JP5479912B2 - 複素環式化合物の非晶質体、それを含む固体分散体、薬剤、およびその製造法 - Google Patents
複素環式化合物の非晶質体、それを含む固体分散体、薬剤、およびその製造法 Download PDFInfo
- Publication number
- JP5479912B2 JP5479912B2 JP2009542608A JP2009542608A JP5479912B2 JP 5479912 B2 JP5479912 B2 JP 5479912B2 JP 2009542608 A JP2009542608 A JP 2009542608A JP 2009542608 A JP2009542608 A JP 2009542608A JP 5479912 B2 JP5479912 B2 JP 5479912B2
- Authority
- JP
- Japan
- Prior art keywords
- group
- alkyl
- solid dispersion
- optionally substituted
- amorphous
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 150000002391 heterocyclic compounds Chemical class 0.000 title claims description 51
- 239000007962 solid dispersion Substances 0.000 title claims description 48
- 238000004519 manufacturing process Methods 0.000 title claims description 22
- 239000003814 drug Substances 0.000 title description 23
- 229940079593 drug Drugs 0.000 title description 22
- 238000000034 method Methods 0.000 claims description 59
- -1 benzylcarbonyl Chemical group 0.000 claims description 58
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 39
- 239000012453 solvate Substances 0.000 claims description 30
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 26
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims description 25
- 150000003839 salts Chemical class 0.000 claims description 25
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 claims description 24
- 238000002360 preparation method Methods 0.000 claims description 24
- 238000001694 spray drying Methods 0.000 claims description 23
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 claims description 22
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 claims description 22
- 238000010298 pulverizing process Methods 0.000 claims description 21
- 229910052757 nitrogen Inorganic materials 0.000 claims description 19
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 claims description 18
- 125000002485 formyl group Chemical group [H]C(*)=O 0.000 claims description 18
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 claims description 18
- 239000001863 hydroxypropyl cellulose Substances 0.000 claims description 18
- 125000004430 oxygen atom Chemical group O* 0.000 claims description 18
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 claims description 14
- 239000008187 granular material Substances 0.000 claims description 14
- 239000002904 solvent Substances 0.000 claims description 14
- 125000004454 (C1-C6) alkoxycarbonyl group Chemical group 0.000 claims description 13
- 125000004890 (C1-C6) alkylamino group Chemical group 0.000 claims description 13
- 125000003277 amino group Chemical group 0.000 claims description 13
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 13
- 125000001424 substituent group Chemical group 0.000 claims description 13
- 239000003826 tablet Substances 0.000 claims description 13
- 239000007788 liquid Substances 0.000 claims description 12
- 125000004043 oxo group Chemical group O=* 0.000 claims description 12
- 125000001028 difluoromethyl group Chemical group [H]C(F)(F)* 0.000 claims description 11
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 claims description 11
- 125000004573 morpholin-4-yl group Chemical group N1(CCOCC1)* 0.000 claims description 11
- 229960003943 hypromellose Drugs 0.000 claims description 10
- 238000002156 mixing Methods 0.000 claims description 10
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 9
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 claims description 8
- 239000000843 powder Substances 0.000 claims description 8
- 239000007884 disintegrant Substances 0.000 claims description 7
- 239000000314 lubricant Substances 0.000 claims description 7
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 7
- 125000004191 (C1-C6) alkoxy group Chemical group 0.000 claims description 6
- 239000002775 capsule Substances 0.000 claims description 6
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 6
- 239000012527 feed solution Substances 0.000 claims description 6
- 229910052736 halogen Inorganic materials 0.000 claims description 6
- 150000002367 halogens Chemical class 0.000 claims description 6
- 238000002844 melting Methods 0.000 claims description 6
- 230000008018 melting Effects 0.000 claims description 6
- 125000004193 piperazinyl group Chemical group 0.000 claims description 6
- 125000000587 piperidin-1-yl group Chemical group [H]C1([H])N(*)C([H])([H])C([H])([H])C([H])([H])C1([H])[H] 0.000 claims description 6
- 125000000719 pyrrolidinyl group Chemical group 0.000 claims description 6
- 238000005507 spraying Methods 0.000 claims description 6
- 238000001035 drying Methods 0.000 claims description 4
- 230000008569 process Effects 0.000 claims description 2
- 150000001875 compounds Chemical class 0.000 description 83
- 238000012360 testing method Methods 0.000 description 68
- 239000007787 solid Substances 0.000 description 29
- 238000002441 X-ray diffraction Methods 0.000 description 16
- 229920000642 polymer Polymers 0.000 description 16
- 239000000203 mixture Substances 0.000 description 15
- 239000008280 blood Substances 0.000 description 13
- 210000004369 blood Anatomy 0.000 description 13
- 239000000126 substance Substances 0.000 description 13
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 12
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 12
- 239000013078 crystal Substances 0.000 description 11
- 238000009472 formulation Methods 0.000 description 11
- 238000000634 powder X-ray diffraction Methods 0.000 description 11
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 10
- 229920002678 cellulose Polymers 0.000 description 8
- 239000001913 cellulose Substances 0.000 description 8
- 230000006872 improvement Effects 0.000 description 8
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 7
- 229920002472 Starch Polymers 0.000 description 7
- 238000010521 absorption reaction Methods 0.000 description 7
- 238000005280 amorphization Methods 0.000 description 7
- 238000010438 heat treatment Methods 0.000 description 7
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 6
- 241000700159 Rattus Species 0.000 description 6
- 238000000227 grinding Methods 0.000 description 6
