CN1213974A - 吸入器具 - Google Patents
吸入器具 Download PDFInfo
- Publication number
- CN1213974A CN1213974A CN96180172A CN96180172A CN1213974A CN 1213974 A CN1213974 A CN 1213974A CN 96180172 A CN96180172 A CN 96180172A CN 96180172 A CN96180172 A CN 96180172A CN 1213974 A CN1213974 A CN 1213974A
- Authority
- CN
- China
- Prior art keywords
- utensil
- passage
- medicine
- outlet
- nest chamber
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000000843 powder Substances 0.000 claims abstract description 22
- 150000001875 compounds Chemical class 0.000 claims abstract description 4
- 239000003814 drug Substances 0.000 claims description 42
- 238000007789 sealing Methods 0.000 claims description 24
- 241000239290 Araneae Species 0.000 claims description 9
- 230000008859 change Effects 0.000 claims description 3
- 230000008602 contraction Effects 0.000 claims description 3
- 230000007246 mechanism Effects 0.000 claims description 3
- ARAIBEBZBOPLMB-UFGQHTETSA-N zanamivir Chemical compound CC(=O)N[C@@H]1[C@@H](N=C(N)N)C=C(C(O)=O)O[C@H]1[C@H](O)[C@H](O)CO ARAIBEBZBOPLMB-UFGQHTETSA-N 0.000 claims description 3
- 229960001028 zanamivir Drugs 0.000 claims description 3
- 229920002725 thermoplastic elastomer Polymers 0.000 claims description 2
- 239000008187 granular material Substances 0.000 description 6
- 238000013467 fragmentation Methods 0.000 description 4
- 238000006062 fragmentation reaction Methods 0.000 description 4
- 238000000465 moulding Methods 0.000 description 4
- 239000002245 particle Substances 0.000 description 4
- GIIZNNXWQWCKIB-UHFFFAOYSA-N Serevent Chemical compound C1=C(O)C(CO)=CC(C(O)CNCCCCCCOCCCCC=2C=CC=CC=2)=C1 GIIZNNXWQWCKIB-UHFFFAOYSA-N 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 239000008393 encapsulating agent Substances 0.000 description 3
- 239000004033 plastic Substances 0.000 description 3
- 229920003023 plastic Polymers 0.000 description 3
- 210000002345 respiratory system Anatomy 0.000 description 3
- XWTYSIMOBUGWOL-UHFFFAOYSA-N (+-)-Terbutaline Chemical compound CC(C)(C)NCC(O)C1=CC(O)=CC(O)=C1 XWTYSIMOBUGWOL-UHFFFAOYSA-N 0.000 description 2
- SNICXCGAKADSCV-JTQLQIEISA-N (-)-Nicotine Chemical compound CN1CCC[C@H]1C1=CC=CN=C1 SNICXCGAKADSCV-JTQLQIEISA-N 0.000 description 2
- KUVIULQEHSCUHY-XYWKZLDCSA-N Beclometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)COC(=O)CC)(OC(=O)CC)[C@@]1(C)C[C@@H]2O KUVIULQEHSCUHY-XYWKZLDCSA-N 0.000 description 2
- -1 acryloyl butyl Chemical group 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- NDAUXUAQIAJITI-UHFFFAOYSA-N albuterol Chemical compound CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1 NDAUXUAQIAJITI-UHFFFAOYSA-N 0.000 description 2
- 229950000210 beclometasone dipropionate Drugs 0.000 description 2
- 238000000354 decomposition reaction Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 229960000289 fluticasone propionate Drugs 0.000 description 2
- WMWTYOKRWGGJOA-CENSZEJFSA-N fluticasone propionate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(OC(=O)CC)[C@@]2(C)C[C@@H]1O WMWTYOKRWGGJOA-CENSZEJFSA-N 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 238000000034 method Methods 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 229960002715 nicotine Drugs 0.000 description 2
- SNICXCGAKADSCV-UHFFFAOYSA-N nicotine Natural products CN1CCCC1C1=CC=CN=C1 SNICXCGAKADSCV-UHFFFAOYSA-N 0.000 description 2
- 229960002052 salbutamol Drugs 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 239000012453 solvate Substances 0.000 description 2
- 229960000195 terbutaline Drugs 0.000 description 2
- VOVIALXJUBGFJZ-KWVAZRHASA-N Budesonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O VOVIALXJUBGFJZ-KWVAZRHASA-N 0.000 description 1
- WJOHZNCJWYWUJD-IUGZLZTKSA-N Fluocinonide Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)COC(=O)C)[C@@]2(C)C[C@@H]1O WJOHZNCJWYWUJD-IUGZLZTKSA-N 0.000 description 1
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 229940057282 albuterol sulfate Drugs 0.000 description 1
- BNPSSFBOAGDEEL-UHFFFAOYSA-N albuterol sulfate Chemical compound OS(O)(=O)=O.CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1.CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1 BNPSSFBOAGDEEL-UHFFFAOYSA-N 0.000 description 1
- 239000003443 antiviral agent Substances 0.000 description 1
- 208000006673 asthma Diseases 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 244000144987 brood Species 0.000 description 1
- 229960004436 budesonide Drugs 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 238000000151 deposition Methods 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- 229920006351 engineering plastic Polymers 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 229960000785 fluocinonide Drugs 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 229960002714 fluticasone Drugs 0.000 description 1
- MGNNYOODZCAHBA-GQKYHHCASA-N fluticasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(O)[C@@]2(C)C[C@@H]1O MGNNYOODZCAHBA-GQKYHHCASA-N 0.000 description 1
- 229960002848 formoterol Drugs 0.000 description 1
- BPZSYCZIITTYBL-UHFFFAOYSA-N formoterol Chemical compound C1=CC(OC)=CC=C1CC(C)NCC(O)C1=CC=C(O)C(NC=O)=C1 BPZSYCZIITTYBL-UHFFFAOYSA-N 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 238000012797 qualification Methods 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 229960004017 salmeterol Drugs 0.000 description 1
- 229920003031 santoprene Polymers 0.000 description 1
- 230000035939 shock Effects 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 239000012808 vapor phase Substances 0.000 description 1
- 229950000339 xinafoate Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M11/00—Sprayers or atomisers specially adapted for therapeutic purposes
- A61M11/001—Particle size control
- A61M11/002—Particle size control by flow deviation causing inertial separation of transported particles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
- A61M15/0046—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier
- A61M15/0048—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier the dosages being arranged in a plane, e.g. on diskettes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0065—Inhalators with dosage or measuring devices
