Abstract
Objective
To evaluate the expression and correlations of liver X receptor alpha (LXRa) and its target gene sterol regulatory element-binding protein-1c (SREBP-1c) in placentas of preeclampsia (PE) and their significance in PE.
Methods
Pregnancies were divided into two groups, 60 cases (29 cases of mild and 31 cases of severe) of PE group and 56 cases of normal group. The level of mRNA and protein of LXRa and SREBP-1c were analyzed by reverse transcription-polymerase chain reaction (RTPCR) and immunohistochemistry (IHC) in the placentas.
Results
RT-PCR and IHC results showed that the mRNA and protein expression of both LXRa and SREBP-1c increased gradually with the extent of PE among normal pregnancy, mild PE and severe PE groups, and the differences were of statistically significance (P<0.01 or P<0.05). There were positive correlations between the expression of LXRa mRNA and SREBP-1c mRNA, also between LXRa mRNA and LXRa protein (r=0.521, P<0.01; r=0.422, P<0.01). The expression of SREBP-1c mRNA positively correlated with its protein level (r=0.598, P<0.01). There were positive correlations between the expression of LXRa protein and SREBP-1c protein (r=0.612, P<0.01).
Conclusion
The expression of LXRa is elevated significantly in placentas of PE patients, and might contribute for promoting the transcription and translation of its target gene SREBP1-c, which is related to the occurrence and development of PE.
1 Introduction
The incidences of preeclampsia (PE) among primipara and multipara are 3%-7% and 1%-3%, respectively [1]. Its pathophysiologic alterations involve lesions of multiple organs and systems, and PE represents as one of the leading causes of maternal mortality.
Liver X receptor alpha (LXRa) is a ligand-activated transcription factor that belongs to the nuclear receptor superfamily. Studies have shown that LXRa is closely related with lipid metabolism, injury of vascular endothelial, proliferation and invasion of trophoblasts. As the key receptor for maintaining homeostasis of total cholesterol (TC) and triglyceride (TG), LXRa acts through transcription regulation of key target genes involved in lipid absorption, transport, synthesis, metabolism and excretion [3]. It is already known that LXRa is expressed in placentas, and compared with normal pregnancies, it is significantly up-regulated in placentas of PE patients, which is positively correlated with the extent of hypoxia [4, 5]. Its abnormal expression may be important for PE progression.
Sterol regulatory element-binding protein-1c (SREBP-1c) belongs to the nuclear transcription factor family that was initially identified from the nuclear extracts of cervical cancer HeLa cells. The mammal genes encoding SREBP include SREBP-1a, -1c and SREBP-2. SREBP-1c and SREBP-2 are the major isoforms expressed in animal tissues, in which SREBP-1c constitutes up to 90% of SREBP-1 in the body. SREBP-1c could activate transcription of multiple genes encoding for enzymes involved in synthesis of TC, TG, fatty acid (FA) and phospholipid, such as 3-hydroxy-3-methylglutaryl-CoA synthase 1 (HMGCS1), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) and squalene epoxidase (SE) that are related with TC synthesis, acetyl-CoA carboxylase alpha (ACC), fatty acid synthase (FAS) and stearoyl-CoA desaturase that are related with FA synthesis. HMGCR is the rate-limiting enzyme for lipid synthesis while ACC is the rate-limiting enzyme for fatty acid synthesis. Thus SREBP -1c plays an irreplaceable role in the process of fat synthesis. Vasarhelyi B et al. had proved that SREBP-1c is a key adjustment factor to keep dynamic balance of fatty acid in placenta through investigating differential gene expression in placentas of PE pregnancies by means of gene chip technology [6]. Besides, SREBP-1c, acyl-CoA synthetase long-chain family member 3 (ACSL3), FAS are all targets of LXRa and are all closely related with lipid metabolism. In particular, SREBP-1c is the chief transcription factor that regulates the transcription of genes involved in fat synthesis, which could be up-regulated by LXRa.
