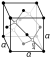Caurbon
The "Scots" that wis uised in this airticle wis written bi a body that haesna a guid grip on the leid. Please mak this airticle mair better gin ye can. |
 Graphite (left) an diamont (richt), twa allotropes o caurbon | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carbon | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | clear (diamond) & black (graphite) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar, std(C) | [12.0096, 12.0116] conventional: 12.011 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carbon in the periodic cairt | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic nummer (Z) | 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 14 (caurbon group) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | p-block | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Reactive nonmetal | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron confeeguration | [He] 2s2 2p2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pheesical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solit | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sublimation pynt | 3915 K (3642 °C, 6588 °F) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | amorphous:[1] 1.8–2.1 g/cm3 diamond: 3.515 g/cm3 graphite: 2.267 g/cm3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treeple pynt | 4600 K, 10800[2][3] kPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat o fusion | 117 (graphite) kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 6.155 (diamond) 8.517 (graphite) J/(mol·K) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −4, −3, −2, −1, 0, +1,[4] +2, +3,[5] +4[6] (a mildly acidic oxide) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.55 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionisation energies |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 77(sp³), 73(sp²), 69(sp) pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 170 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ither properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naitural occurrence | primordial | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creestal structur | diamond cubic (diamond, clear) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed o soond thin rod | 18350 (diamond) m/s (at 20 °C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 0.8 (diamond)[7] µm/(m·K) (at 25 °C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 900-2300 (diamond) 119-165 (graphite) W/(m·K) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic orderin | diamagnetic[8] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 1050 (diamond)[7] GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 478 (diamond)[7] GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 442 (diamond)[7] GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.1 (diamond)[7] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs haurdness | 10 (diamond) 1-2 (graphite) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Nummer | 7440-44-0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diskivery | Egyptians an Sumerians[9] (3750 BC) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Recognized as an element bi | Antoine Lavoisier[10] (1789) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Main isotopes o carbon | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Caurbon (frae Laitin: carbo "coal") is the chemical element wi seembol C an atomic nummer 6. As a member o group 14 on the periodic table, it is nonmetallic an tetravalent—makin fower electrons available tae furm covalent chemical bonds. Thare are three naiturally occurrin isotopes, wi 12C an 13C bein stable, while 14C is radioactive, decayin wi a hauf-life o aboot 5,730 years.[14] Caurbon is ane o the few elements kent syne antiquity.[15]
Thare are several allotropes o caurbon o which the baist kent are graphite, diamond, an amorphous caurbon.[16] The pheesical properties o caurbon vary widely wi the allotropic furm. For example, diamond is heichly transparent, while graphite is opaque an black. Diamond is the hairdest naiturally-occurrin material kent, while graphite is saft enough tae furm a streak on paper (hence its name, frae the Greek wird "γράφω" which means "tae write"). Diamond haes a very law electrical conductivity, while graphite is a very guid conductor. Unner normal condeetions, diamond, caurbon nanotube an graphene hae the heichest thermal conductivities o aw kent materials.
Aw caurbon allotropes are solits unner normal condeetions wi graphite bein the maist thermodynamically stable furm. Thay are chemically resistant an require heich temperatur tae react even wi oxygen. The maist common oxidation state o caurbon in inorganic compounds is +4, while +2 is foond in caurbon monoxide an ither transeetion metal carbonyl complexes. The lairgest soorces o inorganic caurbon are limestanes, dolomites an caurbon dioxide, but signeeficant quantities occur in organic deposits o coal, peat, ile an methane clathrates. Caurbon furms mair compoonds than ony ither element, wi almaist ten million pure organic compoonds describit tae date, which in turn are a tiny fraction o sic compoonds that are theoretically possible unner staundart condeetions.[17]
Caurbon is the 15t maist abundant element in the Yird's crust, an the fowert maist abundant element in the universe bi mass efter hydrogen, helium, an oxygen. It is present in aw kent life furms, an in the human bouk caurbon is the seicont maist abundant element bi mass (aboot 18.5%) efter oxygen.[18] This abundance, thegither wi the unique diversity o organic compoonds an thair unuisual polymer-furmin ability at the temperaturs commonly encoontered on Yird, mak this element the chemical basis o aw kent life.
References
[eedit | eedit soorce]- ↑ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ↑ Haaland, D (1976). "Graphite-liquid-vapor triple point pressure and the density of liquid carbon". Carbon. 14 (6): 357. doi:10.1016/0008-6223(76)90010-5.
- ↑ Savvatimskiy, A (2005). "Measurements of the melting point of graphite and the properties of liquid carbon (a review for 1963–2003)". Carbon. 43 (6): 1115. doi:10.1016/j.carbon.2004.12.027.
- ↑ "Fourier Transform Spectroscopy of the Electronic Transition of the Jet-Cooled CCI Free Radical" (PDF). Retrieved 6 December 2007.
- ↑ "Fourier Transform Spectroscopy of the System of CP" (PDF). Retrieved 6 December 2007.
- ↑ "Carbon: Binary compounds". Retrieved 6 December 2007.
- ↑ a b c d e Properties of diamond, Ioffe Institute Database
- ↑ Magnetic susceptibility of the elements and inorganic compounds, in Handbook of Chemistry and Physics 81st edition, CRC press.
- ↑ "History of Carbon and Carbon Materials - Center for Applied Energy Research - University of Kentucky". Caer.uky.edu. Retrieved 12 September 2008.
- ↑ Senese, Fred (200-09-09). "Who discovered carbon?". Frostburg State University. Retrieved 2007-11-24. Check date values in:
|date=(help) - ↑ "Fourier Transform Spectroscopy of the System of CP" (PDF). Retrieved 6 December 2007.
- ↑ "Fourier Transform Spectroscopy of the Electronic Transition of the Jet-Cooled CCI Free Radical" (PDF). Retrieved 6 December 2007.
- ↑ "Carbon: Binary compounds". Retrieved 6 December 2007.
- ↑ "Carbon – Naturally occurring isotopes". WebElements Periodic Table. Retrieved 9 October 2008.
- ↑ "History of Carbon". Archived frae the original on 1 November 2012. Retrieved 10 Januar 2013.
- ↑ "World of Carbon – Interactive Nano-visulisation in Science & Engineering Education (IN-VSEE)". Archived frae the original on 5 October 2008. Retrieved 9 October 2008.
- ↑ Chemistry Operations (15 December 2003). "Carbon". Los Alamos National Laboratory. Archived frae the original on 13 September 2008. Retrieved 9 October 2008.
- ↑ "Biological Abundance of Elements". The Internet Encyclopedia of Science. Retrieved 9 October 2008.
Freemit airtins
[eedit | eedit soorce]- Carbon on In Our Time at the BBC. (listen now)
- Carbon at The Periodic Table o Videos (Varsity o Nottingham)
- Carbon on Britannica
- Extensive Carbon page at asu.edu Archived 2010-06-18 at the Wayback Machine
- Electrochemical uises o carbon Archived 2001-11-09 at the Library of Congress Web Archives
- Carbon – Super Stuff. Animation wi soond an interactive 3D-models. Archived 2012-11-09 at the Wayback Machine