Molecule of the Month: Citric Acid Cycle
Eight enzymes form a cyclic pathway for energy production and biosynthesis

Eight Reactions
Powerhouse of Energy

Citrate Synthase

Aconitase

Isocitrate Dehydrogenase

2-Oxoglutarate Dehydrogenase Complex

Succinyl-CoA Synthetase

Succinate Dehydrogenase

Fumarase

Malate Dehydrogenase
Exploring the Structure
Allosteric motion of citrate synthase
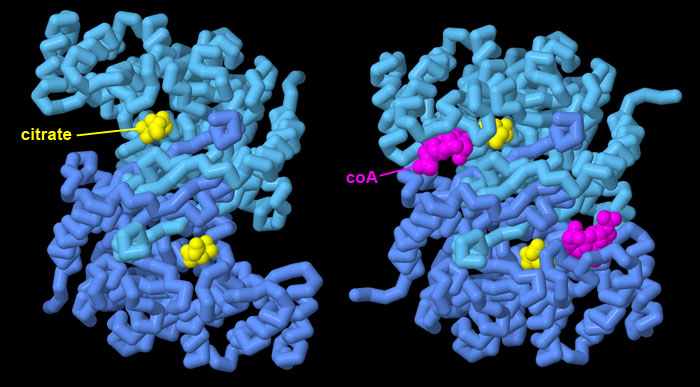
Citrate synthase is an allosteric enzyme that changes shape in the course of its catalytic reaction. The open state, shown here on the left from PDB entry 1cts, captures the substrates and releases the products. In this structure, the product citrate is still bound in the active site. The closed state, shown on the right from PDB entry 2cts, performs the reaction, bringing coenzyme A (magenta) and its attached acetyl group close to oxaloacetate to form citrate. This structure captures the complex after the reaction is completed. Click on the JSmol tab to explore this structural transition in more detail.
Topics for Further Discussion
- In many cases, the citric acid cycle enzymes are very similar when you compare the forms made by different organisms. You can use the Compare Structures tool to look for similarities and differences in the structures available in the PDB.
Related PDB-101 Resources
- Browse Biological Energy
- Browse Enzymes
- Browse Peak Performance
References
- D. Voet and J. G. Voet (2011) Biochemistry, 4th Edition. John Wiley and Sons
- O. Yogev, A. Naamati and O. Pines (2011) Fumarase: a paradigm of dual targeting and dual localized functions. FEBS Journal 278, 4230-4242
- J. Rutter, D. R. Winge and J. D. Schiffman (2010) Succinate dehydrogenase -- assembly, regulation and role in human disease. Mitochondrion 10, 393-401
- M. E. Fraser, K. Hayakawa, M. S. Hume, D. G. Ryan and E. R. Brownie (2006) Interactions of GTP with the ATP-grasp domain of GTP-specific succinyl-CoA synthetase. Journal of Biological Chemistry 281, 11058-11065
- G. E. Murphy and G. J. Jensen (2005) Electron cytotomography of the E. coli pyruvate and 2-oxoglutarate dehydrogenase complexes. Structure 13, 1765-1773
- P. Minarik, N. Tomaskova, M. Kollarova and M. Antalik (2002) Malate dehydrogenases -- structure and function. General Physiology and Biophysics 21, 257-265
October 2012, David Goodsell
http://doi.org/10.2210/rcsb_pdb/mom_2012_10