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 6
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 6
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 6
- 238000001228 spectrum Methods 0.000 description 6
- 238000003860 storage Methods 0.000 description 6
- JIHQDMXYYFUGFV-UHFFFAOYSA-N 1,3,5-triazine Chemical class C1=NC=NC=N1 JIHQDMXYYFUGFV-UHFFFAOYSA-N 0.000 description 5
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 5
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 5
- 125000004432 carbon atom Chemical group C* 0.000 description 5
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 5
- 238000007796 conventional method Methods 0.000 description 5
- 238000000113 differential scanning calorimetry Methods 0.000 description 5
- 239000002552 dosage form Substances 0.000 description 5
- 239000003960 organic solvent Substances 0.000 description 5
- 229940083082 pyrimidine derivative acting on arteriolar smooth muscle Drugs 0.000 description 5
- 150000003230 pyrimidines Chemical class 0.000 description 5
- 239000007921 spray Substances 0.000 description 5
- 235000019698 starch Nutrition 0.000 description 5
- VJHCJDRQFCCTHL-UHFFFAOYSA-N acetic acid 2,3,4,5,6-pentahydroxyhexanal Chemical compound CC(O)=O.OCC(O)C(O)C(O)C(O)C=O VJHCJDRQFCCTHL-UHFFFAOYSA-N 0.000 description 4
- 229910052799 carbon Inorganic materials 0.000 description 4
- 229950008138 carmellose Drugs 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- 201000010099 disease Diseases 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- 239000011521 glass Substances 0.000 description 4
- 229940031703 low substituted hydroxypropyl cellulose Drugs 0.000 description 4
- 239000011734 sodium Substances 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 229920003169 water-soluble polymer Polymers 0.000 description 4
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- 208000023275 Autoimmune disease Diseases 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 3
- 229920002785 Croscarmellose sodium Polymers 0.000 description 3
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 3
- 206010025323 Lymphomas Diseases 0.000 description 3
- 206010028980 Neoplasm Diseases 0.000 description 3
- 239000011575 calcium Substances 0.000 description 3
- 229910052791 calcium Inorganic materials 0.000 description 3
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 3
- 235000013539 calcium stearate Nutrition 0.000 description 3
- 239000008116 calcium stearate Substances 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 229960001681 croscarmellose sodium Drugs 0.000 description 3
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 3
- 238000002425 crystallisation Methods 0.000 description 3
- 230000008025 crystallization Effects 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 208000024200 hematopoietic and lymphoid system neoplasm Diseases 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 235000019359 magnesium stearate Nutrition 0.000 description 3
- 235000010355 mannitol Nutrition 0.000 description 3
- 239000008177 pharmaceutical agent Substances 0.000 description 3
- 238000004321 preservation Methods 0.000 description 3
- 229910052708 sodium Inorganic materials 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- 244000215068 Acacia senegal Species 0.000 description 2
- 229920001817 Agar Polymers 0.000 description 2
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 229920002261 Corn starch Polymers 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- 239000004375 Dextrin Substances 0.000 description 2
- 229920001353 Dextrin Polymers 0.000 description 2
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- 239000001856 Ethyl cellulose Substances 0.000 description 2
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- 229920000084 Gum arabic Polymers 0.000 description 2
- 229920003116 HPC-SSL Polymers 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 238000005481 NMR spectroscopy Methods 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 239000004372 Polyvinyl alcohol Substances 0.000 description 2
- 206010040047 Sepsis Diseases 0.000 description 2
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- HGVNLRPZOWWDKD-UHFFFAOYSA-N ZSTK-474 Chemical compound FC(F)C1=NC2=CC=CC=C2N1C(N=1)=NC(N2CCOCC2)=NC=1N1CCOCC1 HGVNLRPZOWWDKD-UHFFFAOYSA-N 0.000 description 2
- 235000010489 acacia gum Nutrition 0.000 description 2
- 239000000205 acacia gum Substances 0.000 description 2
- 125000000738 acetamido group Chemical group [H]C([H])([H])C(=O)N([H])[*] 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 239000008186 active pharmaceutical agent Substances 0.000 description 2
- 239000008272 agar Substances 0.000 description 2
- 150000001298 alcohols Chemical class 0.000 description 2
- 125000000217 alkyl group Chemical group 0.000 description 2
- 239000012062 aqueous buffer Substances 0.000 description 2
- 125000003118 aryl group Chemical group 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- FUFJGUQYACFECW-UHFFFAOYSA-L calcium hydrogenphosphate Chemical compound [Ca+2].OP([O-])([O-])=O FUFJGUQYACFECW-UHFFFAOYSA-L 0.000 description 2
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 238000013329 compounding Methods 0.000 description 2
- 239000008120 corn starch Substances 0.000 description 2
- 229940099112 cornstarch Drugs 0.000 description 2
- 239000002178 crystalline material Substances 0.000 description 2
- 235000019425 dextrin Nutrition 0.000 description 2
- 235000019700 dicalcium phosphate Nutrition 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- 229940088679 drug related substance Drugs 0.000 description 2
- 235000019325 ethyl cellulose Nutrition 0.000 description 2
- 229920001249 ethyl cellulose Polymers 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 150000008282 halocarbons Chemical class 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 230000002757 inflammatory effect Effects 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 235000010981 methylcellulose Nutrition 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 239000003595 mist Substances 0.000 description 2
- 239000012046 mixed solvent Substances 0.000 description 2
