KR102547232B1 - 올레핀 중합용 촉매의 제조방법 - Google Patents
올레핀 중합용 촉매의 제조방법 Download PDFInfo
- Publication number
- KR102547232B1 KR102547232B1 KR1020190132618A KR20190132618A KR102547232B1 KR 102547232 B1 KR102547232 B1 KR 102547232B1 KR 1020190132618 A KR1020190132618 A KR 1020190132618A KR 20190132618 A KR20190132618 A KR 20190132618A KR 102547232 B1 KR102547232 B1 KR 102547232B1
- Authority
- KR
- South Korea
- Prior art keywords
- aluminum
- group
- transition metal
- carbon atoms
- metal compound
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000003054 catalyst Substances 0.000 title claims abstract description 67
- 150000001336 alkenes Chemical class 0.000 title claims abstract description 47
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 title claims abstract description 45
- 238000004519 manufacturing process Methods 0.000 title claims abstract description 11
- 230000000379 polymerizing effect Effects 0.000 title description 2
- 238000000034 method Methods 0.000 claims abstract description 38
- 238000006116 polymerization reaction Methods 0.000 claims abstract description 35
- 239000012968 metallocene catalyst Substances 0.000 claims abstract description 31
- 229920000098 polyolefin Polymers 0.000 claims abstract description 12
- 230000015572 biosynthetic process Effects 0.000 claims abstract description 9
- 150000003623 transition metal compounds Chemical class 0.000 claims description 171
- CPOFMOWDMVWCLF-UHFFFAOYSA-N methyl(oxo)alumane Chemical compound C[Al]=O CPOFMOWDMVWCLF-UHFFFAOYSA-N 0.000 claims description 112
- 150000001875 compounds Chemical class 0.000 claims description 102
- 125000004432 carbon atom Chemical group C* 0.000 claims description 96
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 49
- -1 tetramethylcyclopentadienyl Chemical group 0.000 claims description 44
- 229910052782 aluminium Inorganic materials 0.000 claims description 41
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 41
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 claims description 21
- 229910052796 boron Inorganic materials 0.000 claims description 21
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 claims description 21
- 239000000377 silicon dioxide Substances 0.000 claims description 19
- YBYIRNPNPLQARY-UHFFFAOYSA-N 1H-indene Natural products C1=CC=C2CC=CC2=C1 YBYIRNPNPLQARY-UHFFFAOYSA-N 0.000 claims description 18
- 125000003454 indenyl group Chemical group C1(C=CC2=CC=CC=C12)* 0.000 claims description 18
- 125000003118 aryl group Chemical group 0.000 claims description 16
- 150000002430 hydrocarbons Chemical group 0.000 claims description 16
- 125000000217 alkyl group Chemical group 0.000 claims description 15
- ZSWFCLXCOIISFI-UHFFFAOYSA-N cyclopentadiene Chemical group C1C=CC=C1 ZSWFCLXCOIISFI-UHFFFAOYSA-N 0.000 claims description 15
- IMFACGCPASFAPR-UHFFFAOYSA-O tributylazanium Chemical compound CCCC[NH+](CCCC)CCCC IMFACGCPASFAPR-UHFFFAOYSA-O 0.000 claims description 14
- 125000003342 alkenyl group Chemical group 0.000 claims description 13
- 125000002877 alkyl aryl group Chemical group 0.000 claims description 13
- 125000003710 aryl alkyl group Chemical group 0.000 claims description 13
- YFTHZRPMJXBUME-UHFFFAOYSA-N tripropylamine Chemical compound CCCN(CCC)CCC YFTHZRPMJXBUME-UHFFFAOYSA-N 0.000 claims description 12
- 125000005843 halogen group Chemical group 0.000 claims description 11
- 239000002002 slurry Substances 0.000 claims description 11
- ZMANZCXQSJIPKH-UHFFFAOYSA-O triethylammonium ion Chemical compound CC[NH+](CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-O 0.000 claims description 10
- DXQXWMYUGOTNGJ-UHFFFAOYSA-N [4-(trifluoromethyl)phenyl]boron Chemical compound [B]C1=CC=C(C(F)(F)F)C=C1 DXQXWMYUGOTNGJ-UHFFFAOYSA-N 0.000 claims description 8
- 229910052736 halogen Inorganic materials 0.000 claims description 8
- CPLXHLVBOLITMK-UHFFFAOYSA-N Magnesium oxide Chemical compound [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 claims description 7
- 150000002367 halogens Chemical class 0.000 claims description 7
- 229910052723 transition metal Inorganic materials 0.000 claims description 7
- 150000003624 transition metals Chemical class 0.000 claims description 7
- KRQUEZVOFNMNFD-UHFFFAOYSA-L Cl[Zr](Cl)C1C=CC=C1C1C=CC2=CC=CC=C12 Chemical compound Cl[Zr](Cl)C1C=CC=C1C1C=CC2=CC=CC=C12 KRQUEZVOFNMNFD-UHFFFAOYSA-L 0.000 claims description 6
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 6
- MCULRUJILOGHCJ-UHFFFAOYSA-N triisobutylaluminium Chemical compound CC(C)C[Al](CC(C)C)CC(C)C MCULRUJILOGHCJ-UHFFFAOYSA-N 0.000 claims description 6
- YWWDBCBWQNCYNR-UHFFFAOYSA-O trimethylphosphanium Chemical compound C[PH+](C)C YWWDBCBWQNCYNR-UHFFFAOYSA-O 0.000 claims description 6
- RIOQSEWOXXDEQQ-UHFFFAOYSA-O triphenylphosphanium Chemical compound C1=CC=CC=C1[PH+](C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-O 0.000 claims description 6
- VPGLGRNSAYHXPY-UHFFFAOYSA-L zirconium(2+);dichloride Chemical compound Cl[Zr]Cl VPGLGRNSAYHXPY-UHFFFAOYSA-L 0.000 claims description 6
- NDJMNNSJDIFFTH-UHFFFAOYSA-L [Cl-].[Cl-].CC1=CC(C(=CC=C2)C=3C=CC=CC=3)=C2C1[Zr+2]([SiH](C)C)C1C(C)=CC2=C1C=CC=C2C1=CC=CC=C1 Chemical compound [Cl-].[Cl-].CC1=CC(C(=CC=C2)C=3C=CC=CC=3)=C2C1[Zr+2]([SiH](C)C)C1C(C)=CC2=C1C=CC=C2C1=CC=CC=C1 NDJMNNSJDIFFTH-UHFFFAOYSA-L 0.000 claims description 5
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 5
- 230000002441 reversible effect Effects 0.000 claims description 5
- VOITXYVAKOUIBA-UHFFFAOYSA-N triethylaluminium Chemical compound CC[Al](CC)CC VOITXYVAKOUIBA-UHFFFAOYSA-N 0.000 claims description 5
- 125000000304 alkynyl group Chemical group 0.000 claims description 4
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 claims description 4
- GGSUCNLOZRCGPQ-UHFFFAOYSA-O diethyl(phenyl)azanium Chemical compound CC[NH+](CC)C1=CC=CC=C1 GGSUCNLOZRCGPQ-UHFFFAOYSA-O 0.000 claims description 4
- 239000000395 magnesium oxide Substances 0.000 claims description 4
- 230000000737 periodic effect Effects 0.000 claims description 4
- 238000002360 preparation method Methods 0.000 claims description 4
- JLTRXTDYQLMHGR-UHFFFAOYSA-N trimethylaluminium Chemical compound C[Al](C)C JLTRXTDYQLMHGR-UHFFFAOYSA-N 0.000 claims description 4
- WCFQIFDACWBNJT-UHFFFAOYSA-N $l^{1}-alumanyloxy(2-methylpropyl)aluminum Chemical compound CC(C)C[Al]O[Al] WCFQIFDACWBNJT-UHFFFAOYSA-N 0.000 claims description 3
- YVSMQHYREUQGRX-UHFFFAOYSA-N 2-ethyloxaluminane Chemical compound CC[Al]1CCCCO1 YVSMQHYREUQGRX-UHFFFAOYSA-N 0.000 claims description 3
- 239000007848 Bronsted acid Substances 0.000 claims description 3
- PLGVIJOQDDMWAO-UHFFFAOYSA-N CCCCN(CCCC)CCCC.FC(C(F)=C(C([Al+2])=C1F)F)=C1F.FC(C(F)=C(C([Al+2])=C1F)F)=C1F.FC(C(F)=C(C([Al+2])=C1F)F)=C1F.FC(C(F)=C(C([Al+2])=C1F)F)=C1F Chemical compound CCCCN(CCCC)CCCC.FC(C(F)=C(C([Al+2])=C1F)F)=C1F.FC(C(F)=C(C([Al+2])=C1F)F)=C1F.FC(C(F)=C(C([Al+2])=C1F)F)=C1F.FC(C(F)=C(C([Al+2])=C1F)F)=C1F PLGVIJOQDDMWAO-UHFFFAOYSA-N 0.000 claims description 3
- JEVCOCKVSCRHMR-UHFFFAOYSA-N CCN(CC)C1=CC=CC=C1.FC(C(F)=C(C([Al+2])=C1F)F)=C1F.FC(C(F)=C(C([Al+2])=C1F)F)=C1F.FC(C(F)=C(C([Al+2])=C1F)F)=C1F.FC(C(F)=C(C([Al+2])=C1F)F)=C1F Chemical compound CCN(CC)C1=CC=CC=C1.FC(C(F)=C(C([Al+2])=C1F)F)=C1F.FC(C(F)=C(C([Al+2])=C1F)F)=C1F.FC(C(F)=C(C([Al+2])=C1F)F)=C1F.FC(C(F)=C(C([Al+2])=C1F)F)=C1F JEVCOCKVSCRHMR-UHFFFAOYSA-N 0.000 claims description 3
- 239000002879 Lewis base Substances 0.000 claims description 3
- SHPVKUQHCZKKRP-UHFFFAOYSA-N [B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.CCCCN(CCCC)CCCC Chemical compound [B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.CCCCN(CCCC)CCCC SHPVKUQHCZKKRP-UHFFFAOYSA-N 0.000 claims description 3
- RPXNIXOOFOQCKJ-UHFFFAOYSA-N [B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.CCNCC Chemical compound [B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.CCNCC RPXNIXOOFOQCKJ-UHFFFAOYSA-N 0.000 claims description 3
- DNFMTFCPAWFGPK-UHFFFAOYSA-L [Cl-].[Cl-].CC1=C2C=CC(C2=CC=C1)C1(C=CC=C1)[Zr+2] Chemical compound [Cl-].[Cl-].CC1=C2C=CC(C2=CC=C1)C1(C=CC=C1)[Zr+2] DNFMTFCPAWFGPK-UHFFFAOYSA-L 0.000 claims description 3
- NSDVYNWCHDHIEY-UHFFFAOYSA-L [Cl-].[Cl-].CC=1C(C2=C3C(=CC=C2C=1)C=CC=C3)C1(C=CC=C1)[Zr+2] Chemical compound [Cl-].[Cl-].CC=1C(C2=C3C(=CC=C2C=1)C=CC=C3)C1(C=CC=C1)[Zr+2] NSDVYNWCHDHIEY-UHFFFAOYSA-L 0.000 claims description 3
- VJIMPANCYFQSPY-UHFFFAOYSA-L [Cl-].[Cl-].CC=1C(C2=CC=CC=C2C=1)C1=C(C(=C(C1(C)[Zr+2])C)C)C Chemical compound [Cl-].[Cl-].CC=1C(C2=CC=CC=C2C=1)C1=C(C(=C(C1(C)[Zr+2])C)C)C VJIMPANCYFQSPY-UHFFFAOYSA-L 0.000 claims description 3
- 229910052795 boron group element Inorganic materials 0.000 claims description 3
- MYBJXSAXGLILJD-UHFFFAOYSA-N diethyl(methyl)alumane Chemical compound CC[Al](C)CC MYBJXSAXGLILJD-UHFFFAOYSA-N 0.000 claims description 3
- JGHYBJVUQGTEEB-UHFFFAOYSA-M dimethylalumanylium;chloride Chemical compound C[Al](C)Cl JGHYBJVUQGTEEB-UHFFFAOYSA-M 0.000 claims description 3
- SHGOGDWTZKFNSC-UHFFFAOYSA-N ethyl(dimethyl)alumane Chemical compound CC[Al](C)C SHGOGDWTZKFNSC-UHFFFAOYSA-N 0.000 claims description 3
- BQBCXNQILNPAPX-UHFFFAOYSA-N methoxy(dimethyl)alumane Chemical compound [O-]C.C[Al+]C BQBCXNQILNPAPX-UHFFFAOYSA-N 0.000 claims description 3
- 230000007935 neutral effect Effects 0.000 claims description 3
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 3
- NDUUEFPGQBSFPV-UHFFFAOYSA-N tri(butan-2-yl)alumane Chemical compound CCC(C)[Al](C(C)CC)C(C)CC NDUUEFPGQBSFPV-UHFFFAOYSA-N 0.000 claims description 3
- SQBBHCOIQXKPHL-UHFFFAOYSA-N tributylalumane Chemical compound CCCC[Al](CCCC)CCCC SQBBHCOIQXKPHL-UHFFFAOYSA-N 0.000 claims description 3
- CMHHITPYCHHOGT-UHFFFAOYSA-N tributylborane Chemical compound CCCCB(CCCC)CCCC CMHHITPYCHHOGT-UHFFFAOYSA-N 0.000 claims description 3
- LALRXNPLTWZJIJ-UHFFFAOYSA-N triethylborane Chemical compound CCB(CC)CC LALRXNPLTWZJIJ-UHFFFAOYSA-N 0.000 claims description 3
- ORYGRKHDLWYTKX-UHFFFAOYSA-N trihexylalumane Chemical compound CCCCCC[Al](CCCCCC)CCCCCC ORYGRKHDLWYTKX-UHFFFAOYSA-N 0.000 claims description 3
- WXRGABKACDFXMG-UHFFFAOYSA-N trimethylborane Chemical compound CB(C)C WXRGABKACDFXMG-UHFFFAOYSA-N 0.000 claims description 3
- LFXVBWRMVZPLFK-UHFFFAOYSA-N trioctylalumane Chemical compound CCCCCCCC[Al](CCCCCCCC)CCCCCCCC LFXVBWRMVZPLFK-UHFFFAOYSA-N 0.000 claims description 3
- JOJQVUCWSDRWJE-UHFFFAOYSA-N tripentylalumane Chemical compound CCCCC[Al](CCCCC)CCCCC JOJQVUCWSDRWJE-UHFFFAOYSA-N 0.000 claims description 3
- JQPMDTQDAXRDGS-UHFFFAOYSA-N triphenylalumane Chemical compound C1=CC=CC=C1[Al](C=1C=CC=CC=1)C1=CC=CC=C1 JQPMDTQDAXRDGS-UHFFFAOYSA-N 0.000 claims description 3
- CNWZYDSEVLFSMS-UHFFFAOYSA-N tripropylalumane Chemical compound CCC[Al](CCC)CCC CNWZYDSEVLFSMS-UHFFFAOYSA-N 0.000 claims description 3
- ZMPKTELQGVLZTD-UHFFFAOYSA-N tripropylborane Chemical compound CCCB(CCC)CCC ZMPKTELQGVLZTD-UHFFFAOYSA-N 0.000 claims description 3
- XDSSGQHOYWGIKC-UHFFFAOYSA-N tris(2-methylpropyl)borane Chemical compound CC(C)CB(CC(C)C)CC(C)C XDSSGQHOYWGIKC-UHFFFAOYSA-N 0.000 claims description 3
- WSITXTIRYQMZHM-UHFFFAOYSA-N tris(4-methylphenyl)alumane Chemical compound C1=CC(C)=CC=C1[Al](C=1C=CC(C)=CC=1)C1=CC=C(C)C=C1 WSITXTIRYQMZHM-UHFFFAOYSA-N 0.000 claims description 3
- VKMQKNJWQNCEQV-UHFFFAOYSA-N (4-methylphenyl)boron Chemical compound [B]C1=CC=C(C)C=C1 VKMQKNJWQNCEQV-UHFFFAOYSA-N 0.000 claims description 2
- GXZJUGGTMPCASB-UHFFFAOYSA-L [Cl-].[Cl-].C1(C=CC2=CC=CC=C12)C1(C(=C(C(=C1C)C)C)C)[Zr+2] Chemical compound [Cl-].[Cl-].C1(C=CC2=CC=CC=C12)C1(C(=C(C(=C1C)C)C)C)[Zr+2] GXZJUGGTMPCASB-UHFFFAOYSA-L 0.000 claims description 2
- 125000003545 alkoxy group Chemical group 0.000 claims description 2
- 125000003837 (C1-C20) alkyl group Chemical group 0.000 claims 1
- XIBZTAIPROXEDH-UHFFFAOYSA-N [B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.CCN(CC)C1=CC=CC=C1 Chemical compound [B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.[B+2]C(C(F)=C(C(F)=C1F)F)=C1F.CCN(CC)C1=CC=CC=C1 XIBZTAIPROXEDH-UHFFFAOYSA-N 0.000 claims 1
- QGHZYTQKNCPPTK-UHFFFAOYSA-N cyclopentylalumane Chemical compound C1(CCCC1)[AlH2] QGHZYTQKNCPPTK-UHFFFAOYSA-N 0.000 claims 1
- HPNMFZURTQLUMO-UHFFFAOYSA-O diethylammonium Chemical compound CC[NH2+]CC HPNMFZURTQLUMO-UHFFFAOYSA-O 0.000 claims 1
- 239000000499 gel Substances 0.000 description 41
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 30
- 230000000052 comparative effect Effects 0.000 description 30
- 239000000203 mixture Substances 0.000 description 24
- 239000002904 solvent Substances 0.000 description 21
- 229910004298 SiO 2 Inorganic materials 0.000 description 15
- LIKMAJRDDDTEIG-UHFFFAOYSA-N 1-hexene Chemical compound CCCCC=C LIKMAJRDDDTEIG-UHFFFAOYSA-N 0.000 description 14
- 229920000642 polymer Polymers 0.000 description 14
- 238000002156 mixing Methods 0.000 description 12
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 9
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 8
- 239000005977 Ethylene Substances 0.000 description 8
- 238000001035 drying Methods 0.000 description 7
- 239000000178 monomer Substances 0.000 description 7
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 6
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 6
- 229920001577 copolymer Polymers 0.000 description 6
- 239000000243 solution Substances 0.000 description 6
- 238000003756 stirring Methods 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 5
- 229920005989 resin Polymers 0.000 description 5
- 239000011347 resin Substances 0.000 description 5
- 239000004215 Carbon black (E152) Substances 0.000 description 4
- OFBQJSOFQDEBGM-UHFFFAOYSA-N Pentane Chemical compound CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 4
- 239000000460 chlorine Substances 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 229930195733 hydrocarbon Natural products 0.000 description 4
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 4
- 238000000465 moulding Methods 0.000 description 4
- 239000012299 nitrogen atmosphere Substances 0.000 description 4
- VSZWPYCFIRKVQL-UHFFFAOYSA-N selanylidenegallium;selenium Chemical compound [Se].[Se]=[Ga].[Se]=[Ga] VSZWPYCFIRKVQL-UHFFFAOYSA-N 0.000 description 4
- 239000010936 titanium Substances 0.000 description 4
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- 229910052801 chlorine Inorganic materials 0.000 description 3
- 238000001125 extrusion Methods 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 238000011068 loading method Methods 0.000 description 3
- 229920002521 macromolecule Polymers 0.000 description 3
- 239000011148 porous material Substances 0.000 description 3
- 239000004711 α-olefin Substances 0.000 description 3
- GWUXLTRGPPIDJA-UHFFFAOYSA-N (4-methylphenyl)alumane Chemical compound CC1=CC=C([AlH2])C=C1 GWUXLTRGPPIDJA-UHFFFAOYSA-N 0.000 description 2
- ZGEGCLOFRBLKSE-UHFFFAOYSA-N 1-Heptene Chemical compound CCCCCC=C ZGEGCLOFRBLKSE-UHFFFAOYSA-N 0.000 description 2
- AFFLGGQVNFXPEV-UHFFFAOYSA-N 1-decene Chemical compound CCCCCCCCC=C AFFLGGQVNFXPEV-UHFFFAOYSA-N 0.000 description 2
- GQEZCXVZFLOKMC-UHFFFAOYSA-N 1-hexadecene Chemical compound CCCCCCCCCCCCCCC=C GQEZCXVZFLOKMC-UHFFFAOYSA-N 0.000 description 2
- KWKAKUADMBZCLK-UHFFFAOYSA-N 1-octene Chemical compound CCCCCCC=C KWKAKUADMBZCLK-UHFFFAOYSA-N 0.000 description 2
- HFDVRLIODXPAHB-UHFFFAOYSA-N 1-tetradecene Chemical compound CCCCCCCCCCCCC=C HFDVRLIODXPAHB-UHFFFAOYSA-N 0.000 description 2
- DCTOHCCUXLBQMS-UHFFFAOYSA-N 1-undecene Chemical compound CCCCCCCCCC=C DCTOHCCUXLBQMS-UHFFFAOYSA-N 0.000 description 2
