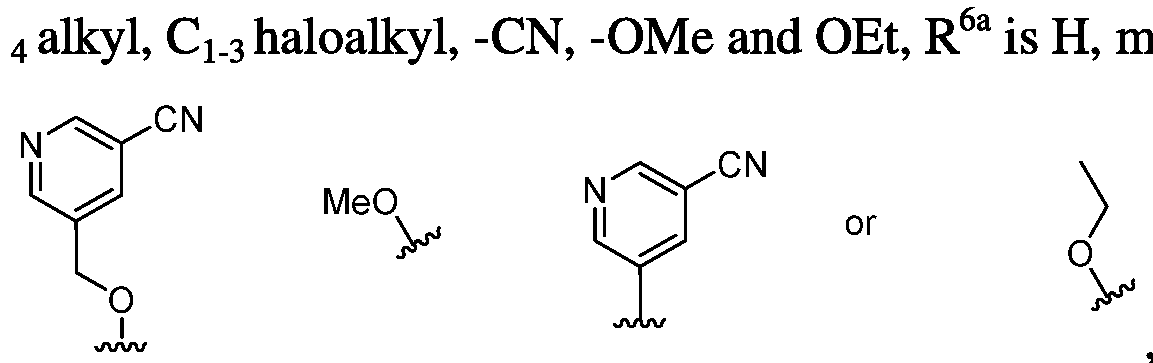WO2018005374A1 - Immunomodulator compounds - Google Patents
Immunomodulator compounds Download PDFInfo
- Publication number
- WO2018005374A1 WO2018005374A1 PCT/US2017/039313 US2017039313W WO2018005374A1 WO 2018005374 A1 WO2018005374 A1 WO 2018005374A1 US 2017039313 W US2017039313 W US 2017039313W WO 2018005374 A1 WO2018005374 A1 WO 2018005374A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- group
- independently selected
- optionally substituted
- haloalkyl
- Prior art date
Links
- 150000001875 compounds Chemical class 0.000 title claims abstract description 221
- 239000002955 immunomodulating agent Substances 0.000 title claims abstract description 12
- 229940121354 immunomodulator Drugs 0.000 title abstract description 6
- 230000002584 immunomodulator Effects 0.000 title description 3
- 150000003839 salts Chemical class 0.000 claims abstract description 91
- 238000000034 method Methods 0.000 claims abstract description 37
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 15
- -1 0-Ru Chemical group 0.000 claims description 143
- 125000004209 (C1-C8) alkyl group Chemical group 0.000 claims description 113
- 125000006648 (C1-C8) haloalkyl group Chemical group 0.000 claims description 101
- 229910052757 nitrogen Inorganic materials 0.000 claims description 94
- 125000001424 substituent group Chemical group 0.000 claims description 88
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 82
- 125000000623 heterocyclic group Chemical group 0.000 claims description 80
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 70
- 229910052739 hydrogen Inorganic materials 0.000 claims description 69
- 125000001072 heteroaryl group Chemical group 0.000 claims description 63
- 125000004043 oxo group Chemical group O=* 0.000 claims description 62
- 229910052736 halogen Inorganic materials 0.000 claims description 61
- 150000002367 halogens Chemical group 0.000 claims description 59
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 59
- 125000003118 aryl group Chemical group 0.000 claims description 57
- 239000000203 mixture Substances 0.000 claims description 57
- 125000005842 heteroatom Chemical group 0.000 claims description 51
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 50
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 50
- 229910052717 sulfur Inorganic materials 0.000 claims description 45
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 claims description 44
- 239000001257 hydrogen Substances 0.000 claims description 44
- 229910052760 oxygen Inorganic materials 0.000 claims description 44
- 125000000565 sulfonamide group Chemical group 0.000 claims description 38
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims description 36
- 125000002947 alkylene group Chemical group 0.000 claims description 35
- 239000003814 drug Substances 0.000 claims description 35
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 31
- 239000003795 chemical substances by application Substances 0.000 claims description 30
- 125000004648 C2-C8 alkenyl group Chemical group 0.000 claims description 28
- 125000002837 carbocyclic group Chemical group 0.000 claims description 27
- 229910003827 NRaRb Inorganic materials 0.000 claims description 25
- 206010028980 Neoplasm Diseases 0.000 claims description 25
- 101710089372 Programmed cell death protein 1 Proteins 0.000 claims description 24
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 24
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 24
- 125000004429 atom Chemical group 0.000 claims description 21
- 125000001544 thienyl group Chemical group 0.000 claims description 21
- 229940024606 amino acid Drugs 0.000 claims description 19
- 235000001014 amino acid Nutrition 0.000 claims description 19
- 150000001413 amino acids Chemical class 0.000 claims description 19
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 claims description 17
- 239000002246 antineoplastic agent Substances 0.000 claims description 16
- 125000003386 piperidinyl group Chemical group 0.000 claims description 16
- 201000011510 cancer Diseases 0.000 claims description 15
- 230000000694 effects Effects 0.000 claims description 15
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 14
- 201000010099 disease Diseases 0.000 claims description 14
- 208000015181 infectious disease Diseases 0.000 claims description 14
- 125000002393 azetidinyl group Chemical group 0.000 claims description 13
- 125000000719 pyrrolidinyl group Chemical group 0.000 claims description 13
- 229940124597 therapeutic agent Drugs 0.000 claims description 13
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 claims description 10
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 10
- 229910052799 carbon Inorganic materials 0.000 claims description 10
- 229940127089 cytotoxic agent Drugs 0.000 claims description 10
- 208000035475 disorder Diseases 0.000 claims description 10
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 9
- 125000004765 (C1-C4) haloalkyl group Chemical group 0.000 claims description 8
- 125000003342 alkenyl group Chemical group 0.000 claims description 8
- 229910052702 rhenium Inorganic materials 0.000 claims description 8
- 125000006376 (C3-C10) cycloalkyl group Chemical group 0.000 claims description 7
- 208000035473 Communicable disease Diseases 0.000 claims description 7
- 239000004037 angiogenesis inhibitor Substances 0.000 claims description 7
- 210000004027 cell Anatomy 0.000 claims description 7
- ZHXTWWCDMUWMDI-UHFFFAOYSA-N dihydroxyboron Chemical compound O[B]O ZHXTWWCDMUWMDI-UHFFFAOYSA-N 0.000 claims description 7
- 229910052731 fluorine Inorganic materials 0.000 claims description 7
- 230000028993 immune response Effects 0.000 claims description 7
- 125000004076 pyridyl group Chemical group 0.000 claims description 7
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 claims description 6
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 claims description 6
- 125000002768 hydroxyalkyl group Chemical group 0.000 claims description 6
- 125000004649 C2-C8 alkynyl group Chemical group 0.000 claims description 5
- 230000003510 anti-fibrotic effect Effects 0.000 claims description 5
- 230000001028 anti-proliverative effect Effects 0.000 claims description 5
- 239000013059 antihormonal agent Substances 0.000 claims description 5
- 239000004599 antimicrobial Substances 0.000 claims description 5
- 229940034982 antineoplastic agent Drugs 0.000 claims description 5
- 239000003443 antiviral agent Substances 0.000 claims description 5
- 125000004452 carbocyclyl group Chemical group 0.000 claims description 5
- 239000002254 cytotoxic agent Substances 0.000 claims description 5
- 231100000599 cytotoxic agent Toxicity 0.000 claims description 5
- 230000014509 gene expression Effects 0.000 claims description 5
- 125000001188 haloalkyl group Chemical group 0.000 claims description 5
- 230000001404 mediated effect Effects 0.000 claims description 5
- 125000003226 pyrazolyl group Chemical group 0.000 claims description 5
- 230000003439 radiotherapeutic effect Effects 0.000 claims description 5
- 238000001959 radiotherapy Methods 0.000 claims description 5
- 229910052701 rubidium Inorganic materials 0.000 claims description 5
- 125000003831 tetrazolyl group Chemical group 0.000 claims description 5
- 125000006583 (C1-C3) haloalkyl group Chemical group 0.000 claims description 4
- 125000006656 (C2-C4) alkenyl group Chemical group 0.000 claims description 4
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 claims description 4
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 claims description 4
- 206010009944 Colon cancer Diseases 0.000 claims description 4
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 claims description 4
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 claims description 4
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 claims description 4
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 claims description 4
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 claims description 4
- 239000004472 Lysine Substances 0.000 claims description 4
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 claims description 4
- 206010061535 Ovarian neoplasm Diseases 0.000 claims description 4
- 206010060862 Prostate cancer Diseases 0.000 claims description 4
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 4
- 208000006265 Renal cell carcinoma Diseases 0.000 claims description 4
- 206010041067 Small cell lung cancer Diseases 0.000 claims description 4
- 208000005718 Stomach Neoplasms Diseases 0.000 claims description 4
- 239000004473 Threonine Substances 0.000 claims description 4
- 208000009956 adenocarcinoma Diseases 0.000 claims description 4
- 206010017758 gastric cancer Diseases 0.000 claims description 4
- 208000014829 head and neck neoplasm Diseases 0.000 claims description 4
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 claims description 4
- 201000001441 melanoma Diseases 0.000 claims description 4
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 4
- 208000003154 papilloma Diseases 0.000 claims description 4
- 125000002098 pyridazinyl group Chemical group 0.000 claims description 4
- 208000000587 small cell lung carcinoma Diseases 0.000 claims description 4
- 201000011549 stomach cancer Diseases 0.000 claims description 4
- 241001631646 Papillomaviridae Species 0.000 claims description 3
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 claims description 3
- 125000005037 alkyl phenyl group Chemical group 0.000 claims description 3
- 125000006404 alkylpyridazinyl group Chemical group 0.000 claims description 3
- 125000006392 alkylpyrimidinyl group Chemical group 0.000 claims description 3
- 230000002708 enhancing effect Effects 0.000 claims description 3
- 210000001035 gastrointestinal tract Anatomy 0.000 claims description 3
- 125000005843 halogen group Chemical group 0.000 claims description 3
- 206010022000 influenza Diseases 0.000 claims description 3
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims description 3
- 125000000714 pyrimidinyl group Chemical group 0.000 claims description 3
- 230000019491 signal transduction Effects 0.000 claims description 3
- 241001529453 unidentified herpesvirus Species 0.000 claims description 3
- 230000003612 virological effect Effects 0.000 claims description 3
- 206010000830 Acute leukaemia Diseases 0.000 claims description 2
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 claims description 2
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 claims description 2
- 208000031261 Acute myeloid leukaemia Diseases 0.000 claims description 2
- 208000003200 Adenoma Diseases 0.000 claims description 2
- 206010001233 Adenoma benign Diseases 0.000 claims description 2
- 201000003076 Angiosarcoma Diseases 0.000 claims description 2
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 claims description 2
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 claims description 2
- 206010004146 Basal cell carcinoma Diseases 0.000 claims description 2
- 206010005949 Bone cancer Diseases 0.000 claims description 2
- 208000018084 Bone neoplasm Diseases 0.000 claims description 2
- 206010006143 Brain stem glioma Diseases 0.000 claims description 2
- 206010006187 Breast cancer Diseases 0.000 claims description 2
- 208000026310 Breast neoplasm Diseases 0.000 claims description 2
- 125000002853 C1-C4 hydroxyalkyl group Chemical group 0.000 claims description 2
- 201000009030 Carcinoma Diseases 0.000 claims description 2
- 208000017897 Carcinoma of esophagus Diseases 0.000 claims description 2
- 206010007953 Central nervous system lymphoma Diseases 0.000 claims description 2
- 201000005262 Chondroma Diseases 0.000 claims description 2
- 208000005243 Chondrosarcoma Diseases 0.000 claims description 2
- 208000006332 Choriocarcinoma Diseases 0.000 claims description 2
- 206010048832 Colon adenoma Diseases 0.000 claims description 2
- 208000001333 Colorectal Neoplasms Diseases 0.000 claims description 2
- 201000005171 Cystadenoma Diseases 0.000 claims description 2
- 201000009051 Embryonal Carcinoma Diseases 0.000 claims description 2
- 208000000461 Esophageal Neoplasms Diseases 0.000 claims description 2
- 201000008808 Fibrosarcoma Diseases 0.000 claims description 2
- 239000004471 Glycine Substances 0.000 claims description 2
- 206010066476 Haematological malignancy Diseases 0.000 claims description 2
- 208000001258 Hemangiosarcoma Diseases 0.000 claims description 2
- 208000002250 Hematologic Neoplasms Diseases 0.000 claims description 2
- 208000005176 Hepatitis C Diseases 0.000 claims description 2
- 208000005331 Hepatitis D Diseases 0.000 claims description 2
- 208000017604 Hodgkin disease Diseases 0.000 claims description 2
- 208000010747 Hodgkins lymphoma Diseases 0.000 claims description 2
- 208000007766 Kaposi sarcoma Diseases 0.000 claims description 2
- 208000008839 Kidney Neoplasms Diseases 0.000 claims description 2
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 claims description 2
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 claims description 2
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 claims description 2
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 claims description 2
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 claims description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 claims description 2
- 208000031671 Large B-Cell Diffuse Lymphoma Diseases 0.000 claims description 2
- 208000018142 Leiomyosarcoma Diseases 0.000 claims description 2
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 claims description 2
- 206010024612 Lipoma Diseases 0.000 claims description 2
- 206010058467 Lung neoplasm malignant Diseases 0.000 claims description 2
- 206010025219 Lymphangioma Diseases 0.000 claims description 2
- 206010052178 Lymphocytic lymphoma Diseases 0.000 claims description 2
- 206010025323 Lymphomas Diseases 0.000 claims description 2
- 208000002030 Merkel cell carcinoma Diseases 0.000 claims description 2
- 206010027406 Mesothelioma Diseases 0.000 claims description 2
- 206010027476 Metastases Diseases 0.000 claims description 2
- 208000034578 Multiple myelomas Diseases 0.000 claims description 2
- 201000003793 Myelodysplastic syndrome Diseases 0.000 claims description 2
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 claims description 2
- 208000002454 Nasopharyngeal Carcinoma Diseases 0.000 claims description 2
- 206010061306 Nasopharyngeal cancer Diseases 0.000 claims description 2
- 206010029266 Neuroendocrine carcinoma of the skin Diseases 0.000 claims description 2
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 claims description 2
- 206010033128 Ovarian cancer Diseases 0.000 claims description 2
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 2
- 208000000821 Parathyroid Neoplasms Diseases 0.000 claims description 2
- 208000002471 Penile Neoplasms Diseases 0.000 claims description 2
- 208000007913 Pituitary Neoplasms Diseases 0.000 claims description 2
- 201000005746 Pituitary adenoma Diseases 0.000 claims description 2
- 206010061538 Pituitary tumour benign Diseases 0.000 claims description 2
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 2
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 claims description 2
- 206010036711 Primary mediastinal large B-cell lymphomas Diseases 0.000 claims description 2
- 208000034541 Rare lymphatic malformation Diseases 0.000 claims description 2
- 208000015634 Rectal Neoplasms Diseases 0.000 claims description 2
- 208000005678 Rhabdomyoma Diseases 0.000 claims description 2
- 206010039491 Sarcoma Diseases 0.000 claims description 2
- 201000010208 Seminoma Diseases 0.000 claims description 2
- 206010040047 Sepsis Diseases 0.000 claims description 2
- 208000000453 Skin Neoplasms Diseases 0.000 claims description 2
- 208000021712 Soft tissue sarcoma Diseases 0.000 claims description 2
- 208000000102 Squamous Cell Carcinoma of Head and Neck Diseases 0.000 claims description 2
- 206010042971 T-cell lymphoma Diseases 0.000 claims description 2
- 208000027585 T-cell non-Hodgkin lymphoma Diseases 0.000 claims description 2
- 208000024313 Testicular Neoplasms Diseases 0.000 claims description 2
- 206010057644 Testis cancer Diseases 0.000 claims description 2
- 208000000728 Thymus Neoplasms Diseases 0.000 claims description 2
- 208000024770 Thyroid neoplasm Diseases 0.000 claims description 2
- 208000023915 Ureteral Neoplasms Diseases 0.000 claims description 2
- 206010046458 Urethral neoplasms Diseases 0.000 claims description 2
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 claims description 2
- 208000002495 Uterine Neoplasms Diseases 0.000 claims description 2
- 201000005969 Uveal melanoma Diseases 0.000 claims description 2
- 201000003761 Vaginal carcinoma Diseases 0.000 claims description 2
- 208000008383 Wilms tumor Diseases 0.000 claims description 2
- 208000024447 adrenal gland neoplasm Diseases 0.000 claims description 2
- 235000004279 alanine Nutrition 0.000 claims description 2
- 125000005213 alkyl heteroaryl group Chemical group 0.000 claims description 2
- 229960001230 asparagine Drugs 0.000 claims description 2
- 235000009582 asparagine Nutrition 0.000 claims description 2
- 208000022362 bacterial infectious disease Diseases 0.000 claims description 2
- 208000003362 bronchogenic carcinoma Diseases 0.000 claims description 2
- 210000003169 central nervous system Anatomy 0.000 claims description 2
- 208000025997 central nervous system neoplasm Diseases 0.000 claims description 2
- 208000019065 cervical carcinoma Diseases 0.000 claims description 2
- 230000001684 chronic effect Effects 0.000 claims description 2
- 208000024207 chronic leukemia Diseases 0.000 claims description 2
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 claims description 2
- 208000029742 colonic neoplasm Diseases 0.000 claims description 2
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 claims description 2
- 208000017763 cutaneous neuroendocrine carcinoma Diseases 0.000 claims description 2
- 208000002445 cystadenocarcinoma Diseases 0.000 claims description 2
- 206010012818 diffuse large B-cell lymphoma Diseases 0.000 claims description 2
- 210000000750 endocrine system Anatomy 0.000 claims description 2
- 201000003914 endometrial carcinoma Diseases 0.000 claims description 2
- 208000021045 exocrine pancreatic carcinoma Diseases 0.000 claims description 2
- 201000001343 fallopian tube carcinoma Diseases 0.000 claims description 2
- 206010016629 fibroma Diseases 0.000 claims description 2
- 201000003444 follicular lymphoma Diseases 0.000 claims description 2
- 208000024386 fungal infectious disease Diseases 0.000 claims description 2
- 208000005017 glioblastoma Diseases 0.000 claims description 2
- 230000012010 growth Effects 0.000 claims description 2
- 201000000459 head and neck squamous cell carcinoma Diseases 0.000 claims description 2
- 201000011066 hemangioma Diseases 0.000 claims description 2
- 208000005252 hepatitis A Diseases 0.000 claims description 2
- 208000002672 hepatitis B Diseases 0.000 claims description 2
- 206010073071 hepatocellular carcinoma Diseases 0.000 claims description 2
- 231100000844 hepatocellular carcinoma Toxicity 0.000 claims description 2
- 208000026278 immune system disease Diseases 0.000 claims description 2
- 208000027866 inflammatory disease Diseases 0.000 claims description 2
- 230000002401 inhibitory effect Effects 0.000 claims description 2
- 201000010260 leiomyoma Diseases 0.000 claims description 2
- 208000032839 leukemia Diseases 0.000 claims description 2
- 206010024627 liposarcoma Diseases 0.000 claims description 2
- 210000004185 liver Anatomy 0.000 claims description 2
- 210000005229 liver cell Anatomy 0.000 claims description 2
- 201000005202 lung cancer Diseases 0.000 claims description 2
- 208000020816 lung neoplasm Diseases 0.000 claims description 2
- 208000012804 lymphangiosarcoma Diseases 0.000 claims description 2
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 2
- 208000026037 malignant tumor of neck Diseases 0.000 claims description 2
- 206010027191 meningioma Diseases 0.000 claims description 2
- 230000009401 metastasis Effects 0.000 claims description 2
- 201000011216 nasopharynx carcinoma Diseases 0.000 claims description 2
- 210000003739 neck Anatomy 0.000 claims description 2
- 208000025402 neoplasm of esophagus Diseases 0.000 claims description 2
- 210000005036 nerve Anatomy 0.000 claims description 2
- 208000008798 osteoma Diseases 0.000 claims description 2
- 201000008968 osteosarcoma Diseases 0.000 claims description 2
- 201000002528 pancreatic cancer Diseases 0.000 claims description 2
- 201000008129 pancreatic ductal adenocarcinoma Diseases 0.000 claims description 2
- 201000010198 papillary carcinoma Diseases 0.000 claims description 2
- 210000002990 parathyroid gland Anatomy 0.000 claims description 2
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 claims description 2
- 208000021310 pituitary gland adenoma Diseases 0.000 claims description 2
- 208000004333 pleomorphic adenoma Diseases 0.000 claims description 2
- 208000016800 primary central nervous system lymphoma Diseases 0.000 claims description 2
- 230000035755 proliferation Effects 0.000 claims description 2
- 206010038038 rectal cancer Diseases 0.000 claims description 2
- 201000001275 rectum cancer Diseases 0.000 claims description 2
- 201000007444 renal pelvis carcinoma Diseases 0.000 claims description 2
- 201000009410 rhabdomyosarcoma Diseases 0.000 claims description 2
- 201000000849 skin cancer Diseases 0.000 claims description 2
- 210000000813 small intestine Anatomy 0.000 claims description 2
- 230000004936 stimulating effect Effects 0.000 claims description 2
- 206010042863 synovial sarcoma Diseases 0.000 claims description 2
- 201000003120 testicular cancer Diseases 0.000 claims description 2
- 210000001685 thyroid gland Anatomy 0.000 claims description 2
- 206010044412 transitional cell carcinoma Diseases 0.000 claims description 2
- 208000022271 tubular adenoma Diseases 0.000 claims description 2
- 230000005747 tumor angiogenesis Effects 0.000 claims description 2
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 claims description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 claims description 2
- 206010046766 uterine cancer Diseases 0.000 claims description 2
- 208000013013 vulvar carcinoma Diseases 0.000 claims description 2
- 102100023990 60S ribosomal protein L17 Human genes 0.000 claims 2
- 229910018828 PO3H2 Inorganic materials 0.000 claims 2
- 229910006074 SO2NH2 Inorganic materials 0.000 claims 1
- 201000008443 lung non-squamous non-small cell carcinoma Diseases 0.000 claims 1
- 238000002360 preparation method Methods 0.000 abstract description 11
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 119
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical class CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 104
- 239000000243 solution Substances 0.000 description 89
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 75
- 239000011541 reaction mixture Substances 0.000 description 65
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 60
- 235000019439 ethyl acetate Nutrition 0.000 description 59
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 58
- 238000003786 synthesis reaction Methods 0.000 description 56
- 230000015572 biosynthetic process Effects 0.000 description 55
- 238000005160 1H NMR spectroscopy Methods 0.000 description 53
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 52
- 125000000250 methylamino group Chemical group [H]N(*)C([H])([H])[H] 0.000 description 52
- 239000000725 suspension Substances 0.000 description 44
- 239000011734 sodium Substances 0.000 description 42
- 238000003818 flash chromatography Methods 0.000 description 39
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 38
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 37
- WIRTYVGMQVIVDM-UHFFFAOYSA-N pyridine-3-carbonitrile Chemical compound N#CC1=C=NC=C[CH]1 WIRTYVGMQVIVDM-UHFFFAOYSA-N 0.000 description 37
- 239000012071 phase Substances 0.000 description 35
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 34
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 32
- DTQVDTLACAAQTR-UHFFFAOYSA-N trifluoroacetic acid Substances OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 31
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 30
- 238000002953 preparative HPLC Methods 0.000 description 28
- 230000002441 reversible effect Effects 0.000 description 28
- 150000002431 hydrogen Chemical class 0.000 description 23
- 102100040678 Programmed cell death protein 1 Human genes 0.000 description 22
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 21
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 21
- 125000000217 alkyl group Chemical group 0.000 description 21
- 239000012044 organic layer Substances 0.000 description 21
- 229940079593 drug Drugs 0.000 description 20
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 20
- NFHFRUOZVGFOOS-UHFFFAOYSA-N Pd(PPh3)4 Substances [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 19
- 238000006243 chemical reaction Methods 0.000 description 18
- 125000000896 monocarboxylic acid group Chemical group 0.000 description 18
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 17
- 239000012043 crude product Substances 0.000 description 17
- 239000000543 intermediate Substances 0.000 description 16
- HXITXNWTGFUOAU-UHFFFAOYSA-N phenylboronic acid Chemical compound OB(O)C1=CC=CC=C1 HXITXNWTGFUOAU-UHFFFAOYSA-N 0.000 description 16
- 239000002904 solvent Substances 0.000 description 15
- 229910001873 dinitrogen Inorganic materials 0.000 description 14
- 150000002148 esters Chemical class 0.000 description 14
- 238000011282 treatment Methods 0.000 description 14
- RWRDLPDLKQPQOW-UHFFFAOYSA-N Pyrrolidine Chemical compound C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 description 13
- 239000002253 acid Substances 0.000 description 13
- 108010074708 B7-H1 Antigen Proteins 0.000 description 12
- 102000008096 B7-H1 Antigen Human genes 0.000 description 12
- 238000001816 cooling Methods 0.000 description 12
- 239000000047 product Substances 0.000 description 12
- 239000003112 inhibitor Substances 0.000 description 11
- 229940002612 prodrug Drugs 0.000 description 11
- 239000000651 prodrug Substances 0.000 description 11
- 239000003039 volatile agent Substances 0.000 description 11
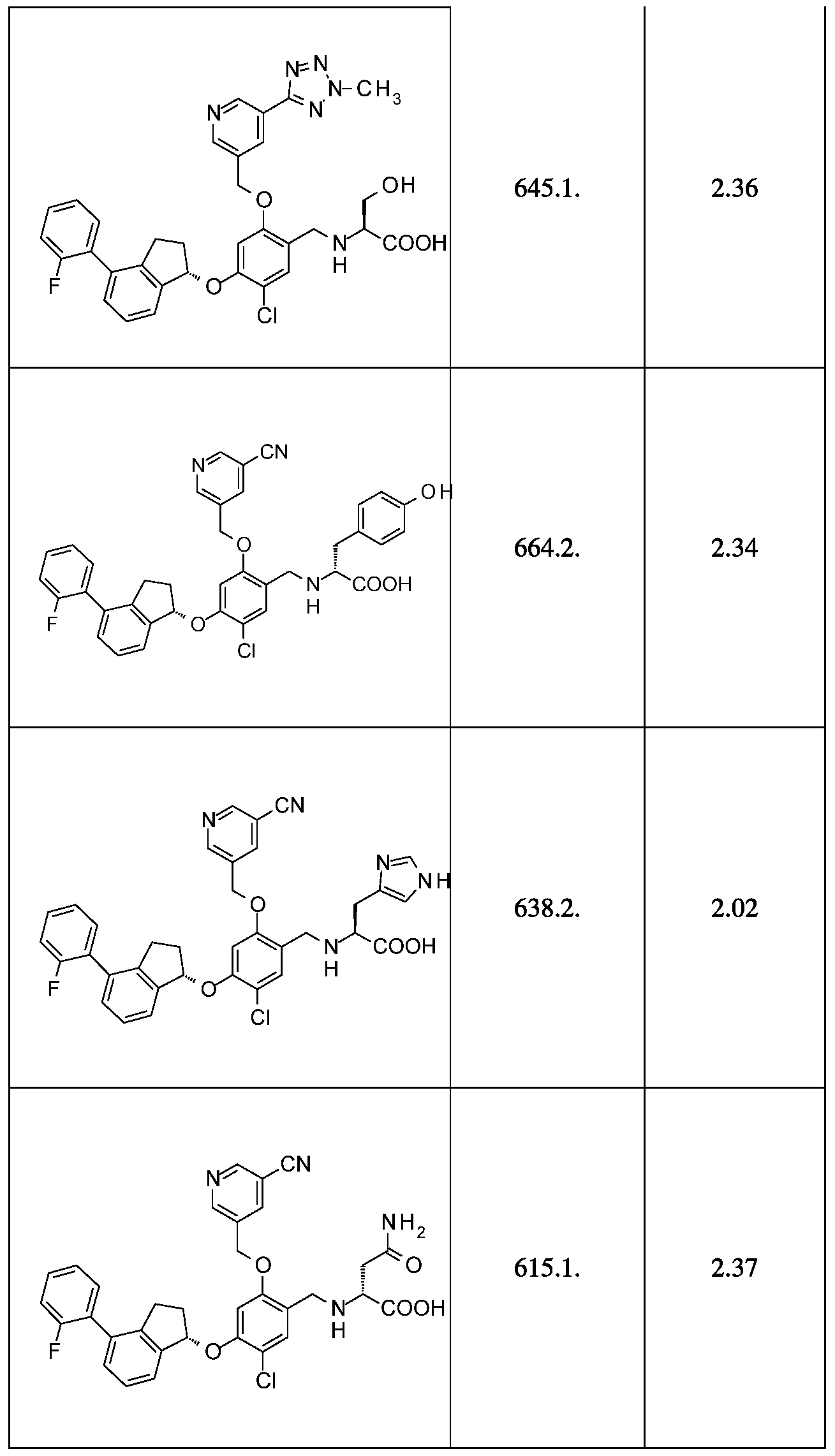
- QHOWYRYZXAXDDS-UHFFFAOYSA-N 5-[[4-chloro-2-formyl-5-[(4-phenyl-2,3-dihydro-1H-inden-1-yl)oxy]phenoxy]methyl]pyridine-3-carbonitrile Chemical compound ClC1=CC(=C(OCC=2C=C(C=NC=2)C#N)C=C1OC1CCC2=C(C=CC=C12)C1=CC=CC=C1)C=O QHOWYRYZXAXDDS-UHFFFAOYSA-N 0.000 description 10
- 239000004480 active ingredient Substances 0.000 description 10
- 229910000024 caesium carbonate Inorganic materials 0.000 description 10
- 125000004432 carbon atom Chemical group C* 0.000 description 10
- 229920006395 saturated elastomer Polymers 0.000 description 10
- 229960001153 serine Drugs 0.000 description 10
- 239000007787 solid Substances 0.000 description 9
- 238000003756 stirring Methods 0.000 description 9
- QCSLIRFWJPOENV-UHFFFAOYSA-N (2-fluorophenyl)boronic acid Chemical compound OB(O)C1=CC=CC=C1F QCSLIRFWJPOENV-UHFFFAOYSA-N 0.000 description 8
- IPOSHVWRFQTHGK-UHFFFAOYSA-N 5-chloro-2,4-dihydroxybenzaldehyde Chemical compound OC1=CC(O)=C(C=O)C=C1Cl IPOSHVWRFQTHGK-UHFFFAOYSA-N 0.000 description 8
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 8
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 8
- 239000012267 brine Substances 0.000 description 8
- 239000003153 chemical reaction reagent Substances 0.000 description 8
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 8
- 230000001225 therapeutic effect Effects 0.000 description 8
- 0 *Cc(c(*)c(*)c(O[C@@](CC1)C(C=C2)=C1C(*)=C*2N)c1*)c1O* Chemical compound *Cc(c(*)c(*)c(O[C@@](CC1)C(C=C2)=C1C(*)=C*2N)c1*)c1O* 0.000 description 7
- 238000009472 formulation Methods 0.000 description 7
- 230000003993 interaction Effects 0.000 description 7
- 230000007935 neutral effect Effects 0.000 description 7
- 150000003254 radicals Chemical class 0.000 description 7
- 239000012321 sodium triacetoxyborohydride Substances 0.000 description 7
- 208000024891 symptom Diseases 0.000 description 7
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 6
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 6
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 6
- 229920001213 Polysorbate 20 Polymers 0.000 description 6
- 239000000796 flavoring agent Substances 0.000 description 6
- 125000004404 heteroalkyl group Chemical group 0.000 description 6
- 125000000592 heterocycloalkyl group Chemical group 0.000 description 6
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 6
- 230000037361 pathway Effects 0.000 description 6
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 description 6
- 229920001223 polyethylene glycol Polymers 0.000 description 6
- 229920000642 polymer Polymers 0.000 description 6
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 6
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 6
- 230000006340 racemization Effects 0.000 description 6
- 210000002966 serum Anatomy 0.000 description 6
- 229910052708 sodium Inorganic materials 0.000 description 6
- 235000015424 sodium Nutrition 0.000 description 6
- 239000003765 sweetening agent Substances 0.000 description 6
- RNXQQZNOSYBFTN-UHFFFAOYSA-N 4-bromo-2,3-dihydro-1h-inden-1-ol Chemical compound C1=CC=C(Br)C2=C1C(O)CC2 RNXQQZNOSYBFTN-UHFFFAOYSA-N 0.000 description 5
- 239000002202 Polyethylene glycol Substances 0.000 description 5
- 150000007513 acids Chemical class 0.000 description 5
- 239000005557 antagonist Substances 0.000 description 5
- 125000002619 bicyclic group Chemical group 0.000 description 5
- 239000007859 condensation product Substances 0.000 description 5
- 235000014113 dietary fatty acids Nutrition 0.000 description 5
- 239000000194 fatty acid Substances 0.000 description 5
- 229930195729 fatty acid Natural products 0.000 description 5
- 150000004665 fatty acids Chemical class 0.000 description 5
- 235000003599 food sweetener Nutrition 0.000 description 5
- 239000004615 ingredient Substances 0.000 description 5
- 230000000155 isotopic effect Effects 0.000 description 5
- VVWRJUBEIPHGQF-MDZDMXLPSA-N propan-2-yl (ne)-n-propan-2-yloxycarbonyliminocarbamate Chemical compound CC(C)OC(=O)\N=N\C(=O)OC(C)C VVWRJUBEIPHGQF-MDZDMXLPSA-N 0.000 description 5
- 230000004850 protein–protein interaction Effects 0.000 description 5
- 230000002285 radioactive effect Effects 0.000 description 5
- 102000005962 receptors Human genes 0.000 description 5
- 108020003175 receptors Proteins 0.000 description 5
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 5
- 125000004434 sulfur atom Chemical group 0.000 description 5
- 239000003826 tablet Substances 0.000 description 5
- UVVYFYLSZIMKMC-UHFFFAOYSA-N 4-bromo-2,3-dihydroinden-1-one Chemical compound BrC1=CC=CC2=C1CCC2=O UVVYFYLSZIMKMC-UHFFFAOYSA-N 0.000 description 4
- IUHPOQYVQPBUBT-UHFFFAOYSA-N 5-(bromomethyl)pyridine-3-carbonitrile Chemical compound BrCC1=CN=CC(C#N)=C1 IUHPOQYVQPBUBT-UHFFFAOYSA-N 0.000 description 4
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 4
- 238000002965 ELISA Methods 0.000 description 4
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 4
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 4
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 4
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 4
- 125000000304 alkynyl group Chemical group 0.000 description 4
