CN102391261A - 一种n-取代噁二嗪类化合物及其制备方法和应用 - Google Patents
一种n-取代噁二嗪类化合物及其制备方法和应用 Download PDFInfo
- Publication number
- CN102391261A CN102391261A CN2011103129260A CN201110312926A CN102391261A CN 102391261 A CN102391261 A CN 102391261A CN 2011103129260 A CN2011103129260 A CN 2011103129260A CN 201110312926 A CN201110312926 A CN 201110312926A CN 102391261 A CN102391261 A CN 102391261A
- Authority
- CN
- China
- Prior art keywords
- oxadiazine
- chloro
- indeno
- methoxycarbonyl
- methyl ester
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- -1 N-substituted dioxazine compound Chemical class 0.000 title claims abstract description 74
- 238000002360 preparation method Methods 0.000 title claims abstract description 18
- 150000001875 compounds Chemical class 0.000 claims abstract description 32
- 238000006243 chemical reaction Methods 0.000 claims abstract description 21
- 230000000694 effects Effects 0.000 claims abstract description 21
- 230000000749 insecticidal effect Effects 0.000 claims abstract description 21
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 claims abstract description 15
- 239000002904 solvent Substances 0.000 claims abstract description 13
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 claims abstract description 10
- 239000001257 hydrogen Substances 0.000 claims abstract description 8
- 229910052739 hydrogen Inorganic materials 0.000 claims abstract description 8
- 239000000126 substance Substances 0.000 claims abstract description 8
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims abstract description 7
- GTCAXTIRRLKXRU-UHFFFAOYSA-N methyl carbamate Chemical compound COC(N)=O GTCAXTIRRLKXRU-UHFFFAOYSA-N 0.000 claims abstract description 7
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 claims abstract description 6
- 125000003118 aryl group Chemical group 0.000 claims abstract description 5
- 238000005984 hydrogenation reaction Methods 0.000 claims abstract description 5
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims abstract description 5
- 238000006482 condensation reaction Methods 0.000 claims abstract description 4
- 239000002994 raw material Substances 0.000 claims abstract description 4
- 125000000714 pyrimidinyl group Chemical group 0.000 claims abstract description 3
- 125000001160 methoxycarbonyl group Chemical group [H]C([H])([H])OC(*)=O 0.000 claims description 119
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 89
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 claims description 53
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 claims description 20
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 claims description 18
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 claims description 18
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 claims description 18
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 17
- 238000000034 method Methods 0.000 claims description 12
- XEKOWRVHYACXOJ-UHFFFAOYSA-N ethyl acetate Substances CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 claims description 10
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 claims description 9
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 claims description 8
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 claims description 8
- KXKVLQRXCPHEJC-UHFFFAOYSA-N acetic acid trimethyl ester Natural products COC(C)=O KXKVLQRXCPHEJC-UHFFFAOYSA-N 0.000 claims description 8
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 claims description 6
- 239000000203 mixture Substances 0.000 claims description 6
- WSLDOOZREJYCGB-UHFFFAOYSA-N 1,2-Dichloroethane Chemical compound ClCCCl WSLDOOZREJYCGB-UHFFFAOYSA-N 0.000 claims description 5
- 238000001953 recrystallisation Methods 0.000 claims description 4
- LUZDYPLAQQGJEA-UHFFFAOYSA-N 2-Methoxynaphthalene Chemical compound C1=CC=CC2=CC(OC)=CC=C21 LUZDYPLAQQGJEA-UHFFFAOYSA-N 0.000 claims description 3
- CDMIQAIIIBPTRK-UHFFFAOYSA-N 2-butoxynaphthalene Chemical compound C1=CC=CC2=CC(OCCCC)=CC=C21 CDMIQAIIIBPTRK-UHFFFAOYSA-N 0.000 claims description 3
- XMIYCZUIHNVBKA-UHFFFAOYSA-N 2-pentoxynaphthalene Chemical compound C1=CC=CC2=CC(OCCCCC)=CC=C21 XMIYCZUIHNVBKA-UHFFFAOYSA-N 0.000 claims description 3
- VTIIVLSJEXKCQD-UHFFFAOYSA-N 2-propan-2-yloxynaphthalene Chemical compound C1=CC=CC2=CC(OC(C)C)=CC=C21 VTIIVLSJEXKCQD-UHFFFAOYSA-N 0.000 claims description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 claims description 3
- FKNQCJSGGFJEIZ-UHFFFAOYSA-N gamma-methylpyridine Natural products CC1=CC=NC=C1 FKNQCJSGGFJEIZ-UHFFFAOYSA-N 0.000 claims description 3
- 238000004440 column chromatography Methods 0.000 claims description 2
- CFHIDWOYWUOIHU-UHFFFAOYSA-N oxomethyl Chemical compound O=[CH] CFHIDWOYWUOIHU-UHFFFAOYSA-N 0.000 claims 30
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 claims 3
- 229960001701 chloroform Drugs 0.000 claims 3
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims 2
- 239000001301 oxygen Substances 0.000 claims 2
- 229910052760 oxygen Inorganic materials 0.000 claims 2
- 238000000746 purification Methods 0.000 claims 2
- 238000000926 separation method Methods 0.000 claims 2
- UDMRXJVASPGFQM-UHFFFAOYSA-N 1-hexoxynaphthalene Chemical compound C1=CC=C2C(OCCCCCC)=CC=CC2=C1 UDMRXJVASPGFQM-UHFFFAOYSA-N 0.000 claims 1
- JOGFTFKRKDIQEK-UHFFFAOYSA-N 1-propoxynaphthalene Chemical compound C1=CC=C2C(OCCC)=CC=CC2=C1 JOGFTFKRKDIQEK-UHFFFAOYSA-N 0.000 claims 1
- 241000409991 Mythimna separata Species 0.000 claims 1
- 239000000460 chlorine Substances 0.000 claims 1
- 229910052801 chlorine Inorganic materials 0.000 claims 1
- OBNCKNCVKJNDBV-UHFFFAOYSA-N ethyl butyrate Chemical compound CCCC(=O)OCC OBNCKNCVKJNDBV-UHFFFAOYSA-N 0.000 claims 1
- 125000004494 ethyl ester group Chemical group 0.000 claims 1
- 229910052740 iodine Inorganic materials 0.000 claims 1
- 239000011630 iodine Substances 0.000 claims 1
- 150000002790 naphthalenes Chemical class 0.000 claims 1
- 125000000636 p-nitrophenyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)[N+]([O-])=O 0.000 claims 1
- 150000003053 piperidines Chemical class 0.000 claims 1
- DSNYFFJTZPIKFZ-UHFFFAOYSA-N propoxybenzene Chemical group CCCOC1=CC=CC=C1 DSNYFFJTZPIKFZ-UHFFFAOYSA-N 0.000 claims 1
- 241001477931 Mythimna unipuncta Species 0.000 abstract description 14
- 239000003054 catalyst Substances 0.000 abstract description 5
- 125000001624 naphthyl group Chemical group 0.000 abstract description 2
- 125000002485 formyl group Chemical group [H]C(*)=O 0.000 description 60
- 238000002844 melting Methods 0.000 description 50
- 230000008018 melting Effects 0.000 description 50
- 238000012216 screening Methods 0.000 description 13
- VBCVPMMZEGZULK-NRFANRHFSA-N indoxacarb Chemical compound C([C@@]1(OC2)C(=O)OC)C3=CC(Cl)=CC=C3C1=NN2C(=O)N(C(=O)OC)C1=CC=C(OC(F)(F)F)C=C1 VBCVPMMZEGZULK-NRFANRHFSA-N 0.000 description 11
- 239000002917 insecticide Substances 0.000 description 11
- 241000238631 Hexapoda Species 0.000 description 9
- 239000005907 Indoxacarb Substances 0.000 description 9
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 8
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 description 8
- 241001124076 Aphididae Species 0.000 description 6
- 241000500437 Plutella xylostella Species 0.000 description 6
- 239000003814 drug Substances 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 0 CC(C1)(C(OC)=O)C(c(cc2)ccc2Cl)=NN1C(*c1ccc(C(F)(F)F)cc1)=O Chemical compound CC(C1)(C(OC)=O)C(c(cc2)ccc2Cl)=NN1C(*c1ccc(C(F)(F)F)cc1)=O 0.000 description 5
- 241000219823 Medicago Species 0.000 description 5
- 235000017587 Medicago sativa ssp. sativa Nutrition 0.000 description 5
- 240000007594 Oryza sativa Species 0.000 description 5
- 235000007164 Oryza sativa Nutrition 0.000 description 5
- 229910052757 nitrogen Inorganic materials 0.000 description 5
- 150000005063 oxadiazines Chemical class 0.000 description 5
- 235000009566 rice Nutrition 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- 229920000742 Cotton Polymers 0.000 description 4
- 230000004071 biological effect Effects 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- OEBRKCOSUFCWJD-UHFFFAOYSA-N dichlorvos Chemical compound COP(=O)(OC)OC=C(Cl)Cl OEBRKCOSUFCWJD-UHFFFAOYSA-N 0.000 description 4
- 229950001327 dichlorvos Drugs 0.000 description 4
- 229920003023 plastic Polymers 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- HRYILSDLIGTCOP-UHFFFAOYSA-N N-benzoylurea Chemical compound NC(=O)NC(=O)C1=CC=CC=C1 HRYILSDLIGTCOP-UHFFFAOYSA-N 0.000 description 3
- 241001556089 Nilaparvata lugens Species 0.000 description 3
- 108010052164 Sodium Channels Proteins 0.000 description 3
- 102000018674 Sodium Channels Human genes 0.000 description 3
- 241000344246 Tetranychus cinnabarinus Species 0.000 description 3
- 241000607479 Yersinia pestis Species 0.000 description 3
- 238000007598 dipping method Methods 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 239000000575 pesticide Substances 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 239000007921 spray Substances 0.000 description 3
- GUMOJENFFHZAFP-UHFFFAOYSA-N 2-Ethoxynaphthalene Chemical compound C1=CC=CC2=CC(OCC)=CC=C21 GUMOJENFFHZAFP-UHFFFAOYSA-N 0.000 description 2
- IPGBTIJXFBAAIM-UHFFFAOYSA-N 2-hexoxynaphthalene Chemical compound C1=CC=CC2=CC(OCCCCCC)=CC=C21 IPGBTIJXFBAAIM-UHFFFAOYSA-N 0.000 description 2
- UEXFDEKTELCPNF-UHFFFAOYSA-N 2-propoxynaphthalene Chemical compound C1=CC=CC2=CC(OCCC)=CC=C21 UEXFDEKTELCPNF-UHFFFAOYSA-N 0.000 description 2
- 241000238876 Acari Species 0.000 description 2
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- 241000239290 Araneae Species 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 2
- 239000005663 Pyridaben Substances 0.000 description 2
- 240000006677 Vicia faba Species 0.000 description 2
- 235000010749 Vicia faba Nutrition 0.000 description 2
- 235000002098 Vicia faba var. major Nutrition 0.000 description 2
- VJMAITQRABEEKP-UHFFFAOYSA-N [6-(phenylmethoxymethyl)-1,4-dioxan-2-yl]methyl acetate Chemical compound O1C(COC(=O)C)COCC1COCC1=CC=CC=C1 VJMAITQRABEEKP-UHFFFAOYSA-N 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 238000004166 bioassay Methods 0.000 description 2
- JLQUFIHWVLZVTJ-UHFFFAOYSA-N carbosulfan Chemical compound CCCCN(CCCC)SN(C)C(=O)OC1=CC=CC2=C1OC(C)(C)C2 JLQUFIHWVLZVTJ-UHFFFAOYSA-N 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 230000010534 mechanism of action Effects 0.000 description 2
- IETRIQRMVUNDTP-NRFANRHFSA-N methyl (4aS)-7-chloro-2-[methoxycarbonyl-[4-(trifluoromethylsulfanyl)phenyl]carbamoyl]-3,5-dihydroindeno[1,2-e][1,3,4]oxadiazine-4a-carboxylate Chemical compound COC(=O)N(C(=O)N1CO[C@]2(CC3=CC(Cl)=CC=C3C2=N1)C(=O)OC)C1=CC=C(SC(F)(F)F)C=C1 IETRIQRMVUNDTP-NRFANRHFSA-N 0.000 description 2
- IODOXLXFXNATGI-UHFFFAOYSA-N methyl naphthalene-2-carboxylate Chemical compound C1=CC=CC2=CC(C(=O)OC)=CC=C21 IODOXLXFXNATGI-UHFFFAOYSA-N 0.000 description 2
- ZWLPBLYKEWSWPD-UHFFFAOYSA-N o-toluic acid Chemical compound CC1=CC=CC=C1C(O)=O ZWLPBLYKEWSWPD-UHFFFAOYSA-N 0.000 description 2
- 238000005457 optimization Methods 0.000 description 2
- DWFZBUWUXWZWKD-UHFFFAOYSA-N pyridaben Chemical compound C1=CC(C(C)(C)C)=CC=C1CSC1=C(Cl)C(=O)N(C(C)(C)C)N=C1 DWFZBUWUXWZWKD-UHFFFAOYSA-N 0.000 description 2
- SZUVGFMDDVSKSI-WIFOCOSTSA-N (1s,2s,3s,5r)-1-(carboxymethyl)-3,5-bis[(4-phenoxyphenyl)methyl-propylcarbamoyl]cyclopentane-1,2-dicarboxylic acid Chemical compound O=C([C@@H]1[C@@H]([C@](CC(O)=O)([C@H](C(=O)N(CCC)CC=2C=CC(OC=3C=CC=CC=3)=CC=2)C1)C(O)=O)C(O)=O)N(CCC)CC(C=C1)=CC=C1OC1=CC=CC=C1 SZUVGFMDDVSKSI-WIFOCOSTSA-N 0.000 description 1
- QFLWZFQWSBQYPS-AWRAUJHKSA-N (3S)-3-[[(2S)-2-[[(2S)-2-[5-[(3aS,6aR)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-[1-bis(4-chlorophenoxy)phosphorylbutylamino]-4-oxobutanoic acid Chemical compound CCCC(NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)C(C)C)P(=O)(Oc1ccc(Cl)cc1)Oc1ccc(Cl)cc1 QFLWZFQWSBQYPS-AWRAUJHKSA-N 0.000 description 1
- SCYULBFZEHDVBN-UHFFFAOYSA-N 1,1-Dichloroethane Chemical compound CC(Cl)Cl SCYULBFZEHDVBN-UHFFFAOYSA-N 0.000 description 1
- NGNBDVOYPDDBFK-UHFFFAOYSA-N 2-[2,4-di(pentan-2-yl)phenoxy]acetyl chloride Chemical compound CCCC(C)C1=CC=C(OCC(Cl)=O)C(C(C)CCC)=C1 NGNBDVOYPDDBFK-UHFFFAOYSA-N 0.000 description 1
- KUZSBKJSGSKPJH-VXGBXAGGSA-N 5-[(9R)-6-[(3R)-3-methylmorpholin-4-yl]-11-oxa-1,3,5-triazatricyclo[7.4.0.02,7]trideca-2,4,6-trien-4-yl]pyrazin-2-amine Chemical compound C[C@@H]1COCCN1c1nc(nc2N3CCOC[C@H]3Cc12)-c1cnc(N)cn1 KUZSBKJSGSKPJH-VXGBXAGGSA-N 0.000 description 1
- 241000251468 Actinopterygii Species 0.000 description 1
- 241000498522 Aphis medicaginis Species 0.000 description 1
- 240000007124 Brassica oleracea Species 0.000 description 1
- 235000003899 Brassica oleracea var acephala Nutrition 0.000 description 1
