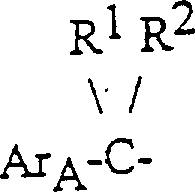CN1147242A - 农药用氟代烯烃 - Google Patents
农药用氟代烯烃 Download PDFInfo
- Publication number
- CN1147242A CN1147242A CN95192851A CN95192851A CN1147242A CN 1147242 A CN1147242 A CN 1147242A CN 95192851 A CN95192851 A CN 95192851A CN 95192851 A CN95192851 A CN 95192851A CN 1147242 A CN1147242 A CN 1147242A
- Authority
- CN
- China
- Prior art keywords
- compound
- phenyl
- formula
- composition
- fluoro
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 230000000361 pesticidal effect Effects 0.000 title abstract description 5
- 150000001875 compounds Chemical class 0.000 claims abstract description 65
- 238000000034 method Methods 0.000 claims abstract description 35
- 238000002360 preparation method Methods 0.000 claims abstract description 21
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims abstract description 17
- 125000000217 alkyl group Chemical group 0.000 claims abstract description 7
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 claims abstract description 5
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims abstract description 4
- 125000003545 alkoxy group Chemical group 0.000 claims abstract description 4
- 229910052736 halogen Inorganic materials 0.000 claims abstract description 4
- 150000002367 halogens Chemical class 0.000 claims abstract description 4
- 229910052739 hydrogen Inorganic materials 0.000 claims abstract description 4
- 239000001257 hydrogen Substances 0.000 claims abstract description 4
- 125000001624 naphthyl group Chemical group 0.000 claims abstract description 3
- 125000001188 haloalkyl group Chemical group 0.000 claims abstract 2
- 239000000203 mixture Substances 0.000 claims description 27
- 229910052731 fluorine Inorganic materials 0.000 claims description 20
- 239000011737 fluorine Substances 0.000 claims description 20
- 239000002689 soil Substances 0.000 claims description 14
- 239000007818 Grignard reagent Substances 0.000 claims description 12
- 241000238631 Hexapoda Species 0.000 claims description 12
- 150000004795 grignard reagents Chemical class 0.000 claims description 12
- 239000003905 agrochemical Substances 0.000 claims description 10
- 239000002917 insecticide Substances 0.000 claims description 5
- 241000607479 Yersinia pestis Species 0.000 claims description 4
- 239000008187 granular material Substances 0.000 claims description 4
- 239000012434 nucleophilic reagent Substances 0.000 claims description 4
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 claims description 4
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 claims description 3
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 3
- 125000003236 benzoyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C(*)=O 0.000 claims description 2
- 238000006555 catalytic reaction Methods 0.000 claims description 2
- 239000000575 pesticide Substances 0.000 claims description 2
- -1 phenoxy, phenyl Chemical group 0.000 abstract description 17
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 abstract description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 60
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 48
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 30
- IAQRGUVFOMOMEM-UHFFFAOYSA-N butene Natural products CC=CC IAQRGUVFOMOMEM-UHFFFAOYSA-N 0.000 description 24
- IAQRGUVFOMOMEM-ONEGZZNKSA-N trans-but-2-ene Chemical compound C\C=C\C IAQRGUVFOMOMEM-ONEGZZNKSA-N 0.000 description 24
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Natural products CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 18
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 15
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 15
- 239000002904 solvent Substances 0.000 description 15
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 12
- 241000257159 Musca domestica Species 0.000 description 11
- 238000012360 testing method Methods 0.000 description 11
- WDYVUKGVKRZQNM-UHFFFAOYSA-N 6-phosphonohexylphosphonic acid Chemical compound OP(O)(=O)CCCCCCP(O)(O)=O WDYVUKGVKRZQNM-UHFFFAOYSA-N 0.000 description 10
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 10
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 10
- 239000002253 acid Substances 0.000 description 10
- ORTQZVOHEJQUHG-UHFFFAOYSA-L copper(II) chloride Chemical compound Cl[Cu]Cl ORTQZVOHEJQUHG-UHFFFAOYSA-L 0.000 description 10
- 229910052749 magnesium Inorganic materials 0.000 description 10
- 239000011777 magnesium Substances 0.000 description 10
- 150000004702 methyl esters Chemical class 0.000 description 10
- 238000004809 thin layer chromatography Methods 0.000 description 10
- 235000019439 ethyl acetate Nutrition 0.000 description 9
- 238000001704 evaporation Methods 0.000 description 9
- 230000008020 evaporation Effects 0.000 description 9
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 9
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 8
- LVZWSLJZHVFIQJ-UHFFFAOYSA-N Cyclopropane Chemical compound C1CC1 LVZWSLJZHVFIQJ-UHFFFAOYSA-N 0.000 description 7
- UCTLHLZWKJIXJI-LXIBVNSESA-N [(3s,8r,9s,10r,13s,14s)-17-chloro-16-formyl-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15-decahydro-1h-cyclopenta[a]phenanthren-3-yl] acetate Chemical compound C([C@@H]12)C[C@]3(C)C(Cl)=C(C=O)C[C@H]3[C@@H]1CC=C1[C@]2(C)CC[C@H](OC(=O)C)C1 UCTLHLZWKJIXJI-LXIBVNSESA-N 0.000 description 7
- 238000003756 stirring Methods 0.000 description 7
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 7
- 240000003291 Armoracia rusticana Species 0.000 description 6
- 235000011330 Armoracia rusticana Nutrition 0.000 description 6
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 6
- 239000007788 liquid Substances 0.000 description 6
- 229910052757 nitrogen Inorganic materials 0.000 description 6
- 238000012545 processing Methods 0.000 description 6
- AHDAKFFMKLQPTD-UHFFFAOYSA-N 1-bromo-3-phenoxybenzene Chemical compound BrC1=CC=CC(OC=2C=CC=CC=2)=C1 AHDAKFFMKLQPTD-UHFFFAOYSA-N 0.000 description 5
- GREWZYRZMVYLBQ-UHFFFAOYSA-N 4-bromo-1-fluoro-2-phenoxybenzene Chemical compound FC1=CC=C(Br)C=C1OC1=CC=CC=C1 GREWZYRZMVYLBQ-UHFFFAOYSA-N 0.000 description 5
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 5
- 241000500437 Plutella xylostella Species 0.000 description 5
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 5
- WETWJCDKMRHUPV-UHFFFAOYSA-N acetyl chloride Chemical compound CC(Cl)=O WETWJCDKMRHUPV-UHFFFAOYSA-N 0.000 description 5
- 239000012346 acetyl chloride Substances 0.000 description 5
- 239000000460 chlorine Substances 0.000 description 5
- 229960003280 cupric chloride Drugs 0.000 description 5
- 125000003963 dichloro group Chemical group Cl* 0.000 description 5
- 239000012280 lithium aluminium hydride Substances 0.000 description 5
- RJBYSQHLLIHSLT-UHFFFAOYSA-N methyl 2-fluoroacetate Chemical compound COC(=O)CF RJBYSQHLLIHSLT-UHFFFAOYSA-N 0.000 description 5
- 239000002808 molecular sieve Substances 0.000 description 5
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 5
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 3
- 150000001336 alkenes Chemical group 0.000 description 3
- 238000001035 drying Methods 0.000 description 3
- 238000000605 extraction Methods 0.000 description 3
- 239000012044 organic layer Substances 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- VEMKTZHHVJILDY-FIWHBWSRSA-N resmethrin Chemical compound CC1(C)[C@H](C=C(C)C)C1C(=O)OCC1=COC(CC=2C=CC=CC=2)=C1 VEMKTZHHVJILDY-FIWHBWSRSA-N 0.000 description 3
- 229940108410 resmethrin Drugs 0.000 description 3
- 239000000741 silica gel Substances 0.000 description 3
- 229910002027 silica gel Inorganic materials 0.000 description 3
- 238000009834 vaporization Methods 0.000 description 3
- 230000008016 vaporization Effects 0.000 description 3
- IKHGUXGNUITLKF-UHFFFAOYSA-N Acetaldehyde Chemical compound CC=O IKHGUXGNUITLKF-UHFFFAOYSA-N 0.000 description 2
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- NBBJYMSMWIIQGU-UHFFFAOYSA-N Propionic aldehyde Chemical compound CCC=O NBBJYMSMWIIQGU-UHFFFAOYSA-N 0.000 description 2
- 240000008042 Zea mays Species 0.000 description 2
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 2
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 2
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 2
- 229910052794 bromium Inorganic materials 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 235000005822 corn Nutrition 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 125000001153 fluoro group Chemical group F* 0.000 description 2