- 239000007900 aqueous suspension Substances 0.000 description 4
- MCQRPQCQMGVWIQ-UHFFFAOYSA-N boron;methylsulfanylmethane Chemical compound [B].CSC MCQRPQCQMGVWIQ-UHFFFAOYSA-N 0.000 description 4
- RYYVLZVUVIJVGH-UHFFFAOYSA-N caffeine Chemical compound CN1C(=O)N(C)C(=O)C2=C1N=CN2C RYYVLZVUVIJVGH-UHFFFAOYSA-N 0.000 description 4
- 239000003638 chemical reducing agent Substances 0.000 description 4
- 239000003086 colorant Substances 0.000 description 4
- 230000008878 coupling Effects 0.000 description 4
- 238000010168 coupling process Methods 0.000 description 4
- 238000005859 coupling reaction Methods 0.000 description 4
- 239000002270 dispersing agent Substances 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 4
- 235000013355 food flavoring agent Nutrition 0.000 description 4
- 238000000338 in vitro Methods 0.000 description 4
- JJWLVOIRVHMVIS-UHFFFAOYSA-N isopropylamine Chemical compound CC(C)N JJWLVOIRVHMVIS-UHFFFAOYSA-N 0.000 description 4
- 239000003446 ligand Substances 0.000 description 4
- OKKJLVBELUTLKV-VMNATFBRSA-N methanol-d1 Chemical compound [2H]OC OKKJLVBELUTLKV-VMNATFBRSA-N 0.000 description 4
- 231100000252 nontoxic Toxicity 0.000 description 4
- 230000003000 nontoxic effect Effects 0.000 description 4
- 239000003921 oil Substances 0.000 description 4
- 235000019198 oils Nutrition 0.000 description 4
- 230000036961 partial effect Effects 0.000 description 4
- 230000001717 pathogenic effect Effects 0.000 description 4
- HDOWRFHMPULYOA-UHFFFAOYSA-N piperidin-4-ol Chemical compound OC1CCNCC1 HDOWRFHMPULYOA-UHFFFAOYSA-N 0.000 description 4
- 239000003755 preservative agent Substances 0.000 description 4
- 238000006268 reductive amination reaction Methods 0.000 description 4
- 239000000375 suspending agent Substances 0.000 description 4
- YAPQBXQYLJRXSA-UHFFFAOYSA-N theobromine Chemical compound CN1C(=O)NC(=O)C2=C1N=CN2C YAPQBXQYLJRXSA-UHFFFAOYSA-N 0.000 description 4
- 238000002560 therapeutic procedure Methods 0.000 description 4
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 4
- 239000000080 wetting agent Substances 0.000 description 4
- RNXQQZNOSYBFTN-SECBINFHSA-N (1r)-4-bromo-2,3-dihydro-1h-inden-1-ol Chemical compound C1=CC=C(Br)C2=C1[C@H](O)CC2 RNXQQZNOSYBFTN-SECBINFHSA-N 0.000 description 3
- LFBCITCRPXFIQR-UHFFFAOYSA-N (2-chloropyrimidin-5-yl)methyl methanesulfonate Chemical compound CS(=O)(=O)OCC=1C=NC(=NC=1)Cl LFBCITCRPXFIQR-UHFFFAOYSA-N 0.000 description 3
- ZIMLRKWQDLVPEK-UHFFFAOYSA-N 1-[4-(4-chloro-3-methoxyphenyl)piperazin-1-yl]-2-[3-(1H-imidazol-2-yl)pyrazolo[3,4-b]pyridin-1-yl]ethanone Chemical compound C1=C(Cl)C(OC)=CC(N2CCN(CC2)C(=O)CN2C3=NC=CC=C3C(C=3NC=CN=3)=N2)=C1 ZIMLRKWQDLVPEK-UHFFFAOYSA-N 0.000 description 3
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 3
- SAUFKLQHUAGUNO-UHFFFAOYSA-N 4-[(4-bromo-2,3-dihydro-1H-inden-1-yl)oxy]-5-chloro-2-hydroxybenzaldehyde Chemical compound BrC1=C2CCC(C2=CC=C1)OC1=CC(=C(C=O)C=C1Cl)O SAUFKLQHUAGUNO-UHFFFAOYSA-N 0.000 description 3
- LUUMLYXKTPBTQR-UHFFFAOYSA-N 4-chloro-n-[5-methyl-2-(7h-pyrrolo[2,3-d]pyrimidine-4-carbonyl)pyridin-3-yl]-3-(trifluoromethyl)benzenesulfonamide Chemical compound C=1C(C)=CN=C(C(=O)C=2C=3C=CNC=3N=CN=2)C=1NS(=O)(=O)C1=CC=C(Cl)C(C(F)(F)F)=C1 LUUMLYXKTPBTQR-UHFFFAOYSA-N 0.000 description 3
- OTSJZOYSLXXFPG-UHFFFAOYSA-N 4-phenyl-2,3-dihydroinden-1-one Chemical compound O=C1CCC2=C1C=CC=C2C1=CC=CC=C1 OTSJZOYSLXXFPG-UHFFFAOYSA-N 0.000 description 3
- JRWROCIMSDXGOZ-UHFFFAOYSA-N 4-tert-butyl-n-[4-chloro-2-(1-oxidopyridin-1-ium-4-carbonyl)phenyl]benzenesulfonamide Chemical compound C1=CC(C(C)(C)C)=CC=C1S(=O)(=O)NC1=CC=C(Cl)C=C1C(=O)C1=CC=[N+]([O-])C=C1 JRWROCIMSDXGOZ-UHFFFAOYSA-N 0.000 description 3
- KHLFHNCFFUQOCC-UHFFFAOYSA-N 5-[[2-(azetidin-1-ylmethyl)-4-chloro-5-[(4-phenyl-2,3-dihydro-1H-inden-1-yl)oxy]phenoxy]methyl]pyridine-3-carbonitrile Chemical compound N1(CCC1)CC1=C(OCC=2C=C(C=NC=2)C#N)C=C(C(=C1)Cl)OC1CCC2=C(C=CC=C12)C1=CC=CC=C1 KHLFHNCFFUQOCC-UHFFFAOYSA-N 0.000 description 3
- JKIGGJJZRLKYQT-UHFFFAOYSA-N 5-[[4-chloro-2-[(3-hydroxyazetidin-1-yl)methyl]-5-[(4-phenyl-2,3-dihydro-1H-inden-1-yl)oxy]phenoxy]methyl]pyridine-3-carbonitrile Chemical compound ClC1=CC(=C(OCC=2C=C(C=NC=2)C#N)C=C1OC1CCC2=C(C=CC=C12)C1=CC=CC=C1)CN1CC(C1)O JKIGGJJZRLKYQT-UHFFFAOYSA-N 0.000 description 3
- QHOWYRYZXAXDDS-HHHXNRCGSA-N 5-[[4-chloro-2-formyl-5-[[(1R)-4-phenyl-2,3-dihydro-1H-inden-1-yl]oxy]phenoxy]methyl]pyridine-3-carbonitrile Chemical compound ClC1=CC(=C(OCC=2C=C(C=NC=2)C#N)C=C1O[C@@H]1CCC2=C(C=CC=C12)C1=CC=CC=C1)C=O QHOWYRYZXAXDDS-HHHXNRCGSA-N 0.000 description 3
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 3
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 3
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 102100034458 Hepatitis A virus cellular receptor 2 Human genes 0.000 description 3
- 101710083479 Hepatitis A virus cellular receptor 2 homolog Proteins 0.000 description 3
- 101000666896 Homo sapiens V-type immunoglobulin domain-containing suppressor of T-cell activation Proteins 0.000 description 3
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 3
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- 101100407308 Mus musculus Pdcd1lg2 gene Proteins 0.000 description 3
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 3
- 108700030875 Programmed Cell Death 1 Ligand 2 Proteins 0.000 description 3
- 102100024213 Programmed cell death 1 ligand 2 Human genes 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 229940126547 T-cell immunoglobulin mucin-3 Drugs 0.000 description 3
- OKJPEAGHQZHRQV-UHFFFAOYSA-N Triiodomethane Natural products IC(I)I OKJPEAGHQZHRQV-UHFFFAOYSA-N 0.000 description 3
- 102100038282 V-type immunoglobulin domain-containing suppressor of T-cell activation Human genes 0.000 description 3
- 241000700605 Viruses Species 0.000 description 3
- 230000029936 alkylation Effects 0.000 description 3
- 238000005804 alkylation reaction Methods 0.000 description 3
- 125000003277 amino group Chemical group 0.000 description 3
- PUKBOVABABRILL-YZNIXAGQSA-N avacopan Chemical compound C1=C(C(F)(F)F)C(C)=CC=C1NC(=O)[C@@H]1[C@H](C=2C=CC(NC3CCCC3)=CC=2)N(C(=O)C=2C(=CC=CC=2C)F)CCC1 PUKBOVABABRILL-YZNIXAGQSA-N 0.000 description 3
- 229950001740 avacopan Drugs 0.000 description 3
- HONIICLYMWZJFZ-UHFFFAOYSA-N azetidine Chemical compound C1CNC1 HONIICLYMWZJFZ-UHFFFAOYSA-N 0.000 description 3
- HUMNYLRZRPPJDN-UHFFFAOYSA-N benzenecarboxaldehyde Natural products O=CC1=CC=CC=C1 HUMNYLRZRPPJDN-UHFFFAOYSA-N 0.000 description 3
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid group Chemical group C(C1=CC=CC=C1)(=O)O WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 3
- 230000037396 body weight Effects 0.000 description 3
- 229910052796 boron Inorganic materials 0.000 description 3
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Chemical compound BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 3
- 239000011575 calcium Substances 0.000 description 3
- 229910052791 calcium Inorganic materials 0.000 description 3
- 239000000460 chlorine Substances 0.000 description 3
- WZHCOOQXZCIUNC-UHFFFAOYSA-N cyclandelate Chemical compound C1C(C)(C)CC(C)CC1OC(=O)C(O)C1=CC=CC=C1 WZHCOOQXZCIUNC-UHFFFAOYSA-N 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 238000000132 electrospray ionisation Methods 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- IJJVMEJXYNJXOJ-UHFFFAOYSA-N fluquinconazole Chemical compound C=1C=C(Cl)C=C(Cl)C=1N1C(=O)C2=CC(F)=CC=C2N=C1N1C=NC=N1 IJJVMEJXYNJXOJ-UHFFFAOYSA-N 0.000 description 3
- 235000019253 formic acid Nutrition 0.000 description 3
- 150000002430 hydrocarbons Chemical group 0.000 description 3
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 230000010354 integration Effects 0.000 description 3
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 3
- 239000002608 ionic liquid Substances 0.000 description 3
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 3
- HXEACLLIILLPRG-RXMQYKEDSA-N l-pipecolic acid Natural products OC(=O)[C@H]1CCCCN1 HXEACLLIILLPRG-RXMQYKEDSA-N 0.000 description 3
- 239000010410 layer Substances 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 229940057995 liquid paraffin Drugs 0.000 description 3
- 239000011777 magnesium Substances 0.000 description 3
- 229910052749 magnesium Inorganic materials 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 239000004006 olive oil Substances 0.000 description 3
- 235000008390 olive oil Nutrition 0.000 description 3
- QNGNSVIICDLXHT-UHFFFAOYSA-N para-ethylbenzaldehyde Natural products CCC1=CC=C(C=O)C=C1 QNGNSVIICDLXHT-UHFFFAOYSA-N 0.000 description 3
- 125000003367 polycyclic group Chemical group 0.000 description 3
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 3
- 239000011591 potassium Substances 0.000 description 3
- 229910052700 potassium Inorganic materials 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 150000003141 primary amines Chemical class 0.000 description 3
- VVWRJUBEIPHGQF-UHFFFAOYSA-N propan-2-yl n-propan-2-yloxycarbonyliminocarbamate Chemical compound CC(C)OC(=O)N=NC(=O)OC(C)C VVWRJUBEIPHGQF-UHFFFAOYSA-N 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 229910052710 silicon Inorganic materials 0.000 description 3
- 150000003384 small molecules Chemical class 0.000 description 3
- 239000004094 surface-active agent Substances 0.000 description 3
- 229960002898 threonine Drugs 0.000 description 3
- 230000009466 transformation Effects 0.000 description 3
- 238000000844 transformation Methods 0.000 description 3
- 125000004417 unsaturated alkyl group Chemical group 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 2
- JJPVZILIOSDMBA-OAHLLOKOSA-N (1R)-4-(2-fluorophenyl)-2,3-dihydro-1H-inden-1-ol Chemical compound FC1=C(C=CC=C1)C1=C2CC[C@H](C2=CC=C1)O JJPVZILIOSDMBA-OAHLLOKOSA-N 0.000 description 2
- UYSMIUXOZPFVJF-OAHLLOKOSA-N (1R)-4-phenyl-2,3-dihydro-1H-inden-1-ol Chemical compound C1(=CC=CC=C1)C1=C2CC[C@H](C2=CC=C1)O UYSMIUXOZPFVJF-OAHLLOKOSA-N 0.000 description 2
- JCKZNMSBFBPDPM-UHFFFAOYSA-N (2-fluoro-3-methoxyphenyl)boronic acid Chemical group COC1=CC=CC(B(O)O)=C1F JCKZNMSBFBPDPM-UHFFFAOYSA-N 0.000 description 2
- PWKKAWWRXCKISN-UHFFFAOYSA-N (5-cyanopyridin-3-yl)methyl methanesulfonate Chemical compound CS(=O)(=O)OCc1cncc(c1)C#N PWKKAWWRXCKISN-UHFFFAOYSA-N 0.000 description 2
- 125000006273 (C1-C3) alkyl group Chemical group 0.000 description 2
- ZORQXIQZAOLNGE-UHFFFAOYSA-N 1,1-difluorocyclohexane Chemical compound FC1(F)CCCCC1 ZORQXIQZAOLNGE-UHFFFAOYSA-N 0.000 description 2
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 2
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 2
- 238000004293 19F NMR spectroscopy Methods 0.000 description 2
- ZFFMLCVRJBZUDZ-UHFFFAOYSA-N 2,3-dimethylbutane Chemical group CC(C)C(C)C ZFFMLCVRJBZUDZ-UHFFFAOYSA-N 0.000 description 2
- YFTHTJAPODJVSL-UHFFFAOYSA-N 2-(1-benzothiophen-5-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Chemical compound O1C(C)(C)C(C)(C)OB1C1=CC=C(SC=C2)C2=C1 YFTHTJAPODJVSL-UHFFFAOYSA-N 0.000 description 2
- MSWZFWKMSRAUBD-IVMDWMLBSA-N 2-amino-2-deoxy-D-glucopyranose Chemical compound N[C@H]1C(O)O[C@H](CO)[C@@H](O)[C@@H]1O MSWZFWKMSRAUBD-IVMDWMLBSA-N 0.000 description 2
- BFSVOASYOCHEOV-UHFFFAOYSA-N 2-diethylaminoethanol Chemical compound CCN(CC)CCO BFSVOASYOCHEOV-UHFFFAOYSA-N 0.000 description 2
- 229940013085 2-diethylaminoethanol Drugs 0.000 description 2
- IZHVBANLECCAGF-UHFFFAOYSA-N 2-hydroxy-3-(octadecanoyloxy)propyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)COC(=O)CCCCCCCCCCCCCCCCC IZHVBANLECCAGF-UHFFFAOYSA-N 0.000 description 2
- DXOSJQLIRGXWCF-UHFFFAOYSA-N 3-fluorocatechol Chemical compound OC1=CC=CC(F)=C1O DXOSJQLIRGXWCF-UHFFFAOYSA-N 0.000 description 2
- SICQQLBGTRPOFT-UHFFFAOYSA-N 4-[(4-bromo-2,3-dihydro-1H-inden-1-yl)oxy]-5-chloro-2-(pyridin-3-ylmethoxy)benzaldehyde Chemical compound BrC1=C2CCC(C2=CC=C1)OC1=CC(=C(C=O)C=C1Cl)OCC=1C=NC=CC=1 SICQQLBGTRPOFT-UHFFFAOYSA-N 0.000 description 2
- SAUFKLQHUAGUNO-OAHLLOKOSA-N 4-[[(1R)-4-bromo-2,3-dihydro-1H-inden-1-yl]oxy]-5-chloro-2-hydroxybenzaldehyde Chemical compound BrC1=C2CC[C@H](C2=CC=C1)OC1=CC(=C(C=O)C=C1Cl)O SAUFKLQHUAGUNO-OAHLLOKOSA-N 0.000 description 2
- BRUGWOMJTQGTHM-UHFFFAOYSA-N 4-bromo-2-fluoro-2,3-dihydroinden-1-one Chemical compound FC1C(C2=CC=CC(=C2C1)Br)=O BRUGWOMJTQGTHM-UHFFFAOYSA-N 0.000 description 2
- HVCNXQOWACZAFN-UHFFFAOYSA-N 4-ethylmorpholine Chemical compound CCN1CCOCC1 HVCNXQOWACZAFN-UHFFFAOYSA-N 0.000 description 2
- UYSMIUXOZPFVJF-UHFFFAOYSA-N 4-phenyl-2,3-dihydro-1h-inden-1-ol Chemical compound OC1CCC2=C1C=CC=C2C1=CC=CC=C1 UYSMIUXOZPFVJF-UHFFFAOYSA-N 0.000 description 2
- TWSZPLLNCGTXBR-UHFFFAOYSA-N 5-[[4-chloro-2-[(2-hydroxyethylamino)methyl]-5-[(4-phenyl-2,3-dihydro-1H-inden-1-yl)oxy]phenoxy]methyl]pyridine-3-carbonitrile Chemical compound ClC1=CC(=C(OCC=2C=C(C=NC=2)C#N)C=C1OC1CCC2=C(C=CC=C12)C1=CC=CC=C1)CNCCO TWSZPLLNCGTXBR-UHFFFAOYSA-N 0.000 description 2
- BDSZUQOQSLPLTI-OAQYLSRUSA-N 5-[[5-[[(1R)-4-bromo-2,3-dihydro-1H-inden-1-yl]oxy]-4-chloro-2-formylphenoxy]methyl]pyridine-3-carbonitrile Chemical compound BrC1=C2CC[C@H](C2=CC=C1)OC=1C(=CC(=C(OCC=2C=C(C=NC=2)C#N)C=1)C=O)Cl BDSZUQOQSLPLTI-OAQYLSRUSA-N 0.000 description 2
- KLSWCTVDYANFKE-UHFFFAOYSA-N 5-chloro-2-hydroxy-4-[(4-phenyl-2,3-dihydro-1H-inden-1-yl)oxy]benzaldehyde Chemical compound ClC=1C(=CC(=C(C=O)C=1)O)OC1CCC2=C(C=CC=C12)C1=CC=CC=C1 KLSWCTVDYANFKE-UHFFFAOYSA-N 0.000 description 2
- KLSWCTVDYANFKE-OAQYLSRUSA-N 5-chloro-2-hydroxy-4-[[(1R)-4-phenyl-2,3-dihydro-1H-inden-1-yl]oxy]benzaldehyde Chemical compound ClC=1C(=CC(=C(C=O)C=1)O)O[C@@H]1CCC2=C(C=CC=C12)C1=CC=CC=C1 KLSWCTVDYANFKE-OAQYLSRUSA-N 0.000 description 2
- LTEBVZAEDVBMGH-UHFFFAOYSA-N 5-chloro-4-[(4-phenyl-2,3-dihydro-1H-inden-1-yl)oxy]-2-(pyridin-3-ylmethoxy)benzaldehyde Chemical compound ClC=1C(=CC(=C(C=O)C=1)OCC=1C=NC=CC=1)OC1CCC2=C(C=CC=C12)C1=CC=CC=C1 LTEBVZAEDVBMGH-UHFFFAOYSA-N 0.000 description 2
- 244000215068 Acacia senegal Species 0.000 description 2
- 235000006491 Acacia senegal Nutrition 0.000 description 2
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 2
- 101150051188 Adora2a gene Proteins 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- 235000003911 Arachis Nutrition 0.000 description 2
- 244000105624 Arachis hypogaea Species 0.000 description 2
- 239000004475 Arginine Substances 0.000 description 2
- 229940122815 Aromatase inhibitor Drugs 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 241000416162 Astragalus gummifer Species 0.000 description 2
- 102100022718 Atypical chemokine receptor 2 Human genes 0.000 description 2
- KWIUHFFTVRNATP-UHFFFAOYSA-N Betaine Natural products C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 2
- 241000283690 Bos taurus Species 0.000 description 2
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Chemical compound CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 2
- 102100025074 C-C chemokine receptor-like 2 Human genes 0.000 description 2
- 108091033409 CRISPR Proteins 0.000 description 2
- 241000282472 Canis lupus familiaris Species 0.000 description 2
- OKTJSMMVPCPJKN-NJFSPNSNSA-N Carbon-14 Chemical compound [14C] OKTJSMMVPCPJKN-NJFSPNSNSA-N 0.000 description 2
- 108010019670 Chimeric Antigen Receptors Proteins 0.000 description 2
- 238000005750 Corey-Bakshi-Shibata reduction reaction Methods 0.000 description 2
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical group [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 2
- 241000282326 Felis catus Species 0.000 description 2
- IAJILQKETJEXLJ-UHFFFAOYSA-N Galacturonsaeure Natural products O=CC(O)C(O)C(O)C(O)C(O)=O IAJILQKETJEXLJ-UHFFFAOYSA-N 0.000 description 2
- 241000224466 Giardia Species 0.000 description 2
- 229920000084 Gum arabic Polymers 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 101000678892 Homo sapiens Atypical chemokine receptor 2 Proteins 0.000 description 2
- LPHGQDQBBGAPDZ-UHFFFAOYSA-N Isocaffeine Natural products CN1C(=O)N(C)C(=O)C2=C1N(C)C=N2 LPHGQDQBBGAPDZ-UHFFFAOYSA-N 0.000 description 2
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 2
- 239000002147 L01XE04 - Sunitinib Substances 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- 239000012359 Methanesulfonyl chloride Substances 0.000 description 2
- KWIUHFFTVRNATP-UHFFFAOYSA-O N,N,N-trimethylglycinium Chemical compound C[N+](C)(C)CC(O)=O KWIUHFFTVRNATP-UHFFFAOYSA-O 0.000 description 2
- UEEJHVSXFDXPFK-UHFFFAOYSA-N N-dimethylaminoethanol Chemical compound CN(C)CCO UEEJHVSXFDXPFK-UHFFFAOYSA-N 0.000 description 2
- HTLZVHNRZJPSMI-UHFFFAOYSA-N N-ethylpiperidine Chemical compound CCN1CCCCC1 HTLZVHNRZJPSMI-UHFFFAOYSA-N 0.000 description 2
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 2
- BPAKGABIKNXYLG-UHFFFAOYSA-N NCCN1N=NNC1=O Chemical compound NCCN1N=NNC1=O BPAKGABIKNXYLG-UHFFFAOYSA-N 0.000 description 2
- 108020005497 Nuclear hormone receptor Proteins 0.000 description 2
- IIWZCJWRNPEHSU-UHFFFAOYSA-N O=C1N(N=NN1)CCNC(OCC1=CC=CC=C1)=O Chemical compound O=C1N(N=NN1)CCNC(OCC1=CC=CC=C1)=O IIWZCJWRNPEHSU-UHFFFAOYSA-N 0.000 description 2
- 241001494479 Pecora Species 0.000 description 2
- 229920000954 Polyglycolide Polymers 0.000 description 2
- 241000288906 Primates Species 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- 229910007161 Si(CH3)3 Inorganic materials 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 230000006044 T cell activation Effects 0.000 description 2
- 210000001744 T-lymphocyte Anatomy 0.000 description 2
- 229920001615 Tragacanth Polymers 0.000 description 2
- YZCKVEUIGOORGS-NJFSPNSNSA-N Tritium Chemical compound [3H] YZCKVEUIGOORGS-NJFSPNSNSA-N 0.000 description 2
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 2
- 235000010489 acacia gum Nutrition 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 239000000556 agonist Substances 0.000 description 2
- IAJILQKETJEXLJ-QTBDOELSSA-N aldehydo-D-glucuronic acid Chemical compound O=C[C@H](O)[C@@H](O)[C@H](O)[C@H](O)C(O)=O IAJILQKETJEXLJ-QTBDOELSSA-N 0.000 description 2
- 125000003545 alkoxy group Chemical group 0.000 description 2
- 150000001350 alkyl halides Chemical class 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 239000000611 antibody drug conjugate Substances 0.000 description 2
- 229940049595 antibody-drug conjugate Drugs 0.000 description 2
- 239000003963 antioxidant agent Substances 0.000 description 2
- 235000006708 antioxidants Nutrition 0.000 description 2
- 239000008346 aqueous phase Substances 0.000 description 2
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 2
- 229960003121 arginine Drugs 0.000 description 2
- 239000003886 aromatase inhibitor Substances 0.000 description 2
- 125000003710 aryl alkyl group Chemical group 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- UQUPQEUNHVVNKW-UHFFFAOYSA-N azetidin-1-ium-3-ol;chloride Chemical compound Cl.OC1CNC1 UQUPQEUNHVVNKW-UHFFFAOYSA-N 0.000 description 2
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 2
- UGUNFSPZJHSINM-UHFFFAOYSA-N benzyl n-(3-chloro-3-oxopropyl)carbamate Chemical compound ClC(=O)CCNC(=O)OCC1=CC=CC=C1 UGUNFSPZJHSINM-UHFFFAOYSA-N 0.000 description 2
- MSWZFWKMSRAUBD-UHFFFAOYSA-N beta-D-galactosamine Natural products NC1C(O)OC(CO)C(O)C1O MSWZFWKMSRAUBD-UHFFFAOYSA-N 0.000 description 2
- 229960003237 betaine Drugs 0.000 description 2
- ZADPBFCGQRWHPN-UHFFFAOYSA-N boronic acid Chemical compound OBO ZADPBFCGQRWHPN-UHFFFAOYSA-N 0.000 description 2
- 229910052794 bromium Inorganic materials 0.000 description 2
- FJDQFPXHSGXQBY-UHFFFAOYSA-L caesium carbonate Chemical compound [Cs+].[Cs+].[O-]C([O-])=O FJDQFPXHSGXQBY-UHFFFAOYSA-L 0.000 description 2
- 229960001948 caffeine Drugs 0.000 description 2
- VJEONQKOZGKCAK-UHFFFAOYSA-N caffeine Natural products CN1C(=O)N(C)C(=O)C2=C1C=CN2C VJEONQKOZGKCAK-UHFFFAOYSA-N 0.000 description 2
- 229910000019 calcium carbonate Inorganic materials 0.000 description 2
- 239000001506 calcium phosphate Substances 0.000 description 2
- 229910000389 calcium phosphate Inorganic materials 0.000 description 2
- 235000011010 calcium phosphates Nutrition 0.000 description 2
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 238000004296 chiral HPLC Methods 0.000 description 2
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 2
- 229960001231 choline Drugs 0.000 description 2
- 229920001577 copolymer Polymers 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 229960004397 cyclophosphamide Drugs 0.000 description 2
- 229910052805 deuterium Inorganic materials 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 235000005911 diet Nutrition 0.000 description 2
- 230000037213 diet Effects 0.000 description 2
- FAMRKDQNMBBFBR-BQYQJAHWSA-N diethyl azodicarboxylate Substances CCOC(=O)\N=N\C(=O)OCC FAMRKDQNMBBFBR-BQYQJAHWSA-N 0.000 description 2
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical group C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 239000000890 drug combination Substances 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- FAMRKDQNMBBFBR-UHFFFAOYSA-N ethyl n-ethoxycarbonyliminocarbamate Chemical compound CCOC(=O)N=NC(=O)OCC FAMRKDQNMBBFBR-UHFFFAOYSA-N 0.000 description 2
- 125000000031 ethylamino group Chemical group [H]C([H])([H])C([H])([H])N([H])[*] 0.000 description 2
- 230000029142 excretion Effects 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 239000007789 gas Substances 0.000 description 2
- 239000007903 gelatin capsule Substances 0.000 description 2
- 229960002442 glucosamine Drugs 0.000 description 2
- 229940097043 glucuronic acid Drugs 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 229940093915 gynecological organic acid Drugs 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 125000004474 heteroalkylene group Chemical group 0.000 description 2
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
- XGIHQYAWBCFNPY-AZOCGYLKSA-N hydrabamine Chemical compound C([C@@H]12)CC3=CC(C(C)C)=CC=C3[C@@]2(C)CCC[C@@]1(C)CNCCNC[C@@]1(C)[C@@H]2CCC3=CC(C(C)C)=CC=C3[C@@]2(C)CCC1 XGIHQYAWBCFNPY-AZOCGYLKSA-N 0.000 description 2
- 238000003384 imaging method Methods 0.000 description 2
- 230000036039 immunity Effects 0.000 description 2
- 125000004129 indan-1-yl group Chemical group [H]C1=C([H])C([H])=C2C(=C1[H])C([H])([H])C([H])([H])C2([H])* 0.000 description 2
- 125000003392 indanyl group Chemical group C1(CCC2=CC=CC=C12)* 0.000 description 2
- 125000003453 indazolyl group Chemical group N1N=C(C2=C1C=CC=C2)* 0.000 description 2
- 125000001041 indolyl group Chemical group 0.000 description 2
- 239000012442 inert solvent Substances 0.000 description 2
- ZCYVEMRRCGMTRW-YPZZEJLDSA-N iodine-125 Chemical compound [125I] ZCYVEMRRCGMTRW-YPZZEJLDSA-N 0.000 description 2
- 229940044173 iodine-125 Drugs 0.000 description 2
- 150000002500 ions Chemical class 0.000 description 2
- 235000010445 lecithin Nutrition 0.000 description 2
- 239000000787 lecithin Substances 0.000 description 2
- 229940067606 lecithin Drugs 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 244000144972 livestock Species 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000004949 mass spectrometry Methods 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- QARBMVPHQWIHKH-UHFFFAOYSA-N methanesulfonyl chloride Chemical compound CS(Cl)(=O)=O QARBMVPHQWIHKH-UHFFFAOYSA-N 0.000 description 2
- 150000004702 methyl esters Chemical class 0.000 description 2
- 239000002480 mineral oil Substances 0.000 description 2
- 235000010446 mineral oil Nutrition 0.000 description 2
- 239000001788 mono and diglycerides of fatty acids Substances 0.000 description 2
- DUWWHGPELOTTOE-UHFFFAOYSA-N n-(5-chloro-2,4-dimethoxyphenyl)-3-oxobutanamide Chemical compound COC1=CC(OC)=C(NC(=O)CC(C)=O)C=C1Cl DUWWHGPELOTTOE-UHFFFAOYSA-N 0.000 description 2
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 125000001624 naphthyl group Chemical group 0.000 description 2
- 239000012299 nitrogen atmosphere Substances 0.000 description 2
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 2
- 239000000346 nonvolatile oil Substances 0.000 description 2
- 239000002674 ointment Substances 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 235000005985 organic acids Nutrition 0.000 description 2
- 239000012074 organic phase Substances 0.000 description 2
- CTSLXHKWHWQRSH-UHFFFAOYSA-N oxalyl chloride Chemical compound ClC(=O)C(Cl)=O CTSLXHKWHWQRSH-UHFFFAOYSA-N 0.000 description 2
- HXEACLLIILLPRG-UHFFFAOYSA-N pipecolic acid Chemical compound OC(=O)C1CCCCN1 HXEACLLIILLPRG-UHFFFAOYSA-N 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 229920000747 poly(lactic acid) Polymers 0.000 description 2
- 239000004633 polyglycolic acid Substances 0.000 description 2
- 239000004626 polylactic acid Substances 0.000 description 2
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 2
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 2
- SCVFZCLFOSHCOH-UHFFFAOYSA-M potassium acetate Chemical compound [K+].CC([O-])=O SCVFZCLFOSHCOH-UHFFFAOYSA-M 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 2
- 229960004919 procaine Drugs 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 235000019260 propionic acid Nutrition 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 150000003212 purines Chemical class 0.000 description 2
- 238000012827 research and development Methods 0.000 description 2
- 229910052703 rhodium Inorganic materials 0.000 description 2
- 125000006413 ring segment Chemical group 0.000 description 2
- 150000003335 secondary amines Chemical class 0.000 description 2
- 235000011069 sorbitan monooleate Nutrition 0.000 description 2
- 239000001593 sorbitan monooleate Substances 0.000 description 2
- 229940035049 sorbitan monooleate Drugs 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 125000003003 spiro group Chemical group 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- CXWXQJXEFPUFDZ-UHFFFAOYSA-N tetralin Chemical compound C1=CC=C2CCCCC2=C1 CXWXQJXEFPUFDZ-UHFFFAOYSA-N 0.000 description 2
- 150000003536 tetrazoles Chemical class 0.000 description 2
- 229960004559 theobromine Drugs 0.000 description 2
- 238000004809 thin layer chromatography Methods 0.000 description 2
- 125000005309 thioalkoxy group Chemical group 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 229910052723 transition metal Inorganic materials 0.000 description 2
- 150000003624 transition metals Chemical class 0.000 description 2
- 238000011269 treatment regimen Methods 0.000 description 2
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 2
- ITMCEJHCFYSIIV-UHFFFAOYSA-M triflate Chemical compound [O-]S(=O)(=O)C(F)(F)F ITMCEJHCFYSIIV-UHFFFAOYSA-M 0.000 description 2
- WJKHJLXJJJATHN-UHFFFAOYSA-N triflic anhydride Chemical compound FC(F)(F)S(=O)(=O)OS(=O)(=O)C(F)(F)F WJKHJLXJJJATHN-UHFFFAOYSA-N 0.000 description 2
- YFTHZRPMJXBUME-UHFFFAOYSA-N tripropylamine Chemical compound CCCN(CCC)CCC YFTHZRPMJXBUME-UHFFFAOYSA-N 0.000 description 2
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 2
- 229910052722 tritium Inorganic materials 0.000 description 2
- 229960000281 trometamol Drugs 0.000 description 2
- 229960005486 vaccine Drugs 0.000 description 2
- 235000015112 vegetable and seed oil Nutrition 0.000 description 2
- 239000008158 vegetable oil Substances 0.000 description 2
- 229910052725 zinc Inorganic materials 0.000 description 2
- 239000011701 zinc Substances 0.000 description 2
- RNXQQZNOSYBFTN-VIFPVBQESA-N (1s)-4-bromo-2,3-dihydro-1h-inden-1-ol Chemical compound C1=CC=C(Br)C2=C1[C@@H](O)CC2 RNXQQZNOSYBFTN-VIFPVBQESA-N 0.000 description 1
- ZDWONVAXOXYOJK-UHFFFAOYSA-N (2-chloro-3-methoxyphenyl)boronic acid Chemical group COC1=CC=CC(B(O)O)=C1Cl ZDWONVAXOXYOJK-UHFFFAOYSA-N 0.000 description 1
- OHMILAMAADHENX-UHFFFAOYSA-N (2-chloropyrimidin-5-yl)methanol Chemical compound OCC1=CN=C(Cl)N=C1 OHMILAMAADHENX-UHFFFAOYSA-N 0.000 description 1
- UXFRBAMOEBPFHN-MBMZGMDYSA-N (2S)-2-[[5-chloro-4-[(4-phenyl-2,3-dihydro-1H-inden-1-yl)oxy]-2-(pyridin-3-ylmethoxy)phenyl]methylamino]-3-hydroxypropanoic acid Chemical compound ClC=1C(=CC(=C(C=1)CN[C@H](C(=O)O)CO)OCC=1C=NC=CC=1)OC1CCC2=C(C=CC=C12)C1=CC=CC=C1 UXFRBAMOEBPFHN-MBMZGMDYSA-N 0.000 description 1
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 1
- YOJXPNNNKZABFE-BXRBKJIMSA-N (2s)-2-amino-3-hydroxypropanoic acid Chemical compound OC[C@H](N)C(O)=O.OC[C@H](N)C(O)=O YOJXPNNNKZABFE-BXRBKJIMSA-N 0.000 description 1
- PZUOEYPTQJILHP-GBXIJSLDSA-N (2s,3r)-2-amino-3-hydroxybutanamide Chemical group C[C@@H](O)[C@H](N)C(N)=O PZUOEYPTQJILHP-GBXIJSLDSA-N 0.000 description 1
- FMMOUBCQFOLVAC-SETSBSEESA-N (3S)-4-[[5-chloro-2-[(5-cyanopyridin-3-yl)methoxy]-4-[[(1R)-4-phenyl-2,3-dihydro-1H-inden-1-yl]oxy]phenyl]methylamino]-3-hydroxybutanoic acid Chemical compound ClC=1C(=CC(=C(C=1)CNC[C@H](CC(=O)O)O)OCC=1C=NC=C(C=1)C#N)O[C@@H]1CCC2=C(C=CC=C12)C1=CC=CC=C1 FMMOUBCQFOLVAC-SETSBSEESA-N 0.000 description 1
- VEEGZPWAAPPXRB-BJMVGYQFSA-N (3e)-3-(1h-imidazol-5-ylmethylidene)-1h-indol-2-one Chemical compound O=C1NC2=CC=CC=C2\C1=C/C1=CN=CN1 VEEGZPWAAPPXRB-BJMVGYQFSA-N 0.000 description 1
- CYEXXDYQJPRMIQ-UHFFFAOYSA-N (5-cyanopyridin-3-yl)boronic acid Chemical compound OB(O)C1=CN=CC(C#N)=C1 CYEXXDYQJPRMIQ-UHFFFAOYSA-N 0.000 description 1
- QUPFKBITVLIQNA-KPKJPENVSA-N (5e)-2-sulfanylidene-5-[[5-[3-(trifluoromethyl)phenyl]furan-2-yl]methylidene]-1,3-thiazolidin-4-one Chemical compound FC(F)(F)C1=CC=CC(C=2OC(\C=C\3C(NC(=S)S/3)=O)=CC=2)=C1 QUPFKBITVLIQNA-KPKJPENVSA-N 0.000 description 1
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 description 1
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- VMKAFJQFKBASMU-QGZVFWFLSA-N (r)-2-methyl-cbs-oxazaborolidine Chemical compound C([C@@H]12)CCN1B(C)OC2(C=1C=CC=CC=1)C1=CC=CC=C1 VMKAFJQFKBASMU-QGZVFWFLSA-N 0.000 description 1
- PAAZPARNPHGIKF-UHFFFAOYSA-N 1,2-dibromoethane Chemical compound BrCCBr PAAZPARNPHGIKF-UHFFFAOYSA-N 0.000 description 1
- WNXJIVFYUVYPPR-UHFFFAOYSA-N 1,3-dioxolane Chemical compound C1COCO1 WNXJIVFYUVYPPR-UHFFFAOYSA-N 0.000 description 1
- 125000000196 1,4-pentadienyl group Chemical group [H]C([*])=C([H])C([H])([H])C([H])=C([H])[H] 0.000 description 1
- YHIIJNLSGULWAA-UHFFFAOYSA-N 1,4-thiazinane 1-oxide Chemical compound O=S1CCNCC1 YHIIJNLSGULWAA-UHFFFAOYSA-N 0.000 description 1
- LXQMHOKEXZETKB-UHFFFAOYSA-N 1-amino-2-methylpropan-2-ol Chemical compound CC(C)(O)CN LXQMHOKEXZETKB-UHFFFAOYSA-N 0.000 description 1
- RWZRJLFTAMZFCM-NSCUHMNNSA-N 1-fluoro-4-[(e)-2-(4-methoxyphenyl)ethenyl]benzene Chemical compound C1=CC(OC)=CC=C1\C=C\C1=CC=C(F)C=C1 RWZRJLFTAMZFCM-NSCUHMNNSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- 125000004214 1-pyrrolidinyl group Chemical group [H]C1([H])N(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- CJXGXVKUSRZGNX-UHFFFAOYSA-N 1h-pyrazol-5-ylmethanamine;hydrochloride Chemical compound Cl.NCC1=CC=NN1 CJXGXVKUSRZGNX-UHFFFAOYSA-N 0.000 description 1
- 125000004206 2,2,2-trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 description 1
- NGNBDVOYPDDBFK-UHFFFAOYSA-N 2-[2,4-di(pentan-2-yl)phenoxy]acetyl chloride Chemical compound CCCC(C)C1=CC=C(OCC(Cl)=O)C(C(C)CCC)=C1 NGNBDVOYPDDBFK-UHFFFAOYSA-N 0.000 description 1
- CDUUKBXTEOFITR-BYPYZUCNSA-N 2-methyl-L-serine Chemical compound OC[C@@]([NH3+])(C)C([O-])=O CDUUKBXTEOFITR-BYPYZUCNSA-N 0.000 description 1
- 125000000094 2-phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 1
- MGADZUXDNSDTHW-UHFFFAOYSA-N 2H-pyran Chemical compound C1OC=CC=C1 MGADZUXDNSDTHW-UHFFFAOYSA-N 0.000 description 1
- ZPSJGADGUYYRKE-UHFFFAOYSA-N 2H-pyran-2-one Chemical compound O=C1C=CC=CO1 ZPSJGADGUYYRKE-UHFFFAOYSA-N 0.000 description 1
- ZHCLIFKUVIFYBY-UHFFFAOYSA-N 2h-tetrazol-5-ylmethanamine Chemical compound NCC1=NN=NN1 ZHCLIFKUVIFYBY-UHFFFAOYSA-N 0.000 description 1