- A61M15/0068—Indicating or counting the number of dispensed doses or of remaining doses
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D83/00—Containers or packages with special means for dispensing contents
- B65D83/04—Containers or packages with special means for dispensing contents for dispensing annular, disc-shaped, spherical or like small articles, e.g. tablets or pills
- B65D83/0445—Containers or packages with special means for dispensing contents for dispensing annular, disc-shaped, spherical or like small articles, e.g. tablets or pills all the articles being stored in individual compartments
- B65D83/0454—Containers or packages with special means for dispensing contents for dispensing annular, disc-shaped, spherical or like small articles, e.g. tablets or pills all the articles being stored in individual compartments the whole forming a circular container with rotating parts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/06—Solids
- A61M2202/064—Powder
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D2583/00—Containers or packages with special means for dispensing contents
- B65D2583/04—For dispensing annular, disc-shaped or spherical or like small articles or tablets
- B65D2583/0404—Indications, e.g. directions for use
- B65D2583/0409—Indications, e.g. directions for use of dates or follow-numbers
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Public Health (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Anesthesiology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Biomedical Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pulmonology (AREA)
- Biophysics (AREA)
- Mechanical Engineering (AREA)
- Medicinal Preparation (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Catching Or Destruction (AREA)
- Manipulator (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
Abstract
一种粉末吸入器具,它包括其内含有一种药理上有效的混合物或药物的壳体、一延伸到壳体内并带有一出口的通道(8)、一向通道分配化合物剂量的计量单元(6、7)以及设置在所述通道内帮助破碎夹带在气流中的粉末团的阻流板(9),使用者通过通道吸药物,以形成通过通道的气流,阻流板(9)包括多个交错排列的板,这些板从通道的两相对侧延伸到通道内,它们与通道侧的夹角小于90°,并朝向出口倾斜,在通道内形成多个收缩,并使通过通道的所述空气流的方向多次改变。
Description
本发明涉及一种吸入器具,借助于这种吸入器具可以将以粉状形式的计量药剂分配给使用者。
已知吸入器具是用于病人呼吸道或肺的药物局部服用的。在英国专利文件GB2178965中描述了这样一种称之为DISKHALER吸入器的器具,这种器具与泡状包结合使用,粉末状药物保持在泡状包中。在使用时,将新的泡状包对准使用部位,然后用一刺穿件通过两个特殊的动作刺破泡状包,使药物能从泡状包中被吸入。
另一种吸入器具在英国专利文件GB2242134中予以描述,这种吸入器具结合可剥离的药包使用,该药包中有几个容纳药物的容器,该吸入器具包括一用于剥开药包的器件,当各容器对正使用部位时剥离件依次打开各容器。这种器具多少有些复杂,并且包含很多部件。
通过吸入服用的药物应该具有控制的粒度,以便获得最大的穿入肺部的效果,优选的粒径为1至10微米,更优选为1至5微米。不幸的是,在这样的粒度范围内的粉末(后面称之为细粉末),例如微粒化的粉末的流动特性通常非常差,这是因为各颗粒之间具有粘合力,该力使得它们很容易结团架桥,而这些桥不易破开形成自由流动。这些特性造成了处理和计量的困难,而处理和测量的困难又反过来影响粉末剂量的精确分送。然而,共同未决的PCT专利文件EP96.03274描述了如果通过仔细选定细小结团颗粒的尺寸,能够利用颗粒之间的粘合力形成自由流动的粉末团。这些粉末团很容易处理,并且可以很便利地用来将这些粉末团充填于器具。
然而,为了向肺中有效的发送,必须在粉末团离开器具之前将其破碎成一种控制的尺寸。
已经发现,如果在粉末吸入器具的嘴部装有一系列阻流板,当使用者吸入时就能将粉末团在汽流中破碎。专利文件EP0237507描述了包括有螺旋通道部分的阻流板,这种阻流板使气流具有旋转螺旋的运动方式。
然而,专利文件EP0237507中的一个缺点是阻流板包括多个部件,这就使得器具制造过程相对复杂和昂贵。
专利文件GB2191718涉及用于分发尼古丁的烟雾器。尼古丁分发器有一设计成分离喷雾的冲击件,使较小的颗粒和蒸汽相围绕阻流板流动,而将较大的颗粒去除。
专利文件PCT/EP93/00582也描述了一种包括阻流板的器具,阻流板用作分离器,如果烟雾中含有较大的颗粒,这些颗粒呈较松散的团的形式,则当它们以足够高的速度冲击阻流板时,这些颗粒团的尺寸就能减小。
在专利文件GB2191718和PCT/EP93/00582中描述的这两组阻流板的缺点是存在大量的粉末沉积,由于阻流板以与气流的流向成90°的方向从器具的壁延伸出,所以这种情况是有可能发生的。这可能会导致更大的粉末团沉积的发生,这种团在后面的吸入中会变松,从而导致剂量的变化。
本发明的一个目的是提供一种刚刚描述过的器具,它易于使用者操作,并且组装起来不过分复杂。本发明的进一步的目的是提供一种器具,它可以充以粉末团,但是发送的却是适合于通过吸入服用的粉末。
按照本发明提供一种吸入器具,它包括一壳体;一出口,使用者可以通过该出口吸入药物;一限定至少一个窝腔的药物容纳器,该窝腔容纳一次剂量的粉末状药物;一封闭件,其具有至少一个由弹性力压迫与药物容纳器接触以封闭至少一个所述窝腔的封闭垫;用于将窝腔移动到与出口对正的位置的装置;以及用于当窝腔与出口对正时提升封闭垫,使其离开药物容纳器,以便打开窝腔的装置。
这种器具易于操作,只需一个特殊的动作就可从中吸入药物。这种器具还可以包括较少的部件。
按照本发明,还提供一种粉末吸入器具,它包括其内含有一种药理上有效的混合物或药物的壳体、一延伸到壳体内并带有一出口的通道、一向通道分配化合物剂量的计量单元以及设置在所述通道内帮助破碎夹带在空气流中的粉末团的阻流板,使用者通过通道吸药物,以产生通过通道的气流,其特征在于:阻流板包括多个交错排列的板,这些板从通道的两相对侧延伸到通道内,与通道侧的夹角小于90°,并朝向出口倾斜,以在通道内形成若干收缩,并使通过通道的所述气流的方向发生多次改变。
通过使用这种结构,可以提供易于制造的阻流板,并使粉末在板上的沉积少,这种隔板在将结团破碎成不连续构成的颗粒以形成可吸入大小的粉末方面具有良好的效果。
这种器具优选具有至少两块板,更优选具有四块板。
优选使所述板相对于通道的纵轴线在空气流方向的倾角小于70°,更优选使该倾角在15°至50°之间。
倒数第二块阻流板取如下形状是适合的,该板沿其上某些点划分成至少两面,第一面连续延伸到通道内,而第二面基本上平行于通道的纵轴线向着出口延伸。这使得空气流能够平行于所述轴线排出,将药物直接喷入使用者的呼吸道,而不是进入其面颊腔。
下面参考附图进一步描述本发明的一实施例,其中:
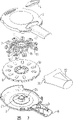
图1是按照本发明的一组装的装置的透视图;
图2是图1所示装置的一分解透视图,图中示出主体部件和基本组件;
图3是一分解透视图,以拆开的形式示出各部件;
图4是一部分剖开的平面视图,示出通过本发明的一优选实施例的空气流;
图5是一透视图,示出通过图4所示装置的空气流;和
图6是一剖视图,示出窝封闭垫处于提起的位置。
图1至3所示的装置包括一主体1,它由一主体基部2和一主体顶部3构成,二者都可由一种比如丙烯酰丁基丙乙烯(acryl-butylstyrene)(ABS)的塑料材料模塑而成。主体顶部3上形成有一窗口4,其目的下面将要描述。主体1限定一适合于容纳一基本组件的主壳体部分和一嘴部8,基本组件包括一剂量环6和辐射架形式的封闭件7;嘴部8形成一从主壳体部分径向延伸出的出口,使用者可以通过该出口吸入药物。从图2中可以看出,主体基部2的嘴部8上形成有多个阻流板9,后面将对此作出解释。
一以盘或剂量环6形式的药物保持器包括一上表面,其上形成若干腔室或窝腔10,用于容纳需要从器具中分发的可吸入粉末状药物。每个窝腔10由一凸唇(未示出)所围绕。所示的剂量环上设有十个窝腔10,这十个窝腔可能适合于容纳十天的药量,也可能容纳足够一个疗程的药量。应当理解,剂量环可能带有多一些或少一些的窝腔,这取决于需容纳的药物量。适用的粉末状药物为,例如用于治疗气喘的药,包括舒喘灵、二丙酸氯地米松、沙美特罗、氟替卡松、福莫特罗、叔丁喘宁、丁地去炎松和9-去氟肤轻松,以及生理上可接受的盐、溶剂化物和酯或其任何组合。优选的药物为舒喘灵、硫酸舒喘灵、沙美特罗、克赛纳弗盐沙美米特罗(xinafoate salmeterol)、丙酸氟替卡松(fluticasonepropionate)、丙酸盐二丙酸氯地米松或其溶剂化物和硫酸盐叔丁喘宁。其它适用的粉末状药物包括抗病毒药物,例如匝纳米维亚(zanamivir)(4-胍-Neu-5-Ac-2-en)。熟悉本领域的技术人员可以理解,如果需要的话,粉末状药物可以是两种或多种有效成分的组合。一次的剂量可以包含一个或多个腔室的含量,腔室的大小取决于需分发的剂量。应该理解药物粉末可以纯粹由一种或多种有效成分构成,也可以有附加载体,例如乳糖粉。
按照在共同未决的PCT专利申请EP96.03274中描述的方法和装置,剂量环6中可以充以成团状的粉末。
剂量环6具有一阶梯状的周边,其目的将在后面予以描述,其中心具有一花键孔,用于与辐射架7接合。剂量环也可以由一种塑料,例如ABS模塑而成。
辐射架7包括一中心环形部分11,多个弹性径向臂12从该部分伸出,弹性径向臂的数量对应于剂量环6中的窝腔10的数量。各径向臂12的端部是一窝腔封闭垫13。多个短弹性钩状薄片14从中心环形部分11沿轴向伸出,并适合于通过与剂量环6中心的花键孔相接合形成一种与剂量环6的咬合连接。辐射架7可以由一种塑料材料模塑而成,例如聚碳酸酯或其它具有所需弹性特征的工程塑料。当辐射架7与剂量环6组装成一剂量元件或基本组件时,钩状薄片14与剂量环6上的花键孔紧密接合,弹性径向臂12的弹性力迫使各窝腔封闭垫13与剂量环6的上表面上的相应的凸起接触,盖住并密封相应的窝腔10,以防止药物从窝腔中逸出以及湿气浸入窝腔。窝腔封闭垫上可以模塑出一直接密封在剂量环6或凸唇28上的光滑平接触面(见图6),也可以设置一种在接触面上形成的热塑弹性体一类的软的密封材料,例如SANTOPRENE(拜尔),来改善密封特性。为了制造窝腔封闭垫上带有不同的密封材料的辐射架,在辐射架上可以模塑出一通道15,该通道形成于中心环形部分11的上表面和各径向臂12内,并模塑出一小孔(未示出),该孔从各径向臂12内的通道15的端部通向窝腔封闭垫13的下侧。然后可以使一种熔融状态的密封材料环通道15流动,并通过孔流到各窝封闭垫13的下侧,以便在冷却后在各垫上形成一种整体垫密封。
窝腔封闭垫13的上表面可以模塑出或印上一数字17或其它指示装置,指示在器具中有多少剂量的药,这在下面将更详细地解释。
在各窝腔封闭垫13的边缘上形成一适合于与主体顶部3整体形成的一斜面20(见图4和5)接合的突舌16,以影响窝腔的开启,这在后面将予以解释。
基本组件安装在主体1的主壳体部分内,如图2和3所示,使主体基部2的轴21位于基本组件的孔内,剂量环的阶梯周边穿入主壳体部分的相对侧上的两隔开部分内。主体基部2上的爪24与剂量环6的下侧上的齿(未示出)接合,以形成一种只允许剂量环在一个方向上运动的棘轮机构。
