JP5203749B2 - 硬化前後における色調変化の少ない光重合性歯科用組成物 - Google Patents
硬化前後における色調変化の少ない光重合性歯科用組成物 Download PDFInfo
- Publication number
- JP5203749B2 JP5203749B2 JP2008052962A JP2008052962A JP5203749B2 JP 5203749 B2 JP5203749 B2 JP 5203749B2 JP 2008052962 A JP2008052962 A JP 2008052962A JP 2008052962 A JP2008052962 A JP 2008052962A JP 5203749 B2 JP5203749 B2 JP 5203749B2
- Authority
- JP
- Japan
- Prior art keywords
- weight
- meth
- dental composition
- parts
- curing
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000000203 mixture Substances 0.000 title claims description 48
- -1 amine compound Chemical class 0.000 claims description 48
- 150000001875 compounds Chemical class 0.000 claims description 30
- 239000000178 monomer Substances 0.000 claims description 27
- VNQXSTWCDUXYEZ-UHFFFAOYSA-N 1,7,7-trimethylbicyclo[2.2.1]heptane-2,3-dione Chemical compound C1CC2(C)C(=O)C(=O)C1C2(C)C VNQXSTWCDUXYEZ-UHFFFAOYSA-N 0.000 claims description 11
- 238000005259 measurement Methods 0.000 claims description 11
- 239000003504 photosensitizing agent Substances 0.000 claims description 10
- VFHVQBAGLAREND-UHFFFAOYSA-N diphenylphosphoryl-(2,4,6-trimethylphenyl)methanone Chemical compound CC1=CC(C)=CC(C)=C1C(=O)P(=O)(C=1C=CC=CC=1)C1=CC=CC=C1 VFHVQBAGLAREND-UHFFFAOYSA-N 0.000 claims description 7
- 229930006711 bornane-2,3-dione Natural products 0.000 claims description 6
- 239000011256 inorganic filler Substances 0.000 claims description 6
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 3
- 125000002947 alkylene group Chemical group 0.000 claims description 3
- 125000004432 carbon atom Chemical group C* 0.000 claims description 3
- 229910052739 hydrogen Inorganic materials 0.000 claims description 3
- 239000001257 hydrogen Substances 0.000 claims description 3
- 239000012766 organic filler Substances 0.000 claims description 3
- 230000009974 thixotropic effect Effects 0.000 claims description 3
- 239000004386 Erythritol Substances 0.000 claims 1
- UNXHWFMMPAWVPI-UHFFFAOYSA-N Erythritol Natural products OCC(O)C(O)CO UNXHWFMMPAWVPI-UHFFFAOYSA-N 0.000 claims 1
- UNXHWFMMPAWVPI-ZXZARUISSA-N erythritol Chemical compound OC[C@H](O)[C@H](O)CO UNXHWFMMPAWVPI-ZXZARUISSA-N 0.000 claims 1
- 229940009714 erythritol Drugs 0.000 claims 1
- 235000019414 erythritol Nutrition 0.000 claims 1
- 238000001723 curing Methods 0.000 description 29
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 28
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 26
- 239000003999 initiator Substances 0.000 description 16
- 239000000463 material Substances 0.000 description 15
- 239000001294 propane Substances 0.000 description 13
- 239000000945 filler Substances 0.000 description 10
- 239000005548 dental material Substances 0.000 description 7
- MPIAGWXWVAHQBB-UHFFFAOYSA-N [3-prop-2-enoyloxy-2-[[3-prop-2-enoyloxy-2,2-bis(prop-2-enoyloxymethyl)propoxy]methyl]-2-(prop-2-enoyloxymethyl)propyl] prop-2-enoate Chemical compound C=CC(=O)OCC(COC(=O)C=C)(COC(=O)C=C)COCC(COC(=O)C=C)(COC(=O)C=C)COC(=O)C=C MPIAGWXWVAHQBB-UHFFFAOYSA-N 0.000 description 6
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 6
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 5
- 239000011521 glass Substances 0.000 description 5
- 238000006116 polymerization reaction Methods 0.000 description 5
- 239000000853 adhesive Substances 0.000 description 4
- 230000001070 adhesive effect Effects 0.000 description 4
- 239000011248 coating agent Substances 0.000 description 4
- 238000000576 coating method Methods 0.000 description 4
- 229910003475 inorganic filler Inorganic materials 0.000 description 4
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 3
- TXBCBTDQIULDIA-UHFFFAOYSA-N 2-[[3-hydroxy-2,2-bis(hydroxymethyl)propoxy]methyl]-2-(hydroxymethyl)propane-1,3-diol Chemical compound OCC(CO)(CO)COCC(CO)(CO)CO TXBCBTDQIULDIA-UHFFFAOYSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 238000010521 absorption reaction Methods 0.000 description 3
- 230000001588 bifunctional effect Effects 0.000 description 3
- 239000005388 borosilicate glass Substances 0.000 description 3
- 239000004568 cement Substances 0.000 description 3
- 239000002131 composite material Substances 0.000 description 3
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 description 3
- 238000002845 discoloration Methods 0.000 description 3
- 229910052736 halogen Inorganic materials 0.000 description 3
- 150000002367 halogens Chemical class 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- 239000000523 sample Substances 0.000 description 3
- 239000004322 Butylated hydroxytoluene Substances 0.000 description 2
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 description 2
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical group C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 2
- QIGBRXMKCJKVMJ-UHFFFAOYSA-N Hydroquinone Chemical compound OC1=CC=C(O)C=C1 QIGBRXMKCJKVMJ-UHFFFAOYSA-N 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 2
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 2
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 2
- WERYXYBDKMZEQL-UHFFFAOYSA-N butane-1,4-diol Chemical compound OCCCCO WERYXYBDKMZEQL-UHFFFAOYSA-N 0.000 description 2
- 229940095259 butylated hydroxytoluene Drugs 0.000 description 2
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- HNRMPXKDFBEGFZ-UHFFFAOYSA-N ethyl trimethyl methane Natural products CCC(C)(C)C HNRMPXKDFBEGFZ-UHFFFAOYSA-N 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- NWVVVBRKAWDGAB-UHFFFAOYSA-N p-methoxyphenol Chemical compound COC1=CC=C(O)C=C1 NWVVVBRKAWDGAB-UHFFFAOYSA-N 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 239000000049 pigment Substances 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 239000003505 polymerization initiator Substances 0.000 description 2
- 229920001296 polysiloxane Polymers 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 150000004756 silanes Chemical class 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- LOEVVVOVKKKPLT-UHFFFAOYSA-N (2,6-dichlorophenyl)-diphenylphosphanylmethanone Chemical compound ClC1=CC=CC(Cl)=C1C(=O)P(C=1C=CC=CC=1)C1=CC=CC=C1 LOEVVVOVKKKPLT-UHFFFAOYSA-N 0.000 description 1
- SUEDCWGEKSLKOM-UHFFFAOYSA-N (2,6-dimethoxyphenyl)-diphenylphosphorylmethanone Chemical compound COC1=CC=CC(OC)=C1C(=O)P(=O)(C=1C=CC=CC=1)C1=CC=CC=C1 SUEDCWGEKSLKOM-UHFFFAOYSA-N 0.000 description 1
- WYTZZXDRDKSJID-UHFFFAOYSA-N (3-aminopropyl)triethoxysilane Chemical compound CCO[Si](OCC)(OCC)CCCN WYTZZXDRDKSJID-UHFFFAOYSA-N 0.000 description 1
- ZNXKLRKQUSZVQY-UHFFFAOYSA-N 1,6-diisocyanatohexane ethyl carbamate 2-(phenoxymethyl)oxirane prop-2-enoic acid Chemical compound OC(=O)C=C.CCOC(N)=O.C1OC1COC1=CC=CC=C1.O=C=NCCCCCCN=C=O ZNXKLRKQUSZVQY-UHFFFAOYSA-N 0.000 description 1
- UKMXWILLHVVZLZ-UHFFFAOYSA-N 2,2-bis(hydroxymethyl)-1-propoxypropane-1,3-diol Chemical compound C(CC)OC(O)C(CO)(CO)CO UKMXWILLHVVZLZ-UHFFFAOYSA-N 0.000 description 1
- XCBBNTFYSLADTO-UHFFFAOYSA-N 2,3-Octanedione Chemical compound CCCCCC(=O)C(C)=O XCBBNTFYSLADTO-UHFFFAOYSA-N 0.000 description 1
- SNFRKUNAXTXJEN-UHFFFAOYSA-N 2,4-diisocyanato-1-methylbenzene ethyl carbamate [2-(hydroxymethyl)-3-prop-2-enoyloxy-2-(prop-2-enoyloxymethyl)propyl] prop-2-enoate Chemical compound NC(=O)OCC.CC=1C(=CC(=CC1)N=C=O)N=C=O.C(C=C)(=O)OCC(COC(C=C)=O)(COC(C=C)=O)CO SNFRKUNAXTXJEN-UHFFFAOYSA-N 0.000 description 1
- KIYAYQYAGXQEKC-UHFFFAOYSA-N 2-(2-methylprop-2-enoyloxy)ethyl 2-methylprop-2-enoate;tetradecane Chemical compound CCCCCCCCCCCCCC.CC(=C)C(=O)OCCOC(=O)C(C)=C KIYAYQYAGXQEKC-UHFFFAOYSA-N 0.000 description 1
- HUEVRTVCIKCHKK-UHFFFAOYSA-N 2-(phenoxymethyl)oxirane toluene Chemical compound CC1=CC=CC=C1.C1OC1COC1=CC=CC=C1 HUEVRTVCIKCHKK-UHFFFAOYSA-N 0.000 description 1
- HWSSEYVMGDIFMH-UHFFFAOYSA-N 2-[2-[2-(2-methylprop-2-enoyloxy)ethoxy]ethoxy]ethyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCOCCOCCOC(=O)C(C)=C HWSSEYVMGDIFMH-UHFFFAOYSA-N 0.000 description 1
- HLGNMOUJXWELKK-UHFFFAOYSA-N 2-[2-[2-[2-[2-[2-[2-[2-[2-(2-methylprop-2-enoyloxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCOCCOCCOCCOCCOCCOCCOCCOCCOC(=O)C(C)=C HLGNMOUJXWELKK-UHFFFAOYSA-N 0.000 description 1
- UEKHZPDUBLCUHN-UHFFFAOYSA-N 2-[[3,5,5-trimethyl-6-[2-(2-methylprop-2-enoyloxy)ethoxycarbonylamino]hexyl]carbamoyloxy]ethyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCOC(=O)NCCC(C)CC(C)(C)CNC(=O)OCCOC(=O)C(C)=C UEKHZPDUBLCUHN-UHFFFAOYSA-N 0.000 description 1
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 description 1
- XDQWJFXZTAWJST-UHFFFAOYSA-N 3-triethoxysilylpropyl prop-2-enoate Chemical compound CCO[Si](OCC)(OCC)CCCOC(=O)C=C XDQWJFXZTAWJST-UHFFFAOYSA-N 0.000 description 1
- UUEWCQRISZBELL-UHFFFAOYSA-N 3-trimethoxysilylpropane-1-thiol Chemical compound CO[Si](OC)(OC)CCCS UUEWCQRISZBELL-UHFFFAOYSA-N 0.000 description 1
- XDLMVUHYZWKMMD-UHFFFAOYSA-N 3-trimethoxysilylpropyl 2-methylprop-2-enoate Chemical compound CO[Si](OC)(OC)CCCOC(=O)C(C)=C XDLMVUHYZWKMMD-UHFFFAOYSA-N 0.000 description 1
- 125000006283 4-chlorobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1Cl)C([H])([H])* 0.000 description 1
- YYVYAPXYZVYDHN-UHFFFAOYSA-N 9,10-phenanthroquinone Chemical compound C1=CC=C2C(=O)C(=O)C3=CC=CC=C3C2=C1 YYVYAPXYZVYDHN-UHFFFAOYSA-N 0.000 description 1
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 1
- 229910002012 Aerosil® Inorganic materials 0.000 description 1
- DKPFZGUDAPQIHT-UHFFFAOYSA-N Butyl acetate Natural products CCCCOC(C)=O DKPFZGUDAPQIHT-UHFFFAOYSA-N 0.000 description 1
- HQLJWZGLXGUBLN-UHFFFAOYSA-N CC(CP(=O)C(=O)c1c(C)ccc(C)c1C)CC(C)(C)C Chemical compound CC(CP(=O)C(=O)c1c(C)ccc(C)c1C)CC(C)(C)C HQLJWZGLXGUBLN-UHFFFAOYSA-N 0.000 description 1
- PBTPIPYHIXYSQU-UHFFFAOYSA-N ClC1=C(C(=O)P(C2=C(C=CC(=C2)C)C)(C(C2=C(C=CC=C2Cl)Cl)=O)=O)C(=CC=C1)Cl.ClC1=C(C(=O)P(C2=CC=CC=C2)(C(C2=C(C=CC=C2Cl)Cl)=O)=O)C(=CC=C1)Cl Chemical compound ClC1=C(C(=O)P(C2=C(C=CC(=C2)C)C)(C(C2=C(C=CC=C2Cl)Cl)=O)=O)C(=CC=C1)Cl.ClC1=C(C(=O)P(C2=CC=CC=C2)(C(C2=C(C=CC=C2Cl)Cl)=O)=O)C(=CC=C1)Cl PBTPIPYHIXYSQU-UHFFFAOYSA-N 0.000 description 1
- DMWINQMHHCNMBM-UHFFFAOYSA-N ClC1=C(C(=O)P(C2=CC=CC3=CC=CC=C23)(C(C2=C(C=CC=C2Cl)Cl)=O)=O)C(=CC=C1)Cl.ClC1=C(C(=O)P(C2=CC=C(C=C2)CCC)(C(C2=C(C=CC=C2Cl)Cl)=O)=O)C(=CC=C1)Cl Chemical compound ClC1=C(C(=O)P(C2=CC=CC3=CC=CC=C23)(C(C2=C(C=CC=C2Cl)Cl)=O)=O)C(=CC=C1)Cl.ClC1=C(C(=O)P(C2=CC=C(C=C2)CCC)(C(C2=C(C=CC=C2Cl)Cl)=O)=O)C(=CC=C1)Cl DMWINQMHHCNMBM-UHFFFAOYSA-N 0.000 description 1
