KR20210078463A - 안정화되고 변형된 비타민 d 방출 제형 및 이의 투여 방법 - Google Patents
안정화되고 변형된 비타민 d 방출 제형 및 이의 투여 방법 Download PDFInfo
- Publication number
- KR20210078463A KR20210078463A KR1020210079948A KR20210079948A KR20210078463A KR 20210078463 A KR20210078463 A KR 20210078463A KR 1020210079948 A KR1020210079948 A KR 1020210079948A KR 20210079948 A KR20210079948 A KR 20210079948A KR 20210078463 A KR20210078463 A KR 20210078463A
- Authority
- KR
- South Korea
- Prior art keywords
- formulation
- hydroxyvitamin
- vitamin
- hours
- compound
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 365
- 238000009472 formulation Methods 0.000 title claims abstract description 321
- 229940046008 vitamin d Drugs 0.000 title claims abstract description 112
- 238000000034 method Methods 0.000 title claims description 96
- JWUBBDSIWDLEOM-NQZHSCJISA-N 25-hydroxy-3 epi cholecalciferol Chemical compound C1([C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](CCCC(C)(C)O)C)=CC=C1C[C@H](O)CCC1=C JWUBBDSIWDLEOM-NQZHSCJISA-N 0.000 claims abstract description 123
- 238000004090 dissolution Methods 0.000 claims abstract description 116
- QYSXJUFSXHHAJI-XFEUOLMDSA-N Vitamin D3 Natural products C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C/C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-XFEUOLMDSA-N 0.000 claims abstract description 113
- -1 vitamin D compound Chemical class 0.000 claims abstract description 112
- 229930003316 Vitamin D Natural products 0.000 claims abstract description 111
- 235000019166 vitamin D Nutrition 0.000 claims abstract description 111
- 239000011710 vitamin D Substances 0.000 claims abstract description 111
- 238000003860 storage Methods 0.000 claims abstract description 75
- 238000013270 controlled release Methods 0.000 claims abstract description 55
- 150000001875 compounds Chemical class 0.000 claims abstract description 42
- KJKIIUAXZGLUND-ICCVIKJNSA-N 25-hydroxyvitamin D2 Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](\C=C\[C@H](C)C(C)(C)O)C)=C\C=C1\C[C@@H](O)CCC1=C KJKIIUAXZGLUND-ICCVIKJNSA-N 0.000 claims abstract description 40
- 229920002678 cellulose Polymers 0.000 claims abstract description 28
- 239000001913 cellulose Substances 0.000 claims abstract description 27
- 235000021318 Calcifediol Nutrition 0.000 claims description 77
- 210000002966 serum Anatomy 0.000 claims description 63
- JWUBBDSIWDLEOM-XHQRYOPUSA-N (3e)-3-[(2e)-2-[1-(6-hydroxy-6-methylheptan-2-yl)-7a-methyl-2,3,3a,5,6,7-hexahydro-1h-inden-4-ylidene]ethylidene]-4-methylidenecyclohexan-1-ol Chemical compound C1CCC2(C)C(C(CCCC(C)(C)O)C)CCC2\C1=C\C=C1/CC(O)CCC1=C JWUBBDSIWDLEOM-XHQRYOPUSA-N 0.000 claims description 59
- 238000011282 treatment Methods 0.000 claims description 53
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 claims description 44
- 238000000338 in vitro Methods 0.000 claims description 40
- 239000011159 matrix material Substances 0.000 claims description 38
- 239000003381 stabilizer Substances 0.000 claims description 35
- 239000001993 wax Substances 0.000 claims description 32
- 239000002775 capsule Substances 0.000 claims description 28
- 235000010980 cellulose Nutrition 0.000 claims description 27
- 230000001965 increasing effect Effects 0.000 claims description 25
- 239000003795 chemical substances by application Substances 0.000 claims description 23
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 claims description 23
- 238000013268 sustained release Methods 0.000 claims description 23
- 239000012730 sustained-release form Substances 0.000 claims description 23
- 238000010521 absorption reaction Methods 0.000 claims description 16
- 239000002552 dosage form Substances 0.000 claims description 16
- 239000012188 paraffin wax Substances 0.000 claims description 15
- 208000020832 chronic kidney disease Diseases 0.000 claims description 14
- 125000000400 lauroyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 13
- 239000002480 mineral oil Substances 0.000 claims description 13
- 239000003995 emulsifying agent Substances 0.000 claims description 12
- 235000010446 mineral oil Nutrition 0.000 claims description 11
- 239000003623 enhancer Substances 0.000 claims description 10
- 229920003086 cellulose ether Polymers 0.000 claims description 8
- 108090000445 Parathyroid hormone Proteins 0.000 claims description 7
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 claims description 7
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 claims description 7
- 230000007812 deficiency Effects 0.000 claims description 6
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 claims description 5
- 229920000609 methyl cellulose Polymers 0.000 claims description 5
- 239000001923 methylcellulose Substances 0.000 claims description 5
- 235000010981 methylcellulose Nutrition 0.000 claims description 5
- 239000008298 dragée Substances 0.000 claims description 4
- 239000000829 suppository Substances 0.000 claims description 4
- 230000002708 enhancing effect Effects 0.000 claims description 3
- 239000001761 ethyl methyl cellulose Substances 0.000 claims description 3
- 235000010944 ethyl methyl cellulose Nutrition 0.000 claims description 3
- 102000003982 Parathyroid hormone Human genes 0.000 claims description 2
- 239000000199 parathyroid hormone Substances 0.000 claims description 2
- 229960001319 parathyroid hormone Drugs 0.000 claims description 2
- JWUBBDSIWDLEOM-UHFFFAOYSA-N 25-Hydroxycholecalciferol Natural products C1CCC2(C)C(C(CCCC(C)(C)O)C)CCC2C1=CC=C1CC(O)CCC1=C JWUBBDSIWDLEOM-UHFFFAOYSA-N 0.000 abstract description 31
- JWUBBDSIWDLEOM-DCHLRESJSA-N 25-Hydroxyvitamin D3 Natural products C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](CCCC(C)(C)O)C)=C/C=C1\C[C@@H](O)CCC1=C JWUBBDSIWDLEOM-DCHLRESJSA-N 0.000 abstract description 21
- 230000008859 change Effects 0.000 description 58
- 150000003710 vitamin D derivatives Chemical class 0.000 description 37
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 26
- 201000010099 disease Diseases 0.000 description 19
- GMRQFYUYWCNGIN-ZVUFCXRFSA-N 1,25-dihydroxy vitamin D3 Chemical compound C1([C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](CCCC(C)(C)O)C)=CC=C1C[C@@H](O)C[C@H](O)C1=C GMRQFYUYWCNGIN-ZVUFCXRFSA-N 0.000 description 18
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 18
- 239000001788 mono and diglycerides of fatty acids Substances 0.000 description 17
- 229940068196 placebo Drugs 0.000 description 17
- 239000000902 placebo Substances 0.000 description 17
- 229940075507 glyceryl monostearate Drugs 0.000 description 15
- 239000011575 calcium Substances 0.000 description 13
- 210000001035 gastrointestinal tract Anatomy 0.000 description 13
- 229910052791 calcium Inorganic materials 0.000 description 12
- 230000000052 comparative effect Effects 0.000 description 12
- 125000005456 glyceride group Chemical group 0.000 description 12
- 239000003981 vehicle Substances 0.000 description 12
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 11
- 230000000694 effects Effects 0.000 description 11
- 239000004322 Butylated hydroxytoluene Substances 0.000 description 10
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 description 10
- 239000013543 active substance Substances 0.000 description 10
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 10
- 229940095259 butylated hydroxytoluene Drugs 0.000 description 10
- JWUBBDSIWDLEOM-DTOXIADCSA-N calcidiol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](CCCC(C)(C)O)C)=C\C=C1\C[C@@H](O)CCC1=C JWUBBDSIWDLEOM-DTOXIADCSA-N 0.000 description 10
- 229960004361 calcifediol Drugs 0.000 description 10
- 239000003814 drug Substances 0.000 description 10
- 238000002844 melting Methods 0.000 description 10
- 230000008018 melting Effects 0.000 description 10
- 239000000047 product Substances 0.000 description 10
- 206010047626 Vitamin D Deficiency Diseases 0.000 description 9
- 238000001990 intravenous administration Methods 0.000 description 9
- 230000009286 beneficial effect Effects 0.000 description 8
- 235000014113 dietary fatty acids Nutrition 0.000 description 8
- 239000000194 fatty acid Substances 0.000 description 8
- 229930195729 fatty acid Natural products 0.000 description 8
- 229940057995 liquid paraffin Drugs 0.000 description 8
- 229910052698 phosphorus Inorganic materials 0.000 description 8
- 208000001132 Osteoporosis Diseases 0.000 description 7
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 7
- 208000035475 disorder Diseases 0.000 description 7
- 239000004615 ingredient Substances 0.000 description 7
- 239000011574 phosphorus Substances 0.000 description 7
- 238000002360 preparation method Methods 0.000 description 7
- 230000002829 reductive effect Effects 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- 208000023275 Autoimmune disease Diseases 0.000 description 6
- 201000004624 Dermatitis Diseases 0.000 description 6
- 201000004681 Psoriasis Diseases 0.000 description 6
- 208000005770 Secondary Hyperparathyroidism Diseases 0.000 description 6
- 239000004480 active ingredient Substances 0.000 description 6
- 210000004369 blood Anatomy 0.000 description 6
- 239000008280 blood Substances 0.000 description 6
- GMRQFYUYWCNGIN-NKMMMXOESA-N calcitriol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](CCCC(C)(C)O)C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C GMRQFYUYWCNGIN-NKMMMXOESA-N 0.000 description 6
- 210000004027 cell Anatomy 0.000 description 6
- 239000012738 dissolution medium Substances 0.000 description 6
- 238000007922 dissolution test Methods 0.000 description 6
- 229940079593 drug Drugs 0.000 description 6
- 230000006872 improvement Effects 0.000 description 6
- 239000003921 oil Substances 0.000 description 6
- 235000019198 oils Nutrition 0.000 description 6
- 239000008194 pharmaceutical composition Substances 0.000 description 6
- 239000003755 preservative agent Substances 0.000 description 6
- 239000007787 solid Substances 0.000 description 6
- 206010028980 Neoplasm Diseases 0.000 description 5
- 102100036893 Parathyroid hormone Human genes 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- 230000001684 chronic effect Effects 0.000 description 5
- 230000007423 decrease Effects 0.000 description 5
- 150000004665 fatty acids Chemical class 0.000 description 5
- 239000007903 gelatin capsule Substances 0.000 description 5
- 239000002609 medium Substances 0.000 description 5
- WWZKQHOCKIZLMA-UHFFFAOYSA-N octanoic acid Chemical compound CCCCCCCC(O)=O WWZKQHOCKIZLMA-UHFFFAOYSA-N 0.000 description 5
- 239000000546 pharmaceutical excipient Substances 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- QYSXJUFSXHHAJI-YRZJJWOYSA-N vitamin D3 Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C\C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-YRZJJWOYSA-N 0.000 description 5
- GFAZGHREJPXDMH-UHFFFAOYSA-N 1,3-dipalmitoylglycerol Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(O)COC(=O)CCCCCCCCCCCCCCC GFAZGHREJPXDMH-UHFFFAOYSA-N 0.000 description 4
- IZHVBANLECCAGF-UHFFFAOYSA-N 2-hydroxy-3-(octadecanoyloxy)propyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)COC(=O)CCCCCCCCCCCCCCCCC IZHVBANLECCAGF-UHFFFAOYSA-N 0.000 description 4
- 206010009944 Colon cancer Diseases 0.000 description 4
- 208000011231 Crohn disease Diseases 0.000 description 4
- 108060006698 EGF receptor Proteins 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 4
- 108010010234 HDL Lipoproteins Proteins 0.000 description 4
- 102000015779 HDL Lipoproteins Human genes 0.000 description 4
- 201000002980 Hyperparathyroidism Diseases 0.000 description 4
- 206010020772 Hypertension Diseases 0.000 description 4
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 4
- 239000004698 Polyethylene Substances 0.000 description 4
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 4
- 230000001154 acute effect Effects 0.000 description 4
- 230000032683 aging Effects 0.000 description 4
- 239000007864 aqueous solution Substances 0.000 description 4
- OGBUMNBNEWYMNJ-UHFFFAOYSA-N batilol Chemical group CCCCCCCCCCCCCCCCCCOCC(O)CO OGBUMNBNEWYMNJ-UHFFFAOYSA-N 0.000 description 4
- 239000011612 calcitriol Substances 0.000 description 4
- 201000011510 cancer Diseases 0.000 description 4
- 230000001276 controlling effect Effects 0.000 description 4
- 230000003247 decreasing effect Effects 0.000 description 4
- 150000002148 esters Chemical class 0.000 description 4
- IPCSVZSSVZVIGE-UHFFFAOYSA-N hexadecanoic acid Chemical compound CCCCCCCCCCCCCCCC(O)=O IPCSVZSSVZVIGE-UHFFFAOYSA-N 0.000 description 4
- 239000012456 homogeneous solution Substances 0.000 description 4
- 239000005556 hormone Substances 0.000 description 4
- 229940088597 hormone Drugs 0.000 description 4
- 238000001727 in vivo Methods 0.000 description 4
- 210000003734 kidney Anatomy 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 238000002483 medication Methods 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 229920000573 polyethylene Polymers 0.000 description 4
- ARIWANIATODDMH-UHFFFAOYSA-N rac-1-monolauroylglycerol Chemical compound CCCCCCCCCCCC(=O)OCC(O)CO ARIWANIATODDMH-UHFFFAOYSA-N 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 230000004044 response Effects 0.000 description 4
- 210000000813 small intestine Anatomy 0.000 description 4
- 239000007901 soft capsule Substances 0.000 description 4
- 229940124597 therapeutic agent Drugs 0.000 description 4
- 230000001225 therapeutic effect Effects 0.000 description 4
- 208000001072 type 2 diabetes mellitus Diseases 0.000 description 4
- MECHNRXZTMCUDQ-RKHKHRCZSA-N vitamin D2 Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)/C=C/[C@H](C)C(C)C)=C\C=C1\C[C@@H](O)CCC1=C MECHNRXZTMCUDQ-RKHKHRCZSA-N 0.000 description 4
- QHZLMUACJMDIAE-SFHVURJKSA-N 1-hexadecanoyl-sn-glycerol Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@@H](O)CO QHZLMUACJMDIAE-SFHVURJKSA-N 0.000 description 3
- 239000000263 2,3-dihydroxypropyl (Z)-octadec-9-enoate Substances 0.000 description 3
- RZRNAYUHWVFMIP-GDCKJWNLSA-N 3-oleoyl-sn-glycerol Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@H](O)CO RZRNAYUHWVFMIP-GDCKJWNLSA-N 0.000 description 3
- 208000024172 Cardiovascular disease Diseases 0.000 description 3
- 208000032928 Dyslipidaemia Diseases 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- 208000031226 Hyperlipidaemia Diseases 0.000 description 3
- 208000017170 Lipid metabolism disease Diseases 0.000 description 3
- 206010025476 Malabsorption Diseases 0.000 description 3
- 208000004155 Malabsorption Syndromes Diseases 0.000 description 3
- 208000029725 Metabolic bone disease Diseases 0.000 description 3
- QHZLMUACJMDIAE-UHFFFAOYSA-N Palmitic acid monoglyceride Natural products CCCCCCCCCCCCCCCC(=O)OCC(O)CO QHZLMUACJMDIAE-UHFFFAOYSA-N 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 239000003963 antioxidant agent Substances 0.000 description 3
- 230000003078 antioxidant effect Effects 0.000 description 3
- 206010012601 diabetes mellitus Diseases 0.000 description 3
- 238000011049 filling Methods 0.000 description 3
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 3
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 3
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 3
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 3
- 239000012729 immediate-release (IR) formulation Substances 0.000 description 3
- 238000003780 insertion Methods 0.000 description 3
- 230000037431 insertion Effects 0.000 description 3
- 230000003834 intracellular effect Effects 0.000 description 3
- 210000004185 liver Anatomy 0.000 description 3
- 239000000155 melt Substances 0.000 description 3
- RZRNAYUHWVFMIP-UHFFFAOYSA-N monoelaidin Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC(O)CO RZRNAYUHWVFMIP-UHFFFAOYSA-N 0.000 description 3
- 239000006186 oral dosage form Substances 0.000 description 3
- 235000019809 paraffin wax Nutrition 0.000 description 3
- 235000019271 petrolatum Nutrition 0.000 description 3
- 229920001223 polyethylene glycol Polymers 0.000 description 3
- 230000002335 preservative effect Effects 0.000 description 3
- 208000007442 rickets Diseases 0.000 description 3
- 238000001179 sorption measurement Methods 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 150000003626 triacylglycerols Chemical class 0.000 description 3
- DRAWQKGUORNASA-UHFFFAOYSA-N (2-hydroxy-3-octadec-9-enoyloxypropyl) octadec-9-enoate Chemical compound CCCCCCCCC=CCCCCCCCC(=O)OCC(O)COC(=O)CCCCCCCC=CCCCCCCCC DRAWQKGUORNASA-UHFFFAOYSA-N 0.000 description 2
- CUNWUEBNSZSNRX-RKGWDQTMSA-N (2r,3r,4r,5s)-hexane-1,2,3,4,5,6-hexol;(z)-octadec-9-enoic acid Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O CUNWUEBNSZSNRX-RKGWDQTMSA-N 0.000 description 2
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical class OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 2
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 2
- ODDSXTDNXBAVPQ-UHFFFAOYSA-N 1,2-dihydroxypropyl hexadecanoate Chemical compound CCCCCCCCCCCCCCCC(=O)OC(O)C(C)O ODDSXTDNXBAVPQ-UHFFFAOYSA-N 0.000 description 2
- 102100027518 1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial Human genes 0.000 description 2
- DMBUODUULYCPAK-UHFFFAOYSA-N 1,3-bis(docosanoyloxy)propan-2-yl docosanoate Chemical compound CCCCCCCCCCCCCCCCCCCCCC(=O)OCC(OC(=O)CCCCCCCCCCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCCCCCCCCCC DMBUODUULYCPAK-UHFFFAOYSA-N 0.000 description 2
- FKOKUHFZNIUSLW-UHFFFAOYSA-N 2-Hydroxypropyl stearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(C)O FKOKUHFZNIUSLW-UHFFFAOYSA-N 0.000 description 2
- ZVTDEEBSWIQAFJ-KHPPLWFESA-N 2-hydroxypropyl (z)-octadec-9-enoate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(C)O ZVTDEEBSWIQAFJ-KHPPLWFESA-N 0.000 description 2
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 2
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 2
- 206010003210 Arteriosclerosis Diseases 0.000 description 2
- 201000001320 Atherosclerosis Diseases 0.000 description 2
- 206010005949 Bone cancer Diseases 0.000 description 2
- 208000020084 Bone disease Diseases 0.000 description 2
- 208000018084 Bone neoplasm Diseases 0.000 description 2
- 201000006474 Brain Ischemia Diseases 0.000 description 2
- 206010006187 Breast cancer Diseases 0.000 description 2
- 208000026310 Breast neoplasm Diseases 0.000 description 2
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 2
- 206010007559 Cardiac failure congestive Diseases 0.000 description 2
- 208000031229 Cardiomyopathies Diseases 0.000 description 2
- 206010007749 Cataract diabetic Diseases 0.000 description 2
- 206010008120 Cerebral ischaemia Diseases 0.000 description 2
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 2
- 208000015943 Coeliac disease Diseases 0.000 description 2
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 2
- 108010015742 Cytochrome P-450 Enzyme System Proteins 0.000 description 2
- 102000002004 Cytochrome P-450 Enzyme System Human genes 0.000 description 2
- 206010012438 Dermatitis atopic Diseases 0.000 description 2
- 206010012442 Dermatitis contact Diseases 0.000 description 2
- 208000002249 Diabetes Complications Diseases 0.000 description 2
- 206010012655 Diabetic complications Diseases 0.000 description 2
- 206010070901 Diabetic dyslipidaemia Diseases 0.000 description 2
- 208000008960 Diabetic foot Diseases 0.000 description 2
- 239000001856 Ethyl cellulose Substances 0.000 description 2
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 2
- 206010016654 Fibrosis Diseases 0.000 description 2
- 208000002705 Glucose Intolerance Diseases 0.000 description 2
- 208000003807 Graves Disease Diseases 0.000 description 2
- 208000015023 Graves' disease Diseases 0.000 description 2
- 208000001204 Hashimoto Disease Diseases 0.000 description 2
- 208000030836 Hashimoto thyroiditis Diseases 0.000 description 2
- 206010019280 Heart failures Diseases 0.000 description 2
- 101000861278 Homo sapiens 1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial Proteins 0.000 description 2
- 208000037147 Hypercalcaemia Diseases 0.000 description 2
- 208000035150 Hypercholesterolemia Diseases 0.000 description 2
- 206010060378 Hyperinsulinaemia Diseases 0.000 description 2
- 206010021024 Hypolipidaemia Diseases 0.000 description 2
- 208000000038 Hypoparathyroidism Diseases 0.000 description 2
- 206010022489 Insulin Resistance Diseases 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 2
- 208000001145 Metabolic Syndrome Diseases 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- PVNIIMVLHYAWGP-UHFFFAOYSA-N Niacin Chemical compound OC(=O)C1=CC=CN=C1 PVNIIMVLHYAWGP-UHFFFAOYSA-N 0.000 description 2
- 206010067482 No adverse event Diseases 0.000 description 2
- 208000008589 Obesity Diseases 0.000 description 2
- 239000005642 Oleic acid Substances 0.000 description 2
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 2
- 206010049088 Osteopenia Diseases 0.000 description 2
- 201000000023 Osteosclerosis Diseases 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 235000021314 Palmitic acid Nutrition 0.000 description 2
- 206010033645 Pancreatitis Diseases 0.000 description 2
- 208000013612 Parathyroid disease Diseases 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical class CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 2
- 206010060862 Prostate cancer Diseases 0.000 description 2
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 2
- 208000015634 Rectal Neoplasms Diseases 0.000 description 2
- 208000017442 Retinal disease Diseases 0.000 description 2
- 206010038923 Retinopathy Diseases 0.000 description 2
- 206010039710 Scleroderma Diseases 0.000 description 2
- 208000021386 Sjogren Syndrome Diseases 0.000 description 2
- 208000000453 Skin Neoplasms Diseases 0.000 description 2
- HVUMOYIDDBPOLL-XWVZOOPGSA-N Sorbitan monostearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O HVUMOYIDDBPOLL-XWVZOOPGSA-N 0.000 description 2
- 235000021355 Stearic acid Nutrition 0.000 description 2
- 206010041969 Steatorrhoea Diseases 0.000 description 2
- 229930182558 Sterol Natural products 0.000 description 2
- 208000006011 Stroke Diseases 0.000 description 2
- 208000007536 Thrombosis Diseases 0.000 description 2
- 206010052779 Transplant rejections Diseases 0.000 description 2
- MECHNRXZTMCUDQ-UHFFFAOYSA-N Vitamin D2 Natural products C1CCC2(C)C(C(C)C=CC(C)C(C)C)CCC2C1=CC=C1CC(O)CCC1=C MECHNRXZTMCUDQ-UHFFFAOYSA-N 0.000 description 2
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 208000002029 allergic contact dermatitis Diseases 0.000 description 2
- 230000008485 antagonism Effects 0.000 description 2
- 230000001773 anti-convulsant effect Effects 0.000 description 2
- 239000001961 anticonvulsive agent Substances 0.000 description 2
- 229960003965 antiepileptics Drugs 0.000 description 2
- 229940034982 antineoplastic agent Drugs 0.000 description 2
- 239000002246 antineoplastic agent Substances 0.000 description 2
- 208000011775 arteriosclerosis disease Diseases 0.000 description 2
- 208000006673 asthma Diseases 0.000 description 2
- 201000008937 atopic dermatitis Diseases 0.000 description 2
- 208000010668 atopic eczema Diseases 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 230000036760 body temperature Effects 0.000 description 2
- 210000000988 bone and bone Anatomy 0.000 description 2
- 229960005084 calcitriol Drugs 0.000 description 2
- 235000020964 calcitriol Nutrition 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 2
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 2
- 229940105329 carboxymethylcellulose Drugs 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 206010008118 cerebral infarction Diseases 0.000 description 2
- 208000026106 cerebrovascular disease Diseases 0.000 description 2
- 208000029742 colonic neoplasm Diseases 0.000 description 2
- 208000010247 contact dermatitis Diseases 0.000 description 2
- 208000029078 coronary artery disease Diseases 0.000 description 2
- 201000001981 dermatomyositis Diseases 0.000 description 2
- 201000007025 diabetic cataract Diseases 0.000 description 2
- 235000005911 diet Nutrition 0.000 description 2
- 238000011978 dissolution method Methods 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 229960002061 ergocalciferol Drugs 0.000 description 2
- 235000019325 ethyl cellulose Nutrition 0.000 description 2
- 229920001249 ethyl cellulose Polymers 0.000 description 2
- 230000004761 fibrosis Effects 0.000 description 2
- 239000003862 glucocorticoid Substances 0.000 description 2
- 230000002641 glycemic effect Effects 0.000 description 2
- 229940074045 glyceryl distearate Drugs 0.000 description 2
- 229940068939 glyceryl monolaurate Drugs 0.000 description 2
- 208000006454 hepatitis Diseases 0.000 description 2
- 231100000283 hepatitis Toxicity 0.000 description 2
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 2
- 230000000148 hypercalcaemia Effects 0.000 description 2
- 208000030915 hypercalcemia disease Diseases 0.000 description 2
- 230000003451 hyperinsulinaemic effect Effects 0.000 description 2
- 201000008980 hyperinsulinism Diseases 0.000 description 2
- 208000006575 hypertriglyceridemia Diseases 0.000 description 2
- 208000029498 hypoalphalipoproteinemia Diseases 0.000 description 2
- 230000003553 hypophosphatemic effect Effects 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 208000027866 inflammatory disease Diseases 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 2
- 208000017169 kidney disease Diseases 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- 201000005202 lung cancer Diseases 0.000 description 2
- 208000020816 lung neoplasm Diseases 0.000 description 2
- 208000023356 medullary thyroid gland carcinoma Diseases 0.000 description 2
- 201000001441 melanoma Diseases 0.000 description 2
- 208000030159 metabolic disease Diseases 0.000 description 2
- 230000004060 metabolic process Effects 0.000 description 2
- 239000002207 metabolite Substances 0.000 description 2
- 201000006417 multiple sclerosis Diseases 0.000 description 2
- 208000010125 myocardial infarction Diseases 0.000 description 2
- 208000031225 myocardial ischemia Diseases 0.000 description 2
- WQEPLUUGTLDZJY-UHFFFAOYSA-N n-Pentadecanoic acid Natural products CCCCCCCCCCCCCCC(O)=O WQEPLUUGTLDZJY-UHFFFAOYSA-N 0.000 description 2
- 201000008383 nephritis Diseases 0.000 description 2
- 201000001119 neuropathy Diseases 0.000 description 2
- 230000007823 neuropathy Effects 0.000 description 2
- 235000020824 obesity Nutrition 0.000 description 2
- GLDOVTGHNKAZLK-UHFFFAOYSA-N octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 description 2
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 2
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 2
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 2
- 235000021313 oleic acid Nutrition 0.000 description 2
- 208000005368 osteomalacia Diseases 0.000 description 2
- 210000000496 pancreas Anatomy 0.000 description 2
- 210000002990 parathyroid gland Anatomy 0.000 description 2
- 201000001245 periodontitis Diseases 0.000 description 2
- 208000033808 peripheral neuropathy Diseases 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- 230000036470 plasma concentration Effects 0.000 description 2
- 201000010065 polycystic ovary syndrome Diseases 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 208000005987 polymyositis Diseases 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 201000009104 prediabetes syndrome Diseases 0.000 description 2
- 230000000069 prophylactic effect Effects 0.000 description 2
- 229940093625 propylene glycol monostearate Drugs 0.000 description 2
- 208000006078 pseudohypoparathyroidism Diseases 0.000 description 2
- GHBFNMLVSPCDGN-UHFFFAOYSA-N rac-1-monooctanoylglycerol Chemical compound CCCCCCCC(=O)OCC(O)CO GHBFNMLVSPCDGN-UHFFFAOYSA-N 0.000 description 2
- 206010038038 rectal cancer Diseases 0.000 description 2
- 201000001275 rectum cancer Diseases 0.000 description 2
- 238000002271 resection Methods 0.000 description 2
- 206010039073 rheumatoid arthritis Diseases 0.000 description 2
- 201000000306 sarcoidosis Diseases 0.000 description 2
- 201000000849 skin cancer Diseases 0.000 description 2
- 239000000344 soap Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 239000001587 sorbitan monostearate Substances 0.000 description 2
- 235000011076 sorbitan monostearate Nutrition 0.000 description 2
- 229940035048 sorbitan monostearate Drugs 0.000 description 2
- 229960005078 sorbitan sesquioleate Drugs 0.000 description 2
- 239000008117 stearic acid Substances 0.000 description 2
- 208000001162 steatorrhea Diseases 0.000 description 2
- 235000003702 sterols Nutrition 0.000 description 2
- 230000000475 sunscreen effect Effects 0.000 description 2
- 239000000516 sunscreening agent Substances 0.000 description 2
- 230000009469 supplementation Effects 0.000 description 2
- 230000002459 sustained effect Effects 0.000 description 2
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 2
- 208000013818 thyroid gland medullary carcinoma Diseases 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 230000032258 transport Effects 0.000 description 2
- UFTFJSFQGQCHQW-UHFFFAOYSA-N triformin Chemical compound O=COCC(OC=O)COC=O UFTFJSFQGQCHQW-UHFFFAOYSA-N 0.000 description 2
- 206010044697 tropical sprue Diseases 0.000 description 2
- 201000008827 tuberculosis Diseases 0.000 description 2
- 238000004704 ultra performance liquid chromatography Methods 0.000 description 2
- 210000002700 urine Anatomy 0.000 description 2
- 208000030402 vitamin D-dependent rickets Diseases 0.000 description 2
- 235000001892 vitamin D2 Nutrition 0.000 description 2
- 239000011653 vitamin D2 Substances 0.000 description 2
- 235000005282 vitamin D3 Nutrition 0.000 description 2
- 239000011647 vitamin D3 Substances 0.000 description 2
- 229940021056 vitamin d3 Drugs 0.000 description 2
- 230000003442 weekly effect Effects 0.000 description 2
- 230000029663 wound healing Effects 0.000 description 2
- XMAYWYJOQHXEEK-OZXSUGGESA-N (2R,4S)-ketoconazole Chemical compound C1CN(C(=O)C)CCN1C(C=C1)=CC=C1OC[C@@H]1O[C@@](CN2C=NC=C2)(C=2C(=CC(Cl)=CC=2)Cl)OC1 XMAYWYJOQHXEEK-OZXSUGGESA-N 0.000 description 1
- YYGNTYWPHWGJRM-UHFFFAOYSA-N (6E,10E,14E,18E)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene Chemical compound CC(C)=CCCC(C)=CCCC(C)=CCCC=C(C)CCC=C(C)CCC=C(C)C YYGNTYWPHWGJRM-UHFFFAOYSA-N 0.000 description 1
- OKMWKBLSFKFYGZ-UHFFFAOYSA-N 1-behenoylglycerol Chemical compound CCCCCCCCCCCCCCCCCCCCCC(=O)OCC(O)CO OKMWKBLSFKFYGZ-UHFFFAOYSA-N 0.000 description 1
- WECGLUPZRHILCT-GSNKCQISSA-N 1-linoleoyl-sn-glycerol Chemical class CCCCC\C=C/C\C=C/CCCCCCCC(=O)OC[C@@H](O)CO WECGLUPZRHILCT-GSNKCQISSA-N 0.000 description 1
- ZGLHBRQAEXKACO-XJRQOBMKSA-N 1alpha,25-dihydroxyvitamin D2 Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](\C=C\[C@H](C)C(C)(C)O)C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C ZGLHBRQAEXKACO-XJRQOBMKSA-N 0.000 description 1
- 208000024827 Alzheimer disease Diseases 0.000 description 1
- 244000144725 Amygdalus communis Species 0.000 description 1
- 235000011437 Amygdalus communis Nutrition 0.000 description 1
- 208000000412 Avitaminosis Diseases 0.000 description 1
- 229940122361 Bisphosphonate Drugs 0.000 description 1
- LKUNXBRZDFMZOK-GFCCVEGCSA-N Capric acid monoglyceride Natural products CCCCCCCCCC(=O)OC[C@H](O)CO LKUNXBRZDFMZOK-GFCCVEGCSA-N 0.000 description 1
- 206010008635 Cholestasis Diseases 0.000 description 1
- 229920001268 Cholestyramine Polymers 0.000 description 1
- 208000013725 Chronic Kidney Disease-Mineral and Bone disease Diseases 0.000 description 1
- 108010004103 Chylomicrons Proteins 0.000 description 1
- 229920002911 Colestipol Polymers 0.000 description 1
- 201000003883 Cystic fibrosis Diseases 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- XWLUWCNOOVRFPX-UHFFFAOYSA-N Fosphenytoin Chemical compound O=C1N(COP(O)(=O)O)C(=O)NC1(C=1C=CC=CC=1)C1=CC=CC=C1 XWLUWCNOOVRFPX-UHFFFAOYSA-N 0.000 description 1
- 239000004354 Hydroxyethyl cellulose Substances 0.000 description 1
- 206010020850 Hyperthyroidism Diseases 0.000 description 1
- 206010021135 Hypovitaminosis Diseases 0.000 description 1
- 201000010538 Lactose Intolerance Diseases 0.000 description 1
- 229920003091 Methocel™ Polymers 0.000 description 1
- 229920003096 Methocel™ K100M Polymers 0.000 description 1
- 208000009793 Milk Hypersensitivity Diseases 0.000 description 1
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 1
- 208000018262 Peripheral vascular disease Diseases 0.000 description 1
- CXOFVDLJLONNDW-UHFFFAOYSA-N Phenytoin Chemical compound N1C(=O)NC(=O)C1(C=1C=CC=CC=1)C1=CC=CC=C1 CXOFVDLJLONNDW-UHFFFAOYSA-N 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- BHEOSNUKNHRBNM-UHFFFAOYSA-N Tetramethylsqualene Natural products CC(=C)C(C)CCC(=C)C(C)CCC(C)=CCCC=C(C)CCC(C)C(=C)CCC(C)C(C)=C BHEOSNUKNHRBNM-UHFFFAOYSA-N 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000011149 active material Substances 0.000 description 1
- 208000024340 acute graft versus host disease Diseases 0.000 description 1
- 210000000577 adipose tissue Anatomy 0.000 description 1
- 230000003679 aging effect Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- OFHCOWSQAMBJIW-AVJTYSNKSA-N alfacalcidol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C OFHCOWSQAMBJIW-AVJTYSNKSA-N 0.000 description 1
- 235000020224 almond Nutrition 0.000 description 1
- 239000010775 animal oil Substances 0.000 description 1
- 239000012736 aqueous medium Substances 0.000 description 1
- 235000013871 bee wax Nutrition 0.000 description 1
- 239000012166 beeswax Substances 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 150000004663 bisphosphonates Chemical class 0.000 description 1
- 230000000125 calcaemic effect Effects 0.000 description 1
- 230000002092 calcimimetic effect Effects 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 229940069978 calcium supplement Drugs 0.000 description 1
- 208000035269 cancer or benign tumor Diseases 0.000 description 1
- FFGPTBGBLSHEPO-UHFFFAOYSA-N carbamazepine Chemical compound C1=CC2=CC=CC=C2N(C(=O)N)C2=CC=CC=C21 FFGPTBGBLSHEPO-UHFFFAOYSA-N 0.000 description 1
- 229960000623 carbamazepine Drugs 0.000 description 1
- 239000004203 carnauba wax Substances 0.000 description 1
- 235000013869 carnauba wax Nutrition 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 208000017760 chronic graft versus host disease Diseases 0.000 description 1
- 239000011247 coating layer Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- GMRWGQCZJGVHKL-UHFFFAOYSA-N colestipol Chemical compound ClCC1CO1.NCCNCCNCCNCCN GMRWGQCZJGVHKL-UHFFFAOYSA-N 0.000 description 1
- 229960002604 colestipol Drugs 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 230000000875 corresponding effect Effects 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 150000005690 diesters Chemical class 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 230000000378 dietary effect Effects 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- PRAKJMSDJKAYCZ-UHFFFAOYSA-N dodecahydrosqualene Natural products CC(C)CCCC(C)CCCC(C)CCCCC(C)CCCC(C)CCCC(C)C PRAKJMSDJKAYCZ-UHFFFAOYSA-N 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- HKXBNHCUPKIYDM-CGMHZMFXSA-N doxercalciferol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)/C=C/[C@H](C)C(C)C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C HKXBNHCUPKIYDM-CGMHZMFXSA-N 0.000 description 1
- 229960000413 doxercalciferol Drugs 0.000 description 1
- 238000009506 drug dissolution testing Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 239000002702 enteric coating Substances 0.000 description 1
- 238000009505 enteric coating Methods 0.000 description 1
- 210000001842 enterocyte Anatomy 0.000 description 1
- 238000006266 etherification reaction Methods 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 238000013265 extended release Methods 0.000 description 1
- 235000013341 fat substitute Nutrition 0.000 description 1
- 239000003778 fat substitute Substances 0.000 description 1
- 235000021323 fish oil Nutrition 0.000 description 1
- 230000009969 flowable effect Effects 0.000 description 1
- 235000012631 food intake Nutrition 0.000 description 1
- 235000020189 fortified milk Nutrition 0.000 description 1
- 229960000693 fosphenytoin Drugs 0.000 description 1
- 229910021485 fumed silica Inorganic materials 0.000 description 1
- 208000020694 gallbladder disease Diseases 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 150000002314 glycerols Chemical class 0.000 description 1
- 229940087068 glyceryl caprylate Drugs 0.000 description 1
- 125000003976 glyceryl group Chemical group [H]C([*])([H])C(O[H])([H])C(O[H])([H])[H] 0.000 description 1
- 229940081618 glyceryl monobehenate Drugs 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 238000001631 haemodialysis Methods 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 230000000322 hemodialysis Effects 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 239000008240 homogeneous mixture Substances 0.000 description 1
- 238000002657 hormone replacement therapy Methods 0.000 description 1
- 230000036571 hydration Effects 0.000 description 1
- 238000006703 hydration reaction Methods 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 239000008172 hydrogenated vegetable oil Substances 0.000 description 1
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 description 1
- 210000003405 ileum Anatomy 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 230000031891 intestinal absorption Effects 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 229960004125 ketoconazole Drugs 0.000 description 1
- 208000019423 liver disease Diseases 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 230000037345 metabolism of vitamins Effects 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- 239000004200 microcrystalline wax Substances 0.000 description 1
- 235000019808 microcrystalline wax Nutrition 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- GOQYKNQRPGWPLP-UHFFFAOYSA-N n-heptadecyl alcohol Natural products CCCCCCCCCCCCCCCCCO GOQYKNQRPGWPLP-UHFFFAOYSA-N 0.000 description 1
- 229960003512 nicotinic acid Drugs 0.000 description 1
- 235000001968 nicotinic acid Nutrition 0.000 description 1
- 239000011664 nicotinic acid Substances 0.000 description 1
- 235000016709 nutrition Nutrition 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-M octanoate Chemical compound CCCCCCCC([O-])=O WWZKQHOCKIZLMA-UHFFFAOYSA-M 0.000 description 1
- 230000000849 parathyroid Effects 0.000 description 1
- 229960000987 paricalcitol Drugs 0.000 description 1
- BPKAHTKRCLCHEA-UBFJEZKGSA-N paricalcitol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](\C=C\[C@H](C)C(C)(C)O)C)=C\C=C1C[C@@H](O)C[C@H](O)C1 BPKAHTKRCLCHEA-UBFJEZKGSA-N 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 230000003285 pharmacodynamic effect Effects 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- DDBREPKUVSBGFI-UHFFFAOYSA-N phenobarbital Chemical compound C=1C=CC=CC=1C1(CC)C(=O)NC(=O)NC1=O DDBREPKUVSBGFI-UHFFFAOYSA-N 0.000 description 1
- 229960002695 phenobarbital Drugs 0.000 description 1
- 229960002036 phenytoin Drugs 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 229920000447 polyanionic polymer Polymers 0.000 description 1
- 208000030761 polycystic kidney disease Diseases 0.000 description 1
- 239000008389 polyethoxylated castor oil Substances 0.000 description 1
- 229920000223 polyglycerol Polymers 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 229940069328 povidone Drugs 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 208000020016 psychiatric disease Diseases 0.000 description 1
- LKUNXBRZDFMZOK-UHFFFAOYSA-N rac-1-monodecanoylglycerol Chemical compound CCCCCCCCCC(=O)OCC(O)CO LKUNXBRZDFMZOK-UHFFFAOYSA-N 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 229940100618 rectal suppository Drugs 0.000 description 1
- 239000006215 rectal suppository Substances 0.000 description 1
- 201000006409 renal osteodystrophy Diseases 0.000 description 1
- JQXXHWHPUNPDRT-WLSIYKJHSA-N rifampicin Chemical compound O([C@](C1=O)(C)O/C=C/[C@@H]([C@H]([C@@H](OC(C)=O)[C@H](C)[C@H](O)[C@H](C)[C@@H](O)[C@@H](C)\C=C\C=C(C)/C(=O)NC=2C(O)=C3C([O-])=C4C)C)OC)C4=C1C3=C(O)C=2\C=N\N1CC[NH+](C)CC1 JQXXHWHPUNPDRT-WLSIYKJHSA-N 0.000 description 1
- 229960001225 rifampicin Drugs 0.000 description 1
- 208000037921 secondary disease Diseases 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- GOLXNESZZPUPJE-UHFFFAOYSA-N spiromesifen Chemical compound CC1=CC(C)=CC(C)=C1C(C(O1)=O)=C(OC(=O)CC(C)(C)C)C11CCCC1 GOLXNESZZPUPJE-UHFFFAOYSA-N 0.000 description 1
- 229940031439 squalene Drugs 0.000 description 1
- TUHBEKDERLKLEC-UHFFFAOYSA-N squalene Natural products CC(=CCCC(=CCCC(=CCCC=C(/C)CCC=C(/C)CC=C(C)C)C)C)C TUHBEKDERLKLEC-UHFFFAOYSA-N 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 230000037072 sun protection Effects 0.000 description 1
- 230000001502 supplementing effect Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 230000001839 systemic circulation Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 230000002485 urinary effect Effects 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 208000030401 vitamin deficiency disease Diseases 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/59—Compounds containing 9, 10- seco- cyclopenta[a]hydrophenanthrene ring systems
- A61K31/592—9,10-Secoergostane derivatives, e.g. ergocalciferol, i.e. vitamin D2
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/59—Compounds containing 9, 10- seco- cyclopenta[a]hydrophenanthrene ring systems
- A61K31/593—9,10-Secocholestane derivatives, e.g. cholecalciferol, i.e. vitamin D3
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/14—Esters of carboxylic acids, e.g. fatty acid monoglycerides, medium-chain triglycerides, parabens or PEG fatty acid esters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
- A61K47/38—Cellulose; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/44—Oils, fats or waxes according to two or more groups of A61K47/02-A61K47/42; Natural or modified natural oils, fats or waxes, e.g. castor oil, polyethoxylated castor oil, montan wax, lignite, shellac, rosin, beeswax or lanolin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/205—Polysaccharides, e.g. alginate, gums; Cyclodextrin
- A61K9/2054—Cellulose; Cellulose derivatives, e.g. hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/28—Dragees; Coated pills or tablets, e.g. with film or compression coating
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/28—Dragees; Coated pills or tablets, e.g. with film or compression coating
- A61K9/2806—Coating materials
- A61K9/2833—Organic macromolecular compounds
- A61K9/286—Polysaccharides, e.g. gums; Cyclodextrin
- A61K9/2866—Cellulose; Cellulose derivatives, e.g. hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/4866—Organic macromolecular compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/18—Drugs for disorders of the alimentary tract or the digestive system for pancreatic disorders, e.g. pancreatic enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/08—Drugs for disorders of the urinary system of the prostate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/04—Antipruritics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/08—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/08—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease
- A61P19/10—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease for osteoporosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/08—Antiepileptics; Anticonvulsants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/12—Ophthalmic agents for cataracts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/02—Nutrients, e.g. vitamins, minerals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/14—Drugs for disorders of the endocrine system of the thyroid hormones, e.g. T3, T4
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/18—Drugs for disorders of the endocrine system of the parathyroid hormones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/18—Drugs for disorders of the endocrine system of the parathyroid hormones
- A61P5/20—Drugs for disorders of the endocrine system of the parathyroid hormones for decreasing, blocking or antagonising the activity of PTH
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/38—Drugs for disorders of the endocrine system of the suprarenal hormones
- A61P5/46—Drugs for disorders of the endocrine system of the suprarenal hormones for decreasing, blocking or antagonising the activity of glucocorticosteroids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/02—Antithrombotic agents; Anticoagulants; Platelet aggregation inhibitors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/04—Inotropic agents, i.e. stimulants of cardiac contraction; Drugs for heart failure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Diabetes (AREA)
- Hematology (AREA)
- Immunology (AREA)
- Physical Education & Sports Medicine (AREA)
- Endocrinology (AREA)
- Rheumatology (AREA)
- Obesity (AREA)
- Cardiology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Heart & Thoracic Surgery (AREA)
- Pulmonology (AREA)
- Dermatology (AREA)
- Urology & Nephrology (AREA)
- Neurology (AREA)
- Ophthalmology & Optometry (AREA)
- Inorganic Chemistry (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Pain & Pain Management (AREA)
- Oncology (AREA)
- Vascular Medicine (AREA)
- Hospice & Palliative Care (AREA)
- Transplantation (AREA)
Abstract
Description
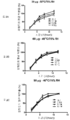
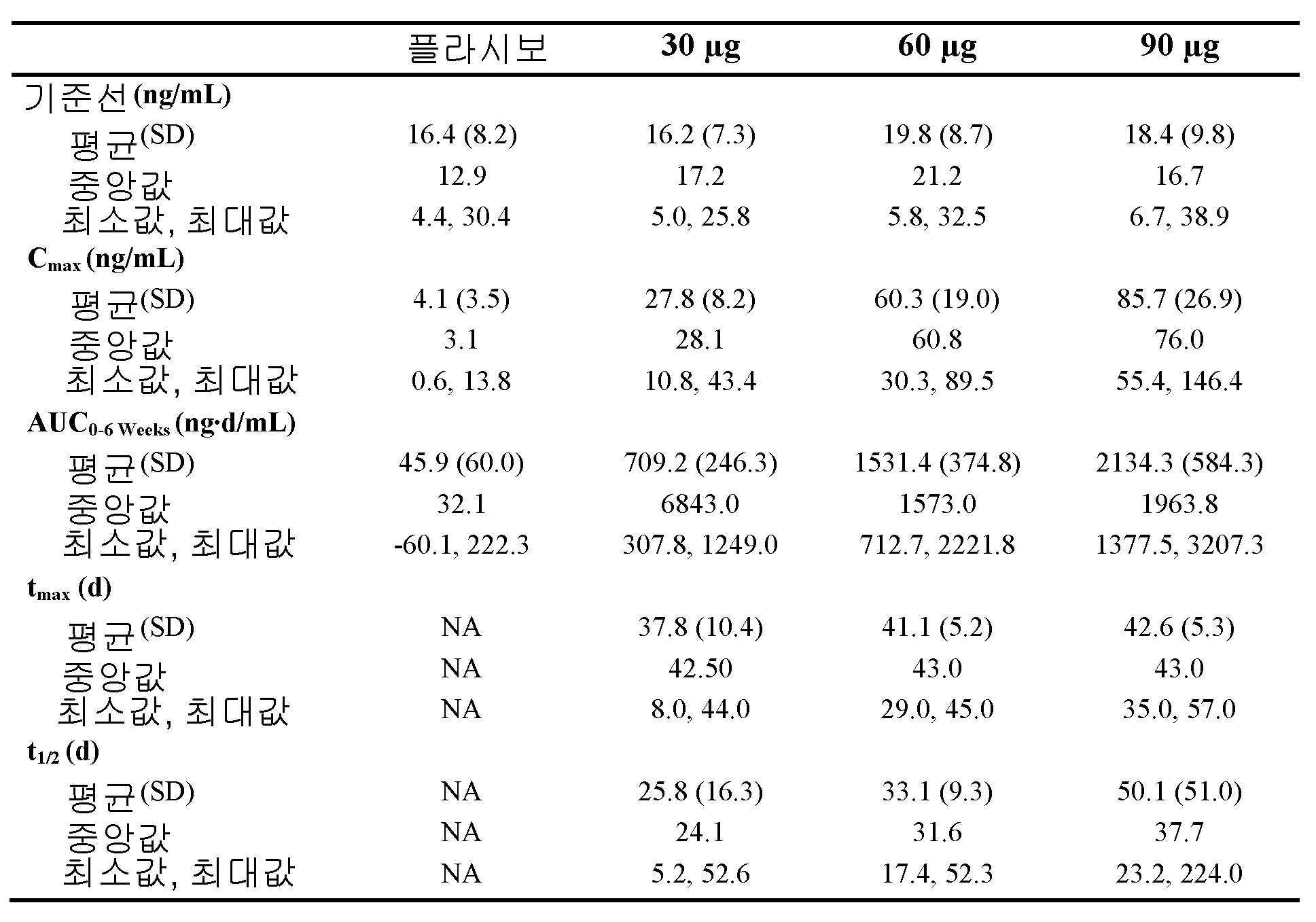
도 2는, 40℃ 및 상대 습도 75%에서 0개월 내지 6개월 동안 저장한 후의, 개시내용에 따른 제형의 용해 프로파일을 나타낸 것이다. 시(hour)로 나타낸 용해 시간은 x-축에 도시하며, 용해된 25-하이드록시비타민 D3의 평균%는 y-축에 도시한다. 도 2A, 2B, 및 2C는 각각, 30 ㎍, 60 ㎍, 및 90 ㎍의 25-하이드록시비타민 D3를 포함하는 제형의 용해 프로파일을 나타낸 것이다.
도 3은, 25℃ 및 상대 습도 60%에서 0개월 내지 12개월 동안 저장한 후의, 제형의 용해 프로파일을 나타낸 것이다. 시(hour)로 나타낸 용해 시간은 x-축에 도시하며, 방출된 25-하이드록시비타민 D3의 평균% 라벨 클레임(Label Claim)은 y-축에 도시한다. 도 3A는, 셀룰로스 화합물을 포함하지 않는 비교 제형의 용해 프로파일을 나타낸 것이다. 도 3B는, 본 개시내용에 따른 안정화된 제형의 용해 프로파일을 나타낸 것이다.
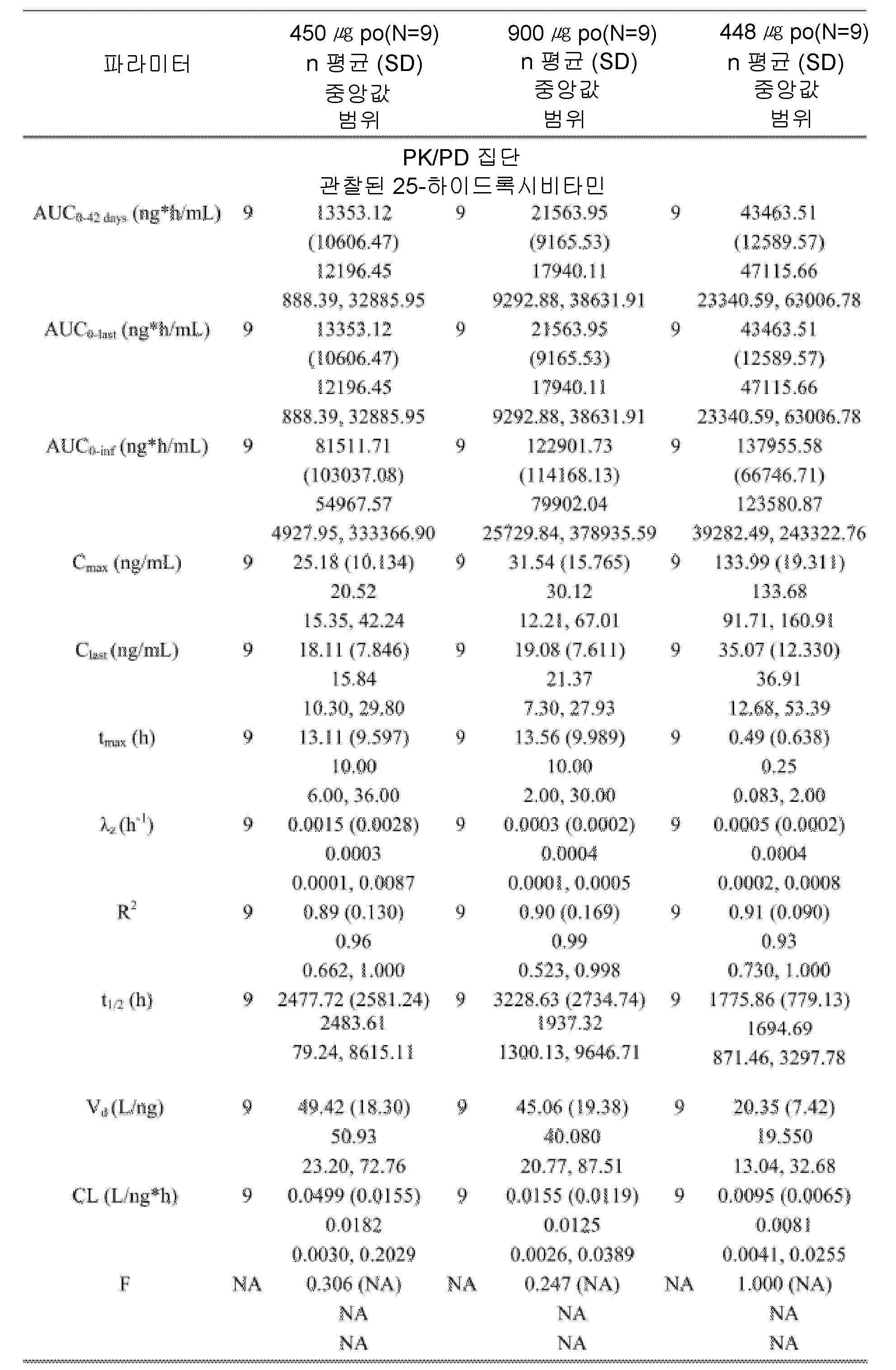
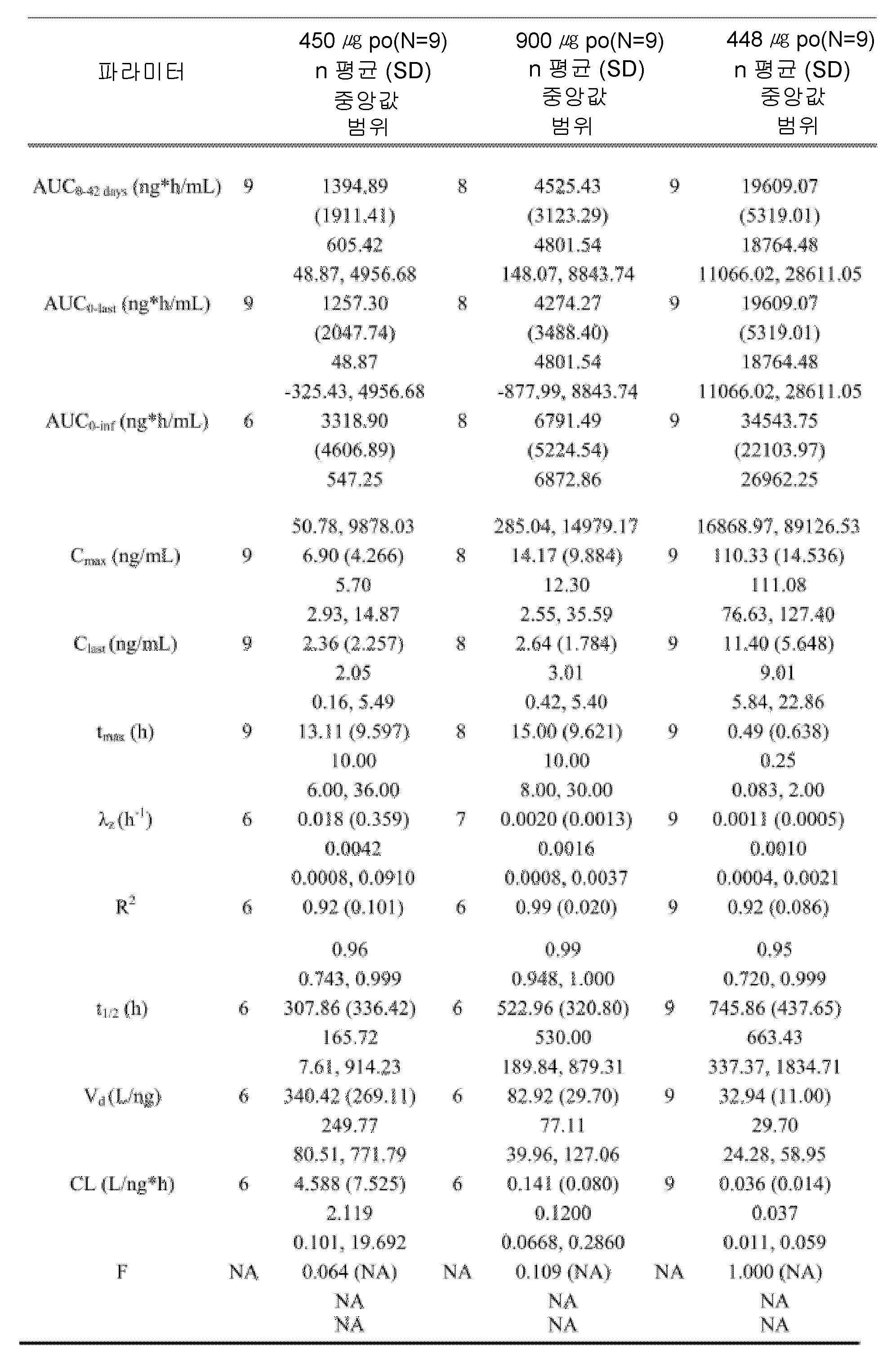
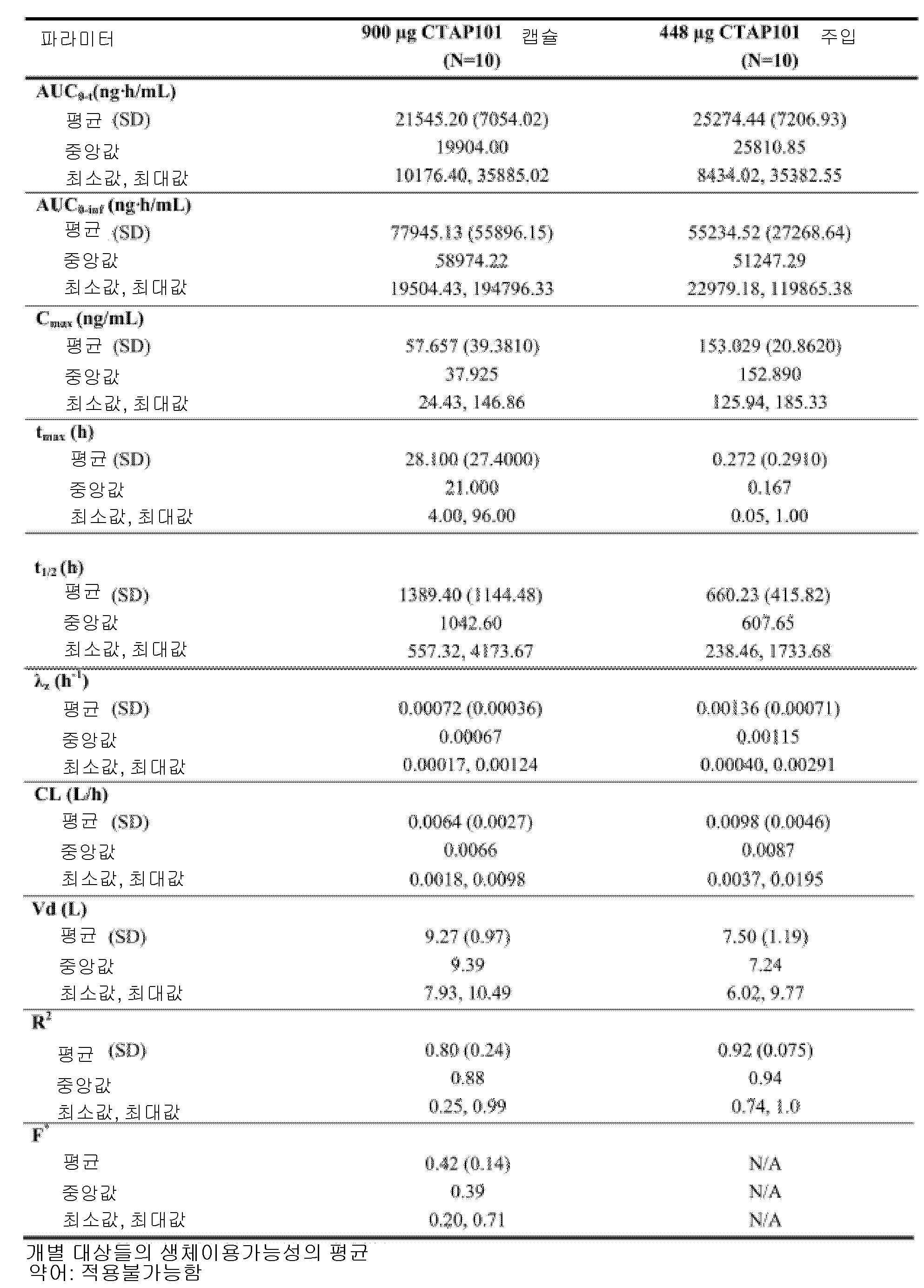
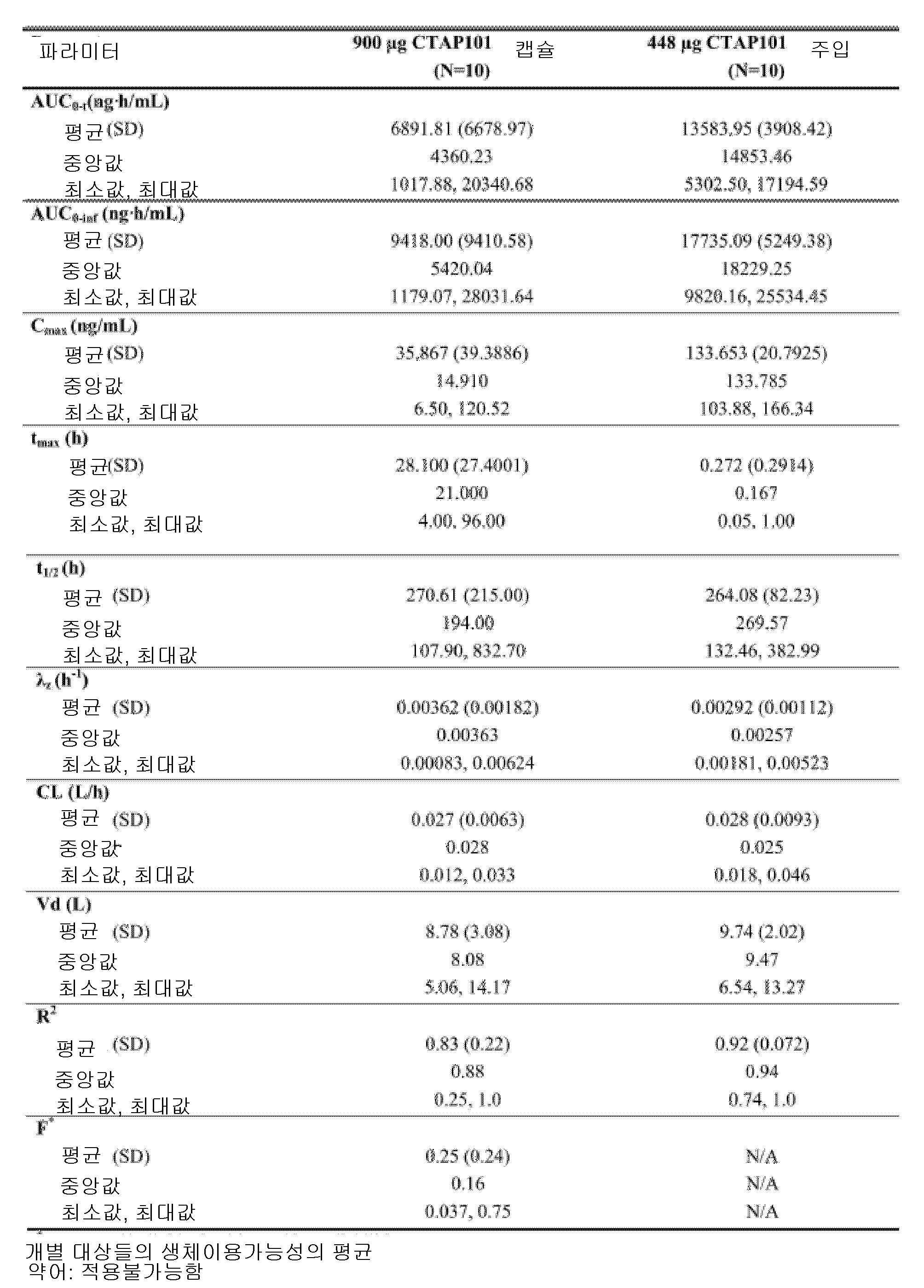
도 4는, 본 개시내용에 따른 제형으로 처리한 실시예 4에서 기술된 환자에 대한 치료군 (PK 집단)에 의한, 결과적인 평균 기준선-조정된(baseline-adjusted) 칼시페다이올 농도를 나타낸 것이다.
도 5는, 본 개시내용에 따른 제형으로 처리한 실시예 4에서 기술된 환자에 대한 치료군 (PK 집단)에 의한 칼시페다이올 농도에 대한 기준선-조정된 PK 파라미터에 대한 결과적인 요약을 나타낸 것이다.
도 6은, 본 개시내용에 따른 제형으로 처리한 실시예 4에서 기술된 환자에 대한 6주간의 치료 (PK 집단) 동안, 결과적인 평균 기준선-조정된 혈청내 1,25-다이하이드록시비타민 D의 농도를 나타낸 것이다.
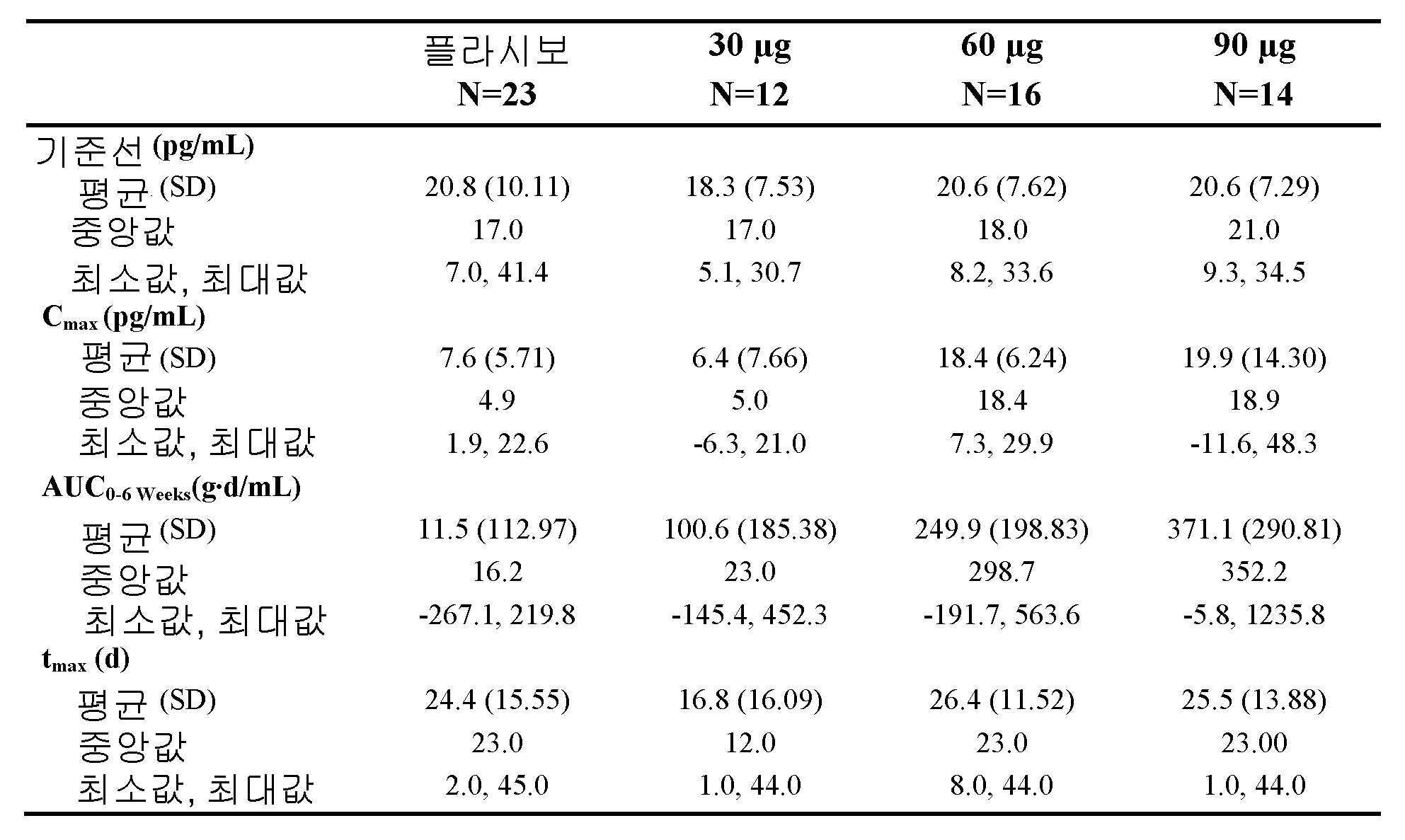
도 7은, 본 개시내용에 따른 제형으로 처리한 실시예 4에서 기술된 환자에 대한 치료군 (PK 집단)에 의한, 혈청내 1,25-다이하이드록시비타민 D에 대한 결과적인 기준선-조정된 반복-투여량 PK 파라미터의 요약을 나타낸 것이다.
도 8은, 본 개시내용에 따른 제형으로 처리한 실시예 4에서 기술된 환자에 대한 6주간의 치료 (PK 집단) 동안, 혈장내 iPTH 농도에서 기준선의 결과적인 평균%를 나타낸 것이다.
도 9는, 본 개시내용에 따른 제형으로 처리한 실시예 4에서 기술된 환자에 대한 치료군 (PK 집단)에 의한, 혈장내 iPTH에 대한 결과적인 기준선-조정된 반복-투여 PK 파라미터의 요약을 나타낸 것이다.
도 10 및 도 11은, 본 개시내용에 따른 제형으로 처리한 실시예 4에서 기술된 환자에 대한 PK 집단에서, 기준선-조정된 칼시페다이올 및 1,25-다이하이드록시비타민 D 노출 (AUC0-6wk)에 대하여, 혈장내 iPTH에 대한 EOT에서 기준선으로부터의 변화%를 나타낸 것이다.
| 시간 (h) | 1개월 | 3개월 | 6개월 | 9개월 | 12개월 | 18개월 | 24개월 |
| 25℃ 및 60% RH에서 저장 | |||||||
| 2 | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% |
| 4 | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% |
| 6 | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% |
| 8 | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% |
| 12 | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% |
| 40℃ 및 75% RH에서 저장 | |||||||
| 2 | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% |
| 4 | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% |
| 6 | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% |
| 8 | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% |
| 12 | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% | 30%, 또는 25%, 또는 20%, 또는 15%, 또는 10% |
| 5℃ / 주위 습도에서 저장한 후의 용해 | |||||||
| 시간 (hour) |
T=0p 슈도 |
T=0f 프레시 | 1개월 (%CV) [T=0p로부터의 변화%] [[T=0f로부터의 변화%]] |
3개월 (% CV) [T=0p로부터의 변화%] [[T=0f로부터의 변화%]] |
6개월 (% CV) [T=0p로부터의 변화%] [[T=0f로부터의 변화%]] |
9개월 (% CV) [T=0p로부터의 변화%] [[T=0f로부터의 변화%]] |
12개월 (% CV) [T=0p로부터의 변화%] [[T=0f로부터의 변화%]] |
| 2 | 6.1 (29.6) |
22.1 (17.2) |
8.9 (13.4) [45.9] [[59.7]] |
15.7 (27.4) [157.4] [[29.0]] |
9.1 (23) [49.2] [[58.8]] |
15.2 (47.2) [149.2] [[31.2]] |
12.7 (16.8) [108.2] [[42.5]] |
| 4 | 14.5 (15.7) |
52.0 (4.6) |
20.7 (19.1) [42.8] [[60.2]] |
22.3 (15.6) [53.8] [[57.1]] |
22.9 (7.1) [57.9] [[56.0]] |
25.2 (19.2) [73.8] [[51.5]] |
24.7 (13.5) [70.3] [[52.5]] |
| 6 | 27.6 (21.2) |
77.9 (4.6) |
35.7 (9.6) [29.3] [[54.2]] |
33.5 (2.6) [21.4] [[57.0]] |
34.1 (7.1) [23.6] [[56.2]] |
36.2 (20.5) [31.2] [[53.5]] |
34.8 (14.6) [26.1] [[55.3]] |
| 8 | 45.7 (23.6) |
96.8 (2.9) |
53.0 (9.9) [16.0] [[45.2]] |
47.4 (3.7) [3.7] [[51.0]] |
46.8 (6) [2.4] [[51.7]] |
47.8 (18.6) [4.6] [[50.6]] |
46.4 (8.9) [1.5] [[52.1]] |
| 12 | 89.7 (15.6) |
112.3 (1.6) |
100.0 (4.8) [11.5] [[11.0]] |
78.9 (8.1) [12.0] [[29.7]] |
76.9 (5.7) [14.3] [[31.5]] |
74.1 (17.3) [17.4] [[34.0]] |
78.8 (5.3) [12.2] [[29.8]] |
| 25℃ / 상대 습도 60%에서 저장한 후의 용해 | |||||||
| 용해 시간 (hour) |
T=0p 슈도 |
T=0f 프레시 |
1개월 (%CV) [T=0p로부터의 변화%] [[T=0f로부터의 변화%]] |
3개월 (% CV) [T=0p로부터의 변화%] [[T=0f로부터의 변화%]] |
6개월 (% CV) [T=0p로부터의 변화%] [[T=0f로부터의 변화%]] |
9개월 (% CV) [T=0p로부터의 변화%] [[T=0f로부터의 변화%]] |
12개월 (% CV) [T=0p로부터의 변화%] [[T=0f로부터의 변화%]] |
| 2 | 6.1 (29.6) |
22.1 (17.2) |
7.6 (7.7) [24.6] [[65.6]] |
10.8 (15.7) [77.0] [[51.1]] |
8.5 (19.2) [39.3] [[61.5]] |
10.8 (15.8) [77.0] [[51.1]] |
13.5 (24.7) [121.3] [[38.9]] |
| 4 | 14.5 (15.7) |
52.0 (4.6) |
18.7 (18.6) [29.0] [[64.0]] |
22.8 (23.7) [57.2] [[56.2]] |
17.9 (11.9) [23.4] [[65.6]] |
21.4 (5.5) [47.6] [[58.8]] |
24.5 (17.4) [69.0] [[52.9]] |
| 6 | 27.6 (21.2) |
77.9 (4.6) |
27.1 (22.7) [1.8] [[65.2]] |
30.7 (29.6) [11.2] [[60.6]] |
23.8 (11.6) [13.8] [[69.4]] |
27.0 (7.3) [2.2] [[65.3]] |
30.0 (15.7) [8.7] [[61.5]] |
| 8 | 45.7 (23.6) |
96.8 (2.9) |
37.1 (18.1) [18/8] [[61.7]] |
40.6 (29.9) [11.2] [[58.1]] |
28.5 (13.2) [37.6] [[70.6]] |
32.3 (6.4) [29.3] [[66.6]] |
35.6 (14.7) [22.1] [[63.2]] |
| 12 | 89.7 (15.6) |
112.3 (1.6) |
61.6 (16.6) [31.3] [[45.1]] |
53.0 (32.2) [40.9] [[52.8]] |
38.5 (12.2) [57.1] [[65.7]] |
38.9 (6) [56.6] [[65.4]] |
44.2 (12.2) [50.7] [[60.6]] |
| 40℃ / 상대 습도 75%에서 저장한 후의 용해 | |||||
| 용해 시간 (hour) |
T=0p 슈도 |
T=0f 프레시 |
1개월 (%CV) [T=0p로부터의 변화%] [[T=0f로부터의 변화%]] |
3개월 (%CV) [T=0p로부터의 변화%] [[T=0f로부터의 변화%]] |
6개월 (%CV) [T=0p로부터의 변화%] [[T=0f로부터의 변화%]] |
| 2 | 6.1 (29.6) |
22.1 (17.2) |
11.7 (50) [91.8] [[47.1]] |
16.9 (36.3) [177.0] [[23.5]] |
1.8 (75) [70.5] [[91.9]] |
| 4 | 14.5 (15.7) |
52.0 (4.6) |
52.0 (45.1) [258.6] [[0]] |
59.8 (31.3) [312.4] [[15.0]] |
36.2 (21.8) [149.7] [[30.4]] |
| 6 | 27.6 (21.2) |
77.9 (4.6) |
87.0 (21.5) [215.2] [[11.7]] |
97.8 (24) [254.3] [[25.5]] |
76.7 (12.3) [177.9] [[1.5]] |
| 8 | 45.7 (23.6) |
96.8 (2.9) |
107.3 (8.1) [134.8] [[10.8]] |
110.9 (13) [142.7] [[14.6]] |
101.2 (6.1) [121.4] [[4.5]] |
| 12 | 89.7 (15.6) |
112.3 (1.6) |
118.7 (1.7) [32.3] [[5.7]] |
115.1 (3.7) [28.3] [[2.5]] |
112.6 (2.1) [25.5] [[0.3]] |
| 40℃에서 72시간 동안 경화한 후의 용해 | ||||
| 용해 시간 (hour) |
T=0 | 2주 % LC [변화%] |
4주 % LC [변화%] |
8주 % LC [변화%] |
| 2 | 17.4 | 12.4 [28.7] |
11.4 [34.5] |
8.2 [52.9] |
| 4 | 53.3 | 46.4 [12.9] |
40.1 [24.8] |
26.6 [50.1] |
| 6 | 86.2 | 76.2 [11.6] |
69.0 [20.0] |
44.8 [48.0] |
| 8 | 103.8 | 102.0 [1.7] |
95.8 [7.7] |
66.6 [35.8] |
| 12 | 115.7 | 110.5 [4.5] |
119.8 [3.5] |
103.3 [10.7] |
| 실온 / 주위 습도에서 저장한 후의 용해 | |||
| 용해 시간 (hour) |
T=0 | 3주 % LC [변화%] |
11주 % LC [변화%] |
| 2 | 15.45 | 14.20 [8.1%] |
12.08 [21.8%] |
| 4 | 36.3 | 38.80 [6.9%] |
37.13 [2.3%] |
| 6 | 56.9 | 62.70 [10.2%] |
59.51 [4.6%] |
| 8 | 71.1 | 71.90 [1.1%] |
69.76 [1.9%] |
| 12 | 89.4 | 91.20 [2.0%] |
89.90 [0.6%] |
| 실온 / 주위 습도에서 저장한 후의 용해 | ||||
| 용해 시간 (hour) |
T=0 | 6주 % LC [변화%] |
13주 % LC [변화%] |
26주 % LC [변화%] |
| 2 | 30.15 | 25.40 [15.8%] |
25.20 [16.4%] |
21.10 [30.0%] |
| 4 | 58.55 | 51.90 [11.4%] |
51.80 [11.5%] |
45.20 [22.8%] |
| 6 | 72.1 | 74.40 [3.2%] |
73.00 [1.2%] |
67.63 [6.2%] |
| 8 | 80.55 | 84.30 [4.7%] |
84.50 [4.9%] |
77.30 [4.0%] |
| 12 | 91.8 | 94.10 [2.5%] |
94.40 [2.8%] |
91.16 [0.7%] |
| 성분 | 양 |
| 25-하이드록시비타민 D3 | 30 ㎍, 60 ㎍, 또는 90 ㎍ |
| 파라핀 왁스 | 20.00 중량% |
| 광유 | 35.36 중량% |
| 하이드록시 프로필 메틸셀룰로스 K100M CR (METHOCEL) | 10.00 중량% |
| 글리세롤 모노스테아레이트 | 22.55 중량% |
| 라우로일 마크로골글리세라이드 및 폴리옥실글리세라이드 (GELUCIRE 44/14) | 9.75 중량% |
| 무수 알코올 | 2.32 중량% |
| BHT | 0.02 중량% |
| 연질 캡슐제 셀 (VEGICAPS) |
| 25℃/ 상대 습도 60%에서 저장한 후의 용해 | ||||||||
|
시간
(hour) |
T=0
%LC (% CV) |
1개월
%LC (% CV) |
3개월
%LC (% CV) |
6개월
%LC (% CV) |
9개월
%LC (% CV) |
12개월
%LC (% CV) |
18개월
%LC (% CV) |
24개월
%LC (% CV) |
| 30 ㎍ 25-하이드록시비타민 D3 (실시예 제형 C) | ||||||||
| 2 | 10.1 (16.1) |
12.9 (25.2) |
14.0 (48.5) |
9.6 (38.3) |
10.5 (18.2) |
13.5 (35.8) |
10.6 (26.5) |
7.0 (47.8) |
| 4 | 43.6 (12.7) |
48.8 (10.9) |
45.9 (29.9) |
36.8 (25) |
37.2 (11.1) |
50.2 (14.8) |
39.3 (22.9) |
39.6 (32.3) |
| 6 | 83.1 (5.7) |
73.4 (9.2) |
72.4 (7.5) |
71.4 (6.8) |
66.7 (10.6) |
70.6 (11.5) |
70.1 (7.2) |
69.3 (12.6) |
| 8 | 96.4 (6.5) |
89.6 (4.4) |
88.4 (4.7) |
88.4 (5.7) |
85.2 (4.9) |
85.4 (9) |
84 (5.1) |
85.7 (8.2) |
| 12 | 115.8 (4.5) |
104.2 (1.9) |
105.6 (1.4) |
106.1 (1.6) |
101.5 (1.5) |
100.8 (3.9) |
100.2 (4.5) |
103 (2.6) |
| 60 ㎍ 25-하이드록시비타민 D3 (실시예 제형 D) | ||||||||
| 2 | 17.5 (20) |
16.3 (27.4) |
16.1 (25.3) |
17.7 (34.7) |
11.2 (20.2) |
14.3 (29.1) |
16.1 (25.8) |
12.4 (52.3) |
| 4 | 53.6 (19.6) |
55.1 (18.8) |
53.8 (12.8) |
55.3 (17.9) |
43.7 (14) |
51 (22.5) |
52.9 (16.7) |
41 (36.6) |
| 6 | 83.9 (7.5) |
78.7 (8.3) |
79.9 (6.6) |
78.4 (4.7) |
72.2 (7.1) |
75 (13) |
72 (9.9) |
64.7 (29.6) |
| 8 | 99.2 (3.9) |
94.3 (5.2) |
97.2 (4.8) |
92.5 (3.3) |
87 (5.5) |
88.9 (6.3) |
84.9 (12.3)* |
81.3 (21.7) |
| 12 | 104.8 (3.3) |
108.7 (1.8) |
111.9 (1.1) |
104.5 (0.5) |
103.1 (0.8) |
104.7 (1.4) |
99.8 (4.6)* |
101.7 (8) |
| 90 ㎍ 25-하이드록시비타민 D3 (실시예 제형 E) | ||||||||
| 2 | 14.9 (19.9) |
14.9 (8.6) |
13.3 (41.2) |
13.5 (23.4) |
14.3 (30.4) |
15 (35.1) |
9.5 (37.1) |
8.5 (53.2) |
| 4 | 49.9 (16.4) |
46.9 (10.2) |
52.4 (18.5) |
49.7 (16.4) |
45.9 (25.6) |
51.8 (14) |
36.8 (34.5) |
34.4 (24.9) |
| 6 | 89.4 (7.2) |
71.4 (4.1) |
81.1 (5.6) |
74.2 (11.1) |
71.6 (17.6) |
77 (4.9) |
64.4 (10.3) |
64.5 (15.1) |
| 8 | 101.7 (2.5) |
84.9 (2.8) |
96.1 (2.3) |
90.8 (6.4) |
89 (9.4) |
91.6 (3.3) |
77.4 (15.7) |
83.6 (11.2) |
| 12 | 103 (2.1) |
99.3 (2.4) |
110 (1.3) |
104.4 (1.1) |
100.5 (2.4) |
104.6 (0.3) |
96.8 (5) |
102.8 (3.4) |
| 시간 (h) |
1개월
변화% |
3개월
변화% |
6개월
변화% |
9개월
변화% |
12개월
변화% |
18개월
변화% |
24개월
변화% |
| 30 ㎍ 25-하이드록시비타민 D3 (실시예 제형 C) | |||||||
| 2 | 27.7% | 38.6% | 5.0% | 4.0% | 33.7% | 5.0% | 30.7% |
| 4 | 11.9% | 5.3% | 15.6% | 14.7% | 15.1% | 9.9% | 9.2% |
| 6 | 11.7% | 12.9% | 14.1% | 19.7% | 15.0% | 15.6% | 16.6% |
| 8 | 7.1% | 8.3% | 8.3% | 11.6% | 11.4% | 12.9% | 11.1% |
| 12 | 10.0% | 8.8% | 8.4% | 12.3% | 13.0% | 13.5% | 11.1% |
| 60 ㎍ 25-하이드록시비타민 D3 (실시예 제형 D) | |||||||
| 2 | 6.9% | 8.0% | 1.1% | 36.0% | 18.3% | 8.0% | 29.1% |
| 4 | 2.8% | 0.4% | 3.2% | 18.5% | 4.9% | 1.3% | 23.5% |
| 6 | 6.2% | 4.8% | 6.6% | 13.9% | 10.6% | 14.2% | 22.9% |
| 8 | 4.9% | 2.0% | 6.8% | 12.3% | 10.4% | 14.4% | 18.0% |
| 12 | 3.7% | 6.8% | 0.3% | 1.6% | 0.1% | 4.8% | 3.0% |
| 90 ㎍ 25-하이드록시비타민 D3 (실시예 제형 E) | |||||||
| 2 | 0.0% | 10.7% | 9.4% | 4.0% | 0.7% | 36.2% | 43.0% |
| 4 | 6.0% | 5.0% | 0.4% | 8.0% | 3.8% | 26.3% | 31.1% |
| 6 | 20.1% | 9.3% | 17.0% | 19.9% | 13.9% | 28.0% | 27.9% |
| 8 | 16.5% | 5.5% | 10.7% | 12.5% | 9.9% | 23.9% | 17.8% |
| 12 | 3.6% | 6.8% | 1.4% | 2.4% | 1.6% | 6.0% | 0.2% |
| 40℃/ 상대 습도 75%에서 저장한 후의 용해 | ||||
|
시간
(hours) |
초기
% LC (%CV) |
1개월
% LC (%CV) |
3개월
% LC (%CV) |
6개월
% LC (%CV) |
| 30 ㎍ 25-하이드록시비타민 D3 (실시예 제형 C) | ||||
| 2 | 10.1 (16.1) |
11.3 (54.3) |
13.5 (53.8) |
11.1 (14.8) |
| 4 | 43.6 (12.7) |
46 (27.1) |
44.4 (23.9) |
40.9 (13.9) |
| 6 | 83.1 (5.7) |
72.7 (9.7) |
62.8 (20.4) |
68.7 (7.3) |
| 8 | 96.4 (6.5) |
88.7 (5) |
76.5 (12.5) |
82.5 (4.1) |
| 12 | 115.8 (4.5) |
103.1 (0.9) |
93.4 (7.6) |
96.4 (2.8) |
| 60 ㎍ 25-하이드록시비타민 D3 (실시예 제형 D) | ||||
| 2 | 17.5 (20) |
12.9 (40.7) |
15.5 (37) |
13.9 (40) |
| 4 | 53.6 (19.6) |
50.7 (18.5) |
54.4 (12) |
49.8 (16.3) |
| 6 | 83.9 (7.5) |
75.8 (9.7) |
78.3 (10.6) |
74.7 (8.1) |
| 8 | 99.2 (3.9) |
91.2 (7.9) |
91.6 (9.1) |
88.5 (6.5) |
| 12 | 104.8 (3.3) |
100.7 (5.1) |
104.1 (6.5) |
101.5 (2.3) |
| 90 ㎍ 25-하이드록시비타민 D3 (실시예 제형 E) | ||||
| 2 | 14.9 (19.9) |
18.5 (32.5) |
10.2 (44.6) |
8.3 (34.7) |
| 4 | 49.9 (16.4) |
50.7 (12..8) |
47 (14.6) |
44.8 (10.6) |
| 6 | 89.4 (7.2) |
74.7 (8) |
72.9 (5.2) |
73 (3.4) |
| 8 | 101.7 (2.5) |
90.5 (5) |
86.6 (4.8) |
87.9 (3) |
| 12 | 103 (2.1) |
100.1 (1.4) |
102.5 (1.6) |
101.0 (1.9) |
| 시간 (hours) |
1개월
변화% |
3개월
변화% |
6개월
변화% |
| 30 ㎍ 25-하이드록시비타민 D3 (실시예 제형 C) | |||
| 2 | 11.9% | 33.7% | 9.9% |
| 4 | 5.5% | 1.8% | 6.2% |
| 6 | 12.5% | 24.4% | 17.3% |
| 8 | 8.0% | 20.6% | 14.4% |
| 12 | 11.0% | 19.3% | 16.8% |
| 60 ㎍ 25-하이드록시비타민 D3 (실시예 제형 D) | |||
| 2 | 26.3% | 11.4% | 20.6% |
| 4 | 5.4% | 1.5% | 7.1% |
| 6 | 9.7% | 6.7% | 11.0% |
| 8 | 8.1% | 7.7% | 10.8% |
| 12 | 3.9% | 0.7% | 3.1% |
| 90 ㎍ 25-하이드록시비타민 D3 (실시예 제형 E) | |||
| 2 | 24.2% | 31.5% | 44.3% |
| 4 | 1.6% | 5.8% | 10.2% |
| 6 | 16.4% | 18.5% | 18.3% |
| 8 | 11.0% | 14.8% | 13.6% |
| 12 | 2.8% | 0.5% | 1.9% |
| 25℃/ 상대 습도 60%에서 저장한 후의 용해 | ||||
|
용해 시간
(hours) |
비교 제형 1 | 실시예 제형 E | ||
| 초기 % LC |
12개월 % LC |
초기 % LC |
12개월 % LC |
|
| 2 | 22.1 | 13.5 | 14.9 | 15.0 |
| 4 | 52.2 | 24.5 | 49.9 | 51.8 |
| 6 | 77.9 | 30.0 | 89.4 | 77.0 |
| 8 | 96.8 | 35.6 | 101.7 | 91.6 |
| 12 | 112.3 | 44.2 | 103.0 | 104.6 |
|
용해 시간
(hours) |
비교 제형 1 초기로부터의 변화% |
실시예 제형 E 초기로부터의 변화% |
| 2 | 38.9% | 0.7% |
| 4 | 53.1% | 3.8% |
| 6 | 61.5% | 13.9% |
| 8 | 63.2% | 9.9% |
| 12 | 60.6% | 1.6% |
Claims (26)
- 25-하이드록시비타민 D2 및 25-하이드록시비타민 D3 중 하나 또는 둘 다 포함하는 비타민 D 화합물의 방출 조절형 제형(controlled release formulation)으로서,
상기 제형은, 상기 비타민 D 화합물을 방출가능하게 결합하며, 조절가능하게 방출하는 매트릭스를 포함하며,
상기 매트릭스는 셀룰로스 유도체를 포함하는, 비타민 D 화합물의 방출 조절형 제형. - 25-하이드록시비타민 D2 및 25-하이드록시비타민 D3 중 하나 또는 둘 다;
왁스 매트릭스; 및
안정화제, 선택적으로는 셀룰로스 화합물로 구성된 혼합물을 포함하는,
안정화된 방출 조절형 비타민 D 화합물 제형. - 제1 항 또는 제2 항에 있어서,
상기 셀룰로스 화합물 또는 셀룰로스 유도체가 셀룰로스 에테르를 포함하는 것을 특징으로 하는, 제형. - 제3 항에 있어서,
상기 셀룰로스 에테르가, 메틸셀룰로스, 하이드록실 프로필 메틸셀룰로스, 하이드록실 에틸 메틸셀룰로스, 하이드록실 에틸 셀룰로스, 하이드록실 프로필 셀룰로스, 또는 이들의 조합으로 이루어진 군으로부터 선택되는 것을 특징으로 하는, 제형. - 제4 항에 있어서,
상기 셀룰로스 에테르가 하이드록실 프로필 메틸셀룰로스인 것을 특징으로 하는, 제형. - 제1 항 또는 제2 항에 있어서,
상기 제형이, 25℃ 및 상대 습도 60%의 저장 조건에 2개월 동안 노출시킨 후 수행된 시험관 내 용해에서, 4시간 이후 소정의 용해 시점에, 25-하이드록시비타민 D를, 저장 조건에 노출시키기 전에 수행된 시험관 내 용해에서 동일한 시점에 방출되는 양과 비교해, 30% 이하의 차이로 방출하는 것을 특징으로 하는, 제형. - 제1 항 또는 제2 항에 있어서,
상기 제형이, 40℃ 및 상대 습도 75%의 저장 조건에 1개월 동안 노출시킨 후 수행된 시험관 내 용해에서, 4시간 이후 소정의 용해 시점에, 25-하이드록시비타민 D를, 저장 조건에 노출시키기 전에 수행된 시험관 내 용해에서 동일한 시점에 방출되는 양과 비교해, 30% 이하의 차이로 방출하는 것을 특징으로 하는, 제형. - 제1 항 또는 제2 항에 있어서,
상기 매트릭스가 방출 조절제, 유화제, 및 흡수 증강제를 포함하는 왁스 매트릭스를 포함하는 것을 특징으로 하는, 제형. - 제8 항에 있어서,
상기 왁스가 파라핀을 포함하는 것을 특징으로 하는, 제형. - 제8 항에 있어서,
상기 유화제의 HLB 값이 7 미만인 것을 특징으로 하는, 제형. - 제8 항에 있어서,
상기 유화제가 글리세롤 모노스테아레이트를 포함하는 것을 특징으로 하는, 제형. - 제8 항에 있어서,
상기 흡수 증강제의 HLB 값이 약 13 내지 약 18인 것을 특징으로 하는, 제형. - 제8 항에 있어서,
상기 흡수 증강제가 라우로일 마크로골글리세라이드와 라우로일 폴리옥실글리세라이드의 혼합물인 것을 특징으로 하는, 제형. - 제1 항 또는 제2 항에 있어서,
상기 비타민 D 화합물이 25-하이드록시비타민 D3를 포함하는 것을 특징으로 하는, 제형. - 제1 항 또는 제2 항에 있어서,
유성 비히클을 추가로 포함하는 것을 특징으로 하는, 제형. - 제15 항에 있어서,
상기 유성 비히클이 광유를 포함하는 것을 특징으로 하는, 제형. - 제8 항에 있어서,
상기 제형이, 파라핀 약 20 중량%, 글리세롤 모노스테아레이트 약 20 중량% 내지 약 25 중량%, 라우로일 마크로골글리세라이드와 라우로일 폴리옥실글리세라이드의 혼합물 약 10 중량%, 광유 약 30 중량% 내지 약 35 중량%, 및 하이드록실 프로필 메틸셀룰로스 약 10 중량% 내지 약 15 중량%를 포함하는 것을 특징으로 하는, 제형. - 제1 항 또는 제2 항에 따른 제형을 포함하는, 캡슐제, 정제, 사세제(sachet), 당의제(dragee), 또는 좌제 형태의 서방성 제형(sustained release dosage form).
- 비타민 D 화합물이, 2시간째에 30% 미만; 6시간째에 45% 초과; 및 12시간째에 80% 초과로 방출되는 용해 프로파일을 특징으로 하는, 제1 항 또는 제2 항에 따른 안정화된 제형(dosage form).
- 파라핀 약 20 중량%, 글리세롤 모노스테아레이트 약 20 중량% 내지 약 25 중량%, 라우로일 마크로골글리세라이드와 라우로일 폴리옥실글리세라이드의 혼합물 약 10 중량%, 광유 약 30 중량% 내지 약 35 중량%, 및 하이드록실 프로필 메틸셀룰로스 약 10 중량% 내지 약 15 중량%를 포함하는, 25-하이드록시비타민 D2 및/또는 25-하이드록시비타민 D3의 안정화된 제형.
- 제1 항, 제2 항, 또는 제20 항 중 어느 한 항에 있어서,
환자에서, 혈청내 25-하이드록시비타민 D의 농도를 높이는 데 사용하기 위한 것임을 특징으로 하는, 제형. - 제1 항, 제2 항, 또는 제20 항 중 어느 한 항에 있어서,
환자에서, 혈청내 1,25-하이드록시비타민 D의 농도를 높이는 데 사용하기 위한 것임을 특징으로 하는, 제형. - 제1 항, 제2 항, 또는 제20 항 중 어느 한 항에 있어서,
환자에서, 혈청내 온전한(intact) 부갑상선 호르몬의 농도를 낮추는 데 사용하기 위한 것임을 특징으로 하는, 제형. - 제1 항, 제2 항, 또는 제20 항 중 어느 한 항에 있어서,
25-하이드록시비타민 D 부족 또는 결핍을 치료하는 데 사용하기 위한 것임을 특징으로 하는, 제형. - 제1 항, 제2 항, 또는 제20 항 중 어느 한 항에 있어서,
2차 부갑상선 기능항진증(secondary hyperparathyodism)을 치료하는 데 사용하기 위한 것임을 특징으로 하는, 제형. - 제1 항, 제2 항, 또는 제20 항 중 어느 한 항에 있어서,
만성 신장 질환을 앓고 있는 환자를 치료하는 데 사용하기 위한 것임을 특징으로 하는, 제형.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361801896P | 2013-03-15 | 2013-03-15 | |
| US61/801,896 | 2013-03-15 | ||
| KR1020190094318A KR20190095216A (ko) | 2013-03-15 | 2019-08-02 | 안정화되고 변형된 비타민 d 방출 제형 및 이의 투여 방법 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020190094318A Division KR20190095216A (ko) | 2013-03-15 | 2019-08-02 | 안정화되고 변형된 비타민 d 방출 제형 및 이의 투여 방법 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20210078463A true KR20210078463A (ko) | 2021-06-28 |
Family
ID=50478969
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020140026781A Active KR101847947B1 (ko) | 2013-03-15 | 2014-03-06 | 안정화되고 변형된 비타민 d 방출 제형 |
| KR1020140151051A Active KR102203003B1 (ko) | 2013-03-15 | 2014-11-03 | 안정화되고 변형된 비타민 d 방출 제형 및 이의 투여 방법 |
| KR1020190094318A Ceased KR20190095216A (ko) | 2013-03-15 | 2019-08-02 | 안정화되고 변형된 비타민 d 방출 제형 및 이의 투여 방법 |
| KR1020210079948A Ceased KR20210078463A (ko) | 2013-03-15 | 2021-06-21 | 안정화되고 변형된 비타민 d 방출 제형 및 이의 투여 방법 |
Family Applications Before (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020140026781A Active KR101847947B1 (ko) | 2013-03-15 | 2014-03-06 | 안정화되고 변형된 비타민 d 방출 제형 |
| KR1020140151051A Active KR102203003B1 (ko) | 2013-03-15 | 2014-11-03 | 안정화되고 변형된 비타민 d 방출 제형 및 이의 투여 방법 |
| KR1020190094318A Ceased KR20190095216A (ko) | 2013-03-15 | 2019-08-02 | 안정화되고 변형된 비타민 d 방출 제형 및 이의 투여 방법 |
Country Status (37)
| Country | Link |
|---|---|
| US (6) | US9861644B2 (ko) |
| EP (4) | EP3888638A1 (ko) |
| JP (4) | JP6492051B2 (ko) |
| KR (4) | KR101847947B1 (ko) |
| CN (2) | CN105246464A (ko) |
| AR (1) | AR095576A1 (ko) |
| AU (2) | AU2014228069B2 (ko) |
| BR (1) | BR112015023658A2 (ko) |
| CA (1) | CA2905409C (ko) |
| CL (1) | CL2015002659A1 (ko) |
| CR (1) | CR20190178A (ko) |
| CY (3) | CY1123167T1 (ko) |
| DE (1) | DE202014011525U1 (ko) |
| DK (3) | DK3332773T3 (ko) |
| EA (2) | EA038867B1 (ko) |
| EC (1) | ECSP23024864A (ko) |
| ES (3) | ES2834900T3 (ko) |
| HK (2) | HK1220362A1 (ko) |
| HR (3) | HRP20201284T1 (ko) |
| HU (3) | HUE055591T2 (ko) |
| IL (2) | IL241456B (ko) |
| LT (3) | LT3332773T (ko) |
| MX (2) | MX2015012625A (ko) |
| MY (1) | MY194092A (ko) |
| NO (1) | NO2021007I1 (ko) |
| PE (1) | PE20151761A1 (ko) |
| PH (2) | PH12015502162A1 (ko) |
| PL (3) | PL3650016T3 (ko) |
| PT (3) | PT3332773T (ko) |
| RS (3) | RS60846B1 (ko) |
| SA (1) | SA515361134B1 (ko) |
| SG (2) | SG11201507323PA (ko) |
| SI (3) | SI2968172T1 (ko) |
| SM (3) | SMT202000651T1 (ko) |
| TW (1) | TWI659753B (ko) |
| UA (1) | UA123386C2 (ko) |
| WO (1) | WO2014143941A1 (ko) |
Families Citing this family (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DK3095447T3 (da) | 2006-02-03 | 2022-01-31 | Opko Renal Llc | Behandling af vitamin d-insufficiens og -mangel med 25-hydroxyvitamin d2 og 25-hydroxyvitamin d3 |
| DK3357496T3 (da) | 2006-06-21 | 2020-05-11 | Opko Ireland Global Holdings Ltd | Terapi ved brug af vitamin d-repletteringsmiddel og vitamin d-hormonsubstitutionsmiddel |
| PL2148661T3 (pl) | 2007-04-25 | 2013-07-31 | Cytochroma Inc | Doustne kompozycje o kontrolowanym uwalnianiu zawierające związek będący witaminą D i woskowy nośnik |
| US11752158B2 (en) | 2007-04-25 | 2023-09-12 | Eirgen Pharma Ltd. | Method of treating vitamin D insufficiency and deficiency |
| EP3636280B1 (en) | 2010-03-29 | 2025-05-14 | EirGen Pharma Ltd. | Methods and compositions for reducing parathyroid levels |
| KR101847947B1 (ko) | 2013-03-15 | 2018-05-28 | 옵코 아이피 홀딩스 Ⅱ 인코포레이티드 | 안정화되고 변형된 비타민 d 방출 제형 |
| US20180085381A1 (en) * | 2014-08-07 | 2018-03-29 | Opko Ireland Global Holdings, Ltd. | Adjunctive Therapy With 25-Hydroxyvitamin D |
| US10220047B2 (en) * | 2014-08-07 | 2019-03-05 | Opko Ireland Global Holdings, Ltd. | Adjunctive therapy with 25-hydroxyvitamin D and articles therefor |
| EP3053598A1 (en) | 2015-02-06 | 2016-08-10 | Faes Farma, S.A. | Calcifediol soft capsules |
| JP7034595B2 (ja) * | 2016-03-23 | 2022-03-14 | 株式会社ファンケル | ビタミンd3安定化組成物 |
| KR20230056790A (ko) * | 2016-03-28 | 2023-04-27 | 옵코 아일랜드 글로벌 홀딩스 리미티드 | 비타민 d 치료 방법 |
| WO2019032746A1 (en) | 2017-08-08 | 2019-02-14 | Fresenius Medical Care Holdings, Inc. | SYSTEMS AND METHODS FOR TREATING AND ESTIMATING THE PROGRESSION OF CHRONIC RENAL DISEASE |
| JP7590184B2 (ja) * | 2018-04-03 | 2024-11-26 | オプコ アイルランド グローバル ホールディングス リミテッド | 肥満外科手術患者におけるカルシフェジオールの使用 |
| BR102018008324A2 (pt) | 2018-04-25 | 2019-11-05 | Laboratorios Ferring Ltda | composição farmacêutica de uso tópico e processo de fabricação de composição farmacêutica de uso tópico |
| CN108902985A (zh) * | 2018-06-08 | 2018-11-30 | 唐飞 | 25-羟基维生素d3在制备保健食品中的应用 |
| KR20210054539A (ko) * | 2018-08-31 | 2021-05-13 | 옵코 아일랜드 글로벌 홀딩스 리미티드 | 비타민 d의 소아용 투여 형태, 이의 제조 및 사용 |
| KR102715550B1 (ko) * | 2018-12-10 | 2024-10-10 | 신성대학교 산학협력단 | 유효성분을 함유하는 왁스 파티클 및 이의 제조방법 |
| CN109632444A (zh) * | 2018-12-24 | 2019-04-16 | 郑州安图生物工程股份有限公司 | 液态25-羟基维生素d校准品稀释液 |
| CN113573714A (zh) * | 2019-02-06 | 2021-10-29 | 艾尔金制药有限公司 | 用骨化二醇控制甲状旁腺功能亢进进展的方法和其中使用的组合物 |
| CN110917169A (zh) * | 2019-12-11 | 2020-03-27 | 正大制药(青岛)有限公司 | 帕立骨化醇胶囊剂及其制备方法 |
| US20230096602A1 (en) | 2020-01-27 | 2023-03-30 | Eirgen Pharma Ltd. | Tetrahydrocyclopenta[b]indole compounds for treatment of renal disease |
| EP4132477A1 (en) | 2020-04-06 | 2023-02-15 | EirGen Pharma Ltd. | 25-hydroxyvitamin d for the treatment of sars-cov-2 infection |
| CA3181945A1 (en) | 2020-05-31 | 2021-12-09 | Eirgen Pharma Ltd. | Hard capsule dosage form and uses thereof |
| CN111991404B (zh) * | 2020-10-10 | 2021-08-13 | 西南医科大学 | 防治真菌感染的复合维生素d及其应用 |
| IT202100015845A1 (it) | 2021-06-17 | 2022-12-17 | I B N Savio S R L | Processo di formulazione della vitamina d / vitamin d formulation process |
| CN115266992A (zh) * | 2022-08-02 | 2022-11-01 | 人福普克药业(武汉)有限公司 | 一种测定维生素d类软胶囊溶出的方法 |
| WO2025094144A1 (en) | 2023-11-02 | 2025-05-08 | Eirgen Pharma Ltd. | Controlling loss of kidney function |
Family Cites Families (273)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998008517A2 (en) | 1996-08-26 | 1998-03-05 | Takeda Chemical Industries, Ltd. | Pharmaceutical composition containing osteogenesis-promoting substance and a polyethylene glycol |
| US3565924A (en) | 1968-07-01 | 1971-02-23 | Wisconsin Alumni Res Found | 25-hydroxycholfcalciferol |
| US3833622A (en) | 1969-03-17 | 1974-09-03 | Upjohn Co | Crystalline 25-hydroxycholecalciferol hydrate and structurally related compounds |
| GB1405088A (en) | 1971-06-03 | 1975-09-03 | Mundipharma Ag | Slow release formulation |
| US3974272A (en) | 1972-09-01 | 1976-08-10 | Merck & Co., Inc. | Palatable cholestyramine coacervate compositions |
| US3880894A (en) | 1974-05-24 | 1975-04-29 | Wisconsin Alumni Res Found | 1,25-Dihydroxyergocalciferol |
| US4004003A (en) | 1974-08-28 | 1977-01-18 | The Upjohn Company | 25-Hydroxycalciferol compounds for treatment of steroid-induced osteoporosis |
| US4335120A (en) | 1979-03-21 | 1982-06-15 | Hoffmann-La Roche Inc. | Administration of biologically active vitamin D3 and vitamin D2 materials |
| US4230701A (en) | 1979-03-21 | 1980-10-28 | Hoffmann-La Roche Inc. | Administration of biologically active vitamin D3 and vitamin D2 materials |
| JPS55139320A (en) | 1979-04-16 | 1980-10-31 | Teijin Ltd | Bone metabolism regulator |
| JPS57188520A (en) | 1981-05-15 | 1982-11-19 | Kureha Chem Ind Co Ltd | Antihyperkalemia |
| US4442093A (en) | 1981-05-15 | 1984-04-10 | Kureha Kagaku Kogyo Kabushiki Kaisha | Method for administering 24,25-dihydroxycholecalciferol to persons suffering from hypercalcemia |
| JPS5832823A (ja) | 1981-08-20 | 1983-02-25 | Chugai Pharmaceut Co Ltd | 脱癌剤 |
| JPS58206524A (ja) | 1982-05-26 | 1983-12-01 | Kureha Chem Ind Co Ltd | 抗腫瘍剤 |
| US4448721A (en) | 1982-09-20 | 1984-05-15 | Wisconsin Alumni Research Foundation | Hydroxyvitamin D2 compounds and process for preparing same |
| US4721613A (en) | 1982-12-13 | 1988-01-26 | Alza Corporation | Delivery system comprising means for shielding a multiplicity of reservoirs in selected environment of use |
| JPS59155309A (ja) * | 1983-02-22 | 1984-09-04 | Teijin Ltd | 活性型ビタミンd↓3類組成物 |
| US4684524A (en) | 1984-03-19 | 1987-08-04 | Alza Corporation | Rate controlled dispenser for administering beneficial agent |
| US4795327A (en) | 1984-03-26 | 1989-01-03 | Forest Laboratories, Inc. | Controlled release solid drug dosage forms based on mixtures of water soluble nonionic cellulose ethers and anionic surfactants |
| US4555364A (en) | 1984-11-01 | 1985-11-26 | Wisconsin Alumni Research Foundation | Method for preparing 1-hydroxyvitamin D compounds |
| US4695591A (en) | 1985-03-29 | 1987-09-22 | Schering Corporation | Controlled release dosage forms comprising hydroxypropylmethylcellulose |
| US4668517A (en) | 1985-04-04 | 1987-05-26 | Norwich Eaton Pharmaceuticals, Inc. | Furazolidone dosage form |
| DE3676235D1 (de) | 1985-06-04 | 1991-01-31 | Teijin Ltd | Arzneizubereitung mit verzoegerter wirkstoffabgabe. |
| JPS61293911A (ja) | 1985-06-24 | 1986-12-24 | Teisan Seiyaku Kk | 徐放化製剤 |
| US4795642A (en) | 1986-05-01 | 1989-01-03 | Pharmacaps, Inc. | Gelatin-encapsulated controlled-release composition |
| US5167965A (en) | 1987-02-09 | 1992-12-01 | The Dow Chemical Company | Palatable cholestyramine granules, tablets and methods for preparation thereof |
| US4892821A (en) | 1987-07-08 | 1990-01-09 | Taisho Pharmaceutical Co., Ltd. | Method for preparing vitamin D compounds |
| US4997824A (en) | 1987-07-22 | 1991-03-05 | Teva Pharmaceutical Industries Ltd. | Combination of cholecalciferol derivatives for the treatment of renal bone disease |
| US5602116A (en) | 1988-08-02 | 1997-02-11 | Bone Care International, Inc. | Method for treating and preventing secondary hyperparathyroidism |
| US5869473A (en) | 1988-08-02 | 1999-02-09 | Bone Care International, Inc. | Method for treating and preventing hyperparathyroidism |
| US5104864A (en) | 1988-08-02 | 1992-04-14 | Bone Care International, Inc. | Method for treating and preventing loss of bone mass |
| JP2893191B2 (ja) | 1988-11-08 | 1999-05-17 | 武田薬品工業株式会社 | 放出制御性マトリックス剤 |
| JP2525478B2 (ja) | 1989-03-01 | 1996-08-21 | 帝人株式会社 | 安定性の改良された活性型ビタミンd▲下3▼類固型製剤 |
| JPH02240024A (ja) | 1989-03-13 | 1990-09-25 | Ss Pharmaceut Co Ltd | 活性型ビタミンd↓3類製剤用組成物 |
| US5026559A (en) | 1989-04-03 | 1991-06-25 | Kinaform Technology, Inc. | Sustained-release pharmaceutical preparation |
| GB9004544D0 (en) | 1990-03-01 | 1990-04-25 | Leo Pharm Prod Ltd | Novel treatment ii |
| JP2845342B2 (ja) | 1990-04-28 | 1999-01-13 | 大正製薬株式会社 | ビタミンd▲下3▼誘導体含有固形製剤組成物 |
| JPH04198129A (ja) * | 1990-11-28 | 1992-07-17 | Sumitomo Pharmaceut Co Ltd | 活性型ビタミンd↓3類含有組成物 |
| JP2893140B2 (ja) | 1990-11-30 | 1999-05-17 | エスエス製薬株式会社 | 安定なビタミンd製剤 |
| JPH04288016A (ja) | 1991-03-14 | 1992-10-13 | Tokai Capsule Kk | 活性型ビタミンd3類軟カプセル剤の製造方法 |
| DE69214704T2 (de) | 1991-04-09 | 1997-04-17 | Takeda Chemical Industries Ltd | Stabilisiertes Vitamin D Arzneimittel |
| US5693615A (en) | 1991-06-05 | 1997-12-02 | The Procter & Gamble Company | Therapeutic compositions for osteoinduction |
| US6011068A (en) | 1991-08-23 | 2000-01-04 | Nps Pharmaceuticals, Inc. | Calcium receptor-active molecules |
| US6313146B1 (en) | 1991-08-23 | 2001-11-06 | Nps Pharmaceuticals, Inc. | Calcium receptor-active molecules |
| US6001884A (en) | 1991-08-23 | 1999-12-14 | Nps Pharmaceuticals, Inc. | Calcium receptor-active molecules |
| US6031003A (en) | 1991-08-23 | 2000-02-29 | Nps Pharmaceuticals, Inc. | Calcium receptor-active molecules |
| US5266331A (en) | 1991-11-27 | 1993-11-30 | Euroceltique, S.A. | Controlled release oxycodone compositions |
| US5472712A (en) | 1991-12-24 | 1995-12-05 | Euroceltique, S.A. | Controlled-release formulations coated with aqueous dispersions of ethylcellulose |
| US5273760A (en) | 1991-12-24 | 1993-12-28 | Euroceltigue, S.A. | Stabilized controlled release substrate having a coating derived from an aqueous dispersion of hydrophobic polymer |
| US5160742A (en) | 1991-12-31 | 1992-11-03 | Abbott Laboratories | System for delivering an active substance for sustained release |
| US5795882A (en) | 1992-06-22 | 1998-08-18 | Bone Care International, Inc. | Method of treating prostatic diseases using delayed and/or sustained release vitamin D formulations |
| NZ254424A (en) | 1992-06-22 | 1997-09-22 | Lunar Corp | Pharmaceutical composition and use of 1 alpha hydroxy-pre-vitamin d |
| US5354743A (en) | 1992-09-15 | 1994-10-11 | Thys Jacobs Susan | Method for the treatment of premenstrual syndrome with vitamin D |
| US5431917A (en) | 1992-10-08 | 1995-07-11 | Japan Elanco Company, Ltd. | Hard capsule for pharmaceutical drugs and method for producing the same |
| US5342626A (en) | 1993-04-27 | 1994-08-30 | Merck & Co., Inc. | Composition and process for gelatin-free soft capsules |
| JP2684587B2 (ja) | 1993-06-21 | 1997-12-03 | 呉羽化学工業株式会社 | 腎性骨異栄養症における骨量減少抑制剤 |
| IL110014A (en) | 1993-07-01 | 1999-11-30 | Euro Celtique Sa | Solid controlled-release oral dosage forms of opioid analgesics |
| US6121469A (en) | 1993-12-23 | 2000-09-19 | The Regents Of The University Of California | Therapeutically effective 1α,25-dihydroxyvitamin D3 analogs |
| JPH07242550A (ja) | 1994-03-02 | 1995-09-19 | Teijin Ltd | 二次性副甲状腺機能亢進症治療剤 |
| IL110117A0 (en) * | 1994-06-24 | 1994-10-07 | Univ Ben Gurion | Pharmaceutical compositions comprising vitamin-d analogs |
| SE9402422D0 (sv) | 1994-07-08 | 1994-07-08 | Astra Ab | New beads for controlled release and a pharmaceutical preparation containing the same |
| JPH10504291A (ja) | 1994-07-22 | 1998-04-28 | ジー.ディー.サール アンド カンパニー | 自己乳化性ドラッグデリバリーシステム |
| JPH0892098A (ja) | 1994-09-27 | 1996-04-09 | Teijin Ltd | 肺結核治療剤 |
| DE69533948T2 (de) | 1994-10-21 | 2005-12-15 | NPS Pharmaceuticals, Inc., Salt Lake City | Kalzium-Rezeptor aktive Verbindungen |
| US5756123A (en) | 1994-12-01 | 1998-05-26 | Japan Elanco Co., Ltd. | Capsule shell |
| US20020183288A1 (en) | 1995-04-03 | 2002-12-05 | Bone Care International, Inc. | Method for treating and preventing hyperparathyroidism |
| US6242434B1 (en) | 1997-08-08 | 2001-06-05 | Bone Care International, Inc. | 24-hydroxyvitamin D, analogs and uses thereof |
| US6376479B1 (en) | 1995-04-03 | 2002-04-23 | Bone Care International, Inc. | Method for treating and preventing hyperparathyroidism |
| US20040043971A1 (en) | 1995-04-03 | 2004-03-04 | Bone Care International, Inc. | Method of treating and preventing hyperparathyroidism with active vitamin D analogs |
| AU717238B2 (en) | 1995-09-21 | 2000-03-23 | Wisconsin Alumni Research Foundation | Calcitriol derivatives and their uses |
| DE19536387A1 (de) | 1995-09-29 | 1997-04-03 | Basf Ag | Verfahren zur Herstellung von vitaminhaltigen festen Zubereitungen |
| DE19549243A1 (de) | 1995-12-21 | 1997-06-26 | Schering Ag | Pharmazeutische Präparate enthaltend Clathrate von Cyclodextrinen und nichtnatürliche Vitamin D-Analoga |
| US5939408A (en) | 1996-05-23 | 1999-08-17 | Hoffman-La Roche Inc. | Vitamin D3 analogs |
| NO971934L (no) | 1996-05-23 | 1997-11-24 | Hoffmann La Roche | Flourinerte vitamin D3 -analoger |
| US5958451A (en) | 1996-09-03 | 1999-09-28 | Yung Shin Pharm Ind. Co., Ltd. | Process for producing porous, controlled-release capsules and encapsulated composition |
| US5976784A (en) | 1996-09-20 | 1999-11-02 | Wisconsin Alumni Research Foundation | Calcitriol derivatives and their uses |
| US6190591B1 (en) | 1996-10-28 | 2001-02-20 | General Mills, Inc. | Embedding and encapsulation of controlled release particles |
| US8828432B2 (en) | 1996-10-28 | 2014-09-09 | General Mills, Inc. | Embedding and encapsulation of sensitive components into a matrix to obtain discrete controlled release particles |
| JPH10158171A (ja) | 1996-12-02 | 1998-06-16 | Kita:Kk | ビタミンd化合物を配合した眼内投与剤 |
| US20020128240A1 (en) | 1996-12-30 | 2002-09-12 | Bone Care International, Inc. | Treatment of hyperproliferative diseases using active vitamin D analogues |
| US6503893B2 (en) | 1996-12-30 | 2003-01-07 | Bone Care International, Inc. | Method of treating hyperproliferative diseases using active vitamin D analogues |
| US20030129194A1 (en) | 1997-02-13 | 2003-07-10 | Bone Care International, Inc. | Targeted therapeutic delivery of vitamin D compounds |
| US6034075A (en) | 1997-03-20 | 2000-03-07 | The Trustees Of Columbia University In The City Of New York | Method of treating polycystic ovarian syndrome |
| US5872113A (en) | 1997-05-16 | 1999-02-16 | Syntex (U.S.A.) Inc. | Fluorinated vitamin D3 analogs |
| WO1999001111A1 (en) | 1997-07-02 | 1999-01-14 | Euro-Celtique, S.A. | Stabilized sustained release tramadol formulations |
| JPH1175863A (ja) | 1997-07-10 | 1999-03-23 | Kyowa Hakko Kogyo Co Ltd | 25−ヒドロキシビタミンD3−1α−水酸化酵素および該酵素をコードするDNA |
| US6096876A (en) | 1997-08-06 | 2000-08-01 | Shriners Hospitals For Children | 1-α-hydroxylase materials and methods |
| AU9376998A (en) | 1997-09-02 | 1999-03-22 | Johns Hopkins University School Of Medicine, The | Vitamin d3 analog loaded polymer formulations for cancer and neurodegenerative disorders |
| US5919986A (en) | 1997-10-17 | 1999-07-06 | Hoffmann-La Roche Inc. | D-homo vitamin D3 derivatives |
| JPH11158074A (ja) | 1997-12-03 | 1999-06-15 | Hayashi Tomie | 高アミノ酸付加活性型ビタミンd強化組成物 |
| US20030059471A1 (en) | 1997-12-15 | 2003-03-27 | Compton Bruce Jon | Oral delivery formulation |
| CA2323782A1 (en) | 1998-03-25 | 1999-09-30 | Cutanogen, Inc. | Methods for prevention and treatment of cancer |
| EP2340840B1 (en) | 1998-03-27 | 2012-08-29 | Oregon Health Sciences University | Pulse dose Vitamin D drug for the treatment of hyperproliferative skin diseases |
| US6197340B1 (en) | 1998-05-28 | 2001-03-06 | Medical Research Institute | Controlled release lipoic acid |
| US8133694B2 (en) | 1998-06-25 | 2012-03-13 | Immundiagnostik Ag | Functional vitamin D derivatives and method of determining 25-hydroxy- and 1α, 25-dihydroxy vitamin D |
| ES2347968T3 (es) | 1998-07-28 | 2010-11-26 | Takeda Pharmaceutical Company Limited | Preparacion solida que se disgrega rapidamente. |
| US6214376B1 (en) | 1998-08-25 | 2001-04-10 | Banner Pharmacaps, Inc. | Non-gelatin substitutes for oral delivery capsules, their composition and process of manufacture |
| ME00850B (me) | 1998-08-27 | 2008-08-07 | Pfizer Health Ab | Nove perle sa kontrolisanim oslobađanjem, postupak za njihovu proizvodnju i višestruka jedinična formulacija koja ih sadrži |
| SE9803871D0 (sv) | 1998-11-11 | 1998-11-11 | Pharmacia & Upjohn Ab | Therapeutic method and formulation |
| US6139875A (en) | 1998-09-29 | 2000-10-31 | Eastman Chemical Company | Aqueous enteric coating composition and low gastric permeability enteric coating |
| JP2002525311A (ja) | 1998-10-01 | 2002-08-13 | エラン ファーマ インターナショナル,リミティド | 徐放性ナノ粒子組成物 |
| DK1119345T3 (da) | 1998-10-09 | 2009-08-03 | Gen Mills Inc | Indkapsling af fölsomme flydende bestanddele i en matrix til opnåelse af adskilte opbevaringsstabile partikler |
| JP3449253B2 (ja) | 1998-10-29 | 2003-09-22 | シオノギクオリカプス株式会社 | 硬質カプセルの製造方法 |
| JP2000206312A (ja) | 1998-11-12 | 2000-07-28 | Olympus Optical Co Ltd | 光学素子 |
| EP1140012B1 (en) | 1998-12-17 | 2004-03-03 | Alza Corporation | Conversion of liquid filled gelatin capsules into controlled release systems by multiple coatings |
| US6342249B1 (en) | 1998-12-23 | 2002-01-29 | Alza Corporation | Controlled release liquid active agent formulation dosage forms |
| US6432936B1 (en) | 1999-01-20 | 2002-08-13 | Wisconsin Alumni Research Foundation | Crystalline 1α-hydroxyvitamin D2 and method of purification thereof |
| US6248363B1 (en) | 1999-11-23 | 2001-06-19 | Lipocine, Inc. | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions |
| ES2614632T3 (es) | 1999-04-01 | 2017-06-01 | Johns Hopkins University | Análogos no calcémicos, antiproliferativos, transcripcionalmente activos de 1-alfa 25-dihidroxi vitamina D3 que contienen azufre |
| US7648826B1 (en) | 1999-04-02 | 2010-01-19 | The Regents Of The University Of California | Detecting CYP24 expression level as a marker for predisposition to cancer |
| DE19916419B4 (de) | 1999-04-08 | 2005-06-16 | Schering Ag | Kombinationspräparat aus Vitamin-D-Metaboliten oder Vitamin-D-Analoga und einem Östrogenpartialagonisten zur Behandlung von Osteoporose |
| WO2000072831A1 (en) | 1999-05-27 | 2000-12-07 | Drugtech Corporation | Nutritional formulations |
| US6340473B1 (en) | 1999-07-07 | 2002-01-22 | R.P. Scherer Technologies, Inc. | Film forming compositions comprising modified starches and iota-carrageenan and methods for manufacturing soft capsules using same |
| US6274169B1 (en) | 1999-08-02 | 2001-08-14 | Abbott Laboratories | Low oxygen content compostions of 1α, 25-dihydroxycholecalciferol |
| US6051567A (en) | 1999-08-02 | 2000-04-18 | Abbott Laboratories | Low oxygen content compositions of 1α, 25-dihydroxycholecalciferol |
| EP1208843B1 (en) | 1999-08-31 | 2010-10-13 | Chugai Seiyaku Kabushiki Kaisha | Soft capsules |
| US20060034937A1 (en) | 1999-11-23 | 2006-02-16 | Mahesh Patel | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions |
| GB0007419D0 (en) | 2000-03-27 | 2000-05-17 | Smithkline Beecham Gmbh | Composition |
| US6375981B1 (en) | 2000-06-01 | 2002-04-23 | A. E. Staley Manufacturing Co. | Modified starch as a replacement for gelatin in soft gel films and capsules |
| CA2414407A1 (en) | 2000-07-18 | 2002-01-24 | Bone Care International, Inc. | Stabilized 1.alpha.-hydroxy vitamin d |
| US6491950B1 (en) | 2000-08-07 | 2002-12-10 | Kos Pharmaceuticals, Inc. | Controlled release pharmaceutical composition |
| EP1323404B1 (en) | 2000-08-29 | 2013-10-16 | Nisshin Kasei Co.,Ltd. | Hard capsule |
| US6887493B2 (en) * | 2000-10-25 | 2005-05-03 | Adi Shefer | Multi component controlled release system for oral care, food products, nutraceutical, and beverages |
| CN101317825A (zh) | 2000-10-30 | 2008-12-10 | 欧罗赛铁克股份有限公司 | 控释氢可酮制剂 |
| US6479649B1 (en) | 2000-12-13 | 2002-11-12 | Fmc Corporation | Production of carrageenan and carrageenan products |
| JP2002302447A (ja) | 2001-04-03 | 2002-10-18 | Shimizu Pharmaceutical Co Ltd | 局所投与用癌治療剤 |
| KR20030096392A (ko) | 2001-05-15 | 2003-12-24 | 워너-램버트 캄파니 엘엘씨 | 나트륨 페니토인 제형 제조를 위한 압축 방법 |
| AU2002315779B2 (en) | 2001-07-05 | 2007-03-22 | Wakunaga Pharmaceutical Co.,Ltd | Soft capsules |
| EP1416919A1 (en) | 2001-07-17 | 2004-05-12 | Teva Pharmaceutical Industries Ltd. | Dosage forms for immediate gastric release of a calcium transport stimulator coupled with delayed gastric release of a bis-phosphonate |
| US6870833B2 (en) | 2001-07-20 | 2005-03-22 | Net2Phone, Inc. | Active voice messaging |
| US7166585B2 (en) | 2001-08-22 | 2007-01-23 | Cytochroma Inc. | 24-Sulfur-substituted analogs of 1α,25-dihydroxy vitamin D3 |
| US7033996B2 (en) | 2001-08-31 | 2006-04-25 | University Of Medicine & Dentistry Of New Jersey | Method for the treatment of vitamin D related disease |
| DE10149674A1 (de) | 2001-10-09 | 2003-04-24 | Apogepha Arzneimittel Gmbh | Orale Darreichungsformen für Propiverin oder seinen pharmazeutisch annehmbaren Salzen mit verlängerter Wirkstoffreisetzung |
| ATE360000T1 (de) | 2001-10-12 | 2007-05-15 | Univ Johns Hopkins | Oxim-analoga von 1-alpha,25-dihydroxy-vitamin-d3 mit geringer calcemischer wirkung |
| US7056655B2 (en) | 2001-11-02 | 2006-06-06 | Scantibodies Laboratory, Inc. | Methods for monitoring and guiding therapeutic suppression of parathyroid hormone in renal patients having secondary hyperparathyroidism |
| US6524788B1 (en) | 2001-11-02 | 2003-02-25 | Thomas L. Cantor | Methods for monitoring and guiding therapeutic suppression of parathyroid hormone in renal patients having secondary hyperparathyroidism |
| ITMI20012366A1 (it) | 2001-11-09 | 2003-05-09 | Farmatron Ltd | Sistemi terapeutici stabilizzati a rilascio immediato e/o modificato per la somministrazione orale di principi attivi e/o eccipienti e/o ali |
| JP4184278B2 (ja) | 2001-11-22 | 2008-11-19 | 森下仁丹株式会社 | 非ゼラチン系カプセル皮膜組成物及びそれを用いたカプセル |
| GB0128415D0 (en) | 2001-11-27 | 2002-01-16 | Univ Sheffield | Medicaments |
| EA008072B1 (ru) | 2001-12-03 | 2007-02-27 | Новацея, Инк. | Фармацевтические составы, содержащие соединения активного витамина d |
| US6627622B2 (en) | 2002-02-18 | 2003-09-30 | Wisconsin Alumni Research Foundation | (20S)-1α-hydroxy-2-methylene-19-nor-bishomopregnacalciferol and its uses |
| FR2829142B1 (fr) | 2001-12-27 | 2004-02-13 | Ulice | Composition filmogene d'heteroxylanes pour la fabrication de capsules ainsi obtenues |
| US7632518B2 (en) | 2002-01-15 | 2009-12-15 | Dsm Ip Assets B.V. | 25-hydroxy vitamin D3 compositions |
| US6949256B2 (en) | 2002-01-18 | 2005-09-27 | Banner Pharmacaps, Inc. | Non-gelatin capsule shell formulation |
| NO20021592D0 (no) | 2002-04-04 | 2002-04-04 | Fmc Biopolymer As | Polysakkaridkapsler og fremgangsmåte ved fremstilling derav |
| US20030190355A1 (en) | 2002-04-05 | 2003-10-09 | Hermelin Marc S. | Modified release minerals |
| AU2003226148A1 (en) * | 2002-04-05 | 2003-10-27 | Merck & Co., Inc. | Method for inhibiting bone resorption with an alendronate and vitamin d formulation |
| CA2481486C (en) | 2002-04-10 | 2012-06-12 | Fred H. Miller | Multi-phase, multi-compartment capsular system |
| WO2003093459A1 (en) | 2002-05-02 | 2003-11-13 | Cytochroma Inc. | Stable cytochrome p450 24 (cyp24) expressing cell line and methods and uses thereof |
| WO2003106411A1 (en) | 2002-06-13 | 2003-12-24 | Johns Hopkins University | 24-sulfoximine vitamin d3 compounds |
| US20060127484A1 (en) | 2002-07-05 | 2006-06-15 | Speirs Christopher J | Controlled release composition |
| TWI336260B (en) | 2002-07-25 | 2011-01-21 | Glaxo Group Ltd | Dosage form suitable for retaining drug substance |
| CN1684670A (zh) | 2002-07-29 | 2005-10-19 | 阿尔扎公司 | 用于控制释放帕潘立酮的方法和剂型 |
| US8268352B2 (en) | 2002-08-05 | 2012-09-18 | Torrent Pharmaceuticals Limited | Modified release composition for highly soluble drugs |
| WO2004028515A1 (en) | 2002-09-26 | 2004-04-08 | Young-Kweon Choi | Matrix type patch for transdermal administration of vitamin d analog and the use thereof |
| US20050101576A1 (en) | 2003-11-06 | 2005-05-12 | Novacea, Inc. | Methods of using vitamin D compounds in the treatment of myelodysplastic syndromes |
| US8999372B2 (en) | 2002-11-14 | 2015-04-07 | Cure Pharmaceutical Corporation | Methods for modulating dissolution, bioavailability, bioequivalence and drug delivery profile of thin film drug delivery systems, controlled-release thin film dosage formats, and methods for their manufacture and use |
| JP2004175750A (ja) | 2002-11-28 | 2004-06-24 | Kose Corp | 皮膚障害抑制剤、皮膚障害改善剤、及びそれらを含有する皮膚外用剤 |
| US20050026877A1 (en) | 2002-12-03 | 2005-02-03 | Novacea, Inc. | Pharmaceutical compositions comprising active vitamin D compounds |
| DE20321698U1 (de) | 2002-12-16 | 2008-12-24 | Teva Pharmaceutical Industries Ltd. | Medikament zur Erhöhung der Bioverfügbarkeit von Alendronat oder einem anderen Bisphosphonat durch Verabreichen einer Vordosis eines Vitamin-D-Derivats |
| GB2411116B (en) | 2002-12-16 | 2009-04-29 | Teva Pharma | Increasing the bioavailability of alendronate by predose administration of alphacalcidol |
| CA2510228C (en) | 2002-12-18 | 2012-10-30 | Johns Hopkins University | 25-so2-substituted analogs of 1.alpha.,25-dihydroxyvitamin d3 |
| KR20050123097A (ko) | 2003-02-11 | 2005-12-29 | 알자 코포레이션 | 변형된 층 형상을 갖는 방법 및 제형 |
| EP1620114A2 (en) | 2003-04-14 | 2006-02-01 | Fmc Corporation | Delivery systems of homogeneous thermoreversible low viscosity polymannan gum films |
| US7816341B2 (en) | 2003-04-14 | 2010-10-19 | Fmc Corporation | Homogeneous, thermoreversible gel containing reduced viscosity carrageenan and products made therefrom |
| WO2004098507A2 (en) | 2003-04-30 | 2004-11-18 | Bioxell S.P.A. | 1,3 aclyated 24-keto-vitamin d3 compounds and methods of use thereof |
| CA2524603C (en) * | 2003-05-07 | 2013-04-02 | Osteologix A/S | Controlled release composition containing a strontium salt |
| EP1479677A1 (en) | 2003-05-19 | 2004-11-24 | Aventis Pharma Deutschland GmbH | New indole derivatives as factor xa inhibitors |
| CA2529495C (en) | 2003-06-16 | 2013-02-05 | Solx, Inc. | Shunt for the treatment of glaucoma |
| ES2379466T3 (es) | 2003-06-26 | 2012-04-26 | Psivida Us, Inc. | Sistema de suministro de fármacos de liberación sostenida bioerosionables |
| AU2003903382A0 (en) | 2003-07-03 | 2003-07-17 | Medvet Science Pty Ltd | Inhibition of calcitriol mediated cyp24 induction screening for compounds therefor and uses thereof |
| US20070155664A1 (en) | 2003-07-04 | 2007-07-05 | Nycomed Danmark A/S | Parathyroid hormone (pth) containing pharmaceutical compositions for oral use |
| US20050124591A1 (en) | 2003-07-29 | 2005-06-09 | Jin Tian | Use of vitamin Ds to treat kidney disease |
| US20050148557A1 (en) | 2003-07-29 | 2005-07-07 | Jin Tian | Use of Vitamin Ds to treat kidney disease |
| DK3395339T3 (da) | 2003-09-12 | 2019-07-22 | Amgen Inc | Hurtigt opløsende formulering af cinacalcet-hcl |
| WO2005051396A2 (en) | 2003-11-25 | 2005-06-09 | Deltanoid Pharmaceuticals, Inc. | Methods for reducing body fat using vitamin d compounds |
| US7427670B2 (en) | 2003-12-19 | 2008-09-23 | Cytochroma Inc. | Cytochrome P450 24 (CYP24) monoclonal antibody and methods and uses thereof |
| EA012570B1 (ru) | 2004-03-10 | 2009-10-30 | Шеринг Акциенгезельшафт | Твердая или полутвердая композиция, содержащая молекулярно диспергированный дроспиренон, и способ ее получения |
| US20060009425A1 (en) | 2004-05-28 | 2006-01-12 | Leticia Delgado-Herrera | Oral formulations of paricalcitol |
| EP1765385A4 (en) | 2004-06-16 | 2008-08-27 | Smart Drug Systems Inc | IMPORTS COMPOSITION WITH DELAYED RELEASE |
| US20050287213A1 (en) | 2004-06-28 | 2005-12-29 | Wong Patrick S | Dosage forms for low solubility and or low dissolution rate free acid pharmaceutical agents |
| US20060019933A1 (en) | 2004-07-22 | 2006-01-26 | David Boardman | Process for preparing stabilized vitamin D |
| CN1993112A (zh) | 2004-07-29 | 2007-07-04 | 赛诺菲-安万特 | 用于具有高度pH依赖性溶解度的活性成分的控释的药物多层片剂 |
| KR101398747B1 (ko) | 2004-08-19 | 2014-05-26 | 디에스엠 아이피 어셋츠 비.브이. | 지용성 물질의 신규한 조성물 |
| US8231896B2 (en) | 2004-11-08 | 2012-07-31 | R.P. Scherer Technologies, Llc | Non-gelatin soft capsule system |
| US7067568B1 (en) | 2004-12-03 | 2006-06-27 | Council Of Scientific And Industrial Research | Process of preparation of biodegradable films from semi refined kappa carrageenan |
| IL166114A0 (en) | 2005-01-03 | 2006-01-15 | Calcident Active Ltd | Long-acting controlled-release pharmaceutical preparation for use in the oral cavity |
| US8318210B2 (en) | 2005-02-28 | 2012-11-27 | Neos Therapeutics, Lp | Compositions and methods of making sustained release liquid formulations |
| DE102005011029A1 (de) | 2005-03-08 | 2006-09-14 | Maria Clementine Martin Klosterfrau Vertriebsgesellschaft Mbh | Zusammensetzung für die perorale Applikation mit gesteuerter Freisetzung von Wirkstoffen |
| US7745226B2 (en) | 2005-04-06 | 2010-06-29 | Quest Diagnostics Investments Incorporated | Methods for detecting vitamin D metabolites |
| PL2985026T3 (pl) | 2005-04-15 | 2023-02-20 | Clarus Therapeutics, Inc. | Farmaceutyczne systemy dostarczania leków hydrofobowych i zawierające je kompozycje |
| US20060257481A1 (en) | 2005-04-21 | 2006-11-16 | Decode Genetics Ehf. | Sustained release formulation and dosing schedule of leukotriene synthesis inhibitor for human therapy |
| US9205047B2 (en) | 2005-04-25 | 2015-12-08 | The Governing Council Of The University Of Toronto | Tunable sustained release of a sparingly soluble hydrophobic therapeutic agent from a hydrogel matrix |
| US20080134937A1 (en) | 2005-05-25 | 2008-06-12 | Joo Hwan Yang | Cellulose hard capsule enhancing mechanical film strength |
| AR055099A1 (es) | 2005-07-28 | 2007-08-08 | Alza Corp | Formulaciones liquidas para la administracion controlada de derivados de bencisoxazol |
| US7895596B2 (en) | 2005-09-13 | 2011-02-22 | Hewlett-Packard Development Company, L.P. | Processor assignment in multi-processor systems |
| WO2007039193A1 (en) | 2005-09-29 | 2007-04-12 | Roche Diagnostics Gmbh | Antibodies against 25-hydroxyvitamin d |
| ITFI20050206A1 (it) | 2005-09-30 | 2007-04-01 | Valpharma Sa | Composizione farmaceutica a rilascio controllato di venlafaxina cloridrato, e processo per la sua preparazione |
| EP1945185B1 (en) | 2005-10-12 | 2016-02-03 | Proventiv Therapeutics, LLC | Methods and articles for treating 25-hydroxyvitamin d insufficiency and deficiency |
| EP1959935A2 (en) | 2005-10-26 | 2008-08-27 | Banner Pharmacaps Inc. | Hydrophilic vehicle-based dual controlled release matrix system as capsule fill |
| WO2007050724A2 (en) | 2005-10-26 | 2007-05-03 | Banner Pharmacaps, Inc. | Lipophilic vehicle-based dual controlled release matrix system as capsule fill |
| EA015170B1 (ru) | 2005-11-01 | 2011-06-30 | СиПи КЕЛКО Ю.С., ИНК. | Высоковязкие диутановые камеди и способы их получения |
| WO2007068287A1 (en) | 2005-12-15 | 2007-06-21 | Laboratoria Qualiphar | Sustained release vitamin preparation |
| MXPA05014091A (es) | 2005-12-20 | 2007-06-20 | Leopoldo De Jesus Espinosa Abdala | Composiciones farmaceuticas que comprenden derivados de esteroides sinteticos, minerales y el metabolito activo de la vitamina d, 1,25(oh)2d3 (calcitriol) para la prevencion y tratamiento de la osteoporosis y el control de los sintomas de la menopaus |
| JP2009525030A (ja) | 2006-01-31 | 2009-07-09 | ヘルス リサーチ インコーポレイテッド | 変化したビタミンd代謝を同定するための方法 |
| US7528122B2 (en) | 2006-02-02 | 2009-05-05 | Wisconsin Alumni Research Foundation | Vitamin D analog—NEL, methods and uses thereof |
| DK3095447T3 (da) | 2006-02-03 | 2022-01-31 | Opko Renal Llc | Behandling af vitamin d-insufficiens og -mangel med 25-hydroxyvitamin d2 og 25-hydroxyvitamin d3 |
| GB0606426D0 (en) | 2006-03-30 | 2006-05-10 | Novartis Ag | Benzimidazole derivatives |
| CA2660613A1 (en) | 2006-06-06 | 2007-12-21 | Fmc Corporation | Kappa-2 carrageenan composition and products made therefrom |
| US20080109983A1 (en) | 2006-11-10 | 2008-05-15 | Kegel, Llc | Zero Turning Radius Lane Maintenance Machine |
| DK3357496T3 (da) | 2006-06-21 | 2020-05-11 | Opko Ireland Global Holdings Ltd | Terapi ved brug af vitamin d-repletteringsmiddel og vitamin d-hormonsubstitutionsmiddel |
| US7387328B2 (en) | 2006-09-07 | 2008-06-17 | Willey Barry A | Cycle windshield |
| EP1912400A1 (en) | 2006-10-10 | 2008-04-16 | Matsushita Electric Industrial Co., Ltd. | Method and apparatus for mobile IP route optimization |
| MX2009004434A (es) | 2006-10-27 | 2009-05-08 | Pfizer Prod Inc | Capsulas duras de hidroxipropilmetilcelulosa y procedimiento de fabricacion. |
| WO2008074144A1 (en) | 2006-12-20 | 2008-06-26 | Genpharm Ulc | A composition containing a bisphosphonic acid in combination with vitamin d |
| US8501717B2 (en) | 2007-02-09 | 2013-08-06 | Merck, Sharp & Dohme Corp. | Methods to treat and/or prevent mucositis |
| US8491937B2 (en) | 2007-02-15 | 2013-07-23 | Wyeth Llc | Stability in vitamin and mineral supplements |
| EP2135345A4 (en) | 2007-03-20 | 2016-12-14 | Electrolock Inc | ROEBEL WINDING WITH CONDUCTIVE FELT |
| US20100144679A1 (en) | 2007-03-21 | 2010-06-10 | Duke University | Medication kits and formulations for preventing, treating or reducing secondary fractures after previous fracture |
| KR100844256B1 (ko) | 2007-03-23 | 2008-07-07 | 코오롱제약주식회사 | 리세드로네이트와 비타민 d를 포함하는 대사성 골질환치료용 약제조성물 및 이의 제조방법 |
| PL2148661T3 (pl) | 2007-04-25 | 2013-07-31 | Cytochroma Inc | Doustne kompozycje o kontrolowanym uwalnianiu zawierające związek będący witaminą D i woskowy nośnik |
| US8592401B2 (en) | 2007-04-25 | 2013-11-26 | Proventiv Therapeutics, Llc | Methods and compounds for vitamin D therapy |
| EP3335712A1 (en) | 2007-04-25 | 2018-06-20 | Opko Renal, LLC | Method of safely and effectively treating and preventing secondary hyperparathyroidism in chronic kidney disease |
| US11752158B2 (en) | 2007-04-25 | 2023-09-12 | Eirgen Pharma Ltd. | Method of treating vitamin D insufficiency and deficiency |
| US20090004284A1 (en) | 2007-06-26 | 2009-01-01 | Watson Pharmaceuticals, Inc. | Controlled release tamsulosin hydrochloride formulation |
| CN201113125Y (zh) * | 2007-07-10 | 2008-09-10 | 富士康(昆山)电脑接插件有限公司 | 电池连接器 |
| KR100836960B1 (ko) | 2007-09-07 | 2008-06-10 | 주식회사 서울제약 | 새로운 나이아신 제어방출형 제제 |
| EP2042165A1 (de) | 2007-09-28 | 2009-04-01 | Swiss Caps Rechte und Lizenzen AG | Hot-Melt-Befüllte Weichkapseln |
| US20090155355A1 (en) | 2007-12-12 | 2009-06-18 | Multi Formulations Ltd. | Particles in a capsule |
| WO2009101136A1 (en) | 2008-02-13 | 2009-08-20 | Dsm Ip Assets B.V. | Treating hyperglycemia with 25-hydroxyvitamin d3 |
| CN101951916A (zh) | 2008-02-13 | 2011-01-19 | 帝斯曼知识产权资产管理有限公司 | 25-羟基-维生素d3用于影响人肌肉生理学的用途 |
| CN101951919A (zh) | 2008-02-13 | 2011-01-19 | 帝斯曼知识产权资产管理有限公司 | 维生素d3和25-羟基-维生素d3用于治疗骨质疏松症和改善骨矿物质密度的组合用途 |
| CN106214683A (zh) | 2008-02-13 | 2016-12-14 | 帝斯曼知识产权资产管理有限公司 | 维生素d和25‑羟基维生素d3的组合 |
| WO2009101131A1 (en) | 2008-02-13 | 2009-08-20 | Dsm Ip Assets B.V. | Treating hypertension with 25-hydroxyvitamin d3 |
| KR101852042B1 (ko) | 2008-04-02 | 2018-04-25 | 사이토크로마 인코포레이티드 | 비타민 d 결핍 및 관련 장애에 유용한 방법, 조성물, 용도 및 키트 |
| US8324191B2 (en) | 2008-07-11 | 2012-12-04 | Biolink Life Sciences, Inc | Combined calcium, magnesium and vitamin D supplements |
| WO2010011906A1 (en) | 2008-07-24 | 2010-01-28 | Wisconsin Alumni Research Foundation | Once-a-week administration of 25-hydroxy vitamin d3 to sustain elevated steady-state pharmacokinetic blood concentration |
| US9757343B2 (en) | 2008-09-24 | 2017-09-12 | Evonik Röhm Gmbh | PH-dependent controlled release pharmaceutical opioid composition with resistance against the influence of ethanol |
| ES2425762T3 (es) | 2008-10-27 | 2013-10-17 | Roquette Freres | Polímero insoluble en agua: revestimientos de película a base de derivados de almidón modificado para la liberación dirigida al colon |
| AU2010206221B2 (en) * | 2009-01-23 | 2016-07-14 | Aziende Chimiche Riunite Angelini Francesco A.C.R.A.F. S.P.A. | Controlled release pharmaceutical or food formulation and process for its preparation |
| CH700543A2 (de) | 2009-03-03 | 2010-09-15 | Innogel Ag | Film auf Basis von Stärke. |
| TR201904204T4 (tr) | 2009-09-10 | 2019-04-22 | Dupont Nutrition Usa Inc | Eklemsiz aljinat kapsülleri. |
| KR101102364B1 (ko) | 2009-09-18 | 2012-01-03 | 한림제약(주) | 비스포스포네이트 유도체 및 고용량의 콜레칼시페롤을 포함하는 약학 조성물 |
| DK2490667T3 (da) | 2009-10-20 | 2020-04-06 | Herbonis Ag | Sammensætning, der omfatter solanum glaucophyllum, til forebyggelse og/eller behandling af hypocalcæmi og til stabilisering af blodcalciumniveauer |
| IT1396937B1 (it) | 2009-11-26 | 2012-12-20 | Bruzzese | Formulazioni di bisfosfonati e vitamina d idonee alla somministrazione intermittente per via intramuscolare e sottocutanea |
| FR2953139B1 (fr) | 2009-11-27 | 2012-04-13 | Servier Lab | Composition pharmaceutique comprenant un sel de strontium, de la vitamine d et une cyclodextrine |
| US8101204B2 (en) | 2010-01-14 | 2012-01-24 | Karl Wei Cao | Hard capsule composition and method of use |
| US8101203B2 (en) | 2010-01-14 | 2012-01-24 | Karl Wei Cao | Hard capsule composition and method of use |
| WO2011095388A1 (en) | 2010-02-04 | 2011-08-11 | Synthon Bv | Tolterodine bead |
| EP3636280B1 (en) | 2010-03-29 | 2025-05-14 | EirGen Pharma Ltd. | Methods and compositions for reducing parathyroid levels |
| WO2012006475A1 (en) | 2010-07-07 | 2012-01-12 | Ardelyx, Inc. | Compounds and methods for inhibiting phosphate transport |
| KR20120005228A (ko) | 2010-07-08 | 2012-01-16 | 주식회사 네비팜 | 골다공증 치료용 비스포스폰산 함유 장용 약학조성물 및 그의 제조방법 |
| EP2600849B1 (en) | 2010-08-04 | 2016-03-30 | R.P. Scherer Technologies, LLC | Film-forming composition for soft capsules |
| US20120135103A1 (en) | 2010-11-30 | 2012-05-31 | Mead Johnson Nutrition Company | Staged Infant Feeding Regimen To Promote Healthy Development And Growth |
| ES2712740T3 (es) | 2010-12-06 | 2019-05-14 | Dsm Ip Assets Bv | Tratamiento de afecciones asociadas con eotaxina incrementada con 25-hidroxivitamina D3 |
| AU2011353202B2 (en) | 2010-12-28 | 2015-07-09 | Future Diagnostics B.V. | Release reagent for vitamin D |
| WO2012117236A1 (en) | 2011-03-02 | 2012-09-07 | D3 Pharma Limited | Vitamin d composition |
| US8992971B2 (en) | 2011-04-20 | 2015-03-31 | Suheung Capsule Co., Ltd. | Non-animal soft capsule shell composition having improved disintegration and shell hardness |
| EP2699250A4 (en) * | 2011-04-20 | 2014-11-12 | Mico Bio Inc | COMPOSITIONS AND METHODS FOR INCREASING IMMUNE RESPONSE |
| CN102771688A (zh) | 2011-05-13 | 2012-11-14 | 富曼实(上海)商贸有限公司 | 可食用液体填充的多糖胶囊 |
| EP2713770B1 (en) | 2011-05-27 | 2017-12-06 | DSM IP Assets B.V. | Extrusion process and extrudate resulting therefrom |
| US20130085121A1 (en) | 2011-09-30 | 2013-04-04 | Jianguo Wang | Pharmaceutical compositions comprising phosphate binder, calcium receptor-active compound and/or active vitamin d |
| US9877929B2 (en) * | 2011-10-13 | 2018-01-30 | Premier Dental Products Company | Topical vitamin D and ubiquinol oral supplement compositions |
| WO2014029953A1 (en) | 2012-08-21 | 2014-02-27 | Cipla Limited | Hot melt extruded (hme) pharmaceutical composition of cinacalcet |
| KR101847947B1 (ko) | 2013-03-15 | 2018-05-28 | 옵코 아이피 홀딩스 Ⅱ 인코포레이티드 | 안정화되고 변형된 비타민 d 방출 제형 |
| PT106978A (pt) | 2013-05-31 | 2014-12-02 | Tecnimede Sociedade Tecnico Medicinal S A | Composição sólida oral contendo ácido ibandrónico e vitamina d |
| EP2815745A1 (en) | 2013-06-21 | 2014-12-24 | Swiss Caps Rechte und Lizenzen AG | Soft shell capsule and process for its manufacture |
| CN103520133B (zh) | 2013-10-26 | 2015-02-04 | 中山市凯博思淀粉材料科技有限公司 | 一种淀粉基软胶囊的制备方法 |
| CN103495176B (zh) | 2013-10-26 | 2015-01-28 | 中山市凯博思淀粉材料科技有限公司 | 一种共混挤出法制备淀粉基软胶囊的方法 |
| TWI645864B (zh) * | 2014-03-14 | 2019-01-01 | 歐科二代智財控股公司 | 經穩定之修飾釋放維他命d調配物及其服用方法 |
| US10220047B2 (en) | 2014-08-07 | 2019-03-05 | Opko Ireland Global Holdings, Ltd. | Adjunctive therapy with 25-hydroxyvitamin D and articles therefor |
| US20180085381A1 (en) | 2014-08-07 | 2018-03-29 | Opko Ireland Global Holdings, Ltd. | Adjunctive Therapy With 25-Hydroxyvitamin D |
| US10998308B2 (en) | 2019-07-22 | 2021-05-04 | Texas Instruments Incorporated | Area-efficient bi-directional ESD structure |
-
2014
- 2014-03-06 KR KR1020140026781A patent/KR101847947B1/ko active Active
- 2014-03-14 SM SM20200651T patent/SMT202000651T1/it unknown
- 2014-03-14 WO PCT/US2014/028132 patent/WO2014143941A1/en active Application Filing
- 2014-03-14 DE DE202014011525.7U patent/DE202014011525U1/de not_active Expired - Lifetime
- 2014-03-14 LT LTEP18155036.9T patent/LT3332773T/lt unknown
- 2014-03-14 RS RS20200963A patent/RS60846B1/sr unknown
- 2014-03-14 EA EA201591809A patent/EA038867B1/ru unknown
- 2014-03-14 HK HK16108396.5A patent/HK1220362A1/zh unknown
- 2014-03-14 DK DK18155036.9T patent/DK3332773T3/da active
- 2014-03-14 CN CN201480024061.XA patent/CN105246464A/zh active Pending
- 2014-03-14 EA EA201991774A patent/EA201991774A1/ru unknown
- 2014-03-14 SI SI201431628T patent/SI2968172T1/sl unknown
- 2014-03-14 EP EP21157479.3A patent/EP3888638A1/en active Pending
- 2014-03-14 PL PL19217651T patent/PL3650016T3/pl unknown
- 2014-03-14 SM SM20200424T patent/SMT202000424T1/it unknown
- 2014-03-14 PT PT181550369T patent/PT3332773T/pt unknown
- 2014-03-14 HU HUE19217651A patent/HUE055591T2/hu unknown
- 2014-03-14 SG SG11201507323PA patent/SG11201507323PA/en unknown
- 2014-03-14 MX MX2015012625A patent/MX2015012625A/es unknown
- 2014-03-14 TW TW105138697A patent/TWI659753B/zh active
- 2014-03-14 AU AU2014228069A patent/AU2014228069B2/en active Active
- 2014-03-14 ES ES18155036T patent/ES2834900T3/es active Active
- 2014-03-14 CR CR20190178A patent/CR20190178A/es unknown
- 2014-03-14 UA UAA201510090A patent/UA123386C2/uk unknown
- 2014-03-14 EP EP18155036.9A patent/EP3332773B8/en active Active
- 2014-03-14 HR HRP20201284TT patent/HRP20201284T1/hr unknown
- 2014-03-14 CA CA2905409A patent/CA2905409C/en active Active
- 2014-03-14 CN CN202010255439.4A patent/CN111346071A/zh active Pending
- 2014-03-14 SI SI201431862T patent/SI3650016T1/sl unknown
- 2014-03-14 ES ES19217651T patent/ES2882567T3/es active Active
- 2014-03-14 MY MYPI2015002281A patent/MY194092A/en unknown
- 2014-03-14 HK HK16108226.1A patent/HK1220128A1/zh unknown
- 2014-03-14 PT PT192176519T patent/PT3650016T/pt unknown
- 2014-03-14 PT PT147170385T patent/PT2968172T/pt unknown
- 2014-03-14 SM SM20210439T patent/SMT202100439T1/it unknown
- 2014-03-14 JP JP2016502712A patent/JP6492051B2/ja active Active
- 2014-03-14 LT LTEP19217651.9T patent/LT3650016T/lt unknown
- 2014-03-14 EP EP14717038.5A patent/EP2968172B8/en active Active
- 2014-03-14 PE PE2015002016A patent/PE20151761A1/es unknown
- 2014-03-14 PL PL18155036T patent/PL3332773T3/pl unknown
- 2014-03-14 EP EP19217651.9A patent/EP3650016B1/en active Active
- 2014-03-14 US US14/213,285 patent/US9861644B2/en active Active
- 2014-03-14 LT LTEP14717038.5T patent/LT2968172T/lt unknown
- 2014-03-14 HU HUE18155036A patent/HUE051923T2/hu unknown
- 2014-03-14 DK DK14717038.5T patent/DK2968172T3/da active
- 2014-03-14 PL PL14717038T patent/PL2968172T3/pl unknown
- 2014-03-14 ES ES14717038T patent/ES2809477T3/es active Active
- 2014-03-14 BR BR112015023658A patent/BR112015023658A2/pt not_active Application Discontinuation
- 2014-03-14 RS RS20210954A patent/RS62176B1/sr unknown
- 2014-03-14 SI SI201431712T patent/SI3332773T1/sl unknown
- 2014-03-14 RS RS20201434A patent/RS61132B1/sr unknown
- 2014-03-14 HU HUE14717038A patent/HUE052014T2/hu unknown
- 2014-03-14 DK DK19217651.9T patent/DK3650016T3/da active
- 2014-03-14 SG SG10201703517VA patent/SG10201703517VA/en unknown
- 2014-03-17 AR ARP140101199A patent/AR095576A1/es not_active Application Discontinuation
- 2014-11-03 KR KR1020140151051A patent/KR102203003B1/ko active Active
-
2015
- 2015-09-10 IL IL241456A patent/IL241456B/en not_active IP Right Cessation
- 2015-09-11 MX MX2020011736A patent/MX2020011736A/es unknown
- 2015-09-14 CL CL2015002659A patent/CL2015002659A1/es unknown
- 2015-09-15 SA SA515361134A patent/SA515361134B1/ar unknown
- 2015-09-15 PH PH12015502162A patent/PH12015502162A1/en unknown
-
2017
- 2017-09-29 US US15/720,948 patent/US10350224B2/en active Active
- 2017-10-27 JP JP2017207982A patent/JP6533268B2/ja active Active
-
2018
- 2018-05-25 US US15/990,354 patent/US10300078B2/en active Active
- 2018-05-25 US US15/990,352 patent/US10357502B2/en active Active
-
2019
- 2019-01-16 AU AU2019200268A patent/AU2019200268B2/en active Active
- 2019-05-23 JP JP2019096924A patent/JP2019135264A/ja active Pending
- 2019-06-21 US US16/448,838 patent/US11253528B2/en active Active
- 2019-08-02 KR KR1020190094318A patent/KR20190095216A/ko not_active Ceased
-
2020
- 2020-05-21 IL IL274841A patent/IL274841A/en unknown
- 2020-08-05 CY CY20201100727T patent/CY1123167T1/el unknown
- 2020-11-24 CY CY20201101109T patent/CY1123568T1/el unknown
- 2020-11-25 HR HRP20201869TT patent/HRP20201869T1/hr unknown
-
2021
- 2021-02-19 NO NO2021007C patent/NO2021007I1/no unknown
- 2021-05-17 PH PH12021551127A patent/PH12021551127A1/en unknown
- 2021-06-21 KR KR1020210079948A patent/KR20210078463A/ko not_active Ceased
- 2021-07-05 JP JP2021111250A patent/JP7282832B2/ja active Active
- 2021-07-30 CY CY20211100683T patent/CY1124393T1/el unknown
- 2021-08-05 HR HRP20211265TT patent/HRP20211265T1/hr unknown
-
2022
- 2022-01-06 US US17/570,194 patent/US20220125806A1/en active Pending
-
2023
- 2023-04-05 EC ECSENADI202324864A patent/ECSP23024864A/es unknown
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20210078463A (ko) | 안정화되고 변형된 비타민 d 방출 제형 및 이의 투여 방법 | |
| TWI743426B (zh) | 經穩定之修飾釋放維他命d調配物及其服用方法 | |
| HK40033528A (en) | Stabilized modified release vitamin d formulation and method of administering same | |
| HK40020409A (en) | Stabilized modified release vitamin d formulation and method of administering same | |
| HK40020409B (en) | Stabilized modified release vitamin d formulation and method of administering same | |
| TWI812891B (zh) | 經穩定之修飾釋放維他命d調配物及其服用方法 | |
| HK1256895B (en) | Stabilized modified release vitamin d formulation and method of administering same | |
| NZ711924B2 (en) | Stabilized modified release vitamin d formulation and method of administering same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| PA0107 | Divisional application |
Comment text: Divisional Application of Patent Patent event date: 20210621 Patent event code: PA01071R01D Filing date: 20190802 Application number text: 1020190094318 |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210721 Comment text: Request for Examination of Application Patent event code: PA02011R04I Patent event date: 20210621 Comment text: Divisional Application of Patent |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20211025 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20220629 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20211025 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |