KR101494594B1 - 약리학적 활성물질의 서방성 지질 초기제제 및 이를 포함하는 약제학적 조성물 - Google Patents
약리학적 활성물질의 서방성 지질 초기제제 및 이를 포함하는 약제학적 조성물 Download PDFInfo
- Publication number
- KR101494594B1 KR101494594B1 KR20120093677A KR20120093677A KR101494594B1 KR 101494594 B1 KR101494594 B1 KR 101494594B1 KR 20120093677 A KR20120093677 A KR 20120093677A KR 20120093677 A KR20120093677 A KR 20120093677A KR 101494594 B1 KR101494594 B1 KR 101494594B1
- Authority
- KR
- South Korea
- Prior art keywords
- sorbitan
- group
- lipid
- sustained
- present
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 150000002632 lipids Chemical class 0.000 title claims abstract description 35
- 238000013268 sustained release Methods 0.000 title claims abstract description 33
- 239000012730 sustained-release form Substances 0.000 title claims abstract description 33
- 239000013543 active substance Substances 0.000 title claims abstract description 28
- 239000008194 pharmaceutical composition Substances 0.000 title claims abstract description 21
- 239000012141 concentrate Substances 0.000 title claims abstract description 8
- 239000000203 mixture Substances 0.000 claims abstract description 85
- 239000004973 liquid crystal related substance Substances 0.000 claims abstract description 59
- -1 sorbitan unsaturated fatty acid ester Chemical class 0.000 claims abstract description 41
- 239000012530 fluid Substances 0.000 claims abstract description 28
- 150000003904 phospholipids Chemical class 0.000 claims abstract description 19
- 235000021122 unsaturated fatty acids Nutrition 0.000 claims abstract description 19
- 239000007791 liquid phase Substances 0.000 claims abstract description 14
- 239000003623 enhancer Substances 0.000 claims abstract description 11
- 230000002209 hydrophobic effect Effects 0.000 claims abstract description 11
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims abstract description 8
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical compound OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 claims description 49
- 239000003814 drug Substances 0.000 claims description 22
- 238000002360 preparation method Methods 0.000 claims description 20
- 238000009472 formulation Methods 0.000 claims description 15
- 239000007924 injection Substances 0.000 claims description 14
- 238000002347 injection Methods 0.000 claims description 14
- 239000007788 liquid Substances 0.000 claims description 12
- 239000003795 chemical substances by application Substances 0.000 claims description 10
- 108090000623 proteins and genes Proteins 0.000 claims description 10
- VYGQUTWHTHXGQB-FFHKNEKCSA-N Retinol Palmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C VYGQUTWHTHXGQB-FFHKNEKCSA-N 0.000 claims description 8
- SESFRYSPDFLNCH-UHFFFAOYSA-N benzyl benzoate Chemical compound C=1C=CC=CC=1C(=O)OCC1=CC=CC=C1 SESFRYSPDFLNCH-UHFFFAOYSA-N 0.000 claims description 8
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 claims description 8
- ZORQXIQZAOLNGE-UHFFFAOYSA-N 1,1-difluorocyclohexane Chemical compound FC1(F)CCCCC1 ZORQXIQZAOLNGE-UHFFFAOYSA-N 0.000 claims description 7
- 125000004432 carbon atom Chemical group C* 0.000 claims description 7
- 102000004169 proteins and genes Human genes 0.000 claims description 7
- 239000001593 sorbitan monooleate Substances 0.000 claims description 7
- 235000011069 sorbitan monooleate Nutrition 0.000 claims description 7
- 229940035049 sorbitan monooleate Drugs 0.000 claims description 7
- ZAKOWWREFLAJOT-CEFNRUSXSA-N D-alpha-tocopherylacetate Chemical compound CC(=O)OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C ZAKOWWREFLAJOT-CEFNRUSXSA-N 0.000 claims description 6
- 125000005907 alkyl ester group Chemical group 0.000 claims description 6
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 claims description 6
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 6
- 229940042585 tocopherol acetate Drugs 0.000 claims description 6
- CUNWUEBNSZSNRX-RKGWDQTMSA-N (2r,3r,4r,5s)-hexane-1,2,3,4,5,6-hexol;(z)-octadec-9-enoic acid Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O CUNWUEBNSZSNRX-RKGWDQTMSA-N 0.000 claims description 4
- 125000003277 amino group Chemical group 0.000 claims description 4
- 229960002903 benzyl benzoate Drugs 0.000 claims description 4
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 4
- 235000012000 cholesterol Nutrition 0.000 claims description 4
- 229940107161 cholesterol Drugs 0.000 claims description 4
- 150000001875 compounds Chemical class 0.000 claims description 4
- 239000000813 peptide hormone Substances 0.000 claims description 4
- 229940108325 retinyl palmitate Drugs 0.000 claims description 4
- 235000019172 retinyl palmitate Nutrition 0.000 claims description 4
- 239000011769 retinyl palmitate Substances 0.000 claims description 4
- 229920006395 saturated elastomer Polymers 0.000 claims description 4
- 229960005078 sorbitan sesquioleate Drugs 0.000 claims description 4
- 150000003626 triacylglycerols Chemical group 0.000 claims description 4
- PORPENFLTBBHSG-MGBGTMOVSA-N 1,2-dihexadecanoyl-sn-glycerol-3-phosphate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(O)=O)OC(=O)CCCCCCCCCCCCCCC PORPENFLTBBHSG-MGBGTMOVSA-N 0.000 claims description 3
- 239000002775 capsule Substances 0.000 claims description 3
- 239000003889 eye drop Substances 0.000 claims description 3
- 239000006210 lotion Substances 0.000 claims description 3
- 238000000034 method Methods 0.000 claims description 3
- 239000002674 ointment Substances 0.000 claims description 3
- 239000007921 spray Substances 0.000 claims description 3
- 239000000725 suspension Substances 0.000 claims description 3
- 229960005486 vaccine Drugs 0.000 claims description 3
- TZCPCKNHXULUIY-RGULYWFUSA-N 1,2-distearoyl-sn-glycero-3-phosphoserine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCC TZCPCKNHXULUIY-RGULYWFUSA-N 0.000 claims description 2
- JZNWSCPGTDBMEW-UHFFFAOYSA-N Glycerophosphorylethanolamin Natural products NCCOP(O)(=O)OCC(O)CO JZNWSCPGTDBMEW-UHFFFAOYSA-N 0.000 claims description 2
- ZWZWYGMENQVNFU-UHFFFAOYSA-N Glycerophosphorylserin Natural products OC(=O)C(N)COP(O)(=O)OCC(O)CO ZWZWYGMENQVNFU-UHFFFAOYSA-N 0.000 claims description 2
- 125000001095 phosphatidyl group Chemical group 0.000 claims description 2
- 150000008104 phosphatidylethanolamines Chemical class 0.000 claims description 2
- 150000003905 phosphatidylinositols Chemical class 0.000 claims description 2
- 108700026220 vif Genes Proteins 0.000 claims description 2
- 239000002537 cosmetic Substances 0.000 claims 3
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 claims 3
- 230000002708 enhancing effect Effects 0.000 claims 2
- IYFATESGLOUGBX-YVNJGZBMSA-N Sorbitan monopalmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O IYFATESGLOUGBX-YVNJGZBMSA-N 0.000 claims 1
- 235000011071 sorbitan monopalmitate Nutrition 0.000 claims 1
- 239000001570 sorbitan monopalmitate Substances 0.000 claims 1
- 229940031953 sorbitan monopalmitate Drugs 0.000 claims 1
- 239000012071 phase Substances 0.000 abstract description 12
- 125000002887 hydroxy group Chemical group [H]O* 0.000 abstract description 7
- 229910052799 carbon Inorganic materials 0.000 abstract description 2
- 125000003010 ionic group Chemical group 0.000 abstract description 2
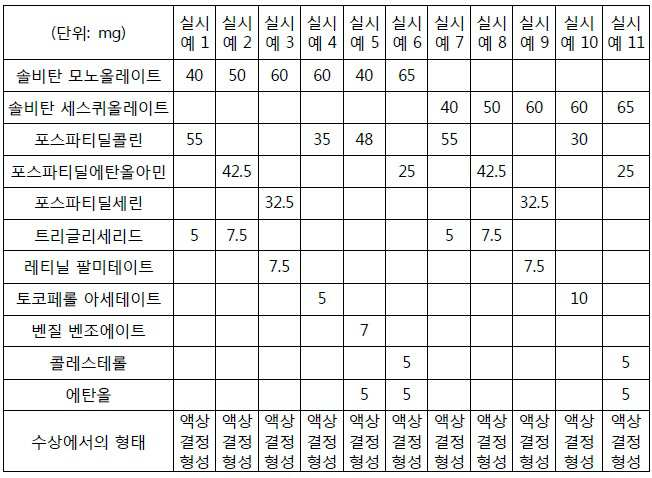
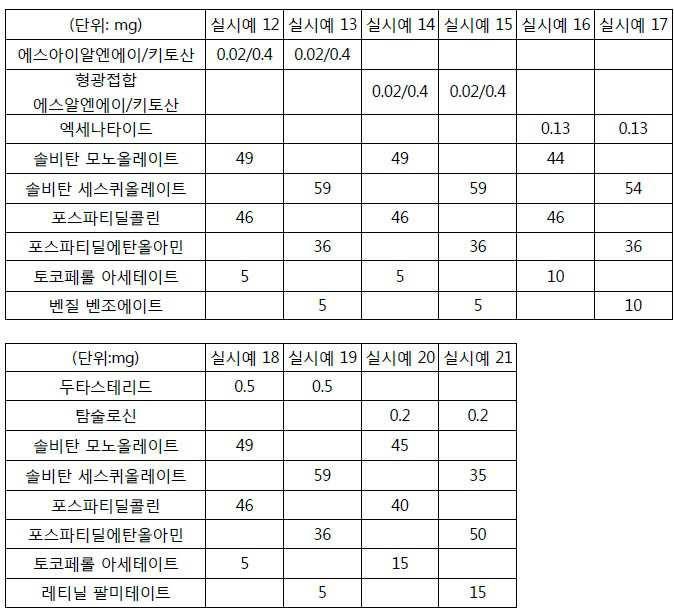
