CN1299646C - Optical-circuit-variable time-domain light-dividing differential wave length spectrometer for detecting tissue content and detection method thereof - Google Patents
Optical-circuit-variable time-domain light-dividing differential wave length spectrometer for detecting tissue content and detection method thereof Download PDFInfo
- Publication number
- CN1299646C CN1299646C CNB2004100193208A CN200410019320A CN1299646C CN 1299646 C CN1299646 C CN 1299646C CN B2004100193208 A CNB2004100193208 A CN B2004100193208A CN 200410019320 A CN200410019320 A CN 200410019320A CN 1299646 C CN1299646 C CN 1299646C
- Authority
- CN
- China
- Prior art keywords
- light
- condenser lens
- circuit
- cpu
- light source
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000001514 detection method Methods 0.000 title claims abstract description 77
- 230000003287 optical effect Effects 0.000 claims abstract description 72
- 238000006243 chemical reaction Methods 0.000 claims abstract description 56
- 238000001228 spectrum Methods 0.000 claims abstract description 46
- 230000008859 change Effects 0.000 claims abstract description 22
- 230000002093 peripheral effect Effects 0.000 claims abstract description 13
- 239000008280 blood Substances 0.000 claims description 34
- 210000004369 blood Anatomy 0.000 claims description 34
- 238000000034 method Methods 0.000 claims description 28
- 238000011896 sensitive detection Methods 0.000 claims description 13
- 230000000694 effects Effects 0.000 claims description 12
- 239000013078 crystal Substances 0.000 claims description 11
- 230000008569 process Effects 0.000 claims description 10
- 238000012545 processing Methods 0.000 claims description 7
- 230000003595 spectral effect Effects 0.000 claims description 6
- 238000000862 absorption spectrum Methods 0.000 claims description 5
- 210000005056 cell body Anatomy 0.000 claims 17
- 230000000877 morphologic effect Effects 0.000 claims 8
- 238000005286 illumination Methods 0.000 claims 5
- 230000009471 action Effects 0.000 claims 2
- 230000005693 optoelectronics Effects 0.000 claims 2
- JBRZTFJDHDCESZ-UHFFFAOYSA-N AsGa Chemical compound [As]#[Ga] JBRZTFJDHDCESZ-UHFFFAOYSA-N 0.000 claims 1
- 230000005540 biological transmission Effects 0.000 claims 1
- 230000008878 coupling Effects 0.000 claims 1
- 238000010168 coupling process Methods 0.000 claims 1
- 238000005859 coupling reaction Methods 0.000 claims 1
- 229910052738 indium Inorganic materials 0.000 claims 1
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 claims 1
- 230000008520 organization Effects 0.000 claims 1
- 238000005259 measurement Methods 0.000 abstract description 22
- 238000001125 extrusion Methods 0.000 abstract description 9
- 210000001519 tissue Anatomy 0.000 description 65
- 239000000306 component Substances 0.000 description 25
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 16
- 239000008103 glucose Substances 0.000 description 16
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 9
- 229910052760 oxygen Inorganic materials 0.000 description 9
- 239000001301 oxygen Substances 0.000 description 9
- 238000012360 testing method Methods 0.000 description 8
- 238000011160 research Methods 0.000 description 7
- 238000010586 diagram Methods 0.000 description 5
- 230000002207 retinal effect Effects 0.000 description 4
- 239000000523 sample Substances 0.000 description 4
- 238000004497 NIR spectroscopy Methods 0.000 description 3
- 238000002835 absorbance Methods 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 238000012805 post-processing Methods 0.000 description 3
- 238000004611 spectroscopical analysis Methods 0.000 description 3
- 230000001360 synchronised effect Effects 0.000 description 3
- 230000000007 visual effect Effects 0.000 description 3
- GYHNNYVSQQEPJS-UHFFFAOYSA-N Gallium Chemical compound [Ga] GYHNNYVSQQEPJS-UHFFFAOYSA-N 0.000 description 2
- 108010073178 Glucan 1,4-alpha-Glucosidase Proteins 0.000 description 2
- 102100022624 Glucoamylase Human genes 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 239000012503 blood component Substances 0.000 description 2
- 238000004364 calculation method Methods 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 229910052733 gallium Inorganic materials 0.000 description 2
- RPQDHPTXJYYUPQ-UHFFFAOYSA-N indium arsenide Chemical compound [In]#[As] RPQDHPTXJYYUPQ-UHFFFAOYSA-N 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 238000012544 monitoring process Methods 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- 102100030497 Cytochrome c Human genes 0.000 description 1
- 108010075031 Cytochromes c Proteins 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 108010054147 Hemoglobins Proteins 0.000 description 1
- 102000001554 Hemoglobins Human genes 0.000 description 1
- 108010064719 Oxyhemoglobins Proteins 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000000443 biocontrol Effects 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 210000003722 extracellular fluid Anatomy 0.000 description 1
- 210000000416 exudates and transudate Anatomy 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 238000000691 measurement method Methods 0.000 description 1
- 238000010339 medical test Methods 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000004445 quantitative analysis Methods 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Landscapes
- Measurement Of The Respiration, Hearing Ability, Form, And Blood Characteristics Of Living Organisms (AREA)
Abstract
本发明公开了一种测量准确、操作方便,并可同时测量多种组织成份的组织成份检测的变光路时域分光差分光谱仪,包括宽带光源、分光装置及其光路、光敏传感器、模拟检测通道、A/D转换模块、挤压装置、CPU及其外围电路;所述的宽带光源的带宽是600~1300nm;所述分光装置及其光路包括分光器件和与其相配套的光路器件;所述分光器件采用AOTF、滤光片或傅立叶分光仪;由上述A/D转换后的信号经CPU处理获得第一组光谱;所述电磁挤压装置对被测人体组织进行施压改变光路长以获得第二组光谱;从所述第一和第二组光谱的差分光谱中得到组织成分中的主要成分的含量。本发明中还公开了上述光谱仪的检测方法。
The invention discloses a variable optical path time-domain spectroscopic difference spectrometer with accurate measurement, convenient operation, and simultaneous measurement of tissue components of various tissue components, including broadband light source, spectroscopic device and its optical path, photosensitive sensor, analog detection channel, A/D conversion module, extrusion device, CPU and its peripheral circuits; the bandwidth of the broadband light source is 600-1300nm; the light splitting device and its optical path include a light splitting device and matching optical path devices; the light splitting device AOTF, filter or Fourier spectrometer is used; the signal after the above A/D conversion is processed by the CPU to obtain the first group of spectra; the electromagnetic extrusion device applies pressure to the measured human tissue to change the optical path length to obtain the A group of spectra; obtaining the content of the main components in the tissue components from the difference spectra of the first and second group of spectra. The invention also discloses a detection method of the spectrometer.
Description
技术领域technical field
本发明涉及一种临床医学检验仪器及方法,特别涉及一种组织成份测量仪器及方法。The invention relates to a clinical medical testing instrument and method, in particular to a tissue component measuring instrument and method.
背景技术Background technique
组织成份的无创检测,对于疾病的诊断和治疗,其重要性和巨大价值是毫无疑问。不仅于此,实现组织成份的无创检测,在信号传感、检测与处理也有极大的学术意义和价值。There is no doubt about the importance and great value of the non-invasive detection of tissue components for the diagnosis and treatment of diseases. Not only that, the realization of non-invasive detection of tissue components also has great academic significance and value in signal sensing, detection and processing.
