WO2020185856A1 - Methods for increasing platelet production - Google Patents
Methods for increasing platelet production Download PDFInfo
- Publication number
- WO2020185856A1 WO2020185856A1 PCT/US2020/022023 US2020022023W WO2020185856A1 WO 2020185856 A1 WO2020185856 A1 WO 2020185856A1 US 2020022023 W US2020022023 W US 2020022023W WO 2020185856 A1 WO2020185856 A1 WO 2020185856A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- vincristine
- megakaryocyte
- vinblastine
- cell
- aurora kinase
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/14—Blood; Artificial blood
- A61K35/19—Platelets; Megacaryocytes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/475—Quinolines; Isoquinolines having an indole ring, e.g. yohimbine, reserpine, strychnine, vinblastine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/14—Blood; Artificial blood
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P41/00—Drugs used in surgical methods, e.g. surgery adjuvants for preventing adhesion or for vitreum substitution
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/04—Antihaemorrhagics; Procoagulants; Haemostatic agents; Antifibrinolytic agents
Definitions
- the field of the invention relates to methods and compositions for increasing platelet production from megakaryocytes.
- Platelets are tiny blood cells that play an integral role in blood clotting.
- platelets which are also called“thrombocytes,” are involved in inflammation, blood vessel growth, and tumor metastasis. The entire platelet population is replaced every 10 days, thus requiring a high amount of ongoing platelet production.
- Platelet supplementation is necessary in a variety of clinical settings including (i) in the treatment of cancer, (ii) surgery, (iii) serious injuries or trauma, and (iv) in the treatment of blood disorders and transplants.
- a megakaryocyte e.g., an in vitro cultured megakaryocyte or immortalized megakaryocyte.
- a method for increasing platelet production from a megakaryocyte comprising: contacting a megakaryocyte (MK) or population thereof with an effective amount of a vinca alkaloid, such as vincristine, vinblastine, vinorelbine, or vindesine, or an Aurora kinase inhibitor (e.g., CCT137690).
- a vinca alkaloid such as vincristine, vinblastine, vinorelbine, or vindesine
- an Aurora kinase inhibitor e.g., CCT137690
- the Aurora kinase inhibitor is CCT137690, ZM447439, Hesperadin, VX-680, barasertib, alisertib, danusertib, AT9283, PF- 03914735, or AMG 900, among others.
- vincristine and/or vinblastine is used in combination with at least one Aurora kinase inhibitor.
- the method comprises contacting with a combination of agents (e.g., at least two, 3, 4, 5, or 6 different agents).
- a combination of agents specifically includes the following: CCT137690 & vincristine, CCT137690 & vinblastine, vinblastine & vincristine, vinblastine & vincristine & CCT137690, vincristine and/or vinblastine & barasertib, vincristine and/or vinblastine & PF03814735, vincristine and/or vinblastine & ZM447434, and the like.
- the effect of the combination of agents is additive.
- the combination of agents produces a synergistic effect.
- a combination comprising an Aurora kinase inhibitor, such as CCT137690, with vincristine can produce a synergistic effect on platelet production.
- the different agents can be contacted with the megakaryocyte or population thereof at the same time, i.e., simultaneously, or the different agents can be contacted with the megakaryocyte or population thereof at different times, i.e., sequentially.
- two or more different agents are contacted with the megakaryocyte or population thereof at the same time.
- the agents e.g., vinblastine and/or vincristine, and the Aurora kinase inhibitor is contacted with the megakaryocyte or population thereof at different times.
- the megakaryocyte or population thereof is contacted with the Aurora kinase inhibitor for a period of time before the megakaryocyte or population thereof is contacted with vinblastine and/or vincristine.
- the time between contacting with the Aurora kinase inhibitor and vinblastine and/or vincristine is from a few minutes to days.
- the time between contacting with the Aurora kinase inhibitor and vinblastine and/or vincristine can be 30 minutes, 60 minutes, 2 hours, 5 hours, 12 hours, 18 hours, 24 hours, 2 days, 3 days, 4 days or more. In some embodiments of this aspect and all other aspects provided herein, the time between contacting with the Aurora kinase inhibitor and vinblastine and/or vincristine is about 3 days.
- the combination of the Aurora kinase inhibitor e.g., CCT137690
- vincristine synergist ically increases platelet production.
- the combination of the Aurora kinase inhibitor e.g., CCT137690
- vinblastine synergist ically increases platelet production.
- control wells e.g., wells containing imMKCLs that were not exposed to CCT137690 and/or vincristine
- agent e.g., CCT137690 or vincristine alone
- combination of agents e.g. CCT137690 and vincristine treated imMKCL wells
- the method is performed in vitro.
- platelet production is increased by at least 10% compared to an untreated control.
- the untreated control comprises a substantially similar megakaryocyte or population thereof that is not contacted with a vinca alkaloid, such as vincristine or vinblastine, or an Aurora kinase inhibitor.
- the increase in platelet production is an increase in platelet number.
- the increase in platelet number is an increase in the number of platelets per megakaryocyte (plate lets/MK).
- the population of megakaryocytes is a homogenous population.
- the effective amount of vincristine, vinblastine and/or the aurora kinase is between O.OImM - IOOmM, inclusive.
- the effective amount of vincristine, vinblastine and/or the aurora kinase is between 0.1mM-5mM.
- the effective amount of vincristine, vinblastine and/or the aurora kinase is between 0.75mM- 1.50mM.
- the Aurora kinase inhibitor comprises CCT137690.
- CCT137690 is a potent inhibitor of Aurora kinases with IC50 values of 0.015, 0.019 and 0.025 mM for Aurora A, Aurora C and Aurora B, respectively.
- CCT137690 possesses antiproliferative activity in various human tumor cell lines.
- the megakaryocyte or population thereof is grown on a substrate.
- the megakaryocyte or population thereof is grown, e.g., on a substrate, for a period time before contacting with vincristine, vinblastine, vinorelbine, or vindesine, or the Aurora kinase inhibitor.
- the megakaryocyte or population thereof can be grown for a few minutes to days before contacting with vincristine, vinblastine, vinorelbine, or vindesine, or the Aurora kinase inhibitor.
- the megakaryocyte or population thereof can be grown for 30 minutes, 60 minutes, 2 hours, 5 hours, 12 hours, 18 hours, 24 hours, 2 days, 3 days, 4 days or more before contacting with vincristine, vinblastine, vinorelbine, or vindesine, or the Aurora kinase inhibitor.
- the megakaryocyte or population thereof can be grown for about 3 days before contacting with vincristine, vinblastine, vinorelbine, or vindesine, or the Aurora kinase inhibitor
- the substrate comprises laminin, fibronectin, Poly-L-Lysine, or methylcellulose.
- the number of platelets is counted using FACS or a burst/spreading assay.
- the megakaryocyte or population thereof is contacted with an effective amount of both vincristine and vinblastine, and optionally an effective amount of an Aurora kinase inhibitor (e.g., CCT137690).
- an Aurora kinase inhibitor e.g., CCT137690.
- the megakaryocyte cell or population thereof is an immortalized megakaryocyte cell or population thereof.
- the vincristine, vinblastine and/or Aurora kinase is contacted with the megakaryocyte cell(s) after induction of terminal differentiation.
- the vincristine, vinblastine, and/or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) at least one day after induction of terminal differentiation.
- the vincristine, vinblastine and/or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) from day 3 after induction of terminal differentiation.
- the vincristine, vinblastine and/or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) until functional platelets are generated.
- the vincristine, vinblastine, and/or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) until a maximal output of functional platelets are generated.
- kits for increasing platelet production comprising: vincristine, vinblastine, and/or an Aurora kinase inhibitor, instructions for use thereof, and at least one of the following: (i) laminin substrate, (ii) megakaryocyte cells, or (iii) positive control comprising human platelets or platelet lysates in a known amount.
- the megakaryocyte cells are immortalized megakaryocyte cells.
- the megakaryocyte or population thereof is grown on a baffled surface.
- the megakaryocyte or population thereof is grown on a flat surface.
- FIG. 1 depicts an exemplary workflow for a high-throughput CV7000 imaging screen to detect platelet generation and pro-platelet phenotype.
- FIG. 2 depicts Fiji based identification and counting of platelet sized particles and megakaryocyte sized imMKCLs from the captured images. Depiction of normal maturation kinetics of megakaryocytes via comparison of Day6 megakaryocyte membrane content vs DNA content, Day6 megakaryocyte circularity vs DNA content, Day6 megakaryocyte Area vs DNA content.
- FIG. 3 depicts Fiji based counting of plate lets/imMKCLs to identify“hit” compounds. Also depicts screening performed to identify pro-platelet phenotype in various drug/compound treated wells.
- FIGs. 4A-4B depicts exemplary data of the “hit” compounds vincristine and vinblastine (FIG. 4A) and identification of“hit” compound-treated wells that produce a significant pro-platelet phenotype from the imMKCLs.
- Initial analyses identify vincristine and vinblastine as “hit” compounds (FIG. 4B).
- FIGs. 5A-5B depicts exemplary methods and data relating to the validation of “hit” compounds. These data show that vincristine and vinblastine treatment results in high platelet yields (FIG. 5A). Also depicted is an exemplary scheme used to validate“hit” compounds in clone 38 imMKCLs in static conditions. FACS based data shows that Vincristine and vinblastine treatment promotes platelet yields by ⁇ 3-4 fold. Vincristine and vinblastine also promote extensive pro-platelet phenotype from the imMKCLs as compared to the corresponding controls (FIG. 5B).
- FIG. 6 shows exemplary data relating to the effect of vincristine and vinblastine on platelet production in static conditions. Platelet count was performed 72h post exposure of clone 38 imMKCLs to vincristine and vinblastine.
- FIGs. 7A-7B shows exemplary data relating to the effects of vincristine and vinblastine on platelet production in shaking conditions. Platelet count was performed 72h post exposure of clone 38 imMKCLs to vincristine and/or vinblastine. This figure also shows the gating strategy used to identify and segregate platelets from contaminating imMKCLs in the culture, and identify live, non-apoptotic, CD41a+ CD42b+ platelets (FIG. 7A). Also depicted is exemplary platelet output in the form of platelet/imMKCL ratio from variously passaged clone 38 imMKCLs with and without incubation of vincristine or vinblastine. Vincristine effects 2-fold increase in platelet output as compared to the control from both flat and baffled surface flasks (FIG. 7B).
- FIG. 8 shows exemplary data relating to vincristine and vinblastine validation, which were identified using the high-throughput imaging library screen.
- FIG. 9 shows exemplary data relating to the temporal treatment of vincristine on platelet production in shaking conditions. Platelet count was performed post-treatment (C-38).
- FIG. 10 depicts an exemplary scheme used to identify compounds in clone 38 imMKCLs that promote endoreplication/endomitosis.
- FACS based data shows that CCT137690, Barasertib, PF03814735 promote endoreplication/endomitosis.
- Figure shows concentration titration as well as various biological replicates depicting that CCT137690 promotes higher endomitosis as compared to other compounds.
- FIG. 11 shows the gating strategy used to identify and segregate singlet imMKCLs on D3 in the absence of DOX from the culture.
- CD41a+CD42b+ megakaryocyte fraction was identified and measurement of ploidy in this CD41a+CD42b+ fraction was performed.
- FIG. 12 shows an exemplary combinatorial and sequential treatment plan of imMKCLs with CCT137690 and vincristine to develop a synergistic or additive effect, as compared to the flat surface imMKCL (untreated) controls, the Baffled surface imMKCL cultures treated with Vincristine and CCT137690 possess ⁇ 14-16-fold higher number of platelets
- FIG. 13 shows an exemplary scheme of“scale up” using the optimized CCT137690 and vincristine treatment plan and PBS biotech bioreactor.
- Treatment of 10 L 7 imMKCLs in lOOmL of differentiation media with the optimized CCT137690 and vincristine treatment plan enables generation of 1.2-1.5c10 L 9 platelets.
- FIG. 14 shows TEM based ultrastructure/organelle comparison of CCT137690 and vincristine treated platelets and fresh donor platelets
- FIG. 15 shows FACS based in vitro activation analyses and comparison of CCT137690 and vincristine treated imMKCL-platelets and fresh donor platelets.
- FIG. 16 shows LTA based aggregation analyses and comparison of CCT137690 and vincristine treated imMKCL-platelets and fresh donor platelets.
- FIG. 17 shows tail vein bleeding time analyses in thrombocytopenic mice (generated via busulphan treatment). This exemplary assay shows the tail vein bleeding times post infusion of CCT137690 and Vincristine treated imMKCL-platelets and fresh donor platelets.
- FIG. 18 Figure shows thrombus incorporation analyses and simultaneous comparison of CCT137690 and Vincristine treated imMKCL-platelets and fresh donor platelets.
- compositions and methods described herein are related, in part, to the discovery that vincristine, vinblastine, and/or Aurora kinase inhibitor can induce platelet production. That is, vincristine, vinblastine, an Aurora kinase inhibitor, or any combination thereof can increase the number of platelets produced by a megakaryocyte or population thereof, as compared to the number of platelets produced by a substantially similar megakaryocyte that is not treated with vincristine, vinblastine, and/or an Aurora kinase inhibitor.
- the term in v/YO-differentiated megakaryocytes refers to megakaryocyte cells that are generated in culture, typically via step-wise differentiation from a precursor cell such as a human embryonic stem cell, an induced pluripotent stem cell, an early mesoderm cell, a lateral plate mesoderm cell or a cardiac progenitor cell.
- the term“in v/Yro-differentiated megakaryocytes” excludes adult human megakaryocytes isolated from a subject (e.g., primary megakaryocytes).
- the in v/Yro-differentiated megakaryocytes are synchronized in culture.
- the term“immortalized megakaryocytes” refers to megakaryocytes that can be expanded or passaged in an in vitro culture.
- markers are used to describe a characteristic and/or phenotype of a cell. Markers can be used for selection of cells comprising characteristics of interest and can vary with specific cells. Markers are characteristics, whether morphological, structural, functional or biochemical (enzymatic) characteristics of the cell of a particular cell type, or molecules expressed by the cell type. In one aspect, such markers are proteins. Such proteins can possess an epitope for antibodies or other binding molecules available in the art. However, a marker can consist of any molecule found in or on a cell, including, but not limited to, proteins (peptides and polypeptides), lipids, polysaccharides, nucleic acids and steroids.
- morphological characteristics or traits include, but are not limited to, shape, size, and nuclear to cytoplasmic ratio.
- functional characteristics or traits include, but are not limited to, the ability to adhere to particular substrates, ability to incorporate or exclude particular dyes, ability to migrate under particular conditions, and the ability to differentiate along particular lineages.
- Markers can be detected by any method available to one of skill in the art. Markers can also be the absence of a morphological characteristic or absence of proteins, lipids etc. Markers can be a combination of a panel of unique characteristics of the presence and/or absence of polypeptides and other morphological or structural characteristics. In one embodiment, the marker is a cell surface marker.
- a stem cell as the term is defined herein, can differentiate to lineage-restricted precursor cells (such as a human hematopoietic progenitor cell), which in turn can differentiate into other types of precursor cells further down the pathway (such as a tissue specific precursor, such as a megakaryocyte progenitor cell), and then to an end-stage differentiated cell, which plays a characteristic role in a certain tissue type, and may or may not retain the capacity to proliferate further.
- lineage-restricted precursor cells such as a human hematopoietic progenitor cell
- a tissue specific precursor such as a megakaryocyte progenitor cell
- end-stage differentiated cell which plays a characteristic role in a certain tissue type, and may or may not retain the capacity to proliferate further.
- the terms“dedifferentiation” or“reprogramming” or“retrodifferentiation” refer to the process that generates a cell that re-expresses a more stem cell phenotype or a less differentiated phenotype than the cell from which it is derived.
- a multipotent cell can be dedifferentiated to a pluripotent cell. That is, dedifferentiation shifts a cell backward along the differentiation spectrum of totipotent cells to fully differentiated cells.
- reversal of the differentiation phenotype of a cell requires artificial manipulation of the cell, for example, by expressing stem cell-specific mRNA and/or proteins. Reprogramming is not typically observed under native conditions in vivo or in vitro.
- the term“somatic cell” refers to any cell other than a germ cell, a cell present in or obtained from a pre-implantation embryo, or a cell resulting from proliferation of such a cell in vitro.
- a somatic cell refers to any cells forming the body of an organism, as opposed to germline cells. Every cell type in the mammalian body— apart from the sperm and ova, the cells from which they are made (gametocytes) and undifferentiated stem cells— is a somatic cell: internal organs, skin, bones, blood, and connective tissue are all substantially made up of somatic cells organism other than an embryo or a fetus or results from proliferation of such a cell in vitro.
- substantially pure refers to a population of cells that is at least about 75%, preferably at least about 85%, more preferably at least about 90%, and most preferably at least about 95% pure, with respect to the cells making up a total cell population.
- the terms "substantially pure” or “homogeneous,” with regard to a population of megakaryocytes or platelets, refers to a population of cells that contain fewer than about 20%, more preferably fewer than about 15%, 10%, 8%, 7%, most preferably fewer than about 5%, 4%, 3%, 2%, 1%, or less than 1%, of cells that are not megakaryocytes or platelets, respectively.
- selection refers to isolating different cell types into one or more populations and collecting the isolated population as a target cell population which is enriched, for example, in a specific target cell. Selection can be performed using positive selection, whereby a target enriched cell population is retained, or negative selection, whereby non-target cell types are discarded (thereby enriching for desired target cell types in the remaining cell population).
- positive selection refers to selection of a desired cell type by retaining the cells of interest.
- positive selection involves the use of an agent to assist in retaining the cells of interest, e.g., use of a positive selection agent such as an antibody which has specific binding affinity for a surface antigen on the desired or target cell.
- positive selection can occur in the absence of a positive selection agent, e.g., in a“touch-free” or closed system, for example, where positive selection of a target cell type is based on any of cell size, density and/or morphology of the target cell type.
- negative selection refers to selection of undesired or non-target cells for depletion or discarding, thereby retaining (and thus enriching) the desired target cell type.
- negative selection involves the use of an agent to assist in selecting undesirable cells for discarding, e.g., use of a negative selection agent such as a monoclonal antibody which has specific binding affinity for a surface antigen on unwanted or non-target cells.
- negative selection does not involve a negative selection agent.
- negative selection can occur in the absence of a negative selection agent, e.g., in a“touch-free” or closed system, for example, where negative selection of an undesired (non-target) cell type to be discarded is based on any of cell size, density and/or morphology of the undesired (non-target) cell type.
- “decrease”,“reduced”,“reduction”, or“inhibit” are all used herein to mean a decrease or lessening of a property, level, or other parameter by a statistically significant amount.
- “reduce,”“reduction” or“decrease” or“inhibit” typically means a decrease by at least 10% as compared to a reference level and can include, for example, a decrease by at least about
- “reduction” or“inhibition” does not encompass a complete inhibition or reduction as compared to a reference level. “Complete inhibition” is a 100% inhibition as compared to a reference level.
- the terms“increased” ‘increase” or“enhance” or“activate” are all used herein to generally mean an increase of a property, level, or other parameter by a statically significant amount; for the avoidance of any doubt, the terms“increased”,“increase” or“enhance” or“activate” means an increase of at least 10% as compared to a reference level, for example an increase of at least about 20%, or at least about 30%, or at least about 40%, or at least about 50%, or at least about 60%, or at least about 70%, or at least about 80%, or at least about 90% or up to and including a 100% increase or any increase between 10-100% as compared to a reference level, or at least about a 2-fold, or at least about a 3 -fold, or at least about a 4-fold, or at least about a 5 -fold or at least about a 10-fold increase, at least about a 20-fold increase, at least about a 50-fold increase, at least about a 100-fold increase
- vinca alkaloid refers to a set of compounds having anti-mitotic and anti-microtubule effects and typically used as chemotherapy agents in the treatment of cancer.
- the original vinca alkaloids were first derived from a plant of the Vinca genus (e.g., Vinca rosea) but newer compounds are synthetic derivatives of the plant compounds.
- Exemplary vinca alkaloids include, but are not limited to, vinblastine, vincristine, vindesine, vinorelbine, vincaminol,ieridine, vinbumine, vinpocetine, minovincine, methoxyminovincine, minovincinine, vincadafformine, desoxyvincaminol and vincamajine.
- Aurora kinase inhibitor refers to a compound(s) that inhibit Aurora kinase, thus affecting downstream cellular processes such as cellular mitosis, phosphorylation of histone H3, etc.
- exemplary Aurora kinase inhibitors include, but are not limited to, CCT137690, ZM447439, Hesperadin, VX-680, barasertib, alisertib, danusertib, AT9283, PF-03914735, or AMG 900, among others.
- the Aurora kinase inhibitor is an Aurora kinase-A inhibitor, an Aurora kinase -B inhibitor or a pan-Aurora kinase inhibitor.
- compositions, methods, and respective component(s) thereof are used in reference to compositions, methods, and respective component(s) thereof, that are essential to the invention, yet open to the inclusion of unspecified elements, whether essential or not.
- the term "consisting essentially of' refers to those elements required for a given embodiment. The term permits the presence of additional elements that do not materially affect the basic and novel or functional characteristic(s) of that embodiment of the invention.
- compositions, methods, and respective components thereof refers to compositions, methods, and respective components thereof as described herein, which are exclusive of any element not recited in that description of the embodiment.
- Platelets are small (e.g., 2-3 um in diameter), irregularly shaped clear cell fragments that are formed from fragmentation of precursor megakaryocytes.
- Megakaryocytes are derived from hematopoietic stem cell precursor cells in the bone marrow in response to thrombopoietin (TPO). TPO induces differentiation of progenitor cells in the bone marrow towards a final megakaryocyte phenotype.
- TPO thrombopoietin
- megakaryocytes develop through the following lineage: CFU-ME (pluripotential hematopoietic stem cell or hemocytoblast) megakaryoblast promegakaryocyte megakaryocyte.
- CFU-ME pluripotential hematopoietic stem cell or hemocytoblast
- megakaryoblast promegakaryocyte megakaryocyte.
- the cell eventually reaches megakaryoblast stage and loses its ability to divide. However, it is still able to replicate its DNA and continue development
- a megakaryocyte for use with the methods and compositions described herein can be an immortalized megakaryocyte or population thereof.
- the immortalized megakaryocyte or population thereof is/are conditionally immortalized, wherein expression of the gene(s) necessary for the immortalized phenotype are controlled using an inducible promoter (e.g., a doxy cy cline controlled promoter).
- stem cells are undifferentiated cells in early stage of development and are capable of giving rise to more cells of the same type or differentiating into a diverse range of cell lineages.
- the main different types of stem cells are human embryonic stem cells (hESC), induced pluripotent stem cells (iPSC), and adult hematopoietic stem cells (HSC).
- the methods and compositions described herein can utilize in vitro derived megakaryocytes to produce platelets in vitro.
- the following describes various stem cells that can be used to prepare a megakaryocyte of a population thereof for the purpose of megakaryopoiesis.
- Stem cells are cells that retain the ability to renew themselves through mitotic cell division and can differentiate into more specialized cell types.
- Three broad types of mammalian stem cells include: embryonic stem (ES) cells that are found in blastocysts, induced pluripotent stem cells (iPSCs) that are reprogrammed from somatic cells, and adult stem cells that are found in adult tissues.
- ES embryonic stem
- iPSCs induced pluripotent stem cells
- Other sources of pluripotent stem cells can include amnion-derived or placental-derived stem cells.
- stem cells can differentiate into all of the specialized embryonic tissues.
- stem cells and progenitor cells act as a repair system for the body, replenishing specialized cells, but also maintain the normal turnover of regenerative organs, such as blood, skin or intestinal tissues.
- Pluripotent stem cells can differentiate into cells derived from any of the three germ layers.
- Megakaryocytes useful in the methods and compositions described herein can be differentiated from both embryonic stem cells and induced pluripotent stem cells, among others.
- the embryonic stem cells or induced pluripotent stem cells are human cells.
- the compositions and methods provided herein do not encompass generation or use of human megakaryocytes or platelets made from cells taken from a viable human embryo.
- Embryonic stem cells and methods for their retrieval are well known in the art and are described, for example, in Trounson A O (Reprod Fertil Dev (2001) 13: 523), Roach M L (Methods Mol Biol (2002) 185: 1), and Smith A G (Annu Rev Cell Dev Biol (2001) 17:435).
- the term "embryonic stem cell” is used to refer to the pluripotent stem cells of the inner cell mass of the embryonic blastocyst (see e.g., US Patent Nos.
- An embryonic stem comprises characteristics including, without limitation, an embryonic stem cell specific gene expression profile, proliferative capacity, differentiation capacity, karyotype, responsiveness to particular culture conditions, and the like.
- Embryonic stem cells are considered to be undifferentiated when they have not committed to a specific differentiation lineage. Such cells display morphological characteristics that distinguish them from differentiated cells of embryo or adult origin. Undifferentiated embryonic stem (ES) cells are easily recognized by those skilled in the art, and typically appear in the two dimensions of a microscopic view in colonies of cells with high nuclear/cytoplasmic ratios and prominent nucleoli.
- the human megakaryocytes and/or platelets described herein are derived from embryonic stem cells. In some embodiments, the human megakaryocytes and/or platelets described herein are not derived from embryonic stem cells or any other cells of embryonic origin.
- Cells derived from embryonic sources can include embryonic stem cells or stem cell lines obtained from a stem cell bank or other recognized depository institution.
- Other means of producing stem cell lines include methods comprising the use of a blastomere cell from an early stage embryo prior to formation of the blastocyst (at around the 8-cell stage). Such techniques correspond to the pre implantation genetic diagnosis technique routinely practiced in assisted reproduction clinics. The single blastomere cell is co-cultured with established ES-cell lines and then separated from them to form fully competent ES cell lines.
- Adult Stem Cells are stem cells derived from tissues (e.g., bone marrow) of a post-natal or post-neonatal organism or from an adult organism.
- An adult stem cell e.g., hematopoietic stem cell
- An adult stem cell is structurally distinct from an embryonic stem cell not only in markers it does or does not express relative to an embryonic stem cell, but also by the presence of epigenetic differences, e.g. differences in DNA methylation patterns.
- the human megakaryocytes and/or platelets described herein are derived from an adult stem cell; in one embodiment, they are derived in vitro from a hematopoietic stem cell (i.e., megakaryocytes do not need to be isolated directly from the bone marrow of an adult).
- iPSCs Induced Pluripotent Stem Cells
- the methods and compositions described herein utilize megakaryocytes that are differentiated in vitro from induced pluripotent stem cells for platelet production.
- An advantage of using iPSCs to generate a megakaryocyte or population thereof for the methods described herein is that the cells can be derived from the same subject to which the resulting platelets are to be administered. That is, a somatic cell can be obtained from a subject, reprogrammed to an induced pluripotent stem cell, and then re- differentiated into a human megakaryocyte for the production of autologous platelets.
- the megakaryocytes and/or platelets are derived from non-autologous sources.
- the use of iPSCs negates the need for cells obtained from an embryonic source.
- the stem cells used to generate megakaryocytes and/or platelets for use in the compositions and methods described herein are not embryonic stem cells.
- Reprogramming is a process of driving the differentiation of a cell backwards to a more undifferentiated or more primitive type of cell. It should be noted that placing many primary cells in culture can lead to some loss of fully differentiated characteristics. However, simply culturing such cells included in the term differentiated cells does not render these cells non-differentiated cells (e.g., undifferentiated cells) or pluripotent cells. The transition of a differentiated cell to pluripotency requires a reprogramming stimulus beyond the stimuli that lead to partial loss of differentiated character when differentiated cells are placed in culture. Reprogrammed cells also have the characteristic of the capacity of extended passaging without loss of growth potential, relative to primary cell parents, which generally have capacity for only a limited number of divisions in culture.
- the cell to be reprogrammed can be either partially or terminally differentiated prior to reprogramming.
- reprogramming encompasses complete reversion of the differentiation state of a differentiated cell (e.g., a somatic cell) to a pluripotent state or a multipotent state.
- reprogramming encompasses complete or partial reversion of the differentiation state of a differentiated cell (e.g., a somatic cell) to an undifferentiated cell (e.g., an embryonic-like cell). Reprogramming can result in expression of particular genes by the cells, the expression of which further contributes to reprogramming.
- reprogramming of a differentiated cell causes the differentiated cell to assume an undifferentiated state with the capacity for self-renewal and differentiation to cells of all three germ cell lineages.
- the resulting cells are referred to as“reprogrammed cells,” or“induced pluripotent stem cells (iPSCs or iPS cells).”
- iPS cells can be generated or derived from terminally differentiated somatic cells, as well as from adult stem cells, or somatic stem cells. That is, a non-pluripotent progenitor cell can be rendered pluripotent or multipotent by reprogramming.
- pluripotent stem cells from somatic cells e.g., any cell of the body with the exclusion of a germ line cell; fibroblasts etc.
- any method that re-programs a somatic cell to the pluripotent phenotype would be appropriate for use in the methods described herein.
- the efficiency of reprogramming i.e., the number of reprogrammed cells derived from a population of starting cells can be enhanced by the addition of various small molecules as shown by Shi, Y., et al (2008) Cell-Stem Cell 2:525-528, Huangfu, D., et al (2008) Nature Biotechnology 26(7):795-797, and Marson, A., et al (2008) Cell-Stem Cell 3: 132-135.
- agents that enhance reprogramming efficiency include soluble Wnt, Wnt conditioned media, BIX- 01294 (a G9a histone methyltransferase), PD0325901 (a MEK inhibitor), DNA methyltransferase inhibitors, histone deacetylase (HDAC) inhibitors, valproic acid, 5'-azacytidine, dexamethasone, suberoylanilide, hydroxamic acid (SAHA), vitamin C, and trichostatin (TSA), among others.
- isolated clones can be tested for the expression of a stem cell marker.
- a stem cell marker can be selected from the non-limiting group including SSEA3, SSEA4, CD9, Nanog, Fbxl5, Ecatl, Esgl, Eras, Gdf3, Fgf4, Cripto, Daxl, Zpf296, Slc2a3, Rexl, Utfl, and Natl.
- a cell that expresses Oct4 or Nanog is identified as pluripotent.
- Methods for detecting the expression of such markers can include, for example, RT-PCR and immunological methods that detect the presence of the encoded polypeptides, such as Western blots or flow cytometric analyses. In some embodiments, detection does not involve only RT-PCR, but also includes detection of protein markers. Intracellular markers may be best identified via RT-PCR, while cell surface markers are readily identified, e.g., by immunocytochemistry.
- the pluripotent stem cell character of isolated cells can be confirmed by tests evaluating the ability of the iPSCs to differentiate to cells of each of the three germ layers.
- teratoma formation in nude mice can be used to evaluate the pluripotent character of the isolated clones.
- the cells are introduced to nude mice and histology and/or immunohistochemistry is performed on a tumor arising from the cells.
- the growth of a tumor comprising cells from all three germ layers, for example, further indicates that the cells are pluripotent stem cells.
- Reprogrammed somatic cells as disclosed herein can express any number of pluripotent cell markers, including: alkaline phosphatase (AP); ABCG2; stage specific embryonic antigen-1 (SSEA- 1); SSEA-3; SSEA-4; TRA-1-60; TRA-1-81; Tra-2-49/6E; ERas/ECAT5, E-cadherin; b-111-tubulin; a-smooth muscle actin (a-SMA); fibroblast growth factor 4 (Fgf4), Cripto, Daxl; zinc finger protein 296 (Zfp296); N-acetyltransferase-1 (Natl); (ES cell associated transcript 1 (ECAT1); ESG1/DPPA5/ECAT2; ECAT3; ECAT6; ECAT7; ECAT8; ECAT9; ECAT10; ECAT15-1; EC ATI 5- 2; Fthl 17; Sall4; undifferentiated embryonic cell transcription factor (Utfl); Rex
- markers can include Dnmt3L; Soxl5; Stat3; Grb2; b-catenin, and Bmil.
- Such cells can also be characterized by the down-regulation of markers characteristic of the somatic cell from which the induced pluripotent stem cell is derived.
- v/Yro-differentiation of megakaryocytes produces an end-result of a cell having the phenotypic and morphological features of a megakaryocyte but that the differentiation steps of in v/Yro-differentiation need not be the same as the differentiation that occurs naturally in the embryo. That is, during differentiation to a megakaryocyte, it is specifically contemplated herein that the step-wise differentiation approach utilized to produce such cells need not proceed through every progenitor cell type that has been identified during embryogenesis and can essentially“skip” over certain stages of development that occur during embryogenesis.
- megakaryocytes undergo a process called endomitosis, where they double their nuclear and cellular contents without cell division.
- endomitosis the megakaryocyte grows to enormous size and can have more than 64 times the normal nuclear contents.