- 230000000749 insecticidal effect Effects 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- YNXOMDRPWQMDQL-UHFFFAOYSA-N 2-(4-chlorophenyl)-2-methylpropanal Chemical compound O=CC(C)(C)C1=CC=C(Cl)C=C1 YNXOMDRPWQMDQL-UHFFFAOYSA-N 0.000 description 1
- BTSIZIIPFNVMHF-UHFFFAOYSA-N 2-penten-1-ol Chemical compound CCC=CCO BTSIZIIPFNVMHF-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 241001136249 Agriotes lineatus Species 0.000 description 1
- 241000218473 Agrotis Species 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 235000016068 Berberis vulgaris Nutrition 0.000 description 1
- 241000335053 Beta vulgaris Species 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 235000007516 Chrysanthemum Nutrition 0.000 description 1
- 244000189548 Chrysanthemum x morifolium Species 0.000 description 1
- 241000254173 Coleoptera Species 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 229920000742 Cotton Polymers 0.000 description 1
- 240000008067 Cucumis sativus Species 0.000 description 1
- 235000010799 Cucumis sativus var sativus Nutrition 0.000 description 1
- XFXPMWWXUTWYJX-UHFFFAOYSA-N Cyanide Chemical compound N#[C-] XFXPMWWXUTWYJX-UHFFFAOYSA-N 0.000 description 1
- 241000489975 Diabrotica Species 0.000 description 1
- 241001529600 Diabrotica balteata Species 0.000 description 1
- 241000255925 Diptera Species 0.000 description 1
- 244000299507 Gossypium hirsutum Species 0.000 description 1
- 241001272567 Hominoidea Species 0.000 description 1
- 101000809257 Homo sapiens Ubiquitin carboxyl-terminal hydrolase 4 Proteins 0.000 description 1
- 239000005909 Kieselgur Substances 0.000 description 1
- 241000254099 Melolontha melolontha Species 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 241000257226 Muscidae Species 0.000 description 1
- 241001608567 Phaedon cochleariae Species 0.000 description 1
- 239000006004 Quartz sand Substances 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 102100038463 Ubiquitin carboxyl-terminal hydrolase 4 Human genes 0.000 description 1
- 210000001015 abdomen Anatomy 0.000 description 1
- 230000000895 acaricidal effect Effects 0.000 description 1
- 239000000642 acaricide Substances 0.000 description 1
- 125000004423 acyloxy group Chemical group 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 239000003849 aromatic solvent Substances 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 125000004093 cyano group Chemical group *C#N 0.000 description 1
- KTUDDNXAXXAXII-UHFFFAOYSA-N cyclopropyl formate Chemical compound O=COC1CC1 KTUDDNXAXXAXII-UHFFFAOYSA-N 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- XYIBRDXRRQCHLP-UHFFFAOYSA-N ethyl acetoacetate Chemical compound CCOC(=O)CC(C)=O XYIBRDXRRQCHLP-UHFFFAOYSA-N 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 239000010440 gypsum Substances 0.000 description 1
- 229910052602 gypsum Inorganic materials 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 239000003999 initiator Substances 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- PNDPGZBMCMUPRI-UHFFFAOYSA-N iodine Chemical compound II PNDPGZBMCMUPRI-UHFFFAOYSA-N 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 229910003002 lithium salt Inorganic materials 0.000 description 1
- 159000000002 lithium salts Chemical class 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 229940031815 mycocide Drugs 0.000 description 1
- 238000005554 pickling Methods 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 239000002728 pyrethroid Substances 0.000 description 1
- 231100000916 relative toxicity Toxicity 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000004575 stone Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 239000012747 synergistic agent Substances 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 229910052723 transition metal Inorganic materials 0.000 description 1
- 150000003624 transition metals Chemical class 0.000 description 1
- 239000004563 wettable powder Substances 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C43/00—Ethers; Compounds having groups, groups or groups
- C07C43/02—Ethers
- C07C43/257—Ethers having an ether-oxygen atom bound to carbon atoms both belonging to six-membered aromatic rings
- C07C43/29—Ethers having an ether-oxygen atom bound to carbon atoms both belonging to six-membered aromatic rings containing halogen
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N31/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic oxygen or sulfur compounds
- A01N31/08—Oxygen or sulfur directly attached to an aromatic ring system
- A01N31/14—Ethers
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Agronomy & Crop Science (AREA)
- Pest Control & Pesticides (AREA)
- Plant Pathology (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Dentistry (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
Abstract
本发明公开了式I的农药化合物,其中R1是氢和R2表示环丙基,或者R1和R2各自表示相同或不同的烷基;ArA表示选择取代的苯基和萘基;ArB表示苯氧基、苯基、苄基或苯甲酰基—取代的苯基,它们可进一步选择取代;双键上的ArA-CR1R2(a)和-CH2ArB基团的构型相互为反式。优选的ArA是取代的苯基;更优选的是在4-(对位)被卤素、烷氧基或卤代烷基取代;本发明还提供了制备式I的农药化合物的方法,该方法包括使含有(a)部分的化合物和含有ArB部分的化合物一起反应形成式I化合物中的(a)和ArB之间的-CH=C(F)CH2-连接。
Description
本发明涉及在烯烃的不饱和位置上有氟取代基的非酯类拟除虫菊酯烯烃农药。
已经证明对常用杀虫剂有抗药性的昆虫种类不断增加,因此,人们在继续研究对易感的和有抗药性的昆虫品系具有新的活性谱的新化合物。
相应的英国专利申请,申请号为GB9219612.0描述和要求保护某些在烯烃的不饱和位置上有氟取代基的芳基环烷基烯烃,该芳基环烷基烯烃显示出作为土壤杀虫剂的应用活性。现在,发现一些新颖的,有3-氟取代基的1-取代,1,4-二芳基-2-丁烯类化合物对一些有害昆虫显示出有价值的活性。
具有杀虫活性的某些1,4-二芳基-2-丁烯类化合物已有报道。USP4 975 451公开了具有杀虫和杀螨活性的环丙基二芳基-2-丁烯类;而日本专利公开号60115545、60193902和60193940报道了1,1-二烷基-1,4-二芳基-2-丁烯类具有杀虫活性。但是没有文献公开或介绍在在烯烃的不饱和位置上有氟取代基。
所以,本发明提供了下式I的农药化合物:
式中:
R1是氢和R2表示环丙基,或者R1和R2各自表示烷基,其中的烷基可以相同或不同;
ArA表示选择取代的苯基和萘基;
ArB表示苯氧基、苯基、苄基或苯甲酰基-取代的苯基,它们可进一步选择取代;
双键上的ArA-CR1R2和-CH2ArB基团的构型相互为反式。
优选的ArA是取代的苯基,优选在4-(对位)由例如卤素、烷氧基或卤代烷氧基取代。
ArB可以是苯氧基、苯基、苄基或苯甲酰基取代的苯基,特别是在3-(间位)上取代;此外,苯基可以,特别是被氟取代,特别是在4-(对位)取代;3-苯氧基苯基和4-氟-3-苯氧基苯基是特别优选的。
相应于本发明的第一组化合物是R1是氢和R2表示环丙基的式I化合物,相应于本发明的第二组化合物是R1和R2各自独立地表示烷基的化合物,优选R1和R2二者均为甲基。
本发明还包括了制备式I的农药化合物的方法,该方法包括使含有-部分的化合物和含有ArB部分的化合物一起反应形成式I化合物中的和ArB之间的-CH=C(F)CH2-连接。优选的方法包括形式为ArB-的亲核试剂与下式化合物
进行催化反应,其中Q表示良好的离去基团。
典型的,该反应在过渡金属催化剂存在下进行,优选使用铜盐或其与锂盐的复合物。
ArB的亲核试剂通常以其式为ArBMgBr的格氏(Grignard)试剂的形式存在,例如ArBLi,以及典型的离去基团Q是卤素,如溴,或酰氧基,如乙酰氧基。所述的铜盐适当的是亚铜盐,特别是卤化物(如溴化物或碘化物)或氰化物。式Li2CuY2Z2的铜的配合物也可以用作催化剂,式中的Y和Z表示氯、溴、碘或氰基。这种转化描述在Erdick,Tetrahedron,1984,40,641-657。
下面的反应路线说明了制备式I化合物的具体方法,在最后阶段Q是乙酰氧基。
格氏试剂ArBMgBr可以多种方法,通过英国专利Nos.2226315和2187731中所述的中间体制备。
式I化合物可用于民居、园林、农业或医药(包括兽用)领域的害虫感染防治。因此本发明还包括含有式I化合物为活性成分,并结合有惰性载体或稀释剂的农药组合物。
适当的稀释剂包括能制成例如可以是颗粒剂、粉剂或乳油的组合物的固体和液体两种稀释剂。适用于制备颗粒剂组合物的稀释剂的实例是多孔材料,如浮石、石膏,或玉米棒,石英砂。适用于制备粉剂的稀释剂的实例是高岭土、膨润土、硅藻土或滑石。制备乳油时,可将各种溶剂,如酮类和芳香族溶剂与一种或多种已知的湿润剂、分散剂或乳化剂一起使用。
固体组合物,特别是颗粒剂,优选含有0.5-15%重量的活性成分;而液体组合物,在用于作物时,可含有低至0.0001-1%重量的活性成分。但例如可湿性粉剂的组合物可含有多达75%的活性成分。
虽然可以推测其它应用取决于需要防治的害虫和感染集中的程度,但是已发现式I化合物特别适合用作土壤杀虫剂,或者用于土壤,或者用于种子的处理。
根据使用的模式不同,本发明的组合物通常以每公顷1-500g活性成分的施用率施用于被感染的地方。
可以理解的是,该组合物可以含有式I化合物和/或其它组分,包括其它农药用材料,如杀虫剂、杀螨剂,或杀真菌剂,或增效剂。
本发明的组合物可用于防治土壤中生长的害虫,例如鞘翅目、鳞翅目和双翅目类的害虫,特别是食根虫、夜盗虫、铁线虫、马陆和冬作种蝇。本发明的组合物可在很多作物如玉米、甜菜、马铃薯和棉花的培育期间施用于土壤和/或种子。
已经发现本发明化合物显示出防治害虫例如家蝇、辣根猿叶甲和小菜蛾的活性。
现参考下面的实施例进一步描述本发明。
多义的测定值由上标a,b标明。在噪声水平以上的未测出的峰用N标出。与氟的偶合常数在括号中标出,单位为Hz。
实施例1
4-(4-氯苯基)-4-环丙基-2-氟丁-2-烯酸甲酯
在氮气中,向搅拌着的酸洗过的锌粉(0.88g)、氯化铜(I)(0.14g)和4A分子筛(2.0g)于无水四氢呋喃(15ml)的混合物之中缓慢加入1-(4-氯苯基)-4-环丙基乙醛(1.07g)(M.Elliott等人,Pestic.Sci.,1980,11,513-525),接着加入乙酐(0.47ml)。在混合物温热至50℃后,滴加二氯一氟乙酸甲酯(0.80g),在50℃继续搅拌4小时。冷却后,混合物用乙醚(150ml)稀释,通过硅藻土床过滤,减压浓缩滤液,残余的油在硅胶上色谱纯化,用乙醚/己烷(1∶9)洗脱,得到4-(4-氯苯基)-4-环丙基-2-氟丁-2-烯酸乙酯(0.92g,64%)。
实施例2
4-环丙基-4-(4-乙氧基苯基)-2-氟丁-2-烯酸甲酯
重复实施例1的方法,用锌粉(4.0g)、氯化铜(I)(0.4g)、4A分子筛(3.2g)、四氢呋喃(40ml)、用M.Elliott等人,Pestic.Sci.,1980,11,513-525所述的方法由(4-乙氧基苯基)甲酸环丙基酯制备的1-环丙基(4-乙氧基苯基)乙醛(3.08g)、乙酐(1.4ml)和二氯一氟乙酸甲酯(3.3g)得到标题化合物(1.6g,39%)。
实施例3
4-(4-氯苯基)-2-氟-4-甲基戊-2-烯酸甲酯
重复实施例1的方法,用锌粉(1.86g)、氯化铜(I)(0.28g)、4A分子筛(2g)、四氢呋喃(30ml)、2-(4-氯苯基)-2-甲基丙醛(1.74g,A.E.Baydar等人,Pestic.Sci.,1988,23,231-246)、乙酐(1.1ml)和二氯一氟乙酸甲酯(1.6g)得到标题化合物(1.4g,20%)。
实施例4
4-(4-乙氧基苯基)-2-氟-4-甲基戊-2-烯酸甲酯
重复实施例1的方法,用锌粉(4g)、氯化铜(I)(0.58g)、4A分子筛(4.4g)、四氢呋喃(40ml)、2-(4-乙氧基苯基)-2-甲基丙醛(3.6g)(A.E.Baydar等人,Pestic.Sci.,1988,23,247-257)、乙酐(2.1ml)和二氯一氟乙酸甲酯(3.5g)得到标题化合物(1.22g,22%)。
实施例5
2-氟-4-甲基-4-(4-三氟甲氧基苯基)戊-2-烯酸甲酯
重复实施例1的方法,用锌粉(3.4g)、氯化铜(I)(0.5g)、4A分子筛(4g)、四氢呋喃(40ml)、2-甲基-(4-三氟甲氧基苯基)丙醛(2.2g)(A.W.Farnham等人,Pestic.Sci.,1990,28,25-34)、乙酐(1.4ml)和二氯一氟乙酸甲酯(2.9g)得到标题化合物(0.49g,16%)。
实施例6
4-(4-氯苯基)-4-环丙基-2-氟丁-2-烯醇
在0℃下,把如实施例1制备的4-(4-氯苯基)-4-环丙基-2-氟丁-2-烯酸甲酯(0.91g)的无水乙醚(20ml)溶液滴加到搅拌着的氢化铝锂(0.34g)的无水乙醚(25ml)悬浮液中,继续搅拌24分钟,同时使混合物温热至室温。加水(4ml),混合物用乙醚(3×20ml)萃取,合并的有机层用水(3×10ml)洗涤,干燥和减压蒸发。残余的油在硅胶上色谱纯化,用乙醚/己烷(2∶3)洗脱,得到4-(4-氯苯基)-4-环丙基-2-氟丁-2-烯醇(0.61g,78%)。
实施例7
4-环丙基-4-(4-乙氧基苯基)-2-氟丁-2-烯醇
重复实施例6的方法,使用4-环丙基-4-(4-乙氧基苯基)-2-氟丁-2-烯酸甲酯(实施例2)(1.2g)、乙醚(40ml)和氢化铝锂(0.2g),得到标题化合物(1.05g,99%)。
实施例8
4-(4-氯苯基)-2-氟-4-甲基-戊-2-烯醇
重复实施例6的方法,使用4-(4-氯苯基)-2-氟-4-甲基-戊-2-烯酸甲酯(实施例3)(0.56g)、乙醚(20ml)和氢化铝锂(0.21g),得到标题化合物(0.35g,92%)。
实施例9
4-(4-乙氧基苯基)-2-氟-4-甲基-戊-2-烯醇
重复实施例6的方法,使用2-氟-4-(4-乙氧基苯基)-4-甲基-戊-2-烯酸甲酯(实施例4)(0.2g)、乙醚(15ml)和氢化铝锂(0.1g),得到标题化合物(0.16g,90%)。
实施例10
2-氟-4-甲基-4-(4-三氟甲氧基苯基)戊-2-烯醇
重复实施例6的方法,使用2-氟-4-甲基-4-(4-三氟甲氧基苯基)戊-2-烯酸甲酯(实施例5)(0.19g)、乙醚(10ml)和氢化铝锂(90mg),得到标题化合物(0.16g,93%)。
实施例11
4-(4-氯苯基)-4-环丙基-2-氟丁-2-烯基乙酸酯
在0℃下,把乙酰氯(1.2ml)缓慢加入搅拌着的4-(4-氯苯基)-4-环丙基-2-氟丁-2-烯醇(实施例6)(0.51g)的苯(30ml)和吡啶(0.24ml)的溶液之中,继续搅拌24小时,同时使混合物温热至室温。加水(2ml),混合物用乙醚(3×20ml)萃取,合并的有机层用水(3×10ml)洗涤,干燥和减压蒸发。残余的油在硅胶上色谱纯化,用乙醚/己烷(1∶4)洗脱,得到4-(4-氯苯基)-4-环丙基-2-氟丁-2-烯基乙酸酯(0.46g,78%)。
实施例12
4-环丙基-4-(4-乙氧基苯基)-2-氟丁-2-烯基乙酸酯
重复实施例11的方法,使用乙酰氯(1.2ml)、4-环丙基-4-(4-乙氧基苯基)-2-氟丁-2-烯醇(实施例7)(0.65g)、苯(34ml)和吡啶(0.24ml)得到标题化合物(0.75g,99%)。
实施例13
4-(4-氯苯基)-2-氟-4-甲基-戊-2-烯基乙酸酯
重复实施例11的方法,使用乙酰氯(0.76ml)、4-(4-氯苯基)-2-氟-4-甲基-戊-2-烯醇(实施例8)(0.34g)、苯(20ml)和吡啶(0.15ml)得到标题化合物(0.35g,87%)。
实施例14
4-(4-乙氧基苯基)-2-氟-4-甲基-戊-2-烯基乙酸酯
重复实施例11的方法,使用乙酰氯(0.17ml)、4-(4-乙氧基苯基)-2-氟-4-甲基-戊-2-烯醇(实施例9)(86mg)、苯(4ml)和吡啶(0.04ml)得到标题化合物(0.1g,99%)。实施例152-氟-4-甲基-4-(4-三氟甲氧基苯基)-2-烯基乙酸酯
重复实施例11的方法,使用乙酰氯(0.41ml)、2-氟-4-甲基-4-(4-三氟甲氧基苯基)-2-烯醇(实施例10)(0.2g)、苯(10ml)和吡啶(0.081ml)得到标题化合物(0.23g,99%)。实施例16(1-(4-氯苯基)-3-氟-4-(3-苯氧基苯基)丁-2-烯基)环丙烷
在氮气中,由3-苯氧基苯基溴(0.3g)于无水四氢呋喃(2ml)和镁(21mg)制备格氏试剂,用碘作引发剂,在大约40℃约反应10分钟。在冷却至-78C后,一边搅拌一边缓慢加入4-(4-氯苯基)-4-环丙基-2-氟丁-2-烯基乙酸酯(实施例11)(0.11g)的四氢呋喃(1ml)溶液,然后使混合物温热至室温过夜。加水(2ml),混合物用乙醚(3×20ml)萃取,合并的有机层用水(3×10ml)洗涤,干燥和减压蒸发。残余的油用制备薄层色谱纯化(溶剂:乙醚/己烷1∶9),然后用制备高效液相色谱纯化(柱:C18;溶剂:甲醇;流速:8ml/min)得到1-(4-氯苯基)-3-氟-4-(3-苯氧基苯基)丁-2-烯基)环丙烷(49.5g,33%)。13CNMR142.9,128.4a,128.7a,131.0,43.6(4),16.6,3.8,4.5,110.0(15),N,38.4(29),138.3,117.3,157.5b,119.1,129.8,123.6,Nb,118.9,129.8,123.3实施例17(1-(4-乙氧基苯基)-3-氟-4-(3-苯氧基苯基)丁-2-烯基)环丙烷
重复实施例16的方法,用由3-苯氧基苯基溴(0.53g)、四氢呋喃(4ml)和镁(38g)制备的格氏试剂,以及4-环丙基-4-(4-乙氧基苯基)-2-氟丁-2-烯基乙酸酯(实施例12)(0.18g)。蒸发后的残余物用制备薄层色谱纯化(溶剂:乙醚/己烷1∶9),得到1-(4-乙氧基苯基)-3-氟-4-(3-苯氧基苯基)丁-2-烯基)环丙烷(66.3g,28%)。13CNMR136.3,128.2,114.3,157.1a,43.2(4),16.8,3.7,4.5,110.6(15),156.9(256),38.8(30),138.6,117.2,157.3a,119.1,129.8,123.6,157.4b,118.9,129.7,123.2和63.4,14.9(OEt)实施例181-(4-氯苯基)-2-氟-4-甲基-1-(3-苯氧基苯基)戊-2-烯
重复实施例16的方法,用由3-苯氧基苯基溴(0.19g)、四氢呋喃(2ml)和镁(22g)制备的格氏试剂,以及1-(4-氯苯基)-2-氟-4-甲基戊-2-烯基乙酸酯(实施例13)(0.12g)。蒸发后的残余物用制备薄层色谱纯化(溶剂:乙醚/己烷1∶9),然后用制备高效液相色谱纯化(柱:C18;溶剂:甲醇;流速:3ml/min)得到标题化合物(38mg,22%)。13CNMR131.4,128.1a,127.1a,148.4,38.5,30.1(3),116.0(9),N,39.1(29),138.6,117.2,157.4b,119.0,129.7,123.6,157.0b,118.9,129.8,123.3实施例191-(4-乙氧基苯基)-2-氟-4-甲基-1-(3-苯氧基苯基)戊-2-烯
重复实施例16的方法,用由3-苯氧基苯基溴(0.31g)、四氢呋喃(2ml)和镁(24mg)制备的格氏试剂,以及1-(4-乙氧基苯基)-2-氟-4-甲基戊-2-烯基乙酸酯(实施例14)(70mg)。蒸发后的残余物用制备薄层色谱纯化(溶剂:乙醚/己烷1∶9),得到标题化合物(44mg,45%)。13CNMR142.0,126.6,113.9,157.1a,38.3,30.2(3),116.6(9),156.7(260),39.2(29),138.9,11,117.1,157.4b,119.1,129.7,123.6,157.1b,118.9,129.7,123.3和63.3,14.9(OEt)实施例202-氟-4-甲基-1-(3-苯氧基苯基)-4-(4-三氟甲氧基苯基)戊-2-烯
重复实施例16的方法,用由3-苯氧基苯基溴(0.3g)、四氢呋喃(2ml)和镁(22mg)制备的格利雅试剂,以及2-氟-4-甲基-4-(4-三氟甲氧基苯基)戊-2-烯基乙酸酯(实施例15)(0.1g)。蒸发后的残余物用制备薄层色谱纯化(溶剂:乙醚/己烷1∶9),得到标题化合物(56mg,42%)。13CNMR148.6,127.0,120.4,157.7a,38.6,30.2(3),115.9(10),157.3(261),39.1(29),138.6,11,117.2,157.5b,119.0,129.8,123.6,151.1a,118.9,129.8,123.3实施例211-(4-氯苯基)-3-氟-4-(4-氟-3-苯氧基苯基)丁-2-烯基)环丙烷
重复实施例16的方法,用由4-氟-3-苯氧基苯基溴(0.3g)、四氢呋喃(2ml)和镁(21mg)制备的格氏试剂,以及4-(4-氯苯基)-4-环丙基-2-氟丁-2-烯基乙酸酯(实施例11)(0.14g)。蒸发后的残余物用制备薄层色谱纯化(溶剂:乙醚/己烷1∶9),然后用制备高效液相色谱纯化(柱:C18;溶剂:甲醇;流速:3ml/min)得到标题化合物(43mg,21%)。13C NMR131.9,128.5a,128.7a,142.8,43.6(4),16.5,3.8,4.5,110.5(15),N,37.9(29),N,121.9,N,N,117.0(20),124.8(6),N,129.7,117.4,123.2实施例221-(4-乙氧基苯基)-3-氟-4-(4-氟-3-苯氧基苯基)丁-2-烯基)环丙烷
重复实施例16的方法,用由4-氟-3-苯氧基苯基溴(0.52g)、四氢呋喃(4ml)和镁(32mg)制备的格氏试剂,以及4-环丙基-4-(4-乙氧基苯基)-2-氟丁-2-烯基乙酸酯(实施例12)(0.2g)。蒸发后的残余物用制备薄层色谱纯化(溶剂:乙醚/己烷1∶9),得到1-(4-乙氧基苯基)-3-氟-4-(3-苯氧基苯基)丁-2-烯基)环丙烷(56mg,20%)。13CNMR136.3,128.2,114.3,157.2a,43.2(4),16.7,3.7,4.5,110.8(14),156.8(255),37.8(30),134.4(4),122.0,143.6(12),153.2(248),116.9(18),124.8(7),157.4a,117.3,129.7,123.2和63.4,14.9(OEt)实施例234-(4-氯苯基)-2-氟-1-(4-氟-3-苯氧基苯基)-4-甲基戊-2-烯
重复实施例16的方法,用由4-氟-3-苯氧基苯基溴(0.36g)、四氢呋喃(4ml)和镁(26mg)制备的格氏试剂,以及4-(4-氯苯基)-2-氟-4-甲基戊-2-烯基乙酸酯(实施例13)(0.1g)。蒸发后的残余物用制备薄层色谱纯化(溶剂:乙醚/己烷1∶9)得到标题化合物(68mg,43%)。13CNMR131.4,127.0a,128.1a,148.3,38.5,30.1(4),116.0(10),156.5(29),38.5(29),133.4,121.8,143.6(12),153.2(248),117.0(18),124.8(8).157.2,117.4,129.7,123.2实施例244-(4-乙氧基苯基)-2-氟-1-(4-氟-3-苯氧基苯基)-4-甲基戊-2-烯
重复实施例16的方法,用由4-氟-3-苯氧基苯基溴(0.28g)、四氢呋喃(2ml)和镁(20mg)制备的格氏试剂,以及4-(4-乙氧基苯基)-2-氟-4-甲基戊-2-烯基乙酸酯(实施例14)(60mg)。蒸发后的残余物用制备薄层色谱纯化(溶剂:乙醚/己烷1∶9),然后用制备高效液相色谱纯化(柱:C18;溶剂:含5%水的甲醇;流速:3ml/min)得到标题化合物(18mg,20%)。13CNMR141.9,121.5,113.9,156.9,38.2,30.2(4),116.6(8),N,38.3(29),121.9,N,N,116.9(18),124.8(7),157.2,117.4,129.7,123.2实施例252-氟-1-(4-氟-3-苯氧基苯基)-4-甲基-4-(4-三氟甲氧基苯基)戊-2-烯
重复实施例16的方法,用由4-氟-3-苯氧基苯基溴(0.4g)、四氢呋喃(4ml)和镁(26mg)制备的格氏试剂,以及2-氟-4-甲基-4-(4-三氟甲氧基苯基)戊-2-烯基乙酸酯(实施例15)(90mg)。蒸发后的残余物用制备薄层色谱纯化(溶剂:乙醚/己烷1∶9),得到标题化合物(40mg,33%)。13CNMR148.4,127.0,120.5,N,38.6,30.2(3),116.1(10),157.1(260),39.5(29),133.4(4),121.8,143.7(12)153.2(248),117.0(18),124.8(7),157.2,117.4,129.8,123.3实施例26生物试验
用下述技术评估对家蝇、辣根猿叶甲、小菜蛾和cornroot worm的农药活性:
家蝇(Musca domestica)
在家蝇的胸部用1微升溶于丙酮的农药滴入处理。每种剂量施用率用15只家蝇,每种试验化合物6种剂量,重复2次。处理之后,将家蝇放置在20℃+1℃的温度下,在处理后24小时和48小时时将它们处死进行评估。以每只家蝇微克农药计算出LD50,由LD50值的反比计算出相对毒性(参见Saeicki等人,Bulletin of World HealthOrganisation,35,893,(1966),Sawicki等人,Entomologia和Exp.Appli.,10253(1967))。辣根猿叶甲(Phaedon cochleariae Fab)
用一微滴给药器将试验化合物的丙酮溶液施用于辣根猿叶甲成虫的腹部,处理后的昆虫放置48小时,然后处死进行评估。每种剂量水平用15只辣根猿叶甲,每种试验化合物5种剂量,重复2次。
如家蝇试验中所作的计算LD50值并由此计算相对效力。小菜蛾(Plutella xylostella)
用0.5μl的农药丙酮溶液处理15只蜕期幼虫。每种剂量施用率用10只幼虫,每种试验化合物5种剂量,重复3次。处理之后,将幼虫放置于大约22℃,5天后不能化蛹时将它们处死进行评估,如家蝇试验中所作的计算LD50值并由此计算相对效力。
对于这三种昆虫类别而言,其相对效力都是以和5-苄基-3-呋喃基甲基(1R)-反式-菊酸酯(苄呋菊酯)作对比计算的,该化合物是已知的对这些昆虫毒性较大的菊酸酯类之一。
对家蝇、辣根猿叶甲和小菜蛾的相对效力(苄呋菊酯=100)在下表1中分别以HF,MB和PX给出。黄瓜条叶甲(cornroot worm)(Diabrotica balteata)
土壤中的残留活性按下面的方法评估:
将溶于1.0ml丙酮的已知量的试验化合物均匀地施用于含10%水份的标准量(22g)的沙土中,1小时后放入10只幼虫。使温度保持在20℃±1℃,48小时后统计死亡率。每种剂量施用率用10只幼虫,每种试验化合物5种剂量,重复2次。应用概率分析的方法计算在标准量的土壤中不同杀虫剂浓度的LD50值。
局部土壤活性的评估是用1μl数滴杀虫剂的甲乙酮溶液处理蜕变后期的幼虫。每种剂量施用率用10只幼虫,每种试验化合物5种剂量,重复3次。20℃,48小时后统计死亡率。如家蝇试验中所作的计算LD50值并由此计算相对效力。
残留和局部活性的试验结果列于下表1:
表1
叶甲(Diabrotica)实施例编号 R1 R2 R X HFa MBa PXa Topb Resc16 H -< Cl H 48 23 -66 .009 .3617 H -< EtO H 9.5 40 160 .024 0.718 Me Me Cl H 9.5 1.6 -12 .0041 .1719 Me Me EtO H 25 4.7 61 .005 .320 Me Me F3CO H 16 13 48 .054 NA21 H -< Cl F 29 90 -180 .005 .0322 H -< EtO F -60 160 -320 .008 .02623 Me Me Cl F 11 5.2 70 .003 .0224 Me Me EtO F 40 24 90 .013 NA25 Me Me F3CO F 13 29 -220 .019 .37a)相对于苄呋菊酯=100的效力b)LD50,以每只幼虫μg计c)LD50,以土壤中含量ppm计
Claims (16)
1.下式I的农药化合物:
式中:
R1是氢和R2表示环丙基,或者R1和R2各自表示烷基,其中的烷基可以相同或不同;
ArA表示选择取代的苯基和萘基;
ArB表示苯氧基、苯基、苄基或苯甲酰基-取代的苯基,它可进一步选择取代;
双键上的ArA-CR1R2和-CH2ArB基团的构型相互为反式。
2.按照权利要求1的化合物,其中ArA是取代的苯基。
3.按照权利要求2的化合物,其中的苯基在4-(对位)被卤素、烷氧基或卤代烷基。
4.按照权利要求1-3中任意一项的化合物,其中的ArB是在3-(间位)上被苯氧基、苯基、苄基或苯甲酰基取代的苯基。
5.按照权利要求4的化合物,其中的苯基是在4-(对位)进一步被氟取代。
7.按照权利要求6的方法,其中包括形式为ArB-的亲核试剂与下式化合物进行催化反应,其中Q表示良好的离去基团。
8.按照权利要求7的方法,亲核试剂以格氏试剂的形式存在。
9.农药组合物,含有农药有效量的权利要求1-5任意一项中定义的式I化合物,还包括可接受的载体或稀释剂。
10.杀虫剂组合物,含有权利要求1-5任意一项中定义的式I化合物,还包括惰性载体或稀释剂。
11.按照权利要求10的组合物,适用于防治生长于土壤中的昆虫害虫。
12.按照权利要求9-11中任意一项的组合物,该组合物是颗粒剂、粉剂或乳油的形式。
13..按照权利要求9-12中任意一项的组合物,该组合物含有0.5-5%重量的式I化合物。
14.权利要求1-5任意一项的化合物或权利要求10-13任意一项的组合物在防治生长于土壤中的昆虫害虫中的应用。
15.防治生长于土壤中的昆虫害虫的方法,包括将权利要求10-13任意一项的组合物施用于土壤或进行种子处理。
16.按照权利要求15的方法,其中施用率为每公顷1-100g活性成分。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB9408605.5 | 1994-04-29 | ||
| GB9408605A GB9408605D0 (en) | 1994-04-29 | 1994-04-29 | Pesticidal fluoroolefins |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN1147242A true CN1147242A (zh) | 1997-04-09 |
Family
ID=10754382
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN95192851A Pending CN1147242A (zh) | 1994-04-29 | 1995-04-26 | 农药用氟代烯烃 |
Country Status (21)
| Country | Link |
|---|---|
| US (1) | US5880162A (zh) |
| EP (1) | EP0757668A1 (zh) |
| JP (1) | JPH09512543A (zh) |
| KR (1) | KR970702836A (zh) |
| CN (1) | CN1147242A (zh) |
| AU (1) | AU2264995A (zh) |
| BG (1) | BG63007B1 (zh) |
| BR (1) | BR9507508A (zh) |
| CA (1) | CA2187918A1 (zh) |
| CZ (1) | CZ310796A3 (zh) |
| FI (1) | FI964343A0 (zh) |
| GB (3) | GB9408605D0 (zh) |
| HU (1) | HU215403B (zh) |
| NO (1) | NO307297B1 (zh) |
| PL (1) | PL179373B1 (zh) |
| RU (1) | RU2154625C2 (zh) |
| SK (1) | SK281501B6 (zh) |
| TR (1) | TR28774A (zh) |
| TW (1) | TW398956B (zh) |
| WO (1) | WO1995029887A1 (zh) |
| ZA (1) | ZA953119B (zh) |
Families Citing this family (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| PT811593E (pt) * | 1996-06-03 | 2002-11-29 | Basf Ag | Agentes insecticidas e acaricidas 1,4-diaril-2-fluor-2-buteno |
| US5849958A (en) * | 1997-03-17 | 1998-12-15 | American Cyanamid Company | 1,4,diaryl-2-fluoro-2-butene insecticidal and acaricidal agents |
| ZA974582B (en) * | 1996-06-03 | 1998-11-26 | American Cyanamid Co | Process and intermediate compounds for the preparation of pesticidal fluoroolefin compounds |
| US5892131A (en) * | 1997-05-29 | 1999-04-06 | American Cyanamid Company | Process for the preparation of pesticidal fluoroolefin compounds |
| US5998673A (en) * | 1996-06-03 | 1999-12-07 | American Cyanamid Company | 1, 4-diaryl-2-fluoro-2-butene insecticidal and acaricidal agents |
| US5962742A (en) * | 1996-06-14 | 1999-10-05 | American Cyanamid Company | Process for the preparation of 5-bromo-2 fluorobenzeneboronic acid |
| US6198008B1 (en) | 1997-06-06 | 2001-03-06 | American Cyanamid Company | Process for the preparation of 5-bromo-2-fluorobenzeneboronic acid |
| GEP20022795B (en) * | 1997-06-19 | 2002-09-25 | American Cyanamid Co | Process and Intermediate Compounds for Preparation of Pesticidal Fluoroolefin Compounds |
| TW467889B (en) * | 1997-11-12 | 2001-12-11 | American Cyanamid Co | 1,4-diaryl-2,3-difluoro-2-butene insecticidal and acaricidal agents |
| IL127856A0 (en) * | 1998-01-30 | 1999-10-28 | American Cyanamid Co | 1,4-Diaryl-3-fluoro-2-butene compounds for protecting animals and humans against attack and infestation by anthropod parasites |
| IL127852A0 (en) * | 1998-01-30 | 1999-10-28 | American Cyanamid Co | Methods and compositions for protecting animals and humans against attack and infestation by arthropod and helminth parasites |
| US6235754B1 (en) | 1998-01-30 | 2001-05-22 | American Cyanamid Company | Methods and compositions for protecting animals and humans against attack and infestation by arthropod and helminth parasites |
| US6646166B1 (en) * | 1998-11-16 | 2003-11-11 | Basf Aktiengesellschaft | Processes for the preparation of 1,4-diaryl-2-fluoro-1,3-butadiene and 1,4-diaryl-2-fluoro 2 butene compounds |
| DE60109914T2 (de) | 2000-05-04 | 2006-02-09 | Basf Ag | 1,4-diaryl-2-fluoro-4-cyano-2-buten-derivate, verfahren zu ihrer herstellung und zwischenprodukte für dieses verfahren |
| CA2421861A1 (en) | 2000-09-12 | 2002-03-21 | Basf Aktiengesellschaft | 1,4-diaryl-2,3-difluoro-2-butene insecticidal and acaricidal agents |
| DE60110123T2 (de) | 2000-09-12 | 2006-03-02 | Basf Ag | Verfahren und zwischenverbindungen zur herstellung von pestiziden fluorolefinverbindungen |
| TWI780112B (zh) * | 2017-03-31 | 2022-10-11 | 美商科迪華農業科技有限責任公司 | 具有殺蟲效用之分子,及其相關之中間物、組成物暨方法 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS60115545A (ja) * | 1983-11-25 | 1985-06-22 | Sumitomo Chem Co Ltd | 炭化水素系化合物を有効成分とする殺虫剤 |
| GB8409195D0 (en) * | 1984-04-09 | 1984-05-16 | Elliott M | Pesticides |
| US4975451A (en) * | 1989-03-02 | 1990-12-04 | Fmc Corporation | Insecticidal cyclopropyl di(aryl) 2-butenes |
| GB9219612D0 (en) * | 1992-09-16 | 1992-10-28 | Khambay Bhupinder P S | Pesticidal fluoroolefins |
-
1994
- 1994-04-29 GB GB9408605A patent/GB9408605D0/en active Pending
-
1995
- 1995-04-18 ZA ZA953119A patent/ZA953119B/xx unknown
- 1995-04-26 HU HU9602967A patent/HU215403B/hu not_active IP Right Cessation
- 1995-04-26 CA CA002187918A patent/CA2187918A1/en not_active Abandoned
- 1995-04-26 AU AU22649/95A patent/AU2264995A/en not_active Abandoned
- 1995-04-26 RU RU96122872/04A patent/RU2154625C2/ru active
- 1995-04-26 CZ CZ963107A patent/CZ310796A3/cs unknown
- 1995-04-26 GB GB9620352A patent/GB2301360A/en not_active Withdrawn
- 1995-04-26 EP EP95915964A patent/EP0757668A1/en not_active Withdrawn
- 1995-04-26 US US08/732,305 patent/US5880162A/en not_active Expired - Fee Related
- 1995-04-26 WO PCT/GB1995/000954 patent/WO1995029887A1/en not_active Application Discontinuation
- 1995-04-26 CN CN95192851A patent/CN1147242A/zh active Pending
- 1995-04-26 BR BR9507508A patent/BR9507508A/pt not_active IP Right Cessation
- 1995-04-26 PL PL95317027A patent/PL179373B1/pl unknown
- 1995-04-26 JP JP7528060A patent/JPH09512543A/ja active Pending
- 1995-04-26 GB GB9508449A patent/GB2288803B/en not_active Expired - Fee Related
- 1995-04-26 SK SK1289-96A patent/SK281501B6/sk unknown
- 1995-04-28 TW TW084104220A patent/TW398956B/zh not_active IP Right Cessation
- 1995-04-28 TR TR00488/95A patent/TR28774A/xx unknown
-
1996
- 1996-10-23 BG BG100934A patent/BG63007B1/bg unknown
- 1996-10-28 NO NO964563A patent/NO307297B1/no not_active IP Right Cessation
- 1996-10-28 FI FI964343A patent/FI964343A0/fi unknown
- 1996-10-29 KR KR1019960706098A patent/KR970702836A/ko not_active Application Discontinuation
Also Published As
| Publication number | Publication date |
|---|---|
| AU2264995A (en) | 1995-11-29 |
| GB9408605D0 (en) | 1994-06-22 |
| FI964343L (fi) | 1996-10-28 |
| HUT76430A (en) | 1997-08-28 |
| HU215403B (hu) | 1998-12-28 |
| GB2288803A (en) | 1995-11-01 |
| SK128996A3 (en) | 1997-06-04 |
| KR970702836A (ko) | 1997-06-10 |
| JPH09512543A (ja) | 1997-12-16 |
| CZ310796A3 (en) | 1997-08-13 |
| GB9508449D0 (en) | 1995-06-14 |
| PL317027A1 (en) | 1997-03-03 |
| US5880162A (en) | 1999-03-09 |
| HU9602967D0 (en) | 1996-12-30 |
| BG63007B1 (bg) | 2001-01-31 |
| GB2301360A (en) | 1996-12-04 |
| NO964563L (no) | 1996-10-28 |
| EP0757668A1 (en) | 1997-02-12 |
| TR28774A (tr) | 1997-02-20 |
| BR9507508A (pt) | 1997-09-02 |
| GB2288803B (en) | 1998-09-16 |
| BG100934A (en) | 1997-12-30 |
| PL179373B1 (en) | 2000-08-31 |
| TW398956B (en) | 2000-07-21 |
| RU2154625C2 (ru) | 2000-08-20 |
| CA2187918A1 (en) | 1995-11-09 |
| ZA953119B (en) | 1996-01-09 |
| GB9620352D0 (en) | 1996-11-13 |
| NO964563D0 (no) | 1996-10-28 |
| FI964343A0 (fi) | 1996-10-28 |
| WO1995029887A1 (en) | 1995-11-09 |
| SK281501B6 (sk) | 2001-04-09 |
| NO307297B1 (no) | 2000-03-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1147242A (zh) | 农药用氟代烯烃 | |
| JPS6355500B2 (zh) | ||
| JPH0212942B2 (zh) | ||
| KR950004892B1 (ko) | 살충제용 에테르의 제조방법 | |
| CN1037098C (zh) | 氟代链烯杀虫剂 | |
| CH658046A5 (fr) | Composes du type arylalkyl ou arylcycloalkyl, procedes de preparation de ces composes et compositions insecticides et acaricides contenant ces composes en tant qu'ingredients actifs. | |
| SE430298B (sv) | Insekticid komposition | |
| EP2203064B1 (en) | Polyenylcyclopropanecarboxylic esters with high insecticidal activity | |
| FR2462419A1 (fr) | Nouveaux cyclopropanecarboxylates de cyclopentenone, leur production et leur utilisation comme insecticides | |
| KR920002126B1 (ko) | 살충제 화합물의 제조방법 | |
| WO1997016067A1 (en) | Compounds for control of whitefly | |
| CN1206395A (zh) | 农药化合物、组合物及其制备方法 | |
| CA2187277C (en) | Novel derivative of ester of carboxylic acid, the process of manufacturing the same, and insecticide and insect proofing agent containing the same | |
| CN1047382C (zh) | 拟除虫菊酯组合物及其应用 | |
| Khambay et al. | The pyrethrins and related compounds. Part XLII: Structure–activity relationships in fluoro‐olefin non‐ester pyrethroids | |
| CN1021434C (zh) | 改进的农药化合物的制备方法 | |
| JPH0372053B2 (zh) | ||
| CN1026649C (zh) | 改进的杀虫剂组合物及其生产方法及用途 | |
| CN1205610A (zh) | 防治白粉虱的化合物 | |
| JPH0328405B2 (zh) | ||
| JPH0135819B2 (zh) | ||
| CN1045772A (zh) | 2-卤代环丙基乙烷衍生物及其制备方法和在杀虫剂上的应用 | |
| JPH04261133A (ja) | 新規芳香族アルカン誘導体、その製造法 およびそれを有効成分とする殺虫、殺ダニ剤 | |
| JPS6221776B2 (zh) | ||
| JPH03115203A (ja) | 2―アリールプロピルエーテル誘導体を含む殺虫、殺ダニ剤 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| ASS | Succession or assignment of patent right |
Owner name: BRITISH TECHNOLOGY GROUP CO., LTD. Free format text: FORMER OWNER: BRITISH TECHNOLOGY GROUP CO., LTD Effective date: 20010821 |
|
| C41 | Transfer of patent application or patent right or utility model | ||
| TA01 | Transfer of patent application right |
Effective date of registration: 20010821 Applicant after: British Technology Group Ltd. Applicant before: British Technology Group Limited |
|
| AD01 | Patent right deemed abandoned | ||
| C20 | Patent right or utility model deemed to be abandoned or is abandoned |