1977年美国科学家Jobsis首次报道了用近红外光观察成年猫脑内氧合血红蛋白、还原血红蛋白和细胞色素c的含量变化的实验结果,揭示了近红外光(700-1300nm)在生物组织内较低的衰减率和用近红外光谱法无创监测组织血氧浓度的可行性。鉴于这一新的无创伤测量方法的极其诱人的应用前景,研究者们做了大量的动物的和人体的实验,从多方面验证了用近红外光谱法监测组织血氧浓度的临床意义。随后,英国London大学的Delpy,美国Duke大学的Jobsis,日本Hokkaido大学的Tamura,Yamamoto,以及日本Omron公司的Shiga等从Lambert-Beer定律出发,通过模型、动物以及人体实验,提出来若干种由吸光度变化推算组织的血氧浓度变化量的演算公式。在测量装置的开发上出现了用普通发光管LED取代激光光源的便携式组织血氧计。然而,由于目前的方法只能给出血氧浓度的变化量或变化趋势,且缺乏通用性,所以都未能进入临床应用。In 1977, the American scientist Jobsis first reported the experimental results of using near-infrared light to observe the changes in the content of oxyhemoglobin, reduced hemoglobin and cytochrome c in the brain of adult cats, revealing that near-infrared light (700-1300nm) has a lower concentration in biological tissues. decay rate and the feasibility of noninvasive monitoring of tissue blood oxygen concentration by near-infrared spectroscopy. In view of the extremely attractive application prospects of this new non-invasive measurement method, researchers have done a large number of animal and human experiments to verify the clinical significance of monitoring tissue blood oxygen concentration with near-infrared spectroscopy. Subsequently, Delpy from the University of London in the UK, Jobsis from Duke University in the United States, Tamura and Yamamoto from Hokkaido University in Japan, and Shiga from Omron Corporation in Japan, etc., started from the Lambert-Beer law, and through models, animal and human experiments, proposed several kinds of absorbance. The calculation formula for calculating the change in the blood oxygen concentration of the tissue. In the development of measuring devices, a portable tissue oximeter that replaces the laser light source with an ordinary luminescent tube LED has appeared. However, because the current methods can only give the change amount or change trend of the blood oxygen concentration, and lack of versatility, they have not been put into clinical application.
80年代,Dhne首次提出了应用近红外分光法进行人体血糖浓度的无创伤测量的方法。近15年以来,美国的Futrex公司、Bio-control公司、New-mexico大学、Iowa大学、西德的Medscience公司、日本的三井金属、日立制作所和松下电器等公司都在这方面进行了不懈的研究。研究方法大体可分为两类,一是利用糖的水溶液模型进行的研究,如美国的Iowa大学Gray W.Small的研究组;另一类是直接测量人体并与抽血测量的结果进行相关比对,如美国的IMI公司等。糖的水溶液模型研究虽在精确测试葡萄糖的分子吸收系数上取得重要进展,但因模型太简单,与人体间的差别太大而难以作为参考。而人体实验虽然可直接验证方法的有效性,但作为归纳定量方法基础的Lambert-Beer定律实际上并不适用于具有强散射特性的人体组织,因此测量的结果难以解释,且不具有通用性与重复性。从检测生物组织化学成份的角度来看,组织血氧浓度同血糖检测面临类似的问题。但是,由于血糖的吸收引起的吸光度变化信号比水分引起的吸光度变化信号要弱得多,目前,血糖的无创光检测技术的研究更多地集中于如何提高测量精度以捡出由血糖含量变化引起的光信号的变化来。所以,尽管由于潜在的巨大经济利益,一些世界上著名大公司在过去的20年间投入了大量的资金进行开发,血糖的无创检测距离实际应用还有一段更长的路要走。相对说来,血液其他成份的无创检测的经济价值要低一点,但难度却更大(由于相对含量低和吸收光谱重叠),国外的相关研究很少,主要集中在血乳酸、激素等成份的测量。而国内就几乎没人进行研究)。由于个体的差异和光谱重叠、测量条件(测量位置、环境温度和压力),即使是国际上已投入巨大人力和财力进行研究的组织血氧和血糖的测量仍然未进入临床实用(仅有脉搏血氧、即动脉血氧已普遍进入临床使用和发挥极其重要的作用),更不用说血液其他成份的无创检测。In the 1980s, Dhne first proposed the method of non-invasive measurement of human blood glucose concentration by using near-infrared spectroscopy. In the past 15 years, companies such as Futrex, Bio-control, New-mexico University, University of Iowa, Medscience in West Germany, Mitsui Metals, Hitachi and Panasonic in Japan have all made unremitting efforts in this regard. Research. The research methods can be roughly divided into two categories, one is the research using the sugar aqueous solution model, such as the research group of Gray W. Small at the University of Iowa in the United States; the other is the direct measurement of the human body and the correlation with the blood measurement results Yes, such as the IMI company in the United States. Although the research on the aqueous model of sugar has made important progress in accurately testing the molecular absorption coefficient of glucose, the model is too simple and too different from the human body to be used as a reference. Although human experiments can directly verify the effectiveness of the method, the Lambert-Beer law, which is the basis of the inductive quantitative method, is actually not applicable to human tissues with strong scattering properties, so the measurement results are difficult to interpret, and they are not universal and comparable. repeatability. From the perspective of detecting biohistochemical components, tissue blood oxygen concentration faces similar problems to blood glucose detection. However, since the absorbance change signal caused by the absorption of blood sugar is much weaker than the absorbance change signal caused by water, at present, the research on the non-invasive optical detection technology of blood sugar is more focused on how to improve the measurement accuracy to pick out the blood sugar caused by the change of blood sugar content. The change of the optical signal comes. Therefore, although some world-renowned large companies have invested a lot of money in development in the past 20 years due to the potential huge economic benefits, the non-invasive detection of blood sugar is still a long way from practical application. Relatively speaking, the economic value of non-invasive detection of other blood components is a little lower, but it is more difficult (due to the low relative content and overlapping absorption spectra), and there are few related studies abroad, mainly focusing on blood lactic acid, hormones and other components. Measurement. And there is almost no research in China). Due to individual differences, overlapping spectra, and measurement conditions (measurement location, ambient temperature, and pressure), even the measurement of tissue blood oxygen and blood glucose, which has invested huge manpower and financial resources internationally, has not yet entered clinical practice (only pulse blood Oxygen, that is, arterial blood oxygen has generally entered clinical use and plays an extremely important role), not to mention the non-invasive detection of other blood components.
中国专利公开号1271562,公开日2000年11月1日,名称是《无创伤自测血糖仪》的中国发明专利申请文件中公开了一种无创伤自测血糖仪,主要由红外光发射管构成的红外光源,通光路部分、光电探测转换器、电通路部分及显示部分构成。显然,采用单一波长的光源是不可能实现在体的无创动脉血糖含量测量的。Chinese Patent Publication No. 1271562, published on November 1, 2000, the Chinese invention patent application document titled "Non-invasive self-test blood glucose meter" discloses a non-invasive self-test blood glucose meter, which is mainly composed of infrared light emitting tubes The infrared light source is composed of an optical path part, a photoelectric detection converter, an electric path part and a display part. Obviously, it is impossible to realize in vivo non-invasive arterial blood glucose measurement with a single wavelength light source.
中国专利公开号1222063,公开日1999年7月07日,名称为《确定血糖浓度的光学方法和装置》的中国发明专利申请中公开了一种用于测量受试者的血糖浓度的方法和装置,其方法包括:a)提供一个光图案,该图案对第一视网膜系统比第二视网膜系统具有更大的刺激量,导致第一:第二的刺激比大于1,其中所说的光图案刺激随第一:第二的刺激比变化的主观视觉特征,和其中所说的第一视网膜系统和第二视网膜系统对所说的光图案的灵敏度随所说的受试者的血糖浓度变化;b)使所说的受试者观察所说光图案的所说的主观视觉特征;和c)使所说的受试者的血糖浓度和所说的主观视觉特征相关。显然,该方法难以客观、定量地测量血糖含量。该专利申请文件中还公开了一种无创血糖测量仪。实现无创伤血糖测量有两种结构:(一)在现有血糖计连接一探头,探头内有氧电极、葡萄糖化酶,探头与气泵连接,将探头紧贴检测者手指,手指上渗出的组织液与葡萄糖化酶作用,血糖计即测得血糖浓度;(二)有一受控的激光器,红外光束经光栅、分光镜分成二束光,一束经手指到达斩波器,一束以参考池到达斩波器,斩波器分别将两束光送至红外接收器,红外接收器将信号送至微处理机,微处理机结合数据库进行运算,显示器显示测量结果。该发明专利申请通过皮肤渗出液的方法操作复杂、测量精度低、测量成份种类少、测量成本高。Chinese Patent Publication No. 1222063, published on July 07, 1999, a Chinese invention patent application titled "Optical Method and Device for Determining Blood Glucose Concentration" discloses a method and device for measuring blood glucose concentration of subjects , the method comprising: a) providing a light pattern that has a greater amount of stimulation to the first retinal system than to the second retinal system, resulting in a first:second stimulation ratio greater than 1, wherein said light pattern stimulates Subjective visual characteristics as a function of first:second stimulus ratio, and wherein sensitivity of said first retinal system and second retinal system to said light pattern varies as a function of blood glucose concentration of said subject; b) causing said subject to observe said subjective visual characteristic of said light pattern; and c) correlating said subject's blood glucose concentration with said subjective visual characteristic. Obviously, this method is difficult to objectively and quantitatively measure the blood sugar content. The patent application document also discloses a non-invasive blood glucose measuring instrument. There are two structures to realize non-invasive blood glucose measurement: (1) Connect a probe to the existing blood glucose meter, the probe has an oxygen electrode, glucoamylase, and the probe is connected to the air pump, and the probe is close to the finger of the tester, and the oozing out of the finger The interstitial fluid interacts with glucoamylase, and the blood glucose meter measures the blood glucose concentration; (2) There is a controlled laser, and the infrared beam is divided into two beams through a grating and a beam splitter. Reaching the chopper, the chopper sends the two beams of light to the infrared receiver respectively, and the infrared receiver sends the signal to the microprocessor, the microprocessor combines the database to perform calculations, and the display shows the measurement results. The invention patent application uses the method of skin exudate with complex operation, low measurement accuracy, few types of measurement components, and high measurement cost.
显然,采用上述现有技术中检测装置操作复杂,测量精度低,测量成份种类少,测量成本高。Apparently, the operation of the detection device in the above-mentioned prior art is complicated, the measurement accuracy is low, the types of measurement components are few, and the measurement cost is high.
发明内容Contents of the invention
为了克服上述现有技术中的不足,本发明提供了一种测量准确、操作方便,并可同时测量多种组织成份的方法及仪器。在本发明下述的描述中涉及到的差分光谱法,其概念是将两组在不同光路下测得的光谱幅值做差,所获得的已经在很大程度上消除了个体差异信息的光谱。另外,本发明描述中涉及到的时域分光法,其概念是指在通过分光器件,在时间域上进行分光,在不同的时刻产生不同波长的单色光。In order to overcome the deficiencies in the above-mentioned prior art, the present invention provides a method and an instrument that are accurate in measurement, easy to operate, and can measure multiple tissue components at the same time. In the differential spectroscopy involved in the following description of the present invention, its concept is to make a difference between two groups of spectral amplitudes measured under different optical paths, and the obtained spectrum has eliminated individual difference information to a large extent . In addition, the concept of the time-domain spectroscopic method involved in the description of the present invention refers to the generation of monochromatic light of different wavelengths at different times by performing light splitting in the time domain through a spectroscopic device.
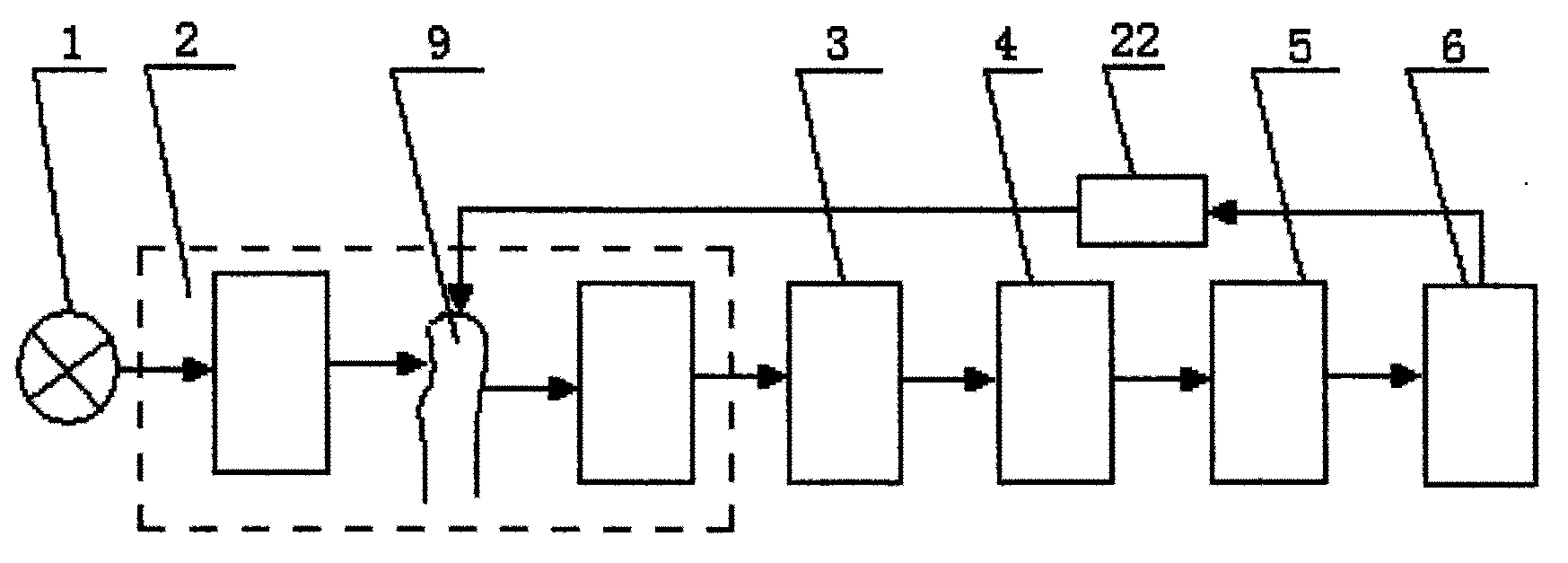
为了解决上述的技术问题,本发明组织成份检测的变光路时域分光差分光谱仪采用的技术方案是,包括宽带光源、分光装置及其光路、光敏传感器、模拟检测通道、A/D转换模块、挤压装置、CPU及其外围电路;所述的宽带光源采用带宽在600~1300nm的可见光乃至近红外光;所述分光装置及其光路包括分光器件和与其相配套的光路器件;所述相配套的光路器件采用下述装置之一:聚光镜、狭缝、小孔;所述分光器件采用AOTF、滤光片或傅立叶分光光谱仪;若所述分光器采用AOTF,则光路中包括宽带光源、第一聚光镜、AOTF分光器件、被测人体组织、第二聚光镜和光敏传感器;所述AOTF的作用是将所述宽带光源的出射光进行聚焦和分光后入射到被测人体组织,并采集出射光,聚焦后输出光束;即,所述宽带光源的出射光经第一聚光镜成为平行光入射到AOTF晶体,在CPU的控制下,AOTF晶体将平行光分时调制成不同波长的单色光入射到被测组织后经第二聚光镜将被测人体组织的出射光聚焦,由处于焦点处的光敏传感器进行光电变换;或若所述分光器采用滤光片时,则光路包括宽带光源、第一聚光镜、滤光片、被测人体组织、第二聚光镜和光敏传感器;所述滤光片的作用是将所述宽带光源的出射光进行聚焦和分光后入射到被测人体组织,并采集出射光,聚焦后输出光束;即,所述宽带光源的出射光经第一聚光镜成为平行光入射到滤光片,在CPU的控制下,滤光片将平行光分时调制成不同波长的单色光入射到被测人体组织后经第二聚光镜将被测人体组织的出射光聚焦,由处于焦点处的光敏传感器进行光电变换;或若所述分光器采用傅立叶分光光谱仪,则光路包括宽带光源、第一聚光镜、被测人体组织、第二聚光镜、傅立叶分光光谱仪和光敏传感器;所述傅立叶分光光谱仪的作用是将宽带光源的出射光聚焦后入射到被测人体组织,并采集出射光,聚焦和分光后输出多个单色光光束;即,所述宽带光源的出射光经过第一聚光镜成为平行光入射到被测人体组织,出射光经第二聚光镜送入傅立叶分光光谱仪,其输出的单色光由光敏传感器进行光电变换;由上述A/D转换后的信号经CPU处理获得第一组光谱;所述电磁挤压装置对被测人体组织进行施压改变光路长以获得第二组光谱;从所述第一和第二组光谱的差分光谱中得到组织成分中的主要成分的含量。In order to solve the above-mentioned technical problems, the technical solution adopted by the time-domain spectroscopic difference spectrometer with variable optical path for tissue composition detection in the present invention is to include a broadband light source, a spectroscopic device and its optical path, a photosensitive sensor, an analog detection channel, an A/D conversion module, a squeeze pressure device, CPU and its peripheral circuits; the broadband light source uses visible light or even near-infrared light with a bandwidth of 600-1300nm; The optical path device adopts one of the following devices: a condenser, a slit, a small hole; the optical splitter adopts an AOTF, an optical filter or a Fourier spectrometer; if the optical splitter adopts an AOTF, the optical path includes a broadband light source, a first condenser , AOTF spectroscopic device, measured human tissue, a second condenser lens and a photosensitive sensor; the function of the AOTF is to focus and split the outgoing light of the broadband light source into the measured human tissue, collect the outgoing light, and focus it Output light beam; that is, the outgoing light of the broadband light source becomes parallel light through the first condenser lens and enters the AOTF crystal. Under the control of the CPU, the AOTF crystal time-divisionally modulates the parallel light into monochromatic light of different wavelengths and enters the tissue under test. Afterwards, the outgoing light of the human tissue to be measured is focused by the second condenser lens, and the photoelectric conversion is performed by the photosensitive sensor at the focal point; film, measured human tissue, second condenser lens and photosensitive sensor; the function of the filter is to focus and split the outgoing light of the broadband light source to enter the measured human tissue, collect the outgoing light, and output it after focusing light beam; that is, the outgoing light of the broadband light source becomes parallel light incident on the optical filter through the first condenser lens, and under the control of the CPU, the optical filter time-divisionally modulates the parallel light into monochromatic light of different wavelengths incident on the measured After the human tissue is focused by the second concentrator, the outgoing light of the human tissue to be measured is converted by the photosensitive sensor at the focal point; Measuring human tissue, a second condenser, a Fourier spectrometer and a photosensitive sensor; the function of the Fourier spectrometer is to focus the outgoing light of the broadband light source into the measured human tissue, collect the outgoing light, focus and split the light, and output multiple Monochromatic light beam; that is, the outgoing light of the broadband light source becomes parallel light incident on the human tissue to be measured through the first condenser, and the outgoing light is sent to the Fourier spectrometer through the second condenser, and the output monochromatic light is detected by the photosensitive sensor. Photoelectric conversion; the signal after the above-mentioned A/D conversion is processed by the CPU to obtain the first group of spectra; the electromagnetic extrusion device applies pressure to the measured human tissue to change the optical path length to obtain the second group of spectra; from the first The content of the main components in the tissue components is obtained from the difference spectrum of the second group of spectra.
所述光敏传感器的作用是进行光电转换,所述光敏传感器采用下述装置之一:光敏管和砷镓铟光敏器件。所述模拟检测通道的作用是将光敏传感器的输出信号转换成与A/D转换模块匹配的电压信号,根据采用的检测方法不同,所述模拟检测通道包括光敏传感器、I/V变换电路、隔直电路、模拟开关、滤波器、相敏检波电路、抗混叠滤波器和A/D转换电路,所述滤波器的中心频率根据波长切换频率设定,取每一波长由AOTF光波的出射频率。所述模拟检测通道中光敏传感器对信号进行光电转换后,通过I/V变换电路可达到一定幅值的电压输出,而且模拟开关的通道数及模拟检测通道的数目,与所采用的经过分光器件分光后的特征光波长数目一致;模拟开关的切换与分光器件的波长切换同步动作,由此将不同波长入射光产生的光电信号分别传输到相应的模拟检测通道中;每一模拟检测通道具有相同的转换模块,包括滤波电路模块、放大电路模块,其输出是对应于某一波长入射光,经过模拟开关调制的高频电压信号;所述相敏检波电路将对应于光强的信号检出,通过模拟开关切换到A/D转换电路,转换成数字信号,供CPU进行后期处理。所述A/D转换模块的作用是将模拟电压信号转换成数字信号,并传递给所述的CPU;所述A/D转换模块包括A/D转换器件及其接口电路;或所述A/D转换模块集成在CPU电路中。所述CPU及其外围电路包括CPU芯片及其最小扩展系统、接口电路、输出电路及装置、人机对话模块、控制电路;所述CPU及其外围电路的作用是接受所述A/D转换模块传递来的数字信号,并进行后期处理和必要的输出,同时对系统进行总体控制和人机对话过程。The function of the photosensitive sensor is to perform photoelectric conversion, and the photosensitive sensor adopts one of the following devices: a photosensitive tube and a gallium indium arsenic photosensitive device. The function of the analog detection channel is to convert the output signal of the photosensitive sensor into a voltage signal matched with the A/D conversion module. According to different detection methods adopted, the analog detection channel includes a photosensitive sensor, an I/V conversion circuit, an isolation Direct circuit, analog switch, filter, phase-sensitive detection circuit, anti-aliasing filter and A/D conversion circuit, the center frequency of the filter is set according to the wavelength switching frequency, and each wavelength is determined by the output frequency of the AOTF light wave . After the photosensitive sensor in the analog detection channel performs photoelectric conversion on the signal, the voltage output of a certain amplitude can be achieved through the I/V conversion circuit, and the number of channels of the analog switch and the number of analog detection channels are different from those of the adopted light splitting device. The number of characteristic light wavelengths after splitting is the same; the switching of the analog switch is synchronized with the wavelength switching of the splitting device, so that the photoelectric signals generated by incident light of different wavelengths are respectively transmitted to the corresponding analog detection channels; each analog detection channel has the same The conversion module includes a filtering circuit module and an amplifying circuit module, the output of which is a high-frequency voltage signal corresponding to a certain wavelength of incident light modulated by an analog switch; the phase-sensitive detection circuit detects the signal corresponding to the light intensity, Switch to the A/D conversion circuit through the analog switch, and convert it into a digital signal for post-processing by the CPU. The function of the A/D conversion module is to convert the analog voltage signal into a digital signal and transmit it to the CPU; the A/D conversion module includes an A/D conversion device and an interface circuit thereof; or the A/D The D conversion module is integrated in the CPU circuit. The CPU and its peripheral circuits include a CPU chip and its minimum expansion system, interface circuit, output circuit and device, man-machine dialogue module, and control circuit; the function of the CPU and its peripheral circuits is to accept the A/D conversion module The transmitted digital signal will be post-processed and necessary output, and at the same time, the overall control of the system and the man-machine dialogue process will be carried out.