窝腔10中可以充填在PCT专利申请PCT EP96.03274中描述的成团状粉末。当这样充填时,对于吸入治疗,基本上是最大可能的吸入基本颗粒量处在可吸入的范围内,该范围小于5微米。为了帮助破碎夹带在空气流中的任何粉末团,在嘴部8中装有一系列阻流板9。后面将要解释,阻流板的位置是很重要的。阻流板可以与主体模塑成一整体,从而能够承受一致的、受控的和可重复的容许极限。可以理解,阻流板的数量和精确尺寸取决于所用的粉末和将粉末团保持在一起的力的大小。例如,已经发现,四个阻流板达到所需的匝纳米维亚(zanamivir)粉末团的破碎。对于熟悉本领域的技术人员来说,很容易对阻流板的尺寸进行实验,以对特定粉末获得所需的流动阻力。
图4和5示出一种阻流板9的优选结构,这种阻流板包括多个交错排列的平板,它们以相对于通道的纵轴线成30°的角度从壳体的相对侧延伸到通道内。多数阻流板超过通道的纵轴线,而最后的阻流板25在空气流动方向上终止于不到轴线处。倒数第二个阻流板26在空气流动方向上具有一平行于通道的纵轴线向着出口延伸的平面27。这使得气流平行于所述轴线流出,直接将药粉发送到使用者的呼吸道内,而不是使用者的面颊腔部。
一嘴盖22适用于当器具不使用时盖住嘴部8,以防止嘴部被灰尘污染。嘴盖带有两个销钉30,它们适合于通过两个槽23穿入主壳体1上的嘴部8的两侧与剂量环的阶梯状周边接合,并从而锁定基本组件,防止器具不使用时的意外移动。要使用器具时,适用者首先要去掉嘴盖22,从而将基本组件解锁。然后使用者通过使剂量环上凸出于主壳体部分上的切开部分之外的阶梯状周边相对于主体1转动剂量环6找到一个窝腔,将其与嘴对正。上面描述的棘轮机构使基本组件只能在一个方向上转动。当基本组件旋转时,下一个窝腔封闭垫13上的突舌16与斜面20接合,当突舌跨在斜面上时,将窝腔封闭垫13提升,使其离开剂量环6的上表面。当窝腔与嘴对正时,围绕着窝腔10的凸唇28(见图6)与出口通道29的侧壁接合,形成对剂量环的运动的增大的阻力,从而清楚地告诉使用者,剂量环已经处于准备发送药物的位置。
当围绕着窝腔10的凸唇28与出口通道29接合时,窝腔封闭垫13处于离开剂量环6的上表面的一升高的位置,如图6所示。在该位置,窝腔封闭垫13直接位于主体顶部3中的窗口4的下面,因此模塑或印在窝腔封闭垫13的上表面上的数字17或其它剂量指示装置可以在窗口4上清楚地看出。这使得使用者看到在器具内还有多少剂药。
现已经准备好发送在窝腔10中所含的药物。使用者通过嘴8吸入。空气通过槽3吸入器具并被引导到窝腔10的上方,如图4和5所示。当空气流过窝腔10时,窝腔中的药粉被空气流中的旋涡夹带,通过嘴部8吸走,并被使用者吸入。
从图5中看出,设置阻流板9是为了破坏空气穿过嘴部的光滑流动,以形成附加的旋涡,使得空气流几次变化方向,从而引起任何粉末团冲击阻流板、壁和其它团,并使空气流收缩,以提高流速。空气循环产生阻流板回流中的涡流,引起进一步的碰撞,帮助粉末团的破碎。图5中表示空气流动的箭头示出这种情况。所有这些作用都促进了空气流中夹带的粉末团的破碎,使粉末具有适合于吸入治疗的形式。平行于通道的纵轴线在空气流动方向上延伸并连接于导数第二阻流板的平面27使空气流能够平行于所述轴线离开嘴部,直接将粉末发送到使用者的呼吸道中,而不是使用者的面颊腔中。
吸入以后,使用者重新盖上嘴盖22,保护并锁闭器具,直到需要吸入下一剂药。
应该理解,本公开只是为了说明的目的,本发明延伸到对其作出的改善、变化和改进。
Claims (27)
1.一种吸入器具,它包括一壳体;一出口,使用者可以通过该出口吸入药物;一限定至少一个窝腔的药物容纳器,该窝腔容纳一次剂量的粉末状药物;一封闭件,其具有至少一个由弹性力压迫与药物容纳器接触以封闭至少一个所述窝腔的封闭垫;用于将窝腔移动到与出口对正的位置的装置;以及用于当窝腔与出口对正时提升封闭垫,使其离开药物容纳器,以便打开窝腔的装置。
2.一种如权利要求1所述的器具,其特征在于:所述药物容纳器包括一可旋转地安装在器具内的盘,至少一个窝腔设置在所述盘的一面上。
3.一种如权利要求1或2所述的器具,其特征在于:各所述封闭垫受到一相应的弹性臂的弹性压迫与药物容纳器接触。
4.一种如权利要求3所述的器具,其特征在于:其包括若干封闭垫,封闭件包括多个弹性臂,它们以辐射架的形状连接在一起。
5.一种如权利要求4所述的器具,其特征在于:所述辐射架由聚碳酸酯制成。
6.一种如前述任一权利要求所述的器具,其特征在于:所述封闭垫包括一种热塑弹性体。
7.一种如权利要求2至6中任一项所述的器具,其特征在于:所述盘从器具的主体中凸出,以提供用于移动至少一个窝腔,使其与出口对正的装置。
8.一种如权利要求7所述的器具,其特征在于:所述盘的周边上带有抓手。
9.一种如权利要求7或8中任一项所述的器具,其特征在于:其还包括一棘轮机构以便只允许盘沿一个方向运动。
10.一种如前述任一权利要求所述的器具,其特征在于:窝腔封闭垫通过与一斜面接合而提高,离开所述盘的表面。
11.一种如前述任一权利要求所述的器具,其特征在于:其还包括一可卸下的嘴盖,当它盖住时与药物容纳器接合,以防止其运动。
12.一种如前述任一权利要求所述的器具,其特征在于:出口包括一通道,其内设置多个交错排列的板,这些板以相对于通道侧小于90°的倾角从通道的两相对侧伸入通道内,并且这些板向出口倾斜,以在通道内形成多个收缩,并多次改变通过通道的气流的方向。
13.一种如权利要求12所述的器具,其特征在于:包括至少两个板。
14.一种如权利要求13所述的器具,其特征在于:包括四个板。
15.一种如权利要求12至14中任一项所述的器具,其特征在于:所述板在气流的方向上相对于通道的纵轴线的倾角小于70°。
16.一种如权利要求15所述的器具,其特征在于:所述板以15至50°的角度倾斜。
17.一种如权利要求16所述的器具,其特征在于:所述板以30°角倾斜。
18.一种如权利要求12至17中任一项所述的器具,其特征在于:倒数第二个板取这样的形状,在沿板的某些点上它划分成至少两个面,第一面连续延伸到通道内,而第二面基本上平行于通道的纵轴线向出口延伸。
19.一种如前述任一权利要求所述的器具,其特征在于:所述窝腔中充以药物。
20.一种粉末吸入器,它包括其内含有一种药物的壳体、一延伸到壳体内并带有一出口的通道、一向通道分配化合物剂量的计量单元以及设置在所述通道内帮助破碎夹带在气流中的粉末团的阻流板,使用者通过通道吸药物,以产生通过通道的气流其特征在于:阻流板包括多个交错排列的板,这些板从通道的两相对侧延伸到通道内,其与通道侧的夹角小于90°,并朝向出口倾斜,在通道内形成多个收缩,并使通过通道的所述气流的方向多次改变。
21.一种如权利要求20所述的器具,其特征在于:包括至少两个板。
22.一种如权利要求21所述的器具,其特征在于:包括四个板。
23.一种如权利要求20至22中任一项所述的器具,其特征在于:所述板在气流的方向上以相对于通道的纵轴线小于70°的倾角倾斜。
24.一种如权利要求23所述的器具,其特征在于:所述板以15至50°的倾角倾斜。
25.一种如权利要求24所述的器具,其特征在于:所述板以30°倾角倾斜。
26.一种如权利要求20至25中任一项所述的器具,其特征在于:倒数第二个板取这样的形状,在沿板的某些点上它划分成至少两个面,第一面连续延伸到通道内,而第二面基本上平行于通道的纵轴线向出口延伸。
27.一种如前述任一权利要求所述的器具,其特征在于:其中的药物为匝纳米维亚(zanamivir)。
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB9600044.3 | 1996-01-03 | ||
| GBGB9600044.3A GB9600044D0 (en) | 1996-01-03 | 1996-01-03 | Inhalatiion device |
| GBGB9625134.3A GB9625134D0 (en) | 1996-12-03 | 1996-12-03 | Powder inhalation device |
| GB9625134.3 | 1996-12-03 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN1213974A true CN1213974A (zh) | 1999-04-14 |
Family
ID=26308412
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN96180172A Pending CN1213974A (zh) | 1996-01-03 | 1996-12-19 | 吸入器具 |
Country Status (22)
| Country | Link |
|---|---|
| US (1) | US6065472A (zh) |
| EP (2) | EP0938907B1 (zh) |
| JP (1) | JP2000503565A (zh) |
| KR (1) | KR19990077014A (zh) |
| CN (1) | CN1213974A (zh) |
| AP (1) | AP9801285A0 (zh) |
| AT (2) | ATE209939T1 (zh) |
| AU (1) | AU725348B2 (zh) |
| BR (1) | BR9612410A (zh) |
| CA (1) | CA2241880A1 (zh) |
| CZ (1) | CZ212598A3 (zh) |
| DE (2) | DE69618624T2 (zh) |
| EA (2) | EA001016B1 (zh) |
| ES (2) | ES2166473T3 (zh) |
| IL (1) | IL125183A0 (zh) |
| IS (1) | IS4787A (zh) |
| MX (1) | MX9805409A (zh) |
| NO (1) | NO983069L (zh) |
| NZ (1) | NZ324374A (zh) |
| PL (1) | PL327616A1 (zh) |
| TR (1) | TR199801265T2 (zh) |
| WO (1) | WO1997025086A2 (zh) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103252007A (zh) * | 2008-06-13 | 2013-08-21 | 曼金德公司 | 干粉吸入器和用于药物输送的系统 |
| CN103764210A (zh) * | 2011-08-19 | 2014-04-30 | 阿普塔尔法国简易股份公司 | 粉末吸入设备 |
| CN106075677A (zh) * | 2016-08-08 | 2016-11-09 | 中山市美捷时包装制品有限公司 | 一种干粉吸入装置的促动机构 |
| CN109310834A (zh) * | 2016-04-18 | 2019-02-05 | 励志私人有限公司 | 用于吸入器的间隔装置 |
| CN110799230A (zh) * | 2017-06-27 | 2020-02-14 | 韩国联合制药株式会社 | 干燥粉末吸入器 |
Families Citing this family (213)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6182655B1 (en) | 1995-12-07 | 2001-02-06 | Jago Research Ag | Inhaler for multiple dosed administration of a pharmacological dry powder |
| US7131441B1 (en) | 1995-12-07 | 2006-11-07 | Skyepharma Ag | Inhaler for multiple dosed administration of a pharmacological dry powder |
| US6006747A (en) * | 1997-03-20 | 1999-12-28 | Dura Pharmaceuticals, Inc. | Dry powder inhaler |
| US6237590B1 (en) * | 1997-09-18 | 2001-05-29 | Delsys Pharmaceutical Corporation | Dry powder delivery system apparatus |
| US20060165606A1 (en) | 1997-09-29 | 2006-07-27 | Nektar Therapeutics | Pulmonary delivery particles comprising water insoluble or crystalline active agents |
| NZ504021A (en) * | 1997-10-17 | 2003-04-29 | Systemic Pulmonary Delivery Lt | Method and apparatus for delivering aerosolized medication having air discharged through air tube directly into plume of aerosolized medication |
| AU5860098A (en) * | 1997-12-23 | 1999-07-19 | Euro-Celtique S.A. | Particle separator for an inhaler |
| NL1008031C2 (nl) * | 1998-01-15 | 1999-07-21 | Pharmachemie Bv | Inrichting voor het inhaleren van medicament. |
| AU749087B2 (en) * | 1998-03-04 | 2002-06-20 | Delsys Pharmaceutical Corporation | Medicament dry powder inhaler dispensing device |
| CN1292714B (zh) * | 1998-03-16 | 2012-08-01 | 诺瓦帝斯公司 | 气溶胶化活性剂的输送 |
| SE9801114D0 (sv) * | 1998-03-30 | 1998-03-30 | Astra Ab | Inhalation device |
| DE19831525A1 (de) * | 1998-07-14 | 2000-01-20 | Pfeiffer Erich Gmbh & Co Kg | Spender für Medien |
| UA73924C2 (en) * | 1998-10-09 | 2005-10-17 | Nektar Therapeutics | Device for delivering active agent formulation to lungs of human patient |
| GB9902493D0 (en) * | 1999-02-05 | 1999-03-24 | Glaxo Group Ltd | Inhalation device |
| US6752148B1 (en) | 1999-02-10 | 2004-06-22 | Delsys Pharmaceutical Company | Medicament dry powder inhaler dispensing device |
| GB9905538D0 (en) | 1999-03-10 | 1999-05-05 | Glaxo Group Ltd | A device |
| DE29908593U1 (de) | 1999-05-14 | 1999-07-22 | Jago Research AG, Hergiswil | Mundstück für einen Inhalator zur Abgabe eines Medikaments |
| CA2373278C (en) * | 1999-06-05 | 2011-04-05 | Innovata Biomed Limited | Medicament delivery device with measuring chamber and dispensing cup |
| US9006175B2 (en) | 1999-06-29 | 2015-04-14 | Mannkind Corporation | Potentiation of glucose elimination |
| US6606992B1 (en) * | 1999-06-30 | 2003-08-19 | Nektar Therapeutics | Systems and methods for aerosolizing pharmaceutical formulations |
| GB9920839D0 (en) * | 1999-09-04 | 1999-11-10 | Innovata Biomed Ltd | Inhaler |
| CN1157236C (zh) * | 1999-10-06 | 2004-07-14 | 劳尔·戈尔德曼 | 呼吸控制的干粉吸入装置和干粉均匀分布在空气中的方法 |
| DE10027631B4 (de) * | 1999-10-06 | 2007-11-29 | Raul Goldemann | Atemzugskontrolliertes Inhalationsgerät für Trockenpulver und Verfahren zum gleichmäßigen Verteilen des Trockenpulvers in Luft |
| GB9924415D0 (en) * | 1999-10-16 | 1999-12-15 | Glaxo Group Ltd | Medicament pack |
| SE517806C2 (sv) * | 1999-11-11 | 2002-07-16 | Microdrug Ag | Doseringsanordning för inhalator |
| US20010035184A1 (en) * | 1999-12-17 | 2001-11-01 | Carlos Schuler | Systems and methods for treating packaged powders |
| US7069929B2 (en) * | 2000-02-01 | 2006-07-04 | Quadrant Technologies Limited | Dry powder inhaler |
| SI1142601T1 (sl) * | 2000-04-07 | 2006-08-31 | Andi Ventis Ltd | Ustnik za inhalator |
| JP4741159B2 (ja) * | 2000-04-07 | 2011-08-03 | アンディベンティス リミテッド | 顆粒吸入器のためのマウスピース |
| US20030026766A1 (en) * | 2000-04-13 | 2003-02-06 | Mark Sanders | Medicaments for treating respiratory disorders comprising formoterol and fluticasone |
| US7871598B1 (en) | 2000-05-10 | 2011-01-18 | Novartis Ag | Stable metal ion-lipid powdered pharmaceutical compositions for drug delivery and methods of use |
| WO2001089616A1 (en) | 2000-05-23 | 2001-11-29 | Glaxo Group Limited | Aerosol container for formulations of salmeterol xinafoate |
| GB0015043D0 (en) | 2000-06-21 | 2000-08-09 | Glaxo Group Ltd | Medicament dispenser |
| TWI224515B (en) * | 2000-06-23 | 2004-12-01 | Norton Healthcare Ltd | Pre-metered dose magazine for breath-actuated dry powder inhaler |
| AR028747A1 (es) * | 2000-06-23 | 2003-05-21 | Norton Health Care Ltd | Desaglomerador para inhalador de polvo seco accionado por la respiracion, un inhalador de polvo seco y un metodo de desaglomeracion de polvo seco. |
| GB0015801D0 (en) * | 2000-06-28 | 2000-08-16 | Innovata Biomed Ltd | Cover |
| EP1172122A1 (en) * | 2000-07-14 | 2002-01-16 | The Technology Partnership Public Limited Company | Dry powder inhaler |
| US6902734B2 (en) | 2000-08-07 | 2005-06-07 | Centocor, Inc. | Anti-IL-12 antibodies and compositions thereof |
| US7288390B2 (en) | 2000-08-07 | 2007-10-30 | Centocor, Inc. | Anti-dual integrin antibodies, compositions, methods and uses |
| UA81743C2 (uk) | 2000-08-07 | 2008-02-11 | Центокор, Инк. | МОНОКЛОНАЛЬНЕ АНТИТІЛО ЛЮДИНИ, ЩО СПЕЦИФІЧНО ЗВ'ЯЗУЄТЬСЯ З ФАКТОРОМ НЕКРОЗУ ПУХЛИН АЛЬФА (ФНПα), ФАРМАЦЕВТИЧНА КОМПОЗИЦІЯ, ЩО ЙОГО МІСТИТЬ, ТА СПОСІБ ЛІКУВАННЯ РЕВМАТОЇДНОГО АРТРИТУ |
| AU2001283546A1 (en) | 2000-08-14 | 2002-02-25 | Advanced Inhalation Research, Inc. | Inhalation device and method |
| US6948492B2 (en) * | 2000-08-15 | 2005-09-27 | University Of Kentucky Research Foundation | Programmable multi-dose intranasal drug delivery device |
| SE517227C2 (sv) * | 2000-09-25 | 2002-05-14 | Microdrug Ag | Inhalator för torrt pulver med folieskärare |
| FI20002363A0 (fi) * | 2000-10-27 | 2000-10-27 | Orion Yhtymae Oyj | Jauheinhalaattori |
| GB0026647D0 (en) * | 2000-10-31 | 2000-12-13 | Glaxo Group Ltd | Medicament dispenser |
| US7077130B2 (en) * | 2000-12-22 | 2006-07-18 | Chrysalis Technologies Incorporated | Disposable inhaler system |
| WO2002053216A2 (en) * | 2001-01-08 | 2002-07-11 | Cipla Limited | An improved device for metered administration of medicament by inhalation |
| US6644309B2 (en) * | 2001-01-12 | 2003-11-11 | Becton, Dickinson And Company | Medicament respiratory delivery device and method |
| US6443152B1 (en) * | 2001-01-12 | 2002-09-03 | Becton Dickinson And Company | Medicament respiratory delivery device |
| US6722364B2 (en) * | 2001-01-12 | 2004-04-20 | Becton, Dickinson And Company | Medicament inhalation delivery devices and methods for using the same |
| EP1359902B1 (en) * | 2001-02-06 | 2007-08-08 | Innovata Biomed Limited | Bimodal dry powder formulation for inhalation |
| US6766799B2 (en) * | 2001-04-16 | 2004-07-27 | Advanced Inhalation Research, Inc. | Inhalation device |
| FI20011317A0 (fi) * | 2001-06-20 | 2001-06-20 | Orion Corp | Jauheinhalaattori |
| WO2003024514A1 (en) * | 2001-09-19 | 2003-03-27 | Advent Pharmaceuticals Pty Ltd | An inhaler |
| US7931022B2 (en) * | 2001-10-19 | 2011-04-26 | Respirks, Inc. | Method and apparatus for dispensing inhalator medicament |
| RU2318829C2 (ru) | 2001-11-14 | 2008-03-10 | Сентокор, Инк. | Антитела против il-6, композиции, способы и применение |
| GB0128148D0 (en) * | 2001-11-23 | 2002-01-16 | Innovata Biomed Ltd | Assembly |
| SI1458360T1 (sl) | 2001-12-19 | 2011-08-31 | Novartis Ag | Pulmonalno dajanje aminoglikozidov |
| US6929798B2 (en) | 2002-02-13 | 2005-08-16 | Immunology Laboratories, Inc. | Compositions and methods for treatment of microbial infections |
| ES2300568T3 (es) | 2002-03-20 | 2008-06-16 | Mannkind Corporation | Aparato de inhalacion. |
| US7185651B2 (en) * | 2002-06-18 | 2007-03-06 | Nektar Therapeutics | Flow regulator for aerosol drug delivery and methods |
| CA2490409A1 (en) | 2002-06-28 | 2004-01-08 | Centocor, Inc. | Mammalian ch1 deleted mimetibodies, compositions, methods and uses |
| AU2008201239B2 (en) * | 2002-10-11 | 2010-02-18 | Otsuka Pharmaceutical Co., Ltd. | Powder inhalation |
| WO2004033010A1 (ja) * | 2002-10-11 | 2004-04-22 | Otsuka Pharmaceutical Co., Ltd. | 粉末吸入器 |
| US6941947B2 (en) * | 2002-12-18 | 2005-09-13 | Quadrant Technologies Limited | Unit dose dry powder inhaler |
| UA89481C2 (uk) | 2003-09-30 | 2010-02-10 | Центокор, Инк. | Еритропоетинові міметичні шарнірно-серцевинні міметитіла людини, композиції, способи та застосування |
| US20070277820A1 (en) * | 2003-10-27 | 2007-12-06 | Timothy Crowder | Blister packages and associated methods of fabricating dry powder drug containment systems |
| WO2005040163A1 (en) | 2003-10-28 | 2005-05-06 | Dr. Reddy's Laboratories Ltd | Heterocyclic compounds that block the effects of advanced glycation end products (age) |
| ITMO20040060A1 (it) * | 2004-03-18 | 2004-06-18 | Roberto Oliva | Inalatore per preparati in polvere |
| WO2005097175A2 (en) | 2004-03-31 | 2005-10-20 | Centocor, Inc. | Human glp-1 mimetibodies, compositions, methods and uses |
| JP2007533387A (ja) * | 2004-04-21 | 2007-11-22 | イノベータ バイオメド リミテッド | 吸入器 |
| GB0409197D0 (en) | 2004-04-24 | 2004-05-26 | Innovata Biomed Ltd | Device |
| BRPI0514263B8 (pt) | 2004-08-20 | 2021-05-25 | Mannkind Corp | método para a síntese de bis-3,6-[4-aminobutil]-2,5-dicetopiperazina n-protegida |
| KR101306384B1 (ko) | 2004-08-23 | 2013-09-09 | 맨카인드 코포레이션 | 약물 전달용 디케토피페라진염, 디케토모르포린염 또는디케토디옥산염 |
| US7393662B2 (en) | 2004-09-03 | 2008-07-01 | Centocor, Inc. | Human EPO mimetic hinge core mimetibodies, compositions, methods and uses |
| US8365725B2 (en) * | 2004-09-13 | 2013-02-05 | Oriel Therapeutics, Inc. | Dry powder inhalers that inhibit agglomeration, related devices and methods |
| JP2006130143A (ja) * | 2004-11-08 | 2006-05-25 | Hitachi Ltd | 吸入式投薬器及び薬剤カートリッジ |
| GB0427856D0 (en) | 2004-12-20 | 2005-01-19 | Glaxo Group Ltd | Maniflod for use in medicament dispenser |
| GB0427858D0 (en) * | 2004-12-20 | 2005-01-19 | Glaxo Group Ltd | Manifold for use in medicament dispenser |
| GB0427853D0 (en) * | 2004-12-20 | 2005-01-19 | Glaxo Group Ltd | Manifold for use in medicament dispenser |
| PE20061324A1 (es) | 2005-04-29 | 2007-01-15 | Centocor Inc | Anticuerpos anti-il-6, composiciones, metodos y usos |
| CN101252951B (zh) | 2005-06-30 | 2011-12-21 | 森托科尔公司 | 抗il-23抗体、组合物、方法和用途 |
| US8763605B2 (en) | 2005-07-20 | 2014-07-01 | Manta Devices, Llc | Inhalation device |
| MX381952B (es) | 2005-09-14 | 2025-03-13 | Mannkind Corp | Metodo para formulacion de farmaco basado en el aumento de la afinidad de agentes activos hacia las superficies de microparticulas cristalinas. |
| DE102005057685A1 (de) * | 2005-12-01 | 2007-06-06 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Inhalator und Speicher für eine trockene Arzneimittelformulierung sowie diesbezügliche Verfahren und Verwendung |
| AR058289A1 (es) | 2005-12-12 | 2008-01-30 | Glaxo Group Ltd | Colector para ser usado en dispensador de medicamento |
| AR058290A1 (es) * | 2005-12-12 | 2008-01-30 | Glaxo Group Ltd | Dispensador de medicamento |
| ES2517420T3 (es) | 2005-12-29 | 2014-11-03 | Janssen Biotech, Inc. | Anticuerpos anti-IL-23 humanos, composiciones, procedimientos y usos |
| JP5599975B2 (ja) | 2006-02-22 | 2014-10-01 | マンカインド コーポレイション | ジケトピペラジン及び活性薬剤を含有する微粒子の製剤特性の改善方法 |
| CA2644679C (en) * | 2006-03-03 | 2013-12-03 | Stc.Unm | Dry powder inhaler with aeroelastic dispersion mechanism |
| PL2068889T3 (pl) | 2006-08-10 | 2020-06-29 | Roy C. Levitt | Anakinra do zastosowania do leczenia zespołu zarostowego zapalenia oskrzelików |
| DE102006047667B3 (de) * | 2006-09-28 | 2008-04-24 | Ing. Erich Pfeiffer Gmbh | Inhalationsvorrichtung |
| US9155849B2 (en) | 2006-10-19 | 2015-10-13 | G Greg Haroutunian | Flow modification device |
| WO2008051471A2 (en) * | 2006-10-19 | 2008-05-02 | Haroutunian Greg G | Flow modification device |
| TW200843794A (en) | 2006-12-21 | 2008-11-16 | Centocor Inc | Use of long-acting GLP-1 receptor agonists to improve insulin sensitivity and lipid profiles |
| US8141551B2 (en) * | 2007-02-16 | 2012-03-27 | Destal Industries, Inc. | Mouthpiece and flow rate controller for intrapulmonary delivery devices |
| WO2008145348A2 (en) * | 2007-06-01 | 2008-12-04 | Boehringer Ingelheim International Gmbh | Dispensing device |
| US8496002B2 (en) * | 2007-06-12 | 2013-07-30 | Civitas Therapeutics, Inc. | Powder inhaler devices |
| BRPI0814248A2 (pt) * | 2007-06-15 | 2015-01-06 | Boehringer Ingelheim Int | Inalador |
| WO2011163272A1 (en) | 2010-06-21 | 2011-12-29 | Mannkind Corporation | Dry powder drug delivery system and methods |
| ES2394589T3 (es) | 2007-12-14 | 2013-02-04 | Aerodesigns, Inc | Suministro de productos alimenticios transformables en aerosol |
| CN101939040B (zh) | 2007-12-20 | 2014-11-05 | 阿斯利康(瑞典)有限公司 | 解聚集粉末的装置和方法 |
| EP2082762A1 (en) * | 2008-01-24 | 2009-07-29 | Boehringer Ingelheim International Gmbh | Inhaler |
| EP2082764A1 (en) | 2008-01-24 | 2009-07-29 | Boehringer Ingelheim International GmbH | Inhaler |
| US8485180B2 (en) | 2008-06-13 | 2013-07-16 | Mannkind Corporation | Dry powder drug delivery system |
| ES2421385T3 (es) | 2008-06-20 | 2013-09-02 | Mannkind Corp | Aparato interactivo y procedimiento para establecer el perfil, en tiempo real, de esfuerzos de inhalación |
| WO2010008523A1 (en) | 2008-07-13 | 2010-01-21 | Map Pharmaceuticals, Inc. | Methods and apparatus for delivering aerosolized medication |
| TWI614024B (zh) | 2008-08-11 | 2018-02-11 | 曼凱公司 | 超快起作用胰島素之用途 |
| KR101781965B1 (ko) | 2008-08-14 | 2017-09-27 | 테바 파마슈티컬즈 오스트레일리아 피티와이 엘티디 | 항-il-12/il-23 항체 |
| CN102245239B (zh) * | 2008-10-08 | 2013-08-14 | 阿斯利康(瑞典)有限公司 | 吸入装置和分配药物的方法 |
| GB0818476D0 (en) * | 2008-10-09 | 2008-11-12 | Vectura Delivery Device Ltd | Inhaler |
| ES2705714T3 (es) | 2008-10-31 | 2019-03-26 | Janssen Biotech Inc | Métodos y usos de dominio de Fibronectina tipo III basado en estructuras de composiciones |
| US8314106B2 (en) | 2008-12-29 | 2012-11-20 | Mannkind Corporation | Substituted diketopiperazine analogs for use as drug delivery agents |
| USD628288S1 (en) | 2009-01-22 | 2010-11-30 | Resmed Limited | Cuff for air delivery tube |
| CN102596992B (zh) | 2009-02-12 | 2015-09-09 | 詹森生物科技公司 | 基于ⅲ型纤连蛋白结构域的支架组合物、方法及用途 |
| US8538707B2 (en) | 2009-03-11 | 2013-09-17 | Mannkind Corporation | Apparatus, system and method for measuring resistance of an inhaler |
| US8883174B2 (en) | 2009-03-25 | 2014-11-11 | The Board Of Regents, The University Of Texas System | Compositions for stimulation of mammalian innate immune resistance to pathogens |
| CN102458543B (zh) | 2009-05-18 | 2015-02-04 | 3M创新有限公司 | 干粉吸入器 |
| MX2011013312A (es) | 2009-06-12 | 2012-01-25 | Mankind Corp | Microparticulas de dicetopiperazina con areas de superficie especificas definidas. |
| WO2011002406A1 (en) | 2009-07-01 | 2011-01-06 | Astrazeneca Ab | Dispenser and method for entraining powder in an airflow |
| IT1395945B1 (it) * | 2009-09-30 | 2012-11-02 | Oliva | Inalatore perfezionato per preparati in polvere |
| EP2496295A1 (en) | 2009-11-03 | 2012-09-12 | MannKind Corporation | An apparatus and method for simulating inhalation efforts |
| US9492625B2 (en) | 2009-11-12 | 2016-11-15 | Stc.Unm | Dry powder inhaler with flutter dispersion member |
| WO2011100403A1 (en) | 2010-02-10 | 2011-08-18 | Immunogen, Inc | Cd20 antibodies and uses thereof |
| WO2011116293A2 (en) * | 2010-03-19 | 2011-09-22 | Manta Devices, Llc | Delivery device and related methods |
| USD635246S1 (en) * | 2010-03-26 | 2011-03-29 | Oriel Therapeutics, Inc. | Dose disk for dry powder inhalers |
| WO2011160119A2 (en) | 2010-06-19 | 2011-12-22 | Memorial Sloan-Kettering Cancer Center | Anti-gd2 antibodies |
| WO2012010877A2 (en) * | 2010-07-21 | 2012-01-26 | Astrazeneca Ab | New device |
| US8372845B2 (en) | 2010-09-17 | 2013-02-12 | Novartis Ag | Pyrazine derivatives as enac blockers |
| DE102010048316B4 (de) * | 2010-10-14 | 2016-02-11 | Primed Halberstadt Medizintechnik Gmbh | Beatmungseinrichtung mit einer Halterung für Sprechventile und/oder Wärme-Feuchtigkeitstauscher ohne Kanülenbefestigung |
| USD666098S1 (en) | 2010-11-01 | 2012-08-28 | Colgate-Palmolive Company | Cap for a container |
| USD666096S1 (en) | 2010-11-01 | 2012-08-28 | Colgate-Palmolive Company | Cap for a container |
| USD666493S1 (en) | 2010-11-01 | 2012-09-04 | Colgate-Palmolive Company | Cap for a container |
| USD666097S1 (en) | 2010-11-01 | 2012-08-28 | Colgate-Palmolive Company | Cap for a container |
| USD666492S1 (en) | 2010-11-01 | 2012-09-04 | Colgate-Palmolive Company | Cap for a container |
| USD666099S1 (en) | 2010-11-01 | 2012-08-28 | Colgate-Palmolive Company | Cap for a container |
| US8561609B2 (en) | 2010-12-07 | 2013-10-22 | Respira Therapeutics, Inc. | Dry powder inhaler |
| JOP20120023B1 (ar) | 2011-02-04 | 2022-03-14 | Novartis Ag | صياغات مساحيق جافة من جسيمات تحتوي على واحد أو اثنين من المواد الفعالة لعلاج امراض ممرات الهواء الانسدادية او الالتهابية |
| RU2563106C2 (ru) * | 2011-03-21 | 2015-09-20 | Симплифайд Солушенз Сведен Аб | Ингаляционное устройство для сухого порошка |
| ES2625858T3 (es) | 2011-04-01 | 2017-07-20 | Mannkind Corporation | Paquete de tipo blíster para cartuchos farmacéuticos |
| WO2012174472A1 (en) | 2011-06-17 | 2012-12-20 | Mannkind Corporation | High capacity diketopiperazine microparticles |
| WO2012175735A1 (en) | 2011-06-23 | 2012-12-27 | Vib Vzw | A20 inhibitors for the treatment of respiratory viral infections |
| CN103945859A (zh) | 2011-10-24 | 2014-07-23 | 曼金德公司 | 用于治疗疼痛的方法和组合物 |
| US10463815B2 (en) | 2012-02-21 | 2019-11-05 | Respira Therapeutics, Inc. | Inhaler to deliver substances for prophylaxis or prevention of disease or injury caused by the inhalation of biological or chemical agents |
| US8809340B2 (en) | 2012-03-19 | 2014-08-19 | Novartis Ag | Crystalline form |
| US10105500B2 (en) * | 2012-05-09 | 2018-10-23 | Virginia Commonwealth University | Dry powder inhaler (DPI) designs for producing aerosols with high fine particle fractions |
| CN108057154B (zh) | 2012-07-12 | 2021-04-16 | 曼金德公司 | 干粉药物输送系统和方法 |
| US9248247B2 (en) * | 2012-07-26 | 2016-02-02 | Nathaniel Gerald Portney | Capsular medication delivery and inhalation device |
| WO2014066856A1 (en) | 2012-10-26 | 2014-05-01 | Mannkind Corporation | Inhalable influenza vaccine compositions and methods |
| JP2015535233A (ja) | 2012-10-31 | 2015-12-10 | ガレクト・バイオテック・エイビイ | ガレクチン−3のガラクトシド阻害剤及び肺線維症のためのその使用 |
| AU2013365742B2 (en) | 2012-12-19 | 2016-11-24 | Novartis Ag | Autotaxin inhibitors |
| TWI726291B (zh) | 2013-01-07 | 2021-05-01 | 英屬維爾京群島商遠東超級實驗室有限公司 | 通過干擾素的經皮和/或經粘膜給藥治療骨癌、皮膚癌、皮下癌、粘膜癌和/或粘膜下癌的方法和組合物 |
| US9452139B2 (en) | 2013-03-14 | 2016-09-27 | Novartis Ag | Respirable agglomerates of porous carrier particles and micronized drug |
| CA2898700C (en) | 2013-03-14 | 2022-07-19 | Novartis Ag | Deamorphization of spray-dried formulations via spray-blending |
| WO2014144763A2 (en) | 2013-03-15 | 2014-09-18 | Memorial Sloan Kettering Cancer Center | High affinity anti-gd2 antibodies |
| WO2014144895A1 (en) | 2013-03-15 | 2014-09-18 | Mannkind Corporation | Microcrystalline diketopiperazine compositions and methods |
| CN105209097B (zh) | 2013-05-17 | 2020-03-31 | 皇家飞利浦有限公司 | 物质输送模块 |
| EP3021834A1 (en) | 2013-07-18 | 2016-05-25 | MannKind Corporation | Heat-stable dry powder pharmaceutical compositions and methods |
| WO2015008229A1 (en) | 2013-07-18 | 2015-01-22 | Novartis Ag | Autotaxin inhibitors |
| SG11201600241RA (en) | 2013-07-18 | 2016-02-26 | Novartis Ag | Autotaxin inhibitors comprising a heteroaromatic ring-benzyl-amide-cycle core |
| EP3030294B1 (en) | 2013-08-05 | 2020-10-07 | MannKind Corporation | Insufflation apparatus |
| DE102014005646B4 (de) * | 2014-01-30 | 2016-05-12 | Klaus-Dieter Beller | Pulverinhalator und Pulverinhalationsset |
| WO2015148905A1 (en) | 2014-03-28 | 2015-10-01 | Mannkind Corporation | Use of ultrarapid acting insulin |
| WO2015162558A1 (en) | 2014-04-24 | 2015-10-29 | Novartis Ag | Autotaxin inhibitors |
| US10286065B2 (en) | 2014-09-19 | 2019-05-14 | Board Of Regents, The University Of Texas System | Compositions and methods for treating viral infections through stimulated innate immunity in combination with antiviral compounds |
| US10561806B2 (en) | 2014-10-02 | 2020-02-18 | Mannkind Corporation | Mouthpiece cover for an inhaler |
| EP3838317A1 (en) | 2015-01-14 | 2021-06-23 | Respira Therapeutics, Inc. | Dry powder inhaler |
| CN107667120B (zh) | 2015-03-17 | 2022-03-08 | 纪念斯隆-凯特林癌症中心 | 抗muc16抗体及其应用 |
| US10765821B2 (en) | 2015-03-19 | 2020-09-08 | Altria Client Services Llc | Vaporizer for vaporizing a constituent of a plant material |
| US10179215B2 (en) | 2015-03-19 | 2019-01-15 | Altria Client Services Llc | Vaporizer for vaporizing a constituent of a plant material |
| UY36889A (es) | 2015-09-09 | 2017-04-28 | Novartis Ag | Moléculas de unión a linfopoyetina estromal timica (tslp) y métodos de uso de las moléculas |
| KR20180042433A (ko) | 2015-09-09 | 2018-04-25 | 노파르티스 아게 | 흉선 기질 림포포이에틴 (tslp)-결합 항체 및 항체의 사용 방법 |
| WO2017103109A1 (en) | 2015-12-18 | 2017-06-22 | Galecto Biotech Ab | Polymorphic forms and process |
| CA3026757A1 (en) | 2016-07-15 | 2018-01-18 | Poseida Therapeutics, Inc. | Chimeric antigen receptors and methods for use |
| KR20190063458A (ko) | 2016-07-15 | 2019-06-07 | 포세이다 테라퓨틱스, 인크. | Muc1에 특이적인 키메라 항원 수용체(cars) 및 그의 사용 방법 |
| WO2018087699A2 (en) | 2016-11-09 | 2018-05-17 | The Board Of Regents Of The University Of Texas System | Methods and compositions for adaptive immune modulation |
| US10569069B2 (en) | 2016-12-14 | 2020-02-25 | Combat Comb, Llc | Applicator for treatments applied to animal skin |
| USD828653S1 (en) | 2016-12-14 | 2018-09-11 | Brandon Penland | Treatment applicator |
| CN110267660A (zh) | 2016-12-16 | 2019-09-20 | 德克萨斯大学系统董事会 | 含布罗莫结构域蛋白4(brd4)的抑制剂 |
| EP3574012A1 (en) | 2017-01-27 | 2019-12-04 | Memorial Sloan Kettering Cancer Center | Bispecific her2 and cd3 binding molecules |
| CA3052095A1 (en) | 2017-01-30 | 2018-08-02 | Janssen Biotech, Inc. | Anti-tnf antibodies, compositions, and methods for the treatment of active psoriatic arthritis |
| KR20240038148A (ko) | 2017-02-07 | 2024-03-22 | 얀센 바이오테크 인코포레이티드 | 활성 강직성 척추염의 치료를 위한 항-tnf 항체, 조성물, 및 방법 |
| MX2019010892A (es) * | 2017-03-15 | 2020-02-12 | Csp Technologies Inc | Inhalador y metodos de uso y de fabricacion del mismo. |
| AU2018393110B2 (en) | 2017-12-20 | 2023-04-27 | Poseida Therapeutics, Inc. | VCAR compositions and methods for use |
| MA52590A (fr) | 2018-05-11 | 2021-03-17 | Janssen Biotech Inc | Méthodes de traitement de la dépression à l'aide d'anticorps il-23 |
| US11389433B2 (en) | 2018-06-18 | 2022-07-19 | Board Of Regents, The University Of Texas System | BRD4 inhibitor treatment of IgE-mediated diseases |
| US20220023553A1 (en) * | 2018-12-04 | 2022-01-27 | Hovione Technology Ltd | Large dose disposable inhaler and method of simple filling |
| CA3126654A1 (en) | 2019-01-15 | 2020-07-23 | Janssen Biotech, Inc. | Anti-tnf antibody compositions and methods for the treatment of juvenile idiopathic arthritis |
| US20220064278A1 (en) | 2019-01-23 | 2022-03-03 | Janssen Biotech, Inc. | Anti-TNF Antibody Compositions for Use in Methods for the Treatment of Psoriatic Arthritis |
| JP2022525179A (ja) | 2019-03-14 | 2022-05-11 | ヤンセン バイオテツク,インコーポレーテツド | 抗tnf抗体組成物を産生するための産生方法 |
| MA55283A (fr) | 2019-03-14 | 2022-01-19 | Janssen Biotech Inc | Procédés de production de compositions d'anticorps anti-tnf |
| EP3938392A1 (en) | 2019-03-14 | 2022-01-19 | Janssen Biotech, Inc. | Methods for producing anti-tnf antibody compositions |
| CN113874073A (zh) | 2019-05-23 | 2021-12-31 | 詹森生物科技公司 | 用针对IL-23和TNFα的抗体的联合疗法治疗炎性肠病的方法 |
| KR20220030952A (ko) | 2019-06-03 | 2022-03-11 | 얀센 바이오테크 인코포레이티드 | 활성 강직성 척추염의 치료를 위한 항-tnf 항체, 조성물, 및 방법 |
| BR112021024425A2 (pt) | 2019-06-03 | 2022-01-18 | Janssen Biotech Inc | Composições de anticorpo anti-tnf e métodos para o tratamento de artrite psoriática |
| USD916361S1 (en) | 2019-06-25 | 2021-04-13 | Altria Client Services Llc | Aerosol-generating capsule |
| US11458262B2 (en) | 2019-06-25 | 2022-10-04 | Altria Client Services Llc | Capsules, heat-not-burn (HNB) aerosol-generating devices, and methods of generating an aerosol |
| WO2021028752A1 (en) | 2019-08-15 | 2021-02-18 | Janssen Biotech, Inc. | Anti-tfn antibodies for treating type i diabetes |
| EP4135758A1 (en) | 2020-04-14 | 2023-02-22 | Poseida Therapeutics, Inc. | Compositions and methods for use in the treatment of cancer |
| WO2021214587A1 (en) | 2020-04-21 | 2021-10-28 | Janssen Biotech, Inc. | Anti-tnf alpha agent for treating viral infections |
| WO2021214588A1 (en) | 2020-04-21 | 2021-10-28 | Janssen Biotech, Inc. | Anti-tnf alpha agent for treating coronavirus infections |
| US12258393B2 (en) | 2020-05-21 | 2025-03-25 | Janssen Biotech, Inc. | Method of treating inflammatory bowel disease with a combination therapy of antibodies to IL-23 and TNF alpha |
| US20230312684A1 (en) | 2020-07-31 | 2023-10-05 | Pinetree Therapeutics, Inc. | Neuropilin and angiotensin converting enzyme 2 fusion peptides for treating viral infections |
| US12193502B2 (en) | 2020-12-30 | 2025-01-14 | Altria Client Services Llc | Capsules including embedded corrugated heater, heat-not-burn (HNB) aerosol-generating devices, and methods of generating an aerosol |
| US12053022B2 (en) | 2021-01-04 | 2024-08-06 | Altria Client Services Llc | Capsules with integrated mouthpieces, heat-not-burn (HNB) aerosol-generating devices, and methods of generating an aerosol |
| US12201148B2 (en) | 2021-01-18 | 2025-01-21 | Altria Client Services Llc | Closed system capsule with airflow, heat-not-burn (HNB) aerosol-generating devices, and methods of generating an aerosol |
| US11910826B2 (en) | 2021-01-18 | 2024-02-27 | Altria Client Services Llc | Heat-not-burn (HNB) aerosol-generating devices and capsules |
| US11789476B2 (en) | 2021-01-18 | 2023-10-17 | Altria Client Services Llc | Heat-not-burn (HNB) aerosol-generating devices including intra-draw heater control, and methods of controlling a heater |
| US12011034B2 (en) | 2021-01-18 | 2024-06-18 | Altria Client Services Llc | Capsules including embedded heaters and heat-not-burn (HNB) aerosol-generating devices |
| WO2023279115A1 (en) | 2021-07-02 | 2023-01-05 | Henry Ford Health System | Compositions comprising endosidin 2 for reducing sars-cov-2 infection |
| IL309997A (en) | 2021-07-09 | 2024-03-01 | Janssen Biotech Inc | Production methods for the production of anti-TNF antibody compositions |
| WO2023281463A1 (en) | 2021-07-09 | 2023-01-12 | Janssen Biotech, Inc. | Manufacturing methods for producing anti-tnf antibody compositions |
| US12127592B2 (en) | 2021-09-20 | 2024-10-29 | Altria Client Services Llc | Capsule validation for heat-not-burn (HNB) aerosol-generating devices |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3288277A (en) * | 1965-09-22 | 1966-11-29 | Richardson Merrell Inc | Dispensing container |
| US3437236A (en) * | 1967-09-26 | 1969-04-08 | Ortho Pharma Corp | Tablet dispensing device |
| PT83094B (pt) | 1985-07-30 | 1993-07-30 | Glaxo Group Ltd | Dispositivos proprios para a administracao de medicamentos a pacientes |
| SE453566B (sv) * | 1986-03-07 | 1988-02-15 | Draco Ab | Anordning vid pulverinhalatorer |
| GB8614805D0 (en) | 1986-06-18 | 1986-07-23 | British American Tobacco Co | Aerosol device |
| GB9004781D0 (en) | 1990-03-02 | 1990-04-25 | Glaxo Group Ltd | Device |
| GB9021433D0 (en) * | 1990-10-02 | 1990-11-14 | Atomic Energy Authority Uk | Power inhaler |
| US5492112A (en) * | 1991-05-20 | 1996-02-20 | Dura Pharmaceuticals, Inc. | Dry powder inhaler |
| GB2264237A (en) * | 1992-02-05 | 1993-08-25 | Robert Edward Newell | An inhaler |
| EP0558879B1 (en) * | 1992-03-04 | 1997-05-14 | Astra Aktiebolag | Disposable inhaler |
| DE4208880A1 (de) * | 1992-03-19 | 1993-09-23 | Boehringer Ingelheim Kg | Separator fuer pulverinhalatoren |
| US5896855A (en) * | 1992-12-24 | 1999-04-27 | Rhone-Poulenc Rorer Limited | Multi dose inhaler apparatus |
| US5388572A (en) * | 1993-10-26 | 1995-02-14 | Tenax Corporation (A Connecticut Corp.) | Dry powder medicament inhalator having an inhalation-activated piston to aerosolize dose and deliver same |
| US5622166A (en) * | 1995-04-24 | 1997-04-22 | Dura Pharmaceuticals, Inc. | Dry powder inhaler delivery system |
-
1996
- 1996-12-19 AT AT99201271T patent/ATE209939T1/de not_active IP Right Cessation
- 1996-12-19 KR KR1019980705145A patent/KR19990077014A/ko not_active Application Discontinuation
- 1996-12-19 EP EP99201271A patent/EP0938907B1/en not_active Expired - Lifetime
- 1996-12-19 DE DE69618624T patent/DE69618624T2/de not_active Expired - Fee Related
- 1996-12-19 EP EP96942494A patent/EP0883414B1/en not_active Expired - Lifetime
- 1996-12-19 AT AT96942494T patent/ATE209053T1/de not_active IP Right Cessation
- 1996-12-19 NZ NZ324374A patent/NZ324374A/xx unknown
- 1996-12-19 EA EA199800556A patent/EA001016B1/ru not_active IP Right Cessation
- 1996-12-19 AU AU11633/97A patent/AU725348B2/en not_active Ceased
- 1996-12-19 AP APAP/P/1998/001285A patent/AP9801285A0/en unknown
- 1996-12-19 WO PCT/GB1996/003141 patent/WO1997025086A2/en not_active Application Discontinuation
- 1996-12-19 ES ES96942494T patent/ES2166473T3/es not_active Expired - Lifetime
- 1996-12-19 PL PL96327616A patent/PL327616A1/xx unknown
- 1996-12-19 CA CA002241880A patent/CA2241880A1/en not_active Abandoned
- 1996-12-19 US US09/101,102 patent/US6065472A/en not_active Expired - Fee Related
- 1996-12-19 ES ES99201271T patent/ES2167987T3/es not_active Expired - Lifetime
- 1996-12-19 IL IL12518396A patent/IL125183A0/xx unknown
- 1996-12-19 CZ CZ982125A patent/CZ212598A3/cs unknown
- 1996-12-19 EA EA199900400A patent/EA001237B1/ru not_active IP Right Cessation
- 1996-12-19 BR BR9612410A patent/BR9612410A/pt not_active Application Discontinuation
- 1996-12-19 DE DE69617754T patent/DE69617754T2/de not_active Expired - Fee Related
- 1996-12-19 TR TR1998/01265T patent/TR199801265T2/xx unknown
- 1996-12-19 CN CN96180172A patent/CN1213974A/zh active Pending
- 1996-12-19 JP JP9524939A patent/JP2000503565A/ja active Pending
-
1998
- 1998-06-29 IS IS4787A patent/IS4787A/is unknown
- 1998-07-02 MX MX9805409A patent/MX9805409A/es unknown
- 1998-07-02 NO NO983069A patent/NO983069L/no not_active Application Discontinuation
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103252007A (zh) * | 2008-06-13 | 2013-08-21 | 曼金德公司 | 干粉吸入器和用于药物输送的系统 |
| CN103252007B (zh) * | 2008-06-13 | 2016-06-22 | 曼金德公司 | 干粉吸入器和用于药物输送的系统 |
| CN103764210A (zh) * | 2011-08-19 | 2014-04-30 | 阿普塔尔法国简易股份公司 | 粉末吸入设备 |
| CN103764210B (zh) * | 2011-08-19 | 2016-07-06 | 阿普塔尔法国简易股份公司 | 粉末吸入设备 |
| CN109310834A (zh) * | 2016-04-18 | 2019-02-05 | 励志私人有限公司 | 用于吸入器的间隔装置 |
| CN106075677A (zh) * | 2016-08-08 | 2016-11-09 | 中山市美捷时包装制品有限公司 | 一种干粉吸入装置的促动机构 |
| CN110799230A (zh) * | 2017-06-27 | 2020-02-14 | 韩国联合制药株式会社 | 干燥粉末吸入器 |
| CN110799230B (zh) * | 2017-06-27 | 2022-04-05 | 韩国联合制药株式会社 | 干燥粉末吸入器 |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1213974A (zh) | 吸入器具 | |
| RU2201768C2 (ru) | Устройство для ингаляции | |
| US8025051B2 (en) | Delivery device | |
| EP1291031B1 (en) | Powdered medicine multi-dose administering device | |
| EP1066849B1 (en) | Device for feeding a constant amount of powder body | |
| HU213495B (en) | Device for inhalation of powdered medicament | |
| NZ534206A (en) | Humidity-tight cartridge for a powder inhaler | |
| WO2008008021A1 (en) | Inhalation system and delivery device for the administration of a drug in the form of dry powder. | |
| AU2004264783A1 (en) | Pre-metered dose magazine for dry powder inhaler | |
| AU2006228033B2 (en) | Gravity-actuated locking mechanism for drug container | |
| AU740228B2 (en) | Inhalation device | |
| CN118414185A (zh) | 粉末吸入器 | |
| CN118434467A (zh) | 粉末吸入器 | |
| CN1162925A (zh) | 吸入装置 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C02 | Deemed withdrawal of patent application after publication (patent law 2001) | ||
| WD01 | Invention patent application deemed withdrawn after publication |