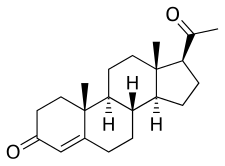
Progesterone
| |
| |
| Names | |
|---|---|
| IUPAC name
(8S,9S,10R,13S,14S,17S)-17-acetyl-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.318 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C21H30O2 | |
| Molar mass | 314.469 g/mol |
| Melting point | 126 |
| log P | 4.04[3] |
| Pharmacology | |
| G03DA04 (WHO) | |
| By mouth, topical/transdermal, vaginal, intramuscular injection, subcutaneous injection, subcutaneous implant | |
| Pharmacokinetics: | |
| OMP: <10%[4][5] | |
| • Albumin: 80% • CBG: 18% • SHBG: <1% • Free: 1–2%[6][7] | |
| Hepatic (CYP2C19, CYP3A4, CYP2C9, 5α-reductase, 3α-HSD, 17α-hydroxylase, 21-hydroxylase, 20α-HSD)[8][9] | |
| OMP: 16–18 hours[4][5][10] IM: 22–26 hours[5][11] SC: 13–18 hours[11] | |
| Renal | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Progesterone is a steroid hormone.
In animals, it is made from cholesterol. It is the base for certain estrogens and androgens. In animals, it is produced by their body mainly during the second half of the menstrual cycle.
During pregnancy it is produced in higher quantities. Willard Myron Allen co-discovered progesterone with George Washington Corner at the University of Rochester Medical School in 1933. Allen discovered the temperature needed to melt it and its weight. He also gave it the name "Progesterone" based on progestational steroidal ketone.[12] In one plant, Juglans regia, progesterone has been found.[13] Also progesterone-like steroids are found in Dioscorea mexicana.[14] Progesterone level is low in children and postmenopausal women.[15]
Biosynthesis
[change | change source]
Related pages
[change | change source]References
[change | change source]- ↑ Adler N, Pfaff D, Goy RW (6 Dec 2012). Handbook of Behavioral Neurobiology Volume 7 Reproduction (1st ed.). New York: Plenum Press. p. 189. ISBN 978-1-4684-4834-4. Retrieved 4 July 2015.
- ↑ "progesterone (CHEBI:17026)". ChEBI. European Molecular Biology Laboratory-EBI. Retrieved 4 July 2015.
- ↑ "Progesterone_msds".
- ↑ 4.0 4.1 Stanczyk FZ (September 2002). "Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception". Reviews in Endocrine & Metabolic Disorders. 3 (3): 211–24. doi:10.1023/A:1020072325818. PMID 12215716. S2CID 27018468.
- ↑ 5.0 5.1 5.2 Simon JA, Robinson DE, Andrews MC, Hildebrand JR, Rocci ML, Blake RE, Hodgen GD (July 1993). "The absorption of oral micronized progesterone: the effect of food, dose proportionality, and comparison with intramuscular progesterone". Fertility and Sterility. 60 (1): 26–33. doi:10.1016/S0015-0282(16)56031-2. PMID 8513955.
- ↑ Fritz MA, Speroff L (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 44–. ISBN 978-1-4511-4847-3.
- ↑ Marshall WJ, Marshall WJ, Bangert SK (2008). Clinical Chemistry. Elsevier Health Sciences. pp. 192–. ISBN 978-0-7234-3455-9.
- ↑ Yamazaki H, Shimada T (October 1997). "Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes". Archives of Biochemistry and Biophysics. 346 (1): 161–9. doi:10.1006/abbi.1997.0302. PMID 9328296.
- ↑ McKay GA, Walters MR (6 February 2013). Lecture Notes: Clinical Pharmacology and Therapeutics. John Wiley & Sons. p. 33. ISBN 978-1-118-34489-7.
- ↑ Zutshi (1 January 2005). Hormones in Obstetrics and Gynaecology. Jaypee Brothers Publishers. p. 74. ISBN 978-81-8061-427-9. Archived from the original on 6 May 2016. Retrieved 5 March 2019.
- ↑ 11.0 11.1 Cometti B (November 2015). "Pharmaceutical and clinical development of a novel progesterone formulation". Acta Obstetricia et Gynecologica Scandinavica. 94 (Suppl 161): 28–37. doi:10.1111/aogs.12765. PMID 26342177. S2CID 31974637.
- ↑ http://pt.wkhealth.com/pt/re/lwwgateway/landingpage.htm;jsessionid=R2dc47m048bxLF2j9vg9nZGkV4LPQ9X4PTkhCqCVdsC7dJrhTPPm!1612949135!181195629!8091!-1?sid=WKPTLP:landingpage&an=00007611-197010000-00012
- ↑ Pauli, Guido F.; Friesen, J. Brent; Gödecke, Tanja; Farnsworth, Norman R.; Glodny, Bernhard (2010). "Occurrence of Progesterone and related animal steroids in two higher plants". Journal of Natural Products. 73 (3): 338–345. doi:10.1021/np9007415. PMID 20108949.
- ↑ http://buynaturalprogesterone.co.uk/
- ↑ "Historical Reference Ranges". Archived from the original on 2009-01-09. Retrieved 2013-03-11.
- ↑ Häggström M, Richfield D (2014). "Diagram of the pathways of human steroidogenesis". WikiJournal of Medicine. 1 (1). doi:10.15347/wjm/2014.005. ISSN 2002-4436.