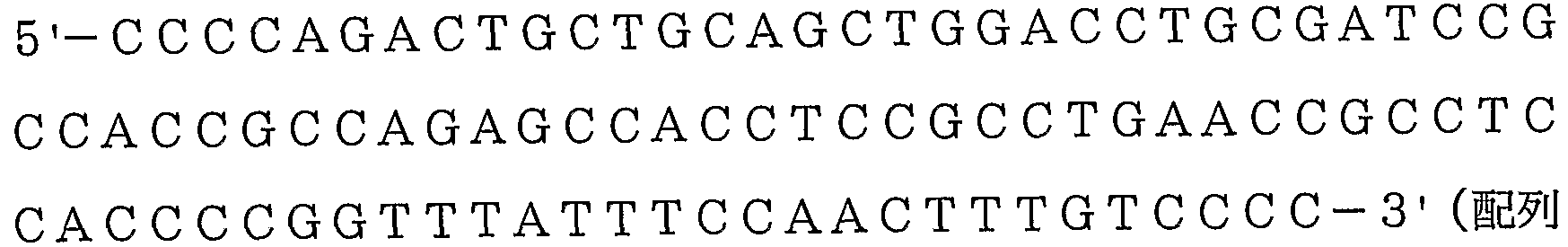WO2004072286A1 - ヒトpd−1に対し特異性を有する物質 - Google Patents
ヒトpd−1に対し特異性を有する物質 Download PDFInfo
- Publication number
- WO2004072286A1 WO2004072286A1 PCT/JP2004/000549 JP2004000549W WO2004072286A1 WO 2004072286 A1 WO2004072286 A1 WO 2004072286A1 JP 2004000549 W JP2004000549 W JP 2004000549W WO 2004072286 A1 WO2004072286 A1 WO 2004072286A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- human
- polypeptide
- antibody
- disease
- fragment
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2818—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD28 or CD152
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/3955—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against proteinaceous materials, e.g. enzymes, hormones, lymphokines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/04—Drugs for disorders of the muscular or neuromuscular system for myasthenia gravis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2809—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against the T-cell receptor (TcR)-CD3 complex
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/10—Cells modified by introduction of foreign genetic material
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P21/00—Preparation of peptides or proteins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/31—Immunoglobulins specific features characterized by aspects of specificity or valency multispecific
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
Definitions
- the present invention relates to the use of a bispecific antibody comprising an antibody having specificity for human PD-1, an antibody against a human T cell receptor complex or a human B cell receptor complex.
- the immune system has acquired a mechanism that can respond to various foreign antigens.
- the mechanism is to bring about diversity of antigen reception by recombination of V (D) J fragments in T cells and B cells.
- V (D) J fragments in T cells and B cells.
- This mechanism resulted in the production of autoreactive lymphocytes at the same time, but these autoreactive lymphocytes were removed by negative selection in the thymus and bone marrow, and also in the periphery, clonal removal was the autoimmune tolerance It is controlled by the mechanism.
- mice that develop autoimmune disease due to single gene deficiency is extremely important for the etiological consideration from the viewpoint of molecular biology.
- CTLA 4 Z—mice that cause lethal systemic lymphocyte infiltration (Science, 1995, 270, 5238, ⁇ ⁇ 985-988, Immunity, 1995, III, 5, p.541-547), SHP—1 deficient mothaten mice (Cell, 1993, 73rd, No.
- PD-1 is a 5 50 type 1 membrane protein belonging to the immunoglobulin family.
- Human PD-1 is composed of 2 8 8 amino acids like mouse PD-1, and has an N-terminal signal peptide (20 amino acids) and a hydrophobic region that is a transmembrane region in the middle (Zambo).
- PD-1 expression is observed in thymocytes upon differentiation from CD 4— CD 8— to CD 4 + CD 8 + cells (Internal Immunology, 1996, 8th). ⁇ , No. 5, pp. 773-780, Journal of Experimental Medicin, 2000, Vol. 191, No. 5, pp. 891-898).
- PD-1 expression in the periphery is activated by stimulation from antigen receptor T cells and B cells (International Immunology, 1996, 8th, 5th, p.765-772) and active bone marrow cells including macrophages.
- PD-1 has ITIM (Immunoreceptor Tyrosine-based Inhibitory Motif) in its intracellular region and is therefore considered a negative regulator of immune responses.
- Lubus-like autoimmune diseases such as glomerulonephritis and arthritis in PD-1-deficient mice (C 5 7 BL / 6 gene background) (international 'immunology' (International Immunology), 1998, No.10, No.10, p.1563 ⁇ : 1572, Immunity, 1999, No.11, No.2, p.141 ⁇ : 151) and PD-1 is autoimmune because it develops dilated cardiomyopathy-like disease (BALB / c gene background) (Science, 2001, Vol.291, No.5502, p.319-322).
- PD-1 is a regulator of various autoimmune diseases and is considered to be one of the causative genes of autoimmune diseases.
- the inventors of the present invention have conducted extensive studies on the assumption that controlling the function of PD-1 can reduce or enhance the immune function, infectious diseases, rejection at the time of transplantation, and tumors. Has arrived at the present invention relating to a substance that controls the function of PD-1.
- TCR T cell receptor
- BCR B cell receptor
- PD-1 negatively regulates various immunocompetent cells such as lymphocytes and myeloid cells in the immune system, and ITIM (immunoreceptor Tyrosine-based Inhibitory Motif) 3 ⁇ 4 in the intracellular region of PD-1
- ITIM immunocompetent cells
- ITIM immunocompetent cell
- ITIM immunocompetent cell
- the present inventors considered that the molecular mechanism of PD-1 inhibitory signaling is the recruitment of phosphatases. Therefore, we came to think that the function of PD-1 can be expressed by positioning PD-1 in the vicinity of TCR and BCR.
- CD3 is a membrane protein expressed in T cells and is one of the complexes that constitute TCR.
- the cDNA encoding the anti-human PD-1 antibody and the cDNA encoding the anti-human TCR antibody were isolated, and the expression vector expressing the fusion protein consisting of the antigen recognition sites of both antibodies.
- the present invention is a.
- a part that recognizes human PD-1 a part that recognizes a membrane protein present in the cell membrane where human PD_1 is expressed, and a substance that has specificity for human PD-1 consisting of a linker,
- the substance recognizing human PD-1 is a substance having specificity for human PD-1 as described in 1 above, wherein the part recognizing human PD-1 is an antibody against human PD-1 or a partial fragment thereof.
- the human PD-1 as described in 1 above comprising an antibody against human PD-1 or a partial fragment thereof, and an antibody against a membrane protein present in a cell membrane expressing human PD-1 or a partial fragment thereof and a linker.
- Antibodies against human PD-1 or partial fragments thereof and T cell receptor complex A bispecific antibody consisting of an antibody against the union or a partial fragment thereof and a linker,
- Bispecific antibody consisting of an antibody against human PD-1 or a partial fragment thereof, an antibody against a B cell receptor complex or a partial fragment thereof and a linker,
- a polypeptide constituting an antibody against human PD-1 which is a polypeptide consisting of the amino acid sequence shown in SEQ ID NO: 2 in a substantially pure form, or a homologue thereof, a fragment thereof or a fragment thereof.
- a polypeptide that constitutes an antibody against human PD-1 and consists of the amino acid sequence shown in SEQ ID NO: 4 in a substantially pure form, a homologue thereof, a fragment thereof, or a homologue thereof. Or a polypeptide comprising an amino acid sequence in which 1 to 10 amino acids of the polypeptide are deleted, substituted and / or added,
- a polypeptide complex comprising the polypeptides described in 1 0 and 1 1 above,
- a polypeptide consisting of the amino acid sequence shown in SEQ ID NO: 11 in substantially pure form, or a homologue thereof, a homologue thereof, a fragment thereof or a fragment thereof, or 1 to 10 amino acids of the polypeptide A polypeptide consisting of an amino acid sequence in which is deleted, substituted and / or added,
- a bispecific antibody according to any one of 7 to 9 above, which is a polypeptide consisting of the amino acid sequence shown in SEQ ID NO: 11 in a substantially pure form or a homologue thereof, a fragment thereof, or Homozygous of the fragment A log, or a polypeptide consisting of an amino acid sequence in which 1 to 10 amino acids of the polypeptide are deleted, substituted and / or added,
- a polynucleotide comprising the base sequence shown in SEQ ID NO: 1, SEQ ID NO: 3 or SEQ ID NO: 9, a homologue thereof or a complementary strand polynucleotide thereof, a fragment thereof or a homologue of the fragment thereof,
- a replication or expression vector comprising the polynucleotide according to 15 or 16 above,
- a method for producing a substance comprising culturing the host cell according to 18 above under conditions for expressing the substance according to any one of 1 to 6 above,
- a method for producing a bispecific antibody comprising culturing the host cell according to 18 above under the conditions for expressing the bispecific antibody according to any of 7 to 9 above,
- a method for producing a polypeptide comprising culturing the host cell according to 18 above under conditions for expressing the polypeptide according to any one of 10 to 14 above,
- composition comprising an amount of the polypeptide described in claim 1 effective in treating and / or preventing a disease given by human PD-1.
- Diseases involving human PD-1 are selected from the group consisting of neurodegenerative diseases, autoimmune diseases, collagen diseases, organ transplant rejection, tumors and infectious diseases 23.
- Neurodegenerative diseases include senile dementia, Al ⁇ heimer's disease, Down's syndrome, Parkinson's disease, Croy's felt'Jakob disease, amyotrophic spinal sclerosis, diabetic neuropathy, Parkinson's syndrome, Huntington's disease, Machadje Cef 23.
- Autoimmune diseases include glomerulonephritis, arthritis, dilated cardiomyopathy-like disease, ulcerative colitis, Sjogren's syndrome, Crohn's disease, systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, dryness, Allergic contact dermatitis, polymyositis, scleroderma, periarteritis nodosa, rheumatic fever, vitiligo vulgaris, insulin-dependent diabetes mellitus, Behcet's disease, Hashimoto's disease, Addison's disease, dermatomyositis, myasthenia gravis , Reiter syndrome, Graves' disease, pernicious anemia, Good Pastaia syndrome, infertility, chronic active hepatitis, pemphigus, autoimmune thrombocytopenic purpura, autoimmune hemolytic anemia and vasculitis
- the part that recognizes human PD-1 in the present invention may be any substance that recognizes human PD_1, such as anti-human PD-1 antibody, anti-human PD-1 antibody fragment, human PD-1 As such, fragments of human PD-1, ligands of human PD-1 (human P DL 1 (Freeman, GJ. 18 others, Journal Experimental Medicine, 2000, 19) No. 7, ⁇ ⁇ 1027 to 1034), human PD-L2, human PD-H3, etc.), fragments thereof, and low-molecular organic compounds.
- human P DL 1 Freeman, GJ. 18 others, Journal Experimental Medicine, 2000, 19
- human PD-L2, human PD-H3, etc. fragments thereof, and low-molecular organic compounds.
- An antibody against human PD_1 or a partial fragment thereof includes a fragment of an anti-human PD-1 antibody or anti-human PD-1 antibody, and is a complete polyclonal or monoclonal antibody, a humanized antibody, a fully human antibody or a shortened version thereof.
- Type eg F (ab) 2 , Fab ', Fab, Fv
- F (ab ′) 2 , Fab ′, Fab, and Fv antibody fragments can be obtained by treating a complete antibody with a protease enzyme and optionally reducing it.
- the cDNA is isolated from the hyperprideoma that produces the antibody, and the antibody or its antibody fragment or antibody fragment is fused with another protein using an expression vector prepared by genetic modification. It can be produced as a protein in the evening.
- the membrane protein present in the cell membrane expressing human PD-1 means the membrane protein expressed on the cell membrane of the same cell as the cell expressing human PD-1, and human PD-1 Any of the membrane proteins present in the cell membranes derived from human-derived primary cultured cells or human cell lines expressing the phenotype are also included. For example, a T cell receptor complex or a B cell receptor complex is preferred.
- the part that recognizes the membrane protein present in the cell membrane expressing human PD-1 is a substance that recognizes the membrane protein expressed on the cell membrane of the same cell as the cell expressing human PD_1.
- antibodies against membrane proteins, fragments of the antibodies, membrane proteins themselves, membrane protein fragments, ligands of membrane proteins, fragments of the ligands, low molecular weight organic compounds that bind to membrane proteins, etc. Can be mentioned.
- the partial fragment is an antibody against a membrane protein expressed on the cell membrane of the same cell as human PD-1 expressing cells or a partial fragment of the antibody, and is a complete polyclonal or monoclonal antibody Any form such as a humanized antibody, a fully human antibody or a truncated form thereof (eg, F (ab ′) 2 , Fab 1 , Fa b, Fv) antibody may be used.
- a T cell receptor complex is a complex composed of at least an ⁇ subunit and a subunit, and a CD 3 composed of an subunit, 5 subunits, a subunit and a subunit. is there.
- the B cell receptor complex is a complex composed of at least membrane-bound immunoglobulin and CD 79 / CD 79/5 subunit.
- the antibody against the T cell receptor complex or a partial fragment thereof may be any antibody that recognizes the T cell receptor complex or a partial fragment thereof, and constitutes a T cell receptor complex. It also includes antibodies to T cell receptors and their respective CD3 subunits, or partial fragments thereof, fully polyclonal or monoclonal antibodies, humanized antibodies, fully human antibodies or their truncated forms (eg F (ab) 2 , Fab ′, Fab, Fv) Any form such as an antibody may be used.
- a monoclonal antibody against CD3 can be produced using an already established hyperpridoma.
- a commercially available anti-CD3 antibody Hiichi CD3 emAb, manufactured by Pharmingen) is also available.
- the antibody against the B cell receptor complex or a partial fragment thereof may be any antibody that recognizes the B cell receptor complex or a partial fragment thereof, and constitutes the B cell receptor complex.
- Antibodies against membrane-bound immunoglobulins and CD 79 subunits or partial fragmentation of the antibodies Any fragment, including fully polyclonal or monoclonal antibodies, humanized antibodies, fully human antibodies or truncated forms thereof (eg, F (ab) 2 , Fab ′, Fab, Fv) antibodies, etc. It may be a form.
- a commercially available anti-BCR antibody can be used.
- an anti-IgG (H + L) polyclonal antibody manufactured by Zymed
- a substance consisting of a part that recognizes human PD-1 and a part that recognizes a membrane protein present in the cell membrane in which human PD_1 is expressed is human PD-1 that is expressed on the cell membrane of the same cell. Means a substance that can bind simultaneously to the extracellular domain of and the extracellular domain of membrane proteins. Specifically, a bispecific protein consisting of an antibody or antibody fragment specific for human PD-1 and an antibody or antibody fragment specific for a membrane protein present in the cell membrane expressing human PD-1. An antibody is mentioned.
- a vice-specific antibody is an asymmetric antibody that has two independent antigen recognition sites with two different antigen specificities.
- the manufacturing method of the bispecific antibody is based on a known chemical method (Nisonoff, A. et al., 1 person, Bios Chemistry and Biophysics.) 1961, volume 90, pp.460-462, Brennan, M. et al., Science (Science), 1985, 299, pp.81-83) are well known.
- two kinds of antibodies are first hydrolyzed by enzymes, then the disulfide bond of the antibody H chain is cleaved with a reducing agent, followed by mixing and reoxidizing dissimilar antibodies.
- a reactive antibody is obtained.
- a crosslinking agent such as glutaraldehyde Ya Karubojiimido been disclosed (JP-2 -1556 JP).
- the peptide linker is preferably 3-12 amino acid residues, but the sequence is not particularly limited (Hudson, PJ., 1 other, Journal of Immunology Medicine, Journal of Immunology Medicine, 1999, No.231, No. 1-2, ⁇ ⁇ 177-: 189).
- a bispecific antibody consisting of an antibody against human PD-1 or a partial fragment thereof and an antibody against a membrane protein present on the cell membrane where human PD-1 is expressed or a partial fragment thereof is expressed on the cell membrane of the same cell. It is an antibody that can bind to the extracellular domain of human PD-1 and the extracellular domain of membrane protein simultaneously.
- a noisy specific antibody can be produced by the following production method.
- Human PD-1 or human-derived membrane protein is used as an immunizing antigen to sensitize animals
- sensitized spleen cells were fusion of sensitized spleen cells with sensitized myeloma cells.
- sensitized antigen human PD-1 or human derived membrane protein
- the obtained anti-human PD-1 antibody and anti-human membrane protein antibody can be prepared by crosslinking with a linker.
- the prepared Fab SH can be prepared by crosslinking with a linker.
- the linker can connect the above-mentioned part that recognizes human PD-1 and the part that recognizes the membrane protein present in the cell membrane where human PD-1 is expressed, maintaining an appropriate distance.
- the linker can connect the above-mentioned part that recognizes human PD-1 and the part that recognizes the membrane protein present in the cell membrane where human PD-1 is expressed, maintaining an appropriate distance.
- peptides, amides and the like can be mentioned.
- linker a commercially available one can be used, and, for example, phenol dimaleimide (manufactured by Aldrich) is available. When both or one of them is a low-molecular-weight organic compound as a substance that recognizes membrane proteins present in the cell membrane where human PD-1 and human PD-1 are expressed,
- human PD-1 or human-derived membrane protein is preferably administered intraperitoneally or in a fat pad to the sensitized animal.
- the sensitized animal is not particularly limited as long as it is an animal from which a monoclonal antibody is generally obtained, such as a mouse or rat. For example, in the case of mice, it is sufficient to administer 10 to 20 antigens at a time.
- Cell fusion in (2) is performed by removing the spleen from the sensitized immunized product immunized in (1) whose antibody titer has increased sufficiently, and suspending the spleen cell suspension according to a conventional method. And then adding polyethylene glycol (preferably PEG4000) at 37 ° C. to the mixture of the obtained splenocytes and myeoma cells derived from the sensitized product.
- polyethylene glycol preferably PEG4000
- Several types of mouse myeloma cells are known, such as P 3 X 63 Ag 8, P 3 / NS 1 / 1—Ag4-1, and SP-2 / 0-Ag-14, all of which are readily available.
- Myeloma cells are useful in HGPRT (hypoxanthine.guanine 'phosphoribosyl' transferase) deficient cell lines that cannot survive in HAT medium (medium containing hypoxanthine, aminopterin and thymidine). Desirably, the cell line itself does not secrete antibodies.
- HGPRT hyperxanthine.guanine 'phosphoribosyl' transferase
- the cell line itself does not secrete antibodies.
- SP-2Z0-Ag-14 is used.
- the resulting cell fusion mixture is then dispensed into a 96 microwell plate at low cell density and cultured in HAT medium. Unfused myeloma cells, myeloma cell-hyperdoma, and unfused spleen cells, and splenocyte-hyperdoma, die in 1 to 2 weeks of culture because the survival conditions are not satisfied. Only hyperidoma with mamma cells grows.
- the screening in (3) is performed by reacting the hybridoma culture supernatant with the immobilized antigen, and quantifying the antibody in the supernatant specifically adsorbed to the antigen using the labeled second antibody. Determine whether it is a hyperprideoma producing antibodies against human PD-1 or human-derived membrane proteins.
- the step (4) is performed by cloning the antibody-producing hyperpridoma according to the soft agar culture method (Monoclonal Antibodies, 1980, 372). In this case, a limiting dilution method can be used.
- the step (5) can be obtained by culturing the cloned hyperpridoma in a normal medium and separating and purifying it from the culture supernatant. To obtain a larger amount of antibody efficiently, the hyperidoma is obtained from a mouse. A method of intraperitoneal administration, growth, and separation and purification from the ascites is used.
- the step (6) can be purified by conventional methods such as salting out, ion exchange chromatography, gel filtration, hydrophobic chromatography, affinity chromatography, etc., but more effectively protein A-Sepharose CL- Affinity chromatography using 4 B (Amersham Biosciences) is used.
- the bispecific antibody of the present invention specifically recognizes human PD-1, it can be used for purification and concentration of human PD-1, such as affinity chromatography.
- the step (7) can be cross-linked by binding, for example, sulfo EMCS (N- (6-maleimidocaproxy) sulfosuccinimide sodium salt) to the amide group or SH (mercapto) group of the antibody as a cross-linking agent.
