 | 2011-05-26 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2011060867A1 (en) | 2009-11-18 | 2011-05-26 | Merck Patent Gmbh | Nitrogen-containing condensed heterocyclic compounds for oleds |
| WO2011066898A1 (en) | 2009-12-05 | 2011-06-09 | Merck Patent Gmbh | Electronic device containing metal complexes |
| WO2011088877A1 (en) | 2010-01-25 | 2011-07-28 | Merck Patent Gmbh | Compounds for electronic devices |
| WO2011116865A1 (en) | 2010-03-25 | 2011-09-29 | Merck Patent Gmbh | Materials for organic electroluminescence devices |
| WO2011137951A1 (en) | 2010-05-04 | 2011-11-10 | Merck Patent Gmbh | Organic electroluminescence devices |
| WO2011157339A1 (en) | 2010-06-15 | 2011-12-22 | Merck Patent Gmbh | Metal complexes |
| WO2012007086A1 (en) | 2010-07-16 | 2012-01-19 | Merck Patent Gmbh | Metal complexes |
| WO2012034627A1 (en) | 2010-09-15 | 2012-03-22 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2012048780A1 (en) | 2010-10-15 | 2012-04-19 | Merck Patent Gmbh | Compounds for electronic devices |
| WO2012048781A1 (en) | 2010-10-15 | 2012-04-19 | Merck Patent Gmbh | Triphenylene-based materials for organic electroluminescent devices |
| US20120187826A1 (en) | 2009-12-21 | 2012-07-26 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element using pyrene derivative |
| WO2012143080A2 (en) | 2011-04-18 | 2012-10-26 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2012150001A1 (en) | 2011-05-05 | 2012-11-08 | Merck Patent Gmbh | Compounds for electronic devices |
| WO2013041176A1 (en) | 2011-09-21 | 2013-03-28 | Merck Patent Gmbh | Carbazole derivatives for organic electroluminescence devices |
| WO2013056776A1 (en) | 2011-10-20 | 2013-04-25 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2013083216A1 (en) | 2011-11-17 | 2013-06-13 | Merck Patent Gmbh | Spiro dihydroacridine derivatives and the use thereof as materials for organic electroluminescence devices |
| WO2013120577A1 (en) | 2012-02-14 | 2013-08-22 | Merck Patent Gmbh | Spirobifluorene compounds for organic electroluminescent devices |
| WO2013185871A1 (en) | 2012-06-12 | 2013-12-19 | Merck Patent Gmbh | Compounds for electronic devices |
| WO2014008982A1 (en) | 2012-07-13 | 2014-01-16 | Merck Patent Gmbh | Metal complexes |
| WO2014015938A1 (en) | 2012-07-23 | 2014-01-30 | Merck Patent Gmbh | Derivatives of 2-diarylaminofluorene and organic electronic compounds containing them |
| WO2014015935A2 (en) | 2012-07-23 | 2014-01-30 | Merck Patent Gmbh | Compounds and organic electronic devices |
| WO2014015937A1 (en) | 2012-07-23 | 2014-01-30 | Merck Patent Gmbh | Compounds and organic electroluminescent devices |
| WO2014023377A2 (en) | 2012-08-07 | 2014-02-13 | Merck Patent Gmbh | Metal complexes |
| WO2014037077A1 (en) | 2012-09-04 | 2014-03-13 | Merck Patent Gmbh | Connections for electronic devices |
| WO2014072017A1 (en) | 2012-11-12 | 2014-05-15 | Merck Patent Gmbh | Materials for electronic devices |
| WO2014094960A1 (en) | 2012-12-21 | 2014-06-26 | Merck Patent Gmbh | Metal complexes |
| WO2014094961A1 (en) | 2012-12-21 | 2014-06-26 | Merck Patent Gmbh | Metal complexes |
| WO2014106522A1 (en) | 2013-01-03 | 2014-07-10 | Merck Patent Gmbh | Materials for electronic devices |
| WO2014111269A2 (en) | 2013-10-14 | 2014-07-24 | Merck Patent Gmbh | Materials for electronic devices |
| WO2015022051A1 (en) | 2013-08-15 | 2015-02-19 | Merck Patent Gmbh | Materials for electronic devices |
| WO2015036074A1 (en) | 2013-09-11 | 2015-03-19 | Merck Patent Gmbh | Metal complexes |
| WO2015082056A1 (en) | 2013-12-06 | 2015-06-11 | Merck Patent Gmbh | Compounds and organic electronic devices |
| WO2015086108A1 (en) | 2013-12-12 | 2015-06-18 | Merck Patent Gmbh | Materials for electronic devices |
| WO2015102118A1 (en) | 2014-02-18 | 2015-07-09 | 学校法人関西学院 | Polycyclic aromatic compound |
