CN1252690A - 杀菌剂活性物质组合 - Google Patents
杀菌剂活性物质组合 Download PDFInfo
- Publication number
- CN1252690A CN1252690A CN98804274A CN98804274A CN1252690A CN 1252690 A CN1252690 A CN 1252690A CN 98804274 A CN98804274 A CN 98804274A CN 98804274 A CN98804274 A CN 98804274A CN 1252690 A CN1252690 A CN 1252690A
- Authority
- CN
- China
- Prior art keywords
- active compound
- group
- formula
- weight ratio
- derivatives
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 230000000855 fungicidal effect Effects 0.000 title claims abstract description 18
- 239000013543 active substance Substances 0.000 title abstract description 7
- 239000000417 fungicide Substances 0.000 title abstract description 5
- 150000001875 compounds Chemical class 0.000 claims description 198
- 239000000203 mixture Substances 0.000 claims description 20
- 239000011701 zinc Substances 0.000 claims description 8
- -1 (6-chloro-3-pyridinyl) -methyl Chemical group 0.000 claims description 7
- 241000233866 Fungi Species 0.000 claims description 7
- 229910052725 zinc Inorganic materials 0.000 claims description 7
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 5
- 229940042055 systemic antimycotics triazole derivative Drugs 0.000 claims description 5
- 239000004606 Fillers/Extenders Substances 0.000 claims description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 4
- 239000000460 chlorine Substances 0.000 claims description 4
- 229910052801 chlorine Inorganic materials 0.000 claims description 4
- 125000001309 chloro group Chemical group Cl* 0.000 claims description 4
- DIOQZVSQGTUSAI-UHFFFAOYSA-N decane Chemical compound CCCCCCCCCC DIOQZVSQGTUSAI-UHFFFAOYSA-N 0.000 claims description 4
- 239000001257 hydrogen Substances 0.000 claims description 4
- 229910052739 hydrogen Inorganic materials 0.000 claims description 4
- 229910052748 manganese Inorganic materials 0.000 claims description 4
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 4
- 239000000126 substance Substances 0.000 claims description 4
- 150000002357 guanidines Chemical class 0.000 claims description 3
- 229940083094 guanine derivative acting on arteriolar smooth muscle Drugs 0.000 claims description 3
- FNQJDLTXOVEEFB-UHFFFAOYSA-N 1,2,3-benzothiadiazole Chemical class C1=CC=C2SN=NC2=C1 FNQJDLTXOVEEFB-UHFFFAOYSA-N 0.000 claims description 2
- LUBJCRLGQSPQNN-UHFFFAOYSA-N 1-Phenylurea Chemical class NC(=O)NC1=CC=CC=C1 LUBJCRLGQSPQNN-UHFFFAOYSA-N 0.000 claims description 2
- 239000005823 Propineb Substances 0.000 claims description 2
- GNVMUORYQLCPJZ-UHFFFAOYSA-M Thiocarbamate Chemical compound NC([S-])=O GNVMUORYQLCPJZ-UHFFFAOYSA-M 0.000 claims description 2
- 150000003936 benzamides Chemical class 0.000 claims description 2
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 claims description 2
- 150000002780 morpholines Chemical class 0.000 claims description 2
- 150000003018 phosphorus compounds Chemical class 0.000 claims description 2
- XKJCHHZQLQNZHY-UHFFFAOYSA-N phthalimide Chemical class C1=CC=C2C(=O)NC(=O)C2=C1 XKJCHHZQLQNZHY-UHFFFAOYSA-N 0.000 claims description 2
- KKMLIVYBGSAJPM-UHFFFAOYSA-L propineb Chemical compound [Zn+2].[S-]C(=S)NC(C)CNC([S-])=S KKMLIVYBGSAJPM-UHFFFAOYSA-L 0.000 claims description 2
- IJIHYLHFNAWUGR-UHFFFAOYSA-L propylene 1,2-bis(dithiocarbamate) Chemical compound [S-]C(=S)NC(C)CNC([S-])=S IJIHYLHFNAWUGR-UHFFFAOYSA-L 0.000 claims description 2
- 229940083082 pyrimidine derivative acting on arteriolar smooth muscle Drugs 0.000 claims description 2
- 150000003230 pyrimidines Chemical class 0.000 claims description 2
- 125000003003 spiro group Chemical group 0.000 claims description 2
- 239000004094 surface-active agent Substances 0.000 claims description 2
- 125000002490 anilino group Chemical class [H]N(*)C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 claims 2
- 238000004519 manufacturing process Methods 0.000 claims 1
- 238000000034 method Methods 0.000 claims 1
- 150000008060 phenylpyrroles Chemical class 0.000 claims 1
- MNHVNIJQQRJYDH-UHFFFAOYSA-N 2-[2-(1-chlorocyclopropyl)-3-(2-chlorophenyl)-2-hydroxypropyl]-1,2-dihydro-1,2,4-triazole-3-thione Chemical compound N1=CNC(=S)N1CC(C1(Cl)CC1)(O)CC1=CC=CC=C1Cl MNHVNIJQQRJYDH-UHFFFAOYSA-N 0.000 abstract description 3
- 241000221785 Erysiphales Species 0.000 description 22
- 241000209140 Triticum Species 0.000 description 20
- 235000021307 Triticum Nutrition 0.000 description 20
- 239000003995 emulsifying agent Substances 0.000 description 19
- 239000002904 solvent Substances 0.000 description 18
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 18
- 230000000694 effects Effects 0.000 description 17
- 230000001681 protective effect Effects 0.000 description 16
- 241000196324 Embryophyta Species 0.000 description 15
- 208000015181 infectious disease Diseases 0.000 description 11
- 238000011081 inoculation Methods 0.000 description 10
- 238000002360 preparation method Methods 0.000 description 10
- 125000002877 alkyl aryl group Chemical group 0.000 description 9
- 239000002552 dosage form Substances 0.000 description 9
- 229920000151 polyglycol Polymers 0.000 description 9
- 239000010695 polyglycol Substances 0.000 description 9
- HIXDQWDOVZUNNA-UHFFFAOYSA-N 2-(3,4-dimethoxyphenyl)-5-hydroxy-7-methoxychromen-4-one Chemical compound C=1C(OC)=CC(O)=C(C(C=2)=O)C=1OC=2C1=CC=C(OC)C(OC)=C1 HIXDQWDOVZUNNA-UHFFFAOYSA-N 0.000 description 8
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 8
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 7
- 239000000843 powder Substances 0.000 description 7
- 230000001225 therapeutic effect Effects 0.000 description 7
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 6
- 239000003905 agrochemical Substances 0.000 description 6
- 238000009472 formulation Methods 0.000 description 6
- 239000008187 granular material Substances 0.000 description 6
- 229910052500 inorganic mineral Inorganic materials 0.000 description 6
- 239000000725 suspension Substances 0.000 description 6
- 230000012010 growth Effects 0.000 description 5
- 239000007788 liquid Substances 0.000 description 5
- 239000011707 mineral Substances 0.000 description 5
- 239000000575 pesticide Substances 0.000 description 5
- 230000002195 synergetic effect Effects 0.000 description 5
- 240000005979 Hordeum vulgare Species 0.000 description 4
- 235000007340 Hordeum vulgare Nutrition 0.000 description 4
- 150000001448 anilines Chemical class 0.000 description 4
- 239000000969 carrier Substances 0.000 description 4
- 201000010099 disease Diseases 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- 239000002689 soil Substances 0.000 description 4
- 239000007921 spray Substances 0.000 description 4
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 3
- UHPMCKVQTMMPCG-UHFFFAOYSA-N 5,8-dihydroxy-2-methoxy-6-methyl-7-(2-oxopropyl)naphthalene-1,4-dione Chemical compound CC1=C(CC(C)=O)C(O)=C2C(=O)C(OC)=CC(=O)C2=C1O UHPMCKVQTMMPCG-UHFFFAOYSA-N 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- 240000008067 Cucumis sativus Species 0.000 description 3
- 235000010799 Cucumis sativus var sativus Nutrition 0.000 description 3
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- 241000223218 Fusarium Species 0.000 description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 3
- 241000221300 Puccinia Species 0.000 description 3
- 241000813090 Rhizoctonia solani Species 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 235000013339 cereals Nutrition 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 239000000975 dye Substances 0.000 description 3
- 239000000839 emulsion Substances 0.000 description 3
- 239000006260 foam Substances 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 239000011572 manganese Substances 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 238000009331 sowing Methods 0.000 description 3
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 229920000742 Cotton Polymers 0.000 description 2
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical class [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 2
- 241001459558 Monographella nivalis Species 0.000 description 2
- 241000131448 Mycosphaerella Species 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- 241000736122 Parastagonospora nodorum Species 0.000 description 2
- 241000317981 Podosphaera fuliginea Species 0.000 description 2
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 2
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 2
- 241001123569 Puccinia recondita Species 0.000 description 2
- 235000019714 Triticale Nutrition 0.000 description 2
- 241000317942 Venturia <ichneumonid wasp> Species 0.000 description 2
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 description 2
- FPIPGXGPPPQFEQ-OVSJKPMPSA-N all-trans-retinol Chemical compound OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-OVSJKPMPSA-N 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 150000002170 ethers Chemical class 0.000 description 2
- 239000007789 gas Substances 0.000 description 2
- 150000002576 ketones Chemical class 0.000 description 2
- 125000003854 p-chlorophenyl group Chemical group [H]C1=C([H])C(*)=C([H])C([H])=C1Cl 0.000 description 2
- 244000052769 pathogen Species 0.000 description 2
- 230000003032 phytopathogenic effect Effects 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 241000228158 x Triticosecale Species 0.000 description 2
- WURBVZBTWMNKQT-UHFFFAOYSA-N 1-(4-chlorophenoxy)-3,3-dimethyl-1-(1,2,4-triazol-1-yl)butan-2-one Chemical compound C1=NC=NN1C(C(=O)C(C)(C)C)OC1=CC=C(Cl)C=C1 WURBVZBTWMNKQT-UHFFFAOYSA-N 0.000 description 1
- VGPIBGGRCVEHQZ-UHFFFAOYSA-N 1-(biphenyl-4-yloxy)-3,3-dimethyl-1-(1,2,4-triazol-1-yl)butan-2-ol Chemical compound C1=NC=NN1C(C(O)C(C)(C)C)OC(C=C1)=CC=C1C1=CC=CC=C1 VGPIBGGRCVEHQZ-UHFFFAOYSA-N 0.000 description 1
- 244000215068 Acacia senegal Species 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- RGCKGOZRHPZPFP-UHFFFAOYSA-N Alizarin Natural products C1=CC=C2C(=O)C3=C(O)C(O)=CC=C3C(=O)C2=C1 RGCKGOZRHPZPFP-UHFFFAOYSA-N 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 241000235349 Ascomycota Species 0.000 description 1
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 1
- 241000221198 Basidiomycota Species 0.000 description 1
- 229940123208 Biguanide Drugs 0.000 description 1
- 241001480061 Blumeria graminis Species 0.000 description 1
- 241000895523 Blumeria graminis f. sp. hordei Species 0.000 description 1
- 241000895502 Blumeria graminis f. sp. tritici Species 0.000 description 1
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- 241001465180 Botrytis Species 0.000 description 1
- 229910021532 Calcite Inorganic materials 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 241000760356 Chytridiomycetes Species 0.000 description 1
- 244000060011 Cocos nucifera Species 0.000 description 1
- 235000013162 Cocos nucifera Nutrition 0.000 description 1
- 229940126062 Compound A Drugs 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- 239000005758 Cyprodinil Substances 0.000 description 1
- 241000221787 Erysiphe Species 0.000 description 1
- 241000223194 Fusarium culmorum Species 0.000 description 1
- 229920000084 Gum arabic Polymers 0.000 description 1
- NLDMNSXOCDLTTB-UHFFFAOYSA-N Heterophylliin A Natural products O1C2COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC2C(OC(=O)C=2C=C(O)C(O)=C(O)C=2)C(O)C1OC(=O)C1=CC(O)=C(O)C(O)=C1 NLDMNSXOCDLTTB-UHFFFAOYSA-N 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- 206010061217 Infestation Diseases 0.000 description 1
- 239000005909 Kieselgur Substances 0.000 description 1
- 241001447252 Leptospora Species 0.000 description 1
- 239000005802 Mancozeb Substances 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- NTIZESTWPVYFNL-UHFFFAOYSA-N Methyl isobutyl ketone Chemical compound CC(C)CC(C)=O NTIZESTWPVYFNL-UHFFFAOYSA-N 0.000 description 1
- UIHCLUNTQKBZGK-UHFFFAOYSA-N Methyl isobutyl ketone Natural products CCC(C)C(C)=O UIHCLUNTQKBZGK-UHFFFAOYSA-N 0.000 description 1
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 1
- 241000228347 Monascus <ascomycete fungus> Species 0.000 description 1
- 244000061176 Nicotiana tabacum Species 0.000 description 1
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- 241000233654 Oomycetes Species 0.000 description 1
- 241000233679 Peronosporaceae Species 0.000 description 1
- 241001503460 Plasmodiophorida Species 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- 239000005828 Pyrimethanil Substances 0.000 description 1
- 206010039509 Scab Diseases 0.000 description 1
- 239000004113 Sepiolite Substances 0.000 description 1
- 241000579741 Sphaerotheca <fungi> Species 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- 239000005864 Sulphur Substances 0.000 description 1
- 241001061127 Thione Species 0.000 description 1
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 1
- 241000228452 Venturia inaequalis Species 0.000 description 1
- BZHJMEDXRYGGRV-UHFFFAOYSA-N Vinyl chloride Chemical class ClC=C BZHJMEDXRYGGRV-UHFFFAOYSA-N 0.000 description 1
- 241000607479 Yersinia pestis Species 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- 230000000895 acaricidal effect Effects 0.000 description 1
- 239000000642 acaricide Substances 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- HFVAFDPGUJEFBQ-UHFFFAOYSA-M alizarin red S Chemical compound [Na+].O=C1C2=CC=CC=C2C(=O)C2=C1C=C(S([O-])(=O)=O)C(O)=C2O HFVAFDPGUJEFBQ-UHFFFAOYSA-M 0.000 description 1
- 150000008055 alkyl aryl sulfonates Chemical class 0.000 description 1
- 150000008051 alkyl sulfates Chemical class 0.000 description 1
- 229940045714 alkyl sulfonate alkylating agent Drugs 0.000 description 1
- 150000008052 alkyl sulfonates Chemical class 0.000 description 1
- 239000011717 all-trans-retinol Substances 0.000 description 1
- 235000019169 all-trans-retinol Nutrition 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 150000001491 aromatic compounds Chemical class 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 229960000892 attapulgite Drugs 0.000 description 1
- 239000000987 azo dye Substances 0.000 description 1
- 229910052796 boron Inorganic materials 0.000 description 1
- 238000009395 breeding Methods 0.000 description 1
- 230000001488 breeding effect Effects 0.000 description 1
- 239000001273 butane Substances 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 150000008422 chlorobenzenes Chemical class 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 239000008199 coating composition Substances 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 239000006184 cosolvent Substances 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- HAORKNGNJCEJBX-UHFFFAOYSA-N cyprodinil Chemical compound N=1C(C)=CC(C2CC2)=NC=1NC1=CC=CC=C1 HAORKNGNJCEJBX-UHFFFAOYSA-N 0.000 description 1
- GUJOJGAPFQRJSV-UHFFFAOYSA-N dialuminum;dioxosilane;oxygen(2-);hydrate Chemical compound O.[O-2].[O-2].[O-2].[Al+3].[Al+3].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O GUJOJGAPFQRJSV-UHFFFAOYSA-N 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 239000010459 dolomite Substances 0.000 description 1
- 229910000514 dolomite Inorganic materials 0.000 description 1
- 239000004495 emulsifiable concentrate Substances 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- FKLFBQCQQYDUAM-UHFFFAOYSA-N fenpiclonil Chemical compound ClC1=CC=CC(C=2C(=CNC=2)C#N)=C1Cl FKLFBQCQQYDUAM-UHFFFAOYSA-N 0.000 description 1
- 239000003337 fertilizer Substances 0.000 description 1
- MUJOIMFVNIBMKC-UHFFFAOYSA-N fludioxonil Chemical compound C=12OC(F)(F)OC2=CC=CC=1C1=CNC=C1C#N MUJOIMFVNIBMKC-UHFFFAOYSA-N 0.000 description 1
- 150000008282 halocarbons Chemical class 0.000 description 1
- 239000004009 herbicide Substances 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- YWTYJOPNNQFBPC-UHFFFAOYSA-N imidacloprid Chemical class [O-][N+](=O)\N=C1/NCCN1CC1=CC=C(Cl)N=C1 YWTYJOPNNQFBPC-UHFFFAOYSA-N 0.000 description 1
- RONFGUROBZGJKP-UHFFFAOYSA-N iminoctadine Chemical compound NC(N)=NCCCCCCCCNCCCCCCCCN=C(N)N RONFGUROBZGJKP-UHFFFAOYSA-N 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000001023 inorganic pigment Substances 0.000 description 1
- 239000002917 insecticide Substances 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- DCYOBGZUOMKFPA-UHFFFAOYSA-N iron(2+);iron(3+);octadecacyanide Chemical compound [Fe+2].[Fe+2].[Fe+2].[Fe+3].[Fe+3].[Fe+3].[Fe+3].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-] DCYOBGZUOMKFPA-UHFFFAOYSA-N 0.000 description 1
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229920005610 lignin Polymers 0.000 description 1
- YKSNLCVSTHTHJA-UHFFFAOYSA-L maneb Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S YKSNLCVSTHTHJA-UHFFFAOYSA-L 0.000 description 1
- 229920000940 maneb Polymers 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical class [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 239000004579 marble Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 239000011785 micronutrient Substances 0.000 description 1
- 235000013369 micronutrients Nutrition 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 229910052750 molybdenum Inorganic materials 0.000 description 1
- 239000011733 molybdenum Substances 0.000 description 1
- 229910052901 montmorillonite Inorganic materials 0.000 description 1
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 1
- OFBQJSOFQDEBGM-UHFFFAOYSA-N n-pentane Natural products CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 1
- 229940042880 natural phospholipid Drugs 0.000 description 1
- 229920005615 natural polymer Polymers 0.000 description 1
- JCXJVPUVTGWSNB-UHFFFAOYSA-N nitrogen dioxide Inorganic materials O=[N]=O JCXJVPUVTGWSNB-UHFFFAOYSA-N 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 238000010422 painting Methods 0.000 description 1
- 229910052625 palygorskite Inorganic materials 0.000 description 1
- 239000006072 paste Substances 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 239000001007 phthalocyanine dye Substances 0.000 description 1
- 239000005648 plant growth regulator Substances 0.000 description 1
- 239000002798 polar solvent Substances 0.000 description 1
- 239000011118 polyvinyl acetate Substances 0.000 description 1
- 229920002689 polyvinyl acetate Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 239000001294 propane Substances 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 229960003351 prussian blue Drugs 0.000 description 1
- 239000013225 prussian blue Substances 0.000 description 1
- 239000008262 pumice Substances 0.000 description 1
- ZLIBICFPKPWGIZ-UHFFFAOYSA-N pyrimethanil Chemical compound CC1=CC(C)=NC(NC=2C=CC=CC=2)=N1 ZLIBICFPKPWGIZ-UHFFFAOYSA-N 0.000 description 1
- 125000000168 pyrrolyl group Chemical group 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- 239000011435 rock Substances 0.000 description 1
- 229910052624 sepiolite Inorganic materials 0.000 description 1
- 235000019355 sepiolite Nutrition 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 1
- BAZVSMNPJJMILC-UHFFFAOYSA-N triadimenol Chemical compound C1=NC=NN1C(C(O)C(C)(C)C)OC1=CC=C(Cl)C=C1 BAZVSMNPJJMILC-UHFFFAOYSA-N 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 238000009369 viticulture Methods 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N37/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids
- A01N37/18—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids containing the group —CO—N<, e.g. carboxylic acid amides or imides; Thio analogues thereof
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N37/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids
- A01N37/18—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids containing the group —CO—N<, e.g. carboxylic acid amides or imides; Thio analogues thereof
- A01N37/20—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids containing the group —CO—N<, e.g. carboxylic acid amides or imides; Thio analogues thereof containing the group, wherein Cn means a carbon skeleton not containing a ring; Thio analogues thereof
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N37/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids
- A01N37/18—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids containing the group —CO—N<, e.g. carboxylic acid amides or imides; Thio analogues thereof
- A01N37/22—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids containing the group —CO—N<, e.g. carboxylic acid amides or imides; Thio analogues thereof the nitrogen atom being directly attached to an aromatic ring system, e.g. anilides
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N37/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids
- A01N37/18—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids containing the group —CO—N<, e.g. carboxylic acid amides or imides; Thio analogues thereof
- A01N37/22—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids containing the group —CO—N<, e.g. carboxylic acid amides or imides; Thio analogues thereof the nitrogen atom being directly attached to an aromatic ring system, e.g. anilides
- A01N37/24—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids containing the group —CO—N<, e.g. carboxylic acid amides or imides; Thio analogues thereof the nitrogen atom being directly attached to an aromatic ring system, e.g. anilides containing at least one oxygen or sulfur atom being directly attached to the same aromatic ring system
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N37/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids
- A01N37/34—Nitriles
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N37/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids
- A01N37/36—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids containing at least one carboxylic group or a thio analogue, or a derivative thereof, and a singly bound oxygen or sulfur atom attached to the same carbon skeleton, this oxygen or sulfur atom not being a member of a carboxylic group or of a thio analogue, or of a derivative thereof, e.g. hydroxy-carboxylic acids
- A01N37/38—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids containing at least one carboxylic group or a thio analogue, or a derivative thereof, and a singly bound oxygen or sulfur atom attached to the same carbon skeleton, this oxygen or sulfur atom not being a member of a carboxylic group or of a thio analogue, or of a derivative thereof, e.g. hydroxy-carboxylic acids having at least one oxygen or sulfur atom attached to an aromatic ring system
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N37/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids
- A01N37/44—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids containing at least one carboxylic group or a thio analogue, or a derivative thereof, and a nitrogen atom attached to the same carbon skeleton by a single or double bond, this nitrogen atom not being a member of a derivative or of a thio analogue of a carboxylic group, e.g. amino-carboxylic acids
- A01N37/50—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids containing at least one carboxylic group or a thio analogue, or a derivative thereof, and a nitrogen atom attached to the same carbon skeleton by a single or double bond, this nitrogen atom not being a member of a derivative or of a thio analogue of a carboxylic group, e.g. amino-carboxylic acids the nitrogen atom being doubly bound to the carbon skeleton
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/24—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with two or more hetero atoms
- A01N43/26—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with two or more hetero atoms five-membered rings
- A01N43/28—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with two or more hetero atoms five-membered rings with two hetero atoms in positions 1,3
- A01N43/30—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with two or more hetero atoms five-membered rings with two hetero atoms in positions 1,3 with two oxygen atoms in positions 1,3, condensed with a carbocyclic ring
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/34—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom
- A01N43/36—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom five-membered rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/34—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom
- A01N43/36—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom five-membered rings
- A01N43/38—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom five-membered rings condensed with carbocyclic rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/54—1,3-Diazines; Hydrogenated 1,3-diazines
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/64—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with three nitrogen atoms as the only ring hetero atoms
- A01N43/647—Triazoles; Hydrogenated triazoles
- A01N43/653—1,2,4-Triazoles; Hydrogenated 1,2,4-triazoles
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
- A01N43/82—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms five-membered rings with three ring hetero atoms
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
- A01N43/84—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms six-membered rings with one nitrogen atom and either one oxygen atom or one sulfur atom in positions 1,4
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
- A01N43/88—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms six-membered rings with three ring hetero atoms
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N47/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid
- A01N47/02—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid the carbon atom having no bond to a nitrogen atom
- A01N47/04—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid the carbon atom having no bond to a nitrogen atom containing >N—S—C≡(Hal)3 groups
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N47/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid
- A01N47/08—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid the carbon atom having one or more single bonds to nitrogen atoms
- A01N47/10—Carbamic acid derivatives, i.e. containing the group —O—CO—N<; Thio analogues thereof
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N47/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid
- A01N47/08—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid the carbon atom having one or more single bonds to nitrogen atoms
- A01N47/10—Carbamic acid derivatives, i.e. containing the group —O—CO—N<; Thio analogues thereof
- A01N47/12—Carbamic acid derivatives, i.e. containing the group —O—CO—N<; Thio analogues thereof containing a —O—CO—N< group, or a thio analogue thereof, neither directly attached to a ring nor the nitrogen atom being a member of a heterocyclic ring
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N47/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid
- A01N47/08—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid the carbon atom having one or more single bonds to nitrogen atoms
- A01N47/10—Carbamic acid derivatives, i.e. containing the group —O—CO—N<; Thio analogues thereof
- A01N47/12—Carbamic acid derivatives, i.e. containing the group —O—CO—N<; Thio analogues thereof containing a —O—CO—N< group, or a thio analogue thereof, neither directly attached to a ring nor the nitrogen atom being a member of a heterocyclic ring
- A01N47/14—Di-thio analogues thereof
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N47/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid
- A01N47/08—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid the carbon atom having one or more single bonds to nitrogen atoms
- A01N47/28—Ureas or thioureas containing the groups >N—CO—N< or >N—CS—N<
- A01N47/32—Ureas or thioureas containing the groups >N—CO—N< or >N—CS—N< containing >N—CO—N< or >N—CS—N< groups directly attached to a cycloaliphatic ring
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N51/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds having the sequences of atoms O—N—S, X—O—S, N—N—S, O—N—N or O-halogen, regardless of the number of bonds each atom has and with no atom of these sequences forming part of a heterocyclic ring
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N53/00—Biocides, pest repellants or attractants, or plant growth regulators containing cyclopropane carboxylic acids or derivatives thereof
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N55/00—Biocides, pest repellants or attractants, or plant growth regulators, containing organic compounds containing elements other than carbon, hydrogen, halogen, oxygen, nitrogen and sulfur
- A01N55/02—Biocides, pest repellants or attractants, or plant growth regulators, containing organic compounds containing elements other than carbon, hydrogen, halogen, oxygen, nitrogen and sulfur containing metal atoms
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N57/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic phosphorus compounds
- A01N57/10—Biocides, pest repellants or attractants, or plant growth regulators containing organic phosphorus compounds having phosphorus-to-oxygen bonds or phosphorus-to-sulfur bonds
- A01N57/12—Biocides, pest repellants or attractants, or plant growth regulators containing organic phosphorus compounds having phosphorus-to-oxygen bonds or phosphorus-to-sulfur bonds containing acyclic or cycloaliphatic radicals
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Agronomy & Crop Science (AREA)
- Pest Control & Pesticides (AREA)
- Plant Pathology (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Dentistry (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
本发明公开了式(Ⅰ)的2-[2-(1-氯代环丙基)-3-(2-氯代苯基)-2-羟基丙基]-2,4-二氢-[1,2,4]-三唑-3-硫酮和本说明书中所列的活性物质组(1)到(24)的新的活性物质组合,它们具有很好的杀真菌性能。
Description
本发明涉及新颖的杀菌活性化合物组合,包括已知的2-[2-(1-氯代环丙基)-3-(2-氯代苯基)-2-羟基丙基]-2,4-二氢-[1,2,4]-三唑-3-硫酮和其它已知的杀菌活性化合物,它们很适合于防治植物病原真菌。
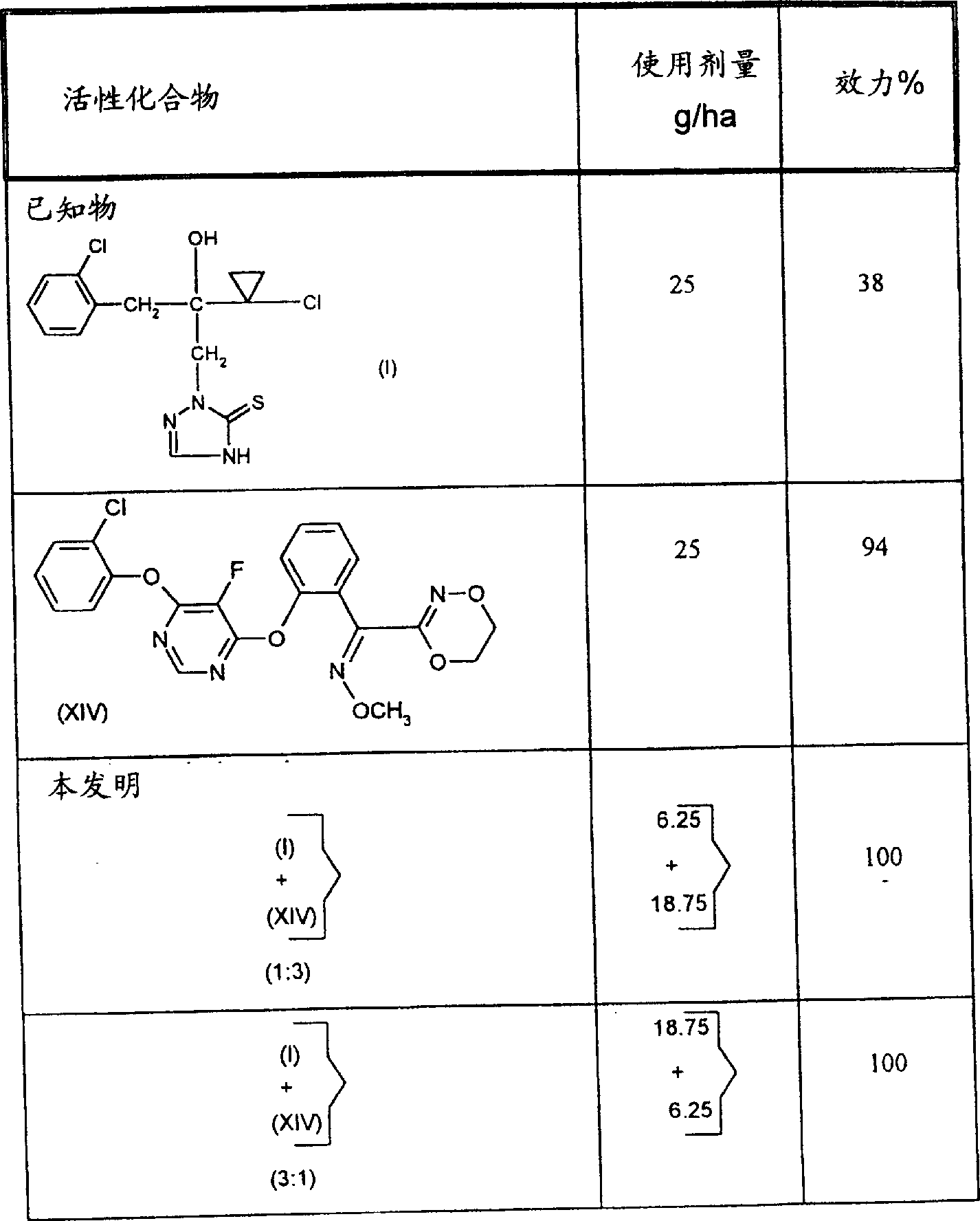
文献中已描述过2-[2-(1-氯代环丙基)-3-(2-氯代苯基)-2-羟基丙基]-2,4-二氢-[1,2,4]-三唑-3-硫酮有较好的杀真菌性能(参见,WO96-16048),但在低使用剂量时,在某些情况下,此化合物的活性并不理想。
此外,据文献报道许多三唑衍生物、苯胺衍生物、二羰基亚胺和其它杂环可用于防治真菌(EP-A 0 040 345,DE-A 2 201 063,DE-A 2 324010,《农药手册》[Pesticide Manual],第9版(1991),第249和827页,US-A 3 903 090和EP-A 0 206 999),在低使用剂量时,这些化合物的活性同样不能令人满意。
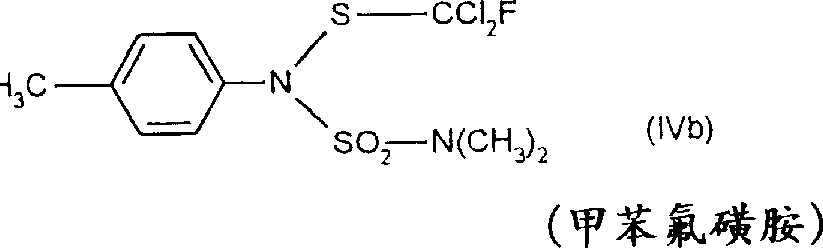
还已知1-[(6-氯-3-吡啶基)-甲基]-N-硝基-2-咪唑烷亚胺可用于动物害虫如昆虫的防治(参见,《农药手册》,第9版(1991),第491页),但迄今未见有关此化合物杀真菌性能的描述。
现已发现,本发明的新颖的活性化合物组合具有非常好的杀真菌性能,它们包括:
式(I)的2-[2-(1-氯代环丙基)-3-(2-氯代苯基)-2-羟基丙基]-2,4-二氢-[1,2,4]-三唑-3-硫酮
和
(1)式(II)的三唑衍生物
和/或
(2)式(III)的三唑衍生物
和/或
其中,R1代表氢或甲基,
和/或
(4)式(V)的N-[1-(4-氯-苯基)-乙基]-2,2-二氯-1-乙基-3-甲基环丙烷-甲酰胺
和/或
(5)式(VI)的亚丙基-1,2-双-(二硫代氨基甲酸)锌
N>=1 (丙森锌)
和/或
(6)式(VII)的至少一种硫代氨基甲酸酯
Me=Zn或Mn,或Zn和Mn的混合物
和/或
(7)式(VIII)的苯胺衍生物
和/或
和/或
和/或
(10)式(XI)的8-叔丁基-2-(N-乙基-N-正丙基-氨基)-甲基-1,4-二氧杂螺[5,4]癸烷
和/或
和/或
和/或
和/或
和/或
其中,R2代表甲基或环丙基
和/或
(16)式(XVII)的苯基衍生物
和/或
和/或
和/或
和/或
其中,R3和R4分别代表氯或一起代表基团-O-CF2-O-
和/或
(21)式(XXII)的1-[(6-氯-3-吡啶基)-甲基]-N-硝基-2-咪唑烷亚胺
和/或
(22)式(XXIII)的苯基脲衍生物
和/或
(23)式(XXIV)的苯甲酰胺衍生物
和/或
其中,m代表整数0到5
R5代表氢(17%到23%)或基团
出人意外的是,本发明活性化合物组合的杀真菌活性比单个活性化合物的活性之和要高得多,因此,其中存在一种不可预知的、真正的协同作用而非活性的简单加和。
式(I)的2-[2-(1-氯代环丙基)-3-(2-氯代苯基)-2-羟基丙基]-2,4-二氢-[1,2,4]-三唑-3-硫酮为已知的(参见,WO 96-16 048),该化合物可以以下式的“硫酮”式存在
或以下式的互变异构体“硫醇”式存在
为简单起见,在本发明中仅给出“硫酮”式。
式(II)包括化合物:
和
1-(4-苯基-苯氧基)-3,3-二甲基-1-(1,2,4-三唑-1-基)-丁烷-2-醇
式(V)的活性化合物很明显有三个不对称取代碳原子,因此产品可能以各种异构体的混合物存在,或以单一异构成分存在。特别优选的化合物有:
和
N-(R)-[1-(4-氯-苯基)-乙基]-(1R)-2,2-二氯-1-乙基-3t-甲基-1r-环丙烷甲酰胺
式(VII)包括化合物:
(VIIa) Me=Zn (代森锌)
(VIIb) Me=Mn (代森锰)
和
(VIIc) (VIIa)和(VIIb)的混合物 (代森锰锌)
式(XVI)包括化合物:
(XVIa) R2=CH3 (嘧霉胺)
和
(XVIb) R2= (嘧菌环胺)
式(XXI)包括化合物:
和
4-(2,2-二氟-1,3-苯并二氧戊环-7-基)-1氢-吡咯-3-腈
式(XXV)的胍衍生物是通用名为双胍辛乙酸盐(guazatine)的混合物。
除了式(I)的活性化合物,存在于本发明的组合中的其它成分也均为已知的。具体地说,它们在下列文献中有描述:
(1) 式(II)的化合物
DE-A 2 201 063
DE-A 2 324 010
(2) 式(III)的化合物
EP-A 0 040 345
(3) 式(IV)的化合物
《农药手册》,第9版(1991),第249和827页
(4) 式(V)的化合物和其单个异构体
EP-A 0 341 475
(5) 式(VI)的化合物
《农药手册》,第9版(1991),第726页
(6) 式(VII)的化合物
《农药手册》,第9版(1991),第529、531和866页
(7) 式(VIII)的化合物
EP-A 0 339 418
(8) 式(IX)的化合物
EP-A 0 472 996
(9) 式(X)的化合物
EP-A 0 313 512
(10) 式(XI)的化合物
EP-A 0 281 842
(11) 式(XII)的化合物
EP-A 0 382 375
(12) 式(XIII)的化合物
EP-A 0 515 901
(13) 式(XIV)的化合物
EP-A196 02 095
(14) 式(XV)的化合物
US-A 3 903 090
(15) 式(XVI)的化合物
EP-A 0 270 111
EP-A 0 310 550
(16) 式(XVII)的化合物
《农药手册》,第9版(1991),第159页
(17) 式(XVIII)的化合物
EP-A 0 219 756
(18) 式(XIX)的化合物
《农药手册》,第9版(1991),第431页
(19) 式(XX)的化合物
《农药手册》,第9版(1991),第443页
(20) 式(XXI)的化合物
EP-A 0 236 272
EP-A 0 206 999
(21) 式(XXII)的化合物
《农药手册》,第9版(1991),第491页
(22) 式(XXIII)的化合物
DE-A 2 732 257
(23) 式(XXIV)的化合物
EP-A 0 600 629
(24) 式(XXII)的物质
《农药手册》,第9版(1991),第461页
除式(I)的活性化合物外,本发明的组合还包含(1)到(24)化合物组中的至少一个活性化合物。另外,可能还包含更多的杀真菌活性成分。
本发明的活性化合物组合中的活性化合物以某种重量比存在时,协同作用尤其明显。然而,活性化合物组合中的活性化合物的重量比有一相对宽的变化范围。一般而言,相对于每份重量的式(I)活性化合物,存在:
0.1至20份重量,优选0.2至10份重量的组(1)活性化合物,
0.1至20份重量,优选0.2至10份重量的组(2)活性化合物,
0.2至150份重量,优选1至100份重量的组(3)活性化合物,
0.1至10份重量,优选0.2至5份重量的组(4)活性化合物,
1至50份重量,优选5至20份重量的组(5)活性化合物,
1至50份重量,优选2至20份重量的组(6)活性化合物,
0.1至50份重量,优选1至30份重量的组(7)活性化合物,
0.2至50份重量,优选1至20份重量的组(8)活性化合物,
0.02至50份重量,优选0.2至10份重量的组(9)活性化合物,
0.1至50份重量,优选0.2至20份重量的组(10)活性化合物,
0.1至50份重量,优选0.2至20份重量的组(11)活性化合物,
0.1至50份重量,优选0.2至20份重量的组(12)活性化合物,
0.1至50份重量,优选0.2至20份重量的组(13)活性化合物,
0.1至50份重量,优选1至30份重量的组(14)活性化合物,
0.1至50份重量,优选0.2至20份重量的组(15)活性化合物,
0.1至50份重量,优选2至20份重量的组(16)活性化合物,
1至20份重量,优选2至10份重量的组(17)活性化合物,
1至50份重量,优选2至20份重量的组(18)活性化合物,
1至50份重量,优选2至20份重量的组(19)活性化合物,
0.1至10份重量,优选0.2至5份重量的组(20)活性化合物,
0.05至20份重量,优选0.1至10份重量的组(21)活性化合物,
0.1至10份重量,优选0.2至5份重量的组(22)活性化合物,
0.1至10份重量,优选0.2至5份重量的组(23)活性化合物,
和/或
0.1至10份重量,优选0.2至5份重量的组(24)活性化合物。
本发明的活性化合物组合有很好的杀真菌性能,可用于防治植物病原真菌,如:根肿菌纲、卵菌纲、壶菌纲、接合菌纲、子囊菌纲、担子菌纲、半知菌纲等。
本发明活性化合物组合尤其适合于防治谷类植物病害如白粉菌、柄锈菌和镰刀菌,和葡萄栽培中的病害如钩丝壳霉病、单轴霉病和葡萄孢,也可用在双子叶作物上防治白粉病和霜霉病真菌以及叶斑中的病原有机物。
在防治所需浓度下植物对活性化合物组合有很好的耐药性,因此可用于植物地上部分、繁殖茎和种子处理。也能用于叶面处理或种子包衣。
本发明活性化合物组合可制成常见剂型,如溶液、乳剂、悬浮剂、粉剂、泡沫剂、糊剂、颗粒剂、气雾剂和包于聚合物中和包于用于种子的包衣组合物中的微囊剂以及超低容量喷雾剂型。
这些型剂可以用已知的方式生产,例如,将活性化合物与扩充剂,即液体溶剂、加压下的液化气和/或固体载体混合,并任选使用表面活性剂,即乳化剂和/或分散剂和/或泡沫形成剂。在用水作扩充剂的情况下,也可以用有机溶剂作助溶剂。适合的液体溶剂主要有:芳族化合物,如二甲苯,甲苯或烷基萘,氯代芳族化合物或氯代脂肪烃,如氯代苯类、氯乙烯类或二氯甲烷,脂族烃,如环己烷或石蜡,例如石油馏份,醇类,如丁醇或乙二醇以及其醚和酯,酮类,如丙酮、甲乙酮、甲基异丁基酮或环己酮,强极性溶剂,如二甲基甲酰胺或二甲基亚砜,以及水。液化气扩充剂或载体应理解为是指在常温和常压下是气体的液体,例如气雾剂抛射剂如卤代烃,或是丁烷、丙烷、氮气和二氧化碳。适合的固体载体是:例如磨碎的天然岩石如高岭土、粘土、滑石、白垩、石英、硅镁土、蒙脱石或硅藻土,和磨碎的合成矿物质,如高分散二氧化硅、矾土和硅酸盐。适合用于颗粒剂的固体载体有:例如压碎并分级的天然矿物质如方解石、大理石、浮石、海泡石和白云石,以及有机和无机粉的合成颗粒,和如下有机物的颗粒:锯木屑、椰壳、玉米穗轴和烟茎。适合的乳化剂和/或泡沫形成剂是:例如非离子和阴离子乳化剂,如聚氧乙烯脂肪酸酯、聚氧乙烯脂肪醇醚,例如,烷芳基聚乙二醇醚,烷基磺酸盐,烷基硫酸盐,芳基磺酸盐以及白蛋白水解产物。适合的分散剂是:例如,木素亚硫酸废液和甲基纤维素。
制剂中可以使用粘合剂如羧甲基纤维素和粉状、颗粒或乳胶形式的天然和合成聚合物,如阿拉伯胶、聚乙烯醇和聚乙酸乙烯酯,以及天然磷脂,如脑磷脂和卵磷脂,和合成磷脂。其它的粘合剂可以是矿物油和植物油。
也可能使用染料,如无机颜料,例如氧化铁、氧化钛和普鲁士蓝,和有机染料,如茜素染料、偶氮染料和金属酞菁染料,和微量营养素如铁、锰、硼、铜、钴、钼和锌的盐。
制剂中通常含有按重量计0.1至95%的活性化合物,优选0.5至90%。
剂型中,本发明活性化合物组合可以以与其它已知活性化合物混合的混合物形式存在,其它已知的活性化合物如杀真菌剂、杀虫剂、杀螨剂和除草剂,也可作为与肥料及植物生长调节剂的混合物。
本发明活性化合物组合可以以其原样、其制剂形式或由此制剂制备的使用形式使用,如直接可用溶液、乳油、乳剂、悬浮剂、可湿性粉剂、可溶性粉剂颗粒剂。它们可以以常规的方式使用,例如通过浇水、喷雾、弥雾、撒施、涂抹,也可以作为用于干种子处理的粉剂、作为用于种子处理的溶液、用于种子处理的水溶性粉剂、用于淤浆处理的水溶性粉剂,或进行包衣。
当使用本发明活性化合物组合时,取决于施用的类型,施用剂量可以在相对宽的范围内变化。在处理植物部分时,活性化合物组合的施用剂量通常在0.1至10,000g/ha,优选10至1000g/ha之间。在处理种子时,活性化合物的施用剂量通常在0.001至50g/kg种子,优选0.01至10g/kg种子之间。在处理土壤时,活性化合物的施用剂量通常在0.1至10,000g/ha,优选1至5000g/ha之间。
从下面的实施例中可以明显看到,本发明活性化合物组合显示很好的杀真菌活性,而单个活性化合物的杀真菌活性较弱,本发明组合的活性超过各化合物活性的简单加和。
当活性化合物组合的杀真菌活性超过各活性化合物单独使用时的活性之和时,则总是存在杀菌剂的协同作用。
两个活性化合物组合后的预期活性可按下面方法计算(参考:Colby,S.R.,“Calculating Synergistic and Antagonistic Responsesof Herbicide Combinations”,Weed 15,(1967),20-22)
如果
X为在m g/ha使用剂量下施用活性化合物A产生的效力,