- 235000011301 Brassica oleracea var capitata Nutrition 0.000 description 1
- 235000001169 Brassica oleracea var oleracea Nutrition 0.000 description 1
- VUZNNDBNYIBEDE-UHFFFAOYSA-N CC(C)(C)OC1OC1Oc1ccc(cccc2)c2c1N(C(N(COC1(C2)C(OC)=O)N=C1c(cc1)c2cc1Cl)=O)C(OC)=O Chemical compound CC(C)(C)OC1OC1Oc1ccc(cccc2)c2c1N(C(N(COC1(C2)C(OC)=O)N=C1c(cc1)c2cc1Cl)=O)C(OC)=O VUZNNDBNYIBEDE-UHFFFAOYSA-N 0.000 description 1
- 241000254173 Coleoptera Species 0.000 description 1
- 241000255777 Lepidoptera Species 0.000 description 1
- 241001556090 Nilaparvata Species 0.000 description 1
- QQWRPXADMUGYKQ-UHFFFAOYSA-N O1NN=CC=C1.O1NN=CC=C1 Chemical compound O1NN=CC=C1.O1NN=CC=C1 QQWRPXADMUGYKQ-UHFFFAOYSA-N 0.000 description 1
- 206010034133 Pathogen resistance Diseases 0.000 description 1
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical group C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 description 1
- 239000006004 Quartz sand Substances 0.000 description 1
- 241001327627 Separata Species 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- 241000985245 Spodoptera litura Species 0.000 description 1
- 241001454294 Tetranychus Species 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- VXSIXFKKSNGRRO-MXOVTSAMSA-N [(1s)-2-methyl-4-oxo-3-[(2z)-penta-2,4-dienyl]cyclopent-2-en-1-yl] (1r,3r)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropane-1-carboxylate;[(1s)-2-methyl-4-oxo-3-[(2z)-penta-2,4-dienyl]cyclopent-2-en-1-yl] (1r,3r)-3-[(e)-3-methoxy-2-methyl-3-oxoprop-1-enyl Chemical class CC1(C)[C@H](C=C(C)C)[C@H]1C(=O)O[C@@H]1C(C)=C(C\C=C/C=C)C(=O)C1.CC1(C)[C@H](/C=C(\C)C(=O)OC)[C@H]1C(=O)O[C@@H]1C(C)=C(C\C=C/C=C)C(=O)C1 VXSIXFKKSNGRRO-MXOVTSAMSA-N 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 238000009395 breeding Methods 0.000 description 1
- 230000001488 breeding effect Effects 0.000 description 1
- 239000000073 carbamate insecticide Substances 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 229940126543 compound 14 Drugs 0.000 description 1
- 229940125898 compound 5 Drugs 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 239000010779 crude oil Substances 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 235000021186 dishes Nutrition 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 125000000816 ethylene group Chemical group [H]C([H])([*:1])C([H])([H])[*:2] 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 229940126181 ion channel inhibitor Drugs 0.000 description 1
- 150000002611 lead compounds Chemical class 0.000 description 1
- 231100000053 low toxicity Toxicity 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 150000004702 methyl esters Chemical class 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 210000002569 neuron Anatomy 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- 239000003986 organophosphate insecticide Substances 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 125000003226 pyrazolyl group Chemical group 0.000 description 1
- HYJYGLGUBUDSLJ-UHFFFAOYSA-N pyrethrin Natural products CCC(=O)OC1CC(=C)C2CC3OC3(C)C2C2OC(=O)C(=C)C12 HYJYGLGUBUDSLJ-UHFFFAOYSA-N 0.000 description 1
- 229940070846 pyrethrins Drugs 0.000 description 1
- 239000002728 pyrethroid Substances 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 238000006798 ring closing metathesis reaction Methods 0.000 description 1
- 238000007142 ring opening reaction Methods 0.000 description 1
- 238000010898 silica gel chromatography Methods 0.000 description 1
- 238000002791 soaking Methods 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
Landscapes
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Nitrogen And Oxygen As The Only Ring Hetero Atoms (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
本发明涉及一种N-取代噁二嗪类化合物及其制备方法和应用,该化合物的化学结构通式(I)如下:
Description
技术领域
本发明涉及一种杀虫剂,尤其是涉及一种具有杀虫活性的N-取代噁二嗪类化合物,其制备方法和应用。
背景技术
噁二嗪类(oxadiazine)杀虫剂茚虫威(indoxacarb)是美国杜邦(Dupont)公司1992年开发的钠通道抑制广谱杀虫剂(W09211249),基于一种新的杀虫作用机制,即阻断神经细胞的钠离子通道,减少和常用杀虫剂(如除虫菊酯、有机磷以及氨基甲酸酯类杀虫剂)发生交互抗性的可能。茚虫威本身是一种前体杀虫剂,通过在昆虫体内代谢去除酰胺氮原子上的甲氧羰基(-COOCH3)后转化为活性化合物,在广谱范围内具有很高的杀虫活性而且融合了环境相容性好、对哺乳动物毒性低和对鸟类、鱼类以及益虫安全的优点,作为替代有机磷杀虫剂的理想品种之一。
茚虫威的先导化合物为苯甲酰脲类杀虫剂RH3421,Philips-Duphar公司在苯甲酰脲类杀虫剂1的基础上运用生物等排理论将1变为2,后来开发出环状结构3。在结构3中A,Z可为多种基团,并且可以同其他原子组成五元或六元环,为了便于合成环状结构3优化为4(PH60-42),可能由于残留的原因,不能满足登记的要求,没有最终工业化。
Ricard Jacobson博士(罗门哈斯公司)于1980年开始针对化合物4,通过结构优化发现化合物5(RH3421),RH3421不仅对鳞翅目、鞘翅目害虫有很好活性,在较低剂量下具有良好防治效果。作用机理新颖,是钠离子通道抑制剂,与苯甲酰脲类杀虫剂的机理完全不同。
由于RH3421具有特异性作用机理,杜邦公司Thomas Stevenson等人开始对RH3421类化合物进行结构优化,在RH3421和化合物6的基础上,将1位上的N原子和3位上的C原子交换,得到化合物7,合成了一些化合物,离体生测结果表明较好的活性。
化合物6的衍生结构8和9,可能是由于氢键的原因,杜邦公司的ThomasStevenson等就设想将化合物物9中的吡唑4位的一个取代基如R2和3位苯环邻位合环即变为结构为10和11的化合物。
通过离体生测发现化合物11的活性优于12b,同时还对吡唑开环化合物12b进行研究,并发现B1为CH2的活性要好于B1为CH2CH2的化合物,但合成的化合物的在活性谱以及残留活性方面均不令人满意,需要改进。通过结构活性分析他们发现环的大小直接影响到生物活性,并将化合物12a和12b进行了组合优化为13。通过研究发现化合物14在一定计量下对鳞翅目害虫具有很好的防治效果,但残留时间过长。
对大量的实验结果数据进行分析,经过进一步优化研究,发现15适宜的残留活性和较好的生态性能,但对哺乳动物具有相对较高的活性。经过进一步的研究发现了茚虫威的结构,尽管茚虫威本身活性较弱但可以被害虫快速代谢为活性很高的茚虫威的合成研制再次表明合成化学是一门取之不尽的技术。
发明内容
本发明的目的就是为了克服上述现有技术存在的缺陷而提供一种N-取代噁二嗪类化合物及其制备方法和应用。
本发明的目的可以通过以下技术方案来实现:一种N-取代噁二嗪类化合物,其特征在于,该化合物的化学结构通式(I)如下:
化学结构通式(I)中Ar为苯环、萘环、吡啶或嘧啶环系。
所述的化合物包括:
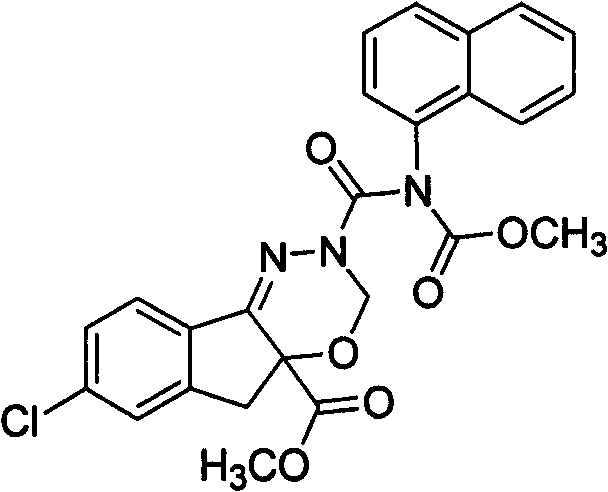
(1)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(吡啶)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-001表示),
(2)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-002表示),
(3)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[2-(4-甲基吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-003表示),
(4)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[2-(6-甲基吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-004表示),
(5)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[2-(5-溴吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-005表示),
(6)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[2-(5-碘吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-006表示),
(8)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[4-(2-氯吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-008表示),
(9)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[3-(2-氯吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-009表示),
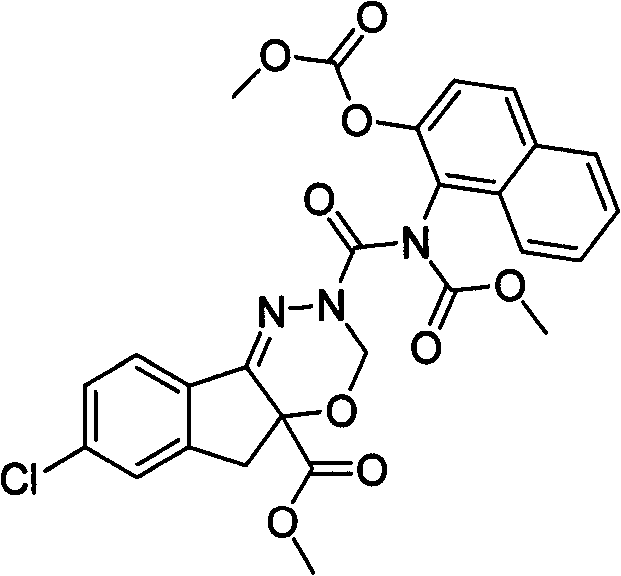
(10)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-甲氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-010表示),
(11)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-乙氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-011表示),
(12)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-正丙氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-012表示),
(13)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-正戊氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-013表示),
(14)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-正己氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-014表示),
(15)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-异丙氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-015表示),
(16)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-正丁氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-016表示),
(17)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[2-(4,6-二甲氧基嘧啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-017表示),
(18)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-特丁酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-018表示),
(19)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-差劲基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-019表示),
(20)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-丁酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-020表示),
(21)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-乙酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-021表示),
(22)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-丙酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-022表示),
(23)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-甲氧甲醚萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-023表示),
(24)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-苯甲酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-024表示),
(25)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-吡喃甲酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-025表示),
(26)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-甲氧甲酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-026表示),
(27)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基2-(4-氯-6-甲氧基嘧啶)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-027表示),
(28)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基2-(4,6-二甲氧基嘧啶)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(用代号SIOC-Y-028表示),
(29)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-甲氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-029表示),
(30)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-乙氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-030表示),
(31)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-正丙氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-031表示),
(32)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-异氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-032表示),
(33)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-正丁氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-033表示),
(34)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-正戊氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-034表示),
(35)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-正己氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-035表示),
(36)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-三氟甲氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-036表示),
(37)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-三氟乙氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-037表示),
(38)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-三氟甲基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-038表示),
(39)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(2-三氟甲氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-039表示),
(40)(S)-7-氯-3,5一二氢-2-[甲氧基羰基2-[(3-三氟甲氧基苯基)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-040表示),
(41)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(2-溴-4-三氟氧基苯基)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-041表示),
(42)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-硝基苯基)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-042表示),
(43)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-乙酰氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-043表示),
(44)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-甲氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-044表示),
(45)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-乙酰基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-045表示),
(46)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-甲酰氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-046表示),
(47)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-三氟甲硫基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-047表示),
(48)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(2,4-二甲氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-048表示),
(49)(S)-(+)-7-氯-3,5-二氢-2-[甲氧基羰基(2,4-二硝基苯基)氨基羰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羟酸甲酯(用代号SIOC-Y-049表示),
(50)(S)-7-氯-3,5-二氢-2-[甲氧基羰基(2-三氟甲氧基-5-氯-苯基)氨基羰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羟酸甲酯(用代号SIOC-Y-050表示),
(51)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4一乙氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯(用代号SIOC-Y-051表示),
(52)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4一甲基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a羧酸甲酯的合成(用代号SIOC-Y-052表示)。
本发明化化合物结构和熔点如下:
一种N-取代噁二嗪类化合物的制备方法,其特征在于,该方法包括以下步骤:
(1)以2-(苄基)-7-氯茚并[1,2-e][1,3,4]]噁二嗪-2,4a(3H,5H)-二羧酸-4a-甲基酯和氢气为原料,在溶剂a中以Pd/C为催化剂a,进行加氢反应,反应温度为20℃~120℃,反应压力为1-15个大气压,反应完毕后得到中间体B;
(2)以(氯羰基)(芳基)氨基甲酸甲酯为中间体C,与步骤(1)得到的中间体B,在溶剂b中,在催化剂b的作用下,进行缩合反应,反应温度为0℃~100℃得到产物N取代噁二嗪类化合物。
步骤(1)所述的溶剂a为乙酸甲酯、乙酸乙酯、甲醇、乙醇、乙腈、二氯甲烷、二氯乙烷、三氯甲烷、甲苯中的一种或两种以上的混合剂。
步骤(1)所述的反应温度为45℃~85℃,反应压力为4-6个大气压。
步骤(2)所述的溶剂b为乙酸甲酯、乙酯乙酸乙酯、甲醇、乙醇、乙腈、二氯甲烷、二氯乙烷、三氯甲烷、甲苯中的一种或两种以上的混合剂。
步骤(2)所述的催化剂b为N,N-二异丙基乙胺、三乙胺、吡啶或哌啶。
步骤(2)所述的反应温度为20℃~60℃。
步骤(2)所得N取代噁二嗪类化合物采用重结晶方法进行分离纯化,或采用柱色谱分离的方法进行分离纯化,所述的重结晶方法采用的溶剂为甲醇、乙醇、异丙醇、乙酸甲酯、乙酸乙酯、乙腈、二氯甲烷、二氯乙烷、三氯甲烷、甲苯、石油醚中的一种或两种以上的混合剂。
一种N-取代噁二嗪类化合物的应用,该化合物用于制备杀虫剂,对粘虫的杀虫活性达到80%~100%。
与现有技术相比,本发明不断对结构(I)进行结构优化和改造,最终研究出一些新的具有高杀虫活性的N取代噁二嗪类化合物Y001-Y052。
本发明涉及有N取代噁二嗪类化合物SIOC-Y-(001-052)对水稻褐飞虱、粘虫(小菜蛾)、红蜘蛛、苜蓿蚜等4个虫靶标的室内生物活性测试表明:化合物SIOC-Y-(001-052)在普筛浓度500mg/L时,其中化合物SIOC-Y-006、014、038、041-043、046、047、049、051对粘虫显示出100%的杀虫活性,036、037、039对粘虫显示出80%的杀虫活性,但对其他3种虫靶标室内杀虫活性较差或无杀虫作用。其余化合物对4种虫靶标室内杀虫活性均较差或无。
活性化合物降低浓度进一步筛选,结果表明SIOC-Y-047对粘虫仍表现出较好的杀虫活性,在浓度20mg/L时杀虫活性仍为100%。其余化合物降低浓度活性明显降低,在浓度20mg/L下室内对粘虫杀虫活性较差或无杀虫作用。结果表明化合物SIOC-Y-047对粘虫和斜纹夜蛾的活性略差与茚虫威,但EC50属于同一数量级水平。
具体实施方式
下面结合具体实施例对本发明进行详细说明:本实施例在以本发明技术方案为前提下进行实施,给出了详细的实施方式和具体的操作过程,但本发明的保护范围不限于下述的实施例。