- QSJXEFYPDANLFS-UHFFFAOYSA-N Diacetyl Chemical group CC(=O)C(C)=O QSJXEFYPDANLFS-UHFFFAOYSA-N 0.000 description 1
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 1
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 1
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- BPQQTUXANYXVAA-UHFFFAOYSA-N Orthosilicate Chemical compound [O-][Si]([O-])([O-])[O-] BPQQTUXANYXVAA-UHFFFAOYSA-N 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- ZTCAGYRXUCWHPV-UHFFFAOYSA-N [(2,6-dimethoxybenzoyl)-(2,5-dimethylphenyl)phosphoryl]-(2,6-dimethoxyphenyl)methanone Chemical compound COC1=CC=CC(OC)=C1C(=O)P(=O)(C=1C(=CC=C(C)C=1)C)C(=O)C1=C(OC)C=CC=C1OC ZTCAGYRXUCWHPV-UHFFFAOYSA-N 0.000 description 1
- QISAYNXDUCNISJ-UHFFFAOYSA-N [(2,6-dimethoxybenzoyl)-phenylphosphoryl]-(2,6-dimethoxyphenyl)methanone Chemical compound COC1=CC=CC(OC)=C1C(=O)P(=O)(C=1C=CC=CC=1)C(=O)C1=C(OC)C=CC=C1OC QISAYNXDUCNISJ-UHFFFAOYSA-N 0.000 description 1
- XRMBQHTWUBGQDN-UHFFFAOYSA-N [2-[2,2-bis(prop-2-enoyloxymethyl)butoxymethyl]-2-(prop-2-enoyloxymethyl)butyl] prop-2-enoate Chemical compound C=CC(=O)OCC(COC(=O)C=C)(CC)COCC(CC)(COC(=O)C=C)COC(=O)C=C XRMBQHTWUBGQDN-UHFFFAOYSA-N 0.000 description 1
- LFOBCPJMYQVUPV-UHFFFAOYSA-N [2-[ethoxycarbonyl-[6-(ethoxycarbonylamino)-3,5,5-trimethylhexyl]amino]-1-(2-methylprop-2-enoyloxy)ethyl] 2-methylprop-2-enoate Chemical compound CCOC(=O)NCC(C)(C)CC(C)CCN(C(=O)OCC)CC(OC(=O)C(C)=C)OC(=O)C(C)=C LFOBCPJMYQVUPV-UHFFFAOYSA-N 0.000 description 1
- KNSXNCFKSZZHEA-UHFFFAOYSA-N [3-prop-2-enoyloxy-2,2-bis(prop-2-enoyloxymethyl)propyl] prop-2-enoate Chemical class C=CC(=O)OCC(COC(=O)C=C)(COC(=O)C=C)COC(=O)C=C KNSXNCFKSZZHEA-UHFFFAOYSA-N 0.000 description 1
- RMKZLFMHXZAGTM-UHFFFAOYSA-N [dimethoxy(propyl)silyl]oxymethyl prop-2-enoate Chemical compound CCC[Si](OC)(OC)OCOC(=O)C=C RMKZLFMHXZAGTM-UHFFFAOYSA-N 0.000 description 1
- IEWRMZSVIVEQCP-UHFFFAOYSA-N [methoxy(phenyl)phosphoryl]-(2,4,6-trimethylphenyl)methanone Chemical compound C=1C=CC=CC=1P(=O)(OC)C(=O)C1=C(C)C=C(C)C=C1C IEWRMZSVIVEQCP-UHFFFAOYSA-N 0.000 description 1
- GUCYFKSBFREPBC-UHFFFAOYSA-N [phenyl-(2,4,6-trimethylbenzoyl)phosphoryl]-(2,4,6-trimethylphenyl)methanone Chemical compound CC1=CC(C)=CC(C)=C1C(=O)P(=O)(C=1C=CC=CC=1)C(=O)C1=C(C)C=C(C)C=C1C GUCYFKSBFREPBC-UHFFFAOYSA-N 0.000 description 1
- AFPRJLBZLPBTPZ-UHFFFAOYSA-N acenaphthoquinone Chemical compound C1=CC(C(C2=O)=O)=C3C2=CC=CC3=C1 AFPRJLBZLPBTPZ-UHFFFAOYSA-N 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 229910052788 barium Inorganic materials 0.000 description 1
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 239000006059 cover glass Substances 0.000 description 1
- 238000006356 dehydrogenation reaction Methods 0.000 description 1
- 125000005442 diisocyanate group Chemical group 0.000 description 1
- HZWAUDKTZOUQPD-UHFFFAOYSA-N diphenylphosphoryl-(2-methylphenyl)methanone Chemical compound CC1=CC=CC=C1C(=O)P(=O)(C=1C=CC=CC=1)C1=CC=CC=C1 HZWAUDKTZOUQPD-UHFFFAOYSA-N 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- FWDBOZPQNFPOLF-UHFFFAOYSA-N ethenyl(triethoxy)silane Chemical compound CCO[Si](OCC)(OCC)C=C FWDBOZPQNFPOLF-UHFFFAOYSA-N 0.000 description 1
- NKSJNEHGWDZZQF-UHFFFAOYSA-N ethenyl(trimethoxy)silane Chemical compound CO[Si](OC)(OC)C=C NKSJNEHGWDZZQF-UHFFFAOYSA-N 0.000 description 1
