CN103524422A - Benzimidazole derivative, and preparation method and purpose thereof - Google Patents
Benzimidazole derivative, and preparation method and purpose thereof Download PDFInfo
- Publication number
- CN103524422A CN103524422A CN201310473947.XA CN201310473947A CN103524422A CN 103524422 A CN103524422 A CN 103524422A CN 201310473947 A CN201310473947 A CN 201310473947A CN 103524422 A CN103524422 A CN 103524422A
- Authority
- CN
- China
- Prior art keywords
- organic layer
- benzimidazole
- dissolve
- structural formula
- acid chloride
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 238000002360 preparation method Methods 0.000 title claims abstract description 13
- 125000003785 benzimidazolyl group Chemical class N1=C(NC2=C1C=CC=C2)* 0.000 title claims abstract 17
- 229940058303 antinematodal benzimidazole derivative Drugs 0.000 claims abstract description 27
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 claims description 48
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 claims description 45
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 32
- 239000012044 organic layer Substances 0.000 claims description 32
- 238000006243 chemical reaction Methods 0.000 claims description 30
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 claims description 24
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 claims description 24
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 23
- 239000000243 solution Substances 0.000 claims description 21
- RGHHSNMVTDWUBI-UHFFFAOYSA-N 4-hydroxybenzaldehyde Chemical compound OC1=CC=C(C=O)C=C1 RGHHSNMVTDWUBI-UHFFFAOYSA-N 0.000 claims description 18
- NGNBDVOYPDDBFK-UHFFFAOYSA-N 2-[2,4-di(pentan-2-yl)phenoxy]acetyl chloride Chemical compound CCCC(C)C1=CC=C(OCC(Cl)=O)C(C(C)CCC)=C1 NGNBDVOYPDDBFK-UHFFFAOYSA-N 0.000 claims description 17
- 239000000543 intermediate Substances 0.000 claims description 16
- 238000003756 stirring Methods 0.000 claims description 16
- 238000000034 method Methods 0.000 claims description 13
- 239000011541 reaction mixture Substances 0.000 claims description 10
- 238000010992 reflux Methods 0.000 claims description 9
- DWAQJAXMDSEUJJ-UHFFFAOYSA-M Sodium bisulfite Chemical compound [Na+].OS([O-])=O DWAQJAXMDSEUJJ-UHFFFAOYSA-M 0.000 claims description 8
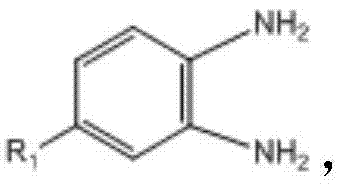
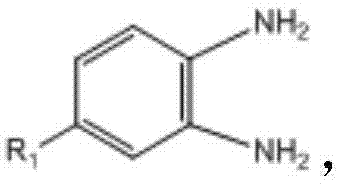
- 150000004987 o-phenylenediamines Chemical class 0.000 claims description 8
- 238000010898 silica gel chromatography Methods 0.000 claims description 8
- 235000010267 sodium hydrogen sulphite Nutrition 0.000 claims description 8
- XQMNBXPNBQFGBP-UHFFFAOYSA-M sodium;(2-hydroxyphenyl)methanesulfonate Chemical compound [Na+].OC1=CC=CC=C1CS([O-])(=O)=O XQMNBXPNBQFGBP-UHFFFAOYSA-M 0.000 claims description 8
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 7
- 239000007864 aqueous solution Substances 0.000 claims description 7
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 claims description 6
- 239000003480 eluent Substances 0.000 claims description 6
- 239000000741 silica gel Substances 0.000 claims description 6
- 229910002027 silica gel Inorganic materials 0.000 claims description 6
- 239000002917 insecticide Substances 0.000 claims description 5
- IAVREABSGIHHMO-UHFFFAOYSA-N 3-hydroxybenzaldehyde Chemical compound OC1=CC=CC(C=O)=C1 IAVREABSGIHHMO-UHFFFAOYSA-N 0.000 claims description 4
- 230000000895 acaricidal effect Effects 0.000 claims description 4
- 239000000642 acaricide Substances 0.000 claims description 4
- 238000001914 filtration Methods 0.000 claims description 4
- 238000005406 washing Methods 0.000 claims description 4
- SMQUZDBALVYZAC-UHFFFAOYSA-N ortho-hydroxybenzaldehyde Natural products OC1=CC=CC=C1C=O SMQUZDBALVYZAC-UHFFFAOYSA-N 0.000 claims description 3
- SMQUZDBALVYZAC-HOSYLAQJSA-N 2-hydroxybenzaldehyde Chemical group OC1=CC=CC=C1[13CH]=O SMQUZDBALVYZAC-HOSYLAQJSA-N 0.000 claims 1
- 230000000844 anti-bacterial effect Effects 0.000 claims 1
- 239000003899 bactericide agent Substances 0.000 claims 1
- 150000002148 esters Chemical class 0.000 claims 1
- 241000238631 Hexapoda Species 0.000 abstract description 14
- 241000243785 Meloidogyne javanica Species 0.000 abstract description 12
- 241000244206 Nematoda Species 0.000 abstract description 12
- 241000238876 Acari Species 0.000 abstract description 11
- 241000196324 Embryophyta Species 0.000 abstract description 11
- 241000607479 Yersinia pestis Species 0.000 abstract description 9
- 244000052616 bacterial pathogen Species 0.000 abstract description 9
- 239000000417 fungicide Substances 0.000 abstract description 5
- 241000243784 Meloidogyne arenaria Species 0.000 abstract description 4
- 241000243787 Meloidogyne hapla Species 0.000 abstract description 4
- 241000243786 Meloidogyne incognita Species 0.000 abstract description 4
- 240000001766 Mycetia javanica Species 0.000 abstract description 3
- 230000000855 fungicidal effect Effects 0.000 abstract description 3
- 230000003071 parasitic effect Effects 0.000 abstract description 3
- 235000017060 Arachis glabrata Nutrition 0.000 abstract description 2
- 244000105624 Arachis hypogaea Species 0.000 abstract description 2
- 235000010777 Arachis hypogaea Nutrition 0.000 abstract description 2
- 235000018262 Arachis monticola Nutrition 0.000 abstract description 2
- 201000010099 disease Diseases 0.000 abstract description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 abstract description 2
- 235000020232 peanut Nutrition 0.000 abstract description 2
- 238000006467 substitution reaction Methods 0.000 abstract 1
- 150000001556 benzimidazoles Chemical class 0.000 description 33
- 239000000203 mixture Substances 0.000 description 17
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 12
- 239000003814 drug Substances 0.000 description 10
- 239000002904 solvent Substances 0.000 description 9
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 8
- 238000005481 NMR spectroscopy Methods 0.000 description 8
- 241000500437 Plutella xylostella Species 0.000 description 8
- 239000007921 spray Substances 0.000 description 8
- 241001124076 Aphididae Species 0.000 description 7
- 241001556089 Nilaparvata lugens Species 0.000 description 7
- 150000001875 compounds Chemical class 0.000 description 7
- -1 sodium p-hydroxybenzene methanesulfonate Chemical compound 0.000 description 7
- 239000011550 stock solution Substances 0.000 description 7
- HYZJCKYKOHLVJF-UHFFFAOYSA-N 1H-benzimidazole Chemical compound C1=CC=C2NC=NC2=C1 HYZJCKYKOHLVJF-UHFFFAOYSA-N 0.000 description 6
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 6
- 241000256186 Anopheles <genus> Species 0.000 description 6
- 229940079593 drug Drugs 0.000 description 6
- 239000003208 petroleum Substances 0.000 description 6
- 229920000136 polysorbate Polymers 0.000 description 6
- 241000344246 Tetranychus cinnabarinus Species 0.000 description 5
- 241001600408 Aphis gossypii Species 0.000 description 4
- 241000256054 Culex <genus> Species 0.000 description 4
- 241000255969 Pieris brassicae Species 0.000 description 4
- 239000012141 concentrate Substances 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 239000012085 test solution Substances 0.000 description 4
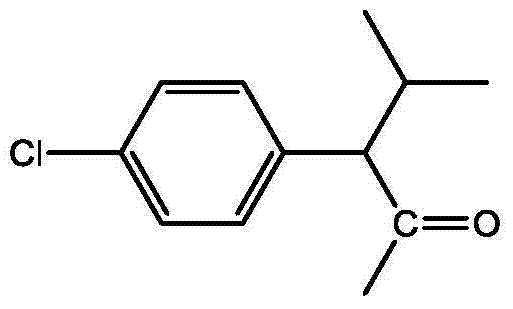
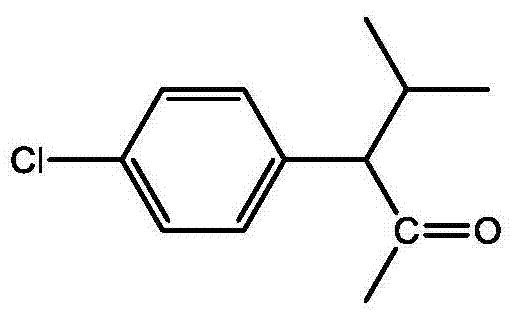
- BWPYAVCZZUTOBY-UHFFFAOYSA-N 2-(4-chlorophenyl)-3-methylbutanoyl chloride Chemical compound CC(C)C(C(Cl)=O)C1=CC=C(Cl)C=C1 BWPYAVCZZUTOBY-UHFFFAOYSA-N 0.000 description 3
- 241000256593 Brachycaudus schwartzi Species 0.000 description 3
- 240000007124 Brassica oleracea Species 0.000 description 3
- 235000003899 Brassica oleracea var acephala Nutrition 0.000 description 3
- 235000011301 Brassica oleracea var capitata Nutrition 0.000 description 3
- 235000001169 Brassica oleracea var oleracea Nutrition 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 3
- 239000004480 active ingredient Substances 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 239000008187 granular material Substances 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 239000008399 tap water Substances 0.000 description 3
- 235000020679 tap water Nutrition 0.000 description 3
- GEYOCULIXLDCMW-UHFFFAOYSA-N 1,2-phenylenediamine Chemical compound NC1=CC=CC=C1N GEYOCULIXLDCMW-UHFFFAOYSA-N 0.000 description 2
- 241000255925 Diptera Species 0.000 description 2
- QUSNBJAOOMFDIB-UHFFFAOYSA-N Ethylamine Chemical compound CCN QUSNBJAOOMFDIB-UHFFFAOYSA-N 0.000 description 2
- 241000258937 Hemiptera Species 0.000 description 2
- 241000255777 Lepidoptera Species 0.000 description 2
- 241000272317 Lipaphis erysimi Species 0.000 description 2
- 241000721621 Myzus persicae Species 0.000 description 2
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 238000007654 immersion Methods 0.000 description 2
- VEFKIGFJSFFFIE-UHFFFAOYSA-M sodium (4-hydroxyphenyl)methanesulfonate Chemical compound [Na+].OC1=CC=C(CS([O-])(=O)=O)C=C1 VEFKIGFJSFFFIE-UHFFFAOYSA-M 0.000 description 2
- 239000002689 soil Substances 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 235000013311 vegetables Nutrition 0.000 description 2
- GLGNXYJARSMNGJ-VKTIVEEGSA-N (1s,2s,3r,4r)-3-[[5-chloro-2-[(1-ethyl-6-methoxy-2-oxo-4,5-dihydro-3h-1-benzazepin-7-yl)amino]pyrimidin-4-yl]amino]bicyclo[2.2.1]hept-5-ene-2-carboxamide Chemical compound CCN1C(=O)CCCC2=C(OC)C(NC=3N=C(C(=CN=3)Cl)N[C@H]3[C@H]([C@@]4([H])C[C@@]3(C=C4)[H])C(N)=O)=CC=C21 GLGNXYJARSMNGJ-VKTIVEEGSA-N 0.000 description 1
- SZUVGFMDDVSKSI-WIFOCOSTSA-N (1s,2s,3s,5r)-1-(carboxymethyl)-3,5-bis[(4-phenoxyphenyl)methyl-propylcarbamoyl]cyclopentane-1,2-dicarboxylic acid Chemical compound O=C([C@@H]1[C@@H]([C@](CC(O)=O)([C@H](C(=O)N(CCC)CC=2C=CC(OC=3C=CC=CC=3)=CC=2)C1)C(O)=O)C(O)=O)N(CCC)CC(C=C1)=CC=C1OC1=CC=CC=C1 SZUVGFMDDVSKSI-WIFOCOSTSA-N 0.000 description 1
- STPKWKPURVSAJF-LJEWAXOPSA-N (4r,5r)-5-[4-[[4-(1-aza-4-azoniabicyclo[2.2.2]octan-4-ylmethyl)phenyl]methoxy]phenyl]-3,3-dibutyl-7-(dimethylamino)-1,1-dioxo-4,5-dihydro-2h-1$l^{6}-benzothiepin-4-ol Chemical compound O[C@H]1C(CCCC)(CCCC)CS(=O)(=O)C2=CC=C(N(C)C)C=C2[C@H]1C(C=C1)=CC=C1OCC(C=C1)=CC=C1C[N+]1(CC2)CCN2CC1 STPKWKPURVSAJF-LJEWAXOPSA-N 0.000 description 1
- WZZBNLYBHUDSHF-DHLKQENFSA-N 1-[(3s,4s)-4-[8-(2-chloro-4-pyrimidin-2-yloxyphenyl)-7-fluoro-2-methylimidazo[4,5-c]quinolin-1-yl]-3-fluoropiperidin-1-yl]-2-hydroxyethanone Chemical compound CC1=NC2=CN=C3C=C(F)C(C=4C(=CC(OC=5N=CC=CN=5)=CC=4)Cl)=CC3=C2N1[C@H]1CCN(C(=O)CO)C[C@@H]1F WZZBNLYBHUDSHF-DHLKQENFSA-N 0.000 description 1
- 238000005160 1H NMR spectroscopy Methods 0.000 description 1
- KWEWNOOZQVJONF-UHFFFAOYSA-N 4-fluorobenzene-1,2-diamine Chemical compound NC1=CC=C(F)C=C1N KWEWNOOZQVJONF-UHFFFAOYSA-N 0.000 description 1
- DGRGLKZMKWPMOH-UHFFFAOYSA-N 4-methylbenzene-1,2-diamine Chemical compound CC1=CC=C(N)C(N)=C1 DGRGLKZMKWPMOH-UHFFFAOYSA-N 0.000 description 1
- 241000239290 Araneae Species 0.000 description 1
- KHJXCKKVEBYYLI-UHFFFAOYSA-N CC(C)(C1C=C(Br)Br)C1C(C)=O Chemical compound CC(C)(C1C=C(Br)Br)C1C(C)=O KHJXCKKVEBYYLI-UHFFFAOYSA-N 0.000 description 1
- ILBDDFPSCLDHEF-UHFFFAOYSA-N CC(C)(C1C=C(Cl)Cl)C1C(C)=O Chemical compound CC(C)(C1C=C(Cl)Cl)C1C(C)=O ILBDDFPSCLDHEF-UHFFFAOYSA-N 0.000 description 1
- 0 CC(C)([C@@]1C=C(C(F)(F)F)Cl)C1*(C)=O Chemical compound CC(C)([C@@]1C=C(C(F)(F)F)Cl)C1*(C)=O 0.000 description 1
- JCKLYLNJESKQRY-UHFFFAOYSA-N CC(C)C(C(C)=O)c(cc1)ccc1Cl Chemical compound CC(C)C(C(C)=O)c(cc1)ccc1Cl JCKLYLNJESKQRY-UHFFFAOYSA-N 0.000 description 1
- LBIKVSSIBIPUSF-UHFFFAOYSA-N CC1(C)C(C)(C)C1C(Oc(cc1)ccc1-c1nc2ccccc2[nH]1)=O Chemical compound CC1(C)C(C)(C)C1C(Oc(cc1)ccc1-c1nc2ccccc2[nH]1)=O LBIKVSSIBIPUSF-UHFFFAOYSA-N 0.000 description 1
- 235000015001 Cucumis melo var inodorus Nutrition 0.000 description 1
- 240000002495 Cucumis melo var. inodorus Species 0.000 description 1
- 239000005909 Kieselgur Substances 0.000 description 1
- 241001522024 Myxus Species 0.000 description 1
- BPQQTUXANYXVAA-UHFFFAOYSA-N Orthosilicate Chemical compound [O-][Si]([O-])([O-])[O-] BPQQTUXANYXVAA-UHFFFAOYSA-N 0.000 description 1
- 241000500441 Plutellidae Species 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 150000008051 alkyl sulfates Chemical class 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 229960000892 attapulgite Drugs 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 238000009395 breeding Methods 0.000 description 1
- 230000001488 breeding effect Effects 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 229910052956 cinnabar Inorganic materials 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 239000003245 coal Substances 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 229940126543 compound 14 Drugs 0.000 description 1
- 229940125758 compound 15 Drugs 0.000 description 1
- GUJOJGAPFQRJSV-UHFFFAOYSA-N dialuminum;dioxosilane;oxygen(2-);hydrate Chemical compound O.[O-2].[O-2].[O-2].[Al+3].[Al+3].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O GUJOJGAPFQRJSV-UHFFFAOYSA-N 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 235000013399 edible fruits Nutrition 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 239000004495 emulsifiable concentrate Substances 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- GFAUNYMRSKVDJL-UHFFFAOYSA-N formyl chloride Chemical compound ClC=O GFAUNYMRSKVDJL-UHFFFAOYSA-N 0.000 description 1
- 239000004009 herbicide Substances 0.000 description 1
- 230000000749 insecticidal effect Effects 0.000 description 1
- 239000012669 liquid formulation Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 239000003094 microcapsule Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- PSZYNBSKGUBXEH-UHFFFAOYSA-M naphthalene-1-sulfonate Chemical compound C1=CC=C2C(S(=O)(=O)[O-])=CC=CC2=C1 PSZYNBSKGUBXEH-UHFFFAOYSA-M 0.000 description 1
- 239000002736 nonionic surfactant Substances 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 229910052625 palygorskite Inorganic materials 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 1
- 239000004546 suspension concentrate Substances 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 239000004563 wettable powder Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D235/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings
- C07D235/02—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings condensed with carbocyclic rings or ring systems
- C07D235/04—Benzimidazoles; Hydrogenated benzimidazoles
- C07D235/18—Benzimidazoles; Hydrogenated benzimidazoles with aryl radicals directly attached in position 2
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/50—1,3-Diazoles; Hydrogenated 1,3-diazoles
- A01N43/52—1,3-Diazoles; Hydrogenated 1,3-diazoles condensed with carbocyclic rings, e.g. benzimidazoles
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N53/00—Biocides, pest repellants or attractants, or plant growth regulators containing cyclopropane carboxylic acids or derivatives thereof
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Dentistry (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Plant Pathology (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Pest Control & Pesticides (AREA)
- Agronomy & Crop Science (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
Abstract
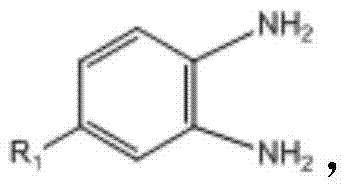
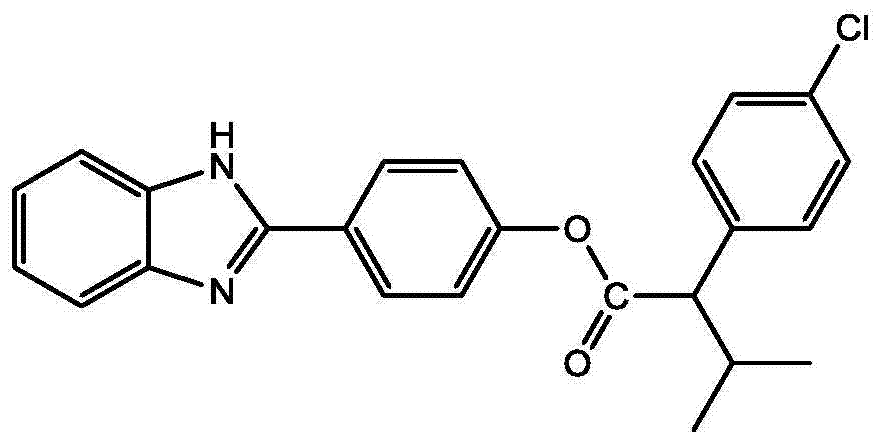
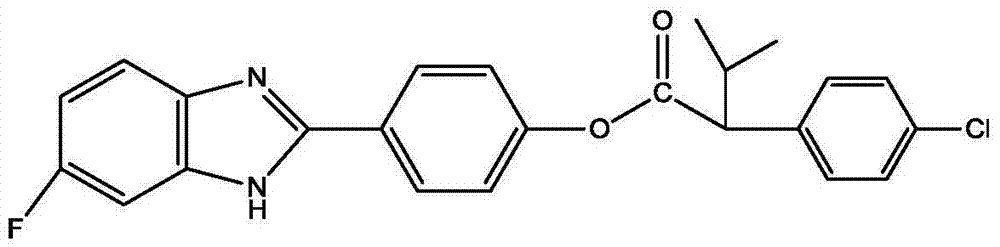
本发明公开了一种苯并咪唑衍生物及其制备方法和用途,其结构式如下:其中,R1选自H、F、Cl、Br、CH3、NO3中的一种,-OR2取代的位置为邻位、间位和对位中的一种。该苯并咪唑衍生物可以用于防治线虫,主要防治植物寄生线虫,如根结线虫,南方根结线虫(M.incognita)、爪哇根结线虫(M.javanica)、花生根结线虫(M.arenaria)和北方根结线虫(M.hapla)。也可以作为杀菌剂,防治多种病原菌。该苯并咪唑衍生物可以有效地控制植物害虫、害螨、线虫和病菌,可以应用在农业上防治病虫害,病原菌。The invention discloses a benzimidazole derivative as well as its preparation method and application. Its structural formula is as follows: Wherein, R 1 is selected from one of H, F, Cl, Br, CH 3 , NO 3 , and the substitution position of -OR 2 is one of ortho, meta and para. The benzimidazole derivatives can be used to prevent and control nematodes, mainly plant parasitic nematodes, such as root-knot nematode, root-knot nematode incognita (M.incognita), root-knot nematode Java (M.javanica), root-knot nematode peanut (M. arenaria) and root-knot nematode (M.hapla). It can also be used as a fungicide to prevent and control a variety of pathogenic bacteria. The benzimidazole derivative can effectively control plant pests, harmful mites, nematodes and germs, and can be used in agriculture to prevent and control diseases and insect pests and pathogenic bacteria.
Description
技术领域technical field
本发明涉及杀虫杀螨技术领域,特别是涉及苯并咪唑衍生物及其制备方法和用途。The invention relates to the technical field of insecticide and acaricide, in particular to benzimidazole derivatives and their preparation methods and applications.
背景技术Background technique
随着经济社会的发展,人们对农业的经济效益要求越来越高,但是农业害虫害螨的危害严重影响了农产品的产量和质量,例如蚜虫能危害多种植物,并且其分泌的蜜露能引起煤污病;小菜蛾危害白菜、甘蓝等多种十字花科植物,影响蔬菜品质,因此杀灭害虫害螨显得越来越重要。With the development of economy and society, people have higher and higher requirements for the economic benefits of agriculture, but the harm of agricultural pests and mites seriously affects the yield and quality of agricultural products. For example, aphids can harm a variety of plants, and the honeydew they secrete can Cause coal pollution; diamondback moth harms cabbage, cabbage and other cruciferous plants, affecting the quality of vegetables, so it is more and more important to kill pests and mites.
发明内容Contents of the invention
有鉴于此,本发明的目的在于提出一种苯并咪唑衍生物及其制备方法和用途,以有效地控制植物害虫、害螨、线虫和病菌。In view of this, the object of the present invention is to propose a kind of benzimidazole derivative and its preparation method and application, to effectively control plant pests, harmful mites, nematodes and germs.
基于上述目的,本发明提供的苯并咪唑衍生物的结构式如下:Based on the above object, the structural formula of the benzimidazole derivative provided by the invention is as follows:
其中,R1选自H、F、Cl、Br、CH3、NO3中的一种,-OR2取代的位置为邻位、间位和对位中的一种,且R2选自Wherein, R 1 is selected from one of H, F, Cl, Br, CH 3 , NO 3 , the position substituted by -OR 2 is one of ortho, meta and para, and R 2 is selected from
和中的一种。 and One of.
可选地,所述苯并咪唑衍生物通过以下步骤制得:Alternatively, the benzimidazole derivatives are prepared through the following steps:
将羟基苯甲醛和亚硫酸氢钠溶于乙醇水溶液中,室温下搅拌0.3-0.8小时,抽滤反应得到的混合物,得到羟基苯甲磺酸酸钠;Dissolving hydroxybenzaldehyde and sodium bisulfite in an aqueous ethanol solution, stirring at room temperature for 0.3-0.8 hours, and suction filtering the reaction mixture to obtain sodium hydroxybenzenemethanesulfonate;
将所述羟基苯甲磺酸酸钠和取代邻苯二胺溶于N,N-二甲基甲酰胺中,回流1-3小时,然后将反应得到的混合物倒入冰中,抽滤,重结晶得中间体;Dissolve the sodium hydroxybenzenemethanesulfonate and substituted o-phenylenediamine in N,N-dimethylformamide, reflux for 1-3 hours, then pour the reaction mixture into ice, suction filter, and re- crystallized intermediates;
将所述中间体和三乙胺溶于二氯甲烷中,冰盐浴冷却至-3~-8℃,将酰氯类化合物溶于二氯甲烷配成酰氯溶液,然后将所述酰氯溶液加入到所述中间体和三乙胺的反应体系中,自然升温至24~28℃,继续搅拌反应1~1.2h;分离反应得到的有机层,然后依次通过水洗、干燥、浓缩和硅胶柱层析分离该有机层,即得到所述苯并咪唑衍生物;Dissolve the intermediate and triethylamine in dichloromethane, cool in an ice-salt bath to -3~-8°C, dissolve the acid chloride compound in dichloromethane to form an acid chloride solution, and then add the acid chloride solution to In the reaction system of the intermediate and triethylamine, the temperature is naturally raised to 24-28°C, and the stirring reaction is continued for 1-1.2h; the organic layer obtained by the reaction is separated, and then washed with water, dried, concentrated and separated by silica gel column chromatography The organic layer obtains the benzimidazole derivative;
其中,所述取代邻苯二胺的结构式为 Wherein, the structural formula of the substituted o-phenylenediamine is
所述中间体的结构式为 The structural formula of the intermediate is
所述酰氯类化合物的结构式为R2-Cl,The structural formula of the acid chloride compound is R 2 -Cl,
R1选自H、F、Cl、Br、CH3、NO3中的一种,R 1 is selected from one of H, F, Cl, Br, CH 3 , NO 3 ,
R2选自 R2 is selected from
和中的一种。 and One of.
本发明还提供制备上述苯并咪唑衍生物的方法,包括以下步骤:The present invention also provides a method for preparing the above-mentioned benzimidazole derivatives, comprising the following steps:
将羟基苯甲醛和亚硫酸氢钠溶于乙醇水溶液中,室温下搅拌0.3-0.8小时,抽滤反应得到的混合物,得到羟基苯甲磺酸酸钠;Dissolving hydroxybenzaldehyde and sodium bisulfite in an aqueous ethanol solution, stirring at room temperature for 0.3-0.8 hours, and suction filtering the reaction mixture to obtain sodium hydroxybenzenemethanesulfonate;
将所述羟基苯甲磺酸酸钠和取代邻苯二胺溶于N,N-二甲基甲酰胺中,回流1-3小时,然后将反应得到的混合物倒入冰中,抽滤,重结晶得中间体;Dissolve the sodium hydroxybenzenemethanesulfonate and substituted o-phenylenediamine in N,N-dimethylformamide, reflux for 1-3 hours, then pour the reaction mixture into ice, suction filter, and re- crystallized intermediates;
将所述中间体和三乙胺溶于二氯甲烷中,冰盐浴冷却至-3~-8℃,将酰氯类化合物溶于二氯甲烷配成酰氯溶液,然后将所述酰氯溶液加入到所述中间体和三乙胺的反应体系中,自然升温至24~28℃,继续搅拌反应1~1.2h;分离反应得到的有机层,然后依次通过水洗、干燥、浓缩和硅胶柱层析分离该有机层,即得到所述苯并咪唑衍生物;Dissolve the intermediate and triethylamine in dichloromethane, cool in an ice-salt bath to -3~-8°C, dissolve the acid chloride compound in dichloromethane to form an acid chloride solution, and then add the acid chloride solution to In the reaction system of the intermediate and triethylamine, the temperature is naturally raised to 24-28°C, and the stirring reaction is continued for 1-1.2h; the organic layer obtained by the reaction is separated, and then washed with water, dried, concentrated and separated by silica gel column chromatography The organic layer obtains the benzimidazole derivative;
其中,所述取代邻苯二胺的结构式为 Wherein, the structural formula of the substituted o-phenylenediamine is
所述中间体的结构式为 The structural formula of the intermediate is
所述酰氯类化合物的结构式为R2-Cl,The structural formula of the acid chloride compound is R 2 -Cl,
R1选自H、F、Cl、Br、CH3、NO3中的一种,R 1 is selected from one of H, F, Cl, Br, CH 3 , NO 3 ,
R2选自 R2 is selected from
和中的一种。 and One of.
可选地,所述羟基苯甲醛选自2-羟基苯甲醛,3-羟基苯甲醛和4-羟基苯甲醛中的一种。Optionally, the hydroxybenzaldehyde is selected from one of 2-hydroxybenzaldehyde, 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde.
可选地,所述乙醇水溶液中乙醇和水的体积比为1:0.9~1.2。Optionally, the volume ratio of ethanol to water in the ethanol aqueous solution is 1:0.9-1.2.
较佳地,在所述水洗有机层的步骤中,采用水洗涤有机层2~4次,每次洗涤所用水的体积为有机层体积的1.3~1.8倍。Preferably, in the step of washing the organic layer with water, the organic layer is washed with water for 2 to 4 times, and the volume of water used for each washing is 1.3 to 1.8 times the volume of the organic layer.
优选地,采用无水硫酸镁干燥所述水洗后的有机层。Preferably, the washed organic layer is dried with anhydrous magnesium sulfate.
可选地,所述硅胶为100~200目,洗脱剂为石油醚和乙酸乙酯,且所述石油醚和乙酸乙酯的体积比为4~1:1。Optionally, the silica gel is 100-200 mesh, the eluent is petroleum ether and ethyl acetate, and the volume ratio of the petroleum ether and ethyl acetate is 4-1:1.
本发明还提供上述苯并咪唑衍生物的用途,所述苯并咪唑衍生物用于杀虫、杀螨和杀菌。The present invention also provides the use of the above-mentioned benzimidazole derivatives, which are used for insecticide, acaricide and fungicide.
从上面所述可以看出,本发明了提供一种新型苯并咪唑衍生物,本发明提供的苯并咪唑衍生物可以用于控制多种农业、园林及仓贮有害的昆虫,如鳞翅目、双翅目、同翅目以及一些无脊椎昆虫,如螨类。具体地:Aphisgossypii(棉蚜)、Myzus persicae(桃蚜)、Lipaphis erysimi(菜蚜)Plutellaxylostella(小菜蛾),Nilaparvata lugens(褐飞虱),Tetranychus cinnabarinus(朱砂叶螨)Pieris brassicae(大菜粉蝶),Anopheles spp.(按蚊属),Culex spp(库蚊属)。也可以用于防治线虫,主要防治植物寄生线虫,如根结线虫,南方根结线虫(M.incognita)、爪哇根结线虫(M.javanica)、花生根结线虫(M.arenaria)和北方根结线虫(M.hapla)。也可以作为杀菌剂,防治多种病原菌。该苯并咪唑衍生物可以有效地控制植物害虫、害螨、线虫和病菌,可以应用在农业上防治病虫害,病原菌。As can be seen from the above, the present invention provides a novel benzimidazole derivative, and the benzimidazole derivative provided by the invention can be used to control various agricultural, garden and storage harmful insects, such as Lepidoptera , Diptera, Homoptera and some invertebrate insects such as mites. Specifically: Aphisgossypii (Cotton aphid), Myzus persicae (Peach aphid), Lipaphis erysimi (Pipella aphid), Plutellaxylostella (Plutella xylostella), Nilaparvata lugens (Brown planthopper), Tetranychus cinnabarinus (Cinnabarinus), Pieris brassicae (Pipella cabbage butterfly), Anopheles spp .(Anopheles), Culex spp (Culex). It can also be used to control nematodes, mainly to control plant parasitic nematodes, such as root-knot nematode, southern root-knot nematode (M.incognita), Javan root-knot nematode (M.javanica), peanut root-knot nematode (M.arenaria) and northern root-knot nematode Knot nematode (M. hapla). It can also be used as a fungicide to prevent and control a variety of pathogenic bacteria. The benzimidazole derivative can effectively control plant pests, harmful mites, nematodes and germs, and can be used in agriculture to prevent and control diseases and insect pests and pathogenic bacteria.
具体实施方式Detailed ways
为使本发明的目的、技术方案和优点更加清楚明白,以下结合具体实施例,对本发明进一步详细说明。In order to make the object, technical solution and advantages of the present invention clearer, the present invention will be further described in detail below in conjunction with specific examples.
本发明提供的苯并咪唑衍生物的结构式如下:The structural formula of the benzimidazole derivative provided by the invention is as follows:
其中,R1选自H、F、Cl、Br、CH3、NO3中的一种,-OR2取代的位置为邻位、间位和对位中的一种,且R2选自Wherein, R 1 is selected from one of H, F, Cl, Br, CH 3 , NO 3 , the position substituted by -OR 2 is one of ortho, meta and para, and R 2 is selected from
和中的一种。 and One of.
部分化合物的具体组合方式参见表1:The specific combinations of some compounds are shown in Table 1:
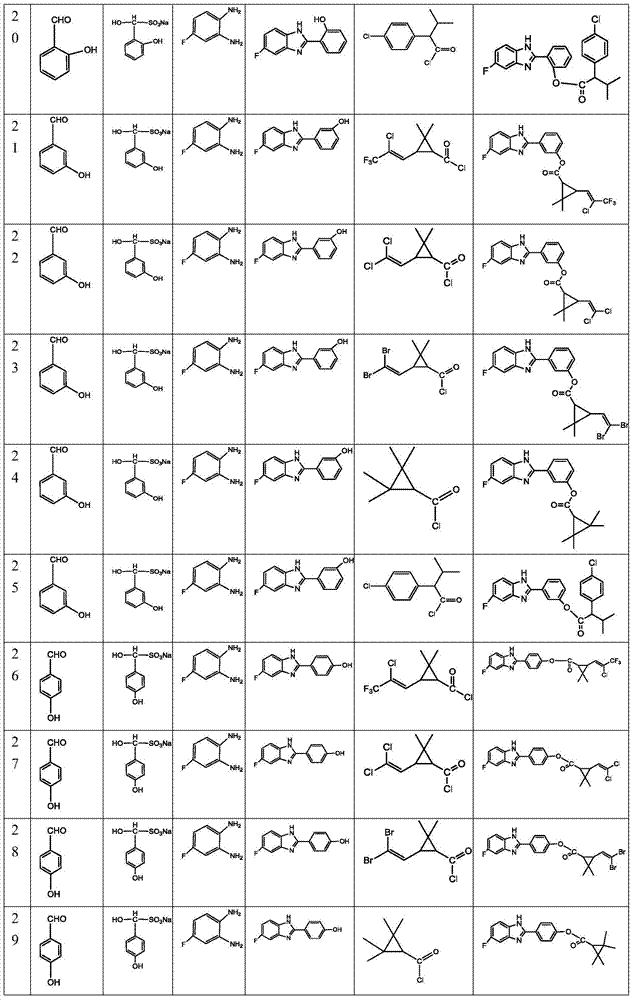
表1Table 1
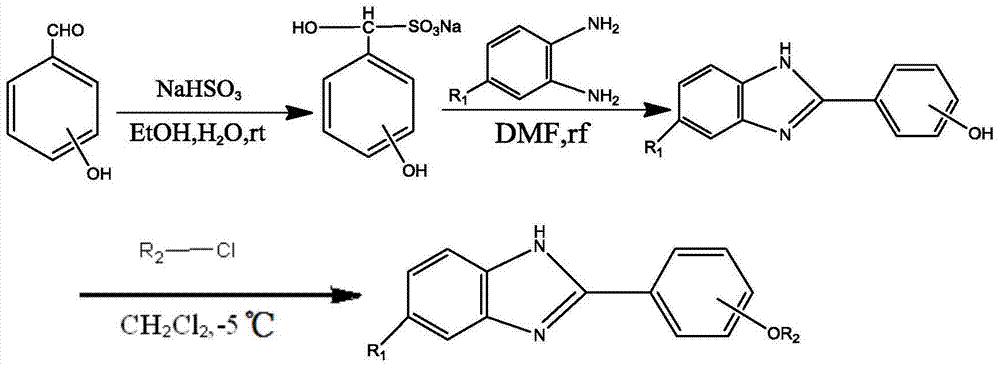
本发明还提供所述苯并咪唑衍生物的制备方法,其合成路线如下:The present invention also provides the preparation method of described benzimidazole derivatives, and its synthetic route is as follows:
其中,R1选自H、F、Cl、Br、CH3、NO3中的一种,-OR2取代的位置为邻位、间位和对位中的一种,且R2选自Wherein, R 1 is selected from one of H, F, Cl, Br, CH 3 , NO 3 , the position substituted by -OR 2 is one of ortho, meta and para, and R 2 is selected from
和中的一种。 and One of.
其中,rt即room temperature室温,rf即reflux回流,ice-salt baths冰盐浴。Among them, rt means room temperature, rf means reflux, and ice-salt baths.
实施例1(化合物14的制备方法)Embodiment 1 (preparation method of compound 14)
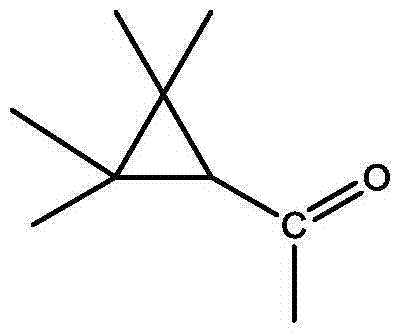
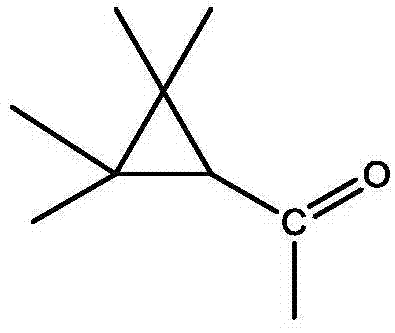
将0.04mol(4.88g)对羟基苯甲醛和0.04mol(4.16g)的亚硫酸氢钠溶于40ml乙醇水溶液中(乙醇和水的体积比为1:1),室温下搅拌0.5h。抽滤反应得到的混合物,得7.7g对羟基苯甲磺酸酸钠,收率为86%。Dissolve 0.04mol (4.88g) of p-hydroxybenzaldehyde and 0.04mol (4.16g) of sodium bisulfite in 40ml of aqueous ethanol (the volume ratio of ethanol and water is 1:1), and stir at room temperature for 0.5h. The mixture obtained by the reaction was filtered by suction to obtain 7.7 g of sodium p-hydroxybenzene methanesulfonate, and the yield was 86%.
将0.01mol(2.26g)对羟基苯甲磺酸钠和0.01mol(1.08g)邻苯二胺溶于30ml的N,N-二甲基甲酰胺中,回流2h;然后将反应得到的混合物倒入冰中,抽滤,重结晶得1.14g2-(2-羟基苯)-1氢-苯并咪唑,收率为54%。Dissolve 0.01mol (2.26g) sodium p-hydroxybenzene methanesulfonate and 0.01mol (1.08g) o-phenylenediamine in 30ml of N,N-dimethylformamide, reflux for 2h; then pour the reaction mixture Pour into ice, filter with suction, and recrystallize to obtain 1.14g of 2-(2-hydroxybenzene)-1hydro-benzimidazole with a yield of 54%.
核磁数据:1HNMR,300MHz,内标TMS,溶剂CDCl3,d:7.93(d,2H),7.57(br,1H),7.55(m,2H),7.21(m,2H),6.93(d,2H),5.35(br,1H)。NMR data: 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 , d: 7.93(d,2H), 7.57(br,1H), 7.55(m,2H), 7.21(m,2H), 6.93(d, 2H), 5.35 (br, 1H).
将4mmol(0.84g)2-(2-羟基苯)-1氢-苯并咪唑和4mmol(0.42g)的三乙胺溶于8ml二氯甲烷中,冰盐浴冷却至-5℃。将4mmol(0.64g)甲氰菊酰氯溶于8ml二氯甲烷配成0.05mol/l的甲氰菊酰氯溶液,将其缓慢加入2-(2-羟基苯)-1氢-苯并咪唑和三乙胺的反应体系中,自然升温至26℃,继续搅拌反应1h。分离反应得到的有机层,用1.5倍体积的水洗涤有机层3次,然后用无水硫酸镁干燥水洗后的有机层,再过滤、浓缩该有机层,最后用硅胶柱层析分离(100-200目硅胶,洗脱剂为石油醚:乙酸乙酯=4:1-1:1),得到产物1.1g,收率82%。所得产物的结构式为:Dissolve 4mmol (0.84g) of 2-(2-hydroxybenzene)-1hydro-benzimidazole and 4mmol (0.42g) of triethylamine in 8ml of dichloromethane, and cool to -5°C in an ice-salt bath. Dissolve 4mmol (0.64g) of cyanoyl chloride in 8ml of dichloromethane to form a 0.05mol/l solution of cyanoyl chloride, and slowly add 2-(2-hydroxybenzene)-1hydro-benzimidazole and tris In the reaction system of ethylamine, the temperature was naturally raised to 26° C., and the stirring reaction was continued for 1 h. Separate the organic layer obtained by the reaction, wash the organic layer 3 times with 1.5 times the volume of water, then dry the washed organic layer with anhydrous magnesium sulfate, filter and concentrate the organic layer, and finally separate it with silica gel column chromatography (100- 200 mesh silica gel, the eluent is petroleum ether: ethyl acetate = 4:1-1:1), the product 1.1g was obtained, and the yield was 82%. The structural formula of the resulting product is:
核磁数据:1HNMR,300MHz,内标TMS,溶剂CDCl3,d:7.92(d,2H),7.59(br,1H),7.53(m,2H),7.21(m,2H),6.93(d,2H),1.07(d,13H)。NMR data: 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 , d: 7.92(d,2H), 7.59(br,1H), 7.53(m,2H), 7.21(m,2H), 6.93(d, 2H), 1.07(d, 13H).
实施例2(化合物15的制备方法)Embodiment 2 (preparation method of compound 15)
将0.04mol(4.88g)对羟基苯甲醛和0.04mol(4.16g)的亚硫酸氢钠溶于50ml乙醇水溶液中(乙醇和水的体积比为1:1.2),室温下搅拌0.6h。抽滤反应得到的混合物,得7.7g对羟基苯甲磺酸酸钠,收率为86%。Dissolve 0.04mol (4.88g) of p-hydroxybenzaldehyde and 0.04mol (4.16g) of sodium bisulfite in 50ml of aqueous ethanol (the volume ratio of ethanol and water is 1:1.2), and stir at room temperature for 0.6h. The mixture obtained by the reaction was filtered by suction to obtain 7.7 g of sodium p-hydroxybenzene methanesulfonate, and the yield was 86%.
将0.01mol(2.26g)对羟基苯甲磺酸钠和0.01mol(1.08g)邻苯二胺溶于35ml的N,N-二甲基甲酰胺中,回流1.5h;然后将反应得到的混合物倒入冰中,抽滤,重结晶得1.14g2-(2-羟基苯)-1氢-苯并咪唑,收率为54%。Dissolve 0.01mol (2.26g) of sodium p-hydroxybenzene methanesulfonate and 0.01mol (1.08g) of o-phenylenediamine in 35ml of N,N-dimethylformamide, and reflux for 1.5h; then the reaction mixture Pour into ice, filter with suction, and recrystallize to obtain 1.14g of 2-(2-hydroxybenzene)-1hydro-benzimidazole with a yield of 54%.
核磁数据:1HNMR,300MHz,内标TMS,溶剂CDCl3,d:7.93(d,2H),7.57(br,1H),7.55(m,2H),7.21(m,2H),6.93(d,2H),5.35(br,1H)。NMR data: 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 , d: 7.93(d,2H), 7.57(br,1H), 7.55(m,2H), 7.21(m,2H), 6.93(d, 2H), 5.35 (br, 1H).
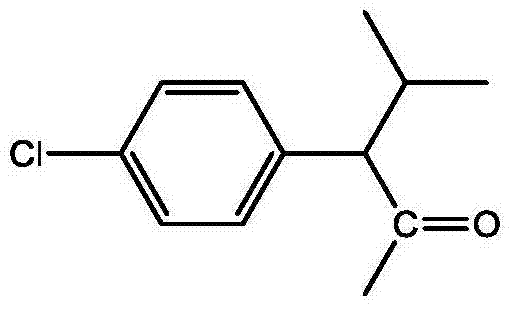
将4mmol(0.84g)2-(2-羟基苯)-1氢-苯并咪唑和4mmol(0.42g)的三乙胺溶于7ml二氯甲烷中,冰盐浴冷却至-7℃。4mmol(0.92g)的3-甲基-2-(4-氯苯基)丁酰氯溶于8ml二氯甲烷配成0.05mol/l的3-甲基-2-(4-氯苯基)丁酰氯溶液,将其缓慢加入2-(2-羟基苯)-1氢-苯并咪唑和三乙胺的反应体系中,自然升温至28℃,继续搅拌反应1.2h。分离反应得到的有机层,用1.8倍体积的水洗涤有机层2次,然后用无水硫酸镁干燥水洗后的有机层,再过滤、浓缩该有机层,最后用硅胶柱层析分离(100-200目硅胶,洗脱剂为石油醚:乙酸乙酯=4:1-1:1),得到产物1.2g,收率74%。所得产物的结构式为:Dissolve 4mmol (0.84g) of 2-(2-hydroxybenzene)-1hydro-benzimidazole and 4mmol (0.42g) of triethylamine in 7ml of dichloromethane, and cool to -7°C in an ice-salt bath. 4mmol (0.92g) of 3-methyl-2-(4-chlorophenyl)butyryl chloride was dissolved in 8ml of dichloromethane to form 0.05mol/l of 3-methyl-2-(4-chlorophenyl)butyl The acid chloride solution was slowly added into the reaction system of 2-(2-hydroxybenzene)-1hydro-benzimidazole and triethylamine, the temperature was naturally raised to 28°C, and the stirring reaction was continued for 1.2h. Separate the organic layer obtained by the reaction, wash the organic layer 2 times with 1.8 times the volume of water, then dry the washed organic layer with anhydrous magnesium sulfate, filter and concentrate the organic layer, and finally separate it with silica gel column chromatography (100- 200 mesh silica gel, the eluent is petroleum ether:ethyl acetate=4:1-1:1), the product 1.2g was obtained, and the yield was 74%. The structural formula of the resulting product is:
核磁数据:1HNMR,300MHz,内标TMS,溶剂CDCl3,d:7.96(d,2H),7.57(br,2H),7.35(m,4H),7.24(m,3H),7.05(d,2H),3.37(s,1H),2.44(m,1H),1.17(t,3H),0.75(m,3H)。NMR data: 1HNMR, 300MHz, internal standard TMS, solvent CDCl3, d: 7.96(d,2H), 7.57(br,2H), 7.35(m,4H), 7.24(m,3H), 7.05(d,2H) , 3.37(s,1H), 2.44(m,1H), 1.17(t,3H), 0.75(m,3H).
实施例3(化合物30的制备方法)Embodiment 3 (preparation method of compound 30)
将0.04mol(4.88g)对羟基苯甲醛和0.04mol(4.16g)的亚硫酸氢钠溶于45ml乙醇水溶液中(乙醇和水的体积比为1:0.9),室温下搅拌0.3h。抽滤反应得到的混合物,得7.7g对羟基苯甲磺酸酸钠,收率为86%。Dissolve 0.04mol (4.88g) of p-hydroxybenzaldehyde and 0.04mol (4.16g) of sodium bisulfite in 45ml of aqueous ethanol (the volume ratio of ethanol and water is 1:0.9), and stir at room temperature for 0.3h. The mixture obtained by the reaction was filtered by suction to obtain 7.7 g of sodium p-hydroxybenzene methanesulfonate, and the yield was 86%.
将0.01mol(2.26g)对羟基苯甲磺酸钠和0.01mol(1.26g)4-氟邻苯二胺溶于30ml的N,N-二甲基甲酰胺中,回流3h;然后将反应得到的混合物倒入冰中,抽滤,重结晶得1.14g2-(2-羟基苯)-1氢-(4-氟)苯并咪唑,收率为50%。Dissolve 0.01mol (2.26g) of sodium p-hydroxybenzenemethanesulfonate and 0.01mol (1.26g) of 4-fluoro-o-phenylenediamine in 30ml of N,N-dimethylformamide and reflux for 3h; then the reaction was obtained The mixture was poured into ice, suction filtered, and recrystallized to obtain 1.14g of 2-(2-hydroxybenzene)-1hydro-(4-fluoro)benzimidazole with a yield of 50%.
核磁数据:1HNMR,300MHz,内标TMS,溶剂CDCl3,d:7.93(d,2H),7.57(br,1H),7.55(m,2H),7.21(m,2H),6.939(d,2H),5.35(br,1H)。NMR data: 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 , d: 7.93(d,2H), 7.57(br,1H), 7.55(m,2H), 7.21(m,2H), 6.939(d, 2H), 5.35 (br, 1H).
将4mmol(0.91g)2-(2-羟基苯)-1氢-(4-氟)苯并咪唑和4mmol(0.42g)的三乙胺溶于10ml二氯甲烷中,冰盐浴冷却至-8℃。将4mmol(0.92g)的3-甲基-2-(4-氯苯基)丁酰氯溶于8ml二氯甲烷配成0.05mol/l的3-甲基-2-(4-氯苯基)丁酰氯溶液,将其缓慢加入2-(2-羟基苯)-1氢-(4-氟)苯并咪唑和三乙胺的反应体系中,自然升温至28℃,继续搅拌反应1.1h。分离反应得到的有机层,用1.3倍体积的水洗涤有机层4次,然后用无水硫酸镁干燥水洗后的有机层,再过滤、浓缩该有机层,最后用硅胶柱层析分离(100-200目硅胶,洗脱剂为石油醚:乙酸乙酯=4:1-1:1),得到产物1.2g,收率74%。所得产物的结构式为:Dissolve 4mmol (0.91g) of 2-(2-hydroxybenzene)-1hydro-(4-fluoro)benzimidazole and 4mmol (0.42g) of triethylamine in 10ml of dichloromethane, and cool in an ice-salt bath to - 8°C. Dissolve 4mmol (0.92g) of 3-methyl-2-(4-chlorophenyl)butyryl chloride in 8ml of dichloromethane to prepare 0.05mol/l of 3-methyl-2-(4-chlorophenyl) Butyryl chloride solution was slowly added into the reaction system of 2-(2-hydroxybenzene)-1hydro-(4-fluoro)benzimidazole and triethylamine, the temperature was naturally raised to 28°C, and the reaction was continued for 1.1h with stirring. Separate the organic layer obtained by the reaction, wash the organic layer 4 times with 1.3 times the volume of water, then dry the washed organic layer with anhydrous magnesium sulfate, filter and concentrate the organic layer, and finally separate it with silica gel column chromatography (100- 200 mesh silica gel, the eluent is petroleum ether:ethyl acetate=4:1-1:1), the product 1.2g was obtained, and the yield was 74%. The structural formula of the resulting product is:
核磁数据:1HNMR,300MHz,内标TMS,溶剂CDCl3,d:7.98(d,2H),7.57(br,2H),7.35(m,4H),7.24(m,3H),7.05(d,2H),3.37(s,1H),2.44(m,1H),1.17(t,3H),0.75(m,3H)。NMR data: 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 , d: 7.98(d,2H), 7.57(br,2H), 7.35(m,4H), 7.24(m,3H), 7.05(d, 2H), 3.37(s, 1H), 2.44(m, 1H), 1.17(t, 3H), 0.75(m, 3H).
实施例4(化合物74的制备方法)Embodiment 4 (preparation method of compound 74)
将0.04mol(4.88g)对羟基苯甲醛和0.04mol(4.16g)的亚硫酸氢钠溶于38ml乙醇水溶液中(乙醇和水的体积比为1:1.1),室温下搅拌0.8h。抽滤反应得到的混合物,得7.7g对羟基苯甲磺酸酸钠,收率为86%。Dissolve 0.04mol (4.88g) of p-hydroxybenzaldehyde and 0.04mol (4.16g) of sodium bisulfite in 38ml of aqueous ethanol (the volume ratio of ethanol and water is 1:1.1), and stir at room temperature for 0.8h. The mixture obtained by the reaction was filtered by suction to obtain 7.7 g of sodium p-hydroxybenzene methanesulfonate, and the yield was 86%.
将0.01mol(2.26g)对羟基苯甲磺酸钠和0.01mol(1.22g)4-甲基邻苯二胺溶于30ml的N,N-二甲基甲酰胺中,回流1h;然后将反应得到的混合物倒入冰中,抽滤,重结晶得1.1g2-(2-羟基苯)-1氢-(4-甲基)苯并咪唑,收率为51%。Dissolve 0.01mol (2.26g) of sodium p-hydroxybenzenemethanesulfonate and 0.01mol (1.22g) of 4-methyl-o-phenylenediamine in 30ml of N,N-dimethylformamide, and reflux for 1h; then the reaction The resulting mixture was poured into ice, filtered with suction, and recrystallized to obtain 1.1 g of 2-(2-hydroxybenzene)-1hydro-(4-methyl)benzimidazole with a yield of 51%.
核磁数据1HNMR,300MHz,内标TMS,溶剂CDCl3,d:7.93(d,2H),7.57(br,1H),7.55(m,2H),7.21(m,2H),6.93(d,2H),5.35(br,1H),2.34(s,3H)。NMR data1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 , d: 7.93( d ,2H), 7.57(br,1H), 7.55(m,2H), 7.21(m,2H), 6.93(d,2H ), 5.35 (br, 1H), 2.34 (s, 3H).
将4mmol(0.89g)2-(2-羟基苯)-1氢-(4-甲基)苯并咪唑和4mmol(0.42g)的三乙胺溶于10ml二氯甲烷中,冰盐浴冷却至-3℃。将4mmol(0.64g)甲氰菊酰氯溶于8ml二氯甲烷配成0.05mol/l的甲氰菊酰氯溶液,将其缓慢加入2-(2-羟基苯)-1氢-(4-甲基)苯并咪唑和三乙胺的反应体系中,自然升温至24℃,继续搅拌反应1.1h。分离反应得到的有机层,用1.5倍体积的水洗涤有机层3次,然后用无水硫酸镁干燥水洗后的有机层,再过滤、浓缩该有机层,最后用硅胶柱层析分离(100-200目硅胶,洗脱剂为石油醚:乙酸乙酯=4:1-1:1),得到产物1.1g,收率71%。所得产物的结构式为:Dissolve 4 mmol (0.89 g) of 2-(2-hydroxybenzene)-1 hydrogen-(4-methyl) benzimidazole and 4 mmol (0.42 g) of triethylamine in 10 ml of dichloromethane, and cool in an ice-salt bath to -3°C. Dissolve 4mmol (0.64g) of fenchromoyl chloride in 8ml of dichloromethane to form a 0.05mol/l methanoyl chloride solution, and slowly add it to 2-(2-hydroxybenzene)-1hydrogen-(4-methyl ) in the reaction system of benzimidazole and triethylamine, the temperature was naturally raised to 24° C., and the stirring reaction was continued for 1.1 h. Separate the organic layer obtained by the reaction, wash the organic layer 3 times with 1.5 times the volume of water, then dry the washed organic layer with anhydrous magnesium sulfate, filter and concentrate the organic layer, and finally separate it with silica gel column chromatography (100- 200 mesh silica gel, the eluent is petroleum ether:ethyl acetate=4:1-1:1), the product 1.1g was obtained, and the yield was 71%. The structural formula of the resulting product is:
核磁数据:1HNMR,300MHz,内标TMS,溶剂CDCl3,d:7.92(d,2H),7.59(br,1H),7.53(m,2H),7.21(m,2H),6.93(d,2H),2.34(s,3H),1.07(d,13H)。NMR data: 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 , d: 7.92(d,2H), 7.59(br,1H), 7.53(m,2H), 7.21(m,2H), 6.93(d, 2H), 2.34(s, 3H), 1.07(d, 13H).
本发明提供的苯并咪唑衍生物可以用于控制多种农业、园林及仓贮有害的昆虫,如鳞翅目、双翅目、同翅目以及一些无脊椎昆虫,如螨类。具体地:Aphis gossypii(棉蚜)、Myzus persicae(桃蚜)、Lipaphis erysimi(菜蚜)Plutella xylostella(小菜蛾),Nilaparvata lugens(褐飞虱),Tetranychuscinnabarinus(朱砂叶螨)Pieris brassicae(大菜粉蝶),Anopheles spp.(按蚊属),Culex spp(库蚊属)。The benzimidazole derivatives provided by the invention can be used to control various agricultural, garden and storage harmful insects, such as Lepidoptera, Diptera, Homoptera and some invertebrate insects, such as mites. Specifically: Aphis gossypii (Cotton aphid), Myzus persicae (Peach aphid), Lipaphis erysimi (Pipella aphid), Plutella xylostella (Plutella xylostella), Nilaparvata lugens (Brown planthopper), Tetranychuscinnabarinus (Cinnabar spider mite), Pieris brassicae (Pipella cabbage butterfly), Anopheles spp. (Anopheles), Culex spp (Culex).
本发明提供的苯并咪唑衍生物可以用于防治线虫,主要防治植物寄生线虫,如根结线虫,南方根结线虫(M.incognita)、爪哇根结线虫(M.javanica)、花生根结线虫(M.arenaria)和北方根结线虫(M.hapla)。The benzimidazole derivatives provided by the present invention can be used for preventing and controlling nematodes, mainly controlling plant parasitic nematodes, such as root-knot nematode, root-knot nematode incognita (M.incognita), root-knot nematode Java (M.javanica), root-knot nematode peanut (M. arenaria) and root-knot nematode (M. hapla).
本发明提供的苯并咪唑衍生物也可以作为杀菌剂,防治多种病原菌。The benzimidazole derivatives provided by the invention can also be used as fungicides to prevent and treat various pathogenic bacteria.
将本发明提供的苯并咪唑衍生物制成多种形式的组合物,如粉剂、可湿性粉剂、粒剂、乳油、悬浮剂等,应用与土壤、植物、种子。为了能够有效地防治有害昆虫、害螨、线虫等,将所述苯并咪唑衍生物制成相应的组合物时,组合物中含有15%-90%质量分数的所述苯并咪唑衍生物。The benzimidazole derivatives provided by the present invention are made into various forms of compositions, such as powders, wettable powders, granules, emulsifiable concentrates, suspension concentrates, etc., and applied to soil, plants, and seeds. In order to effectively control harmful insects, harmful mites, nematodes, etc., when the benzimidazole derivative is made into a corresponding composition, the composition contains 15%-90% of the benzimidazole derivative by mass fraction.
采用本发明提供的苯并咪唑衍生物对几种昆虫和螨类进行生物活性的测定,测定方法为:苯并咪唑衍生物用二甲基亚砜溶解后,采用含0.1%吐温100的水溶液将苯并咪唑衍生物溶液稀释至所需浓度。The benzimidazole derivatives provided by the invention are used to measure the biological activity of several insects and acarids. The assay method is: after the benzimidazole derivatives are dissolved in dimethyl sulfoxide, an aqueous solution containing 0.1% Tween 100 is used Dilute the benzimidazole derivative solution to the desired concentration.
以Myxus persicae(桃蚜),Plutella xylostella(小菜蛾),Nilaparvatalugens(褐飞虱),Tetranychus cinnabarinus(朱砂叶螨)为靶标,采用airbrush喷雾法和浸液法进行杀虫活性鉴定,airbrush喷雾法喷雾压力为10psi(约合0.7kg/cm2),喷液量为0.5ml,处理后48-72h调查靶标的死亡率。Targeting Myxus persicae (Peach aphid), Plutella xylostella (Plutella xylostella), Nilaparvatalugens (BPH), and Tetranychus cinnabarinus (Tetranychus cinnabarinus), the airbrush spray method and immersion method were used to identify the insecticidal activity. The spray pressure of the airbrush spray method was 10psi (approximately 0.7kg/cm 2 ), the spray volume is 0.5ml, and the mortality of the target is investigated 48-72 hours after treatment.
具体操作过程如下:The specific operation process is as follows:
(1)对蚜虫的控制(喷雾法):将20mg苯并咪唑衍生物溶于2ml二甲基亚砜形成10mg/ml的原液,然后用含0.1%吐温100的水溶液将原液稀释至一定浓度。使用手持喷雾器来施用试验溶液,喷洒至含有适当蚜虫(约30-50头蚜虫/叶)的叶片上,直至试验溶液流出叶片两侧。每种化合物每个浓度重复3次。空白对照仅用含0.1%吐温100的水溶液处理。在23℃和相对湿度40%的条件下将叶片保持2-3天,记录每片叶子上蚜虫的总数和死亡数,计算死亡率。(1) Control of aphids (spray method): Dissolve 20mg of benzimidazole derivatives in 2ml of dimethyl sulfoxide to form a 10mg/ml stock solution, and then dilute the stock solution to a certain concentration with an aqueous solution containing 0.1% Tween 100 . Use a hand-held sprayer to apply the test solution onto leaves containing the appropriate aphids (approximately 30-50 aphids/leaf) until the test solution runs off the sides of the leaves. Each compound was replicated 3 times at each concentration. The blank control was only treated with an aqueous solution containing 0.1% Tween 100. Keep the leaves for 2-3 days under the conditions of 23°C and 40% relative humidity, record the total number and dead number of aphids on each leaf, and calculate the mortality rate.
(2)对小菜蛾的控制(浸液法):将20mg苯并咪唑衍生物溶于2ml二甲基亚砜形成10mg/ml的原液,然后用含0.1%吐温100的水溶液将原液稀释至一定浓度。将圆白菜叶片剪成直径7cm左右的圆片,在一定浓度的试验溶液中浸泡5-10s,取出晾干,每片叶片上放置10-20头小菜蛾,保鲜膜封口,每个浓度的药品重复3次。温度24℃,湿度70%,饲养24-48h,记录每个培养皿的总虫数和死亡虫数。(2) Control of Plutella xylostella (immersion method): Dissolve 20mg of benzimidazole derivatives in 2ml of dimethyl sulfoxide to form a 10mg/ml stock solution, and then dilute the stock solution with an aqueous solution containing 0.1% Tween 100 to A certain concentration. Cut the leaves of cabbage into discs with a diameter of about 7cm, soak them in a test solution of a certain concentration for 5-10s, take them out and dry them in the air, place 10-20 diamondback moths on each leaf, seal with plastic wrap, and repeat for each concentration of medicine 3 times. The temperature is 24°C, the humidity is 70%, and the breeding is carried out for 24-48 hours. The total number of insects and the number of dead insects in each petri dish are recorded.
(3)对褐飞虱的控制(喷雾法和系统试验):将秧苗浸入在两部分玻璃圆筒底部的水中,底部使用金属网筛支起秧苗,然后使用透明胶带连接玻璃圆筒的两部分,每个处理用三个圆筒。将20mg苯并咪唑衍生物溶于2ml二甲基亚砜中配成10mg/ml的原液,用含0.1%吐温100的水溶液将原液稀释到不同浓度。将0.5ml的试验溶液和溶剂对照以及空白对照喷雾在玻璃圆筒中。系统试验,将原液加入试验秧苗的水中配成不同浓度。在叶面喷雾和系统试验中,施药3h后将褐飞虱3龄若虫引入圆筒中。处理的试验单元(圆筒)置于温度28℃,湿度70%的生长室中,2天和6天进行观察记录。(3) Control of brown planthopper (spray method and system test): immerse the seedlings in the water at the bottom of the two-part glass cylinder, use a metal mesh screen to support the seedlings at the bottom, and then use scotch tape to connect the two parts of the glass cylinder. Each treatment uses three cylinders. Dissolve 20 mg of benzimidazole derivatives in 2 ml of dimethyl sulfoxide to prepare a 10 mg/ml stock solution, and dilute the stock solution to different concentrations with an aqueous solution containing 0.1% Tween 100. 0.5 ml of the test solution and the solvent control and the blank control were sprayed into the glass cylinder. For the system test, the stock solution was added to the water of the test seedlings to form different concentrations. In the foliar spray and system tests, 3rd instar nymphs of N. lugens were introduced into cylinders 3 h after application. The treated test unit (cylinder) was placed in a growth chamber with a temperature of 28°C and a humidity of 70%, and observations and records were made on day 2 and day 6.
(4)对朱砂叶螨的控制(药膜法):将苯并咪唑衍生物用丙酮稀释成不同浓度的药液,每个浓度3个重复,从稀释好的药液中吸取1.2ml加入1.5ml离心管中,轻轻转动离心管,使药剂在管内壁形成均匀的药膜,倒掉多余的药液,放到通风处待丙酮完全挥发,对照单独用丙酮处理,方法相同。每个药膜管中放置30头健康一致的成螨,室内正常饲养条件下24h,检查死亡率。死亡标准:用毛笔轻触虫体,只有1足动或者完全不动即为死亡。对照死亡率小于10%为有效测定。(4) Control of Tetranychus cinnabarinus (drug film method): dilute the benzimidazole derivatives with acetone into different concentrations of liquid medicine, repeat each concentration 3 times, draw 1.2ml from the diluted liquid medicine and add 1.5 ml centrifuge tube, gently rotate the centrifuge tube to form a uniform drug film on the inner wall of the tube, pour off the excess drug solution, and put it in a ventilated place until the acetone is completely volatilized. The control is treated with acetone alone, and the method is the same. Place 30 healthy and consistent adult mites in each drug-coated tube, and check the mortality rate for 24 hours under normal indoor feeding conditions. Death standard: lightly touch the insect body with a brush, if only one foot moves or does not move at all, it is dead. A control mortality rate of less than 10% was considered valid.
(5)对按蚊幼虫的控制:将苯并咪唑衍生物用含有0.1%吐温的脱氯自来水稀释成不同浓度的药液,将按蚊幼虫在脱氯的自来水中驯化后,分别挑大小一致、活动迅速的3龄幼虫40头,置于不同浓度的药液中,每个浓度重复3次,以未投药的自来水作为空白对照,计算处理2h后幼虫的死亡率。(5) Control of Anopheles larvae: Dilute the benzimidazole derivatives with dechlorinated tap water containing 0.1% Tween into different concentrations of liquid medicine, domesticate the Anopheles larvae in the dechlorinated tap water, and pick the size respectively Forty 3rd-instar larvae with consistent and rapid activities were placed in different concentrations of the drug solution, and each concentration was repeated three times. The tap water without drug application was used as the blank control, and the mortality rate of the larvae after 2 hours of treatment was calculated.
测试结果如下:The test results are as follows:
表2Table 2
需要说明的是,可以将本发明提供的苯并咪唑衍生物以组合物的形式使用,用于杀虫、杀螨,所述组合物包含苯并咪唑衍生物和植物学上可接受的载体。可以以喷雾、局部处理、种子包覆、微胶囊、系统吸收、诱饵等多种形式应用所述组合物,以达到控制昆虫的目的。所述组合物可以是浓缩的固体或液体配方(分散在水中即可使用)或者颗粒配方。所述苯并咪唑衍生物的分散体多数是由苯并咪唑衍生物的浓缩配方制成的水悬浮剂或乳液。这些水可溶性、水悬浮性和可乳化性配方是通常被称作可湿性粉剂或可湿性粒剂。它们是活性化合物(即苯并咪唑衍生物)、惰性载体和表面活性剂的均匀混合物,活性化合物的浓度通常是质量分数的10%-90%。载体通常是蒙脱石粘土、硅藻土、硅酸盐以及凸凹棒粘土,占可湿性粉(粒)剂的0.5%-10%。表面活性剂可以是萘磺酸盐、烷基萘磺酸盐、烷基硫酸酯以及非离子型表面活性剂等。It should be noted that the benzimidazole derivative provided by the present invention can be used in the form of a composition for killing insects and acarids, and the composition comprises a benzimidazole derivative and a phytologically acceptable carrier. The composition can be applied in various forms such as spray, topical treatment, seed coating, microcapsule, systemic absorption, bait, etc., to achieve the purpose of controlling insects. The composition may be a concentrated solid or liquid formulation (dissolved in water for use) or a granular formulation. Most of the dispersions of benzimidazole derivatives are aqueous suspensions or emulsions made from concentrated formulations of benzimidazole derivatives. These water-soluble, water-suspendable and emulsifiable formulations are commonly referred to as wettable powders or wettable granules. They are a uniform mixture of active compounds (ie benzimidazole derivatives), inert carriers and surfactants, and the concentration of active compounds is usually 10%-90% of the mass fraction. The carrier is usually montmorillonite clay, diatomaceous earth, silicate and attapulgite clay, accounting for 0.5%-10% of the wettable powder (granule). The surfactant can be naphthalene sulfonate, alkylnaphthalene sulfonate, alkyl sulfate and non-ionic surfactant.
也可以将所述苯并咪唑衍生物溶于合适的有机溶剂中,以溶液形式再分散使用。杀虫杀螨剂通常以活性成分在液体载体中使用,根据活性成分的浓度来确定使用率,载体通常是水。通常活性成分浓度是10mg/kg-5000mg/kg浓度可良好控制,对于多数化合物10mg/kg-500mg/kg就足够。使用化合物的地点可以是昆虫或螨类聚集的任何地点,如蔬菜作物、水果、观赏植物、建筑物的表面、土壤等。本发明的化合物可以与一种或者多种杀虫剂、杀菌剂和除草剂一起施用,也可以相继施用。The benzimidazole derivatives can also be dissolved in a suitable organic solvent and re-dispersed in the form of a solution for use. Insecticides and acaricides are usually used with active ingredients in a liquid carrier, and the usage rate is determined according to the concentration of the active ingredients. The carrier is usually water. Usually the concentration of the active ingredient is 10mg/kg-5000mg/kg, which can be well controlled, and 10mg/kg-500mg/kg is sufficient for most compounds. The site of application of the compound may be any site where insects or mites gather, such as vegetable crops, fruit, ornamental plants, surfaces of buildings, soil, and the like. The compounds of the invention can be applied together with one or more insecticides, fungicides and herbicides, or sequentially.
由此可见,本发明提供的苯并咪唑衍生物可以有效地控制植物害虫、害螨、线虫和病菌,可以应用在农业上防治病虫害,病原菌。It can be seen that the benzimidazole derivatives provided by the present invention can effectively control plant pests, harmful mites, nematodes and pathogens, and can be applied in agriculture to prevent and control pests and pathogenic bacteria.
所属领域的普通技术人员应当理解:以上所述仅为本发明的具体实施例而已,并不用于限制本发明,凡在本发明的精神和原则之内,所做的任何修改、等同替换、改进等,均应包含在本发明的保护范围之内。Those of ordinary skill in the art should understand that: the above descriptions are only specific embodiments of the present invention, and are not intended to limit the present invention. Any modifications, equivalent replacements, and improvements made within the spirit and principles of the present invention etc., should be included within the protection scope of the present invention.
Claims (9)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201310473947.XA CN103524422B (en) | 2013-10-11 | 2013-10-11 | Benzimidazole derivative, and preparation method and purpose thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201310473947.XA CN103524422B (en) | 2013-10-11 | 2013-10-11 | Benzimidazole derivative, and preparation method and purpose thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN103524422A true CN103524422A (en) | 2014-01-22 |
| CN103524422B CN103524422B (en) | 2015-05-27 |
Family
ID=49926827
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201310473947.XA Expired - Fee Related CN103524422B (en) | 2013-10-11 | 2013-10-11 | Benzimidazole derivative, and preparation method and purpose thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN103524422B (en) |
Cited By (271)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103918704A (en) * | 2014-04-03 | 2014-07-16 | 中国农业科学院植物保护研究所 | Synergetic mixture, synergetic composition and application thereof |
| CN104062413A (en) * | 2014-07-21 | 2014-09-24 | 中国农业科学院蔬菜花卉研究所 | Biological assay method for tetranychids and application of method |
| WO2017072039A1 (en) | 2015-10-26 | 2017-05-04 | Bayer Cropscience Aktiengesellschaft | Condensed bicyclic heterocycle derivatives as pest control agents |
| WO2017093214A1 (en) | 2015-12-03 | 2017-06-08 | Bayer Cropscience Aktiengesellschaft | Mesoionic halogenated 3-(acetyl)-1-[(1,3-thiazol-5-yl)methyl]-1h-imidazo[1,2-a]pyridin-4-ium-2-olate derivatives and related compounds as insecticides |
| WO2017093180A1 (en) | 2015-12-01 | 2017-06-08 | Bayer Cropscience Aktiengesellschaft | Condensed bicyclic heterocycle derivatives as pest control agents |
| CN106918705A (en) * | 2017-01-22 | 2017-07-04 | 贵州勤邦食品安全科学技术有限公司 | Detect test paper and its application of Fenpropathrin |
| WO2017137339A1 (en) | 2016-02-11 | 2017-08-17 | Bayer Cropscience Aktiengesellschaft | Substituted 2-oxyimidazolyl-carboxamides as pest control agents |
| WO2017137338A1 (en) | 2016-02-11 | 2017-08-17 | Bayer Cropscience Aktiengesellschaft | Substituierted 2-(het)aryl-imidazolyl-carboxyamides as pest control agents |
| EP3210468A1 (en) | 2016-02-26 | 2017-08-30 | Bayer CropScience Aktiengesellschaft | Solvent-free formulations of low-melting point agents |
| WO2017144341A1 (en) | 2016-02-23 | 2017-08-31 | Bayer Cropscience Aktiengesellschaft | Condensed bicyclic heterocycle derivatives as pest control agents |
| WO2017146226A1 (en) * | 2016-02-26 | 2017-08-31 | 日本農薬株式会社 | Benzoxazole compound or salt thereof, agricultural/horticultural insecticide containing said compound, and method for using same |
| WO2017157885A1 (en) | 2016-03-16 | 2017-09-21 | Bayer Cropscience Aktiengesellschaft | N-(cyanobenzyl)-6-(cyclopropyl-carbonylamino)-4-(phenyl)-pyridine-2-carboxamide derivatives and related compounds as pesticides and plant protection agents |
| WO2017157735A1 (en) | 2016-03-15 | 2017-09-21 | Bayer Cropscience Aktiengesellschaft | Substituted sulfonyl amides for controlling animal pests |
| WO2017174414A1 (en) | 2016-04-05 | 2017-10-12 | Bayer Cropscience Aktiengesellschaft | Naphthaline-derivatives as pest control agents |
| WO2017178416A1 (en) | 2016-04-15 | 2017-10-19 | Bayer Animal Health Gmbh | Pyrazolopyrimidine derivatives |
| WO2017186536A1 (en) | 2016-04-25 | 2017-11-02 | Bayer Cropscience Aktiengesellschaft | Substituted 2-alkylimidazolyl-carboxamides as pest control agents |
| EP3241830A1 (en) | 2016-05-04 | 2017-11-08 | Bayer CropScience Aktiengesellschaft | Condensed bicyclic heterocyclic derivatives as pesticides |
| EP3245865A1 (en) | 2016-05-17 | 2017-11-22 | Bayer CropScience Aktiengesellschaft | Method for increasing yield in brassicaceae |
| WO2017198454A1 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in cotton |
| WO2017198455A2 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in beta spp. plants |
| WO2017198453A1 (en) | 2016-05-16 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in potato, tomato or alfalfa |
| WO2017198449A1 (en) | 2016-05-15 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in brassicaceae |
| WO2017198452A1 (en) | 2016-05-16 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in soybean |
| WO2017198451A1 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in small grain cereals such as wheat and rice |
| WO2017198450A1 (en) | 2016-05-15 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in maize |
| WO2018015289A1 (en) | 2016-07-19 | 2018-01-25 | Bayer Cropscience Aktiengesellschaft | Condensed bicyclic heterocycle derivatives as pest control agents |
| WO2018019937A1 (en) | 2016-07-29 | 2018-02-01 | Bayer Cropscience Aktiengesellschaft | Formulation comprising a beneficial p. bilaii strain and talc for use in seed treatment |
| WO2018029102A1 (en) | 2016-08-10 | 2018-02-15 | Bayer Cropscience Aktiengesellschaft | Substituted 2-heterocyclyl imidazolyl-carboxamides as pest control agents |
| EP3284739A1 (en) | 2017-07-19 | 2018-02-21 | Bayer CropScience Aktiengesellschaft | Substituted (het) aryl compounds as pesticides |
| WO2018033455A1 (en) | 2016-08-15 | 2018-02-22 | Bayer Cropscience Aktiengesellschaft | Condensed bicyclic heterocycle derivatives as pest control agents |
| WO2018050825A1 (en) | 2016-09-19 | 2018-03-22 | Bayer Cropscience Aktiengesellschaft | Pyrazolo [1,5-a]pyridine derivatives and their use as pesticides |
| EP3305786A2 (en) | 2018-01-22 | 2018-04-11 | Bayer CropScience Aktiengesellschaft | Condensed bicyclic heterocycle derivatives as pesticides |
| WO2018065288A1 (en) | 2016-10-07 | 2018-04-12 | Bayer Cropscience Aktiengesellschaft | 2-[2-phenyl-1-(sulfonyl-methyl)-vinyl]-imidazo-[4,5-b] pyridine derivatives and related compounds as pesticides in plant protection |
| WO2018065292A1 (en) | 2016-10-06 | 2018-04-12 | Bayer Cropscience Aktiengesellschaft | 2-(het)aryl-substituted condensed bicyclic heterocycle derivatives as pest control agents |
| WO2018083288A1 (en) | 2016-11-07 | 2018-05-11 | Bayer Aktiengesellschaft | Substituted sulfonyl amides for controlling animal pests |
| WO2018087036A1 (en) | 2016-11-11 | 2018-05-17 | Bayer Animal Health Gmbh | New anthelmintic quinoline-3-carboxamide derivatives |
| WO2018095953A1 (en) | 2016-11-23 | 2018-05-31 | Bayer Cropscience Aktiengesellschaft | 2-[3-(alkylsulfonyl)-2h-indazol-2-yl]-3h-imidazo[4,5-b]pyridine derivatives and similar compounds as pesticides |
| WO2018104500A1 (en) | 2016-12-09 | 2018-06-14 | Bayer Cropscience Aktiengesellschaft | Plant health effect of purpureocillium lilacinum |
| WO2018108791A1 (en) | 2016-12-16 | 2018-06-21 | Bayer Cropscience Aktiengesellschaft | Thiadiazole derivatives as pesticides |
| WO2018108730A1 (en) | 2016-12-16 | 2018-06-21 | Bayer Aktiengesellschaft | Mesoionic imidazopyridines for use as insecticides |
| WO2018130443A1 (en) | 2017-01-10 | 2018-07-19 | Bayer Aktiengesellschaft | Heterocyclene derivatives as pest control agents |
| WO2018130437A1 (en) | 2017-01-10 | 2018-07-19 | Bayer Aktiengesellschaft | Heterocyclene derivatives as pest control agents |
| WO2018138050A1 (en) | 2017-01-26 | 2018-08-02 | Bayer Aktiengesellschaft | Condensed bicyclic heterocyclene derivatives as pest control agents |
| WO2018141954A1 (en) | 2017-02-06 | 2018-08-09 | Bayer Aktiengesellschaft | Aryl or heteroaryl-substituted imidazo pyridine derivatives and their use as pesticides |
| EP3369320A1 (en) | 2017-03-02 | 2018-09-05 | Bayer CropScience Aktiengesellschaft | Agent for controlling bugs |
| WO2018189077A1 (en) | 2017-04-12 | 2018-10-18 | Bayer Aktiengesellschaft | Mesoionic imidazopyridines for use as insecticides |
| WO2018192872A1 (en) | 2017-04-21 | 2018-10-25 | Bayer Aktiengesellschaft | Mesoionic imidazopyridines as insecticides |
| WO2018197401A1 (en) | 2017-04-27 | 2018-11-01 | Bayer Animal Health Gmbh | New bicyclic pyrazole derivatives |
| WO2018197692A1 (en) | 2017-04-27 | 2018-11-01 | Bayer Aktiengesellschaft | Heteroarylphenylaminoquinolines and analogues |
| WO2018197257A1 (en) | 2017-04-24 | 2018-11-01 | Bayer Aktiengesellschaft | Condensed bicyclic heterocyclic-compound derivatives as pest control agents |
| WO2018202494A1 (en) | 2017-05-02 | 2018-11-08 | Bayer Aktiengesellschaft | 2-(het)aryl-substituted condensed bicyclic heterocyclic derivatives as pest control agents |
| WO2018202712A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylmethylphenoxyquinolines and analogues |
| WO2018202715A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylbenzylbenzimidazoles and analogues |
| WO2018202706A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylheteroaryloxyquinolines and analogues |
| WO2018202501A1 (en) | 2017-05-02 | 2018-11-08 | Bayer Aktiengesellschaft | 2-(het)aryl-substituted condensed bicyclic heterocyclic derivatives as pest control agents |
| WO2018202525A1 (en) | 2017-05-04 | 2018-11-08 | Bayer Cropscience Aktiengesellschaft | Phenoxyethanamine derivatives for controlling pests |
| WO2018202524A1 (en) | 2017-05-04 | 2018-11-08 | Bayer Cropscience Aktiengesellschaft | 2-{[2-(phenyloxymethyl)pyridin-5-yl]oxy}-ethanamin-derivatives and related compounds as pest-control agents e.g. for the protection of plants |
| WO2019002132A1 (en) | 2017-06-30 | 2019-01-03 | Bayer Animal Health Gmbh | New azaquinoline derivatives |
| WO2019007887A1 (en) | 2017-07-06 | 2019-01-10 | Bayer Aktiengesellschaft | INSECTICIDES AND FUNGICIDES ACTIVE COMPOUNDS |
| WO2019025341A1 (en) | 2017-08-04 | 2019-02-07 | Bayer Animal Health Gmbh | Quinoline derivatives for treating infections with helminths |
| CN109336826A (en) * | 2018-11-27 | 2019-02-15 | 盐城锦明药业有限公司 | A kind of chemical synthesis method of 2-hydroxybenzimidazole |
| WO2019035881A1 (en) | 2017-08-17 | 2019-02-21 | Bayer Cropscience Lp | Liquid fertilizer-dispersible compositions and methods thereof |
| WO2019038195A1 (en) | 2017-08-22 | 2019-02-28 | Bayer Aktiengesellschaft | HETEROCYCLEN DERIVATIVES AS PESTICIDES |
| WO2019059412A1 (en) | 2017-09-20 | 2019-03-28 | Mitsui Chemicals Agro, Inc. | Prolonged ectoparasite-controlling agent for animal |
| WO2019068572A1 (en) | 2017-10-04 | 2019-04-11 | Bayer Aktiengesellschaft | HETEROCYCLEN DERIVATIVES AS PESTICIDES |
| EP3473103A1 (en) | 2017-10-17 | 2019-04-24 | Bayer AG | Aqueous suspension concentrates based on 2- [(2,4-dichlorophenyl) -methyl] -4,4 '-dimethyl-3-isoxazolidinone |
| EP3473100A1 (en) | 2017-10-18 | 2019-04-24 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2019076749A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2019076752A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2019076754A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2019076750A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2019076751A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2019092086A1 (en) | 2017-11-13 | 2019-05-16 | Bayer Aktiengesellschaft | Tetrazolylpropyl derivatives and their use as fungicides |
| WO2019105871A1 (en) | 2017-11-29 | 2019-06-06 | Bayer Aktiengesellschaft | Nitrogenous heterocycles as a pesticide |
| WO2019105875A1 (en) | 2017-11-28 | 2019-06-06 | Bayer Aktiengesellschaft | Heterocyclic compounds as pesticides |
| WO2019122319A1 (en) | 2017-12-21 | 2019-06-27 | Bayer Aktiengesellschaft | Trisubstitutedsilylmethylheteroaryloxyquinolines and analogues |
| WO2019155066A1 (en) | 2018-02-12 | 2019-08-15 | Bayer Aktiengesellschaft | Fungicidal oxadiazoles |
| WO2019162228A1 (en) | 2018-02-21 | 2019-08-29 | Bayer Aktiengesellschaft | 1-(5-substituted imidazol-1-yl)but-3-en derivatives and their use as fungicides |
| WO2019162174A1 (en) | 2018-02-21 | 2019-08-29 | Bayer Aktiengesellschaft | Condensed bicyclic heterocyclic derivatives as pest control agents |
| WO2019170626A1 (en) | 2018-03-08 | 2019-09-12 | Bayer Aktiengesellschaft | Use of heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides in plant protection |
| WO2019175046A1 (en) | 2018-03-12 | 2019-09-19 | Bayer Aktiengesellschaft | Condensed bicyclic heterocyclic derivatives as pest control agents |
| EP3545764A1 (en) | 2019-02-12 | 2019-10-02 | Bayer AG | Crystal form of 2-({2-fluoro-4-methyl-5-[(r)-(2,2,2-trifluoroethyl)sulfinyl]phenyl}imino)-3-(2,2,2- trifluoroethyl)-1,3-thiazolidin-4-one |
| WO2019197623A1 (en) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Active ingredient combinations with insecticidal, fungicidal and acaricidal properties |
| WO2019197615A1 (en) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Active ingredient combinations with fungicides, insecticides and acaricidal properties |
| WO2019197371A1 (en) | 2018-04-10 | 2019-10-17 | Bayer Aktiengesellschaft | Oxadiazoline derivatives |
| WO2019197468A1 (en) | 2018-04-12 | 2019-10-17 | Bayer Aktiengesellschaft | N-(cyclopropylmethyl)-5-(methylsulfonyl)-n-{1-[1-(pyrimidin-2-yl)-1h-1,2,4-triazol-5-yl]ethyl}benzamide derivatives and the corresponding pyridine-carboxamide derivatives as pesticides |
| WO2019201921A1 (en) | 2018-04-20 | 2019-10-24 | Bayer Aktiengesellschaft | Heterocyclene derivatives as pest control agents |
| WO2019202077A1 (en) | 2018-04-20 | 2019-10-24 | Bayer Aktiengesellschaft | Heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| WO2019201835A1 (en) | 2018-04-17 | 2019-10-24 | Bayer Aktiengesellschaft | Heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| WO2019206799A1 (en) | 2018-04-25 | 2019-10-31 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| EP3564225A1 (en) | 2019-03-21 | 2019-11-06 | Bayer Aktiengesellschaft | Crystalline form of spiromesifen |
| WO2019215182A1 (en) | 2018-05-09 | 2019-11-14 | Bayer Animal Health Gmbh | New quinoline derivatives |
| WO2019224143A1 (en) | 2018-05-24 | 2019-11-28 | Bayer Aktiengesellschaft | Active ingredient combinations with insecticidal, nematicidal and acaricidal properties |
| EP3586630A1 (en) | 2018-06-28 | 2020-01-01 | Bayer AG | Active compound combinations having insecticidal/acaricidal properties |
| WO2020002189A1 (en) | 2018-06-27 | 2020-01-02 | Bayer Aktiengesellschaft | Active substance combinations |
| WO2020005678A1 (en) | 2018-06-25 | 2020-01-02 | Bayer Cropscience Lp | Seed treatment method |
| WO2020007902A1 (en) | 2018-07-05 | 2020-01-09 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| WO2020020813A1 (en) | 2018-07-25 | 2020-01-30 | Bayer Aktiengesellschaft | Fungicidal active compound combinations |
| WO2020020816A1 (en) | 2018-07-26 | 2020-01-30 | Bayer Aktiengesellschaft | Novel triazole derivatives |
| WO2020021082A1 (en) | 2018-07-27 | 2020-01-30 | Bayer Aktiengesellschaft | Controlled release formulations for agrochemicals |
| WO2020025650A1 (en) | 2018-07-31 | 2020-02-06 | Bayer Aktiengesellschaft | Controlled release formulations with lignin for agrochemicals |
| EP3608311A1 (en) | 2019-06-28 | 2020-02-12 | Bayer AG | Crystalline form a of n-[4-chloro-3-[(1-cyanocyclopropyl)carbamoyl]phenyl]-2-methyl-4-methylsulfonyl-5-(1,1,2,2,2-pentafluoroethyl)pyrazole-3-carboxamide |
| WO2020043650A1 (en) | 2018-08-29 | 2020-03-05 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3620052A1 (en) | 2018-12-12 | 2020-03-11 | Bayer Aktiengesellschaft | Use of phenoxypyridinyl-substituted (1h-1,2,4-triazol-1-yl)alcohols for controlling fungicidal diseases in maize |
| WO2020053282A1 (en) | 2018-09-13 | 2020-03-19 | Bayer Aktiengesellschaft | Heterocyclene derivatives as pest control agents |
| WO2020057939A1 (en) | 2018-09-17 | 2020-03-26 | Bayer Aktiengesellschaft | Use of the fungicide isoflucypram for controlling claviceps purpurea and reducing sclerotia in cereals |
| WO2020070050A1 (en) | 2018-10-01 | 2020-04-09 | Bayer Aktiengesellschaft | Fungicidal 5-substituted imidazol-1-yl carbinol derivatives |
| EP3636644A1 (en) | 2018-10-11 | 2020-04-15 | Bayer Aktiengesellschaft | Mesoionic imidazopyridines as insecticides |
| WO2020079232A1 (en) | 2018-10-20 | 2020-04-23 | Bayer Aktiengesellschaft | Oxetanylphenoxyquinolines and analogues |
| WO2020078839A1 (en) | 2018-10-16 | 2020-04-23 | Bayer Aktiengesellschaft | Active substance combinations |
| WO2020079167A1 (en) | 2018-10-18 | 2020-04-23 | Bayer Aktiengesellschaft | Heteroarylaminoquinolines and analogues |
| WO2020079173A1 (en) | 2018-10-18 | 2020-04-23 | Bayer Aktiengesellschaft | Pyridylphenylaminoquinolines and analogues |
| EP3643711A1 (en) | 2018-10-24 | 2020-04-29 | Bayer Animal Health GmbH | New anthelmintic compounds |
| WO2020109391A1 (en) | 2018-11-28 | 2020-06-04 | Bayer Aktiengesellschaft | Pyridazine (thio)amides as fungicidal compounds |
| WO2020114934A1 (en) | 2018-12-07 | 2020-06-11 | Bayer Aktiengesellschaft | Herbicide compositions |
| WO2020114932A1 (en) | 2018-12-07 | 2020-06-11 | Bayer Aktiengesellschaft | Herbicidal compositions |
| EP3669652A1 (en) | 2018-12-21 | 2020-06-24 | Bayer AG | Active compound combination |
| WO2020127974A1 (en) | 2018-12-21 | 2020-06-25 | Bayer Aktiengesellschaft | 1,3,4-oxadiazoles and their derivatives as new antifungal agents |
| WO2020126980A1 (en) | 2018-12-18 | 2020-06-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2020127780A1 (en) | 2018-12-20 | 2020-06-25 | Bayer Aktiengesellschaft | Heterocyclyl pyridazine as fungicidal compounds |
| EP3679791A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679793A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679789A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679792A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679790A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3701796A1 (en) | 2019-08-08 | 2020-09-02 | Bayer AG | Active compound combinations |
| WO2020173860A1 (en) | 2019-02-26 | 2020-09-03 | Bayer Aktiengesellschaft | Fused bicyclic heterocycle derivatives as pesticides |
| WO2020173861A1 (en) | 2019-02-26 | 2020-09-03 | Bayer Aktiengesellschaft | Condensed bicyclic heterocyclic derivatives as pest control agents |
| WO2020178307A1 (en) | 2019-03-05 | 2020-09-10 | Bayer Aktiengesellschaft | Active compound combination |
| WO2020178067A1 (en) | 2019-03-01 | 2020-09-10 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3708565A1 (en) | 2020-03-04 | 2020-09-16 | Bayer AG | Pyrimidinyloxyphenylamidines and the use thereof as fungicides |
| WO2020182929A1 (en) | 2019-03-13 | 2020-09-17 | Bayer Aktiengesellschaft | Substituted ureas and derivatives as new antifungal agents |
| WO2020187656A1 (en) | 2019-03-15 | 2020-09-24 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3725788A1 (en) | 2019-04-15 | 2020-10-21 | Bayer AG | Novel heteroaryl-substituted aminoalkyl azole compounds as pesticides |
| CN111875545A (en) * | 2020-07-17 | 2020-11-03 | 安阳师范学院 | A kind of directional synthesis method and application of 6-position substituted benzimidazole derivatives |
| WO2020225428A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading ulv formulations for insecticides |
| WO2020225242A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | Active compound combination |
| WO2020231751A1 (en) | 2019-05-10 | 2020-11-19 | Bayer Cropscience Lp | Active compound combinations |
| WO2020229398A1 (en) | 2019-05-14 | 2020-11-19 | Bayer Aktiengesellschaft | (1-alkenyl)-substituted pyrazoles and triazoles as pest control agents |
| EP3750888A1 (en) | 2019-06-12 | 2020-12-16 | Bayer Aktiengesellschaft | Crystalline form a of 1,4-dimethyl-2-[2-(pyridin-3-yl)-2h-indazol-5-yl]-1,2,4-triazolidine-3,5-dione |
| WO2020254488A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and use thereof as fungicides |
| WO2020254490A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Phenoxyphenyl hydroxyisoxazolines and analogues as new antifungal agents |
| WO2020254494A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Fungicidal oxadiazoles |
| WO2020254492A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254486A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254487A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254489A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Benzylphenyl hydroxyisoxazolines and analogues as new antifungal agents |
| WO2020254493A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Thienylhydroxyisoxazolines and derivatives thereof |
| WO2020263812A1 (en) | 2019-06-24 | 2020-12-30 | Auburn University | A bacillus strain and methods of its use for plant growth promotion |
| WO2021001331A1 (en) | 2019-07-03 | 2021-01-07 | Bayer Aktiengesellschaft | Substituted thiophene carboxamides and derivatives thereof as microbicides |
| WO2021001273A1 (en) | 2019-07-04 | 2021-01-07 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2021013721A1 (en) | 2019-07-22 | 2021-01-28 | Bayer Aktiengesellschaft | 5-amino substituted pyrazoles and triazoles as pest control agents |
| WO2021013720A1 (en) | 2019-07-23 | 2021-01-28 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021013719A1 (en) | 2019-07-23 | 2021-01-28 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| EP3771714A1 (en) | 2019-07-30 | 2021-02-03 | Bayer AG | Nitrogen-containing heterocycles as pesticides |
| WO2021018839A1 (en) | 2019-07-30 | 2021-02-04 | Bayer Animal Health Gmbh | Isoquinoline derivatives and their use for the treatment of parasitic infections |
| WO2021048188A1 (en) | 2019-09-11 | 2021-03-18 | Bayer Aktiengesellschaft | Highly effective formulations on the basis of 2-[(2,4-dichlorphenyl)-methyl]-4,4'-dimethyl-3-isoxazolidinones and preemergence herbicides |
| WO2021058659A1 (en) | 2019-09-26 | 2021-04-01 | Bayer Aktiengesellschaft | Rnai-mediated pest control |
| WO2021069575A1 (en) | 2019-10-11 | 2021-04-15 | Bayer Animal Health Gmbh | Heteroaryl-substituted pyrazine derivatives as pesticides |
| WO2021069569A1 (en) | 2019-10-09 | 2021-04-15 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021069567A1 (en) | 2019-10-09 | 2021-04-15 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021089673A1 (en) | 2019-11-07 | 2021-05-14 | Bayer Aktiengesellschaft | Substituted sulfonyl amides for controlling animal pests |
| WO2021097162A1 (en) | 2019-11-13 | 2021-05-20 | Bayer Cropscience Lp | Beneficial combinations with paenibacillus |
| WO2021099303A1 (en) | 2019-11-18 | 2021-05-27 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021105091A1 (en) | 2019-11-25 | 2021-06-03 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021123051A1 (en) | 2019-12-20 | 2021-06-24 | Bayer Aktiengesellschaft | Substituted thiophene carboxamides, thiophene carboxylic acids and derivatives thereof |
| WO2021122986A1 (en) | 2019-12-20 | 2021-06-24 | Bayer Aktiengesellschaft | Thienyloxazolones and analogues |
| EP3845304A1 (en) | 2019-12-30 | 2021-07-07 | Bayer AG | Capsule suspension concentrates based on polyisocyanates and biodegradable amine based cross-linker |
| EP3868207A1 (en) | 2020-02-24 | 2021-08-25 | Bayer Aktiengesellschaft | Encapsulated pyrethroids with improved activity in soil and leaf applications |
| WO2021165195A1 (en) | 2020-02-18 | 2021-08-26 | Bayer Aktiengesellschaft | Heteroaryl-triazole compounds as pesticides |
| WO2021204930A1 (en) | 2020-04-09 | 2021-10-14 | Bayer Animal Health Gmbh | Substituted condensed azines as anthelmintic compounds |
| WO2021209363A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209368A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209366A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209490A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Cyclaminephenylaminoquinolines as fungicides |
| WO2021209365A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209364A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021213978A1 (en) | 2020-04-21 | 2021-10-28 | Bayer Aktiengesellschaft | 2-(het)aryl-substituted condensed heterocyclic derivatives as pest control agents |
| WO2021224323A1 (en) | 2020-05-06 | 2021-11-11 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021224220A1 (en) | 2020-05-06 | 2021-11-11 | Bayer Aktiengesellschaft | Pyridine (thio)amides as fungicidal compounds |
| WO2021228734A1 (en) | 2020-05-12 | 2021-11-18 | Bayer Aktiengesellschaft | Triazine and pyrimidine (thio)amides as fungicidal compounds |
| CN113666875A (en) * | 2021-09-16 | 2021-11-19 | 湖北瑞凯兴科技股份有限公司 | Method for preparing 2-phenylbenzimidazole |
| WO2021233861A1 (en) | 2020-05-19 | 2021-11-25 | Bayer Aktiengesellschaft | Azabicyclic(thio)amides as fungicidal compounds |
| EP3915371A1 (en) | 2020-11-04 | 2021-12-01 | Bayer AG | Active compound combinations and fungicide compositions comprising those |
| EP3915971A1 (en) | 2020-12-16 | 2021-12-01 | Bayer Aktiengesellschaft | Phenyl-s(o)n-phenylamidines and the use thereof as fungicides |
| WO2021245087A1 (en) | 2020-06-04 | 2021-12-09 | Bayer Aktiengesellschaft | Heterocyclyl pyrimidines and triazines as novel fungicides |
| WO2021249995A1 (en) | 2020-06-10 | 2021-12-16 | Bayer Aktiengesellschaft | Azabicyclyl-substituted heterocycles as fungicides |
| WO2021255091A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazoles and their derivatives as fungicides |
| WO2021255169A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines as fungicides |
| WO2021255071A1 (en) | 2020-06-18 | 2021-12-23 | Bayer Aktiengesellschaft | 3-(pyridazin-4-yl)-5,6-dihydro-4h-1,2,4-oxadiazine derivatives as fungicides for crop protection |
| WO2021255170A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines as fungicides |
| WO2021255089A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines and 1,3,4-oxadiazole pyridines as fungicides |
| EP3929189A1 (en) | 2020-06-25 | 2021-12-29 | Bayer Animal Health GmbH | Novel heteroaryl-substituted pyrazine derivatives as pesticides |
| WO2021260017A1 (en) | 2020-06-26 | 2021-12-30 | Bayer Aktiengesellschaft | Aqueous capsule suspension concentrates comprising biodegradable ester groups |
| WO2022002818A1 (en) | 2020-07-02 | 2022-01-06 | Bayer Aktiengesellschaft | Heterocyclene derivatives as pest control agents |
| WO2022033991A1 (en) | 2020-08-13 | 2022-02-17 | Bayer Aktiengesellschaft | 5-amino substituted triazoles as pest control agents |
| WO2022053453A1 (en) | 2020-09-09 | 2022-03-17 | Bayer Aktiengesellschaft | Azole carboxamide as pest control agents |
| WO2022058327A1 (en) | 2020-09-15 | 2022-03-24 | Bayer Aktiengesellschaft | Substituted ureas and derivatives as new antifungal agents |
| EP3974414A1 (en) | 2020-09-25 | 2022-03-30 | Bayer AG | 5-amino substituted pyrazoles and triazoles as pesticides |
| EP3994986A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift and spreading properties |
| EP3994985A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift properties |
| EP3994994A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| EP3994993A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading and ulv tank mix adjuvant formulation |
| EP3994992A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Low drift, rainfastness, high uptake and ulv tank mix adjuvant formulation |
| EP3994989A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, rainfastness and uptake properties |
| EP3994988A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, spreading and rainfastness properties |
| EP3994990A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, spreading and uptake properties |
| EP3994987A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift and uptake properties |
| EP3994995A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| EP3994991A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, spreading, uptake and rainfastness properties |
| WO2022129196A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | Heterobicycle substituted 1,2,4-oxadiazoles as fungicides |
| WO2022129190A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | (hetero)aryl substituted 1,2,4-oxadiazoles as fungicides |
| WO2022129188A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | 1,2,4-oxadiazol-3-yl pyrimidines as fungicides |
| WO2022152728A1 (en) | 2021-01-15 | 2022-07-21 | Bayer Aktiengesellschaft | Herbicidal compositions |
| EP4036083A1 (en) | 2021-02-02 | 2022-08-03 | Bayer Aktiengesellschaft | 5-oxy substituted heterocycles as pesticides |
| WO2022207494A1 (en) | 2021-03-30 | 2022-10-06 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2022207496A1 (en) | 2021-03-30 | 2022-10-06 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2022233777A1 (en) | 2021-05-06 | 2022-11-10 | Bayer Aktiengesellschaft | Alkylamide substituted, annulated imidazoles and use thereof as insecticides |
| WO2022238391A1 (en) | 2021-05-12 | 2022-11-17 | Bayer Aktiengesellschaft | 2-(het)aryl-substituted condensed heterocycle derivatives as pest control agents |
| WO2022238194A1 (en) | 2021-05-10 | 2022-11-17 | Bayer Aktiengesellschaft | Herbicide/safener combination based on safeners from the class of substituted [(1,5-diphenyl-1h-1,2,4-triazol-3-yl)oxy]acetic acids and their salts |
| WO2023017120A1 (en) | 2021-08-13 | 2023-02-16 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2023025682A1 (en) | 2021-08-25 | 2023-03-02 | Bayer Aktiengesellschaft | Novel pyrazinyl-triazole compounds as pesticides |
| EP4144739A1 (en) | 2021-09-02 | 2023-03-08 | Bayer Aktiengesellschaft | Anellated pyrazoles as parasiticides |
| EP4148052A1 (en) | 2021-09-09 | 2023-03-15 | Bayer Animal Health GmbH | New quinoline derivatives |
| WO2023078915A1 (en) | 2021-11-03 | 2023-05-11 | Bayer Aktiengesellschaft | Bis(hetero)aryl thioether (thio)amides as fungicidal compounds |
| WO2023092050A1 (en) | 2021-11-20 | 2023-05-25 | Bayer Cropscience Lp | Beneficial combinations with recombinant bacillus cells expressing a serine protease |
| WO2023099445A1 (en) | 2021-11-30 | 2023-06-08 | Bayer Aktiengesellschaft | Bis(hetero)aryl thioether oxadiazines as fungicidal compounds |
| WO2023110656A1 (en) | 2021-12-15 | 2023-06-22 | Bayer Aktiengesellschaft | Spectroscopic solution for non-destructive quantification of one or more chemical substances in a matrix comprising coating and bulk material in a sample, such as coated seeds, using multivariate data analysis |
| EP4265110A1 (en) | 2022-04-20 | 2023-10-25 | Bayer AG | Water dispersible granules with low melting active ingredients prepared by extrusion |
| WO2023205602A1 (en) | 2022-04-18 | 2023-10-26 | Basf Corporation | High-load agricultural formulations and methods of making same |
| WO2023213626A1 (en) | 2022-05-03 | 2023-11-09 | Bayer Aktiengesellschaft | Use of (5s)-3-[3-(3-chloro-2-fluorophenoxy)-6-methylpyridazin-4-yl]-5-(2-chloro-4-methylbenzyl)-5,6-dihydro-4h-1,2,4-oxadiazine for controlling unwanted microorganisms |
| WO2023213670A1 (en) | 2022-05-03 | 2023-11-09 | Bayer Aktiengesellschaft | Crystalline forms of (5s)-3-[3-(3-chloro-2-fluorophenoxy)-6-methylpyridazin-4-yl]-5-(2-chloro-4-methylbenzyl)-5,6-dihydro-4h-1,2,4-oxadiazine |
| WO2023217619A1 (en) | 2022-05-07 | 2023-11-16 | Bayer Aktiengesellschaft | Low drift aqueous liquid formulations for low, medium, and high spray volume application |
| WO2023237444A1 (en) | 2022-06-06 | 2023-12-14 | Bayer Aktiengesellschaft | Agrochemical formulations comprising crystalline form a of 4-[(6-chloro-3-pyridylmethyl)(2,2-difluoroethyl)amino]furan-2(5h)-one |
| EP4295683A1 (en) | 2022-06-21 | 2023-12-27 | Bayer Aktiengesellschaft | Agrochemical formulations comprising crystalline form a of 4-[(6-chloro-3-pyridylmethyl)(2,2-difluoroethyl)amino]furan-2(5h)-one |
| EP4295688A1 (en) | 2022-09-28 | 2023-12-27 | Bayer Aktiengesellschaft | Active compound combination |
| WO2024013016A1 (en) | 2022-07-11 | 2024-01-18 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024013015A1 (en) | 2022-07-11 | 2024-01-18 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024068517A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068518A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-heteroaryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068519A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068520A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068473A1 (en) | 2022-09-27 | 2024-04-04 | Bayer Aktiengesellschaft | Herbicide/safener combination based on safeners from the class of substituted [(1,5-diphenyl-1h-1,2,4-triazol-3-yl)oxy]acetic acids and their salts |
| EP4353082A1 (en) | 2022-10-14 | 2024-04-17 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024104643A1 (en) | 2022-11-17 | 2024-05-23 | Bayer Aktiengesellschaft | Use of isotianil for controlling plasmodiophora brassica |
| WO2024170472A1 (en) | 2023-02-16 | 2024-08-22 | Bayer Aktiengesellschaft | Herbicidal mixtures |
| WO2024213752A1 (en) | 2023-04-14 | 2024-10-17 | Elanco Animal Health Gmbh | Long-term prevention and/or treatment of a disease by slo-1 inhibitors |
| WO2025026787A1 (en) | 2023-08-01 | 2025-02-06 | Globachem Nv | Plant defense elicitors |
| WO2025026738A1 (en) | 2023-07-31 | 2025-02-06 | Bayer Aktiengesellschaft | 6-[5-(ethylsulfonyl)-1-methyl-1h-imidazol-4-yl]-7-methyl-3-(pentafluoroethyl)-7h-imidazo[4,5-c]pyridazine derivatives as pesticides |
| WO2025026815A1 (en) | 2023-08-01 | 2025-02-06 | Globachem Nv | Insecticidal mixtures |
| WO2025031668A1 (en) | 2023-08-09 | 2025-02-13 | Bayer Aktiengesellschaft | Azaheterobiaryl-substituted 4,5-dihydro-1h-2,4,5-oxadiazines as novel fungicides |
| WO2025032038A1 (en) | 2023-08-09 | 2025-02-13 | Bayer Aktiengesellschaft | Pyridazin-4-yloxadiazines as novel fungicides |
| WO2025040520A1 (en) | 2023-08-21 | 2025-02-27 | Bayer Aktiengesellschaft | Herbicide/safener combinations based on safeners from the class of substituted [(1,5-diphenyl-1h-1,2,4-triazol-3-yl)oxy] acetic acids and herbicides from the class of substituted cyclic diones and the salts thereof |
| WO2025040301A1 (en) | 2023-08-21 | 2025-02-27 | Bayer Aktiengesellschaft | Herbicide/safener combinations based on safeners from the class of substituted [(1,5-diphenyl-1h-1,2,4-triazol-3-yl)oxy] acetic acids and herbicides from the class of substituted cyclic diones and the salts thereof |
| WO2025049555A1 (en) | 2023-08-31 | 2025-03-06 | Oerth Bio Llc | Compositions and methods for targeted inhibition and degradation of proteins in an insect cell |
| WO2025078128A1 (en) | 2023-10-11 | 2025-04-17 | Bayer Aktiengesellschaft | Pyridazin-3-one-4-yloxadiazines as novel fungicides |
| WO2025098875A1 (en) | 2023-11-10 | 2025-05-15 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2025098874A1 (en) | 2023-11-10 | 2025-05-15 | Bayer Aktiengesellschaft | Active compound combinations having fungicidal/insecticidal/acaricidal properties |
| WO2025098876A1 (en) | 2023-11-10 | 2025-05-15 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2025108865A1 (en) | 2023-11-23 | 2025-05-30 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2025114238A1 (en) | 2023-12-01 | 2025-06-05 | Bayer Aktiengesellschaft | Solid agrochemical formulations comprising crystalline form a of 4-[(6-chloro-3-pyridylmethyl)(2,2- difluoroethyl)amino]furan-2(5h)-one |
| WO2025119860A1 (en) | 2023-12-05 | 2025-06-12 | Bayer Aktiengesellschaft | Liquid agrochemical formulations comprising flupyradifurone |
| WO2025132148A1 (en) | 2023-12-21 | 2025-06-26 | Bayer Aktiengesellschaft | Adjuvant compositions for agrochemical applications |
| WO2025168777A1 (en) | 2024-02-08 | 2025-08-14 | Bayer Aktiengesellschaft | Use of isoxazolinecarboxamide for selective weed control in pineapple |
| WO2025168620A1 (en) | 2024-02-07 | 2025-08-14 | Bayer Aktiengesellschaft | Heteroaryl-substituted 4,5-dihydro-1h-2,4,5-oxadiazines as novel fungicides |
| WO2025186065A1 (en) | 2024-03-05 | 2025-09-12 | Bayer Aktiengesellschaft | Heteroaryl-substituted (aza)quinoxaline derivatives as pesticides |
| WO2025190927A1 (en) | 2024-03-14 | 2025-09-18 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| CN120817945A (en) * | 2025-09-17 | 2025-10-21 | 山东成欣生物科技有限公司 | A pesticide composition for controlling pests and diseases and its preparation process |
| WO2025257121A1 (en) | 2024-06-12 | 2025-12-18 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2025257122A1 (en) | 2024-06-12 | 2025-12-18 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2025257242A1 (en) | 2024-06-14 | 2025-12-18 | Bayer Aktiengesellschaft | Heterocyclyl-substituted aryl- and heteroarylpyrazines and salts thereof, and the use thereof as herbicidal active substances |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4988734A (en) * | 1988-05-07 | 1991-01-29 | Bayer Aktiengesellschaft | Fungicidal stereoisomers of N-(R)-(1-aryl-ethyl)-1-alkyl-2,2,-dichloro-cyclopropanecarboxamides |
| CN1918988A (en) * | 2005-08-26 | 2007-02-28 | 沈阳化工研究院 | Sterilizing and insecticidal composition |
| CN101039578A (en) * | 2004-10-12 | 2007-09-19 | 拜尔农作物科学股份公司 | Fungicidal active ingredient combinations containing fluoxastrobin |
| CN101237777A (en) * | 2005-06-09 | 2008-08-06 | 拜尔农作物科学股份公司 | active substance combination |
-
2013
- 2013-10-11 CN CN201310473947.XA patent/CN103524422B/en not_active Expired - Fee Related
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4988734A (en) * | 1988-05-07 | 1991-01-29 | Bayer Aktiengesellschaft | Fungicidal stereoisomers of N-(R)-(1-aryl-ethyl)-1-alkyl-2,2,-dichloro-cyclopropanecarboxamides |
| CN101039578A (en) * | 2004-10-12 | 2007-09-19 | 拜尔农作物科学股份公司 | Fungicidal active ingredient combinations containing fluoxastrobin |
| CN101237777A (en) * | 2005-06-09 | 2008-08-06 | 拜尔农作物科学股份公司 | active substance combination |
| CN1918988A (en) * | 2005-08-26 | 2007-02-28 | 沈阳化工研究院 | Sterilizing and insecticidal composition |
Non-Patent Citations (5)
| Title |
|---|
| 华纯: "拟除虫菊酯类农药的进展和剂型", 《世界农药》 * |
| 宋倩等: "生物电子等排原理在农药与医药开发中的应用", 《河北工业科技》 * |
| 尹江平等: "除虫菊酯类化学的发展与未来", 《世界农药》 * |
| 王元正: "农药设计中的活性子叠加法", 《化学世界》 * |
| 王平等: "原位氧化法制备2-苯基苯并咪唑", 《江苏化工》 * |
Cited By (319)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103918704B (en) * | 2014-04-03 | 2015-08-12 | 中国农业科学院植物保护研究所 | Synergistic mixtures, cooperative compositions and purposes |
| CN103918704A (en) * | 2014-04-03 | 2014-07-16 | 中国农业科学院植物保护研究所 | Synergetic mixture, synergetic composition and application thereof |
| CN104062413A (en) * | 2014-07-21 | 2014-09-24 | 中国农业科学院蔬菜花卉研究所 | Biological assay method for tetranychids and application of method |
| WO2017072039A1 (en) | 2015-10-26 | 2017-05-04 | Bayer Cropscience Aktiengesellschaft | Condensed bicyclic heterocycle derivatives as pest control agents |
| WO2017093180A1 (en) | 2015-12-01 | 2017-06-08 | Bayer Cropscience Aktiengesellschaft | Condensed bicyclic heterocycle derivatives as pest control agents |
| WO2017093214A1 (en) | 2015-12-03 | 2017-06-08 | Bayer Cropscience Aktiengesellschaft | Mesoionic halogenated 3-(acetyl)-1-[(1,3-thiazol-5-yl)methyl]-1h-imidazo[1,2-a]pyridin-4-ium-2-olate derivatives and related compounds as insecticides |
| WO2017137339A1 (en) | 2016-02-11 | 2017-08-17 | Bayer Cropscience Aktiengesellschaft | Substituted 2-oxyimidazolyl-carboxamides as pest control agents |
| WO2017137338A1 (en) | 2016-02-11 | 2017-08-17 | Bayer Cropscience Aktiengesellschaft | Substituierted 2-(het)aryl-imidazolyl-carboxyamides as pest control agents |
| WO2017144341A1 (en) | 2016-02-23 | 2017-08-31 | Bayer Cropscience Aktiengesellschaft | Condensed bicyclic heterocycle derivatives as pest control agents |
| WO2017146226A1 (en) * | 2016-02-26 | 2017-08-31 | 日本農薬株式会社 | Benzoxazole compound or salt thereof, agricultural/horticultural insecticide containing said compound, and method for using same |
| US10575525B2 (en) | 2016-02-26 | 2020-03-03 | Nihon Nohyaku Co., Ltd. | Benzoxazole compound or salt thereof, agricultural and horticultural insecticide comprising the compound, and method for using the insecticide |
| WO2017144497A1 (en) | 2016-02-26 | 2017-08-31 | Bayer Cropscience Aktiengesellschaft | Solvent-free formulations of low-melting active substances |
| JPWO2017146226A1 (en) * | 2016-02-26 | 2018-12-13 | 日本農薬株式会社 | Benzoxazole compounds or salts thereof, agricultural and horticultural insecticides containing the compounds, and methods of use thereof |
| EP3210468A1 (en) | 2016-02-26 | 2017-08-30 | Bayer CropScience Aktiengesellschaft | Solvent-free formulations of low-melting point agents |
| WO2017157735A1 (en) | 2016-03-15 | 2017-09-21 | Bayer Cropscience Aktiengesellschaft | Substituted sulfonyl amides for controlling animal pests |
| WO2017157885A1 (en) | 2016-03-16 | 2017-09-21 | Bayer Cropscience Aktiengesellschaft | N-(cyanobenzyl)-6-(cyclopropyl-carbonylamino)-4-(phenyl)-pyridine-2-carboxamide derivatives and related compounds as pesticides and plant protection agents |
| WO2017174414A1 (en) | 2016-04-05 | 2017-10-12 | Bayer Cropscience Aktiengesellschaft | Naphthaline-derivatives as pest control agents |
| WO2017178416A1 (en) | 2016-04-15 | 2017-10-19 | Bayer Animal Health Gmbh | Pyrazolopyrimidine derivatives |
| WO2017186536A1 (en) | 2016-04-25 | 2017-11-02 | Bayer Cropscience Aktiengesellschaft | Substituted 2-alkylimidazolyl-carboxamides as pest control agents |
| EP3241830A1 (en) | 2016-05-04 | 2017-11-08 | Bayer CropScience Aktiengesellschaft | Condensed bicyclic heterocyclic derivatives as pesticides |
| WO2017198450A1 (en) | 2016-05-15 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in maize |
| WO2017198449A1 (en) | 2016-05-15 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in brassicaceae |
| WO2017198452A1 (en) | 2016-05-16 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in soybean |
| WO2017198453A1 (en) | 2016-05-16 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in potato, tomato or alfalfa |
| WO2017198454A1 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in cotton |
| WO2017198455A2 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in beta spp. plants |
| EP3245865A1 (en) | 2016-05-17 | 2017-11-22 | Bayer CropScience Aktiengesellschaft | Method for increasing yield in brassicaceae |
| WO2017198451A1 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in small grain cereals such as wheat and rice |
| WO2018015289A1 (en) | 2016-07-19 | 2018-01-25 | Bayer Cropscience Aktiengesellschaft | Condensed bicyclic heterocycle derivatives as pest control agents |
| WO2018019937A1 (en) | 2016-07-29 | 2018-02-01 | Bayer Cropscience Aktiengesellschaft | Formulation comprising a beneficial p. bilaii strain and talc for use in seed treatment |
| WO2018029102A1 (en) | 2016-08-10 | 2018-02-15 | Bayer Cropscience Aktiengesellschaft | Substituted 2-heterocyclyl imidazolyl-carboxamides as pest control agents |
| WO2018033455A1 (en) | 2016-08-15 | 2018-02-22 | Bayer Cropscience Aktiengesellschaft | Condensed bicyclic heterocycle derivatives as pest control agents |
| WO2018050825A1 (en) | 2016-09-19 | 2018-03-22 | Bayer Cropscience Aktiengesellschaft | Pyrazolo [1,5-a]pyridine derivatives and their use as pesticides |
| WO2018065292A1 (en) | 2016-10-06 | 2018-04-12 | Bayer Cropscience Aktiengesellschaft | 2-(het)aryl-substituted condensed bicyclic heterocycle derivatives as pest control agents |
| WO2018065288A1 (en) | 2016-10-07 | 2018-04-12 | Bayer Cropscience Aktiengesellschaft | 2-[2-phenyl-1-(sulfonyl-methyl)-vinyl]-imidazo-[4,5-b] pyridine derivatives and related compounds as pesticides in plant protection |
| WO2018083288A1 (en) | 2016-11-07 | 2018-05-11 | Bayer Aktiengesellschaft | Substituted sulfonyl amides for controlling animal pests |
| US10889573B2 (en) | 2016-11-11 | 2021-01-12 | Bayer Animal Health Gmbh | Anthelmintic quinoline-3-carboxamide derivatives |
| WO2018087036A1 (en) | 2016-11-11 | 2018-05-17 | Bayer Animal Health Gmbh | New anthelmintic quinoline-3-carboxamide derivatives |
| US11505545B2 (en) | 2016-11-11 | 2022-11-22 | Bayer Animal Health Gmbh | Anthelmintic quinoline-3-carboxamide derivatives |
| WO2018095953A1 (en) | 2016-11-23 | 2018-05-31 | Bayer Cropscience Aktiengesellschaft | 2-[3-(alkylsulfonyl)-2h-indazol-2-yl]-3h-imidazo[4,5-b]pyridine derivatives and similar compounds as pesticides |
| WO2018104500A1 (en) | 2016-12-09 | 2018-06-14 | Bayer Cropscience Aktiengesellschaft | Plant health effect of purpureocillium lilacinum |
| WO2018108791A1 (en) | 2016-12-16 | 2018-06-21 | Bayer Cropscience Aktiengesellschaft | Thiadiazole derivatives as pesticides |
| WO2018108730A1 (en) | 2016-12-16 | 2018-06-21 | Bayer Aktiengesellschaft | Mesoionic imidazopyridines for use as insecticides |
| WO2018130443A1 (en) | 2017-01-10 | 2018-07-19 | Bayer Aktiengesellschaft | Heterocyclene derivatives as pest control agents |
| WO2018130437A1 (en) | 2017-01-10 | 2018-07-19 | Bayer Aktiengesellschaft | Heterocyclene derivatives as pest control agents |
| CN106918705A (en) * | 2017-01-22 | 2017-07-04 | 贵州勤邦食品安全科学技术有限公司 | Detect test paper and its application of Fenpropathrin |
| WO2018138050A1 (en) | 2017-01-26 | 2018-08-02 | Bayer Aktiengesellschaft | Condensed bicyclic heterocyclene derivatives as pest control agents |
| WO2018141954A1 (en) | 2017-02-06 | 2018-08-09 | Bayer Aktiengesellschaft | Aryl or heteroaryl-substituted imidazo pyridine derivatives and their use as pesticides |
| EP3369320A1 (en) | 2017-03-02 | 2018-09-05 | Bayer CropScience Aktiengesellschaft | Agent for controlling bugs |
| WO2018189077A1 (en) | 2017-04-12 | 2018-10-18 | Bayer Aktiengesellschaft | Mesoionic imidazopyridines for use as insecticides |
| WO2018192872A1 (en) | 2017-04-21 | 2018-10-25 | Bayer Aktiengesellschaft | Mesoionic imidazopyridines as insecticides |
| WO2018197257A1 (en) | 2017-04-24 | 2018-11-01 | Bayer Aktiengesellschaft | Condensed bicyclic heterocyclic-compound derivatives as pest control agents |
| WO2018197692A1 (en) | 2017-04-27 | 2018-11-01 | Bayer Aktiengesellschaft | Heteroarylphenylaminoquinolines and analogues |
| WO2018197401A1 (en) | 2017-04-27 | 2018-11-01 | Bayer Animal Health Gmbh | New bicyclic pyrazole derivatives |
| US11130768B2 (en) | 2017-04-27 | 2021-09-28 | Bayer Animal Health Gmbh | Bicyclic pyrazole derivatives |
| WO2018202494A1 (en) | 2017-05-02 | 2018-11-08 | Bayer Aktiengesellschaft | 2-(het)aryl-substituted condensed bicyclic heterocyclic derivatives as pest control agents |
| WO2018202501A1 (en) | 2017-05-02 | 2018-11-08 | Bayer Aktiengesellschaft | 2-(het)aryl-substituted condensed bicyclic heterocyclic derivatives as pest control agents |
| WO2018202715A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylbenzylbenzimidazoles and analogues |
| WO2018202712A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylmethylphenoxyquinolines and analogues |
| WO2018202706A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylheteroaryloxyquinolines and analogues |
| WO2018202525A1 (en) | 2017-05-04 | 2018-11-08 | Bayer Cropscience Aktiengesellschaft | Phenoxyethanamine derivatives for controlling pests |
| US11827616B2 (en) | 2017-05-04 | 2023-11-28 | Discovery Purchaser Corporation | Heterocyclic compounds as pesticides |
| WO2018202524A1 (en) | 2017-05-04 | 2018-11-08 | Bayer Cropscience Aktiengesellschaft | 2-{[2-(phenyloxymethyl)pyridin-5-yl]oxy}-ethanamin-derivatives and related compounds as pest-control agents e.g. for the protection of plants |
| EP3400801A1 (en) | 2017-05-10 | 2018-11-14 | Bayer CropScience Aktiengesellschaft | Plant health effect of purpureocillium lilacinum |
| WO2019002132A1 (en) | 2017-06-30 | 2019-01-03 | Bayer Animal Health Gmbh | New azaquinoline derivatives |
| WO2019007887A1 (en) | 2017-07-06 | 2019-01-10 | Bayer Aktiengesellschaft | INSECTICIDES AND FUNGICIDES ACTIVE COMPOUNDS |
| EP3284739A1 (en) | 2017-07-19 | 2018-02-21 | Bayer CropScience Aktiengesellschaft | Substituted (het) aryl compounds as pesticides |
| WO2019025341A1 (en) | 2017-08-04 | 2019-02-07 | Bayer Animal Health Gmbh | Quinoline derivatives for treating infections with helminths |
| WO2019035881A1 (en) | 2017-08-17 | 2019-02-21 | Bayer Cropscience Lp | Liquid fertilizer-dispersible compositions and methods thereof |
| WO2019038195A1 (en) | 2017-08-22 | 2019-02-28 | Bayer Aktiengesellschaft | HETEROCYCLEN DERIVATIVES AS PESTICIDES |
| WO2019059412A1 (en) | 2017-09-20 | 2019-03-28 | Mitsui Chemicals Agro, Inc. | Prolonged ectoparasite-controlling agent for animal |
| WO2019068572A1 (en) | 2017-10-04 | 2019-04-11 | Bayer Aktiengesellschaft | HETEROCYCLEN DERIVATIVES AS PESTICIDES |
| EP3473103A1 (en) | 2017-10-17 | 2019-04-24 | Bayer AG | Aqueous suspension concentrates based on 2- [(2,4-dichlorophenyl) -methyl] -4,4 '-dimethyl-3-isoxazolidinone |
| WO2019076744A1 (en) | 2017-10-17 | 2019-04-25 | Bayer Aktiengesellschaft | AQUEOUS SUSPENSION CONCENTRATES BASED ON 2 - [(2,4-DICHLORPHENYL) -METHYL] -4,4'-DIMETHYL-3-ISOXAZOLIDINONE |
| WO2019076749A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2019076750A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2019076751A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2019076754A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2019076752A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3473100A1 (en) | 2017-10-18 | 2019-04-24 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2019092086A1 (en) | 2017-11-13 | 2019-05-16 | Bayer Aktiengesellschaft | Tetrazolylpropyl derivatives and their use as fungicides |
| WO2019105875A1 (en) | 2017-11-28 | 2019-06-06 | Bayer Aktiengesellschaft | Heterocyclic compounds as pesticides |
| WO2019105871A1 (en) | 2017-11-29 | 2019-06-06 | Bayer Aktiengesellschaft | Nitrogenous heterocycles as a pesticide |
| WO2019122319A1 (en) | 2017-12-21 | 2019-06-27 | Bayer Aktiengesellschaft | Trisubstitutedsilylmethylheteroaryloxyquinolines and analogues |
| EP3305786A2 (en) | 2018-01-22 | 2018-04-11 | Bayer CropScience Aktiengesellschaft | Condensed bicyclic heterocycle derivatives as pesticides |
| WO2019155066A1 (en) | 2018-02-12 | 2019-08-15 | Bayer Aktiengesellschaft | Fungicidal oxadiazoles |
| WO2019162228A1 (en) | 2018-02-21 | 2019-08-29 | Bayer Aktiengesellschaft | 1-(5-substituted imidazol-1-yl)but-3-en derivatives and their use as fungicides |
| WO2019162174A1 (en) | 2018-02-21 | 2019-08-29 | Bayer Aktiengesellschaft | Condensed bicyclic heterocyclic derivatives as pest control agents |
| WO2019170626A1 (en) | 2018-03-08 | 2019-09-12 | Bayer Aktiengesellschaft | Use of heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides in plant protection |
| WO2019175045A1 (en) | 2018-03-12 | 2019-09-19 | Bayer Aktiengesellschaft | Condensed bicyclic heterocyclic derivatives as pest control agents |
| WO2019175046A1 (en) | 2018-03-12 | 2019-09-19 | Bayer Aktiengesellschaft | Condensed bicyclic heterocyclic derivatives as pest control agents |
| WO2019197371A1 (en) | 2018-04-10 | 2019-10-17 | Bayer Aktiengesellschaft | Oxadiazoline derivatives |
| WO2019197468A1 (en) | 2018-04-12 | 2019-10-17 | Bayer Aktiengesellschaft | N-(cyclopropylmethyl)-5-(methylsulfonyl)-n-{1-[1-(pyrimidin-2-yl)-1h-1,2,4-triazol-5-yl]ethyl}benzamide derivatives and the corresponding pyridine-carboxamide derivatives as pesticides |
| EP3904350A1 (en) | 2018-04-12 | 2021-11-03 | Bayer Aktiengesellschaft | N-(cyclopropylmethyl)-5-(methylsulfonyl)-n-{1-[1-(pyrimidin-2-yl)-1h-1,2,4-triazol-5-yl]ethyl}benzamide derivatives and the corresponding pyridine-carboxamide derivatives as pesticides |
| EP3904349A2 (en) | 2018-04-12 | 2021-11-03 | Bayer Aktiengesellschaft | N-(cyclopropylmethyl)-5-(methylsulfonyl)-n-{1-[1-(pyrimidin-2-yl)-1h-1,2,4-triazol-5-yl]ethyl}heterocyclyl amide derivatives and similar compounds as pesticides |
| WO2019197623A1 (en) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Active ingredient combinations with insecticidal, fungicidal and acaricidal properties |
| WO2019197615A1 (en) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Active ingredient combinations with fungicides, insecticides and acaricidal properties |
| EP4670505A2 (en) | 2018-04-13 | 2025-12-31 | Bayer Aktiengesellschaft | Active ingredient combinations with insecticidal, nematicidal and acaricidal properties |
| WO2019201835A1 (en) | 2018-04-17 | 2019-10-24 | Bayer Aktiengesellschaft | Heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| WO2019201921A1 (en) | 2018-04-20 | 2019-10-24 | Bayer Aktiengesellschaft | Heterocyclene derivatives as pest control agents |
| WO2019202077A1 (en) | 2018-04-20 | 2019-10-24 | Bayer Aktiengesellschaft | Heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| EP3919486A1 (en) | 2018-04-25 | 2021-12-08 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| WO2019206799A1 (en) | 2018-04-25 | 2019-10-31 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| WO2019215182A1 (en) | 2018-05-09 | 2019-11-14 | Bayer Animal Health Gmbh | New quinoline derivatives |
| WO2019224143A1 (en) | 2018-05-24 | 2019-11-28 | Bayer Aktiengesellschaft | Active ingredient combinations with insecticidal, nematicidal and acaricidal properties |
| WO2020005678A1 (en) | 2018-06-25 | 2020-01-02 | Bayer Cropscience Lp | Seed treatment method |
| WO2020002189A1 (en) | 2018-06-27 | 2020-01-02 | Bayer Aktiengesellschaft | Active substance combinations |
| EP3586630A1 (en) | 2018-06-28 | 2020-01-01 | Bayer AG | Active compound combinations having insecticidal/acaricidal properties |
| US12516037B2 (en) | 2018-07-05 | 2026-01-06 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| US11952359B2 (en) | 2018-07-05 | 2024-04-09 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| US11884643B2 (en) | 2018-07-05 | 2024-01-30 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| WO2020007902A1 (en) | 2018-07-05 | 2020-01-09 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| WO2020007905A1 (en) | 2018-07-05 | 2020-01-09 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| WO2020007904A1 (en) | 2018-07-05 | 2020-01-09 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| WO2020020813A1 (en) | 2018-07-25 | 2020-01-30 | Bayer Aktiengesellschaft | Fungicidal active compound combinations |
| WO2020020816A1 (en) | 2018-07-26 | 2020-01-30 | Bayer Aktiengesellschaft | Novel triazole derivatives |
| WO2020021082A1 (en) | 2018-07-27 | 2020-01-30 | Bayer Aktiengesellschaft | Controlled release formulations for agrochemicals |
| WO2020025650A1 (en) | 2018-07-31 | 2020-02-06 | Bayer Aktiengesellschaft | Controlled release formulations with lignin for agrochemicals |
| WO2020043650A1 (en) | 2018-08-29 | 2020-03-05 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2020053282A1 (en) | 2018-09-13 | 2020-03-19 | Bayer Aktiengesellschaft | Heterocyclene derivatives as pest control agents |
| WO2020057939A1 (en) | 2018-09-17 | 2020-03-26 | Bayer Aktiengesellschaft | Use of the fungicide isoflucypram for controlling claviceps purpurea and reducing sclerotia in cereals |
| WO2020070050A1 (en) | 2018-10-01 | 2020-04-09 | Bayer Aktiengesellschaft | Fungicidal 5-substituted imidazol-1-yl carbinol derivatives |
| EP3636644A1 (en) | 2018-10-11 | 2020-04-15 | Bayer Aktiengesellschaft | Mesoionic imidazopyridines as insecticides |
| WO2020078839A1 (en) | 2018-10-16 | 2020-04-23 | Bayer Aktiengesellschaft | Active substance combinations |
| WO2020079173A1 (en) | 2018-10-18 | 2020-04-23 | Bayer Aktiengesellschaft | Pyridylphenylaminoquinolines and analogues |
| WO2020079167A1 (en) | 2018-10-18 | 2020-04-23 | Bayer Aktiengesellschaft | Heteroarylaminoquinolines and analogues |
| WO2020079232A1 (en) | 2018-10-20 | 2020-04-23 | Bayer Aktiengesellschaft | Oxetanylphenoxyquinolines and analogues |
| EP3643711A1 (en) | 2018-10-24 | 2020-04-29 | Bayer Animal Health GmbH | New anthelmintic compounds |
| WO2020083971A2 (en) | 2018-10-24 | 2020-04-30 | Bayer Animal Health Gmbh | New anthelmintic compounds |
| US12435068B2 (en) | 2018-10-24 | 2025-10-07 | Elanco Animal Health Gmbh | Anthelmintic compounds |
| CN109336826A (en) * | 2018-11-27 | 2019-02-15 | 盐城锦明药业有限公司 | A kind of chemical synthesis method of 2-hydroxybenzimidazole |
| WO2020109391A1 (en) | 2018-11-28 | 2020-06-04 | Bayer Aktiengesellschaft | Pyridazine (thio)amides as fungicidal compounds |
| WO2020114932A1 (en) | 2018-12-07 | 2020-06-11 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2020114934A1 (en) | 2018-12-07 | 2020-06-11 | Bayer Aktiengesellschaft | Herbicide compositions |
| EP3620052A1 (en) | 2018-12-12 | 2020-03-11 | Bayer Aktiengesellschaft | Use of phenoxypyridinyl-substituted (1h-1,2,4-triazol-1-yl)alcohols for controlling fungicidal diseases in maize |
| WO2020126980A1 (en) | 2018-12-18 | 2020-06-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2020127780A1 (en) | 2018-12-20 | 2020-06-25 | Bayer Aktiengesellschaft | Heterocyclyl pyridazine as fungicidal compounds |
| WO2020127974A1 (en) | 2018-12-21 | 2020-06-25 | Bayer Aktiengesellschaft | 1,3,4-oxadiazoles and their derivatives as new antifungal agents |
| EP3669652A1 (en) | 2018-12-21 | 2020-06-24 | Bayer AG | Active compound combination |
| EP3679791A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679790A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679789A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679792A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679793A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3545764A1 (en) | 2019-02-12 | 2019-10-02 | Bayer AG | Crystal form of 2-({2-fluoro-4-methyl-5-[(r)-(2,2,2-trifluoroethyl)sulfinyl]phenyl}imino)-3-(2,2,2- trifluoroethyl)-1,3-thiazolidin-4-one |
| WO2020173860A1 (en) | 2019-02-26 | 2020-09-03 | Bayer Aktiengesellschaft | Fused bicyclic heterocycle derivatives as pesticides |
| WO2020173861A1 (en) | 2019-02-26 | 2020-09-03 | Bayer Aktiengesellschaft | Condensed bicyclic heterocyclic derivatives as pest control agents |
| WO2020178067A1 (en) | 2019-03-01 | 2020-09-10 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2020178307A1 (en) | 2019-03-05 | 2020-09-10 | Bayer Aktiengesellschaft | Active compound combination |
| WO2020182929A1 (en) | 2019-03-13 | 2020-09-17 | Bayer Aktiengesellschaft | Substituted ureas and derivatives as new antifungal agents |
| WO2020187656A1 (en) | 2019-03-15 | 2020-09-24 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3564225A1 (en) | 2019-03-21 | 2019-11-06 | Bayer Aktiengesellschaft | Crystalline form of spiromesifen |
| WO2020212235A1 (en) | 2019-04-15 | 2020-10-22 | Bayer Animal Health Gmbh | Novel heteroaryl-substituted aminoalkyl azole compounds as pesticides |
| EP3725788A1 (en) | 2019-04-15 | 2020-10-21 | Bayer AG | Novel heteroaryl-substituted aminoalkyl azole compounds as pesticides |
| WO2020225435A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading and uptake ulv formulations |
| WO2020225438A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High uptake and rainfastness ulv formulations |
| WO2020225437A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | Ulv formulations with enhanced uptake |
| WO2020225439A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | Ulv formulations with enhanced rainfastness |
| WO2020225242A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | Active compound combination |
| WO2020225440A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading and rainfastness ulv formulations |
| WO2020225434A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading ulv formulations for agrochemical compounds ii |
| WO2020225436A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading, uptake and rainfastness ulv formulations |
| WO2020225428A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading ulv formulations for insecticides |
| WO2020231751A1 (en) | 2019-05-10 | 2020-11-19 | Bayer Cropscience Lp | Active compound combinations |
| WO2020229398A1 (en) | 2019-05-14 | 2020-11-19 | Bayer Aktiengesellschaft | (1-alkenyl)-substituted pyrazoles and triazoles as pest control agents |
| EP3750888A1 (en) | 2019-06-12 | 2020-12-16 | Bayer Aktiengesellschaft | Crystalline form a of 1,4-dimethyl-2-[2-(pyridin-3-yl)-2h-indazol-5-yl]-1,2,4-triazolidine-3,5-dione |
| WO2020254488A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and use thereof as fungicides |
| WO2020254486A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254489A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Benzylphenyl hydroxyisoxazolines and analogues as new antifungal agents |
| WO2020254487A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254493A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Thienylhydroxyisoxazolines and derivatives thereof |
| WO2020254492A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254494A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Fungicidal oxadiazoles |
| WO2020254490A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Phenoxyphenyl hydroxyisoxazolines and analogues as new antifungal agents |
| WO2020263812A1 (en) | 2019-06-24 | 2020-12-30 | Auburn University | A bacillus strain and methods of its use for plant growth promotion |
| EP3608311A1 (en) | 2019-06-28 | 2020-02-12 | Bayer AG | Crystalline form a of n-[4-chloro-3-[(1-cyanocyclopropyl)carbamoyl]phenyl]-2-methyl-4-methylsulfonyl-5-(1,1,2,2,2-pentafluoroethyl)pyrazole-3-carboxamide |
| WO2021001331A1 (en) | 2019-07-03 | 2021-01-07 | Bayer Aktiengesellschaft | Substituted thiophene carboxamides and derivatives thereof as microbicides |
| EP4378314A2 (en) | 2019-07-04 | 2024-06-05 | Bayer AG | Herbicidal compositions |
| WO2021001273A1 (en) | 2019-07-04 | 2021-01-07 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2021013721A1 (en) | 2019-07-22 | 2021-01-28 | Bayer Aktiengesellschaft | 5-amino substituted pyrazoles and triazoles as pest control agents |
| WO2021013720A1 (en) | 2019-07-23 | 2021-01-28 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021013719A1 (en) | 2019-07-23 | 2021-01-28 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021018839A1 (en) | 2019-07-30 | 2021-02-04 | Bayer Animal Health Gmbh | Isoquinoline derivatives and their use for the treatment of parasitic infections |
| EP3771714A1 (en) | 2019-07-30 | 2021-02-03 | Bayer AG | Nitrogen-containing heterocycles as pesticides |
| EP3701796A1 (en) | 2019-08-08 | 2020-09-02 | Bayer AG | Active compound combinations |
| WO2021048188A1 (en) | 2019-09-11 | 2021-03-18 | Bayer Aktiengesellschaft | Highly effective formulations on the basis of 2-[(2,4-dichlorphenyl)-methyl]-4,4'-dimethyl-3-isoxazolidinones and preemergence herbicides |
| WO2021058659A1 (en) | 2019-09-26 | 2021-04-01 | Bayer Aktiengesellschaft | Rnai-mediated pest control |
| WO2021069569A1 (en) | 2019-10-09 | 2021-04-15 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021069567A1 (en) | 2019-10-09 | 2021-04-15 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021069575A1 (en) | 2019-10-11 | 2021-04-15 | Bayer Animal Health Gmbh | Heteroaryl-substituted pyrazine derivatives as pesticides |
| WO2021089673A1 (en) | 2019-11-07 | 2021-05-14 | Bayer Aktiengesellschaft | Substituted sulfonyl amides for controlling animal pests |
| WO2021097162A1 (en) | 2019-11-13 | 2021-05-20 | Bayer Cropscience Lp | Beneficial combinations with paenibacillus |
| WO2021099303A1 (en) | 2019-11-18 | 2021-05-27 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021105091A1 (en) | 2019-11-25 | 2021-06-03 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021123051A1 (en) | 2019-12-20 | 2021-06-24 | Bayer Aktiengesellschaft | Substituted thiophene carboxamides, thiophene carboxylic acids and derivatives thereof |
| WO2021122986A1 (en) | 2019-12-20 | 2021-06-24 | Bayer Aktiengesellschaft | Thienyloxazolones and analogues |
| WO2021136758A1 (en) | 2019-12-30 | 2021-07-08 | Bayer Aktiengesellschaft | Aqueous capsule suspension concentrates based on polyurea shell material containing polyfunctional aminocarboxylic esters |
| EP3845304A1 (en) | 2019-12-30 | 2021-07-07 | Bayer AG | Capsule suspension concentrates based on polyisocyanates and biodegradable amine based cross-linker |
| WO2021165195A1 (en) | 2020-02-18 | 2021-08-26 | Bayer Aktiengesellschaft | Heteroaryl-triazole compounds as pesticides |
| WO2021170527A1 (en) | 2020-02-24 | 2021-09-02 | Bayer Aktiengesellschaft | Encapsulated pyrethroids with improved effictiveness in soil and leaf applications |
| EP3868207A1 (en) | 2020-02-24 | 2021-08-25 | Bayer Aktiengesellschaft | Encapsulated pyrethroids with improved activity in soil and leaf applications |
| EP3708565A1 (en) | 2020-03-04 | 2020-09-16 | Bayer AG | Pyrimidinyloxyphenylamidines and the use thereof as fungicides |
| WO2021204930A1 (en) | 2020-04-09 | 2021-10-14 | Bayer Animal Health Gmbh | Substituted condensed azines as anthelmintic compounds |
| WO2021209364A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209366A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209490A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Cyclaminephenylaminoquinolines as fungicides |
| WO2021209365A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209363A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209368A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021213978A1 (en) | 2020-04-21 | 2021-10-28 | Bayer Aktiengesellschaft | 2-(het)aryl-substituted condensed heterocyclic derivatives as pest control agents |
| WO2021224323A1 (en) | 2020-05-06 | 2021-11-11 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021224220A1 (en) | 2020-05-06 | 2021-11-11 | Bayer Aktiengesellschaft | Pyridine (thio)amides as fungicidal compounds |
| WO2021228734A1 (en) | 2020-05-12 | 2021-11-18 | Bayer Aktiengesellschaft | Triazine and pyrimidine (thio)amides as fungicidal compounds |
| WO2021233861A1 (en) | 2020-05-19 | 2021-11-25 | Bayer Aktiengesellschaft | Azabicyclic(thio)amides as fungicidal compounds |
| WO2021245087A1 (en) | 2020-06-04 | 2021-12-09 | Bayer Aktiengesellschaft | Heterocyclyl pyrimidines and triazines as novel fungicides |
| WO2021249995A1 (en) | 2020-06-10 | 2021-12-16 | Bayer Aktiengesellschaft | Azabicyclyl-substituted heterocycles as fungicides |
| WO2021255071A1 (en) | 2020-06-18 | 2021-12-23 | Bayer Aktiengesellschaft | 3-(pyridazin-4-yl)-5,6-dihydro-4h-1,2,4-oxadiazine derivatives as fungicides for crop protection |
| WO2021255089A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines and 1,3,4-oxadiazole pyridines as fungicides |
| WO2021255170A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines as fungicides |
| WO2021255091A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazoles and their derivatives as fungicides |
| WO2021255169A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines as fungicides |
| WO2021259997A1 (en) | 2020-06-25 | 2021-12-30 | Bayer Animal Health Gmbh | Novel heteroaryl-substituted pyrazine derivatives as pesticides |
| EP3929189A1 (en) | 2020-06-25 | 2021-12-29 | Bayer Animal Health GmbH | Novel heteroaryl-substituted pyrazine derivatives as pesticides |
| WO2021260017A1 (en) | 2020-06-26 | 2021-12-30 | Bayer Aktiengesellschaft | Aqueous capsule suspension concentrates comprising biodegradable ester groups |
| WO2022002818A1 (en) | 2020-07-02 | 2022-01-06 | Bayer Aktiengesellschaft | Heterocyclene derivatives as pest control agents |
| CN111875545A (en) * | 2020-07-17 | 2020-11-03 | 安阳师范学院 | A kind of directional synthesis method and application of 6-position substituted benzimidazole derivatives |
| WO2022033991A1 (en) | 2020-08-13 | 2022-02-17 | Bayer Aktiengesellschaft | 5-amino substituted triazoles as pest control agents |
| WO2022053453A1 (en) | 2020-09-09 | 2022-03-17 | Bayer Aktiengesellschaft | Azole carboxamide as pest control agents |
| WO2022058327A1 (en) | 2020-09-15 | 2022-03-24 | Bayer Aktiengesellschaft | Substituted ureas and derivatives as new antifungal agents |
| EP3974414A1 (en) | 2020-09-25 | 2022-03-30 | Bayer AG | 5-amino substituted pyrazoles and triazoles as pesticides |
| EP3915371A1 (en) | 2020-11-04 | 2021-12-01 | Bayer AG | Active compound combinations and fungicide compositions comprising those |
| WO2022096685A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift and spreading properties |
| WO2022096696A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| EP3994987A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift and uptake properties |
| EP3994995A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| EP3994991A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, spreading, uptake and rainfastness properties |
| WO2022096686A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift properties |
| WO2022096695A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| WO2022096687A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift and uptake properties |
| WO2022096691A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, uptake and rainfastness properties |
| WO2022096692A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, spreading, uptake and rainfastness properties |
| WO2022096690A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, spreading and uptake properties |
| WO2022096694A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading and ulv tank mix adjuvant formulation |
| EP3994988A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, spreading and rainfastness properties |
| WO2022096688A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, spreading and rainfastness properties |
| WO2022096693A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Low drift, rainfastness, high uptake and ulv tank mix adjuvant formulation |
| EP3994992A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Low drift, rainfastness, high uptake and ulv tank mix adjuvant formulation |
| EP3994990A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, spreading and uptake properties |
| EP3994986A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift and spreading properties |
| EP3994985A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift properties |
| EP3994989A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, rainfastness and uptake properties |
| EP3994994A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| EP3994993A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading and ulv tank mix adjuvant formulation |
| EP3915971A1 (en) | 2020-12-16 | 2021-12-01 | Bayer Aktiengesellschaft | Phenyl-s(o)n-phenylamidines and the use thereof as fungicides |
| WO2022129188A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | 1,2,4-oxadiazol-3-yl pyrimidines as fungicides |
| WO2022129190A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | (hetero)aryl substituted 1,2,4-oxadiazoles as fungicides |
| WO2022129196A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | Heterobicycle substituted 1,2,4-oxadiazoles as fungicides |
| WO2022152728A1 (en) | 2021-01-15 | 2022-07-21 | Bayer Aktiengesellschaft | Herbicidal compositions |
| EP4036083A1 (en) | 2021-02-02 | 2022-08-03 | Bayer Aktiengesellschaft | 5-oxy substituted heterocycles as pesticides |
| WO2022207496A1 (en) | 2021-03-30 | 2022-10-06 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2022207494A1 (en) | 2021-03-30 | 2022-10-06 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2022233777A1 (en) | 2021-05-06 | 2022-11-10 | Bayer Aktiengesellschaft | Alkylamide substituted, annulated imidazoles and use thereof as insecticides |
| WO2022238194A1 (en) | 2021-05-10 | 2022-11-17 | Bayer Aktiengesellschaft | Herbicide/safener combination based on safeners from the class of substituted [(1,5-diphenyl-1h-1,2,4-triazol-3-yl)oxy]acetic acids and their salts |
| WO2022238391A1 (en) | 2021-05-12 | 2022-11-17 | Bayer Aktiengesellschaft | 2-(het)aryl-substituted condensed heterocycle derivatives as pest control agents |
| WO2023017120A1 (en) | 2021-08-13 | 2023-02-16 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2023025682A1 (en) | 2021-08-25 | 2023-03-02 | Bayer Aktiengesellschaft | Novel pyrazinyl-triazole compounds as pesticides |
| EP4144739A1 (en) | 2021-09-02 | 2023-03-08 | Bayer Aktiengesellschaft | Anellated pyrazoles as parasiticides |
| WO2023036821A1 (en) | 2021-09-09 | 2023-03-16 | Bayer Animal Health Gmbh | New quinoline derivatives |
| EP4148052A1 (en) | 2021-09-09 | 2023-03-15 | Bayer Animal Health GmbH | New quinoline derivatives |
| CN113666875A (en) * | 2021-09-16 | 2021-11-19 | 湖北瑞凯兴科技股份有限公司 | Method for preparing 2-phenylbenzimidazole |
| WO2023078915A1 (en) | 2021-11-03 | 2023-05-11 | Bayer Aktiengesellschaft | Bis(hetero)aryl thioether (thio)amides as fungicidal compounds |
| WO2023092050A1 (en) | 2021-11-20 | 2023-05-25 | Bayer Cropscience Lp | Beneficial combinations with recombinant bacillus cells expressing a serine protease |
| WO2023099445A1 (en) | 2021-11-30 | 2023-06-08 | Bayer Aktiengesellschaft | Bis(hetero)aryl thioether oxadiazines as fungicidal compounds |
| WO2023110656A1 (en) | 2021-12-15 | 2023-06-22 | Bayer Aktiengesellschaft | Spectroscopic solution for non-destructive quantification of one or more chemical substances in a matrix comprising coating and bulk material in a sample, such as coated seeds, using multivariate data analysis |
| WO2023205602A1 (en) | 2022-04-18 | 2023-10-26 | Basf Corporation | High-load agricultural formulations and methods of making same |
| EP4265110A1 (en) | 2022-04-20 | 2023-10-25 | Bayer AG | Water dispersible granules with low melting active ingredients prepared by extrusion |
| WO2023203009A1 (en) | 2022-04-20 | 2023-10-26 | Bayer Aktiengesellschaft | Water dispersible granules with low melting active ingredients prepared by extrusion |
| WO2023213670A1 (en) | 2022-05-03 | 2023-11-09 | Bayer Aktiengesellschaft | Crystalline forms of (5s)-3-[3-(3-chloro-2-fluorophenoxy)-6-methylpyridazin-4-yl]-5-(2-chloro-4-methylbenzyl)-5,6-dihydro-4h-1,2,4-oxadiazine |
| WO2023213626A1 (en) | 2022-05-03 | 2023-11-09 | Bayer Aktiengesellschaft | Use of (5s)-3-[3-(3-chloro-2-fluorophenoxy)-6-methylpyridazin-4-yl]-5-(2-chloro-4-methylbenzyl)-5,6-dihydro-4h-1,2,4-oxadiazine for controlling unwanted microorganisms |
| WO2023217619A1 (en) | 2022-05-07 | 2023-11-16 | Bayer Aktiengesellschaft | Low drift aqueous liquid formulations for low, medium, and high spray volume application |
| WO2023237444A1 (en) | 2022-06-06 | 2023-12-14 | Bayer Aktiengesellschaft | Agrochemical formulations comprising crystalline form a of 4-[(6-chloro-3-pyridylmethyl)(2,2-difluoroethyl)amino]furan-2(5h)-one |
| EP4295683A1 (en) | 2022-06-21 | 2023-12-27 | Bayer Aktiengesellschaft | Agrochemical formulations comprising crystalline form a of 4-[(6-chloro-3-pyridylmethyl)(2,2-difluoroethyl)amino]furan-2(5h)-one |
| WO2024013016A1 (en) | 2022-07-11 | 2024-01-18 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024013015A1 (en) | 2022-07-11 | 2024-01-18 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024068473A1 (en) | 2022-09-27 | 2024-04-04 | Bayer Aktiengesellschaft | Herbicide/safener combination based on safeners from the class of substituted [(1,5-diphenyl-1h-1,2,4-triazol-3-yl)oxy]acetic acids and their salts |
| WO2024068519A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068520A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068517A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068518A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-heteroaryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| EP4295688A1 (en) | 2022-09-28 | 2023-12-27 | Bayer Aktiengesellschaft | Active compound combination |
| EP4353082A1 (en) | 2022-10-14 | 2024-04-17 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024104643A1 (en) | 2022-11-17 | 2024-05-23 | Bayer Aktiengesellschaft | Use of isotianil for controlling plasmodiophora brassica |
| WO2024170472A1 (en) | 2023-02-16 | 2024-08-22 | Bayer Aktiengesellschaft | Herbicidal mixtures |
| WO2024213752A1 (en) | 2023-04-14 | 2024-10-17 | Elanco Animal Health Gmbh | Long-term prevention and/or treatment of a disease by slo-1 inhibitors |
| WO2025026738A1 (en) | 2023-07-31 | 2025-02-06 | Bayer Aktiengesellschaft | 6-[5-(ethylsulfonyl)-1-methyl-1h-imidazol-4-yl]-7-methyl-3-(pentafluoroethyl)-7h-imidazo[4,5-c]pyridazine derivatives as pesticides |
| WO2025026787A1 (en) | 2023-08-01 | 2025-02-06 | Globachem Nv | Plant defense elicitors |
| WO2025026815A1 (en) | 2023-08-01 | 2025-02-06 | Globachem Nv | Insecticidal mixtures |
| WO2025032038A1 (en) | 2023-08-09 | 2025-02-13 | Bayer Aktiengesellschaft | Pyridazin-4-yloxadiazines as novel fungicides |
| WO2025031668A1 (en) | 2023-08-09 | 2025-02-13 | Bayer Aktiengesellschaft | Azaheterobiaryl-substituted 4,5-dihydro-1h-2,4,5-oxadiazines as novel fungicides |
| WO2025040520A1 (en) | 2023-08-21 | 2025-02-27 | Bayer Aktiengesellschaft | Herbicide/safener combinations based on safeners from the class of substituted [(1,5-diphenyl-1h-1,2,4-triazol-3-yl)oxy] acetic acids and herbicides from the class of substituted cyclic diones and the salts thereof |
| WO2025040301A1 (en) | 2023-08-21 | 2025-02-27 | Bayer Aktiengesellschaft | Herbicide/safener combinations based on safeners from the class of substituted [(1,5-diphenyl-1h-1,2,4-triazol-3-yl)oxy] acetic acids and herbicides from the class of substituted cyclic diones and the salts thereof |
| WO2025049555A1 (en) | 2023-08-31 | 2025-03-06 | Oerth Bio Llc | Compositions and methods for targeted inhibition and degradation of proteins in an insect cell |
| WO2025078128A1 (en) | 2023-10-11 | 2025-04-17 | Bayer Aktiengesellschaft | Pyridazin-3-one-4-yloxadiazines as novel fungicides |
| WO2025098876A1 (en) | 2023-11-10 | 2025-05-15 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2025098875A1 (en) | 2023-11-10 | 2025-05-15 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2025098874A1 (en) | 2023-11-10 | 2025-05-15 | Bayer Aktiengesellschaft | Active compound combinations having fungicidal/insecticidal/acaricidal properties |
| WO2025108865A1 (en) | 2023-11-23 | 2025-05-30 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2025114238A1 (en) | 2023-12-01 | 2025-06-05 | Bayer Aktiengesellschaft | Solid agrochemical formulations comprising crystalline form a of 4-[(6-chloro-3-pyridylmethyl)(2,2- difluoroethyl)amino]furan-2(5h)-one |
| WO2025119860A1 (en) | 2023-12-05 | 2025-06-12 | Bayer Aktiengesellschaft | Liquid agrochemical formulations comprising flupyradifurone |
| WO2025132148A1 (en) | 2023-12-21 | 2025-06-26 | Bayer Aktiengesellschaft | Adjuvant compositions for agrochemical applications |
| WO2025168620A1 (en) | 2024-02-07 | 2025-08-14 | Bayer Aktiengesellschaft | Heteroaryl-substituted 4,5-dihydro-1h-2,4,5-oxadiazines as novel fungicides |
| WO2025168777A1 (en) | 2024-02-08 | 2025-08-14 | Bayer Aktiengesellschaft | Use of isoxazolinecarboxamide for selective weed control in pineapple |
| WO2025186065A1 (en) | 2024-03-05 | 2025-09-12 | Bayer Aktiengesellschaft | Heteroaryl-substituted (aza)quinoxaline derivatives as pesticides |
| WO2025190927A1 (en) | 2024-03-14 | 2025-09-18 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2025257121A1 (en) | 2024-06-12 | 2025-12-18 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2025257122A1 (en) | 2024-06-12 | 2025-12-18 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2025257242A1 (en) | 2024-06-14 | 2025-12-18 | Bayer Aktiengesellschaft | Heterocyclyl-substituted aryl- and heteroarylpyrazines and salts thereof, and the use thereof as herbicidal active substances |
| CN120817945A (en) * | 2025-09-17 | 2025-10-21 | 山东成欣生物科技有限公司 | A pesticide composition for controlling pests and diseases and its preparation process |
| CN120817945B (en) * | 2025-09-17 | 2025-12-16 | 山东成欣生物科技有限公司 | A pesticide composition for pest and disease control and its preparation process |
Also Published As
| Publication number | Publication date |
|---|---|
| CN103524422B (en) | 2015-05-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN103524422B (en) | Benzimidazole derivative, and preparation method and purpose thereof | |
| KR102445236B1 (en) | m-diamide-based compound and its preparation and application | |
| WO2021043115A1 (en) | Piperic acid derivative and application thereof | |
| JP7138781B2 (en) | Benzamide compound and use thereof | |
| CN111662269B (en) | 1-pyridyl pyrazole amide compound and preparation method and application thereof | |
| BRPI0712930A2 (en) | pesticide containing pyridyl methanamine derivative or its salt | |
| BRPI0714572A2 (en) | compounds, process for preparing compounds, agricultural composition, process for treating harmful phytopathogenic fungi, use of compounds, methods to combat arthropod pests, to protect crops from attack or infestation by arthropod pests, to protect seed from pest infestation arthropods and from infestation of shoots and seedling roots by arthropod pests, and to protect non-living materials from attack or infestation by arthropod and seed pests | |
| WO2020156512A1 (en) | Aromatic (hetero)cyclic ether compound having insecticidal activity, preparation method therefor, and application thereof | |
| JPH04230670A (en) | Agent for controlling noxious organism | |
| WO2014187298A1 (en) | N-pyridine amide compound, preparation method therefor, and application thereof | |
| CN103483313A (en) | Novel hydrazide derivatives as well as preparation method and application thereof | |
| WO2024061178A1 (en) | Compound containing five-membered heterocycle, preparation method therefor, and application thereof | |
| CN104151309B (en) | Preparation and application containing 1,3,4 thiadiazoles Pyrazole Oxime Esters | |
| CN106349223B (en) | The preparation method and application of the pyrazoles oxime ether compound of the sulfide based structural containing pyrimidine | |
| CN103755700A (en) | Novel pyrazol amides compound and application thereof | |
| CN106432081A (en) | Preparation method and application of pyrazole oxime ether compound containing 4-Cl-3-ethyl-1-methylpyrazole structure | |
| WO2012065568A1 (en) | Azo heterocyclic compound having insecticidal activity, and preparation and applications thereof | |
| CN105693710A (en) | Preparation and application of pyrazole oxime ether compound with 5-(4-fluorophenyl) isoxazole structure | |
| JPH04128275A (en) | N-benzylamides and insecticidal miticide containing the compound as active component | |
| CN111662283B (en) | Imidazopyridine compound and intermediate, preparation method and application thereof | |
| CN109232550B (en) | A kind of compound containing 3-chloro-5-trifluoromethylpyridyl-1,3,4-oxadiazol-2-one and application thereof | |
| CN104892482A (en) | Preparation and application of ester group-containing N substituted aryl pyrrole derivatives | |
| CN116924952A (en) | A kind of sulfur-containing amide compound and its preparation method and application | |
| CN114716408A (en) | Bisamide derivative containing aromatic amide and preparation method and application thereof | |
| CN120172906B (en) | Preparation and application of trifluoroethanethiol heterocyclic derivatives |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| CF01 | Termination of patent right due to non-payment of annual fee | ||
| CF01 | Termination of patent right due to non-payment of annual fee |
Granted publication date: 20150527 Termination date: 20171011 |