- sulfo EMCS N- (6-maleimidocaproxy) sulfosuccinimide sodium salt
- SH mercapto
- Pepsin digested Fiab) 2 is the usual method, For example, it can be purified by salting out, ion exchange chromatography, gel filtration, hydrophobic chromatography, affinity chromatography, etc., but more effectively gel using Cefacryl S-200 (Amersham Biosciences) Filtration is used.
- step (9) 2-mercaptoethanol is added to F (ab) 2 and reduced at 30 ° C for 30 minutes.
- Reduced Fab SH can be purified by conventional methods such as salting out, ion exchange chromatography, gel filtration, hydrophobic chromatography, affinity chromatography, etc. More effectively, The gel filtration used is used.
- a linker is bound to the Fab SH fraction of one antibody.
- Any crosslinking agent can be used as long as it can bind to the mercapto (SH) group of Fab SH .
- phenylene maleimide is added and allowed to react at room temperature for 30 minutes.
- the resulting bivalent specific substance can be purified by conventional methods such as salting out, ion exchange chromatography, gel filtration, hydrophobic chromatography, affinity chromatography, etc. For this, gel filtration using Cefacryl S-2200 is used.
- the antibody obtained in the step (6) can be used as it is, or can be appropriately labeled by a conventional method (for example, pyotinylated label, FITC label, etc.).
- a conventional method for example, pyotinylated label, FITC label, etc.
- an enzyme-labeled secondary antibody or a piotinylated antibody the enzyme-labeled streptavidin is added, and then the specific binding between the antibody and the antigen is measured in the presence of a chromophore product. Measure with By using this accessory system, small molecules that specifically recognize PD-1 or membrane proteins can be obtained.
- the process of (12) is the step of obtaining a low molecule obtained when one is an antibody or Fab.
- an appropriate functional group into the antibody, it can be bound to an antibody or Fab.
- a maleimide group is introduced, it can be bound to an antibody or Fab mercapto (SH) group.
- SH Fab mercapto
- each antibody cDNA is isolated from the hybridoma producing monoclonal antibodies against each antigen, and both cDNAs or their cDNA fragments are fused by gene recombination.
- a bispecific antibody can be produced from the host cell.
- a bispecific antibody comprising an antibody against human PD-1 or a partial fragment thereof and an antibody against a membrane protein present in the cell membrane expressing human PD_1 or a partial fragment thereof
- Anti-human PD_1 monoclonal antibody, anti-membrane protein monoclonal antibody antibody gene is isolated from each hyperpridoma producing antibody
- the produced protein can be separated and purified.
- the step (1) comprises a step of isolating RNA from hyperidoma cells and isolating cDNA encoding the antibody gene or a partial peptide thereof.
- total NA total RNA
- mRNA total RNA
- the process of isolating total NA (total RNA) or mRNA from a hyperidoma cell is a known method (hereinafter, unless otherwise specified, Sambrook, J. et al., 2 authors, Molecular Cloning (Molecular Cloning), 1989, Cold Spring Can be performed according to 2 methods from Harbor Laboratory or FMAusubel, Current Protocol (Method described in Current Protocol in Molecular Biology).
- a synthetic DNA primer having a partial base sequence of the antibody protein of the present invention is used as a means for cloning the cDNA encoding the antibody gene of the present invention or a partial peptide thereof.
- Polymerase Chain Reaction Polymerase Chain Reaction; In the following, it is abbreviated as PCR method.
- a DNA fragment that is amplified by or incorporated into an appropriate vector is a DNA fragment or synthetic DNA that encodes part or all of the antibody protein of the present invention. It can be sorted by high pre-labeling with the ones used and labeled.
- the hybridization method can be performed according to a known method.
- the antibody gene can also be amplified directly by using reverse RNA polymerase chain reaction (hereinafter abbreviated as RT-PCR method) using total RNA or mRNA.
- Examples of expression systems (host-vector system) for producing peptides using genetic recombination techniques include expression systems for bacteria, yeast, insect cells and mammalian cells.
- vector systems include plasmids derived from E. coli (eg, PBR322, pBR325, pUC 12, pUC 13), plasmids derived from Bacillus subtilis (eg, pUB 110, pTP 5, pC 194), yeast-derived plasmids (eg, p SH 19, pSH 1 5), bacteriophage such as human phage, retrovirus, vaccinia virus, animal virus such as baculovirus, etc.
- plasmids derived from E. coli eg, PBR322, pBR325, pUC 12, pUC 13
- Bacillus subtilis eg, pUB 110, pTP 5, pC 194
- yeast-derived plasmids eg, p SH 19, pSH 1 5
- bacteriophage such as human phage, retrovirus, vaccinia virus, animal virus such as baculovirus, etc.
- the promoter used in the present invention may be any promoter as long as it is suitable for the host used for gene expression.
- SR promoter, SV40 promoter, LTR promoter, CMV promoter, HSV-TK promoter and the like can be mentioned.
- CMV (cytomegalovirus) promoter, SR promoter and the like are preferable.
- the host is Escherichia coli, trp promoter overnight, lac promoter overnight, recA promoter, human PL promoter overnight, lpp promoter, T7 promoter, etc. are preferable, and the host is Bacillus. If it is a fungus, SP 01 promoter, SP02 promoter, pen nP promoter, etc. are preferred. If the host is yeast, PH05 promoter, PGK promoter, GAP promoter, ADH A promoter is preferred. When the host is an insect cell, a polyhedrin promoter, a P10 promoter, etc. are preferable.
- the expression vector contains an enhancer, a splicing signal, a poly A addition signal, a selection marker, an SV40 replication origin (hereinafter sometimes abbreviated as SV40 ori), and the like.
- a selection marker include dihydrofolate reductase (hereinafter abbreviated as dhfr) gene (methotrexate (MTX) resistance), ampicillin resistance gene (hereinafter abbreviated as Amp r). ), Neomycin resistance gene (hereinafter sometimes abbreviated as Ne or G418 resistance), and the like.
- a signal sequence suitable for the host is added to the N-terminal side of the protein of the present invention.
- the host is Escherichia, PhoA ⁇ signal sequence, OmpA ⁇ signal sequence, etc.
- the host is Bacillus
- the host is yeast, signal sequence, subtilisin ⁇ signal sequence, etc.
- MFa signal sequence, SUC2 signal sequence, etc. if the host is an animal cell, the insulin signal sequence, the signal sequence, antibody signal sequence, antibody molecule signal sequence, etc. It is available.
- a transformant can be produced using the thus constructed vector containing DNA encoding the protein of the present invention.
- the Escherichia bacterium transformed with the expression vector is cultured in an appropriate medium, and the desired peptide can be obtained from the microbial cells.
- a bacterial signal peptide for example, a signal peptide of pe 1B
- the peptide of interest can be secreted during periplasm.
- it can produce fusion proteins with other peptides.
- a cDNA encoding the target protein is used to transform a suitable mammalian cell as a host cell using an appropriate expression vector, and a transformant is appropriately used. By culturing in a suitable medium, the desired peptide is secreted into the culture medium.
- Examples of host cells that can be used include Escherichia bacteria, Bacillus bacteria, yeast, insect cells, insects, and animal cells.
- Specific examples of the genus Escherichia include, for example, Escherichia coli K12, DH1, JM103, JA221, HB101, C600, JM109, DH5, and DH5.
- Examples of Bacillus bacteria include Bacillus subtilis Mill 4.
- yeast include Saccharomyces cerevisiae AH22, AH22R—, NA87-1 1 A DKD-5 D 20B-12, (Saccharomyces pombe) NCYC 19 13, NCYC 2036, Pichiapastoris KM 71, etc. are used.
- Insect cells for example, when the virus is Ac NPV, a larval cell line derived from a night stealer (Spodoptera fragiperdaCell; S f cell), MG 1 cell derived from the midgut of Trichoplusia ni, derived from eggs of Trichoplusia ni High Five TM cells, cells derived from Mamestra brassicae or cells derived from Estigmene acrea are used.
- S f cells include S f 9 cells (ATCCCRL1711), Sf 21 cells (Vaughn, JL, In Vivo, 1977, No.
- CHO cell Chinese hamster cell CHO
- CHO cell dhfr gene-deficient Chinese hamster cell CHO
- CHO (dhf r-) cells Mouse L cells, mouse At T-20, mouse myeloma cells, rat GH3, HEK 293 T cells, human FL cells, etc.
- Insect cells or insects can be transformed, for example, according to the method described in Bio / Technology, 1988, Vol. 6, ⁇ ⁇ 47-55.
- transforming animal cells for example, "Cell engineering separate volume 8 new cell engineering experiment protocol", Shujunsha, 1995, No. 263, Virology, 1973, No. 52, This can be done according to the method described in No. 456.
- the peptide obtained as described above can be purified by usual methods such as salting out, ion exchange chromatography, gel filtration, hydrophobic chromatography, affinity chromatography and the like.
- polypeptide consisting of the amino acid sequence shown in SEQ ID NO: 2, SEQ ID NO: 4 or SEQ ID NO: 11 which is a substantially pure form of the present invention generally means 90% or more of the polypeptide at the time of production, for example, 9 A polypeptide having an amino acid sequence which is 5, 98 or 99% homologous.
- the homologue of the polypeptide consisting of the amino acid sequence shown in SEQ ID NO: 2, SEQ ID NO: 4 or SEQ ID NO: 11 in substantially pure form is generally at least 20 and preferably at least 30 An amino acid sequence that is at least 70%, preferably at least 80% or 90%, more preferably 95% or more homologous in, for example, 40, 60 or 100 consecutive amino acid regions. It has a polypeptide.
- a fragment of the polypeptide consisting of the amino acid sequence shown in SEQ ID NO: 2, SEQ ID NO: 4 or SEQ ID NO: 11 in substantially pure form is at least 10 amino acids, preferably at least 15 amino acids
- polypeptides comprising 20, 25, 30, 40, 50 or 60 amino acid moieties, and their homologues are generally at least 10 amino acids, preferably at least 15 amino acids, for example 20 , 2 5, 3 0, 4 0, 5 0 or 60 series
- a peptide is one or more amino acids in the amino acid sequence (preferably about 1 to 25, preferably about 1 to 10, more preferably a number (1 to 5)).
- a polypeptide consisting of an amino acid sequence in which one or several amino acids of the polypeptide consisting of the amino acid sequence shown in SEQ ID NO: 2, SEQ ID NO: 4 or SEQ ID NO: 11 shown in substantially pure form are Includes an amino acid sequence in which one or more amino acids are substituted in the amino acid sequence (preferably about 1 to 25, preferably about 1 to 10, more preferably several (1 to 5)) It is a polypeptide.
- Peptides are those in which one or more amino acids are inserted into the amino acid sequence (preferably about 1 to 25, preferably about 1 to 10, more preferably a number (1 to 5)).
- the homologue of the polynucleotide consisting of the DNA base sequence shown in SEQ ID NO: 1, SEQ ID NO: 3 or SEQ ID NO: 9 is generally at least 20, preferably at least 30, for example 40, 60 or 1
- a fragment of a polynucleotide comprising the DNA base sequence shown in SEQ ID NO: 1, SEQ ID NO: 3 or SEQ ID NO: 9 is at least 20 bases, preferably at least 30 bases, such as 40, 50, 60 or 10 It includes a polynucleotide consisting of a 0 base moiety.
- the homologue of the fragment is generally at least 20, preferably at least 30, for example 40, 60 or 100 consecutive base sequence regions, at least 70%, preferably Is a polynucleotide that is at least 80 or 90% homologous, more preferably 95% or more homologous.
- DNA that hybridizes with DNA containing the nucleotide sequence shown in SEQ ID NO: 1, SEQ ID NO: 3 or SEQ ID NO: 9 under highly stringent conditions include SEQ ID NO: 1, SEQ ID NO: 3 or SEQ ID NO: 9, respectively.
- Hybridization can be performed by a known method or a method similar thereto, for example, by J. Sambrook, Molecular Cloning 2nd edition, Cold Spring Harbor Labratry (ColdSpring Harbor Labratry). It can be carried out according to the method described.
- the high stringent conditions are, for example, a sodium concentration of about 19 to 40 mM, preferably about 19 to 20 mM, and a temperature of about 50 to 70 ° (preferably about 60 to Indicates conditions at 65 ° C, especially when the sodium concentration is about 19 mM and the temperature is about 65 ° C.
- the substance having specificity for human PD-1 of the present invention is used for the treatment and Z or prevention of the following diseases.
- Substances having specificity for human PD-1 of the present invention include, for example, neurodegenerative diseases (senile dementia, Alzheimer's disease, Down's syndrome, Parkinson's disease, Croy's felt 'Jakob disease, amyotrophic spinal sclerosis, It is useful for the treatment and / or prevention of diseases such as diabetic neuropathy, Parkinson's syndrome, Huntington's disease, Machadojesef's disease, amyotrophic lateral sclerosis, Croy's felt-Jakob disease).
- neurodegenerative diseases senile dementia, Alzheimer's disease, Down's syndrome, Parkinson's disease, Croy's felt 'Jakob disease, amyotrophic spinal sclerosis.
- the substance having specificity for human PD-1 of the present invention is associated with PD-1 and a disease caused by an enhanced immune response, such as an autoimmune disease (glomerulonephritis, arthritis, dilated myocardium).
- an autoimmune disease glomerulonephritis, arthritis, dilated myocardium.
- Symptom-like disease ulcerative colitis, Siegren's syndrome, Crohn's disease, systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, dryness, allergic contact dermatitis, multiple myositis, scleroderma, nodular periarterial Inflammation, rheumatic fever, vitiligo vulgaris, insulin-dependent diabetes mellitus, Behcet's disease, Hashimoto's disease, Addison's disease, dermatomyositis, myasthenia gravis, Reiter's syndrome, Dale's disease, pernicious anemia, Good
- the substance having specificity for human PD-1 of the present invention is collagen disease (systemic eritematodes, rheumatoid arthritis, generalized scleroderma, generalized progressive sclerosis, dermatomyositis, It is useful for the treatment and / or prevention of diseases such as polymyositis, Siegren's syndrome, mixed connective tissue disease, nodular polyarteritis, and rheumatic fever.
- collagen disease systemic eritematodes, rheumatoid arthritis, generalized scleroderma, generalized progressive sclerosis, dermatomyositis.
- the substance having specificity for human PD-1 of the present invention includes organ transplant rejection reaction, allergic disease, and further, PD-1 is involved, and diseases caused by decreased immune reaction, for example, Treatment of diseases such as tumors and infections And / or useful for prevention.
- the substance having specificity for human PD-1 of the present invention is used for the above purpose, it is usually administered systemically or locally in an oral or parenteral form.
- Dosage varies depending on age, weight, symptoms, therapeutic effect, administration method, treatment time, etc., but is usually in the range of O.lm g to 10 O mg per adult per day. Orally administered several times to several times, or parenterally (preferably intravenously administered) once to several times a day in the range of O.Olm g to 3 O mg per adult Or administered intravenously in the range of 1 to 24 hours per day.
- the dosage varies depending on various conditions, and therefore, an amount smaller than the above dosage may be sufficient, or may be necessary beyond the range.
- the compound of the present invention When the compound of the present invention is administered, it is used as a solid preparation for internal use for oral administration, a solution for internal use, and an injection, external preparation, suppository for parenteral administration and the like.
- solid preparations for internal use for oral administration include tablets, pills, capsules, powders, and condyles.
- Capsules include hard capsules and soft capsules.
- one or more active substances are left as they are or excipients (lactose, mannitol, glucose, microcrystalline cellulose, starch, etc.), binders (hydroxypropylcellulose, Polyvinylpyrrolidone, magnesium aluminate metasilicate, etc.), disintegrants (calcium glycol glycolate, etc.), lubricants (magnesium stearate, etc.), stabilizers, solubilizers (glutamic acid, aspartic acid, etc.), etc. They are mixed and formulated according to conventional methods.
- coating agent sucrose, gelatin, hydroxypropylcellulose, hydroxypropylmethyl cell It may be covered with roast sulfate, etc., or may be covered with two or more layers. Also included are capsules of absorbable substances such as gelatin.
- Liquid preparations for internal use for oral administration include pharmaceutically acceptable solutions, suspensions, emulsions, syrups, elixirs and the like.
- a solution one or more active substances are dissolved, suspended or emulsified in a commonly used diluent (purified water, ethanol or a mixture thereof).
- this liquid preparation may contain a wetting agent, a suspending agent, an emulsifier, a sweetening agent, a flavoring agent, a fragrance, a preservative, a buffering agent and the like.
- injections for parenteral administration include solutions, suspensions, emulsions, and solid injections that are used by dissolving or suspending in solutions for use.
- injectables are used by dissolving, suspending or emulsifying one or more active substances in a solvent.
- the solvent include distilled water for injection, physiological saline, vegetable oil, propylene glycol, polyethylene glycol, alcohols such as ethanol, and combinations thereof.
- this injection contains a stabilizer, a solubilizer (glutamate, aspartate, polysorbate 80 (registered trademark), etc.), a suspending agent, an emulsifier, a soothing agent, a buffer, a preservative, etc. You may go out. They are sterilized in the final step or prepared by aseptic manipulation.
- aseptic solid preparations such as lyophilized products can be produced and used by dissolving them in sterilized or sterile distilled water for injection or other solvents before use.
- compositions for parenteral administration include one or more active substances, externally formulated liquids, ointments, coatings, inhalants, sprays, suppositories and vagina Pessari etc. for internal administration are included.
- sprays may contain a buffer that provides isotonicity with stabilizers such as sodium bisulfite, for example, isotonic agents such as sodium chloride, sodium citrate, or citrate. You may contain.
- stabilizers such as sodium bisulfite
- isotonic agents such as sodium chloride, sodium citrate, or citrate. You may contain.
- the production method of the prescription agent is described in detail, for example, in US Pat. Nos. 2868691 and 3095355.
- PD-1 Since PD-1 is involved in the immune reaction, it is involved in the immune reaction by measuring the expression of human PD-1 using the substance having specificity for human PD-1 of the present invention. It can be used for screening of substances to be used.
- the substance having specificity for human PD-1 of the present invention includes a part that recognizes human PD-1, a part that recognizes a membrane protein present in a cell membrane expressing human PD-1, and a linker. Therefore, it is an excellent substance that can specifically recognize human PD-1 and membrane proteins and transmit human PD-1 signals.
- FIG. 1 is a diagram showing the structure of a vice-specific antibody expression vector.
- Figure 2 shows that A and B are X 63 cell surface antigens and (CD 3 (+) / PD) forcibly expressing (CD 3 (—) / PD-1 (+) cells), respectively, of bispecific antibodies.
- -1 (1) Reactivity against human peripheral blood mononuclear cell (PBMC) surface antigens.
- PBMC peripheral blood mononuclear cell
- the filled area of the histogram shows control IgG, and the open area is a graph showing the distribution of P D-1 or CD 3 positive cells.
- FIG. 3 is a graph showing the effect of a vice-specific antibody on the proliferation response of activated human peripheral blood T cells.
- “one” indicates the control IgG addition group
- “BsAb” indicates the bispecific antibody addition group
- the vertical axis indicates the measured absorbance, and indicates P ⁇ 0.05 in the significant difference test.
- J 1 10 High Pridoma cDNA Library and CD 3 Antibody Hyper Pridoma cDNA Library were prepared using Ready-To-Go You-Prime First-Strand Beads (trade name: purchased from Amersham Falmacia).
- cDNA was prepared from (total RNA) by the oligo dT prime method. The operation and procedure were in accordance with the attachment.
- a DNA encoding a bispecific antibody was prepared by ligating the cDNA isolated in Example 1.
- the ligation of the anti-human PD-1 antibody IG heavy chain cDNA (SEQ ID NO: 1) and the anti-human CD3 antibody Ig g light chain cDNA (SEQ ID NO: 7) was linked to the linker No. 1 (SEQ ID NO: 19) and No. 2 ( Fragment 1 was prepared by PCR reaction using SEQ ID NO: 20) and primers No. 1 (SEQ ID NO: 14) and No. 2 (SEQ ID NO: 15) (FIG. 1).
- fragment 1 was prepared by PCR reaction using No. 3 (SEQ ID NO: 16) and No. 4 (SEQ ID NO: 17) (Fig. 1).
- fragment 1 and fragment 2 are linked by linker No. 5, 5 (SEQ ID NO: 23) and ⁇ ⁇ 6 (SEQ ID NO: 24) and primer No. 5 (SEQ ID NO: 18) and ⁇ ⁇ 4 (SEQ ID NO: 22). ) was used to produce fragment 3 (Fig. 1), and its nucleotide sequence was determined (SEQ ID NO: 9).
