KR20150064093A - 이중 glp1/글루카곤 작용제로서의 엑센딘-4 유도체 - Google Patents
이중 glp1/글루카곤 작용제로서의 엑센딘-4 유도체 Download PDFInfo
- Publication number
- KR20150064093A KR20150064093A KR1020157010088A KR20157010088A KR20150064093A KR 20150064093 A KR20150064093 A KR 20150064093A KR 1020157010088 A KR1020157010088 A KR 1020157010088A KR 20157010088 A KR20157010088 A KR 20157010088A KR 20150064093 A KR20150064093 A KR 20150064093A
- Authority
- KR
- South Korea
- Prior art keywords
- carboxy
- amino acid
- acid residue
- residue selected
- butyryl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 239000000556 agonist Substances 0.000 title claims description 21
- 230000009977 dual effect Effects 0.000 title claims description 6
- JUFFVKRROAPVBI-PVOYSMBESA-N chembl1210015 Chemical class C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(=O)N[C@H]1[C@@H]([C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO[C@]3(O[C@@H](C[C@H](O)[C@H](O)CO)[C@H](NC(C)=O)[C@@H](O)C3)C(O)=O)O2)O)[C@@H](CO)O1)NC(C)=O)C(=O)NCC(=O)NCC(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 JUFFVKRROAPVBI-PVOYSMBESA-N 0.000 title description 40
- 102000051325 Glucagon Human genes 0.000 title description 29
- MASNOZXLGMXCHN-ZLPAWPGGSA-N glucagon Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C1=CC=CC=C1 MASNOZXLGMXCHN-ZLPAWPGGSA-N 0.000 title description 29
- 229960004666 glucagon Drugs 0.000 title description 29
- 108060003199 Glucagon Proteins 0.000 title description 27
- 101100337060 Caenorhabditis elegans glp-1 gene Proteins 0.000 title 1
- 238000011282 treatment Methods 0.000 claims abstract description 55
- 206010012601 diabetes mellitus Diseases 0.000 claims abstract description 42
- 208000008589 Obesity Diseases 0.000 claims abstract description 37
- 235000020824 obesity Nutrition 0.000 claims abstract description 37
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 21
- 235000012631 food intake Nutrition 0.000 claims abstract description 19
- 230000037406 food intake Effects 0.000 claims abstract description 17
- 208000001145 Metabolic Syndrome Diseases 0.000 claims abstract description 13
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 claims abstract description 13
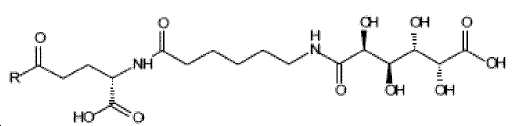
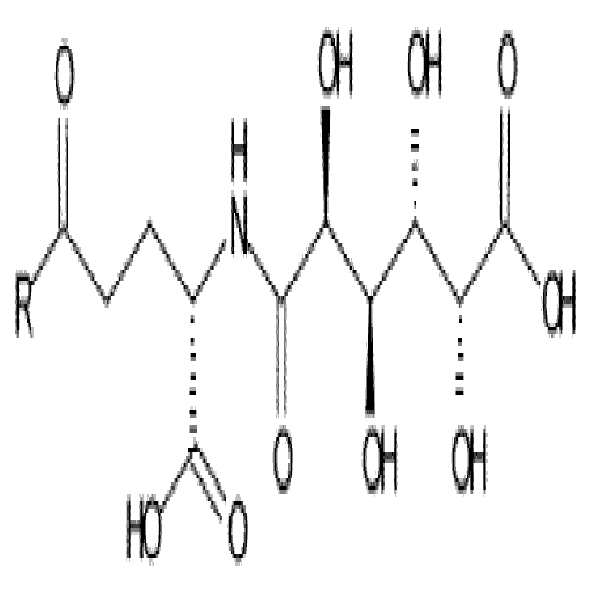
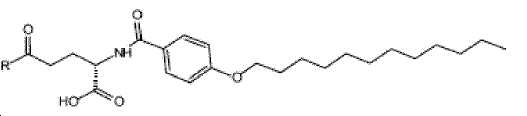
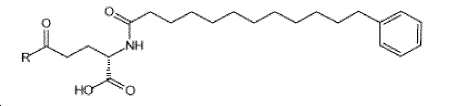
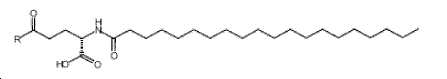
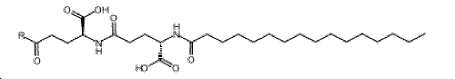
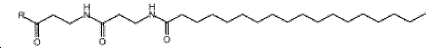
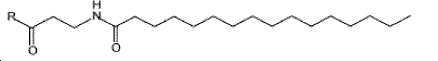
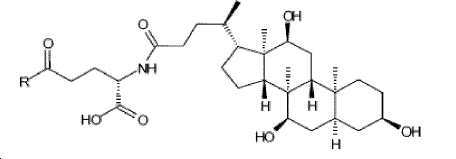
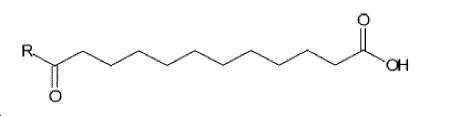
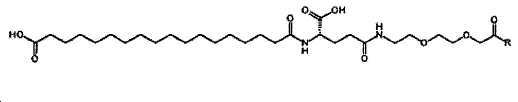
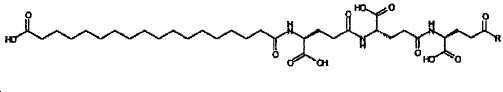
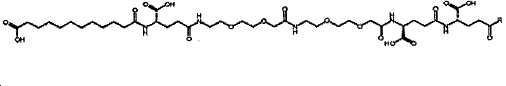
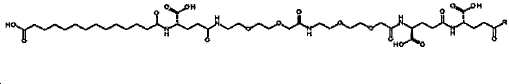
- -1 (S) -4-carboxy-4-octadecanoylamino-butyryl- Chemical group 0.000 claims description 408
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 351
- 125000000539 amino acid group Chemical group 0.000 claims description 177
- MTCFGRXMJLQNBG-UWTATZPHSA-N D-Serine Chemical compound OC[C@@H](N)C(O)=O MTCFGRXMJLQNBG-UWTATZPHSA-N 0.000 claims description 165
- 150000001875 compounds Chemical class 0.000 claims description 147
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 111
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 claims description 72
- 239000008103 glucose Substances 0.000 claims description 72
- 238000000034 method Methods 0.000 claims description 47
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 claims description 43
- 102100040918 Pro-glucagon Human genes 0.000 claims description 40
- DTHNMHAUYICORS-KTKZVXAJSA-N Glucagon-like peptide 1 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1N=CNC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 DTHNMHAUYICORS-KTKZVXAJSA-N 0.000 claims description 36
- 150000001413 amino acids Chemical class 0.000 claims description 33
- 150000003839 salts Chemical class 0.000 claims description 32
- 239000003112 inhibitor Substances 0.000 claims description 26
- 125000001312 palmitoyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 25
- QNAYBMKLOCPYGJ-UWTATZPHSA-N D-alanine Chemical compound C[C@@H](N)C(O)=O QNAYBMKLOCPYGJ-UWTATZPHSA-N 0.000 claims description 23
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 claims description 20
- 102000004877 Insulin Human genes 0.000 claims description 19
- 108090001061 Insulin Proteins 0.000 claims description 19
- 239000013543 active substance Substances 0.000 claims description 18
- 229940125396 insulin Drugs 0.000 claims description 18
- 230000001965 increasing effect Effects 0.000 claims description 17
- 239000000203 mixture Substances 0.000 claims description 17
- 102000005962 receptors Human genes 0.000 claims description 17
- 108020003175 receptors Proteins 0.000 claims description 17
- 208000001072 type 2 diabetes mellitus Diseases 0.000 claims description 17
- 239000003814 drug Substances 0.000 claims description 16
- 241000024188 Andala Species 0.000 claims description 15
- 239000008194 pharmaceutical composition Substances 0.000 claims description 15
- 239000012453 solvate Substances 0.000 claims description 14
- 230000004580 weight loss Effects 0.000 claims description 14
- 201000010099 disease Diseases 0.000 claims description 13
- 206010020772 Hypertension Diseases 0.000 claims description 12
- 125000000217 alkyl group Chemical group 0.000 claims description 12
- 239000005557 antagonist Substances 0.000 claims description 11
- 108010086246 Glucagon-Like Peptide-1 Receptor Proteins 0.000 claims description 10
- 229940044601 receptor agonist Drugs 0.000 claims description 10
- 239000000018 receptor agonist Substances 0.000 claims description 10
- 239000000126 substance Substances 0.000 claims description 10
- 102100032882 Glucagon-like peptide 1 receptor Human genes 0.000 claims description 9
- 229910052736 halogen Inorganic materials 0.000 claims description 9
- 150000002367 halogens Chemical class 0.000 claims description 9
- 230000002265 prevention Effects 0.000 claims description 9
- 229920006395 saturated elastomer Polymers 0.000 claims description 9
- 201000001320 Atherosclerosis Diseases 0.000 claims description 8
- 210000004899 c-terminal region Anatomy 0.000 claims description 8
- 125000004432 carbon atom Chemical group C* 0.000 claims description 8
- 239000003937 drug carrier Substances 0.000 claims description 8
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 claims description 8
- 125000005842 heteroatom Chemical group 0.000 claims description 8
- 229910052760 oxygen Inorganic materials 0.000 claims description 8
- 229910052717 sulfur Inorganic materials 0.000 claims description 8
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 claims description 7
- 125000002015 acyclic group Chemical group 0.000 claims description 7
- 238000007306 functionalization reaction Methods 0.000 claims description 7
- 210000001035 gastrointestinal tract Anatomy 0.000 claims description 7
- 208000015122 neurodegenerative disease Diseases 0.000 claims description 7
- 125000000734 D-serino group Chemical group [H]N([H])[C@@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 claims description 6
- 238000009472 formulation Methods 0.000 claims description 6
- 230000002496 gastric effect Effects 0.000 claims description 6
- 229930195734 saturated hydrocarbon Natural products 0.000 claims description 6
- 241000282414 Homo sapiens Species 0.000 claims description 5
- 150000002430 hydrocarbons Chemical group 0.000 claims description 5
- 230000006698 induction Effects 0.000 claims description 5
- 238000004519 manufacturing process Methods 0.000 claims description 5
- 239000002464 receptor antagonist Substances 0.000 claims description 5
- 229940044551 receptor antagonist Drugs 0.000 claims description 5
- QNAYBMKLOCPYGJ-UHFFFAOYSA-N D-alpha-Ala Natural products CC([NH3+])C([O-])=O QNAYBMKLOCPYGJ-UHFFFAOYSA-N 0.000 claims description 4
- 208000032928 Dyslipidaemia Diseases 0.000 claims description 4
- 229940089838 Glucagon-like peptide 1 receptor agonist Drugs 0.000 claims description 4
- 108010041872 Islet Amyloid Polypeptide Chemical class 0.000 claims description 4
- 102000036770 Islet Amyloid Polypeptide Human genes 0.000 claims description 4
- 108010092277 Leptin Proteins 0.000 claims description 4
- 102000016267 Leptin Human genes 0.000 claims description 4
- 208000017170 Lipid metabolism disease Diseases 0.000 claims description 4
- 206010033307 Overweight Diseases 0.000 claims description 4
- 125000003118 aryl group Chemical group 0.000 claims description 4
- 229960001058 bupropion Drugs 0.000 claims description 4
- SNPPWIUOZRMYNY-UHFFFAOYSA-N bupropion Chemical compound CC(C)(C)NC(C)C(=O)C1=CC=CC(Cl)=C1 SNPPWIUOZRMYNY-UHFFFAOYSA-N 0.000 claims description 4
- 208000029078 coronary artery disease Diseases 0.000 claims description 4
- 230000003247 decreasing effect Effects 0.000 claims description 4
- 230000003111 delayed effect Effects 0.000 claims description 4
- 229960003692 gamma aminobutyric acid Drugs 0.000 claims description 4
- BTCSSZJGUNDROE-UHFFFAOYSA-N gamma-aminobutyric acid Chemical compound NCCCC(O)=O BTCSSZJGUNDROE-UHFFFAOYSA-N 0.000 claims description 4
- 239000003877 glucagon like peptide 1 receptor agonist Substances 0.000 claims description 4
- 201000001421 hyperglycemia Diseases 0.000 claims description 4
- 229940039781 leptin Drugs 0.000 claims description 4
- NRYBAZVQPHGZNS-ZSOCWYAHSA-N leptin Chemical compound O=C([C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CC(C)C)CCSC)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CS)C(O)=O NRYBAZVQPHGZNS-ZSOCWYAHSA-N 0.000 claims description 4
- SLXKOJJOQWFEFD-UHFFFAOYSA-N 6-aminohexanoic acid Chemical compound NCCCCCC(O)=O SLXKOJJOQWFEFD-UHFFFAOYSA-N 0.000 claims description 3
- 229940127291 Calcium channel antagonist Drugs 0.000 claims description 3
- 101800001586 Ghrelin Proteins 0.000 claims description 3
- 102400000442 Ghrelin-28 Human genes 0.000 claims description 3
- 206010018429 Glucose tolerance impaired Diseases 0.000 claims description 3
- 108091016366 Histone-lysine N-methyltransferase EHMT1 Proteins 0.000 claims description 3
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 claims description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N acetic acid Substances CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 claims description 3
- 235000019789 appetite Nutrition 0.000 claims description 3
- 230000036528 appetite Effects 0.000 claims description 3
- UCMIRNVEIXFBKS-UHFFFAOYSA-N beta-alanine Chemical compound NCCC(O)=O UCMIRNVEIXFBKS-UHFFFAOYSA-N 0.000 claims description 3
- 125000004122 cyclic group Chemical group 0.000 claims description 3
- 230000006735 deficit Effects 0.000 claims description 3
- GNKDKYIHGQKHHM-RJKLHVOGSA-N ghrelin Chemical compound C([C@H](NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)CN)COC(=O)CCCCCCC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1N=CNC=1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C1=CC=CC=C1 GNKDKYIHGQKHHM-RJKLHVOGSA-N 0.000 claims description 3
- 239000004026 insulin derivative Substances 0.000 claims description 3
- 230000036186 satiety Effects 0.000 claims description 3
- 235000019627 satiety Nutrition 0.000 claims description 3
- 229930195735 unsaturated hydrocarbon Natural products 0.000 claims description 3
- HSINOMROUCMIEA-FGVHQWLLSA-N (2s,4r)-4-[(3r,5s,6r,7r,8s,9s,10s,13r,14s,17r)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]-2-methylpentanoic acid Chemical compound C([C@@]12C)C[C@@H](O)C[C@H]1[C@@H](CC)[C@@H](O)[C@@H]1[C@@H]2CC[C@]2(C)[C@@H]([C@H](C)C[C@H](C)C(O)=O)CC[C@H]21 HSINOMROUCMIEA-FGVHQWLLSA-N 0.000 claims description 2
- 102000006902 5-HT2C Serotonin Receptor Human genes 0.000 claims description 2
- 108010072553 5-HT2C Serotonin Receptor Proteins 0.000 claims description 2
- 239000005541 ACE inhibitor Substances 0.000 claims description 2
- 101100460776 Arabidopsis thaliana NPY2 gene Proteins 0.000 claims description 2
- 101100460788 Arabidopsis thaliana NPY5 gene Proteins 0.000 claims description 2
- 208000032841 Bulimia Diseases 0.000 claims description 2
- 206010006550 Bulimia nervosa Diseases 0.000 claims description 2
- 102100031151 C-C chemokine receptor type 2 Human genes 0.000 claims description 2
- 101710149815 C-C chemokine receptor type 2 Proteins 0.000 claims description 2
- 206010007558 Cardiac failure chronic Diseases 0.000 claims description 2
- 102100036869 Diacylglycerol O-acyltransferase 1 Human genes 0.000 claims description 2
- 108050004099 Diacylglycerol O-acyltransferase 1 Proteins 0.000 claims description 2
- 206010061818 Disease progression Diseases 0.000 claims description 2
- 102100026148 Free fatty acid receptor 1 Human genes 0.000 claims description 2
- 208000002705 Glucose Intolerance Diseases 0.000 claims description 2
- 102000003638 Glucose-6-Phosphatase Human genes 0.000 claims description 2
- 108010086800 Glucose-6-Phosphatase Proteins 0.000 claims description 2
- 229920002527 Glycogen Polymers 0.000 claims description 2
- 101000912510 Homo sapiens Free fatty acid receptor 1 Proteins 0.000 claims description 2
- 101001098868 Homo sapiens Proprotein convertase subtilisin/kexin type 9 Proteins 0.000 claims description 2
- DEFJQIDDEAULHB-IMJSIDKUSA-N L-alanyl-L-alanine Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(O)=O DEFJQIDDEAULHB-IMJSIDKUSA-N 0.000 claims description 2
- 244000137850 Marrubium vulgare Species 0.000 claims description 2
- 101150111774 NPY5R gene Proteins 0.000 claims description 2
- 102000018886 Pancreatic Polypeptide Human genes 0.000 claims description 2
- 102100038955 Proprotein convertase subtilisin/kexin type 9 Human genes 0.000 claims description 2
- 229940123518 Sodium/glucose cotransporter 2 inhibitor Drugs 0.000 claims description 2
- 229940123464 Thiazolidinedione Drugs 0.000 claims description 2
- 102000008316 Type 4 Melanocortin Receptor Human genes 0.000 claims description 2
- 108010021436 Type 4 Melanocortin Receptor Proteins 0.000 claims description 2
- 101710204865 Tyrosine-protein phosphatase 1 Proteins 0.000 claims description 2
- 238000010521 absorption reaction Methods 0.000 claims description 2
- 230000002378 acidificating effect Effects 0.000 claims description 2
- 239000000670 adrenergic alpha-2 receptor antagonist Substances 0.000 claims description 2
- 239000000674 adrenergic antagonist Substances 0.000 claims description 2
- 239000002333 angiotensin II receptor antagonist Substances 0.000 claims description 2
- 229940044094 angiotensin-converting-enzyme inhibitor Drugs 0.000 claims description 2
- 229940127218 antiplatelet drug Drugs 0.000 claims description 2
- 235000021407 appetite control Nutrition 0.000 claims description 2
- 239000002876 beta blocker Substances 0.000 claims description 2
- 229940097320 beta blocking agent Drugs 0.000 claims description 2
- 239000003613 bile acid Substances 0.000 claims description 2
- 239000011230 binding agent Substances 0.000 claims description 2
- 238000002591 computed tomography Methods 0.000 claims description 2
- 239000002934 diuretic Substances 0.000 claims description 2
- 229940030606 diuretics Drugs 0.000 claims description 2
- 239000002792 enkephalinase inhibitor Substances 0.000 claims description 2
- 229940096919 glycogen Drugs 0.000 claims description 2
- 230000002209 hydrophobic effect Effects 0.000 claims description 2
- 230000005764 inhibitory process Effects 0.000 claims description 2
- 230000037356 lipid metabolism Effects 0.000 claims description 2
- 210000004185 liver Anatomy 0.000 claims description 2
- AHLBNYSZXLDEJQ-FWEHEUNISA-N orlistat Chemical compound CCCCCCCCCCC[C@H](OC(=O)[C@H](CC(C)C)NC=O)C[C@@H]1OC(=O)[C@H]1CCCCCC AHLBNYSZXLDEJQ-FWEHEUNISA-N 0.000 claims description 2
- 229960001243 orlistat Drugs 0.000 claims description 2
- 238000007911 parenteral administration Methods 0.000 claims description 2
- 239000003428 phospholipase inhibitor Substances 0.000 claims description 2
- 239000000106 platelet aggregation inhibitor Substances 0.000 claims description 2
- 229960003611 pramlintide Drugs 0.000 claims description 2
- 108010029667 pramlintide Proteins 0.000 claims description 2
- NRKVKVQDUCJPIZ-MKAGXXMWSA-N pramlintide acetate Chemical compound C([C@@H](C(=O)NCC(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CS)NC(=O)[C@@H](N)CCCCN)[C@@H](C)O)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 NRKVKVQDUCJPIZ-MKAGXXMWSA-N 0.000 claims description 2
- 238000011084 recovery Methods 0.000 claims description 2
- UNAANXDKBXWMLN-UHFFFAOYSA-N sibutramine Chemical compound C=1C=C(Cl)C=CC=1C1(C(N(C)C)CC(C)C)CCC1 UNAANXDKBXWMLN-UHFFFAOYSA-N 0.000 claims description 2
- 229960004425 sibutramine Drugs 0.000 claims description 2
- 125000003696 stearoyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 2
- 238000002560 therapeutic procedure Methods 0.000 claims description 2
- 101710198884 GATA-type zinc finger protein 1 Proteins 0.000 claims 3
- CMDCQWBXYHWZTH-UHFFFAOYSA-N [4-amino-2-(propylamino)-1,3-thiazol-5-yl]-pyridin-3-ylmethanol Chemical compound S1C(NCCC)=NC(N)=C1C(O)C1=CC=CN=C1 CMDCQWBXYHWZTH-UHFFFAOYSA-N 0.000 claims 3
- UPCIBFUJJLCOQG-UHFFFAOYSA-L ethyl-[2-[2-[ethyl(dimethyl)azaniumyl]ethyl-methylamino]ethyl]-dimethylazanium;dibromide Chemical compound [Br-].[Br-].CC[N+](C)(C)CCN(C)CC[N+](C)(C)CC UPCIBFUJJLCOQG-UHFFFAOYSA-L 0.000 claims 2
- 102000040430 polynucleotide Human genes 0.000 claims 2
- 108091033319 polynucleotide Proteins 0.000 claims 2
- 239000002157 polynucleotide Substances 0.000 claims 2
- SFLSHLFXELFNJZ-QMMMGPOBSA-N (-)-norepinephrine Chemical compound NC[C@H](O)C1=CC=C(O)C(O)=C1 SFLSHLFXELFNJZ-QMMMGPOBSA-N 0.000 claims 1
- ZOBPZXTWZATXDG-UHFFFAOYSA-N 1,3-thiazolidine-2,4-dione Chemical compound O=C1CSC(=O)N1 ZOBPZXTWZATXDG-UHFFFAOYSA-N 0.000 claims 1
- 125000004198 2-fluorophenyl group Chemical group [H]C1=C([H])C(F)=C(*)C([H])=C1[H] 0.000 claims 1
- IGRCWJPBLWGNPX-UHFFFAOYSA-N 3-(2-chlorophenyl)-n-(4-chlorophenyl)-n,5-dimethyl-1,2-oxazole-4-carboxamide Chemical compound C=1C=C(Cl)C=CC=1N(C)C(=O)C1=C(C)ON=C1C1=CC=CC=C1Cl IGRCWJPBLWGNPX-UHFFFAOYSA-N 0.000 claims 1
- SWLAMJPTOQZTAE-UHFFFAOYSA-N 4-[2-[(5-chloro-2-methoxybenzoyl)amino]ethyl]benzoic acid Chemical class COC1=CC=C(Cl)C=C1C(=O)NCCC1=CC=C(C(O)=O)C=C1 SWLAMJPTOQZTAE-UHFFFAOYSA-N 0.000 claims 1
- HFDKKNHCYWNNNQ-YOGANYHLSA-N 75976-10-2 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@@H](NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](C)N)C(C)C)[C@@H](C)O)C1=CC=C(O)C=C1 HFDKKNHCYWNNNQ-YOGANYHLSA-N 0.000 claims 1
- 102000005666 Apolipoprotein A-I Human genes 0.000 claims 1
- 108010059886 Apolipoprotein A-I Proteins 0.000 claims 1
- 101001025319 Arabidopsis thaliana High mobility group B protein 1 Proteins 0.000 claims 1
- 101100460782 Arabidopsis thaliana NPY4 gene Proteins 0.000 claims 1
- OBMZMSLWNNWEJA-XNCRXQDQSA-N C1=CC=2C(C[C@@H]3NC(=O)[C@@H](NC(=O)[C@H](NC(=O)N(CC#CCN(CCCC[C@H](NC(=O)[C@@H](CC4=CC=CC=C4)NC3=O)C(=O)N)CC=C)NC(=O)[C@@H](N)C)CC3=CNC4=C3C=CC=C4)C)=CNC=2C=C1 Chemical compound C1=CC=2C(C[C@@H]3NC(=O)[C@@H](NC(=O)[C@H](NC(=O)N(CC#CCN(CCCC[C@H](NC(=O)[C@@H](CC4=CC=CC=C4)NC3=O)C(=O)N)CC=C)NC(=O)[C@@H](N)C)CC3=CNC4=C3C=CC=C4)C)=CNC=2C=C1 OBMZMSLWNNWEJA-XNCRXQDQSA-N 0.000 claims 1
- PWDLDBWXTVILPC-WGAVTJJLSA-N CC(C)(N)CC1=CC=CC=C1.C1O[C@@]2(COS(N)(=O)=O)OC(C)(C)O[C@H]2[C@@H]2OC(C)(C)O[C@@H]21 Chemical compound CC(C)(N)CC1=CC=CC=C1.C1O[C@@]2(COS(N)(=O)=O)OC(C)(C)O[C@H]2[C@@H]2OC(C)(C)O[C@@H]21 PWDLDBWXTVILPC-WGAVTJJLSA-N 0.000 claims 1
- 229940123239 Cholesterol synthesis inhibitor Drugs 0.000 claims 1
- 101000783577 Dendroaspis angusticeps Thrombostatin Proteins 0.000 claims 1
- 101000783578 Dendroaspis jamesoni kaimosae Dendroaspin Proteins 0.000 claims 1
- 102100040134 Free fatty acid receptor 4 Human genes 0.000 claims 1
- 102000027487 Fructose-Bisphosphatase Human genes 0.000 claims 1
- 108010017464 Fructose-Bisphosphatase Proteins 0.000 claims 1
- 102100025353 G-protein coupled bile acid receptor 1 Human genes 0.000 claims 1
- 229940100607 GPR119 agonist Drugs 0.000 claims 1
- 229930183217 Genin Natural products 0.000 claims 1
- 229940123232 Glucagon receptor agonist Drugs 0.000 claims 1
- 206010019708 Hepatic steatosis Diseases 0.000 claims 1
- 101000890672 Homo sapiens Free fatty acid receptor 4 Proteins 0.000 claims 1
- 101000857733 Homo sapiens G-protein coupled bile acid receptor 1 Proteins 0.000 claims 1
- 101000887427 Homo sapiens Probable G-protein coupled receptor 142 Proteins 0.000 claims 1
- 102100021711 Ileal sodium/bile acid cotransporter Human genes 0.000 claims 1
- 101710156096 Ileal sodium/bile acid cotransporter Proteins 0.000 claims 1
- 102000000853 LDL receptors Human genes 0.000 claims 1
- 108010001831 LDL receptors Proteins 0.000 claims 1
- 229940127470 Lipase Inhibitors Drugs 0.000 claims 1
- 229940126033 PPAR agonist Drugs 0.000 claims 1
- 101710176384 Peptide 1 Proteins 0.000 claims 1
- 102100038824 Peroxisome proliferator-activated receptor delta Human genes 0.000 claims 1
- 229940122907 Phosphatase inhibitor Drugs 0.000 claims 1
- 102100039861 Probable G-protein coupled receptor 142 Human genes 0.000 claims 1
- 108091006300 SLC2A4 Proteins 0.000 claims 1
- 108091006277 SLC5A1 Proteins 0.000 claims 1
- 108091006269 SLC5A2 Proteins 0.000 claims 1
- 102000058090 Sodium-Glucose Transporter 1 Human genes 0.000 claims 1
- 102000058081 Sodium-Glucose Transporter 2 Human genes 0.000 claims 1
- 102000004117 Somatostatin receptor 3 Human genes 0.000 claims 1
- 108090000674 Somatostatin receptor 3 Proteins 0.000 claims 1
- 229940100389 Sulfonylurea Drugs 0.000 claims 1
- 101000983124 Sus scrofa Pancreatic prohormone precursor Proteins 0.000 claims 1
- 229940121886 Thyroid hormone receptor agonist Drugs 0.000 claims 1
- 102100033451 Thyroid hormone receptor beta Human genes 0.000 claims 1
- KJADKKWYZYXHBB-XBWDGYHZSA-N Topiramic acid Chemical compound C1O[C@@]2(COS(N)(=O)=O)OC(C)(C)O[C@H]2[C@@H]2OC(C)(C)O[C@@H]21 KJADKKWYZYXHBB-XBWDGYHZSA-N 0.000 claims 1
- 238000002835 absorbance Methods 0.000 claims 1
- OIPILFWXSMYKGL-UHFFFAOYSA-N acetylcholine Chemical compound CC(=O)OCC[N+](C)(C)C OIPILFWXSMYKGL-UHFFFAOYSA-N 0.000 claims 1
- 229960004373 acetylcholine Drugs 0.000 claims 1
- 239000000048 adrenergic agonist Substances 0.000 claims 1
- 229940126157 adrenergic receptor agonist Drugs 0.000 claims 1
- 108090000861 alpha Adrenergic Receptors Proteins 0.000 claims 1
- 102000004305 alpha Adrenergic Receptors Human genes 0.000 claims 1
- 229940121369 angiogenesis inhibitor Drugs 0.000 claims 1
- 239000004037 angiogenesis inhibitor Substances 0.000 claims 1
- 239000002220 antihypertensive agent Substances 0.000 claims 1
- 229940030600 antihypertensive agent Drugs 0.000 claims 1
- 229940125388 beta agonist Drugs 0.000 claims 1
- 125000002619 bicyclic group Chemical group 0.000 claims 1
- 239000000480 calcium channel blocker Substances 0.000 claims 1
- 230000001906 cholesterol absorption Effects 0.000 claims 1
- 239000000544 cholinesterase inhibitor Substances 0.000 claims 1
- CYQFCXCEBYINGO-IAGOWNOFSA-N delta1-THC Chemical compound C1=C(C)CC[C@H]2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3[C@@H]21 CYQFCXCEBYINGO-IAGOWNOFSA-N 0.000 claims 1
- HSUGRBWQSSZJOP-RTWAWAEBSA-N diltiazem Chemical compound C1=CC(OC)=CC=C1[C@H]1[C@@H](OC(C)=O)C(=O)N(CCN(C)C)C2=CC=CC=C2S1 HSUGRBWQSSZJOP-RTWAWAEBSA-N 0.000 claims 1
- 229960004166 diltiazem Drugs 0.000 claims 1
- 230000005750 disease progression Effects 0.000 claims 1
- 230000003028 elevating effect Effects 0.000 claims 1
- 210000002950 fibroblast Anatomy 0.000 claims 1
- 239000003626 gastrointestinal polypeptide Substances 0.000 claims 1
- 229940124828 glucokinase activator Drugs 0.000 claims 1
- 229940125425 inverse agonist Drugs 0.000 claims 1
- 229950004994 meglitinide Drugs 0.000 claims 1
- 108700008455 metreleptin Proteins 0.000 claims 1
- 229960000668 metreleptin Drugs 0.000 claims 1
- 229960002748 norepinephrine Drugs 0.000 claims 1
- SFLSHLFXELFNJZ-UHFFFAOYSA-N norepinephrine Natural products NCC(O)C1=CC=C(O)C(O)=C1 SFLSHLFXELFNJZ-UHFFFAOYSA-N 0.000 claims 1
- 229940012843 omega-3 fatty acid Drugs 0.000 claims 1
- 235000020660 omega-3 fatty acid Nutrition 0.000 claims 1
- 239000004031 partial agonist Substances 0.000 claims 1
- 239000002307 peroxisome proliferator activated receptor agonist Substances 0.000 claims 1
- 108091008765 peroxisome proliferator-activated receptors β/δ Proteins 0.000 claims 1
- 239000003358 phospholipase A2 inhibitor Substances 0.000 claims 1
- 239000000651 prodrug Substances 0.000 claims 1
- 229940002612 prodrug Drugs 0.000 claims 1
- YROXIXLRRCOBKF-UHFFFAOYSA-N sulfonylurea Chemical class OC(=N)N=S(=O)=O YROXIXLRRCOBKF-UHFFFAOYSA-N 0.000 claims 1
- 230000009885 systemic effect Effects 0.000 claims 1
- 108091008762 thyroid hormone receptors ß Proteins 0.000 claims 1
- 229960004394 topiramate Drugs 0.000 claims 1
- 108010011459 Exenatide Proteins 0.000 abstract description 27
- 230000009467 reduction Effects 0.000 abstract description 5
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 426
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 419
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 222
- UMHUHHJMEXNSIV-CIUDSAMLSA-N Asp-Leu-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC(O)=O UMHUHHJMEXNSIV-CIUDSAMLSA-N 0.000 description 178
- GNRMAQSIROFNMI-IXOXFDKPSA-N Phe-Thr-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O GNRMAQSIROFNMI-IXOXFDKPSA-N 0.000 description 178
- FDMKYQQYJKYCLV-GUBZILKMSA-N Pro-Pro-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 FDMKYQQYJKYCLV-GUBZILKMSA-N 0.000 description 178
- UQFYNFTYDHUIMI-WHFBIAKZSA-N Ser-Gly-Ala Chemical compound OC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](N)CO UQFYNFTYDHUIMI-WHFBIAKZSA-N 0.000 description 177
- JXFLPKSDLDEOQK-JHEQGTHGSA-N Gln-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCC(N)=O JXFLPKSDLDEOQK-JHEQGTHGSA-N 0.000 description 175
- QQUJSUFWEDZQQY-AVGNSLFASA-N Lys-Gln-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(O)=O)CCCCN QQUJSUFWEDZQQY-AVGNSLFASA-N 0.000 description 175
- HAOUOFNNJJLVNS-BQBZGAKWSA-N Gly-Pro-Ser Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O HAOUOFNNJJLVNS-BQBZGAKWSA-N 0.000 description 126
- 108010034529 leucyl-lysine Proteins 0.000 description 121
- HGJREIGJLUQBTJ-SZMVWBNQSA-N Glu-Trp-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC(C)C)C(O)=O HGJREIGJLUQBTJ-SZMVWBNQSA-N 0.000 description 120
- PCJOFZYFFMBZKC-PCBIJLKTSA-N Asp-Phe-Ile Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O PCJOFZYFFMBZKC-PCBIJLKTSA-N 0.000 description 103
- 108010076324 alanyl-glycyl-glycine Proteins 0.000 description 101
- GXCSUJQOECMKPV-CIUDSAMLSA-N Arg-Ala-Gln Chemical compound C[C@H](NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O GXCSUJQOECMKPV-CIUDSAMLSA-N 0.000 description 96
- 108010009298 lysylglutamic acid Proteins 0.000 description 96
- 229940024606 amino acid Drugs 0.000 description 92
- 235000001014 amino acid Nutrition 0.000 description 92
- 108010003700 lysyl aspartic acid Proteins 0.000 description 80
- 210000004369 blood Anatomy 0.000 description 65
- 239000008280 blood Substances 0.000 description 65
- LEHPJMKVGFPSSP-ZQINRCPSSA-N Ile-Glu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)[C@@H](C)CC)C(O)=O)=CNC2=C1 LEHPJMKVGFPSSP-ZQINRCPSSA-N 0.000 description 61
- 241000699670 Mus sp. Species 0.000 description 56
- XFIHDSBIPWEYJJ-YUMQZZPRSA-N Lys-Ala-Gly Chemical compound OC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN XFIHDSBIPWEYJJ-YUMQZZPRSA-N 0.000 description 55
- 108010051307 glycyl-glycyl-proline Proteins 0.000 description 55
- BUEFQXUHTUZXHR-LURJTMIESA-N Gly-Gly-Pro zwitterion Chemical compound NCC(=O)NCC(=O)N1CCC[C@H]1C(O)=O BUEFQXUHTUZXHR-LURJTMIESA-N 0.000 description 54