- WOXXJEVNDJOOLV-UHFFFAOYSA-N ethenyl-tris(2-methoxyethoxy)silane Chemical compound COCCO[Si](OCCOC)(OCCOC)C=C WOXXJEVNDJOOLV-UHFFFAOYSA-N 0.000 description 1
- HLBOJHQDGVDWPU-UHFFFAOYSA-N ethyl carbamate;[2-(hydroxymethyl)-3-prop-2-enoyloxy-2-(prop-2-enoyloxymethyl)propyl] prop-2-enoate;5-isocyanato-1-(isocyanatomethyl)-1,3,3-trimethylcyclohexane Chemical compound CCOC(N)=O.CC1(C)CC(N=C=O)CC(C)(CN=C=O)C1.C=CC(=O)OCC(CO)(COC(=O)C=C)COC(=O)C=C HLBOJHQDGVDWPU-UHFFFAOYSA-N 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- APURLPHDHPNUFL-UHFFFAOYSA-M fluoroaluminum Chemical compound [Al]F APURLPHDHPNUFL-UHFFFAOYSA-M 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 239000005350 fused silica glass Substances 0.000 description 1
- 238000001879 gelation Methods 0.000 description 1
- 239000002241 glass-ceramic Substances 0.000 description 1
- XXMIOPMDWAUFGU-UHFFFAOYSA-N hexane-1,6-diol Chemical compound OCCCCCCO XXMIOPMDWAUFGU-UHFFFAOYSA-N 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 230000001678 irradiating effect Effects 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 238000000691 measurement method Methods 0.000 description 1
- AUHZEENZYGFFBQ-UHFFFAOYSA-N mesitylene Substances CC1=CC(C)=CC(C)=C1 AUHZEENZYGFFBQ-UHFFFAOYSA-N 0.000 description 1
- 238000000034 method Methods 0.000 description 1
- 210000000214 mouth Anatomy 0.000 description 1
- SLCVBVWXLSEKPL-UHFFFAOYSA-N neopentyl glycol Chemical compound OCC(C)(C)CO SLCVBVWXLSEKPL-UHFFFAOYSA-N 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- FZUGPQWGEGAKET-UHFFFAOYSA-N parbenate Chemical compound CCOC(=O)C1=CC=C(N(C)C)C=C1 FZUGPQWGEGAKET-UHFFFAOYSA-N 0.000 description 1
- WXZMFSXDPGVJKK-UHFFFAOYSA-N pentaerythritol Chemical compound OCC(CO)(CO)CO WXZMFSXDPGVJKK-UHFFFAOYSA-N 0.000 description 1
- TZMFJUDUGYTVRY-UHFFFAOYSA-N pentane-2,3-dione Chemical compound CCC(=O)C(C)=O TZMFJUDUGYTVRY-UHFFFAOYSA-N 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 238000000016 photochemical curing Methods 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- UIIMBOGNXHQVGW-UHFFFAOYSA-N sodium;hydron;carbonate Chemical compound [Na+].OC(O)=O UIIMBOGNXHQVGW-UHFFFAOYSA-N 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229910052712 strontium Inorganic materials 0.000 description 1
- CIOAGBVUUVVLOB-UHFFFAOYSA-N strontium atom Chemical compound [Sr] CIOAGBVUUVVLOB-UHFFFAOYSA-N 0.000 description 1
- 150000003440 styrenes Chemical class 0.000 description 1
- 238000004381 surface treatment Methods 0.000 description 1
- 239000012756 surface treatment agent Substances 0.000 description 1
- GQIUQDDJKHLHTB-UHFFFAOYSA-N trichloro(ethenyl)silane Chemical compound Cl[Si](Cl)(Cl)C=C GQIUQDDJKHLHTB-UHFFFAOYSA-N 0.000 description 1
- ZIBGPFATKBEMQZ-UHFFFAOYSA-N triethylene glycol Chemical compound OCCOCCOCCO ZIBGPFATKBEMQZ-UHFFFAOYSA-N 0.000 description 1
- 125000004205 trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 description 1
- YUYCVXFAYWRXLS-UHFFFAOYSA-N trimethoxysilane Chemical compound CO[SiH](OC)OC YUYCVXFAYWRXLS-UHFFFAOYSA-N 0.000 description 1
- 239000006097 ultraviolet radiation absorber Substances 0.000 description 1
- 239000005050 vinyl trichlorosilane Substances 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 229910052724 xenon Inorganic materials 0.000 description 1