- Each PCR reaction was performed in two steps. The first PCR reaction was repeated 20 times at 94 ° C for 30 seconds, 40 ° C for 30 seconds, and 72 ° C for 50 seconds. The second PCR reaction was the first PCR reaction. Using the reaction solution as a vertical sample, the temperature operation was repeated 30 times at 94 ° C for 30 seconds, at 50 ° C for 30 seconds, and at 72 ° C for 50 seconds.
- bispecific antibody is IipofectAMNE-plus (trade name: Invito
- the human kidney cell line 293T (ATCC Number: CRL-1 1268) into which J110-CD3scDb-pSec / hygroA was introduced was purchased. After culturing for 4 days after the introduction of the expression vector gene, the culture supernatant was recovered. The culture supernatant was sterilized by filtration through a 0.22 II1 PVDF filter, dialyzed in a 40% PEG 20000 solution, and concentrated. The concentrated supernatant was purified using HiTrapChelatingHP column (trade name: purchased from Amersham Falmacia).
- HiTrapChelatingHP column (trade name: purchased from Amersham Falmacia).
- the confirmation of the activity of the bispecific antibody was evaluated as an effect on the proliferation response of activated human peripheral blood T cells.
- PBMC peripheral blood of healthy humans using a Lymphoprep Tube (trade name: purchased from HYCOMED PHARMA). The operation and procedure were in accordance with the attachment. The separated cells are then lysed with hemolysis buffer (0.8% NH 4 C 1, 0.1% KC0 3 , ImM EDTA), and erythrocytes were hemolyzed. Subsequently, T cells purified using Nylon Fiber Column T (trade name: purchased from Roche) were suspended in a medium (RPMI 1640 medium containing 10% urine fetal serum).
- a medium RPMI 1640 medium containing 10% urine fetal serum
- the bispecific antibody significantly reduced the proliferation activity of activated human peripheral blood T cells.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Medicinal Chemistry (AREA)
- Immunology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Genetics & Genomics (AREA)
- Biomedical Technology (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Neurology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Microbiology (AREA)
- Neurosurgery (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
- Diabetes (AREA)
- Endocrinology (AREA)
- Dermatology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Mycology (AREA)
- Epidemiology (AREA)
- Rheumatology (AREA)
- Hematology (AREA)
- Cardiology (AREA)
- Emergency Medicine (AREA)
Abstract
Description
Claims
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP10176748.1A EP2270051B1 (en) | 2003-01-23 | 2004-01-22 | Antibody specific for human PD-1 and CD3 |
| JP2005504930A JP4532409B2 (ja) | 2003-01-23 | 2004-01-22 | ヒトpd−1に対し特異性を有する物質 |
| EP04704324.5A EP1591527B1 (en) | 2003-01-23 | 2004-01-22 | Substance specific to human pd-1 |
| US10/543,323 US7563869B2 (en) | 2003-01-23 | 2004-01-22 | Substance specific to human PD-1 |
| US12/469,188 US7998479B2 (en) | 2003-01-23 | 2009-05-20 | Substance specific to human PD-1 |
| US13/169,278 US8246955B2 (en) | 2003-01-23 | 2011-06-27 | Substance specific to human PD-1 |
| US13/548,807 US8951518B2 (en) | 2003-01-23 | 2012-07-13 | Substance specific to human PD-1 |
| US14/584,664 US9783609B2 (en) | 2003-01-23 | 2014-12-29 | Substance specific to human PD-1 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2003014793 | 2003-01-23 | ||
| JP2003-14793 | 2003-01-23 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/543,323 A-371-Of-International US7563869B2 (en) | 2003-01-23 | 2004-01-22 | Substance specific to human PD-1 |
| US12/469,188 Continuation US7998479B2 (en) | 2003-01-23 | 2009-05-20 | Substance specific to human PD-1 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2004072286A1 true WO2004072286A1 (ja) | 2004-08-26 |
Family
ID=32866196
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2004/000549 WO2004072286A1 (ja) | 2003-01-23 | 2004-01-22 | ヒトpd−1に対し特異性を有する物質 |
Country Status (6)
| Country | Link |
|---|---|
| US (5) | US7563869B2 (ja) |
| EP (2) | EP2270051B1 (ja) |
| JP (4) | JP4532409B2 (ja) |
| KR (1) | KR20050107399A (ja) |
| ES (1) | ES2729974T3 (ja) |
| WO (1) | WO2004072286A1 (ja) |
Cited By (191)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006121168A1 (en) * | 2005-05-09 | 2006-11-16 | Ono Pharmaceutical Co., Ltd. | Human monoclonal antibodies to programmed death 1(pd-1) and methods for treating cancer using anti-pd-1 antibodies alone or in combination with other immunotherapeutics |
| WO2007122815A1 (ja) * | 2006-04-14 | 2007-11-01 | Ono Pharmaceutical Co., Ltd. | Bir1に対する二価抗体 |
| WO2010027423A2 (en) | 2008-08-25 | 2010-03-11 | Amplimmune, Inc. | Compositions of pd-1 antagonists and methods of use |
| US7989595B2 (en) | 2005-06-20 | 2011-08-02 | Genentech, Inc. | Compositions and methods for the diagnosis and treatment of tumor |
| US20110287533A1 (en) * | 2009-01-30 | 2011-11-24 | Ab Biosciences, Inc. | Novel lowered affinity antibodies and uses therefor |
| WO2013022091A1 (ja) | 2011-08-11 | 2013-02-14 | 小野薬品工業株式会社 | Pd-1アゴニストからなる自己免疫疾患治療剤 |
| US8497072B2 (en) | 2005-11-30 | 2013-07-30 | Abbott Laboratories | Amyloid-beta globulomer antibodies |
| US8691224B2 (en) | 2005-11-30 | 2014-04-08 | Abbvie Inc. | Anti-Aβ globulomer 5F7 antibodies |
| WO2014122271A1 (en) | 2013-02-07 | 2014-08-14 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for predicting the survival time of patients suffering from diffuse large b-cell lymphomas |
| US8877190B2 (en) | 2006-11-30 | 2014-11-04 | Abbvie Inc. | Aβ conformer selective anti-Aβ globulomer monoclonal antibodies |
| US8895004B2 (en) | 2007-02-27 | 2014-11-25 | AbbVie Deutschland GmbH & Co. KG | Method for the treatment of amyloidoses |
| US8987419B2 (en) | 2010-04-15 | 2015-03-24 | AbbVie Deutschland GmbH & Co. KG | Amyloid-beta binding proteins |
| US9062101B2 (en) | 2010-08-14 | 2015-06-23 | AbbVie Deutschland GmbH & Co. KG | Amyloid-beta binding proteins |
| US9067999B1 (en) | 2002-07-03 | 2015-06-30 | Ono Pharmaceutical Co., Ltd. | Immunopotentiative composition |
| WO2015119944A1 (en) | 2014-02-04 | 2015-08-13 | Incyte Corporation | Combination of a pd-1 antagonist and an ido1 inhibitor for treating cancer |
| US9176150B2 (en) | 2003-01-31 | 2015-11-03 | AbbVie Deutschland GmbH & Co. KG | Amyloid beta(1-42) oligomers, derivatives thereof and antibodies thereto, methods of preparation thereof and use thereof |
| WO2015181624A2 (en) | 2014-05-28 | 2015-12-03 | Idenix Pharmaceuticals, Inc | Nucleoside derivatives for the treatment of cancer |
| US9212224B2 (en) | 2012-05-15 | 2015-12-15 | Bristol-Myers Squibb Company | Antibodies that bind PD-L1 and uses thereof |
| US9226958B2 (en) | 2010-10-01 | 2016-01-05 | University Of Georgia Research Foundation, Inc. | Use of Listeria vaccine vectors to reverse vaccine unresponsiveness in parasitically infected individuals |
| US20160075782A1 (en) | 2005-07-01 | 2016-03-17 | E.R. Squibb & Sons, L. L. C. | Human monoclonal antibodies to programmed death ligand 1 (pd-l1) |
| WO2016057898A1 (en) | 2014-10-10 | 2016-04-14 | Idera Pharmaceuticals, Inc. | Treatment of cancer using tlr9 agonist with checkpoint inhibitors |
| WO2016089830A1 (en) | 2014-12-05 | 2016-06-09 | Merck Sharp & Dohme Corp. | Novel tricyclic compounds as inhibitors of mutant idh enzymes |
| WO2016089797A1 (en) | 2014-12-05 | 2016-06-09 | Merck Sharp & Dohme Corp. | Novel tricyclic compounds as inhibitors of mutant idh enzymes |
| WO2016089833A1 (en) | 2014-12-05 | 2016-06-09 | Merck Sharp & Dohme Corp. | Novel tricyclic compounds as inhibitors of mutant idh enzymes |
| WO2016140717A1 (en) | 2015-03-04 | 2016-09-09 | Merck Sharp & Dohme Corp. | Combination of a pd-1 antagonist and a vegfr/fgfr/ret tyrosine kinase inhibitor for treating cancer |
| WO2016141209A1 (en) | 2015-03-04 | 2016-09-09 | Merck Sharp & Dohme Corp. | Combination of a pd-1 antagonist and eribulin for treating cancer |
| US9463227B2 (en) | 2011-03-11 | 2016-10-11 | Advaxis, Inc. | Listeria-based adjuvants |
| WO2016189055A1 (en) | 2015-05-27 | 2016-12-01 | Idenix Pharmaceuticals Llc | Nucleotides for the treatment of cancer |
| WO2016196173A1 (en) | 2015-05-29 | 2016-12-08 | Merck Sharp & Dohme Corp. | Combination of a pd-1 antagonist and cpg-c type oligonucleotide for treating cancer |
| WO2017007700A1 (en) | 2015-07-06 | 2017-01-12 | Iomet Pharma Ltd. | Pharmaceutical compound |
| WO2017026532A1 (ja) * | 2015-08-10 | 2017-02-16 | 国立大学法人富山大学 | 抗原特異的モノクローナル抗体作製方法 |
| WO2017027646A1 (en) | 2015-08-13 | 2017-02-16 | Merck Sharp & Dohme Corp. | Cyclic di-nucleotide compounds as sting agonists |
| WO2017055404A1 (en) | 2015-10-02 | 2017-04-06 | F. Hoffmann-La Roche Ag | Bispecific antibodies specific for pd1 and tim3 |
| WO2017055443A1 (en) | 2015-10-02 | 2017-04-06 | F. Hoffmann-La Roche Ag | Anti-pd1 antibodies and methods of use |
| US9644212B2 (en) | 2008-05-19 | 2017-05-09 | Advaxis, Inc. | Dual delivery system for heterologous antigens |
| US9650639B2 (en) | 2008-05-19 | 2017-05-16 | Advaxis, Inc. | Dual delivery system for heterologous antigens |
| WO2017093933A1 (en) | 2015-12-03 | 2017-06-08 | Glaxosmithkline Intellectual Property Development Limited | Cyclic purine dinucleotides as modulators of sting |
| WO2017098421A1 (en) | 2015-12-08 | 2017-06-15 | Glaxosmithkline Intellectual Property Development Limited | Benzothiadiazine compounds |
| US9683048B2 (en) | 2014-01-24 | 2017-06-20 | Novartis Ag | Antibody molecules to PD-1 and uses thereof |
| WO2017106062A1 (en) | 2015-12-15 | 2017-06-22 | Merck Sharp & Dohme Corp. | Novel compounds as indoleamine 2,3-dioxygenase inhibitors |
| WO2017106061A1 (en) | 2015-12-14 | 2017-06-22 | Macrogenics, Inc. | Bispecific molecules having immunoreactivity with pd-1 and ctla-4, and methods of use thereof |
| WO2017153952A1 (en) | 2016-03-10 | 2017-09-14 | Glaxosmithkline Intellectual Property Development Limited | 5-sulfamoyl-2-hydroxybenzamide derivatives |
| WO2017155981A1 (en) | 2016-03-07 | 2017-09-14 | Massachusetts Institute Of Technology | Protein-chaperoned t-cell vaccines |
| WO2017160754A1 (en) | 2016-03-15 | 2017-09-21 | Mersana Therapeutics,Inc. | Napi2b-targeted antibody-drug conjugates and methods of use thereof |
| WO2017160599A1 (en) | 2016-03-14 | 2017-09-21 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Use of cd300b antagonists to treat sepsis and septic shock |
| WO2017175147A1 (en) | 2016-04-07 | 2017-10-12 | Glaxosmithkline Intellectual Property Development Limited | Heterocyclic amides useful as protein modulators |
| WO2017175156A1 (en) | 2016-04-07 | 2017-10-12 | Glaxosmithkline Intellectual Property Development Limited | Heterocyclic amides useful as protein modulators |
| WO2017191545A1 (en) | 2016-05-05 | 2017-11-09 | Glaxosmithkline Intellectual Property (No.2) Limited | Enhancer of zeste homolog 2 inhibitors |
| EP3243832A1 (en) | 2016-05-13 | 2017-11-15 | F. Hoffmann-La Roche AG | Antigen binding molecules comprising a tnf family ligand trimer and pd1 binding moiety |
| WO2017212425A1 (en) | 2016-06-08 | 2017-12-14 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds as atf4 pathway inhibitors |
| WO2017212423A1 (en) | 2016-06-08 | 2017-12-14 | Glaxosmithkline Intellectual Property Development Limited | Chemcical compounds |
| WO2018015879A1 (en) | 2016-07-20 | 2018-01-25 | Glaxosmithkline Intellectual Property Development Limited | Isoquinoline derivatives as perk inhibitors |
| WO2018027039A1 (en) | 2016-08-03 | 2018-02-08 | Nextcure, Inc. | Compositions and methods for modulating lair signal transduction |
| WO2018037595A1 (ja) * | 2016-08-26 | 2018-03-01 | 哲治 奥野 | 微小血管血流低減剤およびその利用 |
| US9907849B2 (en) | 2014-07-18 | 2018-03-06 | Advaxis, Inc. | Combination of a PD-1 antagonist and a listeria-based vaccine for treating prostate cancer |
| WO2018053434A1 (en) | 2016-09-16 | 2018-03-22 | The Johns Hopkins University | Protein nanocages with enhanced mucus penetration for targeted tissue and intracellular delivery |
| WO2018067423A1 (en) | 2016-10-04 | 2018-04-12 | Merck Sharp & Dohme Corp. | BENZO[b]THIOPHENE COMPOUNDS AS STING AGONISTS |
| WO2018071792A1 (en) | 2016-10-14 | 2018-04-19 | Merck Sharp & Dohme Corp. | Combination of a pd-1 antagonist and eribulin for treating urothelial cancer |
| WO2018091542A1 (en) | 2016-11-21 | 2018-05-24 | Idenix Pharmaceuticals Llc | Cyclic phosphate substituted nucleoside derivatives for the treatment of liver diseases |
| WO2018098269A2 (en) | 2016-11-23 | 2018-05-31 | Mersana Therapeutics, Inc. | Peptide-containing linkers for antibody-drug conjugates |
| WO2018100534A1 (en) | 2016-12-01 | 2018-06-07 | Glaxosmithkline Intellectual Property Development Limited | Combination therapy |
| WO2018100535A1 (en) | 2016-12-01 | 2018-06-07 | Glaxosmithkline Intellectual Property Development Limited | Combination therapy |
| US10016617B2 (en) | 2009-11-11 | 2018-07-10 | The Trustees Of The University Of Pennsylvania | Combination immuno therapy and radiotherapy for the treatment of Her-2-positive cancers |
| US10058599B2 (en) | 2012-03-12 | 2018-08-28 | Advaxis, Inc. | Suppressor cell function inhibition following Listeria vaccine treatment |
| WO2018154520A1 (en) | 2017-02-27 | 2018-08-30 | Glaxosmithkline Intellectual Property Development Limited | Heterocyclic amides as kinase inhibitors |
| US10064898B2 (en) | 2011-03-11 | 2018-09-04 | Advaxis, Inc. | Listeria-based adjuvants |
| WO2018160538A1 (en) | 2017-02-28 | 2018-09-07 | Mersana Therapeutics, Inc. | Combination therapies of her2-targeted antibody-drug conjugates |
| WO2018185043A1 (en) | 2017-04-05 | 2018-10-11 | F. Hoffmann-La Roche Ag | Bispecific antibodies specifically binding to pd1 and lag3 |
| WO2018225033A1 (en) | 2017-06-09 | 2018-12-13 | Glaxosmithkline Intellectual Property Development Limited | Combination therapy |
| WO2018226336A1 (en) | 2017-06-09 | 2018-12-13 | Providence Health & Services - Oregon | Utilization of cd39 and cd103 for identification of human tumor reactive cells for treatment of cancer |
| WO2018225093A1 (en) | 2017-06-07 | 2018-12-13 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds as atf4 pathway inhibitors |
| US10160806B2 (en) | 2014-06-26 | 2018-12-25 | Macrogenics, Inc. | Covalently bonded diabodies having immunoreactivity with PD-1 and LAG-3, and methods of use thereof |
| USRE47194E1 (en) | 2007-05-08 | 2019-01-08 | Genentech, Inc. | Cysteine engineered anti-MUC16 antibodies and antibody drug conjugates |
| WO2019008507A1 (en) | 2017-07-03 | 2019-01-10 | Glaxosmithkline Intellectual Property Development Limited | 2- (4-CHLOROPHENOXY) -N - ((1- (2- (4-CHLOROPHENOXY) ETHYNAZETIDIN-3-YL) METHYL) ACETAMIDE DERIVATIVES AND RELATED COMPOUNDS AS INHIBITORS OF ATF4 FOR THE TREATMENT OF CANCER AND D OTHER DISEASES |
| WO2019008506A1 (en) | 2017-07-03 | 2019-01-10 | Glaxosmithkline Intellectual Property Development Limited | N- (3- (2- (4-CHLOROPHENOXY) ACETAMIDO) BICYCLO [1.1.1] PENTAN-1-YL) -2-CYCLOBUTANE-1-CARBOXAMIDE DERIVATIVES AND RELATED COMPOUNDS AS ATF4 INHIBITORS FOR THE TREATMENT OF CANCER AND OTHER DISEASES |
| WO2019021208A1 (en) | 2017-07-27 | 2019-01-31 | Glaxosmithkline Intellectual Property Development Limited | USEFUL INDAZOLE DERIVATIVES AS PERK INHIBITORS |
| USRE47223E1 (en) | 2005-06-20 | 2019-02-05 | Genentech, Inc. | Compositions and methods for the diagnosis and treatment of tumor |
| WO2019049061A1 (en) | 2017-09-07 | 2019-03-14 | Glaxosmithkline Intellectual Property Development Limited | 5- (1H-BENZO [D] IMIDAZO-2-YL) -PYRIDIN-2-AMINE AND 5- (3H-IMIDAZO [4,5-B] PYRIDIN-6-YL) -PYRIDIN-2- DERIVATIVES AMINE AS HISTONE ACETYLTRANSFERASE INHIBITORS OF C-MYC AND P300 / CBP FOR THE TREATMENT OF CANCER |
| EP3456346A1 (en) | 2015-07-30 | 2019-03-20 | MacroGenics, Inc. | Pd-1 and lag-3 binding molecules and methods of use thereof |
