CN1262267A - 20位改性的维生素d系列衍生物,其制备方法和其药剂 - Google Patents
20位改性的维生素d系列衍生物,其制备方法和其药剂 Download PDFInfo
- Publication number
- CN1262267A CN1262267A CN99126473A CN99126473A CN1262267A CN 1262267 A CN1262267 A CN 1262267A CN 99126473 A CN99126473 A CN 99126473A CN 99126473 A CN99126473 A CN 99126473A CN 1262267 A CN1262267 A CN 1262267A
- Authority
- CN
- China
- Prior art keywords
- methyl
- nmr
- formula
- title compound
- cdcl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 239000003814 drug Substances 0.000 title abstract description 7
- 150000003710 vitamin D derivatives Chemical class 0.000 title abstract description 7
- 235000019166 vitamin D Nutrition 0.000 title description 7
- 239000011710 vitamin D Substances 0.000 title description 7
- 229930003316 Vitamin D Natural products 0.000 title description 6
- QYSXJUFSXHHAJI-XFEUOLMDSA-N Vitamin D3 Natural products C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C/C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-XFEUOLMDSA-N 0.000 title description 6
- 229940046008 vitamin d Drugs 0.000 title description 5
- 229940079593 drug Drugs 0.000 title description 3
- 125000001181 organosilyl group Chemical group [SiH3]* 0.000 claims description 29
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 claims description 25
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N EtOH Substances CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 9
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 7
- 239000001301 oxygen Substances 0.000 claims description 6
- 229910052760 oxygen Inorganic materials 0.000 claims description 6
- 239000013067 intermediate product Substances 0.000 claims description 5
- FDPIMTJIUBPUKL-UHFFFAOYSA-N pentan-3-one Chemical compound CCC(=O)CC FDPIMTJIUBPUKL-UHFFFAOYSA-N 0.000 claims 2
- 229960003328 benzoyl peroxide Drugs 0.000 claims 1
- 238000002360 preparation method Methods 0.000 abstract description 9
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 abstract description 5
- 201000010099 disease Diseases 0.000 abstract description 4
- 230000003463 hyperproliferative effect Effects 0.000 abstract description 3
- 229940124597 therapeutic agent Drugs 0.000 abstract description 2
- 230000001225 therapeutic effect Effects 0.000 abstract 1
- 150000001875 compounds Chemical class 0.000 description 164
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 125
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 102
- 238000005160 1H NMR spectroscopy Methods 0.000 description 90
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical class [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 72
- 239000000243 solution Substances 0.000 description 72
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 69
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 51
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 44
- 239000006260 foam Substances 0.000 description 42
- FPGGTKZVZWFYPV-UHFFFAOYSA-M tetrabutylammonium fluoride Chemical compound [F-].CCCC[N+](CCCC)(CCCC)CCCC FPGGTKZVZWFYPV-UHFFFAOYSA-M 0.000 description 40
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 36
- 239000011780 sodium chloride Substances 0.000 description 35
- 239000012230 colorless oil Substances 0.000 description 34
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 33
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 33
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 30
- 239000000460 chlorine Substances 0.000 description 26
- 239000002904 solvent Substances 0.000 description 26
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 22
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 22
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 21
- 229910052938 sodium sulfate Inorganic materials 0.000 description 21
- 235000011152 sodium sulphate Nutrition 0.000 description 21
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 21
- 239000007818 Grignard reagent Substances 0.000 description 20
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 20
- 238000006243 chemical reaction Methods 0.000 description 20
- 150000004795 grignard reagents Chemical class 0.000 description 20
- 239000011777 magnesium Substances 0.000 description 20
- 229910052749 magnesium Inorganic materials 0.000 description 20
- LEHBURLTIWGHEM-UHFFFAOYSA-N pyridinium chlorochromate Chemical compound [O-][Cr](Cl)(=O)=O.C1=CC=[NH+]C=C1 LEHBURLTIWGHEM-UHFFFAOYSA-N 0.000 description 20
- 238000003756 stirring Methods 0.000 description 20
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 19
- 238000010898 silica gel chromatography Methods 0.000 description 19
- IJOOHPMOJXWVHK-UHFFFAOYSA-N chlorotrimethylsilane Chemical compound C[Si](C)(C)Cl IJOOHPMOJXWVHK-UHFFFAOYSA-N 0.000 description 18
- SHFJWMWCIHQNCP-UHFFFAOYSA-M hydron;tetrabutylazanium;sulfate Chemical compound OS([O-])(=O)=O.CCCC[N+](CCCC)(CCCC)CCCC SHFJWMWCIHQNCP-UHFFFAOYSA-M 0.000 description 18
- 239000012074 organic phase Substances 0.000 description 18
- 229910052799 carbon Inorganic materials 0.000 description 17
- 125000000026 trimethylsilyl group Chemical group [H]C([H])([H])[Si]([*])(C([H])([H])[H])C([H])([H])[H] 0.000 description 17
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 16
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 16
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 16
- 239000000126 substance Substances 0.000 description 16
- ZCSHNCUQKCANBX-UHFFFAOYSA-N lithium diisopropylamide Chemical compound [Li+].CC(C)[N-]C(C)C ZCSHNCUQKCANBX-UHFFFAOYSA-N 0.000 description 14
- 238000012360 testing method Methods 0.000 description 14
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 12
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 12
- -1 α-hydroxypropyl Chemical group 0.000 description 12
- 229910052786 argon Inorganic materials 0.000 description 11
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 11
- 235000011121 sodium hydroxide Nutrition 0.000 description 11
- 239000011612 calcitriol Substances 0.000 description 10
- 229960005084 calcitriol Drugs 0.000 description 10
- 239000001257 hydrogen Substances 0.000 description 10
- 229910052739 hydrogen Inorganic materials 0.000 description 10
- 238000000034 method Methods 0.000 description 10
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 9
- 239000005051 trimethylchlorosilane Substances 0.000 description 9
- MZRVEZGGRBJDDB-UHFFFAOYSA-N N-Butyllithium Chemical compound [Li]CCCC MZRVEZGGRBJDDB-UHFFFAOYSA-N 0.000 description 8
- XXROGKLTLUQVRX-UHFFFAOYSA-N allyl alcohol Chemical compound OCC=C XXROGKLTLUQVRX-UHFFFAOYSA-N 0.000 description 8
- RDHPKYGYEGBMSE-UHFFFAOYSA-N bromoethane Chemical compound CCBr RDHPKYGYEGBMSE-UHFFFAOYSA-N 0.000 description 8
- GMRQFYUYWCNGIN-NKMMMXOESA-N calcitriol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](CCCC(C)(C)O)C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C GMRQFYUYWCNGIN-NKMMMXOESA-N 0.000 description 8
- 235000020964 calcitriol Nutrition 0.000 description 8
- 239000011575 calcium Substances 0.000 description 8
- 210000004027 cell Anatomy 0.000 description 8
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 8
- 238000000926 separation method Methods 0.000 description 8
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 7
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical class [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 7
- 239000002585 base Substances 0.000 description 7
- 239000012043 crude product Substances 0.000 description 7
- 150000002576 ketones Chemical class 0.000 description 7
- LPNYRYFBWFDTMA-UHFFFAOYSA-N potassium tert-butoxide Chemical compound [K+].CC(C)(C)[O-] LPNYRYFBWFDTMA-UHFFFAOYSA-N 0.000 description 7
- 210000003491 skin Anatomy 0.000 description 7
- UUIQMZJEGPQKFD-UHFFFAOYSA-N Methyl butyrate Chemical compound CCCC(=O)OC UUIQMZJEGPQKFD-UHFFFAOYSA-N 0.000 description 6
- RJUFJBKOKNCXHH-UHFFFAOYSA-N Methyl propionate Chemical compound CCC(=O)OC RJUFJBKOKNCXHH-UHFFFAOYSA-N 0.000 description 6
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 6
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 6
- MWPLVEDNUUSJAV-UHFFFAOYSA-N anthracene Chemical compound C1=CC=CC2=CC3=CC=CC=C3C=C21 MWPLVEDNUUSJAV-UHFFFAOYSA-N 0.000 description 6
- 229910052791 calcium Inorganic materials 0.000 description 6
- 150000001721 carbon Chemical group 0.000 description 6
- 239000003153 chemical reaction reagent Substances 0.000 description 6
- 238000004587 chromatography analysis Methods 0.000 description 6
- 125000006239 protecting group Chemical group 0.000 description 6
- 238000000746 purification Methods 0.000 description 6
- 229910000104 sodium hydride Inorganic materials 0.000 description 6
- 239000007858 starting material Substances 0.000 description 6
- XVMSFILGAMDHEY-UHFFFAOYSA-N 6-(4-aminophenyl)sulfonylpyridin-3-amine Chemical compound C1=CC(N)=CC=C1S(=O)(=O)C1=CC=C(N)C=N1 XVMSFILGAMDHEY-UHFFFAOYSA-N 0.000 description 5
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 5
- OKJPEAGHQZHRQV-UHFFFAOYSA-N Triiodomethane Natural products IC(I)I OKJPEAGHQZHRQV-UHFFFAOYSA-N 0.000 description 5
- 125000000217 alkyl group Chemical group 0.000 description 5
- 229910052794 bromium Inorganic materials 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- 239000000543 intermediate Substances 0.000 description 5
- 125000001160 methoxycarbonyl group Chemical group [H]C([H])([H])OC(*)=O 0.000 description 5
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 5
- 239000000203 mixture Substances 0.000 description 5
- 239000013558 reference substance Substances 0.000 description 5
- 239000000725 suspension Substances 0.000 description 5
- 230000002194 synthesizing effect Effects 0.000 description 5
- BNWCETAHAJSBFG-UHFFFAOYSA-N tert-butyl 2-bromoacetate Chemical compound CC(C)(C)OC(=O)CBr BNWCETAHAJSBFG-UHFFFAOYSA-N 0.000 description 5
- KRHYYFGTRYWZRS-UHFFFAOYSA-N Fluorane Chemical compound F KRHYYFGTRYWZRS-UHFFFAOYSA-N 0.000 description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 4
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- 239000002253 acid Substances 0.000 description 4
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 4
- 239000006285 cell suspension Substances 0.000 description 4
- 150000002431 hydrogen Chemical class 0.000 description 4
- NUJOXMJBOLGQSY-UHFFFAOYSA-N manganese dioxide Chemical compound O=[Mn]=O NUJOXMJBOLGQSY-UHFFFAOYSA-N 0.000 description 4
- VMGAPWLDMVPYIA-HIDZBRGKSA-N n'-amino-n-iminomethanimidamide Chemical compound N\N=C\N=N VMGAPWLDMVPYIA-HIDZBRGKSA-N 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- BOLDJAUMGUJJKM-LSDHHAIUSA-N renifolin D Natural products CC(=C)[C@@H]1Cc2c(O)c(O)ccc2[C@H]1CC(=O)c3ccc(O)cc3O BOLDJAUMGUJJKM-LSDHHAIUSA-N 0.000 description 4
- 125000003808 silyl group Chemical class [H][Si]([H])([H])[*] 0.000 description 4
- 239000011701 zinc Substances 0.000 description 4
- CYNYIHKIEHGYOZ-UHFFFAOYSA-N 1-bromopropane Chemical compound CCCBr CYNYIHKIEHGYOZ-UHFFFAOYSA-N 0.000 description 3
- IKHGUXGNUITLKF-UHFFFAOYSA-N Acetaldehyde Chemical compound CC=O IKHGUXGNUITLKF-UHFFFAOYSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 3
- 208000006386 Bone Resorption Diseases 0.000 description 3
- 208000037147 Hypercalcaemia Diseases 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- FNJSWIPFHMKRAT-UHFFFAOYSA-N Monomethyl phthalate Chemical compound COC(=O)C1=CC=CC=C1C(O)=O FNJSWIPFHMKRAT-UHFFFAOYSA-N 0.000 description 3
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 3
- 239000012298 atmosphere Substances 0.000 description 3
- SIPUZPBQZHNSDW-UHFFFAOYSA-N bis(2-methylpropyl)aluminum Chemical compound CC(C)C[Al]CC(C)C SIPUZPBQZHNSDW-UHFFFAOYSA-N 0.000 description 3
- 230000024279 bone resorption Effects 0.000 description 3
- 230000024245 cell differentiation Effects 0.000 description 3
- 229910052801 chlorine Inorganic materials 0.000 description 3
- 230000004069 differentiation Effects 0.000 description 3
- 238000001035 drying Methods 0.000 description 3