- UAIUNKRWKOVEES-UHFFFAOYSA-N 3,3',5,5'-tetramethylbenzidine Chemical compound CC1=C(N)C(C)=CC(C=2C=C(C)C(N)=C(C)C=2)=C1 UAIUNKRWKOVEES-UHFFFAOYSA-N 0.000 description 1
- UZGLOGCJCWBBIV-UHFFFAOYSA-N 3-(chloromethyl)pyridin-1-ium;chloride Chemical compound Cl.ClCC1=CC=CN=C1 UZGLOGCJCWBBIV-UHFFFAOYSA-N 0.000 description 1
- GEVGRLPYQJTKKS-UHFFFAOYSA-N 3-(phenylmethoxycarbonylamino)propanoic acid Chemical compound OC(=O)CCNC(=O)OCC1=CC=CC=C1 GEVGRLPYQJTKKS-UHFFFAOYSA-N 0.000 description 1
- YYVSXUAJMARYHP-UHFFFAOYSA-N 3-amino-2,2-dimethylpropanoic acid;hydrochloride Chemical compound Cl.NCC(C)(C)C(O)=O YYVSXUAJMARYHP-UHFFFAOYSA-N 0.000 description 1
- 125000000474 3-butynyl group Chemical group [H]C#CC([H])([H])C([H])([H])* 0.000 description 1
- GQKDZDYQXPOXEM-UHFFFAOYSA-N 3-chlorocatechol Chemical group OC1=CC=CC(Cl)=C1O GQKDZDYQXPOXEM-UHFFFAOYSA-N 0.000 description 1
- JVQIKJMSUIMUDI-UHFFFAOYSA-N 3-pyrroline Chemical compound C1NCC=C1 JVQIKJMSUIMUDI-UHFFFAOYSA-N 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-M 4-hydroxybenzoate Chemical compound OC1=CC=C(C([O-])=O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-M 0.000 description 1
- GFOAHABINHRDKL-UHFFFAOYSA-N 5-(aminomethyl)pyrrolidin-2-one Chemical compound NCC1CCC(=O)N1 GFOAHABINHRDKL-UHFFFAOYSA-N 0.000 description 1
- MNDSDSKHCOGXRZ-HHHXNRCGSA-N 5-[[4-chloro-5-[[(1R)-4-(2-fluorophenyl)-2,3-dihydro-1H-inden-1-yl]oxy]-2-formylphenoxy]methyl]pyridine-3-carbonitrile Chemical compound ClC1=CC(=C(OCC=2C=C(C=NC=2)C#N)C=C1O[C@@H]1CCC2=C(C=CC=C12)C1=C(C=CC=C1)F)C=O MNDSDSKHCOGXRZ-HHHXNRCGSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical group [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- 208000030507 AIDS Diseases 0.000 description 1
- 241000235389 Absidia Species 0.000 description 1
- 241000224424 Acanthamoeba sp. Species 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 208000006400 Arbovirus Encephalitis Diseases 0.000 description 1
- 241000228212 Aspergillus Species 0.000 description 1
- 102100022716 Atypical chemokine receptor 3 Human genes 0.000 description 1
- 102100029822 B- and T-lymphocyte attenuator Human genes 0.000 description 1
- 241000223848 Babesia microti Species 0.000 description 1
- 241000304886 Bacilli Species 0.000 description 1
- 241000193738 Bacillus anthracis Species 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 241001235572 Balantioides coli Species 0.000 description 1
- 241000228405 Blastomyces dermatitidis Species 0.000 description 1
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- 208000003508 Botulism Diseases 0.000 description 1
- OEDJQURAYJIGLX-UHFFFAOYSA-N BrC1=C2CC(C(C2=CC=C1)O)F Chemical compound BrC1=C2CC(C(C2=CC=C1)O)F OEDJQURAYJIGLX-UHFFFAOYSA-N 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- 102100031172 C-C chemokine receptor type 1 Human genes 0.000 description 1
- 101710149814 C-C chemokine receptor type 1 Proteins 0.000 description 1
- 102100031151 C-C chemokine receptor type 2 Human genes 0.000 description 1
- 101710149815 C-C chemokine receptor type 2 Proteins 0.000 description 1
- 102100024167 C-C chemokine receptor type 3 Human genes 0.000 description 1
- 101710149862 C-C chemokine receptor type 3 Proteins 0.000 description 1
- 102100037853 C-C chemokine receptor type 4 Human genes 0.000 description 1
- 101710149863 C-C chemokine receptor type 4 Proteins 0.000 description 1
- 102100035875 C-C chemokine receptor type 5 Human genes 0.000 description 1
- 101710149870 C-C chemokine receptor type 5 Proteins 0.000 description 1
- 102100036301 C-C chemokine receptor type 7 Human genes 0.000 description 1
- 102100036305 C-C chemokine receptor type 8 Human genes 0.000 description 1
- 102100036166 C-X-C chemokine receptor type 1 Human genes 0.000 description 1
- 102100028989 C-X-C chemokine receptor type 2 Human genes 0.000 description 1
- 102100028990 C-X-C chemokine receptor type 3 Human genes 0.000 description 1
- 102100031650 C-X-C chemokine receptor type 4 Human genes 0.000 description 1
- 102100031658 C-X-C chemokine receptor type 5 Human genes 0.000 description 1
- 102100025618 C-X-C chemokine receptor type 6 Human genes 0.000 description 1
- FGUUSXIOTUKUDN-IBGZPJMESA-N C1(=CC=CC=C1)N1C2=C(NC([C@H](C1)NC=1OC(=NN=1)C1=CC=CC=C1)=O)C=CC=C2 Chemical compound C1(=CC=CC=C1)N1C2=C(NC([C@H](C1)NC=1OC(=NN=1)C1=CC=CC=C1)=O)C=CC=C2 FGUUSXIOTUKUDN-IBGZPJMESA-N 0.000 description 1
- 102100032957 C5a anaphylatoxin chemotactic receptor 1 Human genes 0.000 description 1
- 101710098483 C5a anaphylatoxin chemotactic receptor 1 Proteins 0.000 description 1
- WOCZEORBWVGRNT-UHFFFAOYSA-N CC(NCc1ccn[nH]1)I Chemical compound CC(NCc1ccn[nH]1)I WOCZEORBWVGRNT-UHFFFAOYSA-N 0.000 description 1
- 102100036008 CD48 antigen Human genes 0.000 description 1
- ICJYZOCGKJOEPX-UHFFFAOYSA-N CN(CC1)CC1(C(O)=O)O Chemical compound CN(CC1)CC1(C(O)=O)O ICJYZOCGKJOEPX-UHFFFAOYSA-N 0.000 description 1
- FLVFPAIGVBQGET-RXMQYKEDSA-N CN(CC1)C[C@@H]1O Chemical compound CN(CC1)C[C@@H]1O FLVFPAIGVBQGET-RXMQYKEDSA-N 0.000 description 1
- NKDYHCPYSXTJNL-UHFFFAOYSA-N CNCC(CC1)NC1=O Chemical compound CNCC(CC1)NC1=O NKDYHCPYSXTJNL-UHFFFAOYSA-N 0.000 description 1
- 238000010354 CRISPR gene editing Methods 0.000 description 1
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 1
- 102000008203 CTLA-4 Antigen Human genes 0.000 description 1
- 229940045513 CTLA4 antagonist Drugs 0.000 description 1
- INDRPMONSGHHBH-GBXIJSLDSA-N C[C@H]([C@@H](C(N)=O)NI)O Chemical compound C[C@H]([C@@H](C(N)=O)NI)O INDRPMONSGHHBH-GBXIJSLDSA-N 0.000 description 1
- PUEHCTDTWVEIMF-GBXIJSLDSA-N C[C@H]([C@@H](C(O)=O)NI)O Chemical compound C[C@H]([C@@H](C(O)=O)NI)O PUEHCTDTWVEIMF-GBXIJSLDSA-N 0.000 description 1
- GAWIXWVDTYZWAW-UHFFFAOYSA-N C[CH]O Chemical group C[CH]O GAWIXWVDTYZWAW-UHFFFAOYSA-N 0.000 description 1
- 102000000905 Cadherin Human genes 0.000 description 1
- 108050007957 Cadherin Proteins 0.000 description 1
- 206010058019 Cancer Pain Diseases 0.000 description 1
- 241000222120 Candida <Saccharomycetales> Species 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- OKTJSMMVPCPJKN-OUBTZVSYSA-N Carbon-13 Chemical compound [13C] OKTJSMMVPCPJKN-OUBTZVSYSA-N 0.000 description 1
- HBWCTPVLNCHQDO-INIZCTEOSA-N Cc(cc(C=O)c(O)c1)c1O[C@@H](CC1)c2c1c(Br)ccc2 Chemical compound Cc(cc(C=O)c(O)c1)c1O[C@@H](CC1)c2c1c(Br)ccc2 HBWCTPVLNCHQDO-INIZCTEOSA-N 0.000 description 1
- 241000606161 Chlamydia Species 0.000 description 1
- KZBUYRJDOAKODT-UHFFFAOYSA-N Chlorine Chemical compound ClCl KZBUYRJDOAKODT-UHFFFAOYSA-N 0.000 description 1
- 206010008631 Cholera Diseases 0.000 description 1
- 241000223205 Coccidioides immitis Species 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 241000709687 Coxsackievirus Species 0.000 description 1
- 201000007336 Cryptococcosis Diseases 0.000 description 1
- 241000221204 Cryptococcus neoformans Species 0.000 description 1
- 241000295636 Cryptosporidium sp. Species 0.000 description 1
- 229920000858 Cyclodextrin Polymers 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- 241000725619 Dengue virus Species 0.000 description 1
- 241001466953 Echovirus Species 0.000 description 1
- 241000224432 Entamoeba histolytica Species 0.000 description 1
- 241000709661 Enterovirus Species 0.000 description 1
- 241000991587 Enterovirus C Species 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 241000283073 Equus caballus Species 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- CWYNVVGOOAEACU-UHFFFAOYSA-N Fe2+ Chemical compound [Fe+2] CWYNVVGOOAEACU-UHFFFAOYSA-N 0.000 description 1
- 241000710831 Flavivirus Species 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- 102100031351 Galectin-9 Human genes 0.000 description 1
- 101710121810 Galectin-9 Proteins 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 244000068988 Glycine max Species 0.000 description 1
- 235000010469 Glycine max Nutrition 0.000 description 1
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 1
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 1
- 102000004457 Granulocyte-Macrophage Colony-Stimulating Factor Human genes 0.000 description 1
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 208000031886 HIV Infections Diseases 0.000 description 1
- 208000037357 HIV infectious disease Diseases 0.000 description 1
- 206010019799 Hepatitis viral Diseases 0.000 description 1
- 241000228404 Histoplasma capsulatum Species 0.000 description 1
- 101000678890 Homo sapiens Atypical chemokine receptor 3 Proteins 0.000 description 1
- 101000798902 Homo sapiens Atypical chemokine receptor 4 Proteins 0.000 description 1
- 101000864344 Homo sapiens B- and T-lymphocyte attenuator Proteins 0.000 description 1
- 101000777558 Homo sapiens C-C chemokine receptor type 10 Proteins 0.000 description 1
- 101000716068 Homo sapiens C-C chemokine receptor type 6 Proteins 0.000 description 1
- 101000716065 Homo sapiens C-C chemokine receptor type 7 Proteins 0.000 description 1
- 101000716063 Homo sapiens C-C chemokine receptor type 8 Proteins 0.000 description 1
- 101000716070 Homo sapiens C-C chemokine receptor type 9 Proteins 0.000 description 1
- 101000947174 Homo sapiens C-X-C chemokine receptor type 1 Proteins 0.000 description 1
- 101000916050 Homo sapiens C-X-C chemokine receptor type 3 Proteins 0.000 description 1
- 101000922348 Homo sapiens C-X-C chemokine receptor type 4 Proteins 0.000 description 1
- 101000922405 Homo sapiens C-X-C chemokine receptor type 5 Proteins 0.000 description 1
- 101000856683 Homo sapiens C-X-C chemokine receptor type 6 Proteins 0.000 description 1
- 101000716130 Homo sapiens CD48 antigen Proteins 0.000 description 1
- 101001117317 Homo sapiens Programmed cell death 1 ligand 1 Proteins 0.000 description 1
- 101000611936 Homo sapiens Programmed cell death protein 1 Proteins 0.000 description 1
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 1
- 101000669447 Homo sapiens Toll-like receptor 4 Proteins 0.000 description 1
- 241000598436 Human T-cell lymphotropic virus Species 0.000 description 1
- 241000701044 Human gammaherpesvirus 4 Species 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- XQFRJNBWHJMXHO-RRKCRQDMSA-N IDUR Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(I)=C1 XQFRJNBWHJMXHO-RRKCRQDMSA-N 0.000 description 1
- WRYCSMQKUKOKBP-UHFFFAOYSA-N Imidazolidine Chemical compound C1CNCN1 WRYCSMQKUKOKBP-UHFFFAOYSA-N 0.000 description 1
- 102000037982 Immune checkpoint proteins Human genes 0.000 description 1
- 108091008036 Immune checkpoint proteins Proteins 0.000 description 1
- 108010002352 Interleukin-1 Proteins 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- 108010018951 Interleukin-8B Receptors Proteins 0.000 description 1
- 241000701460 JC polyomavirus Species 0.000 description 1
- 241000588748 Klebsiella Species 0.000 description 1
- 125000000769 L-threonyl group Chemical group [H]N([H])[C@]([H])(C(=O)[*])[C@](O[H])(C([H])([H])[H])[H] 0.000 description 1
- 239000005517 L01XE01 - Imatinib Substances 0.000 description 1
- 102000017578 LAG3 Human genes 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 101150030213 Lag3 gene Proteins 0.000 description 1
- 241001245510 Lambia <signal fly> Species 0.000 description 1
- 241000589248 Legionella Species 0.000 description 1
- 208000007764 Legionnaires' Disease Diseases 0.000 description 1
- 241000222722 Leishmania <genus> Species 0.000 description 1
- 241000222727 Leishmania donovani Species 0.000 description 1
- 206010024238 Leptospirosis Diseases 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 208000016604 Lyme disease Diseases 0.000 description 1
- 108010046938 Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102000007651 Macrophage Colony-Stimulating Factor Human genes 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 241000712079 Measles morbillivirus Species 0.000 description 1
- 108010061593 Member 14 Tumor Necrosis Factor Receptors Proteins 0.000 description 1
- BZLVMXJERCGZMT-UHFFFAOYSA-N Methyl tert-butyl ether Chemical compound COC(C)(C)C BZLVMXJERCGZMT-UHFFFAOYSA-N 0.000 description 1
- 241000235395 Mucor Species 0.000 description 1
- 241000235388 Mucorales Species 0.000 description 1
- 241000711386 Mumps virus Species 0.000 description 1
- XFJYSOHNDNIVLK-KYJUHHDHSA-N N#Cc1cc(COc(c(CN[C@@H](CO)C(O)=O)c2)cc(O[C@@H]3c4cccc(-c5ccc6OCCOc6c5F)c4CC3)c2Cl)cc(C#N)c1 Chemical compound N#Cc1cc(COc(c(CN[C@@H](CO)C(O)=O)c2)cc(O[C@@H]3c4cccc(-c5ccc6OCCOc6c5F)c4CC3)c2Cl)cc(C#N)c1 XFJYSOHNDNIVLK-KYJUHHDHSA-N 0.000 description 1
- 238000005481 NMR spectroscopy Methods 0.000 description 1
- 241000224438 Naegleria fowleri Species 0.000 description 1
- 206010061309 Neoplasm progression Diseases 0.000 description 1
- 241001126259 Nippostrongylus brasiliensis Species 0.000 description 1
- 101710163270 Nuclease Proteins 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium on carbon Substances [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 1
- 241000526686 Paracoccidioides brasiliensis Species 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 208000037581 Persistent Infection Diseases 0.000 description 1
- 229940049937 Pgp inhibitor Drugs 0.000 description 1
- 206010035148 Plague Diseases 0.000 description 1
- 241000223810 Plasmodium vivax Species 0.000 description 1
- 241000233872 Pneumocystis carinii Species 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 229920002565 Polyethylene Glycol 400 Polymers 0.000 description 1
- 229920001030 Polyethylene Glycol 4000 Polymers 0.000 description 1
- 229920001710 Polyorthoester Polymers 0.000 description 1
- 208000006994 Precancerous Conditions Diseases 0.000 description 1
- 241000588769 Proteus <enterobacteria> Species 0.000 description 1
- 241000125945 Protoparvovirus Species 0.000 description 1
- 241000589516 Pseudomonas Species 0.000 description 1
- WHBMMWSBFZVSSR-UHFFFAOYSA-N R3HBA Natural products CC(O)CC(O)=O WHBMMWSBFZVSSR-UHFFFAOYSA-N 0.000 description 1
- 241000711798 Rabies lyssavirus Species 0.000 description 1
- 241000725643 Respiratory syncytial virus Species 0.000 description 1
- 241000606651 Rickettsiales Species 0.000 description 1
- 241000702670 Rotavirus Species 0.000 description 1
- 241000710799 Rubella virus Species 0.000 description 1
- 241000607142 Salmonella Species 0.000 description 1
- 241000607720 Serratia Species 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 241001149962 Sporothrix Species 0.000 description 1
- 241000295644 Staphylococcaceae Species 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 241000282898 Sus scrofa Species 0.000 description 1
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 1
- 238000010459 TALEN Methods 0.000 description 1
- 206010043376 Tetanus Diseases 0.000 description 1
- 102100039360 Toll-like receptor 4 Human genes 0.000 description 1
- 241000223996 Toxoplasma Species 0.000 description 1
- 108010043645 Transcription Activator-Like Effector Nucleases Proteins 0.000 description 1
- 241000223105 Trypanosoma brucei Species 0.000 description 1
- 241000223109 Trypanosoma cruzi Species 0.000 description 1
- 102100028785 Tumor necrosis factor receptor superfamily member 14 Human genes 0.000 description 1
- 241000700618 Vaccinia virus Species 0.000 description 1
- 241000607479 Yersinia pestis Species 0.000 description 1
- 108010017070 Zinc Finger Nucleases Proteins 0.000 description 1
- WERKSKAQRVDLDW-ANOHMWSOSA-N [(2s,3r,4r,5r)-2,3,4,5,6-pentahydroxyhexyl] (z)-octadec-9-enoate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO WERKSKAQRVDLDW-ANOHMWSOSA-N 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 125000000218 acetic acid group Chemical group C(C)(=O)* 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 239000011149 active material Substances 0.000 description 1
- 125000002252 acyl group Chemical group 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 150000001335 aliphatic alkanes Chemical class 0.000 description 1
- 229930013930 alkaloid Natural products 0.000 description 1
- 150000003797 alkaloid derivatives Chemical class 0.000 description 1
- 125000004450 alkenylene group Chemical group 0.000 description 1
- 125000003282 alkyl amino group Chemical group 0.000 description 1
- 125000005907 alkyl ester group Chemical group 0.000 description 1
- 125000005189 alkyl hydroxy group Chemical group 0.000 description 1
- 125000004414 alkyl thio group Chemical group 0.000 description 1
- 125000005237 alkyleneamino group Chemical group 0.000 description 1
- 125000005238 alkylenediamino group Chemical group 0.000 description 1
- 125000005530 alkylenedioxy group Chemical group 0.000 description 1
- 125000005529 alkyleneoxy group Chemical group 0.000 description 1
- 125000004419 alkynylene group Chemical group 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 239000012491 analyte Substances 0.000 description 1
- 229940121369 angiogenesis inhibitor Drugs 0.000 description 1
- 229940045799 anthracyclines and related substance Drugs 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000001093 anti-cancer Effects 0.000 description 1
- 230000003474 anti-emetic effect Effects 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000002111 antiemetic agent Substances 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 229940041181 antineoplastic drug Drugs 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 206010003246 arthritis Diseases 0.000 description 1
- 125000005165 aryl thioxy group Chemical group 0.000 description 1
- 125000004104 aryloxy group Chemical group 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000003190 augmentative effect Effects 0.000 description 1
- 208000007456 balantidiasis Diseases 0.000 description 1
- 235000013871 bee wax Nutrition 0.000 description 1
- 239000012166 beeswax Substances 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 125000004603 benzisoxazolyl group Chemical group O1N=C(C2=C1C=CC=C2)* 0.000 description 1
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000003354 benzotriazolyl group Chemical group N1N=NC2=C1C=CC=C2* 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- GPRLTFBKWDERLU-UHFFFAOYSA-N bicyclo[2.2.2]octane Chemical compound C1CC2CCC1CC2 GPRLTFBKWDERLU-UHFFFAOYSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 238000002306 biochemical method Methods 0.000 description 1
- 229920002988 biodegradable polymer Polymers 0.000 description 1
- 239000004621 biodegradable polymer Substances 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- 239000004305 biphenyl Chemical group 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- AEILLAXRDHDKDY-UHFFFAOYSA-N bromomethylcyclopropane Chemical group BrCC1CC1 AEILLAXRDHDKDY-UHFFFAOYSA-N 0.000 description 1
- 235000019437 butane-1,3-diol Nutrition 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 239000012876 carrier material Substances 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 150000005829 chemical entities Chemical class 0.000 description 1
- 239000007910 chewable tablet Substances 0.000 description 1
- 229940112822 chewing gum Drugs 0.000 description 1
- 235000015218 chewing gum Nutrition 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 125000000259 cinnolinyl group Chemical group N1=NC(=CC2=CC=CC=C12)* 0.000 description 1
- 229940110456 cocoa butter Drugs 0.000 description 1
- 235000019868 cocoa butter Nutrition 0.000 description 1
- 239000003240 coconut oil Substances 0.000 description 1
- 235000019864 coconut oil Nutrition 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 125000000596 cyclohexenyl group Chemical group C1(=CCCCC1)* 0.000 description 1
- GCUVBACNBHGZRS-UHFFFAOYSA-N cyclopenta-1,3-diene cyclopenta-2,4-dien-1-yl(diphenyl)phosphane iron(2+) Chemical compound [Fe++].c1cc[cH-]c1.c1cc[c-](c1)P(c1ccccc1)c1ccccc1 GCUVBACNBHGZRS-UHFFFAOYSA-N 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 230000002939 deleterious effect Effects 0.000 description 1
- 125000004663 dialkyl amino group Chemical group 0.000 description 1
- ZOCHARZZJNPSEU-UHFFFAOYSA-N diboron Chemical compound B#B ZOCHARZZJNPSEU-UHFFFAOYSA-N 0.000 description 1
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 description 1
- 239000012897 dilution medium Substances 0.000 description 1
- 206010013023 diphtheria Diseases 0.000 description 1
- 238000000375 direct analysis in real time Methods 0.000 description 1
- 208000037765 diseases and disorders Diseases 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 238000012063 dual-affinity re-targeting Methods 0.000 description 1
- 239000007911 effervescent powder Substances 0.000 description 1
- 239000007938 effervescent tablet Substances 0.000 description 1
- 239000003974 emollient agent Substances 0.000 description 1
- 238000004945 emulsification Methods 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 229940007078 entamoeba histolytica Drugs 0.000 description 1
- 125000004494 ethyl ester group Chemical group 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 239000002360 explosive Substances 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- 229940083124 ganglion-blocking antiadrenergic secondary and tertiary amines Drugs 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 229940074045 glyceryl distearate Drugs 0.000 description 1
- 229940075507 glyceryl monostearate Drugs 0.000 description 1
- 239000002748 glycoprotein P inhibitor Substances 0.000 description 1
- 239000007902 hard capsule Substances 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- FBPFZTCFMRRESA-UHFFFAOYSA-N hexane-1,2,3,4,5,6-hexol Chemical compound OCC(O)C(O)C(O)C(O)CO FBPFZTCFMRRESA-UHFFFAOYSA-N 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000003667 hormone antagonist Substances 0.000 description 1
- 102000048776 human CD274 Human genes 0.000 description 1
- 102000048362 human PDCD1 Human genes 0.000 description 1
- 208000033519 human immunodeficiency virus infectious disease Diseases 0.000 description 1
- WJRBRSLFGCUECM-UHFFFAOYSA-N hydantoin Chemical compound O=C1CNC(=O)N1 WJRBRSLFGCUECM-UHFFFAOYSA-N 0.000 description 1
- 229940091173 hydantoin Drugs 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 239000000017 hydrogel Substances 0.000 description 1
- RCCPEORTSYDPMB-UHFFFAOYSA-N hydroxy benzenecarboximidothioate Chemical compound OSC(=N)C1=CC=CC=C1 RCCPEORTSYDPMB-UHFFFAOYSA-N 0.000 description 1
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 1
- KTUFNOKKBVMGRW-UHFFFAOYSA-N imatinib Chemical compound C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 KTUFNOKKBVMGRW-UHFFFAOYSA-N 0.000 description 1
- 229960002411 imatinib Drugs 0.000 description 1
- YLMAHDNUQAMNNX-UHFFFAOYSA-N imatinib methanesulfonate Chemical compound CS(O)(=O)=O.C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 YLMAHDNUQAMNNX-UHFFFAOYSA-N 0.000 description 1
- YAMHXTCMCPHKLN-UHFFFAOYSA-N imidazolidin-2-one Chemical compound O=C1NCCN1 YAMHXTCMCPHKLN-UHFFFAOYSA-N 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 125000004857 imidazopyridinyl group Chemical class N1C(=NC2=C1C=CC=N2)* 0.000 description 1
- 230000004957 immunoregulator effect Effects 0.000 description 1
- 230000001024 immunotherapeutic effect Effects 0.000 description 1
- YIAPLDFPUUJILH-UHFFFAOYSA-N indan-1-ol Chemical class C1=CC=C2C(O)CCC2=C1 YIAPLDFPUUJILH-UHFFFAOYSA-N 0.000 description 1
- 125000003406 indolizinyl group Chemical group C=1(C=CN2C=CC=CC12)* 0.000 description 1
- 239000003701 inert diluent Substances 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 229940102223 injectable solution Drugs 0.000 description 1
- 229940102213 injectable suspension Drugs 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- HVTICUPFWKNHNG-BJUDXGSMSA-N iodoethane Chemical group [11CH3]CI HVTICUPFWKNHNG-BJUDXGSMSA-N 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- KQNPFQTWMSNSAP-UHFFFAOYSA-N isobutyric acid Chemical compound CC(C)C(O)=O KQNPFQTWMSNSAP-UHFFFAOYSA-N 0.000 description 1
- 125000000904 isoindolyl group Chemical group C=1(NC=C2C=CC=CC12)* 0.000 description 1
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 1
- 125000005956 isoquinolyl group Chemical group 0.000 description 1
- 125000001786 isothiazolyl group Chemical group 0.000 description 1
- 125000000842 isoxazolyl group Chemical group 0.000 description 1
- 235000015110 jellies Nutrition 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 229940043355 kinase inhibitor Drugs 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 229960003881 letrozole Drugs 0.000 description 1
- HPJKCIUCZWXJDR-UHFFFAOYSA-N letrozole Chemical compound C1=CC(C#N)=CC=C1C(N1N=CN=C1)C1=CC=C(C#N)C=C1 HPJKCIUCZWXJDR-UHFFFAOYSA-N 0.000 description 1
- 201000002364 leukopenia Diseases 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- PWPJGUXAGUPAHP-UHFFFAOYSA-N lufenuron Chemical compound C1=C(Cl)C(OC(F)(F)C(C(F)(F)F)F)=CC(Cl)=C1NC(=O)NC(=O)C1=C(F)C=CC=C1F PWPJGUXAGUPAHP-UHFFFAOYSA-N 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 201000004792 malaria Diseases 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- LVWZTYCIRDMTEY-UHFFFAOYSA-N metamizole Chemical compound O=C1C(N(CS(O)(=O)=O)C)=C(C)N(C)N1C1=CC=CC=C1 LVWZTYCIRDMTEY-UHFFFAOYSA-N 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 230000011278 mitosis Effects 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 125000002950 monocyclic group Chemical group 0.000 description 1
- 125000006682 monohaloalkyl group Chemical group 0.000 description 1
- 125000004573 morpholin-4-yl group Chemical group N1(CCOCC1)* 0.000 description 1
- 125000002757 morpholinyl group Chemical group 0.000 description 1
- 239000002324 mouth wash Substances 0.000 description 1
- YAHBUVWRSTZBAX-UHFFFAOYSA-N n-(2-aminoethyl)prop-2-enamide;hydrochloride Chemical compound Cl.NCCNC(=O)C=C YAHBUVWRSTZBAX-UHFFFAOYSA-N 0.000 description 1
- LBWFXVZLPYTWQI-IPOVEDGCSA-N n-[2-(diethylamino)ethyl]-5-[(z)-(5-fluoro-2-oxo-1h-indol-3-ylidene)methyl]-2,4-dimethyl-1h-pyrrole-3-carboxamide;(2s)-2-hydroxybutanedioic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O.CCN(CC)CCNC(=O)C1=C(C)NC(\C=C/2C3=CC(F)=CC=C3NC\2=O)=C1C LBWFXVZLPYTWQI-IPOVEDGCSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003136 n-heptyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 208000004235 neutropenia Diseases 0.000 description 1
- OSTGTTZJOCZWJG-UHFFFAOYSA-N nitrosourea Chemical compound NC(=O)N=NO OSTGTTZJOCZWJG-UHFFFAOYSA-N 0.000 description 1
- 231100000344 non-irritating Toxicity 0.000 description 1
- UMRZSTCPUPJPOJ-KNVOCYPGSA-N norbornane Chemical compound C1C[C@H]2CC[C@@H]1C2 UMRZSTCPUPJPOJ-KNVOCYPGSA-N 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- TVMXDCGIABBOFY-UHFFFAOYSA-N octane Chemical compound CCCCCCCC TVMXDCGIABBOFY-UHFFFAOYSA-N 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- 238000011275 oncology therapy Methods 0.000 description 1
- 244000309459 oncolytic virus Species 0.000 description 1
- 229940041672 oral gel Drugs 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 description 1
- 229960001756 oxaliplatin Drugs 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- PIBWKRNGBLPSSY-UHFFFAOYSA-L palladium(II) chloride Chemical compound Cl[Pd]Cl PIBWKRNGBLPSSY-UHFFFAOYSA-L 0.000 description 1
- 125000001312 palmitoyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 244000045947 parasite Species 0.000 description 1
- 239000004031 partial agonist Substances 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- JLFNLZLINWHATN-UHFFFAOYSA-N pentaethylene glycol Chemical compound OCCOCCOCCOCCOCCO JLFNLZLINWHATN-UHFFFAOYSA-N 0.000 description 1
- 239000008024 pharmaceutical diluent Substances 0.000 description 1
- 230000003285 pharmacodynamic effect Effects 0.000 description 1
- OJMIONKXNSYLSR-UHFFFAOYSA-N phosphorous acid Chemical class OP(O)O OJMIONKXNSYLSR-UHFFFAOYSA-N 0.000 description 1
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 1
- XKJCHHZQLQNZHY-UHFFFAOYSA-N phthalimide Chemical compound C1=CC=C2C(=O)NC(=O)C2=C1 XKJCHHZQLQNZHY-UHFFFAOYSA-N 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- XUWHAWMETYGRKB-UHFFFAOYSA-N piperidin-2-one Chemical compound O=C1CCCCN1 XUWHAWMETYGRKB-UHFFFAOYSA-N 0.000 description 1
- 230000036470 plasma concentration Effects 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 239000002798 polar solvent Substances 0.000 description 1
- 229920000768 polyamine Polymers 0.000 description 1
- 229920001610 polycaprolactone Polymers 0.000 description 1
- 229920002721 polycyanoacrylate Polymers 0.000 description 1
- 239000008389 polyethoxylated castor oil Substances 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 125000006684 polyhaloalkyl group Polymers 0.000 description 1
- 229940115272 polyinosinic:polycytidylic acid Drugs 0.000 description 1
- 229920000656 polylysine Polymers 0.000 description 1
- 108091033319 polynucleotide Proteins 0.000 description 1
- 102000040430 polynucleotide Human genes 0.000 description 1
- 239000002157 polynucleotide Substances 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 1
- 229920006324 polyoxymethylene Polymers 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 238000002600 positron emission tomography Methods 0.000 description 1
- 235000011056 potassium acetate Nutrition 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- ZQSBPSWEMOKULO-JEDNCBNOSA-N propan-2-yl (2s)-2-amino-3-hydroxypropanoate;hydrochloride Chemical compound Cl.CC(C)OC(=O)[C@@H](N)CO ZQSBPSWEMOKULO-JEDNCBNOSA-N 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 235000018102 proteins Nutrition 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 238000000425 proton nuclear magnetic resonance spectrum Methods 0.000 description 1
- 125000001042 pteridinyl group Chemical group N1=C(N=CC2=NC=CN=C12)* 0.000 description 1
- 125000000561 purinyl group Chemical group N1=C(N=C2N=CNC2=C1)* 0.000 description 1
- 125000003373 pyrazinyl group Chemical group 0.000 description 1
- USPWKWBDZOARPV-UHFFFAOYSA-N pyrazolidine Chemical compound C1CNNC1 USPWKWBDZOARPV-UHFFFAOYSA-N 0.000 description 1
- STIKETVNLGXQCS-UHFFFAOYSA-N pyridazin-3-ylmethanol Chemical compound OCC1=CC=CN=N1 STIKETVNLGXQCS-UHFFFAOYSA-N 0.000 description 1
- GDSUSSVBFDAOCB-UHFFFAOYSA-N pyridazin-3-ylmethyl methanesulfonate Chemical compound CS(=O)(=O)OCC=1N=NC=CC=1 GDSUSSVBFDAOCB-UHFFFAOYSA-N 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 125000005344 pyridylmethyl group Chemical group [H]C1=C([H])C([H])=C([H])C(=N1)C([H])([H])* 0.000 description 1
- HNJBEVLQSNELDL-UHFFFAOYSA-N pyrrolidin-2-one Chemical compound O=C1CCCN1 HNJBEVLQSNELDL-UHFFFAOYSA-N 0.000 description 1
- 125000000168 pyrrolyl group Chemical group 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 230000000171 quenching effect Effects 0.000 description 1
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 description 1
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 1
- 125000005493 quinolyl group Chemical group 0.000 description 1
- 125000001567 quinoxalinyl group Chemical group N1=C(C=NC2=CC=CC=C12)* 0.000 description 1
- SBYHFKPVCBCYGV-UHFFFAOYSA-N quinuclidine Chemical compound C1CC2CCN1CC2 SBYHFKPVCBCYGV-UHFFFAOYSA-N 0.000 description 1
- 231100000336 radiotoxic Toxicity 0.000 description 1
- 230000001690 radiotoxic effect Effects 0.000 description 1
- 210000000664 rectum Anatomy 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 231100000205 reproductive and developmental toxicity Toxicity 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 238000004007 reversed phase HPLC Methods 0.000 description 1
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 description 1
- 229940081974 saccharin Drugs 0.000 description 1
- 235000019204 saccharin Nutrition 0.000 description 1
- 239000000901 saccharin and its Na,K and Ca salt Substances 0.000 description 1
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical compound O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 239000012279 sodium borohydride Substances 0.000 description 1
- 229910000033 sodium borohydride Inorganic materials 0.000 description 1
- 229910001467 sodium calcium phosphate Inorganic materials 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- BEOOHQFXGBMRKU-UHFFFAOYSA-N sodium cyanoborohydride Chemical compound [Na+].[B-]C#N BEOOHQFXGBMRKU-UHFFFAOYSA-N 0.000 description 1
- HRZFUMHJMZEROT-UHFFFAOYSA-L sodium disulfite Chemical compound [Na+].[Na+].[O-]S(=O)S([O-])(=O)=O HRZFUMHJMZEROT-UHFFFAOYSA-L 0.000 description 1
- 229940001584 sodium metabisulfite Drugs 0.000 description 1
- 235000010262 sodium metabisulphite Nutrition 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- 239000007901 soft capsule Substances 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 239000012089 stop solution Substances 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- WINHZLLDWRZWRT-ATVHPVEESA-N sunitinib Chemical compound CCN(CC)CCNC(=O)C1=C(C)NC(\C=C/2C3=CC(F)=CC=C3NC\2=O)=C1C WINHZLLDWRZWRT-ATVHPVEESA-N 0.000 description 1
- 229960001796 sunitinib Drugs 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 229940034785 sutent Drugs 0.000 description 1
- 238000002636 symptomatic treatment Methods 0.000 description 1
- 210000001258 synovial membrane Anatomy 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 125000005247 tetrazinyl group Chemical group N1=NN=NC(=C1)* 0.000 description 1
- 231100001274 therapeutic index Toxicity 0.000 description 1
- 125000001113 thiadiazolyl group Chemical group 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- BRNULMACUQOKMR-UHFFFAOYSA-N thiomorpholine Chemical compound C1CSCCN1 BRNULMACUQOKMR-UHFFFAOYSA-N 0.000 description 1
- IBBLKSWSCDAPIF-UHFFFAOYSA-N thiopyran Chemical compound S1C=CC=C=C1 IBBLKSWSCDAPIF-UHFFFAOYSA-N 0.000 description 1
- 125000000341 threoninyl group Chemical group [H]OC([H])(C([H])([H])[H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 206010043554 thrombocytopenia Diseases 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 238000003325 tomography Methods 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 230000037317 transdermal delivery Effects 0.000 description 1
- 125000004306 triazinyl group Chemical group 0.000 description 1
- 125000001425 triazolyl group Chemical group 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- SEDZOYHHAIAQIW-UHFFFAOYSA-N trimethylsilyl azide Chemical compound C[Si](C)(C)N=[N+]=[N-] SEDZOYHHAIAQIW-UHFFFAOYSA-N 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- 230000005751 tumor progression Effects 0.000 description 1
- 241000701161 unidentified adenovirus Species 0.000 description 1
- 241000712461 unidentified influenza virus Species 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 238000010626 work up procedure Methods 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/075—Ethers or acetals
- A61K31/085—Ethers or acetals having an ether linkage to aromatic ring nuclear carbon
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/01—Hydrocarbons
- A61K31/015—Hydrocarbons carbocyclic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/02—Halogenated hydrocarbons
- A61K31/025—Halogenated hydrocarbons carbocyclic
- A61K31/03—Halogenated hydrocarbons carbocyclic aromatic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/275—Nitriles; Isonitriles
- A61K31/277—Nitriles; Isonitriles having a ring, e.g. verapamil
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/4439—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a five-membered ring with nitrogen as a ring hetero atom, e.g. omeprazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
- A61K31/4523—Non condensed piperidines, e.g. piperocaine containing further heterocyclic ring systems
- A61K31/4545—Non condensed piperidines, e.g. piperocaine containing further heterocyclic ring systems containing a six-membered ring with nitrogen as a ring hetero atom, e.g. pipamperone, anabasine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/50—Pyridazines; Hydrogenated pyridazines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/34—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyesters, polyamino acids, polysiloxanes, polyphosphazines, copolymers of polyalkylene glycol or poloxamers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C215/00—Compounds containing amino and hydroxy groups bound to the same carbon skeleton
- C07C215/02—Compounds containing amino and hydroxy groups bound to the same carbon skeleton having hydroxy groups and amino groups bound to acyclic carbon atoms of the same carbon skeleton
- C07C215/04—Compounds containing amino and hydroxy groups bound to the same carbon skeleton having hydroxy groups and amino groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton being saturated
- C07C215/06—Compounds containing amino and hydroxy groups bound to the same carbon skeleton having hydroxy groups and amino groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton being saturated and acyclic
- C07C215/12—Compounds containing amino and hydroxy groups bound to the same carbon skeleton having hydroxy groups and amino groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton being saturated and acyclic the nitrogen atom of the amino group being further bound to hydrocarbon groups substituted by hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C229/00—Compounds containing amino and carboxyl groups bound to the same carbon skeleton
- C07C229/02—Compounds containing amino and carboxyl groups bound to the same carbon skeleton having amino and carboxyl groups bound to acyclic carbon atoms of the same carbon skeleton
- C07C229/04—Compounds containing amino and carboxyl groups bound to the same carbon skeleton having amino and carboxyl groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton being acyclic and saturated
- C07C229/22—Compounds containing amino and carboxyl groups bound to the same carbon skeleton having amino and carboxyl groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton being acyclic and saturated the carbon skeleton being further substituted by oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C229/00—Compounds containing amino and carboxyl groups bound to the same carbon skeleton
- C07C229/02—Compounds containing amino and carboxyl groups bound to the same carbon skeleton having amino and carboxyl groups bound to acyclic carbon atoms of the same carbon skeleton
- C07C229/34—Compounds containing amino and carboxyl groups bound to the same carbon skeleton having amino and carboxyl groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton containing six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C235/00—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms
- C07C235/02—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to acyclic carbon atoms and singly-bound oxygen atoms bound to the same carbon skeleton
- C07C235/04—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to acyclic carbon atoms and singly-bound oxygen atoms bound to the same carbon skeleton the carbon skeleton being acyclic and saturated
- C07C235/18—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to acyclic carbon atoms and singly-bound oxygen atoms bound to the same carbon skeleton the carbon skeleton being acyclic and saturated having at least one of the singly-bound oxygen atoms further bound to a carbon atom of a six-membered aromatic ring, e.g. phenoxyacetamides
- C07C235/20—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to acyclic carbon atoms and singly-bound oxygen atoms bound to the same carbon skeleton the carbon skeleton being acyclic and saturated having at least one of the singly-bound oxygen atoms further bound to a carbon atom of a six-membered aromatic ring, e.g. phenoxyacetamides having the nitrogen atoms of the carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C235/00—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms
- C07C235/42—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings and singly-bound oxygen atoms bound to the same carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C255/00—Carboxylic acid nitriles
- C07C255/49—Carboxylic acid nitriles having cyano groups bound to carbon atoms of six-membered aromatic rings of a carbon skeleton
- C07C255/54—Carboxylic acid nitriles having cyano groups bound to carbon atoms of six-membered aromatic rings of a carbon skeleton containing cyano groups and etherified hydroxy groups bound to the carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C255/00—Carboxylic acid nitriles
- C07C255/49—Carboxylic acid nitriles having cyano groups bound to carbon atoms of six-membered aromatic rings of a carbon skeleton
- C07C255/58—Carboxylic acid nitriles having cyano groups bound to carbon atoms of six-membered aromatic rings of a carbon skeleton containing cyano groups and singly-bound nitrogen atoms, not being further bound to other hetero atoms, bound to the carbon skeleton
- C07C255/59—Carboxylic acid nitriles having cyano groups bound to carbon atoms of six-membered aromatic rings of a carbon skeleton containing cyano groups and singly-bound nitrogen atoms, not being further bound to other hetero atoms, bound to the carbon skeleton the carbon skeleton being further substituted by singly-bound oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C317/00—Sulfones; Sulfoxides
- C07C317/16—Sulfones; Sulfoxides having sulfone or sulfoxide groups and singly-bound oxygen atoms bound to the same carbon skeleton
- C07C317/22—Sulfones; Sulfoxides having sulfone or sulfoxide groups and singly-bound oxygen atoms bound to the same carbon skeleton with sulfone or sulfoxide groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C317/00—Sulfones; Sulfoxides
- C07C317/44—Sulfones; Sulfoxides having sulfone or sulfoxide groups and carboxyl groups bound to the same carbon skeleton
- C07C317/48—Sulfones; Sulfoxides having sulfone or sulfoxide groups and carboxyl groups bound to the same carbon skeleton the carbon skeleton being further substituted by singly-bound nitrogen atoms, not being part of nitro or nitroso groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C43/00—Ethers; Compounds having groups, groups or groups
- C07C43/02—Ethers
- C07C43/03—Ethers having all ether-oxygen atoms bound to acyclic carbon atoms
- C07C43/14—Unsaturated ethers
- C07C43/164—Unsaturated ethers containing six-membered aromatic rings
- C07C43/168—Unsaturated ethers containing six-membered aromatic rings containing six-membered aromatic rings and other rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D205/00—Heterocyclic compounds containing four-membered rings with one nitrogen atom as the only ring hetero atom
- C07D205/02—Heterocyclic compounds containing four-membered rings with one nitrogen atom as the only ring hetero atom not condensed with other rings
- C07D205/04—Heterocyclic compounds containing four-membered rings with one nitrogen atom as the only ring hetero atom not condensed with other rings having no double bonds between ring members or between ring members and non-ring members
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D211/00—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings
- C07D211/04—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D211/06—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D211/36—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D211/40—Oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/24—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D213/28—Radicals substituted by singly-bound oxygen or sulphur atoms
- C07D213/30—Oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/61—Halogen atoms or nitro radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/78—Carbon atoms having three bonds to hetero atoms, with at the most one bond to halogen, e.g. ester or nitrile radicals
- C07D213/79—Acids; Esters
- C07D213/80—Acids; Esters in position 3
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/78—Carbon atoms having three bonds to hetero atoms, with at the most one bond to halogen, e.g. ester or nitrile radicals
- C07D213/84—Nitriles
- C07D213/85—Nitriles in position 3
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D237/00—Heterocyclic compounds containing 1,2-diazine or hydrogenated 1,2-diazine rings
- C07D237/02—Heterocyclic compounds containing 1,2-diazine or hydrogenated 1,2-diazine rings not condensed with other rings
- C07D237/06—Heterocyclic compounds containing 1,2-diazine or hydrogenated 1,2-diazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members
- C07D237/08—Heterocyclic compounds containing 1,2-diazine or hydrogenated 1,2-diazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/28—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to ring carbon atoms
- C07D239/30—Halogen atoms or nitro radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/28—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to ring carbon atoms
- C07D239/32—One oxygen, sulfur or nitrogen atom
- C07D239/42—One nitrogen atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D319/00—Heterocyclic compounds containing six-membered rings having two oxygen atoms as the only ring hetero atoms
- C07D319/10—1,4-Dioxanes; Hydrogenated 1,4-dioxanes
- C07D319/14—1,4-Dioxanes; Hydrogenated 1,4-dioxanes condensed with carbocyclic rings or ring systems
- C07D319/16—1,4-Dioxanes; Hydrogenated 1,4-dioxanes condensed with carbocyclic rings or ring systems condensed with one six-membered ring
- C07D319/18—Ethylenedioxybenzenes, not substituted on the hetero ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F5/00—Compounds containing elements of Groups 3 or 13 of the Periodic Table
- C07F5/02—Boron compounds
- C07F5/025—Boronic and borinic acid compounds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F9/00—Compounds containing elements of Groups 5 or 15 of the Periodic Table
- C07F9/02—Phosphorus compounds
- C07F9/547—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom
- C07F9/553—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom having one nitrogen atom as the only ring hetero atom
- C07F9/576—Six-membered rings
- C07F9/58—Pyridine rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/357—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having two or more oxygen atoms in the same ring, e.g. crown ethers, guanadrel
- A61K31/36—Compounds containing methylenedioxyphenyl groups, e.g. sesamin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/02—Systems containing only non-condensed rings with a three-membered ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2602/00—Systems containing two condensed rings
- C07C2602/02—Systems containing two condensed rings the rings having only two atoms in common
- C07C2602/04—One of the condensed rings being a six-membered aromatic ring
- C07C2602/08—One of the condensed rings being a six-membered aromatic ring the other ring being five-membered, e.g. indane
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Definitions
- PD-1 Programmed cell death- 1
- CD28 CD28 superfamily that delivers negative signals upon interaction with its two ligands, PD-L1 or PD-L2.
- PD-1 and its ligands are broadly expressed and exert a wide range of immunoregulatory roles in T cells activation and tolerance.
- PD-1 and its ligands are involved in attenuating infectious immunity and tumor immunity, and facilitating chronic infection and tumor progression.
- the present disclosure further provides pharmaceutical compositions containing one or more of these compounds, as well as methods associated with preparation and use of such compounds.
- the compounds are used in therapeutic methods to treat diseases associated with the PD-1/PD-L1 pathway.
- the terms "about” and “approximately” shall generally mean an acceptable degree of error for the quantity measured given the nature or precision of the measurements. Typical, exemplary degrees of error are within 20 percent (%), preferably within 10%, and more preferably within 5% of a given value or range of values. Alternatively, and particularly in biological systems, the terms “about” and “approximately” may mean values that are within an order of magnitude, preferably within 5-fold and more preferably within 2-fold of a given value. Numerical quantities given herein are approximate unless stated otherwise, meaning that the term “about” or “approximately” can be inferred when not expressly stated.
- alkyl by itself or as part of another substituent, means, unless otherwise stated, a straight or branched chain hydrocarbon radical, having the number of carbon atoms designated (i.e. C s means one to eight carbons).
- alkyl groups include methyl, ethyl, n-propyl, isopropyl, n-butyl, t-butyl, isobutyl, sec-butyl, n-pentyl, n-hexyl, n-heptyl, n- octyl, and the like.
- alkyl in its broadest sense is also meant to include those unsaturated groups such as alkenyl and alkynyl groups.
- alkenyl refers to an unsaturated alkyl group having one or more double bonds.
- alkynyl refers to an unsaturated alkyl group having one or more triple bonds. Examples of such unsaturated alkyl groups include vinyl, 2-propenyl, crotyl, 2-isopentenyl, 2-(butadienyl), 2,4-pentadienyl, 3- (1,4-pentadienyl), ethynyl, 1- and 3-propynyl, 3-butynyl, and the higher homologs and isomers.
- cycloalkyl refers to hydrocarbon rings having the indicated number of ring atoms (e.g., C3.6cycloalkyl) and being fully saturated or having no more than one double bond between ring vertices.
- Cycloalkyl is also meant to refer to bicyclic and polycyclic hydrocarbon rings such as, for example, bicyclo[2.2.1]heptane, bicyclo[2.2.2]octane, etc.
- the bicyclic or polycyclic rings may be fused, bridged, spiro or a combination thereof.
- heterocycloalkyl refers to a cycloalkyl group that contain from one to five heteroatoms selected from N, O, and S, wherein the nitrogen and sulfur atoms are optionally oxidized, and the nitrogen atom(s) are optionally quaternized.
- the heterocycloalkyl may be a monocyclic, a bicyclic or a polycylic ring system.
- the bicyclic or polycyclic rings may be fused, bridged, spiro or a combination thereof.
- C 4-12 heterocyclyl refers to a heterocycloalkyl moiety having from 5 to 12 ring members where at least one of the ring members is a heteroatom.
- heterocycloalkyl groups include
- pyrrolidine imidazolidine, pyrazolidine, butyrolactam, valerolactam, imidazolidinone, hydantoin, dioxolane, phthalimide, piperidine, 1,4-dioxane, morpholine, thiomorpholine, thiomorpholine-S-oxide, tWomorpholine-S,S-oxide, piperazine, pyran, pyridone, 3-pyrroline, thiopyran, pyrone, tetrahydrofuran, tetrhydrothiophene, quinuclidine, and the like.
- heterocycloalkyl group can be attached to the remainder of the molecule through a ring carbon or a heteroatom.
- alkylene by itself or as part of another substituent means a divalent radical derived from an alkane, as exemplified by -CH 2 CH 2 CH 2 CH 2 -.
- an alkyl (or alkylene) group will have from 1 to 24 carbon atoms, with those groups having 10 or fewer carbon atoms being preferred in the present disclosure.
- a “lower alkyl” or “lower alkylene” is a shorter chain alkyl or alkylene group, generally having four or fewer carbon atoms.
- alkenylene and “alkynylene” refer to the unsaturated forms of "alkylene” having double or triple bonds, respectively.
- heteroalkyl by itself or in combination with another term, means, unless otherwise stated, a stable straight or branched chain, or cyclic hydrocarbon radical, or
- heteroatoms consisting of the stated number of carbon atoms and from one to three heteroatoms selected from the group consisting of O, N, Si and S, and wherein the nitrogen and sulfur atoms may optionally be oxidized and the nitrogen heteroatom may optionally be quaternized.
- the heteroatom(s) O, N and S may be placed at any interior position of the heteroalkyl group.
- the heteroatom Si may be placed at any position of the heteroalkyl group, including the position at which the alkyl group is attached to the remainder of the molecule.
- heteroalkenyl and “heteroalkynyl” by itself or in combination with another term, means, unless otherwise stated, an alkenyl group or alkynyl group, respectively, that contains the stated number of carbons and having from one to three heteroatoms selected from the group consisting of O, N, Si and S, and wherein the nitrogen and sulfur atoms may optionally be oxidized and the nitrogen heteroatom may optionally be quaternized.
- the heteroatom(s) O, N and S may be placed at any interior position of the heteroalkyl group.
- heteroalkylene by itself or as part of another substituent means a divalent radical, saturated or unsaturated or polyunsaturated, derived from heteroalkyl, as exemplified by -CH 2 -CH 2 -S-CH 2 CH 2 - and -CH 2 -S-CH 2 -CH 2 -NH-CH 2 -
- heteroatoms can also occupy either or both of the chain termini (e.g., alkyleneoxy,
- alkylenedioxy, alkyleneamino, alkylenediamino, and the like are used in their conventional sense, and refer to those alkyl groups attached to the remainder of the molecule via an oxygen atom, an amino group, or a sulfur atom, respectively. Additionally, for dialkylamino groups, the alkyl portions can be the same or different and can also be combined to form a 3-7 membered ring with the nitrogen atom to which each is attached. Accordingly, a group represented as -NR a R b is meant to include piperidinyl, pyrrolidinyl, morpholinyl, azetidinyl and the like.
- halo or halogen
- substituents mean, unless otherwise stated, a fluorine, chlorine, bromine, or iodine atom.
- terms such as “haloalkyl,” are meant to include monohaloalkyl and polyhaloalkyl.
- Ci- 4 haloalkyl is mean to include trifluoromethyl, 2,2,2-trifluoroethyl, 4-chlorobutyl, 3- bromopropyl, and the like.
- hydroxyalkyl or "alkyl-OH” refers to an alkyl group, as defined above, where at least one of the hydrogen atoms is replaced with a hydroxy group.
- alkyl group hydroxyalkyl groups can have any suitable number of carbon atoms, such as C 1-6 .
- alkylhydroxy groups include, but are not limited to, hydroxy-methyl, hydroxyethyl (where the hydroxy is in the 1- or 2-position), hydroxypropyl (where the hydroxy is in the 1-, 2- or 3-position), etc.
- C 1-3 alkyl-guanidinyl refers to a C 1-3 alkyl group, as defined above, where at least one of the hydrogen atoms is replaced with a guanidinyl group ( -NC(NH)NH 2 ).
- C 1-3 alkyl-guanidinyl refers a C 1-3 alkyl group where one of the hydrogen atoms is replaced with a guanidinyl.
- aryl means, unless otherwise stated, a polyunsaturated, typically aromatic, hydrocarbon group which can be a single ring or multiple rings (up to three rings) which are fused together or linked covalently.
- heteroaryl refers to aryl groups (or rings) that contain from one to five heteroatoms selected from N, O, and S, wherein the nitrogen and sulfur atoms are optionally oxidized, and the nitrogen atom(s) are optionally quaternized.
- a heteroaryl group can be attached to the remainder of the molecule through a heteroatom.
- Cs-io heteroaryl refers to a heteroaryl moiety having from 5 to 10 ring members where at least one of the ring members is a heteroatom.
- aryl groups include phenyl, naphthyl and biphenyl
- heteroaryl groups include pyridyl, pyridazinyl, pyrazinyl, pyrimindinyl, triazinyl, quinolinyl, quinoxalinyl, quinazolinyl, cinnolinyl, phthalaziniyl, benzotriazinyl, purinyl, benzimidazolyl, benzopyrazolyl, benzotriazolyl, benzisoxazolyl, isobenzofuryl, isoindolyl, indolizinyl, benzotriazinyl,
- Carbocyclic ring or “carbocyclyl” refers to cyclic moieties with only carbon atoms as ring vertices. Carbocyclic ring moieties are saturated or unsaturated and can be aromatic. Generally, carbocyclic moieties have from 3 to 10 ring members. Carbocylic moieties with multiple ring structure (e.g. bicyclic) can include a cycloalkyl ring fused to a aromatic ring (e.g. 1,2,3,4-tetrahydronaphthalene). Thus, carboclicic rings include cyclopentyl, cyclohexenyl, naphthyl, and 1,2,3,4-tetrahydronaphthyl. The term “heterocyclic ring” refers to both
- heterocycloalkyl and “heteroaryl” moieties.
- heterocyclic rings are saturated or unsaturated and can be aromatic.
- heterocyclic rings are 4 to 10 ring members and include piperidiyl, tetrazinyl, pyrazolo, and indolyl.
- aryl when used in combination with other terms ⁇ e.g., aryloxy, arylthioxy, arylalkyl) includes both aryl and heteroaryl rings as defined above.
- arylalkyl is meant to include those radicals in which an aryl group is attached to an alkyl group ⁇ e.g., benzyl, phenethyl, pyridylmethyl and the like).
- alkyl in some embodiments, will include both substituted and unsubstituted forms of the indicated radical. Preferred substituents for each type of radical are provided below.
- aryl and heteroaryl will refer to substituted or unsubstituted versions as provided below, while the term “alkyl” and related aliphatic radicals is meant to refer to unsubstituted version, unless indicated to be substituted.
- R', R" and R'" each independently refer to hydrogen, unsubstituted Q-s alkyl, unsubstituted heteroalkyl, unsubstituted aryl, aryl substituted with 1-3 halogens, unsubstituted Ci- 8 alkyl, Ci- 8 alkoxy or Ci- 8 thioalkoxy groups, or unsubstituted aryl-Ci- 4 alkyl groups.
- R' and R" are attached to the same nitrogen atom, they can be combined with the nitrogen atom to form a 3-, 4- , 5-, 6-, or 7-membered ring.
- -NR'R is meant to include 1-pyrrolidinyl and 4- morpholinyl.
- Two of the substituents on adjacent atoms of the aryl or heteroaryl ring may optionally be replaced with a substituent of the formula -T-C(0)-(CH 2 ) q -U-, wherein T and U are independently -NH-, -0-, -CH 2 - or a single bond, and q is an integer of from 0 to 2.
- two of the substituents on adjacent atoms of the aryl or heteroaryl ring may optionally be replaced with a substituent of the formula -A-(CH 2 ) r B-, wherein A and B are independently -CH 2 -, -0-, -NH-, -S-, -S(O)-, -S(0) 2 -, -S(0) 2 NR'- or a single bond, and r is an integer of from 1 to 3.
- One of the single bonds of the new ring so formed may optionally be replaced with a double bond.
- two of the substituents on adjacent atoms of the aryl or heteroaryl ring may optionally be replaced with a substituent of the formula -(CH 2 ) s -X-(CH 2 ) t - , where s and t are independently integers of from 0 to 3, and X is -0-, -NR'-, -S-, -S(O)-, -
- heteroatom is meant to include oxygen (O), nitrogen (N), sulfur (S) and silicon (Si).
- ionic liquid refers to any liquid that contains mostly ions.
- ionic liquid refers to the salts whose melting point is relatively low (e.g., below 250 °C).
- ionic liquids include but are not limited to l-butyl-3- methylimidazolium tetrafluoroborate, l-hexyl-3-methylimidazolium tetrafluoroborate, l-octyl-3- methylimidazolium tetrafluoroborate, l-nonyl-3-methylimidazolium tetrafluoroborate, 1-decyl- 3-methylimidazolium tetrafluoroborate, l-hexyl-3-methylimidazolium hexafluorophosphate and l-hexyl-3-methylimidazolium bromide, and the like.
- patient and “subject” include primates (especially humans), domesticated companion animals (such as dogs, cats, horses, and the like) and livestock (such as cattle, pigs, sheep, and the like).
- livestock such as cattle, pigs, sheep, and the like.
- treating encompasses both disease-modifying treatment and symptomatic treatment, either of which may be prophylactic (i.e., before the onset of symptoms, in order to prevent, delay or reduce the severity of symptoms) or therapeutic (i.e., after the onset of symptoms, in order to reduce the severity and/or duration of symptoms).
- salts are meant to include salts of the active compounds which are prepared with relatively nontoxic acids or bases, depending on the particular substituents found on the compounds described herein.
- base addition salts can be obtained by contacting the neutral form of such compounds with a sufficient amount of the desired base, either neat or in a suitable inert solvent.
- salts derived from pharmaceutically- acceptable inorganic bases include aluminum, ammonium, calcium, copper, ferric, ferrous, lithium, magnesium, manganic, manganous, potassium, sodium, zinc and the like.
- Salts derived from pharmaceutically-acceptable organic bases include salts of primary, secondary and tertiary amines, including substituted amines, cyclic amines, naturally-occuring amines and the like, such as arginine, betaine, caffeine, choline, ⁇ , ⁇ '-dibenzylethylenediamine, diethylamine, 2- diethylaminoethanol, 2-dimethylaminoethanol, ethanolamine, ethylenediamine, N- ethylmorpholine, N-ethylpiperidine, glucamine, glucosamine, histidine, hydrabamine, isopropylamine, lysine, methylglucamine, morpholine, piperazine, piperadine, polyamine resins, procaine, purines, theobromine, triethylamine, trimethylamine, tripropylamine, tromethamine and the like.
- acid addition salts can be obtained by contacting the neutral form of such compounds with a sufficient amount of the desired acid, either neat or in a suitable inert solvent.
- suitable inert solvent examples include those derived from inorganic acids like hydrochloric, hydrobromic, nitric, carbonic, monohydrogencarbonic, phosphoric,
- the neutral forms of the compounds may be regenerated by contacting the salt with a base or acid and isolating the parent compound in the conventional manner.
- the parent form of the compound differs from the various salt forms in certain physical properties, such as solubility in polar solvents, but otherwise the salts are equivalent to the parent form of the compound for the purposes of the present disclosure.
- Certain compounds of the present disclosure can exist in unsolvated forms as well as solvated forms, including hydrated forms. In general, the solvated forms are equivalent to unsolvated forms and are intended to be encompassed within the scope of the present disclosure. Certain compounds of the present disclosure may exist in multiple crystalline or amorphous forms. In general, all physical forms are equivalent for the uses contemplated by the present disclosure and are intended to be within the scope of the present disclosure.
- Certain compounds of the present disclosure possess asymmetric carbon atoms (optical centers) or double bonds; the racemates, diastereomers, geometric isomers, regioisomers and individual isomers (e.g., separate enantiomers) are all intended to be encompassed within the scope of the present disclosure.
- the compounds of the present disclosure may also contain unnatural proportions of atomic isotopes at one or more of the atoms that constitute such compounds.
- the compounds may be radiolabeled with radioactive isotopes, such as for example tritium ( 3 H), iodine-125 ( 125 I) or carbon-14 ( 14 C).
- the compounds of the present disclosure may be prepared such that any number of hydrogen atoms are replaced with a deuterium ( H) isotope.
- the compounds of the present disclosure may also contain unnatural proportions of atomic isotopes at one or more of the atoms that constitute such compounds. Unnatural proportions of an isotope may be defined as ranging from the amount found in nature to an amount consisting of 100% of the atom in question.
- the compounds may incorporate radioactive isotopes, such as for example tritium ( 3 H), iodine-125 ( 125 I) or carbon-14 ( 14 C), or non-radioactive isotopes, such as deuterium ( H) or carbon- 13 ( C).
- radioactive isotopes such as for example tritium ( 3 H), iodine-125 ( 125 I) or carbon-14 ( 14 C), or non-radioactive isotopes, such as deuterium ( H) or carbon- 13 ( C).
- isotopic variants of the compounds of the disclosure may find additional utility, including but not limited to, as diagnostic and/or imaging reagents, or as cytotoxic/radiotoxic therapeutic agents.
- isotopic variants of the compounds of the disclosure can have altered pharmacokinetic and pharmacodynamic characteristics which can contribute to enhanced safety, tolerability or efficacy during treatment. All isotopic variations of the compounds of the present disclosure, whether radioactive or not, are intended to be encompassed within the scope
- the present disclosure provides compounds having the formula ( ⁇ )
- R 1 is selected from the group consisting of halogen, C 5-8 cycloalkyl, C 6-1 o aryl and thienyl,
- each R x is independently selected from the group consisting of halogen, -CN, -R c , -C0 2 R a ,
- each X 1 is a C 1-4 alkylene
- each R a and R b is independently selected from hydrogen, C 1-8 alkyl, and C 1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O or S, wherein the five or six-membered ring is optionally substituted with oxo
- each R c is independently selected from the group consisting of C 1-8 alkyl, C 2-8 alkenyl, C 2-8 alkynyl and C 1-8 haloalkyl; and optionally when two R x substituents are on adjacent atoms, they are combined to form a fused five, six
- each R 2a , R 2b and R 2c is independently selected from the group consisting of H, halogen, -CN, -R d , -C0 2 R e , -CONR ⁇ , -C(0)R e , -OC(0)NR e R f , -NR f C(0)R e , -NR f C(0) 2 R d ,
- each X 2 is a C 1-4 alkylene; each R e and R f is independently selected from hydrogen, C 1-8 alkyl, and C 1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six- membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O and S, and optionally substituted with oxo; each R d is independently selected from the group consisting of C 1-8 alkyl, C 2-8 alkenyl, and C 1-8 haloalkyl;
- R 3 is selected from the group consisting of -NR g R h and C 4-12 heterocyclyl, wherein the C 4-12 heterocyclyl is optionally substituted with 1 to 6 R y ;
- each R y is independently selected from the group consisting of
- R j and R k is independently selected from hydrogen, C 1-8 alkyl optionally substituted with 1 to 2 substituents selected from OH, S0 2 NH 2 , CONH 2 , CONOH, P0 3 H 2 , COO-C 1-8 alkyl or C0 2 H, and C 1-8 haloalkyl optionally substituted with 1 to 2 substituents selected from OH, S0 2 NH 2 , CONH 2 , CONOH, P0 3 H 2 , COO-C 1-8 alkyl or C0 2 H, and C 1-8 haloalkyl optionally substituted with 1 to 2 substituents selected from OH, S0 2 NH 2 , CONH 2 , CONOH, P0 3 H 2 , COO-C 1-8 alkyl or C0 2 H, or when attached to the same nitrogen atom R* and R k can be combined with the nitrogen atom to form a five or six- membered ring having from 0 to 2 additional heteroatoms as ring members selected from N
- R h combined with the N to which it is attached is a mono-, di- or tri-peptide comprising 1-3 natural amino acids and 0-2 non-natural amino acids, wherein
- the non-natural aminoacids have an alpha carbon substituent selected from the group consisting of C 2-4 hydroxyalkyl, C 1-3 alkyl-guanidinyl, and C 1-4 alkyl-heteroaryl, the alpha carbon of each natural or non-natural amino acids are optionally further substituted with a methyl group, and
- the terminal moiety of the mono-, di-, or tri-peptide is selected from the group consisting of C(0)OH, C(0)0-C 1-6 alkyl, and P0 3 H 2 , wherein
- R ⁇ and R* 2 are each independently selected from the group consisting of H, C 1-6 alkyl, and C 1-4 hydroxyalkyl;
- the C 1-8 alkyl portions of R h are optionally further substituted with from 1 to 3 substituents independently selected from OH, COOH, S0 2 NH 2 , CONH 2 , CONOH, COO- C 1-8 alkyl, P0 3 H 2 and C 5- 6 heteroaryl optionally substituted with 1 to 2 C 1-3 alkyl substituents,
- the Cio carbocyclyl, Cs-io heteroaryl and the C 6-1 o aryl portions of R h are optionally substituted with 1 to 3 substituents independently selected from OH, B(OH) 2 , COOH, S0 2 NH 2 , CONH 2 , CONOH, P0 3 H 2 , COO-C 1-8 alkyl, C 1-4 alkyl, C 1-4 alkyl-OH, C 1-4 alkyl- S0 2 NH 2 , C 1-4 alkyl CONH 2 , C 1-4 alkyl-CONOH, C 1-4 alkyl- P0 3 H 2 , C ⁇ alkyl-COOH, and phenyl and the C 4-8 heterocyclyl and C 3-1 o cycloalkyl portions of R h are optionally substituted with 1 to 4 R w substituents;
- each R w substituent is independently selected from C 1-4 alkyl, C 1-4 alkyl-OH, C 1-4 alkyl-COOH, C 1-4 alkyl-S0 2 NH 2 , C 1-4 alkyl CONH 2 , C 1-4 alkyl- CONOH, C 1-4 alkyl-P0 3 H, OH, COO- C 1-8 alkyl, COOH, S0 2 NH 2 , CONH 2 , CONOH, P0 3 H 2 and oxo;
- R 4 is selected from the group consisting of 0-C 1-8 alkyl, 0-C 1-8 haloalkyl, 0-C 1-8 alkyl-R z , C 6-1 o aryl, Cs-io heteroaryl , -0-C 1-4 alkyl-Ce-ioaryl and -0-C 1-4 alkyl-Cs-io heteroaryl, wherein the C 6-1 o aryl and the Cs-io heteroaryl are optionally substituted with 1 to 5 R z ;
- each R z is independently selected from the group consisting of halogen, -CN, -R m , -C0 2 R n ,
- heterocyclic ring is optionally substituted with 1 to 5 R l , wherein each R l is independently selected from the group consisting of C 1-8 alkyl,
- each X 3 is a C 1-4 alkylene; each R n and R p is independently selected from hydrogen, C 1-8 alkyl, and C 1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O or S, and optionally substituted with oxo; each R m is independently selected from the group consisting of C 1-8 alkyl, C 2-8 alkenyl, and C 1-8 haloalkyl; and optionally when two R z substituents are on adjacent atoms, they are combined to form a fused five or six-membered carbocyclic or heterocyclic ring optionally substituted with oxo;
- n 0, 1, 2 or 3;
- each R 5 is independently selected from the group consisting of halogen, -CN, -R q , -C0 2 R r ,
- R 6a is selected from the group consisting of H, C 1-4 alkyl and C 1-4 haloalkyl; each R 6b is independently selected from the group consisting of F, C 1-4 alkyl, 0-R u , C 1-4
- haloalkyl NR U R V , wherein each R u and R v is independently selected from hydrogen, C 1-8 alkyl, and C 1-8 haloalkyl, or when attached to the same mtrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O or S, and optionally substituted with oxo; and m is 0, 1, 2, 3 or 4
- the present disclosure provides compounds having the formula (ID
- R 1 is selected from the group consisting of C 6-1 o aryl and thienyl, wherein the C 6 .
- thienyl are optionally substituted with 1 to 5 R x substituents
- x is independently selected from the group consisting of halogen, -CN, -R c , -C0 2 R a , -CONR a R b , -C(0)R a , -OC(0)NR a R b , -NR b C(0)R a , -NR b C(0) 2 R c , -NR a -C(0)NR a R b , -NR a R b , -OR a , -O-X ⁇ OR 3 , -O- X ⁇ COzR 3 , -O-X ⁇ CONR'R", -X ⁇ OR 3 , -X ⁇ NRH", - X ⁇ COzR 3 , -X ⁇ CONR ⁇ ", -SF 5 , -S(0) 2 NR a R b , wherein each X 1 is a C 1-4 alkylene; each R a and R b is independently selected from hydrogen, C 1-8 alkyl, and C
- each R 2a , R 2b and R 2c is independently selected from the group consisting of H, halogen, -CN, -R d , -C0 2 R e , -CONR ⁇ , -C(0)R e , -OC(0)NR e R f , -NR f C(0)R e , -NR f C(0) 2 R d ,
- each X 2 is a C 1-4 alkylene; each R e and R f is independently selected from hydrogen, C 1-8 alkyl, and C 1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O or S, and optionally substituted with oxo; each R d is independently selected from the group consisting of C 1-8 alkyl, C 2-8 alkenyl, and C 1-8 haloalkyl;
- R 3 is selected from the group consisting of NR g R h and C 4-12 heterocyclyl wherein the C 4-12
- heterocyclyl is optionally substituted with 1 to 6 R y ;
- each R y is independently selected from the group consisting of halogen, -CN, -R 1 , -C0 2 R J ,
- each R 1 is independently selected from the group consisting of C 1-8 alkyl, C 2-8 alkenyl, and C 1-8 haloalkyl each of which may be optionally substituted with OH, S0 2 NH 2 , CONH 2 , CONOH, P0 3 H 2 , COO-C 1-8 alkyl or C0 2 H;
- R g is selected from the group consisting of H, C 1-8 haloalkyl and C 1-8 alkyl;
- COO-C 1-8 alkyl C 1-4 alkyl, C 1-4 alkyl-OH, C 1-4 alkyl-S0 2 NH 2 , C 1-4 alkyl CONH 2 , C 1-4 alkyl- CONOH, C 1-4 alkyl- P0 3 H 2 , and C 1-4 alkyl-COOH, and wherein the C 4-8 heterocyclyl and C 3-1 o cycloalkyl are optionally substituted with 1 to 3 R w substituents;
- each R w substituent is independently selected from C 1-4 alkyl, C 1-4 alkyl-OH, C 1-4 alkyl-COOH, C 1-4 alkyl-S0 2 NH 2 , C 1-4 alkyl CONH 2 , C 1-4 alkyl- CONOH, C 1-4 alkyl-P0 3 H, OH, COO-
- R 4 is selected from the group consisting of 0-C 1-8 alkyl, 0-C 1-8 haloalkyl, 0-C 1-8 alkyl-R z , C 6-1 o aryl, Cs-io heteroaryl , -0-C 1-4 alkyl-Ce-ioaryl and -0-C 1-4 alkyl-Cs-io heteroaryl, wherein the C 6-1 o aryl and the C 5-1 o heteroaryl are optionally substituted with 1 to 5 R z ;
- each R z is independently selected from the group consisting of halogen, -CN, -R m , -C0 2 R n ,
- each X 3 is a C 1-4 alkylene; each R n and R p is independently selected from hydrogen, C 1-8 alkyl, and C 1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O or S, and optionally substituted with oxo; each R m is independently selected from the group consisting of C 1-8 alkyl, C 2-8 alkenyl, and C 1-8 haloalkyl; and optionally when two R z substituents are on adjacent atoms, they are combined to form a fused five or six-membered carbocyclic or heterocyclic ring optionally substituted with oxo;
- n 0, 1, 2 or 3;
- each R 5 is independently selected from the group consisting of halogen, -CN, -R q , -C0 2 R r ,
- each X 4 is a C 1-4 alkylene; each R r and R s is independently selected from hydrogen, C 1-8 alkyl, and C 1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O or S, and optionally substitute
- R 6a is selected from the group consisting of H, C 1-4 alkyl and C 1-4 haloalkyl;
- each R 6b is independently selected from the group consisting of F, C 1-4 alkyl, 0-R u , C 1-4
- haloalkyl NR U R V , wherein each R u and R v is independently selected from hydrogen, C 1-8 alkyl, and C 1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O or S, and optionally substituted with oxo; and
- the compound, or a pharmaceutically acceptable salt thereof has the formula (Ila)
- the compound, or a pharmaceutically acceptable salt thereof having the formula (lib) having the formula (lib)
- R 1 is selected from the group consisting of phenyl and thienyl, wherein the phenyl and thienyl are optionally substituted with 1 to 5 R x substituents. In some embodiments, R 1 is phenyl optionally substituted with 1 or 2 R x wherein each R x is
- R 1 is phenyl optionally substituted with F.
- R 1 is selected from the group consisting of:
- each R a , R and R c is independently selected from the group consisting of H, halogen, -CN, -R d , -NR3 ⁇ 4 f , -OR e , -X 2 -OR e , -X 2 -NR e R f , wherein X 2 is C 1-4 alkylene; each R e and R f is independently selected from hydrogen, C 1-8 alkyl, and C 1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O or S, and optionally substituted with oxo; each R d is independently selected from the group consisting of C 1-8 alkyl, C 2-8 alkenyl, and C 1-8 haloalkyl.
- R 2b and R 2c are both H and R 2a is selected from the group consisting of halogen, C 1-4 alkyl, C 2-4 alkenyl, C 1-3 haloalkyl, -CN, -OMe and OEt. In some embodiments, R 2b and R 2c are both H and R 2a is halogen. In some embodiments, R 2b and R 2c are both H and R 2a is Cl.
- n 0, 1 or 2 and each R 5 is independently selected from the group consisting of halogen, -CN, -R q , -NR r R s , and -OR r , wherein each R r and R s is
- R a is H.
- m is 0.
- R ⁇ is selected from the group consisting of F, C 1-4 alkyl, 0-R u , C 1-4 haloalkyl and NR U R V , wherein each R u and R v is independently selected from hydrogen, C 1-8 alkyl, and C 1-8 haloalkyl.
- m is 1 and R 6b is F.
- R 4 is selected from the group consisting of 0-C 1-4 alkyl, 0-C 1-6 alkyl-R z , C 6-1 o aryl, C 5-1 o heteroaryl , -0-C 1-4 alkyl-C 6-1 oaryl and -0-C 1-4 alkyl-Cs.io heteroaryl, wherein the C 6-1 o aryl and the C 5-1 o heteroaryl are optionally substituted with 1 to 2 R z , wherein each R z is independently selected from the group consisting of halogen, -CN, -R m , -CO ⁇ ",
- R 4 is selected from the group consisting of 0-C 1-4 alkyl, 0-C 1-6 alkyl-CN, phenyl, pyridinyl , -0-C 1-2 alkyl-pyridinyl, -0-C 1-2 alkyl-pyrimidinyl, -O- C 1-2 alkyl- pyridazinyl, and -0-C 1-2 alkyl-phenyl, wherein the pyridinyl, phenyl, pyrimidinyl and pyridazinyl is optionally substituted with 1 to 2 R z , wherein each R z is independently selected from the group consisting of halogen, -CN, -C0 2 R n , -NR n R p , -OR n , and piperidinyl optionally substituted with OH. [0046] In some embodiments, R is selected from the group consisting of halogen, -CN, -C0 2 R n ,
- R 3 is selected from the group consisting of NR g R h and C 4-6 heterocyclyl wherein the C 4-6 heterocyclyl is optionally substituted with 1 to 3 R y , wherein R g is selected from the group consisting of H, C 1-8 haloalkyl and C 1-8 alkyl, and wherein R h is -C 1-8 alkyl substituted with from 1 to 3 substituents independently selected from OH, COOH, S0 2 NH 2 , CONH 2 , CONOH, COO-C 1-8 alkyl, C 5-6 heteroaryl, C 5-6 heterocyclyl and P0 3 H 2 , wherein the C 5- 6 heteroaryl and the C 5- 6 heterocyclyl are optionally substituted with 1 to 3 substituents independently selected from OH, B(OH) 2 , COOH, S0 2 NH 2 , CONH 2 , CONOH,
- R is selected from the group consisting of azetidinyl, pyrrolidinyl and piperidinyl, wherein the azetidinyl, pyrrolidinyl or piperidinyl is linked through the nitrogen atom and wherein the azetidinyl, pyrrolidinyl or piperidinyl is optionally substituted with 1 to 3 R y , wherein each R y is independently selected from the group consisting of -C0 2 H, CONOH, P0 3 H 2 , OH, S0 2 NH 2 , CONH 2 , and COO-Ci. 8 alkyl.
- R 3 is NHR h , wherein R h is -C 1-8 alkyl substituted with from 1 to 2 substituents independently selected from OH, COOH, CONH 2 , P0 3 H 2 , tetrazolyl, tetrazolonyl, and pyrazolyl.
- R is selected from the group consisting of:
- R 3 is -NR g R h .
- R h combined with the N to which it is attached is a mono-, di- or tri-peptide comprising 1-3 natural amino acids and 0-2 non-natural amino acids, wherein
- the non-natural aminoacids have an alpha carbon substituent selected from the group consisting of C 2-4 hydroxyalkyl, C 1-3 alkyl-guanidinyl, and C 1-4 alkly-heteroaryl, the alpha carbon of each natural or non-natural amino acids are optionally further substituted with a methyl group, and
- each natural amino acid of R h is independently selected from the group consisting of serine, alanine, glycine, lysine, argining, threonine, phenylalanine, tyrosine, asparatate, asparagine, histidine, and leucine.
- R 1 is phenyl optionally substituted with 1 to 3 R x
- R 6a is H
- R 4 is selected from the group consisting of 0-C 1-4 alkyl, 0-C 1-6 alkyl-CN, phenyl, pyridinyl , -0-C 1-2 alkyl-pyridinyl, -0-C 1-2 alkyl-pyrimidinyl, -0-C 1-2 alkyl- pyridazinyl, and -0-C 1-2 alkyl-phenyl, wherein the pyridinyl, phenyl, pyrimidinyl and pyridazinyl is optionally substituted with 1 to 2 R z , wherein each R z is independently selected from the group consisting of halogen, -CN, -C0 2 R n , -NR n R p , -OR n , and piperidinyl optionally substituted with OH, and R 3
- C5 -6 heteroaryl and the C5 -6 heterocyclyl are optionally substituted with 1 to 3 substituents independently selected from OH, B(OH) 2 , COOH, S0 2 NH 2 , CONH 2 , CONOH, P0 3 H 2 , COO-C 1-8 alkyl, C 1-4 alkyl, C 1-4 alkyl-OH, C 1-4 alkyl-S0 2 NH 2 , C 1-4 alkyl CONH 2 , C 1-4 alkyl-CONOH, C 1-4 alkyl- P0 3 H 2 , and C 1-4 alkyl-COOH and wherein the C5-6 heterocyclyl is additionally optionally substituted with oxo.
- R 1 is phenyl optionally substituted with 1 or 2 R x wherein each R x is independently selected from halogen, C 1-8 alkyl, 0-C 1-8 alkyl, 0-C 1-8 haloalkyl, -NR a R b , and CN, wherein R 2b and R 2c are both H, R 2a is selected from the group consisting of halogen, C 1-4 0, n is 0, R 4 is
- R is selected from the group consisting of NHR h , azetidinyl, pyrrolidinyl and piperidinyl, wherein the azetidinyl, pyrrolidinyl or piperidinyl is linked through the nitrogen atom and wherein the azetidinyl, pyrrolidinyl or piperidinyl is optionally substituted with 1 to 3 R y , wherein each R y is independently selected from the group consisting of C0 2 H, CONOH, P0 3 H 2 , OH, S0 2 NH 2 , CONH 2 , and COO-Q.
- R h is C 1-8 alkyl substituted with from 1 to 2 substituents independently selected from OH, COOH, CONH 2 , P0 3 H 2 , tetrazolyl, tetrazolonyl, and pyrazolyl.
- R 2a is halogen.
- the compound, or a pharmaceutically acceptable salt thereof is selected from the compounds of Table 2 having an activity of ++ or +++.
- the compound, or a pharmaceutically acceptable salt thereof is selected from the compounds of Table 2 having an activity of +++.
- the compound, or a pharmaceutically acceptable salt thereof is selected from the compounds of Table 2 having an activity of ++.
- the compound, or a pharmaceutically acceptable salt thereof is selected from the compounds of Table 2 having an activity of +.
- the compound, or a pharmaceutically acceptable salt thereof is selected from the compounds of Table 3 having an activity of ++ or +++. In some embodiments, the compound, or a pharmaceutically acceptable salt thereof, is selected from the compounds of Table 3 having an activity of +++. In some embodiments, the compound, or a pharmaceutically acceptable salt thereof, is selected from the compounds of Table 3 having an activity of ++. In some embodiments, the compound, or a pharmaceutically acceptable salt thereof, is selected from the compounds of Table 3 having an activity of +.
- R 1 is selected from the group consisting of C 6-1 o aryl and thienyl, wherein the C 6-1 o aryl and
- thienyl are optionally substituted with 1 to 5 R x substituents
- each R x is independently selected from the group consisting of halogen, -CN, -R c ,
- each X 1 is a C 1-4 alkylene
- each R a and R b is independently selected from hydrogen, C 1-8 alkyl, and C 1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O or S, and optionally substituted with oxo
- each R c is independently selected from the group consisting of C 1-8 alkyl, C 2-8 alkenyl, and C 1-8 haloalkyl; and optionally when two R x substituents are on adjacent atoms, they are combined to form a fused five or six- membered carbocyclic or heterocyclic ring optionally substituted with oxo;
- each R 2a , R 2b , and R 2c is independently selected from the group consisting of H, halogen, -CN, -R d , -C0 2 R e , -CONR ⁇ , -C(0)R e , -OC(0)NR e R f , -NR f C(0)R e , -NR f C(0) 2 R d ,
- each X 2 is a C 1-4 alkylene; each R e and R f is independently selected from hydrogen, C 1-8 alkyl, and C 1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O or S, and optionally substituted with oxo; each R d is independently selected from the group consisting of C 1-8 alkyl, C 2-8 alkenyl, and C 1-8 haloalkyl; R 3 is selected from the group consisting of -NR g R h and C 4-8 heterocyclyl wherein the C 4-8 heterocyclyl is optionally substituted with 1 to 6 R y ;
- R g is selected from H or C 1-8 alkyl
- R h is selected from C 1-8 alkyl, C 1-8 haloalkyl, -C 1-8 alkyl-C 4-8 heterocyclyl, -Ci.salkyl-C5.i0
- Ci. 8 alkyl-OH, and Ci. 8 alkyl-COOH wherein the Ci. 8 alkyl is optionally substituted with OH or COOH, wherein the Cs.io heteroaryl is optionally substituted with 1 to 3 substituents independently selected from OH, COOH, Ci. 4 alkyl, C 1-4 alkyl-OH, and C 1-4 alkyl-COOH, and wherein the C 4-8 heterocyclyl is optionally substituted with 1 to 3 R w substituents;
- each R w substituent is independently selected from C 1-4 alkyl, C 1-4 alkyl-OH, C 1-4 alkyl-COOH and oxo;
- each R y is independently selected from the group consisting of halogen, -CN, -R 1 , -C0 2 R J ,
- each R* and R k is independently selected from hydrogen, C 1-8 alkyl optionally substituted with OH or C0 2 H, and C 1-8 haloalkyl optionally substituted with OH or C0 2 H, or when attached to the same nitrogen atom R* and R k can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O or S, and optionally substituted with oxo; each R 1 is independently selected from the group consisting of C 1-8 alkyl, C 2-8 alkenyl, and C 1-8 haloalkyl which may be
- R 4a is selected from wherein the C 6-1 oaryl and the C5. 10 heteroaryl are optionally substituted with 1 to 5 R z ;
- each R z is independently selected from the group consisting of halogen, -CN, -R m , -C0 2 R n , -CONR n R p , -C(0)R n , -OC(0)NR n R p , -NR n C(0)R p , -NR n C(0) 2 R m ,
- each X 3 is a C1-4 alkylene
- each R n and R p is independently selected from hydrogen, C 1-8 alkyl, and C 1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O or S, and optionally substituted with oxo
- each R m is independently selected from the group consisting of C 1-8 alkyl, C 2-8 alkenyl, and C 1-8 haloalkyl; and optionally when two R z substituents are on adjacent atoms, they are combined to form a fused five or six- membered carbocyclic or heterocyclic ring optionally substituted with oxo;
- n 0, 1, 2 or 3;
- each R 5 is independently selected from the group consisting of halogen, -CN, -R q ,
- each X 4 is a C 1-4 alkylene; each R r and R s is independently selected from hydrogen, C 1-8 alkyl, and C 1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O or S, and optionally substituted with oxo; each R q is independently selected from the group consisting of C 1-8 alkyl, and C 1-8 haloalkyl.
- R 1 is selected from the group consisting of C 6-1 o aryl and thienyl, wherein the C 6-1 o aryl and thienyl are optionally substituted with 1 to 5 R x substituents.
- R 1 is selected from the group consisting of phenyl and thienyl, wherein the phenyl and thienyl are optionally substituted with 1 to 5 R x substituents.
- R 1 is phenyl substituted with 1 to 5 R x substituents.
- R 1 is unsubstituted phenyl.
- each R 2a , R 2b , and R 2c is independently selected from the group consisting of H, halogen, -CN, -R d , -NR3 ⁇ 4 f , -OR e , -X 2 -OR e , -X 2 -NR3 ⁇ 4 f , wherein X 2 is C 1-4 alkylene; each R e and R f is independently selected from hydrogen, C 1-8 alkyl, and C 1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O or S, and optionally substituted with oxo; each R d is independently selected from the group consisting of C 1-8 alkyl, C 2-8 alkenyl, and C 1-8 haloalkyl.
- each R 2a , R 2b and R 2c is independently selected from the group consisting of halogen, C 1-4 alkyl, C 2-4 alkenyl, C 1-3 haloalkyl, -CN, -OMe and OEt.
- R 2b and R 2c are both H and R 2a is selected from the group consisting of halogen, C 1-4 alkyl, C 2-4 alkenyl, C 1-3 haloalkyl, -CN, -OMe and OEt. [0063] In some embodiments, R 2b and R 2c are both H and R 2a is halogen.
- R 2b and R 2c are both H and R 2a is CI.
- n is 0, 1 or 2. In some embodiments, n is 0 or 1. In some embodiments, n is 0. [0066] In some embodiments, R 3 is selected from the group consisting of NR g R h and C 4-8 heterocyclyl wherein R g is H and wherein the C 4-8 heterocyclyl is linked through a N and is optionally substituted with 1 to 6 R y .
- R 3 is selected from the group consisting of NR g R h and C 4-8 heterocyclyl wherein R g is H, R h is selected from -C 1-8 alkyl-tetrazole, -C 1-8 alkyl-pyrazole, -Q.
- R 3 is selected from the group consisting of NR g R h and C 4-8 heterocyclyl wherein R g is H, R h is selected from -C 1-4 alkyl-tetrazole, -C 1-4 alkyl-pyrazole, -Q.
- R is selected from the group consisting of:
- R is selected from the group consisting of:
- R 4a is selected from -C 1-2 alkyl-C6 -1 oaryl and -C 1-2 alkyl-C5. 10 heteroaryl, wherein the Ce-ioaryl and the Cs-io heteroaryl are optionally substituted with 1 to 5 R z .
- R 4a is -C 1-2 alkyl-C5.6 heteroaryl, wherein the C5 -6 heteroaryl is optionally substituted with 1 to 3 R z .
- R 4a is -CH 2 -C 5-1 o heteroaryl optionally substituted with 1 to 3 R z .
- R 4a is -CH 2 -C5 -6 heteroaryl optionally substituted with 1 to 3 R z . In some embodiments, R 4a is -CH 2 -pyridinyl optionally substituted with 1 to 2 R z .
- R 4a is ° r
- the pharmaceutically acceptable salts are selected from ammonium, calcium, magnesium, potassium, sodium, zinc, arginine, betaine, caffeine, choline, ⁇ , ⁇ '-dibenzylethylenediamine, diethylamine, 2-diethylaminoethanol, 2- dimethylaminoethanol, ethanolamine, ethylenediamine, N-ethylmorpholine, N-ethylpiperidine, glucamine, glucosamine, histidine, hydrabamine, isopropylamine, lysine, methylglucamine, morpholine, piperazine, piperadine, procaine, purines, theobromine, triethylamine,
- the pharmaceutically acceptable salts are selected from ammonium, calcium, magnesium, potassium, sodium, hydrochloric, carbonic, monohydrogencarbonic, phosphoric, monohydrogenphosphoric, dihydrogenphosphoric, acetic, propionic, isobutyric, malonic, benzoic, succinic, suberic, fumaric, mandelic, phthalic, benzenesulfomc, p-tolylsulfonic, citric, tartaric, methanesulfonic, arginate, glucuronic acid and galactunoric acids.
- the pharmaceutically acceptable salts are selected from ammonium, calcium, magnesium, potassium, sodium, hydrochloric, carbonic, monohydrogencarbonic, phosphoric, monohydrogenphosphoric, dihydrogenphosphoric, acetic, propionic, isobutyric, malonic, benzoic, succinic, suberic, fumaric, mandelic, phthalic, benzenesulfomc, p-
- the pharmaceutically acceptable salts are sodium or hydrochloric.
- the present disclosure provides compounds which are in a prodrug form.
- Prodrugs of the compounds described herein are those compounds that readily undergo chemical changes under physiological conditions to provide the compounds of the present disclosure.
- prodrugs can be converted to the compounds of the present disclosure by chemical or biochemical methods in an ex vivo environment. For example, prodrugs can be slowly converted to the compounds of the present disclosure when placed in a transdermal patch reservoir with a suitable enzyme or chemical reagent.
- An ester may be used as a prodrug for the corresponding carboxylic acid.
- a C ⁇ . ⁇ o alkyl ester or a C 1-10 haloalkyl ester may be used as a prodrug for the corresponding carboxylic acid.
- the following esters may be used: ter-butyl ester, methyl ester, ethyl ester, isopropyl ester.
- ester prodrugs may be used as R groups such as threonine or serine prodrug esters which are linked to the rest of the molecule through their nitrogen. More specifically, the following prodrugs may be used for R :
- compositions of those compounds will typically contain a pharmaceutical carrier or diluent.
- composition as used herein is intended to encompass a product comprising the specified ingredients in the specified amounts, as well as any product which results, directly or indirectly, from combination of the specified ingredients in the specified amounts.
- pharmaceutically acceptable it is meant the carrier, diluent or excipient must be compatible with the other ingredients of the formulation and not deleterious to the recipient thereof.
- a pharmaceutical composition comprising a compound of the present disclosure including a compound of Formula (II), (Ha), (Hb), (I), (la), or (lb) or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable excipient, is provided.
- the pharmaceutical composition further comprises one or more additional therapeutic agents.
- the one or more additional therapeutic agent is selected from the group consisting of an antimicrobial agent, an antiviral agent, a cytotoxic agent, a gene expression modulatory agent, a chemotherapeutic agent, an anti-cancer agent, an anti-angiogenic agent, an immunotherapeutic agent, an anti-hormonal agent, an anti-fibrotic agent, radiotherapy, a radiotherapeutic agent, an anti-neoplastic agent, and an anti-proliferation agent.
- the one or more additional therapeutic agent is selected from the group consisting of one or more of CCX354, CCX9588, CCX140, CCX872, CCX598, CCX6239, CCX9664, CCX2553, CCX 2991, CCX282, CCX025, CCX507, CCX430, CCX765, CCX224, CCX662, CCX650, CCX832, CCX168, and CCX168-M1.
- compositions for the administration of the compounds of this disclosure may conveniently be presented in unit dosage form and may be prepared by any of the methods well known in the art of pharmacy and drug delivery. All methods include the step of bringing the active ingredient into association with the carrier which constitutes one or more accessory ingredients.
- the pharmaceutical compositions are prepared by uniformly and intimately bringing the active ingredient into association with a liquid carrier or a finely divided solid carrier or both, and then, if necessary, shaping the product into the desired formulation.
- the active object compound is included in an amount sufficient to produce the desired effect upon the process or condition of diseases.
- compositions containing the active ingredient may be in a form suitable for oral use, for example, as tablets, troches, lozenges, aqueous or oily suspensions, dispersible powders or granules, emulsions and self-emulsifications as described in U.S. Patent Application 2002-0012680, hard or soft capsules, syrups, elixirs, solutions, buccal patch, oral gel, chewing gum, chewable tablets, effervescent powder and effervescent tablets.
- compositions intended for oral use may be prepared according to any method known to the art for the manufacture of pharmaceutical compositions and such compositions may contain one or more agents selected from the group consisting of sweetening agents, flavoring agents, coloring agents, antioxidants and preserving agents in order to provide pharmaceutically elegant and palatable preparations.
- Tablets contain the active ingredient in admixture with non-toxic pharmaceutically acceptable excipients which are suitable for the manufacture of tablets.
- excipients may be for example, inert diluents, such as cellulose, silicon dioxide, aluminum oxide, calcium carbonate, sodium carbonate, glucose, mannitol, sorbitol, lactose, calcium phosphate or sodium phosphate; granulating and disintegrating agents, for example, corn starch, or alginic acid; binding agents, for example PVP, cellulose, PEG, starch, gelatin or acacia, and lubricating agents, for example magnesium stearate, stearic acid or talc.
- the tablets may be uncoated or they may be coated, enterically or otherwise, by known techniques to delay disintegration and absorption in the gastrointestinal tract and thereby provide a sustained action over a longer period.
- a time delay material such as glyceryl monostearate or glyceryl distearate may be employed. They may also be coated by the techniques described in the U.S. Pat. Nos. 4,256,108; 4,166,452; and 4,265,874 to form osmotic therapeutic tablets for control release.
- Formulations for oral use may also be presented as hard gelatin capsules wherein the active ingredient is mixed with an inert solid diluent, for example, calcium carbonate, calcium phosphate or kaolin, polyethylene glycol (PEG) of various average sizes (e.g., PEG400, PEG4000) and certain surfactants such as cremophor or solutol, or as soft gelatin capsules wherein the active ingredient is mixed with water or an oil medium, for example peanut oil, liquid paraffin, or olive oil.
- emulsions can be prepared with a non-water miscible ingredient such as oils and stabilized with surfactants such as mono- or di-glycerides, PEG esters and the like.
- Aqueous suspensions contain the active materials in admixture with excipients suitable for the manufacture of aqueous suspensions.
- excipients are suspending agents, for example sodium carboxymethylcellulose, methylcellulose, hydroxy-propylmethylcellulose, sodium alginate, polyvinyl-pyrrolidone, gum tragacanth and gum acacia; dispersing or wetting agents may be a naturally-occurring phosphatide, for example lecithin, or condensation products of an alkylene oxide with fatty acids, for example polyoxy-ethylene stearate, or condensation products of ethylene oxide with long chain aliphatic alcohols, for example heptadecaethyleneoxycetanol, or condensation products of ethylene oxide with partial esters derived from fatty acids and a hexitol such as polyoxyethylene sorbitol monooleate, or condensation products of ethylene oxide with partial esters derived from fatty acids and hexitol anhydrides, for example polyethylene sorbit
- the aqueous suspensions may also contain one or more preservatives, for example ethyl, or n-propyl, p-hydroxybenzoate, one or more coloring agents, one or more flavoring agents, and one or more sweetening agents, such as sucrose or saccharin.
- preservatives for example ethyl, or n-propyl, p-hydroxybenzoate
- coloring agents for example ethyl, or n-propyl, p-hydroxybenzoate
- coloring agents for example ethyl, or n-propyl, p-hydroxybenzoate
- flavoring agents for example ethyl, or n-propyl, p-hydroxybenzoate
- sweetening agents such as sucrose or saccharin.
- Oily suspensions may be formulated by suspending the active ingredient in a vegetable oil, for example arachis oil, olive oil, sesame oil or coconut oil, or in a mineral oil such as liquid paraffin.
- the oily suspensions may contain a thickening agent, for example beeswax, hard paraffin or cetyl alcohol. Sweetening agents such as those set forth above, and flavoring agents may be added to provide a palatable oral preparation. These compositions may be preserved by the addition of an anti-oxidant such as ascorbic acid.
- Dispersible powders and granules suitable for preparation of an aqueous suspension by the addition of water provide the active ingredient in admixture with a dispersing or wetting agent, suspending agent and one or more preservatives.
- a dispersing or wetting agent exemplified by those already mentioned above.
- Additional excipients for example sweetening, flavoring and coloring agents, may also be present.
- the pharmaceutical compositions of the disclosure may also be in the form of oil-in- water emulsions.
- the oily phase may be a vegetable oil, for example olive oil or arachis oil, or a mineral oil, for example liquid paraffin or mixtures of these.
- Suitable emulsifying agents may be naturally-occurring gums, for example gum acacia or gum tragacanth, naturally-occurring phosphatides, for example soy bean, lecithin, and esters or partial esters derived from fatty acids and hexitol anhydrides, for example sorbitan monooleate, and condensation products of the said partial esters with ethylene oxide, for example polyoxyethylene sorbitan monooleate.
- the emulsions may also contain sweetening and flavoring agents.
- Syrups and elixirs may be formulated with sweetening agents, for example glycerol, propylene glycol, sorbitol or sucrose. Such formulations may also contain a demulcent, a preservative and flavoring and coloring agents. Oral solutions can be prepared in combination with, for example, cyclodextrin, PEG and surfactants.
- sweetening agents for example glycerol, propylene glycol, sorbitol or sucrose.
- Such formulations may also contain a demulcent, a preservative and flavoring and coloring agents.
- Oral solutions can be prepared in combination with, for example, cyclodextrin, PEG and surfactants.
- the pharmaceutical compositions may be in the form of a sterile injectable aqueous or oleagenous suspension.
- This suspension may be formulated according to the known art using those suitable dispersing or wetting agents and suspending agents which have been mentioned above.
- the sterile injectable preparation may also be a sterile injectable solution or suspension in a non-toxic parenterally-acceptable diluent or solvent, for example as a solution in 1,3-butane diol.
- the acceptable vehicles and solvents that may be employed are water, Ringer's solution and isotonic sodium chloride solution.
- sterile, fixed oils are conventionally employed as a solvent or suspending medium.
- any bland fixed oil may be employed including synthetic mono- or diglycerides.
- fatty acids such as oleic acid find use in the preparation of injectables.
- the compounds of the present disclosure may also be administered in the form of suppositories for rectal administration of the drug.
- These compositions can be prepared by mixing the drug with a suitable non-irritating excipient which is solid at ordinary temperatures but liquid at the rectal temperature and will therefore melt in the rectum to release the drug.
- a suitable non-irritating excipient which is solid at ordinary temperatures but liquid at the rectal temperature and will therefore melt in the rectum to release the drug.
- Such materials include cocoa butter and polyethylene glycols.
- the compounds can be administered via ocular delivery by means of solutions or ointments.
- transdermal delivery of the subject compounds can be accomplished by means of iontophoretic patches and the like.
- creams, ointments, jellies, solutions or suspensions, etc., containing the compounds of the present disclosure are employed.
- topical application is also meant to include the use of mouth washes and gargles.
- the compounds of this disclosure may also be coupled a carrier that is a suitable polymers as targetable drug carriers.
- suitable polymers can include polyvinylpyrrolidone, pyran copolymer, polyhydroxy-propyl-methacrylamide-phenol, polyhydroxyethyl-aspartamide-phenol, or polyethyleneoxide-polylysine substituted with palmitoyl residues.
- the compounds of the disclosure may be coupled to a carrier that is a class of biodegradable polymers useful in achieving controlled release of a drug, for example polylactic acid, polyglycolic acid, copolymers of polylactic and polyglycolic acid, polyepsilon caprolactone, polyhydroxy butyric acid, polyorthoesters, polyacetals, polydihydropyrans, polycyanoacrylates and cross linked or amphipathic block copolymers of hydrogels.
- Polymers and semipermeable polymer matrices may be formed into shaped articles, such as valves, stents, tubing, prostheses and the like.
- the compound of the disclosure is coupled to a polymer or semipermeable polymer matrix that is formed as a stent or stent-graft device.
- the compounds of the disclosure may be used as immunomodulators.
- the compounds of the disclosure may be used as agonists, antagonists, partial agonists, inverse agonists, inhibitors of PD-1 and/or PD-L1 in a variety of contexts, both in vitro and in vivo.
- the compounds of the disclosure may be used as inhibitors of the PD-1/PD-L1 protein protein interaction.
- the compounds of the disclosure may be used as inhibitors of PD-L1.
- the compounds of the disclosure may be used as inhibitors of the CD80/PD-L1 protein protein interaction.
- the compounds of the disclosure may be used to inhibit the interaction between PD-1 and PD-L1 and/or PD-1 and CD80 and/or PD-1 and PD-L2 in vitro or in vivo. In some embodiments, the compounds of the disclosure may be used to inhibit VISTA and/or TIM-3. In some embodiments, the compounds of the disclosure may be inhibitors of the PD-l/PD-Ll protein protein interaction and inhibitors of VISTA and/or TIM-3.
- the compounds of the disclosure may be inhibitors of CTLA-4 and/or BTLA and/or LAG-3 and/or KLRG-1 and/or 2B4 and/or CD 160 and/or HVEM and/or CD48 and/or E-cadherin and/or MHC-II and/or galectin-9 and/or CD86 and/or PD-L2 and/or VISTA and/or TIM-3 and/or CD80.
- the compounds of the disclosure may be contacted with the receptor they interact with, in aqueous solution and under conditions otherwise suitable for binding of the ligand to the receptor.
- the receptor may be present in suspension (e.g., in an isolated membrane or cell preparation), in a cultured or isolated cell, or in a tissue or organ.
- the amount of the compounds of the disclosure contacted with the receptor should be sufficient to inhibit the PD-l/PD-Ll binding in vitro as measured, for example, using an ELISA.
- the receptor may be present in solution or suspension, in a cultured or isolated cell preparation or within a patient.
- the compounds of the present disclosure are useful for restoring and augmenting T cell activation. In some embodiments, the compounds of the present disclosure are useful for enhancing an immune response in a patient. In some embodiments, the compounds of the present disclosure are useful for treating, preventing, or slowing the progression of diseases or disorders in a variety of therapeutic areas, such as cancer and infectious diseases.
- the compounds of the present disclosure can be used for treating patients suffering from conditions that are responsive to PD-l/PD-Ll protein protein interaction modulation.
- a method of modulating an immune response mediated by the PD-1 signaling pathway in a subject comprising administering to the subject a therapeutically effective amount of a compound of the present disclosure including a compound of Formula Formula (II), (Ha), (lib), (I), (la), or (lb), or a pharmaceutically acceptable salt thereof or a composition comprising a compound of the present disclosure including a compound of Formula (II), (Ha), (lib), (I), (la), or (lb), or a pharmaceutically acceptable salt thereof, is provided.
- a method of enhancing, stimulating, modulating and/or increasing the immune response in a subject in need thereof comprising administering to the subject a therapeutically effective amount of a compound of the present disclosure including a compound of Formula ( ⁇ ), (Ila), (lib), (I), (la), or (lb), or a pharmaceutically acceptable salt thereof or a composition of a compound of the present disclosure including a compound of Formula (II), (Ha), (lib), (I), (la), or (lb), or a pharmaceutically acceptable salt thereof, is provided.
- a method of inhibiting growth, proliferation, or metastasis of cancer cells in a subject in need thereof comprising administering to the subject a therapeutically effective amount of a compound of the present disclosure including a compound of Formula (II), (Ila), (Hb), (I), (la), or (lb), or a pharmaceutically acceptable salt thereof or a composition of a compound of the present disclosure including a compound of Formula (II), (Ha), (lib), (I), (la), or (lb), or a pharmaceutically acceptable salt thereof, is provided.
- a method of treating a subject in need thereof comprising administering to the subject a therapeutically effective amount of a compound of the present disclosure including a compound of Formula (II), (Ha), (lib), (I), (la), or (lb), or a
- the subject suffers from a disease or disorder selected from the group consisting of an infectious disease, a bacterial infectious disease, a viral infectious disease a fungal infectious disease, a solid tumor, a hematological malignancy, an immune disorder, an inflammatory disease, and cancer.
- a disease or disorder selected from the group consisting of an infectious disease, a bacterial infectious disease, a viral infectious disease a fungal infectious disease, a solid tumor, a hematological malignancy, an immune disorder, an inflammatory disease, and cancer.
- the disease or disorder is selected from the group consisting of melanoma, glioblastoma, esophagus tumor, nasopharyngeal carcinoma, uveal melanoma, lymphoma, lymphocytic lymphoma, primary CNS lymphoma, T-cell lymphoma, diffuse large B-cell lymphoma, primary mediastinal large B-cell lymphoma, prostate cancer, castration-resistant prostate cancer, chronic myelocytic leukemia, Kaposi's sarcoma fibrosarcoma, liposarcoma, chondrosarcoma, osteogenic sarcoma, angiosarcoma, lymphangiosarcoma, synovioma, , meningioma, leiomyosarcoma, rhabdomyosarcoma, sarcoma of soft tissue, sarcoma, sepsis, biliary tumor, basal cell carcinoma,
- NSCLC colorectal cancer, ovarian cancer, gastric cancer, hepatocellular carcinoma, pancreatic carcinoma, pancreatic cancer, Pancreatic ductal adenocarcinoma, squamous cell carcinoma of the head and neck, cancer of the head or neck, gastrointestinal tract, stomach cancer, HTV, Hepatitis A, Hepatitis B, Hepatitis C, hepatitis D, herpes viruses, papillomaviruses, influenza, bone cancer, skin cancer, rectal cancer, cancer of the anal region, testicular cancer, carcinoma of the fallopian tubes, carcinoma of the endometrium, carcinoma of the cervix, carcinoma of the vagina, carcinoma of the vulva, cancer of the esophagus, cancer of the small intestine, cancer of the endocrine system, cancer of the urethra, cancer of the penis, cancer of the bladder, cancer of the kidney, cancer of the ureter, carcinoma of the renal pelvis, neoplasm of the
- cystadenocarcinoma bronchogenic carcinoma, renal cell carcinoma, transitional cell carcinoma, choriocarcinoma, seminoma, embryonal carcinoma, wilm's tumor, pleomorphic adenoma, liver cell papilloma, renal tubular adenoma, cystadenoma, papilloma, adenoma, leiomyoma, rhabdomyoma, hemangioma, lymphangioma, osteoma, chondroma, lipoma and fibroma.
- a therapeutically effective amount of one or more additional therapeutic agents is further administered to the subject.
- the one or more additional therapeutic agents is selected from the group consisting of an antimicrobial agent, an antiviral agent, a cytotoxic agent, a gene expression modulatory agent, a chemotherapeutic agent, an anti-cancer agent, an anti-angiogenic agent, an immunotherapeutic agent, an anti-hormonal agent, an anti-fibrotic agent, radiotherapy, a radiotherapeutic agent, an anti-neoplastic agent, and an anti-proliferation agent.
- the one or more additional therapeutic agent is selected from the group consisting of one or more of CCX354, CCX9588, CCX140, CCX872, CCX598, CCX6239, CCX9664, CCX2553, CCX 2991, CCX282, CCX025, CCX507, CCX430, CCX765, CCX224, CCX662, CCX650, CCX832, CCX168, and CCX168-M1.
- the compounds of the present disclosure may be used to inhibit an infectious disease.
- the infectious disease includes but is not limited to HTV, Influenza, Herpes, Giardia, Malaria, Leishmania, the pathogenic infection by the virus Hepatitis (A, B, and C), herpes virus (e.g., VZV, HSV-I, HAV-6, HSV-II, and CMV, Epstein Barr virus), adenovirus, influenza virus, flaviviruses, echovirus, rhinovirus, coxsackie virus, cornovirus, respiratory syncytial virus, mumps virus, rotavirus, measles virus, rubella virus, parvovirus, vaccinia virus, HTLV virus, dengue virus, papillomavirus, molluscum virus, poliovirus, rabies virus, JC virus and arboviral encephalitis virus, pathogenic infection by the bacteria chlamydia, rickettsial bacteria, mycobacteri
- herpes virus
- coli legionella, diphtheria, salmonella, bacilli, cholera, tetanus, botulism, anthrax, plague, leptospirosis, and Lyme's disease bacteria, pathogenic infection by the fungi Candida (albicans, krusei, glabrata, tropicalis, etc.),
- Cryptococcus neoformans Aspergillus (fumigatus, niger, etc.), Genus Mucorales (mucor, absidia, rhizophus), Sporothrix schenkii, Blastomyces dermatitidis, Paracoccidioides brasiliensis, Coccidioides immitis and Histoplasma capsulatum, and pathogenic infection by the parasites Entamoeba histolytica, Balantidium coli, Naegleriafowleri, Acanthamoeba sp., Giardia lambia, Cryptosporidium sp., Pneumocystis carinii, Plasmodium vivax, Babesia microti, Trypanosoma brucei, Trypanosoma cruzi, Leishmania donovani, Toxoplasma gondi, Nippostrongylus brasiliensis. [0104] In some embodiments, the compounds of the present
- Treatment methods provided herein include, in general, administration to a patient an effective amount of one or more compounds provided herein.
- Suitable patients include those patients suffering from or susceptible to (i.e., prophylactic treatment) a disorder or disease identified herein.
- Typical patients for treatment as described herein include mammals, particularly primates, especially humans.
- Other suitable patients include domesticated companion animals such as a dog, cat, horse, and the like, or a livestock animal such as cattle, pig, sheep and the like.
- treatment methods provided herein comprise administering to a patient an effective amount of a compound one or more compounds provided herein.
- the compound(s) of the disclosure are preferably administered to a patient (e.g., a human) intravenously, orally or topically.
- the effective amount may be an amount sufficient to modulate the PD-1/PD-L1 interaction and/or an amount sufficient to reduce or alleviate the symptoms presented by the patient.
- the amount administered is sufficient to yield a plasma concentration of the compound (or its active metabolite, if the compound is a pro-drug) high enough to sufficient to modulate the PD-1/PD-L1 interaction.
- Treatment regimens may vary depending on the compound used and the particular condition to be treated; for treatment of most disorders, a frequency of administration of 4 times daily or less is preferred. In general, a dosage regimen of 2 times daily is more preferred, with once a day dosing particularly preferred. It will be understood, however, that the specific dose level and treatment regimen for any particular patient will depend upon a variety of factors including the activity of the specific compound employed, the age, body weight, general health, sex, diet, time of administration, route of administration, rate of excretion, drug combination (i.e., other drugs being administered to the patient) and the severity of the particular disease undergoing therapy, as well as the judgment of the prescribing medical practitioner. In general, the use of the minimum dose sufficient to provide effective therapy is preferred. Patients may generally be monitored for therapeutic effectiveness using medical or veterinary criteria suitable for the condition being treated or prevented.
- a concomitant medicine comprising the compounds of the present disclosure and other drug may be administered as a combination preparation in which both components are contained in a single formulation, or administered as separate formulations.
- the administration by separate formulations includes simultaneous administration and administration with some time intervals.
- the compound of the present disclosure can be administered first, followed by another drug or another drug can be administered first, followed by the compound of the present disclosure.
- the administration method of the respective drugs may be the same or different.
- the dosage of the other drug can be properly selected, based on a dosage that has been clinically used.
- the compounding ratio of the compound of the present disclosure and the other drug can be properly selected according to age and weight of a subject to be administered, administration method, administration time, disorder to be treated, symptom and combination thereof.
- the other drug may be used in an amount of 0.01 to 100 parts by mass, based on 1 part by mass of the compound of the present disclosure.
- the other drug may be a combination of two or more kind of arbitrary drugs in a proper proportion.
- the compounds described herein may be used or combined with one or more therapeutic agent such as an antimicrobial agent, an antiviral agent, a cytotoxic agent, a gene expression modulatory agent, a chemotherapeutic agent, an anti-cancer agent, an anti-angiogenic agent, an immunotherapeutic agent, an anti-hormonal agent, an anti-fibrotic agent, radiotherapy, a radiotherapeutic agent, an anti-neoplastic agent, and an anti-proliferation agent.
- therapeutic agents may be in the forms of compounds, antibodies, polypeptides, or
- the compounds described herein may be used or combined with one or more of a therapeutic antibody, a bispecific antibody and "antibody-like" therapeutic protein (such as DARTs®, Duobodies®, Bites®, XmAbs®, TandAbs ®, Fab derivatives), an antibody-drug conjugate (ADC), a virus, an oncolytic virus, gene modifiers or editors such as CRISPR
- chemotherapeutics include an alkylation agent, nitrosourea agent, antimetabolite, anticancer antibiotics, vegetable-origin alkaloid, topoisomerase inhibitor, hormone drug, hormone antagonist, aromatase inhibitor, P-glycoprotein inhibitor, platinum complex derivative, other immunotherapeutic drugs and other anticancer drugs.
- the compounds described herein may be used or combined with a cancer treatment adjunct, such as a leucopenia (neutropenia) treatment drug, thrombocytopenia treatment drug, antiemetic and cancer pain intervention drug, concomitantly or in a mixture form.
- a cancer treatment adjunct such as a leucopenia (neutropenia) treatment drug, thrombocytopenia treatment drug, antiemetic and cancer pain intervention drug, concomitantly or in a mixture form.
- the compounds described herein may be used or combined with a kinase inhibitor.
- the compounds of the present disclosure can be used with other immunomodulators and/or a potentiating agent concomitantly or in a mixture form.
- the immunomodulator include various cytokines, vaccines and adjuvants.
- these cytokines, vaccines and adjuvants that stimulates immune responses include but not limited to GM-CSF, M-CSF, G-CSF, interferon-a, beta, or gamma, IL-1, IL-2, IL- 3, IL-12, Poly (I:C) and CPG.
- the potentiating agents include cyclophosphamide and analogs of cyclophosphamide, anti- TGF and imatinib (Gleevac), a mitosis inhibitor, such as paclitaxel, Sunitinib (Sutent) or other antiangiogenic agents, an aromatase inhibitor, such as letrozole, an A2a adenosine receptor (A2AR) antagonist, an angiogenesis inhibitor, anthracyclines, oxaliplatin, doxorubicin, TLR4 antagonists, and IL- 18 antagonists.
- a mitosis inhibitor such as paclitaxel, Sunitinib (Sutent) or other antiangiogenic agents
- an aromatase inhibitor such as letrozole
- A2a adenosine receptor (A2AR) antagonist an angiogenesis inhibitor
- anthracyclines oxaliplatin
- doxorubicin TLR4 antagonists
- the compounds described herein may be used or combined with one or more modulator of CCR1, CCR2, CCR3, CCR4, CCR5, CCR6, CCR7, CCR8, CCR9, CCR10, CCR11, CXCR1, CXCR2, CXCR3, CXCR4, CXCR5, CXCR6, CXCR7, ChemR23, C5aR, C5a, and C5.
- the modulator is an antagonist.
- the compounds described herein may be used or combined with one or more of CCX354, CCX9588, CCX140, CCX872, CCX598, CCX6239, CCX9664, CCX2553, CCX 2991, CCX282, CCX025, CCX507, CCX430, CCX765, CCX224, CCX662, CCX650, CCX832, CCX168, and CCX168-M1.
- Dosage levels of the order of from about 0.1 mg to about 140 mg per kilogram of body weight per day are useful in the treatment or preventions of conditions involving the PD-l/PD- LI interaction (about 0.5 mg to about 7 g per human patient per day).
- the amount of active ingredient that may be combined with the carrier materials to produce a single dosage form will vary depending upon the host treated and the particular mode of administration. Dosage unit forms will generally contain between from about 1 mg to about 500 mg of an active ingredient.
- concentration of 5 ng (nanograms)/mL-10 ⁇ g (micrograms)/mL serum more preferably sufficient compound to achieve a serum concentration of 20 ng-1 ⁇ g/ml serum should be administered, most preferably sufficient compound to achieve a serum concentration of 50 ng/ml-200 ng/ml serum should be administered.
- concentration of 5 ng (nanograms)/mL-10 ⁇ g (micrograms)/mL serum more preferably sufficient compound to achieve a serum concentration of 20 ng-1 ⁇ g/ml serum should be administered, most preferably sufficient compound to achieve a serum concentration of 50 ng/ml-200 ng/ml serum should be administered.
- For direct injection into the synovium (for the treatment of arthritis) sufficient compounds should be administered to achieve a local concentration of approximately 1 micromolar.
- Frequency of dosage may also vary depending on the compound used and the particular disease treated. However, for treatment of most disorders, a dosage regimen of 4 times daily, three times daily, or less is preferred, with a dosage regimen of once daily or 2 times daily being particularly preferred. It will be understood, however, that the specific dose level for any particular patient will depend upon a variety of factors including the activity of the specific compound employed, the age, body weight, general health, sex, diet, time of administration, route of administration, and rate of excretion, drug combination (i.e., other drugs being administered to the patient), the severity of the particular disease undergoing therapy, and other factors, including the judgment of the prescribing medical practitioner.
- the compounds of the disclosure can be used in a variety of non-pharmaceutical in vitro and in vivo application.
- the compounds of the disclosure may also be used as positive controls in assays for PD-1/PD-L1 interaction activity, i.e., as standards for determining the ability of a candidate agent to bind to PD-1 and/or PD-L1, or as radiotracers for positron emission tomography (PET) imaging or for single photon emission computerized tomography (SPECT).
- PET positron emission tomography
- SPECT single photon emission computerized tomography
- kits comprising a compound of the present disclosure or pharmaceutically acceptable salts thereof and instructions for use.
- the kit can further contain at least one additional reagent.
- Kits typically include a label indicating the intended use of the contents of the kit.
- the term label includes any writing, or recorded material supplied on or with the kit, or which otherwise accompanies the kit.
- the embodiments are also directed to processes and intermediates useful for preparing the subject compounds or pharmaceutically acceptable salts thereof.
- Coupling at the 4-position of the indane ring can be accomplished via transition metal mediated coupling using the appropriate 4-bromoindanol and a boronic acid or ester.
- the ether bond can be formed using appropriate reagents such as triphenyl phosphine and diisopropyl or diethyl azodicarboxylate.
- Alkylation of the phenol intermediate can be achieved using the corresponding alkyl halide or mesylate reagent.
- the following reductive amination can be accomplished using an appropriate primary or secondary amine (shown as H 2 N-R') and a reducing agent such as sodium cyanoborohydride or sodium
- the 4-Bromoindanone compound can be enantioselectively reduced to its optically pure 4-bromoindanol derivative using a chiral reducing agent containing boron. Coupling at the 4-position of the indane ring can be accomplished via transition metal mediated coupling using the 4-bromoindanol and boronic acid or ester. In the subsequent step, the ether bond can be formed using reagents such as triphenyl phosphine and diisopropyl or diethyl azodicarboxylate (in this case, the reaction leads to an inversion of configuration, however, some racemization was observed).
- Alkylation of the phenol intermediate can be achieved using the appropriate alkyl halide or mesylate reagent.
- the reductive amination can be accomplished using the appropriate primary or secondary amine (shown as H 2 N-R') and a reducing agent such as sodium
- Mass spectrometry results are reported as the ratio of mass over charge. In the examples, a single m z value is reported for the M+H (or, as noted, M-H) ion containing the most common atomic isotopes. Isotope patterns correspond to the expected formula in all cases.
- Electrospray ionization (ESI) mass spectrometry analysis was conducted on a Hewlett-Packard MSD electrospray mass spectrometer using the HP1100 HPLC for sample delivery. Normally the analyte was dissolved in methanol or C3 ⁇ 4CN at 0.1 mg/mL and 1 microliter was infused with the delivery solvent into the mass spectrometer, which scanned from 100 to 1000 Daltons. All compounds could be analyzed in the positive or negative ESI mode, using acetonitrile / water with 1% formic acid as the delivery solvent.
- TLC Thin layer chromatography.
- Compounds within the scope of this disclosure can be synthesized as described below, using a variety of reactions known to the skilled artisan. One skilled in the art will also recognize that alternative methods may be employed to synthesize the target compounds of this disclosure, and that the approaches described within the body of this document are not exhaustive, but do provide broadly applicable and practical routes to compounds of interest.
- Certain molecules claimed in this patent can exist in different enantiomeric and diastereomeric forms and all such variants of these compounds are claimed unless a specific enantiomer is specified.
- the detailed description of the experimental procedures used to synthesize key compounds in this text lead to molecules that are described by the physical data identifying them as well as by the structural depictions associated with them.
- Step a To a solution of 4-bromoindan-l-ol (500 mg, 2.34 mmol) in DME (10 mL) was added phenylboronic acid (286 mg, 2.34 mmol), K 2 C0 3 (969 mg, 7.02 mmol) and the resulting suspension was bubbled with nitrogen gas for one minute. Pd(PPh 3 ) 4 (271 mg, 0.234 mmol) was then added and the reaction mixture was bubbled with nitrogen gas for an additional minute and stirred at 80 °C overnight. The reaction mixture was diluted with EtOAc (30 mL), washed with water (30 mL), brine (30 mL), dried (Na 2 S0 4 ) and concentrated in vacuo.
- Step b To a solution of 4-phenylindan-l-ol (418 mg, 1.99 mmol) in THF (5 mL) at room temperature was added 5-chloro-2,4-dihydroxy-benzaldehyde (309 mg, 1.791 mmol) and PPh 3 (521 mg, 1.99 mmol).
- Step c To a solution of 5-chloro-2-hydroxy-4-(4-phenylindan-l-yl)oxy-benzaldehyde (100 mg, 0.274 mmol) in DMF (5 mL) was added 5-(bromomethyl)pyridine-3-carbonitrile (108 mg, 0.549 mmol) followed by Cs 2 C0 3 (178 mg, 0.549 mmol). The resulting suspension was then stirred at 65 °C for 2 h. The reaction mixture was diluted with EtOAc (20 mL), washed with water (20 mL), dried (MgS0 4 ), concentrated in vacuo.
- Step d To a solution of 5-[[4-chloro-2-formyl-5-(4-phenylindan-l-yl)oxy- phenoxy]methyl]pyridine-3-carbonitrile (50 mg, xx mmol) in DMF (2 mL) was added (2S)-2- amino-3-hydroxy-propanoic acid (100 mg) and Na(OAc) 3 BH (100 mg, xx mmol), and the resulting suspension was stirred at room temperature overnight. The reaction mixture was diluted with 2: 1 CHC1 3 /IPA (30 mL), washed with water (15 mL), dried (MgS0 4 ), and concentrated in vacuo. The crude residue was purified by reverse phase preparative HPLC (CH 3 CN-H 2 0 with
- Step a To a solution of 4-bromoindan-l-one (3 g, 2.34 mmol) in DME (15 mL) was added phenylboronic acid (1.73 g, 14.2 mmol) and K 2 C0 3 (5.9 g, 42.6 mmol). The resulting suspension was bubbled with nitrogen gas for one minute before Pd(PPh 3 ) 4 (1.64 g, 1.42 mmol) was added. The reaction mixture was bubbled with nitrogen gas for an additional minute and subsequently stirred at 75 °C overnight. The mixture was diluted with EtOAc (100 mL), washed with water (50 mL), brine (50 mL), dried (MgS0 4 ), and concentrated in vacuo.
- Step b To GS (-)-2-Methyl-CBS (Corey-Bakshi-Shibata)-oxazaborolidine (900 ⁇ ., 0.887 mmol, 1 M in THF) was added BH 3 » DMS (443 ⁇ ., 0.887 mmol, 2 M solution in THF) under nitrogen atmosphere and the reaction mixture was stirred at room temperature for 10 minutes. The reaction was diluted with CH 2 C1 2 (5 mL).followed by the addition of BH 3 » DMS (16.3 mL, 32.52 mmol, 2 M solution in THF).
- Step c To a solution of (lR)-4-phenylindan-l-ol (840 mg, 4.0 mmol) in THF (10 mL) at room temperature was added 5-chloro-2,4-dihydroxy-benzaldehyde (690 mg, 4.0 mmol), followed by PPh 3 (1.05 g, 4 mmol), and the resulting solution was cooled to 0 °C. DIAD (808 mg, 4.0 mmol) in THF (3 mL) was added slowly dropwise and the resulting solution was allowed to warm to room temperature with stirring.
- Step d To a solution of 5-chloro-2-hydroxy-4-[(lS)-4-phenylindan-l-yl]oxy- benzaldehyde (178 mg, 0.489 mmol) in DMF (5 mL) was added 5-(bromomethyl)pyridine-3- carbonitrile (192 mg, 0.978 mmol) and Cs 2 C0 3 (318 mg, 0.978 mmol) and the resulting suspension was then stirred at 75 °C for 2 h. The reaction mixture was diluted with EtOAc (30 mL), washed with water (20 mL), dried (MgS0 4 ), and concentrated in vacuo.
- Step e To a solution of 5-[[4-chloro-2-formyl-5-[(15)-4-phenylindan-l-yl]oxy- phenoxy]methyl]pyridine-3-carbonitrile (66 mg, 0.1375 mmol) in DMF (4 mL) was added (25)- 2-amino-3-hydroxy-propanoic acid (33 mg, 0.275 mmol), AcOH (20 ⁇ , 0.1375 mmol), followed by NaCNBH 3 (20 mg, 0.206 mmol). The resulting mixture was stirred at room temperature overnight before it was diluted with 2:1 CHC1 3 /IPA (30 mL), washed with water (15 mL), dried (MgS0 4 ), and concentrated in vacuo.
- Step a To (R)-(+)-2-methyl-CBS (Corey-Bakshi-Shibata)-oxazaborolidine (900 ⁇ ., 0.887 mmol, 1 M solution in THF) under nitrogen atmosphere was added BH 3 » DMS (443 ⁇ _, 0.887 mmol, 2 M solution in THF) at room temperature and stirred for 10 minutes. The reaction mixture was diluted with CH 2 C1 2 (5 mL) and BH 3 » DMS (16.3 mL, 32.52 mmol, 2 M solution in THF) was added before cooling to -20 °C.
- Step b To a solution of (l;S)-4-phenylindan-l-ol (840 mg, 4 mmol) in THF (10 mL) at room temperature was added 5-chloro-2,4-dihydroxy-benzaldehyde (690 mg, 4 mmol) followed by PPh 3 (1.05 g, 4 mmol). The resulting solution was cooled to 0 °C before DIAD (808 mg, 4 mmol) in THF 3 mL) was added slowly dropwise. The solution was allowed warm to room temperature and stirred for 12 h.
- Step c To a solution of 5-chloro-2-hydroxy-4-[(lR)-4-phenylindan-l-yl]oxy- benzaldehyde (340 mg, 0.934 mmol) in DMF (5 mL) was added 5-(bromomethyl)pyridine-3- carbonitrile (366 mg, 1.868 mmol), followed by Cs 2 C0 3 (607 mg, 1.868 mmol). The resulting suspension was then stirred at 75 °C for 2 h. Reaction mixture was diluted with EtOAc (30 mL), washed with water (20 mL), dried (MgS0 4 ), and concentrated in vacuo.
- Step d To a solution of 5-[[4-chloro-2-formyl-5-[(lR)-4-phenylindan-l-yl]oxy- phenoxy]methyl]pyridine-3-carbonitrile (304 mg, 0.633 mmol) in DMF (5 mL) was added (25)- 2-amino-3-hydroxy-propanoic acid (301 mg, 2.53 mmol) and AcOH (152 ⁇ L, 2.53 mmol), followed by NaCNBH 3 (159 mg, 2.53 mmol), and the resulting suspension was stirred at room temperature overnight.
- reaction mixture was diluted with 2:1 CH.CI3/IPA (30 mL) and washed with water (15 mL), dried (MgS0 4 ), concentrated in vacuo and purified by reverse phase preparative HPLC (CH 3 CN-H 2 0 with 0.1% TFA) to obtain 5-[[4-chloro-5-(4-phenylindan-l-yl)oxy-2-[(lH- tetrazol-5-ylmethylaniino)methyl]phenoxy]methyl]pyridine-3-carbonitrile.
- reaction mixture was diluted with 2: 1 CHC1 3 /IPA (30 mL) and washed with water (15 mL), dried (MgS0 4 ), concentrated in vacuo and purified by reverse phase preparative HPLC (CH 3 CN-H 2 0 with 0.1% TFA) to obtain 5- [ [4-chloro-2- [(2-hydroxyethylamino)methyl]-5-(4-phenylindan- 1 -yl)oxy- phenoxy]methyl]pyridine-3-carbonitrile.
- reaction mixture was diluted with 2:1 CHC1 3 /IPA (30 mL) and washed with water (15 mL), dried (MgS0 4 ), concentrated in vacuo and purified by reverse phase preparative HPLC (CH 3 CN-H 2 0 with 0.1% TFA) to (2-S')-l-[[5-chloro-2-[(5-cyano-3-pyridyl)methoxy]-4-(4-phenylindan-l- yl)oxy-phenyl]methyl]piperidine-2-carboxylic acid.
- reaction mixture was diluted with 2:1 CHCls/IPA (30 mL) and washed with water (15 mL), dried (MgS0 4 ), concentrated in vacuo and purified by reverse phase preparative HPLC (CH 3 CN-H 2 0 with 0.1% TFA) to obtain 5-[[2-(azetidin-l-ylmethyl)-4-chloro-5-(4-phenylindan-l-yl)oxy- phenoxy]methyl]pyridine-3-carbonitrile.
- Step a Oxalyl chloride (5.7 mL, 67.26 mmol) was slowly added to 3- (benzyloxycarbonylamino)propanoic acid (5 g, 22.42 mmol) dissolved in CH 2 C1 2 (75 mL) at room temperature followed by few drops of DMF to catalyze the reaction (gas evolution was observed immediately). After 2 h, the reaction mixture was concentrated in vacuo. Additional CH 2 C1 2 (50 mL) was added and concentrated in vacuo followed by drying on high vacuum pump to obtain benzyl N-(3-chloro-3-oxo-propyl)carbamate which was used as such in the next step without any further purification. MS (after quenching the acid chloride with MeOH): (ES) m/z calculated for C 12 H 15 N0 4 Na [Methyl ester, M+Na] + 260.1, found 260.3.
- Step b A safety notice for the procedure: Azide compounds are potentially explosive. This reaction was performed behind a blast shield. TMSN 3 (2.4 mL, 18 mmol) was slowly added to benzyl N-(3-chloro-3-oxo-propyl)carbamate (723 mg, 6 mmol) at room temperature (gas evolution was observed). The resulting reaction mixture was heated and stirred overnight at 100 °C. Volatiles were removed in vacuo and the crude product was directly purified by flash chromatography (Si0 2 , 80% EtOAc in hexanes) to obtain benzyl N-[2-(5-oxo-lH-tetrazol-4- yl)ethyl]carbamate. MS: (ES) m/z calculated for CnH 14 N 5 0 3 [ ⁇ + ⁇ ] + 264.1, found 264.4 (also observed significant peak for [M+Na] + ).
- Step c To benzyl N-[2-(5-oxo-lH-tetrazol-4-yl)ethyl]carbamate (250 mg, 0.95 mmol) in MeOH (10 mL) was added 10% Pd/C (200 mg) in a Parr shaker flask, the resulting suspension was purged twice with hydrogen gas and agitated at room temperature under hydrogen gas (60 psi) for one hour. The reaction mixture was filtered through a pad of Celite, washed with MeOH (15 mL) and concentrated in vacuo to obtain 4-(2-aminoethyl)-lH-tetrazol-5-one which was used as such in the next step without any further purification. MS: (ES) m/z calculated for C 3 H 8 N 5 0 [M+H] + 130.1, found 130.3.
- Step d To a solution of 5-[[4-chloro-2-formyl-5-[(l 1 S)-4-phenylindan-l-yl]oxy- phenoxy]methyl]pyridine-3-carbonitrile (100 mg, 0.208 mmol) in DMF (4 mL) was added 4-(2- aminoethyl)-lH-tetrazol-5-one (50 mg, 0.387 mmol), AcOH (50 ⁇ L, 0.53 mmol) followed by Na(OAc) 3 BH (90 mg, 0.424 mmol) and the resulting suspension was stirred at room temperature overnight.
- reaction mixture was diluted with 2:1 CHC1 3 /IPA (30 mL) and washed with water (15 mL), dried (MgS0 4 ) concentrated in vacuo and purified by reverse phase preparative HPLC (CH 3 CN-H 2 0 with 0.1% TFA) to obtain 5-[[4-chloro-2-[[2-(5-oxo-lH-tetrazol-4- yl)ethylamino]methyl]-5-[(15 -4-phenylindan-l-yl]oxy-phenoxy]methyl]pyridine-3-carbonitril ⁇ er: -3.5:1.
- Step a To a solution of 4-bromoindan-l-ol (5.3 g, 24.91 mmol) in THF (30 mL) at room temperature was added 5-chloro-2,4-dihydroxy-benzaldehyde (4.3 g, 24.91 mmol) followed by PPh 3 (6.5 g, 24.91 mmol) and the resulting solution was cooled in an ice-bath. DIAD (5.03 g, 24.91 mmol) in THF (10 mL) was added slowly dropwise at 0 °C and the resulting solution was allowed to warm to room temperature with stirring.
- DIAD 5.03 g, 24.91 mmol
- Step b To a solution of 4-(4-bromoindan-l-yl)oxy-5-chloro-2-hydroxy-benzaldehyde (250 mg, 0.683 mmol) in DMF (3 mL) was added 3-(chloromethyl)pyridine hydrochloride (225 mg, 1.37 mmol) followed by Cs 2 C0 3 (444 mg, 1.37 mmol). The resulting suspension was stirred at 75 °C for 2 h.
- Step c To a solution of 4-(4-bromoindan-l-yl)oxy-5-chloro-2-(3- pyridylmethoxy)benzaldehyde (312 mg, 0.683 mmol) in DME (5 mL) was added phenylboronic acid (150 mg, 1.02 mmol), K 2 C0 3 (283 mg, 2.05 mmol) and the resulting suspension was bubbled with nitrogen gas for one minute. Pd(PPh 3 ) 4 (80 mg, 0.0683 mmol) was then added, bubbled the reaction mixture with nitrogen gas for additional minute and stirred at 80 °C overnight.
- Step d To a solution of 5-chloro-4-(4-phenylindan-l-yl)oxy-2-(3- pyridylmethoxy)benzaldehyde (55 mg) in DMF (3 mL) was added (25)-2-amino-3-hydroxy- propanoic acid (100 mg) followed by Na(OAc) 3 BH (100 mg) and the resulting suspension was stirred at room temperature overnight.
- reaction mixture was diluted with 2:1 CRCl ⁇ WA (30 mL) and washed with water (15 mL), dried (MgS0 4 ) concentrated in vacuo and purified by reverse phase preparative HPLC (CH 3 CN-H 2 0 with 0.1% TFA) to obtain (2S)-2-[[5-chloro-4- (4-phenylindan-l-yl)oxy-2-(3-pyridylmethoxy)phenyl]methylamino]-3-hydroxy-propanoic acid.
- reaction mixture was diluted with 2: 1 CHC1 3 /IPA (30 mL) and washed with water (15 mL), dried (MgS0 4 ), concentrated in vacuo and purified by reverse phase preparative HPLC (CH 3 CN-H 2 0 with 0.1% TFA) to obtain 5-[[4- chloro-5-[(15)-4-phenylindan-l-yl]oxy-2-[(lH-pyrazol-5- ylmethylamino)methyl]phenoxy]methyl]pyridine-3-carbonitrile, er: -3.5:1.
- Step a To a 500 mL three-neck round bottom flask equipped with an internal thermometer under nitrogen was added (5)-(-)-2-methyl-CBS-oxazaborolidine (7.1 mL, 7.1 mmol, 1M THF) and borane-dimethyl sulfide (3.6 mL, 7.2 mmol, 2M THF) at room temperature. The mixture was stirred for 10 min then diluted with dichloromethane (60 mL). Borane- dimethyl sulfide (130 mL, 260 mmol, 2M THF) was added at room temperature and the mixture was cooled to -30 °C. A solution of 4-bromoindan-l-one (13.6 g, 64.4 mmol) in
- Step b To a cooled (0 °C) solution of (R)-4-bromoindan-l-ol (11.2 g, 52.6 mmol), 5- chloro-2,4-dihydroxy-benzaldehyde (9.1 g, 52.6 mmol), and triphenylphosphine (13.8 g, 52.6 mmol) in THF (100 mL) was slowly added diisopropyl azodicarboxylate (10.3 mL, 52.6 mmol) in THF (25 mL). The mixture was allowed to gradually warm to room temperature for three days.
- Step c To a solution of 4-[(15)-4-bromoindan-l-yl]oxy-5-chloro-2-hydroxy- benzaldehyde (2.0 g, 5.4 mmol) in DMF (12 mL) was added (5-cyano-3-pyridyl)methyl methanesulfonate (1.5 g, 7.1 mmol), followed by Cs 2 C0 3 (3.5 g, 11 mmol). The resulting suspension was stirred at 75 °C for 2 h. After cooling to room temperature, the reaction mixture was diluted with EtOAc (50 mL) and washed with water (20 mL). The aqueous layer was re- extracted with EtOAc (2 x 25 mL).
- Step d To a solution of 5-[[5-[(l 1 S)-4-bromoindan-l-yl]oxy-4-chloro-2-formyl- phenoxy]methyl]pyridine-3-carbonitrile (0.83 g, 1.7 mmol) in 1,2-dimethoxyethane (10 mL) was added phenylboronic acid (0.22 g, 1.8 mmol), aqueous 2M K 2 C0 3 (1.3 mL, 2.6 mmol) and the resulting mixture was bubbled with nitrogen gas for a few minutes.
- Step e To a solution of 5-[[4-chloro-2-formyl-5-[(l 1 S)-4-phenylindan-l-yl]oxy- phenoxy]methyl]pyridine-3-carbonitrile (150 mg, 0.31 mmol) in DMF (3 mL) was added azetidin-3-ol hydrochloride (130 mg, 1.2 mmol), triethylamine (0.40 mL, 2.9 mmol), acetic acid (0.20 mL, 2.9 mmol), and sodium triacetoxyborohydride (190 mg, 0.88 mmol). After stirring at room temperature overnight, the reaction mixture was diluted with 2:1 CHCl 3 /i-PrOH (30 mL) and washed with water (15 mL). The organic layer was dried (MgS0 4 ), filtered, and
- Step a To a cold (0 °C) solution of pyridazin-3-ylmethanol (500 mg, 4.5 mmol) and triethylamine (1.26 mL,9.1 mmol) in CH 2 C1 2 (5 mL) was added methanesulfonyl chloride (0.60 mL, 7.8 mmol) by dropwise addition. The resulting mixture was allowed to warm to room temperature and stirred for 1 h. The reaction mixture was added to water and the organic phase was separated. The aqueous phase was extracted with EtOAc, and solvent was removed from the combined organic layers in vacuo. The crude residue was purified by flash chromatography (Si0 2 , 50% EtOAc in hexanes) to obtain (2-chloropyrimidin-5-yl)methyl methanesulfonate.
- Step b To a solution of 5-chloro-4-[(15)-4-(2-fluorophenyl)indan-l-yl]oxy-2-hydroxy- benzaldehyde (150 mg, 0.393 mmol) and pyridazin-3-ylmethyl methanesulfonate
- Step c To a solution of 5-chloro-4-[(l 1 S)-4-(2-fluorophenyl)indan-l-yl]oxy-2- (pyridazin-3-ylmethoxy)benzaldehyde (50 mg, 0.1 mmol) in DMF (3 mL) was added (2S,3R)-2- amino-3-hydroxy-butanoic acid (100 mg, 0.57 mmol) Na(OAc) 3 BH (100 mg, 0.49 mmol) and acetic acid (0.10 mL, 1.8 mmol). The resulting suspension was stirred at 45 °C for overnight.
- the reaction mixture was diluted with 2:1 CHCl 3 /i-PrOH (5 mL), washed with water (1 mL), and concentrated in vacuo.
- the crude residue was purified by reverse phase preparative HPLC (CH 3 CN-H 2 0 with 0.1% TFA). The fractions were combined and diluted with 2:1 CHCl 3 /i- PrOH (30 mL).
- the reaction mixture was diluted with 2:1 CHCi i-PrOH (5 mL), washed with water (1 mL), and concentrated in vacuo.
- the crude residue was purified by reverse phase preparative HPLC (CH 3 CN-H 2 0 with 0.1% TFA). The fractions were combined and diluted with 2:1 CHCl 3 /i-PrOH (30 mL).
- the crude residue was purified by reverse phase preparative HPLC (CH 3 CN-H 2 0 with 0.1% TFA) to obtain 1- methylethyl (2-S -2-[[5-chloro-2-[(5-cyano-3-pyridyl)methoxy]-4-[(l-S -4-(2-fluorophenyl)indan- l-yl]oxy-phenyl]methylamino]-3-hydroxy-propanoate as a trifluoroacetic acid salt.
- the salt was neutralized by passing the purified fractions through an Agilent Technologies PL-HC03 MP SPE cartridge to obtain the neutral form, dr: -3.5:1.
- Step a To a solution of 4-bromoindan-l-one (10 g, 47 mmol) dissolved in methanol (110 mL) was added l-chloromethyl-4-fluoro-l,4 ⁇ diazoniabicyclo[2,2.2]octane
- Step b To a solution of 4-bromo-2-fluoroindan-l-one (2.0 g, 8.7 mmol) in ethanol (40 mL) was added sodium borohydride (380 mg, 10 mmol). The mixture was stirred for 10 minutes at room temperature, then quenched with the addition of saturated aqueous sodium bicarbonate (10 mL). Ethanol was removed in vacuo, and the residue was extracted with ethyl acetate, washed with brine, dried over sodium sulfate, filtered and concentrated. The crude solid was purified by flash chromatography to obtain 4-bromo-2-fluoroindan-l-ol. MS: (ES) m/z
- Step c To a cooled (0 °C) solution of re -(lR,2R)-4-bromo-2-fluoroindan-l-ol (1.2 g, 5.3 mmol), 5-chloro-2,4-dihydroxybenzaldehyde (0.96 g, 5.6 mmol),and triphenylphosphine (1.5 g, 5.7 mmol) in THF (40 mL) was slowly added DIAD (1.2 g, 5.6 mmol) in THF (40 mL). The mixture was allowed to warm to room temperature and stirred for 16 h.
- Step d To a solution of 4-[re -(lR,2R)-4-bromo-2-fluoro-indan-l-yl]oxy-5-chloro-2- hydroxy-benzaldehyde (340 mg, 0.87 mmol) in DMF (4 mL) was added (5-cyano-3- pyridyl)methyl methanesulfonate (300 mg, 1.4 mmol), followed by Cs 2 C0 3 (1.0 g, 3.1 mmol). The resulting suspension was stirred at 50 °C for 30 min. The reaction mixture was diluted with dichloromethane and washed with water, and the organic layer was concentrated in vacuo.
- Step e To a solution of 5-[[5-[re -(lR,2R)-4-bromo-2-fluoro-indan-l-yl]oxy-4-chloro- 2-formyl-phenoxy]methyl]pyridine-3-carbonitrile (270 mg, 0.54 mmol) in DME (5 mL) was added 2-flurophenylboronic acid (120 mg, 0.86 mmol), K 2 C0 3 (240 mg, 1.7 mmol) and the resulting mixture was bubbled with nitrogen gas for a few minutes. Pd(PPh 3 ) 4 (110 mg, 0.096 mmol) was then added and the reaction mixture was stirred at 80 °C for 2 h.
- Step f To a solution of 5-[[4-chloro-5-[re -(lR,2R)-2-fluoro-4-(2-fluorophenyl)indan- l-yl]oxy-2-formyl-phenoxy]methyl]pyridine-3-carbonitrile (50 mg, 0.097 mmol) in DMF (2 mL) was added L-serine (100 mg, 0.95 mmol), Na(OAc) 3 BH (105 mg, 0.50 mmol) and acetic acid (0.10 mL, 1.8 mmol). The resulting mixture was stirred at 50 °C for 2 h. .
- reaction mixture was diluted with 2:1 CHCl 3 /i-PrOH (5 mL), washed with water (1 mL), and concentrated in vacuo.
- the crude residue was purified by reverse phase preparative HPLC (CH 3 CN-H 2 0 with 0.1% TFA) to obtain (2S)-2-[[5-chloro-2-[(5-cyano-3-pyridyl)methoxy]-4-[re -(lR,2R)-2-fluoro- 4-(2-fluorophenyl)indan-l-yl]oxy-phenyl]methylamino]-3-hydroxy-propanoic acid.
- purified HPLC fractions were basified with sodium bicarbonate, and solvent was removed in vacuo.
- Step a To a cold (0 °C) solution of (2-chloropyrimidin-5-yl)methanol (710 mg, 4.9 mmol) and triethylamine (1.8 mL, 13 mmol) in EtOAc (20 mL) was added methanesulfonyl chloride (0.60 mL, 7.8 mmol) by dropwise addition. The resulting mixture was allowed to warm to room temperature and stirred for 2 days. The reaction mixture was added to water and the organic phase was separated. The aqueous phase was extracted with EtOAc, and solvent was removed from the combined organic layers in vacuo.
- Step b To a solution of 5-chloro-4-[(lS)-4-(2-fluorophenyl)indan-l-yl]oxy-2-hydroxy- benzaldehyde (200 mg, 0.52 mmol) and (2-chloropyrimidin-5-yl)methyl methanesulfonate (200 mg, 0.90 mmol) in DMF (2 mL) was added cesium carbonate (400 mg, 1.2 mmol). The mixture was stirred at 40 °C overnight.
- Step c To a solution of 5-chloro-2-[(2-chloropyrimidin-5-yl)methoxy]-4-[(lS)-4-(2- fluorophenyl)indan-l-yl]oxy-benzaldehyde (50 mg, 0.098 mmol) in THF (1 mL) in a 4 mL glass vial was added 7M ammonia in methanol (1.4 mL, 9.8 mmol). The vial was secured with a teflon-lined screwcap and placed in an aluminum heating block maintained at 100 °C for four hours.
- Step d To a solution of crude 2-[(2-aminopyrimidin-5-yl)methoxy]-5-chloro-4-[(lS)- 4-(2-fluorophenyl)indan-l-yl]oxy-benzaldehyde (50 mg, 0.1 mmol) in NMP (1 mL) was added 4-hydroxypiperidine (113 mg, 1.1 mmol), Na(OAc) 3 BH (125 mg, 0.59 mmol), and acetic acid (0.075 mL, 1.3 mmol). The mixture was stirred at room temperature overnight followed by an additional 6 h at 50 °C. The resulting suspension was stirred at room temperature for 3.5 hours.
- reaction mixture was diluted with 2:1 CHCl 3 /i-PrOH (5 mL), washed with water (1 mL), and concentrated in vacuo.
- the crude residue was purified by reverse phase preparative HPLC (CH 3 CN-H 2 0 with 0.1% TFA) to obtain l-[[2-[(2-aminopyrimidin-5-yl)methoxy]-5-chloro-4- [(lS)-4-(2-fluorophenyl)indan-l-yl]oxy-phenyl]methyl]piperidin-4-ol as a trifluoroacetic acid salt.
- Step a To a solution of 5-[[5-[(l 1 S)-4-bromoindan-l-yl]oxy-4-chloro-2-formyl- phenoxy]methyl]pyridine-3-carbonitrile (570 mg, 1.2 mmol) in 1,2-dimethoxyethane (10 mL) was added 2-fluorophenylboronic acid (250 mg, 1.8 mmol), aqueous 2M K 2 C0 3 (1.20mL, 3.5 mmol) and the resulting mixture was bubbled with nitrogen gas for a few minutes.
- Tetrakis(triphenylphosphine)palladium(0) 140 mg, 0.12 mmol was then added and the reaction mixture was stirred at 80 °C for 2 h. After cooling to room temperature, the reaction mixture was diluted with EtOAc (30 mL) and washed with water (30 mL) and brine (30 mL). SThe organic layer was dried (Na 2 S0 4 ), filtered, and concentrated in vacuo.
- Step b To a solution of 5-[[4-chloro-5-[(l 1 S)-4-(2-fluorophenyl)indan-l-yl]oxy-2- formyl-phenoxy]methyl]pyridine-3-carbonitrile (50 mg, 0.10 mmol) in DMF (3 mL) was added L-serine (100 mg, 0.95 mmol) and sodium triacetoxyborohydride (150 mg, 0.71 mmol). The resulting suspension was stirred at room temperature overnight. The reaction mixture was diluted with 2:1 CHCl 3 /i ' -PrOH (30 mL), washed with water (15 mL), dried (MgS0 4 ), and concentrated in vacuo.
- Step a To a solution of 4-[(15)-4-bromoindan-l-yl]oxy-5-chloro-2-hydroxy- benzaldehyde (200 mg, 0.54 mmol) in DMF (1 mL) was added iodomethane (130 ⁇ _, 2.1 mmol), followed by Cs 2 C0 3 (360 mg, 1.1 mmol). The resulting suspension was stirred at 40 °C for 1 h. After cooling to room temperature, the reaction was diluted with dichloromethane (15 mL) and washed with water (20 mL). The aqueous layer was re-extracted with dichloromethane (2 x 20 mL).
- Step b To a solution of 4-[(15)-4-bromoindan-l-yl]oxy-5-chloro-2-methoxy- benzaldehyde (210 mg, 0.54 mmol) in DME (5 mL) was added phenylboronic acid (79 mg, 0.65 mmol), aqueous 2M K 2 C0 3 (0.41 mL, 0.81 mmol) and the resulting mixture was bubbled with nitrogen gas for a few minutes. Tetrakis(triphenylphosphine)palladium(0) (31 mg, 0.81 mmol) was then added and the reaction mixture was stirred at 80 °C overnight.
- Step c To a solution of 5-chloro-2-methoxy-4-[(15)-4-phenylindan-l-yl]oxy- benzaldehyde (100 mg, 0.26 mmol) in DMF (3 mL) was added L-serine (100 mg, 0.95 mmol) and sodium triacetoxyborohydride (150 mg, 0.71 mmol). The resulting suspension was stirred at room temperature overnight. The reaction mixture was diluted with 2:1 CHCl 3 /i-PrOH (30 mL), washed with water (15 mL), dried (MgS0 4 ), filtered, and concentrated in vacuo.
- Step a To a solution of 3-fluorocatechol (5.30 g, 41.2 mmol) and K 2 C0 3 (17.1 g, 124 mmol) in DMF (50 mL) was added 1,2-dibromoethane (3.90 mL, 45.3 mmol) and the mixture was left to stir at room temperature for 4 days. Water (50 mL) was added and the mixture was extracted with EtOAc (3 x 50 mL). The combined organic layers were dried (MgS0 4 ), filtered, and concentrated in vacuo.
- Step b To a cooled (0 °C) solution of 5-fluoro-2,3-dihydro-l,4-benzodioxine (1.0 g, 6.5 mmol) in methanol (25 mL) was added bromine (1.2 g, 0.40 mL, 7.8 mmol), and the resulting mixture was allowed to warm to room temperature. After stirring for 24 h, saturated aqeous sodium metabisulfite (lOOmL) was added and the solution was extracted with
- Step c To a solution of 6-bromo-5-fluoro-2,3-dihydro-l,4-benzodioxine (705 mg, 3.02 mmol), bw(pinacolato)diboron (1.53 g, 6.04 mmol), and potassium acetate (890 mg, 9.06 mmol) in 1,4-dioxane (15mL) was added [l, -bw(diphenylphosphino)ferrocene]dichloropalladium(II) complex with dichloromethane (244 mg, 0.299 mmol). The mixture was heated at 100 °C and stirred for 3 h.
- Step d To a solution of 5-[[5-[(l 1 S)-4-bromoindan-l-yl]oxy-4-chloro-2-formyl- phenoxy]methyl]pyridine-3-carbonitrile (100 mg, 0.20 mmol) in 1,2-dimethoxyethane (4 mL) and aqueous 2M K 2 C0 3 (0.40 mL, 0.80 mmol) was added 2-(5-fluoro-2,3-dihydro-l,4- benzodioxin-6-yl)-4,4,5,5-tetramethyl-l,3,2-dioxaborolane (120 mg, 0.41 mmol) and the resulting mixture was bubbled with nitrogen gas for a few minutes.
- Tetrakis(triphenylphosphine)palladium(0) 25 mg, 0.020 mmol was then added and the reaction mixture was stirred at 80 °C ofor 1 h. After cooling to room temperature, the reaction mixture was diluted with EtOAc (30 mL) and washed with water (20 mL). The organic layer was dried (MgS0 4 ), filtered, and concentrated in vacuo.
- Step e To a solution of 5-[[4-chloro-5-[(15)-4-(5-fluoro-2,3-dihydro-l,4-benzodioxin- 6-yl)indan-l-yl]oxy-2-formyl-phenoxy]methyl]pyridine-3-carbonitrile (47 mg, 0.084 mmol) in DMF (3 mL) was added L-serine (70 mg, 0.67 mmol) and sodium triacetoxyborohydride (90 mg, 0.42mmol). The resulting suspension was stirred at room temperature overnight.
- reaction mixture was diluted with 2:1 CHCi i-PrOH (30 mL), washed with water (15 mL), dried (MgS0 4 ), and concentrated in vacuo.
- the crude residue was purified by reverse phase preparative HPLC (CH 3 CN-H 2 0 with 0.1% TFA) to obtain (2-S -2-[[5-chloro-2-[(5-cyano-3- pyridyl)methoxy]-4-[(15 -4-(5-fluoro-2,3-dihydro-l,4-benzodioxin-6-yl)indan-l-yl]oxy- phenyl]methylamino]-3-hydroxy-propanoic acid as a di-trifluoroacetic acid salt, dr: -3.5:1. .
- Step a To a solution of (R)-4-bromoindan-l-ol (5.0 g, 24 mmol) in 1,2- dimethoxyethane (50 mL) and water (30 mL) was added 2-fluorophenylboronic acid (4.3, 31 mmol)and K 2 C0 3 (8.1 g, 59 mmol) and the resulting mixture was bubbled with nitrogen gas for a few minutes . Tetrakis(triphenylphosphine)palladium(0) (0.8 lg, 0.71 mmol) was added, and the reaction mixture was stirred at 80 °C overnight.
- Step b To a cooled (0 °C) solution of (R)-4-(2-fluorophenyl)indan-l-ol (5.4 g, 24 mmol), 5-chloro-2,4-dihydroxy-benzaldehyde (4.1 g, 24 mmol), and triphenylphosphine (6.2 g, 24 mmol) in THF (100 mL) was slowly added diisopropyl azodicarboxylate (4.8 g, 24 mmol) in THF (10 mL). The mixture was allowed to gradually warm to room temperature for two days.
- Step c To a cooled (-78 °C) solution of 5-chloro-4-[(l£ 4-(2-fluorophenyl)indan-l- yl]oxy-2-hydroxy-benzaldehyde (1.0 g, 2.6 mmol) in dichloromethane (10 mL) was sequentially added pyridine (1.0 mL, 12 mmol) and triflic anhydride (0.87 mL, 5.2 mmol). The reaction mixture was allowed to warm to room temperature. After 2 h, the reaction was quenched by the careful addition of few milliliters of saturated aqueous NaHC0 3 .
- Step d To a solution of [4-chloro-5-[(l 1 S)-4-(2-fluorophenyl)indan-l-yl]oxy-2-formyl- phenyl] trifluoromethanesulfonate (100 mg, 0.19 mmol) in 1,2-dimethoxyethane (3 mL) and 2M
- Step e To a solution of (5-[4-chloro-5-[(l-S')-4-(2-fluorophenyl)indan-l-yl]oxy-2- formyl-phenyl]pyridine-3-carbonitrile (30 mg, 0.064 mmol) in DMF (2 mL) was added L-serine (60 mg, 0.57 mmol) and sodium triacetoxyborohydride (60 mg, 0.28 mmol). The resulting suspension was stirred at room temperature overnight. The reaction mixture was diluted with 2:1 CHCl 3 /i ' -PrOH (30 mL), washed with water (15 mL), dried (MgS0 4 ), filtered, and concentrated in vacuo.
- Step a To a 1-L three-neck round bottom flask equipped with an internal thermometer under nitrogen was added (R)-(+)-2-methyl-CBS-oxazaborolidine (3.2mL, 3.2 mmol, 1M THF) and borane-dimethyl sulfide (1.6 mL, 3.2 mmol, 2M THF) at room temperature. The mixture was stirred for 10 min then diluted with dichloromethane (100 mL). Borane-dimethyl sulfide (60 mL, 120 mmol, 2M THF) was added at room temperature and the mixture was cooled to -30 °C.
- Step b To a cooled (0 °C) solution of (;S)-4-bromoindan-l-ol (1.7 g, 7.9 mmol), 5- chloro-2,4-dihydroxy-benzaldehyde (1.3g, 7.9 mmol), and triphenylphosphine (2.1 g, 7.9 mmol) in THF (25 mL) was slowly added diisopropyl azodicarboxylate (1.7 mL, 8.7 mmol) in THF (5 mL). The mixture was allowed to gradually warm to room temperature for three days. The volatiles were removed in vacuo and the resulting crude residue was purified by flash
- Step c To a solution of 4-[(lR)-4-bromoindan-l-yl]oxy-5-chloro-2-hydroxy- benzaldehyde (0.84 g, 2.29 mmol) in DMF (12 mL) was added 5-(bromomethyl)nicotinonitrile (0.54 g, 2.75 mmol), followed by Cs 2 C0 3 (1.5 g, 4.58 mmol). After stirring at room temperature overnight, the reaction mixture was diluted with 2: 1 CHCl 3 /i-PrOH (30 mL) and washed with water (20 mL). The aqueous layer was re-extracted with 2:1 CHCl 3 /i-PrOH (2 x 15 mL).
- Step d To a solution of 5-[[5-[(lR)-4-bromoindan-l-yl]oxy-4-chloro-2-formyl- phenoxy]methyl]pyridine-3-carbonitrile (282 mg, 0.58 mmol) in 1,2-dimethoxyethane (4 mL) was added 2-fluorophenylboronic acid (122 mg, 0.87 mmol), aqueous 2M K 2 C0 3 (1.30 mL,
- Tetrakis(triphenylphosphine)palladium(0) (100 mg, 0.086 mmol) was then added and the reaction mixture was stirred at 80 °C overnight. After cooling to room temperature, the reaction mixture was diluted with EtOAc (30 mL) and washed with water (30 mL) and brine (30 mL). The organic layer was dried (Na 2 S0 4 ), filtered, and concentrated in vacuo.
- Step e To a solution of 5-[[4-chloro-2-formyl-5-[(lR)-4-phenylindan-l-yl]oxy- phenoxy]methyl]pyridine-3-carbonitrile (31 mg, 0.062 mmol) in DMF (2 mL) was added L- threonine (50 mg, 0.42 mmol) and sodium triacetoxyborohydride (100 mg, 0.47 mmol). After stirring at room temperature overnight, the reaction mixture was concentrated and the crude residue was purified by reverse phase preparative HPLC (CH 3 CN-H 2 0 with 0.1% TFA). The fractions were combined and diluted with 2:1 CHCl 3 /i-PrOH (30 mL).
- Example 36 Synthesis of (25)-2-[[5-chloro-4-[(15)-4-(5-chloro-2 ⁇ -dihydro-l,4- benzodioxin-6-yl)indan-l-yl]oxy-2-[(5-cyano-3 ⁇ yridyl)methoxy]phenyl]methylamino]-3- hydroxy-propanoic acid
- Example 46 Synthesis of (5-chloro-4-(((5)-4-(5-fluoro-2,3-dihydrobenzo[b][l,4]dioxin-6- yl)-2,3-dihydro-lH-inden-l-yl)oxy)-2-((5-(methylsulfonyl)pyridin-3-yl)methoxy)benzyl)-L- serine
- Plates were coated with 1 ⁇ g/mL of human PD-L1 (obtained from R&D) in PBS overnight at 4 °C. The wells were then blocked with PBS containing 2% BSA in PBS (W/V) with 0.05 % TWEEN-20 for 1 hour at 37 °C. The plates were washed 3 times with PBS/0.05% TWEEN-20 and the samples were diluted to 1:5 in dilution medium in the ELISA plates. Human PD-1 and biotin O ⁇ g/mL (ACRO Biosystems) were added and incubated for lhour at 37 °C then washed 3 times with PBS/0.05% TWEEN-20.
- human PD-1 and biotin O ⁇ g/mL ACRO Biosystems
- a second block was added with PBS containing 2% BSA in PBS (W/V)/0.05% TWEEN-20 for 10 min at 37 °C and was washed 3 times with PBS/0.05% TWEEN-20.
- Streptavidin -HRP was added for 1 hour at 37 °C then washed 3 times with PBS/0.05% TWEEN-20.
- TMB substrate was added and reacted for 20 min at 37 °C.
- a stop solution (2 N aqueous H 2 S0 4 ) was added. The absorbance was read at 450 nm using a micro-plate spectrophotometer. The results are shown in Tables 2 and 3.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pain & Pain Management (AREA)
- Rheumatology (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Inorganic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Heterocyclic Compounds That Contain Two Or More Ring Oxygen Atoms (AREA)
- Hydrogenated Pyridines (AREA)
- Pyridine Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
Description
Claims
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201780052652.1A CN109803651B (en) | 2016-06-27 | 2017-06-26 | Immunomodulatory compounds |
| KR1020197002639A KR102401963B1 (en) | 2016-06-27 | 2017-06-26 | Immunomodulatory compounds |
| CA3029256A CA3029256A1 (en) | 2016-06-27 | 2017-06-26 | Immunomodulator compounds |
| AU2017289038A AU2017289038B2 (en) | 2016-06-27 | 2017-06-26 | Immunomodulator compounds |
| JP2018567603A JP7185532B2 (en) | 2016-06-27 | 2017-06-26 | immunomodulatory compounds |
| ES17821019T ES2979332T3 (en) | 2016-06-27 | 2017-06-26 | Immunomodulatory compounds |
| EP17821019.1A EP3474845B1 (en) | 2016-06-27 | 2017-06-26 | Immunomodulator compounds |
| IL263752A IL263752B (en) | 2016-06-27 | 2018-12-17 | Immunomodulator compounds |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662355119P | 2016-06-27 | 2016-06-27 | |
| US62/355,119 | 2016-06-27 | ||
| US201662440100P | 2016-12-29 | 2016-12-29 | |
| US62/440,100 | 2016-12-29 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018005374A1 true WO2018005374A1 (en) | 2018-01-04 |
Family
ID=60786614
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2017/039313 WO2018005374A1 (en) | 2016-06-27 | 2017-06-26 | Immunomodulator compounds |
Country Status (11)
| Country | Link |
|---|---|
| US (3) | US10639284B2 (en) |
| EP (1) | EP3474845B1 (en) |
| JP (1) | JP7185532B2 (en) |
| KR (1) | KR102401963B1 (en) |
| CN (1) | CN109803651B (en) |
| AU (1) | AU2017289038B2 (en) |
| CA (1) | CA3029256A1 (en) |
| ES (1) | ES2979332T3 (en) |
| IL (1) | IL263752B (en) |
| TW (1) | TWI758300B (en) |
| WO (1) | WO2018005374A1 (en) |
Cited By (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019060820A1 (en) | 2017-09-25 | 2019-03-28 | Chemocentryx, Inc. | Combination therapy using a chemokine receptor 2 (ccr2) antagonist and a pd-1/pd-l1 inhibitor |
| CN110092745A (en) * | 2018-01-29 | 2019-08-06 | 广州丹康医药生物有限公司 | A kind of compound and its application containing aromatic ring |
| WO2019165043A2 (en) | 2018-02-22 | 2019-08-29 | Chemocentryx, Inc. | Indane-amines as pd-l1 antagonists |
| WO2019169123A1 (en) * | 2018-03-01 | 2019-09-06 | Bristol-Myers Squibb Company | Compounds useful as immunomodulators |
| WO2019232319A1 (en) | 2018-05-31 | 2019-12-05 | Peloton Therapeutics, Inc. | Compositions and methods for inhibiting cd73 |
| WO2020014643A1 (en) * | 2018-07-13 | 2020-01-16 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| US10710986B2 (en) | 2018-02-13 | 2020-07-14 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| WO2020192570A1 (en) | 2019-03-22 | 2020-10-01 | 上海再极医药科技有限公司 | Small-molecule inhibitor of pd-1/pd-l1, pharmaceutical composition thereof with pd-l1 antibody, and application of same |
| CN112105353A (en) * | 2018-01-08 | 2020-12-18 | 凯莫森特里克斯股份有限公司 | Methods of treating solid tumors with CCR2 antagonists |
| WO2021007386A1 (en) | 2019-07-10 | 2021-01-14 | Chemocentryx, Inc. | Indanes as pd-l1 inhibitors |
| US10899735B2 (en) | 2018-04-19 | 2021-01-26 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| CN112601526A (en) * | 2018-08-29 | 2021-04-02 | 凯莫森特里克斯股份有限公司 | Combination therapy using C-C chemokine receptor 4(CCR4) antagonists and one or more checkpoint inhibitors |
| WO2021076691A1 (en) | 2019-10-16 | 2021-04-22 | Chemocentryx, Inc. | Heteroaryl-biphenyl amides for the treatment of pd-l1 diseases |
| WO2021076688A1 (en) | 2019-10-16 | 2021-04-22 | Chemocentryx, Inc. | Heteroaryl-biphenyl amines for the treatment of pd-l1 diseases |
| US11130740B2 (en) | 2017-04-25 | 2021-09-28 | Arbutus Biopharma Corporation | Substituted 2,3-dihydro-1H-indene analogs and methods using same |
| US11142521B2 (en) | 2011-12-01 | 2021-10-12 | Chemocentryx, Inc. | Substituted anilines as CCR(4) antagonists |
| WO2021228000A1 (en) | 2020-05-11 | 2021-11-18 | 上海长森药业有限公司 | Preparation of biaryl ring-linked aromatic heterocyclic derivative as immunomodulator and use thereof |
| US11236085B2 (en) | 2018-10-24 | 2022-02-01 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| US11266643B2 (en) | 2019-05-15 | 2022-03-08 | Chemocentryx, Inc. | Triaryl compounds for treatment of PD-L1 diseases |
| EP3843711A4 (en) * | 2018-08-29 | 2022-08-03 | ChemoCentryx, Inc. | Methods of treating cancer with small molecule pd-l1 inhibitors |
| US11485708B2 (en) | 2019-06-20 | 2022-11-01 | Chemocentryx, Inc. | Compounds for treatment of PD-L1 diseases |
| JP2022547200A (en) * | 2019-09-09 | 2022-11-10 | シャンハイ ロングウッド バイオファルマシューティカルズ カンパニー リミテッド | Aromatic heterocyclic compound having a tricyclic structure, and its preparation method and application |
| US11986466B2 (en) | 2018-01-08 | 2024-05-21 | Chemocentryx, Inc. | Methods of treating solid tumors with CCR2 antagonists |
| US12054484B2 (en) | 2015-05-21 | 2024-08-06 | Chemocentryx, Inc. | Substituted tetrahydropyrans as CCR2 modulators |
| US12083118B2 (en) | 2018-03-29 | 2024-09-10 | Arbutus Biopharma Corporation | Substituted 1,1′-biphenyl compounds, analogues thereof, and methods using same |
| US12116417B2 (en) | 2017-11-14 | 2024-10-15 | GC Cell Corporation | Anti-HER2 antibody or antigen-binding fragment thereof, and chimeric antigen receptor comprising same |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7185532B2 (en) * | 2016-06-27 | 2022-12-07 | ケモセントリックス,インコーポレイティド | immunomodulatory compounds |
| KR102647257B1 (en) * | 2017-07-28 | 2024-03-13 | 케모센트릭스, 인크. | immunomodulator compounds |
| US10392405B2 (en) | 2017-08-08 | 2019-08-27 | Chemocentryx, Inc. | Macrocyclic immunomodulators |
| CN110128415B (en) * | 2019-05-31 | 2022-03-25 | 沈阳药科大学 | Indoline compounds used as immunomodulators and preparation method thereof |
| CA3194742A1 (en) | 2020-09-10 | 2022-03-17 | Nammi Therapeutics, Inc. | Formulated and/or co-formulated liposome compositions containing pd-1 antagonist prodrugs useful in the treatment of cancer and methods thereof |
| CN114437051A (en) * | 2021-09-18 | 2022-05-06 | 药康众拓(江苏)医药科技有限公司 | Biphenyl compound, preparation method and application thereof |
Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1268002A (en) | 1917-09-12 | 1918-05-28 | William M Goodwin | Measuring instrument. |
| US4166452A (en) | 1976-05-03 | 1979-09-04 | Generales Constantine D J Jr | Apparatus for testing human responses to stimuli |
| US4256108A (en) | 1977-04-07 | 1981-03-17 | Alza Corporation | Microporous-semipermeable laminated osmotic system |
| US4265874A (en) | 1980-04-25 | 1981-05-05 | Alza Corporation | Method of delivering drug with aid of effervescent activity generated in environment of use |
| US20100292227A1 (en) * | 2009-05-15 | 2010-11-18 | Boehringer Ingleheim International Gmbh | Inhibitors of human immunodeficiency virus replication |
| WO2015034820A1 (en) | 2013-09-04 | 2015-03-12 | Bristol-Myers Squibb Company | Compounds useful as immunomodulators |
| WO2015033299A1 (en) | 2013-09-06 | 2015-03-12 | Aurigene Discovery Technologies Limited | 1,2,4-oxadiazole derivatives as immunomodulators |
| WO2015033301A1 (en) | 2013-09-06 | 2015-03-12 | Aurigene Discovery Technologies Limited | 1,3,4-oxadiazole and 1,3,4-thiadiazole derivatives as immunomodulators |
| WO2015160641A2 (en) | 2014-04-14 | 2015-10-22 | Bristol-Myers Squibb Company | Compounds useful as immunomodulators |
| WO2017066227A1 (en) | 2015-10-15 | 2017-04-20 | Bristol-Myers Squibb Company | Compounds useful as immunomodulators |
| WO2017070089A1 (en) | 2015-10-19 | 2017-04-27 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US20170145025A1 (en) | 2015-11-19 | 2017-05-25 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2017106634A1 (en) | 2015-12-17 | 2017-06-22 | Incyte Corporation | N-phenyl-pyridine-2-carboxamide derivatives and their use as pd-1/pd-l1 protein/protein interaction modulators |
Family Cites Families (75)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IL120266A (en) | 1996-02-28 | 2005-05-17 | Pfizer | Use of estrogen antagonists and estrogen agonists in the preparation of medicaments for inhibiting pathological conditions |
| RU2456273C2 (en) | 2006-03-31 | 2012-07-20 | Новартис Аг | New compounds |
| WO2008008059A1 (en) | 2006-07-12 | 2008-01-17 | Locus Pharmaceuticals, Inc. | Anti-cancer agents ans uses thereof |
| US20080194557A1 (en) | 2007-01-18 | 2008-08-14 | Joseph Barbosa | Methods and compositions for the treatment of pain, inflammation and cancer |
| EP2185539A4 (en) * | 2007-08-03 | 2011-07-20 | Boehringer Ingelheim Int | Viral polymerase inhibitors |
| EP2368876A1 (en) * | 2010-03-01 | 2011-09-28 | Sanofi | Derivatives of aminoindanes, their preparation and their application in therapeutics |
| TW201329025A (en) * | 2011-11-01 | 2013-07-16 | Astex Therapeutics Ltd | Pharmaceutical compound |
| US9308236B2 (en) | 2013-03-15 | 2016-04-12 | Bristol-Myers Squibb Company | Macrocyclic inhibitors of the PD-1/PD-L1 and CD80(B7-1)/PD-L1 protein/protein interactions |
| KR20160036568A (en) * | 2013-07-18 | 2016-04-04 | 노파르티스 아게 | Aminomethyl-biaryl derivatives as complement factor d inhibitors and uses thereof |
| WO2016088082A1 (en) | 2014-12-05 | 2016-06-09 | Novartis Ag | Amidomethyl-biaryl derivatives complement factor d inhibitors and uses thereof |
| SG10202005790VA (en) | 2015-12-22 | 2020-07-29 | Incyte Corp | Heterocyclic compounds as immunomodulators |
| EP3190103A1 (en) | 2016-01-08 | 2017-07-12 | Rijksuniversiteit Groningen | Inhibitors of the pd-1/pd-l1 protein/protein interaction |
| RU2745195C2 (en) | 2016-04-07 | 2021-03-22 | Кемосентрикс, Инк. | Reduction of tumor mass by injecting ccr1 antagonists in combination with pd-1 inhibitors or pd-l1 inhibitors |
| AR108396A1 (en) | 2016-05-06 | 2018-08-15 | Incyte Corp | HETEROCYCLIC COMPOUNDS AS IMMUNOMODULATORS |
| KR102364344B1 (en) | 2016-05-23 | 2022-02-18 | 인스티튜드 오브 머테리아 메디카, 차이니스 아케데미 오브 메디컬 싸이언시스 | Nicotinyl alcohol ether derivatives, methods for their preparation, and pharmaceutical compositions and uses thereof |
| TW201808902A (en) | 2016-05-26 | 2018-03-16 | 美商英塞特公司 | Heterocyclic compounds as immunomodulators |
| CN109890819B (en) | 2016-06-20 | 2022-11-22 | 因赛特公司 | Heterocyclic compounds as immunomodulators |
| JP7185532B2 (en) | 2016-06-27 | 2022-12-07 | ケモセントリックス,インコーポレイティド | immunomodulatory compounds |
| EP3483142A1 (en) | 2016-07-05 | 2019-05-15 | Guangzhou Maxinovel Pharmaceuticals Co., Ltd. | Aromatic acetylene or aromatic ethylene compound, intermediate, preparation method, pharmaceutical composition and use thereof |
| WO2018009505A1 (en) | 2016-07-08 | 2018-01-11 | Bristol-Myers Squibb Company | 1,3-dihydroxy-phenyl derivatives useful as immunomodulators |
| MA45669A (en) | 2016-07-14 | 2019-05-22 | Incyte Corp | HETEROCYCLIC COMPOUNDS USED AS IMMUNOMODULATORS |
| ES2941716T3 (en) | 2016-08-29 | 2023-05-25 | Incyte Corp | Heterocyclic compounds as immunomodulators |
| US10144706B2 (en) | 2016-09-01 | 2018-12-04 | Bristol-Myers Squibb Company | Compounds useful as immunomodulators |
| US20180065917A1 (en) | 2016-09-02 | 2018-03-08 | Polaris Pharmaceuticals, Inc. | Immune checkpoint inhibitors, compositions and methods thereof |
| US10882844B2 (en) | 2016-12-20 | 2021-01-05 | Bristol-Myers Squibb Company | Compounds useful as immunomodulators |
| KR102696516B1 (en) | 2016-12-22 | 2024-08-22 | 인사이트 코포레이션 | Benzoxazole derivatives as immunomodulators |
| US10806785B2 (en) | 2016-12-22 | 2020-10-20 | Incyte Corporation | Immunomodulator compounds and methods of use |
| ES2874756T3 (en) | 2016-12-22 | 2021-11-05 | Incyte Corp | Triazolo [1,5-A] pyridine derivatives as immunomodulators |
| WO2018119263A1 (en) | 2016-12-22 | 2018-06-28 | Incyte Corporation | Heterocyclic compounds derivatives as pd-l1 internalization inducers |
| ES2899402T3 (en) | 2016-12-22 | 2022-03-11 | Incyte Corp | Pyridine derivatives as immunomodulators |
| JP7303108B2 (en) | 2016-12-22 | 2023-07-04 | インサイト・コーポレイション | Bicyclic heteroaromatic compounds as immunomodulators |
| WO2018121560A1 (en) | 2016-12-29 | 2018-07-05 | 深圳微芯生物科技有限责任公司 | Urea compound and preparation method and application thereof |
| CN108395443B (en) | 2017-02-04 | 2021-05-04 | 广州丹康医药生物有限公司 | Cyclic compounds inhibiting programmed death receptor ligand 1 and uses thereof |
| EP3601258B1 (en) | 2017-03-27 | 2023-08-30 | Bristol-Myers Squibb Company | Substituted isoquionline derivatives as immunomudulators |
| JOP20180040A1 (en) * | 2017-04-20 | 2019-01-30 | Gilead Sciences Inc | Pd-1/pd-l1 inhibitors |
| TW201841896A (en) | 2017-04-26 | 2018-12-01 | 大陸商南京聖和藥業股份有限公司 | Heterocyclic compound serving as pd-l1 inhibitor |
| CN108863963B (en) | 2017-05-08 | 2022-05-27 | 南京圣和药物研发有限公司 | Heterocyclic compounds as PD-L1 inhibitors |
| KR102647257B1 (en) * | 2017-07-28 | 2024-03-13 | 케모센트릭스, 인크. | immunomodulator compounds |
| US10392405B2 (en) | 2017-08-08 | 2019-08-27 | Chemocentryx, Inc. | Macrocyclic immunomodulators |
| WO2019034172A1 (en) | 2017-08-18 | 2019-02-21 | 上海轶诺药业有限公司 | Compound having pd-l1 inhibitory activity, preparation method therefor and use thereof |
| US11492375B2 (en) | 2017-10-03 | 2022-11-08 | Bristol-Myers Squibb Company | Cyclic peptide immunomodulators |
| CN109665968B (en) | 2017-10-16 | 2022-02-22 | 四川科伦博泰生物医药股份有限公司 | Fused ring compound, preparation method and application thereof |
| CN109678796B (en) | 2017-10-19 | 2023-01-10 | 上海长森药业有限公司 | PD-1/PD-L1 small molecule inhibitor and preparation method and application thereof |
| CN109721527B (en) | 2017-10-27 | 2024-03-12 | 广州丹康医药生物有限公司 | Novel anti-PD-L1 compound, application thereof and composition containing same |
| CA3080677A1 (en) | 2017-11-06 | 2019-05-09 | Jubilant Prodel LLC | Pyrimidine derivatives as inhibitors of pd1/pd-l1 activation |
| WO2019120297A1 (en) | 2017-12-22 | 2019-06-27 | 上海海雁医药科技有限公司 | Immunomodulator and preparation method and medical use thereof |
| EP3733659B1 (en) | 2017-12-29 | 2023-12-20 | Guangzhou Maxinovel Pharmaceuticals Co., Ltd. | Aromatic vinyl or aromatic ethyl derivative, preparation method therefor, intermediate, pharmaceutical composition, and application |
| EP3743425B1 (en) | 2018-01-23 | 2024-11-27 | Bristol-Myers Squibb Company | 2,8-diacyl-2,8-diazaspiro[5.5]undecane compounds useful as immunomodulators |
| CN110092745B (en) * | 2018-01-29 | 2022-12-30 | 广州丹康医药生物有限公司 | Compound containing aromatic ring and application thereof |
| WO2019149183A1 (en) | 2018-02-05 | 2019-08-08 | 上海和誉生物医药科技有限公司 | Biaryl derivative, preparation method thereof and pharmaceutical application thereof |
| KR102708681B1 (en) | 2018-02-13 | 2024-09-26 | 길리애드 사이언시즈, 인코포레이티드 | Pd-1/pd-l1 inhibitors |
| EP3755311A4 (en) | 2018-02-22 | 2021-11-10 | ChemoCentryx, Inc. | Indane-amines as pd-l1 antagonists |
| KR102758923B1 (en) * | 2018-03-01 | 2025-01-24 | 브리스톨-마이어스 스큅 컴퍼니 | Compounds useful as immunomodulators |
| WO2019174533A1 (en) | 2018-03-13 | 2019-09-19 | 广东东阳光药业有限公司 | Small molecule pd-1/pd-l1 inhibitor and use thereof in drugs |
| WO2019175897A1 (en) | 2018-03-13 | 2019-09-19 | Jubilant Biosys Limited | Bicyclic compounds as inhibitors of pd1/pd-l1 interaction/activation |
| MX2020010321A (en) | 2018-03-30 | 2021-01-08 | Incyte Corp | Heterocyclic compounds as immunomodulators. |
| WO2019192506A1 (en) | 2018-04-03 | 2019-10-10 | Betta Pharmaceuticals Co., Ltd | Immunomodulators, compositions and methods thereof |
| KR102591947B1 (en) | 2018-04-19 | 2023-10-25 | 길리애드 사이언시즈, 인코포레이티드 | PD-1/PD-L1 inhibitors |
| JP7328995B2 (en) | 2018-05-11 | 2023-08-17 | インサイト・コーポレイション | Tetrahydro-imidazo[4,5-C]pyridine derivatives as PD-L1 immunomodulators |
| CN111886219B (en) | 2018-07-11 | 2023-04-25 | 上海和誉生物医药科技有限公司 | Immunosuppressant, preparation method and pharmaceutical application thereof |
| CN112384500B (en) | 2018-07-12 | 2024-05-14 | 贝达药业股份有限公司 | Immunomodulator, composition and preparation method thereof |
| SG11202012425QA (en) | 2018-07-13 | 2021-01-28 | Gilead Sciences Inc | Pd-1/pd-l1 inhibitors |
| US20210269440A1 (en) | 2018-07-19 | 2021-09-02 | Betta Pharmaceuticals Co., Ltd | Immunomodulators, compositions and methods thereof |
| US20210300909A1 (en) | 2018-07-19 | 2021-09-30 | Betta Pharmaceuticals Co., Ltd | Immunomodulators, compositions and methods thereof |
| WO2020025030A1 (en) | 2018-08-01 | 2020-02-06 | 上海轶诺药业有限公司 | Preparation and application of aromatic compound having immunoregulatory function |
| WO2020047035A1 (en) | 2018-08-29 | 2020-03-05 | Chemocentryx, Inc. | Methods of treating cancer with small molecule pd-l1 inhibitors |
| CN109336857A (en) | 2018-11-13 | 2019-02-15 | 南方医科大学 | A kind of flavonoid containing substituted biphenyl and application thereof |
| CN109438263A (en) | 2018-11-28 | 2019-03-08 | 南方医科大学 | A kind of naphthalene and its application containing substituted biphenyl |
| CN109503546A (en) | 2019-01-10 | 2019-03-22 | 南方医科大学 | A kind of two methyl phenyl ethers anisole of resorcinol and its application |
| CN109776377B (en) | 2019-02-01 | 2021-08-24 | 沈阳药科大学 | Indoline compounds and preparation method and application thereof |
| CN109776445B (en) | 2019-03-28 | 2022-12-06 | 中国药科大学 | Benzoxadiazole compound and its preparation method and medical application |
| SG11202112310TA (en) | 2019-05-15 | 2021-12-30 | Chemocentryx Inc | Triaryl compounds for treatment of pd-l1 diseases |
| CN110200959A (en) | 2019-05-30 | 2019-09-06 | 天津科技大学 | A kind of application of flavone compound |
| CN110128415B (en) | 2019-05-31 | 2022-03-25 | 沈阳药科大学 | Indoline compounds used as immunomodulators and preparation method thereof |
| JP2022539830A (en) | 2019-07-10 | 2022-09-13 | ケモセントリックス,インコーポレイティド | Indane as a PD-L1 inhibitor |
-
2017
- 2017-06-26 JP JP2018567603A patent/JP7185532B2/en active Active
- 2017-06-26 KR KR1020197002639A patent/KR102401963B1/en active Active
- 2017-06-26 AU AU2017289038A patent/AU2017289038B2/en active Active
- 2017-06-26 WO PCT/US2017/039313 patent/WO2018005374A1/en unknown
- 2017-06-26 US US15/633,569 patent/US10639284B2/en active Active
- 2017-06-26 CN CN201780052652.1A patent/CN109803651B/en active Active
- 2017-06-26 CA CA3029256A patent/CA3029256A1/en active Pending
- 2017-06-26 EP EP17821019.1A patent/EP3474845B1/en active Active
- 2017-06-26 ES ES17821019T patent/ES2979332T3/en active Active
- 2017-06-27 TW TW106121437A patent/TWI758300B/en active
-
2018
- 2018-12-17 IL IL263752A patent/IL263752B/en unknown
-
2020
- 2020-01-23 US US16/751,019 patent/US11426364B2/en active Active
-
2022
- 2022-06-13 US US17/838,400 patent/US11793771B2/en active Active
Patent Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1268002A (en) | 1917-09-12 | 1918-05-28 | William M Goodwin | Measuring instrument. |
| US4166452A (en) | 1976-05-03 | 1979-09-04 | Generales Constantine D J Jr | Apparatus for testing human responses to stimuli |
| US4256108A (en) | 1977-04-07 | 1981-03-17 | Alza Corporation | Microporous-semipermeable laminated osmotic system |
| US4265874A (en) | 1980-04-25 | 1981-05-05 | Alza Corporation | Method of delivering drug with aid of effervescent activity generated in environment of use |
| US20100292227A1 (en) * | 2009-05-15 | 2010-11-18 | Boehringer Ingleheim International Gmbh | Inhibitors of human immunodeficiency virus replication |
| WO2015034820A1 (en) | 2013-09-04 | 2015-03-12 | Bristol-Myers Squibb Company | Compounds useful as immunomodulators |
| WO2015033299A1 (en) | 2013-09-06 | 2015-03-12 | Aurigene Discovery Technologies Limited | 1,2,4-oxadiazole derivatives as immunomodulators |
| WO2015033301A1 (en) | 2013-09-06 | 2015-03-12 | Aurigene Discovery Technologies Limited | 1,3,4-oxadiazole and 1,3,4-thiadiazole derivatives as immunomodulators |
| WO2015160641A2 (en) | 2014-04-14 | 2015-10-22 | Bristol-Myers Squibb Company | Compounds useful as immunomodulators |
| WO2017066227A1 (en) | 2015-10-15 | 2017-04-20 | Bristol-Myers Squibb Company | Compounds useful as immunomodulators |
| WO2017070089A1 (en) | 2015-10-19 | 2017-04-27 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US20170145025A1 (en) | 2015-11-19 | 2017-05-25 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2017106634A1 (en) | 2015-12-17 | 2017-06-22 | Incyte Corporation | N-phenyl-pyridine-2-carboxamide derivatives and their use as pd-1/pd-l1 protein/protein interaction modulators |
Non-Patent Citations (4)
| Title |
|---|
| ANALYTICAL CHEMISTRY, vol. 86, no. 5, 2014, pages 2429 - 2435 |
| BERGE, S.M. ET AL.: "Pharmaceutical Salts", JOURNAL OF PHARMACEUTICAL SCIENCE, vol. 66, 1977, pages 1 - 19, XP002675560, DOI: 10.1002/jps.2600660104 |
| HYUN-TAK JIN ET AL., CURR TOP MICROBIOL IMMUNOL, vol. 350, 2011, pages 17 - 37 |
| RD HARVEY, CLINICAL PHARMACOLOGY AND THERAPEUTICS, vol. 96, no. 2, 2014, pages 214 - 223 |
Cited By (65)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11142521B2 (en) | 2011-12-01 | 2021-10-12 | Chemocentryx, Inc. | Substituted anilines as CCR(4) antagonists |
| US11884654B2 (en) | 2011-12-01 | 2024-01-30 | Chemocentryx, Inc. | Substituted anilines as CCR(4) antagonists |
| US12054484B2 (en) | 2015-05-21 | 2024-08-06 | Chemocentryx, Inc. | Substituted tetrahydropyrans as CCR2 modulators |
| US11130740B2 (en) | 2017-04-25 | 2021-09-28 | Arbutus Biopharma Corporation | Substituted 2,3-dihydro-1H-indene analogs and methods using same |
| JP2020535144A (en) * | 2017-09-25 | 2020-12-03 | ケモセントリックス,インコーポレイティド | Combination therapy with chemokine receptor 2 (CCR2) antagonist and PD-1 / PD-L1 inhibitor |
| US11304952B2 (en) | 2017-09-25 | 2022-04-19 | Chemocentryx, Inc. | Combination therapy using a chemokine receptor 2 (CCR2) antagonist and a PD-1/PD-L1 inhibitor |
| WO2019060820A1 (en) | 2017-09-25 | 2019-03-28 | Chemocentryx, Inc. | Combination therapy using a chemokine receptor 2 (ccr2) antagonist and a pd-1/pd-l1 inhibitor |
| JP7453139B2 (en) | 2017-09-25 | 2024-03-19 | ケモセントリックス,インコーポレイティド | Combination therapy using chemokine receptor 2 (CCR2) antagonists and PD-1/PD-L1 inhibitors |
| US12116417B2 (en) | 2017-11-14 | 2024-10-15 | GC Cell Corporation | Anti-HER2 antibody or antigen-binding fragment thereof, and chimeric antigen receptor comprising same |
| CN112105353B (en) * | 2018-01-08 | 2024-04-19 | 凯莫森特里克斯股份有限公司 | Methods of treating solid tumors with CCR2 antagonists |
| CN112105353A (en) * | 2018-01-08 | 2020-12-18 | 凯莫森特里克斯股份有限公司 | Methods of treating solid tumors with CCR2 antagonists |
| US11154556B2 (en) | 2018-01-08 | 2021-10-26 | Chemocentryx, Inc. | Methods of treating solid tumors with CCR2 antagonists |
| US11986466B2 (en) | 2018-01-08 | 2024-05-21 | Chemocentryx, Inc. | Methods of treating solid tumors with CCR2 antagonists |
| CN110092745A (en) * | 2018-01-29 | 2019-08-06 | 广州丹康医药生物有限公司 | A kind of compound and its application containing aromatic ring |
| CN110092745B (en) * | 2018-01-29 | 2022-12-30 | 广州丹康医药生物有限公司 | Compound containing aromatic ring and application thereof |
| US11555029B2 (en) | 2018-02-13 | 2023-01-17 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| US10710986B2 (en) | 2018-02-13 | 2020-07-14 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| WO2019165043A2 (en) | 2018-02-22 | 2019-08-29 | Chemocentryx, Inc. | Indane-amines as pd-l1 antagonists |
| WO2019165043A3 (en) * | 2018-02-22 | 2020-04-30 | Chemocentryx, Inc. | Indane-amines as pd-l1 antagonists |
| US11759458B2 (en) | 2018-02-22 | 2023-09-19 | Chemocentryx, Inc. | Indane-amines as PD-L1 antagonists |
| JP7387616B2 (en) | 2018-02-22 | 2023-11-28 | ケモセントリックス,インコーポレイティド | Indan-amines as PD-L1 antagonists |
| JP2021513996A (en) * | 2018-02-22 | 2021-06-03 | ケモセントリックス,インコーポレイティド | Indane-amine as a PD-L1 antagonist |
| EP3755311A4 (en) * | 2018-02-22 | 2021-11-10 | ChemoCentryx, Inc. | Indane-amines as pd-l1 antagonists |
| WO2019169123A1 (en) * | 2018-03-01 | 2019-09-06 | Bristol-Myers Squibb Company | Compounds useful as immunomodulators |
| JP2021516232A (en) * | 2018-03-01 | 2021-07-01 | ブリストル−マイヤーズ スクイブ カンパニーBristol−Myers Squibb Company | Compounds useful as immunomodulators |
| KR102758923B1 (en) | 2018-03-01 | 2025-01-24 | 브리스톨-마이어스 스큅 컴퍼니 | Compounds useful as immunomodulators |
| JP7326306B2 (en) | 2018-03-01 | 2023-08-15 | ブリストル-マイヤーズ スクイブ カンパニー | Compounds Useful as Immunomodulators |
| US11578054B2 (en) | 2018-03-01 | 2023-02-14 | Bristol-Myers Squibb Company | Compounds useful as immunomodulators |
| CN112041312A (en) * | 2018-03-01 | 2020-12-04 | 百时美施贵宝公司 | Compounds used as immunomodulators |
| KR20200127007A (en) * | 2018-03-01 | 2020-11-09 | 브리스톨-마이어스 스큅 컴퍼니 | Compounds useful as immunomodulators |
| US12083118B2 (en) | 2018-03-29 | 2024-09-10 | Arbutus Biopharma Corporation | Substituted 1,1′-biphenyl compounds, analogues thereof, and methods using same |
| US10899735B2 (en) | 2018-04-19 | 2021-01-26 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| WO2019232319A1 (en) | 2018-05-31 | 2019-12-05 | Peloton Therapeutics, Inc. | Compositions and methods for inhibiting cd73 |
| EP4234030A3 (en) * | 2018-07-13 | 2023-10-18 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| JP2021530500A (en) * | 2018-07-13 | 2021-11-11 | ギリアード サイエンシーズ, インコーポレイテッド | PD-1 / PD-L1 inhibitor |
| JP7105359B2 (en) | 2018-07-13 | 2022-07-22 | ギリアード サイエンシーズ, インコーポレイテッド | PD-1/PD-L1 inhibitor |
| CN112399874B (en) * | 2018-07-13 | 2024-03-22 | 吉利德科学公司 | PD-1/PD-L1 inhibitors |
| KR102625712B1 (en) | 2018-07-13 | 2024-01-19 | 길리애드 사이언시즈, 인코포레이티드 | PD-1/PD-L1 inhibitors |
| WO2020014643A1 (en) * | 2018-07-13 | 2020-01-16 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| US10774071B2 (en) | 2018-07-13 | 2020-09-15 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| KR20210031936A (en) * | 2018-07-13 | 2021-03-23 | 길리애드 사이언시즈, 인코포레이티드 | PD-1/PD-L1 inhibitor |
| AU2019301811B2 (en) * | 2018-07-13 | 2022-05-26 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| CN112399874A (en) * | 2018-07-13 | 2021-02-23 | 吉利德科学公司 | PD-1/PD-L1 inhibitors |
| IL280831B1 (en) * | 2018-08-29 | 2024-01-01 | Chemocentryx Inc | Combination therapy using c-c chemokine receptor 4 (ccr4) antagonists and one or more immune checkpoint inhibitors |
| JP2021536442A (en) * | 2018-08-29 | 2021-12-27 | ケモセントリックス,インコーポレイティド | Combination therapy with CC chemokine receptor 4 (CCR4) antagonists and one or more checkpoint inhibitors |
| US12011439B2 (en) | 2018-08-29 | 2024-06-18 | Chemocentryx, Inc. | Combination therapy using C—C chemokine receptor 4 (CCR4) antagonists and one or more immune checkpoint inhibitors |
| JP7554180B2 (en) | 2018-08-29 | 2024-09-19 | ケモセントリックス,インコーポレイティド | Combination therapy using a C-C chemokine receptor 4 (CCR4) antagonist and one or more checkpoint inhibitors |
| CN112601526A (en) * | 2018-08-29 | 2021-04-02 | 凯莫森特里克斯股份有限公司 | Combination therapy using C-C chemokine receptor 4(CCR4) antagonists and one or more checkpoint inhibitors |
| IL280831B2 (en) * | 2018-08-29 | 2024-05-01 | Chemocentryx Inc | Combination therapy with C-C antagonists such as cytokine receptor 4 (CCR4) and one or more immune checkpoint inhibitors |
| EP3843734A4 (en) * | 2018-08-29 | 2022-05-25 | ChemoCentryx, Inc. | Combination therapy using c-c chemokine receptor 4 (ccr4) antagonists and one or more immune checkpoint inhibitors |
| US11446289B2 (en) | 2018-08-29 | 2022-09-20 | Chemocentryx, Inc. | Combination therapy using C-C chemokine receptor 4 (CCR4) antagonists and one or more immune checkpoint inhibitors |
| EP3843711A4 (en) * | 2018-08-29 | 2022-08-03 | ChemoCentryx, Inc. | Methods of treating cancer with small molecule pd-l1 inhibitors |
| US11236085B2 (en) | 2018-10-24 | 2022-02-01 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| WO2020192570A1 (en) | 2019-03-22 | 2020-10-01 | 上海再极医药科技有限公司 | Small-molecule inhibitor of pd-1/pd-l1, pharmaceutical composition thereof with pd-l1 antibody, and application of same |
| US11266643B2 (en) | 2019-05-15 | 2022-03-08 | Chemocentryx, Inc. | Triaryl compounds for treatment of PD-L1 diseases |
| EP3986392A4 (en) * | 2019-06-20 | 2023-07-12 | ChemoCentryx, Inc. | Compounds for treatment of pd-l1 diseases |
| US11485708B2 (en) | 2019-06-20 | 2022-11-01 | Chemocentryx, Inc. | Compounds for treatment of PD-L1 diseases |
| US11872217B2 (en) | 2019-07-10 | 2024-01-16 | Chemocentryx, Inc. | Indanes as PD-L1 inhibitors |
| WO2021007386A1 (en) | 2019-07-10 | 2021-01-14 | Chemocentryx, Inc. | Indanes as pd-l1 inhibitors |
| JP2022547200A (en) * | 2019-09-09 | 2022-11-10 | シャンハイ ロングウッド バイオファルマシューティカルズ カンパニー リミテッド | Aromatic heterocyclic compound having a tricyclic structure, and its preparation method and application |
| WO2021076691A1 (en) | 2019-10-16 | 2021-04-22 | Chemocentryx, Inc. | Heteroaryl-biphenyl amides for the treatment of pd-l1 diseases |
| US11866429B2 (en) | 2019-10-16 | 2024-01-09 | Chemocentryx, Inc. | Heteroaryl-biphenyl amines for the treatment of PD-L1 diseases |
| WO2021076688A1 (en) | 2019-10-16 | 2021-04-22 | Chemocentryx, Inc. | Heteroaryl-biphenyl amines for the treatment of pd-l1 diseases |
| US11713307B2 (en) | 2019-10-16 | 2023-08-01 | Chemocentryx, Inc. | Heteroaryl-biphenyl amides for the treatment of PD-L1 diseases |
| WO2021228000A1 (en) | 2020-05-11 | 2021-11-18 | 上海长森药业有限公司 | Preparation of biaryl ring-linked aromatic heterocyclic derivative as immunomodulator and use thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| JP7185532B2 (en) | 2022-12-07 |
| AU2017289038B2 (en) | 2021-07-22 |
| JP2019527202A (en) | 2019-09-26 |
| US20180008554A1 (en) | 2018-01-11 |
| ES2979332T3 (en) | 2024-09-25 |
| CN109803651A (en) | 2019-05-24 |
| IL263752B (en) | 2022-04-01 |
| KR102401963B1 (en) | 2022-05-25 |
| US20230117425A1 (en) | 2023-04-20 |
| IL263752A (en) | 2019-01-31 |
| KR20190053836A (en) | 2019-05-20 |
| US11426364B2 (en) | 2022-08-30 |
| CN109803651B (en) | 2022-05-31 |
| EP3474845A1 (en) | 2019-05-01 |
| CA3029256A1 (en) | 2018-01-04 |
| TW201803844A (en) | 2018-02-01 |
| US11793771B2 (en) | 2023-10-24 |
| EP3474845B1 (en) | 2024-03-20 |
| TWI758300B (en) | 2022-03-21 |
| AU2017289038A1 (en) | 2019-02-07 |
| US20210052514A1 (en) | 2021-02-25 |
| US10639284B2 (en) | 2020-05-05 |
| EP3474845A4 (en) | 2020-02-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2017289038B2 (en) | Immunomodulator compounds | |
| AU2018313744B2 (en) | Macrocyclic immunomodulators | |
| AU2018306619B2 (en) | Immunomodulator compounds | |
| US11266643B2 (en) | Triaryl compounds for treatment of PD-L1 diseases | |
| US11759458B2 (en) | Indane-amines as PD-L1 antagonists | |
| US11713307B2 (en) | Heteroaryl-biphenyl amides for the treatment of PD-L1 diseases | |
| AU2020294781A1 (en) | Compounds for treatment of PD-L1 diseases |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17821019 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 3029256 Country of ref document: CA |
|
| ENP | Entry into the national phase |
Ref document number: 2018567603 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20197002639 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2017289038 Country of ref document: AU Date of ref document: 20170626 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2017821019 Country of ref document: EP Effective date: 20190128 |