本实施例采用2-(苄基)-7-氯茚并[1,2-e][1,3,4]]噁二嗪-2,4a(3H,5H)-二羧酸-4a-甲基酯(A)为原料在Pd/C催化下进行加氢反应,加氢还原产物7-氯-2,3,4a,5-四氢茚并[1,2-e][1,3,4]]噁二嗪-4a-甲酸甲酯(B)再与(氯羰基)(芳基)氨基甲酸甲酯中间体(C)进行缩合反应等反应步骤制备N取代噁二嗪类化合物,反应式中Bn表示苄基,Ar表示芳基。
以下为本发明实施例的详细描述。
7-氯-2,3,4a,5-四氢茚并[1,2-e][1,3,4]]噁二嗪-4a-甲酸甲酯(B)的制备
在50ml带导气管的三口反应瓶中,加入乙酸甲酯(20ml),2-(苄基)-7-氯茚并[1,2-e][1,3,4]噁二嗪-2,4a(3H,5H)-二羧酸4a-甲基酯(200mg),10%Pd/C(30mg),氢气保护下反应30分钟;加入制得的酰氯(C),加入N,N-二异丙基乙胺(200mg),同时撤掉氢气保护,改换氮气保护,反应12-18小时;撤掉氮气保护,水洗,用二氯甲烷萃取,无水硫酸镁干燥,浓缩,并进行后处理得N取代噁二嗪类化合物。
本发明结构通式(I)化合物的制备方法包括以下两个反应进行,反应式如下:
实施例1
(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(吡啶)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(SIOC-Y-001)的制备
在50ml反应瓶中加入乙酸甲酯(20ml),10%Pd/C(50mg)和2-(苄基)-7-氯茚并[1,2-e][1,3,4]噁二嗪-2,4a(3H,5H)-二羧酸4a-甲基酯(A)(200mg,0.5mmol),氢气保护下反应30分钟;加入(氯羰基)(2-吡啶)氨基甲酸甲酯(130mg,0.6mmol)的乙酸甲酯溶液(10ml),加入N,N-二异丙基乙胺(200mg,1.5mmol)同时撤掉氢气保护,改换氮气保护,反应12-18小时;撤掉氮气保护,水洗,用二氯甲烷萃取,无水硫酸镁干燥,浓缩,经硅胶柱层析分离,得淡黄色固体161.1mg,产率69.5%,熔点:150-152℃。
同理得SIOC-Y-002至SIOC-Y-052化合物。
实施例2.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(SIOC-Y-002)的制备。
熔点:81-83℃。
1H NMR(300MHz,CDCl3)δ:8.14(d,J=7.5Hz,1H),7.96-7.83(m,2H),7.66-7.44(m,4H),7.36-7.25(m,3H),5.70(d,J=9.0Hz,1H),5.26(d,J=9.6Hz,1H),3.76-3.64(m,6H),3.48(d,J=16.5Hz,1H),3.27(d,J=16.8Hz,1H)。
3.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[2-(4-甲基吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯(SIOC-Y-003)
熔点:169-171℃。
1H NMR(300MHz,CDCl3)δ:8.17(d,J=4.8Hz,1H),7.43(s,1H),7.38(d,J=8.7Hz,1H),7.30-7.22(m,2H),6.89(d,J=5.1Hz,1H),5.82(d,J=9.9Hz,1H),5.24(d,J=9.6Hz,1H),3.74(s,3H),3.70(s,3H),3.45(d,J=16.5Hz,1H),3.22(d,J=16.2Hz,1H),2.36(s,3H)。
4.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[2-(6-甲基吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:84-86℃。
1H NMR(300MHz,CDCl3)δ:7.59(t,J=7.8Hz,1H),7.42(d,J=8.4Hz,1H),7.35(d,J=8.4Hz,1H),7.31-7.21(m,2H),6.90(d,J=7.5Hz,1H),5.82(d,J=10.2Hz,1H),5.24(d,J=10.2Hz,1H),3.77(s,3H),3.71(s,3H),3.45(d,J=16.5Hz,1H),3.23(d,J=16.2Hz,1H),2.39(s,3H)。
5.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[2-(5-溴吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:127-129℃。
1H NMR(300MHz,CDCl3)δ:8.33(s,1H),7.83(dd,J=8.7,2.7Hz,1H),7.63(d,J=8.7Hz,1H),7.37(d,J=8.7Hz,1H),7.32-7.22(m,2H),5.84(d,J=10.2Hz,1H),5.26(d,J=10.2Hz,1H),3.78(s,3H),3.72(s,3H),3.47(d,J=16.2Hz,1H),3.25(d,J=16.2Hz,1H)。
6.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[2-(5-碘吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:154-156℃。
1H NMR(300MHz,CDCl3)δ:8.47(s,1H),7.99(dd,J=8.4,1.8Hz,1H),7.54(d,J=9.0Hz,1H),7.36(d,J=8.7Hz,1H),7.31-7.22(m,2H),5.83(d,J=9.6Hz,1H),5.25(d,J=10.2Hz,1H),3.77(s,3H),3.72(s,3H),3.46(d,J=16.2Hz,1H),3.25(d,J=16.2Hz,1H)。
8.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[4-(2-氯吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:63-66℃。
1H NMR(300MHz,CDCl3)δ:8.38(s,1H),7.67(dd,J=11.4,2.1Hz,1H),7.50(d,J=8.1Hz,1H),7.40-7.28(m,3H),5.72(d,J=9.3Hz,1H),5.21(d,J=9.9Hz,1H),3.75(s,3H),3.69(s,3H),3.49(d,J=16.2Hz,1H),3.26(d,J=16.5Hz,1H)。
9.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[3-(2-氯吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:91-93℃。
1H NMR(300MHz,CDCl3)δ:8.33(dd,J=4.5,1.8Hz,1H),7.67(dd,J=7.8,1.5Hz,1H),7.49(d,J=8.4Hz,1H),7.37-7.27(m,3H),5.64(d,J=9.6Hz,1H),5.23(d,J=9.3Hz,1H),3.77(s,3H),3.71(s,3H),3.46(d,J=16.8Hz,1H),3.23(d,J=16.5Hz,1H)。
10.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-甲氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:105-107℃。
1H NMR(300MHz,CDCl3)δ:7.98-7.74(m,3H),7.60-7.24(m,6H),5.63(d,J=9.3Hz,1H),5.30(d,J=9.3Hz,1H),3.76-3.67(m,9H),3.43(d,J=10.2Hz,1H),3.28(d,J=9.0Hz,1H)。
11.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-乙氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:75-77℃。
1H NMR(300MHz,CDCl3)δ:7.94-7.74(m,3H),7.59-7.23(m,6H),5.78-5.64(m,1H),5.33-5.15(m,1H),4.26-4.10(m,2H),3.76-3.63(m,6H),3.55-3.37(m,1H),3.33-3.14(m,1H),1.43-1.28(m,3H)。
12.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-正丙氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:77-79℃
1H NMR(300MHz,CDCl3)δ:7.98-7.72(m,3H),7.58-7.23(m,6H),5.78-5.64(m,1H),5.33-5.16(m,1H),4.15-3.99(m,2H),3.77-3.64(m,6H),3.53-3.38(m,1H),3.31-3.17(m,1H),1.88-1.62(m,2H),1.12-0.92(m,3H)。
13.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-正戊氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:55-57℃
1H NMR(300MHz,CDCl3)δ:7.96-7.74(m,3H),7.59-7.24(m,6H),5.78-5.64(m,1H),5.31-5.16(m,1H),4.19-4.02(m,2H),3.77-3.64(m,6H),3.53-3.39(m,1H),3.31-3.18(m,1H),1.90-1.62(m,2H),1.53-1.17(m,4H),0.99-0.78(m,3H)。
14.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-正己氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:62-64℃
1H NMR(300MHz,CDCl3)δ:7.92-7.73(m,3H),7.57-7.20(m,6H),5.77-5.62(m,1H),5.30-5.15(m,1H),4.18-4.03(m,2H),3.76-3.63(m,6H),3.52-3.39(m,1H),3.32-3.18(m,1H),1.85-1.62(m,2H),1.51-1.12(m,6H),0.94-0.78(m,3H)。
15.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-异丙氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
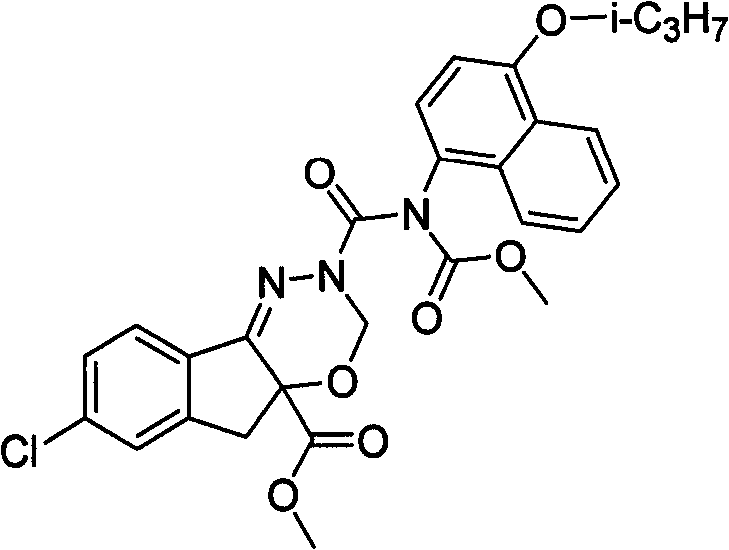
熔点:72-74℃
1H NMR(300MHz,CDCl3)δ:7.95-7.71(m,3H),7.63-7.21(m,6H),5.80-5.66(m,1H),5.33-5.16(m,1H),4.79-4.59(m,1H),3.74-3.63(m,6H),3.50-3.37(m,1H),3.29-3.16(m,1H),1.38-1.23(m,6H)。
16(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-正丁氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:79-81℃。
1H NMR(300MHz,CDCl3)δ:7.96-7.74(m,3H),7.59-7.23(m,6H),5.78-5.64(m,1H),5.33-5.14(m,1H),4.20-4.05(m,2H),3.77-3.64(m,6H),3.55-3.37(m,1H),3.34-3.15(m,1H),1.86-1.55(m,4H),1.05-0.88(m,3H)。
17.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[2-(4,6-二甲氧基嘧啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
1H NMR(300MHz,CDCl3)δ:7.36(d,J=9.0Hz,1H),7.30-7.22(m,2H),6.81(s,1H),5.85(d,J=9.9Hz,1H),3.76(s,3H),3.71(s,3H),3.45(d,J=16.5Hz,1H),3.22(d,J=15.9Hz,1H),2.43(s,6H)。
18.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-特丁酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:103-104℃
1H NMR(300MHz,CDCl3)δ:7.94-7.72(m,3H),7.56-7.25(m,6H),5.72-5.60(m,1H),5.33-5.14(m,1H),3.77-3.59(m,6H),3.54-3.37(m,1H),3.34-3.17(m,1H),1.26-1.10(m,9H)。
19.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-差劲基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:107-109℃
1H NMR(300MHz,CDCl3)δ:8.18(s,1H),8.00(t,J=8.1Hz,2H),7.74(d,J=8.1Hz,1H)7.66-7.47(m,5H),6.52(brs,1H),5.71(d,J=9.6Hz,1H),5.08(d,J=9.9Hz,1H),3.77(s,3H),3.74(s,3H),3.52(d,J=15.9Hz,1H),3.33(d,J=15.6Hz,1H)。
20.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-丁酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:85-87℃
1H NMR(300MHz,CDCl3)δ:8.07-7.80(m,4H),7.63-7.45(m,2H),7.35-7.15(m,3H),5.75-5.60(m,1H),5.27-5.13(m,1H),3.73-3.67(m,6H),3.48-3.37(m,1H),3.27-3.17(m,1H),2.62-2.44(m,2H),1.84-1.57(m,4H),0.91-0.83(m,3H)。
21.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-乙酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:93-95℃
1H NMR(300MHz,CDCl3)δ:8.08-7.80(m,4H),7.68-7.44(m,3H),7.40-7.16(m,2H),5.74-5.62(m,1H),5.29-5.16(m,1H),3.74-3.61(m,6H),3.42-3.33(m,1H),3.27-3.15(m,1H),2.33-2.16(m,3H)。
22.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-丙酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:87-89℃
1H NMR(300MHz,CDCl3)δ:8.07-7.79(m,4H),7.65-7.45(m,2H),7.36-7.14(m,3H),5.75-5.58(m,1H),5.28-5.12(m,1H),3.75-3.57(m,6H),3.49-3.35(m,1H),3.29-3.14(m,1H),2.68-2.46(m,2H),1.31-1.18(m,3H)。
23.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-甲氧甲醚萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:71-73℃
1H NMR(300MHz,CDCl3)δ:8.01-7.74(m,3H),7.63-7.33(m,4H),7.32-7.22(m,2H),5.75-5.60(m,1H),5.35-6.15(m,3H),3.80-3.63(m,6H),3.54-3.38(m,4H),3.30-3.18(m,1H)。
24.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-苯甲酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:109-111℃
1H NMR(300MHz,CDCl3)δ:8.33-7.85(m,6H),7.69-6.81(m,8H),5.78-5.57(m,1H),5.23-5.08(m,3H),3.73-3.49(m,6H),3.47-2.81(m,2H)。
25.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-吡喃甲酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:80-82℃
1H NMR(300MHz,CDCl3)δ:8.11-7.85(m,4H),7.75-7.37(m,6H),7.31-7.16(m,2H),5.78-5.61(m,1H),5.26-5.16(m,1H),3.75-3.57(m,6H),3.54-3.03(m,2H)。
26.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-甲氧甲酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:97-99℃
1H NMR(300MHz,CDCl3)δ:8.10-7.80(m,4H),7.66-7.47(m,3H),7.45-7.30(m,2H),5.75-5.64(m,1H),5.26-5.18(m,1H),3.79-3.64(m,6H),3.53-3.40(m,1H),3.37-3.24(m,1H)。
27.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基2-(4-氯-6-甲氧基嘧啶)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:61-63℃
1H NMR(300MHz,CDCl3)δ:7.42-7.24(m,3H),6.49(s,1H),5.83(d,J=9.9Hz,1H),3.91(s,3H),3.81(s,3H),3.71(s,3H),3.47(d,J=16.2Hz,1H),3.23(d,J=16.2Hz,1H).
28.(S)-7-氯-3,5-二氢-2-[[甲氧基羰基2-(4,6-二甲氧基嘧啶)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯
熔点:58-60℃
1H NMR(300MHz,CDCl3)δ:7.40-7.22(m,3H),5.83(d,J=10.2Hz,1H),5.77(s,1H),5.22(d,J=9.9Hz,1H),3.85(s,6H),3.80(s,3H),3.69(s,3H),3.46(d,J=16.5Hz,1H),3.21(d,J=16.2Hz,1H)。
29.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-甲氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:87-88℃
1H NMR(300MHz,CDCl3)δ:8.41-8.23(m,3H),8.09(d,J=9.6Hz,1H),7.66-7.39(m,5H),7.37-7.28(m,2H),5.68(t,J=11.1Hz,1H),5.24(t,J=11.4Hz,1H),3.99(s,3H),3.70(s,6H),3.47(d,J=16.5Hz,1H),3.25(d,J=16.5Hz,1H)。
30.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-乙氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:75-77℃
1H NMR(300MHz,CDCl3)δ:8.39-8.27(m,1H),8.08(d,J=7.8Hz,1H),7.64-7.27(m,6H),6.82-6.71(m,1H),5.68(t,J=10.5Hz,1H),5.24(t,J=10.5Hz,1H),4.27-4.13(m,2H),3.72-3.64(s,6H),3.47-3.36(m,1H),3.32-3.18(m,1H),1.58-1.46(m,3H)。
31.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-正丙氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:57-59℃
1H NMR(300MHz,CDCl3)δ:8.40-8.27(m,1H),8.07(d,J=8.1Hz,1H),7.63-7.21(m,6H),6.82-6.71(m,1H),5.67(t,J=12.3Hz,1H),5.23(t,J=9.9Hz,1H),4.16-4.00(m,2H),3.73-3.63(m,6H),3.36-3.26(m,1H),3.18-3.07(m,1H),1.18-0.96(m,5H)。
32.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-异氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:74-76℃
1H NMR(300MHz,CDCl3)δ:8.41-8.27(m,1H),8.09(d,J=7.8Hz,1H),7.65-7.41(m,4H),7.38-7.30(m,2H),6.80(d,J=7.8Hz,1H),5.72(t,J=10.2Hz,1H),5.27(t,J=10.2Hz,1H),4.83-4.68(m,1H),3.75-3.67(s,6H),3.49(d,J=16.2Hz,1H),3.28(d,J=16.2Hz,1H),1.46(s,6H)。
33.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-正丁氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:67-69℃
1H NMR(300MHz,CDCl3)δ:8.42-8.27(m,1H),8.09(d,J=8.7Hz,1H),7.65-7.26(m,6H),6.83-6.73(m,1H),5.69(t,J=10.2Hz,1H),5.25(t,J=10.2Hz,1H),4.22-4.08(m,2H),3.75-3.67(s,6H),3.49(d,J=16.2Hz,1H),3.27(d,J=16.2Hz,1H),2.00-1.84(m,2H),1.70-1.53(m,2H),1.09-0.98(m,3H)。
34.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-正戊氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:95-97℃
1H NMR(300MHz,CDCl3)δ:8.40-8.28(m,1H),8.09(d,J=7.5Hz,1H),7.66-7.29(m,6H),6.84-6.73(m,1H),5.70(t,J=9.0Hz,1H),5.25(t,J=9.0Hz,1H),4.14(t,J=6.0Hz,2H),3.71(s,6H),3.49(d,J=16.2Hz,1H),3.27(d,J=16.2Hz,1H),1.99-1.87(m,2H),1.62-1.36(m,4H),0.98(t,J=6.9Hz,3H)。
35.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-正己氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:91-93℃
1H NMR(300MHz,CDCl3)δ:8.42-8.28(m,1H),8.09(d,J=9.0Hz,1H),7.64-7.26(m,6H),6.85-6.73(m,1H),5.69(t,J=11.4Hz,1H),5.26(t,J=11.4Hz,1H),4.22-4.09(m,2H),3.75-3.67(s,6H),3.49(d,J=16.5Hz,1H),3.27(d,J=16.5Hz,1H),2.01-1.86(m,2H),1.66-1.49(m,2H),1.47-1.34(m,4H),0.99-0.81(m,3H)。
36.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-三氟甲氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
1H NMR(300MHz,CDCl3)δ:8.27-8.14(m,2H),7.71-7.28(m,7H),5.69(d,J=9.6Hz,1H),5.26(d,J=9.6Hz,1H)3.73(s,6H),3.50(d,J=16.5Hz,1H),3.28(d,J=16.5Hz,1H)。
37.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-三氟乙氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:65-67℃
1H NMR(300MHz,CDCl3)δ:8.41-8.26(m,1H),8.14(d,J=8.1Hz,1H),7.71-7.25(m,6H),6.79(d,J=8.1Hz,1H),5.80-5.64(m,1H),5.36-5.18(m,1H),5.54(q,J=7.8Hz,2H),5.51(d,J=16.2Hz,1H),5.29(d,J=16.2Hz,1H),3.73(s,6H)。
38.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-三氟甲基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:96-98℃
1H NMR(300MHz,CDCl3)δ:8.33-8.20(m,2H),7.90(d,J=7.8Hz,1H),7.76-7.63(m,2H),7.58(d,J=7.8Hz,1H),7.38-7.29(m,3H),5.71(d,J=8.4Hz,1H),5.28(d,J=8.4Hz,1H),3.74(s,6H),3.50(d,J=16.8Hz,1H),3.28(d,J=16.8Hz,1H)。
39.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(2-三氟甲氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
1H NMR(300MHz,CDCl3)δ:8.22(d,J=9.0Hz,1H),7.97-7.81(m,2H),7.73(s,1H),7.62-7.26(m,5H),5.71(d,J=9.0Hz,1H),5.27(d,J=9.0Hz,1H),3.75(s,6H),3.50(d,J=16.5Hz,1H),3.27(d,J=16.5Hz,1H).
熔点:91-93℃
40.(S)-7-氯-3,5一二氢-2-[甲氧基羰基2-[(3-三氟甲氧基苯基)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:91-93℃
1H NMR(300MHz,CDCl3)δ:8.13(s,1H),8.02-7.86(m,2H),7.67(d,J=7.5Hz,1H),7.56(d,J=7.5Hz,1H),7.53-7.30(m,5H),5.83-5.60(m,1H),5.40-5.15(m,5H),3.71(s,6H),3.52(d,J=16.2Hz,1H),3.39-3.20(m,1H).