- 238000000605 extraction Methods 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- 125000002485 formyl group Chemical class [H]C(*)=O 0.000 description 3
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 3
- 230000000148 hypercalcaemia Effects 0.000 description 3
- 208000030915 hypercalcemia disease Diseases 0.000 description 3
- 230000001965 increasing effect Effects 0.000 description 3
- 229910052740 iodine Inorganic materials 0.000 description 3
- 210000002540 macrophage Anatomy 0.000 description 3
- 239000002674 ointment Substances 0.000 description 3
- 239000007800 oxidant agent Substances 0.000 description 3
- AUONHKJOIZSQGR-UHFFFAOYSA-N oxophosphane Chemical compound P=O AUONHKJOIZSQGR-UHFFFAOYSA-N 0.000 description 3
- 125000004115 pentoxy group Chemical group [*]OC([H])([H])C([H])([H])C([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 3
- 239000000825 pharmaceutical preparation Substances 0.000 description 3
- 102000005962 receptors Human genes 0.000 description 3
- 108020003175 receptors Proteins 0.000 description 3
- 229920006395 saturated elastomer Polymers 0.000 description 3
- 235000017557 sodium bicarbonate Nutrition 0.000 description 3
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 3
- 239000012312 sodium hydride Substances 0.000 description 3
- 125000003718 tetrahydrofuranyl group Chemical group 0.000 description 3
- 239000003104 tissue culture media Substances 0.000 description 3
- 102000009310 vitamin D receptors Human genes 0.000 description 3
- 108050000156 vitamin D receptors Proteins 0.000 description 3
- MECHNRXZTMCUDQ-RKHKHRCZSA-N vitamin D2 Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)/C=C/[C@H](C)C(C)C)=C\C=C1\C[C@@H](O)CCC1=C MECHNRXZTMCUDQ-RKHKHRCZSA-N 0.000 description 3
- 239000011647 vitamin D3 Substances 0.000 description 3
- 229940021056 vitamin d3 Drugs 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- FKHQVTZBKCBVOA-UHFFFAOYSA-N 1,2,3,3a,5,6,7,7a-octahydroinden-4-one Chemical compound O=C1CCCC2CCCC12 FKHQVTZBKCBVOA-UHFFFAOYSA-N 0.000 description 2
- QBLJMYITBNHQOT-UHFFFAOYSA-N 4-bromo-1,1,1-trimethoxybutane Chemical compound COC(OC)(OC)CCCBr QBLJMYITBNHQOT-UHFFFAOYSA-N 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 2
- 229920002307 Dextran Polymers 0.000 description 2
- 229910010082 LiAlH Inorganic materials 0.000 description 2
- 241000699670 Mus sp. Species 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 201000004681 Psoriasis Diseases 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- 238000006932 Simmons-Smith cyclopropanation reaction Methods 0.000 description 2
- 206010040799 Skin atrophy Diseases 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 125000002252 acyl group Chemical group 0.000 description 2
- 125000002947 alkylene group Chemical group 0.000 description 2
- SMZOGRDCAXLAAR-UHFFFAOYSA-N aluminium isopropoxide Chemical compound [Al+3].CC(C)[O-].CC(C)[O-].CC(C)[O-] SMZOGRDCAXLAAR-UHFFFAOYSA-N 0.000 description 2
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 2
- 125000004106 butoxy group Chemical group [*]OC([H])([H])C([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 2
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 150000001668 calcitriol derivatives Chemical class 0.000 description 2
- 125000001589 carboacyl group Chemical group 0.000 description 2
- 150000001735 carboxylic acids Chemical class 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 239000003638 chemical reducing agent Substances 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 239000006071 cream Substances 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 230000005595 deprotonation Effects 0.000 description 2
- 238000010537 deprotonation reaction Methods 0.000 description 2
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 2
- 210000002615 epidermis Anatomy 0.000 description 2
- 239000000706 filtrate Substances 0.000 description 2
- 238000011010 flushing procedure Methods 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 125000003707 hexyloxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 2
- BNRNAKTVFSZAFA-UHFFFAOYSA-N hydrindane Chemical compound C1CCCC2CCCC21 BNRNAKTVFSZAFA-UHFFFAOYSA-N 0.000 description 2
- 230000000121 hypercalcemic effect Effects 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 208000032839 leukemia Diseases 0.000 description 2
- 229910052744 lithium Inorganic materials 0.000 description 2
- AHNJTQYTRPXLLG-UHFFFAOYSA-N lithium;diethylazanide Chemical compound [Li+].CC[N-]CC AHNJTQYTRPXLLG-UHFFFAOYSA-N 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000004060 metabolic process Effects 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 2
- 239000012038 nucleophile Substances 0.000 description 2
- 125000004430 oxygen atom Chemical group O* 0.000 description 2
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 2
- 229940127557 pharmaceutical product Drugs 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- 125000002572 propoxy group Chemical group [*]OC([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 2
- 239000011541 reaction mixture Substances 0.000 description 2
- 239000000741 silica gel Substances 0.000 description 2
- 229910002027 silica gel Inorganic materials 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 239000012279 sodium borohydride Substances 0.000 description 2
- 229910000033 sodium borohydride Inorganic materials 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 125000001412 tetrahydropyranyl group Chemical group 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- KPZSTOVTJYRDIO-UHFFFAOYSA-K trichlorocerium;heptahydrate Chemical compound O.O.O.O.O.O.O.Cl[Ce](Cl)Cl KPZSTOVTJYRDIO-UHFFFAOYSA-K 0.000 description 2
- VFJYIHQDILEQNR-UHFFFAOYSA-M trimethylsulfanium;iodide Chemical compound [I-].C[S+](C)C VFJYIHQDILEQNR-UHFFFAOYSA-M 0.000 description 2
- QYSXJUFSXHHAJI-YRZJJWOYSA-N vitamin D3 Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C\C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-YRZJJWOYSA-N 0.000 description 2
- 229910052725 zinc Inorganic materials 0.000 description 2
- FMCAFXHLMUOIGG-JTJHWIPRSA-N (2s)-2-[[(2r)-2-[[(2s)-2-[[(2r)-2-formamido-3-sulfanylpropanoyl]amino]-3-methylbutanoyl]amino]-3-(4-hydroxy-2,5-dimethylphenyl)propanoyl]amino]-4-methylsulfanylbutanoic acid Chemical compound O=CN[C@@H](CS)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(=O)N[C@@H](CCSC)C(O)=O)CC1=CC(C)=C(O)C=C1C FMCAFXHLMUOIGG-JTJHWIPRSA-N 0.000 description 1
- GMRQFYUYWCNGIN-ZVUFCXRFSA-N 1,25-dihydroxy vitamin D3 Chemical compound C1([C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](CCCC(C)(C)O)C)=CC=C1C[C@@H](O)C[C@H](O)C1=C GMRQFYUYWCNGIN-ZVUFCXRFSA-N 0.000 description 1
- VOKRYNAPUFSUKA-UHFFFAOYSA-N 1-bromobutane-1,1-diol Chemical compound CCCC(O)(O)Br VOKRYNAPUFSUKA-UHFFFAOYSA-N 0.000 description 1
- YQTCQNIPQMJNTI-UHFFFAOYSA-N 2,2-dimethylpropan-1-one Chemical group CC(C)(C)[C]=O YQTCQNIPQMJNTI-UHFFFAOYSA-N 0.000 description 1
- JWUBBDSIWDLEOM-UHFFFAOYSA-N 25-Hydroxycholecalciferol Natural products C1CCC2(C)C(C(CCCC(C)(C)O)C)CCC2C1=CC=C1CC(O)CCC1=C JWUBBDSIWDLEOM-UHFFFAOYSA-N 0.000 description 1
- 239000003872 25-hydroxy-cholecalciferol Substances 0.000 description 1
- VXEUGLRMYAXWKM-UHFFFAOYSA-N 3-phenyl-1h-imidazole-2-thione Chemical compound S=C1NC=CN1C1=CC=CC=C1 VXEUGLRMYAXWKM-UHFFFAOYSA-N 0.000 description 1
- 208000002874 Acne Vulgaris Diseases 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- ROFVEXUMMXZLPA-UHFFFAOYSA-N Bipyridyl Chemical compound N1=CC=CC=C1C1=CC=CC=N1 ROFVEXUMMXZLPA-UHFFFAOYSA-N 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- 235000021318 Calcifediol Nutrition 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 239000012027 Collins reagent Substances 0.000 description 1
- 241001125840 Coryphaenidae Species 0.000 description 1
- YFPJFKYCVYXDJK-UHFFFAOYSA-N Diphenylphosphine oxide Chemical compound C=1C=CC=CC=1[P+](=O)C1=CC=CC=C1 YFPJFKYCVYXDJK-UHFFFAOYSA-N 0.000 description 1
- 206010013457 Dissociation Diseases 0.000 description 1
- KRHYYFGTRYWZRS-UHFFFAOYSA-M Fluoride anion Chemical compound [F-] KRHYYFGTRYWZRS-UHFFFAOYSA-M 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 208000005016 Intestinal Neoplasms Diseases 0.000 description 1
- 125000003580 L-valyl group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(C([H])([H])[H])(C([H])([H])[H])[H] 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 101100272976 Panax ginseng CYP716A53v2 gene Proteins 0.000 description 1
- 101710146873 Receptor-binding protein Proteins 0.000 description 1
- DHXVGJBLRPWPCS-UHFFFAOYSA-N Tetrahydropyran Chemical compound C1CCOCC1 DHXVGJBLRPWPCS-UHFFFAOYSA-N 0.000 description 1
- MECHNRXZTMCUDQ-UHFFFAOYSA-N Vitamin D2 Natural products C1CCC2(C)C(C(C)C=CC(C)C(C)C)CCC2C1=CC=C1CC(O)CCC1=C MECHNRXZTMCUDQ-UHFFFAOYSA-N 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- 206010000496 acne Diseases 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 125000004423 acyloxy group Chemical group 0.000 description 1
- 230000001464 adherent effect Effects 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical group 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000012300 argon atmosphere Substances 0.000 description 1
- 125000003435 aroyl group Chemical group 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- ZZCNKSMCIZCVDR-UHFFFAOYSA-N barium(2+);dioxido(dioxo)manganese Chemical compound [Ba+2].[O-][Mn]([O-])(=O)=O ZZCNKSMCIZCVDR-UHFFFAOYSA-N 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 150000001559 benzoic acids Chemical class 0.000 description 1
- PASDCCFISLVPSO-UHFFFAOYSA-N benzoyl chloride Chemical compound ClC(=O)C1=CC=CC=C1 PASDCCFISLVPSO-UHFFFAOYSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 238000004820 blood count Methods 0.000 description 1
- 150000001649 bromium compounds Chemical class 0.000 description 1
- JWUBBDSIWDLEOM-DTOXIADCSA-N calcidiol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](CCCC(C)(C)O)C)=C\C=C1\C[C@@H](O)CCC1=C JWUBBDSIWDLEOM-DTOXIADCSA-N 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 125000002837 carbocyclic group Chemical group 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 150000001805 chlorine compounds Chemical class 0.000 description 1
- 238000011097 chromatography purification Methods 0.000 description 1
- 230000001609 comparable effect Effects 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 125000004956 cyclohexylene group Chemical group 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- HQWPLXHWEZZGKY-UHFFFAOYSA-N diethylzinc Chemical compound CC[Zn]CC HQWPLXHWEZZGKY-UHFFFAOYSA-N 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- KPUWHANPEXNPJT-UHFFFAOYSA-N disiloxane Chemical compound [SiH3]O[SiH3] KPUWHANPEXNPJT-UHFFFAOYSA-N 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 208000018459 dissociative disease Diseases 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 230000000668 effect on calcium Effects 0.000 description 1
- 235000013601 eggs Nutrition 0.000 description 1
- 239000012039 electrophile Substances 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 150000002118 epoxides Chemical class 0.000 description 1
- 229960002061 ergocalciferol Drugs 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- 238000006266 etherification reaction Methods 0.000 description 1
- CHDFNIZLAAFFPX-UHFFFAOYSA-N ethoxyethane;oxolane Chemical group CCOCC.C1CCOC1 CHDFNIZLAAFFPX-UHFFFAOYSA-N 0.000 description 1
- 125000004494 ethyl ester group Chemical group 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 239000012894 fetal calf serum Substances 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000003205 fragrance Substances 0.000 description 1
- FVIZARNDLVOMSU-UHFFFAOYSA-N ginsenoside K Natural products C1CC(C2(CCC3C(C)(C)C(O)CCC3(C)C2CC2O)C)(C)C2C1C(C)(CCC=C(C)C)OC1OC(CO)C(O)C(O)C1O FVIZARNDLVOMSU-UHFFFAOYSA-N 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 150000004820 halides Chemical class 0.000 description 1
- 125000005843 halogen group Chemical group 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- UQEAIHBTYFGYIE-UHFFFAOYSA-N hexamethyldisiloxane Chemical compound C[Si](C)(C)O[Si](C)(C)C UQEAIHBTYFGYIE-UHFFFAOYSA-N 0.000 description 1
- RCBVKBFIWMOMHF-UHFFFAOYSA-L hydroxy-(hydroxy(dioxo)chromio)oxy-dioxochromium;pyridine Chemical compound C1=CC=NC=C1.C1=CC=NC=C1.O[Cr](=O)(=O)O[Cr](O)(=O)=O RCBVKBFIWMOMHF-UHFFFAOYSA-L 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 201000002313 intestinal cancer Diseases 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 238000006317 isomerization reaction Methods 0.000 description 1
- 125000003253 isopropoxy group Chemical group [H]C([H])([H])C([H])(O*)C([H])([H])[H] 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 231100001231 less toxic Toxicity 0.000 description 1
- YNESATAKKCNGOF-UHFFFAOYSA-N lithium bis(trimethylsilyl)amide Chemical compound [Li+].C[Si](C)(C)[N-][Si](C)(C)C YNESATAKKCNGOF-UHFFFAOYSA-N 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 150000004682 monohydrates Chemical class 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 150000002829 nitrogen Chemical class 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 239000012299 nitrogen atmosphere Substances 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 239000012434 nucleophilic reagent Substances 0.000 description 1
- 235000016709 nutrition Nutrition 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 239000008194 pharmaceutical composition Substances 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 235000015497 potassium bicarbonate Nutrition 0.000 description 1
- 229910000028 potassium bicarbonate Inorganic materials 0.000 description 1
- 239000011736 potassium bicarbonate Substances 0.000 description 1
- TYJJADVDDVDEDZ-UHFFFAOYSA-M potassium hydrogencarbonate Chemical compound [K+].OC([O-])=O TYJJADVDDVDEDZ-UHFFFAOYSA-M 0.000 description 1
- LJCNRYVRMXRIQR-UHFFFAOYSA-L potassium sodium tartrate Chemical compound [Na+].[K+].[O-]C(=O)C(O)C(O)C([O-])=O LJCNRYVRMXRIQR-UHFFFAOYSA-L 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- NPRDHMWYZHSAHR-UHFFFAOYSA-N pyridine;trioxochromium Chemical compound O=[Cr](=O)=O.C1=CC=NC=C1.C1=CC=NC=C1 NPRDHMWYZHSAHR-UHFFFAOYSA-N 0.000 description 1
- ZDYVRSLAEXCVBX-UHFFFAOYSA-N pyridinium p-toluenesulfonate Chemical compound C1=CC=[NH+]C=C1.CC1=CC=C(S([O-])(=O)=O)C=C1 ZDYVRSLAEXCVBX-UHFFFAOYSA-N 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 125000004469 siloxy group Chemical group [SiH3]O* 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- CQLFBEKRDQMJLZ-UHFFFAOYSA-M silver acetate Chemical compound [Ag+].CC([O-])=O CQLFBEKRDQMJLZ-UHFFFAOYSA-M 0.000 description 1
- 229940071536 silver acetate Drugs 0.000 description 1
- WBHQBSYUUJJSRZ-UHFFFAOYSA-M sodium bisulfate Chemical compound [Na+].OS([O-])(=O)=O WBHQBSYUUJJSRZ-UHFFFAOYSA-M 0.000 description 1
- 229910000342 sodium bisulfate Inorganic materials 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000004611 spectroscopical analysis Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 239000008174 sterile solution Substances 0.000 description 1
- 239000003270 steroid hormone Substances 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 229940037128 systemic glucocorticoids Drugs 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- WMOVHXAZOJBABW-UHFFFAOYSA-N tert-butyl acetate Chemical compound CC(=O)OC(C)(C)C WMOVHXAZOJBABW-UHFFFAOYSA-N 0.000 description 1
- FGTJJHCZWOVVNH-UHFFFAOYSA-N tert-butyl-[tert-butyl(dimethyl)silyl]oxy-dimethylsilane Chemical compound CC(C)(C)[Si](C)(C)O[Si](C)(C)C(C)(C)C FGTJJHCZWOVVNH-UHFFFAOYSA-N 0.000 description 1
- BCNZYOJHNLTNEZ-UHFFFAOYSA-N tert-butyldimethylsilyl chloride Chemical compound CC(C)(C)[Si](C)(C)Cl BCNZYOJHNLTNEZ-UHFFFAOYSA-N 0.000 description 1
- 125000001981 tert-butyldimethylsilyl group Chemical group [H]C([H])([H])[Si]([H])(C([H])([H])[H])[*]C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 125000000037 tert-butyldiphenylsilyl group Chemical group [H]C1=C([H])C([H])=C([H])C([H])=C1[Si]([H])([*]C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- IMNIMPAHZVJRPE-UHFFFAOYSA-N triethylenediamine Chemical compound C1CN2CCN1CC2 IMNIMPAHZVJRPE-UHFFFAOYSA-N 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 235000001892 vitamin D2 Nutrition 0.000 description 1
- 239000011653 vitamin D2 Substances 0.000 description 1
- 235000005282 vitamin D3 Nutrition 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C49/00—Ketones; Ketenes; Dimeric ketenes; Ketonic chelates
- C07C49/587—Unsaturated compounds containing a keto groups being part of a ring
- C07C49/753—Unsaturated compounds containing a keto groups being part of a ring containing ether groups, groups, groups, or groups
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C401/00—Irradiation products of cholesterol or its derivatives; Vitamin D derivatives, 9,10-seco cyclopenta[a]phenanthrene or analogues obtained by chemical preparation without irradiation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C45/00—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds
- C07C45/27—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by oxidation
- C07C45/30—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by oxidation with halogen containing compounds, e.g. hypohalogenation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C45/00—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds
- C07C45/27—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by oxidation
- C07C45/30—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by oxidation with halogen containing compounds, e.g. hypohalogenation
- C07C45/305—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by oxidation with halogen containing compounds, e.g. hypohalogenation with halogenochromate reagents, e.g. pyridinium chlorochromate
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C49/00—Ketones; Ketenes; Dimeric ketenes; Ketonic chelates
- C07C49/385—Saturated compounds containing a keto group being part of a ring
- C07C49/517—Saturated compounds containing a keto group being part of a ring containing ether groups, groups, groups, or groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C69/00—Esters of carboxylic acids; Esters of carbonic or haloformic acids
- C07C69/013—Esters of alcohols having the esterified hydroxy group bound to a carbon atom of a ring other than a six-membered aromatic ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F7/00—Compounds containing elements of Groups 4 or 14 of the Periodic Table
- C07F7/02—Silicon compounds
- C07F7/08—Compounds having one or more C—Si linkages
- C07F7/18—Compounds having one or more C—Si linkages as well as one or more C—O—Si linkages
- C07F7/1804—Compounds having Si-O-C linkages
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Public Health (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Diabetes (AREA)
- Hematology (AREA)
- Immunology (AREA)
- Emergency Medicine (AREA)
- Endocrinology (AREA)
- Obesity (AREA)
- Oncology (AREA)
- Dermatology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Abstract
本发明涉及20-位改性的式(Ⅰ)维生素D-衍生物,式中X,Y,Z,R1,R2和R3如说明书所述,其制备方法及其作为皮肤过量增生疾病治疗剂的应用。
Description
本发明涉及式I维生素D衍生物及其制备方法,该方法中的中间产物,含该化合物的药剂及其在制药过程中的应用其中Y名为氢或1~9碳烷酰基或芳酰基;Z为氢,羟基或1~9碳烷酰基;X各为氢或两个X共同为环外甲基;R1和R2分别为氢,1~4碳烷基,共同为亚甲基或与季碳原子20共同成为环丙基单元,其中两个X为亚甲基时,R1和R2不是甲基;R3各为氢或1~15碳直链或支链烷基,三氟甲基或与叔碳原子共同形成的饱和或不饱和碳环或杂环3,4,5或6圆环;L为
,其中A为亚甲基或氧-,硫-或氢化物-或1-4碳烷基-取代的氮原子,而B为亚烷基-(CH2)n-,其中n=1,2,3,4,5或6并且任何亚甲基可被氧原子代替或L为其中D为第20和第22碳原子之间的直接键,亚甲基或1,2-次乙二基桥键(E-双键),E和F分别为氢或共同为第二键(E-双键),而G为直接键或亚烷基-(CH2)n-,其中n=1,2,3,4,5或6,任何亚甲基可被氧原子代替并且各亚甲基可被羟基或卤原子(氟,氯,溴)取代。
可作Y和Z的酰基或酰氧基尤其衍生自饱和羧酸或苯甲酸。
烷基R3可为甲基,乙基或丙基并且可为与叔碳原子共同形成的环丙或环戊环。
特别优选化合物为:
(5Z,7E)-(1S,3R)-26,27-二甲基-23-氧杂-9,10-断胆甾-5,7,10(19),20-四烯-1,3,25-三醇,
(5Z,7E)-(1S,3R)-26,27-二乙基-23-氧杂-9,10-断胆甾-5,7,10(19),20-四烯-1,3,25-三醇,
(7E)-(1R,3R)-20,26,27-三甲基-23-氧杂-19-降-9,10-断胆甾-5,7-二烯-1,3,25-三醇,
(5Z,7E)-(1S,3R)-26,27-二甲基-20,21-亚甲基-23-氧杂-9,10-断胆甾-5,7,10(19)-三烯-1,3,2 5-三醇,
(5Z,7E)-(1S,3R)-23-氧杂-9,10-断胆甾-5,7,10(19),20-四烯-1,3,25-三醇,
(5Z,7E)-(1S,3R)-24-(3-羟基-3-甲基丁基)-23-氧杂-9,10-断胆-5,7,10(19),20-四烯-1,3-二醇,
(5Z,7E)-(1S,3R)-24-(3-乙基-3-羟戊基)-23-氧杂-9,10-断胆-5,7,10(19),20-四烯-1,3-二醇,
(5Z,7E)-(1S,3R)-26,27-二乙基-20,21-亚甲基-23-氧杂-9,10-断胆甾-5,7,10(19)-三烯-1,3,25-三醇,
(7E)-(1R,3R)-23-氧杂-19-降-9,10-断胆甾-5,7,20-三烯-1,3,25-三醇
(5Z,7E)-(1S,3R)-20,21-亚甲基-23-氧杂-9,10-断胆甾-5,7,10(19)-三烯-1,3,25-三醇,
(5Z,7E)-(1S,3R)-24-(3-羟基-3-甲基丁基)-20,21-亚甲基-23-氧杂-9,10-断胆-5,7,10(19)-三烯-1,3-二醇,
(5Z,7E)-(1S,3R)-24-(3-乙基-3-羟戊基)-20,21-亚甲基-23-氧杂-9,10-断胆-5,7,10(19)-三烯-1,3-二醇,
(7E)-(1R,3R)-20-甲基-19-降-23-氧杂-9,10-断胆甾-5,7-二烯-1,3,25-三醇,
(7E)-(1R,3R)-26,27-乙基-20-甲基-19-降-23-氧杂-9,10-断胆甾-5,7-二烯-1,3,25-三醇,
(7E)-(1R,3R)-24-(3-羟基-3-甲基丁基)-20-甲基-19-降-23-氧杂-9,10-断胆-5,7-二烯-1,3-二醇,
(7E)-(1R,3R)-24-(3-乙基-3-羟戊基)-20-甲基-19-降-23-氧杂-9,10-断胆-5,7-二烯-1,3-二醇
(7E)-(1R,3R)-26,27-二甲基-19-降-23-氧杂-9,10-断胆甾-5,7,20-三烯-1,3,25-三醇,
(7E)-(1R,3R)-26,27-二乙基-19-降-23-氧杂-9,10-断胆甾-5,7,20-三烯-1,3,25-三醇,
(7E)-(1R,3R)-24-(3-羟基-3-甲基丁基)-19-降-23-氧杂-9,10-断胆-5,7,20-三烯-1,3-二醇,
(7E)-(1R,3R)-24-(3-乙基-3-羟戊基)-19-降-23-氧杂-9,10-断胆-5,7,20-三烯-1,3-二醇,
(5Z,7E,22E)-(1S,3R)-24-(2-羟基-2-甲基丙氧基)-9,10-断胆-5,7,10(19),20,22-五烯-1,3-二醇,
(5Z,7E,22E)-(1S,3R)-24-(2-乙基-2-羟丁氧基)-9,10-断胆-5,7,10(19),20,22-五烯-1,3-二醇,
(7E,22E)-(1R,3R)-24-(2-羟基-2-甲基丙氧基)-19-降-9,10-断胆-5,7,20,22-四烯-1,3-二醇,
(7E,22E)-(1R,3R)-24-(2-乙基-2-羟丁氧基)-19-降-9,10-断胆-5,7,20,22-四烯-1,3-二醇。
天然维生素D2和D3(见通式Vit.D)本身无生物活性,可先在肝中于25-位或在肾中于1-位羟基化后转化为其生物活性代谢物,维生素D2和D3的作用在于稳定血浆-Ca++和血浆-磷酸盐水平,可阻止血浆-Ca++水平下降。
麦角骨化醇:Ra=Rb=H,Rc=CH3 维生素D2
双键C-22/23
胆钙化〔甾〕醇:Ra=Rb=Rc=H 维生素D3
25-羟基胆钙化〔甾〕醇:Ra=Rc=H,Rb=OH
1a-羟基胆钙化〔甾〕醇:Ra=OH,Rb=Rc=H
1a-二羟基胆钙化〔甾〕醇:Ra=Rb=OH,Rc=H钙三醇
除了对钙和磷酸盐代谢是有显著作用外,维生素D2和D3及其衍生物还是有阻止细胞增生和细胞分化作用(H.F.De Luca,TheMetabolism and Function of Vitamin D inBiochemistry of Steroid Hormones,Hrsg.H.L.J.Makin,2nd Edition,Blackwell ScientificPublications 1984,S.71-116)。
维生素D应用中有过量问题(高钙血)。
24-位羟基化1α-胆钙化〔甾〕醇已见于DE-A-2526981,其毒性低于相应的非羟基化1α-胆钙化〔甾〕醇,羟基化化合物可选择性活化肠内钙吸收并且骨吸收作用弱于1α-胆钙化〔甾〕醇。
国际专利申请WO 87/00834所述24-羟基维生素D类似物可用于治疗不正常细胞增生和/或细胞分化引起的人和动物障碍。
对于多种1,25-二羟基同型维生素D衍生物,有关骨吸收作用和HL-60细胞分化性能的分离最近已由Deluca报到,其中体外骨吸收作用为体内钙活动的直接参数。
本发明化合物的维生素D活性可由钙三醇受体试验确定,其中可用取自小猪肠的专-性受体蛋进行(M.C.Dame,E.A.Pierce,H.F.Deluca,Proc.Natl.Acad.Sci.USA 82,7825(1985))。
含受体结合蛋白在试管中于4℃有和没有试验物质存在下用反应体积为0.270ml的3H-钙三醇(5×10-10mol/l)保持2小时,为将游离的和结合受体的钙三醇分离可进行木炭-葡聚糖吸收,其中各试管中加250ml木炭-葡聚糖悬浮液并4℃保持20分钟后试样在10000×g下4℃离心分离5分钟,上层清液倾析出来并平衡1小时后在Picofluor 15TM中用β-计数器测定。
用不同浓度的试验物质以及对比物质(未标示钙三醇)在恒定浓度的参考物质(3H-钙三醇)情况下所得竞争曲线相互关联起来而得出竞争因子(KF)。
该因子定为达到50%竞争性所需各试验物质和对比物质浓度之商:
为确定各种钙三醇衍生物的急性高钙血作用,可进行以下试验。
参考物质(溶剂培养基),对比物质(1,25(OH)2-D3=钙三醇)和试验物质作用可通过在10只天然鼠(140-170g)组中皮下一次注射来进行试验,该试验期间鼠关在特殊的笼中以确定水和矿物质的排泄,两次收集尿样(16h和22h)。口服钙剂(6.5%α-羟丙基纤维素中0.1mM钙,5ml/只动物)以补充因取消饲料而缺少的钙吸入量,试验结束时断头杀死各动物并且为确定血清钙质而使其失血,而为了体内进行初级过筛临床检查,可试验各标准剂量(200μg/kg),选定物质的结果可通过剂量-作用关系而保证得到。
高钙血作用体现在与参考物质比较而提高的血清钙水平值。
物质组和参考组间及试验物质和对比物质间的显著差别可用适宜统计方法保证得到,结果以剂量关系DR(DR=可比作用下试验物质剂量/对比物质剂量的因子)给出。
钙三醇类似物的分化刺激作用必要时可定量得到。
定量收集方法在文献中已知(Mangelsdorf,D.J.etal.,J.Cell.Biol.98;391-398(1984)),其中体外用钙三醇治疗人白血病细胞(PromyelozytenzellinieHL 60),诱发这些细胞分化成巨噬细胞。
HL 60-细胞用组织培养基(RPMI-10%胎儿牛犊血清)37℃在5%CO2空气气氛中培养。
试验中细胞离心分离后在无酚红的组织培养基中制成浓度为2.0×105个细胞/ml的悬浮液,再将试验物质溶于乙醇并用无酚红组织培养基稀释到要求浓度,然后将稀释液与细胞悬浮液以1∶10的比例混合并将100ml这种加有试验物质的细胞悬浮液吸移到96孔板凹槽内而参考细胞悬浮液中类似加溶剂。
在5%CO2空气气氛中37℃保持96小时后向96孔板凹槽内的细胞悬浮液中吸移入100ml NBT-TPA液(Nitrobl-autetrazolium(NBT)加料终浓度1mg/ml,Tetradecanoylphorbolmyristat-13-acetat(TPA)加料终浓度2×10-7mol/l)。
在37℃和5%CO2空气气氛中保持2小时,由于细胞内氧自由基释放而得以由TPA刺激,在分化成巨噬细胞的细胞中NBT还原成不溶性甲_。
反应结束时从96孔板凹槽中吸出物料并加甲醇使粘附细胞固定后干燥,为使形成的细胞内甲_晶体溶解而在各凹槽中吸移入100μl氢氧化钾(2val/l)和100μl二甲基甲酰胺并超声波辐射1分钟,而甲浓度由光谱测定法在650nm下测定。
HL60-细胞分化成巨噬细胞的诱导参数为形成的甲_浓度,该结果有时也以上述剂量关系DR给出。
钙三醇受体试验以及HL60-细胞诱导分化剂量关系和高钙组剂量关系测定结果总结如下:
(5Z,7E)-(1S,3R)-24-(3-乙基-3-羟戊基)-23-氧杂-9,10-断胆-5,7,10(19),20-四烯-1,3-二醇(16)
(5Z,7E)-(1S,3R)-26,27-二乙基-20,21-亚甲基-23-氧杂-9,10-断胆甾-5,7,10(19)-三烯-1,3,25-三醇(21)
(7E)-(1R,3R)-20,26,27-三甲基-19-降-23-氧杂-9,10-断胆甾-5,7-二烯-1,3,25-三醇(40)
(7E)-(1R,3R)-24-(3-乙基-3-羟戊基)-19-降-23-氧杂-9,10-断胆-5,7,20-三烯-1,3-二醇(87)
(5Z,7E,22E)-(1S,3R)-24-(2-羟基-2-甲基丙氧基)-9,10-断胆-5,7,10(19),20,22-五烯-1,3-二醇(92)
(5Z,7E,22E)-(1S,3R)-24-(2-乙基-2-羟丁氧基)-9,10-断胆-5,7,10(19),20,22-五烯-1,3-二醇(93)
对比化合物:钙三醇
选定化合物的生物数据:化合物 KF(受体) DR(HL 60) DR(高钙血)钙三醇 1 1 116 1.6 0.3 3021 2.2 10 10040 1.5 0.2 587 6.3 1 10092 8.3 1.5 10093 2.2 1 100
通过降低高钙血危险,本发明物质可以优选公式用于制备过量增生疾病的治疗药剂,如皮肤过量增生病(牛皮癣)和恶性肿瘤(白血病,肠癌和乳腺癌)以及痤疮(J.Invest.Dermatol.,Vol.92,No.3,1989),本发明化合物也可用于治疗和预防免疫系统失衡疾病,如自身免疫疾病,包括糖尿病和移植过程中的排斥现象(WO-A-91/00855)。在本发明特别优选的方案中可在治疗前明确所涉及器官中的钙三醇受体。
而且出人意料地发现,通过大鼠、小鼠和海豚皮肤上局部应用来发明化合物可增加皮肤潮红并加大表皮层厚度,其中皮肤潮红的增加可通过提高由色彩测定仪定量测得的皮肤表面红色值而达到,以典型方式间隔24小时3次用药(剂量0.003%)可使红色值提高约1.5倍,而表皮层厚度的加大可用组织切法定量测得,表面层增生细胞(皮肤周期的S-阶段细胞)数目可经导流血细胞计数法测得,其提高因子一般约6。
本发明化合物的这些性解可用药物制剂在萎缩皮肤上显示出来,如在皮肤自然变化过程中,由于光照增强或用糖〔肾上腺〕皮质激素治疗造成的药物诱发皮肤萎缩,会出现早期皮肤变异。
因此,本发明还涉及药物制剂,其中含至少一种式(I)化合物以及药用载体。
这些化合物可在药用溶剂中配制成溶液或在适宜药用溶剂或载体中配制成乳液,悬浮液或分散液或用固体载体按已知方式配制成丸,片或胶囊,而局部用药时这些化合物优选配制成乳膏或软膏或宜于局部应用的常规剂型,而且各些配分还可含其它非毒性药用助剂如稳定剂,抗氧化剂,粘合剂,色素,乳化剂或矫味剂。这些化合物优选服用方式可为注射或静脉输注适宜无菌溶液或经营养途径口服或以乳膏,软膏,洗剂或宜经皮用药的膏剂局部应用,如见于EP-A0387077。
日服用剂量0.1-1000μg/人/天,优选1.0-500μg/人/天。
本发明化合物可按已知“钙三醇”制剂用于治疗牛皮癣的用药方式给药。
此外,本发明还涉及式(I)化合物在药剂制备过程中的应用。
式(I)化合物,特别是其制备过程中必须的初始化合物可用新方法制得,本发明也涉及这些化合物的制备方法。
以下式I′化合物由式(I)化合物衍生,其中式I中标为X的两个取代基形成环外亚甲基。
Me3S+I-或Me3S+(O)I-类型试剂用碱如叔丁醇钾(KOtBu),NaH或KH脱质子可制得Schwefelyliden,再用其反应可得式VIII化合物,其中C-20上的立体化学结构不必是单一的。
用碱如二异丙氨化锂(LDA),二乙氨化锂(LiNEt2),双(三甲基甲硅烷基)氨化锂(LiN(TMS)2)或异丙醇铝(Al(OiPr)3重排环氧化物VIII可得烯丙醇IX,该化合物可灵活转化成式(I)化合物。
为合成式(I′)中R1和R2共同为亚甲基,L为
且A为氧的化合物,可将式IX化合物用式X化合物醚化其中L为离去基如Br,I,CH3C6H4SO2O;B为亚烷基-(CH2)n-,其中n=1,2或3;R为1-8碳直链或支链烷基以及R4和R5可各为OR基或R4和R5共同为氧,从而得到式XI化合物。
在其羰基上加式XII亲核试剂
R3-M (XII)其中R3为1-5碳直链或支链烷基且M为MgHal(Hal=Cl,Br,I)或碱金属原子(Li,Na,K),从而形成式XIII化合物其中Z′为羟基。
甲硅烷基分离后自由羟基必要时部分或全部用链烷酰氯,-溴或-酸酐或苯甲酰氯酯化,其中烷酰基部分含1-9碳。
例如可在所谓的“三重线增敏剂”存在下经紫外光照射而将式XIII化合物转化成式XIV化合物,其中本发明为此可用蒽,然后将5,6-双键的Pi-键分开,A-环绕5,6-单键转180℃并重新形成5,6-双键即可再次得到5,6-双键立体异构本。
然后脱除已有的羟基保护基,如用氟化四正丁铵进行并且必要时用相应酰卤(酰氯,酰溴)或酸酐按常见方法将自由羟基部分或全部酯化。
为合成式I′中R1和R2与季碳原子20共同为环丙基且L为的化合物,烯丙醇IX先类似于式XIII化合物与式XIV化合物的反应经光化学法异构化成式XV化合物后与I-CH2-Zn-I类型的金属有机试剂反应(Simmons-Smith反应)而形成式XVI化合物,其中用Zn/Cu,Zn/Ag或Et2Zn(二乙基锌)与CH2I2反应制成金属有机试剂。
再类似于上述反应将式XVI化合物经式XVII和XVIII中间产物转化成式IXX化合物,其中B,Q和Z′定义和分解性如上述。 为合成式I″中式I的两个X为氢且L为
或,可采用会聚合成法(CD-部分和A-部分分别建立)。
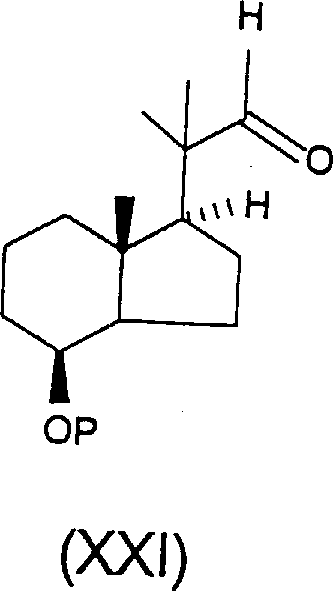
初始物料可用文献中已知的式XX醛(H.H.Inhoffen etal.Chem.Ber.91,780(1958),Chem.Ber.92,1772(1959))。其中P为氢,1-9碳烷酰基,四氢吡喃基或四氢呋喃基,烷基-或芳基-取代或烷基-和芳基-取代(混合取代)甲硅烷基,其烷酰基优选来自直链或支链饱和羧酸,优选为乙酰基或新戊酰基,而甲硅烷基尤其可举出叔丁基二甲基甲硅烷基,三甲基甲硅烷基,叔丁基二苯基甲硅烷基或三苯基甲硅烷基。
为合成式I″中R1和R2各为亚甲基的化合物,可用碱如NaH,KH,二异丙氨化锂(LDA)或叔丁醇钾(KOtBu)脱质子并与亲电子试剂CH3X(X=Cl,Br,I,CH3C6H4SO2O)反应而形成式XXI化合物。
式XXIV化合物中必要时存在的保护基脱除后得式XXV化合物,其中酰基保护基情况下在碱性条件(K2CO3/甲醇,KOH或NaOH/甲醇)下进行,在甲硅烷基保护基情况下用氟化物试剂(氟化四丁铵,HF,HF/吡啶)进行并在四氢吡喃或四氢呋喃醚保护基情况下用酸催化(对甲苯磺酸,PPTS,离子交换剂)进行,其仲羟基用氧化剂(PCC,PDC,Collins反应剂,BaMnO4)氧化并且其叔羟基Z′可被保护而成为例如甲硅烷基醚,如三甲基甲硅烷基醚,其中生成式XXVI化合物其中Z″为甲硅烷氧基,如三甲基甲硅烷氧基,四氢吡喃基或四氢呋喃基。
通过与用碱如正丁基锂(BuLi)或二异丙氨化锂(LDA)产生的文献中已知的膦氧化物XXVII(H.F.Deluca,TetrahedronLett.32,7663(1991))的阴离子反应,其中Q为烷基-或芳基-取代的甲硅烷基,从而得到式XXVIII化合物,其保护基Q和Z″可如上述脱除并必要时使自由羟基酰化。
为合成式I″中R1和R2共同为亚甲基或与季碳原子20共同形成环丙基单元的化合物,可用文献中已知的式XX醛类似于式VII化合物的制备过程分解成式V酮,其中P如上述。
类似于式VII→VIII→IX的顺序用式V化合物经式XXIX中间产物转化成式VI烯丙醇。
如上所述仅将式XXXIV酮与文献中已知的式XXVII膦氧化物偶联即可得式XXXV化合物,其保护基可如上述脱除并且其自由羟基必要时可酰化。为合成式I′中L为
D为20和22碳间的直接键,E和F为E-双键且G为CH2-O-CH2单元的化合物,可用氧化剂(二氧化锰,氯铬酸吡啶鎓,锰酸钡)将式XV醇氧化成式XXXVI醛,氧化剂亦可用重铬酸吡啶鎓。
用碱(NaH,KH,二异丙氨化锂,叔丁醇钾)脱质子化产生的式XXXVII膦酸盐阴离子进行Wadsworth-Emmons反应,
(RO)2P(O)-CH2-COOR1 (XXXVII)其中R和R1分别为1-9碳直链或支链烷基或苯基,从而产生式XXXVIII化合物,其酯基可用还原剂(NaBH4,NaBH4/CeCl3,LiAlH4,氢化二异丁铝〔DIBAH〕)还原成式XXXIX醇。再用上述式X化合物醚化可得式XL化合物。
在其羰基上加上述式R3-M(XII)亲核试剂可生成式XLI化合物,其中B,Q和Z′定义和分解性如上述。
为合成I″中L为
,式I中X为氢,而D,E和F以及G定义如上述的化合物,可按类似于上述合成式XLI化合物的方法将式VI烯丙醇氧化成式XLII化合物。
本发明尤其涉及以下中间化合物:
(5E,7E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-20-亚甲基-9,10-断孕甾-5,7,10(19)-三烯-21-醇,
(5Z,7E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-20-亚甲基-9,10-断孕甾-5,7,10(19)-三烯-21-醇,
〔1S-(1,3aβ,4α,7aα)〕-1-〔4-(乙酰氧基)-7a-甲基八氢-1H-茚-1-基〕乙酮(ethanon),
〔1R(1α,3aβ,4α,7aα)〕-4-〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-7a-甲基-β-亚甲基八氢-1H-茚-1-乙醇。
以下实施例详述本发明。
实施例
1.(5E,7E)-(1S,3R,20R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-20,21-桥氧-20-甲基-9,10-断孕甾-5,7,10(19)-三烯(2)
氩气下将3.1g(3.84mmol)(5E,7E)-(1S,3R)-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-9,10-断孕甾-5,7,10(19)-三烯-20-酮(1)(双TBDMS醚,见WO 90/09991,Leo PharmaceuticalProducts)溶于70ml N,N-二甲基甲酰胺(DMF)后加入1.06g(5.2mmol)碘化三甲基锍,冷至0℃后分批加入0.51g(5.2mmol)叔丁醇钾,0℃15分钟后加饱和氯化钠液,用乙酸乙酯萃取后有机相用氯化钠液多次洗涤,硫酸钠干燥并分出溶剂后经硅胶(己烷/乙酸乙酯)提纯剩余物而得2.2g无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,58ppm(s,3H,H-18);0,89 u.0,94(2x s,je 9H,Si-t-Butyl);1,32(s,3H,H-21);2,31 u.2,50(2x d,J=5Hz,je 1H,H-22 u.H-22’);4,19(m,1H,H-3);4,59(t,J=5,5Hz,1H,H-1);4,70u.4,82(2x s,je 1H,H-19 u.H-19’);5,57 u.6,31(2x d,J=11Hz,je1H,H-6 u.H-7);7,12-7,68(m,20H,Si-Phenyl)[Es wird durchg_ngig dieSteroid-Nummerierung verwendet]
2.(5E,7E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-20-亚甲基-9,10-断孕甾-5,7,10(19)-三烯-21-醇(3)
氩气下将0.28g(3.8mmol)二乙胺溶于35ml乙醚并0℃加入2.4ml(3.8mmol)正丁基锂液(己烷中1.6M),该温度下30分钟后滴加0.72g(0.88mmol)(2)的5ml乙醚液并0℃搅拌1小时后室温搅拌1小时,再加氯化钠液,用乙酸乙酯萃取后有机相用氯化钠液洗涤,硫酸钠干燥后剩余物浓缩并用硅胶色谱(己烷/乙酸乙酯)提纯而得360mg无色泡沫状题示化合物以及280mg初始物料。1H-NMR(CDCl3):δ=0,45ppm(s,3H,H-18);0,99 u.1,00(2x s,je 9H,Si-t-Butyl);4,08 u.4,17(2x d,J=14,5Hz,je 1H,H-22 u.H-22’);4,29(m,1H,H-3);4,65(m,1H,H-1);4,75 u.4,90(2x s,je 1H,H-19 u.H-19’);5,03 u.5,23(2x s,je 1H,H-21 u.H-21’);5,67 u.6,39(2xd,J=11Hz,je 1H,H-6 u.H-7);7,20-7,62(m,20H,Si-Phenyl)
3.(5E,7E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-23-氧杂-9,10-断胆-5,7,10(19),20-四烯-24-羧酸-1,1-二甲基乙基酯(4)
将800mg(0.97mmol)(3)加入3ml甲苯中并氩气下加入4.6ml氢氧化钠水溶液(25%),1.45g(7.4mmol)溴乙酸叔丁酯和22mg硫酸氢四丁铵,室温搅拌过夜后将混合物倒入氯化钠液中,乙酸乙酯萃取,用氯化钠液洗涤有机相,硫酸钠干燥并分出溶剂后硅胶(己烷/乙酸乙酯)提纯粗产品而得640mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,43ppm(s,3H,H-18);0,96 u.0,98(2x s,je 9H,Si-t-Butyl);1,50(s,9H,t-Butylester);3,98(s,2H,H-24);4,02(sbr,2H,H-22);4,29(m,1H,H-3);4,63(m,1H,H-1);4,72 u.4,89(2x s,je 1H,H-19 u.H-19’),5,07 u.5,23(2x s,je 1H,H-21 u.H-21’);5,65u.6,39(2x d,J=11Hz,je 1H,H-6 u.H-7);7,24-7,65(m,20H,Si-Phenyl)IR(KBr):v=1750cm-1
4.(5E,7E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-23-氧杂-9,10-断胆甾-5,7,10(19),20-四烯-25-醇(5)
在5ml乙醚中用90mg(3.7mmol)镁屑和522mg(3.7mmol)碘甲烷制成格利雅试剂,0℃仅加350mg(0.37mmol)(4)的2ml四氢呋喃(THF)溶液并室温搅拌1小时后用氯化铵液水解,用乙酸乙酯萃取水相,用氯化钠液洗涤有机相并用硫酸钠干燥,分出溶剂后硅胶色谱(己烷/乙酸乙酯)提纯剩余物而得125mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,43ppm(s,3H,H-18);0,98(s,18H,Si-t-Butyl);1,20(s,6H,H-26 u.H-27);3,21(d,J=9,5Hz,1H,H-24);3,28(d,J=9,5Hz,1H,H-24’);3,94(d,J=12,5Hz,1H,H-22);4,01(d,J=12,5Hz,1H,H-22’);4,30(m,1H,H-3);4,53(m,1H,H-1);4,69 u.4,89(2xs,je 1H,H-19 u.H-19’);5,02 u.5,20(2x s,je 1H,H-21 u.H-21’);5,67 u.6,41(2x d,J=11Hz,je 1H,H-6 u.H-7);7,25-7,65(m,20H,Si-Phenyl)
5.(5E,7E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-26,27-二甲基-23-氧杂-9,10-断胆甾-5,7,10(19),20-四烯-25-醇(6)
在5ml THF中氩气下用350mg(3.2mmol)溴乙烷和78mg(3.2mmol)镁屑制成格利雅试剂并同于4与310mg(0.33mmol)(4)反应而得270mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,46ppm(s,3H,H-18);0,90(t,J=7Hz,6H,H-28 u.H-29);0,96 u.0,98(2x s,je 9H,Si-t-Butyl);1,56(q,J=7Hz,4H,H-26 u.H-27);3,27 u.3,32(2x d,J=10Hz,je 1H,H-24 u.H-24’);3,92 u.4,01(2x d,J=13Hz,je 1H,H-22 u.H-22’);4,29(m,1H,H-3);4,64(m,1H,H-1);4,73 u.4,90(2x s,je 1H,H-19 u.H-19’);5,03 u.5,20(2x s,je 1H,H-21 u.H-21’);5,65 u.6,39(2x d,J=11Hz,je 1H,H-6 u.H-7);7,24-7,64(m,20H,Si-Phenyl)
6.(5E,7E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-26,27-二乙基-23-氧杂-9,10-断胆甾-5,7,10(19),20-四烯-25-醇(7)
在5ml THF中氩气下用390mg(3.2mmol)1-溴丙烷和78mg(3.2mmol)镁屑制成格利雅试剂并同于4与310mg(0.33mmol)(4)反应而得265mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,44ppm(s,3H,H-18);0,92(t,J=7Hz,6H,H-30 u.H-31);0,96 u.0,98(2x s,je 9H,Si-t-Butyl);3,24 u.3,30(2x d,J=11Hz,je 1H,H-24 u.H-24’);3,91 u.4,00(2x d,J=13Hz,je 1H,H-22 u.H-22’);4,29(m,1H,H-3);4,63(m,1H,H-1);4,73 u.4,90(2xs,je 1H,H-19 u.H-19’);5,02 u.5,20(2x s,je 1H,H-21 u.H-21’);5,65 u.6,39(2x d,J=11Hz,je 1H,H-6 u.H-7);7,23-7,63(m,20H,Si-Phenyl)
7.(5Z,7E)-(1S,3R)-23-氧杂-9,10-断胆甾-5,7,10(19),20-四烯-1,3,25-三醇(8)
在Pyrex-Tauchreaktor中将125mg(0.14mmol)(5),25mg蒽和5μl三乙胺溶于80ml甲苯中并在氮气氛中用高压水银灯(Philips HPK 125)照射15分钟,浓缩后将剩余物溶于20ml THF中,加2.1ml氟化四丁铵液(THF中1M)并60℃氩气下搅拌1小时后将反应混合物搅拌加入饱和碳酸氢钠液,用乙酸乙酯萃取,硫酸钠干燥并分出溶剂,再用硅胶色谱(己烷/乙酸乙酯)多次提纯而得25mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,40ppm(s,3H,H-18);1,12(s,6H,H-26 u.H-27);3,12(d,J=9,5Hz,1H,H-24);3,18(d,J=9,5Hz,1H,H-24’);3,83(d,J=14Hz,1H,H-22);3,92(d,J=14Hz,1H,H-22’);4,08(m,1H,H-3);4,31(m,1H,H-1);4,89(s,2H,H-19 u.H-21);5,10(s,1H,H-21’);5,25(s,1H,H-19’);5,98 u.6,29(2x d,J=11Hz,je 1H,H-6 u.H-7)
8.(5Z,7E)-(1S,3R)-26,27-二甲基-23-氧杂-9,10-断胆甾-5,7,10(19),20-四烯-1,3,25-三醇(9)
类似于7用270mg(0.2 9mmol)(6)反应并相应提纯后得41mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,49ppm(s,3H,H-18);0,88 u.0,90(2xt,J=7Hz,je 3H,H-28 u.H-29);1,51(q,J=7Hz,4H,H-26 u.H-27);3,22 u.3,30(2x d,J=9,5Hz,je 1H,H-24 u.H-24’);3,90 u.3,98(2x d,J=14Hz,je 1H,H-22 u.H-22’);4,18(m,1H,H-3);4,39(m,1H,H-1);4,98(s,2H,H-19 u.H-21);5,18(s,1H,H-21’);5,30(s,1H,H-19’);6,05 u.6,38(2x d,J=11Hz,je 1H,H-6 u.H-7)
9.(5Z,7E)-(1S,3R)-26,27-二乙基-23-氧杂-9,10-断胆甾-5,7,10(19),20-四烯-1,3,25-三醇(10)
类似于7用265mg(0.24mmol)(7)反应并相应提纯后得38mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,48ppm(s,3H,H-18);0,90(t,J=7Hz,6H,H-30 u.H-31);3,22 u.3,28(2x d,J=9,5Hz,H-24 u.H-24’);3,89 u.3,98(2x d,J=14Hz,H-22 u.H-22’);4,18(m,1H,H-3);4,39(m 1H,H-1);4,98(s,2H,H-19 u.H-21);5,17(s,1H,H-21’);5,30(s,1H,H-19’);6,04 u.6,38(2x d,J=11Hz,je 1H,H-6 u.H-7)
10.(5Z,7E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-20-亚甲基-9,10-断孕甾-5,7,10(19)-三烯-21-醇(11)
将500mg(0.61mmol)(3)溶于80ml甲苯中,与80mg(0.44mmol)蒽和15μl三乙胺反应并在7所述装置中照射18分钟,处理并提纯而得450mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,43ppm(s,3H,H-18);0,95 u.1,00(2x s,je 9H,Si-t-Butyl);4,05 u.4,15(2x d,J=14,5Hz,je 1H,H-22 u.H-22’);4,25(m,1H,H-3);4,55(m,1H,H-1);4,83(s,1H,H-19);5,00(s,1H,H-21);5,08(s,1H,H-19’);5,21(s,1H,H-21’);6,02 u.6,10(2x d,J=11Hz,je 1H,H-6 u.H-7);7,15-7,68(m,20H,Si-Phenyl)
11.3-〔(5Z,7E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-23-氧杂-9,10-断胆-5,7,10(19),20-四烯-24-基〕丙酸甲酯(12)
将500mg(0.61mmol)(11)溶于1ml甲苯中并与2.8ml苛性钠水溶液(25%),12mg硫酸氢四丁铵和681mg(1.83mmol)4-溴原丁酸三甲酯并室温搅拌过夜后倒入氯化钠液中,用乙酸乙酯萃取,用氯化钠液洗涤有机相,硫酸钠干燥后分出溶剂,色谱提纯而得180mg无色泡沫状题示化合物以及130mg未反应离析物。1H-NMR(CDCl3):δ=0.42ppm(s,3H,H-18);0,92 u.1,00(2x s,je 9H,Si-t-Butyl);2,47(t,J=7Hz,2H,H-26);3,70(s,3H,COOMe);3,46(m,2H,H-24);3,91(s,2H,H-22);4,24(m,1H,H-3);4,55(m,1H,H-1);4,83(s,1H,H-19);4,98(s,1H,H-21);5,08(s,1H,H-19’);5,17(s,1H,H-21’);6,03 u.6,10(2x d,J=11Hz,je 1H,H-6 u.H-7);7,22-7,70(m,20H,Si-Phenyl)
12.(5Z,7E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-24-(3-羟基-3-甲基丁基-23-氧杂-9,10-断胆-5,7,10(19),20-四烯(13)
在5ml乙醚中用185mg(1.3mmol)碘甲烷和31mg(1.3mmol)镁屑制成格利雅试剂并同于4与120mg(0.13mmol)(12)反应而得60mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,42ppm(s,3H,H-18);0,91 u.1,00(2x s,je 9H,Si-t-Butyl);1,23(s,6H,H-28 u.H-29);3,48(m,2H,H-24);3,92(s,2H,H-22);4,24(m,1H,H-3);4,54(m,1H,H-3);4,83(s,1H,H-19);5,00(s,1H,H-21);5,09(s,1H,H-19’);5,19(s,1H,H-21’);6,01 u.6,09(2x d,J=11Hz,H-6 u.H-7);7,22-7,68(m,20H,Si-Phenyl)
13.(5Z,7E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-24-(3-乙基-3-羟戊基)-23-氧杂-9,10-断胆-5,7,10(19),20-四烯(14)
在5ml THF中用142mg(1.3mmol)溴乙烷和31mg(1.3mmol)镁屑制成格利雅试剂并同于4与120mg(0.13mmol)(12)反应而得70mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,42ppm(s,3H,H-18);0,88(t,J=7Hz,6H,H-30 u.H-31);0,92 u.1,00(2x s,je 9H,Si-t-Butyl);1,49(q,J=7Hz,H-28 u.H-29);3,46(m,2H,H-24);3,91(s,2H,H-22);4,24(m,1H,H-3);4,54(m,1H,H-1);4,82(s,1H,H-19);4,98(s,1H,H-21);5,08(s,1H,H-19’);5,18(s,1H,H-21’);6,01 u.6,09(2x d,J=11Hz,H-6 u.H-7);7,23-7,69(m,20H,Si-Phenyl)
14.(5Z,7E)-(1S,3R)-24-(3-羟基-3-甲基丁基)-23-氧杂-9,10-断胆-5,7,10(19),20-四烯-1,3-二醇(15)
将57mg(0.062mmol)(13)溶于5ml THF中并加0.67ml氟化四丁铵液(THF中1M)后60℃搅拌1小时,再加氯化钠液后用乙酸乙酯萃取,用氯化钠液洗涤有机相,硫酸钠干燥并分出溶剂,硅胶色谱(己烷/乙酸乙酯)多次提纯而得9mg无色泡沫状题示化合物1H-NMR(CD2Cl2):δ=0,47ppm(s,3H,H-18);1,19(s,6H,H-28 u.H-29);3,42(m,2H,H-24);3,89(s,2H,H-22);4,18(m,1H,H-3);4,39(m,1H,H-1);4,97(s,2H,H-19 u.H-21);5,17(s,1H,H-19’);5,30(s,1H,H-21’);6,05 u.6,29(2x d,J=11Hz,je 1H,H-6 u.H-7)
15.(5Z,7E)-(1S,3R)-24-(3-乙基-3-羟戊基)-23-氧杂-9,10-断胆-5,7,10(19),20-四烯-1,3-二醇(6)