| WO2019053617A1 (en) | 2017-09-12 | 2019-03-21 | Glaxosmithkline Intellectual Property Development Limited | CHEMICAL COMPOUNDS |
| WO2019070047A1 (ja) | 2017-10-06 | 2019-04-11 | 小野薬品工業株式会社 | 二重特異性抗体 |
| WO2019069270A1 (en) | 2017-10-05 | 2019-04-11 | Glaxosmithkline Intellectual Property Development Limited | GENERATOR STIMULATOR MODULATORS (STING) INTERFERON |
| WO2019069269A1 (en) | 2017-10-05 | 2019-04-11 | Glaxosmithkline Intellectual Property Development Limited | INTERFERON GENE STIMULATOR MODULATORS USEFUL IN THE TREATMENT OF HIV |
| WO2019089412A1 (en) | 2017-11-01 | 2019-05-09 | Merck Sharp & Dohme Corp. | Novel substituted tetrahydroquinolin compounds as indoleamine 2,3-dioxygenase (ido) inhibitors |
| WO2019099294A1 (en) | 2017-11-14 | 2019-05-23 | Merck Sharp & Dohme Corp. | Novel substituted biaryl compounds as indoleamine 2,3-dioxygenase (ido) inhibitors |
| WO2019099314A1 (en) | 2017-11-14 | 2019-05-23 | Merck Sharp & Dohme Corp. | Novel substituted biaryl compounds as indoleamine 2,3-dioxygenase (ido) inhibitors |
| WO2019104289A1 (en) | 2017-11-27 | 2019-05-31 | Mersana Therapeutics, Inc. | Pyrrolobenzodiazepine antibody conjugates |
| US10323091B2 (en) | 2015-09-01 | 2019-06-18 | Agenus Inc. | Anti-PD-1 antibodies and methods of use thereof |
| EP3498734A1 (en) | 2014-02-04 | 2019-06-19 | Pfizer Inc | Combination of a pd-1 antagonist and a vegfr inhibitor for treating cancer |
| WO2019126691A1 (en) | 2017-12-21 | 2019-06-27 | Mersana Therapeutics, Inc. | Pyrrolobenzodiazepine antibody conjugates |
| WO2019147670A1 (en) | 2018-01-23 | 2019-08-01 | Nextcure, Inc. | B7-h4 antibodies and methods of use thereof |
| WO2019149716A1 (en) | 2018-01-31 | 2019-08-08 | F. Hoffmann-La Roche Ag | Bispecific antibodies comprising an antigen-binding site binding to lag3 |
| WO2019156199A1 (ja) | 2018-02-09 | 2019-08-15 | 小野薬品工業株式会社 | 二重特異性抗体 |
| WO2019193541A1 (en) | 2018-04-06 | 2019-10-10 | Glaxosmithkline Intellectual Property Development Limited | Bicyclic aromatic ring derivatives of formula (i) as atf4 inhibitors |
| WO2019193540A1 (en) | 2018-04-06 | 2019-10-10 | Glaxosmithkline Intellectual Property Development Limited | Heteroaryl derivatives of formula (i) as atf4 inhibitors |
| WO2019195124A1 (en) | 2018-04-03 | 2019-10-10 | Merck Sharp & Dohme Corp. | Benzothiophenes and related compounds as sting agonists |
| US10472419B2 (en) | 2014-01-31 | 2019-11-12 | Novartis Ag | Antibody molecules to TIM-3 and uses thereof |
| WO2019219820A1 (en) | 2018-05-16 | 2019-11-21 | Ctxt Pty Limited | Substituted condensed thiophenes as modulators of sting |
| WO2019231870A1 (en) | 2018-05-31 | 2019-12-05 | Merck Sharp & Dohme Corp. | Novel substituted [1.1.1] bicyclo compounds as indoleamine 2,3-dioxygenase inhibitors |
| WO2019245890A1 (en) | 2018-06-20 | 2019-12-26 | Merck Sharp & Dohme Corp. | Arginase inhibitors and methods of use |
| WO2020012339A1 (en) | 2018-07-09 | 2020-01-16 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds |
| WO2020023268A1 (en) | 2018-07-24 | 2020-01-30 | Amgen Inc. | Combination of lilrb1/2 pathway inhibitors and pd-1 pathway inhibitors |
| WO2020031107A1 (en) | 2018-08-08 | 2020-02-13 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds |
| US10570204B2 (en) | 2013-09-26 | 2020-02-25 | The Medical College Of Wisconsin, Inc. | Methods for treating hematologic cancers |
| WO2020047345A1 (en) | 2018-08-31 | 2020-03-05 | Yale University | Compositions and methods of using cell-penetrating antibodies in combination with immune checkpoint modulators |
| WO2020044206A1 (en) | 2018-08-29 | 2020-03-05 | Glaxosmithkline Intellectual Property Development Limited | Heterocyclic amides as kinase inhibitors for use in the treatment cancer |
| WO2020055702A1 (en) | 2018-09-13 | 2020-03-19 | Merck Sharp & Dohme Corp. | Combination of pd-1 antagonist and lag3 antagonist for treating non-microsatellite instablity-high/proficient mismatch repair colorectal cancer |
| WO2020086476A1 (en) | 2018-10-22 | 2020-04-30 | Glaxosmithkline Intellectual Property Development Limited | Dosing |
| WO2020092385A1 (en) | 2018-10-29 | 2020-05-07 | Mersana Therapeutics, Inc. | Cysteine engineered antibody-drug conjugates with peptide-containing linkers |
| WO2020092183A1 (en) | 2018-11-01 | 2020-05-07 | Merck Sharp & Dohme Corp. | Novel substituted pyrazole compounds as indoleamine 2,3-dioxygenase inhibitors |
| WO2020096871A1 (en) | 2018-11-06 | 2020-05-14 | Merck Sharp & Dohme Corp. | Novel substituted tricyclic compounds as indoleamine 2,3-dioxygenase inhibitors |
| WO2020102804A2 (en) | 2018-11-16 | 2020-05-22 | Arqule, Inc. | Pharmaceutical combination for treatment of cancer |
| WO2020106558A1 (en) | 2018-11-20 | 2020-05-28 | Merck Sharp & Dohme Corp. | Substituted amino triazolopyrimidine and amino triazolopyrazine adenosine receptor antagonists, pharmaceutical compositions and their use |
| WO2020106560A1 (en) | 2018-11-20 | 2020-05-28 | Merck Sharp & Dohme Corp. | Substituted amino triazolopyrimidine and amino triazolopyrazine adenosine receptor antagonists, pharmaceutical compositions and their use |
| WO2020112581A1 (en) | 2018-11-28 | 2020-06-04 | Merck Sharp & Dohme Corp. | Novel substituted piperazine amide compounds as indoleamine 2, 3-dioxygenase (ido) inhibitors |
| WO2020110056A1 (en) | 2018-11-30 | 2020-06-04 | Glaxosmithkline Intellectual Property Development Limited | Compounds useful in hiv therapy |
| WO2020112700A1 (en) | 2018-11-30 | 2020-06-04 | Merck Sharp & Dohme Corp. | 9-substituted amino triazolo quinazoline derivatives as adenosine receptor antagonists, pharmaceutical compositions and their use |
| WO2020205688A1 (en) | 2019-04-04 | 2020-10-08 | Merck Sharp & Dohme Corp. | Inhibitors of histone deacetylase-3 useful for the treatment of cancer, inflammation, neurodegeneration diseases and diabetes |
| WO2020204152A1 (ja) | 2019-04-04 | 2020-10-08 | 小野薬品工業株式会社 | 二重特異性抗体 |
| US10800846B2 (en) | 2015-02-26 | 2020-10-13 | Merck Patent Gmbh | PD-1/PD-L1 inhibitors for the treatment of cancer |
| WO2020232375A1 (en) | 2019-05-16 | 2020-11-19 | Silicon Swat, Inc. | Oxoacridinyl acetic acid derivatives and methods of use |
| WO2020232378A1 (en) | 2019-05-16 | 2020-11-19 | Silicon Swat, Inc. | Benzo[b][1,8]naphthyridine acetic acid derivatives and methods of use |
| WO2020239558A1 (en) | 2019-05-24 | 2020-12-03 | Pfizer Inc. | Combination therapies using cdk inhibitors |
| US10869924B2 (en) | 2015-06-16 | 2020-12-22 | Merck Patent Gmbh | PD-L1 antagonist combination treatments |
| WO2020260547A1 (en) | 2019-06-27 | 2020-12-30 | Rigontec Gmbh | Design method for optimized rig-i ligands |
| WO2021006199A1 (ja) | 2019-07-05 | 2021-01-14 | 小野薬品工業株式会社 | Pd-1/cd3二重特異性タンパク質による血液がん治療 |
| WO2021009365A1 (en) | 2019-07-18 | 2021-01-21 | Ctxt Pty Limited | Benzothiophene, thienopyridine and thienopyrimidine derivatives for the modulation of sting |
| WO2021009362A1 (en) | 2019-07-18 | 2021-01-21 | Ctxt Pty Limited | Benzothiophene, thienopyridine and thienopyrimidine derivatives for the modulation of sting |
| US10899840B2 (en) | 2014-02-04 | 2021-01-26 | Pfizer Inc. | Combination of a PD-1 antagonist and a 4-1BB agonist for treating cancer |
| WO2021020416A1 (ja) | 2019-07-30 | 2021-02-04 | 小野薬品工業株式会社 | 二重特異性抗体 |
| WO2021020336A1 (ja) | 2019-07-26 | 2021-02-04 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 腫瘍治療用医薬組成物 |
| WO2021025140A1 (ja) | 2019-08-08 | 2021-02-11 | 小野薬品工業株式会社 | 二重特異性タンパク質 |
| WO2021058711A2 (en) | 2019-09-27 | 2021-04-01 | Glaxosmithkline Intellectual Property Development Limited | Antigen binding proteins |
| WO2021083060A1 (zh) | 2019-10-28 | 2021-05-06 | 中国科学院上海药物研究所 | 五元杂环氧代羧酸类化合物及其医药用途 |
| WO2021086909A1 (en) | 2019-10-29 | 2021-05-06 | Eisai R&D Managment Co., Ltd. | Combination of a pd-1 antagonist, a vegfr/fgfr/ret tyrosine kinase inhibitor and a cbp/beta-catenin inhibitor for treating cancer |
| WO2021108025A1 (en) | 2019-11-26 | 2021-06-03 | Massachusetts Institute Of Technology | Cell-based cancer vaccines and cancer therapies |
| WO2021113679A1 (en) | 2019-12-06 | 2021-06-10 | Mersana Therapeutics, Inc. | Dimeric compounds as sting agonists |
| WO2021119753A1 (en) | 2019-12-18 | 2021-06-24 | Ctxt Pty Limited | Compounds |
| US11072653B2 (en) | 2015-06-08 | 2021-07-27 | Macrogenics, Inc. | LAG-3-binding molecules and methods of use thereof |
| WO2021209356A1 (en) | 2020-04-14 | 2021-10-21 | Glaxosmithkline Intellectual Property Development Limited | Combination treatment for cancer |
| WO2021209357A1 (en) | 2020-04-14 | 2021-10-21 | Glaxosmithkline Intellectual Property Development Limited | Combination treatment for cancer involving anti-icos and anti-pd1 antibodies, optionally further involving anti-tim3 antibodies |
| WO2021222188A1 (en) | 2020-04-27 | 2021-11-04 | Seagen Inc. | Anti-cd40 antibody combination treatment for cancer |
| WO2021226003A1 (en) | 2020-05-06 | 2021-11-11 | Merck Sharp & Dohme Corp. | Il4i1 inhibitors and methods of use |
| US11174315B2 (en) | 2015-10-08 | 2021-11-16 | Macrogenics, Inc. | Combination therapy for the treatment of cancer |
| WO2021231350A1 (en) | 2020-05-13 | 2021-11-18 | Massachusetts Institute Of Technology | Compositions of polymeric microdevices and their use in cancer immunotherapy |
| WO2021248065A1 (en) | 2020-06-05 | 2021-12-09 | Theraly Fibrosis, Inc. | Trail compositions with reduced immunogenicity |
| WO2022049526A1 (en) | 2020-09-02 | 2022-03-10 | Pharmabcine Inc. | Combination therapy of a pd-1 antagonist and an antagonist for vegfr-2 for treating patients with cancer |
| US11274154B2 (en) | 2016-10-06 | 2022-03-15 | Pfizer Inc. | Dosing regimen of avelumab for the treatment of cancer |
| US11285131B2 (en) | 2017-08-04 | 2022-03-29 | Merck Sharp & Dohme Corp. | Benzo[b]thiophene STING agonists for cancer treatment |
| US11312772B2 (en) | 2017-08-04 | 2022-04-26 | Merck Sharp & Dohme Corp. | Combinations of PD-1 antagonists and benzo [b] thiophene STING agonists for cancer treatment |
| WO2022092085A1 (ja) | 2020-10-28 | 2022-05-05 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 腫瘍治療用医薬組成物 |
| US11344620B2 (en) | 2014-09-13 | 2022-05-31 | Novartis Ag | Combination therapies |
| WO2022130206A1 (en) | 2020-12-16 | 2022-06-23 | Pfizer Inc. | TGFβr1 INHIBITOR COMBINATION THERAPIES |
| WO2022165403A1 (en) | 2021-02-01 | 2022-08-04 | Yale University | Chemotherapeutic bioadhesive particles with immunostimulatory molecules for cancer treatment |
| US11413331B2 (en) | 2017-04-03 | 2022-08-16 | Hoffmann-La Roche Inc. | Immunoconjugates |
| WO2022181515A1 (ja) * | 2021-02-26 | 2022-09-01 | 国立大学法人京都大学 | Pd-1シグナル阻害剤の併用療法 |
| WO2022185160A1 (en) | 2021-03-02 | 2022-09-09 | Glaxosmithkline Intellectual Property Development Limited | Substituted pyridines as dnmt1 inhibitors |
| US11453697B1 (en) | 2015-08-13 | 2022-09-27 | Merck Sharp & Dohme Llc | Cyclic di-nucleotide compounds as sting agonists |
| WO2022208353A1 (en) | 2021-03-31 | 2022-10-06 | Glaxosmithkline Intellectual Property Development Limited | Antigen binding proteins and combinations thereof |
| US11466047B2 (en) | 2017-05-12 | 2022-10-11 | Merck Sharp & Dohme Llc | Cyclic di-nucleotide compounds as sting agonists |
| WO2022217123A2 (en) | 2021-04-08 | 2022-10-13 | Nurix Therapeutics, Inc. | Combination therapies with cbl-b inhibitor compounds |
| WO2022232333A1 (en) | 2021-04-30 | 2022-11-03 | Merck Sharp & Dohme Llc | Il4i1 inhibitors and methods of use |
| WO2022256534A1 (en) | 2021-06-03 | 2022-12-08 | Synthorx, Inc. | Head and neck cancer combination therapy comprising an il-2 conjugate and pembrolizumab |
| WO2022261018A1 (en) | 2021-06-07 | 2022-12-15 | Providence Health & Services - Oregon | Cxcr5, pd-1, and icos expressing tumor reactive cd4 t cells and their use |
| US11542505B1 (en) | 2018-04-20 | 2023-01-03 | Merck Sharp & Dohme Llc | Substituted RIG-I agonists: compositions and methods thereof |
| US11559504B2 (en) | 2017-06-02 | 2023-01-24 | The Penn State Research Foundation | Ceramide nanoliposomes, compositions and methods of using for immunotherapy |
| WO2023010080A1 (en) | 2021-07-30 | 2023-02-02 | Seagen Inc. | Treatment for cancer |
| WO2023012147A1 (en) | 2021-08-03 | 2023-02-09 | F. Hoffmann-La Roche Ag | Bispecific antibodies and methods of use |
| WO2023057882A1 (en) | 2021-10-05 | 2023-04-13 | Pfizer Inc. | Combinations of azalactam compounds with a pd-1 axis binding antagonist for the treatment of cancer |
| WO2023079428A1 (en) | 2021-11-03 | 2023-05-11 | Pfizer Inc. | Combination therapies using tlr7/8 agonist |
| US11685761B2 (en) | 2017-12-20 | 2023-06-27 | Merck Sharp & Dohme Llc | Cyclic di-nucleotide compounds as sting agonists |
| WO2023122573A1 (en) | 2021-12-20 | 2023-06-29 | Synthorx, Inc. | Head and neck cancer combination therapy comprising an il-2 conjugate and pembrolizumab |
| US11702430B2 (en) | 2018-04-03 | 2023-07-18 | Merck Sharp & Dohme Llc | Aza-benzothiophene compounds as STING agonists |
| WO2023230554A1 (en) | 2022-05-25 | 2023-11-30 | Pfizer Inc. | Combination of a braf inhibitor, an egfr inhibitor, and a pd-1 antagonist for the treatment of braf v600e-mutant, msi-h/dmmr colorectal cancer |
| WO2023230541A1 (en) | 2022-05-27 | 2023-11-30 | Viiv Healthcare Company | Piperazine derivatives useful in hiv therapy |
| WO2024040264A1 (en) | 2022-08-19 | 2024-02-22 | Massachusetts Institute Of Technology | Compositions and methods for targeting dendritic cell lectins |
| WO2024043319A1 (ja) | 2022-08-26 | 2024-02-29 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 抗体薬物複合体 |
| US11939343B2 (en) | 2019-08-02 | 2024-03-26 | Mersana Therapeutics, Inc. | Sting agonist compounds and methods of use |
| US11946094B2 (en) | 2017-12-10 | 2024-04-02 | Augusta University Research Institute, Inc. | Combination therapies and methods of use thereof |
| WO2024081736A2 (en) | 2022-10-11 | 2024-04-18 | Yale University | Compositions and methods of using cell-penetrating antibodies |
| US11993653B2 (en) | 2016-12-07 | 2024-05-28 | Agenus Inc. | Antibodies and methods of use thereof |
| WO2024112867A1 (en) | 2022-11-23 | 2024-05-30 | University Of Georgia Research Foundation, Inc. | Compositions and methods of use thereof for increasing immune responses |
| US12029724B2 (en) | 2016-04-28 | 2024-07-09 | Eisai R&D Management Co., Ltd. | Method for inhibiting tumor growth |
| US12036204B2 (en) | 2019-07-26 | 2024-07-16 | Eisai R&D Management Co., Ltd. | Pharmaceutical composition for treating tumor |
| US12042560B2 (en) | 2009-03-30 | 2024-07-23 | Eisai R&D Management Co., Ltd. | Liposome composition |
| WO2024206357A1 (en) | 2023-03-29 | 2024-10-03 | Merck Sharp & Dohme Llc | Il4i1 inhibitors and methods of use |
| US12152019B2 (en) | 2018-10-17 | 2024-11-26 | Merck Sharp & Dohme Llc | Arylalkyl pyrazole compounds as indoleamine 2,3-dioxygenase inhibitors |
| US12156870B2 (en) | 2020-04-02 | 2024-12-03 | Mersana Therapeutics, Inc. | Antibody drug conjugates comprising sting agonists |
| WO2025049858A1 (en) | 2023-09-01 | 2025-03-06 | Amgen Inc. | Molecules for treatment of cancer |
| US12252535B2 (en) | 2014-03-14 | 2025-03-18 | Novartis Ag | Antibody molecules to LAG-3 and uses thereof |
| US12257340B2 (en) | 2019-10-15 | 2025-03-25 | Agensys, Inc. | Pharmaceutical compositions comprising anti-191P4D12 antibody drug conjugates and methods of use thereof |
Families Citing this family (119)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2392477A1 (en) | 1999-11-30 | 2001-06-07 | Mayo Foundation For Medical Education And Research | B7-h1, a novel immunoregulatory molecule |
| US7030219B2 (en) * | 2000-04-28 | 2006-04-18 | Johns Hopkins University | B7-DC, Dendritic cell co-stimulatory molecules |
| US7432351B1 (en) | 2002-10-04 | 2008-10-07 | Mayo Foundation For Medical Education And Research | B7-H1 variants |
| EP2270051B1 (en) * | 2003-01-23 | 2019-05-15 | Ono Pharmaceutical Co., Ltd. | Antibody specific for human PD-1 and CD3 |
