CN115197245A - Kras inhibitor and preparation method thereof - Google Patents
Kras inhibitor and preparation method thereof Download PDFInfo
- Publication number
- CN115197245A CN115197245A CN202210373726.4A CN202210373726A CN115197245A CN 115197245 A CN115197245 A CN 115197245A CN 202210373726 A CN202210373726 A CN 202210373726A CN 115197245 A CN115197245 A CN 115197245A
- Authority
- CN
- China
- Prior art keywords
- compound
- pharmaceutically acceptable
- acceptable salt
- deuterium
- hydroxy
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 229940124785 KRAS inhibitor Drugs 0.000 title abstract description 4
- 238000002360 preparation method Methods 0.000 title description 61
- 150000001875 compounds Chemical class 0.000 claims abstract description 154
- 150000003839 salts Chemical class 0.000 claims abstract description 65
- -1 cyano, hydroxy Chemical group 0.000 claims description 213
- 229910052805 deuterium Inorganic materials 0.000 claims description 63
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 claims description 62
- 125000000623 heterocyclic group Chemical group 0.000 claims description 53
- 125000000217 alkyl group Chemical group 0.000 claims description 50
- 229910052736 halogen Inorganic materials 0.000 claims description 49
- 150000002367 halogens Chemical class 0.000 claims description 49
- 229910052739 hydrogen Inorganic materials 0.000 claims description 45
- 239000001257 hydrogen Substances 0.000 claims description 41
- 125000003118 aryl group Chemical group 0.000 claims description 38
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 28
- 239000008194 pharmaceutical composition Substances 0.000 claims description 24
- 238000006467 substitution reaction Methods 0.000 claims description 20
- 239000000460 chlorine Substances 0.000 claims description 18
- 125000000449 nitro group Chemical class [O-][N+](*)=O 0.000 claims description 18
- 125000004429 atom Chemical group 0.000 claims description 17
- 125000000171 (C1-C6) haloalkyl group Chemical group 0.000 claims description 15
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 12
- 102200006539 rs121913529 Human genes 0.000 claims description 12
- 206010028980 Neoplasm Diseases 0.000 claims description 11
- 125000003601 C2-C6 alkynyl group Chemical group 0.000 claims description 10
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 10
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 10
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 claims description 9
- 229910052801 chlorine Inorganic materials 0.000 claims description 9
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 claims description 9
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 claims description 8
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 claims description 8
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 claims description 8
- 229910052794 bromium Inorganic materials 0.000 claims description 8
- 229910052731 fluorine Inorganic materials 0.000 claims description 8
- 239000011737 fluorine Substances 0.000 claims description 8
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 claims description 8
- 201000011510 cancer Diseases 0.000 claims description 7
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 7
- 201000010099 disease Diseases 0.000 claims description 6
- 208000035475 disorder Diseases 0.000 claims description 6
- 239000003814 drug Substances 0.000 claims description 6
- 125000000304 alkynyl group Chemical group 0.000 claims description 5
- 125000004431 deuterium atom Chemical group 0.000 claims description 5
- 230000000155 isotopic effect Effects 0.000 claims description 5
- 125000000882 C2-C6 alkenyl group Chemical group 0.000 claims description 4
- 206010009944 Colon cancer Diseases 0.000 claims description 4
- 208000001333 Colorectal Neoplasms Diseases 0.000 claims description 4
- 125000001028 difluoromethyl group Chemical group [H]C(F)(F)* 0.000 claims description 3
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims description 3
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 claims description 3
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 2
- 208000015634 Rectal Neoplasms Diseases 0.000 claims description 2
- 125000001246 bromo group Chemical group Br* 0.000 claims description 2
- 125000001309 chloro group Chemical group Cl* 0.000 claims description 2
- 125000001153 fluoro group Chemical group F* 0.000 claims description 2
- AQYSYJUIMQTRMV-UHFFFAOYSA-N hypofluorous acid Chemical compound FO AQYSYJUIMQTRMV-UHFFFAOYSA-N 0.000 claims description 2
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 2
- 201000002528 pancreatic cancer Diseases 0.000 claims description 2
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 2
- 206010038038 rectal cancer Diseases 0.000 claims description 2
- 201000001275 rectum cancer Diseases 0.000 claims description 2
- 238000004519 manufacturing process Methods 0.000 claims 2
- 230000002265 prevention Effects 0.000 claims 2
- 150000005840 aryl radicals Chemical class 0.000 claims 1
- 150000002431 hydrogen Chemical class 0.000 claims 1
- 125000001424 substituent group Chemical group 0.000 abstract description 31
- 239000000203 mixture Substances 0.000 abstract description 29
- 238000000034 method Methods 0.000 abstract description 13
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 78
- 238000006243 chemical reaction Methods 0.000 description 64
- 239000000243 solution Substances 0.000 description 55
- 239000012043 crude product Substances 0.000 description 43
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 42
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 38
- 125000000753 cycloalkyl group Chemical group 0.000 description 37
- 125000004435 hydrogen atom Chemical class [H]* 0.000 description 37
- 125000001313 C5-C10 heteroaryl group Chemical group 0.000 description 32
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 31
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 29
- 235000019439 ethyl acetate Nutrition 0.000 description 27
- 230000002829 reductive effect Effects 0.000 description 26
- 125000004765 (C1-C4) haloalkyl group Chemical group 0.000 description 22
- 238000005481 NMR spectroscopy Methods 0.000 description 22
- 125000004432 carbon atom Chemical group C* 0.000 description 20
- 239000003480 eluent Substances 0.000 description 20
- 125000006552 (C3-C8) cycloalkyl group Chemical group 0.000 description 19
- 238000001914 filtration Methods 0.000 description 19
- 238000003818 flash chromatography Methods 0.000 description 19
- 229910052757 nitrogen Inorganic materials 0.000 description 19
- 239000003208 petroleum Substances 0.000 description 19
- 238000010898 silica gel chromatography Methods 0.000 description 19
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 18
- 238000001035 drying Methods 0.000 description 18
- 239000012074 organic phase Substances 0.000 description 18
- 229910052799 carbon Inorganic materials 0.000 description 17
- 125000004043 oxo group Chemical group O=* 0.000 description 16
- 230000015572 biosynthetic process Effects 0.000 description 15
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 15
- 239000007858 starting material Substances 0.000 description 15
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 14
- 238000003786 synthesis reaction Methods 0.000 description 14
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 14
- 125000001072 heteroaryl group Chemical group 0.000 description 13
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 11
- 125000004093 cyano group Chemical group *C#N 0.000 description 11
- JWAZRIHNYRIHIV-UHFFFAOYSA-N 2-naphthol Chemical compound C1=CC=CC2=CC(O)=CC=C21 JWAZRIHNYRIHIV-UHFFFAOYSA-N 0.000 description 10
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 10
- 150000001721 carbon Chemical group 0.000 description 10
- 125000004433 nitrogen atom Chemical group N* 0.000 description 10
- 238000003756 stirring Methods 0.000 description 10
- AICOOMRHRUFYCM-ZRRPKQBOSA-N oxazine, 1 Chemical compound C([C@@H]1[C@H](C(C[C@]2(C)[C@@H]([C@H](C)N(C)C)[C@H](O)C[C@]21C)=O)CC1=CC2)C[C@H]1[C@@]1(C)[C@H]2N=C(C(C)C)OC1 AICOOMRHRUFYCM-ZRRPKQBOSA-N 0.000 description 9
- 239000011541 reaction mixture Substances 0.000 description 9
- 125000006272 (C3-C7) cycloalkyl group Chemical group 0.000 description 8
- KZPYGQFFRCFCPP-UHFFFAOYSA-N 1,1'-bis(diphenylphosphino)ferrocene Chemical compound [Fe+2].C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1 KZPYGQFFRCFCPP-UHFFFAOYSA-N 0.000 description 8
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 8
- 125000003545 alkoxy group Chemical group 0.000 description 8
- 125000002947 alkylene group Chemical group 0.000 description 8
- 229910052717 sulfur Inorganic materials 0.000 description 8
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 7
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 7
- 239000012295 chemical reaction liquid Substances 0.000 description 7
- 239000007789 gas Substances 0.000 description 7
- GVOISEJVFFIGQE-YCZSINBZSA-N n-[(1r,2s,5r)-5-[methyl(propan-2-yl)amino]-2-[(3s)-2-oxo-3-[[6-(trifluoromethyl)quinazolin-4-yl]amino]pyrrolidin-1-yl]cyclohexyl]acetamide Chemical compound CC(=O)N[C@@H]1C[C@H](N(C)C(C)C)CC[C@@H]1N1C(=O)[C@@H](NC=2C3=CC(=CC=C3N=CN=2)C(F)(F)F)CC1 GVOISEJVFFIGQE-YCZSINBZSA-N 0.000 description 7
- 229910052760 oxygen Inorganic materials 0.000 description 7
- 239000000126 substance Substances 0.000 description 7
- 238000004809 thin layer chromatography Methods 0.000 description 7
- 125000005330 8 membered heterocyclic group Chemical group 0.000 description 6
- 102100030708 GTPase KRas Human genes 0.000 description 6
- 101000584612 Homo sapiens GTPase KRas Proteins 0.000 description 6
- 125000000852 azido group Chemical group *N=[N+]=[N-] 0.000 description 6
- 229940125773 compound 10 Drugs 0.000 description 6
- CSJLBAMHHLJAAS-UHFFFAOYSA-N diethylaminosulfur trifluoride Chemical compound CCN(CC)S(F)(F)F CSJLBAMHHLJAAS-UHFFFAOYSA-N 0.000 description 6
- 125000005842 heteroatom Chemical group 0.000 description 6
- 239000000543 intermediate Substances 0.000 description 6
- ZLVXBBHTMQJRSX-VMGNSXQWSA-N jdtic Chemical compound C1([C@]2(C)CCN(C[C@@H]2C)C[C@H](C(C)C)NC(=O)[C@@H]2NCC3=CC(O)=CC=C3C2)=CC=CC(O)=C1 ZLVXBBHTMQJRSX-VMGNSXQWSA-N 0.000 description 6
- 239000000047 product Substances 0.000 description 6
- 229920006395 saturated elastomer Polymers 0.000 description 6
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 5
- 125000003342 alkenyl group Chemical group 0.000 description 5
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 5
- 125000002768 hydroxyalkyl group Chemical group 0.000 description 5
- 230000000670 limiting effect Effects 0.000 description 5
- ZSFLPNDWUWXOGM-UHFFFAOYSA-N naphthalen-2-yl formate Chemical compound C1=CC=CC2=CC(OC=O)=CC=C21 ZSFLPNDWUWXOGM-UHFFFAOYSA-N 0.000 description 5
- 239000001301 oxygen Substances 0.000 description 5
- 238000002953 preparative HPLC Methods 0.000 description 5
- 125000006413 ring segment Chemical group 0.000 description 5
- 239000000741 silica gel Substances 0.000 description 5
- 229910002027 silica gel Inorganic materials 0.000 description 5
- 239000002904 solvent Substances 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- UNILWMWFPHPYOR-KXEYIPSPSA-M 1-[6-[2-[3-[3-[3-[2-[2-[3-[[2-[2-[[(2r)-1-[[2-[[(2r)-1-[3-[2-[2-[3-[[2-(2-amino-2-oxoethoxy)acetyl]amino]propoxy]ethoxy]ethoxy]propylamino]-3-hydroxy-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-3-[(2r)-2,3-di(hexadecanoyloxy)propyl]sulfanyl-1-oxopropan-2-yl Chemical compound O=C1C(SCCC(=O)NCCCOCCOCCOCCCNC(=O)COCC(=O)N[C@@H](CSC[C@@H](COC(=O)CCCCCCCCCCCCCCC)OC(=O)CCCCCCCCCCCCCCC)C(=O)NCC(=O)N[C@H](CO)C(=O)NCCCOCCOCCOCCCNC(=O)COCC(N)=O)CC(=O)N1CCNC(=O)CCCCCN\1C2=CC=C(S([O-])(=O)=O)C=C2CC/1=C/C=C/C=C/C1=[N+](CC)C2=CC=C(S([O-])(=O)=O)C=C2C1 UNILWMWFPHPYOR-KXEYIPSPSA-M 0.000 description 4
- HIHOEGPXVVKJPP-JTQLQIEISA-N 5-fluoro-2-[[(1s)-1-(5-fluoropyridin-2-yl)ethyl]amino]-6-[(5-methyl-1h-pyrazol-3-yl)amino]pyridine-3-carbonitrile Chemical compound N([C@@H](C)C=1N=CC(F)=CC=1)C(C(=CC=1F)C#N)=NC=1NC=1C=C(C)NN=1 HIHOEGPXVVKJPP-JTQLQIEISA-N 0.000 description 4
- 125000002853 C1-C4 hydroxyalkyl group Chemical group 0.000 description 4
- RGSFGYAAUTVSQA-UHFFFAOYSA-N Cyclopentane Chemical compound C1CCCC1 RGSFGYAAUTVSQA-UHFFFAOYSA-N 0.000 description 4
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 4
- 125000001931 aliphatic group Chemical group 0.000 description 4
- 150000001413 amino acids Chemical group 0.000 description 4
- 239000012298 atmosphere Substances 0.000 description 4
- 238000004296 chiral HPLC Methods 0.000 description 4
- 125000000000 cycloalkoxy group Chemical group 0.000 description 4
- 238000000605 extraction Methods 0.000 description 4
- 125000004474 heteroalkylene group Chemical group 0.000 description 4
- 125000005844 heterocyclyloxy group Chemical group 0.000 description 4
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 4
- 125000002950 monocyclic group Chemical group 0.000 description 4
- 230000035772 mutation Effects 0.000 description 4
- KDLHZDBZIXYQEI-UHFFFAOYSA-N palladium Substances [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 4
- 239000012071 phase Substances 0.000 description 4
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 4
- 238000007789 sealing Methods 0.000 description 4
- 239000011593 sulfur Substances 0.000 description 4
- RBWMYPKAPIYTJQ-VMBFOHBNSA-N (1R,2S,5S)-6,6-dimethyl-N-[(2S)-1-oxo-3-[(3S)-2-oxopyrrolidin-3-yl]propan-2-yl]-3-[2-[4-(trifluoromethoxy)phenoxy]acetyl]-3-azabicyclo[3.1.0]hexane-2-carboxamide Chemical compound CC1([C@@H]2[C@H]1[C@H](N(C2)C(=O)COC3=CC=C(C=C3)OC(F)(F)F)C(=O)N[C@@H](C[C@@H]4CCNC4=O)C=O)C RBWMYPKAPIYTJQ-VMBFOHBNSA-N 0.000 description 3
- AOSZTAHDEDLTLQ-AZKQZHLXSA-N (1S,2S,4R,8S,9S,11S,12R,13S,19S)-6-[(3-chlorophenyl)methyl]-12,19-difluoro-11-hydroxy-8-(2-hydroxyacetyl)-9,13-dimethyl-6-azapentacyclo[10.8.0.02,9.04,8.013,18]icosa-14,17-dien-16-one Chemical compound C([C@@H]1C[C@H]2[C@H]3[C@]([C@]4(C=CC(=O)C=C4[C@@H](F)C3)C)(F)[C@@H](O)C[C@@]2([C@@]1(C1)C(=O)CO)C)N1CC1=CC=CC(Cl)=C1 AOSZTAHDEDLTLQ-AZKQZHLXSA-N 0.000 description 3
- GHYOCDFICYLMRF-UTIIJYGPSA-N (2S,3R)-N-[(2S)-3-(cyclopenten-1-yl)-1-[(2R)-2-methyloxiran-2-yl]-1-oxopropan-2-yl]-3-hydroxy-3-(4-methoxyphenyl)-2-[[(2S)-2-[(2-morpholin-4-ylacetyl)amino]propanoyl]amino]propanamide Chemical compound C1(=CCCC1)C[C@@H](C(=O)[C@@]1(OC1)C)NC([C@H]([C@@H](C1=CC=C(C=C1)OC)O)NC([C@H](C)NC(CN1CCOCC1)=O)=O)=O GHYOCDFICYLMRF-UTIIJYGPSA-N 0.000 description 3
- PHDIJLFSKNMCMI-ITGJKDDRSA-N (3R,4S,5R,6R)-6-(hydroxymethyl)-4-(8-quinolin-6-yloxyoctoxy)oxane-2,3,5-triol Chemical compound OC[C@@H]1[C@H]([C@@H]([C@H](C(O1)O)O)OCCCCCCCCOC=1C=C2C=CC=NC2=CC=1)O PHDIJLFSKNMCMI-ITGJKDDRSA-N 0.000 description 3
- QFLWZFQWSBQYPS-AWRAUJHKSA-N (3S)-3-[[(2S)-2-[[(2S)-2-[5-[(3aS,6aR)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-[1-bis(4-chlorophenoxy)phosphorylbutylamino]-4-oxobutanoic acid Chemical compound CCCC(NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)C(C)C)P(=O)(Oc1ccc(Cl)cc1)Oc1ccc(Cl)cc1 QFLWZFQWSBQYPS-AWRAUJHKSA-N 0.000 description 3
- DMQYDVBIPXAAJA-VHXPQNKSSA-N (3z)-5-[(1-ethylpiperidin-4-yl)amino]-3-[(3-fluorophenyl)-(5-methyl-1h-imidazol-2-yl)methylidene]-1h-indol-2-one Chemical compound C1CN(CC)CCC1NC1=CC=C(NC(=O)\C2=C(/C=3NC=C(C)N=3)C=3C=C(F)C=CC=3)C2=C1 DMQYDVBIPXAAJA-VHXPQNKSSA-N 0.000 description 3
- FRJJJAKBRKABFA-TYFAACHXSA-N (4r,6s)-6-[(e)-2-[6-chloro-4-(4-fluorophenyl)-2-propan-2-ylquinolin-3-yl]ethenyl]-4-hydroxyoxan-2-one Chemical compound C(\[C@H]1OC(=O)C[C@H](O)C1)=C/C=1C(C(C)C)=NC2=CC=C(Cl)C=C2C=1C1=CC=C(F)C=C1 FRJJJAKBRKABFA-TYFAACHXSA-N 0.000 description 3
- UVNPEUJXKZFWSJ-LMTQTHQJSA-N (R)-N-[(4S)-8-[6-amino-5-[(3,3-difluoro-2-oxo-1H-pyrrolo[2,3-b]pyridin-4-yl)sulfanyl]pyrazin-2-yl]-2-oxa-8-azaspiro[4.5]decan-4-yl]-2-methylpropane-2-sulfinamide Chemical compound CC(C)(C)[S@@](=O)N[C@@H]1COCC11CCN(CC1)c1cnc(Sc2ccnc3NC(=O)C(F)(F)c23)c(N)n1 UVNPEUJXKZFWSJ-LMTQTHQJSA-N 0.000 description 3
- LVHOHZHTZXRVRJ-CMDGGOBGSA-N (e)-3-(3-methoxyphenyl)-n-(3,4,5-trimethoxyphenyl)prop-2-enamide Chemical compound COC1=CC=CC(\C=C\C(=O)NC=2C=C(OC)C(OC)=C(OC)C=2)=C1 LVHOHZHTZXRVRJ-CMDGGOBGSA-N 0.000 description 3
- JNPGUXGVLNJQSQ-BGGMYYEUSA-M (e,3r,5s)-7-[4-(4-fluorophenyl)-1,2-di(propan-2-yl)pyrrol-3-yl]-3,5-dihydroxyhept-6-enoate Chemical compound CC(C)N1C(C(C)C)=C(\C=C\[C@@H](O)C[C@@H](O)CC([O-])=O)C(C=2C=CC(F)=CC=2)=C1 JNPGUXGVLNJQSQ-BGGMYYEUSA-M 0.000 description 3
- ONBQEOIKXPHGMB-VBSBHUPXSA-N 1-[2-[(2s,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-4,6-dihydroxyphenyl]-3-(4-hydroxyphenyl)propan-1-one Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1OC1=CC(O)=CC(O)=C1C(=O)CCC1=CC=C(O)C=C1 ONBQEOIKXPHGMB-VBSBHUPXSA-N 0.000 description 3
- RZFJBSIAXYEPBX-UHFFFAOYSA-N 1-[4-[4-[2-[4-chloro-3-(diethylsulfamoyl)anilino]pyrimidin-4-yl]pyridin-2-yl]phenyl]-3-methylurea Chemical compound C1=C(Cl)C(S(=O)(=O)N(CC)CC)=CC(NC=2N=C(C=CN=2)C=2C=C(N=CC=2)C=2C=CC(NC(=O)NC)=CC=2)=C1 RZFJBSIAXYEPBX-UHFFFAOYSA-N 0.000 description 3
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 3
- QBXVXKRWOVBUDB-GRKNLSHJSA-N ClC=1C(=CC(=C(CN2[C@H](C[C@H](C2)O)C(=O)O)C1)OCC1=CC(=CC=C1)C#N)OCC1=C(C(=CC=C1)C1=CC2=C(OCCO2)C=C1)C Chemical compound ClC=1C(=CC(=C(CN2[C@H](C[C@H](C2)O)C(=O)O)C1)OCC1=CC(=CC=C1)C#N)OCC1=C(C(=CC=C1)C1=CC2=C(OCCO2)C=C1)C QBXVXKRWOVBUDB-GRKNLSHJSA-N 0.000 description 3
- 108020004705 Codon Proteins 0.000 description 3
- 229940126657 Compound 17 Drugs 0.000 description 3
- BZLVMXJERCGZMT-UHFFFAOYSA-N Methyl tert-butyl ether Chemical compound COC(C)(C)C BZLVMXJERCGZMT-UHFFFAOYSA-N 0.000 description 3
- NELWQUQCCZMRPB-UBPLGANQSA-N [(2r,3r,4r,5r)-4-acetyloxy-5-(4-amino-5-ethenyl-2-oxopyrimidin-1-yl)-2-methyloxolan-3-yl] acetate Chemical compound CC(=O)O[C@@H]1[C@H](OC(C)=O)[C@@H](C)O[C@H]1N1C(=O)N=C(N)C(C=C)=C1 NELWQUQCCZMRPB-UBPLGANQSA-N 0.000 description 3
- LNUFLCYMSVYYNW-ZPJMAFJPSA-N [(2r,3r,4s,5r,6r)-2-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[[(3s,5s,8r,9s,10s,13r,14s,17r)-10,13-dimethyl-17-[(2r)-6-methylheptan-2-yl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-yl]oxy]-4,5-disulfo Chemical compound O([C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1C[C@@H]2CC[C@H]3[C@@H]4CC[C@@H]([C@]4(CC[C@@H]3[C@@]2(C)CC1)C)[C@H](C)CCCC(C)C)[C@H]1O[C@H](COS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]1OS(O)(=O)=O LNUFLCYMSVYYNW-ZPJMAFJPSA-N 0.000 description 3
- 150000001336 alkenes Chemical class 0.000 description 3
- 125000003282 alkyl amino group Chemical group 0.000 description 3
- SRVFFFJZQVENJC-IHRRRGAJSA-N aloxistatin Chemical compound CCOC(=O)[C@H]1O[C@@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCCC(C)C SRVFFFJZQVENJC-IHRRRGAJSA-N 0.000 description 3
- 239000011324 bead Substances 0.000 description 3
- UNXISIRQWPTTSN-UHFFFAOYSA-N boron;2,3-dimethylbutane-2,3-diol Chemical compound [B].[B].CC(C)(O)C(C)(C)O UNXISIRQWPTTSN-UHFFFAOYSA-N 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 229940125904 compound 1 Drugs 0.000 description 3
- 229940125797 compound 12 Drugs 0.000 description 3
- 229940126142 compound 16 Drugs 0.000 description 3
- 229940125782 compound 2 Drugs 0.000 description 3
- 229940126214 compound 3 Drugs 0.000 description 3
- 229940125898 compound 5 Drugs 0.000 description 3
- 238000007796 conventional method Methods 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 239000000706 filtrate Substances 0.000 description 3
- 125000004438 haloalkoxy group Chemical group 0.000 description 3
- 238000004128 high performance liquid chromatography Methods 0.000 description 3
- KQNYTTDHCMFOME-UHFFFAOYSA-N methyl n-[[3-[(4-tert-butylpiperazin-1-yl)methyl]-8-fluoro-2-phenylquinoline-4-carbonyl]amino]-n-phenylcarbamate Chemical compound C=1C=CC=CC=1N(C(=O)OC)NC(=O)C(C1=CC=CC(F)=C1N=C1C=2C=CC=CC=2)=C1CN1CCN(C(C)(C)C)CC1 KQNYTTDHCMFOME-UHFFFAOYSA-N 0.000 description 3
- MUJNAWXXOJRNGK-UHFFFAOYSA-N n-[3-(6-methyl-1,2,3,4-tetrahydrocarbazol-9-yl)propyl]cyclohexanamine Chemical compound C1=2CCCCC=2C2=CC(C)=CC=C2N1CCCNC1CCCCC1 MUJNAWXXOJRNGK-UHFFFAOYSA-N 0.000 description 3
- PFGVNLZDWRZPJW-OPAMFIHVSA-N otamixaban Chemical compound C([C@@H](C(=O)OC)[C@@H](C)NC(=O)C=1C=CC(=CC=1)C=1C=C[N+]([O-])=CC=1)C1=CC=CC(C(N)=N)=C1 PFGVNLZDWRZPJW-OPAMFIHVSA-N 0.000 description 3
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 3
- 125000003367 polycyclic group Chemical group 0.000 description 3
- SCVFZCLFOSHCOH-UHFFFAOYSA-M potassium acetate Chemical compound [K+].CC([O-])=O SCVFZCLFOSHCOH-UHFFFAOYSA-M 0.000 description 3
- 125000000719 pyrrolidinyl group Chemical group 0.000 description 3
- TZSZZENYCISATO-WIOPSUGQSA-N rodatristat Chemical compound CCOC(=O)[C@@H]1CC2(CN1)CCN(CC2)c1cc(O[C@H](c2ccc(Cl)cc2-c2ccccc2)C(F)(F)F)nc(N)n1 TZSZZENYCISATO-WIOPSUGQSA-N 0.000 description 3
- DVWOYOSIEJRHKW-UIRZNSHLSA-M sodium (2S)-2-[[(2S)-2-[[(4,4-difluorocyclohexyl)-phenylmethoxy]carbonylamino]-4-methylpentanoyl]amino]-1-hydroxy-3-[(3S)-2-oxopyrrolidin-3-yl]propane-1-sulfonate Chemical compound FC1(CCC(CC1)C(OC(=O)N[C@H](C(=O)N[C@H](C(S(=O)(=O)[O-])O)C[C@H]1C(NCC1)=O)CC(C)C)C1=CC=CC=C1)F.[Na+] DVWOYOSIEJRHKW-UIRZNSHLSA-M 0.000 description 3
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 3
- GLGNXYJARSMNGJ-VKTIVEEGSA-N (1s,2s,3r,4r)-3-[[5-chloro-2-[(1-ethyl-6-methoxy-2-oxo-4,5-dihydro-3h-1-benzazepin-7-yl)amino]pyrimidin-4-yl]amino]bicyclo[2.2.1]hept-5-ene-2-carboxamide Chemical compound CCN1C(=O)CCCC2=C(OC)C(NC=3N=C(C(=CN=3)Cl)N[C@H]3[C@H]([C@@]4([H])C[C@@]3(C=C4)[H])C(N)=O)=CC=C21 GLGNXYJARSMNGJ-VKTIVEEGSA-N 0.000 description 2
- SZUVGFMDDVSKSI-WIFOCOSTSA-N (1s,2s,3s,5r)-1-(carboxymethyl)-3,5-bis[(4-phenoxyphenyl)methyl-propylcarbamoyl]cyclopentane-1,2-dicarboxylic acid Chemical compound O=C([C@@H]1[C@@H]([C@](CC(O)=O)([C@H](C(=O)N(CCC)CC=2C=CC(OC=3C=CC=CC=3)=CC=2)C1)C(O)=O)C(O)=O)N(CCC)CC(C=C1)=CC=C1OC1=CC=CC=C1 SZUVGFMDDVSKSI-WIFOCOSTSA-N 0.000 description 2
- DIXMBHMNEHPFCX-MCMMXHMISA-N (2r)-2-[5-[6-amino-5-[(1r)-1-[5-fluoro-2-(triazol-2-yl)phenyl]ethoxy]pyridin-3-yl]-4-methyl-1,3-thiazol-2-yl]propane-1,2-diol Chemical compound O([C@H](C)C=1C(=CC=C(F)C=1)N1N=CC=N1)C(C(=NC=1)N)=CC=1C=1SC([C@](C)(O)CO)=NC=1C DIXMBHMNEHPFCX-MCMMXHMISA-N 0.000 description 2
- HPJGEESDHAUUQR-SKGSPYGFSA-N (2s)-2-[[(2s)-5-(diaminomethylideneamino)-2-[[(2s)-1-[(2s)-5-(diaminomethylideneamino)-2-[[(2s)-2-[[(2s)-3-naphthalen-2-yl-2-(3-pyridin-3-ylpropanoylamino)propanoyl]amino]-3-phenylpropanoyl]amino]pentanoyl]pyrrolidine-2-carbonyl]amino]pentanoyl]amino]buta Chemical compound NC(=O)C[C@@H](C(N)=O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=C2C=CC=CC2=CC=1)NC(=O)CCC=1C=NC=CC=1)CC1=CC=CC=C1 HPJGEESDHAUUQR-SKGSPYGFSA-N 0.000 description 2
- VUDZSIYXZUYWSC-DBRKOABJSA-N (4r)-1-[(2r,4r,5r)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-4-hydroxy-1,3-diazinan-2-one Chemical compound FC1(F)[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)N[C@H](O)CC1 VUDZSIYXZUYWSC-DBRKOABJSA-N 0.000 description 2
- AXQACEQYCPKDMV-RZAWKFBISA-N (4r,4as,7ar,12bs)-3-(cyclopropylmethyl)-4a,7,9-trihydroxy-n-[2-(3-phenyl-1,2,4-oxadiazol-5-yl)propan-2-yl]-1,2,4,5,7a,13-hexahydro-4,12-methanobenzofuro[3,2-e]isoquinoline-6-carboxamide Chemical compound N1([C@@H]2CC=3C4=C(C(=CC=3)O)O[C@H]3C(O)=C(C[C@]2(O)[C@]34CC1)C(=O)NC(C)(C)C=1ON=C(N=1)C=1C=CC=CC=1)CC1CC1 AXQACEQYCPKDMV-RZAWKFBISA-N 0.000 description 2
- VAVHMEQFYYBAPR-ITWZMISCSA-N (e,3r,5s)-7-[4-(4-fluorophenyl)-1-phenyl-2-propan-2-ylpyrrol-3-yl]-3,5-dihydroxyhept-6-enoic acid Chemical compound CC(C)C1=C(\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)C(C=2C=CC(F)=CC=2)=CN1C1=CC=CC=C1 VAVHMEQFYYBAPR-ITWZMISCSA-N 0.000 description 2
- YKYWUHHZZRBGMG-JWTNVVGKSA-N 1-methyl-2-[[(1r,5s)-6-[[5-(trifluoromethyl)pyridin-2-yl]methoxymethyl]-3-azabicyclo[3.1.0]hexan-3-yl]methyl]benzimidazole Chemical compound C1([C@@H]2CN(C[C@@H]21)CC=1N(C2=CC=CC=C2N=1)C)COCC1=CC=C(C(F)(F)F)C=N1 YKYWUHHZZRBGMG-JWTNVVGKSA-N 0.000 description 2
- WGABOZPQOOZAOI-UHFFFAOYSA-N 2-[4-[[(3,5-dimethoxy-4-methylbenzoyl)-(3-phenylpropyl)amino]methyl]phenyl]acetic acid Chemical compound COC1=C(C)C(OC)=CC(C(=O)N(CCCC=2C=CC=CC=2)CC=2C=CC(CC(O)=O)=CC=2)=C1 WGABOZPQOOZAOI-UHFFFAOYSA-N 0.000 description 2
- TXEBWPPWSVMYOA-UHFFFAOYSA-N 4-[3-[(1-amino-2-chloroethyl)amino]propyl]-1-[[3-(2-chlorophenyl)phenyl]methyl]-5-hydroxyimidazolidin-2-one Chemical compound NC(CCl)NCCCC1NC(=O)N(Cc2cccc(c2)-c2ccccc2Cl)C1O TXEBWPPWSVMYOA-UHFFFAOYSA-N 0.000 description 2
- KUZSBKJSGSKPJH-VXGBXAGGSA-N 5-[(9R)-6-[(3R)-3-methylmorpholin-4-yl]-11-oxa-1,3,5-triazatricyclo[7.4.0.02,7]trideca-2,4,6-trien-4-yl]pyrazin-2-amine Chemical compound C[C@@H]1COCCN1c1nc(nc2N3CCOC[C@H]3Cc12)-c1cnc(N)cn1 KUZSBKJSGSKPJH-VXGBXAGGSA-N 0.000 description 2
- 208000010507 Adenocarcinoma of Lung Diseases 0.000 description 2
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- NZSQBRZWARZNQH-ZWOACCQCSA-N C1(CC1)NC(=O)O[C@H]1C(C2CC[C@]3([C@@]4(CC[C@@]5(C(C4CCC3[C@]2(CC1)C)[C@@H](CC5)[C@H](C)O)C(=O)O)C)C)(C)C Chemical compound C1(CC1)NC(=O)O[C@H]1C(C2CC[C@]3([C@@]4(CC[C@@]5(C(C4CCC3[C@]2(CC1)C)[C@@H](CC5)[C@H](C)O)C(=O)O)C)C)(C)C NZSQBRZWARZNQH-ZWOACCQCSA-N 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- PMPVIKIVABFJJI-UHFFFAOYSA-N Cyclobutane Chemical compound C1CCC1 PMPVIKIVABFJJI-UHFFFAOYSA-N 0.000 description 2
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 2
- LVZWSLJZHVFIQJ-UHFFFAOYSA-N Cyclopropane Chemical compound C1CC1 LVZWSLJZHVFIQJ-UHFFFAOYSA-N 0.000 description 2
- OKKJLVBELUTLKV-MZCSYVLQSA-N Deuterated methanol Chemical compound [2H]OC([2H])([2H])[2H] OKKJLVBELUTLKV-MZCSYVLQSA-N 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- 229940126204 KRAS G12D inhibitor Drugs 0.000 description 2
- ZNSPHKJFQDEABI-NZQKXSOJSA-N Nc1nc(O[C@H](c2ccc(Cl)cc2-c2ccccc2)C(F)(F)F)cc(n1)N1CCC2(CN[C@@H](C2)C(O)=O)CC1 Chemical compound Nc1nc(O[C@H](c2ccc(Cl)cc2-c2ccccc2)C(F)(F)F)cc(n1)N1CCC2(CN[C@@H](C2)C(O)=O)CC1 ZNSPHKJFQDEABI-NZQKXSOJSA-N 0.000 description 2
- QQONPFPTGQHPMA-UHFFFAOYSA-N Propene Chemical group CC=C QQONPFPTGQHPMA-UHFFFAOYSA-N 0.000 description 2
- KYQCOXFCLRTKLS-UHFFFAOYSA-N Pyrazine Chemical compound C1=CN=CC=N1 KYQCOXFCLRTKLS-UHFFFAOYSA-N 0.000 description 2
- 102100032929 Son of sevenless homolog 1 Human genes 0.000 description 2
- 101710146001 Son of sevenless homolog 1 Proteins 0.000 description 2
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 239000012300 argon atmosphere Substances 0.000 description 2
- 239000007853 buffer solution Substances 0.000 description 2
- AEULIVPVIDOLIN-UHFFFAOYSA-N cep-11981 Chemical compound C1=C2C3=C4CNC(=O)C4=C4C5=CN(C)N=C5CCC4=C3N(CC(C)C)C2=CC=C1NC1=NC=CC=N1 AEULIVPVIDOLIN-UHFFFAOYSA-N 0.000 description 2
- 239000012069 chiral reagent Substances 0.000 description 2
- 238000004587 chromatography analysis Methods 0.000 description 2
- 238000004440 column chromatography Methods 0.000 description 2
- 229940126543 compound 14 Drugs 0.000 description 2
- 229940125758 compound 15 Drugs 0.000 description 2
- 229940125876 compound 15a Drugs 0.000 description 2
- 229940126212 compound 17a Drugs 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 230000006837 decompression Effects 0.000 description 2
- 238000001212 derivatisation Methods 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 125000001188 haloalkyl group Chemical group 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- DMEGYFMYUHOHGS-UHFFFAOYSA-N heptamethylene Natural products C1CCCCCC1 DMEGYFMYUHOHGS-UHFFFAOYSA-N 0.000 description 2
- 125000004404 heteroalkyl group Chemical group 0.000 description 2
- 238000005984 hydrogenation reaction Methods 0.000 description 2
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 description 2
- 239000005457 ice water Substances 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 230000014759 maintenance of location Effects 0.000 description 2
- 125000002757 morpholinyl group Chemical group 0.000 description 2
- 239000012452 mother liquor Substances 0.000 description 2
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 2
- DOWVMJFBDGWVML-UHFFFAOYSA-N n-cyclohexyl-n-methyl-4-(1-oxidopyridin-1-ium-3-yl)imidazole-1-carboxamide Chemical compound C1=NC(C=2C=[N+]([O-])C=CC=2)=CN1C(=O)N(C)C1CCCCC1 DOWVMJFBDGWVML-UHFFFAOYSA-N 0.000 description 2
- 125000001624 naphthyl group Chemical group 0.000 description 2
- 239000012299 nitrogen atmosphere Substances 0.000 description 2
- RLOWWWKZYUNIDI-UHFFFAOYSA-N phosphinic chloride Chemical compound ClP=O RLOWWWKZYUNIDI-UHFFFAOYSA-N 0.000 description 2
- 125000003386 piperidinyl group Chemical group 0.000 description 2
- 125000003226 pyrazolyl group Chemical group 0.000 description 2
- 125000004076 pyridyl group Chemical group 0.000 description 2
- 125000000714 pyrimidinyl group Chemical group 0.000 description 2
- HNJBEVLQSNELDL-UHFFFAOYSA-N pyrrolidin-2-one Chemical compound O=C1CCCN1 HNJBEVLQSNELDL-UHFFFAOYSA-N 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 125000003003 spiro group Chemical group 0.000 description 2
- 125000001544 thienyl group Chemical group 0.000 description 2
- WJKHJLXJJJATHN-UHFFFAOYSA-N triflic anhydride Chemical compound FC(F)(F)S(=O)(=O)OS(=O)(=O)C(F)(F)F WJKHJLXJJJATHN-UHFFFAOYSA-N 0.000 description 2
- 238000007039 two-step reaction Methods 0.000 description 2
- HBENZIXOGRCSQN-VQWWACLZSA-N (1S,2S,6R,14R,15R,16R)-5-(cyclopropylmethyl)-16-[(2S)-2-hydroxy-3,3-dimethylpentan-2-yl]-15-methoxy-13-oxa-5-azahexacyclo[13.2.2.12,8.01,6.02,14.012,20]icosa-8(20),9,11-trien-11-ol Chemical compound N1([C@@H]2CC=3C4=C(C(=CC=3)O)O[C@H]3[C@@]5(OC)CC[C@@]2([C@@]43CC1)C[C@@H]5[C@](C)(O)C(C)(C)CC)CC1CC1 HBENZIXOGRCSQN-VQWWACLZSA-N 0.000 description 1
- LJIOTBMDLVHTBO-CUYJMHBOSA-N (2s)-2-amino-n-[(1r,2r)-1-cyano-2-[4-[4-(4-methylpiperazin-1-yl)sulfonylphenyl]phenyl]cyclopropyl]butanamide Chemical compound CC[C@H](N)C(=O)N[C@]1(C#N)C[C@@H]1C1=CC=C(C=2C=CC(=CC=2)S(=O)(=O)N2CCN(C)CC2)C=C1 LJIOTBMDLVHTBO-CUYJMHBOSA-N 0.000 description 1
- 125000004973 1-butenyl group Chemical group C(=CCC)* 0.000 description 1
- 125000006023 1-pentenyl group Chemical group 0.000 description 1
- 125000006017 1-propenyl group Chemical group 0.000 description 1
- 125000000530 1-propynyl group Chemical group [H]C([H])([H])C#C* 0.000 description 1
- 238000005160 1H NMR spectroscopy Methods 0.000 description 1
- AVRPFRMDMNDIDH-UHFFFAOYSA-N 1h-quinazolin-2-one Chemical compound C1=CC=CC2=NC(O)=NC=C21 AVRPFRMDMNDIDH-UHFFFAOYSA-N 0.000 description 1
- JENUMEXEVAAAJX-SNVBAGLBSA-N 2-(3,5-dimethyl-1,2,4-triazol-1-yl)-1-[(2r)-2-methyl-4-[2-(trifluoromethyl)-4-[2-(trifluoromethyl)pyrimidin-5-yl]-1,3-thiazol-5-yl]piperazin-1-yl]ethanone Chemical compound C([C@H]1C)N(C2=C(N=C(S2)C(F)(F)F)C=2C=NC(=NC=2)C(F)(F)F)CCN1C(=O)CN1N=C(C)N=C1C JENUMEXEVAAAJX-SNVBAGLBSA-N 0.000 description 1
- IOOWNWLVCOUUEX-WPRPVWTQSA-N 2-[(3r,6s)-2-hydroxy-3-[(2-thiophen-2-ylacetyl)amino]oxaborinan-6-yl]acetic acid Chemical compound OB1O[C@H](CC(O)=O)CC[C@@H]1NC(=O)CC1=CC=CS1 IOOWNWLVCOUUEX-WPRPVWTQSA-N 0.000 description 1
- 125000004974 2-butenyl group Chemical group C(C=CC)* 0.000 description 1
- 125000004493 2-methylbut-1-yl group Chemical group CC(C*)CC 0.000 description 1
- 125000001622 2-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C(*)C([H])=C([H])C2=C1[H] 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- 125000001494 2-propynyl group Chemical group [H]C#CC([H])([H])* 0.000 description 1
- 125000004975 3-butenyl group Chemical group C(CC=C)* 0.000 description 1
- 125000000474 3-butynyl group Chemical group [H]C#CC([H])([H])C([H])([H])* 0.000 description 1
- NHQDETIJWKXCTC-UHFFFAOYSA-N 3-chloroperbenzoic acid Chemical compound OOC(=O)C1=CC=CC(Cl)=C1 NHQDETIJWKXCTC-UHFFFAOYSA-N 0.000 description 1
- HRADVHZVMOMEPU-UHFFFAOYSA-N 3-iodopyrrolidine-2,5-dione Chemical compound IC1CC(=O)NC1=O HRADVHZVMOMEPU-UHFFFAOYSA-N 0.000 description 1
- 125000003542 3-methylbutan-2-yl group Chemical group [H]C([H])([H])C([H])(*)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 125000006042 4-hexenyl group Chemical group 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- OSJRWZLWCLWVJF-UHFFFAOYSA-N 7H-pyrido[4,3-d]pyrimidin-8-one Chemical class O=C1CN=Cc2cncnc12 OSJRWZLWCLWVJF-UHFFFAOYSA-N 0.000 description 1
- MITGKKFYIJJQGL-UHFFFAOYSA-N 9-(4-chlorobenzoyl)-6-methylsulfonyl-2,3-dihydro-1H-carbazol-4-one Chemical compound ClC1=CC=C(C(=O)N2C3=CC=C(C=C3C=3C(CCCC2=3)=O)S(=O)(=O)C)C=C1 MITGKKFYIJJQGL-UHFFFAOYSA-N 0.000 description 1
- QUMCIHKVKQYNPA-RUZDIDTESA-N C1(CCCCC1)CN1[C@@H](C=2N(C=3C=NC(=NC1=3)NC1=C(C=C(C(=O)NC3CCN(CC3)C)C=C1)OC)C(=NN=2)C)CC Chemical compound C1(CCCCC1)CN1[C@@H](C=2N(C=3C=NC(=NC1=3)NC1=C(C=C(C(=O)NC3CCN(CC3)C)C=C1)OC)C(=NN=2)C)CC QUMCIHKVKQYNPA-RUZDIDTESA-N 0.000 description 1
- 125000000041 C6-C10 aryl group Chemical group 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 1
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N Dimethyl sulfoxide Chemical compound [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- 230000005526 G1 to G0 transition Effects 0.000 description 1
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Natural products NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 239000007821 HATU Substances 0.000 description 1
- 101000801643 Homo sapiens Retinal-specific phospholipid-transporting ATPase ABCA4 Proteins 0.000 description 1
- 101000652482 Homo sapiens TBC1 domain family member 8 Proteins 0.000 description 1
- 101150105104 Kras gene Proteins 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- HPKJGHVHQWJOOT-ZJOUEHCJSA-N N-[(2S)-3-cyclohexyl-1-oxo-1-({(2S)-1-oxo-3-[(3S)-2-oxopyrrolidin-3-yl]propan-2-yl}amino)propan-2-yl]-1H-indole-2-carboxamide Chemical compound C1C(CCCC1)C[C@H](NC(=O)C=1NC2=CC=CC=C2C=1)C(=O)N[C@@H](C[C@H]1C(=O)NCC1)C=O HPKJGHVHQWJOOT-ZJOUEHCJSA-N 0.000 description 1
- JCXJVPUVTGWSNB-UHFFFAOYSA-N Nitrogen dioxide Chemical compound O=[N]=O JCXJVPUVTGWSNB-UHFFFAOYSA-N 0.000 description 1
- 108700020796 Oncogene Proteins 0.000 description 1
- 101150003085 Pdcl gene Proteins 0.000 description 1
- PCNDJXKNXGMECE-UHFFFAOYSA-N Phenazine Natural products C1=CC=CC2=NC3=CC=CC=C3N=C21 PCNDJXKNXGMECE-UHFFFAOYSA-N 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 108091000080 Phosphotransferase Proteins 0.000 description 1
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 1
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 1
- 102000001788 Proto-Oncogene Proteins c-raf Human genes 0.000 description 1
- 108010029869 Proto-Oncogene Proteins c-raf Proteins 0.000 description 1
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 1
- 102100033617 Retinal-specific phospholipid-transporting ATPase ABCA4 Human genes 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- 206010041067 Small cell lung cancer Diseases 0.000 description 1
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical class [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 1
- 102100030302 TBC1 domain family member 8 Human genes 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 230000001594 aberrant effect Effects 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- YLEIFZAVNWDOBM-ZTNXSLBXSA-N ac1l9hc7 Chemical compound C([C@H]12)C[C@@H](C([C@@H](O)CC3)(C)C)[C@@]43C[C@@]14CC[C@@]1(C)[C@@]2(C)C[C@@H]2O[C@]3(O)[C@H](O)C(C)(C)O[C@@H]3[C@@H](C)[C@H]12 YLEIFZAVNWDOBM-ZTNXSLBXSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 239000012445 acidic reagent Substances 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 150000001335 aliphatic alkanes Chemical class 0.000 description 1
- 125000004414 alkyl thio group Chemical group 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- 238000003016 alphascreen Methods 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 238000011914 asymmetric synthesis Methods 0.000 description 1
- HONIICLYMWZJFZ-UHFFFAOYSA-N azetidine Chemical compound C1CNC1 HONIICLYMWZJFZ-UHFFFAOYSA-N 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000004619 benzopyranyl group Chemical group O1C(C=CC2=C1C=CC=C2)* 0.000 description 1
- 125000001164 benzothiazolyl group Chemical group S1C(=NC2=C1C=CC=C2)* 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- UORVGPXVDQYIDP-UHFFFAOYSA-N borane Chemical class B UORVGPXVDQYIDP-UHFFFAOYSA-N 0.000 description 1
- ZADPBFCGQRWHPN-UHFFFAOYSA-N boronic acid Chemical compound OBO ZADPBFCGQRWHPN-UHFFFAOYSA-N 0.000 description 1
- FJDQFPXHSGXQBY-UHFFFAOYSA-L caesium carbonate Chemical compound [Cs+].[Cs+].[O-]C([O-])=O FJDQFPXHSGXQBY-UHFFFAOYSA-L 0.000 description 1
- 229910000024 caesium carbonate Inorganic materials 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- PBAYDYUZOSNJGU-UHFFFAOYSA-N chelidonic acid Natural products OC(=O)C1=CC(=O)C=C(C(O)=O)O1 PBAYDYUZOSNJGU-UHFFFAOYSA-N 0.000 description 1
- 235000019504 cigarettes Nutrition 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 230000006552 constitutive activation Effects 0.000 description 1
- DOBRDRYODQBAMW-UHFFFAOYSA-N copper(i) cyanide Chemical compound [Cu+].N#[C-] DOBRDRYODQBAMW-UHFFFAOYSA-N 0.000 description 1
- 125000005366 cycloalkylthio group Chemical group 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000002188 cycloheptatrienyl group Chemical group C1(=CC=CC=CC1)* 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000003678 cyclohexadienyl group Chemical group C1(=CC=CCC1)* 0.000 description 1
- 125000000596 cyclohexenyl group Chemical group C1(=CCCCC1)* 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000000640 cyclooctyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- NXQGGXCHGDYOHB-UHFFFAOYSA-L cyclopenta-1,4-dien-1-yl(diphenyl)phosphane;dichloropalladium;iron(2+) Chemical compound [Fe+2].Cl[Pd]Cl.[CH-]1C=CC(P(C=2C=CC=CC=2)C=2C=CC=CC=2)=C1.[CH-]1C=CC(P(C=2C=CC=CC=2)C=2C=CC=CC=2)=C1 NXQGGXCHGDYOHB-UHFFFAOYSA-L 0.000 description 1
- 125000002433 cyclopentenyl group Chemical group C1(=CCCC1)* 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 150000001975 deuterium Chemical group 0.000 description 1
- 125000004852 dihydrofuranyl group Chemical group O1C(CC=C1)* 0.000 description 1
- 125000005047 dihydroimidazolyl group Chemical group N1(CNC=C1)* 0.000 description 1
- 125000005052 dihydropyrazolyl group Chemical group N1(NCC=C1)* 0.000 description 1
- 125000005054 dihydropyrrolyl group Chemical group [H]C1=C([H])C([H])([H])C([H])([H])N1* 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 239000012065 filter cake Substances 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- 150000002332 glycine derivatives Chemical class 0.000 description 1
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000000592 heterocycloalkyl group Chemical group 0.000 description 1
- 125000004468 heterocyclylthio group Chemical group 0.000 description 1
- 125000005980 hexynyl group Chemical group 0.000 description 1
- 125000002632 imidazolidinyl group Chemical group 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 125000003392 indanyl group Chemical group C1(CCC2=CC=CC=C12)* 0.000 description 1
- 125000003453 indazolyl group Chemical group N1N=C(C2=C1C=CC=C2)* 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- PNDPGZBMCMUPRI-UHFFFAOYSA-N iodine Chemical compound II PNDPGZBMCMUPRI-UHFFFAOYSA-N 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- HVTICUPFWKNHNG-UHFFFAOYSA-N iodoethane Chemical class CCI HVTICUPFWKNHNG-UHFFFAOYSA-N 0.000 description 1
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical class IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 1
- 239000002608 ionic liquid Substances 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000003384 isochromanyl group Chemical group C1(OCCC2=CC=CC=C12)* 0.000 description 1
- 238000006317 isomerization reaction Methods 0.000 description 1
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000002183 isoquinolinyl group Chemical group C1(=NC=CC2=CC=CC=C12)* 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 125000000842 isoxazolyl group Chemical group 0.000 description 1
- JFOZKMSJYSPYLN-QHCPKHFHSA-N lifitegrast Chemical compound CS(=O)(=O)C1=CC=CC(C[C@H](NC(=O)C=2C(=C3CCN(CC3=CC=2Cl)C(=O)C=2C=C3OC=CC3=CC=2)Cl)C(O)=O)=C1 JFOZKMSJYSPYLN-QHCPKHFHSA-N 0.000 description 1
- YNESATAKKCNGOF-UHFFFAOYSA-N lithium bis(trimethylsilyl)amide Chemical compound [Li+].C[Si](C)(C)[N-][Si](C)(C)C YNESATAKKCNGOF-UHFFFAOYSA-N 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- LVWZTYCIRDMTEY-UHFFFAOYSA-N metamizole Chemical compound O=C1C(N(CS(O)(=O)=O)C)=C(C)N(C)N1C1=CC=CC=C1 LVWZTYCIRDMTEY-UHFFFAOYSA-N 0.000 description 1
- BGXZIBSLBRKDTP-UHFFFAOYSA-N methyl 9-(4-chloroanilino)-[1,3]thiazolo[5,4-f]quinazoline-2-carboximidate Chemical compound C12=C3SC(C(=N)OC)=NC3=CC=C2N=CN=C1NC1=CC=C(Cl)C=C1 BGXZIBSLBRKDTP-UHFFFAOYSA-N 0.000 description 1
- KTMKRRPZPWUYKK-UHFFFAOYSA-N methylboronic acid Chemical compound CB(O)O KTMKRRPZPWUYKK-UHFFFAOYSA-N 0.000 description 1
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 239000011259 mixed solution Substances 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- FMASTMURQSHELY-UHFFFAOYSA-N n-(4-fluoro-2-methylphenyl)-3-methyl-n-[(2-methyl-1h-indol-4-yl)methyl]pyridine-4-carboxamide Chemical compound C1=CC=C2NC(C)=CC2=C1CN(C=1C(=CC(F)=CC=1)C)C(=O)C1=CC=NC=C1C FMASTMURQSHELY-UHFFFAOYSA-N 0.000 description 1
- PHHRKRGXWSEXFZ-UHFFFAOYSA-N n-(pyridin-3-ylmethyl)-3-[[2-[(2,3,4-trifluorophenoxy)methyl]-1,3-benzoxazol-4-yl]oxy]propan-1-amine Chemical compound FC1=C(F)C(F)=CC=C1OCC(OC1=CC=C2)=NC1=C2OCCCNCC1=CC=CN=C1 PHHRKRGXWSEXFZ-UHFFFAOYSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- WOOWBQQQJXZGIE-UHFFFAOYSA-N n-ethyl-n-propan-2-ylpropan-2-amine Chemical compound CCN(C(C)C)C(C)C.CCN(C(C)C)C(C)C WOOWBQQQJXZGIE-UHFFFAOYSA-N 0.000 description 1
- HBEDNENASUYMPO-LJQANCHMSA-N n-hydroxy-4-[[(2r)-3-oxo-2-(thiophen-2-ylmethyl)-2,4-dihydroquinoxalin-1-yl]methyl]benzamide Chemical compound C1=CC(C(=O)NO)=CC=C1CN1C2=CC=CC=C2NC(=O)[C@H]1CC1=CC=CS1 HBEDNENASUYMPO-LJQANCHMSA-N 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- JWAZRIHNYRIHIV-UHFFFAOYSA-M naphthalen-2-olate Chemical compound C1=CC=CC2=CC([O-])=CC=C21 JWAZRIHNYRIHIV-UHFFFAOYSA-M 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 239000002773 nucleotide Substances 0.000 description 1
- 125000003729 nucleotide group Chemical group 0.000 description 1
- 231100000590 oncogenic Toxicity 0.000 description 1
- 230000002246 oncogenic effect Effects 0.000 description 1
- 238000011275 oncology therapy Methods 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 125000003566 oxetanyl group Chemical group 0.000 description 1
- 201000008129 pancreatic ductal adenocarcinoma Diseases 0.000 description 1
- 125000003538 pentan-3-yl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000005981 pentynyl group Chemical group 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 102000020233 phosphotransferase Human genes 0.000 description 1
- 125000004193 piperazinyl group Chemical group 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 125000002568 propynyl group Chemical group [*]C#CC([H])([H])[H] 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 125000003373 pyrazinyl group Chemical group 0.000 description 1
- 125000002098 pyridazinyl group Chemical group 0.000 description 1
- PLZDHJUUEGCXJH-UHFFFAOYSA-N pyrido[4,3-d]pyrimidine Chemical compound C1=NC=C2C=NC=CC2=N1 PLZDHJUUEGCXJH-UHFFFAOYSA-N 0.000 description 1
- 125000000168 pyrrolyl group Chemical group 0.000 description 1
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 1
- 102000016914 ras Proteins Human genes 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 208000000587 small cell lung carcinoma Diseases 0.000 description 1
- MFRIHAYPQRLWNB-UHFFFAOYSA-N sodium tert-butoxide Chemical compound [Na+].CC(C)(C)[O-] MFRIHAYPQRLWNB-UHFFFAOYSA-N 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 125000005931 tert-butyloxycarbonyl group Chemical group [H]C([H])([H])C(OC(*)=O)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 125000003718 tetrahydrofuranyl group Chemical group 0.000 description 1
- 125000001712 tetrahydronaphthyl group Chemical group C1(CCCC2=CC=CC=C12)* 0.000 description 1
- 125000005958 tetrahydrothienyl group Chemical group 0.000 description 1
- CZDYPVPMEAXLPK-UHFFFAOYSA-N tetramethylsilane Chemical compound C[Si](C)(C)C CZDYPVPMEAXLPK-UHFFFAOYSA-N 0.000 description 1
- 125000003831 tetrazolyl group Chemical group 0.000 description 1
- VLLMWSRANPNYQX-UHFFFAOYSA-N thiadiazole Chemical compound C1=CSN=N1.C1=CSN=N1 VLLMWSRANPNYQX-UHFFFAOYSA-N 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 125000004568 thiomorpholinyl group Chemical group 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 125000001425 triazolyl group Chemical group 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- JQSHBVHOMNKWFT-DTORHVGOSA-N varenicline Chemical compound C12=CC3=NC=CN=C3C=C2[C@H]2C[C@@H]1CNC2 JQSHBVHOMNKWFT-DTORHVGOSA-N 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D519/00—Heterocyclic compounds containing more than one system of two or more relevant hetero rings condensed among themselves or condensed with a common carbocyclic ring system not provided for in groups C07D453/00 or C07D455/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F5/00—Compounds containing elements of Groups 3 or 13 of the Periodic Table
- C07F5/02—Boron compounds
- C07F5/025—Boronic and borinic acid compounds
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
The present disclosure relates to a Kras inhibitor and a method for preparing the same. Specifically, the disclosure relates to a compound shown as a formula I or a pharmaceutically acceptable salt thereof, and also relates to a composition and a medical application thereof, wherein the definition of a specific substituent group is described in the specification.
Description
Technical Field
The disclosure belongs to the field of medicines, and relates to a Kras inhibitor, and a preparation method, a composition and medical application thereof.
Background
The Kirsten Rat Sarcoma 2 virus oncogene homolog ("kras") is a small gtpase and RAS series of cancer tissues. KRAS is used as a cyclic molecular switch to cycle between inactive (GDP bond) and active (GTP-bound) states to receive upstream cellular signals from multiple tyrosine kinases to downstream effectors to regulate various processes, including cell proliferation. Aberrant expression of KRA, which accounts for 20% of all cancers and oncogenic cancers, stabilizes GTP binding and results in constitutive activation of krahs and downstream signals, reported in 25-30% of lung adenocarcinomas. Single nucleotide substitutions at codons 12 and 13 of the KRAS primary amino acid sequence resulting in codon mutations comprise about 40% of KRAS-driven mutations in lung adenocarcinomas. 25.0% of KRAS G12D mutations accounted for 25.0% of patients with pancreatic ductal adenocarcinoma, 13.3% of all patients with colorectal cancer, 10.1% of patients with colorectal cancer, 4.1% of all patients with non-small cell lung cancer, and 1.7% of all patients with small cell lung cancer. The well-known role of KRAS in malignancies and the frequent involvement of KRAS in various tumour types make KRAS a highly attractive target for the pharmaceutical industry for cancer therapy, and prior art WO2021041671 discloses a class of KRA G12D inhibitors.
Disclosure of Invention
The present disclosure provides a compound of formula I or a pharmaceutically acceptable salt thereof,
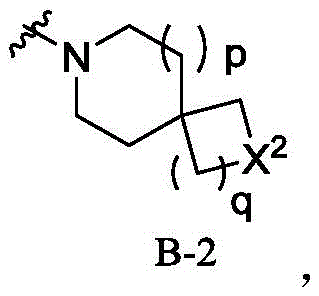
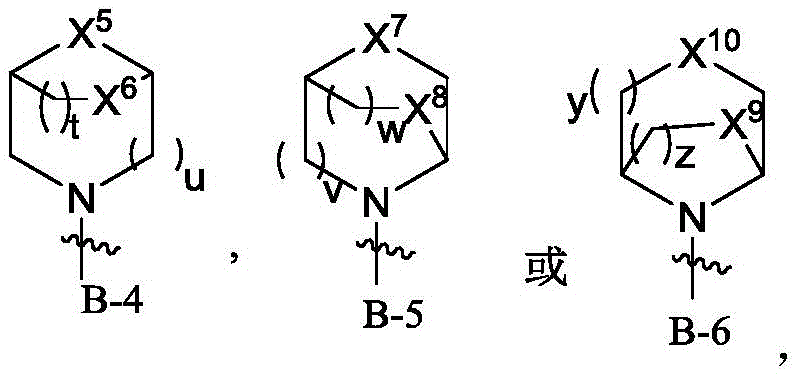
wherein R is 1 Is heterocyclic radical containing at least 1N atom, the heterocyclic radical containing at least 1N atom is selected from B-1, B-2, B-3, B-4, B-5 or B-6,
said X is 1 、X 2 、X 3 、X 4 、X 5 、X 6 、X 7 、X 8 、X 9 、X 10 Each independently selected from-CR a R b- 、-O-、-S-、-NR c -;
n, p, q, r, s, t, u, v, w, y, z are each independently selected from 0,1,2,3 or 4;
Said heterocyclyl containing at least 1N atom being optionally selected from 0,1,2,3,4,5 or 6R d Substitution;
x is selected from N or CR e ;
L 1 Is selected from-CH 2 R f -,-OR f -,-SR f -,-R f NR g R h -,-COR f -,-CONR g R h ,-SO 2 NR g R h Said R is f Is selected from-CR f1 R f2 -、-CR f1 R f2 -CR f3 R f4 -or-CR f1 R f2 -CR f3 R f4 -CR f5 R f6 -;
R 2 None, or 3-to 12-membered selected from at least 1 nitrogen atomA 5-to 10-membered heteroaryl group containing at least 1 nitrogen atom; said 3-12 membered heterocyclyl or 5-10 membered heteroaryl is optionally independently substituted with 0,1,2,3,4,5, or 6R i Substitution;
cy and the atom connected with Cy form 5-7 membered cycloalkyl, 5-7 membered heterocyclic group, 5-7 membered aryl, 5-7 membered nitrogen-containing heteroaryl;
m is selected from 0,1,2,3,4,5 or 6;
R 3 selected from hydrogen, deuterium, halogen, cyano, hydroxy, azido, nitro, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 1-6 Alkoxy radical, C 1-4 Hydroxyalkyl, oxo, C 3-8 Cycloalkyl, 3-8 membered heterocyclyl; or two R 3 A cycloalkyl group having 3 to 6 members formed with the carbon atom to which it is attached;
L 2 selected from the group consisting of a bond, -S-, -C (O) -, -CR f1 R f2 -;
Ar is selected from C 6-10 Aryl, 5-10 membered heteroaryl, said Ar optionally substituted with 1-6R j Substitution;
R a 、R b 、R c 、R i 、R j each independently selected from hydrogen, deuterium, halogen, cyano, hydroxy, azido, -B (OH) 2 Nitro group, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 1-6 Alkoxy radical, C 1-4 Hydroxyalkyl radical, C 2-6 Alkenyl radical, C 2-6 Alkynyl, oxo, = NR a1 ,-SR a1 、-OR a1 、-NR a1 R a2 、-COR a2 、-CONR a1 R a2 、-C(O)OR a2 、-N(R a2 )-C(O)R a2 、-N(R a2 )-C(O)NR a1 R a2 、-N(R a2 )-C(O)OR a2 、-N(R a2 )-SO 2 R a2 、-SO 2 R a2 、-SO 2 OR a2 、-SO 2 NR a1 R a2 、-O-SO 2 NR a1 R a2 、-O(CO)NR a1 R a2 、C 3-8 Cycloalkyl, 3-to 8-membered heterocyclic group, C 6-10 Aryl, 5-10 membered heteroaryl, C 3-8 Cycloalkyl substitution-C 1-4 Alkyl, 3-8 membered heterocyclyl substituted-C 1-4 Alkyl radical, C 6-10 Aryl substituted-C 1-4 Alkyl, 5-10 membered heteroaryl substituted-C 1-4 An alkyl group;
said C is 3-8 Cycloalkyl, 3-to 8-membered heterocyclic group, C 6-10 Aryl, 5-to 10-membered heteroaryl, C 3-8 Cycloalkyl substituted by-C 1-4 Alkyl, 3-8 membered heterocyclyl substituted-C 1-4 Alkyl radical, C 6-10 Aryl substituted-C 1-4 Alkyl, 5-10 membered heteroaryl substituted-C 1-4 Alkyl is optionally substituted by 1-3 substituents selected from halogen, cyano, hydroxy, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 1-6 Alkoxy radical, C 1-4 Hydroxyalkyl, said 5-10 membered heteroaryl containing 1-5 heteroatoms, each independently selected from S, N and O;
Wherein R is 4 Or R 5 Each independently selected from hydrogen, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 1-6 Alkoxy radical, C 1-4 A hydroxyalkyl group;
X 11 selected from-O-or-NR a1 -;
R 6 Is selected from-OR a1 or-NR a1 R a2 ;
Wherein L is 3 Selected from alkylene or heteroalkylene groups containing 1 to 6 carbons;
X 12 is selected from-O-or-NR a1 -;
X 13 Selected from oxygen or sulfur;
R 7 is selected from-OR a1 or-NR a1 R a2 ;
R d Selected from hydrogen, deuterium, halogen, cyano, hydroxy, azido, nitro, C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl, C 1-4 alkoxy-C 1-4 Alkyl, -NR a1 R a2 、-SR a1 、-OR a1 、-COR a2 ;
R e Selected from hydrogen, deuterium, hydroxy, C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl radical, C 1-4 alkoxy-C 1-4 Alkyl, -NR a1 R a2 、C(O)NH 2 ;
R g Selected from H, C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl, C 1-4 alkoxy-C 1-4 Alkyl radicals, C (O) NH 2 ;
R h Selected from H, C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl radical, C 1-4 alkoxy-C 1-4 Alkyl radical, C 6-10 Aryl, 5-to 10-membered heteroaryl, said C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl, C 1-4 alkoxy-C 1-4 Alkyl radical, C 6-10 Aryl, 5-10 membered heteroaryl optionally substituted by 1-3 substituents selected from halogen, cyano, hydroxy, C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 1-4 alkoxy-C 1-4 Alkyl substituent substitution;
R a1 selected from hydrogen, C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl radical, C 1-4 alkoxy-C 1-4 Alkyl radicals, C (O) NH 2 ;
R a2 Selected from hydrogen, C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl, C 1-4 alkoxy-C 1-4 Alkyl radical, C 6-10 Aryl, 5-to 10-membered heteroaryl, wherein C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl radical, C 1-4 alkoxy-C 1-4 Alkyl radical, C 6-10 Aryl, 5-10 membered heteroaryl optionally substituted by 1-3 substituents selected from halogen, cyano, hydroxy, C 1-4 Alkyl radical, C 1-4 Haloalkyl, C 1-4 Alkylamino, hydroxy-C 1-4 Alkyl radical, C 1-4 alkoxy-C 1-4 Alkyl substitution; or
R a1 And R a2 A heterocyclic group having 3 to 8 members as the nitrogen atom bonded thereto;
the R is f1 、R f2 、R f3 、R f4 、R f5 、R f6 Each independently selected from hydrogen, deuterium, halogen, hydroxy, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 1-6 Alkoxy, or optionally R f1 And R f2 、R f3 And R f4 、R f5 And R f6 The carbon atom connected with the heterocyclic ring forms 3-6 membered cycloalkyl or 3-6 membered heterocyclic group, the 3-6 membered cycloalkyl or 3-6 membered heterocyclic group is optionally substituted by 1-3 groups selected from deuterium, halogen, hydroxyl, C 1-6 Alkyl substituents.
In alternative embodiments, the present disclosure provides a compound of formula I, or a pharmaceutically acceptable salt thereof,
wherein R is 1 Is a heterocyclic group containing at least 1N atom, the heterocyclic group containing at least 1N atom is selected from B-1, B-2, B-3, B-4, B-5 or B-6,
said X is 1 、X 2 、X 3 、X 4 、X 5 、X 6 、X 7 、X 8 、X 9 、X 10 Each independently selected from-CR a R b- 、-O-、-S-、-NR c -;
n, p, q, r, s, t, u, v, w, y, z are each independently selected from 0,1,2,3 or 4;
Said heterocyclyl containing at least 1N atom is optionally selected from 0,1,2,3,4,5 or 6R d Substitution;
x is selected from N or CR e ;
L 1 Is selected from-CH 2 R f -,-OR f -,-SR f -,-R f NR g R h -,-COR f -,-CONR g R h ,-SO 2 NR g R h Said R is f Is selected from-CR f1 R f2 -、-CR f1 R f2 -CR f3 R f4 -or-CR f1 R f2 -CR f3 R f4 -CR f5 R f6 -;
R 2 Is free of, or selected from the group consisting of 3-12 membered heterocyclyl containing at least 1 nitrogen atom, 5-10 membered heteroaryl containing at least 1 nitrogen atom; said 3-12 membered heterocyclyl or 5-10 membered heteroaryl is optionally independently substituted with 0,1,2,3,4,5, or 6R i Substitution;
cy forms a 5-7 membered cycloalkyl group, a 5-7 membered heterocyclyl group, a 5-7 membered aryl group, a 5-7 membered nitrogen-containing heteroaryl group with the atom to which it is attached;
m is selected from 0,1,2,3,4,5 or 6;
R 3 selected from hydrogen, deuterium, halogen, cyano, hydroxy, azido, nitro, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 1-6 Alkoxy radical, C 1-4 Hydroxyalkyl, oxo, C 3-8 Cycloalkyl, 3-8 membered heterocyclyl; or two R 3 A cycloalkyl group having 3 to 6 members formed with a carbon atom bonded thereto;
L 2 selected from the group consisting of a bond, -S-, -C (O) -, -CR f1 R f2 -;
Ar is selected from C 6-10 Aryl, 5-10 membered heteroaryl, said Ar is optionally substituted with 1-6R j Substitution;
R a 、R b 、R c 、R i 、R j each independently selected from hydrogen, deuterium, halogen, cyano, hydroxy, azido, nitro, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 1-6 Alkoxy radical, C 1-4 Hydroxyalkyl radical, C 2-6 Alkenyl radical, C 2-6 Alkynyl, oxo, = NR a1 ,-SR a1 、-OR a1 、-NR a1 R a2 、-COR a2 、-CONR a1 R a2 、-C(O)OR a2 、-N(R a2 )-C(O)R a2 、-N(R a2 )-C(O)NR a1 R a2 、-N(R a2 )-C(O)OR a2 、-N(R a2 )-SO 2 R a2 、-SO 2 R a2 、-SO 2 OR a2 、-SO 2 NR a1 R a2 、-O-SO 2 NR a1 R a2 、-O(CO)NR a1 R a2 、C 3-8 Cycloalkyl, 3-to 8-membered heterocyclic group, C 6-10 Aryl, 5-to 10-membered heteroaryl, C 3-8 Cycloalkyl substituted by-C 1-4 Alkyl, 3-8 membered heterocyclyl substituted-C 1-4 Alkyl, C6-10 aryl substituted-C 1-4 Alkyl, 5-10 membered heteroaryl substituted-C 1-4 An alkyl group;
said C is 3-8 Cycloalkyl, 3-to 8-membered heterocyclic group, C 6-10 Aryl, 5-to 10-membered heteroaryl, C 3-8 Cycloalkyl substituted by-C 1-4 Alkyl, 3-8 membered heterocyclyl substituted-C 1-4 Alkyl radical, C 6-10 Aryl substituted-C 1-4 Alkyl, 5-10 membered heteroaryl substituted-C 1-4 Alkyl is optionally substituted by 1-3 substituents selected from halogen, cyano, hydroxy, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 1-6 Alkoxy radical, C 1-4 Hydroxyalkyl, said 5-10 membered heteroaryl containing 1-5 heteroatoms, each independently selected from S, N and O;
R d selected from hydrogen, deuterium, halogen, cyano, hydroxy, azido, nitro, C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl radical, C 1-4 alkoxy-C 1-4 Alkyl, -NR a1 R a2 、-SR a1 、-OR a1 、-COR a2 ;
R e Selected from hydrogen, deuterium, hydroxy, C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl radical, C 1-4 alkoxy-C 1-4 Alkyl, -NR a1 R a2 、C(O)NH 2 ;
R g Selected from H, C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl, C 1-4 alkoxy-C 1-4 Alkyl, C (O) NH 2 ;
R h Selected from H, C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl radical, C 1-4 alkoxy-C 1-4 Alkyl radical, C 6-10 Aryl, 5-to 10-membered heteroaryl, said C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl radical, C 1-4 alkoxy-C 1-4 Alkyl radical, C 6-10 Aryl, 5-10 membered heteroaryl optionally substituted by 1-3 substituents selected from halogen, cyano, hydroxy, C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 1-4 alkoxy-C 1-4 Alkyl substituent substitution;
R a1 selected from hydrogen, C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl radical, C 1-4 alkoxy-C 1-4 Alkyl radicals, C (O) NH 2 ;
R a2 Selected from hydrogen, C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl, C 1-4 alkoxy-C 1-4 Alkyl radical, C 6-10 Aryl, 5-to 10-membered heteroaryl, wherein C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl radical, C 1-4 alkoxy-C 1-4 Alkyl radical, C 6-10 Aryl, 5-to 10-membered heteroaryl is optionally substituted with1-3 groups selected from halogen, cyano, hydroxy, C 1-4 Alkyl radical, C 1-4 Haloalkyl, C 1-4 Alkylamino, hydroxy-C 1-4 Alkyl radical, C 1-4 alkoxy-C 1-4 Alkyl substitution;
the R is f1 、R f2 、R f3 、R f4 、R f5 、R f6 Each independently selected from hydrogen, deuterium, halogen, hydroxy, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 1-6 Alkoxy, or optionally R f1 And R f2 、R f3 And R f4 、R f5 And R f6 The carbon atoms connected with the heterocyclic ring form a 3-6 membered cycloalkyl or 3-6 membered heterocyclic ring, the 3-6 membered cycloalkyl or 3-6 membered heterocyclic ring is optionally substituted by 1-3 groups selected from deuterium, halogen, hydroxyl, C 1-6 Alkyl substituents.
In some embodiments, the compound, or a pharmaceutically acceptable salt thereof, has the structure of formula II-1 or II-2,
in some embodiments, the compound, or a pharmaceutically acceptable salt thereof, has the structure of formulae III-1 to III-58,
said R is k R of a compound of the same formula I or a pharmaceutically acceptable salt thereof c 。
In some embodiments, a compound of formula I, or a pharmaceutically acceptable salt thereof, has the structure
In some embodiments, the compound of formula I or a pharmaceutically acceptable salt thereof, the compound of formula II-1 or II-2 or a pharmaceutically acceptable salt thereof, the compound of formulae III-1 to III-54, or a pharmaceutically acceptable salt thereof, wherein L 1 Is selected from-CH 2 R f -,-OR f -,-SR f -,-COR f -,-CONR g R h Is optionally-CH 2 R f -,-OR f -,-SR f -,-CONR g R h 。
In alternative embodiments, R is f Is selected from-CR f1 R f2 -、-CR f1 R f2 -CR f3 R f4 -, optionally-CR f1 R f2 -。
In alternative embodiments, R is f1 、R f2 、R f3 、R f4 、R f5 、R f6 Each independently selected from hydrogen, deuterium, halogen, C 1-6 Alkyl, or optionally R f1 And R f2 、R f3 And R f4 、R f5 And R f6 The carbon atom connected with the heterocyclic ring forms 3-6 membered cycloalkyl or 3-6 membered heterocyclic group, the 3-6 membered cycloalkyl or 3-6 membered heterocyclic group is optionally substituted by 1-3 groups selected from deuterium, halogen, hydroxyl, C 1-6 Alkyl substituents.
In alternative embodiments, R is f1 、R f2 、R f3 、R f4 、R f5 、R f6 Each independently selected from hydrogen, deuterium, fluorine, chlorine, bromine, iodine, methyl, ethyl, n-propyl, isopropyl, tert-butyl, optionally hydrogen, deuterium, fluorine, methyl or ethyl.
In alternative embodiments, R is f1 、R f2 、R f3 、R f4 、R f5 、R f6 The carbon atom to which it is attached constitutes a 3-to 6-membered cycloalkyl group, said 3-The 6-membered cycloalkyl is optionally substituted by 1-3 substituents selected from deuterium, halogen, hydroxy, C 1-6 Substituted with alkyl, optionally said R f1 、R f2 、R f3 、R f4 、R f5 、R f6 The carbon atoms connected with the compound form 3-membered, 4-membered and five-membered cycloalkyl.
In some embodiments, the compound of formula I or a pharmaceutically acceptable salt thereof, the compound of formula II-1 or II-2 or a pharmaceutically acceptable salt thereof, the compound of formulae III-1 to III-54, or a pharmaceutically acceptable salt thereof, wherein R is 2 Selected from the group consisting of 3-12 membered heterocyclic group having at least 1 nitrogen atom, 5-10 membered heteroaryl group having at least 1 nitrogen atom, said 3-12 membered heterocyclic group having at least 1 nitrogen atom, said 5-10 membered heteroaryl group having at least 1 nitrogen atom is selected from the group consisting of hexahydro-1H-pyrrolizinyl group, hexahydro-1H-pyrrolizin-3-onyl group, hexahydro-1H-pyrrolo [2,1-c ] group][1,4]Oxazinyl, octahydroindolizinyl, hexahydropyrrolizine-4-1H-oxide, azetidine, pyrrolidinyl, pyrrolidin-2-one, oxetanyl, piperidinyl, l-azabicyclo [2.2.1 ] oxide]Heptyl, morpholinyl, oxa-5-azabicyclo [2.2.1]Heptyl-5-yl, thiopyranyl or 2',3' -dihydrospiro [ cyclopropane-1, 1' -indenyl]Alternatively, hexahydro-1H-pyrrolizinyl, alternatively hexahydro-1H-pyrrolizinyl or pyrrolidinyl may be used.
In alternative embodiments, R is i Selected from hydrogen, halogen, deuterium, hydroxy, amino, oxo, C 1-6 Alkyl radical, C 1-6 Haloalkyl group
Wherein L is 3 Selected from alkylene or heteroalkylene groups containing 1 to 6 carbons;
X 12 is selected from-O-or-NR a1 -;
X 13 Selected from oxygen or sulfur;
R 7 is selected from-OR a1 or-NR a1 R a2 ;
R a1 And R a2 Each independently selected from hydrogen and C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl radical, C 1-4 alkoxy-C 1-4 Alkyl, or R a1 And R a2 A heterocyclic group having 3 to 8 members as the nitrogen atom bonded thereto;
in alternative embodiments, R i Selected from deuterium, halogen, methyl, ethyl, n-propyl, isopropyl, tert-butyl or
In alternative embodiments, R is i Selected from hydrogen, halogen, deuterium, hydroxy, amino, oxo, C 1-6 Alkyl radical, C 1-6 Haloalkyl, optionally deuterium, halogen, methyl, ethyl, n-propyl, isopropyl, tert-butyl.
In alternative embodiments, the-L 1 -R 2 Is selected from
In alternative embodiments, the-L 1 -R 2 Is selected from
In some embodiments, the compound of formula I or a pharmaceutically acceptable salt thereof, the compound of formula II-1 or II-2 or a pharmaceutically acceptable salt thereof, the compound of formulae III-1 to III-54, or a pharmaceutically acceptable salt thereof, and Ar is selected from C 6-10 Aryl, 5-to 10-membered heteroaryl, said C 6-10 Aryl or 5-to 10-membered heteroaryl is selected from phenyl, naphthyl, 1,2,3, 4-tetrahydronaphthyl, 2, 3-dihydro-1H-indenyl, pyridyl, pyridazinyl, pyrazinyl, pyrimidinyl, quinolinyl, indazolyl, indolylIsoquinolinyl, isoxazolyl, thienyl, triazolyl, pyrazolyl, benzothiazolyl, pyridone, quinolinonyl, isoquinolinyl, quinazolinedionyl, pyrazinonyl, pyrimidinonyl, pyrimidinedionyl, pyridazinonyl, quinazolinone, benzofuranyl, benzodioxazolyl, naphthyridonyl, benzopyranyl, isochromanyl, said Ar optionally substituted with 1-6R j And (4) substitution.
In alternative embodiments, ar is selected from C 6-10 Aryl, 5-to 10-membered heteroaryl, said C 6-10 Aryl or 5-to 10-membered heteroaryl is selected from
In alternative embodiments, ar is selected from C 6-10 Aryl, 5-to 10-membered heteroaryl, said C 6-10 Aryl or 5-to 10-membered heteroaryl is selected from Ar is optionally substituted with 1-6R j And (4) substitution.
In alternative embodiments, R is j Selected from halogen, deuterium, hydroxy, C 2-6 Alkenyl radical, C 2-6 Alkynyl, -B (OH) 2 Difluoromethyl, hydroxymethyl or R j Is composed of
Wherein R is 4 Or R 5 Each independently selected from hydrogen, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 1-6 Alkoxy radical, C 1-4 A hydroxyalkyl group;
X 11 selected from-O-or-NR a1 -;
R 6 Is selected from-OR a1 or-NR a1 R a2 ;
R a1 And R a2 Each independently selected from hydrogen and C 1-4 Alkyl radical, C 1-4 Haloalkyl, hydroxy-C 1-4 Alkyl radical, C 3-8 Cycloalkyl radical, C 1-4 alkoxy-C 1-4 Alkyl, or R a1 And R a2 The nitrogen atom connected with the heterocyclic group forms a 3-8-membered heterocyclic group;
in alternative embodiments R j Selected from deuterium, hydroxy, fluoro, chloro, bromo, C 2-6 Alkenyl radical, C 2-6 Alkynyl, -B (OH) 2 Difluoromethyl, hydroxymethyl or
In alternative embodiments, R is j Selected from halogen, deuterium, hydroxy, C 2-6 Alkenyl radical, C 2-6 Alkynyl, preferably deuterium, hydroxy, fluorine, chlorine, bromine, C 2-6 Alkenyl radical, C 2-6 Alkynyl.
In some embodiments, the compound of formula I, or a pharmaceutically acceptable salt thereof, is IV-1,
each substituent is defined in a compound shown as a formula I or a pharmaceutically acceptable salt thereof, a compound shown as a formula II-1 or II-2 or a pharmaceutically acceptable salt thereof, and compounds shown as formulas III-1 to III-54 or pharmaceutically acceptable salts thereof.
In alternative embodiments, ar is selected from
In alternative embodiments, ar is selected from
In alternative embodiments, ar is selected from
In alternative embodiments, ar is selected from
In alternative embodiments, ar is selected from
In alternative embodiments, ar is selected from
In alternative embodiments, the R is 1 Is composed ofB-1 is optionally selected from 0,1,2,3,4,5 or 6R d Substituted, said X 1 Is selected from-NR c -。
In alternative embodiments, R is 1 Is composed ofB-2 is optionally selected from 0,1,2,3,4,5 or 6R d Substituted, said X 2 Is selected from-NR c -。
In alternative embodiments, R is 1 Is composed ofB-3 is optionally selected from 0,1,2,3,4,5 or 6R d Substituted, said X 2 、X 4 Is selected from-NR c -、-CR a R b- 。
In alternative embodiments, the R is 1 Is selected from
Each of B-4, B-5, B-6 is independently optionally substituted with 0,1,2,3,4,5 or 6R d Substituted, said X 5 、X 6 、X 7 、X 8 、X 9 、X 10 Each independently selected from-NR c -、-CR a R b- 。
In alternative embodiments, the R is d Selected from hydrogen, halogen, deuterium, hydroxy, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 1-6 Alkoxy radical, C 1-4 Hydroxyalkyl radical, C 2-6 Alkenyl radical, C 2-6 Alkynyl, oxo, -SR a1 、-OR a1 、-NR a1 R a2 、-COR a2 、C 3-8 Cycloalkyl, 3-to 8-membered heterocyclic group, C 3-8 Cycloalkyl substituted by-C 1-4 Alkyl, 3-8 membered heterocyclyl substituted-C 1-4 An alkyl group;
said C is 3-8 Cycloalkyl, 3-to 8-membered heterocyclic group, C 3-8 Cycloalkyl substituted by-C 1-4 Alkyl, 3-8 membered heterocyclyl substituted-C 1-4 Alkyl is optionally substituted by 1-3 substituents selected from halogen, cyano, hydroxy, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 1-6 Alkoxy radical, C 1-4 A hydroxyalkyl group;
in alternative embodiments, R is d Selected from hydrogen, halogen, deuterium, C 1-6 Alkyl radical, C 3-8 A cycloalkyl group;
in alternative embodiments, R is d Selected from hydrogen, fluorine, chlorine, bromine, iodine, methyl, ethyl, n-propyl, isopropyl, tert-butyl, cyclopropane, cyclobutane, cyclopentane, cyclohexane.
In alternative embodiments, R is c Selected from hydrogen, deuterium, C 1-6 Alkyl radical, C 3-8 Cycloalkyl, 3-to 8-membered heterocyclyl, optionally hydrogen, deuteromethyl, ethyl, n-propyl, isopropylT-butyl, cyclopropane, cyclobutane, cyclopentane, cyclohexane.
In alternative embodiments, the R is a 、R b Each independently selected from hydrogen, deuterium, hydroxy, amino, halogen, C 1-6 Alkyl radical, C 3-8 Cycloalkyl, 3-8 membered heterocyclyl.
In alternative embodiments, R is a 、R b Each independently selected from hydrogen.
In some embodiments, B-2 is selected from
In some embodiments, B-3 is selected from
In some embodiments, B-4, B-5, B-6 are selected from
In some embodiments, the R is 1 Is selected from
In some embodiments, the R is 3 Selected from hydrogen, deuterium, halogen, cyano, hydroxy, C 1-6 Alkyl, preferably hydrogen, deuterium, fluorine, chlorine, bromine, iodine, methyl, ethyl, n-propyl, isopropyl, tert-butyl.
In alternative embodiments, the L is 2 Selected from the group consisting of bonds.
In alternative embodiments, the L is 2 Is selected from-S-.
In alternative embodiments, the L is 2 Is selected from-C (O) -.
In alternative embodiments, the L is 2 Is selected from-CR f1 R f2 -; the R is f1 、R f2 Each independently selected from hydrogen, deuterium, halogen, C 1-6 Alkyl, or optionally R f1 And R f2 The carbon atom connected with the heterocyclic ring forms 3-6 membered cycloalkyl or 3-6 membered heterocyclic group, the 3-6 membered cycloalkyl or 3-6 membered heterocyclic group is optionally substituted by 1-3 groups selected from deuterium, halogen, hydroxyl, C 1-6 Substituted with alkyl, optionally, said R f1 、R f2 Each independently selected from hydrogen, deuterium, fluorine, chlorine, bromine, iodine, methyl, ethyl, n-propyl, isopropyl, tert-butyl; or said R is f1 、R f2 The carbon atom connected with the cycloalkyl is 3-6 membered cycloalkyl, and the 3-6 membered cycloalkyl is optionally substituted by 1-3 substituents selected from deuterium, halogen, hydroxyl and C 1-6 Substituted with alkyl, optionally said R f1 、R f2 The carbon atoms connected with the compound form 3-membered, 4-membered and five-membered naphthenic base.
In some embodiments, the compound, or pharmaceutically acceptable salt thereof, is
Each substituent group is defined in a compound shown as a formula I or a pharmaceutically acceptable salt thereof, a compound shown as a formula II-1 or II-2 or a pharmaceutically acceptable salt thereof, compounds shown as formulas III-1 to III-54 or a pharmaceutically acceptable salt thereof, and a compound shown as an IV-1 or a pharmaceutically acceptable salt thereof.
In some embodiments, the compound or pharmaceutically acceptable salt thereof is
Each substituent group is defined in a compound shown as a formula I or a pharmaceutically acceptable salt thereof, a compound shown as a formula II-1 or II-2 or a pharmaceutically acceptable salt thereof, compounds shown as formulas III-1 to III-54 or a pharmaceutically acceptable salt thereof, and a compound shown as an IV-1 or a pharmaceutically acceptable salt thereof.
In an alternative embodiment, the compound of formula I or a pharmaceutically acceptable salt thereof is
In alternative embodiments, the compound of formula I, or a pharmaceutically acceptable salt thereof, is
Or a pharmaceutically acceptable salt thereof.
In another aspect, the disclosure provides an isotopic substitution of the compound, or a pharmaceutically acceptable salt thereof, with the isotopic atom being deuterium.
In alternative embodiments, the abundance of deuterium atoms is greater than 20%, optionally the abundance of deuterium atoms is greater than 50%, optionally the abundance of deuterium atoms is greater than 90%, optionally the abundance of deuterium atoms is greater than 95%.
In another aspect, the present disclosure provides a pharmaceutical composition comprising at least one therapeutically effective amount of the above compound or a pharmaceutically acceptable salt thereof and a pharmaceutically acceptable excipient.
In some embodiments, the unit dose of the pharmaceutical composition is from 0.001mg to 1000mg.
In certain embodiments, the pharmaceutical composition comprises from 0.01 to 99.99% of the compound of formula I, or a pharmaceutically acceptable salt thereof, based on the total weight of the composition.
In certain embodiments, the pharmaceutical composition comprises 0.1-99.9% of the aforementioned compound or a pharmaceutically acceptable salt thereof.
In certain embodiments, the pharmaceutical composition comprises 0.5% to 99.5% of the aforementioned compound or a pharmaceutically acceptable salt thereof.
In certain embodiments, the pharmaceutical composition comprises 1% to 99% of the aforementioned compound or a pharmaceutically acceptable salt thereof.
In certain embodiments, the pharmaceutical composition comprises from 2% to 98% of a compound of the foregoing formula or a pharmaceutically acceptable salt thereof.
In certain embodiments, the pharmaceutical composition comprises from 0.01% to 99.99% of a pharmaceutically acceptable excipient, based on the total weight of the composition.
In certain embodiments, the pharmaceutical composition contains 0.1% to 99.9% of a pharmaceutically acceptable excipient.
In certain embodiments, the pharmaceutical composition contains 0.5% to 99.5% of a pharmaceutically acceptable excipient.
In certain embodiments, the pharmaceutical composition contains 1% to 99% of a pharmaceutically acceptable excipient.
In certain embodiments, the pharmaceutical composition contains 2% to 98% of a pharmaceutically acceptable excipient.
In another aspect, the present disclosure provides a method of preventing and/or treating a patient having a disease or disorder associated with KRAS G12D comprising administering to the patient a therapeutically effective amount of a compound described above or a pharmaceutically acceptable salt thereof or a pharmaceutical composition described above.
In another aspect, the present disclosure provides a method for preventing and/or treating a patient having cancer, comprising administering to the patient a therapeutically effective amount of the above-described compound or a pharmaceutically acceptable salt thereof or the above-described pharmaceutical composition.
In another aspect, the present disclosure provides a use of the above compound or a pharmaceutically acceptable salt thereof or the above pharmaceutical composition for preparing a medicament for preventing and/or treating a disease or disorder associated with KRAS G12D.
In another aspect, the present disclosure provides a use of the above compound or a pharmaceutically acceptable salt thereof or the above pharmaceutical composition for the preparation of a medicament for preventing and/or treating cancer.
In another aspect, the present disclosure provides a method of inhibiting KRA G12D activity in a cell using a compound described above or a pharmaceutically acceptable salt thereof or a pharmaceutical composition described above.
In another aspect, the present disclosure provides a use of the above compound or a pharmaceutically acceptable salt thereof or the above pharmaceutical composition for medical treatment.
In another aspect, the present disclosure provides a use of the above compound or a pharmaceutically acceptable salt thereof, or the above pharmaceutical composition as a KRAS G12D inhibitor.
The cancer described in the present disclosure is selected from non-small cell lung cancer, colorectal cancer, rectal cancer or pancreatic cancer.
In another aspect, the pharmaceutically acceptable salts of the compounds described in this disclosure are selected from inorganic or organic salts.
"KRAS G12D" refers to a mutant form of mammalian KRA protein containing an amino acid substitution of glycine at amino acid position 12. The assignment of amino acid codons and the residual positions of human KRA is based on the amino acid sequence identified by Uniprotkb/Swiss-prot P01116: variantp. Gly12ax.
"KRAS G12D inhibitor" refers to a compound of the invention represented by formula (I) as described herein. These compounds are capable of negatively modulating or inhibiting all or a portion of the enzymatic activity of KRAS G12D.
"KRAS G12D-associated disease or disorder" relates to a disease or disorder that has or is associated with or mediated by a KRAS G12D mutation. A non-limiting example of a KRAS G12D-associated disease or disorder is KRAS G12D-associated cancer.
The disclosed compounds may exist in specific geometric or stereoisomeric forms. The present disclosure contemplates all such compounds, including cis and trans isomers, (-) -and (+) -enantiomers, (R) -and (S) -enantiomers, diastereomers, (D) -isomers, (L) -isomers, as well as racemic and other mixtures thereof, such as enantiomerically or diastereomerically enriched mixtures, all of which fall within the scope of the present disclosure. Additional asymmetric carbon atoms may be present in substituents such as alkyl groups. All such isomers, as well as mixtures thereof, are included within the scope of the present disclosure. The compounds of the present disclosure containing asymmetric carbon atoms can be isolated in optically active pure form or in racemic form. The optically active pure form can be resolved from a racemic mixture or synthesized by using chiral starting materials or chiral reagents.
Optically active (R) -and (S) -isomers as well as D and L isomers can be prepared by chiral synthesis or chiral reagents or other conventional techniques. If one of the enantiomers of a compound of the present disclosure is desired, it can be prepared by asymmetric synthesis or derivatization with a chiral auxiliary, wherein the resulting diastereomeric mixture is separated and the auxiliary group is cleaved to provide the pure desired enantiomer. Alternatively, where the molecule contains a basic functional group (e.g., amino) or an acidic functional group (e.g., carboxyl), diastereomeric salts are formed with an appropriate optically active acid or base, followed by diastereomeric resolution by conventional methods known in the art, and the pure enantiomers are recovered. Furthermore, separation of enantiomers and diastereomers is typically accomplished by using chromatography employing a chiral stationary phase, optionally in combination with chemical derivatization (e.g., carbamate formation from amines).
In the chemical structure of the compounds described in the present disclosure, a bondDenotes an unspecified configuration, i.e. a bond if a chiral isomer is present in the chemical structureCan be thatOrOr at the same time containAndtwo configurations. Key with a key bodyIndicates unspecified configurations, including cis (E) or trans (Z) configurations.
The compounds and intermediates of the present disclosure may also exist in different tautomeric forms, and all such forms are included within the scope of the present disclosure. The term "tautomer" or "tautomeric form" refers to structural isomers of different energies that can interconvert via a low energy barrier. For example, proton tautomers (also referred to as proton transfer tautomers) include interconversion via proton migration, such as keto-enol and imine-enamine, lactam-lactam isomerizations. An example of a lactam-lactam equilibrium is between A and B as shown below.
All compounds in this disclosure can be drawn as form a or form B. All tautomeric forms are within the scope of the disclosure. The nomenclature of the compounds does not exclude any tautomers.
The present disclosure also includes certain isotopically-labeled compounds of the present disclosure which are identical to those recited herein, but for the fact that one or more atoms are replaced by an atom having an atomic mass or mass number different from the atomic mass or mass number usually found in nature. Examples of isotopes that can be incorporated into compounds of the disclosure include isotopes of hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur, fluorine, iodine, and chlorine, such as respectively 2 H、 3 H、 11 C、 13 C、 14 C、 13 N、 15 N、 15 O、 17 O、 18 O、 31 P、 32 P、 35 S、 18 F、 123 I、 125 I and 36 cl, and the like.
Unless otherwise indicated, when a position is specifically designated as deuterium (D), that position is understood to be deuterium having an abundance that is at least 1000 times greater than the natural abundance of deuterium (which is 0.015%) (i.e., at least 10% deuterium incorporation). The compound of examples can have a natural abundance of deuterium greater than that of deuterium of at least 1000 times the abundance of deuterium, deuterium of at least 2000 times the abundance of deuterium, deuterium of at least 3000 times the abundance of deuterium, deuterium of at least 4000 times the abundance of deuterium, deuterium of at least 5000 times the abundance of deuterium, deuterium of at least 6000 times the abundance of deuterium, or deuterium of greater abundance. The disclosure also includes various deuterated forms of the compounds of formula (I). Each available hydrogen atom attached to a carbon atom may be independently replaced by a deuterium atom. The person skilled in the art is able to synthesize the compounds of formula (I) in deuterated form with reference to the relevant literature. Commercially available deuterated starting materials can be used in preparing the deuterated forms of the compounds of formula (I), or they can be synthesized using conventional techniques using deuterated reagents including, but not limited to, deuterated boranes, trideuteroborane tetrahydrofuran solutions, deuterated lithium aluminum hydrides, deuterated iodoethanes, deuterated iodomethanes, and the like.
Interpretation of terms:
"pharmaceutically acceptable excipient" includes, but is not limited to, any adjuvant, carrier, excipient, glidant, sweetener, diluent, preservative, dye/colorant, flavoring agent, surfactant, wetting agent, dispersing agent, suspending agent, stabilizing agent, isotonic agent, solvent, or emulsifying agent that has been approved by the U.S. food and drug administration for use in humans or livestock animals.
"alkyl" refers to a saturated aliphatic hydrocarbon group, including straight and branched chain groups of 1 to 20 carbon atoms. Alkyl groups having 1 to 12 carbon atoms are preferred, and alkyl groups having 1 to 6 carbon atoms are more preferred. Non-limiting examples include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, t-butylA group, sec-butyl, n-pentyl, 1-dimethylpropyl, 1, 2-dimethylpropyl, 2-dimethylpropyl, 1-ethylpropyl, 2-methylbutyl, 3-methylbutyl, and various branched isomers thereof, and the like. Alkyl groups may be substituted or unsubstituted, and when substituted, the substituents may be substituted at any available point of attachment, preferably one or more groups independently selected from halogen, hydroxy, oxo, nitro, cyano, C 1-6 Alkyl radical, C 1-6 Alkoxy radical, C 3-7 Cycloalkyl, 3-12 membered heterocyclyl, and the like.
"Heteroalkyl" refers to one or more-CH groups in an alkyl group 2 -substituted by a heteroatom selected from N, O and S; wherein said alkyl is as defined above; heteroalkyl groups may be substituted or unsubstituted, and when substituted, substituents may be substituted at any available point of attachment, preferably independently optionally one or more substituents selected from H atoms, D atoms, halogens, alkyl groups, alkoxy groups, haloalkyl groups, haloalkoxy groups, cycloalkyloxy groups, heterocyclyloxy groups, hydroxy groups, hydroxyalkyl groups, cyano groups, amino groups, nitro groups, cycloalkyl groups, heterocyclyl groups, aryl groups, heteroaryl groups.
The term "alkylene" refers to a saturated straight or branched chain aliphatic hydrocarbon group having 2 residues derived from the parent alkane by removal of two hydrogen atoms from the same carbon atom or two different carbon atoms, and is a straight or branched chain group containing 1 to 20 carbon atoms, preferably 1 to 12 (e.g., 1,2,3,4,5, 6,7,8, 9, 10, 11, and 12) carbon atoms, more preferably an alkylene group containing 1 to 6 carbon atoms. Non-limiting examples of alkylene groups include, but are not limited to, methylene (-CH) 2 -), 1-ethylene (-CH (CH) 3 ) -), 1, 2-ethylene (-CH) 2 CH 2 ) -, 1-propylene (-CH (CH) 2 CH 3 ) -), 1, 2-propylene (-CH) 2 CH(CH 3 ) -), 1, 3-propylene (-CH) 2 CH 2 CH 2 -) 1, 4-butylene (-CH 2 CH 2 CH 2 CH 2 -) and the like. The alkylene group may be substituted or unsubstituted, and when substituted, the substituent may be substituted at any available point of attachment, preferably independentlyAnd (b) one or more substituents selected from the group consisting of alkyl, alkenyl, alkynyl, alkoxy, haloalkoxy, cycloalkyloxy, heterocyclyloxy, alkylthio, alkylamino, halogen, mercapto, hydroxy, nitro, cyano, cycloalkyl, heterocyclyl, aryl, heteroaryl, cycloalkoxy, heterocyclyloxy, cycloalkylthio, heterocyclylthio, and oxo are optionally substituted.
"Heteroalkylene" refers to one or more-CH groups in an alkylene group 2 -is replaced by a heteroatom selected from N, O and S; wherein said alkylene is as defined above; heteroalkylene groups may be substituted or unsubstituted and, when substituted, substituents may be substituted at any available point of attachment, preferably the substituents are independently optionally one or more substituents selected from H atoms, D atoms, halogen, alkyl, alkoxy, haloalkyl, haloalkoxy, cycloalkyloxy, heterocyclyloxy, hydroxy, hydroxyalkyl, cyano, amino, nitro, cycloalkyl, heterocyclyl, aryl, heteroaryl.
"alkenyl" includes branched and straight chain olefins having 2 to 12 carbon atoms or olefins containing aliphatic hydrocarbon groups. E.g. "C 2-6 Alkenyl "means alkenyl having 2,3,4,5 or 6 carbon atoms. Examples of alkenyl groups include, but are not limited to, vinyl, allyl, 1-propenyl, 1-butenyl, 2-butenyl, 3-butenyl, 2-methylbut-2-enyl, 3-methylbut-1-enyl, 1-pentenyl, 3-pentenyl, and 4-hexenyl. Alkenyl groups may be substituted or unsubstituted, and when substituted, substituents may be substituted at any available point of attachment, preferably one or more groups independently selected from halogen, hydroxy, oxo, nitro, cyano, C 1-6 Alkyl radical, C 1-6 Alkoxy radical, C 3-7 Cycloalkyl, 3-12 membered heterocyclyl, and the like.
"alkynyl" includes branched and straight chain alkynyl groups having 2 to 12 carbon atoms or olefins containing aliphatic hydrocarbon groups, or if the specified number of carbon atoms is specified, that particular number is intended. For example, ethynyl, propynyl (e.g., 1-propynyl, 2-propynyl), 3-butynyl, pentynyl, hexynyl and 1-methylpent-2-ynyl groups. Alkynyl groups may be substituted or unsubstituted, when substitutedWhere substituents may be substituted at any available point of attachment, preferably one or more groups independently selected from halogen, hydroxy, oxo, nitro, cyano, C 1-6 Alkyl radical, C 1-6 Alkoxy radical, C 3-7 Cycloalkyl, 3-12 membered heterocyclyl, and the like.
The term "cycloalkyl" refers to a saturated or partially unsaturated monocyclic or polycyclic cyclic hydrocarbon substituent, the cycloalkyl ring containing from 3 to 20 carbon atoms, preferably from 3 to 12 carbon atoms, more preferably from 3 to 6 carbon atoms. Non-limiting examples of monocyclic cycloalkyl groups include cyclopropyl, cyclobutyl, cyclopentyl, cyclopentenyl, cyclohexyl, cyclohexenyl, cyclohexadienyl, cycloheptyl, cycloheptatrienyl, cyclooctyl, and the like; polycyclic cycloalkyl groups include spiro, fused and bridged cycloalkyl groups.
The cycloalkyl ring may be fused to an aryl, heteroaryl or heterocycloalkyl ring, where the ring to which the parent structure is attached is a cycloalkyl, non-limiting examples of which include indanyl, tetrahydronaphthyl, benzocycloheptanyl, and the like. Cycloalkyl groups may be optionally substituted or unsubstituted, and when substituted, the substituents are preferably one or more groups independently selected from halogen, hydroxy, oxo, nitro, cyano, C 1-6 Alkyl radical, C 1-6 Alkoxy radical, C 3-7 Cycloalkyl, 3-12 membered heterocyclyl, and the like.
The term "heterocyclyl" refers to a saturated or partially unsaturated monocyclic or polycyclic cyclic hydrocarbon substituent comprising 3 to 20 ring atoms wherein one or more of the ring atoms is selected from nitrogen, oxygen or S (O) m (wherein m is an integer of 0 to 2) but does not include a cyclic moiety of-O-O-, -O-S-, or-S-S-, the remaining ring atoms being carbon. Preferably 3 to 12 ring atoms, of which 1 to 4 are heteroatoms; more preferably from 3 to 8 ring atoms. Non-limiting examples of monocyclic heterocyclyl groups include pyrrolidinyl, imidazolidinyl, tetrahydrofuranyl, tetrahydrothienyl, dihydroimidazolyl, dihydrofuranyl, dihydropyrazolyl, dihydropyrrolyl, piperidinyl, piperazinyl, morpholinyl, thiomorpholinyl, homopiperazinyl, and the like. Polycyclic heterocyclic groups include spiro, fused and bridged heterocyclic groups. Non-limiting examples of "heterocyclyl" include:
The heterocyclyl ring may be fused to an aryl, heteroaryl or cycloalkyl ring, wherein the ring to which the parent structure is attached is heterocyclyl, non-limiting examples of which include:
The heterocyclyl group may be optionally substituted or unsubstituted and when substituted the substituents are preferably one or more groups independently selected from halogen, hydroxy, oxo, nitro, cyano, C 1-6 Alkyl radical, C 1-6 Alkoxy radical, C 3-7 Cycloalkyl, 3-12 membered heterocyclyl, and the like.
The term "aryl" refers to a 6 to 14 membered, all carbon monocyclic or fused polycyclic (i.e., rings which share adjacent pairs of carbon atoms) group having a conjugated pi-electron system, preferably 6 to 12 membered, such as phenyl and naphthyl. The aryl ring may be fused to a heteroaryl, heterocyclyl or cycloalkyl ring, wherein the ring attached to the parent structure is an aryl ring, non-limiting examples of which include:
aryl groups may be substituted or unsubstituted, and when substituted, the substituents are preferably one or more groups independently selected from halogen, hydroxy, oxo, nitro, cyano, C 1-6 Alkyl radical, C 1-6 Alkoxy radical, C 3-7 Cycloalkyl, 3-12 membered heterocyclic group, etc., preferably phenyl.
The term "heteroaryl" refers to 5 to 14 rings containing 1 to 4 heteroatomsA heteroaromatic system of atoms, wherein the heteroatoms are selected from oxygen, sulfur and nitrogen. The heteroaryl group is preferably 6 to 12-membered, more preferably 5-or 6-membered. For example. Non-limiting examples thereof include: imidazolyl, furyl, thienyl, thiazolyl, pyrazolyl, oxazolyl, pyrrolyl, tetrazolyl, pyridyl, pyrimidinyl, thiadiazole, pyrazine,and so on.
The heteroaryl ring may be fused to an aryl, heterocyclyl or cycloalkyl ring, wherein the ring joined together with the parent structure is a heteroaryl ring, non-limiting examples of which include:
heteroaryl groups may be optionally substituted or unsubstituted, and when substituted, the substituents are preferably one or more groups independently selected from halogen, hydroxy, oxo, nitro, cyano, C 1-6 Alkyl radical, C 1-6 Alkoxy radical, C 3-7 Cycloalkyl, 3-12 membered heterocyclyl, and the like.
The term "alkoxy" refers to-O- (alkyl) and-O- (unsubstituted cycloalkyl), wherein alkyl is as defined above. Non-limiting examples of alkoxy groups include: methoxy, ethoxy, propoxy, butoxy, cyclopropoxy, cyclobutoxy, cyclopentyloxy, cyclohexyloxy. Alkoxy groups may be optionally substituted or unsubstituted, and when substituted, the substituents are preferably one or more groups independently selected from halogen, hydroxy, oxo, nitro, cyano, C 1-6 Alkyl radical, C 1-6 Alkoxy radical, C 3-7 Cycloalkyl, 3-12 membered heterocyclyl, and the like.
The term "hydroxy" refers to an-OH group.
The term "halogen" refers to fluorine, chlorine, bromine or iodine.
The term "amino" refers to the group-NH 2 。
The term "cyano" refers to — CN.
Term "Nitro "means-NO 2 。
The term "oxo" refers to the = O substituent.
"optional" or "optionally" means that the subsequently described event or circumstance may, but need not, occur, and that the description includes instances where the event or circumstance occurs or does not. For example, "a heterocyclic group optionally substituted with an alkyl" means that an alkyl may, but need not, be present, and the description includes the case where the heterocyclic group is substituted with an alkyl and the heterocyclic group is not substituted with an alkyl.
"substituted" means that one or more, preferably up to 5, more preferably 1 to 3, hydrogen atoms in a group are independently substituted with a corresponding number of substituents. It goes without saying that the substituents are only in their possible chemical positions, and that the person skilled in the art is able to determine (experimentally or theoretically) possible or impossible substitutions without undue effort. For example, an amino or hydroxyl group having a free hydrogen may be unstable in combination with a carbon atom having an unsaturated (e.g., olefinic) bond.
"pharmaceutical composition" means a mixture containing one or more compounds described herein, or a physiologically acceptable salt or prodrug thereof, in admixture with other chemical components, as well as other components such as physiologically acceptable carriers and excipients. The purpose of the pharmaceutical composition is to facilitate administration to an organism, facilitate absorption of the active ingredient and exert biological activity.
Detailed Description
The present disclosure is further described below with reference to examples, but these examples do not limit the scope of the present disclosure.
Experimental procedures, in which specific conditions are not noted in the examples of the present disclosure, are generally performed under conventional conditions, or under conditions recommended by manufacturers of raw materials or commercial products. Reagents of specific sources are not indicated, and are conventional reagents purchased in the market.
NMR shift (. Delta.) of 10 -6 The units in (ppm) are given. NMR was measured by Bruker AVANCE-400 NMR spectrometer using deuterated dimethyl sulfoxide (DMSO-d) 6 ) Deuterated chloroform (CDCl) 3 ) Deuterated methanol (CD) 3 OD), internal standard Tetramethylsilane (TMS).
MS was measured using a Shimadzu 2010Mass Spectrometer or Agilent 6110A MSD Mass Spectrometer.
HPLC measurements were performed using Shimadzu LC-20A systems, shimadzu LC-2010HT series, or Agilent 1200LC HPLC (Ultimate XB-C18.3.0 × 150mm column or xtate C18.1 × 30mm column).
Chiral HPLC assay using Chiralpak IC-3 x 4.6mm i.d.,3um, chiralpak AD-3 x 4.6mm i.d.,3um, chiralpak AS-3 x 4.6mm i.d.,3 μm, chiralCel OD-3 x 4.6mm i.d.,3um, chiralCel OD-100 x 4.6mm i.d.,3 μm, chiralCel OJ-H150 x 4.6mm i.d, 5um, chiralCel OJ-3. X4.6 mm i.d, 3um chromatography column; the thin-layer chromatography silica gel plate adopts a cigarette platform yellow sea HSGF254 or Qingdao GF254 silica gel plate, the specification of the silica gel plate used by the thin-layer chromatography (TLC) is 0.15 mm-0.2 mm, and the specification of the thin-layer chromatography separation and purification product is 0.4 mm-0.5 mm.
The column chromatography generally uses 100-200 mesh, 200-300 mesh or 300-400 mesh silica gel of Futai Huanghai silica gel as a carrier.
The chiral preparative column used DAICEL CHIRALPAK IC (250mm. About.30mm, 10 um) or Phenomenex-Amylose-1 (250mm. About.30mm, 5 um).
The CombiFlash rapid preparation instrument uses CombiFlash Rf150 (TELEDYNE ISCO).
Average inhibition rate of kinase and IC 50 The values were determined with a NovoStar microplate reader (BMG, germany).
Known starting materials of the present disclosure may be synthesized using or according to methods known in the art, or may be purchased from companies such as ABCR GmbH & co.kg, acros Organics, aldrich Chemical Company, nephelo Chemical science and technology (Accela ChemBio Inc), dare chemicals, and the like.
In the examples, the reaction can be carried out in an argon atmosphere or a nitrogen atmosphere, unless otherwise specified.
An argon atmosphere or nitrogen atmosphere means that the reaction flask is connected to a balloon of argon or nitrogen with a volume of about 1L.
The hydrogen atmosphere refers to a reaction flask connected with a hydrogen balloon with a volume of about 1L.
The pressure hydrogenation reaction used a Parr 3916EKX type hydrogenator and a Qinglan QL-500 type hydrogen generator or HC2-SS type hydrogenator.
The hydrogenation reaction was usually evacuated and charged with hydrogen and repeated 3 times.
A CEM Discover-S908860 type microwave reactor was used for the microwave reaction.
In the examples, the solution means an aqueous solution unless otherwise specified.
In the examples, the reaction temperature is, unless otherwise specified, from 20 ℃ to 30 ℃ at room temperature.
The monitoring of the progress of the reaction in the examples employed Thin Layer Chromatography (TLC), a developing solvent used for the reaction, a system of eluents for column chromatography used for purifying compounds and a developing solvent system for thin layer chromatography including: a: dichloromethane/methanol system, B: n-hexane/ethyl acetate system, C: petroleum ether/ethyl acetate system, D: the volume ratio of petroleum ether/ethyl acetate/methanol, E: tetrahydrofuran/petroleum ether, solvent is adjusted according to the polarity of the compound, and small amount of basic or acidic reagent such as triethylamine and acetic acid can be added for adjustment.
The abbreviations used in the following experiments have the following meanings:
DCM is dichloromethane; DIEA N, N-diisopropylethylamine Boc: a tert-butoxycarbonyl group; pd (dppf) Cl 2 : [1,1' -bis (diphenylphosphino) ferrocene]Palladium dichloride; 1,4-dioxane:1, 4-dioxane; cs 2 CO 3 : cesium carbonate; NIS is iodosuccinimide; DAST is diethylaminosulfur trifluoride; tf (f) 2 O: trifluoromethanesulfonic anhydride
Example 1
4- (4- (2, 5-diazabicyclo [2.2.2] oct-2-yl) -8-fluoro-2- (((2R, 7aS) -2-fluorotetrahydro-1H-bispyridopyridin-7 a (5H) -yl) methoxy) pyrido [4,3-d ] pyrimidin-7-yl) naphthalen-2-ol (Compound 1)
The first step is as follows: preparation of Compound 1c
Reference is made to the preparation of Compound 1a (WO 2021/041671 A1). To a solution of compound 1a (300.0 mg, 1.19mmol) and DIEA (921.5mg, 7.13mmol) in DCM (5 mL) at-40 ℃ was added compound 1b (252.3mg, 1.19mmol), and the mixture was stirred at that temperature for 0.5 hour. Water (20 mL) was added to the reaction mixture, extracted with DCM (15 mL. Times.3), and the combined organic phases were extracted with anhydrous Na 2 SO 4 Drying, filtration and concentration under reduced pressure gave crude compound 1c (450.0 mg, crude). The crude product was used directly in the next reaction.
MS(ESI):m/z=428.0[M+H] +
The second step: preparation of Compound 1e
To a solution of compound 1c (450.0 mg, crude) in 1,4-dioxane (10 mL) was added compound 1d (334.5mg, 2.10mmol) and DIEA (921.5mg, 7.13mmol). The reaction solution is heated to 80 ℃ and stirred for 16 hours, and the reaction solution is concentrated under vacuum and reduced pressure to obtain a crude product. The crude product is purified by silica gel chromatography flash column chromatography, and the eluent is: 10-100% Petroleum ether of ethyl acetate to give Compound 1e (400.0 mg).
MS(ESI):m/z=551.1[M+H] +
The third step: preparation of Compound 1g
To a solution of compound 1e (150.0 mg, 0.27mmol) in 1,4-dioxane (8 mL) was added compound 1f (95.6 mg, 0.35mmol), pd (dppf) Cl under Ar gas 2 (20.2mg,0.03mmol),Cs 2 CO 3 (266.1, 0.82mmol) and H 2 O (2 mL). Heat to 100 ℃ and stir for 2 hours. Vacuum concentrating the reaction solution under reduced pressure to obtain a crude product, purifying the crude product by silica gel chromatography flash column chromatography, wherein the eluent is as follows: 5% methanol in dichloromethane to give 1g of the compound (80.0 mg, yield 44.6%).
MS(ESI):m/z=659.3[M+H] +
The fourth step: preparation of Compound 1
To CH containing 1g (80.0 mg, 0.12mmol) of the compound dissolved therein was added 3 CN (1.0 mL) was added to HCl/1,4-dioxane (4M, 8mL). Stirred at room temperature for 0.5 h. Vacuum decompression concentration of the reaction liquid to obtain coarse productPreparative HPLC (basic mobile phase) gave compound 1 (17.10 mg, 25.2% yield).
MS(ESI):m/z=559.2[M+H] +
1 H NMR:(400MHz,DMSO)δ10.02(s,1H),9.27(s,1H),7.81(d,J=8.2Hz,1H),7.51(d,J=8.5Hz,1H),7.48-7.42(m,1H),7.30(d,J=2.5Hz,1H),7.28-7.21(m,2H),5.40-5.17(m,1H),4.98(s,1H),4.34-4.22(m,2H),4.21-4.14(m,1H),4.10-4.03(m,1H),3.27-3.25(m,1H),3.26-3.25(m,2H),3.11-3.09(m,2H),3.02(s,1H),2.88-2.77(m,1H),2.21-2.10(m,2H),2.09-1.95(m,4H),1.89-1.74(m,4H)
Example 2
4- (8-fluoro-2- (((2R, 7aS) -2-fluorotetrahydro-1H-bispyrrolidin-7 a (5H) -yl) methoxy) -4- ((1R, 5S) -8-methyl-3, 8-diazabicyclo [3.2.1] octan-3-yl) pyrido [4,3-d ] pyrimidin-7-yl) naphthalen-2-ol formate (Compound 2)
The first step is as follows: preparation of Compound 2b
To a solution of compound 1a (200.0 mg, 0.79mmol) and DIEA (614.2mg, 4.75mmol) in DCM (5 mL) at-40 deg.C was added compound 2a (100.0 mg, 0.79mmol), and the mixture was stirred at this temperature for 0.5 h. Water (10 mL) was added to the reaction, extracted with DCM (10 mL. Times.3), and the combined organic phases were extracted with anhydrous Na 2 SO 4 Drying, filtration and concentration under reduced pressure gave crude compound 2b (278.0 mg, crude). The crude product was used directly in the next reaction.
MS(ESI):m/z=341.9[M+H] +
The second step is that: preparation of Compound 2c
To a solution of compound 2b (278.0 mg, crude) in 1,4-dioxane (5 mL) was added compound 1d (258.7 mg, 1.63mmol) and DIEA (226.6 mg, 1.75mmol). The reaction solution is heated to 95 ℃ and stirred for 12 hours, and the reaction solution is decompressed and concentrated in vacuum to obtain a crude product. The crude product is purified by silica gel chromatography flash column chromatography, and the eluent is: 1-30% Petroleum ether of tetrahydrofuran to give Compound 2c (280.0 mg).
MS(ESI):m/z=465.1[M+H] +
The third step: preparation of Compound 2
To a solution of compound 2c (280.0 mg, 0.60mmol) in 1,4-dioxane (7.5 mL) was added compound 1f (211.5 mg, 0.78mmol), pd (dppf) Cl under protection of Ar gas 2 (44.7mg,0.06mmol),Cs 2 CO 3 (588.6mg, 1.81mmol) and H 2 O (2 mL). Heated to 110 ℃ and stirred for 1.5 hours. The reaction was concentrated under reduced pressure in vacuo to give the crude product, which was subjected to preparative HPLC (acidic mobile phase) to give compound 2 (28.2 mg, yield 8.18%).
MS(ESI):m/z=573.1[M+H] +
1 H NMR:(400MHz,CD 3 OD)δ9.19(s,1H),8.43(s,1H),7.79(d,J=8.2Hz,1H),7.55(d,J=8.2Hz,1H),7.48-7.42(m,1H),7.32(d,J=2.3Hz,1H),7.29-7.23(m,2H),5.62-5.42(m,1H),4.74(dd,J=5.9,11.6Hz,2H),4.60(q,J=11.8Hz,2H),3.93-3.85(m,2H),3.83-3.66(m,3H),3.62(s,2H),3.41-3.34(m,1H),2.69-2.46(m,5H),2.42-2.34(m,1H),2.31-2.23(m,2H),2.19-2.12(m,3H),1.86-1.81(m,2H)
Example 3
4- (4- (3, 6-diazabicyclo [3.1.1] heptan-3-yl) -8-fluoro-2- (((2R, 7aS) -2-fluorotetrahydro-1H-bispyridyl-7 a (5H) -yl) methoxy) pyrido [4,3-d ] pyrimidin-7-yl) naphthalen-2-olate (compound 3)
Preparation of compound 3 reference is made to the synthesis of compound 1. Starting material compound 1a (200.0 mg, 0.79mmol) and 3a (157.1mg, 0.79mmol) were reacted in four steps to give compound 3 (11.3 mg).
m/z=545.1[M+H] +
1 H NMR:(400MHz,CD 3 OD)δ9.43(s,1H),8.34(s,1H),7.67(d,J=8.3Hz,1H),7.42(d,J=8.3Hz,1H),7.34(t,J=7.4Hz,1H),7.21(d,J=2.1Hz,1H),7.17-7.10(m,2H),5.52-5.17(m,1H),4.60-4.37(m,5H),4.24(d,J=5.4Hz,2H),3.64-3.43(m,3H),2.97-2.80(m,1H),2.54-2.33(m,2H),2.28-2.07(m,3H),2.0-1.92(m,1H),1.88-1.77(m,1H),1.31–1.19(m,2H)
Example 4
4- (8-fluoro-2- (((2R, 7aS) -2-fluorotetrahydro-1H-bispolypylidin-7 a (5H) -yl) methoxy) -4- (2, 7-azospiro [3.5] nonan-7-yl) pyrido [4,3-d ] pyrimidin-7-yl) naphthalen-2-ol (Compound 4)
Preparation of compound 4 reference is made to the synthesis of compound 1. Starting material compound 1a (200.0 mg, 0.79mmol) and 4a (179.3 mg, 0.792mmol) were reacted in four steps to give compound 4 (29.34 mg).
m/z=573.3[M+H] +
1 H NMR:(400MHz,DMSO-d 6 )δ9.13(s,1H),8.36(br,1H),7.80(d,J=8.3Hz,1H),7.53(d,J=8.5Hz,1H),7.43(t,J=7.5Hz,1H),7.29(d,J=2.3Hz,1H),7.26-7.21(m,2H),5.38-5.18(m,1H),4.15(d,J=10.4Hz,1H),4.05(d,J=10.4Hz,1H),3.89(s,4H),3.76(s,4H),3.15-2.99(m,4H),2.87-2.79(m,1H),2.15-1.92(m,7H),1.90-1.72(m,3H)
Example 5
4- (4- (3, 5-dimethylpiperazin-1-yl) -8-fluoro-2- (((2R, 7aS) -2-fluorotetrahydro-1H-bispyridyl-7 a (5H) -yl) methoxy) pyrido [4,3-d ] pyrimidin-7-yl) naphthalen-2-ol (compound 5)
Preparation of compound 5 reference is made to the synthesis of compound 1. Starting material compound 1a (200.0mg, 0.79mmol) and 5a (169.8mg, 0.79mmol) were reacted in four steps to give compound 5 (13.0 mg).
m/z=561.3[M+H] +
1H NMR(400MHz,DMSO-d 6 )δ9.17(s,1H),8.21(s,1H),7.81(d,J=8.3Hz,1H),7.56(d,J=8.5Hz,1H),7.45(t,J=7.4Hz,1H),7.32-7.20(m,3H),5.42-5.16(m,1H),4.47(d,J=11.6Hz,2H),4.47(d,J=12Hz,1H),4.16(d,J=12Hz,1H),3.15-2.77(m,9H),2.19-1.99(m,3H),1.92-1.73(m,3H),1.11-1.09(m,6H)
Example 6
4- (4- ((1R, 5S) -3, 9-azobis [3.3.1] nonan-3-yl) -8-fluoro-2- (((2R, 7aS) -2-fluorotetrahydro-1H-bispyridyl-7 a (5H) -yl) methoxy) pyrido [4,3-d ] pyrimidin-7-yl) naphthalen-2-ol (Compound 6)
Preparation of compound 6 reference is made to the synthesis of compound 1. Starting material compound 1a (200.0mg, 0.79mmol) and 6a (169.8mg, 0.79mmol) were reacted in four steps to give compound 6 (13.0 mg).
MS(ESI):m/z=573.2[M+H] +
1 H NMR:(400MHz,DMSO-d 6 )δ9.23(s,1H),8.23(s,1H),7.81(d,J=8.3Hz,1H),7.56(d,J=8.3Hz,1H),7.45(t,J=7.4Hz,1H),7.32-7.21(m,3H),5.38-5.20(m,1H),4.70–4.67(m,2H),4.18(d,J=10.4Hz,1H),4.10(d,J=10.4Hz,1H),3.81(d,J=12.6Hz,2H),3.34(s,2H),3.16-3.00(m,3H),2.87-2.79(m,1H),2.20-2.11(m,1H),2.11-1.91(m,3H),1.89-1.71(m,8H),1.52(s,1H)
Example 7
4- (4- ((1R, 5S) -3, 8-azobis [3.2.1] octan-3-yl) -8-fluoro-2- (1- ((S) -1-methylpyrrol-2-yl) cyclopropyloxypyrido [4,3-d ] pyrimidin-7-yl) naphthalen-2-ol formate (Compound 7)
Preparation of compound 7 reference is made to the synthesis of compound 1. Starting material compound 7a (200.0 mg,0.79mmol, prepared by the method disclosed in patent application "intermediate 5 on page 82 of the specification of WO2021041671A 1") and 7b (179.3 mg, 0.79mmol) were reacted in four steps to give compound 7 (4.7 mg).
MS(ESI):m/z=565.4[M+H] +
1 H NMR:(400MHz,CD 3 OD)δ8.74(s,1H),8.48(s,1H),7.81(d,J=7.7Hz,1H),7.51(d,J=6.2Hz,1H),7.42-7.36(m,1H),7.32(d,J=2.4Hz,1H),7.14(d,J=2.4Hz,1H),4.60–4.56(m,3H),4.16(s,2H),3.76(d,J=12.0Hz,4H),3.43(s,1H),3.26(s,3H),3.06(s,2H),2.87–2.85(m,1H),2.26-2.08(m,5H),1.83-1.61(m,4H)
Example 8
4- (4- ((1R, 5S) -3, 8-azobis [3.2.1] octan-3-yl) -2- (((2R, 7aS) -2-fluorotetrahydro-1H-bispyridazin-7 a (5H) -yl) methoxy) -7- (3-methoxynaphthyl-1-yl) pyrimidine [4,5-d ] pyridazin-8 (7H) -one (Compound 8)
The first step is as follows: preparation of Compound 8b
To a solution of compound 8a (10.0 g,44.8 mmol) in DMF (100 mL) at 15 ℃ was added K 2 CO 3 (18.6g, 134.5mmol). Stirred at this temperature for 3 hours. Water (10 mL) was added to the reaction, extracted with AcOEt (100 mL. Times.3), and the combined organic phases were extracted with anhydrous Na 2 SO 4 Drying, filtration and concentration under reduced pressure gave crude compound 8b (278.0 mg, crude). The crude product was used directly in the next reaction.
The second step: preparation of Compound 8d
To H dissolved with Compound 8b (6.00 g) under Ar gas protection 2 O (7.5 mL) solution was added t-BuONa (4.86g, 50.6 mmol), t-BuBrettPos Pd G3 (2164.7mg, 2.5mmol), and Compound 8c (2 mL). Heated to 45 ℃ and stirred for 16 hours. The reaction was filtered and the filtrate was concentrated under reduced pressure to give crude 8d (6.51 g, crude).
MS(ESI):m/z=288.9[M+H] +
The third step: preparation of Compound 8e
Compound 8d (6.00g, 20.8mmol) was dissolved in HCl/1,4-dioxane (4M, 10mL). Stirred at room temperature for 0.5 h. The reaction was concentrated under reduced pressure in vacuo to give crude 8e (3.91 g), which was used directly in the next reaction.
MS(ESI):m/z=188.9[M+H] +
Fourth step/fifth step: preparation of Compound 8i
To a solution of compound 8e (3.00 g) in DMF (30 mL) at 20 deg.C was added compound 8f (3.56g, 15.9 mmol). Stirred at this temperature for 4 hours. Then, compound 8h (2.70g, 12.7 mmol) and DIEA (4.11g, 31.8mmol) were added to the reaction mixture, and stirring was continued for 3 hours. Water (100 mL) was added to the reaction, extraction was performed with AcOEt (100 mL. Times.3), and the combined organic phases were extracted with anhydrous Na 2 SO 4 Drying, filtering, and vacuum concentrating to obtain crude compound. The crude product is purified by silica gel chromatography flash column chromatography, and the eluent is: ethyl acetate 10% petroleum ether gave compound 8i (5.81 g).
MS(ESI):m/z=569.3[M+H] +
And a sixth step: preparation of Compound 8j
To a solution of compound 8i (5.00g, 8.80mmol) in MeOH at room temperature were added TEA (2.4 mL,17.6 mmol) and Pd (dppf) Cl 2 (651.7mg, 0.88mmol). The mixture was heated to 100 ℃ under CO (2M Pa) and stirred for 12 hours. Vacuum concentrating the reaction solution to obtain a crude product, purifying the crude product by silica gel chromatography flash column chromatography, wherein the eluent is as follows: ethyl acetate 10% petroleum ether afforded Compound 8j (1.31 g). MS (ESI) m/z =593.2[ M + H ]] +
The seventh step: preparation of Compound 8k
A solution of compound 8j (500.0 mg, 0.84mmol) in AcOH (20 mL) was stirred at 110 ℃ for 15min. Adding saturated Na to the reaction solution 2 CO 3 Aqueous solution (20 mL), extracted with AcOEt (20 mL. Times.3), and the combined organic phases extracted with anhydrous Na 2 SO 4 Drying, filtration and concentration under reduced pressure gave crude compound 8k (461.3 mg). The crude product was used directly in the next reaction.
MS(ESI):m/z=561.2[M+H] +
Eighth step: preparation of Compound 8l
To a solution of compound 8k (500.0mg, 0.89mmol) in DCM (20 mL) was added m-CPBA (384.7mg, 2.23mmol), and the mixture was stirred at 0 ℃ for 40min. Adding saturated NaHCO into the reaction solution 3 (30 mL), extraction with AcOEt (30 mL. Times.3), and combined organic phases over anhydrous Na 2 SO 4 Drying, filtration and concentration under reduced pressure gave 8l (431.1 mg) of the crude compound. The crude product was used directly in the next reaction.
MS(ESI):m/z=577.1[M+H] +
The ninth step: preparation of Compound 8m
To a solution of compound 8l (400 mg) in THF (20 mL) was added compound 1d (220.9mg, 1.39mmol). LiHMDS (0.14mL, 0.76mmol) was added to the reaction mixture at 0 ℃ under Ar gas shield. The reaction solution was stirred at 0 ℃ for 30min, warmed to room temperature, and stirred for 1 hour. Adding saturated NH to the reaction solution 4 Cl (30 mL), extraction with AcOEt (30 mL. Times.3), and combined organic phases over anhydrous Na 2 SO 4 Drying, filtration and concentration under reduced pressure gave the crude compound 8m (219.5 mg).
MS(ESI):m/z=672.3[M+H] +
The tenth step: preparation of Compound 8
Compound 8m (100.0 mg,) was dissolved in HCl/1,4-dioxane (4M, 8.0 mL). Stirred at room temperature for 0.5 h. The reaction was concentrated under reduced pressure in vacuo to give a crude product, which was subjected to preparative HPLC to give compound 8 (4.29 mg).
MS(ESI):m/z=572.2[M+H] +
1 H NMR:(400MHz,CD 3 CN)δ8.28(s,1H),7.92(d,J=8.3Hz,1H),7.57-7.43(m,3H),7.39-7.31(m,1H),7.26(d,J=2.3Hz,1H),5.38-5.16(m,1H),4.34(s,2H),4.21(d,J=10.8Hz,1H),4.13(d,J=10.8Hz,1H),3.96(s,3H),3.61-3.50(m,4H),3.24-3.11(m,3H),3.96-2.85(m,1H),2.15-2.03(m,4H),1.92-1.81(m,2H),1.76-1.63(m,4H)
Example 9
4- ((1R, 5S) -3, 8-azobis [3.2.1] nonan-3-yl) -N- ((R) -1- (dimethylamino) propan-2-yl) -7- (3-hydroxynaphthalen-1-yl) -5,6,7, 8-tetrahydro-1, 7-naphthyridine-2-carboxamide formate (Compound 9)
The first step is as follows: preparation of Compound 9c
Compound 9a (10.0 g, 72.4mmol) and compound 9b ((20.2 g, 17.38mmol) were added to a reaction flask at room temperature, and stirred for 10min 3 (150 mL), heated to 100 ℃ and stirred for 1h. Water (25 mL) was added to the reaction mixture, which was extracted with DCM (20 mL. Times.3), and the combined organic phases were extracted with anhydrous Na 2 SO 4 Drying, filtering, and vacuum concentrating to obtain crude compound. The crude product is purified by silica gel chromatography flash column chromatography, and the eluent is: ethyl acetate with 25% petroleum ether to give compound 9c (3.00g, 17.5% yield).
MS(ESI):m/z=236.9[M+H] +
The second step is that: preparation of Compound 9d
To a solution of compound 9c (3.30G) in 1,4-dioxane (60 mL) was added compound 8h (3.55g, 16.7 mmol), ruphos-Pd-G3 (1.17g, 1.39mmol) and Cs 2 CO 3 (11.3g, 34.9mmol). Stirring at 80 deg.C under Ar gas for 16 hr. After the reaction solution is filtered, the filtrate is concentrated under vacuum to obtain a crude product. The crude product is purified by silica gel chromatography flash column chromatography, and the eluent is: 25-40% Ethyl acetate with petroleum ether to give compound 9d (2.8 g).
MS(ESI):m/z=399.1[M+H] +
The third step: preparation of Compound 9e
To a solution of compound 9d (1.60g, 4.02mmol) in AcOH (10 mL) was added compound NaBH 3 CN (720.6 mg,12.0 mmol). Stirring for 10min at 20 ℃ under the protection of Ar gas. Water (10 mL) was added to the reaction mixture, which was extracted with DCM (15 mL. Times.3), and the combined organic phases were extracted with anhydrous Na 2 SO 4 Drying, filtration and concentration under reduced pressure gave crude compound 9e (1.20 g). The crude product was used directly in the next reaction.
MS(ESI):m/z=403.2[M+H] +
The fourth step: preparation of Compound 9h
To a solution of compound 9e (400 mg) in 1,4-dioxane (60 mL) were added compound 9f (318.6 mg, 1.19mmol), ruphos-Pd-G3 (3.1mg, 0.10mmol) and Cs 2 CO 3 (971.4mg, 2.98mmol). Stirring at 80 deg.C under Ar gas for 10 hr. After the reaction solution is filtered, the filtrate is concentrated under vacuum to obtain a crude product. The crude product is purified by silica gel chromatography flash column chromatography, and the eluent is: 8-15% ethyl acetate in petroleum ether to give compound 9h (200mg, 0.34mmol).
MS(ESI):m/z=589.5[M+H] +
The fifth step: preparation of Compound 9i
To a solution of compound 9H (200.0 mg, 0.34mmol) in THF/H 2 To the O (1,4mL) solution, liOH-H was added 2 O (28.2mg, 0.67mmol). Stirring was carried out at 20 ℃ for 0.5 hour. Vacuum concentrating the reaction solution, adding H 2 O (15 mL) and adjusted the pH to 3-4 with 1N HCl. The solution was extracted with DCM (10 mL. Times.3), and the combined organic phases were extracted with anhydrous Na 2 SO 4 Drying, filtration and concentration under reduced pressure gave crude compound 9i (180 mg). The crude product was used directly in the next reaction.
MS(ESI):m/z=575.4[M+H] +
And a sixth step: preparation of Compound 9k
To a solution of compound 9i (180.0 mg) in DMF (3 mL) was added compound 9j (82.2mg, 0.47mmol), DIEA (0.26mL, 1.57mmol) and HATU (178.6 mg, 0.47mmol). The mixture was stirred at 20 ℃ for 1 hour. The reaction mixture was concentrated under reduced pressure, saturated Na2CO3 (15 mL) was added, and extraction was performed with DCM (15 mL. Times.3)And combining the organic phases with anhydrous Na 2 SO 4 Drying, filtration and concentration under reduced pressure gave crude 9k (190 mg) of the compound. The crude product was used directly in the next reaction.
MS(ESI):m/z=659.6[M+H] +
The seventh step: preparation of Compound 9
To CH in which Compound 9k (100.0 mg) is dissolved 3 CN (1 mL) was added to HCl/1,4-dioxane (4M, 6.0 mL). Stir at room temperature for 15min. The reaction was concentrated under reduced pressure in vacuo to give a crude product, which was subjected to preparative HPLC to give compound 9 (41.96 mg).
MS(ESI):m/z=515.1[M+H] +
1 H NMR:(400MHz,CD 3 OD)δ8.50(s,1H),8.08(d,J=8.4Hz,1H),7.72-7.59(m,2H),7.38(t,J=7.3Hz,1H),7.30-7.22(m,1H),6.89(d,J=1.7Hz,1H),6.83(d,J=2.0Hz,1H),4.54-4.48(m,1H),4.40(s,2H),4.11(s,2H),3.44–3.40(m,4H),3.29-3.20(m,2H),3.16(s,2H),3.11-3.01(m,1H),2.85–2.82(m,1H),2.63(s,6H),2.31(d,J=7.5Hz,2H),2.14(s,2H),1.30(d,J=6.6Hz,3H)
Example 10
4- (4- (2, 5-diazabicyclo [2.2.2] octan-2-yl) -8-fluoro-2- (((2R) -2-fluorotetrahydro-1H-bispyridyl-7 a (5H) -yl) methoxy) pyrido [4,3-d ] pyrimidin-7-yl) -5-ethynyl-6-fluoronaphthalen-2-ol (compound 10)
Preparation of compound 10c reference is made to the synthesis of compound 1. Starting materials 1e (300mg, 0.54mmol) and 10a (334.8mg, 0.65mmol, prepared by the method disclosed in the patent application "intermediate 15 on page 96 of the specification in WO2021041671A 1") were reacted in two steps to give compound 10c (320.0 mg).
Preparation of compound 10:
to a solution of compound 10c (310.0 mg, 0.410mmol) in DMF (6.0 mL) was added CsF (311.0 mg, 2.05mmol) at room temperature. The reaction solution was heated to 85 ℃ and stirred for 1.5h. The reaction was filtered, concentrated under reduced pressure in vacuo to give crude which was purified by preparative HPLC to give compound 10 (36.7 mg, 15.1% yield)
Chiral HPLC resolution of the compound 10 is carried out to obtain a compound 10-1 and a compound 10-2
Single configuration compound 10-1:
MS(ESI)m/z=601.4[M+H] +
chiral HPLC analysis: retention time 1.975 min, chiral purity: 100% (chromatographic column: chiralCel IC-3 100 x 4.6mm,3um with protective column; mobile phase: A: CO) 2 ;B:Methanol(0.05%DEA)
1 H NMR:(400MHz,DMSO-d 6 )
δ9.13(br s,1H),8.23(br s,1H),7.98(dd,J=6.0,9.2Hz,1H),7.47(t,J=8.9Hz,1H),7.40(d,J=2.3Hz,1H),7.18(br s,1H),5.37-5.18(m,1H),4.91(s,1H),4.14-4.01(m,4H),3.44-3.43(m,1H),3.26-3.19(m,2H),3.09(br d,J=7.6Hz,2H),3.02(br s,1H),2.86-2.82(m,1H),2.25-1.72(m,10H)
Single configuration compound 10-2:
MS(ESI):m/z=601.4[M+H] +
chiral HPLC analysis: retention time 4.088 min, chiral purity: 94.5% (chromatographic column: chiralCel IC-3 100X 4.6mm,3um with guard column; mobile phase: A: CO) 2 ;B:Methanol(0.05%DEA)
1 H NMR:(400MHz,DMSO-d 6 )δ9.13(s,1H),8.23(s,1H),7.98(dd,1H),7.47(t,1H),7.40(d,1H),7.18(s,1H),5.37-5.18(m,1H),4.91(s,1H),4.14-4.01(m,4H),3.44-3.19(m,3H),3.09(m,2H),3.02(s,1H),2.86-2.82(m,1H),2.25-1.72(m,10H)
Example 11
4- (1R, 5S) -3, 8-diazabicyclo [3.2.1] octan-3-yl) -2- (((2R, 7aS) -2-fluorotetrahydro-1H-bispyridyl-7 a (5H) -yl) methoxy) -7- (naphthalen-1-yl) pyrido [4,3-d ] pyrimidine-8-carbonitrile (Compound 11)
The first step is as follows: preparation of Compound 11b
To DMSO (100 mL) in which compound 11a (12.0 g,57.8 mmol) was dissolved at room temperature was added CuCN (15.5 g,173.5 mmol). The reaction solution is heated to 130 ℃ and stirred for 10-12h. Cooling the reaction liquid to 15 ℃, and adding NH into the reaction liquid 4 OH (250 mL), stirred for 10min, extracted with EtOAc (200 mL. Times.2). The combined organic phases were combined with anhydrous Na 2 SO 4 Drying, filtration and concentration under reduced pressure gave crude 11b (2.2 g, crude) of the compound. The crude product was used directly in the next reaction.
MS(ESI):m/z=154.3[M+H] +
The second step is that: preparation of Compound 11c
To CH in which Compound 11b (2.00 g, crude) was dissolved at room temperature 3 CN (20 mL) was added NIS (4.40g, 19.5 mmol) and TsOH (123.9mg, 0.65mmol). The temperature of the reaction solution is raised to 70 ℃, and the reaction solution is stirred for 10 to 12 hours. The reaction mixture was concentrated under reduced pressure in vacuo, and then extracted with water (50 mL) and EtOAc (50 mL. Times.2). The combined organic phases were combined with anhydrous Na 2 SO 4 Drying, filtration and concentration under reduced pressure gave crude 11c (2.20 g, crude). The crude product is purified by silica gel chromatography flash column chromatography, and the eluent is: 10% Ethyl acetate in petroleum ether to give Compound 11c (2.10 g).
MS(ESI):m/z=279.8[M+H] +
The third step: preparation of Compound 11d
Compound 11c (1.00g, 3.58mmol), TEA (1.09g, 10.7 mmol) and PdCl were added to ethanol (10 mL) at room temperature 2 (PPh 3 ) 2 (251.1mg, 0.36mmol) was heated to 80 ℃ under CO (50 psi) and stirred for 16h. Filtering the reaction solution, and concentrating under reduced pressure to obtain a crude product. The crude product is purified by silica gel chromatography flash column chromatography, and the eluent is: 20% Ethyl acetate in petroleum ether to give compound 11d (440.0 mg, yield 54.5%).
The fourth step: preparation of Compound 11f
At room temperature,to 1,4-dioxane/H 2 O (4 mL, 3) 2 CO 3 (1.30g, 3.99mmol) and Pd (dppf) Cl 2 . Heating to 100 ℃ under Ar atmosphere, and stirring for 2h. Water (10 mL) was added to the reaction solution, and the mixture was extracted with EtOAc (15 mL. Times.2). The combined organic phases were combined with anhydrous Na 2 SO 4 Drying, filtering, and vacuum concentrating to obtain crude compound. The crude product is purified by silica gel chromatography flash column chromatography, and the eluent is: petroleum ether of 30% ethyl acetate gave compound 11f (300.0 mg, 71.1% yield).
MS(ESI):m/z=318.2[M+H] +
The fifth step: preparation of Compound 11g
At room temperature, trichloroacetylcarbonyl isocyanate (0.19mL, 1.58mmol) was added to THF (3 mL) in which compound 11f (250.0 mg, 0.79mmol) was dissolved. The reaction was stirred at room temperature for 12h. Vacuum decompression concentration of the reaction liquid to obtain coarse product. The crude product was ground and MTBE (10.0 mL) was added, stirred for 10min and filtered to give 11g (346.0 mg, yield 86.8%).
MS(ESI):m/z=505.1[M+H] +
And a sixth step: preparation of Compound 11h
At 25 ℃ 11g (340.0 mg, 0.67mmol) of the compound was added to 4M NH 3 MeOH (5 mL) was stirred for 1h. Vacuum concentrating the reaction liquid to obtain coarse product. MTBE (10.0 mL) was added, the mixture was stirred for 10min, and the mixture was filtered to obtain compound 11h (170.0 mg, yield 80.5%)
MS(ESI):m/z=314.9[M+H] +
The seventh step: preparation of Compound 11i
Compound 11h (160.0 mg, 0.51mmol) was dissolved in POCl 3 (2 mL), DIEA (0.25mL, 1.53mmol) was added dropwise thereto. The temperature is increased to 110 ℃, and the mixture is stirred for 2 hours. Water (20 mL) was added to the reaction solution, and the mixture was extracted with EtOAc (15 mL. Times.2). The combined organic phases were combined with anhydrous Na 2 SO 4 Drying, filtration and concentration under reduced pressure gave crude compound 11i (130.0 mg, crude).
MS(ESI):m/z=351.1[M+H] +
Eighth step: preparation of Compound 11j
DIEA (294.9mg, 2.282mmol) and compound 8h (72.5mg, 0.34mmol) were added to DCM (0.5 mL) containing compound 11i (120.0 mg, crude) dissolved therein at-40 deg.C and stirred at this temperature for 30min. Water (20 mL) was added to the reaction solution, and the mixture was extracted with EtOAc (15 mL. Times.2). The crude product is purified by silica gel chromatography flash column chromatography, and the eluent is: ethyl acetate to give Compound 11j (170.0 mg).
MS(ESI):m/z=527.3[M+H] +
The ninth step: preparation of Compound 11k
To 1,4-dioxane (2.0 mL) containing compound 11j (120.0 mg, 0.23mmol) was added DIEA (88.3mg, 0.683mmol) and compound 1d (72.5mg, 0.34mmol) at room temperature. The temperature is increased to 80 ℃, and the mixture is stirred for 12 hours. Water (10 mL) was added to the reaction solution, and the mixture was extracted with EtOAc (15 mL. Times.3). The crude product is purified by silica gel chromatography flash column chromatography, and the eluent is: 10% methanol in dichloromethane gave compound 11k (60.0 mg, yield 40.5%).
MS(ESI):m/z=650.2[M+H] +
The tenth step: preparation of Compound 11
To CH in which compound 11k (45.0 mg, 0.07mmol) was dissolved at 0 deg.C 3 CN (2.5 mL) was added to 4M HCl/dioxane (2 mL) and stirred for 1h. The reaction was concentrated under reduced pressure to give a crude product, which was subjected to preparative HPLC to give compound 11 (10.8 mg, yield 28.4%).
MS(ESI):m/z=550.1[M+H] +
1 H NMR(400MHz,DMSO-d 6 )δ9.43(s,1H),8.13(d,1H),8.06(d,1H),7.75-7.65(m,3H),7.63-7.57(m,1H),7.54-7.48(m,1H),5.45-5.07(m,1H),4.56(m,2H),4.27-4.06(m,2H),4.02-3.52(m,3H),3.16-2.98(m,3H),2.89-2.74(m,1H),2.21-1.95(m,3H),1.90-1.65(m,7H)
Example 12
4- (1R, 5S) -3, 8-diazabicyclo [3.2.1] octan-3-yl) -7- (3- (difluoromethyl) naphthalen-1-yl) -8-fluoro-2- ((tetrahydro-1H-bispyridyl-7 a (5H) -yl) methoxy) pyrido [4,3-d ] pyrimidine (Compound 12)
The first step is as follows: preparation of Compound 12b-2
To a solution of compound 12b-1 (500.00mg, 2.13mmol) in DCM (5.0 mL) was slowly added DAST (3.43g, 21.3mmol) at room temperature, at which temperature N 2 Stirring for 12h under protection. The reaction mixture was poured into ice water (30 mL) at 0 ℃ and extracted with DCM (20 mL. Times.3). The combined organic phases were combined with anhydrous Na 2 SO 4 Drying, filtering, and vacuum concentrating to obtain crude compound. The crude product is purified by silica gel chromatography flash column chromatography, and the eluent is: 10% Ethyl acetate in petroleum ether to give compound 12b-2 (430.0 mg, yield 78.6%).
The second step is that: preparation of Compound 12b
To 1,4-dioxane (5.0 mL) in which compound 12b-2 (380.0 mg, 1.48mmol) was dissolved at room temperature were added pinacol diboron (563.0 mg, 2.22mmol), KOAc (290.1mg, 2.95mmol) and Pd (dppf) Cl 2 (109.6mg, 0.148mmol). The reaction solution is heated to 110 ℃ and stirred for 12h. Water (10 mL) was added to the reaction solution, and the mixture was extracted with EtOAc (15 mL. Times.2). The organic phases were combined and washed with anhydrous Na 2 SO 4 Drying, filtering, and vacuum concentrating to obtain crude compound. The crude product is purified by silica gel chromatography flash column chromatography, and the eluent is: 20% Ethyl acetate in petroleum ether to give compound 12b (340.0 mg, yield 75.6%).
1 H NMR:(400MHz,CD 3 OD)δ8.78(d,J=8.3Hz,1H),8.13(s,2H),7.98-7.92(m,1H),7.63-7.51(m,2H),7.08-6.74(m,1H),1.44(s,12H)
Preparation of compound 12 reference is made to the synthesis of compound 1. Compound 12 (26.5 mg) was obtained by reacting the starting materials 12a (200.0mg, 0.38mmol, which was obtained by the method disclosed in the patent application "WO2021041671A1, page 83, intermediate 6", and 12b (148.3mg, 0.49mmol) in two steps.
MS(ESI):m/z=575.2[M+H] +
1 H NMR:(400MHz,DMSO-d 6 )δ9.21(s,1H),8.21-8.17(m,2H),7.82-7.77(m,2H),7.73-7.60(m,2H),7.45-7.14(m,1H),4.53–4.50(m,2H),4.21(d,2H),3.70(s,4H),3.09(s,2H),2.77-2.67(m,2H),1.99-1.94(m,2H),1.93-1.77(m,4H),1.71(s,6H)
Example 13
4- (4- (1R, 5S) -3, 8-diazabicyclo [3.2.1] octan-3-yl) -8-fluoro-2- ((tetrahydro-1H-bispyrolidin-7 a (5H) -yl) deuterated methoxy-d 2) pyrido [4,3-d ] pyrimidin-7-yl) -5-ethynylnaphthalene-2-ol (compound 13)
Preparation of compound 13 a:
LiAlD was added to THF (10 mL) containing compound 7a (1 g, 5.91mmol) at 0 deg.C 4 (744.2mg, 17.7 mmol), heating to 20 deg.C, and stirring for 16h. Cooling to 0 ℃, and slowly adding D into the reaction solution 2 The reaction was quenched with O (1.0 mL). Vacuum concentration to obtain crude product, which is directly used for next reaction.
1 H NMR:(400MHz,CDCl 3 )δ3.05-2.93(m,2H),2.69-2.57(m,2H),1.88-1.64(m,6H),1.61-1.50(m,2H)
Preparation of compound 13 reference is made to the synthesis of compound 10. Compound 13 (4.7 mg) was obtained by a four-step reaction of starting material 7a (500.0mg, 1.17mmol, prepared by the method disclosed in the patent application "WO2021041671A1, page 82, intermediate 5", and 13a (334.4mg, 2.34mmol). (Compound 7d was prepared by a method disclosed in the patent application "intermediate 17 on page 102 of the description in WO2021041671A 1")
MS(ESI):m/z=567.3[M+H] +
1 H NMR:(400MHz,DMSO-d 6 )δ9.04(s,1H),8.27(s,1H),7.88(dd,1H),7.49-7.38(m,2H),7.34(d,1H),7.12(d,1H),4.52(d,1H),4.35(d,2H),3.71-3.57(m,6H),3.11-2.99(m,2H),2.71-2.67(m,2H),1.99-1.63(m,12H)
Example 14
4- (1R, 5S) -3, 8-diazabicyclo [3.2.1] octan-3-yl) -7- (8-chloronaphthalen-1-yl) -2- (((2R, 7aS) -2-fluorotetrahydro-1H-bispyridopyridin-7 a (5H) -yl) methoxy) pyrido [4,3-d ] pyrimidin-8 (7H) -one compounds 14)
The first step is as follows: synthesis of Compound 14b
At room temperature and CO 2 Under atmosphere, compound is added into ionic liquid [ HDBU +][TFE-](6 mL) was stirred for 8h. Water (5 mL) was added to the reaction mixture, which was filtered and the filter cake was washed with MTBE (10 mL) to afford compound 14b.
MS(ESI):m/z=339.9[M+H] +
The second step is that: synthesis of Compound 14c
Compound 10 (600.0mg, 339.7mmol) was dissolved in POCl at room temperature 3 To (4 mL) DIEA (1.46mL, 8.83mmol) was added slowly. The reaction solution is heated to 100 ℃ and stirred for 12h. The reaction was slowly poured into ice water (10 mL), filtered, and compound 14c (600.0 mg, yield 90.2%) was dried under reduced pressure
MS(ESI):m/z=377.8[M+H] +
Preparation of compound 14 reference is made to the synthesis of compound 10. Starting material 14c (600.0 mg, 1.59mmol) and 8h (507.3 mg, 2.39mmol) were reacted in three steps to give compound 14 (400.0 mg).
MS(ESI):m/z=575.1[M+H] +
1 H NMR:(400MHz,DMSO-d 6 )δ8.21(d,1H),8.11(d,1H),7.74(t,1H),7.68-7.63(m,1H),7.61-7.53(m,2H),7.29(d,1H),6.57(d,1H),5.41-5.11(m,1H),4.21-3.94(m,4H),3.65(s,1H),3.47-3.36(m,4H),3.15-3.03(m,2H),3.01(s,1H),2.86-2.77(m,1H),2.18-1.93(m,3H),1.88-1.67(m,7H)
Example 15
4- (4- (1R, 5S) -3, 8-diazabicyclo [3.2.1] octan-3-yl) -8-fluoro-2- (((2R, 7aS) -2-fluorotetrahydro-1H-bispyridyl-7 a (5H) -yl) methoxy) pyrido [4,3-d ] pyrimidin-7-yl) -5-ethynyl-6-fluoronaphthalen-2-yl) boronic acid (compound 15)
Synthesis of Compound 15a was carried out by the method disclosed in WO2021041671A1, description No. 456 or example 252 ".
The first step is as follows: preparation of Compound 15b
DIEA (244.3mg, 1.89mmol) and Tf were added to DCM (2 mL) containing compound 15a (540.0 mg, 0.63mmol) at-40 deg.C 2 O (244.2mg, 0.95mmol), stirred for 2h. Vacuum concentrating the reaction liquid to obtain coarse product. The crude product is purified by silica gel chromatography flash column chromatography, and the eluent is: 25-50% Ethyl acetate in petroleum ether to give compound 15b (400.0 mg, yield 64.2%).
MS(ESI):m/z=989.3[M+H] +
The second step: preparation of Compound 15c
At room temperature N 2 To 1,4-dioxane (2.0 mL) containing compound 15b (400.0 mg, 0.40mmol) was added pinacol diboron (205.4 mg, 0.81mmol), pd (dppf) Cl 2 (30.0 mg, 0.04mmol) and KOAc (138.9mg, 1.42mmol). The reaction solution is heated to 120 ℃ and stirred for 12h. The reaction solution is concentrated under vacuum and reduced pressure to obtain a crude product. The crude product is purified by silica gel chromatography flash column chromatography, and the eluent is: 25-50% Petroleum ether of ethyl acetate to afford Compound 15c (260.0 mg, 66.5% yield).
MS(ESI):m/z=967.9[M+H] +
The third step: preparation of Compound 15d
To DCM (7.0 mL) containing compound 15c (400.0 mg, 0.40mmol) dissolved therein was added methylboronic acid (80.5 mg, 1.34mmol) and TFA (0.33 mL) at room temperature. At N 2 The mixture was stirred at this temperature for 2h under an atmosphere. The reaction solution was concentrated under vacuum to give crude product 15d.
MS(ESI):m/z=885.6[M+H] +
Synthesis of Compound 15 (12.26 mg) A method for reference Compound 10 was prepared from Compound 15d.
MS(ESI):m/z=629.1[M+H] + δ10.90(s,1H),9.41(s,1H),9.22-9.08(m,2H),8.46(s,1H),8.23(dd,1H),7.60(t,1H),5.68-5.47(m,1H),4.70(d,1H),4.66-4.52(m,3H),4.26-4.19(m,2H),3.96(d,1H),3.93-3.82(m,4H),3.76(m,4H),2.29-2.09(m,3H),2.06-1.89(m,5H)
Example 16
9- (4- (1R, 5S) -3, 8-diazabicyclo [3.2.1] octan-3-yl) -8-fluoro-2- ((tetrahydro-1H-bispyrolidin-7 a (5H) -yl) methoxy) pyrido [4,3-d ] pyrimidin-7-yl) -9H-carbazole (compound 16)
Preparation of compound 16 reference is made to the synthesis of compound 1. From starting material 12a (200.0 mg, 0.38mmol) and 16a (75.3 mg, 0.45mmol), compound 16 (83.5 mg) was obtained in a two-step reaction.
MS(ESI):m/z=564.2[M+H] +
1 H NMR:(400MHz,CD 3 OD)δ9.16(s,1H),8.17(d,2H),7.51-7.44(m,4H),7.37-7.32(m,2H),4.76(d,2H),4.67(s,2H),3.93(s,2H),3.86(d,2H),3.70(m,2H),3.30-3.24(m,2H),2.38-2.30(m,2H),2.28-2.15(m,4H),2.15-2.06(m,2H),2.04-1.87(m,4H)
Example 17
(4- (4- (1R, 5S) -3, 8-diazabicyclo [3.2.1] octan-3-yl) -8-fluoro-2- ((tetrahydro-1H-bispyridyl-7 a (5H) -yl) methoxy) pyrido [4,3-d ] pyrimidin-7-yl) naphthalen-2-yl) methanol (Compound 17)
The first step is as follows: preparation of Compound 17a-1
At room temperature N 2 To 1,4-dioxane (1.0 mL) containing compound 12a (400.0 mg, 1.70mmol) was added pinacol diboron (648.1mg, 2.55mmol), pd (dppf) Cl 2 (126.2mg, 0.17mmol) and KOAc (333.9mg, 3.40mmol). The reaction solution was heated to 110 ℃ and stirred for 12h. Vacuum concentrating the reaction liquid to obtain coarse product. Crude product is obtained byPurifying by silica gel chromatography and flash column chromatography, wherein the eluent is as follows: 20% Ethyl acetate in petroleum ether to give compound 17a-1 (160.0 mg, yield 33.3%).
MS(ESI):m/z=282.9[M+H] +
The second step: preparation of Compound 17a
To MeOH (5 mL) in which compound 17a-1 (400.0 mg, 1.42mmol) was dissolved at 0 deg.C was added NaBH 4 (95.8mg, 2.84mmol) and stirred for 1h. Water (10 mL) was added to the reaction solution, and the mixture was extracted with EtOAc (15 mL. Times.3). The organic phases were combined and washed with anhydrous Na 2 SO 4 Drying, filtering, and vacuum concentrating to obtain crude compound. The crude product is purified by silica gel chromatography flash column chromatography, and the eluent is: 20% Ethyl acetate in petroleum ether to give compound 17a (120.0 mg, yield 29.8%).
Preparation of compound 17 reference is made to the synthesis of compound 1. Compound 17 (21.8 mg) was obtained from starting material 12a (200.0mg, 0.38 and 17a (138.6mg, 0.488mmol) by a two-step reaction.
MS(ESI):m/z=555.5[M+H] +
1 H NMR:(400MHz,DMSO-d 6 )δ9.19(s,1H),8.04-7.97(m,2H),7.70-7.69(m,1H),7.63(s,1H),7.58-7.52(m,1H),7.49-7.43(m,1H),4.74(s,2H),4.50(d,2H),4.17(d,2H),3.67(s,4H),3.06(s,2H),2.68(s,2H),2.02-1.62(m,12H)
Example 18
(4- (4- (1R, 5S) -8-oxa-3-azabicyclo [3.2.1] octan-3-yl) -8-fluoro-2- ((tetrahydro-1H-bispyridyl-7 a (5H) -yl) methoxy) pyrido [4,3-d ] pyrimidin-7-yl) -5-ethynylnaphthalene-2-ol (compound 18)
Preparation of compound 18 reference is made to the synthesis of compound 10. Starting materials 1a (300.0mg, 1.19mmol) and 18a (142.2mg, 0.95mmol) were reacted in five steps to obtain compound 18 (15.2 mg).
MS(ESI):m/z=566.3[M+H] +
1 H NMR:(400MHz,CD 3 OD)δ9.04(s,1H),7.83(d,1H),7.51(d,1H),7.44-7.37(m,1H),7.34(d,1H),7.16(d,1H),4.71-4.43(m,5H),3.83-3.82(m,2H),3.40-3.33(m,2H),3.01(s,1H),3.00-2.92(m,2H),2.22-2.13(m,2H),2.12-1.96(m,6H),1.96-1.82(m,5H)
Test example 1, activity test
Experimental protocol
1. Preparation of Compounds
According to the compound well plate profile, samples were loaded using ECHO, with a maximum concentration of 10000nM, 3-fold dilution, 10 doses, using 0.5% DMSO as solvent, and wells were re-plated.
2. Experimental procedures
1) Preparing a 160nM His-K-RasG12D mother liquor by using a buffer solution;
2) Adding 5uL His-K-RasG12D protein mother liquor into the corresponding Plate hole in Plate 1; sealing plate, and rapidly centrifuging at 1000rpm for 1 minute; incubating at room temperature for 120min;
3) Preparing a mixed solution of SOS-1 and GTP by using a buffer solution on ice;
4) Adding a mixture of 5Ul SOS-1 and GTP to the corresponding plate well; sealing plate, centrifuging at 1000rpm for one minute; incubating at room temperature for 60min;
5) Add 5uL GST c-Raf and receptor beads mixture to the corresponding plate well; sealing plate, centrifuging at 1000rpm for one minute; incubating at room temperature for 60min;
6) Add 5uL of donor bead mixture to the corresponding well (the donor beads need to be protected from light); sealing plate, centrifuging at 1000rpm for one minute; incubate at room temperature for 60min.
3. Read plate using Envision 3
| Optical filter | AlphaScreen emission 570-Em Slot 8 |
| Mirror image | AlphaScreen-Slot 4 |
| Total test time (ms) | 600 |
| Excitation time (ms) | 150 |
| Test mode | By rows,bi-directional |
| Test mode | Optiplate-384 |
Table 1.
Claims (15)
1. A compound of formula III-2 or a pharmaceutically acceptable salt thereof,
wherein R is 1 Is a heterocyclic group containing at least 1N atom, and the heterocyclic group containing at least 1N atom is selected from
The R is 3 Selected from deuterium, halogen, cyano, hydroxy, C 1-6 An alkyl group;
m is selected from 0,1,2,3 or 4;
L 2 is a bond;
ar is selected from C 6-10 Aryl, said Ar is optionally substituted with 1-6R j Substituted, R j Each independently selected from deuterium, halogen, cyano, hydroxy, -B (OH) 2 Nitro, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 1-6 Alkoxy radical, C 1-4 Hydroxyalkyl radical, C 2-6 Alkenyl or C 2-6 An alkynyl group;
3. The compound according to any one of claims 1-2, or a pharmaceutically acceptable salt thereof, wherein Ar is selected from C 6-10 Aryl radical, said C 6-10 Aryl is selected fromAr is optionally substituted with 1-6R j Substitution; preferably, said C 6-10 Aryl isAr is optionally substituted with 1-6R j Substitution;
said R is j As defined in claim 1.
4. A compound according to any one of claims 1-3, or a pharmaceutically acceptable salt thereof, wherein R j Selected from deuterium, hydroxy, fluoro, chloro, bromo, C 2-6 Alkenyl radical, C 2-6 Alkynyl, -B (OH) 2 And difluoromethyl.
5. A compound according to any one of claims 1-4, or a pharmaceutically acceptable salt thereof, wherein R j Selected from halogen, deuterium, hydroxy, C 2-6 Alkenyl or C 2-6 Alkynyl, preferably deuterium, hydroxy, fluorine, chlorine, bromine, C 2-6 Alkenyl or C 2-6 Alkynyl.
7. The compound according to any one of claims 1-32, or a pharmaceutically acceptable salt thereof, wherein R is 3 Selected from deuterium, halogen, cyano, hydroxy, C 1-6 Alkyl, preferably hydrogen, deuterium, fluorine, chlorine, bromine, iodine, methyl, ethyl, n-propyl, isopropyl or tert-butyl.
10. An isotopic substitution of the compound of any one of claims 1 to 9, or a pharmaceutically acceptable salt thereof, with the isotopic atom being deuterium.
11. The isotopic substitution of the compound or pharmaceutically acceptable salt thereof according to claim 10, wherein the abundance of said deuterium atoms is greater than 20%, preferably greater than 50%, more preferably greater than 90%, and most preferably greater than 95%.
12. A pharmaceutical composition comprising a therapeutically effective amount of at least one compound of any one of claims 1-11, or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable excipient.
13. Use of a compound of any one of claims 1-11, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition of claim 12, in the manufacture of a medicament for the prevention and/or treatment of a disease or disorder associated with KRAS G12D.
14. Use of a compound according to any one of claims 1 to 11, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition according to claim 12, in the manufacture of a medicament for the prevention and/or treatment of cancer.
15. The use of claim 14, wherein the cancer is selected from non-small cell lung cancer, colorectal cancer, rectal cancer, or pancreatic cancer.
Applications Claiming Priority (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2021103835370 | 2021-04-09 | ||
| CN202110383537 | 2021-04-09 | ||
| CN202110485881 | 2021-04-30 | ||
| CN2021104858810 | 2021-04-30 | ||
| CN202110730131 | 2021-06-29 | ||
| CN2021107301315 | 2021-06-29 | ||
| CN2021107904420 | 2021-07-13 | ||
| CN202110790442 | 2021-07-13 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN115197245A true CN115197245A (en) | 2022-10-18 |
Family
ID=83574334
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202210373726.4A Pending CN115197245A (en) | 2021-04-09 | 2022-04-07 | Kras inhibitor and preparation method thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN115197245A (en) |
Cited By (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN115785124A (en) * | 2021-09-10 | 2023-03-14 | 润佳(苏州)医药科技有限公司 | KRAS G12D inhibitors and uses thereof |
| CN116157401A (en) * | 2021-04-28 | 2023-05-23 | 浙江海正药业股份有限公司 | Heterocyclic derivatives and their preparation and use |
| WO2023098426A1 (en) * | 2021-12-02 | 2023-06-08 | 上海和誉生物医药科技有限公司 | 7-(naphthalene-l-yl)pyrido[4,3-d]pyrimidine derivatives, preparation method therefor, and use thereof |
| CN116284055A (en) * | 2021-09-10 | 2023-06-23 | 润佳(苏州)医药科技有限公司 | A kind of KRAS inhibitor and its use |
| WO2023134465A1 (en) * | 2022-01-11 | 2023-07-20 | 上海艾力斯医药科技股份有限公司 | Nitrogen-containing heterocyclic compound, and preparation method therefor, intermediate thereof and use thereof |
| WO2023138583A1 (en) * | 2022-01-21 | 2023-07-27 | 上海湃隆生物科技有限公司 | Heterocyclic compound, pharmaceutical composition and use thereof |
| WO2024119277A1 (en) * | 2022-12-08 | 2024-06-13 | Risen (Suzhou) Pharma Tech Co., Ltd. | Kras inhibitors and pharmaceutical uses thereof |
| US12059425B2 (en) | 2022-08-05 | 2024-08-13 | Kumquat Biosciences Inc. | Heterocyclic compounds and uses thereof |
| WO2024206858A1 (en) | 2023-03-30 | 2024-10-03 | Revolution Medicines, Inc. | Compositions for inducing ras gtp hydrolysis and uses thereof |
| WO2024227091A1 (en) * | 2023-04-26 | 2024-10-31 | Ranok Therapeutics (Hangzhou) Co. Ltd. | Diazabicyclooctane inhibitors of kras (g12d) and uses |
| WO2024229406A1 (en) | 2023-05-04 | 2024-11-07 | Revolution Medicines, Inc. | Combination therapy for a ras related disease or disorder |
| WO2025034702A1 (en) | 2023-08-07 | 2025-02-13 | Revolution Medicines, Inc. | Rmc-6291 for use in the treatment of ras protein-related disease or disorder |
| WO2025080946A2 (en) | 2023-10-12 | 2025-04-17 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2025171296A1 (en) | 2024-02-09 | 2025-08-14 | Revolution Medicines, Inc. | Ras inhibitors |
| US12448400B2 (en) | 2023-09-08 | 2025-10-21 | Gilead Sciences, Inc. | KRAS G12D modulating compounds |
| WO2025237091A1 (en) * | 2024-05-17 | 2025-11-20 | 中山优理生物医药有限公司 | Preparation method for spiro compound, and intermediate thereof |
| WO2025240847A1 (en) | 2024-05-17 | 2025-11-20 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2025255438A1 (en) | 2024-06-07 | 2025-12-11 | Revolution Medicines, Inc. | Methods of treating a ras protein-related disease or disorder |
| WO2025265060A1 (en) | 2024-06-21 | 2025-12-26 | Revolution Medicines, Inc. | Therapeutic compositions and methods for managing treatment-related effects |
| WO2026006747A1 (en) | 2024-06-28 | 2026-01-02 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2026015790A1 (en) | 2024-07-12 | 2026-01-15 | Revolution Medicines, Inc. | Methods of treating a ras related disease or disorder |
| WO2026015801A1 (en) | 2024-07-12 | 2026-01-15 | Revolution Medicines, Inc. | Methods of treating a ras related disease or disorder |
| WO2026015825A1 (en) | 2024-07-12 | 2026-01-15 | Revolution Medicines, Inc. | Use of ras inhibitor for treating pancreatic cancer |
| WO2026015796A1 (en) | 2024-07-12 | 2026-01-15 | Revolution Medicines, Inc. | Methods of treating a ras related disease or disorder |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2020146613A1 (en) * | 2019-01-10 | 2020-07-16 | Mirati Therapeutics, Inc. | Kras g12c inhibitors |
| WO2021041671A1 (en) * | 2019-08-29 | 2021-03-04 | Mirati Therapeutics, Inc. | Kras g12d inhibitors |
| WO2022042630A1 (en) * | 2020-08-26 | 2022-03-03 | InventisBio Co., Ltd. | Heteroaryl compounds, preparation methods and uses thereof |
| WO2022060583A1 (en) * | 2020-09-03 | 2022-03-24 | Revolution Medicines, Inc. | Use of sos1 inhibitors to treat malignancies with shp2 mutations |
| WO2022061251A1 (en) * | 2020-09-18 | 2022-03-24 | Plexxikon Inc. | Compounds and methods for kras modulation and indications therefor |
-
2022
- 2022-04-07 CN CN202210373726.4A patent/CN115197245A/en active Pending
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2020146613A1 (en) * | 2019-01-10 | 2020-07-16 | Mirati Therapeutics, Inc. | Kras g12c inhibitors |
| WO2021041671A1 (en) * | 2019-08-29 | 2021-03-04 | Mirati Therapeutics, Inc. | Kras g12d inhibitors |
| WO2022042630A1 (en) * | 2020-08-26 | 2022-03-03 | InventisBio Co., Ltd. | Heteroaryl compounds, preparation methods and uses thereof |
| WO2022060583A1 (en) * | 2020-09-03 | 2022-03-24 | Revolution Medicines, Inc. | Use of sos1 inhibitors to treat malignancies with shp2 mutations |
| WO2022061251A1 (en) * | 2020-09-18 | 2022-03-24 | Plexxikon Inc. | Compounds and methods for kras modulation and indications therefor |
Cited By (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116157401A (en) * | 2021-04-28 | 2023-05-23 | 浙江海正药业股份有限公司 | Heterocyclic derivatives and their preparation and use |
| CN116157401B (en) * | 2021-04-28 | 2025-06-20 | 浙江海正药业股份有限公司 | Heterocyclic derivatives and preparation methods and uses thereof |
| CN115785124B (en) * | 2021-09-10 | 2024-11-08 | 润佳(苏州)医药科技有限公司 | KRAS G12D inhibitors and uses thereof |
| CN116284055A (en) * | 2021-09-10 | 2023-06-23 | 润佳(苏州)医药科技有限公司 | A kind of KRAS inhibitor and its use |
| CN116284055B (en) * | 2021-09-10 | 2025-09-23 | 润佳(苏州)医药科技有限公司 | A KRAS inhibitor and its use |
| CN115785124A (en) * | 2021-09-10 | 2023-03-14 | 润佳(苏州)医药科技有限公司 | KRAS G12D inhibitors and uses thereof |
| WO2023098426A1 (en) * | 2021-12-02 | 2023-06-08 | 上海和誉生物医药科技有限公司 | 7-(naphthalene-l-yl)pyrido[4,3-d]pyrimidine derivatives, preparation method therefor, and use thereof |
| WO2023134465A1 (en) * | 2022-01-11 | 2023-07-20 | 上海艾力斯医药科技股份有限公司 | Nitrogen-containing heterocyclic compound, and preparation method therefor, intermediate thereof and use thereof |
| WO2023138583A1 (en) * | 2022-01-21 | 2023-07-27 | 上海湃隆生物科技有限公司 | Heterocyclic compound, pharmaceutical composition and use thereof |
| US12059425B2 (en) | 2022-08-05 | 2024-08-13 | Kumquat Biosciences Inc. | Heterocyclic compounds and uses thereof |
| WO2024119277A1 (en) * | 2022-12-08 | 2024-06-13 | Risen (Suzhou) Pharma Tech Co., Ltd. | Kras inhibitors and pharmaceutical uses thereof |
| WO2024206858A1 (en) | 2023-03-30 | 2024-10-03 | Revolution Medicines, Inc. | Compositions for inducing ras gtp hydrolysis and uses thereof |
| WO2024227091A1 (en) * | 2023-04-26 | 2024-10-31 | Ranok Therapeutics (Hangzhou) Co. Ltd. | Diazabicyclooctane inhibitors of kras (g12d) and uses |
| WO2024229406A1 (en) | 2023-05-04 | 2024-11-07 | Revolution Medicines, Inc. | Combination therapy for a ras related disease or disorder |
| WO2025034702A1 (en) | 2023-08-07 | 2025-02-13 | Revolution Medicines, Inc. | Rmc-6291 for use in the treatment of ras protein-related disease or disorder |
| US12448400B2 (en) | 2023-09-08 | 2025-10-21 | Gilead Sciences, Inc. | KRAS G12D modulating compounds |
| WO2025080946A2 (en) | 2023-10-12 | 2025-04-17 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2025171296A1 (en) | 2024-02-09 | 2025-08-14 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2025237091A1 (en) * | 2024-05-17 | 2025-11-20 | 中山优理生物医药有限公司 | Preparation method for spiro compound, and intermediate thereof |
| WO2025240847A1 (en) | 2024-05-17 | 2025-11-20 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2025255438A1 (en) | 2024-06-07 | 2025-12-11 | Revolution Medicines, Inc. | Methods of treating a ras protein-related disease or disorder |
| WO2025265060A1 (en) | 2024-06-21 | 2025-12-26 | Revolution Medicines, Inc. | Therapeutic compositions and methods for managing treatment-related effects |
| WO2026006747A1 (en) | 2024-06-28 | 2026-01-02 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2026015790A1 (en) | 2024-07-12 | 2026-01-15 | Revolution Medicines, Inc. | Methods of treating a ras related disease or disorder |
| WO2026015801A1 (en) | 2024-07-12 | 2026-01-15 | Revolution Medicines, Inc. | Methods of treating a ras related disease or disorder |
| WO2026015825A1 (en) | 2024-07-12 | 2026-01-15 | Revolution Medicines, Inc. | Use of ras inhibitor for treating pancreatic cancer |
| WO2026015796A1 (en) | 2024-07-12 | 2026-01-15 | Revolution Medicines, Inc. | Methods of treating a ras related disease or disorder |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN115197245A (en) | Kras inhibitor and preparation method thereof | |
| CN111704611B (en) | Aryl spiro SHP2 inhibitor compound, preparation method and application | |
| AU2020337938B2 (en) | KRas G12D inhibitors | |
| CN112300194B (en) | Condensed ring pyridone compounds, preparation method and application | |
| CN111153901B (en) | Nitrogen-containing fused heterocyclic SHP2 inhibitor compound, preparation method and application | |
| CN104837829B (en) | Inhibitor compound | |
| JP6726677B2 (en) | Substituted 2-H-pyrazole derivatives as anticancer agents | |
| WO2023151621A1 (en) | Compound having anti-kras mutant tumor activity | |
| WO2024002373A1 (en) | Substituted pyrimidine-fused ring inhibitor, method for preparing same, and use thereof | |
| TW202417455A (en) | Novel triheterocyclic compounds and pharmaceutical composition | |
| CN112300196A (en) | Piperidine condensed ring compound, preparation method and application | |
| CN115427035B (en) | ENL/AF9 YEATS inhibitors | |
| TW202102509A (en) | Compounds targeting prmt5 | |
| EP4206197B1 (en) | Preparation method for novel rho-related protein kinase inhibitor and intermediate in preparation method | |
| EP3556758B1 (en) | 1,2-dihydro-1,6-naphthyridin-2-one derivatives as cdk4/6 inhibitors | |
| CN114805311B (en) | Spiroindene | |
| CN118994158A (en) | Nitrogen-containing heterocyclic compound, preparation method and application | |
| JP2021501738A (en) | Aminopyrazolopyrimidine-containing macrocycles and their pharmaceutical compositions, as well as their use | |
| CN116323562B (en) | A class of compounds having kinase inhibitory activity | |
| JP7663968B2 (en) | WDR5 inhibitors and modulators | |
| JP7675136B2 (en) | Macrolide derivatives, their production method and use | |
| WO2023224894A9 (en) | Macrocycles as lrrk2 inhibitors, pharmaceutical compositions, and uses thereof | |
| JP2022538901A (en) | Pyrazolone condensed pyrimidine compound, its production method and use | |
| WO2022174765A1 (en) | Fused ring compound as wee-1 inhibitor | |
| CN114790206A (en) | Cyclin-dependent kinase inhibitor and preparation method thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| WD01 | Invention patent application deemed withdrawn after publication | ||
| WD01 | Invention patent application deemed withdrawn after publication |
Application publication date: 20221018 |