| WO2015104045A1 (en) | 2014-01-13 | 2015-07-16 | Merck Patent Gmbh | Metal complexes |
| WO2015117718A1 (en) | 2014-02-05 | 2015-08-13 | Merck Patent Gmbh | Metal complexes |
| WO2015131976A1 (en) | 2014-03-07 | 2015-09-11 | Merck Patent Gmbh | Materials for electronic devices |
| WO2015158411A1 (en) | 2014-04-14 | 2015-10-22 | Merck Patent Gmbh | Materials for electronic devices |
| WO2015158409A1 (en) | 2014-04-16 | 2015-10-22 | Merck Patent Gmbh | Materials for electronic devices |
| WO2015169412A1 (en) | 2014-05-05 | 2015-11-12 | Merck Patent Gmbh | Materials for organic light emitting devices |
| WO2016015810A1 (en) | 2014-07-29 | 2016-02-04 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2016015815A1 (en) | 2014-07-28 | 2016-02-04 | Merck Patent Gmbh | Metal complexes |
| WO2016023608A1 (en) | 2014-08-13 | 2016-02-18 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2016078738A1 (en) | 2014-11-18 | 2016-05-26 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2016087017A1 (en) | 2014-12-01 | 2016-06-09 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2016102048A1 (en) | 2014-12-22 | 2016-06-30 | Merck Patent Gmbh | Materials for electronic devices |
| WO2016124304A1 (en) | 2015-02-03 | 2016-08-11 | Merck Patent Gmbh | Metal complexes |
| WO2016131521A1 (en) | 2015-02-16 | 2016-08-25 | Merck Patent Gmbh | Spirobifluorene derivative-based materials for electronic devices |
| WO2016152418A1 (en) | 2015-03-25 | 2016-09-29 | 学校法人関西学院 | Polycyclic aromatic compound and light emission layer-forming composition |
| WO2016150544A1 (en) | 2015-03-25 | 2016-09-29 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2017025165A1 (en) | 2015-08-12 | 2017-02-16 | Merck Patent Gmbh | Materials for electronic devices |
| WO2017028941A1 (en) | 2015-08-14 | 2017-02-23 | Merck Patent Gmbh | Phenoxazine derivatives for organic electroluminescent devices |
| WO2017028940A1 (en) | 2015-08-14 | 2017-02-23 | Merck Patent Gmbh | Phenoxazine derivatives for organic electroluminescent devices |
| WO2017032439A1 (en) | 2015-08-25 | 2017-03-02 | Merck Patent Gmbh | Metal complexes |
| WO2017036573A1 (en) | 2015-08-28 | 2017-03-09 | Merck Patent Gmbh | Compounds for electronic devices |
| WO2017036574A1 (en) | 2015-08-28 | 2017-03-09 | Merck Patent Gmbh | 6,9,15,18-tetrahydro-s-indaceno[1,2-b:5,6-b']difluorene derivatives and use thereof in electronic devices |
| WO2017148565A1 (en) | 2016-03-03 | 2017-09-08 | Merck Patent Gmbh | Materials for organic electroluminescence devices |
| WO2018011186A1 (en) | 2016-07-14 | 2018-01-18 | Merck Patent Gmbh | Metal complexes |
| WO2018041769A1 (en) | 2016-08-30 | 2018-03-08 | Merck Patent Gmbh | Binuclear and trinuclear metal complexes composed of two inter-linked tripodal hexadentate ligands for use in electroluminescent devices |
| WO2018095397A1 (en) | 2016-11-23 | 2018-05-31 | 广州华睿光电材料有限公司 | Organic compound containing boron and uses thereof, organic mixture, and organic electronic device |
| WO2018178001A1 (en) | 2017-03-29 | 2018-10-04 | Merck Patent Gmbh | Metal complexes |
| WO2019004248A1 (en) | 2017-06-30 | 2019-01-03 | 住友化学株式会社 | Macromolecular compound and light-emitting element using same |
| WO2019020538A1 (en) | 2017-07-25 | 2019-01-31 | Merck Patent Gmbh | METALLIC COMPLEXES |
| WO2019115423A1 (en) | 2017-12-13 | 2019-06-20 | Merck Patent Gmbh | Metal complexes |
| WO2019132040A1 (en) | 2017-12-28 | 2019-07-04 | 出光興産株式会社 | Novel compound and organic electroluminescence element |
| WO2019158453A1 (en) | 2018-02-13 | 2019-08-22 | Merck Patent Gmbh | Metal complexes |
| US20200161552A1 (en) | 2018-11-21 | 2020-05-21 | Sfc Co., Ltd. | Indolocarbazole derivatives and organic electroluminescent devices using the same |
