CN113940997B - 一种双特异性抗体的稳定制剂 - Google Patents
一种双特异性抗体的稳定制剂 Download PDFInfo
- Publication number
- CN113940997B CN113940997B CN202111566377.XA CN202111566377A CN113940997B CN 113940997 B CN113940997 B CN 113940997B CN 202111566377 A CN202111566377 A CN 202111566377A CN 113940997 B CN113940997 B CN 113940997B
- Authority
- CN
- China
- Prior art keywords
- antibody
- bispecific antibody
- aqueous composition
- nkg
- seq
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000002360 preparation method Methods 0.000 title abstract description 16
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 claims abstract description 39
- 239000007853 buffer solution Substances 0.000 claims abstract description 15
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 claims abstract description 15
- 229920000053 polysorbate 80 Polymers 0.000 claims abstract description 15
- 229930006000 Sucrose Natural products 0.000 claims abstract description 11
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 claims abstract description 11
- 239000005720 sucrose Substances 0.000 claims abstract description 11
- 238000002347 injection Methods 0.000 claims abstract description 8
- 239000007924 injection Substances 0.000 claims abstract description 8
- 102000008096 B7-H1 Antigen Human genes 0.000 claims abstract 5
- 108010074708 B7-H1 Antigen Proteins 0.000 claims abstract 5
- 230000027455 binding Effects 0.000 claims description 48
- 239000000203 mixture Substances 0.000 claims description 31
- 239000000427 antigen Substances 0.000 claims description 30
- 102000036639 antigens Human genes 0.000 claims description 30
- 108091007433 antigens Proteins 0.000 claims description 30
- 102100022682 NKG2-A/NKG2-B type II integral membrane protein Human genes 0.000 claims description 28
- 101150069255 KLRC1 gene Proteins 0.000 claims description 25
- 101100404845 Macaca mulatta NKG2A gene Proteins 0.000 claims description 25
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 claims description 22
- 239000000872 buffer Substances 0.000 claims description 17
- 239000011780 sodium chloride Substances 0.000 claims description 11
- 239000003223 protective agent Substances 0.000 claims description 8
- 239000004094 surface-active agent Substances 0.000 claims description 6
- 239000007979 citrate buffer Substances 0.000 claims description 5
- 238000009472 formulation Methods 0.000 claims description 4
- 239000004615 ingredient Substances 0.000 claims 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 abstract description 30
- 238000012360 testing method Methods 0.000 abstract description 29
- 238000001514 detection method Methods 0.000 abstract description 11
- 230000004071 biological effect Effects 0.000 abstract description 7
- 238000005286 illumination Methods 0.000 abstract description 4
- 239000003381 stabilizer Substances 0.000 abstract description 2
- 210000004027 cell Anatomy 0.000 description 29
- 239000012634 fragment Substances 0.000 description 26
- 238000001542 size-exclusion chromatography Methods 0.000 description 20
- 108090000623 proteins and genes Proteins 0.000 description 19
- 235000018102 proteins Nutrition 0.000 description 18
- 102000004169 proteins and genes Human genes 0.000 description 18
- 239000000523 sample Substances 0.000 description 17
- 238000004255 ion exchange chromatography Methods 0.000 description 14
- 150000001413 amino acids Chemical class 0.000 description 13
- 239000002245 particle Substances 0.000 description 12
- 238000013368 capillary electrophoresis sodium dodecyl sulfate analysis Methods 0.000 description 11
- 230000000694 effects Effects 0.000 description 11
- 230000003993 interaction Effects 0.000 description 10
- 238000011835 investigation Methods 0.000 description 10
- 239000003446 ligand Substances 0.000 description 10
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 10
- 229940068968 polysorbate 80 Drugs 0.000 description 10
- 235000001014 amino acid Nutrition 0.000 description 9
- 238000010494 dissociation reaction Methods 0.000 description 9
- 230000005593 dissociations Effects 0.000 description 9
- 238000000034 method Methods 0.000 description 8
- 102100028970 HLA class I histocompatibility antigen, alpha chain E Human genes 0.000 description 7
- 101000986085 Homo sapiens HLA class I histocompatibility antigen, alpha chain E Proteins 0.000 description 7
- 101001109508 Homo sapiens NKG2-A/NKG2-B type II integral membrane protein Proteins 0.000 description 7
- 206010028980 Neoplasm Diseases 0.000 description 7
- 210000001744 T-lymphocyte Anatomy 0.000 description 7
- 230000008859 change Effects 0.000 description 7
- 230000002829 reductive effect Effects 0.000 description 7
- 210000004881 tumor cell Anatomy 0.000 description 7
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 6
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 5
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 5
- 230000000903 blocking effect Effects 0.000 description 5
- 238000002474 experimental method Methods 0.000 description 5
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 5
- 210000000822 natural killer cell Anatomy 0.000 description 5
- 238000005457 optimization Methods 0.000 description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 5
- 101001117317 Homo sapiens Programmed cell death 1 ligand 1 Proteins 0.000 description 4
- 238000003556 assay Methods 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 102000048776 human CD274 Human genes 0.000 description 4
- 102000057310 human KLRC1 Human genes 0.000 description 4
- 238000009169 immunotherapy Methods 0.000 description 4
- 230000006641 stabilisation Effects 0.000 description 4
- 238000011105 stabilization Methods 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 4
- 101000971513 Homo sapiens Natural killer cells antigen CD94 Proteins 0.000 description 3
- 102000018071 Immunoglobulin Fc Fragments Human genes 0.000 description 3
- 108010091135 Immunoglobulin Fc Fragments Proteins 0.000 description 3
- 102100021462 Natural killer cells antigen CD94 Human genes 0.000 description 3
- 230000002776 aggregation Effects 0.000 description 3
- 238000004220 aggregation Methods 0.000 description 3
- 108010069205 aspartyl-phenylalanine Proteins 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- UQLDLKMNUJERMK-UHFFFAOYSA-L di(octadecanoyloxy)lead Chemical compound [Pb+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O UQLDLKMNUJERMK-UHFFFAOYSA-L 0.000 description 3
- 239000012636 effector Substances 0.000 description 3
- 238000000684 flow cytometry Methods 0.000 description 3
- 230000006870 function Effects 0.000 description 3
- 210000002865 immune cell Anatomy 0.000 description 3
- 230000002401 inhibitory effect Effects 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 210000000581 natural killer T-cell Anatomy 0.000 description 3
- 229920000136 polysorbate Polymers 0.000 description 3
- 108020003175 receptors Proteins 0.000 description 3
- 102000005962 receptors Human genes 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- MNQMTYSEKZHIDF-GCJQMDKQSA-N Asp-Thr-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O MNQMTYSEKZHIDF-GCJQMDKQSA-N 0.000 description 2
- 210000001266 CD8-positive T-lymphocyte Anatomy 0.000 description 2
- YQPFCZVKMUVZIN-AUTRQRHGSA-N Glu-Val-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O YQPFCZVKMUVZIN-AUTRQRHGSA-N 0.000 description 2
- BYYNJRSNDARRBX-YFKPBYRVSA-N Gly-Gln-Gly Chemical compound NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O BYYNJRSNDARRBX-YFKPBYRVSA-N 0.000 description 2
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 2
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 2
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 2
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 2
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 2
- PDIDTSZKKFEDMB-UWVGGRQHSA-N Lys-Pro-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O PDIDTSZKKFEDMB-UWVGGRQHSA-N 0.000 description 2
- 108010079364 N-glycylalanine Proteins 0.000 description 2
- 101710094000 Programmed cell death 1 ligand 1 Proteins 0.000 description 2
- 102100024216 Programmed cell death 1 ligand 1 Human genes 0.000 description 2
- 108091007744 Programmed cell death receptors Proteins 0.000 description 2
- 102000001708 Protein Isoforms Human genes 0.000 description 2
- 108010029485 Protein Isoforms Proteins 0.000 description 2
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 2
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 2
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 2
- OWFGFHQMSBTKLX-UFYCRDLUSA-N Val-Tyr-Tyr Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)N OWFGFHQMSBTKLX-UFYCRDLUSA-N 0.000 description 2
- 230000021736 acetylation Effects 0.000 description 2
- 238000006640 acetylation reaction Methods 0.000 description 2
- 108010069020 alanyl-prolyl-glycine Proteins 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 230000009830 antibody antigen interaction Effects 0.000 description 2
- 230000008827 biological function Effects 0.000 description 2
- 210000004899 c-terminal region Anatomy 0.000 description 2
- 238000005251 capillar electrophoresis Methods 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- 235000018417 cysteine Nutrition 0.000 description 2
- 230000001086 cytosolic effect Effects 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 239000013604 expression vector Substances 0.000 description 2
- 102000037865 fusion proteins Human genes 0.000 description 2
- 108020001507 fusion proteins Proteins 0.000 description 2
- 230000014509 gene expression Effects 0.000 description 2
- 230000013595 glycosylation Effects 0.000 description 2
- 238000006206 glycosylation reaction Methods 0.000 description 2
- VPZXBVLAVMBEQI-UHFFFAOYSA-N glycyl-DL-alpha-alanine Natural products OC(=O)C(C)NC(=O)CN VPZXBVLAVMBEQI-UHFFFAOYSA-N 0.000 description 2
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Natural products NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 2
- 201000010536 head and neck cancer Diseases 0.000 description 2
- 208000014829 head and neck neoplasm Diseases 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 108091008042 inhibitory receptors Proteins 0.000 description 2
- 210000001165 lymph node Anatomy 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 230000002503 metabolic effect Effects 0.000 description 2
- 239000011859 microparticle Substances 0.000 description 2
- 238000004806 packaging method and process Methods 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 230000035755 proliferation Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- 238000013207 serial dilution Methods 0.000 description 2
- 230000009870 specific binding Effects 0.000 description 2
- 238000012289 standard assay Methods 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 108010044292 tryptophyltyrosine Proteins 0.000 description 2
- 239000013598 vector Substances 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- YYSWCHMLFJLLBJ-ZLUOBGJFSA-N Ala-Ala-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YYSWCHMLFJLLBJ-ZLUOBGJFSA-N 0.000 description 1
- KQFRUSHJPKXBMB-BHDSKKPTSA-N Ala-Ala-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)C)C(O)=O)=CNC2=C1 KQFRUSHJPKXBMB-BHDSKKPTSA-N 0.000 description 1
- JBGSZRYCXBPWGX-BQBZGAKWSA-N Ala-Arg-Gly Chemical compound OC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](N)C)CCCN=C(N)N JBGSZRYCXBPWGX-BQBZGAKWSA-N 0.000 description 1
- AWAXZRDKUHOPBO-GUBZILKMSA-N Ala-Gln-Lys Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(O)=O AWAXZRDKUHOPBO-GUBZILKMSA-N 0.000 description 1
- PAIHPOGPJVUFJY-WDSKDSINSA-N Ala-Glu-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O PAIHPOGPJVUFJY-WDSKDSINSA-N 0.000 description 1
- BEMGNWZECGIJOI-WDSKDSINSA-N Ala-Gly-Glu Chemical compound [H]N[C@@H](C)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(O)=O BEMGNWZECGIJOI-WDSKDSINSA-N 0.000 description 1
- ARHJJAAWNWOACN-FXQIFTODSA-N Ala-Ser-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O ARHJJAAWNWOACN-FXQIFTODSA-N 0.000 description 1
- CREYEAPXISDKSB-FQPOAREZSA-N Ala-Thr-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O CREYEAPXISDKSB-FQPOAREZSA-N 0.000 description 1
- VKKYFICVTYKFIO-CIUDSAMLSA-N Arg-Ala-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N VKKYFICVTYKFIO-CIUDSAMLSA-N 0.000 description 1
- HKRXJBBCQBAGIM-FXQIFTODSA-N Arg-Asp-Ser Chemical compound C(C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CO)C(=O)O)N)CN=C(N)N HKRXJBBCQBAGIM-FXQIFTODSA-N 0.000 description 1
- FEZJJKXNPSEYEV-CIUDSAMLSA-N Arg-Gln-Ala Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O FEZJJKXNPSEYEV-CIUDSAMLSA-N 0.000 description 1
- AOHKLEBWKMKITA-IHRRRGAJSA-N Arg-Phe-Ser Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N AOHKLEBWKMKITA-IHRRRGAJSA-N 0.000 description 1
- PRLPSDIHSRITSF-UNQGMJICSA-N Arg-Phe-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PRLPSDIHSRITSF-UNQGMJICSA-N 0.000 description 1
- ADPACBMPYWJJCE-FXQIFTODSA-N Arg-Ser-Asp Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O ADPACBMPYWJJCE-FXQIFTODSA-N 0.000 description 1
- KMFPQTITXUKJOV-DCAQKATOSA-N Arg-Ser-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O KMFPQTITXUKJOV-DCAQKATOSA-N 0.000 description 1
- WCZXPVPHUMYLMS-VEVYYDQMSA-N Arg-Thr-Asp Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O WCZXPVPHUMYLMS-VEVYYDQMSA-N 0.000 description 1
- XYBJLTKSGFBLCS-QXEWZRGKSA-N Asp-Arg-Val Chemical compound NC(N)=NCCC[C@@H](C(=O)N[C@@H](C(C)C)C(O)=O)NC(=O)[C@@H](N)CC(O)=O XYBJLTKSGFBLCS-QXEWZRGKSA-N 0.000 description 1
- YNQIDCRRTWGHJD-ZLUOBGJFSA-N Asp-Asn-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC(O)=O YNQIDCRRTWGHJD-ZLUOBGJFSA-N 0.000 description 1
- VHQOCWWKXIOAQI-WDSKDSINSA-N Asp-Gln-Gly Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O VHQOCWWKXIOAQI-WDSKDSINSA-N 0.000 description 1
- VFUXXFVCYZPOQG-WDSKDSINSA-N Asp-Glu-Gly Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O VFUXXFVCYZPOQG-WDSKDSINSA-N 0.000 description 1
- QNFRBNZGVVKBNJ-PEFMBERDSA-N Asp-Ile-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)O)N QNFRBNZGVVKBNJ-PEFMBERDSA-N 0.000 description 1
- GGRSYTUJHAZTFN-IHRRRGAJSA-N Asp-Pro-Tyr Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CC(=O)O)N)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O GGRSYTUJHAZTFN-IHRRRGAJSA-N 0.000 description 1
- BRRPVTUFESPTCP-ACZMJKKPSA-N Asp-Ser-Glu Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O BRRPVTUFESPTCP-ACZMJKKPSA-N 0.000 description 1
- GYNUXDMCDILYIQ-QRTARXTBSA-N Asp-Val-Trp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)[C@H](CC(=O)O)N GYNUXDMCDILYIQ-QRTARXTBSA-N 0.000 description 1
- 101150091609 CD274 gene Proteins 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- YFXFOZPXVFPBDH-VZFHVOOUSA-N Cys-Ala-Thr Chemical compound C[C@@H](O)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)CS)C(O)=O YFXFOZPXVFPBDH-VZFHVOOUSA-N 0.000 description 1
- SZQCDCKIGWQAQN-FXQIFTODSA-N Cys-Arg-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(O)=O SZQCDCKIGWQAQN-FXQIFTODSA-N 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 108700024394 Exon Proteins 0.000 description 1
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 1
- OYTPNWYZORARHL-XHNCKOQMSA-N Gln-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)N)N OYTPNWYZORARHL-XHNCKOQMSA-N 0.000 description 1
- IKFZXRLDMYWNBU-YUMQZZPRSA-N Gln-Gly-Arg Chemical compound NC(=O)CC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCN=C(N)N IKFZXRLDMYWNBU-YUMQZZPRSA-N 0.000 description 1
- FQCILXROGNOZON-YUMQZZPRSA-N Gln-Pro-Gly Chemical compound NC(=O)CC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O FQCILXROGNOZON-YUMQZZPRSA-N 0.000 description 1
- CGYDXNKRIMJMLV-GUBZILKMSA-N Glu-Arg-Glu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O CGYDXNKRIMJMLV-GUBZILKMSA-N 0.000 description 1
- PAQUJCSYVIBPLC-AVGNSLFASA-N Glu-Asp-Phe Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 PAQUJCSYVIBPLC-AVGNSLFASA-N 0.000 description 1
- ZYRXTRTUCAVNBQ-GVXVVHGQSA-N Glu-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N ZYRXTRTUCAVNBQ-GVXVVHGQSA-N 0.000 description 1
- YMUFWNJHVPQNQD-ZKWXMUAHSA-N Gly-Ala-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)CN YMUFWNJHVPQNQD-ZKWXMUAHSA-N 0.000 description 1
- UQJNXZSSGQIPIQ-FBCQKBJTSA-N Gly-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)CN UQJNXZSSGQIPIQ-FBCQKBJTSA-N 0.000 description 1
- MIIVFRCYJABHTQ-ONGXEEELSA-N Gly-Leu-Val Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O MIIVFRCYJABHTQ-ONGXEEELSA-N 0.000 description 1
- IMRNSEPSPFQNHF-STQMWFEESA-N Gly-Ser-Trp Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=CC=CC=C12)C(=O)O IMRNSEPSPFQNHF-STQMWFEESA-N 0.000 description 1
- NVTPVQLIZCOJFK-FOHZUACHSA-N Gly-Thr-Asp Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O NVTPVQLIZCOJFK-FOHZUACHSA-N 0.000 description 1
- ZZWUYQXMIFTIIY-WEDXCCLWSA-N Gly-Thr-Leu Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O ZZWUYQXMIFTIIY-WEDXCCLWSA-N 0.000 description 1
- MYXNLWDWWOTERK-BHNWBGBOSA-N Gly-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN)O MYXNLWDWWOTERK-BHNWBGBOSA-N 0.000 description 1
- UMRIXLHPZZIOML-OALUTQOASA-N Gly-Trp-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CC2=CNC3=CC=CC=C32)NC(=O)CN UMRIXLHPZZIOML-OALUTQOASA-N 0.000 description 1
- GNNJKUYDWFIBTK-QWRGUYRKSA-N Gly-Tyr-Asp Chemical compound [H]NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(O)=O)C(O)=O GNNJKUYDWFIBTK-QWRGUYRKSA-N 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 208000002250 Hematologic Neoplasms Diseases 0.000 description 1
- MWWOPNQSBXEUHO-ULQDDVLXSA-N His-Arg-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CN=CN1 MWWOPNQSBXEUHO-ULQDDVLXSA-N 0.000 description 1
- 101100407305 Homo sapiens CD274 gene Proteins 0.000 description 1
- OTSVBELRDMSPKY-PCBIJLKTSA-N Ile-Phe-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(=O)N)C(=O)O)N OTSVBELRDMSPKY-PCBIJLKTSA-N 0.000 description 1
- JHNJNTMTZHEDLJ-NAKRPEOUSA-N Ile-Ser-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O JHNJNTMTZHEDLJ-NAKRPEOUSA-N 0.000 description 1
- ZGKVPOSSTGHJAF-HJPIBITLSA-N Ile-Tyr-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CO)C(=O)O)N ZGKVPOSSTGHJAF-HJPIBITLSA-N 0.000 description 1
- 102000037982 Immune checkpoint proteins Human genes 0.000 description 1
- 108091008036 Immune checkpoint proteins Proteins 0.000 description 1
- 108060003951 Immunoglobulin Proteins 0.000 description 1
- 102100037850 Interferon gamma Human genes 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102000002698 KIR Receptors Human genes 0.000 description 1
- 108010043610 KIR Receptors Proteins 0.000 description 1
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 1
- TYYLDKGBCJGJGW-UHFFFAOYSA-N L-tryptophan-L-tyrosine Natural products C=1NC2=CC=CC=C2C=1CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 TYYLDKGBCJGJGW-UHFFFAOYSA-N 0.000 description 1
- CQGSYZCULZMEDE-UHFFFAOYSA-N Leu-Gln-Pro Natural products CC(C)CC(N)C(=O)NC(CCC(N)=O)C(=O)N1CCCC1C(O)=O CQGSYZCULZMEDE-UHFFFAOYSA-N 0.000 description 1
- FEHQLKKBVJHSEC-SZMVWBNQSA-N Leu-Glu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC(C)C)C(O)=O)=CNC2=C1 FEHQLKKBVJHSEC-SZMVWBNQSA-N 0.000 description 1
- KIZIOFNVSOSKJI-CIUDSAMLSA-N Leu-Ser-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N KIZIOFNVSOSKJI-CIUDSAMLSA-N 0.000 description 1
- MVJRBCJCRYGCKV-GVXVVHGQSA-N Leu-Val-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O MVJRBCJCRYGCKV-GVXVVHGQSA-N 0.000 description 1
- AIMGJYMCTAABEN-GVXVVHGQSA-N Leu-Val-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O AIMGJYMCTAABEN-GVXVVHGQSA-N 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- IXHKPDJKKCUKHS-GARJFASQSA-N Lys-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCCN)N IXHKPDJKKCUKHS-GARJFASQSA-N 0.000 description 1
- UWKNTTJNVSYXPC-CIUDSAMLSA-N Lys-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN UWKNTTJNVSYXPC-CIUDSAMLSA-N 0.000 description 1
- QUCDKEKDPYISNX-HJGDQZAQSA-N Lys-Asn-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QUCDKEKDPYISNX-HJGDQZAQSA-N 0.000 description 1
- AIRZWUMAHCDDHR-KKUMJFAQSA-N Lys-Leu-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O AIRZWUMAHCDDHR-KKUMJFAQSA-N 0.000 description 1
- RPWTZTBIFGENIA-VOAKCMCISA-N Lys-Thr-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O RPWTZTBIFGENIA-VOAKCMCISA-N 0.000 description 1
- UGCIQUYEJIEHKX-GVXVVHGQSA-N Lys-Val-Glu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O UGCIQUYEJIEHKX-GVXVVHGQSA-N 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- CAODKDAPYGUMLK-FXQIFTODSA-N Met-Asn-Ser Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O CAODKDAPYGUMLK-FXQIFTODSA-N 0.000 description 1
- SJDQOYTYNGZZJX-SRVKXCTJSA-N Met-Glu-Leu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O SJDQOYTYNGZZJX-SRVKXCTJSA-N 0.000 description 1
- SPSSJSICDYYTQN-HJGDQZAQSA-N Met-Thr-Gln Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(N)=O SPSSJSICDYYTQN-HJGDQZAQSA-N 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 102000018656 Mitogen Receptors Human genes 0.000 description 1
- 108010052006 Mitogen Receptors Proteins 0.000 description 1
- 101150110881 NKG2A gene Proteins 0.000 description 1
- 108091005461 Nucleic proteins Chemical group 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- MRWOVVNKSXXLRP-IHPCNDPISA-N Phe-Ser-Trp Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O MRWOVVNKSXXLRP-IHPCNDPISA-N 0.000 description 1
- KLYYKKGCPOGDPE-OEAJRASXSA-N Phe-Thr-Leu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O KLYYKKGCPOGDPE-OEAJRASXSA-N 0.000 description 1
- UIMCLYYSUCIUJM-UWVGGRQHSA-N Pro-Gly-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 UIMCLYYSUCIUJM-UWVGGRQHSA-N 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- UCOYFSCEIWQYNL-FXQIFTODSA-N Ser-Cys-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCSC)C(O)=O UCOYFSCEIWQYNL-FXQIFTODSA-N 0.000 description 1
- SQBLRDDJTUJDMV-ACZMJKKPSA-N Ser-Glu-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O SQBLRDDJTUJDMV-ACZMJKKPSA-N 0.000 description 1
- UQFYNFTYDHUIMI-WHFBIAKZSA-N Ser-Gly-Ala Chemical compound OC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](N)CO UQFYNFTYDHUIMI-WHFBIAKZSA-N 0.000 description 1
- YMTLKLXDFCSCNX-BYPYZUCNSA-N Ser-Gly-Gly Chemical compound OC[C@H](N)C(=O)NCC(=O)NCC(O)=O YMTLKLXDFCSCNX-BYPYZUCNSA-N 0.000 description 1
- UIGMAMGZOJVTDN-WHFBIAKZSA-N Ser-Gly-Ser Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O UIGMAMGZOJVTDN-WHFBIAKZSA-N 0.000 description 1
- QYSFWUIXDFJUDW-DCAQKATOSA-N Ser-Leu-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O QYSFWUIXDFJUDW-DCAQKATOSA-N 0.000 description 1
- GJFYFGOEWLDQGW-GUBZILKMSA-N Ser-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CO)N GJFYFGOEWLDQGW-GUBZILKMSA-N 0.000 description 1
- YUJLIIRMIAGMCQ-CIUDSAMLSA-N Ser-Leu-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YUJLIIRMIAGMCQ-CIUDSAMLSA-N 0.000 description 1
- AZWNCEBQZXELEZ-FXQIFTODSA-N Ser-Pro-Ser Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O AZWNCEBQZXELEZ-FXQIFTODSA-N 0.000 description 1
- XJDMUQCLVSCRSJ-VZFHVOOUSA-N Ser-Thr-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O XJDMUQCLVSCRSJ-VZFHVOOUSA-N 0.000 description 1
- BCAVNDNYOGTQMQ-AAEUAGOBSA-N Ser-Trp-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)NCC(O)=O BCAVNDNYOGTQMQ-AAEUAGOBSA-N 0.000 description 1
- GSCVDSBEYVGMJQ-SRVKXCTJSA-N Ser-Tyr-Asp Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CO)N)O GSCVDSBEYVGMJQ-SRVKXCTJSA-N 0.000 description 1
- LGIMRDKGABDMBN-DCAQKATOSA-N Ser-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CO)N LGIMRDKGABDMBN-DCAQKATOSA-N 0.000 description 1
- 108010003723 Single-Domain Antibodies Proteins 0.000 description 1
- 210000000662 T-lymphocyte subset Anatomy 0.000 description 1
- YDWLCDQXLCILCZ-BWAGICSOSA-N Thr-His-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O YDWLCDQXLCILCZ-BWAGICSOSA-N 0.000 description 1
- FQPDRTDDEZXCEC-SVSWQMSJSA-N Thr-Ile-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O FQPDRTDDEZXCEC-SVSWQMSJSA-N 0.000 description 1
- GXUWHVZYDAHFSV-FLBSBUHZSA-N Thr-Ile-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GXUWHVZYDAHFSV-FLBSBUHZSA-N 0.000 description 1
- IJVNLNRVDUTWDD-MEYUZBJRSA-N Thr-Leu-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O IJVNLNRVDUTWDD-MEYUZBJRSA-N 0.000 description 1
- BIBYEFRASCNLAA-CDMKHQONSA-N Thr-Phe-Gly Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CC=CC=C1 BIBYEFRASCNLAA-CDMKHQONSA-N 0.000 description 1
- RVMNUBQWPVOUKH-HEIBUPTGSA-N Thr-Ser-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O RVMNUBQWPVOUKH-HEIBUPTGSA-N 0.000 description 1
- YRJOLUDFVAUXLI-GSSVUCPTSA-N Thr-Thr-Asp Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC(O)=O YRJOLUDFVAUXLI-GSSVUCPTSA-N 0.000 description 1
- LECUEEHKUFYOOV-ZJDVBMNYSA-N Thr-Thr-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@@H](N)[C@@H](C)O LECUEEHKUFYOOV-ZJDVBMNYSA-N 0.000 description 1
- MNYNCKZAEIAONY-XGEHTFHBSA-N Thr-Val-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O MNYNCKZAEIAONY-XGEHTFHBSA-N 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- AZBIIKDSDLVJAK-VHWLVUOQSA-N Trp-Ile-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)N AZBIIKDSDLVJAK-VHWLVUOQSA-N 0.000 description 1
- BOMYCJXTWRMKJA-RNXOBYDBSA-N Trp-Phe-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC2=CC=CC=C2)C(=O)O)NC(=O)[C@H](CC3=CNC4=CC=CC=C43)N BOMYCJXTWRMKJA-RNXOBYDBSA-N 0.000 description 1
- MBLJBGZWLHTJBH-SZMVWBNQSA-N Trp-Val-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 MBLJBGZWLHTJBH-SZMVWBNQSA-N 0.000 description 1
- YJQCOFNZVFGCAF-UHFFFAOYSA-N Tunicamycin II Natural products O1C(CC(O)C2C(C(O)C(O2)N2C(NC(=O)C=C2)=O)O)C(O)C(O)C(NC(=O)C=CCCCCCCCCC(C)C)C1OC1OC(CO)C(O)C(O)C1NC(C)=O YJQCOFNZVFGCAF-UHFFFAOYSA-N 0.000 description 1
- 108091005956 Type II transmembrane proteins Proteins 0.000 description 1
- CKKFTIQYURNSEI-IHRRRGAJSA-N Tyr-Asn-Arg Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 CKKFTIQYURNSEI-IHRRRGAJSA-N 0.000 description 1
- QOIKZODVIPOPDD-AVGNSLFASA-N Tyr-Cys-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(O)=O QOIKZODVIPOPDD-AVGNSLFASA-N 0.000 description 1
- QUILOGWWLXMSAT-IHRRRGAJSA-N Tyr-Gln-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O QUILOGWWLXMSAT-IHRRRGAJSA-N 0.000 description 1
- AKLNEFNQWLHIGY-QWRGUYRKSA-N Tyr-Gly-Asp Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)NCC(=O)N[C@@H](CC(=O)O)C(=O)O)N)O AKLNEFNQWLHIGY-QWRGUYRKSA-N 0.000 description 1
- NKUGCYDFQKFVOJ-JYJNAYRXSA-N Tyr-Leu-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 NKUGCYDFQKFVOJ-JYJNAYRXSA-N 0.000 description 1
- DDRBQONWVBDQOY-GUBZILKMSA-N Val-Ala-Arg Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O DDRBQONWVBDQOY-GUBZILKMSA-N 0.000 description 1
- LTFLDDDGWOVIHY-NAKRPEOUSA-N Val-Ala-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](C)NC(=O)[C@H](C(C)C)N LTFLDDDGWOVIHY-NAKRPEOUSA-N 0.000 description 1
- DIOSYUIWOQCXNR-ONGXEEELSA-N Val-Lys-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O DIOSYUIWOQCXNR-ONGXEEELSA-N 0.000 description 1
- SSYBNWFXCFNRFN-GUBZILKMSA-N Val-Pro-Ser Chemical compound CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O SSYBNWFXCFNRFN-GUBZILKMSA-N 0.000 description 1
- JQTYTBPCSOAZHI-FXQIFTODSA-N Val-Ser-Cys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N JQTYTBPCSOAZHI-FXQIFTODSA-N 0.000 description 1
- USXYVSTVPHELAF-RCWTZXSCSA-N Val-Thr-Met Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](C(C)C)N)O USXYVSTVPHELAF-RCWTZXSCSA-N 0.000 description 1
- HTONZBWRYUKUKC-RCWTZXSCSA-N Val-Thr-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O HTONZBWRYUKUKC-RCWTZXSCSA-N 0.000 description 1
- RSEIVHMDTNNEOW-JYJNAYRXSA-N Val-Trp-Cys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CS)C(=O)O)N RSEIVHMDTNNEOW-JYJNAYRXSA-N 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 239000008351 acetate buffer Substances 0.000 description 1
- 238000001467 acupuncture Methods 0.000 description 1
- 108010008685 alanyl-glutamyl-aspartic acid Proteins 0.000 description 1
- 230000000735 allogeneic effect Effects 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- 230000009435 amidation Effects 0.000 description 1
- 238000007112 amidation reaction Methods 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 108010008355 arginyl-glutamine Proteins 0.000 description 1
- 108010062796 arginyllysine Proteins 0.000 description 1
- 108010040443 aspartyl-aspartic acid Proteins 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000003196 chaotropic effect Effects 0.000 description 1
- 238000012412 chemical coupling Methods 0.000 description 1
- 238000007385 chemical modification Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000012875 competitive assay Methods 0.000 description 1
- 230000004154 complement system Effects 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 230000009918 complex formation Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 150000001945 cysteines Chemical class 0.000 description 1
- 108010016616 cysteinylglycine Proteins 0.000 description 1
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 1
- 230000006240 deamidation Effects 0.000 description 1
- 239000007857 degradation product Substances 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000001212 derivatisation Methods 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- 230000017188 evasion or tolerance of host immune response Effects 0.000 description 1
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 1
- 230000022244 formylation Effects 0.000 description 1
- 238000006170 formylation reaction Methods 0.000 description 1
- 238000013467 fragmentation Methods 0.000 description 1
- 238000006062 fragmentation reaction Methods 0.000 description 1
- 108010090037 glycyl-alanyl-isoleucine Proteins 0.000 description 1
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 1
- 108010010096 glycyl-glycyl-tyrosine Proteins 0.000 description 1
- 108010089804 glycyl-threonine Proteins 0.000 description 1
- 108010015792 glycyllysine Proteins 0.000 description 1
- 108010037850 glycylvaline Proteins 0.000 description 1
- 230000005931 immune cell recruitment Effects 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000036737 immune function Effects 0.000 description 1
- 108091008915 immune receptors Proteins 0.000 description 1
- 102000027596 immune receptors Human genes 0.000 description 1
- 230000006058 immune tolerance Effects 0.000 description 1
- 102000018358 immunoglobulin Human genes 0.000 description 1
- 238000007689 inspection Methods 0.000 description 1
- 238000006317 isomerization reaction Methods 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 108010009298 lysylglutamic acid Proteins 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 108010005942 methionylglycine Proteins 0.000 description 1
- 230000004001 molecular interaction Effects 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 150000007523 nucleic acids Chemical group 0.000 description 1
- 239000002773 nucleotide Substances 0.000 description 1
- 125000003729 nucleotide group Chemical group 0.000 description 1
- 230000010355 oscillation Effects 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 230000006320 pegylation Effects 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 108010070409 phenylalanyl-glycyl-glycine Proteins 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 229950008882 polysorbate Drugs 0.000 description 1
- 230000008092 positive effect Effects 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 108010020755 prolyl-glycyl-glycine Proteins 0.000 description 1
- 108010004914 prolylarginine Proteins 0.000 description 1
- 230000006337 proteolytic cleavage Effects 0.000 description 1
- 238000003127 radioimmunoassay Methods 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000008261 resistance mechanism Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 238000007423 screening assay Methods 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 238000011476 stem cell transplantation Methods 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 238000010257 thawing Methods 0.000 description 1
- 108010033670 threonyl-aspartyl-tyrosine Proteins 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 108091005703 transmembrane proteins Proteins 0.000 description 1
- 102000035160 transmembrane proteins Human genes 0.000 description 1
- ZHSGGJXRNHWHRS-VIDYELAYSA-N tunicamycin Chemical compound O([C@H]1[C@@H]([C@H]([C@@H](O)[C@@H](CC(O)[C@@H]2[C@H]([C@@H](O)[C@@H](O2)N2C(NC(=O)C=C2)=O)O)O1)O)NC(=O)/C=C/CC(C)C)[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(C)=O ZHSGGJXRNHWHRS-VIDYELAYSA-N 0.000 description 1
- MEYZYGMYMLNUHJ-UHFFFAOYSA-N tunicamycin Natural products CC(C)CCCCCCCCCC=CC(=O)NC1C(O)C(O)C(CC(O)C2OC(C(O)C2O)N3C=CC(=O)NC3=O)OC1OC4OC(CO)C(O)C(O)C4NC(=O)C MEYZYGMYMLNUHJ-UHFFFAOYSA-N 0.000 description 1
- 108010003137 tyrosyltyrosine Proteins 0.000 description 1
- 238000011179 visual inspection Methods 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/12—Carboxylic acids; Salts or anhydrides thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/26—Carbohydrates, e.g. sugar alcohols, amino sugars, nucleic acids, mono-, di- or oligo-saccharides; Derivatives thereof, e.g. polysorbates, sorbitan fatty acid esters or glycyrrhizin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2827—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against B7 molecules, e.g. CD80, CD86
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/31—Immunoglobulins specific features characterized by aspects of specificity or valency multispecific
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Molecular Biology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Engineering & Computer Science (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Epidemiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Peptides Or Proteins (AREA)
Abstract
本发明针对双特异性抗体与天然抗体分子结构上的差异,以物理状态、微观结构、生物活性三个方面的指标为依据,优化双特异性抗体稳定性制剂的组分。以人源化重组抗NKG2A和PD‑L1双特异性抗体为例,优化的稳定性制剂以pH5.5‑6.5的醋酸缓冲液或柠檬酸缓冲液为缓冲体系、以4%蔗糖为稳定剂、添加0.005‑0.08%的Tween‑80。高温试验、振摇试验、反复冻融试验、光照试验检测结果表明本发明的稳定制剂能够维持水针剂的外观和物理状态,保持双特异性抗体分子的结构稳定和生物活性。
Description
技术领域
本发明涉及抗体药物领域,具体涉及一种适用于双特异性抗体的稳定制剂,特别是涉及一种人源化重组抗NKG2A和PD-L1双特异性抗体的水针制剂及其制备方法。
背景技术
近几年来,肿瘤免疫治疗取得了前所未有的成功,仍只有少数患者表现出持久的疗效。改善临床响应以及克服耐药机制是肿瘤免疫治疗领域正面临的挑战,而阻断其它抑制性免疫受体可能是一种可行的策略。
NKG2A(killer cell lectin like receptor C1)又名KLRC1或CD159A,是一类II型跨膜蛋白,属于NKG2/CD94自然杀伤细胞凝集素受体家族。NKG2A在胞外具有识别碳水化合物的结构域CRD(Carbohydrate-recognitiondomain,通常由115-130个氨基酸组成,含2-3个二硫键,有2-3个N连接的糖基化位点,其配体识别的过程往往是Ca2+依赖性的)。NKG2A主要在NK细胞、NKT细胞以及T细胞中表达;相对分子质量为43000,由233个氨基酸组成,其胞外区有135个氨基酸。NKG2A通过与其配体HLA-E的相互作用来抑制免疫细胞的激活。HLA-E广泛表达于头颈癌、肺癌、前列腺癌以及结直肠癌等多种肿瘤细胞表面,而免疫细胞释放的IFN-γ会进一步上调HLA-E的表达 (J Clin Invest. 2019; 129(5): 2094-2106)。与其它受体-配体(如PD-1/PD-L1)类似,NKG2A/HLA-E这对免疫检查点也是肿瘤免疫逃逸的重要信号通路,阻断NKG2A/HLA-E之间的相互作用成为了肿瘤免疫治疗领域一个非常有潜力的靶点。目前有多家公司(如Innate Pharma/Novo Nordisk/AstraZeneca 和 ChemPartner)开发了针对NKG2A的单克隆抗体,通过阻断NKG2A/HLA-E的相互作用来提高NK细胞以及T细胞的免疫活性来杀伤肿瘤细胞。
最新的研究发现,NKG2A与PD-1在头颈癌及黑色素瘤浸润性的CD8+ T细胞上共表达,同时阻断NKG2A/HLA-E及PD-1/PD-L1两条信号通路有很强的协同抗肿瘤作用(Cell.2018; 175: 1-13, Cell. 2018; 175: 1744-1755)。同时针对多种靶点进行治疗对提高肿瘤免疫治疗的响应率及降低免疫耐受有积极的作用。目前针对NKG2A的治疗性抗体对抗原的亲和力不足、结合NKG2A单靶点对肿瘤的治疗效果不佳,全球范围内尚没有针对NKG2A的上市药物。法国Paoli-Calmettes研究院正在推进一项人源化抗NKG2A单抗IPH2201与同种异体干细胞移植联用治疗血液恶性肿瘤的I期临床安全性实验(NCT02921685)。申请人已经通过构建轻重链突变抗体库进行亲和力提高和/或解离常数改善的突变抗体,将突变抗体的CDRs区构建人Fab重链基因表达载体和含人κ亚类轻链恒定区基因的哺乳动物细胞表达载体中,将亲和力成熟抗体的重链载体和轻链载体交叉配对,筛选获得抗NKG2A的突变Fab抗体,连接人抗体Fc段。将抗PD-L1纳米抗体通过linker连接到Fc段的C端,获得对NKG2A和PD-L1具有双特异性的抗体。
与普通抗体相比,双特异抗体具有特异性好、靶向性强、起效剂量低、毒副作用小等优点,在肿瘤的临床治疗意义重大。人源化重组双特异性抗体为生物大分子,结构复杂,在生产和贮存过程中,易发生聚集、变性、沉淀等物理变化及异构化、脱酰胺和氧化等化学变化,这些改变会影响产品的安全性及有效性,因此需要一种稳定的制剂来保证抗体用于患者体内前仍具有治疗所需的生物活性。目前市场上尚没有专门针对双特异性抗体的稳定制剂,大都直接使用天然抗体分子稳定制剂。虽然双特异性抗体或多或少具有一般抗体分子的组件,然而双特异性抗体还具备以下三点与一般抗体分子不同之处:(1)双特异性抗体是人工设计的分子,并非天然存在的分子,人工设计的因素导致双特异性抗体分子面临更多不确定的降解、聚集、代谢影响。(2)双特异性抗体结构与天然抗体结构存在差异,这种分子结构上的差异导致了在双特异性分子在分子量、等电点、酶切位点等理化参数上不同于天然抗体分子。(3)双特异性抗体分子在活性和功能上不同于天然抗体分子,双特异性抗体分子要同时维持两种特异性结合活性,必须使两种抗原结合位点都维持具有功能的结构,特别是当两种抗原结合位点分别位于两端时,例如Fab-Fc-sdA(Fab段通过重链C端连接于抗体Fc段的N端、纳米抗体通过N端连接于抗体Fc端的C端),必须维持整个分子的结构才能具有活性。
发明内容
为解决上述问题,本发明针对双特异性抗体与天然抗体分子结构上的差异,以物理状态、微观结构、生物活性三个方面的指标为依据,优化双特异性抗体稳定性制剂的组分。以人源化重组抗NKG2A和PD-L1双特异性抗体为例,优化的稳定性制剂以pH5.5-6.5的醋酸缓冲液或柠檬酸缓冲液为缓冲体系、以4%蔗糖为稳定剂、添加0.005-0.08%的Tween-80。高温试验、振摇试验、反复冻融试验、光照试验检测结果表明本发明的稳定制剂能够维持水针剂的外观和物理状态,保持双特异性抗体分子的结构稳定和生物活性。
具体而言:
一方面,本发明提供一种水性组合物,其包含:
缓冲液:10-20mM的醋酸缓冲液或柠檬酸缓冲液;
保护剂:4%(w/v)的蔗糖;
表面活性剂:0.005-0.08%(w/v)的Tween-80;
所述水性组合物的pH为5.5-6.5。
进一步,本发明所述的水性组合物,其特征在于:所述缓冲液为10mM的醋酸缓冲液,所述水性组合物的pH为5.5。
进一步,本发明所述的水性组合物,其特征在于:所述缓冲液为20mM的柠檬酸缓冲液,所述水性组合物的pH为6.0-6.5,优选6.2;并且所述水性组合物中还包含50mM的NaCl。
进一步,本发明所述的水性组合物,其特征在于:所述水性组合物中还包含至少一种双特异性抗体,所述双特异性抗体的浓度为10-50mg/mL,优选20mg/mL。
进一步,本发明所述的水性组合物,其特征在于所述双特异性抗体包括提供第一特异性的Fab、提供第二特异性的纳米抗体结构域;其中,所述Fab段通过其重链C端连接于抗体Fc段的N端,所述纳米抗体结构域通过其N端连接于抗体Fc端的C端。
进一步,本发明所述的水性组合物,其特征在于所述双特异性抗体是人源化重组抗NKG2A和PD-L1双特异性抗体,其中SEQ ID NO:1所示轻链可变区和SEQ ID NO:2所示重链可变区形成抗NKG2A的抗原结合位点;SEQ ID NO:3所示纳米抗体结构域形成抗PD-L1特异性。
第二方面,本发明还提供前述任一项水性组合物在稳定双特异性抗体和/或制备双特异性抗体制剂中的应用。
进一步,本发明所述的应用,其特征在于所述双特异性抗体包括提供第一特异性的Fab、提供第二特异性的纳米抗体结构域;其中,所述Fab段通过其重链C端连接于抗体Fc段的N端,所述纳米抗体结构域通过其N端连接于抗体Fc端的C端。
第三方面,本发明还提供前述任一项水性组合物在提高双特异性抗体高温稳定性、振摇稳定性、冻融稳定性、和/或光照稳定性中的应用。
进一步,本发明所述的应用,其特征在于所述双特异性抗体包括提供第一特异性的Fab、提供第二特异性的纳米抗体结构域;其中,所述Fab段通过其重链C端连接于抗体Fc段的N端,所述纳米抗体结构域通过其N端连接于抗体Fc端的C端。
第四方面,本发明提供人源化重组抗NKG2A和PD-L1双特异性抗体的水针制剂,包括:
双特异抗体 10-50mg/ml
柠檬酸缓冲液 20mM
氯化钠 50mM
蔗糖 4%(w/v)
聚山梨酯80 0.005%-0.08%(w/v)
pH 5.5-6.5
为更好理解本发明,首先定义一些术语。其他定义则贯穿具体实施方式部分而列出。
术语“NKG2A”是存在于NK、NKT和T细胞亚组中的抑制性受体,NKG2A (OMIM161555,其全部公开内容通过引用结合于本文中)是转录物的NKG2组的成员(Houchins,等(1991) J. Exp. Med. 173:1017-1020)。NKG2A由跨度25kb显示出一些差别剪接的7个外显子编码。NKG2A与CD94一起形成发现于NK细胞、α/β T细胞、γ/δ T细胞和NKT细胞的亚组的表面上的异二聚体抑制性受体CD94/NKG2A。与抑制性KIR受体类似,其在其胞质结构域具有ITIM。如用于本文中,“NKG2A”指NKG2A基因或编码的蛋白的任何变体、衍生物或同种型(isoform)。还包括与野生型全长NKG2A共享一个或多个生物学性质或功能,并共享至少70%、80%、90%、95%、96%、97%、98%、99%或更高核苷酸或氨基酸同一性的任何核酸或蛋白序列。人NKG2A在3个结构域中包含233个氨基酸,其中胞质结构域包含残基1-70,跨膜区包含残基71-93,和胞外区包含残基94-233。
术语“PD-L1”,即PD-L1(programmed death ligand 1)全称程序性死亡受体配体1,也称为表面抗原分化簇274(cluster of differentiation 274,CD274)或 B7同源体(B7homolog 1,B7-H1),由CD274基因编码,是PD-1(programmed cell death 1,程序性死亡受体1)的配体。PD-L1 是大小为 40kDa 的第一型跨膜蛋白,表达在T细胞、B细胞等免疫细胞以及肿瘤细胞上,正常情形下免疫系统会对聚集在淋巴结或脾脏的外来抗原产生反应,促发具抗原特异性的细胞毒杀性T细胞(CD8+ Tcell增生)。当肿瘤细胞膜上的PD-L1与T细胞等免疫细胞上的PD-1结合后,肿瘤细胞发出抑制性信号,减低淋巴结CD8+ T细胞的增殖,进而导致T细胞不能识别肿瘤细胞和对肿瘤细胞产生杀伤作用,机体的免疫功能受到抑制。
术语“特异性”是指在蛋白和/或其他生物异质群体中确定是否存在所述蛋白。因此,在所指定的条件下,特定的配体/抗原与特定的受体/抗体结合,并且并不以显著的量与样本中存在的其它蛋白结合。
本文中的术语“抗体”意在包括全长抗体及其任何抗原结合片段(即,抗原结合部分)或单链。全长抗体是包含至少两条重(H)链和两条轻(L)链的糖蛋白,重链和轻链由二硫键连接。各重链由重链可变区(简称VH)和重链恒定区构成。重链恒定区由三个结构域构成,即CH1、CH2和CH3。各轻链由轻链可变区(简称VL)和轻链恒定区构成。轻链恒定区由一个结构域CL构成。VH和VL区还可以划分为称作互补决定区(CDR)的高变区,其由较为保守的框架区(FR)区分隔开。各VH和VL由三个CDR以及四个FR构成,从氨基端到羧基端以FR1、CDR1、FR2、CDR2、FR3、CDR3、FR4的顺序排布。重链和轻链的可变区包含与抗原相互作用的结合域。抗体的恒定区可以介导免疫球蛋白与宿主组织或因子的结合,包括多种免疫系统细胞(例如,效应细胞)和传统补体系统的第一组分(C1q)。
术语“双特异性抗体”(bispecific antibodies),一种可与相同或不同抗原上的不同表位结合的抗体结构。因此,双特异性抗体能够桥连两种不同的分子,起到将效应分子、效应细胞、病毒和药物载体系统招募至靶标结构的作用。双特异性抗体这种可同时识别两种不同分子(受体和/或配体) 的特点,提高了抗体的选择性和功能性亲和力。
术语“单克隆抗体”或“单抗”或“单克隆抗体组成”是指单一分子组成的抗体分子制品。单克隆抗体组成呈现出对于特定表位的单一结合特异性和亲和力。
本文中的术语,抗体的“抗原结合片段”(或简称为抗体部分),是指抗体的保持有特异结合抗原能力的一个或多个片段。已证实,抗体的抗原结合功能可以通过全长抗体的片段来实施。包含在抗体的“抗原结合部分”中的结合片段的例子包括(i)Fab片段,由VL、VH、CL和CH1构成的单价片段;(ii)F(ab′)2片段,包含铰链区二硫桥连接的两个Fab片段的二价片段;(iii)由VH和CH1构成的Fd片段;(iv)由抗体单臂VL和VH构成的Fv片段;(v)由VH构成的dAb片段(Ward et al.,(1989)Nature 341:544-546);(vi)分离的互补决定区(CDR);以及(vii)纳米抗体,一种包含单可变结构域和两个恒定结构域的重链可变区。此外,尽管Fv片段的两个结构域VL和VH由不同的基因编码,它们可以通过重组法经由使两者成为单蛋白链的合成接头而连接,其中VL和VH区配对形成单价分子(称为单链Fc(scFv);参见例如Bird et al.,(1988)Science 242:423-426;and Huston et al.,(1988)Proc.Natl.Acad.Sci.USA 85:5879-5883)。这些单链抗体也意在包括在术语涵义中。这些抗体片段可以通过本领域技术人员已知的常用技术而得到,且片段可以通过与完整抗体相同的方式进行功能筛选。
本发明的抗原结合片段包括能够特异性结合抗原的那些。抗体结合片段的实例包括例如但不限于Fab、Fab'、F(ab')2、Fv片段、单链Fv(scFv)片段和单结构域片段。
Fab片段含有轻链的恒定结构域和重链的第一恒定结构域(CH1)。Fab'片段与Fab片段的不同之处在于在重链CH1结构域的羧基末端处的少数残基的添加,包括来自抗体铰链区的一个或多个半胱氨酸。通过切割在F(ab')2胃蛋白酶消化产物的铰链半胱氨酸处的二硫键产生Fab'片段。抗体片段的另外化学偶联是本领域普通技术人员已知的。Fab和F(ab')2片段缺乏完整抗体的片段可结晶(Fc)区,从动物的循环中更快速地清除,并且可能具有比完整抗体更少的非特异性组织结合(参见例如,Wahl等人,1983,J. Nucl. Med. 24:316)。
如本领域通常理解的,“Fc”区是不包含抗原特异性结合区的抗体的片段可结晶恒定区。在IgG、IgA和IgD抗体同种型中,Fc区由两个相同的蛋白质片段组成,衍生自抗体的两条重链的第二和第三恒定结构域(分别为CH2和CH3结构域)。IgM和IgE Fc区在每条多肽链中含有三个重链恒定结构域(CH2、CH3和CH4结构域)。
“单结构域片段”,sdA(single domain antibody)由抗原显示出足够亲和力的单个VH或VL结构域组成。在一个具体实施方案中,单结构域片段是骆驼化的(参见例如,Riechmann,1999,Journal ofImmunological Methods 231:25–38)。
本发明的抗NKG2A抗体包括衍生化抗体。例如,衍生化抗体通常通过糖基化、乙酰化、聚乙二醇化、磷酸化、酰胺化、通过已知保护/封闭基团的衍生化、蛋白酶解切割、与细胞配体或其它蛋白质的连接来修饰。可以通过已知技术进行众多化学修饰中的任一种,所述技术包括但不限于特定的化学切割、乙酰化、甲酰化、衣霉素的代谢合成等。另外,衍生物可以含有一种或多种非天然氨基酸,例如,使用ambrx技术(参见例如,Wolfson,2006,Chem.Biol. 13(10):1011-2)。
术语“结合亲和力”在本文中用作为两个分子(例如,抗体或其片段,和抗原)之间非共价相互作用强度的度量。术语“结合亲和力”用于描述单价相互作用(内在活性)。经由单价相互作用的两个分子(例如,抗体或其片段,和抗原)之间的结合亲和力,可通过测定解离常数(KD)来定量测定。继而,可通过对复合物形成和解离动力学的测量,例如通过SPR方法,来测定KD。对应于单价复合物缔合和解离的速率常数,被分别称为缔合速率常数ka(或kon)和解离速率常数kd(或koff)。KD通过等式KD = kd / ka与ka和kd相联系。根据以上定义,与不同分子相互作用相关的结合亲和力,例如不同抗体对于给定的抗原的结合亲和力的比较,可通过比较各个抗体/抗原复合物的KD值进行比较。类似地,可通过测定,并比较感兴趣的相互作用(例如抗体和抗原之间的特异性相互作用)的KD值与不感兴趣的相互作用的KD值,来评价相互作用的特异性。通过众所周知的方法可直接测定该解离常数的值,例如,通过评价配体(例如抗体)对靶的结合能力的标准测定是本领域已知的,并包括例如ELISA、蛋白质印迹、RIA和流式细胞术分析。通过本领域已知的标准测定,例如SPR,还可评价抗体的结合动力学和结合亲和力。可进行竞争性结合测定,其中,将抗体与靶的结合,和该靶的另外的配体(例如另外的抗体)与该靶结合,进行比较。
术语“高亲和性”对于IgG抗体而言,是指对于抗原的KD为1.0x 10-6M以下,优选5.0x 10-8M以下,更优选1.0x 10-8M以下、5.0x 10-9M以下,更优选1.0x 10-9M以下。对于其他抗体亚型,“高亲和性”结合可能会变化。例如,IgM亚型的“高亲和性”结合是指KD为10-6M以下,优选10-7M以下,更优选10-8M以下。
术语“Kassoc”或“Ka”是指特定抗体-抗原相互作用的结合速率,而术语“Kdis”或“Kd”是指特定抗体-抗原相互作用的离解速率。术语“KD”是指解离常数,由Kd与Ka比(Kd/Ka)得到,并以摩尔浓度(M)表示。抗体的KD值可以通过领域内已知的方法确定。优选的确定抗体KD的方式是使用表面等离子共振仪(SPR)测得的,优选使用生物传感系统例如BiacoreTM系统测得。
术语“EC50”,又叫半最大效应浓度,是指引起50%最大效应的抗体浓度。
与现有技术相比,本发明的技术方案具有以下优点:
第一、针对Fab-Fc-sdA型四价双特异性抗体的结构特点进行物理状态、化学状态、生物功能的检测。以水针剂物理形态的改变、四价双特异性抗体分子结构的改变、生物活性的改变为依据,对影响其稳定性的缓冲剂、渗透压调节剂、保护剂、表面活性剂的种类和含量进行了优化,以提高其生产、贮存、运输的稳定性。
第二、采用全面的稳定性指标,提高了双特异性抗体在多种理化条件改变下的稳定性。根据导致Fab-Fc-sdA型四价双特异性抗体不稳定的理化因素,通过水针剂各组分、含量的优化,全面提高了双特异性抗体高温稳定性、振摇稳定性、冻融稳定性、光照稳定性。
第三、采用多角度的检测方法。根据双特异性抗体发生聚集或降解产物在体积、电荷等方面的改变,采用体积排阻色谱(SEC,Size Exclusion Chromatograph)、离子交换色谱(IEC,Ion-exchange chromatography)、非还原十二烷基硫酸钠毛细管电泳(NR CE-SDS,Non-Redundant Capillary Electrophoresis Sodium Dodecyl Sulfate)多角度检测双特异性抗体微观分子的改变。
附图说明
通过阅读下文优选实施方式的详细描述,各种其他的优点和益处对于本领域普通技术人员将变得清楚明了。附图仅用于示出优选实施方式的目的,而并不认为是对本发明的限制。而且在整个附图中,用相同的参考符号表示相同的部件。在附图中:
图1:FACS 分析6MW3411对NK92 细胞表面NKG2A 结合活性。
图2:FACS 检测6MW3411与MDA-MB-231 细胞表面PD-L1 结合活性分析。
具体实施方式
应当理解,可以以各种形式实现本公开而不应被这里阐述的实施方式所限制。相反,提供这些实施方式是为了能够更透彻地理解本公开,并且能够将本公开的范围完整的传达给本领域的技术人员。
实施例1. 抗体制剂筛选实验
1.1 pH及缓冲体系的优化
根据分子特点将人源化重组抗NKG2A和PD-L1双特异性抗体(其中该双抗抗NKG2A轻链可变区氨基酸序列为SEQ ID NO.1;抗NKG2A重链可变区氨基酸序列为SEQ ID NO.2;抗PD-L1单可变域抗体VHH-F2可变区氨基酸序列为SEQ ID NO.3;后续简称为6MW3411)置换至以下目的缓冲液中,分装,进行稳定性考察。缓冲液体系如表1所示。
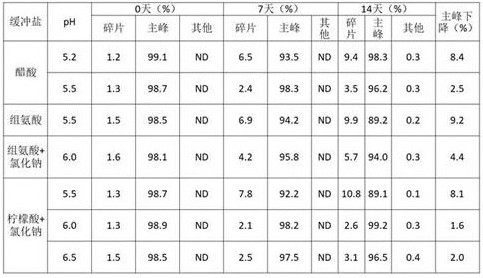
表1. pH及缓冲体系的优化
将6MW3411样品置换至上述缓冲体系后,通过外观检查发现柠檬酸体系中的样品在静置后出现分层现象,组氨酸在pH>6.0时出现沉淀现象。因此,暂以醋酸缓冲液为基础对其它参数进行优化,同时对柠檬酸缓冲液和组氨酸缓冲液通过添加促溶剂等进行改进。
1.2双特异性抗体浓度优化
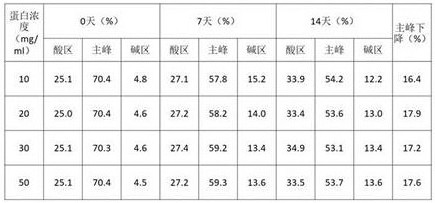
上述实验表明在醋酸条件下蛋白未产生沉淀现象,因此在10mM醋酸pH5.5条件下考察不同蛋白浓度的稳定性。将6MW3411双抗置换至不同蛋白浓度中,放置40℃进行高温强制条件试验,并在0天、7天、14天时取样进行SEC、IEC、NR CE-SDS检测。实验结果如下:
表2. 不同蛋白浓度高温试验SEC考察结果
表3. 不同蛋白浓度高温试验IEC考察结果
表4. 不同蛋白浓度高温试验NR CE-SDS考察结果
表2-4的结果显示在40℃条件放置14天后,6MW3411样品的SEC纯度、NR-CE纯度及CEX主峰均出现下降,而且浓度越高各关键质量属性下降的幅度越显著;说明较高的蛋白浓度不利于6MW3411的稳定,样品更容易聚集、断裂及产生电荷异质性。鉴于上述结果,综合考虑临床用药方案,后续将围绕20mg/ml的浓度进行进一步考察。
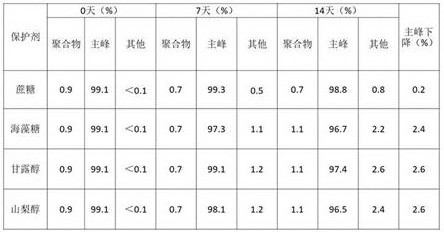
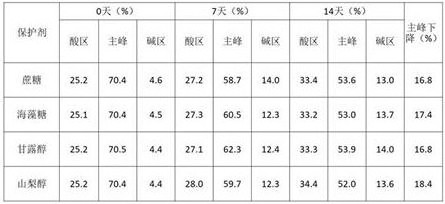
1.3保护剂的优化
在10mM醋酸pH5.5条件下考察不同保护剂种类中6MW3411的稳定性。将6MW3411双抗置换至不同保护剂中,放置40℃进行高温强制条件试验,并在0天、7天、14天时取样进行SEC、IEC、NR CE-SDS检测。实验结果如下:
表5. 不同保护剂高温试验SEC考察结果
表6. 不同保护剂高温试验IEC考察结果
表7. 不同保护剂高温试验NR CE-SDS考察结果
表5-7的结果显示40℃条件放置14天后,6MW3411样品的SEC主峰、NR-CE主峰和CEX主峰均出现下降。海藻糖、甘露醇和山梨醇中的样品SEC纯度、NR-CE纯度及CEX主峰下降幅度显著高于蔗糖中的样品,说明蔗糖有利于维持6MW3411样品的稳定性。因此,后续研究采用蔗糖作为6MW3411的保护剂。
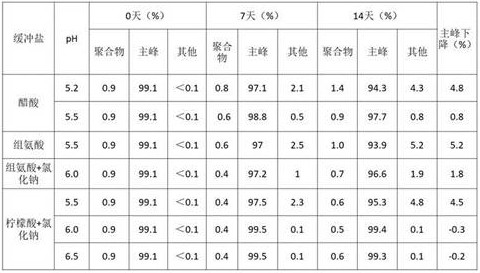
1.4氯化钠对缓冲液稳定功能的影响
前期稳定性考察表明在组氨酸和柠檬酸中蛋白容易沉淀,因此在两种缓冲体系中添加氯化钠促进溶解,以醋酸为对照,将6MW3411双抗置换至不同的缓冲体系中,放置40℃进行高温强制条件试验,并在0天、7天、14天时取样进行SEC、IEC、NR CE-SDS检测。实验结果如下:
表8. 不同缓冲液pH高温试验SEC考察结果
表9. 不同缓冲液pH高温试验IEC考察结果
表10. 不同缓冲液pH高温试验NR CE-SDS考察结果
表8-10的结果显示40℃条件放置14天后,6MW3411样品的SEC主峰、NR-CE主峰和CEX主峰均出现下降。其中醋酸PH5.2、组氨酸PH5.5和柠檬酸+氯化钠PH5.5中,SEC纯度和NR-CE纯度显著降低;柠檬酸pH大于6.0时,SEC纯度和NR-CE纯度的变化幅度小于醋酸和组氨酸。在pH6.5中,CEX主峰下降较明显,其他条件中CEX电荷异质性变化没有显著差异。综合上述结果,后续选择柠檬酸+氯化钠6.0左右进行下一步考察。
本发明通过对比上述1.4和1.1的试验,意外发现尽管单因素试验中柠檬酸缓冲液对双特异性抗体的稳定能力较差、导致抗体水针剂静置分层,但是在添加50mM氯化钠、pH>6.0的条件下柠檬酸缓冲液的稳定效果是最优的、主峰下降值最小。
1.5表面活性剂选择
将6MW3411样品置换至20mM柠檬酸、4%蔗糖、50mM氯化钠,pH6.2的缓冲溶液中,蛋白浓度20mg/ml,并向置换好的样品中加入不同比例的聚山梨酯80。制备好的样品反复冻融1次、3次、5次、震荡1天、3天及40℃放置14天后经MFI测定样品中的不溶性微粒。结果如表11-12所示。
表11. 不同比例聚山梨酯80冻融样品不溶性微粒检测结果
表12. 不同比例聚山梨酯80震荡及高温样品不溶性微粒结果
表11-12的结果显示不含聚山梨酯80的样品未进行任何处理即出现大量颗粒,出现肉眼可见的颗粒,说明表面活性剂对6MW3411稳定性很重要。不同比例聚山梨酯80中的样品在冻融5次后,0.005%聚山梨酯80中的样品微粒显著增加;震荡3天和40℃放置14天后,0.005%和0.01%聚山梨酯80中的样品微粒均显著增加;40℃放置14后,当聚山梨酯80浓度≥0.04%时,≥5μm的颗粒低于500个/ml,≥10μm的颗粒低于100个/ml。因此,有效抑制6MW3411样品产生不溶性微粒的合适聚山梨酯80浓度为0.05%±0.01%。
综上所述,选取20mg/ml的蛋白浓度、20mM柠檬酸、50mM氯化钠、4%蔗糖、pH 6.0±0.2。同时,添加0.05%的聚山梨酯80作为6MW3411的表面活性剂。
实施例二:处方影响因素试验
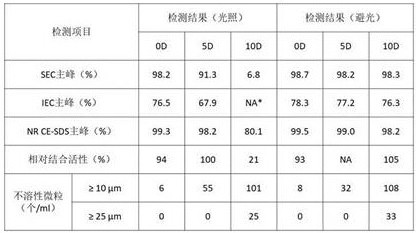
2.1高温试验
将置换好的样品在40℃条件下放置7天、14天和28天后,检测样品的SEC、IEC、NRCE-SDS、相对结合活性和不溶性微粒,结果如表13所示。结果显示,高温条件下SEC纯度和NR-CE纯度轻微降低,IEC主峰明显降低,不溶性微粒少量增加,表明6MW3411对高温敏感,样品应该低温保存。
表13. 6MW3411优选处方高温试验结果
*指未进行检测
2.2振摇试验
将置换好的样品在10℃,200 rpm条件下分别振摇0天、1天、3天后,检测样品的SEC、IEC、NR CE-SDS、相对结合活性和不溶性微粒,结果如表14所示。结果显示,各检测指标均未发生显著变化,表明在优选处方中,6MW3411双抗对振荡不敏感,稳定性良好。
表14. 6MW3411优选处方振摇试验结果
*指未进行检测
2.3反复冻融试验
将置换好的样品在-40℃ ~ 4℃条件下分别冻融0次、1次、3次和5次后,检测样品的SEC、IEC、NR CE-SDS、相对结合活性和不溶性微粒,结果如表15所示。结果显示,各检测指标均未发生显著变化,表明6MW3411双抗在优选处方中,对反复冻融不敏感,稳定性良好。
表15. 6MW3411优选处方反复冻融试验结果
*指未进行检测
2.4光照试验
将置换好的样品在4500 lx ± 500 lx条件下分别光照0天、5天和10天(同时将样品置于普通的纸质包装盒中作为避光对照组),检测样品的SEC、IEC、NR CE-SDS、相对结合活性和不溶性微粒检测,结果如表16所示。结果显示,光照试验中,6MW3411双抗的SEC和IEC主峰含量显著降低、NR-CE主峰有明显下降,相对结合活性显著降低;而避光对照组中,所有检测指标均未见显著变化。表明光照对优选处方中的6MW3411双抗有影响,而普通纸质包装盒即可起到避光作用。
表16. 6MW3411优选处方光照试验结果
*指未进行检测
实施例3. 6MW3411抗体分子亲和力分析
利用Fortebio公司的Octet QKe system仪器,采用6MW3411抗体Fc段的捕获抗体(AHC)生物探针捕获抗体Fc段的方法,分别测定6MW341对NKG2A和PD-L1的亲和力。将6MW3411用PBS缓冲液稀释至4μg/ml,流经AHC探针表面,时间为240s。使用人NKG2A胞外区融合蛋白或人PD-L1胞外区融合蛋白作为流动相,结合时间300s,解离时间300s。实验完毕,扣除空白对照响应值,用软件进行1:1 Langmuir 结合模式拟合,计算抗原抗体结合的动力学常数。
6MW3411与人NKG2A和PD-L1重组蛋白的亲和力(KD)参数如表17所示,6MW3411可以与NKG2A和PD-L1有效结合。
表17. 6MW3411与人NKG2A/PD-L1重组蛋白亲和力测定结果
实施例4.6MW3411对细胞表面NKG2A和PD-L1结合活性分析
分别利用NK92细胞和MBA-MD-231细胞,评估6MW3411与细胞表面NKG2A和PD‐L1结合情况。
具体地,将天然细胞NK92悬液与标记AF488的6MW3411及对照抗体NC‐IgG4(Mix‐n‐Stain CF488A Antibody Labeling Kit:Cat.MX488AS100‐1KT,sigma)在4℃孵育40 min,细胞浓度为:1×105cells/样品,抗体终浓度:55 nM起始3倍连续稀释8个梯度。以冰冷PBS(含0.05%吐温)洗涤细胞3次后上机检测。PBST洗涤细胞3次后通过流式细胞仪(型号B49007AD,SNAW31211,BECKMAN COULTER)检测细胞的平均荧光强度(MFI),以检测人源化抗体与天然细胞NK92的结合能力。结果如图1所示,6MW3411能与NK92细胞表面的NKG2A抗体特异性结合。
另一方面,将MDA-MB-231细胞悬液与抗体梯度稀释的检测抗体6MW3411和阴性对照抗体NC-IgG4在4℃孵育60 min,细胞浓度为:1×105cells/样品,抗体终浓度为10 nM起始,3倍连续稀释10个梯度。以冰冷PBST(0.05%吐温)洗涤细胞3次后,加入1:100稀释的羊抗人IgG‐FITC(Cat.:F9512,Sigma)并在4℃孵育30min。PBST洗涤细胞3次后通过流式细胞仪检测细胞的平均荧光强度(MFI),以检测人源化抗体与MDA-MB-231细胞表面的人PD-L1结合能力。结果如图2所示,双特异性抗体均保持了与细胞表面PD-L1结合活性。
本发明中涉及的氨基酸序列如下:
SEQ ID NO.1:抗NKG2A轻链可变区氨基酸序列
DIQMTQSPSSLSASVGDRVTITCRASENIYSYAAWYQQKPGKAPKLLIYNRKTLAEGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQHRYGTPRTFGGGTKVEIK
SEQ ID NO.2:抗NKG2A重链可变区氨基酸序列
EVQLVQSGAEVKKPGASVKVSCKASSYDFSWYWINWVRQAPGQGLEWMGAIDPYDSETHYAQKLQGRVTMTTDTSTSTAYMELRSLRSDDTAVYYCARGGYDFDQGTLYWFFDVWGQGTTVTVSS
SEQ ID NO.3:抗PD-L1单可变域抗体VHH-F2可变区氨基酸序列
EVQLVESGGGLVQPGGSLRLSCAASRDSDEGASCMGWFRQAPGKEREGVAIIFNAGERTDYGDSVKGRFTISRDNAKNTLYLQMNSLRAEDTAVYYCATVWCGSWVARSWGQGTLVTVSS
以上所述,仅为本发明较佳的具体实施方式,但本发明的保护范围并不局限于此,任何熟悉本技术领域的技术人员在本发明揭露的技术范围内,可轻易想到的变化或替换,都应涵盖在本发明的保护范围之内。因此,本发明的保护范围应以所述权利要求的保护范围为准。
序列表
<110> 迈威(上海)生物科技股份有限公司
<120> 一种双特异性抗体的稳定制剂
<160> 3
<170> SIPOSequenceListing 1.0
<210> 1
<211> 107
<212> PRT
<213> 人工序列(Artificial Sequence)
<400> 1
Asp Ile Gln Met Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly
1 5 10 15
Asp Arg Val Thr Ile Thr Cys Arg Ala Ser Glu Asn Ile Tyr Ser Tyr
20 25 30
Ala Ala Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile
35 40 45
Tyr Asn Arg Lys Thr Leu Ala Glu Gly Val Pro Ser Arg Phe Ser Gly
50 55 60
Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro
65 70 75 80
Glu Asp Phe Ala Thr Tyr Tyr Cys Gln His Arg Tyr Gly Thr Pro Arg
85 90 95
Thr Phe Gly Gly Gly Thr Lys Val Glu Ile Lys
100 105
<210> 2
<211> 125
<212> PRT
<213> 人工序列(Artificial Sequence)
<400> 2
Glu Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly Ala
1 5 10 15
Ser Val Lys Val Ser Cys Lys Ala Ser Ser Tyr Asp Phe Ser Trp Tyr
20 25 30
Trp Ile Asn Trp Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met
35 40 45
Gly Ala Ile Asp Pro Tyr Asp Ser Glu Thr His Tyr Ala Gln Lys Leu
50 55 60
Gln Gly Arg Val Thr Met Thr Thr Asp Thr Ser Thr Ser Thr Ala Tyr
65 70 75 80
Met Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Gly Gly Tyr Asp Phe Asp Gln Gly Thr Leu Tyr Trp Phe Phe
100 105 110
Asp Val Trp Gly Gln Gly Thr Thr Val Thr Val Ser Ser
115 120 125
<210> 3
<211> 120
<212> PRT
<213> 人工序列(Artificial Sequence)
<400> 3
Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly
1 5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Arg Asp Ser Asp Glu Gly Ala
20 25 30
Ser Cys Met Gly Trp Phe Arg Gln Ala Pro Gly Lys Glu Arg Glu Gly
35 40 45
Val Ala Ile Ile Phe Asn Ala Gly Glu Arg Thr Asp Tyr Gly Asp Ser
50 55 60
Val Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ala Lys Asn Thr Leu
65 70 75 80
Tyr Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr
85 90 95
Cys Ala Thr Val Trp Cys Gly Ser Trp Val Ala Arg Ser Trp Gly Gln
100 105 110
Gly Thr Leu Val Thr Val Ser Ser
115 120
Claims (7)
1.一种水性组合物,所述组合物成分由如下构成:
缓冲液:10-20mM的柠檬酸缓冲液;
保护剂:4%(w/v)的蔗糖;
表面活性剂:0.005-0.08%(w/v)的Tween-80;以及50mM的NaCl,和
一种双特异性抗体;
所述双特异性抗体是人源化重组抗NKG2A和PD-L1双特异性抗体,其包括SEQ ID NO:1所示轻链可变区和SEQ ID NO:2所示重链可变区形成的抗NKG2A特异性抗原结合位点; SEQID NO:3所示纳米抗体结构域形成的抗PD-L1特异性抗原结合位点;
所述水性组合物的pH为5.5-6.5。
2.如权利要求1所述的水性组合物,其特征在于:所述缓冲液为20mM的柠檬酸缓冲液,所述水性组合物的pH为6.0-6.5。
3.如权利要求2所述的水性组合物,其特征在于:所述水性组合物的pH为6.2。
4.如权利要求1所述的水性组合物,其特征在于:所述双特异性抗体的浓度为10-50mg/mL。
5.如权利要求4所述的水性组合物,其特征在于:所述双特异性抗体的浓度为20mg/mL。
6.如权利要求4所述的水性组合物,其特征在于所述双特异性抗体包括提供NKG2A特异性的Fab、提供PD-L1特异性的纳米抗体结构域;其中,所述Fab段通过其重链C端连接于抗体Fc段的N端,所述纳米抗体结构域通过其N端连接于抗体Fc端的C端。
7.人源化重组抗NKG2A和PD-L1双特异性抗体的水针制剂,包括:
双特异性抗体 10-50mg/ml
柠檬酸缓冲液 20mM
氯化钠 50mM
蔗糖 4%(w/v)
Tween-80 0.005-0.08%(w/v)
pH 5.5-6.5
所述双特异性抗体是人源化重组抗NKG2A和PD-L1双特异性抗体,其包括SEQ ID NO:1所示轻链可变区和SEQ ID NO:2所示重链可变区形成的抗NKG2A特异性抗原结合位点; SEQID NO:3所示纳米抗体结构域形成的抗PD-L1特异性抗原结合位点。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202111566377.XA CN113940997B (zh) | 2021-12-21 | 2021-12-21 | 一种双特异性抗体的稳定制剂 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202111566377.XA CN113940997B (zh) | 2021-12-21 | 2021-12-21 | 一种双特异性抗体的稳定制剂 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN113940997A CN113940997A (zh) | 2022-01-18 |
| CN113940997B true CN113940997B (zh) | 2022-04-08 |
Family
ID=79339440
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202111566377.XA Active CN113940997B (zh) | 2021-12-21 | 2021-12-21 | 一种双特异性抗体的稳定制剂 |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN113940997B (zh) |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106999581A (zh) * | 2014-11-07 | 2017-08-01 | 诺华股份有限公司 | 含有高浓度抗vegf抗体的稳定蛋白质溶液剂型 |
| WO2017180769A1 (en) * | 2016-04-13 | 2017-10-19 | Capten Therapeutics Inc. | Small molecules for immunogenic treatment of cancer |
| CN110974958A (zh) * | 2019-12-25 | 2020-04-10 | 北京东方百泰生物科技有限公司 | 一种抗pd-l1单克隆抗体的注射制剂 |
| CN113527488A (zh) * | 2020-04-22 | 2021-10-22 | 迈威(上海)生物科技股份有限公司 | 一种靶向人程序性死亡配体1(pd-l1)的单可变域抗体及其衍生物 |
| CN113583127A (zh) * | 2020-04-30 | 2021-11-02 | 迈威(上海)生物科技股份有限公司 | 一种靶向nkg2a和pd-l1的双特异性抗体及应用 |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GEP20217249B (en) * | 2016-06-30 | 2021-04-26 | Inc Celltrion | Stable liquid pharmaceutical preparation |
-
2021
- 2021-12-21 CN CN202111566377.XA patent/CN113940997B/zh active Active
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106999581A (zh) * | 2014-11-07 | 2017-08-01 | 诺华股份有限公司 | 含有高浓度抗vegf抗体的稳定蛋白质溶液剂型 |
| WO2017180769A1 (en) * | 2016-04-13 | 2017-10-19 | Capten Therapeutics Inc. | Small molecules for immunogenic treatment of cancer |
| CN110974958A (zh) * | 2019-12-25 | 2020-04-10 | 北京东方百泰生物科技有限公司 | 一种抗pd-l1单克隆抗体的注射制剂 |
| CN113527488A (zh) * | 2020-04-22 | 2021-10-22 | 迈威(上海)生物科技股份有限公司 | 一种靶向人程序性死亡配体1(pd-l1)的单可变域抗体及其衍生物 |
| CN113583127A (zh) * | 2020-04-30 | 2021-11-02 | 迈威(上海)生物科技股份有限公司 | 一种靶向nkg2a和pd-l1的双特异性抗体及应用 |
Non-Patent Citations (1)
| Title |
|---|
| Potential prognostic value of PD‑L1 and NKG2A expression in Indonesian patients with skin nodular melanoma;Ridwan Dwi Saputro et al.;《BMC Research Notes》;20210528;第14卷;第1-6页 * |
Also Published As
| Publication number | Publication date |
|---|---|
| CN113940997A (zh) | 2022-01-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN113286817B (zh) | Dll3结合蛋白及使用方法 | |
| EP3818086B1 (en) | Treatment and prevention of cancer using her3 antigen-binding molecules | |
| KR102593409B1 (ko) | Her3 항원 결합 분자 | |
| EP3645570B1 (en) | Vista antigen-binding molecules | |
| CN113412281B (zh) | Btn3a结合蛋白及其用途 | |
| JP7436711B2 (ja) | 抗sirp-アルファ抗体 | |
| WO2021219048A1 (zh) | 一种靶向nkg2a和pd-l1的双特异性抗体及应用 | |
| US11117964B2 (en) | Anti-KIR3DL1 antibodies | |
| WO2022082014A2 (en) | Skin targeted immunotolerance | |
| JP2022505925A (ja) | 抗tim-3抗体 | |
| CN110167959A (zh) | 用于监测对免疫检查点抑制剂pd1和pd-l1的抗体治疗的免疫测定和工程化蛋白 | |
| CN112442123B (zh) | 抗cd47的单克隆抗体及其用途 | |
| CN111808190B (zh) | 结合pd-1的抗体 | |
| CN113940997B (zh) | 一种双特异性抗体的稳定制剂 | |
| TW202330026A (zh) | 用於療法之抗btn3a活化抗體及il2促效劑之組合 | |
| WO2023031435A1 (en) | Treatment and prevention of cancer using her3 antigen-binding molecules | |
| WO2021000953A1 (zh) | 治疗肿瘤的物质和方法 | |
| WO2020156507A1 (zh) | 抗pd-l1的新型抗体及其用途 | |
| CN114085291B (zh) | 一种降低或消除重组蛋白cex酸性峰的方法 | |
| HK40063855A (zh) | 治療腫瘤的物質和方法 | |
| TW202241957A (zh) | 抗pd-1抗體及其用途 | |
| TW202241958A (zh) | 抗pd-l1抗體及其應用 | |
| HK40033623A (zh) | 結合pd-1的抗體 | |
| JP2024513473A (ja) | Dpep-1結合物質および使用の方法 | |
| KR20210090172A (ko) | Epn1을 표적화하는 항체 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |