Abstract
Tuberculosis is still the most frequent granulomatous laryngeal disease. Absence of pathognomonic symptoms and change in clinical pattern frequently leads to misdiagnosis and delayed treatment. Hoarseness is the commonest symptom of laryngeal tuberculosis and constitutional symptoms are usually rare. However dysphonia can be caused by many other more common conditions. Hoarseness can be a symptom of organic (nodules and polyps of vocal folds, tumors, vocal fold paresis) or functional (functional dysphonia, laryngeal conversion disorder, paradoxical vocal folds motion) conditions. Rarely systemic diseases as amyloidosis, sarcoidosis, Wegener’s granulomatosis or tuberculosis can cause vocal dysfunction too. That is why laryngeal tuberculosis is often forgotten in case of persistent hoarseness. In this article, we present a case of a young previously healthy woman, complaining of persistent hoarseness with no other leading symptoms. Though endoscopic image suggested a malignancy, histology showed granulomatous lesion. Detailed examination revealed laryngeal and pulmonary tuberculosis resistant to rifampicin. Conclusion: Dysphonia can be the only one symptom of laryngeal tuberculosis. The disease should be taken into consideration when a patient complains of persistent hoarseness in order to avoid delays in treatment and spread of infection.
1 Introduction
Tuberculosis is an infectious disease characterized by the formation of granulomas in the infected tissues. It is still one of the most contagious diseases worldwide and is ranked as the second leading cause of death from an infection, secondary to human immunodeficiency virus (HIV) [1, 2]. A major decline in new tuberculosis cases and deaths was seen globally over the past two decades – the mortality rate decreased by 41% since 1990 [2]. Despite this, the extent of tuberculosis remains significant: according to the World Health Organization, there were 8, 7 million new cases of tuberculosis in 2011. What is more, 3, 7% of newly infected and 20% of previously treated patients are estimated to have multidrug resistant tuberculosis [2]. As the incidence depends heavily on the living conditions, an alarming statistic is observed in many developing countries in Central and Eastern Europe and even more in poor Asia and Africa countries [2, 3].
Tuberculosis usually affects the lungs; nevertheless, other organs can be affected too. The disease is spread in the air, however, via the blood, it can give rise to the different clinical manifestations of extra pulmonary tuberculosis. Laryngeal tuberculosis is estimated to occur in less than 1% of tuberculosis cases, yet still remains one of the most frequent granulomatous diseases of the larynx [1]. Hoarseness is the most common symptom of laryngeal tuberculosis; however, tuberculosis itself is a very rare cause of this voice disorder.
Dysphonia or hoarseness is a disorder, characterized by altered vocal quality, pitch, loudness or vocal effort and is one of the most frequent and earliest laryngological symptoms of many different laryngeal and extralaryngeal diseases [4]. Clark A. Rosen classified dysphonia into four different categories: functional voice disorders, organic voice disorders, movement disorders, and hoarseness caused by systemic diseases [5]. Numerous different infectious and non infectious diseases comprise the fourth group of causes: reflux laryngitis, Parkinson’s disease, Wegener’s granulomatosis, amyloidosis, sarcoidosis as well as tuberculosis fall into this group [5]. The diagnosis of laryngeal tuberculosis is difficult for it has no pathognomonic symptoms or physical findings [6]. In the case we present, dysphonia was the only one symptom of rifampicin resistant tuberculosis; moreover, it was diagnosed and properly treated in time. We would like to alert physicians attention to the increasing rate of this rare and serious laryngeal infection, accentuate its behavioral changes and diagnostic difficulties.
2 Case report
A 30-year-old, earlier completely healthy woman addressed to laryngologist complaining of persistent dysphonia lasting for about 12 months. Vocal function tended to worsen in the evening, no provocative or relieving factors as well as no systemic symptoms were mentioned. Prior medical history was unremarkable. Stroboscopy of the vocal cords was performed showing granuloma on the right vocal cord, causing decreased movement and diminished mucous wave in the 2nd and 3rd third of the right vocal cord (Fig. 1). The patient was diagnosed with chronic hyperplastic laryngitis and recommended to undergo surgical treatment with histological evaluation of the mass.