| SI1810026T1 (en) | 2004-10-06 | 2018-08-31 | Mayo Foundation For Medical Education And Research | B7-H1 and PD-1 in the treatment of renal cell carcinoma |
| KR100728962B1 (ko) * | 2004-11-08 | 2007-06-15 | 주식회사 하이닉스반도체 | 지르코늄산화막을 갖는 반도체소자의 캐패시터 및 그 제조방법 |
| KR100670747B1 (ko) | 2005-11-28 | 2007-01-17 | 주식회사 하이닉스반도체 | 반도체소자의 캐패시터 제조 방법 |
| US20090215084A1 (en) * | 2006-01-05 | 2009-08-27 | Mayo Foundation For Medical Education And Research | B7-h1 and b7-h4 in cancer |
| US20100015642A1 (en) * | 2006-01-05 | 2010-01-21 | Kwon Eugene D | B7-h1 and survivin in cancer |
| WO2007124361A2 (en) * | 2006-04-20 | 2007-11-01 | Mayo Foundation For Medical Education And Research | Soluble b7-h1 |
| ME02093B (me) | 2007-06-18 | 2014-04-30 | N V Organon | Antitijela za humani receptor programirane smrti pd-1 |
| WO2009029342A2 (en) | 2007-07-13 | 2009-03-05 | The Johns Hopkins University | B7-dc variants |
| US8454960B2 (en) | 2008-01-03 | 2013-06-04 | The Scripps Research Institute | Multispecific antibody targeting and multivalency through modular recognition domains |
| KR101658247B1 (ko) | 2008-01-03 | 2016-09-22 | 더 스크립스 리서치 인스티튜트 | 모듈 인식 도메인을 통한 항체 표적화 |
| US8557243B2 (en) | 2008-01-03 | 2013-10-15 | The Scripps Research Institute | EFGR antibodies comprising modular recognition domains |
| US8574577B2 (en) | 2008-01-03 | 2013-11-05 | The Scripps Research Institute | VEGF antibodies comprising modular recognition domains |
| US8557242B2 (en) | 2008-01-03 | 2013-10-15 | The Scripps Research Institute | ERBB2 antibodies comprising modular recognition domains |
| US20110020325A1 (en) * | 2008-02-29 | 2011-01-27 | Kwon Eugene D | Methods for reducing granulomatous inflammation |
| BRPI0917891A2 (pt) * | 2008-08-25 | 2015-11-24 | Amplimmune Inc | antagonistas de pd-1 e métodos de utilização dos mesmos |
| CA2738252C (en) * | 2008-09-26 | 2018-05-01 | Dana-Farber Cancer Institute, Inc. | Human anti-pd-1, pd-l1, and pd-l2 antibodies and uses therefor |
| SG2014006878A (en) * | 2008-11-12 | 2014-04-28 | Merck Sharp & Dohme | ßGI-IGG INTRON FOR ENHANCED ANTI-IGF1 R EXPRESSION |
| KR20190069615A (ko) | 2008-12-09 | 2019-06-19 | 제넨테크, 인크. | 항-pd-l1 항체 및 t 세포 기능을 향상시키기 위한 그의 용도 |
| US8563694B2 (en) | 2009-07-31 | 2013-10-22 | Medarex, Inc. | Fully human antibodies to BTLA |
| AU2010319531A1 (en) * | 2009-11-10 | 2012-05-24 | Amgen Inc. | Anti-c-MPL antibodies |
| WO2012009705A1 (en) | 2010-07-15 | 2012-01-19 | Zyngenia, Inc. | Ang-2 binding complexes and uses thereof |
| US11066483B2 (en) | 2010-11-30 | 2021-07-20 | Chugai Seiyaku Kabushiki Kaisha | Cytotoxicity-inducing therapeutic agent |
| CN103429264A (zh) | 2011-03-31 | 2013-12-04 | 默沙东公司 | 针对人程序性死亡受体pd-1的抗体的稳定制剂和有关的治疗 |
| EP2699264B1 (en) | 2011-04-20 | 2018-03-14 | Medlmmune, LLC | Antibodies and other molecules that bind b7-h1 and pd-1 |
| ES2704038T3 (es) | 2011-05-24 | 2019-03-13 | Zyngenia Inc | Complejos multiespecíficos multivalentes y monovalentes y sus usos |
| US9494597B2 (en) | 2012-04-02 | 2016-11-15 | Ab Biosciences, Inc. | Human control antibodies and uses therefor |
| US9302005B2 (en) | 2013-03-14 | 2016-04-05 | Mayo Foundation For Medical Education And Research | Methods and materials for treating cancer |
| EA038918B1 (ru) | 2013-03-15 | 2021-11-09 | Зинджения, Инк. | Пептид, связывающий рецептор эпидермального фактора роста, мультиспецифические комплексы, содержащие пептид и антитела, и их применение |
| SI2992017T1 (sl) | 2013-05-02 | 2021-04-30 | Anaptysbio, Inc. | Protitelesa, usmerjena proti programirani smrti-1 (PD-1) |
| WO2014194293A1 (en) | 2013-05-30 | 2014-12-04 | Amplimmune, Inc. | Improved methods for the selection of patients for pd-1 or b7-h4 targeted therapies, and combination therapies thereof |
| CN107090041B (zh) | 2013-09-13 | 2018-11-16 | 百济神州有限公司 | 抗pd1抗体及其作为治疗剂与诊断剂的用途 |
| US10259875B2 (en) | 2013-10-01 | 2019-04-16 | Mayo Foundation For Medical Education And Research | Methods for treating cancer in patients with elevated levels of BIM |
| SG11201604738TA (en) | 2013-12-12 | 2016-07-28 | Shanghai Hengrui Pharm Co Ltd | Pd-1 antibody, antigen-binding fragment thereof, and medical application thereof |
| CA2936244A1 (en) * | 2014-01-21 | 2015-07-30 | Medimmune, Llc | Compositions and methods for modulating and redirecting immune responses |
| TWI681969B (zh) * | 2014-01-23 | 2020-01-11 | 美商再生元醫藥公司 | 針對pd-1的人類抗體 |
| TWI680138B (zh) | 2014-01-23 | 2019-12-21 | 美商再生元醫藥公司 | 抗pd-l1之人類抗體 |
| US10618963B2 (en) | 2014-03-12 | 2020-04-14 | Yeda Research And Development Co. Ltd | Reducing systemic regulatory T cell levels or activity for treatment of disease and injury of the CNS |
| US9394365B1 (en) | 2014-03-12 | 2016-07-19 | Yeda Research And Development Co., Ltd | Reducing systemic regulatory T cell levels or activity for treatment of alzheimer's disease |
| US10519237B2 (en) | 2014-03-12 | 2019-12-31 | Yeda Research And Development Co. Ltd | Reducing systemic regulatory T cell levels or activity for treatment of disease and injury of the CNS |
| KR102248804B1 (ko) | 2014-03-12 | 2021-05-11 | 예다 리서치 앤드 디벨럽먼트 캄파니 리미티드 | Cns의 질환 및 손상을 치료하기 위한 전신적 조절 t 세포 수준 또는 활성의 감소 |
| WO2015156268A1 (ja) | 2014-04-07 | 2015-10-15 | 中外製薬株式会社 | 免疫活性化抗原結合分子 |
| CA2947157A1 (en) * | 2014-05-13 | 2015-11-19 | Chugai Seiyaku Kabushiki Kaisha | T cell-redirected antigen-binding molecule for cells having immunosuppression function |
| WO2015179654A1 (en) | 2014-05-22 | 2015-11-26 | Mayo Foundation For Medical Education And Research | Distinguishing antagonistic and agonistic anti b7-h1 antibodies |
| TWI687438B (zh) | 2014-07-03 | 2020-03-11 | 英屬開曼群島商百濟神州生物科技有限公司 | 抗pd-l1抗體及其作為治療及診斷之用途 |
| RU2711141C2 (ru) | 2014-07-22 | 2020-01-15 | СиБи ТЕРЕПЬЮТИКС, ИНК. | Антитела против pd-1 |
| EP3171896A4 (en) | 2014-07-23 | 2018-03-21 | Mayo Foundation for Medical Education and Research | Targeting dna-pkcs and b7-h1 to treat cancer |
| CA2957258C (en) | 2014-08-05 | 2023-11-07 | MabQuest SA | Immunological reagents |
| US9982052B2 (en) | 2014-08-05 | 2018-05-29 | MabQuest, SA | Immunological reagents |
| CA2956399A1 (en) | 2014-08-05 | 2016-02-11 | Cb Therapeutics, Inc. | Anti-pd-l1 antibodies |
| CA2955676A1 (en) | 2014-08-25 | 2016-03-03 | Pfizer Inc. | Combination of a pd-1 antagonist and an alk inhibitor for treating cancer |
| EP3215182B1 (en) | 2014-11-05 | 2023-01-04 | The Regents of The University of California | Combination immunotherapy |
| TWI595006B (zh) | 2014-12-09 | 2017-08-11 | 禮納特神經系統科學公司 | 抗pd-1抗體類和使用彼等之方法 |
| US10828353B2 (en) | 2015-01-31 | 2020-11-10 | The Trustees Of The University Of Pennsylvania | Compositions and methods for T cell delivery of therapeutic molecules |
| CA2975362A1 (en) | 2015-02-06 | 2016-08-11 | Navigo Proteins Gmbh | Egfr binding proteins comprising ubiquitin muteins |
| US20180030140A1 (en) * | 2015-02-06 | 2018-02-01 | Navigo Proteins Gmbh | Novel binding proteins comprising a ubiquitin mutein and antibodies or antibody fragments |
| EP3770171A1 (en) | 2015-04-03 | 2021-01-27 | XOMA Technology Ltd. | Treatment of cancer using inhibitors of tgf-beta and pd-1 |
| PT3283508T (pt) | 2015-04-17 | 2021-06-21 | Alpine Immune Sciences Inc | Proteínas imuno modulatórias com afinidades afináveis |
| CA2988602A1 (en) | 2015-06-12 | 2016-12-15 | Macrogenics, Inc. | Combination therapy for the treatment of cancer |
| CA2991976A1 (en) | 2015-07-13 | 2017-01-19 | Cytomx Therapeutics, Inc. | Anti-pd-1 antibodies, activatable anti-pd-1 antibodies, and methods of use thereof |
| BR112018000477A2 (pt) | 2015-07-16 | 2018-09-18 | Navigo Proteins Gmbh | proteína de ligação de imunoglobulina não natural, composição, uso da proteína de ligação de ig não natural, método de purificação de afinidade de imunoglobulinas, método de geração de uma proteína de ligação de imunoglobulina não natural, molécula de ácido nucleico, vetor, célula hospedeira ou um hospedeiro não humano, e método para a produção de uma proteína de ligação de imunoglobulina não natural |
| US10584152B2 (en) | 2015-07-20 | 2020-03-10 | Navigo Proteins Gmbh | Binding proteins based on di-ubiquitin muteins and methods for generation |
| WO2017024465A1 (en) | 2015-08-10 | 2017-02-16 | Innovent Biologics (Suzhou) Co., Ltd. | Pd-1 antibodies |
| US10428145B2 (en) | 2015-09-29 | 2019-10-01 | Celgene Corporation | PD-1 binding proteins and methods of use thereof |
| WO2017075045A2 (en) | 2015-10-30 | 2017-05-04 | Mayo Foundation For Medical Education And Research | Antibodies to b7-h1 |
| WO2017086367A1 (ja) | 2015-11-18 | 2017-05-26 | 中外製薬株式会社 | 免疫抑制機能を有する細胞に対するt細胞リダイレクト抗原結合分子を用いた併用療法 |
| WO2017086419A1 (ja) | 2015-11-18 | 2017-05-26 | 中外製薬株式会社 | 液性免疫応答の増強方法 |
| MD3394103T2 (ro) | 2015-12-22 | 2023-11-30 | Regeneron Pharma | Combinație de anticorpi anti-PD-1 și anticorpi bispecifici anti-CD20/anti-CD3 pentru tratamentul cancerului |
| US11214617B2 (en) | 2016-01-22 | 2022-01-04 | MabQuest SA | Immunological reagents |
| US10294299B2 (en) | 2016-01-22 | 2019-05-21 | MabQuest SA | Immunological reagents |
| CN109310780A (zh) | 2016-05-04 | 2019-02-05 | 纳维格蛋白质有限公司 | 包含肽接头的用于化学部分位点-特异性偶联的靶向化合物 |
| TWI822521B (zh) | 2016-05-13 | 2023-11-11 | 美商再生元醫藥公司 | 藉由投予pd-1抑制劑治療皮膚癌之方法 |
| SG11201811600PA (en) * | 2016-06-30 | 2019-01-30 | Oncorus Inc | Pseudotyped oncolytic viral delivery of therapeutic polypeptides |
| NZ749997A (en) | 2016-07-05 | 2022-11-25 | Beigene Ltd | Combination of a pd-l antagonist and a raf inhibitor for treating cancer |
| US11230576B2 (en) | 2016-08-11 | 2022-01-25 | Navigo Proteins Gmbh | Alkaline stable immunoglobulin-binding proteins |
| EP4353747A3 (en) | 2016-08-19 | 2024-06-26 | BeiGene Switzerland GmbH | Combination of zanubrutinib with an anti-cd20 or an anti-pd-1 antibody for use in treating cancer |
| CN110121352B (zh) | 2016-09-01 | 2020-12-11 | 嵌合体生物工程公司 | Gold优化的car t-细胞 |
| AU2017326329B2 (en) | 2016-09-14 | 2023-10-26 | Abbvie Biotherapeutics Inc. | Anti-pd-1 antibodies and their uses |
| JP2019534859A (ja) * | 2016-09-19 | 2019-12-05 | セルジーン コーポレイション | Pd−1結合タンパク質を使用して白斑を治療する方法 |
| SG11201901950TA (en) | 2016-09-19 | 2019-04-29 | Celgene Corp | Methods of treating immune disorders using pd-1 binding proteins |
| EP4360714A3 (en) | 2016-09-21 | 2024-07-24 | Nextcure, Inc. | Antibodies for siglec-15 and methods of use thereof |
| CN110035769A (zh) | 2016-09-21 | 2019-07-19 | 奈斯科尔公司 | 针对siglec-15的抗体及其使用方法 |
| MA46724A (fr) | 2016-11-01 | 2021-04-21 | Anaptysbio Inc | Anticorps dirigés contre la mort programmée 1 (pd -1) |
| WO2018129559A1 (en) | 2017-01-09 | 2018-07-12 | Tesaro, Inc. | Methods of treating cancer with anti-pd-1 antibodies |
| EP3573989A4 (en) | 2017-01-25 | 2020-11-18 | Beigene, Ltd. | CRYSTALLINE FORMS OF (S) -7- (1- (BUT-2-YNOYL) -PIPERIDINE-4-YL) -2- (4-PHENOXYPHENYL) -4,5,6,7-TETRAHYDROPYRAZOLO [1,5-A ] PYRIMIDINE-3-CARBOXAMIDE, MANUFACTURING AND USES THEREOF |
| CN117442719A (zh) | 2017-02-21 | 2024-01-26 | 瑞泽恩制药公司 | 用于治疗肺癌的抗pd-1抗体 |
| US11603407B2 (en) | 2017-04-06 | 2023-03-14 | Regeneron Pharmaceuticals, Inc. | Stable antibody formulation |
| EP3618871A4 (en) | 2017-05-02 | 2021-01-06 | Merck Sharp & Dohme Corp. | ANTI-LAG3 ANTIBODIES ETCO-FORMULATIONS ANTI-LAG3 ANTIBODIES AND ANTI-PD-1 ANTIBODIES |
| JOP20190260A1 (ar) | 2017-05-02 | 2019-10-31 | Merck Sharp & Dohme | صيغ ثابتة لأجسام مضادة لمستقبل الموت المبرمج 1 (pd-1) وطرق استخدامها |
| JP2020525411A (ja) | 2017-06-26 | 2020-08-27 | ベイジーン リミテッド | 肝細胞癌のための免疫療法 |
| US11021540B2 (en) | 2017-09-07 | 2021-06-01 | Augusta University Research Institute, Inc. | Antibodies to programmed cell death protein 1 |
| US11753458B2 (en) | 2017-10-10 | 2023-09-12 | Alpine Immune Sciences, Inc. | CTLA-4 variant immunomodulatory proteins and uses thereof |
| EP3706804B1 (en) | 2017-11-07 | 2022-02-23 | Navigo Proteins GmbH | Fusion proteins with specificity for ed-b and long serum half-life for diagnosis or treatment of cancer |
| CN111801334B (zh) | 2017-11-29 | 2023-06-09 | 百济神州瑞士有限责任公司 | 使用包含btk抑制剂的组合治疗惰性或侵袭性b-细胞淋巴瘤 |
| JP2021508477A (ja) | 2017-12-29 | 2021-03-11 | オンコラス, インコーポレイテッド | 治療用ポリペプチドの腫瘍溶解性ウイルス送達 |
| US11324774B2 (en) | 2018-01-05 | 2022-05-10 | Augusta University Research Institute, Inc. | Compositions of oral alkaline salts and metabolic acid inducers and uses thereof |
| WO2019169229A1 (en) | 2018-03-01 | 2019-09-06 | Nextcure, Inc. | Klrg1 binding compositions and methods of use thereof |
| US12048745B2 (en) | 2018-05-01 | 2024-07-30 | Augusta University Research Institute, Inc. | Methods for detecting and reversing immune therapy resistance |
| IL280915B1 (en) | 2018-08-30 | 2025-02-01 | Hcw Biologics Inc | Single-chain chimeric polypeptides and uses thereof |
| KR20210143718A (ko) | 2019-01-17 | 2021-11-29 | 조지아 테크 리서치 코포레이션 | 산화된 콜레스테롤을 함유하는 약물 전달 시스템 |
| EP3924521A4 (en) | 2019-02-15 | 2023-03-29 | IncellDx, Inc. | Assaying bladder-associated samples, identifying and treating bladder-associated neoplasia, and kits for use therein |
| RU2731293C1 (ru) | 2019-04-12 | 2020-09-01 | Игорь Петрович Белецкий | Способ получения генно-модифицированных линий клеток натуральных киллеров с нокаутированным геном PD-1 и повышенной экспрессией белков семейства Фактора Некроза Опухолей для иммунотерапии онкологических заболеваний |
| EP3725370A1 (en) | 2019-04-19 | 2020-10-21 | ImmunoBrain Checkpoint, Inc. | Modified anti-pd-l1 antibodies and methods and uses for treating a neurodegenerative disease |
| JP7137696B2 (ja) | 2019-05-14 | 2022-09-14 | プロヴェンション・バイオ・インコーポレイテッド | 1型糖尿病を予防するための方法および組成物 |
| KR20220041080A (ko) | 2019-06-18 | 2022-03-31 | 얀센 사이언시즈 아일랜드 언리미티드 컴퍼니 | B형 간염 바이러스(hbv) 백신 및 항-pd-1 또는 항-pc-l1 항체의 조합 |
| CN114206379A (zh) | 2019-06-18 | 2022-03-18 | 爱尔兰詹森科学公司 | 乙型肝炎病毒(hbv)疫苗和抗pd-1抗体的组合 |
| US11897950B2 (en) | 2019-12-06 | 2024-02-13 | Augusta University Research Institute, Inc. | Osteopontin monoclonal antibodies |
| IL295077A (en) | 2020-02-11 | 2022-09-01 | Hcw Biologics Inc | Methods of treating age-related and inflammatory diseases |
| CN115362169A (zh) | 2020-02-11 | 2022-11-18 | Hcw生物科技公司 | 色谱树脂以及其用途 |
| US20210268022A1 (en) * | 2020-02-11 | 2021-09-02 | HCW Biologics, Inc. | Methods of activating regulatory t cells |
| US12024545B2 (en) | 2020-06-01 | 2024-07-02 | HCW Biologics, Inc. | Methods of treating aging-related disorders |
| WO2021252917A2 (en) | 2020-06-11 | 2021-12-16 | Provention Bio, Inc. | Methods and compositions for preventing type 1 diabetes |
| WO2021263167A2 (en) | 2020-06-26 | 2021-12-30 | Amgen Inc. | Il-10 muteins and fusion proteins thereof |
| WO2022051448A1 (en) | 2020-09-03 | 2022-03-10 | Regeneron Pharmaceuticals, Inc. | Methods of treating cancer pain by administering a pd-1 inhibitor |
| AU2023212994A1 (en) | 2022-01-28 | 2024-08-08 | Georgiamune Inc. | Antibodies to programmed cell death protein 1 that are pd-1 agonists |
| WO2024228167A1 (en) | 2023-05-03 | 2024-11-07 | Iox Therapeutics Inc. | Inkt cell modulator liposomal compositions and methods of use |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001064249A1 (en) * | 2000-02-28 | 2001-09-07 | Chugai Seiyaku Kabushiki Kaisha | Tissue decomposition inhibitor |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| BE555319A (ja) | 1956-03-21 | 1900-01-01 | ||
| US3095355A (en) | 1961-10-12 | 1963-06-25 | Revlon | Aerosol composition |
| US4474893A (en) | 1981-07-01 | 1984-10-02 | The University of Texas System Cancer Center | Recombinant monoclonal antibodies |
| JPH021556A (ja) | 1988-06-09 | 1990-01-05 | Snow Brand Milk Prod Co Ltd | ハイブリッド抗体及びその作製方法 |
| JP3454275B2 (ja) | 1992-06-05 | 2003-10-06 | 佑 本庶 | プログラムされた細胞死に関連した新規なポリペプチドおよびそれをコードするdna |
| JPH07291996A (ja) | 1994-03-01 | 1995-11-07 | Yuu Honshiyo | ヒトにおけるプログラムされた細胞死に関連したポリペプチド、それをコードするdna、そのdnaからなるベクター、そのベクターで形質転換された宿主細胞、そのポリペプチドの抗体、およびそのポリペプチドまたはその抗体を含有する薬学的組成物 |
| US6111079A (en) * | 1995-06-05 | 2000-08-29 | Bionebraska, Inc. | Lead binding polypeptides and nucleotides coding therefore |
| US5932449A (en) * | 1996-02-01 | 1999-08-03 | The United States Of America As Represented By The Secretary Of The Army | Detection of botulinum toxin |
| CZ121599A3 (cs) | 1998-04-09 | 1999-10-13 | Aventis Pharma Deutschland Gmbh | Jednořetězcová molekula vázající několik antigenů, způsob její přípravy a léčivo obsahující tuto molekulu |
| BR0013581A (pt) * | 1999-08-23 | 2002-07-02 | Dana Faber Cancer Inst Inc | Pd-1, um receptor para b7-4, e usos para isto |
| JP3871326B2 (ja) | 2000-11-15 | 2007-01-24 | 小野薬品工業株式会社 | Pd−1欠損マウスおよびその用途 |
| AR036993A1 (es) * | 2001-04-02 | 2004-10-20 | Wyeth Corp | Uso de agentes que modulan la interaccion entre pd-1 y sus ligandos en la submodulacion de respuestas inmunologicas |
| EP1445264B1 (en) * | 2001-07-31 | 2011-09-14 | Ono Pharmaceutical Co., Ltd. | Substance specific to pd-1 |
| EP2270051B1 (en) * | 2003-01-23 | 2019-05-15 | Ono Pharmaceutical Co., Ltd. | Antibody specific for human PD-1 and CD3 |
-
2004
- 2004-01-22 EP EP10176748.1A patent/EP2270051B1/en not_active Expired - Lifetime
- 2004-01-22 JP JP2005504930A patent/JP4532409B2/ja not_active Expired - Lifetime
- 2004-01-22 KR KR1020057013393A patent/KR20050107399A/ko not_active Application Discontinuation
- 2004-01-22 ES ES10176748T patent/ES2729974T3/es not_active Expired - Lifetime
- 2004-01-22 US US10/543,323 patent/US7563869B2/en active Active
- 2004-01-22 EP EP04704324.5A patent/EP1591527B1/en not_active Expired - Lifetime
- 2004-01-22 WO PCT/JP2004/000549 patent/WO2004072286A1/ja active Application Filing
-
2009
- 2009-05-20 US US12/469,188 patent/US7998479B2/en not_active Expired - Lifetime
-
2010
- 2010-04-26 JP JP2010100455A patent/JP2010229134A/ja active Pending
-
2011
- 2011-06-27 US US13/169,278 patent/US8246955B2/en not_active Expired - Fee Related
-
2012
- 2012-07-13 US US13/548,807 patent/US8951518B2/en not_active Expired - Lifetime
- 2012-11-22 JP JP2012256828A patent/JP5892913B2/ja not_active Expired - Lifetime
-
2014
- 2014-12-29 US US14/584,664 patent/US9783609B2/en not_active Expired - Lifetime
-
2015
- 2015-12-08 JP JP2015239678A patent/JP6157574B2/ja not_active Expired - Lifetime
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001064249A1 (en) * | 2000-02-28 | 2001-09-07 | Chugai Seiyaku Kabushiki Kaisha | Tissue decomposition inhibitor |
Non-Patent Citations (5)
| Title |
|---|
| ALT M. ET AL.: "Novel tetravalent and bispecific IgG-like antibody molecules combining single-chain diabodies with the immunoglobulin gamma1 Fc or CH3 region", FEBS LETTERS, vol. 454, 1999, pages 90 - 94, XP002120438 * |
| CARTER L.L. ET AL.: "PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2", EUR. J. IMMUNOL., vol. 32, 2002, pages 634 - 643, XP002980901 * |
| HUDSON P.J. ET AL.: "High avidity scFv multimers; diabodies and triabodies", JOURNAL OF IMMUNOLOGICAL METHODS, vol. 231, 1999, pages 177 - 189, XP004186084 * |
| OZKAYNAK E. ET AL.: "Programmed Death-1 Targeting Can Promote Allograft Survival", THE JOURNAL OF IMMUNOLOGY, vol. 169, 2002, pages 6546 - 6553, XP002980100 * |
| See also references of EP1591527A4 * |
Cited By (306)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9439962B2 (en) | 2002-07-03 | 2016-09-13 | Ono Pharmaceutical Co., Ltd. | Immunopotentiative composition |
| US9073994B2 (en) | 2002-07-03 | 2015-07-07 | Ono Pharmaceutical Co., Ltd. | Immunopotentiative composition |
| US9067999B1 (en) | 2002-07-03 | 2015-06-30 | Ono Pharmaceutical Co., Ltd. | Immunopotentiative composition |
| US9393301B2 (en) | 2002-07-03 | 2016-07-19 | Ono Pharmaceutical Co., Ltd. | Immunopotentiative composition |
| US9402899B2 (en) | 2002-07-03 | 2016-08-02 | Ono Pharmaceutical Co., Ltd. | Immunopotentiative composition |
| US10464976B2 (en) | 2003-01-31 | 2019-11-05 | AbbVie Deutschland GmbH & Co. KG | Amyloid β(1-42) oligomers, derivatives thereof and antibodies thereto, methods of preparation thereof and use thereof |
| US9176150B2 (en) | 2003-01-31 | 2015-11-03 | AbbVie Deutschland GmbH & Co. KG | Amyloid beta(1-42) oligomers, derivatives thereof and antibodies thereto, methods of preparation thereof and use thereof |
| US10441655B2 (en) | 2005-05-09 | 2019-10-15 | Ono Pharmaceutical Co., Ltd. | Monoclonal antibodies to programmed death 1 (PD-1) |
| US9358289B2 (en) | 2005-05-09 | 2016-06-07 | Ono Pharmaceutical Co., Ltd. | Methods for treating cancer using anti-PD-1 antibodies in combination with anti-CTLA-4 antibodies |
| US9084776B2 (en) | 2005-05-09 | 2015-07-21 | E.R. Squibb & Sons, L.L.C. | Methods for treating cancer using anti-PD-1 antibodies |
| JP2017052784A (ja) * | 2005-05-09 | 2017-03-16 | 小野薬品工業株式会社 | Programmed Death 1(PD−1)に対するヒトモノクローナル抗体および抗PD−1抗体単独または他の免疫療法と併用した癌治療方法 |
| WO2006121168A1 (en) * | 2005-05-09 | 2006-11-16 | Ono Pharmaceutical Co., Ltd. | Human monoclonal antibodies to programmed death 1(pd-1) and methods for treating cancer using anti-pd-1 antibodies alone or in combination with other immunotherapeutics |
| JP2009155338A (ja) * | 2005-05-09 | 2009-07-16 | Ono Pharmaceut Co Ltd | ProgrammedDeath1(PD−1)に対するヒトモノクローナル抗体および抗PD−1抗体単独または他の免疫療法と併用した癌治療方法 |
| US9492539B2 (en) | 2005-05-09 | 2016-11-15 | Ono Pharmaceutical Co., Ltd. | Monoclonal antibodies to Programmed Death 1 (PD-1) |
| US9492540B2 (en) | 2005-05-09 | 2016-11-15 | Ono Pharmaceutical Co., Ltd. | Methods for treating cancer using anti-PD-1 antibodies |
| US8779105B2 (en) | 2005-05-09 | 2014-07-15 | Medarex, L.L.C. | Monoclonal antibodies to programmed death 1 (PD-1) |
| JP2012158605A (ja) * | 2005-05-09 | 2012-08-23 | Ono Pharmaceut Co Ltd | ProgrammedDeath1(PD−1)に対するヒトモノクローナル抗体および抗PD−1抗体単独または他の免疫療法と併用した癌治療方法 |
| US8008449B2 (en) | 2005-05-09 | 2011-08-30 | Medarex, Inc. | Human monoclonal antibodies to programmed death 1 (PD-1) and methods for treating cancer using anti-PD-1 antibodies alone or in combination with other immunotherapeutics |
| US9387247B2 (en) | 2005-05-09 | 2016-07-12 | Ono Pharmaceutical Co., Ltd. | Monoclonal antibodies to programmed death 1 (PD-1) |
| US7989595B2 (en) | 2005-06-20 | 2011-08-02 | Genentech, Inc. | Compositions and methods for the diagnosis and treatment of tumor |
| USRE47223E1 (en) | 2005-06-20 | 2019-02-05 | Genentech, Inc. | Compositions and methods for the diagnosis and treatment of tumor |
| US8449883B2 (en) | 2005-06-20 | 2013-05-28 | Genentech, Inc. | Compositions and methods for the diagnosis and treatment of tumor |
| US9580507B2 (en) | 2005-07-01 | 2017-02-28 | E.R. Squibb & Sons, L. L. C. | Human monoclonal antibodies to programmed death ligand 1 (PD-L1) |
| US9580505B2 (en) | 2005-07-01 | 2017-02-28 | E.R. Squibb & Sons, L. L. C. | Human monoclonal antibodies to programmed death ligand 1 (PD-L1) |
| US20160075782A1 (en) | 2005-07-01 | 2016-03-17 | E.R. Squibb & Sons, L. L. C. | Human monoclonal antibodies to programmed death ligand 1 (pd-l1) |
| US10208109B2 (en) | 2005-11-30 | 2019-02-19 | Abbvie Inc. | Monoclonal antibodies against amyloid beta protein and uses thereof |
| US10323084B2 (en) | 2005-11-30 | 2019-06-18 | Abbvie Inc. | Monoclonal antibodies against amyloid beta protein and uses thereof |
| US10538581B2 (en) | 2005-11-30 | 2020-01-21 | Abbvie Inc. | Anti-Aβ globulomer 4D10 antibodies |
| US9540432B2 (en) | 2005-11-30 | 2017-01-10 | AbbVie Deutschland GmbH & Co. KG | Anti-Aβ globulomer 7C6 antibodies |
| US8497072B2 (en) | 2005-11-30 | 2013-07-30 | Abbott Laboratories | Amyloid-beta globulomer antibodies |
| US8691224B2 (en) | 2005-11-30 | 2014-04-08 | Abbvie Inc. | Anti-Aβ globulomer 5F7 antibodies |
| WO2007122815A1 (ja) * | 2006-04-14 | 2007-11-01 | Ono Pharmaceutical Co., Ltd. | Bir1に対する二価抗体 |
| US9394360B2 (en) | 2006-11-30 | 2016-07-19 | Abbvie Inc. | Aβ conformer selective anti-Aβ globulomer monoclonal antibodies |
| US9359430B2 (en) | 2006-11-30 | 2016-06-07 | Abbvie Inc. | Abeta conformer selective anti-Abeta globulomer monoclonal antibodies |
| US9951125B2 (en) | 2006-11-30 | 2018-04-24 | Abbvie Inc. | Aβ conformer selective anti-Aβ globulomer monoclonal antibodies |
| US8877190B2 (en) | 2006-11-30 | 2014-11-04 | Abbvie Inc. | Aβ conformer selective anti-Aβ globulomer monoclonal antibodies |
| US8895004B2 (en) | 2007-02-27 | 2014-11-25 | AbbVie Deutschland GmbH & Co. KG | Method for the treatment of amyloidoses |
| USRE47194E1 (en) | 2007-05-08 | 2019-01-08 | Genentech, Inc. | Cysteine engineered anti-MUC16 antibodies and antibody drug conjugates |
| US9644212B2 (en) | 2008-05-19 | 2017-05-09 | Advaxis, Inc. | Dual delivery system for heterologous antigens |
| US9650639B2 (en) | 2008-05-19 | 2017-05-16 | Advaxis, Inc. | Dual delivery system for heterologous antigens |
| US8609089B2 (en) | 2008-08-25 | 2013-12-17 | Amplimmune, Inc. | Compositions of PD-1 antagonists and methods of use |
| WO2010027423A2 (en) | 2008-08-25 | 2010-03-11 | Amplimmune, Inc. | Compositions of pd-1 antagonists and methods of use |
| EP2927240A1 (en) | 2008-08-25 | 2015-10-07 | Amplimmune, Inc. | Compositions of pd-1 antagonists and methods of use |
| US8709416B2 (en) | 2008-08-25 | 2014-04-29 | Amplimmune, Inc. | Compositions of PD-1 antagonists and methods of use |
| US8530629B2 (en) * | 2009-01-30 | 2013-09-10 | Ab Biosciences, Inc. | Lowered affinity antibodies and uses therefor |
| US20110287533A1 (en) * | 2009-01-30 | 2011-11-24 | Ab Biosciences, Inc. | Novel lowered affinity antibodies and uses therefor |
| US12042560B2 (en) | 2009-03-30 | 2024-07-23 | Eisai R&D Management Co., Ltd. | Liposome composition |
| US10016617B2 (en) | 2009-11-11 | 2018-07-10 | The Trustees Of The University Of Pennsylvania | Combination immuno therapy and radiotherapy for the treatment of Her-2-positive cancers |
| US8987419B2 (en) | 2010-04-15 | 2015-03-24 | AbbVie Deutschland GmbH & Co. KG | Amyloid-beta binding proteins |
| US9822171B2 (en) | 2010-04-15 | 2017-11-21 | AbbVie Deutschland GmbH & Co. KG | Amyloid-beta binding proteins |
| US10047121B2 (en) | 2010-08-14 | 2018-08-14 | AbbVie Deutschland GmbH & Co. KG | Amyloid-beta binding proteins |
| US9062101B2 (en) | 2010-08-14 | 2015-06-23 | AbbVie Deutschland GmbH & Co. KG | Amyloid-beta binding proteins |
| US9943590B2 (en) | 2010-10-01 | 2018-04-17 | The Trustees Of The University Of Pennsylvania | Use of Listeria vaccine vectors to reverse vaccine unresponsiveness in parasitically infected individuals |
| US9226958B2 (en) | 2010-10-01 | 2016-01-05 | University Of Georgia Research Foundation, Inc. | Use of Listeria vaccine vectors to reverse vaccine unresponsiveness in parasitically infected individuals |
| US9463227B2 (en) | 2011-03-11 | 2016-10-11 | Advaxis, Inc. | Listeria-based adjuvants |
| US10064898B2 (en) | 2011-03-11 | 2018-09-04 | Advaxis, Inc. | Listeria-based adjuvants |
| US10647770B2 (en) | 2011-08-11 | 2020-05-12 | Ono Pharmaceutical Co., Ltd. | Therapeutic agent for autoimmune diseases comprising PD-1 agonist |
| EP3939613A1 (en) | 2011-08-11 | 2022-01-19 | ONO Pharmaceutical Co., Ltd. | Therapeutic agent for autoimmune diseases comprising pd-1 agonist |
| US9701749B2 (en) | 2011-08-11 | 2017-07-11 | Ono Pharmaceutical Co., Ltd. | Therapeutic agent for autoimmune diseases comprising PD-1 agonist |
| WO2013022091A1 (ja) | 2011-08-11 | 2013-02-14 | 小野薬品工業株式会社 | Pd-1アゴニストからなる自己免疫疾患治療剤 |
| US10058599B2 (en) | 2012-03-12 | 2018-08-28 | Advaxis, Inc. | Suppressor cell function inhibition following Listeria vaccine treatment |
| US10316090B2 (en) | 2012-05-15 | 2019-06-11 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting PD-1/PD-L1 signaling |
| US10266595B2 (en) | 2012-05-15 | 2019-04-23 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting PD-1/PD-L1 signaling |
| US10584170B2 (en) | 2012-05-15 | 2020-03-10 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting PD-1/PD-L1 signaling |
| US10138299B2 (en) | 2012-05-15 | 2018-11-27 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting PD-1/PD-L1 signaling |
| US10604575B2 (en) | 2012-05-15 | 2020-03-31 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting PD-1/PD-L1 signaling |
| US9212224B2 (en) | 2012-05-15 | 2015-12-15 | Bristol-Myers Squibb Company | Antibodies that bind PD-L1 and uses thereof |
| US10308714B2 (en) | 2012-05-15 | 2019-06-04 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting PD-1/PD-L1 signaling |
| US10323092B2 (en) | 2012-05-15 | 2019-06-18 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting PD-1/PD-L1 signaling |
| US10323093B2 (en) | 2012-05-15 | 2019-06-18 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting PD-1/PD-L1 signaling |
| US10316091B2 (en) | 2012-05-15 | 2019-06-11 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting PD-1/PD-L1 signaling |
| US10266594B1 (en) | 2012-05-15 | 2019-04-23 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting PD-1/PD-L1 signaling |
| US10072082B2 (en) | 2012-05-15 | 2018-09-11 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting PD-1/PD-L1 signaling |
| US10266596B1 (en) | 2012-05-15 | 2019-04-23 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting PD-1/PD-L1 signaling |
| US10577423B2 (en) | 2012-05-15 | 2020-03-03 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting PD-1/PD-L1 signaling |
| US9856320B2 (en) | 2012-05-15 | 2018-01-02 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting PD-1/PD-L1 signaling |
| WO2014122271A1 (en) | 2013-02-07 | 2014-08-14 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for predicting the survival time of patients suffering from diffuse large b-cell lymphomas |
| US10570204B2 (en) | 2013-09-26 | 2020-02-25 | The Medical College Of Wisconsin, Inc. | Methods for treating hematologic cancers |
| US11708412B2 (en) | 2013-09-26 | 2023-07-25 | Novartis Ag | Methods for treating hematologic cancers |
| US11827704B2 (en) | 2014-01-24 | 2023-11-28 | Novartis Ag | Antibody molecules to PD-1 and uses thereof |
| US9815898B2 (en) | 2014-01-24 | 2017-11-14 | Novartis Ag | Antibody molecules to PD-1 and uses thereof |
| US10752687B2 (en) | 2014-01-24 | 2020-08-25 | Novartis Ag | Antibody molecules to PD-1 and uses thereof |
| US9683048B2 (en) | 2014-01-24 | 2017-06-20 | Novartis Ag | Antibody molecules to PD-1 and uses thereof |
| US10981990B2 (en) | 2014-01-31 | 2021-04-20 | Novartis Ag | Antibody molecules to TIM-3 and uses thereof |
| US10472419B2 (en) | 2014-01-31 | 2019-11-12 | Novartis Ag | Antibody molecules to TIM-3 and uses thereof |
| US11155620B2 (en) | 2014-01-31 | 2021-10-26 | Novartis Ag | Method of detecting TIM-3 using antibody molecules to TIM-3 |
| US10899840B2 (en) | 2014-02-04 | 2021-01-26 | Pfizer Inc. | Combination of a PD-1 antagonist and a 4-1BB agonist for treating cancer |
| EP3498734A1 (en) | 2014-02-04 | 2019-06-19 | Pfizer Inc | Combination of a pd-1 antagonist and a vegfr inhibitor for treating cancer |
| US10570202B2 (en) | 2014-02-04 | 2020-02-25 | Pfizer Inc. | Combination of a PD-1 antagonist and a VEGFR inhibitor for treating cancer |
| WO2015119944A1 (en) | 2014-02-04 | 2015-08-13 | Incyte Corporation | Combination of a pd-1 antagonist and an ido1 inhibitor for treating cancer |
| EP3971209A1 (en) | 2014-02-04 | 2022-03-23 | Pfizer Inc. | Combination of a pd-1 antagonist and a vegfr inhibitor for treating cancer |
| US12252535B2 (en) | 2014-03-14 | 2025-03-18 | Novartis Ag | Antibody molecules to LAG-3 and uses thereof |
| WO2015181624A2 (en) | 2014-05-28 | 2015-12-03 | Idenix Pharmaceuticals, Inc | Nucleoside derivatives for the treatment of cancer |
| US11098119B2 (en) | 2014-06-26 | 2021-08-24 | Macrogenics, Inc. | Covalently bonded diabodies having immunoreactivity with PD-1 and LAG-3, and methods of use thereof |
| US10160806B2 (en) | 2014-06-26 | 2018-12-25 | Macrogenics, Inc. | Covalently bonded diabodies having immunoreactivity with PD-1 and LAG-3, and methods of use thereof |
| US9907849B2 (en) | 2014-07-18 | 2018-03-06 | Advaxis, Inc. | Combination of a PD-1 antagonist and a listeria-based vaccine for treating prostate cancer |
| US11344620B2 (en) | 2014-09-13 | 2022-05-31 | Novartis Ag | Combination therapies |
| WO2016057898A1 (en) | 2014-10-10 | 2016-04-14 | Idera Pharmaceuticals, Inc. | Treatment of cancer using tlr9 agonist with checkpoint inhibitors |
| EP4029508A1 (en) | 2014-10-10 | 2022-07-20 | Idera Pharmaceuticals, Inc. | Treatment of cancer using tlr9 agonists and checkpoint inhibitors |
| WO2016089797A1 (en) | 2014-12-05 | 2016-06-09 | Merck Sharp & Dohme Corp. | Novel tricyclic compounds as inhibitors of mutant idh enzymes |
| WO2016089830A1 (en) | 2014-12-05 | 2016-06-09 | Merck Sharp & Dohme Corp. | Novel tricyclic compounds as inhibitors of mutant idh enzymes |
| WO2016089833A1 (en) | 2014-12-05 | 2016-06-09 | Merck Sharp & Dohme Corp. | Novel tricyclic compounds as inhibitors of mutant idh enzymes |
| US10800846B2 (en) | 2015-02-26 | 2020-10-13 | Merck Patent Gmbh | PD-1/PD-L1 inhibitors for the treatment of cancer |
| US12083112B2 (en) | 2015-03-04 | 2024-09-10 | Eisai R&D Management Co., Ltd. | Combination of a PD-1 antagonist and a VEGFR/FGFR/RET tyrosine kinase inhibitor for treating cancer |
| WO2016141209A1 (en) | 2015-03-04 | 2016-09-09 | Merck Sharp & Dohme Corp. | Combination of a pd-1 antagonist and eribulin for treating cancer |
| US10945990B2 (en) | 2015-03-04 | 2021-03-16 | Eisai R&D Management Co., Ltd. | Combination of a PD-1 antagonist and eribulin for treating cancer |
| WO2016141218A1 (en) | 2015-03-04 | 2016-09-09 | Merck Sharp & Dohme Corp. | Combination of a pd-1 antagonist and a vegfr/fgfr/ret tyrosine kinase inhibitor for treating cancer |
| US11547705B2 (en) | 2015-03-04 | 2023-01-10 | Merck Sharp & Dohme Llc | Combination of a PD-1 antagonist and a VEGF-R/FGFR/RET tyrosine kinase inhibitor for treating cancer |
| WO2016140717A1 (en) | 2015-03-04 | 2016-09-09 | Merck Sharp & Dohme Corp. | Combination of a pd-1 antagonist and a vegfr/fgfr/ret tyrosine kinase inhibitor for treating cancer |
| US10815264B2 (en) | 2015-05-27 | 2020-10-27 | Southern Research Institute | Nucleotides for the treatment of cancer |
| WO2016189055A1 (en) | 2015-05-27 | 2016-12-01 | Idenix Pharmaceuticals Llc | Nucleotides for the treatment of cancer |
| US10751412B2 (en) | 2015-05-29 | 2020-08-25 | Merck Sharp & Dohme Corp. | Combination of a PD-1 antagonist and CPG-C type oligonucleotide for treating cancer |
| EP3892284A1 (en) | 2015-05-29 | 2021-10-13 | Merck Sharp & Dohme Corp. | Combination of a pd-1 antagonist and cpg-c type oligonucleotide for treating cancer |
| US11918648B2 (en) | 2015-05-29 | 2024-03-05 | Merck Sharp & Dohme Llc | Combination of a PD-1 antagonist and CpG-C type oligonucleotide for treating cancer |
| WO2016196173A1 (en) | 2015-05-29 | 2016-12-08 | Merck Sharp & Dohme Corp. | Combination of a pd-1 antagonist and cpg-c type oligonucleotide for treating cancer |
| US11072653B2 (en) | 2015-06-08 | 2021-07-27 | Macrogenics, Inc. | LAG-3-binding molecules and methods of use thereof |
| US11858991B2 (en) | 2015-06-08 | 2024-01-02 | Macrogenics, Inc. | LAG-3-binding molecules and methods of use thereof |
| US10869924B2 (en) | 2015-06-16 | 2020-12-22 | Merck Patent Gmbh | PD-L1 antagonist combination treatments |
| WO2017007700A1 (en) | 2015-07-06 | 2017-01-12 | Iomet Pharma Ltd. | Pharmaceutical compound |
| US11623959B2 (en) | 2015-07-30 | 2023-04-11 | Macrogenics, Inc. | PD-1-binding molecules and methods of use thereof |
| EP3456346A1 (en) | 2015-07-30 | 2019-03-20 | MacroGenics, Inc. | Pd-1 and lag-3 binding molecules and methods of use thereof |
| EP3981792A1 (en) | 2015-07-30 | 2022-04-13 | MacroGenics, Inc. | Pd-1-binding molecules and methods of use thereof |
| US10577422B2 (en) | 2015-07-30 | 2020-03-03 | Macrogenics, Inc. | PD-1-binding molecules and methods of use thereof |
| EP4450088A2 (en) | 2015-07-30 | 2024-10-23 | MacroGenics, Inc. | Pd-1-binding molecules and methods of use thereof |
| US10416165B2 (en) | 2015-08-10 | 2019-09-17 | National University Corporation University Of Toyama | Method for producing antigen specific monoclonal antibody |
| WO2017026532A1 (ja) * | 2015-08-10 | 2017-02-16 | 国立大学法人富山大学 | 抗原特異的モノクローナル抗体作製方法 |
| US10766919B2 (en) | 2015-08-13 | 2020-09-08 | Merck Sharp & Dohme Corp. | Cyclic di-nucleotide compounds as sting agonists |
| WO2017027645A1 (en) | 2015-08-13 | 2017-02-16 | Merck Sharp & Dohme Corp. | Cyclic di-nucleotide compounds as sting agonists |
| WO2017027646A1 (en) | 2015-08-13 | 2017-02-16 | Merck Sharp & Dohme Corp. | Cyclic di-nucleotide compounds as sting agonists |
| US11453697B1 (en) | 2015-08-13 | 2022-09-27 | Merck Sharp & Dohme Llc | Cyclic di-nucleotide compounds as sting agonists |
| US10106574B2 (en) | 2015-08-13 | 2018-10-23 | Merck Sharp & Dohme Corp. | Cyclic di-nucleotide compounds as sting agonists |
| US10759825B2 (en) | 2015-08-13 | 2020-09-01 | Merck Sharp & Dohme Corp. | Cyclic di-nucleotide compounds as STING agonists |
| US10738074B2 (en) | 2015-08-13 | 2020-08-11 | Merck Sharp & Dohme Corp. | Cyclic di-nucleotide compounds as STING agonists |
| US11345755B2 (en) | 2015-09-01 | 2022-05-31 | Agenus Inc. | Anti-PD-1 antibodies and methods of use thereof |
| US10450373B2 (en) | 2015-09-01 | 2019-10-22 | Agenus Inc. | Anti-PD-1 antibodies and methods of use thereof |
| US10323091B2 (en) | 2015-09-01 | 2019-06-18 | Agenus Inc. | Anti-PD-1 antibodies and methods of use thereof |
| WO2017055443A1 (en) | 2015-10-02 | 2017-04-06 | F. Hoffmann-La Roche Ag | Anti-pd1 antibodies and methods of use |
| US10287352B2 (en) | 2015-10-02 | 2019-05-14 | Hoffman-La Roche Inc. | Bispecific antibodies specific for PD1 and TIM3 |
| WO2017055404A1 (en) | 2015-10-02 | 2017-04-06 | F. Hoffmann-La Roche Ag | Bispecific antibodies specific for pd1 and tim3 |
| US11130810B2 (en) | 2015-10-02 | 2021-09-28 | Hoffmann-La Roche Inc. | Bispecific antibodies specific for PD1 and TIM3 |
| US11174315B2 (en) | 2015-10-08 | 2021-11-16 | Macrogenics, Inc. | Combination therapy for the treatment of cancer |
| WO2017093933A1 (en) | 2015-12-03 | 2017-06-08 | Glaxosmithkline Intellectual Property Development Limited | Cyclic purine dinucleotides as modulators of sting |
| EP3366691A1 (en) | 2015-12-03 | 2018-08-29 | GlaxoSmithKline Intellectual Property Development Limited | Cyclic purine dinucleotides as modulators of sting |
| WO2017098421A1 (en) | 2015-12-08 | 2017-06-15 | Glaxosmithkline Intellectual Property Development Limited | Benzothiadiazine compounds |
| US11840571B2 (en) | 2015-12-14 | 2023-12-12 | Macrogenics, Inc. | Methods of using bispecific molecules having immunoreactivity with PD-1 and CTLA-4 |
| WO2017106061A1 (en) | 2015-12-14 | 2017-06-22 | Macrogenics, Inc. | Bispecific molecules having immunoreactivity with pd-1 and ctla-4, and methods of use thereof |
| US10954301B2 (en) | 2015-12-14 | 2021-03-23 | Macrogenics, Inc. | Bispecific molecules having immunoreactivity with PD-1 and CTLA-4, and methods of use thereof |
| WO2017106062A1 (en) | 2015-12-15 | 2017-06-22 | Merck Sharp & Dohme Corp. | Novel compounds as indoleamine 2,3-dioxygenase inhibitors |
| WO2017155981A1 (en) | 2016-03-07 | 2017-09-14 | Massachusetts Institute Of Technology | Protein-chaperoned t-cell vaccines |
| WO2017153952A1 (en) | 2016-03-10 | 2017-09-14 | Glaxosmithkline Intellectual Property Development Limited | 5-sulfamoyl-2-hydroxybenzamide derivatives |
| WO2017160599A1 (en) | 2016-03-14 | 2017-09-21 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Use of cd300b antagonists to treat sepsis and septic shock |
| WO2017160754A1 (en) | 2016-03-15 | 2017-09-21 | Mersana Therapeutics,Inc. | Napi2b-targeted antibody-drug conjugates and methods of use thereof |
| EP4302782A2 (en) | 2016-03-15 | 2024-01-10 | Mersana Therapeutics, Inc. | Napi2b-targeted antibody-drug conjugates and methods of use thereof |
| WO2017175147A1 (en) | 2016-04-07 | 2017-10-12 | Glaxosmithkline Intellectual Property Development Limited | Heterocyclic amides useful as protein modulators |
| EP4032885A1 (en) | 2016-04-07 | 2022-07-27 | GlaxoSmithKline Intellectual Property Development Limited | Heterocyclic amides useful as protein modulators |
| WO2017175156A1 (en) | 2016-04-07 | 2017-10-12 | Glaxosmithkline Intellectual Property Development Limited | Heterocyclic amides useful as protein modulators |
| US12029724B2 (en) | 2016-04-28 | 2024-07-09 | Eisai R&D Management Co., Ltd. | Method for inhibiting tumor growth |
| WO2017191545A1 (en) | 2016-05-05 | 2017-11-09 | Glaxosmithkline Intellectual Property (No.2) Limited | Enhancer of zeste homolog 2 inhibitors |
| EP3243832A1 (en) | 2016-05-13 | 2017-11-15 | F. Hoffmann-La Roche AG | Antigen binding molecules comprising a tnf family ligand trimer and pd1 binding moiety |
| WO2017212425A1 (en) | 2016-06-08 | 2017-12-14 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds as atf4 pathway inhibitors |
| WO2017212423A1 (en) | 2016-06-08 | 2017-12-14 | Glaxosmithkline Intellectual Property Development Limited | Chemcical compounds |
| WO2018015879A1 (en) | 2016-07-20 | 2018-01-25 | Glaxosmithkline Intellectual Property Development Limited | Isoquinoline derivatives as perk inhibitors |
| WO2018027039A1 (en) | 2016-08-03 | 2018-02-08 | Nextcure, Inc. | Compositions and methods for modulating lair signal transduction |
| WO2018037595A1 (ja) * | 2016-08-26 | 2018-03-01 | 哲治 奥野 | 微小血管血流低減剤およびその利用 |
| US11382953B2 (en) | 2016-08-26 | 2022-07-12 | Tetsuji Okuno | Microvascular blood flow decreasing agent and use thereof |
| JPWO2018037595A1 (ja) * | 2016-08-26 | 2019-06-20 | 哲治 奥野 | 微小血管血流低減剤およびその利用 |
| US11090391B2 (en) | 2016-09-16 | 2021-08-17 | The Johns Hopkins University | Protein nanocages with enhanced mucus penetration for targeted tissue and intracellular delivery |
| WO2018053434A1 (en) | 2016-09-16 | 2018-03-22 | The Johns Hopkins University | Protein nanocages with enhanced mucus penetration for targeted tissue and intracellular delivery |
| WO2018067423A1 (en) | 2016-10-04 | 2018-04-12 | Merck Sharp & Dohme Corp. | BENZO[b]THIOPHENE COMPOUNDS AS STING AGONISTS |
| US10730849B2 (en) | 2016-10-04 | 2020-08-04 | Merck Sharp & Dohme Corp. | Benzo[b]thiophene compounds as STING agonists |
| US10414747B2 (en) | 2016-10-04 | 2019-09-17 | Merck Sharp & Dohme Corp. | Benzo[b]thiophene compounds as sting agonists |
| US10703738B2 (en) | 2016-10-04 | 2020-07-07 | Merck Sharp & Dohme Corp. | Benzo[b]thiophene compounds as STING agonists |
| US11274154B2 (en) | 2016-10-06 | 2022-03-15 | Pfizer Inc. | Dosing regimen of avelumab for the treatment of cancer |
| WO2018071792A1 (en) | 2016-10-14 | 2018-04-19 | Merck Sharp & Dohme Corp. | Combination of a pd-1 antagonist and eribulin for treating urothelial cancer |
| WO2018091542A1 (en) | 2016-11-21 | 2018-05-24 | Idenix Pharmaceuticals Llc | Cyclic phosphate substituted nucleoside derivatives for the treatment of liver diseases |
| US11730748B2 (en) | 2016-11-21 | 2023-08-22 | Msd International Gmbh | Cyclic phosphate substituted nucleoside derivatives for the treatment of liver diseases |
| WO2018098269A2 (en) | 2016-11-23 | 2018-05-31 | Mersana Therapeutics, Inc. | Peptide-containing linkers for antibody-drug conjugates |
| WO2018100535A1 (en) | 2016-12-01 | 2018-06-07 | Glaxosmithkline Intellectual Property Development Limited | Combination therapy |
| WO2018100534A1 (en) | 2016-12-01 | 2018-06-07 | Glaxosmithkline Intellectual Property Development Limited | Combination therapy |
| US11993653B2 (en) | 2016-12-07 | 2024-05-28 | Agenus Inc. | Antibodies and methods of use thereof |
| WO2018154520A1 (en) | 2017-02-27 | 2018-08-30 | Glaxosmithkline Intellectual Property Development Limited | Heterocyclic amides as kinase inhibitors |
| WO2018160538A1 (en) | 2017-02-28 | 2018-09-07 | Mersana Therapeutics, Inc. | Combination therapies of her2-targeted antibody-drug conjugates |
| US11413331B2 (en) | 2017-04-03 | 2022-08-16 | Hoffmann-La Roche Inc. | Immunoconjugates |
| US12023368B2 (en) | 2017-04-03 | 2024-07-02 | Hoffmann-La Roche Inc. | Immunoconjugates |
| WO2018185043A1 (en) | 2017-04-05 | 2018-10-11 | F. Hoffmann-La Roche Ag | Bispecific antibodies specifically binding to pd1 and lag3 |
| US11285207B2 (en) | 2017-04-05 | 2022-03-29 | Hoffmann-La Roche Inc. | Bispecific antibodies specifically binding to PD1 and LAG3 |
| US11466047B2 (en) | 2017-05-12 | 2022-10-11 | Merck Sharp & Dohme Llc | Cyclic di-nucleotide compounds as sting agonists |
| US11559504B2 (en) | 2017-06-02 | 2023-01-24 | The Penn State Research Foundation | Ceramide nanoliposomes, compositions and methods of using for immunotherapy |
| WO2018225093A1 (en) | 2017-06-07 | 2018-12-13 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds as atf4 pathway inhibitors |
| WO2018226336A1 (en) | 2017-06-09 | 2018-12-13 | Providence Health & Services - Oregon | Utilization of cd39 and cd103 for identification of human tumor reactive cells for treatment of cancer |
| EP4461825A2 (en) | 2017-06-09 | 2024-11-13 | Providence Health & Services - Oregon | Tumor-infiltrating t-cells for use in the treatment of cancer |
| WO2018225033A1 (en) | 2017-06-09 | 2018-12-13 | Glaxosmithkline Intellectual Property Development Limited | Combination therapy |
| WO2019008507A1 (en) | 2017-07-03 | 2019-01-10 | Glaxosmithkline Intellectual Property Development Limited | 2- (4-CHLOROPHENOXY) -N - ((1- (2- (4-CHLOROPHENOXY) ETHYNAZETIDIN-3-YL) METHYL) ACETAMIDE DERIVATIVES AND RELATED COMPOUNDS AS INHIBITORS OF ATF4 FOR THE TREATMENT OF CANCER AND D OTHER DISEASES |
| WO2019008506A1 (en) | 2017-07-03 | 2019-01-10 | Glaxosmithkline Intellectual Property Development Limited | N- (3- (2- (4-CHLOROPHENOXY) ACETAMIDO) BICYCLO [1.1.1] PENTAN-1-YL) -2-CYCLOBUTANE-1-CARBOXAMIDE DERIVATIVES AND RELATED COMPOUNDS AS ATF4 INHIBITORS FOR THE TREATMENT OF CANCER AND OTHER DISEASES |
| WO2019021208A1 (en) | 2017-07-27 | 2019-01-31 | Glaxosmithkline Intellectual Property Development Limited | USEFUL INDAZOLE DERIVATIVES AS PERK INHIBITORS |
| US11312772B2 (en) | 2017-08-04 | 2022-04-26 | Merck Sharp & Dohme Corp. | Combinations of PD-1 antagonists and benzo [b] thiophene STING agonists for cancer treatment |
| US11285131B2 (en) | 2017-08-04 | 2022-03-29 | Merck Sharp & Dohme Corp. | Benzo[b]thiophene STING agonists for cancer treatment |
| WO2019049061A1 (en) | 2017-09-07 | 2019-03-14 | Glaxosmithkline Intellectual Property Development Limited | 5- (1H-BENZO [D] IMIDAZO-2-YL) -PYRIDIN-2-AMINE AND 5- (3H-IMIDAZO [4,5-B] PYRIDIN-6-YL) -PYRIDIN-2- DERIVATIVES AMINE AS HISTONE ACETYLTRANSFERASE INHIBITORS OF C-MYC AND P300 / CBP FOR THE TREATMENT OF CANCER |
| WO2019053617A1 (en) | 2017-09-12 | 2019-03-21 | Glaxosmithkline Intellectual Property Development Limited | CHEMICAL COMPOUNDS |
| WO2019069269A1 (en) | 2017-10-05 | 2019-04-11 | Glaxosmithkline Intellectual Property Development Limited | INTERFERON GENE STIMULATOR MODULATORS USEFUL IN THE TREATMENT OF HIV |
| WO2019069270A1 (en) | 2017-10-05 | 2019-04-11 | Glaxosmithkline Intellectual Property Development Limited | GENERATOR STIMULATOR MODULATORS (STING) INTERFERON |
| JP7230819B2 (ja) | 2017-10-06 | 2023-03-01 | 小野薬品工業株式会社 | 二重特異性抗体 |
| JPWO2019070047A1 (ja) * | 2017-10-06 | 2020-10-22 | 小野薬品工業株式会社 | 二重特異性抗体 |
| WO2019070047A1 (ja) | 2017-10-06 | 2019-04-11 | 小野薬品工業株式会社 | 二重特異性抗体 |
| WO2019089412A1 (en) | 2017-11-01 | 2019-05-09 | Merck Sharp & Dohme Corp. | Novel substituted tetrahydroquinolin compounds as indoleamine 2,3-dioxygenase (ido) inhibitors |
| WO2019099314A1 (en) | 2017-11-14 | 2019-05-23 | Merck Sharp & Dohme Corp. | Novel substituted biaryl compounds as indoleamine 2,3-dioxygenase (ido) inhibitors |
| WO2019099294A1 (en) | 2017-11-14 | 2019-05-23 | Merck Sharp & Dohme Corp. | Novel substituted biaryl compounds as indoleamine 2,3-dioxygenase (ido) inhibitors |