本发明中,用于组织成份检测的变光路时域分光差分的测量方法包括以下步骤:第一步骤,连接好包括宽带光源、分光装置及其光路、光敏传感器、模拟检测通道、A/D转换模块、CPU及其外围电路所组成的用于组织成份检测的变光路时域分光差分光谱仪;所述宽带光源采用带宽在600~1300nm的可见光乃至近红外光;第二步骤,将被测人体组织以自由状态置于所述光路中;第三步骤,根据光路中所采用的分光器件是AOTF,或滤光镜,或傅立叶分光光谱仪不同,对入射光的处理过程是下述情形之一:当光路中的分光器件采用AOTF时,宽带光源的出射光经第一聚光镜进行聚焦和分光后,成为平行光入射到AOTF晶体,在CPU的控制下,AOTF晶体将平行光分时调制成不同波长的单色光入射到被测组织上,采集所述出射光经第二聚光镜聚焦后输出光束;或当光路中的分光器件采用滤光片时,首先,将宽带光源的出射光经第一聚光镜进行聚焦和分光后,将单色光分时入射到被测人体组织,所述第二聚光镜采集通过被测人体组织的出射光聚焦后输出光束;或当光路中的分光器件采用傅立叶分光光谱仪时,首先,将宽带光源的出射光经第一聚光镜聚焦后入射到被测人体组织,其出射光经过第二聚光镜送入傅立叶分光光谱仪,所述傅立叶分光光谱仪对出射光进行采集、聚焦和分光,并输出多个单色光光束;所述光敏传感器对上述输出的光束进行光电变换;第四步骤,上述变换后的信号进入模拟检测通道,通过I/V变换电路达到一定幅值的电压输出;所述模拟开关的通道数及模拟检测通道的数目,与所采用的经过分光器件分光后的特征光波长数目一致;所述模拟开关的切换与分光器件的波长切换同步动作,由不同波长入射光所产生的光电信号分别传输到相应的模拟检测通道中;每一模拟检测通道具有相同的转换模块包括滤波电路模块和相敏检波电路模块,其输出是对应于某一波长入射光,经过模拟开关调制的高频电压信号;相敏检波电路将光谱信号检出,由此,完成分时采集不同模拟检测通道的输出信号,并通过模拟开关切换到A/D转换电路,转换成数字信号;第五步骤,将上述A/D转换的数字信号送入CPU进行处理,首先,将表征不同波长差分光谱的数据分离,即将源自于分光器件同一波长出射光的信号,组合在一起,形成与该波长相对应的光电信号描记序列。其次,提取每组信号的幅值,从而获得一组光谱;第六步骤,应用电磁挤压装置向被测人体组织施加压力,以改变光路长,然后,重复上述第三、第四和第五步骤过程,即可检测出被测人体组织的另一组吸收光谱;第七步骤,将上述两组光谱相减获得差分光谱;然后,通过化学计量方法,从差分光谱中计算得出被测人体动脉血液中的主要成分的含量。In the present invention, the method for measuring the variable optical path time domain spectroscopic difference for tissue component detection includes the following steps: the first step is to connect the broadband light source, spectroscopic device and its optical path, photosensitive sensor, analog detection channel, and A/D conversion. A variable optical path time-domain spectroscopic difference spectrometer for tissue component detection composed of a module, a CPU and its peripheral circuits; the broadband light source uses visible light or even near-infrared light with a bandwidth of 600-1300nm; the second step is to test human tissue Place it in the optical path in a free state; the third step, according to the optical splitting device adopted in the optical path is AOTF, or optical filter, or Fourier spectrometer is different, the processing process of incident light is one of the following situations: when When the light splitting device in the optical path adopts AOTF, the outgoing light of the broadband light source is focused and split by the first condenser lens, and becomes parallel light and enters the AOTF crystal. Under the control of the CPU, the AOTF crystal time-shares the parallel light into different wavelengths Monochromatic light is incident on the tissue to be measured, and the emitted light is collected and output after being focused by the second condenser lens; or when the spectroscopic device in the optical path uses a filter, firstly, the outgoing light of the broadband light source is processed by the first condenser lens. After focusing and splitting, the monochromatic light is time-divided and incident on the measured human tissue, and the second condenser lens collects the outgoing light passing through the measured human tissue and focuses it to output the light beam; or when the light splitting device in the optical path adopts a Fourier spectrometer, First, the outgoing light of the broadband light source is focused by the first condenser lens and then incident on the human tissue to be measured, and the outgoing light is sent to the Fourier spectrometer through the second condenser lens, and the Fourier spectrometer collects, focuses and splits the outgoing light, and output a plurality of monochromatic light beams; the photosensitive sensor performs photoelectric conversion on the above-mentioned output beams; the fourth step, the above-mentioned converted signal enters the analog detection channel, and reaches a voltage output of a certain amplitude through the I/V conversion circuit; The number of channels of the analog switch and the number of analog detection channels are consistent with the number of characteristic light wavelengths adopted after being split by the optical splitter; The generated photoelectric signals are respectively transmitted to the corresponding analog detection channels; each analog detection channel has the same conversion module including a filter circuit module and a phase-sensitive detection circuit module, and its output corresponds to a certain wavelength of incident light, which is modulated by an analog switch The high-frequency voltage signal; the phase-sensitive detection circuit detects the spectral signal, thereby completing the time-sharing acquisition of the output signals of different analog detection channels, and switching to the A/D conversion circuit through the analog switch, and converting them into digital signals; the fifth The first step is to send the above-mentioned A/D converted digital signal into the CPU for processing. First, separate the data representing the differential spectrum of different wavelengths, that is, combine the signals originating from the outgoing light of the same wavelength of the optical splitting device to form a Corresponding photoelectric signal tracing sequence. Secondly, the amplitude of each group of signals is extracted to obtain a group of spectra; the sixth step is to apply pressure to the measured human tissue with an electromagnetic extrusion device to change the optical path length, and then repeat the third, fourth and fifth above In the process of steps, another group of absorption spectra of the measured human tissue can be detected; the seventh step is to subtract the above two groups of spectra to obtain the difference spectrum; The content of major components in arterial blood.
与现有技术相比,本发明组织成份检测的变光路时域分光差分光谱仪及检测方法的有益效果是:由于本发明的差分光谱仪采用差分检测方法,能够大大削减个体差异影响。因此,其测量准确、操作方便,并可同时测量多种组织成份。Compared with the prior art, the beneficial effect of the variable optical path time-domain spectroscopic difference spectrometer and detection method of the present invention is: since the differential spectrometer of the present invention adopts the differential detection method, the influence of individual differences can be greatly reduced. Therefore, it is accurate in measurement, easy to operate, and can measure multiple tissue components at the same time.
附图说明Description of drawings
图1是本发明差分光谱测量仪器的结构框图;Fig. 1 is the structural block diagram of differential spectrum measuring instrument of the present invention;
图2是本发明差分光谱测量仪器中采用AOTF时的光路示意图;Fig. 2 is the optical path schematic diagram when AOTF is adopted in the differential spectrum measuring instrument of the present invention;
图3是本发明差分光谱测量仪器中采用滤光片时的光路示意图;Fig. 3 is a schematic diagram of the optical path when using a filter in the differential spectrum measuring instrument of the present invention;
图4是本发明差分光谱测量仪器中采用傅立叶分光光谱仪时的光路示意图;Fig. 4 is the optical path schematic diagram when adopting Fourier transform spectrometer in differential spectrum measuring instrument of the present invention;
图5是本发明差分光谱测量仪器的电气框图;Fig. 5 is the electrical block diagram of differential spectrum measuring instrument of the present invention;
图6是用本发明差分光谱测量仪器进行测量时的工作流程图。Fig. 6 is a flow chart of the process of measuring with the differential spectrum measuring instrument of the present invention.
下面是本发明说明书附图中主要附图标记的说明。The following is an explanation of the main reference signs in the drawings of the specification of the present invention.
1——宽带光源 2——分光装置及其光路1——Broadband
3——光敏传感器 4——模拟检测通道3——Photosensitive sensor 4——Analog detection channel
5——A/D转换模块 6——CPU5——A/
7——第一聚光镜 8——AOTF分光器件7——First Condenser 8——AOTF Spectroscopic Device
9——被测人体组织 10——第二聚光镜9——Tested
11——傅立叶分光光谱仪 13——隔直电路11——Fourier transform spectrometer 13——DC blocking circuit
15——滤光片 16——I/V变换电路15——Optical filter 16——I/V conversion circuit
17——模拟开关 19——滤波器17——analog switch 19——filter
21——A/D转换器 22——挤压装置21——A/
25——相敏检波电路 31——抗混叠滤波器25——phase sensitive detection circuit 31——anti-aliasing filter
具体实施方式Detailed ways
下面结合附图对本发明用于组织成份检测的变光路时域分光差分光谱仪及检测方法做进一步详细说明。The variable optical path time-domain spectroscopic difference spectrometer and detection method for tissue component detection of the present invention will be further described in detail below in conjunction with the accompanying drawings.
如图1至图5所示,本发明用于组织成份检测的变光路时域分光差分光谱仪,包括宽带光源1、分光装置及其光路2、光敏传感器3、模拟检测通道4、A/D转换模块5、挤压装置22、CPU6及其外围电路;所述的宽带光源1采用带宽在600~1300nm的可见光乃至近红外光;所述分光装置及其光路2包括分光器件和与其相配套的光路器件;所述相配套的光路器件可以采用聚光镜,还可以采用狭缝或小孔;所述分光器件采用AOTF8,还可以滤光片15或傅立叶分光光谱仪11;若所述分光器采用AOTF8,则光路中包括宽带光源1、第一聚光镜7、AOTF分光器件8、被测人体组织9、第二聚光镜10和光敏传感器3;所述AOTF8的作用是将所述宽带光源1的出射光进行聚焦和分光后入射到被测人体组织9,并采集出射光,聚焦后输出光束;即,所述宽带光源1的出射光经第一聚光镜7成为平行光入射到AOTF晶体,在CPU6的控制下,AOTF晶体将平行光分时调制成不同波长的单色光入射到被测组织9后经第二聚光镜10将被测人体组织9的出射光聚焦,由处于焦点处的光敏传感器3进行光电变换;或若所述分光器采用滤光片15时,则光路包括宽带光源1、第一聚光镜7、滤光片15、被测人体组织9、第二聚光镜10和光敏传感器3;所述滤光片15的作用是将所述宽带光源1的出射光进行聚焦和分光后入射到被测人体组织9,并采集出射光,聚焦后输出光束;即,所述宽带光源1的出射光经第一聚光镜7成为平行光入射到滤光片15,在CPU6的控制下,滤光片15将平行光分时调制成不同波长的单色光入射到被测人体组织9后经第二聚光镜10将被测人体组织9的出射光聚焦,由处于焦点处的光敏传感器3进行光电变换;或若所述分光器采用傅立叶分光光谱仪11,则光路包括宽带光源1、第一聚光镜7、被测人体组织9、第二聚光镜10、傅立叶分光光谱仪11和光敏器件3;所述傅立叶分光光谱仪11的作用是将宽带光源1的出射光聚焦后入射到被测人体组织9,并采集出射光,聚焦和分光后输出多个单色光光束;即,所述宽带光源1的出射光经过第一聚光镜7成为平行光入射到被测人体组织9,出射光经第二聚光镜10送入傅立叶分光光谱仪11,其输出的单色光由光敏传感器3进行光电变换;As shown in Figures 1 to 5, the variable optical path time-domain spectroscopic difference spectrometer for tissue component detection of the present invention includes a broadband light source 1, a spectroscopic device and its optical path 2, a photosensitive sensor 3, an analog detection channel 4, and an A/D conversion Module 5, extrusion device 22, CPU6 and its peripheral circuits; the broadband light source 1 uses visible light or even near-infrared light with a bandwidth of 600-1300nm; the light splitting device and its optical path 2 include a light splitting device and an optical path matching it device; the matching optical path device can adopt condenser lens, can also adopt slit or aperture; described spectroscopic device adopts AOTF8, can also optical filter 15 or Fourier transform spectrometer 11; If described spectroscope adopts AOTF8, then Include broadband light source 1, the first condenser lens 7, AOTF spectroscopic device 8, measured human tissue 9, the second condenser lens 10 and photosensitive sensor 3 in the light path; The effect of described AOTF8 is to focus and After splitting the light, it enters the measured human tissue 9, collects the outgoing light, and outputs the light beam after focusing; that is, the outgoing light of the broadband light source 1 becomes parallel light through the first condenser lens 7 and enters the AOTF crystal. Under the control of the CPU6, the AOTF The crystal time-divisionally modulates the parallel light into monochromatic light of different wavelengths incident on the tissue under
由上述A/D转换后的信号经CPU6处理获得第一组光谱;所述电磁挤压装置22对被测人体组织9进行施压改变光路长以获得第二组光谱;从所述第一和第二组光谱的差分光谱中得到组织成分中的主要成分的含量。The signal after the above-mentioned A/D conversion is processed by the CPU6 to obtain the first group of spectra; the
所述光敏传感器3的作用是进行光电转换,所述光敏传感器3可以采用光敏管,还可以采用砷镓铟光敏器件。The function of the
所述模拟检测通道4的作用是将光敏传感器3的输出信号转换成与A/D转换模块5匹配的电压信号,根据采用的检测方法不同,所述模拟检测通道4包括光敏传感器3、I/V变换电路16、隔直电路13、模拟开关17、滤波器19、相敏检波电路25、抗混叠滤波器31和A/D转换器21,所述滤波器19的中心频率根据波长切换频率设定,取每一波长由AOTF光波的出射频率。The effect of the analog detection channel 4 is to convert the output signal of the
所述模拟检测通道4中光敏传感器3对信号进行光电转换后,通过I/V变换电路16可达到一定幅值的电压输出,而且模拟开关17的通道数及模拟检测通道4的数目,与所采用的经过分光器件分光后的特征光波长数目一致;模拟开关17的切换与分光器件的波长切换同步动作,由此将不同波长入射光产生的光电信号分别传输到相应的模拟检测通道4中;每一模拟检测通道4具有相同的转换模块,包括滤波电路模块、放大电路模块,其输出是对应于某一波长入射光,经过模拟开关17调制的高频电压信号;所述相敏检波电路25将对应于光强的信号检出,通过模拟开关17切换到A/D转换电路21,转换成数字信号,供CPU6进行后期处理。After the
所述A/D转换模块5的作用是将模拟电压信号转换成数字信号,并传递给所述的CPU6;所述A/D转换模块5包括A/D转换器件及其接口电路;或所述A/D转换模块5集成在CPU电路中。The effect of described A/
所述CPU6及其外围电路包括CPU芯片及其最小扩展系统、接口电路、输出电路及装置、人机对话模块、控制电路;所述CPU6及其外围电路的作用是接受所述A/D转换模块5传递来的数字信号,并进行后期处理和必要的输出,同时对系统进行总体控制和人机对话过程。Described CPU6 and its peripheral circuit comprise CPU chip and minimum expansion system thereof, interface circuit, output circuit and device, man-machine dialogue module, control circuit; The effect of described CPU6 and its peripheral circuit is to accept described A/
本发明用于组织成份检测的变光路时域分光差分光谱仪的工作流程如图1和图6所示,由宽带光源1发出所需波长的光,经过第一聚光镜7聚焦102,由分光器件进行时域分光后入射到被测人体组织103,由所述光敏传感器3接收出射光并转换成电信号104,然后,经模拟检测通道4中的电路部分进行转换,即,经模拟开关送入相应的模拟检测通道105,用相应的模拟检测通道实现信号变换106;由A/D转换模块5对各模拟检测通道的光电脉搏波进行A/D转换,将其转换成数字信号107。A/D转换的结果将由以CPU为核心的数据处理系统进行后期处理。即,首先,将表征不同差分脉搏波的数据分离,产生脉搏波描记数列108,如果采用n个特征波长,则第mn+i(i=1,2,……,n;m=0,1,2,……)个数据共同表征第i个特征波长对应的光谱信息;其次,提取每个波形的特征波幅值作为对应于每个入射光波长的光谱幅值,即可获得第一组光谱,在提取光谱幅值时,可根据所获得的数据提取脉搏信号的峰峰值或交流成份的基波分量幅值作为光谱的特征幅值,亦可提取吸收光谱的平均幅值作为光谱的特征幅值109。应用电磁挤压装置22对被测人体组织9进行施压,以改变光路长,然后重复上述过程,以获得第二组光谱110;将上述两组光谱相减,获得差分光谱111;获得差分光谱后,采用化学计量方法从差分光谱中计算得到组织中的主要成份的含量112。The working process of the variable optical path time-domain spectroscopic difference spectrometer used for tissue component detection of the present invention is shown in Figure 1 and Figure 6, the light of the required wavelength is emitted by the
本发明用于组织成份检测的变光路时域分光差分光谱仪的检测方法包括以下步骤:The detection method of the variable optical path time-domain spectroscopic difference spectrometer used for tissue component detection of the present invention comprises the following steps:
第一步骤,连接好包括宽带光源1、分光装置及其光路2、光敏传感器3、模拟检测通道4、A/D转换模块5、CPU及其外围电路6所组成的用于组织成份检测的变光路时域分光差分光谱仪;所述宽带光源1采用带宽在600~1300nm的可见光乃至近红外光;The first step is to connect the variable for tissue component detection composed of
第二步骤,将被测人体组织9以自由状态置于所述光路中;In the second step, placing the measured
第三步骤,根据光路中所采用的分光器件是AOTF,或滤光镜,或傅立叶分光光谱仪不同,对入射光的处理过程是下述情形之一:In the third step, depending on whether the spectroscopic device used in the optical path is an AOTF, or a filter, or a Fourier spectrometer, the processing of the incident light is one of the following situations:
当光路中的分光器件采用AOTF8时,宽带光源1的出射光经第一聚光镜7进行聚焦和分光后,成为平行光入射到AOTF晶体,在CPU6的控制下,AOTF晶体将平行光分时调制成不同波长的单色光入射到被测组织9上,采集所述出射光经第二聚光镜10聚焦后输出光束;或When the light splitting device in the optical path adopts AOTF8, the outgoing light of the
当光路中的分光器件采用滤光片15时,首先,将宽带光源1的出射光经第一聚光镜7进行聚焦和分光后,将单色光分时入射到被测人体组织9,所述第二聚光镜10采集通过被测人体组织9的出射光聚焦后输出光束;或When the spectroscopic device in the optical path adopts the
当光路中的分光器件采用傅立叶分光光谱仪11时,首先,将宽带光源1的出射光经第一聚光镜7聚焦后入射到被测人体组织9,其出射光经过第二聚光镜10送入傅立叶分光光谱仪11,所述傅立叶分光光谱仪11对出射光进行采集、聚焦和分光,并输出多个单色光光束;When the light-splitting device in the optical path adopts the
所述光敏传感器3对上述输出的光束进行光电变换;The
第四步骤,上述变换后的信号进入模拟检测通道4,通过I/V变换电路16达到一定幅值的电压输出;所述模拟开关17的通道数及模拟检测通道4的数目,与所采用的经过分光器件分光后的特征光波长数目一致;所述模拟开关17的切换与分光器件的波长切换同步动作,由不同波长入射光所产生的光电信号分别传输到相应的模拟检测通道4中;每一模拟检测通道4具有相同的转换模块包括滤波电路模块和相敏检波电路模块,其输出是对应于某一波长入射光,经过模拟开关17调制的高频电压信号;相敏检波电路25将光谱信号检出,由此,完成分时采集不同模拟检测通道4的输出信号,并通过模拟开关17切换到A/D转换电路21,转换成数字信号;In the fourth step, the above-mentioned converted signal enters the analog detection channel 4, and reaches a certain amplitude voltage output through the I/V conversion circuit 16; the number of channels of the analog switch 17 and the number of analog detection channels 4 are the same as those used The number of characteristic light wavelengths after the light splitting by the light splitting device is consistent; the switching of the analog switch 17 is synchronized with the wavelength switching of the light splitting device, and the photoelectric signals generated by incident light of different wavelengths are respectively transmitted to the corresponding analog detection channels 4; An analog detection channel 4 has the same conversion module including a filter circuit module and a phase-sensitive detection circuit module, and its output is a high-frequency voltage signal corresponding to a certain wavelength of incident light modulated by an analog switch 17; the phase-sensitive detection circuit 25 converts the spectrum Signal detection, thus, complete the time-sharing acquisition of the output signals of different analog detection channels 4, and switch to the A/D conversion circuit 21 through the analog switch 17, and convert them into digital signals;
第五步骤,将上述A/D转换的数字信号送入CPU6进行处理,首先,将表征不同波长差分光谱的数据分离,即将源自于分光器件同一波长出射光的信号,组合在一起,形成与该波长相对应的光电信号描记序列。其次,提取每组信号的幅值,从而获得一组光谱;In the fifth step, the above-mentioned A/D converted digital signal is sent to the CPU6 for processing. First, the data representing the differential spectrum of different wavelengths is separated, that is, the signals originating from the outgoing light of the same wavelength of the optical splitting device are combined together to form a The photoelectric signal corresponding to this wavelength traces the sequence. Second, extract the amplitude of each set of signals to obtain a set of spectra;
第六步骤,应用电磁挤压装置22向被测人体组织9施加压力,以改变光路长,然后,重复上述第三、第四和第五步骤过程,即可检测出被测人体组织9的另一组吸收光谱;In the sixth step, the
第七步骤,将上述两组光谱相减获得差分光谱;然后,通过化学计量方法,从差分光谱中计算得出被测人体动脉血液中的主要成分的含量。In the seventh step, the above two groups of spectra are subtracted to obtain a differential spectrum; then, the content of the main components in the measured human arterial blood is calculated from the differential spectrum by a stoichiometric method.
尽管上面结合附图对本发明进行了描述,但是本发明并不局限于上述的具体实施方式,上述的具体实施方式仅仅是示意性的,而不是限制性的,本领域的普通技术人员在本发明的启示下,在不脱离本发明宗旨和权利要求所保护的范围情况下,还可以作出很多变形,这些均属于本发明的保护之内。Although the present invention has been described above in conjunction with the accompanying drawings, the present invention is not limited to the above-mentioned specific embodiments, and the above-mentioned specific embodiments are only illustrative, rather than restrictive. Under the enlightenment of the present invention, many modifications can be made without departing from the purpose of the present invention and the scope of protection of the claims, and these all belong to the protection of the present invention.
Claims (7)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CNB2004100193208A CN1299646C (en) | 2004-05-21 | 2004-05-21 | Optical-circuit-variable time-domain light-dividing differential wave length spectrometer for detecting tissue content and detection method thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CNB2004100193208A CN1299646C (en) | 2004-05-21 | 2004-05-21 | Optical-circuit-variable time-domain light-dividing differential wave length spectrometer for detecting tissue content and detection method thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN1579327A CN1579327A (en) | 2005-02-16 |
| CN1299646C true CN1299646C (en) | 2007-02-14 |
Family
ID=34581865
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNB2004100193208A Expired - Fee Related CN1299646C (en) | 2004-05-21 | 2004-05-21 | Optical-circuit-variable time-domain light-dividing differential wave length spectrometer for detecting tissue content and detection method thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN1299646C (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102187200A (en) * | 2008-04-04 | 2011-09-14 | 高露洁-棕榄公司 | Analysis of substrates having agents deposited thereon |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN108593593A (en) * | 2018-04-24 | 2018-09-28 | 深圳市英谱科技有限公司 | Serial double infrared spectrum Woundless blood sugar measuring devices |
| CN108318428A (en) * | 2018-05-16 | 2018-07-24 | 德州尧鼎光电科技有限公司 | A kind of photoelectric sensing measuring device |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998034097A1 (en) * | 1997-01-31 | 1998-08-06 | University College London | Determination of the ratio of absorption coefficients at different wavelengths in a scattering medium |
| WO2000053085A1 (en) * | 1999-03-10 | 2000-09-14 | Optiscan Biomedical Corporation | Solid-state non-invasive absorption spectrometer |
| WO2001060248A1 (en) * | 1999-04-06 | 2001-08-23 | Argose, Inc. | Non-invasive tissue glucose level monitoring |
| CN1384348A (en) * | 2002-06-18 | 2002-12-11 | 天津大学 | Non-wound artirial blood component measuring instrument and method |
-
2004
- 2004-05-21 CN CNB2004100193208A patent/CN1299646C/en not_active Expired - Fee Related
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998034097A1 (en) * | 1997-01-31 | 1998-08-06 | University College London | Determination of the ratio of absorption coefficients at different wavelengths in a scattering medium |
| WO2000053085A1 (en) * | 1999-03-10 | 2000-09-14 | Optiscan Biomedical Corporation | Solid-state non-invasive absorption spectrometer |
| WO2001060248A1 (en) * | 1999-04-06 | 2001-08-23 | Argose, Inc. | Non-invasive tissue glucose level monitoring |
| CN1384348A (en) * | 2002-06-18 | 2002-12-11 | 天津大学 | Non-wound artirial blood component measuring instrument and method |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102187200A (en) * | 2008-04-04 | 2011-09-14 | 高露洁-棕榄公司 | Analysis of substrates having agents deposited thereon |
| US8803095B2 (en) | 2008-04-04 | 2014-08-12 | Colgate-Palmolive Company | Analysis of substrates having agents deposited thereon |
| US8895929B2 (en) | 2008-04-04 | 2014-11-25 | Colgate-Palmolive Company | Analysis of substrates having agents deposited thereon |
Also Published As
| Publication number | Publication date |
|---|---|
| CN1579327A (en) | 2005-02-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN101933809B (en) | Multiband reflection spectrum noninvasive blood component measuring device and method | |
| JPH11506202A (en) | Method for minimizing scatter and improving tissue sampling in non-invasive examination and imaging | |
| CN1022513C (en) | liver function test device | |
| CN103190917B (en) | Laser Raman technique-based glucometer | |
| CN101799412A (en) | Near infrared spectral transmission method and device for non-invasive measurement of blood sugar for human body | |
| CN107242855A (en) | A kind of biological tissue's dynamic modulation spectral measurement device and method | |
| CN204666826U (en) | The continuous safety check imaging device of a kind of Terahertz | |
| CN1299636C (en) | Double-probe differential wavelength spectrometer for detecting tissue content and detection method | |
| CN1215814C (en) | Cerebral blood oxygen saturation detector | |
| CN108634964A (en) | A kind of non-invasive blood sugar instrument based on spectrum | |
| CN1299646C (en) | Optical-circuit-variable time-domain light-dividing differential wave length spectrometer for detecting tissue content and detection method thereof | |
| CN1579321A (en) | Airspace light-diving differential wavelength spectro meter for detecting artery blood content and detection method thereof | |
| Shulei et al. | Non-invasive blood glucose measurement scheme based on near-infrared spectroscopy | |
| CN111803085A (en) | Noninvasive hemoglobin concentration level measuring device based on color characteristics | |
| CN1384348A (en) | Non-wound artirial blood component measuring instrument and method | |
| CN104224198A (en) | Multi-function portable noninvasive medical treatment detection device and method based on Raman scattering | |
| CN1672628A (en) | Dynamic spectrometry instrument with multiple wavelength LED | |
| CN1297232C (en) | Optical-circuit-variable airspace light-dividing differencial wavelength spectometer for detecting tissue content and detection method thereof | |
| CN205215187U (en) | Skin detecting system based on it is multispectral | |
| CN1579322A (en) | Time-domain light-deivisding differential wavelength spectro meter for detecting artery blood content and detection method thereof | |
| CN114886421B (en) | Near infrared-based high-precision noninvasive blood glucose concentration detection system and method | |
| CN208031214U (en) | The measuring device of Non-invasive detection hemoglobin level | |
| CN205913354U (en) | A non-invasive blood glucose detection device | |
| CN108593593A (en) | Serial double infrared spectrum Woundless blood sugar measuring devices | |
| CN114081483A (en) | A Tissue Blood Flow and Blood Oxygen Saturation Measurement Method Based on Deep Learning Diffusion Correlation Spectroscopy |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| C19 | Lapse of patent right due to non-payment of the annual fee | ||
| CF01 | Termination of patent right due to non-payment of annual fee |