41.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(2-溴-4-三氟氧基苯基)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:55-57℃
1H NMR(300MHz,CDCl3)δ:7.64-7.49(m,2H),7.43-7.29(m,3H),7.20(d,J=7.8Hz,1H),5.66(d,J=9.0Hz,1H),5.24(d,J=9.9Hz,1H),3.76(s,3H),3.73(s,3H),3.49(d,J=16.8Hz,1H),3.26(d,J=16.5Hz,1H)。
42.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-硝基苯基)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:69-71℃
1H NMR(300MHz,CDCl3)δ:8.25(d,J=7.8Hz,2H),7.53-7.45(m,3H),7.38-7.31(m,2H),5.77(d,J=10.2Hz,1H),5.25(d,J=9.6Hz,1H),3.78(s,3H),3.74(s,3H),3.52(d,J=15.9Hz,1H),3.28(d,J=16.5Hz,1H)。
43.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-乙酰氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:49-51℃
1H NMR(300MHz,CDCl3)δ:8.10-7.98(m,2H),7.46(d,J=8.4Hz,1H),7.40-7.24(m,4H),5.70(d,J=9.6Hz,1H),5.21(d,J=9.9Hz,1H),4.40-4.26(m,2H),3.79-3.60(m,6H),3.45(d,J=15.9Hz,1H),3.21(d,J=16.2Hz,1H),1.41-1.27(m,3H)。
44.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-甲氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:49-51℃
1H NMR(300MHz,CDCl3)δ:7.98(d,J=8.4Hz,2H),7.50(d,J=8.1Hz,1H),7.42(d,J=8.7Hz,2H),7.33(d,J=8.1Hz,2H),5.75(d,J=10.2Hz,1H),5.24(d,J=9.9Hz,1H),3.76(s,3H),3.73(s,3H),3.50(d,J=16.2Hz,1H),3.27(d,J=16.2Hz,1H)。
45.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-乙酰基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:64-66℃
1H NMR(300MHz,CDCl3)δ:8.03(d,J=8.7Hz,2H),7.48(d,J=8.1Hz,1H),7.43-7.28(m,4H),5.73(d,J=9.9Hz,1H),5.22(d,J=9.9Hz,1H),3.90(s,3H),3.75(s,3H),3.70(s,3H),3.58(d,J=16.8Hz,1H),3.25(d,J=16.8Hz,1H)。
46.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-甲酰氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:69-71℃
1H NMR(300MHz,CDCl3)δ:8.03(d,J=8.7Hz,2H),7.48(d,J=8.1Hz,1H),7.43-7.28(m,4H),5.73(d,J=9.9Hz,1H),5.22(d,J=9.9Hz,1H),3.90(s,3H),3.75(s,3H),3.70(s,3H),3.58(d,J=16.8Hz,1H),3.25(d,J=16.8Hz,1H)。
47.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-三氟甲硫基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:36-38℃
1H NMR(300MHz,CDCl3)δ:7.65(d,J=8.4Hz,2H),7.48(d,J=8.4Hz,1H),7.42-7.29(m,4H),5.74(d,J=9.6Hz,1H),5.22(d,J=9.6Hz,1H),3.74(s,3H),3.71(s,3H),3.49(d,J=16.5Hz,1H),3.26(d,J=16.5Hz,1H)。
48.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(2,4-二甲氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
熔点:78-80℃
1H NMR(300MHz,CDCl3)δ:7.50(d,J=8.1Hz,1H),7.34-7.25(m,2H),6.92-6.76(m,3H),5.68(d,J=9.6Hz,1H),5.21(d,J=9.6Hz,1H),3.78(s,3H),3.73(s,3H),3.71(s,6H),3.47(d,J=16.5Hz,1H),3.25(d,J=16.5Hz,1H)。
49.(S)-(+)-7-氯-3,5-二氢-2-[甲氧基羰基(2,4-二硝基苯基)氨基羰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羟酸甲酯
熔点:77-79℃
1H NMR(300MHz,CDCl3)δ:8.47(d,J=8.1Hz,1H),7.61(d,J=8.4Hz,1H),7.44(d,J=8.17Hz,1H),7.39-7.30(m,3H),5.66(d,J=9.6Hz,1H),5.25(d,J=9.6Hz,1H),3.78(s,3H),3.74(s,3H),3.49(d,J=16.5Hz,1H),3.26(d,J=17.1Hz,1H)。
50.(S)-7-氯-3,5-二氢-2-[甲氧基羰基(2-三氟甲氧基-5-氯-苯基)氨基羰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羟酸甲酯
熔点:46-48℃
1H NMR(300MHz,CDCl3)δ:7.50(d,J=8.7Hz,1H),7.44(d,J=2.1Hz,1H),7.41-7.31(m,3H),7.30-7-26(m,1H),5.69(d,J=9.6Hz,1H),5.24(d,J=9.3Hz,1H),3.74(s,63H),3.51(d,J=16.8Hz,1H),3.29(d,J=16.8Hz,1H)。
熔点:47-49℃。
51.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4一乙氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯
1H NMR(300MHz,CDCl3)δ:7.56(d,J=8.4Hz,1H),7.35-7.24(m,4H),6.89(s,1H),6.86(s,1H),5.69(d,J=9.6Hz,1H),5.19(d,J=10.2Hz,1H),4.04-3.97(m,2H),3.71(s,6H),3.48(d,J=16.2Hz,1H),3.26(d,J=16.5Hz,1H),1.41-1.37(m,3H)
熔点:137-139℃。
52.(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4一甲基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a羧酸甲酯的合成
熔点:151-153℃
1H NMR(300MHz,CDCl3)δ:7.56(d,J=8.1Hz,1H),7.35(d,J=8.1Hz,1H),7.32(s,1H),7.28-7.17(m,4H),5.71(d,J=9.9Hz,1H),5.22(d,J=9.9Hz,1H),3.72(s,3H),3.72(s,3H),3.50(d,J=16.8Hz,1H),3.27(d,J=16.5Hz,1H),2.35(s,3H)。
杀虫剂室内生物活性测定
1材料与方法
1.1试验药剂
SIOC-Y-(001-052)原药;
98%敌敌畏原油(张家港市新宇化工厂)、95%哒螨灵原药(安徽金泰农药化工有限公司)、95%丁硫克百威原药(浙江禾田化工有限公司)。86.5%茚虫威原药(浙江禾田化工有限公司)
1.2试验靶标
稻褐飞虱(Nilaparvata legen)、粘虫(Mythima separata)、小菜蛾(Plutellaxylostella)、红蜘蛛(Tetranychus cinnabarnus)、苜蓿蚜(Aphis medicagini)。以上虫种为室内常年累代饲养或从田间采集室内饲养多代虫种。
1.3仪器设备
电子分析天平、培养皿、移液枪、Potter喷雾塔、毛笔等。
1.4药剂处理
用分析天平(0.0001g)称取一定质量的原药,加含有0.1%吐温-80的溶剂(DMF),配制成1%制剂。称取一定质量的制剂,加蒸馏水(点滴法稀释用丙酮)稀释配制成测定所需浓度的药液。
根据创制农药生物活性评价SOP(杀虫剂卷),普筛浓度选择500mg/L。
1.5试验方法
1.5.1水稻褐飞虱筛选——培养皿苗虫定量喷雾法
将水稻苗用白石英沙固定于培养皿内,接用CO2麻醉3龄中期若虫,置于POTTER喷雾塔下喷雾。喷雾后用透明塑料杯罩住,标记后放于观察室内。72h后检查结果。以敌敌畏和空白CK为对照。
1.5.2粘虫筛选——浸苗饲喂法
将玉米叶在药液中充分浸润后自然阴干,放入培养皿中,接3龄中期幼虫,加盖标记后置于观察室内。72h后检查结果。以敌敌畏和空白CK为对照。
1.5.3小菜蛾筛选——浸渍法
将甘蓝片剪下,打孔成圆片,然后浸于药液中20s,放于Φ9cm塑料培养皿内(5片/皿),接小菜蛾2龄幼虫15头/皿,放一张滤纸,加盖。置于26℃室内培养,72h后检查结果。试验重复4次。以尖头镊子轻触虫体,无反应视为死虫。以敌敌畏和空白CK为对照。
1.5.4朱砂叶螨筛选——浸渍法
将蚕豆叶片打成叶碟,背面朝上放在小块棉花上,置于塑料培养皿内,加少量水,接朱砂叶螨成螨。待成螨于叶片上稳定后,将叶片在药液中充分浸润5s后迅速用吸水纸吸去叶片表面水滴,重新置于棉花上,风干。72h后检查结果。以哒螨灵和空白CK为对照。
1.5.5苜蓿蚜筛选——浸渍法
将蚕豆叶片剪去两端,背面朝上放在小块棉花上,置于塑料培养皿内,加少量水,接苜蓿蚜成蚜以产若蚜。24h后去除成蚜,继续培养2d后将叶片在药液中充分浸润5s后,重新置于棉花上,自然凉干。24h后检查结果。以丁硫克百威和空白CK为对照。
1.6试验统计和进筛标准
统计各个处理的死虫数和活虫数,计算死亡率(Abbott’s公式)。
2结果与分析
化合物SIOC-Y-(001-052)对水稻褐飞虱、粘虫(小菜蛾)、红蜘蛛、苜蓿蚜等4个虫靶标的室内生物活性筛选结果表明:化合物SIOC-Y-(001-040)在普筛浓度500mg/L时,SIOC-Y-006、014、038、039、041、042、043、046、047、049对粘虫显示出100%的杀虫活性,036对粘虫显示出80%的杀虫活性。进一步筛选时,0047对粘虫仍表现出较好的杀虫活性,在浓度100mg/L和20mg/L时杀虫活性仍为100%。因此,建议SIOC-Y-006、014、036、038、039、041、042、043、046、047、049进行下一步筛选验证;其它化合物对水稻褐飞虱、粘虫、朱砂叶螨、苜蓿蚜等4个虫靶标均无明显活性。(筛选试验结果见附表1)
附表1:化合物杀虫活性筛选结果(死亡率,%)
Claims (10)
2.根据权利要求1所述的一种N-取代噁二嗪类化合物,其特征在于,所述的化合物包括:
(1)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(吡啶)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(2)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(3)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[2-(4-甲基吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(4)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[2-(6-甲基吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(5)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[2-(5-溴吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(6)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[2-(5-碘吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(8)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[4-(2-氯吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(9)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[3-(2-氯吡啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(10)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-甲氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(11)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-乙氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(12)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-正丙氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(13)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-正戊氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(14)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-正己氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(15)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-异丙氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(16)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-正丁氧基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(17)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基[2-(4,6-二甲氧基嘧啶)]氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(18)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-特丁酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(19)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-差劲基萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(20)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-丁酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(21)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-乙酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(22)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-丙酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(23)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-甲氧甲醚萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(24)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-苯甲酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(25)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-吡喃甲酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(26)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基(2-甲氧甲酸酯萘)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(27)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基2-(4-氯-6-甲氧基嘧啶)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(28)(S)-7-氯-3,5-二氢-2-[[甲氧基羰基2-(4,6-二甲氧基嘧啶)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羧酸甲酯,
(29)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-甲氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(30)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-乙氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(31)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-正丙氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(32)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-异氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(33)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-正丁氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(34)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-正戊氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(35)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-正己氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(36)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-三氟甲氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(37)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-三氟乙氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(38)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-三氟甲基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(39)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(2-三氟甲氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(40)(S)-7-氯-3,5一二氢-2-[甲氧基羰基2-[(3-三氟甲氧基苯基)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(41)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(2-溴-4-三氟氧基苯基)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(42)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-硝基苯基)氨基]甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(43)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-乙酰氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(44)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-甲氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(45)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-乙酰基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(46)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-甲酰氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(47)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4-三氟甲硫基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(48)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(2,4-二甲氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(49)(S)-(+)-7-氯-3,5-二氢-2-[甲氧基羰基(2,4-二硝基苯基)氨基羰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羟酸甲酯,
(50)(S)-7-氯-3,5-二氢-2-[甲氧基羰基(2-三氟甲氧基-5-氯-苯基)氨基羰基]茚并[1,2-e][1,3,4-]噁二嗪-4a(3H)-羟酸甲酯,
(51)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4一乙氧基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a-羧酸甲酯,
(52)(S)-7-氯-3,5一二氢-2-[甲氧基羰基(4一甲基苯基)氨基甲酰基]茚并[1,2-e][1,3,4-]噁二嗪-4a羧酸甲酯的合成。
3.一种权利要求1或2所述的一种N-取代噁二嗪类化合物的制备方法,其特征在于,该方法包括以下步骤:
(1)以2-(苄基)-7-氯茚并[1,2-e][1,3,4]]噁二嗪-2,4a(3H,5H)-二羧酸-4a-甲基酯和氢气为原料,在溶剂a中以Pd/C为催化剂a,进行加氢反应,反应温度为20℃~120℃,反应压力为1-15个大气压,反应完毕后得到中间体B;
(2)以(氯羰基)(芳基)氨基甲酸甲酯为中间体C,与步骤(1)得到的中间体B,在溶剂b中,在催化剂b的作用下,进行缩合反应,反应温度为0℃~100℃得到产物N取代噁二嗪类化合物。
4.根据权利要求3所述的一种N-取代噁二嗪类化合物的制备方法,其特征在于,步骤(1)所述的溶剂a为乙酸甲酯、乙酸乙酯、甲醇、乙醇、乙腈、二氯甲烷、二氯乙烷、三氯甲烷、甲苯中的一种或两种以上的混合剂。
5.根据权利要求3所述的一种N-取代噁二嗪类化合物的制备方法,其特征在于,步骤(1)所述的反应温度为45℃~85℃,反应压力为4-6个大气压。
6.根据权利要求3所述的一种N-取代噁二嗪类化合物的制备方法,其特征在于,步骤(2)所述的溶剂b为乙酸甲酯、乙酯乙酸乙酯、甲醇、乙醇、乙腈、二氯甲烷、二氯乙烷、三氯甲烷、甲苯中的一种或两种以上的混合剂。
7.根据权利要求3所述的一种N-取代噁二嗪类化合物的制备方法,其特征在于,步骤(2)所述的催化剂b为N,N-二异丙基乙胺、三乙胺、吡啶或哌啶。
8.根据权利要求3所述的一种N-取代噁二嗪类化合物的制备方法,其特征在于,步骤(2)所述的反应温度为20℃~60℃。
9.根据权利要求3所述的一种N-取代噁二嗪类化合物的制备方法,其特征在于,步骤(2)所得N取代噁二嗪类化合物采用重结晶方法进行分离纯化,或采用柱色谱分离的方法进行分离纯化,所述的重结晶方法采用的溶剂为甲醇、乙醇、异丙醇、乙酸甲酯、乙酸乙酯、乙腈、二氯甲烷、二氯乙烷、三氯甲烷、甲苯、石油醚中的一种或两种以上的混合剂。
10.一种权利要求1或2所述的一种N-取代噁二嗪类化合物的应用,其特征在于,该化合物用于制备杀虫剂,对粘虫的杀虫活性达到80%~100%。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2011103129260A CN102391261A (zh) | 2011-10-14 | 2011-10-14 | 一种n-取代噁二嗪类化合物及其制备方法和应用 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2011103129260A CN102391261A (zh) | 2011-10-14 | 2011-10-14 | 一种n-取代噁二嗪类化合物及其制备方法和应用 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN102391261A true CN102391261A (zh) | 2012-03-28 |
Family
ID=45858665
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN2011103129260A Pending CN102391261A (zh) | 2011-10-14 | 2011-10-14 | 一种n-取代噁二嗪类化合物及其制备方法和应用 |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN102391261A (zh) |
Cited By (251)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104151260A (zh) * | 2014-08-26 | 2014-11-19 | 常州大学 | 一种新型噁二嗪类杀虫剂sioc-y-047的制备方法 |
| CN105330661A (zh) * | 2015-10-30 | 2016-02-17 | 青岛科技大学 | 一种n-芳甲基取代的噁二嗪硝基亚胺衍生物及其用途 |
| WO2017072039A1 (de) | 2015-10-26 | 2017-05-04 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2017093214A1 (de) | 2015-12-03 | 2017-06-08 | Bayer Cropscience Aktiengesellschaft | Mesolonische halogenierte 3-(acetyl)-1-[(1,3-thiazol-5-yl)methyl]-1h-imidazo[1,2-a]pyridin-4-ium-2-olat derivate und verwandte verbindungen als insektizide |
| WO2017093180A1 (de) | 2015-12-01 | 2017-06-08 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2017137338A1 (de) | 2016-02-11 | 2017-08-17 | Bayer Cropscience Aktiengesellschaft | Substituierte 2-(het)aryl-imidazolyl-carboxyamide als schädlingsbekämpfungsmittel |
| WO2017137339A1 (de) | 2016-02-11 | 2017-08-17 | Bayer Cropscience Aktiengesellschaft | Substituierte 2-oxyimidazolyl-carboxamide als schädlingsbekämpfungsmittel |
| EP3210468A1 (de) | 2016-02-26 | 2017-08-30 | Bayer CropScience Aktiengesellschaft | Lösungsmittelfreie formulierungen von niedrig schmelzenden wirkstoffen |
| WO2017144341A1 (de) | 2016-02-23 | 2017-08-31 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2017157735A1 (de) | 2016-03-15 | 2017-09-21 | Bayer Cropscience Aktiengesellschaft | Substituierte sulfonylamide zur bekämpfung tierischer schädlinge |
| WO2017157885A1 (de) | 2016-03-16 | 2017-09-21 | Bayer Cropscience Aktiengesellschaft | N-(cyanbenzyl)-6-(cyclopropylcarbonylamino)-4-(phenyl)-pyridin-2-carboxamid-derivate und verwandte verbindungen als pestizide pflanzenschutzmittel |
| WO2017174414A1 (de) | 2016-04-05 | 2017-10-12 | Bayer Cropscience Aktiengesellschaft | Naphthalin-derivate als schädlingsbekämpfungsmittel |
| WO2017178416A1 (en) | 2016-04-15 | 2017-10-19 | Bayer Animal Health Gmbh | Pyrazolopyrimidine derivatives |
| WO2017186536A1 (de) | 2016-04-25 | 2017-11-02 | Bayer Cropscience Aktiengesellschaft | Substituierte 2-alkylimidazolyl-carboxamide als schädlingsbekämpfungsmittel |
| EP3241830A1 (de) | 2016-05-04 | 2017-11-08 | Bayer CropScience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| EP3245865A1 (en) | 2016-05-17 | 2017-11-22 | Bayer CropScience Aktiengesellschaft | Method for increasing yield in brassicaceae |
| WO2017198450A1 (en) | 2016-05-15 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in maize |
| WO2017198451A1 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in small grain cereals such as wheat and rice |
| WO2017198449A1 (en) | 2016-05-15 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in brassicaceae |
| WO2017198452A1 (en) | 2016-05-16 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in soybean |
| WO2017198454A1 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in cotton |
| WO2017198455A2 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in beta spp. plants |
| WO2017198453A1 (en) | 2016-05-16 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in potato, tomato or alfalfa |
| WO2018015289A1 (de) | 2016-07-19 | 2018-01-25 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018019937A1 (en) | 2016-07-29 | 2018-02-01 | Bayer Cropscience Aktiengesellschaft | Formulation comprising a beneficial p. bilaii strain and talc for use in seed treatment |
| WO2018029102A1 (de) | 2016-08-10 | 2018-02-15 | Bayer Cropscience Aktiengesellschaft | Substituierte 2-heterocyclyl-imidazolyl-carboxamide als schädlingsbekämpfungsmittel |
| EP3284739A1 (de) | 2017-07-19 | 2018-02-21 | Bayer CropScience Aktiengesellschaft | Substituierte (het)arylverbindungen als schädlingsbekämpfungsmittel |
| WO2018033455A1 (de) | 2016-08-15 | 2018-02-22 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018050825A1 (de) | 2016-09-19 | 2018-03-22 | Bayer Cropscience Aktiengesellschaft | Pyrazolo[1,5-a]pyridin- derivative und ihre verwendung als schädlingsbekämpfungsmittel |
| EP3305786A2 (de) | 2018-01-22 | 2018-04-11 | Bayer CropScience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018065288A1 (de) | 2016-10-07 | 2018-04-12 | Bayer Cropscience Aktiengesellschaft | 2-[2-phenyl-1-(sulfonylmethyl)vinyl]-imidazo[4,5-b]pyridin-derivate und verwandte verbindungen als schädlingsbekämpfungsmittel im pflanzenschutz |
| WO2018065292A1 (de) | 2016-10-06 | 2018-04-12 | Bayer Cropscience Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte bicyclische heterocyclen-derivate als schädlings-bekämpfungsmittel |
| WO2018083288A1 (de) | 2016-11-07 | 2018-05-11 | Bayer Aktiengesellschaft | Substituierte sulfonylamide zur bekämpfung tierischer schädlinge |
| WO2018087036A1 (en) | 2016-11-11 | 2018-05-17 | Bayer Animal Health Gmbh | New anthelmintic quinoline-3-carboxamide derivatives |
| WO2018095953A1 (de) | 2016-11-23 | 2018-05-31 | Bayer Cropscience Aktiengesellschaft | 2-[3-(alkylsulfonyl)-2h-indazol-2-yl]-3h-imidazo[4,5-b]pyridin-derivate und ähnliche verbindungen als schädlingsbekämpfungsmittel |
| WO2018104500A1 (en) | 2016-12-09 | 2018-06-14 | Bayer Cropscience Aktiengesellschaft | Plant health effect of purpureocillium lilacinum |
| WO2018108791A1 (en) | 2016-12-16 | 2018-06-21 | Bayer Cropscience Aktiengesellschaft | Thiadiazole derivatives as pesticides |
| WO2018108730A1 (de) | 2016-12-16 | 2018-06-21 | Bayer Aktiengesellschaft | Mesoionische imidazopyridine als insektizide |
| CN108250156A (zh) * | 2018-01-29 | 2018-07-06 | 华南农业大学 | 一种肉桂酸噁二嗪衍生物及其制备方法和应用 |
| WO2018130437A1 (de) | 2017-01-10 | 2018-07-19 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018130443A1 (de) | 2017-01-10 | 2018-07-19 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018138050A1 (de) | 2017-01-26 | 2018-08-02 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018141954A1 (de) | 2017-02-06 | 2018-08-09 | Bayer Aktiengesellschaft | Aryl- oder heteroaryl-substituierte imidazopyridinderivate und deren anwendung als schädlingsbekämpfungsmittel |
| EP3369320A1 (de) | 2017-03-02 | 2018-09-05 | Bayer CropScience Aktiengesellschaft | Wirkstoff zur bekämpfung von wanzen |
| WO2018189077A1 (de) | 2017-04-12 | 2018-10-18 | Bayer Aktiengesellschaft | Mesoionische imidazopyridine als insektizide |
| WO2018192872A1 (de) | 2017-04-21 | 2018-10-25 | Bayer Aktiengesellschaft | Mesoionische imidazopyridine als insektizide |
| WO2018197692A1 (en) | 2017-04-27 | 2018-11-01 | Bayer Aktiengesellschaft | Heteroarylphenylaminoquinolines and analogues |
| WO2018197401A1 (en) | 2017-04-27 | 2018-11-01 | Bayer Animal Health Gmbh | New bicyclic pyrazole derivatives |
| WO2018197257A1 (de) | 2017-04-24 | 2018-11-01 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018202525A1 (en) | 2017-05-04 | 2018-11-08 | Bayer Cropscience Aktiengesellschaft | Phenoxyethanamine derivatives for controlling pests |
| WO2018202712A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylmethylphenoxyquinolines and analogues |
| WO2018202524A1 (de) | 2017-05-04 | 2018-11-08 | Bayer Cropscience Aktiengesellschaft | 2-{[2-(phenyloxymethyl)pyridin-5-yl]oxy}-ethanamin-derivate und verwandte verbindungen als schädlingsbekämpfungsmittel z.b. für den pflanzenschutz |
| WO2018202494A1 (de) | 2017-05-02 | 2018-11-08 | Bayer Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018202706A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylheteroaryloxyquinolines and analogues |
| WO2018202715A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylbenzylbenzimidazoles and analogues |
| WO2018202501A1 (de) | 2017-05-02 | 2018-11-08 | Bayer Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019002132A1 (en) | 2017-06-30 | 2019-01-03 | Bayer Animal Health Gmbh | NEW AZAQUINOLINE DERIVATIVES |
| WO2019007887A1 (de) | 2017-07-06 | 2019-01-10 | Bayer Aktiengesellschaft | Insektizide und fungizide wirkstoffkombinationen |
| WO2019025341A1 (en) | 2017-08-04 | 2019-02-07 | Bayer Animal Health Gmbh | QUINOLINE DERIVATIVES FOR THE TREATMENT OF INFECTIONS BY HELMINTHES |
| WO2019035881A1 (en) | 2017-08-17 | 2019-02-21 | Bayer Cropscience Lp | DISPERSIBLE COMPOSITIONS IN LIQUID FERTILIZER AND ASSOCIATED METHODS |
| WO2019038195A1 (de) | 2017-08-22 | 2019-02-28 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019059412A1 (en) | 2017-09-20 | 2019-03-28 | Mitsui Chemicals Agro, Inc. | AGENT FOR EXTENDED CONTROL OF ECTOPARASITES FOR ANIMAL |
| WO2019068572A1 (de) | 2017-10-04 | 2019-04-11 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| EP3473100A1 (en) | 2017-10-18 | 2019-04-24 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3473103A1 (de) | 2017-10-17 | 2019-04-24 | Bayer AG | Wässrige suspensionskonzentrate auf basis von 2-[(2,4-dichlorphenyl)-methyl]-4,4'-dimethyl-3-isoxazolidinon |
| WO2019076749A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS HAVING INSECTICIDAL / ACARICIDE PROPERTIES |
| WO2019076751A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS HAVING INSECTICIDAL / ACARICIDE PROPERTIES |
| WO2019076752A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS AYNT INSECTICIDE / ACARICIDE PROPERTIES |
| WO2019076750A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS HAVING INSECTICIDE PROPERTIES7ACARICIDES |
| WO2019076754A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS HAVING INSECTICIDAL / ACARICIDE PROPERTIES |
| WO2019092086A1 (en) | 2017-11-13 | 2019-05-16 | Bayer Aktiengesellschaft | Tetrazolylpropyl derivatives and their use as fungicides |
| WO2019105875A1 (en) | 2017-11-28 | 2019-06-06 | Bayer Aktiengesellschaft | Heterocyclic compounds as pesticides |
| WO2019105871A1 (de) | 2017-11-29 | 2019-06-06 | Bayer Aktiengesellschaft | Stickstoffhaltige heterocyclen als schädlingsbekämpfungsmittel |
| WO2019122319A1 (en) | 2017-12-21 | 2019-06-27 | Bayer Aktiengesellschaft | Trisubstitutedsilylmethylheteroaryloxyquinolines and analogues |
| WO2019155066A1 (en) | 2018-02-12 | 2019-08-15 | Bayer Aktiengesellschaft | Fungicidal oxadiazoles |
| WO2019162174A1 (de) | 2018-02-21 | 2019-08-29 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019162228A1 (en) | 2018-02-21 | 2019-08-29 | Bayer Aktiengesellschaft | 1-(5-substituted imidazol-1-yl)but-3-en derivatives and their use as fungicides |
| WO2019170626A1 (en) | 2018-03-08 | 2019-09-12 | Bayer Aktiengesellschaft | Use of heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides in plant protection |
| WO2019175046A1 (de) | 2018-03-12 | 2019-09-19 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| EP3545764A1 (en) | 2019-02-12 | 2019-10-02 | Bayer AG | Crystal form of 2-({2-fluoro-4-methyl-5-[(r)-(2,2,2-trifluoroethyl)sulfinyl]phenyl}imino)-3-(2,2,2- trifluoroethyl)-1,3-thiazolidin-4-one |
| WO2019197468A1 (en) | 2018-04-12 | 2019-10-17 | Bayer Aktiengesellschaft | N-(cyclopropylmethyl)-5-(methylsulfonyl)-n-{1-[1-(pyrimidin-2-yl)-1h-1,2,4-triazol-5-yl]ethyl}benzamide derivatives and the corresponding pyridine-carboxamide derivatives as pesticides |
| WO2019197371A1 (en) | 2018-04-10 | 2019-10-17 | Bayer Aktiengesellschaft | Oxadiazoline derivatives |
| WO2019197623A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit insektiziden, nematiziden und akariziden eigenschaften |
| WO2019197615A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit fungiziden, insektiziden und akariziden eigenschaften |
| WO2019201835A1 (en) | 2018-04-17 | 2019-10-24 | Bayer Aktiengesellschaft | Heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| WO2019202077A1 (en) | 2018-04-20 | 2019-10-24 | Bayer Aktiengesellschaft | Heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| WO2019201921A1 (de) | 2018-04-20 | 2019-10-24 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019206799A1 (en) | 2018-04-25 | 2019-10-31 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| EP3564225A1 (en) | 2019-03-21 | 2019-11-06 | Bayer Aktiengesellschaft | Crystalline form of spiromesifen |
| WO2019215182A1 (en) | 2018-05-09 | 2019-11-14 | Bayer Animal Health Gmbh | New quinoline derivatives |
| WO2019224143A1 (de) | 2018-05-24 | 2019-11-28 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit insektiziden, nematiziden und akariziden eigenschaften |
| EP3586630A1 (en) | 2018-06-28 | 2020-01-01 | Bayer AG | Active compound combinations having insecticidal/acaricidal properties |
| WO2020005678A1 (en) | 2018-06-25 | 2020-01-02 | Bayer Cropscience Lp | Seed treatment method |
| WO2020002189A1 (de) | 2018-06-27 | 2020-01-02 | Bayer Aktiengesellschaft | Wirkstoffkombinationen |
| WO2020007904A1 (en) | 2018-07-05 | 2020-01-09 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| WO2020020813A1 (en) | 2018-07-25 | 2020-01-30 | Bayer Aktiengesellschaft | Fungicidal active compound combinations |
| WO2020020816A1 (en) | 2018-07-26 | 2020-01-30 | Bayer Aktiengesellschaft | Novel triazole derivatives |
| WO2020021082A1 (en) | 2018-07-27 | 2020-01-30 | Bayer Aktiengesellschaft | Controlled release formulations for agrochemicals |
| WO2020025650A1 (en) | 2018-07-31 | 2020-02-06 | Bayer Aktiengesellschaft | Controlled release formulations with lignin for agrochemicals |
| EP3608311A1 (en) | 2019-06-28 | 2020-02-12 | Bayer AG | Crystalline form a of n-[4-chloro-3-[(1-cyanocyclopropyl)carbamoyl]phenyl]-2-methyl-4-methylsulfonyl-5-(1,1,2,2,2-pentafluoroethyl)pyrazole-3-carboxamide |
| WO2020043650A1 (en) | 2018-08-29 | 2020-03-05 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3620052A1 (en) | 2018-12-12 | 2020-03-11 | Bayer Aktiengesellschaft | Use of phenoxypyridinyl-substituted (1h-1,2,4-triazol-1-yl)alcohols for controlling fungicidal diseases in maize |
| WO2020053282A1 (de) | 2018-09-13 | 2020-03-19 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2020057939A1 (en) | 2018-09-17 | 2020-03-26 | Bayer Aktiengesellschaft | Use of the fungicide isoflucypram for controlling claviceps purpurea and reducing sclerotia in cereals |
| WO2020070050A1 (en) | 2018-10-01 | 2020-04-09 | Bayer Aktiengesellschaft | Fungicidal 5-substituted imidazol-1-yl carbinol derivatives |
| EP3636644A1 (de) | 2018-10-11 | 2020-04-15 | Bayer Aktiengesellschaft | Mesoionische imidazopyridine als insektizide |
| WO2020079173A1 (en) | 2018-10-18 | 2020-04-23 | Bayer Aktiengesellschaft | Pyridylphenylaminoquinolines and analogues |
| WO2020078839A1 (de) | 2018-10-16 | 2020-04-23 | Bayer Aktiengesellschaft | Wirkstoffkombinationen |
| WO2020079232A1 (en) | 2018-10-20 | 2020-04-23 | Bayer Aktiengesellschaft | Oxetanylphenoxyquinolines and analogues |
| WO2020079167A1 (en) | 2018-10-18 | 2020-04-23 | Bayer Aktiengesellschaft | Heteroarylaminoquinolines and analogues |
| EP3643711A1 (en) | 2018-10-24 | 2020-04-29 | Bayer Animal Health GmbH | New anthelmintic compounds |
| WO2020109391A1 (en) | 2018-11-28 | 2020-06-04 | Bayer Aktiengesellschaft | Pyridazine (thio)amides as fungicidal compounds |
| WO2020114934A1 (de) | 2018-12-07 | 2020-06-11 | Bayer Aktiengesellschaft | Herbizide zusammensetzungen |
| WO2020114932A1 (de) | 2018-12-07 | 2020-06-11 | Bayer Aktiengesellschaft | Herbizide zusammensetzungen |
| EP3669652A1 (en) | 2018-12-21 | 2020-06-24 | Bayer AG | Active compound combination |
| WO2020126980A1 (en) | 2018-12-18 | 2020-06-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2020127780A1 (en) | 2018-12-20 | 2020-06-25 | Bayer Aktiengesellschaft | Heterocyclyl pyridazine as fungicidal compounds |
| WO2020127974A1 (en) | 2018-12-21 | 2020-06-25 | Bayer Aktiengesellschaft | 1,3,4-oxadiazoles and their derivatives as new antifungal agents |
| EP3679792A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679789A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679791A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679793A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679790A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3701796A1 (en) | 2019-08-08 | 2020-09-02 | Bayer AG | Active compound combinations |
| WO2020173861A1 (de) | 2019-02-26 | 2020-09-03 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2020173860A1 (en) | 2019-02-26 | 2020-09-03 | Bayer Aktiengesellschaft | Fused bicyclic heterocycle derivatives as pesticides |
| WO2020178307A1 (en) | 2019-03-05 | 2020-09-10 | Bayer Aktiengesellschaft | Active compound combination |
| WO2020178067A1 (en) | 2019-03-01 | 2020-09-10 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3708565A1 (en) | 2020-03-04 | 2020-09-16 | Bayer AG | Pyrimidinyloxyphenylamidines and the use thereof as fungicides |
| WO2020182929A1 (en) | 2019-03-13 | 2020-09-17 | Bayer Aktiengesellschaft | Substituted ureas and derivatives as new antifungal agents |
| WO2020187656A1 (en) | 2019-03-15 | 2020-09-24 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3725788A1 (en) | 2019-04-15 | 2020-10-21 | Bayer AG | Novel heteroaryl-substituted aminoalkyl azole compounds as pesticides |
| WO2020225438A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High uptake and rainfastness ulv formulations |
| WO2020225242A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | Active compound combination |
| WO2020229398A1 (de) | 2019-05-14 | 2020-11-19 | Bayer Aktiengesellschaft | (1-alkenyl)-substituierte pyrazole und triazole als schädlingsbekämpfungsmittel |
| WO2020231751A1 (en) | 2019-05-10 | 2020-11-19 | Bayer Cropscience Lp | Active compound combinations |
| EP3750888A1 (en) | 2019-06-12 | 2020-12-16 | Bayer Aktiengesellschaft | Crystalline form a of 1,4-dimethyl-2-[2-(pyridin-3-yl)-2h-indazol-5-yl]-1,2,4-triazolidine-3,5-dione |
| WO2020254490A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Phenoxyphenyl hydroxyisoxazolines and analogues as new antifungal agents |
| WO2020254494A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Fungicidal oxadiazoles |
| WO2020254486A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254489A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Benzylphenyl hydroxyisoxazolines and analogues as new antifungal agents |
| WO2020254493A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Thienylhydroxyisoxazolines and derivatives thereof |
| WO2020254488A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and use thereof as fungicides |
| WO2020254487A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254492A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020263812A1 (en) | 2019-06-24 | 2020-12-30 | Auburn University | A bacillus strain and methods of its use for plant growth promotion |
| WO2021001273A1 (de) | 2019-07-04 | 2021-01-07 | Bayer Aktiengesellschaft | Herbizide zusammensetzungen |
| WO2021001331A1 (en) | 2019-07-03 | 2021-01-07 | Bayer Aktiengesellschaft | Substituted thiophene carboxamides and derivatives thereof as microbicides |
| WO2021013719A1 (en) | 2019-07-23 | 2021-01-28 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021013720A1 (en) | 2019-07-23 | 2021-01-28 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021013721A1 (de) | 2019-07-22 | 2021-01-28 | Bayer Aktiengesellschaft | 5-amino substituierte pyrazole und triazole als schädlingsbekämpfungsmittel |
| EP3771714A1 (de) | 2019-07-30 | 2021-02-03 | Bayer AG | Stickstoffhaltige heterocyclen als schädlingsbekämpfungsmittel |
| WO2021018839A1 (en) | 2019-07-30 | 2021-02-04 | Bayer Animal Health Gmbh | Isoquinoline derivatives and their use for the treatment of parasitic infections |
| WO2021048188A1 (de) | 2019-09-11 | 2021-03-18 | Bayer Aktiengesellschaft | Hochwirksame formulierungen auf basis von 2-[(2;4-dichlorphenyl)-m ethyl|-4,4'-dimethyl- 3-isoxazolidinone sowie vorauflaufherbiziden |
| WO2021058659A1 (en) | 2019-09-26 | 2021-04-01 | Bayer Aktiengesellschaft | Rnai-mediated pest control |
| WO2021069569A1 (en) | 2019-10-09 | 2021-04-15 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021069575A1 (en) | 2019-10-11 | 2021-04-15 | Bayer Animal Health Gmbh | Heteroaryl-substituted pyrazine derivatives as pesticides |
| WO2021069567A1 (en) | 2019-10-09 | 2021-04-15 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021089673A1 (de) | 2019-11-07 | 2021-05-14 | Bayer Aktiengesellschaft | Substituierte sulfonylamide zur bekämpfung tierischer schädlinge |
| WO2021097162A1 (en) | 2019-11-13 | 2021-05-20 | Bayer Cropscience Lp | Beneficial combinations with paenibacillus |
| WO2021099303A1 (en) | 2019-11-18 | 2021-05-27 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021105091A1 (en) | 2019-11-25 | 2021-06-03 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021123051A1 (en) | 2019-12-20 | 2021-06-24 | Bayer Aktiengesellschaft | Substituted thiophene carboxamides, thiophene carboxylic acids and derivatives thereof |
| WO2021122986A1 (en) | 2019-12-20 | 2021-06-24 | Bayer Aktiengesellschaft | Thienyloxazolones and analogues |
| EP3845304A1 (en) | 2019-12-30 | 2021-07-07 | Bayer AG | Capsule suspension concentrates based on polyisocyanates and biodegradable amine based cross-linker |
| EP3868207A1 (de) | 2020-02-24 | 2021-08-25 | Bayer Aktiengesellschaft | Verkapselte pyrethroide mit verbesserter wirksamkeit bei boden- und blattanwendungen |
| WO2021165195A1 (en) | 2020-02-18 | 2021-08-26 | Bayer Aktiengesellschaft | Heteroaryl-triazole compounds as pesticides |
| WO2021204930A1 (en) | 2020-04-09 | 2021-10-14 | Bayer Animal Health Gmbh | Substituted condensed azines as anthelmintic compounds |
| WO2021209365A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209364A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209366A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209368A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209490A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Cyclaminephenylaminoquinolines as fungicides |
| WO2021209363A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021213978A1 (de) | 2020-04-21 | 2021-10-28 | Bayer Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2021224220A1 (en) | 2020-05-06 | 2021-11-11 | Bayer Aktiengesellschaft | Pyridine (thio)amides as fungicidal compounds |
| WO2021224323A1 (en) | 2020-05-06 | 2021-11-11 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021228734A1 (en) | 2020-05-12 | 2021-11-18 | Bayer Aktiengesellschaft | Triazine and pyrimidine (thio)amides as fungicidal compounds |
| WO2021233861A1 (en) | 2020-05-19 | 2021-11-25 | Bayer Aktiengesellschaft | Azabicyclic(thio)amides as fungicidal compounds |
| EP3915971A1 (en) | 2020-12-16 | 2021-12-01 | Bayer Aktiengesellschaft | Phenyl-s(o)n-phenylamidines and the use thereof as fungicides |
| EP3915371A1 (en) | 2020-11-04 | 2021-12-01 | Bayer AG | Active compound combinations and fungicide compositions comprising those |
| WO2021245087A1 (en) | 2020-06-04 | 2021-12-09 | Bayer Aktiengesellschaft | Heterocyclyl pyrimidines and triazines as novel fungicides |
| WO2021249995A1 (en) | 2020-06-10 | 2021-12-16 | Bayer Aktiengesellschaft | Azabicyclyl-substituted heterocycles as fungicides |
| WO2021255091A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazoles and their derivatives as fungicides |
| WO2021255089A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines and 1,3,4-oxadiazole pyridines as fungicides |
| WO2021255170A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines as fungicides |
| WO2021255071A1 (en) | 2020-06-18 | 2021-12-23 | Bayer Aktiengesellschaft | 3-(pyridazin-4-yl)-5,6-dihydro-4h-1,2,4-oxadiazine derivatives as fungicides for crop protection |
| WO2021255169A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines as fungicides |
| EP3929189A1 (en) | 2020-06-25 | 2021-12-29 | Bayer Animal Health GmbH | Novel heteroaryl-substituted pyrazine derivatives as pesticides |
| WO2021260017A1 (en) | 2020-06-26 | 2021-12-30 | Bayer Aktiengesellschaft | Aqueous capsule suspension concentrates comprising biodegradable ester groups |
| WO2022002818A1 (de) | 2020-07-02 | 2022-01-06 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2022033991A1 (de) | 2020-08-13 | 2022-02-17 | Bayer Aktiengesellschaft | 5-amino substituierte triazole als schädlingsbekämpfungsmittel |
| WO2022053453A1 (de) | 2020-09-09 | 2022-03-17 | Bayer Aktiengesellschaft | Azolcarboxamide als schädlingsbekämpfungsmittel |
| WO2022058327A1 (en) | 2020-09-15 | 2022-03-24 | Bayer Aktiengesellschaft | Substituted ureas and derivatives as new antifungal agents |
| EP3974414A1 (de) | 2020-09-25 | 2022-03-30 | Bayer AG | 5-amino substituierte pyrazole und triazole als schädlingsbekämpfungsmittel |
| EP3994992A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Low drift, rainfastness, high uptake and ulv tank mix adjuvant formulation |
| EP3994985A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift properties |
| EP3994988A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, spreading and rainfastness properties |
| EP3994987A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift and uptake properties |
| EP3994989A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, rainfastness and uptake properties |
| EP3994993A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading and ulv tank mix adjuvant formulation |
| EP3994995A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| EP3994986A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift and spreading properties |
| EP3994994A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| EP3994990A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, spreading and uptake properties |
| EP3994991A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, spreading, uptake and rainfastness properties |
| WO2022129196A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | Heterobicycle substituted 1,2,4-oxadiazoles as fungicides |
| WO2022129188A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | 1,2,4-oxadiazol-3-yl pyrimidines as fungicides |
| WO2022129190A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | (hetero)aryl substituted 1,2,4-oxadiazoles as fungicides |
| WO2022152728A1 (de) | 2021-01-15 | 2022-07-21 | Bayer Aktiengesellschaft | Herbizide zusammensetzungen |
| EP4036083A1 (de) | 2021-02-02 | 2022-08-03 | Bayer Aktiengesellschaft | 5-oxy substituierte hetereozyklen, als schädlingsbekämpfungsmittel |
| WO2022207494A1 (en) | 2021-03-30 | 2022-10-06 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2022207496A1 (en) | 2021-03-30 | 2022-10-06 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2022233777A1 (en) | 2021-05-06 | 2022-11-10 | Bayer Aktiengesellschaft | Alkylamide substituted, annulated imidazoles and use thereof as insecticides |
| WO2022238194A1 (de) | 2021-05-10 | 2022-11-17 | Bayer Aktiengesellschaft | Herbizid/safener-kombinationen basierend auf safenern aus der klasse der substituierten [(1,5-diphenyl1h-1,2,4-triazol-3-yl)oxy]essigsäuren sowie deren salze |
| WO2022238391A1 (de) | 2021-05-12 | 2022-11-17 | Bayer Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2023017120A1 (en) | 2021-08-13 | 2023-02-16 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2023025682A1 (en) | 2021-08-25 | 2023-03-02 | Bayer Aktiengesellschaft | Novel pyrazinyl-triazole compounds as pesticides |
| EP4144739A1 (de) | 2021-09-02 | 2023-03-08 | Bayer Aktiengesellschaft | Anellierte pyrazole als schädlingsbekämpfungsmittel |
| WO2023036821A1 (en) | 2021-09-09 | 2023-03-16 | Bayer Animal Health Gmbh | New quinoline derivatives |
| WO2023078915A1 (en) | 2021-11-03 | 2023-05-11 | Bayer Aktiengesellschaft | Bis(hetero)aryl thioether (thio)amides as fungicidal compounds |
| WO2023092050A1 (en) | 2021-11-20 | 2023-05-25 | Bayer Cropscience Lp | Beneficial combinations with recombinant bacillus cells expressing a serine protease |
| WO2023099445A1 (en) | 2021-11-30 | 2023-06-08 | Bayer Aktiengesellschaft | Bis(hetero)aryl thioether oxadiazines as fungicidal compounds |
| WO2023110656A1 (en) | 2021-12-15 | 2023-06-22 | Bayer Aktiengesellschaft | Spectroscopic solution for non-destructive quantification of one or more chemical substances in a matrix comprising coating and bulk material in a sample, such as coated seeds, using multivariate data analysis |
| EP4265110A1 (en) | 2022-04-20 | 2023-10-25 | Bayer AG | Water dispersible granules with low melting active ingredients prepared by extrusion |
| WO2023205602A1 (en) | 2022-04-18 | 2023-10-26 | Basf Corporation | High-load agricultural formulations and methods of making same |
| WO2023213626A1 (en) | 2022-05-03 | 2023-11-09 | Bayer Aktiengesellschaft | Use of (5s)-3-[3-(3-chloro-2-fluorophenoxy)-6-methylpyridazin-4-yl]-5-(2-chloro-4-methylbenzyl)-5,6-dihydro-4h-1,2,4-oxadiazine for controlling unwanted microorganisms |
| WO2023213670A1 (en) | 2022-05-03 | 2023-11-09 | Bayer Aktiengesellschaft | Crystalline forms of (5s)-3-[3-(3-chloro-2-fluorophenoxy)-6-methylpyridazin-4-yl]-5-(2-chloro-4-methylbenzyl)-5,6-dihydro-4h-1,2,4-oxadiazine |
| WO2023217619A1 (en) | 2022-05-07 | 2023-11-16 | Bayer Aktiengesellschaft | Low drift aqueous liquid formulations for low, medium, and high spray volume application |
| WO2023237444A1 (en) | 2022-06-06 | 2023-12-14 | Bayer Aktiengesellschaft | Agrochemical formulations comprising crystalline form a of 4-[(6-chloro-3-pyridylmethyl)(2,2-difluoroethyl)amino]furan-2(5h)-one |
| EP4295688A1 (en) | 2022-09-28 | 2023-12-27 | Bayer Aktiengesellschaft | Active compound combination |
| EP4295683A1 (en) | 2022-06-21 | 2023-12-27 | Bayer Aktiengesellschaft | Agrochemical formulations comprising crystalline form a of 4-[(6-chloro-3-pyridylmethyl)(2,2-difluoroethyl)amino]furan-2(5h)-one |
| WO2024013015A1 (en) | 2022-07-11 | 2024-01-18 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024013016A1 (en) | 2022-07-11 | 2024-01-18 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024068519A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068517A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068518A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-heteroaryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068520A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068473A1 (de) | 2022-09-27 | 2024-04-04 | Bayer Aktiengesellschaft | Herbizid/safener-kombinationen basierend auf safenern aus der klasse der substituierten [(1,5-diphenyl1h-1,2,4-triazol-3-yl)oxy]essigsäuren sowie deren salze |
| EP4353082A1 (en) | 2022-10-14 | 2024-04-17 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024104643A1 (en) | 2022-11-17 | 2024-05-23 | Bayer Aktiengesellschaft | Use of isotianil for controlling plasmodiophora brassica |
| WO2024170472A1 (en) | 2023-02-16 | 2024-08-22 | Bayer Aktiengesellschaft | Herbicidal mixtures |
| WO2024213752A1 (en) | 2023-04-14 | 2024-10-17 | Elanco Animal Health Gmbh | Long-term prevention and/or treatment of a disease by slo-1 inhibitors |
| WO2025026815A1 (en) | 2023-08-01 | 2025-02-06 | Globachem Nv | Insecticidal mixtures |
| WO2025026738A1 (en) | 2023-07-31 | 2025-02-06 | Bayer Aktiengesellschaft | 6-[5-(ethylsulfonyl)-1-methyl-1h-imidazol-4-yl]-7-methyl-3-(pentafluoroethyl)-7h-imidazo[4,5-c]pyridazine derivatives as pesticides |
| WO2025026787A1 (en) | 2023-08-01 | 2025-02-06 | Globachem Nv | Plant defense elicitors |
| WO2025032038A1 (en) | 2023-08-09 | 2025-02-13 | Bayer Aktiengesellschaft | Pyridazin-4-yloxadiazines as novel fungicides |
| WO2025031668A1 (en) | 2023-08-09 | 2025-02-13 | Bayer Aktiengesellschaft | Azaheterobiaryl-substituted 4,5-dihydro-1h-2,4,5-oxadiazines as novel fungicides |
| WO2025040301A1 (de) | 2023-08-21 | 2025-02-27 | Bayer Aktiengesellschaft | Herbizid/safener-kombinationen basierend auf safenern aus der klasse der substituierten [(1,5-diphenyl-1h-1,2,4-triazol-3-yl)oxy]essigsäuren und herbiziden aus der klasse der substituierten cyclischen dione sowie deren salze |
| WO2025040520A1 (de) | 2023-08-21 | 2025-02-27 | Bayer Aktiengesellschaft | Herbizid/safener-kombinationen basierend auf safenern aus der klasse der substituierten [(1,5-diphenyl-1h-1,2,4-triazol-3-yl)oxy]essigsäuren und herbiziden aus der klasse der substituierten cyclischen dione sowie deren salze |
| WO2025049555A1 (en) | 2023-08-31 | 2025-03-06 | Oerth Bio Llc | Compositions and methods for targeted inhibition and degradation of proteins in an insect cell |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1992011249A1 (en) * | 1990-12-21 | 1992-07-09 | E.I. Du Pont De Nemours And Company | Arthropodicidal carboxanilides |
| CN1146205A (zh) * | 1994-04-20 | 1997-03-26 | 纳幕尔杜邦公司 | 杀节肢动物的噁二嗪的制备 |
| WO1998005656A1 (en) * | 1996-08-05 | 1998-02-12 | E.I. Du Pont De Nemours And Company | Processes for preparing indeno[1,2-e][1,3,4]oxadiazine-dicarboxylates |
| WO1999063825A1 (en) * | 1998-06-10 | 1999-12-16 | E.I. Du Pont De Nemours And Company | Arthropodicidal carboxanilides |
| CN1663384A (zh) * | 2005-03-11 | 2005-09-07 | 王正权 | 一种杀虫剂及其制备方法 |
| CN101723913A (zh) * | 2009-12-03 | 2010-06-09 | 湖南化工研究院 | 具有杀菌活性的o取代噁二嗪类化合物及其制备方法和用途 |
-
2011
- 2011-10-14 CN CN2011103129260A patent/CN102391261A/zh active Pending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1992011249A1 (en) * | 1990-12-21 | 1992-07-09 | E.I. Du Pont De Nemours And Company | Arthropodicidal carboxanilides |
| CN1146205A (zh) * | 1994-04-20 | 1997-03-26 | 纳幕尔杜邦公司 | 杀节肢动物的噁二嗪的制备 |
| WO1998005656A1 (en) * | 1996-08-05 | 1998-02-12 | E.I. Du Pont De Nemours And Company | Processes for preparing indeno[1,2-e][1,3,4]oxadiazine-dicarboxylates |
| WO1999063825A1 (en) * | 1998-06-10 | 1999-12-16 | E.I. Du Pont De Nemours And Company | Arthropodicidal carboxanilides |
| CN1663384A (zh) * | 2005-03-11 | 2005-09-07 | 王正权 | 一种杀虫剂及其制备方法 |
| CN101723913A (zh) * | 2009-12-03 | 2010-06-09 | 湖南化工研究院 | 具有杀菌活性的o取代噁二嗪类化合物及其制备方法和用途 |
Non-Patent Citations (2)
| Title |
|---|
| KEITH D. WING 等: "Bioactivation and mode of action of the oxadiazine indoxacarb in insects", 《CROP PROTECTION》 * |
| 丁宁,等: "噁二嗪类杀虫剂茚虫威的研究进展", 《农药学学》 * |
Cited By (292)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104151260A (zh) * | 2014-08-26 | 2014-11-19 | 常州大学 | 一种新型噁二嗪类杀虫剂sioc-y-047的制备方法 |
| WO2017072039A1 (de) | 2015-10-26 | 2017-05-04 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| CN105330661A (zh) * | 2015-10-30 | 2016-02-17 | 青岛科技大学 | 一种n-芳甲基取代的噁二嗪硝基亚胺衍生物及其用途 |
| WO2017093180A1 (de) | 2015-12-01 | 2017-06-08 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2017093214A1 (de) | 2015-12-03 | 2017-06-08 | Bayer Cropscience Aktiengesellschaft | Mesolonische halogenierte 3-(acetyl)-1-[(1,3-thiazol-5-yl)methyl]-1h-imidazo[1,2-a]pyridin-4-ium-2-olat derivate und verwandte verbindungen als insektizide |
| WO2017137338A1 (de) | 2016-02-11 | 2017-08-17 | Bayer Cropscience Aktiengesellschaft | Substituierte 2-(het)aryl-imidazolyl-carboxyamide als schädlingsbekämpfungsmittel |
| WO2017137339A1 (de) | 2016-02-11 | 2017-08-17 | Bayer Cropscience Aktiengesellschaft | Substituierte 2-oxyimidazolyl-carboxamide als schädlingsbekämpfungsmittel |
| WO2017144341A1 (de) | 2016-02-23 | 2017-08-31 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| EP3210468A1 (de) | 2016-02-26 | 2017-08-30 | Bayer CropScience Aktiengesellschaft | Lösungsmittelfreie formulierungen von niedrig schmelzenden wirkstoffen |
| WO2017144497A1 (de) | 2016-02-26 | 2017-08-31 | Bayer Cropscience Aktiengesellschaft | Lösungsmittelfreie formulierungen von niedrig schmelzenden wirkstoffen |
| WO2017157735A1 (de) | 2016-03-15 | 2017-09-21 | Bayer Cropscience Aktiengesellschaft | Substituierte sulfonylamide zur bekämpfung tierischer schädlinge |
| WO2017157885A1 (de) | 2016-03-16 | 2017-09-21 | Bayer Cropscience Aktiengesellschaft | N-(cyanbenzyl)-6-(cyclopropylcarbonylamino)-4-(phenyl)-pyridin-2-carboxamid-derivate und verwandte verbindungen als pestizide pflanzenschutzmittel |
| WO2017174414A1 (de) | 2016-04-05 | 2017-10-12 | Bayer Cropscience Aktiengesellschaft | Naphthalin-derivate als schädlingsbekämpfungsmittel |
| WO2017178416A1 (en) | 2016-04-15 | 2017-10-19 | Bayer Animal Health Gmbh | Pyrazolopyrimidine derivatives |
| WO2017186536A1 (de) | 2016-04-25 | 2017-11-02 | Bayer Cropscience Aktiengesellschaft | Substituierte 2-alkylimidazolyl-carboxamide als schädlingsbekämpfungsmittel |
| EP3241830A1 (de) | 2016-05-04 | 2017-11-08 | Bayer CropScience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2017198450A1 (en) | 2016-05-15 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in maize |
| WO2017198449A1 (en) | 2016-05-15 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in brassicaceae |
| WO2017198453A1 (en) | 2016-05-16 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in potato, tomato or alfalfa |
| WO2017198452A1 (en) | 2016-05-16 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in soybean |
| WO2017198451A1 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in small grain cereals such as wheat and rice |
| WO2017198454A1 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in cotton |
| WO2017198455A2 (en) | 2016-05-17 | 2017-11-23 | Bayer Cropscience Nv | Method for increasing yield in beta spp. plants |
| EP3245865A1 (en) | 2016-05-17 | 2017-11-22 | Bayer CropScience Aktiengesellschaft | Method for increasing yield in brassicaceae |
| WO2018015289A1 (de) | 2016-07-19 | 2018-01-25 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018019937A1 (en) | 2016-07-29 | 2018-02-01 | Bayer Cropscience Aktiengesellschaft | Formulation comprising a beneficial p. bilaii strain and talc for use in seed treatment |
| WO2018029102A1 (de) | 2016-08-10 | 2018-02-15 | Bayer Cropscience Aktiengesellschaft | Substituierte 2-heterocyclyl-imidazolyl-carboxamide als schädlingsbekämpfungsmittel |
| WO2018033455A1 (de) | 2016-08-15 | 2018-02-22 | Bayer Cropscience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018050825A1 (de) | 2016-09-19 | 2018-03-22 | Bayer Cropscience Aktiengesellschaft | Pyrazolo[1,5-a]pyridin- derivative und ihre verwendung als schädlingsbekämpfungsmittel |
| WO2018065292A1 (de) | 2016-10-06 | 2018-04-12 | Bayer Cropscience Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte bicyclische heterocyclen-derivate als schädlings-bekämpfungsmittel |
| WO2018065288A1 (de) | 2016-10-07 | 2018-04-12 | Bayer Cropscience Aktiengesellschaft | 2-[2-phenyl-1-(sulfonylmethyl)vinyl]-imidazo[4,5-b]pyridin-derivate und verwandte verbindungen als schädlingsbekämpfungsmittel im pflanzenschutz |
| WO2018083288A1 (de) | 2016-11-07 | 2018-05-11 | Bayer Aktiengesellschaft | Substituierte sulfonylamide zur bekämpfung tierischer schädlinge |
| WO2018087036A1 (en) | 2016-11-11 | 2018-05-17 | Bayer Animal Health Gmbh | New anthelmintic quinoline-3-carboxamide derivatives |
| US11505545B2 (en) | 2016-11-11 | 2022-11-22 | Bayer Animal Health Gmbh | Anthelmintic quinoline-3-carboxamide derivatives |
| US10889573B2 (en) | 2016-11-11 | 2021-01-12 | Bayer Animal Health Gmbh | Anthelmintic quinoline-3-carboxamide derivatives |
| WO2018095953A1 (de) | 2016-11-23 | 2018-05-31 | Bayer Cropscience Aktiengesellschaft | 2-[3-(alkylsulfonyl)-2h-indazol-2-yl]-3h-imidazo[4,5-b]pyridin-derivate und ähnliche verbindungen als schädlingsbekämpfungsmittel |
| WO2018104500A1 (en) | 2016-12-09 | 2018-06-14 | Bayer Cropscience Aktiengesellschaft | Plant health effect of purpureocillium lilacinum |
| WO2018108791A1 (en) | 2016-12-16 | 2018-06-21 | Bayer Cropscience Aktiengesellschaft | Thiadiazole derivatives as pesticides |
| WO2018108730A1 (de) | 2016-12-16 | 2018-06-21 | Bayer Aktiengesellschaft | Mesoionische imidazopyridine als insektizide |
| WO2018130443A1 (de) | 2017-01-10 | 2018-07-19 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018130437A1 (de) | 2017-01-10 | 2018-07-19 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018138050A1 (de) | 2017-01-26 | 2018-08-02 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018141954A1 (de) | 2017-02-06 | 2018-08-09 | Bayer Aktiengesellschaft | Aryl- oder heteroaryl-substituierte imidazopyridinderivate und deren anwendung als schädlingsbekämpfungsmittel |
| EP3369320A1 (de) | 2017-03-02 | 2018-09-05 | Bayer CropScience Aktiengesellschaft | Wirkstoff zur bekämpfung von wanzen |
| WO2018189077A1 (de) | 2017-04-12 | 2018-10-18 | Bayer Aktiengesellschaft | Mesoionische imidazopyridine als insektizide |
| WO2018192872A1 (de) | 2017-04-21 | 2018-10-25 | Bayer Aktiengesellschaft | Mesoionische imidazopyridine als insektizide |
| WO2018197257A1 (de) | 2017-04-24 | 2018-11-01 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018197692A1 (en) | 2017-04-27 | 2018-11-01 | Bayer Aktiengesellschaft | Heteroarylphenylaminoquinolines and analogues |
| WO2018197401A1 (en) | 2017-04-27 | 2018-11-01 | Bayer Animal Health Gmbh | New bicyclic pyrazole derivatives |
| US11130768B2 (en) | 2017-04-27 | 2021-09-28 | Bayer Animal Health Gmbh | Bicyclic pyrazole derivatives |
| WO2018202494A1 (de) | 2017-05-02 | 2018-11-08 | Bayer Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018202501A1 (de) | 2017-05-02 | 2018-11-08 | Bayer Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2018202712A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylmethylphenoxyquinolines and analogues |
| WO2018202706A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylheteroaryloxyquinolines and analogues |
| WO2018202715A1 (en) | 2017-05-03 | 2018-11-08 | Bayer Aktiengesellschaft | Trisubstitutedsilylbenzylbenzimidazoles and analogues |
| WO2018202525A1 (en) | 2017-05-04 | 2018-11-08 | Bayer Cropscience Aktiengesellschaft | Phenoxyethanamine derivatives for controlling pests |
| US11827616B2 (en) | 2017-05-04 | 2023-11-28 | Discovery Purchaser Corporation | Heterocyclic compounds as pesticides |
| WO2018202524A1 (de) | 2017-05-04 | 2018-11-08 | Bayer Cropscience Aktiengesellschaft | 2-{[2-(phenyloxymethyl)pyridin-5-yl]oxy}-ethanamin-derivate und verwandte verbindungen als schädlingsbekämpfungsmittel z.b. für den pflanzenschutz |
| EP3400801A1 (en) | 2017-05-10 | 2018-11-14 | Bayer CropScience Aktiengesellschaft | Plant health effect of purpureocillium lilacinum |
| WO2019002132A1 (en) | 2017-06-30 | 2019-01-03 | Bayer Animal Health Gmbh | NEW AZAQUINOLINE DERIVATIVES |
| WO2019007887A1 (de) | 2017-07-06 | 2019-01-10 | Bayer Aktiengesellschaft | Insektizide und fungizide wirkstoffkombinationen |
| EP3284739A1 (de) | 2017-07-19 | 2018-02-21 | Bayer CropScience Aktiengesellschaft | Substituierte (het)arylverbindungen als schädlingsbekämpfungsmittel |
| WO2019025341A1 (en) | 2017-08-04 | 2019-02-07 | Bayer Animal Health Gmbh | QUINOLINE DERIVATIVES FOR THE TREATMENT OF INFECTIONS BY HELMINTHES |
| WO2019035881A1 (en) | 2017-08-17 | 2019-02-21 | Bayer Cropscience Lp | DISPERSIBLE COMPOSITIONS IN LIQUID FERTILIZER AND ASSOCIATED METHODS |
| WO2019038195A1 (de) | 2017-08-22 | 2019-02-28 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019059412A1 (en) | 2017-09-20 | 2019-03-28 | Mitsui Chemicals Agro, Inc. | AGENT FOR EXTENDED CONTROL OF ECTOPARASITES FOR ANIMAL |
| WO2019068572A1 (de) | 2017-10-04 | 2019-04-11 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| EP3473103A1 (de) | 2017-10-17 | 2019-04-24 | Bayer AG | Wässrige suspensionskonzentrate auf basis von 2-[(2,4-dichlorphenyl)-methyl]-4,4'-dimethyl-3-isoxazolidinon |
| WO2019076744A1 (de) | 2017-10-17 | 2019-04-25 | Bayer Aktiengesellschaft | Wässrige suspensionskonzentrate auf basis von 2-[(2,4-dichlorphenyl)-methyl]-4,4'-dimethyl-3-isoxazolidinone |
| WO2019076754A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS HAVING INSECTICIDAL / ACARICIDE PROPERTIES |
| WO2019076749A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS HAVING INSECTICIDAL / ACARICIDE PROPERTIES |
| WO2019076750A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS HAVING INSECTICIDE PROPERTIES7ACARICIDES |
| WO2019076751A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS HAVING INSECTICIDAL / ACARICIDE PROPERTIES |
| WO2019076752A1 (en) | 2017-10-18 | 2019-04-25 | Bayer Aktiengesellschaft | COMBINATIONS OF ACTIVE COMPOUNDS AYNT INSECTICIDE / ACARICIDE PROPERTIES |
| EP3473100A1 (en) | 2017-10-18 | 2019-04-24 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2019092086A1 (en) | 2017-11-13 | 2019-05-16 | Bayer Aktiengesellschaft | Tetrazolylpropyl derivatives and their use as fungicides |
| WO2019105875A1 (en) | 2017-11-28 | 2019-06-06 | Bayer Aktiengesellschaft | Heterocyclic compounds as pesticides |
| WO2019105871A1 (de) | 2017-11-29 | 2019-06-06 | Bayer Aktiengesellschaft | Stickstoffhaltige heterocyclen als schädlingsbekämpfungsmittel |
| WO2019122319A1 (en) | 2017-12-21 | 2019-06-27 | Bayer Aktiengesellschaft | Trisubstitutedsilylmethylheteroaryloxyquinolines and analogues |
| EP3305786A2 (de) | 2018-01-22 | 2018-04-11 | Bayer CropScience Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| CN108250156B (zh) * | 2018-01-29 | 2021-07-09 | 华南农业大学 | 一种肉桂酸噁二嗪衍生物及其制备方法和应用 |
| CN108250156A (zh) * | 2018-01-29 | 2018-07-06 | 华南农业大学 | 一种肉桂酸噁二嗪衍生物及其制备方法和应用 |
| WO2019155066A1 (en) | 2018-02-12 | 2019-08-15 | Bayer Aktiengesellschaft | Fungicidal oxadiazoles |
| WO2019162174A1 (de) | 2018-02-21 | 2019-08-29 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019162228A1 (en) | 2018-02-21 | 2019-08-29 | Bayer Aktiengesellschaft | 1-(5-substituted imidazol-1-yl)but-3-en derivatives and their use as fungicides |
| WO2019170626A1 (en) | 2018-03-08 | 2019-09-12 | Bayer Aktiengesellschaft | Use of heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides in plant protection |
| WO2019175046A1 (de) | 2018-03-12 | 2019-09-19 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019175045A1 (de) | 2018-03-12 | 2019-09-19 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019197371A1 (en) | 2018-04-10 | 2019-10-17 | Bayer Aktiengesellschaft | Oxadiazoline derivatives |
| EP3904350A1 (en) | 2018-04-12 | 2021-11-03 | Bayer Aktiengesellschaft | N-(cyclopropylmethyl)-5-(methylsulfonyl)-n-{1-[1-(pyrimidin-2-yl)-1h-1,2,4-triazol-5-yl]ethyl}benzamide derivatives and the corresponding pyridine-carboxamide derivatives as pesticides |
| EP3904349A2 (en) | 2018-04-12 | 2021-11-03 | Bayer Aktiengesellschaft | N-(cyclopropylmethyl)-5-(methylsulfonyl)-n-{1-[1-(pyrimidin-2-yl)-1h-1,2,4-triazol-5-yl]ethyl}heterocyclyl amide derivatives and similar compounds as pesticides |
| WO2019197468A1 (en) | 2018-04-12 | 2019-10-17 | Bayer Aktiengesellschaft | N-(cyclopropylmethyl)-5-(methylsulfonyl)-n-{1-[1-(pyrimidin-2-yl)-1h-1,2,4-triazol-5-yl]ethyl}benzamide derivatives and the corresponding pyridine-carboxamide derivatives as pesticides |
| WO2019197623A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit insektiziden, nematiziden und akariziden eigenschaften |
| WO2019197615A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit fungiziden, insektiziden und akariziden eigenschaften |
| WO2019201835A1 (en) | 2018-04-17 | 2019-10-24 | Bayer Aktiengesellschaft | Heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| WO2019202077A1 (en) | 2018-04-20 | 2019-10-24 | Bayer Aktiengesellschaft | Heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| WO2019201921A1 (de) | 2018-04-20 | 2019-10-24 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2019206799A1 (en) | 2018-04-25 | 2019-10-31 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| EP3919486A1 (en) | 2018-04-25 | 2021-12-08 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole and heteroaryl-tetrazole compounds as pesticides |
| WO2019215182A1 (en) | 2018-05-09 | 2019-11-14 | Bayer Animal Health Gmbh | New quinoline derivatives |
| WO2019224143A1 (de) | 2018-05-24 | 2019-11-28 | Bayer Aktiengesellschaft | Wirkstoffkombinationen mit insektiziden, nematiziden und akariziden eigenschaften |
| WO2020005678A1 (en) | 2018-06-25 | 2020-01-02 | Bayer Cropscience Lp | Seed treatment method |
| WO2020002189A1 (de) | 2018-06-27 | 2020-01-02 | Bayer Aktiengesellschaft | Wirkstoffkombinationen |
| EP3586630A1 (en) | 2018-06-28 | 2020-01-01 | Bayer AG | Active compound combinations having insecticidal/acaricidal properties |
| US11884643B2 (en) | 2018-07-05 | 2024-01-30 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| WO2020007902A1 (en) | 2018-07-05 | 2020-01-09 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| US11952359B2 (en) | 2018-07-05 | 2024-04-09 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| WO2020007905A1 (en) | 2018-07-05 | 2020-01-09 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| WO2020007904A1 (en) | 2018-07-05 | 2020-01-09 | Bayer Aktiengesellschaft | Substituted thiophenecarboxamides and analogues as antibacterials agents |
| WO2020020813A1 (en) | 2018-07-25 | 2020-01-30 | Bayer Aktiengesellschaft | Fungicidal active compound combinations |
| WO2020020816A1 (en) | 2018-07-26 | 2020-01-30 | Bayer Aktiengesellschaft | Novel triazole derivatives |
| WO2020021082A1 (en) | 2018-07-27 | 2020-01-30 | Bayer Aktiengesellschaft | Controlled release formulations for agrochemicals |
| WO2020025650A1 (en) | 2018-07-31 | 2020-02-06 | Bayer Aktiengesellschaft | Controlled release formulations with lignin for agrochemicals |
| WO2020043650A1 (en) | 2018-08-29 | 2020-03-05 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2020053282A1 (de) | 2018-09-13 | 2020-03-19 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2020057939A1 (en) | 2018-09-17 | 2020-03-26 | Bayer Aktiengesellschaft | Use of the fungicide isoflucypram for controlling claviceps purpurea and reducing sclerotia in cereals |
| WO2020070050A1 (en) | 2018-10-01 | 2020-04-09 | Bayer Aktiengesellschaft | Fungicidal 5-substituted imidazol-1-yl carbinol derivatives |
| EP3636644A1 (de) | 2018-10-11 | 2020-04-15 | Bayer Aktiengesellschaft | Mesoionische imidazopyridine als insektizide |
| WO2020078839A1 (de) | 2018-10-16 | 2020-04-23 | Bayer Aktiengesellschaft | Wirkstoffkombinationen |
| WO2020079167A1 (en) | 2018-10-18 | 2020-04-23 | Bayer Aktiengesellschaft | Heteroarylaminoquinolines and analogues |
| WO2020079173A1 (en) | 2018-10-18 | 2020-04-23 | Bayer Aktiengesellschaft | Pyridylphenylaminoquinolines and analogues |
| WO2020079232A1 (en) | 2018-10-20 | 2020-04-23 | Bayer Aktiengesellschaft | Oxetanylphenoxyquinolines and analogues |
| WO2020083971A2 (en) | 2018-10-24 | 2020-04-30 | Bayer Animal Health Gmbh | New anthelmintic compounds |
| EP3643711A1 (en) | 2018-10-24 | 2020-04-29 | Bayer Animal Health GmbH | New anthelmintic compounds |
| WO2020109391A1 (en) | 2018-11-28 | 2020-06-04 | Bayer Aktiengesellschaft | Pyridazine (thio)amides as fungicidal compounds |
| WO2020114934A1 (de) | 2018-12-07 | 2020-06-11 | Bayer Aktiengesellschaft | Herbizide zusammensetzungen |
| WO2020114932A1 (de) | 2018-12-07 | 2020-06-11 | Bayer Aktiengesellschaft | Herbizide zusammensetzungen |
| EP3620052A1 (en) | 2018-12-12 | 2020-03-11 | Bayer Aktiengesellschaft | Use of phenoxypyridinyl-substituted (1h-1,2,4-triazol-1-yl)alcohols for controlling fungicidal diseases in maize |
| WO2020126980A1 (en) | 2018-12-18 | 2020-06-25 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2020127780A1 (en) | 2018-12-20 | 2020-06-25 | Bayer Aktiengesellschaft | Heterocyclyl pyridazine as fungicidal compounds |
| EP3669652A1 (en) | 2018-12-21 | 2020-06-24 | Bayer AG | Active compound combination |
| WO2020127974A1 (en) | 2018-12-21 | 2020-06-25 | Bayer Aktiengesellschaft | 1,3,4-oxadiazoles and their derivatives as new antifungal agents |
| EP3679791A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679790A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679793A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679792A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3679789A1 (en) | 2019-01-08 | 2020-07-15 | Bayer AG | Active compound combinations |
| EP3545764A1 (en) | 2019-02-12 | 2019-10-02 | Bayer AG | Crystal form of 2-({2-fluoro-4-methyl-5-[(r)-(2,2,2-trifluoroethyl)sulfinyl]phenyl}imino)-3-(2,2,2- trifluoroethyl)-1,3-thiazolidin-4-one |
| WO2020173861A1 (de) | 2019-02-26 | 2020-09-03 | Bayer Aktiengesellschaft | Kondensierte bicyclische heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2020173860A1 (en) | 2019-02-26 | 2020-09-03 | Bayer Aktiengesellschaft | Fused bicyclic heterocycle derivatives as pesticides |
| WO2020178067A1 (en) | 2019-03-01 | 2020-09-10 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| WO2020178307A1 (en) | 2019-03-05 | 2020-09-10 | Bayer Aktiengesellschaft | Active compound combination |
| WO2020182929A1 (en) | 2019-03-13 | 2020-09-17 | Bayer Aktiengesellschaft | Substituted ureas and derivatives as new antifungal agents |
| WO2020187656A1 (en) | 2019-03-15 | 2020-09-24 | Bayer Aktiengesellschaft | Active compound combinations having insecticidal/acaricidal properties |
| EP3564225A1 (en) | 2019-03-21 | 2019-11-06 | Bayer Aktiengesellschaft | Crystalline form of spiromesifen |
| WO2020212235A1 (en) | 2019-04-15 | 2020-10-22 | Bayer Animal Health Gmbh | Novel heteroaryl-substituted aminoalkyl azole compounds as pesticides |
| EP3725788A1 (en) | 2019-04-15 | 2020-10-21 | Bayer AG | Novel heteroaryl-substituted aminoalkyl azole compounds as pesticides |
| WO2020225436A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading, uptake and rainfastness ulv formulations |
| WO2020225439A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | Ulv formulations with enhanced rainfastness |
| WO2020225428A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading ulv formulations for insecticides |
| WO2020225435A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading and uptake ulv formulations |
| WO2020225438A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High uptake and rainfastness ulv formulations |
| WO2020225434A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading ulv formulations for agrochemical compounds ii |
| WO2020225242A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | Active compound combination |
| WO2020225440A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | High spreading and rainfastness ulv formulations |
| WO2020225437A1 (en) | 2019-05-08 | 2020-11-12 | Bayer Aktiengesellschaft | Ulv formulations with enhanced uptake |
| WO2020231751A1 (en) | 2019-05-10 | 2020-11-19 | Bayer Cropscience Lp | Active compound combinations |
| WO2020229398A1 (de) | 2019-05-14 | 2020-11-19 | Bayer Aktiengesellschaft | (1-alkenyl)-substituierte pyrazole und triazole als schädlingsbekämpfungsmittel |
| EP3750888A1 (en) | 2019-06-12 | 2020-12-16 | Bayer Aktiengesellschaft | Crystalline form a of 1,4-dimethyl-2-[2-(pyridin-3-yl)-2h-indazol-5-yl]-1,2,4-triazolidine-3,5-dione |
| WO2020254490A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Phenoxyphenyl hydroxyisoxazolines and analogues as new antifungal agents |
| WO2020254492A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254487A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254488A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and use thereof as fungicides |
| WO2020254493A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Thienylhydroxyisoxazolines and derivatives thereof |
| WO2020254489A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Benzylphenyl hydroxyisoxazolines and analogues as new antifungal agents |
| WO2020254486A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Hydroxyisoxazolines and derivatives thereof |
| WO2020254494A1 (en) | 2019-06-21 | 2020-12-24 | Bayer Aktiengesellschaft | Fungicidal oxadiazoles |
| WO2020263812A1 (en) | 2019-06-24 | 2020-12-30 | Auburn University | A bacillus strain and methods of its use for plant growth promotion |
| EP3608311A1 (en) | 2019-06-28 | 2020-02-12 | Bayer AG | Crystalline form a of n-[4-chloro-3-[(1-cyanocyclopropyl)carbamoyl]phenyl]-2-methyl-4-methylsulfonyl-5-(1,1,2,2,2-pentafluoroethyl)pyrazole-3-carboxamide |
| WO2021001331A1 (en) | 2019-07-03 | 2021-01-07 | Bayer Aktiengesellschaft | Substituted thiophene carboxamides and derivatives thereof as microbicides |
| EP4378314A2 (de) | 2019-07-04 | 2024-06-05 | Bayer AG | Herbizide zusammensetzungen |
| WO2021001273A1 (de) | 2019-07-04 | 2021-01-07 | Bayer Aktiengesellschaft | Herbizide zusammensetzungen |
| WO2021013721A1 (de) | 2019-07-22 | 2021-01-28 | Bayer Aktiengesellschaft | 5-amino substituierte pyrazole und triazole als schädlingsbekämpfungsmittel |
| WO2021013720A1 (en) | 2019-07-23 | 2021-01-28 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021013719A1 (en) | 2019-07-23 | 2021-01-28 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| EP3771714A1 (de) | 2019-07-30 | 2021-02-03 | Bayer AG | Stickstoffhaltige heterocyclen als schädlingsbekämpfungsmittel |
| WO2021018839A1 (en) | 2019-07-30 | 2021-02-04 | Bayer Animal Health Gmbh | Isoquinoline derivatives and their use for the treatment of parasitic infections |
| EP3701796A1 (en) | 2019-08-08 | 2020-09-02 | Bayer AG | Active compound combinations |
| WO2021048188A1 (de) | 2019-09-11 | 2021-03-18 | Bayer Aktiengesellschaft | Hochwirksame formulierungen auf basis von 2-[(2;4-dichlorphenyl)-m ethyl|-4,4'-dimethyl- 3-isoxazolidinone sowie vorauflaufherbiziden |
| WO2021058659A1 (en) | 2019-09-26 | 2021-04-01 | Bayer Aktiengesellschaft | Rnai-mediated pest control |
| WO2021069567A1 (en) | 2019-10-09 | 2021-04-15 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021069569A1 (en) | 2019-10-09 | 2021-04-15 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021069575A1 (en) | 2019-10-11 | 2021-04-15 | Bayer Animal Health Gmbh | Heteroaryl-substituted pyrazine derivatives as pesticides |
| WO2021089673A1 (de) | 2019-11-07 | 2021-05-14 | Bayer Aktiengesellschaft | Substituierte sulfonylamide zur bekämpfung tierischer schädlinge |
| WO2021097162A1 (en) | 2019-11-13 | 2021-05-20 | Bayer Cropscience Lp | Beneficial combinations with paenibacillus |
| WO2021099303A1 (en) | 2019-11-18 | 2021-05-27 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021105091A1 (en) | 2019-11-25 | 2021-06-03 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021122986A1 (en) | 2019-12-20 | 2021-06-24 | Bayer Aktiengesellschaft | Thienyloxazolones and analogues |
| WO2021123051A1 (en) | 2019-12-20 | 2021-06-24 | Bayer Aktiengesellschaft | Substituted thiophene carboxamides, thiophene carboxylic acids and derivatives thereof |
| WO2021136758A1 (en) | 2019-12-30 | 2021-07-08 | Bayer Aktiengesellschaft | Aqueous capsule suspension concentrates based on polyurea shell material containing polyfunctional aminocarboxylic esters |
| EP3845304A1 (en) | 2019-12-30 | 2021-07-07 | Bayer AG | Capsule suspension concentrates based on polyisocyanates and biodegradable amine based cross-linker |
| WO2021165195A1 (en) | 2020-02-18 | 2021-08-26 | Bayer Aktiengesellschaft | Heteroaryl-triazole compounds as pesticides |
| EP3868207A1 (de) | 2020-02-24 | 2021-08-25 | Bayer Aktiengesellschaft | Verkapselte pyrethroide mit verbesserter wirksamkeit bei boden- und blattanwendungen |
| WO2021170527A1 (de) | 2020-02-24 | 2021-09-02 | Bayer Aktiengesellschaft | Verkapselte pyrethroide mit verbesserter wirksamkeit bei boden- und blattanwendungen |
| EP3708565A1 (en) | 2020-03-04 | 2020-09-16 | Bayer AG | Pyrimidinyloxyphenylamidines and the use thereof as fungicides |
| WO2021204930A1 (en) | 2020-04-09 | 2021-10-14 | Bayer Animal Health Gmbh | Substituted condensed azines as anthelmintic compounds |
| WO2021209363A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209365A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209364A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209366A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021209490A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Cyclaminephenylaminoquinolines as fungicides |
| WO2021209368A1 (en) | 2020-04-16 | 2021-10-21 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2021213978A1 (de) | 2020-04-21 | 2021-10-28 | Bayer Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2021224323A1 (en) | 2020-05-06 | 2021-11-11 | Bayer Aktiengesellschaft | Novel heteroaryl-triazole compounds as pesticides |
| WO2021224220A1 (en) | 2020-05-06 | 2021-11-11 | Bayer Aktiengesellschaft | Pyridine (thio)amides as fungicidal compounds |
| WO2021228734A1 (en) | 2020-05-12 | 2021-11-18 | Bayer Aktiengesellschaft | Triazine and pyrimidine (thio)amides as fungicidal compounds |
| WO2021233861A1 (en) | 2020-05-19 | 2021-11-25 | Bayer Aktiengesellschaft | Azabicyclic(thio)amides as fungicidal compounds |
| WO2021245087A1 (en) | 2020-06-04 | 2021-12-09 | Bayer Aktiengesellschaft | Heterocyclyl pyrimidines and triazines as novel fungicides |
| WO2021249995A1 (en) | 2020-06-10 | 2021-12-16 | Bayer Aktiengesellschaft | Azabicyclyl-substituted heterocycles as fungicides |
| WO2021255071A1 (en) | 2020-06-18 | 2021-12-23 | Bayer Aktiengesellschaft | 3-(pyridazin-4-yl)-5,6-dihydro-4h-1,2,4-oxadiazine derivatives as fungicides for crop protection |
| WO2021255091A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazoles and their derivatives as fungicides |
| WO2021255089A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines and 1,3,4-oxadiazole pyridines as fungicides |
| WO2021255170A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines as fungicides |
| WO2021255169A1 (en) | 2020-06-19 | 2021-12-23 | Bayer Aktiengesellschaft | 1,3,4-oxadiazole pyrimidines as fungicides |
| EP3929189A1 (en) | 2020-06-25 | 2021-12-29 | Bayer Animal Health GmbH | Novel heteroaryl-substituted pyrazine derivatives as pesticides |
| WO2021259997A1 (en) | 2020-06-25 | 2021-12-30 | Bayer Animal Health Gmbh | Novel heteroaryl-substituted pyrazine derivatives as pesticides |
| WO2021260017A1 (en) | 2020-06-26 | 2021-12-30 | Bayer Aktiengesellschaft | Aqueous capsule suspension concentrates comprising biodegradable ester groups |
| WO2022002818A1 (de) | 2020-07-02 | 2022-01-06 | Bayer Aktiengesellschaft | Heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2022033991A1 (de) | 2020-08-13 | 2022-02-17 | Bayer Aktiengesellschaft | 5-amino substituierte triazole als schädlingsbekämpfungsmittel |
| WO2022053453A1 (de) | 2020-09-09 | 2022-03-17 | Bayer Aktiengesellschaft | Azolcarboxamide als schädlingsbekämpfungsmittel |
| WO2022058327A1 (en) | 2020-09-15 | 2022-03-24 | Bayer Aktiengesellschaft | Substituted ureas and derivatives as new antifungal agents |
| EP3974414A1 (de) | 2020-09-25 | 2022-03-30 | Bayer AG | 5-amino substituierte pyrazole und triazole als schädlingsbekämpfungsmittel |
| EP3915371A1 (en) | 2020-11-04 | 2021-12-01 | Bayer AG | Active compound combinations and fungicide compositions comprising those |
| EP3994985A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift properties |
| EP3994987A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift and uptake properties |
| EP3994995A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| EP3994986A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift and spreading properties |
| EP3994994A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| EP3994990A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, spreading and uptake properties |
| EP3994991A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, spreading, uptake and rainfastness properties |
| WO2022096686A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift properties |
| WO2022096695A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| WO2022096688A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, spreading and rainfastness properties |
| WO2022096691A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, uptake and rainfastness properties |
| WO2022096687A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift and uptake properties |
| WO2022096694A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading and ulv tank mix adjuvant formulation |
| WO2022096693A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Low drift, rainfastness, high uptake and ulv tank mix adjuvant formulation |
| WO2022096692A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, spreading, uptake and rainfastness properties |
| WO2022096690A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift, spreading and uptake properties |
| WO2022096685A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Agrochemical composition with improved drift and spreading properties |
| WO2022096696A1 (en) | 2020-11-08 | 2022-05-12 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading, high uptake and ulv tank mix adjuvant formulation |
| EP3994989A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, rainfastness and uptake properties |
| EP3994992A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Low drift, rainfastness, high uptake and ulv tank mix adjuvant formulation |
| EP3994988A1 (en) | 2020-11-08 | 2022-05-11 | Bayer AG | Agrochemical composition with improved drift, spreading and rainfastness properties |
| EP3994993A1 (en) | 2020-11-08 | 2022-05-11 | Bayer Aktiengesellschaft | Low drift, rainfastness, high spreading and ulv tank mix adjuvant formulation |
| EP3915971A1 (en) | 2020-12-16 | 2021-12-01 | Bayer Aktiengesellschaft | Phenyl-s(o)n-phenylamidines and the use thereof as fungicides |
| WO2022129196A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | Heterobicycle substituted 1,2,4-oxadiazoles as fungicides |
| WO2022129190A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | (hetero)aryl substituted 1,2,4-oxadiazoles as fungicides |
| WO2022129188A1 (en) | 2020-12-18 | 2022-06-23 | Bayer Aktiengesellschaft | 1,2,4-oxadiazol-3-yl pyrimidines as fungicides |
| WO2022152728A1 (de) | 2021-01-15 | 2022-07-21 | Bayer Aktiengesellschaft | Herbizide zusammensetzungen |
| EP4036083A1 (de) | 2021-02-02 | 2022-08-03 | Bayer Aktiengesellschaft | 5-oxy substituierte hetereozyklen, als schädlingsbekämpfungsmittel |
| WO2022207494A1 (en) | 2021-03-30 | 2022-10-06 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2022207496A1 (en) | 2021-03-30 | 2022-10-06 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2022233777A1 (en) | 2021-05-06 | 2022-11-10 | Bayer Aktiengesellschaft | Alkylamide substituted, annulated imidazoles and use thereof as insecticides |
| WO2022238194A1 (de) | 2021-05-10 | 2022-11-17 | Bayer Aktiengesellschaft | Herbizid/safener-kombinationen basierend auf safenern aus der klasse der substituierten [(1,5-diphenyl1h-1,2,4-triazol-3-yl)oxy]essigsäuren sowie deren salze |
| WO2022238391A1 (de) | 2021-05-12 | 2022-11-17 | Bayer Aktiengesellschaft | 2-(het)aryl-substituierte kondensierte heterocyclen-derivate als schädlingsbekämpfungsmittel |
| WO2023017120A1 (en) | 2021-08-13 | 2023-02-16 | Bayer Aktiengesellschaft | Active compound combinations and fungicide compositions comprising those |
| WO2023025682A1 (en) | 2021-08-25 | 2023-03-02 | Bayer Aktiengesellschaft | Novel pyrazinyl-triazole compounds as pesticides |
| EP4144739A1 (de) | 2021-09-02 | 2023-03-08 | Bayer Aktiengesellschaft | Anellierte pyrazole als schädlingsbekämpfungsmittel |
| WO2023036821A1 (en) | 2021-09-09 | 2023-03-16 | Bayer Animal Health Gmbh | New quinoline derivatives |
| WO2023078915A1 (en) | 2021-11-03 | 2023-05-11 | Bayer Aktiengesellschaft | Bis(hetero)aryl thioether (thio)amides as fungicidal compounds |
| WO2023092050A1 (en) | 2021-11-20 | 2023-05-25 | Bayer Cropscience Lp | Beneficial combinations with recombinant bacillus cells expressing a serine protease |
| WO2023099445A1 (en) | 2021-11-30 | 2023-06-08 | Bayer Aktiengesellschaft | Bis(hetero)aryl thioether oxadiazines as fungicidal compounds |
| WO2023110656A1 (en) | 2021-12-15 | 2023-06-22 | Bayer Aktiengesellschaft | Spectroscopic solution for non-destructive quantification of one or more chemical substances in a matrix comprising coating and bulk material in a sample, such as coated seeds, using multivariate data analysis |
| WO2023205602A1 (en) | 2022-04-18 | 2023-10-26 | Basf Corporation | High-load agricultural formulations and methods of making same |
| EP4265110A1 (en) | 2022-04-20 | 2023-10-25 | Bayer AG | Water dispersible granules with low melting active ingredients prepared by extrusion |
| WO2023203009A1 (en) | 2022-04-20 | 2023-10-26 | Bayer Aktiengesellschaft | Water dispersible granules with low melting active ingredients prepared by extrusion |
| WO2023213626A1 (en) | 2022-05-03 | 2023-11-09 | Bayer Aktiengesellschaft | Use of (5s)-3-[3-(3-chloro-2-fluorophenoxy)-6-methylpyridazin-4-yl]-5-(2-chloro-4-methylbenzyl)-5,6-dihydro-4h-1,2,4-oxadiazine for controlling unwanted microorganisms |
| WO2023213670A1 (en) | 2022-05-03 | 2023-11-09 | Bayer Aktiengesellschaft | Crystalline forms of (5s)-3-[3-(3-chloro-2-fluorophenoxy)-6-methylpyridazin-4-yl]-5-(2-chloro-4-methylbenzyl)-5,6-dihydro-4h-1,2,4-oxadiazine |
| WO2023217619A1 (en) | 2022-05-07 | 2023-11-16 | Bayer Aktiengesellschaft | Low drift aqueous liquid formulations for low, medium, and high spray volume application |
| WO2023237444A1 (en) | 2022-06-06 | 2023-12-14 | Bayer Aktiengesellschaft | Agrochemical formulations comprising crystalline form a of 4-[(6-chloro-3-pyridylmethyl)(2,2-difluoroethyl)amino]furan-2(5h)-one |
| EP4295683A1 (en) | 2022-06-21 | 2023-12-27 | Bayer Aktiengesellschaft | Agrochemical formulations comprising crystalline form a of 4-[(6-chloro-3-pyridylmethyl)(2,2-difluoroethyl)amino]furan-2(5h)-one |
| WO2024013015A1 (en) | 2022-07-11 | 2024-01-18 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024013016A1 (en) | 2022-07-11 | 2024-01-18 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024068473A1 (de) | 2022-09-27 | 2024-04-04 | Bayer Aktiengesellschaft | Herbizid/safener-kombinationen basierend auf safenern aus der klasse der substituierten [(1,5-diphenyl1h-1,2,4-triazol-3-yl)oxy]essigsäuren sowie deren salze |
| WO2024068518A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-heteroaryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068517A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| WO2024068519A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| EP4295688A1 (en) | 2022-09-28 | 2023-12-27 | Bayer Aktiengesellschaft | Active compound combination |
| WO2024068520A1 (en) | 2022-09-28 | 2024-04-04 | Bayer Aktiengesellschaft | 3-(hetero)aryl-5-chlorodifluoromethyl-1,2,4-oxadiazole as fungicide |
| EP4353082A1 (en) | 2022-10-14 | 2024-04-17 | Bayer Aktiengesellschaft | Herbicidal compositions |
| WO2024104643A1 (en) | 2022-11-17 | 2024-05-23 | Bayer Aktiengesellschaft | Use of isotianil for controlling plasmodiophora brassica |
| WO2024170472A1 (en) | 2023-02-16 | 2024-08-22 | Bayer Aktiengesellschaft | Herbicidal mixtures |
| WO2024213752A1 (en) | 2023-04-14 | 2024-10-17 | Elanco Animal Health Gmbh | Long-term prevention and/or treatment of a disease by slo-1 inhibitors |
| WO2025026738A1 (en) | 2023-07-31 | 2025-02-06 | Bayer Aktiengesellschaft | 6-[5-(ethylsulfonyl)-1-methyl-1h-imidazol-4-yl]-7-methyl-3-(pentafluoroethyl)-7h-imidazo[4,5-c]pyridazine derivatives as pesticides |
| WO2025026815A1 (en) | 2023-08-01 | 2025-02-06 | Globachem Nv | Insecticidal mixtures |
| WO2025026787A1 (en) | 2023-08-01 | 2025-02-06 | Globachem Nv | Plant defense elicitors |
| WO2025032038A1 (en) | 2023-08-09 | 2025-02-13 | Bayer Aktiengesellschaft | Pyridazin-4-yloxadiazines as novel fungicides |
| WO2025031668A1 (en) | 2023-08-09 | 2025-02-13 | Bayer Aktiengesellschaft | Azaheterobiaryl-substituted 4,5-dihydro-1h-2,4,5-oxadiazines as novel fungicides |
| WO2025040301A1 (de) | 2023-08-21 | 2025-02-27 | Bayer Aktiengesellschaft | Herbizid/safener-kombinationen basierend auf safenern aus der klasse der substituierten [(1,5-diphenyl-1h-1,2,4-triazol-3-yl)oxy]essigsäuren und herbiziden aus der klasse der substituierten cyclischen dione sowie deren salze |
| WO2025040520A1 (de) | 2023-08-21 | 2025-02-27 | Bayer Aktiengesellschaft | Herbizid/safener-kombinationen basierend auf safenern aus der klasse der substituierten [(1,5-diphenyl-1h-1,2,4-triazol-3-yl)oxy]essigsäuren und herbiziden aus der klasse der substituierten cyclischen dione sowie deren salze |
| WO2025049555A1 (en) | 2023-08-31 | 2025-03-06 | Oerth Bio Llc | Compositions and methods for targeted inhibition and degradation of proteins in an insect cell |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN102391261A (zh) | 一种n-取代噁二嗪类化合物及其制备方法和应用 | |
| CA3150249C (en) | Piperonylic acid derivative and application thereof | |
| US5290754A (en) | Triazine derivative and a herbicide comprising the same as an effective ingredient | |
| RU2041220C1 (ru) | Производное гидразина, инсектицидная композиция, способ борьбы с вредными насекомыми, способ получения производного гидразина | |
| KR900002063B1 (ko) | 피라졸 옥심 유도체 | |
| HUE027959T2 (en) | Anthranilic acid diamide derivative with heteroaromatic and heterocyclic substituents | |
| da Silva et al. | Thiosemicarbazones as Aedes aegypti larvicidal | |
| CN102153498B (zh) | 一种白藜芦醇类似物及其制备方法和应用 | |
| JP2751309B2 (ja) | ピラゾール類およびこれを有効成分とする殺虫、殺ダニ、殺菌剤 | |
| CN114605324A (zh) | 异噁唑啉取代的苯甲酰胺类衍生物及其制备方法与用途 | |
| CN103201266A (zh) | 芳氧基二卤丙烯醚类化合物与应用 | |
| CN107266511A (zh) | 一类新颖的5‑肟酯B2a结构的化合物及其制备方法与应用 | |
| CN108341808B (zh) | 一种噁二唑连吡唑类化合物及其用途 | |
| WO2009036235A2 (en) | Insecticidal carbamates exhibiting species-selective inhibition of acetylcholinesterase (ache) | |
| CN100503602C (zh) | 用包括四唑的仲胺取代的苯并吡喃衍生物、其制备方法以及含有它们的药物组合物 | |
| CN110551148B (zh) | 含硅酰基乙腈化合物及其制备方法与应用 | |
| CN105348296B (zh) | 一类2‑取代芳基呋喃并香豆素衍生物及其应用 | |
| CN111205223B (zh) | 喹啉类衍生物及其制备方法与用途 | |
| CN101423501A (zh) | 含杂环5-苯基-2,4-戊二烯-1-酮类化合物及其制备方法与应用 | |
| CN103275073B (zh) | 2-(1,2,4-三唑-1-甲基)-2-(苯并呋喃-5-基)-1,3-二氧戊环及其应用 | |
| CN110655512B (zh) | 二芳基乙烯类化合物及其制备和用途 | |
| US5494888A (en) | 6-chloro-2-(4,6-dimethoxypyrimidin-2-yl)oxybenzoic acid imino ester derivatives, processes for their production and a method for their application as herbicides | |
| CN101255147A (zh) | 螺螨酯衍生物及其合成方法和用途 | |
| Hwang et al. | New 2‐phenyl‐4, 5, 6, 7‐tetrahydro‐2H‐indazole derivatives as paddy field herbicides | |
| CN111226956A (zh) | 3,6-二取代咪唑[1,2-b]哒嗪类衍生物在制备杀菌剂中的应用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C12 | Rejection of a patent application after its publication | ||
| RJ01 | Rejection of invention patent application after publication |
Application publication date: 20120328 |