- FHNFHKCVQCLJFQ-UHFFFAOYSA-N xenon atom Chemical compound [Xe] FHNFHKCVQCLJFQ-UHFFFAOYSA-N 0.000 description 1
- 238000004383 yellowing Methods 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K6/00—Preparations for dentistry
- A61K6/60—Preparations for dentistry comprising organic or organo-metallic additives
- A61K6/62—Photochemical radical initiators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K6/00—Preparations for dentistry
- A61K6/20—Protective coatings for natural or artificial teeth, e.g. sealings, dye coatings or varnish
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K6/00—Preparations for dentistry
- A61K6/30—Compositions for temporarily or permanently fixing teeth or palates, e.g. primers for dental adhesives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K6/00—Preparations for dentistry
- A61K6/80—Preparations for artificial teeth, for filling teeth or for capping teeth
- A61K6/884—Preparations for artificial teeth, for filling teeth or for capping teeth comprising natural or synthetic resins
- A61K6/887—Compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Plastic & Reconstructive Surgery (AREA)
- Biophysics (AREA)
- Dental Preparations (AREA)
- Chemical & Material Sciences (AREA)
- Polymerisation Methods In General (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
Description
[1] 光増感剤としてアミン化合物を実質的に含まず、(ビス)アシルホスフィンオキサイド化合物0.01〜10重量部、α−ジケトン化合物0.01〜10重量部、および重合性モノマー100重量部を含むことを特徴とする可視光重合性歯科用組成物;
[2] (ビス)アシルホスフィンオキサイド化合物が2,4,6−トリメチルベンゾイルジフェニルホスフィンオキサイドであることを特徴とする前記[1]に記載の可視光重合性歯科用組成物;
[3] α−ジケトン化合物がカンファーキノンであることを特徴とする前記[1]または[2]に記載の可視光重合性歯科用組成物;
[4] 重合性モノマーとしてジペンタエリスリトールヘキサアクリレートを含むことを特徴とする前記[1]ないし[3]のいずれか1に記載の可視光重合性歯科用組成物;
[5] 重合性モノマーとして、一般式(I):
[6] 一般式(I)中のXがエチレンオキサイド基のみからなる繰り返し単位であって、繰り返し単位数が9〜23であることを特徴とする前記[5]に記載の可視光重合性歯科用組成物;
[7] 一般式(I)で表される重合性モノマーを、重合性モノマーの全量に対して5〜90重量%含むことを特徴とする前記[5]または[6]に記載の可視光重合性歯科用組成物;
[8] さらに、無機または有機フィラーの少なくとも1種類を0.1〜90重量部含有することを特徴とする前記[1]〜[7]のいずれか1に記載の可視光重合性歯科用組成物;
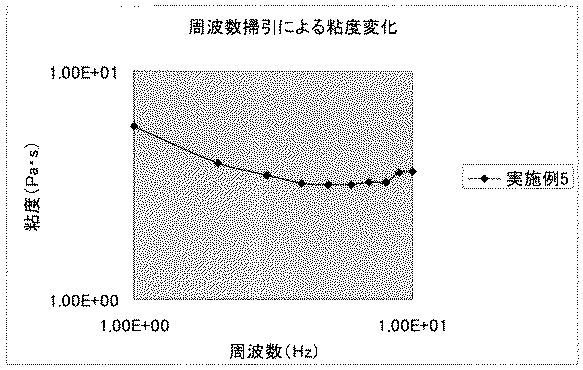
[9] 23℃におけるプレートプレート系粘度測定(ギャップ0.500mm)、開始応力1.00E+1Paで測定した周波数掃引7〜1Hz時において2〜8Pa・Sの粘度、および、周波数7〜1Hzにおいて粘度の最小値と最大値の比が1.1以上のチクソトロピー性を有することを特徴とする前記[1]〜[8]のいずれか1に記載の可視光重合性歯科用組成物
を提供する。
本発明の歯科用組成物およびそれに用いる光重合開始剤は、光増感剤として実質的にアミン化合物を含まないことを特徴とする。ここで、「光増感剤として実質的にアミン化合物を含まない」とは、光増感剤としてのアミン化合物を能動的に組成物に配合しないということを意味し、他成分原料に由来して受動的に配合されるアミン化合物はこの定義に含まれない。具体的には、本発明の光重合開始剤としては0〜1重量部、可視光重合性歯科用組成物としては0〜0.1重量部のアミン化合物が含まれることは許容される。
光増感剤(安定剤、還元剤)であるアミン化合物を歯科用組成物に添加するとアミン特有の不快臭を有し、使用者にとって不快感を与える。さらに硬化後の色調に褐色をもたらすため、審美的に致命的な変色を生じる。
重合禁止剤としてはハイドロキノン、ハイドロキノンモノメチルエーテル、ブチル化ヒドロキシトルエンなどが挙げられるが、その中でもハイドロキノンモノメチルエーテルおよびブチル化ヒドロキシトルエンが好ましい。
また、溶剤としては、水、エタノール、i−プロパノール、アセトン、ジメチルスルホキシド、ジメチルホルムアミド、酢酸エチル、酢酸ブチルなどが挙げられる。
CQ:dl−カンファーキノン
APO:2,4,6−トリメチルベンゾイル−ジフェニルホスフィンオキサイド
DMBE:4−ジメチルアミノ安息香酸エチル
DPH:ジペンタエリスリトールヘキサアクリレート
UDMA:ジメタクリロキシエチル−2,2,4−トリメチルヘキサメチレンジウレタン
14G:テトラデカンエチレングリコールジメタクリレート(繰り返し単位数n=14)
9G:ノナエチレングリコールジメタクリレート(繰り返し単位数n=9)
3G:トリエチレングリコールジメタクリレート(繰り返し単位数n=3)
R−8200:アエロジルR−8200(日本アエロジル社製)
薄層表面硬化性評価
調製した各種光硬化性組成物を練板紙上に1滴採取し、筆で薄く(厚さ約0.1mm)拡げた。各種光重合器で光照射し、手感により薄層表面硬化性を確認した。2種の歯科用光照射器は、ハロゲンランプ照射器(Hal)はソリディライト[株式会社松風社製](1分間照射)、LED照射器(LED)としてSPEKTRA LED[シュッツデンタル社製] (2分間照射)を使用した。
◎:表面の未反応モノマー量が極めて少なく、極めて高い薄層表面硬化性を示す。
○:表面の未反応モノマー量が少なく、高い薄層表面硬化性を示す。
×:表面の未反応モノマー量が認められ、低い薄層表面硬化性を示す。
調製した各種光硬化性組成物をステンレス製リング(内径15mm、厚さ0.5mm)内に入れ、2枚のカバーガラスで上下方向から圧接し、分光測色計CM−2002(コニカミノルタ社製)により測色(L*a*b*表色系)し、硬化前の色調とした。次に、光重合器(ソリディライト、株式会社松風社製)で表裏両面1分間ずつ光照射した後測色を行った。硬化前と硬化後の色差ΔE*およびΔb*を算出した。ΔE*とΔb*は以下のように算出される。
ISO10477に従い、キセノンランプに色温度変換フィルタを挿入した光源を使用し、測定方法もISO規格に準じて実施した。スライドガラス上に試料を約30mg採取し、光源下でゲル化するまでの時間を測定した。
測定条件を以下に記載する。
1)試料間隙が0.500mmとなるように平板−平板治具をセットする。
2)測定環境を温度23℃、大気圧下とする。
3)開始応力は1.00E+1Paで測定する。
4)周波数10〜1[Hz]における粘度を測定する。
5)横軸に周波数、縦軸に粘度を対数プロットし、粘度の最大値を最小値で除したものをチクソトロピー比としその比が1.1以上でチクソトロピー性があると判定する。
Claims (4)
- 一般式(I)で表される重合性モノマーを、重合性モノマーの全量に対して5〜90重量部含むことを特徴とする請求項1記載の可視光重合性歯科用組成物。
- さらに、無機または有機フィラーの少なくとも1種類を0.1〜90重量部含有することを特徴とする請求項1または2記載の可視光重合性歯科用組成物。
- 23℃におけるプレートプレート系粘度測定(ギャップ0.500mm)、開始応力1.00E+1Paで測定した周波数掃引7〜1Hz時において2〜8Pa・Sの粘度、および、周波数7〜1Hzにおいて粘度の最小値と最大値の比が1.1以上のチクソトロピー性を有することを特徴とする請求項1〜3のいずれか1項に記載の可視光重合性歯科用組成物。
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2008052962A JP5203749B2 (ja) | 2008-03-04 | 2008-03-04 | 硬化前後における色調変化の少ない光重合性歯科用組成物 |
| CN2009101263450A CN101524314B (zh) | 2008-03-04 | 2009-03-03 | 固化前后色调很少变化的光聚合性牙科用组合物 |
| DE102009011536.6A DE102009011536B4 (de) | 2008-03-04 | 2009-03-03 | Photopolymerisierbare Dentalzusammensetzung mit weniger Änderung im Farbton vor und nach dem Härten |
| US12/379,926 US7872058B2 (en) | 2008-03-04 | 2009-03-04 | Photopolymerizable dental composition with less change in color tone before and after curing |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2008052962A JP5203749B2 (ja) | 2008-03-04 | 2008-03-04 | 硬化前後における色調変化の少ない光重合性歯科用組成物 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2009209078A JP2009209078A (ja) | 2009-09-17 |
| JP5203749B2 true JP5203749B2 (ja) | 2013-06-05 |
Family
ID=40936547
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008052962A Active JP5203749B2 (ja) | 2008-03-04 | 2008-03-04 | 硬化前後における色調変化の少ない光重合性歯科用組成物 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US7872058B2 (ja) |
| JP (1) | JP5203749B2 (ja) |
| CN (1) | CN101524314B (ja) |
| DE (1) | DE102009011536B4 (ja) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5702044B2 (ja) * | 2008-03-04 | 2015-04-15 | 株式会社松風 | モノマーによって色調変化を抑えた光硬化性歯科用組成物 |
| US8697769B2 (en) | 2010-09-30 | 2014-04-15 | Voco Gmbh | Lacquer composition comprising a monomer with a polyalicyclic structure element |
| DE102012212429A1 (de) | 2012-07-16 | 2014-01-16 | Voco Gmbh | Dentalhandgerät, Verfahren und Verwendung desselben zum Aushärten lichthärtbaren Materials |
| EP3801360A1 (en) * | 2018-06-06 | 2021-04-14 | 3M Innovative Properties Company | Hardenable dental compositions comprising basic core material encapsulated in an inorganic shell and dispensing devices therewith |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1408265A (en) | 1971-10-18 | 1975-10-01 | Ici Ltd | Photopolymerisable composition |
| DE2909994A1 (de) | 1979-03-14 | 1980-10-02 | Basf Ag | Acylphosphinoxidverbindungen, ihre herstellung und verwendung |
| US4265723A (en) | 1979-07-06 | 1981-05-05 | Basf Aktiengesellschaft | Photocurable molding, impregnating and coating compositions |
| DE3443221A1 (de) | 1984-11-27 | 1986-06-05 | ESPE Fabrik pharmazeutischer Präparate GmbH, 8031 Seefeld | Bisacylphosphinoxide, ihre herstellung und verwendung |
| JP2629060B2 (ja) * | 1990-05-25 | 1997-07-09 | 株式会社クラレ | 光重合性歯科用表面被覆材 |
| FR2664801B1 (fr) | 1990-07-20 | 1994-09-30 | Duret M & Fils | Garniture souple pour sieges, procede de fabrication d'une telle garniture et son utilisation pour le garnissage d'une ossature de siege. |
| DE4137076A1 (de) | 1991-11-12 | 1993-05-13 | Ivoclar Ag | Dentalklebstoff |
| EP0627476B1 (en) | 1992-12-21 | 1999-08-04 | Adell Co., Ltd. | Photopolymerization initiator composition for visible ray polymerizable adhesive |
| US5663264A (en) * | 1993-12-27 | 1997-09-02 | Hitachi Chemical Company, Ltd. | Transparent resin and plastic lens |
| TW381106B (en) * | 1994-09-02 | 2000-02-01 | Ciba Sc Holding Ag | Alkoxyphenyl-substituted bisacylphosphine oxides |
| EP0789721B1 (en) | 1994-10-31 | 2001-02-28 | Minnesota Mining And Manufacturing Company | Visible-light curable epoxy system with enhanced depth of cure |
| JP4204098B2 (ja) | 1998-06-26 | 2009-01-07 | 株式会社トクヤマ | 光硬化性歯科用修復材料 |
| JP4427133B2 (ja) * | 1998-07-03 | 2010-03-03 | サンメディカル株式会社 | 歯科接着性組成物 |
| US6730715B2 (en) | 2001-07-06 | 2004-05-04 | Pentron Clinical Technologies, Llc | Dental restorative composition, dental restoration, and a method of use thereof |
| JP4615260B2 (ja) * | 2004-06-23 | 2011-01-19 | クラレメディカル株式会社 | 小窩裂溝填塞用キット |
| JP2009507041A (ja) | 2005-09-02 | 2009-02-19 | デンツプライ インターナショナル インコーポレーテッド | シーラント用のナノメートルサイズのシリカ粒子を含む歯科用組成物 |
| DE102007035735A1 (de) | 2006-08-16 | 2008-02-21 | Ivoclar Vivadent Ag | Pastenförmige, polymerisierbare Dentalmassen und Verfahren zu deren Herstellung |
-
2008
- 2008-03-04 JP JP2008052962A patent/JP5203749B2/ja active Active
-
2009
- 2009-03-03 CN CN2009101263450A patent/CN101524314B/zh active Active
- 2009-03-03 DE DE102009011536.6A patent/DE102009011536B4/de active Active
- 2009-03-04 US US12/379,926 patent/US7872058B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CN101524314B (zh) | 2013-03-13 |
| DE102009011536A1 (de) | 2009-09-10 |
| DE102009011536B4 (de) | 2019-03-21 |
| JP2009209078A (ja) | 2009-09-17 |
| US7872058B2 (en) | 2011-01-18 |
| CN101524314A (zh) | 2009-09-09 |
| US20090227700A1 (en) | 2009-09-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7084182B2 (en) | Photopolymerization initiator | |
| CN102037024B (zh) | 光聚合性组合物 | |
| JP6517226B2 (ja) | 歯科用組成物 | |
| AU2016335612A1 (en) | Dental composition | |
| JP5268478B2 (ja) | 歯科用硬化性材料キット | |
| CN113993497B (zh) | 固化性组合物和包含其的光造形用树脂组合物 | |
| WO2018074380A1 (ja) | 光学的立体造形用組成物 | |
| JP5203749B2 (ja) | 硬化前後における色調変化の少ない光重合性歯科用組成物 | |
| KR102572754B1 (ko) | 치과용 조성물 | |
| WO2007105296A1 (ja) | アシルフォスフィンオキサイド基を有するカンファーキノン誘導体、それを含有する光重合触媒および光・化学重合触媒ならびにそれらを含有する硬化性組成物 | |
| JP5702044B2 (ja) | モノマーによって色調変化を抑えた光硬化性歯科用組成物 | |
| JPH06345614A (ja) | 歯科用充填修復材料及び義歯床用樹脂組成物 | |
| JP4204098B2 (ja) | 光硬化性歯科用修復材料 | |
| JP5478860B2 (ja) | 光重合開始剤及び該光重合開始剤を含む歯科用コンポジットレジン | |
| KR20220029592A (ko) | 경화성 조성물 및 그것으로 이루어지는 광 조형용 수지 조성물 | |
| JP4712532B2 (ja) | 可視光線重合開始剤 | |
| JP5882082B2 (ja) | 光重合性組成物 | |
| JP2704967B2 (ja) | 歯科用光硬化性修復材料 | |
| US20070015845A1 (en) | Dental resin composition, method of manufacture, and method of use thereof | |
| JP5305617B2 (ja) | 光重合性組成物 | |
| JP2019069916A (ja) | 歯科補綴物 | |
| JP2022033644A (ja) | 歯科用硬化性組成物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100915 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20110927 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20121204 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130121 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20130205 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20130214 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 Ref document number: 5203749 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20160222 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |