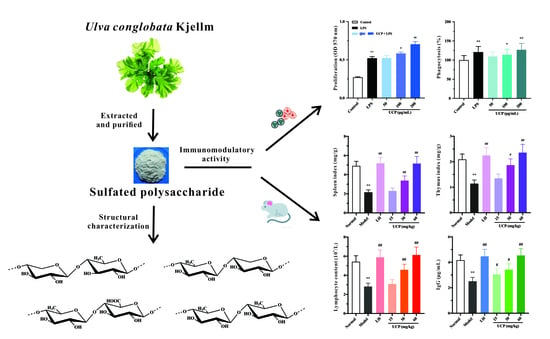
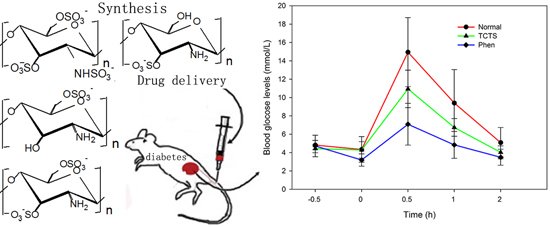
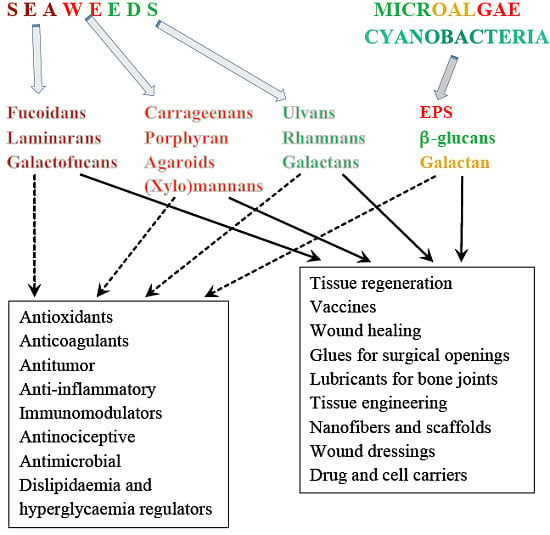
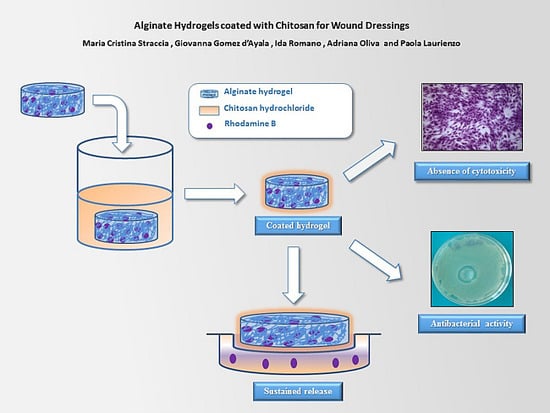
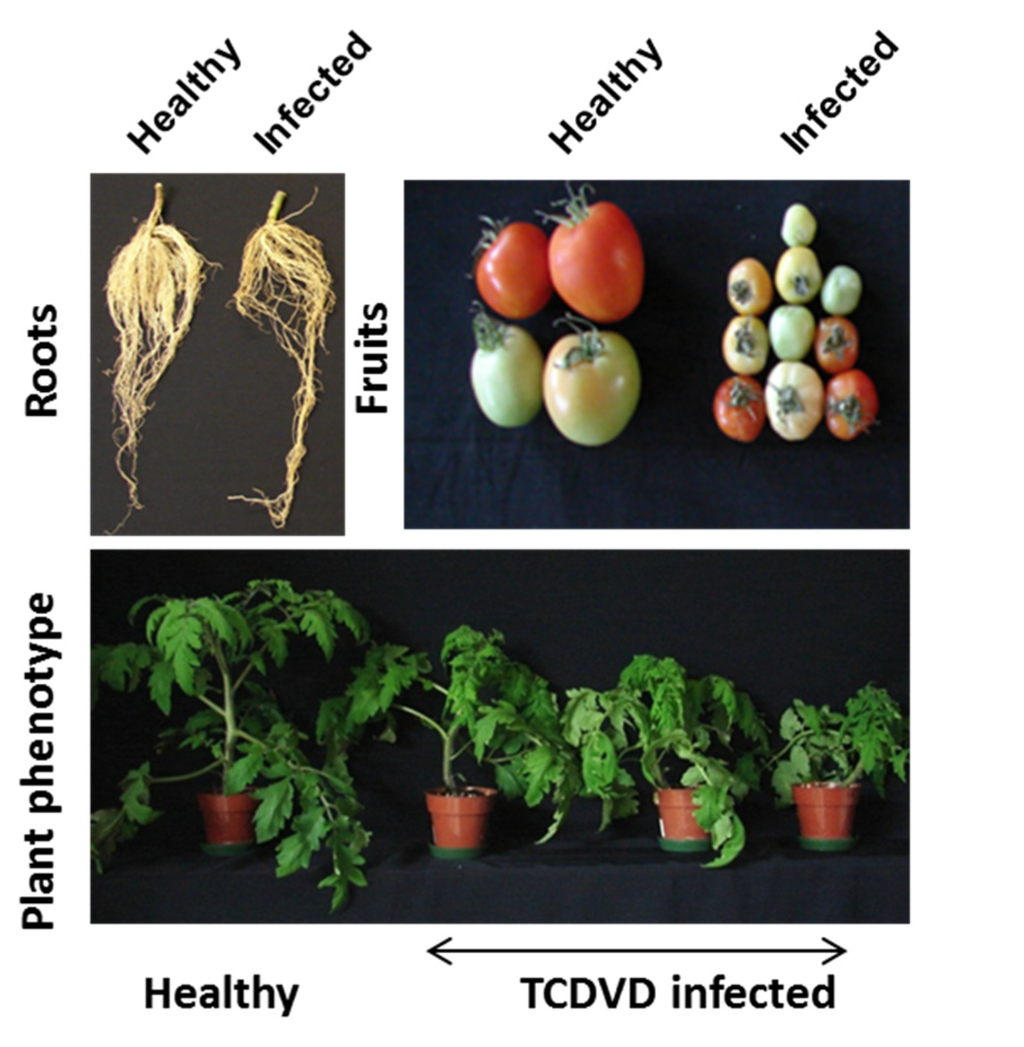
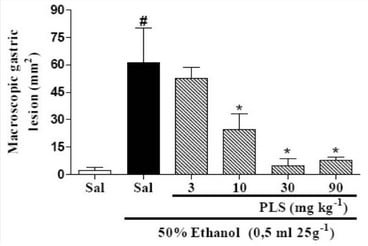
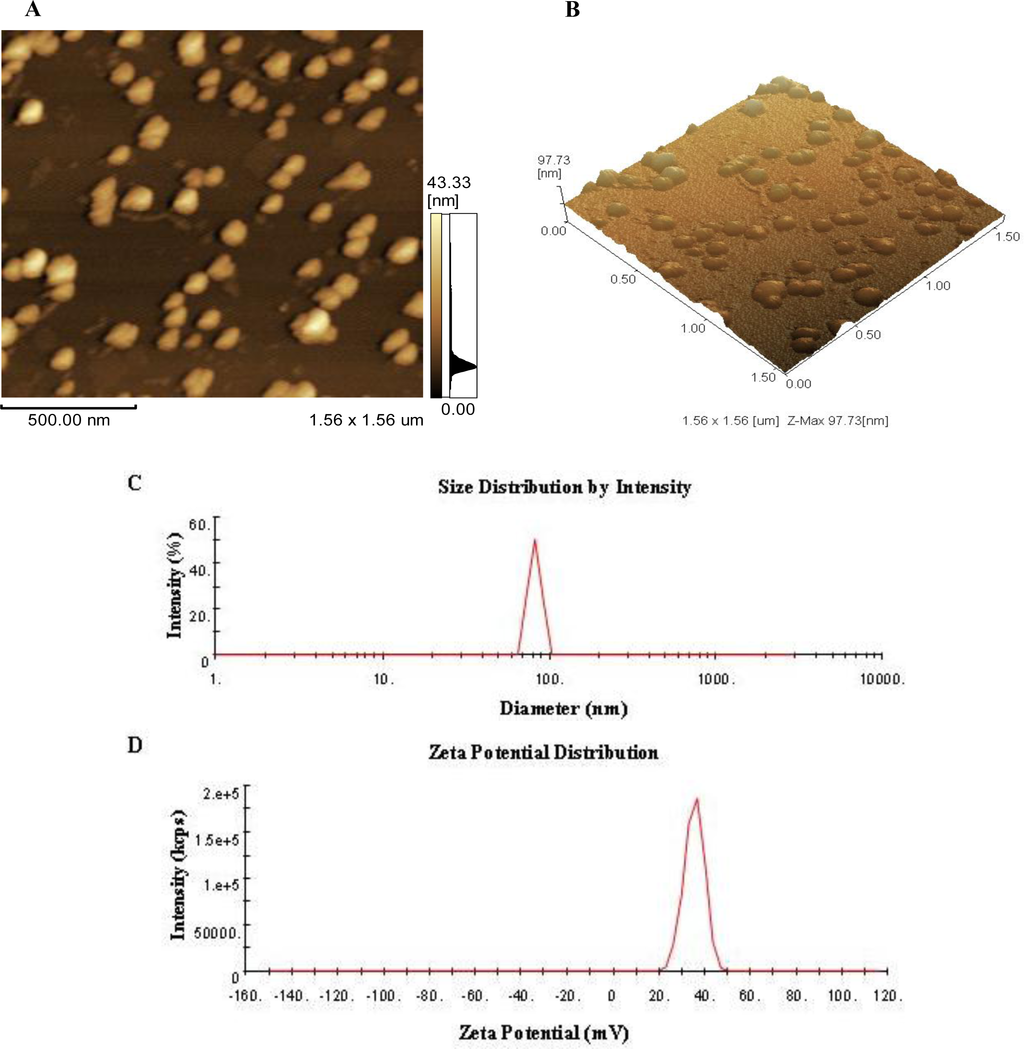
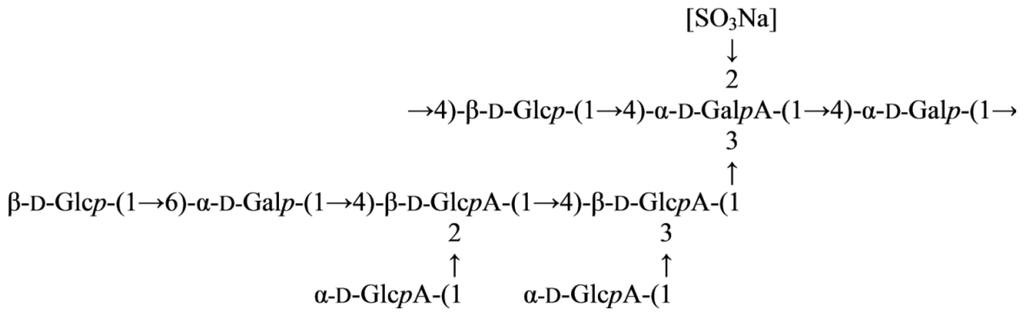<p>Single-factor test. (<b>a</b>) NaOH mass fraction. (<b>b</b>) Solid-to-liquid ratio. (<b>c</b>) Ultrasound time. (<b>d</b>) Ultrasonic power. (<b>e</b>) Water bath time. The data are expressed as mean ± standard deviation of at least 3 independent experiments (3 replicates).</p> Full article ">Figure 2
<p>The effects of mass fraction of NaOH, ultrasonic power, and solid–liquid ratio on the extraction rate of polysaccharide were studied. A: mass fraction of NaOH; B: ultrasonic power; C: solid–liquid ratio.</p> Full article ">Figure 3
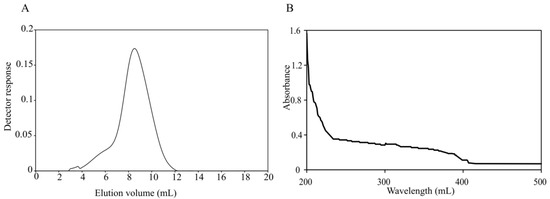
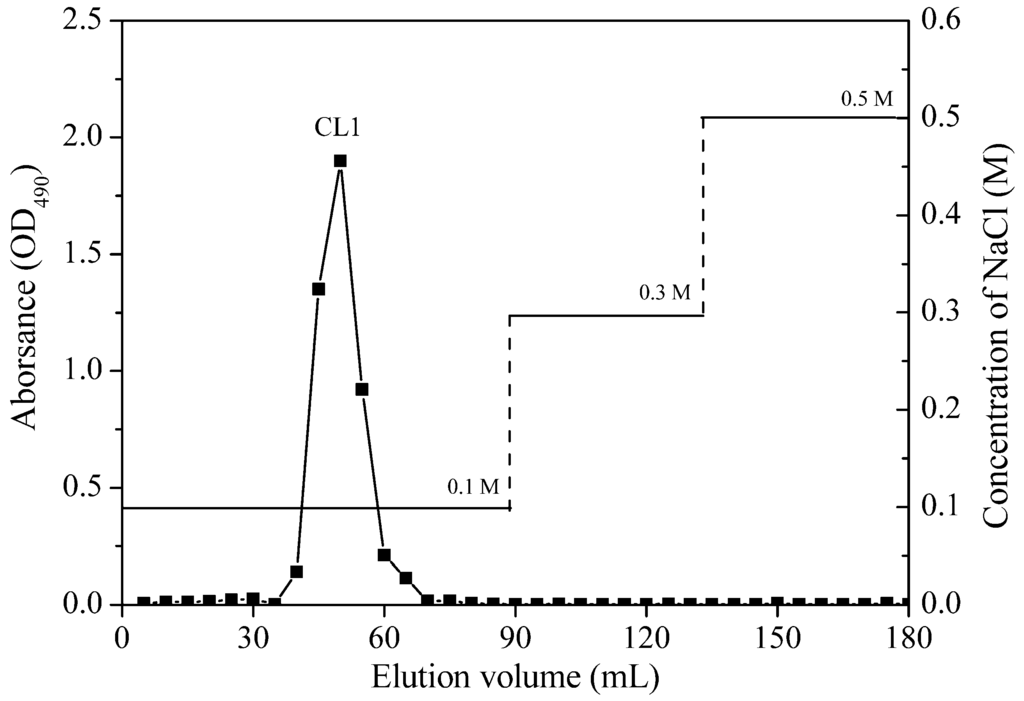
<p>The elution profile of crude polysaccharide from <span class="html-italic">C. reinhardtii</span> on DEAE-cellulose anion exchange chromatography column. The black curve represents the elution curve of polysaccharides measured by the anthrone sulfuric acid method. The red curve represents different concentrations of NaCl.</p> Full article ">Figure 4
<p>Ultraviolet spectra of <span class="html-italic">C. reinhardtii</span> polysaccharides. There is no absorption peak at 260 nm or 280 nm.</p> Full article ">Figure 5
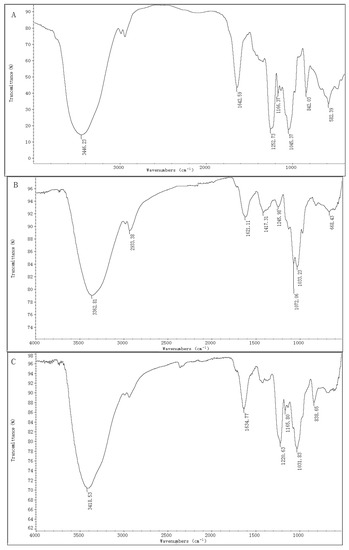
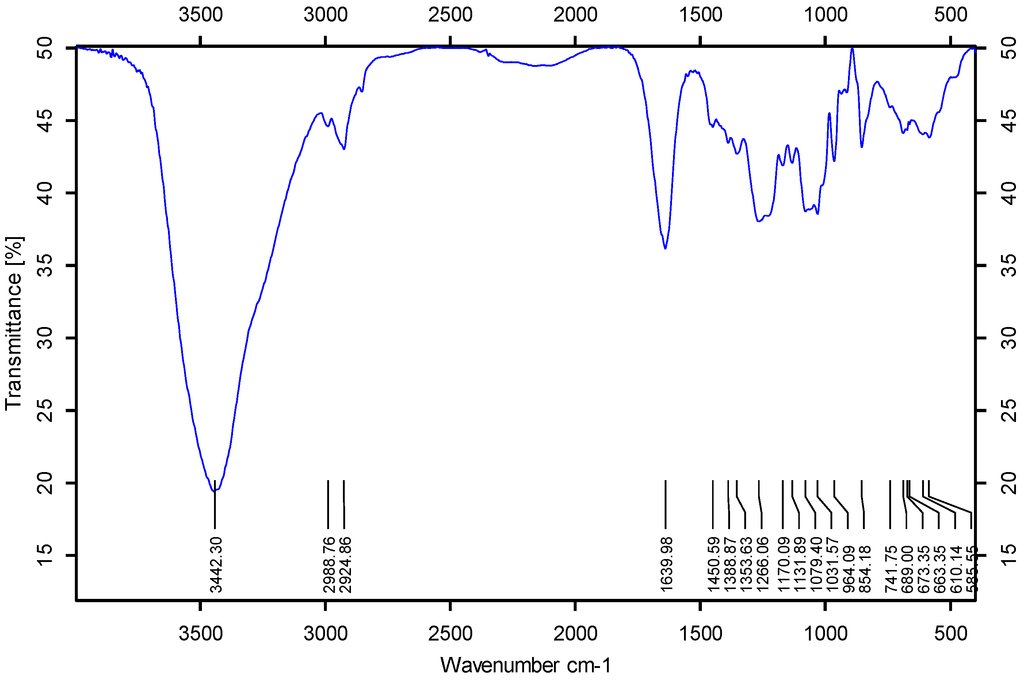
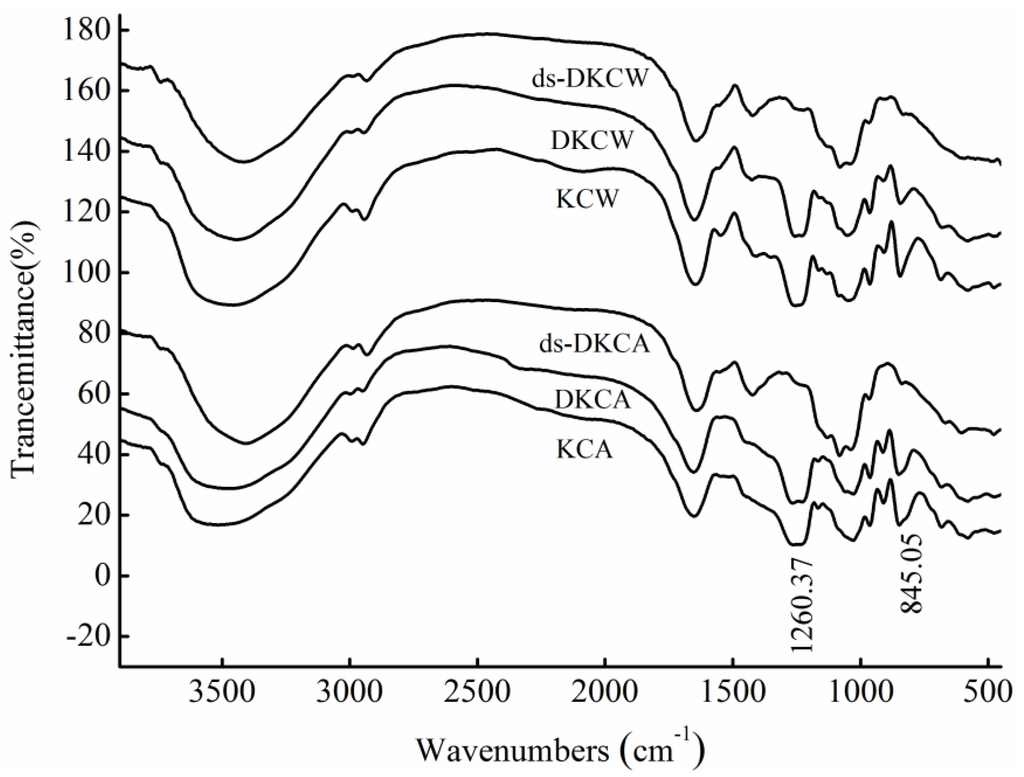
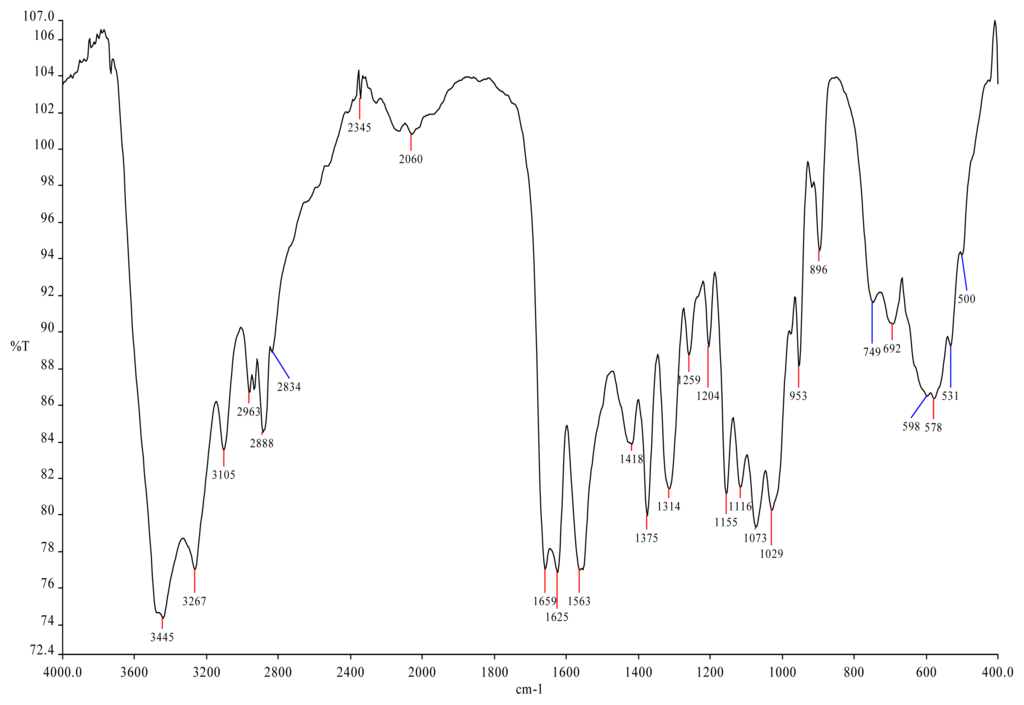
<p>Infrared spectra of four purified polysaccharides. (<b>a</b>) CRP-1. (<b>b</b>) CRP-2. (<b>c</b>) CRP-3. (<b>d</b>) CRP-4.</p> Full article ">Figure 6
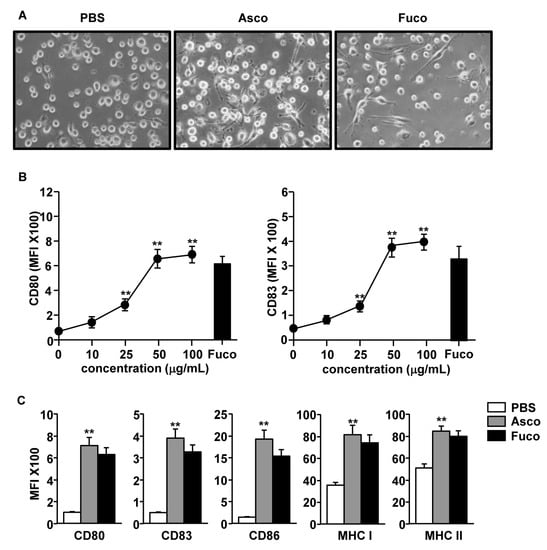
<p>Toxic effect of <span class="html-italic">C. reinhardtii</span> polysaccharide on Vero cells. There was no cytotoxicity to Vero cells after treatment with CRP-1, CRP-2, CRP-3 and CRP-4 for 24 h. The data are expressed as mean ± standard deviation of at least 3 independent experiments (3 replicates).</p> Full article ">Figure 7
<p>Effects of <span class="html-italic">C. reinhardtii</span> polysaccharide on the proliferation of different tumor cells. HCT-116, HeLa, and HepG2 cells were treated with four purified polysaccharides for 24 h, and the proliferation of tumor cells was inhibited. (<b>a</b>) HCT-116 cells. (<b>b</b>) HeLa cells. (<b>c</b>) HepG-2 cells. The data are expressed as mean ± standard deviation of at least 3 independent experiments (3 replicates).</p> Full article ">Figure 8
<p>The effect of CRP-4 on the morphology of cervical cancer HeLa cells. (<b>a</b>) Control; (<b>b</b>) 0.1 mg/mL CRP-4; (<b>c</b>) 0.3 mg/mL CRP-4; (<b>d</b>) 0.5 mg/mL CRP-4; (<b>e</b>) 1 mg/mL CRP-4. After HeLa cells were treated with CRP-4 for 24 h, HeLa cells showed different morphological changes compared with the control group, including morphological abnormalities, increased cell gap, cell shrinkage, blurred contour, and darkening of luster. Microscope magnification is 200×.</p> Full article ">Figure 9
<p>Effect of <span class="html-italic">C. reinhardtii</span> polysaccharide on migration of cervical cancer HeLa cells. Compared with the control group, the migration ability of HeLa cells was inhibited after treatment with four purified polysaccharides for 24 h. Microscope magnification is 100×.</p> Full article ">Figure 10
<p>The effects of four purified polysaccharides on the migration of cervical cancer HeLa cells. The scratches were quantitatively analyzed by Image J 1.8 software and similar results were obtained from two other independent experiments. The data are expressed as mean ± standard deviation of at least 3 independent experiments (3 replicates). An asterisk (*) indicates a significant difference (<span class="html-italic">p</span> < 0.05), while three asterisks (***) indicate a highly significant difference (<span class="html-italic">p</span> < 0.01).</p> Full article ">Figure 11
<p>DAPI staining was used to observe the effect of <span class="html-italic">C. reinhardtii</span> polysaccharide on the apoptosis morphology of cervical cancer HeLa cells. Red arrows represent chromatin condensation, nuclear fragmentation, formation of apoptotic bodies, bright blue aggregates or dots.</p> Full article ">