| WO2020208051A1 (en) | 2019-04-11 | 2020-10-15 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2021089450A1 (en) | 2019-11-04 | 2021-05-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| CN114195796A (en) * | 2021-12-20 | 2022-03-18 | 常州大学 | Deep blue thermal exciton luminescent material, preparation method and application thereof |
-
2024
- 2024-05-21 WO PCT/EP2024/063872 patent/WO2024240725A1/en active Pending
Patent Citations (157)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5061569A (en) | 1990-07-26 | 1991-10-29 | Eastman Kodak Company | Electroluminescent device with organic electroluminescent medium |
| WO1995009147A1 (en) | 1993-09-29 | 1995-04-06 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element and arylenediamine derivative |
| EP0652273A1 (en) | 1993-11-09 | 1995-05-10 | Shinko Electric Industries Co. Ltd. | Organic material for electroluminescent device and electroluminescent device |
| EP0676461A2 (en) | 1994-04-07 | 1995-10-11 | Hoechst Aktiengesellschaft | Spiro compounds and their application as electroluminescence materials |
| JP2000053957A (en) | 1998-06-23 | 2000-02-22 | Koto Gijutsu Kenkyuin Kenkyu Kumiai | Novel organometallic luminescent material and organic electroluminescent device containing the same |
| WO2000070655A2 (en) | 1999-05-13 | 2000-11-23 | The Trustees Of Princeton University | Very high efficiency organic light emitting devices based on electrophosphorescence |
| WO2001041512A1 (en) | 1999-12-01 | 2001-06-07 | The Trustees Of Princeton University | Complexes of form l2mx as phosphorescent dopants for organic leds |
| WO2001049806A1 (en) | 1999-12-31 | 2001-07-12 | Lg Chemical Co., Ltd | Electronic device comprising organic compound having p-type semiconducting characteristics |
| EP1205527A1 (en) | 2000-03-27 | 2002-05-15 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device |
| US6392250B1 (en) | 2000-06-30 | 2002-05-21 | Xerox Corporation | Organic light emitting devices having improved performance |
| WO2002002714A3 (en) | 2000-06-30 | 2002-10-24 | Du Pont | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| WO2002015645A1 (en) | 2000-08-11 | 2002-02-21 | The Trustees Of Princeton University | Organometallic compounds and emission-shifting organic electrophosphorescence |
| EP1191613A2 (en) | 2000-09-26 | 2002-03-27 | Canon Kabushiki Kaisha | Luminescence device, display apparatus and metal coordination compound |
| EP1191614A2 (en) | 2000-09-26 | 2002-03-27 | Canon Kabushiki Kaisha | Luminescence device and metal coordination compound therefor |
| EP1191612A2 (en) | 2000-09-26 | 2002-03-27 | Canon Kabushiki Kaisha | Luminescence device, display apparatus and metal coordination compound |
| US6803720B2 (en) | 2000-12-15 | 2004-10-12 | Universal Display Corporation | Highly stable and efficient OLEDs with a phosphorescent-doped mixed layer architecture |
| WO2002072714A1 (en) | 2001-03-10 | 2002-09-19 | Covion Organic Semiconductors Gmbh | Solutions and dispersions of organic semiconductors |
| WO2003019694A2 (en) | 2001-08-24 | 2003-03-06 | Covion Organic Semiconductors Gmbh | Solutions of polymer semiconductors |
| WO2003060956A2 (en) | 2002-01-18 | 2003-07-24 | Lg Chem, Ltd. | New material for transporting electrons and organic electroluminescent display using the same |
| WO2004013080A1 (en) | 2002-08-01 | 2004-02-12 | Covion Organic Semiconductors Gmbh | Spirobifluorene derivatives, their preparation and uses thereof |
| EP1553154A1 (en) | 2002-08-23 | 2005-07-13 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device and anthracene derivative |
| WO2004028217A1 (en) | 2002-09-20 | 2004-04-01 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element |
| WO2004058911A2 (en) | 2002-12-23 | 2004-07-15 | Covion Organic Semiconductors Gmbh | Organic electroluminescent element |
| WO2004081017A1 (en) | 2003-03-11 | 2004-09-23 | Covion Organic Semiconductors Gmbh | Metal complexes |
| WO2004080975A1 (en) | 2003-03-13 | 2004-09-23 | Idemitsu Kosan Co., Ltd. | Nitrogen-containing heterocycle derivative and organic electroluminescent element using the same |
| JP2004288381A (en) | 2003-03-19 | 2004-10-14 | Konica Minolta Holdings Inc | Organic electroluminescent element |
| WO2004093207A2 (en) | 2003-04-15 | 2004-10-28 | Covion Organic Semiconductors Gmbh | Mixtures of matrix materials and organic semiconductors capable of emission, use of the same and electronic components containing said mixtures |
| EP1617710A1 (en) | 2003-04-23 | 2006-01-18 | Konica Minolta Holdings, Inc. | Material for organic electroluminescent device, organic electroluminescent device, illuminating device and display |
| EP1617711A1 (en) | 2003-04-23 | 2006-01-18 | Konica Minolta Holdings, Inc. | Organic electroluminescent device and display |
| WO2005011013A1 (en) | 2003-07-21 | 2005-02-03 | Covion Organic Semiconductors Gmbh | Organic electroluminescent element |
| WO2005019373A2 (en) | 2003-08-19 | 2005-03-03 | Basf Aktiengesellschaft | Transition metal complexes comprising carbene ligands serving as emitters for organic light-emitting diodes (oled's) |
| WO2005033244A1 (en) | 2003-09-29 | 2005-04-14 | Covion Organic Semiconductors Gmbh | Metal complexes |
| WO2005039246A1 (en) | 2003-09-30 | 2005-04-28 | Konica Minolta Holdings, Inc. | Organic electroluminescent device, illuminating device, and display |
| US20050069729A1 (en) | 2003-09-30 | 2005-03-31 | Konica Minolta Holdings, Inc. | Organic electroluminescent element, illuminator, display and compound |
| WO2005084081A1 (en) | 2004-02-20 | 2005-09-09 | Merck Patent Gmbh | Organic electronic devices |
| WO2005084082A1 (en) | 2004-02-20 | 2005-09-09 | Merck Patent Gmbh | Organic electronic devices |
| EP1731584A1 (en) | 2004-03-31 | 2006-12-13 | Konica Minolta Holdings, Inc. | Organic electroluminescent device material, organic electroluminescent device, display and illuminating device |
| WO2005111172A2 (en) | 2004-05-11 | 2005-11-24 | Merck Patent Gmbh | Novel material mixtures for use in electroluminescence |
| US20050258742A1 (en) | 2004-05-18 | 2005-11-24 | Yui-Yi Tsai | Carbene containing metal complexes as OLEDs |
| EP1749809A1 (en) | 2004-05-27 | 2007-02-07 | Idemitsu Kosan Co., Ltd. | Asymmetric pyrene derivative and organic electroluminescent device using same |
| JP2005347160A (en) | 2004-06-04 | 2005-12-15 | Konica Minolta Holdings Inc | Organic electroluminescence element, lighting device and display device |
| WO2006005627A1 (en) | 2004-07-15 | 2006-01-19 | Merck Patent Gmbh | Oligomeric derivatives of spirobifluorene, their preparation and use |
| WO2006048268A1 (en) | 2004-11-06 | 2006-05-11 | Merck Patent Gmbh | Organic electroluminescent device |
| EP1661888A1 (en) | 2004-11-29 | 2006-05-31 | Samsung SDI Co., Ltd. | Phenylcarbazole-based compound and organic electroluminescent device employing the same |
| WO2006097208A1 (en) | 2005-03-16 | 2006-09-21 | Merck Patent Gmbh | Novel materials for organic electroluminescent devices |
| WO2006100896A1 (en) | 2005-03-18 | 2006-09-28 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative and organic electroluminescence device utilizing the same |
| WO2006108497A1 (en) | 2005-04-14 | 2006-10-19 | Merck Patent Gmbh | Compounds for organic electronic devices |
| WO2006117052A1 (en) | 2005-05-03 | 2006-11-09 | Merck Patent Gmbh | Organic electroluminescent device and boric acid and borinic acid derivatives used therein |
| WO2006122630A1 (en) | 2005-05-20 | 2006-11-23 | Merck Patent Gmbh | Compounds for organic electronic devices |
| WO2006131192A1 (en) | 2005-06-09 | 2006-12-14 | Merck Patent Gmbh | New materials for organic electroluminescence devices |