- ZRHDPZAAWLXXIR-SRVKXCTJSA-N Leu-Lys-Ala Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O ZRHDPZAAWLXXIR-SRVKXCTJSA-N 0.000 description 53
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 52
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 51
- 108010031719 prolyl-serine Proteins 0.000 description 48
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 47
- HQVDJTYKCMIWJP-YUMQZZPRSA-N Lys-Asn-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O HQVDJTYKCMIWJP-YUMQZZPRSA-N 0.000 description 41
- 229920005989 resin Polymers 0.000 description 40
- 239000011347 resin Substances 0.000 description 40
- JKPGHIQCHIIRMS-AVGNSLFASA-N Gln-Asp-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)N)N JKPGHIQCHIIRMS-AVGNSLFASA-N 0.000 description 39
- 230000000694 effects Effects 0.000 description 36
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 35
- 101800000224 Glucagon-like peptide 1 Proteins 0.000 description 31
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 31
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 30
- FZIJIFCXUCZHOL-CIUDSAMLSA-N Lys-Ala-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN FZIJIFCXUCZHOL-CIUDSAMLSA-N 0.000 description 30
- 108010025153 lysyl-alanyl-alanine Proteins 0.000 description 30
- FUOOLUPWFVMBKG-UHFFFAOYSA-N 2-Aminoisobutyric acid Chemical compound CC(C)(N)C(O)=O FUOOLUPWFVMBKG-UHFFFAOYSA-N 0.000 description 29
- 239000002904 solvent Substances 0.000 description 28
- 102000004196 processed proteins & peptides Human genes 0.000 description 25
- 108010063919 Glucagon Receptors Proteins 0.000 description 24
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 24
- 102100040890 Glucagon receptor Human genes 0.000 description 23
- 230000015572 biosynthetic process Effects 0.000 description 23
- 229960001519 exenatide Drugs 0.000 description 22
- 238000003786 synthesis reaction Methods 0.000 description 21
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 21
- 238000007920 subcutaneous administration Methods 0.000 description 20
- 230000037396 body weight Effects 0.000 description 19
- YJIUYQKQBBQYHZ-ACZMJKKPSA-N Gln-Ala-Ala Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(O)=O YJIUYQKQBBQYHZ-ACZMJKKPSA-N 0.000 description 18
- 108010044940 alanylglutamine Proteins 0.000 description 18
- 125000003277 amino group Chemical group 0.000 description 18
- 125000003275 alpha amino acid group Chemical group 0.000 description 17
- 241001465754 Metazoa Species 0.000 description 15
- 125000003088 (fluoren-9-ylmethoxy)carbonyl group Chemical group 0.000 description 14
- SYRTUBLKWNDSDK-DKIMLUQUSA-N Leu-Phe-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O SYRTUBLKWNDSDK-DKIMLUQUSA-N 0.000 description 14
- 210000004027 cell Anatomy 0.000 description 14
- 238000012360 testing method Methods 0.000 description 14
- DTQVDTLACAAQTR-UHFFFAOYSA-N trifluoroacetic acid Substances OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 14
- ODKSFYDXXFIFQN-BYPYZUCNSA-N L-arginine Chemical compound OC(=O)[C@@H](N)CCCN=C(N)N ODKSFYDXXFIFQN-BYPYZUCNSA-N 0.000 description 13
- 238000011421 subcutaneous treatment Methods 0.000 description 13
- 238000011785 NMRI mouse Methods 0.000 description 12
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 11
- 230000004913 activation Effects 0.000 description 11
- 230000001154 acute effect Effects 0.000 description 11
- 235000009200 high fat diet Nutrition 0.000 description 11
- 235000018102 proteins Nutrition 0.000 description 11
- 102000004169 proteins and genes Human genes 0.000 description 11
- 108090000623 proteins and genes Proteins 0.000 description 11
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 10
- YSDQQAXHVYUZIW-QCIJIYAXSA-N Liraglutide Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCC)C(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=C(O)C=C1 YSDQQAXHVYUZIW-QCIJIYAXSA-N 0.000 description 10
- 210000000577 adipose tissue Anatomy 0.000 description 10
- 230000002950 deficient Effects 0.000 description 10
- 102000005861 leptin receptors Human genes 0.000 description 10
- 108010019813 leptin receptors Proteins 0.000 description 10
- 125000006239 protecting group Chemical group 0.000 description 10
- 238000010532 solid phase synthesis reaction Methods 0.000 description 10
- SHAUZYVSXAMYAZ-JYJNAYRXSA-N Gln-Leu-Phe Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)NC(=O)[C@H](CCC(=O)N)N SHAUZYVSXAMYAZ-JYJNAYRXSA-N 0.000 description 9
- 108010019598 Liraglutide Proteins 0.000 description 9
- 206010002022 amyloidosis Diseases 0.000 description 9
- 230000008859 change Effects 0.000 description 9
- 238000002953 preparative HPLC Methods 0.000 description 9
- NWZSZGALRFJKBT-KNIFDHDWSA-N (2s)-2,6-diaminohexanoic acid;(2s)-2-hydroxybutanedioic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O.NCCCC[C@H](N)C(O)=O NWZSZGALRFJKBT-KNIFDHDWSA-N 0.000 description 8
- BUANFPRKJKJSRR-ACZMJKKPSA-N Ala-Ala-Gln Chemical compound C[C@H]([NH3+])C(=O)N[C@@H](C)C(=O)N[C@H](C([O-])=O)CCC(N)=O BUANFPRKJKJSRR-ACZMJKKPSA-N 0.000 description 8
- 238000001061 Dunnett's test Methods 0.000 description 8
- 239000004472 Lysine Substances 0.000 description 8
- 241000699666 Mus <mouse, genus> Species 0.000 description 8
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 8
- 239000004480 active ingredient Substances 0.000 description 8
- 150000001408 amides Chemical class 0.000 description 8
- 235000005911 diet Nutrition 0.000 description 8
- 210000003736 gastrointestinal content Anatomy 0.000 description 8
- IPCSVZSSVZVIGE-UHFFFAOYSA-N hexadecanoic acid Chemical compound CCCCCCCCCCCCCCCC(O)=O IPCSVZSSVZVIGE-UHFFFAOYSA-N 0.000 description 8
- IKDUDTNKRLTJSI-UHFFFAOYSA-N hydrazine monohydrate Substances O.NN IKDUDTNKRLTJSI-UHFFFAOYSA-N 0.000 description 8
- 125000005647 linker group Chemical group 0.000 description 8
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 8
- 229960002701 liraglutide Drugs 0.000 description 8
- 238000005259 measurement Methods 0.000 description 8
- 239000002953 phosphate buffered saline Substances 0.000 description 8
- 239000002994 raw material Substances 0.000 description 8
- 239000003643 water by type Substances 0.000 description 8
- IXHPIPUIOSSAIS-NSHDSACASA-N (2s)-2-[(2-methylpropan-2-yl)oxycarbonylamino]-3-[1-[(2-methylpropan-2-yl)oxycarbonyl]imidazol-4-yl]propanoic acid Chemical group CC(C)(C)OC(=O)N[C@H](C(O)=O)CC1=CN(C(=O)OC(C)(C)C)C=N1 IXHPIPUIOSSAIS-NSHDSACASA-N 0.000 description 7
- 239000012043 crude product Substances 0.000 description 7
- 208000035475 disorder Diseases 0.000 description 7
- 230000030136 gastric emptying Effects 0.000 description 7
- 125000000623 heterocyclic group Chemical group 0.000 description 7
- 238000004128 high performance liquid chromatography Methods 0.000 description 7
- 239000000047 product Substances 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- RUFHOVYUYSNDNY-ACZMJKKPSA-N Glu-Ala-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCC(O)=O RUFHOVYUYSNDNY-ACZMJKKPSA-N 0.000 description 6
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- 239000002253 acid Substances 0.000 description 6
- 230000009471 action Effects 0.000 description 6
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 6
- 238000000540 analysis of variance Methods 0.000 description 6
- 238000004458 analytical method Methods 0.000 description 6
- 239000003795 chemical substances by application Substances 0.000 description 6
- 235000014113 dietary fatty acids Nutrition 0.000 description 6
- 229940079593 drug Drugs 0.000 description 6
- 238000002474 experimental method Methods 0.000 description 6
- 229930195729 fatty acid Natural products 0.000 description 6
- 239000000194 fatty acid Substances 0.000 description 6
- 150000004665 fatty acids Chemical class 0.000 description 6
- 229930195712 glutamate Natural products 0.000 description 6
- 238000011068 loading method Methods 0.000 description 6
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 description 6
- 238000010647 peptide synthesis reaction Methods 0.000 description 6
- 230000004584 weight gain Effects 0.000 description 6
- 235000019786 weight gain Nutrition 0.000 description 6
- NNQHEEQNPQYPGL-FXQIFTODSA-N Gln-Ala-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(O)=O NNQHEEQNPQYPGL-FXQIFTODSA-N 0.000 description 5
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 5
- 238000005481 NMR spectroscopy Methods 0.000 description 5
- IVDFVBVIVLJJHR-LKXGYXEUSA-N Thr-Ser-Asp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O IVDFVBVIVLJJHR-LKXGYXEUSA-N 0.000 description 5
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 5
- 125000000753 cycloalkyl group Chemical group 0.000 description 5
- 230000037213 diet Effects 0.000 description 5
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 5
- 238000005516 engineering process Methods 0.000 description 5
- 238000000338 in vitro Methods 0.000 description 5
- 238000001727 in vivo Methods 0.000 description 5
- 230000000968 intestinal effect Effects 0.000 description 5
- 229930182817 methionine Natural products 0.000 description 5
- 230000028327 secretion Effects 0.000 description 5
- 239000007787 solid Substances 0.000 description 5
- 235000000891 standard diet Nutrition 0.000 description 5
- 230000001225 therapeutic effect Effects 0.000 description 5
- OGNSCSPNOLGXSM-UHFFFAOYSA-N 2,4-diaminobutyric acid Chemical compound NCCC(N)C(O)=O OGNSCSPNOLGXSM-UHFFFAOYSA-N 0.000 description 4
- 206010002091 Anaesthesia Diseases 0.000 description 4
- KXDHJXZQYSOELW-UHFFFAOYSA-N Carbamic acid Chemical class NC(O)=O KXDHJXZQYSOELW-UHFFFAOYSA-N 0.000 description 4
- HTQBXNHDCUEHJF-XWLPCZSASA-N Exenatide Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)NCC(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 HTQBXNHDCUEHJF-XWLPCZSASA-N 0.000 description 4
- RGXXLQWXBFNXTG-CIUDSAMLSA-N Gln-Arg-Ala Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(O)=O RGXXLQWXBFNXTG-CIUDSAMLSA-N 0.000 description 4
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical compound NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 4
- 208000013016 Hypoglycemia Diseases 0.000 description 4
- 108010057186 Insulin Glargine Proteins 0.000 description 4
- COCFEDIXXNGUNL-RFKWWTKHSA-N Insulin glargine Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@H]1CSSC[C@H]2C(=O)N[C@H](C(=O)N[C@@H](CO)C(=O)N[C@H](C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3C=CC(O)=CC=3)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3NC=NC=3)NC(=O)[C@H](CO)NC(=O)CNC1=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(=O)NCC(O)=O)=O)CSSC[C@@H](C(N2)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)CN)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C=CC=CC=1)C(C)C)C1=CN=CN1 COCFEDIXXNGUNL-RFKWWTKHSA-N 0.000 description 4
- 125000000998 L-alanino group Chemical group [H]N([*])[C@](C([H])([H])[H])([H])C(=O)O[H] 0.000 description 4
- VCHVSKNMTXWIIP-SRVKXCTJSA-N Leu-Lys-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O VCHVSKNMTXWIIP-SRVKXCTJSA-N 0.000 description 4
- GAOJCVKPIGHTGO-UWVGGRQHSA-N Lys-Arg-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O GAOJCVKPIGHTGO-UWVGGRQHSA-N 0.000 description 4
- QUCDKEKDPYISNX-HJGDQZAQSA-N Lys-Asn-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QUCDKEKDPYISNX-HJGDQZAQSA-N 0.000 description 4
- XIZQPFCRXLUNMK-BZSNNMDCSA-N Lys-Leu-Phe Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)NC(=O)[C@H](CCCCN)N XIZQPFCRXLUNMK-BZSNNMDCSA-N 0.000 description 4
- 235000021314 Palmitic acid Nutrition 0.000 description 4
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 4
- 230000037005 anaesthesia Effects 0.000 description 4
- 239000002585 base Substances 0.000 description 4
- 239000003153 chemical reaction reagent Substances 0.000 description 4
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 4
- 230000008878 coupling Effects 0.000 description 4
- 238000010168 coupling process Methods 0.000 description 4
- 238000005859 coupling reaction Methods 0.000 description 4
- 230000002354 daily effect Effects 0.000 description 4
- 239000007857 degradation product Substances 0.000 description 4
- 230000001419 dependent effect Effects 0.000 description 4
- 238000011156 evaluation Methods 0.000 description 4
- 229910052731 fluorine Inorganic materials 0.000 description 4
- 238000002868 homogeneous time resolved fluorescence Methods 0.000 description 4
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 4
- 230000002218 hypoglycaemic effect Effects 0.000 description 4
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 4
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 4
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- WQEPLUUGTLDZJY-UHFFFAOYSA-N n-Pentadecanoic acid Natural products CCCCCCCCCCCCCCC(O)=O WQEPLUUGTLDZJY-UHFFFAOYSA-N 0.000 description 4
- 238000006722 reduction reaction Methods 0.000 description 4
- 210000000813 small intestine Anatomy 0.000 description 4
- 239000007790 solid phase Substances 0.000 description 4
- 238000007619 statistical method Methods 0.000 description 4
- 239000006228 supernatant Substances 0.000 description 4
- 108010061238 threonyl-glycine Proteins 0.000 description 4
- VDWWLJRQDNTHJB-MXAMYCJDSA-N (2s)-2-[[(2s,3r)-2-[[2-[[(2s)-5-amino-2-[[(2s)-2-[[(2s)-2-amino-3-(1h-imidazol-5-yl)propanoyl]amino]-3-hydroxypropanoyl]amino]-5-oxopentanoyl]amino]acetyl]amino]-3-hydroxybutanoyl]amino]-3-phenylpropanoic acid Chemical compound C([C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CN=CN1 VDWWLJRQDNTHJB-MXAMYCJDSA-N 0.000 description 3
- GOPWHXPXSPIIQZ-FQEVSTJZSA-N (4s)-4-(9h-fluoren-9-ylmethoxycarbonylamino)-5-[(2-methylpropan-2-yl)oxy]-5-oxopentanoic acid Chemical compound C1=CC=C2C(COC(=O)N[C@@H](CCC(O)=O)C(=O)OC(C)(C)C)C3=CC=CC=C3C2=C1 GOPWHXPXSPIIQZ-FQEVSTJZSA-N 0.000 description 3
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 3
- AIRYAONNMGRCGJ-FHFVDXKLSA-N CC[C@H](C)[C@H](NC(=O)CN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H]1CSSC[C@@H]2NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc3c[nH]cn3)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)Cc3ccccc3)C(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](Cc3c[nH]cn3)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC2=O)C(=O)N[C@@H](CC(N)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N2CCC[C@H]2C(=O)N[C@@H]([C@@H](C)O)C(O)=O)NC1=O)[C@@H](C)O)[C@@H](C)CC Chemical compound CC[C@H](C)[C@H](NC(=O)CN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H]1CSSC[C@@H]2NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc3c[nH]cn3)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)Cc3ccccc3)C(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](Cc3c[nH]cn3)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC2=O)C(=O)N[C@@H](CC(N)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N2CCC[C@H]2C(=O)N[C@@H]([C@@H](C)O)C(O)=O)NC1=O)[C@@H](C)O)[C@@H](C)CC AIRYAONNMGRCGJ-FHFVDXKLSA-N 0.000 description 3
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- 102000016622 Dipeptidyl Peptidase 4 Human genes 0.000 description 3
- 108010067722 Dipeptidyl Peptidase 4 Proteins 0.000 description 3
- JZJGEKDPWVJOLD-QEWYBTABSA-N Glu-Phe-Ile Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O JZJGEKDPWVJOLD-QEWYBTABSA-N 0.000 description 3
- 239000007821 HATU Substances 0.000 description 3
- 241000270431 Heloderma suspectum Species 0.000 description 3
- BDFCIKANUNMFGB-PMVVWTBXSA-N His-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC1=CN=CN1 BDFCIKANUNMFGB-PMVVWTBXSA-N 0.000 description 3
- 101001040075 Homo sapiens Glucagon receptor Proteins 0.000 description 3
- 101001015516 Homo sapiens Glucagon-like peptide 1 receptor Proteins 0.000 description 3
- AMSSKPUHBUQBOQ-SRVKXCTJSA-N Leu-Ser-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)O)N AMSSKPUHBUQBOQ-SRVKXCTJSA-N 0.000 description 3
- LTXREWYXXSTFRX-QGZVFWFLSA-N Linagliptin Chemical compound N=1C=2N(C)C(=O)N(CC=3N=C4C=CC=CC4=C(C)N=3)C(=O)C=2N(CC#CC)C=1N1CCC[C@@H](N)C1 LTXREWYXXSTFRX-QGZVFWFLSA-N 0.000 description 3
- QIJVAFLRMVBHMU-KKUMJFAQSA-N Lys-Asp-Phe Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O QIJVAFLRMVBHMU-KKUMJFAQSA-N 0.000 description 3
- GCMWRRQAKQXDED-IUCAKERBSA-N Lys-Glu-Gly Chemical compound [NH3+]CCCC[C@H]([NH3+])C(=O)N[C@@H](CCC([O-])=O)C(=O)NCC([O-])=O GCMWRRQAKQXDED-IUCAKERBSA-N 0.000 description 3
- DUTMKEAPLLUGNO-JYJNAYRXSA-N Lys-Glu-Phe Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O DUTMKEAPLLUGNO-JYJNAYRXSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 206010028980 Neoplasm Diseases 0.000 description 3
- 208000006011 Stroke Diseases 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 230000010933 acylation Effects 0.000 description 3
- 238000005917 acylation reaction Methods 0.000 description 3
- 125000002947 alkylene group Chemical group 0.000 description 3
- 229940124277 aminobutyric acid Drugs 0.000 description 3
- 238000010171 animal model Methods 0.000 description 3
- 210000000227 basophil cell of anterior lobe of hypophysis Anatomy 0.000 description 3
- 210000004204 blood vessel Anatomy 0.000 description 3
- 210000004556 brain Anatomy 0.000 description 3
- 229910052794 bromium Inorganic materials 0.000 description 3
- 239000000460 chlorine Substances 0.000 description 3
- 229910052801 chlorine Inorganic materials 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000002648 combination therapy Methods 0.000 description 3
- 230000000052 comparative effect Effects 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- 230000000378 dietary effect Effects 0.000 description 3
- 231100000673 dose–response relationship Toxicity 0.000 description 3
- 210000003890 endocrine cell Anatomy 0.000 description 3
- 210000003743 erythrocyte Anatomy 0.000 description 3
- 108010006664 gamma-glutamyl-glycyl-glycine Proteins 0.000 description 3
- 230000014509 gene expression Effects 0.000 description 3
- 239000004220 glutamic acid Substances 0.000 description 3
- 125000000592 heterocycloalkyl group Chemical group 0.000 description 3
- NPZTUJOABDZTLV-UHFFFAOYSA-N hydroxybenzotriazole Substances O=C1C=CC=C2NNN=C12 NPZTUJOABDZTLV-UHFFFAOYSA-N 0.000 description 3
- 230000001976 improved effect Effects 0.000 description 3
- 239000012535 impurity Substances 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 230000002401 inhibitory effect Effects 0.000 description 3
- PBGKTOXHQIOBKM-FHFVDXKLSA-N insulin (human) Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@H]1CSSC[C@H]2C(=O)N[C@H](C(=O)N[C@@H](CO)C(=O)N[C@H](C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3C=CC(O)=CC=3)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3NC=NC=3)NC(=O)[C@H](CO)NC(=O)CNC1=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O)=O)CSSC[C@@H](C(N2)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)CN)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C=CC=CC=1)C(C)C)C1=CN=CN1 PBGKTOXHQIOBKM-FHFVDXKLSA-N 0.000 description 3
- 230000003870 intestinal permeability Effects 0.000 description 3
- 230000003834 intracellular effect Effects 0.000 description 3
- 229910052740 iodine Inorganic materials 0.000 description 3
- 210000003734 kidney Anatomy 0.000 description 3
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 108020004707 nucleic acids Proteins 0.000 description 3
- 102000039446 nucleic acids Human genes 0.000 description 3
- 150000007523 nucleic acids Chemical class 0.000 description 3
- 210000000496 pancreas Anatomy 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 3
- 239000008363 phosphate buffer Substances 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 238000011321 prophylaxis Methods 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 210000002784 stomach Anatomy 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- 125000000923 (C1-C30) alkyl group Chemical group 0.000 description 2
- MYRTYDVEIRVNKP-UHFFFAOYSA-N 1,2-Divinylbenzene Chemical compound C=CC1=CC=CC=C1C=C MYRTYDVEIRVNKP-UHFFFAOYSA-N 0.000 description 2
- BDNKZNFMNDZQMI-UHFFFAOYSA-N 1,3-diisopropylcarbodiimide Chemical compound CC(C)N=C=NC(C)C BDNKZNFMNDZQMI-UHFFFAOYSA-N 0.000 description 2
- HNSDLXPSAYFUHK-UHFFFAOYSA-N 1,4-bis(2-ethylhexyl) sulfosuccinate Chemical compound CCCCC(CC)COC(=O)CC(S(O)(=O)=O)C(=O)OCC(CC)CCCC HNSDLXPSAYFUHK-UHFFFAOYSA-N 0.000 description 2
- PECYZEOJVXMISF-UHFFFAOYSA-N 3-aminoalanine Chemical compound [NH3+]CC(N)C([O-])=O PECYZEOJVXMISF-UHFFFAOYSA-N 0.000 description 2
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 2
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 2
- 108010088751 Albumins Proteins 0.000 description 2
- 102000009027 Albumins Human genes 0.000 description 2
- 208000024827 Alzheimer disease Diseases 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 239000004215 Carbon black (E152) Substances 0.000 description 2
- 208000024172 Cardiovascular disease Diseases 0.000 description 2
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 2
- JVHXJTBJCFBINQ-ADAARDCZSA-N Dapagliflozin Chemical compound C1=CC(OCC)=CC=C1CC1=CC([C@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O2)O)=CC=C1Cl JVHXJTBJCFBINQ-ADAARDCZSA-N 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 2
- LCDDAGSJHKEABN-MLGOLLRUSA-N Evogliptin Chemical compound C1CNC(=O)[C@@H](COC(C)(C)C)N1C(=O)C[C@H](N)CC1=CC(F)=C(F)C=C1F LCDDAGSJHKEABN-MLGOLLRUSA-N 0.000 description 2
- LFIVHGMKWFGUGK-IHRRRGAJSA-N Gln-Glu-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCC(=O)N)N LFIVHGMKWFGUGK-IHRRRGAJSA-N 0.000 description 2
- HPCOBEHVEHWREJ-DCAQKATOSA-N Gln-Lys-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O HPCOBEHVEHWREJ-DCAQKATOSA-N 0.000 description 2
- UQHGAYSULGRWRG-WHFBIAKZSA-N Glu-Ser Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CO)C(O)=O UQHGAYSULGRWRG-WHFBIAKZSA-N 0.000 description 2
- KKBWDNZXYLGJEY-UHFFFAOYSA-N Gly-Arg-Pro Natural products NCC(=O)NC(CCNC(=N)N)C(=O)N1CCCC1C(=O)O KKBWDNZXYLGJEY-UHFFFAOYSA-N 0.000 description 2
- LLWQVJNHMYBLLK-CDMKHQONSA-N Gly-Thr-Phe Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O LLWQVJNHMYBLLK-CDMKHQONSA-N 0.000 description 2
- 239000007995 HEPES buffer Substances 0.000 description 2
- 102000005548 Hexokinase Human genes 0.000 description 2
- 108700040460 Hexokinases Proteins 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 206010061218 Inflammation Diseases 0.000 description 2
- 206010022489 Insulin Resistance Diseases 0.000 description 2
- FYZPCMFQCNBYCY-WIWKJPBBSA-N Insulin degludec Chemical compound CC[C@H](C)[C@H](NC(=O)CN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H]1CSSC[C@@H]2NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc3c[nH]cn3)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)Cc3ccccc3)C(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](Cc3c[nH]cn3)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC2=O)C(=O)N[C@@H](CC(N)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]([C@@H](C)O)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CCCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCC(O)=O)C(O)=O)C(O)=O)NC1=O)[C@@H](C)O)[C@@H](C)CC FYZPCMFQCNBYCY-WIWKJPBBSA-N 0.000 description 2
- XVVOERDUTLJJHN-UHFFFAOYSA-N Lixisenatide Chemical compound C=1NC2=CC=CC=C2C=1CC(C(=O)NC(CC(C)C)C(=O)NC(CCCCN)C(=O)NC(CC(N)=O)C(=O)NCC(=O)NCC(=O)N1C(CCC1)C(=O)NC(CO)C(=O)NC(CO)C(=O)NCC(=O)NC(C)C(=O)N1C(CCC1)C(=O)N1C(CCC1)C(=O)NC(CO)C(=O)NC(CCCCN)C(=O)NC(CCCCN)C(=O)NC(CCCCN)C(=O)NC(CCCCN)C(=O)NC(CCCCN)C(=O)NC(CCCCN)C(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)CC)NC(=O)C(NC(=O)C(CC(C)C)NC(=O)C(CCCNC(N)=N)NC(=O)C(NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(CCC(O)=O)NC(=O)C(CCC(O)=O)NC(=O)C(CCSC)NC(=O)C(CCC(N)=O)NC(=O)C(CCCCN)NC(=O)C(CO)NC(=O)C(CC(C)C)NC(=O)C(CC(O)=O)NC(=O)C(CO)NC(=O)C(NC(=O)C(CC=1C=CC=CC=1)NC(=O)C(NC(=O)CNC(=O)C(CCC(O)=O)NC(=O)CNC(=O)C(N)CC=1NC=NC=1)C(C)O)C(C)O)C(C)C)CC1=CC=CC=C1 XVVOERDUTLJJHN-UHFFFAOYSA-N 0.000 description 2
- DGAAQRAUOFHBFJ-CIUDSAMLSA-N Lys-Asn-Ala Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(O)=O DGAAQRAUOFHBFJ-CIUDSAMLSA-N 0.000 description 2
- AAORVPFVUIHEAB-YUMQZZPRSA-N Lys-Asp-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O AAORVPFVUIHEAB-YUMQZZPRSA-N 0.000 description 2
- XOQMURBBIXRRCR-SRVKXCTJSA-N Lys-Lys-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCCCN XOQMURBBIXRRCR-SRVKXCTJSA-N 0.000 description 2
- YUAXTFMFMOIMAM-QWRGUYRKSA-N Lys-Lys-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O YUAXTFMFMOIMAM-QWRGUYRKSA-N 0.000 description 2
- SBQDRNOLGSYHQA-YUMQZZPRSA-N Lys-Ser-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SBQDRNOLGSYHQA-YUMQZZPRSA-N 0.000 description 2
- 102000003729 Neprilysin Human genes 0.000 description 2
- 108090000028 Neprilysin Proteins 0.000 description 2
- 208000018737 Parkinson disease Diseases 0.000 description 2
- 102000035195 Peptidases Human genes 0.000 description 2
- 108091005804 Peptidases Proteins 0.000 description 2
- 101800001672 Peptide YY(3-36) Proteins 0.000 description 2
- PCWLNNZTBJTZRN-AVGNSLFASA-N Pro-Pro-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 PCWLNNZTBJTZRN-AVGNSLFASA-N 0.000 description 2
- 108010058003 Proglucagon Proteins 0.000 description 2
- YASAKCUCGLMORW-UHFFFAOYSA-N Rosiglitazone Chemical compound C=1C=CC=NC=1N(C)CCOC(C=C1)=CC=C1CC1SC(=O)NC1=O YASAKCUCGLMORW-UHFFFAOYSA-N 0.000 description 2
- 206010039710 Scleroderma Diseases 0.000 description 2
- AEGUWTFAQQWVLC-BQBZGAKWSA-N Ser-Gly-Arg Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O AEGUWTFAQQWVLC-BQBZGAKWSA-N 0.000 description 2
- 235000021355 Stearic acid Nutrition 0.000 description 2
- 239000002535 acidifier Substances 0.000 description 2
- 108010047495 alanylglycine Proteins 0.000 description 2
- GZCGUPFRVQAUEE-SLPGGIOYSA-N aldehydo-D-glucose Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O GZCGUPFRVQAUEE-SLPGGIOYSA-N 0.000 description 2
- 125000001931 aliphatic group Chemical group 0.000 description 2
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 2
- 230000003113 alkalizing effect Effects 0.000 description 2
- 206010002026 amyotrophic lateral sclerosis Diseases 0.000 description 2
- 238000005571 anion exchange chromatography Methods 0.000 description 2
- 230000003178 anti-diabetic effect Effects 0.000 description 2
- 239000003524 antilipemic agent Substances 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 108010029539 arginyl-prolyl-proline Proteins 0.000 description 2
- 239000012131 assay buffer Substances 0.000 description 2
- 230000003143 atherosclerotic effect Effects 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 230000001588 bifunctional effect Effects 0.000 description 2
- 238000009395 breeding Methods 0.000 description 2
- 230000001488 breeding effect Effects 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 2
- 229960003834 dapagliflozin Drugs 0.000 description 2
- 230000007850 degeneration Effects 0.000 description 2
- 238000010511 deprotection reaction Methods 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical compound C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 2
- 230000006806 disease prevention Effects 0.000 description 2
- 239000003480 eluent Substances 0.000 description 2
- 230000002708 enhancing effect Effects 0.000 description 2
- 229950011259 evogliptin Drugs 0.000 description 2
- 239000013604 expression vector Substances 0.000 description 2
- 235000019253 formic acid Nutrition 0.000 description 2
- 208000020694 gallbladder disease Diseases 0.000 description 2
- 108010036598 gastric inhibitory polypeptide receptor Proteins 0.000 description 2
- 230000014101 glucose homeostasis Effects 0.000 description 2
- 235000013922 glutamic acid Nutrition 0.000 description 2
- 210000003128 head Anatomy 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 210000002216 heart Anatomy 0.000 description 2
- 208000019622 heart disease Diseases 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 2
- 230000004054 inflammatory process Effects 0.000 description 2
- 108010050259 insulin degludec Proteins 0.000 description 2
- 229960002869 insulin glargine Drugs 0.000 description 2
- 230000003914 insulin secretion Effects 0.000 description 2
- 210000000936 intestine Anatomy 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 229940060975 lantus Drugs 0.000 description 2
- 230000002045 lasting effect Effects 0.000 description 2
- UGOZVNFCFYTPAZ-IOXYNQHNSA-N levemir Chemical compound CCCCCCCCCCCCCC(=O)NCCCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]([C@@H](C)O)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@H]1NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=2C=CC(O)=CC=2)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=2N=CNC=2)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=2N=CNC=2)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC=2C=CC=CC=2)C(C)C)CSSC[C@@H]2NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CN)[C@@H](C)CC)C(C)C)CSSC[C@H](NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@H](CO)NC(=O)[C@H]([C@@H](C)O)NC2=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H](CSSC1)C(=O)N[C@@H](CC(N)=O)C(O)=O)CC1=CC=C(O)C=C1 UGOZVNFCFYTPAZ-IOXYNQHNSA-N 0.000 description 2
- 229960002397 linagliptin Drugs 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- 229960001093 lixisenatide Drugs 0.000 description 2
- 108010004367 lixisenatide Proteins 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 208000030159 metabolic disease Diseases 0.000 description 2
- 238000012544 monitoring process Methods 0.000 description 2
- 125000001624 naphthyl group Chemical group 0.000 description 2
- 230000004770 neurodegeneration Effects 0.000 description 2
- 125000004433 nitrogen atom Chemical group N* 0.000 description 2
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 2
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 2
- MKMPWKUAHLTIBJ-ISTRZQFTSA-N omarigliptin Chemical compound C1([C@H]2OC[C@@H](C[C@@H]2N)N2CC3=CN(N=C3C2)S(=O)(=O)C)=CC(F)=CC=C1F MKMPWKUAHLTIBJ-ISTRZQFTSA-N 0.000 description 2
- 238000001543 one-way ANOVA Methods 0.000 description 2
- 229940125395 oral insulin Drugs 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 239000000813 peptide hormone Substances 0.000 description 2
- 239000008024 pharmaceutical diluent Substances 0.000 description 2
- 229940124531 pharmaceutical excipient Drugs 0.000 description 2
- 239000008055 phosphate buffer solution Substances 0.000 description 2
- HYAFETHFCAUJAY-UHFFFAOYSA-N pioglitazone Chemical compound N1=CC(CC)=CC=C1CCOC(C=C1)=CC=C1CC1C(=O)NC(=O)S1 HYAFETHFCAUJAY-UHFFFAOYSA-N 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- WGYKZJWCGVVSQN-UHFFFAOYSA-N propylamine Chemical compound CCCN WGYKZJWCGVVSQN-UHFFFAOYSA-N 0.000 description 2
- 238000005070 sampling Methods 0.000 description 2
- 108010033693 saxagliptin Proteins 0.000 description 2
- QGJUIPDUBHWZPV-SGTAVMJGSA-N saxagliptin Chemical compound C1C(C2)CC(C3)CC2(O)CC13[C@H](N)C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21 QGJUIPDUBHWZPV-SGTAVMJGSA-N 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- 201000002859 sleep apnea Diseases 0.000 description 2
- 238000013112 stability test Methods 0.000 description 2
- 238000012430 stability testing Methods 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 239000008117 stearic acid Substances 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 230000008685 targeting Effects 0.000 description 2
- RMMXLENWKUUMAY-UHFFFAOYSA-N telmisartan Chemical compound CCCC1=NC2=C(C)C=C(C=3N(C4=CC=CC=C4N=3)C)C=C2N1CC(C=C1)=CC=C1C1=CC=CC=C1C(O)=O RMMXLENWKUUMAY-UHFFFAOYSA-N 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- 208000019553 vascular disease Diseases 0.000 description 2
- 238000005303 weighing Methods 0.000 description 2
- XUFXOAAUWZOOIT-SXARVLRPSA-N (2R,3R,4R,5S,6R)-5-[[(2R,3R,4R,5S,6R)-5-[[(2R,3R,4S,5S,6R)-3,4-dihydroxy-6-methyl-5-[[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)-1-cyclohex-2-enyl]amino]-2-oxanyl]oxy]-3,4-dihydroxy-6-(hydroxymethyl)-2-oxanyl]oxy]-6-(hydroxymethyl)oxane-2,3,4-triol Chemical compound O([C@H]1O[C@H](CO)[C@H]([C@@H]([C@H]1O)O)O[C@H]1O[C@@H]([C@H]([C@H](O)[C@H]1O)N[C@@H]1[C@@H]([C@@H](O)[C@H](O)C(CO)=C1)O)C)[C@@H]1[C@@H](CO)O[C@@H](O)[C@H](O)[C@H]1O XUFXOAAUWZOOIT-SXARVLRPSA-N 0.000 description 1
- ZWASRJHIEFYJGL-BFUOFWGJSA-N (2r)-1,1,1-trifluoro-2-[3-[(2r)-4-[4-fluoro-2-(trifluoromethyl)phenyl]-2-methylpiperazin-1-yl]sulfonylphenyl]propan-2-ol Chemical compound C([C@H]1C)N(C=2C(=CC(F)=CC=2)C(F)(F)F)CCN1S(=O)(=O)C1=CC=CC([C@@](C)(O)C(F)(F)F)=C1 ZWASRJHIEFYJGL-BFUOFWGJSA-N 0.000 description 1
- ADOHASQZJSJZBT-SANMLTNESA-N (2s)-2-(9h-fluoren-9-ylmethoxycarbonylamino)-3-[1-[(2-methylpropan-2-yl)oxycarbonyl]indol-3-yl]propanoic acid Chemical compound C12=CC=CC=C2N(C(=O)OC(C)(C)C)C=C1C[C@@H](C(O)=O)NC(=O)OCC1C2=CC=CC=C2C2=CC=CC=C21 ADOHASQZJSJZBT-SANMLTNESA-N 0.000 description 1
- BUBGAUHBELNDEW-SFHVURJKSA-N (2s)-2-(9h-fluoren-9-ylmethoxycarbonylamino)-4-methylsulfanylbutanoic acid Chemical compound C1=CC=C2C(COC(=O)N[C@@H](CCSC)C(O)=O)C3=CC=CC=C3C2=C1 BUBGAUHBELNDEW-SFHVURJKSA-N 0.000 description 1
- OTKXCALUHMPIGM-FQEVSTJZSA-N (2s)-2-(9h-fluoren-9-ylmethoxycarbonylamino)-5-[(2-methylpropan-2-yl)oxy]-5-oxopentanoic acid Chemical compound C1=CC=C2C(COC(=O)N[C@@H](CCC(=O)OC(C)(C)C)C(O)=O)C3=CC=CC=C3C2=C1 OTKXCALUHMPIGM-FQEVSTJZSA-N 0.000 description 1
- UMRUUWFGLGNQLI-QFIPXVFZSA-N (2s)-2-(9h-fluoren-9-ylmethoxycarbonylamino)-6-[(2-methylpropan-2-yl)oxycarbonylamino]hexanoic acid Chemical compound C1=CC=C2C(COC(=O)N[C@@H](CCCCNC(=O)OC(C)(C)C)C(O)=O)C3=CC=CC=C3C2=C1 UMRUUWFGLGNQLI-QFIPXVFZSA-N 0.000 description 1
- KSDTXRUIZMTBNV-INIZCTEOSA-N (2s)-2-(9h-fluoren-9-ylmethoxycarbonylamino)butanedioic acid Chemical compound C1=CC=C2C(COC(=O)N[C@@H](CC(=O)O)C(O)=O)C3=CC=CC=C3C2=C1 KSDTXRUIZMTBNV-INIZCTEOSA-N 0.000 description 1
- QWXZOFZKSQXPDC-NSHDSACASA-N (2s)-2-(9h-fluoren-9-ylmethoxycarbonylamino)propanoic acid Chemical compound C1=CC=C2C(COC(=O)N[C@@H](C)C(O)=O)C3=CC=CC=C3C2=C1 QWXZOFZKSQXPDC-NSHDSACASA-N 0.000 description 1
- XFJAMQQAAMJFGB-ZQGJOIPISA-N (2s,3r,4r,5s,6r)-2-[3-(2,3-dihydro-1,4-benzodioxin-6-ylmethyl)-4-ethylphenyl]-6-(hydroxymethyl)oxane-3,4,5-triol Chemical compound C1=C(CC=2C=C3OCCOC3=CC=2)C(CC)=CC=C1[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O XFJAMQQAAMJFGB-ZQGJOIPISA-N 0.000 description 1
- BTCRKOKVYTVOLU-SJSRKZJXSA-N (2s,3r,4r,5s,6r)-2-[4-chloro-3-[[4-(2-cyclopropyloxyethoxy)phenyl]methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1C1=CC=C(Cl)C(CC=2C=CC(OCCOC3CC3)=CC=2)=C1 BTCRKOKVYTVOLU-SJSRKZJXSA-N 0.000 description 1
- JSYGLDMGERSRPC-FQUUOJAGSA-N (2s,4s)-4-fluoro-1-[2-[[(1r,3s)-3-(1,2,4-triazol-1-ylmethyl)cyclopentyl]amino]acetyl]pyrrolidine-2-carbonitrile Chemical compound C1[C@@H](F)C[C@@H](C#N)N1C(=O)CN[C@H]1C[C@@H](CN2N=CN=C2)CC1 JSYGLDMGERSRPC-FQUUOJAGSA-N 0.000 description 1
- NGJOFQZEYQGZMB-KTKZVXAJSA-N (4S)-5-[[2-[[(2S,3R)-1-[[(2S)-1-[[(2S,3R)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-5-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[2-[[(1S)-4-carbamimidamido-1-carboxybutyl]amino]-2-oxoethyl]amino]-1-oxohexan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-2-oxoethyl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-3-carboxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-2-oxoethyl]amino]-4-[[(2S)-2-[[(2S)-2-amino-3-(1H-imidazol-4-yl)propanoyl]amino]propanoyl]amino]-5-oxopentanoic acid Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 NGJOFQZEYQGZMB-KTKZVXAJSA-N 0.000 description 1
- 125000006702 (C1-C18) alkyl group Chemical group 0.000 description 1
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 1
- DHBXNPKRAUYBTH-UHFFFAOYSA-N 1,1-ethanedithiol Chemical compound CC(S)S DHBXNPKRAUYBTH-UHFFFAOYSA-N 0.000 description 1
- OAAZMUGLOXGVNH-UHFFFAOYSA-N 1-(3-hydroxyazetidin-1-yl)-2-(6-hydroxy-2-phenyl-2-adamantyl)ethanone Chemical compound C1C(O)CN1C(=O)CC1(C=2C=CC=CC=2)C2CC(C3O)CC1CC3C2 OAAZMUGLOXGVNH-UHFFFAOYSA-N 0.000 description 1
- ASOKPJOREAFHNY-UHFFFAOYSA-N 1-Hydroxybenzotriazole Chemical compound C1=CC=C2N(O)N=NC2=C1 ASOKPJOREAFHNY-UHFFFAOYSA-N 0.000 description 1
- DFPYXQYWILNVAU-UHFFFAOYSA-N 1-hydroxybenzotriazole Chemical compound C1=CC=C2N(O)N=NC2=C1.C1=CC=C2N(O)N=NC2=C1 DFPYXQYWILNVAU-UHFFFAOYSA-N 0.000 description 1
- FGRBYDKOBBBPOI-UHFFFAOYSA-N 10,10-dioxo-2-[4-(N-phenylanilino)phenyl]thioxanthen-9-one Chemical compound O=C1c2ccccc2S(=O)(=O)c2ccc(cc12)-c1ccc(cc1)N(c1ccccc1)c1ccccc1 FGRBYDKOBBBPOI-UHFFFAOYSA-N 0.000 description 1
- NCYCYZXNIZJOKI-IOUUIBBYSA-N 11-cis-retinal Chemical compound O=C/C=C(\C)/C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C NCYCYZXNIZJOKI-IOUUIBBYSA-N 0.000 description 1
- HOZZVEPRYYCBTO-UHFFFAOYSA-N 2-(9h-fluoren-9-ylmethoxycarbonylamino)-2-methylpropanoic acid Chemical compound C1=CC=C2C(COC(=O)NC(C)(C)C(O)=O)C3=CC=CC=C3C2=C1 HOZZVEPRYYCBTO-UHFFFAOYSA-N 0.000 description 1
- NDKDFTQNXLHCGO-UHFFFAOYSA-N 2-(9h-fluoren-9-ylmethoxycarbonylamino)acetic acid Chemical compound C1=CC=C2C(COC(=O)NCC(=O)O)C3=CC=CC=C3C2=C1 NDKDFTQNXLHCGO-UHFFFAOYSA-N 0.000 description 1
- NFTMKHWBOINJGM-UHFFFAOYSA-N 2-[1-(5-ethylpyrimidin-2-yl)piperidin-4-yl]-4-[[4-(tetrazol-1-yl)phenoxy]methyl]-1,3-thiazole Chemical compound N1=CC(CC)=CN=C1N1CCC(C=2SC=C(COC=3C=CC(=CC=3)N3N=NN=C3)N=2)CC1 NFTMKHWBOINJGM-UHFFFAOYSA-N 0.000 description 1
- AYKLXGCULGWUJX-UHFFFAOYSA-N 2-[5-chloro-2-[[1-[(3,4-difluorophenyl)methyl]-4-[(4-methylsulfonylphenyl)methyl]pyrrole-2-carbonyl]amino]-1,3-thiazol-4-yl]acetic acid Chemical compound C1=CC(S(=O)(=O)C)=CC=C1CC1=CN(CC=2C=C(F)C(F)=CC=2)C(C(=O)NC=2SC(Cl)=C(CC(O)=O)N=2)=C1 AYKLXGCULGWUJX-UHFFFAOYSA-N 0.000 description 1
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 1
- APIXJSLKIYYUKG-UHFFFAOYSA-N 3 Isobutyl 1 methylxanthine Chemical compound O=C1N(C)C(=O)N(CC(C)C)C2=C1N=CN2 APIXJSLKIYYUKG-UHFFFAOYSA-N 0.000 description 1
- FODHWOBAQBTTFS-UHFFFAOYSA-N 3-[4-[2-(2-methylphenyl)ethynyl]phenyl]propanoic acid Chemical compound CC1=CC=CC=C1C#CC1=CC=C(CCC(O)=O)C=C1 FODHWOBAQBTTFS-UHFFFAOYSA-N 0.000 description 1
- OCNBSSLDAIWTKS-UHFFFAOYSA-N 3-[[[3,5-bis(trifluoromethyl)phenyl]methyl-(2-methyltetrazol-5-yl)amino]methyl]-n,n-bis(cyclopropylmethyl)-8-methylquinolin-2-amine Chemical compound C1CC1CN(CC1CC1)C=1N=C2C(C)=CC=CC2=CC=1CN(C1=NN(C)N=N1)CC1=CC(C(F)(F)F)=CC(C(F)(F)F)=C1 OCNBSSLDAIWTKS-UHFFFAOYSA-N 0.000 description 1
- ACUIFAAXWDLLTR-UHFFFAOYSA-N 4-(9h-fluoren-9-ylmethoxycarbonylamino)butanoic acid Chemical compound C1=CC=C2C(COC(=O)NCCCC(=O)O)C3=CC=CC=C3C2=C1 ACUIFAAXWDLLTR-UHFFFAOYSA-N 0.000 description 1
- XWBXJBSVYVJAMZ-UHFFFAOYSA-N 4-[4-(2-adamantylcarbamoyl)-5-tert-butylpyrazol-1-yl]benzoic acid Chemical compound CC(C)(C)C1=C(C(=O)NC2C3CC4CC(C3)CC2C4)C=NN1C1=CC=C(C(O)=O)C=C1 XWBXJBSVYVJAMZ-UHFFFAOYSA-N 0.000 description 1
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 1
- ALQLYJHDBAKLBB-UHFFFAOYSA-N 4-dodecoxybenzoic acid Chemical compound CCCCCCCCCCCCOC1=CC=C(C(O)=O)C=C1 ALQLYJHDBAKLBB-UHFFFAOYSA-N 0.000 description 1
- AYJRTVVIBJSSKN-UHFFFAOYSA-N 5-[4-[[6-(4-methylsulfonylphenyl)pyridin-3-yl]oxymethyl]piperidin-1-yl]-3-propan-2-yl-1,2,4-oxadiazole Chemical compound CC(C)C1=NOC(N2CCC(COC=3C=NC(=CC=3)C=3C=CC(=CC=3)S(C)(=O)=O)CC2)=N1 AYJRTVVIBJSSKN-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- 208000023769 AA amyloidosis Diseases 0.000 description 1
- 208000023761 AL amyloidosis Diseases 0.000 description 1
- 208000022385 ALys amyloidosis Diseases 0.000 description 1
- 208000004611 Abdominal Obesity Diseases 0.000 description 1
- MQIGTEQXYCRLGK-BQBZGAKWSA-N Ala-Gly-Pro Chemical compound C[C@H](N)C(=O)NCC(=O)N1CCC[C@H]1C(O)=O MQIGTEQXYCRLGK-BQBZGAKWSA-N 0.000 description 1
- YXXPVUOMPSZURS-ZLIFDBKOSA-N Ala-Trp-Leu Chemical compound C1=CC=C2C(C[C@@H](C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)[C@H](C)N)=CNC2=C1 YXXPVUOMPSZURS-ZLIFDBKOSA-N 0.000 description 1
- 229940077274 Alpha glucosidase inhibitor Drugs 0.000 description 1
- 208000031277 Amaurotic familial idiocy Diseases 0.000 description 1
- 206010002027 Amyotrophy Diseases 0.000 description 1
- 206010059245 Angiopathy Diseases 0.000 description 1
- VBFJESQBIWCWRL-DCAQKATOSA-N Arg-Ala-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCNC(N)=N VBFJESQBIWCWRL-DCAQKATOSA-N 0.000 description 1
- IIAXFBUTKIDDIP-ULQDDVLXSA-N Arg-Leu-Phe Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O IIAXFBUTKIDDIP-ULQDDVLXSA-N 0.000 description 1
- MFDPBZAFCRKYEY-LAEOZQHASA-N Asp-Val-Gln Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O MFDPBZAFCRKYEY-LAEOZQHASA-N 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- XUKUURHRXDUEBC-KAYWLYCHSA-N Atorvastatin Chemical compound C=1C=CC=CC=1C1=C(C=2C=CC(F)=CC=2)N(CC[C@@H](O)C[C@@H](O)CC(O)=O)C(C(C)C)=C1C(=O)NC1=CC=CC=C1 XUKUURHRXDUEBC-KAYWLYCHSA-N 0.000 description 1
- XUKUURHRXDUEBC-UHFFFAOYSA-N Atorvastatin Natural products C=1C=CC=CC=1C1=C(C=2C=CC(F)=CC=2)N(CCC(O)CC(O)CC(O)=O)C(C(C)C)=C1C(=O)NC1=CC=CC=C1 XUKUURHRXDUEBC-UHFFFAOYSA-N 0.000 description 1
- 238000012935 Averaging Methods 0.000 description 1
- 102000040350 B family Human genes 0.000 description 1
- 108091072128 B family Proteins 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 208000014644 Brain disease Diseases 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- 206010068597 Bulbospinal muscular atrophy congenital Diseases 0.000 description 1
- 239000002083 C09CA01 - Losartan Substances 0.000 description 1
- 239000004072 C09CA03 - Valsartan Substances 0.000 description 1
- 239000002081 C09CA05 - Tasosartan Substances 0.000 description 1
- 239000002053 C09CA06 - Candesartan Substances 0.000 description 1
- 239000005537 C09CA07 - Telmisartan Substances 0.000 description 1
- BDDMZWCXJSGFAX-UHFFFAOYSA-N CN(C)C(O[S+]1C(C=CC=C2)=C2N=C1)=[N+](C)C Chemical compound CN(C)C(O[S+]1C(C=CC=C2)=C2N=C1)=[N+](C)C BDDMZWCXJSGFAX-UHFFFAOYSA-N 0.000 description 1
- 101100512078 Caenorhabditis elegans lys-1 gene Proteins 0.000 description 1
- 101100505161 Caenorhabditis elegans mel-32 gene Proteins 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- XTNGUQKDFGDXSJ-ZXGKGEBGSA-N Canagliflozin Chemical compound CC1=CC=C([C@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O2)O)C=C1CC(S1)=CC=C1C1=CC=C(F)C=C1 XTNGUQKDFGDXSJ-ZXGKGEBGSA-N 0.000 description 1
- 229940123158 Cannabinoid CB1 receptor antagonist Drugs 0.000 description 1
- 208000002177 Cataract Diseases 0.000 description 1
- 102000003908 Cathepsin D Human genes 0.000 description 1
- 108090000258 Cathepsin D Proteins 0.000 description 1
- 206010065941 Central obesity Diseases 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 229940122502 Cholesterol absorption inhibitor Drugs 0.000 description 1
- 229920001268 Cholestyramine Polymers 0.000 description 1
- 208000020406 Creutzfeldt Jacob disease Diseases 0.000 description 1
- 208000003407 Creutzfeldt-Jakob Syndrome Diseases 0.000 description 1
- 208000010859 Creutzfeldt-Jakob disease Diseases 0.000 description 1
- 208000035902 Critical illness myopathy Diseases 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- 229920000858 Cyclodextrin Polymers 0.000 description 1
- 201000003883 Cystic fibrosis Diseases 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 206010012289 Dementia Diseases 0.000 description 1
- 208000007342 Diabetic Nephropathies Diseases 0.000 description 1
- 208000032131 Diabetic Neuropathies Diseases 0.000 description 1
- 206010012689 Diabetic retinopathy Diseases 0.000 description 1
- ZAFNJMIOTHYJRJ-UHFFFAOYSA-N Diisopropyl ether Chemical compound CC(C)OC(C)C ZAFNJMIOTHYJRJ-UHFFFAOYSA-N 0.000 description 1
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 1
- 108010016626 Dipeptides Proteins 0.000 description 1
- 102000003779 Dipeptidyl-peptidases and tripeptidyl-peptidases Human genes 0.000 description 1
- 108090000194 Dipeptidyl-peptidases and tripeptidyl-peptidases Proteins 0.000 description 1
- 206010013954 Dysphoria Diseases 0.000 description 1
- 206010014486 Elevated triglycerides Diseases 0.000 description 1
- 208000032274 Encephalopathy Diseases 0.000 description 1
- 108010043222 Exubera Proteins 0.000 description 1
- 208000034846 Familial Amyloid Neuropathies Diseases 0.000 description 1
- 206010016202 Familial Amyloidosis Diseases 0.000 description 1
- BZCALJIHZVNMGJ-HSZRJFAPSA-N Fasiglifam Chemical compound CC1=CC(OCCCS(C)(=O)=O)=CC(C)=C1C1=CC=CC(COC=2C=C3OC[C@@H](CC(O)=O)C3=CC=2)=C1 BZCALJIHZVNMGJ-HSZRJFAPSA-N 0.000 description 1
- 108010049003 Fibrinogen Proteins 0.000 description 1
- 102000008946 Fibrinogen Human genes 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- 229930091371 Fructose Natural products 0.000 description 1
- 239000005715 Fructose Substances 0.000 description 1
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 1
- 102000003688 G-Protein-Coupled Receptors Human genes 0.000 description 1
- 108090000045 G-Protein-Coupled Receptors Proteins 0.000 description 1
- 108091007911 GSKs Proteins 0.000 description 1
- ZWPRRQZNBDYKLH-VIFPVBQESA-N Gemigliptin Chemical compound C([C@@H](N)CC(=O)N1CC2=C(C(=NC(=N2)C(F)(F)F)C(F)(F)F)CC1)N1CC(F)(F)CCC1=O ZWPRRQZNBDYKLH-VIFPVBQESA-N 0.000 description 1
- 208000034826 Genetic Predisposition to Disease Diseases 0.000 description 1
- 208000010412 Glaucoma Diseases 0.000 description 1
- QMVCEWKHIUHTSD-GUBZILKMSA-N Gln-Met-Glu Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CCC(=O)N)N QMVCEWKHIUHTSD-GUBZILKMSA-N 0.000 description 1
- NCWOMXABNYEPLY-NRPADANISA-N Glu-Ala-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O NCWOMXABNYEPLY-NRPADANISA-N 0.000 description 1
- 102400000322 Glucagon-like peptide 1 Human genes 0.000 description 1
- 101800004295 Glucagon-like peptide 1(7-36) Proteins 0.000 description 1
- 102400000326 Glucagon-like peptide 2 Human genes 0.000 description 1
- 101800000221 Glucagon-like peptide 2 Proteins 0.000 description 1
- 102000030595 Glucokinase Human genes 0.000 description 1
- 108010021582 Glucokinase Proteins 0.000 description 1
- 102000004103 Glycogen Synthase Kinases Human genes 0.000 description 1
- 102100030488 HEAT repeat-containing protein 6 Human genes 0.000 description 1
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 1
- 206010018910 Haemolysis Diseases 0.000 description 1
- 239000012981 Hank's balanced salt solution Substances 0.000 description 1
- 102000001554 Hemoglobins Human genes 0.000 description 1
- 108010054147 Hemoglobins Proteins 0.000 description 1
- AFPFGFUGETYOSY-HGNGGELXSA-N His-Ala-Glu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O AFPFGFUGETYOSY-HGNGGELXSA-N 0.000 description 1
- HAPWZEVRQYGLSG-IUCAKERBSA-N His-Gly-Glu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(O)=O HAPWZEVRQYGLSG-IUCAKERBSA-N 0.000 description 1
- 101000886868 Homo sapiens Gastric inhibitory polypeptide Proteins 0.000 description 1
- 101000990566 Homo sapiens HEAT repeat-containing protein 6 Proteins 0.000 description 1
- 101000876418 Homo sapiens Laforin Proteins 0.000 description 1
- 101000801684 Homo sapiens Phospholipid-transporting ATPase ABCA1 Proteins 0.000 description 1
- 108010090613 Human Regular Insulin Proteins 0.000 description 1
- 102000013266 Human Regular Insulin Human genes 0.000 description 1
- 208000023105 Huntington disease Diseases 0.000 description 1
- 208000031226 Hyperlipidaemia Diseases 0.000 description 1
- 229940077672 Ileal bile acid transport inhibitor Drugs 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 208000005531 Immunoglobulin Light-chain Amyloidosis Diseases 0.000 description 1
- 206010021518 Impaired gastric emptying Diseases 0.000 description 1
- 208000029400 Inclusion myopathy Diseases 0.000 description 1
- 108010089308 Insulin Detemir Proteins 0.000 description 1
- 108010065920 Insulin Lispro Proteins 0.000 description 1
- 208000027747 Kennedy disease Diseases 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- QOOWRKBDDXQRHC-BQBZGAKWSA-N L-lysyl-L-alanine Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN QOOWRKBDDXQRHC-BQBZGAKWSA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- 238000008214 LDL Cholesterol Methods 0.000 description 1
- 102100035192 Laforin Human genes 0.000 description 1
- 241001071861 Lethrinus genivittatus Species 0.000 description 1
- JLWZLIQRYCTYBD-IHRRRGAJSA-N Leu-Lys-Arg Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O JLWZLIQRYCTYBD-IHRRRGAJSA-N 0.000 description 1
- WXUOJXIGOPMDJM-SRVKXCTJSA-N Leu-Lys-Asn Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O WXUOJXIGOPMDJM-SRVKXCTJSA-N 0.000 description 1
- KCXUCYYZNZFGLL-SRVKXCTJSA-N Lys-Ala-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O KCXUCYYZNZFGLL-SRVKXCTJSA-N 0.000 description 1
- UWKNTTJNVSYXPC-CIUDSAMLSA-N Lys-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN UWKNTTJNVSYXPC-CIUDSAMLSA-N 0.000 description 1
- CKSBRMUOQDNPKZ-SRVKXCTJSA-N Lys-Gln-Met Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O CKSBRMUOQDNPKZ-SRVKXCTJSA-N 0.000 description 1
- LPAJOCKCPRZEAG-MNXVOIDGSA-N Lys-Glu-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCCCN LPAJOCKCPRZEAG-MNXVOIDGSA-N 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- 238000006845 Michael addition reaction Methods 0.000 description 1
- 102100031545 Microsomal triglyceride transfer protein large subunit Human genes 0.000 description 1
- IBAQFPQHRJAVAV-ULAWRXDQSA-N Miglitol Chemical compound OCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO IBAQFPQHRJAVAV-ULAWRXDQSA-N 0.000 description 1
- 206010049816 Muscle tightness Diseases 0.000 description 1
- 208000021642 Muscular disease Diseases 0.000 description 1
- 208000023178 Musculoskeletal disease Diseases 0.000 description 1
- 201000003793 Myelodysplastic syndrome Diseases 0.000 description 1
- 201000009623 Myopathy Diseases 0.000 description 1
- PVNIIMVLHYAWGP-UHFFFAOYSA-N Niacin Chemical compound OC(=O)C1=CC=CN=C1 PVNIIMVLHYAWGP-UHFFFAOYSA-N 0.000 description 1
- 108091028043 Nucleic acid sequence Proteins 0.000 description 1
- 108010036875 ORMD-0801 Proteins 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 1
- 239000005480 Olmesartan Substances 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- 102000016979 Other receptors Human genes 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 108700020479 Pancreatic hormone Proteins 0.000 description 1
- 208000005764 Peripheral Arterial Disease Diseases 0.000 description 1
- 208000030831 Peripheral arterial occlusive disease Diseases 0.000 description 1
- 208000018262 Peripheral vascular disease Diseases 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 208000001280 Prediabetic State Diseases 0.000 description 1
- 206010036673 Primary amyloidosis Diseases 0.000 description 1
- SBVPYBFMIGDIDX-SRVKXCTJSA-N Pro-Pro-Pro Chemical compound OC(=O)[C@@H]1CCCN1C(=O)[C@H]1N(C(=O)[C@H]2NCCC2)CCC1 SBVPYBFMIGDIDX-SRVKXCTJSA-N 0.000 description 1
- 108010076181 Proinsulin Proteins 0.000 description 1
- 102000003946 Prolactin Human genes 0.000 description 1
- 108010057464 Prolactin Proteins 0.000 description 1
- LCTONWCANYUPML-UHFFFAOYSA-M Pyruvate Chemical compound CC(=O)C([O-])=O LCTONWCANYUPML-UHFFFAOYSA-M 0.000 description 1
- 208000007014 Retinitis pigmentosa Diseases 0.000 description 1
- 102100040756 Rhodopsin Human genes 0.000 description 1
- 108090000820 Rhodopsin Proteins 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- RYMZZMVNJRMUDD-UHFFFAOYSA-N SJ000286063 Natural products C12C(OC(=O)C(C)(C)CC)CC(C)C=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 RYMZZMVNJRMUDD-UHFFFAOYSA-N 0.000 description 1
- 101100379247 Salmo trutta apoa1 gene Proteins 0.000 description 1
- 208000034189 Sclerosis Diseases 0.000 description 1
- 206010039811 Secondary amyloidosis Diseases 0.000 description 1
- DLSWIYLPEUIQAV-UHFFFAOYSA-N Semaglutide Chemical compound CCC(C)C(NC(=O)C(Cc1ccccc1)NC(=O)C(CCC(O)=O)NC(=O)C(CCCCNC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CCC(NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)NC(=O)C(C)NC(=O)C(C)NC(=O)C(CCC(N)=O)NC(=O)CNC(=O)C(CCC(O)=O)NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(O)cc1)NC(=O)C(CO)NC(=O)C(CO)NC(=O)C(NC(=O)C(CC(O)=O)NC(=O)C(CO)NC(=O)C(NC(=O)C(Cc1ccccc1)NC(=O)C(NC(=O)CNC(=O)C(CCC(O)=O)NC(=O)C(C)(C)NC(=O)C(N)Cc1cnc[nH]1)C(C)O)C(C)O)C(C)C)C(=O)NC(C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CC(C)C)C(=O)NC(C(C)C)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CCCNC(N)=N)C(=O)NCC(O)=O DLSWIYLPEUIQAV-UHFFFAOYSA-N 0.000 description 1
- MIJWOJAXARLEHA-WDSKDSINSA-N Ser-Gly-Glu Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCC(O)=O MIJWOJAXARLEHA-WDSKDSINSA-N 0.000 description 1
- WSTIOCFMWXNOCX-YUMQZZPRSA-N Ser-Gly-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CO)N WSTIOCFMWXNOCX-YUMQZZPRSA-N 0.000 description 1
- 208000027583 Serpinopathy Diseases 0.000 description 1
- 102000000070 Sodium-Glucose Transport Proteins Human genes 0.000 description 1
- 108010080361 Sodium-Glucose Transport Proteins Proteins 0.000 description 1
- 102000019355 Synuclein Human genes 0.000 description 1
- 108050006783 Synuclein Proteins 0.000 description 1
- 208000032859 Synucleinopathies Diseases 0.000 description 1
- 208000034799 Tauopathies Diseases 0.000 description 1
- 208000033781 Thyroid carcinoma Diseases 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- YLGQHMHKAASRGJ-WDSOQIARSA-N Trp-Leu-Met Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)N YLGQHMHKAASRGJ-WDSOQIARSA-N 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- BSCBBPKDVOZICB-KKUMJFAQSA-N Tyr-Leu-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O BSCBBPKDVOZICB-KKUMJFAQSA-N 0.000 description 1
- KSCVLGXNQXKUAR-JYJNAYRXSA-N Tyr-Leu-Glu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O KSCVLGXNQXKUAR-JYJNAYRXSA-N 0.000 description 1
- NHOVZGFNTGMYMI-KKUMJFAQSA-N Tyr-Ser-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 NHOVZGFNTGMYMI-KKUMJFAQSA-N 0.000 description 1
- DIOSYUIWOQCXNR-ONGXEEELSA-N Val-Lys-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O DIOSYUIWOQCXNR-ONGXEEELSA-N 0.000 description 1
- PZTZYZUTCPZWJH-FXQIFTODSA-N Val-Ser-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PZTZYZUTCPZWJH-FXQIFTODSA-N 0.000 description 1
- FZNCGRZWXLXZSZ-CIQUZCHMSA-N Voglibose Chemical compound OCC(CO)N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O FZNCGRZWXLXZSZ-CIQUZCHMSA-N 0.000 description 1
- 239000003875 Wang resin Substances 0.000 description 1
- 208000006269 X-Linked Bulbo-Spinal Atrophy Diseases 0.000 description 1
- 102400000752 Xenin Human genes 0.000 description 1
- NERFNHBZJXXFGY-UHFFFAOYSA-N [4-[(4-methylphenyl)methoxy]phenyl]methanol Chemical compound C1=CC(C)=CC=C1COC1=CC=C(CO)C=C1 NERFNHBZJXXFGY-UHFFFAOYSA-N 0.000 description 1
- 229960002632 acarbose Drugs 0.000 description 1
- XUFXOAAUWZOOIT-UHFFFAOYSA-N acarviostatin I01 Natural products OC1C(O)C(NC2C(C(O)C(O)C(CO)=C2)O)C(C)OC1OC(C(C1O)O)C(CO)OC1OC1C(CO)OC(O)C(O)C1O XUFXOAAUWZOOIT-UHFFFAOYSA-N 0.000 description 1
- 108010076089 accutase Proteins 0.000 description 1
- 229940095602 acidifiers Drugs 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 125000002252 acyl group Chemical group 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 210000001789 adipocyte Anatomy 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 210000004100 adrenal gland Anatomy 0.000 description 1
- 229960004733 albiglutide Drugs 0.000 description 1
- OGWAVGNOAMXIIM-UHFFFAOYSA-N albiglutide Chemical compound O=C(O)C(NC(=O)CNC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)CNC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)CNC(=O)C(NC(=O)CNC(=O)C(N)CC=1(N=CNC=1))CCC(=O)O)C(O)C)CC2(=CC=CC=C2))C(O)C)CO)CC(=O)O)C(C)C)CO)CO)CC3(=CC=C(O)C=C3))CC(C)C)CCC(=O)O)CCC(=O)N)C)C)CCCCN)CCC(=O)O)CC4(=CC=CC=C4))C(CC)C)C)CC=6(C5(=C(C=CC=C5)NC=6)))CC(C)C)C(C)C)CCCCN)CCCNC(=N)N OGWAVGNOAMXIIM-UHFFFAOYSA-N 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 230000029936 alkylation Effects 0.000 description 1