In our study, we applied RT-PCR and IHC methods to investigate the expression of LXRa and its target gene SREBP-1c and their correlations, to explore the clinical value of these two molecules in the pathology of PE. Our results would help to understand more about PE placental pathologic changes and its pathogenesis mechanisms.
2 Materials and Methods
2.1 Patients
Samples were obtained from ll6 cases of pregnancies undergoing cesarean section at our hospital from 2011 Oct to 2012 Aug, including 60 cases of PE pregnancy group (29 mild cases and 31 severe cases) and 56 cases of normal pregnancy group. No women with chronic hypertension, diabetes, liver or kidney diseases or other diseases that influence lipid metabolism were included. The body mass indexes (BMI) of these candidates are all in the normal range. No other complications occured during their pregnancies. All subjects had cesarian deliveries. The diagnostic standard of PE was according to the 7th edition of the “Obstetrics and Gynecology” [2].
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
Informed consent: Informed consent has been obtained from all individuals included in this study.
2.2 Methods
2.2.1 Tissue samples
All tissue samples were obtained during cesarean section, and two pieces of placental tissues, of approximately 1cm3, were immediately taken off the maternal side. Omit the infarcted, organized and calcificated regions. One copy of placenta tissues were fixed in 10% neutral paraform aldehyde for 24hrs after repeatedly rinsed by physiological saline, followed by conventional paraffin embedding and serial section at a thickness of 2.5μm. The other copy of placenta tissues were put into the RNA enzyme-inactivated cryopreserved tubes and snap-frozen in liquid nitrogen within 5 minutes ready for RNA extraction.
2.2.2 RT-PCR method for detecting the mRNA expression level of LXRa and SREBP-1c in placentas
(1) Total RNA of placentas was isolated as per the instructions of the Trizol kit (ordered from the American Invitrogen company). (2) cDNA was synthesized according to the reverse transcription kit (ordered from the Fermentas company). (3) PCR amplification procedures: All set up to 20 μl reaction system; PCR reaction conditions for LXRa, SREBP-1c and the internal reference gene β-actin were all as follows: 94oC 5min; 36 cycles of 94oC 30sec, 58oC 30sec, 72oC 4min; 72oC 7min. Primers were all designed and synthesized by Shanghai Sangon biological engineering technology service co., LTD. The sequences of forward (F) and reverse (R) primers for LXRa, SREBP-1c and β-actin were as follows respectively: L LXRa-F: 5’-GCGAGGGCTGCAAGGGATTCT-3’, LXRa-R: 5’-ATGGGCCAAGGCGTGACGCG-3’, with the product length of 376 bp; SREBP-1c-F: 5’-GCCTATTTAACCCACCCTATG-3’, SREBP-1c-R: 5’-TGGCACTGACTCTTCCTTGAT-3’, with the product length of 251bp; β-actin-F: 5’-CTGGGACGACATGGAGAAAA-3’, β-actin-R: 5’-AAGGAAGGCTGGAAGAGTGC-3’, with the product length of 564bp. (4) The above RT-PCR products were further run for gel electrophoresis, imaging and quantification by the Quantity One software to determine the ratios (RIs) of absorbance value of purpose stripes with that of the internal reference gene β-actin, which were considered as the relative gene expression levels in each sample (experiments were repeated 3 times for each gene).
2.2.3 IHC technology for detecting the protein expression of LXRa and SREBP-1c in placentas
Experiments were performed by using the 2nd general 2-step detection system (non-biotin, PV-9000 purchased from Beijing Zhongshan Golden Bridge Company) according to the instructions of the manufacturer. The concentrations of working liquid of rabbit anti-human polyclonal antibody LXRa and SREBP-1c were 1:100, 1:200 diluted in PBS, respectively. PBS was used instead of primary antibody in the negative control group. Yellow and/or brownish-yellow granules appeared within cells or matrix was considered as positive cells. Ten 400× high power fields were randomly shot for each section. Integral optical density (IOD) was calculated by Image-Pro plus 6.0 software for each images, and the mean value of IOD was used as the relative expression value of the indicated proteins in placentas (experiments were repeated 3 times for each protein).
2.3 Statistical analysis
Statistical analysis was performed with SPSS version 17.0. All data were expressed as ± s. The comparison between measurement data sets was done by t-test. Bivariate correlation analysis was performed by using Pearman linear correlation analysis.
3 Results
3.1 Clinical characters
Among the objects of our study, there were no significant differences in age and BMI between the PE groups and the normal group (P>0.05). However, there were significant statistic differences in gestational age, systolic pressure, diastolic pressure, and birth weight between the indicated two groups (P<0.01 or P<0.05) (Table 1).
Clinical characteristics of the control and PE patient groups
| Group | n | Age/year | gestational age/week | BMI/(Kg/m2) | systolic pressure/mmHg | diastolic pressure/mmHg | birth weight/Kg |
|---|---|---|---|---|---|---|---|
Normal | 56 | 28.83±4.65 | 39.61±1.10 | 29.66±3.61 | 113.23±9.87 | 72.77±7.21 | 3.31±0.28 |
| PE | 60 | 28.72±4.86 | 37.62±1.38[b][**] | 30.84±4.65 | 155.24±9.88[b][**] | 98.24±7.81[b][**] | 2.75±0.39[b][**] |
| Mild PE | 29 | 29.13±4.12 | 38.12±0.91[b][**] | 30.61±3.91 | 145.21±7.98[b][**] | 94.68±6.69[b][**] | 2.98±0.30 |
| Severe PE | 31 | 28.22±4.11 | 37.14±1.20[a][**] | 30.96±4.23 | 165.23±8.91[a][**] | 102.33±6.12[a][**] | 2.61±0.29[a][**] |
Note:
P values
3.2 Expression of LXRa and SREBP-1c in placenta tissues of pregnancies
3.2.1
As shown by RT-PCR, the mRNA levels of LXRa and SREBP-1c were gradually upregulated along the normal pregnancy group, mild PE and severe PE groups (Figure 1-2). The differences were of statistical significance (P<0.01 or P<0.05) (Table 2).