| EP1905754A1 (en) | 2005-07-06 | 2008-04-02 | Idemitsu Kosan Co., Ltd. | Pyrene derivative and organic electroluminescence device making use of the same |
| WO2007063754A1 (en) | 2005-12-01 | 2007-06-07 | Nippon Steel Chemical Co., Ltd. | Compound for organic electroluminescent element and organic electroluminescent element |
| WO2007065678A1 (en) | 2005-12-08 | 2007-06-14 | Merck Patent Gmbh | Novel materials for organic electroluminiescent devices |
| WO2007065550A1 (en) | 2005-12-08 | 2007-06-14 | Merck Patent Gmbh | Novel materials for organic electroluminescent devices |
| WO2007110129A1 (en) | 2006-03-24 | 2007-10-04 | Merck Patent Gmbh | New materials for organic electroluminescent devices |
| WO2007137725A1 (en) | 2006-05-31 | 2007-12-06 | Merck Patent Gmbh | New materials for organic electroluminescent devices |
| WO2007140847A1 (en) | 2006-06-02 | 2007-12-13 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2008006449A1 (en) | 2006-07-11 | 2008-01-17 | Merck Patent Gmbh | Novel materials for organic electroluminescent devices |
| WO2008056746A1 (en) | 2006-11-09 | 2008-05-15 | Nippon Steel Chemical Co., Ltd. | Compound for organic electroluminescent device and organic electroluminescent device |
| WO2008086851A1 (en) | 2007-01-18 | 2008-07-24 | Merck Patent Gmbh | Carbazole derivatives for organc electroluminescent devices |
| WO2008145239A2 (en) | 2007-05-29 | 2008-12-04 | Merck Patent Gmbh | Benzanthracene derivatives for organic electroluminescent devices |
| WO2009062578A1 (en) | 2007-11-12 | 2009-05-22 | Merck Patent Gmbh | Organic electroluminescent devices comprising azomethine-metal complexes |
| WO2009100925A1 (en) | 2008-02-13 | 2009-08-20 | Merck Patent Gmbh | Novel materials for organic electroluminescent devices |
| WO2009146770A2 (en) | 2008-06-05 | 2009-12-10 | Merck Patent Gmbh | Electronic device comprising metal complexes |
| WO2010006680A1 (en) | 2008-07-18 | 2010-01-21 | Merck Patent Gmbh | Materials for organic electroluminescence devices |
| WO2010012328A1 (en) | 2008-07-29 | 2010-02-04 | Merck Patent Gmbh | Compounds for electronic devices |
| WO2010015307A1 (en) | 2008-08-04 | 2010-02-11 | Merck Patent Gmbh | Electronic devices comprising metal complexes having isonitrile ligands |
| WO2010015306A1 (en) | 2008-08-08 | 2010-02-11 | Merck Patent Gmbh, | Organic electroluminescence device |
| WO2010031485A1 (en) | 2008-09-22 | 2010-03-25 | Merck Patent Gmbh | Materials for organic electroluminescence devices |
| WO2010054730A1 (en) | 2008-11-11 | 2010-05-20 | Merck Patent Gmbh | Organic electroluminescent devices |
| WO2010054729A2 (en) | 2008-11-11 | 2010-05-20 | Merck Patent Gmbh | Materials for organic electroluminescence devices |
| WO2010054731A1 (en) | 2008-11-13 | 2010-05-20 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2010054728A1 (en) | 2008-11-13 | 2010-05-20 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2010072300A1 (en) | 2008-12-22 | 2010-07-01 | Merck Patent Gmbh | Organic electroluminescent device comprising triazine derivatives |
| WO2010086089A1 (en) | 2009-02-02 | 2010-08-05 | Merck Patent Gmbh | Metal complexes |
| WO2010099852A1 (en) | 2009-03-02 | 2010-09-10 | Merck Patent Gmbh | Metal complexes having azaborol ligands and electronic device having the same |
| WO2010102709A1 (en) | 2009-03-13 | 2010-09-16 | Merck Patent Gmbh | Materials for organic electroluminescence devices |
| WO2010108579A1 (en) | 2009-03-23 | 2010-09-30 | Merck Patent Gmbh | Organic electroluminescent device |
| WO2010136109A1 (en) | 2009-05-29 | 2010-12-02 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2011000455A1 (en) | 2009-06-30 | 2011-01-06 | Merck Patent Gmbh | Materials for organic electroluminescence devices |
| WO2011032626A1 (en) | 2009-09-16 | 2011-03-24 | Merck Patent Gmbh | Metal complexes |