Y为在n g/ha使用剂量下施用活性化合物B产生的效力,
E为在m+n g/ha使用剂量下施用活性化合物A+B产生的效力,
则
效力以%计算,0%是指效力与对照的相当,100%是指没有观察到侵染。
如果实际的杀真菌活性超过了计算值,则组合的活性是超加合的,即,存在协同作用。在此情况下,实际观察到的效力一定比按上述公式计算得到的期望效力(E)值要大。
下面的实施例举例说明本发明。
实施例1
单丝壳菌试验(黄瓜)/保护性
溶剂: 47份重量的丙酮
乳化剂: 3份重量的烷基芳基聚乙二醇醚
为了制得合适的活性化合物,将一份按重量计的活性化合物或活性化合物的组合与上述量的溶剂和乳化剂混合,并用水稀释到所需浓度,或者将活性化合物或活性化合物组合的商品剂型用水稀释到所需浓度。
为了测试保护性活性,将幼苗用所述使用剂量的活性化合物制剂喷雾,待喷液层变干后,将幼苗用单丝壳菌(Sphaerotheca fuliginea)的孢子悬浮液接种,然后把黄瓜苗置于温度约23℃、空气湿度约70%的温室中。
接种10天后进行效果评价,0%是指效力与对照的相当,100%是指没有观察到侵染。
活性化合物、使用剂量和试验结果列于下表中:表1单丝壳菌试验(黄瓜)/保护性表1(续)单丝壳菌试验(黄瓜)/保护性表1(续)单丝壳菌试验(黄瓜)/保护性表1(续)单丝壳菌试验(黄瓜)/保护性表1(续)单丝壳菌试验(黄瓜)/保护性表1(续)单丝壳菌试验(黄瓜)/保护性表1(续)单丝壳菌试验(黄瓜)/保护性表1(续)单丝壳菌试验(黄瓜)/保护性表1(续)单丝壳菌试验(黄瓜)/保护性表1(续)单丝壳菌试验(黄瓜)/保护性实测值=实测的效力计算值=用Colby式计算得的效力
实施例2
黑星菌试验(苹果)/保护性
溶剂: 47份重量的丙酮
乳化剂: 3份重量的烷基芳基聚乙二醇醚
为了制得合适的活性化合物,将一份按重量计的活性化合物或活性化合物的组合与上述量的溶剂和乳化剂混合,并用水稀释到所需浓度,或者将活性化合物或活性化合物组合的商品剂型用水稀释到所需浓度。
为了测试保护性活性,将幼苗用所述使用剂量的活性化合物制剂喷雾,待喷液层变干后,将幼苗用苹果黑星病(Venturia inaequalis)的病原有机物的分生孢子悬浮液接种,并将其置于温度约20℃、空气湿度为100%的保温室中1天。
然后将试验植物置于温度约21℃、空气湿度约90%的温室中。
接种12天后进行效果评价,0%是指效力与对照的相当,100%是指没有观察到侵染。
活性化合物、使用剂量和试验结果列于下表中:表2黑星菌试验(苹果)/保护性实测值=实测的效力计算值=用Colby式计算得的效力
实施例3
白粉菌试验(大麦)/治疗性
溶剂: 10份重量的N-甲基-吡咯烷酮
乳化剂: 0.6份重量的烷基芳基聚乙二醇醚
为了制得合适的活性化合物,将一份按重量计的活性化合物或活性化合物的组合与上述量的溶剂和乳化剂混合,并用水稀释到所需浓度,或者将活性化合物或活性化合物组合的商品剂型用水稀释到所需浓度。
为了测试治疗性活性,将幼苗用大麦白粉菌(Erysiphe graminisf.sp.hordei)孢子喷粉。接种48小时后,将幼苗用所述使用剂量的活性化合物制剂喷雾。
然后将试验植物置于温度约20℃、空气湿度约80%的温室中以促进白粉病斑的生长。
接种7天后进行效果评价,0%是指效力与对照的相当,100%是指没有观察到侵染。
实施例4
白粉菌试验(大麦)/保护性
溶剂: 10份重量的N-甲基-吡咯烷酮
乳化剂: 0.6份重量的烷基芳基聚乙二醇醚
为了制得合适的活性化合物,将一份按重量计的活性化合物或活性化合物的组合与上述量的溶剂和乳化剂混合,并用水稀释到所需浓度,或者将活性化合物或活性化合物组合的商品剂型用水稀释到所需浓度。
为了测试保护性活性,将幼苗用所述使用剂量的活性化合物制剂喷雾。
待喷液层变干后,将幼苗用大麦白粉菌(Erysiphe graminis f.sp.hordei)的孢子喷粉。
然后将试验植物置于温度约20℃、空气湿度约80%的温室中以促进白粉病斑的生长。
接种7天后进行效果评价,0%是指效力与对照的相当,100%是指没有观察到侵染。
实施例5
白粉菌试验(小麦)/治疗性
溶剂: 10份重量的N-甲基-吡咯烷酮
乳化剂: 0.6份重量的烷基芳基聚乙二醇醚
为了制得合适的活性化合物,将一份按重量计的活性化合物或活性化合物的组合与上述量的溶剂和乳化剂混合,并用水稀释到所需浓度,或者将活性化合物或活性化合物组合的商品剂型用水稀释到所需浓度。
为了测试治疗性活性,将幼苗用小麦白粉菌(Erysiphe graminisf.sp.tritici)孢子喷粉。接种48小时后,将幼苗用所述使用剂量的活性化合物制剂喷雾。
然后将试验植物置于温度约20℃、空气湿度约80%的温室中以促进白粉病斑的生长。
接种7天后进行效果评价,0%是指效力与对照的相当,100%是指没有观察到侵染。
活性化合物、使用剂量和试验结果列于下表中:表5白粉菌试验(小麦)/治疗性表5(续)白粉菌试验(小麦)/治疗性表5(续)白粉菌试验(小麦)/治疗性表5(续)白粉菌试验(小麦)/治疗性表5(续)白粉菌试验(小麦)/治疗性
实施例6
白粉菌试验(小麦)/保护性
溶剂: 10份重量的N-甲基-吡咯烷酮
乳化剂: 0.6份重量的烷基芳基聚乙二醇醚
为了制得合适的活性化合物,将一份按重量计的活性化合物或活性化合物的组合与上述量的溶剂和乳化剂混合,并用水稀释到所需浓度,或者将活性化合物或活性化合物组合的商品剂型用水稀释到所需浓度。
为了测试保护性活性,将幼苗用所述使用剂量的活性化合物制剂喷雾。
待喷液层变干后,将幼苗用小麦白粉菌(Erysiphe graminis f.sp.tritici)的孢子喷粉。
然后将试验植物置于温度约20℃、空气湿度约80%的温室中以促进白粉病斑的生长。
接种7天后进行效果评价,0%是指效力与对照的相当,100%是指没有观察到侵染。
实施例7
小球腔菌试验(小麦)/保护性
溶剂: 10份重量的N-甲基-吡咯烷酮
乳化剂: 0.6份重量的烷基芳基聚乙二醇醚
为了制得合适的活性化合物,将一份按重量计的活性化合物或活性化合物的组合与上述量的溶剂和乳化剂混合,并用水稀释到所需浓度,或者将活性化合物或活性化合物组合的商品剂型用水稀释到所需浓度。
为了测试保护性活性,将幼苗用所述使用剂量的活性化合物制剂喷雾,待喷液层变干后,将幼苗用小球腔菌(Leptosphaeria nodorum)孢子悬浮液喷雾。并将其置于温度20℃、空气湿度为100%的保温室中48小时。
然后将试验植物置于温度约15℃、空气湿度约80%的温室中。
接种10天后进行效果评价,0%是指效力与对照的相当,100%是指没有观察到侵染。
实施例8
柄锈菌试验(小麦)/保护性
溶剂: 10份重量的N-甲基-吡咯烷酮
乳化剂: 0.6份重量的烷基芳基聚乙二醇醚
为了制得合适的活性化合物,将一份按重量计的活性化合物或活性化合物的组合与上述量的溶剂和乳化剂混合,并用水稀释到所需浓度,或者将活性化合物或活性化合物组合的商品剂型用水稀释到所需浓度。
为了测试保护性活性,将幼苗用柄锈菌(Puccinia recondita)在0.1%的琼脂水溶液中的孢子悬浮液接种,待喷液层变干后,将幼苗用所述使用剂量的活性物质喷雾,并将其置于温度20℃、空气湿度为100%的保温室中24小时。
然后将试验植物置于温度约20℃、空气湿度约80%的温室中,以促进锈病斑的生长。
接种10天后进行效果评价,0%是指效力与对照的相当,100%是指没有观察到侵染。
实施例9
黄色镰孢菌(Fusarium culmorum)试验(小麦)/种子处理
活性化合物以干种子拌种剂施用。该制剂可通过将单独的活性化合物或组合的活性化合物与磨细的矿物质混合来制备,得到能在种子表面均匀分布的细粉状混合物。
种子拌种时,将侵染种子和种子拌种剂在封闭的玻璃烧瓶内摇动3分钟。
2×100粒小麦播种于1厘米深的标准土壤中,在温度约18℃、空气湿度约95%的温室内培养,种子栽培箱每日需15小时光照。
播种3周后,评价麦苗的病症,0%是指效力与对照的相当,100%是指没有观察到侵染。
实施例10
雪腐镰孢菌(Fusarium nivale)试验(黑小麦)/种子处理
活性化合物以干种子拌种剂施用。该制剂可通过将单独的活性化合物或组合的活性化合物与磨细的矿物质混合来制备,得到能在种子表面均匀分布的细粉状混合物。
种子拌种时,将侵染种子和种子拌种剂在封闭的玻璃烧瓶内摇动3分钟。
2×100粒小麦播种于1厘米深的标准土壤中,在温度约10℃、空气湿度约95%的温室内培养,种子栽培箱每日需15小时光照。
播种3周后,评价麦苗的病症,0%是指效力与对照的相当,100%是指没有观察到侵染。
实施例11
立枯丝核菌(Rhizoctonia solani)试验(棉花)/种子处理
活性化合物以干种子拌种剂施用。该制剂可通过将单独的活性化合物或组合的活性化合物与磨细的矿物质混合来制备,得到能在种子表面均匀分布的细粉状混合物。
种子拌种时,将侵染种子和种子拌种剂在封闭的玻璃烧瓶内摇动3分钟。
2×50粒棉花籽播种于2厘米深的感染立枯丝核菌的标准土壤中,在种子托盘上于温度约22℃的温室内培养,种子栽培箱每日需15小时光照。
播种8天后进行评价。0%是指效力与对照的相当,100%是指没有观察到侵染。
活性化合物、使用剂量和试验结果列于下表中:表11立枯丝核菌试验(棉花)/种子处理
Claims (5)
1.杀真菌组合物,其特征在于,它们含有如下活性化合物组合:
和
和/或
(2)式(III)的三唑衍生物
和/或
其中,R1代表氢或甲基,
和/或
和/或
n>=1 (丙森锌)
和/或
Me=Zn或Mn
或Zn和Mn的混合物
和/或
(7)式(VIII)的苯胺衍生物
和/或
和/或
(9)式(X)的苯并噻二唑衍生物
和/或
和/或
和/或
和/或
(13)式(XIV)的化合物
和/或
和/或
其中,R2代表甲基或环丙基
和/或
和/或
(17)式(XVIII)的吗啉衍生物
和/或
和/或
(19)式(XX)的磷化合物
和/或
其中,R3和R4分别代表氯或一起代表基团-O-CF2-O-
和/或
和/或
和/或
和/或
其中,m代表整数0到5
2.根据权利要求1的组合物,其特征在于,其中,在活性化合物组合中,式(I)的化合物与其它物质的重量比为:
与组(1)的活性化合物重量比在1∶0.1到1∶20之间,
与组(2)的活性化合物重量比在1∶0.1到1∶20之间,
与组(3)的活性化合物重量比在1∶0.2到1∶150之间,
与组(4)的活性化合物重量比在1∶0.1到1∶10之间,
与组(5)的活性化合物重量比在1∶1到1∶50之间,
与组(6)的活性化合物重量比在1∶1到1∶50之间,
与组(7)的活性化合物重量比在1∶0.1到1∶50之间,
与组(8)的活性化合物重量比在1∶0.2到1∶50之间,
与组(9)的活性化合物重量比在1∶0.02到1∶50之间,
与组(10)的活性化合物重量比在1∶0.1到1∶50之间,
与组(11)的活性化合物重量比在1∶0.1到1∶50之间,
与组(12)的活性化合物重量比在1∶0.1到1∶50之间,
与组(13)的活性化合物重量比在1∶0.1到1∶50之间,
与组(14)的活性化合物重量比在1∶0.1到1∶50之间,
与组(15)的活性化合物重量比在1∶0.1到1∶50之间,
与组(16)的活性化合物重量比在1∶1到1∶50之间,
与组(17)的活性化合物重量比在1∶1到1∶20之间,
与组(18)的活性化合物重量比在1∶1到1∶50之间,
与组(19)的活性化合物重量比在1∶1到1∶50之间,
与组(20)的活性化合物重量比在1∶0.1到1∶10之间,
与组(21)的活性化合物重量比在1∶0.05到1∶20之间,
与组(22)的活性化合物重量比在1∶0.1到1∶10之间,
与组(23)的活性化合物重量比在1∶0.1到1∶10之间和
与组(24)的活性化合物重量比在1∶0.1到1∶10之间。
3.防治真菌的方法,其特征在于,将权利要求1中的活性化合物组合施用于真菌和/或它们的栖息地。
4.权利要求1中的活性化合物组合用防治真菌的应用。
5.制备杀真菌组合物的方法,其特征在于,将权利要求1中的活性化合物组合与扩充剂和/或表面活性剂混合。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19716257A DE19716257A1 (de) | 1997-04-18 | 1997-04-18 | Fungizide Wirkstoffkombination |
| DE19716257.6 | 1997-04-18 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN1252690A true CN1252690A (zh) | 2000-05-10 |
| CN1109499C CN1109499C (zh) | 2003-05-28 |
Family
ID=7826916
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN98804274A Expired - Lifetime CN1109499C (zh) | 1997-04-18 | 1998-04-06 | 杀菌剂活性物质组合 |
Country Status (31)
| Country | Link |
|---|---|
| US (8) | US6306850B1 (zh) |
| EP (1) | EP0975219B1 (zh) |
| JP (1) | JP4094067B2 (zh) |
| KR (1) | KR100525469B1 (zh) |
| CN (1) | CN1109499C (zh) |
| AT (1) | ATE214230T1 (zh) |
| AU (1) | AU727186B2 (zh) |
| BR (1) | BR9809100B1 (zh) |
| CA (7) | CA2800471C (zh) |
| CZ (1) | CZ296701B6 (zh) |
| DE (3) | DE19716257A1 (zh) |
| DK (1) | DK0975219T3 (zh) |
| EA (1) | EA002598B1 (zh) |
| EE (1) | EE03657B1 (zh) |
| ES (1) | ES2172143T3 (zh) |
| FR (4) | FR06C0041I2 (zh) |
| HK (1) | HK1026822A1 (zh) |
| HU (1) | HU227139B1 (zh) |
| ID (1) | ID22820A (zh) |
| IL (1) | IL131900A (zh) |
| NL (2) | NL350024I2 (zh) |
| NZ (1) | NZ500367A (zh) |
| PL (1) | PL191483B1 (zh) |
| PT (1) | PT975219E (zh) |
| SI (1) | SI0975219T1 (zh) |
| SK (1) | SK284214B6 (zh) |
| TR (1) | TR199902400T2 (zh) |
| TW (1) | TW505504B (zh) |
| UA (1) | UA55451C2 (zh) |
| WO (1) | WO1998047367A1 (zh) |
| ZA (1) | ZA983236B (zh) |
Cited By (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN100403901C (zh) * | 2002-03-07 | 2008-07-23 | 巴斯福股份公司 | 基于三唑的杀真菌混合物 |
| CN102258033A (zh) * | 2011-08-19 | 2011-11-30 | 陕西美邦农药有限公司 | 一种含丙硫菌唑与三唑类的新型农药组合物 |
| CN102258034A (zh) * | 2011-08-30 | 2011-11-30 | 陕西美邦农药有限公司 | 一种含丙硫菌唑与三唑类的杀菌组合物 |
| CN102273462A (zh) * | 2011-09-05 | 2011-12-14 | 陕西美邦农药有限公司 | 一种含丙硫菌唑与三唑类的新型农药组合物 |
| CN102273461A (zh) * | 2011-08-25 | 2011-12-14 | 陕西美邦农药有限公司 | 一种含丙硫菌唑与三唑类的增效农药组合物 |
| CN102349516A (zh) * | 2011-08-18 | 2012-02-15 | 陕西美邦农药有限公司 | 一种含丙硫菌唑与嘧菌环胺的杀菌组合物 |
| CN101184396B (zh) * | 2005-05-24 | 2012-05-30 | 拜尔农作物科学股份公司 | 杀真菌活性化合物结合物 |
| CN102657186A (zh) * | 2012-04-24 | 2012-09-12 | 杭州宇龙化工有限公司 | 一种含有噻呋酰胺与丙硫菌唑的杀菌组合物 |
| CN102972420A (zh) * | 2012-12-18 | 2013-03-20 | 广西农喜作物科学有限公司 | 一种含丙硫菌唑的杀菌组合物 |
| CN103314964A (zh) * | 2012-03-20 | 2013-09-25 | 陕西韦尔奇作物保护有限公司 | 一种含环酰菌胺与三唑类的农药组合物 |
| CN103461362A (zh) * | 2013-09-05 | 2013-12-25 | 江苏龙灯化学有限公司 | 一种杀菌活性成分组合物 |
| CN103563910A (zh) * | 2013-10-23 | 2014-02-12 | 江苏丰登农药有限公司 | 一种含丙硫菌唑和井冈霉素的杀菌组合物及其应用 |
| CN103651425A (zh) * | 2012-09-18 | 2014-03-26 | 陕西美邦农药有限公司 | 一种含醚菌胺的农药组合物 |
| CN103651371A (zh) * | 2012-09-07 | 2014-03-26 | 陕西美邦农药有限公司 | 一种含苯酰菌胺的高效杀菌组合物 |
| CN103783046A (zh) * | 2013-12-09 | 2014-05-14 | 海利尔药业集团股份有限公司 | 一种含有丙硫菌唑与嘧霉胺的杀菌组合物 |
| CN103931623A (zh) * | 2014-04-30 | 2014-07-23 | 海利尔药业集团股份有限公司 | 一种含有硝苯菌酯与丙硫菌唑的杀菌组合物 |
| CN103957703A (zh) * | 2011-11-14 | 2014-07-30 | 江苏龙灯化学有限公司 | 处理真菌感染的方法、杀真菌组合物及其用途 |
| CN104012555A (zh) * | 2014-06-25 | 2014-09-03 | 深圳诺普信农化股份有限公司 | 一种农用杀菌组合物 |
| CN104026141A (zh) * | 2014-06-27 | 2014-09-10 | 深圳诺普信农化股份有限公司 | 一种农用杀菌组合物 |
| CN104621158A (zh) * | 2013-11-15 | 2015-05-20 | 南京华洲药业有限公司 | 一种含丙硫菌唑和丙森锌的杀菌组合物及其应用 |
| CN104621131A (zh) * | 2013-11-15 | 2015-05-20 | 南京华洲药业有限公司 | 一种含丙硫菌唑和恶唑菌酮的杀菌组合物及其应用 |
| CN104642334A (zh) * | 2013-11-15 | 2015-05-27 | 南京华洲药业有限公司 | 一种含丙硫菌唑和嘧菌环胺的杀菌组合物及其应用 |
| CN104642331A (zh) * | 2013-11-15 | 2015-05-27 | 南京华洲药业有限公司 | 一种含丙硫菌唑和戊唑醇的杀菌组合物及其应用 |
| CN104642330A (zh) * | 2013-11-15 | 2015-05-27 | 南京华洲药业有限公司 | 一种含丙硫菌唑和三唑酮的杀菌组合物及其应用 |
| CN104904723A (zh) * | 2014-03-11 | 2015-09-16 | 江苏龙灯化学有限公司 | 一种杀菌组合物 |
| CN106417311A (zh) * | 2015-08-12 | 2017-02-22 | 四川利尔作物科学有限公司 | 种子处理剂组合物及其应用 |
| CN107242242A (zh) * | 2017-08-02 | 2017-10-13 | 广东广康生化科技股份有限公司 | 包含灭菌丹和丙硫菌唑的杀菌组合物 |
| CN108477193A (zh) * | 2018-07-03 | 2018-09-04 | 广东广康生化科技股份有限公司 | 一种含灭菌丹与腈菌唑的农药组合物 |
| CN108477192A (zh) * | 2018-07-03 | 2018-09-04 | 广东广康生化科技股份有限公司 | 一种含灭菌丹与己唑醇的高效杀菌组合物 |
| CN109042699A (zh) * | 2018-07-19 | 2018-12-21 | 广东广康生化科技股份有限公司 | 包含灭菌丹与代森锌的增效杀菌组合物 |
| WO2019153897A1 (en) * | 2018-02-09 | 2019-08-15 | Jiangsu Rotam Chemistry Co., Ltd | Fungicidal composition and the use thereof in controlling undesired fungal infestations |
| CN114206114A (zh) * | 2019-06-05 | 2022-03-18 | Upl有限责任公司 | 用于防治植物中的发酵壳针孢感染的杀真菌剂组合物 |
Families Citing this family (85)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE4426753A1 (de) * | 1994-07-28 | 1996-02-01 | Bayer Ag | Mittel zur Bekämpfung von Pflanzenschädlingen |
| DE19716257A1 (de) | 1997-04-18 | 1998-10-22 | Bayer Ag | Fungizide Wirkstoffkombination |
| NZ508749A (en) * | 1998-06-10 | 2002-06-28 | Bayer Ag | Agents for combating plant pests containing imidacloprid [1-(6-chloro-3-pyridylmethyl)-2-nitroiminoimidazolidine] |
| DE10019758A1 (de) * | 2000-04-20 | 2001-10-25 | Bayer Ag | Fungizide Wirkstoffkombinationen |
| DE10140108A1 (de) | 2001-08-16 | 2003-03-06 | Bayer Cropscience Ag | Fungizide Wirkstoffkombinationen |
| WO2003035005A2 (en) * | 2001-10-26 | 2003-05-01 | University Of Connecticut | Heteroindanes: a new class of potent cannabimimetic ligands |
| FR2832031A1 (fr) * | 2001-11-14 | 2003-05-16 | Aventis Cropscience Sa | Composition fongicide a base d'au moins un derive de pyridylmethylbenzamide et d'au moins un derive de type valinamide |
| KR100951210B1 (ko) * | 2002-03-01 | 2010-04-05 | 바스프 에스이 | 프로티오코나졸 및 스트로빌루린 유도체를 기재로 하는살진균제 혼합물 |
| DE50309843D1 (de) | 2002-03-01 | 2008-06-26 | Basf Se | Fungizide mischungen auf der basis von prothioconazol |
| EP2008518A3 (de) | 2002-03-08 | 2011-01-19 | Basf Se | Fungizide Mischungen auf der Basis von Prothioconazol mit einem Insektizid |
| IL163708A0 (en) * | 2002-03-21 | 2005-12-18 | Basf Ag | Fungicidal mixtures |
| AU2013202522B2 (en) * | 2002-03-21 | 2015-04-09 | Basf Se | Fungicidal mixtures |
| DE10228103A1 (de) * | 2002-06-24 | 2004-01-15 | Bayer Cropscience Ag | Fungizide Wirkstoffkombinationen |
| DE10228104A1 (de) * | 2002-06-24 | 2004-01-15 | Bayer Cropscience Ag | Fungizide Wirkstoffkombination |
| DE10228102A1 (de) * | 2002-06-24 | 2004-01-15 | Bayer Cropscience Ag | Fungizide Wirkstoffkombinationen |
| CN1274218C (zh) * | 2002-07-10 | 2006-09-13 | 巴斯福股份公司 | 基于二噻农的杀真菌混合物 |
| DE10233171A1 (de) * | 2002-07-22 | 2004-02-12 | Bayer Cropscience Ag | Kristallmodifikation II des 2-[2-(Chlor-cyclopropyl)-3-(2-chlorphenyl)-2-hydroxy-propyl]-2,4dihydro-3H-1,2,4-triazol-3-thions |
| DE10335183A1 (de) * | 2003-07-30 | 2005-02-24 | Bayer Cropscience Ag | Fungizide Wirkstoffkombinationen |
| DE10341945A1 (de) * | 2003-09-11 | 2005-04-21 | Bayer Cropscience Ag | Verwendung von fungiziden Mitteln zur Beizung von Saatgut |
| DE10347090A1 (de) | 2003-10-10 | 2005-05-04 | Bayer Cropscience Ag | Synergistische fungizide Wirkstoffkombinationen |
| DE10347440A1 (de) * | 2003-10-13 | 2005-05-04 | Bayer Cropscience Ag | Synergistische insektizide Mischungen |
| DE10349503A1 (de) | 2003-10-23 | 2005-05-25 | Bayer Cropscience Ag | Fungizide Wirkstoffkombinationen |
| DE10352264A1 (de) * | 2003-11-08 | 2005-06-09 | Bayer Cropscience Ag | Fungizide Wirkstoffkombination |
| AU2004294711B2 (en) * | 2003-12-04 | 2011-05-12 | Bayer Intellectual Property Gmbh | Active substance combinations having insecticidal properties |
| KR100870174B1 (ko) * | 2003-12-04 | 2008-11-24 | 바이엘 크롭사이언스 아게 | 살충 및 살비성을 가지는 활성 물질 배합물 |
| EP1563731A1 (en) * | 2004-02-12 | 2005-08-17 | Bayer CropScience S.A. | Fungicidal composition comprising a pyridylethylbenzamide derivative and a compound capable of inhibiting the ergosterol biosynthesis |
| DE102004020840A1 (de) * | 2004-04-27 | 2005-11-24 | Bayer Cropscience Ag | Verwendung von Alkylcarbonsäureamiden als Penetrationsförderer |
| EP1606999A1 (de) * | 2004-06-18 | 2005-12-21 | Bayer CropScience AG | Saatgutbehandlungsmittel für Soja |
| DE102004045242A1 (de) * | 2004-09-17 | 2006-03-23 | Bayer Cropscience Ag | Synergistische fungizide Wirkstoffkombinationen |
| DE102004049041A1 (de) * | 2004-10-08 | 2006-04-13 | Bayer Cropscience Ag | Fungizide Wirkstoffkombinationen |
| DE102004049761A1 (de) * | 2004-10-12 | 2006-04-13 | Bayer Cropscience Ag | Fungizide Wirkstoffkombinationen |
| DE102005015677A1 (de) * | 2005-04-06 | 2006-10-12 | Bayer Cropscience Ag | Synergistische fungizide Wirkstoffkombinationen |
| EP1917856A1 (en) * | 2006-11-01 | 2008-05-07 | Syngeta Participations AG | Pesticidal compositions comprising an azole, a phenylamide and azoxystrobin |
| GB0508993D0 (en) * | 2005-05-03 | 2005-06-08 | Syngenta Participations Ag | Pesticidal compositions |
| WO2006131230A2 (de) | 2005-06-09 | 2006-12-14 | Bayer Cropscience Ag | Wirkstoffkombinationen |
| US20060293381A1 (en) * | 2005-06-23 | 2006-12-28 | Kaihei Kojima | Fungicidal effect by regulating signal transduction pathways |
| DE102005035300A1 (de) * | 2005-07-28 | 2007-02-01 | Bayer Cropscience Ag | Synergistische fungizide Wirkstoffkombinationen |
| BRPI0615645B1 (pt) * | 2005-09-09 | 2017-01-31 | Bayer Cropscience Ag | formulações fungicidas sólidas, seus empregos e seu processo de preparação, e agente |
| US20090018176A1 (en) * | 2005-09-13 | 2009-01-15 | Bayer Cropscience Ag | Fungicide composition comprising an arylamidine derivative and two known fungicide compounds |
| SI1763998T1 (sl) * | 2005-09-16 | 2007-10-31 | Syngenta Participations Ag | Fungicidni sestavki |
| AR055657A1 (es) * | 2005-09-29 | 2007-08-29 | Syngenta Participations Ag | Composiciones fungicidas |
| WO2007045455A1 (en) * | 2005-10-20 | 2007-04-26 | Syngenta Participations Ag | Fungicidal compositions |
| EP1776864A1 (en) | 2005-10-20 | 2007-04-25 | Syngenta Participations AG | Fungicidal compositions |
| WO2007048534A1 (en) * | 2005-10-26 | 2007-05-03 | Syngenta Participations Ag | Fungicidal compositions |
| KR20080095285A (ko) * | 2006-02-14 | 2008-10-28 | 바스프 에스이 | 유해 진균 방제용 트리아졸에 대한 완화제로서 미량 영양소를 사용하는 방법 |
| DE102006026106A1 (de) * | 2006-05-11 | 2007-11-15 | Isp Biochema Schwaben Gmbh | Flüssiges Konzentrat für die Filmkonservierung |
| DE102006031976A1 (de) * | 2006-07-11 | 2008-01-17 | Bayer Cropscience Ag | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| DE102006031978A1 (de) * | 2006-07-11 | 2008-01-17 | Bayer Cropscience Ag | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| US20090281157A1 (en) * | 2006-07-11 | 2009-11-12 | Bayer Cropscience Ag | Active Ingredient Combinations With Insecticidal and Acaricidal Properties |
| WO2008020998A2 (en) * | 2006-08-08 | 2008-02-21 | Bayer Cropscience Lp | Method of improving plant growth by reducing viral infections |
| BRPI0719154A2 (pt) * | 2006-11-24 | 2014-02-04 | Bayer Crospscience Ag | Combinações de substâncias ativas fungicidas ternárias. |
| BRPI0807066A8 (pt) * | 2007-02-02 | 2018-06-05 | Bayer Cropscience Ag | Combinações sinergísticas de compostos ativos fungicidas |
| AU2008226090B2 (en) * | 2007-03-09 | 2013-12-05 | Syngenta Participations Ag | Ternary fungicidal compositions |
| DE102008029252A1 (de) | 2007-06-22 | 2008-12-24 | Bayer Cropscience Ag | Verwendung von fungizide Wirkstoffkombinationen zur Bekämpfung von Fusarien |
| EP2036438A1 (en) * | 2007-09-12 | 2009-03-18 | Bayer CropScience AG | Post-harvest treatment |
| EP2242370A2 (en) * | 2008-02-05 | 2010-10-27 | Basf Se | Pesticidal mixtures |
| US8683346B2 (en) * | 2008-11-17 | 2014-03-25 | Sap Portals Israel Ltd. | Client integration of information from a supplemental server into a portal |
| WO2010081646A2 (de) * | 2009-01-15 | 2010-07-22 | Bayer Cropscience Aktiengesellschaft | Fungizide wirkstoffkombinationen |
| EP2269454A1 (en) * | 2009-06-24 | 2011-01-05 | Bayer CropScience AG | Combinations of fungicidally active yeast and fungicides |
| JP5655080B2 (ja) * | 2009-10-07 | 2015-01-14 | ダウ アグロサイエンシィズ エルエルシー | 穀類において真菌を制御するための5−フルオロシトシンを含有する相乗的殺真菌組成物 |
| PL2512244T3 (pl) | 2009-12-16 | 2014-11-28 | Bayer Ip Gmbh | Kombinacja związków czynnych zawierająca prochinazyd, biksafen i ewentualnie protiokonazol |
| WO2011076688A2 (en) | 2009-12-21 | 2011-06-30 | Bayer Cropscience Ag | Synergistic combination of prothioconazole and metominostrobin |
| EP2377397A1 (de) | 2010-04-14 | 2011-10-19 | Bayer CropScience AG | Verwendung fungizider Wirkstoffe zur Kontrolle von Mykosen an Palmengewächsen |
| CA2808333C (en) * | 2010-08-11 | 2016-02-16 | Randy Allen Myers | Method of improving plant growth by reducing fungal infections |
| CN102258039A (zh) * | 2011-08-26 | 2011-11-30 | 陕西美邦农药有限公司 | 一种含丙硫菌唑与硫代氨基甲酸酯类的杀菌组合物 |
| JP2013119537A (ja) * | 2011-12-08 | 2013-06-17 | Sumitomo Chemical Co Ltd | 有害生物の防除方法 |
| CN103563913B (zh) * | 2012-08-04 | 2015-11-04 | 南京华洲药业有限公司 | 一种含噻呋酰胺和丙硫菌唑的杀菌组合物及其应用 |
| CA2892699C (en) | 2012-11-30 | 2023-01-24 | Bayer Cropscience Ag | Ternary fungicidal combinations comprising pyrazole carboxamides |
| CN104621130A (zh) * | 2013-11-15 | 2015-05-20 | 南京华洲药业有限公司 | 一种含丙硫菌唑和嘧菌酯的杀菌组合物及其应用 |
| GB2532700B (en) * | 2013-11-26 | 2016-08-31 | Upl Ltd | A method for controlling rust |
| EP3154352A1 (en) | 2014-06-11 | 2017-04-19 | Bayer CropScience Aktiengesellschaft | Active compound combinations comprising proquinazid and spiroxamine and optionally prothioconazole |
| BR102014031250A2 (pt) * | 2014-12-12 | 2016-06-14 | Upl Do Brasil Indústria E Comércio De Insumos Agropecuários S A | método antirresistência |
| UY36432A (es) * | 2014-12-16 | 2016-06-30 | Bayer Cropscience Ag | Combinaciones de compuestos activos que comprenden un derivado de (tio) carboxamida y compuesto(s) fungicida(s) |
| US10952433B2 (en) | 2015-03-31 | 2021-03-23 | Kop-Coat, Inc. | Solutions for enhancing the effectiveness of insecticides and fungicides on living plants and related methods |
| US20160286798A1 (en) * | 2015-03-31 | 2016-10-06 | Kop-Coat, Inc. | Solutions for enhancing the effectiveness of insecticides and fungicides on living plants and related methods |
| EP3341718B1 (en) * | 2015-08-25 | 2022-06-08 | Life Technologies Corporation | Deep microwell design and method of making the same |
| WO2017072013A1 (en) | 2015-10-27 | 2017-05-04 | Bayer Cropscience Aktiengesellschaft | Composition comprising a safener, a fungicide and metalaxyl |
| EP3432718A1 (en) | 2016-03-24 | 2019-01-30 | Bayer CropScience Aktiengesellschaft | Method to control septoria leaf blotch caused by resistant zymoseptoria tritici strains |
| WO2017162564A1 (en) | 2016-03-24 | 2017-09-28 | Bayer Cropscience Aktiengesellschaft | Method to control septoria leaf blotch caused by resistant zymoseptoria tritici strains |
| EP3432717A1 (en) | 2016-03-24 | 2019-01-30 | Bayer CropScience Aktiengesellschaft | Method to control septoria leaf blotch caused by resistant zymoseptoria tritici strains |
| CN106342821A (zh) * | 2016-08-25 | 2017-01-25 | 安徽美兰农业发展股份有限公司 | 一种丙硫菌唑和戊唑醇复配悬浮剂及其制备方法 |
| EP3403504B1 (en) | 2017-05-16 | 2020-12-30 | Rotam Agrochem International Company Limited | Fungicidal composition containing prothioconazole and chlorothalonil |
| US11272706B2 (en) | 2017-06-12 | 2022-03-15 | Upl Limited | Anti-resistance method |
| WO2020231751A1 (en) | 2019-05-10 | 2020-11-19 | Bayer Cropscience Lp | Active compound combinations |
| EP3753408A1 (en) * | 2019-06-21 | 2020-12-23 | Rotam Agrochem International Company Limited | A synergistic fungicidal composition |
Family Cites Families (53)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US599845A (en) * | 1898-03-01 | Water-wheel bucket | ||
| US3903090A (en) | 1969-03-19 | 1975-09-02 | Sumitomo Chemical Co | Novel n-(3,5-dihalophenyl)-imide compounds |
| US4147791A (en) | 1972-01-11 | 1979-04-03 | Bayer Aktiengesellschaft | 1-Substituted-1,2,4-triazole fungicidal compositions and methods for combatting fungi that infect or attack plants |
| US3912752A (en) | 1972-01-11 | 1975-10-14 | Bayer Ag | 1-Substituted-1,2,4-triazoles |
| US4048318A (en) | 1972-01-11 | 1977-09-13 | Bayer Aktiengesellschaft | 1-Substituted-1,2,4-triazole fungicides |
| DE2324010C3 (de) | 1973-05-12 | 1981-10-08 | Bayer Ag, 5090 Leverkusen | 1-Substituierte 2-Triazolyl-2-phenoxyäthanol-Verbindungen, Verfahren zu ihrer Herstellung und ihre Verwendung zur Bekämpfung von Pilzen |
| JPS5312844A (en) | 1976-07-20 | 1978-02-04 | Nippon Tokushu Noyaku Seizo Kk | Nn44halogenobenzyllnnmethyl*or nonsubstitutedd*cycloalkylln**phenylurea or thiourea compounds* their preparation and fungicides containing the same as active constituents |
| AU542623B2 (en) | 1980-05-16 | 1985-02-28 | Bayer Aktiengesellschaft | 1-hydroxyethyl-azole derivatives |
| US4705800A (en) | 1985-06-21 | 1987-11-10 | Ciba-Geigy Corporation | Difluorbenzodioxyl cyanopyrrole microbicidal compositions |
| DE3689506D1 (de) | 1985-10-09 | 1994-02-17 | Shell Int Research | Neue Acrylsäureamide. |
| DE3770657D1 (de) | 1986-03-04 | 1991-07-18 | Ciba Geigy Ag | Fungizide verwendung eines cyanopyrrol-derivates. |
| JPH0784445B2 (ja) | 1986-12-03 | 1995-09-13 | クミアイ化学工業株式会社 | ピリミジン誘導体および農園芸用殺菌剤 |
| EP0313512B1 (de) | 1987-08-21 | 1992-11-25 | Ciba-Geigy Ag | Benzothiadiazole und ihre Verwendung in Verfahren und Mitteln gegen Pflanzenkrankheiten |
| DE3881320D1 (de) | 1987-09-28 | 1993-07-01 | Ciba Geigy Ag | Schaedlingsbekaempfungsmittel. |
| US5153200A (en) | 1987-09-28 | 1992-10-06 | Ciba-Geigy Corporation | Pesticides |
| DE3814505A1 (de) | 1988-04-29 | 1989-11-09 | Bayer Ag | Substituierte cycloalkyl- bzw. heterocyclyl-carbonsaeureanilide |
| DE3815728A1 (de) | 1988-05-07 | 1989-11-16 | Bayer Ag | Stereoisomere von n-(r)-(1-aryl-ethyl)-1-alkyl-2,2-dichlor- cyclopropancarbonsaeureamiden |
| PH11991042549B1 (zh) * | 1990-06-05 | 2000-12-04 | ||
| US5453531A (en) | 1990-08-25 | 1995-09-26 | Bayer Aktiengesellschaft | Substituted valinamide derivatives |
| DE4117371A1 (de) | 1991-05-28 | 1992-12-03 | Basf Ag | Antimykotische mittel, die phenylessigsaeurederivate enthalten |
| DE4139637A1 (de) | 1991-12-02 | 1993-06-03 | Bayer Ag | Fungizide wirkstoffkombinationen |
| US5304572A (en) | 1992-12-01 | 1994-04-19 | Rohm And Haas Company | N-acetonylbenzamides and their use as fungicides |
| TW286264B (zh) * | 1994-05-20 | 1996-09-21 | Ciba Geigy Ag | |
| DE19528046A1 (de) * | 1994-11-21 | 1996-05-23 | Bayer Ag | Triazolyl-Derivate |
| BE1008947A3 (nl) * | 1994-12-01 | 1996-10-01 | Dsm Nv | Werkwijze voor de bereiding van condensatieproducten van melamine. |
| MY115814A (en) * | 1995-06-16 | 2003-09-30 | Bayer Ip Gmbh | Crop protection compositions |
| DE19602095A1 (de) | 1996-01-22 | 1997-07-24 | Bayer Ag | Halogenpyrimidine |
| US6297236B1 (en) * | 1998-04-06 | 2001-10-02 | Bayer Aktiengesellschaft | Fungicide active substance combinations |
| DE19716257A1 (de) * | 1997-04-18 | 1998-10-22 | Bayer Ag | Fungizide Wirkstoffkombination |
| DE19857963A1 (de) * | 1998-12-16 | 2000-06-21 | Bayer Ag | Agrochemische Formulierungen |
| DE19933938A1 (de) * | 1999-07-20 | 2001-01-25 | Bayer Ag | Fungizide Wirkstoffkombinationen |
| TR200201544T2 (tr) * | 1999-12-13 | 2002-11-21 | Bayer Aktiengesellschaft | Fungusid aktivitesine sahip bileşik kombinasyonları. |
| KR100951210B1 (ko) * | 2002-03-01 | 2010-04-05 | 바스프 에스이 | 프로티오코나졸 및 스트로빌루린 유도체를 기재로 하는살진균제 혼합물 |
| CA2778803C (en) * | 2002-03-07 | 2014-06-10 | Basf Aktiengesellschaft | Fungicidal mixtures based on triazoles |
| DE10228102A1 (de) * | 2002-06-24 | 2004-01-15 | Bayer Cropscience Ag | Fungizide Wirkstoffkombinationen |
| DE10228104A1 (de) * | 2002-06-24 | 2004-01-15 | Bayer Cropscience Ag | Fungizide Wirkstoffkombination |
| DE10228103A1 (de) * | 2002-06-24 | 2004-01-15 | Bayer Cropscience Ag | Fungizide Wirkstoffkombinationen |
| KR100480442B1 (ko) * | 2002-08-17 | 2005-04-06 | 한국과학기술연구원 | 미량도핑에 의한 고효율 백색 유기 발광 물질 및 이를이용한 전기발광소자 |
| DE10329714A1 (de) * | 2003-07-02 | 2005-01-20 | Bayer Cropscience Ag | Agrochemischen Formulierungen |
| DE10335183A1 (de) * | 2003-07-30 | 2005-02-24 | Bayer Cropscience Ag | Fungizide Wirkstoffkombinationen |
| DE10341945A1 (de) * | 2003-09-11 | 2005-04-21 | Bayer Cropscience Ag | Verwendung von fungiziden Mitteln zur Beizung von Saatgut |
| DE10347090A1 (de) * | 2003-10-10 | 2005-05-04 | Bayer Cropscience Ag | Synergistische fungizide Wirkstoffkombinationen |
| DE102004020840A1 (de) * | 2004-04-27 | 2005-11-24 | Bayer Cropscience Ag | Verwendung von Alkylcarbonsäureamiden als Penetrationsförderer |
| DE102004029972A1 (de) * | 2004-06-21 | 2006-01-05 | Bayer Cropscience Ag | Beizmittel zur Bekämpfung von phytopathogenen Pilzen |
| DE102004045242A1 (de) * | 2004-09-17 | 2006-03-23 | Bayer Cropscience Ag | Synergistische fungizide Wirkstoffkombinationen |
| DE102004049041A1 (de) * | 2004-10-08 | 2006-04-13 | Bayer Cropscience Ag | Fungizide Wirkstoffkombinationen |
| DE102004049761A1 (de) * | 2004-10-12 | 2006-04-13 | Bayer Cropscience Ag | Fungizide Wirkstoffkombinationen |
| DE102005023835A1 (de) * | 2005-05-24 | 2006-12-07 | Bayer Cropscience Ag | Fungizide Wirkstoffkombination |
| DE102005035300A1 (de) * | 2005-07-28 | 2007-02-01 | Bayer Cropscience Ag | Synergistische fungizide Wirkstoffkombinationen |
| BRPI0615645B1 (pt) * | 2005-09-09 | 2017-01-31 | Bayer Cropscience Ag | formulações fungicidas sólidas, seus empregos e seu processo de preparação, e agente |
| EP2036438A1 (en) * | 2007-09-12 | 2009-03-18 | Bayer CropScience AG | Post-harvest treatment |
| EP2269454A1 (en) * | 2009-06-24 | 2011-01-05 | Bayer CropScience AG | Combinations of fungicidally active yeast and fungicides |
| AR077956A1 (es) * | 2009-09-14 | 2011-10-05 | Bayer Cropscience Ag | Combinaciones de compuestos activos |
-
1997
- 1997-04-18 DE DE19716257A patent/DE19716257A1/de not_active Withdrawn
-
1998
- 1998-04-02 TW TW087104935A patent/TW505504B/zh not_active IP Right Cessation
- 1998-04-06 CA CA2800471A patent/CA2800471C/en not_active Expired - Lifetime
- 1998-04-06 DE DE122008000040C patent/DE122008000040I2/de active Active
- 1998-04-06 CA CA2905473A patent/CA2905473C/en not_active Expired - Lifetime
- 1998-04-06 ES ES98922647T patent/ES2172143T3/es not_active Expired - Lifetime
- 1998-04-06 CN CN98804274A patent/CN1109499C/zh not_active Expired - Lifetime
- 1998-04-06 EE EEP199900500A patent/EE03657B1/xx not_active IP Right Cessation
- 1998-04-06 EP EP98922647A patent/EP0975219B1/de not_active Expired - Lifetime
- 1998-04-06 CA CA2721222A patent/CA2721222C/en not_active Expired - Lifetime
- 1998-04-06 DE DE59803337T patent/DE59803337D1/de not_active Expired - Lifetime
- 1998-04-06 SK SK143599A patent/SK284214B6/sk not_active IP Right Cessation
- 1998-04-06 BR BRPI9809100-0A patent/BR9809100B1/pt not_active IP Right Cessation
- 1998-04-06 EA EA199900916A patent/EA002598B1/ru not_active IP Right Cessation
- 1998-04-06 PT PT98922647T patent/PT975219E/pt unknown
- 1998-04-06 AT AT98922647T patent/ATE214230T1/de active
- 1998-04-06 NZ NZ500367A patent/NZ500367A/xx not_active IP Right Cessation
- 1998-04-06 IL IL13190098A patent/IL131900A/en not_active IP Right Cessation
- 1998-04-06 SI SI9830134T patent/SI0975219T1/xx unknown
- 1998-04-06 CZ CZ0369699A patent/CZ296701B6/cs not_active IP Right Cessation
- 1998-04-06 CA CA2600538A patent/CA2600538C/en not_active Expired - Lifetime
- 1998-04-06 JP JP54492298A patent/JP4094067B2/ja not_active Expired - Lifetime
- 1998-04-06 AU AU75220/98A patent/AU727186B2/en not_active Expired
- 1998-04-06 DK DK98922647T patent/DK0975219T3/da active
- 1998-04-06 US US09/402,866 patent/US6306850B1/en not_active Expired - Lifetime
- 1998-04-06 CA CA2846948A patent/CA2846948C/en not_active Expired - Lifetime
- 1998-04-06 KR KR10-1999-7009141A patent/KR100525469B1/ko not_active IP Right Cessation
- 1998-04-06 CA CA002286772A patent/CA2286772C/en not_active Expired - Lifetime
- 1998-04-06 PL PL336226A patent/PL191483B1/pl unknown
- 1998-04-06 HU HU0001682A patent/HU227139B1/hu active Protection Beyond IP Right Term
- 1998-04-06 CA CA2944940A patent/CA2944940C/en not_active Expired - Lifetime
- 1998-04-06 WO PCT/EP1998/001986 patent/WO1998047367A1/de active IP Right Grant
- 1998-04-06 TR TR1999/02400T patent/TR199902400T2/xx unknown
- 1998-04-06 ID IDW991231A patent/ID22820A/id unknown
- 1998-04-17 ZA ZA983236A patent/ZA983236B/xx unknown
- 1998-06-04 UA UA99116269A patent/UA55451C2/uk unknown
-
2000
- 2000-09-25 HK HK00106059A patent/HK1026822A1/xx not_active IP Right Cessation
-
2001
- 2001-04-26 US US09/843,396 patent/US20020173529A1/en not_active Abandoned
-
2005
- 2005-12-21 NL NL350024C patent/NL350024I2/nl unknown
-
2006
- 2006-12-01 FR FR06C0041C patent/FR06C0041I2/fr active Active
- 2006-12-15 NL NL350031C patent/NL350031I2/nl unknown
-
2009
- 2009-06-10 US US12/481,947 patent/US8637534B2/en not_active Expired - Fee Related
-
2010
- 2010-10-15 FR FR10C0046C patent/FR10C0046I2/fr active Active
- 2010-12-06 FR FR10C0051C patent/FR10C0051I2/fr active Active
-
2013
- 2013-01-15 US US13/742,052 patent/US8846738B2/en not_active Expired - Fee Related
- 2013-03-05 US US13/786,295 patent/US20130296389A1/en not_active Abandoned
- 2013-11-08 US US14/075,149 patent/US9253982B2/en not_active Expired - Fee Related
-
2014
- 2014-08-25 US US14/467,653 patent/US9445601B2/en not_active Expired - Fee Related
-
2015
- 2015-08-17 FR FR15C0057C patent/FR15C0057I2/fr active Active
- 2015-12-28 US US14/979,997 patent/US20160106105A1/en not_active Abandoned
Cited By (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN100403901C (zh) * | 2002-03-07 | 2008-07-23 | 巴斯福股份公司 | 基于三唑的杀真菌混合物 |
| CN101184396B (zh) * | 2005-05-24 | 2012-05-30 | 拜尔农作物科学股份公司 | 杀真菌活性化合物结合物 |
| CN102349516A (zh) * | 2011-08-18 | 2012-02-15 | 陕西美邦农药有限公司 | 一种含丙硫菌唑与嘧菌环胺的杀菌组合物 |
| CN102258033A (zh) * | 2011-08-19 | 2011-11-30 | 陕西美邦农药有限公司 | 一种含丙硫菌唑与三唑类的新型农药组合物 |
| CN102273461A (zh) * | 2011-08-25 | 2011-12-14 | 陕西美邦农药有限公司 | 一种含丙硫菌唑与三唑类的增效农药组合物 |
| CN102258034A (zh) * | 2011-08-30 | 2011-11-30 | 陕西美邦农药有限公司 | 一种含丙硫菌唑与三唑类的杀菌组合物 |
| CN102273462A (zh) * | 2011-09-05 | 2011-12-14 | 陕西美邦农药有限公司 | 一种含丙硫菌唑与三唑类的新型农药组合物 |
| CN103957703A (zh) * | 2011-11-14 | 2014-07-30 | 江苏龙灯化学有限公司 | 处理真菌感染的方法、杀真菌组合物及其用途 |
| CN103314964A (zh) * | 2012-03-20 | 2013-09-25 | 陕西韦尔奇作物保护有限公司 | 一种含环酰菌胺与三唑类的农药组合物 |
| CN102657186A (zh) * | 2012-04-24 | 2012-09-12 | 杭州宇龙化工有限公司 | 一种含有噻呋酰胺与丙硫菌唑的杀菌组合物 |
| CN103651371A (zh) * | 2012-09-07 | 2014-03-26 | 陕西美邦农药有限公司 | 一种含苯酰菌胺的高效杀菌组合物 |
| CN103651425A (zh) * | 2012-09-18 | 2014-03-26 | 陕西美邦农药有限公司 | 一种含醚菌胺的农药组合物 |
| CN103651425B (zh) * | 2012-09-18 | 2015-12-16 | 陕西美邦农药有限公司 | 一种含醚菌胺的农药组合物 |
| CN102972420A (zh) * | 2012-12-18 | 2013-03-20 | 广西农喜作物科学有限公司 | 一种含丙硫菌唑的杀菌组合物 |
| CN103461362A (zh) * | 2013-09-05 | 2013-12-25 | 江苏龙灯化学有限公司 | 一种杀菌活性成分组合物 |
| CN103461362B (zh) * | 2013-09-05 | 2014-12-24 | 江苏龙灯化学有限公司 | 一种杀菌活性成分组合物 |
| CN103563910A (zh) * | 2013-10-23 | 2014-02-12 | 江苏丰登农药有限公司 | 一种含丙硫菌唑和井冈霉素的杀菌组合物及其应用 |
| CN103563910B (zh) * | 2013-10-23 | 2015-04-01 | 江苏丰登作物保护股份有限公司 | 一种含丙硫菌唑和井冈霉素的杀菌组合物及其应用 |
| CN104642331A (zh) * | 2013-11-15 | 2015-05-27 | 南京华洲药业有限公司 | 一种含丙硫菌唑和戊唑醇的杀菌组合物及其应用 |
| CN104642330A (zh) * | 2013-11-15 | 2015-05-27 | 南京华洲药业有限公司 | 一种含丙硫菌唑和三唑酮的杀菌组合物及其应用 |
| CN104621158A (zh) * | 2013-11-15 | 2015-05-20 | 南京华洲药业有限公司 | 一种含丙硫菌唑和丙森锌的杀菌组合物及其应用 |
| CN104621131A (zh) * | 2013-11-15 | 2015-05-20 | 南京华洲药业有限公司 | 一种含丙硫菌唑和恶唑菌酮的杀菌组合物及其应用 |
| CN104642334A (zh) * | 2013-11-15 | 2015-05-27 | 南京华洲药业有限公司 | 一种含丙硫菌唑和嘧菌环胺的杀菌组合物及其应用 |
| CN103783046A (zh) * | 2013-12-09 | 2014-05-14 | 海利尔药业集团股份有限公司 | 一种含有丙硫菌唑与嘧霉胺的杀菌组合物 |
| CN104904723A (zh) * | 2014-03-11 | 2015-09-16 | 江苏龙灯化学有限公司 | 一种杀菌组合物 |
| CN103931623A (zh) * | 2014-04-30 | 2014-07-23 | 海利尔药业集团股份有限公司 | 一种含有硝苯菌酯与丙硫菌唑的杀菌组合物 |
| CN104012555A (zh) * | 2014-06-25 | 2014-09-03 | 深圳诺普信农化股份有限公司 | 一种农用杀菌组合物 |
| CN104026141A (zh) * | 2014-06-27 | 2014-09-10 | 深圳诺普信农化股份有限公司 | 一种农用杀菌组合物 |
| CN104026141B (zh) * | 2014-06-27 | 2015-08-12 | 深圳诺普信农化股份有限公司 | 一种农用杀菌组合物 |
| CN106417311A (zh) * | 2015-08-12 | 2017-02-22 | 四川利尔作物科学有限公司 | 种子处理剂组合物及其应用 |
| CN107242242A (zh) * | 2017-08-02 | 2017-10-13 | 广东广康生化科技股份有限公司 | 包含灭菌丹和丙硫菌唑的杀菌组合物 |
| WO2019153897A1 (en) * | 2018-02-09 | 2019-08-15 | Jiangsu Rotam Chemistry Co., Ltd | Fungicidal composition and the use thereof in controlling undesired fungal infestations |
| CN111601506A (zh) * | 2018-02-09 | 2020-08-28 | 江苏龙灯化学有限公司 | 杀真菌组合物及其在控制不希望的真菌感染中的用途 |
| CN108477193A (zh) * | 2018-07-03 | 2018-09-04 | 广东广康生化科技股份有限公司 | 一种含灭菌丹与腈菌唑的农药组合物 |
| CN108477192A (zh) * | 2018-07-03 | 2018-09-04 | 广东广康生化科技股份有限公司 | 一种含灭菌丹与己唑醇的高效杀菌组合物 |
| CN109042699A (zh) * | 2018-07-19 | 2018-12-21 | 广东广康生化科技股份有限公司 | 包含灭菌丹与代森锌的增效杀菌组合物 |
| CN114206114A (zh) * | 2019-06-05 | 2022-03-18 | Upl有限责任公司 | 用于防治植物中的发酵壳针孢感染的杀真菌剂组合物 |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1109499C (zh) | 杀菌剂活性物质组合 | |
| CN1162083C (zh) | 含有咪蚜胺和杀真菌剂的组合物及其应用 | |
| CN1031971C (zh) | 包含(亚苄基)-唑基甲基环烷烃及其杀菌剂组合物 | |
| CN1053905C (zh) | 噁二嗪衍生物、其制备方法和用途及制备其用的中间体 | |
| CN1152007C (zh) | 杀虫剂 | |
| CN1067063C (zh) | 取代的羧酰苯胺衍生物和含其作为活性成分的植物疾病控制剂 | |
| CN1083437C (zh) | 2-[1’,2’,4’-三唑-3’-基氧基亚甲基]酰苯胺类、它们的制备和应用 | |
| CN1016484B (zh) | 含取代的四唑啉酮的组合物 | |
| CN1566113A (zh) | 防治植物害虫的组合物 | |
| CN1636455A (zh) | 保护作物的组合物及控制与预防植物病害的方法 | |
| CN1019260B (zh) | 含喹唑啉衍生物的杀真菌剂 | |
| CN1915019A (zh) | 杀菌剂组合物 | |
| CN1096446C (zh) | N-(邻位取代苄氧基)亚胺衍生物及其用作杀真菌剂、杀螨剂或杀虫剂的用途 | |
| CN1040388C (zh) | 增效除草剂 | |
| CN87103906A (zh) | 新的嘧啶衍生物的制备和应用 | |
| CN1091426A (zh) | 吲唑衍生物 | |
| CN1026697C (zh) | 含β-苯氧基腈的杀菌组合物及其制备方法和应用 | |
| CN1325268A (zh) | 含噻吩并[2,3-d]嘧啶-4-酮的杀真菌混剂 | |
| CN1214717C (zh) | 杀真菌混合物 | |
| CN1206914C (zh) | 含o-苄基肟醚衍生物的农药及其应用 | |
| CN1061406A (zh) | 杀霉的3,3-双烷硫基-2-吡啶基丙烯酸衍生物 | |
| CN1023753C (zh) | 含咪唑烷衍生物的杀虫剂 | |
| CN1675996A (zh) | 水稻作物的杀真菌组合物和水稻作物的病害防治方法 | |
| CN1075478A (zh) | 唑类衍生物除草剂和杀菌剂 | |
| CN1039009C (zh) | 噁唑啉衍生物及其制备方法和杀虫、杀螨或杀真菌组合物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| ASS | Succession or assignment of patent right |
Owner name: BAYER INTELLECTUAL PROPERTY GMBH Free format text: FORMER OWNER: BAYER AKTIENGESELLSCHAFT Effective date: 20150604 |
|
| C41 | Transfer of patent application or patent right or utility model | ||
| TR01 | Transfer of patent right |
Effective date of registration: 20150604 Address after: German Monheim Patentee after: Bayer Pharma Aktiengesellschaft Address before: Germany Leverkusen Patentee before: Bayer Aktiengesellschaft |
|
| CX01 | Expiry of patent term | ||
| CX01 | Expiry of patent term |
Granted publication date: 20030528 |