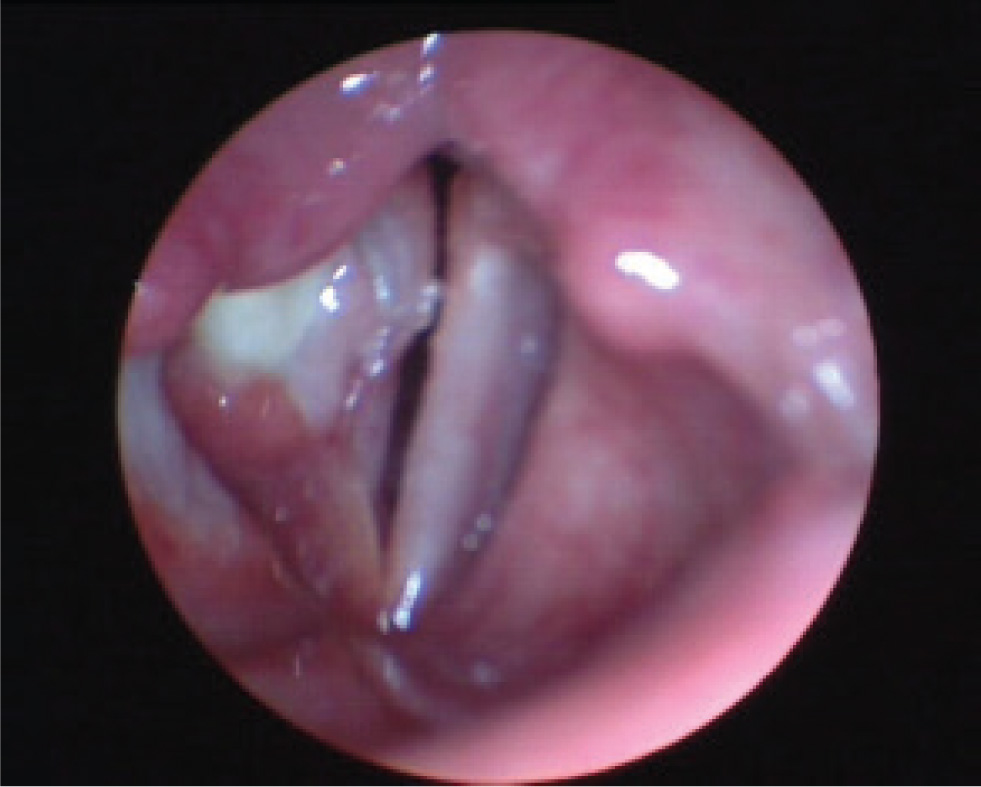
Videostroboscopy before the surgery: granuloma on 2nd and 3rd thirds of the right vocal cord.
Microscopic view during the surgery suggested the need of rapid intraoperative histological examination. The answer came: granulation tissue. Hyperplastic tissues of the right vocal cord were removed with CO2 laser and sent for further detailed histological examination. Suspecting tuberculosis, initial anteroposterior chest X-ray was made showing nodules on the apex of the left lung. The patient was referred to the pulmonologist. As there were no clinical signs of pulmonary disease, CT scan and fibrobronchoscopy with bronchoalveolar lavage and BACTEC were done to differentiate between focal pulmonary tuberculosis and cancer. Fibrobronchoscopy demonstrated obturated subsegmential B1-2c bronchus: diameter was narrowed by mucous outgrowths (similar to granulative tissue), covered with whitish necrotic substance. Final histopathologic diagnosis came one week after the surgery: chronic granulomatous – necrotic laryngitis (might be of tubercular origin). Two pulmonary CT scans made with 1, 5 month interval showed negative dynamics – some of the nodules on the apex of the left lung were enlarged and new ones had appeared (Fig. 2).

Chest CT scan: comparing two CT scan images, negative dynamics are seen in the latter, some of the nodes in the apical lobe of the left lung are enlarged and new ones had appeared. 5
No tuberculosis mycobacterium bacillus was found in bronchoalveolar lavage. In order to rule out Wegener’s granulomatosis, autoimmune markers were taken – all of them negative. Bronchoalveolar lavage was repeated, and samples taken for Xpert MTB/Rif genetic test. Tuberculosis mycobacterium resistant to rifampicin was found. Esophagogastroduodenoscopy was performed to rule out associated gastrointestinal involvement – no macroscopic signs of gastrointestinal tuberculosis were detected. Final diagnosis was made: infiltrative pulmonary and laryngeal tuberculosis, a new case. The patient was admitted to the hospital and a combination of medications (Ethambutol 1,6g, Cycloserine 0,75g, Prothionamide 0,75g, Moxifloxacin 0,4g for 708 days; Para-Aminosalicylic acid 8,0g for 259 days and Capreomycin 1,0g for 202 days) was prescribed for the next two years.
Four months after the surgery, during a visit to the laryngologist, stroboscopy demonstrated normal vocal cord movement, reduced mucous wave in the 2nd and 3rd third of the right vocal cord and no pathologic masses on the right vocal cord (Fig 3).
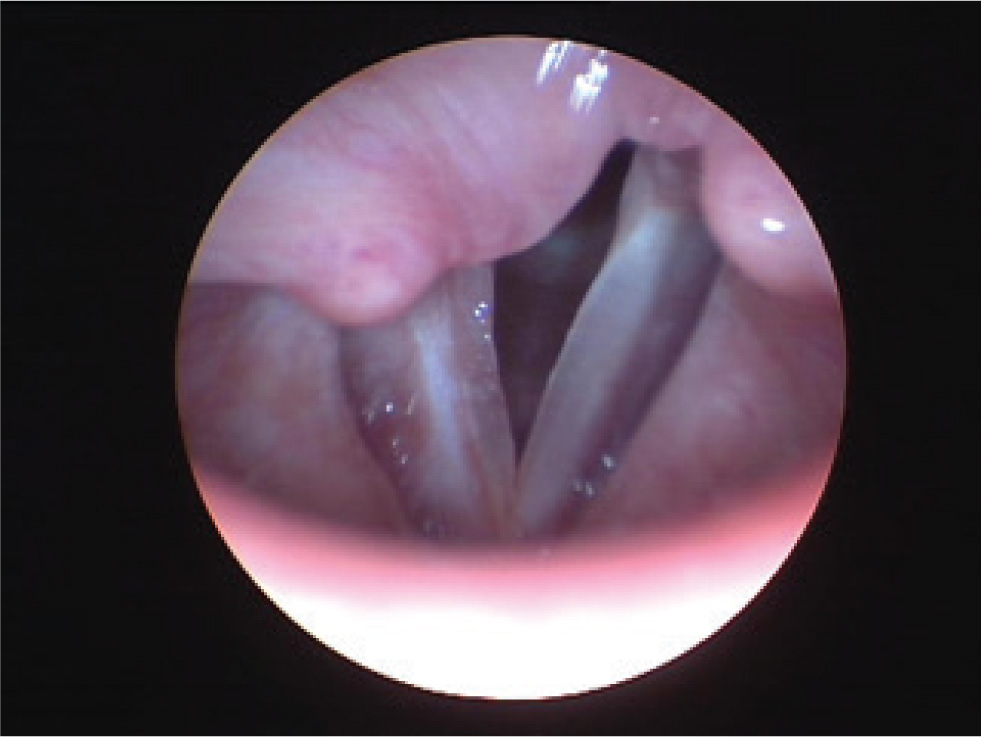
Videostroboscopy after the surgery: normal vocal cord movement, no pathologic masses seen on the right vocal cord.
Ethical approval
The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
Informed consent
Informed consent has been obtained from all individuals included in this study.
3 Discussion
Today, laryngeal tuberculosis is becoming a more frequent manifestation of tubercular disease. Unfortunately, it is rarely taken into consideration as clinical signs of laryngeal tuberculosis have changed over the past decades (Table 1). Therefore, it is important for otolaryngologists to be aware of the changes in the mode of this rare but serious infection.
Changes in clinical pattern of laryngeal tuberculosis
| Feature | Earlier | Today |
|---|---|---|
| Mean age (years) | 20 – 30 | 40 – 60 |
| Signs | Dyspnoea, pulmonary or constitutional symptoms (fever, weight loss, night sweat, fatigue) | Hoarseness, followed by odynophagia and dysphagia |
| Part of larynx affected | Posterior larynx (epiglottis, arytenoids, interarytenoid) | Vocal cord involvement – the most common; Anterior larynx twice as often as posterior |
| Type of lesions | Severe ulcerative or granulomatous | Hypertrophic, exophytic or polypoid |
| Way of mycobacterium spread | Bronchogenic | Hematogenous or lymphatic spread is becoming more often |
| Pulmonary involvement | Usually advanced pulmonary infection | No evidence of pulmonary disease, normal chest X-ray |
In the preantibiotic era, laryngeal tuberculosis was the most prevalent disease of the larynx in the world [7, 8]. Based on the literature, at the beginning of the 20th century, approximately 25–40% of all infected patients suffered from laryngeal tuberculosis [9, 10]. It is important to mention that involvement of the larynx was considered as preterminal event and was usually a complication of advanced pulmonary tuberculosis [8, 11]. Currently, larynx is affected only in 1% of all cases of tuberculosis, with a 2% mortality rate [3, 8, 10, 12]. Decrease in the rate of laryngeal tuberculosis was associated with introduction of antituberculous medications, improvement in living standards and worldwide prevention programs. Notwithstanding, the incidence of tuberculosis, as well as laryngeal tuberculosis, is increasing due to immunosuppressive conditions, HIV infection, immigration and resistance of mycobacterium strains. Formerly, the disease mostly affected young people about 20 – 30 years of age, no sex distribution was noticed [9, 13]. Nowadays, according to clinical studies, there is a shift to the older age, the disease is frequently diagnosed in 40 – 60 years of age, and males tend to be affected more. According to Kim, male to female ratio is 1, 9:1 [8], Bailey – 3,6: 1 [14], Ling Ling – 6: 1 [13].
Laryngeal tuberculosis may result from direct bronchogenic, haematogenic or lymphogenic spread. Previously, infection tended to spread directly along the airway and was almost always associated with advanced pulmonary infection. In that case, acid fast bacilli in the sputum infect laryngeal mucosa via direct contact. That way, the posterior part of the larynx is affected most often with disease gradually progressing to the other parts of the larynx. Currently, about 6% of infected patients have no evidence of pulmonary disease [10]. Some authors report laryngeal tuberculosis to be diagnosed for up to 40,6% of patients with normal lungs [15]. This suggests haematogenic and lymphogenic spread to be responsible for the development of laryngeal tuberculosis nowadays. What is more, some authors claim haematogenic spread is responsible for more than half of laryngeal tuberculosis cases [16]. Consequently, much more lesions are found in the anterior regions of the larynx, vocal cords being affected most often [10].
The classical lesions of the laryngeal tuberculosis described in the past were: diffuse whitish edema or ulcerated lesions and chondritis [13]. Today, according to the literature, there is a wider variety of lesions observed. They may be divided into four types: granulomatous, ulcerative, polypoid and nonspecific. Currently, hypertrophic, exophytic and polypoid lesions are more frequent than ulcerative or granulomatous, aggravating distinction between laryngeal tuberculosis and carcinoma. According to Lim and colleagues, granulomatous and polypoid lesions were usually single, while ulcerative lesions were found to be multiple. Moreover, examining patients with inactive pulmonary tuberculosis or those with healthy lungs, mostly single lesions of polypoid or nonspecific type were seen. Relatively more patients with active pulmonary tuberculosis had granulomatous or ulcerative lesions [9]. Bailey reported that in 73% of cases the lesions were unilateral [14]. Comparing the data, characteristic localization of the lesions changed: posterior larynx (arytenoids and interarytenoid notch) was the commonest site earlier, but the anterior half of the larynx is affected now twice as often as posterior [12].
Clinical symptoms of laryngeal tuberculosis seem to have changed a lot too, aggravating early diagnosis of the disease. While previously constitutional symptoms were predominant (fever, weight loss, night sweat, fatigue, haemoptysis and dyspnoea), today the major symptoms are: hoarseness followed by odynophagia and dysphagia. According to Lim, hoarseness was the main symptom in 96, 9% of cases, followed by odynophagia in 40% [9], Ling Ling reported hoarseness as the chief complaint in 71,4% of patients [13]. Based on the literature, odynophagia (painful dysphagia) should be kept in mind as an important factor for differential diagnosis, being less typical for cancer and more usual for laryngeal tuberculosis [9, 10].
Though extrapulmonary tuberculosis is usually not infectious, laryngeal involvement is associated with the high infectiousness of the disease because of the release of acid fast bacilli to the exhaled air. Long before, it was estimated that infectiousness is determined by the acid-fast bacilli smear status, treatment status, and frequency of cough. Smear positive persons expectorate 108 – 1010 bacilli per day [17], and Braden in his article reported that tuberculin conversions occurred among classmates who were in contact to a person with laryngeal tuberculosis for less than 5 hours [18]. However, treatment of the disease decreases contagiousness by decreasing the number of bacilli expectorated as well as by introducing antibiotic into the infectious droplet nuclei [17]. Therefore, it is clear that the later the laryngeal tuberculosis is diagnosed, the bigger the risk of disease trasmittion. Early diagnosis is crucial in disease transmission control. Nonetheless, despite of these signs and symptoms described above, there are no pathognomonic characteristics typical of laryngeal tuberculosis, thus, the diagnosis can be easily delayed.
When laryngeal tuberculosis is suspected and sputum microscopy is negative, biopsy from the lesion is recommended. That is especially useful when malignancy cannot be ruled out [12]. Once tuberculosis is diagnosed, antituberculous treatment should be prompt in order to prevent chronic complications. During the treatment, laryngeal lesions should resolve and surgery is reserved only for cases of airway compromise and for chronic complications (posterior glottic stenosis, vocal cord paralysis when cricoarytenoid joint or recurrent laryngeal nerve are affected).
4 Conclusions
Laryngeal tuberculosis is an uncommon condition, however it still occurs. Since there are no pathognomonic features indicative of this disease and clinical patterns of this condition are continuously changing, it can mimic many other diseases, especially malignancies. If misdiagnosed, laryngeal tuberculosis may cause severe consequences for the patient and anyone he comes into contact with. Therefore, otolaryngologists should always consider the possibility of laryngeal tuberculosis in case of persistent dysphonia, even if there are no other suggestive systemic symptoms.
Conflict of interest statement: Authors state no conflict of interest
References
[1] Gonzalez-Martin J., Garcia-Garcia J.M., Anibarro L., Vidal R., Esteban J., Blanquer R. et al., Consensus Document on the Diagnosis, Treatment and Prevention of Tuberculosis, Arch Bronconeumol, 2010, 46 (5), 255–27410.1016/S1579-2129(10)70061-6Search in Google Scholar
[2] World health organization, Global tuberculosis report 2012, Switzerland, WHO Press, 2012Search in Google Scholar
[3] Topak M., Oysu C., Yelken K., Sahin-Yilmaz A., Kulekci M., Laryngeal involvement in patients with active pulmonary tuberculosis, Eur Arch Otorhinolaryngol, 2008, 265, 327–33010.1007/s00405-007-0459-xSearch in Google Scholar
[4] Schwartz S.R., Cohen S.M., Dailey S.H., Rosenfeld R.M., Deutsch E.S., Gillespie M.B. et al, Clinical practice guideline: Hoarseness (Dysphonia), Otolaryngol-Head Neck Surg, 2009, 141 (3S2), S1-S3110.1016/j.otohns.2009.06.744Search in Google Scholar
[5] Banjara H., Mungutwar V., Singh D., Gupta A., Hoarseness of voice: a retrospective study of 251 cases, Int J Phonosurg Laryngol, 2011, 1(1), 21–2710.5005/jp-journals-10023-1006Search in Google Scholar
[6] Ozudogru E., Cakli H., Altuntas E.E., Gurbuuz M.K., Effects of laryngeal tuberculosis on vocal fold functions; case report, Acta Otorhinolaryngol Ital, 2005, 25(6), 374–377Search in Google Scholar
[7] Auerbach O., Laryngeal tuberculosis, Arch Otolaryngol, 1946, 44, 191–20110.1001/archotol.1946.00680060208008Search in Google Scholar
[8] Yencha M.W., Linfesty R., Blackmon A., Laryngeal Tuberculosis, Am J Otolaryngol, 2000, 21, 122–12610.1016/S0196-0709(00)85010-3Search in Google Scholar
[9] Lim J.Y., Kim K.M., Choi E.C., Kim Y.H., Kim H.S., Choi H.S., Current clinical propensity of laryngeal tuberculosis: review of 60 cases, Eur Arch Otorhinolaryngol, 2006, 263, 838–84210.1007/s00405-006-0063-5Search in Google Scholar
[10] Rizzo P.B., Da Mosto M.C., Clari M., Scotton P.G., Vaglia A., Marchiori C., Laryngeal tuberculosis: and often forgotten diagnosis, Int J Infect Dis, 2003, 7, 129–13110.1016/S1201-9712(03)90008-7Search in Google Scholar
[11] Smulders Y.E., De Bondt B.J., Lacko M., Hodge J.A., Kross K.W., Laryngeal tuberculosis presenting as supraglottic carcinoma: a case report and review of the literature, Journal of Medical Case Reports, 2009, 3, 928810.1186/1752-1947-3-9288Search in Google Scholar PubMed PubMed Central
[12] Nalini B., Vinayak S., Tuberculosis in ear, nose, and throat practice: its presentation and diagnosis, Am J Otolaryngol, 2006, 27, 39–4510.1016/j.amjoto.2005.07.005Search in Google Scholar PubMed
[13] Ling L., Zhou S.H., Wang S.Q., Changing trends in the clinical features of laryngeal tuberculosis: a report of 19 cases, International Journal of Infectious Diseases, 2010, 14, e230-e23510.1016/j.ijid.2009.05.002Search in Google Scholar PubMed
[14] Bailey C.M., Windle-Taylor P.C., Tuberculous laryngitis: A series of 37 patients, Laryngoscope, 1981, 91, 93–10010.1288/00005537-198101000-00014Search in Google Scholar PubMed
[15] Shin J.E., Nam S.Y., Yoo S.J., Kim S.Y., Changing trends in clinical manifestations of laryngeal tuberculosis, Laryngoscope, 2000, 110, 1950–195310.1097/00005537-200011000-00034Search in Google Scholar PubMed
[16] Kiakojuri K., Hasanjani Roushan M.R., Laryngeal tuberculosis without pulmonary involvement, Caspian J Intern Med, 2012, 3(1), 397–399Search in Google Scholar
[17] Sepkowitz K.A., How contagious is tuberculosis? Clin Infect Dis, 1996, 23, 965-6210.1093/clinids/23.5.954Search in Google Scholar PubMed
[18] Braden C.R., Infectiousness of a university student with laryngeal and cavitary tuberculosis, Clin Infect Dis, 1995, 21, 565–7010.1093/clinids/21.3.565Search in Google Scholar PubMed
© 2016 Iveta Paulauskienė et al.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