| WO2011042107A2 (en) | 2009-10-08 | 2011-04-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2011054442A2 (en) | 2009-11-06 | 2011-05-12 | Merck Patent Gmbh | Materials for electronic devices |
| WO2011057706A2 (en) | 2009-11-14 | 2011-05-19 | Merck Patent Gmbh | Materials for electronic devices |
| WO2011060877A2 (en) | 2009-11-17 | 2011-05-26 | Merck Patent Gmbh | Materials for organic light emitting devices |
| WO2011060859A1 (en) | 2009-11-17 | 2011-05-26 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2011060867A1 (en) | 2009-11-18 | 2011-05-26 | Merck Patent Gmbh | Nitrogen-containing condensed heterocyclic compounds for oleds |
| WO2011066898A1 (en) | 2009-12-05 | 2011-06-09 | Merck Patent Gmbh | Electronic device containing metal complexes |
| US20120187826A1 (en) | 2009-12-21 | 2012-07-26 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element using pyrene derivative |
| WO2011088877A1 (en) | 2010-01-25 | 2011-07-28 | Merck Patent Gmbh | Compounds for electronic devices |
| WO2011116865A1 (en) | 2010-03-25 | 2011-09-29 | Merck Patent Gmbh | Materials for organic electroluminescence devices |
| WO2011137951A1 (en) | 2010-05-04 | 2011-11-10 | Merck Patent Gmbh | Organic electroluminescence devices |
| WO2011157339A1 (en) | 2010-06-15 | 2011-12-22 | Merck Patent Gmbh | Metal complexes |
| WO2012007086A1 (en) | 2010-07-16 | 2012-01-19 | Merck Patent Gmbh | Metal complexes |
| WO2012034627A1 (en) | 2010-09-15 | 2012-03-22 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2012048780A1 (en) | 2010-10-15 | 2012-04-19 | Merck Patent Gmbh | Compounds for electronic devices |
| WO2012048781A1 (en) | 2010-10-15 | 2012-04-19 | Merck Patent Gmbh | Triphenylene-based materials for organic electroluminescent devices |
| WO2012143080A2 (en) | 2011-04-18 | 2012-10-26 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2012150001A1 (en) | 2011-05-05 | 2012-11-08 | Merck Patent Gmbh | Compounds for electronic devices |
| WO2013041176A1 (en) | 2011-09-21 | 2013-03-28 | Merck Patent Gmbh | Carbazole derivatives for organic electroluminescence devices |
| WO2013056776A1 (en) | 2011-10-20 | 2013-04-25 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2013083216A1 (en) | 2011-11-17 | 2013-06-13 | Merck Patent Gmbh | Spiro dihydroacridine derivatives and the use thereof as materials for organic electroluminescence devices |
| WO2013120577A1 (en) | 2012-02-14 | 2013-08-22 | Merck Patent Gmbh | Spirobifluorene compounds for organic electroluminescent devices |
| WO2013185871A1 (en) | 2012-06-12 | 2013-12-19 | Merck Patent Gmbh | Compounds for electronic devices |
| WO2014008982A1 (en) | 2012-07-13 | 2014-01-16 | Merck Patent Gmbh | Metal complexes |
| WO2014015938A1 (en) | 2012-07-23 | 2014-01-30 | Merck Patent Gmbh | Derivatives of 2-diarylaminofluorene and organic electronic compounds containing them |
| WO2014015935A2 (en) | 2012-07-23 | 2014-01-30 | Merck Patent Gmbh | Compounds and organic electronic devices |
| WO2014015937A1 (en) | 2012-07-23 | 2014-01-30 | Merck Patent Gmbh | Compounds and organic electroluminescent devices |
| WO2014023377A2 (en) | 2012-08-07 | 2014-02-13 | Merck Patent Gmbh | Metal complexes |
| WO2014037077A1 (en) | 2012-09-04 | 2014-03-13 | Merck Patent Gmbh | Connections for electronic devices |
| WO2014072017A1 (en) | 2012-11-12 | 2014-05-15 | Merck Patent Gmbh | Materials for electronic devices |
| WO2014094960A1 (en) | 2012-12-21 | 2014-06-26 | Merck Patent Gmbh | Metal complexes |
| WO2014094961A1 (en) | 2012-12-21 | 2014-06-26 | Merck Patent Gmbh | Metal complexes |
| WO2014106522A1 (en) | 2013-01-03 | 2014-07-10 | Merck Patent Gmbh | Materials for electronic devices |
| WO2015022051A1 (en) | 2013-08-15 | 2015-02-19 | Merck Patent Gmbh | Materials for electronic devices |
| WO2015036074A1 (en) | 2013-09-11 | 2015-03-19 | Merck Patent Gmbh | Metal complexes |
| WO2014111269A2 (en) | 2013-10-14 | 2014-07-24 | Merck Patent Gmbh | Materials for electronic devices |
| WO2015082056A1 (en) | 2013-12-06 | 2015-06-11 | Merck Patent Gmbh | Compounds and organic electronic devices |
| WO2015086108A1 (en) | 2013-12-12 | 2015-06-18 | Merck Patent Gmbh | Materials for electronic devices |
| WO2015104045A1 (en) | 2014-01-13 | 2015-07-16 | Merck Patent Gmbh | Metal complexes |
| WO2015117718A1 (en) | 2014-02-05 | 2015-08-13 | Merck Patent Gmbh | Metal complexes |
| WO2015102118A1 (en) | 2014-02-18 | 2015-07-09 | 学校法人関西学院 | Polycyclic aromatic compound |
| WO2015131976A1 (en) | 2014-03-07 | 2015-09-11 | Merck Patent Gmbh | Materials for electronic devices |
| WO2015158411A1 (en) | 2014-04-14 | 2015-10-22 | Merck Patent Gmbh | Materials for electronic devices |
| WO2015158409A1 (en) | 2014-04-16 | 2015-10-22 | Merck Patent Gmbh | Materials for electronic devices |
| WO2015169412A1 (en) | 2014-05-05 | 2015-11-12 | Merck Patent Gmbh | Materials for organic light emitting devices |
| WO2016015815A1 (en) | 2014-07-28 | 2016-02-04 | Merck Patent Gmbh | Metal complexes |
| WO2016015810A1 (en) | 2014-07-29 | 2016-02-04 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2016023608A1 (en) | 2014-08-13 | 2016-02-18 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2016078738A1 (en) | 2014-11-18 | 2016-05-26 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2016087017A1 (en) | 2014-12-01 | 2016-06-09 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2016102048A1 (en) | 2014-12-22 | 2016-06-30 | Merck Patent Gmbh | Materials for electronic devices |
| WO2016124304A1 (en) | 2015-02-03 | 2016-08-11 | Merck Patent Gmbh | Metal complexes |
| WO2016131521A1 (en) | 2015-02-16 | 2016-08-25 | Merck Patent Gmbh | Spirobifluorene derivative-based materials for electronic devices |
| WO2016152418A1 (en) | 2015-03-25 | 2016-09-29 | 学校法人関西学院 | Polycyclic aromatic compound and light emission layer-forming composition |
| WO2016150544A1 (en) | 2015-03-25 | 2016-09-29 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2017025165A1 (en) | 2015-08-12 | 2017-02-16 | Merck Patent Gmbh | Materials for electronic devices |
| WO2017028941A1 (en) | 2015-08-14 | 2017-02-23 | Merck Patent Gmbh | Phenoxazine derivatives for organic electroluminescent devices |
| WO2017028940A1 (en) | 2015-08-14 | 2017-02-23 | Merck Patent Gmbh | Phenoxazine derivatives for organic electroluminescent devices |
| WO2017032439A1 (en) | 2015-08-25 | 2017-03-02 | Merck Patent Gmbh | Metal complexes |
| WO2017036573A1 (en) | 2015-08-28 | 2017-03-09 | Merck Patent Gmbh | Compounds for electronic devices |
| WO2017036574A1 (en) | 2015-08-28 | 2017-03-09 | Merck Patent Gmbh | 6,9,15,18-tetrahydro-s-indaceno[1,2-b:5,6-b']difluorene derivatives and use thereof in electronic devices |
| WO2017148565A1 (en) | 2016-03-03 | 2017-09-08 | Merck Patent Gmbh | Materials for organic electroluminescence devices |
| WO2017148564A1 (en) | 2016-03-03 | 2017-09-08 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2018011186A1 (en) | 2016-07-14 | 2018-01-18 | Merck Patent Gmbh | Metal complexes |
| WO2018041769A1 (en) | 2016-08-30 | 2018-03-08 | Merck Patent Gmbh | Binuclear and trinuclear metal complexes composed of two inter-linked tripodal hexadentate ligands for use in electroluminescent devices |
| WO2018095397A1 (en) | 2016-11-23 | 2018-05-31 | 广州华睿光电材料有限公司 | Organic compound containing boron and uses thereof, organic mixture, and organic electronic device |
| WO2018178001A1 (en) | 2017-03-29 | 2018-10-04 | Merck Patent Gmbh | Metal complexes |
| WO2019004248A1 (en) | 2017-06-30 | 2019-01-03 | 住友化学株式会社 | Macromolecular compound and light-emitting element using same |
| WO2019020538A1 (en) | 2017-07-25 | 2019-01-31 | Merck Patent Gmbh | METALLIC COMPLEXES |
| WO2019115423A1 (en) | 2017-12-13 | 2019-06-20 | Merck Patent Gmbh | Metal complexes |
| WO2019132040A1 (en) | 2017-12-28 | 2019-07-04 | 出光興産株式会社 | Novel compound and organic electroluminescence element |
| WO2019158453A1 (en) | 2018-02-13 | 2019-08-22 | Merck Patent Gmbh | Metal complexes |
| US20200161552A1 (en) | 2018-11-21 | 2020-05-21 | Sfc Co., Ltd. | Indolocarbazole derivatives and organic electroluminescent devices using the same |
| WO2020208051A1 (en) | 2019-04-11 | 2020-10-15 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2021089450A1 (en) | 2019-11-04 | 2021-05-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| CN114195796A (en) * | 2021-12-20 | 2022-03-18 | 常州大学 | Deep blue thermal exciton luminescent material, preparation method and application thereof |
Non-Patent Citations (6)
| Title |
|---|
| B. R. HOJO ET AL., J. MATER. CHEM. C, vol. 10, 2022, pages 13871 |
| D. BHATTACHARYYA ET AL., ORG. LETT., vol. 23, 2021, pages 869 |
| HOJO RYOGA ET AL: "Deep-blue emission and thermally activated delayed fluorescence via Dimroth rearrangement of tris(triazolo)triazines", JOURNAL OF MATERIALS CHEMISTRY C, vol. 10, no. 37, 12 May 2022 (2022-05-12), GB, pages 13871 - 13877, XP093193900, ISSN: 2050-7526, DOI: 10.1039/D2TC01153K * |
| JOCHEM MATTHIAS ET AL: "Experimental and theoretical investigation on the thermal isomerization reaction of tristriazolotriazines", JOURNAL OF PHYSICAL ORGANIC CHEMISTRY., vol. 35, no. 7, 30 March 2022 (2022-03-30), GB, XP093193907, ISSN: 0894-3230, DOI: 10.1002/poc.4346 * |
| T. RIETH ET AL., MOLECULES, vol. 25, 2020, pages 5761 |
| Y. SHIROTA ET AL., CHEM. REV., vol. 107, no. 4, 2007, pages 953 - 1010 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3080229B1 (en) | Materials for electronic devices | |
| WO2015197156A1 (en) | Materials for organic electroluminescent devices | |
| EP3060623A1 (en) | Materials for electronic devices | |
| EP3197982A1 (en) | Materials for organic electroluminescent devices | |
| WO2017041874A1 (en) | Materials for organic electroluminescent devices | |
| WO2016128103A1 (en) | Electronic device containing cyclic lactams | |
| WO2023094412A1 (en) | Materials for electronic devices | |
| WO2023052272A1 (en) | Materials for electronic devices | |
| DE102010024897A1 (en) | Materials for organic electroluminescent devices | |
| EP4640022A1 (en) | Materials for electronic devices | |
| WO2024013004A1 (en) | Materials for electronic devices | |
| EP4077315B1 (en) | Materials for organic electroluminescent apparatus | |
| EP4409620A1 (en) | Materials for electronic devices | |
| WO2023052313A1 (en) | Materials for electronic devices | |
| EP4452909A1 (en) | Process for preparing deuterated organic compounds | |
| EP4192832A1 (en) | Materials for organic electroluminescent devices | |
| WO2024240725A1 (en) | Tris[1,2,4]triazolo[1,5-a:1',5'-c:1'',5''-e][1,3,5]triazine derivatives for use in organic electroluminescent devices | |
| WO2025132551A1 (en) | Materials for electronic devices | |
| WO2023152346A1 (en) | Materials for electronic devices | |
| WO2025210013A1 (en) | Compounds for electronic devices, in particular compounds for oleds | |
| WO2024218109A1 (en) | Materials for electronic devices | |
| WO2025045842A1 (en) | Materials for organic light-emitting devices | |
| WO2025021855A1 (en) | Materials for organic light-emitting devices and organic sensors | |
| WO2024194264A1 (en) | Materials for organic electroluminescent devices | |
| WO2025045851A1 (en) | Materials for organic light-emitting devices |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 24725903 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2024725903 Country of ref document: EP |