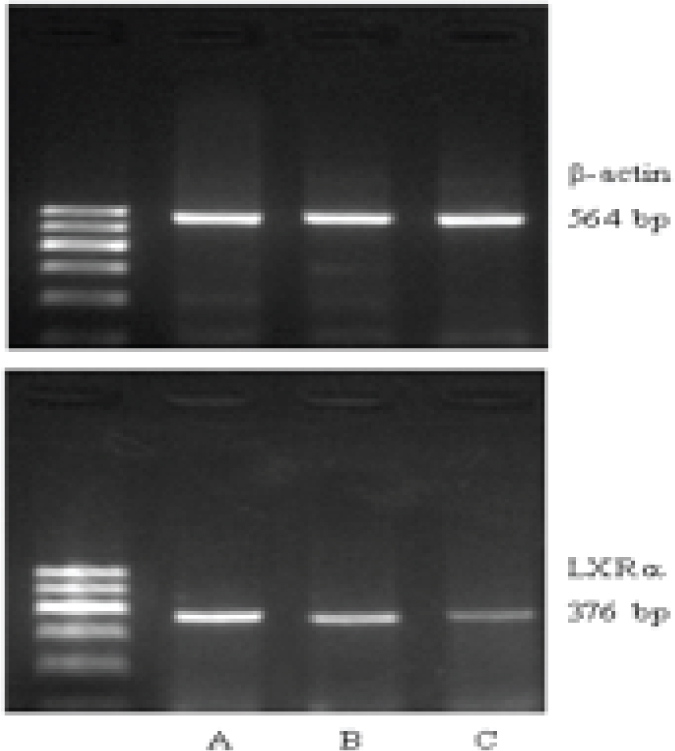
LXRa mRNA expression in placentas of each group (A: severe PE, B: mild PE, C: normal)
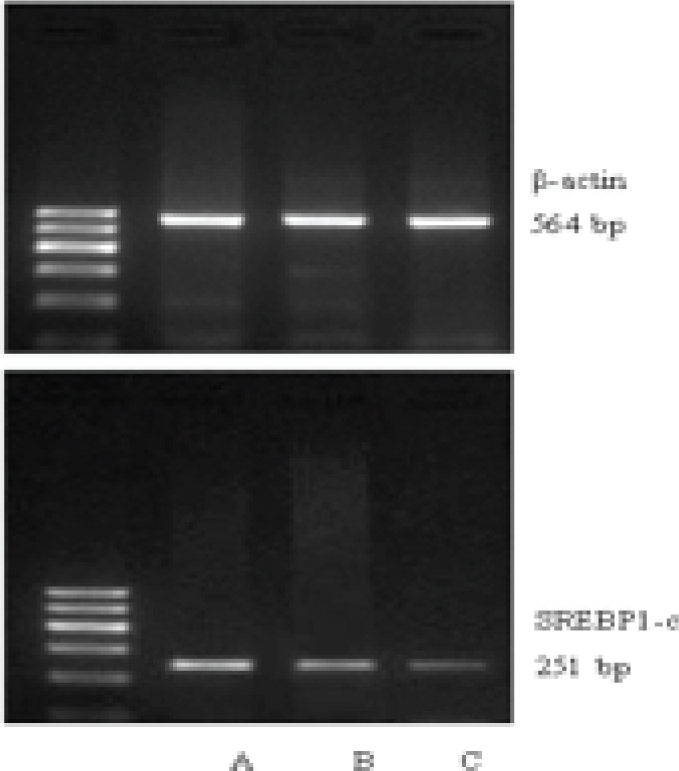
SREBP-1c mRNA expression in placentas of each group (A: severe PE, B: mild PE, C: normal)
The expression levels of LXRa and SREBP-1c in placentas of each group
| Group | n | LXRa mRNA | SREBP-1c mRNA | LXRa protein | SREBP-1c protein |
|---|---|---|---|---|---|
| Normal | 56 | 0.33±0.12 | 0.36±0.17 | 65887.56±43863.11 | 51667.78±15874.12 |
| PE | 60 | 0.55±0.21[b][**] | 0.56±0.19[b][**] | 96874.18±30124.16[b][**] | 71845.64±14667.12[b][**] |
| Mild PE | 29 | 0.48±0.14[b][*] | 0.49±0.15[b][*] | 87142.11±12846.11[b][**] | 65892.15±10651.41[b][*] |
| Severe PE | 31 | 0.62±0.16[a][**] | 0.60±0.18[a][*] | 106871.12±20121.11[a][*] | 76741.229±12684.11[b][*] |
Note:
P values
3.2.2
The results from the IHC staining showed that LXRa and SREBP-1c were mainly located in cell membrane and cytoplasm of cytotrophoblasts, syncytiotrophoblasts and vascular endothelial cells. They were hardly observed in cell membrane or cytoplasm of villus stroma core and the decidual cells. Yellow or brown staining indicated positive expression. The protein levels of LXRa and SREBP-1c emerged gradually increased expression along the severity of PE (Figure 3). Also, LXRa and SREBP-1c protein levels were gradually upregulated along the normal pregnancy group, mild PE and severe PE groups. The differences were of statistical significance (P<0.01 or P<0.05) (Table 2).
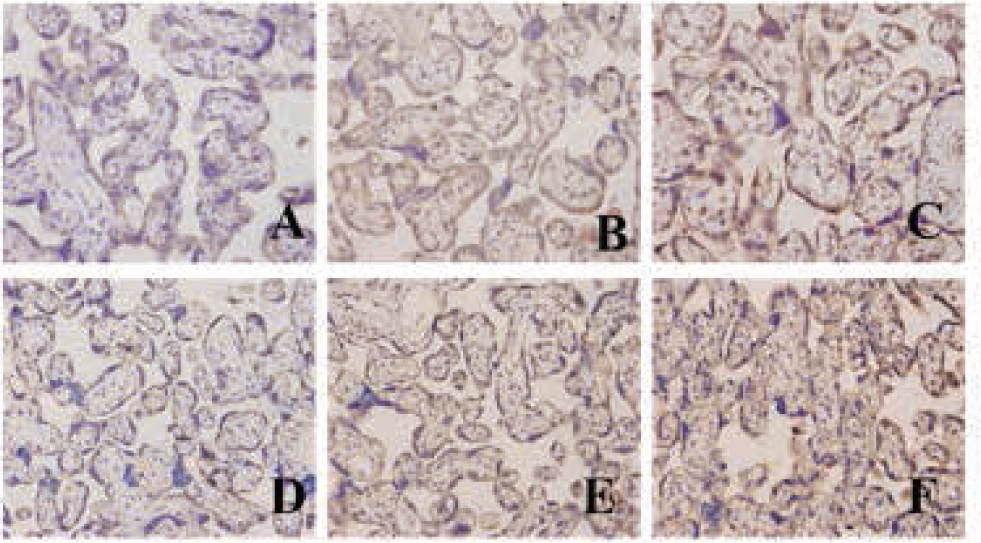
The protein of LXRa and SREBP-1c expression in different tissue (A: LXRa protein expression in placenta of a normal pregnancy; B: LXRa protein expression in a mild PE placenta; C: LXRa protein expression in a severe PE placenta; D: SREBP-1c protein expression in placenta of a normal pregnancy; E: SREBP-1c protein expression in a mild PE placenta; F: REBP-1c protein expression in a severe PE placenta)
3.2.3
After that, the correlation of LXRa and SREBP-1c expression was calculated: LXRa mRNA expression positively correlated with SREBP-1c mRNA and LXRa protein (r=0.521, P<0.01; r=0.422, P<0.01). The expression of SREBP-1c mRNA positively correlated with its protein level (r=0.598, P<0.01). There were positive correlations between the expression of LXRa protein and SREBP-1c protein (r=0.612, P<0.01) (Table 3).
4 Discussion
It is well known that SREBP-1c is a target of LXRa. The promoter of SREBP-1c gene contains the LXR reaction element (LXRE). LXRa orms obligate heterodimers with the nuclear receptors retinoid X receptors (RXRs) and thereafter binds to the LXRE in the upstream promoter of SREBP-1c gene, thus controling the transcription of SREBP-1c, and further regulate expression of the downstream genes involved in de novo fatty acid metabolism, TG synthesis and cholesterol homeostasis. It was shown that ligands or agonists of LXR could elevate the protein levels of LXR and SREBP-1, meanwhile upregulating the mRNA expression of ACC and FAS. As an agonist of LXRa, T0901317 could enhance the promoter activity of SREBP-1c by three times and upregulate its expression, thus to induce significantly increased serum levels of TC, TG, low density lipoprotein cholesterol (LDL-C) and very low density lipoprotein cholesterol (VLDL-C) [7-9]. Schultz JR et al. reported that treating mice with oralagonists of LXRa led to increased levels of serum TC, TG and VLDL. The mRNA expression of hepatic SREBP-1 and SCD-1 showed dose-dependent upregulation, twofold for ACC, fourfold for FAS and nonuple for SCD-l in detail. However, in LXRa knockout mice, the transcription or expression of these above genes was decreased. Antagonists of LXR such as PP2P and 23f could partially antagonize raised TG by LXRa [11].
In the present study, we found that mRNA and protein expression of both LXRa and SREBP-1c significantly increased gradually with the extent of PE among normal pregnancy, mild PE and severe PE groups, indicating that up-regulated LXRa could contribute for promoting the transcription and translation of the target gene, SREBP-1c, which further influences the synthesis of enzymes involved in lipid metabolism. However, we were still not confident about the direct regulation of SREBP-1c by LXRa. Although it is well characterized in other systems, we did not provide more evidence about their direct interaction. Hence, we just said that these two molecules were correlated with each other, at least reflected by their similar expression pattern in PE. Future mechanical studies are on the way to investigate the regulating mechanisms. We believe that better understanding the LXRa/SREBP-1c axis in PE would benefit the overcoming of abnormal lipid deposition within vascular endothelial cells and cytotrophoblast. This impairs recasting of the uterine spiral artery, causing shallow implantation of the trophocyte in placenta, eventually induces ischemic anoxia of uterus and placenta, finally leads to the higher incidence of the maternal complications and perinatal mortality.
Taken together, LXRa and SREBP-1c in placentas tissues might mediate the lipid metabolism disorder of the pregnancies, and play a regulatory role during PE. Future study on inhibitors or antagonists of LXRa/SREBP-1c and effective controlling their expression in placentas might provide novel clues for preventing and treating PE.
Conflict of interest statement
Authors state no conflict of interest
References
[1] Uzan J, Carbonnel M, Piconne O, et al. Pre-eclampsia: pathophysiology, diagnosis, and management [J]. Vasc Health Risk Manag, 2011, 7:467-47410.2147/VHRM.S20181Search in Google Scholar PubMed PubMed Central
[2] Yasuda T, Grillot D, Billheimer JT, et al. Tissue-specific liver X receptor activation promotes macrophage reverse cholesterol transport in vivo [J]. Arterioscler Thromb Vasc Biol, 2010, 30(4):781-78610.1161/ATVBAHA.109.195693Search in Google Scholar PubMed PubMed Central
[3] Plosch T, Gellhaus A, van Straten EM, et al. The liver X receptor (LXR) and its target gene ABCA1 are regulated upon low oxygen in human trophoblast cells: a reason for alterations in preeclampsia? [J]. Placenta, 2010, 31(10):910-91810.1016/j.placenta.2010.07.009Search in Google Scholar PubMed
[4] Wang J, Ding LF, Shang LX, et al. Study on changes of liver X receptor a in maternal serum and placenta in hypertensive disorder complicating pregnancy. [J]. Chinese Journal of Practical Gynecology and Obstetrics, 2011, 27(2):148-150Search in Google Scholar
[5] Vasarhelyi B, Cseh A, Kocsis I, et al. Three mechanisms in the pathogenesis of pre-eclampsia suggested by over-represented transcription factor- binding sites detected with comparative promoter analysis [J]. Mol Hum Repord, 2006, 12(1): 31-3410.1093/molehr/gal001Search in Google Scholar PubMed
[6] Le J. Gynecology and Obstetrics [M]. The seventh edition. Beijing people’s medical publishing house, 2008:94Search in Google Scholar
[7] Xu L, Bai Q, Rodriguez-Agudo D, et al. Regulation of hepatocyte lipid metabolism and inflammatory response by 25-hydroxycholesterol and 25-hydroxycholesterol -3-sulfate [J]. Lipids, 2010, 45(9:821-83210.1007/s11745-010-3451-ySearch in Google Scholar PubMed
[8] Okazaki H, Goldstein JL, Brown MS, et al. LXR-SREBP-1c-phospholipid transfer protein axis controls very low density lipoprotein (VLDL) particle size [J]. J Biol Chem, 2010, 285(9):6801- 681010.1074/jbc.M109.079459Search in Google Scholar PubMed PubMed Central
[9] Hong C, Walczak R, Dhamko H, et al. Constitutive activation of LXR in macrophag- es regulates metabolic and inflammatory gene expression: identification of ARL7 as a direct target [J]. 2011, 52(3):531-53910.1194/jlr.M010686Search in Google Scholar PubMed PubMed Central
[10] Schultz JR, Tu H, Luk A, Repa JJ, et al. Role of LXRs in control of lipogenesis [J]. Genes Dev, 2000, 14(22):2831-238810.1101/gad.850400Search in Google Scholar PubMed PubMed Central
[11] Motoshima K, Noguchi-Yachide T, Sugita K, et al. Separation of alpha-glucosidase- inhibitory and liver X receptor-antagonistic activities of phenethylphenylphthalimide analogs and generation of LXRalpha-selective antagonists [J]. Bioorg Med Chem, 2009, 17(14):5001-501410.1016/j.bmc.2009.05.066Search in Google Scholar PubMed
© 2016 Li Jianhua et al.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