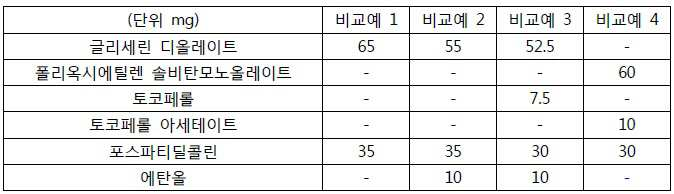
- 230000000052 comparative effect Effects 0.000 description 19
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 17
- 229940079593 drug Drugs 0.000 description 15
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 13
- 235000014113 dietary fatty acids Nutrition 0.000 description 13
- 230000000694 effects Effects 0.000 description 13
- 239000000194 fatty acid Substances 0.000 description 13
- 229930195729 fatty acid Natural products 0.000 description 13
- 238000001727 in vivo Methods 0.000 description 13
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 10
- GVJHHUAWPYXKBD-UHFFFAOYSA-N d-alpha-tocopherol Natural products OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 9
- 238000002474 experimental method Methods 0.000 description 9
- 239000000463 material Substances 0.000 description 9
- 229920001606 poly(lactic acid-co-glycolic acid) Polymers 0.000 description 9
- 108010011459 Exenatide Proteins 0.000 description 8
- HTQBXNHDCUEHJF-XWLPCZSASA-N Exenatide Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)NCC(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 HTQBXNHDCUEHJF-XWLPCZSASA-N 0.000 description 8
- 239000013078 crystal Substances 0.000 description 8
- 229960001519 exenatide Drugs 0.000 description 8
- 239000000126 substance Substances 0.000 description 8
- 239000003960 organic solvent Substances 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 6
- 150000004665 fatty acids Chemical class 0.000 description 6
- 235000011187 glycerol Nutrition 0.000 description 6
- 239000007787 solid Substances 0.000 description 6
- 238000005728 strengthening Methods 0.000 description 6
- RBNPOMFGQQGHHO-UWTATZPHSA-N D-glyceric acid Chemical compound OC[C@@H](O)C(O)=O RBNPOMFGQQGHHO-UWTATZPHSA-N 0.000 description 5
- 230000006399 behavior Effects 0.000 description 5
- 210000004027 cell Anatomy 0.000 description 5
- 238000000338 in vitro Methods 0.000 description 5
- 235000019198 oils Nutrition 0.000 description 5
- 206010061218 Inflammation Diseases 0.000 description 4
- 241000700159 Rattus Species 0.000 description 4
- 239000003405 delayed action preparation Substances 0.000 description 4
- 150000002148 esters Chemical class 0.000 description 4
- 125000005456 glyceride group Chemical group 0.000 description 4
- 229940088597 hormone Drugs 0.000 description 4
- 239000005556 hormone Substances 0.000 description 4
- 230000004054 inflammatory process Effects 0.000 description 4
- 239000002502 liposome Substances 0.000 description 4
- 239000002609 medium Substances 0.000 description 4
- 239000003921 oil Substances 0.000 description 4
- 239000002245 particle Substances 0.000 description 4
- 239000000546 pharmaceutical excipient Substances 0.000 description 4
- 230000000144 pharmacologic effect Effects 0.000 description 4
- 239000002904 solvent Substances 0.000 description 4
- RZRNAYUHWVFMIP-KTKRTIGZSA-N 1-oleoylglycerol Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(O)CO RZRNAYUHWVFMIP-KTKRTIGZSA-N 0.000 description 3
- ZWGOKSXVMODEMQ-UHFFFAOYSA-N 3,7,11,15-tetramethylhexadecyl 2,3-dihydroxypropanoate Chemical compound CC(C)CCCC(C)CCCC(C)CCCC(C)CCOC(=O)C(O)CO ZWGOKSXVMODEMQ-UHFFFAOYSA-N 0.000 description 3
- 239000006145 Eagle's minimal essential medium Substances 0.000 description 3
- 229920001214 Polysorbate 60 Polymers 0.000 description 3
- 229930003427 Vitamin E Natural products 0.000 description 3
- 239000008346 aqueous phase Substances 0.000 description 3
- 238000006065 biodegradation reaction Methods 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000000604 cryogenic transmission electron microscopy Methods 0.000 description 3
- 125000005313 fatty acid group Chemical group 0.000 description 3
- 150000002191 fatty alcohols Chemical class 0.000 description 3
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 3
- 239000007943 implant Substances 0.000 description 3
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 3
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 3
- 229920000053 polysorbate 80 Polymers 0.000 description 3
- 239000004094 surface-active agent Substances 0.000 description 3
- 230000002459 sustained effect Effects 0.000 description 3
- 229930003799 tocopherol Natural products 0.000 description 3
- 235000010384 tocopherol Nutrition 0.000 description 3
- 229960001295 tocopherol Drugs 0.000 description 3
- 239000011732 tocopherol Substances 0.000 description 3
- 239000011709 vitamin E Substances 0.000 description 3
- 229940046009 vitamin E Drugs 0.000 description 3
- 235000019165 vitamin E Nutrition 0.000 description 3
- GVJHHUAWPYXKBD-IEOSBIPESA-N α-tocopherol Chemical compound OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-IEOSBIPESA-N 0.000 description 3
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- 102000003815 Interleukin-11 Human genes 0.000 description 2
- 108090000177 Interleukin-11 Proteins 0.000 description 2
- 239000004166 Lanolin Substances 0.000 description 2
- RJKFOVLPORLFTN-LEKSSAKUSA-N Progesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C)CC2 RJKFOVLPORLFTN-LEKSSAKUSA-N 0.000 description 2
- 206010060862 Prostate cancer Diseases 0.000 description 2
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- MUMGGOZAMZWBJJ-DYKIIFRCSA-N Testostosterone Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 MUMGGOZAMZWBJJ-DYKIIFRCSA-N 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 210000001124 body fluid Anatomy 0.000 description 2
- 239000010839 body fluid Substances 0.000 description 2
- 238000002425 crystallisation Methods 0.000 description 2
- 230000008025 crystallization Effects 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- 238000009509 drug development Methods 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 230000001747 exhibiting effect Effects 0.000 description 2
- 239000012091 fetal bovine serum Substances 0.000 description 2
- UPWGQKDVAURUGE-UHFFFAOYSA-N glycerine monooleate Natural products CCCCCCCCC=CCCCCCCCC(=O)OC(CO)CO UPWGQKDVAURUGE-UHFFFAOYSA-N 0.000 description 2
- 239000008384 inner phase Substances 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 229940074383 interleukin-11 Drugs 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 229940039717 lanolin Drugs 0.000 description 2
- 235000019388 lanolin Nutrition 0.000 description 2
- 239000000693 micelle Substances 0.000 description 2
- 239000004530 micro-emulsion Substances 0.000 description 2
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid group Chemical group C(CCCCCCC\C=C/CCCCCCCC)(=O)O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 2
- 229940046166 oligodeoxynucleotide Drugs 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 239000002504 physiological saline solution Substances 0.000 description 2
- 238000010254 subcutaneous injection Methods 0.000 description 2
- 239000007929 subcutaneous injection Substances 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- 230000001960 triggered effect Effects 0.000 description 2
- 150000003712 vitamin E derivatives Chemical class 0.000 description 2
- RBNPOMFGQQGHHO-UHFFFAOYSA-N -2,3-Dihydroxypropanoic acid Natural products OCC(O)C(O)=O RBNPOMFGQQGHHO-UHFFFAOYSA-N 0.000 description 1
- VOXZDWNPVJITMN-ZBRFXRBCSA-N 17β-estradiol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 VOXZDWNPVJITMN-ZBRFXRBCSA-N 0.000 description 1
- NALREUIWICQLPS-UHFFFAOYSA-N 7-imino-n,n-dimethylphenothiazin-3-amine;hydrochloride Chemical compound [Cl-].C1=C(N)C=C2SC3=CC(=[N+](C)C)C=CC3=NC2=C1 NALREUIWICQLPS-UHFFFAOYSA-N 0.000 description 1
- 108010064733 Angiotensins Proteins 0.000 description 1
- 102000015427 Angiotensins Human genes 0.000 description 1
- CEUORZQYGODEFX-UHFFFAOYSA-N Aripirazole Chemical compound ClC1=CC=CC(N2CCN(CCCCOC=3C=C4NC(=O)CCC4=CC=3)CC2)=C1Cl CEUORZQYGODEFX-UHFFFAOYSA-N 0.000 description 1
- 108010001478 Bacitracin Proteins 0.000 description 1
- 102000015081 Blood Coagulation Factors Human genes 0.000 description 1
- 108010039209 Blood Coagulation Factors Proteins 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 101800004538 Bradykinin Proteins 0.000 description 1
- 102400000967 Bradykinin Human genes 0.000 description 1
- 229920001661 Chitosan Polymers 0.000 description 1
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 1
- 229930105110 Cyclosporin A Natural products 0.000 description 1
- 108010036949 Cyclosporine Proteins 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 102400001368 Epidermal growth factor Human genes 0.000 description 1
- 101800003838 Epidermal growth factor Proteins 0.000 description 1
- 102000003951 Erythropoietin Human genes 0.000 description 1
- 108090000394 Erythropoietin Proteins 0.000 description 1
- 102400000921 Gastrin Human genes 0.000 description 1
- 108010052343 Gastrins Proteins 0.000 description 1
- 102400000321 Glucagon Human genes 0.000 description 1
- 108060003199 Glucagon Proteins 0.000 description 1
- 108010088406 Glucagon-Like Peptides Proteins 0.000 description 1
- 244000068988 Glycine max Species 0.000 description 1
- 235000010469 Glycine max Nutrition 0.000 description 1
- 108010026389 Gramicidin Proteins 0.000 description 1
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 1
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 1
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102000004457 Granulocyte-Macrophage Colony-Stimulating Factor Human genes 0.000 description 1
- 108010051696 Growth Hormone Proteins 0.000 description 1
- 239000000095 Growth Hormone-Releasing Hormone Substances 0.000 description 1
- QXZGBUJJYSLZLT-UHFFFAOYSA-N H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH Natural products NC(N)=NCCCC(N)C(=O)N1CCCC1C(=O)N1C(C(=O)NCC(=O)NC(CC=2C=CC=CC=2)C(=O)NC(CO)C(=O)N2C(CCC2)C(=O)NC(CC=2C=CC=CC=2)C(=O)NC(CCCN=C(N)N)C(O)=O)CCC1 QXZGBUJJYSLZLT-UHFFFAOYSA-N 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 108010033040 Histones Proteins 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- ZCVMWBYGMWKGHF-UHFFFAOYSA-N Ketotifene Chemical compound C1CN(C)CCC1=C1C2=CC=CC=C2CC(=O)C2=C1C=CS2 ZCVMWBYGMWKGHF-UHFFFAOYSA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- 239000000232 Lipid Bilayer Substances 0.000 description 1
- 108010046938 Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102000007651 Macrophage Colony-Stimulating Factor Human genes 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- UCHDWCPVSPXUMX-TZIWLTJVSA-N Montelukast Chemical compound CC(C)(O)C1=CC=CC=C1CC[C@H](C=1C=C(\C=C\C=2N=C3C=C(Cl)C=CC3=CC=2)C=CC=1)SCC1(CC(O)=O)CC1 UCHDWCPVSPXUMX-TZIWLTJVSA-N 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 108010025020 Nerve Growth Factor Proteins 0.000 description 1
- 102000015336 Nerve Growth Factor Human genes 0.000 description 1
- AZLIXMDAMOHKAG-CVBJKYQLSA-N OCC(O)CO.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O Chemical compound OCC(O)CO.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O AZLIXMDAMOHKAG-CVBJKYQLSA-N 0.000 description 1
- 101800000989 Oxytocin Proteins 0.000 description 1
- 102400000050 Oxytocin Human genes 0.000 description 1
- XNOPRXBHLZRZKH-UHFFFAOYSA-N Oxytocin Natural products N1C(=O)C(N)CSSCC(C(=O)N2C(CCC2)C(=O)NC(CC(C)C)C(=O)NCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(CCC(N)=O)NC(=O)C(C(C)CC)NC(=O)C1CC1=CC=C(O)C=C1 XNOPRXBHLZRZKH-UHFFFAOYSA-N 0.000 description 1
- 235000019482 Palm oil Nutrition 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 108010079943 Pentagastrin Proteins 0.000 description 1
- 108010038512 Platelet-Derived Growth Factor Proteins 0.000 description 1
- 102000010780 Platelet-Derived Growth Factor Human genes 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 108010057464 Prolactin Proteins 0.000 description 1
- 102100024819 Prolactin Human genes 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 235000019484 Rapeseed oil Nutrition 0.000 description 1
- 102100028255 Renin Human genes 0.000 description 1
- 108090000783 Renin Proteins 0.000 description 1
- 235000019485 Safflower oil Nutrition 0.000 description 1
- 108020004459 Small interfering RNA Proteins 0.000 description 1
- 102100022831 Somatoliberin Human genes 0.000 description 1
- 101710142969 Somatoliberin Proteins 0.000 description 1
- 102100038803 Somatotropin Human genes 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- 229930182558 Sterol Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 235000019486 Sunflower oil Nutrition 0.000 description 1
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 description 1
- 108010012944 Tetragastrin Proteins 0.000 description 1
- 229910004338 Ti-S Inorganic materials 0.000 description 1
- GXBMIBRIOWHPDT-UHFFFAOYSA-N Vasopressin Natural products N1C(=O)C(CC=2C=C(O)C=CC=2)NC(=O)C(N)CSSCC(C(=O)N2C(CCC2)C(=O)NC(CCCN=C(N)N)C(=O)NCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(CCC(N)=O)NC(=O)C1CC1=CC=CC=C1 GXBMIBRIOWHPDT-UHFFFAOYSA-N 0.000 description 1
- 108010004977 Vasopressins Proteins 0.000 description 1
- 102000002852 Vasopressins Human genes 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 239000011149 active material Substances 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 238000005054 agglomeration Methods 0.000 description 1
- 230000004931 aggregating effect Effects 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 229960002932 anastrozole Drugs 0.000 description 1
- YBBLVLTVTVSKRW-UHFFFAOYSA-N anastrozole Chemical compound N#CC(C)(C)C1=CC(C(C)(C#N)C)=CC(CN2N=CN=C2)=C1 YBBLVLTVTVSKRW-UHFFFAOYSA-N 0.000 description 1
- 230000000692 anti-sense effect Effects 0.000 description 1
- 239000003472 antidiabetic agent Substances 0.000 description 1
- 229940125708 antidiabetic agent Drugs 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- KBZOIRJILGZLEJ-LGYYRGKSSA-N argipressin Chemical compound C([C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CSSC[C@@H](C(N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N1)=O)N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(N)=O)C1=CC=CC=C1 KBZOIRJILGZLEJ-LGYYRGKSSA-N 0.000 description 1
- 229960004372 aripiprazole Drugs 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 229960003071 bacitracin Drugs 0.000 description 1
- 229930184125 bacitracin Natural products 0.000 description 1
- CLKOFPXJLQSYAH-ABRJDSQDSA-N bacitracin A Chemical compound C1SC([C@@H](N)[C@@H](C)CC)=N[C@@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]1C(=O)N[C@H](CCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2N=CNC=2)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)NCCCC1 CLKOFPXJLQSYAH-ABRJDSQDSA-N 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 229920002988 biodegradable polymer Polymers 0.000 description 1
- 239000004621 biodegradable polymer Substances 0.000 description 1
- 239000013060 biological fluid Substances 0.000 description 1
- 239000012620 biological material Substances 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000003114 blood coagulation factor Substances 0.000 description 1
- 229940019700 blood coagulation factors Drugs 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- QXZGBUJJYSLZLT-FDISYFBBSA-N bradykinin Chemical compound NC(=N)NCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(=O)NCC(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CO)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)CCC1 QXZGBUJJYSLZLT-FDISYFBBSA-N 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- AOXOCDRNSPFDPE-UKEONUMOSA-N chembl413654 Chemical compound C([C@H](C(=O)NCC(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@H](CCSC)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC=1C=CC=CC=1)C(N)=O)NC(=O)[C@@H](C)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H]1N(CCC1)C(=O)CNC(=O)[C@@H](N)CCC(O)=O)C1=CC=C(O)C=C1 AOXOCDRNSPFDPE-UKEONUMOSA-N 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 229960001265 ciclosporin Drugs 0.000 description 1
- 230000005757 colony formation Effects 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 235000005687 corn oil Nutrition 0.000 description 1
- 239000002285 corn oil Substances 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 239000002385 cottonseed oil Substances 0.000 description 1
- 229930182912 cyclosporin Natural products 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 230000009849 deactivation Effects 0.000 description 1
- 239000007857 degradation product Substances 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 235000013601 eggs Nutrition 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 229940116977 epidermal growth factor Drugs 0.000 description 1
- 229940105423 erythropoietin Drugs 0.000 description 1
- 229930182833 estradiol Natural products 0.000 description 1
- 229960005309 estradiol Drugs 0.000 description 1
- 210000003722 extracellular fluid Anatomy 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000010685 fatty oil Substances 0.000 description 1
- PJMPHNIQZUBGLI-UHFFFAOYSA-N fentanyl Chemical compound C=1C=CC=CC=1N(C(=O)CC)C(CC1)CCN1CCC1=CC=CC=C1 PJMPHNIQZUBGLI-UHFFFAOYSA-N 0.000 description 1
- 229960002428 fentanyl Drugs 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- DBEPLOCGEIEOCV-WSBQPABSSA-N finasteride Chemical compound N([C@@H]1CC2)C(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H](C(=O)NC(C)(C)C)[C@@]2(C)CC1 DBEPLOCGEIEOCV-WSBQPABSSA-N 0.000 description 1
- 229960004039 finasteride Drugs 0.000 description 1
- 235000021323 fish oil Nutrition 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 229960004666 glucagon Drugs 0.000 description 1
- MASNOZXLGMXCHN-ZLPAWPGGSA-N glucagon Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C1=CC=CC=C1 MASNOZXLGMXCHN-ZLPAWPGGSA-N 0.000 description 1
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 1
- 229960004905 gramicidin Drugs 0.000 description 1
- ZWCXYZRRTRDGQE-SORVKSEFSA-N gramicidina Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CC=3C4=CC=CC=C4NC=3)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CC=3C4=CC=CC=C4NC=3)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CC=3C4=CC=CC=C4NC=3)NC(=O)[C@H](C(C)C)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](C(C)C)NC(=O)[C@H](C)NC(=O)[C@H](NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC=O)C(C)C)CC(C)C)C(=O)NCCO)=CNC2=C1 ZWCXYZRRTRDGQE-SORVKSEFSA-N 0.000 description 1
- 239000000122 growth hormone Substances 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 229920001477 hydrophilic polymer Polymers 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 229940079322 interferon Drugs 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 229960004958 ketotifen Drugs 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 229960004616 medroxyprogesterone Drugs 0.000 description 1
- FRQMUZJSZHZSGN-HBNHAYAOSA-N medroxyprogesterone Chemical compound C([C@@]12C)CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2CC[C@]2(C)[C@@](O)(C(C)=O)CC[C@H]21 FRQMUZJSZHZSGN-HBNHAYAOSA-N 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 235000021243 milk fat Nutrition 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 235000021281 monounsaturated fatty acids Nutrition 0.000 description 1
- 229960005127 montelukast Drugs 0.000 description 1
- 210000004400 mucous membrane Anatomy 0.000 description 1
- DQCKKXVULJGBQN-XFWGSAIBSA-N naltrexone Chemical compound N1([C@@H]2CC3=CC=C(C=4O[C@@H]5[C@](C3=4)([C@]2(CCC5=O)O)CC1)O)CC1CC1 DQCKKXVULJGBQN-XFWGSAIBSA-N 0.000 description 1
- 229960003086 naltrexone Drugs 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 229940053128 nerve growth factor Drugs 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 229960005017 olanzapine Drugs 0.000 description 1
- KVWDHTXUZHCGIO-UHFFFAOYSA-N olanzapine Chemical compound C1CN(C)CCN1C1=NC2=CC=CC=C2NC2=C1C=C(C)S2 KVWDHTXUZHCGIO-UHFFFAOYSA-N 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 229960004114 olopatadine Drugs 0.000 description 1
- JBIMVDZLSHOPLA-LSCVHKIXSA-N olopatadine Chemical compound C1OC2=CC=C(CC(O)=O)C=C2C(=C/CCN(C)C)\C2=CC=CC=C21 JBIMVDZLSHOPLA-LSCVHKIXSA-N 0.000 description 1
- 230000002188 osteogenic effect Effects 0.000 description 1
- XNOPRXBHLZRZKH-DSZYJQQASA-N oxytocin Chemical compound C([C@H]1C(=O)N[C@H](C(N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CSSC[C@H](N)C(=O)N1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)=O)[C@@H](C)CC)C1=CC=C(O)C=C1 XNOPRXBHLZRZKH-DSZYJQQASA-N 0.000 description 1
- 229960001723 oxytocin Drugs 0.000 description 1
- 239000002540 palm oil Substances 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 229960000444 pentagastrin Drugs 0.000 description 1
- ANRIQLNBZQLTFV-DZUOILHNSA-N pentagastrin Chemical compound C([C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1[C]2C=CC=CC2=NC=1)NC(=O)CCNC(=O)OC(C)(C)C)CCSC)C(N)=O)C1=CC=CC=C1 ANRIQLNBZQLTFV-DZUOILHNSA-N 0.000 description 1
- 239000008177 pharmaceutical agent Substances 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 229940067631 phospholipid Drugs 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 108091033319 polynucleotide Proteins 0.000 description 1
- 102000040430 polynucleotide Human genes 0.000 description 1
- 239000002157 polynucleotide Substances 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 229950008882 polysorbate Drugs 0.000 description 1
- OXCMYAYHXIHQOA-UHFFFAOYSA-N potassium;[2-butyl-5-chloro-3-[[4-[2-(1,2,4-triaza-3-azanidacyclopenta-1,4-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol Chemical compound [K+].CCCCC1=NC(Cl)=C(CO)N1CC1=CC=C(C=2C(=CC=CC=2)C2=N[N-]N=N2)C=C1 OXCMYAYHXIHQOA-UHFFFAOYSA-N 0.000 description 1
- 230000001376 precipitating effect Effects 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 239000000186 progesterone Substances 0.000 description 1
- 229960003387 progesterone Drugs 0.000 description 1
- 229940097325 prolactin Drugs 0.000 description 1
- 150000003180 prostaglandins Chemical class 0.000 description 1
- 239000008213 purified water Substances 0.000 description 1
- 229960001534 risperidone Drugs 0.000 description 1
- RAPZEAPATHNIPO-UHFFFAOYSA-N risperidone Chemical compound FC1=CC=C2C(C3CCN(CC3)CCC=3C(=O)N4CCCCC4=NC=3C)=NOC2=C1 RAPZEAPATHNIPO-UHFFFAOYSA-N 0.000 description 1
- 235000005713 safflower oil Nutrition 0.000 description 1
- 239000003813 safflower oil Substances 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 238000007086 side reaction Methods 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- FBOUYBDGKBSUES-VXKWHMMOSA-N solifenacin Chemical compound C1([C@H]2C3=CC=CC=C3CCN2C(O[C@@H]2C3CCN(CC3)C2)=O)=CC=CC=C1 FBOUYBDGKBSUES-VXKWHMMOSA-N 0.000 description 1
- 229960003855 solifenacin Drugs 0.000 description 1
- NHXLMOGPVYXJNR-ATOGVRKGSA-N somatostatin Chemical class C([C@H]1C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CSSC[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2C3=CC=CC=C3NC=2)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(=O)N1)[C@@H](C)O)NC(=O)CNC(=O)[C@H](C)N)C(O)=O)=O)[C@H](O)C)C1=CC=CC=C1 NHXLMOGPVYXJNR-ATOGVRKGSA-N 0.000 description 1
- 229940075620 somatostatin analogue Drugs 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 150000003432 sterols Chemical class 0.000 description 1
- 235000003702 sterols Nutrition 0.000 description 1
- 238000012916 structural analysis Methods 0.000 description 1
- 239000002600 sunflower oil Substances 0.000 description 1
- 210000004243 sweat Anatomy 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 229960001967 tacrolimus Drugs 0.000 description 1
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 description 1
- IEHKWSGCTWLXFU-IIBYNOLFSA-N tadalafil Chemical compound C1=C2OCOC2=CC([C@@H]2C3=C([C]4C=CC=CC4=N3)C[C@H]3N2C(=O)CN(C3=O)C)=C1 IEHKWSGCTWLXFU-IIBYNOLFSA-N 0.000 description 1
- 229960000835 tadalafil Drugs 0.000 description 1
- 239000003760 tallow Substances 0.000 description 1
- 210000001138 tear Anatomy 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 229960003604 testosterone Drugs 0.000 description 1
- RGYLYUZOGHTBRF-BIHRQFPBSA-N tetragastrin Chemical compound C([C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C2=CC=CC=C2NC=1)CCSC)C(N)=O)C1=CC=CC=C1 RGYLYUZOGHTBRF-BIHRQFPBSA-N 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 230000000472 traumatic effect Effects 0.000 description 1
- UFTFJSFQGQCHQW-UHFFFAOYSA-N triformin Chemical compound O=COCC(OC=O)COC=O UFTFJSFQGQCHQW-UHFFFAOYSA-N 0.000 description 1
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 229960003726 vasopressin Drugs 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 208000016261 weight loss Diseases 0.000 description 1
- 230000004580 weight loss Effects 0.000 description 1
- 239000010698 whale oil Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/107—Emulsions ; Emulsion preconcentrates; Micelles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/22—Heterocyclic compounds, e.g. ascorbic acid, tocopherol or pyrrolidones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/16—Amides, e.g. hydroxamic acids
- A61K31/18—Sulfonamides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/56—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids
- A61K31/58—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids containing heterocyclic rings, e.g. danazol, stanozolol, pancuronium or digitogenin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/713—Double-stranded nucleic acids or oligonucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/22—Hormones
- A61K38/26—Glucagons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/10—Alcohols; Phenols; Salts thereof, e.g. glycerol; Polyethylene glycols [PEG]; Poloxamers; PEG/POE alkyl ethers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/14—Esters of carboxylic acids, e.g. fatty acid monoglycerides, medium-chain triglycerides, parabens or PEG fatty acid esters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/24—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing atoms other than carbon, hydrogen, oxygen, halogen, nitrogen or sulfur, e.g. cyclomethicone or phospholipids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/26—Carbohydrates, e.g. sugar alcohols, amino sugars, nucleic acids, mono-, di- or oligo-saccharides; Derivatives thereof, e.g. polysorbates, sorbitan fatty acid esters or glycyrrhizin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
- A61K9/0024—Solid, semi-solid or solidifying implants, which are implanted or injected in body tissue
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/107—Emulsions ; Emulsion preconcentrates; Micelles
- A61K9/1075—Microemulsions or submicron emulsions; Preconcentrates or solids thereof; Micelles, e.g. made of phospholipids or block copolymers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/0008—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'non-active' part of the composition delivered, e.g. wherein such 'non-active' part is not delivered simultaneously with the 'active' part of the composition
- A61K48/0016—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'non-active' part of the composition delivered, e.g. wherein such 'non-active' part is not delivered simultaneously with the 'active' part of the composition wherein the nucleic acid is delivered as a 'naked' nucleic acid, i.e. not combined with an entity such as a cationic lipid
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Pharmacology & Pharmacy (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Dermatology (AREA)
- Dispersion Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Immunology (AREA)
- Endocrinology (AREA)
- Zoology (AREA)
- Gastroenterology & Hepatology (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Inorganic Chemistry (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
[도 2]는 실시예 14의 약리학적 활성물질의 in vitro에서의 방출 거동을 나타낸 것이다.
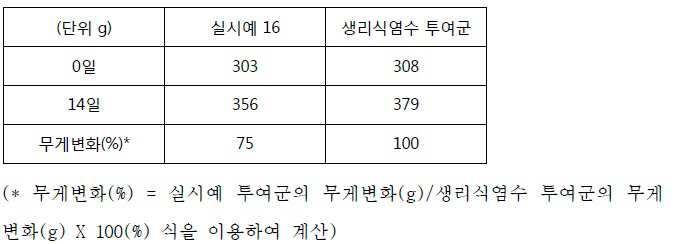
[도 3]은 실시예 16 및 비교예 5의 약리학적 활성물질의 in vivo 방출 거동을 나타낸 것이다.
[도 4]는 실시예 4 및 비교예 4의 수성유체 상에서의 상변화 거동을 나타낸 것이다.
[도 5]는 실시예 4의 제제의 액상결정구조를 Cryo TEM 사진으로 확인한 것이다.
Claims (15)
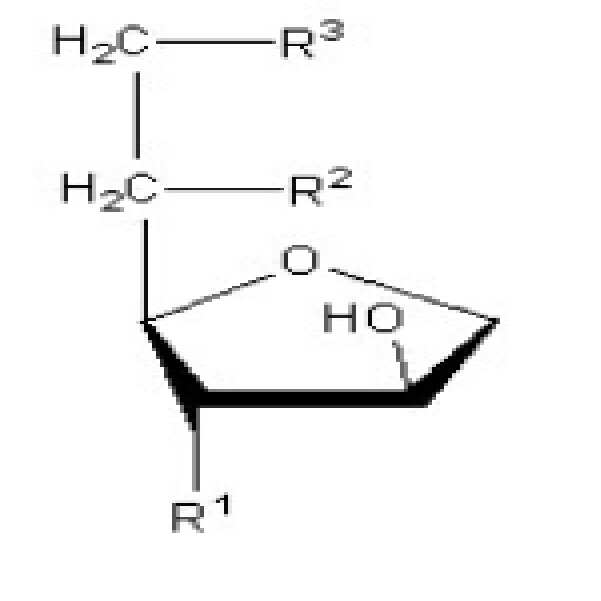
- a) 솔비탄 모노올레이트, 솔비탄 모노리놀레이트, 솔비탄 모노팔미톨레이트, 솔비탄 모노미리스톨레이트, 솔비탄 세스퀴올레이트, 솔비탄 세스퀴리놀레이트, 솔비탄 세스퀴팔미톨레이트, 솔비탄 세스퀴미리스톨레이트, 솔비탄 디올레이트, 솔비탄 디리놀레이트, 솔비탄 디팔미톨레이트, 솔비탄 디미리스톨레이트 및 이들의 혼합물 중에서 선택된 솔비탄 불포화지방산 에스터(sorbitan unsaturated fatty acid ester);
b) 포스포리피드(phospholipid); 및
c) 카르복실기 또는 아민기의 이온화기를 가지지 않고 소수성 부분은 탄소수가 15개 내지 40개의 트리아실기를 가지거나 탄소링 구조를 가지는 액상결정 강화제를 포함하고 수성유체가 없는 상태에서 지질 액상이며 수성유체 상에서 비층상 액상결정을 형성하는 서방성 지질 초기제제(pre-concentrate). - 삭제
- 제 1 항에 있어서, 솔비탄 불포화지방산 에스터는 솔비탄 모노올레이트, 솔비탄 모노리놀레이트, 솔비탄 모노팔미톨레이트, 솔비탄 모노미리스톨레이트 및 이들의 혼합물 중에서 선택된 것인 서방성 지질 초기제제.
- 제 1 항에 있어서, 포스포리피드는 포화 또는 불포화된 탄소수가 4 내지 30인 알킬 에스터 그룹을 가지는 포스파티딜콜린(phosphatidylcholine), 포스파티딜에탄올아민(phosphatidylethanolamine), 포스파티딜세린(phosphatidylserine), 포스파티딜글리세린(phosphatidylglycerine), 포스파티딜이노시톨(phosphatidylinositol), 포스파티딘산(phosphatidic acid), 스핑고미엘린(sphingomyelin) 및 이들의 혼합물 중에서 선택된 것인 서방성 지질 초기제제.
- 제 1 항에 있어서, 액상결정 강화제가 트리글리세리드, 레티닐 팔미테이트, 토코페롤 아세테이트, 콜레스테롤, 벤질 벤조에이트 및 이들의 혼합물 중에서 선택된 것인 서방성 지질 초기 제제.
- 제 1 항에 있어서, 액상결정 강화제가 트리글리세리드, 레티닐 팔미테이트, 토코페롤 아세테이트, 콜레스테롤 및 이들의 혼합물 중에서 선택된 것인 서방성 지질 초기 제제.
- 제 1 항에 있어서, 액상결정 강화제가 토코페롤 아세테이트인 서방성 지질 초기 제제.
- 제 1 항, 및 제 3 항 내지 제 7 항 중 어느 한 항에 있어서, a) 및 b)의 중량비가 10:1 내지 1:10인 서방성 지질 초기제제.
- 제 1 항, 및 제 3 항 내지 제 7 항 중 어느 한 항에 있어서, a) + b) 및 c)의 중량비가 100:1 내지 1:1인 서방성 지질 초기제제.
- 제 1 항, 및 제 3 항 내지 제 7 항 어느 한 항의 지질 초기제제에서, d) 단백질, 펩티드, 백신, 유전자, 비-펩티드 호르몬, 합성의약품 및 이들의 혼합물 중에서 선택된 약리활성 물질을 추가로 포함하는 약제학적 조성물.
- 제 10 항에 있어서, 지질 초기제제의 구성성분인 a) 및 b)의 중량비가 10:1 내지 1:10인 약제학적 조성물.
- 제 10 항에 있어서, 지질 초기제제의 구성성분인 a) + b) 및 c)의 중량비가 100:1 내지 1:1인 약제학적 조성물.
- 제 10 항에 있어서, 약제학적 조성물이 주사제, 연고제, 겔제, 로션제, 캡슐제, 정제, 액제, 현탁제, 분무제, 흡입제, 점안제, 점착제, 첩부제 중에서 선택된 제형인 약제학적 조성물.
- a) 솔비탄 모노올레이트, 솔비탄 모노리놀레이트, 솔비탄 모노팔미톨레이트, 솔비탄 모노미리스톨레이트, 솔비탄 세스퀴올레이트, 솔비탄 세스퀴리놀레이트, 솔비탄 세스퀴팔미톨레이트, 솔비탄 세스퀴미리스톨레이트, 솔비탄 디올레이트, 솔비탄 디리놀레이트, 솔비탄 디팔미톨레이트, 솔비탄 디미리스톨레이트 및 이들의 혼합물 중에서 선택된 솔비탄 불포화지방산 에스터(sorbitan unsaturated fatty acid ester);
b) 포화 또는 불포화된 탄소수가 4 내지 30인 알킬 에스터 그룹을 가지는 포스포리피드(phospholipid); 및
c) 카르복실기 또는 아민기의 이온화기를 가지지 않고 소수성 부분은 탄소수가 15개 내지 40개의 트리아실기를 가지거나 탄소링 구조를 가지는 액상결정 강화제를 포함하고 수성유체가 없는 상태에서 지질 액상이며 수성유체 상에서 비층상 액상결정을 형성하는 서방성 지질 초기제제(pre-concentrate). - a) 솔비탄 모노올레이트, 솔비탄 모노리놀레이트, 솔비탄 모노팔미톨레이트, 솔비탄 모노미리스톨레이트, 솔비탄 세스퀴올레이트, 솔비탄 세스퀴리놀레이트, 솔비탄 세스퀴팔미톨레이트, 솔비탄 세스퀴미리스톨레이트, 솔비탄 디올레이트, 솔비탄 디리놀레이트, 솔비탄 디팔미톨레이트, 솔비탄 디미리스톨레이트 및 이들의 혼합물 중에서 선택된 솔비탄 불포화지방산 에스터(sorbitan unsaturated fatty acid ester);
b) 포화 또는 불포화된 탄소수가 4 내지 30인 알킬 에스터 그룹을 가지는 포스포리피드(phospholipid); 및
c) 트리글리세리드, 레티닐 팔미테이트, 토코페롤 아세테이트, 콜레스테롤, 벤질 벤조에이트 및 이들의 혼합물 중에서 선택된 액상결정 강화제를 포함하고 수성유체가 없는 상태에서 지질 액상이며 수성유체 상에서 비층상 액상결정을 형성하는 서방성 지질 초기제제(pre-concentrate).
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020110087160 | 2011-08-30 | ||
| KR20110087160 | 2011-08-30 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20130024804A KR20130024804A (ko) | 2013-03-08 |
| KR101494594B1 true KR101494594B1 (ko) | 2015-02-23 |
Family
ID=47756584
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR20120093677A Active KR101494594B1 (ko) | 2011-08-30 | 2012-08-27 | 약리학적 활성물질의 서방성 지질 초기제제 및 이를 포함하는 약제학적 조성물 |
Country Status (21)
| Country | Link |
|---|---|
| US (1) | US9526787B2 (ko) |
| EP (1) | EP2750667B1 (ko) |
| JP (1) | JP5981997B2 (ko) |
| KR (1) | KR101494594B1 (ko) |
| CN (1) | CN103764127B (ko) |
| AU (1) | AU2012302422B2 (ko) |
| BR (1) | BR112014004967B1 (ko) |
| CA (1) | CA2845784C (ko) |
| DK (1) | DK2750667T3 (ko) |
| ES (1) | ES2661487T3 (ko) |
| HU (1) | HUE036150T2 (ko) |
| LT (1) | LT2750667T (ko) |
| MX (1) | MX351430B (ko) |
| NO (1) | NO2750667T3 (ko) |
| PH (1) | PH12014500449B1 (ko) |
| PL (1) | PL2750667T3 (ko) |
| PT (1) | PT2750667T (ko) |
| RU (1) | RU2632433C2 (ko) |
| SI (1) | SI2750667T1 (ko) |
| TR (1) | TR201802984T4 (ko) |
| WO (1) | WO2013032207A1 (ko) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017069533A1 (en) * | 2015-10-20 | 2017-04-27 | Mirae Pharm Co.,Ltd. | Oral liquid formulation of tadalafil |
| WO2020171491A1 (ko) | 2019-02-18 | 2020-08-27 | (주)아이엠디팜 | 서방성 지질 전구 제제 및 이를 포함하는 지질 용액 형태의 서방성 주사용 약학 조성물 |
| WO2022215899A1 (ko) | 2021-04-08 | 2022-10-13 | 주식회사 티온랩테라퓨틱스 | 서방성 지질 전구 제제 |
Families Citing this family (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101383324B1 (ko) | 2011-11-10 | 2014-04-28 | 주식회사 종근당 | 신규한 유전자 전달용 조성물 |
| EP3936133A1 (en) | 2011-11-23 | 2022-01-12 | TherapeuticsMD, Inc. | Natural combination hormone replacement formulations and therapies |
| US9301920B2 (en) | 2012-06-18 | 2016-04-05 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US20150196640A1 (en) | 2012-06-18 | 2015-07-16 | Therapeuticsmd, Inc. | Progesterone formulations having a desirable pk profile |
| US10806697B2 (en) | 2012-12-21 | 2020-10-20 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US20130338122A1 (en) | 2012-06-18 | 2013-12-19 | Therapeuticsmd, Inc. | Transdermal hormone replacement therapies |
| US10806740B2 (en) | 2012-06-18 | 2020-10-20 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US10537581B2 (en) | 2012-12-21 | 2020-01-21 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10471072B2 (en) | 2012-12-21 | 2019-11-12 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US9180091B2 (en) | 2012-12-21 | 2015-11-10 | Therapeuticsmd, Inc. | Soluble estradiol capsule for vaginal insertion |
| US10568891B2 (en) | 2012-12-21 | 2020-02-25 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11246875B2 (en) | 2012-12-21 | 2022-02-15 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11266661B2 (en) | 2012-12-21 | 2022-03-08 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| KR101586789B1 (ko) * | 2012-12-28 | 2016-01-19 | 주식회사 종근당 | 양이온성 약리학적 활성물질의 서방성 지질 초기제제 및 이를 포함하는 약제학적 조성물 |
| KR101586791B1 (ko) | 2012-12-28 | 2016-01-19 | 주식회사 종근당 | GnRH 유도체의 서방성 지질 초기제제 및 이를 포함하는 약제학적 조성물 |
| KR101586790B1 (ko) * | 2012-12-28 | 2016-01-19 | 주식회사 종근당 | 음이온성 약리학적 활성물질의 서방성 지질 초기제제 및 이를 포함하는 약제학적 조성물 |
| HUE033790T2 (en) | 2013-01-31 | 2017-12-28 | Chong Kun Dang Pharmaceutical Corp | Biaryl- or heterocyclic biaryl-substituted cyclohexene derivative compounds as cetp inhibitors |
| KR101601035B1 (ko) * | 2013-02-28 | 2016-03-08 | 주식회사 종근당 | 키토산 및 액상결정 형성 물질을 포함하는 유전자 전달용 조성물 |
| AR100562A1 (es) | 2014-05-22 | 2016-10-12 | Therapeuticsmd Inc | Composición farmacéutica de estradiol y progesterona para terapia de reemplazo hormonal |
| AU2015296609A1 (en) | 2014-07-29 | 2016-12-22 | Therapeuticsmd, Inc. | Transdermal cream |
| US20180000943A1 (en) * | 2014-12-23 | 2018-01-04 | Camurus Ab | Controlled-release formulations |
| US10328087B2 (en) | 2015-07-23 | 2019-06-25 | Therapeuticsmd, Inc. | Formulations for solubilizing hormones |
| WO2017173044A1 (en) | 2016-04-01 | 2017-10-05 | Therapeuticsmd Inc. | Steroid hormone compositions in medium chain oils |
| AU2017239645A1 (en) | 2016-04-01 | 2018-10-18 | Therapeuticsmd, Inc. | Steroid hormone pharmaceutical composition |
| US20210307365A1 (en) * | 2016-11-08 | 2021-10-07 | North Carolina State University | Encapsulation of nutritional and/or compounds for controlled release and enhancing their bioavailability by limiting chemical or microbial exposure |
| CA3082708A1 (en) * | 2017-11-15 | 2019-05-23 | Rhythm Pharmaceuticals, Inc. | Sustained release peptide formulations |
| AU2021299156C1 (en) * | 2020-06-30 | 2025-02-06 | Chong Kun Dang Pharmaceutical Corp. | Injectable composition comprising GnRH analogue |
| EP4378452A4 (en) | 2021-07-27 | 2025-07-23 | Farnex Incorporated | NON-LAMELLAR LIQUID CRYSTAL FORMING COMPOSITION AND ITS USE |
| KR20230065921A (ko) * | 2021-11-05 | 2023-05-12 | (주)아이엠디팜 | 신규한 서방성 지질 전구 제제 및 이를 포함하는 서방성 주사용 약학 조성물 |
| EP4553129A1 (en) * | 2023-11-09 | 2025-05-14 | Oleon N.V. | Emulsifier combination to enhance stability of invert emulsion drilling muds |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5858398A (en) * | 1994-11-03 | 1999-01-12 | Isomed Inc. | Microparticular pharmaceutical compositions |
Family Cites Families (50)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0224987B1 (en) | 1985-11-29 | 1992-04-15 | Biomatrix, Inc. | Drug delivery systems based on hyaluronan, derivatives thereof and their salts and method of producing same |
| DE3706036A1 (de) | 1987-02-25 | 1988-09-08 | Basf Ag | Polyacetale, verfahren zu deren herstellung aus dialdehyden und polyolcarbonsaeuren und verwendung der polyacetale |
| MY107937A (en) | 1990-02-13 | 1996-06-29 | Takeda Chemical Industries Ltd | Prolonged release microcapsules. |
| GB9302492D0 (en) | 1993-02-09 | 1993-03-24 | Procter & Gamble | Cosmetic compositions |
| SE518578C2 (sv) | 1994-06-15 | 2002-10-29 | Gs Dev Ab | Lipidbaserad komposition |
| EP0711556A1 (de) | 1994-11-09 | 1996-05-15 | Ciba-Geigy Ag | Intravenöse Lösungen für ein Staurosporinderivat |
| WO1996032930A1 (en) | 1995-04-18 | 1996-10-24 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Liposome drug-loading method and composition |
| US5736152A (en) | 1995-10-27 | 1998-04-07 | Atrix Laboratories, Inc. | Non-polymeric sustained release delivery system |
| WO1998047487A1 (en) | 1997-04-17 | 1998-10-29 | Dumex-Alpharma A/S | A novel bioadhesive drug delivery system based on liquid crystals |
| CN1138591C (zh) | 1997-09-09 | 2004-02-18 | 莱奥特罗皮克治疗公司 | 包覆颗粒以及其制备和使用方法 |
| WO1999033490A1 (fr) | 1997-12-26 | 1999-07-08 | Yamanouchi Pharmaceutical Co., Ltd. | Compositions medicinales a liberation prolongee |
| TWI241915B (en) | 1998-05-11 | 2005-10-21 | Ciba Sc Holding Ag | A method of preparing a pharmaceutical end formulation using a nanodispersion |
| US6565874B1 (en) | 1998-10-28 | 2003-05-20 | Atrix Laboratories | Polymeric delivery formulations of leuprolide with improved efficacy |
| IE990935A1 (en) | 1999-11-08 | 2002-04-03 | Procter & Gamble | Cosmetic Compositions |
| AU781682B2 (en) * | 2000-03-20 | 2005-06-09 | Zoetis Services Llc | Sustained-release compositions for parenteral administration |
| US20030070679A1 (en) | 2001-06-01 | 2003-04-17 | Boehringer Ingelheim Pharma Kg | Capsules containing inhalable tiotropium |
| EP1429726A2 (en) | 2001-09-06 | 2004-06-23 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | A method for preparing liposome formulations with a predefined release profile |
| JP2003252748A (ja) * | 2002-03-05 | 2003-09-10 | Kanebo Ltd | 美白用化粧料 |
| US7731947B2 (en) * | 2003-11-17 | 2010-06-08 | Intarcia Therapeutics, Inc. | Composition and dosage form comprising an interferon particle formulation and suspending vehicle |
| AU2005324794C1 (en) | 2003-11-07 | 2010-12-02 | Camurus Ab | Somatostatin analogue formulations |
| DK1682091T3 (en) | 2003-11-07 | 2017-05-15 | Camurus Ab | COMPOSITIONS OF LIPIDS AND CATIONIC PEPTIDES |
| US20050118206A1 (en) * | 2003-11-14 | 2005-06-02 | Luk Andrew S. | Surfactant-based gel as an injectable, sustained drug delivery vehicle |
| US20050106214A1 (en) | 2003-11-14 | 2005-05-19 | Guohua Chen | Excipients in drug delivery vehicles |
| TW200529890A (en) | 2004-02-10 | 2005-09-16 | Takeda Pharmaceutical | Sustained-release preparations |
| WO2005110360A2 (en) | 2004-05-18 | 2005-11-24 | Phares Pharmaceutical Research N.V. | Compositions for injection |
| EP1768650B1 (en) | 2004-06-04 | 2008-07-16 | Camurus Ab | Liquid depot formulations |
| EP1848403B8 (en) | 2005-01-14 | 2010-05-19 | Camurus Ab | Topical bioadhesive formulations |
| CA2595385C (en) * | 2005-01-21 | 2011-01-25 | Camurus Ab | Pharmaceutical lipid compositions |
| GB0501364D0 (en) * | 2005-01-21 | 2005-03-02 | Camurus Ab | Compositions |
| US20060182790A1 (en) * | 2005-02-17 | 2006-08-17 | Flor Mayoral | Dermal medicaments application enhancer |
| FR2903602B1 (fr) * | 2006-07-12 | 2012-10-26 | Seppic Sa | Formulation injectable a liberation prolongee de principes actifs, procede pour sa preparation |
| US20080102128A1 (en) | 2006-07-28 | 2008-05-01 | Flamel Technologies, Inc. | Modified-release microparticles based on amphiphilic copolymer and on active principles(s) and pharmaceutical formulations comprising them |
| CA2685534A1 (en) * | 2007-04-30 | 2008-11-06 | Living Proof, Inc. | Use of matrix metalloproteinase inhibitors in skin care |
| US20100129456A1 (en) | 2007-05-14 | 2010-05-27 | Ltt Bio-Pharma Co., Ltd. | Sustained-release nanoparticle containing low-molecular-weight drug with negatively charged group |
| GB0711656D0 (en) | 2007-06-15 | 2007-07-25 | Camurus Ab | Formulations |
| GB0716385D0 (en) | 2007-08-22 | 2007-10-03 | Camurus Ab | Formulations |
| CA2714514C (en) | 2008-02-08 | 2016-08-16 | Qps Llc | Non-polymeric compositions for controlled drug delivery |
| WO2009114959A1 (zh) | 2008-03-20 | 2009-09-24 | 中国人民解放军军事医学科学院毒物药物研究所 | 可注射用缓释药物制剂及其制备方法 |
| AU2010227549B2 (en) | 2009-03-25 | 2014-02-27 | Novartis Ag | Pharmaceutical composition containing a drug and siRNA |
| CN101904814A (zh) | 2009-06-04 | 2010-12-08 | 上海恒瑞医药有限公司 | 制备载药乳剂的方法 |
| KR20110056042A (ko) | 2009-11-20 | 2011-05-26 | 주식회사유한양행 | 종양세포 표적지향을 위한 나노 입자 및 이의 제조방법 |
| KR101319420B1 (ko) | 2011-03-18 | 2013-10-17 | 한남대학교 산학협력단 | 서방형 방출이 가능한 수용성 양이온 약물 전달 시스템 |
| JP6054291B2 (ja) | 2011-05-31 | 2016-12-27 | 株式会社ナノエッグ | 親油性化合物を高濃度で含有する液晶組成物の製造方法及びその方法によって製造された液晶組成物 |
| KR101383324B1 (ko) | 2011-11-10 | 2014-04-28 | 주식회사 종근당 | 신규한 유전자 전달용 조성물 |
| KR101586790B1 (ko) | 2012-12-28 | 2016-01-19 | 주식회사 종근당 | 음이온성 약리학적 활성물질의 서방성 지질 초기제제 및 이를 포함하는 약제학적 조성물 |
| KR101586791B1 (ko) | 2012-12-28 | 2016-01-19 | 주식회사 종근당 | GnRH 유도체의 서방성 지질 초기제제 및 이를 포함하는 약제학적 조성물 |
| KR101586789B1 (ko) | 2012-12-28 | 2016-01-19 | 주식회사 종근당 | 양이온성 약리학적 활성물질의 서방성 지질 초기제제 및 이를 포함하는 약제학적 조성물 |
| HUE033790T2 (en) | 2013-01-31 | 2017-12-28 | Chong Kun Dang Pharmaceutical Corp | Biaryl- or heterocyclic biaryl-substituted cyclohexene derivative compounds as cetp inhibitors |
| KR101601035B1 (ko) | 2013-02-28 | 2016-03-08 | 주식회사 종근당 | 키토산 및 액상결정 형성 물질을 포함하는 유전자 전달용 조성물 |
| JP6117430B2 (ja) | 2013-04-29 | 2017-04-19 | チョン クン ダン ファーマシューティカル コーポレーション | 選択的ヒストン脱アセチル化酵素抑制剤としての新規化合物およびこれを含む薬剤学的組成物 |
-
2012
- 2012-08-27 KR KR20120093677A patent/KR101494594B1/ko active Active
- 2012-08-28 MX MX2014002131A patent/MX351430B/es active IP Right Grant
- 2012-08-28 SI SI201231230T patent/SI2750667T1/en unknown
- 2012-08-28 ES ES12826818.2T patent/ES2661487T3/es active Active
- 2012-08-28 NO NO12826818A patent/NO2750667T3/no unknown
- 2012-08-28 HU HUE12826818A patent/HUE036150T2/hu unknown
- 2012-08-28 CN CN201280041992.1A patent/CN103764127B/zh active Active
- 2012-08-28 PT PT128268182T patent/PT2750667T/pt unknown
- 2012-08-28 PL PL12826818T patent/PL2750667T3/pl unknown
- 2012-08-28 PH PH1/2014/500449A patent/PH12014500449B1/en unknown
- 2012-08-28 DK DK12826818.2T patent/DK2750667T3/en active
- 2012-08-28 US US14/241,696 patent/US9526787B2/en active Active
- 2012-08-28 LT LTEP12826818.2T patent/LT2750667T/lt unknown
- 2012-08-28 RU RU2014112189A patent/RU2632433C2/ru active
- 2012-08-28 AU AU2012302422A patent/AU2012302422B2/en active Active
- 2012-08-28 CA CA2845784A patent/CA2845784C/en active Active
- 2012-08-28 EP EP12826818.2A patent/EP2750667B1/en active Active
- 2012-08-28 TR TR2018/02984T patent/TR201802984T4/tr unknown
- 2012-08-28 WO PCT/KR2012/006855 patent/WO2013032207A1/en active Application Filing
- 2012-08-28 JP JP2014528270A patent/JP5981997B2/ja active Active
- 2012-08-28 BR BR112014004967-0A patent/BR112014004967B1/pt active IP Right Grant
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5858398A (en) * | 1994-11-03 | 1999-01-12 | Isomed Inc. | Microparticular pharmaceutical compositions |
Non-Patent Citations (2)
| Title |
|---|
| Guo Rong 외 2명. Complex Lamellar Structure of Polyoxyethylene 20 Sorbitan Oleate and a Fatty Acid/Lecithin Lamellar Liquid Crystal. Langmuir. 12, pp.4286~4291. (1996) * |
| Guo Rong 외 2명. Complex Lamellar Structure of Polyoxyethylene 20 Sorbitan Oleate and a Fatty Acid/Lecithin Lamellar Liquid Crystal. Langmuir. 12, pp.4286~4291. (1996)* |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017069533A1 (en) * | 2015-10-20 | 2017-04-27 | Mirae Pharm Co.,Ltd. | Oral liquid formulation of tadalafil |
| WO2020171491A1 (ko) | 2019-02-18 | 2020-08-27 | (주)아이엠디팜 | 서방성 지질 전구 제제 및 이를 포함하는 지질 용액 형태의 서방성 주사용 약학 조성물 |
| WO2022215899A1 (ko) | 2021-04-08 | 2022-10-13 | 주식회사 티온랩테라퓨틱스 | 서방성 지질 전구 제제 |
| KR20220139791A (ko) | 2021-04-08 | 2022-10-17 | 주식회사 티온랩테라퓨틱스 | 서방성 지질 전구 제제 |
Also Published As
| Publication number | Publication date |
|---|---|
| DK2750667T3 (en) | 2018-03-05 |
| NZ622165A (en) | 2015-10-30 |
| JP2014527545A (ja) | 2014-10-16 |
| EP2750667B1 (en) | 2017-12-06 |
| US20140206616A1 (en) | 2014-07-24 |
| RU2632433C2 (ru) | 2017-10-04 |
| PH12014500449B1 (en) | 2018-09-21 |
| ES2661487T3 (es) | 2018-04-02 |
| US9526787B2 (en) | 2016-12-27 |
| PT2750667T (pt) | 2018-01-19 |
| CA2845784A1 (en) | 2013-03-07 |
| BR112014004967B1 (pt) | 2022-03-15 |
| CN103764127B (zh) | 2017-08-22 |
| CA2845784C (en) | 2016-11-22 |
| LT2750667T (lt) | 2018-03-26 |
| JP5981997B2 (ja) | 2016-08-31 |
| PH12014500449A1 (en) | 2014-04-14 |
| CN103764127A (zh) | 2014-04-30 |
| KR20130024804A (ko) | 2013-03-08 |
| HUE036150T2 (hu) | 2018-06-28 |
| SI2750667T1 (en) | 2018-04-30 |
| EP2750667A4 (en) | 2015-06-03 |
| EP2750667A1 (en) | 2014-07-09 |
| BR112014004967A2 (pt) | 2017-03-21 |
| RU2014112189A (ru) | 2015-10-10 |
| MX2014002131A (es) | 2014-09-15 |
| AU2012302422B2 (en) | 2015-10-08 |
| WO2013032207A1 (en) | 2013-03-07 |
| TR201802984T4 (tr) | 2018-03-21 |
| PL2750667T3 (pl) | 2018-05-30 |
| NO2750667T3 (ko) | 2018-05-05 |
| AU2012302422A1 (en) | 2014-03-27 |
| MX351430B (es) | 2017-10-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101494594B1 (ko) | 약리학적 활성물질의 서방성 지질 초기제제 및 이를 포함하는 약제학적 조성물 | |
| KR101586789B1 (ko) | 양이온성 약리학적 활성물질의 서방성 지질 초기제제 및 이를 포함하는 약제학적 조성물 | |
| JP6078660B2 (ja) | GnRH誘導体の徐放性脂質初期製剤およびこれを含む薬剤学的組成物 | |
| AU2013371098B2 (en) | Sustained-release lipid pre-concentrate of anionic pharmacologically active substances and pharmaceutical composition comprising the same | |
| JP2019510048A (ja) | 週1回又は隔週1回の投与に適したリラグルチドの粘弾性ゲル | |
| AU2021299156B2 (en) | Injectable composition comprising GnRH analogue | |
| NZ622165B2 (en) | Sustained-release lipid pre-concentrate of pharmacologically active substance and pharmaceutical composition comprising the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| PA0109 | Patent application |
Patent event code: PA01091R01D Comment text: Patent Application Patent event date: 20120827 |
|
| PA0201 | Request for examination | ||
| PG1501 | Laying open of application | ||
| N231 | Notification of change of applicant | ||
| PN2301 | Change of applicant |
Patent event date: 20140128 Comment text: Notification of Change of Applicant Patent event code: PN23011R01D |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20140328 Patent event code: PE09021S01D |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
Patent event code: PE07011S01D Comment text: Decision to Grant Registration Patent event date: 20141210 |
|
| GRNT | Written decision to grant | ||
| PR0701 | Registration of establishment |
Comment text: Registration of Establishment Patent event date: 20150212 Patent event code: PR07011E01D |
|
| PR1002 | Payment of registration fee |
Payment date: 20150212 End annual number: 3 Start annual number: 1 |
|
| PG1601 | Publication of registration | ||
| FPAY | Annual fee payment |
Payment date: 20171025 Year of fee payment: 4 |
|
| PR1001 | Payment of annual fee |
Payment date: 20171025 Start annual number: 4 End annual number: 4 |
|
| FPAY | Annual fee payment |
Payment date: 20181206 Year of fee payment: 5 |
|
| PR1001 | Payment of annual fee |
Payment date: 20181206 Start annual number: 5 End annual number: 5 |
|
| FPAY | Annual fee payment |
Payment date: 20191223 Year of fee payment: 6 |
|
| PR1001 | Payment of annual fee |
Payment date: 20191223 Start annual number: 6 End annual number: 6 |
|
| PR1001 | Payment of annual fee |
Payment date: 20201228 Start annual number: 7 End annual number: 7 |
|
| PR1001 | Payment of annual fee |
Payment date: 20211227 Start annual number: 8 End annual number: 8 |
|
| PR1001 | Payment of annual fee |
Payment date: 20221214 Start annual number: 9 End annual number: 9 |
|
| PR1001 | Payment of annual fee |
Payment date: 20231220 Start annual number: 10 End annual number: 10 |
|
| PR1001 | Payment of annual fee |
Payment date: 20241219 Start annual number: 11 End annual number: 11 |