- WSSSPWUEQFSQQG-UHFFFAOYSA-N 4-methyl-1-pentene Chemical compound CC(C)CC=C WSSSPWUEQFSQQG-UHFFFAOYSA-N 0.000 description 2
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 2
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 2
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 2
- 239000004698 Polyethylene Substances 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 2
- XFNJQHPCDTWSNM-UHFFFAOYSA-L [Cl-].[Cl-].C[SiH](C)[Zr+2](C1C(=CC2=C(C=CC=C12)C1=CC=C(C=C1)C(C)(C)C)C)C1(C(=C(C(=C1)C)C)C)C Chemical compound [Cl-].[Cl-].C[SiH](C)[Zr+2](C1C(=CC2=C(C=CC=C12)C1=CC=C(C=C1)C(C)(C)C)C)C1(C(=C(C(=C1)C)C)C)C XFNJQHPCDTWSNM-UHFFFAOYSA-L 0.000 description 2
- XOMCHMPRFJYGBF-UHFFFAOYSA-L [Cl-].[Cl-].C[SiH](C)[Zr+2](C1C(=CC2=C(C=CC=C12)C1=CC=CC=C1)C)C1(C(=C(C(=C1)C)C)C)C Chemical compound [Cl-].[Cl-].C[SiH](C)[Zr+2](C1C(=CC2=C(C=CC=C12)C1=CC=CC=C1)C)C1(C(=C(C(=C1)C)C)C)C XOMCHMPRFJYGBF-UHFFFAOYSA-L 0.000 description 2
- 150000001338 aliphatic hydrocarbons Chemical group 0.000 description 2
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 2
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 125000001309 chloro group Chemical group Cl* 0.000 description 2
- MVPPADPHJFYWMZ-UHFFFAOYSA-N chlorobenzene Chemical compound ClC1=CC=CC=C1 MVPPADPHJFYWMZ-UHFFFAOYSA-N 0.000 description 2
- 229910052681 coesite Inorganic materials 0.000 description 2
- 229910052906 cristobalite Inorganic materials 0.000 description 2
- 238000004132 cross linking Methods 0.000 description 2
- DIOQZVSQGTUSAI-UHFFFAOYSA-N decane Chemical compound CCCCCCCCCC DIOQZVSQGTUSAI-UHFFFAOYSA-N 0.000 description 2
- MWNKMBHGMZHEMM-UHFFFAOYSA-N dimethylalumanylium;ethanolate Chemical compound CCO[Al](C)C MWNKMBHGMZHEMM-UHFFFAOYSA-N 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- QUSNBJAOOMFDIB-UHFFFAOYSA-O ethylaminium Chemical compound CC[NH3+] QUSNBJAOOMFDIB-UHFFFAOYSA-O 0.000 description 2
- 238000010528 free radical solution polymerization reaction Methods 0.000 description 2
- 229910052735 hafnium Inorganic materials 0.000 description 2
- VBJZVLUMGGDVMO-UHFFFAOYSA-N hafnium atom Chemical compound [Hf] VBJZVLUMGGDVMO-UHFFFAOYSA-N 0.000 description 2
- 229920001519 homopolymer Polymers 0.000 description 2
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- YWAKXRMUMFPDSH-UHFFFAOYSA-N pentene Chemical compound CCCC=C YWAKXRMUMFPDSH-UHFFFAOYSA-N 0.000 description 2
- 229920000573 polyethylene Polymers 0.000 description 2
- 229920005672 polyolefin resin Polymers 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 235000012239 silicon dioxide Nutrition 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 229910052682 stishovite Inorganic materials 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 229910052719 titanium Inorganic materials 0.000 description 2
- PYLGJXLKFZZEBJ-UHFFFAOYSA-N tricyclopentylalumane Chemical compound C1CCCC1[Al](C1CCCC1)C1CCCC1 PYLGJXLKFZZEBJ-UHFFFAOYSA-N 0.000 description 2
- 229910052905 tridymite Inorganic materials 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- PMJHHCWVYXUKFD-SNAWJCMRSA-N (E)-1,3-pentadiene Chemical compound C\C=C\C=C PMJHHCWVYXUKFD-SNAWJCMRSA-N 0.000 description 1
- CRSBERNSMYQZNG-UHFFFAOYSA-N 1 -dodecene Natural products CCCCCCCCCCC=C CRSBERNSMYQZNG-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 125000003860 C1-C20 alkoxy group Chemical group 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- SNRUBQQJIBEYMU-UHFFFAOYSA-N Dodecane Natural products CCCCCCCCCCCC SNRUBQQJIBEYMU-UHFFFAOYSA-N 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 150000001639 boron compounds Chemical class 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 238000005266 casting Methods 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 150000001925 cycloalkenes Chemical class 0.000 description 1
- 125000000058 cyclopentadienyl group Chemical group C1(=CC=CC1)* 0.000 description 1
- 150000001993 dienes Chemical class 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 229940069096 dodecene Drugs 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 238000012685 gas phase polymerization Methods 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 150000002469 indenes Chemical class 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910001629 magnesium chloride Inorganic materials 0.000 description 1
- 239000011259 mixed solution Substances 0.000 description 1
- TVMXDCGIABBOFY-UHFFFAOYSA-N n-Octanol Natural products CCCCCCCC TVMXDCGIABBOFY-UHFFFAOYSA-N 0.000 description 1
- ZCYXXKJEDCHMGH-UHFFFAOYSA-N nonane Chemical compound CCCC[CH]CCCC ZCYXXKJEDCHMGH-UHFFFAOYSA-N 0.000 description 1
- BKIMMITUMNQMOS-UHFFFAOYSA-N normal nonane Natural products CCCCCCCCC BKIMMITUMNQMOS-UHFFFAOYSA-N 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 125000000538 pentafluorophenyl group Chemical group FC1=C(F)C(F)=C(*)C(F)=C1F 0.000 description 1
- PMJHHCWVYXUKFD-UHFFFAOYSA-N piperylene Natural products CC=CC=C PMJHHCWVYXUKFD-UHFFFAOYSA-N 0.000 description 1
- 229920006254 polymer film Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 239000002516 radical scavenger Substances 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 239000004460 silage Substances 0.000 description 1
- 125000005373 siloxane group Chemical group [SiH2](O*)* 0.000 description 1
- 229920006302 stretch film Polymers 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 238000009827 uniform distribution Methods 0.000 description 1
- 239000010457 zeolite Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F4/00—Polymerisation catalysts
- C08F4/42—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors
- C08F4/44—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides
- C08F4/60—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides together with refractory metals, iron group metals, platinum group metals, manganese, rhenium technetium or compounds thereof
- C08F4/62—Refractory metals or compounds thereof
- C08F4/64—Titanium, zirconium, hafnium or compounds thereof
- C08F4/659—Component covered by group C08F4/64 containing a transition metal-carbon bond
- C08F4/65904—Component covered by group C08F4/64 containing a transition metal-carbon bond in combination with another component of C08F4/64
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F4/00—Polymerisation catalysts
- C08F4/02—Carriers therefor
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F4/00—Polymerisation catalysts
- C08F4/02—Carriers therefor
- C08F4/025—Metal oxides
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F4/00—Polymerisation catalysts
- C08F4/42—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors
- C08F4/44—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides
- C08F4/60—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides together with refractory metals, iron group metals, platinum group metals, manganese, rhenium technetium or compounds thereof
- C08F4/62—Refractory metals or compounds thereof
- C08F4/64—Titanium, zirconium, hafnium or compounds thereof
- C08F4/659—Component covered by group C08F4/64 containing a transition metal-carbon bond
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F4/00—Polymerisation catalysts
- C08F4/42—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors
- C08F4/44—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides
- C08F4/60—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides together with refractory metals, iron group metals, platinum group metals, manganese, rhenium technetium or compounds thereof
- C08F4/62—Refractory metals or compounds thereof
- C08F4/64—Titanium, zirconium, hafnium or compounds thereof
- C08F4/659—Component covered by group C08F4/64 containing a transition metal-carbon bond
- C08F4/65908—Component covered by group C08F4/64 containing a transition metal-carbon bond in combination with an ionising compound other than alumoxane, e.g. (C6F5)4B-X+
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F4/00—Polymerisation catalysts
- C08F4/42—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors
- C08F4/44—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides
- C08F4/60—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides together with refractory metals, iron group metals, platinum group metals, manganese, rhenium technetium or compounds thereof
- C08F4/62—Refractory metals or compounds thereof
- C08F4/64—Titanium, zirconium, hafnium or compounds thereof
- C08F4/659—Component covered by group C08F4/64 containing a transition metal-carbon bond
- C08F4/65912—Component covered by group C08F4/64 containing a transition metal-carbon bond in combination with an organoaluminium compound
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F4/00—Polymerisation catalysts
- C08F4/42—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors
- C08F4/44—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides
- C08F4/60—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides together with refractory metals, iron group metals, platinum group metals, manganese, rhenium technetium or compounds thereof
- C08F4/62—Refractory metals or compounds thereof
- C08F4/64—Titanium, zirconium, hafnium or compounds thereof
- C08F4/659—Component covered by group C08F4/64 containing a transition metal-carbon bond
- C08F4/65916—Component covered by group C08F4/64 containing a transition metal-carbon bond supported on a carrier, e.g. silica, MgCl2, polymer
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F4/00—Polymerisation catalysts
- C08F4/42—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors
- C08F4/44—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides
- C08F4/60—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides together with refractory metals, iron group metals, platinum group metals, manganese, rhenium technetium or compounds thereof
- C08F4/62—Refractory metals or compounds thereof
- C08F4/64—Titanium, zirconium, hafnium or compounds thereof
- C08F4/659—Component covered by group C08F4/64 containing a transition metal-carbon bond
- C08F4/6592—Component covered by group C08F4/64 containing a transition metal-carbon bond containing at least one cyclopentadienyl ring, condensed or not, e.g. an indenyl or a fluorenyl ring
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F4/00—Polymerisation catalysts
- C08F4/42—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors
- C08F4/44—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides
- C08F4/60—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides together with refractory metals, iron group metals, platinum group metals, manganese, rhenium technetium or compounds thereof
- C08F4/62—Refractory metals or compounds thereof
- C08F4/64—Titanium, zirconium, hafnium or compounds thereof
- C08F4/659—Component covered by group C08F4/64 containing a transition metal-carbon bond
- C08F4/6592—Component covered by group C08F4/64 containing a transition metal-carbon bond containing at least one cyclopentadienyl ring, condensed or not, e.g. an indenyl or a fluorenyl ring
- C08F4/65922—Component covered by group C08F4/64 containing a transition metal-carbon bond containing at least one cyclopentadienyl ring, condensed or not, e.g. an indenyl or a fluorenyl ring containing at least two cyclopentadienyl rings, fused or not
- C08F4/65927—Component covered by group C08F4/64 containing a transition metal-carbon bond containing at least one cyclopentadienyl ring, condensed or not, e.g. an indenyl or a fluorenyl ring containing at least two cyclopentadienyl rings, fused or not two cyclopentadienyl rings being mutually bridged
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Crystallography & Structural Chemistry (AREA)
- Transition And Organic Metals Composition Catalysts For Addition Polymerization (AREA)
Abstract
Description
| MAO 함량(중량%) | 담지 순서 | ||
| MAO1 | MAO2 | ||
| 대조예 | 7.5 | 5 | (M1+M2+MAO1+MAO2)/SiO2 |
| 실시예 1 | 7.5 | 5 | (M1+MAO1)/SiO2/(M2+MAO2) |
| 실시예 2 | 7.5 | 5 | (M1+MAO1)/SiO2/MAO2/M2 |
| 실시예 3 | 7.5 | 5 | (M1+MAO1)/SiO2/M2/MAO2 |
| 실시예 4 | 7.5 | 5 | (M1+MAO1)/(SiO2+MAO2)/M2 |
| 실시예 5 | 7.5 | 5 | (SiO2+MAO1)/M1/(M2+MAO2) |
| 실시예 6 | 7.5 | 5 | (SiO2+MAO1)/M1/MAO2/M2 |
| 실시예 7 | 7.5 | 5 | (SiO2+MAO1)/(M1+MAO2)/M2 |
| 실시예 8 | 7.5 | 5 | (SiO2+MAO1)/M1/M2/MAO2 |
| 비교예 1 | 7.5 | 5 | (M2+MAO2)/SiO2/(M1+MAO1) |
| 비교예 2 | 7.5 | 5 | (M2+MAO2)/SiO2/MAO1/M1 |
| 비교예 3 | 7.5 | 5 | (M1+½M2+MAO1)/SiO2/(½M2+MAO2) |
| 비교예 4 | 7.5 | 5 | (½M1+MAO1)/SiO2/(½M1+M2+MAO2) |
| 비교예 5 | 7.5 | 5 | (SiO2+MAO2)/M2/(M1+MAO1) |
| 비교예 6 | 7.5 | 5 | (SiO2+MAO2)/M2/MAO1/M1 |
| 비교예 7 | 7.5 | 5 | (SiO2+MAO1)/(M1+½M2)/(½M2+MAO2) |
| 비교예 8 | 7.5 | 5 | (SiO2+MAO1)/(M1+M2+MAO2) |
| 촉매 활성 (gPE/gCat-hr) |
전체 젤의 개수 | 미세 젤의 개수 | 대조예 대비 젤의 수준(%) | ||
| 전체 젤 | 미세 젤 | ||||
| 대조예 | 3,120 | 120,340 | 8,450 | - | - |
| 실시예 1 | 3,170 | 10,659 | 1,409 | 8.9 | 16.7 |
| 실시예 2 | 3,190 | 11,098 | 1,345 | 9.2 | 15.9 |
| 실시예 3 | 3,071 | 15,478 | 1,430 | 12.9 | 16.9 |
| 실시예 4 | 3,170 | 16,783 | 1,903 | 13.9 | 22.5 |
| 실시예 5 | 3,220 | 11,536 | 1,524 | 9.6 | 18.0 |
| 실시예 6 | 3,087 | 10,863 | 1,302 | 9.0 | 12.0 |
| 실시예 7 | 3,790 | 14,950 | 1,473 | 12.4 | 17.4 |
| 실시예 8 | 3,470 | 12,830 | 1,347 | 10.7 | 15.9 |
| 비교예 1 | 3,220 | 114,598 | 7,982 | 95.2 | 94.5 |
| 비교예 2 | 3,098 | 124,038 | 7,684 | 103 | 90.9 |
| 비교예 3 | 3,170 | 178,235 | 9,309 | 148 | 110 |
| 비교예 4 | 3,120 | 172,203 | 9,237 | 143 | 109 |
| 비교예 5 | 2,920 | 104,638 | 9,982 | 87.0 | 118 |
| 비교예 6 | 3,087 | 124,029 | 8,125 | 103 | 96.2 |
| 비교예 7 | 3,098 | 154,245 | 8,120 | 128 | 96.1 |
| 비교예 8 | 3,470 | 161,398 | 9,109 | 134 | 108 |
Claims (16)
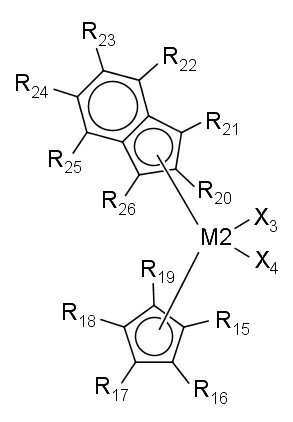
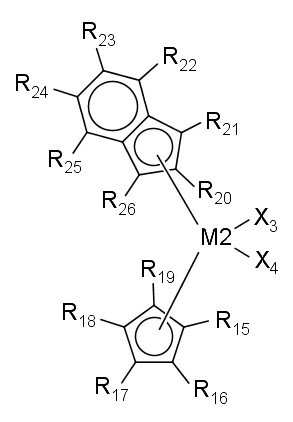
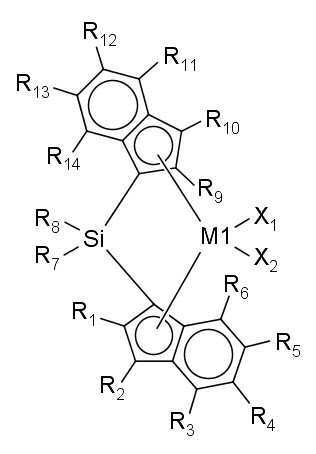
- (1) 아래 화학식 1로 표시되는 제1 전이금속 화합물을 (1a) 제1 조촉매 화합물과 혼합한 후 담체와 접촉시키거나, (1b) 제1 조촉매 화합물과 담체의 슬러리와 접촉시켜, 제1 전이금속 화합물의 담지 촉매를 얻는 단계; 및 (2) 제2 조촉매 화합물과 아래 화학식 2로 표시되는 제2 전이금속 화합물을 이 순서대로, 역순으로, 또는 동시에 단계 (1)에서 얻어진 제1 전이금속 화합물의 담지 촉매와 접촉시켜, 올레핀 중합용 혼성 메탈로센 담지 촉매를 얻는 단계를 포함하는 올레핀 중합용 혼성 메탈로센 담지 촉매의 제조방법으로서, 제1 전이금속 화합물이 단계 (2)에서 사용되지 않고, 제2 전이금속 화합물이 단계 (1)에서 사용되지 않는, 젤의 생성이 억제된 폴리올레핀을 제조할 수 있는 올레핀 중합용 혼성 메탈로센 담지 촉매의 제조방법:
[화학식 1]
[화학식 2]
위 화학식 1과 2에서, M1과 M2는 각각 독립적으로 원소 주기율표의 4족 전이금속이고;
X1 내지 X4는 각각 독립적으로 할로겐 원자, 탄소수 1~20의 알킬기, 탄소수 2~20의 알케닐기, 탄소수 2~20의 알키닐기, 탄소수 6~20의 아릴기, 탄소수 7~40의 알킬아릴기, 탄소수 7~40의 아릴알킬기, 탄소수 1~20의 알킬아미도기, 또는 탄소수 6~20의 아릴아미도기이고;
R1 내지 R6과 R9 내지 R14는 각각 독립적으로 수소 원자, 치환 또는 비치환된 탄소수 1~20의 알킬기, 치환 또는 비치환된 탄소수 2~20의 알케닐기, 치환 또는 비치환된 탄소수 6~20의 아릴기, 치환 또는 비치환된 탄소수 7~40의 알킬아릴기, 또는 치환 또는 비치환된 탄소수 7~40의 아릴알킬기이고, 서로 연결되어 고리를 형성할 수 있으며;
R7과 R8은 각각 독립적으로 치환 또는 비치환된 탄소수 1~20의 알킬기, 치환 또는 비치환된 탄소수 2~20의 알케닐기, 치환 또는 비치환된 탄소수 6~20의 아릴기, 치환 또는 비치환된 탄소수 7~40의 알킬아릴기 또는 치환 또는 비치환된 탄소수 7~40의 아릴알킬기이고, 서로 연결되어 고리를 형성할 수 있으며;
R1 내지 R6과 결합하는 인덴과 R9 내지 R14와 결합하는 인덴은 서로 같은 구조이고, R1 내지 R6과 결합하는 인덴과 R9 내지 R14와 결합하는 인덴은 서로 Si와 연결되어 있는 다리 구조를 형성하며;
R15 내지 R26은 각각 독립적으로 수소 원자, 치환 또는 비치환된 탄소수 1~20의 알킬기, 치환 또는 비치환된 탄소수 2~20의 알케닐기, 치환 또는 비치환된 탄소수 6~20의 아릴기, 치환 또는 비치환된 탄소수 7~40의 알킬아릴기, 또는 치환 또는 비치환된 탄소수 7~40의 아릴알킬기이고, 서로 연결되어 고리를 형성할 수 있으며;
R15 내지 R19와 결합하는 사이클로펜타디엔과 R20 내지 R26과 결합하는 인덴은 서로 다른 구조를 가지는 비대칭 구조이며, R15 내지 R19와 결합하는 사이클로펜타디엔과 R20 내지 R26과 결합하는 인덴은 서로 연결되어 있지 않은 비다리 구조를 형성한다. - 제1항에 있어서, 제2 전이금속 화합물이 [인데닐(사이클로펜타디에닐)]지르코늄 디클로라이드, [4-메틸인데닐(싸이클로펜타디에닐)]지르코늄 디클로라이드, [인데닐(테트라메틸싸이클로펜타디에닐)]지르코늄 디클로라이드, [2-메틸인데닐(테트라메틸싸이클로펜타디에닐)]지르코늄 디클로라이드, [2-메틸벤조인데닐(싸이클로펜타디에닐)]지르코늄 디클로라이드 및 [4,5-벤조인데닐(테트라메틸싸이클로펜타디에닐)]지르코늄 디클로라이드로 구성되는 군으로부터 선택되는 적어도 하나를 포함하는 올레핀 중합용 혼성 메탈로센 담지 촉매의 제조방법.
- 제1항에 있어서, 제2 조촉매 화합물이 단계 (1)에서 제1 전이금속 화합물 또는 담체와 혼합되는 올레핀 중합용 혼성 메탈로센 담지 촉매의 제조방법.
- 제1항에 있어서, 조촉매 화합물이 아래 화학식 3으로 표시되는 화합물, 아래 화학식 4로 표시되는 화합물 및 아래 화학식 5로 표시되는 화합물로 구성되는 군으로부터 선택되는 하나 이상인 올레핀 중합용 혼성 메탈로센 담지 촉매의 제조방법:
[화학식 3]
[화학식 4]
[L-H]+[Z(A)4]- 또는 [L]+[Z(A)4]-
위 화학식 3에서, n은 2 이상의 정수이고, Ra는 할로겐 원자, C1-20 탄화수소기 또는 할로겐으로 치환된 C1-20 탄화수소기이고,
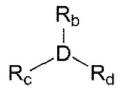
위 화학식 4에서, D는 알루미늄(Al) 또는 보론(B)이고, Rb, Rc 및 Rd는 각각 독립적으로 할로겐 원자, C1-20 탄화수소기, 할로겐으로 치환된 C1-20 탄화수소기 또는 C1-20 알콕시기이며,
위 화학식 5에서, L은 중성 또는 양이온성 루이스 염기이고, [L-H]+ 및 [L]+는 브뢴스테드 산이며, Z는 13족 원소이고, A는 각각 독립적으로 치환 또는 비치환된 C6-20 아릴기이거나 치환 또는 비치환된 C1-20 알킬기이다. - 제5항에 있어서, 화학식 3으로 표시되는 화합물이 메틸알루미녹산, 에틸알루미녹산, 이소부틸알루미녹산 및 부틸알루미녹산으로 구성되는 군으로부터 선택되는 적어도 하나인 올레핀 중합용 혼성 메탈로센 담지 촉매의 제조방법.
- 제5항에 있어서, 화학식 4로 표시되는 화합물이 트리메틸알루미늄, 트리에틸알루미늄, 트리이소부틸알루미늄, 트리프로필알루미늄, 트리부틸알루미늄, 디메틸클로로알루미늄, 트리이소프로필알루미늄, 트리-s-부틸알루미늄, 트리사이클로펜틸알루미늄, 트리펜틸알루미늄, 트리이소펜틸알루미늄, 트리헥실알루미늄, 트리옥틸알루미늄, 에틸디메틸알루미늄, 메틸디에틸알루미늄, 트리페닐알루미늄, 트리-p-톨릴알루미늄, 디메틸알루미늄메톡시드, 디메틸알루미늄에톡시드, 트리메틸보론, 트리에틸보론, 트리이소부틸보론, 트리프로필보론 및 트리부틸보론으로 구성되는 군으로부터 선택되는 적어도 하나인 올레핀 중합용 혼성 메탈로센 담지 촉매의 제조방법.
- 제5항에 있어서, 화학식 5로 표시되는 화합물이 트리에틸암모니움테트라페닐보론, 트리부틸암모니움테트라페닐보론, 트리메틸암모니움테트라페닐보론, 트리프로필암모니움테트라페닐보론, 트리메틸암모니움테트라(p-톨릴)보론, 트리메틸암모니움테트라(o,p-디메틸페닐)보론, 트리부틸암모니움테트라(p-트리플로로메틸페닐)보론, 트리메틸암모니움테트라(p-트리플로로메틸페닐)보론, 트리부틸암모니움테트라펜타플로로페닐보론, N,N-디에틸아닐리니움테트라페닐보론, N,N-디에틸아닐리니움테트라펜타플로로페닐보론, 디에틸암모니움테트라펜타플로로페닐보론, 트리페닐포스포늄테트라페닐보론, 트리메틸포스포늄테트라페닐보론, 트리에틸암모니움테트라페닐알루미늄, 트리부틸암모니움테트라페닐알루미늄, 트리메틸암모니움테트라페닐알루미늄, 트리프로필암모니움테트라페닐알루미늄, 트리메틸암모니움테트라(p-톨릴)알루미늄, 트리프로필암모니움테트라(p-톨릴)알루미늄, 트리에틸암모니움테트라(o,p-디메틸페닐)알루미늄, 트리부틸암모니움테트라(p-트리플로로메틸페닐)알루미늄, 트리메틸암모니움테트라(p-트리플로로메틸페닐)알루미늄, 트리부틸암모니움테트라펜타플로로페닐알루미늄, N,N-디에틸아닐리니움테트라페닐알루미늄, N,N-디에틸아닐리니움테트라펜타플로로페닐알루미늄, 디에틸암모니움테트라펜타테트라페닐알루미늄, 트리페닐포스포늄테트라페닐알루미늄, 트리메틸포스포늄테트라페닐알루미늄, 트리프로필암모니움테트라(p-톨릴)보론, 트리에틸암모니움테트라(o,p-디메틸페닐)보론, 트리페닐카보니움테트라(p-트리플로로메틸페닐)보론 및 트리페닐카보니움테트라펜타플로로페닐보론으로 구성되는 군으로부터 선택되는 적어도 하나인 올레핀 중합용 혼성 메탈로센 담지 촉매의 제조방법.
- 제1항에 있어서, 제1 조촉매 화합물과 제2 조촉매 화합물이 동일한 올레핀 중합용 혼성 메탈로센 담지 촉매의 제조방법.
- 제6항에 있어서, 제1 조촉매 화합물과 제2 조촉매 화합물이 메틸알루미녹산인 올레핀 중합용 혼성 메탈로센 담지 촉매의 제조방법.
- 제1항에 있어서, 담체가 실리카, 알루미나 및 마그네시아로 구성되는 군으로부터 선택되는 적어도 하나를 포함하는 올레핀 중합용 혼성 메탈로센 담지 촉매의 제조방법.
- 제11항에 있어서, 제1 전이금속 화합물, 제2 전이금속 화합물, 제1 조촉매 화합물 및 제2 조촉매 화합물이 단일 종의 담체에 담지되는 올레핀 중합용 혼성 메탈로센 담지 촉매의 제조방법.
- 제12항에 있어서, 제1 전이금속 화합물, 제2 전이금속 화합물, 제1 조촉매 화합물 및 제2 조촉매 화합물이 실리카에 담지되는 올레핀 중합용 혼성 메탈로센 담지 촉매의 제조방법.
- 제11항에 있어서, 담체에 담지되는 제1 전이금속 화합물과 제2 전이금속 화합물의 총량이 담체 1 g을 기준으로 0.001~1 mmole이며, 담체에 담지되는 조촉매 화합물의 총량이 담체 1 g을 기준으로 2~15 mmole인 올레핀 중합용 혼성 메탈로센 담지 촉매의 제조방법.
- 제1항에 있어서, 제1 전이금속 화합물과 제2 전이금속 화합물이 20:1~1:20의 중량비로 사용되는 올레핀 중합용 혼성 메탈로센 담지 촉매의 제조방법.
- 제1항 내지 제15항 중 어느 한 항의 올레핀 중합용 혼성 메탈로센 담지 촉매의 제조방법에 의해 제조된 올레핀 중합용 혼성 메탈로센 담지 촉매.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020190132618A KR102547232B1 (ko) | 2019-10-24 | 2019-10-24 | 올레핀 중합용 촉매의 제조방법 |
| PCT/KR2020/014127 WO2021080249A1 (ko) | 2019-10-24 | 2020-10-16 | 올레핀 중합용 촉매의 제조방법 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020190132618A KR102547232B1 (ko) | 2019-10-24 | 2019-10-24 | 올레핀 중합용 촉매의 제조방법 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20210048677A KR20210048677A (ko) | 2021-05-04 |
| KR102547232B1 true KR102547232B1 (ko) | 2023-06-26 |
Family
ID=75619465
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020190132618A Active KR102547232B1 (ko) | 2019-10-24 | 2019-10-24 | 올레핀 중합용 촉매의 제조방법 |
Country Status (2)
| Country | Link |
|---|---|
| KR (1) | KR102547232B1 (ko) |
| WO (1) | WO2021080249A1 (ko) |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014210937A (ja) | 2004-01-21 | 2014-11-13 | シェブロン フィリップス ケミカル カンパニー エルピー | 機械方向(md)エレメンドルフ引裂強度に優れたフィルム樹脂製造用デュアルメタロセン触媒 |
| WO2015123165A2 (en) | 2014-02-11 | 2015-08-20 | Univation Technologies, Llc | Producing polyolefin products with improved stiffness, toughness, and processability |
| KR101711788B1 (ko) | 2016-03-09 | 2017-03-14 | 한화케미칼 주식회사 | 혼성 촉매 조성물, 이의 제조방법, 및 이를 이용하여 제조된 폴리올레핀 |
| KR101725004B1 (ko) * | 2016-04-27 | 2017-04-18 | 한화케미칼 주식회사 | 혼성 담지 메탈로센 촉매 및 이를 이용한 가공성이 우수한 폴리올레핀 수지 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6432860B1 (en) | 1999-03-22 | 2002-08-13 | Fina Technology, Inc. | Supported metallocene catalysts |
| US20070049711A1 (en) | 2005-09-01 | 2007-03-01 | Chi-I Kuo | Catalyst compositions comprising support materials having an improved particle-size distribution |
| US8119553B2 (en) | 2007-09-28 | 2012-02-21 | Chevron Phillips Chemical Company Lp | Polymerization catalysts for producing polymers with low melt elasticity |
| KR102180532B1 (ko) * | 2016-11-28 | 2020-11-18 | 주식회사 엘지화학 | 내환경 응력 균열성이 우수한 폴리올레핀의 제조 방법 |
-
2019
- 2019-10-24 KR KR1020190132618A patent/KR102547232B1/ko active Active
-
2020
- 2020-10-16 WO PCT/KR2020/014127 patent/WO2021080249A1/ko active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014210937A (ja) | 2004-01-21 | 2014-11-13 | シェブロン フィリップス ケミカル カンパニー エルピー | 機械方向(md)エレメンドルフ引裂強度に優れたフィルム樹脂製造用デュアルメタロセン触媒 |
| WO2015123165A2 (en) | 2014-02-11 | 2015-08-20 | Univation Technologies, Llc | Producing polyolefin products with improved stiffness, toughness, and processability |
| KR101711788B1 (ko) | 2016-03-09 | 2017-03-14 | 한화케미칼 주식회사 | 혼성 촉매 조성물, 이의 제조방법, 및 이를 이용하여 제조된 폴리올레핀 |
| KR101725004B1 (ko) * | 2016-04-27 | 2017-04-18 | 한화케미칼 주식회사 | 혼성 담지 메탈로센 촉매 및 이를 이용한 가공성이 우수한 폴리올레핀 수지 |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2021080249A1 (ko) | 2021-04-29 |
| KR20210048677A (ko) | 2021-05-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7470495B2 (ja) | ポリオレフィン重合触媒組成物、ポリオレフィンの製造方法及びポリオレフィン樹脂 | |
| KR102272245B1 (ko) | 올레핀 중합용 촉매 및 이를 이용하여 제조된 올레핀계 중합체 | |
| JP7577211B2 (ja) | オレフィン系重合体、それにより製造されたフィルム、およびその製造方法 | |
| JP7590580B2 (ja) | オレフィン系重合体およびその製造方法 | |
| KR102285143B1 (ko) | 올레핀 중합용 촉매의 제조방법 | |
| KR102547229B1 (ko) | 올레핀 중합용 혼성 촉매의 제조방법, 올레핀 중합용 혼성 촉매 및 올레핀계 중합체 | |
| KR20200077888A (ko) | 올레핀 중합용 촉매의 제조방법, 올레핀 중합용 촉매 및 올레핀계 중합체 | |
| KR102285140B1 (ko) | 올레핀 중합용 촉매의 제조방법 | |
| KR102547232B1 (ko) | 올레핀 중합용 촉매의 제조방법 | |
| KR102652274B1 (ko) | 올레핀계 중합체 및 그 제조방법 | |
| JP7577212B2 (ja) | オレフィン系重合体およびその製造方法 | |
| KR102579816B1 (ko) | 올레핀계 중합체 및 그 제조방법 | |
| KR102287064B1 (ko) | 올레핀 중합용 촉매의 제조방법 | |
| KR102619077B1 (ko) | 올레핀계 중합체의 제조방법 | |
| KR102778099B1 (ko) | 올레핀계 중합체의 제조방법 및 이를 이용하여 제조된 올레핀계 중합체 | |
| KR20210108665A (ko) | 혼성 촉매 조성물, 이를 포함하는 촉매 및 이들의 제조방법 | |
| KR20220090839A (ko) | 혼성 촉매 조성물, 이를 포함하는 촉매 및 이를 이용한 올레핀계 중합체의 제조방법 | |
| JP7603162B2 (ja) | オレフィン系重合体、それより製造されたフィルム、およびその製造方法 | |
| KR100310758B1 (ko) | 과립형수지의제조방법 | |
| KR20180075318A (ko) | 담지 메탈로센 촉매의 제조방법 | |
| KR20210128041A (ko) | 혼성 촉매 조성물, 이를 포함하는 촉매 및 이들의 제조방법 | |
| KR20220055863A (ko) | 신규한 메탈로센 화합물, 이를 포함하는 촉매 조성물 및 이를 이용한 올레핀 중합체의 제조 방법 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0109 | Patent application |
Patent event code: PA01091R01D Comment text: Patent Application Patent event date: 20191024 |
|
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210127 Comment text: Request for Examination of Application Patent event code: PA02011R01I Patent event date: 20191024 Comment text: Patent Application |
|
| PG1501 | Laying open of application | ||
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20221019 Patent event code: PE09021S01D |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
Patent event code: PE07011S01D Comment text: Decision to Grant Registration Patent event date: 20230412 |
|
| GRNT | Written decision to grant | ||
| PR0701 | Registration of establishment |
Comment text: Registration of Establishment Patent event date: 20230620 Patent event code: PR07011E01D |
|
| PR1002 | Payment of registration fee |
Payment date: 20230621 End annual number: 3 Start annual number: 1 |
|
| PG1601 | Publication of registration |