- the increase of nuclear contents, or polyploidy plays a fundamental role in the platelet formation by allowing the cell to produce the large amounts of proteins and organelles necessary for platelet formation and function.
- mature megakaryocytes also have vast quantities of extra cell membrane with which to make platelets. Inducing polyploidization or maturation of megakaryocytes in vitro can be achieved using the following reagents alone or in different combinations.
- Overexpression of Ectopic genes It is desirable to generate self-renewing megakaryocytes progenitor cells that can be quickly matured in order to initiate platelet production.
- self-renewing megakaryocyte progenitor cells are immortalized cells.
- the control of the maturation of megakaryocytes from self-renewing cells is achieved by overexpression of one or more megakaryocyte maturation markers, the expression of which is under the control of an inducible promoter.
- a doxycycline (dox) -inducible promoter can be used to regulate expression of a megakaryocyte maturation marker such that in the presence of doxy cy cline the maturation marker is expressed (or vice versa).
- One system for maturing megakaryocytes in vitro uses a lentivirus to express the transcription factors MYC and BMI1, and one or more pro-survival proteins (e.g., Bcl-xL or BCL2L1) under the control of a dox-inducible promoter.
- a dox-inducible promoter e.g., Bcl-xL or BCL2L1
- a second system employs controlled expression of GATA1 by a dox-inducible promoter.
- the megakaryocyte progenitor cells Under conditions where GATA1 expression is repressed, the megakaryocyte progenitor cells self-renew and when GATA1 expression is induced the cells mature into megakaryocytes that release platelets.
- Other genes that can be targeted to modulate maturation of megakaryocytes include, but are not limited to, gpl30/interleukin-6-dependent genes, Notch, stromal cell-derived factor 1 (SDFl)/fibroblast growth factor 4-dependent signaling pathways and interleukin 1 -alpha signaling pathways. It is specifically contemplated herein that vincristine and/or vinblastine can be used in combination with any of these systems, or alternatively in isolation, to increase the efficiency of platelet production.
- Rho Rock inhibitors The final steps of megakaryocyte cell division require regulation of actin and myosin to form the cleavage furrow and contractile ring. The inhibition of actin and myosin during cytokinesis allows megakaryocytes to replicate DNA material without undergoing cell division.
- the Rho/Rock pathway signals through myosin light chain (MLC) and filamin and activates both stress fibers and lamellipodia formation.
- Y27632 inhibits the Rho/Rock pathway and consequently inhibits myosin activation and the contractile ring formation, presumably allowing the megakaryocyte to undergo polyploidization.
- Rho/Rock inhibitors include, but are not limited to, Y27632, thiazovivin, GSK429286A, fasudil HC1, Y39983, Wf-536, SLx-2119, Azabenzimidazole-aminofurazans, DE-104, and H-1152P.
- NIC Nicotinamide Decreases in p53 activity are responsible for accelerated DNA synthesis, higher ploidy and delayed apoptosis. NIC increases p53 activity and thus increases endomitosis and megakaryocyte polyploidization.
- Src inhibitors The inhibition of Src family kinases increases megakaryocyte polyploidization through the Lyn Fyn pathway and inhibition of actin polymerization.
- Exemplary Src inhibitors include, but are not limited to, saracatinib (AZD0530), bosutinib (SKI-606), danusertib (PHA- 739358), NVP-BHG712, quercetin (sophoretin), PCI-32765, KX2-391, AP23846, and PP2.
- Aurora-B inhibitors are responsible for controlling microtubule formation and subsequent chromosome separation during mitosis. Its inhibition increases microtubule destruction through stathmin and mitotic centromere-associated kinesin (MCAK) action.
- MCAK mitotic centromere-associated kinesin
- Aurora-B kinase inhibitors include, but are not limited to, AMG 900, AT9283, Aurora A Inhibitor I, AZD1152, AZD1152-HQPA (barasertib), CCT129202, CYC 116, danusertib (PHA-739358), ENMD-2076, GSK1070916, hesperadin, JNJ-7706621, KW-2449, MLN8054, MLN8237 (alisertib), PF-03814735, PHA-680632, SNS-314, TAK-901, VX-680 (MK-0457, tozasertib), and ZM-447439.
- MLCK Myosin light chain kinase
- MLC Myosin light chain kinase
- MLCK inhibitors include, but are not limited to, A3 HC1, Go 7874 HC1, InSolutionTM K-252a (Nocardiopsis sp.), K-252a (Nocardiopsis sp.), K-252b (Nocardiopsis sp.), ML-7 HC1, ML-9 HC1, MLCK inhibitor peptide 18, piceatannol, and staurosporine ( Streptomyces sp).
- PMA Protein kinase C
- Blebbistatin inhibits myosin II and consequently the last steps of cytokinesis and cell division, thus allowing the cell to undergo polyploidization and increase the nuclear material.
- Other stimulators of megakaryocyte maturation include the transcription factor‘all trans retinoic acid,’ and the histone deacetylase inhibitor valproic acid.
- Racl activation, CDC42 activation, hirudin and c-Myc inhibition also increase proplatelet formation.
- some physical stimuli can induce megakaryocyte maturation and polyploidization, such as an increased oxygen concentration during culture, culturing at a temperature higher than conventional culturing temperature (e.g., 39°C).
- the successful maturation of megakaryocytes from pluripotent stem cells can be determined by morphology (e.g., presence of DNA polyploidy (e.g., up to 128N), increase in cell size (up to a diameter of 60 um), increased granularity, and the presence of intracellular organelles reminiscent of pro-platelets), or by the presence of cell surface markers (e.g., CD41, CXCR4, or the TPO receptor, among others).
- morphology e.g., presence of DNA polyploidy (e.g., up to 128N), increase in cell size (up to a diameter of 60 um), increased granularity, and the presence of intracellular organelles reminiscent of pro-platelets), or by the presence of cell surface markers (e.g., CD41, CXCR4, or the TPO receptor, among others).
- Cell surface markers are useful with the methods and compositions described herein to identify the differentiation or dedifferentiation state of a cell. Both cell surface markers and intracellular markers can be detected, for example, using an antibody for binding, e.g., cell surface markers or by PCR for intracellular markers. In some embodiments, antibodies or similar agents specific for a given marker, or set of markers, can be used to separate and isolate the desired cells using fluorescent activated cell sorting (FACS), panning methods, magnetic particle selection, particle sorter selection and other methods known to persons skilled in the art, including density separation (Xu et al. (2002) Circ. Res. 91:501; U.S.S.N. 20030022367) and separation based on other physical properties (Doevendans et al. (2000) J. Mol. Cell. Cardiol. 32:839-851).
- FACS fluorescent activated cell sorting
- negative selection can be performed, including selecting and removing cells with undesired markers or characteristics, for example fibroblast markers, epithelial cell markers etc.
- Undifferentiated ES cells express genes that can be used as markers to detect the presence of undifferentiated cells.
- Exemplary ES cell markers include stage -specific embryonic antigen (SSEA)- 3, SSEA-4, TRA-I-60, TRA-1-81, alkaline phosphatase or those described in e.g., U.S.S.N. 2003/0224411; or Bhattacharya (2004) Blood 103(8):2956-64, each herein incorporated by reference in their entirety.
- Exemplary markers expressed on cardiac progenitor cells include, but are not limited to, TMEM88, GATA4, ISL1, MYL4, and NKX2-5.
- the methods described herein relate to increasing the amount of platelets produced in vitro from megakaryocytes or the efficiency of platelet production from cultured megakaryocytes by contacting a megakaryocyte cell or population thereof with an effective amount of vincristine, vinblastine, and/or an Aurora kinase inhibitor.
- An “effective amount” refers to an amount of vincristine, vinblastine, and/or an Aurora kinase inhibitor that increases the number of platelets produced from a given megakaryocyte (e.g., platelet/megakaryocyte) by at least 10% as compared to a megakaryocyte grown under substantially identical conditions but in the absence of vincristine, vinblastine, and/or an Aurora kinase inhibitor.
- an effective amount of vincristine, vinblastine, and/or an Aurora kinase inhibitor can increase the number of platelets produced by at least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 75%, at least 80%, at least 90%, at least 95%, at least 99%, at least 1-fold, at least 2-fold, at least 5-fold, at least 10- fold, at least 25 -fold, at least 50-fold, at least 100-fold or more as compared to a megakaryocyte not treated with vincristine, vinblastine, and/or an Aurora kinase inhibitor.
- the effective amount of vincristine, vinblastine, and/or the Aurora kinase inhibitor is within the range of 4nM - 100 mM, inclusive.
- the range of vincristine, vinblastine, and/or the Aurora kinase inhibitor for use with the methods and compositions described herein is: 0.04mM - 50 mM, for example, 0.04-40 mM, 0.04-30 mM, 0.04-20 mM, 0.04- 10 mM, 0.04-5 mM, 0.04-4 mM, 0.004-3 mM, 0.04-2 mM, 0.04-1 mM, 0.04-0.01 mM, 0.04-0-0.75 mM, 0.1-40 mM, 0.5-40 mM, 1-40 mM, 5-40 mM, 10-40mM, 20-40 mM, 30-40 mM, 0.1-5 mM, 0.1-4 mM, 0.1-3 mM, 0.1-2 m
- the vincristine, vinblastine, and/or an Aurora kinase inhibitor is contacted with the megakaryocyte cells at day 0 of a platelet biogenesis protocol, such as the protocol provided herein in the working Examples.
- the vincristine, vinblastine, and/or Aurora kinase inhibitor is contacted with the megakaryocyte cells at day 1, 2, 3, 4, 5, or 6 of a platelet biogenesis protocol.
- the vincristine, vinblastine, and/or Aurora kinase inhibitor is contacted with the megakaryocyte cells from day 3 to the end of the platelet biogenesis protocol (e.g., from days 3-6, or days 3-5, or days 3-7).
- the vincristine, vinblastine, and/or Aurora kinase inhibitor is contacted with the megakaryocyte cells for at least 6h, at least 12h, at least 24h, at least 2 days, at least 3 days, at least 4 days, at least 5 days or more (e.g., until the end of the platelet biogenesis protocol).
- the Aurora kinase inhibitor is an Aurora B kinase inhibitor.
- Aurora-B kinase inhibitors include, but are not limited to, AMG 900, AT9283, Aurora A Inhibitor I, AZD1152, AZD1152-HQPA (barasertib), CCT129202, CYC116, danusertib (PHA-739358), ENMD- 2076, GSK1070916, hesperadin, JNJ-7706621, KW-2449, MLN8054, MLN8237 (alisertib), PF- 03814735, PHA-680632, SNS-314, TAK-901, VX-680 (MK-0457, tozasertib), and ZM-447439.
- formulations of vincristine, vinblastine, and/or Aurora kinase inhibitors are preferably non-toxic to cultured cells and do not substantially alter cell viability, cell growth rate etc.
- Any cell culture medium and/or supplements can be used to optimize the growth and maintenance of self-renewing megakaryocyte progenitor cells or immortalized megakaryocytes for the purpose of generating platelets in vitro. Such methods of optimization are known to those of skill in the art or can be determined using techniques known in the art. It is also contemplated herein that the cells can be cultured on a desired substrate, such as laminin, fibronectin, poly-lysine or methy cellulose.
- the number of platelets produced under a given set of conditions can be determined using FACS or a megakaryocyte bursting assay as described herein in the Examples section.
- FACS counting can be performed on the basis of size; that is FACS can be used to sort platelets from megakaryocytes based on size.
- suitable cell surface markers useful for counting platelets include, but are not limited to, CD41, CD61 and/or CD62.
- a composition i.e., a synergistic composition, comprising vincristine and/or vinblastine in combination with an Aurora kinase inhibitor.
- a composition comprising an effective amount of vincristine and/or vinblastine and an effective amount of an Aurora kinase inhibitor.
- the composition comprises vincristine and an Aurora kinase inhibitor.
- vincristine and an Aurora kinase inhibitor selected from the group consisting of CCT137690, ZM447439, Hesperadin, VX-680, barasertib, alisertib, danusertib, AT9283, PF- 03914735, or AMG 900.
- the composition comprises vincristine and the Aurora kinase inhibitor CCT137690.
- the composition comprises vinblastine and an Aurora kinase inhibitor.
- vinblastine and an Aurora kinase inhibitor selected from the group consisting of CCT137690, ZM447439, Hesperadin, VX-680, barasertib, alisertib, danusertib, AT9283, PF- 03914735, or AMG 900.
- the composition comprises vinblastine and the Aurora kinase inhibitor CCT137690.
- the composition further comprises megakaryocyte cell(s). It is noted that the megakaryocytes can be cultured megakaryocytes, e.g., immortalized and/or matured megakaryocytes. In some other embodiments, the composition further comprises plate let(s). In still other embodiments, the composition further comprises both megakaryocyte cell(s) and platelet(s).
- the methods and compositions for increasing the number and/or efficiency of platelet production using vincristine and/or vinblastine can be performed in a large-scale manner, for example, in a bioreactor.
- the specific methods for increasing the scale of platelet production are not critical to the invention and as such any method known in the art can be used in combination with the methods described herein.
- kits for enhancing platelet production from cultured megakaryocytes are described herein. Described herein are kit components that can be included in one or more of the kits described herein.
- the kit comprises an effective amount of vincristine, vinblastine, and/or Aurora kinase inhibitor formulated for use with cultured megakaryocytes (e.g., immortalized and/or matured megakaryocytes).
- cultured megakaryocytes e.g., immortalized and/or matured megakaryocytes.
- vincristine, vinblastine, and/or Aurora kinase inhibitor can be supplied in a lyophilized form or a concentrated form that can diluted prior to use with cultured cells.
- Preferred formulations include those that are non-toxic to the cells and/or does not affect growth rate or viability etc.
- vincristine, vinblastine, and/or Aurora kinase inhibitor can be supplied in aliquots or in unit doses.
- the kit further comprises a vector comprising a nucleic acid encoding a gene to mature megakaryocytes under the control of an inducible promoter for maturing megakaryocytes in vitro.
- kits includes the components described herein, e.g., a composition comprising vincristine and/or vinblastine, a composition(s) that includes a vector comprising e.g., a megakaryocyte maturation marker (e.g., GATA-1, CD42b etc.) under the control of an inducible promoter (e.g., doxycycline promoter), a doxycycline composition, and one or more maturation compounds as described throughout the specification.
- kits can optionally include one or more agents that permit the detection of residual stem cells or a set thereof.
- the kit optionally comprises informational material.
- kits provided herein comprise an aliquot of immortalized megakaryocytes and optionally a preferred medium for growth thereof.
- the kit can also contain a substrate for coating culture dishes, such as laminin, fibronectin, Poly-L-Lysine, or methylcellulose.
- the compositions in the kit can be provided in a watertight or gas tight container which in some embodiments is substantially free of other components of the kit.
- a vincristine, vinblastine, and/or Aurora kinase inhibitor composition can be supplied in more than one container, e.g., it can be supplied in a container having sufficient reagent for a predetermined number of differentiation reactions, e.g., 1, 2, 3 or greater.
- One or more components as described herein can be provided in any form, e.g., liquid, dried or lyophilized form. It is preferred that the components described herein are substantially pure and/or sterile.
- the liquid solution preferably is an aqueous solution, with a sterile aqueous solution being preferred.
- the informational material can be descriptive, instructional, marketing or other material that relates to the methods described herein.
- the informational material of the kits is not limited in its form.
- the informational material can include information about production of the megakaryocytes and/or platelets, concentration, date of expiration, batch or production site information, and so forth.
- the informational material relates to methods for using or administering the components of the kit.
- the kit can include a component for the detection of a marker for human cells, ES cells, iPS cells, hematopoietic progenitor cells, immature megakaryocytes, mature megakaryocytes, platelets etc.
- the kit can include one or more antibodies that bind a cell marker, or primers for an RT-PCR or PCR reaction, e.g., a semi -quantitative or quantitative RT-PCR or PCR reaction.
- Such components can be used to assess the activation of megakaryocyte-specific markers or the loss of ES cell, iPSC, or adult stem cell markers.
- the detection reagent is an antibody, it can be supplied in dry preparation, e.g., lyophilized, or in a solution.
- the antibody or other detection reagent can be linked to a label, e.g., a radiological, fluorescent (e.g., GFP) or colorimetric label for use in detection.
- a label e.g., a radiological, fluorescent (e.g., GFP) or colorimetric label for use in detection.
- the detection reagent is a primer, it can be supplied in dry preparation, e.g., lyophilized, or in a solution.
- the kit will typically be provided with its various elements included in one package, e.g., a fiber-based, e.g., a cardboard, or polymeric, e.g., a Styrofoam box.