类似于14将67mg(0.07mmol)(14)与0.76ml氟化四丁铵液在5ml THF中反应后提纯而得11mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,47ppm(s,3H,H-18);0,84(t,J=7Hz,6H,H-30 u.H-31);1,47(q,J=7Hz,H-28 u.H.29);3,40(m,2H,H-24);3,89(m,2H,H-22);4,18(m,1H,H-3);4,39(m,1H,H-1);4,97(s,2H,H-19 u.H-21);5,16(s,1H,H-19’);5,30(s,1H,H-21’);6,04 u.6,38(2x d,J=11Hz,H-6 u.H-7)
16.(5Z,7E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-20,21-亚甲基-9,10-断孕甾-5,7,10(19)-三烯-20-甲醇(17)
用锌粉和乙酸银类似于J.M.Conia et al.(Synthesis549(1972))制成锌/银试剂,氩气下仅将98mg(1.5mmol)试剂加入5ml乙醚中并缓慢滴加268mg(1mmol)二碘甲烷后将反应液稍为煮沸,室温搅拌30分钟并加入200mg(0.24mmol)(11)的5ml乙醚液,室温再搅拌1小时后加0.2ml吡啶,滤除产生的沉淀后用乙酸乙酯稀释滤液,有机相用碳酸氢钠和氯化钠液洗涤,硫酸钠干燥并分出溶剂,剩余物经硅胶色谱(己烷/乙酸乙酯)提纯而得65mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,20ppm(m,1H,Cyclopropyl);0,34(m,2H,Cyclopropyl);0,55(s,3H,H-18);0,66(m,1H,Cyclopropyl);0,92 u.0,99(2x s,je 9H,Si-t-Butyl);3,04 u.3,92(2xdbr,J=10,5Hz,je 1H,H-22 u.H-22’);4,23(m,1H,H-3);4,54(m,1H,H-1);4,82 u.5,09(2xs,je 1H,H-19 u.H-19’);5,98 u.6,10(2x d,J=11Hz,je 1H,H-6 u.H-7);7,22-7,68(m,20H,Si-Phenyl)
17.(5Z,7E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-20,21-亚甲基-23-氧杂-9,10-断胆-5,7,10(19)-三烯-24-羧酸-1,1-二甲基乙基酯(18)
类似于3在1ml甲苯中将130mg(17)与0.16g(0.81mmol)溴乙酸叔丁酯,0.7ml氢氧化钠水溶液和3mg硫酸氢四丁铵反应后提纯而得80mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,28-0,42ppm(m,3H,Cyclopropyl);0,53(s,3H,H-18);0,62(m,1H,Cyclopropyl);0,90 u.0,98(2x s,je 9H,Si-t-Butyl);1,50(s,9H,t-Butylester);2,99(dbr,J=10,5Hz,1H,H-22);3,72(d,J=10Hz,1H,H-24);3,90(dbr,J=10,5Hz,1H,H-22’);3,90(d,J=10Hz,1H,H-24’);4,25(m,1H,H-3);4,55(m,1H,H-1);4,82 u.5,08(2x s,je 1H,H-19 u.H-19’);5,99 u.6,12(2x d,J=11Hz,je 1H,H-6 u.H-7);7,28-7,68(m,20H,Si-Phenyl)
18.(5Z,7E)-(1S,3R)-20,21-亚甲基-23-氧杂-9,10-断胆甾-5,7,10(19)-三烯-1,3,25-三醇(19)
在5ml乙醚中用170mg(1.2mmol)碘甲烷和30mg(1.2mmol)镁屑制成格利雅试剂并同于4与120mg(0.13mmol)(18)反应,所得粗产物同于14直接与1.1ml氟化四丁铵的THF液反应并色谱提纯而得14mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,14-0,30ppm(m,3H,Cyclopropyl);0,54(s,3H,H-18);1,02(m,1H,Cyclopropyl);1,18(s,6H,H-26 u.H-27);2,82(d,J=10Hz,1H,H-22);3,05(d,J=9,5Hz,1H,H-24);3,17(d,J=9,5Hz,1H,H-24’);3,68(d,J=10Hz,1H,H-22’);4,09(m,1H,H-3);4,30(m,1H,H-1);4,88 u.5,21(2x s,je 1H,H-19 u.H-19’);5,91 u.6,28(2x d,J=11Hz,je 1H,H-6 u.H-7)
19.(5Z,7E)-(1S,3R)-26,27-二甲基-20,21-亚甲基-23-氧杂-9,10-断胆甾-5,7,10(19)-三烯-1,3,25-三醇(20)
用19.4 mg(0.8mmol)镁屑和88mg(0.8mmol)溴乙烷制成格利雅试剂并同于4与80mg(0.1mmol)(18)反应,所得粗产物溶于5ml THF并同于14与0.85ml氟化四丁铵的THF液反应后经硅胶色谱(己烷/乙酸乙酯)多次提纯而得22mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,21-0,40ppm(m,3H,Cyclopropyl);0,52(s,3H,H-18);0,85(t,J=7Hz,6H,H-28 u.H-29);0,89(m,1H,Cyclopropyl);1,49(q,J=7Hz,4H,H-26 u.H-27);2,88(dbr,J=10,5Hz,1H,H-22);3,17(d,J=10Hz,1H,H-24);3,26(d,J=10Hz,1H,H-24’);3,72(dbr,J=10,5Hz,1H,H-22’);4,17(m,1H,H-3);4,39(m,1H,H-1);4,97 u.5,29(2x s,je 1H,H-19 u.H-19’);6,00 u.6,38(2x d,J=11Hz,je 1H,H-6 u.H-7)
20.(5Z,7E)-(1S,3R)-26,27-二乙基-20,21-亚甲基-23-氧杂-9,10-断胆甾-5,7,10(19)-三烯-1,3,25-三醇(21)
在5ml THF中用163mg(1.3mmol)1-溴丙烷和32mg(1.3mmol)镁屑制成格利雅试剂并同于4与125mg(0.13mmol)(18)反应,所得粗产物同于14与0.72ml氟化四丁铵液在5ml THF中反应并多次色谱提纯而得26mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,20-0,37ppm(m,3H,Cyclopropyl);0,90(t,J=7Hz,6H,H-30 u.H-31);1,08(m,1H,Cyclopropyl);2,87(d,J=10Hz,1H,H-22);3,13(d,J=9,5Hz,1H,H-24);3,23(d,J=9,5Hz,1H,H-24’);3,70(d,J=10Hz,1H,H-22’);4,17(m,1H,H-3);4,37(m,1H,H-1);4,93 u.5,28(2x s,je 1H,H-19 u.H-19’);5,98 u.6,35(2x d,J=11Hz,je 1H,H-6 u.H-7)
21. 3-〔(5Z,7E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-20,21-亚甲基-23-氧杂-9,10-断胆-5,7,10(19)-三烯-24-基〕丙酸甲酯(22)
400mg(0.48mmol)(17),2.2ml苛性钠水溶液(25%),10mg硫酸氢四丁铵和536mg(1.44mmol)4-溴原丁酸三甲酯在1ml甲苯中同于11反应,提纯而得100mg无色泡沫状题示化合物以及310mg初始物料。1H-NMR(CDCl3):δ=0,18-0,35ppm(m,3H,Cyclopropyl);0,47(s,3H,H-18);0,53(m,1H,Cyclopropyl);0,88 u.0,97(2x s,je 9H,Si-t-Butyl);2,37(t,J=7Hz,2H,H-26);2,74(d,J=10,5Hz,1H,H-22);3,35(m,2H,H-24);3,60(s,3H,COOMe);3,62(d,J=10,5Hz,1H,H-22’);4,17(m,1H,H-3);4,48(m,1H,H-1);4,76 u.5,02(2x s,je 1H,H-19 u.H-19’);5,90u.6.02(2x d,J=11Hz,je 1H,H-6 u.H-7);7,20-7,60(m,20H,Si-Phenyl)
22.(5Z,7E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-24-(3-乙基-3-羟戊基)-20,21-亚甲基-23-氧杂-9,10-断胆-5,7,10(19)-三烯(23)
在5ml THF中用305mg(2.8mmol)溴乙烷和68mg(2.8mmol)镁屑制成格利雅试剂并同于4与145mg(0.15mmol)(22)反应而得103mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,28-0,45ppm(m,3H,Cyclopropyl);0,52(s,3H,H-18);0,61(m,1H,Cyclopropyl);0,88(t,J=7Hz,6H,H-30 u.H-31);0,90 u.0,99(2x s,je 9H,Si-t-Butyl);2,86(d,J=10Hz,1H,H-22);3,39(m,2H,H-24);3,70(d,J=10Hz,1H,H-22’);4,23(m,1H,H-3);4,54(t,J=6Hz,1H,H-1);4,82 u.5,09(2x s,je 1H,H-19 u.H-19’);5,98 u,6,09(2x d,J=11Hz,je 1H,H-6 u.H-7);7,22-7,68(m,20H,Si-Phenyl)
23.(5Z,7E)-(1S,3R)-24-(3-乙基-3-羟戊基)-20,21-亚甲基-23-氧杂-9,10-断胆-5,7,10(19)-三烯-1,3-二醇(24)
在5ml THF中同于11将100mg(0.1mmol)(23)与1ml氟化四丁铵反应并提纯而得21mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,10-0,29ppm(m,3H,Cyclopropyl);0,51(s,3H,H-18);0,78(t,J=7Hz,H-30 u.H-31);1,01(m,1H,Cyclopropyl);1,38(q,J=7Hz,4H,H-28 u.H-29);2,78(d,J=10Hz,1H,H-22);3,28(m,2H,H-24);3,58(d,J=10Hz,1H,H-22’);4,09(m,1H,H-3);4,30(m,1H,H-1);4,88 u.5,22(2x s,je 1H,H-19);5,91 u.6,29(2x d,J=11Hz,je 1H,H-6 u.H-7)
24.〔1S-(1α,3aβ,4α,7aα)〕-4-(乙酰氧基)八氢-α,α,7a-三甲基-1H-茚-1-乙醛(25)
在120ml THF中用900mg(30mmol)氢化钠(80%)制成悬浮液并氢气下0℃滴入6.3g(25mmol)〔1R-〔1α(S*),3aβ,4α,7aα〕〕-4-(乙酰氧基)-α,7a-二甲基八氢-1H-茚-1-乙醛(H.H.Inhoffen etal.Chem.Ber.91,780(1958),Chem.Ber.92,1772(1959))的60ml THF液,30分钟后再滴加19.65g(75mmol)甲基碘并50℃搅拌6小时,冷却后将反应混合物倒入氯化钠液中,用乙酸乙酯萃取,有机相用氯化钠液洗涤后硫酸钠干燥并分出溶剂,剩余物经硅胶色谱(己烷/乙酸乙酯)提纯而得3.2g无色油状题示化合物。1H-NMR(CDCl3):δ=0,82ppm(s,3H,H-18);1,05 u.1,08(2x s,je 3H,H-21 u.C-20-Methyl);1,99(s,3H,OAc);5,09(m,1H,H-8);9,60(s,1H,H-22)IR(Film):v=1725,1710cm-1
25.〔1S-(1α,3aβ,4α,7aα)〕-4-(乙酰氧基)八氢-β,β,7a-三甲基-1H-茚-1-乙醇(26)
将350mg(1.3mmol)(25)溶于5ml THF和5ml甲醇中并加入193mg(1.4mmol)三氯化铈七水合物,0℃分批加入仅46mg(1.2mmol)硼氢化钠并再搅拌1小时,然后用氯化钠液水解,用乙酸乙酯萃取,有机相用氯化钠液洗涤后用硫酸钠干燥并浓缩,剩余物经硅胶色谱(己烷/乙酸乙酯)提纯而得285mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,90ppm(s,3H,H-18);1,00 u.1,01(2x s,je 3H,H-21 u.C-20-Methyl);2,05(s,3H,OAc);3,29 u.3,37(2x d,J=10,5Hz,je 1H,H-22 u.H-22’);5,16(m,1H,H-8)IR(Film):v=1725cm-1
26.〔1S-(1α,3aβ,4α,7aα)〕-1,1-二甲基乙基-〔2-〔4-(乙酰氧基)-7a-甲基八氢-1H-茚-1-基〕-2-甲基丙氧基〕乙酸酯(27)
将3.04g(11.3mmol)(26)溶于40ml甲苯中,氩气下加11.9(61.3mmol)溴乙酸叔丁酯,33.9ml氢氧化钠水溶液(25%)和172mg硫酸氢四丁铵,室温搅拌48小时后倒入氯化钠液,用乙酸乙酯萃取后,有机相用氯化钠液洗涤后用硫酸钠干燥并分出溶剂,剩余物用硅胶色谱(己烷/乙酸乙酯)提纯而得1.1g无色油状题示化合物以及1.93g初始物料。1H-NMR(CDCl3):δ=0,88ppm(s,3H,H-18);0,93 u.0,99(2x s,je 3H,H-21 u.C-20-Methyl);1,41(s,9H,t-Butylester);1,99(s,3H,OAc);3,03 u.3,20(2x d,J=9Hz,je1H,H-22 u.H-22’);3,82 u.3,90(2x d,J=16Hz,je 1H,H-24 u.H-24’);5,09(m,1H,H-8)
27.〔1S-(1α,3aβ,4α,7aα)〕-1-〔1,1-二甲基-2-(2-乙基-2-羟丁氧基)乙基〕-7a-甲基八氢-1H-茚-4-醇(28)
在20ml THF中用10.8g(100mmol)溴乙烷和928mg(88mmol)镁屑制成格利雅试剂并0℃加1.1g(2.8mmol)(27)的39ml THF溶液,室温搅拌1小时后将反应混合物倒入饱和氯化铵液中,用乙酸乙酯萃取后有机相用氯化钠液洗涤,用硫酸钠干燥并蒸出溶剂后粗产物经硅胶色谱(己烷/乙酸乙酯)提纯而得995mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,88ppm(s,3H,H-18);0,90(t,J=7Hz,6H,H-28 u.H-29);1,00 u.1,07(2x s,je 3H,H-21 u.C-20-Methyl);1,51(q,J=7Hz,4H,H-26 u.H-27);3,11 u.3,16(2xd,J=9,5Hz,je 1H,H-22 u.H-22’);3,21 u.3,27(2x d,J=9,5Hz,je 1H,H-24 u.H-24’);4,09(m,1H,H-8)
28.〔1S-(1α,3aβ,4α,7aα)〕-1-〔1,1-二甲基-2-(2-羟基-2-甲基丙氧基)乙基〕-7a-甲基八氢-1H-茚-4-醇(29)
在20ml乙醚中用2.04g(14.4mmol)碘甲烷和350mg(14.4mmol)镁屑制成格利雅试剂并同于27与690mg(1.8mmol)(27)反应而得410mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,87ppm(s,1H,H-18);0,94 u.1,00(2x s,je 3H,H-21 u.C-20-Methyl);1,15(s,3H,H-26 u.H-27);2,31(sbr,1H,OH);3,09(s,2H,H-22);3,11(d,J=9,5Hz,1H,H-24);3,18(d,J=9,5Hz,1H,H-24’);4,02(m,1H,H-8)
29.〔1S-(1α,3aβ,4α,7aα)〕-1-〔1,1-二甲基-2-(2-羟基-2-丙基戊氧基)乙基〕-7a-甲基八氢-1H-茚-4-醇(30)
在20ml THF中用1.97g(14.4mmol)1-溴丙烷和350mg(14.4mmol)镁屑制成格利雅试剂并同于27与690mg(1.8mmol)(27)反应而得630mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,82ppm(s,3H,H-18);0,84(t,J=7Hz,6H,H-30 u.H-31);0,93 u.0,98(2x s,je 3H,H-21 u.C-20-Methyl);2,29(t,J=6Hz,1H,OH);3,07(s,2H,H-22);3,12(d,J=9,5Hz,1H,H-24);3,18(d,J=9,5Hz,1H,H-24’);4,01(m,1H,H-8)
30.〔1S-(1α,3aβ,7aα)〕-1-〔1,1-二甲基-2-(2-乙基-2-羟丁氧基)乙基〕-7a-甲基八氢-4H-茚-4-酮(31)
氩气下将890mg(2.8mmol)(28)的10ml二氯甲烷液滴入1.41g(6.6mmol)氯铬酸吡啶鎓的50ml二氯甲烷悬浮液中,室温2小时后用乙醚稀释,用Celite多次过滤后分出溶剂,剩余物用硅胶色谱(己烷/乙酸乙酯)提纯而得696mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,72ppm(s,3H,H-18);0,90(t,J=7Hz,6H,H-28 u.H-29);0,92 u.1,02(2x s,je 3H,H-21 u.C-20-Methyl);1,52(q,J=7Hz,4H,H-26 u.H-27);3,18(s,2H,H-22);3,22 u.3,28(2x d,J=9,5Hz,je 1H,H-24 u.H-24’)
31.〔1S-(1α,3aβ,7aα)〕-1-〔1,1-二甲基-2-(2-羟基-2-甲基丙氧基)乙基〕-7a-甲基八氢-4H-茚-4-酮(32)
在20ml二氯甲烷中同于30将410mg(1.37mmol)(29)与379mg(1.76mmol)氯铬酸吡啶鎓反应而得273mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,76ppm(s,3H,H-18);0,88 u.0,97(2x s,je 1H,H-21 u.C-20-Methyl);1,15(s,6H,H-26 u.H-27);2,36(dd,J=10,5,7,5Hz,1H,H-14);3,12(s,2H,H-22);3,12(d,J=9,5Hz,1H,H-24);3,19(d,J=9,5Hz,1H,H-24’)
32.〔1S-(1α,3aβ,7aα)〕-1-〔1,1-二甲基-2-(2-羟基-2-丙基戊氧基)乙基〕-7a-甲基八氢-4H-茚-4-酮(33)
在20ml二氯甲烷中同于30将640mg(1.83mmol)(30)与503mg(2.34mmol)氯铬酸吡啶鎓反应而得386mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,75ppm(s,3H,H-18);0,86(t,J=7Hz,6H,H-30 u.H-31);0,86 u.0,95(2x s,je 3H,H-21 u.C-20-Methyl);2,35(dd,J=10,5,7,5Hz,1H,H-14);3,08(s,2H,H-22);3,13(d,J=9,5,2H,H-22);3,18(d,J=9,5Hz,1H,H-22’)
33.〔1S-(1α,3aβ,7aα)〕-1-〔1,1-二甲基-2-〔2-乙基-2-〔(三甲基甲硅烷基)氧代〕丁氧基〕乙基〕-7a-甲基八氢-4H-茚-4-酮(34)
氩气下将696mg(2.1mmol)(31),571mg(8.4mmol)咪唑和456mg(4.2mmol)三甲基氯硅烷溶于10mlDMF中并室温搅拌过夜,然后加氯化钠液,用乙酸乙酯萃取,有机相用氯化钠液洗涤,用硫酸钠干燥并分出溶剂后剩余物经硅胶色谱(己烷/乙酸乙酯)提纯而得766mg无色油状题示化合物。1H-NMR(CD2Cl2):δ=0,10ppm(s,9H,SiMe3);0,70(s,3H,H-18);0,82(t,J=7Hz,6H,H-28 u.H-29);0,91 u.1,03(2x s,je 3H,H-21 u.C-20-Methyl);1,53(q,J=7Hz,4H,H-26 u.H-27);3,05 u.3,13(2x d,J=9Hz,je 1H,H-22 u.H-22’);3,20 u.3,24(2x d,J=9,5Hz,je 1H,H-24 u.H-24’)
34.〔1S-(1α,3aβ,7aα)〕-1-〔1,1-二甲基-2-〔2-甲基-2-〔(三甲基甲硅烷基)氧代〕丙氧基〕乙基〕-7a-甲基八氢-4H-茚-4-酮(35)
在30ml乙醚中同于33将270mg(0.92mmol)(32)与300mg(2.76mmol)三甲基氯硅烷,248mg(3.59mmol)咪唑和0.37ml吡啶反应而得272mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,11ppm(s,9H,SiMe3);0,72(s,3H,H-18);0,91 u.1,02(2x s,je 3H,H-21 u.C-20-Methyl);1,23(s,6H,H-26 u.H-27);2,41(dd,J=10,5,7,5Hz,1H,H-14);3,11(d,J=9,5Hz,1H,H-22);3,14(2x d,J=9,5Hz,je 1H,H-22’u.H-24);3,19(d,J=9,5Hz,1H,H-24’)
35.〔1S-(1α,3aβ,7aα)〕-1-〔1,1-二甲基-2-〔2-丙基-2-〔(三甲基甲硅烷基)氧代〕戊氧基〕乙基〕-7a-甲基八氢-4H-茚-4-酮(36)
在30ml乙醚中同于33将383mg(1.10mmol)(33)与358mg(3.30mmol)三甲基氯硅烷,296mg(4.29mmol)咪唑和0.44ml吡啶反应而得386mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,11ppm(s,9H,SiMe3);0,73(s,3H,H-18);0,90(t,J=7Hz,6H,H-30u.H-31);0,91 u.1,03(2x s,je 3H,H-21 u.C-20-Methyl);2,42(dd,J=10,5,7,5Hz,1H,H-14);2,92 u.3,00(2x d,J=9,5Hz,je 1H,H-22 u.H-22’);3,08 u.3,12(2x d,J=9,5Hz,je1H,H-24 u.H-24’)
36.(7E)-(1R,3R)-1,3-双〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-20,26,27-三甲基-25-〔(三甲基甲硅烷基)氧代〕-19-降-23-氧杂-9,10-断胆甾-5,7-二烯(37)
将200mg(0.35mmol)(3R-反式)-〔2-〔3,5-双〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕亚环己基〕乙基〕二苯基膦氧化物〔H.F.Deluca et al.Tetrahedron Lett.32,7663(1991))溶于10mlTHF中并氩气下冷至-70℃,然后仅滴加0.21ml(0.36mmol)正丁基锂液(己烷中1.6M),5分钟后滴入277mg(0.7mmol)(34)的4ml THF液并于该温度下搅拌30分钟,之后用酒石酸钾-钠/碳酸氢钾液水解,用乙酸乙酯萃取,有机相用氯化钠液洗涤,用硫酸钠干燥并分出溶剂,剩余物经硅胶色谱(己烷/乙酸乙酯)提纯而得80mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,00ppm(s,12H,SiMe2);0,07(s,9H,SiMe3);0,58(s,3H,H-18);0,80(t,J=7Hz,6H,H-28 u.H-29);0,82(s,18H,Si-t-Butyl);0,88 u.0,98(2x s,je 3H,H-21u.C-20-Methyl);2,98 u.3,08(2x d,J=9Hz,je 1H,H-22 u.H-22’);3,12 u.3,18(2x d,J=9,5Hz,je 1H,H-24 u.H-24’);4,02(m,2H,H-1 u.H-3);5,76 u.6,12(2x d,J=11Hz,je 1H,H-6u.H-7)
37.(7E)-(1R,3R)-1,3-双〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-20-甲基-25-〔(三甲基甲硅烷基)氧代〕-19-降-23-氧杂-9,10-断胆甾-5,7-二烯(38)
类似于36用61mg(0.16mmol)(33)反应而得75mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,04,0,05,0,11ppm(3x s,21H,SiMe);0,62(s,3H,H-18);0,87 u.0,88(2x s,je 9H,Si-t-Butyl);0,90 u.1,01(2x s,je 3H,H-21 u.C-20-Methyl);1,22(s,6H,H-26 u.H-27);3,10(d,J=9,5Hz,H-22);3,12(d,J=9,5Hz,1H,H-24);3,17(d,J=9,5Hz,1H,H-22’);3,18(d,J=9,5Hz,1H,H-24’);4,08(m,2H,H-1 u.H-3);5,80 u.6,18(2x d,J=11Hz,je 1H,H-6 u.H-7)
38.(7E)-(1R,3R)-1,3-双〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-26,27-二乙基-20-甲基-25-〔(三甲基甲硅烷基)氧代〕-19-降-23-氧杂-9,10-断胆甾-5,7-二烯(39)
类似于36用126g(0.30mmol)(35)反应而得193mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,04,0,05,0,10ppm(3x s,21H,SiMe);0,62(s,3H,H-18);0,88(t,J=7Hz,6H,H-30 u.H-31);0,87(2x s,je 9H,Si-t-butyl);0,92 u.1,02(2x s,je 3H,H-21 u.C-20-Methyl);3,03 u.3,12(2x d,J=9,5Hz,je 1H,H-22);3,18 u.3,21(2x d,J=9,5Hz,je 1H,H-24);4,08(m,2H,H-1 u-H-3);5,81 u.6,18(2x d,J=11Hz,je 1H,H-6 u-H-7)
39.(7E)-(1R,3R)-20,26,27-三甲基-19-降-23-氧杂-9,10-断胆甾-5,7-二烯-1,3,25-三醇(40)
将80mg(0.106mmol)(37)溶于12ml THF中并氩气下加183mg(0.58mmol)氟化四丁铵后55℃搅拌2小时,然后加入氯化钠液,用乙酸乙酯萃取,有机相用氯化钠液洗涤,用硫酸钠干燥并分出溶剂,剩余物经硅胶色谱(己烷/乙酸乙酯)提纯而得24mg题示化合物无色晶体。1H-NMR(CD2Cl2):δ=0,63ppm(s,3H,H-18);0,85(t,J=7Hz,6H,H-28 u.H-29);0,93 u.1,00(2x s,je 3H,H-21 u.C-20-Methyl);1,49(q,J=7Hz,4H,H-26 u.H-27);3,15 u.3,17(2xd,J=9Hz,je 1H,H-22 u.H-22’);3,22 u.3,27(2x d,J=9,5Hz,je 1H,H-24 u.H-24’);3,98 u.4,07(2x m,je 1H,H-1 u.H-3);5,85 u.6,28(d,J=11Hz,je 1H,H-6 u.H-7)UV(MeOH):λmax=251nm,Fp:155℃
40.(7E)-(1R,3R)-20-甲基-19-降-23氧杂-9,10-断胆甾-5,7-二烯-1,3,25-三醇(41)
在5ml THF中同于39将72mg(0.10mmol)(38)与234mg(0.75mmol)氟化四丁铵反应并提纯而得29mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,57ppm(s,3H,H-18);0,87 u.0,94(2x s,je 3H,H-21 u.C-20-Methyl);1,10(s,6H,H-26 u.H-27);3,09(d,J=9,5Hz,1H,H-24);3,10(s,2H,H-22);3,13(d,J=9,5Hz,1H,H-24’);3,91 u.3,99(2x m,je 1H,H-1 u.H-3);5,77 u.6,20(d,J=11Hz,je 1H,H-6 u.H-7)
41.(7E)-(1R,3R)-26,27-二乙基-20-甲基-19-降-23-氧杂-9,10-断胆甾-5,7-二烯-1,3,25-三醇(42)
在12ml THF中同于39将190mg(0.24mmol)(39)与571mg(1.83mmol)氟化四丁铵反应并提纯而得87mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,57ppm(s,3H,H-18);0,84(t,J=7Hz,6H,H-30 u.H-31);0,85 u.0,92(2x s,je 3H,H-21 u.C-20-Methyl);3,08(s,2H,H-22);3,11(d,J=9,5Hz,1H,H-24);3,17(d,J=9,5Hz,1H,H-24’);3,91 u.3,99(2x m,je 1H,H-1 u.H-3);5,77 u.6,20(2x d,J=11Hz,je 1H,H-6 u.H-7)
42.〔1S-(1α,3aβ,4α,7aα)〕-4-〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕八氢-α,α,7a-三甲基-1H-茚-乙醛(43)
在130ml THF中同于24将9.2g(28.34mmol)〔1R-〔1α(S*),3aβ,4α,7aα〕〕-α,7a-二甲基-4-〔〔二甲基-(1,1-二甲基乙基)甲硅烷基〕氧代〕八氢-1H-茚-1-乙醛(W.G.Dauben et al.Tetranedron Lett.30,6m(1989)与1.02g(34.05mmol)氢化钠(80%)和12.07g(85.03mmol)碘甲烷反应而得7.89g无色油状题示化合物。1H-NMR(CDCl3):δ=0,01ppm(2x s,je 3H,SiMe);0,88(s,9H,Si-t-Butyl);0,98(s,3H,H-18);1,09 u.1,12(2x s,je 3H,H-21 u.C-20-Methyl);4,01(m,1H,H-8);9,68(s,1H,H-22)
43.〔1S-(1α,3a β,4α,7aα)〕-4-〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕八氢-β,β,7a-三甲基-1H-茚-1-乙醇(44)
在27ml THF/27ml甲醇中同于25将3.5g(10.33mmol)(43)与1.53g(11.1mmol)三氯化铈七水合物和365mg(9.53mmol)硼氢化钠反应而得2.36g无色油状题示化合物。1H-NMR(CDCl3):δ=0,01ppm(2x s,je 3H,SiMe);0,89(s,9H,Si-t-Butyl);0,89(s,3H,H-18);0,99 u.1,05(2x s,je 3H,H-21 u.H-20-Methyl);1,60(t,J=5Hz,1H,OH);3,30(dd,J=11,5,5Hz,1H,H-22);3,36(dd,J=11,5Hz,1H,H-22’);4,00(m,1H,H-8)
44.〔1S-(1α,3aβ,4α,7aα)〕-4-〔2-〔4-〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-7a-甲基八氢-1H-茚-1-基〕-2-甲基丙氧基〕丁酸甲酯(45)
在9.3ml苛性钠溶液(25%)和3ml甲苯中同于11将2.36g(6.93mmol)(44)与6.3g(27.7mmol)4-溴原丁酸三甲酯和366mg硫酸氢四丁铵反应而得3.14g无色油状题示化合物。1H-NMR(CDCl3):δ=0,01ppm(2x s,je 3H,SiMe);0,89(s,9H,Si-t-Butyl);0,89(s,3H,H-18);0,98 u.1,03(2x s,je 3H,H-21 u.C-20-Methyl);2,41(t,J=7Hz,2H,H-26);3,03(d,J=9Hz,1H,H-22);3,10(d,J=9Hz,1H,H-22’);3,38(t,J=7Hz,2H,H-24);3,70(s,3H,COOMe);4,00(m,1H,H-8)
45.〔1S-(1α,3aβ,4α,7aα)〕-5-〔2-〔4-〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-7a-甲基八氢-1H-茚-1-基〕-2-甲基丙氧基〕-2-甲基-2-戊醇(46)
在10ml乙醚中用1.21g(8.5mmol)碘甲烷和206mg(8.5mmol)镁屑制成格利雅试剂并同于27与750mg(1.7mmol)(45)反应而得453mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,02ppm(2x s,je 3H,SiMe);0,89(s,9H,Si-t-Butyl);0,92(s,3H,H-18);1,00 u.1,04(2x s,je 3H,H-21 u.C-20-Me);1,22(s,6H,H-28 u.H-29);3,09(d,J=9,5Hz,1H,H-22);3,18(d,J=9,5Hz,1H,H-22’);3,42(t,J=7Hz,2H,H-24);4,00(m,1H,H-8)
46.〔1S-(1α,3aβ,4α,7aα)〕-1-〔2-〔4-〔〔二甲基(1,1-甲基乙基)甲硅烷基〕氧代〕-7a-甲基八氢-1H-茚-1-基〕-2-甲基丙氧基〕-4-乙基-4-己醇(47)
在20ml THF中用935mg(8.5mmol)溴乙烷和206mg(8.5mmol)镁屑制成格利雅试剂并同于27与750mg(1.7mmol)(45)反应而得412mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,01ppm(2x s,je 3H,SiMe);0,88(t,J=7Hz,6H,H-30 u.H-31);0,89(s,9H,Si-t-Butyl);0,90(s,3H,H-18);1,00 u.1,04(2x s,je 3H,H-21 u.C-20-Methyl);1,48(q,J=7Hz,4H,H-28 u.H-29);3,07(d,J=9,5Hz,1H,H-22);3,14(d,J=9,5Hz,1H,H-22’);3,40(t,J=7Hz,2H,H-24);3,99(m,1H,H-8)
47.〔1α-(1α,3aβ,4α,7aα)〕-1-〔1,1-二甲基-〔2-(4-羟基-4-甲基戊氧基)乙基〕-7a-甲基八氢-1H-茚-4-醇(48)
在8.2ml THF/乙腈(1∶1)中将400mg(0.91mmol)(46)与4.1ml氢氟酸(40%)室温搅拌30分钟后用苛性钠稀溶液中和,用乙酸乙酯萃取,有机相用氯化钠液洗涤并用硫酸钠干燥,分出溶剂后的剩余物色谱提纯而得266mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,84ppm(s,3H,H-18);0,92 u.1,00(2x s,je 3H,H-21 u.C-20-Methyl);1,17(s,6H,H-28 u.H-29);3,01 u.3.09(2x d,J=9,5Hz,je 1H,H-22 u.H-22’);3,36(t,J=7Hz,2H,H-24);4,00(m,1H,H-8)
48.〔1S-(1α,3aβ,4α,7aα)〕-1-〔1,1-二甲基-〔2-(4-乙基-4-羟己氧基)乙基〕-7a-甲基八氢-1H-茚-4-醇(49)
在7.8ml THF/乙腈(1∶1)同于47将412mg(0.87mmol)(47)与3.9ml氢氟酸(40%)反应而得251mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,80ppm(t,J=7Hz,6H,H-30 u.H-31);0,82(s,3H,H-18);0,93 u.0,99(2x s,je 3H,H-21 u.C-20-Methyl);1,42(q,J=7Hz,4H,H-28 u.H-29);3,00 u.3,08(2xd,J=9,5Hz,je 1H,H-22 u.H-22’);3,33(t,J=7Hz,2H,H-24);4,00(m,1H,H-8)
49.〔1S-(1α,3aβ,7aα)〕-1-〔1,1-二甲基-〔2-(4-羟基-4-甲基戊氧基)乙基〕-7a-甲基八氢-4H-茚-4-酮(50)
在16ml二氯甲烷中同于30将260mg(0.80mmol)(48)与240mg(1.12mmol)氯铬酸吡啶鎓反应而得201mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,69ppm(s,3H,H-18);0,88 u.1,01(2x s,je 3H,H-21 u.C-20-Methyl);1,22(s,6H,H-28 u.H-29);2,42(dd,J=10,5,7,5Hz,1H,H-14);3,07 u.3,13(2x d,J=9,5Hz,je 1H,H-22 u.H-22’);3,39(t,J=7Hz,2H,H-24)
50.〔1S-〔1α,3aβ,7aα)〕-1-〔1,1-二甲基-〔2-(4-乙基-4-羟己氧基)乙基〕-7a-甲基八氢-4H-茚-4-酮(51)
在16ml二氯甲烷中同于30将251mg(0.71mmol)(49)与212mg(0.99mmol)氯铬酸吡啶鎓反应而得183mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,70ppm(s,3H,H-18);0,86(t,J=7Hz,6H,H-30 u.H-31);0,90 u.1,01(2x s,je 3H,H-21 u.H-20-Methyl);1,48(q,J=7Hz,4H,H-28 u.H-29);2,42(dd,J=10,5,7,5Hz,1H,H-14);3,08 u.3,15(2x d,J=9,5Hz,je 1H,H-22 u.H-22’);3,40(t,J=7Hz,2H,H-24)
51.〔1S-(1α,3aβ,7aα)〕-1-〔1,1-二甲基-〔2-〔4-甲基-〔(三甲基甲硅烷基)氧代〕戊氧基〕乙基〕-7a-甲基八氢-4H-茚-4-酮(52)
在15ml乙醚中同于33将201mg(0.62mmol)(50)与205mg(1.86mmol)三甲基氯硅烷,167mg(2.42mmol)咪唑和0.25ml吡啶反应而得194mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,11ppm(s,9H,SiMe3);0,82(s,3H,H-18);0,90 u.1,01(2x s,je 3H,H-21 u.C-20-Methyl);1,22(s,6H,H-29 u.H-30);2,43(dd,J=10,5,7,5Hz,1H,H-14);3,07u.3,12(2x d,J=9,5Hz,je 1H,H-22);3,37(t,J=7Hz,2H,H-24)
52.〔1S-(1α,3aβ,7aα)〕-1-〔1,1-二甲基-〔2-〔4-乙基-4-〔(三甲基甲硅烷基)氧代〕己氧基〕乙基〕-7a-甲基八氢-4H-茚-4-酮(53)
在15ml乙醚中同于33将183mg(0.52mmol)(51)与171mg(1.56mmol)三甲基氯硅烷,140mg(2.03mmol)咪唑和0.21ml吡啶反应而得178mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,10ppm(s,9H,SiMe3);0,70(s,3H,H-18);0,82(t,J=7Hz,6H,H-30u.H-31);0,91 u.1,00(2x s,je 3H,H-21 u.C-20-Methyl);2,42(dd,J=10,5,7,5Hz,1H,H-14);3,05 u.3,11(2x d,J=9,5Hz,je 1H,H-22);3,35(t,J=7Hz,2H,H-24)
53.(7E)-(1R,3R)-1,3-双〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-20-甲基-24-〔3-甲基-3-〔(三甲基甲硅烷基)氧代〕丁基〕-19-降-23-氧杂-9,10-断胆-5,7-二烯(54)
类似于36用100mg(0.25mmol)(52)反应而得130mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,01 u.0,08ppm(2x s,21H,SiMe3 u.SiMe);0,58(s,3H,H-18);0,82(s,18H,Si-t-Butyl);0,86 u.0,97(2x s,je 3H,H-21 u.C-20-Methyl);1,18(s,6H,H-28 u.H-29);3,01 u.3,09(2x d,J=9,5Hz,je 1H,H-22 u.H-22’);3,32(t,J=7Hz,2H,H-24);4,03(m,2H,H-1 u.H-3);5,76 u.6,12(2x d,J=11Hz,je 1H,H-6 u.H-7)
54.(7E)-(1R,3R)-1,3-双〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-20-甲基-24-〔3-乙基-3-〔(三甲基甲硅烷基)氧代〕戊基〕-19-降-23-氧杂-9,10-断胆-5,7-二烯(55)
类似于36用100mg(0.24mmol)(53)反应而得111mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,02 u.0,10ppm(2x s,21H,SiMe3 u.SiMe);0,56(s,3H,H-18);0,79(t,J=7Hz,6H,H-30 u.H-31);0,83(s,18H,Si-t-Butyl);0,85 u.0,95(2x s,je 3H,H-21 u.C-20-Methyl);3,02 u.3,08(2x d,J=9,5Hz,je 1H,H-22 u.H-22’);3,26(t,J=7Hz,2H,H-24);3,99(m,2H,H-1 u.H-3);5,70 u.6,07(2x d,J=11Hz,je 1H,H-6 u.H-7)
55.(7E)-(1R,3R)-24-(3-羟基-3-甲基丁基)-20-甲基-19-降-23-氧杂-9,10-断胆-5,7-二烯-1,3-二醇(56)
在10ml THF中同于39将125mg(0.17mmol)(54)与424mg(1.36mmol)氟化四丁铵反应而得56mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,55ppm(s,3H,H-18);0,82 u.0,90(2x s,je 3H,H-21 u.C-20-Methyl);1,10(s,6H,H-28 u.H-29);3,00 u.3,08(2x d,J=9,5Hz,je 1H,H-22 u.H-22’);3,30(t,J=7Hz,2H,H-24);3,91 u.3,98(2x m,je 1H,H-1 u.H-3);5,77 u.6,20(2x d,J=11Hz,je 1H,H-6 u.H-7)
56.(7E)-(1R,3R)-24-(3-乙基-3-羟戊基)-20-甲基-19-降-23-氧杂-9,10-断胆-5,7-二烯-1,3-二醇(57)
在10ml THF中同于39将106mg(0.14mmol)(55)与340mg(1.09mmol)氟化四丁铵反应而得57mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,54ppm(s,3H,H-18);0,77(t,J=7Hz,6H,H-30 u.H-31);0,81 u.0,90(2x s,je 3H,H-21 u.C-20-Methyl);1,38(q,J=7Hz,4H,H-28 u.H-29);3,00 u.3,08(2xd,J=9,5Hz,je 1H,H-22 u.H-22’);3,30(t,J=7Hz,2H,H-24);3,91 u.3,98(2x m.je 1H,H-1u.H-3);5,77 u.6,20(2x d,J=11Hz,je 1H,H-6 u.H-7)
57.〔1S-(1α,3aβ,4α,7aα)〕-1-〔4-(乙酰氧基)-7a-甲基八氢-1H-茚-1-基〕乙酮(ethanon)(58)
将19.2g(76.1mmol)〔1R-〔1α(S*),3aβ,4α,7aα〕〕-4-(乙酰氧基)-α,7a-二甲基八氢-1H-茚-1-乙醛(见24)溶于1000ml DMF中并加7.59g(66.5mmol)1,4-二氮双环〔2.2.2〕辛烷,1.14g(5.7mmol)乙酸酮(II)一水合物和909mg(5.7mmol)2,2′-联吡啶后加热到70℃并同时导入氧气,24小时后倒入氯化钠液中,用乙酸乙酯萃取,用氯化钠液洗涤,硫酸钠干燥并分出溶剂剩余物经硅胶色谱(己烷/乙酸乙酯)提纯而得12.75g无色油状题示化合物。1H-NMR(CDCl3):δ=0,83ppm(s,3H,H-18);2,03(s,3H,H-21);2,12(s,3H,OAc);2,50(t,J=9,5Hz,1H,H-17);5,19(m,1H,H-8)
58.〔1S-(1α,3aβ,4α,7aα)〕-7a-甲基-1-(2-甲基-2-环氧乙烷基)八氢-1H-茚-4-酮(59)
将12.75g(53.5mmol)(58)溶于400mlDMF中并加入15.2g(86mmol)碘化三甲基锍,冷至0℃后分批加入29.8g(267mmol)叔丁氧钾,室温搅拌24小时后加入氯化钠液,用乙酸乙酯萃取,有机相用氯化钠液洗涤,硫酸钠干燥并分出溶剂后粗产物经硅胶色谱(己烷/乙酸乙酯作洗涤剂)提纯而得7.91g无色油状题示化合物。1H-NMR(CDCl3):δ=1,00ppm(s,3H,H-18);1,30(s,3H,H-21);2,25(d,J=5Hz,1H,H-22);2,43(d,J=5Hz,1H,H-22’);4,02(m,1H,H-8)
59.〔1S-(1α,3aβ,4α,7aα)〕-4-〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-7a-甲基-1-(2-甲基-2-环氧乙烷基)八氢-1H-茚(60)
向7.91g(37.6mmol)(59)的200ml DMF液中加10.19g(150mmol)咪唑和11.25g(75mmol)叔丁基二甲基甲硅烷基氯并室温搅拌过夜,然后倒入氯化钠液中,用乙酸乙酯萃取,有机相用氯化钠液洗涤,用硫酸钠干燥并分出溶剂后剩余物溶于50ml乙醚中并0℃滴入二乙基氨化锂液中〔2.77g(37.8mmol)二乙胺,24.28ml正丁基锂液(己烷中1.6M)〕,再室温搅拌24小时,加氯化钠液,用乙酸乙酯萃取,有机相用氯化钠液洗涤,硫酸钠干燥并分出溶剂后色谱提纯而得8.64g无色油状题示化合物。1H-NMR(CDCl3):δ=0,01 u.0,10ppm(2x s,je 3H,SiMe);0,88(s,9H,Si-t-Butyl);0,90(s,3H,H-18);1,36(s,3H,H-21);2,31(d,J=5Hz,H-22);2,50(d,J=5Hz,H-22’);4,00(m,1H,H-8)
60.〔1S-(1α,3aβ,4α,7aα)〕-4-〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-7a-甲基-β-亚甲基八氢-1H-茚-1-乙醇(61)
将8.46g(26.06mmol)(60)溶于500ml甲苯中并加10.8g(53.2mmol)异丙醇铝后120℃搅拌过夜,冷却后加水/异丙醇,过滤后滤液浓缩,剩余物经硅胶色谱(己烷/乙酸乙酯)提纯而得7.56g题示化合物。1H-NMR(CDCl3):δ=0,01 u.0,02ppm(2x s,je 3H,SiMe);0,80(s,3H,H-18);0,89(s,9H,Si-t-Butyl);2,05(t,J=9,5Hz,1H,H-17);4,00(dbr,J=15Hz,1H,H-22);4,02(m,1H,H-8);4,10(dd,J=15,5Hz,1H,H-22’);4,92 u.5,20(2x s,je 1H,H-21)
61.〔1S-〔1α,3aβ,4α,7aα〕〕-1,1-二甲基乙基-2-〔4-〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-7a-甲基八氢-1H-茚-1-基〕-2-丙烯氧基〕乙酸酯(62)
在18ml甲苯中同于3将2g(6.16mmol)与27.6ml苛性钠溶液(25%),129mg硫酸氢四丁铵和5.96g(30.55mmol)溴乙酸叔丁酯而得2.67g无色油状题示化合物。1H-NMR(CDCl3):δ=0,01ppm(2x s,je 3H,SiMe);0,80(s,3H,H-18);0,90(s,9H,Si-t-Butyl);1,50(s,9H,t-Butylester);3,93(s,2H,H-24);3,98(s,2H,H-22);4,02(m,1H,H-8);4,98 u.5,22(2x s,je 1H,H-21)
62.〔1S-〔1α,3aβ,4α,7aα〕〕-3-〔〔〔2-〔4-〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-7a-甲基八氢-1H-茚-1-基〕-2-丙烯基〕氧代〕甲基〕-3-戊醇(63)
在30ml THF中用1.34g(12.32mmol)溴乙烷和300mg(12.3mmol)镁屑制成格利雅试剂并同于4与2.67g(6.24mmol)(62)反应而得2.58g无色油状题示化合物,不经提纯而用于进一步反应。
63.〔1S-〔1α,3aβ,4α,7aα〕〕-1-〔2-〔4-〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-7a-甲基八氢-1H-茚-1-基〕-2-丙烯氧基〕-2-甲基-2-丙醇(64)
在100ml乙醚中用2.52g(21.3mmol)碘甲烷和517mg(21.3mmol)镁屑制成格利雅试剂并同于4与3.4g(7.7mmol)(62)反应而得2.65g题示化合物。1H-NMR(CDCl3):δ=0,01ppm(2x s,je 3H,SiMe);0,80(s,3H,H-18);0,88(s,9H,Si-t-Butyl);1,21(s,6H,H-26 u.H-27);3,19 u.3,25(2x d,J=9Hz,je 1H,H-24 u.H-24’);3,89 u.3,96(2x d,J=13Hz,je 1H,H-22 u.H-22’);4,01(m,1H,H-8);4,93 u.5,18(2x s,je1H,H-21 u.H-21’)
64.〔1S-〔1α,3aβ,4α,7aα〕〕-1-〔2-(2-乙基-2-羟丁氧基)-1-亚甲基乙基〕-7a-甲基八氢-1H-茚-4-醇(65)
将2.58g(6.07mmol)(63)溶于60ml THF中,加12.4ml HF/吡啶(70%)并室温搅拌3天后倒入硫酸氢钠液,用乙酸乙酯萃取,有机相用碳酸氢钠液和氯化钠液洗涤并用硫酸钠干燥,分出溶剂后色谱提纯而得750mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,78ppm(s,3H,H-18);0,81(t,J=7Hz,6H,H-28 u.H-29);1,48(q,J=7Hz,4H,H-26 u.H-27);3,17 u.3,23(2x d,J=9Hz,je 1H,H-24 u.H-24’);3,81 u.3,90(2x d,J=12,5Hz,je 1H,H-22 u.H-22’);4,03(m,1H,H-8);4,89 u.5,14(2x s,je 1H,H-21 u.H-21’)
65.〔1S-〔1α,3aβ,4α,7aα〕〕-1-〔2-(2-羟基-2-甲基丙氧基)-1-亚甲基乙基〕-7a-甲基八氢-1H-茚-4-醇(66)
在38ml乙腈和30ml THF中将2g(5mmol)(64)与30ml HF(40%)反应并室温搅拌3小时后小心加入碳酸氢钠液,用乙酸乙酯萃取,用氯化钠液洗涤并用硫酸钠干燥,分出溶剂后色谱提纯而得1.25g无色油状题示化合物。1H-NMR(CDCl3):δ=0,85ppm(s,3H,H-18);1,22(s,6H,H-26 u.H-27);3,21 u.3,28(2x d,J=9Hz,je 1H,H-24 u.H-24’);3,91 u.3,98(2x d,J=13Hz,je 1H,H-22 u.H-22’);4,10(m,1H,H-8);4,97 u.5,22(2x s,je 1H,H-21 u.H-21’)
66.〔1S-〔1α,3aβ,7aα〕〕-1-〔2-(2-乙基-2-羟丁氧基)-1-亚甲基乙基〕-7a-甲基八氢-4 H-茚-4-酮(67)
在50ml二氯甲烷中同于30将720mg(2.40mmol)(65)与755mg(3.51mmol)氯铬酸吡啶鎓反应而得522mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,52ppm(s,3H,H-18);0,80(t,J=7Hz,6H,H-28 u.H-29);1,21(s,1H,OH);1,48(q,J=7Hz,4H,H-26 u.H-27);3,18 u.3,23(2x d,J=9,5Hz,je 1H,H-24 u.H-24’);3,84 u.3,95(2x d,J=13Hz,je 1H,H-22 u.H-22’);4,96 u.5,16(2x s,je 1H,H-21 u.H-21’)
67.〔1S-〔1α,3aβ,7aα〕〕-1-〔2-(2-羟基-2-甲基丙氧基)-1-亚甲基乙基〕-7a-甲基八氢-4H-茚-4-酮(68)
在40ml二氯甲烷中同于30将600mg(2.12mmol)(66)与645mg(3mmol)氯铬酸吡啶鎓反应而得439mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,58ppm(s,3H,H-18);1,22(s,6H,H-26 u.H-27);2,57(dd,J=10,5,7,5Hz,1H,H-14);3,22 u.3,28(2x d,J=9Hz,je 1H,H-24 u.H-24’);3,95 u.4,04(2x d,J=13Hz,je 1H,H-22 u.H-22’);5,02 u.5,23(2x s,je 1H,H-21 u.H-21’)
68.〔1S-〔1α,3aβ,7aα〕〕-1-〔2-〔〔2-乙基-2-〔(三甲基甲硅烷基)氧代〕丁氧基〕-1-亚甲基乙基〕-7a-甲基八氢-4H-茚-4-酮(69)
在50ml乙醚中同于33将522mg(1.75mmol)(67)与554mg(5.1mmol)三甲基氯硅烷,459mg(6.63mmol)咪唑和0.7ml吡啶反应而得343mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,01ppm(s,9H,SiMe3);0,48(s,3H,H-18);0,77(t,J=7Hz,6H,H-28u.H-29);3,11 u.3,18(2x d,J=9,5Hz,je 1H,H-24 u.H-24’);3,75 u.3,87(2x d,J=13Hz,je1H,H-22 u.H-22’);4,91 u.5,16(2x s,je 1H,H-21 u.H-21’)
69.〔1S-〔1α,3aβ,7aα〕〕-7a-甲基-1-〔2-〔〔2-甲基-2-〔(三甲基甲硅烷基)氧代〕丙氧基〕-1-亚甲基乙基〕八氢-4H-茚-4-酮(70)
在50ml乙醚中同于33将420mg(1.5mmol)(68)与325mg(3mmol)三甲基氯硅烷。408mg(6mmol)咪唑和0.6ml吡啶反应而得445mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,10ppm(s,9H,SiMe3);0,58(s,3H,H-18);1,25(s,6H,H-26 u.H-27);2,57(dd,J=10,5,7,5Hz,1H,H-14);3,18 u.3,22(2x d,J=9Hz,je 1H,H-24 u.H-24’);3,92 u.4,00(2x d,J=13Hz,je 1H,H-22 u.H-22’);5,01 u.5,24(2x s,je 1H,H-21 u.H-21’)
70.(7E)-(1R,3R)-1,3-双〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-26,27-二甲基-25-(三甲基甲硅烷基)氧代-19-降-23-氧杂-9,10-断胆甾-5,7,20-三烯(71)
类似于36用300mg(0.81mmol)(69)反应而得508mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,06 u.0.09ppm(2x s,12H u.9H,SiMe);0,47(s,3H,H-18);0,83 u.0,84(2x s,je 9H,Si-t-Butyl);0,85(t,J=7Hz,6H,H-28 u.H-29);1,52(q,J=7Hz,4H,H-26u.H-27);3,20 u.3,27(2x d,J=9,5Hz,je 1H,H-24 u.H-24’);3,84 u.3,91(2x d,J=13Hz,je1H,H-22 u.H-22’);4,09(m,2H,H-1 u.H-3);4,97 u.5,20(2x s,je 1H,H-21 u.H-21’);5,83u.6,18(2x d,J=11Hz,je 1H,H-6 u.H-7)
71.(7E)-(1R,3R)-1,3-双〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-25-(三甲基甲硅烷基)氧代-19-降-23-氧杂-9,10-断胆甾-5,7,20-三烯(72)
类似于36用352mg(1mmol)(70)反应而得50mg无色泡沫状题示化合物,直接用于下一步反应。
72.(7E)-(1R,3R)-26,27-二甲基-19-降-23-氧杂-9,10-断胆甾-5,7,20-三烯-1,3,25-三醇(73)
在45ml THF中同于39将500mg(0.68mmol)(71)与1.70g(5.44mmol)氟化四丁铵反应而得217mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,40ppm(s,3H,H-18);0,82(t,J=7Hz,6H,H-28 u.H-29);1,48(q,J=7Hz,4H,H-26 u.H-27);3,18 u.3,23(2x d,J=9Hz,je 1H,H-24 u.H-24’);3,84 u.3,92(2x d,J=13Hz,je 1H,H-22 u.H-22’);3,99 u.4,06(2x m,je 1H,H-1 u.H-3);4,92 u.5,11(2x s,je 1H,H-21 u.H-21’);5,81 u.6,24(2x d,J=11Hz,je 1H,H-6 u.H-7)
73.(7E)-(1R,3R)-19-降-23-氧杂-9,10-断胆甾-5,7,20-三烯-1,3,25-三醇(74)
在10ml THF中同于39将50mg(0.07mmol)(72)与121mg(0.385mmol)氟化四丁铵反应而得22.5mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,40ppm(s,3H,H-18);1,10(s,6H,H-26 u.H-27);3,12 u.3,18(2xd,J=9Hz,je 1H,H-24 u.H-24’);3,85 u.3,92(2x d,J=13Hz,je 1H,H-22 u.H-22’);3,93 u.4,00(2x m,je 1H,H-1 u.H-3);4,91 u.5,10(2x s,je 1H,H-21 u.H-21’);5,70 u.6,20(2x d,J=11Hz,je 1H,H-6 u.H-7)
74.〔1S-〔1α,3aβ,4α,7aα〕〕-4-〔2-〔4-〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-7a-甲基八氢-1H-茚-1-基〕-2-丙烯氧基〕丁酸甲酯(75)
类似于11将2.30g(7.09mmol)(61)与6.45g(28.4mmol)4-溴原丁酸三甲酯,9.5ml苛性钠溶液(50%)和375mg硫酸氢四丁铵反应而得2.31g无色油状题示化合物。1H-NMR(CDCl3):δ=0,01ppm(2x s,je 3H,SiMe);0,80(s,3H,H-18);0,89(s,9H,Si-t-Butyl);2,42(t,J=6,5Hz,2H,H-26);3,40(t,J=6,5Hz,2H,H-24);3,68(s,3H,COOMe);3,85(s,2H,H-22);4,01(m,1H,H-8);4,92 u.5,18(2x s,je 1H,H-21 u.H-21’)
75.〔1S-〔1α,3aβ,4α,7aα〕〕-1-〔2-〔4-〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-7a-甲基八氢-1H-茚-1-基〕-2-丙烯氧基〕-4-甲基-4-戊醇(76)
在15ml乙醚中用1.15g(8.1mmol)碘甲烷和196mg(8.1mmol)镁屑制成格利雅试剂并用于4与1.15g(2.7mmol)(75)反应而得934mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,01ppm(2x s,je 3H,SiMe);0,80(s,3H,H-18);0,88(s,9H,Si-t-Butyl);1,21(s,6H,H-28 u.H-29);3,42(m,2H,H-24);3,88(s,2H,H-22);4,01(m,1H,H-8);4,92 u.5,18(2x s,je 1H,H-21 u.H-21’)
76.〔1S-〔1α,3aβ,4α,7aα〕〕-1-〔2-〔4-〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-7a-甲基八氢-1H-茚-1-基〕-2-丙烯氧基〕-4-乙基-4-己醇(77)
在15ml THF中用882mg(8.1mmol)溴乙烷和196mg(8.1mmol)镁屑制成格利雅试剂并同于4与1.15g(2.7mmol)(75)反应而得734mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,01ppm(2x s,je 3H,SiMe);0,80(s,3H,H-18);0,88(t,J=7Hz,6H,H-30 u.H-31);0,89(s,9H,Si-t-Butyl);1,48(q,J=7Hz,4H,H-28 u.H-29);3,42(m,2H,H-24);3,88(s,2H,H-22);4,02(m,1H,H-8);4,93 u.5,20(2x s,je 1H,H-21 u.H-21’)
77.〔1S-〔1α,3aβ,4α,7aα〕〕-1-〔2-(4-羟基-4-甲基戊氧基)-1-亚甲基乙基〕-7a-甲基八氢-1H-茚-4-醇(78)
在30ml THF中同于64将580mg(1.37mmol)(76)与4.41ml HF/吡啶(70%)反应而得156mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,78ppm(s,3H,H-18);1,18(s,6H,H-28 u.H-29);3,48(m,2H,H-24);3,82(s,2H,H-22);4,03(m,1H,H-8);4,89 u.5,15(2x s,je 1H,H-21 u.H-21’)
78.〔1S-〔1α,3aβ,4α,7aα〕〕-1-〔2-(4-乙基-4-羟己氧基)-1-亚甲基乙基〕-7a-甲基八氢-1H-茚-4-醇(79)
在35ml THF中同于64将734mg(1.62mmol)(77)与5.22ml HF/吡啶(70%)反应而得200mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,77ppm(s,3H,H-18);0,78(t,J=7Hz,6H,H-30 u.H-31);1,40(q,J=7Hz,4H,H-28 u.H-29);3,34(m,2H,H-24);3,70(s,2H,H-22);4,03(m,1H,H-8);4,88u.5,14(2x s,je 1H,H-21 u.H-21’)
79.〔1S-〔1α,3aβ,7aα〕〕-1-〔2-(4-羟基-4-甲基戊氧基)-1-亚甲基乙基〕-7a-甲基八氢-4H-茚-4-酮(80)
在10ml二氯甲烷中同于29将150mg(0.48mmol)(78)与144mg(0.67mmol)氯铬酸吡啶鎓反应而得124mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,58ppm(s,3H,H-18);1,23(s,6H,H-28 u.H-29);2,57(dd,J=10,5,7,5Hz,1H,H-14);3,42 u.3,50(2x dt,J=9,7Hz,je 1H,H-24 u.H-24’);3,90 u.3,97(2x d,J=12,5Hz,je 1H H-22 u.H-22’);5,02 u.5,24(2x s,je 1H,H-21 u.H-21’)
80.〔1S-〔1α,3aβ,7aα〕〕-1-〔2-(4-乙基-4-羟己氧基)-1-亚甲基乙基〕-7a-甲基八氢-4H-茚-4-酮(81)
在10ml二氯甲烷中同于30将200mg(0.59mmol)(79)与177mg(0.83ml)氯铬酸吡啶鎓反应而得145mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,58ppm(s,3H,H-18);0,88(t,J=7Hz,6H,H-30 u.H-31);1,50(q,J=7Hz,4H,H-28 u.H-29);2,58(dd,J=10,5,7,5Hz,1H,H-14);3,40 u.3,48(2x dt,J=9,7Hz,je 1H,H-24 u.H-24’);3,89 u.3,96(d,J=12,5Hz,je 1H,H-22 u.H-22’);5,01 u.5,23(2xs,je 1H,H-21 u.H-21’)
81.〔1S-〔1α,3aβ,7aα〕〕-1-〔2-〔4-甲基-4-〔(三甲基甲硅烷基)氧代〕戊氧基〕-1-亚甲基乙基〕-7a-甲基八氢-4H-茚-4-酮(82)
在10ml乙醚中同于33将120mg(0.39mmol)(80)与127mg(1.17mmol)三甲基氯硅烷,105mg(1.52mmol)咪唑和0.16ml吡啶反应而得119mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,10ppm(s,9H,SiMe3);0,58(s,3H,H-18);1,12(s,6H,H-28 u.H-29);2,57(dd,J=10,5,7,5Hz,1H,H-14);3,38 u.3,43(2x dt,J=9,7Hz,je 1H,H-24 u.H-24’);3,88 u.3,96(2x d,J=12,5Hz,je 1H,H-22 u.H-22’);5,01 u.5,23(2x s,je 1H,H-21u.H-21’)
82.〔1S-〔1α,3aβ,7aα〕〕-1-〔2-〔4-乙基-4-〔(三甲基甲硅烷基)氧代〕己氧基〕-1-亚甲基乙基〕-7a-甲基八氢-4H-茚-4-酮(83)
在10ml乙醚中同于33将140mg(0.42mmol)(81)与136mg(1.26mmol)三甲基氯硅烷,113mg(1.64mmol)咪唑和0.17ml吡啶反应而得136mg无色油状题示化合物。1H-NMR(CDCl3):δ=0,01ppm(s,9H,MeSi);0,49(s,3H,H-18);0,71(t,J=7Hz,6H,H-30u.H-31);1,39(q,J=7Hz,4H,H-28 u.H-29);2,47(dd,J=10,5,7,5Hz,1H,H-14);3,26 u.3,33(2x dt,J=9,7Hz,je 1H,H-24 u.H-24’);3,79 u.3,87(2x d,J=12,5Hz,je 1H,H-22 U.H-22’);4,91 u.5,14(2x s,je 1H,H-21 u.H-21’)
83.(7E)-(1R,3R)-1,3-双〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-24-〔3-甲基-3-〔(三甲基甲硅烷基)氧代〕丁基〕-19-降-23-氧杂-9,10-断胆-5,7,20-三烯(84)
类似于36用110mg(0.29mmol)(82)反应而得125mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,01 u.0,07ppm(2x s,12H u.9H,SiMe);0,41(s,3H,H-18);0,79 u.0,80(2x s,Si-t-Butyl);1,18(s,6H,H-28 u.H-29);3,37(m,2H,H-24);3,88(s,2H,H-22);4,04(m,2H,H-1 u.H-3);4,93 u.5,15(2x s,je 1H,H-21 u.H-21’);5,79 u.6,12(d,J=11Hz,je 1H,H-6 u.H-7)
84.(7E)-(1R,3R)-1,3-双〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧代〕-24-〔3-乙基-3-〔(三甲基甲硅烷基)氧代〕戊基〕-19-降-23-氧杂-9,10-断胆-5,7,20-三烯(85)
类似于36用126mg(0.31mmol)(83)反应而得150mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,01 u.0,05ppm(2x s,12H u.9H,SiMe);0,38(s,3H,H-18);0,79 u.0,80(2x s,je 9H,Si-t-Butyl);0,83(t,J=7Hz,6H,H-30 u.H-31);1,38(q,J=7Hz,4H,H-28u.H-29);3,30(m,2H,H-24);3,81(s,2H,H-22);4,00(m,2H,H-1 u.H-3);4,89 u.5,10(2xs,je 1H,H-21 u.H-21’);5,74 u.6,08(2x d,J=11Hz,je 1H,H-6 u.H-7)
85.(7E)-(1R,3R)-24-(3-羟基-3-甲基丁基)-19-降-23-氧杂-9,10-断胆-5,7,20-三烯-1,3-二醇(86)
在10ml THF中同于39将110mg(0.15mmol)(84)与374mg(1.2mmol)氟化四丁铵反应而得53mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,38ppm(s,3H,H-18);1,12(s,6H,H-28 u.H-29);3,33(m,2H,H-24);3,81(s,2H,H-22);3,91 u.3,98(2x m,je 1H,H-1 u.H.3);4,90 u.5,10(2x s,je 1H,H-21 u.H-21’);5,80 u.6,21(2x d,J=11Hz,je 1H,H-6 u.H-7)
86.(7E)-(1R,3R)-24-(3-乙基-3-羟戊基)-19-降-23-氧杂-9,10-断胆-5,7,20-三烯-1,3-二醇(87)
在10ml THF中同于39将132mg(0.17mmol)(85)与424mg(1.36mmol)氟化四丁铵反应而得59mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,39ppm(s,3H,H-18);0,77(t,J=7Hz,6H,H-30 u.H-31);1,38(q,J=7Hz,4H,H-28 u.H-29);3,32(m,2H,H-24);3,80(s,2H,H-22);3,91 u.3,98(2x m,je1H,H-1 u.H-3);4,89 u.5,09(2x s,je 1H,H-21 u.H-21’);5,80 u.6,21(2x d,J=11Hz,je1H,H-6 u.H-7)
87.(5Z,7E)-(1S,3R)-1,3-双〔〔1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-20-甲酰基-9,10-断孕甾-5,7,10(19),20-四烯(88)
将2.8g(3.36mmol)(11)溶于100ml二氯甲烷中并加11。6g(133mmol)二氧化锰,室温搅拌1小时后经Celite吸滤,分出溶剂而得2.5g无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,35ppm(s,3H,H-18);0,92 u.0,99(2x s,je 9H,Si-t-Butyl);4,23(m,1H,H-3);4,55(m,1H,H-1);4,83 u.5,10(2x s,je 1H,H-19 u.H-19’);6,02 u.6,09(2x d,J=11Hz,je 1H,H-6 u.H-7);6,11 u.6,32(2x s,je 1H,H-21 u.H-21’);7,23-7,69(m,20H,Si-Phenyl);9,58(s,1H,H-22)
88.(5Z,7E,22E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-9,10-断胆-5,7,10(19),20,22-五烯-24-酸甲酯(89)
将182mg(6mmol)氢化钠(80%)加入10mlTHF中并室温加825ml(4.8mmol)膦酸二甲基(甲氧羰基)甲基酯的20ml THF液,30分钟后滴加1.2g(1.5mmol)(88)并搅拌过夜,冰冷情况下仅滴入饱和氯化钠液,用乙酸乙酯萃取,有机相用氯化钠液洗涤,硫酸钠干燥后剩余物经硅胶色谱(己烷/乙酸乙酯作洗脱剂)提纯而得470mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,39ppm(s,3H,H-18);0,93 u.0,99(2x s,je 9H,Si-t-Butyl);3,79(s,3H,COOMe);4,25(m,1H,H-3);4,57(m,1H,H-1);4,83 u.5,10(2x s,je 1H,H-19 u.H-19’);5,35 u.5,55(2x s,je 1H,H-21 u.H-21’);6,05(d,J=15,5Hz,1H,H-23);6,09 u.6,20(2x d,J=11Hz,je 1H,H-6 u.H-7);7,24-7,69(m,20H,Si-Phenyl)
89.(5Z,7E,22E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-9,10-断胆-5,7,10(19),20,22-五烯-24-醇(90)
将470mg(0.52mmol)(89)加入60mlTHF中并0℃加2.16ml DIBAH液(甲苯中1.3M),0℃搅拌1小时后用甲苯稀释,加0.1ml异丙醇和1.05ml水并室温搅拌30分钟,过滤分出溶剂后剩余物经硅胶色谱提纯而得450mg无色泡沫状题示物质。1H-NMR(CDCl3):δ=0,40ppm(s,3H,H-18);0,94 u.0,99(2x s,je 9H,Si-t-Butyl);4,20(m,3H,H-3 u.H-24);4,57(t,J=5,5Hz,1H,H-1);4,83(s,1H,H-19);5,00(s,1H,H-21);5,10(s,1H,H-19’);5,22(s,1H,H-21’);6,00(dt,J=16,5,5Hz,1H,H-23);6,01 u.6,09(2x d,J=11Hz,je 1H,H-6 u.H-7);6,27(d,J=16Hz,1H,H-22);7,12-7,70(m,20H,Si-Phenyl)
90.〔(5Z,7E,22E)-(1S,3R)-1,3-双〔〔(1,1-二甲基乙基)二苯基甲硅烷基〕氧代〕-9,10-断胆-5,7,10(19),20,22-五烯-24-氧代〕乙酸-1,1-二甲基乙基酯(91)
类似于3将450mg(0.53mmol)(90)与2.7ml苛性钠溶液(25%),11mg硫酸氢四丁铵和760mg(3.9mmol)溴乙酸叔丁酯反应而得340mg无色泡沫状题示化合物。1H-NMR(CDCl3):δ=0,40ppm(s,3H,H-18);0,94 u.1,12(2x s,je 9H,Si-t-Butyl);1,28(s,9H,t-Butylester);3,98(s,2H,H-26);4,15(d,J=5,5Hz,2H,H-24);4,25(m,1H,H-3);4,56(m,1H,H-1);4,84(s,1H,H-19);5,01(s,1H,H-21);5,10(s,1H,H-19’);5,22(s,1H,H-21’);5,90(dt,J=15,5,5,5Hz,1H,H-23);6,28(d,J=15,5Hz,1H,H-22);6,03 u.6,10(2x d,J=11Hz,je 1H,H-6 u.H-7);7,24-7,70(m,20H,Si-Phenyl)
91.(5Z,7E,22E)-(1S,3R)-24-(2-羟基-2-甲基丙氧基)-9,10-断胆-5,7,10(19),20,22-五烯-1,3-二醇(92)
在5ml乙醚中用397mg(2.8mmol)碘甲烷和69mg(2.8mmol)镁屑制成格利雅试剂并同于4与340mg(0.35mmol)(91)反应而得210mg粗产物,将其溶于5ml THF中并同于14与1ml氟化四丁铵液(THF中1M)反应,提纯分离后得60mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,42ppm(s,3H,H-18);1,20(s,6H,H-28 u.H-29);3,25(s,2H,H-26);4,08(d,J=5,5Hz,2H,H-24);4,13(m,1H,H-3);4,38(m,1H,H-1);4,97(s,1H,H-19);5,00 u.5,20(2x s,je 1H,H-21 u.H-21’);5,30(s,1H,H-19’);5,90(dt,J=15,5,5,5Hz,1H,H-23);6,05 u.6,35(2x d,J=11Hz,je 1H,H-6 u.H-7);6,25(d,J=15,5Hz,1H,H-22)
92.(5Z,7E,22E)-(1S,3R)-24-(2-乙基-2-羟丁氧基)-9,10-断胆-5,7,10(19),20,22-五烯-1,3-二醇(93)
在5ml THF中用136mg(125mmol)碘甲烷和30mg(1.25mmol)镁屑制成格利雅试剂并同于4与120mg(0.12mmol)(92)反应而得110mg粗产物,将其溶于5ml THF中并同于14与1ml氟化四丁铵液(THF中1M)反应,提纯分离后得21mg无色泡沫状题示化合物。1H-NMR(CD2Cl2):δ=0,37ppm(s,3H,H-18);0,78(t,J=7Hz,6H,H-30 u.H-31);1,40(q,J=7Hz,4H,H-28 u.H-29);3,20(s,2H,H-26);3,97(d,J=5,5Hz,2H,H-24);4,10(m,1H,H-3);4,31(m,1H,H-1);4,89(s,1H,H-19);4,91 u.5,12(2x s,je 1H,H-21 u.H-21’);5,23(s,1H,H-19’);5,81(dt,J=15,5,5,5Hz,1H,H-24);5,98 u.6,30(2x d,J=11Hz,je 1H,H-6 u.H-7);6,18(d,J=15,5Hz,1H,H-22)
Claims (1)
1.中间产物
〔1S-(1α,3aβ,4α,7aα)〕-1-〔4-(乙酰氧基)-7a-甲基八氢-1H-茚-1-基〕乙酮,
〔1R-(1α,3aβ,4α,7aα)〕-4-〔〔二甲基(1,1-二甲基乙基)甲硅烷基〕氧基〕-7a-甲基-β-亚甲基八氢-1H-茚-1-乙醇。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DEP4220757.6 | 1992-06-24 | ||
| DE4220757A DE4220757A1 (de) | 1992-06-24 | 1992-06-24 | Derivate in der Vitamin D-Reihe mit Modifikationen in der 20-Position, Verfahren zu ihrer Herstellung, Zwischenprodukte für dieses Verfahren, diese Derivate enthaltende pharmazeutische Präparate sowie deren Verwendung zur Herstellung von Arzneimitteln |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN93107406A Division CN1055921C (zh) | 1992-06-24 | 1993-06-23 | 20位改性的维生素d系列衍生物,其制备方法和其药剂 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN1262267A true CN1262267A (zh) | 2000-08-09 |
| CN1173909C CN1173909C (zh) | 2004-11-03 |
Family
ID=6461752
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN93107406A Expired - Fee Related CN1055921C (zh) | 1992-06-24 | 1993-06-23 | 20位改性的维生素d系列衍生物,其制备方法和其药剂 |
| CNB991264738A Expired - Fee Related CN1173909C (zh) | 1992-06-24 | 1999-12-23 | 20位改性的维生素d系列衍生物,其制备方法和其药剂 |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN93107406A Expired - Fee Related CN1055921C (zh) | 1992-06-24 | 1993-06-23 | 20位改性的维生素d系列衍生物,其制备方法和其药剂 |
Country Status (24)
| Country | Link |
|---|---|
| US (2) | US5585368A (zh) |
| EP (1) | EP0647219B1 (zh) |
| JP (1) | JPH08501531A (zh) |
| KR (1) | KR100300274B1 (zh) |
| CN (2) | CN1055921C (zh) |
| AT (1) | ATE140918T1 (zh) |
| AU (1) | AU679685B2 (zh) |
| CA (1) | CA2138989A1 (zh) |
| CZ (1) | CZ282151B6 (zh) |
| DE (2) | DE4220757A1 (zh) |
| DK (1) | DK0647219T3 (zh) |
| ES (1) | ES2093440T3 (zh) |
| FI (1) | FI111720B (zh) |
| GR (1) | GR3020974T3 (zh) |
| HU (1) | HUT70563A (zh) |
| IL (1) | IL106128A0 (zh) |
| MX (1) | MX9303799A (zh) |
| NO (1) | NO306614B1 (zh) |
| NZ (1) | NZ253698A (zh) |
| PL (1) | PL174024B1 (zh) |
| SK (1) | SK281244B6 (zh) |
| TW (1) | TW255883B (zh) |
| WO (1) | WO1994000428A1 (zh) |
| ZA (1) | ZA934550B (zh) |
Families Citing this family (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5830885A (en) * | 1992-03-12 | 1998-11-03 | The Johns Hopkins University | Antiproliferative vitamin D3 hybrids |
| AU666563B2 (en) * | 1992-08-07 | 1996-02-15 | Wisconsin Alumni Research Foundation | Preparation of 19-nor-vitamin D compounds |
| US5449668A (en) * | 1993-06-04 | 1995-09-12 | Duphar International Research B.V. | Vitamin D compounds and method of preparing these compounds |
| IL112759A0 (en) * | 1994-02-25 | 1995-05-26 | Khepri Pharmaceuticals Inc | Novel cysteine protease inhibitors |
| DK0755922T3 (da) * | 1994-04-11 | 2000-11-13 | Chugai Pharmaceutical Co Ltd | 22-Thiavitamin D3-derivat |
| DK0717034T3 (da) * | 1994-12-14 | 1999-10-18 | Duphar Int Res | Vitamin D-forbindelser og fremgangsmåde til fremstilling af disse forbindelser |
| US6242434B1 (en) | 1997-08-08 | 2001-06-05 | Bone Care International, Inc. | 24-hydroxyvitamin D, analogs and uses thereof |
| CA2175881A1 (en) * | 1995-05-09 | 1996-11-10 | Andrzej Kutner | Vitamin d compounds and methods of preparing these compounds |
| DE19619036A1 (de) | 1996-04-30 | 1997-11-13 | Schering Ag | Neue Vitamin D-Derivate mit carbo- oder heterocyclischen Substituenten an C-25, Verfahren zu ihrer Herstellung und die Verwendung zur Herstellung von Arzneimitteln |
| SG70010A1 (en) * | 1996-05-23 | 2000-01-25 | Hoffmann La Roche | Fluorinated vitamin d3 analogs |
| AU743399B2 (en) * | 1997-06-25 | 2002-01-24 | Teijin Limited | Vitamin d3 derivatives and remedies for inflammatory respiratory diseases prepared from the same |
| DE69814109T2 (de) * | 1997-09-08 | 2004-02-26 | F. Hoffmann-La Roche Ag | 1,3-dihydroxy-20,20-dialkyl-vitamin d3 analoga |
| DE19744127B4 (de) * | 1997-10-01 | 2006-10-05 | Schering Ag | Neue Vitamin D-Derivate mit Cyclopropylringen in den Seitenketten, Verfahren und Zwischenprodukte zu ihrer Herstellung sowie ihre Verwendung zur Herstellung von Arzneimitteln |
| US5972917A (en) * | 1998-05-29 | 1999-10-26 | Bone Care Int Inc | 1 α-hydroxy-25-ene-vitamin D, analogs and uses thereof |
| DE19935771A1 (de) * | 1999-07-23 | 2001-02-01 | Schering Ag | Neue Vitamin D-Derivate mit cyclischen Substrukturen in den Seitenketten, Verfahren und Zwischenprodukte zu ihrer Herstellung und die Verwendung zur Herstellung von Arzneimitteln |
| EP1314427A4 (en) * | 2000-08-17 | 2006-05-03 | Chugai Pharmaceutical Co Ltd | THERAPEUTIC AGENT FOR THE TREATMENT OF OSTEOPOROSIS |
| US7491711B2 (en) * | 2001-09-21 | 2009-02-17 | Roche Palo Alto Llc | Methods of treatment using 3-desoxy vitamin D3 analogs |
| WO2004046097A1 (en) * | 2002-11-18 | 2004-06-03 | Teva Pharmaceutical Industries Ltd. | A crystallization method for purification of calcipotriene |
| US7094775B2 (en) | 2004-06-30 | 2006-08-22 | Bone Care International, Llc | Method of treating breast cancer using a combination of vitamin D analogues and other agents |
| TW200800156A (en) * | 2005-09-23 | 2008-01-01 | Bioxell Spa | 1, 25-dihydroxy, 20-cyclopropyl, 26, 27-deuteroalkyl vitamin D3 compounds and methods of use thereof |
| MX2009000660A (es) | 2006-07-17 | 2009-04-08 | Glaxosmithkline Biolog Sa | Vacuna de influenza. |
| US7888339B2 (en) | 2008-07-10 | 2011-02-15 | Wisconsin Alumni Research Foundation | 2-methylene-20(21)-dehydro-19-nor-vitamin D analogs |
| CR20170434A (es) | 2009-01-27 | 2017-11-21 | Berg Llc | VITAMINA D3 Y ANALOGOS DE LA MISMA PARA ALIVIAR EFECTOS SECUNDARIOS ASOCIADOS CON LA QUIMIOTERAPIA. (Divisional 2011-0445) |
| KR101791828B1 (ko) | 2009-08-14 | 2017-10-31 | 베르그 엘엘씨 | 탈모증 치료용 비타민 d3 및 이의 유사체들 |
| CN103073607B (zh) * | 2013-02-26 | 2014-09-03 | 昆明理工大学 | 12β-羟基雄甾-4,6,8(9),13(14)-四烯-3,11,16-三酮及其应用 |
| CN103073608B (zh) * | 2013-02-26 | 2014-09-03 | 昆明理工大学 | 雄甾-4,6,8(9),13(14)-四烯-3,11,16-三酮及其应用 |
| AU2014274099A1 (en) | 2013-05-29 | 2015-12-24 | Berg Llc | Preventing or mitigating chemotherapy induced alopecia using vitamin D |
| US10548908B2 (en) * | 2016-09-15 | 2020-02-04 | Nostopharma, LLC | Compositions and methods for preventing and treating heterotopic ossification and pathologic calcification |
Family Cites Families (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4617297A (en) * | 1982-02-12 | 1986-10-14 | Hoffmann-La Roche Inc. | Method of treating disease states by the administration of 1α,25,26-trihydroxycholecalciferol |
| US4594432A (en) * | 1983-01-28 | 1986-06-10 | Hoffman-La Roche Inc. | Process for the synthesis of 1α,23(S),25-trihydroxycholecalciferol and 1α,23(R),25-trihydroxycholecalciferol |
| US4719205A (en) * | 1984-01-30 | 1988-01-12 | Wisconsin Alumini Research Foundation | Side-chain unsaturated 1-hydroxyvitamin D compounds |
| JPS6144860A (ja) * | 1984-08-10 | 1986-03-04 | Taisho Pharmaceut Co Ltd | 1α.25−ジヒドロキシ−26.27−ジメチルビタミンD↓3 |
| EP0184112B1 (en) * | 1984-11-27 | 1990-05-09 | Chugai Seiyaku Kabushiki Kaisha | Novel vitamin d derivatives and process for producing the same |
| US4612308A (en) * | 1984-11-29 | 1986-09-16 | Hoffmann-La Roche Inc. | 25,26-Dehydro-1α,23(S,R)-dihydroxycholecalciferol and its epimers |
| ATE45347T1 (de) * | 1985-05-30 | 1989-08-15 | Taisho Pharmaceutical Co Ltd | Vitamin d3-derivate. |
| US4832875A (en) * | 1985-11-21 | 1989-05-23 | Taisho Pharmaceutical Co., Ltd. | Vitamin D3 derivative |
| US4804502A (en) * | 1988-01-20 | 1989-02-14 | Hoffmann-La Roche Inc. | Vitamin D compounds |
| US4851401A (en) * | 1988-07-14 | 1989-07-25 | Wisconsin Alumni Research Foundation | Novel cyclopentano-vitamin D analogs |
| US4970203A (en) * | 1988-12-09 | 1990-11-13 | Deluca Hector F | Method for improving reproductive functions in mammals |
| NZ232734A (en) * | 1989-03-09 | 1991-11-26 | Wisconsin Alumni Res Found | 19-nor vitamin d derivatives and pharmaceutical compositions |
| CA1333616C (en) * | 1989-03-09 | 1994-12-20 | Hector F. Deluca | 19-nor-vitamin d compounds |
| GB8915770D0 (en) * | 1989-07-10 | 1989-08-31 | Leo Pharm Prod Ltd | Chemical compounds |
| US5260290A (en) * | 1990-02-14 | 1993-11-09 | Wisconsin Alumni Research Foundation | Homologated vitamin D2 compounds and the corresponding 1α-hydroxylated derivatives |
| DE4011682A1 (de) * | 1990-04-06 | 1991-10-10 | Schering Ag | 24-oxa-derivate in der vitamin d-reihe |
| EP0521550B1 (en) * | 1991-07-05 | 1996-09-18 | Duphar International Research B.V | Vitamin D compound, method of preparing this compound and intermediate therefor |
| DE4141746A1 (de) * | 1991-12-13 | 1993-06-17 | Schering Ag | 20-methyl-substituierte vitamin d-derivate |
| US5200536A (en) * | 1992-02-10 | 1993-04-06 | Daikin Industries Ltd. | Fluorine-containing vitamin D3 analogues |
| US5194431A (en) * | 1992-07-08 | 1993-03-16 | Wisconsin Alumni Research Foundation | 24-cyclopropane vitamin D derivatives |
| IL107185A (en) * | 1992-10-06 | 1998-02-22 | Schering Ag | History of 52-carboxylic acid, processes for their preparation and pharmaceutical preparations containing them |
-
1992
- 1992-06-24 DE DE4220757A patent/DE4220757A1/de not_active Withdrawn
-
1993
- 1993-06-23 CN CN93107406A patent/CN1055921C/zh not_active Expired - Fee Related
- 1993-06-24 DE DE59303365T patent/DE59303365D1/de not_active Expired - Lifetime
- 1993-06-24 US US08/080,841 patent/US5585368A/en not_active Expired - Fee Related
- 1993-06-24 PL PL93306813A patent/PL174024B1/pl not_active IP Right Cessation
- 1993-06-24 WO PCT/EP1993/001620 patent/WO1994000428A1/de active IP Right Grant
- 1993-06-24 NZ NZ253698A patent/NZ253698A/en unknown
- 1993-06-24 JP JP6502048A patent/JPH08501531A/ja active Pending
- 1993-06-24 IL IL106128A patent/IL106128A0/xx unknown
- 1993-06-24 DK DK93914720.3T patent/DK0647219T3/da active
- 1993-06-24 ZA ZA934550A patent/ZA934550B/xx unknown
- 1993-06-24 HU HU9403771A patent/HUT70563A/hu unknown
- 1993-06-24 KR KR1019940704723A patent/KR100300274B1/ko not_active Expired - Fee Related
- 1993-06-24 AT AT93914720T patent/ATE140918T1/de not_active IP Right Cessation
- 1993-06-24 CA CA002138989A patent/CA2138989A1/en not_active Abandoned
- 1993-06-24 SK SK1584-94A patent/SK281244B6/sk unknown
- 1993-06-24 MX MX9303799A patent/MX9303799A/es not_active IP Right Cessation
- 1993-06-24 AU AU45007/93A patent/AU679685B2/en not_active Ceased
- 1993-06-24 ES ES93914720T patent/ES2093440T3/es not_active Expired - Lifetime
- 1993-06-24 CZ CZ943276A patent/CZ282151B6/cs not_active IP Right Cessation
- 1993-06-24 EP EP93914720A patent/EP0647219B1/de not_active Expired - Lifetime
- 1993-08-12 TW TW082106461A patent/TW255883B/zh not_active IP Right Cessation
-
1994
- 1994-12-23 NO NO945034A patent/NO306614B1/no unknown
- 1994-12-23 FI FI946073A patent/FI111720B/fi not_active IP Right Cessation
-
1996
- 1996-06-14 US US08/664,121 patent/US5700791A/en not_active Expired - Fee Related
- 1996-09-06 GR GR960402337T patent/GR3020974T3/el unknown
-
1999
- 1999-12-23 CN CNB991264738A patent/CN1173909C/zh not_active Expired - Fee Related
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1262267A (zh) | 20位改性的维生素d系列衍生物,其制备方法和其药剂 | |
| CN1042026C (zh) | 维生素d系列的25羧酸衍生物、其制法及中间产物、含该衍生物的药物及其药用 | |
| CN1036200C (zh) | A-去甲甾类-3-羧酸衍生物的制备方法 | |
| CN1176072C (zh) | 维生素d3类似物 | |
| CN1043883C (zh) | 维生素d类似物,含有其的药物组合物及其医药用途 | |
| CN1358193A (zh) | 用于治疗的具有c-17烷基侧链和芳香a-环的甾族化合物 | |
| JPH06321894A (ja) | 2−位に置換基を有する19−ノル−ビタミンd3 化合物 | |
| CN1099385A (zh) | 维生素d化合物和这些化合物的制备方法 | |
| CN1130899A (zh) | 维生素d的新型结构类似物 | |
| CN1125436A (zh) | 维生素d酰胺衍生物 | |
| CN1231439C (zh) | 在膦配体存在下经铑催化的开环反应制备的新的氢化萘化合物 | |
| CN1230166A (zh) | 隐杯伞素类抗肿瘤剂 | |
| JP3589664B2 (ja) | ビタミンd系列の22−エン−25−オキサ−誘導体,該化合物の製造法,該誘導体を含有する薬学的調剤ならびに薬剤としての該調剤の使用 | |
| CN1133621C (zh) | 环己二醇衍生物 | |
| CN1771226A (zh) | 2-亚丙基-19-去甲维生素d化合物 | |
| CN1993342A (zh) | 用于制备软海绵素b的中间体 | |
| CN1402735A (zh) | 新颖的雄激素 | |
| CN1029400C (zh) | 侧链系维生素d衍生物的制备方法 | |
| CN1075809C (zh) | 新的维生素d类似物 | |
| CN1167678C (zh) | 新颖的维生素d类似物 | |
| CN1705643A (zh) | 维生素d类似物,包含所述类似物的组合物及其用途 | |
| CN1272840A (zh) | 在侧链中带有环丙基环的新型维生素d衍生物,其制备方法和中间产物以及在制备药物中的应用 | |
| CN1241999A (zh) | 维生素d3衍生物 | |
| CN1252791A (zh) | 芳基断-胆二烯衍生物 | |
| CN1172476A (zh) | 丙烯酮衍生物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C06 | Publication | ||
| PB01 | Publication | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| C19 | Lapse of patent right due to non-payment of the annual fee | ||
| CF01 | Termination of patent right due to non-payment of annual fee |