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 201000008968 osteosarcoma Diseases 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 229920002451 polyvinyl alcohol Polymers 0.000 description 2
- 229920001592 potato starch Polymers 0.000 description 2
- 229940116317 potato starch Drugs 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 229940100486 rice starch Drugs 0.000 description 2
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 2
- 239000008247 solid mixture Substances 0.000 description 2
- 229960002920 sorbitol Drugs 0.000 description 2
- 230000006641 stabilisation Effects 0.000 description 2
- 238000011105 stabilization Methods 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- VZGDMQKNWNREIO-UHFFFAOYSA-N tetrachloromethane Chemical compound ClC(Cl)(Cl)Cl VZGDMQKNWNREIO-UHFFFAOYSA-N 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 229940100445 wheat starch Drugs 0.000 description 2
- CDJCXRGNYXKUIN-LIOBNPLQSA-N (2S,3R)-4-[2-[2-(difluoromethyl)benzimidazol-1-yl]-6-morpholin-4-ylpyrimidin-4-yl]-2,3-dimethylmorpholine 4-[2-[2-(difluoromethyl)benzimidazol-1-yl]-6-morpholin-4-ylpyrimidin-4-yl]morpholine Chemical compound FC(C1=NC2=C(N1C1=NC(=CC(=N1)N1CCOCC1)N1CCOCC1)C=CC=C2)F.FC(C2=NC1=C(N2C2=NC(=CC(=N2)N2[C@@H]([C@@H](OCC2)C)C)N2CCOCC2)C=CC=C1)F CDJCXRGNYXKUIN-LIOBNPLQSA-N 0.000 description 1
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 1
- SCYULBFZEHDVBN-UHFFFAOYSA-N 1,1-Dichloroethane Chemical compound CC(Cl)Cl SCYULBFZEHDVBN-UHFFFAOYSA-N 0.000 description 1
- IJGXOPYYSVJWJP-UHFFFAOYSA-N 1-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)benzimidazole-2,4-diamine Chemical compound NC1=NC2=C(N)C=CC=C2N1C(N=1)=NC(N2CCOCC2)=NC=1N1CCOCC1 IJGXOPYYSVJWJP-UHFFFAOYSA-N 0.000 description 1
- PKDPUENCROCRCH-UHFFFAOYSA-N 1-piperazin-1-ylethanone Chemical compound CC(=O)N1CCNCC1 PKDPUENCROCRCH-UHFFFAOYSA-N 0.000 description 1
- IAJLTMBBAVVMQO-UHFFFAOYSA-N 1h-benzimidazol-2-ylmethanol Chemical compound C1=CC=C2NC(CO)=NC2=C1 IAJLTMBBAVVMQO-UHFFFAOYSA-N 0.000 description 1
- LRYSHDDWCVYSPV-UHFFFAOYSA-N 2-(difluoromethyl)-1-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)benzimidazol-4-amine Chemical compound FC(F)C1=NC=2C(N)=CC=CC=2N1C(N=1)=NC(N2CCOCC2)=NC=1N1CCOCC1 LRYSHDDWCVYSPV-UHFFFAOYSA-N 0.000 description 1
- WEZPCORGIAYVCL-UHFFFAOYSA-N 2-(difluoromethyl)-1-[4-(2,2-dimethyl-1,3-oxazolidin-3-yl)-6-morpholin-4-yl-1,3,5-triazin-2-yl]benzimidazol-5-ol 2-(difluoromethyl)-1-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)benzimidazol-4-ol Chemical compound FC(C1=NC2=C(N1C1=NC(=NC(=N1)N1CCOCC1)N1CCOCC1)C=CC=C2O)F.FC(C2=NC1=C(N2C2=NC(=NC(=N2)N2C(OCC2)(C)C)N2CCOCC2)C=CC(=C1)O)F WEZPCORGIAYVCL-UHFFFAOYSA-N 0.000 description 1
- ZIAVNTRJSBSAQL-UHFFFAOYSA-N 2-(difluoromethyl)-1-[4-(2,2-dimethylmorpholin-4-yl)-6-morpholin-4-ylpyrimidin-2-yl]benzimidazol-4-ol 1-[4-(2,2-dimethylmorpholin-4-yl)-6-morpholin-4-ylpyrimidin-2-yl]benzimidazole-2,4-diamine Chemical compound NC1=NC2=C(N1C1=NC(=CC(=N1)N1CC(OCC1)(C)C)N1CCOCC1)C=CC=C2N.FC(C2=NC1=C(N2C2=NC(=CC(=N2)N2CC(OCC2)(C)C)N2CCOCC2)C=CC=C1O)F ZIAVNTRJSBSAQL-UHFFFAOYSA-N 0.000 description 1
- OVOPODKPWBSNHV-OLZOCXBDSA-N 2-(difluoromethyl)-1-[4-[(2s,3r)-2,3-dimethylmorpholin-4-yl]-6-morpholin-4-yl-1,3,5-triazin-2-yl]benzimidazol-5-ol Chemical compound C[C@@H]1[C@H](C)OCCN1C1=NC(N2CCOCC2)=NC(N2C3=CC=C(O)C=C3N=C2C(F)F)=N1 OVOPODKPWBSNHV-OLZOCXBDSA-N 0.000 description 1
- LLHRNYKBWUIXFG-UHFFFAOYSA-N 2-(difluoromethyl)-3-(4,6-dimorpholin-4-ylpyrimidin-2-yl)benzimidazol-5-amine Chemical compound C12=CC(N)=CC=C2N=C(C(F)F)N1C(N=1)=NC(N2CCOCC2)=CC=1N1CCOCC1 LLHRNYKBWUIXFG-UHFFFAOYSA-N 0.000 description 1
- FNUYDLJAQXFNGR-UHFFFAOYSA-N 2-(difluoromethyl)-3-[4-(2,2-dimethylmorpholin-4-yl)-6-morpholin-4-yl-1,3,5-triazin-2-yl]benzimidazol-5-ol Chemical compound C1COC(C)(C)CN1C1=NC(N2CCOCC2)=NC(N2C3=CC(O)=CC=C3N=C2C(F)F)=N1 FNUYDLJAQXFNGR-UHFFFAOYSA-N 0.000 description 1
- ICVBBVHKXQZGRY-UHFFFAOYSA-N 2-amino-1-[4-(2,2-dimethylmorpholin-4-yl)-6-morpholin-4-yl-1,3,5-triazin-2-yl]benzimidazol-4-ol Chemical compound C1COC(C)(C)CN1C1=NC(N2CCOCC2)=NC(N2C3=CC=CC(O)=C3N=C2N)=N1 ICVBBVHKXQZGRY-UHFFFAOYSA-N 0.000 description 1
- YXGZPUKZUSJEJT-XFULWGLBSA-N 4-[2-[2-(difluoromethyl)benzimidazol-1-yl]-6-morpholin-4-ylpyrimidin-4-yl]-2-methylmorpholine (5R)-4-[2-[2-(difluoromethyl)benzimidazol-1-yl]-6-morpholin-4-ylpyrimidin-4-yl]-2,2,5-trimethylmorpholine Chemical compound FC(C1=NC2=C(N1C1=NC(=CC(=N1)N1CCOCC1)N1CC(OC[C@H]1C)(C)C)C=CC=C2)F.FC(C2=NC1=C(N2C2=NC(=CC(=N2)N2CC(OCC2)C)N2CCOCC2)C=CC=C1)F YXGZPUKZUSJEJT-XFULWGLBSA-N 0.000 description 1
- XDGJRXIJORDCQT-UHFFFAOYSA-N 4-[2-[2-(difluoromethyl)benzimidazol-1-yl]-6-thiomorpholin-4-ylpyrimidin-4-yl]morpholine Chemical compound FC(F)C1=NC2=CC=CC=C2N1C(N=1)=NC(N2CCOCC2)=CC=1N1CCSCC1 XDGJRXIJORDCQT-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 206010000830 Acute leukaemia Diseases 0.000 description 1
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- RPSYEHUAMDSSCN-FVMQXDBGSA-N C[C@@H]1OCCN([C@@H]1C)C1=NC(=NC(=C1)N1CCOCC1)N1C(=NC2=C1C=CC=C2)CF.FC(C2=NC1=C(N2C2=NC(=CC(=N2)N2CCOCC2)N2CC(OC[C@@H]2C)(C)C)C=CC=C1)F Chemical compound C[C@@H]1OCCN([C@@H]1C)C1=NC(=NC(=C1)N1CCOCC1)N1C(=NC2=C1C=CC=C2)CF.FC(C2=NC1=C(N2C2=NC(=CC(=N2)N2CCOCC2)N2CC(OC[C@@H]2C)(C)C)C=CC=C1)F RPSYEHUAMDSSCN-FVMQXDBGSA-N 0.000 description 1
- 201000009030 Carcinoma Diseases 0.000 description 1
- 229920000623 Cellulose acetate phthalate Polymers 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 206010009900 Colitis ulcerative Diseases 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 206010010744 Conjunctivitis allergic Diseases 0.000 description 1
- 241000711573 Coronaviridae Species 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- 206010012438 Dermatitis atopic Diseases 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 206010014733 Endometrial cancer Diseases 0.000 description 1
- 206010014759 Endometrial neoplasm Diseases 0.000 description 1
- RMNFKICSDODEAK-VJIXHXHOSA-N FC(C1=NC2=C(N1C1=NC(=CC(=N1)N1CC(OCC1)(C)C)N1CCOCC1)C=CC=C2)F.FC(C2=NC1=C(N2C2=NC(=CC(=N2)N2[C@H]([C@@H](OCC2)C)C)N2CCOCC2)C=CC=C1)F Chemical compound FC(C1=NC2=C(N1C1=NC(=CC(=N1)N1CC(OCC1)(C)C)N1CCOCC1)C=CC=C2)F.FC(C2=NC1=C(N2C2=NC(=CC(=N2)N2[C@H]([C@@H](OCC2)C)C)N2CCOCC2)C=CC=C1)F RMNFKICSDODEAK-VJIXHXHOSA-N 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 206010018364 Glomerulonephritis Diseases 0.000 description 1
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical class OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical class Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-L Malonate Chemical compound [O-]C(=O)CC([O-])=O OFOBLEOULBTSOW-UHFFFAOYSA-L 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 229920000057 Mannan Polymers 0.000 description 1
- 208000001940 Massive Hepatic Necrosis Diseases 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- 229920000881 Modified starch Polymers 0.000 description 1
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 description 1
- MOMWZCRZHFZFLA-UHFFFAOYSA-N NC1=NC2=C(N1C1=NC(=CC(=N1)N1CC(OCC1)(C)C)N1CCOCC1)C=CC=C2O.NC2=NC1=C(N2C2=NC(=CC(=N2)N2CCOCC2)N2CCOCC2)C=CC=C1N Chemical compound NC1=NC2=C(N1C1=NC(=CC(=N1)N1CC(OCC1)(C)C)N1CCOCC1)C=CC=C2O.NC2=NC1=C(N2C2=NC(=CC(=N2)N2CCOCC2)N2CCOCC2)C=CC=C1N MOMWZCRZHFZFLA-UHFFFAOYSA-N 0.000 description 1
- BZTNNRDCIHYDKM-IPQKMFTISA-N NC1=NC2=C(N1C1=NC(=CC(=N1)N1[C@H]([C@@H](OCC1)C)C)N1CCOCC1)C=CC=C2.NC2=NC1=C(N2C2=NC(=CC(=N2)N2[C@@H]([C@@H](OCC2)C)C)N2CCOCC2)C=CC=C1 Chemical compound NC1=NC2=C(N1C1=NC(=CC(=N1)N1[C@H]([C@@H](OCC1)C)C)N1CCOCC1)C=CC=C2.NC2=NC1=C(N2C2=NC(=CC(=N2)N2[C@@H]([C@@H](OCC2)C)C)N2CCOCC2)C=CC=C1 BZTNNRDCIHYDKM-IPQKMFTISA-N 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 206010033645 Pancreatitis Diseases 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 206010035226 Plasma cell myeloma Diseases 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-N Propionic acid Chemical class CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 239000004373 Pullulan Substances 0.000 description 1
- 229920001218 Pullulan Polymers 0.000 description 1
- LCTONWCANYUPML-UHFFFAOYSA-M Pyruvate Chemical compound CC(=O)C([O-])=O LCTONWCANYUPML-UHFFFAOYSA-M 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 206010039085 Rhinitis allergic Diseases 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- 201000003176 Severe Acute Respiratory Syndrome Diseases 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- 208000021386 Sjogren Syndrome Diseases 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- 206010041067 Small cell lung cancer Diseases 0.000 description 1
- 201000009594 Systemic Scleroderma Diseases 0.000 description 1
- 206010042953 Systemic sclerosis Diseases 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- 201000006704 Ulcerative Colitis Diseases 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- SHUVKSZVCVHWSX-CVEARBPZSA-N [1-[4-[(2s,3r)-2,3-dimethylmorpholin-4-yl]-6-morpholin-4-ylpyrimidin-2-yl]benzimidazol-2-yl]methanol Chemical compound C[C@@H]1[C@H](C)OCCN1C1=CC(N2CCOCC2)=NC(N2C3=CC=CC=C3N=C2CO)=N1 SHUVKSZVCVHWSX-CVEARBPZSA-N 0.000 description 1
- NOSVBMJRSAGRRH-GARXDOFDSA-N [1-[6-[(2s,3r)-2,3-dimethylmorpholin-4-yl]-2-[2-(hydroxymethyl)benzimidazol-1-yl]pyrimidin-4-yl]pyrrolidin-2-yl]methanol Chemical compound C[C@@H]1[C@H](C)OCCN1C1=CC(N2C(CCC2)CO)=NC(N2C3=CC=CC=C3N=C2CO)=N1 NOSVBMJRSAGRRH-GARXDOFDSA-N 0.000 description 1
- DHKHKXVYLBGOIT-UHFFFAOYSA-N acetaldehyde Diethyl Acetal Natural products CCOC(C)OCC DHKHKXVYLBGOIT-UHFFFAOYSA-N 0.000 description 1
- 150000001242 acetic acid derivatives Chemical class 0.000 description 1
- GAMPNQJDUFQVQO-UHFFFAOYSA-N acetic acid;phthalic acid Chemical compound CC(O)=O.OC(=O)C1=CC=CC=C1C(O)=O GAMPNQJDUFQVQO-UHFFFAOYSA-N 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 125000003545 alkoxy group Chemical group 0.000 description 1
- 208000002205 allergic conjunctivitis Diseases 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- 208000030961 allergic reaction Diseases 0.000 description 1
- 208000028004 allergic respiratory disease Diseases 0.000 description 1
- 201000010105 allergic rhinitis Diseases 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 208000006673 asthma Diseases 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 208000024998 atopic conjunctivitis Diseases 0.000 description 1
- 201000008937 atopic dermatitis Diseases 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 238000000498 ball milling Methods 0.000 description 1
- 235000013871 bee wax Nutrition 0.000 description 1
- 239000012166 beeswax Substances 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 229940050390 benzoate Drugs 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- BFAKENXZKHGIGE-UHFFFAOYSA-N bis(2,3,5,6-tetrafluoro-4-iodophenyl)diazene Chemical compound FC1=C(C(=C(C(=C1F)I)F)F)N=NC1=C(C(=C(C(=C1F)F)I)F)F BFAKENXZKHGIGE-UHFFFAOYSA-N 0.000 description 1
- 230000036765 blood level Effects 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 238000010322 bone marrow transplantation Methods 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 244000309464 bull Species 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 239000004203 carnauba wax Substances 0.000 description 1
- 235000013869 carnauba wax Nutrition 0.000 description 1
- 229940081734 cellulose acetate phthalate Drugs 0.000 description 1
- 235000013339 cereals Nutrition 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 208000024207 chronic leukemia Diseases 0.000 description 1
- 229940114081 cinnamate Drugs 0.000 description 1
- 229940001468 citrate Drugs 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 229960000913 crospovidone Drugs 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 238000001938 differential scanning calorimetry curve Methods 0.000 description 1
- 238000002050 diffraction method Methods 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- FSBVERYRVPGNGG-UHFFFAOYSA-N dimagnesium dioxido-bis[[oxido(oxo)silyl]oxy]silane hydrate Chemical compound O.[Mg+2].[Mg+2].[O-][Si](=O)O[Si]([O-])([O-])O[Si]([O-])=O FSBVERYRVPGNGG-UHFFFAOYSA-N 0.000 description 1
- QKIUAMUSENSFQQ-UHFFFAOYSA-N dimethylazanide Chemical compound C[N-]C QKIUAMUSENSFQQ-UHFFFAOYSA-N 0.000 description 1
- 229910001873 dinitrogen Inorganic materials 0.000 description 1
- BNIILDVGGAEEIG-UHFFFAOYSA-L disodium hydrogen phosphate Chemical compound [Na+].[Na+].OP([O-])([O-])=O BNIILDVGGAEEIG-UHFFFAOYSA-L 0.000 description 1
- 235000019800 disodium phosphate Nutrition 0.000 description 1
- 229910000397 disodium phosphate Inorganic materials 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 238000001647 drug administration Methods 0.000 description 1
- 239000013583 drug formulation Substances 0.000 description 1
- 238000007908 dry granulation Methods 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 239000002702 enteric coating Substances 0.000 description 1
- 238000009505 enteric coating Methods 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 239000003889 eye drop Substances 0.000 description 1
- 229940012356 eye drops Drugs 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 230000004761 fibrosis Effects 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000004503 fine granule Substances 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 229940050411 fumarate Drugs 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 1
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 1
- 150000004677 hydrates Chemical class 0.000 description 1
- 150000003840 hydrochlorides Chemical class 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 1
- 229920000639 hydroxypropylmethylcellulose acetate succinate Polymers 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 230000001506 immunosuppresive effect Effects 0.000 description 1
- 229940125721 immunosuppressive agent Drugs 0.000 description 1
- 239000003018 immunosuppressive agent Substances 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000031891 intestinal absorption Effects 0.000 description 1
- 238000007917 intracranial administration Methods 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 210000001503 joint Anatomy 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 239000000391 magnesium silicate Substances 0.000 description 1
- 229910052919 magnesium silicate Inorganic materials 0.000 description 1
- 235000019792 magnesium silicate Nutrition 0.000 description 1
- 229940049920 malate Drugs 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N malic acid Chemical compound OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 229920003145 methacrylic acid copolymer Polymers 0.000 description 1
- 229940117841 methacrylic acid copolymer Drugs 0.000 description 1
- XLJIMMGLNGRDER-UHFFFAOYSA-N methyl n-[1-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)benzimidazol-2-yl]carbamate Chemical compound COC(=O)NC1=NC2=CC=CC=C2N1C(N=1)=NC(N2CCOCC2)=NC=1N1CCOCC1 XLJIMMGLNGRDER-UHFFFAOYSA-N 0.000 description 1
- 239000004570 mortar (masonry) Substances 0.000 description 1
- 208000025113 myeloid leukemia Diseases 0.000 description 1
- 201000000050 myeloid neoplasm Diseases 0.000 description 1
- HVLDQYDHOWKYTC-UHFFFAOYSA-N n-[1-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)benzimidazol-2-yl]formamide Chemical compound O=CNC1=NC2=CC=CC=C2N1C(N=1)=NC(N2CCOCC2)=NC=1N1CCOCC1 HVLDQYDHOWKYTC-UHFFFAOYSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 210000003928 nasal cavity Anatomy 0.000 description 1
- 208000014500 neuronal tumor Diseases 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- 208000002154 non-small cell lung carcinoma Diseases 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000008816 organ damage Effects 0.000 description 1
- 150000003891 oxalate salts Chemical class 0.000 description 1
- 125000000160 oxazolidinyl group Chemical group 0.000 description 1
- 239000006201 parenteral dosage form Substances 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 238000010951 particle size reduction Methods 0.000 description 1
- 235000010987 pectin Nutrition 0.000 description 1
- 239000001814 pectin Substances 0.000 description 1
- 229920001277 pectin Polymers 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 229940127557 pharmaceutical product Drugs 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 230000036470 plasma concentration Effects 0.000 description 1
- 208000008423 pleurisy Diseases 0.000 description 1
- 229940100467 polyvinyl acetate phthalate Drugs 0.000 description 1
- 235000013809 polyvinylpolypyrrolidone Nutrition 0.000 description 1
- 229920000523 polyvinylpolypyrrolidone Polymers 0.000 description 1
- 229940069328 povidone Drugs 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 235000019423 pullulan Nutrition 0.000 description 1
- 238000010926 purge Methods 0.000 description 1
- 239000008213 purified water Substances 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 206010039073 rheumatoid arthritis Diseases 0.000 description 1
- YGSDEFSMJLZEOE-UHFFFAOYSA-M salicylate Chemical compound OC1=CC=CC=C1C([O-])=O YGSDEFSMJLZEOE-UHFFFAOYSA-M 0.000 description 1
- 229960001860 salicylate Drugs 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 201000000849 skin cancer Diseases 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 208000000587 small cell lung carcinoma Diseases 0.000 description 1
- 238000000371 solid-state nuclear magnetic resonance spectroscopy Methods 0.000 description 1
- 238000000935 solvent evaporation Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- 238000004808 supercritical fluid chromatography Methods 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 238000002076 thermal analysis method Methods 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- 125000005505 thiomorpholino group Chemical group 0.000 description 1
- 206010043778 thyroiditis Diseases 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- WBYWAXJHAXSJNI-VOTSOKGWSA-M trans-cinnamate Chemical compound [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
- A61K9/1652—Polysaccharides, e.g. alginate, cellulose derivatives; Cyclodextrin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1682—Processes
- A61K9/1694—Processes resulting in granules or microspheres of the matrix type containing more than 5% of excipient
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/205—Polysaccharides, e.g. alginate, gums; Cyclodextrin
- A61K9/2054—Cellulose; Cellulose derivatives, e.g. hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/06—Anti-spasmodics, e.g. drugs for colics, esophagic dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/18—Drugs for disorders of the alimentary tract or the digestive system for pancreatic disorders, e.g. pancreatic enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/02—Nasal agents, e.g. decongestants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/04—Antipruritics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/08—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing three or more hetero rings
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Epidemiology (AREA)
- Pulmonology (AREA)
- Immunology (AREA)
- Rheumatology (AREA)
- Dermatology (AREA)
- Physical Education & Sports Medicine (AREA)
- Oncology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Communicable Diseases (AREA)
- Hematology (AREA)
- Pain & Pain Management (AREA)
- Ophthalmology & Optometry (AREA)
- Otolaryngology (AREA)
- Urology & Nephrology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Medicinal Preparation (AREA)
Description
各記号・用語の定義、意味または例を以下に説明する。
化学式中の記号については、以下のように定義する。
「C1−C6」とは限定がなければ炭素数1〜6個を有する基を意味する。
「C1−C6アルキル」としては、例えば、メチル、エチル、n−プロピル、iso−プロピル、n−ブチル、tert−ブチル、n−ペンチル、n−ヘキシル等の直鎖又は分枝鎖状のアルキル基が挙げられる。
「ヒドロキシC1−C6アルキル」とは、上記「C1−C6アルキル」で定義される基の何れかの炭素原子にヒドロキシ基が結合した基を意味する。
「オキソC1−C6アルキル」とは、上記「C1−C6アルキル」で定義される基の何れかの炭素原子にオキソ基が結合した基を意味する。
「C1−C6アルキルアミノ」とは、アミノ基に上記「C1−C6アルキル」で定義される基の何れかが結合した基を意味する。
「C1−C6アルコキシ」としては、例えば、メトキシ、エトキシ、n−プロポキシ、iso−プロポキシ、n−ブトキシ、tert−ブトキシ、n−ペンチルオキシ、n−ヘキシルオキシ等の直鎖又は分枝鎖状のアルコキシ基が挙げられる。
「C1−C6アルコキシカルボニル」とは、カルボニル基に上記「C1−C6アルコキシ」で定義される基の何れかが結合した基を意味する。
「芳香族カルボニル」とは、カルボニル基に任意の芳香族(例えば、フェニル、チエニル、フリル等)基が結合した基を意味する。
「置換カルバモイル」とは、メチルカルバモイル、フェニルカルバモイル等の、アルキル(例えば、上述のC1−C6アルキルなど)または上述の芳香族基で置換されたカルバモイル基を意味する。
以下、本発明の実施形態について、説明する。
本発明のある実施形態は、一般式(I):
・R1、R2の何れかがヒドロキシル基である上記の複素環式化合物。
・R1、R2の何れかがヒドロキシル基であり、R3がジフルオロメチルである上記の複素環式化合物。
・R1、R2の何れも水素であり、R3がジフルオロメチルである上記の複素環式化合物。
・R6が4-アセチルピペラジンである上記の複素環式化合物。
・R1がジフルオロメチルである上記の複素環式化合物。
・R1がジフルオロメチル、R2が1〜3個のメチルで置換されていてもよいモルホリノ、R3およびR4がそれぞれ水素原子又はメチルである上記の複素環式化合物。
・R1がジフルオロメチル、R2が1〜3個のメチルで置換されていてもよいモルホリノ、R3およびR4が水素原子、R5がアミノ又は水酸基である上記の複素環式化合物。
・R1がヒドロキシメチルである上記の複素環式化合物。
・R1がヒドロキシメチル、R2が1〜2個のメチルで置換されていてもよいモルホリノ、R3およびR4がそれぞれ水素原子又はメチルである上記の複素環式化合物。
・R1がアミノ、ホルミルアミノ又はアセチルアミノである上記の複素環式化合物。
・R1がアミノ、ホルミルアミノ又はアセチルアミノ、R2が1〜2個のメチルで置換されていてもよいモルホリノ、R3およびR4がそれぞれ水素原子である上記の複素環式化合物。
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−(cis−2,3−ジメチルモルホリノ)−6−モルホリノピリミジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4,6−ジモルホリノピリミジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−モルホリノ−6−チオモルホリノピリミジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−(trans−2,3−ジメチルモルホリノ)−6−モルホリノピリミジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−(2,2−ジメチルモルホリノ)−6−モルホリノピリミジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−(2−メチルモルホリノ)−6−モルホリノピリミジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−モルホリノ−6−[2,2,5(R)−トリメチルモルホリノ]ピリミジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−モルホリノ−6−[2,2,5(S)−トリメチルモルホリノ]ピリミジン
・4−(cis−2,3−ジメチルモルホリノ)−2−(2−フルオロメチルベンズイミダゾール−1−イル)−6−モルホリノピリミジン
・2−(2−アミノベンズイミダゾール−1−イル)−4−(cis−2,3−ジメチルモルホリノ)−6−モルホリノピリミジン
・2−(2−アミノベンズイミダゾール−1−イル)−4−(trans−2,3−ジメチルモルホリノ)−6−モルホリノピリミジン
・4−(cis−2,3−ジメチルモルホリノ)−2−(2−ヒドロキシメチルベンズイミダゾール−1−イル)−6−モルホリノピリミジン
・4−(cis−2,3−ジメチルモルホリノ)−2−(2−ヒドロキシメチルベンズイミダゾール−1−イル)−6−ピペリジノピリミジン
・4−(cis−2,3−ジメチルモルホリノ)−2−(2−ヒドロキシメチルベンズイミダゾール−1−イル)−6−(2−ヒドロキシメチルピロリジン−1−イル)ピリミジン
・2−(6−アミノ−2−ジフルオロメチルベンズイミダゾール−1−イル)−4,6−ジモルホリノピリミジン
・2−(6−アミノ−2−ジフルオロメチルベンズイミダゾール−1−イル)−4−(cis−2,3−ジメチルモルホリノ)−6−モルホリノピリミジン
・2−(2−ジフルオロメチル−5−ヒドロキシベンズイミダゾール−1−イル)−4,6−ジモルホリノピリミジン
・2−(2−ジフルオロメチル−4−ヒドロキシベンズイミダゾール−1−イル)−4−(2,2−ジメチルモルホリノ)−6−モルホリノピリミジン
・2−(2,4−ジアミノベンズイミダゾール−1−イル)−4−(2,2−ジメチルモルホリノ)−6−モルホリノピリミジン
・2−(2,4−ジアミノベンズイミダゾール−1−イル)−4,6−ジモルホリノピリミジン
・2−(2−アミノ−4−ヒドロキシベンズイミダゾール−1−イル)−4−(2,2−ジメチルモルホリノ)−6−モルホリノピリミジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−(cis−2,3−ジメチルモルホリノ)−6−モルホリノ−1,3,5−トリアジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−(trans−2,3−ジメチルモルホリノ)−6−モルホリノ−1,3,5−トリアジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−(2,2−ジメチルモルホリノ)−6−モルホリノ−1,3,5−トリアジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−モルホリノ−6−チオモルホリノ−1,3,5−トリアジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−(2−メチルモルホリノ)−6−モルホリノ−1,3,5−トリアジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−(trans−2,5−ジメチルモルホリノ)−6−モルホリノ−1,3,5−トリアジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4,6−ジモルホリノ−1,3,5−トリアジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−モルホリノ−6−[2,2,5(R)−トリメチルモルホリノ]−1,3,5−トリアジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−モルホリノ−6−(テトラヒドロ−1,4−チアジン−1−オキソ−4−イル)−1,3,5−トリアジン
・2−(2−アセチルアミノベンズイミダゾール−1−イル)−4,6−ジモルホリノ−1,3,5−トリアジン
・2−(2−アセチルアミノベンズイミダゾール−1−イル)−4−(trans−2,3−ジメチルモルホリノ)−6−モルホリノピリミジン
・2−(2−ホルミルアミノベンズイミダゾール−1−イル)−4,6−ジモルホリノ−1,3,5−トリアジン
・2−(2−プロピオニルアミノベンズイミダゾール−1−イル)−4−(trans−2,3−ジメチルモルホリノ)−6−モルホリノ−1,3,5−トリアジン
・2−(trans−2,3−ジメチルモルホリノ)−4−(2−ホルミルアミノベンズイミダゾール−1−イル)−6−モルホリノ−1,3,5−トリアジン
・4−(trans−2,3−ジメチルモルホリノ)−2−(2−ホルミルアミノベンズイミダゾール−1−イル)−6−モルホリノピリミジン
・2−(cis−2,6−ジメチルモルホリノ)−4−(2−ホルミルアミノベンズイミダゾール−1−イル)−6−モルホリノ−1,3,5−トリアジン
・2−(2−メトキシカルボニルアミノベンズイミダゾール−1−イル)−4,6−ジモルホリノ−1,3,5−トリアジン
・2−(2−アミノベンズイミダゾール−1−イル)−4,6−ジモルホリノ−1,3,5−トリアジン
・2−(2−アミノベンズイミダゾール−1−イル)−4−(trans−2,3−ジメチルモルホリノ)−6−モルホリノ−1,3,5−トリアジン
・2−(2−アミノベンズイミダゾール−1−イル)−4−(cis−2,3−ジメチルモルホリノ)−6−ピペリジノ−1,3,5−トリアジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−モルホリノ−6−ピペリジノ−1,3,5−トリアジン
・2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4−(trans−2,3−ジメチルモルホリノ)−6−(2−ヒドロキシメチルピロリジン−1−イル)−1,3,5−トリアジン
・2−(6−アミノ−2−ジフルオロメチルベンズイミダゾール−1−イル)−4−(2,2−ジメチルモルホリノ)−6−モルホリノ−1,3,5−トリアジン
・2−(6−アミノ−2−ジフルオロメチルベンズイミダゾール−1−イル)−4−(cis−2,3−ジメチルモルホリノ)−6−モルホリノ−1,3,5−トリアジン
・2−(4−アミノ−2−ジフルオロメチルベンズイミダゾール−1−イル)−4,6−ジモルホリノ−1,3,5−トリアジン
・2−(2−ジフルオロメチル−5−ヒドロキシベンズイミダゾール−1−イル)−4−(2,3−cis−ジメチルモルホリノ)−6−モルホリノ−1,3,5−トリアジン
・2−(2−ジフルオロメチル−6−ヒドロキシベンズイミダゾール−1−イル)−4−(2,2−ジメチルモルホリノ)−6−モルホリノ−1,3,5−トリアジン
・2−(2−ジフルオロメチル−5−ヒドロキシベンズイミダゾール−1−イル)−4−(2,2−ジメチルオキサゾリジン−3−イル)−6−モルホリノ−1,3,5−トリアジン
・2−(2−ジフルオロメチル−4−ヒドロキシベンズイミダゾール−1−イル)−4,6−ジモルホリノ−1,3,5−トリアジン
・2−(2−ジフルオロメチル−4−ヒドロキシベンズイミダゾール−1−イル)−4−(2,2−ジメチルモルホリノ)−6−モルホリノ−1,3,5−トリアジン
・2−(2,4−ジアミノベンズイミダゾール−1−イル)−4−(2,2−ジメチルモルホリノ)−6−モルホリノ−1,3,5−トリアジン
・2−(2,4−ジアミノベンズイミダゾール−1−イル)−4,6−ジモルホリノ−1,3,5−トリアジン
・2−(2−アミノ−4−ヒドロキシベンズイミダゾール−1−イル)−4−(2,2−ジメチルモルホリノ)−6−モルホリノ−1,3,5−トリアジン
上記の複素環式化合物は、当業者であれば、公知の種々の反応を組み合わせて合成することができる(例えば、特許文献1〜6を参照のこと)。例えば、2−(2−ジフルオロメチルベンズイミダゾール−1−イル)−4,6−ジモルホリノ−1,3,5−トリアジンは、特許文献3の実施例に記載の方法によって、合成することができる。
さらに、上記の複素環式化合物は、水和物、溶媒和物または薬学的に許容される塩としての酸付加塩などの形態をとってもよい。
溶融法は、薬物と水溶性高分子担体の融点降下を利用し両者を加熱して溶融させた後、これを冷却・固化・粉砕して固体分散体を得る方法[Chem.Pharm.Bull,9,866(1961)]や、薬物を比較的融点の低い水溶性高分子に加熱溶解させ、これを冷却・固化・粉砕して固体分散体を得る方法[Int.J.Pharm,47,51(1988)]がある。
なお、当然のことながら、式(II)の複素環式化合物も同様の方法で製造することができる。
また、乾燥の際には、さらに減圧を行ってもよい。この際の圧力としては、1気圧(1013.25 hPa)未満の圧力を有する減圧が好ましいがこれに限られない。
また、試験化合物(2‐(2‐ジフルオロメチルベンズイミダゾール)‐4,6‐ジモルホリノ‐1,3,5‐トリアジン)は、特許文献3の実施例に記載の方法に従って合成した。
〔実施例1〕
(噴霧乾燥法による試験化合物非晶質体の調製)
300ml容量のマイヤーに塩化メチレン121.6gとメタノール30.4gを入れた。ここに試験化合物と、HPCとを溶解させ、試験化合物の5%溶液を調製した。排熱温度が60℃付近となるように入熱温度をコントロールし、フィード液を噴霧圧が一定になるように、スプレーした。得られた調製物は、実験用減圧乾燥機を用いて、室温で一晩減圧乾燥した。
(噴霧乾燥法による試験化合物非晶質体の調製)
実施例1と同様の方法で、HPCをPVPまたはHPMCに変更して作業を行った。その際に、試験化合物とそれらの固体高分子との比率を1:1.5、1:2.0、1:2.5、1:5で実施した。
(X線回折法(XRD)による試験化合物非晶質体の解析)
常法により得られた試験化合物の結晶を粉末X線回折法により解析したところ、2θ=5〜14、特に2θ=7〜10に幾つかの特徴的な回折角のピークが観察された(図1)。これに対し、実施例1および2で得られた調製物を、粉末X線回折法(XRD)で解析したところ、結晶体に特徴的な回折角のピークは観察されず、これらの調製物では試験化合物は非晶質化した状態であることが明らかとなった(図2)。
(混合粉砕法による試験化合物非晶質体の調製)
試験化合物及びHPMCを各0.75g秤取し、石川式攪拌擂潰機(AGA型、石川工場)にて2時間混合粉砕した。
〔実施例5〕
(混合粉砕法による試験化合物非晶質体の調製)
実施例3と同様の方法で、HPMCをHPC、PVPもしくはD−マンニトールに変更して混合粉砕した。
(溶媒留去法による試験化合物非晶質体の調製)
試験化合物2.4gおよびHPC(L)6.0gを秤量し、塩化メチレン300mLに溶解した。エバポレータにより有機溶媒を減圧溜去して試験化合物の固体分散体を得た。
(各試験化合物試料の投与における血中濃度の測定)
7週齢のSD系雄性ラットを日本チャールス・リバー(株)より購入し、1週間の予備飼育をした後に試験に供した。薬物投与16時間前から絶食し、実施例1、4および5で得られた各調製物を、試験化合物として100mg/5ml/kgとなるように蒸留水で用時懸濁して経口投与した。これらの製剤を投与後15分から24あるいは30時間まで経時的にヘパリン処理したガラス採血管を用いて尾静脈から採血し、遠心分離(3,000rpm、10min、4℃)して血漿を得、試験化合物の血中濃度を調べた(図3)。
(長期間経過後の試験化合物非晶質体の状態変化)
試験化合物の非晶質体の状態変化を調べるために、試験化合物とHPMCとの配合比1:2.5の噴霧乾燥法による固体分散体を一定の条件下で保存し、実施例3と同様に粉末X線回折により、試験化合物非晶質体の状態変化を解析した。結果を表1に示した。その結果、密栓したガラス容器に密閉状態で保存した場合、25℃や40℃保存6ヵ月目でも結晶化を示す回折角のピークは認められず、試験化合物の非晶質体は、薬剤として用いるに際しては、十分に安定であることが明らかとなった。
(試験化合物非晶質体を含む500mg錠剤の製造)
試験化合物非晶質体175mg 、崩壊剤(低置換度ヒドロキシプロピルセルロース、クロスカルメロースナトリウム、カルメロースカルシウム等)150mg、滑沢剤(ステアリン酸カルシウム、ステアリン酸マグネシウム等)2.5mgを室温によりV型混合機で15分間混合し、ローラーコンパクターを用いて乾式造粒を行い造粒顆粒を得た。
得られた造粒顆粒を篩を用いて適度な粒度に整粒し、これに室温にて賦形剤(結晶セルロース、ブドウ糖、白糖、乳糖、D−マンニトール、D−ソルビトール等)170mg、滑沢剤(ステアリン酸カルシウム、ステアリン酸マグネシウム等)2.5mgを添加してV型混合機で15分間混合し打錠用顆粒を得た。
この打錠用顆粒をロータリー打錠機を用いて1錠500mgの錠剤に成型した。
試験化合物非晶質体 175mg
崩壊剤 150mg
賦形剤 170mg
滑沢剤 5mg
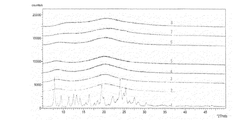
本錠剤をガラス瓶に充填し、密栓後40℃(75%RH)に6ヶ月保存後の非晶質安定性を調べた。試験化合物非晶質体500mg錠製造時のXRDは図9に示したように、結晶化に起因する2θ=7〜10.0のピークが全く認められず、図10に示す崩壊剤、賦形剤および滑沢剤に起因するピークのみが観察された。6ヶ月保存後のXRDにおいても2θ=7〜10.0のピークが全く認められないことから、本錠剤中の試験化合物も非晶質状態で安定に保存されていることが明らかとなった(図11)。
Claims (8)
- 一般式(I):
ヒドロキシプロピルセルロース又はヒプロメロースとを含んでなる固体分散体であって、
前記複素環式化合物、その水和物、その溶媒和物、もしくはその製薬学的に許容される塩と、ヒドロキシプロピルセルロース又はヒプロメロースとの質量比が、1:0.1〜1:10である、固体分散体。 - 前記非晶質体が噴霧乾燥法、混合粉砕法、溶融法又は、溶媒法により製造される請求項1に記載の固体分散体。
- 前記質量比が、1:1〜1:5である、請求項1又は2に記載の固体分散体。
- 前記質量比が、1:1〜1:4である、請求項1又は2に記載の固体分散体。
- 前記質量比が、1:2〜1:2.5である、請求項1又は2に記載の固体分散体。
- 賦形剤、崩壊剤及び滑沢剤をさらに含む、請求項1ないし5の何れか一項に記載の固体分散体。
- 請求項1ないし6の何れか一項に記載の固体分散体を含み、散剤、細粒、顆粒、錠剤、又はカプセル剤の形態で提供される製剤。
- 一般式(I):
ヒドロキシプロピルセルロース又はヒプロメロースとを含んでなる固体分散体の製造方法であって、
前記複素環式化合物、その水和物、その溶媒和物、もしくはその製薬学的に許容される塩を溶媒に溶解し、フィード液を調製する工程、
前記フィード液をスプレーする工程、
スプレーされたフィード液を乾燥し、前記非晶質体を得る工程、および
前記複素環式化合物、その水和物、その溶媒和物、もしくはその製薬学的に許容される塩と、前記ヒドロキシプロピルセルロース又はヒプロメロースとの質量比が、1:0.1〜1:10となるように、前記非晶質体を、前記ヒドロキシプロピルセルロース又はヒプロメロースと混合する工程、
を含む製造方法。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2009542608A JP5479912B2 (ja) | 2007-11-22 | 2008-11-21 | 複素環式化合物の非晶質体、それを含む固体分散体、薬剤、およびその製造法 |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2007303332 | 2007-11-22 | ||
| JP2007303332 | 2007-11-22 | ||
| PCT/JP2008/071259 WO2009066775A1 (ja) | 2007-11-22 | 2008-11-21 | 複素環式化合物の非晶質体、それを含む固体分散体、薬剤、およびその製造法 |
| JP2009542608A JP5479912B2 (ja) | 2007-11-22 | 2008-11-21 | 複素環式化合物の非晶質体、それを含む固体分散体、薬剤、およびその製造法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JPWO2009066775A1 JPWO2009066775A1 (ja) | 2011-04-07 |
| JP5479912B2 true JP5479912B2 (ja) | 2014-04-23 |
Family
ID=40667597
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009542608A Active JP5479912B2 (ja) | 2007-11-22 | 2008-11-21 | 複素環式化合物の非晶質体、それを含む固体分散体、薬剤、およびその製造法 |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US8227463B2 (ja) |
| EP (2) | EP2218718A1 (ja) |
| JP (1) | JP5479912B2 (ja) |
| KR (1) | KR101576695B1 (ja) |
| CN (1) | CN101883765B (ja) |
| AU (1) | AU2008327095B2 (ja) |
| CA (1) | CA2706536C (ja) |
| DK (1) | DK2409975T3 (ja) |
| ES (1) | ES2539714T3 (ja) |
| HK (1) | HK1163079A1 (ja) |
| PT (1) | PT2409975E (ja) |
| TW (1) | TWI482768B (ja) |
| WO (1) | WO2009066775A1 (ja) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7280111B2 (ja) | 2019-05-28 | 2023-05-23 | ファナック株式会社 | モータ制御装置 |
Families Citing this family (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10022352B2 (en) | 2006-04-07 | 2018-07-17 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| SI3091011T1 (en) | 2006-04-07 | 2018-06-29 | Vertex Pharmaceuticals Incorporated | Modulators of ATP conveyor belt conveyors |
| US7645789B2 (en) | 2006-04-07 | 2010-01-12 | Vertex Pharmaceuticals Incorporated | Indole derivatives as CFTR modulators |
| US8563573B2 (en) | 2007-11-02 | 2013-10-22 | Vertex Pharmaceuticals Incorporated | Azaindole derivatives as CFTR modulators |
| JP5766177B2 (ja) | 2009-03-27 | 2015-08-19 | ベトディーシー,インコーポレイテッド | ピリミジニル及び1,3,5−トリアジニルベンゾイミダゾールスルホンアミド及びガンの療法におけるその使用 |
| EP2451802A1 (en) | 2009-07-07 | 2012-05-16 | Pathway Therapeutics, Inc. | Pyrimidinyl and 1,3,5-triazinyl benzimidazoles and their use in cancer therapy |
| CN109081804B (zh) * | 2010-03-25 | 2021-12-10 | 弗特克斯药品有限公司 | 环丙烷甲酰胺的固体形式 |
| US8802868B2 (en) | 2010-03-25 | 2014-08-12 | Vertex Pharmaceuticals Incorporated | Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxo1-5-yl)-N-(1-(2,3-dihydroxypropyl-6-fluoro-2-(1-hydroxy-2-methylpropan2-yl)-1H-Indol-5-yl)-Cyclopropanecarboxamide |
| KR20130056244A (ko) | 2010-04-22 | 2013-05-29 | 버텍스 파마슈티칼스 인코포레이티드 | 시클로알킬카르복스아미도-인돌 화합물의 제조 방법 |
| JP5850576B2 (ja) * | 2010-07-06 | 2016-02-03 | 富士化学工業株式会社 | ボセンタン固体分散体 |
| EA023931B1 (ru) * | 2010-08-10 | 2016-07-29 | Астеллас Фарма Инк. | Гетероциклическое соединение |
| JP6042406B2 (ja) | 2011-03-28 | 2016-12-14 | メイ プハルマ,インコーポレーテッド | (α−置換アラルキルアミノ及びヘテロアリールアルキルアミノ)ピリミジニル及び1,3,5−トリアジニルベンズイミダゾール、それらを含む医薬組成物、並びに増殖性疾患の治療で使用するためのこれらの化合物 |
| EP2872122A1 (en) | 2012-07-16 | 2015-05-20 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions of (r)-1-(2,2-diflurorbenzo[d][1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof |
| HUE039062T2 (hu) | 2014-04-15 | 2018-12-28 | Vertex Pharma | Gyógyszerészeti készítmények cisztás fibrózis transzmembrán konduktancia regulátor által mediált betegségek kezelésére |
| US9481653B2 (en) | 2014-09-12 | 2016-11-01 | Knopp Biosciences Llc | Benzoimidazol-1,2-yl amides as Kv7 channel activators |
| CA2987867C (en) | 2015-06-09 | 2023-06-27 | Capsugel Belgium Nv | Formulations to achieve rapid dissolution of drug from spray-dried dispersions in capsules |
| US20190307886A1 (en) * | 2016-12-23 | 2019-10-10 | University Of Copenhagen | A co-amorphous form of a substance and a protein |
| MX2019013862A (es) | 2017-05-23 | 2020-01-20 | Mei Pharma Inc | Terapia de combinacion. |
| CA3072476A1 (en) | 2017-08-14 | 2019-02-21 | Mei Pharma, Inc. | Combination therapy |
| SG11202008030WA (en) | 2018-03-19 | 2020-10-29 | Knopp Biosciences Llc | Kv7 channel activators compositions and methods of use |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005095389A1 (ja) * | 2004-03-31 | 2005-10-13 | Zenyaku Kogyo Kabushiki Kaisha | 複素環式化合物及びそれを有効成分とする抗悪性腫瘍剤 |
| WO2006095906A1 (ja) * | 2005-03-11 | 2006-09-14 | Zenyaku Kogyo Kabushikikaisha | 複素環式化合物を有効成分とする免疫抑制剤及び抗腫瘍剤 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100563514B1 (ko) | 1997-07-24 | 2006-03-27 | 젠야쿠코교가부시키가이샤 | 헤테로고리 화합물 및 이를 유효성분으로 하는 항종양제 |
| WO2000043385A1 (fr) | 1999-01-25 | 2000-07-27 | Zenyaku Kogyo Kabushiki Kaisha | Composes heterocycliques et agents antitumoraux les comprenant en tant que principe actif |
| AU2002253619B2 (en) | 2001-04-27 | 2007-05-17 | Zenyaku Kogyo Kabushiki Kaisha | Heterocyclic compound and antitumor agent containing the same active ingredients |
| JP4493503B2 (ja) | 2002-10-25 | 2010-06-30 | 全薬工業株式会社 | 複素環式化合物及びそれを有効成分とする抗腫瘍剤 |
-
2008
- 2008-11-21 PT PT111684619T patent/PT2409975E/pt unknown
- 2008-11-21 US US12/744,160 patent/US8227463B2/en not_active Expired - Fee Related
- 2008-11-21 JP JP2009542608A patent/JP5479912B2/ja active Active
- 2008-11-21 TW TW097145093A patent/TWI482768B/zh not_active IP Right Cessation
- 2008-11-21 CA CA2706536A patent/CA2706536C/en not_active Expired - Fee Related
- 2008-11-21 KR KR1020107013811A patent/KR101576695B1/ko active IP Right Grant
- 2008-11-21 WO PCT/JP2008/071259 patent/WO2009066775A1/ja active Application Filing
- 2008-11-21 EP EP08852868A patent/EP2218718A1/en not_active Withdrawn
- 2008-11-21 AU AU2008327095A patent/AU2008327095B2/en not_active Ceased
- 2008-11-21 EP EP11168461.9A patent/EP2409975B1/en not_active Not-in-force
- 2008-11-21 DK DK11168461.9T patent/DK2409975T3/en active
- 2008-11-21 CN CN200880117078.4A patent/CN101883765B/zh active Active
- 2008-11-21 ES ES11168461.9T patent/ES2539714T3/es active Active
-
2012
- 2012-04-12 HK HK12103623.5A patent/HK1163079A1/xx not_active IP Right Cessation
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005095389A1 (ja) * | 2004-03-31 | 2005-10-13 | Zenyaku Kogyo Kabushiki Kaisha | 複素環式化合物及びそれを有効成分とする抗悪性腫瘍剤 |
| WO2006095906A1 (ja) * | 2005-03-11 | 2006-09-14 | Zenyaku Kogyo Kabushikikaisha | 複素環式化合物を有効成分とする免疫抑制剤及び抗腫瘍剤 |
Non-Patent Citations (3)
| Title |
|---|
| JPN6009001011; NUERNBERG, E et al.: 'Modifizierung der physikalischen und biopharmazeutischen Eigenschaften von Arzneistoffen durch Sprue' Chemische Industrie Vol.33, No.12, 1981, p.794-796 * |
| JPN6009001013; 竹内洋文 外: '特集 粉粒体技術の進展 粒子設計と製造' ケミカルエンジニヤリング Vol.37, No.6, 1992, p.496-501 * |
| JPN6009001016; 岡本浩一: '製剤関係分野シンポジウムレポート' PHARM TECH JAPAN 8月号 Vol.20, No.9, p.1783-1785 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7280111B2 (ja) | 2019-05-28 | 2023-05-23 | ファナック株式会社 | モータ制御装置 |
Also Published As
| Publication number | Publication date |
|---|---|
| TWI482768B (zh) | 2015-05-01 |
| EP2409975B1 (en) | 2015-05-27 |
| US8227463B2 (en) | 2012-07-24 |
| KR101576695B1 (ko) | 2015-12-10 |
| KR20100108533A (ko) | 2010-10-07 |
| TW200930712A (en) | 2009-07-16 |
| AU2008327095A1 (en) | 2009-05-28 |
| WO2009066775A1 (ja) | 2009-05-28 |
| CN101883765A (zh) | 2010-11-10 |
| EP2218718A1 (en) | 2010-08-18 |
| CA2706536A1 (en) | 2009-05-28 |
| HK1163079A1 (en) | 2012-09-07 |
| AU2008327095B2 (en) | 2013-07-25 |
| US20100249063A1 (en) | 2010-09-30 |
| JPWO2009066775A1 (ja) | 2011-04-07 |
| DK2409975T3 (en) | 2015-08-03 |
| CA2706536C (en) | 2016-04-19 |
| ES2539714T3 (es) | 2015-07-03 |
| PT2409975E (pt) | 2015-07-27 |
| CN101883765B (zh) | 2014-10-01 |
| EP2409975A1 (en) | 2012-01-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5479912B2 (ja) | 複素環式化合物の非晶質体、それを含む固体分散体、薬剤、およびその製造法 | |
| AU2015238857B2 (en) | Propane- I-sulfonic acid {3- [5- (4 -chloro-phenyl) -1H-pyrrolo [2, 3-b] pyridine-3-carbonyl] -2, 4-difluoro-pheny l } -amide compositions and uses thereof | |
| JP6906308B2 (ja) | 4環性化合物の非晶質体 | |
| KR101118930B1 (ko) | 페닐알라닌 유도체의 고체 분산체 또는 고체 분산체 의약제제 | |
| EP1793824B1 (en) | New pharmaceutical compositions comprising 4-(4-(3-(4-chloro-3-trifluoromethyl-phenyl)-ureido)-3-fluoro-phenoxy)-pyridine-2-carboxylic acid for the treatment of hyper-proliferative disorders | |
| JP6878398B2 (ja) | Parp阻害剤固形医薬剤型及びその使用 | |
| JP2011530532A (ja) | 固体分子分散物中のhcvプロテアーゼインヒビターの薬学的処方物 | |
| WO2006087919A1 (ja) | 難水溶性物質含有微細化組成物 | |
| CN108472291A (zh) | 用于治疗恶性肿瘤的组合疗法 | |
| WO2019107412A1 (ja) | 固体分散体 | |
| CN116685308A (zh) | 无定形固体分散体 | |
| US20240216347A1 (en) | Bezuclastinib formulations | |
| TW202110827A (zh) | 吡唑化合物之非吸濕性結晶鹽及其醫藥組合物及用途 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20110819 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130319 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130520 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20140204 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20140213 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5479912 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |