WO2024201327A1 - Novel combinations comprising konjac mannan, their compositions and uses thereof - Google Patents
Novel combinations comprising konjac mannan, their compositions and uses thereof Download PDFInfo
- Publication number
- WO2024201327A1 WO2024201327A1 PCT/IB2024/052961 IB2024052961W WO2024201327A1 WO 2024201327 A1 WO2024201327 A1 WO 2024201327A1 IB 2024052961 W IB2024052961 W IB 2024052961W WO 2024201327 A1 WO2024201327 A1 WO 2024201327A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- salts
- water
- kgm
- sodium
- potassium
- Prior art date
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 381
- 229940025902 konjac mannan Drugs 0.000 title description 5
- 229920002752 Konjac Polymers 0.000 claims abstract description 80
- 239000000252 konjac Substances 0.000 claims abstract description 76
- 235000001206 Amorphophallus rivieri Nutrition 0.000 claims abstract description 75
- 229920002581 Glucomannan Polymers 0.000 claims abstract description 74
- LUEWUZLMQUOBSB-FSKGGBMCSA-N (2s,3s,4s,5s,6r)-2-[(2r,3s,4r,5r,6s)-6-[(2r,3s,4r,5s,6s)-4,5-dihydroxy-2-(hydroxymethyl)-6-[(2r,4r,5s,6r)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxan-3-yl]oxy-4,5-dihydroxy-2-(hydroxymethyl)oxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol Chemical compound O[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@@H](O[C@@H]2[C@H](O[C@@H](OC3[C@H](O[C@@H](O)[C@@H](O)[C@H]3O)CO)[C@@H](O)[C@H]2O)CO)[C@H](O)[C@H]1O LUEWUZLMQUOBSB-FSKGGBMCSA-N 0.000 claims abstract description 72
- 229940046240 glucomannan Drugs 0.000 claims abstract description 72
- 235000010485 konjac Nutrition 0.000 claims abstract description 72
- 239000007787 solid Substances 0.000 claims abstract description 71
- 235000013361 beverage Nutrition 0.000 claims abstract description 28
- 238000000034 method Methods 0.000 claims abstract description 26
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 claims abstract description 18
- 239000008280 blood Substances 0.000 claims abstract description 11
- 210000004369 blood Anatomy 0.000 claims abstract description 11
- 235000000346 sugar Nutrition 0.000 claims abstract description 11
- 230000008569 process Effects 0.000 claims abstract description 10
- 235000012000 cholesterol Nutrition 0.000 claims abstract description 9
- 230000004580 weight loss Effects 0.000 claims abstract description 9
- 230000009467 reduction Effects 0.000 claims abstract description 7
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 claims description 247
- 229910001868 water Inorganic materials 0.000 claims description 180
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 176
- 239000000843 powder Substances 0.000 claims description 150
- 235000002639 sodium chloride Nutrition 0.000 claims description 143
- 235000010489 acacia gum Nutrition 0.000 claims description 115
- 150000003839 salts Chemical class 0.000 claims description 113
- 239000001785 acacia senegal l. willd gum Substances 0.000 claims description 98
- 239000012530 fluid Substances 0.000 claims description 82
- 235000015165 citric acid Nutrition 0.000 claims description 77
- 244000247812 Amorphophallus rivieri Species 0.000 claims description 75
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 claims description 75
- 229910052708 sodium Inorganic materials 0.000 claims description 59
- PJAHUDTUZRZBKM-UHFFFAOYSA-K potassium citrate monohydrate Chemical class O.[K+].[K+].[K+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O PJAHUDTUZRZBKM-UHFFFAOYSA-K 0.000 claims description 49
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 claims description 48
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 claims description 46
- UIIMBOGNXHQVGW-UHFFFAOYSA-M sodium bicarbonate Substances [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 claims description 46
- 235000013336 milk Nutrition 0.000 claims description 39
- 239000008267 milk Substances 0.000 claims description 39
- 210000004080 milk Anatomy 0.000 claims description 39
- 239000011734 sodium Substances 0.000 claims description 39
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 claims description 36
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 claims description 36
- 150000001261 hydroxy acids Chemical class 0.000 claims description 36
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 claims description 32
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 claims description 32
- QEEAPRPFLLJWCF-UHFFFAOYSA-K potassium citrate (anhydrous) Chemical class [K+].[K+].[K+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O QEEAPRPFLLJWCF-UHFFFAOYSA-K 0.000 claims description 32
- 239000000126 substance Substances 0.000 claims description 31
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 claims description 30
- 239000001110 calcium chloride Substances 0.000 claims description 29
- 229960002713 calcium chloride Drugs 0.000 claims description 29
- 229910001628 calcium chloride Inorganic materials 0.000 claims description 29
- 235000011148 calcium chloride Nutrition 0.000 claims description 29
- 229910052700 potassium Inorganic materials 0.000 claims description 28
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 claims description 27
- 229910052791 calcium Inorganic materials 0.000 claims description 27
- 229960003975 potassium Drugs 0.000 claims description 27
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 claims description 26
- 235000015114 espresso Nutrition 0.000 claims description 26
- 239000011591 potassium Substances 0.000 claims description 26
- 235000017557 sodium bicarbonate Nutrition 0.000 claims description 26
- 229910000030 sodium bicarbonate Inorganic materials 0.000 claims description 26
- TYJJADVDDVDEDZ-UHFFFAOYSA-M potassium hydrogencarbonate Chemical compound [K+].OC([O-])=O TYJJADVDDVDEDZ-UHFFFAOYSA-M 0.000 claims description 25
- 239000011780 sodium chloride Substances 0.000 claims description 24
- 235000002906 tartaric acid Nutrition 0.000 claims description 24
- 239000011975 tartaric acid Substances 0.000 claims description 24
- 240000007154 Coffea arabica Species 0.000 claims description 23
- 239000011575 calcium Substances 0.000 claims description 23
- 229910001629 magnesium chloride Inorganic materials 0.000 claims description 23
- 235000011147 magnesium chloride Nutrition 0.000 claims description 23
- 229910002092 carbon dioxide Inorganic materials 0.000 claims description 22
- 235000008939 whole milk Nutrition 0.000 claims description 22
- 235000010323 ascorbic acid Nutrition 0.000 claims description 21
- 235000015115 caffè latte Nutrition 0.000 claims description 21
- 235000016213 coffee Nutrition 0.000 claims description 21
- 235000013353 coffee beverage Nutrition 0.000 claims description 21
- 235000013365 dairy product Nutrition 0.000 claims description 21
- 239000007788 liquid Substances 0.000 claims description 21
- 244000299461 Theobroma cacao Species 0.000 claims description 20
- 235000005764 Theobroma cacao ssp. cacao Nutrition 0.000 claims description 20
- 235000005767 Theobroma cacao ssp. sphaerocarpum Nutrition 0.000 claims description 20
- 235000001046 cacaotero Nutrition 0.000 claims description 20
- 235000015497 potassium bicarbonate Nutrition 0.000 claims description 20
- 229910000028 potassium bicarbonate Inorganic materials 0.000 claims description 20
- 239000011736 potassium bicarbonate Substances 0.000 claims description 20
- 239000001508 potassium citrate Substances 0.000 claims description 20
- 235000018102 proteins Nutrition 0.000 claims description 20
- 102000004169 proteins and genes Human genes 0.000 claims description 20
- 108090000623 proteins and genes Proteins 0.000 claims description 20
- HRXKRNGNAMMEHJ-UHFFFAOYSA-K trisodium citrate Chemical class [Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O HRXKRNGNAMMEHJ-UHFFFAOYSA-K 0.000 claims description 20
- 240000002319 Citrus sinensis Species 0.000 claims description 19
- 235000005976 Citrus sinensis Nutrition 0.000 claims description 19
- 229960005069 calcium Drugs 0.000 claims description 19
- -1 zinc ascorbate monohydrate salts Chemical class 0.000 claims description 19
- 235000013399 edible fruits Nutrition 0.000 claims description 18
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 claims description 18
- 239000003921 oil Substances 0.000 claims description 18
- 235000019198 oils Nutrition 0.000 claims description 18
- 108010046377 Whey Proteins Proteins 0.000 claims description 17
- 230000037396 body weight Effects 0.000 claims description 17
- RYYVLZVUVIJVGH-UHFFFAOYSA-N caffeine Chemical compound CN1C(=O)N(C)C(=O)C2=C1N=CN2C RYYVLZVUVIJVGH-UHFFFAOYSA-N 0.000 claims description 17
- 239000012141 concentrate Substances 0.000 claims description 16
- 235000011164 potassium chloride Nutrition 0.000 claims description 16
- 239000001103 potassium chloride Substances 0.000 claims description 16
- 235000015870 tripotassium citrate Nutrition 0.000 claims description 16
- 239000003086 colorant Substances 0.000 claims description 15
- 239000000284 extract Substances 0.000 claims description 15
- PHOQVHQSTUBQQK-SQOUGZDYSA-N D-glucono-1,5-lactone Chemical compound OC[C@H]1OC(=O)[C@H](O)[C@@H](O)[C@@H]1O PHOQVHQSTUBQQK-SQOUGZDYSA-N 0.000 claims description 14
- 230000001476 alcoholic effect Effects 0.000 claims description 14
- 235000012209 glucono delta-lactone Nutrition 0.000 claims description 14
- 239000000182 glucono-delta-lactone Substances 0.000 claims description 14
- 229960003681 gluconolactone Drugs 0.000 claims description 14
- AVTYONGGKAJVTE-OLXYHTOASA-L potassium L-tartrate Chemical compound [K+].[K+].[O-]C(=O)[C@H](O)[C@@H](O)C([O-])=O AVTYONGGKAJVTE-OLXYHTOASA-L 0.000 claims description 14
- 239000001472 potassium tartrate Substances 0.000 claims description 14
- 229940111695 potassium tartrate Drugs 0.000 claims description 14
- 235000011005 potassium tartrates Nutrition 0.000 claims description 14
- 102000007544 Whey Proteins Human genes 0.000 claims description 13
- 239000001509 sodium citrate Substances 0.000 claims description 13
- 235000021119 whey protein Nutrition 0.000 claims description 13
- 238000002156 mixing Methods 0.000 claims description 12
- 150000007524 organic acids Chemical class 0.000 claims description 12
- 235000010378 sodium ascorbate Nutrition 0.000 claims description 12
- PPASLZSBLFJQEF-RKJRWTFHSA-M sodium ascorbate Substances [Na+].OC[C@@H](O)[C@H]1OC(=O)C(O)=C1[O-] PPASLZSBLFJQEF-RKJRWTFHSA-M 0.000 claims description 12
- 229960005055 sodium ascorbate Drugs 0.000 claims description 12
- 239000000176 sodium gluconate Substances 0.000 claims description 12
- 235000012207 sodium gluconate Nutrition 0.000 claims description 12
- 229940005574 sodium gluconate Drugs 0.000 claims description 12
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 claims description 11
- 230000002378 acidificating effect Effects 0.000 claims description 11
- 239000000796 flavoring agent Substances 0.000 claims description 11
- 235000019634 flavors Nutrition 0.000 claims description 11
- 235000003599 food sweetener Nutrition 0.000 claims description 11
- 235000013384 milk substitute Nutrition 0.000 claims description 11
- 235000020245 plant milk Nutrition 0.000 claims description 11
- 239000003765 sweetening agent Substances 0.000 claims description 11
- WMBWREPUVVBILR-WIYYLYMNSA-N (-)-Epigallocatechin-3-o-gallate Chemical compound O([C@@H]1CC2=C(O)C=C(C=C2O[C@@H]1C=1C=C(O)C(O)=C(O)C=1)O)C(=O)C1=CC(O)=C(O)C(O)=C1 WMBWREPUVVBILR-WIYYLYMNSA-N 0.000 claims description 10
- 239000001100 (2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chroman-4-one Substances 0.000 claims description 10
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 claims description 10
- QUQPHWDTPGMPEX-UHFFFAOYSA-N Hesperidine Natural products C1=C(O)C(OC)=CC=C1C1OC2=CC(OC3C(C(O)C(O)C(COC4C(C(O)C(O)C(C)O4)O)O3)O)=CC(O)=C2C(=O)C1 QUQPHWDTPGMPEX-UHFFFAOYSA-N 0.000 claims description 10
- HLCFGWHYROZGBI-JJKGCWMISA-M Potassium gluconate Chemical class [K+].OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O HLCFGWHYROZGBI-JJKGCWMISA-M 0.000 claims description 10
- QUQPHWDTPGMPEX-UTWYECKDSA-N aurantiamarin Natural products COc1ccc(cc1O)[C@H]1CC(=O)c2c(O)cc(O[C@@H]3O[C@H](CO[C@@H]4O[C@@H](C)[C@H](O)[C@@H](O)[C@H]4O)[C@@H](O)[C@H](O)[C@H]3O)cc2O1 QUQPHWDTPGMPEX-UTWYECKDSA-N 0.000 claims description 10
- 235000015117 caffè macchiato Nutrition 0.000 claims description 10
- APSNPMVGBGZYAJ-GLOOOPAXSA-N clematine Natural products COc1cc(ccc1O)[C@@H]2CC(=O)c3c(O)cc(O[C@@H]4O[C@H](CO[C@H]5O[C@@H](C)[C@H](O)[C@@H](O)[C@H]5O)[C@@H](O)[C@H](O)[C@H]4O)cc3O2 APSNPMVGBGZYAJ-GLOOOPAXSA-N 0.000 claims description 10
- 229940025878 hesperidin Drugs 0.000 claims description 10
- QUQPHWDTPGMPEX-QJBIFVCTSA-N hesperidin Chemical compound C1=C(O)C(OC)=CC=C1[C@H]1OC2=CC(O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@@H](CO[C@H]4[C@@H]([C@H](O)[C@@H](O)[C@H](C)O4)O)O3)O)=CC(O)=C2C(=O)C1 QUQPHWDTPGMPEX-QJBIFVCTSA-N 0.000 claims description 10
- VUYDGVRIQRPHFX-UHFFFAOYSA-N hesperidin Natural products COc1cc(ccc1O)C2CC(=O)c3c(O)cc(OC4OC(COC5OC(O)C(O)C(O)C5O)C(O)C(O)C4O)cc3O2 VUYDGVRIQRPHFX-UHFFFAOYSA-N 0.000 claims description 10
- 229940057917 medium chain triglycerides Drugs 0.000 claims description 10
- ARGKVCXINMKCAZ-UHFFFAOYSA-N neohesperidine Natural products C1=C(O)C(OC)=CC=C1C1OC2=CC(OC3C(C(O)C(O)C(CO)O3)OC3C(C(O)C(O)C(C)O3)O)=CC(O)=C2C(=O)C1 ARGKVCXINMKCAZ-UHFFFAOYSA-N 0.000 claims description 10
- ZZZCUOFIHGPKAK-UHFFFAOYSA-N D-erythro-ascorbic acid Natural products OCC1OC(=O)C(O)=C1O ZZZCUOFIHGPKAK-UHFFFAOYSA-N 0.000 claims description 9
- 229930003268 Vitamin C Natural products 0.000 claims description 9
- 229940093797 bioflavonoids Drugs 0.000 claims description 9
- 235000015155 buttermilk Nutrition 0.000 claims description 9
- 235000020152 coffee milk drink Nutrition 0.000 claims description 9
- 235000015897 energy drink Nutrition 0.000 claims description 9
- 235000020280 flat white Nutrition 0.000 claims description 9
- 235000020509 fortified beverage Nutrition 0.000 claims description 9
- 235000011389 fruit/vegetable juice Nutrition 0.000 claims description 9
- 239000004310 lactic acid Substances 0.000 claims description 9
- 235000014655 lactic acid Nutrition 0.000 claims description 9
- 235000020282 macchiato Nutrition 0.000 claims description 9
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 claims description 9
- 235000021489 probiotic drink Nutrition 0.000 claims description 9
- 235000013570 smoothie Nutrition 0.000 claims description 9
- 235000019154 vitamin C Nutrition 0.000 claims description 9
- 239000011718 vitamin C Substances 0.000 claims description 9
- 235000008924 yoghurt drink Nutrition 0.000 claims description 9
- 102000008186 Collagen Human genes 0.000 claims description 8
- 108010035532 Collagen Proteins 0.000 claims description 8
- 239000011668 ascorbic acid Substances 0.000 claims description 8
- 229960005070 ascorbic acid Drugs 0.000 claims description 8
- 235000015116 cappuccino Nutrition 0.000 claims description 8
- 239000001569 carbon dioxide Substances 0.000 claims description 8
- BVKZGUZCCUSVTD-UHFFFAOYSA-N carbonic acid Chemical class OC(O)=O BVKZGUZCCUSVTD-UHFFFAOYSA-N 0.000 claims description 8
- 229920001436 collagen Polymers 0.000 claims description 8
- 239000008187 granular material Substances 0.000 claims description 8
- 150000002632 lipids Chemical class 0.000 claims description 8
- 239000001433 sodium tartrate Substances 0.000 claims description 8
- 229960002167 sodium tartrate Drugs 0.000 claims description 8
- 235000011004 sodium tartrates Nutrition 0.000 claims description 8
- PPASLZSBLFJQEF-RXSVEWSESA-M sodium-L-ascorbate Chemical compound [Na+].OC[C@H](O)[C@H]1OC(=O)C(O)=C1[O-] PPASLZSBLFJQEF-RXSVEWSESA-M 0.000 claims description 8
- 235000019156 vitamin B Nutrition 0.000 claims description 8
- 239000011720 vitamin B Substances 0.000 claims description 8
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 claims description 7
- VFLDPWHFBUODDF-FCXRPNKRSA-N Curcumin Natural products C1=C(O)C(OC)=CC(\C=C\C(=O)CC(=O)\C=C\C=2C=C(OC)C(O)=CC=2)=C1 VFLDPWHFBUODDF-FCXRPNKRSA-N 0.000 claims description 7
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 claims description 7
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 claims description 7
- 239000001630 malic acid Substances 0.000 claims description 7
- 235000011090 malic acid Nutrition 0.000 claims description 7
- 238000004519 manufacturing process Methods 0.000 claims description 7
- 229940088594 vitamin Drugs 0.000 claims description 7
- 229930003231 vitamin Natural products 0.000 claims description 7
- 235000013343 vitamin Nutrition 0.000 claims description 7
- 239000011782 vitamin Substances 0.000 claims description 7
- 229940056904 zinc ascorbate Drugs 0.000 claims description 7
- AEQDJSLRWYMAQI-UHFFFAOYSA-N 2,3,9,10-tetramethoxy-6,8,13,13a-tetrahydro-5H-isoquinolino[2,1-b]isoquinoline Chemical compound C1CN2CC(C(=C(OC)C=C3)OC)=C3CC2C2=C1C=C(OC)C(OC)=C2 AEQDJSLRWYMAQI-UHFFFAOYSA-N 0.000 claims description 6
- 244000144725 Amygdalus communis Species 0.000 claims description 6
- 235000011437 Amygdalus communis Nutrition 0.000 claims description 6
- LPHGQDQBBGAPDZ-UHFFFAOYSA-N Isocaffeine Natural products CN1C(=O)N(C)C(=O)C2=C1N(C)C=N2 LPHGQDQBBGAPDZ-UHFFFAOYSA-N 0.000 claims description 6
- 229920000388 Polyphosphate Chemical class 0.000 claims description 6
- CXJAAWRLVGAKDV-UHFFFAOYSA-N acetyltaurine Chemical class CC(=O)NCCS(O)(=O)=O CXJAAWRLVGAKDV-UHFFFAOYSA-N 0.000 claims description 6
- 235000020224 almond Nutrition 0.000 claims description 6
- 235000010208 anthocyanin Nutrition 0.000 claims description 6
- 229930002877 anthocyanin Natural products 0.000 claims description 6
- 239000004410 anthocyanin Substances 0.000 claims description 6
- 150000004636 anthocyanins Chemical class 0.000 claims description 6
- 229960001948 caffeine Drugs 0.000 claims description 6
- VJEONQKOZGKCAK-UHFFFAOYSA-N caffeine Natural products CN1C(=O)N(C)C(=O)C2=C1C=CN2C VJEONQKOZGKCAK-UHFFFAOYSA-N 0.000 claims description 6
- 235000021388 linseed oil Nutrition 0.000 claims description 6
- 239000000944 linseed oil Substances 0.000 claims description 6
- 235000013824 polyphenols Nutrition 0.000 claims description 6
- 239000001205 polyphosphate Chemical class 0.000 claims description 6
- 235000011176 polyphosphates Nutrition 0.000 claims description 6
- CDBYLPFSWZWCQE-UHFFFAOYSA-L sodium carbonate Substances [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 claims description 6
- MHJJUOJOAJLYBS-ZBRNBAAYSA-N (2s)-2-aminopropanoic acid;(2s)-pyrrolidine-2-carboxylic acid Chemical compound C[C@H](N)C(O)=O.OC(=O)[C@@H]1CCCN1 MHJJUOJOAJLYBS-ZBRNBAAYSA-N 0.000 claims description 5
- 235000007319 Avena orientalis Nutrition 0.000 claims description 5
- 244000075850 Avena orientalis Species 0.000 claims description 5
- 229910019142 PO4 Inorganic materials 0.000 claims description 5
- QYSXJUFSXHHAJI-XFEUOLMDSA-N Vitamin D3 Natural products C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C/C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-XFEUOLMDSA-N 0.000 claims description 5
- AGBQKNBQESQNJD-UHFFFAOYSA-N alpha-Lipoic acid Natural products OC(=O)CCCCC1CCSS1 AGBQKNBQESQNJD-UHFFFAOYSA-N 0.000 claims description 5
- 235000019270 ammonium chloride Nutrition 0.000 claims description 5
- 235000019197 fats Nutrition 0.000 claims description 5
- 239000010452 phosphate Substances 0.000 claims description 5
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical class [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 claims description 5
- 150000008442 polyphenolic compounds Chemical class 0.000 claims description 5
- 239000004224 potassium gluconate Substances 0.000 claims description 5
- 235000013926 potassium gluconate Nutrition 0.000 claims description 5
- 229960003189 potassium gluconate Drugs 0.000 claims description 5
- 229910000029 sodium carbonate Inorganic materials 0.000 claims description 5
- 244000025254 Cannabis sativa Species 0.000 claims description 4
- 235000012766 Cannabis sativa ssp. sativa var. sativa Nutrition 0.000 claims description 4
- 235000012765 Cannabis sativa ssp. sativa var. spontanea Nutrition 0.000 claims description 4
- 240000000560 Citrus x paradisi Species 0.000 claims description 4
- 235000013162 Cocos nucifera Nutrition 0.000 claims description 4
- 244000060011 Cocos nucifera Species 0.000 claims description 4
- WMBWREPUVVBILR-UHFFFAOYSA-N GCG Natural products C=1C(O)=C(O)C(O)=CC=1C1OC2=CC(O)=CC(O)=C2CC1OC(=O)C1=CC(O)=C(O)C(O)=C1 WMBWREPUVVBILR-UHFFFAOYSA-N 0.000 claims description 4
- 235000010469 Glycine max Nutrition 0.000 claims description 4
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims description 4
- 240000007594 Oryza sativa Species 0.000 claims description 4
- 235000007164 Oryza sativa Nutrition 0.000 claims description 4
- 108010009736 Protein Hydrolysates Proteins 0.000 claims description 4
- REFJWTPEDVJJIY-UHFFFAOYSA-N Quercetin Chemical compound C=1C(O)=CC(O)=C(C(C=2O)=O)C=1OC=2C1=CC=C(O)C(O)=C1 REFJWTPEDVJJIY-UHFFFAOYSA-N 0.000 claims description 4
- QNVSXXGDAPORNA-UHFFFAOYSA-N Resveratrol Natural products OC1=CC=CC(C=CC=2C=C(O)C(O)=CC=2)=C1 QNVSXXGDAPORNA-UHFFFAOYSA-N 0.000 claims description 4
- 235000001484 Trigonella foenum graecum Nutrition 0.000 claims description 4
- 244000250129 Trigonella foenum graecum Species 0.000 claims description 4
- 239000005862 Whey Substances 0.000 claims description 4
- WHMDKBIGKVEYHS-IYEMJOQQSA-L Zinc gluconate Chemical class [Zn+2].OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O.OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O WHMDKBIGKVEYHS-IYEMJOQQSA-L 0.000 claims description 4
- 239000004480 active ingredient Substances 0.000 claims description 4
- 229940047036 calcium ascorbate Drugs 0.000 claims description 4
- 239000004227 calcium gluconate Substances 0.000 claims description 4
- 235000013927 calcium gluconate Nutrition 0.000 claims description 4
- 229960004494 calcium gluconate Drugs 0.000 claims description 4
- 229940057801 calcium lactate pentahydrate Drugs 0.000 claims description 4
- JCFHGKRSYPTRSS-UHFFFAOYSA-N calcium;2-hydroxypropanoic acid;hydrate Chemical compound O.[Ca].CC(O)C(O)=O JCFHGKRSYPTRSS-UHFFFAOYSA-N 0.000 claims description 4
- 235000009120 camo Nutrition 0.000 claims description 4
- 235000005607 chanvre indien Nutrition 0.000 claims description 4
- 239000003240 coconut oil Substances 0.000 claims description 4
- 235000019864 coconut oil Nutrition 0.000 claims description 4
- 235000012754 curcumin Nutrition 0.000 claims description 4
- 239000004148 curcumin Substances 0.000 claims description 4
- 229940109262 curcumin Drugs 0.000 claims description 4
- VFLDPWHFBUODDF-UHFFFAOYSA-N diferuloylmethane Natural products C1=C(O)C(OC)=CC(C=CC(=O)CC(=O)C=CC=2C=C(OC)C(O)=CC=2)=C1 VFLDPWHFBUODDF-UHFFFAOYSA-N 0.000 claims description 4
- 235000011869 dried fruits Nutrition 0.000 claims description 4
- 230000000694 effects Effects 0.000 claims description 4
- 239000011487 hemp Substances 0.000 claims description 4
- 239000004337 magnesium citrate Substances 0.000 claims description 4
- 235000002538 magnesium citrate Nutrition 0.000 claims description 4
- 229960005336 magnesium citrate Drugs 0.000 claims description 4
- AIOKQVJVNPDJKA-ZZMNMWMASA-L magnesium;(2r)-2-[(1s)-1,2-dihydroxyethyl]-4-hydroxy-5-oxo-2h-furan-3-olate Chemical class [Mg+2].OC[C@H](O)[C@H]1OC(=O)C(O)=C1[O-].OC[C@H](O)[C@H]1OC(=O)C(O)=C1[O-] AIOKQVJVNPDJKA-ZZMNMWMASA-L 0.000 claims description 4
- IAKLPCRFBAZVRW-XRDLMGPZSA-L magnesium;(2r,3s,4r,5r)-2,3,4,5,6-pentahydroxyhexanoate;hydrate Chemical class O.[Mg+2].OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O.OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O IAKLPCRFBAZVRW-XRDLMGPZSA-L 0.000 claims description 4
- 235000001968 nicotinic acid Nutrition 0.000 claims description 4
- 229960003512 nicotinic acid Drugs 0.000 claims description 4
- 239000011664 nicotinic acid Substances 0.000 claims description 4
- 229910000027 potassium carbonate Inorganic materials 0.000 claims description 4
- 235000011181 potassium carbonates Nutrition 0.000 claims description 4
- PHZLMBHDXVLRIX-UHFFFAOYSA-M potassium lactate Chemical compound [K+].CC(O)C([O-])=O PHZLMBHDXVLRIX-UHFFFAOYSA-M 0.000 claims description 4
- 235000011085 potassium lactate Nutrition 0.000 claims description 4
- 239000001521 potassium lactate Substances 0.000 claims description 4
- 229960001304 potassium lactate Drugs 0.000 claims description 4
- LJCNRYVRMXRIQR-OLXYHTOASA-L potassium sodium L-tartrate Chemical compound [Na+].[K+].[O-]C(=O)[C@H](O)[C@@H](O)C([O-])=O LJCNRYVRMXRIQR-OLXYHTOASA-L 0.000 claims description 4
- 229940074439 potassium sodium tartrate Drugs 0.000 claims description 4
- OTYBMLCTZGSZBG-UHFFFAOYSA-L potassium sulfate Chemical class [K+].[K+].[O-]S([O-])(=O)=O OTYBMLCTZGSZBG-UHFFFAOYSA-L 0.000 claims description 4
- CONVKSGEGAVTMB-RXSVEWSESA-M potassium-L-ascorbate Chemical class [K+].OC[C@H](O)[C@H]1OC(=O)C(O)=C1[O-] CONVKSGEGAVTMB-RXSVEWSESA-M 0.000 claims description 4
- GZWNUORNEQHOAW-UHFFFAOYSA-M potassium;2-aminoacetate Chemical class [K+].NCC([O-])=O GZWNUORNEQHOAW-UHFFFAOYSA-M 0.000 claims description 4
- LXNHXLLTXMVWPM-UHFFFAOYSA-N pyridoxine Chemical compound CC1=NC=C(CO)C(CO)=C1O LXNHXLLTXMVWPM-UHFFFAOYSA-N 0.000 claims description 4
- 235000021283 resveratrol Nutrition 0.000 claims description 4
- 229940016667 resveratrol Drugs 0.000 claims description 4
- 235000009566 rice Nutrition 0.000 claims description 4
- 235000011088 sodium lactate Nutrition 0.000 claims description 4
- 239000001540 sodium lactate Substances 0.000 claims description 4
- 229940005581 sodium lactate Drugs 0.000 claims description 4
- 235000011006 sodium potassium tartrate Nutrition 0.000 claims description 4
- 229910052938 sodium sulfate Inorganic materials 0.000 claims description 4
- 235000011152 sodium sulphate Nutrition 0.000 claims description 4
- 235000001019 trigonella foenum-graecum Nutrition 0.000 claims description 4
- PLSARIKBYIPYPF-UHFFFAOYSA-H trimagnesium dicitrate Chemical compound [Mg+2].[Mg+2].[Mg+2].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O.[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O PLSARIKBYIPYPF-UHFFFAOYSA-H 0.000 claims description 4
- WGIWBXUNRXCYRA-UHFFFAOYSA-H trizinc;2-hydroxypropane-1,2,3-tricarboxylate Chemical compound [Zn+2].[Zn+2].[Zn+2].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O.[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O WGIWBXUNRXCYRA-UHFFFAOYSA-H 0.000 claims description 4
- VUTPBYRVUIEEGC-UHFFFAOYSA-L zinc 2-aminoacetate hydrate Chemical class O.[Zn+2].NCC([O-])=O.NCC([O-])=O VUTPBYRVUIEEGC-UHFFFAOYSA-L 0.000 claims description 4
- 239000011746 zinc citrate Substances 0.000 claims description 4
- 235000006076 zinc citrate Nutrition 0.000 claims description 4
- 229940068475 zinc citrate Drugs 0.000 claims description 4
- UOXSXMSTSYWNMH-UHFFFAOYSA-L zinc;2-aminoacetate Chemical compound [Zn+2].NCC([O-])=O.NCC([O-])=O UOXSXMSTSYWNMH-UHFFFAOYSA-L 0.000 claims description 4
- HZUKVFGMACOUEH-UHFFFAOYSA-L zinc;2-hydroxypropanoate;dihydrate Chemical compound O.O.[Zn+2].CC(O)C([O-])=O.CC(O)C([O-])=O HZUKVFGMACOUEH-UHFFFAOYSA-L 0.000 claims description 4
- PHIQHXFUZVPYII-LURJTMIESA-O (S)-carnitinium Chemical compound C[N+](C)(C)C[C@@H](O)CC(O)=O PHIQHXFUZVPYII-LURJTMIESA-O 0.000 claims description 3
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 claims description 3
- 239000001606 7-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxy-5-hydroxy-2-(4-hydroxyphenyl)chroman-4-one Substances 0.000 claims description 3
- 235000002568 Capsicum frutescens Nutrition 0.000 claims description 3
- 235000000882 Citrus x paradisi Nutrition 0.000 claims description 3
- 241000195493 Cryptophyta Species 0.000 claims description 3
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 3
- PVNIIMVLHYAWGP-UHFFFAOYSA-N Niacin Chemical compound OC(=O)C1=CC=CN=C1 PVNIIMVLHYAWGP-UHFFFAOYSA-N 0.000 claims description 3
- 235000000556 Paullinia cupana Nutrition 0.000 claims description 3
- 240000003444 Paullinia cupana Species 0.000 claims description 3
- 229930003316 Vitamin D Natural products 0.000 claims description 3
- CANRESZKMUPMAE-UHFFFAOYSA-L Zinc lactate Chemical compound [Zn+2].CC(O)C([O-])=O.CC(O)C([O-])=O CANRESZKMUPMAE-UHFFFAOYSA-L 0.000 claims description 3
- FNAQSUUGMSOBHW-UHFFFAOYSA-H calcium citrate Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O.[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O FNAQSUUGMSOBHW-UHFFFAOYSA-H 0.000 claims description 3
- 239000001354 calcium citrate Substances 0.000 claims description 3
- 229960004256 calcium citrate Drugs 0.000 claims description 3
- MKJXYGKVIBWPFZ-UHFFFAOYSA-L calcium lactate Chemical compound [Ca+2].CC(O)C([O-])=O.CC(O)C([O-])=O MKJXYGKVIBWPFZ-UHFFFAOYSA-L 0.000 claims description 3
- 235000011086 calcium lactate Nutrition 0.000 claims description 3
- 239000001527 calcium lactate Substances 0.000 claims description 3
- 229960002401 calcium lactate Drugs 0.000 claims description 3
- YKPUWZUDDOIDPM-SOFGYWHQSA-N capsaicin Natural products COC1=CC(CNC(=O)CCCC\C=C\C(C)C)=CC=C1O YKPUWZUDDOIDPM-SOFGYWHQSA-N 0.000 claims description 3
- 125000005587 carbonate group Chemical group 0.000 claims description 3
- 235000020230 cinnamon extract Nutrition 0.000 claims description 3
- 229930003949 flavanone Natural products 0.000 claims description 3
- 235000011981 flavanones Nutrition 0.000 claims description 3
- 150000002208 flavanones Chemical class 0.000 claims description 3
- FTSSQIKWUOOEGC-RULYVFMPSA-N fructooligosaccharide Chemical compound OC[C@H]1O[C@@](CO)(OC[C@@]2(OC[C@@]3(OC[C@@]4(OC[C@@]5(OC[C@@]6(OC[C@@]7(OC[C@@]8(OC[C@@]9(OC[C@@]%10(OC[C@@]%11(O[C@H]%12O[C@H](CO)[C@@H](O)[C@H](O)[C@H]%12O)O[C@H](CO)[C@@H](O)[C@@H]%11O)O[C@H](CO)[C@@H](O)[C@@H]%10O)O[C@H](CO)[C@@H](O)[C@@H]9O)O[C@H](CO)[C@@H](O)[C@@H]8O)O[C@H](CO)[C@@H](O)[C@@H]7O)O[C@H](CO)[C@@H](O)[C@@H]6O)O[C@H](CO)[C@@H](O)[C@@H]5O)O[C@H](CO)[C@@H](O)[C@@H]4O)O[C@H](CO)[C@@H](O)[C@@H]3O)O[C@H](CO)[C@@H](O)[C@@H]2O)[C@@H](O)[C@@H]1O FTSSQIKWUOOEGC-RULYVFMPSA-N 0.000 claims description 3
- 229940107187 fructooligosaccharide Drugs 0.000 claims description 3
- 235000013569 fruit product Nutrition 0.000 claims description 3
- 229940093915 gynecological organic acid Drugs 0.000 claims description 3
- 239000000413 hydrolysate Substances 0.000 claims description 3
- 229930005346 hydroxycinnamic acid Natural products 0.000 claims description 3
- DEDGUGJNLNLJSR-UHFFFAOYSA-N hydroxycinnamic acid group Chemical class OC(C(=O)O)=CC1=CC=CC=C1 DEDGUGJNLNLJSR-UHFFFAOYSA-N 0.000 claims description 3
- 235000010359 hydroxycinnamic acids Nutrition 0.000 claims description 3
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 claims description 3
- 235000010445 lecithin Nutrition 0.000 claims description 3
- 239000000787 lecithin Substances 0.000 claims description 3
- 229940067606 lecithin Drugs 0.000 claims description 3
- DFPMSGMNTNDNHN-ZPHOTFPESA-N naringin Chemical compound O[C@@H]1[C@H](O)[C@@H](O)[C@H](C)O[C@H]1O[C@H]1[C@H](OC=2C=C3O[C@@H](CC(=O)C3=C(O)C=2)C=2C=CC(O)=CC=2)O[C@H](CO)[C@@H](O)[C@@H]1O DFPMSGMNTNDNHN-ZPHOTFPESA-N 0.000 claims description 3
- 229940052490 naringin Drugs 0.000 claims description 3
- 229930019673 naringin Natural products 0.000 claims description 3
- 235000020660 omega-3 fatty acid Nutrition 0.000 claims description 3
- 235000005985 organic acids Nutrition 0.000 claims description 3
- 235000020183 skimmed milk Nutrition 0.000 claims description 3
- 235000013337 tricalcium citrate Nutrition 0.000 claims description 3
- 235000019166 vitamin D Nutrition 0.000 claims description 3
- 239000011710 vitamin D Substances 0.000 claims description 3
- 229940046008 vitamin d Drugs 0.000 claims description 3
- 150000003722 vitamin derivatives Chemical class 0.000 claims description 3
- 230000037221 weight management Effects 0.000 claims description 3
- 239000011576 zinc lactate Substances 0.000 claims description 3
- 235000000193 zinc lactate Nutrition 0.000 claims description 3
- 229940050168 zinc lactate Drugs 0.000 claims description 3
- WWRJFSIRMWUMAE-ZZMNMWMASA-L zinc;(2r)-2-[(1s)-1,2-dihydroxyethyl]-3-hydroxy-5-oxo-2h-furan-4-olate Chemical compound [Zn+2].OC[C@H](O)[C@H]1OC(=O)C(O)=C1[O-].OC[C@H](O)[C@H]1OC(=O)C(O)=C1[O-] WWRJFSIRMWUMAE-ZZMNMWMASA-L 0.000 claims description 3
- PFTAWBLQPZVEMU-DZGCQCFKSA-N (+)-catechin Chemical compound C1([C@H]2OC3=CC(O)=CC(O)=C3C[C@@H]2O)=CC=C(O)C(O)=C1 PFTAWBLQPZVEMU-DZGCQCFKSA-N 0.000 claims description 2
- PFTAWBLQPZVEMU-ZFWWWQNUSA-N (+)-epicatechin Natural products C1([C@@H]2OC3=CC(O)=CC(O)=C3C[C@@H]2O)=CC=C(O)C(O)=C1 PFTAWBLQPZVEMU-ZFWWWQNUSA-N 0.000 claims description 2
- PFTAWBLQPZVEMU-UKRRQHHQSA-N (-)-epicatechin Chemical compound C1([C@H]2OC3=CC(O)=CC(O)=C3C[C@H]2O)=CC=C(O)C(O)=C1 PFTAWBLQPZVEMU-UKRRQHHQSA-N 0.000 claims description 2
- FYGDTMLNYKFZSV-URKRLVJHSA-N (2s,3r,4s,5s,6r)-2-[(2r,4r,5r,6s)-4,5-dihydroxy-2-(hydroxymethyl)-6-[(2r,4r,5r,6s)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1[C@@H](CO)O[C@@H](OC2[C@H](O[C@H](O)[C@H](O)[C@H]2O)CO)[C@H](O)[C@H]1O FYGDTMLNYKFZSV-URKRLVJHSA-N 0.000 claims description 2
- PHIQHXFUZVPYII-ZCFIWIBFSA-N (R)-carnitine Chemical compound C[N+](C)(C)C[C@H](O)CC([O-])=O PHIQHXFUZVPYII-ZCFIWIBFSA-N 0.000 claims description 2
- 241000383638 Allium nigrum Species 0.000 claims description 2
- 241000208223 Anacardiaceae Species 0.000 claims description 2
- 229920002498 Beta-glucan Polymers 0.000 claims description 2
- 241001626537 Bifidobacterium psychraerophilum Species 0.000 claims description 2
- GHOKWGTUZJEAQD-UHFFFAOYSA-N Chick antidermatitis factor Natural products OCC(C)(C)C(O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-UHFFFAOYSA-N 0.000 claims description 2
- 244000223760 Cinnamomum zeylanicum Species 0.000 claims description 2
- 108010016626 Dipeptides Proteins 0.000 claims description 2
- CITFYDYEWQIEPX-UHFFFAOYSA-N Flavanol Natural products O1C2=CC(OCC=C(C)C)=CC(O)=C2C(=O)C(O)C1C1=CC=C(O)C=C1 CITFYDYEWQIEPX-UHFFFAOYSA-N 0.000 claims description 2
- 235000002956 Gynostemma pentaphyllum Nutrition 0.000 claims description 2
- 240000006509 Gynostemma pentaphyllum Species 0.000 claims description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 claims description 2
- 241000925032 Lactobacillus harbinensis Species 0.000 claims description 2
- 241000186604 Lactobacillus reuteri Species 0.000 claims description 2
- 241000408747 Lepomis gibbosus Species 0.000 claims description 2
- 235000004431 Linum usitatissimum Nutrition 0.000 claims description 2
- 240000006240 Linum usitatissimum Species 0.000 claims description 2
- 102100024295 Maltase-glucoamylase Human genes 0.000 claims description 2
- 102000014171 Milk Proteins Human genes 0.000 claims description 2
- 108010011756 Milk Proteins Proteins 0.000 claims description 2
- CWEZAWNPTYBADX-UHFFFAOYSA-N Procyanidin Natural products OC1C(OC2C(O)C(Oc3c2c(O)cc(O)c3C4C(O)C(Oc5cc(O)cc(O)c45)c6ccc(O)c(O)c6)c7ccc(O)c(O)c7)c8c(O)cc(O)cc8OC1c9ccc(O)c(O)c9 CWEZAWNPTYBADX-UHFFFAOYSA-N 0.000 claims description 2
- ZVOLCUVKHLEPEV-UHFFFAOYSA-N Quercetagetin Natural products C1=C(O)C(O)=CC=C1C1=C(O)C(=O)C2=C(O)C(O)=C(O)C=C2O1 ZVOLCUVKHLEPEV-UHFFFAOYSA-N 0.000 claims description 2
- HWTZYBCRDDUBJY-UHFFFAOYSA-N Rhynchosin Natural products C1=C(O)C(O)=CC=C1C1=C(O)C(=O)C2=CC(O)=C(O)C=C2O1 HWTZYBCRDDUBJY-UHFFFAOYSA-N 0.000 claims description 2
- JZRWCGZRTZMZEH-UHFFFAOYSA-N Thiamine Natural products CC1=C(CCO)SC=[N+]1CC1=CN=C(C)N=C1N JZRWCGZRTZMZEH-UHFFFAOYSA-N 0.000 claims description 2
- 235000004240 Triticum spelta Nutrition 0.000 claims description 2
- 240000003834 Triticum spelta Species 0.000 claims description 2
- 229930003571 Vitamin B5 Natural products 0.000 claims description 2
- 108010028144 alpha-Glucosidases Proteins 0.000 claims description 2
- 235000014121 butter Nutrition 0.000 claims description 2
- FAPWYRCQGJNNSJ-UBKPKTQASA-L calcium D-pantothenic acid Chemical compound [Ca+2].OCC(C)(C)[C@@H](O)C(=O)NCCC([O-])=O.OCC(C)(C)[C@@H](O)C(=O)NCCC([O-])=O FAPWYRCQGJNNSJ-UBKPKTQASA-L 0.000 claims description 2
- 229960002079 calcium pantothenate Drugs 0.000 claims description 2
- 235000017663 capsaicin Nutrition 0.000 claims description 2
- 229960002504 capsaicin Drugs 0.000 claims description 2
- 235000020226 cashew nut Nutrition 0.000 claims description 2
- ADRVNXBAWSRFAJ-UHFFFAOYSA-N catechin Natural products OC1Cc2cc(O)cc(O)c2OC1c3ccc(O)c(O)c3 ADRVNXBAWSRFAJ-UHFFFAOYSA-N 0.000 claims description 2
- 235000005487 catechin Nutrition 0.000 claims description 2
- 239000000212 ceratonia siliqua l. fruit extract Substances 0.000 claims description 2
- 229940046374 chromium picolinate Drugs 0.000 claims description 2
- GJYSUGXFENSLOO-UHFFFAOYSA-N chromium;pyridine-2-carboxylic acid Chemical compound [Cr].OC(=O)C1=CC=CC=N1.OC(=O)C1=CC=CC=N1.OC(=O)C1=CC=CC=N1 GJYSUGXFENSLOO-UHFFFAOYSA-N 0.000 claims description 2
- 229950001002 cianidanol Drugs 0.000 claims description 2
- LPTRNLNOHUVQMS-UHFFFAOYSA-N epicatechin Natural products Cc1cc(O)cc2OC(C(O)Cc12)c1ccc(O)c(O)c1 LPTRNLNOHUVQMS-UHFFFAOYSA-N 0.000 claims description 2
- 235000012734 epicatechin Nutrition 0.000 claims description 2
- 150000002206 flavan-3-ols Chemical class 0.000 claims description 2
- 235000011987 flavanols Nutrition 0.000 claims description 2
- 229930003935 flavonoid Natural products 0.000 claims description 2
- 150000002215 flavonoids Chemical class 0.000 claims description 2
- 235000017173 flavonoids Nutrition 0.000 claims description 2
- HVQAJTFOCKOKIN-UHFFFAOYSA-N flavonol Natural products O1C2=CC=CC=C2C(=O)C(O)=C1C1=CC=CC=C1 HVQAJTFOCKOKIN-UHFFFAOYSA-N 0.000 claims description 2
- 150000002216 flavonol derivatives Chemical class 0.000 claims description 2
- 235000011957 flavonols Nutrition 0.000 claims description 2
- 235000004426 flaxseed Nutrition 0.000 claims description 2
- 229940094952 green tea extract Drugs 0.000 claims description 2
- 235000020688 green tea extract Nutrition 0.000 claims description 2
- 235000021539 instant coffee Nutrition 0.000 claims description 2
- 229910052742 iron Inorganic materials 0.000 claims description 2
- 229960003284 iron Drugs 0.000 claims description 2
- MWDZOUNAPSSOEL-UHFFFAOYSA-N kaempferol Natural products OC1=C(C(=O)c2cc(O)cc(O)c2O1)c3ccc(O)cc3 MWDZOUNAPSSOEL-UHFFFAOYSA-N 0.000 claims description 2
- 235000019136 lipoic acid Nutrition 0.000 claims description 2
- 235000021239 milk protein Nutrition 0.000 claims description 2
- 235000020733 paullinia cupana extract Nutrition 0.000 claims description 2
- 235000009048 phenolic acids Nutrition 0.000 claims description 2
- 150000007965 phenolic acids Chemical class 0.000 claims description 2
- 239000006041 probiotic Substances 0.000 claims description 2
- 235000018291 probiotics Nutrition 0.000 claims description 2
- 229920002414 procyanidin Polymers 0.000 claims description 2
- 235000020236 pumpkin seed Nutrition 0.000 claims description 2
- RADKZDMFGJYCBB-UHFFFAOYSA-N pyridoxal hydrochloride Natural products CC1=NC=C(CO)C(C=O)=C1O RADKZDMFGJYCBB-UHFFFAOYSA-N 0.000 claims description 2
- 235000005875 quercetin Nutrition 0.000 claims description 2
- 229960001285 quercetin Drugs 0.000 claims description 2
- 235000021286 stilbenes Nutrition 0.000 claims description 2
- 150000001629 stilbenes Chemical class 0.000 claims description 2
- 235000019157 thiamine Nutrition 0.000 claims description 2
- KYMBYSLLVAOCFI-UHFFFAOYSA-N thiamine Chemical compound CC1=C(CCO)SCN1CC1=CN=C(C)N=C1N KYMBYSLLVAOCFI-UHFFFAOYSA-N 0.000 claims description 2
- 229960003495 thiamine Drugs 0.000 claims description 2
- 239000011721 thiamine Substances 0.000 claims description 2
- 229960002663 thioctic acid Drugs 0.000 claims description 2
- 229940052016 turmeric extract Drugs 0.000 claims description 2
- 235000020240 turmeric extract Nutrition 0.000 claims description 2
- 239000008513 turmeric extract Substances 0.000 claims description 2
- 239000011675 vitamin B5 Substances 0.000 claims description 2
- 235000009492 vitamin B5 Nutrition 0.000 claims description 2
- 235000019158 vitamin B6 Nutrition 0.000 claims description 2
- 239000011726 vitamin B6 Substances 0.000 claims description 2
- 235000005282 vitamin D3 Nutrition 0.000 claims description 2
- 239000011647 vitamin D3 Substances 0.000 claims description 2
- QYSXJUFSXHHAJI-YRZJJWOYSA-N vitamin D3 Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C\C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-YRZJJWOYSA-N 0.000 claims description 2
- 229940011671 vitamin b6 Drugs 0.000 claims description 2
- 229940021056 vitamin d3 Drugs 0.000 claims description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-M L-ascorbate Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1[O-] CIWBSHSKHKDKBQ-JLAZNSOCSA-M 0.000 claims 2
- 241000186012 Bifidobacterium breve Species 0.000 claims 1
- 240000001046 Lactobacillus acidophilus Species 0.000 claims 1
- 240000007472 Leucaena leucocephala Species 0.000 claims 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 claims 1
- 235000017803 cinnamon Nutrition 0.000 claims 1
- 235000015872 dietary supplement Nutrition 0.000 abstract description 2
- 239000013589 supplement Substances 0.000 abstract description 2
- 241001312219 Amorphophallus konjac Species 0.000 abstract 1
- 239000000499 gel Substances 0.000 description 44
- 239000002253 acid Substances 0.000 description 41
- 238000009472 formulation Methods 0.000 description 36
- 239000006260 foam Substances 0.000 description 19
- 239000000047 product Substances 0.000 description 19
- 244000215068 Acacia senegal Species 0.000 description 18
- 229920000084 Gum arabic Polymers 0.000 description 18
- 239000004376 Sucralose Substances 0.000 description 18
- 239000004615 ingredient Substances 0.000 description 18
- 230000007935 neutral effect Effects 0.000 description 18
- BAQAVOSOZGMPRM-QBMZZYIRSA-N sucralose Chemical compound O[C@@H]1[C@@H](O)[C@@H](Cl)[C@@H](CO)O[C@@H]1O[C@@]1(CCl)[C@@H](O)[C@H](O)[C@@H](CCl)O1 BAQAVOSOZGMPRM-QBMZZYIRSA-N 0.000 description 18
- 235000019408 sucralose Nutrition 0.000 description 18
- 230000015572 biosynthetic process Effects 0.000 description 16
- 150000000994 L-ascorbates Chemical class 0.000 description 15
- 239000000205 acacia gum Substances 0.000 description 15
- 230000000052 comparative effect Effects 0.000 description 15
- CPLXHLVBOLITMK-UHFFFAOYSA-N Magnesium oxide Chemical compound [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 14
- 206010033307 Overweight Diseases 0.000 description 14
- 150000005846 sugar alcohols Chemical class 0.000 description 14
- OCUCCJIRFHNWBP-IYEMJOQQSA-L Copper gluconate Chemical class [Cu+2].OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O.OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O OCUCCJIRFHNWBP-IYEMJOQQSA-L 0.000 description 13
- 235000008504 concentrate Nutrition 0.000 description 13
- 229940086066 potassium hydrogencarbonate Drugs 0.000 description 13
- 239000004386 Erythritol Substances 0.000 description 12
- UNXHWFMMPAWVPI-UHFFFAOYSA-N Erythritol Natural products OCC(O)C(O)CO UNXHWFMMPAWVPI-UHFFFAOYSA-N 0.000 description 12
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 12
- 235000009499 Vanilla fragrans Nutrition 0.000 description 12
- 235000012036 Vanilla tahitensis Nutrition 0.000 description 12
- 235000019414 erythritol Nutrition 0.000 description 12
- UNXHWFMMPAWVPI-ZXZARUISSA-N erythritol Chemical compound OC[C@H](O)[C@H](O)CO UNXHWFMMPAWVPI-ZXZARUISSA-N 0.000 description 12
- 229940009714 erythritol Drugs 0.000 description 12
- 238000003756 stirring Methods 0.000 description 12
- 229940058023 trisodium citrate anhydrous Drugs 0.000 description 11
- 244000290333 Vanilla fragrans Species 0.000 description 10
- BITYAPCSNKJESK-UHFFFAOYSA-N potassiosodium Chemical compound [Na].[K] BITYAPCSNKJESK-UHFFFAOYSA-N 0.000 description 10
- 206010013911 Dysgeusia Diseases 0.000 description 9
- 235000013305 food Nutrition 0.000 description 9
- XLYOFNOQVPJJNP-ZSJDYOACSA-N heavy water Substances [2H]O[2H] XLYOFNOQVPJJNP-ZSJDYOACSA-N 0.000 description 9
- 229940083542 sodium Drugs 0.000 description 9
- CLJTZNIHUYFUMR-UHFFFAOYSA-M sodium;hydrogen carbonate;2-hydroxypropane-1,2,3-tricarboxylic acid Chemical compound [Na+].OC([O-])=O.OC(=O)CC(O)(C(O)=O)CC(O)=O CLJTZNIHUYFUMR-UHFFFAOYSA-M 0.000 description 9
- 150000007513 acids Chemical class 0.000 description 8
- 239000000654 additive Substances 0.000 description 8
- 230000000996 additive effect Effects 0.000 description 8
- 238000004090 dissolution Methods 0.000 description 8
- 230000035622 drinking Effects 0.000 description 8
- 239000007968 orange flavor Substances 0.000 description 8
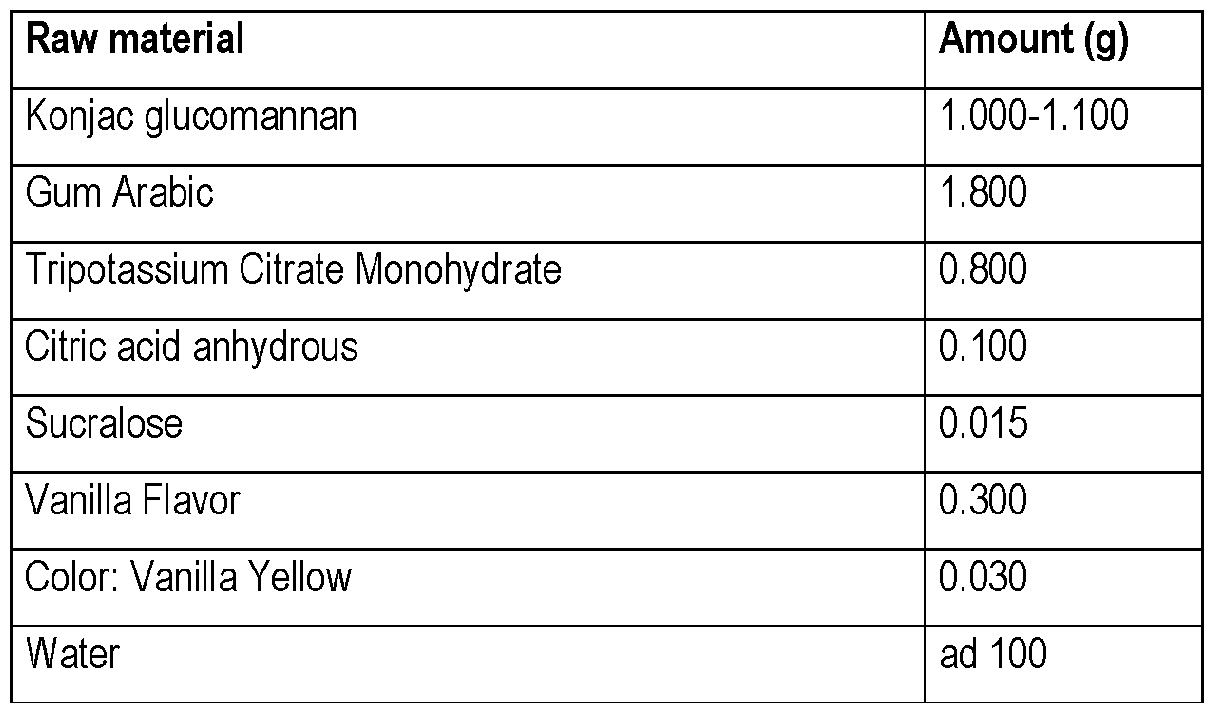
- 239000002994 raw material Substances 0.000 description 8
- 238000011161 development Methods 0.000 description 7
- 239000011521 glass Substances 0.000 description 7
- 235000019823 konjac gum Nutrition 0.000 description 7
- 239000000395 magnesium oxide Substances 0.000 description 7
- 239000004094 surface-active agent Substances 0.000 description 7
- RGHNJXZEOKUKBD-SQOUGZDYSA-N D-gluconic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 description 6
- 244000235659 Rubus idaeus Species 0.000 description 6
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 6
- 235000019625 fat content Nutrition 0.000 description 6
- 239000000835 fiber Substances 0.000 description 6
- 235000013312 flour Nutrition 0.000 description 6
- XMGQYMWWDOXHJM-UHFFFAOYSA-N limonene Chemical compound CC(=C)C1CCC(C)=CC1 XMGQYMWWDOXHJM-UHFFFAOYSA-N 0.000 description 6
- 239000002245 particle Substances 0.000 description 6
- 235000011007 phosphoric acid Nutrition 0.000 description 6
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 5
- AUNGANRZJHBGPY-SCRDCRAPSA-N Riboflavin Chemical compound OC[C@@H](O)[C@@H](O)[C@@H](O)CN1C=2C=C(C)C(C)=CC=2N=C2C1=NC(=O)NC2=O AUNGANRZJHBGPY-SCRDCRAPSA-N 0.000 description 5
- 244000078534 Vaccinium myrtillus Species 0.000 description 5
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 5
- 150000003841 chloride salts Chemical class 0.000 description 5
- 230000037406 food intake Effects 0.000 description 5
- 210000001035 gastrointestinal tract Anatomy 0.000 description 5
- 230000036541 health Effects 0.000 description 5
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 5
- 229940038773 trisodium citrate Drugs 0.000 description 5
- 239000008371 vanilla flavor Substances 0.000 description 5
- 235000006491 Acacia senegal Nutrition 0.000 description 4
- 241000196324 Embryophyta Species 0.000 description 4
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 4
- 229920002472 Starch Polymers 0.000 description 4
- 235000003095 Vaccinium corymbosum Nutrition 0.000 description 4
- 235000017537 Vaccinium myrtillus Nutrition 0.000 description 4
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 4
- 239000002585 base Substances 0.000 description 4
- 235000021014 blueberries Nutrition 0.000 description 4
- 238000005516 engineering process Methods 0.000 description 4
- 239000003925 fat Substances 0.000 description 4
- 229960001031 glucose Drugs 0.000 description 4
- FYQGBXGJFWXIPP-UHFFFAOYSA-N hydroprene Chemical compound CCOC(=O)C=C(C)C=CCC(C)CCCC(C)C FYQGBXGJFWXIPP-UHFFFAOYSA-N 0.000 description 4
- 229960002635 potassium citrate Drugs 0.000 description 4
- 235000011082 potassium citrates Nutrition 0.000 description 4
- 235000021013 raspberries Nutrition 0.000 description 4
- 235000019698 starch Nutrition 0.000 description 4
- 239000008107 starch Substances 0.000 description 4
- 235000007425 Aronia melanocarpa Nutrition 0.000 description 3
- 240000005662 Aronia melanocarpa Species 0.000 description 3
- AUNGANRZJHBGPY-UHFFFAOYSA-N D-Lyxoflavin Natural products OCC(O)C(O)C(O)CN1C=2C=C(C)C(C)=CC=2N=C2C1=NC(=O)NC2=O AUNGANRZJHBGPY-UHFFFAOYSA-N 0.000 description 3
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Natural products OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 3
- 244000228451 Stevia rebaudiana Species 0.000 description 3
- LUKBXSAWLPMMSZ-OWOJBTEDSA-N Trans-resveratrol Chemical compound C1=CC(O)=CC=C1\C=C\C1=CC(O)=CC(O)=C1 LUKBXSAWLPMMSZ-OWOJBTEDSA-N 0.000 description 3
- 150000001413 amino acids Chemical class 0.000 description 3
- 230000009286 beneficial effect Effects 0.000 description 3
- 239000008373 coffee flavor Substances 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 239000006071 cream Substances 0.000 description 3
- 238000005187 foaming Methods 0.000 description 3
- 239000000174 gluconic acid Substances 0.000 description 3
- 235000012208 gluconic acid Nutrition 0.000 description 3
- 239000008103 glucose Substances 0.000 description 3
- 235000019822 konjac glucomannane Nutrition 0.000 description 3
- 150000003893 lactate salts Chemical class 0.000 description 3
- 239000008101 lactose Substances 0.000 description 3
- 229960001375 lactose Drugs 0.000 description 3
- 239000002417 nutraceutical Substances 0.000 description 3
- 235000021436 nutraceutical agent Nutrition 0.000 description 3
- HELXLJCILKEWJH-NCGAPWICSA-N rebaudioside A Chemical compound O([C@H]1[C@H](O)[C@@H](CO)O[C@H]([C@@H]1O[C@H]1[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O1)O)O[C@]12C(=C)C[C@@]3(C1)CC[C@@H]1[C@@](C)(CCC[C@]1([C@@H]3CC2)C)C(=O)O[C@H]1[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O1)O)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O HELXLJCILKEWJH-NCGAPWICSA-N 0.000 description 3
- 229960002477 riboflavin Drugs 0.000 description 3
- 230000036186 satiety Effects 0.000 description 3
- 235000019627 satiety Nutrition 0.000 description 3
- 239000008247 solid mixture Substances 0.000 description 3
- 150000003892 tartrate salts Chemical class 0.000 description 3
- GVJHHUAWPYXKBD-UHFFFAOYSA-N (±)-α-Tocopherol Chemical compound OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 2
- FPIPGXGPPPQFEQ-UHFFFAOYSA-N 13-cis retinol Natural products OCC=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-UHFFFAOYSA-N 0.000 description 2
- 241000251468 Actinopterygii Species 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-M Aminoacetate Chemical class NCC([O-])=O DHMQDGOQFOQNFH-UHFFFAOYSA-M 0.000 description 2
- 239000004382 Amylase Substances 0.000 description 2
- 102000013142 Amylases Human genes 0.000 description 2
- 108010065511 Amylases Proteins 0.000 description 2
- 240000006063 Averrhoa carambola Species 0.000 description 2
- 235000010082 Averrhoa carambola Nutrition 0.000 description 2
- 241000167854 Bourreria succulenta Species 0.000 description 2
- 240000006432 Carica papaya Species 0.000 description 2
- 235000009467 Carica papaya Nutrition 0.000 description 2
- 241000207199 Citrus Species 0.000 description 2
- 235000005979 Citrus limon Nutrition 0.000 description 2
- 244000131522 Citrus pyriformis Species 0.000 description 2
- 241001672694 Citrus reticulata Species 0.000 description 2
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical class OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 2
- WQZGKKKJIJFFOK-QTVWNMPRSA-N D-mannopyranose Chemical compound OC[C@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-QTVWNMPRSA-N 0.000 description 2
- 206010056465 Food craving Diseases 0.000 description 2
- 244000070406 Malus silvestris Species 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 240000005561 Musa balbisiana Species 0.000 description 2
- XVNDVDJDPPYFKN-UHFFFAOYSA-K O.C(CC(O)(C(=O)[O-])CC(=O)[O-])(=O)[O-].[K+].[K+].[K+].C(CC(O)(C(=O)O)CC(=O)O)(=O)O Chemical compound O.C(CC(O)(C(=O)[O-])CC(=O)[O-])(=O)[O-].[K+].[K+].[K+].C(CC(O)(C(=O)O)CC(=O)O)(=O)O XVNDVDJDPPYFKN-UHFFFAOYSA-K 0.000 description 2
- 235000010582 Pisum sativum Nutrition 0.000 description 2
- 240000004713 Pisum sativum Species 0.000 description 2
- 235000012377 Salvia columbariae var. columbariae Nutrition 0.000 description 2
- 240000005481 Salvia hispanica Species 0.000 description 2
- 235000001498 Salvia hispanica Nutrition 0.000 description 2
- 235000021307 Triticum Nutrition 0.000 description 2
- 244000098338 Triticum aestivum Species 0.000 description 2
- 244000263375 Vanilla tahitensis Species 0.000 description 2
- FPIPGXGPPPQFEQ-BOOMUCAASA-N Vitamin A Natural products OC/C=C(/C)\C=C\C=C(\C)/C=C/C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-BOOMUCAASA-N 0.000 description 2
- 235000014787 Vitis vinifera Nutrition 0.000 description 2
- 240000006365 Vitis vinifera Species 0.000 description 2
- 235000019631 acid taste sensations Nutrition 0.000 description 2
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 2
- FPIPGXGPPPQFEQ-OVSJKPMPSA-N all-trans-retinol Chemical compound OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-OVSJKPMPSA-N 0.000 description 2
- 235000019418 amylase Nutrition 0.000 description 2
- 244000052616 bacterial pathogen Species 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 235000021324 borage oil Nutrition 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 229940077731 carbohydrate nutrients Drugs 0.000 description 2
- 150000001720 carbohydrates Chemical class 0.000 description 2
- 235000014633 carbohydrates Nutrition 0.000 description 2
- UUUDMEBRZTWNAO-UHFFFAOYSA-N carbonic acid;2-hydroxypropane-1,2,3-tricarboxylic acid Chemical compound OC(O)=O.OC(=O)CC(O)(C(O)=O)CC(O)=O UUUDMEBRZTWNAO-UHFFFAOYSA-N 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 239000005018 casein Substances 0.000 description 2
- BECPQYXYKAMYBN-UHFFFAOYSA-N casein, tech. Chemical compound NCCCCC(C(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(CC(C)C)N=C(O)C(CCC(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(C(C)O)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(COP(O)(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(N)CC1=CC=CC=C1 BECPQYXYKAMYBN-UHFFFAOYSA-N 0.000 description 2
- 235000021240 caseins Nutrition 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 235000019693 cherries Nutrition 0.000 description 2
- 239000007910 chewable tablet Substances 0.000 description 2
- 229940068682 chewable tablet Drugs 0.000 description 2
- 235000014167 chia Nutrition 0.000 description 2
- 229940001468 citrate Drugs 0.000 description 2
- 229940013145 citric acid / tartaric acid Drugs 0.000 description 2
- 150000001860 citric acid derivatives Chemical class 0.000 description 2
- 235000020971 citrus fruits Nutrition 0.000 description 2
- 210000001072 colon Anatomy 0.000 description 2
- 235000009508 confectionery Nutrition 0.000 description 2
- 235000013601 eggs Nutrition 0.000 description 2
- 235000008524 evening primrose extract Nutrition 0.000 description 2
- 239000010475 evening primrose oil Substances 0.000 description 2
- 229940089020 evening primrose oil Drugs 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- YMTINGFKWWXKFG-UHFFFAOYSA-N fenofibrate Chemical compound C1=CC(OC(C)(C)C(=O)OC(C)C)=CC=C1C(=O)C1=CC=C(Cl)C=C1 YMTINGFKWWXKFG-UHFFFAOYSA-N 0.000 description 2
- 235000019688 fish Nutrition 0.000 description 2
- 235000021323 fish oil Nutrition 0.000 description 2
- 230000009969 flowable effect Effects 0.000 description 2
- 235000013373 food additive Nutrition 0.000 description 2
- 239000002778 food additive Substances 0.000 description 2
- 230000014509 gene expression Effects 0.000 description 2
- 235000021472 generally recognized as safe Nutrition 0.000 description 2
- 229910052500 inorganic mineral Inorganic materials 0.000 description 2
- 150000002596 lactones Chemical class 0.000 description 2
- 125000000311 mannosyl group Chemical group C1([C@@H](O)[C@@H](O)[C@H](O)[C@H](O1)CO)* 0.000 description 2
- 235000012054 meals Nutrition 0.000 description 2
- 230000004060 metabolic process Effects 0.000 description 2
- 235000021243 milk fat Nutrition 0.000 description 2
- 235000010755 mineral Nutrition 0.000 description 2
- 239000011707 mineral Substances 0.000 description 2
- 150000007522 mineralic acids Chemical class 0.000 description 2
- 235000008390 olive oil Nutrition 0.000 description 2
- 239000004006 olive oil Substances 0.000 description 2
- 238000004806 packaging method and process Methods 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- AKEKKCGPLHMFCI-UHFFFAOYSA-L potassium sodium hydrogen carbonate Chemical compound [Na+].[K+].OC([O-])=O.OC([O-])=O AKEKKCGPLHMFCI-UHFFFAOYSA-L 0.000 description 2
- 244000144977 poultry Species 0.000 description 2
- 235000013594 poultry meat Nutrition 0.000 description 2
- 230000001737 promoting effect Effects 0.000 description 2
- 239000003642 reactive oxygen metabolite Substances 0.000 description 2
- 238000012827 research and development Methods 0.000 description 2
- 235000019192 riboflavin Nutrition 0.000 description 2
- 239000002151 riboflavin Substances 0.000 description 2
- 238000004088 simulation Methods 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- 230000008719 thickening Effects 0.000 description 2
- SOBHUZYZLFQYFK-UHFFFAOYSA-K trisodium;hydroxy-[[phosphonatomethyl(phosphonomethyl)amino]methyl]phosphinate Chemical compound [Na+].[Na+].[Na+].OP(O)(=O)CN(CP(O)([O-])=O)CP([O-])([O-])=O SOBHUZYZLFQYFK-UHFFFAOYSA-K 0.000 description 2
- 235000015112 vegetable and seed oil Nutrition 0.000 description 2
- 235000019155 vitamin A Nutrition 0.000 description 2
- 239000011719 vitamin A Substances 0.000 description 2
- 150000003710 vitamin D derivatives Chemical class 0.000 description 2
- 229940045997 vitamin a Drugs 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- SPFMQWBKVUQXJV-BTVCFUMJSA-N (2r,3s,4r,5r)-2,3,4,5,6-pentahydroxyhexanal;hydrate Chemical compound O.OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O SPFMQWBKVUQXJV-BTVCFUMJSA-N 0.000 description 1
- WVXRAFOPTSTNLL-NKWVEPMBSA-N 2',3'-dideoxyadenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@H]1CC[C@@H](CO)O1 WVXRAFOPTSTNLL-NKWVEPMBSA-N 0.000 description 1
- PYMYPHUHKUWMLA-UHFFFAOYSA-N 2,3,4,5-tetrahydroxypentanal Chemical compound OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 description 1
- MIDXCONKKJTLDX-UHFFFAOYSA-N 3,5-dimethylcyclopentane-1,2-dione Chemical compound CC1CC(C)C(=O)C1=O MIDXCONKKJTLDX-UHFFFAOYSA-N 0.000 description 1
- CYDQOEWLBCCFJZ-UHFFFAOYSA-N 4-(4-fluorophenyl)oxane-4-carboxylic acid Chemical compound C=1C=C(F)C=CC=1C1(C(=O)O)CCOCC1 CYDQOEWLBCCFJZ-UHFFFAOYSA-N 0.000 description 1
- PXRKCOCTEMYUEG-UHFFFAOYSA-N 5-aminoisoindole-1,3-dione Chemical compound NC1=CC=C2C(=O)NC(=O)C2=C1 PXRKCOCTEMYUEG-UHFFFAOYSA-N 0.000 description 1
- PVXPPJIGRGXGCY-TZLCEDOOSA-N 6-O-alpha-D-glucopyranosyl-D-fructofuranose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)C(O)(CO)O1 PVXPPJIGRGXGCY-TZLCEDOOSA-N 0.000 description 1
- 229920000107 Acetylated distarch adipate Polymers 0.000 description 1
- 244000298715 Actinidia chinensis Species 0.000 description 1
- 235000009434 Actinidia chinensis Nutrition 0.000 description 1
- 235000009436 Actinidia deliciosa Nutrition 0.000 description 1
- WSVLPVUVIUVCRA-KPKNDVKVSA-N Alpha-lactose monohydrate Chemical compound O.O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O WSVLPVUVIUVCRA-KPKNDVKVSA-N 0.000 description 1
- 235000009027 Amelanchier alnifolia Nutrition 0.000 description 1
- 240000003278 Amelanchier canadensis Species 0.000 description 1
- 235000007087 Amelanchier canadensis Nutrition 0.000 description 1
- 235000001428 Amelanchier x grandiflora Nutrition 0.000 description 1
- 235000007119 Ananas comosus Nutrition 0.000 description 1
- 244000099147 Ananas comosus Species 0.000 description 1
- 244000021317 Annona cherimola Species 0.000 description 1
- 235000007747 Annona muricata Nutrition 0.000 description 1
- 240000004749 Annona muricata Species 0.000 description 1
- 241001444063 Aronia Species 0.000 description 1
- 240000004161 Artocarpus altilis Species 0.000 description 1
- 235000002672 Artocarpus altilis Nutrition 0.000 description 1
- 235000008725 Artocarpus heterophyllus Nutrition 0.000 description 1
- 244000025352 Artocarpus heterophyllus Species 0.000 description 1
- 235000006264 Asimina triloba Nutrition 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 235000004936 Bromus mango Nutrition 0.000 description 1
- 241000195940 Bryophyta Species 0.000 description 1
- FERIUCNNQQJTOY-UHFFFAOYSA-M Butyrate Chemical compound CCCC([O-])=O FERIUCNNQQJTOY-UHFFFAOYSA-M 0.000 description 1
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Natural products CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 235000017350 Carissa Nutrition 0.000 description 1
- 240000004927 Carissa macrocarpa Species 0.000 description 1
- 235000009241 Cereus peruvianus Nutrition 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 244000241235 Citrullus lanatus Species 0.000 description 1
- 235000012828 Citrullus lanatus var citroides Nutrition 0.000 description 1
- 235000008733 Citrus aurantifolia Nutrition 0.000 description 1
- 235000002320 Citrus hystrix Nutrition 0.000 description 1
- 240000000981 Citrus hystrix Species 0.000 description 1
- 244000175448 Citrus madurensis Species 0.000 description 1
- 235000001759 Citrus maxima Nutrition 0.000 description 1
- 244000276331 Citrus maxima Species 0.000 description 1
- 241000333459 Citrus x tangelo Species 0.000 description 1
- 235000002187 Coffea robusta Nutrition 0.000 description 1
- 235000017788 Cydonia oblonga Nutrition 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- LKDRXBCSQODPBY-JDJSBBGDSA-N D-allulose Chemical compound OCC1(O)OC[C@@H](O)[C@@H](O)[C@H]1O LKDRXBCSQODPBY-JDJSBBGDSA-N 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 240000001008 Dimocarpus longan Species 0.000 description 1
- 235000006025 Durio zibethinus Nutrition 0.000 description 1
- 240000000716 Durio zibethinus Species 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 235000009008 Eriobotrya japonica Nutrition 0.000 description 1
- 244000061508 Eriobotrya japonica Species 0.000 description 1
- 235000000235 Euphoria longan Nutrition 0.000 description 1
- 244000233576 Feijoa sellowiana Species 0.000 description 1
- 235000012068 Feijoa sellowiana Nutrition 0.000 description 1
- 235000017317 Fortunella Nutrition 0.000 description 1
- 240000009088 Fragaria x ananassa Species 0.000 description 1
- 235000017048 Garcinia mangostana Nutrition 0.000 description 1
- 240000006053 Garcinia mangostana Species 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 229920002148 Gellan gum Polymers 0.000 description 1
- DTHNMHAUYICORS-KTKZVXAJSA-N Glucagon-like peptide 1 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1N=CNC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 DTHNMHAUYICORS-KTKZVXAJSA-N 0.000 description 1
- 101800000224 Glucagon-like peptide 1 Proteins 0.000 description 1
- 241001091440 Grossulariaceae Species 0.000 description 1
- 229920002907 Guar gum Polymers 0.000 description 1
- 101001012669 Homo sapiens Melanoma inhibitory activity protein 2 Proteins 0.000 description 1
- 229920003012 Hydroxypropyl distarch phosphate Polymers 0.000 description 1
- 240000009012 Hylocereus polyrhizus Species 0.000 description 1
- 235000001680 Hylocereus polyrhizus Nutrition 0.000 description 1
- 235000018481 Hylocereus undatus Nutrition 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- 240000003589 Impatiens walleriana Species 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 241001195326 Lactobacillus hordei Species 0.000 description 1
- 240000006024 Lactobacillus plantarum Species 0.000 description 1
- 235000009770 Lemaireocereus queretaroensis Nutrition 0.000 description 1
- 244000108452 Litchi chinensis Species 0.000 description 1
- 229920000161 Locust bean gum Polymers 0.000 description 1
- 241000219745 Lupinus Species 0.000 description 1
- 240000003394 Malpighia glabra Species 0.000 description 1
- 235000014837 Malpighia glabra Nutrition 0.000 description 1
- 235000005087 Malus prunifolia Nutrition 0.000 description 1
- 235000011430 Malus pumila Nutrition 0.000 description 1
- 235000015103 Malus silvestris Nutrition 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 240000007228 Mangifera indica Species 0.000 description 1
- 235000014826 Mangifera indica Nutrition 0.000 description 1
- 244000061354 Manilkara achras Species 0.000 description 1
- 235000011339 Manilkara zapota Nutrition 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 102100029778 Melanoma inhibitory activity protein 2 Human genes 0.000 description 1
- 241000736262 Microbiota Species 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 241000218231 Moraceae Species 0.000 description 1
- 235000008708 Morus alba Nutrition 0.000 description 1
- 235000003805 Musa ABB Group Nutrition 0.000 description 1
- 235000018290 Musa x paradisiaca Nutrition 0.000 description 1
- 235000016392 Myrciaria paraensis Nutrition 0.000 description 1
- 244000002791 Myrciaria paraensis Species 0.000 description 1
- 244000183331 Nephelium lappaceum Species 0.000 description 1
- 235000015742 Nephelium litchi Nutrition 0.000 description 1
- 208000008589 Obesity Diseases 0.000 description 1
- 240000001439 Opuntia Species 0.000 description 1
- 235000013389 Opuntia humifusa var. humifusa Nutrition 0.000 description 1
- 235000000370 Passiflora edulis Nutrition 0.000 description 1
- 244000288157 Passiflora edulis Species 0.000 description 1
- 235000008673 Persea americana Nutrition 0.000 description 1
- 244000025272 Persea americana Species 0.000 description 1
- 235000009230 Physalis pubescens Nutrition 0.000 description 1
- 235000002491 Physalis viscosa Nutrition 0.000 description 1
- 240000001558 Physalis viscosa Species 0.000 description 1
- 235000015266 Plantago major Nutrition 0.000 description 1
- 102100040918 Pro-glucagon Human genes 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- HDSBZMRLPLPFLQ-UHFFFAOYSA-N Propylene glycol alginate Chemical compound OC1C(O)C(OC)OC(C(O)=O)C1OC1C(O)C(O)C(C)C(C(=O)OCC(C)O)O1 HDSBZMRLPLPFLQ-UHFFFAOYSA-N 0.000 description 1
- 235000009827 Prunus armeniaca Nutrition 0.000 description 1
- 244000018633 Prunus armeniaca Species 0.000 description 1
- 235000005805 Prunus cerasus Nutrition 0.000 description 1
- 240000002878 Prunus cerasus Species 0.000 description 1
- 240000005809 Prunus persica Species 0.000 description 1
- 235000006029 Prunus persica var nucipersica Nutrition 0.000 description 1
- 235000006040 Prunus persica var persica Nutrition 0.000 description 1
- 244000017714 Prunus persica var. nucipersica Species 0.000 description 1
- 235000009226 Prunus puddum Nutrition 0.000 description 1
- 235000014441 Prunus serotina Nutrition 0.000 description 1
- 241000508269 Psidium Species 0.000 description 1
- 235000014360 Punica granatum Nutrition 0.000 description 1
- 244000294611 Punica granatum Species 0.000 description 1
- 235000014443 Pyrus communis Nutrition 0.000 description 1
- 240000001987 Pyrus communis Species 0.000 description 1
- 235000009411 Rheum rhabarbarum Nutrition 0.000 description 1
- 244000299790 Rheum rhabarbarum Species 0.000 description 1
- 235000001537 Ribes X gardonianum Nutrition 0.000 description 1
- 235000001535 Ribes X utile Nutrition 0.000 description 1
- 235000002357 Ribes grossularia Nutrition 0.000 description 1
- 235000016954 Ribes hudsonianum Nutrition 0.000 description 1
- 240000001890 Ribes hudsonianum Species 0.000 description 1
- 235000001466 Ribes nigrum Nutrition 0.000 description 1
- 235000016919 Ribes petraeum Nutrition 0.000 description 1
- 244000281247 Ribes rubrum Species 0.000 description 1
- 235000002355 Ribes spicatum Nutrition 0.000 description 1
- 241001412173 Rubus canescens Species 0.000 description 1
- 235000016554 Rubus chamaemorus Nutrition 0.000 description 1
- 240000006831 Rubus chamaemorus Species 0.000 description 1
- 235000017848 Rubus fruticosus Nutrition 0.000 description 1
- 235000011034 Rubus glaucus Nutrition 0.000 description 1
- 241000870397 Rubus hybrid cultivar Species 0.000 description 1
- 235000009122 Rubus idaeus Nutrition 0.000 description 1
- 235000009184 Spondias indica Nutrition 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- 235000019486 Sunflower oil Nutrition 0.000 description 1
- 235000004298 Tamarindus indica Nutrition 0.000 description 1
- 240000004584 Tamarindus indica Species 0.000 description 1
- 240000007313 Tilia cordata Species 0.000 description 1
- 235000011941 Tilia x europaea Nutrition 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- 240000001717 Vaccinium macrocarpon Species 0.000 description 1
- 235000017606 Vaccinium vitis idaea Nutrition 0.000 description 1
- 244000077923 Vaccinium vitis idaea Species 0.000 description 1
- 229930003779 Vitamin B12 Natural products 0.000 description 1
- 229930003471 Vitamin B2 Natural products 0.000 description 1
- 229930003427 Vitamin E Natural products 0.000 description 1
- 229930003448 Vitamin K Natural products 0.000 description 1
- 235000009754 Vitis X bourquina Nutrition 0.000 description 1
- 235000012333 Vitis X labruscana Nutrition 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 238000005054 agglomeration Methods 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 229910000288 alkali metal carbonate Inorganic materials 0.000 description 1
- 150000008041 alkali metal carbonates Chemical class 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 235000021407 appetite control Nutrition 0.000 description 1
- 239000010242 baoji Substances 0.000 description 1
- 235000021028 berry Nutrition 0.000 description 1
- WQZGKKKJIJFFOK-RWOPYEJCSA-N beta-D-mannose Chemical compound OC[C@H]1O[C@@H](O)[C@@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-RWOPYEJCSA-N 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 235000021029 blackberry Nutrition 0.000 description 1
- 235000021152 breakfast Nutrition 0.000 description 1
- 235000020299 breve Nutrition 0.000 description 1
- LLSDKQJKOVVTOJ-UHFFFAOYSA-L calcium chloride dihydrate Chemical compound O.O.[Cl-].[Cl-].[Ca+2] LLSDKQJKOVVTOJ-UHFFFAOYSA-L 0.000 description 1
- 229940052299 calcium chloride dihydrate Drugs 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 235000013736 caramel Nutrition 0.000 description 1
- 150000005323 carbonate salts Chemical class 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 235000010418 carrageenan Nutrition 0.000 description 1
- 229920001525 carrageenan Polymers 0.000 description 1
- 235000012547 cherimoya Nutrition 0.000 description 1
- 150000001844 chromium Chemical class 0.000 description 1
- AGVAZMGAQJOSFJ-WZHZPDAFSA-M cobalt(2+);[(2r,3s,4r,5s)-5-(5,6-dimethylbenzimidazol-1-yl)-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl] [(2r)-1-[3-[(1r,2r,3r,4z,7s,9z,12s,13s,14z,17s,18s,19r)-2,13,18-tris(2-amino-2-oxoethyl)-7,12,17-tris(3-amino-3-oxopropyl)-3,5,8,8,13,15,18,19-octamethyl-2 Chemical compound [Co+2].N#[C-].[N-]([C@@H]1[C@H](CC(N)=O)[C@@]2(C)CCC(=O)NC[C@@H](C)OP(O)(=O)O[C@H]3[C@H]([C@H](O[C@@H]3CO)N3C4=CC(C)=C(C)C=C4N=C3)O)\C2=C(C)/C([C@H](C\2(C)C)CCC(N)=O)=N/C/2=C\C([C@H]([C@@]/2(CC(N)=O)C)CCC(N)=O)=N\C\2=C(C)/C2=N[C@]1(C)[C@@](C)(CC(N)=O)[C@@H]2CCC(N)=O AGVAZMGAQJOSFJ-WZHZPDAFSA-M 0.000 description 1
- 235000020247 cow milk Nutrition 0.000 description 1
- 235000021019 cranberries Nutrition 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 229960000673 dextrose monohydrate Drugs 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 235000013325 dietary fiber Nutrition 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 235000013804 distarch phosphate Nutrition 0.000 description 1
- 239000001245 distarch phosphate Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 238000004945 emulsification Methods 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 235000021035 energy-restricted diet Nutrition 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 239000008369 fruit flavor Substances 0.000 description 1
- 235000021255 galacto-oligosaccharides Nutrition 0.000 description 1
- 150000003271 galactooligosaccharides Chemical class 0.000 description 1
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 235000010492 gellan gum Nutrition 0.000 description 1
- 239000000216 gellan gum Substances 0.000 description 1
- 125000002791 glucosyl group Chemical group C1([C@H](O)[C@@H](O)[C@H](O)[C@H](O1)CO)* 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 229960005150 glycerol Drugs 0.000 description 1
- 235000015810 grayleaf red raspberry Nutrition 0.000 description 1
- 239000000665 guar gum Substances 0.000 description 1
- 235000010417 guar gum Nutrition 0.000 description 1
- 229960002154 guar gum Drugs 0.000 description 1
- 244000005709 gut microbiome Species 0.000 description 1
- 239000008240 homogeneous mixture Substances 0.000 description 1
- 235000020603 homogenised milk Nutrition 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 239000000416 hydrocolloid Substances 0.000 description 1
- DVROLKBAWTYHHD-UHFFFAOYSA-N hydroxy propyl distarch phosphate Chemical compound OC1C(O)C(OC)OC(CO)C1OC(O)CCOC1C(OC2C(C(O)C(OC3C(C(OP(O)(=O)OC4C(C(O)C(OC)OC4CO)O)C(C)OC3CO)O)OC2COC2C(C(O)C(OC)C(CO)O2)O)O)OC(CO)C(OC)C1O DVROLKBAWTYHHD-UHFFFAOYSA-N 0.000 description 1
- 235000013825 hydroxy propyl distarch phosphate Nutrition 0.000 description 1
- 239000001310 hydroxy propyl distarch phosphate Substances 0.000 description 1
- 235000013828 hydroxypropyl starch Nutrition 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 229920000831 ionic polymer Polymers 0.000 description 1
- 235000015110 jellies Nutrition 0.000 description 1
- 239000008274 jelly Substances 0.000 description 1
- 239000000832 lactitol Substances 0.000 description 1
- VQHSOMBJVWLPSR-JVCRWLNRSA-N lactitol Chemical compound OC[C@H](O)[C@@H](O)[C@@H]([C@H](O)CO)O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O VQHSOMBJVWLPSR-JVCRWLNRSA-N 0.000 description 1
- 235000010448 lactitol Nutrition 0.000 description 1
- 229960003451 lactitol Drugs 0.000 description 1
- 229960001021 lactose monohydrate Drugs 0.000 description 1
- 235000020307 latte macchiato Nutrition 0.000 description 1
- 239000004571 lime Substances 0.000 description 1
- 235000010420 locust bean gum Nutrition 0.000 description 1
- 239000000711 locust bean gum Substances 0.000 description 1
- 235000004213 low-fat Nutrition 0.000 description 1
- 229940074358 magnesium ascorbate Drugs 0.000 description 1
- 239000001755 magnesium gluconate Substances 0.000 description 1
- 235000015778 magnesium gluconate Nutrition 0.000 description 1
- 229960003035 magnesium gluconate Drugs 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- VQHSOMBJVWLPSR-WUJBLJFYSA-N maltitol Chemical compound OC[C@H](O)[C@@H](O)[C@@H]([C@H](O)CO)O[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O VQHSOMBJVWLPSR-WUJBLJFYSA-N 0.000 description 1
- 235000010449 maltitol Nutrition 0.000 description 1
- 239000000845 maltitol Substances 0.000 description 1
- 229940035436 maltitol Drugs 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 235000020124 milk-based beverage Nutrition 0.000 description 1
- 235000011929 mousse Nutrition 0.000 description 1
- 235000019659 mouth feeling Nutrition 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 235000020824 obesity Nutrition 0.000 description 1
- 229920001542 oligosaccharide Polymers 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 239000000123 paper Substances 0.000 description 1
- 239000001814 pectin Substances 0.000 description 1
- 229920001277 pectin Polymers 0.000 description 1
- 235000010987 pectin Nutrition 0.000 description 1
- SHUZOJHMOBOZST-UHFFFAOYSA-N phylloquinone Natural products CC(C)CCCCC(C)CCC(C)CCCC(=CCC1=C(C)C(=O)c2ccccc2C1=O)C SHUZOJHMOBOZST-UHFFFAOYSA-N 0.000 description 1
- 229940023488 pill Drugs 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229910052939 potassium sulfate Inorganic materials 0.000 description 1
- 235000011151 potassium sulphates Nutrition 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 235000010409 propane-1,2-diol alginate Nutrition 0.000 description 1
- 239000000770 propane-1,2-diol alginate Substances 0.000 description 1
- 239000003531 protein hydrolysate Substances 0.000 description 1
- 235000021075 protein intake Nutrition 0.000 description 1
- 235000007861 rambutan Nutrition 0.000 description 1
- 229920005604 random copolymer Polymers 0.000 description 1
- 235000021580 ready-to-drink beverage Nutrition 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 235000021391 short chain fatty acids Nutrition 0.000 description 1
- 150000004666 short chain fatty acids Chemical class 0.000 description 1
- 229940001593 sodium carbonate Drugs 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000011122 softwood Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 235000013826 starch sodium octenyl succinate Nutrition 0.000 description 1
- 239000001334 starch sodium octenyl succinate Substances 0.000 description 1
- 235000021012 strawberries Nutrition 0.000 description 1
- 238000005728 strengthening Methods 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 229940005741 sunflower lecithin Drugs 0.000 description 1
- 239000002600 sunflower oil Substances 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 239000008399 tap water Substances 0.000 description 1
- 235000020679 tap water Nutrition 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 235000019163 vitamin B12 Nutrition 0.000 description 1
- 239000011715 vitamin B12 Substances 0.000 description 1
- 235000019164 vitamin B2 Nutrition 0.000 description 1
- 239000011716 vitamin B2 Substances 0.000 description 1
- 235000020798 vitamin C status Nutrition 0.000 description 1
- 235000019165 vitamin E Nutrition 0.000 description 1
- 229940046009 vitamin E Drugs 0.000 description 1
- 239000011709 vitamin E Substances 0.000 description 1
- 235000019168 vitamin K Nutrition 0.000 description 1
- 239000011712 vitamin K Substances 0.000 description 1
- 150000003721 vitamin K derivatives Chemical class 0.000 description 1
- 229940046010 vitamin k Drugs 0.000 description 1
- 229920001285 xanthan gum Polymers 0.000 description 1
- 235000010493 xanthan gum Nutrition 0.000 description 1
- 239000000230 xanthan gum Substances 0.000 description 1
- 229940082509 xanthan gum Drugs 0.000 description 1
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 1
- 239000001060 yellow colorant Substances 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L29/00—Foods or foodstuffs containing additives; Preparation or treatment thereof
- A23L29/20—Foods or foodstuffs containing additives; Preparation or treatment thereof containing gelling or thickening agents
- A23L29/206—Foods or foodstuffs containing additives; Preparation or treatment thereof containing gelling or thickening agents of vegetable origin
- A23L29/244—Foods or foodstuffs containing additives; Preparation or treatment thereof containing gelling or thickening agents of vegetable origin from corms, tubers or roots, e.g. glucomannan
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L19/00—Products from fruits or vegetables; Preparation or treatment thereof
- A23L19/01—Instant products; Powders; Flakes; Granules
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L19/00—Products from fruits or vegetables; Preparation or treatment thereof
- A23L19/10—Products from fruits or vegetables; Preparation or treatment thereof of tuberous or like starch containing root crops
- A23L19/115—Konjak; Konntaku
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L2/00—Non-alcoholic beverages; Dry compositions or concentrates therefor; Preparation or treatment thereof
- A23L2/385—Concentrates of non-alcoholic beverages
- A23L2/39—Dry compositions
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L2/00—Non-alcoholic beverages; Dry compositions or concentrates therefor; Preparation or treatment thereof
- A23L2/40—Effervescence-generating compositions
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L2/00—Non-alcoholic beverages; Dry compositions or concentrates therefor; Preparation or treatment thereof
- A23L2/52—Adding ingredients
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L2/00—Non-alcoholic beverages; Dry compositions or concentrates therefor; Preparation or treatment thereof
- A23L2/52—Adding ingredients
- A23L2/68—Acidifying substances
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L29/00—Foods or foodstuffs containing additives; Preparation or treatment thereof
- A23L29/20—Foods or foodstuffs containing additives; Preparation or treatment thereof containing gelling or thickening agents
- A23L29/206—Foods or foodstuffs containing additives; Preparation or treatment thereof containing gelling or thickening agents of vegetable origin
- A23L29/25—Exudates, e.g. gum arabic, gum acacia, gum karaya or tragacanth
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/30—Dietetic or nutritional methods, e.g. for losing weight
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/715—Polysaccharides, i.e. having more than five saccharide radicals attached to each other by glycosidic linkages; Derivatives thereof, e.g. ethers, esters
- A61K31/736—Glucomannans or galactomannans, e.g. locust bean gum, guar gum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L2/00—Non-alcoholic beverages; Dry compositions or concentrates therefor; Preparation or treatment thereof
- A23L2/52—Adding ingredients
- A23L2/58—Colouring agents
Definitions
- the present invention relates to novel solid fast dissolving or fast dispersing combinations containing Konjac mannan, to processes for preparing drinkable compositions comprising them, to drinkable compositions comprising them and to their uses as a food supplement/beverage supplement, in particular, but not limited to, in the managing of weight loss or overweight reduction, and in cholesterol and blood sugar levels control in a subject in need.
- Konjac is an Asian plant with a starchy root rich in fiber called glucomannan.
- Konjac mannan or Konjac glucomannan
- Konjac Mannan is a high molecular weight, water-soluble, non-ionic glucomannan found in tubers of the Amorphophallus konjac plant (Konjac Mannan - an overview Science Direct Topics; Handbook of Hydrocolloids (Second Edition), 2009.
- Konjac flour is produced from the corms of the Amorphophallus konjac plant, a tropical perennial crop.
- the corms of the plant contain about 30%-50% glucomannan gum.
- the resulting powder is konjac flour.
- the flour constitutes 60%-80% of the dried root corm.
- the corms grow below ground and are harvested at 2 kg for commercial processing. They are then washed and cut into thin slices, which are then dried and dry-milled into powder. The powder is separated, usually by cyclone or wind separation, producing crude konjac flour.
- Glucomannans in which some of the mannose residues are replaced by D-glucose are found in softwoods, tubers, and bulbs.
- Konjac glucomannan is a linear random copolymer of (1-4) linked beta-D-mannose and beta-D-glucose. It has mannose and glucose units at molar ratio of 1.6:1 with a low degree of acetyl groups (approximately 1 acetyl group per 17 residues) at the C-6 position. The degree of water solubility is controlled by the presence of the acetyl units.
- KGM The natural polysaccharide Konjac glucomannan
- KGM The natural polysaccharide Konjac glucomannan
- Glucomannan whose glucose and mannose units are connected by p-1 ,4-linkages, with a low degree of acetyl groups at the side chain C-6 position, as shown in Formula (I):
- the p-1 ,4-linkages are not digested by human enzymes of the microbiota in the human Gl tract. This is due to the p-glycosidic linkages between the mannose and glucose molecules, which cannot be hydrolysed by pancreatic amylase or salivary amylase. This means that it passes into the colon without being digested. Once it is in the colon, it is fermented by the native flora to produce glucomannan-oligosaccharides. Because it is not digested immediately, konjac glucomannan can increase feelings of satiety, leading the consumer to eat less.
- KGM has been widely used in food industry as it has been authorized as a food additive in Europe and classified as GRAS (Generally Recognized as Safe) by the FDA (Food and Drug Administration.
- the KGM powder used as such shows too many drawbacks.
- 1g KGM powder (high molecular weight) in 99 g water at a temperature of 25°C under constant stirring with, for example, a spoon it can take up to 10-15 minutes to form a gel in water.
- it is preferred to use a high molecular weight KGM and due to this high molecular weight the dissolution or gel formation of the KGM powder in water is time consuming for a consumer, and the formation of lumps (agglomeration of KGM in water) is a further disadvantage.
- the resulting gel is very stiff and does not flow easily out of the drinking glass to the mouth.
- the gel colour is grayish/grey, therefore it is not very appealing, and its viscosity makes difficult to swallow it.
- EFSA to reduce overweight it has to be in-taken three times a day (at 1 g level, 3x1g/day); it is therefore self-evident that the above drawbacks are not in favour of the compliance.
- the patent application CN105901269A discloses a Konjac tablet candy for weight management.
- the patent application CN1799410A discloses a diet fiber composition comprising Konjac glucomannan and Gum arabic in in the form of a capsule or chewable tablet.
- the patent application CN108041570A discloses a food additive comprising Konjac gum, Arabic gum and polyphosphate salts to be added to a food material for obtaining a good emulsification, stability, thickening, water retention.
- the technical problem that the present invention addresses and solves is to provide a solid fast dissolving or fast dispersing combination or composition of high molecular weight KGM, useful for preparing, for example, RTD beverage or another water-based fluid product for oral use containing high molecular weight KGM, that is easy to prepare (also with the only aid of a spoon), stable, easy to swallow and with an appealing mouthfeel, taste and colour.
- the solid (e.g, powder) compositions for instant (or RTD, ready to drink) beverage according to the present invention are particularly advantageous and offer great compliance for those taking them. They are easy to prepare, stable, easy to swallow and suitable for a wide range of individuals, including the elderly and children. Usually they can be poured into water, or another water-based fluid, and quickly and easily, with the only aid of a spoon, transformed into a drinkable fluid having an appealing mouthfeel, taste and colour. BRIEF DESCRIPTION OF THE DRAWINGS
- Figure 1 shows a simulation of fluid KGM behaviour after ingestion. After the ingestion of a water formulation comprising KGM, the water penetrates into the tissue or blood stream in the body. To simulate the vanishing of water from the formulation a series of experiments was made; gel formation occurs again when the water is no longer a significant part of the formulation and gel formation lowers food craving.
- the Applicant addresses and solves the above requirements by providing an innovative combination that allows KGM powder composition to be quickly and easily diluted in water, or another water-based fluid, resulting in a drinkable, stable and easy-to-take composition.
- the KGM powder composition of the invention by adding a fluid, leads preferably to:
- an effervescent fluid KGM composition e.g., if the KGM powder composition contains an effervescent system
- the term “fluid” may have, for example, the meaning of water, a translucent/transparent or colored water-based system, a milk (white fluid), a fluid similar to milk (milk substitute, plant-based milk, non dairy substitute, non-dairy beverage, instant milk), a beverage (non-alcoholic, alcoholic), a drink (fruit drink, energy drink, fortified drink, herbal drink, sport drink, yogurt drink, buttermilk drink, probiotic drink), a nectar, a juice, a coffee, a cappuccino, a smoothie, a macchiato, a cafe au lait, a coffee milk, a frapuccino, an espresso, an espresso macchiato, a latte espresso, a flat white, a caffee latte, a cacao.
- fluid gel may have, for example, the meaning of a system that is not a stiff gel (not flowable) but a flowable system (e.g., from a beverage glass so that a consumer can easily drink it).
- a fluid gel is a translucent/transparent gel (water based KGM system) or a white composition (milk-like appearance) with higher viscositiy so that a fluid gel-like KGM composition results that is drinkable) or a colored fluid gel KGM composition so that a colored fluid gel KGM composition results that is drinkable.
- a fluid gel of the invention may contain an effervescent system (e.g., mousse-like transparent or white or colored appearance).
- a mousse is a soft, light, and airy system, that may contain bubbles of C02 to give it a light and airy texture.
- fluid composition and “fluid gel composition” mean that they flow easily out from the drinking glass (or other containers) to the mouth of a consumer or from a spoon to the mouth of the consumer.
- fast dissolving or fast dispersing means that, when a solid powder combination or composition of the invention is poured into a water-based fluid at a temperature from 18 °C to 40°C, preferably from 18°C to 25 °C, and stirred, preferably with the aid of a spoon, it easily gives, within a time from 10 seconds to 5 minutes, preferably from 1 minute to 3 minutes, a solution or a homogeneous fine dispersion free of lumps, insoluble bottom residue, precipitates.
- the Applicant has found a powder combination or composition comprising KGM that is able to be quickly and easily diluted in a water-based fluid (e.g., water) and to reduce the formation of lumps, improve the flow behaviour of a water-based composition out of a drinking glass, and reduce the viscosity of KGM powder when diluted in water or another water-based fluid, improving the mouthfeel of KGM, its taste, and the colour of the final drinkable composition.
- a water-based fluid e.g., water
- the subject-matter of the present invention relates to a solid combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with at least one further dissolving or dispersing aid component (i) to (ix) which is selected from group comprising or, alternatively, consisting of: i. at least one arabic gum and at least one water-soluble salt, as defined in the present invention; and/or ii. at least one arabic gum, at least one water-soluble salt, as defined in the present invention, and at least one acid; preferably at least one hydroxy acid, as defined in the present invention; more preferably, citric acid and/or tartaric acid; and/or ill.
- KGM Konjac glucomannan
- ix which is selected from group comprising or, alternatively, consisting of: i. at least one arabic gum and at least one water-soluble salt, as defined in the present invention; and/or ii. at least one arabic gum, at least one water-
- At least one sugar alcohol preferably at least one sugar alcohol erythritol, as defined in the present invention, and at least one component selected from i. to ix. (not vii); and/or viii. at least one acid, preferably at least one hydroxy acid; and/or ix. at least one arabic gum and at least one acid, preferably at least one hydroxy acid, or their mixtures thereof, preferably in combination with one or more flavours and/or colorants and/or sweetener and/or oils (lipids) and/or surfactants and/or actives as defined above.
- component herein includes either a single substance or mixture of substances thereof.
- Konjac glucomannan is a known and commercially available compound having for example CAS RN No 37220-17-0. It is also referred to as Konjac gum, Konjac Glucomannan, Glucomannan Konjac Jelly, Konnyaku and Amorphophallus Konjac Root Mannan.
- KGM is for example in powder form
- Konjac gum may be indicated with E425(i) or E425(ii), both contain Konjac glucomannan.
- a high molecular weight KGM (e.g., ⁇ 10 6 Da, preferably e.g., from ⁇ 10 6 Da to 10 s Da, more preferably e.g., from ⁇ 10 6 Da to 10 7 Da) forms a gel in water which leads to feeling of satiety after ingestion (e.g., 1 g KGM).
- said high molecular weight Konjac glucomannan (KGM) has a molecular weight equal to or bigger than 10 6 Da; preferably, has a molecular weight comprised from bigger than 10 6 Da to 10 8 Da; more preferably, has a molecular weight comprised from 10 7 Da to 10 8 Da.
- a high molecular weight KGM than a low molecular weight KGM.
- a high molecular weight KGM with a viscosity value comprised from 20,000 cps to 100,000 cps, preferably comprised from 25,000 cps to 80,000 cps, more preferably comprised from 30,000 cps (30,000 mPas) to 60,000 cps, even more preferably comprised from 35,000 cps to 50,000 cps, for example bigger than 36,000 cps.
- the solid fast dissolving or fast dispersing composition of the present invention can be mixed with a drinkable liquid or a drinkable fluid gel like composition, which may be selected from water, milk (e.g., cow milk), a milk substitute (plant based milk, non dairy substitute, dairy beverage), a carbonated fluid, a beverage, a drink (fruit drink, energy drink, fortified drink, herbal drink, sport drink, yogurt drink, buttermilk drink, probiotic drink), a nectar, a juice, a smoothie, a macchiato, a cafe au lait, a caffee latte, a coffee milk, a frapuccino, an espresso, an espresso macchiato, a latte espresso, a flat white, a cacao without forming lumps.
- milk e.g., cow milk
- a milk substitute plant based milk, non dairy substitute, dairy beverage
- a carbonated fluid e.g., a beverage
- a drink fruit drink, energy drink, fortified drink, herbal drink, sport
- Arabic gum is also a known and commercially available compound, and it is also referred to as acacia gum, and preferably it is also in powder form. Arabic gum is the dried resin of acacia Senegal and related species.
- said at least one water-soluble salt mentioned in the component (i), (ii), (iii), (iv) and (v) above is more preferably selected from the group comprising or, alternatively, consisting of citrate salts, ascorbate salts, gluconate salts, chloride salts, lactate salts, tartrate salts, sulfate salts, glycinate/bisglycinate salts, acetyl taurinate salts, or mixtures thereof; more preferably, said at least one water-soluble salt mentioned in the component (i), (ii), (iii), (iv) and (v) above is selected from water-soluble alkali-metal salts and/or water soluble alkali-earth metal salts such as for example citrate salts, ascorbate salt, gluconate salts, chloride salts, lactate salts, tartrate salts, or their mixtures thereof.
- chloride salts include sodium chloride, potassium chloride, calcium chloride
- said at least one water-soluble salt mentioned in the component (i), (ii), (iii), (iv) and (v) above may be selected from a tripotassium citrate salt, more preferably may be a tripotassium citrate and/or tripotassium citrate monohydrate (company Pharmavit or from Magnesia/Jungbunzlauer) or trisodium citrate anhydrous (company Magnesia/Jungbunzlauer) or magnesium citrate (company Pharmavit) or zinc citrate or their mixtures thereof.
- a tripotassium citrate salt more preferably may be a tripotassium citrate and/or tripotassium citrate monohydrate (company Pharmavit or from Magnesia/Jungbunzlauer) or trisodium citrate anhydrous (company Magnesia/Jungbunzlauer) or magnesium citrate (company Pharmavit) or zinc citrate or their mixtures thereof.
- said at least one water-soluble salt mentioned in the component (i), (ii), (iii) and (iv) above may be selected from a sodium and/or potassium ascorbate salt (Jebsen&Jessen) and/or calcium and/or magnesium ascorbate (company Global Calcium) and/or zinc ascorbate monohydrate (company BioIla Chemicals GmbH) or their mixtures thereof.
- a sodium and/or potassium ascorbate salt Jebsen&Jessen
- calcium and/or magnesium ascorbate company Global Calcium
- zinc ascorbate monohydrate company BioIla Chemicals GmbH
- said at least one water-soluble salt mentioned in the component (i), (ii), (iii), (iv) and (v) above may be selected from a sodium and/or potassium gluconate salt (company Magnesia/Jungbunzlauer) and/or calcium and/or magnesium gluconate (company Global Calcium) and/or zinc gluconate salt (company Magnesia/Jungbunzlauer) or their mixtures thereof.
- said at least one water-soluble salt mentioned in the component (i), (II), (Hi), (iv) and (v) above may be selected from an alkali-earth metal halogenide salt; e.g., an alkali-earth chloride salt (e.g., CaCfe x 2H2O, company Magnesia/Jungbunzlauer) or (e.g., MgCh x 6H2O, company Magnesia/Jungbunzlauer) or hydrate water free versions like calcium chloride salt or a magnesium chloride or their mixtures thereof.
- an alkali-earth metal halogenide salt e.g., an alkali-earth chloride salt (e.g., CaCfe x 2H2O, company Magnesia/Jungbunzlauer) or (e.g., MgCh x 6H2O, company Magnesia/Jungbunzlauer) or hydrate water free versions like calcium chloride salt
- said at least one water-soluble salt mentioned in the component (I), (II), (ill), (iv) and (v) above may be selected from an alkali chloride salt, e.g., sodium chloride, potassium chloride, ammonium chloride, or their mixtures thereof.
- an alkali chloride salt e.g., sodium chloride, potassium chloride, ammonium chloride, or their mixtures thereof.
- said at least one water-soluble salt mentioned in the component (I), (II), (ill), (iv) and (v) above may be selected from lactate salts, more preferably may be a calcium lactate pentahydrate (company Global Calcium) and/or sodium lactate (company Magnesia/Jungbunzlauer) and/or potassium lactate and/or zinc lactate dihydrate, or their mixtures thereof.
- said at least one water-soluble salt mentioned in the component (I), (II), (ill), (iv) and (v) above may be selected from tartrate salts, more preferably may be a potassium tartrate x 0.5 H2O (company Dr. Paul Lohmann) and/or potassium sodium tartrate x 4 H2O, or their mixtures thereof.
- said at least one water-soluble salt mentioned in the component (I), (II), (ill), (iv) and (v) above may be selected from sulfate salts, more preferably may be a sodium and/or potassium sulfate.
- said at least one water-soluble salt mentioned in the component (I), (II), (ill), (iv) and (v) above may be selected from glycinate salts, more preferably may be a sodium and/or potassium glycinate.
- said at least one water-soluble salt mentioned in the component (I), (II), (ill), (iv) and (v) above may be selected from bisglycinate salts, more preferably may be a zinc bisglycinate or zinc bisglycinate monohydrate (company BioIla Chemicals GmbH).
- said at least one water-soluble salt mentioned in the component (i), (ii), (Hi), (iv) and (v) above may be selected from acetyl taurinate salts, more preferably may be a calcium and/or magnesium acetyl taurinate (company Synapharm).
- said at least one acid, more preferably at least one hydroxy acid, mentioned in the components (II), (Hi), (in the components iv. and v. , here is an acid that forms the effervescent system together with a base), (viii) and (lx) above may be selected from at least one organic and/or inorganic acids, more preferably may be a hydroxy acid such as, for example, citric acid, lactic acid, ascorbic acid, tartaric acid, malic acid or other acids like phosphoric acid or glucono-delta-lactone (GdL -is a lactone that rapidly dissolves in water, and subsequently slowly hydrolyses to gluconic acid) and/or mixtures thereof; more preferably, said an acid mentioned in the component (ii), (iii), (in iv and v., here is an acid that forms the effervescent system together with a base), (viii) and (lx) above may be a hydroxy acid, most preferably
- said an effervescent system mentioned in the components (iv) and (v) above comprises or, alternatively, consists of at least one base that can release carbon dioxide.
- said an effervescent system comprises or, alternatively, consists of at least one basic substance (i.e., an organic and/or inorganic base) capable of releasing carbon dioxide in the presence of an acidic substance (i.e. , an organic and/or inorganic acid) preferably in the presence of a composition or formulation containing water.
- an organic and/or inorganic base i.e., an organic and/or inorganic base
- an acidic substance i.e. , an organic and/or inorganic acid
- said base may be a carbonate and/or bicarbonate salt, more preferably may be a sodium and/or potassium carbonate salt or sodium and/or potassium bicarbonate salt.
- said an effervescent system mentioned in the components (iv) and (v) above comprises or, alternatively, consists of a sodium and/or potassium bicarbonate salt (also referred to as sodium or potassium hydrogen carbonate salt, NaHCOs or KHCO3) and citric acid.
- a sodium and/or potassium bicarbonate salt also referred to as sodium or potassium hydrogen carbonate salt, NaHCOs or KHCO3
- citric acid also referred to as sodium or potassium hydrogen carbonate salt, NaHCOs or KHCO3
- said at least one water-soluble salt mentioned in the components (iv) and (v) above may be selected from alkali-metal carbonate salts; more preferably, may be a sodium carbonate and/or sodium hydrogen carbonate salt (Solvay Chemicals International: BICAR® FOOD, A+E. Fischer-Chemie GmbH & Co. KG, Wiesbaden, Germany, Carl Dicke GmbH & Co KG, Monchengladbach, Germany) and/or potassium hydrogen carbonate (company Dr. Paul Lohmann) or their mixtures thereof
- the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with at least one arable gum, and at least one water-soluble salt, with the provision that said at least one water-soluble salt is not a phosphate or polyphosphate salt.
- KGM Konjac glucomannan
- the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with an arabie gum, at least one water-soluble salt, and at least one hydroxy acid, such as preferably citric acid, lactic acid, ascorbic acid, tartaric acid, malic acid or other acids like phosphoric acid or glucono-delta-lactone (GdL).
- KGM Konjac glucomannan
- GdL glucono-delta-lactone
- the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with at least one water- soluble salt and, optionally, at least one acid, more preferably at leastone hydroxy acid, with the provision that said at least one water-soluble salt is not a phosphate or polyphosphate salt.
- KGM Konjac glucomannan
- the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with an effervescent system and, optionally, at least one water-soluble salt.
- KGM Konjac glucomannan
- the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with an effervescent system, an arabic gum and, optionally, at least one water-soluble salt.
- KGM Konjac glucomannan
- the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with at least one sugar alcohol; more preferably, at least one sugar alcohol erythritol.
- KGM Konjac glucomannan
- the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with at least one sugar alcohol; more preferably, at least one sugar alcohol erythritol, and at least one component selected from I. to lx., (not vii.).
- KGM Konjac glucomannan
- the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with at least one acid, preferably at least one hydroxy acid; more preferably selected from citric acid and/or tartaric acid.
- KGM Konjac glucomannan
- the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with at least one arabic gum and at least one acid, preferably at least one hydroxy acid, selected from citric acid and/or tartaric acid.
- KGM Konjac glucomannan
- the solid fast dissolving or fast dispersing combination of the invention may be present in a composition for oral use such as a solid pharmaceutical, nutraceutical or food composition which comprises an aforementioned combination (i) to (ix) as above defined, and (x) optionally, at least one flavour and/or colorant and/or sweetener, and/or a carrier or at least one excipient pharma or food grade.
- a composition for oral use such as a solid pharmaceutical, nutraceutical or food composition which comprises an aforementioned combination (i) to (ix) as above defined, and (x) optionally, at least one flavour and/or colorant and/or sweetener, and/or a carrier or at least one excipient pharma or food grade.
- composition'' herein includes pharmaceutical, nutraceutical or food compositions or compositions for medical devices under the Eli Reg. 745/2017.
- flavours and/or colorants and/or sweeteners may be of synthetic or natural origin.
- components (x) may be selected from fruit flavours, such as vanilla, orange, blueberry, lemon, and the like; and sweeteners such as sucralose and/or stevia and/or erythritol.
- Colorants may be any colorant which makes the more appealing the composition when mixed up with water, for instance yellow colorants, such as vanilla yellow and the like.
- Colorants may also be selected from active-coloured ingredients, as it will be disclosed in detail in the following.
- All the components used in the solid fast dissolving or fast dispersing combination and composition of the present invention are physiologically acceptable having a food or pharmaceutical grade.
- All the components of the solid fast dissolving or fast dispersing composition of the present invention are in a powder form and/or in a granular form, to give a solid fast dissolving or fast dispersing composition of the present invention in the form of a mixture of powders and/or granules.
- solid fast dissolving or fast dispersing composition and “solid fast dissolving or fast dispersing combination” are intended to refer to and include any solid composition or combination of the present invention in the form of a loose and/or free-flowing mixture of powders and/or granules, i.e. it is not a in the form of a tablet, a chewable tablet, a pill, a candy, a capsule, etc.
- composition of the invention comprises or, alternatively, consists of:
- (F) at least one sugar alcohol erythritol and at least one composition selected from (A), (B), (C), (D'), (D"), (G) and/or (H); or
- compositions from (A) to (H) in combination with one or more flavours and/or colorants and/or sweetener and/or oils (lipids) and/or surfactants and/or actives as defined above.
- composition (A) comprises or, alternatively, consists of KGM, an arabic gum, and a tripotassium citrate salt; even more preferably, a tripotassium citrate monohydrate salt.
- composition (B) comprises or, alternatively, consists of KGM, an arabic gum, a tripotassium citrate salt; more preferably, a tripotassium citrate monohydrate salt, and citric acid.
- composition (C) comprises or, alternatively, consists of KGM, sodium and/or potassium gluconate salt and, optionally, a tripotassium citrate salt; more preferably, a tripotassium citrate monohydrate salt.
- composition (D’) comprises or, alternatively, consists of KGM, a sodium and/or potassium (bi)carbonate, and citric acid or tartraric acid (or a mixture citric acid/tartaric acid).
- composition (D”) comprises or, alternatively, consists of KGM, an arabic gum, a sodium and/or potassium (bi)carbonate, and citric acid or tartraric acid (or a mixture citric acid/tartaric acid).
- composition (E) comprises or, alternatively, consists of KGM and erythritol.
- composition (F) comprises or, alternatively, consists of erythritol and at least one of the compositions selected from (A), (B), (C), (D-), (D"), (G) and/ or (H).
- composition (G) comprises or, alternatively, consists of KGM and citric acid and/or tartaric acid. More preferably, composition (H) comprises or, alternatively, consists of KGM, arabic gum and citric acid and/or tartaric acid.
- the weight ratio KGM/Arabic gum/salt is the range comprised from 1 : 1 :0.1 to 1 : 15:7, more preferably in the range comprised from 1 :1.5:0.5 to 1 :2:2, for instance in the range from 1 : 1 , 8:0, 8 to 1 : 1 , 8: 1.6, wherein the salt is preferably a tripotassium citrate, more preferably a tripotassium citrate monohydrate.
- Tripotassium citrate salt may be replaced by the other salts as defined above, for instance gluconates and ascorbates, such as gluconates (sodium, potassium, calcium), ascorbates (sodium), potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate anhydrous, magnesium chloride x 6H2O, calcium chloride x 2H2O (or hydrate water free versions of magnesium chloride, calcium chloride), sodium chloride, potassium chloride. Mixtures of salts may also be used.
- gluconates and ascorbates such as gluconates (sodium, potassium, calcium), ascorbates (sodium), potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate anhydrous, magnesium chloride x 6H2O, calcium chloride x 2H2O (or hydrate water free versions of magnesium chloride, calcium chloride), sodium chloride, potassium chloride. Mixtures of salts may also be
- Tripotassium citrate salt may be replaced by the other salts as defined above, for instance gluconates and ascorbates, such as gluconates (sodium, potassium, calcium), ascorbates (sodium), potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate anhydrous, magnesium chloride x 6H2O, calcium chloride x 2H2O (or water free versions of magnesium chloride, calcium chloride), sodium chloride, potassium chloride. Mixtures of salts may also be used.
- gluconates and ascorbates such as gluconates (sodium, potassium, calcium), ascorbates (sodium), potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate anhydrous, magnesium chloride x 6H2O, calcium chloride x 2H2O (or water free versions of magnesium chloride, calcium chloride), sodium chloride, potassium chloride. Mixtures of salts may also be used.
- the citric acid may be replaced by other acids such as hydroxy acid like lactic acid, ascorbic acid, tartaric acid, malic acid or phosphoric acid or glucono-delta-lactone (GdL) which rapidly dissolves, and subsequently slowly hydrolyses to gluconic acid) or mixtures of acids
- compositions (A) showed a more neutral pH
- compositions (B) also comprising citric acid, showed pH ⁇ 6.5.
- arabic gum is a fibre
- it is possible to use according to the present invention for example in a drink, 9 g for example of a powder composition of KGM/Arabic gum/ at least one water-soluble salt, preferably in a weight ratio of 1 :9:1.6.
- Drinking this composition delivers 10 g fibre/drink or 30 g/day (3 drinks).
- the composition of KGM/Arabic gum/tripotassium citrate monohydrate 1 :9:1.6 is fluid and delivers 10 g of fibre/or 30 g/day (3 drinks). Even higher amounts of Arabic gum in a drink are also possible.
- lipophilic and/or surface-active components as a powder and/or granule (actives, oils, lipids and/or surfactants) to the system of (A) KGM/Arabic gum/at least one water-soluble salt leads to a milk-like product (instant milk) or milk alternatives product (milk substitute products, non dairy substitutes, plant-based milks, non-dairy beverage) or cocoa-like product at a approximately neutral pH range.
- the lipophilic and/or surface-active component can be added a) as a powder and/or granule or b) as a liquid, more preferably as a powder and/or granule.
- Examples of powders a1) full cream milk powder, a2) coffee powder, a3) wheat germs, a4) almond flour, a5) cacao powder, a6) MCT powder, a7) omega-3 powder, a8) oatmeal/oatflour powder, or a9) soymeal/soyflour powder.
- Examples of liquid oils b1) Linseed oil + EPA +DHA (liquid omega 3, containing ALA, EPA and DHA)
- liquid oils examples include flaxseed oil, fish oil, borage oil, blackcurrent seed oil, evening primrose oil, olive oil, MCT oils (e.g. coconut oil).
- the weight ratio KGM/water soluble salt is in the range comprised from 1/0.5 to 1/15, more preferably in the range of 1 :6, even more preferably 1 :3 for example with tripotassium citrate monohydrate.
- the pH of such a composition is around about 7.4 ⁇ 2 (at20.5°C).
- Tripotassium citrate monohydrate salt may be replaced by the other water-soluble salts as defined above, for instance gluconates and ascorbates, such as gluconates (sodium, potassium, calcium), ascorbates (sodium), potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate anhydrous, magnesium chloride x 6H2O, calcium chloride x 2H2O (or water free versions of magnesium chloride, calcium chloride), sodium chloride, potassium chloride. Mixtures of salts may also be used.
- gluconates and ascorbates such as gluconates (sodium, potassium, calcium), ascorbates (sodium), potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate anhydrous, magnesium chloride x 6H2O, calcium chloride x 2H2O (or water free versions of magnesium chloride, calcium chloride), sodium chloride, potassium chloride. Mixtures of salts may also
- said composition D’ comprises or, alternatively, consists of KGM and an effervescent system, as defined above, and optionally a salt as defined above.
- said composition D' comprises or, alternatively, consists of KGM/sodium hydrogen carbon ate/ci trie acid and/or tartaric acid, and/or KGM/potassium hydrogen carbonate/citric acid and/or tartaric acid.
- the weight ratio of KGM/sodium and/or potassium hydrogen carbonate salt/citric and/or tartaric acid is in the range comprised from 1/0.1/0.06/ to 1/10/23, more preferably in the range comprised from 1/5/2.5 to 1/5/12, even more preferably in the range of 1.00/0.1/0.23, or more preferably 1/(0.1-0.60)/(0.1-1 .8), or more preferably 1/0.6/(0.25-1 .4), wherein the water-soluble salt in the composition D' is preferably tripotassium citrate, more preferably tripotassium citrate monohydrate.
- the optional added salt is in the range from 0.1 g to 15 g.
- said composition D comprises or, alternatively, consists of KGM/Arabic gum/sodium hydrogen carbonate/citric acid (and/or tartaric acid) and, optionally, at least one salt selected from the group comprising or, alternatevily, consisting of tripotassium citrate, preferably tripotassium citrate monohydrate, gluconates (sodium, potassium, calcium), ascorbates (sodium and/or potassium), sodium and/or potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate, preferably trisodium citrate anhydrous, magnesium chloride x 6H2O, calcium chloride X2H2O (or water free versions of magnesium chloride, calcium chloride), sodium chloride, potassium chloride; or said composition D” comprises or, alternatively, consists of KGM/Arabic gum/potassium hydrogen carbonate/citric acid (and/or tartaric acid) and, optionally, a salt selected from the group copm
- the weight ratio of KGM/Arabic gum/sodium or potassium (bi)carbonateZcitric acid (and/or tartaric acid) is in the range comprised from 1/0.1/0.15/0 1 to 1/15/0.8/1.8, more preferably:
- the arable gum may be present at 1.4 or 1.5 or 1.6 or 1.7 weight ratio based on 100 g (e.g., 1.4 means 1.4%).
- arabic gum >1 .7 gives a fluid composition.
- a higher amount of arabic gum which may be in the range (weight ratio) from 10 to 50, preferably from 15 to 40, more preferably from 20 to 30 by weight for 1.0 KGM.
- said composition D comprises or, alternatively, consists of KGM/arabic gum/sodium and/or potassium (bi)carbonatefci trie acid (and/or tartaric acid)/at least one salt; preferably, with a weight ratio of:
- Tripotassium citrate and tripotassium citrate monohydrate salt may be replaced by the other water-soluble salts, as defined above, selected from the group copmprising or, alternatevily, consisting of tripotassium citrate, preferably tripotassium citrate monohydrate, gluconates (sodium, potassium, calcium), ascorbates (sodium and/or potassium), sodium and/or potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate, preferably trisodium citrate anhydrous, magnesium chloride x 6H 2 O, calcium chloride x 2H 2 O (or water free versions of magnesium chloride, calcium chloride) sodium chloride, potassium chloride. Mixtures of salts may also be used.
- Citric acid may be replaced by other acids, preferred a hydroxy acid such as lactic acid, ascorbic acid, tartaric acid, malic acid or by other acids like phosphoric acid or glucono-delta-lactone (GdL) or mixtures of the acids mentioned above.
- a hydroxy acid such as lactic acid, ascorbic acid, tartaric acid, malic acid or by other acids like phosphoric acid or glucono-delta-lactone (GdL) or mixtures of the acids mentioned above.
- composition D may further comprises at least one lipophilic and/or surface-active component such as, for example, actives, oils, lipids and/or surfactants; for example, the composition D” may comprises:
- KGM/arabic gum/sodium and/or potassium (bi) carbonate/hydroxy acid KGM/arabic gum/sodium and/or potassium (bi) carbonate/hydroxy acid
- KGM/arabic gum/sodium and/or potassium (bi) carbonate/citric acid preferably with a weight ratio of:
- KGM Arabic gum Carbonate Citric acid (Active, Oil, lipid, Surfactant) 1.0: (15.0-40): (0.15-0.8): (0.15-1.5): (0.5-80.0) or, KGM Arabic gum Carbonate Citric acid MCT-C8
- compositions showed a faster dissolution in water, less lump formation, a better flow behaviour from a beverage drinking glass to the mouth, a better mouthfeel, an improved viscosity when mixed with water, contrary to the extreme high viscosity of KGM alone in a liquid, preferably but not limited to, water.
- the subject matter of the present invention relates to a solid fast dissolving or fast dispersing composition
- a solid fast dissolving or fast dispersing composition comprising or, alternatively, consisting of, a high molecular weight Konjac glucomannan (KGM) and an arabic gum in combination with at least one further dissolving or dispersing aid component which is selected from the group comprising or, alternatively, consisting of:
- At least one water-soluble salt at least one water-soluble salt, with the provision that said at least one water-soluble salt is not a phosphate or polyphosphate salt;
- an effervescent system comprising or, alternatively, consisting of at least one basic substance capable of releasing carbon dioxide in the presence of at least one acidic substance and, optionally, at least one water-soluble salt; or
- (H) at least one organic acid, preferably at least one hydroxy acid; and/or their mixtures thereof.
- said high molecular weight Konjac glucomannan has a molecular weight equal to or bigger than 10 s Da; preferably, has a molecular weight comprised from bigger than 10 6 Da to 10 8 Da; more preferably, has a molecular weight comprised from 10 7 Da to 10 8 Da; and/or said high molecular weight Konjac glucomannan (KGM) has viscosity value preferably comprised from 20,000 cps to 100,000 cps, preferably comprised from 25,000 cps to 80,000 cps, more preferably comprised from 30,000 cps to 60,000 cps, even more preferably comprised from 35,000 cps to 50,000 cps, and preferably has glucomannan content comprised from bigger than 85% to 98%, more preferably from 90% and 95%.
- said at least one water-soluble salt is preferably selected from the group comprising or, alternatively, consisting of:
- citrate salts preferably, sodium and/or potassium citrate salts; more preferably tripotassium citrate salts, tripotassium citrate monohydrate salts, trisodium citrate salts, trisodium citate anhydrous salts, magnesium citrate, calcium citrate, zinc citrate, or their mixtures thereof;
- - ascorbate salts preferably, sodium and/or potassium ascorbate salts, calcium and/or magnesium ascorbate salts, zinc ascorbate, zinc ascorbate monohydrate salts, or their mixtures thereof;
- gluconate salts preferably, sodium and/or potassium gluconate salts, calcium and/or magnesium gluconate salts, zinc gluconate salt, or their mixtures thereof;
- chloride salts preferably, sodium chloride, potassium chloride, calcium chloride, magnesium chloride, ammonium chloride, or their mixture thereof.
- lactate salts preferably, calcium lactate, calcium lactate pentahydrate, sodium and/or potassium lactate, zinc lactate, zinc lactate dihydrate, or their mixtures thereof;
- - tartrate salts preferably, sodium and/or potassium tartrate, potassium sodium tartrate, or their mixtures thereof;
- - sulfate salts preferably, sodium and/or potassium sulfate salts
- - glycinate salts preferably, sodium and/or potassium glycinate salts
- said at least one organic acid is selected from the group comprising or, alternatevily, consisting of citric acid, lactic acid, ascorbic acid, tartaric acid, malic acid, or phosphoric acid or glucono-delta-lactone (GdL).
- said effervescent system comprises or, alternatively, consists of at least one basic substance capable of releasing carbon dioxide in the presence of at least one acidic substance, wherein said basic substance is selected from carbonate or bicarbonate salts; more preferably, sodium and/or potassium carbonate, sodium and/or potassium bicarbonate, or their mixtures thereof; and said at least one acidic substance is selected from organic acids; more preferably, hydroxy acids such as citric acid and/or tartaric acid.
- said solid fast dissolving or fast dispersing composition comprises or, altervatively, consists of (i) KGM, arabic gum, tripotassium citrate; more preferably, tripotassium citrate monohydrate, and citric acid, or (ii) KGM, arabic gum, sodium and/or potassium (bi)carbonate, and citric acid.
- the subject matter of the present invention relates to a process for preparing a drinkable compositition comprising the steps of: a) putting into contact a solid fast dissolving or fast dispersing composition of the invention with a water-based fluid, which is selected from water, a beverage, a milk, a milk substitute, a plant-based milk, non dairy substitute, nondairy beverage, instant milk; non-alcoholic, alcoholic, a fruit drink, energy drink, fortified drink, herbal drink, sport drink, yogurt drink, buttermilk drink, probiotic drink, a nectar, a juice or a cappuccino, a smoothie, a coffee, a macchiato, a cafe au lait, a coffee milk, a frapuccino, an espresso, an espresso macchiato, a latte espresso, a flat white, a caffee latte, a cacao, or mixtures thereof; b) mixing, preferably with the aid of a spoon, for a time from a water-based fluid
- the subject matter of the present invention relates to a drinkable composition obtained by mixing the solid fast dissolving or fast dispersing combination, as described above, with water, a beverage, a milk, a milk substitute, a plant-based milk, non dairy substitute, non-dairy beverage, instant milk; nonalcoholic, alcoholic, a fruit drink, energy drink, fortified drink, herbal drink, sport drink, yogurt drink, buttermilk drink, probiotic drink, a nectar, a juice or a cappuccino, a smoothie, a coffee, a macchiato, a cafe au lait, a coffee milk, a frapuccino, an espresso, an espresso macchiato, a latte espresso, a flat white, a caffee latte, a cacao, or mixture thereof.
- the subject matter of the present invention relates to the solid fast dissolving or fast dispersing composition, as described above, for use in a method for managing weight loss or overweight reduction in a subject in need.
- the subject matter of the present invention relates to the solid fast dissolving or fast dispersing composition, as described above, for use in a method to promote body weight loss in a healthy subject, or to reduce body weight in an overweight and/or obese subjects.
- the subject matter of the present invention relates to the solid fast dissolving or fast dispersing composition, as described above, for use in a method for reducing cholesterol and blood sugar levels in a subject in need.
- the subject matter of the present invention relates to the drinkable composition, as described above, for use in a method for managing weight loss or overweight reduction in a subject in need. According to one its aspects, the subject matter of the present invention relates to the drinkable composition, as described above, for use in a method to promote body weight loss in a healthy subject, or to reduce body weight in an overweight and/or obese subjects.
- the subject matter of the present invention relates to the drinkable composition, as described above, for use in a method for reducing cholesterol and blood sugar levels in a subject in need.
- the subject matter of the present invention relates to a solid fast dissolving or fast dispersing composition
- a solid fast dissolving or fast dispersing composition comprising or, alternatively, consisting of, a high molecular weight Konjac glucomannan (KGM) in combination with at least one further dissolving or dispersing aid component which is selected from the group comprising or, alternatively, consisting of:
- At least one effervescent system comprising or, alternatively, consisting of at least one basic substance capable of releasing carbon dioxide in the presence of at least one acidic substance and, optionally, at least one water- soluble salt;
- (G) at least one acid, preferably at least one hydroxy acid; and/or their mixtures thereof.
- said high molecular weight Konjac glucomannan has a molecular weight equal to or bigger than 10 5 Da; preferably, has a molecular weight comprised from bigger than 10 6 Da to 10 8 Da; more preferably, has a molecular weight comprised from 1 O 7 Da to 10 8 Da; and/or said high molecular weight Konjac glucomannan (KGM) has viscosity value preferably comprised from 20,000 cps to 100,000 cps, preferably comprised from 25,000 cps to 80,000 cps, more preferably comprised from 30,000 cps to 60,000 cps, even more preferably comprised from 35,000 cps to 50,000 cps, and preferably has glucomannan content comprised from bigger than 85% to 98%, more preferably from 90% and 95%.
- said at least one water-soluble salt is preferably selected from the group comprising or, alternatively, consisting of:
- citrate salts preferably, sodium and/or potassium citrate salts; more preferably tripotassium citrate salts, tripotassium citrate monohydrate salts, trisodium citrate salts, trisodium citate anhydrous salts, magnesium citrate, calcium citrate, zinc citrate, or their mixtures thereof;
- - ascorbate salts preferably, sodium and/or potassium ascorbate salts, calcium and/or magnesium ascorbate salts, zinc ascorbate, zinc ascorbate monohydrate salts, or their mixtures thereof;
- gluconate salts preferably, sodium and/or potassium gluconate salts, calcium and/or magnesium gluconate salts, zinc gluconate salt, or their mixtures thereof;
- chloride salts preferably, sodium chloride, potassium chloride, calcium chloride, magnesium chloride, ammonium chloride, or their mixture thereof.
- lactate salts preferably, calcium lactate, calcium lactate pentahydrate, sodium and/or potassium lactate, zinc lactate, zinc lactate dihydrate, or their mixtures thereof;
- - tartrate salts preferably, sodium and/or potassium tartrate, potassium sodium tartrate, or their mixtures thereof;
- - sulfate salts preferably, sodium and/or potassium sulfate salts
- - glycinate salts preferably, sodium and/or potassium glycinate salts
- said at least one organic acid is selected from the group comprising or, alternatevily, consisting of citric acid, lactic acid, ascorbic acid, tartaric acid, malic acid, or phosphoric acid or glucono-delta-lactone (GdL).
- said effervescent system comprises or, alternatively, consists of at least one basic substance capable of releasing carbon dioxide in the presence of at least one acidic substance, wherein said basic substance is selected from carbonate or bicarbonate salts; more preferably, sodium and/or potassium carbonate, sodium and/or potassium bicarbonate, or their mixtures thereof; and said at least one acidic substance is selected from organic acids; more preferably, hydroxy acids such as citric acid and/or tartaric acid.
- the subject matter of the present invention relates to a process for preparing a drinkable compositition comprising the steps of: a) putting into contact a solid fast dissolving or fast dispersing composition of the invention with a water-based fluid, which is selected from water, a beverage, a milk, a milk substitute, a plant-based milk, non dairy substitute, nondairy beverage, instant milk; non-alcoholic, alcoholic, a fruit drink, energy drink, fortified drink, herbal drink, sport drink, yogurt drink, buttermilk drink, probiotic drink, a nectar, a juice or a cappuccino, a smoothie, a coffee, a macchiato, a cafe au lait, a coffee milk, a frapuccino, an espresso, an espresso macchiato, a latte espresso, a flat white, a caffee latte, a cacao, or mixtures thereof; b) mixing, preferably with the aid of a spoon, for a time from a water-based fluid
- the subject matter of the present invention relates to a drinkable composition obtained by mixing the solid fast dissolving or fast dispersing combination, as described above, with water, a beverage, a milk, a milk substitute, a plant-based milk, non dairy substitute, non-dairy beverage, instant milk; nonalcoholic, alcoholic, a fruit drink, energy drink, fortified drink, herbal drink, sport drink, yogurt drink, buttermilk drink, probiotic drink, a nectar, a juice or a cappuccino, a smoothie, a coffee, a macchiato, a cafe au lait, a coffee milk, a frapuccino, an espresso, an espresso macchiato, a latte espresso, a flat white, a caffee latte, a cacao, or mixtures thereof.
- the subject matter of the present invention relates to the solid fast dissolving or fast dispersing composition, as described above, for use in a method for managing weight loss or overweight reduction in a subject in need.
- the subject matter of the present invention relates to the solid fast dissolving or fast dispersing composition, as described above, for use in a method to promote body weight loss in a healthy subject, or to reduce body weight in an overweight and/or obese subjects.
- the subject matter of the present invention relates to the solid fast dissolving or fast dispersing composition, as described above, for use in a method for reducing cholesterol and blood sugar levels in a subject in need.
- the subject matter of the present invention relates to the drinkable composition, as described above, for use in a method for managing weight loss or overweight reduction in a subject in need
- the subject matter of the present invention relates to the drinkable composition, as described above, for use in a method to promote body weight loss in a healthy subject, or to reduce body weight in an overweight and/or obese subjects.
- the subject matter of the present invention relates to the drinkable composition, as described above, for use in a method for reducing cholesterol and blood sugar levels in a subject in need
- Coffee powder is added to KGM/Arabic gum/water soluble salt and poured into milk.
- a fluid composition results with a white appearance and neutral oatmeal taste impression d)
- Full cream milk powder and a freeze-dried fruit are added to KGM/Arabic gum/water soluble salt
- This raspberry containing powder mixture (1.0g/1.8g/0.8g/30g/2.0g) was added to water in a beverage glass and stirred with a spoon.
- the fluid mixture was allowed to stand for 10 min for the rehydration of the raspberries.
- a red raspberry containing fluid milk drink (with KGM) was obtained (pH: 6.8, 25°C).
- KGM arabic gum, at least one water-soluble salt, as defined above, and at least one hydroxy acid, as defined above.
- a powder mixture of KGM/Arabic gum/citric acid/ tripotassium citrate monohydrate makes easier the dissolving process of the KGM and arabic gum in water by simply stirring the composition with a spoon in water. Immediately, a fluid is obtained (pH 3.95 (20.7°C)). No lump formation (compared to KGM pure in water). The consumer can drink it immediately. More neutral compositions (neutral aftertaste, e.g., pH 5.7 (21.1 °C) can be obtained by changing the relative ratios citrate/hydroxy acid ((1.0:1.5:07:0.1).
- the salt is preferably selected from the group copmprising or, alternatevily, consisting of tripotassium citrate, preferably tripotassium citrate monohydrate, gluconates (sodium, potassium, calcium), ascorbates (sodium and/or potassium), sodium and/or potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate, preferably trisodium citrate anhydrous, magnesium chloride x 6H2O, calcium chloride x 2H2O (or water free versions of magnesium chloride, calcium chloride) sodium chloride, potassium chloride.
- a powder mixture of KGM/Arabic gum/Tripotassium citrate monohydrate/citric acid/Full cream milk powder (1.00 g/1.50 g/1 ,5g/0.1 g/3.00 g) was added to 92.9 g of water and stirred with a spoon.
- a fluid composition results (pH 6.24, 19.6°C) with a white appearance. Increasing the citric acid content leads to more acidic formulations.
- cacoa powder Plaine Arome (Fat content: 22-24%)
- full cream milk powder 3.0g/1.2g
- KGM/Arabic gum/water soluble salt /hydroxy acid e.g., citric acid
- Cacao powder is available from e.g., Barry Callebout Anlagen GmbH, im Mediapark 8a, 50670 Koln (with different fat contents, e.g., “Noir lntense”: 10- 12%, “Plaine Arome” or “Extra brute”: 22-24%, “Rouge Ultime”20-22%, “Legere”: 1%.
- a powder mixture of KGM/Arabic gum/Tripotassium citrate monohydrate/citric acid/Full cream powder/Cacao powder (Plaine Arome (Fat content: 22-24%)) (1.0 g/1.5g/1.5g/0.1g/3.0g/1.2g) was added to 91.7 g of water and stirred with a spoon.
- a fluid composition results (pH 6.6, 20.5°C) with a brown appearance. Due to the combination of full cream milk/cacao powder the product has a good cacao taste.
- KGM a water-soluble salt and, optionally, a hydroxy acid as defined above.
- Astonishingly powder mixtures of KGM/water soluble salt facilitates the dissolving process of the high molecular weight KGM in water. Immediately a fluid is obtained by simply stirring the composition with a spoon. No lump formation (compared to KGM pure in water). Small KGM particles are visible in water directly after the mixture was prepared.
- KGM/sodium gluconate/water (1g/3g/96g) has a pH of 7.43 (20.5°C) and a more neutral taste.
- Adding citric acid (e.g., 0.25 g) gives a pH of 4.44 (20.1 °C) in a formulation of KGM/sodium gluconate/water/citric acid (1 g/3g/0.25g/95.50g/0.25g) and a little bit more acid taste perception.
- a composition of KG M/Tri potassium citrate monohydrate/citric acid can be formulated at more neutral pH (citric acid: 0 g, pH: 7 4, 20 5°C) or by addition of citric acid the desired pH can be found (g water: 100g - g raw materials/ingredients)
- a powder mixture of KGM/sodium chloride (1 g/0,5g) up to KGM/sodium chloride (1 g/2g) leads to a fast-dissolving process of the KGM (water: 100 g - g of raw materials).
- KGM/water soluble salt/water formulations are translucent to transparent, for example the high molecular weight KGM/sodium gluconate/water system is translucent to transparent.
- High molecular weight KGM/water systems are more greyish and translucent/not transparent (see introduction) Consumers that prefer a translucent to transparent KGM fluid or a more translucent/transparent KGM fluid gel will like such compositions.
- Adding a water soluble natural or synthetic color to such formulations results in the corresponding colored translucent to transparent fluids or colored translucent to transparent fluid gels.
- Adding a water-soluble flavour and/or a sweetener like sucralose, stevia is additionly possible.
- Compositions (D) e.g., D’ and D” become effervescent when mixed with water, through the CO2 development due to the following reaction: MeHCOa + (H00CCH 2 ) 2 C(0H)C00H -> C0 2 + Me-citrate; Me being Na or K.
- Me Hydrogen carbonate + acid e.g., citric acid
- KGM/Me hydrogen carbonate/citric acid such as for example: a) KGM/sodium hydrogen carbonate/citric acid.
- a higher content of the effervescent system e.g., NaHCOa/citric acid
- KGM/potassium hydrogen carbonate/citric acid e.g., NaHCOa/citric acid
- the freedom to operate at different neutralisation levels and the usage of the corresponding sodium or potassium hydrogen carbonate salt is relevant for at least six reasons. 1) Some consumers prefer an acid taste of a product (or the combination acid combined with sweetness from a sweetener). The formulator will formulate the KGM product at a lower pH value in this case. 2) The stability of added actives is in some cases better in an acid (more neutral) than in a more neutral (acid) pH. The formulator will formulate at the corresponding preferred pH value (acid or more neutral pH value). 3) The color impression of an active is different at acid pH values versus a more neutral pH (e.g., for actives like antocyanins).
- the color is brighter in an acid environment that the formulator will formulate at a lower pH value the KGM product.
- the consumer (obese adult, obese child) is interested in a KGM product with (a lot of) foam and an acid aftertaste after dissolving the KGM powder mixture.
- a high amount of the hydrogen carbonate salt e.g 0.4 g to 3.0 g potassium hydrogen carbonate
- a foam development is observed in the presence of 1.00 g KGM; 0.77g citric acid (for 0.40 g potassium hydrogen carbonate) or in the presence of 5.80 g citric acid (for 3.00 g potassium hydrogen carbonate).
- the pH of such formulations in the range of pH 3.7-4.0.
- the consumer (obese adult, obese child) is interested in a KGM product with (a lot of) foam and a more neutral aftertaste after dissolving the KGM powder mixture.
- a high amount of the hydrogen carbonate salt e.g. 0.4g to 3.0 g potassium hydrogen carbonate
- a foam development and neutral aftertaste (pH 6.1) is observed.
- the consumer in a sample with 1.00 g KGM, 1.92 g citric acid, 300 g potassium hydrogen carbonate
- the consumer consumes too much sodium or not enough potassium and/or has a high blood pressure problem.
- it is preferred to formulate with the potassium hydrogen carbonate salt instead of the sodium hydrogen carbonate salt or a mixture of potassium and sodium hydrogen carbonate can be used.
- an effervescent system e.g. sodium (potassium) hydrogen carbonate, a hydroxy acid (in this case: citric acid) and the formation of the resulting sodium citrate (or the resulting potassium citrate) are a “very fast dissolver” or a “very fast suspending” combination for a KGM composition.
- a powder mixture of KGM/Arabic gum/sodium hydrogen carbonate/citric acid A powder mixture of KGM/Arabic gum/sodium hydrogen carbonate/citric acid.
- the dissolved C02, arabic gum and the resulting formation of the salt lead to a fast dissolution process by simply stirring the composition with a spoon and the composition stays fluid or gives a fluid gel
- the resulting salt is potassium citrate instead of sodium citrate
- Weight ratios of 1.0/1 .2/0 3/0 7 gives a foam that disappears after a while and dissolves quickly the powder ingredients.
- a fluid is obtained (pH 3.92, 19.9°C) by simply stirring the composition with a spoon. The consumer can drink it immediately. If he prefers to wait a while (e.g., 10-15 min) a drinkable fluid gel results that he can drink.
- the company selling the drink should disclose “drink immediately after mixing to get a fluid” or alternatively “drink the fluid gel after 10 to 15 min after mixing”.
- the composition has an acid aftertaste.
- Weight ratios of 1 .0/1 .2/0.3/0.23 gives less foam that disappears after a while and dissolves quickly the powder ingredients.
- a fluid is obtained by simply stirring the composition with a spoon. (pH 5.9, (19.6°C)).
- the consumer can drink it immediately. If he prefers to wait a while (e.g., 10-15 min) a drinkable fluid gel results that he can drink.
- the company selling the drink should disclose “drink immediately after mixing to get a fluid” or alternatively “drink the fluid gel after 10 to 15 min after mixing”.
- the composition has a more neutral aftertaste.
- Weight ratios of 1.0/1.4/0.26/0.6 or 1.0/1.8/0.5/1.0 gives a foam that disappears after a while and dissolves quickly the powder ingredients by simply stirring the composition with a spoon. Immediately a fluid is obtained. (pH: 3.92 (20.7°C).
- the composition has an acid aftertaste.
- Higher concentrations of Arabic gum relative to KGM gives fluid systems (e.g., KGM/Gum Arabic: 1 : 1.5 or 1 :1.6 or 1 :1.7 etc.).
- an effervescent system e.g., sodium (potassium) hydrogen carbonate, a hydroxy acid (in this case: citric acid) and the resulting sodium citrate (or the resulting potassium citrate) are a “very fast dissolver” or a “very fast suspending” combination for a KGM/Arabic gum composition and reduces the viscosity of KGM (>1.4).
- lipophilic and/or surface-active components as a powder (actives, oils, lipids and/or surfactants) to the effervescent system of KGM/Arabic gum/sodium or potassium (bi) carbonate/hydroxy acid (e g., citric acid) and, optionally, a water-soluble salt (D") leads to a milk-like product or foamed milk-like product (instant milk, or foamed instant milk) or milk alternatives product (milk substitute products, non dairy substitutes, plant based milks, nondairy beverage) or cocoa-like product.
- the lipophilic (and/or surface active) component can be added as a) a powder or b) a liquid.
- powders are: a1) full cream milk powder, a2) coffee powder (expresso etc.), a3) wheat germs, a4) almond flour, a5) cacao powder, a6) MCT powder, a7) omega-3 powder, a8) oatmeal/oatflour powder, a9) soymeal/soyflour powder.
- liquid oils examples include: b1) Linseed oil + EPA + DHA (liquid omega 3, containing ALA, EPA and DHA); examples of other liquid oils are flaxseed oil, fish oil, borage oil, blackcurrent seed oil, evening primrose oil, olive oil, MCT oils (e.g., coconut oil).
- Linseed oil + EPA + DHA liquid omega 3, containing ALA, EPA and DHA
- examples of other liquid oils are flaxseed oil, fish oil, borage oil, blackcurrent seed oil, evening primrose oil, olive oil, MCT oils (e.g., coconut oil).
- An instant full cream milk powder (lactose free) is available from Saputo, Australia (Instant Full cream Lactose Free powder 25 kg). It contains a mixture of fat (minimum 28%), proteins (at least 35%, calcium (1g/100g).
- a powder mixture of KGM/Arabic gum/hydrogen carbonate/citric acid/full cream milk powder (1 ,00g/1 ,80g/0.3g/0.23g/3.60g was added to 9307 g of water and stirred with a spoon
- a fluid composition results (pH: 6.2 (19.7°C) with a white appearance Due to the full cream milk powder the product has a rich mouthfeeling.
- the effervescent system was adjusted to a total content of 0 53g/100g so that a consumer may notice a small amount of foam.
- Using higher amounts of the effervescent system gives a foaming milk like composition.
- no external foam equipment or a small handheld electric mixer producing this foamed milk is necessary (the foam comes from the development of CO2).
- a composition comprising KGM/Arabic gum/sodium or potassium (bi) carbonate/hydroxy acid (e.g., citric acid) can be added to a full cream milk powder.
- KGM/Arabic gum/sodium or potassium (bi) carbonate/hydroxy acid e.g., citric acid
- NaHCO3 part of the effervescent system
- KHCO3 potassium can be delivered as potassium citrate.
- a combination of NaHCO3/KHCO3 delivers both minerals for a consumer.
- Typcial vitamins are B-vitamins like vitamin B2 (riboflavin) vitamin B12 (riboflavin), the B-vitamin complex (all B-Vitamins), vitamin A, vitamin D, vitamin E, vitamin K or vitamin C.
- Proteins like whey (concentrates, isolates, hydrolysate) or from other protein sources (already mentioned in the patent) can be added.
- the composition can be a foamed milk (total effervescent content (0.88 g/100 g or 0.53/100 g) or a milk were the foam is not visible for a consumer.
- Other ingredients like skim milk, whole milk, anhydrous milk fat can be used and are also available from Saputo Dairy Australia Pty Ltd.
- a ready to drink liquid coffee formulation with a powder composition of instant full cream milk powder, coffee powder (espresso) and an effervescent system that release CO2 for the development of a foam, KGM and arabic gum can be prepared. Additional ingredients are usually sugars, a coffee flavour, caramel, salts, colorants.
- the foam comes from the effervescent sytem (CO2 development) instead from a device that, gives the foam (air).
- actives e.g., proteins like whey proteins or other proteins like milk, egg, casein, pea, animal, fish, poultry, soy, almond, chia, hemp, rice, collagen, vegan collagen, a-lactalbumin
- actives e.g., proteins like whey proteins or other proteins like milk, egg, casein, pea, animal, fish, poultry, soy, almond, chia, hemp, rice, collagen, vegan collagen, a-lactalbumin
- Body height 1.70 m, weight: 80 kg, BMI: 28 kg/m2;
- the person would have a BMI of 22 kg/m2 at a body weight of 63 kg, i.e., normal weight.
- 50 g of proteins can be supplemented with three drinks/day (16.6 g/drink).
- Powder mixture comprising of KGM/Arabic gum/effervescent system/whey protein concentrate.
- compositions of the invention can be easily used by the consumers at home. Indeed, a consumer may just use a spoon as "stirrer”, which is sufficient to make the KGM more fluid thanks to the further components of the composition.
- An alternative to a spoon is a small handheld electric mixer. If a consumer has a problem to dissolve the KGM composition the company selling the KGM composition should give the advice to use a milk foamer, manual model or handheld stick frothers (e.g., Nursingaufschaumer FINO, rotation speed 12.000 U/Min, sacred-Nr. : 12720, GEFU GmbH, Braukweg 28, 59889 Eslohe, Germany). In this case the device is used to stir the composition (instead of foaming, in this case there is no foam development). On the other hand, in industrial processes, a typical industrial stirrer can be used.
- composition of the invention may also comprise additional active ingredients or compositions, for instance, substances effective in promoting weight loss. All the components of the compositions of the invention are commercially available.
- the water phase may be represented by tap water, sparkling water or still water.
- the water phase may contain (e.g. for industrial processes) water soluble ingredients like polyols, sugars, acids (hydroxy acids, citric acid, lactic acid, tartartic acid, solutions (e.g. gluconic acid, 50%, lactic acid solutions) water soluble flavors, water soluble vitamins (e.g.
- B-Vitamins, Vitamin C and derivatives water soluble salts, water soluble dextrins, water soluble proteins, protein concentrates, protein isolates, protein hydrolysates from whey, milk, egg, casein, pea, animal, fish, poultry, soy, almond, chia, hemp, rice, lentins, lupins, collagen, vegan collagen, water soluble amino acids, water soluble surfactants, water soluble colors, water soluble sweetener, (e.g.
- stevia, sucralose, erythritol polymers like xanthan gum, sodium carboxymethylcellulose, pectins, high methoxyl pectin, carrageenans, cellulose gum, gellan gum, guar gum, locust bean gum, propylene glycol alginate, gum tragacanth, microcrystalline cellulose, distarch phosphate, acetylated starch, acetylated distarch adipate, hydroxypropyl distarch phosphate, hydroxypropyl starches, starch sodium octenyl succinate, fructooligosaccharide, galacto-oligosaccharides, water soluble powders (like cacao powder, milk powder etc.), carbonate salts, hydrogen carbonate salts, water soluble minerals, lactones like glucono deltalacton, dextrose monohydrate, mannitol, xylit, allulose, maltitol, lactose monohydrate, g
- an arable gum available in the market is for example a raw material that is water soluble, pH 4-5, loss of drying ⁇ 10%, Ash ⁇ 4%, viscosity (25% in water) of 60-180 mPas (Benecke GmbH, Hamburg, Germany, Norevo GmbH, Germany, Nexira, France).
- a highly emulsifying Type 4810, or Type 4810, or Type 4810, or Type 4810 (Benecke GmbH) can be used in accordance with the present invention.
- Other sources of Arabic gum are from the company Nexira (Tradenames: Efistab AA, SuperStab, Eficacia, InstaGum AX, Instagum AA, Instagum AP).
- a KGM as a raw material with starch content ⁇ 1- 3%, preferred ⁇ 3%, protein content ⁇ 1.5-3.0%, preferred ⁇ 3.0%, pH 5-7, Ash ⁇ 5%, glucomannan content >85-90%, preferred >90%, more preferred from 90% to 98%, for example 92%-95%, viscosity from 26,000 to 36,000 mPas, preferred > 36,000 mPas, more preferred from 36,000 to 80,000 mPas, particle size from 90 to 200 mesh, preferably 120 to 200 mesh.
- KGM is available from FH Diedrichs & Ludwig Post GmbH, Mannheim, Germany, Neupert Ingredients, Garstedt, Germany, C.E. Roeper GmbH, Hamburg, Germany, Baoji Konjac Chemical Co. Ltd, China.
- a KGM E425(i) and/or E425(ii) can bused in accordance with the present invention.
- a Konjac glucomannan (KGM) commercially available into the market having preferably CAS RN No. 37220-17-0.
- KGM Konjac glucomannan
- it may be used a Konjac gum preferably indicated with E425(i) or E425(ii), both containing Konjac glucomannan.
- a high molecular weight KGM e.g., >10 6 Da, preferably e.g., from >10 6 Da to 10 e Da, more preferably e g., from >10 6 Da to 10 7 Da).
- a high molecular weight KGM with a viscosity value comprised from 20,000 ops to 100,000 ops, preferably comprised from 25,000 cps to 80,000 cps, more preferably comprised from 30,000 cps (30,000 mPas) to 60,000 cps, even more preferably comprised from 35,000 cps to 50,000 cps, for example bigger than 36,000 cps.
- Sodium hydrogen carbonate salt is available fromSolvay Chemicals International: BICAR® FOOD, A+E. Fischer- Chemie GmbH & Co. KG, Wiesbaden, Germany, Carl Dicke GmbH & Co KG, Mbnchengladbach, Germany) and potassium hydrogen carbonate is available from company Dr. Paul Lohmann).
- vanilla flavor is available from Sensient, Geesthacht, Germany.
- Blood orange flavour is available from OlbrichtArom GmbH & Co. KG, Leisnig, Germany.
- Blue berry flavor is available from IMCD Kunststoff GmbH.
- Color is avalable from Vanilla Yellow P-WS, Sensient, Geesthacht, Germany.
- Sucralose is available from Atlantic Chemicals Trading GmbH, Hamburg/Germany.
- Citric acid is available from Jungbunzlauer (“Citronensaure Anhydrat”).
- subject-matter of the invention is a solid pharmaceutical, nutraceutical or food composition or a composition for medical device under EU Reg. 745/2017 which comprises the solid fast dissolving or fast dispersing combination of the invention, as above defined, and a) optionally, at least flavours and/or colorants and/or sweeteners; and b) optionally further (active) ingredients.
- Illustrative and non-limiting examples of such (active) ingredients that can be combined with the solid fast dissolving or fast dispersing composition of the present invention containing KGM and Arabic gum are the following: Antocyanines such as “Brainberry” from company Bioactor, Aronia melanocarpa extract (25% Anthocyanins) or antocyanins/polyphenols mixtures (“Berriesence”) from MOB Ming Chyi Biotechnology Ltd.
- Antocyanines such as “Brainberry” from company Bioactor, Aronia melanocarpa extract (25% Anthocyanins) or antocyanins/polyphenols mixtures (“Berriesence”) from MOB Ming Chyi Biotechnology Ltd.
- Carob bean extract and fructooligosaccharide such as “CSAT+” is available from company Pharmactive Biotech Products.
- Black garlic such as “ABG10+” is available from company Pharmactive Biotech Products.
- Coffein such as “Caffeine, 98%'' is available from company OmniActive Health Technologies or “CaffXtend” is available from Nutriventia (Mumbai, India) or from soluble coffee extracts such as “Arabica kraftig, 23-10234”, “Espresso, aromatisch (Arabica/Robusta-Blend), 23-10236”,” Arabica mild, 23-10232”, “Espresso, medium, Robusta, 23-10235”, “Robusta, kraftig, 23-10233”, “Robusta, mild, 23-10231” are available from Deutscheset Ex Cool GmbH, Hamburg, Germany.
- Cacao powder such as “Noir Intense”, “Plaine Arome”, “Extra brute”, “Rouge Ultime”, “Legere”: 1% are available from Barry Callebout Kunststoff GmbH, Im Mediapark 8a, 50670 Kbln.
- Actives in cacao are polyphenols like flavanols (epicatechin, catechin, and procyanidins) and flavonols (quercetin), anthocyanins, phenolic acids, and stilbenes.
- coffein is in guarana (“Guarana Extract PDR & N. Caff BLDR22%-904”) is available from OmniActive Health Technologies.
- Collagens are available from the companies Gelita AG, Rousselot, Weishardt, Nitta Gelatin. Abyss or vegan collagens are available from company MCB Ming Chyi Biotechnology Ltd (“CollaGem”) or from INB GmbH (“Vollagen”) or from Vecollal/TCI (“VeCollal”).
- Chromium salts like “Chromium picolinate” are available from Vertellus, Indianapolis, USA.
- Niacin is available from Wegochem Europe B.V., Netherlands.
- Alpha Lipoic acid such as “Alipure” is available from Alzchem Group AG, Germany.
- Whey hydrolysate with an active dipeptide AP (Alanine-Proline) which inhibits the alpha glucosidase's activity such as “Pep2Dia” is available from Ingredia Dairy experts
- Cinnamon or cinnamon extracts are available from Flavex Naturextrakte GmbH, Rehlingen, Germany (Zimtrinden Ceylanicum CO2-se Exschreib, Nr. 034.001).
- Curcumin is available from the company Gencor (“HydroCurc® Liquid Grade 10%”) or from company OmniActive Health Technologies (“Curcuwin Ultra+ Curcuminoids 20%” or “CurcuWIN Curcumin DNS HB Pdr 20%/EU”) or from company Natural remedies, India (“Turmacin”), or from company Nutriventia, Mumbai, India (“Water Dispersible Turmeric Extract Powder 60N” or “unstain”).
- Vitamins lipophilic like Vitamin A, D, E, K
- B-vitamins or the “B-vitamin complex” can be added to the formulations.
- Thiamine Vitamin B-1
- Vitamin B6 helps the body cells convert carbohydrates into energy.
- Vitamin B6 can help with weight loss by boosting metabolism.
- vitamin C, niacin, iron, calcium, and vitamin B5 can help to speed up metabolism.
- a slow-release vitamin C formulation is “C-Fence” which is available from Nutriventia, Mumbai, India.
- a heat stabilized vitamin C is “Stabilized Vitamin C 80” which is available from Nutriventia Mumbai, India.
- Oat powder is available as Hafermehl Vollkorn (Art Nr. 4.1), Rubin Miihle GmbH, Germany.
- Dried fruits and/or freeze dried fruits and/or individually quick frozen (IQF) fruits and/or frozen fruit products such as A ai, Acerola, Apple, Apricot, Aronia, Avocado, Banana, Berry mixes, Blackberry, Blackcurrant, Black Cherries, Blood Oranges, Blueberry, Boysenberry, Breadfruit, Camu Camu, Carambola, Carissa, Cherimoya, Cherries, Cherries Morello, Citrus, Cloudberry, Corinthians, Crabapples, Cranberries, Currants, Dates, Durian, Feijoa, Figs, Gooseberries, Grape, Grapefruit, Groundcherries, Guarana, Guava, Jackfruit, Juneberry, Kaffir Lime I Combava, Kiwi, Kumquats, Langsart, Lemon, Lime, Lingonberry, Longan, Loquats, Lychee, Mandarin, Mango, Mangosteen, Marionberry, Melon, Mint, Mulberries, Nectarine, Oranges, Papaya, Passion Fruit, Pawpaw, Peaches,
- Dried fruits and/or freeze-dried fruits and/or individually quick frozen (IQF) fruits and/or frozen fruit products are available, for example, from TALI e.K., Steinweg 1, 34298 Helsa, Germany or Allfood GmbH-Handels-technik mbH, Bavariaring 2, D-80336 Munchen or FRUKTIA GmbH, Haferplanetary 10A, 28357 Bremen, Germany or Buxtrade GmbH, An den Geestergen 1 , 21614 Buxtehude.
- Raspberries whole freeze dried (Himbeeren ganz gefriergetrocknet) are available from Buxtrade GmbH, Germany.
- Carnitin, Carnitin Hydrochloride such as “L-Carnitin HCI” are available from Liaoning Koncepnutra Co Ltd.
- Resveratrol such as “ResveraFen” is available from Akay Natural Ingredients Private Limited, containing resveratrol, de-bitterised fenugreek powder rich in soluble dietary fibre, Sunflower lecithin and/or Sunflower oil; or Resveratrol such as “Veri-Sperse®” from Gencor/pharmaco biotechnologies.
- Capsaicin such as “Capsifen” is available from Akay Natural Ingredients Private Limited, chili extract, fenugreek powder, Cellulose powder and/or Lecithin, gum acacia; or “Capsimax” from OmniActive Health Technologies.
- Omega-3 such as “DHA Algae Powder DAF” is available from MOB Ming Chyi Biotechnology Ltd.
- Citrus Sinensis fruit extract (Anthocyanins, Hydroxycinnamic acids, Flavanones, Vitamin C) such as “Morosil” or “Red orange complex” are available from Bionap.
- Weight Management Actives such as Flavonoids like Bioflavonoids are available from Citrus sinensis (Hesperidin) and Caffeine.
- Bioflavonoids from citrus sinensis & citrus paradisi Hesperidin >80%; Naringin >5%
- Bioflavonoids from citrus sinensis & citrus paradisi such as “Microbiomex®” are available from Bioactor or bioflavonoids from citrus sinensis (hesperidin > 90%) (“Cordiart”) from Bioactor.
- Vitamin D (Vitamin D3) powder 100000 I U/G is available from company Echemi.
- Beta-glucane is available from company Leiber (“Yestimmun Beta Glucan”).
- Green Tea extract (EGCG,” Teavigo”) or ECG (“Sunphenon ECG”), or EGC “Sunphenon XLB-100”) or EC (“Sunphenon EC”) all are available from Taiyo.
- Oils, lipids like MCT (Medium chain triglycerides), like “Powder MCT C8 coconut 70% vegan” are available from Bioriginal or “Medium Chain Triglycerides (MCT-CTS or MCT-CPH”) oil powder from MCB, or Sumbia - Keto-HiMCT (high in C8 fatty acids) from Nutriventia (Mumbai, India), skim milk and whole milk and full cream milk powders from Saputo, Australia (“Instant Full cream Lactose Free powder 25 kg”), anhydrous milk, butter fat or liquid oils such as linseed oil, coconut oil.
- MCT-CTS or MCT-CPH Medium Chain Triglycerides
- Carallum Fimbriata Extract such as “Slimaluma” are available from Gencor Pacific Limited.
- Whey proteins hydrolysates, concentrates, isolates
- Whey protein concentrate 80 Instant are available from Vivi Solution.
- Milk protein concentrates like MPC 80/85 are available from Saputo Dairy Australia Pty Ltd.
- Probiotics like B. psychraerophilum Q5 (DSM 33131) + L. hordei KLL19 (DSM 33127) + L. harbinensis G8 (DSM 33126) such as "premix novaMETABOLISM” are available from Probionova or Bifidobactehum breve B-3 from Morinaga Milk Industry, a mixture of Lacidophilus PBS066-DSM 24936 (Synbalance LA) and L. reuteri PBS072- DSM25175 (SynBalance LR) and L. plantarum PBS067-DSM24937 (Synbalance LP) with the tradename MetSyn are available from company Synbalance, Italy
- ROS reactive oxygen species
- composition of the invention also solves this problem so that any other useful substance may be added to the composition of the invention, provided that said substance is chemically compatible with the other components of the composition of the invention.
- Said colorants may also be active coloured ingredient, such as MorosilTM (comprising anthocyanins, hydroxycinnamic acids, flavanones, and ascorbic acid) or Brainberry® (comprising cyanidin-3-O-glycosides from Aronia melanocarpa'j without adding colorants.
- MorosilTM comprising anthocyanins, hydroxycinnamic acids, flavanones, and ascorbic acid
- Brainberry® comprising cyanidin-3-O-glycosides from Aronia melanocarpa'j without adding colorants.
- composition of the invention having the correct pH, i.e., pH ⁇ 6, so that said active-coloured ingredients may develop their nice red colour (both ingredients do not show a red colour at pH 7 or higher).
- any further conventional excipient may be added to the composition, provided it is chemically compatible with the other components of the composition of the invention.
- the solid fast dissolving or fast dispersing composition of the invention is a mixture of powders and/or granules which must be mixed with suitable water-based fluid, as above indicated, before intake.
- the solid fast dissolving or fast dispersing composition of the invention is to be administered by oral route, after having been mixed with water or another water-based fluid
- a drinkable composition obtained by mixing the solid fast dissolving or fast dispersing composition of the invention with water or another water-based fluid is another subject-matter of the invention.
- subject-matter of the invention is a liquid/fluid composition comprising the solid fast dissolving or fast dispersing combination or the composition of the invention, and a suitable drinkable liquid, preferably, but not limited to, water.
- the drinkable composition of the invention may be prepared by diluting the solid fast dissolving or fast dispersing combination or the composition of the invention with a suitable liquid, preferably water.
- the subject matter of the present invention relates to a process for preparing a drinkable compositition comprising the steps of: a) putting into contact a solid fast dissolving or fast dispersing composition of the invention with a water-based fluid, which is selected from water, a beverage, a milk, a milk substitute, a plant-based milk, non dairy substitute, nondairy beverage, instant milk; non-alcoholic, alcoholic, a fruit drink, energy drink, fortified drink, herbal drink, sport drink, yogurt drink, buttermilk drink, probiotic drink, a nectar, a juice or a cappuccino, a smoothie, a coffee, a macchiato, a cafe au lait, a coffee milk, a frapuccino, an espresso, an espresso macchiato, a latte espresso, a flat white, a caffee latte, a cacao, or mixtures thereof; b) mixing, preferably with the aid of a spoon, for a time from a water-based fluid
- the solid fast dissolving or fast dispersing compositions and/or the drinkable compositions of the invention are for use in a subject, said subject being a mammal, preferably a human being.
- a solid fast dissolving or fast dispersing composition of the invention may be prepared by simply blending the solid powder components of the composition and then may be packaged in multi-dose container or as a unit dose form, such as a sachet of a stick-pack.
- the mixture of powders and/or granules of the invention can be part of a two-part system (where the powder is separated from the fluid phase, e.g., in a cap of a biphase container), a can, a cardboard can, paper can, plastic packaging boxes, biodegradable packaging containers.
- the solid fast dissolving or fast dispersing compositions and/or the drinkable compositions of the invention are useful in the management of body weight, especially in the treatment and/or prevention of overweight and obesity, and also for lowering cholesterol and blood sugar levels.
- solid fast dissolving or fast dispersing compositions and/or the drinkable compositions of the invention for use in reducing body weight in overweight and obese subjects.
- the use of the solid fast dissolving or fast dispersing composition of the invention in promoting body weight loss in a healthy subject is not a therapeutic one.
- solid fast dissolving or fast dispersing compositions and/or the drinkable compositions of the invention for use in lowering cholesterol and blood sugar levels in a subject.
- the invention also relates to a method for reducing body weight which comprises administering to a subject in need thereof an effective amount of a solid fast dissolving or fast dispersing composition and/or a drinkable composition of the invention.
- the solid fast dissolving or fast dispersing composition of the invention is generally administered at a dose of about 1-5 g/day of KGM, preferably 2-4 g/day of KGM, more preferably about 3g /day of KGM, preferably in the context of an energy restricted diet.
- the solid fast dissolving or fast dispersing composition of the invention is administered at a dose of 1 g/day of KGM three times per day.
- FIG. 1 shows a simulation of fluid KGM behaviour after ingestion.
- Konjac Glucomannan powder was added to water in a beverage glass and stirred with a spoon. Lump formation was observed. A greyish, lump containing gel formulation was obtained that is not appealing for a consumer. Drinking of this formulation is impossible due to the high viscosity of 36,000 mPas. The gel does not flow so that a consumer can not drink it from the beverage container. Furthermore the taste of this Konjac glucomannan gel is very bad.
- Morosil only partially show their red color at neutral pH.
- Konjac Glucomannan Gum Arabic, Tripotassium Citrate Monohydrate, Citric acid anhydrous, Brainberry (Aronia melanocarpa extract), Sucralose and blueberry flavor were mixed and the powder mixture was added to water and stirred with a spoon.
- the formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1).
- Konjac Glucomannan Gum Arabic, Tripotassium Citrate Monohydrate, Citric acid anhydrous, Watts'up (bioflavaonoids from citrus sinensis (Hesperidin), caffeine, sucralose the color additive (Vanilla Yellow), and orange flavor were mixed, and the powder mixture was added to water and stirred with a spoon.
- the formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1).
- Konjac Glucomannan and Tripotassium Citrate Monohydrate were mixed and the powder mixture was added to water and stirred with a spoon.
- the formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1). It is a fluid. It gives a fluid gel after 10-15 minutes.
- Konjac Glucomannan and Potassium Gluconate were mixed, and the powder mixture was added to water and stirred with a spoon.
- the formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1). It is a fluid. It gives a fluid gel after 10-15 minutes.
- Konjac Glucomannan Gum Arabic, Tripotassium Citrate Monohydrate, Citric acid anhydrous, Bioflavonoids from citrus sinensis & citrus paradisi, Sucralose, Color additive (Vanilla Yellow) and Blood orange flavor were mixed and the powder mixture was added to water and stirred with a spoon. The mixture gives a small foam (C02 release) that breaks down after a while. The formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1).
- Konjac Glucomannan Gum Arabic, Sodium hydrogen carbonate, Citric acid anhydrous, Sucralose, Whey protein concentrate 80 (Whey protein concentrate), Morosil (Citrus Sinensis fruit extract), Blood orange flavor were mixed, and the powder mixture was added to water and stirred with a spoon. The mixture gives a small foam (CO2 release) that breaks down after a while.
- the formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1).
- Konjac Glucomannan, sodium hydrogen carbonate, Citric acid anhydrous were mixed and the powder mixture was added to water and stirred with a spoon.
- Very fast dissolution of Konjac Glucomannan Due to the dissolved CO2 a creamy taste result.
- Konjac Glucomannan Sodium hydrogen carbonate, Citric acid anhydrous and Tripotassium Citrate Monohydrate were mixed, and the powder mixture was added to water and stirred with a spoon. Very fast dissolution of Konjac. A fluid is obtained. After 15 min due to the dissolved CO2 a creamy taste result. A smooth gel (drinkable) result.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Polymers & Plastics (AREA)
- Food Science & Technology (AREA)
- Nutrition Science (AREA)
- Diabetes (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Obesity (AREA)
- Hematology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- Dispersion Chemistry (AREA)
- Emergency Medicine (AREA)
- Child & Adolescent Psychology (AREA)
- Molecular Biology (AREA)
- Epidemiology (AREA)
- Mycology (AREA)
- Endocrinology (AREA)
- Coloring Foods And Improving Nutritive Qualities (AREA)
Abstract
The present invention relates to novel solid fast dissolving or fast dispersing combinations containing Konjac glucomannan, to processes for preparing drinkable compositions comprising them, to drinkable compositions comprising them and to their use as a food supplement/beverage supplement in particular, but not limited to, in the managing of weight loss or overweight reduction, and in cholesterol and blood sugar levels control in a subject in need.
Description
NOVEL COMBINATIONS COMPRISING KONJAC MANNAN, THEIR COMPOSITIONS AND USES THEREOF
The present invention relates to novel solid fast dissolving or fast dispersing combinations containing Konjac mannan, to processes for preparing drinkable compositions comprising them, to drinkable compositions comprising them and to their uses as a food supplement/beverage supplement, in particular, but not limited to, in the managing of weight loss or overweight reduction, and in cholesterol and blood sugar levels control in a subject in need.
Konjac is an Asian plant with a starchy root rich in fiber called glucomannan. Konjac mannan (or Konjac glucomannan) is a high molecular weight, water-soluble, non-ionic glucomannan found in tubers of the Amorphophallus konjac plant (Konjac Mannan - an overview Science Direct Topics; Handbook of Hydrocolloids (Second Edition), 2009.
Konjac flour is produced from the corms of the Amorphophallus konjac plant, a tropical perennial crop. The corms of the plant contain about 30%-50% glucomannan gum. When the corms are dried and milled, the resulting powder is konjac flour. The flour constitutes 60%-80% of the dried root corm. The corms grow below ground and are harvested at 2 kg for commercial processing. They are then washed and cut into thin slices, which are then dried and dry-milled into powder. The powder is separated, usually by cyclone or wind separation, producing crude konjac flour. Glucomannans in which some of the mannose residues are replaced by D-glucose are found in softwoods, tubers, and bulbs.
Konjac glucomannan (KGM) is a linear random copolymer of (1-4) linked beta-D-mannose and beta-D-glucose. It has mannose and glucose units at molar ratio of 1.6:1 with a low degree of acetyl groups (approximately 1 acetyl group per 17 residues) at the C-6 position. The degree of water solubility is controlled by the presence of the acetyl units.
The natural polysaccharide Konjac glucomannan (hereinafter referred to as KGM; see, for example Formula (I)) is a water-soluble and non-ionic polymer which is mainly composed of glucomannan, whose glucose and mannose units are connected by p-1 ,4-linkages, with a low degree of acetyl groups at the side chain C-6 position, as shown in Formula (I):
The p-1 ,4-linkages are not digested by human enzymes of the microbiota in the human Gl tract. This is due to the
p-glycosidic linkages between the mannose and glucose molecules, which cannot be hydrolysed by pancreatic amylase or salivary amylase. This means that it passes into the colon without being digested. Once it is in the colon, it is fermented by the native flora to produce glucomannan-oligosaccharides. Because it is not digested immediately, konjac glucomannan can increase feelings of satiety, leading the consumer to eat less.
KGM has been widely used in food industry as it has been authorized as a food additive in Europe and classified as GRAS (Generally Recognized as Safe) by the FDA (Food and Drug Administration.
However, despite its interesting beneficial properties, the KGM powder used as such shows too many drawbacks. For example, after adding 1g KGM powder (high molecular weight) in 99 g water at a temperature of 25°C under constant stirring with, for example, a spoon it can take up to 10-15 minutes to form a gel in water. To have a healthy effect from the KGM, it is preferred to use a high molecular weight KGM and due to this high molecular weight, the dissolution or gel formation of the KGM powder in water is time consuming for a consumer, and the formation of lumps (agglomeration of KGM in water) is a further disadvantage. Furthermore, the resulting gel is very stiff and does not flow easily out of the drinking glass to the mouth.
Further, it has a very bad mouthfeel, and the taste of the resulting gel is not acceptable. In addition to the above, the gel colour is grayish/grey, therefore it is not very appealing, and its viscosity makes difficult to swallow it. According to EFSA, to reduce overweight it has to be in-taken three times a day (at 1 g level, 3x1g/day); it is therefore self-evident that the above drawbacks are not in favour of the compliance.
Therefore, it remains very high a need to provide an effective solution for the administration of KGM, which overcomes the drawbacks and inconveniences showed above which is easy to be prepared and is able to meet a level of compliance much higher.
The patent application CN105901269A discloses a Konjac tablet candy for weight management.
The patent application CN1799410A discloses a diet fiber composition comprising Konjac glucomannan and Gum arabic in in the form of a capsule or chewable tablet.
The patent application CN108041570A discloses a food additive comprising Konjac gum, Arabic gum and polyphosphate salts to be added to a food material for obtaining a good emulsification, stability, thickening, water retention.
The technical problem that the present invention addresses and solves is to provide a solid fast dissolving or fast dispersing combination or composition of high molecular weight KGM, useful for preparing, for example, RTD beverage or another water-based fluid product for oral use containing high molecular weight KGM, that is easy to prepare (also with the only aid of a spoon), stable, easy to swallow and with an appealing mouthfeel, taste and colour.
The solid (e.g, powder) compositions for instant (or RTD, ready to drink) beverage according to the present invention are particularly advantageous and offer great compliance for those taking them. They are easy to prepare, stable, easy to swallow and suitable for a wide range of individuals, including the elderly and children. Usually they can be poured into water, or another water-based fluid, and quickly and easily, with the only aid of a spoon, transformed into a drinkable fluid having an appealing mouthfeel, taste and colour.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows a simulation of fluid KGM behaviour after ingestion. After the ingestion of a water formulation comprising KGM, the water penetrates into the tissue or blood stream in the body. To simulate the vanishing of water from the formulation a series of experiments was made; gel formation occurs again when the water is no longer a significant part of the formulation and gel formation lowers food craving.
DETAILED DESCRIPTION OF THE INVENTION
As a result of extensive research and development, the Applicant addresses and solves the above requirements by providing an innovative combination that allows KGM powder composition to be quickly and easily diluted in water, or another water-based fluid, resulting in a drinkable, stable and easy-to-take composition.
The KGM powder composition of the invention, by adding a fluid, leads preferably to:
- a fluid KGM composition, or
- a fluid gel KGM composition, or
- an effervescent fluid KGM composition (e.g., if the KGM powder composition contains an effervescent system), or
- an effervescent fluid gel KGM composition (e.g., if the KGM powder composition contains an effervescent system). In the context of the present invention, the term “fluid" may have, for example, the meaning of water, a translucent/transparent or colored water-based system, a milk (white fluid), a fluid similar to milk (milk substitute, plant-based milk, non dairy substitute, non-dairy beverage, instant milk), a beverage (non-alcoholic, alcoholic), a drink (fruit drink, energy drink, fortified drink, herbal drink, sport drink, yogurt drink, buttermilk drink, probiotic drink), a nectar, a juice, a coffee, a cappuccino, a smoothie, a macchiato, a cafe au lait, a coffee milk, a frapuccino, an espresso, an espresso macchiato, a latte espresso, a flat white, a caffee latte, a cacao.
In the context of the present invention, the term “fluid gel” may have, for example, the meaning of a system that is not a stiff gel (not flowable) but a flowable system (e.g., from a beverage glass so that a consumer can easily drink it). A fluid gel is a translucent/transparent gel (water based KGM system) or a white composition (milk-like appearance) with higher viscositiy so that a fluid gel-like KGM composition results that is drinkable) or a colored fluid gel KGM composition so that a colored fluid gel KGM composition results that is drinkable.
Preferably, a fluid gel of the invention may contain an effervescent system (e.g., mousse-like transparent or white or colored appearance). A mousse is a soft, light, and airy system, that may contain bubbles of C02 to give it a light and airy texture.
In the context of the present invention, the term “fluid composition” and “fluid gel composition” mean that they flow easily out from the drinking glass (or other containers) to the mouth of a consumer or from a spoon to the mouth of the consumer.
In the context of the present invention, the expression “fast dissolving or fast dispersing” means that, when a solid powder combination or composition of the invention is poured into a water-based fluid at a temperature from 18 °C to 40°C, preferably from 18°C to 25 °C, and stirred, preferably with the aid of a spoon, it easily gives, within a time from 10 seconds to 5 minutes, preferably from 1 minute to 3 minutes, a solution or a homogeneous fine dispersion
free of lumps, insoluble bottom residue, precipitates.
The Applicant has found a powder combination or composition comprising KGM that is able to be quickly and easily diluted in a water-based fluid (e.g., water) and to reduce the formation of lumps, improve the flow behaviour of a water-based composition out of a drinking glass, and reduce the viscosity of KGM powder when diluted in water or another water-based fluid, improving the mouthfeel of KGM, its taste, and the colour of the final drinkable composition.
According to one of its aspects, the subject-matter of the present invention relates to a solid combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with at least one further dissolving or dispersing aid component (i) to (ix) which is selected from group comprising or, alternatively, consisting of: i. at least one arabic gum and at least one water-soluble salt, as defined in the present invention; and/or ii. at least one arabic gum, at least one water-soluble salt, as defined in the present invention, and at least one acid; preferably at least one hydroxy acid, as defined in the present invention; more preferably, citric acid and/or tartaric acid; and/or ill. at least one water-soluble salt and, optionally, at least one acid; preferably, at least one hydroxy acid, as defined in the present invention; and/or iv. at least one effervescent system, as defined in the present invention and, optionally, at least one water- soluble salt, as defined in the present invention; and/or v. at least one arabic gum, an effervescent system, as defined in the present invention and, optionally, at least one water-soluble salt, as defined in the present invention; and/or vi. at least one sugar alcohol, preferably at least one sugar alcohol erythritol, as defined in the present invention; and/or vii. at least one sugar alcohol, preferably at least one sugar alcohol erythritol, as defined in the present invention, and at least one component selected from i. to ix. (not vii); and/or viii. at least one acid, preferably at least one hydroxy acid; and/or ix. at least one arabic gum and at least one acid, preferably at least one hydroxy acid, or their mixtures thereof, preferably in combination with one or more flavours and/or colorants and/or sweetener and/or oils (lipids) and/or surfactants and/or actives as defined above.
With reference to said at least one further component (i) to (ix), the term "component" herein includes either a single substance or mixture of substances thereof.
Konjac glucomannan (KGM) is a known and commercially available compound having for example CAS RN No 37220-17-0. It is also referred to as Konjac gum, Konjac Glucomannan, Glucomannan Konjac Jelly, Konnyaku and Amorphophallus Konjac Root Mannan. Preferably, KGM is for example in powder form For example, Konjac gum may be indicated with E425(i) or E425(ii), both contain Konjac glucomannan. It is known that a high molecular weight KGM (e.g., ^106 Da, preferably e.g., from ^106 Da to 10s Da, more preferably e.g., from ^106 Da to 107 Da) forms a gel in water which leads to feeling of satiety after ingestion (e.g., 1 g KGM).
For example, said high molecular weight Konjac glucomannan (KGM) has a molecular weight equal to or bigger than 106 Da; preferably, has a molecular weight comprised from bigger than 106 Da to 108 Da; more preferably, has a molecular weight comprised from 107 Da to 108 Da.
In the present invention, it is preferred to use a high molecular weight KGM than a low molecular weight KGM. Preferably, it is used a high molecular weight KGM with a viscosity value comprised from 20,000 cps to 100,000 cps, preferably comprised from 25,000 cps to 80,000 cps, more preferably comprised from 30,000 cps (30,000 mPas) to 60,000 cps, even more preferably comprised from 35,000 cps to 50,000 cps, for example bigger than 36,000 cps.
Advantageously, the solid fast dissolving or fast dispersing composition of the present invention can be mixed with a drinkable liquid or a drinkable fluid gel like composition, which may be selected from water, milk (e.g., cow milk), a milk substitute (plant based milk, non dairy substitute, dairy beverage), a carbonated fluid, a beverage, a drink (fruit drink, energy drink, fortified drink, herbal drink, sport drink, yogurt drink, buttermilk drink, probiotic drink), a nectar, a juice, a smoothie, a macchiato, a cafe au lait, a caffee latte, a coffee milk, a frapuccino, an espresso, an espresso macchiato, a latte espresso, a flat white, a cacao without forming lumps.
Arabic gum is also a known and commercially available compound, and it is also referred to as acacia gum, and preferably it is also in powder form. Arabic gum is the dried resin of acacia Senegal and related species.
Preferably, said at least one water-soluble salt mentioned in the component (i), (ii), (iii), (iv) and (v) above is more preferably selected from the group comprising or, alternatively, consisting of citrate salts, ascorbate salts, gluconate salts, chloride salts, lactate salts, tartrate salts, sulfate salts, glycinate/bisglycinate salts, acetyl taurinate salts, or mixtures thereof; more preferably, said at least one water-soluble salt mentioned in the component (i), (ii), (iii), (iv) and (v) above is selected from water-soluble alkali-metal salts and/or water soluble alkali-earth metal salts such as for example citrate salts, ascorbate salt, gluconate salts, chloride salts, lactate salts, tartrate salts, or their mixtures thereof. For example, chloride salts include sodium chloride, potassium chloride, calcium chloride and ammonium chloride.
Preferably, said at least one water-soluble salt mentioned in the component (i), (ii), (iii), (iv) and (v) above may be selected from a tripotassium citrate salt, more preferably may be a tripotassium citrate and/or tripotassium citrate monohydrate (company Pharmavit or from Magnesia/Jungbunzlauer) or trisodium citrate anhydrous (company Magnesia/Jungbunzlauer) or magnesium citrate (company Pharmavit) or zinc citrate or their mixtures thereof.
Preferably, said at least one water-soluble salt mentioned in the component (i), (ii), (iii) and (iv) above may be selected from a sodium and/or potassium ascorbate salt (Jebsen&Jessen) and/or calcium and/or magnesium ascorbate (company Global Calcium) and/or zinc ascorbate monohydrate (company BioIla Chemicals GmbH) or their mixtures thereof.
Preferably, said at least one water-soluble salt mentioned in the component (i), (ii), (iii), (iv) and (v) above may be selected from a sodium and/or potassium gluconate salt (company Magnesia/Jungbunzlauer) and/or calcium and/or magnesium gluconate (company Global Calcium) and/or zinc gluconate salt (company Magnesia/Jungbunzlauer) or their mixtures thereof.
Preferably, said at least one water-soluble salt mentioned in the component (i), (II), (Hi), (iv) and (v) above may be selected from an alkali-earth metal halogenide salt; e.g., an alkali-earth chloride salt (e.g., CaCfe x 2H2O, company Magnesia/Jungbunzlauer) or (e.g., MgCh x 6H2O, company Magnesia/Jungbunzlauer) or hydrate water free versions like calcium chloride salt or a magnesium chloride or their mixtures thereof.
Preferably, said at least one water-soluble salt mentioned in the component (I), (II), (ill), (iv) and (v) above may be selected from an alkali chloride salt, e.g., sodium chloride, potassium chloride, ammonium chloride, or their mixtures thereof.
Preferably, said at least one water-soluble salt mentioned in the component (I), (II), (ill), (iv) and (v) above may be selected from lactate salts, more preferably may be a calcium lactate pentahydrate (company Global Calcium) and/or sodium lactate (company Magnesia/Jungbunzlauer) and/or potassium lactate and/or zinc lactate dihydrate, or their mixtures thereof.
Preferably, said at least one water-soluble salt mentioned in the component (I), (II), (ill), (iv) and (v) above may be selected from tartrate salts, more preferably may be a potassium tartrate x 0.5 H2O (company Dr. Paul Lohmann) and/or potassium sodium tartrate x 4 H2O, or their mixtures thereof.
Preferably, said at least one water-soluble salt mentioned in the component (I), (II), (ill), (iv) and (v) above may be selected from sulfate salts, more preferably may be a sodium and/or potassium sulfate.
Preferably, said at least one water-soluble salt mentioned in the component (I), (II), (ill), (iv) and (v) above may be selected from glycinate salts, more preferably may be a sodium and/or potassium glycinate.
Preferably, said at least one water-soluble salt mentioned in the component (I), (II), (ill), (iv) and (v) above may be selected from bisglycinate salts, more preferably may be a zinc bisglycinate or zinc bisglycinate monohydrate (company BioIla Chemicals GmbH).
Preferably, said at least one water-soluble salt mentioned in the component (i), (ii), (Hi), (iv) and (v) above may be selected from acetyl taurinate salts, more preferably may be a calcium and/or magnesium acetyl taurinate (company Synapharm).
Preferably, said at least one acid, more preferably at least one hydroxy acid, mentioned in the components (II), (Hi), (in the components iv. and v. , here is an acid that forms the effervescent system together with a base), (viii) and (lx) above may be selected from at least one organic and/or inorganic acids, more preferably may be a hydroxy acid such as, for example, citric acid, lactic acid, ascorbic acid, tartaric acid, malic acid or other acids like phosphoric acid or glucono-delta-lactone (GdL -is a lactone that rapidly dissolves in water, and subsequently slowly hydrolyses to gluconic acid) and/or mixtures thereof; more preferably, said an acid mentioned in the component (ii), (iii), (in iv and v., here is an acid that forms the effervescent system together with a base), (viii) and (lx) above may be a hydroxy acid, most preferably it may be a citric acid or tartartic acid.
Preferably, said an effervescent system mentioned in the components (iv) and (v) above comprises or, alternatively, consists of at least one base that can release carbon dioxide.
According to the present invention, said an effervescent system comprises or, alternatively, consists of at least one basic substance (i.e., an organic and/or inorganic base) capable of releasing carbon dioxide in the presence of an
acidic substance (i.e. , an organic and/or inorganic acid) preferably in the presence of a composition or formulation containing water.
Preferably, said base may be a carbonate and/or bicarbonate salt, more preferably may be a sodium and/or potassium carbonate salt or sodium and/or potassium bicarbonate salt.
More preferably, said an effervescent system mentioned in the components (iv) and (v) above comprises or, alternatively, consists of a sodium and/or potassium bicarbonate salt (also referred to as sodium or potassium hydrogen carbonate salt, NaHCOs or KHCO3) and citric acid.
Preferably, said at least one water-soluble salt mentioned in the components (iv) and (v) above may be selected from alkali-metal carbonate salts; more preferably, may be a sodium carbonate and/or sodium hydrogen carbonate salt (Solvay Chemicals International: BICAR® FOOD, A+E. Fischer-Chemie GmbH & Co. KG, Wiesbaden, Germany, Carl Dicke GmbH & Co KG, Monchengladbach, Germany) and/or potassium hydrogen carbonate (company Dr. Paul Lohmann) or their mixtures thereof
Preferably, (A) the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with at least one arable gum, and at least one water-soluble salt, with the provision that said at least one water-soluble salt is not a phosphate or polyphosphate salt.
Preferably, (B) the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with an arabie gum, at least one water-soluble salt, and at least one hydroxy acid, such as preferably citric acid, lactic acid, ascorbic acid, tartaric acid, malic acid or other acids like phosphoric acid or glucono-delta-lactone (GdL).
Preferably, (C) the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with at least one water- soluble salt and, optionally, at least one acid, more preferably at leastone hydroxy acid, with the provision that said at least one water-soluble salt is not a phosphate or polyphosphate salt.
Preferably, (D’) the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with an effervescent system and, optionally, at least one water-soluble salt.
Preferably, (D”) the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with an effervescent system, an arabic gum and, optionally, at least one water-soluble salt.
Preferably, (E) the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with at least one sugar alcohol; more preferably, at least one sugar alcohol erythritol.
Preferably, (F) the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with at least one sugar alcohol; more preferably, at least one sugar alcohol erythritol, and at least one component selected from I. to lx.,
(not vii.).
Preferably, (G) the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with at least one acid, preferably at least one hydroxy acid; more preferably selected from citric acid and/or tartaric acid.
Preferably, (H) the invention refers to a solid fast dissolving or fast dispersing combination (or composition) comprising or, alternatively, consisting of Konjac glucomannan (KGM) in combination with at least one arabic gum and at least one acid, preferably at least one hydroxy acid, selected from citric acid and/or tartaric acid.
The solid fast dissolving or fast dispersing combination of the invention may be present in a composition for oral use such as a solid pharmaceutical, nutraceutical or food composition which comprises an aforementioned combination (i) to (ix) as above defined, and (x) optionally, at least one flavour and/or colorant and/or sweetener, and/or a carrier or at least one excipient pharma or food grade.
Even if not expressly disclosed, the term "composition'' herein includes pharmaceutical, nutraceutical or food compositions or compositions for medical devices under the Eli Reg. 745/2017.
Preferably, flavours and/or colorants and/or sweeteners may be of synthetic or natural origin. Preferably, components (x) may be selected from fruit flavours, such as vanilla, orange, blueberry, lemon, and the like; and sweeteners such as sucralose and/or stevia and/or erythritol.
Colorants may be any colorant which makes the more appealing the composition when mixed up with water, for instance yellow colorants, such as vanilla yellow and the like.
Colorants may also be selected from active-coloured ingredients, as it will be disclosed in detail in the following.
All the components used in the solid fast dissolving or fast dispersing combination and composition of the present invention are physiologically acceptable having a food or pharmaceutical grade.
All the components of the solid fast dissolving or fast dispersing composition of the present invention are in a powder form and/or in a granular form, to give a solid fast dissolving or fast dispersing composition of the present invention in the form of a mixture of powders and/or granules.
In the context of the present invention, the expressions “solid fast dissolving or fast dispersing composition” and “solid fast dissolving or fast dispersing combination” are intended to refer to and include any solid composition or combination of the present invention in the form of a loose and/or free-flowing mixture of powders and/or granules, i.e. it is not a in the form of a tablet, a chewable tablet, a pill, a candy, a capsule, etc.
Preferably, the composition of the invention comprises or, alternatively, consists of:
(A) KGM, arabic gum, and at least one water-soluble salt as defined above; or
(B) KGM, arabic gum, at least one water-soluble salt as defined above, and at least one hydroxy acid as defined above, preferably citric acid and/or tartaric acid; or
(C) KGM, at least one water-soluble salt as defined above and, optionally, at least one hydroxy acid as defined above; or
(D') KGM and an effervescent system as defined above and, optionally, at least one water-soluble salt as defined above; or
(D”) KGM, arabic gum, an effervescent system as defined above and, optionally, at least one water-soluble salt as defined above; or
(E) KGM and the sugar alcohol erythritol; or
(F) at least one sugar alcohol erythritol and at least one composition selected from (A), (B), (C), (D'), (D"), (G) and/or (H); or
(G) KGM and at least one hydroxy acid; or
(H) KGM, arabic gum and at least one hydroxy acid; or
(I) any one of the above compositions from (A) to (H) in combination with one or more flavours and/or colorants and/or sweetener and/or oils (lipids) and/or surfactants and/or actives as defined above.
More preferably, composition (A) comprises or, alternatively, consists of KGM, an arabic gum, and a tripotassium citrate salt; even more preferably, a tripotassium citrate monohydrate salt.
More preferably, composition (B) comprises or, alternatively, consists of KGM, an arabic gum, a tripotassium citrate salt; more preferably, a tripotassium citrate monohydrate salt, and citric acid.
More preferably, composition (C) comprises or, alternatively, consists of KGM, sodium and/or potassium gluconate salt and, optionally, a tripotassium citrate salt; more preferably, a tripotassium citrate monohydrate salt.
More preferably, composition (D’) comprises or, alternatively, consists of KGM, a sodium and/or potassium (bi)carbonate, and citric acid or tartraric acid (or a mixture citric acid/tartaric acid).
More preferably, composition (D”) comprises or, alternatively, consists of KGM, an arabic gum, a sodium and/or potassium (bi)carbonate, and citric acid or tartraric acid (or a mixture citric acid/tartaric acid).
More preferably, composition (E) comprises or, alternatively, consists of KGM and erythritol.
More preferably, composition (F) comprises or, alternatively, consists of erythritol and at least one of the compositions selected from (A), (B), (C), (D-), (D"), (G) and/ or (H).
More preferably, composition (G) comprises or, alternatively, consists of KGM and citric acid and/or tartaric acid. More preferably, composition (H) comprises or, alternatively, consists of KGM, arabic gum and citric acid and/or tartaric acid.
Preferably, in said composition (A) the weight ratio KGM/Arabic gum/salt is the range comprised from 1 : 1 :0.1 to 1 : 15:7, more preferably in the range comprised from 1 :1.5:0.5 to 1 :2:2, for instance in the range from 1 : 1 , 8:0, 8 to 1 : 1 , 8: 1.6, wherein the salt is preferably a tripotassium citrate, more preferably a tripotassium citrate monohydrate. Tripotassium citrate salt may be replaced by the other salts as defined above, for instance gluconates and ascorbates, such as gluconates (sodium, potassium, calcium), ascorbates (sodium), potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate anhydrous, magnesium chloride x 6H2O, calcium chloride x 2H2O (or hydrate water free versions of magnesium chloride, calcium chloride), sodium chloride, potassium chloride. Mixtures of salts may also be used.
Preferably, in said composition (B) the weight ratio KGM/arabic gum/salt/citric acid (or acid) is in the range comprised from 1/1/0.1/0.1 to 1/15/7/5, more preferably in the range comprised from 1/1/0.2/0.2 to 1/7.5/5/4, even more preferably in the range comprised from 1/1.5/0.3/0.3 to 1/2/4/3, for istance from 1/1.8/0.4/0.3 to 1/1 .8/1/2, or
from 1/1.8/0.4/0.4 to 1/1.8/0.8/1, for istance 1/1.8/0.4/0.4, wherein the water soluble salt is preferably tripotassium citrate, more preferably tripotassium citrate monohydrate. Tripotassium citrate salt may be replaced by the other salts as defined above, for instance gluconates and ascorbates, such as gluconates (sodium, potassium, calcium), ascorbates (sodium), potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate anhydrous, magnesium chloride x 6H2O, calcium chloride x 2H2O (or water free versions of magnesium chloride, calcium chloride), sodium chloride, potassium chloride. Mixtures of salts may also be used.
The citric acid may be replaced by other acids such as hydroxy acid like lactic acid, ascorbic acid, tartaric acid, malic acid or phosphoric acid or glucono-delta-lactone (GdL) which rapidly dissolves, and subsequently slowly hydrolyses to gluconic acid) or mixtures of acids
For example, compositions (A) showed a more neutral pH, while compositions (B), also comprising citric acid, showed pH< 6.5.
It was observed that the addition of at least one water-soluble salt as defined above to a powder mixture of KGM/Arabic gum (e.g., 1 g/1 g) resulted astonishingly to a faster dissolution process (suspending process) of KGM/Arabic gum in water than without the salt (e.g., a powder mixture of KGM/Arabic gum/tripotassium citrate monohydrate, preferably 1.0 g/1.0 g/1 .8 g in 96.2 g of water. Further, the addition of a salt, for example 1.8 g of tripotassium citrate monohydrate, it reduces the viscosity. A composition of a powder mixture comprising KGM/Arabic gum/ at least one water-soluble salt, for example in a weight ratio of 1/3.6/0.5, gives a fluid instead of a fluid gel (e.g., KGM/Arabic gum/tripotassium citrate monohydrate). Furthermore, a higher amount of arabic gum gives a nice mouthfeel.
Since arabic gum is a fibre, it is possible to use according to the present invention, for example in a drink, 9 g for example of a powder composition of KGM/Arabic gum/ at least one water-soluble salt, preferably in a weight ratio of 1 :9:1.6. Drinking this composition delivers 10 g fibre/drink or 30 g/day (3 drinks). The German organization “Deutsche Gesellschaft fur Ernahrung” https://www.dge.de/wissenschaft/referenzwerte/ballaststoffe/ recommends 30 g fibre/adults/day. The composition of KGM/Arabic gum/tripotassium citrate monohydrate 1 :9:1.6 is fluid and delivers 10 g of fibre/or 30 g/day (3 drinks). Even higher amounts of Arabic gum in a drink are also possible.
Preferably, the addition of lipophilic and/or surface-active components as a powder and/or granule (actives, oils, lipids and/or surfactants) to the system of (A) KGM/Arabic gum/at least one water-soluble salt leads to a milk-like product (instant milk) or milk alternatives product (milk substitute products, non dairy substitutes, plant-based milks, non-dairy beverage) or cocoa-like product at a approximately neutral pH range. The lipophilic and/or surface-active component can be added a) as a powder and/or granule or b) as a liquid, more preferably as a powder and/or granule.
Examples of powders: a1) full cream milk powder, a2) coffee powder, a3) wheat germs, a4) almond flour, a5) cacao powder, a6) MCT powder, a7) omega-3 powder, a8) oatmeal/oatflour powder, or a9) soymeal/soyflour powder. Examples of liquid oils: b1) Linseed oil + EPA +DHA (liquid omega 3, containing ALA, EPA and DHA)
Examples of other liquid oils are flaxseed oil, fish oil, borage oil, blackcurrent seed oil, evening primrose oil, olive oil, MCT oils (e.g. coconut oil).
Preferably, in said composition (C) the weight ratio KGM/water soluble salt is in the range comprised from 1/0.5 to 1/15, more preferably in the range of 1 :6, even more preferably 1 :3 for example with tripotassium citrate monohydrate. The pH of such a composition is around about 7.4 ± 2 (at20.5°C). Tripotassium citrate monohydrate salt may be replaced by the other water-soluble salts as defined above, for instance gluconates and ascorbates, such as gluconates (sodium, potassium, calcium), ascorbates (sodium), potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate anhydrous, magnesium chloride x 6H2O, calcium chloride x 2H2O (or water free versions of magnesium chloride, calcium chloride), sodium chloride, potassium chloride. Mixtures of salts may also be used.
Preferably, said composition D’ comprises or, alternatively, consists of KGM and an effervescent system, as defined above, and optionally a salt as defined above.
More preferably, said composition D' comprises or, alternatively, consists of KGM/sodium hydrogen carbon ate/ci trie acid and/or tartaric acid, and/or KGM/potassium hydrogen carbonate/citric acid and/or tartaric acid.
Preferably, in said composition D' the weight ratio of KGM/sodium and/or potassium hydrogen carbonate salt/citric and/or tartaric acid is in the range comprised from 1/0.1/0.06/ to 1/10/23, more preferably in the range comprised from 1/5/2.5 to 1/5/12, even more preferably in the range of 1.00/0.1/0.23, or more preferably 1/(0.1-0.60)/(0.1-1 .8), or more preferably 1/0.6/(0.25-1 .4), wherein the water-soluble salt in the composition D' is preferably tripotassium citrate, more preferably tripotassium citrate monohydrate. Preferably, the optional added salt is in the range from 0.1 g to 15 g.
Tripotassium citrate monohydrate salt may be replaced by the other water-soluble salts, as defined above, for instance gluconates and ascorbates, such as gluconates (sodium, potassium, calcium), ascorbates (sodium), potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate anhydrous, magnesium chloride x 6H2O, calcium chloride x 2H2O, (or water free versions of magnesium chloride, calcium chloride), sodium chloride, potassium chloride. Mixtures of salts may also be used.
Preferably, said composition D” comprises or, alternatively, consists of KGM/Arabic gum/sodium hydrogen carbonate/citric acid (and/or tartaric acid) and, optionally, at least one salt selected from the group comprising or, alternatevily, consisting of tripotassium citrate, preferably tripotassium citrate monohydrate, gluconates (sodium, potassium, calcium), ascorbates (sodium and/or potassium), sodium and/or potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate, preferably trisodium citrate anhydrous, magnesium chloride x 6H2O, calcium chloride X2H2O (or water free versions of magnesium chloride, calcium chloride), sodium chloride, potassium chloride; or said composition D” comprises or, alternatively, consists of KGM/Arabic gum/potassium hydrogen carbonate/citric acid (and/or tartaric acid) and, optionally, a salt selected from the group copmprising or, alternatevily, consisting of tripotassium citrate, preferably tripotassium citrate monohydrate, gluconates (sodium, potassium, calcium), ascorbates (sodium and/or potassium), sodium and/or potassium tartrate x O 5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate, preferably trisodium citrate anhydrous, magnesium chloride x 6H2O, calcium chloride x 2H2O (or water free versions of magnesium chloride, calcium chloride) sodium chloride, potassium chloride; or said composition D” comprises or, alternatively, consists of KGM/ Arabic gum, mixture of
sodium and potassium hydrogen carbonate/ citric acid (and/or tartaric acid) and, optionally, a salt selected from the group copmprising or, alternatevily, consisting of tripotassium citrate, preferably tripotassium citrate monohydrate, gluconates (sodium, potassium, calcium), ascorbates (sodium and/or potassium), sodium and/or potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate, preferably trisodium citrate anhydrous, magnesium chloride x 6H2O, calcium chloride x 2H2O (or water free versions of magnesium chloride, calcium chloride) sodium chloride, potassium chloride.
Preferably, in said composition D” the weight ratio of KGM/Arabic gum/sodium or potassium (bi)carbonateZcitric acid (and/or tartaric acid) is in the range comprised from 1/0.1/0.15/0 1 to 1/15/0.8/1.8, more preferably:
KGM Arabic gum Sodium Bicarbonate Citric acid
1 0: 15.0: (0.15-0.8): (0.15-1.5)
KGM Arabic gum Sodium Bicarbonate Citric acid
1 0: 15.0: 0 2: 0.38
KGM Arabic gum Sodium Bicarbonate Citric acid
1.0: (0.1-0.5):mim (0.3-0.6): (0.19-0.70)
KGM Arabic gum Sodium Bicarbonate Citric acid
1.0: 1.0: (0.3-0.6): (0.19 - 0.70)
KGM Arabic gum Sodium Bicarbonate Citric acid
1.0: 1.2: (0.3-0.4) (0.23-0.92)
KGM Arabic gum Sodium Bicarbonate Citric acid
1.0: 1.2: (0.3): (0.70)
KGM Arabic gum Sodium Bicarbonate Citric acid
1.0: 1.4: (0.15-0.6): (0.10 - 1.0)
KGM Arabic gum Sodium Bicarbonate Citric acid
1.0: 1.4: (0.225-0,263): (0.17- 0.60)
KGM Arabic gum Sodium Bicarbonate Citric acid
1.0: 1.8: 0.23: (0.175-0.53)
Preferably, if less arabic gum is present it makes the composition more fluid to fluid-gel like, more preferably the arable gum may be present at 1.4 or 1.5 or 1.6 or 1.7 weight ratio based on 100 g (e.g., 1.4 means 1.4%).
Preferably, arabic gum >1 .7 gives a fluid composition.
Preferably, in case of a drinkable liquid or a drinkable fluid gel like composition, with a high liphophilic content i.e., a high fat content, is preferred to use a higher amount of arabic gum which may be in the range (weight ratio) from 10 to 50, preferably from 15 to 40, more preferably from 20 to 30 by weight for 1.0 KGM.
Preferably, said composition D” comprises or, alternatively, consists of KGM/arabic gum/sodium and/or potassium (bi)carbonatefci trie acid (and/or tartaric acid)/at least one salt; preferably, with a weight ratio of:
KGM Arabic gum (bi)Carbonate Citric acid Salt
1.0: (0.1-15.0): (0.15-0.8): (0.10-1.80): (0.1-7.0)
more preferably, with a weight ratio of:
KGM Arabic gum (bi)Carbonate Citric acid Salt
1.0: 1.0: (0.3-0.6): (0.7- 2.0): (0.25-7.00) even more preferably, with a weight ratio of
KGM Arabic gum (bi)Carbonate Citric acid Salt
1.0: 1.0: (0.3-0.6): (0.7- 2.0): (0.25-3.00) for instance,
KGM Arabic gum (bi)Carbonate Citric acid Tripotassium citrate monohydrate
1 0: 1.0: (0.3-0 6): (0.7- 2.0): (0.25-3 00) for instance,
KGM Arabic gum (bi)Carbonate Citric acid Tripotassium citrate monohydrate
1 0: 1.0: (0.3-0 6): (0.7- 2.0): (0.25-3 00)
Tripotassium citrate and tripotassium citrate monohydrate salt may be replaced by the other water-soluble salts, as defined above, selected from the group copmprising or, alternatevily, consisting of tripotassium citrate, preferably tripotassium citrate monohydrate, gluconates (sodium, potassium, calcium), ascorbates (sodium and/or potassium), sodium and/or potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate, preferably trisodium citrate anhydrous, magnesium chloride x 6H 2O, calcium chloride x 2H2O (or water free versions of magnesium chloride, calcium chloride) sodium chloride, potassium chloride. Mixtures of salts may also be used. Citric acid may be replaced by other acids, preferred a hydroxy acid such as lactic acid, ascorbic acid, tartaric acid, malic acid or by other acids like phosphoric acid or glucono-delta-lactone (GdL) or mixtures of the acids mentioned above.
Preferably, the composition D” may further comprises at least one lipophilic and/or surface-active component such as, for example, actives, oils, lipids and/or surfactants; for example, the composition D” may comprises:
KGM/arabic gum/sodium and/or potassium (bi) carbonate/hydroxy acid, or
KGM/arabic gum/sodium and/or potassium (bi) carbonate/citric acid, preferably with a weight ratio of:
KGM Arabic gum Carbonate Citric acid (Active, Oil, lipid, Surfactant) 1.0: (15.0-40): (0.15-0.8): (0.15-1.5): (0.5-80.0) or, KGM Arabic gum Carbonate Citric acid MCT-C8
1 0: (1.0-5.0): 0 45: 1.37: (0.5-4.0)
It was observed that the above compositions showed a faster dissolution in water, less lump formation, a better flow behaviour from a beverage drinking glass to the mouth, a better mouthfeel, an improved viscosity when mixed with water, contrary to the extreme high viscosity of KGM alone in a liquid, preferably but not limited to, water.
Accorging to one of its aspects, the subject matter of the present invention relates to a solid fast dissolving or fast dispersing composition comprising or, alternatively, consisting of, a high molecular weight Konjac glucomannan (KGM) and an arabic gum in combination with at least one further dissolving or dispersing aid component which is
selected from the group comprising or, alternatively, consisting of:
(A) at least one water-soluble salt, with the provision that said at least one water-soluble salt is not a phosphate or polyphosphate salt; or
(B) at least one water-soluble salt and at least one organic acid, preferably at least one hydroxy acid; or
(D”) an effervescent system comprising or, alternatively, consisting of at least one basic substance capable of releasing carbon dioxide in the presence of at least one acidic substance and, optionally, at least one water-soluble salt; or
(H) at least one organic acid, preferably at least one hydroxy acid; and/or their mixtures thereof.
Preferably, said high molecular weight Konjac glucomannan (KGM) has a molecular weight equal to or bigger than 10s Da; preferably, has a molecular weight comprised from bigger than 106 Da to 108 Da; more preferably, has a molecular weight comprised from 107 Da to 108 Da; and/or said high molecular weight Konjac glucomannan (KGM) has viscosity value preferably comprised from 20,000 cps to 100,000 cps, preferably comprised from 25,000 cps to 80,000 cps, more preferably comprised from 30,000 cps to 60,000 cps, even more preferably comprised from 35,000 cps to 50,000 cps, and preferably has glucomannan content comprised from bigger than 85% to 98%, more preferably from 90% and 95%.
Preferably, said at least one water-soluble salt is preferably selected from the group comprising or, alternatively, consisting of:
- citrate salts; preferably, sodium and/or potassium citrate salts; more preferably tripotassium citrate salts, tripotassium citrate monohydrate salts, trisodium citrate salts, trisodium citate anhydrous salts, magnesium citrate, calcium citrate, zinc citrate, or their mixtures thereof;
- ascorbate salts; preferably, sodium and/or potassium ascorbate salts, calcium and/or magnesium ascorbate salts, zinc ascorbate, zinc ascorbate monohydrate salts, or their mixtures thereof;
- gluconate salts; preferably, sodium and/or potassium gluconate salts, calcium and/or magnesium gluconate salts, zinc gluconate salt, or their mixtures thereof;
- chloride salts; preferably, sodium chloride, potassium chloride, calcium chloride, magnesium chloride, ammonium chloride, or their mixture thereof.
- lactate salts; preferably, calcium lactate, calcium lactate pentahydrate, sodium and/or potassium lactate, zinc lactate, zinc lactate dihydrate, or their mixtures thereof;
- tartrate salts; preferably, sodium and/or potassium tartrate, potassium sodium tartrate, or their mixtures thereof;
- sulfate salts; preferably, sodium and/or potassium sulfate salts;
- glycinate salts; preferably, sodium and/or potassium glycinate salts;
- bisglycinate salts; preferably, zinc bisglycinate or zinc bisglycinate monohydrate salts;
- acetyl taurinate salts; preferably, calcium and/or magnesium acetyl taurinate salts; or their mixturs thereof. Preferably, said at least one organic acid is selected from the group comprising or, alternatevily, consisting of citric acid, lactic acid, ascorbic acid, tartaric acid, malic acid, or phosphoric acid or glucono-delta-lactone (GdL).
Preferably, said effervescent system comprises or, alternatively, consists of at least one basic substance capable of releasing carbon dioxide in the presence of at least one acidic substance, wherein said basic substance is selected from carbonate or bicarbonate salts; more preferably, sodium and/or potassium carbonate, sodium and/or potassium bicarbonate, or their mixtures thereof; and said at least one acidic substance is selected from organic acids; more preferably, hydroxy acids such as citric acid and/or tartaric acid.
More preferably, said solid fast dissolving or fast dispersing composition comprises or, altervatively, consists of (i) KGM, arabic gum, tripotassium citrate; more preferably, tripotassium citrate monohydrate, and citric acid, or (ii) KGM, arabic gum, sodium and/or potassium (bi)carbonate, and citric acid.
According to one of its aspects, the subject matter of the present invention relates to a process for preparing a drinkable compositition comprising the steps of: a) putting into contact a solid fast dissolving or fast dispersing composition of the invention with a water-based fluid, which is selected from water, a beverage, a milk, a milk substitute, a plant-based milk, non dairy substitute, nondairy beverage, instant milk; non-alcoholic, alcoholic, a fruit drink, energy drink, fortified drink, herbal drink, sport drink, yogurt drink, buttermilk drink, probiotic drink, a nectar, a juice or a cappuccino, a smoothie, a coffee, a macchiato, a cafe au lait, a coffee milk, a frapuccino, an espresso, an espresso macchiato, a latte espresso, a flat white, a caffee latte, a cacao, or mixtures thereof; b) mixing, preferably with the aid of a spoon, for a time from 10 seconds to 5 minutes, preferably from 1 minute to 3 minutes, at a temperature from 18 °C to 40°C, preferably from 18°C to 25 °C.
According to one of its aspects, the subject matter of the present invention relates to a drinkable composition obtained by mixing the solid fast dissolving or fast dispersing combination, as described above, with water, a beverage, a milk, a milk substitute, a plant-based milk, non dairy substitute, non-dairy beverage, instant milk; nonalcoholic, alcoholic, a fruit drink, energy drink, fortified drink, herbal drink, sport drink, yogurt drink, buttermilk drink, probiotic drink, a nectar, a juice or a cappuccino, a smoothie, a coffee, a macchiato, a cafe au lait, a coffee milk, a frapuccino, an espresso, an espresso macchiato, a latte espresso, a flat white, a caffee latte, a cacao, or mixture thereof.
According to one its aspects, the subject matter of the present invention relates to the solid fast dissolving or fast dispersing composition, as described above, for use in a method for managing weight loss or overweight reduction in a subject in need.
According to one its aspects, the subject matter of the present invention relates to the solid fast dissolving or fast dispersing composition, as described above, for use in a method to promote body weight loss in a healthy subject, or to reduce body weight in an overweight and/or obese subjects.
According to one its aspects, the subject matter of the present invention relates to the solid fast dissolving or fast dispersing composition, as described above, for use in a method for reducing cholesterol and blood sugar levels in a subject in need.
According to one its aspects, the subject matter of the present invention relates to the drinkable composition, as described above, for use in a method for managing weight loss or overweight reduction in a subject in need.
According to one its aspects, the subject matter of the present invention relates to the drinkable composition, as described above, for use in a method to promote body weight loss in a healthy subject, or to reduce body weight in an overweight and/or obese subjects.
According to one its aspects, the subject matter of the present invention relates to the drinkable composition, as described above, for use in a method for reducing cholesterol and blood sugar levels in a subject in need.
According to one its aspects, the subject matter of the present invention relates to a solid fast dissolving or fast dispersing composition comprising or, alternatively, consisting of, a high molecular weight Konjac glucomannan (KGM) in combination with at least one further dissolving or dispersing aid component which is selected from the group comprising or, alternatively, consisting of:
(C) at least one water-soluble salt and, optionally, at least one acid; preferably, at least one hydroxy acid, with the provision that said at least one water-soluble salt is not a phosphate or polyphosphate salt; and/or
(D1) at least one effervescent system comprising or, alternatively, consisting of at least one basic substance capable of releasing carbon dioxide in the presence of at least one acidic substance and, optionally, at least one water- soluble salt; and/or
(E) at least one sugar alcohol, preferably at least one sugar alcohol erythritol; and/or
(F) at least one sugar alcohol, preferably at least one sugar alcohol erythritol, and at least one component selected from (C) and (D1); and/or
(G) at least one acid, preferably at least one hydroxy acid; and/or their mixtures thereof.
Preferably, said high molecular weight Konjac glucomannan (KGM) has a molecular weight equal to or bigger than 105 Da; preferably, has a molecular weight comprised from bigger than 106 Da to 108 Da; more preferably, has a molecular weight comprised from 1 O7 Da to 108 Da; and/or said high molecular weight Konjac glucomannan (KGM) has viscosity value preferably comprised from 20,000 cps to 100,000 cps, preferably comprised from 25,000 cps to 80,000 cps, more preferably comprised from 30,000 cps to 60,000 cps, even more preferably comprised from 35,000 cps to 50,000 cps, and preferably has glucomannan content comprised from bigger than 85% to 98%, more preferably from 90% and 95%.
Preferably, said at least one water-soluble salt is preferably selected from the group comprising or, alternatively, consisting of:
- citrate salts; preferably, sodium and/or potassium citrate salts; more preferably tripotassium citrate salts, tripotassium citrate monohydrate salts, trisodium citrate salts, trisodium citate anhydrous salts, magnesium citrate, calcium citrate, zinc citrate, or their mixtures thereof;
- ascorbate salts; preferably, sodium and/or potassium ascorbate salts, calcium and/or magnesium ascorbate salts, zinc ascorbate, zinc ascorbate monohydrate salts, or their mixtures thereof;
- gluconate salts; preferably, sodium and/or potassium gluconate salts, calcium and/or magnesium gluconate salts, zinc gluconate salt, or their mixtures thereof;
- chloride salts; preferably, sodium chloride, potassium chloride, calcium chloride, magnesium chloride, ammonium
chloride, or their mixture thereof.
- lactate salts; preferably, calcium lactate, calcium lactate pentahydrate, sodium and/or potassium lactate, zinc lactate, zinc lactate dihydrate, or their mixtures thereof;
- tartrate salts; preferably, sodium and/or potassium tartrate, potassium sodium tartrate, or their mixtures thereof;
- sulfate salts; preferably, sodium and/or potassium sulfate salts;
- glycinate salts; preferably, sodium and/or potassium glycinate salts;
- bisglycinate salts; preferably, zinc bisglycinate or zinc bisglycinate monohydrate salts;
- acetyl taurinate salts; preferably, calcium and/or magnesium acetyl taurinate salts; or their mixturs thereof. Preferably, said at least one organic acid is selected from the group comprising or, alternatevily, consisting of citric acid, lactic acid, ascorbic acid, tartaric acid, malic acid, or phosphoric acid or glucono-delta-lactone (GdL).
Preferably, said effervescent system comprises or, alternatively, consists of at least one basic substance capable of releasing carbon dioxide in the presence of at least one acidic substance, wherein said basic substance is selected from carbonate or bicarbonate salts; more preferably, sodium and/or potassium carbonate, sodium and/or potassium bicarbonate, or their mixtures thereof; and said at least one acidic substance is selected from organic acids; more preferably, hydroxy acids such as citric acid and/or tartaric acid.
According to one of its aspects, the subject matter of the present invention relates to a process for preparing a drinkable compositition comprising the steps of: a) putting into contact a solid fast dissolving or fast dispersing composition of the invention with a water-based fluid, which is selected from water, a beverage, a milk, a milk substitute, a plant-based milk, non dairy substitute, nondairy beverage, instant milk; non-alcoholic, alcoholic, a fruit drink, energy drink, fortified drink, herbal drink, sport drink, yogurt drink, buttermilk drink, probiotic drink, a nectar, a juice or a cappuccino, a smoothie, a coffee, a macchiato, a cafe au lait, a coffee milk, a frapuccino, an espresso, an espresso macchiato, a latte espresso, a flat white, a caffee latte, a cacao, or mixtures thereof; b) mixing, preferably with the aid of a spoon, for a time from 10 seconds to 5 minutes, preferably from 1 minute to 3 minutes, at a temperature from 18 °C to 40°C, preferably from 18°C to 25 °C.
According to one of its aspects, the subject matter of the present invention relates to a drinkable composition obtained by mixing the solid fast dissolving or fast dispersing combination, as described above, with water, a beverage, a milk, a milk substitute, a plant-based milk, non dairy substitute, non-dairy beverage, instant milk; nonalcoholic, alcoholic, a fruit drink, energy drink, fortified drink, herbal drink, sport drink, yogurt drink, buttermilk drink, probiotic drink, a nectar, a juice or a cappuccino, a smoothie, a coffee, a macchiato, a cafe au lait, a coffee milk, a frapuccino, an espresso, an espresso macchiato, a latte espresso, a flat white, a caffee latte, a cacao, or mixtures thereof.
According to one its aspects, the subject matter of the present invention relates to the solid fast dissolving or fast dispersing composition, as described above, for use in a method for managing weight loss or overweight reduction in a subject in need.
According to one its aspects, the subject matter of the present invention relates to the solid fast dissolving or fast
dispersing composition, as described above, for use in a method to promote body weight loss in a healthy subject, or to reduce body weight in an overweight and/or obese subjects.
According to one its aspects, the subject matter of the present invention relates to the solid fast dissolving or fast dispersing composition, as described above, for use in a method for reducing cholesterol and blood sugar levels in a subject in need.
According to one its aspects, the subject matter of the present invention relates to the drinkable composition, as described above, for use in a method for managing weight loss or overweight reduction in a subject in need
According to one its aspects, the subject matter of the present invention relates to the drinkable composition, as described above, for use in a method to promote body weight loss in a healthy subject, or to reduce body weight in an overweight and/or obese subjects.
According to one its aspects, the subject matter of the present invention relates to the drinkable composition, as described above, for use in a method for reducing cholesterol and blood sugar levels in a subject in need
Experimental part
1 . KGM, arabic gum, and at least one water-soluble salt as defined above. a) Fullcream milk powder is added to a system of KGM/Arabic gum/water soluble salt.
A powder mixture of KGM/Arabic gum/Tripotassium citrate monohydrate/Full cream milk powder with a fat content of 3.5%-4.2% (1 .0 g/1.8 g/0.8 g/2.0 g) was poured in water (100 g water - 5.6 g of raw materials) and stirred with a spoon. A fluid composition results (pH 7.8 (20.3°C) with a white appearance and neutral taste impression. b) Coffee powder is added to KGM/Arabic gum/water soluble salt and poured into milk.
A powder mixture of KGM/Arabic gum/Tripotassium citrate monohydrate/coffee powder (Robusta, mild, 23-10231, coffein content: 3.5-5.5% from Deutsche Extrakt Kaffee GmbH, Hamburg, Germany)/sucralose (1g/2.6g/0.8g/1.5g/0.015g) is added to 94.085g of a low fat homogenized milk (fat content: 1.5%, available in German supermarket EDEKA, https://www.supermarktcheck.de/edeka/sortiment/h-milch-1-5/). A fluid brown composition results (taste similar to a milk coffee from a fridge in a german supermarket (EDEKA), taste like e.g. , “Starbucks” caffee latte, “Emmi” caffee latte, “Gut und gunstig” latte espresso, “Muller” latte macchiato, “Muller" espresso macchiato). c) Oat powder is added to KGM/Arabic gum/water soluble salt
A powder mixture of KGM/Gum arabic/Tripotassium citrate monohydrate/oat meal powder/vanilla flavor/Sucralose (1 ,0g/2.7g/1.6g/2.5g/0.4g/0.015g) is added to water (91 ,585g) and stirred with a spoon.
A fluid composition results with a white appearance and neutral oatmeal taste impression d) Full cream milk powder and a freeze-dried fruit are added to KGM/Arabic gum/water soluble salt
A powder mixture of KGM, Gum Arabic, Tripotassium citrate/Full cream milk powder (1 ,0g/1 ,8g/0.8g/3.0g) was mixed and whole freeze-dried raspberries (2g) were added to the powder mixture. This raspberry containing powder mixture (1.0g/1.8g/0.8g/30g/2.0g) was added to water in a beverage glass and stirred with a spoon.
The fluid mixture was allowed to stand for 10 min for the rehydration of the raspberries.
A red raspberry containing fluid milk drink (with KGM) was obtained (pH: 6.8, 25°C).
2. KGM, arabic gum, at least one water-soluble salt, as defined above, and at least one hydroxy acid, as defined above.
A powder mixture of KGM/Arabic gum/citric acid/ tripotassium citrate monohydrate (1.0: 1.5:0.4:0.4) makes easier the dissolving process of the KGM and arabic gum in water by simply stirring the composition with a spoon in water. Immediately, a fluid is obtained (pH 3.95 (20.7°C)). No lump formation (compared to KGM pure in water). The consumer can drink it immediately. More neutral compositions (neutral aftertaste, e.g., pH 5.7 (21.1 °C) can be obtained by changing the relative ratios citrate/hydroxy acid ((1.0:1.5:07:0.1).
In the KGM/Arabic gum system with at least one water-soluble salt, the salt is preferably selected from the group copmprising or, alternatevily, consisting of tripotassium citrate, preferably tripotassium citrate monohydrate, gluconates (sodium, potassium, calcium), ascorbates (sodium and/or potassium), sodium and/or potassium tartrate x O.5H2O, potassium sodium tartrate-4-hydrate, trisodium citrate, preferably trisodium citrate anhydrous, magnesium chloride x 6H2O, calcium chloride x 2H2O (or water free versions of magnesium chloride, calcium chloride) sodium chloride, potassium chloride.
3. KGM/Arabic gum/Tripotassium citrate monohydrate/citric acid/Full cream milk powder.
A powder mixture of KGM/Arabic gum/Tripotassium citrate monohydrate/citric acid/Full cream milk powder (1.00 g/1.50 g/1 ,5g/0.1 g/3.00 g) was added to 92.9 g of water and stirred with a spoon. A fluid composition results (pH 6.24, 19.6°C) with a white appearance. Increasing the citric acid content leads to more acidic formulations.
Adding cacoa powder (Plaine Arome (Fat content: 22-24%)) and full cream milk powder (3.0g/1.2g) to the system of KGM/Arabic gum/water soluble salt /hydroxy acid (e.g., citric acid). Cacao powder is available from e.g., Barry Callebout Deutschland GmbH, im Mediapark 8a, 50670 Koln (with different fat contents, e.g., “Noir lntense”: 10- 12%, “Plaine Arome” or “Extra brute": 22-24%, “Rouge Ultime”20-22%, “Legere": 1%.
A powder mixture of KGM/Arabic gum/Tripotassium citrate monohydrate/citric acid/Full cream powder/Cacao powder (Plaine Arome (Fat content: 22-24%)) (1.0 g/1.5g/1.5g/0.1g/3.0g/1.2g) was added to 91.7 g of water and stirred with a spoon. A fluid composition results (pH 6.6, 20.5°C) with a brown appearance. Due to the combination of full cream milk/cacao powder the product has a good cacao taste.
4. KGM, a water-soluble salt and, optionally, a hydroxy acid as defined above.
Astonishingly powder mixtures of KGM/water soluble salt (e.g., 1 :6 or 1 :3 or 1 :1) facilitates the dissolving process of the high molecular weight KGM in water. Immediately a fluid is obtained by simply stirring the composition with a spoon. No lump formation (compared to KGM pure in water). Small KGM particles are visible in water directly after the mixture was prepared. The formation of these small particles in comparison to the pure KGM/water system is an advantage because these particles are in fluid state (salt/water) instead of a thick gel that is formed in case of the KGM/water combination After a while from these fluid particles starts the conversion to a fluid gel The consumer can drink the fluid immediately If he prefers to wait a while (e.g., 10-15 min) a drinkable fluid gel results that he can drink. Sodium and potassium gluconate or tripotassium citrate monohydrate, trisodium citrate anhydrous, sodium ascorbate, potassium tartrate x 0,5 H20, potassium sodium tartrate-4-hydrate or magnesium chloride x 6 H2O or the corresponding calcium chloride and sodium chloride or potassium chloride are examples
that work very well. Furthermore, combinations with water soluble salts are also possible. It is evident, that these salts or salt combinations have ‘‘very fast dissolver" or ‘‘very fast suspending” properties for KGM.
It is possible to formulate at a more neutral pH or in an acid range, e.g., a mixture KGM/sodium gluconate/water (1g/3g/96g) has a pH of 7.43 (20.5°C) and a more neutral taste. Adding citric acid (e.g., 0.25 g) gives a pH of 4.44 (20.1 °C) in a formulation of KGM/sodium gluconate/water/citric acid (1 g/3g/0.25g/95.50g/0.25g) and a little bit more acid taste perception.
A composition of KG M/Tri potassium citrate monohydrate/citric acid can be formulated at more neutral pH (citric acid: 0 g, pH: 7 4, 20 5°C) or by addition of citric acid the desired pH can be found (g water: 100g - g raw materials/ingredients)
For example:
KG M/Tri potassium citrate monohydrate/citric acid (1g/3g/1g): pH = 4.9 (20.3°C);
KG M/Tri potassium citrate monohydrate/citric acid (1g/2g/2g): pH = 3.9 (20.2°C);
KG M/Tri potassium citrate monohydrate/citric acid (1g/1 g/3g): pH = 3.0 (19.8°C);
KG M/Tri potassium citrate monohydrate/citric acid (1g/0.5g/3.5g): pH = 2.5 (19.9°C).
In case of a system KGM/sodium ascorbate/water (1g/3g/96g) the formulation has an added benefit due to the sodium ascorbate. Sodium ascorbate serves as an antioxidant, helping to protect the cells from damage. Furthermore, a study in 2005 describes that individuals with adequate vitamin C status oxidize 30% more fat during a moderate exercise (J Am Coll Nutr 2005 Jun; 24(3): 158-65).
A very cheap compositon with sodium chloride or potassium chloride can be obtained.
A powder mixture of KGM/sodium chloride (1 g/0,5g) up to KGM/sodium chloride (1 g/2g) leads to a fast-dissolving process of the KGM (water: 100 g - g of raw materials).
Higher amounts of sodium chloride (e.g., 3%) leads to salty compositions (that might be not acceptable for some consumers). It is therefore preferred to formulate below 2% of sodium chloride. A powder mixture of KGM/sodium chloride/citric acid (1g/1g/1g) leads to a fast-dissolving process of KGM with an acid aftertaste whereas mixtures of KGM/sodium chloride/citric acid (1 g/1 g/0.06g up to 1 ,00g) leads to a more neutral tasting (0.06g) or slightly acid tasting compositions that do not have a salty aftertaste. A composition of KGM/sodium chloride/citric acid/water (1 g/1g/1 g/97 g water) has a pH of 2.4 (25°C) in comparison to pH = 3.75 (25°C) for a KGM/sodium chloride/citric acid/water (1 g/1 g/0,06g/97.4g) composition.
Furthermore, it was observed that KGM/water soluble salt/water formulations are translucent to transparent, for example the high molecular weight KGM/sodium gluconate/water system is translucent to transparent. High molecular weight KGM/water systems are more greyish and translucent/not transparent (see introduction) Consumers that prefer a translucent to transparent KGM fluid or a more translucent/transparent KGM fluid gel will like such compositions. Adding a water soluble natural or synthetic color to such formulations results in the corresponding colored translucent to transparent fluids or colored translucent to transparent fluid gels. Adding a water-soluble flavour and/or a sweetener like sucralose, stevia is additionly possible.
5. (D1) KGM and an effervescent system, as defined above, and optionally at least one water-soluble salt as defined
above.
Compositions (D) e.g., D’ and D” become effervescent when mixed with water, through the CO2 development due to the following reaction: MeHCOa + (H00CCH2)2C(0H)C00H -> C02 + Me-citrate; Me being Na or K. Effervescent system: Me Hydrogen carbonate + acid (e.g., citric acid).
6. KGM/Me hydrogen carbonate/citric acid (powder mixture) such as for example: a) KGM/sodium hydrogen carbonate/citric acid.
Surprisingly in a powder mixture of KGM/sodium hydrogen carbonate/citric acid (1.0:0.6g:0.4g) a very fast dissolution of the KGM in water was observed by simply stirring the composition with a spoon, no lump formation. The formulation is immediately liquid (water: 10Og-g raw materials). A representative example is also KGM/sodium hydrogen carbonate/citric acid (1g/0.3 g/ 0.7 g). Avery fast dissolution of the KGM was observed simply stirring the composition with a spoon Due to the dissolved CO2 a creamy taste is obtained in the form of a smooth (drinkable) gel. A higher content of the effervescent system (e.g., NaHCOa/citric acid) for 1 g of KGM is possible (e.g., 0.6g/1 4g etc.). b) KGM/potassium hydrogen carbonate/citric acid.
It is possible to achieve different pH values (for 1 g KGM), e.g., it was observed that more citric acid (e.g., 5.75 g) gives with potassium hydrogen carbonate (3.00 g) a pH of 3.7 and the usage of less citric acid (1 .92 g) gives with potassium carbonate (3.00 g) a pH of 6.1. Water content = 100 g - g of the powder raw material mixture.
The freedom to operate at different neutralisation levels and the usage of the corresponding sodium or potassium hydrogen carbonate salt is relevant for at least six reasons. 1) Some consumers prefer an acid taste of a product (or the combination acid combined with sweetness from a sweetener). The formulator will formulate the KGM product at a lower pH value in this case. 2) The stability of added actives is in some cases better in an acid (more neutral) than in a more neutral (acid) pH. The formulator will formulate at the corresponding preferred pH value (acid or more neutral pH value). 3) The color impression of an active is different at acid pH values versus a more neutral pH (e.g., for actives like antocyanins). If the color is brighter in an acid environment that the formulator will formulate at a lower pH value the KGM product. 4) The consumer (obese adult, obese child) is interested in a KGM product with (a lot of) foam and an acid aftertaste after dissolving the KGM powder mixture. In case of a high amount of the hydrogen carbonate salt (e.g 0.4 g to 3.0 g potassium hydrogen carbonate) a foam development is observed in the presence of 1.00 g KGM; 0.77g citric acid (for 0.40 g potassium hydrogen carbonate) or in the presence of 5.80 g citric acid (for 3.00 g potassium hydrogen carbonate). The pH of such formulations in the range of pH 3.7-4.0. If costs of the final formulations have to be considered the lower boundaries of the hydrogen carbonate salt will be used. 5) The consumer (obese adult, obese child) is interested in a KGM product with (a lot of) foam and a more neutral aftertaste after dissolving the KGM powder mixture. In case of a high amount of the hydrogen carbonate salt (e.g. 0.4g to 3.0 g potassium hydrogen carbonate) a foam development and neutral aftertaste (pH 6.1) is observed. For example, in a sample with 1.00 g KGM, 1.92 g citric acid, 300 g potassium hydrogen carbonate 6) The consumer (obese adult, obese child) consumes too much sodium or not enough potassium and/or has a high blood pressure problem. In this case it is preferred to formulate with the potassium
hydrogen carbonate salt instead of the sodium hydrogen carbonate salt or a mixture of potassium and sodium hydrogen carbonate can be used.
It is evident from the above that an effervescent system (e.g. sodium (potassium) hydrogen carbonate, a hydroxy acid (in this case: citric acid) and the formation of the resulting sodium citrate (or the resulting potassium citrate) are a “very fast dissolver” or a “very fast suspending” combination for a KGM composition.
7. (D”) KGM, an effervescent system as defined above, and arabic gum, and optionally a water-soluble salt as defined above.
A powder mixture of KGM/Arabic gum/sodium hydrogen carbonate/citric acid.
Surprisingly in this case the dissolved C02, arabic gum and the resulting formation of the salt (sodium citrate) lead to a fast dissolution process by simply stirring the composition with a spoon and the composition stays fluid or gives a fluid gel In case of potassium hydrogen carbonate the resulting salt is potassium citrate instead of sodium citrate a) Weight ratios of 1.0/1 .2/0 3/0 7 gives a foam that disappears after a while and dissolves quickly the powder ingredients. Immediately a fluid is obtained (pH 3.92, 19.9°C) by simply stirring the composition with a spoon. The consumer can drink it immediately. If he prefers to wait a while (e.g., 10-15 min) a drinkable fluid gel results that he can drink. The company selling the drink should disclose “drink immediately after mixing to get a fluid" or alternatively “drink the fluid gel after 10 to 15 min after mixing”. The composition has an acid aftertaste. b) Weight ratios of 1 .0/1 .2/0.3/0.23 gives less foam that disappears after a while and dissolves quickly the powder ingredients. Immediately a fluid is obtained by simply stirring the composition with a spoon. (pH 5.9, (19.6°C)). The consumer can drink it immediately. If he prefers to wait a while (e.g., 10-15 min) a drinkable fluid gel results that he can drink. The company selling the drink should disclose “drink immediately after mixing to get a fluid" or alternatively “drink the fluid gel after 10 to 15 min after mixing”. The composition has a more neutral aftertaste. c) Weight ratios of 1.0/1.4/0.26/0.6 or 1.0/1.8/0.5/1.0 gives a foam that disappears after a while and dissolves quickly the powder ingredients by simply stirring the composition with a spoon. Immediately a fluid is obtained. (pH: 3.92 (20.7°C). The composition has an acid aftertaste. Higher concentrations of Arabic gum relative to KGM gives fluid systems (e.g., KGM/Gum Arabic: 1 : 1.5 or 1 :1.6 or 1 :1.7 etc.).
It is evident from the above that an effervescent system (e.g., sodium (potassium) hydrogen carbonate, a hydroxy acid (in this case: citric acid) and the resulting sodium citrate (or the resulting potassium citrate) are a “very fast dissolver” or a “very fast suspending” combination for a KGM/Arabic gum composition and reduces the viscosity of KGM (>1.4).
The addition of lipophilic and/or surface-active components as a powder (actives, oils, lipids and/or surfactants) to the effervescent system of KGM/Arabic gum/sodium or potassium (bi) carbonate/hydroxy acid (e g., citric acid) and, optionally, a water-soluble salt (D") leads to a milk-like product or foamed milk-like product (instant milk, or foamed instant milk) or milk alternatives product (milk substitute products, non dairy substitutes, plant based milks, nondairy beverage) or cocoa-like product. The lipophilic (and/or surface active) component can be added as a) a powder or b) a liquid.
Examples of powders are: a1) full cream milk powder, a2) coffee powder (expresso etc.), a3) wheat germs, a4)
almond flour, a5) cacao powder, a6) MCT powder, a7) omega-3 powder, a8) oatmeal/oatflour powder, a9) soymeal/soyflour powder.
Examples of liquid oils are: b1) Linseed oil + EPA + DHA (liquid omega 3, containing ALA, EPA and DHA); examples of other liquid oils are flaxseed oil, fish oil, borage oil, blackcurrent seed oil, evening primrose oil, olive oil, MCT oils (e.g., coconut oil).
8. KGM/Arabic gum/hydrogen carbonate/citric acid/Full cream milk powder.
An instant full cream milk powder (lactose free) is available from Saputo, Australia (Instant Full cream Lactose Free powder 25 kg). It contains a mixture of fat (minimum 28%), proteins (at least 35%, calcium (1g/100g). A powder mixture of KGM/Arabic gum/hydrogen carbonate/citric acid/full cream milk powder (1 ,00g/1 ,80g/0.3g/0.23g/3.60g was added to 9307 g of water and stirred with a spoon A fluid composition results (pH: 6.2 (19.7°C) with a white appearance Due to the full cream milk powder the product has a rich mouthfeeling. In this case the effervescent system was adjusted to a total content of 0 53g/100g so that a consumer may notice a small amount of foam. Using higher amounts of the effervescent system gives a foaming milk like composition. Interestingly no external foam equipment or a small handheld electric mixer producing this foamed milk is necessary (the foam comes from the development of CO2).
Alternatively, a composition comprising KGM/Arabic gum/sodium or potassium (bi) carbonate/hydroxy acid (e.g., citric acid) can be added to a full cream milk powder. In case of NaHCO3 (part of the effervescent system) sodium is already available for the consumer as sodium citrate. In case of KHCO3 potassium can be delivered as potassium citrate. A combination of NaHCO3/KHCO3 delivers both minerals for a consumer. Typcial vitamins are B-vitamins like vitamin B2 (riboflavin) vitamin B12 (riboflavin), the B-vitamin complex (all B-Vitamins), vitamin A, vitamin D, vitamin E, vitamin K or vitamin C. Proteins like whey (concentrates, isolates, hydrolysate) or from other protein sources (already mentioned in the patent) can be added. By adjusting the amount of the effervescent system, the composition can be a foamed milk (total effervescent content (0.88 g/100 g or 0.53/100 g) or a milk were the foam is not visible for a consumer. Other ingredients like skim milk, whole milk, anhydrous milk fat can be used and are also available from Saputo Dairy Australia Pty Ltd.
9. KGM/Arabic gum/hydrogen carbonate/citric acid/coffee powder.
A ready to drink liquid coffee formulation with a powder composition of instant full cream milk powder, coffee powder (espresso) and an effervescent system that release CO2 for the development of a foam, KGM and arabic gum can be prepared. Additional ingredients are usually sugars, a coffee flavour, caramel, salts, colorants. It was observed that a formulation of KGM/Arabic gum/full cream milk powder, sodium hydrogen carbonate, citric acid, Jacobs Espresso powder (Jacobs Douwe Egbert) la coffee flavour/sucralose (1 g/1 .8 g/1.8 g/0.6 g/0.37 g/0.6 g/0.3 g/0.015 g) that was added to 93.38 g of water and stirred with a spoon at room temperature 20°C leads to a foaming composition with a typical coffee aroma.
The foam comes from the effervescent sytem (CO2 development) instead from a device that, gives the foam (air).
Changing the amount of the effervescent system and/or changing the milk fat content, and/or
the amount of amount/type of coffein, (100% Robusta, 100% Arabica and/or mixture of Robusta and Arabica or espresso versions) and/or the type of coffee powder (agglomerated, spraydried, freeze-dried), taste (mild, strong, expresso-typical) and/or certification of the coffee powder (Rainforst alliance, bio-certified) and/or type of coffee flavour and/or the choice of sweetener is possible to those skilled in the art.
Proteins
They are usually difficult to combine actives (e.g., proteins like whey proteins or other proteins like milk, egg, casein, pea, animal, fish, poultry, soy, almond, chia, hemp, rice, collagen, vegan collagen, a-lactalbumin) in high concentrations together with thickening ingredients like a high molecular weight KGM. Drinking a combination of KGM/Arabic gum/effervescent system does not provide a lot of energy for consumers because it is the intent to lose weight.
If it is the attention of the consumer not to have a breakfast or meal after drinking the KGM composition of the invention, he/she might be interested to get a little bit of energy from the KGM drink.
It was found that low to high amounts of proteins powders (concentrates, isolates, hydrolysates) like whey proteins can be added to a powder mixture of KGM/Arabic gum/effervescent system. The complete composition of KGM/Arabic gum/effervescent system/ protein can be dissolved quickly by stirring it with a spoon. Furthermore, a lack of proteins and amino acids in overweight persons is discussed in the literature which can lead to the effect that these consumers eat more fats and carbohydrates. Specific amino acids in the plasma affect the secretion of gastric hormones such as glucagon-like peptide-1 which influence satiety and appetite control.
Typical protein requirements according to Deutsche Gesellschaft fur Ernahrung E.V. may be found in https://www.dge.de/wissenschaft/faqs/protein/.
Calculation of protein intake per day in case of overweight:
Body height: 1.70 m, weight: 80 kg, BMI: 28 kg/m2;
Body weight at a BMI of 22 kg/m2= 22 kg/m2 * 80 kg / 28 kg/m2 = 63 kg.
The person would have a BMI of 22 kg/m2 at a body weight of 63 kg, i.e., normal weight.
The recommended intake of protein is therefore calculated: 0.8 g/kg body weight/day * 63 kg= 50 g protein/day.
Thus, 50 g of proteins can be supplemented with three drinks/day (16.6 g/drink).
Powder mixture comprising of KGM/Arabic gum/effervescent system/whey protein concentrate.
Interestingly and surprisingly, it was easy to add 17 g of whey protein concentrate in powder form (protein from company Vivi solutions (VIVI Solutions GmbH, Im Holde 25 D-48499 Salzbergen: Whey protein concentrate 80 Instant.): Whey protein concentrate 80 Instant) to a powder mixture of KGM/Arabic gum/sodium hydrogen carbonate/citric acid (1 g/1.8 g/0.5 g/1.0 g). (Water: 100g - g of the powder raw materials) and to dissolve this powder composition in water by simply stirring the composition with a spoon The effervescent system helps to dissolve the high amount of the whey protein concentrate. The final composition has a very creamy taste.
General remarks
The compositions of the invention can be easily used by the consumers at home. Indeed, a consumer may just use a spoon as "stirrer”, which is sufficient to make the KGM more fluid thanks to the further components of the
composition. An alternative to a spoon is a small handheld electric mixer. If a consumer has a problem to dissolve the KGM composition the company selling the KGM composition should give the advice to use a milk foamer, manual model or handheld stick frothers (e.g., Milchaufschaumer FINO, rotation speed 12.000 U/Min, Artikel-Nr. : 12720, GEFU GmbH, Braukweg 28, 59889 Eslohe, Germany). In this case the device is used to stir the composition (instead of foaming, in this case there is no foam development). On the other hand, in industrial processes, a typical industrial stirrer can be used.
Besides the above disclosed components, the composition of the invention may also comprise additional active ingredients or compositions, for instance, substances effective in promoting weight loss. All the components of the compositions of the invention are commercially available.
The water phase may be represented by tap water, sparkling water or still water. The water phase may contain (e.g. for industrial processes) water soluble ingredients like polyols, sugars, acids (hydroxy acids, citric acid, lactic acid, tartartic acid, solutions (e.g. gluconic acid, 50%, lactic acid solutions) water soluble flavors, water soluble vitamins (e.g. B-Vitamins, Vitamin C and derivatives), water soluble salts, water soluble dextrins, water soluble proteins, protein concentrates, protein isolates, protein hydrolysates from whey, milk, egg, casein, pea, animal, fish, poultry, soy, almond, chia, hemp, rice, lentins, lupins, collagen, vegan collagen, water soluble amino acids, water soluble surfactants, water soluble colors, water soluble sweetener, (e.g. stevia, sucralose, erythritol), polymers like xanthan gum, sodium carboxymethylcellulose, pectins, high methoxyl pectin, carrageenans, cellulose gum, gellan gum, guar gum, locust bean gum, propylene glycol alginate, gum tragacanth, microcrystalline cellulose, distarch phosphate, acetylated starch, acetylated distarch adipate, hydroxypropyl distarch phosphate, hydroxypropyl starches, starch sodium octenyl succinate, fructooligosaccharide, galacto-oligosaccharides, water soluble powders (like cacao powder, milk powder etc.), carbonate salts, hydrogen carbonate salts, water soluble minerals, lactones like glucono deltalacton, dextrose monohydrate, mannitol, xylit, allulose, maltitol, lactose monohydrate, glycerol, glucose syrup, water soluble starch and starch hydrolysates, sucrose, trehalose, lactitol, isomaltulose and water soluble actives.
Generally, 5-20 parts per weight of the composition of the invention is mixed with 80-95 parts per weight of liquid. Preferably, an arable gum (Acacia Senegal) available in the market is for example a raw material that is water soluble, pH 4-5, loss of drying <10%, Ash < 4%, viscosity (25% in water) of 60-180 mPas (Benecke GmbH, Hamburg, Germany, Norevo GmbH, Germany, Nexira, France). For example, a highly emulsifying Type 4810, or Type 4810, or Type 4810, or Type 4810 (Benecke GmbH) can be used in accordance with the present invention. Other sources of Arabic gum are from the company Nexira (Tradenames: Efistab AA, SuperStab, Eficacia, InstaGum AX, Instagum AA, Instagum AP).
For example, a KGM as a raw material with starch content <1- 3%, preferred <3%, protein content <1.5-3.0%, preferred <3.0%, pH 5-7, Ash <5%, glucomannan content >85-90%, preferred >90%, more preferred from 90% to 98%, for example 92%-95%, viscosity from 26,000 to 36,000 mPas, preferred > 36,000 mPas, more preferred from 36,000 to 80,000 mPas, particle size from 90 to 200 mesh, preferably 120 to 200 mesh. For example, KGM is available from FH Diedrichs & Ludwig Post GmbH, Mannheim, Germany, Neupert Ingredients, Garstedt, Germany,
C.E. Roeper GmbH, Hamburg, Germany, Baoji Konjac Chemical Co. Ltd, China. For example, a KGM E425(i) and/or E425(ii) can bused in accordance with the present invention.
According to the present invention, it may be used a Konjac glucomannan (KGM) commercially available into the market having preferably CAS RN No. 37220-17-0. For example, it may be used a Konjac gum preferably indicated with E425(i) or E425(ii), both containing Konjac glucomannan.
According to the present invention, it is used a high molecular weight KGM (e.g., >106 Da, preferably e.g., from >106 Da to 10e Da, more preferably e g., from >106 Da to 107 Da).
According to the present invention, it is used a high molecular weight KGM with a viscosity value comprised from 20,000 ops to 100,000 ops, preferably comprised from 25,000 cps to 80,000 cps, more preferably comprised from 30,000 cps (30,000 mPas) to 60,000 cps, even more preferably comprised from 35,000 cps to 50,000 cps, for example bigger than 36,000 cps.
Sodium hydrogen carbonate salt is available fromSolvay Chemicals International: BICAR® FOOD, A+E. Fischer- Chemie GmbH & Co. KG, Wiesbaden, Germany, Carl Dicke GmbH & Co KG, Mbnchengladbach, Germany) and potassium hydrogen carbonate is available from company Dr. Paul Lohmann).
Vanilla flavor is available from Sensient, Geesthacht, Germany.
Blood orange flavour is available from OlbrichtArom GmbH & Co. KG, Leisnig, Germany.
Orange Spray dried Flavor is available from Sensient, Geesthacht, Germany.
Blue berry flavor is available from IMCD Deutschland GmbH.
Color is avalable from Vanilla Yellow P-WS, Sensient, Geesthacht, Germany.
Sucralose is available from Atlantic Chemicals Trading GmbH, Hamburg/Germany.
Citric acid is available from Jungbunzlauer ("Citronensaure Anhydrat”).
According to another of its aspects, subject-matter of the invention is a solid pharmaceutical, nutraceutical or food composition or a composition for medical device under EU Reg. 745/2017 which comprises the solid fast dissolving or fast dispersing combination of the invention, as above defined, and a) optionally, at least flavours and/or colorants and/or sweeteners; and b) optionally further (active) ingredients.
Illustrative and non-limiting examples of such (active) ingredients that can be combined with the solid fast dissolving or fast dispersing composition of the present invention containing KGM and Arabic gum are the following: Antocyanines such as “Brainberry” from company Bioactor, Aronia melanocarpa extract (25% Anthocyanins) or antocyanins/polyphenols mixtures (“Berriesence”) from MOB Ming Chyi Biotechnology Ltd.
Carob bean extract and fructooligosaccharide such as “CSAT+" is available from company Pharmactive Biotech Products.
Black garlic such as “ABG10+” is available from company Pharmactive Biotech Products.
Coffein: such as “Caffeine, 98%'' is available from company OmniActive Health Technologies or “CaffXtend" is available from Nutriventia (Mumbai, India) or from soluble coffee extracts such as “Arabica kraftig, 23-10234”, “Espresso, aromatisch (Arabica/Robusta-Blend), 23-10236”,” Arabica mild, 23-10232”, "Espresso, medium, Robusta, 23-10235”, “Robusta, kraftig, 23-10233”, “Robusta, mild, 23-10231” are available from Deutsche Kaffee
Extrakt GmbH, Hamburg, Germany.
Cacao powder such as “Noir Intense”, “Plaine Arome”, “Extra brute”, “Rouge Ultime”, “Legere”: 1% are available from Barry Callebout Deutschland GmbH, Im Mediapark 8a, 50670 Kbln. Actives in cacao are polyphenols like flavanols (epicatechin, catechin, and procyanidins) and flavonols (quercetin), anthocyanins, phenolic acids, and stilbenes. Furthermore coffein is in guarana (“Guarana Extract PDR & N. Caff BLDR22%-904") is available from OmniActive Health Technologies.
Collagens are available from the companies Gelita AG, Rousselot, Weishardt, Nitta Gelatin. Abyss or vegan collagens are available from company MCB Ming Chyi Biotechnology Ltd (“CollaGem”) or from INB GmbH (“Vollagen”) or from Vecollal/TCI (“VeCollal”).
Chromium salts like “Chromium picolinate” are available from Vertellus, Indianapolis, USA
Niacin is available from Wegochem Europe B.V., Netherlands.
Alpha Lipoic acid such as “Alipure” is available from Alzchem Group AG, Germany.
Pumpkin seed protein powder is available from Bioriginal, Netherland.
Whey hydrolysate with an active dipeptide AP (Alanine-Proline) which inhibits the alpha glucosidase's activity such as “Pep2Dia” is available from Ingredia Dairy experts
Cinnamon or cinnamon extracts are available from Flavex Naturextrakte GmbH, Rehlingen, Germany (Zimtrinden Ceylanicum CO2-se Extrakt, Nr. 034.001).
Curcumin is available from the company Gencor (“HydroCurc® Liquid Grade 10%”) or from company OmniActive Health Technologies (“Curcuwin Ultra+ Curcuminoids 20%” or “CurcuWIN Curcumin DNS HB Pdr 20%/EU") or from company Natural remedies, India (“Turmacin”), or from company Nutriventia, Mumbai, India (“Water Dispersible Turmeric Extract Powder 60N” or “unstain”).
Vitamins (lipophilic like Vitamin A, D, E, K) and B-vitamins or the “B-vitamin complex" can be added to the formulations. Thiamine (Vitamin B-1), for example, helps the body cells convert carbohydrates into energy. Furthermore, Vitamin B6 can help with weight loss by boosting metabolism. Additionally, vitamin C, niacin, iron, calcium, and vitamin B5 can help to speed up metabolism. A slow-release vitamin C formulation is “C-Fence” which is available from Nutriventia, Mumbai, India. A heat stabilized vitamin C is “Stabilized Vitamin C 80” which is available from Nutriventia Mumbai, India.
Powders from almond, cashews, soy, rice, hemp, coconut, oats, linseed, spelt.
Oat powder is available as Hafermehl Vollkorn (Art Nr. 4.1), Rubin Miihle GmbH, Germany.
Dried fruits and/or freeze dried fruits and/or individually quick frozen (IQF) fruits and/or frozen fruit products, such as A ai, Acerola, Apple, Apricot, Aronia, Avocado, Banana, Berry mixes, Blackberry, Blackcurrant, Black Cherries, Blood Oranges, Blueberry, Boysenberry, Breadfruit, Camu Camu, Carambola, Carissa, Cherimoya, Cherries, Cherries Morello, Citrus, Cloudberry, Corinthians, Crabapples, Cranberries, Currants, Dates, Durian, Feijoa, Figs, Gooseberries, Grape, Grapefruit, Groundcherries, Guarana, Guava, Jackfruit, Juneberry, Kaffir Lime I Combava, Kiwi, Kumquats, Langsart, Lemon, Lime, Lingonberry, Longan, Loquats, Lychee, Mandarin, Mango, Mangosteen, Marionberry, Melon, Mint, Mulberries, Nectarine, Oranges, Papaya, Passion Fruit, Pawpaw, Peaches, Pear,
Pineapple, Pitahaya, Plantain, Plum, Prune, Pomegranate, Prickly Pear, Pummelo, Quince, Raisins, Rambutan, Raspberries, Rhubarb, Sapodilla, Sour cherries, Soursop, Starfruit, Strawberries, Sultana, Tamarind, Tangelo, Tangerine and Watermelon.
Dried fruits and/or freeze-dried fruits and/or individually quick frozen (IQF) fruits and/or frozen fruit products are available, for example, from TALI e.K., Steinweg 1, 34298 Helsa, Germany or Allfood Lebensmittel-Handels- Gesellschaft mbH, Bavariaring 2, D-80336 Munchen or FRUKTIA GmbH, Haferwende 10A, 28357 Bremen, Germany or Buxtrade GmbH, An den Geestergen 1 , 21614 Buxtehude.
For example, Raspberries, whole freeze dried (Himbeeren ganz gefriergetrocknet) are available from Buxtrade GmbH, Germany.
Polyphenols and the like such as “WATTS' UP'' are available from company Bioactor, Bioflavonoids from Citrus sinensis (Hesperidin).
Carnitin, Carnitin Hydrochloride such as “L-Carnitin HCI” are available from Liaoning Koncepnutra Co Ltd.
Resveratrol such as "ResveraFen” is available from Akay Natural Ingredients Private Limited, containing resveratrol, de-bitterised fenugreek powder rich in soluble dietary fibre, Sunflower lecithin and/or Sunflower oil; or Resveratrol such as “Veri-Sperse®” from Gencor/pharmaco biotechnologies.
Capsaicin such as “Capsifen” is available from Akay Natural Ingredients Private Limited, Chili extract, fenugreek powder, Cellulose powder and/or Lecithin, gum acacia; or “Capsimax” from OmniActive Health Technologies.
Omega-3, such as “DHA Algae Powder DAF” is available from MOB Ming Chyi Biotechnology Ltd
Complexes from Citrus Sinensis fruit extract (Anthocyanins, Hydroxycinnamic acids, Flavanones, Vitamin C) such as “Morosil” or “Red orange complex” are available from Bionap.
Combination of Weight Management Actives, such as Flavonoids like Bioflavonoids are available from Citrus sinensis (Hesperidin) and Caffeine.
Bioflavonoids from citrus sinensis & citrus paradisi (Hesperidin >80%; Naringin >5%) such as “Microbiomex®” are available from Bioactor or bioflavonoids from citrus sinensis (hesperidin > 90%) (“Cordiart”) from Bioactor.
Vitamin D (Vitamin D3) powder 100000 I U/G is available from company Echemi.
Beta-glucane is available from company Leiber (“Yestimmun Beta Glucan”).
Green Tea extract (EGCG," Teavigo”) or ECG (“Sunphenon ECG”), or EGC “Sunphenon XLB-100”) or EC (“Sunphenon EC”) all are available from Taiyo.
Oils, lipids like MCT (Medium chain triglycerides), like “Powder MCT C8 coconut 70% vegan” are available from Bioriginal or “Medium Chain Triglycerides (MCT-CTS or MCT-CPH”) oil powder from MCB, or Sumbia - Keto-HiMCT (high in C8 fatty acids) from Nutriventia (Mumbai, India), skim milk and whole milk and full cream milk powders from Saputo, Australia (“Instant Full cream Lactose Free powder 25 kg”), anhydrous milk, butter fat or liquid oils such as linseed oil, coconut oil.
Carallum Fimbriata Extract such as “Slimaluma” are available from Gencor Pacific Limited.
Extract from Gynostemma pentaphyllum such as “Active AMP” is available from Gencor Pacific Limited
Whey proteins (hydrolysates, concentrates, isolates), such as “Whey protein concentrate 80 Instant” are available
from Vivi Solution.
Milk protein concentrates like MPC 80/85 are available from Saputo Dairy Australia Pty Ltd.
Probiotics like B. psychraerophilum Q5 (DSM 33131) + L. hordei KLL19 (DSM 33127) + L. harbinensis G8 (DSM 33126) such as "premix novaMETABOLISM” are available from Probionova or Bifidobactehum breve B-3 from Morinaga Milk Industry, a mixture of Lacidophilus PBS066-DSM 24936 (Synbalance LA) and L. reuteri PBS072- DSM25175 (SynBalance LR) and L. plantarum PBS067-DSM24937 (Synbalance LP) with the tradename MetSyn are available from company Synbalance, Italy
In particular, the active ingredients from citrus extracts (Hesperidin, Naringin) have been specifically designed to support gut and immune health by beneficially shifting the gut microbiome composition, lowering gut inflammation, and strengthening the gut barrier.
They are particularly beneficial because there is a greater production of Short Chain Fatty Acids - mainly butyrate and propionate - essential compounds for the gut barrier maintenance and regulation of the immune system Furthermore, reactive oxygen species (ROS) are reduced by Microbiomex®.
Usually, it is difficult to get a homogenous mixture of KGM and the above substances. The composition of the invention also solves this problem so that any other useful substance may be added to the composition of the invention, provided that said substance is chemically compatible with the other components of the composition of the invention.
As said, the solid fast dissolving or fast dispersing composition of the invention may also comprise colorants, to improve the appearance of the composition when mixed with water.
Said colorants may also be active coloured ingredient, such as Morosil™ (comprising anthocyanins, hydroxycinnamic acids, flavanones, and ascorbic acid) or Brainberry® (comprising cyanidin-3-O-glycosides from Aronia melanocarpa'j without adding colorants.
To that purpose, it is important to select the composition of the invention having the correct pH, i.e., pH < 6, so that said active-coloured ingredients may develop their nice red colour (both ingredients do not show a red colour at pH 7 or higher).
Accordingly, when the above active-coloured ingredients are added to the composition of the invention to provide both a further beneficial activity and colour, then it is important that they are added to compositions (B), having pH lower than 6.
If needed or desired, any further conventional excipient may be added to the composition, provided it is chemically compatible with the other components of the composition of the invention.
As said, the solid fast dissolving or fast dispersing composition of the invention is a mixture of powders and/or granules which must be mixed with suitable water-based fluid, as above indicated, before intake.
As said, the solid fast dissolving or fast dispersing composition of the invention is to be administered by oral route, after having been mixed with water or another water-based fluid
A drinkable composition obtained by mixing the solid fast dissolving or fast dispersing composition of the invention with water or another water-based fluid is another subject-matter of the invention.
So, according to another of its aspects, subject-matter of the invention is a liquid/fluid composition comprising the solid fast dissolving or fast dispersing combination or the composition of the invention, and a suitable drinkable liquid, preferably, but not limited to, water.
The drinkable composition of the invention may be prepared by diluting the solid fast dissolving or fast dispersing combination or the composition of the invention with a suitable liquid, preferably water.
According to one of its aspects, the subject matter of the present invention relates to a process for preparing a drinkable compositition comprising the steps of: a) putting into contact a solid fast dissolving or fast dispersing composition of the invention with a water-based fluid, which is selected from water, a beverage, a milk, a milk substitute, a plant-based milk, non dairy substitute, nondairy beverage, instant milk; non-alcoholic, alcoholic, a fruit drink, energy drink, fortified drink, herbal drink, sport drink, yogurt drink, buttermilk drink, probiotic drink, a nectar, a juice or a cappuccino, a smoothie, a coffee, a macchiato, a cafe au lait, a coffee milk, a frapuccino, an espresso, an espresso macchiato, a latte espresso, a flat white, a caffee latte, a cacao, or mixtures thereof; b) mixing, preferably with the aid of a spoon, for a time from 10 seconds to 5 minutes, preferably from 1 minute to 3 minutes, at a temperature from 18 °C to 40°C, preferably from 18°C to 25 °C.
The solid fast dissolving or fast dispersing compositions and/or the drinkable compositions of the invention are for use in a subject, said subject being a mammal, preferably a human being.
A solid fast dissolving or fast dispersing composition of the invention may be prepared by simply blending the solid powder components of the composition and then may be packaged in multi-dose container or as a unit dose form, such as a sachet of a stick-pack.
The mixture of powders and/or granules of the invention can be part of a two-part system (where the powder is separated from the fluid phase, e.g., in a cap of a biphase container), a can, a cardboard can, paper can, plastic packaging boxes, biodegradable packaging containers.
The solid fast dissolving or fast dispersing compositions and/or the drinkable compositions of the invention are useful in the management of body weight, especially in the treatment and/or prevention of overweight and obesity, and also for lowering cholesterol and blood sugar levels.
According to another of its aspects, it is a subject-matter of the invention the solid fast dissolving or fast dispersing compositions and/or the drinkable compositions of the invention for use in reducing body weight in overweight and obese subjects.
According to another of its aspects, it is a subject-matter of the invention the use of the solid fast dissolving or fast dispersing composition of the invention in promoting body weight loss in a healthy subject. In this case, the use is not a therapeutic one.
According to another of its aspects, it is a subject-matter of the invention the solid fast dissolving or fast dispersing compositions and/or the drinkable compositions of the invention for use in lowering cholesterol and blood sugar levels in a subject.
The invention also relates to a method for reducing body weight which comprises administering to a subject in need
thereof an effective amount of a solid fast dissolving or fast dispersing composition and/or a drinkable composition of the invention.
The solid fast dissolving or fast dispersing composition of the invention is generally administered at a dose of about 1-5 g/day of KGM, preferably 2-4 g/day of KGM, more preferably about 3g /day of KGM, preferably in the context of an energy restricted diet. For instance, the solid fast dissolving or fast dispersing composition of the invention is administered at a dose of 1 g/day of KGM three times per day.
Representative examples of the solid fast dissolving or fast dispersing compositions and drinkable compositions obtained therefrom of the invention are reported in the Experimental Section which follows, for illustrative purposes only.
The following are representative practical examples.
EXPERIMENTAL SECTION
Evaluation of viscosity
After the ingestion of a water formulation comprising KGM, the water penetrates into the tissue or blood stream in the body. To simulate the vanishing of water from the formulation a series of experiments was made; gel formation occurs again when the water is no longer a significant part of the formulation and gel formation lowers food craving. Figure 1 shows a simulation of fluid KGM behaviour after ingestion.
With Reference to Figure 1 , an increase in the viscosity is expected leading to a higher viscous composition/gel, depending on the amount of water. This can be simulated by formulating a usually fluid composition (e.g., KGM/Arabic gum/salt tripotassium citrate monohydrate/colorant Vanilla Yellow P-WS from Sensient/water 1g/1.8g/0.8g/0.06/96.3g) by taking out the water (composition with 80%, 60%, 40%, 20% of water instead of 96.3 g of water). Indeed, an increase of viscosity and gel formation was observed in the samples from 80% to 20% of water.
Examples of compositions
Representative Examples of solid compositions in powder form of the invention in mixed with water q.s. to 100 g. The viscosity behaviour of the compositions of the Examples was observed.
Table 1
Konjac Glucomannan powder was added to water in a beverage glass and stirred with a spoon. Lump formation was observed. A greyish, lump containing gel formulation was obtained that is not appealing for a consumer. Drinking of this formulation is impossible due to the high viscosity of 36,000 mPas. The gel does not flow so that a consumer can not drink it from the beverage container. Furthermore the taste of this Konjac glucomannan gel is very bad.
Example 2
Table 2
Konjac Glucomannan, Gum arable, Tripotassium Citrate Monohydrate, Citric acid anhydrous, Morosil, Sucralose,
Blood orange flavor were mixed, and the powder mixture was added to water and stirred with a spoon. The formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1).
A low pH formulation with a red color is obtained. This acid pH is important because active ingredients such as
Morosil only partially show their red color at neutral pH.
Table 3 Konjac Glucomannan, Gum arabic, Tripotassium Citrate Monohydrate, Citric acid anhydrous, Sucralose, Vanilla Flavor, Color additive (Vanilla Yellow) were mixed, and the powder mixture was added to water and stirred with a spoon. The formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1).
Example 4
Table 4
Konjac Glucomannan, Gum arable urn and Tripotassium Citrate Monohydrate were mixed and the powder mixture was added to water and stirred with a spoon. The formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1).
Table 5
Konjac Glucomannan, Gum Arabic, Tripotassium Citrate Monohydrate, Citric acid anhydrous, Brainberry (Aronia melanocarpa extract), Sucralose and blueberry flavor were mixed and the powder mixture was added to water and stirred with a spoon. The formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1).
Table 6
Konjac Glucomannan, Gum Arabic, Tripotassium Citrate Monohydrate, Citric acid anhydrous, Watts'up (bioflavaonoids from citrus sinensis (Hesperidin), caffeine, sucralose the color additive (Vanilla Yellow), and orange flavor were mixed, and the powder mixture was added to water and stirred with a spoon. The formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1).
Table 7
Konjac Glucomannan and Tripotassium Citrate Monohydrate were mixed and the powder mixture was added to water and stirred with a spoon. The formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1). It is a fluid. It gives a fluid gel after 10-15 minutes.
Table 8
Konjac Glucomannan and Potassium Gluconate were mixed, and the powder mixture was added to water and stirred with a spoon. The formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1). It is a fluid. It gives a fluid gel after 10-15 minutes.
Konjac Glucomannan, Gum Arabic, Sodium Hydrogen Carbonate, Citric acid anhydrous, Sucralose, Vanilla Flavor and the color additive (Vanilla Yellow) were mixed, and the powder mixture was added to water and stirred with a spoon. The mixture gives a foam (CO2 release) that breaks down after a while. The formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1).
Konjac Glucomannan, Gum Arabic, Sodium Ascorbate, Citric acid anhydrous, Sucralose, Blood orange flavor, Color additive (Vanilla Yellow) were mixed, and the powder mixture was added to water and stirred with a spoon The formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1).
Table 11
Konjac Glucomannan, Gum Arabic, Calcium chloride Dihydrate, Citric acid anhydrous, Sucralose, Blood orange
flavor, Color additive (Vanilla Yellow) were mixed, and the powder mixture was added to water and stirred with a spoon. The formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative
Example 1).
Konjac Glucomannan, Gum Arabic, DHA Algae Powder DAF, Sodium hydrogen carbonate, Citric acid anhydrous, Sucralose, Vanilla flavor, Color additive (Vanilla Yellow) were mixed and the powder mixture was added to water and stirred with a spoon. The formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1). Example 13
Table 13
Konjac Glucomannan, Gum Arabic, Capsifen (Chili extract, fenugreek powder, Cellulose powder, Lecithin, gum acacia), Sodium hydrogen carbonate, Citric acid anhydrous, Sucralose, Orange Flavour, Color additive (Vanilla Yellow) were mixed and the powder mixture was added to water and stirred with a spoon. The mixture gives a small foam (CO2 release) that breaks down after a while. The formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1). The Capsifen particles are homogenous suspended in the mixture directly after the preparation.
Table 14
Konjac Glucomannan, Gum Arabic, Tripotassium Citrate Monohydrate, Citric acid anhydrous, Bioflavonoids from citrus sinensis & citrus paradisi, Sucralose, Color additive (Vanilla Yellow) and Blood orange flavor were mixed and the powder mixture was added to water and stirred with a spoon. The mixture gives a small foam (C02 release) that breaks down after a while. The formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1).
Table 15
Konjac Glucomannan, Gum Arabic, Sodium hydrogen carbonate, Citric acid anhydrous, Sucralose, Whey protein concentrate 80 (Whey protein concentrate), Morosil (Citrus Sinensis fruit extract), Blood orange flavor were mixed, and the powder mixture was added to water and stirred with a spoon. The mixture gives a small foam (CO2 release) that breaks down after a while. The formulation has a lower viscosity then 1g pure Konjac Glucomannan in 99 g water (Comparative Example 1).
Table 16
Konjac Glucomannan, sodium hydrogen carbonate, Citric acid anhydrous were mixed and the powder mixture was added to water and stirred with a spoon. Very fast dissolution of Konjac Glucomannan Due to the dissolved CO2 a creamy taste result. A smooth gel (drinkable) results.
Table 17
Konjac Glucomannan, Sodium hydrogen carbonate, Citric acid anhydrous and Tripotassium Citrate Monohydrate were mixed, and the powder mixture was added to water and stirred with a spoon. Very fast dissolution of Konjac.
A fluid is obtained. After 15 min due to the dissolved CO2 a creamy taste result. A smooth gel (drinkable) result.
Table 18
A test conducted on milk and coffee is reported. In practice, a solid composition was prepared, in accordance with the present invention, containing 1% KGM and a mild Robusta coffee powder (1 5 g/100 g; caffeine 52.5-82.5 mg/100 g). Subsequently, said composition was added to milk. A fluid composition of milk and coffee with good color, odor, taste and free of lumps was immediately obtained.
Claims
1 . A solid fast dissolving or fast dispersing composition comprising or, alternatively, consisting of a high molecular weight Konjac glucomannan (KGM) and an arable gum in combination with at least one further component which is selected from the group comprising or, alternatively, consisting of:
(A) at least one water-soluble salt, with the provision that said at least one water-soluble salt is not a phosphate or polyphosphate salt; or
(B) at least one water-soluble salt and at least one organic acid, preferably at least one hydroxy acid; or
(D”) an effervescent system comprising or, alternatively, consisting of at least one basic substance capable of releasing carbon dioxide in the presence of at least one acidic substance and, optionally, at least one water-soluble salt; or
(H) at least one organic acid, preferably at least one hydroxy acid; and/or their mixtures thereof, wherein said solid fast dissolving or fast dispersing composition is in the form of a mixture of powders and/or granules.
2. The solid fast dissolving or fast dispersing composition according to claim 1, wherein said high molecular weight Konjac glucomannan (KGM) has a molecular weight equal to or bigger than 106 Da; preferably, has a molecular weight comprised from bigger than 106 Da to 108 Da; more preferably, has a molecular weight comprised from 107 Da to 10s Da.
3. The solid fast dissolving or fast dispersing composition according to claim 1 or 2, wherein said high molecular weight Konjac glucomannan (KGM) has viscosity value preferably comprised from 20,000 cps to 100,000 cps, preferably comprised from 25,000 cps to 80,000 cps, more preferably comprised from 30,000 cps to 60,000 cps, even more preferably comprised from 35,000 cps to 50,000 cps, and preferably has glucomannan content comprised from bigger than 85% to 98%, more preferably from 90% and 95%.
4. The solid fast dissolving or fast dispersing composition according to any one of claims 1-3, wherein said at least one water-soluble salt is preferably selected from the group comprising or, alternatively, consisting of:
- citrate salts; preferably, sodium and/or potassium citrate salts; more preferably tripotassium citrate salts, tripotassium citrate monohydrate salts, trisodium citrate salts, trisodium citrate anhydrous salts, magnesium citrate, calcium citrate, zinc citrate, or their mixtures thereof;
- ascorbate salts; preferably, sodium and/or potassium ascorbate salts, calcium and/or magnesium ascorbate salts, zinc ascorbate, zinc ascorbate monohydrate salts, or their mixtures thereof;
- gluconate salts; preferably, sodium and/or potassium gluconate salts, calcium and/or magnesium gluconate salts, zinc gluconate salt, or their mixtures thereof;
- chloride salts; preferably, sodium chloride, potassium chloride, calcium chloride, magnesium chloride, ammonium chloride, or their mixture thereof.
- lactate salts; preferably, calcium lactate, calcium lactate pentahydrate, sodium and/or potassium lactate, zinc lactate, zinc lactate dihydrate, or their mixtures thereof;
- tartrate salts; preferably, sodium and/or potassium tartrate, potassium sodium tartrate, or their mixtures thereof;
- sulfate salts; preferably, sodium and/or potassium sulfate salts;
- glycinate salts; preferably, sodium and/or potassium glycinate salts;
- bisglycinate salts; preferably, zinc bisglycinate or zinc bisglycinate monohydrate salts;
- acetyl taurinate salts; preferably, calcium and/or magnesium acetyl taurinate salts; or their mixturs thereof.
5. The solid fast dissolving or fast dispersing composition according to any one of claims 1-4, wherein said at least one organic acid is selected from the group comprising or, alternatevily, consisting of citric acid, lactic acid, ascorbic acid, tartaric acid, malic acid, or glucono-delta-lactone (GdL).
6 The solid fast dissolving or fast dispersing composition according to any one of claims 1-5, wherein said effervescent system comprises or, alternatively, consists of at least one basic substance capable of releasing carbon dioxide in the presence of at least one acidic substance, wherein said basic substance is selected from carbonate or bicarbonate salts; more preferably, sodium and/or potassium carbonate, sodium and/or potassium bicarbonate, or their mixtures thereof; and said at least one acidic substance is selected from organic acids; preferably, hydroxy acids such as citric acid and/or tartaric acid.
7. The solid fast dissolving or fast dispersing composition according to any one of claims 1-6, wherein said composition comprises or, altervatively, consists of
(i) KGM, arabic gum, tripotassium citrate, more preferably, tripotassium citrate monohydrate, and, optionally, citric acid; or
(ii) KGM, arabic gum, sodium and/or potassium (bi)carbonate and citric acid and, optionally; at least one water- soluble salt preferably selected from tri potassium citrate; more preferably, tripotassium citrate monohydrate, sodium ascorbate, calcium chloride, preferably calcium chloride x 2 H20, potassium gluconate, sodium gluconate, and mixture thereof; or
(iii) KGM, arabic gum and citric acid and, optionally, at least one water-soluble salt preferably selected from tripotassium citrate; more preferably, tripotassium citrate monohydrate, sodium ascorbate, calcium chloride, preferably calcium chloride x 2 H20, potassium gluconate, sodium gluconate, and mixture thereof; or
(iv) KGM, arabic gum and at least one water-soluble salt preferably selected from tripotassium citrate; more preferably, tripotassium citrate monohydrate, sodium ascorbate, calcium chloride, preferably calcium chloride x 2 H20, potassium gluconate, sodium gluconate, and mixture thereof and, optionally, citric acid.
8. The solid fast dissolving or fast dispersing composition according to any one of claims 1-7, wherein said composition may further comprise a) optionally, at least one flavours and/or colorant and/or sweetener; and/or and/or mixtures thereof; and/or b) optionally, at least one further active ingredient which is selected from:
- antocyanines or antocyanins/polyphenols mixtures;
- carob bean extract and fructooligosaccharide;
- black garlic such as ABG10+;
- coffein such as Caffeine, 98% or CaffXtend or soluble coffee extracts such as Arabica kraftig, 23-10234, Espresso, aromatisch (Arabica/Robusta-Blend), 23-10236, Arabica mild, 23-10232, Espresso, medium, Robusta, 23-10235,
Robusta, kraftig, 23-10233, Robusta, mild, 23-10231 ;
- cacao powder such as Noir Intense, Plaine Arome, Extra brute, Rouge Ultime, Legere 1%; preferably, actives in cacao are polyphenols like flavanols such as epicatechin, catechin, and procyanidins, and flavonols such as quercetin, anthocyanins, phenolic acids, and stilbenes; coffein is in guarana Guarana Extract PDR & N.Caff BLDR22%-904;
- collagens, and vegan collagens;
- chromium salts such as chromium picolinate;
- niacin;
- alpha lipoic acid such as Alipure;
- pumpkin seed protein powder;
- whey hydrolysate with an active dipeptide AP (Alanine-Proline) which inhibits the alpha glucosidase's activity such as Pep2Dia;
- cinnamon or cinnamon extracts;
- curcumin such as HydroCurc® Liquid Grade 10%, or Curcuwin Ultra+ Curcuminoids 20%, or CurcuWIN Curcumin DNS HB Pdr 20%/EU, or Turmacin, or Water Dispersible Turmeric Extract Powder 60N;
- vitamins (lipophilic vitamins such as A, D, E, K, and B-vitamins or the B-vitamin complex; or thiamine (Vitamin B- 1), vitamin B6, vitamin C, niacin, iron, calcium, and vitamin B5;
- powders from almond, cashews, soy, rice, hemp, coconut, oats, linseed, spelt;
- dried fruits and/or freeze-dried fruits and/or individually quick frozen (IQF) fruits and/or frozen fruit products;
- polyphenols and the like such as WATTS' UP;
- bioflavonoids from Citrus sinensis (Hesperidin);
- carnitin, carnitin hydrochloride such as L-Carnitin HCI;
- resveratrol such as ResveraFen, or Veri-Sperse®;
- capsaicin such as Capsifen, chili extract, fenugreek powder, cellulose powder and/or lecithin, acacia; or capsimax;
- omega-3, such as DHA Algae Powder DAF;
- complexes from Citrus Sinensis fruit extract (anthocyanins, hydroxycinnamic acids, flavanones, vitamin C) such as Morosil or Red orange complex;
- combination of weight management actives, such as flavonoids like bioflavonoids (Hesperidin) and caffeine;
- bioflavonoids from citrus sinensis & citrus paradisi (Hesperidin >80%; Naringin >5%) such as “Microbiomex®’, or bioflavonoids from citrus sinensis (hesperidin > 90%) Cordiart;
- vitamin D (vitamin D3) powder 100000 IU/G;
- beta-glucane such as Yestimmun Beta Glucan;
- green tea extract (EGCG, Teavigo) or ECG (Sunphenon ECG), or EGC (Sunphenon XLB-100) or EC (Sunphenon EC;
- oils, lipids like MCT (medium chain triglycerides) such as Powder MCT C8 coconut 70% vegan, or medium chain triglycerides (MCT-CTS or MCT-CPH”) oil powder from MCB, or skim milk and whole milk and full cream milk
powders, anhydrous milk, butter fat or liquid oils such as linseed oil, coconut oil;
- carallum fimbriata extract such as Slimaluma;
- extract from Gynostemma pentaphyl lum such as Active AMP;
- whey proteins (hydrolysates, concentrates, isolates), such as whey protein concentrate 80 I;
- milk protein concentrates such as MPG 80/85;
- probiotics such as B. psychraerophilum Q5 (DSM 33131) or L. horde! KLL19 (DSM 33127) or L. harbinensis G8 (DSM 33126) and /or Bifidobacterium breve B-3, or a mixture of L. acidophilus PBS066-DSM 24936 and L. reuteri PBS072-DSM25175 and L plantarum PBS067-DSM24937; and mixtures thereof.
9 A process for preparing a drinkable compositition comprising the steps of: a) putting into contact a solid fast dissolving or fast dispersing composition according to any one of claims 1-8 with a water-based fluid, which is preferably selected from water, a beverage, a milk, a milk substitute, a plant-based milk, non dairy substitute, non-dairy beverage, instant milk; non-alcoholic, alcoholic, a fruit drink, energy drink, fortified drink, herbal drink, sport drink, yogurt drink, buttermilk drink, probiotic drink, a nectar, a juice or a cappuccino, a smoothie, a coffee, a macchiato, a cafe au lait, a coffee milk, a frapuccino, an espresso, an espresso macchiato, a latte espresso, a flat white, a caffee latte, a cacao, or mixtures thereof; b) mixing, preferably with the aid of a spoon, for a time from 10 seconds to 5 minutes, preferably from 1 minute to 3 minutes, at a temperature from 18 °C to 40°C, preferably from 18°C to 25 °C.
10. A drinkable composition obtained by the process according to claim 9 comprising a solid fast dissolving or fast dispersing composition according to any one of claims 1 -8 with a water-based fluid, which is preferably selected from water, a beverage, a milk, a milk substitute, a plant-based milk, non dairy substitute, non-dairy beverage, instant milk; non-alcoholic, alcoholic, a fruit drink, energy drink, fortified drink, herbal drink, sport drink, yogurt drink, buttermilk drink, probiotic drink, a nectar, a juice or a cappuccino, a smoothie, a coffee, a macchiato, a cafe au lait, a coffee milk, a frapuccino, an espresso, an espresso macchiato, a latte espresso, a flat white, a caffee latte, a cacao, or mixtures thereof.
11. The solid fast dissolving or fast dispersing composition according to any one of claims 1-8, or the drinkable composition according to claim 10, for use in a method for managing weight loss or overweight reduction in a subject in need.
12. The solid fast dissolving or fast dispersing composition according to any one of claims 1-8, or the drinkable composition according to claim 10, for use in a method to promote body weight loss in a healthy subject, or to reduce body weight in an overweight and/or obese subjects.
13. The solid fast dissolving or fast dispersing composition according to any one of claims 1-8, or the drinkable composition according to claim 10, for use in a method for reducing cholesterol and blood sugar levels in a subject in need.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IT202300005823 | 2023-03-27 | ||
| IT102023000005823 | 2023-03-27 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2024201327A1 true WO2024201327A1 (en) | 2024-10-03 |
Family
ID=86604515
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IB2024/052961 WO2024201327A1 (en) | 2023-03-27 | 2024-03-27 | Novel combinations comprising konjac mannan, their compositions and uses thereof |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2024201327A1 (en) |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1799410A (en) | 2005-01-07 | 2006-07-12 | 周宓 | Diet fiber composition with blood lipid-reducing blood sugar-decreasing functions and its production method |
| CN105901269A (en) | 2016-04-28 | 2016-08-31 | 张雅萍 | Konjac tablet candy for weight management and preparation method of konjac tablet candy for weight management |
| CN108041570A (en) | 2017-12-06 | 2018-05-18 | 连云港西都生化有限公司 | A kind of composite food additive |
| WO2022013120A1 (en) * | 2020-07-15 | 2022-01-20 | Société des Produits Nestlé S.A. | Compositions to promote swallowing safety and efficiency |
-
2024
- 2024-03-27 WO PCT/IB2024/052961 patent/WO2024201327A1/en unknown
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1799410A (en) | 2005-01-07 | 2006-07-12 | 周宓 | Diet fiber composition with blood lipid-reducing blood sugar-decreasing functions and its production method |
| CN105901269A (en) | 2016-04-28 | 2016-08-31 | 张雅萍 | Konjac tablet candy for weight management and preparation method of konjac tablet candy for weight management |
| CN108041570A (en) | 2017-12-06 | 2018-05-18 | 连云港西都生化有限公司 | A kind of composite food additive |
| WO2022013120A1 (en) * | 2020-07-15 | 2022-01-20 | Société des Produits Nestlé S.A. | Compositions to promote swallowing safety and efficiency |
Non-Patent Citations (6)
| Title |
|---|
| ANONYMOUS: "Shape shots", 28 May 2022 (2022-05-28), www.foodspring.es, pages 1 - 4, XP093176436, Retrieved from the Internet <URL:https://web.archive.org/web/20220528011441/https://www.foodspring.es/shots-para-bajar-de-peso#product-ingredients> [retrieved on 20240619] * |
| DATABASE GNPD [online] MINTEL; 31 May 2022 (2022-05-31), ANONYMOUS: "Refreshing White Grape Flavoured Energy Jelly Drink", XP093175521, retrieved from https://www.gnpd.com/sinatra/recordpage/9624672/ Database accession no. 9624672 * |
| FARBSTEIN DAN ET AL: "HDL dysfunction in diabetes: causes and possible treatments", vol. 10, no. 3, 1 March 2012 (2012-03-01), pages 356 - 361, XP093087587, Retrieved from the Internet <URL:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3332215/> [retrieved on 20231002], DOI: 10.1586/erc.11.182 * |
| J AM COLL NUTR, vol. 24, no. 37220-17-0, June 2005 (2005-06-01), pages 158 - 65 |
| KONJAC MANNAN: "Handbook of Hydrocolloids", 2009 |
| MARTÍNEZ GINÉS B HERNÁNDEZ ET AL: "Nutritional and bioactive compounds of commercialized algae powders used as food supplements", vol. 24, no. 2, 7 November 2017 (2017-11-07), pages 172 - 182, XP093086807, Retrieved from the Internet <URL:https://pubmed.ncbi.nlm.nih.gov/29110539/> [retrieved on 20230929], DOI: 10.1177/1082013217740000 * |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN103202417B (en) | Absorbable agar jelly | |
| US20040096547A1 (en) | Healthy alternative ready-to-drink energy beverage | |
| RU2317731C2 (en) | Composition for preparing of instantaneous beverages | |
| RU2363269C2 (en) | Amorphous water-soluble calcium citrate salts and its manufacturing and usage method | |
| CA2863613C (en) | Low calorie drink tablet | |
| ES2795668T3 (en) | Anti-regurgitation composition that preserves intestinal transit | |
| ES2759021T3 (en) | Powder mix | |
| JP4352354B2 (en) | Solid instant jelly mix | |
| US20070190223A1 (en) | Soy/whey protein recovery composition | |
| JP2014532725A (en) | Concentrated prunes and prebiotic formulations as laxatives and food supplements | |
| TW200936060A (en) | Induced viscosity nutritional emulsions comprising a carbohydrate-surfactant complex | |
| CN101642272A (en) | Millet drink and manufacturing method thereof | |
| ES2911350T3 (en) | Concentrated liquid drink with soluble fibers and method of supplying soluble fiber | |
| WO2024201327A1 (en) | Novel combinations comprising konjac mannan, their compositions and uses thereof | |
| WO2018047621A1 (en) | Olive oil composition for beverage, olive oil beverage, and method for manufacturing same | |
| WO2024201329A1 (en) | Novel combinations comprising konjac mannan, their compositions and uses thereof | |
| JP3620757B2 (en) | Liquid food and drink with solids | |
| KR100885512B1 (en) | Method for preparing agar jelly containing antihypertensive functional peptide | |
| KR101061933B1 (en) | How to prepare vitamin nutrients | |
| RU2486843C1 (en) | Dry ingredients mixture for mousses preparation | |
| RU2322159C1 (en) | Vegetable concentrate "apogey" | |
| ES2781428T3 (en) | Calcium absorption accelerating agent | |
| JP2006342061A (en) | Mineral-containing aqueous solution composition | |
| KR20070012281A (en) | Hangover Chlorella Drink | |
| CZ32393U1 (en) | A powder mixture for preparing a beverage or for thickening food |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 24720293 Country of ref document: EP Kind code of ref document: A1 |