| US11638760B2 (en) | 2017-11-27 | 2023-05-02 | Mersana Therapeutics, Inc. | Pyrrolobenzodiazepine antibody conjugates |
| WO2019104289A1 (en) | 2017-11-27 | 2019-05-31 | Mersana Therapeutics, Inc. | Pyrrolobenzodiazepine antibody conjugates |
| US11946094B2 (en) | 2017-12-10 | 2024-04-02 | Augusta University Research Institute, Inc. | Combination therapies and methods of use thereof |
| US11685761B2 (en) | 2017-12-20 | 2023-06-27 | Merck Sharp & Dohme Llc | Cyclic di-nucleotide compounds as sting agonists |
| WO2019126691A1 (en) | 2017-12-21 | 2019-06-27 | Mersana Therapeutics, Inc. | Pyrrolobenzodiazepine antibody conjugates |
| WO2019147670A1 (en) | 2018-01-23 | 2019-08-01 | Nextcure, Inc. | B7-h4 antibodies and methods of use thereof |
| WO2019149716A1 (en) | 2018-01-31 | 2019-08-08 | F. Hoffmann-La Roche Ag | Bispecific antibodies comprising an antigen-binding site binding to lag3 |
| JP7363828B2 (ja) | 2018-02-09 | 2023-10-18 | 小野薬品工業株式会社 | 二重特異性抗体 |
| JP2021063142A (ja) * | 2018-02-09 | 2021-04-22 | 小野薬品工業株式会社 | 二重特異性抗体 |
| US12091461B2 (en) | 2018-02-09 | 2024-09-17 | Ono Pharmaceutical Co., Ltd. | Bispecific antibody |
| JPWO2019156199A1 (ja) * | 2018-02-09 | 2021-01-28 | 小野薬品工業株式会社 | 二重特異性抗体 |
| KR20200119244A (ko) | 2018-02-09 | 2020-10-19 | 오노 야꾸힝 고교 가부시키가이샤 | 이중 특이성 항체 |
| WO2019156199A1 (ja) | 2018-02-09 | 2019-08-15 | 小野薬品工業株式会社 | 二重特異性抗体 |
| US11091550B2 (en) | 2018-02-09 | 2021-08-17 | Ono Pharmaceutical Co., Ltd. | Bispecific antibody |
| US11702430B2 (en) | 2018-04-03 | 2023-07-18 | Merck Sharp & Dohme Llc | Aza-benzothiophene compounds as STING agonists |
| WO2019195124A1 (en) | 2018-04-03 | 2019-10-10 | Merck Sharp & Dohme Corp. | Benzothiophenes and related compounds as sting agonists |
| US10793557B2 (en) | 2018-04-03 | 2020-10-06 | Merck Sharp & Dohme Corp. | Sting agonist compounds |
| WO2019193541A1 (en) | 2018-04-06 | 2019-10-10 | Glaxosmithkline Intellectual Property Development Limited | Bicyclic aromatic ring derivatives of formula (i) as atf4 inhibitors |
| WO2019193540A1 (en) | 2018-04-06 | 2019-10-10 | Glaxosmithkline Intellectual Property Development Limited | Heteroaryl derivatives of formula (i) as atf4 inhibitors |
| US11542505B1 (en) | 2018-04-20 | 2023-01-03 | Merck Sharp & Dohme Llc | Substituted RIG-I agonists: compositions and methods thereof |
| US11613525B2 (en) | 2018-05-16 | 2023-03-28 | Ctxt Pty Limited | Substituted condensed thiophenes as modulators of sting |
| WO2019219820A1 (en) | 2018-05-16 | 2019-11-21 | Ctxt Pty Limited | Substituted condensed thiophenes as modulators of sting |
| WO2019231870A1 (en) | 2018-05-31 | 2019-12-05 | Merck Sharp & Dohme Corp. | Novel substituted [1.1.1] bicyclo compounds as indoleamine 2,3-dioxygenase inhibitors |
| WO2019245890A1 (en) | 2018-06-20 | 2019-12-26 | Merck Sharp & Dohme Corp. | Arginase inhibitors and methods of use |
| WO2020012339A1 (en) | 2018-07-09 | 2020-01-16 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds |
| WO2020023268A1 (en) | 2018-07-24 | 2020-01-30 | Amgen Inc. | Combination of lilrb1/2 pathway inhibitors and pd-1 pathway inhibitors |
| WO2020031107A1 (en) | 2018-08-08 | 2020-02-13 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds |
| WO2020044206A1 (en) | 2018-08-29 | 2020-03-05 | Glaxosmithkline Intellectual Property Development Limited | Heterocyclic amides as kinase inhibitors for use in the treatment cancer |
| WO2020047345A1 (en) | 2018-08-31 | 2020-03-05 | Yale University | Compositions and methods of using cell-penetrating antibodies in combination with immune checkpoint modulators |
| WO2020055702A1 (en) | 2018-09-13 | 2020-03-19 | Merck Sharp & Dohme Corp. | Combination of pd-1 antagonist and lag3 antagonist for treating non-microsatellite instablity-high/proficient mismatch repair colorectal cancer |
| US12152019B2 (en) | 2018-10-17 | 2024-11-26 | Merck Sharp & Dohme Llc | Arylalkyl pyrazole compounds as indoleamine 2,3-dioxygenase inhibitors |
| WO2020086479A1 (en) | 2018-10-22 | 2020-04-30 | Glaxosmithkline Intellectual Property Development Limited | Dosing |
| WO2020086476A1 (en) | 2018-10-22 | 2020-04-30 | Glaxosmithkline Intellectual Property Development Limited | Dosing |
| WO2020092385A1 (en) | 2018-10-29 | 2020-05-07 | Mersana Therapeutics, Inc. | Cysteine engineered antibody-drug conjugates with peptide-containing linkers |
| WO2020092183A1 (en) | 2018-11-01 | 2020-05-07 | Merck Sharp & Dohme Corp. | Novel substituted pyrazole compounds as indoleamine 2,3-dioxygenase inhibitors |
| WO2020096871A1 (en) | 2018-11-06 | 2020-05-14 | Merck Sharp & Dohme Corp. | Novel substituted tricyclic compounds as indoleamine 2,3-dioxygenase inhibitors |
| WO2020102804A2 (en) | 2018-11-16 | 2020-05-22 | Arqule, Inc. | Pharmaceutical combination for treatment of cancer |
| WO2020106558A1 (en) | 2018-11-20 | 2020-05-28 | Merck Sharp & Dohme Corp. | Substituted amino triazolopyrimidine and amino triazolopyrazine adenosine receptor antagonists, pharmaceutical compositions and their use |
| WO2020106560A1 (en) | 2018-11-20 | 2020-05-28 | Merck Sharp & Dohme Corp. | Substituted amino triazolopyrimidine and amino triazolopyrazine adenosine receptor antagonists, pharmaceutical compositions and their use |
| WO2020112581A1 (en) | 2018-11-28 | 2020-06-04 | Merck Sharp & Dohme Corp. | Novel substituted piperazine amide compounds as indoleamine 2, 3-dioxygenase (ido) inhibitors |
| EP4342473A2 (en) | 2018-11-30 | 2024-03-27 | GlaxoSmithKline Intellectual Property Development Limited | Compounds useful in hiv therapy |
| WO2020110056A1 (en) | 2018-11-30 | 2020-06-04 | Glaxosmithkline Intellectual Property Development Limited | Compounds useful in hiv therapy |
| WO2020112700A1 (en) | 2018-11-30 | 2020-06-04 | Merck Sharp & Dohme Corp. | 9-substituted amino triazolo quinazoline derivatives as adenosine receptor antagonists, pharmaceutical compositions and their use |
| WO2020204152A1 (ja) | 2019-04-04 | 2020-10-08 | 小野薬品工業株式会社 | 二重特異性抗体 |
| WO2020205688A1 (en) | 2019-04-04 | 2020-10-08 | Merck Sharp & Dohme Corp. | Inhibitors of histone deacetylase-3 useful for the treatment of cancer, inflammation, neurodegeneration diseases and diabetes |
| WO2020232375A1 (en) | 2019-05-16 | 2020-11-19 | Silicon Swat, Inc. | Oxoacridinyl acetic acid derivatives and methods of use |
| WO2020232378A1 (en) | 2019-05-16 | 2020-11-19 | Silicon Swat, Inc. | Benzo[b][1,8]naphthyridine acetic acid derivatives and methods of use |
| WO2020239558A1 (en) | 2019-05-24 | 2020-12-03 | Pfizer Inc. | Combination therapies using cdk inhibitors |
| WO2020260547A1 (en) | 2019-06-27 | 2020-12-30 | Rigontec Gmbh | Design method for optimized rig-i ligands |
| WO2021006199A1 (ja) | 2019-07-05 | 2021-01-14 | 小野薬品工業株式会社 | Pd-1/cd3二重特異性タンパク質による血液がん治療 |
| EP3998081A4 (en) * | 2019-07-05 | 2023-07-12 | Ono Pharmaceutical Co., Ltd. | TREATMENT OF BLOOD CANCER WITH PD-1/CD3 DUAL SPECIFICITY PROTEIN |
| JP7533330B2 (ja) | 2019-07-05 | 2024-08-14 | 小野薬品工業株式会社 | Pd-1/cd3二重特異性タンパク質による血液がん治療 |
| JP2021014449A (ja) * | 2019-07-05 | 2021-02-12 | 小野薬品工業株式会社 | Pd−1/cd3二重特異性タンパク質による血液がん治療 |
| WO2021009362A1 (en) | 2019-07-18 | 2021-01-21 | Ctxt Pty Limited | Benzothiophene, thienopyridine and thienopyrimidine derivatives for the modulation of sting |
| WO2021009365A1 (en) | 2019-07-18 | 2021-01-21 | Ctxt Pty Limited | Benzothiophene, thienopyridine and thienopyrimidine derivatives for the modulation of sting |
| US12036204B2 (en) | 2019-07-26 | 2024-07-16 | Eisai R&D Management Co., Ltd. | Pharmaceutical composition for treating tumor |
| US11083705B2 (en) | 2019-07-26 | 2021-08-10 | Eisai R&D Management Co., Ltd. | Pharmaceutical composition for treating tumor |
| WO2021020336A1 (ja) | 2019-07-26 | 2021-02-04 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 腫瘍治療用医薬組成物 |
| WO2021020416A1 (ja) | 2019-07-30 | 2021-02-04 | 小野薬品工業株式会社 | 二重特異性抗体 |
| US11939343B2 (en) | 2019-08-02 | 2024-03-26 | Mersana Therapeutics, Inc. | Sting agonist compounds and methods of use |
| WO2021025140A1 (ja) | 2019-08-08 | 2021-02-11 | 小野薬品工業株式会社 | 二重特異性タンパク質 |
| WO2021058711A2 (en) | 2019-09-27 | 2021-04-01 | Glaxosmithkline Intellectual Property Development Limited | Antigen binding proteins |
| US12257340B2 (en) | 2019-10-15 | 2025-03-25 | Agensys, Inc. | Pharmaceutical compositions comprising anti-191P4D12 antibody drug conjugates and methods of use thereof |
| WO2021083060A1 (zh) | 2019-10-28 | 2021-05-06 | 中国科学院上海药物研究所 | 五元杂环氧代羧酸类化合物及其医药用途 |
| WO2021086909A1 (en) | 2019-10-29 | 2021-05-06 | Eisai R&D Managment Co., Ltd. | Combination of a pd-1 antagonist, a vegfr/fgfr/ret tyrosine kinase inhibitor and a cbp/beta-catenin inhibitor for treating cancer |
| WO2021108025A1 (en) | 2019-11-26 | 2021-06-03 | Massachusetts Institute Of Technology | Cell-based cancer vaccines and cancer therapies |
| WO2021113679A1 (en) | 2019-12-06 | 2021-06-10 | Mersana Therapeutics, Inc. | Dimeric compounds as sting agonists |
| WO2021119753A1 (en) | 2019-12-18 | 2021-06-24 | Ctxt Pty Limited | Compounds |
| US12156870B2 (en) | 2020-04-02 | 2024-12-03 | Mersana Therapeutics, Inc. | Antibody drug conjugates comprising sting agonists |
| WO2021209356A1 (en) | 2020-04-14 | 2021-10-21 | Glaxosmithkline Intellectual Property Development Limited | Combination treatment for cancer |
| WO2021209357A1 (en) | 2020-04-14 | 2021-10-21 | Glaxosmithkline Intellectual Property Development Limited | Combination treatment for cancer involving anti-icos and anti-pd1 antibodies, optionally further involving anti-tim3 antibodies |
| WO2021222188A1 (en) | 2020-04-27 | 2021-11-04 | Seagen Inc. | Anti-cd40 antibody combination treatment for cancer |
| WO2021226003A1 (en) | 2020-05-06 | 2021-11-11 | Merck Sharp & Dohme Corp. | Il4i1 inhibitors and methods of use |
| WO2021231350A1 (en) | 2020-05-13 | 2021-11-18 | Massachusetts Institute Of Technology | Compositions of polymeric microdevices and their use in cancer immunotherapy |
| WO2021248065A1 (en) | 2020-06-05 | 2021-12-09 | Theraly Fibrosis, Inc. | Trail compositions with reduced immunogenicity |
| WO2022049526A1 (en) | 2020-09-02 | 2022-03-10 | Pharmabcine Inc. | Combination therapy of a pd-1 antagonist and an antagonist for vegfr-2 for treating patients with cancer |
| WO2022092085A1 (ja) | 2020-10-28 | 2022-05-05 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 腫瘍治療用医薬組成物 |
| WO2022130206A1 (en) | 2020-12-16 | 2022-06-23 | Pfizer Inc. | TGFβr1 INHIBITOR COMBINATION THERAPIES |
| WO2022165403A1 (en) | 2021-02-01 | 2022-08-04 | Yale University | Chemotherapeutic bioadhesive particles with immunostimulatory molecules for cancer treatment |
| WO2022181515A1 (ja) * | 2021-02-26 | 2022-09-01 | 国立大学法人京都大学 | Pd-1シグナル阻害剤の併用療法 |
| WO2022185160A1 (en) | 2021-03-02 | 2022-09-09 | Glaxosmithkline Intellectual Property Development Limited | Substituted pyridines as dnmt1 inhibitors |
| WO2022208353A1 (en) | 2021-03-31 | 2022-10-06 | Glaxosmithkline Intellectual Property Development Limited | Antigen binding proteins and combinations thereof |
| WO2022217123A2 (en) | 2021-04-08 | 2022-10-13 | Nurix Therapeutics, Inc. | Combination therapies with cbl-b inhibitor compounds |
| WO2022232333A1 (en) | 2021-04-30 | 2022-11-03 | Merck Sharp & Dohme Llc | Il4i1 inhibitors and methods of use |
| WO2022256534A1 (en) | 2021-06-03 | 2022-12-08 | Synthorx, Inc. | Head and neck cancer combination therapy comprising an il-2 conjugate and pembrolizumab |
| WO2022261018A1 (en) | 2021-06-07 | 2022-12-15 | Providence Health & Services - Oregon | Cxcr5, pd-1, and icos expressing tumor reactive cd4 t cells and their use |
| WO2023010080A1 (en) | 2021-07-30 | 2023-02-02 | Seagen Inc. | Treatment for cancer |
| WO2023012147A1 (en) | 2021-08-03 | 2023-02-09 | F. Hoffmann-La Roche Ag | Bispecific antibodies and methods of use |
| WO2023057882A1 (en) | 2021-10-05 | 2023-04-13 | Pfizer Inc. | Combinations of azalactam compounds with a pd-1 axis binding antagonist for the treatment of cancer |
| WO2023079428A1 (en) | 2021-11-03 | 2023-05-11 | Pfizer Inc. | Combination therapies using tlr7/8 agonist |
| WO2023122573A1 (en) | 2021-12-20 | 2023-06-29 | Synthorx, Inc. | Head and neck cancer combination therapy comprising an il-2 conjugate and pembrolizumab |
| WO2023230554A1 (en) | 2022-05-25 | 2023-11-30 | Pfizer Inc. | Combination of a braf inhibitor, an egfr inhibitor, and a pd-1 antagonist for the treatment of braf v600e-mutant, msi-h/dmmr colorectal cancer |
| WO2023230541A1 (en) | 2022-05-27 | 2023-11-30 | Viiv Healthcare Company | Piperazine derivatives useful in hiv therapy |
| WO2024040264A1 (en) | 2022-08-19 | 2024-02-22 | Massachusetts Institute Of Technology | Compositions and methods for targeting dendritic cell lectins |
| WO2024043319A1 (ja) | 2022-08-26 | 2024-02-29 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 抗体薬物複合体 |
| WO2024081736A2 (en) | 2022-10-11 | 2024-04-18 | Yale University | Compositions and methods of using cell-penetrating antibodies |
| WO2024112867A1 (en) | 2022-11-23 | 2024-05-30 | University Of Georgia Research Foundation, Inc. | Compositions and methods of use thereof for increasing immune responses |
| WO2024206357A1 (en) | 2023-03-29 | 2024-10-03 | Merck Sharp & Dohme Llc | Il4i1 inhibitors and methods of use |
| WO2025049858A1 (en) | 2023-09-01 | 2025-03-06 | Amgen Inc. | Molecules for treatment of cancer |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2270051B1 (en) | 2019-05-15 |
| KR20050107399A (ko) | 2005-11-11 |
| US20080025979A1 (en) | 2008-01-31 |
| EP2270051A2 (en) | 2011-01-05 |
| ES2729974T3 (es) | 2019-11-07 |
| US8951518B2 (en) | 2015-02-10 |
| JP4532409B2 (ja) | 2010-08-25 |
| JP6157574B2 (ja) | 2017-07-05 |
| US20090263865A1 (en) | 2009-10-22 |
| JP2016033169A (ja) | 2016-03-10 |
| EP1591527B1 (en) | 2015-08-26 |
| EP1591527A4 (en) | 2006-05-24 |
| US8246955B2 (en) | 2012-08-21 |
| EP2270051A3 (en) | 2011-10-19 |
| US7998479B2 (en) | 2011-08-16 |
| US9783609B2 (en) | 2017-10-10 |
| US7563869B2 (en) | 2009-07-21 |
| JPWO2004072286A1 (ja) | 2006-06-01 |
| JP2010229134A (ja) | 2010-10-14 |
| US20150118234A1 (en) | 2015-04-30 |
| EP1591527A1 (en) | 2005-11-02 |
| JP5892913B2 (ja) | 2016-03-23 |
| US20110280878A1 (en) | 2011-11-17 |
| JP2013082712A (ja) | 2013-05-09 |
| US20130164294A1 (en) | 2013-06-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2004072286A1 (ja) | ヒトpd−1に対し特異性を有する物質 | |
| US11472882B2 (en) | Anti-B7-H4 antibody, antigen-binding fragment thereof and pharmaceutical use thereof | |
| US10077305B2 (en) | Antibodies against PD-1 and uses thereof | |
| JP4249013B2 (ja) | Pd−1に対し特異性を有する物質 | |
| CN109843927B (zh) | 抗b7-h3抗体、其抗原结合片段及其医药用途 | |
| TW201735947A (zh) | 用於癌之治療的細胞傷害誘導治療劑 | |
| KR20100100935A (ko) | 소양증 치료제 | |
| JPWO2019156199A1 (ja) | 二重特異性抗体 | |
| KR20200123164A (ko) | Lag3 및 pd1에 결합하는 치료 분자 | |
| WO2021100022A1 (en) | Anti-pd-l1/anti-b7-h3 multispecific antibodies and uses thereof | |
| CN110938146A (zh) | Tim-3单域抗体及其应用 | |
| TWI862565B (zh) | 雙特異性抗體 | |
| EP3938397A1 (en) | Small shedding blocking agents | |
| WO2021020416A1 (ja) | 二重特異性抗体 | |
| CA3238936A1 (en) | Antibodies against ctla-4 and methods of use thereof | |
| JP2022060182A (ja) | 二重特異性抗体を有効成分として含む医薬組成物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NA NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): BW GH GM KE LS MW MZ SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LU MC NL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2005504930 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020057013393 Country of ref document: KR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 10543323 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2004704324 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 2004704324 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 1020057013393 Country of ref document: KR |
|
| WWP | Wipo information: published in national office |
Ref document number: 10543323 Country of ref document: US |