- the enclosure can be configured so as to maintain a temperature differential between the interior and the exterior, e.g., it can provide insulating properties to keep the reagents at a preselected temperature for a preselected time.
- the present invention may be as defined in any one of the following numbered paragraphs.
- a method for increasing platelet production from a megakaryocyte comprising: contacting a megakaryocyte (MK) or population thereof with an effective amount of vincristine, vinblastine, an Aurora kinase inhibitor, or a combination thereof.
- MK megakaryocyte
- the substrate comprises laminin, fibronectin, Poly-L-Lysine, or methylcellulose.
- Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) until functional platelets are generated.
- a kit for increasing platelet production comprising: vincristine, vinblastine, and/or Aurora kinase inhibitor, instructions for use thereof, and at least one of the following: (i) Laminin substrate, (ii) Megakaryocyte cells, or (iii) a positive control comprising human platelet lysates in a known amount.
- EXAMPLE 1 Vincristine and vinblastine stimulate megakaryocyte maturation and platelet production
- hiPSCs human pluripotent stem cells
- hiPSCs human pluripotent stem cells
- PLT platelet
- This assay was used to screen approximately 4,000 chemical genetics compounds, each at four different concentrations, and image processing and analysis scripts were developed to quantify the maturation of imMKCLs and the production of pro- PLTs and PLTs. About 300,000 images from over 20,000 wells were acquired, processed and analyzed using FIJI scripts, and the resulting data evaluated using MATLAB scripts.
- This primary screen identified ⁇ 30 compounds (Hit compounds) that substantially promoted a pro-PLT phenotype or the output of PLT-like particles.
- a secondary validation screen was performed on the hit compounds at 11 different doses ranging from 4hM-100mM. The 15 compounds that elicited a dose dependent increase of PLT release were selected for further evaluation.
- EXAMPLE 2 Novel High-throughput live-cell imaging assays to identify and validate agents that promote megakaryocyte maturation and trigger platelet release
- EXAMPLE 3 Exemplary protocol for generation of synergistic effects of CCT137690 and Vincristine
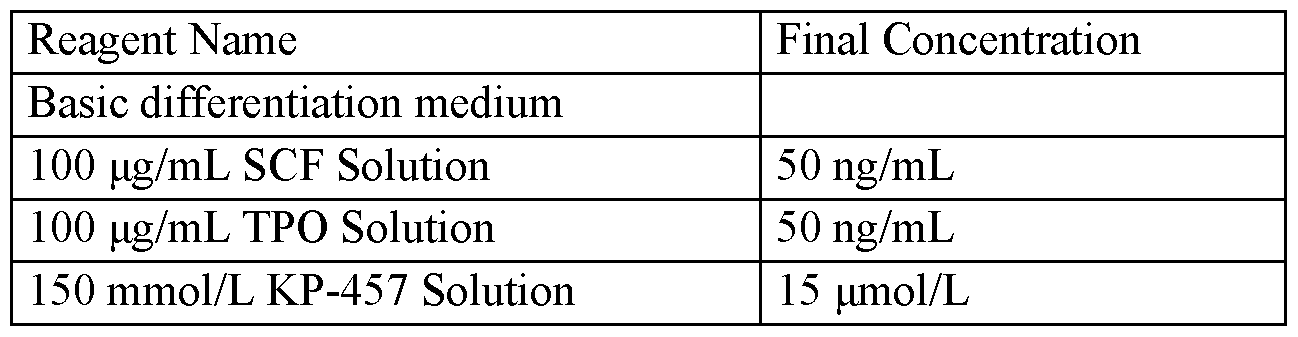
- the cells are diluted to a concentration of 100,000cells/mL in CCT137690 (Aurora Kinase inhibitor) (working concentration of 90nM) SeV2 differentiation medium. Transfer the differentiation culture to orbital shaker Erlenmeyer flask or PBS biotech Bioreactor vessel.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Hematology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Developmental Biology & Embryology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Zoology (AREA)
- Virology (AREA)
- Immunology (AREA)
- Biomedical Technology (AREA)
- Biotechnology (AREA)
- Cell Biology (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Surgery (AREA)
- Diabetes (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Abstract
Provided herein are methods and compositions for enhancing platelet production from a megakaryocyte cell or population thereof. Provided herein, in part, are methods and compositions comprising the use of vincristine and/or vinblastine in enhancing platelet production from a megakaryocyte (e.g., an in vitro cultured megakaryocyte or immortalized megakaryocyte). In one embodiment of this aspect and all other aspects provided herein, vincristine and/or vinblastine is used in combination with at least one Aurora kinase inhibitor.
Description
METHODS FOR INCREASING PLATELET PRODUCTION
CROSS-REFERENCE TO RELATED APPLICATION
[0001] This application is an International Application which designated the U.S., and which claims the benefit under 35 U.S.C. § 119(e) of U.S. Provisional Application No. 62/816,612 filed on March 11, 2019, the contents of which are incorporated herein by reference in their entireties.
FIELD OF THE INVENTION
[0002] The field of the invention relates to methods and compositions for increasing platelet production from megakaryocytes.
BACKGROUND
[0003] Platelets are tiny blood cells that play an integral role in blood clotting. In addition, platelets, which are also called“thrombocytes,” are involved in inflammation, blood vessel growth, and tumor metastasis. The entire platelet population is replaced every 10 days, thus requiring a high amount of ongoing platelet production.
[0004] Platelet supplementation is necessary in a variety of clinical settings including (i) in the treatment of cancer, (ii) surgery, (iii) serious injuries or trauma, and (iv) in the treatment of blood disorders and transplants.
[0005] Unfortunately, there is a shortage of platelet donors and platelets have a limited shelf life once they are isolated from a donor. Thus, there is a need for new methods for generating platelets that are independent of conventional platelet donation methods.
SUMMARY
[0006] Provided herein, in part, are methods and compositions comprising the use of vincristine and/or vinblastine in enhancing platelet production from a megakaryocyte (e.g., an in vitro cultured megakaryocyte or immortalized megakaryocyte).
[0007] Accordingly, in one aspect provided herein is a method for increasing platelet production from a megakaryocyte, the method comprising: contacting a megakaryocyte (MK) or population thereof with an effective amount of a vinca alkaloid, such as vincristine, vinblastine, vinorelbine, or vindesine, or an Aurora kinase inhibitor (e.g., CCT137690). In one embodiment of this aspect and all other aspects provided herein, the vinca alkaloid is vincristine and/or vinblastine. In other embodiments of this aspect and all other aspects provided herein, the Aurora kinase inhibitor is CCT137690, ZM447439, Hesperadin, VX-680, barasertib, alisertib, danusertib, AT9283, PF- 03914735, or AMG 900, among others.
[0008] In one embodiment of this aspect and all other aspects provided herein, vincristine and/or vinblastine is used in combination with at least one Aurora kinase inhibitor.
[0009] In one embodiment of this aspect and all other aspects provided herein, the method comprises contacting with a combination of agents (e.g., at least two, 3, 4, 5, or 6 different agents). In this embodiment, a combination of agents specifically includes the following: CCT137690 & vincristine, CCT137690 & vinblastine, vinblastine & vincristine, vinblastine & vincristine & CCT137690, vincristine and/or vinblastine & barasertib, vincristine and/or vinblastine & PF03814735, vincristine and/or vinblastine & ZM447434, and the like. In certain embodiments, the effect of the combination of agents is additive. In other embodiments, the combination of agents produces a synergistic effect. In particular, a combination comprising an Aurora kinase inhibitor, such as CCT137690, with vincristine can produce a synergistic effect on platelet production.
[0010] It is noted that when a combination of agents is used, the different agents can be contacted with the megakaryocyte or population thereof at the same time, i.e., simultaneously, or the different agents can be contacted with the megakaryocyte or population thereof at different times, i.e., sequentially. Thus, in some embodiments of this aspect and all other aspects provided herein, two or more different agents are contacted with the megakaryocyte or population thereof at the same time.
[0011] In some embodiments of this aspect and all other aspects provided herein, the agents, e.g., vinblastine and/or vincristine, and the Aurora kinase inhibitor is contacted with the megakaryocyte or population thereof at different times. For example, the megakaryocyte or population thereof is contacted with the Aurora kinase inhibitor for a period of time before the megakaryocyte or population thereof is contacted with vinblastine and/or vincristine. Generally, the time between contacting with the Aurora kinase inhibitor and vinblastine and/or vincristine is from a few minutes to days. For example, the time between contacting with the Aurora kinase inhibitor and vinblastine and/or vincristine can be 30 minutes, 60 minutes, 2 hours, 5 hours, 12 hours, 18 hours, 24 hours, 2 days, 3 days, 4 days or more. In some embodiments of this aspect and all other aspects provided herein, the time between contacting with the Aurora kinase inhibitor and vinblastine and/or vincristine is about 3 days.
[0012] In one embodiment of this aspect and all other aspects provided herein, the combination of the Aurora kinase inhibitor (e.g., CCT137690) with vincristine synergistically increases platelet production. In another embodiment of this aspect and all other aspects provided herein, the combination of the Aurora kinase inhibitor (e.g., CCT137690) with vinblastine synergistically increases platelet production. To assess synergy, the number of platelets produced from control wells (e.g., wells containing imMKCLs that were not exposed to CCT137690 and/or vincristine) were compared to the number of platelets generated in wells treated individually with each agent (e.g., CCT137690 or vincristine alone) or wells treated with the combination of agents (e.g. CCT137690 and vincristine treated imMKCL wells). Using this method, synergy of platelet production was detected for the combination treatment of CCD 137690 with vincristine, which resulted in platelet counts that were 8- 10-fold higher as compared to the platelet counts from untreated control imMKCLs. In contrast, treatment with either agent alone (i.e., CCT137690 or vincristine)
demonstrated only a 2-4 fold increase in platelet counts, thus an additive effect of these two agents would be expected to be approximately a 4-8 fold increase. Thus, an 8-10 fold increase in platelet production comprises a synergistic effect.
[0013] In one embodiment of this aspect and all other aspects provided herein, the method is performed in vitro.
[0014] In another embodiment of this aspect and all other aspects provided herein, platelet production is increased by at least 10% compared to an untreated control.
[0015] In another embodiment of this aspect and all other aspects provided herein, the untreated control comprises a substantially similar megakaryocyte or population thereof that is not contacted with a vinca alkaloid, such as vincristine or vinblastine, or an Aurora kinase inhibitor.
[0016] In another embodiment of this aspect and all other aspects provided herein, the increase in platelet production is an increase in platelet number.
[0017] In another embodiment of this aspect and all other aspects provided herein, the increase in platelet number is an increase in the number of platelets per megakaryocyte (plate lets/MK).
[0018] In another embodiment of this aspect and all other aspects provided herein, the population of megakaryocytes is a homogenous population.
[0019] In another embodiment of this aspect and all other aspects provided herein, the effective amount of vincristine, vinblastine and/or the aurora kinase is between O.OImM - IOOmM, inclusive.
[0020] In another embodiment of this aspect and all other aspects provided herein, the effective amount of vincristine, vinblastine and/or the aurora kinase is between 0.1mM-5mM.
[0021] In another embodiment of this aspect and all other aspects provided herein, the effective amount of vincristine, vinblastine and/or the aurora kinase is between 0.75mM- 1.50mM.
[0022] In another embodiment of this aspect and all other aspects provided herein, the Aurora kinase inhibitor comprises CCT137690. CCT137690 is a potent inhibitor of Aurora kinases with IC50 values of 0.015, 0.019 and 0.025 mM for Aurora A, Aurora C and Aurora B, respectively. CCT137690 possesses antiproliferative activity in various human tumor cell lines. CCT137690 also inhibits in vitro phosphorylation of histone H3, is a moderate inhibitor of the hERG ion-channel (IC50=3.0 mM) and also inhibits histone H3 and TACC3 phosphorylation (Aurora B and A substrates, respectively) in HCT116 and HeLa cells. Continuous exposure of tumor cells to CCT137690 promotes multipolar spindle formation, chromosome misalignment, polyploidy and apoptosis. One of skill in the art can easily calculate an appropriate effective dose using the IC50 values provided herein and knowledge known in the field.
[0023] In another embodiment of this aspect and all other aspects provided herein, the megakaryocyte or population thereof is grown on a substrate.
[0024] In some embodiments of this aspect and all other aspects provided herein, the megakaryocyte or population thereof is grown, e.g., on a substrate, for a period time before contacting with
vincristine, vinblastine, vinorelbine, or vindesine, or the Aurora kinase inhibitor. Generally, the megakaryocyte or population thereof can be grown for a few minutes to days before contacting with vincristine, vinblastine, vinorelbine, or vindesine, or the Aurora kinase inhibitor. For example, the megakaryocyte or population thereof can be grown for 30 minutes, 60 minutes, 2 hours, 5 hours, 12 hours, 18 hours, 24 hours, 2 days, 3 days, 4 days or more before contacting with vincristine, vinblastine, vinorelbine, or vindesine, or the Aurora kinase inhibitor. In some embodiments of this aspect and all other aspects provided herein, the megakaryocyte or population thereof can be grown for about 3 days before contacting with vincristine, vinblastine, vinorelbine, or vindesine, or the Aurora kinase inhibitor
[0025] In another embodiment of this aspect and all other aspects provided herein, the substrate comprises laminin, fibronectin, Poly-L-Lysine, or methylcellulose.
[0026] In another embodiment of this aspect and all other aspects provided herein, the number of platelets is counted using FACS or a burst/spreading assay.
[0027] In another embodiment of this aspect and all other aspects provided herein, the megakaryocyte or population thereof is contacted with an effective amount of both vincristine and vinblastine, and optionally an effective amount of an Aurora kinase inhibitor (e.g., CCT137690).
[0028] In another embodiment of this aspect and all other aspects provided herein, the megakaryocyte cell or population thereof is an immortalized megakaryocyte cell or population thereof.
[0029] In another embodiment of this aspect and all other aspects provided herein, the vincristine, vinblastine and/or Aurora kinase is contacted with the megakaryocyte cell(s) after induction of terminal differentiation.
[0030] In another embodiment of this aspect and all other aspects provided herein, the vincristine, vinblastine, and/or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) at least one day after induction of terminal differentiation.
[0031] In another embodiment of this aspect and all other aspects provided herein, the vincristine, vinblastine and/or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) from day 3 after induction of terminal differentiation.
[0032] In another embodiment of this aspect and all other aspects provided herein, the vincristine, vinblastine and/or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) until functional platelets are generated.
[0033] In another embodiment of this aspect and all other aspects provided herein, the vincristine, vinblastine, and/or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) until a maximal output of functional platelets are generated.
[0034] Another aspect provided herein relates to a kit for increasing platelet production comprising: vincristine, vinblastine, and/or an Aurora kinase inhibitor, instructions for use thereof, and at least one
of the following: (i) laminin substrate, (ii) megakaryocyte cells, or (iii) positive control comprising human platelets or platelet lysates in a known amount.
[0035] In one embodiment of this aspect and all other aspects provided herein, the megakaryocyte cells are immortalized megakaryocyte cells.
[0036] In some embodiments of this aspect and all other aspects provided herein, the megakaryocyte or population thereof is grown on a baffled surface.
[0037] In some embodiments of this aspect and all other aspects provided herein, the megakaryocyte or population thereof is grown on a flat surface.
BRIEF DESCRIPTION OF THE FIGURES
[0038] FIG. 1 depicts an exemplary workflow for a high-throughput CV7000 imaging screen to detect platelet generation and pro-platelet phenotype.
[0039] FIG. 2 depicts Fiji based identification and counting of platelet sized particles and megakaryocyte sized imMKCLs from the captured images. Depiction of normal maturation kinetics of megakaryocytes via comparison of Day6 megakaryocyte membrane content vs DNA content, Day6 megakaryocyte circularity vs DNA content, Day6 megakaryocyte Area vs DNA content.
[0040] FIG. 3 depicts Fiji based counting of plate lets/imMKCLs to identify“hit” compounds. Also depicts screening performed to identify pro-platelet phenotype in various drug/compound treated wells.
[0041] FIGs. 4A-4B depicts exemplary data of the “hit” compounds vincristine and vinblastine (FIG. 4A) and identification of“hit” compound-treated wells that produce a significant pro-platelet phenotype from the imMKCLs. Initial analyses identify vincristine and vinblastine as “hit” compounds (FIG. 4B).
[0042] FIGs. 5A-5B depicts exemplary methods and data relating to the validation of “hit” compounds. These data show that vincristine and vinblastine treatment results in high platelet yields (FIG. 5A). Also depicted is an exemplary scheme used to validate“hit” compounds in clone 38 imMKCLs in static conditions. FACS based data shows that Vincristine and vinblastine treatment promotes platelet yields by ~3-4 fold. Vincristine and vinblastine also promote extensive pro-platelet phenotype from the imMKCLs as compared to the corresponding controls (FIG. 5B).
[0043] FIG. 6 shows exemplary data relating to the effect of vincristine and vinblastine on platelet production in static conditions. Platelet count was performed 72h post exposure of clone 38 imMKCLs to vincristine and vinblastine.
[0044] FIGs. 7A-7B shows exemplary data relating to the effects of vincristine and vinblastine on platelet production in shaking conditions. Platelet count was performed 72h post exposure of clone 38 imMKCLs to vincristine and/or vinblastine. This figure also shows the gating strategy used to identify and segregate platelets from contaminating imMKCLs in the culture, and identify live, non-apoptotic, CD41a+ CD42b+ platelets (FIG. 7A). Also depicted is exemplary platelet output in the form of
platelet/imMKCL ratio from variously passaged clone 38 imMKCLs with and without incubation of vincristine or vinblastine. Vincristine effects 2-fold increase in platelet output as compared to the control from both flat and baffled surface flasks (FIG. 7B).
[0045] FIG. 8 shows exemplary data relating to vincristine and vinblastine validation, which were identified using the high-throughput imaging library screen.
[0046] FIG. 9 shows exemplary data relating to the temporal treatment of vincristine on platelet production in shaking conditions. Platelet count was performed post-treatment (C-38).
[0047] FIG. 10 depicts an exemplary scheme used to identify compounds in clone 38 imMKCLs that promote endoreplication/endomitosis. FACS based data shows that CCT137690, Barasertib, PF03814735 promote endoreplication/endomitosis. Figure shows concentration titration as well as various biological replicates depicting that CCT137690 promotes higher endomitosis as compared to other compounds.
[0048] FIG. 11 shows the gating strategy used to identify and segregate singlet imMKCLs on D3 in the absence of DOX from the culture. CD41a+CD42b+ megakaryocyte fraction was identified and measurement of ploidy in this CD41a+CD42b+ fraction was performed.
[0049] FIG. 12 shows an exemplary combinatorial and sequential treatment plan of imMKCLs with CCT137690 and vincristine to develop a synergistic or additive effect, as compared to the flat surface imMKCL (untreated) controls, the Baffled surface imMKCL cultures treated with Vincristine and CCT137690 possess ~14-16-fold higher number of platelets
[0050] FIG. 13 shows an exemplary scheme of“scale up” using the optimized CCT137690 and vincristine treatment plan and PBS biotech bioreactor. Treatment of 10L7 imMKCLs in lOOmL of differentiation media with the optimized CCT137690 and vincristine treatment plan enables generation of 1.2-1.5c10L9 platelets.
[0051] FIG. 14 shows TEM based ultrastructure/organelle comparison of CCT137690 and vincristine treated platelets and fresh donor platelets
[0052] FIG. 15 shows FACS based in vitro activation analyses and comparison of CCT137690 and vincristine treated imMKCL-platelets and fresh donor platelets.
[0053] FIG. 16 shows LTA based aggregation analyses and comparison of CCT137690 and vincristine treated imMKCL-platelets and fresh donor platelets.
[0054] FIG. 17 shows tail vein bleeding time analyses in thrombocytopenic mice (generated via busulphan treatment). This exemplary assay shows the tail vein bleeding times post infusion of CCT137690 and Vincristine treated imMKCL-platelets and fresh donor platelets.
[0055] FIG. 18 Figure shows thrombus incorporation analyses and simultaneous comparison of CCT137690 and Vincristine treated imMKCL-platelets and fresh donor platelets.
DETAILED DESCRIPTION
[0056] The compositions and methods described herein are related, in part, to the discovery that vincristine, vinblastine, and/or Aurora kinase inhibitor can induce platelet production. That is, vincristine, vinblastine, an Aurora kinase inhibitor, or any combination thereof can increase the number of platelets produced by a megakaryocyte or population thereof, as compared to the number of platelets produced by a substantially similar megakaryocyte that is not treated with vincristine, vinblastine, and/or an Aurora kinase inhibitor.
Definitions
[0057] As used herein, the term in v/YO-differentiated megakaryocytes” refers to megakaryocyte cells that are generated in culture, typically via step-wise differentiation from a precursor cell such as a human embryonic stem cell, an induced pluripotent stem cell, an early mesoderm cell, a lateral plate mesoderm cell or a cardiac progenitor cell. In one embodiment, the term“in v/Yro-differentiated megakaryocytes” excludes adult human megakaryocytes isolated from a subject (e.g., primary megakaryocytes). In some embodiments, the in v/Yro-differentiated megakaryocytes are synchronized in culture.
[0058] As used herein, the term“immortalized megakaryocytes” refers to megakaryocytes that can be expanded or passaged in an in vitro culture.
[0059] The term "marker" as used herein is used to describe a characteristic and/or phenotype of a cell. Markers can be used for selection of cells comprising characteristics of interest and can vary with specific cells. Markers are characteristics, whether morphological, structural, functional or biochemical (enzymatic) characteristics of the cell of a particular cell type, or molecules expressed by the cell type. In one aspect, such markers are proteins. Such proteins can possess an epitope for antibodies or other binding molecules available in the art. However, a marker can consist of any molecule found in or on a cell, including, but not limited to, proteins (peptides and polypeptides), lipids, polysaccharides, nucleic acids and steroids. Examples of morphological characteristics or traits include, but are not limited to, shape, size, and nuclear to cytoplasmic ratio. Examples of functional characteristics or traits include, but are not limited to, the ability to adhere to particular substrates, ability to incorporate or exclude particular dyes, ability to migrate under particular conditions, and the ability to differentiate along particular lineages. Markers can be detected by any method available to one of skill in the art. Markers can also be the absence of a morphological characteristic or absence of proteins, lipids etc. Markers can be a combination of a panel of unique characteristics of the presence and/or absence of polypeptides and other morphological or structural characteristics. In one embodiment, the marker is a cell surface marker.
[0060] In the context of cell ontogeny, the term "differentiate", or "differentiating" is a relative term that indicates a "differentiated cell" is a cell that has progressed further down the developmental pathway than its precursor cell. Thus, in some embodiments, a stem cell as the term is defined herein, can differentiate to lineage-restricted precursor cells (such as a human hematopoietic progenitor cell),
which in turn can differentiate into other types of precursor cells further down the pathway (such as a tissue specific precursor, such as a megakaryocyte progenitor cell), and then to an end-stage differentiated cell, which plays a characteristic role in a certain tissue type, and may or may not retain the capacity to proliferate further. Methods for in vitro differentiation of stem cells to megakaryocytes are known in the art and/or described herein below. Also provided herein are methods for in vitro differentiation of megakaryocytes to platelets.
[0061] As used herein, the terms“dedifferentiation" or“reprogramming" or“retrodifferentiation" refer to the process that generates a cell that re-expresses a more stem cell phenotype or a less differentiated phenotype than the cell from which it is derived. For example, a multipotent cell can be dedifferentiated to a pluripotent cell. That is, dedifferentiation shifts a cell backward along the differentiation spectrum of totipotent cells to fully differentiated cells. Typically, reversal of the differentiation phenotype of a cell requires artificial manipulation of the cell, for example, by expressing stem cell-specific mRNA and/or proteins. Reprogramming is not typically observed under native conditions in vivo or in vitro.
[0062] As used herein, the term“somatic cell” refers to any cell other than a germ cell, a cell present in or obtained from a pre-implantation embryo, or a cell resulting from proliferation of such a cell in vitro. Stated another way, a somatic cell refers to any cells forming the body of an organism, as opposed to germline cells. Every cell type in the mammalian body— apart from the sperm and ova, the cells from which they are made (gametocytes) and undifferentiated stem cells— is a somatic cell: internal organs, skin, bones, blood, and connective tissue are all substantially made up of somatic cells organism other than an embryo or a fetus or results from proliferation of such a cell in vitro.
[0063] The term "substantially pure," or “homogeneous” with respect to a particular cell population, refers to a population of cells that is at least about 75%, preferably at least about 85%, more preferably at least about 90%, and most preferably at least about 95% pure, with respect to the cells making up a total cell population. That is, the terms "substantially pure" or "homogeneous,” with regard to a population of megakaryocytes or platelets, refers to a population of cells that contain fewer than about 20%, more preferably fewer than about 15%, 10%, 8%, 7%, most preferably fewer than about 5%, 4%, 3%, 2%, 1%, or less than 1%, of cells that are not megakaryocytes or platelets, respectively.
[0064] The term“separation” or“selection” as used herein refers to isolating different cell types into one or more populations and collecting the isolated population as a target cell population which is enriched, for example, in a specific target cell. Selection can be performed using positive selection, whereby a target enriched cell population is retained, or negative selection, whereby non-target cell types are discarded (thereby enriching for desired target cell types in the remaining cell population).
[0065] The term“positive selection” as used herein refers to selection of a desired cell type by retaining the cells of interest. In some embodiments, positive selection involves the use of an agent to assist in retaining the cells of interest, e.g., use of a positive selection agent such as an antibody which
has specific binding affinity for a surface antigen on the desired or target cell. In some embodiments, positive selection can occur in the absence of a positive selection agent, e.g., in a“touch-free” or closed system, for example, where positive selection of a target cell type is based on any of cell size, density and/or morphology of the target cell type.
[0066] The term“negative selection” as used herein refers to selection of undesired or non-target cells for depletion or discarding, thereby retaining (and thus enriching) the desired target cell type. In some embodiments, negative selection involves the use of an agent to assist in selecting undesirable cells for discarding, e.g., use of a negative selection agent such as a monoclonal antibody which has specific binding affinity for a surface antigen on unwanted or non-target cells. In some embodiments, negative selection does not involve a negative selection agent. In some embodiments, negative selection can occur in the absence of a negative selection agent, e.g., in a“touch-free” or closed system, for example, where negative selection of an undesired (non-target) cell type to be discarded is based on any of cell size, density and/or morphology of the undesired (non-target) cell type.
[0067] The terms“decrease”,“reduced”,“reduction”, or“inhibit” are all used herein to mean a decrease or lessening of a property, level, or other parameter by a statistically significant amount. In some embodiments,“reduce,”“reduction" or“decrease" or“inhibit” typically means a decrease by at least 10% as compared to a reference level and can include, for example, a decrease by at least about
10%, at least about 20%, at least about 25%, at least about 30%, at least about 35%, at least about
40%, at least about 45%, at least about 50%, at least about 55%, at least about 60%, at least about
65%, at least about 70%, at least about 75%, at least about 80%, at least about 85%, at least about
90%, at least about 95%, at least about 98%, at least about 99% , or more. As used herein,“reduction” or“inhibition” does not encompass a complete inhibition or reduction as compared to a reference level. “Complete inhibition” is a 100% inhibition as compared to a reference level.
[0068] The terms“increased" ‘increase" or“enhance" or“activate" are all used herein to generally mean an increase of a property, level, or other parameter by a statically significant amount; for the avoidance of any doubt, the terms“increased",“increase" or“enhance" or“activate" means an increase of at least 10% as compared to a reference level, for example an increase of at least about 20%, or at least about 30%, or at least about 40%, or at least about 50%, or at least about 60%, or at least about 70%, or at least about 80%, or at least about 90% or up to and including a 100% increase or any increase between 10-100% as compared to a reference level, or at least about a 2-fold, or at least about a 3 -fold, or at least about a 4-fold, or at least about a 5 -fold or at least about a 10-fold increase, at least about a 20-fold increase, at least about a 50-fold increase, at least about a 100-fold increase, at least about a 1000-fold increase or more as compared to a reference level.
[0069] As used herein the term“vinca alkaloid” refers to a set of compounds having anti-mitotic and anti-microtubule effects and typically used as chemotherapy agents in the treatment of cancer. The original vinca alkaloids were first derived from a plant of the Vinca genus (e.g., Vinca rosea) but newer compounds are synthetic derivatives of the plant compounds. Exemplary vinca alkaloids
include, but are not limited to, vinblastine, vincristine, vindesine, vinorelbine, vincaminol, vineridine, vinbumine, vinpocetine, minovincine, methoxyminovincine, minovincinine, vincadafformine, desoxyvincaminol and vincamajine.
[0070] As used herein, the term“Aurora kinase inhibitor” refers to a compound(s) that inhibit Aurora kinase, thus affecting downstream cellular processes such as cellular mitosis, phosphorylation of histone H3, etc. Exemplary Aurora kinase inhibitors include, but are not limited to, CCT137690, ZM447439, Hesperadin, VX-680, barasertib, alisertib, danusertib, AT9283, PF-03914735, or AMG 900, among others. In some embodiments, the Aurora kinase inhibitor is an Aurora kinase-A inhibitor, an Aurora kinase -B inhibitor or a pan-Aurora kinase inhibitor.
[0071] As used herein the term "comprising" or "comprises" is used in reference to compositions, methods, and respective component(s) thereof, that are essential to the invention, yet open to the inclusion of unspecified elements, whether essential or not.
[0072] As used herein the term "consisting essentially of' refers to those elements required for a given embodiment. The term permits the presence of additional elements that do not materially affect the basic and novel or functional characteristic(s) of that embodiment of the invention.
[0073] The term "consisting of' refers to compositions, methods, and respective components thereof as described herein, which are exclusive of any element not recited in that description of the embodiment.
[0074] As used in this specification and the appended claims, the singular forms“a,” "an," and "the" include plural references unless the context clearly dictates otherwise. Thus for example, references to "the method" includes one or more methods, and/or steps of the type described herein and/or which will become apparent to those persons skilled in the art upon reading this disclosure and so forth.
Megakaryocyte and Platelet Biology
[0075] Platelets are small (e.g., 2-3 um in diameter), irregularly shaped clear cell fragments that are formed from fragmentation of precursor megakaryocytes. Megakaryocytes are derived from hematopoietic stem cell precursor cells in the bone marrow in response to thrombopoietin (TPO). TPO induces differentiation of progenitor cells in the bone marrow towards a final megakaryocyte phenotype. Typically, megakaryocytes develop through the following lineage: CFU-ME (pluripotential hematopoietic stem cell or hemocytoblast) megakaryoblast promegakaryocyte megakaryocyte. The cell eventually reaches megakaryoblast stage and loses its ability to divide. However, it is still able to replicate its DNA and continue development, becoming polyploid. The cytoplasm continues to expand and the DNA complement can increase to greater than 64 N.
[0076] Only when a megakaryocyte is mature does it begin the process of producing platelets. TPO also plays a role in inducing the megakaryocyte to form small proto-platelet processes. Platelets are held within internal membranes within the cytoplasm of the megakaryocytes. There are two
proposed mechanisms for platelet release: (i) proto-platelet processes break up explosively to become platelets, and (ii) the cell forms platelet ribbons into blood vessels. The ribbons are formed via pseudopodia and they are able to continuously emit platelets into circulation. In either scenario, each of these proto-platelet processes can give rise to 2000-5000 new platelets upon breakup. Overall, more than 75% of these newly-produced platelets will remain in circulation while the remainder will be sequestered by the spleen.
[0077] For the purpose of generating in vitro- derived platelets, it may be necessary to produce synchronous megakaryocytes. One method to ensure that the megakaryocytes are synchronous is to generate them in vitro from pluripotent stem cells.
[0078] A megakaryocyte for use with the methods and compositions described herein can be an immortalized megakaryocyte or population thereof. In some embodiments, the immortalized megakaryocyte or population thereof is/are conditionally immortalized, wherein expression of the gene(s) necessary for the immortalized phenotype are controlled using an inducible promoter (e.g., a doxy cy cline controlled promoter).
Stem Cells
[0079] There has been much interest in the possibility of using stem cells to produce platelets in the laboratory for clinical use. Stem cells are undifferentiated cells in early stage of development and are capable of giving rise to more cells of the same type or differentiating into a diverse range of cell lineages. The main different types of stem cells are human embryonic stem cells (hESC), induced pluripotent stem cells (iPSC), and adult hematopoietic stem cells (HSC).
[0080] The methods and compositions described herein can utilize in vitro derived megakaryocytes to produce platelets in vitro. The following describes various stem cells that can be used to prepare a megakaryocyte of a population thereof for the purpose of megakaryopoiesis.
[0081] Stem cells are cells that retain the ability to renew themselves through mitotic cell division and can differentiate into more specialized cell types. Three broad types of mammalian stem cells include: embryonic stem (ES) cells that are found in blastocysts, induced pluripotent stem cells (iPSCs) that are reprogrammed from somatic cells, and adult stem cells that are found in adult tissues. Other sources of pluripotent stem cells can include amnion-derived or placental-derived stem cells. In a developing embryo, stem cells can differentiate into all of the specialized embryonic tissues. In adult organisms, stem cells and progenitor cells act as a repair system for the body, replenishing specialized cells, but also maintain the normal turnover of regenerative organs, such as blood, skin or intestinal tissues. Pluripotent stem cells can differentiate into cells derived from any of the three germ layers.
[0082] Megakaryocytes useful in the methods and compositions described herein can be differentiated from both embryonic stem cells and induced pluripotent stem cells, among others. In one embodiment, the embryonic stem cells or induced pluripotent stem cells are human cells. Alternatively, in some embodiments, the compositions and methods provided herein do not
encompass generation or use of human megakaryocytes or platelets made from cells taken from a viable human embryo.
[0083] Embryonic stem cells and methods for their retrieval are well known in the art and are described, for example, in Trounson A O (Reprod Fertil Dev (2001) 13: 523), Roach M L (Methods Mol Biol (2002) 185: 1), and Smith A G (Annu Rev Cell Dev Biol (2001) 17:435). The term "embryonic stem cell" is used to refer to the pluripotent stem cells of the inner cell mass of the embryonic blastocyst (see e.g., US Patent Nos. 5843780, 6200806) An embryonic stem comprises characteristics including, without limitation, an embryonic stem cell specific gene expression profile, proliferative capacity, differentiation capacity, karyotype, responsiveness to particular culture conditions, and the like. Embryonic stem cells are considered to be undifferentiated when they have not committed to a specific differentiation lineage. Such cells display morphological characteristics that distinguish them from differentiated cells of embryo or adult origin. Undifferentiated embryonic stem (ES) cells are easily recognized by those skilled in the art, and typically appear in the two dimensions of a microscopic view in colonies of cells with high nuclear/cytoplasmic ratios and prominent nucleoli. In one embodiment, the human megakaryocytes and/or platelets described herein are derived from embryonic stem cells. In some embodiments, the human megakaryocytes and/or platelets described herein are not derived from embryonic stem cells or any other cells of embryonic origin.
[0084] Cells derived from embryonic sources can include embryonic stem cells or stem cell lines obtained from a stem cell bank or other recognized depository institution. Other means of producing stem cell lines include methods comprising the use of a blastomere cell from an early stage embryo prior to formation of the blastocyst (at around the 8-cell stage). Such techniques correspond to the pre implantation genetic diagnosis technique routinely practiced in assisted reproduction clinics. The single blastomere cell is co-cultured with established ES-cell lines and then separated from them to form fully competent ES cell lines.
[0085] Adult Stem Cells: Adult stem cells are stem cells derived from tissues (e.g., bone marrow) of a post-natal or post-neonatal organism or from an adult organism. An adult stem cell (e.g., hematopoietic stem cell) is structurally distinct from an embryonic stem cell not only in markers it does or does not express relative to an embryonic stem cell, but also by the presence of epigenetic differences, e.g. differences in DNA methylation patterns. In one embodiment, the human megakaryocytes and/or platelets described herein are derived from an adult stem cell; in one embodiment, they are derived in vitro from a hematopoietic stem cell (i.e., megakaryocytes do not need to be isolated directly from the bone marrow of an adult).
[0086] Induced Pluripotent Stem Cells (iPSCs): In some embodiments, the methods and compositions described herein utilize megakaryocytes that are differentiated in vitro from induced pluripotent stem cells for platelet production. An advantage of using iPSCs to generate a megakaryocyte or population thereof for the methods described herein is that the cells can be derived
from the same subject to which the resulting platelets are to be administered. That is, a somatic cell can be obtained from a subject, reprogrammed to an induced pluripotent stem cell, and then re- differentiated into a human megakaryocyte for the production of autologous platelets. In some embodiments, the megakaryocytes and/or platelets are derived from non-autologous sources. In addition, the use of iPSCs negates the need for cells obtained from an embryonic source. Thus, in one embodiment, the stem cells used to generate megakaryocytes and/or platelets for use in the compositions and methods described herein are not embryonic stem cells.
[0087] Although differentiation is generally irreversible under physiological contexts, several methods have been developed in recent years to reprogram somatic cells to induced pluripotent stem cells. Exemplary methods are known to those of skill in the art and are described briefly herein below.
[0088] Reprogramming is a process of driving the differentiation of a cell backwards to a more undifferentiated or more primitive type of cell. It should be noted that placing many primary cells in culture can lead to some loss of fully differentiated characteristics. However, simply culturing such cells included in the term differentiated cells does not render these cells non-differentiated cells (e.g., undifferentiated cells) or pluripotent cells. The transition of a differentiated cell to pluripotency requires a reprogramming stimulus beyond the stimuli that lead to partial loss of differentiated character when differentiated cells are placed in culture. Reprogrammed cells also have the characteristic of the capacity of extended passaging without loss of growth potential, relative to primary cell parents, which generally have capacity for only a limited number of divisions in culture.
[0089] The cell to be reprogrammed can be either partially or terminally differentiated prior to reprogramming. In some embodiments, reprogramming encompasses complete reversion of the differentiation state of a differentiated cell (e.g., a somatic cell) to a pluripotent state or a multipotent state. In some embodiments, reprogramming encompasses complete or partial reversion of the differentiation state of a differentiated cell (e.g., a somatic cell) to an undifferentiated cell (e.g., an embryonic-like cell). Reprogramming can result in expression of particular genes by the cells, the expression of which further contributes to reprogramming. In certain embodiments described herein, reprogramming of a differentiated cell (e.g., a somatic cell) causes the differentiated cell to assume an undifferentiated state with the capacity for self-renewal and differentiation to cells of all three germ cell lineages. The resulting cells are referred to as“reprogrammed cells,” or“induced pluripotent stem cells (iPSCs or iPS cells).” iPS cells can be generated or derived from terminally differentiated somatic cells, as well as from adult stem cells, or somatic stem cells. That is, a non-pluripotent progenitor cell can be rendered pluripotent or multipotent by reprogramming.
[0090] The specific approach or method used to generate pluripotent stem cells from somatic cells (e.g., any cell of the body with the exclusion of a germ line cell; fibroblasts etc.) is not critical to the claimed invention. Thus, any method that re-programs a somatic cell to the pluripotent phenotype would be appropriate for use in the methods described herein.
[0091] The efficiency of reprogramming (i.e., the number of reprogrammed cells) derived from a
population of starting cells can be enhanced by the addition of various small molecules as shown by Shi, Y., et al (2008) Cell-Stem Cell 2:525-528, Huangfu, D., et al (2008) Nature Biotechnology 26(7):795-797, and Marson, A., et al (2008) Cell-Stem Cell 3: 132-135. Some non-limiting examples of agents that enhance reprogramming efficiency include soluble Wnt, Wnt conditioned media, BIX- 01294 (a G9a histone methyltransferase), PD0325901 (a MEK inhibitor), DNA methyltransferase inhibitors, histone deacetylase (HDAC) inhibitors, valproic acid, 5'-azacytidine, dexamethasone, suberoylanilide, hydroxamic acid (SAHA), vitamin C, and trichostatin (TSA), among others.
[0092] To confirm the induction of pluripotent stem cells for use with the methods described herein, isolated clones can be tested for the expression of a stem cell marker. Such expression in a cell derived from a somatic cell identifies the cells as induced pluripotent stem cells. Stem cell markers can be selected from the non-limiting group including SSEA3, SSEA4, CD9, Nanog, Fbxl5, Ecatl, Esgl, Eras, Gdf3, Fgf4, Cripto, Daxl, Zpf296, Slc2a3, Rexl, Utfl, and Natl. In one embodiment, a cell that expresses Oct4 or Nanog is identified as pluripotent. Methods for detecting the expression of such markers can include, for example, RT-PCR and immunological methods that detect the presence of the encoded polypeptides, such as Western blots or flow cytometric analyses. In some embodiments, detection does not involve only RT-PCR, but also includes detection of protein markers. Intracellular markers may be best identified via RT-PCR, while cell surface markers are readily identified, e.g., by immunocytochemistry.
[0093] The pluripotent stem cell character of isolated cells can be confirmed by tests evaluating the ability of the iPSCs to differentiate to cells of each of the three germ layers. As one example, teratoma formation in nude mice can be used to evaluate the pluripotent character of the isolated clones. The cells are introduced to nude mice and histology and/or immunohistochemistry is performed on a tumor arising from the cells. The growth of a tumor comprising cells from all three germ layers, for example, further indicates that the cells are pluripotent stem cells.
[0094] Reprogrammed somatic cells as disclosed herein can express any number of pluripotent cell markers, including: alkaline phosphatase (AP); ABCG2; stage specific embryonic antigen-1 (SSEA- 1); SSEA-3; SSEA-4; TRA-1-60; TRA-1-81; Tra-2-49/6E; ERas/ECAT5, E-cadherin; b-111-tubulin; a-smooth muscle actin (a-SMA); fibroblast growth factor 4 (Fgf4), Cripto, Daxl; zinc finger protein 296 (Zfp296); N-acetyltransferase-1 (Natl); (ES cell associated transcript 1 (ECAT1); ESG1/DPPA5/ECAT2; ECAT3; ECAT6; ECAT7; ECAT8; ECAT9; ECAT10; ECAT15-1; EC ATI 5- 2; Fthl 17; Sall4; undifferentiated embryonic cell transcription factor (Utfl); Rexl; p53; G3PDH; telomerase, including TERT; silent X chromosome genes; Dnmt3a; Dnmt3b; TRIM28; F-box containing protein 15 (Fbxl5); Nanog/ECAT4; Oct3/4; Sox2; Klf4; c-Myc; Esrrb; TDGF1; GABRB3; Zfp42, FoxD3; GDF3; CYP25A1; developmental pluripotency-associated 2 (DPPA2); T-cell lymphoma breakpoint 1 (Tell); DPPA3/Stella; DPPA4; other general markers for pluripotency, etc. Other markers can include Dnmt3L; Soxl5; Stat3; Grb2; b-catenin, and Bmil. Such cells can also be
characterized by the down-regulation of markers characteristic of the somatic cell from which the induced pluripotent stem cell is derived.
In vitro Differentiation to Megakaryocytes
[0095] The methods and compositions described herein use in vitro differentiated megakaryocytes for the production of platelets or platelet-like particles. Methods for the differentiation of megakaryocytes from ESCs or IPSCs are known in the art. (See, e.g., Takayama et al. Methods Mol Biol 788:205-217 (2012); Liu et al. Stem Cells Transl Med 4(4):309-319 (2015); Moreau et al. Nature Communications 7 (2016) doi: 10.1038/ncommsl 1208; Chagraoui et al. PLOS One 7(3):e32981 (2012); Eto et al. PNAS 99(20): 12819-12824 (2002); US2014/0127815; US2016//0002586; the contents of each of which are incorporated herein by reference in their entirety). These approaches use various factors and conditions to activate and guide differentiation programs leading to their respective cell types.
[0096] As will be appreciated by those of skill in the art, in v/Yro-differentiation of megakaryocytes produces an end-result of a cell having the phenotypic and morphological features of a megakaryocyte but that the differentiation steps of in v/Yro-differentiation need not be the same as the differentiation that occurs naturally in the embryo. That is, during differentiation to a megakaryocyte, it is specifically contemplated herein that the step-wise differentiation approach utilized to produce such cells need not proceed through every progenitor cell type that has been identified during embryogenesis and can essentially“skip” over certain stages of development that occur during embryogenesis.
Maturation of Megakaryocytes In Vitro
[0097] In vivo megakaryocytes undergo a process called endomitosis, where they double their nuclear and cellular contents without cell division. Through endomitosis, the megakaryocyte grows to enormous size and can have more than 64 times the normal nuclear contents. The increase of nuclear contents, or polyploidy, plays a fundamental role in the platelet formation by allowing the cell to produce the large amounts of proteins and organelles necessary for platelet formation and function. Importantly, mature megakaryocytes also have vast quantities of extra cell membrane with which to make platelets. Inducing polyploidization or maturation of megakaryocytes in vitro can be achieved using the following reagents alone or in different combinations.
[0098] Overexpression of Ectopic genes: It is desirable to generate self-renewing megakaryocytes progenitor cells that can be quickly matured in order to initiate platelet production. In some embodiments, such self-renewing megakaryocyte progenitor cells are immortalized cells. Typically, the control of the maturation of megakaryocytes from self-renewing cells is achieved by overexpression of one or more megakaryocyte maturation markers, the expression of which is under the control of an inducible promoter. For example, a doxycycline (dox) -inducible promoter can be
used to regulate expression of a megakaryocyte maturation marker such that in the presence of doxy cy cline the maturation marker is expressed (or vice versa). Several approaches for inducing the maturation of self-renewing or immortalized megakaryocytes are summarized herein, however the means for maturing megakaryocytes in vitro is not critical to the invention. That is, any method for maturing megakaryocytes known in the art can be combined with the methods described herein to increase efficiency of platelet production in vitro.
[0099] One system for maturing megakaryocytes in vitro uses a lentivirus to express the transcription factors MYC and BMI1, and one or more pro-survival proteins (e.g., Bcl-xL or BCL2L1) under the control of a dox-inducible promoter. In the presence of dox, the immortalized megakaryocyte progenitors self-renew, and when the dox is removed the progenitors mature into megakaryocytes and release platelets. A second system employs controlled expression of GATA1 by a dox-inducible promoter. Under conditions where GATA1 expression is repressed, the megakaryocyte progenitor cells self-renew and when GATA1 expression is induced the cells mature into megakaryocytes that release platelets. Other genes that can be targeted to modulate maturation of megakaryocytes include, but are not limited to, gpl30/interleukin-6-dependent genes, Notch, stromal cell-derived factor 1 (SDFl)/fibroblast growth factor 4-dependent signaling pathways and interleukin 1 -alpha signaling pathways. It is specifically contemplated herein that vincristine and/or vinblastine can be used in combination with any of these systems, or alternatively in isolation, to increase the efficiency of platelet production.
[00100] Rho Rock inhibitors. The final steps of megakaryocyte cell division require regulation of actin and myosin to form the cleavage furrow and contractile ring. The inhibition of actin and myosin during cytokinesis allows megakaryocytes to replicate DNA material without undergoing cell division. The Rho/Rock pathway signals through myosin light chain (MLC) and filamin and activates both stress fibers and lamellipodia formation. Y27632 inhibits the Rho/Rock pathway and consequently inhibits myosin activation and the contractile ring formation, presumably allowing the megakaryocyte to undergo polyploidization. Exemplary Rho/Rock inhibitors include, but are not limited to, Y27632, thiazovivin, GSK429286A, fasudil HC1, Y39983, Wf-536, SLx-2119, Azabenzimidazole-aminofurazans, DE-104, and H-1152P.
[00101 ] Nicotinamide (NIC). Decreases in p53 activity are responsible for accelerated DNA synthesis, higher ploidy and delayed apoptosis. NIC increases p53 activity and thus increases endomitosis and megakaryocyte polyploidization.
[00102] Src inhibitors. The inhibition of Src family kinases increases megakaryocyte polyploidization through the Lyn Fyn pathway and inhibition of actin polymerization. Exemplary Src inhibitors include, but are not limited to, saracatinib (AZD0530), bosutinib (SKI-606), danusertib (PHA- 739358), NVP-BHG712, quercetin (sophoretin), PCI-32765, KX2-391, AP23846, and PP2.
[00103] Aurora-B inhibitors. Aurora-B is responsible for controlling microtubule formation and subsequent chromosome separation during mitosis. Its inhibition increases microtubule destruction
through stathmin and mitotic centromere-associated kinesin (MCAK) action. Exemplary Aurora-B kinase inhibitors include, but are not limited to, AMG 900, AT9283, Aurora A Inhibitor I, AZD1152, AZD1152-HQPA (barasertib), CCT129202, CYC 116, danusertib (PHA-739358), ENMD-2076, GSK1070916, hesperadin, JNJ-7706621, KW-2449, MLN8054, MLN8237 (alisertib), PF-03814735, PHA-680632, SNS-314, TAK-901, VX-680 (MK-0457, tozasertib), and ZM-447439.
[00104] A vb? Light Chain Kinase Inhibitors. Myosin light chain kinase (MLCK) is involved in late stages of myosin stimulation; it acts through MLC and is responsible for stress fiber activation and lamellipodia formation. Exemplary MLCK inhibitors include, but are not limited to, A3 HC1, Go 7874 HC1, InSolution™ K-252a (Nocardiopsis sp.), K-252a (Nocardiopsis sp.), K-252b (Nocardiopsis sp.), ML-7 HC1, ML-9 HC1, MLCK inhibitor peptide 18, piceatannol, and staurosporine ( Streptomyces sp).
[00105 \Phorbol 12-myristate 13-acetate (PMA). Protein kinase C (PKC) is involved in megakaryocyte differentiation and growth and its activation through PMA increases cell ploidy.
[00106] Blebbistatin. Blebbistatin inhibits myosin II and consequently the last steps of cytokinesis and cell division, thus allowing the cell to undergo polyploidization and increase the nuclear material.
[00107] Other stimulators of megakaryocyte maturation include the transcription factor‘all trans retinoic acid,’ and the histone deacetylase inhibitor valproic acid. Racl activation, CDC42 activation, hirudin and c-Myc inhibition also increase proplatelet formation.
[00108] In addition, some physical stimuli can induce megakaryocyte maturation and polyploidization, such as an increased oxygen concentration during culture, culturing at a temperature higher than conventional culturing temperature (e.g., 39°C).
[00109] The successful maturation of megakaryocytes from pluripotent stem cells can be determined by morphology (e.g., presence of DNA polyploidy (e.g., up to 128N), increase in cell size (up to a diameter of 60 um), increased granularity, and the presence of intracellular organelles reminiscent of pro-platelets), or by the presence of cell surface markers (e.g., CD41, CXCR4, or the TPO receptor, among others).
[00110] Cell surface markers are useful with the methods and compositions described herein to identify the differentiation or dedifferentiation state of a cell. Both cell surface markers and intracellular markers can be detected, for example, using an antibody for binding, e.g., cell surface markers or by PCR for intracellular markers. In some embodiments, antibodies or similar agents specific for a given marker, or set of markers, can be used to separate and isolate the desired cells using fluorescent activated cell sorting (FACS), panning methods, magnetic particle selection, particle sorter selection and other methods known to persons skilled in the art, including density separation (Xu et al. (2002) Circ. Res. 91:501; U.S.S.N. 20030022367) and separation based on other physical properties (Doevendans et al. (2000) J. Mol. Cell. Cardiol. 32:839-851).
[00111] In some embodiments, negative selection can be performed, including selecting and removing cells with undesired markers or characteristics, for example fibroblast markers, epithelial cell markers
etc. Undifferentiated ES cells express genes that can be used as markers to detect the presence of undifferentiated cells. Exemplary ES cell markers include stage -specific embryonic antigen (SSEA)- 3, SSEA-4, TRA-I-60, TRA-1-81, alkaline phosphatase or those described in e.g., U.S.S.N. 2003/0224411; or Bhattacharya (2004) Blood 103(8):2956-64, each herein incorporated by reference in their entirety. Exemplary markers expressed on cardiac progenitor cells include, but are not limited to, TMEM88, GATA4, ISL1, MYL4, and NKX2-5.
Formulations for Increasing Platelet Production
[00112] The methods described herein relate to increasing the amount of platelets produced in vitro from megakaryocytes or the efficiency of platelet production from cultured megakaryocytes by contacting a megakaryocyte cell or population thereof with an effective amount of vincristine, vinblastine, and/or an Aurora kinase inhibitor. An “effective amount” refers to an amount of vincristine, vinblastine, and/or an Aurora kinase inhibitor that increases the number of platelets produced from a given megakaryocyte (e.g., platelet/megakaryocyte) by at least 10% as compared to a megakaryocyte grown under substantially identical conditions but in the absence of vincristine, vinblastine, and/or an Aurora kinase inhibitor. For example, an effective amount of vincristine, vinblastine, and/or an Aurora kinase inhibitor can increase the number of platelets produced by at least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 75%, at least 80%, at least 90%, at least 95%, at least 99%, at least 1-fold, at least 2-fold, at least 5-fold, at least 10- fold, at least 25 -fold, at least 50-fold, at least 100-fold or more as compared to a megakaryocyte not treated with vincristine, vinblastine, and/or an Aurora kinase inhibitor.
[00113] In some embodiments, the effective amount of vincristine, vinblastine, and/or the Aurora kinase inhibitor is within the range of 4nM - 100 mM, inclusive. In other embodiments, the range of vincristine, vinblastine, and/or the Aurora kinase inhibitor for use with the methods and compositions described herein is: 0.04mM - 50 mM, for example, 0.04-40 mM, 0.04-30 mM, 0.04-20 mM, 0.04- 10 mM, 0.04-5 mM, 0.04-4 mM, 0.004-3 mM, 0.04-2 mM, 0.04-1 mM, 0.04-0.01 mM, 0.04-0-0.75 mM, 0.1-40 mM, 0.5-40 mM, 1-40 mM, 5-40 mM, 10-40mM, 20-40 mM, 30-40 mM, 0.1-5 mM, 0.1-4 mM, 0.1-3 mM, 0.1-2 mM, 0.1-1 mM, 0.5-5 mM, 0.5-4 mM, 0.5-3 mM, 0.5-2 mM, 0.5-1 mM, 0.75-1.5 mM, 0.5-1.5 mM, 15-30mM, or any range therebetween. In one embodiment, the effective amount of vincristine, vinblastine, and/or the Aurora kinase inhibitor is about 1 mM.
[00114] In some embodiments, the vincristine, vinblastine, and/or an Aurora kinase inhibitor is contacted with the megakaryocyte cells at day 0 of a platelet biogenesis protocol, such as the protocol provided herein in the working Examples. In other embodiments, the vincristine, vinblastine, and/or Aurora kinase inhibitor is contacted with the megakaryocyte cells at day 1, 2, 3, 4, 5, or 6 of a platelet biogenesis protocol. In one embodiment, the vincristine, vinblastine, and/or Aurora kinase inhibitor is contacted with the megakaryocyte cells from day 3 to the end of the platelet biogenesis protocol (e.g.,
from days 3-6, or days 3-5, or days 3-7). In some embodiments, the vincristine, vinblastine, and/or Aurora kinase inhibitor is contacted with the megakaryocyte cells for at least 6h, at least 12h, at least 24h, at least 2 days, at least 3 days, at least 4 days, at least 5 days or more (e.g., until the end of the platelet biogenesis protocol).
[00115] In some embodiments, the Aurora kinase inhibitor is an Aurora B kinase inhibitor. Exemplary Aurora-B kinase inhibitors include, but are not limited to, AMG 900, AT9283, Aurora A Inhibitor I, AZD1152, AZD1152-HQPA (barasertib), CCT129202, CYC116, danusertib (PHA-739358), ENMD- 2076, GSK1070916, hesperadin, JNJ-7706621, KW-2449, MLN8054, MLN8237 (alisertib), PF- 03814735, PHA-680632, SNS-314, TAK-901, VX-680 (MK-0457, tozasertib), and ZM-447439.
[00116] As will be appreciated by one of skill in the art, formulations of vincristine, vinblastine, and/or Aurora kinase inhibitors are preferably non-toxic to cultured cells and do not substantially alter cell viability, cell growth rate etc. Any cell culture medium and/or supplements can be used to optimize the growth and maintenance of self-renewing megakaryocyte progenitor cells or immortalized megakaryocytes for the purpose of generating platelets in vitro. Such methods of optimization are known to those of skill in the art or can be determined using techniques known in the art. It is also contemplated herein that the cells can be cultured on a desired substrate, such as laminin, fibronectin, poly-lysine or methy cellulose.
[00117] The number of platelets produced under a given set of conditions can be determined using FACS or a megakaryocyte bursting assay as described herein in the Examples section. FACS counting can be performed on the basis of size; that is FACS can be used to sort platelets from megakaryocytes based on size. Alternatively, suitable cell surface markers useful for counting platelets include, but are not limited to, CD41, CD61 and/or CD62.
[00118] As the data in the Examples section demonstrates, vincristine and/or vinblastine when used in combination with an Aurora kinase inhibitor, synergistically increase platelet production. As used herein, the term "synergistic" means that the increase in platelet production achieved with compositions comprising vincristine and/or vinblastine in combination with an Aurora kinase inhibitor is greater than the sum of the effects that result from using vincristine, vinblastine or the Aurora kinase inhibitor alone. Accordingly, in one aspect provided herein is a composition, i.e., a synergistic composition, comprising vincristine and/or vinblastine in combination with an Aurora kinase inhibitor. For example, a composition comprising an effective amount of vincristine and/or vinblastine and an effective amount of an Aurora kinase inhibitor.
[00119] In some embodiments, the composition comprises vincristine and an Aurora kinase inhibitor. For example, vincristine and an Aurora kinase inhibitor selected from the group consisting of CCT137690, ZM447439, Hesperadin, VX-680, barasertib, alisertib, danusertib, AT9283, PF- 03914735, or AMG 900. In one embodiment, the composition comprises vincristine and the Aurora kinase inhibitor CCT137690.
[00120] In some other embodiments, the composition comprises vinblastine and an Aurora kinase inhibitor. For example, vinblastine and an Aurora kinase inhibitor selected from the group consisting of CCT137690, ZM447439, Hesperadin, VX-680, barasertib, alisertib, danusertib, AT9283, PF- 03914735, or AMG 900. In one embodiment, the composition comprises vinblastine and the Aurora kinase inhibitor CCT137690.
[00121] In some embodiments, the composition further comprises megakaryocyte cell(s). It is noted that the megakaryocytes can be cultured megakaryocytes, e.g., immortalized and/or matured megakaryocytes. In some other embodiments, the composition further comprises plate let(s). In still other embodiments, the composition further comprises both megakaryocyte cell(s) and platelet(s).
Large Scale Platelet Production
[00122] It is specifically contemplated herein that the methods and compositions for increasing the number and/or efficiency of platelet production using vincristine and/or vinblastine can be performed in a large-scale manner, for example, in a bioreactor. The specific methods for increasing the scale of platelet production are not critical to the invention and as such any method known in the art can be used in combination with the methods described herein.
[00123] Some non-limiting examples of large-scale platelet production methods can be found in US2012/0238020, US2018/0104692, US2012/0315338, the contents of each of which are incorporated by reference in their entirety.
Kits
[00124] Another aspect of the technology described herein relates to kits for enhancing platelet production from cultured megakaryocytes, among others. Described herein are kit components that can be included in one or more of the kits described herein.
[00125] In some embodiments, the kit comprises an effective amount of vincristine, vinblastine, and/or Aurora kinase inhibitor formulated for use with cultured megakaryocytes (e.g., immortalized and/or matured megakaryocytes). As will be appreciated by one of skill in the art, vincristine, vinblastine, and/or Aurora kinase inhibitor can be supplied in a lyophilized form or a concentrated form that can diluted prior to use with cultured cells. Preferred formulations include those that are non-toxic to the cells and/or does not affect growth rate or viability etc. vincristine, vinblastine, and/or Aurora kinase inhibitor can be supplied in aliquots or in unit doses.
[00126] In some embodiments the kit further comprises a vector comprising a nucleic acid encoding a gene to mature megakaryocytes under the control of an inducible promoter for maturing megakaryocytes in vitro.
[00127] In some embodiments, the components described herein can be provided singularly or in any combination as a kit. The kit includes the components described herein, e.g., a composition comprising vincristine and/or vinblastine, a composition(s) that includes a vector comprising e.g., a
megakaryocyte maturation marker (e.g., GATA-1, CD42b etc.) under the control of an inducible promoter (e.g., doxycycline promoter), a doxycycline composition, and one or more maturation compounds as described throughout the specification. Such kits can optionally include one or more agents that permit the detection of residual stem cells or a set thereof. In addition, the kit optionally comprises informational material. In some embodiments, the kits provided herein comprise an aliquot of immortalized megakaryocytes and optionally a preferred medium for growth thereof. The kit can also contain a substrate for coating culture dishes, such as laminin, fibronectin, Poly-L-Lysine, or methylcellulose.
[00128] In some embodiments, the compositions in the kit can be provided in a watertight or gas tight container which in some embodiments is substantially free of other components of the kit. For example, a vincristine, vinblastine, and/or Aurora kinase inhibitor composition can be supplied in more than one container, e.g., it can be supplied in a container having sufficient reagent for a predetermined number of differentiation reactions, e.g., 1, 2, 3 or greater. One or more components as described herein can be provided in any form, e.g., liquid, dried or lyophilized form. It is preferred that the components described herein are substantially pure and/or sterile. When the components described herein are provided in a liquid solution, the liquid solution preferably is an aqueous solution, with a sterile aqueous solution being preferred.
[00129] The informational material can be descriptive, instructional, marketing or other material that relates to the methods described herein. The informational material of the kits is not limited in its form. In one embodiment, the informational material can include information about production of the megakaryocytes and/or platelets, concentration, date of expiration, batch or production site information, and so forth. In one embodiment, the informational material relates to methods for using or administering the components of the kit.
[00130] The kit can include a component for the detection of a marker for human cells, ES cells, iPS cells, hematopoietic progenitor cells, immature megakaryocytes, mature megakaryocytes, platelets etc. In addition, the kit can include one or more antibodies that bind a cell marker, or primers for an RT-PCR or PCR reaction, e.g., a semi -quantitative or quantitative RT-PCR or PCR reaction. Such components can be used to assess the activation of megakaryocyte-specific markers or the loss of ES cell, iPSC, or adult stem cell markers. If the detection reagent is an antibody, it can be supplied in dry preparation, e.g., lyophilized, or in a solution. The antibody or other detection reagent can be linked to a label, e.g., a radiological, fluorescent (e.g., GFP) or colorimetric label for use in detection. If the detection reagent is a primer, it can be supplied in dry preparation, e.g., lyophilized, or in a solution.
[00131] The kit will typically be provided with its various elements included in one package, e.g., a fiber-based, e.g., a cardboard, or polymeric, e.g., a Styrofoam box. The enclosure can be configured so as to maintain a temperature differential between the interior and the exterior, e.g., it can provide insulating properties to keep the reagents at a preselected temperature for a preselected time.
[00132] The present invention may be as defined in any one of the following numbered paragraphs.
[00133] 1. A method for increasing platelet production from a megakaryocyte, the method comprising: contacting a megakaryocyte (MK) or population thereof with an effective amount of vincristine, vinblastine, an Aurora kinase inhibitor, or a combination thereof.
[00134] 2. The method of paragraph 1, wherein the Aurora kinase inhibitor comprises CCT137690.
[00135] 3. The method of paragraph 1 or 2, wherein vincristine and/or vinblastine is used in combination with the Aurora kinase inhibitor.
[00136] 4. The method of paragraph 3, wherein the combination of the Aurora kinase inhibitor with either vincristine or vinblastine synergistically increases platelet production.
[00137] 5. The method of any one of paragraphs 1-4, wherein the method is performed in vitro.
[00138] 6. The method of any one of paragraphs 1-3 or 5, wherein platelet production is increased by at least 10% compared to an untreated control.
[00139] 7. The method of paragraph 3, wherein the untreated control comprises a substantially similar megakaryocyte or population thereof that is not contacted with vincristine or vinblastine.
[00140] 8. The method of any one of paragraphs 1-7, wherein the increase in platelet production is an increase in platelet number.
[00141] 9. The method of paragraph 8, wherein the increase in platelet number is an increase in the number of platelets per megakaryocyte (plate lets/MK).
[00142] 10. The method of any one of paragraphs 1-9, wherein the population of megakaryocytes is a homogenous population.
[00143] 11. The method of any one of paragraphs 1-10, wherein the effective amount of vincristine, vinblastine and/orthe aurora kinase is between O.OImM - IOOmM, inclusive.
[00144] 12. The method of paragraph 11, wherein the effective amount of vincristine, vinblastine and/orthe aurora kinase is between 0.1mM-5mM.
[00145] 13. The method of paragraph 12, wherein the effective amount of vincristine, vinblastine and/or the aurora kinase is between 0.75mM- 1.50mM.
[00146] 14. The method of any one of paragraphs 1-13, wherein the megakaryocyte or population thereof is grown on a substrate.
[00147] 15. The method of paragraph 14, wherein the substrate comprises laminin, fibronectin, Poly-L-Lysine, or methylcellulose.
[00148] 16. The method of any one of paragraphs 1-15, wherein the number of platelets is counted using FACS or a burst/spreading assay.
[00149] 17. The method of any one of paragraphs 1-16, wherein the megakaryocyte or population thereof is contacted with an effective amount of both vincristine and vinblastine, and optionally the Aurora kinase inhibitor.
[00150] 18. The method of any one of paragraphs 1-17, wherein the megakaryocyte cell or population thereof is an immortalized megakaryocyte cell or population thereof.
[00151] 19. The method of any one of paragraphs 1-18, wherein the vincristine, vinblastine, and/or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) after induction of terminal differentiation.
[00152] 20. The method of any one of paragraphs 1-19, wherein the vincristine, vinblastine, or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) at least one day after induction of terminal differentiation.
[00153] 21. The method of paragraph 20, wherein the vincristine, vinblastine, and/or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) from day 3 after induction of terminal differentiation.
[00154] 22. The method of any one of paragraphs 1-21, wherein the vincristine, vinblastine and/or
Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) until functional platelets are generated.
[00155] 23. The method of paragraph 19, wherein the vincristine, vinblastine, and/or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) until a maximal output of functional platelets are generated.
[00156] 24. A kit for increasing platelet production comprising: vincristine, vinblastine, and/or Aurora kinase inhibitor, instructions for use thereof, and at least one of the following: (i) Laminin substrate, (ii) Megakaryocyte cells, or (iii) a positive control comprising human platelet lysates in a known amount.
[00157] 25. The kit of paragraph 24, wherein the megakaryocyte cells are immortalized megakaryocyte cells.
EXAMPLES
EXAMPLE 1: Vincristine and vinblastine stimulate megakaryocyte maturation and platelet production
[00158] Using human pluripotent stem cells (hiPSCs) to produce universal donor type platelets in a scalable and cost-efficient manner remains challenging. Moreover, the mechanisms and signals that orchestrate megakaryocyte (MK) maturation and/or platelet (PLT) production have been the focus of numerous studies, yet they remain poorly understood. Consequently, in vitro PLT yields per MK as well as the quality of these PLTs are often low compared to their in vivo counterparts. During terminal differentiation, MKs become polyploid and generate PLTs.
[00159] The ability to mass-produce PLTs for clinical purposes or to study MK maturation and PLT production in detail has been limited by difficulties obtaining pure and homogeneous MKs as well as quantifying the rate, extent and dynamics of their maturation and pro-PLT production. To address these issues, conditionally immortalized hiPSC-derived MKs (imMKCLs) were used. A novel high- throughput, quantitative live-cell image-based assay using the Yokogawa (CV7000) automated confocal system was used that allowed us to measure MK maturation as well as pro-PLT phenotype and PLT production under live culture conditions. This assay was used to screen approximately 4,000 chemical genetics compounds, each at four different concentrations, and image processing and analysis scripts were developed to quantify the maturation of imMKCLs and the production of pro- PLTs and PLTs. About 300,000 images from over 20,000 wells were acquired, processed and analyzed using FIJI scripts, and the resulting data evaluated using MATLAB scripts. This primary screen identified ~30 compounds (Hit compounds) that substantially promoted a pro-PLT phenotype or the output of PLT-like particles. A secondary validation screen was performed on the hit compounds at 11 different doses ranging from 4hM-100mM. The 15 compounds that elicited a dose dependent increase of PLT release were selected for further evaluation. The effect of 15 candidate compounds on PLT production and PLT quality were then evaluated in 12 well format via flow cytometry. Of the 15 analyzed candidate compounds, two compounds (Vincristine and Vinblastine) augmented the yield of highly functional PLTs by a factor of 3-4-fold. Next, flow cytometry based temporal and concentration titrations were performed to identify the optimal conditions for maximal PLT generation. It was shown that ImM treatment of vincristine and vinblastine during a specific time window (from day 3 after induction of terminal differentiation) generated a maximal output of functional PLTs from imMKCLs. Furthermore, since vincristine and vinblastine target tubulins and microtubule structures it was further tested if other, chemically unrelated tubulin/microtubule binding molecules could also augment MK maturation and PLT production. However, none of the tested unrelated tubulin/microtubule active compounds were active in these assays, indicating that the positive effects observed when vincristine or vinblastine were added are specific to this class of tubulin/microtubule-binding molecules, and possibly to a select sub-set of vinca-alkaloid type microtubule inhibitors.
EXAMPLE 2: Novel High-throughput live-cell imaging assays to identify and validate agents that promote megakaryocyte maturation and trigger platelet release
[00160] A novel high-throughput, quantitative live-cell time-course imaging assay using the Yokogawa (CV7000) automated confocal system was developed that allowed us to measure MK maturation and pro-PLT production under live culture conditions for extended periods of time and at single cell resolution. Using this system, it was observed that PLT production is not synchronized between individual MKs and that only a fraction of MKs produces significant amounts of pro-PLTs,
indicating that MK-maturation and PLT-release promoting pathways are sub-optimally engaged under conventional culture conditions.
[00161] Next, a 1536 well plate based high content imaging chemical screening assay was developed and used to screen -4200 compounds, each at 4 different concentrations, for their ability to promote MK polyploidization and pro-PLT production. Over 60,000 images were processed and analyzed using FIJI and Matlab scripts. In addition to known MK maturation promoting compounds, such as PKC activators and SRC inhibitors, this screen identified novel pathways not previously implicated in MK biology as well as novel chemical entities with yet to be determined targets that induce a more synchronized and productive maturation of MKs.
EXAMPLE 3: Exemplary protocol for generation of synergistic effects of CCT137690 and Vincristine
[00162] 1.1 Centrifuge the expanding imMKCLs containing cell culture media at 400xg for 5 minutes, at RT (Room Temperature).
[00163] 1.2 Remove the supernatant via aspiration and gently re-suspend the cells in lmL of warm PBS (-/-) solution. Then add 49mL of PBS (-/-) solution to make the volume to 50mL.
[00164] 1.3 Centrifuge the imMKCLs containing PBS (-/-) solution at 400xg for 5 minutes, at RT (Room Temperature).
[00165] 1.4 Repeat the wash procedure 2 more times.
[00166] 1.5 Post PBS (-/-) solution washing, re-suspend the cells in lmL of basal Sev2 differentiation medium. The composition of the basal SeV2 differentiation media is in Table 2. Count the cells via FACS.
[00167] 1.6 Then, the cells are diluted to a concentration of 100,000cells/mL in CCT137690 (Aurora Kinase inhibitor) (working concentration of 90nM) SeV2 differentiation medium. Transfer the differentiation culture to orbital shaker Erlenmeyer flask or PBS biotech Bioreactor vessel.
[00168] 1.7 The differentiation cultures were incubated in the humidified incubators at 37°C from dayO to Day3 on orbital shaker at 50 RPM or on PBS biotech Bioreactor platform at 50 RPM.
[00169] 1.8 On Day 3, bring the cultures to the laminar hood and centrifuge differentiating imMKCLs containing differentiation culture media at 400xg for 5 minutes, at RT (Room Temperature).
[00170] 1.9 Resuspend the imMKCL pellet in fresh CCT137690 [90nM] containing differentiation culture medium. Aliquot lOOuL for FACS based counting of imMKCLs via FACS.
[00171] 1.10 Post FACS based counting of imMKCLs resuspend the imMKCLs at the density of 100,000cells/mL in CCT137690 [90nM] +Vincristine [ImM] containing differentiation culture medium. Transfer the differentiation culture to the same orbital shaker Erlenmeyer flask or PBS biotech Bioreactor vessel.
[00172] 1.10 The differentiation cultures were incubated in the humidified incubators at 37°C from day3 to Day6 on orbital shaker at 100 RPM or on PBS biotech Bioreactor platform at 95 RPM.
Table 1: SeV2 expansion media
Claims
1. A method for increasing platelet production from a megakaryocyte, the method comprising: contacting a megakaryocyte (MK) or population thereof with an effective amount of vincristine, vinblastine, an Aurora kinase inhibitor, or a combination thereof.
2. The method of claim 1, wherein the Aurora kinase inhibitor cisCCT137690.
3. The method of claim 1 or 2, wherein vincristine and/or vinblastine is used in combination with the Aurora kinase inhibitor.
4. The method of claim 3, wherein the combination of the Aurora kinase inhibitor with either vincristine or vinblastine synergistically increases platelet production.
5. The method of any one of claims 1-4, wherein the method is performed in vitro.
6. The method of any one of claims 1-3 or 5, wherein platelet production is increased by at least 10% compared to an untreated control.
7. The method of claim 3, wherein the untreated control comprises a substantially similar
megakaryocyte or population thereof that is not contacted with vincristine or vinblastine.
8. The method of any one of claims 1-7, wherein the increase in platelet production is an increase in platelet number.
9. The method of claim 8, wherein the increase in platelet number is an increase in the number of platelets per megakaryocyte (plate lets/MK).
10. The method of any one of claims 1-9, wherein the population of megakaryocytes is a
homogenous population.
11. The method of any one of claims 1-10, wherein the effective amount of vincristine, vinblastine and/or the aurora kinase is between O.OImM - IOOmM, inclusive.
12. The method of claim 11, wherein the effective amount of vincristine, vinblastine and/or the aurora kinase is between 0.1mM-5mM.
13. The method of claim 12, wherein the effective amount of vincristine, vinblastine and/or the aurora kinase is between 0.75mM- 1.50mM.
14. The method of any one of claims 1-13, wherein the megakaryocyte or population thereof is grown on a substrate.
15. The method of claim 14, wherein the substrate comprises laminin, fibronectin, Poly-L-Lysine, or methylcellulose.
16. The method of any one of claims 1-15, wherein the number of platelets is counted using FACS or a burst/spreading assay.
17. The method of any one of claims 1-16, wherein the megakaryocyte or population thereof is contacted with an effective amount of both vincristine and vinblastine, and optionally the Aurora kinase inhibitor.
18. The method of any one of claims 1-17, wherein the megakaryocyte cell or population thereof is an immortalized megakaryocyte cell or population thereof.
19. The method of any one of claims 1-18, wherein the vincristine, vinblastine, and/or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) after induction of terminal differentiation.
20. The method of any one of claims 1-19, wherein the vincristine, vinblastine, or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) at least one day after induction of terminal differentiation.
21. The method of claim 20, wherein the vincristine, vinblastine, and/or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) from day 3 after induction of terminal differentiation.
22. The method of any one of claims 1-21, wherein the vincristine, vinblastine and/or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) until functional platelets are generated.
23. The method of claim 19, wherein the vincristine, vinblastine, and/or Aurora kinase inhibitor is contacted with the megakaryocyte cell(s) until a maximal output of functional platelets are generated.
24. A kit for increasing platelet production comprising: vincristine, vinblastine, and/or Aurora kinase inhibitor, instructions for use thereof, and at least one of the following:
(i) laminin substrate,
(ii) megakaryocyte cells, or
(iii) positive control comprising human platelet lysates in a known amount.
25. The kit of claim 24, wherein the megakaryocyte cells are immortalized megakaryocyte cells.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201962816612P | 2019-03-11 | 2019-03-11 | |
| US62/816,612 | 2019-03-11 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2020185856A1 true WO2020185856A1 (en) | 2020-09-17 |
Family
ID=72427581
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2020/022023 Ceased WO2020185856A1 (en) | 2019-03-11 | 2020-03-11 | Methods for increasing platelet production |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2020185856A1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024120329A1 (en) * | 2022-12-06 | 2024-06-13 | 苏州血霁生物科技有限公司 | Method for differentiating platelets, culture medium, and use |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5571686A (en) * | 1994-04-14 | 1996-11-05 | Massachusetts Institute Of Technology | Method of using megapoietin for prolonging the survival & viability of platlets |
| WO2006001954A2 (en) * | 2004-05-20 | 2006-01-05 | Puget Sound Blood Center And Program | Methods for promoting the formation of platelets and for treating blood and bone marrow disorders |
| WO2013190320A1 (en) * | 2012-06-21 | 2013-12-27 | Cancer Research Technology Limited | Imidazopyridines as inhibitors of aurora kinase and/or flt3 |
| US20150148345A1 (en) * | 2013-11-26 | 2015-05-28 | Gilead Sciences, Inc. | Therapies for treating myeloproliferative disorders |
| US20160002599A1 (en) * | 2013-02-08 | 2016-01-07 | Kyoto University | Production methods for megakaryocytes and platelets |
| US20170121682A1 (en) * | 2011-03-18 | 2017-05-04 | New York Blood Center, Inc. | Megakaryocyte and platelet production from stem cells |
| US20180318353A1 (en) * | 2012-12-21 | 2018-11-08 | Astellas Institute For Regenerative Medicine | Methods for production of platelets from pluripotent stem cells and compositions thereof |
| WO2020006539A1 (en) * | 2018-06-29 | 2020-01-02 | Platelet Biogenesis, Inc. | Compositions for drug delivery and methods of use thereof |
-
2020
- 2020-03-11 WO PCT/US2020/022023 patent/WO2020185856A1/en not_active Ceased
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5571686A (en) * | 1994-04-14 | 1996-11-05 | Massachusetts Institute Of Technology | Method of using megapoietin for prolonging the survival & viability of platlets |
| WO2006001954A2 (en) * | 2004-05-20 | 2006-01-05 | Puget Sound Blood Center And Program | Methods for promoting the formation of platelets and for treating blood and bone marrow disorders |
| US20170121682A1 (en) * | 2011-03-18 | 2017-05-04 | New York Blood Center, Inc. | Megakaryocyte and platelet production from stem cells |
| WO2013190320A1 (en) * | 2012-06-21 | 2013-12-27 | Cancer Research Technology Limited | Imidazopyridines as inhibitors of aurora kinase and/or flt3 |
| US20180318353A1 (en) * | 2012-12-21 | 2018-11-08 | Astellas Institute For Regenerative Medicine | Methods for production of platelets from pluripotent stem cells and compositions thereof |
| US20160002599A1 (en) * | 2013-02-08 | 2016-01-07 | Kyoto University | Production methods for megakaryocytes and platelets |
| US20150148345A1 (en) * | 2013-11-26 | 2015-05-28 | Gilead Sciences, Inc. | Therapies for treating myeloproliferative disorders |
| WO2020006539A1 (en) * | 2018-06-29 | 2020-01-02 | Platelet Biogenesis, Inc. | Compositions for drug delivery and methods of use thereof |
Non-Patent Citations (1)
| Title |
|---|
| PAUL P. CARBONE, M.D., VINCENT BONO, M.D., EMIL FREI, III, M.D., CLYDE O. BRINDLEY, M.D.: "Clinical Studies with Vincristine", BLOOD, vol. 21, no. 5, 31 May 1963 (1963-05-31), pages 640 - 647, XP055739863 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024120329A1 (en) * | 2022-12-06 | 2024-06-13 | 苏州血霁生物科技有限公司 | Method for differentiating platelets, culture medium, and use |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5984217B2 (en) | Preparation of induced pluripotent stem cells from a small amount of peripheral blood | |
| CN103380212B (en) | A cell culture platform for single-cell sorting and enhanced IPSC reprogramming | |
| US8709805B2 (en) | Canine iPS cells and method of producing same | |
| WO2022253022A1 (en) | Method for detecting ipsc residues based on single-cell sequencing data analysis | |
| US20200248138A1 (en) | Method for inducing differentiation of pluripotent stem cells into germline stem cell-like cells | |
| EP2603583B1 (en) | Method of inducing differentiation from pluripotent stem cells to germ cells | |
| UA129013C2 (en) | Method for culture of cells | |
| CN104673741A (en) | High-efficiency non-integrated human iPSC induction platform | |
| EP4251735A1 (en) | Methods and cellular structures | |
| US8759098B2 (en) | Method for cloning pluripotent stem cells | |
| WO2020185856A1 (en) | Methods for increasing platelet production | |
| US12492372B2 (en) | Induced stem cells | |
| US20120009601A1 (en) | Methods and compositions for reprogramming cells | |
| JP6516280B2 (en) | Method for establishing iPS cells and method for long-term maintenance of stem cells | |
| WO2025035461A1 (en) | In-vitro blastoid induction and use of blastoid | |
| HK40068498B (en) | Cell culture platform for single cell sorting and enhanced reprogramming of ipscs | |
| CN116761882A (en) | induced stem cells | |
| KR20250021862A (en) | Method for inducing reprogramming of alveolar type 1 cell into alveolar type 2 cell using Rhosin | |
| JP2020174644A (en) | Cancer stem cell or cell population containing the same, method for producing the same and cancer treating agent screening method | |
| Chappell | Novel roles for MYC in the maintainence of pluripotency | |
| US20150258084A1 (en) | Stem cell compositions and methods of their use |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 20769313 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 20769313 Country of ref document: EP Kind code of ref document: A1 |