- 238000005804 alkylation reaction Methods 0.000 description 1
- 125000005275 alkylenearyl group Chemical group 0.000 description 1
- ZSBOMTDTBDDKMP-OAHLLOKOSA-N alogliptin Chemical compound C=1C=CC=C(C#N)C=1CN1C(=O)N(C)C(=O)C=C1N1CCC[C@@H](N)C1 ZSBOMTDTBDDKMP-OAHLLOKOSA-N 0.000 description 1
- 239000003888 alpha glucosidase inhibitor Substances 0.000 description 1
- 102000030484 alpha-2 Adrenergic Receptor Human genes 0.000 description 1
- 108020004101 alpha-2 Adrenergic Receptor Proteins 0.000 description 1
- 229940126575 aminoglycoside Drugs 0.000 description 1
- 125000004202 aminomethyl group Chemical group [H]N([H])C([H])([H])* 0.000 description 1
- 201000000921 amyloid tumor Diseases 0.000 description 1
- 229950009977 anagliptin Drugs 0.000 description 1
- LDXYBEHACFJIEL-HNNXBMFYSA-N anagliptin Chemical compound C=1N2N=C(C)C=C2N=CC=1C(=O)NCC(C)(C)NCC(=O)N1CCC[C@H]1C#N LDXYBEHACFJIEL-HNNXBMFYSA-N 0.000 description 1
- 229940126317 angiotensin II receptor antagonist Drugs 0.000 description 1
- 230000008485 antagonism Effects 0.000 description 1
- 230000006909 anti-apoptosis Effects 0.000 description 1
- 229940127003 anti-diabetic drug Drugs 0.000 description 1
- 230000003276 anti-hypertensive effect Effects 0.000 description 1
- 239000003529 anticholesteremic agent Substances 0.000 description 1
- 229940127226 anticholesterol agent Drugs 0.000 description 1
- 239000003472 antidiabetic agent Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 229940112930 apidra Drugs 0.000 description 1
- RCHHVVGSTHAVPF-ZPHPLDECSA-N apidra Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@H]1CSSC[C@H]2C(=O)N[C@H](C(=O)N[C@@H](CO)C(=O)N[C@H](C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3C=CC(O)=CC=3)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3N=CNC=3)NC(=O)[C@H](CO)NC(=O)CNC1=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O)=O)CSSC[C@@H](C(N2)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)CN)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C=CC=CC=1)C(C)C)C1=CNC=N1 RCHHVVGSTHAVPF-ZPHPLDECSA-N 0.000 description 1
- 239000002830 appetite depressant Substances 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- 125000000613 asparagine group Chemical group N[C@@H](CC(N)=O)C(=O)* 0.000 description 1
- 229940009098 aspartate Drugs 0.000 description 1
- 108010068265 aspartyltyrosine Proteins 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 229960005370 atorvastatin Drugs 0.000 description 1
- 230000001746 atrial effect Effects 0.000 description 1
- 125000002393 azetidinyl group Chemical group 0.000 description 1
- RROBIDXNTUAHFW-UHFFFAOYSA-N benzotriazol-1-yloxy-tris(dimethylamino)phosphanium Chemical compound C1=CC=C2N(O[P+](N(C)C)(N(C)C)N(C)C)N=NC2=C1 RROBIDXNTUAHFW-UHFFFAOYSA-N 0.000 description 1
- IIBYAHWJQTYFKB-UHFFFAOYSA-N bezafibrate Chemical compound C1=CC(OC(C)(C)C(O)=O)=CC=C1CCNC(=O)C1=CC=C(Cl)C=C1 IIBYAHWJQTYFKB-UHFFFAOYSA-N 0.000 description 1
- 229960000516 bezafibrate Drugs 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 239000000090 biomarker Substances 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- 239000004305 biphenyl Substances 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 229940012191 bupropion / naltrexone Drugs 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 235000019577 caloric intake Nutrition 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 229960000932 candesartan Drugs 0.000 description 1
- SGZAIDDFHDDFJU-UHFFFAOYSA-N candesartan Chemical compound CCOC1=NC2=CC=CC(C(O)=O)=C2N1CC(C=C1)=CC=C1C1=CC=CC=C1C1=NN=N[N]1 SGZAIDDFHDDFJU-UHFFFAOYSA-N 0.000 description 1
- 239000003555 cannabinoid 1 receptor antagonist Substances 0.000 description 1
- 230000023852 carbohydrate metabolic process Effects 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 125000005392 carboxamide group Chemical group NC(=O)* 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 206010061592 cardiac fibrillation Diseases 0.000 description 1
- 230000003293 cardioprotective effect Effects 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 150000001767 cationic compounds Chemical class 0.000 description 1
- 238000000423 cell based assay Methods 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 239000003354 cholesterol ester transfer protein inhibitor Substances 0.000 description 1
- 125000003016 chromanyl group Chemical group O1C(CCC2=CC=CC=C12)* 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- ASARMUCNOOHMLO-WLORSUFZSA-L cobalt(2+);[(2r,3s,4r,5s)-5-(5,6-dimethylbenzimidazol-1-yl)-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl] [(2s)-1-[3-[(1r,2r,3r,4z,7s,9z,12s,13s,14z,17s,18s,19r)-2,13,18-tris(2-amino-2-oxoethyl)-7,12,17-tris(3-amino-3-oxopropyl)-3,5,8,8,13,15,18,19-octamethyl-2 Chemical compound [Co+2].[N-]([C@@H]1[C@H](CC(N)=O)[C@@]2(C)CCC(=O)NC[C@H](C)OP([O-])(=O)O[C@H]3[C@H]([C@H](O[C@@H]3CO)N3C4=CC(C)=C(C)C=C4N=C3)O)\C2=C(C)/C([C@H](C\2(C)C)CCC(N)=O)=N/C/2=C\C([C@H]([C@@]/2(CC(N)=O)C)CCC(N)=O)=N\C\2=C(C)/C2=N[C@]1(C)[C@@](C)(CC(N)=O)[C@@H]2CCC(N)=O ASARMUCNOOHMLO-WLORSUFZSA-L 0.000 description 1
- 230000001054 cortical effect Effects 0.000 description 1
- 239000006184 cosolvent Substances 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000000640 cyclooctyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 230000006240 deamidation Effects 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 208000033679 diabetic kidney disease Diseases 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 125000004177 diethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 125000002147 dimethylamino group Chemical group [H]C([H])([H])N(*)C([H])([H])[H] 0.000 description 1
- UXGNZZKBCMGWAZ-UHFFFAOYSA-N dimethylformamide dmf Chemical compound CN(C)C=O.CN(C)C=O UXGNZZKBCMGWAZ-UHFFFAOYSA-N 0.000 description 1
- 229940090124 dipeptidyl peptidase 4 (dpp-4) inhibitors for blood glucose lowering Drugs 0.000 description 1
- 238000001647 drug administration Methods 0.000 description 1
- 229940127257 dual PPAR agonist Drugs 0.000 description 1
- 229940125542 dual agonist Drugs 0.000 description 1
- 229960005175 dulaglutide Drugs 0.000 description 1
- 108010005794 dulaglutide Proteins 0.000 description 1
- 235000005686 eating Nutrition 0.000 description 1
- 238000002330 electrospray ionisation mass spectrometry Methods 0.000 description 1
- 239000003974 emollient agent Substances 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 238000005265 energy consumption Methods 0.000 description 1
- RIZMRRKBZQXFOY-UHFFFAOYSA-N ethion Chemical group CCOP(=S)(OCC)SCSP(=S)(OCC)OCC RIZMRRKBZQXFOY-UHFFFAOYSA-N 0.000 description 1
- NHWGPUVJQFTOQX-UHFFFAOYSA-N ethyl-[2-[2-[ethyl(dimethyl)azaniumyl]ethyl-methylamino]ethyl]-dimethylazanium Chemical compound CC[N+](C)(C)CCN(C)CC[N+](C)(C)CC NHWGPUVJQFTOQX-UHFFFAOYSA-N 0.000 description 1
- 230000003203 everyday effect Effects 0.000 description 1
- 229940012151 exubera Drugs 0.000 description 1
- OLNTVTPDXPETLC-XPWALMASSA-N ezetimibe Chemical compound N1([C@@H]([C@H](C1=O)CC[C@H](O)C=1C=CC(F)=CC=1)C=1C=CC(O)=CC=1)C1=CC=C(F)C=C1 OLNTVTPDXPETLC-XPWALMASSA-N 0.000 description 1
- YMTINGFKWWXKFG-UHFFFAOYSA-N fenofibrate Chemical compound C1=CC(OC(C)(C)C(=O)OC(C)C)=CC=C1C(=O)C1=CC=C(Cl)C=C1 YMTINGFKWWXKFG-UHFFFAOYSA-N 0.000 description 1
- 229960002297 fenofibrate Drugs 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 229940125753 fibrate Drugs 0.000 description 1
- 230000002600 fibrillogenic effect Effects 0.000 description 1
- 229940012952 fibrinogen Drugs 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 238000002825 functional assay Methods 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 229960002458 gemigliptin Drugs 0.000 description 1
- 238000001415 gene therapy Methods 0.000 description 1
- 208000004104 gestational diabetes Diseases 0.000 description 1
- 210000004907 gland Anatomy 0.000 description 1
- 108010063245 glucagon-like peptide 1 (7-36)amide Proteins 0.000 description 1
- TWSALRJGPBVBQU-PKQQPRCHSA-N glucagon-like peptide 2 Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O)[C@@H](C)CC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)CC)C1=CC=CC=C1 TWSALRJGPBVBQU-PKQQPRCHSA-N 0.000 description 1
- 150000002303 glucose derivatives Chemical class 0.000 description 1
- 230000009229 glucose formation Effects 0.000 description 1
- 230000010030 glucose lowering effect Effects 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 230000020169 heat generation Effects 0.000 description 1
- 230000008588 hemolysis Effects 0.000 description 1
- 230000002440 hepatic effect Effects 0.000 description 1
- 108010045383 histidyl-glycyl-glutamic acid Proteins 0.000 description 1
- 230000003054 hormonal effect Effects 0.000 description 1
- 229940038661 humalog Drugs 0.000 description 1
- 229940103471 humulin Drugs 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M hydrogensulfate Chemical compound OS([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- 125000004356 hydroxy functional group Chemical group O* 0.000 description 1
- 239000002471 hydroxymethylglutaryl coenzyme A reductase inhibitor Substances 0.000 description 1
- 206010020718 hyperplasia Diseases 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 201000008319 inclusion body myositis Diseases 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 229910001411 inorganic cation Inorganic materials 0.000 description 1
- 229960003948 insulin detemir Drugs 0.000 description 1
- 108700039926 insulin glulisine Proteins 0.000 description 1
- 229960002068 insulin lispro Drugs 0.000 description 1
- 230000002608 insulinlike Effects 0.000 description 1
- 230000031146 intracellular signal transduction Effects 0.000 description 1
- 229940121068 invokana Drugs 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 238000006317 isomerization reaction Methods 0.000 description 1
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 1
- 206010023365 keratopathy Diseases 0.000 description 1
- 229940043355 kinase inhibitor Drugs 0.000 description 1
- 229940102988 levemir Drugs 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 229960004773 losartan Drugs 0.000 description 1
- KJJZZJSZUJXYEA-UHFFFAOYSA-N losartan Chemical compound CCCCC1=NC(Cl)=C(CO)N1CC1=CC=C(C=2C(=CC=CC=2)C=2[N]N=NN=2)C=C1 KJJZZJSZUJXYEA-UHFFFAOYSA-N 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229950009585 melogliptin Drugs 0.000 description 1
- XZWYZXLIPXDOLR-UHFFFAOYSA-N metformin Chemical compound CN(C)C(=N)NC(N)=N XZWYZXLIPXDOLR-UHFFFAOYSA-N 0.000 description 1
- 229960003105 metformin Drugs 0.000 description 1
- 229960001110 miglitol Drugs 0.000 description 1
- 125000002950 monocyclic group Chemical group 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 125000002757 morpholinyl group Chemical group 0.000 description 1
- 239000012452 mother liquor Substances 0.000 description 1
- 230000004899 motility Effects 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- 208000031225 myocardial ischemia Diseases 0.000 description 1
- CMWYAOXYQATXSI-UHFFFAOYSA-N n,n-dimethylformamide;piperidine Chemical compound CN(C)C=O.C1CCNCC1 CMWYAOXYQATXSI-UHFFFAOYSA-N 0.000 description 1
- BNPZTRDIESRGTC-IXYVTWBDSA-N n-[2-(dimethylamino)ethyl]-2-methyl-2-[4-[4-[[2-methyl-5-[(2s,3r,4r,5s,6r)-3,4,5-trihydroxy-6-methylsulfanyloxan-2-yl]phenyl]methyl]phenyl]butanoylamino]propanamide Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](SC)O[C@H]1C1=CC=C(C)C(CC=2C=CC(CCCC(=O)NC(C)(C)C(=O)NCCN(C)C)=CC=2)=C1 BNPZTRDIESRGTC-IXYVTWBDSA-N 0.000 description 1
- 230000009826 neoplastic cell growth Effects 0.000 description 1
- 229940117337 nesina Drugs 0.000 description 1
- 230000004112 neuroprotection Effects 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 229960003512 nicotinic acid Drugs 0.000 description 1
- 235000001968 nicotinic acid Nutrition 0.000 description 1
- 239000011664 nicotinic acid Substances 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 230000000269 nucleophilic effect Effects 0.000 description 1
- 208000001797 obstructive sleep apnea Diseases 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- 235000021313 oleic acid Nutrition 0.000 description 1
- 229960005117 olmesartan Drugs 0.000 description 1
- VTRAEEWXHOVJFV-UHFFFAOYSA-N olmesartan Chemical compound CCCC1=NC(C(C)(C)O)=C(C(O)=O)N1CC1=CC=C(C=2C(=CC=CC=2)C=2NN=NN=2)C=C1 VTRAEEWXHOVJFV-UHFFFAOYSA-N 0.000 description 1
- 229950000074 omarigliptin Drugs 0.000 description 1
- 229940001450 onglyza Drugs 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 150000002892 organic cations Chemical class 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- 201000008482 osteoarthritis Diseases 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 210000002571 pancreatic alpha cell Anatomy 0.000 description 1
- 210000000277 pancreatic duct Anatomy 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 229940083256 peripheral vasodilators nicotinic acid and derivative Drugs 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 125000001792 phenanthrenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3C=CC12)* 0.000 description 1
- NMHMNPHRMNGLLB-UHFFFAOYSA-N phloretic acid Chemical compound OC(=O)CCC1=CC=C(O)C=C1 NMHMNPHRMNGLLB-UHFFFAOYSA-N 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 229930029653 phosphoenolpyruvate Natural products 0.000 description 1
- DTBNBXWJWCWCIK-UHFFFAOYSA-N phosphoenolpyruvic acid Chemical compound OC(=O)C(=C)OP(O)(O)=O DTBNBXWJWCWCIK-UHFFFAOYSA-N 0.000 description 1
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 229960005095 pioglitazone Drugs 0.000 description 1
- 125000003386 piperidinyl group Chemical group 0.000 description 1
- 230000001817 pituitary effect Effects 0.000 description 1
- 229940096701 plain lipid modifying drug hmg coa reductase inhibitors Drugs 0.000 description 1
- 229940068918 polyethylene glycol 400 Drugs 0.000 description 1
- 108700002854 polyethylene glycol(B1)- insulin Proteins 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 229920005990 polystyrene resin Polymers 0.000 description 1
- 230000008092 positive effect Effects 0.000 description 1
- 230000000291 postprandial effect Effects 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 201000009104 prediabetes syndrome Diseases 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- GCYXWQUSHADNBF-AAEALURTSA-N preproglucagon 78-108 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1N=CNC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 GCYXWQUSHADNBF-AAEALURTSA-N 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 229940097325 prolactin Drugs 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- WPDCHTSXOPUOII-UHFFFAOYSA-N propan-2-yl 4-[5-methoxy-6-[(2-methyl-6-methylsulfonylpyridin-3-yl)amino]pyrimidin-4-yl]oxypiperidine-1-carboxylate Chemical compound N1=CN=C(OC2CCN(CC2)C(=O)OC(C)C)C(OC)=C1NC1=CC=C(S(C)(=O)=O)N=C1C WPDCHTSXOPUOII-UHFFFAOYSA-N 0.000 description 1
- 235000019833 protease Nutrition 0.000 description 1
- 230000006920 protein precipitation Effects 0.000 description 1
- 229940024999 proteolytic enzymes for treatment of wounds and ulcers Drugs 0.000 description 1
- 239000012264 purified product Substances 0.000 description 1
- 125000000719 pyrrolidinyl group Chemical group 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 108700027806 rGLP-1 Proteins 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 108091006084 receptor activators Proteins 0.000 description 1
- 238000006268 reductive amination reaction Methods 0.000 description 1
- 230000011514 reflex Effects 0.000 description 1
- 230000008844 regulatory mechanism Effects 0.000 description 1
- 230000003979 response to food Effects 0.000 description 1
- 210000001525 retina Anatomy 0.000 description 1
- 210000003994 retinal ganglion cell Anatomy 0.000 description 1
- 238000004007 reversed phase HPLC Methods 0.000 description 1
- 229960004586 rosiglitazone Drugs 0.000 description 1
- 210000003079 salivary gland Anatomy 0.000 description 1
- 229960004937 saxagliptin Drugs 0.000 description 1
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical compound O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 description 1
- 201000000980 schizophrenia Diseases 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 108010060325 semaglutide Proteins 0.000 description 1
- 229950011186 semaglutide Drugs 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 208000007056 sickle cell anemia Diseases 0.000 description 1
- 229960002855 simvastatin Drugs 0.000 description 1
- RYMZZMVNJRMUDD-HGQWONQESA-N simvastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)C(C)(C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 RYMZZMVNJRMUDD-HGQWONQESA-N 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- MUZRGKSNUTWRAF-UHFFFAOYSA-M sodium;2-[4-[4-[5-[[6-(trifluoromethyl)pyridin-3-yl]amino]pyridin-2-yl]phenyl]cyclohexyl]acetate Chemical compound [Na+].C1CC(CC(=O)[O-])CCC1C1=CC=C(C=2N=CC(NC=3C=NC(=CC=3)C(F)(F)F)=CC=2)C=C1 MUZRGKSNUTWRAF-UHFFFAOYSA-M 0.000 description 1
- 230000000392 somatic effect Effects 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 208000002320 spinal muscular atrophy Diseases 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 208000023516 stroke disease Diseases 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 229940124530 sulfonamide Drugs 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 229960000651 tasosartan Drugs 0.000 description 1
- ADXGNEYLLLSOAR-UHFFFAOYSA-N tasosartan Chemical compound C12=NC(C)=NC(C)=C2CCC(=O)N1CC(C=C1)=CC=C1C1=CC=CC=C1C=1N=NNN=1 ADXGNEYLLLSOAR-UHFFFAOYSA-N 0.000 description 1
- 229960005187 telmisartan Drugs 0.000 description 1
- 125000005931 tert-butyloxycarbonyl group Chemical group [H]C([H])([H])C(OC(*)=O)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- TUNFSRHWOTWDNC-HKGQFRNVSA-N tetradecanoic acid Chemical compound CCCCCCCCCCCCC[14C](O)=O TUNFSRHWOTWDNC-HKGQFRNVSA-N 0.000 description 1
- 150000001467 thiazolidinediones Chemical class 0.000 description 1
- HNKJADCVZUBCPG-UHFFFAOYSA-N thioanisole Chemical compound CSC1=CC=CC=C1 HNKJADCVZUBCPG-UHFFFAOYSA-N 0.000 description 1
- 150000003568 thioethers Chemical class 0.000 description 1
- 201000002510 thyroid cancer Diseases 0.000 description 1
- 208000013077 thyroid gland carcinoma Diseases 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- IWYJYHUNXVAVAA-OAHLLOKOSA-N trelagliptin Chemical compound C=1C(F)=CC=C(C#N)C=1CN1C(=O)N(C)C(=O)C=C1N1CCC[C@@H](N)C1 IWYJYHUNXVAVAA-OAHLLOKOSA-N 0.000 description 1
- 229950010728 trelagliptin Drugs 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 125000002221 trityl group Chemical group [H]C1=C([H])C([H])=C([H])C([H])=C1C([*])(C1=C(C(=C(C(=C1[H])[H])[H])[H])[H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- GXPHKUHSUJUWKP-UHFFFAOYSA-N troglitazone Chemical compound C1CC=2C(C)=C(O)C(C)=C(C)C=2OC1(C)COC(C=C1)=CC=C1CC1SC(=O)NC1=O GXPHKUHSUJUWKP-UHFFFAOYSA-N 0.000 description 1
- 229960001641 troglitazone Drugs 0.000 description 1
- GXPHKUHSUJUWKP-NTKDMRAZSA-N troglitazone Natural products C([C@@]1(OC=2C(C)=C(C(=C(C)C=2CC1)O)C)C)OC(C=C1)=CC=C1C[C@H]1SC(=O)NC1=O GXPHKUHSUJUWKP-NTKDMRAZSA-N 0.000 description 1
- 229960004799 tryptophan Drugs 0.000 description 1
- 238000007492 two-way ANOVA Methods 0.000 description 1
- 230000002485 urinary effect Effects 0.000 description 1
- IBIDRSSEHFLGSD-UHFFFAOYSA-N valinyl-arginine Natural products CC(C)C(N)C(=O)NC(C(O)=O)CCCN=C(N)N IBIDRSSEHFLGSD-UHFFFAOYSA-N 0.000 description 1
- 229960004699 valsartan Drugs 0.000 description 1
- SJSNUMAYCRRIOM-QFIPXVFZSA-N valsartan Chemical compound C1=CC(CN(C(=O)CCCC)[C@@H](C(C)C)C(O)=O)=CC=C1C1=CC=CC=C1C1=NN=N[N]1 SJSNUMAYCRRIOM-QFIPXVFZSA-N 0.000 description 1
- 239000013598 vector Substances 0.000 description 1
- 229940007428 victoza Drugs 0.000 description 1
- SYOKIDBDQMKNDQ-XWTIBIIYSA-N vildagliptin Chemical compound C1C(O)(C2)CC(C3)CC1CC32NCC(=O)N1CCC[C@H]1C#N SYOKIDBDQMKNDQ-XWTIBIIYSA-N 0.000 description 1
- 229960001254 vildagliptin Drugs 0.000 description 1
- 229960001729 voglibose Drugs 0.000 description 1
- 239000007762 w/o emulsion Substances 0.000 description 1
- 208000027121 wild type ATTR amyloidosis Diseases 0.000 description 1
- IDHVLSACPFUBDY-QCDLPZBNSA-N xenin Chemical compound C([C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CN=CN1 IDHVLSACPFUBDY-QCDLPZBNSA-N 0.000 description 1
- 108010006643 xenin 25 Proteins 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/22—Hormones
- A61K38/26—Glucagons
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/575—Hormones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/22—Hormones
- A61K38/2264—Obesity-gene products, e.g. leptin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/22—Hormones
- A61K38/28—Insulins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/30—Drugs for disorders of the nervous system for treating abuse or dependence
- A61P25/36—Opioid-abuse
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/575—Hormones
- C07K14/605—Glucagons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Endocrinology (AREA)
- Gastroenterology & Hepatology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Zoology (AREA)
- Diabetes (AREA)
- Epidemiology (AREA)
- Obesity (AREA)
- Immunology (AREA)
- Genetics & Genomics (AREA)
- Hematology (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Toxicology (AREA)
- Heart & Thoracic Surgery (AREA)
- Cardiology (AREA)
- Addiction (AREA)
- Emergency Medicine (AREA)
- Neurosurgery (AREA)
- Urology & Nephrology (AREA)
- Vascular Medicine (AREA)
- Child & Adolescent Psychology (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Psychiatry (AREA)
Abstract
Description
| 구조 | IUPAC | 명칭 |
| (S)-4-카르복시-4-헥사데카노일아미노-부티릴- | γE-x53 | |
| (S)-4-카르복시-4-옥타데카노일아미노부티릴- | γE-x70 | |
| 4-헥사데카노일아미노-부티릴- | GABA-x53 | |
| 4-{3-[(R)-2,5,7,8-테트라메틸-2-((4R,8R)-4,8,12-트리메틸-트리데실)-크로만-6-일옥시카르보닐]-프로피오닐아미노}-부티릴- | GABA-x60 | |
| 4-옥타데카노일아미노-부티릴- | GABA-x70 | |
| 4-((Z)-옥타데크-9-엔오일아미노)-부티릴- | GABA-x74 | |
| 6-[(4,4-디페닐-시클로헥실옥시)-하이드록시-포스포릴옥시]-헥사노일- | 포스포1 | |
| 헥사데카노일- | x53 | |
| (S)-4-카르복시-4-(15-카르복시-펜타데카노일아미노)-부티릴- | x52 | |
| (S)-4-카르복시-4-{3-[3-((2S,3R,4S,5R)-5-카르복시-2,3,4,5-테트라하이드록시-펜타노일아미노)-프로피오닐아미노]-프로피오닐아미노}-부티릴- | γE-x59 | |
| (S)-4-카르복시-4-{3-[(R)-2,5,7,8-테트라메틸-2-((4R,8R)-4,8,12-트리메틸-트리데실)-크로만-6-일옥시카르보닐]-프로피오닐아미노}-부티릴- | γE-x60 | |
| (S)-4-카르복시-4-((9Z,12Z)-옥타데카-9,12-디에노일아미노)-부티릴- | γE-x61 | |
| (S)-4-카르복시-4-[6-((2S,3R,4S,5R)-5-카르복시-2,3,4,5-테트라하이드록시-펜타노일아미노)-헥사노일아미노]-부티릴- | γE-x64 | |
| (S)-4-카르복시-4-((2S,3R,4S,5R)-5-카르복시-2,3,4,5-테트라하이드록시-펜타노일아미노)-부티릴- | γE-x65 | |
| (S)-4-카르복시-4-테트라데카노일아미노-부티릴- | γE-x69 | |
| (S)-4-(11-벤질옥시카르보닐-운데카노일아미노)-4-카르복시-부티릴- | γE-x72 | |
| (S)-4-카르복시-4-[11-((2S,3R,4R,5R)-2,3,4,5,6-펜타하이드록시-헥실카르바모일)-운데카노일아미노]-부티릴- | γE-x73 | |
| (S)-4-카르복시-4-((Z)-옥타데크-9-엔오일아미노)-부티릴- | γE-x74 | |
| (S)-4-카르복시-4-(4-도데실옥시-벤조일아미노)-부티릴- | γE-x75 | |
| (S)-4-카르복시-4-헤니코사노일아미노-부티릴- | γE-x76 | |
| (S)-4-카르복시-4-도코사노일아미노-부티릴- | γE-x77 | |
| (S)-4-카르복시-4-((Z)-노나데크-10-엔오일아미노)-부티릴- | γE-x79 | |
| (S)-4-카르복시-4-(4-데실옥시-벤조일아미노)-부티릴- | γE-x80 | |
| (S)-4-카르복시-4-[(4'-옥틸옥시-비페닐-4-카르보닐)-아미노]-부티릴- | γE-x81 | |
| (S)-4-카르복시-4-(12-페닐-도데카노일아미노)-부티릴- | γE-x82 | |
| (S)-4-카르복시-4-아이코사노일아미노-부티릴- | γE-x95 | |
| (S)-4-카르복시-4-((S)-4-카르복시-4-헥사데카노일아미노-부티릴아미노)-부티릴- | γE-γE-x53 | |
| (S)-4-카르복시-4-((S)-4-카르복시-4-옥타데카노일아미노-부티릴아미노)-부티릴- | γE-γE-x70 | |
| 3-(3-옥타데카노일아미노-프로피오닐아미노)-프로피오닐- | β-Ala-β-Ala-x70 | |
| 3-(3-헥사데카노일아미노-프로피오닐아미노)-프로피오닐- | β-Ala-β-Ala-x53 | |
| 3-헥사데카노일아미노-프로피오닐- | β-Ala-x53 | |
| (S)-4-카르복시-4-[(R)-4-((3R,5S,7R,8R,9R,10S,12S,13R,14R,17R)-3,7,12-트리하이드록시-8,10,13-트리메틸-헥사데카하이드로-시클로펜타[a]페난트렌-17-일)-펜타노일아미노]-부티릴- | γE-x16 | |
| (S)-4-카르복시-4-[(R)-4-((3R,5R,8R,9S,10S,13R,14S,17R)-3-하이드록시-10,13-디메틸-헥사데카하이드로-시클로펜타[a]페난트렌-17-일)-펜타노일아미노]-부티릴- | γE-x19 | |
| (S)-4-카르복시-4-((9S,10R)-9,10,16-트리하이드록시-헥사데카노일아미노)-부티릴- | γE-x25 | |
| 테트라데카노일- | x69 | |
| 11-카르복시-운데카노일- | x71 | |
| 11-벤질옥시카르보닐-운데카노일- | x72 | |
| (S)-4-카르복시-4-((S)-4-카르복시-4-테트라데카노일아미노-부티릴아미노)-부티릴- | γE-γE-x69 | |
| 6-[하이드록시-(나프탈렌-2-일옥시)-포스포릴옥시]-헥사노일- | 포스포2 | |
| 6-[하이드록시-(5-페닐-펜틸옥시)-포스포릴옥시]-헥사노일- | 포스포3 | |
| 4-(나프탈렌-2-설포닐아미노)-4-옥소-부티릴- | 설폰아미드1 | |
| 4-(비페닐-4-설포닐아미노)-4-옥소-부티릴- | 설폰아미드2 | |
| (S)-4-카르복시-4-{(S)-4-카르복시-4-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴- | x100 | |
| (S)-4-카르복시-4-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴- | x101 | |
| (S)-4-카르복시-2-{(S)-4-카르복시-2-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴- | x102 | |
| (S)-4-카르복시-2-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴- | x103 | |
| (S)-4-카르복시-4-{(S)-4-카르복시-4-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴- | x104 | |
| (S)-4-카르복시-4-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴- | x105 | |
| (S)-4-카르복시-2-{(S)-4-카르복시-2-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴- | x106 | |
| (S)-4-카르복시-2-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴- | x107 | |
| 2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸- | x108 | |
| 2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸 | x109 | |
| (S)-4-카르복시-4-((S)-4-카르복시-4-{(S)-4-카르복시-4-[(S)-4-카르복시-4-(19-카르복시-노나데카노일아미노)-부티릴아미노]-부티릴아미노}-부티릴아미노)-부티릴 | x110 | |
| 2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(16-1H-테트라졸-5-일-헥사데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸- | x111 | |
| 2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(16-카르복시-헥사데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸- | x112 | |
| (S)-4-카르복시-4-{(S)-4-카르복시-4-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-부티릴아미노}-부티릴- | x113 | |
| (S)-4-카르복시-4-((S)-4-카르복시-4-{2-[2-(2-{2-[2-(2-{(S)-4-카르복시-4-[10-(4-카르복시-페녹시)-데카노일아미노]-부티릴아미노}-에톡시)-에톡시]-아세틸아미노}-에톡시)-에톡시]-아세틸아미노}-부티릴- | x114 | |
| (S)-4-카르복시-4-{(S)-4-카르복시-4-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(7-카르복시-헵타노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴- | x115 | |
| (S)-4-카르복시-4-{(S)-4-카르복시-4-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(11-카르복시-운데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴- | x116 | |
| (S)-4-카르복시-4-{(S)-4-카르복시-4-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(13-카르복시-트리데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴- | x117 | |
| (S)-4-카르복시-4-{(S)-4-카르복시-4-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(15-카르복시-펜타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴- | x118 | |
| (S)-4-카르복시-4-{(S)-4-카르복시-4-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(19-카르복시-노나데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아노]-부티릴아미노}-부티릴- | x119 |
a) (위 배출에 대한 지표로서) 잔류 위 내용물에 미치는 SEQ ID NO: 97 및 리라글루타이드(모두 0.02 mg/kg, 피하)의 효과
b) 소장 이동성에 미치는 SEQ ID NO: 97 및 리라글루타이드(모두 0.02 mg/kg, 피하)의 효과
c) (위 배출에 대한 지표로서) 잔류 위 내용물에 미치는, 0.02 및 0.002 mg/kg, 피하에서의 SEQ ID NO: 97의 효과
d) 소장 이동성에 미치는, 0.02 및 0.002 mg/kg, 피하에서의 SEQ ID NO: 97의 효과
도 2. 암컷 NMRI-마우스에서 22시간 식품 섭취에 미치는, 0.1 및 0.01 mg/kg, 피하에서의 SEQ ID NO: 97의 효과. 데이터는 평균 + SEM이다. *p<0.05.
도 3. 암컷의 식이로 유도한 비만 C57BL/6NCrl 마우스(고지방 식이 9개월)의 혈당에 미치는 SEQ ID NO: 97의 피하 투여의 급성 효과. 데이터는 평균 + SEM이다. *p<0.05.
도 4. 암컷의 렙틴-수용체 결핍 당뇨성 db/db 마우스의 혈당에 미치는 SEQ ID NO: 97의 피하 투여의 급성 효과. 데이터는 평균 + SEM이다. *p<0.05.
도 5. 암컷의 렙틴-수용체 결핍 당뇨성 db/db 마우스에서 4주간의 SEQ ID NO: 97을 이용한 피하 치료 전과 후의 글루코오스 수준. 데이터는 평균 + SEM이다.
도 6. 암컷의 렙틴-수용체 결핍 당뇨성 db/db 마우스에서 4주간의 SEQ ID NO: 97을 이용한 피하 치료 전과 후의 HbA1c 수준. 데이터는 평균 + SEM이다.
도 7. 수컷의 고지방 급이 C57BL/6N Crl 마우스에서 3주간의 SEQ ID NO: 24를 이용한 피하 치료 중의 체중 발달. 데이터는 평균 + SEM이다.
도 8. 수컷의 고지방 급이 C57BL/6N Crl 마우스에서 3주간의 SEQ ID NO: 24를 이용한 피하 치료 중의 상대적인 체중 변화 %. 데이터는 평균 + SEM이다.
도 9. 수컷의 고지방 급이 C57BL/6N Crl 마우스에서 3주간의 SEQ ID NO: 24를 이용한 치료 전과 후의, 브루커(Bruker) 미니스펙을 이용하여 핵자기 공명(NMR)으로 측정한 총 지방 질량의 결정. 데이터는 평균 + SEM이다.
도 10. 암컷의 렙틴-수용체 결핍 당뇨성 db/db 마우스의 혈당에 미치는 SEQ ID NO: 24의 피하 투여의 급성 효과. 데이터는 평균 + SEM이다.
도 11. 암컷의 렙틴-수용체 결핍 당뇨성 db/db 마우스에서 4주간의 SEQ ID NO: 24를 이용한 피하 치료 전과 후의 글루코오스 수준. 데이터는 평균 + SEM이다.
도 12. 암컷의 렙틴-수용체 결핍 당뇨성 db/db 마우스에서 4주간의 SEQ ID NO: 24를 이용한 피하 치료 전과 후의 HbA1c 수준. 데이터는 평균 + SEM이다.
| 합성된 펩타이드의 목록 및 계산된 분자량 대 발견된 분자량의 비교 | ||
| SEQ ID NO | 계산된 질량 | 발견된 질량 |
| 4 | 4553.1 | 4552.4 |
| 5 | 4422.0 | 4421.4 |
| 6 | 5046.9 | 5046.8 |
| 7 | 4396.0 | 4395.1 |
| 8 | 4610.2 | 4609.8 |
| 9 | 4518.1 | 4518.2 |
| 10 | 4624.2 | 4624.6 |
| 11 | 4425.0 | 4424.4 |
| 12 | 4352.0 | 4351.2 |
| 13 | 4395.0 | 4394.1 |
| 14 | 4396.9 | 4396.0 |
| 15 | 4395.0 | 4394.4 |
| 16 | 4483.0 | 4482.0 |
| 17 | 4483.0 | 4483.2 |
| 18 | 4439.9 | 4439.1 |
| 19 | 4481.1 | 4480.5 |
| 20 | 4440.9 | 4440.0 |
| 21 | 4439.0 | 4438.2 |
| 22 | 4468.0 | 4467.9 |
| 23 | 4537.2 | 4536.5 |
| 24 | 4440.0 | 4439.5 |
| 25 | 4438.0 | 4437.4 |
| 26 | 4468.1 | 4467.2 |
| 27 | 4466.1 | 4465.3 |
| 28 | 4454.0 | 4454.0 |
| 29 | 4438.1 | 4437.3 |
| 30 | 4426.0 | 4425.9 |
| 31 | 4424.0 | 4423.9 |
| 32 | 4310.9 | 4310.3 |
| 33 | 4308.9 | 4308.3 |
| 34 | 4468.0 | 4467.9 |
| 35 | 4439.9 | 4439.4 |
| 36 | 4438.0 | 4437.3 |
| 37 | 4454.0 | 4453.9 |
| 38 | 4452.0 | 4451.9 |
| 39 | 4425.9 | 4425.9 |
| 40 | 4468.0 | 4467.4 |
| 41 | 4466.0 | 4465.4 |
| 42 | 4310.8 | 4310.3 |
| 43 | 4308.9 | 4308.3 |
| 44 | 4468.0 | 4467.4 |
| 45 | 4494.1 | 4493.4 |
| 46 | 4423.0 | 4422.3 |
| 47 | 4482.0 | 4482.0 |
| 48 | 4466.1 | 4465.4 |
| 49 | 4597.1 | 4596.4 |
| 50 | 4424.0 | 4423.5 |
| 51 | 4496.1 | 4495.2 |
| 52 | 4625.2 | 4626.0 |
| 53 | 4452.1 | 4452.0 |
| 54 | 4509.1 | 4509.0 |
| 55 | 4494.0 | 4493.7 |
| 56 | 4450.0 | 4449.6 |
| 57 | 4742.4 | 4741.6 |
| 58 | 4698.4 | 4698.0 |
| 59 | 4538.2 | 4538.3 |
| 60 | 4552.2 | 4552.1 |
| 61 | 4508.1 | 4507.7 |
| 62 | 4490.0 | 4490.2 |
| 63 | 4474.0 | 4474.3 |
| 64 | 4474.0 | 4474.3 |
| 65 | 4496.1 | 4495.5 |
| 66 | 4338.9 | 4338.4 |
| 67 | 4496.1 | 4495.7 |
| 68 | 4551.2 | 4550.5 |
| 69 | 4422.1 | 4421.5 |
| 70 | 4466.1 | 4465.5 |
| 71 | 4539.1 | 4538.8 |
| 72 | 4525.0 | 4524.8 |
| 73 | 4562.1 | 4561.5 |
| 74 | 4539.1 | 4538.4 |
| 75 | 4510.1 | 4509.4 |
| 76 | 4381.0 | 4380.3 |
| 77 | 4551.1 | 4550.5 |
| 78 | 4553.1 | 4552.7 |
| 79 | 4567.1 | 4566.7 |
| 80 | 4583.1 | 4582.4 |
| 81 | 4454.0 | 4453.5 |
| 82 | 4696.3 | 4695.8 |
| 83 | 4567.1 | 4566.7 |
| 84 | 4596.2 | 4595.4 |
| 85 | 4610.2 | 4609.7 |
| 86 | 4513.0 | 4512.8 |
| 87 | 4624.2 | 4623.4 |
| 88 | 4623.2 | 4622.5 |
| 89 | 4856.5 | 4856.3 |
| 90 | 4554.1 | 4553.7 |
| 91 | 4646.1 | 4645.8 |
| 92 | 4626.2 | 4625.5 |
| 93 | 4596.1 | 4595.4 |
| 94 | 4596.1 | 4595.3 |
| 95 | 4610.2 | 4609.5 |
| 96 | 4640.2 | 4639.8 |
| 97 | 4582.1 | 4581.7 |
| 98 | 4651.3 | 4651.1 |
| 99 | 4672.3 | 4672.1 |
| 100 | 4638.3 | 4638.0 |
| 101 | 4638.3 | 4638.2 |
| 102 | 4652.2 | 4652.2 |
| 103 | 4664.2 | 4663.7 |
| 104 | 4830.4 | 4830.3 |
| 105 | 5711.5 | 5711.2 |
| 106 | 4806.6 | 4806.5 |
| 107 | 4766.5 | 4766.0 |
| 108 | 4792.6 | 4792.6 |
| 109 | 4834.6 | 4834.5 |
| 110 | 4778.5 | 4778.9 |
| 111 | 4724.3 | 4723.9 |
| 112 | 4595.2 | 4594.7 |
| 113 | 4637.2 | 4636.7 |
| 114 | 4508.1 | 4507.7 |
| 115 | 4580.1 | 4579.4 |
| 116 | 4596.1 | 4595.4 |
| 117 | 4594.2 | 4593.4 |
| 118 | 4539.1 | 4538.6 |
| 119 | 4424.0 | 4423.4 |
| 120 | 4553.1 | 4552.5 |
| 121 | 4466.1 | 4466.0 |
| 122 | 4337.0 | 4336.5 |
| 123 | 4511.0 | 4511.0 |
| 124 | 4525.1 | 4525.0 |
| 125 | 4624.2 | 4623.7 |
| 126 | 4652.2 | 4651.7 |
| 127 | 4638.2 | 4637.7 |
| 128 | 4555.1 | 4554.3 |
| 129 | 4569.1 | 4568.6 |
| 131 | 4381.0 | 4380.9 |
| 133 | 4506.2 | 4505.4 |
| 134 | 4470.0 | 4470.0 |
| 135 | 4484.0 | 4484.0 |
| 136 | 4468.1 | 4468.0 |
| 137 | 4463.0 | 4462.4 |
| 138 | 4475.2 | 4475.8 |
| 139 | 4495.2 | 4495.6 |
| 140 | 4555.1 | 4554.0 |
| 142 | 4482.1 | 4481.4 |
| 143 | 4468.0 | 4467.0 |
| 144 | 4440.0 | 4439.1 |
| 145 | 4442.0 | 4440.0 |
| 146 | 4468.0 | 4466.1 |
| 147 | 4441.0 | 4438.8 |
| 148 | 4464.1 | 4462.2 |
| 149 | 4506.2 | 4505.4 |
| 150 | 4453.1 | 4453.6 |
| 151 | 4468.0 | 4467.9 |
| 152 | 4593.2 | 4592.1 |
| 153 | 4506.2 | 4505.1 |
| 155 | 4423.9 | 4423.9 |
| 156 | 4452.0 | 4451.9 |
| 157 | 4454.0 | 4453.9 |
| 158 | 4464.1 | 4462.8 |
| 159 | 4506.2 | 4504.8 |
| 161 | 4581.2 | 4580.7 |
| 162 | 4565.2 | 4564.2 |
| 163 | 4567.1 | 4566.4 |
| 164 | 4468.1 | 4468.0 |
| 166 | 4541.1 | 4540.8 |
| 173 | 4442.0 | 4441.9 |
| 174 | 4609.2 | 4608.3 |
| 175 | 4595.2 | 4594.8 |
| 183 | 4214.6 | 4214.1 |
| 184 | 4188.6 | 4190.7 |
| 185 | 4259.7 | 4259.0 |
| 186 | 4231.7 | 4231.0 |
| 187 | 4188.6 | 4188.4 |
| 188 | 4174.6 | 4172.0 |
| 189 | 4075.5 | 4074.8 |
| 190 | 4145.6 | 4145.1 |
| 191 | 4057.4 | 4056.2 |
| 192 | 4043.4 | 4043.4 |
| 193 | 4043.4 | 4043.2 |
| 196 | 4496.1 | 4494.4 |
| 197 | 4577.3 | 4575.6 |
| 198 | 4563.2 | 4561.2 |
| 199 | 4593.2 | 4591.2 |
| 200 | 4591.3 | 4589.7 |
| 201 | 4548.3 | 4546.2 |
| 202 | 4536.2 | 4534.0 |
| 203 | 4534.2 | 4532.4 |
| 204 | 4548.3 | 4546.2 |
| 205 | 4591.3 | 4590.4 |
| 206 | 4565.3 | 4567.0 |
| 207 | 4710.3 | 4710.6 |
| 208 | 4562.1 | 4559.6 |
| 209 | 4620.3 | 4618.8 |
| 210 | 4618.4 | 4616.1 |
| 211 | 4533.3 | 4532.4 |
| 212 | 4575.3 | 4573.5 |
| 213 | 4493.1 | 4493.4 |
| 214 | 4521.1 | 4523.4 |
| 215 | 4535.2 | 4536.9 |
| 217 | 4544.2 | 4545.0 |
| 219 | 4546.2 | 4545.3 |
| 221 | 4495.1 | 4494.4 |
| 222 | 4523.1 | 4522.4 |
| 226 | 4622.2 | 4621.6 |
| 227 | 4631.2 | 4629.6 |
| 비슷한 방식으로 합성될 수 있는 펩타이드의 목록 |
| SEQ ID NO |
| 130 |
| 132 |
| 141 |
| 154 |
| 160 |
| 165 |
| 167 |
| 168 |
| 169 |
| 170 |
| 171 |
| 172 |
| 176 |
| 177 |
| 178 |
| 179 |
| 180 |
| 181 |
| 182 |
| 218 |
| 223 |
| 228 |
| 229 |
| 화학적 안정성 및 용해도 | ||||
| SEQ ID NO | 안정성 | 용해도 [mg/ml] | ||
| pH4.5 | pH7.4 | pH4.5 | pH7.4 | |
| 35 | 100 | 100 | >1000 | >1000 |
| 36 | 99.7 | 100 | >1000 | >1000 |
| 44 | 99.1 | 99.4 | >1000 | >1000 |
| 24 | 100 | 100 | >1000 | >1000 |
| 25 | 99.6 | 99.6 | >1000 | >1000 |
| 66 | 100 | 98.1 | >1000 | >1000 |
| 82 | 98.4 | 99.9 | >1000 | >1000 |
| 126 | 99.5 | 91.4 | >1000 | >1000 |
| 85 | 95.9 | 85.8 | >1000 | >968.6 |
| 97 | 99.5 | 96.5 | >2000 | >2000 |
| 70 | 98.2 | 97.5 | >1000 | >1000 |
| 4 | 99.5 | 98.8 | >815 | >910 |
| 117 | 98.3 | 87.2 | >1000 | >1000 |
| 121 | 100 | 90.5 | >1000 | >980 |
| 195 | 0 | >985 | ||
| GLP-1 및 글루카곤 수용체에서 엑센딘-4 유도체의 EC50 값(pM로 나타냄) | ||
| SEQ ID NO | EC50 hGLP-1R | EC50 h글루카곤-R |
| 2 | 0.7 | > 10000000 |
| 3 | 56.6 | 1.0 |
| 4 | 5 | 4 |
| 5 | 11 | 109 |
| 6 | 141 | 18.9 |
| 7 | 3.5 | 20.7 |
| 8 | 6.3 | 2.3 |
| 9 | 2.2 | 4.1 |
| 10 | 9.2 | 1.7 |
| 11 | 3.6 | 25.7 |
| 12 | 4.6 | 263 |
| 13 | 3.1 | 281 |
| 14 | 4.6 | 94.7 |
| 15 | 6.6 | 176 |
| 16 | 2.8 | 117 |
| 17 | 1.7 | 93.1 |
| 18 | 2.6 | 152 |
| 19 | 1.9 | 104 |
| 20 | 3.8 | 104 |
| 21 | 3.8 | 144 |
| 22 | 1.1 | 2.4 |
| 23 | 5.6 | 126 |
| 24 | 1.9 | 9.4 |
| 25 | 4.2 | 40.6 |
| 26 | 5.1 | 5.4 |
| 27 | 7.7 | 25.1 |
| 28 | 5.5 | 12.6 |
| 29 | 5.9 | 87.9 |
| 30 | 3.2 | 7 |
| 31 | 1.7 | 9.3 |
| 32 | 10.2 | 188 |
| 33 | 11.2 | 473 |
| 34 | 1.5 | 6.7 |
| 35 | 1.5 | 14.2 |
| 36 | 2.7 | 45.9 |
| 37 | 1.5 | 12.9 |
| 38 | 2.9 | 53.1 |
| 39 | 2.7 | 7.6 |
| 40 | 2.6 | 4.8 |
| 41 | 3.3 | 20.7 |
| 42 | 10.2 | 199 |
| 43 | 4.1 | 443 |
| 44 | 2.7 | 12 |
| 45 | 7.5 | 19.9 |
| 46 | 3.2 | 25.1 |
| 47 | 2.2 | 10.3 |
| 48 | 5.9 | 53.6 |
| 49 | 1.1 | 2.9 |
| 50 | 3.3 | 11.1 |
| 51 | 2.7 | 3 |
| 52 | 1.9 | 2 |
| 53 | 5.4 | 6.5 |
| 54 | 4.8 | 4 |
| 55 | 5.4 | 15.8 |
| 56 | 4.5 | 29.3 |
| 57 | 45 | 8 |
| 58 | 45.6 | 15.1 |
| 59 | 7.9 | 6.8 |
| 60 | 38.4 | 19.3 |
| 61 | 5.3 | 16 |
| 62 | 3.9 | 10.6 |
| 63 | 4.9 | 8.4 |
| 64 | 3.1 | 6.9 |
| 65 | 5 | 5.6 |
| 66 | 8.4 | 113 |
| 67 | 15.7 | 3 |
| 68 | 7.9 | 5.7 |
| 69 | 44.8 | 52.4 |
| 70 | 6.5 | 40.9 |
| 71 | 20.5 | 5.6 |
| 72 | 25.9 | 386 |
| 73 | 4.1 | 1.7 |
| 74 | 4.2 | 1.3 |
| 75 | 11.1 | 12.5 |
| 76 | 44.9 | 162 |
| 77 | 4.3 | 11.9 |
| 78 | 17.8 | 1.6 |
| 79 | 23.3 | 7.5 |
| 80 | 5.8 | 1 |
| 81 | 48 | 7.1 |
| 82 | 11.7 | 4.7 |
| 83 | 53.9 | 41.3 |
| 84 | 8.1 | 4.3 |
| 85 | 8.1 | 10.4 |
| 86 | 4.9 | 3.5 |
| 87 | 3 | 1.3 |
| 88 | 2.4 | 1.6 |
| 89 | 35.6 | 13.7 |
| 90 | 8.8 | 3.7 |
| 91 | 15.1 | 8.9 |
| 92 | 26 | 1 |
| 93 | 10.7 | 2.6 |
| 94 | 5.2 | 2.1 |
| 95 | 20.6 | 9.2 |
| 96 | 74.3 | 3.4 |
| 97 | 3.5 | 1 |
| 98 | 9.6 | 1.4 |
| 99 | 15.9 | 2.6 |
| 100 | 13.5 | 2 |
| 101 | 9.8 | 1.7 |
| 102 | 7.2 | 1.1 |
| 103 | 10.1 | 1.7 |
| 104 | 6.5 | 1.1 |
| 105 | 7.9 | 1 |
| 106 | 210 | 10.5 |
| 107 | 188 | 37.8 |
| 108 | 197 | 9 |
| 109 | 430 | 28.6 |
| 110 | 213 | 7.2 |
| 111 | 8.1 | 2.5 |
| 112 | 33.6 | 21.1 |
| 113 | 11.4 | 5.4 |
| 114 | 62.3 | 31.1 |
| 115 | 2.4 | 1.9 |
| 116 | 6 | 3.6 |
| 117 | 3.8 | 16.5 |
| 118 | 15.3 | 4.3 |
| 119 | 30.8 | 41.2 |
| 121 | 6.1 | 23.7 |
| 122 | 24.9 | 156 |
| 123 | 2.6 | 9.7 |
| 124 | 3 | 8.4 |
| 125 | 31.4 | 6.9 |
| 126 | 6.6 | 6.8 |
| 127 | 14.7 | 9.4 |
| 128 | 6.2 | 1.6 |
| 129 | 14.8 | 4.1 |
| 131 | 9.1 | 24.9 |
| 138 | 5.5 | 9.2 |
| 140 | 1.3 | 1.5 |
| 142 | 4.1 | 2.1 |
| 150 | 6 | 35.5 |
| 152 | 3.2 | 2.3 |
| 155 | 2.5 | 25.1 |
| 156 | 2.9 | 12.5 |
| 161 | 5 | 2.4 |
| 162 | 3.1 | 2.4 |
| 173 | 5.7 | 5.9 |
| 174 | 2.6 | 1.9 |
| 175 | 2.5 | 3.1 |
| 196 | 7.8 | 1.8 |
| 197 | 6.8 | 5.8 |
| 198 | 8.2 | 2.4 |
| 199 | 10.1 | 7.2 |
| 200 | 4.6 | 4.4 |
| 201 | 22.7 | 29.6 |
| 202 | 26.2 | 6.9 |
| 203 | 34.9 | 13.1 |
| 204 | 34.1 | 12.5 |
| 205 | 12.3 | 5.2 |
| 206 | 3.2 | 12.5 |
| 207 | 1.1 | 1.2 |
| 208 | 2.0 | 1.3 |
| 209 | 5.4 | 1.9 |
| 210 | 6.7 | 3.0 |
| 211 | 15.5 | 26.4 |
| 212 | 14.1 | 6.6 |
| 213 | 2.7 | 59.1 |
| 214 | 4.2 | 16.0 |
| 215 | 5.3 | 42.6 |
| 216 | 4.7 | 19.5 |
| 217 | 4.3 | 2.1 |
| 219 | 2.1 | 3.7 |
| 220 | 2.0 | 2.3 |
| 221 | 1.5 | 9.2 |
| 222 | 1.8 | 2.9 |
| 226 | 1.4 | 19.1 |
| 227 | 1.4 | 1.1 |
| 엑센딘-4 유도체의 약물동력학 프로파일 | ||
| SEQ ID NO | T1/2 [h] | Cmax [ng/ml] |
| 35 | 3.6 | 4910 |
| 36 | 3.8 | 5260 |
| 44 | 3.4 | 2450 |
| 24 | 3.7 | 6560 |
| 8 | 3.3 | 2680 |
| 126 | 1.5 | 3160 |
| 97 | 3.2 | 2000 |
| 4 | 2.8 | 3590 |
| 117 | 2.7 | 5000 |
| 5 | 1.7 | 3180 |
| 4주 치료 기간에 걸친 DIO 마우스의 체중 변화(평균 ± SEM) | ||
| 예(용량) | 전체 체중 변화(g) | 체지방 변화(g) |
| 표준 식이 대조군 | -0.7 ± 0.5 | -1.1 ± 0.5 |
| 고지방 식이 대조군 | 1.3 ± 0.5 | 1.0 ± 0.4 |
| SEQ ID NO: 97 (3 μg/kg) | -0.9 ± 1.0 | -0.5 ± 0.8 |
| SEQ ID NO: 97 (10 μg/kg) | -3.0 ± 1.4* | -2.5 ± 1.0* |
| SEQ ID NO: 97 (100 μg/kg) | -2.3 ± 0.9* | -2.4 ± 0.8* |
| 14번 위치에 비 기능화된 아미노산을 포함하는 엑센딘-4 유도체 대 14번 위치에 기능화된 아미노산을 포함하는 엑센딘-4 유도체의 비교. GLP-1 및 글루카곤 수용체에서의 EC50 값을 pM로 나타내었다(M=메티오닌, K=리신, Nle=노르류신, γE-x53=(S)-4-카르복시-4-헥사데카노일아미노-부티릴-, Ac=아세테이트) | |||
| SEQ ID NO | EC50 hGLP-1R | EC50 h글루카곤-R | 14번 위치의 잔기 |
| 182 | 5.8 | 419.0 | M |
| 115 | 2.4 | 1.9 | K(γE-x53) |
| 183 | 1020.0 | 916.0 | K |
| 97 | 6.8 | 1.2 | K(γE-x53) |
| 194 | 159.0 | 1290.0 | K(Ac) |
| 184 | 85.7 | 991.0 | M |
| 4 | 5.0 | 4.0 | K(γE-x53) |
| 185 | 75.7 | 262.0 | M |
| 125 | 31.4 | 6.9 | K(γE-x53) |
| 186 | 102.0 | 590.0 | M |
| 84 | 8.1 | 4.3 | K(γE-x53) |
| 187 | 152.0 | 195.0 | M |
| 78 | 17.8 | 1.6 | K(γE-x53) |
| 188 | 89.6 | 186.0 | M |
| 74 | 4.2 | 1.3 | K(γE-x53) |
| 189 | 5.6 | 1680.0 | M |
| 24 | 2.0 | 9.8 | K(γE-x53) |
| 190 | 21.3 | 1560.0 | M |
| 75 | 11.1 | 12.5 | K(γE-x53) |
| 192 | 6.8 | 478 | Nle |
| 30 | 3.2 | 7.0 | K(γE-x53) |
| 224 | 1.3 | 2930 | L |
| 216 | 4.7 | 19.5 | K(γE-x70) |
| 225 | 0.7 | 2870 | L |
| 215 | 5.3 | 42.6 | K(γE-x70) |
| 3주 처리 기간에 걸친 DIO 마우스의 체중 변화 | ||
| 예 (용량) | 전체 체중 변화(g) | 체지방 변화(g) |
| 표준 식이 대조군 | 0.02 ± 0.2 | -0.02 ± 0.22 |
| 고지방 식이 대조군 | -0.5 ± 0.3 | -0.8 ± 0.3 |
| SEQ ID NO: 24 (0.5 μg/kg 1일 2회) |
-0.9 ± 0.4 | -0.09 ± 0.3 |
| SEQ ID NO: 24 (1.5 μg/kg 1일 2회) |
-6.9 ± 0.7 | -3.9 ± 0.5 |
| SEQ ID NO: 24 (5 μg/kg 1일 2회) |
-7.4 ± 0.8 | -4.4 ± 0.7 |
| SEQ ID NO: 24 (15 μg/kg 1일 2회) |
-9.1 ± 0.7 | -6.7 ± 0.4 |
Claims (28)
- 화학식 (I)을 가지고 있는 펩타이드 화합물 또는 이의 염 또는 용매 화합물:
R1 - Z - R2 (I)
(화학식 (I)에서, Z는 화학식 (II)를 가지고 있는 펩타이드 모이어티로서,
His-X2-X3-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-X14-X15-X16-X17-X18-Ala-X20-X21-Phe-Ile-Glu-Trp-Leu-Lys-X28-X29-Gly-Pro-Ser-Ser-Gly-X35-Pro-Pro-Pro-X39-X40 (II)
X2는 Ser, D-Ser 및 Aib로부터 선택된 아미노산 잔기를 나타내고,
X3은 Gln, His 및 α-아미노-기능화된 Gln으로부터 선택된 아미노산 잔기를 나타내는데, 이때, Gln은 α-NH2 기의 H가 (C1-C4)-알킬로 치환된다는 점에서 기능화될 수 있고,
X14는 -NH2 기가 있는 곁사슬을 가지고 있는 아미노산 잔기를 나타내는데, 이때, -NH2 곁사슬 기는 -C(O)-R5, -C(O)O-R5, -C(O)NH-R5, -S(O)2-R5 또는 R5로, 바람직하게는 -C(O)-R5로 기능화되고, 이때, R5는 최대 50개 또는 최대 100개의 탄소 원자 및 선택적으로 할로겐, N, O, S 및/또는 P로부터 선택된 헤테로원자를 포함하는 모이어티일 수 있고,
X15는 Glu 및 Asp으로부터 선택된 아미노산 잔기를 나타내고,
X16은 Ser, Glu 및 Lys으로부터 선택된 아미노산 잔기를 나타내고,
X17은 Arg, Glu, Gln, Leu, Aib 및 Lys으로부터 선택된 아미노산 잔기를 나타내고,
X18은 Arg, Ala 및 Lys으로부터 선택된 아미노산 잔기를 나타내고,
X20은 Gln, Arg, Lys, His, Glu 및 Aib으로부터 선택된 아미노산 잔기를 나타내고,
X21은 Asp, Leu 및 Glu으로부터 선택된 아미노산 잔기를 나타내고,
X28은 Asn, Arg, Lys, Aib, Ser, Glu, Ala 및 Asp으로부터 선택된 아미노산 잔기를 나타내고,
X29는 Gly, Ala, D-Ala 및 Thr으로부터 선택된 아미노산 잔기를 나타내고,
X35는 Ala, Glu, Arg 및 Lys으로부터 선택된 아미노산 잔기를 나타내고,
X39는 Ser을 나타내거나 존재하지 않고,
X40은 부존재하거나 -NH2 기가 있는 곁사슬을 가지고 있는 아미노산을 나타내는데, 이때, -NH2 곁사슬 기는 선택적으로 -C(O)-R5, -C(O)O-R5, -C(O)NH-R5, -S(O)2-R5 또는 R5로, 바람직하게는 -C(O)-R5로 기능화되며, 이때, R5는 최대 50개 또는 최대 100개의 탄소 원자 및 선택적으로 할로겐, N, O, S 및/또는 P로부터 선택된 헤테로원자를 포함하는 모이어티일 수 있고,
R1은 펩타이드 화합물의 N 말단 기를 나타내고, NH2 및 단일 또는 이기능화된 NH2로부터 선택되고,
R2는 펩타이드 화합물의 C 말단 기를 나타내고,
(i) OH 또는 기능화된 OH 및
(ii) NH2 또는 단일 또는 이기능화된 NH2로부터 선택된다). - 제1항에 있어서,
X14는 Lys, Orn, Dab 및 Dap으로부터 선택된 아미노산 잔기를 나타내는데, 이때, -NH2 곁사슬 기는 -C(O)-R5로 기능화되고,
X40은 Lys, Orn, Dab 및 Dap으로부터 선택된 아미노산 잔기를 나타내는데, 이때, -NH2 곁사슬 기는 -C(O)-R5로 기능화될 수 있고,
R5는 비고리형의 선형 또는 가지형 (C4-C30) 포화 또는 불포화 탄화수소 기, 및/또는 고리형 포화, 불포화 또는 방향족 기로부터 선택된 친유성 모이어티로서, 친유성 모이어티는 모든 입체이성질체 형태의 (β-Ala)1-4, (γ-Glu)1-4, (ε-Ahx)1-4, 또는 (GABA)1-4로부터 선택된 링커에 의해 -NH2 곁사슬 기에 부착될 수 있는 것인 화합물. - 제1항 또는 제2항에 있어서,
X14는 Lys, Orn, Dab 및 Dap으로부터 선택된 아미노산 잔기를 나타내는데, 이때, -NH2 곁사슬 기는 -C(O)-R5로 기능화되고,
X40은 Lys, Orn, Dab 및 Dap으로부터 선택된 아미노산 잔기를 나타내는데, 이때, -NH2 곁사슬 기는 -C(O)-R5로 기능화될 수 있고, -C(O)-R5는 (S)-4-카르복시-4-헥사데카노일아미노-부티릴-, (S)-4-카르복시-4-옥타데카노일아미노-부티릴-,4-헥사데카노일아미노-부티릴-, 4-{3-[(R)-2,5,7,8-테트라메틸-2-((4R,8R)-4,8,12-트리메틸-트리데실)-크로만-6-일옥시카르보닐]-프로피오닐아미노}-부티릴-, 4-옥타데카노일아미노-부티릴-, 4-((Z)-옥타데크-9-엔오일아미노)-부티릴-, 6-[(4,4-디페닐-시클로헥실옥시)-하이드록시-포스포릴옥시]-헥사노일-, 헥사데카노일-, (S)-4-카르복시-4-(15-카르복시-펜타데카노일아미노)-부티릴-, (S)-4-카르복시-4-{3-[3-((2S,3R,4S,5R)-5-카르복시-2,3,4,5-테트라하이드록시-펜타노일아미노)-프로피오닐아미노]-프로피오닐아미노}-부티릴, (S)-4-카르복시-4-{3-[(R)-2,5,7,8-테트라메틸-2-((4R,8R)-4,8,12-트리메틸-트리데실)-크로만-6-일옥시카르보닐]-프로피오닐아미노}-부티릴-, (S)-4-카르복시-4-((9Z,12Z)-옥타데카-9,12-디에노일아미노)-부티릴-, (S)-4-카르복시-4-[6-((2S,3R,4S,5R)-5-카르복시-2,3,4,5-테트라하이드록시-펜타노일아미노)-헥사노일아미노]-부티릴, (S)-4-카르복시-4-((2S,3R,4S,5R)-5-카르복시-2,3,4,5-테트라하이드록시-펜타노일아미노)-부티릴, (S)-4-카르복시-4-테트라데카노일아미노-부티릴-, (S)-4-(11-벤질옥시카르보닐-운데카노일아미노)-4-카르복시-부티릴, (S)-4-카르복시-4-[11-((2S,3R,4R,5R)-2,3,4,5,6-펜타하이드록시-헥실카르바모일)-운데카노일아미노]-부티릴-, (S)-4-카르복시-4-((Z)-옥타데크-9-엔오일아미노)-부티릴-, (S)-4-카르복시-4-(4-도데실옥시-벤조일아미노)-부티릴-, (S)-4-카르복시-4-헤니코사노일아미노-부티릴-, (S)-4-카르복시-4-도코사노일아미노-부티릴-, (S)-4-카르복시-4-((Z)-노나데크-10-엔오일아미노)-부티릴-, (S)-4-카르복시-4-(4-데실옥시-벤조일아미노)-부티릴-, (S)-4-카르복시-4-[(4'-옥틸옥시-비페닐-4-카르보닐)-아미노]-부티릴-, (S)-4-카르복시-4-(12-페닐-도데카노일아미노)-부티릴-, (S)-4-카르복시-4-아이코사노일아미노-부티릴-, (S)-4-카르복시-4-((S)-4-카르복시-4-헥사데카노일아미노-부티릴아미노)-부티릴-, (S)-4-카르복시-4-((S)-4-카르복시-4-옥타데카노일아미노-부티릴아미노)-부티릴-, 3-(3-옥타데카노일아미노-프로피오닐아미노)-프로피오닐-, 3-(3-헥사데카노일아미노-프로피오닐아미노)-프로피오닐-, 3-헥사데카노일아미노-프로피오닐-, (S)-4-카르복시-4-[(R)-4-((3R,5S,7R,8R,9R,10S,12S,13R,14R,17R)-3,7,12-트리하이드록시-8,10,13-트리메틸-헥사데카하이드로-시클로펜타[a]페난트렌-17-일)-펜타노일아미노]-부티릴-, (S)-4-카르복시-4-[(R)-4-((3R,5R,8R,9S,10S,13R,14S,17R)-3-하이드록시-10,13-디메틸-헥사데카하이드로-시클로펜타[a]페난트렌-17-일)-펜타노일아미노]-부티릴-, (S)-4-카르복시-4-((9S,10R)-9,10,16-트리하이드록시-헥사데카노일아미노)-부티릴-, 테트라데카노일-, 11-카르복시-운데카노일-, 11-벤질옥시카르보닐-운데카노일, (S)-4-카르복시-4-((S)-4-카르복시-4-테트라데카노일아미노-부티릴아미노)-부티릴-, 6-[하이드록시-(나프탈렌-2-일옥시)-포스포릴옥시]-헥사노일-, 6-[하이드록시-(5-페닐-펜틸옥시)-포스포릴옥시]-헥사노일-, 4-(나프탈렌-2-설포닐아미노)-4-옥소-부티릴-, 4-(비페닐-4-설포닐아미노)-4-옥소-부티릴-, (S)-4-카르복시-4-{(S)-4-카르복시-4-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴-, (S)-4-카르복시-4-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴, (S)-4-카르복시-2-{(S)-4-카르복시-2-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴, (S)-4-카르복시-2-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴, (S)-4-카르복시-4-{(S)-4-카르복시-4-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴, (S)-4-카르복시-4-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴,(S)-4-카르복시-2-{(S)-4-카르복시-2-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴, (S)-4-카르복시-2-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴, 2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸-, 2-(2-{2-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸, (S)-4-카르복시-4-((S)-4-카르복시-4-{(S)-4-카르복시-4-[(S)-4-카르복시-4-(19-카르복시-노나데카노일아미노)-부티릴아미노]-부티릴아미노}-부티릴아미노)-부티릴, 2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(16-1H-테트라졸-5-일-헥사데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸, 2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(16-카르복시-헥사데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸, (S)-4-카르복시-4-{(S)-4-카르복시-4-[(S)-4-카르복시-4-(17-카르복시-헵타데카노일아미노)-부티릴아미노]-부티릴아미노}-부티릴, (S)-4-카르복시-4-((S)-4-카르복시-4-{2-[2-(2-{2-[2-(2-{(S)-4-카르복시-4-[10-(4-카르복시-페녹시)-데카노일아미노]-부티릴아미노}-에톡시)-에톡시]-아세틸아미노}-에톡시)-에톡시]-아세틸아미노}-부티릴, (S)-4-카르복시-4-{(S)-4-카르복시-4-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(7-카르복시-헵타노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴, (S)-4-카르복시-4-{(S)-4-카르복시-4-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(11-카르복시-운데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴, (S)-4-카르복시-4-{(S)-4-카르복시-4-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(13-카르복시-트리데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴, (S)-4-카르복시-4-{(S)-4-카르복시-4-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(15-카르복시-펜타데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴, 및 (S)-4-카르복시-4-{(S)-4-카르복시-4-[2-(2-{2-[2-(2-{2-[(S)-4-카르복시-4-(19-카르복시-노나데카노일아미노)-부티릴아미노]-에톡시}-에톡시)-아세틸아미노]-에톡시}-에톡시)-아세틸아미노]-부티릴아미노}-부티릴로부터 선택되는 것인 화합물. - 제1항 내지 제3항 중 어느 한 항에 있어서,
R1은 NH2,
R2는 NH2 또는
R1 및 R2는 NH2인 것인 화합물. - 제1항 내지 제4항 중 어느 한 항에 있어서,
X14는 Lys으로, 이는 -C(O)R5 기로 기능화되며, R5는 제1항 내지 제3항 중 어느 한 항에 기술된 바와 같은 것인 화합물. - 제1항 내지 제5항 중 어느 한 항에 있어서,
X14는 Lys으로, 이는 -C(O)R5 기로 기능화되며, R5는 친유성 모이어티, -NH2 곁사슬 기에 직접적으로 부착된, 또는 모든 입체이성질체 형태의 β-Ala, γ-Glu, β-Ala-β-Ala 및 γ-Glu-γ-Glu의 군으로부터 선택된 링커에 의해 -NH2 곁사슬 기에 부착된, 비고리형의 선형 또는 가지형 (C12-C22) 포화 탄화수소 기를 포함하는 것인 화합물. - 제1항 내지 제6항 중 어느 한 항에 있어서,
X2는 Ser, D-Ser 및 Aib로부터 선택된 아미노산 잔기를 나타내고,
X3은 Gln, His 및 α-아미노-기능화된 Gln으로부터 선택된 아미노산 잔기를 나타내는데, 이때, Gln은 α-NH2 기의 H가 (C1-C4)-알킬로 치환된다는 점에서 기능화될 수 있고,
X14는 Lys, Orn, Dab 및 Dap로부터 선택된 아미노산 잔기를 나타내는데, 이때, -NH2 곁사슬 기는 -C(O)-R5로 기능화되고,
X15는 Glu 및 Asp으로부터 선택된 아미노산 잔기를 나타내고,
X16은 Ser, Lys 및 Glu으로부터 선택된 아미노산 잔기를 나타내고,
X17은 Arg, Glu, Gln, Leu 및 Lys으로부터 선택된 아미노산 잔기를 나타내고,
X18은 Arg 및 Ala으로부터 선택된 아미노산 잔기를 나타내고,
X20은 Gln, Arg, Lys 및 Aib으로부터 선택된 아미노산 잔기를 나타내고,
X21은 Asp, Leu 및 Glu으로부터 선택된 아미노산 잔기를 나타내고,
X28은 Asn, Arg, Lys, Aib, Ser, Glu, Asp 및 Ala으로부터 선택된 아미노산 잔기를 나타내고,
X29는 Gly, Ala, D-Ala 및 Thr으로부터 선택된 아미노산 잔기를 나타내고,
X35는 Ala 또는 Glu으로부터 선택된 아미노산 잔기를 나타내고,
X39는 Ser 또는 부존재하고,
X40은 부존재하거나 Lys을 나타내는데, 이때, -NH2 곁사슬 기는 -C(O)-R5로 기능화될 수 있고,
-C(O)-R5은 제2항에서 정의된 바와 같은 것인 화합물. - 제1항 내지 제7항 중 어느 한 항에 있어서,
X2는 D-Ser 및 Aib으로부터 선택된 아미노산 잔기를 나타내고,
X3은 Gln을 나타내고,
X14는 Lys 및 Orn으로부터 선택된 아미노산 잔기를 나타내는데, 이때, -NH2 곁사슬 기는 -C(O)-R5로 기능화되고,
X15는 Glu 및 Asp으로부터 선택된 아미노산 잔기를 나타내고,
X16은 Ser 및 Glu으로부터 선택된 아미노산 잔기를 나타내고,
X17은 Arg, Gln 및 Lys으로부터 선택된 아미노산 잔기를 나타내고,
X18은 Arg 및 Ala으로부터 선택된 아미노산 잔기를 나타내고,
X20은 Gln, Arg, Lys 및 Aib으로부터 선택된 아미노산 잔기를 나타내고,
X21은 Asp, Leu 및 Glu으로부터 선택된 아미노산 잔기를 나타내고,
X28은 Asn, Arg, Lys, Aib, Ser 및 Ala으로부터 선택된 아미노산 잔기를 나타내고,
X29는 Gly, Ala 또는 Thr으로부터 선택된 아미노산 잔기를 나타내고,
X35는 Ala을 나타내고,
X39는 Ser 또는 부존재하고,
X40은 부존재하거나 Lys을 나타내는데, 이때, -NH2 곁사슬 기는 -C(O)-R5로 기능화될 수 있고,
-C(O)-R5은 제2항에서 정의된 바와 같은 것인 화합물. - 제1항 내지 제8항 중 어느 한 항에 있어서,
X20은 Gln, Lys 및 Aib로부터 선택된 아미노산 잔기를 나타내는 것인 화합물. - 제1항 내지 제9항 중 어느 한 항에 있어서,
X2는 D-Ser 및 Aib으로부터 선택된 아미노산 잔기를 나타내고,
X3은 Gln을 나타내고,
X14는 Lys을 나타내는데, 이때, -NH2 곁사슬 기는 3-(3-옥타데카노일아미노-프로피오닐-아미노)-프로피오닐-, 4-헥사데카노일아미노-부티릴-, 4-{3-[(R)-2,5,7,8-테트라메틸-2-((4R,8R)-4,8,12-트리메틸-트리데실)-크로만-6-일옥시카르보닐]-프로피오닐아미노}-부티릴-, 4-옥타데카노일아미노-부티릴-, 4-((Z)-옥타데크-9-엔오일아미노)-부티릴-, 헥사데카노일-, (S)-4-카르복시-4-((Z)-옥타데크-9-엔오일아미노)-부티릴-, (S)-4-카르복시-4-(4-도데실옥시-벤조일아미노)-부티릴-, (S)-4-카르복시-4-헤니코사노일아미노-부티릴-, (S)-4-카르복시-4-도코사노일아미노-부티릴-, (S)-4-카르복시-4-((Z)-노나데크-10-엔오일아미노)-부티릴-, (S)-4-카르복시-4-(4-데실옥시-벤조일아미노)-부티릴-, (S)-4-카르복시-4-[(4'-옥틸옥시-비페닐-4-카르보닐)-아미노]-부티릴-, (S)-4-카르복시-4-(12-페닐-도데카노일아미노)-부티릴-, (S)-4-카르복시-4-((S)-4-카르복시-4-헥사데카노일아미노-부티릴아미노)-부티릴-, (S)-4-카르복시-4-((S)-4-카르복시-4-옥타데카노일아미노-부티릴아미노)-부티릴-, (S)-4-카르복시-4-{3-[(R)-2,5,7,8-테트라메틸-2-((4R,8R)-4,8,12-트리메틸-트리데실)-크로만-6-일옥시카르보닐]-프로피오닐아미노}-부티릴-, (S)-4-카르복시-4-((9Z,12Z)-옥타데카-9,12-디에노일아미노)-부티릴-, (S)-4-카르복시-4-옥타데카노일아미노-부티릴- 및 (S)-4-카르복시-4-헥사데카노일아미노-부티릴-로부터 선택된 기 중 하나로 기능화되고,
X15는 Glu을 나타내고,
X16은 Ser을 나타내고,
X17은 Arg, Gln 및 Lys으로부터 선택된 아미노산 잔기를 나타내고,
X18은 Ala을 나타내고,
X20은 Gln을 나타내고,
X21은 Asp을 나타내고,
X28은 Ala을 나타내고,
X29는 Gly을 나타내고,
X35는 Ala을 나타내고,
X39는 Ser이고,
X40은 부존재하는 것인 화합물. - 제1항 내지 제10항 중 어느 한 항에 있어서,
X2는 Aib을 나타내고,
X3은 Gln을 나타내고,
X14는 Lys을 나타내는데, 이때, -NH2 곁사슬 기는 특히 (S)-4-카르복시-4-헥사데카노일아미노-부티릴- 및 (S)-4-카르복시-4-옥타데카노일아미노-부티릴-로 기능화되고,
X15는 Asp 및 Glu으로부터 선택된 아미노산 잔기를 나타내고,
X16은 Ser 및 Glu으로부터 선택된 아미노산 잔기를 나타내고,
X17은 Gln 및 Lys으로부터 선택된 아미노산 잔기를 나타내고,
X18은 Ala을 나타내고,
X20은 Gln 및 Lys으로부터 선택된 아미노산 잔기를 나타내고,
X21은 Asp 및 Leu으로부터 선택된 아미노산 잔기를 나타내고,
X28은 Ala을 나타내고,
X29는 Gly 및 D-Ala으로부터 선택된 아미노산 잔기를 나타내고,
X35는 Ala을 나타내고,
X39는 Ser이고,
X40은 부존재하는 것인 화합물. - 제1항 내지 제11항 중 어느 한 항에 있어서,
X2는 D-Ser을 나타내고,
X3은 Gln을 나타내고,
X14는 Lys을 나타내는데, 이때, -NH2 곁사슬 기는 특히 (S)-4-카르복시-4-헥사데카노일아미노-부티릴- 또는 헥사데카노일-로 기능화되고,
X15는 Glu 및 Asp으로부터 선택된 아미노산 잔기를 나타내고,
X16은 Ser 및 Glu으로부터 선택된 아미노산 잔기를 나타내고,
X17은 Arg, Glu, Lys 및 Aib으로부터 선택된 아미노산 잔기를 나타내고,
X18은 Arg, Lys 및 Ala으로부터 선택된 아미노산 잔기를 나타내고,
X20은 Gln, Lys 및 Aib으로부터 선택된 아미노산 잔기를 나타내고,
X21은 Asp 및 Leu으로부터 선택된 아미노산 잔기를 나타내고,
X28은 Ala 및 Asn으로부터 선택된 아미노산 잔기를 나타내고,
X29는 Gly을 나타내고,
X35는 Ala을 나타내고,
X39는 Ser이고,
X40은 부존재하는 것인 화합물. - 제1항 내지 제12항 중 어느 한 항에 있어서,
X2는 Aib 및 D-Ser으로부터 선택된 아미노산 잔기를 나타내고,
X3은 Gln 및 His으로부터 선택된 아미노산 잔기를 나타내고,
X14는 Lys을 나타내는데, 이때, -NH2 곁사슬 기는 (S)-4-카르복시-4-헥사데카노일아미노-부티릴-, (S)-4-카르복시-4-옥타데카노일아미노-부티릴-, (S)-4-카르복시-4-((S)-4-카르복시-4-헥사데카노일아미노-부티릴아미노)-부티릴-, (S)-4-카르복시-4-((S)-4-카르복시-4-옥타데카노일아미노-부티릴아미노)-부티릴-, 3-(3-옥타데카노일아미노-프로피오닐아미노)-프로피오닐-, 3-(3-헥사데카노일아미노-프로피오닐아미노)-프로피오닐-, (S)-4-카르복시-4-헤니코사노일아미노-부티릴-, 4-헥사데카노일아미노-부티릴- 및 4-옥타데카노일아미노-부티릴-로부터 선택된 기 중 하나로 기능화되고,
X15는 Asp 및 Glu으로부터 선택된 아미노산 잔기를 나타내고,
X16은 Ser 및 Glu으로부터 선택된 아미노산 잔기를 나타내고,
X17은 Arg, Gln, Lys, Aib 및 Leu으로부터 선택된 아미노산 잔기를 나타내고,
X18은 Arg 및 Ala으로부터 선택된 아미노산 잔기를 나타내고,
X20은 Gln, Aib 및 Lys으로부터 선택된 아미노산 잔기를 나타내고,
X21은 Asp, Glu 및 Lys으로부터 선택된 아미노산 잔기를 나타내고,
X28은 Asn, Ser, Aib, Ala 및 Arg으로부터 선택된 아미노산 잔기를 나타내고,
X29는 Gly, Thr, Ala 및 D-Ala으로부터 선택된 아미노산 잔기를 나타내고,
X35는 Ala을 나타내고,
X39는 Ser을 나타내고,
X40은 부존재하는 것인 화합물. - 제1항 내지 제13항 중 어느 한 항에 있어서,
14번 위치의 기능화된 Lys은 ε-아미노 기에서 -C(O)-R5로 기능화되고, -C(O)-R5는 (S)-4-카르복시-4-헥사데카노일-아미노-부티릴, (S)-4-카르복시-4-옥타데카노일아미노-부티릴, 헥사데카노일 또는 옥타데카노일인 것인 화합물. - 제14항에 있어서,
X2는 Aib 및 D-Ser으로부터 선택된 아미노산 잔기를 나타내고,
X3은 Gln을 나타내고,
X14는 Lys을 나타내는데, 이때, -NH2 곁사슬 기는 (S)-4-카르복시-4-헥사데카노일-아미노-부티릴, (S)-4-카르복시-4-옥타데카노일아미노-부티릴, 헥사데카노일 및 옥타데카노일로부터 선택된 기 중 하나로 기능화되고,
X15는 Glu을 나타내고,
X16은 Ser을 나타내고,
X17은 Arg, Gln 및 Lys으로부터 선택된 아미노산 잔기를 나타내고,
X18은 Ala을 나타내고,
X20은 Gln을 나타내고,
X21은 Asp을 나타내고,
X28은 Ala을 나타내고,
X29는 Gly을 나타내고,
X35는 Ala을 나타내고,
X39는 Ser을 나타내고,
X40은 부존재하는 것인 화합물. - 제1항 내지 제15항 중 어느 한 항에 있어서,
X2는 Aib을 나타내고,
X3은 Gln을 나타내고,
X14는 Lys을 나타내는데, 이때, -NH2 곁사슬 기는 특히 (S)-4-카르복시-4-헤니코사노일아미노-부티릴- 및 (S)-4-카르복시-4-옥타데카노일아미노-부티릴-로 기능화되고,
X15는 Asp을 나타내고,
X16은 Lys 및 Glu으로부터 선택된 아미노산 잔기를 나타내고,
X17은 Arg 및 Glu으로부터 선택된 아미노산 잔기를 나타내고,
X18은 Ala 및 Arg으로부터 선택된 아미노산 잔기를 나타내고,
X20은 Gln 및 Lys으로부터 선택된 아미노산 잔기를 나타내고,
X21은 Asp 및 Leu으로부터 선택된 아미노산 잔기를 나타내고,
X28은 Ala을 나타내고,
X29는 Gly 및 D-Ala으로부터 선택된 아미노산 잔기를 나타내고,
X35는 Ala을 나타내고,
X39는 Ser이고,
X40은 부존재하는 것인 화합물. - 제1항 내지 제16항 중 어느 한 항에 있어서, SEQ ID NO. 4~181의 화합물, 또는 이의 염 또는 용매 화합물로부터 선택된 화합물.
- 제1항 내지 제16항 중 어느 한 항에 있어서, SEQ ID NO. 4~181, 196~223, 226~229의 화합물, 또는 이의 염 또는 용매 화합물로부터 선택된 화합물.
- 제1항 내지 제18항 중 어느 한 항에 있어서, 산성 pH 값에서, 예컨대, 25℃에서 pH 4.5에서, 및/또는 생리적 pH 값에서, 예컨대, 25℃에서 pH 7.4에서 높은 용해도를 나타내며, 이때, 상기 pH 값 및/또는 pH 값들에서의 용해도는 특히 적어도 0.5 mg/ml, 또는 적어도 1.0 mg/ml를 나타내는 화합물.
- 제1항 내지 제19항 중 어느 한 항에 있어서, 의약, 특히 인간 의약에 이용하기 위한 화합물.
- 제20항에 있어서, 적어도 하나의 약학적으로 허용 가능한 담체와 함께 약학적 조성물 내에 존재하는 활성 물질로서 이용하기 위한 화합물.
- 제20항 또는 제21항에 있어서, 적어도 하나의 추가적인, 치료적으로 활성을 나타내는 물질과 함께 사용하고, 추가적인, 치료적으로 활성을 나타내는 물질은 인슐린 및 인슐린 유도체 시리즈, GLP-1, GLP-1 유사체 및 GLP-1 수용체 작용제, 고분자 결합된 GLP-1 및 GLP-1 유사체, 이중적인 GLP1/GIP 작용제, PYY3-36 또는 이의 유사체, 췌장 폴리펩타이드 또는 이의 유사체, 글루카곤 수용체 작용제, GIP 수용체 작용제 또는 길항제, 그렐린 길항제 또는 역 작용제, 제닌 및 이의 유사체, DDP-IV 저해제, SGLT2 저해제, 이중적인 SGLT2 / SGLT1 저해제, 비구아니드 티아졸리딘디온, 이중적인 PPAR 작용제, 설포닐우레아, 메글리티니드, 알파-글루코시다아제 저해제, 아밀린 및 아밀린 유사체, GPR119 작용제, GPR40 작용제, GPR120 작용제, GPR142 작용제, 전신성 또는 저흡수성 TGR5 작용제, 사이클로세트, 11-베타-HSD 저해제, 글루코키나아제 활성화제, DGAT 저해제, 단백질 티로신포스파타아제 1 저해제, 글루코오스-6-포스파타아제 저해제, 프룩토오스-1,6-비스포스파타아제 저해제, 글리코겐 포스포릴라아제 저해제, 포스포에놀 피루브산 카르복시키나아제 저해제, 글리코겐 합성효소 키나아제 저해제, 피루브산 탈수소효소 키나아제 저해제, 알파2-길항제, CCR-2 길항제, 글루코오스 운반체-4의 조절제, 소마토스타틴 수용체 3 작용제, HMG-CoA-환원효소 저해제, 피브레이트, 니코틴산 및 이의 유도체, 니코틴산 수용체 1 작용제, PPAR-(알파, 감마 또는 알파/감마) 작용제 또는 조절제, PPAR-델타 작용제, ACAT 저해제, 콜레스테롤 흡수 저해제, 담즙산 결합 물질, IBAT 저해제, MTP 저해제, PCSK9 조절제, 간 선택적 갑상선 호르몬 수용체 베타 작용제의 LDL 수용체 상향조절제(up-regulator), HDL 상승 화합물, 지질 대사 조절제, PLA2 저해제, ApoA-I 증진제, 갑상선 호르몬 수용체 작용제, 콜레스테롤 합성 저해제, 오메가-3 지방산 및 이의 유도체, 시부트라민, 테소펜신, 오를리스타트, CB-1 수용체 길항제, MCH-1 길항제, MC4 수용체 작용제 및 부분적 작용제, NPY5 또는 NPY2 길항제, NPY4 작용제, 베타-3-작용제, 렙틴 또는 렙틴 미메틱스, 5HT2c 수용체 작용제, 또는 부프로피온/날트렉손(CONTRAVE), 부프로피온/조니사마이드(EMPATIC), 부프로피온/펜터민 또는 프람린타이드/메트레렙틴, QNEXA(펜터민+ 토피라메이트)의 병용과 같은 비만 치료를 위한 활성 물질, 리파아제 저해제, 혈관형성 저해제, H3 길항제, AgRP 저해제, 삼중 모노아민 흡수 저해제(노르에피네프린 및 아세틸콜린), MetAP2 저해제, 칼슘 채널 차단제 딜티아젬의 비강 제형, 섬유모세포 성장인자 수용체 4의 생성에 대한 안티센스, 프로히비틴 표적화 펩타이드-1, 안지오텐신 II 수용체 길항제, ACE 저해제, ECE 저해제, 이뇨제, 베타 차단제, 칼슘 길항제, 중심 작용성 혈압항진제, 알파-2-아드레날린 작용성 수용체 길항제, 중성 엔도펩티다아제 저해제, 혈소판 응집 저해제와 같은, 높은 혈압, 만성 심부전 또는 죽상 동맥 경화증에 영향을 미치는 약물로부터 선택되는 것인 화합물.
- 제20항 또는 제21항에 있어서, 적어도 하나의 추가적인, 치료적으로 활성을 나타내는 물질과 함께 사용하고, 추가적인, 치료적으로 활성을 나타내는 물질은 특히 GLP-1 화합물 및/또는 인슐린성 화합물 및/또는 위장 펩타이드인 것인 화합물.
- 제20항 또는 제21항에 있어서, 적어도 하나의 추가적인, 치료적으로 활성을 나타내는 물질과 함께 사용하고, 추가적인, 치료적으로 활성을 나타내는 물질은 특히 인슐린 또는 인슐린 유도체인 것인 화합물.
- 제20항 또는 제21항에 있어서, 약학적 조성물은 비경구 투여용, 바람직하게는 단일 용량을 주사할 수 있는 형태, 특히 펜 형태인 것인 화합물.
- 제20항 내지 제25항 중 어느 한 항에 있어서, 고혈당증, 제2형 당뇨병, 글루코오스 내성 손상, 제1형 당뇨병, 비만, 대사 증후군 및 신경퇴행성 장애의 치료 또는 예방, 특히, 제2형 당뇨병에서의 질병 진행의 지연 또는 예방, 대사 증후군 치료, 비만 치료 또는 과체중 예방, 식품 섭취 감소, 에너지 소비 증가, 체중 감소, 글루코오스 내성 손상(IGT)으로부터 제2형 당뇨병으로의 진행 지연; 제2형 당뇨병으로부터 인슐린 필요성 당뇨병으로의 진행 지연; 식욕 조절; 포만감 유도; 성공적인 체중 감량 후 체중 회복 방지; 과체중 또는 비만과 관련된 질병 또는 상태 치료; 식욕 이상 항진증 치료; 폭식증 치료; 죽상 동맥 경화증, 고혈압, IGT, 이상지질혈증, 관상 동맥성 질병, 간 지방증 치료, 베타 차단제 중독 치료, 기법, 예컨대, X선, CT 스캐닝 및 NMR 스캐닝을 이용한 위장관 조사와 연결하여 유용한, 위장관의 이동성 억제를 위한 사용을 위한 화합물.
- 제20항 내지 제26항 중 어느 한 항에 있어서, 고혈당증, 제2형 당뇨병, 비만 및 대사 증후군의 치료 또는 예방 또는 체중 감소를 위한 화합물.
- 제20항 내지 제26항 중 어느 한 항에 있어서, 비만과 당뇨병의 동시 치료를 위한 화합물.
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP12306232.5 | 2012-10-09 | ||
| EP12306232 | 2012-10-09 | ||
| EP13305222 | 2013-02-27 | ||
| EP13305222.5 | 2013-02-27 | ||
| PCT/EP2013/070882 WO2014056872A1 (en) | 2012-10-09 | 2013-10-08 | Exendin-4 derivatives as dual glp1/glucagon agonists |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20150064093A true KR20150064093A (ko) | 2015-06-10 |
| KR102179751B1 KR102179751B1 (ko) | 2020-11-17 |
Family
ID=49304983
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020157010088A Active KR102179751B1 (ko) | 2012-10-09 | 2013-10-08 | 이중 glp1/글루카곤 작용제로서의 엑센딘-4 유도체 |
Country Status (38)
| Country | Link |
|---|---|
| US (3) | US9365632B2 (ko) |
| EP (1) | EP2906595B1 (ko) |
| JP (1) | JP6373270B2 (ko) |
| KR (1) | KR102179751B1 (ko) |
| CN (1) | CN104837864B (ko) |
| AR (1) | AR092925A1 (ko) |
| AU (1) | AU2013328802B2 (ko) |
| BR (1) | BR112015007685A2 (ko) |
| CA (1) | CA2887272C (ko) |
| CL (2) | CL2015000811A1 (ko) |
| CR (1) | CR20150200A (ko) |
| CY (1) | CY1119987T1 (ko) |
| DK (1) | DK2906595T3 (ko) |
| DO (1) | DOP2015000073A (ko) |
| EA (1) | EA030023B1 (ko) |
| ES (1) | ES2647418T3 (ko) |
| GT (1) | GT201500081A (ko) |
| HR (1) | HRP20171726T1 (ko) |
| HU (1) | HUE037150T2 (ko) |
| IL (1) | IL237641B (ko) |
| LT (1) | LT2906595T (ko) |
| MX (1) | MX359533B (ko) |
| MY (1) | MY168749A (ko) |
| NO (1) | NO2906595T3 (ko) |
| NZ (1) | NZ706898A (ko) |
| PE (1) | PE20150900A1 (ko) |
| PH (1) | PH12015500688B1 (ko) |
| PL (1) | PL2906595T3 (ko) |
| PT (1) | PT2906595T (ko) |
| RS (1) | RS56515B1 (ko) |
| SG (1) | SG11201501770WA (ko) |
| SI (1) | SI2906595T1 (ko) |
| TN (1) | TN2015000101A1 (ko) |
| TW (1) | TWI613213B (ko) |
| UA (1) | UA116217C2 (ko) |
| UY (1) | UY35072A (ko) |
| WO (1) | WO2014056872A1 (ko) |
| ZA (1) | ZA201501694B (ko) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20200037358A (ko) * | 2017-08-09 | 2020-04-08 | 사노피 | 지방간 질환 및 지방간염의 치료에서의 glp-1/글루카곤 수용체 작용제 |
| KR20200069225A (ko) | 2018-12-06 | 2020-06-16 | 경상대학교산학협력단 | 지속형 엑센딘-4 및 이의 용도 |
Families Citing this family (43)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9255154B2 (en) | 2012-05-08 | 2016-02-09 | Alderbio Holdings, Llc | Anti-PCSK9 antibodies and use thereof |
| UA116217C2 (uk) | 2012-10-09 | 2018-02-26 | Санофі | Пептидна сполука як подвійний агоніст рецепторів glp1-1 та глюкагону |
| HK1211231A1 (en) | 2012-12-21 | 2016-05-20 | Sanofi | Exendin-4 derivatives |
| EP3080154B1 (en) | 2013-12-13 | 2018-02-07 | Sanofi | Dual glp-1/gip receptor agonists |
| TW201609795A (zh) | 2013-12-13 | 2016-03-16 | 賽諾菲公司 | 作為雙重glp-1/gip受體促效劑的艾塞那肽-4(exendin-4)胜肽類似物 |
| TW201609797A (zh) | 2013-12-13 | 2016-03-16 | 賽諾菲公司 | 雙重glp-1/升糖素受體促效劑 |
| WO2015086730A1 (en) | 2013-12-13 | 2015-06-18 | Sanofi | Non-acylated exendin-4 peptide analogues |
| TW201625668A (zh) | 2014-04-07 | 2016-07-16 | 賽諾菲公司 | 作為胜肽性雙重glp-1/昇糖素受體激動劑之艾塞那肽-4衍生物 |
| TW201625669A (zh) | 2014-04-07 | 2016-07-16 | 賽諾菲公司 | 衍生自艾塞那肽-4(Exendin-4)之肽類雙重GLP-1/升糖素受體促效劑 |
| TW201625670A (zh) | 2014-04-07 | 2016-07-16 | 賽諾菲公司 | 衍生自exendin-4之雙重glp-1/升糖素受體促效劑 |
| US9932381B2 (en) | 2014-06-18 | 2018-04-03 | Sanofi | Exendin-4 derivatives as selective glucagon receptor agonists |
| AR105319A1 (es) | 2015-06-05 | 2017-09-27 | Sanofi Sa | Profármacos que comprenden un conjugado agonista dual de glp-1 / glucagón conector ácido hialurónico |
| WO2016198624A1 (en) | 2015-06-12 | 2016-12-15 | Sanofi | Exendin-4 derivatives as trigonal glp-1/glucagon/gip receptor agonists |
| WO2016198628A1 (en) | 2015-06-12 | 2016-12-15 | Sanofi | Non-acylated exendin-4 derivatives as dual glp-1/glucagon receptor agonists |
| AR104932A1 (es) * | 2015-06-22 | 2017-08-23 | Lilly Co Eli | Compuestos co-agonistas del glucagón y péptido-1 similar al glugacón (glp-1) |
| TW201706291A (zh) | 2015-07-10 | 2017-02-16 | 賽諾菲公司 | 作為選擇性肽雙重glp-1/升糖素受體促效劑之新毒蜥外泌肽(exendin-4)衍生物 |
| TWI622596B (zh) | 2015-10-26 | 2018-05-01 | 美國禮來大藥廠 | 升糖素受體促效劑 |
| US11046743B2 (en) * | 2015-12-14 | 2021-06-29 | Antaros Medical Ab | Selective glucagon receptor agonists comprising a chelating moiety for imaging purposes |
| TW201821434A (zh) | 2016-10-10 | 2018-06-16 | 法商賽諾菲公司 | 製備包含親脂性修飾的離胺酸側鏈的肽的方法 |
| TW201833132A (zh) | 2016-12-02 | 2018-09-16 | 法商賽諾菲公司 | 作為肽類glp1/升糖素/gip三重受體激動劑之新穎化合物 |
| AR110299A1 (es) | 2016-12-02 | 2019-03-13 | Sanofi Sa | Conjugados que comprenden un agonista dual de glp-1 / glucagón, un conector y ácido hialurónico |
| TW201833131A (zh) * | 2016-12-02 | 2018-09-16 | 法商賽諾菲公司 | 作為胜肽類glp1/升糖素/gip受體促效劑之新化合物 |
| AR110988A1 (es) | 2017-02-21 | 2019-05-22 | Sanofi Sa | Compuestos de azetidina como moduladores de gpr119 para el tratamiento de la diabetes, la obesidad, la dislipidemia y trastornos relacionados |
| US11590206B2 (en) | 2017-12-21 | 2023-02-28 | Sanofi | Liquid pharmaceutical composition |
| HRP20240749T1 (hr) | 2018-04-05 | 2024-09-13 | Sun Pharmaceutical Industries Limited | Novi analozi glp-1 |
| EP3774838B1 (en) | 2018-04-10 | 2022-08-10 | Sanofi-Aventis Deutschland GmbH | Lixisenatide synthesis with capping |
| US11560402B2 (en) | 2018-04-10 | 2023-01-24 | Sanofi-Aventis Deutschland Gmbh | Method for cleavage of solid phase-bound peptides from the solid phase |
| TWI829687B (zh) | 2018-05-07 | 2024-01-21 | 丹麥商諾佛 儂迪克股份有限公司 | 包含glp-1促效劑與n-(8-(2-羥基苯甲醯基)胺基)辛酸之鹽的固體組成物 |
| CN110452952B (zh) * | 2018-05-08 | 2024-01-16 | 宜昌东阳光长江药业股份有限公司 | 一种glp-1类似物生物活性的检测方法 |
| UY38249A (es) | 2018-05-30 | 2019-12-31 | Sanofi Sa | Productos conjugados que comprenden un agonista del receptor triple de glp-1/glucagón/gip, un conector y ácido hialurónico |
| US20220323548A1 (en) * | 2019-08-13 | 2022-10-13 | Anygen Co., Ltd. | Exenatide analog and use thereof |
| AU2021229621B2 (en) | 2020-03-06 | 2023-08-31 | Sanofi | Peptides as selective GIP receptor agonists |
| CN115484972B (zh) | 2020-04-24 | 2025-07-01 | 勃林格殷格翰国际有限公司 | 作为长效glp-1/升糖素受体激动剂以治疗脂肪肝疾病及脂肪肝炎的升糖素类似物 |
| AR124395A1 (es) * | 2020-12-17 | 2023-03-22 | Intarcia Therapeutics Inc | Agonistas del receptor del polipéptido tipo glucagón 1 (glp-1) de acción prolongada y métodos de uso de los mismos |
| CN117355297A (zh) * | 2021-05-20 | 2024-01-05 | 燃脂医药股份有限公司 | 用于诱导褐色脂肪生成的方法和组合物 |
| CA3226846A1 (en) | 2021-07-30 | 2023-02-02 | Boehringer Ingelheim International Gmbh | Dose regimen for long-acting glp1/glucagon receptor agonists |
| EP4399224A1 (en) | 2021-09-06 | 2024-07-17 | Sanofi | New peptides as potent and selective gip receptor agonists |
| CN116606367A (zh) * | 2022-05-31 | 2023-08-18 | 南京盛德瑞尔医药科技有限公司 | 长效Exendin-9-39及其在低血糖治疗中的应用和作为治疗低血糖的药物 |
| EP4536272A1 (en) * | 2022-06-07 | 2025-04-16 | Helicore Biopharma, Inc. | Compositions and methods for treating postural tachycardia syndrome |
| WO2024165571A2 (en) | 2023-02-06 | 2024-08-15 | E-Therapeutics Plc | Inhibitors of expression and/or function |
| WO2025125576A2 (en) | 2023-12-15 | 2025-06-19 | E-Therapeutics Plc | Inhibitors of expression and/or function |
| WO2025133348A1 (en) | 2023-12-22 | 2025-06-26 | E-Therapeutics Plc | Inhibitors of expression and/or function |
| US12303604B1 (en) | 2024-10-16 | 2025-05-20 | Currax Pharmaceuticals Llc | Pharmaceutical formulations comprising naltrexone and/or bupropion |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011016030A1 (en) * | 2009-08-03 | 2011-02-10 | Technion Research & Development Foundation Ltd. | Hydrogen production by an autothermal heat exchanger packed-bed membrane gas reformer |
| US20120178670A1 (en) * | 2009-07-13 | 2012-07-12 | Zealand Pharma A/S | Acylated glucagon analogues |
| US8268781B2 (en) * | 2004-09-03 | 2012-09-18 | Philipps-Universitat Marburg | Peptide derivatives of exendin-4 |
Family Cites Families (432)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NZ250844A (en) | 1993-04-07 | 1996-03-26 | Pfizer | Treatment of non-insulin dependant diabetes with peptides; composition |
| US6284727B1 (en) | 1993-04-07 | 2001-09-04 | Scios, Inc. | Prolonged delivery of peptides |
| US5424286A (en) | 1993-05-24 | 1995-06-13 | Eng; John | Exendin-3 and exendin-4 polypeptides, and pharmaceutical compositions comprising same |
| US5641757A (en) | 1994-12-21 | 1997-06-24 | Ortho Pharmaceutical Corporation | Stable 2-chloro-2'-deoxyadenosine formulations |
| EP2016950B1 (en) | 1996-08-08 | 2011-01-05 | Amylin Pharmaceuticals, Inc. | Pharmaceutical composition comprising an exendin-4 peptide |
| US6458924B2 (en) | 1996-08-30 | 2002-10-01 | Novo Nordisk A/S | Derivatives of GLP-1 analogs |
| ES2283025T3 (es) | 1996-08-30 | 2007-10-16 | Novo Nordisk A/S | Derivados de glp-1.1. |
| DE69831673C5 (de) | 1997-01-07 | 2015-01-22 | Amylin Pharmaceuticals, Llc | Verwendung von exedinen und deren antagonisten zur verminderung der lebensmittelaufnahme |
| US6410511B2 (en) | 1997-01-08 | 2002-06-25 | Amylin Pharmaceuticals, Inc. | Formulations for amylin agonist peptides |
| US7312196B2 (en) | 1997-01-08 | 2007-12-25 | Amylin Pharmaceuticals, Inc. | Formulations for amylin agonist peptides |
| US6723530B1 (en) | 1997-02-05 | 2004-04-20 | Amylin Pharmaceuticals, Inc. | Polynucleotides encoding proexendin, and methods and uses thereof |
| BR9811866A (pt) | 1997-08-08 | 2000-08-15 | Amylin Pharmaceuticals Inc | Compostos agonistas de exendina |
| US7157555B1 (en) | 1997-08-08 | 2007-01-02 | Amylin Pharmaceuticals, Inc. | Exendin agonist compounds |
| US7223725B1 (en) | 1997-11-14 | 2007-05-29 | Amylin Pharmaceuticals, Inc. | Exendin agonist compounds |
| ATE383867T1 (de) | 1997-11-14 | 2008-02-15 | Amylin Pharmaceuticals Inc | Neuartige exendin agonisten |
| BR9815670A (pt) | 1997-11-14 | 2000-10-17 | Amylin Pharmaceuticals Inc | Compostos agonistas de exendina |
| US7220721B1 (en) | 1997-11-14 | 2007-05-22 | Amylin Pharmaceuticals, Inc. | Exendin agonist peptides |
| DE69840106D1 (de) | 1998-01-09 | 2008-11-20 | Amylin Pharmaceuticals Inc | Formulierungen für amylin peptidagonisten mit insulin |
| US6703359B1 (en) | 1998-02-13 | 2004-03-09 | Amylin Pharmaceuticals, Inc. | Inotropic and diuretic effects of exendin and GLP-1 |
| AU3247799A (en) | 1998-02-27 | 1999-09-15 | Novo Nordisk A/S | Glp-1 derivatives of glp-1 and exendin with protracted profile of action |
| EP1950224A3 (en) | 1998-03-09 | 2008-12-17 | Zealand Pharma A/S | Pharmacologically active peptide conjugates having a reduced tendency towards enzymatic hydrolysis |
| AU2612599A (en) | 1998-03-13 | 1999-10-11 | Novo Nordisk A/S | Stabilized aqueous peptide solutions |
| US6998387B1 (en) | 1998-03-19 | 2006-02-14 | Amylin Pharmaceuticals, Inc. | Human appetite control by glucagon-like peptide receptor binding compounds |
| EP1419783A3 (en) | 1998-06-12 | 2005-01-12 | Amylin Pharmaceuticals, Inc. | Use of a composition comprising an exendin or a compound derived therefrom and a pharmaceutical carrier |
| US7056734B1 (en) | 1998-08-10 | 2006-06-06 | The United States Of America As Represented By The Department Of Health And Human Services, Nih | Differentiation of non-insulin producing cells into insulin producing cells by GLP-1 or exendin-4 and uses thereof |
| AU765584B2 (en) | 1998-09-17 | 2003-09-25 | Eli Lilly And Company | Protein formulations |
| US7259136B2 (en) | 1999-04-30 | 2007-08-21 | Amylin Pharmaceuticals, Inc. | Compositions and methods for treating peripheral vascular disease |
| US6284725B1 (en) | 1998-10-08 | 2001-09-04 | Bionebraska, Inc. | Metabolic intervention with GLP-1 to improve the function of ischemic and reperfused tissue |
| US6429197B1 (en) | 1998-10-08 | 2002-08-06 | Bionebraska, Inc. | Metabolic intervention with GLP-1 or its biologically active analogues to improve the function of the ischemic and reperfused brain |
| ATE307603T1 (de) | 1998-12-22 | 2005-11-15 | Lilly Co Eli | Lagerstabile flüssige zusammensetzungen von glucagon-ähnlichem peptid-1 |
| ES2244416T5 (es) | 1999-01-14 | 2020-01-03 | Amylin Pharmaceuticals Llc | Formulaciones novedosas de agonistas de la exendina y métodos de administración de los mismos |
| US20030087820A1 (en) | 1999-01-14 | 2003-05-08 | Young Andrew A. | Novel exendin agonist formulations and methods of administration thereof |
| PT1143989E (pt) | 1999-01-14 | 2007-03-30 | Amylin Pharmaceuticals Inc | Exendinas para supressão de glucagon |
| US7399489B2 (en) | 1999-01-14 | 2008-07-15 | Amylin Pharmaceuticals, Inc. | Exendin analog formulations |
| US6451974B1 (en) | 1999-03-17 | 2002-09-17 | Novo Nordisk A/S | Method of acylating peptides and novel acylating agents |
| EP1956000B1 (en) | 1999-03-17 | 2016-10-05 | Novo Nordisk A/S | Acylating agents useful for acylating peptides |
| US6924264B1 (en) | 1999-04-30 | 2005-08-02 | Amylin Pharmaceuticals, Inc. | Modified exendins and exendin agonists |
| JP2002544127A (ja) | 1999-04-30 | 2002-12-24 | アミリン・ファーマシューティカルズ,インコーポレイテッド | 修飾されたエキセンジンおよびエキセンジン・アゴニスト |
| US6887470B1 (en) | 1999-09-10 | 2005-05-03 | Conjuchem, Inc. | Protection of endogenous therapeutic peptides from peptidase activity through conjugation to blood components |
| US6849714B1 (en) | 1999-05-17 | 2005-02-01 | Conjuchem, Inc. | Protection of endogenous therapeutic peptides from peptidase activity through conjugation to blood components |
| US6514500B1 (en) | 1999-10-15 | 2003-02-04 | Conjuchem, Inc. | Long lasting synthetic glucagon like peptide {GLP-!} |
| AU754770B2 (en) | 1999-05-17 | 2002-11-21 | Conjuchem Biotechnologies Inc. | Long lasting insulinotropic peptides |
| US6482799B1 (en) | 1999-05-25 | 2002-11-19 | The Regents Of The University Of California | Self-preserving multipurpose ophthalmic solutions incorporating a polypeptide antimicrobial |
| US6506724B1 (en) | 1999-06-01 | 2003-01-14 | Amylin Pharmaceuticals, Inc. | Use of exendins and agonists thereof for the treatment of gestational diabetes mellitus |
| US6344180B1 (en) | 1999-06-15 | 2002-02-05 | Bionebraska, Inc. | GLP-1 as a diagnostic test to determine β-cell function and the presence of the condition of IGT and type II diabetes |
| US6528486B1 (en) | 1999-07-12 | 2003-03-04 | Zealand Pharma A/S | Peptide agonists of GLP-1 activity |
| US6972319B1 (en) | 1999-09-28 | 2005-12-06 | Bayer Pharmaceuticals Corporation | Pituitary adenylate cyclase activating peptide (PACAP)receptor 3 (R3) agonists and their pharmacological methods of use |
| GB9930882D0 (en) | 1999-12-30 | 2000-02-23 | Nps Allelix Corp | GLP-2 formulations |
| US20030036504A1 (en) | 2000-01-10 | 2003-02-20 | Amylin Pharmaceuticals, Inc. | Use of exendins and agonists thereof for modulation of triglyceride levels and treatment of dyslipidemia |
| JP2003526671A (ja) | 2000-03-14 | 2003-09-09 | レストラゲン,インコーポレイテッド | 幽門洞−十二指腸運動性に対するグルカゴン様ペプチド‐1(7−36)の作用 |
| WO2001087322A2 (en) | 2000-05-17 | 2001-11-22 | Bionebraska, Inc. | Peptide pharmaceutical formulations |
| CN103356996A (zh) | 2000-05-19 | 2013-10-23 | 埃米林药品公司 | Glp-1用于制备治疗急性冠脉综合征的药物的用途 |
| US7138546B2 (en) | 2000-08-18 | 2006-11-21 | Emisphere Technologies, Inc. | Compounds and compositions for delivering active agents |
| US7507714B2 (en) | 2000-09-27 | 2009-03-24 | Bayer Corporation | Pituitary adenylate cyclase activating peptide (PACAP) receptor 3 (R3) agonists and their pharmacological methods of use |
| MXPA02006118A (es) | 2000-10-20 | 2004-08-23 | Amylin Pharmaceuticals Inc | Tratamiento de la invernacion del miocardio y cardiopatia diabetica con un peptido glp-1. |
| CN1483041A (zh) | 2000-12-07 | 2004-03-17 | Glp-1融合蛋白 | |
| EP2186824A3 (en) | 2000-12-13 | 2010-09-22 | Eli Lilly & Company | Chronic treatment regimen using glucagon-like insulinotropic peptides |
| GB2371227A (en) | 2001-01-10 | 2002-07-24 | Grandis Biotech Gmbh | Crystallisation - resistant aqueous growth hormone formulations |
| US6573237B2 (en) | 2001-03-16 | 2003-06-03 | Eli Lilly And Company | Protein formulations |
| CN1162446C (zh) | 2001-05-10 | 2004-08-18 | 上海华谊生物技术有限公司 | 促胰岛素分泌肽衍生物 |
| EP1542712A2 (en) | 2001-06-01 | 2005-06-22 | Eli Lilly And Company | Glp-1 formulations with protracted time action |
| DK1412384T3 (da) | 2001-06-28 | 2008-04-28 | Novo Nordisk As | Stabil formulering af modificeret GLP-1 |
| EP1485707B1 (en) | 2001-07-16 | 2009-01-14 | caprotec bioanalytics GmbH | Capture compounds, collections thereof and methods for analyzing the proteome and complex compositions |
| ATE408414T1 (de) | 2001-07-31 | 2008-10-15 | Us Gov Health & Human Serv | Glp 1 exendin 4 peptidanaloga und deren verwendungen |
| US7238663B2 (en) | 2001-08-28 | 2007-07-03 | Eli Lilly And Company | Pre-mixes of GLP-1 and basal insulin |
| US7179788B2 (en) | 2001-10-19 | 2007-02-20 | Eli Lilly And Company | Biphasic mixtures of GLP-1 and insulin |
| WO2003059934A2 (en) | 2001-12-21 | 2003-07-24 | Human Genome Sciences, Inc. | Albumin fusion proteins |
| CA2471363C (en) | 2001-12-21 | 2014-02-11 | Human Genome Sciences, Inc. | Albumin fusion proteins |
| US7105489B2 (en) | 2002-01-22 | 2006-09-12 | Amylin Pharmaceuticals, Inc. | Methods and compositions for treating polycystic ovary syndrome |
| AU2003208945B2 (en) | 2002-02-20 | 2008-05-01 | Emisphere Technologies, Inc | Method for administering GLP-1 molecules |
| WO2003072060A2 (en) | 2002-02-27 | 2003-09-04 | Immunex Corporation | Polypeptide formulation |
| US20040209255A1 (en) | 2002-03-11 | 2004-10-21 | Hk Pharmaceuticals, Inc. | Compounds and methods for analyzing the proteome |
| US7141240B2 (en) | 2002-03-12 | 2006-11-28 | Cedars-Sinai Medical Center | Glucose-dependent insulin-secreting cells transfected with a nucleotide sequence encoding GLP-1 |
| WO2003084563A1 (en) | 2002-04-04 | 2003-10-16 | Novo Nordisk A/S | Glp-1 agonist and cardiovascular complications |
| US20050164925A1 (en) | 2002-04-10 | 2005-07-28 | Joseph Anthony Jakubowski And Thurman Dwight Mc Kinney | Treatment of gastroparesis |
| US6861236B2 (en) | 2002-05-24 | 2005-03-01 | Applied Nanosystems B.V. | Export and modification of (poly)peptides in the lantibiotic way |
| EP1515749B1 (en) | 2002-06-14 | 2012-08-15 | Novo Nordisk A/S | Combined use of a modulator of cd3 and a glp-1 compound |
| US20040037826A1 (en) | 2002-06-14 | 2004-02-26 | Michelsen Birgitte Koch | Combined use of a modulator of CD3 and a GLP-1 compound |
| DE10227232A1 (de) | 2002-06-18 | 2004-01-15 | Aventis Pharma Deutschland Gmbh | Saure Insulinzubereitungen mit verbesserter Stabilität |
| EP1525219B1 (en) | 2002-07-04 | 2009-05-27 | Zealand Pharma A/S | Glp-1 and methods for treating diabetes |
| US20070065469A1 (en) | 2002-07-09 | 2007-03-22 | Michael Betz | Liquid formulations with high concentration of human growth hormone (high) comprising glycine |
| WO2004004780A1 (en) | 2002-07-09 | 2004-01-15 | Sandoz Ag | Liquid formulations with high concentration of human growth hormone (hgh) comprising phenol |
| US20080260838A1 (en) | 2003-08-01 | 2008-10-23 | Mannkind Corporation | Glucagon-like peptide 1 (glp-1) pharmaceutical formulations |
| CA2493478C (en) | 2002-08-01 | 2014-11-18 | Mannkind Corporation | Cell transport compositions and uses thereof |
| US20040038865A1 (en) | 2002-08-01 | 2004-02-26 | Mannkind Corporation | Cell transport compositions and uses thereof |
| EP2058311A3 (de) | 2002-08-21 | 2011-04-13 | Boehringer Ingelheim Pharma GmbH & Co. KG | 8-[3-amino-piperidin-1-yl]-xanthine, deren Herstellung und deren Verwendung als Arzneimittel |
| US7407955B2 (en) | 2002-08-21 | 2008-08-05 | Boehringer Ingelheim Pharma Gmbh & Co., Kg | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions |
| CA2500295A1 (en) | 2002-10-02 | 2004-04-29 | Zealand Pharma A/S | Stabilized exendin-4 compounds |
| US6969702B2 (en) | 2002-11-20 | 2005-11-29 | Neuronova Ab | Compounds and methods for increasing neurogenesis |
| DK1583541T3 (da) | 2002-11-20 | 2011-04-11 | Neuronova Ab | Forbindelser og fremgangsmåder til at øge neurogenese |
| US20050209142A1 (en) | 2002-11-20 | 2005-09-22 | Goran Bertilsson | Compounds and methods for increasing neurogenesis |
| JP2006514035A (ja) | 2002-12-17 | 2006-04-27 | アミリン・ファーマシューティカルズ,インコーポレイテッド | 心不整脈の予防および治療 |
| US20040209803A1 (en) | 2002-12-19 | 2004-10-21 | Alain Baron | Compositions for the treatment and prevention of nephropathy |
| US7790681B2 (en) | 2002-12-17 | 2010-09-07 | Amylin Pharmaceuticals, Inc. | Treatment of cardiac arrhythmias with GLP-1 receptor ligands |
| GB0300571D0 (en) | 2003-01-10 | 2003-02-12 | Imp College Innovations Ltd | Modification of feeding behaviour |
| PL1620118T3 (pl) | 2003-04-08 | 2014-11-28 | Yeda Res & Dev | Leki odwracalnie pegylowane |
| WO2004089985A1 (en) | 2003-04-11 | 2004-10-21 | Novo Nordisk A/S | Stable pharmaceutical compositions |
| ATE549028T1 (de) | 2003-05-15 | 2012-03-15 | Tufts College | Stabile analoga von glp-1 |
| US7947261B2 (en) | 2003-05-23 | 2011-05-24 | Nektar Therapeutics | Conjugates formed from polymer derivatives having particular atom arrangements |
| KR101128320B1 (ko) | 2003-05-23 | 2012-04-12 | 넥타르 테라퓨틱스 | 아미도카르보네이트 연결기를 갖는 peg 유도체 |
| US8008255B2 (en) | 2003-05-30 | 2011-08-30 | Amylin Pharmaceuticals, Inc. | Methods and compositions for enhanced transmucosal delivery of peptides and proteins |
| ATE529126T1 (de) | 2003-06-03 | 2011-11-15 | Novo Nordisk As | Stabilisierte pharmazeutische peptid zusammensetzungen |
| JP2007524592A (ja) | 2003-06-03 | 2007-08-30 | ノボ・ノルデイスク・エー/エス | 安定化された薬学的ペプチド組成物 |
| KR101308912B1 (ko) | 2003-06-03 | 2013-09-23 | 노보 노르디스크 에이/에스 | 안정화된 약학적 펩티드 조성물 |
| CA2527743A1 (en) | 2003-06-03 | 2004-12-09 | Novo Nordisk A/S | Stabilized pharmaceutical peptide compositions |
| US8921311B2 (en) | 2003-08-01 | 2014-12-30 | Mannkind Corporation | Method for treating hyperglycemia |
| EP2107069B1 (en) | 2003-08-05 | 2013-01-16 | Novo Nordisk A/S | Novel insulin derivatives |
| RU2006103860A (ru) | 2003-08-21 | 2006-07-27 | Ново Нордиск А/С (DK) | Способ разделения полипептидов, содержащих рацемическую аминокислоту |
| WO2005021022A2 (en) | 2003-09-01 | 2005-03-10 | Novo Nordisk A/S | Stable formulations of peptides |
| US20060247167A1 (en) | 2003-09-01 | 2006-11-02 | Novo Nordisk A/S | Stable formulations of peptides |
| JP2007537981A (ja) | 2003-09-19 | 2007-12-27 | ノボ ノルディスク アクティーゼルスカブ | 新規の血漿タンパク質親和性タグ |
| JP4800959B2 (ja) | 2003-11-13 | 2011-10-26 | ノヴォ ノルディスク アー/エス | 糖尿病及び過食症を治療するためのglp−1ペプチド及び短時間作用型インスリンペプチドを含む、非経口投与用の可溶性医薬組成物 |
| US20060287221A1 (en) | 2003-11-13 | 2006-12-21 | Novo Nordisk A/S | Soluble pharmaceutical compositions for parenteral administration comprising a GLP-1 peptide and an insulin peptide of short time action for treatment of diabetes and bulimia |
| US20050281879A1 (en) | 2003-11-14 | 2005-12-22 | Guohua Chen | Excipients in drug delivery vehicles |
| US20050106214A1 (en) | 2003-11-14 | 2005-05-19 | Guohua Chen | Excipients in drug delivery vehicles |
| WO2005081619A2 (en) | 2003-11-20 | 2005-09-09 | Neuronova Ab | Compounds and methods for increasing neurogenesis |
| CN113304250A (zh) | 2003-11-20 | 2021-08-27 | 诺和诺德股份有限公司 | 对于生产和用于注射装置中是最佳的含有丙二醇的肽制剂 |
| AU2004295023A1 (en) | 2003-12-03 | 2005-06-16 | Novo Nordisk A/S | Single-chain insulin |
| CA2549011A1 (en) | 2003-12-10 | 2005-06-30 | Nektar Therapeutics Al, Corporation | Compositions comprising two different populations of polymer-active agent conjugates |
| US20050143303A1 (en) | 2003-12-26 | 2005-06-30 | Nastech Pharmaceutical Company Inc. | Intranasal administration of glucose-regulating peptides |
| US20060210614A1 (en) | 2003-12-26 | 2006-09-21 | Nastech Pharmaceutical Company Inc. | Method of treatment of a metabolic disease using intranasal administration of exendin peptide |
| CN1938334A (zh) | 2004-01-30 | 2007-03-28 | 瓦拉塔药品公司 | Glp-1激动剂和胃泌素化合物的联合使用 |
| CN1961000B (zh) | 2004-02-11 | 2011-05-04 | 安米林药品公司 | 具有可选择特性的杂合多肽 |
| CA2556226A1 (en) | 2004-02-11 | 2006-08-10 | Amylin Pharmaceuticals, Inc. | Amylin family peptides and methods for making and using them |
| US8076288B2 (en) | 2004-02-11 | 2011-12-13 | Amylin Pharmaceuticals, Inc. | Hybrid polypeptides having glucose lowering activity |
| WO2005077094A2 (en) | 2004-02-11 | 2005-08-25 | Amylin Pharmaceuticals, Inc. | Pancreatic polypeptide family motifs and polypeptides comprising the same |
| US7399744B2 (en) | 2004-03-04 | 2008-07-15 | Amylin Pharmaceuticals, Inc. | Methods for affecting body composition |
| US7456254B2 (en) | 2004-04-15 | 2008-11-25 | Alkermes, Inc. | Polymer-based sustained release device |
| US20060110423A1 (en) | 2004-04-15 | 2006-05-25 | Wright Steven G | Polymer-based sustained release device |
| AU2004319756C1 (en) | 2004-04-15 | 2014-02-20 | Alkermes Pharma Ireland Limited | Polymer-based sustained release device |
| WO2005117584A2 (en) | 2004-05-28 | 2005-12-15 | Amylin Pharmaceuticals, Inc | Improved transmucosal delivery of peptides and proteins |
| US20090069226A1 (en) | 2004-05-28 | 2009-03-12 | Amylin Pharmaceuticals, Inc. | Transmucosal delivery of peptides and proteins |
| EP1758575A1 (en) | 2004-06-11 | 2007-03-07 | Novo Nordisk A/S | Counteracting drug-induced obesity using glp-1 agonists |
| US20070021346A1 (en) | 2005-05-26 | 2007-01-25 | Ewing William R | N-terminally modified GLP-1 receptor modulators |
| CN103223160B (zh) | 2004-07-19 | 2015-02-18 | 比奥孔有限公司 | 胰岛素-低聚物共轭物,制剂及其用途 |
| JP2008507280A (ja) | 2004-07-21 | 2008-03-13 | アンブレツクス・インコーポレイテツド | 非天然コードアミノ酸を用いた生合成ポリペプチド |
| CA2575756A1 (en) | 2004-08-03 | 2006-02-16 | Biorexis Technology, Inc. | Combination therapy using transferrin fusion proteins comprising glp-1 |
| ES2442223T3 (es) | 2004-08-31 | 2014-02-10 | Novo Nordisk A/S | Uso de tris(hidroximetil) aminometano para la estabilización de péptidos, polipéptidos y proteínas |
| JP2008513384A (ja) | 2004-09-17 | 2008-05-01 | ノボ ノルディスク アクティーゼルスカブ | インスリンおよびインスリン分泌性ペプチドを含有する医薬組成物 |
| WO2006039336A2 (en) | 2004-10-01 | 2006-04-13 | Ramscor, Inc. | Conveniently implantable sustained release drug compositions |
| JP5107713B2 (ja) | 2004-10-07 | 2012-12-26 | ノヴォ ノルディスク アー/エス | 遅延性のエキセンディン−4化合物 |
| US7595294B2 (en) | 2004-10-08 | 2009-09-29 | Transition Therapeutics, Inc. | Vasoactive intestinal polypeptide pharmaceuticals |
| US20090036355A1 (en) | 2004-10-13 | 2009-02-05 | Sanjay Bhanot | Antisense Modulation of PTP1B Expression |
| US7442682B2 (en) | 2004-10-19 | 2008-10-28 | Nitto Denko Corporation | Transepithelial delivery of peptides with incretin hormone activities |
| WO2006051103A2 (en) | 2004-11-12 | 2006-05-18 | Novo Nordisk A/S | Stable formulations of peptides |
| CN101056650A (zh) | 2004-11-12 | 2007-10-17 | 诺和诺德公司 | 促胰岛素肽的稳定制剂 |
| ES2735533T3 (es) | 2004-11-12 | 2019-12-19 | Novo Nordisk As | Formulaciones estables de GLP-1 |
| CN101128487B (zh) | 2004-12-02 | 2012-10-10 | 杜门蒂斯有限公司 | 靶向血清白蛋白和glp-1或pyy的双特异性结构域抗体 |
| SG158141A1 (en) | 2004-12-13 | 2010-01-29 | Amylin Pharmaceuticals Inc | Pancreatic polypeptide family motifs, polypeptides and methods comprising the same |
| ES2357089T5 (es) | 2004-12-21 | 2014-02-24 | Nektar Therapeutics | Reactivos de tiol polimérico estabilizados |
| MX2007007565A (es) | 2004-12-22 | 2007-07-24 | Lilly Co Eli | Formulaciones de proteina de fusion analoga del peptido-1similar al glucagon (glp-1). |
| CA2599594A1 (en) | 2004-12-24 | 2006-07-13 | Amylin Pharmaceuticals, Inc. | Use of glp-1 and agonists thereof to prevent cardiac myocyte apoptosis |
| EP1845105A4 (en) | 2005-01-14 | 2009-02-18 | Wuxi Grandchamp Pharmaceutical | MODIFIED EXENDINES AND CORRESPONDING USES |
| US8716221B2 (en) | 2005-01-14 | 2014-05-06 | Wuxi Grandchamp Pharmaceutical Technology Co., Ltd. | Modified exendins and uses thereof |
| WO2006082588A2 (en) | 2005-02-07 | 2006-08-10 | Pharmalight Inc. | Method and device for ophthalmic administration of active pharmaceutical ingredients |
| JP2008530130A (ja) | 2005-02-11 | 2008-08-07 | アミリン・ファーマシューティカルズ,インコーポレイテッド | Gip類似体および選択可能な特性を備えるハイブリッドポリペプチド |
| US8263545B2 (en) | 2005-02-11 | 2012-09-11 | Amylin Pharmaceuticals, Inc. | GIP analog and hybrid polypeptides with selectable properties |
| WO2006097535A2 (en) | 2005-03-18 | 2006-09-21 | Novo Nordisk A/S | Peptide agonists of the glucagon family with secretin like activity |
| DK1888103T3 (da) | 2005-04-11 | 2012-04-23 | Amylin Pharmaceuticals Inc | Anvendelse af glp-1, exendin og agonister deraf til forsinkelse eller forhindring af kardial remodellering |
| WO2006114396A1 (en) | 2005-04-24 | 2006-11-02 | Novo Nordisk A/S | Injection device |
| WO2006125763A1 (en) | 2005-05-25 | 2006-11-30 | Novo Nordisk A/S | Stabilized polypeptide formulations |
| US8546326B2 (en) | 2005-06-06 | 2013-10-01 | Camurus Ab | Glp-1 analogue formulations |
| GB0511986D0 (en) | 2005-06-13 | 2005-07-20 | Imp College Innovations Ltd | Novel compounds and their effects on feeding behaviour |
| ATE553124T1 (de) * | 2005-06-13 | 2012-04-15 | Imp Innovations Ltd | Oxyntomodulinanaloga und ihre wirkungen auf das fressverhalten |
| BRPI0611872B8 (pt) | 2005-06-16 | 2021-05-25 | Nektar Therapeutics | reagente polimérico, conjugado, método para preparação de um conjugado e composição farmacêutica |
| BRPI0614257A2 (pt) | 2005-08-04 | 2011-03-15 | Nektar Therapeutics Al Corp | conjugados de uma porção de g-csf e um polìmero |
| CN101277722A (zh) | 2005-08-06 | 2008-10-01 | 王庆华 | 用于预防和治疗ⅰ型糖尿病的组合物及方法 |
| EP2330124B1 (en) | 2005-08-11 | 2015-02-25 | Amylin Pharmaceuticals, LLC | Hybrid polypeptides with selectable properties |
| WO2007024700A2 (en) | 2005-08-19 | 2007-03-01 | Amylin Pharmaceuticals, Inc. | Exendin for treating diabetes and reducing body weight |
| WO2007033372A2 (en) | 2005-09-14 | 2007-03-22 | Mannkind Corporation | Method of drug formulation based on increasing the affinity of crystalline microparticle surfaces for active agents |
| KR101368525B1 (ko) | 2005-09-20 | 2014-03-06 | 노파르티스 아게 | 저혈당 증상을 저하시키기 위한 dpp-ⅳ 억제제의 용도 |
| US8759290B2 (en) | 2005-10-18 | 2014-06-24 | Biocon Limited | Oral glucagon-like peptide conjugates for metabolic diseases |
| WO2007047922A2 (en) | 2005-10-19 | 2007-04-26 | Smartcells, Inc. | Polymer-drug conjugates |
| CN101534846B (zh) | 2005-11-07 | 2014-11-05 | 印第安纳大学研究及科技有限公司 | 显示生理学溶解性和稳定性的胰高血糖素类似物 |
| US8039432B2 (en) | 2005-11-09 | 2011-10-18 | Conjuchem, Llc | Method of treatment of diabetes and/or obesity with reduced nausea side effect |
| JP2009518315A (ja) | 2005-12-02 | 2009-05-07 | エムディーアールエヌエー,インコーポレイテッド | グルコース調節ペプチドの上皮透過性を増大させるための製剤処方 |
| WO2007064691A1 (en) | 2005-12-02 | 2007-06-07 | Nabil Habib Lab | Treatment of cancer and other diseases |
| RU2008124109A (ru) | 2005-12-08 | 2010-01-20 | МДРНА, Инк. (US) | Чресслизистая доставка стабилизированных композиций эксендина |
| WO2007075534A2 (en) | 2005-12-16 | 2007-07-05 | Nektar Therapeutics Al, Corporation | Polymer conjugates of glp-1 |
| US8841255B2 (en) | 2005-12-20 | 2014-09-23 | Duke University | Therapeutic agents comprising fusions of vasoactive intestinal peptide and elastic peptides |
| US20130172274A1 (en) | 2005-12-20 | 2013-07-04 | Duke University | Methods and compositions for delivering active agents with enhanced pharmacological properties |
| JP2009525946A (ja) | 2005-12-20 | 2009-07-16 | デューク・ユニヴァーシティ | 増強された薬理学的性質を有する活性物質を送達するための方法および組成物 |
| SI1984009T1 (sl) | 2006-01-18 | 2013-02-28 | Qps, Llc | Farmacevtski sestavki z izboljĺ ano stabilnostjo |
| AU2007212147A1 (en) | 2006-02-03 | 2007-08-16 | Medimmune, Llc | Protein formulations |
| US7704953B2 (en) | 2006-02-17 | 2010-04-27 | Mdrna, Inc. | Phage displayed cell binding peptides |
| CA2646598C (en) | 2006-03-21 | 2014-08-19 | Amylin Pharmaceuticals, Inc. | Peptide-peptidase inhibitor conjugates and methods of using same |
| RU2419452C2 (ru) | 2006-04-13 | 2011-05-27 | Ипсен Фарма С.А.С. | ФАРМАЦЕВТИЧЕСКИЕ КОМПОЗИЦИИ hGLP-1, ЭКСЕНДИНА-4 И ИХ АНАЛОГОВ |
| KR20150042304A (ko) | 2006-04-14 | 2015-04-20 | 맨카인드 코포레이션 | 글루카곤 유사 펩타이드 1 (glp-1) 약제학적 제제 |
| PE20110235A1 (es) | 2006-05-04 | 2011-04-14 | Boehringer Ingelheim Int | Combinaciones farmaceuticas que comprenden linagliptina y metmorfina |
| WO2007133778A2 (en) | 2006-05-12 | 2007-11-22 | Amylin Pharmaceuticals, Inc. | Methods to restore glycemic control |
| EA200870575A1 (ru) * | 2006-05-26 | 2009-08-28 | Амилин Фармасьютикалз, Инк. | Композиции и способы лечения застойной сердечной недостаточности |
| EP2021014A1 (en) | 2006-05-26 | 2009-02-11 | Brystol-Myers Squibb Company | Sustained release glp-1 receptor modulators |
| WO2007148345A2 (en) | 2006-06-21 | 2007-12-27 | Biocon Limited | A method of producing biologically active polypeptide having insulinotropic activity |
| PL2494959T3 (pl) | 2006-07-05 | 2015-06-30 | Foamix Pharmaceuticals Ltd | Nośnik ze spienialnego kwasu dikarboksylowego oraz kompozycje farmaceutyczne z nośnikiem |
| AU2007278994B2 (en) | 2006-07-24 | 2013-08-15 | Biorexis Pharmaceutical Corporation | Exendin fusion proteins |
| US7928186B2 (en) | 2006-08-02 | 2011-04-19 | Phoenix Pharmaceuticals, Inc. | Cell permeable bioactive peptide conjugates |
| AU2007281433A1 (en) | 2006-08-04 | 2008-02-07 | Nastech Pharmaceutical Company Inc. | Compositions for intranasal delivery of human insulin and uses thereof |
| KR101200728B1 (ko) | 2006-08-09 | 2012-11-13 | 인타르시아 세라퓨틱스 인코포레이티드 | 삼투성 전달 시스템 및 피스톤 조립체 |
| US8497240B2 (en) | 2006-08-17 | 2013-07-30 | Amylin Pharmaceuticals, Llc | DPP-IV resistant GIP hybrid polypeptides with selectable properties |
| EP2057188B1 (en) | 2006-08-17 | 2013-07-31 | Amylin Pharmaceuticals, LLC | Dpp-iv resistant gip hybrid polypeptides with selectable properties |
| WO2008023050A1 (en) * | 2006-08-25 | 2008-02-28 | Novo Nordisk A/S | Acylated exendin-4 compounds |
| CN102827284B (zh) | 2006-11-14 | 2015-07-29 | 上海仁会生物制药股份有限公司 | 带有聚乙二醇基团的Exendin或其类似物及其制剂和用途 |
| EP2124883A2 (en) | 2006-12-12 | 2009-12-02 | Amylin Pharmaceuticals, Inc. | Pharmaceutical formulations and methods for making the same |
| TWI428346B (zh) | 2006-12-13 | 2014-03-01 | Imp Innovations Ltd | 新穎化合物及其等對進食行為影響 |
| US20090098130A1 (en) * | 2007-01-05 | 2009-04-16 | Bradshaw Curt W | Glucagon-like protein-1 receptor (glp-1r) agonist compounds |
| CA2674354A1 (en) | 2007-01-05 | 2008-07-17 | Indiana University Research And Technology Corporation | Glucagon analogs exhibiting enhanced solubility in physiological ph buffers |
| CN101663317A (zh) | 2007-01-05 | 2010-03-03 | CovX科技爱尔兰有限公司 | 胰高血糖素样蛋白-1受体glp-1r激动剂化合物 |
| RU2432361C2 (ru) | 2007-01-05 | 2011-10-27 | КовЭкс Текнолоджиз Айэлэнд Лимитед | Соединения агонисты рецептора глюкагоноподобного белка-1 (glp-1r) |
| WO2008098212A2 (en) | 2007-02-08 | 2008-08-14 | Diobex, Inc. | Extended release formulations of glucagon and other peptides and proteins |
| BRPI0807728A2 (pt) | 2007-02-15 | 2012-04-17 | Univ Indiana Res & Tech Corp | co-agonistas de receptor glucagon/glp-1 |
| US8420598B2 (en) | 2007-04-20 | 2013-04-16 | B & L Delipharm Corp. | Mono modified exendin with polyethylene glycol or its derivatives and uses thereof |
| ES2402172T3 (es) | 2007-04-23 | 2013-04-29 | Intarcia Therapeutics, Inc | Formulación en suspensión de péptidos insulinotrópicos y usos de los mismos |
| WO2008134425A1 (en) | 2007-04-27 | 2008-11-06 | Cedars-Sinai Medical Center | Use of glp-1 receptor agonists for the treatment of gastrointestinal disorders |
| US7829664B2 (en) | 2007-06-01 | 2010-11-09 | Boehringer Ingelheim International Gmbh | Modified nucleotide sequence encoding glucagon-like peptide-1 (GLP-1), nucleic acid construct comprising same for production of glucagon-like peptide-1 (GLP-1), human cells comprising said construct and insulin-producing constructs, and methods of use thereof |
| US9353170B2 (en) | 2007-06-08 | 2016-05-31 | Sanofi-Aventis Deutschland Gmbh | Long-acting transient polymer conjugates of exendin |
| ATE520714T1 (de) | 2007-06-15 | 2011-09-15 | Zealand Pharma As | Glucagonanaloga |
| KR20100020516A (ko) | 2007-07-10 | 2010-02-22 | 일라이 릴리 앤드 캄파니 | Glp-1-fc 융합 단백질 제제 |
| EP2185178B1 (en) | 2007-08-03 | 2017-08-23 | Eli Lilly And Company | Use of an fgf-21 compound and a glp-1 compound for the treatment of obesity |
| CN101366692A (zh) | 2007-08-15 | 2009-02-18 | 江苏豪森药业股份有限公司 | 一种稳定的艾塞那肽制剂 |
| ES2534434T3 (es) | 2007-08-30 | 2015-04-22 | Curedm Group Holdings, Llc | Composiciones y métodos de uso de péptidos proislotes y análogos de los mismos |
| CN101842109B (zh) | 2007-09-05 | 2014-01-29 | 诺沃-诺迪斯克有限公司 | 用a-b-c-d-衍生的肽和它们的治疗用途 |
| US20100256056A1 (en) | 2007-09-07 | 2010-10-07 | Zheng Xin Dong | Analogues of exendin-4 and exendin-3 |
| BRPI0818872A2 (pt) | 2007-10-24 | 2015-05-05 | Mannkind Corp | Método para prevenir efeitos adversos por glp-1 |
| JP5813323B2 (ja) | 2007-10-24 | 2015-11-17 | マンカインド コーポレイション | 活性薬剤の送達方法 |
| US8785396B2 (en) | 2007-10-24 | 2014-07-22 | Mannkind Corporation | Method and composition for treating migraines |
| MX2010004298A (es) | 2007-10-30 | 2010-05-03 | Univ Indiana Res & Tech Corp | Compuestos que exhiben actividad antagonista de glucagon y agonista de glp-1. |
| AU2008318986B2 (en) | 2007-10-30 | 2014-12-04 | Indiana University Research And Technology Corporation | Glucagon antagonists |
| MX2010005245A (es) | 2007-11-16 | 2010-06-01 | Novo Nordisk As | Composiciones farmaceuticas que comprenden peptidos glp-1 o exendin-4 y un peptido de insulina basal. |
| US8710002B2 (en) | 2007-11-23 | 2014-04-29 | Michael Rothkopf | Methods of enhancing diabetes resolution |
| CN101444618B (zh) | 2007-11-26 | 2012-06-13 | 杭州九源基因工程有限公司 | 含有艾塞那肽的药物制剂 |
| CN102026666B (zh) | 2007-12-11 | 2013-10-16 | 常山凯捷健生物药物研发(河北)有限公司 | 促胰岛素肽缀合物制剂 |
| PL2229407T3 (pl) | 2008-01-09 | 2017-06-30 | Sanofi-Aventis Deutschland Gmbh | Nowe pochodne insuliny o ekstremalnie spowolnionym profilu czasu działania |
| EP2249853A4 (en) | 2008-01-30 | 2012-12-26 | Univ Indiana Res & Tech Corp | ESTER BASED PEPTIDE PRODRUGS |
| HRP20191052T1 (hr) | 2008-02-01 | 2019-11-01 | Ascendis Pharma As | Predlijek koji obuhvaća samocijepajuću poveznicu |
| MX349413B (es) | 2008-02-06 | 2017-07-19 | Biocon Ltd | Medios de fermentacion y procedimientos para los mismos. |
| WO2009114959A1 (zh) | 2008-03-20 | 2009-09-24 | 中国人民解放军军事医学科学院毒物药物研究所 | 可注射用缓释药物制剂及其制备方法 |
| US20110034385A1 (en) | 2008-04-07 | 2011-02-10 | National Institute Of Immunology | Compositions Useful for the Treatment of Diabetes and Other Chronic Disorder |
| KR20110007614A (ko) | 2008-05-07 | 2011-01-24 | 메리온 리서치 Ⅲ 리미티드 | GnRH 관련 화합물의 조성물 및 제조 방법 |
| WO2009143285A2 (en) | 2008-05-21 | 2009-11-26 | Amylin Pharmaceuticals, Inc. | Exendins to lower cholestrol and triglycerides |
| JP2011523052A (ja) | 2008-05-23 | 2011-08-04 | アミリン・ファーマシューティカルズ,インコーポレイテッド | Glp−1受容体アゴニスト・バイオアッセイ |
| KR101629154B1 (ko) | 2008-06-13 | 2016-06-21 | 맨카인드 코포레이션 | 건조 분말 흡입기 및 약물 투여 시스템 |
| US8485180B2 (en) | 2008-06-13 | 2013-07-16 | Mannkind Corporation | Dry powder drug delivery system |
| EA020326B9 (ru) | 2008-06-17 | 2015-03-31 | Индиана Юниверсити Рисерч Энд Текнолоджи Корпорейшн | Агонисты смешанного действия на основе глюкозозависимого инсулинотропного пептида для лечения нарушений обмена веществ и ожирения |
| BRPI0914889A2 (pt) | 2008-06-17 | 2015-11-24 | Otsuka Chemical Co Ltd | peptídeo glp-1 adicionado de cadeia oligossacarídica |
| CA2727161A1 (en) | 2008-06-17 | 2009-12-23 | Indiana University Research And Technology Corporation | Glucagon analogs exhibiting enhanced solubility and stability physiological ph buffers |
| EP2676673B1 (en) | 2008-06-17 | 2016-11-16 | Indiana University Research and Technology Corporation | Glucagon/glp-1 receptor co-agonists |
| CN102131516B (zh) | 2008-06-27 | 2016-03-16 | 杜克大学 | 包含弹性蛋白样肽的治疗剂 |
| EP2303247A4 (en) | 2008-07-21 | 2012-08-01 | Syneron Medical Ltd | TRANSDERMALES SYSTEM FOR THE EXTENDED RELEASE OF INCRETINS AND INCRETIN MIMETIC PEPTIDES |
| WO2010013012A2 (en) | 2008-08-01 | 2010-02-04 | Lund University Bioscience Ab | Novel polypeptides and uses thereof |
| CN101670096B (zh) | 2008-09-11 | 2013-01-16 | 杭州九源基因工程有限公司 | 含有艾塞那肽的药物制剂 |
| EP3228320B1 (de) | 2008-10-17 | 2019-12-18 | Sanofi-Aventis Deutschland GmbH | Kombination von einem insulin und einem glp-1-agonisten |
| DK2373681T3 (en) | 2008-12-10 | 2017-04-24 | Glaxosmithkline Llc | PHARMACEUTICAL COMPOSITIONS OF ALBIGLUTID |
| MX2011006314A (es) | 2008-12-15 | 2011-09-22 | Zealand Pharma As | Analogos de glucagon. |
| DK2370462T3 (da) | 2008-12-15 | 2014-09-08 | Zealand Pharma As | Glucagon-analoger |
| EA020537B1 (ru) | 2008-12-15 | 2014-11-28 | Зилэнд Фарма А/С | Аналоги глюкагона |
| CA2747155A1 (en) | 2008-12-15 | 2010-06-24 | Zealand Pharma A/S | Glucagon analogues |
| MX2011006524A (es) | 2008-12-19 | 2011-08-17 | Univ Indiana Res & Tech Corp | Profarmacos de peptido de la superfamilia de glucagon basado en amida. |
| CN101538323B (zh) | 2009-01-13 | 2012-05-09 | 深圳翰宇药业股份有限公司 | 一种纯化艾塞那肽的方法 |
| WO2010096052A1 (en) | 2009-02-19 | 2010-08-26 | Merck Sharp & Dohme Corp. | Oxyntomodulin analogs |
| AU2010221254B2 (en) | 2009-03-04 | 2014-04-03 | Mannkind Corporation | An improved dry powder drug delivery system |
| WO2010100241A1 (en) | 2009-03-05 | 2010-09-10 | Sanofi-Aventis Deutschland Gmbh | Drug delivery device with retractable needle |
| US8642544B2 (en) | 2009-04-01 | 2014-02-04 | Amylin Pharmaceuticals, Llc | N-terminus conformationally constrained GLP-1 receptor agonist compounds |
| WO2010118034A2 (en) | 2009-04-06 | 2010-10-14 | Board Of Regents, The University Of Texas System | Cyclic peptide analogues for non-invasive imaging of pancreatic beta-cells |
| AU2010239861B2 (en) | 2009-04-22 | 2013-07-11 | Alteogen, Inc | In vivo half life increased fusion protein or peptide maintained by sustained in vivo release, and method for increasing in vivo half-life using same |
| CN101870728A (zh) | 2009-04-23 | 2010-10-27 | 派格生物医药(苏州)有限公司 | 新型Exendin变体及其缀合物 |
| CN101559041B (zh) | 2009-05-19 | 2014-01-15 | 中国科学院过程工程研究所 | 粒径均一的多肽药物缓释微球或微囊制剂及制备方法 |
| CN102438679B (zh) | 2009-05-20 | 2016-03-09 | 赛诺菲-安万特德国有限公司 | 用于药物递送装置中药物容纳筒的塞子 |
| WO2010133676A1 (en) | 2009-05-20 | 2010-11-25 | Sanofi-Aventis Deutschland Gmbh | A system comprising a drug delivery device and a cartridge provided with a bung and a method of identifying the cartridge |
| EP2435061A4 (en) | 2009-05-28 | 2013-03-27 | Amylin Pharmaceuticals Inc | DAMPING GLP-1 RECEPTOR AGONIST COMPOUNDS |
| US20120172298A1 (en) | 2009-06-11 | 2012-07-05 | Novo Nordisk A/S | Glp-1 and fgf21 combinations for treatment of diabetes type 2 |
| EP2443146B1 (en) | 2009-06-16 | 2016-10-05 | Indiana University Research And Technology Corporation | Gip receptor-active glucagon compounds |
| CA2766537A1 (en) | 2009-07-02 | 2011-01-06 | Angiochem Inc. | Multimeric peptide conjugates and uses thereof |
| CN101601646B (zh) | 2009-07-22 | 2011-03-23 | 南京凯瑞尔纳米生物技术有限公司 | 治疗糖尿病的鼻腔滴剂及其制备方法 |
| WO2011011675A1 (en) | 2009-07-23 | 2011-01-27 | Zelos Therapeutics, Inc. | Pharmaceutically acceptable formulations/compositions for peptidyl drugs |
| EP2459228B1 (en) | 2009-07-31 | 2020-03-04 | Sanofi-Aventis Deutschland GmbH | Prodrugs comprising an insulin linker conjugate |
| WO2011017835A1 (en) | 2009-08-11 | 2011-02-17 | Nanjing University | Preparation method of protein or peptide nanoparticles for in vivo drug delivery by unfolding and refolding |
| CN101993485B (zh) | 2009-08-20 | 2013-04-17 | 重庆富进生物医药有限公司 | 促胰岛素分泌肽类似物同源二聚体及其用途 |
| US20120148586A1 (en) * | 2009-08-27 | 2012-06-14 | Joyce Ching Tsu Chou | Glucagon-like protein-1 receptor (glp-1r) agonists for treating autoimmune disorders |
| JP2013506628A (ja) | 2009-09-30 | 2013-02-28 | グラクソ グループ リミテッド | 延長された半減期を有する薬物融合物及びコンジュゲート |
| WO2011049713A2 (en) | 2009-10-22 | 2011-04-28 | Biodel Inc. | Stabilized glucagon solutions |
| US20110097386A1 (en) | 2009-10-22 | 2011-04-28 | Biodel, Inc. | Stabilized glucagon solutions |
| US9610329B2 (en) | 2009-10-22 | 2017-04-04 | Albireo Pharma, Inc. | Stabilized glucagon solutions |
| BR112012011467A2 (pt) | 2009-10-30 | 2019-09-24 | Otsuka Chemical Co Ltd | forma glicosilada de análogo ao glp-1 antigênico. |
| PT2496583E (pt) | 2009-11-02 | 2015-01-14 | Pfizer | Derivados de dioxa-biciclo[3.2.1]octano-2,3,4-triol |
| EP2496249B1 (en) | 2009-11-03 | 2016-03-09 | Amylin Pharmaceuticals, LLC | Glp-1 receptor agonist for use in treating obstructive sleep apnea |
| BR112012011403B8 (pt) | 2009-11-13 | 2021-05-25 | Sanofi Aventis Deutschland | composição farmacêutica líquida compreendendo um agonista glp-1 e metionina e uso da mesma |
| CA2780460C (en) | 2009-11-13 | 2018-09-04 | Sanofi-Aventis Deutschland Gmbh | Pharmaceutical composition comprising a glp-1 agonist, an insulin and methionine |
| KR102029560B1 (ko) | 2009-12-15 | 2019-10-07 | 씨리우스 테라퓨틱스, 엘엘씨 | 대사성 질환의 치료를 위한 ppar 절약형 티아졸리딘디온염 |
| EP2512475A1 (en) | 2009-12-15 | 2012-10-24 | Metabolic Solutions Development Company LLC | Ppar-sparing thiazolidinediones and combinations for the treatment of obesity and other metabolic diseases |
| PL2512470T3 (pl) | 2009-12-15 | 2017-06-30 | Octeta Therapeutics, Llc | Oszczędzające ppar tiazolidynodiony i połączenia do leczenia chorób neurodegeneracyjnych |
| AU2010340058A1 (en) | 2009-12-15 | 2012-06-21 | Metabolic Solutions Development Company, Llc | PPAR-sparing thiazolidinediones and combinations for the treatment of diabetes mellitus and other metabolic diseases |
| EP2513141B1 (en) | 2009-12-16 | 2017-03-01 | Novo Nordisk A/S | Glp-1 analogues and derivatives |
| EP2512503A4 (en) | 2009-12-18 | 2013-08-21 | Univ Indiana Res & Tech Corp | COAGONISTS OF GLUCAGON / GLP-1 RECEPTOR |
| CN102933200B (zh) | 2009-12-18 | 2015-11-25 | 莱迪杜德制药公司 | 包含磷脂的单相凝胶组合物 |
| CN101798588B (zh) | 2009-12-21 | 2015-09-09 | 上海仁会生物制药股份有限公司 | Glp-1受体激动剂生物学活性测定方法 |
| JO2976B1 (en) | 2009-12-22 | 2016-03-15 | ايلي ليلي اند كومباني | Axentomodulin polypeptide |
| AR079344A1 (es) | 2009-12-22 | 2012-01-18 | Lilly Co Eli | Analogo peptidico de oxintomodulina, composicion farmaceutica que lo comprende y uso para preparar un medicamento util para tratar diabetes no insulinodependiente y/u obesidad |
| US20130157953A1 (en) | 2010-01-20 | 2013-06-20 | Zealand Pharma A/S | Treatment of cardiac conditions |
| CA2788304A1 (en) | 2010-01-27 | 2011-08-04 | Indiana University Research And Technology Corporation | Glucagon antagonist - gip agonist conjugates and compositions for the treatment of metabolic disorders and obesity |
| WO2011092326A1 (en) | 2010-02-01 | 2011-08-04 | Sanofi-Aventis Deutschland Gmbh | Cartridge holder, drug delivery device and method for securing a cartridge in a cartridge holder |
| WO2011109784A1 (en) | 2010-03-05 | 2011-09-09 | Conjuchem, Llc | Formulation of insulinotropic peptide conjugates |
| KR20130018410A (ko) * | 2010-03-26 | 2013-02-21 | 노보 노르디스크 에이/에스 | 새로운 글루카곤 유사체 |
| AR080592A1 (es) | 2010-03-26 | 2012-04-18 | Lilly Co Eli | Peptido con actividad para el gip-r y glp-1-r, formulacion famaceutica que lo comprende, su uso para preparar un medicamento util para el tratamiento de diabetes mellitus y para inducir la perdida de peso |
| ES2661228T3 (es) | 2010-05-13 | 2018-03-28 | Indiana University Research And Technology Corporation | Péptidos de la superfamilia de glucagón que muestran actividad de receptor nuclear de hormona |
| US9145451B2 (en) | 2010-05-13 | 2015-09-29 | Indiana University Research And Technology Corporation | Glucagon superfamily peptides exhbiting G protein coupled receptor activity |
| AU2011202239C1 (en) | 2010-05-19 | 2017-03-16 | Sanofi | Long-acting formulations of insulins |
| BR112012029280A2 (pt) | 2010-05-20 | 2016-11-29 | Glaxo Group Ltd | variante de domínio variável único de imunoglobulina antialbumina sérica, imunoglobulina anti-sa, ligando multiespecífico, proteína de fusão, composição, ácido nucleico, vetor, célula hospedeira isolada, e, uso de uma variante, ligando multiespecífico ou proteína de fusão |
| EP2578726B1 (en) | 2010-05-31 | 2018-10-17 | JTEKT Corporation | Method for manufacturing a coated member |
| US9252665B2 (en) | 2010-06-01 | 2016-02-02 | Honda Motor Co., Ltd. | DC-DC converter control apparatus |
| WO2011156407A2 (en) | 2010-06-09 | 2011-12-15 | Amylin Pharmaceuticals, Inc. | Glp-1 receptor agonists to treat pancre-atitis |
| CN101891823B (zh) | 2010-06-11 | 2012-10-03 | 北京东方百泰生物科技有限公司 | 一种Exendin-4及其类似物融合蛋白 |
| US8636711B2 (en) | 2010-06-14 | 2014-01-28 | Legacy Emanuel Hospital & Health Center | Stabilized glucagon solutions and uses therefor |
| JP6385673B2 (ja) | 2010-06-21 | 2018-09-05 | マンカインド コーポレイション | 乾燥粉末薬物送達システム |
| UY33462A (es) | 2010-06-23 | 2012-01-31 | Zealand Pharma As | Analogos de glucagon |
| WO2011162830A2 (en) | 2010-06-24 | 2011-12-29 | Biousian Biosystems, Inc. | Glucagon-like peptide-1 glycopeptides |
| EP2588126A4 (en) | 2010-06-24 | 2015-07-08 | Univ Indiana Res & Tech Corp | AMID-BASED GLUCAGON SUPERFAMILY PEPTIDE PRODRUGS |
| WO2011163473A1 (en) | 2010-06-25 | 2011-12-29 | Indiana University Research And Technology Corporation | Glucagon analogs exhibiting enhanced solubility and stability in physiological ph buffers |
| WO2012012352A2 (en) | 2010-07-19 | 2012-01-26 | Amidebio, Llc | Modified peptides and proteins |
| WO2012012460A1 (en) | 2010-07-19 | 2012-01-26 | Xeris Pharmaceuticals, Inc. | Stable glucagon formulations for the treatment of hypoglycemia |
| WO2012015975A2 (en) | 2010-07-28 | 2012-02-02 | Amylin Pharmaceuticals, Inc. | Glp-1 receptor agonist compounds having stabilized regions |
| CN102397558B (zh) | 2010-09-09 | 2013-08-14 | 中国人民解放军军事医学科学院毒物药物研究所 | Exendin-4类似物的定位聚乙二醇化修饰物及其用途 |
| EP2438930A1 (en) | 2010-09-17 | 2012-04-11 | Sanofi-Aventis Deutschland GmbH | Prodrugs comprising an exendin linker conjugate |
| JP6121330B2 (ja) | 2010-09-28 | 2017-04-26 | アミリン・ファーマシューティカルズ, リミテッド・ライアビリティ・カンパニーAmylin Pharmaceuticals, Llc | 作用持続時間が増した改変ポリペプチド |
| JP5894174B2 (ja) | 2010-11-03 | 2016-03-23 | アレコー リミテッド | グルカゴンを含む新規組成物 |
| BR112013011549B1 (pt) | 2010-11-09 | 2022-01-04 | Mannkind Corporation | Composições farmacêuticas compreendendo um agonista do receptor de serotonina e uso das mesmas para tratar sintomas associados com enxaqueca |
| EP2460552A1 (en) | 2010-12-06 | 2012-06-06 | Sanofi-Aventis Deutschland GmbH | Drug delivery device with locking arrangement for dose button |
| CN102552883B (zh) | 2010-12-09 | 2014-02-19 | 天津药物研究院 | 一种多肽复合物、药物组合物、其制备方法和应用 |
| KR101925620B1 (ko) | 2010-12-16 | 2018-12-05 | 노보 노르디스크 에이/에스 | Glp-1 아고니스트 및 n-(8-(2-히드록시벤조일)아미노)카프릴산의 염을 포함하는 고체 조성물 |
| JP2014504588A (ja) | 2010-12-22 | 2014-02-24 | アミリン・ファーマシューティカルズ,リミテッド・ライアビリティ・カンパニー | 膵島細胞移植のためのglp−1受容体アゴニスト |
| ES2713952T3 (es) | 2010-12-22 | 2019-05-24 | Univ Indiana Res & Tech Corp | Análogos de glucagón que muestran actividad de receptor de GIP |
| CN102532301B (zh) | 2010-12-31 | 2014-09-03 | 上海医药工业研究院 | 一类新型的Exendin-4类似物及其制备方法 |
| US20120208755A1 (en) | 2011-02-16 | 2012-08-16 | Intarcia Therapeutics, Inc. | Compositions, Devices and Methods of Use Thereof for the Treatment of Cancers |
| CN102100906A (zh) | 2011-02-18 | 2011-06-22 | 深圳翰宇药业股份有限公司 | 一种艾塞那肽的药用制剂及其制备方法 |
| US8697644B2 (en) | 2011-03-10 | 2014-04-15 | Xeris Pharmaceuticals, Inc. | Stable formulations for parenteral injection of peptide drugs |
| CN102718858B (zh) | 2011-03-29 | 2014-07-02 | 天津药物研究院 | 胰高血糖素样肽-1类似物单体、二聚体及其制备方法与应用 |
| CN106928341B (zh) | 2011-03-30 | 2021-06-01 | 上海仁会生物制药股份有限公司 | 定点单取代聚乙二醇化Exendin类似物及其制备方法 |
| DK2694095T3 (en) | 2011-04-05 | 2018-05-28 | Longevity Biotech Inc | COMPOSITIONS COMPREHENSIVE GLUCAGON ANALOGS AND METHODS FOR PREPARING AND USING THE SAME |
| US9480751B2 (en) | 2011-04-11 | 2016-11-01 | Yeda Research And Development Co. Ltd. | Albumin binding probes and drug conjugates thereof |
| WO2012150503A2 (en) | 2011-05-03 | 2012-11-08 | Zealand Pharma A/S | Glu-glp-1 dual agonist signaling-selective compounds |
| CN102766204B (zh) | 2011-05-05 | 2014-10-15 | 天津药物研究院 | 胰高血糖素样肽-1突变体多肽及其制备方法和其应用 |
| JP6284471B2 (ja) | 2011-05-18 | 2018-02-28 | メデリス ダイアビーティーズ,エルエルシー | インスリン抵抗性のための改善されたペプチドの調合薬 |
| JP2014521594A (ja) | 2011-05-25 | 2014-08-28 | アミリン・ファーマシューティカルズ,リミテッド・ライアビリティ・カンパニー | 長持続期間デュアルホルモンコンジュゲート |
| UA113626C2 (xx) | 2011-06-02 | 2017-02-27 | Композиція для лікування діабету, що містить кон'югат інсуліну тривалої дії та кон'югат інсулінотропного пептиду тривалої дії | |
| BR112013031794B1 (pt) | 2011-06-10 | 2020-11-10 | Hanmi Science Co., Ltd | peptídeo derivados de oxintomodulina e seu uso, polinucleotídeo, composição farmacêutica e seu uso e método para prevenção ou tratamento da obesidade compreendendo os mesmos |
| WO2012167744A1 (en) | 2011-06-10 | 2012-12-13 | Beijing Hanmi Pharmaceutical Co., Ltd. | Glucose dependent insulinotropic polypeptide analogs, pharmaceutical compositions and use thereof |
| CN103974715A (zh) | 2011-06-17 | 2014-08-06 | 哈洛齐梅公司 | 乙酰透明质酸降解酶的稳定制剂 |
| CA3080189C (en) | 2011-06-17 | 2023-01-17 | Hanmi Science Co., Ltd. | A conjugate comprising oxyntomodulin and an immunoglobulin fragment, and use thereof |
| JP6184404B2 (ja) | 2011-06-22 | 2017-08-23 | インディアナ ユニヴァーシティ リサーチ アンド テクノロジー コーポレイション | グルカゴン/glp−1レセプターコアゴニスト |
| HRP20170890T1 (hr) | 2011-06-22 | 2017-09-08 | Indiana University Research And Technology Corporation | Suagonisti glukagonskog/glp-1 receptora |
| CN103906528A (zh) | 2011-06-24 | 2014-07-02 | 安米林药品有限责任公司 | 用glp-1受体激动剂的缓释制剂治疗糖尿病的方法 |
| KR101357117B1 (ko) | 2011-06-28 | 2014-02-06 | 비앤엘델리팜 주식회사 | 폴리에틸렌글라이콜 또는 이의 유도체로 페길화된 엑센딘-4 유사체, 이의 제조방법 및 이를 유효성분으로 함유하는 당뇨병 예방 또는 치료용 약학적 조성물 |
| US9944687B2 (en) | 2011-07-04 | 2018-04-17 | Imperial Innovations Limited | Compounds and their effects on feeding behaviour |
| WO2013009545A1 (en) | 2011-07-08 | 2013-01-17 | Amylin Pharmaceuticals, Inc. | Engineered polypeptides having enhanced duration of action with reduced immunogenicity |
| KR101810055B1 (ko) | 2011-08-10 | 2017-12-18 | 아도시아 | 하나 이상의 유형의 기저 인슐린의 주사액 |
| JP6169079B2 (ja) | 2011-08-24 | 2017-07-26 | フェーズバイオ ファーマシューティカルズ,インコーポレイテッド | 徐放のための活性剤の製剤 |
| CN103189389B (zh) | 2011-09-03 | 2017-08-11 | 深圳市健元医药科技有限公司 | 新的glp‑ⅰ类似物及其制备方法和用途 |
| CA2849673A1 (en) | 2011-09-23 | 2013-03-28 | Novo Nordisk A/S | Novel glucagon analogues |
| KR101967941B1 (ko) | 2011-10-28 | 2019-04-10 | 사노피-아벤티스 도이칠란트 게엠베하 | 제2형 당뇨병 치료 프로토콜 |
| CN102363633B (zh) | 2011-11-16 | 2013-11-20 | 天津拓飞生物科技有限公司 | 胰高血糖素样肽-1突变体多肽及其制备方法、药物组合物和其应用 |
| WO2013074910A1 (en) | 2011-11-17 | 2013-05-23 | Indiana University Research And Technology Corporation | Glucagon superfamily peptides exhibiting glucocorticoid receptor activity |
| CA2852716C (en) | 2011-11-29 | 2015-11-17 | Jurox Pty Ltd | Methods of preserving injectable pharmaceutical compositions comprising a cyclodextrin and a hydrophobic drug |
| EP2791160B1 (en) | 2011-12-16 | 2022-03-02 | ModernaTX, Inc. | Modified mrna compositions |
| WO2013093720A2 (en) | 2011-12-22 | 2013-06-27 | Pfizer Inc. | Anti-diabetic compounds |
| AP2014007797A0 (en) | 2011-12-23 | 2014-07-31 | Boehringer Ingelheim Int | Glucagon analogues |
| WO2013101749A1 (en) | 2011-12-29 | 2013-07-04 | Latitude Pharmaceuticals, Inc. | Stabilized glucagon nanoemulsions |
| EP2814461B1 (fr) | 2012-01-09 | 2019-07-24 | Adocia | Solution injectable a ph 7 comprenant au moins une insuline basale dont le pi est compris entre 5,8 et 8,5 et un co-polyaminoacide substitue |
| EP2844269A4 (en) | 2012-03-28 | 2016-01-06 | Amylin Pharmaceuticals Llc | TRANSMUCOSAL ADMINISTRATION OF MANIPULATED POLYPEPTIDES |
| WO2013148871A1 (en) | 2012-03-28 | 2013-10-03 | Amylin Pharmaceuticals, Llc | Engineered polypeptides |
| CA2868393A1 (en) | 2012-04-02 | 2013-10-10 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of oncology-related proteins and peptides |
| HK1206612A1 (en) | 2012-04-02 | 2016-01-15 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of secreted proteins |
| CN102649947A (zh) | 2012-04-20 | 2012-08-29 | 无锡和邦生物科技有限公司 | 一种用于测定glp-1及其功能类似物生物活性的细胞株及其应用 |
| WO2013163162A1 (en) | 2012-04-24 | 2013-10-31 | Amylin Pharmaceuticals, Llc | Site-specific enzymatic modification of exendins and analogs thereof |
| US20130289241A1 (en) | 2012-04-26 | 2013-10-31 | Shanghai Ambiopharm, Inc. | Method for preparing exenatide |
| US8901484B2 (en) | 2012-04-27 | 2014-12-02 | Sanofi-Aventis Deutschland Gmbh | Quantification of impurities for release testing of peptide products |
| WO2013182217A1 (en) | 2012-04-27 | 2013-12-12 | Sanofi-Aventis Deutschland Gmbh | Quantification of impurities for release testing of peptide products |
| US9453064B2 (en) | 2012-05-03 | 2016-09-27 | Zealand Pharma A/S | Glucagon-like-peptide-2 (GLP-2) analogues |
| CA2872314C (en) | 2012-05-03 | 2021-08-31 | Zealand Pharma A/S | Gip-glp-1 dual agonist compounds and methods |
| EP2664374A1 (en) | 2012-05-15 | 2013-11-20 | F. Hoffmann-La Roche AG | Lysin-glutamic acid dipeptide derivatives |
| CN103421094A (zh) | 2012-05-24 | 2013-12-04 | 上海医药工业研究院 | 一种具有epo类似活性的多肽化合物 |
| US20150174209A1 (en) | 2012-05-25 | 2015-06-25 | Amylin Pharmaceuticals. Llc | Insulin-pramlintide compositions and methods for making and using them |
| AR091422A1 (es) | 2012-06-14 | 2015-02-04 | Sanofi Sa | Analogos peptidicos de la exendina 4 |
| AR091478A1 (es) | 2012-06-21 | 2015-02-04 | Univ Indiana Res & Tech Corp | Analogos de glucagon que exhiben actividad de receptor de gip (peptido insulinotropico dependiente de glucosa) |
| WO2013192129A1 (en) | 2012-06-21 | 2013-12-27 | Indiana University Research And Technology Corporation | Glucagon analogs exhibiting gip receptor activity |
| MX354163B (es) | 2012-07-12 | 2018-02-15 | Mannkind Corp | Sistemas y métodos de suministro de fármaco en polvo seco. |
| AR091866A1 (es) | 2012-07-23 | 2015-03-04 | Zealand Pharma As | Analogos del glucagon |
| AR094821A1 (es) | 2012-07-25 | 2015-09-02 | Hanmi Pharm Ind Co Ltd | Formulación líquida de un conjugado de péptido insulinotrópico de acción prolongada |
| KR101968344B1 (ko) | 2012-07-25 | 2019-04-12 | 한미약품 주식회사 | 옥신토모듈린 유도체를 포함하는 고지혈증 치료용 조성물 |
| AR092862A1 (es) | 2012-07-25 | 2015-05-06 | Hanmi Pharm Ind Co Ltd | Formulacion liquida de insulina de accion prolongada y un peptido insulinotropico y metodo de preparacion |
| US20150258198A1 (en) | 2012-08-14 | 2015-09-17 | Wockhardt Limited | Pharmaceutical microparticulate compositions of polypeptides |
| US20150224176A1 (en) | 2012-08-14 | 2015-08-13 | Wockhardt Limited | Pharmaceutical microparticulate compositions of polypeptides |
| CN102816244A (zh) | 2012-08-23 | 2012-12-12 | 无锡和邦生物科技有限公司 | 一种Exendin-4肽与人血清白蛋白HSA的融合蛋白及其制备方法 |
| CN102827270A (zh) | 2012-09-13 | 2012-12-19 | 无锡和邦生物科技有限公司 | 一种聚乙二醇化艾塞那肽衍生物及其用途 |
| US9546205B2 (en) | 2012-09-17 | 2017-01-17 | Imperial Innovations Limited | Peptide analogues of glucagon and GLP1 |
| TWI608013B (zh) | 2012-09-17 | 2017-12-11 | 西蘭製藥公司 | 升糖素類似物 |
| AR092873A1 (es) | 2012-09-26 | 2015-05-06 | Cadila Healthcare Ltd | Peptidos como agonistas triples de los receptores de gip, glp-1 y glugagon |
| UA116217C2 (uk) | 2012-10-09 | 2018-02-26 | Санофі | Пептидна сполука як подвійний агоніст рецепторів glp1-1 та глюкагону |
| KR101993393B1 (ko) | 2012-11-06 | 2019-10-01 | 한미약품 주식회사 | 옥신토모듈린 유도체를 포함하는 당뇨병 또는 비만성 당뇨병 치료용 조성물 |
| TWI816739B (zh) | 2012-11-06 | 2023-10-01 | 南韓商韓美藥品股份有限公司 | 包含調酸素及免疫球蛋白片段之蛋白質接合物的液態製劑 |
| JP6525456B2 (ja) | 2012-11-20 | 2019-06-05 | メデリス ダイアビーティーズ,エルエルシー | インスリン抵抗性のための改善されたペプチド製剤 |
| TWI674270B (zh) | 2012-12-11 | 2019-10-11 | 英商梅迪繆思有限公司 | 用於治療肥胖之升糖素與glp-1共促效劑 |
| HK1211231A1 (en) | 2012-12-21 | 2016-05-20 | Sanofi | Exendin-4 derivatives |
| CN103908657A (zh) | 2012-12-31 | 2014-07-09 | 复旦大学附属华山医院 | 胰升糖素样肽-1类似物在制备眼科疾病药物中的用途 |
| JP2016512213A (ja) | 2013-03-14 | 2016-04-25 | メディミューン リミテッド | 肥満の治療のためのペグ化グルカゴン及びglp−1コアゴニスト |
| WO2014158900A1 (en) | 2013-03-14 | 2014-10-02 | Indiana University Research And Technology Corporation | Insulin-incretin conjugates |
| WO2014152460A2 (en) | 2013-03-15 | 2014-09-25 | Indiana University Research And Technology Corporation | Prodrugs with prolonged action |
| MY174727A (en) | 2013-04-18 | 2020-05-11 | Novo Nordisk As | Stable, protracted glp-1/glucagon receptor co-agonists for medical use |
| JP2014227368A (ja) | 2013-05-21 | 2014-12-08 | 国立大学法人帯広畜産大学 | 糖尿病および高血糖状態の処置のためのグルカゴンアナログ |
| CN103304660B (zh) | 2013-07-12 | 2016-08-10 | 上海昂博生物技术有限公司 | 一种利拉鲁肽的合成方法 |
| CN103405753B (zh) | 2013-08-13 | 2016-05-11 | 上海仁会生物制药股份有限公司 | 稳定的促胰岛素分泌肽水针药物组合物 |
| CN119119233A (zh) | 2013-10-17 | 2024-12-13 | 西兰制药公司 | 酰化胰高血糖素类似物 |
| US9988429B2 (en) | 2013-10-17 | 2018-06-05 | Zealand Pharma A/S | Glucagon analogues |
| WO2015067716A1 (en) | 2013-11-06 | 2015-05-14 | Zealand Pharma A/S | Glucagon-glp-1-gip triple agonist compounds |
| TW201609797A (zh) | 2013-12-13 | 2016-03-16 | 賽諾菲公司 | 雙重glp-1/升糖素受體促效劑 |
| WO2015086730A1 (en) | 2013-12-13 | 2015-06-18 | Sanofi | Non-acylated exendin-4 peptide analogues |
| TW201609795A (zh) | 2013-12-13 | 2016-03-16 | 賽諾菲公司 | 作為雙重glp-1/gip受體促效劑的艾塞那肽-4(exendin-4)胜肽類似物 |
| EP3080151A1 (en) | 2013-12-13 | 2016-10-19 | Sanofi | Exendin-4 peptide analogues |
| EP3080155A1 (en) | 2013-12-13 | 2016-10-19 | Sanofi | Exendin-4 peptide analogues as dual glp-1/glucagon receptor agonists |
| EP3080154B1 (en) | 2013-12-13 | 2018-02-07 | Sanofi | Dual glp-1/gip receptor agonists |
| CN103665148B (zh) | 2013-12-17 | 2016-05-11 | 中国药科大学 | 一种可口服给药的降糖多肽及其制法和用途 |
| CN103980358B (zh) | 2014-01-03 | 2016-08-31 | 杭州阿诺生物医药科技股份有限公司 | 一种制备利拉鲁肽的方法 |
| WO2015104311A1 (en) | 2014-01-09 | 2015-07-16 | Sanofi | Stabilized glycerol free pharmaceutical formulations of insulin analogues and/or insulin derivatives |
| US9839692B2 (en) | 2014-01-09 | 2017-12-12 | Sanofi | Stabilized pharmaceutical formulations of insulin analogues and/or insulin derivatives |
| GB201404002D0 (en) | 2014-03-06 | 2014-04-23 | Imp Innovations Ltd | Novel compounds |
| TW201625668A (zh) | 2014-04-07 | 2016-07-16 | 賽諾菲公司 | 作為胜肽性雙重glp-1/昇糖素受體激動劑之艾塞那肽-4衍生物 |
| TW201625669A (zh) | 2014-04-07 | 2016-07-16 | 賽諾菲公司 | 衍生自艾塞那肽-4(Exendin-4)之肽類雙重GLP-1/升糖素受體促效劑 |
| TW201625670A (zh) | 2014-04-07 | 2016-07-16 | 賽諾菲公司 | 衍生自exendin-4之雙重glp-1/升糖素受體促效劑 |
| US9932381B2 (en) | 2014-06-18 | 2018-04-03 | Sanofi | Exendin-4 derivatives as selective glucagon receptor agonists |
| CN104926934B (zh) | 2014-09-23 | 2016-11-09 | 蒋先兴 | 胃泌酸调节素类似物 |
| EP3204408B1 (en) | 2014-10-10 | 2020-05-06 | Novo Nordisk A/S | Stable glp-1 based glp-1/glucagon receptor co-agonists |
| TW201625672A (zh) | 2014-10-24 | 2016-07-16 | 默沙東藥廠 | 升糖素及glp-1受體之共促效劑 |
| WO2016198604A1 (en) | 2015-06-12 | 2016-12-15 | Sanofi | Exendin-4 derivatives as dual glp-1 /glucagon receptor agonists |
| WO2016198624A1 (en) | 2015-06-12 | 2016-12-15 | Sanofi | Exendin-4 derivatives as trigonal glp-1/glucagon/gip receptor agonists |
-
2013
- 2013-08-10 UA UAA201504488A patent/UA116217C2/uk unknown
- 2013-10-07 AR ARP130103627A patent/AR092925A1/es unknown
- 2013-10-08 EP EP13773767.2A patent/EP2906595B1/en active Active
- 2013-10-08 MY MYPI2015000584A patent/MY168749A/en unknown
- 2013-10-08 EA EA201590715A patent/EA030023B1/ru not_active IP Right Cessation
- 2013-10-08 NZ NZ706898A patent/NZ706898A/en not_active IP Right Cessation
- 2013-10-08 JP JP2015535054A patent/JP6373270B2/ja active Active
- 2013-10-08 KR KR1020157010088A patent/KR102179751B1/ko active Active
- 2013-10-08 CA CA2887272A patent/CA2887272C/en active Active
- 2013-10-08 BR BR112015007685A patent/BR112015007685A2/pt not_active IP Right Cessation
- 2013-10-08 DK DK13773767.2T patent/DK2906595T3/da active
- 2013-10-08 ES ES13773767.2T patent/ES2647418T3/es active Active
- 2013-10-08 PE PE2015000458A patent/PE20150900A1/es unknown
- 2013-10-08 HR HRP20171726TT patent/HRP20171726T1/hr unknown
- 2013-10-08 RS RS20171151A patent/RS56515B1/sr unknown
- 2013-10-08 PL PL13773767T patent/PL2906595T3/pl unknown
- 2013-10-08 SG SG11201501770WA patent/SG11201501770WA/en unknown
- 2013-10-08 LT LTEP13773767.2T patent/LT2906595T/lt unknown
- 2013-10-08 AU AU2013328802A patent/AU2013328802B2/en not_active Ceased
- 2013-10-08 SI SI201330838T patent/SI2906595T1/sl unknown
- 2013-10-08 NO NO13773767A patent/NO2906595T3/no unknown
- 2013-10-08 HU HUE13773767A patent/HUE037150T2/hu unknown
- 2013-10-08 WO PCT/EP2013/070882 patent/WO2014056872A1/en active Application Filing
- 2013-10-08 TW TW102136267A patent/TWI613213B/zh not_active IP Right Cessation
- 2013-10-08 CN CN201380064117.XA patent/CN104837864B/zh active Active
- 2013-10-08 PT PT137737672T patent/PT2906595T/pt unknown
- 2013-10-08 MX MX2015004531A patent/MX359533B/es active IP Right Grant
- 2013-10-09 UY UY35072A patent/UY35072A/es not_active Application Discontinuation
- 2013-10-09 US US14/049,597 patent/US9365632B2/en active Active
-
2015
- 2015-03-09 IL IL237641A patent/IL237641B/en active IP Right Grant
- 2015-03-11 ZA ZA2015/01694A patent/ZA201501694B/en unknown
- 2015-03-17 TN TNP2015000101A patent/TN2015000101A1/fr unknown
- 2015-03-24 DO DO2015000073A patent/DOP2015000073A/es unknown
- 2015-03-26 PH PH12015500688A patent/PH12015500688B1/en unknown
- 2015-03-27 GT GT201500081A patent/GT201500081A/es unknown
- 2015-03-31 CL CL2015000811A patent/CL2015000811A1/es unknown
- 2015-04-17 CR CR20150200A patent/CR20150200A/es unknown
-
2016
- 2016-04-15 US US15/130,647 patent/US20160220643A1/en not_active Abandoned
- 2016-08-24 CL CL2016002137A patent/CL2016002137A1/es unknown
-
2017
- 2017-11-14 CY CY20171101194T patent/CY1119987T1/el unknown
- 2017-12-11 US US15/837,958 patent/US10758592B2/en active Active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8268781B2 (en) * | 2004-09-03 | 2012-09-18 | Philipps-Universitat Marburg | Peptide derivatives of exendin-4 |
| US20120178670A1 (en) * | 2009-07-13 | 2012-07-12 | Zealand Pharma A/S | Acylated glucagon analogues |
| WO2011016030A1 (en) * | 2009-08-03 | 2011-02-10 | Technion Research & Development Foundation Ltd. | Hydrogen production by an autothermal heat exchanger packed-bed membrane gas reformer |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20200037358A (ko) * | 2017-08-09 | 2020-04-08 | 사노피 | 지방간 질환 및 지방간염의 치료에서의 glp-1/글루카곤 수용체 작용제 |
| KR20200069225A (ko) | 2018-12-06 | 2020-06-16 | 경상대학교산학협력단 | 지속형 엑센딘-4 및 이의 용도 |
| US12157761B2 (en) | 2018-12-06 | 2024-12-03 | Industry-Academic Cooperation Foundation Gyeongsang National University | Long-acting exendin-4 and use thereof |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102179751B1 (ko) | 이중 glp1/글루카곤 작용제로서의 엑센딘-4 유도체 | |
| US10253079B2 (en) | Functionalized Exendin-4 derivatives | |
| AU2015243611B2 (en) | Dual GLP-1 / glucagon receptor agonists derived from exendin-4 | |
| AU2015243610B2 (en) | Exendin-4 derivatives as peptidic dual GLP-1 / glucagon receptor agonists | |
| KR20150023690A (ko) | 엑센딘-4 펩타이드 유사체 | |
| HK1209766B (en) | Exendin-4 derivatives as dual glp1/glucagon agonists | |
| OA17436A (en) | Functionalized exendin-4 derivatives. | |
| OA17287A (en) | New indanyloxydihydrobenzofuranylacetic acids |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20150420 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20181005 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20200207 Patent event code: PE09021S01D |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
Patent event code: PE07011S01D Comment text: Decision to Grant Registration Patent event date: 20200902 |
|
| GRNT | Written decision to grant | ||
| PR0701 | Registration of establishment |
Comment text: Registration of Establishment Patent event date: 20201111 Patent event code: PR07011E01D |
|
| PR1002 | Payment of registration fee |
Payment date: 20201112 End annual number: 3 Start annual number: 1 |
|
| PG1601 | Publication of registration | ||
| PR1001 | Payment of annual fee |
Payment date: 20240927 Start annual number: 5 End annual number: 5 |