CN111808019A - 一种并环化合物及其应用 - Google Patents
一种并环化合物及其应用 Download PDFInfo
- Publication number
- CN111808019A CN111808019A CN202010932125.3A CN202010932125A CN111808019A CN 111808019 A CN111808019 A CN 111808019A CN 202010932125 A CN202010932125 A CN 202010932125A CN 111808019 A CN111808019 A CN 111808019A
- Authority
- CN
- China
- Prior art keywords
- halogen
- pain
- formula
- pharmaceutically acceptable
- independently
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 150000001875 compounds Chemical class 0.000 title claims abstract description 62
- 229910052736 halogen Inorganic materials 0.000 claims abstract description 50
- 150000002367 halogens Chemical class 0.000 claims abstract description 41
- 150000003839 salts Chemical class 0.000 claims abstract description 31
- 125000005843 halogen group Chemical group 0.000 claims abstract 9
- 208000002193 Pain Diseases 0.000 claims description 45
- 230000036407 pain Effects 0.000 claims description 37
- 239000003814 drug Substances 0.000 claims description 24
- 208000004296 neuralgia Diseases 0.000 claims description 19
- 239000000126 substance Substances 0.000 claims description 17
- 229940079593 drug Drugs 0.000 claims description 16
- 208000021722 neuropathic pain Diseases 0.000 claims description 16
- 125000000217 alkyl group Chemical group 0.000 claims description 14
- 125000003545 alkoxy group Chemical group 0.000 claims description 13
- 229910052794 bromium Inorganic materials 0.000 claims description 9
- 229910052801 chlorine Inorganic materials 0.000 claims description 9
- 229910052731 fluorine Inorganic materials 0.000 claims description 9
- 125000004430 oxygen atom Chemical group O* 0.000 claims description 9
- 201000010099 disease Diseases 0.000 claims description 8
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 8
- 229910052740 iodine Inorganic materials 0.000 claims description 8
- -1 methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, isobutoxy, sec-butoxy Chemical group 0.000 claims description 8
- 238000002360 preparation method Methods 0.000 claims description 6
- 239000000664 voltage gated sodium channel blocking agent Substances 0.000 claims description 6
- 208000000094 Chronic Pain Diseases 0.000 claims description 5
- 108010053752 Voltage-Gated Sodium Channels Proteins 0.000 claims description 5
- 102000016913 Voltage-Gated Sodium Channels Human genes 0.000 claims description 5
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 5
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 claims description 4
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 claims description 4
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 claims description 4
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 claims description 4
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 claims description 4
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 4
- 206010065390 Inflammatory pain Diseases 0.000 claims description 3
- 206010028391 Musculoskeletal Pain Diseases 0.000 claims description 3
- 208000005298 acute pain Diseases 0.000 claims description 3
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 3
- 230000002401 inhibitory effect Effects 0.000 claims description 3
- 230000000968 intestinal effect Effects 0.000 claims description 3
- 201000006417 multiple sclerosis Diseases 0.000 claims description 3
- 208000011580 syndromic disease Diseases 0.000 claims description 3
- 125000004863 4-trifluoromethoxyphenyl group Chemical group [H]C1=C([H])C(OC(F)(F)F)=C([H])C([H])=C1* 0.000 claims description 2
- 206010058019 Cancer Pain Diseases 0.000 claims description 2
- 206010021639 Incontinence Diseases 0.000 claims description 2
- 208000004550 Postoperative Pain Diseases 0.000 claims description 2
- 230000006793 arrhythmia Effects 0.000 claims description 2
- 206010003119 arrhythmia Diseases 0.000 claims description 2
- 238000000338 in vitro Methods 0.000 claims description 2
- 239000008194 pharmaceutical composition Substances 0.000 claims description 2
- 125000004213 tert-butoxy group Chemical group [H]C([H])([H])C(O*)(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 2
- 125000000876 trifluoromethoxy group Chemical group FC(F)(F)O* 0.000 claims description 2
- 208000009935 visceral pain Diseases 0.000 claims description 2
- 150000002391 heterocyclic compounds Chemical class 0.000 claims 3
- 239000002671 adjuvant Substances 0.000 claims 1
- 230000000903 blocking effect Effects 0.000 abstract description 5
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 26
- 210000004027 cell Anatomy 0.000 description 22
- 239000012071 phase Substances 0.000 description 22
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 17
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 15
- 230000015572 biosynthetic process Effects 0.000 description 15
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 14
- 238000003786 synthesis reaction Methods 0.000 description 14
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 13
- 230000000694 effects Effects 0.000 description 13
- 239000000543 intermediate Substances 0.000 description 13
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 12
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 12
- 239000002253 acid Substances 0.000 description 11
- 239000000203 mixture Substances 0.000 description 10
- 238000005160 1H NMR spectroscopy Methods 0.000 description 9
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 9
- 201000008482 osteoarthritis Diseases 0.000 description 9
- 238000012360 testing method Methods 0.000 description 9
- PCLIMKBDDGJMGD-UHFFFAOYSA-N N-bromosuccinimide Chemical compound BrN1C(=O)CCC1=O PCLIMKBDDGJMGD-UHFFFAOYSA-N 0.000 description 8
- 238000006243 chemical reaction Methods 0.000 description 8
- 239000000460 chlorine Substances 0.000 description 8
- 239000012074 organic phase Substances 0.000 description 8
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 7
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 6
- 238000001816 cooling Methods 0.000 description 6
- 108091006146 Channels Proteins 0.000 description 5
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 5
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 5
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 5
- 239000003153 chemical reaction reagent Substances 0.000 description 5
- HEDRZPFGACZZDS-MICDWDOJSA-N deuterated chloroform Substances [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 5
- 238000000746 purification Methods 0.000 description 5
- 239000011734 sodium Substances 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- 238000011282 treatment Methods 0.000 description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 5
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical group [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- OKKJLVBELUTLKV-MZCSYVLQSA-N Deuterated methanol Chemical compound [2H]OC([2H])([2H])[2H] OKKJLVBELUTLKV-MZCSYVLQSA-N 0.000 description 4
- UGJMXCAKCUNAIE-UHFFFAOYSA-N Gabapentin Chemical compound OC(=O)CC1(CN)CCCCC1 UGJMXCAKCUNAIE-UHFFFAOYSA-N 0.000 description 4
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 4
- 108010052164 Sodium Channels Proteins 0.000 description 4
- 102000018674 Sodium Channels Human genes 0.000 description 4
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 4
- 125000004432 carbon atom Chemical group C* 0.000 description 4
- 238000001035 drying Methods 0.000 description 4
- 239000012530 fluid Substances 0.000 description 4
- 238000000034 method Methods 0.000 description 4
- CTSLXHKWHWQRSH-UHFFFAOYSA-N oxalyl chloride Chemical compound ClC(=O)C(Cl)=O CTSLXHKWHWQRSH-UHFFFAOYSA-N 0.000 description 4
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 230000002829 reductive effect Effects 0.000 description 4
- 239000011780 sodium chloride Substances 0.000 description 4
- 229910052938 sodium sulfate Inorganic materials 0.000 description 4
- 239000011550 stock solution Substances 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical class CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 4
- 229910021595 Copper(I) iodide Inorganic materials 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 3
- LFTLOKWAGJYHHR-UHFFFAOYSA-N N-methylmorpholine N-oxide Chemical compound CN1(=O)CCOCC1 LFTLOKWAGJYHHR-UHFFFAOYSA-N 0.000 description 3
- 239000007832 Na2SO4 Substances 0.000 description 3
- 229910002666 PdCl2 Inorganic materials 0.000 description 3
- 208000010261 Small Fiber Neuropathy Diseases 0.000 description 3
- 206010073928 Small fibre neuropathy Diseases 0.000 description 3
- 229940035676 analgesics Drugs 0.000 description 3
- 239000000730 antalgic agent Substances 0.000 description 3
- FJDQFPXHSGXQBY-UHFFFAOYSA-L caesium carbonate Chemical compound [Cs+].[Cs+].[O-]C([O-])=O FJDQFPXHSGXQBY-UHFFFAOYSA-L 0.000 description 3
- 238000004113 cell culture Methods 0.000 description 3
- DOBRDRYODQBAMW-UHFFFAOYSA-N copper(i) cyanide Chemical compound [Cu+].N#[C-] DOBRDRYODQBAMW-UHFFFAOYSA-N 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 239000012458 free base Substances 0.000 description 3
- 230000028161 membrane depolarization Effects 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- MZRVEZGGRBJDDB-UHFFFAOYSA-N n-Butyllithium Substances [Li]CCCC MZRVEZGGRBJDDB-UHFFFAOYSA-N 0.000 description 3
- 231100000252 nontoxic Toxicity 0.000 description 3
- 230000003000 nontoxic effect Effects 0.000 description 3
- 238000000554 physical therapy Methods 0.000 description 3
- AYXYPKUFHZROOJ-ZETCQYMHSA-N pregabalin Chemical compound CC(C)C[C@H](CN)CC(O)=O AYXYPKUFHZROOJ-ZETCQYMHSA-N 0.000 description 3
- 229960001233 pregabalin Drugs 0.000 description 3
- 208000017692 primary erythermalgia Diseases 0.000 description 3
- 230000002441 reversible effect Effects 0.000 description 3
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Substances ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 3
- DTQVDTLACAAQTR-UHFFFAOYSA-N trifluoroacetic acid Substances OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 3
- GHYOCDFICYLMRF-UTIIJYGPSA-N (2S,3R)-N-[(2S)-3-(cyclopenten-1-yl)-1-[(2R)-2-methyloxiran-2-yl]-1-oxopropan-2-yl]-3-hydroxy-3-(4-methoxyphenyl)-2-[[(2S)-2-[(2-morpholin-4-ylacetyl)amino]propanoyl]amino]propanamide Chemical compound C1(=CCCC1)C[C@@H](C(=O)[C@@]1(OC1)C)NC([C@H]([C@@H](C1=CC=C(C=C1)OC)O)NC([C@H](C)NC(CN1CCOCC1)=O)=O)=O GHYOCDFICYLMRF-UTIIJYGPSA-N 0.000 description 2
- ZEUITGRIYCTCEM-KRWDZBQOSA-N (S)-duloxetine Chemical compound C1([C@@H](OC=2C3=CC=CC=C3C=CC=2)CCNC)=CC=CS1 ZEUITGRIYCTCEM-KRWDZBQOSA-N 0.000 description 2
- KZPYGQFFRCFCPP-UHFFFAOYSA-N 1,1'-bis(diphenylphosphino)ferrocene Chemical compound [Fe+2].C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1 KZPYGQFFRCFCPP-UHFFFAOYSA-N 0.000 description 2
- WSLDOOZREJYCGB-UHFFFAOYSA-N 1,2-Dichloroethane Chemical compound ClCCCl WSLDOOZREJYCGB-UHFFFAOYSA-N 0.000 description 2
- ONBQEOIKXPHGMB-VBSBHUPXSA-N 1-[2-[(2s,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-4,6-dihydroxyphenyl]-3-(4-hydroxyphenyl)propan-1-one Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1OC1=CC(O)=CC(O)=C1C(=O)CCC1=CC=C(O)C=C1 ONBQEOIKXPHGMB-VBSBHUPXSA-N 0.000 description 2
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 2
- NHQDETIJWKXCTC-UHFFFAOYSA-N 3-chloroperbenzoic acid Chemical compound OOC(=O)C1=CC=CC(Cl)=C1 NHQDETIJWKXCTC-UHFFFAOYSA-N 0.000 description 2
- SFHYNDMGZXWXBU-LIMNOBDPSA-N 6-amino-2-[[(e)-(3-formylphenyl)methylideneamino]carbamoylamino]-1,3-dioxobenzo[de]isoquinoline-5,8-disulfonic acid Chemical compound O=C1C(C2=3)=CC(S(O)(=O)=O)=CC=3C(N)=C(S(O)(=O)=O)C=C2C(=O)N1NC(=O)N\N=C\C1=CC=CC(C=O)=C1 SFHYNDMGZXWXBU-LIMNOBDPSA-N 0.000 description 2
- 206010067484 Adverse reaction Diseases 0.000 description 2
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- KZMGYPLQYOPHEL-UHFFFAOYSA-N Boron trifluoride etherate Chemical compound FB(F)F.CCOCC KZMGYPLQYOPHEL-UHFFFAOYSA-N 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- 239000007995 HEPES buffer Substances 0.000 description 2
- 206010019233 Headaches Diseases 0.000 description 2
- 101000654356 Homo sapiens Sodium channel protein type 10 subunit alpha Proteins 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- 238000005481 NMR spectroscopy Methods 0.000 description 2
- 206010028813 Nausea Diseases 0.000 description 2
- 206010028980 Neoplasm Diseases 0.000 description 2
- 208000005890 Neuroma Diseases 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 206010034620 Peripheral sensory neuropathy Diseases 0.000 description 2
- 208000032140 Sleepiness Diseases 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- 102100031374 Sodium channel protein type 10 subunit alpha Human genes 0.000 description 2
- 206010041349 Somnolence Diseases 0.000 description 2
- 230000006838 adverse reaction Effects 0.000 description 2
- 239000001961 anticonvulsive agent Substances 0.000 description 2
- 229910052786 argon Inorganic materials 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- MUALRAIOVNYAIW-UHFFFAOYSA-N binap Chemical compound C1=CC=CC=C1P(C=1C(=C2C=CC=CC2=CC=1)C=1C2=CC=CC=C2C=CC=1P(C=1C=CC=CC=1)C=1C=CC=CC=1)C1=CC=CC=C1 MUALRAIOVNYAIW-UHFFFAOYSA-N 0.000 description 2
- 230000005540 biological transmission Effects 0.000 description 2
- 210000000988 bone and bone Anatomy 0.000 description 2
- 229910000024 caesium carbonate Inorganic materials 0.000 description 2
- AIYUHDOJVYHVIT-UHFFFAOYSA-M caesium chloride Chemical compound [Cl-].[Cs+] AIYUHDOJVYHVIT-UHFFFAOYSA-M 0.000 description 2
- HUCVOHYBFXVBRW-UHFFFAOYSA-M caesium hydroxide Chemical compound [OH-].[Cs+] HUCVOHYBFXVBRW-UHFFFAOYSA-M 0.000 description 2
- 201000011510 cancer Diseases 0.000 description 2
- 239000003990 capacitor Substances 0.000 description 2
- 210000000170 cell membrane Anatomy 0.000 description 2
- 239000006285 cell suspension Substances 0.000 description 2
- 229940125797 compound 12 Drugs 0.000 description 2
- 229940126142 compound 16 Drugs 0.000 description 2
- 239000006059 cover glass Substances 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 206010012601 diabetes mellitus Diseases 0.000 description 2
- 208000002173 dizziness Diseases 0.000 description 2
- 238000002651 drug therapy Methods 0.000 description 2
- 206010013781 dry mouth Diseases 0.000 description 2
- 229960002866 duloxetine Drugs 0.000 description 2
- 230000002526 effect on cardiovascular system Effects 0.000 description 2
- 235000019439 ethyl acetate Nutrition 0.000 description 2
- 210000003722 extracellular fluid Anatomy 0.000 description 2
- 229960002870 gabapentin Drugs 0.000 description 2
- 230000002496 gastric effect Effects 0.000 description 2
- 231100000869 headache Toxicity 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 230000008693 nausea Effects 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 2
- 239000003208 petroleum Substances 0.000 description 2
- 229910000027 potassium carbonate Inorganic materials 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 238000012827 research and development Methods 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- 210000001044 sensory neuron Anatomy 0.000 description 2
- 201000005572 sensory peripheral neuropathy Diseases 0.000 description 2
- QZAYGJVTTNCVMB-UHFFFAOYSA-N serotonin Chemical compound C1=C(O)C=C2C(CCN)=CNC2=C1 QZAYGJVTTNCVMB-UHFFFAOYSA-N 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 208000020431 spinal cord injury Diseases 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 238000010998 test method Methods 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 238000004809 thin layer chromatography Methods 0.000 description 2
- 230000000472 traumatic effect Effects 0.000 description 2
- SZUVGFMDDVSKSI-WIFOCOSTSA-N (1s,2s,3s,5r)-1-(carboxymethyl)-3,5-bis[(4-phenoxyphenyl)methyl-propylcarbamoyl]cyclopentane-1,2-dicarboxylic acid Chemical compound O=C([C@@H]1[C@@H]([C@](CC(O)=O)([C@H](C(=O)N(CCC)CC=2C=CC(OC=3C=CC=CC=3)=CC=2)C1)C(O)=O)C(O)=O)N(CCC)CC(C=C1)=CC=C1OC1=CC=CC=C1 SZUVGFMDDVSKSI-WIFOCOSTSA-N 0.000 description 1
- QFLWZFQWSBQYPS-AWRAUJHKSA-N (3S)-3-[[(2S)-2-[[(2S)-2-[5-[(3aS,6aR)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-[1-bis(4-chlorophenoxy)phosphorylbutylamino]-4-oxobutanoic acid Chemical compound CCCC(NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)C(C)C)P(=O)(Oc1ccc(Cl)cc1)Oc1ccc(Cl)cc1 QFLWZFQWSBQYPS-AWRAUJHKSA-N 0.000 description 1
- UNILWMWFPHPYOR-KXEYIPSPSA-M 1-[6-[2-[3-[3-[3-[2-[2-[3-[[2-[2-[[(2r)-1-[[2-[[(2r)-1-[3-[2-[2-[3-[[2-(2-amino-2-oxoethoxy)acetyl]amino]propoxy]ethoxy]ethoxy]propylamino]-3-hydroxy-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-3-[(2r)-2,3-di(hexadecanoyloxy)propyl]sulfanyl-1-oxopropan-2-yl Chemical compound O=C1C(SCCC(=O)NCCCOCCOCCOCCCNC(=O)COCC(=O)N[C@@H](CSC[C@@H](COC(=O)CCCCCCCCCCCCCCC)OC(=O)CCCCCCCCCCCCCCC)C(=O)NCC(=O)N[C@H](CO)C(=O)NCCCOCCOCCOCCCNC(=O)COCC(N)=O)CC(=O)N1CCNC(=O)CCCCCN\1C2=CC=C(S([O-])(=O)=O)C=C2CC/1=C/C=C/C=C/C1=[N+](CC)C2=CC=C(S([O-])(=O)=O)C=C2C1 UNILWMWFPHPYOR-KXEYIPSPSA-M 0.000 description 1
- IUVCFHHAEHNCFT-INIZCTEOSA-N 2-[(1s)-1-[4-amino-3-(3-fluoro-4-propan-2-yloxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]ethyl]-6-fluoro-3-(3-fluorophenyl)chromen-4-one Chemical compound C1=C(F)C(OC(C)C)=CC=C1C(C1=C(N)N=CN=C11)=NN1[C@@H](C)C1=C(C=2C=C(F)C=CC=2)C(=O)C2=CC(F)=CC=C2O1 IUVCFHHAEHNCFT-INIZCTEOSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 206010001497 Agitation Diseases 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- 208000019901 Anxiety disease Diseases 0.000 description 1
- 208000006820 Arthralgia Diseases 0.000 description 1
- 208000008035 Back Pain Diseases 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- OJRUSAPKCPIVBY-KQYNXXCUSA-N C1=NC2=C(N=C(N=C2N1[C@H]3[C@@H]([C@@H]([C@H](O3)COP(=O)(CP(=O)(O)O)O)O)O)I)N Chemical compound C1=NC2=C(N=C(N=C2N1[C@H]3[C@@H]([C@@H]([C@H](O3)COP(=O)(CP(=O)(O)O)O)O)O)I)N OJRUSAPKCPIVBY-KQYNXXCUSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 108090000312 Calcium Channels Proteins 0.000 description 1
- 102000003922 Calcium Channels Human genes 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 208000023890 Complex Regional Pain Syndromes Diseases 0.000 description 1
- 206010010774 Constipation Diseases 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- 208000025962 Crush injury Diseases 0.000 description 1
- 206010012335 Dependence Diseases 0.000 description 1
- 208000032131 Diabetic Neuropathies Diseases 0.000 description 1
- 208000027534 Emotional disease Diseases 0.000 description 1
- 208000001640 Fibromyalgia Diseases 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 239000007821 HATU Substances 0.000 description 1
- 208000031886 HIV Infections Diseases 0.000 description 1
- 208000037357 HIV infectious disease Diseases 0.000 description 1
- 208000007514 Herpes zoster Diseases 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- 208000004454 Hyperalgesia Diseases 0.000 description 1
- 208000008454 Hyperhidrosis Diseases 0.000 description 1
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 1
- 208000005615 Interstitial Cystitis Diseases 0.000 description 1
- 206010023230 Joint stiffness Diseases 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 208000002472 Morton Neuroma Diseases 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- 208000028389 Nerve injury Diseases 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- 206010030124 Oedema peripheral Diseases 0.000 description 1
- 208000008558 Osteophyte Diseases 0.000 description 1
- 208000004983 Phantom Limb Diseases 0.000 description 1
- 206010056238 Phantom pain Diseases 0.000 description 1
- 206010036376 Postherpetic Neuralgia Diseases 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 206010037779 Radiculopathy Diseases 0.000 description 1
- 229910006124 SOCl2 Inorganic materials 0.000 description 1
- 208000008765 Sciatica Diseases 0.000 description 1
- 208000033712 Self injurious behaviour Diseases 0.000 description 1
- FKNQFGJONOIPTF-UHFFFAOYSA-N Sodium cation Chemical compound [Na+] FKNQFGJONOIPTF-UHFFFAOYSA-N 0.000 description 1
- 239000005708 Sodium hypochlorite Substances 0.000 description 1
- 208000006011 Stroke Diseases 0.000 description 1
- 206010042458 Suicidal ideation Diseases 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- 239000004098 Tetracycline Substances 0.000 description 1
- 229940123445 Tricyclic antidepressant Drugs 0.000 description 1
- 206010047700 Vomiting Diseases 0.000 description 1
- 208000003728 Vulvodynia Diseases 0.000 description 1
- 206010069055 Vulvovaginal pain Diseases 0.000 description 1
- 208000021017 Weight Gain Diseases 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 108010084455 Zeocin Proteins 0.000 description 1
- 150000001242 acetic acid derivatives Chemical class 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000036982 action potential Effects 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 230000009692 acute damage Effects 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 210000003766 afferent neuron Anatomy 0.000 description 1
- 206010053552 allodynia Diseases 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 238000002266 amputation Methods 0.000 description 1
- 230000036592 analgesia Effects 0.000 description 1
- 230000000202 analgesic effect Effects 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 229940125681 anticonvulsant agent Drugs 0.000 description 1
- 239000000935 antidepressant agent Substances 0.000 description 1
- 238000011225 antiretroviral therapy Methods 0.000 description 1
- 230000036506 anxiety Effects 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 206010003246 arthritis Diseases 0.000 description 1
- 230000002567 autonomic effect Effects 0.000 description 1
- JPNZKPRONVOMLL-UHFFFAOYSA-N azane;octadecanoic acid Chemical class [NH4+].CCCCCCCCCCCCCCCCCC([O-])=O JPNZKPRONVOMLL-UHFFFAOYSA-N 0.000 description 1
- 150000007514 bases Chemical class 0.000 description 1
- 150000001558 benzoic acid derivatives Chemical class 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- IPWKHHSGDUIRAH-UHFFFAOYSA-N bis(pinacolato)diboron Chemical compound O1C(C)(C)C(C)(C)OB1B1OC(C)(C)C(C)(C)O1 IPWKHHSGDUIRAH-UHFFFAOYSA-N 0.000 description 1
- 230000036772 blood pressure Effects 0.000 description 1
- 230000036760 body temperature Effects 0.000 description 1
- UNXISIRQWPTTSN-UHFFFAOYSA-N boron;2,3-dimethylbutane-2,3-diol Chemical compound [B].[B].CC(C)(O)C(C)(C)O UNXISIRQWPTTSN-UHFFFAOYSA-N 0.000 description 1
- 210000003461 brachial plexus Anatomy 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 125000004106 butoxy group Chemical group [*]OC([H])([H])C([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 238000001460 carbon-13 nuclear magnetic resonance spectrum Methods 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 208000003295 carpal tunnel syndrome Diseases 0.000 description 1
- 230000008355 cartilage degradation Effects 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 1
- 208000037976 chronic inflammation Diseases 0.000 description 1
- 230000006020 chronic inflammation Effects 0.000 description 1
- 150000001860 citric acid derivatives Chemical class 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 229940125904 compound 1 Drugs 0.000 description 1
- 229940125773 compound 10 Drugs 0.000 description 1
- 229940126543 compound 14 Drugs 0.000 description 1
- 229940125758 compound 15 Drugs 0.000 description 1
- 229940125782 compound 2 Drugs 0.000 description 1
- 229940126214 compound 3 Drugs 0.000 description 1
- 229940125898 compound 5 Drugs 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 230000001143 conditioned effect Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- RFKZUAOAYVHBOY-UHFFFAOYSA-M copper(1+);acetate Chemical compound [Cu+].CC([O-])=O RFKZUAOAYVHBOY-UHFFFAOYSA-M 0.000 description 1
- LSXDOTMGLUJQCM-UHFFFAOYSA-M copper(i) iodide Chemical compound I[Cu] LSXDOTMGLUJQCM-UHFFFAOYSA-M 0.000 description 1
- NXQGGXCHGDYOHB-UHFFFAOYSA-L cyclopenta-1,4-dien-1-yl(diphenyl)phosphane;dichloropalladium;iron(2+) Chemical compound [Fe+2].Cl[Pd]Cl.[CH-]1C=CC(P(C=2C=CC=CC=2)C=2C=CC=CC=2)=C1.[CH-]1C=CC(P(C=2C=CC=CC=2)C=2C=CC=CC=2)=C1 NXQGGXCHGDYOHB-UHFFFAOYSA-L 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 238000001647 drug administration Methods 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 206010013663 drug dependence Diseases 0.000 description 1
- 238000000132 electrospray ionisation Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- DEFVIWRASFVYLL-UHFFFAOYSA-N ethylene glycol bis(2-aminoethyl)tetraacetic acid Chemical compound OC(=O)CN(CC(O)=O)CCOCCOCCN(CC(O)=O)CC(O)=O DEFVIWRASFVYLL-UHFFFAOYSA-N 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000012091 fetal bovine serum Substances 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 150000004675 formic acid derivatives Chemical class 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-L fumarate(2-) Chemical class [O-]C(=O)\C=C\C([O-])=O VZCYOOQTPOCHFL-OWOJBTEDSA-L 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 125000001188 haloalkyl group Chemical group 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 208000033519 human immunodeficiency virus infectious disease Diseases 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 238000002169 hydrotherapy Methods 0.000 description 1
- 230000037315 hyperhidrosis Effects 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 210000002977 intracellular fluid Anatomy 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical class OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 1
- OWFXIOWLTKNBAP-UHFFFAOYSA-N isoamyl nitrite Chemical compound CC(C)CCON=O OWFXIOWLTKNBAP-UHFFFAOYSA-N 0.000 description 1
- 125000003253 isopropoxy group Chemical group [H]C([H])([H])C([H])(O*)C([H])([H])[H] 0.000 description 1
- ZLVXBBHTMQJRSX-VMGNSXQWSA-N jdtic Chemical compound C1([C@]2(C)CCN(C[C@@H]2C)C[C@H](C(C)C)NC(=O)[C@@H]2NCC3=CC(O)=CC=C3C2)=CC=CC(O)=C1 ZLVXBBHTMQJRSX-VMGNSXQWSA-N 0.000 description 1
- 238000011813 knockout mouse model Methods 0.000 description 1
- 150000003893 lactate salts Chemical class 0.000 description 1
- 230000002045 lasting effect Effects 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- DLEDOFVPSDKWEF-UHFFFAOYSA-N lithium butane Chemical compound [Li+].CCC[CH2-] DLEDOFVPSDKWEF-UHFFFAOYSA-N 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 150000002688 maleic acid derivatives Chemical class 0.000 description 1
- 150000004701 malic acid derivatives Chemical class 0.000 description 1
- 238000001819 mass spectrum Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-M methanesulfonate group Chemical class CS(=O)(=O)[O-] AFVFQIVMOAPDHO-UHFFFAOYSA-M 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 239000002808 molecular sieve Substances 0.000 description 1
- 229910000402 monopotassium phosphate Inorganic materials 0.000 description 1
- 235000019796 monopotassium phosphate Nutrition 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 125000003506 n-propoxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 1
- 210000005036 nerve Anatomy 0.000 description 1
- 230000008764 nerve damage Effects 0.000 description 1
- 210000000653 nervous system Anatomy 0.000 description 1
- 239000002547 new drug Substances 0.000 description 1
- 230000003040 nociceptive effect Effects 0.000 description 1
- 210000000929 nociceptor Anatomy 0.000 description 1
- 239000002767 noradrenalin uptake inhibitor Substances 0.000 description 1
- 229940127221 norepinephrine reuptake inhibitor Drugs 0.000 description 1
- 239000000014 opioid analgesic Substances 0.000 description 1
- 229940005483 opioid analgesics Drugs 0.000 description 1
- 229940124636 opioid drug Drugs 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 230000008058 pain sensation Effects 0.000 description 1
- 230000008533 pain sensitivity Effects 0.000 description 1
- PIBWKRNGBLPSSY-UHFFFAOYSA-L palladium(II) chloride Chemical compound Cl[Pd]Cl PIBWKRNGBLPSSY-UHFFFAOYSA-L 0.000 description 1
- YJVFFLUZDVXJQI-UHFFFAOYSA-L palladium(ii) acetate Chemical compound [Pd+2].CC([O-])=O.CC([O-])=O YJVFFLUZDVXJQI-UHFFFAOYSA-L 0.000 description 1
- 230000001314 paroxysmal effect Effects 0.000 description 1
- 230000010412 perfusion Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 230000002688 persistence Effects 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 238000001050 pharmacotherapy Methods 0.000 description 1
- CWCMIVBLVUHDHK-ZSNHEYEWSA-N phleomycin D1 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC[C@@H](N=1)C=1SC=C(N=1)C(=O)NCCCCNC(N)=N)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C CWCMIVBLVUHDHK-ZSNHEYEWSA-N 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- PJNZPQUBCPKICU-UHFFFAOYSA-N phosphoric acid;potassium Chemical compound [K].OP(O)(O)=O PJNZPQUBCPKICU-UHFFFAOYSA-N 0.000 description 1
- 230000002980 postoperative effect Effects 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 238000002953 preparative HPLC Methods 0.000 description 1
- 238000012746 preparative thin layer chromatography Methods 0.000 description 1
- XUWVIABDWDTJRZ-UHFFFAOYSA-N propan-2-ylazanide Chemical compound CC(C)[NH-] XUWVIABDWDTJRZ-UHFFFAOYSA-N 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- BBFCIBZLAVOLCF-UHFFFAOYSA-N pyridin-1-ium;bromide Chemical group Br.C1=CC=NC=C1 BBFCIBZLAVOLCF-UHFFFAOYSA-N 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 230000000171 quenching effect Effects 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- 206010039073 rheumatoid arthritis Diseases 0.000 description 1
- 230000000630 rising effect Effects 0.000 description 1
- 150000003873 salicylate salts Chemical class 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000003772 serotonin uptake inhibitor Substances 0.000 description 1
- 230000037321 sleepiness Effects 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- SUKJFIGYRHOWBL-UHFFFAOYSA-N sodium hypochlorite Chemical compound [Na+].Cl[O-] SUKJFIGYRHOWBL-UHFFFAOYSA-N 0.000 description 1
- 229910001415 sodium ion Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical class O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 230000003238 somatosensory effect Effects 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 208000005198 spinal stenosis Diseases 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 208000011117 substance-related disease Diseases 0.000 description 1
- 150000003890 succinate salts Chemical class 0.000 description 1
- 238000011477 surgical intervention Methods 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 150000003892 tartrate salts Chemical class 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 235000019364 tetracycline Nutrition 0.000 description 1
- 150000003522 tetracyclines Chemical class 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- 239000003029 tricyclic antidepressant agent Substances 0.000 description 1
- 206010044652 trigeminal neuralgia Diseases 0.000 description 1
- 238000002604 ultrasonography Methods 0.000 description 1
- 210000001170 unmyelinated nerve fiber Anatomy 0.000 description 1
- PNVNVHUZROJLTJ-UHFFFAOYSA-N venlafaxine Chemical compound C1=CC(OC)=CC=C1C(CN(C)C)C1(O)CCCCC1 PNVNVHUZROJLTJ-UHFFFAOYSA-N 0.000 description 1
- 229960004688 venlafaxine Drugs 0.000 description 1
- 230000008673 vomiting Effects 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 239000003643 water by type Substances 0.000 description 1
- 230000004584 weight gain Effects 0.000 description 1
- 235000019786 weight gain Nutrition 0.000 description 1
- 239000012224 working solution Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/72—Nitrogen atoms
- C07D213/75—Amino or imino radicals, acylated by carboxylic or carbonic acids, or by sulfur or nitrogen analogues thereof, e.g. carbamates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/06—Antiarrhythmics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Cardiology (AREA)
- Pain & Pain Management (AREA)
- Neurology (AREA)
- Urology & Nephrology (AREA)
- Heart & Thoracic Surgery (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Vascular Medicine (AREA)
- Rheumatology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
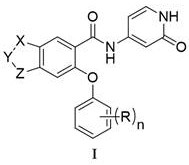
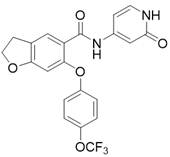
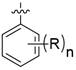
本发明公开了一种并环化合物及其应用。本发明一种如式I所示的并环化合物或其药学上可接受的盐;其中,X为‑CH2‑或‑O‑;Y为‑CR1R2‑;R1和R2独立地为H或卤素;Z为‑CH2‑或‑O‑;n为1或2;R独立地为卤素、C1~C4的烷基、或、卤素取代的C1~C4的烷氧基。该并环化合物具有较佳的NaV1.8阻滞活性。
Description
技术领域
本发明涉及一种并环化合物及其应用。
背景技术
疼痛是临床上最常见的症状之一,是继呼吸、脉搏、血压和体温之后的第五生命体征,严重影响患者的生活质量。据统计,2018年全球镇痛药市场约为360亿美元,预计2023年将达到560亿美元。其中急性中重度主要依赖于阿片类药物,占镇痛药市场份额的三分之二左右,预计未来将以2.5%的年复合增长率稳定增长。而以神经病理性疼痛(neuropathicpain)和关节炎疼痛为主的慢性疼痛患者数量逐年增加,预计市场将呈现18%左右的年复合增长率,是驱动未来十年全球疼痛市场持续增长的主要推动力。
神经病理性疼痛是由于外周躯体感觉神经系统的损伤或疾病导致的一种慢性疼痛,其症状包括自发性疼痛以及对正常无害刺激产生的痛觉超敏。诱发神经病理性疼痛的常见病因包括:糖尿病、带状疱疹、脊髓损伤、脑卒中、多发性硬化、癌症、HIV感染、腰或颈神经根性神经病变和创伤或术后神经损害等等。骨关节炎又称退化性关节炎,是由多种因素引起的骨关节软骨退化,能导致关节骨表面凸凹不平,并有可能形成骨刺,临床表现主要是关节疼痛和关节僵硬。长期疼痛不但影响患者睡眠、工作和生活能力,还会增加抑郁或焦虑等感情障碍的发病率,因此给患者家庭及社会带来沉重的经济负担。
根据国际疼痛学会神经病理性疼痛特别小组(NeuPSIG)发布的数据,神经病理性疼痛患病率约3.3%-8.2%。据此推算,仅我国国内就有至少5千万以上患者。2017年,美国、日本和欧盟五大市场(法国、德国、意大利、西班牙和英国)共有3050万例神经病理性疼痛患者,并呈逐年上升趋势。神经病理性疼痛是最难治疗的疾病之一,目前大多数治疗方案仍不能达到令人满意的效果。有报道指出,能通过药物治疗而及时止痛的门诊患者仅有14.9%,即约85%的疼痛病人并没有得到及时有效的药物治疗,因而一些病人不得不寻求手术介入性治疗。目前临床上用于神经病理性疼痛治疗的一线药物主要是钙离子通道调节剂(如普瑞巴林、加巴喷丁)、三环类抗抑郁药和5-羟色胺、去甲肾上腺素再摄取抑制药(如度洛西汀、文拉法辛等抗惊厥、抗抑郁的药物)。这些药疗效有限并伴随有各种不良反应。度洛西汀是神经病理性疼痛治疗的一线用药之一,主要副作用包括胃肠道反应、恶心、嗜睡、口干、多汗和头晕等,由此导致的停药率达到15%-20%。抗癫痫药物加巴喷丁和普瑞巴林是治疗神经病理性疼痛的主要药物,会引起头晕、嗜睡、周围性水肿、体重增加、虚弱、头痛和口干等诸多不良反应。近年来还发现普瑞巴林会导致极少部分患者出现药物使用相关的自杀观念和自伤行为。
骨关节炎患者数量庞大,预计目前全世界骨关节炎患者超过4亿,中国患者人数已过亿。骨关节炎疼痛目前也没有有效的治疗方法。临床上有物理疗法、药物疗法和手术治疗。物理疗法包括热疗,水疗,超声和按摩等,另外辅助用具减少关节压力缓解疼痛,但效果均有限,大部分依然需要依赖药物进行治疗。这些药物均存在不同程度的副作用。非甾体类抗炎药只适用于轻中度疼痛,而且有胃肠道副作用和心脑血管方面的风险。阿片类镇痛药用于重度疼痛,但有明显的恶心呕吐、便秘和药物依赖等副作用,不适合长期服用。因此,研发靶向新靶点新机制以及安全有效的镇痛药物,满足未被满足的临床需求,具有重要的经济意义和社会意义。
近年来的研究成果逐步揭示了钠离子通道亚型1.8(NaV1.8)在痛觉的发生和传递方面起重要作用。 NaV1.8是一种电压门控钠离子通道,主要表达在包括感觉神经元在内的传入神经元上,通过控制钠离子进出细胞,在维持伤害性感觉神经元的兴奋性、动作电位的发放和持续以及痛觉敏感性的调节等方面,发挥着重要作用。NaV1.8激活性突变病人会出现小纤维神经病变(主要负责痛觉传递的Aδ纤维和无髓纤维C型纤维受损)导致的阵发性疼痛。慢性炎症和糖尿病等疾病会引起NaV1.8表达增加或性质改变从而敏化伤害感受神经元,引起多种疼痛。而NaV1.8基因敲除小鼠对痛觉不敏感。
随着Nav1.8在慢性疼痛中地位的确定,基于此靶点的药物研究也日益火热,目前国际上有一个小分子阻滞剂处于临床2期,其他多个小分子阻滞剂及抗体在进行临床前开发,国内尚无其他针对该靶点的新药研发。处于研发前端的是美国福泰(Vertex)公司的小分子NaV1.8阻滞剂VX-150,目前已在骨性关节炎、急性疼痛及小纤维神经病变导致疼痛的患者中进行了2期临床试验,并且所有三项研究均获得阳性结果,表明抑制NaV1.8活性可以缓解包括神经病理性疼痛在内的多种疼痛。目前VX-150获得了美国FDA突破性疗法认定,用于治疗中度至重度疼痛,再次证明NaV1.8是镇痛很有潜力的靶点。另外,NaV1.8 阻滞剂的作用机理及二期临床实验表明,其适应广泛,包括神经病理性疼痛、骨关节炎疼痛和急性损伤疼痛等多种疼痛;安全性相对高,没有成瘾性,也没有非甾体类抗炎药的胃肠道副作用及心脑血管方面的副作用;可以与其他镇痛药联用,增强疗效,降低副作用。
发明内容
本发明所要解决的技术问题是现有的NaV1.8阻滞剂的种类较少,为此,本发明提供了一种并环化合物及其应用。该并环化合物具有较佳的NaV1.8阻滞活性。
本发明提供了一种如式I所示的并环化合物或其药学上可接受的盐;
其中,X为-CH2-或-O-;
Y为-CR1R2-;R1和R2独立地为H或卤素;
Z为-CH2-或-O-;
n为1或2;
R独立地为卤素、C1~C4的烷基、或、卤素取代的C1~C4的烷氧基。
在某一方案中,所述的如式I所示的并环化合物或其药学上可接受的盐里,某些基团的定义如下所述,未涉及的基团的定义如前述任一方案所述(以下简称为“在某一方案中”):
当所述的R1独立地为卤素时,所述的卤素可为F、Cl、Br或I。
在某一方案中,当所述的R1独立地为卤素时,所述的卤素可为F。
在某一方案中,当所述的R2独立地为卤素时,所述的卤素可为F、Cl、Br或I。
在某一方案中,当所述的R2独立地为卤素时,所述的卤素可为F。
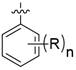
在某一方案中,当n为1时,所述的R可位于氧原子的邻位、间位或对位。
在某一方案中,当n为1时,所述的R可位于氧原子的对位。
在某一方案中,当n为2时,所述的R可独立地位于氧原子的邻位、间位或对位。
在某一方案中,当n为2时,所述的R可位于氧原子的邻位和对位。
在某一方案中,当所述的R独立地为卤素时,所述的卤素可为F、Cl、Br或I。
在某一方案中,当所述的R独立地为卤素时,所述的卤素可为F。
在某一方案中,当所述的R独立地为C1~C4的烷基时,所述的C1~C4的烷基可为甲基、乙基、正丙基、异丙基、正丁基、异丁基、仲丁基或叔丁基。
在某一方案中,当所述的R独立地为C1~C4的烷基时,所述的C1~C4的烷基可为甲基。
在某一方案中,当所述的R独立地为卤素取代的C1~C4的烷氧基时,所述的卤素可为F、Cl、Br或I。
在某一方案中,当所述的R独立地为卤素取代的C1~C4的烷氧基时,所述的卤素可为F。
在某一方案中,当所述的R独立地为卤素取代的C1~C4的烷氧基时,所述的卤素的个数可为1个、2个或3个。
在某一方案中,当所述的R独立地为卤素取代的C1~C4的烷氧基时,所述的卤素的个数可为3个。
在某一方案中,当所述的R独立地为卤素取代的C1~C4的烷氧基时,所述的C1~C4的烷氧基可为甲氧基、乙氧基、正丙氧基、异丙氧基、正丁氧基、异丁氧基、仲丁氧基或叔丁氧基。
在某一方案中,当所述的R独立地为卤素取代的C1~C4的烷氧基时,所述的C1~C4的烷氧基可为甲氧基。
在某一方案中,当所述的R独立地为卤素取代的C1~C4的烷氧基时,所述的卤素取代的C1~C4的烷氧基可为三氟甲氧基。
在某一方案中,X为-CH2-;Y为-CH2-;Z为-CH2-。
在某一方案中,所述的如式I所示的并环化合物或其药学上可接受的盐里,所述的如式I所示的并环化合物可为下述任一化合物:
本发明还提供了一种药物组合物,其包括物质A和药用辅料;所述的物质A为上述的如式I所示的并环化合物或其药学上可接受的盐。
本发明还提供了一种物质A在制备电压门控型钠通道阻滞剂中的应用;所述的物质A为上述的如式I所示的并环化合物或其药学上可接受的盐。
在所述的应用的某一方案中,所述的电压门控型钠通道可为NaV1.8。
在所述的应用的某一方案中,所述的电压门控型钠通道阻滞剂可为在体外使用的电压门控型钠通道阻滞剂。
本发明还提供了一种物质A在制备药物中的应用;所述的药物为用于抑制电压门控型钠通道的药物;所述的物质A为上述的如式I所示的并环化合物或其药学上可接受的盐。
在所述的应用的某一方案中,所述的电压门控型钠通道可为NaV1.8。
本发明还提供了一种物质A在制备药物中的应用;所述的物质A为上述的如式I所示的并环化合物或其药学上可接受的盐;
所述的药物为用于治疗下组疾病中的一种或多种的药物:慢性疼痛、肠痛、神经性疼痛、肌肉骨骼痛、急性疼痛、炎性疼痛、癌症疼痛、原发性疼痛、手术后疼痛、内脏痛、多发性硬化症、夏-马-图三氏综合症、失禁和心律失常。
在所述的应用的某一方案中,所述的肠痛可为发炎性肠病疼痛、克罗恩病疼痛或间质性膀胱炎疼痛。
在所述的应用的某一方案中,所述的神经性疼痛可为疱疹后神经痛、糖尿病性神经痛、痛性HIV相关性感觉神经病、三叉神经痛(例如三叉自主神经性头痛)、口灼伤综合症、截肢术后疼痛、幻痛、痛性神经瘤、创伤性神经瘤、Morton神经瘤、神经挤压损伤、脊管狭窄、腕管综合症、神经根痛、坐骨神经痛、神经撕脱伤、臂丛撕脱伤、复杂性区域疼痛综合症、药物疗法引起的神经痛、癌症化学疗法引起的神经痛、抗逆转录病毒疗法引起的神经痛、脊髓损伤后疼痛、原发性小纤维神经病或原发性感觉神经病。
在所述的应用的某一方案中,所述的肌肉骨骼痛可为骨关节炎疼痛、背痛、冷痛、烧伤疼痛或牙痛。
在所述的应用的某一方案中,所述的炎性疼痛可为类风湿性关节炎疼痛或外阴痛。
在所述的应用的某一方案中,所述的原发性疼痛可为纤维肌痛。
定义
本发明中所用的下列术语和符号具有如下所述的含义,其所处的上下文中另有说明除外。
术语“卤素”指氟(F)、氯(Cl)、溴(Br)或碘(I)。
术语“烷基”指具有1-4个碳原子,例如具有1、2或3个碳原子的直链或支链饱和一价烃基。例如,“C1~C4的烷基”表示具有1-4个碳原子的烷基;“C1~C3的烷基”表示具有1-3个碳原子的烷基。烷基的实例包括但不限于甲基(“Me”)、乙基(“Et”)、丙基如正丙基(“n-Pr”)或异丙基(“i-Pr”)、丁基如正丁基(“n-Bu”)、异丁基(“i-Bu”)、仲丁基(“s-Bu”)或叔丁基(“t-Bu”)等。无论术语“烷基”是单独使用、还是作为其它基团如卤代烷基、烷氧基等的一部分,均适用该定义。
术语“烷氧基”表示通过一个氧原子连接到分子的其余部分的烷基基团。C1~C4的烷氧基的实例包括但不限于甲氧基、乙氧基、丙氧基 (包括正丙氧基和异丙氧基)、丁氧基(包括n-丁氧基、异丁氧基、s-丁氧基和t-丁氧基)等。
术语“药学上可接受的”指无毒的、生物学上可耐受的、适合给个体施用的。
术语“药学上可接受的盐”指式I化合物的无毒的、生物学上可耐受的适合给个体施用的酸加成盐或碱加成盐,包括但不限于:式I化合物与无机酸形成的酸加成盐,例如盐酸盐、氢溴酸盐、碳酸盐、碳酸氢盐、磷酸盐、硫酸盐、亚硫酸盐、硝酸盐等;以及式I化合物与有机酸形成的酸加成盐,例如甲酸盐、乙酸盐、苹果酸盐、马来酸盐、富马酸盐、酒石酸盐、琥珀酸盐、柠檬酸盐、乳酸盐、甲磺酸盐、对甲苯磺酸盐、2-羟基乙磺酸盐、苯甲酸盐、水杨酸盐、硬脂酸盐和与式HOOC-(CH2)n-COOH (其中n是0-4)的链烷二羧酸形成的盐等。“药学上可接受的盐”也包括带有酸性基团的式I化合物与药学上可接受的阳离子如钠、钾、钙、铝、锂和铵形成的碱加成盐。
此外,如果本发明所述的化合物是以酸加成盐的形式得到的,其游离碱形式可以通过碱化该酸加成盐的溶液获得。相反地,如果产物是游离碱形式,则其酸加成盐、特别是药学上可接受的酸加成盐可以按照由碱性化合物制备酸加成盐的常规操作(通过将游离碱溶于合适的溶剂并且用酸处理该溶液)来得到。本领域技术人员无需过多实验即可确定各种可用来制备无毒的药学上可接受的酸加成盐的合成方法。
术语“治疗”指给患有疾病或者具有所述疾病的症状的个体施用一种或多种药物物质、特别是本发明所述的式I化合物和/或其药学上可接受的盐,用以治愈、缓解、减轻、改变、医治、改善、改进或影响所述疾病或者所述疾病的症状。
当涉及化学反应时,术语“处理”、“接触”和“反应”指在适当的条件下加入或混合两种或更多种试剂,以产生所示的和/或所需的产物。应当理解的是,产生所示的和/或所需的产物的反应可能不一定直接来自最初加入的两种试剂的组合,即,在混合物中可能存在生成的一个或多个中间体,这些中间体最终导致了所示的和/或所需的产物的形成。
本发明所用的未具体定义的技术和科学术语具有本发明所属领域的技术人员通常理解的含义。
在不违背本领域常识的基础上,上述各优选条件,可任意组合,即得本发明各较佳实例。
本发明所用试剂和原料均市售可得。
本发明的积极进步效果在于:该并环化合物具有较佳的NaV1.8阻滞活性。
具体实施方式
下面通过实施例的方式进一步说明本发明,但并不因此将本发明限制在所述的实施例范围之中。下列实施例中未注明具体条件的实验方法,按照常规方法和条件,或按照商品说明书选择。
除非另外说明,否则百分比和份数是重量百分比和重量份数。除非另外说明,否则液体的比为体积比。
以下实施例中所用的实验材料和试剂如无特别说明均可从市售渠道获得。
在下列实施例中,1H-NMR谱是用Bluker AVANCE III HD 400MHz核磁共振仪记录的;13C-NMR谱是用Bluker AVANCE III HD 400MHz核磁共振仪记录的,化学位移以δ(ppm)表示;质谱是用Agilent 1260 (ESI)型或Shimadzu LCMS-2020 (ESI型)或Agilent 6215(ESI)型质谱仪记录的;反相制备型HPLC分离是用Agilent 1290 紫外引导的全自动纯化系统 (Xtimate ®Prep C18 OBDTM 21.2*250mm 10μm 柱 )或 用Gilson GX281 紫外引导的全自动纯化系统 (xBridge ®Prep C18 OBDTM 19*250mm 10μm 柱 ) 或Waters QDa引导的全自动纯化系统(SunFire ®Prep C18 OBD 29*250mm 10μm柱)进行的。
其中,化学式或英文字母缩写代表的试剂中文名称表如下:
Aq代表水溶液;Ar代表氩气;br代表宽峰;BF3.Et2O代表三氟化硼乙醚;BINAP代表1,1’-联萘-2,2’-双苯磷;B2Pin2代表联硼酸频那醇酯;℃代表摄氏度;CO代表一氧化碳;CD3OD代表氘代甲醇;CDCl3代表氘代氯仿;conc.代表浓;(COCl)2代表草酰氯;Cs2CO3代表碳酸铯;CuAc代表醋酸亚铜;CuCN代表氰化亚铜;CuI代表碘化亚铜;d代表二重峰;DCE代表1,2-二氯乙烷;DCM代表二氯甲烷;Dioxane或1,4-dioxane代表二氧六环;DIPEA或DIEA代表N,N-二异丙基乙胺;DMF代表二甲基甲酰胺;DMSO代表二甲基亚砜;EA或EtOAc代表乙酸乙酯;ESI代表电喷雾电离;g代表克;h代表小时;H2O代表水;HATU代表1-[双(二甲基氨基)亚甲基]-1H-1,2,3-三唑并[4,5-b]吡啶鎓3-氧化物六氟磷酸盐;HPLC代表高效液相色谱法;K2CO3代表碳酸钾;KH2PO4代表磷酸二氢钾;LCMS代表液相色谱法-质谱法联用;LDA代表二异丙基氨基锂;LiOH代表氢氧化锂;m代表多重峰;m/z代表质荷比;MeCN、ACN或CH3CN代表乙腈;m-CPBA代表间氯过氧苯甲酸;MeOH代表甲醇;min代表分钟;mg代表毫克;mL代表毫升;mmol代表毫摩尔;N2代表氮气;Na2CO3代表碳酸钠;NaCl代表氯化钠;NaClO2代表次氯酸钠;NaHCO3代表碳酸氢钠;NaOH代表氢氧化钠;Na2SO4代表硫酸钠;NBS代表N-溴代丁二酰亚胺;n-BuLi代表丁基锂;NH4Cl代表氯化铵;NMO代表N-甲基-N-氧化吗啉;NMP代表N-甲基-2-吡咯烷酮;Pd(AcO)2代表醋酸钯;Pd(dppf)Cl2或PdCl2(dppf)代表1,1’-双(二苯基膦基)二茂铁二氯化钯;PE代表石油醚;p-TsOH代表对甲苯磺酸;Py-HBr代表吡啶氢溴酸盐;r.t.或RT代表室温;s代表单峰;SOCl2代表二氯亚砜;t代表三重峰;TEA代表三乙胺;TFA代表三氟乙酸;TLC代表薄层色谱法;THF代表四氢呋喃;Toluene或tol.代表甲苯。
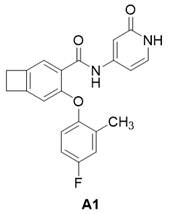
实施例A1
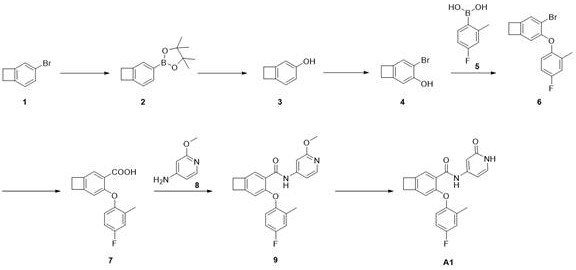
步骤1,中间体2的合成
化合物1(10.0 g,54.63 mmol),B2Pin2(16.7 g, 65.56 mmol),PdCl2dppf(399.0 mg,0.55 mmol),K2CO3(22.7 g, 163.89 mmol),1,4-二氧六环(200 mL)混合。Ar置换3次,110oC反应16 h。冷却至室温,加入石油醚(500 mL), 水洗(500 mL),饱和NaCl洗(500 mL),无水Na2SO4干燥,正相柱纯化(PE/EA=0-100%)得化合物2,11.58 g,LCMS: m/z 231.3(M+H)+。
步骤2,中间体3的合成
化合物2(11.6 g,50.32 mmol),NMO (23.6 g, 201.29 mmol)混合后加入1.4-二氧六环(60 mL),加热至80 oC反应16 h,减压浓缩后,正相柱纯化(PE/EA=0-100%)得化合物3,4.8 g。1H NMR (400 MHz, DMSO-d 6 ) δ 9.04 (s, 1H), 6.84 (d, J = 7.9 Hz, 1H), 6.58(dd, J = 7.9, 2.1 Hz, 1H), 6.53 (d, J = 1.6 Hz, 1H), 3.02 – 2.98 (m, 4H)。
步骤3,中间体4的合成
化合物3(4.8 g,39.95 mmol),溶于乙腈(50 mL),分批加入NBS (7.11 g, 39.95mmol), 冷却至0 oC反应1 h。减压浓缩后正相柱纯化(PE/EA=0-100%)得化合物4,6.45 g。1HNMR (400 MHz, DMSO-d 6 ) δ 9.92 (s, 1H), 7.20 (s, 1H), 6.78 (s, 1H), 3.09-3.04(m, 4H)。
步骤4,中间体6的合成
化合物4(1.3 g,6.53 mmol),化合物5(2.0 g,13.1 mmol),CuAc (1.2 g, 6.53mmol), DCM(50 mL), TEA(3.3 g,32.1 mmol)和4A分子筛(600 mg)混合,室温反应48 h。加入DCM(100 mL),搅拌30分钟,过滤,减压浓缩,正相柱纯化(PE/EA=0-100%)得化合物6,200mg。1H NMR (400 MHz, CDCl3) δ 7.29 (s, 1H), 7.26 (s, 1H), 6.95 (dd, J = 9.0,2.8 Hz, 1H), 6.81 (ddd, J = 8.0, 5.7, 2.7 Hz, 1H), 6.72 (dd, J = 8.9, 4.9 Hz,1H), 6.50 (s, 1H), 3.24 – 3.09 (m, 2H), 3.08 – 2.98 (m, 2H)。
步骤5,中间体7的合成
化合物6(165.0 mg,0.54 mmol),PdCl2dppf(41.0 mg, 0.056 mmol), BINAP(15.0mg, 0.024 mmol),1,4-二氧六环/H2O(10 mL/5 mL), TEA(271.0 mg,2.6 mmol)混合,氩气置换,通入CO,升温至100oC反应18 h。反应液浓缩后正相柱纯化(PE/EA=0-100%)得化合物7,14.0 mg。LCMS: m/z 273.1(M+H)+。
步骤6,中间体9的合成
化合物7(14.0 mg,0.051 mmol)溶于DCM(3 mL), DMF(2滴),于0 oC滴加草酰氯(0.2mL)。室温反应0.5 h。减压旋干,加入DCM(3 mL), 降温到0 oC,加入DIEA(10.0 mg, 0.077mmol),加入化合物8(8.0 mg,0.067 mmol),室温反应1 h。LCMS检测反应完成。反应液浓缩后经制备TLC板纯化(PE/EA = 4:1)分离得到化合物9,6.0 mg。LCMS: m/z 379.2(M+H)+。
步骤7,化合物A1的合成
化合物9(6.0 mg, 0.016 mmol)DMF(0.5 mL)和Py-HBr(51.0 mg, 0.317 mmol)混合100 oC反应2 h。冷却过滤,反相制备(流动相A为乙腈,流动相B为含有0.05%的NH4HCO3的水溶液,流动相A的体积百分比为5%~95%),得化合物A1。2.10 mg。LCMS: m/z 365.1(M+H)+。1HNMR (400 MHz, CD3OD) δ 8.48 (s, 1H), 7.36 (d, J = 6.1 Hz, 2H), 7.05 – 6.96(m, 2H), 6.91 – 6.76 (m, 2H), 6.66 (dd, J = 7.2, 2.1 Hz, 1H), 6.55 (s, 1H),3.18 (dd, J = 12.1, 5.0 Hz, 4H), 2.23 (s, 3H)。
实施例A2
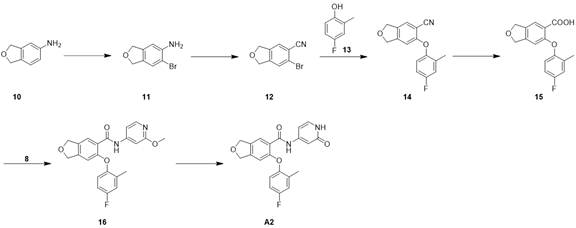
步骤1,中间体11的合成
化合物10(4.0 g,29.6 mmol)加入到MeCN(50 mL)中,冷却到-10°C,分批次加入NBS(5.79 g,32.6 mmol),-10°C下反应2 h,LCMS检测反应完毕,加水50 mL,升至室温,加入EA50 mL,搅拌30 min,分层,水相用EA(50 mL*2)萃取,合并有机相,有机相依次用 H2O(50mL*2)洗涤,饱和NaCl洗(50 mL), 无水Na2SO4干燥。有机相旋干,正相柱纯化(PE/EA=0-100%)得化合物11,4.1 g. 1H NMR (400 MHz, DMSO-d 6 ) δ7.27 (s, 1H), 6.64 (s, 1H),4.99–4.97(m, 4H), 1.91(s, 2H)。
步骤2,中间体12的合成
CuCN(1.8 g,19.5 mmol)溶于无水DMSO(20 mL)中,升温至60°C,在此温度下加入亚硝酸异戊酯(3.4 g,29.3 mmol),滴加中间体11/DMSO(2.1 g/5 mL,9.8 mmol),加完后在60°C下搅拌1h,取样LCMS检测原料反应完,冷却到40-50°C,加入5N HCl(20 mL)淬灭,冷却至室温,EA(40 mL*2)萃取,合并有机相,有机相依次用 H2O(50 mL*2)洗涤,饱和NaCl洗(40mL),无水Na2SO4干燥。有机相浓缩后正相柱纯化(PE/EA=0-100%)得化合物12,400mg。1H NMR(400 MHz, DMSO-d 6 ) δ7.57 (s, 1H), 7.53(s, 1H), 5.12–5.07(m, 4H)。
步骤3,中间体14的合成
化合物12(400 mg,1.8 mmol),化合物13(340 mg,2.7 mmol)和Cs2CO3 (1.2 g,3.6mmol),CuI(162 mg,0.9 mmol)混合于甲苯(10 mL)中,氮气置换,微波150℃反应3 h,冷却至室温。过滤后浓缩,正相柱纯化(PE/EA=0-100%)得化合物14,230 mg。1H NMR (400 MHz,DMSO-d 6 ) δ7.58-7.49 (m, 2H), 7.03-6.93(m, 2H), 6.49(s, 1H), 5.15–5.06(m, 4H),2.22(s, 3H)。
步骤4,中间体15的合成
化合物14(230 mg,0.85 mmol),NaOH(170 mg,4.27 mmol),乙醇 10 mL,水 3 mL混合,80°C反应60 h, LCMS检测反应完成后,降至室温,加入5N HCl 调节PH至4~5,EA(20 mL*3)萃取,合并有机相,饱和食盐水洗(40 mL),无水硫酸钠干燥。有机相旋干,正相柱纯化(PE/EA=0-100%)得化合物15,120 mg。LCMS: m/z 289.0(M+H)+。
步骤5,中间体16的合成
化合物15(120 mg,0.42 mmol)溶于DCM (10mL)中,冷却至0°C,加入2滴DMF,滴加(COCl)2(114 mg,0.9 mmol)反应1h后旋干。加入DCM(10 mL),冷却至0°C,加入DIPEA(220mg,1.71mmol),化合物8(100 mg,0.8 mmol),室温反应2h,LCMS检测,原料反应完,加入饱和的NaHCO3调节PH至弱碱性,用EA(20 mL*2)萃取,合并有机相用饱和NaCl洗(20 mL),无水Na2SO4干燥,滤液浓缩后正相柱纯化(PE/EA=0-100%)得化合物16,100 mg。LCMS: m/z395.1(M+H)+。
步骤6,化合物A2的合成
化合物16(60 mg,0.15 mmol)溶到DMF(3 mL)中,加入Py-HBr(480 mg,3.0 mmol),100°C反应3h。冷却过滤,反相制备(流动相A为乙腈,流动相B为含有0.05%的NH4HCO3的水溶液,流动相A的体积百分比为5%~95%),得化合物A2。31 mg。LCMS: m/z 381.3(M+H)+。1H NMR (400MHz, DMSO-d 6 ) δ 11.21 (s, 1H), 10.42 (s, 1H),7.54 (s, 1H), 7.29 –7.27 (d, J=7.2Hz, 1H), 7.19–7.15 (m, 1H), 705–6.98 (m, 2H), 6.76–6.74 (m, 2H), 6.41-6.38(m, 1H), 4.99(s, 2H), 4.93(s, 2H), 2.17(s, 3H)。
类似于实施例A2的合成,合成了下列实施例A3-A6,如下表1所示:
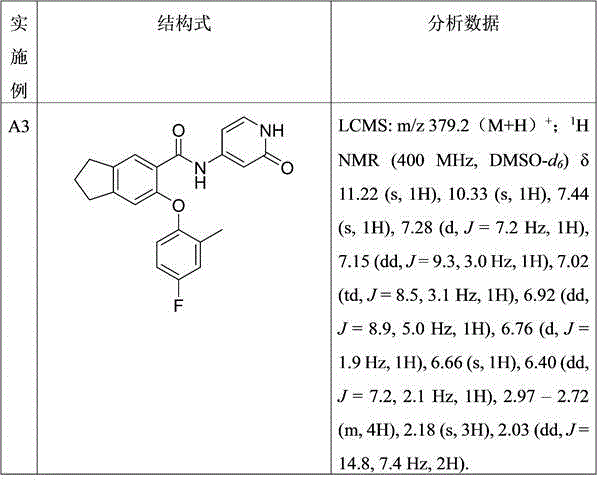
表1:实施例A3-A6
效果实施例:
一.本发明化合物对钠离子通道1.8(NaV1.8)的阻滞活性
1.测试方法:膜片钳技术检测化合物对电压门控钠离子通道(NaV)1.1~1.8亚型电流的影响
2.给药制剂的配制和分析
2.1给药制剂储液配制方法
对照:称量合适体积的DMSO作为储液。
测试化合物:称量合适质量的化合物(实际量=理论浓度*体积×分子量/纯度),根据公式,计算出所需的DMSO的体积,然后换算出最终所需的DMSO的质量。之后将粉末用称量的DMSO溶解。根据最终的DMSO使用量计算出实际的储液浓度,一般地实际储液浓度与理论浓度略有差异。
2.2 给药制剂工作液配制方法及浓度
NaV通道电流测试之前,将对照和测试化合物储液稀释到10 mL细胞外液中作为工作液,并超声20 min。
3. 实验系统
3.1 细胞培养
1)稳定表达NaV1.8通道的CHO细胞系具体信息如下:SCN10A: NM_006514。
2)细胞在含有10%胎牛血清以及10 µg/mL Blasticidin、200 µg/mL HygromycinB及100 µg/mL Zeocin的HAM’S/F-12培养基中培养,培养温度为37 ℃,二氧化碳浓度为5%。
3)细胞传代:除去旧培养基并用PBS洗一次,然后加入1 mL 0.25 %-Trypsin-EDTA溶液,37 ℃孵育1.5 min。当细胞从皿底脱离,加入5 mL 37 ℃预热的完全培养基。将细胞悬液用吸管轻轻吹打使聚集的细胞分离。将细胞悬液转移至无菌的离心管中,1000 rpm离心5 min收集细胞。扩增或维持培养,将细胞接种于6厘米细胞培养皿,每个细胞培养皿接种细胞量为2.5*105 cells(最终体积:5 mL)。
4)为维持细胞的电生理活性,细胞密度必须不能超过80%。
5)膜片钳检测,实验之前细胞用0.25%-Trypsin-EDTA分离,以每孔8*103细胞的密度接种到预先放好盖玻片的24孔板中(最终体积:500 µL),加入四环素,第二天进行实验检测。
3.2. 电生理溶液
细胞外液: 140 mM NaCl,3.5mM KCl,2mM CaCl2,10mM HEPES,1.25mM NaH2PO4,1mMMgCl2,10mM Glucose, pH=7.4 ( NaOH)。
细胞内液: 50mM CsCl,10mM NaCl,10mM HEPES ,20mM EGTA,60mM CsF,pH=7.2(CsOH)。
4. 试验方法
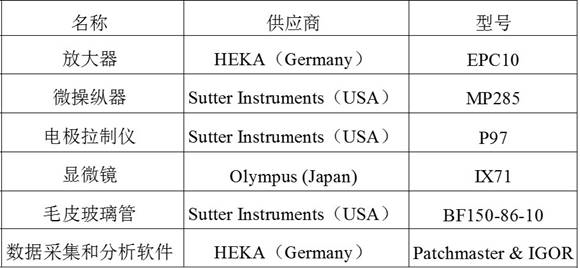
4.1 仪器如下表2所示
表2:采用的仪器的供应商及其型号
4.2 膜片钳检测
全细胞膜片钳记录NaV通道电流的电压刺激方案如下:首先将细胞的膜电位钳制在-130mV, 然后以10mv的阶跃间隔,将电压阶跃至-40mV或者-20mV,持续8s。钳制电压维持在-120mV,每隔20秒重复采集数据。测量其内向电流的峰值振幅,确定其半失活电压。
细胞钳制电位设定在-120mV。钠电流的静息和半失活抑制使用双脉冲模式来测量。双脉冲模式由两个持续50ms的0mV去极化测试脉(TP1以及TP2)完成。两个去极化脉冲之间的条件电压,设定在半失活电压附近(持续8s)。在给与第二个去极化脉冲之前, 将细胞膜电位钳制到-120mv, 持续20ms以使得未结合化合物,且处于失活状态的通道得到恢复。以20s的间隔重复采集数据,并测量两个测试脉冲处的电流峰值。
实验数据由EPC-10 放大器(HEKA)进行采集并储存于PatchMaster(HEKA)软件中(软件版本:v2x73.2)。
用微电极拉制仪(P97,Sutter Instruments)将毛细玻璃管(BF150-86-10,SutterInstruments)拉制成记录电极。在倒置显微镜(IX71)下操纵微电极操纵仪(MP285)将记录电极接触到细胞上,给予负压抽吸,形成GΩ封接。形成GΩ封接后进行快速电容补偿,然后继续给予负压,吸破细胞膜,形成全细胞记录模式。然后进行慢速电容的补偿并记录膜电容及串联电阻,不给予漏电补偿。
当全细胞记录的Nav通道电流稳定后开始给药,每个药物浓度作用至5分钟(或者电流至稳定)后检测下一个浓度,每一个测试化合物检测多个浓度。将铺有细胞的盖玻片置于倒置显微中的记录浴槽中,测试化合物以及不含化合物的外液利用重力灌流的方法从低浓度到高浓度依次流经记录小室从而作用于细胞,在记录中利用真空泵进行液体交换。每一个细胞在不含化合物的外液中检测到的电流作为自己的对照组。独立重复检测多个细胞。所有电生理实验在室温下进行。
4.3 数据分析
首先将每一个药物浓度作用后的电流和空白对照电流标准化,然后计算每一个药物浓度对应的阻滞率。对每一个浓度计算平均数和标准误差,以上所有数值利用MicrosoftExcel 2013计算获得。此外通过IGOR软件运用以下的方程计算每种化合物的半抑制浓度:阻滞率=1/【1+(IC50/c)h】。
用以上方程对剂量依赖效应进行非线性拟合,其中c代表药物浓度,IC50为半抑制浓度,h代表希尔系数。曲线拟合以及IC50的计算利用IGOR软件完成(软件版本:6.0.1.0)。
在本实施例中测定了本发明的化合物对NaV1.8的半数阻滞活性(IC50)如下表3中所示,其中:
表3:本发明的化合物在一定浓度时对NaV1.8的阻滞率
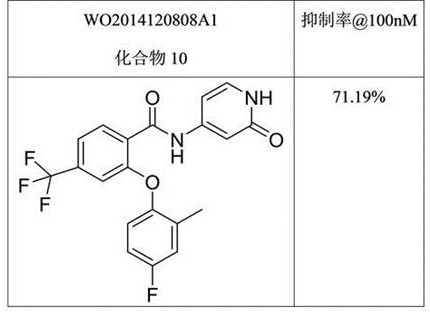
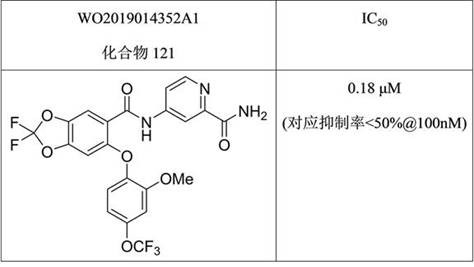
相比下表4和表5所列现有技术中的两个化合物,本发明中的化合物如A3等具有明显活性优势。
表4:现有技术中化合物在一定浓度时对NaV1.8的阻滞率
表5:现有技术中化合物在一定浓度时对NaV1.8的阻滞率
在本发明提及的所有文献都在本发明中引用作为参考,就如同每一篇文献被单独引用作为参考那样。此外应理解,在阅读了本发明的上述讲授内容之后,本领域技术人员可以对本发明作各种改动或修改,这些等价形式同样落于本发明所附权利要求书所限定的范围。
Claims (10)
2.如权利要求1所述的如式I所示的并环化合物或其药学上可接受的盐,其特征在于,X为-CH2-;Y为-CH2-;Z为-CH2-。
3.如权利要求1或2所述的如式I所示的并环化合物或其药学上可接受的盐,其特征在于,当所述的R1独立地为卤素时,所述的卤素为F、Cl、Br或I;
和/或,当所述的R2独立地为卤素时,所述的卤素为F、Cl、Br或I;
和/或,当n为1时,所述的R位于氧原子的邻位、间位或对位;
和/或,当n为2时,所述的R独立地位于氧原子的邻位、间位或对位;
和/或,当所述的R独立地为卤素时,所述的卤素为F、Cl、Br或I;
和/或,当所述的R独立地为C1~C4的烷基时,所述的C1~C4的烷基为甲基、乙基、正丙基、异丙基、正丁基、异丁基、仲丁基或叔丁基;
和/或,当所述的R独立地为卤素取代的C1~C4的烷氧基时,所述的卤素为F、Cl、Br或I;
和/或,当所述的R独立地为卤素取代的C1~C4的烷氧基时,所述的卤素的个数为1个、2个或3个;
和/或,当所述的R独立地为卤素取代的C1~C4的烷氧基时,所述的C1~C4的烷氧基为甲氧基、乙氧基、正丙氧基、异丙氧基、正丁氧基、异丁氧基、仲丁氧基或叔丁氧基。
4.如权利要求3所述的如式I所示的并环化合物或其药学上可接受的盐,其特征在于,当所述的R1独立地为卤素时,所述的卤素为F;
和/或,当所述的R2独立地为卤素时,所述的卤素为F;
和/或,当n为1时,所述的R位于氧原子的对位;
和/或,当n为2时,所述的R位于氧原子的邻位和对位;
和/或,当所述的R独立地为卤素时,所述的卤素为F;
和/或,当所述的R独立地为C1~C4的烷基时,所述的C1~C4的烷基为甲基;
和/或,当所述的R独立地为卤素取代的C1~C4的烷氧基时,所述的卤素取代的C1~C4的烷氧基为三氟甲氧基。
7.一种药物组合物,其包括物质A和药用辅料;所述的物质A为如权利要求1~6中任一项所述的如式I所示的并环化合物或其药学上可接受的盐。
8.一种物质A在制备电压门控型钠通道阻滞剂或药物中的应用;所述的物质A为如权利要求1~6中任一项所述的如式I所示的并环化合物或其药学上可接受的盐;所述的药物为用于抑制电压门控型钠通道的药物。
9.如权利要求8所述的应用,其特征在于,所述的电压门控型钠通道为NaV1.8;
和/或,所述的电压门控型钠通道阻滞剂为在体外使用的电压门控型钠通道阻滞剂。
10.一种物质A在制备药物中的应用;所述的物质A为如权利要求1~6中任一项所述的如式I所示的并环化合物或其药学上可接受的盐;
所述的药物为用于治疗下组疾病中的一种或多种的药物:慢性疼痛、肠痛、神经性疼痛、肌肉骨骼痛、急性疼痛、炎性疼痛、癌症疼痛、原发性疼痛、手术后疼痛、内脏痛、多发性硬化症、夏-马-图三氏综合症、失禁和心律失常。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010932125.3A CN111808019B (zh) | 2020-09-08 | 2020-09-08 | 一种并环化合物及其应用 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010932125.3A CN111808019B (zh) | 2020-09-08 | 2020-09-08 | 一种并环化合物及其应用 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN111808019A true CN111808019A (zh) | 2020-10-23 |
| CN111808019B CN111808019B (zh) | 2020-11-27 |
Family
ID=72860012
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202010932125.3A Active CN111808019B (zh) | 2020-09-08 | 2020-09-08 | 一种并环化合物及其应用 |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN111808019B (zh) |
Cited By (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112225695A (zh) * | 2020-12-15 | 2021-01-15 | 上海济煜医药科技有限公司 | 一种氮氧化合物及其制备方法和用途 |
| WO2022256708A1 (en) | 2021-06-04 | 2022-12-08 | Vertex Pharmaceuticals Incorporated | Solid dosage forms and dosing regimens comprising (2r,3s,4s,5r)-4-[[3-(3,4-difluoro-2-methoxy-phenyl)-4,5-dimethyl-5-(trifluoromethyl) tetrahydrofuran-2-carbonyl]amino]pyridine-2-carboxamide |
| WO2022256676A1 (en) | 2021-06-04 | 2022-12-08 | Vertex Pharmaceuticals Incorporated | Substituted tetrahydrofuran analogs as modulators of sodium channels |
| WO2022256842A1 (en) | 2021-06-04 | 2022-12-08 | Vertex Pharmaceuticals Incorporated | Hydroxy and (halo)alkoxy substituted tetrahydrofurans as modulators of sodium channels |
| WO2022256622A1 (en) | 2021-06-04 | 2022-12-08 | Vertex Pharmaceuticals Incorporated | N-(hydroxyalkyl (hetero)aryl) tetrahydrofuran carboxamides as modulators of sodium channels |
| WO2022256702A1 (en) | 2021-06-04 | 2022-12-08 | Vertex Pharmaceuticals Incorporated | Substituted tetrahydrofuran-2-carboxamides as modulators of sodium channels |
| WO2022256679A1 (en) | 2021-06-04 | 2022-12-08 | Vertex Pharmaceuticals Incorporated | N-(hydroxyalkyl (hetero)aryl) tetrahydrofuran carboxamide analogs as modulators of sodium channels |
| WO2023138599A1 (zh) | 2022-01-18 | 2023-07-27 | 成都康弘药业集团股份有限公司 | 芳香并环类Nav1.8抑制剂及其用途 |
| WO2023205468A1 (en) | 2022-04-22 | 2023-10-26 | Vertex Pharmaceuticals Incorporated | Heteroaryl compounds for the treatment of pain |
| WO2023205778A1 (en) | 2022-04-22 | 2023-10-26 | Vertex Pharmaceuticals Incorporated | Heteroaryl compounds for the treatment of pain |
| WO2023205463A1 (en) | 2022-04-22 | 2023-10-26 | Vertex Pharmaceuticals Incorporated | Heteroaryl compounds for the treatment of pain |
| WO2023205465A1 (en) | 2022-04-22 | 2023-10-26 | Vertex Pharmaceuticals Incorporated | Heteroaryl compounds for the treatment of pain |
| WO2024104464A1 (zh) * | 2022-11-18 | 2024-05-23 | 武汉人福创新药物研发中心有限公司 | 酰胺类化合物作为Nav1.8抑制剂 |
| WO2024123815A1 (en) | 2022-12-06 | 2024-06-13 | Vertex Pharmaceuticals Incorporated | Process for the synthesis of substituted tetrahydrofuran modulators of sodium channels |
| WO2025090516A1 (en) | 2023-10-23 | 2025-05-01 | Vertex Pharmaceuticals Incorporated | Methods of preparing compounds for treating pain and solid forms thereof |
| WO2025090465A1 (en) | 2023-10-23 | 2025-05-01 | Vertex Pharmaceuticals Incorporated | Heteroaryl compounds for the treatment of pain |
| WO2025090511A1 (en) | 2023-10-23 | 2025-05-01 | Vertex Pharmaceuticals Incorporated | Methods of preparing modulators of sodium channels and solid forms of the same for treating pain |
| WO2025090480A1 (en) | 2023-10-23 | 2025-05-01 | Vertex Pharmaceuticals Incorporated | Heteroaryl compounds for the treatment of pain |
| WO2025122953A1 (en) | 2023-12-07 | 2025-06-12 | Vertex Pharmaceuticals Incorporated | Dosing regimens and formulations of suzetrigine for use in the treatment of acute and chronic pain |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5254592A (en) * | 1991-12-31 | 1993-10-19 | Sterling Drug Inc. | Multiply substituted anilines, phenols and pyridines-immunomodulating agents |
| CN105026373A (zh) * | 2013-01-31 | 2015-11-04 | 沃泰克斯药物股份有限公司 | 作为钠通道调节剂的吡啶酮酰胺 |
| CN105814067A (zh) * | 2013-12-13 | 2016-07-27 | 沃泰克斯药物股份有限公司 | 作为钠通道调节剂的吡啶酮酰胺的前药 |
| CN110740993A (zh) * | 2017-05-16 | 2020-01-31 | 沃泰克斯药物股份有限公司 | 用作钠通道调节剂的氘代吡啶酮酰胺及其前药 |
| CN111065383A (zh) * | 2017-07-11 | 2020-04-24 | 沃泰克斯药物股份有限公司 | 用作钠通道调节剂的羧酰胺 |
| WO2020146682A1 (en) * | 2019-01-10 | 2020-07-16 | Vertex Pharmaceuticals Incorporated | Carboxamides as modulators of sodium channels |
-
2020
- 2020-09-08 CN CN202010932125.3A patent/CN111808019B/zh active Active
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5254592A (en) * | 1991-12-31 | 1993-10-19 | Sterling Drug Inc. | Multiply substituted anilines, phenols and pyridines-immunomodulating agents |
| CN105026373A (zh) * | 2013-01-31 | 2015-11-04 | 沃泰克斯药物股份有限公司 | 作为钠通道调节剂的吡啶酮酰胺 |
| CN105814067A (zh) * | 2013-12-13 | 2016-07-27 | 沃泰克斯药物股份有限公司 | 作为钠通道调节剂的吡啶酮酰胺的前药 |
| CN110740993A (zh) * | 2017-05-16 | 2020-01-31 | 沃泰克斯药物股份有限公司 | 用作钠通道调节剂的氘代吡啶酮酰胺及其前药 |
| CN111065383A (zh) * | 2017-07-11 | 2020-04-24 | 沃泰克斯药物股份有限公司 | 用作钠通道调节剂的羧酰胺 |
| WO2020146682A1 (en) * | 2019-01-10 | 2020-07-16 | Vertex Pharmaceuticals Incorporated | Carboxamides as modulators of sodium channels |
Non-Patent Citations (1)
| Title |
|---|
| 李海涛: "新型Nav1.8抑制剂的设计、合成及活性评价", 《湘潭大学硕士学位论文》 * |
Cited By (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112225695A (zh) * | 2020-12-15 | 2021-01-15 | 上海济煜医药科技有限公司 | 一种氮氧化合物及其制备方法和用途 |
| US12258333B2 (en) | 2021-06-04 | 2025-03-25 | Vertex Pharmaceuticals Incorporated | N-(hydroxyalkyl (hetero)aryl) tetrahydrofuran carboxamides as modulators of sodium channels |
| WO2022256676A1 (en) | 2021-06-04 | 2022-12-08 | Vertex Pharmaceuticals Incorporated | Substituted tetrahydrofuran analogs as modulators of sodium channels |
| WO2022256842A1 (en) | 2021-06-04 | 2022-12-08 | Vertex Pharmaceuticals Incorporated | Hydroxy and (halo)alkoxy substituted tetrahydrofurans as modulators of sodium channels |
| WO2022256622A1 (en) | 2021-06-04 | 2022-12-08 | Vertex Pharmaceuticals Incorporated | N-(hydroxyalkyl (hetero)aryl) tetrahydrofuran carboxamides as modulators of sodium channels |
| WO2022256702A1 (en) | 2021-06-04 | 2022-12-08 | Vertex Pharmaceuticals Incorporated | Substituted tetrahydrofuran-2-carboxamides as modulators of sodium channels |
| WO2022256679A1 (en) | 2021-06-04 | 2022-12-08 | Vertex Pharmaceuticals Incorporated | N-(hydroxyalkyl (hetero)aryl) tetrahydrofuran carboxamide analogs as modulators of sodium channels |
| WO2022256708A1 (en) | 2021-06-04 | 2022-12-08 | Vertex Pharmaceuticals Incorporated | Solid dosage forms and dosing regimens comprising (2r,3s,4s,5r)-4-[[3-(3,4-difluoro-2-methoxy-phenyl)-4,5-dimethyl-5-(trifluoromethyl) tetrahydrofuran-2-carbonyl]amino]pyridine-2-carboxamide |
| US11827627B2 (en) | 2021-06-04 | 2023-11-28 | Vertex Pharmaceuticals Incorporated | N-(hydroxyalkyl (hetero)aryl) tetrahydrofuran carboxamides as modulators of sodium channels |
| WO2023138599A1 (zh) | 2022-01-18 | 2023-07-27 | 成都康弘药业集团股份有限公司 | 芳香并环类Nav1.8抑制剂及其用途 |
| WO2023205463A1 (en) | 2022-04-22 | 2023-10-26 | Vertex Pharmaceuticals Incorporated | Heteroaryl compounds for the treatment of pain |
| WO2023205778A1 (en) | 2022-04-22 | 2023-10-26 | Vertex Pharmaceuticals Incorporated | Heteroaryl compounds for the treatment of pain |
| WO2023205468A1 (en) | 2022-04-22 | 2023-10-26 | Vertex Pharmaceuticals Incorporated | Heteroaryl compounds for the treatment of pain |
| WO2023205465A1 (en) | 2022-04-22 | 2023-10-26 | Vertex Pharmaceuticals Incorporated | Heteroaryl compounds for the treatment of pain |
| WO2024104464A1 (zh) * | 2022-11-18 | 2024-05-23 | 武汉人福创新药物研发中心有限公司 | 酰胺类化合物作为Nav1.8抑制剂 |
| WO2024123815A1 (en) | 2022-12-06 | 2024-06-13 | Vertex Pharmaceuticals Incorporated | Process for the synthesis of substituted tetrahydrofuran modulators of sodium channels |
| WO2025090516A1 (en) | 2023-10-23 | 2025-05-01 | Vertex Pharmaceuticals Incorporated | Methods of preparing compounds for treating pain and solid forms thereof |
| WO2025090465A1 (en) | 2023-10-23 | 2025-05-01 | Vertex Pharmaceuticals Incorporated | Heteroaryl compounds for the treatment of pain |
| WO2025090511A1 (en) | 2023-10-23 | 2025-05-01 | Vertex Pharmaceuticals Incorporated | Methods of preparing modulators of sodium channels and solid forms of the same for treating pain |
| WO2025090480A1 (en) | 2023-10-23 | 2025-05-01 | Vertex Pharmaceuticals Incorporated | Heteroaryl compounds for the treatment of pain |
| WO2025122953A1 (en) | 2023-12-07 | 2025-06-12 | Vertex Pharmaceuticals Incorporated | Dosing regimens and formulations of suzetrigine for use in the treatment of acute and chronic pain |
Also Published As
| Publication number | Publication date |
|---|---|
| CN111808019B (zh) | 2020-11-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN111808019B (zh) | 一种并环化合物及其应用 | |
| CN112225695B (zh) | 一种氮氧化合物及其制备方法和用途 | |
| CN112457294B (zh) | 一种作为NaV1.8阻滞剂的化合物及其制备方法和用途 | |
| US11498900B2 (en) | Salts of an LSD1 inhibitor | |
| US11512064B2 (en) | Salts of an LSD1 inhibitor and processes for preparing the same | |
| CN118146148A (zh) | 吡啶氮氧化合物及其制备方法和用途 | |
| EP2949647B1 (en) | Deuterated phenyl amino pyrimidine compound and pharmaceutical composition containing same | |
| JP2016169161A (ja) | 新規イミダゾピリジン化合物 | |
| CN110143961A (zh) | 一种基于vhl配体诱导bet降解的吡咯并吡啶酮类双功能分子化合物 | |
| CN113943285B (zh) | 作为ret抑制剂的化合物 | |
| EP4353724A1 (en) | Compound as cdk kinase inhibitor and use thereof | |
| CN116375707A (zh) | Menin抑制剂及其用途 | |
| JP7129728B2 (ja) | Fgfr4阻害剤として使用される縮環誘導体 | |
| CN110483547B (zh) | 二氢青蒿素的简单酚类偶联物、合成方法及应用 | |
| EP3831822A1 (en) | Polysubstituted benzene compound and preparation method and use thereof | |
| CN115368306B (zh) | 含四氢异喹啉类结构的hdac抑制剂、组合物及其用途 | |
| CN104326963A (zh) | 一类二氢吲哚类化合物、其制备方法、药物组合物及应用 | |
| CN114634521A (zh) | Dna-pk选择性抑制剂及其制备方法和用途 | |
| CN112047848A (zh) | 多巴胺d2受体选择性激动剂及其应用 | |
| HK40061625A (zh) | 吡啶氮氧化合物及其製備方法和用途 | |
| CN115353489A (zh) | 一种氘代酰胺类衍生物及其应用 | |
| CN108290842B (zh) | 一种取代的喹啉化合物及其药物组合物 | |
| WO2023286768A1 (en) | Hydrogenated quinoxalines | |
| CN105272936B (zh) | 一类氮芳基苯并噻唑类parp抑制剂及其制备方法和用途 | |
| US20250179092A1 (en) | Pyrido[1,2-a]pyrimidin-4-one derivatives |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |