WO2009070597A2 - Fusion proteins for delivery of gdnf to the cns - Google Patents
Fusion proteins for delivery of gdnf to the cns Download PDFInfo
- Publication number
- WO2009070597A2 WO2009070597A2 PCT/US2008/084718 US2008084718W WO2009070597A2 WO 2009070597 A2 WO2009070597 A2 WO 2009070597A2 US 2008084718 W US2008084718 W US 2008084718W WO 2009070597 A2 WO2009070597 A2 WO 2009070597A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- gdnf
- fusion protein
- bbb
- brain
- bdnf
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/475—Growth factors; Growth regulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/6811—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being a protein or peptide, e.g. transferrin or bleomycin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6847—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a hormone or a hormone-releasing or -inhibiting factor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/02—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by the carrier, i.e. characterised by the agent or material covalently linked or complexing the radioactive nucleus
- A61K51/04—Organic compounds
- A61K51/08—Peptides, e.g. proteins, carriers being peptides, polyamino acids, proteins
- A61K51/088—Peptides, e.g. proteins, carriers being peptides, polyamino acids, proteins conjugates with carriers being peptides, polyamino acids or proteins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/02—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by the carrier, i.e. characterised by the agent or material covalently linked or complexing the radioactive nucleus
- A61K51/04—Organic compounds
- A61K51/08—Peptides, e.g. proteins, carriers being peptides, polyamino acids, proteins
- A61K51/10—Antibodies or immunoglobulins; Fragments thereof, the carrier being an antibody, an immunoglobulin or a fragment thereof, e.g. a camelised human single domain antibody or the Fc fragment of an antibody
- A61K51/1024—Antibodies or immunoglobulins; Fragments thereof, the carrier being an antibody, an immunoglobulin or a fragment thereof, e.g. a camelised human single domain antibody or the Fc fragment of an antibody against hormones, hormone-releasing or hormone-inhibiting factors
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2869—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against hormone receptors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/33—Fusion polypeptide fusions for targeting to specific cell types, e.g. tissue specific targeting, targeting of a bacterial subspecies
Definitions
- the present invention represents an advance in providing accessibility of the CNS for molecular entities whose ability to cross the blood brain barrier is limited.
- the invention provides a composition comprising a neurotherapeutic peptide comprising an amino acid sequence which is at least 80% (e.g., 95%) identical to the amino acid sequence of mature human GDNF (SEQ ID NO: 52) covalently linked to a structure that is capable of crossing the blood brain barrier (BBB) (e.g., an antibody).
- BBB blood brain barrier
- the structure that is capable of crossing the BBB crosses the BBB on an endogenous BBB receptor.
- the endogenous BBB receptor is the insulin receptor, transferrin receptor, leptin receptor, lipoprotein receptor, and the IGF receptor.
- the structure that is capable of crossing the BBB is a monoclonal antibody.
- the monoclonal antibody is a chimeric monoclonal antibody.
- the chimeric antibody contains sufficient human sequences to avoid significant immunogenic reaction when administered to a human.
- the above-mentioned neurotherapeutic peptide is covalently linked at its amino terminus to the carboxy terminus of the heavy chain of the MAb.
- the neurotherapeutic peptide of the above-mentioned composition comprises the amino acid sequence of mature human GDNF.
- compositions for treating a neurological disorder comprising a neurotherapeutic peptide comprising an amino acid sequence which is at least 80% identical to the amino acid sequence of mature human GDNF covalently linked to an immunoglobulin that is capable of crossing the blood brain barrier, wherein the composition is capable of crossing the BBB in an amount that is effective in treating the neurological disorder.
- a mammalian cell comprising the just-mentioned composition.
- a neurotherapeutic peptide comprising an amino acid sequence which is at least 80% identical to the amino acid sequence of mature human GDNF (SEQ ID NO: 52) from the peripheral circulation across the BBB in an effective amount, comprising peripherally administering to an individual the GDNF covalently attached to a structure that crosses the BBB, under conditions where the agent covalently attached to a structure that crosses the BBB is transported across the BBB in an effective amount.
- a method for treating a CNS disorder in an individual comprising peripherally administering to the individual an effective amount of a composition comprising a neurotherapeutic peptide comprising an amino acid sequence which is at least 80% (e.g., 95%) identical to the amino acid sequence of human GDNF covalently attached to a structure capable of crossing the BBB.
- the neurotherapeutic peptide comprises the amino acid sequence of human GDNF.
- the administering is oral, intravenous, intramuscular, subcutaneous, intraperitoneal, rectal, transbuccal, intranasal, transdermal, or inhalation administration.
- the CNS disorder is an acute CNS disorder (e.g., spinal cord injury, brain injury focal brain ischemia and global brain ischemia), hi other embodiments, the CNS disorder is a chronic CNS disorder (e.g., a chronic neurodegenerative disease, drug addiction, or alcohol addiction). In some embodiments, the chronic neurodegenerative disease is Parkinson's disease or a motor neuron disease (e.g., amyotrophic lateral sclerosis). In some embodiments, the individual to be treated is administered about 1 to about 100 mg of the composition used in above-mentioned method.
- acute CNS disorder e.g., spinal cord injury, brain injury focal brain ischemia and global brain ischemia
- the CNS disorder is a chronic CNS disorder (e.g., a chronic neurodegenerative disease, drug addiction, or alcohol addiction).
- the chronic neurodegenerative disease is Parkinson's disease or a motor neuron disease (e.g., amyotrophic lateral sclerosis).
- the individual to be treated is administered about 1 to
- composition comprising a neurotherapeutic peptide comprising an amino acid sequence which is at least 80% identical to the amino acid sequence of human GDNF covalently linked to an immunoglobulin, wherein the neurotherapeutic peptide has a serum half-life that is an average of at least about 5 -fold greater than the serum half- life of the neurotherapeutic peptide alone, hi some embodiments, the immunoglobulin is an antibody to an endogenous BBB receptor (e.g., the insulin receptor, transferrin receptor, leptin receptor, lipoprotein receptor, or the IGF receptor).
- the neurotherapeutic peptide comprises the amino acid sequence of human GDNF.
- a method for treating substance abuse in an individual comprising administering to the individual an effective amount of a composition comprising a neurotherapeutic peptide comprising an amino acid sequence which is at least 80% identical to the amino acid sequence of mature human GDNF (SEQ ID NO: 52) covalently attached to a structure capable of crossing the BBB.
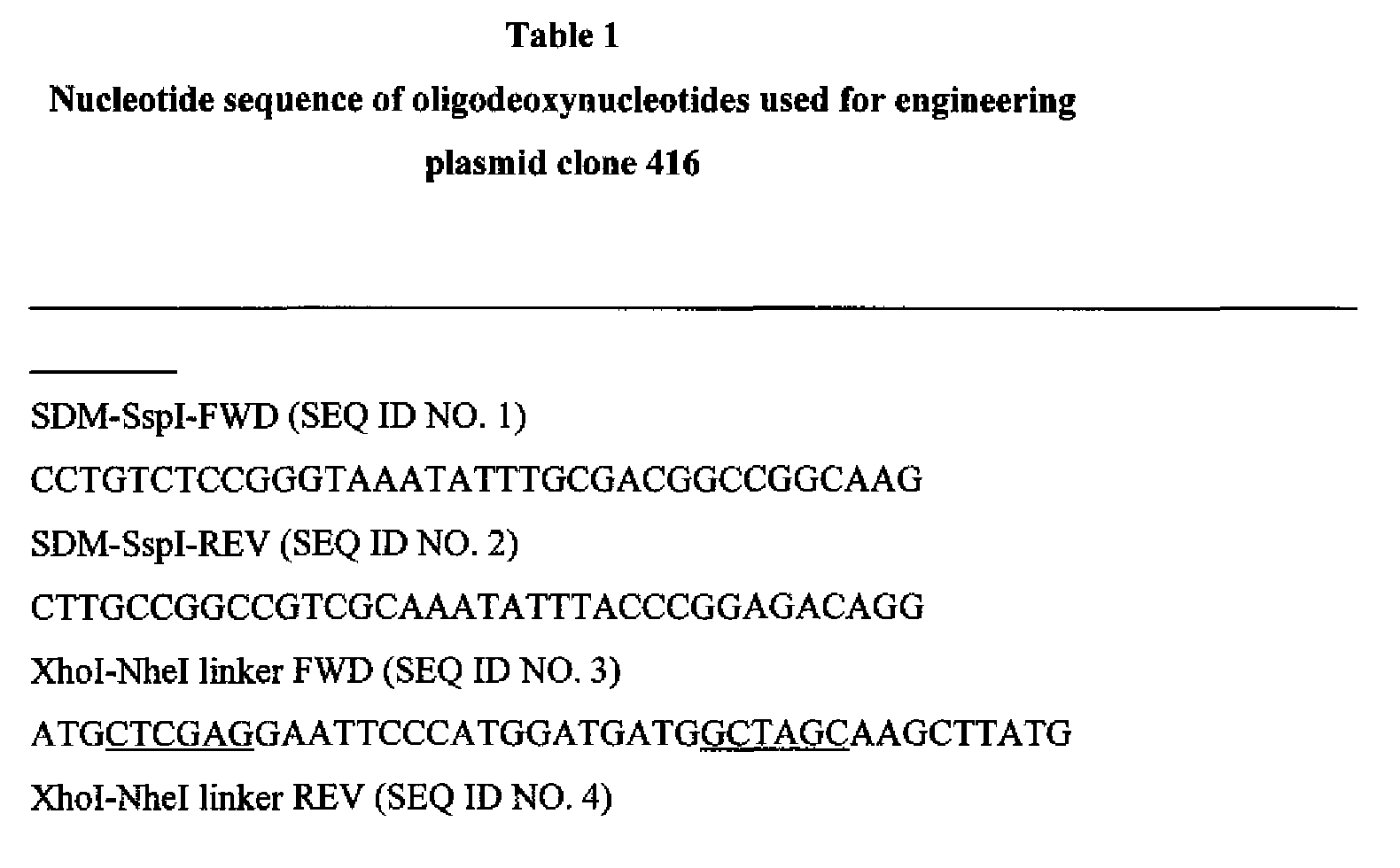
- Figure 1 Diagram showing genetic engineering of a eukaryotic expression vector encoding a fusion gene comprised of the variable region of the heavy chain (VH) of the chimeric HIRMAb, a genomic fragment encoding the constant region of human IgGl, which is comprised of 4 regions (CHl, hinge, CH2, and CH3), and the cDNA for the BDNF variant (vBDNF). Transcription of the gene is driven by the human IgGl promoter (PRO). This vector produces the heavy chain (HC) of the fusion protein.
- VH variable region of the heavy chain
- vBDNF cDNA for the BDNF variant
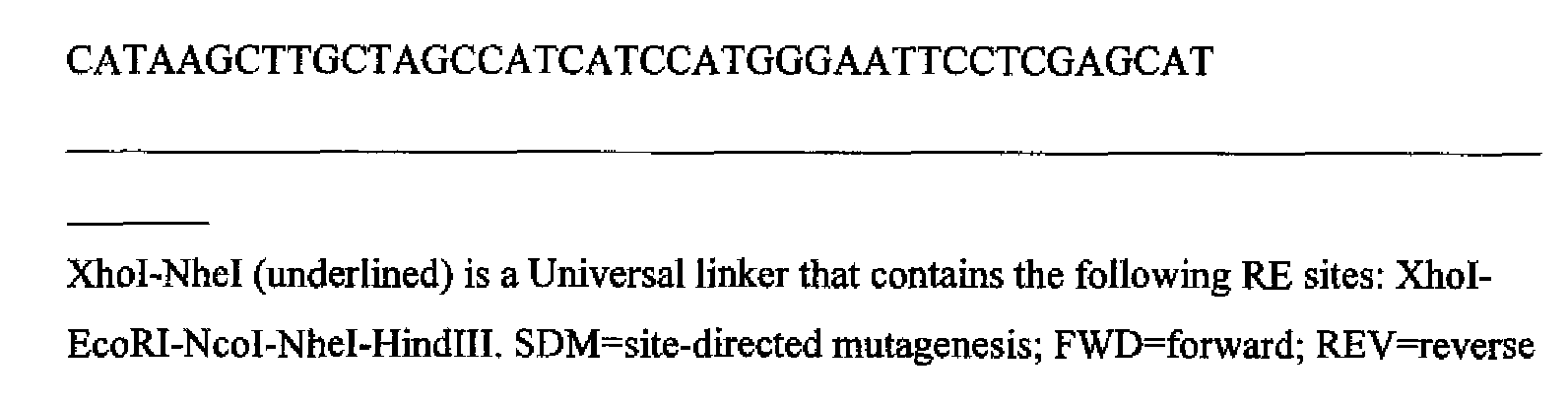
- Figure 2 Diagram showing genetic engineering of a bacterial expression plasmid encoding vBDNF cDNA with modified 5 1 - and 3'-linkers.
- Figure 3 Ethidium bromide stained agarose gels showing size of various constructs that are intermediates in construction of a tandem vector that produces the fusion protein.
- Lanes 1-2 plasmid from figure 2 digested with Nrul showing 0.4 kb vBDNF and 3.5 kb vector backbone.
- Lane 3 MW size standards ranging from 1.4-0.1 kb.
- Lane 4 MW size standards ranging from 23-0.6 kb.
- Lane 1 the 0.4 kb vBDNF cDNA is produced by the polymerase chain reaction (PCR) using cDNA reverse transcribed from polyA ⁇ RNA isolated from human U87 glioma cells; the PCR primer sequences are given in Table 2, Lanes 2 and 3: same MW size standards as shown in panel A.
- C lane 1 : clone 416 following digestion with Nhel and BamHI; lane 2: negative clone; lane 3: clone 400 following digestion with Nhel and BamHI: lanes 4 and 5: same MW size standards as shown in panel A.
- the 3-amino acid linker between the HIRMAb HC and the vBDNF is shown, as well as the new stop codon at the carboxyl terminus of vBDNF.
- Figure 5 Nucleotide sequence (SEQ ID NO: 23) of fusion protein HC gene cloned into plasmid 416. Italics: human IgGl constant region introns; bold font: human IgGl exon sequence; underline font: vBDNF.
- FIG. 1 Amino acid sequence (SEQ ID NO: 24) of the fusion protein HC.
- the 19 amino acid signal peptide is underlined, as is the 3-amino acid linker between the CH3 region and the vBDNF.
- the N-linked glycosylation consensus sequence within CH2 is underlined.
- FIG. 7 The amino acid sequence (SEQ ID NO: 25) of the different domains of the fusion protein HC are shown.
- Figure 8 Diagram showing production of the intronless eukaryotic expression vector, clone 422a, which encodes the fusion protein HC.
- the fusion protein HC cDNA was produced by PCR from cDNA generated by reverse transcriptase of RNA isolated from myeloma cells transfected with clone 416.
- Figure 9 (A) Nucleotide sequence (SEQ ID NO: 26) of the fusion protein HC cDNA inserted in clone 422a. (B) (SEQ ID NOS 27 & 28) Amino acid sequence of the fusion protein HC that is deduced from the nucleotide sequence shown in panel A. The sequence of the signal peptide is underlined.
- FIG. 10 Diagram showing production of the intronless eukaryotic expression vector, clone 423 a, which encodes the fusion protein LC.
- the fusion protein LC cDNA was produced by PCR from cDNA generated by reverse transcriptase of RNA isolated from myeloma cells transfected with an expression vector producing the LC gene that was derived from chromosomal fragment encoding intron/exon sequence of the human kappa LC gene with the VL of the chimeric HIRMAb LC.
- FIG. 11 Nucleotide sequence (SEQ ID NO: 29) of the fusion protein LC cDNA inserted in clone 423a.
- B (SEQ ID NOS 30 & 31) Amino acid sequence of the fusion protein LC that is deduced from the nucleotide sequence shown in panel A, The sequence of the signal peptide is underlined.
- Figure 12 Diagram showing the construction of a tandem vector encoding the HC
- the TV was engineered from the cDNA expression vectors, clones 422a and 423 a, for the HC and LC, respectively, as well as from a bacterial expression plasmid encoding the expression cassette for mouse DHFR.
- Figures 13A and 13B Nucleotide sequence (SEQ ID NO: 32) of the fusion protein HC gene and LC gene, and the DHFR genes incorporated in the tandem vector.
- Figure 14 Deduced amino acid sequence of the fusion protein HC based on tandem vector nucleotide sequence analysis (SEQ ID NOS 33 & 34). The signal peptide sequence is underlined.
- Figure 15 Deduced amino acid sequence of the fusion protein LC based on tandem vector nucleotide sequence analysis (SEQ ID NO 35 & 36). The signal peptide sequence is underlined.
- Figure 16 Deduced amino acid sequence of the DHFR based on tandem vector nucleotide sequence analysis (SEQ ID NO 37 & 38).
- Figure 17 Viable and total cell density of CHO cells in bioreactor maintained continuously for 50 days; the CHO cells had been stably transfected with the tandem vector encoding the fusion protein.
- Figure 18 Structure of fusion protein, a bi-functional molecule that both (a) binds to the human BBB human insulin receptor (HIR) to enable transport across the BBB from blood, and (b) binds to the trkB on neurons to induce neuroprotection.
- HIR human BBB human insulin receptor
- Figure 19 Correlation of 2 different 'sandwich 1 immunoassays, where the secondary antibody is either directed against the Fc region of human IgGl (x-axis) or against human BDNF (y-axis). The primary antibody in either assay is directed against the human kappa light chain. The measured level of fusion protein in CHO cell conditioned medium is the same whether the anti-Fc or the anti-BDNF antibody is used.
- Figure 20 Reducing (left) and non-reducing (right) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of chimeric HIRMAb and fusion protein.
- SDS-PAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis
- the size of the light chain, 30 kDa is identical for chimeric HIRMAb and the fusion protein; the size of the heavy chain of fusion protein is about 15 kDa larger than the chimeric HIRMAb heavy chain, owing to the presence of the BDNF.
- the chimeric HIRMAb and the fusion protein migrate as single hetero-tetrameric species with molecular weights of 180 and 200 kDa, respectively.
- Figure 21 (Left panel) Western blot with anti-human IgG primary antibody.
- the size of the heavy chain of the fusion protein and the chimeric HIRMAb is 64 kDa and 50 kDa, respectively, and the size of the light chain for either the fusion protein or the chimeric HIRMAb is 25 kDa.
- MW standards STDS
- FIG. 22 Isoelectric focusing (IEF) of isoelectric point (pi) standards (lane 1), chimeric
- HIRMAb (lanes 2 and 4), BDNF (lane 3), and fusion protein (lane 5).
- BDNF is highly cationic with a pI>10
- the pi of the fusion protein approximates the pi of the chimeric HIRMAb, which is about 8.5, and close to the theoretical pi of the fusion protein.
- FIG. 23 (A) Outline for human insulin receptor (HER) competitive ligand binding assay
- FIG. 24 (A) Design of trkB competitive ligand binding assay (CLBA).
- CLBA trkB competitive ligand binding assay
- the advantage of the PEG linker is that this modification eliminates the high non-specific binding (NSB) of the cationic BDNF to the ELISA wells, which gives an assay with a high signal/noise ratio.
- the binding of the BDNF-PEG 20C0 -biotin to the trkB ECD was detected with a peroxidase system using avidin and biotinylated peroxidase.
- B The binding of the BDNF-PEG 2000 -biotin to the trkB ECD is competitively displaced by recombinant BDNF.
- FIG. 25 (A) Design of hypoxia-reoxygenation neuroprotection assay in human neural
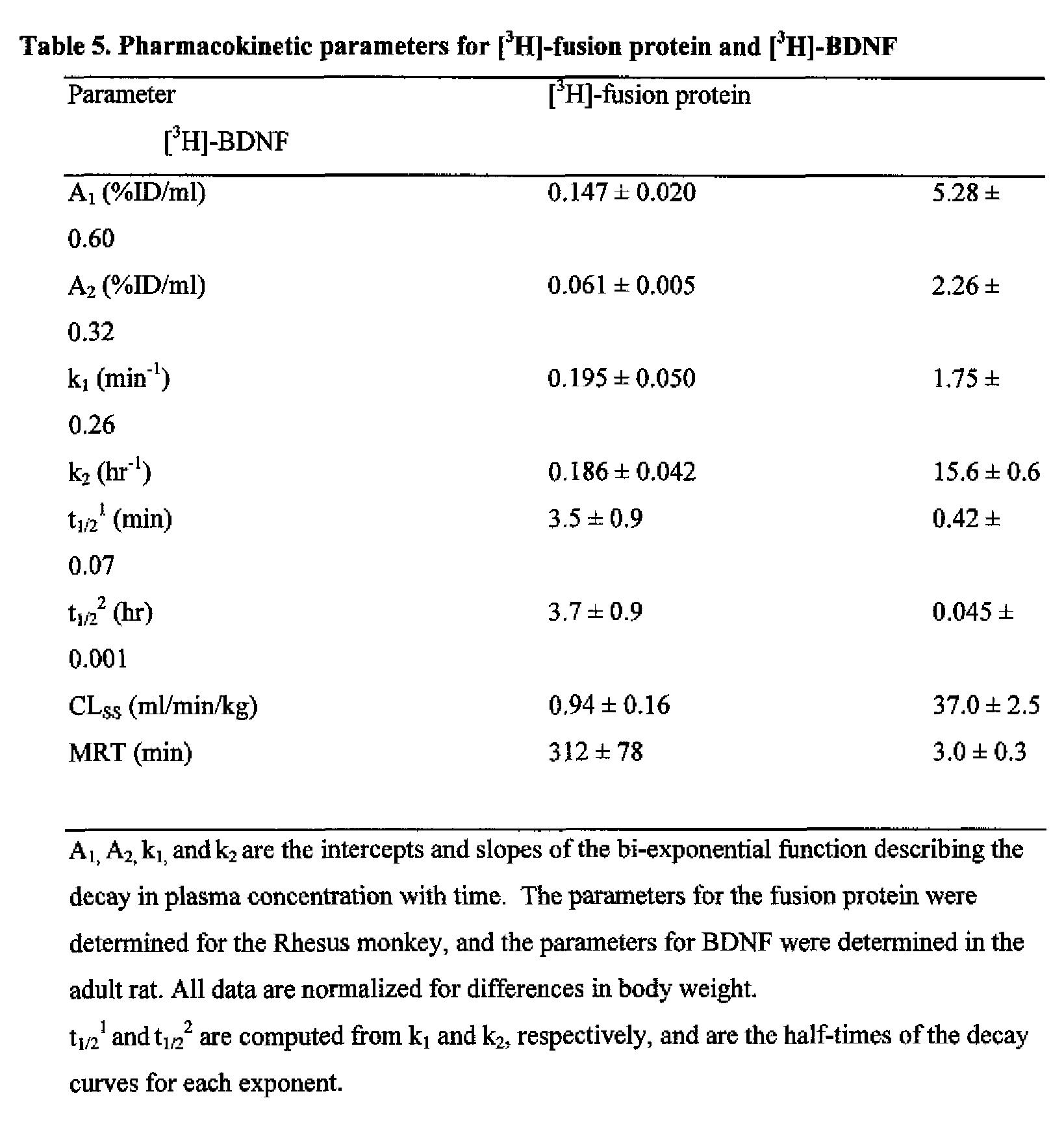
- FIG. 27 Pharmacokinetics and brain uptake of fusion protein in the adult Rhesus monkey.
- A The serum concentration of [ 3 H]-fusion protein, or [ Iz5 l]-murine HIRMAb, is plotted vs. time after a single intravenous injection of either protein in anesthetized adult Rhesus monkeys.
- B The serum radioactivity that is precipitable by trichloroacetic acid (TCA) is plotted vs time after a single intravenous injection of either [ 3 H]-fusion protein in the anesthetized adult Rhesus monkey, or [ 3 H]-BDNF in the anesthetized adult rat.
- TCA trichloroacetic acid
- C Capillary depletion analysis of brain distribution at 180 minutes after a single intravenous injection of either [ 3 H]-fusion protein, or [ 3 H]-mouse IgG2a, in the anesthetized adult Rhesus monkey.
- D Primate brain concentrations of fusion protein at 180 minutes after an intravenous injection of 373 ⁇ g fusion protein, as compared to the endogenous primate brain concentration of BDNF.
- FIG. 28 Schematic illustration of the protein formed by fusion of the amino terminus of the mature GDNF to the carboxyl terminus of the CH3 region of the heavy chain of the chimeric HIRMAb.
- the fusion protein is a bi-functional molecule: the fusion protein binds the HIR, at the BBB, to mediate transport into the brain, and binds the GFR ⁇ l, to mediate GDNF biologic effects in brain behind the BBB.
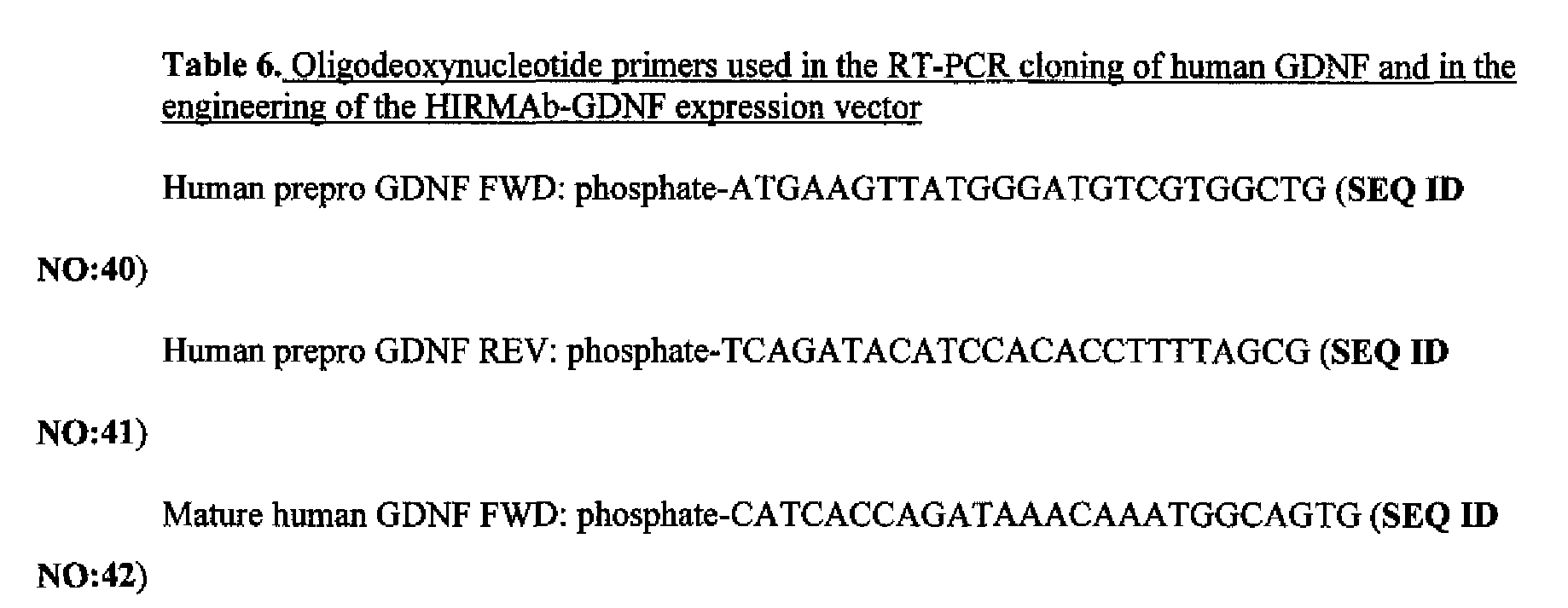
- FIG. 29 Ethidium bromide stain of agarose gel of human GDNF cDNA (lane 1), which was produced by PCR from cDNA produced by reverse transcription of RNA from human U87 glial cells, and GDNF-specific ODN primers (Table 6). Lane 2: DNA sizing standards.
- B Genetic engineering of pHIRMAb-GDNF, the eukaryotic expression plasmid encoding the fusion protein of GDNF and the heavy chain (HC) of the chimeric HIRMAb. The fusion gene is 5'- flanked by the cytomegalovirus (CMV) promoter and 3 '-flanked by the bovine growth hormone polyA (pA) sequence.
- CMV cytomegalovirus
- pA bovine growth hormone polyA
- Figure 30 Reducing SDS-PAGE and Coomasie blue staining of protein A affinity purified, COS-cell chimeric HIRMAb and the HIRMAb-GDNF fusion protein. Both are purified to homogeneity and are comprised of a heavy chain and a light chain.
- the molecular weight (MW) of the heavy chain (HC) of the HIRMAb-GDNF fusion protein is about 15 kDa larger than the MW of the HC of the chimeric HIRMAb, owing to fusion of the 15 kDa GDNF.
- FIG. 31 Western blot with either anti-human (h) IgG primary antibody (left) or an anti- human GDNF primary antiserum (right).
- the immunoreactivity of the COS cell derived HIRMAb- GDNF fusion protein is compared to the chimeric HIRMAb and to recombinant GDNF.
- Both the HIRMAb-GDNF fusion protein and the HIRMAb have identical light chains on the anti-hlgG Western.
- the HIRMAb-GDNF fusion heavy chain reacts with both the anti-hlgG and the anti- human GDNF antibody, whereas the HIRMAb heavy chain only reacts with the anti-hlgG antibody.
- the size of the HIRMAb-GDNF fusion heavy chain, 70 kDa is about 15 kDa larger than the size of the heavy chain of the HlRMAb, owing to the fusion of the 15 kDa GDNF to the 55 kDa HIRMAb heavy chain.
- Figure 32 Binding of either the COS cell-derived chimeric HIRMAb or the HIRMAb-
- GDNF fusion protein to the HIR extracellular domain is saturable.
- the ED50 of HIRMAb- GDNF binding to the HIR ECD is comparable to the ED50 of the binding of the chimeric HIRMAb.
- FIG. 33 (A) Outline of GFR ⁇ l receptor binding assay.
- the GFR ⁇ 1 :Fc fusion protein is captured by a mouse anti-human (MAH) Fc antibody.
- the GDNF, or HIRMAb-GDNF fusion protein binds to the GFR ⁇ l, and this binding is detected with a goat anti-GDNF antibody and a rabbit anti-goat (RAG) antibody conjugated to alkaline phosphatase (AP).
- B Binding of either the COS cell derived chimeric HIRMAb or the HIRMAb-GDNF fusion protein to the GFR ⁇ l extracellular domain (ECD) is saturable.
- the ED50 of HIRMAb-GDNF binding to the GFR ⁇ l ECD is comparable to the ED50 of the binding of recombinant GDNF. There is no binding to the GFR ⁇ l by the chimeric HIRMAb.
- Figure 34 (A) Binding by either GDNF or the HIRMAb-GDNF fusion protein to the
- GFR ⁇ l on the cell membrane of c-ret kinase transfected human neural SK-N-MC cells activates the tyrosine hydroxylase (TH) 5 '-flanking sequence (FS), which drives luciferase gene expression.
- TH tyrosine hydroxylase
- FS 5 '-flanking sequence
- Both GDNF and the COS cell-derived HIRMAb-GDNF fusion protein activate luciferase gene expression in a saturable manner, with ED50 values comparable to GFR ⁇ l binding ( Figure 33B). There is no activation of luciferase gene expression by the chimeric HIRMAb.
- FIG. 35 Effect of intra-cerebral injection of the HIRMAb-GDNF versus saline on stroke volume in rats.
- Serial coronal sections of rat brain at 24 hours after permanent MCAO and intra-cerebral saline (B) or HIRMAb-GDNF fusion protein (C) are stained with 2% 2,3,5- triphenyltetrazolium chloride (TTC).
- TTC 2,3,5- triphenyltetrazolium chloride
- the polypeptide is comprised of a signal peptide, followed by the variable region of the heavy chain (VH) of the chimeric HIRMAb.
- VH variable region of the heavy chain
- the 3 CDRs and 4 FRs are shown.
- the VH region is followed by the human IgGl constant region, which is comprised of the CHl, hinge, CH2, and CH3 domains, followed by a 2-amino acid (Ser-Ser) linker, followed by the mature human GDNF.
- the single N-linked glycosylation site within the CH2 region, and the 2 N- linked glycosylation sites within the GDNF are underlined with the asparagine (N) residues in bold font.
- Cysteine (C) residues which form inter-chain disulfide bonds are shown, and include a linkage between the HC hinge region and the light chain (LC), the HC hinge region and the paired HC, the HC CH2 region and the paired HC, and the GDNF-GDNF linkage.
- LC light chain
- FIG. 37 Tandem vector (TV) encoding multiple genes on a single piece of DNA.
- GDNF cDNA produced by PCR, is subcloned into the Hpal site of the universal tandem vector (UTV) to generate the HIRMAb-GDNF TV, which allows for expression in CHO cells of the fusion heavy chain (HC) gene, the light chain (LC) gene, and the dihydrofolate reductase (DHFR) gene.
- UUV universal tandem vector
- Figure 38 Domain structure of the light chain of the HlRMAb.
- Figure 39 Western blot with a primary antibody against either human IgG (A) or human
- GDNF (B).
- the CHO-derived chimeric HIRMAb and the CHO-derived HIRMAb-GDNF fusion protein are applied in panel A, and the HIRMAb, the HIRMAb-GDNF fusion protein, and GDNF are applied in panel B.
- the migration of molecular weight standards is shown hi lane 1 of panel A.
- Figure 40 The affinity of the CHO-derived HIRMAb and the CHO-derived HIRMAb-
- GDNF fusion protein for binding to the extracellular domain of the human insulin receptor (HIR) is measured in this ELISA format.
- the concentration of antibody that causes 50% saturation of binding, the ED50, was determined by non-linear regression analysis.
- the 2 proteins have the same affinity for the HIR, indicating fusion of GDNF to the C-terminus of the heavy chain of the HIRMAb does not affect binding to the HIR.
- Figure 41 Size exclusion high performance liquid chromatography of the HIRMAb-
- GDNF fusion protein shows the absence of aggregates in the CHO-derived protein.
- Figure 42 Native polyacrylamide gel electrophoresis of the HIRMAb, control human
- IgGl or the HIRMAb-GDNF fusion protein shows the absence of aggregates in the CHO-derived fusion protein.
- FIG. 43 (A) Light micrograph of isolated human brain capillaries, used as an in vitro model system of the human BBB. (B) Binding of the 3 H-HIRMAb-GDNF fusion protein to the isolated brain capillaries is inhibited by the murine HIRMAb. The component of HIRMAb-GDNF uptake by the capillaries that is resistant to HlRMAb inhibition represents endocytosed fusion protein.
- the blood brain barrier is a limiting factor in the delivery of many peripherally- administered agents to the central nervous system.
- the present invention addresses three factors that are important in delivering an agent across the BBB to the CNS: 1) a pharmacokinetic profile for the agent that allows sufficient tune in the peripheral circulation for the agent to have enough contact with the BBB to traverse it; 2) modification of the agent to allow it to cross the BBB; and 3) retention of activity of the agent once across the BBB.
- fusion structures e.g., fusion proteins
- an agent e.g., a therapeutic agent
- the invention provides compositions and methods that utilize an agent covalently linked to a structure capable of crossing the blood brain barrier (BBB).
- BBB blood brain barrier
- the compositions and methods are useful in transporting agents, e.g. therapeutic agents such as neurotherapeutic agents, from the peripheral blood and across the BBB into the CNS.
- Neurotherapeutic agents useful in the invention include neurotrophins, e.g. brain-derived neurotrophic factor (BDNF) and glial-derived neurotrophic factor (GDNF).
- BDNF brain-derived neurotrophic factor
- GDNF glial-derived neurotrophic factor
- the structure that is capable of crossing the BBB is capable of binding to an endogenous BBB receptor mediated transport system and crossing the BBB.
- the structure that is capable of crossing the BBB is an antibody, e.g., a monoclonal antibody (MAb) such as a chimeric MAb.
- MAb monoclonal antibody
- the invention provides a fusion protein that includes a structure capable of crossing the BBB covalently linked to a peptide that is active in the central nervous system (CNS), where the structure capable of crossing the blood brain barrier and the peptide that is active in the central nervous system each retain a proportion (e.g., 10-100%) of their activities or their binding affinities for their respective receptors, compared to their activities or their binding affinities for their respective receptors as separate entities.
- a proportion e.g., 10-100%
- the invention provides a composition containing a cationic therapeutic peptide covalently linked to an immunoglobulin, where the cationic therapeutic peptide in the composition has a serum and/or circulating half-life that is an average of at least about five fold greater than the serum and/or circulating half-life of the cationic therapeutic peptide alone.
- the invention also provides nucleic acids coding for peptides and proteins.
- the invention provides a single nucleic acid sequence that contains a gene coding for a light chain of an immunoglobulin and a gene coding for a fusion protein made up of a heavy chain of the immunoglobulin covalently linked to a peptide.
- the peptide of the fusion protein is a therapeutic peptide, e.g., a neurotherapeutic peptide such as a neurotrophin.
- the invention also provides vectors containing the nucleic acids of the invention, and cells containing the vectors.
- an immunoglobulin fusion protein where the fusion protein contains an immunoglobulin heavy chain fused to a therapeutic agent
- the methods include integrating into a eukaryotic cell a single tandem expression vector in which both the immunoglobulin light chain gene and the gene for the immunoglobulin heavy chain fused to the therapeutic agent are incorporated into a single piece of DNA.
- the invention further provides therapeutic compositions, such as pharmaceutical compositions that contain an agent covalently linked to a structure capable of crossing the blood brain barrier (BBB) and a pharmaceutically acceptable excipient.
- BBB blood brain barrier
- the invention provides a composition for treating a neurological disorder that includes a BDNF or a GDNF (e.g., human GDNF) covalently linked to an immunoglobulin that is capable of crossing the blood brain barrier, wherein the composition is capable of crossing the BBB in an amount that is effective in treating the neurological disorder.
- BDNF BDNF
- GDNF e.g., human GDNF
- the invention also provides methods for treating a neurological disorder in an individual that include peripherally administering to the individual an effective amount of one or more of the compositions of the invention, optionally in combination with other therapy for the disorder.
- an “agent” includes any substance that is useful in producing an effect, including a physiological or biochemical effect in an organism.
- a “therapeutic agent” is a substance that produces or is intended to produce a therapeutic effect, i.e., an effect that leads to amelioration, prevention, and/or complete or partial cure of a disorder.
- a “therapeutic effect,” as that term is used herein, also includes the production of a condition that is better than the average or normal condition in an individual that is not suffering from a disorder, i.e., a supranormal effect such as improved cognition, memory, mood, or other characteristic attributable at least in part to the functioning of the CNS, compared to the normal or average state.
- a “neurotherapeutic agent” is an agent that produces a therapeutic effect in the CNS.
- a “therapeutic peptide” includes therapeutic agents that consists of a peptide.
- a “cationic therapeutic peptide” encompasses therapeutic peptides whose isoelectric point is above about 7.4, in some embodiments, above about 8, 8.5, 9, 9.5, 10, 10.5, 11, 11.5, 12, or above about 12.5.
- a subcategory of cationic therapeutic peptides is cationic neurotherapeutic peptides.
- a "peptide that is active in the central nervous system (CNS)” includes peptides that have an effect when administered to the CNS.
- the effect may be a therapeutic effect or a non-therapeutic effect, e.g., a diagnostic effect or an effect useful hi research. If the effect is a therapeutic effect, then the peptide is also a therapeutic peptide,
- a therapeutic peptide that is also a peptide that is active in the CNS is encompassed by the term "neurotherapeutic peptide," as used herein.
- Treatment includes achieving a therapeutic benefit and/or a prophylactic benefit.
- therapeutic benefit is meant eradication or amelioration of the underlying disorder or condition being treated.
- therapeutic benefit includes partial or complete halting of the progression of the disorder, or partial or complete reversal of the disorder.
- a therapeutic benefit is achieved with the eradication or amelioration of one or more of the physiological or psychological symptoms associated with the underlying condition such that an improvement is observed in the patient, notwithstanding the fact that the patient may still be affected by the condition.
- a prophylactic benefit of treatment includes prevention of a condition, retarding the progress of a condition (e.g., slowing the progression of a neurological disorder), or decreasing the likelihood of occurrence of a condition.
- “treating” or “treatment” includes prophylaxis.
- the term "effective amount" can be an amount sufficient to effect beneficial or desired results, such as beneficial or desired clinical results, or enhanced cognition, memory, mood, or other desired CNS results.
- An effective amount is also an amount that produces a prophylactic effect, e.g., an amount that delays, reduces, or eliminates the appearance of a pathological or undesired condition.
- Such conditions of the CNS include dementia, neurodegenerative diseases as described herein, suboptimal memory or cognition, mood disorders, general CNS aging, or other undesirable conditions.
- An effective amount can be administered in one or more administrations.
- an “effective amount” of a composition of the invention is an amount that is sufficient to palliate, ameliorate, stabilize, reverse or slow the progression of a disorder, e.g., a neurological disorder.
- An “effective amount” may be of any of the compositions of the invention used alone or in conjunction with one or more agents used to treat a disease or disorder.
- An “effective amount” of a therapeutic agent within the meaning of the present invention will be determined by a patient's attending physician or veterinarian. Such amounts are readily ascertained by one of ordinary skill in the art and will a therapeutic effect when administered in accordance with the present invention.
- Factors which influence what a therapeutically effective amount will be include, the specific activity of the therapeutic agent being used, the type of disorder (e.g., acute vs. chronic neurological disorder), tune elapsed since the initiation of the disorder, and the age, physical condition, existence of other disease states, and nutritional status of the individual being treated. Additionally, other medication the patient may be receiving will affect the determination of the therapeutically effective amount of the therapeutic agent to administer.
- the specific activity of the therapeutic agent being used e.g., the type of disorder (e.g., acute vs. chronic neurological disorder), tune elapsed since the initiation of the disorder, and the age, physical condition, existence of other disease states, and nutritional status of the individual being treated. Additionally, other medication the patient may be receiving will affect the determination of the therapeutically effective amount of the therapeutic agent to administer.
- a "subject” or an “individual,” as used herein, is an animal, for example, a mammal. In some embodiments a “subject” or an “individual” is a human. In some embodiments, the subject suffers from a neurological disorder.
- an agent is "administered peripherally" or “peripherally administered.”
- these terms refer to any form of administration of an agent, e.g., a therapeutic agent, to an individual mat is not direct administration to the CNS, i.e., that brings the agent in contact with the non-brain side of the blood-brain barrier.
- Peripheral administration includes intravenous, subcutaneous, intramuscular, intraperitoneal, transdermal, inhalation, transbuccal, intranasal, rectal, and oral administration.
- a "pharmaceutically acceptable carrier” or “pharmaceutically acceptable excipienf ' herein refers to any carrier that does not itself induce the production of antibodies harmful to the individual receiving the composition. Such carriers are well known to those of ordinary skill in the art. A thorough discussion of pharmaceutically acceptable carriers/excipients can be found in Remington 's Pharmaceutical Sciences, Gennaro, AR, ed., 20th edition, 2000: Williams and Wilkins PA, USA..
- Exemplary pharmaceutically acceptable carriers can include salts, for example, mineral acid salts such as hydrochlorides, hydrobromides, phosphates, sulfates, and the like; and the salts of organic acids such as acetates, propionates, malonates, benzoates, and the like.
- compositions of the invention may be provided in liquid form, and formulated in saline based aqueous solution of varying pH (5-8), with or without detergents such polysorbate-80 at 0.01-1%, or carbohydrate additives, such mannitol, sorbitol, or trehalose.
- Commonly used buffers include histidine, acetate, phosphate, or citrate.
- a "recombinant host cell” or “host cell” refers to a cell that includes an exogenous polynucleotide, regardless of the method used for insertion, for example, direct uptake, transduction, f-mating, or other methods known in the art to create recombinant host cells.
- the exogenous polynucleotide may be maintained as a nonintegrated vector, for example, a plasmid, or alternatively, may be integrated into the host genome.
- polypeptide peptide
- protein protein
- polypeptide polymer of amino acid residues. That is, a description directed to a polypeptide applies equally to a description of a peptide and a description of a protein, and vice versa.
- the terms apply to naturally occurring ammo acid polymers as well as amino acid polymers in which one or more amino acid residues is a non-naturally occurring amino acid, e.g., an amino acid analog.
- the terms encompass amino acid chains of any length, including full length proteins (Le., antigens), wherein the amino acid residues are linked by covalent peptide bonds.
- amino acid refers to naturally occurring and non-naturally occurring amino acids, as well as amino acid analogs and amino acid mimetics that function in a manner similar to the naturally occurring amino acids.
- Naturally encoded amino acids are the 20 common amino acids (alanine, arginine, asparagine, aspartic acid, cysteine, glutamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, and valine) and pyrolysine and selenocysteine.
- Amino acid analogs refers to compounds that have the same basic chemical structure as a naturally occurring amino acid, i. e., an ⁇ carbon that is bound to a hydrogen, a carboxyl group, an amino group, and an R group, such as, homoserine, norleucine, methionine sulfoxide, methionine methyl sulfonium.
- Such analogs have modified R groups (such as, norleucine) or modified peptide backbones, but retain the same basic chemical structure as a naturally occurring amino acid.
- Amino acids may be referred to herein by either their commonly known three letter symbols or by the one-letter symbols recommended by the IUPAC-IUB Biochemical Nomenclature Commission. Nucleotides, likewise, may be referred to by their commonly accepted single-letter codes.
- nucleic acid refers to deoxyribonucleotides, deoxyribonucleosides, ribonucleosides, or ribonucleotides and polymers thereof in either single- or double-stranded form. Unless specifically limited, the term encompasses nucleic acids containing known analogues of natural nucleotides which have similar binding properties as the reference nucleic acid and are metabolized in a manner similar to naturally occurring nucleotides. Unless specifically limited otherwise, the term also refers to oligonucleotide analogs including PNA (peptidonucleic acid), analogs of DNA used in antisense technology (phosphorothioates, phosphoroamidates, and the like).
- PNA peptidonucleic acid
- analogs of DNA used in antisense technology phosphorothioates, phosphoroamidates, and the like.
- nucleic acid sequence also implicitly encompasses conservatively modified variants thereof, including, but not limited to, degenerate codon substitutions, and complementary sequences as well as the sequence explicitly indicated.
- degenerate codon substitutions may be achieved by generating sequences in which the third position of one or more selected (or all) codons is substituted with mixed-base and/or deoxyinosine residues (Batzer et al., Nucleic Acid Res. 19:5081 (1991); Ohtsuka et al., J, Biol, Chem. 260.-2605-2608 (1985); and Cassol et al. (1992); Rossolini et al., MoI. Cell. Probes 8:91-98 (1994)).
- isolated and purified refer to a material that is substantially or essentially removed from or concentrated in its natural environment.
- an isolated nucleic acid may be one that is separated from the nucleic acids that normally flank it or other nucleic acids or components (proteins, lipids, etc..) in a sample.
- a polypeptide is purified if it is substantially removed from or concentrated in its natural environment. Methods for purification and isolation of nucleic acids and peptides are well known in the art. III. The blood brain barrier
- the invention provides compositions and methods that utilize an agent covalently linked to a structure capable of crossing the blood brain barrier (BBB).
- BBB blood brain barrier
- the compositions and methods are useful in transporting agents, e.g. therapeutic agents such as neurotherapeutic agents, from the peripheral blood and across the BBB into the CNS.
- therapeutic agents e.g. neurotherapeutic agents
- the "blood-brain barrier” refers to the barrier between the peripheral circulation and the brain and spinal cord which is formed by tight junctions within the brain capillary endothelial plasma membranes, creates an extremely tight barrier that restricts the transport of molecules into the brain, even molecules as small as urea, molecular weight of 60 Da.
- the blood-brain barrier within the brain, the blood-spinal cord barrier within the spinal cord, and the blood-retinal barrier within the retina, are contiguous capillary barriers within the central nervous system (CNS), and are collectively referred to as the blood-brain barrier or BBB,
- the BBB is a limiting step in the development of new neurotherapeutics, diagnostics, and research tools for the brain and CNS.
- large molecule therapeutics such as recombinant proteins, antisense drugs, gene medicines, monoclonal antibodies, or RNA interference (RNAi)- based drugs, do not cross the BBB in pharmacologically significant amounts. While it is generally assumed that small molecule drugs can cross the BBB, in fact, ⁇ 2% of all small molecule drugs are active in the brain owing to the lack transport across the BBB.
- a molecule must be lipid soluble and have a molecular weight less than 400 Daltons (Da) in order to cross the BBB in pharmacologically significant amounts, and the vast majority of small molecules do not have these dual molecular characteristics. Therefore, most potentially therapeutic, diagnostic, or research molecules do not cross the BBB in pharmacologically active amounts.
- invasive transcranial drug delivery strategies are used, such as intracerebro-ventricular (ICV) infusion, intracerebral (IC) administration, and convection enhanced diffusion (CED). Transcranial drug delivery to the brain is expensive, invasive, and largely ineffective.
- the ICV route delivers BDNF only to the ependymal surface of the brain, not into brain parenchyma, which is typical for drugs given by the ICV route.
- a neurotrophin such as nerve growth factor (NGF)
- NGF nerve growth factor
- the CED of neurotrophin results in preferential fluid flow through the white matter tracts of brain, which causes demyelination, and astrogliosis.
- the present invention offers an alternative to these highly invasive and generally unsatisfactory methods for bypassing the BBB, allowing agents, e.g., neuroprotective factors to cross the BBB from the peripheral blood. It is based on the use of endogenous transport systems present in the BBB to provide a mechanism to transport a desired substance from the peripheral blood to the CNS.
- the invention provides compositions that include a structure that binds to a BBB receptor mediated transport system, coupled to an agent for which transport across the BBB is desired, e.g., a neurotherapeutic agent.
- the compositions and methods of the invention may utilize any suitable structure that is capable of transport by the selected endogenous BBB receptor-mediated transport system, and that is also capable of attachment to the desired agent.
- the structure is an antibody.
- the antibody is a monoclonal antibody (MAb), e.g., a chimeric MAb.
- Endogenous BBB receptor-mediated transport systems The BBB has been shown to have specific receptors that allow the transport from the blood to the brain of several macromolecules; these transporters are suitable as transporters for compositions of the invention.
- Endogenous BBB receptor-mediated transport systems useful in the invention include those that transport insulin, transferrin, insulin-like growth factors 1 and 2 (IGFl and IGF2), leptin, and lipoproteins.
- the invention utilizes a structure that is capable of crossing the BBB via the endogenous insulin BBB receptor-mediated transport system, e.g., the human endogenous insulin BBB receptor-mediated transport system.
- MTH molecular Trojan horses
- the MTH can be an antibody to a receptor of the transport system, e.g., the insulin receptor.
- the antibody is a monoclonal antibody (MAb).
- the MAb is a chimeric MAb.
- antibodies are particularly useful in embodiments of the invention, especially
- the MTH is a MAb to the human insulin receptor (HIR) on the human BBB.
- HIR human insulin receptor
- the HIR MAb binds an exofacial epitope on the human BBB HIR and this binding enables the MAb to traverse the BBB via a transport reaction that is mediated by the human BBB insulin receptor.
- an antibody includes reference to any molecule, whether naturally-occurring, artificially induced, or recombinant, which has specific immunoreactive activity.
- an antibody is a protein that includes two molecules, each molecule having two different polypeptides, the shorter of which functions as the light chains of the antibody and the longer of which polypeptides function as the heavy chains of the antibody.
- an antibody will include at least one variable region from a heavy or light chain.
- the antibody may comprise combinations of variable regions. The combination may include more than one variable region of a light chain or of a heavy chain.
- the antibody may also include variable regions from one or more light chains in combination with variable regions of one or more heavy chains.
- an antibody can be an immunoglobulin molecule obtained by in vitro or in vivo generation of the humoral response, and includes both polyclonal and monoclonal antibodies.
- the present invention includes antigen binding fragments of the antibodies described herein, such as Fab, Fab', F(ab) 2 , and Fv fragments, fragments comprised of one or more CDRs, single-chain antibodies (e.g., single chain Fv fragments (scFv)), disulfide stabilized (dsFv) Fv fragments, heteroconjugate antibodies (e.g., bispecific antibodies), pFv fragments, heavy chain monomers or dimers, light chain monomers or dimers, and ditners consisting of one heavy chain and one light chain.
- Fab single chain Fv fragments
- dsFv disulfide stabilized
- Such antibody fragments may be produced by chemical methods, e.g., by cleaving an intact antibody with a protease, such as pepsin or papain, or via recombinant DNA techniques, e.g., by using host cells transformed with truncated heavy and/or light chain genes. Synthetic methods of generating such fragments are also contemplated. Heavy and light chain monomers may similarly be produced by treating an intact antibody with a reducing agent, such as dithiothreitol or beta-mercaptoethanol, or by using host cells transformed with DNA encoding either the desired heavy chain or light chain or both.
- An antibody immunologically reactive with a particular antigen can be generated in vivo or by recombinant methods such as selection of libraries of recombinant antibodies in phage or similar vectors.
- a "chimeric" antibody includes an antibody derived from a combination of different mammals.
- the mammal may be, for example, a rabbit, a mouse, a rat, a goat, or a human.
- the combination of different mammals includes combinations of fragments from human and mouse sources.
- an antibody of the present invention is a monoclonal antibody
- MAb typically a human monoclonal antibody.
- Such antibodies are obtained from transgenic mice that have been "engineered” to produce specific human antibodies in response to antigenic challenge.
- elements of the human heavy and light chain locus are introduced into strains of mice derived from embryonic stem cell lines that contain targeted disruptions of the endogenous heavy chain and light chain loci.
- the transgenic mice can synthesize human antibodies specific for human antigens, and the mice can be used to produce human antibody-secreting hybridomas.
- a chimeric MAb is preferred that contains enough human sequence that it is not significantly immunogenic when administered to humans, e.g., at least about 80% human and about 20% mouse, or about 85% human and about 15% mouse, or about 90% human and about 10% mouse, or about 95% human and 5% mouse, or greater than about 95% human and less than about 5% mouse.
- Chimeric antibodies to the human BBB insulin receptor with sufficient human sequences for use in the invention are described in, e.g., Coloma et al. (2000) Pharm. Res. 17: 266- 274, which is incorporated by reference herein in its entirety.
- HIR MAb A more highly humanized form of the HIR MAb can also be engineered, and the humanized HIRMAb has activity comparable to the murine HIRMAb and can be used in embodiments of the invention. See, e.g., U.S. Patent Application Publication No. 20040101904, filed 11/27/02, incorporated by reference herein in its entirety.
- Antibodies used in the invention may be glycosylated or non-glycosylated. If the antibody is glycosylated, any pattern of glycosylation that does not significantly affect the function of the antibody may be used. Glycosylation can occur in the pattern typical of the cell in which the antibody is made, and may vary from cell type to cell type.
- the glycosylation pattern of a monoclonal antibody produced by a mouse myeloma cell can be different than the glycosylation pattern of a monoclonal antibody produced by a transfected Chinese hamster ovary (CHO) cell.
- the antibody is glycosylated in the pattern produced by a transfected Chinese hamster ovary (CHO) cell.
- a genetically engineered HIR MAb with the desired level of human sequences, is fused to an agent for which transport across the BBB is desired, e.g., a neurotherapeutic agent such as a neurotrophin such as human BDNF, to produce a recombinant fusion protein that is a bi-functional molecule.
- a neurotherapeutic agent such as a neurotrophin such as human BDNF
- the recombinant therapeutic neuroprotective factor/HERMAb is able to both (i) cross the human BBB, via transport on the BBB HIR, and (ii) activate the factor's target, e.g., neuronal BDNF receptor, trkB, to cause neurotherapeutic effects once inside the brain, following peripheral administration.
- the agent for which transport across the BBB is desired may be any suitable substance for introduction into the CNS.
- the agent is a substance for which transport across the BBB is desired, which does not, in its native form, cross the BBB in significant amounts.
- the agent may be, e.g., a therapeutic agent, a diagnostic agent, or a research agent.
- Diagnostic agents include peptide radiopharmaceuticals, such as radiolabeled epidermal growth factor (EGF) for imaging brain cancer (Kurihara and Pardridge (1999) Cam, Res. 54: 6159-6163), and amyloid peptides for imaging brain amyloid such as in Alzheimers disease (Lee et al (2002) J. Cereb. Blood Flow Metabol. 22: 223-231).
- the agent is a therapeutic agent, such as a neurotherapeutic agent.
- a therapeutic agent such as a neurotherapeutic agent.
- potentially useful therapeutic protein agents include recombinant enzymes for lysosomal storage disorders (see, e.g., U.S. Patent Application Publication No. 20050142141, filed 2/17/05, incorporated by reference herein in its entirety), monoclonal antibodies that either mimic an endogenous peptide or block the action of an endogenous peptide, polypeptides for brain disorders, such as secretin for autism (Ratliff-Schaub et al (2005) Autism 9: 256-265), opioid peptides for drug or alchol addiction (Cowen et al, (2004) J. Neurochem.
- the agent is a neurotrophic factor, also referred to herein as a "neurotrophin.”
- the invention provides compositions and methods that utilize a neurotrophin.
- a single neurotrophin may be used.
- combinations of neurotrophins are used.
- the invention utilizes a brain- derived neurotrophic factor (BDNF).
- BDNF brain- derived neurotrophic factor
- the invention utilizes a glial-derived neurotrophic factor.
- neurotrophic factors are neuroprotective in brain, but do not cross the blood-brain barrier. These factors are suitable for use in the compositions and methods of the invention and include brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), neurotrophin-4/5, fibroblast growth factor (FGF)-2 and other FGFs, neurotrophin (NT)-3, erythropoietin (EPO), hepatocyte growth factor (HGF), epidermal growth factor (EGF), transforming growth factor (TGF)- ⁇ , TGF- ⁇ , vascular endothelial growth factor (VEGF), interleukin-1 receptor antagonist (IL- lra), ciliary neurotrophic factor (CNTF), glial-derived neurotrophic factor (GDNF) 5 neurturin, platelet-derived growth factor (PDGF), heregulin, neuregulin, artemin, persephin, interleukins, granulocyte-colony stimulating factor (CSF), granulocyte-macrophage, BD
- neurotrophins that are used as precursors for fusion proteins that cross the BBB are those that naturally form dimeric structures, similar to BDNF.
- Certain neurotrophins such as BDNF or NT-3 may form hetero-dimeric structures, and in some embodiments the invention provides a fusion protein constructed of one neurotrophin monomer fused to one chain (e.g., a light or heavy chain) of an antibody, e.g., of the HIRMAb, and another neurotrophin monomer fused to the second chain (e.g., a light or heavy chain) of the antibody.
- the molecular weight range of recombinant proteins that may be fused to the molecular Trojan horse ranges from 1000 Daltons to 500,000 Daltons.
- One particularly useful neurotrophin in embodiments of the invention is brain-derived neurotrophic factor (BDNF).
- BDNF brain-derived neurotrophic factor
- AD Alzheimer's disease
- PD Parkinson's disease
- HD Huntington's disease
- ALS amyotrophic lateral sclerosis
- BDNF blood-brain barrier
- BDNF BDNF
- the lack of utility of BDNF as a CNS therapeutic following peripheral administration is expected and follows from the limiting role that is played by the BBB in the development of neurotherapeutics, especially large molecule drugs such as BDNF.
- BDNF does not cross the BBB, and the lack of transport of the neuiotrophin across the BBB prevents the molecule from being pharmacologically active in the brain following peripheral administration.
- the lack of BDNF transport across the BBB means that the neurotrophin must be directly injected into the brain across the skull bone to be pharmacologically active in the CNS.
- the BDNF when the BDNF is fused to a Trojan horse such as the HIR MAb, this neurotrophin is now able to enter brain from blood following a non-invasive peripheral route of administration such as intravenous intramuscular, subcutaneous, intraperitoneal, or even oral administration. Owing to the BBB transport properties of this new class of molecule, it is not necessary to administer the BDNF directly into the CNS with an invasive delivery procedure requiring penetration of the skull or spinal canal.
- the reformulated ftision protein of the BDNF variant and the HIR MAb now enables entry of BDNF into the brain from the blood, and the development of BDNF as a neurotherapeutic for human diseases.
- BDNF used in various embodiments of the invention may include pharmaceutically acceptable salts and prodrugs, and prodrugs of the salts, polymorphs, hydrates, solvates, biologically-active fragments, biologically active variants and stereoisomers of the naturally-occurring BDNF, as well as agonist, mimetic, and antagonist variants of the naturally- occurring BDNF and polypeptide fusions thereof.
- Variants that include one or more deletions, substitutions, or insertions in the natural sequence of the BDNF, in particular truncated versions of the native BDNF comprising deletion of one or more amino acids at the amino terminus, carboxyl terminus, or both, may also be used in certain embodiments.
- the invention utilizes a carboxy-truncated variant of the native
- BDNF e.g., a variant in which 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more than 10 amino acids are absent from the carboxy-terminus of the BDNF.
- BDNF variants include the complete 119 amino acid BDNF, the 117 or 118 amino acid variant with a truncated carboxyl terminus, variants with a truncated amino terminus, or variants with up to about a 20, 30, or 40% change in amino acid composition, as long as the fusion protein variant still binds to the brain neuroprotection receptor with high affinity.
- additional fusion protein variants can be produced with the substitution of amino acids within either the framework region (FR) or the complementarity determining region (CDR) of either the light chain or the heavy chain of the Ab, e.g., HIRMAb, as long as the fusion protein binds with high affinity to the endogenous receptor, e.g., HIR to promote transport across the human BBB.
- Additional fusion protein variants can be produced by changing the composition or length of the linker peptide separating the fusion protein from the HIRMAb.
- the full-length 119 a.a. sequence of BDNF is utilized (SEQ ID NO:
- a one amino-acid carboxy-truncated variant of BDNF is utilized (amino acids 1-118 of SEQ ID NO: 39).
- a two amino-acid carboxy-truncated variant of BDNF is utilized (amino acids 1-117 of SEQ ID NO: 39).
- a three amino-acid carboxy-truncated variant of BDNF is utilized (amino acids 1-116 of SEQ ID NO: 39).
- a four amino-acid carboxy-truncated variant of BDNF is utilized (amino acids 1-115 of SEQ ID NO: 39).
- a five amino-acid carboxy-truncated variant of BDNF is utilized (amino acids 1-114 of SEQ ID NO: 39).
- the sequence of human BDNF is given in SEQ ID NO: 39.
- the invention utilizes a BDNF that is about 60, 70, 80, 90, 95, 99, or 100% identical with the sequence of SEQ ID NO: 39, or a truncated version thereof, e.g., the 117 or 118 amino acid variant with a one- or two-amino acid truncated carboxyl terminus, or variants with a truncated amino terminus.
- the invention utilizes a two amino-acid carboxy-truncated 117 amino acid variant human BDNF with a sequence that is at least about 60, 70, 80, 90, 95, 99 or 100% identical to the sequence of amino acids 466-582 of SEQ ID NO: 24. In some embodiments, the invention utilizes a two amino-acid carboxy-truncated human 117 amino acid BDNF with a sequence that includes amino acids 466-582 of SEQ ID NO: 24.
- BDNFs useful in the invention include peptides having at least about 60%, at least about 70%, at least about 80%, at least about 90%, at least about 95%, at least about 99%, or greater than 95% or greater than 99% sequence identity, e.g., 100% sequence identity, to the amino acid sequences disclosed herein.
- GDNF glial-derived neurotrophic factor
- Another particularly useful neurotrophin in embodiments of the invention is glial-derived neurotrophic factor (GDNF).
- GDNF is a neurotrophic factor that can be used in the treatment of many acute and chronic brain diseases.
- the lack of transport of GDNF across the BBB has prevented the development of this molecule as a neurotherapeutic for the brain and spinal cord.
- Acute brain conditions that can be treated by the GDNF-containing compositions described herein include, but are not limited to, spinal cord injury, brain injury (e.g., traumatic brain injury), focal brain ischemia, and global brain ischemia.
- Chronic brain conditions that can be treated by the GDNF-containing compositions described herein include, but are not limited to neurodegenerative disease such as Parkinson's disease, motor neuron disease (e.g., spinal cord motor neuron disease such as amyotrophic lateral sclerosis); and substance abuse, e.g., addiction, including drug addiction and alchohol addiction.
- neurodegenerative disease such as Parkinson's disease, motor neuron disease (e.g., spinal cord motor neuron disease such as amyotrophic lateral sclerosis); and substance abuse, e.g., addiction, including drug addiction and alchohol addiction.
- the forms of GDNF used in various embodiments of the invention may include acceptable salts and prodrugs, and prodrugs of the salts, polymorphs, hydrates, solvates, biologically-active fragments, biologically active variants and stereoisomers of the naturally-occurring GDNF (e.g., human GDNF and mature human GDNF), as well as agonist, mimetic, and antagonist variants of the naturally-occurring GDNF and polypeptide fusions thereof.
- Variants that include one or more deletions, substitutions, or insertions in the natural sequence of the GDNF may also be used in certain embodiments, insofar as the variants retain binding and/or activation of GFR ⁇ l .
- GDNF The structure-function relationship of GDNF and its ability to bind and activate GFR ⁇ l has been studied extensively. See, e.g., Eketjal et al (1999), EMBO J, 18(21):5901-5910; and Baloh et al (2000), J Biol Chem, 275(5):3412-3420.
- mutations known to be particularly sensitive to mutation include, e.g., the following residues in rat GDNF: D52, E61, E62, Dl 16, 164, Ll 14, Ll 18, Y120, 1122, and ClOl.
- Functional assays for GDNF are known in the art. See, e.g., Eketjal et al, or Baloh et al supra. Functional assays for GDNF are also described in Example 15 herein.
- additional fusion protein variants can be produced with the substitution of amino acids within either the framework region (FR) or the complementarity determining region (CDR) of either the light chain or the heavy chain of the Ab, e.g., HIRMAb, as long as the fusion protein binds with high affinity to the endogenous receptor, e.g., HIR to promote transport across the human BBB.
- Additional fusion protein variants can be produced by changing the composition or length of the linker peptide separating the fusion protein from the HIRMAb.
- the full-length 211 a.a. sequence of human prepro GDNF is utilized
- the invention utilizes a GDNF that is about 60, 70, 80, 90, 95, 99, or 100% identical with the sequence of SEQ ID NO:52.
- GDNFs useful in the invention include peptides having at least about 60%, at least about 70%, at least about 80%, at least about 90%, at least about 95%, at least about 99%, or greater man 95% or greater than 99% sequence identity, e.g., 100% sequence identity, to the amino acid sequences disclosed herein
- Percent sequence identity is determined by conventional methods. See, for example,
- FASTA Pearson and Lipman is a suitable protein alignment method for examining the level of identity shared by an amino acid sequence disclosed herein and the amino acid sequence of another peptide.
- the FASTA algorithm is described by Pearson and Lipman, Proc. Nat'lAcad. Set USA 85:2444 (1988), and by Pearson, Meth. En ⁇ ymol. 183:63 (1990).
- the ten regions with the highest density of identities are then rescored by comparing the similarity of all paired amino acids using an amino acid substitution matrix, and the ends of the regions are "trimmed" to include only those residues that contribute to the highest score.
- the trimmed initial regions are examined to determine whether the regions can be joined to form an approximate alignment with gaps.
- the highest scoring regions of the two amino acid sequences are aligned using a modification of the Needleman-Wunsch- Sellers algorithm (Needleman and Wunsch, J. MoL Biol, 48:444 (1970); Sellers, SIAM J. Appl. Math. 26:787 (1974)), which allows for amino acid insertions and deletions.
- the present invention also includes peptides having a conservative amino acid change, compared with an amino acid sequence disclosed herein. Many such changes have been described specifically. More generally, for example, variants can be obtained that contain one or more amino acid substitutions of SEQ ID NO: 52.
- sequence variants include conservative amino acid substitutions, e.g., an alkyl amino acid is substituted for an alkyl amino acid in a GDNF peptide amino acid sequence, an aromatic amino acid is substituted for an aromatic amino acid in a GDNF peptide amino acid sequence, a sulfur-containing amino acid is substituted for a sulfur- containing amino acid in a GDNF peptide amino acid sequence, a hydroxy-containing amino acid is substituted for a hydroxy-containing amino acid in a GDNF peptide amino acid sequence, an acidic amino acid is substituted for an acidic amino acid in a GDNF peptide amino acid sequence, a basic amino acid is substituted for a basic amino acid in GDNF peptide amino acid sequence, or a dibasic monocarboxylic amino acid is substituted for a dibasic monocarboxylic amino acid in a GDNF peptide amino acid sequence.
- conservative amino acid substitutions e.g., an alkyl amino acid is substituted for an al
- a "conservative amino acid substitution” is illustrated by a substitution among amino acids within each of the following groups: (1) glycine, alanine, valine, leucine, and isoleucine, (2) phenylalanine, tyrosine, and tryptophan, (3) serine and threonine, (4) aspartate and glutamate, (5) glutamine and asparagine, and (6) lysine, arginine and histidine.
- the BLOSUM62 table is an amino acid substitution matrix derived from about 2,000 local multiple alignments of protein sequence segments, representing highly conserved regions of more than 500 groups of related proteins (Henikoff and Henikoff, Proc. Nat'lAcad.
- the BLOSUM62 substitution frequencies can be used to define conservative amino acid substitutions that may be introduced into the amino acid sequences of the present invention.
- conservative amino acid substitution preferably refers to a substitution represented by a BLOSUM62 value of greater than - 1.
- an amino acid substitution is conservative if the substitution is characterized by a BLOSUM62 value of 0, 1, 2, or 3.
- preferred conservative amino acid substitutions are characterized by a BLOSUM62 value of at least 1 (e.g., 1, 2 or 3), while more preferred conservative amino acid substitutions are characterized by a BLOSUM62 value of at least 2 (e.g., 2 or 3).
- amino acid sequences may include additional residues, such as additional N- or C-terminal amino acids, and yet still be essentially as set forth in one of the sequences disclosed herein, so long as the sequence retains sufficient biological protein activity to be functional in the compositions and methods of the invention.
- GDNF sequence variants e.g., variants of SEQ ID NO: 52
- mutation tolerance prediction programs can be used to greatly reduce the number of non-functional sequence variants that would be generated by strictly random mutagenesis.
- Various programs for predicting the effects of amino acid substitutions in a protein sequence on protein function e.g., SIFT, PolyPhen, PANTHER PSEC, PMUT, and TopoSNP are described in, e.g., Henikoff et al., (2006), Anna. Rev. Genomics Hum. Genet., 7:61-80..
- compositions of the invention are useful in one or more of: increasing serum half-life of a cationic compound, transporting an agent across the BBB, and/or retaining activity of the agent once transported across the BBB.
- the invention provides compositions containing a neurotherapeutic agent covalently linked to a structure that is capable of crossing the blood brain barrier (BBB), where the composition is capable of producing an average elevation of concentration in the brain of the neurotherapeutic agent of at least about 1 , 2, 3, 4, 5, 10, 20, 30, 40, or 50 ng/gram brain following peripheral administration.
- BBB blood brain barrier
- the invention also provides compositions containing an agent that is covalently linked to a chimeric MAb to the human BBB insulin receptor.
- the invention further provides a fusion protein containing a structure capable of crossing the BBB, covalently linked to a peptide that is active in the central nervous system (CNS), where the structure capable of crossing the blood brain barrier and the peptide that is active in the central nervous system each retain an average of at least about 10, 20, 30, 40, 50, 60, 70, 80, 90, 95, 99, or 100% of their activities, compared to their activities as separate entities.
- the invention further provides compositions that increase the serum half-life of cationic substances.
- the invention also provides pharmaceutical compositions that contain one or more compositions of the invention and a pharmaceutically acceptable excipient.
- the invention provides compositions containing a neurotherapeutic agent covalently linked to a structure that is capable of crossing the blood brain barrier (BBB), where the composition is capable of producing an average elevation of concentration in the brain of the neurotherapeutic agent of at least about 1, 2, 3, 4, 5, 10, 20, 30, 40, or 50 ng/gram brain following peripheral administration.
- BBB blood brain barrier
- Elevation of the agent is an increase in the brain concentration of the agent compared to the concentration of the agent administered alone (i.e., not covalently linked to a structure that is capable of crossing the BBB). In the case of agents for which only a small amount of the agent alone normally crosses the BBB, “elevation” may be an increase in the agent compared to resting brain levels.
- Amage refers to the mean of at least three, four, five, or more than five measurements, preferably in different individuals. The individual in which the elevation is measured is a mammal, such as a rat, or, preferably, a primate, e.g., a monkey. An example of measurements of elevation of the level of a neurotherapeutic agent (BDNF) is given in Example 7.
- BDNF neurotherapeutic agent
- the structure that is capable of crossing the BBB utilizes an endogenous BBB receptor mediated transport system, such as a system that utilizes the insulin receptor, transferrin receptor, leptin receptor, LDL receptor, or IGF receptor.
- the endogenous BBB receptor mediated transport system is the insulin BBB receptor mediated transport system.
- the structure that is capable of crossing the BBB is an antibody, e.g., a monoclonal antibody (MAb) such as a chimeric MAb.
- the antibody can be a chimeric antibody with sufficient human sequence that it is suitable for administration to a human.
- the antibody can be glycosylated or nonglycosylated; in some embodiments, the antibody is glycosylated, e.g., in a glycosylation pattern produced by its synthesis in a CHO cell.
- the covalent linkage between the antibody and the neurotherapeutic agent may be a linkage between any suitable portion of the antibody and the neurotherapeutic agent, as long as it allows the antibody-agent fusion to cross the blood brain barrier and the neurotherapeutic agent to retain a therapeutically useful portion of its activity within the CNS.
- the covalent link is between one or more light chains of the antibody and the neurotherapeutic agent
- a peptide neurotherapeutic agent e.g., a neurotrophin such as GDNF
- the peptide can be covalently linked by its carboxy or amino terminus to the carboxy or amino terminus of the light chain (LC) or heavy chain (HC) of the antibody.
- any suitable linkage may be used, e.g., carboxy terminus of light chain to amino terminus of peptide, carboxy terminus of heavy chain to amino terminus of peptide, amino terminus of light chain to amino terminus of peptide, amino terminus of heavy chain to amino terminus of peptide, carboxy terminus of light chain to carboxy terminus of peptide, carboxy terminus of heavy chain to carboxy terminus of peptide, amino terminus of light chain to carboxy terminus of peptide, or amino terminus of heavy chain to carboxy terminus of peptide.
- the linkage is from the carboxy terminus of the HC to the amino terminus of the peptide.
- the invention utilizes BDNF, either the native form or truncated variants. Strikingly, it has been found that fusion proteins of these forms of BDNF retain foil transport and activity. This is surprising because the neurotrophin is translated in vivo in cells as a prepro form and the prepro-BDNF is then converted into mature BDNF following cleavage of the prepro peptide from the amino terminus of the BDNF.
- the prepro BDNF In order to preserve the prepro form of the BDNF, and the subsequent cleavability of the prepro peptide, it would seem to be necessary to fuse the prepro BDNF to the amino terminus of either the HC or the LC of the targeting MAb. This could, however, inhibit the binding of the MAb for the target antigen, since the complementarity determining regions (CDR) of the heavy chain or light chain of the MAb molecule, which comprise the antigen binding site of the MAb, are situated near the amino terminus of the heavy chain or light chains of the antibody. Therefore, fusion of the prepro-neurotrophin to the amino terminus of the antibody chains is expected to result in not only impairment of antibody activity, but also an impairment of antibody folding following translation.
- CDR complementarity determining regions
- the present invention shows the unexpected finding that it is possible to fuse the mature form of a neurotrophin, such as a BDNF variant (vBDNF), to the carboxyl terminus of the heavy chain of the HIR MAb.
- a neurotrophin such as a BDNF variant (vBDNF)
- vBDNF BDNF variant
- the invention utilizes GDNF (e.g., mature human GDNF) or a sequence variant of GDNF as described herein..
- GDNF e.g., mature human GDNF
- sequence variant of GDNF as described herein.
- more than one molecule of the same neurotherapeutic agent is attached to the structure that crosses the BBB.
- one molecule of the neurotrophin is attached to each heavy chain, naturally producing a structure that is ideal for homodimer formation. This is the case for compositions containing BDNF or GDNF.
- Neurotrophins such as BDNF or GDNF require an obligatory formation of a homo-dimeric structure to be biologically active, and to bind with high affinity to the cognate receptor, e.g. TrkB or GFR ⁇ l .
- a naturally occurring homo-dimeric structure between two BDNF molecules is formed when the neurotrophin is fused to a carboxyl terminus of the CH3 region of an IgG molecule, as illustrated in Figure 18. Without being bound by theory, it is thought that this may account for the unexpected finding of essentially 100% of activity for the BDNF when bound to the IgG (see, e.g., Fig. 24).
- more than one type of neurotherapeutic agent can be attached to the structure that is capable of crossing the blood brain barrier.
- 2, 3, 4, 5, 6, 7, 8, 9, 10, or more than 10 different neurotherapeutic agents may be attached to the structure that is capable of crossing the blood brain barrier.
- 2 different neurotrophins are attached to an antibody to an endogenous BBB receptor-mediated transport system. Any combination of neurotrophins may be used. Particularly useful in some embodiments of the invention are neurotrophins used as precursors for fusion proteins that cross the BBB are those that naturally form dimeric structures, similar to BDNF or GDNF.
- Certain neurotrophins such as BDNF or NT-3 may form hetero-dimeric structures, and in some embodiments the invention provides a fusion protein constructed of one neurotrophin monomer fused to one chain (e.g., heavy chain) of an antibody, e.g., of the HIRMAb, and another neurotrophin monomer fused to the second chain of the antibody.
- the molecular weight range of recombinant proteins that may be fused to the molecular Trojan horse ranges from 1000 Daltons to 500,000 Daltons.
- more than one type of structure capable of crossing the BBB e.g., molecular Trojan horse
- the different structures may be covalently attached to a single neurotherapeutic agent, e.g., a single neurotrophin such as GDNF, or multiple neurotherapeutics, e.g., multiple neurotrophins, or any combination thereof.
- a single neurotrophin such as GDNF
- multiple neurotherapeutics e.g., multiple neurotrophins, or any combination thereof.
- the neuroprotective recombinant protein can be fused to multiple molecular Trojan horses that undergo receptor- mediated transport across the blood-brain barrier, including monoclonal antibodies to the insulin receptor, transferrin receptor, insulin-like growth factor (IGF) receptor, or the low density lipoprotein (LDL) receptor or the endogenous ligand, including insulin, transferrin, the IGFs, or LDL.
- Ligands that traverse the blood-brain barrier via absorptive-mediated transport may also be used as molecular Trojan horses including cationic proteins, or carbohydrate bearing proteins that bind to membrane lectins.
- the molecular weight range of molecular Trojan horses is 1000 Daltons to 500,000 Daltons.
- the covalent linkage between the structure capable of crossing the BBB and the neurotherapeutic agent may be direct, e.g., a peptide bond between the terminal amino acid of one peptide and the terminal amino acid of the other peptide to which it is linked, or indirect, via a linker.
- a linker may be any suitable linker, e.g., a peptide linker.
- a peptide linker it may be 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more than 10 amino acids in length. In some embodiments, a three amino acid linker is used.
- the linker has the sequence ser-ser-met.
- the covalent linkage may be cleavable, however this is not a requirement for activity of the system in some embodiments; indeed, an advantage of these embodiments of the present invention is that the fusion protein, without cleavage, is partially or fully active both for transport and for activity once across the BBB.
- a noncovalent attachment may be used.
- An example of noncovalent attachment of the MTH, e.g., MAb, to the large molecule therapeutic neuroprotective factor is avidin/streptavidin-biotin attachment.
- avidin/streptavidin-biotin attachment is further described in U.S. Patent Application No. 10/858,729, entitled “Anti-growth factor receptor avidin fusion proteins as universal vectors for drug delivery," filed April 21, 2005, which is hereby incorporated by reference in its entirety.
- the neurotherapeutic agent may be any suitable neurotherapeutic agent, such as a neurotrophin.
- the neurotherapeutic agent is a neurotrophin such as brain derived neurotrophic factor (BDNF), nerve growth factor (NGF), neurotrophin-4/5, fibroblast growth factor (FGF)-2 and other FGFs, neurotrophin (NT>3, erythropoietin (EPO), hepatocyte growth factor (HGF), epidermal growth factor (EGF), transforming growth factor (TGF)- ⁇ , TGF- ⁇ , vascular endothelial growth factor (VEGF), interleukin-1 receptor antagonist (IL- Ira), ciliary neurotrophic factor (CNTF), glial-derived neurotrophic factor (GDNF), neurturin, platelet-derived growth factor (PDGF), heregulin, neuregulin, artemin, persephin, interleukins, granulocyte-colony stimulating factor (CSF), granulocyte-macrophage-C
- BDNF brain
- the neurotrophin is BDNF.
- the BDNF may be native BDNF or a variant BDNF. Some embodiments utilize a two amino acid carboxyl-truncated variant.
- the BDNF can be a human BDNF.
- the BDNF contains a sequence that is about 60, 70, 80, 90, 95, 99, or 100% identical to the sequence of amino acids 466-582 of SEQ ID NO: 24.
- the invention provides compositions containing a neurotherapeutic agent covalently linked to a structure that is capable of crossing the BBB where the composition is capable of producing an average elevation of concentration in the brain of the neurotherapeutic agent of at least about 1, 2, 3, 4, 5, 10, 20, 30, 40, or 50 ng/gram brain following peripheral administration, where the neurotherapeutic agent is a neurotrophin and the structure that is capable of crossing the BBB is a MAb to an endogenous BBB receptor mediated transport system.
- the antibody can be glycosylated or nonglycosylated; in some embodiments, the antibody is glycosylated, e.g., in a glycosylation pattern produced by its synthesis in a CHO cell.
- the neurotrophin is GDNF 5 e.g., a mature human GDNF (SEQ ID NO:52) or a sequence variant thereof.
- the MAb can be an antibody to the insulin BBB receptor mediated transport system, e.g., a chimeric MAb.
- the antibody can be a chimeric antibody with sufficient human sequence that it is suitable for administration to a human, e.g., at least about 80% human sequence, e.g., 85%, 90%, 95%, or another percent human amino acid sequence from about 80% to about 100% human sequence.
- the insulin receptor is a human insulin receptor and the GDNF is a human GDNF.
- the GDNF contains a sequence that is about 60, 70, 80, 90, 95, 99, or 100% identical to the sequence of SEQ ID NO: 44.
- the GDNF can be covalently linked at its amino terminus to the carboxy terminus of the heavy chain of the MAb, optionally with a linker between the termini, such as the three amino-acid linker ser-ser- met, or the two amino acid linker ser-ser.
- the heavy chain of the MAb contains a sequence that is about 60, 70, 80, 90, 95, 99, or 100% identical to amino acids 20-462 of SEQ ID NO: 24.
- the light chain of the MAb contains a sequence that is about 60, 70, 80, 90, 95, 99, or 100% identical to amino acids 21-234 of SEQ ID NO: 36.
- the invention also provides compositions containing an agent that is covalently linked to a chimeric MAb to the human BBB insulin receptor.
- the heavy chain of the MAb is covalently linked to the agent to form a fusion protein.
- the agent can be any agent described herein, i.e., any agent for which transport across the BBB is desired.
- the agent is a therapeutic agent, such as a neurotherapeutic agent as described herein, e.g., a neurotrophin such as GDNF Strikingly, it has been found that multifunctional fusion proteins of the invention, e.g., difunctional fusion proteins, retain a high proportion of the activity of the separate portions, e.g., the portion that is capable of crossing the BBB and the portion that is active in the CNS.
- a neurotherapeutic agent as described herein, e.g., a neurotrophin such as GDNF Strikingly
- multifunctional fusion proteins of the invention e.g., difunctional fusion proteins, retain a high proportion of the activity of the separate portions, e.g., the portion that is capable of crossing the BBB and the portion that is active in the CNS.
- the invention further provides a fusion protein containing a structure capable of crossing the BBB, covalently linked to a peptide that is active in the central nervous system (CNS), where the structure capable of crossing the BBB and the peptide that is active in the central nervous system each retain an average of at least about 10, 20, 30, 40, 50, 60, 70, 80, 90, 95, 99, or 100% of their activities, compared to their activities as separate entities.
- the structure capapble of crossing the BBB, and the peptide that is active in the central nervous system each retain about 20% to about 80% of their activities (e.g., about 30% to about 70, or about 40% to about 60%) compared to their activities as separate entities.
- the invention provides a fusion protein containing a structure capable of crossing the BBB, covalently linked to a peptide that is active in the central nervous system (CNS), where the structure capable of crossing the blood brain barrier and the peptide that is active in the central nervous system each retain an average of at least about 50% of their activities, compared to their activities as separate entities.
- the invention provides a fusion protein containing a structure capable of crossing the BBB, covalently linked to a peptide that is active in the central nervous system (CNS), where the structure capable of crossing the blood brain barrier and the peptide that is active in the central nervous system each retain an average of at least about 60% of their activities, compared to their activities as separate entities.
- the invention provides a fusion protein containing a structure capable of crossing the BBB, covalently linked to a peptide that is active in the central nervous system (CNS), where the structure capable of crossing the blood brain barrier and the peptide that is active in the central nervous system each retain an average of at least about 70% of their activities, compared to their activities as separate entities.
- the invention provides a fusion protein containing a structure capable of crossing the BBB, covalently linked to a peptide that is active in the central nervous system (CNS), where the structure capable of crossing the blood brain barrier and the peptide that is active in the central nervous system each retain an average of at least about 80% of their activities, compared to their activities as separate entities.
- the invention provides a fusion protein containing a structure capable of crossing the BBB, covalently linked to a peptide that is active in the central nervous system (CNS), where the structure capable of crossing the blood brain barrier and the peptide that is active in the central nervous system each retain an average of at least about 90% of their activities, compared to their activities as separate entities.
- CNS central nervous system
- the structure capable of crossing the blood brain barrier retains at least about 10, 20, 30, 40, 50, 60, 70, 80, 90, 95, 99, or 100% of its activity, compared to its activity as a separate entity, and the peptide that is active in the central nervous system retains at least about 10, 20, 30, 40, 50, 60, 70, 80, 90, 95, 99, or 100% of its activity, compared to its activity as a separate entity.
- activity includes physiological activity, e.g., ability to cross the BBB and/or therapeutic activity and also binding affinity of the structures for their respective receptors.
- Transport of the structure capable of crossing the BBB across the BBB may be compared for the structure alone and for the structure as part of a fusion structure of the invention by standard methods.
- pharmacokinetics and brain uptake of the fusion structure e.g., fusion protein
- a model animal e.g., a mammal such as a primate
- Such techniques are illustrated in Example 7, which demonstrates pharmacokinetics and brain uptake of a fusion protein of the invention by the adult Rhesus monkey.
- standard models for the function of an agent e.g.
- the therapeutic or protective function of a therapeutic agent may also be used to compare the function of the agent alone and the function of the agent as part of a fusion structure of the invention. See, e.g., Example 5, which demonstrates the activity of a neurotrophin alone and the same neurotrophin bound to a fusion protein in a model system (hypoxia-reoxygenation in human neural cells).
- Example 5 which demonstrates the activity of a neurotrophin alone and the same neurotrophin bound to a fusion protein in a model system (hypoxia-reoxygenation in human neural cells).
- the fusion protein of the invention retained about 100% of the transport ability and the therapeutic function of its individual components, i.e., a structure capable of crossing the BBB (a MAb to the human insulin receptor) and a therapeutic agent (BDNF).
- BBB a MAb to the human insulin receptor
- BDNF therapeutic agent
- binding affinity for receptors may be used as a marker of activity. Binding affinity for the receptor is compared for the structure alone and for the structure when part of the fusion protein.
- a suitable type of binding affinity assay is the competitive ligand binding assay (CLBA).
- CLBA competitive ligand binding assay
- a CLBA may be used both to assay the affinity of the MAb for its receptor and the neurotrophin for its receptor, either as part of the fusion protein or as separate entities, and percentage affinity calculated.
- the peptide that is active in the CNS is highly ionic, e.g., cationic, causing a high degree of non-specific binding
- suitable measures should be taken to eliminate the nonspecific binding. See, e.g., Example 4. "Average" measurements are the average of at least three separate measurements.
- the structure capable of crossing the blood brain barrier crosses the BBB on an endogenous BBB receptor-mediated transporter, such as a transporter selected from the group consisting of the insulin transporter, the transferrin transporter, the lept ⁇ i transporter, the LDL transporter, and the IGF receptor.
- the endogenous BBB receptor-mediated transporter is selected from the group consisting of the insulin transporter and the transferrin transporter.
- the endogenous BBB receptor- mediated transporter is the insulin transporter, e.g., the human insulin transporter.
- the structure capable of crossing the BBB can be an antibody, e.g., a MAb such as a chimeric MAb.
- the antibody can be an antibody to an endogenous BBB receptor-mediated transporter, as described herein.
- the peptide that is active in the CNS can be a neurotherapeutic agent, e.g., a neurotrophin.
- the neurotrophin is selected from the group consisting of brain-derived neurotrophic factor, nerve growth factor (NGF), neurotrophin-4/5, fibroblast growth factor (FGF)-2 and other FGFs, neurotrophin (NT)-3, erythropoietin (EPO), hepatocyte growth factor (HGF), epidermal growth factor (EGF), transforming growth factor (TGF)- ⁇ , TGF- ⁇ , vascular endothelial growth factor (VEGF), interleukin-1 receptor antagonist (IL- Ira), ciliary neurotrophic factor (CNTF), glial-derived neurotrophic factor (GDNF), neurturin, platelet-derived growth factor (PDGF), heregulin, neuregulin, artemin, persephin, interleukins
- the neurotrophin is BDNF such as a truncated BDNF, e.g., a carboxyl-tnuicated BDNF.
- the carboxyl-truncated BDNF is lacking the two carboxyl terminal amino acids in some embodiments.
- the structure capable of crossing the BBB and the neurotherapeutic agent are covalently linked by a peptide linker in some embodiments.
- the invention provides compositions that increase the serum half- life of cationic substances.
- One limitation for many current therapeutics, especially cationic therapeutic peptides is their rapid clearance from the circulation.
- the positive charge on the cationic substance such as cationic peptides, rapidly interacts with negative charges on cell membranes, which triggers an absorptive-mediated endocytosis into the cell, particularly liver and spleen.
- This is true not only for neurotherapeutics (where rapid clearance means only limited contact with the BBB and thus limited ability to cross the BBB) but for other agents as well, such as cationic import peptides such as the tat peptide, or cationic proteins (e.g. protamine, polylysine, polyarginine) that bind nucleic acids, or cationic proteins such as avidin that bind biotinylated drugs.
- fusion compositions of the invention that include a cationic therapeutic peptide covalently linked to an immunoglobulin show greatly enhanced serum half-life compared to the same peptide when it was not covalently part of a fusion immunoglobulin.
- a highly cationic protein e.g., BDNF
- an immunoglobulin e.g. HIRMAb 5
- the invention provides composition comprising a cationic therapeutic peptide covIERly linked to an immunoglobulin, wherein the cationic therapeutic peptide in the composition has a serum half-life that is an average of at least about 1.5, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 100, or more than about 100-fold greater than the serum half-life of the cationic therapeutic peptide alone.
- the invention provides a composition comprising a cationic therapeutic peptide covIERly linked to an immunoglobulin, wherein the cationic therapeutic peptide in the composition has a mean residence time (MRT) in the serum that is an average of at least about 1.5, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 100, or more than about 100-fold greater than the serum half-life of the cationic therapeutic peptide alone.
- MRT mean residence time
- the invention provides composition comprising a cationic therapeutic peptide covIERly linked to an immunoglobulin, wherein the cationic therapeutic peptide in the composition has a systemic clearance rate that is an average of at least about 1.5, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 100, or more than about 100-fold slower than the systemic clearance rate of the cationic therapeutic peptide alone.
- the invention provides composition comprising a cationic therapeutic peptide covIERly linked to an immunoglobulin, wherein the cationic therapeutic peptide in the composition has average blood level after peripheral administration that is an average of at least about 1.5, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 100, or more than about 100- fold greater than the average blood level after peripheral administration of the cationic therapeutic peptide alone.
- the cationic therapeutic peptide comprises a neurotherapeutic agent.
- neurotherapeutic agents that are cationic peptides interferons, interleukins, cytokines, or growth factors with an isoelectric point (pi) above 8.
- the neurotherapeutic agent is a neurotrophin.
- Cationic peptide neurotrophins include BDNF, GDNF, NT-3, NT-4/5, NGF, and FGF-2.
- the neurotrophin is BDNF or GDNF.
- the immunoglobulin is an antibody to an endogenous BBB receptor-mediated transport system.
- the endogenous BBB receptor-mediated transport system is selected from the group consisting of the insulin BBB transport system, the BBB transferrin receptor, the BBB leptin receptor, the BBB IGF receptor, or the BBB lipoprotein receptor.
- the antibody is an antibody to the endogenous insulin BBB receptor-mediated transport system. Antibodies can be any suitable antibody as described herein.
- compositions that contain one or more compositions of the invention and a pharmaceutically acceptable excipient.
- a pharmaceutically acceptable carrier/excipient can be found in Remington 's Pharmaceutical Sciences, Gennaro, AR, ed., 20th edition, 2000: Williams and Wilkins PA, USA.
- Pharmaceutical compostions of the invention include compositions suitable for administration via any peripheral route, including intravenous, subcutaneous, intrmuscular, intraperitoneal injection; oral, rectal, transbuccal, pulmonary, transdermal, intranasal, or any other suitable route of peripheral administration.
- compositions of the invention are particular suited for injection, e.g., as a pharmaceutical composition for intravenous, subcutaneous, intramuscular, or intraperitona ⁇ administration.
- Aqueous compositions of the present invention comprise an effective amount of a composition of the present invention, which may be dissolved or dispersed in a pharmaceutically acceptable carrier or aqueous medium.
- pharmaceutically or pharmacologically acceptable refer to molecular entities and compositions that do not produce an adverse, allergic or other untoward reaction when administered to an animal, e.g., a human, as appropriate.
- pharmaceutically acceptable carrier includes any and all solvents, dispersion media, coatings, antibacterial and antifungal agents, isotonic and absorption delaying agents and the like.
- the use of such media and agents for pharmaceutically active substances is well known in the art. Except insofar as any conventional media or agent is incompatible with the active ingredient, its use in the therapeutic compositions is contemplated. Supplementary active ingredients can also be incorporated into the compositions.
- Exemplary pharmaceutically acceptable carriers for injectable compositions can include salts, for example, mineral acid salts such as hydrochlorides, hydrobromides, phosphates, sulfates, and the like; and the salts of organic acids such as acetates, propionates, malonates, benzoates, and the like.
- compositions of the invention may be provided in liquid form, and formulated in saline based aqueous solution of varying pH (5-8), with or without detergents such polysorbate-80 at 0.01-1%, or carbohydrate additives, such mannitol, sorbitol, or trehalose.
- Commonly used buffers include histidine, acetate, phosphate, or citrate.
- these preparations can contain a preservative to prevent the growth of microorganisms.
- the prevention of the action of microorganisms can be brought about by various antibacterial and antifungal agents, for example, parabens, chlorobutanol; phenol, sorbic acid, thimerosal, and the like, In many cases, it will be preferable to include isotonic agents, for example, sugars or sodium chloride.
- Prolonged absorption of the injectable compositions can be brought about by the use in the compositions of agents delaying absorption, for example, aluminum monostearate, and gelatin.
- compositions For human administration, preparations meet sterility, pyrogenicity, general safety, and purity standards as required by FDA and other regulatory agency standards.
- the active compounds will generally be formulated for parenteral administration, e.g., formulated for injection via the intravenous, intramuscular, subcutaneous, intralesional, or intraperitoneal routes.
- parenteral administration e.g., formulated for injection via the intravenous, intramuscular, subcutaneous, intralesional, or intraperitoneal routes.
- the preparation of an aqueous composition that contains an active component or ingredient will be known to those of skill in the art in light of the present disclosure.
- such compositions can be prepared as injectables, either as liquid solutions or suspensions; solid forms suitable for use in preparing solutions or suspensions upon the addition of a liquid prior to injection can also be prepared; and the preparations can also be emulsified.
- Sterile injectable solutions are prepared by incorporating the active compounds in the required amount in the appropriate solvent with various of the other ingredients enumerated above, as required, followed by filtered sterilization.
- dispersions are prepared by incorporating the various sterilized active ingredients into a sterile vehicle which contains the basic dispersion medium and the required other ingredients from those enumerated above.
- methods of preparation include vacuum- drying and freeze-drying techniques which yield a powder of the active ingredient plus any additional desired ingredient from a previously sterile-filtered solution thereof.
- solutions Upon formulation, solutions will be administered in a manner compatible with the dosage formulation and in such amount as is therapeutically effective.
- the formulations are easily administered in a variety of dosage forms, such as the type of injectable solutions described above, but drug release capsules and the like can also be employed
- unit dose refers to physically discrete units suitable for use in a subject, each unit containing a predetermined-quantity of the therapeutic composition calculated to produce the desired responses, discussed above, in association with its administration, i.e., the appropriate route and treatment regimen.
- the quantity to be administered both according to number of treatments and unit dose, depends on the subject to be treated, the state of the subject and the protection desired. The person responsible for administration will, in any event, determine the appropriate dose for the individual subject.
- the active therapeutic agents may be formulated within a mixture to comprise about
- a dosage of about 2.5 to about 25 mg of a fusion protein of the invention is used as a unit dose for administration to a human, e.g., about 2.5 to about 25 mg of a fusion protein of GDNF and a HIR MAb.
- intradermal administration See U.S. Pat. Nos. 5,997,501 ; 5,848,991; and 5,527,288)
- pulmonary administration See U.S. Pat. Nos. 6,361,760; 6,060,069; and 6,041,775
- buccal administration See U.S. Pat. Nos. 6,375,975; and 6,284,262
- transdermal administration See U.S. Pat. Nos. 6,348,210; and 6,322,808
- transmucosal administration See U.S. Pat. No.
- nasal solutions are usually aqueous solutions designed to be administered to the nasal passages in drops or sprays. Nasal solutions are prepared so that they are similar in many respects to nasal secretions. Thus, the aqueous nasal solutions usually are isotonic and slightly buffered to maintain a pH of 5.5 to 6.5.
- antimicrobial preservatives similar to those used in ophthalmic preparations and appropriate drug stabilizers, if required, may be included in the formulation.
- Various commercial nasal preparations are known and include, for example, antibiotics and antihistamines and are used for asthma prophylaxis.
- Additional formulations which are suitable for other modes of administration, include suppositories and pessaries.
- a rectal pessary or suppository may also be used.
- Suppositories are solid dosage forms of various weights and shapes, usually medicated, for insertion into the rectum or the urethra. After insertion, suppositories soften, melt or dissolve in the cavity fluids.
- traditional binders and carriers generally include, for example, polyalkylene glycols or triglycerides; such suppositories may be formed from mixtures containing the active ingredient in any suitable range, e.g., in the range of 0.5% to 10%, preferably l%-2%.
- Oral formulations include such normally employed excipients as, for example, pharmaceutical grades of mannitol, lactose, starch, magnesium stearate, sodium saccharine, cellulose, magnesium carbonate and the like. These compositions take the form of solutions, suspensions, tablets, pills, capsules, sustained release formulations, or powders.
- oral pharmaceutical compositions will comprise an inert diluent or assimilable edible carrier, or they may be enclosed in a hard or soft shell gelatin capsule, or they may be compressed into tablets, or they may be incorporated directly with the food of the diet.
- the active compounds may be incorporated with excipients and used in the form of ingestible tablets, buccal tables, troches, capsules, elixirs, suspensions, syrups, wafers, and the like.
- Such compositions and preparations can contain at least 0.1% of active compound.
- the percentage of the compositions and preparations may, of course, be varied, and may conveniently be between about 2 to about 75% of the weight of the unit, or between about 25-60%.
- the amount of active compounds in such therapeutically useful compositions is such that a suitable dosage will be obtained.
- the tablets, troches, pills, capsules and the like may also contain the following: a binder, such as gum tragacanth, acacia, cornstarch, or gelatin; excipients, such as dicalcium phosphate; a disintegrating agent, such as corn starch, potato starch, alginic acid and the like; a lubricant, such as magnesium stearate; and a sweetening agent, such as sucrose, lactose or saccharin may be added or a flavoring agent, such as peppermint, oil of wintergreen, or cherry flavoring.
- a binder such as gum tragacanth, acacia, cornstarch, or gelatin
- excipients such as dicalcium phosphate
- a disintegrating agent such as corn starch, potato starch, alginic acid and the like
- a lubricant such as magnesium stearate
- a sweetening agent such as sucrose, lactose or saccharin may be added or
- tablets, pills, or capsules may be coated with shellac, sugar or both.
- a syrup of elixir may contain the active compounds sucrose as a sweetening agent, methylene and propyl parabens as preservatives, a dye and flavoring, such as cherry or orange flavor.
- an oral pharmaceutical composition may be enterically coated to protect the active ingredients from the environment of the stomach; enteric coating methods and formulations are well-known in the art. VI. Nucleic acids, vectors, cells, and manufacture.
- the invention also provides nucleic acids, vectors, cells, and methods of production.
- the invention provides nucleic acids that code for proteins or peptides of the invention.
- the invention provides a single nucleic acid sequence containing a first sequence coding for a light chain of an immunoglobulin and second sequence coding a heavy chain of the immunoglobulin, where either the first sequence also codes for a peptide that is expressed as a fusion protein of the peptide covalently linked to the light chain, or the second sequence also codes for a peptide that is expressed as a fusion protein of the peptide covalently linked to the heavy chain.
- the invention provides nucleic acid sequences, and in some embodiments the invention provides nucleic acid sequences that are at least about 60, 70, 80, 90, 95, 99, or 100% identical to a particular nucleotide sequence.
- the invention provides a nucleic acid containing a first sequence that is at least about 60, 70, 80, 90, 95, 99, or 100% identical to SEQ ID NO:45and a second sequence that is at least about 60, 70, 80, 90, 95, 99, or 100% identical to nucleotides 1396-1746 of SEQ ID NO: 33.
- the invention provides a nucleic acid containing a sequence that is at least about 60, 70, 80, 90, 95, 99, or 100% identical to SEQ ID NO:45.
- sequence comparison For sequence comparison, of two nucleic acids, typically one sequence acts as a reference sequence, to which test sequences are compared.
- test and reference sequences are entered into a computer, subsequence coordinates are designated, if necessary, and sequence algorithm program parameters are designated. Default program parameters can be used, or alternative parameters can be designated.
- sequence comparison algorithm then calculates the percent sequence identities for the test sequences relative to the reference sequence, based on the program parameters.
- a “comparison window”, as used herein, includes reference to a segment of any one of the number of contiguous positions selected from the group consisting of from 20 to 600, usually about 50 to about 200, more usually about 100 to about 150 in which a sequence may be compared to a reference sequence of the same number of contiguous positions after the two sequences are optimally aligned.
- Methods of alignment of sequences for comparison are well-known in the art.
- Optimal alignment of sequences for comparison can be conducted, including but not limited to, by the local homology algorithm of Smith and Waterman (1970) Adv. Appt. Math. 2:482c, by the homology alignment algorithm of Need ⁇ eman and Wunsch (1970) J. MoI. Biol.
- the BLAST algorithm parameters W, T, and X determine the sensitivity and speed of the alignment.
- the BLAST algorithm is typically performed with the "low complexity" filter turned off.
- the BLAST algorithm also performs a statistical analysis of the similarity between two sequences ⁇ see, e.g., Karlin and Altschul (1993) Proc. Natl. Acad. ScL USA 90:5873-5787).
- BLAST algorithm One measure of similarity provided by the BLAST algorithm is the smallest sum probability (P(N)), which provides an indication of the probability by which a match between two nucleotide or amino acid sequences would occur by chance.
- P(N) the smallest sum probability
- a nucleic acid is considered similar to a reference sequence if the smallest sum probability in a comparison of the test nucleic acid to the reference nucleic acid is less than about 0.2, more preferably less than about 0.01, and most preferably less than about 0.001.
- the invention provides nucleic acids that code for any of the peptides of the invention.
- the invention provides a single nucleic acid sequence containing a gene coding for a light chain of an immunoglobulin and a gene coding for a fusion protein, where the fusion protein includes a heavy chain of the immunoglobulin covalently linked to a peptide.
- the peptide is a therapeutic peptide.
- the peptide is a neurotherapeutic peptide, e.g., a neurotrophin such as BDNF or GDNF (e.g., mature human GDNF).
- the BDNF is a two amino acid carboxy-truncated BDNF.
- the immunoglobulin is an IgG.
- the IgG is a MAb, such as a chimeric MAb.
- the antibody can be an antibody to a transport system, e.g., an endogenous BBB receptor-mediated transport system such as the endogenous BBB receptor-mediated insulin transport system.
- the endogenous BBB receptor-mediated insulin transport system is a human endogenous BBB receptor-mediated insulin transport system and wherein the peptide to which the immunoglobulin heavy chain is covalently linked is human BDNF or human GDNF.
- any suitable peptide, neurotherapeutic peptide, neurotrophin, BDNF, GDNF (e.g., mature human GDNF), antibody, monoclonal antibody, or chimeric antibody, as described herein, may be coded for by the nucleic acid, combined as a fusion protein and coded for in a single nucleic acid sequence.
- any combination of suitable codons may be used to code for the desired fusion protein.
- other elements useful in recombinant technology such as promoters, termination signals, and the like, may also be included in the nucleic acid sequence. Such elements are well-known in the art.
- all nucleic acid sequences described and claimed herein include the complement of the sequence. [00151]
- the nucleic acid codes for a BDNF, e.g., a variant BDNF, or GDNF
- the BDNF contains a sequence that is about 60, 70,80, 90, 95, 99, or 100% identical to the sequence of amino acids 466-582 of SEQ ID NO: 24.
- the GDNF contains a sequence that is at least about 60, 70,80, 90, 95, 99, or 100% identical to the sequence of SEQ ID NO:52.
- the amino acid sequence of the encoded GDNF consists essentially of SEQ ID NO:52.
- the nucleic acid codes for a fusion protein comprising an amino acid sequence that is at least about 60, 70,80, 90, 95, 99, or 100% identical to the sequence of SEQ ID NO:46.
- the encoded nucleic acid comprises the amino acid sequence of SEQ ID NO:46.
- the BDNF or GDNF is linked at its amino terminus to carboxy terminus of the heavy chain of the immunoglobulin, e.g., MAb.
- the heavy chain of the MAb can comprise a sequence that is about 60, 70, 80, 90, 95, 99 or 100% identical to amino acids 20-462 of SEQ ID NO: 24.
- the light chain of the immunoglobulin comprises a sequence that is about 60, 70, 80, 90, 95, 99 or 100% identical to amino acids 21-234 of SEQ ID NO: 36.
- the nucleic acid can further contain a nucleic acid sequence that codes for a peptide linker between the heavy chain of the MAb and the BDNF or GDNF.
- the linker is S-S-M.
- the nucleic acid may further contain a nucleic acid sequence coding for a signal peptide, wherein the signal peptide is linked to the heavy chain. Any suitable signal peptide, as known in the art or subsequently developed, may be used.
- the signal peptide attached to the heavy chain comprises a sequence that is about 60, 70, 80, 90, 95, 99, or 100% identical to amino acids 1-19 of SEQ ID NO: 24.
- the nucleic acid contains a nucleic acid sequence coding for another signal peptide, wherein the other signal peptide is linked to the light chain.
- the signal peptide linked to the light chain can comprise a sequence that is about 60, 70, 80, 90, 95, 99, or 100% identical to amino acids 1-20 of SEQ ID NO: 36.
- the nucleic acid can contain a nucleic acid sequence coding for a selectable marker.
- the selectable marker is DHFR.
- the sequence of the DHFR can be about 60, 70, 80, 90, 95, 99, or 100% identical to amino acids 1-187 of SEQ ID NO: 38.
- the invention provides a nucleic acid comprising a first sequence that codes for a neurotherapeutic peptide, e.g., a neurotrophin such as BDNF, in the same open reading frame as a second sequence that codes for an immunoglobulin component.
- the immunoglobulin component can be, e.g., a light chain or a heavy chain, e.g., that is at least about 60, 70, 80, 90, 95, 99, or 100% identical to nucleotides 58-1386-of SEQ ID NO: 33 and a second sequence that is at least about 60, 70, 80, 90, 95, 99, or 100% identical to nucleotides 1396-1746 of SEQ ID NO: 33.
- the nucleic acid also contains a third sequence that is at least about 60, 70, 80, 90, 95, 99, or 100% identical to nucleotides 61-702 of SEQ ID NO: 35.
- the nucleic acid further contains a fourth sequence that codes for a first signal peptide and a fifth sequence that codes for a second signal peptide.
- the fourth sequence is at least about 60, 70, 80, 90, 95, 99, or 100% identical to nucleotides 1-57 of SEQ ID NO: 33 and the fifth sequence is at least about 60, 70, 80, 90, 95, 99, or 100% identical to nucleotides 1-60 of SEQ ID NO: 35.
- the nucleic acid further contains a sequence that codes for a selectable marker, such as dihydrofolate reductase (DHFR).
- DHFR dihydrofolate reductase
- the sequence that codes for the DHFR is at least about 60, 70, 80, 90, 95, 99, or 100% identical to nucleotides 1-561 of SEQ ID NO: 37.
- the invention also provides vectors.
- the vector can contain any of the nucleic acid sequences described herein.
- the invention provides a single tandem expression vector containing nucleic acid coding for an antibody heavy chain fused to a peptide, e.g., a therapeutic peptide such as a neurotrophin, and nucleic acid coding for a light chain of the antibody, all incorporated into a single piece of nucleic acid, e.g., a single piece of DNA.
- the singie tandem vector can also include one or more selection and/or amplification genes.
- a method of making an exemplary vector of the invention is provided in the Examples. However, any suitable techniques, as known in the art, may be used to construct the vector.
- the use of a single tandem vector has several advantages over previous techniques.
- the transfection of a eukaryotic cell line with immunoglobulin G (IgG) genes generally involves the co- transfection of the cell line with separate plasmids encoding the heavy chain (HC) and the light chain (LC) comprising the IgG.
- the gene encoding the recombinant therapeutic protein may be fused to either the HC or LC gene.
- this co- transfection approach makes it difficult to select a cell line that has equally high integration of both the HC and LC-fusion genes, or the HC-fusion and LC genes.
- the approach to manufacturing the fusion protein utilized in certain embodiments of the invention is the production of a cell line that is stably transfected with a single plasmid DNA that contains all the required genes on a single strand of DNA, including the HC-fusion protein gene, the LC gene, the selection gene, e.g. neo, and the amplification gene, e.g. the dihydrofolate reductase gene.
- the HC-fusion gene, the LC gene, the neo gene, and the DHFR gene are all under the control of separate, but tandem promoters and separate but tandem transcription termination sequences. Therefore, all genes are equally integrated into the host cell genome, including the fusion gene of the therapeutic protein and either the HC or LC IgG gene.
- the invention further provides cells that incorporate one or more of the vectors of the invention.
- the cell may be a prokaryotic cell or a eukaryotic cell.
- the cell is a eukaryotic cell.
- the cell is a mouse myeloma hybridoma cell.
- the cell is a Chinese hamster ovary (CHO) cell. Exemplary methods for incorporation of the vector(s) into the cell are given in the Examples. However, any suitable techniques, as known in the art, may be used to incorporate the vector(s) into the cell.
- the invention provides a cell capable of expressing an immunoglobulin fusion protein, where the cell is a cell into which has been introduced a single tandem expression vector, where both the immunoglobulin light chain gene and the gene for the immunoglobulin heavy chain fused to the therapeutic agent, are incorporated into a single piece of nucleic acid, e.g., DNA.
- the invention provides a cell capable of expressing an immunoglobulin fusion protein, where the cell is a cell into which has been stably transfected a single tandem expression vector, where both the immunoglobulin heavy chain gene and the gene for the immunoglobulin light chain fused to the therapeutic agent, are incorporated into a single piece of nucleic acid, e.g., DNA.
- the introduction of the tandem vector may be by, e.g., permanent integration into the chromsomal nucleic acid, or by, e.g., introduction of an episomal genetic element.
- the invention provides methods of manufacture.
- the invention provides a method of manufacturing an immunoglobulin fusion protein, where the fusion protein contains an immunoglobulin heavy chain fused to a therapeutic agent, by introducing into a eukaryotic cell a single tandem expression vector, where both the immunoglobulin light chain gene and the gene for the immunoglobulin heavy chain fused to the therapeutic agent, are incorporated into a single piece of nucleic acid, e.g., DNA.
- the invention provides a method of manufacturing an immunoglobulin fusion protein, where the fusion protein contains an immunoglobulin light chain fused to a therapeutic agent, by Iy introducing into a eukaryotic cell a single tandem expression vector, where both the immunoglobulin heavy chain gene and the gene for the immunoglobulin light chain fused to the therapeutic agent, are incorporated into a single piece of nucleic acid, e.g., DNA.
- the introduction of the vector is accomplished by integration into the host cell genome.
- the introduction of the vector is accomplished by introduction of an episomal genetic element containing the vector into the host cell. Episomal genetic elements are well-known in the art
- the therapeutic agent is a neurotherapeutic agent.
- the single piece of nucleic acid further includes one or more genes for selectable markers. In some embodiments, the single piece of nucleic acid further includes one or more amplification genes.
- the immunoglobulin is an IgG, e.g., a MAb such as a chimeric MAb.
- the methods may further include expressing the immunoglobulin fusion protein, and/or purifying the immunoglobulin fusion protein. Exemplary methods for manufacture, including expression and purification, are given in the Examples.
- any suitable techniques may be used to manufacture, optionally express, and purify the proteins.
- These include non-recombinant techniques of protein synthesis, such as solid phase synthesis, manual or automated, as first developed by Merrifield and described by Stewart et al. in Solid Phase Peptide Synthesis (1984).
- Chemical synthesis joins the amino acids in the predetermined sequence starting at the C-terminus.
- Basic solid phase methods require coupling the C-terminal protected ⁇ -amino acid to a suitable insoluble resin support.
- Amino acids for synthesis require protection on the ⁇ -amino group to ensure proper peptide bond formation with the preceding residue (or resin support).
- the ⁇ -amino protecting group is removed to allow the addition of the next residue.
- ⁇ -protecting groups have been described, see Stewart et al. in Solid Phase Peptide Synthesis (1984), with the acid labile, urethane-based tertiary-butyloxycarbonyl (Boc) being the historically preferred.
- Other protecting groups, and the related chemical strategies may be used, including the base labile 9-fluorenylmethyloxycarbonyl (FMOC).
- FMOC base labile 9-fluorenylmethyloxycarbonyl
- the reactive amino acid sidechain functional groups require blocking until the synthesis is completed.
- the complex array of functional blocking groups, along with strategies and limitations to their use, have been reviewed by Bodansky in Peptide Synthesis (1976) and, Stewart et al. in Solid Phase Peptide Synthesis (1984).
- Solid phase synthesis is initiated by the coupling of the described C-terminal ⁇ -protected amino acid residue. Coupling requires activating agents, such as dicyclohexycarbodiimide (DCC) with or without l-hydroxybenzo-triazole (HOBT), diisopropylcarbodiimide (DIIPC), or ethyldimethylaminopropylcarbodiimide (EDC).
- DCC dicyclohexycarbodiimide
- DIIPC diisopropylcarbodiimide
- EDC ethyldimethylaminopropylcarbodiimide
- the ⁇ -amino protected group is removed by trifluoroacetic acid (25% or greater) in dichloromethane in the case of acid labile tertiary-butyloxycarbonyl (Boc) groups.
- a neutralizing step with triethylamine (10%) in dichloro-methane recovers the free amine (versus the salt).
- the cycle of deprotection, neutralization and coupling, with intermediate wash steps, is repeated in order to extend the protected peptide chain.
- Each protected amino acid is introduced in excess (three to five fold) with equimolar amounts of coupling reagent in suitable solvent.
- reagents are applied to cleave the peptide form the resin and to remove the side chain blocking groups.
- Anhydrous hydrogen fluoride (HF) cleaves the acid labile tertiary-butyloxycarbonyl (Boc) chemistry groups.
- nucleophilic scavengers such as dimethylsulf ⁇ de and anisole, are included to avoid side reactions especially on side chain functional groups.
- the invention also provides methods.
- the invention provides methods for transport of an agent active in the CNS across the BBB in an effective amount.
- the invention provides therapeutic, diagnostic, or research methods. Diagnostic methods include the development of peptide radiopharmaceuticals capable of transport across the BBB, such as the fusion of a peptide ligand, or peptidomimetic MAb for an endogenous receptor in the brain, followed by the radiolabelling of the fusion protein, followed by systemic administration, and external imaging of the localization within the brain of the peptide radiopharmaceutical.
- neurotrophins such as BDNF or GDNF were injected directly into the brain to achieve a therapeutic effect, because the neurotrophin does not cross the BBB. Therefore, it is not expected that neurotrophic factors will have beneficial effects on brain disorders following the peripheral (intravenous, subcutaneous) administration of these molecules,
- neurotherapeutics can be developed as drugs for peripheral routes of administration, providing the neurotherapeutic is enabled to cross the BBB.
- Attachment of the neurotherapeutic e.g. a neurotrophin such as BDNF or GDNF to a MTH, e.g., the chimeric HIRMAb
- a neurotrophin such as BDNF or GDNF
- MTH e.g., the chimeric HIRMAb
- a neurotrophin such as BDNF or GDNF
- MTH e.g., the chimeric HIRMAb
- the neurotherapeutics offers a new approach to the non-invasive delivery of neurotherapeutics to the CNS in animals, e.g., mammals such as humans for the treatment of acute brain and spinal cord conditions, such as focal brain ischemia, global brain ischemia, and spinal cord injury, and chronic treatment of neurodegenerative disease, including prion diseases, Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), ALS,
- the invention provides methods of transport of an agent active in the CNS from the peripheral circulation across the BBB in an effective amount, where the agent is covalently attached to a structure that crosses the BBB, and where the agent alone is not transported across the BBB in an effective amount.
- the invention provides methods of transport of neurotherapeutic agent from the peripheral circulation across the BBB in a therapeutically effective amount, where the neurotherapeutic agent is covalently attached to a structure that crosses the BBB, and where the neurotherapeutic agent alone is not transported across the BBB in a therapeutically effective amount
- the invention also provides, in some embodiments, methods of treatment of disorders of the CNS by peripheral administration of an effective amount of a therapeutic agent, e.g., a neurotherapeutic agent covalently linked to a structure that is capable of crossing the BBB, where the agent alone is not capable of crossing the BBB in an effective amount when administered peripherally.
- a therapeutic agent e.g., a neurotherapeutic agent covalently linked to a structure that is capable of crossing the BBB, where the agent alone is not capable of crossing the BBB in an effective amount when administered peripherally.
- the CNS disorder is an acute disorder, and, in some cases, may require only a single administration of the agent.
- the CNS disorder is a chronic disorder and may require more than one administration of the agent.
- the effective amount e.g., therapeutically effective amount is such that a concentration in the brain is reached of at least about 0.001, 0.01, 0.1, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 35, 40, 45, 50, 100, or more than 100 ng/gram brain.
- a therapeutically effective amount e.g., of a neurotrophin such as BDNF or GDNF, is such that a brain level is achieved of about 0.1 to 1000, or about 1-100, or about 5-50 ng/g brain.
- the neurotherapeutic agent is a neurotrophin
- the neurotrophin is selected from the group consisting of BDNF, nerve growth factor (NGF), neurotrophin-4/5, fibroblast growth factor (FGF)-2 and other FGFs, neurotrophin (NT)-3, erythropoietin (EPO), hepatocyte growth factor (HGF), epidermal growth factor (EGF), transforming growth factor (TGF)- ⁇ , TGF- ⁇ , vascular endothelial growth factor (VEGF), interleukin-1 receptor antagonist (IL- Ira), ciliary neurotrophic factor (CNTF), glial-derived neurotrophic factor (GDNF), neurturin, platelet-derived growth factor (PDGF), heregulin, neuregulin, artemin, persephin, interleukins, gramilocyte-colony stimulating factor (CSF), granulocyte-macrophage-CSF, netrins, cardiotrophin-1, hedgehogs,
- BDNF nerve growth factor
- the invention provides methods of treating a disorder of the CNS by peripherally administering to an individual in need of such treatment an effective amount of a neurotrophin, where the neurotrophin is capable of crossing the BBB to produce an average elevation of neurotrophin concentration in the brain of at least about 0.1, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 35, 40, 45, 50, 100, or more than 100 ng/gram brain following said peripheral administration, and where the neurotrophin remains at the elevated level for about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more than 10 days after a single administration.
- the neurotrophin remains at a level of greater than about 1 ng/g brain, or about 2 ng/g brain, or about 5 ng/g brain for about 2, 3, 4, 5, 6, 7, 8, 9, 10, or more than 10 days after a single administration.
- the neurotrophin is BDNF, including truncated versions thereof.
- the invention provides methods of treating a disorder of the CNS by peripherally administering to an individual in need of such treatment an effective amount of a composition of the invention.
- peripheral administration includes any method of administration that is not direct administration into the CNS, i.e., that does not involve physical penetration or disruption of the BBB.
- Peripheral administration includes, but is not limited to, intravenous intramuscular, subcutaneous, intraperitoneal, intranasal, transbuccal, transdermal, rectal, transalveolar (inhalation), or oral administration. Any suitable composition of the invention, as described herein, may be used.
- the composition is a neurotrophin covalently linked to a chimeric HIR-MAb.
- the neurotrophin is a BDNF.
- the BDNF is a variant as described herein, such as a carboxyl- terminal truncated variant.
- the neurotrophin is a GDNF (e.g., mature human GDNF).
- the disorder is an acute disorder.
- Acute disorders of the CNS include focal brain ischemia, global brain ischemia, brain trauma, spinal cord injury, acute infections, status epilepticus, migrane headache, acute psychosis, suicidal depression, and acute anxiety/phobia.
- the disorder is a chronic disorder. Chronic disorders of the CNS include chronic neurodegeneration, retinal degeneration, depression, chronic affective disorders, lysosmal storage disorders, chronic infections of the brain, brain cancer, stroke rehabilitation, inborn errors of metabolism, autism, mental retardation.
- Chronic neurodegeneration includes neurodegenerative diseases such as prion diseases, Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), transverse myelitis, motor neuron disease, Pick's disease, tuberous sclerosis, lysosomal storage disorders, Canavan's disease, Rett's syndrome, spinocerebellar ataxias, Friedreich's ataxia, optic atrophy, and retinal degeneration, and aging of the CNS.
- AD Alzheimer's disease
- PD Parkinson's disease
- HD Huntington's disease
- MS multiple sclerosis
- ALS amyotrophic lateral sclerosis
- transverse myelitis motor neuron disease
- Pick's disease tuberous sclerosis
- lysosomal storage disorders Canavan's disease
- Rett's syndrome spinocerebellar ataxias
- Friedreich's ataxia optic atrophy
- the invention provides methods of treatment of the retina, or for treatment or prevention of blindness.
- the retina like the brain, is protected from the blood by the blood-retinal barrier (BRB).
- BBB blood-retinal barrier
- the insulin receptor is expressed on both the BBB and the BRB, and the HIRMAb has been shown to deliver therapeutics to the retina via RMT across the BRB.
- BDNF is neuroprotective in retinal degeneration, but it was necessary to inject the neurotrophin directly into the eyeball, because BDNF does not cross the BRB.
- fusion proteins of the invention are used to treat retinal degeneration and blindness with a route of administration no more invasive than an intravenous or subcutaneous injection, because the HIRMAb delivers the BDNF across the BRB, so that the neurotrophin is exposed to retinal neural cells from the blood compartment.
- the invention provides a method of treatment for depression.
- a subset of patients with depression may have a brain deficiency of BDNF, and the correlation of single nucleotide polymorphisms (SNPs) with affective disorders has been reported.
- SNPs single nucleotide polymorphisms
- the direct injection of BDNF into the brain has durable anti-depressant effects in rodent model.
- the BDNF must be injected directly into the brain, because the neurotrophin does not cross the BBB.
- the invention provides a method for treating depression by chronic administration of a fusion protein of the invention, thus elevating the brain levels of BDNF and being therapeutic in those patients with depression and a reduced production of brain BDNF.
- compositions of the invention are typically administered in a single dose, e.g., an intravenous dose, of about 0.01-1000 mg, or about 0.05-500 mg, or about 0.1-100 mg, or about 1-100 mg, or about 0.5-50 mg, or about 5-50 mg, or about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 15, 20, 25, 30, 25, 40, 45, 50, 60, 70, 80, 90, or 100 mg.
- an intravenous dose of about 0.01-1000 mg, or about 0.05-500 mg, or about 0.1-100 mg, or about 1-100 mg, or about 0.5-50 mg, or about 5-50 mg, or about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 15, 20, 25, 30, 25, 40, 45, 50, 60, 70, 80, 90, or 100 mg.
- formulations of the invention may be provided in liquid form, and formulated in saline based aqueous solution of varying pH (5-8), with or without detergents such ⁇ olysorbate-80 at 0.01-1%, or carbohydrate additives, such mannitol, sorbitol, or trehalose.
- Commonly used buffers include histidine, acetate, phosphate, or citrate.
- Dosages for humans can be calculated from appropriate animal data.
- human dosing of a BDNF-MAB conjugate is based on pre-clinical pharmacokinetic studies, and these measurements have been performed in 2 species, rats, and Rhesus monkeys.
- Prior work in 3 models of cerebral ischemia in rats demonstrated the range of effective doses of the BDNF-MAb conjugate is 5-50 ⁇ g/rat or 20-200 ⁇ g/kg of BDNF in the form of the BDNF-MAb conjugate. Since the BDNF component of the fusion protein molecule is 16%, and the HIRMAb component is 84%, the dose of fusion protein is 6-fold greater than the equivalent BDNF dose.
- the human brain is 1200 grams, then >1% of the injected dose is delivered to the human brain, which is a level of brain uptake comparable to small molecules.
- the expected 60 min plasma concentration is 250- 2500 ng/ml of fusion protein
- the expected 60 min brain concentration is 25-250 ng/g of fusion protein, which is equivalent to 4-40 ng/gram brain of BDNF.
- the 5 mg and 25 mg fusion protein doses in humans will produce a brain concentration of the BDNF that is neuroprotective in either global or regional brain ischemia. Since the BDNF comprises 16% of the fusion protein, the effective doses of BDNF administered to humans is 0.4 or 4.0 mg, respectively, for the 2.5 or 25 mg dose of fusion protein.
- the fusion protein may also be formulated for chronic use for the treatment of a chronic
- CNS disorder e.g., neurodegenerative disease, stroke or brain/spinal cord injury rehabilitation, or depression.
- Chronic treatment may involve daily, weekly, bi-weekly administration of the composition of the invention, e.g., fusion protein either intravenously, intra-muscularly, or subcutaneous in formulations similar to that used for acute treatment.
- the composition, e.g., fusion protein may be formulated as part of a bio-degradable polymer, and administered on a monthly schedule.
- Combination therapies The composition of the invention, e.g., fusion protein may be administered as part of a combination therapy.
- the combination therapy involves the administration of a composition of the invention in combination with another therapy for the CNS disorder being treated.
- composition of the invention is used in combination with another CNS disorder method or composition
- any combination of the composition of the invention and the additional method or composition may be used.
- the two may be administered simultaneously, consecutively, in overlapping durations, in similar, the same, or different frequencies, etc.
- a composition will be used that contains a composition of the invention in combination with one or more other CNS disorder treatment agents.
- CNS disorder treatment agents that may be used in methods of the invention include, without limitation, thromolytic therapy for stroke, amyloid-directed therapy for Alzheimers disease, dopamine restoration therapy for Parkinsons disease, RNA interference therapy for genetic disorders, cancer, or infections, and anticonvulsant therapy for epilepsy. Dosages, routes of administration, administration regimes, and the like for these agents are well-known in the art.
- the composition e.g., fusion protein is co-administered to the patient with another medication, either within the same formulation or as a separate composition.
- the fusion protein could be formulated with another fusion protein that is also designed to deliver across the human biood-brain barrier a recombinant protein other than BDNF (e.g., GDNF).
- BDNF e.g., GDNF
- the fusion protein may be formulated in combination with other large or small molecules.
- compositions of the invention may be provided as a kit that includes the formulation, e.g., fusion protein in a container and in suitable packaging.
- the composition can be provided in a dry powder form, in solid form (i.e., lyophilized), in solution, or in suspension. If the composition is a protein, to the proteins may have been added emulsifiers, salts, preservatives, other proteins, nucleic acids, protease inhibitors, antibiotics, perfumes, polysaccharides, adhesive agents, polymers, microfibrils, oils, etc.
- the composition is packaged for transport, storage and/or use by a consumer.
- kits of the invention may also include written materials, including instructions for use, results of clinical studies, desired outcome and expected course of treatment, information about precautions and side effects, and the like.
- the kits may optionally further contain other components, such as gloves, scissors, tape, implements for disposal of used vials and other waste, masks, antiseptic, antibiotics, and the like.
- a eukaryotic expression vector encoding the heavy chain (HC) of the fusion protein is outlined in Figure 1.
- the final fusion protein HC expression vector was designated pHIRMAb-BDNF, or clone 416.
- This vector was designed to produce a fusion protein, comprised of a BDNF variant fused to the HC of the HIRMAb.
- Either BDNF or a variant of BDNF (vBDNF) can be fused to the HIRMAb.
- the vBDNF differs from native human BDNF by substitution of certain amino acids, such as a vBDNF where the 2 amino acids at the carboxyl terminus of BDNF are absent in vBDNF.
- the clone 416 plasmid was derived from clone 400, which produces the HC of the chimeric form of the HIRMAb, and a cDNA encoding mature human vBDNF, which was produced as described in Figure 2.
- Clone 400 encodes a chimeric human IgGl that is derived from a chromosomal fragment encoding the human IgGl constant region, and is comprised of both intron and exon sequences.
- the HC gene of the chimeric HIRMAb in clone 400 was subcloned at the BamHI site of the pCR II plasmid to facilitate engineering of the stop codon located at the 3 '-end of the CH3 region by site directed mutagenesis (SDM).
- the engineering of the stop codon located at the end of the CH3 region was performed by site-directed mutagenesis to produce a Sspl site.
- the Sspl site allows for insertion of the vBDNF cDNA ( Figure 3) by blunt-end ligation into clone 400 to form clone 415.
- SDM was performed using the QuickChange SDM kit (Stratagene, CA).
- Sense and complementary mutagenic primers were designed in a way that the CH3 stop codon (aaTGAg) is mutated to Sspl site (aaTATt).
- primers contained 15 nucleotides of the stop codon 5'- and 3 '-surrounding region; the sequence of these primers, designated SDM-SspI forward (FWD) and reverse (REV) are given in Table 1.
- Plasmid clone 405 (-Pro-VH) now carries the single Sspl site introduced by SDM.
- the human vBDNF cDNA was subcloned at Sspl to form an intermediate plasmid named clone 414 (not shown).
- the complete fusion protein HC expression cassette was then reconstructed by subcloning of the PRO-VH fragment previously deleted to form clone 415.
- the fusion protein HC gene was then subcloned in the eukaryotic expression vector, clone 400, at the BamHI site to form clone 416.
- vBDNF cDNA was produced by PCR via either of 2 equivalent approaches.
- a prokaryotic expression plasmid, pHTBSOl, isolated as an expressed sequence tag (EST), and encoding human BDNF was digested with BamHI and BpII, and gel purified, and re- ligated with T4 ligase and the 5'-end linker to produce clone 412 ( Figure 2).
- the sequence of the forward and reverse ODNs used to produce the 5'-end linker are given in Table 2.
- Clone 412 was then digested with Xhol and BsiWI, and gel purified, and re-ligated with
- T4 iigase and the 3'end linker to produce clone 413 ( Figure 2).
- the sequence of the forward and reverse ODNs used to produce the 3 '-end linker are given in Table 2.
- the BDNF cDNA was produced by PCR from cDNA derived by reverse transcription of polyA+RNA isolated from human U87 glioma cells, which produce neurotrophins.
- the primers used to produce the vBDNF by PCR from the U87-derived cDNA are given in Table 2. This PCR produced the expected 0.4 kb vBDNF cDNA ( Figure 3B).
- Both plasmids produce a 6 kb vector backbone (upper of 3 bands in lanes 1 and 3), and a 2.5 kb promoter region (lower of 3 bands in lanes 1 and 3). However, the size of the middle band is 0.4 kb larger for clone 416, as compared to clone 400 (middle band, lanes 1 and 3). A negative clone is shown in lane 2 of Figure 3C.
- the signal peptide is underlined; the cysteine (C) residues within the constant region that form inter- or intra-chain disulfide bridges are shown in bold font; the serine-serine-methionine (SSM) linker between the CH3 region of the IgG and the vBDNF is underlined; the single N-linked glycosylation site, at the asparagine residue within CH2 in shown by bold underlined font ( Figure 6).
- the amino acid sequences of the individual domains of the fusion protein HC protein are given in Figure 7.
- the vBDNF domain of the fusion protein is comprised of 117 amino acids.
- Clone 416 plasmid DNA was electroporated into mouse myeloma cells that had previously been transfected with an expression plasmid encoding the light chain (LC) of the chimeric HIRMAb. Since the vBDNF is fused only to the HC, there is no modification of the LC of the chimeric HIRMAb.
- media from 96-well plates were screened with an ELISA comprised of 2 anti-human IgG antibodies; one antibody is directed against the heavy chain of human IgGl, and the other antibody is directed against human kappa light chains.
- Myeloma clones encoding for intact fusion protein were isolated, and propagated in a 10 L bioreactor.
- the production levels of the fusion protein were low. This low production was attributed to several factors, including (i) transfection of the myeloma line by 3 separate expression plasmids encoding the heavy chain gene, the light chain gene, and the antibiotic resistance gene; and (ii) the use of genomic fragment of the heavy and light chain genes with large intronic sequences. Therefore, the fusion protein expression plasmid was re-engineered with the following features:
- PCR polymerase chain reaction
- the promoter driving the expression of the fusion protein HC and LC genes was changed from the human IgG promoters to the cytomegalovirus (CMV) promoter, to enable transfection of non-myeloma cells, such as Chinese hamster ovary (CHO) cells
- CMV cytomegalovirus
- the tandem vector encoding fusion protein contains a gene encoding for the dihydrofolate reductase (DHFR) gene, under a separate SV40 promoter, to allow for methotrexate (MTX) selection of CHO lines which contain amplification of the genome in the region of the insertion of the expression vector.
- DHFR dihydrofolate reductase
- MTX methotrexate
- Eukaryotic expression plasmids carrying the CMV promoter and the bovine growth hormone (BGH) poly-A (pA) transcription termination sequences, and designated pCD, were digested with Nhel and Xhol and re-ligated with T4 ligase and an Nhel-EcoRV-Kpnl-Scal-BamHI- Xhol linker, as shown in Figure 8.
- the sequence of the forward and reverse ODNs used to produce this linker are given in Table 3.
- PCR was used to produce the cDNA form of the fusion protein HC gene, using the forward and reverse primers shown in Table 3, and high fidelity Pfu DNA polymerase.
- the fusion protein LC cDNA was produced by PCR from the myeloma derived cDNA, and the sequences of the forward and reverse PCR primers used to amplify the fusion protein LC cDNA are given in Table 3.
- the cDNA was applied to an 0.8% agarose gel, and all amplifications yielded a single product, a 1.8 kb fusion protein HC cDNA (lane 1, Figure 3D), and a 0.7 kb fusion protein LC cDNA (lane 2, Figure 3D).
- the fusion protein HC PCR product was digested with Sspl and BamHI and subckmed into CD-linker to produce the clone 422a (Figure 8), which is an intronless eukaryotic expression plasmid encoding the fusion protein HC cDNA.
- Clone 422a was analyzed by restriction endonuclease using Nhel; digestion with this enzyme, which has a site in the new multiple cloning region of the pCD vector, produced the expected 0.4 kb fragment corresponding to the fiision protein heavy chain variable region (VH) cDNA (lanes 1-4, Figure 3E).
- FIG. 9A The nucleotide sequence of the fusion protein HC cDNA encoded by clone 422a is shown in Figure 9A, which shows the intron sequences present in clone 416 ( Figure 5) have been deleted by the PCR of processed myeloma RNA.
- Figure 9B The amino acid sequence encoded by the fusion protein HC cDNA is given in Figure 9B, and this ammo acid sequence is identical to that produced by the genomic fragment in clone 416 ( Figure 6).
- the fusion protein LC PCR product was digested with EcoRV and Xhol and subcloned into CD-linker to produce the clone 423a ( Figure 10), which is an intronless eukaryotic expression plasmid encoding the fusion protein LC cDNA.
- Clone 423a was analyzed by restriction endonuclease using EcoRV and BamHI; digestion with these enzymes, which have a site in the new multiple cloning region of the pCD vector, produced the expected 0.7 kb fragment corresponding to the fusion protein LC cDNA (lanes 1-5, Figure 3F).
- the nucleotide sequence of the fusion protein LC cDNA encoded by clone 423a is shown in Figure 1 IA, which shows the intron sequences have been deleted by the PCR of processed myeloma RNA.
- the amino acid sequence encoded by the fusion protein LC cDNA is shown in Figure 1 IB.
- Clones 422a and 423a were the precursors to the fusion protein tandem vector, as outlined in Figure 12. In 2 steps, clone 422a was subjected to SDM to introduce an EcoRI site at the 3'-end of the fusion protein HC expression cassette; the sequences of the forward and reverse SDM primers are given in Table 4.
- step 2 the mutated clone 422a was digested with EcoRI, blunt-ended, and re-ligated with the EcoRI-Hindlll-Notl-Xcal linker to produce clone 422a-I ( Figure 12).
- the sequence of the ODNs used to produce this EcoRI linker are given in Table 4.
- Clone 422a-I was digested with EcoRI and Hindlll, and closed with T4 ligase in the presence of the fusion protein LC expression cassette to produce clone 422a-II ( Figure 12).
- the fusion protein LC expression cassette was generated by digestion of clone pBS-LC-1 with EcoRI and Hindlll.
- Clone pB S-LC-I was produced from EcoRV-digested pBS (Bluescript), T4 ligase, and the fusion protein LC expression cassette produced by digestion of clone 423a with Sspl ( Figure 12).
- a mouse DHFR expression cassette containing the SV40 promoter and the hepatitis C virus polyA region, was produced from the pFR400 plasmid (designated pDHFR) by digestion of the plasmid with Smal and Sail ( Figure 12).
- the final fusion protein tandem vector was produced by subcloning the DHFR expression cassette into Xcal digested clone 422a-II followed by closure with T4 ligase ( Figure 12).
- the fusion protein tandem vector was analyzed by restriction endonuclease, and the 11 kb plasmid was linearized by Pvul (lane 1, Figure 3G).
- the 1.8 kb fusion protein LC and 1.5 kb DHFR expression cassettes, and the 8 kb vector backbone including the fusion protein HC expression cassette were released by digestion with EcoRI and HindIII (lane 2, Figure 3G).
- the tandem vector was subjected to DNA sequencing in both directions, and the nucleotide sequence, and the deduced amino acid sequence of the fusion protein HC, the fusion protein LC, and the DHFR genes are shown in Figures 14, 15, and 16, respectively.
- the calculated MW of the fusion protein HC and LC are 62,220 and 25,760 Da, respectively, not accounting for any carbohydrate content of the fusion protein HC.
- the MTX-selected cell line was grown in T 175 flasks and then transferred to a 2OL bioreactor with a 1OL volume of CHO cell serum free medium (SFM).
- SFM CHO cell serum free medium
- the CHO cells were maintained at high density in excess of 10 million viable cells/mL for nearly 50 days in perfusion mode in the bioreactor.
- the secretion by these cells of the fusion protein was detected by ELISA using antibodies to either human IgG or to human BDNF.
- the fusion protein is a 1: 1 fusion of the vBDNF to the carboxyl terminus of the HIRMAb heavy chain, which results in formation of the fusion protein heavy chain. This heavy chain complexes with the light chain, as shown in Figure 18.
- the fusion protein should react equally well to 3 antibodies directed against: (i) the human IgGl HC, (ii) the human kappa LC; or (iii) human BDNF.
- FIG 19 there is a direct correlation in measurement of the fusion protein in the CHO cell medium depending on whether anti-human IgG or anti-human BDNF antibodies are used in the ELISA.
- ICC immunocytochemistry
- Example 3 Purification and characterization of bioreactor produced fusion protein.
- the conditioned medium obtained from the bioreactor under perfusion mode was passed through a 1 ⁇ m filter, and the medium collected in a 200 L Bioprocess container under sterile conditions, which were maintained at 4°C in a glass door refrigerator contiguous with the bioreactor. Then, 200 L batches of conditioned medium were passed through 1 ⁇ m and 0.4 ⁇ m pre-filters for the removal of cell debris. The medium was then concentrated with tangential flow filtration (TFF).
- TMF tangential flow filtration
- the TFF system was a Pellicon 2 model from Millipore and was comprised of five 0.5 m 2 filtration cassettes with a 30 kDa molecular weight cutoff and a total surface area of 2.5 m 2 .
- a transmembrane gradient of 15 PSI was produced, which results in a reduction in volume of the 200 L to 2 L within 2 hours.
- CHO cell host protein (CHOP) was monitored at A280 with a Shimadzu detector.
- the residual CHOP was separated from the fusion protein with a linear NaCl gradient from 0 to 1 M NaCl.
- the fusion protein peak was pooled and buffer exchanged and concentrated with a Millipore diafiltration unit with a 30 kDa molecular weight cutoff.
- the final concentrated antibody solution was sterile filtered (0.22 ⁇ m) and stored at 4°C.
- the fusion protein was purified to homogeneity on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), as demonstrated in Figure 20.
- the size of the fusion protein heavy chain was 68 kDa as compared to the size of the HIRMAb heavy chain, which was 54 kDa.
- the difference between the size of the fusion protein and HIRMAb heavy chains reflects the added vBDNF monomer (14 kDa) fused to each heavy chain of the fusion protein.
- the fusion protein reacts with both anti-human IgG antibodies and anti-human BDNF antibodies on Western blotting with the expected molecular weight size of the immunoreactive bands (Figure 21).
- Isoelectric focusing (IEF) shows the isoelectric point (pi) of recombinant BDNF was highly cationic with a pI>10 ( Figure 22).
- the observed pi of the fusion protein was 8.5, and approximates the pi of the HIRMAb ( Figure 22).
- the observed pi of the fusion protein, 8.5 was consistent with the calculated pi, which is 9.04 and 5.27 for the fusion protein HC and LC, respectively (http://scansite.mit.edu/).
- the fusion protein is bi-functionat and binds with high affinity to both the human insulin receptor and to the human trkB receptor.
- the affinity of the fusion protein for the HIR extracellular domain (ECD) was determined with a competitive ligand binding assay (CLBA) using the lectin affinity purified HIR ECD.
- CHO cells transfected with the HIR ECD were grown in serum free media (SFM), and the HIR ECD was purified with a wheat germ agglutinin affinity column.
- the HIR ECD was plated on Nunc-Maxisorb 96 well dishes and the binding of the murine HIRMAb to the HIR ECD was detected by radioactivity measurements following addition of [ 125 I] murine HIRMAb as the ligand in the binding assay ( Figure 23A).
- the binding of the [ 125 I] murine HIRMAb to the HIR ECD was displaced by the addition of unlabeled fusion protein or HIRMAb as demonstrated in Figure 23B.
- the CLBA shows comparable binding of the HIRMAb or the fusion protein.
- TrkB CLBA was designed for measurement of the affinity of the fusion protein for recombinant human TrkB ECD.
- the design of a TrkB CLBA was made difficult by the cationic nature of BDNF, which causes a high degree of nonspecific binding in the assay and this reduces the sensitivity of the assay.
- the nonspecific binding of BDNF could be eliminated by conjugation of 2000 Da polyethyleneglycol (PEG) to the protein.
- PEG polyethyleneglycol
- a bifunctional PEG molecule, biotin-PEG 2000 - hydrazide (Hz) was commercially obtained, and conjugated to BDNF to produce BDNF-PEG 2000 - biotin, as outlined in Figure 24A; this molecule was used as the "tracer" in the CLBA.
- TrkB ECD was absorbed to ELISA plates and binding of BDNF-PEG 2C00 -biotin to the TrkB was detected colorimetrically with avidin and biotin peroxidase (Figure 24A).
- Prior studies showed the ELISA signal (A490) was directly proportional to the amount of TrkB added to the well.
- the assay had a very low blank and the A490 was ⁇ 0.04 when no TrkB is plated.
- the binding of the BDNF-PEG 2000 -biotin to the TrkB was competitively displaced by the recombinant BDNF (Figure 24B) or the fusion protein (Figure 24C).
- the Scatchard analysis of the binding data using nonlinear regression analysis allowed for the computation of the Ki of binding of either BDNF or fusion protein to TrkB, as shown in Figure 24B and 24C, respectively.
- the affinity of the fusion protein for TrkB was not statistically different from the affinity of the recombinant BDNF ( Figure 19 B,C).
- the nonspecific binding (NSB) of the assay was comparable for either BDNF or the fusion protein.
- the NSB likely represents nonlinear cooperative binding of the neurotrophin to the TrkB extracellular domain.
- the TrkB CLBA results shown in Figure 24 indicate the affinity of fusion protein for the TrkB receptor was not changed following fusion of the vBDNF to the carboxyi terminus of the HIRMAb heavy chain.
- Neurotrophins such as BDNF require an obligatory formation of a homo-dimeric structure to be biologically active, and to bind with high affinity to the cognate receptor, e.g. TrkB.
- a naturally occurring homo-dimeric structure between two BDNF molecules was formed when the neurotrophin was fused to a carboxyi terminus of the CH3 region of an IgG molecule, as illustrated in Figure 18.
- the surprising observation of the maintenance of the high affinity binding of BDNF for TrkB ( Figure 24), despite fusion to the HIRMAb heavy chain ( Figure 18), is consistent with the fact that BDNF normally binds to TrkB as a dimer.
- Human neural cells subjected to hypoxia are neuroprotcctcd by the fusion protein with equal activity as recombinant BDNF.
- Human SH-SY5Y neural cells were exposed to 10 uM retinoic acid for 7 days, which induces gene expression of trkB, the BDNF receptor. The cells were then exposed to 16 hours of oxygen deprivation in a sealed chamber, with oxygen sensor. Excitotoxic neural damage was then induced by 4 hours of re-oxygenation (Figure 25A). During this 4 hour re-oxygenation period, the cells were exposed to either no treatment or equi-molar concentrations of human recombinant BDNF or fusion protein. As shown in Figure 25B 5 the fusion protein was equipotent with native human BDNF with respect to inducing neuroprotection in human neural cells exposed to excitoxic ischemia-re-oxygenation.
- the fusion protein is bound by the insulin receptor of the human BBB, because the murine HIRMAb (mHIRMAb) also inhibits binding of [ 3 H] -fusion protein to the human BBB.
- the KD is ⁇ 1 nM, which indicate the fusion protein binds the HIR on the human BBB with very high affinity.
- Example 7 Pharmacokinetics and brain uptake of fusion protein by the adult Rhesus monkey.
- the fusion protein was tritiated with [ 3 H]-N-succinimidyl propionate to a specific activity of 2.0 ⁇ Ci/ ⁇ g.
- the serum glucose of the anesthetized, overnight-fasted primate was constant throughout the 180 min study period, and averaged 72 ⁇ 2 mg%, which indicates that the administration of the HIRMAb based fusion protein caused no interference of the endogenous insulin receptor, and had no effect on glycemia control.
- FIG. 27A The plasma pharmacokinetics analysis ( Figure 27A) shows that the fusion protein of the genetically engineered HLRMAb and the BDNF is removed from blood at the same rate as the original murine HIRMAb. This is an important finding, because it shows that the fusion of BDNF, a highly cationic protein, to the HIRMAb does not accelerate the blood clearance of the HIRMAb.
- Prior work shows that the attachment of the cationic BDNF to a monoclonal antibody greatly accelerates the blood clearance of the antibody, owing to the cationic nature of the BDNF, which greatly enhances hepatic uptake.
- FIG. 27A shows that when the cationic BDNF was re-engineered as an IgG fusion protein, the plasma pharmacokinetics was dominated by the IgG moiety, and that the blood level of the BDNF remains high for a prolonged period.
- FIG. 27B show that when BDNF was re-formulated as an IgG fusion protein, the metabolic stability of the neurotrophin in blood was greatly enhanced, as compared to the native BDNF. Owing to its cationic nature, the native BDNF was rapidly removed from blood, and was rapidly degraded into TCA-soluble radioactive metabolites ⁇ Figure 27B). However, the TCA- insoluble form of the labeled fusion protein remains high during the 3 hours after an intravenous injection in the primate (Figure 27B).
- the data in Figures 27A,B show the advantages of re- engineering a neurotrophin pharmaceutical as a fusion protein. The native neurotrophin was rapidly removed from blood and was rapidly degraded. However, the plasma pharmacokinetics profile, and metabolic stability profile, of the neurotrophin resemble those of an IgG molecule, when the IgG- neurotrophm fusion protein was produced.
- Capillary depletion analysis separates the brain vasculature from the post-vascular supernatant, and allows detection of the transport of a drug through the BBB and into brain, as opposed to simple sequestration of the drug by the brain vasculature.
- the brain V D of the post-vascular supernatant of the [ 3 H]-fusion protein was equal to the V n of the brain homogenate ( Figure 27C), which indicates the fusion protein was transported through the BBB and into brain parenchyma.
- the brain V D of the fusion protein was converted into ng fusion protein per gram brain, based on the specific activity of the [ 3 H]-fusion protein, and this allowed for calculation of the total mass of fusion protein in the brain, 24 ⁇ 1 ng/g, as shown in Figure 27D.
- This value is > 10-fold higher than the endogenous brain concentration of BDNF in the adult primate (45). Therefore, the administration of a dose of 373 ⁇ g to a 5.2 kg Rhesus monkey, which is equal to a normalized dose of 72 ⁇ g/kg of fusion protein, results in a marked increase in the brain concentration of BDNF.
- the data shows that: (1) the plasma mean residence time (MRT) of the fusion protein, 312 minutes, was 100-fold greater than the MRT for native BDNF, which was 3.0 minutes, and (2) the systemic clearance of the fusion protein, 0.94 mL/min/kg, was 39-fold slower than the systemic clearance of the BDNF, which was 37 mL/min/kg.
- MRT plasma mean residence time
- systemic clearance of the fusion protein 0.94 mL/min/kg
- fusion of the BDNF to the molecular Trojan horse had 2 benefits: (1) the molecular Trojan horse carried the BDNF across the blood-brain barrier (BBB), whereas the BDNF alone cannot cross the BBB, and (2) the molecular Trojan horse prevented the rapid loss from blood of the neurotrophin; BDNF by itself lasts only about 3 minutes in the blood. Both of these properties serve to enhance the pharmacological effect of the BDNF in brain following administration into the blood stream. See, e.g., Table 5.
- Example 8 Neuroprotection in regional brain ischemia by conjugates of BDNF and a BBB molecular Trojan horse. Numerous attempts have been made to develop neuroprotective agents for the treatment of acute stroke. There have been no successes to date because the neuroprotective drugs are either too toxic, in the case of certain small molecules, or ineffective, because the drag does not cross the BBB.
- BDNF is neuroprotective when injected directly in the brain in parallel with experimental stroke in rodents and regional brain ischemia. The BDNF must be injected across the skull bone into the brain, because this large molecule drug does not cross the BBB.
- the neurotrophin was attached to a mouse MAb to the rat transferrin receptor (TfR).
- TfR rat transferrin receptor
- This peptidomimetic MAb carries BDNF across the BBB, and the combined BDNF-MAb conjugate is highly neuroprotective following delayed intravenous administration in experimental stroke, because the BDNF is able to cross the BBB and enter the brain from blood.
- the BDNF activates its cognate receptor, trkB, which then induces neuroprotection in ischemic neurons, and stops the apoptotic death cycle.
- the neuroprotective effect of the BDNF-MAb conjugate demonstrates a dose response effect, a time response effect, and is long-lasting, as the neuroprotection at 7 days is identical to the neuroprotection at 1 day after a single intravenous administration of the BDNF-MAb conjugate. See, e.g., Zhang and Pardridge (2001) Brain Res. 889:49-56, and Zhang and Pardridge (2001) Stroke 32:1378-1374, which are incorporated by reference herein in their entirety.
- the fusion protein would also be neuroprotective in human stroke, since the BDNF is fused to an MAb to the HIR, which rapidly binds to both the human BBB in vitro, and is rapidly transported across the primate BBB in vivo.
- Example 9 Neuroprotection in global brain ischemia of conjugates of BDNF and a BBB molecular Trojan horse.
- the direct injection of BDNF into the brain is also neuroprotective in transient forebrain ischemia (TFI), such as might occur after a cardiac arrest.
- TFI transient forebrain ischemia
- intravenous BDNF is not neuroprotective in TFI, because the BDNF does not cross the BBB, and because the BBB is intact in the early hours after TFI, when neuroprotection is still possible.
- BDNF intravenous BDNF was neuroprotective in TFI if the BDNF was attached to a mouse MAb against the rat transferrin receptor (TfR), which acts as a molecular Trojan horse to ferry the BDNF across the BBB and into brain.
- TfR rat transferrin receptor
- Adult rats were subjected to TFI, which resulted in a flat-line electroencephalogram (EEG) for approximately a 10-minute period.
- EEG electroencephalogram
- the animals were resuscitated and then administered 1 of 4 different therapeutics intravenously: (a) buffer, (b) unconjugated BDNF, (c) the receptor specific MAb without the BDNF attached, and (d) the BDNF-MAb conjugate.
- BDNF is neuroprotective in brain and spinal cord injury if the neurotrophin can access brain cells.
- BDNF is neuroprotective in brain injury, providing the neurotrophin is injected directly through the skull bone, because BDNF does not cross the BBB.
- BDNF is also neuroprotective in brain subjected to excitotoxic injury by neurotoxins, and is neuroprotective in brain infected with the human immune deficiency virus (HIV)-I.
- HIV human immune deficiency virus
- BDNF is also neuroprotective in acute spinal cord injury; however, the BDNF must be administered by direct infusion into the spinal canal, because the BDNF does not cross the blood-spinal cord barrier, which is the same as the BBB in the forebrain.
- the intravenous administration of BDNF would not be neuroprotective, because the BDNF does not cross the BBB, and the BBB is intact in brain injury in the early hours after the injury, when neuroprotection is still possible.
- the BDNF fusion protein would be neuroprotective in these conditions following intravenous administration, because the BDNF is fused to the BBB molecular Trojan horse, and is able to penetrate the brain and spinal cord from the blood following peripheral administration.
- BDNF is neuroprotective in chronic neurodegenerative conditions of brain if the neurotrophin can access brain cells.
- Neurotrophins such as BDNF can be developed as drugs for peripheral routes of administration, providing the neurotrophin is enabled to cross the BBB.
- Fusion of BDNF to the chimeric HIRMAb offers a new approach to the non-invasive delivery of BDNF to the brain in humans for the chronic treatment of neurodegenerative disease, including prion diseases, Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), ALS, transverse myelitis, motor neuron disease, Pick's disease, tuberous sclerosis, lysosomal storage disorders, Canavan's disease, Rett's syndrome, spinocerebellar ataxias, Friedreich's ataxia, optic atrophy, and retinal degeneration, and brain aging.
- AD Alzheimer's disease
- PD Parkinson's disease
- HD Huntington's disease
- ALS transverse myelitis
- motor neuron disease Pick's disease, tuberous sclerosis, lysosomal storage disorders
- Canavan's disease Rett's syndrome
- spinocerebellar ataxias Friedreich's ataxia
- optic atrophy and retinal degeneration
- BDNF as a therapeutic in retinal degeneration and blindness.
- the retina like the brain, is protected from the blood by the blood-retinal barrier (BRB).
- BBB blood-retinal barrier
- the insulin receptor is expressed on both the BBB and the BRB, and the HIRMAb has been shown to deliver therapeutics to the retina via RMT across the BRB (Zhang et al, (2003) MoL Ther. 7: 11-18).
- BDNF is neuroprotective in retinal degeneration, but it was necessary to inject the neurotrophin directly into the eyeball, because BDNF does not cross the BRB.
- the fusion protein could be used to treat retinal degeneration and blindness with a route of administration no more invasive than an intravenous or subcutaneous injection, because the HIRMAb would deliver the BDNF across the BRB, so that the neurotrophin would be exposed to retinal neural cells from the blood compartment.
- BDNF as a therapeutic for depression.
- a subset of patients with depression may have a brain deficiency of BDNF, and the correlation of single nucleotide polymorphisms (SNPs) with affective disorders has been reported.
- SNPs single nucleotide polymorphisms
- the direct injection of BDNF into the brain has durable antidepressant effects in rodent model.
- the BDNF must be injected directly into the brain, because the neurotrophin does not cross the BBB.
- the chronic administration of the fusion protein would provide a means for elevating the brain levels of BDNF, and may be therapeutic in those patients with depression and a reduced production of brain BDNF.
- Example 14 Method of manufacturing IgG fusion proteins.
- the trans fection of a eukaryotic cell line with immunoglobulin G (IgG) genes generally involves the co-transfection of the cell line with separate plasmids encoding the heavy chain (HC) and the light chain (LC) comprising the IgG.
- the gene encoding the recombinant therapeutic protein may be fused to either the HC or LC gene.
- this co-transfection approach makes it difficult to select a cell line that has equally high integration of both the HC and LC-fusion genes, or the HC- fusion and LC genes.
- the preferred approach to manufacturing the fusion protein is the production of a cell line that is transfected with a single plasmid DNA that contains all the required genes on a single strand of DNA, including the HC-fusion protein gene, the LC gene, the selection gene, e.g. neo, and the amplification gene, e.g. the dihydrofolate reductase gene.
- the HC-fusion gene, the LC gene, the neo gene, and the DHFR gene are all under the control of separate, but tandem promoters and separate but tandem transcription termination sequences. Therefore, all genes are equally integrated into the host cell genome, including the fusion gene of the therapeutic protein and either the HC or LC IgG gene.
- Glial-derived neurotrophic factor is a potent neuroprotective neurotrophin (Lin et al, Science 260:1130- 1132 (1993)).
- GDNF could be developed as a neuroprotective drug for acute conditions, such as acute ischemic stroke, or chronic conditions, such as Parkinson's disease, or motor neuron disease, or post-stroke neural repair (Lapchak et al, Neuroscience 78:61-72 (1997); Kitagawa et al, Stroke 29:1417-1422 (1998); Bonn, Exp. Neurol. 190:263-275 (2004); Kobayashi, et al.
- GDNF blood-brain barrier
- BBB blood-brain barrier
- both approaches were limited by poor GDNF penetration into the brain, as well as adverse events related to the invasive drug delivery system.
- a BBB molecular Trojan horse is an endogenous peptide, or receptor-specific peptidomimetic monoclonal antibody (MAb) that undergoes receptor-mediated transport across the BBB via endogenous peptide receptors, such as the transferrin receptor or the insulin receptor.
- MAb receptor-specific peptidomimetic monoclonal antibody
- Prior work has developed genetically engineered chimeric or humanized MAb's to the human insulin receptor (HIR), as molecular Trojan horses for the human brain (Boado et al, Biotechnol. Bioeng.
- the non-transportable protein therapeutic is fused to the heavy chain (HC) of the HIRMAb, It is essential that the genetically engineered fusion protein retain bi-functionality, and both bind with high affinity to the HIR, to cause BBB transport, and bind to the cognate receptor on brain cells, to induce the desired pharmacologic effect, once the fusion protein penetrates the brain.
- HC heavy chain
- HIRMAb and human GDNF The amino terminus of mature human GDNF is fused to the carboxyl terminus of the HC of the HIRMAb ( Figure 28).
- the bi- functionality of the HIRMAb-GDNF fusion protein is evaluated with receptor binding assays for both the HIR and the human GDNF receptor (GFR)- ⁇ l .
- the GDNF biologic activity of the fusion protein is also evaluated with bio-assays using 2 human neural cell lines, the SH-SY5 Y line, and the SK-N-MC line.
- the in vivo neuroprotective effects of the HIRMAb-GDNF fusion protein is demonstrated in rat brain using the middle cerebral artery occlusion model (MCAO) of acute ischemic stroke.
- MCAO middle cerebral artery occlusion model
- Met-"1-Ile211 was cloned by the polymerase chain reaction (PCR) using the oligodexoynucleotides (ODNs) described in Table 6 and cDNA derived from reverse transcription of polyA+RNA isolated from human U87 glial cells. DNA sequence analysis was performed and the nucleic acid and amino acid sequences of the cloned human prepro GDNF are given in SEQ ID NOs :43 and 44, respectively.
- the GDNF cDNA was cloned by PCR using 25 ng polyA+RNA-derived cDNA, 0.2 ⁇ M forward and reverse ODN primers (Table 6), 0.2 mM deoxynucleosidetriphosphates, and 2.5 U PMJltra DNA polymerase (Stratagene, San Diego, CA) in a 50 ⁇ l Pfu buffer (Stratagene).
- the amplification was performed in a Mastercycler temperature cycler (Eppendorf, Hamburg, Germany) with an initial denaturing step of 95°C for 2 min followed by 30 cycles of denaturing at 95°C for 30 sec, annealing at 55°C for 30 sec and amplification at 72°C for 1 min; followed by a final incubation at 72°C for 10 min.
- PCR products were resolved in 0.8% agarose gel electrophoresis, and the expected major single band of -0.6 kb corresponding to the human GDNF cDNA was produced ( Figure 29A).
- the human prepro GDNF cDNA was subcloned into the pcDNA3.1 eukaryotic expression plasmid, and was designated pCD-GDNF. The engineering of this plasmid was validated by DNA sequencing, and by expression of immunoreactive GDNF in COS cells transfected with pCD-GDNF.
- pHIRMAb-HC plasmid encodes the HC of the chimeric HIRMAb, and dual transfection of COS cells with this plasmid and a light chain (LC) expression plasmid, pHIRMAb- LC, allows for transient expression of either the chimeric HIRMAb, or a fusion protein, in COS cells.
- LC light chain
- the mature human GDNF forward (FWD) PCR primer (Table 6) introduces "CA" nucleotides to maintain the open reading frame and to introduce a Ser-Ser linker between the carboxyl terminus of the CH3 region of the HIRMAb HC and the amino terminus of the GDNF.
- the fusion of the GDNF monomer to the carboxyl terminus of each HC is depicted in Figure 28. This design sterically restricts the GDNF to a dimeric configuration, which replicates the native dimeric conformation of GDNF that binds to the GFR ⁇ l (Xu et al, 1998; Eketjall et aL 1999).
- the GDNF reverse (REV) PCR primer introduces a stop codon, "TGA,” immediately after the terminal isoleucine of the mature GDNF protein, and it is identical to the human prepro GDNF REV ODN primer (Table 6) used in the cloning of the human prepro GDNF cDNA.
- the engineered pHIRMAb-GDNF expression vector was validated by DNA sequencing.
- the nucleic acid and amino acid sequences for fusion heavy chain (SEQ ID NOs 45 and 46) are as follow:
- MDWTWRVFCLLAVAPGAHSQVQLQQSGPELVKPGAL VKISCKASGYTFTNYDIHWVKQRPGQGLEW IGWIYPGDGSTKYNE KFKGKATLTAD KSSSTAYMHLSSLTSEKSAVYFCAREWAYWGQGTLVTVSA ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLS SVVTVPSSSLGTQTYICNVNHKPSNTKVDK-CVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKD TI ⁇ ISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRWSVLTVLHQDWLN GKEYKCKVSNKALPAPI ⁇ KTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEW ESNGQPENNYKTTPPVLDSDGSFF
- HIRMAb HC and LC cDNA expression cassettes are driven by the cytomegalovirus
- CMV bovine growth hormone
- BGH bovine growth hormone polyadenylation
- Figure 29B The engineering of the universal pHIRMAb-HC vector was performed by insertion of a single Hpal site at the end of the HIRMAb HC CH3 open reading frame (orf) by site directed mutagenesis (SDM). Transient expression of HIRMAb-GDNF fusion protein in COS cells
- COS cells were dual transfected with pHIRMAb-LC and pHIRM Ab-HC-GDNF using
- Lipofectamine 2000 with a ratio of 1:2.5, ug DNA:uL Lipofectamine. Following transfection, the cells were cultured in serum-free VP-SFM (Invitrogen, Carlsbad, CA). The conditioned serum free medium was collected at 3 and 7 days. The fusion protein was purified by protein A affinity chromatography. Human IgG and GDNF ELISAs
- the affinity of the fusion protein for the HIR extracellular domain was determined with an ELISA using the lectin affinity purified HIR ECD.
- CHO cells transfected with the HIR ECD were grown in serum free media (SFM), and the HIR ECD was purified with a wheat germ agglutinin affinity column.
- the HIR ECD (0.2 ⁇ g/well) was plated on Immulon 2 high binding 96- well plates, and the binding of the chimeric HfRMAb, the HIRMAb-GDNF fusion protein, or human IgGl to the HIR ECD was detected with a biotinylated goat anti-human IgG (H+L) antibody (0.3 ⁇ g/well), and the ABC Elite detection system (Vector Labs).
- the concentration that caused 50% binding to the HIR ECD, the ED50 was determined by non-linear regression analysis using the WinNonlin software.
- Binding of recombinant GDNF (Pepro ⁇ tech, Rocky Hill, NJ), the chimeric HIRMAb, or the HIRMAb-GDNF fusion protein to the ECD of recombinant human GFR ⁇ l was measured with an ELISA.
- a mouse anti-human (MAH) IgG (Zymed-Invitrogen) was plated in 96-well plates overnight at 2 ⁇ g/well. Following aspiration, washing, and blocking with 1% bovine serum albumin (BSA), the Fc fusion of the human GFR ⁇ l ECD (R&D Systems, Minneapolis, MN) was plated at 0.4 ⁇ g/well.
- SK-N-MC bio-assay Following an incubation at room temperature (RT) for 60 min, the wells were washed with PBST, and the GDNF, antibody, or fusion protein was plated for 2 hours at RT. Following washing in PBST, a goat anti-GDNF antibody (R&D Systems) was plated at 0.4 ug/well for 30 min at RT. Following washing in PBST, a conjugate of alkaline phosphatase (AP) and a rabbit anti-goat (RAG) IgG(H+L) (Vector Labs) was plated and detection at 405 nm was performed with an ELISA plate reader after color development with para-nitrophenylphosphate (Sigma Chemical Co.). SK-N-MC bio-assay
- Human neural SK-N-MC cells were dual transfected with the c-ret kinase and a luciferase reporter plasmid under the influence of the 5'-flanking sequence (FS) of the rat tyrosine hydroxylase (TH) gene.
- the cells were grown in collagen coated 24-well dishes to 70% confluency in Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) and 400 ⁇ g/mL G418. To begin the assay, the medium was aspirated, and 400 ⁇ L/well of fresh DMEM/ 10% FBS was added along with either recombinant GDNF or fusion protein.
- DMEM Dulbecco's modified Eagle medium
- FBS fetal bovine serum
- the permanent middle cerebral artery occlusion (MCAO) model was performed as described previously in adult Sprague-Dawley rats (Zhang and Pardridge, Brain Res. 889:49-56 (2001)).
- the right middle cerebral artery of the anesthetized rat was occluded by insertion of an intra-luminal 4-0 nylon suture; the suture was coated in 0.1% poly-L-lysine and dried at 60°C for 1 hour.
- the rat was treated with an intra-cerebral injection of either HIRMAb-GDNF fusion protein (130 ⁇ g in l0 ⁇ L) or an equal volume of saline.
- the dose of fusion protein is equivalent to a dose of GDNF of 22 ⁇ g, which is a dose that causes pharmacological effects in the brain (Sullivan et al, 1998).
- the drug was injected into the brain under stereotaxic guidance with the following coordinates: 0.2 mm posterior to bregma, 4.0 mm lateral to the midline, and 5.0 mm deep from the dural surface.
- the arterial nylon suture was left in place for permanent occlusion of the middle cerebral artery.
- a total of 20 rats were used for the study; 9 rats (5 treated with saline and 4 treated with HIRMAb-GDNF) expired prematurely and were excluded from the study.
- the rats were allowed to recover, and were euthanized 24 hours later, and coronal sections of brain were prepared with a rat brain matrix (ASI Instruments, Warren, MI), followed by staining with 2% 2,3,5-triphenyltetrazolium chloride (TTC), as described previously (Zhang and Pardridge, Brain Res. 889:49-56 (2001)).
- TTC stains healthy brain red, and infarcted brain is colorless.
- the stained brain sections were scanned on a UMAX PowerLook II flatbed scanner, and the area of the hemispheric infarct volumes was determined with the N1H Image software.
- the volume of the infarct was computed from the area of the infarcted zone and length (2 mm) of the coronal slices of brain hemisphere (Zhang and Pardridge, Brain Res. 889:49-56 (2001)).
- the scanned color image was converted to an inverted grayscale image in Photoshop, so that infarcted brain appears black and healthy brain appears white.
- the neurologic deficit was determined 24 hours after the occlusion, as described previously (Zhang and Pardridge, Stroke 32:1378-1384 (200I)), and scored as follows: 0, no deficit; 1, failure to extend contralateral forepaw fully; 2, decreased grip of contralateral forelimb while tail is pulled; 3, spontaneous circling to left; 4, walks only when stimulated with decreased level of consciousness. RESULTS
- DNA sequencing of the expression cassette of the pCD-GDNF encompassed 1,752 nucleotides (nt), including a 715 nt CMV promoter, a 636 nt prepro GDNF open reading frame, and a 401 nt BGH sequence, which predicted a 211 amino acid human preproGDNF protein, including a 19 amino acid signal peptide with 100% identity with the known sequence for human GDNF (P39905).
- Transfection of COS cells with pCD-GDNF resulted in high levels of immunoreactive GDNF the medium at 3 and 7 days following transfection.
- the cDNA corresponding to the 134 amino acid mature GDNF was amplified by PCR using the ODNs in Table 6 and the pCD-GDNF as template, and this cDNA was subcloned into the Hpal site of the pHIRM Ab-HC plasmid, as outlined in Figure 29B.
- DNA sequencing of the expression cassette of the pHIRMAb-GDNF plasmid encompassed 2,890 nt, including a 714 nt CMV promoter, a 9 nt full Kozak site (GCCGCCACC), a 1,797 nt HIRMAb HC-GDNF fusion protein open reading frame, and a 370 nt BGH sequence.
- the plasmid encoded for a 598 amino acid protein comprised of a 19 amino acid IgG signal peptide, the 443 amino acid HIRMAb HC, a 2 amino acid linker (Ser-Ser), and the 134 amino acid human GDNF minus the signal peptide or propeptide.
- the predicted molecular weight of the heavy chain fusion protein, minus glycosylation, is 63,860 Da, with a predicted isoelectric point (pi) of 9.03.
- the concentration of the HIRMAb-GDNF fusion protein the medium was the same irrespective of whether the human IgG or GDNF ELISA is used.
- the HIRMAb-GDNF fusion protein was purified by protein A affinity chromatography.
- the size of the light chain (LC) was the same for both the HIRMAb and the HIRMAb-GDNF fusion protein ( Figure 30).
- the size of the heavy chain (HC) of the fusion protein was about 15 kDa larger than the HC of the HIRMAb ( Figure 30).
- the LC of either the HIRMAb or the HIRMAb-GDNF fusion protein reacted equally on the Western with a primary antibody directed against the human IgG (H+L), as shown in Figure 31.
- the size of the HC of the fusion protein was about 15 kDa larger than the size of the HC of the HIRMAb on both Western blots using either the anti-human IgG primary antibody ( Figure 31) or the anti-human GDNF primary antibody ( Figure 31).
- the anti-GDNF primary antibody reacts with the HC of the fusion protein, and with recombinant GDNF, but does not react with the HIRMAb ( Figure 31).
- the human neural SK-N-MC cell line was dual transfected with the c-ret kinase, and with a luciferase expression plasmid under the influence of the 5 '-flanking sequence (FS) of the rat TH promoter.
- FS 5 '-flanking sequence
- Addition of GDNF to the medium activates the TH promoter via activation of the GFR ⁇ l/c-ret kinase system, and this leads to increased luciferase expression (Figure 34A).
- GDNF increased luciferase expression with an ED50 of 1.03 ⁇ 0.31 nM
- the HIRMAb-GDNF fusion protein also increased luciferase expression with an ED50 of 1.68 ⁇ 0.45 nM ( Figure 34B).
- Both GDNF and the HIRMAb-GDNF fusion protein activated cell division of human neural SH-S Y5 Y cells following retinoic acid differentiation, and both proteins increased cell division about 50% over a 5 day incubation incubation period (Table 8).
- FIG. 35B and 35C A representative TTC stain of the brain 24 hours after permanent MCAO and intra-cerebral injection of either saline or the HIRMAb-GDNF fusion protein is shown in Figures 35B and 35C, respectively.
- GDNF is a member of the transforming growth factor (TGF)- ⁇ gene family, along with other neurotrophins, such as neuturin, persephin, or artemin.
- GDNF reacts with its cognate receptor, GFR ⁇ I, as a homo-dimer (Eketjall et al, 1999). Therefore, the GDNF fusion construct described in this work ( Figure 28) places GDNF in a dimeric configuration that mimicked the conformation of the neurotrophin at the receptor.
- the prepro GDNF could be fused to the amino terminus of the HIRMAb HC. However, this would interfere with HIRMAb binding to the HIR, which would impair transport of the fusion protein across the BBB via the insulin receptor.
- the mature GDNF protein folded into a biologically active conformation, despite the absence of the prepro GDNF peptide in the fusion protein described in this work.
- the goal of fusion protein engineering was to retain the bi-functional characteristics of the fusion construct, and this was accomplished in the case of the HIRMAb-GDNF fusion protein.
- the fusion protein was secreted in high amounts to the medium by COS cells co- transfected with the HC expression plasmid, pHIRMAb-GDNF ( Figure 29B), and the LC expression plasmid, pHIRMAb-LC (Table 7).
- the HC of the fusion protein is about 15 kDa larger than the HC of the chimeric HIRMAb, based on either SDS-PAGE gels with Coomassie blue staining (Figure 30), or on Western blot analysis using primary antibodies to either human IgG or human GDNF ( Figure 31).
- the anti-human IgG antibody reacts equally with the LC of the fusion protein or the chimeric HIRMAb, since both proteins use the same LC.
- Both the HIRMAb-GDNF fusion protein and the HIRMAb bind with high affinity to the HIR, and with comparable affinity (Figure 32).
- the HIRMAb-GDNF fusion protein retains high affinity binding to the human GFR ⁇ 1 receptor, and the affinity constant of binding is comparable to that of recombinant GDNF ( Figure 33B).
- the high affinity binding of the HIRMAb-GDNF fusion protein to the GFR ⁇ l was translated into biological activity in two different human neural cell lines.
- the SH-SH5Y cell line expresses the GFR ⁇ l 1, but does not express the c-ret kinase in the undifferentiated state (Xiao et al, J. Neurochem. 82:701-808 (2002)).
- Both the GFR ⁇ l and the c-ret kinase must be expressed to enable GDNF activation of a neural cell (Cik et al, 2000).
- differentiation of SH-SH5Y cells by retinoic acid causes an induction of the expression of the c-ret kinase (Xiao et al, J. Neurochem. 82:701-808 (2002)).
- the SH-S Y5 Y cells demonstrate enhanced proliferation hi response to either recombinant human GDNF or the HIRMAb-GDNF fusion protein (Table 8).
- the SK-N-MC human neural cell line also expresses the GFR ⁇ l receptor, but not the c-ret kinase (Hirata and Kiuchi, Brain Res. 983:1-12 (2003)). However, following co-transfection of these neural cells with a c-ret kinase cDNA and a plasmid encoding luciferase under the influence of 2 kb of the 5'-FS of the rat TH promoter, these cells respond to GDNF (Tanaka et al, Brain res. Brain Res. Protoc. 11 : 119-122 (2003)).
- the extracellular GDNF binds the GFR ⁇ l, which activates the c-ret kinase, which induces a signal transduction cascade leading to activation of the TH promoter. Accordingly, both recombinant GDNF and the HIRMAb-GDNF fusion protein caused an approximate 500% increase in cellular luciferase enzyme activity.
- the GDNF ED50 in the luciferase assay is 1.0 ⁇ 0.1 nM, and the HIRMAb-GDNF ED50 in this assay is 1.7 ⁇ 0.5 nM ( Figure 34B).
- ED50 values are identical to the ED50 of saturable binding of either GDNF or the HIRMAb-GDNF fusion protein in the GFR ⁇ l binding assay ( Figure 33B). This correlation indicates that the rate-limiting step in activation of neural pathways by GDNF is binding to the cell membrane GFR ⁇ l receptor.
- FIG. 35B A representative TTC stain of the rat brain at 24 hours after the permanent MCAO is shown in Figure 35B, for the saline treated rat, and in Figure 35C for the HIRMAb-GDNF fusion protein treated rat.
- the reduction in stroke volume was correlated with a functional improvement as the neurologic deficit was reduced from 3.0 ⁇ 0,3 to 1.3 ⁇ 0.6 (p ⁇ 0.05) in the rats treated with the HIRMAb-GDNF fusion protein as compared to the saline treated rats.
- the HIRMAb-GDNF fusion protein could not be delivered to rat brain in the MCAO model following intravenous administration, because the HIRMAb part of the fusion protein is not reactive with the rodent insulin receptor.
- the HIRMAb is active in Old World primates, such as the Rhesus monkey (Pardridge et al, Pharm. Res. 12:807-816 (1995)).
- fusion proteins of the HIRMAb and brain-derived neurotrophic factor Boado et al, Biotechnol. Bioeng. 97:1376-1386 (2007)
- a single chain Fv antibody Boado et al, Bioconjug Chem.
- the expected brain concentration of the fusion protein is about 25 ng/gram human brain, which is equivalent to 5 ng/gram of GDNF, since the fusion protein is about 20% GDNF and 80% HIRMAb.
- the concentration of GDNF in the human brain is 0.2-1 ng/gram (Wiesenhofer et al, Acta Neuropathol (Berl) 99:131-137 (2000)). Therefore, the dosing of 25 mg of the HIRMAb-GDNF fusion protein to a 60 kg human could result in pharmacologically significant increase in GDNF in the brain. In experimental Parkinson's disease, pharmacological effects are achieved with just a 3-fold increase in brain GDNF concentration (Eslamboli et al, J.
- HIRMAb-GDNF fusion protein represents a re-engineering of this neurotrophin to enable transport across the human BBB in vivo.
- the fusion protein can be administered by systemic injection to humans for treatment of multiple neurologic disorders, including stroke, neural repair, Parkinson's disease, or motor neuron disease.
- Example 16 Expression of HIRMAb-GDNF fusion protein following stable transfection of CHO cells
- a tandem vector (TV) encoding the HIRMAb-GDNF fusion protein was engineered as shown in Figure 37, and is designated HIRMAb-GDNF TV,
- the TV contains on a single piece of DNA the fusion heavy chain (HC), the light chain (LC), and the gene for DHFR.
- DNA sequencing of the entire 6,300+ nucleotides (nt) of the HIRMAb-GDNF TV using custom oligodeoxynucleotides (ODNs) showed the sequence was comprised of 6,342 nt (SEQ ID NO. 47), which included the following domains:
- CMV cytomegalovirus
- BGH bovine growth hormone
- pA polyA
- the HIRMAb-GDNF TV also included the expression cassette encoding neo, the neomycin resistance gene, to enable selection with G418 ( Figure 37). It was necessary to include the HC fusion gene, the LC gene, and the DHFR gene on a single piece of DNA, or tandem vector ( Figure 37) to allow for equally high expression of all 3 genes in the transfected CHO cell.
- the HIRMAb-GDNF TV sequence encoded for a 234 AA LC protein (SEQ ID NO 49), which was comprised of a 20 AA IgG signal peptide, and the 214 AA HIRMAb LC.
- the predicted MW of the LC was 23,398 Da and the predicted pi of the LC protein was 5.45.
- the HIRMAb-GDNF TV sequence encoded for a DHFR protein (SEQ ID NO 50), that had an AA sequence 100% identical with the known AA sequence of murine DHFR.
- the domain structure of the HC is shown in Figure 36 and the domain structure of the LC is shown in Figure 38. Electroporation of CHO cells.
- DG44 CHO cells were grown in serum-free medium (SFM), containing IX HT supplement (hypoxanthine and thymidine).
- SFM serum-free medium
- IX HT supplement hyperxanthine and thymidine.
- DG44 CHO cells (5 x 10 6 viable cells) were electroporated with 5 ⁇ g Pvul-linearized TV plasmid DNA. The cell-DNA suspension is then incubated for 10 min on ice. Cells are electroporated with pre-set protocol for CHO cells, i.e., square wave with pulse of 15 msec and 160 volts. After electroporation, cells are incubated for 10 min on ice.
- the cell suspension is transferred to 50 ml culture medium and plated at 125 ⁇ l per well in 40 x 96-well plates (10,000 cells per well), and 4,000 wells per study. Selection and amplification with methotrexate and screening IgG ELISA.
- transfected cell lines are initially selected with G418.
- the TV also contains the gene for DHFR, so the transfected cells were also selected with 20 nM methotrexate (MTX) and HT-deficient medium.
- MTX methotrexate
- HT-deficient medium Once visible colonies are detected at about 21 days after EP, the conditioned medium was sampled for human IgG by ELISA, Plates were removed from the incubator and transferred to the sterile hood where 100 ⁇ L samples were taken from each well using a Precision Pipettor system and transferred into a sterile 96-well tissue culture plate, which was then used for the human IgG ELISA.
- the media taken from the EP plates for the ELISA was replaced with 100 ⁇ L of SFM and cells were returned to the incubator at 37 °C and 8% CO 2 .
- Wells with high human IgG signals in the ELISA were transferred from the 96-well plate to a 24-well plate with 1 mL of SFM.
- the 24-well plates were returned to the incubator at 37 °C and 8% CO 2 .
- the following week IgG ELISA was performed on the clones in the 24-well plates. This was repeated through the 6- well plates to T75 flasks and finally to 60 mL and 125 mL square plastic bottles on an orbital shaker.
- the cells adapted to the 60 mL bottle on the orbital shaker at 120RPM they were transferred into a 125 mL plastic square bottle with 12 mL of SFM.
- the final MTX concentration was 80 nM
- the medium IgG concentration which is a measure of HIRMAb-GDNF fusion protein in the medium was >10 mg/L at a cell density of 10 6 /mL. Dilutional cloning of CHO cells.
- Clones selected for dilutional cloning were removed from the orbital shaker in the incubator and transferred to the sterile hood.
- the cells were diluted to 500 mL in F-12K medium with 5% dialyzed fetal bovine serum (d-FBS) and penicillin/streptomycin, and the final dilution was 8 cells per mL, so that 4,000 wells in 40 x 96-well plates were plated at a cell density of 1 cell per well (CPW).
- CPW cell density of 1 cell per well
- the plates were then returned to the incubator at 37 °C and 8% CO 2 .
- the cells diluted to 1 cell/well cannot survive without serum.
- DC plates were removed from the incubator and transferred to the sterile hood where 125 ⁇ L of F-12K medium with 5% dialyzed fetal bovine serum (d-FBS) was added to each well.
- the selection media contained 5% d-FBS, 30 nM MTX and 0.25 mg/mL Geneticin.
- aliquots from each of the 4,000 wells were removed for human IgG ELISA, using robotics equipment.
- DC plates were removed from the incubator and transferred to the sterile hood, where 100 ⁇ L of media was removed per well of the 96-well plate and transferred into a new, sterile sample 96-well plate using an 8-channel pipettor or a Precision Pipettor system. ELISA screening of 4,000 wells.
- HIRMAb-GDNF fusion protein was purified to homogeneity based on reducing and non-reducing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS- PAGE).
- SDS- PAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis
- a characteristic "fingerprint" of the HIRMAb-GDNF fusion protein is the detection of 3 heavy chain glycoforms, which arise from differential glycosylation of the GDNF part of the fusion protein. This pattern is reproducible from run to run.
- GDNF and human IgG Western blot The CHO derived HIRMAb-GDNF fusion protein was electrophoresed on a 12% SDS-PAGE gel and blotted to nitrocellulose for Western blotting with primary antibodies to either human IgG (left panel, Figure 39), or to human GDNF (right panel, Figure 39). Both the anti-human IgG antibody and the anti-human GDNF antibody reacted specifically with the heavy chain of the HIRMAb-GDNF fusion protein. CH2 region and 2 sites within the GDNF region of the fusion protein heavy chain.
- the neutral monosaccharide content (moles monosaccharide/mole fusion protein) was 7.7 for N-acetyl glucosamine, 5.3 for mannose, 2.3 for galactose, 1.6 for fucose, and 0.19 for N-acetyl galactosamine.
- These 4 sugars are organized as 1 of 4 different terminal structures, designated GO, Gl(1,6), Gl(1,3), or G2, depending on whether there is 0, 1, or 2 terminal galactose moieties, respectively.
- the profile for AGT-190 is GO>G1>G2, which is typical of CHO-derived recombinant proteins, and indicative of relative reduced terminal galactosylation.
- the glycosylation of HIRMAb-GDNF fusion protein on at least 3 different asparagine residues explains the heterogeneity of the heavy chain on Western blotting ( Figure 39).
- Parkinson's disease is a neurodegenerative condition that affects the dopaminergic neurons of the nigral-striatal tract.
- GDNF is a potent trophic factor for these dopaminergic neurons.
- the intra-cerebral injection of the GDNF protein into the brain of rats with experimental PD can protect these neurons, and blocks further axotomy of the fibers projecting from the substantia nigra to the striatum. Accordingly, recombinant human GDNF has been developed as a new therapeutic for PD. However, GDNF does not cross the BBB.
- GDNF was administered via a transcranial route using intra-cerebroventricular (ICV) injection.
- ICV intra-cerebroventricular
- GDNF delivered by ICV injection was found to not penetrate into the brain parenchyma and was ineffective in patients with PD, and this delivery approach was abandoned.
- GDNF has been administered by convection enhanced diffusion (CED).
- CED convection enhanced diffusion
- a reservoir holding the GDNF is implanted in the abdomen, and a catheter is run through the skin from the reservoir to the brain via a transcranial passage.
- the reservoir has a pump that continuously pumps the GDNF fluid into the brain.
- the trans-BBB delivery route has two unique advantages.
- the GDNF can be given by a non-invasive systemic injection, such as an intravenous, subcutaneous, or infra-muscular injection, and no neurosurgical procedure is required.
- the GDNF enters the brain via the trans-vascular route.
- the GDNF Since every neuron in the brain is perfused by its own blood vessel, the GDNF is delivered to every cell comprising the nigra-striatal tract of brain.
- the GDNF could be given on a weekly or bi-weekly basis to patients with PD at doses ranging from 1, 3, 10, 30, or 100 mg.
- the considerations in Example 7 suggest that 25-30 mg may be a preferred dose.
- GDNF is highly neuroprotective in experimental stroke (Kitagawa et al, Stroke 29:1417-
- GDNF receptor GFR ⁇ l
- Activation of the GDNF receptor, GFR ⁇ l inhibits apoptosis in the neuron that is induced by an ischemic event.
- the demonstration of GDNF's neuroprotective effects in experimental stroke have uniformly involved intra-cerebral injection of the neurotrophin, because the GDNF does not cross the BBB, and because the BBB is intact in the first 5 hours after stroke, when the rescue of dying neurons is still possible.
- the HIRMAb-GDNF is a re-engineered form of GDNF that is both equi-potent with GDNF as a neuroprotective agent, and is able to cross the human BBB following systemic administration.
- the patient suffering from an acute stroke could be rapidly treated with intravenous HIRMAb-GDNF in the emergency room.
- the HIRMAb- GDNF fusion protein will rapidly enter the brain and activate GFR ⁇ l receptors on neurons to inhibit the apoptosis cycle induced by the stroke.
- chronic treatment with systemic HIRMAb-GDNF fusion protein could be therapeutic in the rehabilitation phase of stroke, since the intra-cerebral infusion of GDNF into the post-stroke brain induces neurogenesis, and neural repair in the post-stroke period (Kobayashi et al, 2006).
- GDNF amyotrophic lateral sclerosis
- SMA spinal muscular atrophy
- GDNF is highly neuroprotective of spinal cord motor neurons (Bonn supra). However, it is unlikely that GDNF will be developed as a drug for ALS or SMA, given the failed clinical trials of other neurotrophic factors for these conditions.
- Both BDNF and ciliary neurotrophic factor (CNTF) were administered to ALS patients in the 1990s by subcutaneous administration. The subcutaneous administration phase III clinical trials failed, because BDNF and CNTF, like GDNF, do not cross the BBB.
- HlRMAb fusion proteins cross the blood-spinal cord barrier, which as been demonstrated by brain scanning of adult Rhesus monkeys following intravenous administration of the HIRMAb fusion protein (Boado et al, Biotechnoi Bioeng, 97(6): 1376-1386 (2007); Boado et al, Bioconjug. Chem. 18:447-455 (2007)).
- Example 20
- GDNF promotes neural repair in the period following acute experimental brain injury, such as with a lateral fluid percussion injury (Bakshi et al, Ever. J. Neurosci. 23:2119-2134 (2006)). GDNF does not cross the BBB, and the BBB is intact in the days/weeks after head injury, when the brain attempts to heal from the acute insult. Therefore, the beneficial effects of GDNF on acute head injury could only be demonstrated by the intra-cerebral injection of genetically modified stem cells that secrete GDNF. Although the trans-cranial injection approach may be feasible in the rat brain, the human brain is 1000-fold larger than a rat brain.
- Diffusion of the GDNF from the depot injection site becomes limiting, and the GDNF cannot penetrate to the wound area, beyond the local injection site.
- the GDNF is delivered to brain via the trans-vascular route, then every injured neuron in the brain is exposed to the GDNF.
- the latter is feasible with the weekly, biweekly, or daily systemic administration of the HIRMAb-GDNF fusion protein during the post- injury repair and rehabilitation period.
- GDNF vascular endothelial growth factor
- GDNF can promote neural repair following experimental spinal cord injury (Lu et al, J. Neurotrauma 19: 1081- 1090 (2002)).
- the opening of the BBB following spinal cord injury is only transient, it is not possible to treat spinal cord injury with systemic GDNF administration.
- the GDNF expressing plasmid DNA was injected directly into the rat spinal cord following the acute spinal cord injury.
- the size of the spinal cord in humans is 1000-fold larger than that of the rat Therefore, it is not possible to distribute GDNF to the entire lesioned area following direct spinal injection of the GDNF therapeutic in humans.
- GDNF is delivered to all injured neurons Ln the spinal cord following a trans-vascular delivery, and this is possible with the systemic administration of the HIRMAb-GDNF fusion protein.
- the intra-cerebral injection of GDNF results in treatment of animals addicted to either opioid, or non-opioid drugs.
- the intra-cerebral injection of GDNF decreases cocaine self-aministration in rats (Green-Sadan et al, Eur. J. Neurosci., 18: 2093-2098, 2003).
- the intra-cerebral injection of GDNF decreases ethanol self-administration (He et al, J. Neurosci., 25: 619-628, 2005).
- the GDNF has to be administered via a trans-cranial route, because GDNF does not cross the BBB.
- the drug, ibogaine decreases withdrawl symptoms to chronic opioid drugs, cocaine, or alchohol, and works by increasing GDNF in regions of the brain such as the ventral tegmental area (VTA) (He and Ron, FASEB J, 20: E1820-D1827, 2006).
- VTA ventral tegmental area
- ibogaine is a small molecule that crosses the BBB, its application in the treatment of addiction is limited by severe side effects.
- GDNF is implicated in another study related to cocaine addiction.
- Lys Arg lie Gly Trp Arg Phe He Arg He Asp Thr Ser Cys VaI Cys
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Engineering & Computer Science (AREA)
- Medicinal Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- Molecular Biology (AREA)
- Endocrinology (AREA)
- Optics & Photonics (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Physics & Mathematics (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Toxicology (AREA)
- Zoology (AREA)
- Gastroenterology & Hepatology (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Description
FUSION PROTEINS FOR DELIVERY OF GDNF TO THE CNS
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application in a continϊation-in-part of U.S. Patent Application No. 11/245,546 filed
October 7, 2005. This application also claims priority to U.S. Provisional Patent Application serial No. 60/990,290 filed November 26, 2007, the contents of which are incorporated herein by reference in their entirety.
BACKGROUND OF THE INVENTION
[0002] Neurological disorders represent a major cause of mortality and disability worldwide.
Despite extensive progress, current treatment options remain limited in some aspects. One major reason for this limitation is that the brain is unique in allowing only select access to molecules. While this is a useful protective mechanism, it also means that many potentially beneficial molecular entities do not have access to the central nervous system (CNS), and thus are unable to exert a therapeutic effect in many neurological disorders or other conditions of the CNS. The present invention represents an advance in providing accessibility of the CNS for molecular entities whose ability to cross the blood brain barrier is limited.
SUMMARY OF THE INVENTION
[0003] In one aspect, the invention provides a composition comprising a neurotherapeutic peptide comprising an amino acid sequence which is at least 80% (e.g., 95%) identical to the amino acid sequence of mature human GDNF (SEQ ID NO: 52) covalently linked to a structure that is capable of crossing the blood brain barrier (BBB) (e.g., an antibody). In some embodiments, the structure that is capable of crossing the BBB crosses the BBB on an endogenous BBB receptor. In some embodiments, the endogenous BBB receptor is the insulin receptor, transferrin receptor, leptin receptor, lipoprotein receptor, and the IGF receptor. In some embodiments, the structure that is capable of crossing the BBB is a monoclonal antibody. In some embodiments, the monoclonal antibody is a chimeric monoclonal antibody. In one embodiment, the chimeric antibody contains sufficient human sequences to avoid significant immunogenic reaction when administered to a human, In some embodiments, the above-mentioned neurotherapeutic peptide is covalently linked at its amino terminus to the carboxy terminus of the heavy chain of the MAb. In one embodiment, the neurotherapeutic peptide of the above-mentioned composition comprises the amino acid sequence of mature human GDNF.
[0004] In a further aspect provided herein is a composition for treating a neurological disorder comprising a neurotherapeutic peptide comprising an amino acid sequence which is at least 80% identical to the amino acid sequence of mature human GDNF covalently linked to an immunoglobulin that is capable of crossing the blood brain barrier, wherein the composition is capable of crossing the BBB in an amount that is effective in treating the neurological disorder. In
some embodiments provided herein is a mammalian cell comprising the just-mentioned composition.
[0005] In another aspect provided herein is a method of transport of a neurotherapeutic peptide comprising an amino acid sequence which is at least 80% identical to the amino acid sequence of mature human GDNF (SEQ ID NO: 52) from the peripheral circulation across the BBB in an effective amount, comprising peripherally administering to an individual the GDNF covalently attached to a structure that crosses the BBB, under conditions where the agent covalently attached to a structure that crosses the BBB is transported across the BBB in an effective amount.
[0006] In yet another aspect provided herein is a method for treating a CNS disorder in an individual comprising peripherally administering to the individual an effective amount of a composition comprising a neurotherapeutic peptide comprising an amino acid sequence which is at least 80% (e.g., 95%) identical to the amino acid sequence of human GDNF covalently attached to a structure capable of crossing the BBB. In one embodiment, the neurotherapeutic peptide comprises the amino acid sequence of human GDNF. In some embodiments, the administering is oral, intravenous, intramuscular, subcutaneous, intraperitoneal, rectal, transbuccal, intranasal, transdermal, or inhalation administration. In some embodiments, the CNS disorder is an acute CNS disorder (e.g., spinal cord injury, brain injury focal brain ischemia and global brain ischemia), hi other embodiments, the CNS disorder is a chronic CNS disorder (e.g., a chronic neurodegenerative disease, drug addiction, or alcohol addiction). In some embodiments, the chronic neurodegenerative disease is Parkinson's disease or a motor neuron disease (e.g., amyotrophic lateral sclerosis). In some embodiments, the individual to be treated is administered about 1 to about 100 mg of the composition used in above-mentioned method.
[0007] In yet another aspect provided herein is a composition comprising a neurotherapeutic peptide comprising an amino acid sequence which is at least 80% identical to the amino acid sequence of human GDNF covalently linked to an immunoglobulin, wherein the neurotherapeutic peptide has a serum half-life that is an average of at least about 5 -fold greater than the serum half- life of the neurotherapeutic peptide alone, hi some embodiments, the immunoglobulin is an antibody to an endogenous BBB receptor (e.g., the insulin receptor, transferrin receptor, leptin receptor, lipoprotein receptor, or the IGF receptor). In one embodiment, the neurotherapeutic peptide comprises the amino acid sequence of human GDNF.
[0008] In a further aspect provided herein is a method for treating substance abuse in an individual, comprising administering to the individual an effective amount of a composition comprising a neurotherapeutic peptide comprising an amino acid sequence which is at least 80% identical to the amino acid sequence of mature human GDNF (SEQ ID NO: 52) covalently attached to a structure capable of crossing the BBB.
INCORPORATION BY REFERENCE
[0009] All publications and patent applications mentioned in this specification are herein incorporated by reference to the same extent as if each individual publication or patent application was specifically and individually indicated to be incorporated by reference. BRIEF DESCRIPTION OF THE DRAWINGS
[0010] The novel features of the invention are set forth with particularity in the appended claims.
A better understanding of the features and advantages of the present invention will be obtained by reference to the following detailed description that sets forth illustrative embodiments, in which the principles of the invention are utilized, and the accompanying drawings of which:
[0011] Figure 1. Diagram showing genetic engineering of a eukaryotic expression vector encoding a fusion gene comprised of the variable region of the heavy chain (VH) of the chimeric HIRMAb, a genomic fragment encoding the constant region of human IgGl, which is comprised of 4 regions (CHl, hinge, CH2, and CH3), and the cDNA for the BDNF variant (vBDNF). Transcription of the gene is driven by the human IgGl promoter (PRO). This vector produces the heavy chain (HC) of the fusion protein.
[0012] Figure 2. Diagram showing genetic engineering of a bacterial expression plasmid encoding vBDNF cDNA with modified 51- and 3'-linkers.
[0013] Figure 3. Ethidium bromide stained agarose gels showing size of various constructs that are intermediates in construction of a tandem vector that produces the fusion protein. (A) Lanes 1-2: plasmid from figure 2 digested with Nrul showing 0.4 kb vBDNF and 3.5 kb vector backbone. Lane 3: MW size standards ranging from 1.4-0.1 kb. Lane 4: MW size standards ranging from 23-0.6 kb. (B) Lane 1: the 0.4 kb vBDNF cDNA is produced by the polymerase chain reaction (PCR) using cDNA reverse transcribed from polyA÷ RNA isolated from human U87 glioma cells; the PCR primer sequences are given in Table 2, Lanes 2 and 3: same MW size standards as shown in panel A. (C) lane 1 : clone 416 following digestion with Nhel and BamHI; lane 2: negative clone; lane 3: clone 400 following digestion with Nhel and BamHI: lanes 4 and 5: same MW size standards as shown in panel A. (D) PCR fragments of DNA encoding fusion protein HC (lane 1) and LC (lane 2); lanes 3-4: same MW size standards as shown in panel A. (E) lanes 1-4: 4 different but identical copies of clone 422a following digestion with Nhel, showing release of 0.4 kb fusion protein HC variable region (VH) cDNA; lanes 5-6: same MW size standards as shown in panel A. (F) lanes 1- 4: 5 different but identical copies of clone 423a following digestion with EcoRV and BamHI, showing release of 0.7 kb entire LC cDNA; lanes 5-6: same MW size standards as shown in panel A. (G) Restriction endonuclease mapping of tandem vector (Figure 12) with Pvul (lane 1), and EcoRI-Hindlll (lane 2). Pvul (single cut) produced the expected linear DNA band of ~11 kb. Digestion with EcoRI and HindIII releases both the fusion protein light chain (i.e. 1.8 kb) and DHFR (i.e. 1.5 kb) expression cassettes. The ~8 kb band represents the backbone vector with the fusion protein heavy chain expression cassette; lanes 3-4: same MW size standards as shown in panel A, albeit in reverse order.
[0014] Figure 4. Nucleotide (SEQ ID NO: 21) and amino acid (SEQ ID NO: 22) sequence of fusion site between carboxyl terminus of the fusion protein HC and the amino terminus of the vBDNF. The 3-amino acid linker between the HIRMAb HC and the vBDNF is shown, as well as the new stop codon at the carboxyl terminus of vBDNF.
[0015] Figure 5. Nucleotide sequence (SEQ ID NO: 23) of fusion protein HC gene cloned into plasmid 416. Italics: human IgGl constant region introns; bold font: human IgGl exon sequence; underline font: vBDNF.
[0016] Figure 6. Amino acid sequence (SEQ ID NO: 24) of the fusion protein HC. The 19 amino acid signal peptide is underlined, as is the 3-amino acid linker between the CH3 region and the vBDNF. The N-linked glycosylation consensus sequence within CH2 is underlined.
[0017] Figure 7. The amino acid sequence (SEQ ID NO: 25) of the different domains of the fusion protein HC are shown.
[0018] Figure 8. Diagram showing production of the intronless eukaryotic expression vector, clone 422a, which encodes the fusion protein HC. The fusion protein HC cDNA was produced by PCR from cDNA generated by reverse transcriptase of RNA isolated from myeloma cells transfected with clone 416.
[0019] Figure 9. (A) Nucleotide sequence (SEQ ID NO: 26) of the fusion protein HC cDNA inserted in clone 422a. (B) (SEQ ID NOS 27 & 28) Amino acid sequence of the fusion protein HC that is deduced from the nucleotide sequence shown in panel A. The sequence of the signal peptide is underlined.
[0020] Figure 10. Diagram showing production of the intronless eukaryotic expression vector, clone 423 a, which encodes the fusion protein LC. The fusion protein LC cDNA was produced by PCR from cDNA generated by reverse transcriptase of RNA isolated from myeloma cells transfected with an expression vector producing the LC gene that was derived from chromosomal fragment encoding intron/exon sequence of the human kappa LC gene with the VL of the chimeric HIRMAb LC.
[0021] Figure 11. (A) Nucleotide sequence (SEQ ID NO: 29) of the fusion protein LC cDNA inserted in clone 423a. (B) (SEQ ID NOS 30 & 31) Amino acid sequence of the fusion protein LC that is deduced from the nucleotide sequence shown in panel A, The sequence of the signal peptide is underlined.
[0022] Figure 12, Diagram showing the construction of a tandem vector encoding the HC and
LC genes of the fusion protein. The TV was engineered from the cDNA expression vectors, clones 422a and 423 a, for the HC and LC, respectively, as well as from a bacterial expression plasmid encoding the expression cassette for mouse DHFR.
[0023] Figures 13A and 13B. Nucleotide sequence (SEQ ID NO: 32) of the fusion protein HC gene and LC gene, and the DHFR genes incorporated in the tandem vector.
[0024] Figure 14. Deduced amino acid sequence of the fusion protein HC based on tandem vector nucleotide sequence analysis (SEQ ID NOS 33 & 34). The signal peptide sequence is underlined.
[0025] Figure 15. Deduced amino acid sequence of the fusion protein LC based on tandem vector nucleotide sequence analysis (SEQ ID NO 35 & 36). The signal peptide sequence is underlined.
[0026] Figure 16. Deduced amino acid sequence of the DHFR based on tandem vector nucleotide sequence analysis (SEQ ID NO 37 & 38).
[0027] Figure 17. Viable and total cell density of CHO cells in bioreactor maintained continuously for 50 days; the CHO cells had been stably transfected with the tandem vector encoding the fusion protein.
[0028] Figure 18. Structure of fusion protein, a bi-functional molecule that both (a) binds to the human BBB human insulin receptor (HIR) to enable transport across the BBB from blood, and (b) binds to the trkB on neurons to induce neuroprotection.
[0029] Figure 19. Correlation of 2 different 'sandwich1 immunoassays, where the secondary antibody is either directed against the Fc region of human IgGl (x-axis) or against human BDNF (y-axis). The primary antibody in either assay is directed against the human kappa light chain. The measured level of fusion protein in CHO cell conditioned medium is the same whether the anti-Fc or the anti-BDNF antibody is used.
[0030] Figure 20. Reducing (left) and non-reducing (right) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of chimeric HIRMAb and fusion protein. Under reducing conditions, the size of the light chain, 30 kDa, is identical for chimeric HIRMAb and the fusion protein; the size of the heavy chain of fusion protein is about 15 kDa larger than the chimeric HIRMAb heavy chain, owing to the presence of the BDNF. Under non-reducing conditions, the chimeric HIRMAb and the fusion protein migrate as single hetero-tetrameric species with molecular weights of 180 and 200 kDa, respectively.
[0031] Figure 21. (Left panel) Western blot with anti-human IgG primary antibody. The size of the heavy chain of the fusion protein and the chimeric HIRMAb is 64 kDa and 50 kDa, respectively, and the size of the light chain for either the fusion protein or the chimeric HIRMAb is 25 kDa. (Right panel) Western blot with anti-human BDNF antibody, which reacts with either fusion protein or BDNF, but not with chimeric HIRMAb. MW standards (STDS) are shown on the right side.
[0032] Figure 22. Isoelectric focusing (IEF) of isoelectric point (pi) standards (lane 1), chimeric
HIRMAb (lanes 2 and 4), BDNF (lane 3), and fusion protein (lane 5). Whereas BDNF is highly cationic with a pI>10, the pi of the fusion protein approximates the pi of the chimeric HIRMAb, which is about 8.5, and close to the theoretical pi of the fusion protein.
[0033] Figure 23. (A) Outline for human insulin receptor (HER) competitive ligand binding assay
(CLBA). The HIR extracellular domain (ECD) is bound by the [125l]-labeled murine HIRMAb, and this binding is competitively displaced by either the chimeric HIRMAb or the fusion protein, as
shown in Panel B. (B) Displacement of binding of [125l]-labeled murine HIRMAb to the HIR ECD by either chimeric HIRMAb or fusion protein. The affinity of the chimeric HIRMAb to the HIR ECD is high, and the affinity of the fusion protein for the HIR ECD is not significantly different from that of the chimeric HIRMAb. These results show that the fusion of the vBDNF to the carboxyl terminus of the chimeric HIRMAb heavy chain does not impair binding of the fusion protein to the HIR.
[0034] Figure 24. (A) Design of trkB competitive ligand binding assay (CLBA). The advantage of the PEG linker is that this modification eliminates the high non-specific binding (NSB) of the cationic BDNF to the ELISA wells, which gives an assay with a high signal/noise ratio. The binding of the BDNF-PEG20C0-biotin to the trkB ECD was detected with a peroxidase system using avidin and biotinylated peroxidase. (B) The binding of the BDNF-PEG2000-biotin to the trkB ECD is competitively displaced by recombinant BDNF. This binding data was analyzed by non-linear regression analysis to yield the K| of BDNF binding, 3.5 ± 1.3 pmol/well and the NSB parameter. (C) The binding of the BDNF-PEG20ύ0-biotin to the trkB ECD is competitively displaced by the fusion protein. This binding data was analyzed by non-linear regression analysis to yield the K1 of fusion protein binding, 2.2 ± 1.2 pmol/well, which is not significantly different than the Ki for native BDNF. These data show that the affinity of the fusion protein for the trkB receptor is equal to that of native BDNF.
[0035] Figure 25. (A) Design of hypoxia-reoxygenation neuroprotection assay in human neural
SH-SY5Y cells, Exposure of the cells to retinoic acid for 7 days causes an up-regulation in the gene expression of trkB, the BDNF receptor. (B) Neuroprotection assay based on the measurement of mitochondrial respiration with 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT). The maximal neuroprotection is established with 4 nM BDNF, and 4 nM fusion protein yields a comparable level of neuroprotection in human neural cells. The MTT level does not return to that of non-hypoxic cells, because only about 50% of the cells induce trkB in response to retinoic acid.
[0036] Figure 26. (A) Light micrograph of capillaries isolated from human brain, used as an in vitro model system of the human BBB. (B) Radio-receptor assay of binding of [3H]-fusion protein to the HIR on the human BBB; the binding is self-inhibited by unlabeled fusion protein. Fitting the saturation data to a Scatchard plot with a non-linear regression analysis yields the binding parameters: KD= 0.55 ± 0.07 nM, B1110x = 1.35 ± 0.10 pmol/mgp.
[0037] Figure 27. Pharmacokinetics and brain uptake of fusion protein in the adult Rhesus monkey. (A) The serum concentration of [3H]-fusion protein, or [Iz5l]-murine HIRMAb, is plotted vs. time after a single intravenous injection of either protein in anesthetized adult Rhesus monkeys. (B) The serum radioactivity that is precipitable by trichloroacetic acid (TCA) is plotted vs time after a single intravenous injection of either [3H]-fusion protein in the anesthetized adult Rhesus monkey, or [3H]-BDNF in the anesthetized adult rat. (C) Capillary depletion analysis of brain distribution at 180 minutes after a single intravenous injection of either [3H]-fusion protein, or [3H]-mouse IgG2a,
in the anesthetized adult Rhesus monkey. (D) Primate brain concentrations of fusion protein at 180 minutes after an intravenous injection of 373 μg fusion protein, as compared to the endogenous primate brain concentration of BDNF.
[0038] Figure 28. Schematic illustration of the protein formed by fusion of the amino terminus of the mature GDNF to the carboxyl terminus of the CH3 region of the heavy chain of the chimeric HIRMAb. The fusion protein is a bi-functional molecule: the fusion protein binds the HIR, at the BBB, to mediate transport into the brain, and binds the GFRαl, to mediate GDNF biologic effects in brain behind the BBB.
[0039] Figure 29. (A) Ethidium bromide stain of agarose gel of human GDNF cDNA (lane 1), which was produced by PCR from cDNA produced by reverse transcription of RNA from human U87 glial cells, and GDNF-specific ODN primers (Table 6). Lane 2: DNA sizing standards. (B) Genetic engineering of pHIRMAb-GDNF, the eukaryotic expression plasmid encoding the fusion protein of GDNF and the heavy chain (HC) of the chimeric HIRMAb. The fusion gene is 5'- flanked by the cytomegalovirus (CMV) promoter and 3 '-flanked by the bovine growth hormone polyA (pA) sequence.
[0040] Figure 30. Reducing SDS-PAGE and Coomasie blue staining of protein A affinity purified, COS-cell chimeric HIRMAb and the HIRMAb-GDNF fusion protein. Both are purified to homogeneity and are comprised of a heavy chain and a light chain. The molecular weight (MW) of the heavy chain (HC) of the HIRMAb-GDNF fusion protein is about 15 kDa larger than the MW of the HC of the chimeric HIRMAb, owing to fusion of the 15 kDa GDNF.
[0041] Figure 31. Western blot with either anti-human (h) IgG primary antibody (left) or an anti- human GDNF primary antiserum (right). The immunoreactivity of the COS cell derived HIRMAb- GDNF fusion protein is compared to the chimeric HIRMAb and to recombinant GDNF. Both the HIRMAb-GDNF fusion protein and the HIRMAb have identical light chains on the anti-hlgG Western. The HIRMAb-GDNF fusion heavy chain reacts with both the anti-hlgG and the anti- human GDNF antibody, whereas the HIRMAb heavy chain only reacts with the anti-hlgG antibody. The size of the HIRMAb-GDNF fusion heavy chain, 70 kDa, is about 15 kDa larger than the size of the heavy chain of the HlRMAb, owing to the fusion of the 15 kDa GDNF to the 55 kDa HIRMAb heavy chain.
[0042] Figure 32. Binding of either the COS cell-derived chimeric HIRMAb or the HIRMAb-
GDNF fusion protein to the HIR extracellular domain (ECD) is saturable. The ED50 of HIRMAb- GDNF binding to the HIR ECD is comparable to the ED50 of the binding of the chimeric HIRMAb.
[0043] Figure 33. (A) Outline of GFRαl receptor binding assay. The GFRα 1 :Fc fusion protein is captured by a mouse anti-human (MAH) Fc antibody. The GDNF, or HIRMAb-GDNF fusion protein, binds to the GFRαl, and this binding is detected with a goat anti-GDNF antibody and a rabbit anti-goat (RAG) antibody conjugated to alkaline phosphatase (AP). (B) Binding of either the
COS cell derived chimeric HIRMAb or the HIRMAb-GDNF fusion protein to the GFRαl extracellular domain (ECD) is saturable. The ED50 of HIRMAb-GDNF binding to the GFRαl ECD is comparable to the ED50 of the binding of recombinant GDNF. There is no binding to the GFRαl by the chimeric HIRMAb.
[0044] Figure 34. (A) Binding by either GDNF or the HIRMAb-GDNF fusion protein to the
GFRαl on the cell membrane of c-ret kinase transfected human neural SK-N-MC cells activates the tyrosine hydroxylase (TH) 5 '-flanking sequence (FS), which drives luciferase gene expression. (B) Both GDNF and the COS cell-derived HIRMAb-GDNF fusion protein activate luciferase gene expression in a saturable manner, with ED50 values comparable to GFRαl binding (Figure 33B). There is no activation of luciferase gene expression by the chimeric HIRMAb.
[0045] Figure 35. (A) Effect of intra-cerebral injection of the HIRMAb-GDNF versus saline on stroke volume in rats. Serial coronal sections of rat brain at 24 hours after permanent MCAO and intra-cerebral saline (B) or HIRMAb-GDNF fusion protein (C) are stained with 2% 2,3,5- triphenyltetrazolium chloride (TTC). A representative stain is shown for 1 rat from each treatment group. In these inverted grayscale images, the infarcted brain is biack, and the healthy brain is white. Most rostral section is top and most caudal section is bottom.
[0046] Figure 36. Domain structure and amino acid sequence of the heavy chain (HC) of the
HIRMAb-GDNF fusion protein. The polypeptide is comprised of a signal peptide, followed by the variable region of the heavy chain (VH) of the chimeric HIRMAb. The 3 CDRs and 4 FRs are shown. The VH region is followed by the human IgGl constant region, which is comprised of the CHl, hinge, CH2, and CH3 domains, followed by a 2-amino acid (Ser-Ser) linker, followed by the mature human GDNF. The single N-linked glycosylation site within the CH2 region, and the 2 N- linked glycosylation sites within the GDNF are underlined with the asparagine (N) residues in bold font. Cysteine (C) residues, which form inter-chain disulfide bonds are shown, and include a linkage between the HC hinge region and the light chain (LC), the HC hinge region and the paired HC, the HC CH2 region and the paired HC, and the GDNF-GDNF linkage.
[0047] Figure 37. Tandem vector (TV) encoding multiple genes on a single piece of DNA. The
GDNF cDNA, produced by PCR, is subcloned into the Hpal site of the universal tandem vector (UTV) to generate the HIRMAb-GDNF TV, which allows for expression in CHO cells of the fusion heavy chain (HC) gene, the light chain (LC) gene, and the dihydrofolate reductase (DHFR) gene.
[0048] Figure 38. Domain structure of the light chain of the HlRMAb.
[0049] Figure 39. Western blot with a primary antibody against either human IgG (A) or human
GDNF (B). The CHO-derived chimeric HIRMAb and the CHO-derived HIRMAb-GDNF fusion protein are applied in panel A, and the HIRMAb, the HIRMAb-GDNF fusion protein, and GDNF are applied in panel B. The migration of molecular weight standards is shown hi lane 1 of panel A.
[0050] Figure 40. The affinity of the CHO-derived HIRMAb and the CHO-derived HIRMAb-
GDNF fusion protein for binding to the extracellular domain of the human insulin receptor (HIR) is
measured in this ELISA format. The concentration of antibody that causes 50% saturation of binding, the ED50, was determined by non-linear regression analysis. The 2 proteins have the same affinity for the HIR, indicating fusion of GDNF to the C-terminus of the heavy chain of the HIRMAb does not affect binding to the HIR.
[0051] Figure 41. Size exclusion high performance liquid chromatography of the HIRMAb-
GDNF fusion protein shows the absence of aggregates in the CHO-derived protein.
[0052] Figure 42. Native polyacrylamide gel electrophoresis of the HIRMAb, control human
IgGl, or the HIRMAb-GDNF fusion protein shows the absence of aggregates in the CHO-derived fusion protein.
[0053] Figure 43. (A) Light micrograph of isolated human brain capillaries, used as an in vitro model system of the human BBB. (B) Binding of the 3H-HIRMAb-GDNF fusion protein to the isolated brain capillaries is inhibited by the murine HIRMAb. The component of HIRMAb-GDNF uptake by the capillaries that is resistant to HlRMAb inhibition represents endocytosed fusion protein.
DETAILED DESCRIPTION OF THE INVENTION
I. Introduction
II. Definitions
III. The blood brain barrier
A. Transport systems
B. Structures that bind to a blood brain barrier receptor-mediated transport system
IV. Agents for transport across the blood brain barrier
A. Neurotrophins
B. Brain-derived neurotrophic factor
C. Glial-derived neurotrophic factor
V. Compositions
VI. Nucleic acids, vectors, cells, and manufacture
A. Nucleic acids
B. Vectors
C. Cells
D. Manufacture
VII. Methods
VIII. Kits
Abbreviations
AA amino acid
ALS amyotrophic lateral sclerosis
AP alkaline phosphatase
BBB blood-brain barrier
BCA bicinchoninic acid
BDNF brain derived neurotrophic factor
BGH bovine growth hormone
Bmax dose causing maximal effect
BSA bovine serum albumin
C cysteine
CDR compementarity determining region
CED convection enhanced diffusion
CHO Chinese hamster ovary
CMV cytomegalovirus
CNTF ciliary neurotrophic factor
DC dilutional cloning
DHFR dihydrofolate reductase
ECD extracellular domain
ED50 effective dose causing 50% saturation
FR framework region
FS flanking sequence
FWD forward
GDNF glial derived neurotrophic factor
GFR GDNF receptor
HC heavy chain
HIR human insulin receptor
HIRMAb MAb to HIR
HIRMAb-GDNF fusion protein of HIRMAb and GDNF
HPLC high pressure liquid chromatography
HT hypoxanthine-thymidine
ICV intra-cerebroventricular
ID injected dose
IgG immunoglobulin G
LC light chain
MAb monoclonal antibody
MAH mouse anti-human IgG
MCAO middle cerebral artery occlusion
MTX methotrexate
MW molecular weight
N asparagine nt nucleotide
ODN oligodeoxynucleotide pA poly-adenylation
PAGE poiyacrylamide gel electrophoresis
PBS phosphate buffered saline
PBST PBS plus Tween-20
PCR polymerase chain reaction
PD Parkinson's disease pi isoelectric point
RAG rabbit anti-goat IgG
REV reverse
RNase A ribonuclease A
RT reverse transcriptase
RT room temperature
SDM site-directed mutagenesis
SDS sodium dodecyl sulfate
SEC size exclusion chromatography
Ser serine
SFM serum free medium
SMA spinal muscular atrophy
TH tyrosine hydroxylase
TTC triphenyltetrazolium chloride
TV tandem vector
UTV universal TV
VH variable region of heavy chain
VL variable region of light chain
I. Introduction
[0054] The blood brain barrier is a limiting factor in the delivery of many peripherally- administered agents to the central nervous system. The present invention addresses three factors that are important in delivering an agent across the BBB to the CNS: 1) a pharmacokinetic profile for the agent that allows sufficient tune in the peripheral circulation for the agent to have enough contact with the BBB to traverse it; 2) modification of the agent to allow it to cross the BBB; and 3) retention of activity of the agent once across the BBB. Various aspects of the invention address these factors, by providing fusion structures (e.g., fusion proteins) of an agent (e.g., a therapeutic agent) covatently linked to a structure that causes the agent to have increased serum half life, to be transported across the BBB, and/or to retain some or all of its activity in the brain while still attached to the structure.
[0055] Accordingly, in one aspect, the invention provides compositions and methods that utilize an agent covalently linked to a structure capable of crossing the blood brain barrier (BBB). The compositions and methods are useful in transporting agents, e.g. therapeutic agents such as neurotherapeutic agents, from the peripheral blood and across the BBB into the CNS. Neurotherapeutic agents useful in the invention include neurotrophins, e.g. brain-derived neurotrophic factor (BDNF) and glial-derived neurotrophic factor (GDNF). In some embodiments, the structure that is capable of crossing the BBB is capable of binding to an endogenous BBB receptor mediated transport system and crossing the BBB. In some embodiments, the structure that is capable of crossing the BBB is an antibody, e.g., a monoclonal antibody (MAb) such as a chimeric MAb.
[0056] In some embodiments, the invention provides a fusion protein that includes a structure capable of crossing the BBB covalently linked to a peptide that is active in the central nervous system (CNS), where the structure capable of crossing the blood brain barrier and the peptide that is active in the central nervous system each retain a proportion (e.g., 10-100%) of their activities or
their binding affinities for their respective receptors, compared to their activities or their binding affinities for their respective receptors as separate entities.
[0057] In another aspect, the invention provides a composition containing a cationic therapeutic peptide covalently linked to an immunoglobulin, where the cationic therapeutic peptide in the composition has a serum and/or circulating half-life that is an average of at least about five fold greater than the serum and/or circulating half-life of the cationic therapeutic peptide alone.
[0058] The invention also provides nucleic acids coding for peptides and proteins. In some embodiments, the invention provides a single nucleic acid sequence that contains a gene coding for a light chain of an immunoglobulin and a gene coding for a fusion protein made up of a heavy chain of the immunoglobulin covalently linked to a peptide. In some embodiments the peptide of the fusion protein is a therapeutic peptide, e.g., a neurotherapeutic peptide such as a neurotrophin. The invention also provides vectors containing the nucleic acids of the invention, and cells containing the vectors. Further provided are methods of manufacturing an immunoglobulin fusion protein, where the fusion protein contains an immunoglobulin heavy chain fused to a therapeutic agent, where the methods include integrating into a eukaryotic cell a single tandem expression vector in which both the immunoglobulin light chain gene and the gene for the immunoglobulin heavy chain fused to the therapeutic agent are incorporated into a single piece of DNA.
[0059] The invention further provides therapeutic compositions, such as pharmaceutical compositions that contain an agent covalently linked to a structure capable of crossing the blood brain barrier (BBB) and a pharmaceutically acceptable excipient. In some embodiments, the invention provides a composition for treating a neurological disorder that includes a BDNF or a GDNF (e.g., human GDNF) covalently linked to an immunoglobulin that is capable of crossing the blood brain barrier, wherein the composition is capable of crossing the BBB in an amount that is effective in treating the neurological disorder.
[0060] The invention also provides methods for treating a neurological disorder in an individual that include peripherally administering to the individual an effective amount of one or more of the compositions of the invention, optionally in combination with other therapy for the disorder. II. Definitions
[0061] As used herein, an "agent" includes any substance that is useful in producing an effect, including a physiological or biochemical effect in an organism. A "therapeutic agent" is a substance that produces or is intended to produce a therapeutic effect, i.e., an effect that leads to amelioration, prevention, and/or complete or partial cure of a disorder. A "therapeutic effect," as that term is used herein, also includes the production of a condition that is better than the average or normal condition in an individual that is not suffering from a disorder, i.e., a supranormal effect such as improved cognition, memory, mood, or other characteristic attributable at least in part to the functioning of the CNS, compared to the normal or average state. A "neurotherapeutic agent" is an agent that produces a therapeutic effect in the CNS. A "therapeutic peptide" includes therapeutic agents that consists of a peptide. A "cationic therapeutic peptide" encompasses therapeutic peptides
whose isoelectric point is above about 7.4, in some embodiments, above about 8, 8.5, 9, 9.5, 10, 10.5, 11, 11.5, 12, or above about 12.5. A subcategory of cationic therapeutic peptides is cationic neurotherapeutic peptides.
[0062] As used herein, a "peptide that is active in the central nervous system (CNS)" includes peptides that have an effect when administered to the CNS. The effect may be a therapeutic effect or a non-therapeutic effect, e.g., a diagnostic effect or an effect useful hi research. If the effect is a therapeutic effect, then the peptide is also a therapeutic peptide, A therapeutic peptide that is also a peptide that is active in the CNS is encompassed by the term "neurotherapeutic peptide," as used herein.
[0063] "Treatment" or "treating" as used herein includes achieving a therapeutic benefit and/or a prophylactic benefit. By therapeutic benefit is meant eradication or amelioration of the underlying disorder or condition being treated. For example, in an individual with a neurological disorder, therapeutic benefit includes partial or complete halting of the progression of the disorder, or partial or complete reversal of the disorder. Also, a therapeutic benefit is achieved with the eradication or amelioration of one or more of the physiological or psychological symptoms associated with the underlying condition such that an improvement is observed in the patient, notwithstanding the fact that the patient may still be affected by the condition. A prophylactic benefit of treatment includes prevention of a condition, retarding the progress of a condition (e.g., slowing the progression of a neurological disorder), or decreasing the likelihood of occurrence of a condition. As used herein, "treating" or "treatment" includes prophylaxis.
[0064] As used herein, the term "effective amount" can be an amount sufficient to effect beneficial or desired results, such as beneficial or desired clinical results, or enhanced cognition, memory, mood, or other desired CNS results. An effective amount is also an amount that produces a prophylactic effect, e.g., an amount that delays, reduces, or eliminates the appearance of a pathological or undesired condition. Such conditions of the CNS include dementia, neurodegenerative diseases as described herein, suboptimal memory or cognition, mood disorders, general CNS aging, or other undesirable conditions. An effective amount can be administered in one or more administrations. In terms of treatment, an "effective amount" of a composition of the invention is an amount that is sufficient to palliate, ameliorate, stabilize, reverse or slow the progression of a disorder, e.g., a neurological disorder. An "effective amount" may be of any of the compositions of the invention used alone or in conjunction with one or more agents used to treat a disease or disorder. An "effective amount" of a therapeutic agent within the meaning of the present invention will be determined by a patient's attending physician or veterinarian. Such amounts are readily ascertained by one of ordinary skill in the art and will a therapeutic effect when administered in accordance with the present invention. Factors which influence what a therapeutically effective amount will be include, the specific activity of the therapeutic agent being used, the type of disorder (e.g., acute vs. chronic neurological disorder), tune elapsed since the initiation of the disorder, and the age, physical condition, existence of other disease states, and
nutritional status of the individual being treated. Additionally, other medication the patient may be receiving will affect the determination of the therapeutically effective amount of the therapeutic agent to administer.
[0065] A "subject" or an "individual," as used herein, is an animal, for example, a mammal. In some embodiments a "subject" or an "individual" is a human. In some embodiments, the subject suffers from a neurological disorder.
[0066] In some embodiments, an agent is "administered peripherally" or "peripherally administered." As used herein, these terms refer to any form of administration of an agent, e.g., a therapeutic agent, to an individual mat is not direct administration to the CNS, i.e., that brings the agent in contact with the non-brain side of the blood-brain barrier. "Peripheral administration," as used herein, includes intravenous, subcutaneous, intramuscular, intraperitoneal, transdermal, inhalation, transbuccal, intranasal, rectal, and oral administration.
[0067] A "pharmaceutically acceptable carrier" or "pharmaceutically acceptable excipienf ' herein refers to any carrier that does not itself induce the production of antibodies harmful to the individual receiving the composition. Such carriers are well known to those of ordinary skill in the art. A thorough discussion of pharmaceutically acceptable carriers/excipients can be found in Remington 's Pharmaceutical Sciences, Gennaro, AR, ed., 20th edition, 2000: Williams and Wilkins PA, USA.. Exemplary pharmaceutically acceptable carriers can include salts, for example, mineral acid salts such as hydrochlorides, hydrobromides, phosphates, sulfates, and the like; and the salts of organic acids such as acetates, propionates, malonates, benzoates, and the like. For example, compositions of the invention may be provided in liquid form, and formulated in saline based aqueous solution of varying pH (5-8), with or without detergents such polysorbate-80 at 0.01-1%, or carbohydrate additives, such mannitol, sorbitol, or trehalose. Commonly used buffers include histidine, acetate, phosphate, or citrate.
[0068] A "recombinant host cell" or "host cell" refers to a cell that includes an exogenous polynucleotide, regardless of the method used for insertion, for example, direct uptake, transduction, f-mating, or other methods known in the art to create recombinant host cells. The exogenous polynucleotide may be maintained as a nonintegrated vector, for example, a plasmid, or alternatively, may be integrated into the host genome.
[0069] The terms "polypeptide," "peptide" and "protein" are used interchangeably herein to refer to a polymer of amino acid residues. That is, a description directed to a polypeptide applies equally to a description of a peptide and a description of a protein, and vice versa. The terms apply to naturally occurring ammo acid polymers as well as amino acid polymers in which one or more amino acid residues is a non-naturally occurring amino acid, e.g., an amino acid analog. As used herein, the terms encompass amino acid chains of any length, including full length proteins (Le., antigens), wherein the amino acid residues are linked by covalent peptide bonds.
[0070] The term "amino acid" refers to naturally occurring and non-naturally occurring amino acids, as well as amino acid analogs and amino acid mimetics that function in a manner similar to
the naturally occurring amino acids. Naturally encoded amino acids are the 20 common amino acids (alanine, arginine, asparagine, aspartic acid, cysteine, glutamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, and valine) and pyrolysine and selenocysteine. Amino acid analogs refers to compounds that have the same basic chemical structure as a naturally occurring amino acid, i. e., an α carbon that is bound to a hydrogen, a carboxyl group, an amino group, and an R group, such as, homoserine, norleucine, methionine sulfoxide, methionine methyl sulfonium. Such analogs have modified R groups (such as, norleucine) or modified peptide backbones, but retain the same basic chemical structure as a naturally occurring amino acid.
[0071] Amino acids may be referred to herein by either their commonly known three letter symbols or by the one-letter symbols recommended by the IUPAC-IUB Biochemical Nomenclature Commission. Nucleotides, likewise, may be referred to by their commonly accepted single-letter codes.
[0072] The term "nucleic acid" refers to deoxyribonucleotides, deoxyribonucleosides, ribonucleosides, or ribonucleotides and polymers thereof in either single- or double-stranded form. Unless specifically limited, the term encompasses nucleic acids containing known analogues of natural nucleotides which have similar binding properties as the reference nucleic acid and are metabolized in a manner similar to naturally occurring nucleotides. Unless specifically limited otherwise, the term also refers to oligonucleotide analogs including PNA (peptidonucleic acid), analogs of DNA used in antisense technology (phosphorothioates, phosphoroamidates, and the like). Unless otherwise indicated, a particular nucleic acid sequence also implicitly encompasses conservatively modified variants thereof, including, but not limited to, degenerate codon substitutions, and complementary sequences as well as the sequence explicitly indicated. Specifically, degenerate codon substitutions may be achieved by generating sequences in which the third position of one or more selected (or all) codons is substituted with mixed-base and/or deoxyinosine residues (Batzer et al., Nucleic Acid Res. 19:5081 (1991); Ohtsuka et al., J, Biol, Chem. 260.-2605-2608 (1985); and Cassol et al. (1992); Rossolini et al., MoI. Cell. Probes 8:91-98 (1994)).
[0073] The terms "isolated" and "purified" refer to a material that is substantially or essentially removed from or concentrated in its natural environment. For example, an isolated nucleic acid may be one that is separated from the nucleic acids that normally flank it or other nucleic acids or components (proteins, lipids, etc..) in a sample. In another example, a polypeptide is purified if it is substantially removed from or concentrated in its natural environment. Methods for purification and isolation of nucleic acids and peptides are well known in the art. III. The blood brain barrier
[0074] In one aspect, the invention provides compositions and methods that utilize an agent covalently linked to a structure capable of crossing the blood brain barrier (BBB). The compositions and methods are useful in transporting agents, e.g. therapeutic agents such as
neurotherapeutic agents, from the peripheral blood and across the BBB into the CNS. As used herein, the "blood-brain barrier" refers to the barrier between the peripheral circulation and the brain and spinal cord which is formed by tight junctions within the brain capillary endothelial plasma membranes, creates an extremely tight barrier that restricts the transport of molecules into the brain, even molecules as small as urea, molecular weight of 60 Da. The blood-brain barrier within the brain, the blood-spinal cord barrier within the spinal cord, and the blood-retinal barrier within the retina, are contiguous capillary barriers within the central nervous system (CNS), and are collectively referred to as the blood-brain barrier or BBB,
[0075] The BBB is a limiting step in the development of new neurotherapeutics, diagnostics, and research tools for the brain and CNS. In general, large molecule therapeutics such as recombinant proteins, antisense drugs, gene medicines, monoclonal antibodies, or RNA interference (RNAi)- based drugs, do not cross the BBB in pharmacologically significant amounts. While it is generally assumed that small molecule drugs can cross the BBB, in fact, <2% of all small molecule drugs are active in the brain owing to the lack transport across the BBB. A molecule must be lipid soluble and have a molecular weight less than 400 Daltons (Da) in order to cross the BBB in pharmacologically significant amounts, and the vast majority of small molecules do not have these dual molecular characteristics. Therefore, most potentially therapeutic, diagnostic, or research molecules do not cross the BBB in pharmacologically active amounts. So as to bypass the BBB, invasive transcranial drug delivery strategies are used, such as intracerebro-ventricular (ICV) infusion, intracerebral (IC) administration, and convection enhanced diffusion (CED). Transcranial drug delivery to the brain is expensive, invasive, and largely ineffective. The ICV route delivers BDNF only to the ependymal surface of the brain, not into brain parenchyma, which is typical for drugs given by the ICV route. The IC administration of a neurotrophin, such as nerve growth factor (NGF), only delivers drug to the local injection site, owing to the Jow efficiency of drug diffusion within the brain. The CED of neurotrophin results in preferential fluid flow through the white matter tracts of brain, which causes demyelination, and astrogliosis.
[0076] The present invention offers an alternative to these highly invasive and generally unsatisfactory methods for bypassing the BBB, allowing agents, e.g., neuroprotective factors to cross the BBB from the peripheral blood. It is based on the use of endogenous transport systems present in the BBB to provide a mechanism to transport a desired substance from the peripheral blood to the CNS.
A. Transport systems
[0077] In some embodiments, the invention provides compositions that include a structure that binds to a BBB receptor mediated transport system, coupled to an agent for which transport across the BBB is desired, e.g., a neurotherapeutic agent. The compositions and methods of the invention may utilize any suitable structure that is capable of transport by the selected endogenous BBB receptor-mediated transport system, and that is also capable of attachment to the desired agent. In
some embodiments, the structure is an antibody. In some embodiment the antibody is a monoclonal antibody (MAb), e.g., a chimeric MAb.
[0078] Endogenous BBB receptor-mediated transport systems The BBB has been shown to have specific receptors that allow the transport from the blood to the brain of several macromolecules; these transporters are suitable as transporters for compositions of the invention. Endogenous BBB receptor-mediated transport systems useful in the invention include those that transport insulin, transferrin, insulin-like growth factors 1 and 2 (IGFl and IGF2), leptin, and lipoproteins. In some embodiments, the invention utilizes a structure that is capable of crossing the BBB via the endogenous insulin BBB receptor-mediated transport system, e.g., the human endogenous insulin BBB receptor-mediated transport system.
B. Structures that bind to a BBB receptor mediated transport system
[0079] One noninvasive approach for the delivery of drugs to the CNS is to attach the agent of interest to a structure, e.g., molecule that binds with receptors on the BBB. The structure then serves as a vector for transport of the agent across the BBB. Such structures are referred to herein as "molecular Trojan horses (MTH)." Typically, though not necessarily, a MTH is an exogenous peptide or peptidomimetic moiety (e.g., a MAb ) capable of binding to an endogenous BBB receptor mediated transport system that traverses the BBB on the endogenous BBB receptor- mediated transport system. In certain embodiments, the MTH can be an antibody to a receptor of the transport system, e.g., the insulin receptor. In some embodiments, the antibody is a monoclonal antibody (MAb). In some embodiments, the MAb is a chimeric MAb. Thus, despite the fact that Abs normally are excluded from the brain, they can be an effective vehicle for the delivery of molecules into the brain parenchyma if they have specificity for receptors on the BBB.
[0080] Accordingly, antibodies are particularly useful in embodiments of the invention, especially
MAbs. Certain receptor-specific MAbs may mimic the endogenous ligand and function as a MTH and traverse a plasma membrane barrier via transport on the specific receptor system. In certain embodiments, the MTH is a MAb to the human insulin receptor (HIR) on the human BBB. The HIR MAb binds an exofacial epitope on the human BBB HIR and this binding enables the MAb to traverse the BBB via a transport reaction that is mediated by the human BBB insulin receptor.
[0081] An "antibody," as that term is used herein, includes reference to any molecule, whether naturally-occurring, artificially induced, or recombinant, which has specific immunoreactive activity. Generally, though not necessarily, an antibody is a protein that includes two molecules, each molecule having two different polypeptides, the shorter of which functions as the light chains of the antibody and the longer of which polypeptides function as the heavy chains of the antibody. Normally, as used herein, an antibody will include at least one variable region from a heavy or light chain. Additionally, the antibody may comprise combinations of variable regions. The combination may include more than one variable region of a light chain or of a heavy chain. The antibody may also include variable regions from one or more light chains in combination with variable regions of one or more heavy chains. An antibody can be an immunoglobulin molecule obtained by in vitro or
in vivo generation of the humoral response, and includes both polyclonal and monoclonal antibodies. Furthermore, the present invention includes antigen binding fragments of the antibodies described herein, such as Fab, Fab', F(ab)2, and Fv fragments, fragments comprised of one or more CDRs, single-chain antibodies (e.g., single chain Fv fragments (scFv)), disulfide stabilized (dsFv) Fv fragments, heteroconjugate antibodies (e.g., bispecific antibodies), pFv fragments, heavy chain monomers or dimers, light chain monomers or dimers, and ditners consisting of one heavy chain and one light chain. Such antibody fragments may be produced by chemical methods, e.g., by cleaving an intact antibody with a protease, such as pepsin or papain, or via recombinant DNA techniques, e.g., by using host cells transformed with truncated heavy and/or light chain genes. Synthetic methods of generating such fragments are also contemplated. Heavy and light chain monomers may similarly be produced by treating an intact antibody with a reducing agent, such as dithiothreitol or beta-mercaptoethanol, or by using host cells transformed with DNA encoding either the desired heavy chain or light chain or both. An antibody immunologically reactive with a particular antigen can be generated in vivo or by recombinant methods such as selection of libraries of recombinant antibodies in phage or similar vectors.
[0082] A "chimeric" antibody includes an antibody derived from a combination of different mammals. The mammal may be, for example, a rabbit, a mouse, a rat, a goat, or a human. The combination of different mammals includes combinations of fragments from human and mouse sources.
[0083] In some embodiments, an antibody of the present invention is a monoclonal antibody
(MAb), typically a human monoclonal antibody. Such antibodies are obtained from transgenic mice that have been "engineered" to produce specific human antibodies in response to antigenic challenge. In this technique, elements of the human heavy and light chain locus are introduced into strains of mice derived from embryonic stem cell lines that contain targeted disruptions of the endogenous heavy chain and light chain loci. The transgenic mice can synthesize human antibodies specific for human antigens, and the mice can be used to produce human antibody-secreting hybridomas.
[0084] For use in humans, a chimeric MAb is preferred that contains enough human sequence that it is not significantly immunogenic when administered to humans, e.g., at least about 80% human and about 20% mouse, or about 85% human and about 15% mouse, or about 90% human and about 10% mouse, or about 95% human and 5% mouse, or greater than about 95% human and less than about 5% mouse. Chimeric antibodies to the human BBB insulin receptor with sufficient human sequences for use in the invention are described in, e.g., Coloma et al. (2000) Pharm. Res. 17: 266- 274, which is incorporated by reference herein in its entirety. A more highly humanized form of the HIR MAb can also be engineered, and the humanized HIRMAb has activity comparable to the murine HIRMAb and can be used in embodiments of the invention. See, e.g., U.S. Patent Application Publication No. 20040101904, filed 11/27/02, incorporated by reference herein in its entirety.
[0085] Antibodies used in the invention may be glycosylated or non-glycosylated. If the antibody is glycosylated, any pattern of glycosylation that does not significantly affect the function of the antibody may be used. Glycosylation can occur in the pattern typical of the cell in which the antibody is made, and may vary from cell type to cell type. For example, the glycosylation pattern of a monoclonal antibody produced by a mouse myeloma cell can be different than the glycosylation pattern of a monoclonal antibody produced by a transfected Chinese hamster ovary (CHO) cell. In some embodiments, the antibody is glycosylated in the pattern produced by a transfected Chinese hamster ovary (CHO) cell.
[0086] Accordingly, in some embodiments, a genetically engineered HIR MAb, with the desired level of human sequences, is fused to an agent for which transport across the BBB is desired, e.g., a neurotherapeutic agent such as a neurotrophin such as human BDNF, to produce a recombinant fusion protein that is a bi-functional molecule. The recombinant therapeutic neuroprotective factor/HERMAb is able to both (i) cross the human BBB, via transport on the BBB HIR, and (ii) activate the factor's target, e.g., neuronal BDNF receptor, trkB, to cause neurotherapeutic effects once inside the brain, following peripheral administration.
IV. Agents for Transport Across the BBB
[0087] The agent for which transport across the BBB is desired may be any suitable substance for introduction into the CNS. Generally, the agent is a substance for which transport across the BBB is desired, which does not, in its native form, cross the BBB in significant amounts. The agent may be, e.g., a therapeutic agent, a diagnostic agent, or a research agent. Diagnostic agents include peptide radiopharmaceuticals, such as radiolabeled epidermal growth factor (EGF) for imaging brain cancer (Kurihara and Pardridge (1999) Cam, Res. 54: 6159-6163), and amyloid peptides for imaging brain amyloid such as in Alzheimers disease (Lee et al (2002) J. Cereb. Blood Flow Metabol. 22: 223-231). In some embodiments, the agent is a therapeutic agent, such as a neurotherapeutic agent. Apart from neurotrophins, potentially useful therapeutic protein agents include recombinant enzymes for lysosomal storage disorders (see, e.g., U.S. Patent Application Publication No. 20050142141, filed 2/17/05, incorporated by reference herein in its entirety), monoclonal antibodies that either mimic an endogenous peptide or block the action of an endogenous peptide, polypeptides for brain disorders, such as secretin for autism (Ratliff-Schaub et al (2005) Autism 9: 256-265), opioid peptides for drug or alchol addiction (Cowen et al, (2004) J. Neurochem. 89: 273-285), or neuropeptides for appetite control (Jethwa et al (2005) Am. J. Physiol. 289: E301-305). In some embodiments, the agent is a neurotrophic factor, also referred to herein as a "neurotrophin." Thus, in some embodiments, the invention provides compositions and methods that utilize a neurotrophin. In some embodiments, a single neurotrophin may be used. In others, combinations of neurotrophins are used. In some embodiments, the invention utilizes a brain- derived neurotrophic factor (BDNF). In other embodiments, the invention utilizes a glial-derived neurotrophic factor.
A. Neurotrophins
[0088] Many neurotrophic factors are neuroprotective in brain, but do not cross the blood-brain barrier. These factors are suitable for use in the compositions and methods of the invention and include brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), neurotrophin-4/5, fibroblast growth factor (FGF)-2 and other FGFs, neurotrophin (NT)-3, erythropoietin (EPO), hepatocyte growth factor (HGF), epidermal growth factor (EGF), transforming growth factor (TGF)-α, TGF-β, vascular endothelial growth factor (VEGF), interleukin-1 receptor antagonist (IL- lra), ciliary neurotrophic factor (CNTF), glial-derived neurotrophic factor (GDNF)5 neurturin, platelet-derived growth factor (PDGF), heregulin, neuregulin, artemin, persephin, interleukins, granulocyte-colony stimulating factor (CSF), granulocyte-macrophage-CSF, netrins, cardiotrophin- 1, hedgehogs, leukemia inhibitory factor (LIF), midkine, pleiotrophin, bone morphogenetic proteins (BMPs), netrins, saposins, semaphorins, and stem cell factor (SCF). Particularly useful in some embodiments of the invention utilizing neurotrophins that are used as precursors for fusion proteins that cross the BBB are those that naturally form dimeric structures, similar to BDNF. Certain neurotrophins such as BDNF or NT-3 may form hetero-dimeric structures, and in some embodiments the invention provides a fusion protein constructed of one neurotrophin monomer fused to one chain (e.g., a light or heavy chain) of an antibody, e.g., of the HIRMAb, and another neurotrophin monomer fused to the second chain (e.g., a light or heavy chain) of the antibody. Typically, the molecular weight range of recombinant proteins that may be fused to the molecular Trojan horse ranges from 1000 Daltons to 500,000 Daltons.
B. Brain-Derived Neurotrophic Factor
[0089] One particularly useful neurotrophin in embodiments of the invention is brain-derived neurotrophic factor (BDNF). In experimental models of chronic neurodegenerative disease such as prion diseases, Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), or amyotrophic lateral sclerosis (ALS), the direct intracerebral injection of BDNF is neuroprotective.
[0090] In studies demonstrating the pharmacologic efficacy of BDNF in experimental brain disease, it is necessary to administer the neurotrophin directly into the brain following a transcranial drug delivery procedure. The transcranial drug delivery is required because BDNF does not cross the brain capillary wall, which forms the blood-brain barrier (BBB) in vivo. Owing to the lack of transport of BDNF across the BBB, it is not possible for the neurotrophin to enter the CNS, including the brain or spinal cord, following a peripheral administration unless the BBB is experimentally disrupted. Ciinical trials showed that subcutaneous administration of BDNF was not effective in the treatment of chronic neurodegenerative conditions, which derives from the lack of transport of BDNF across the BBB. The lack of utility of BDNF as a CNS therapeutic following peripheral administration is expected and follows from the limiting role that is played by the BBB in the development of neurotherapeutics, especially large molecule drugs such as BDNF. BDNF
does not cross the BBB, and the lack of transport of the neuiotrophin across the BBB prevents the molecule from being pharmacologically active in the brain following peripheral administration. The lack of BDNF transport across the BBB means that the neurotrophin must be directly injected into the brain across the skull bone to be pharmacologically active in the CNS. However, when the BDNF is fused to a Trojan horse such as the HIR MAb, this neurotrophin is now able to enter brain from blood following a non-invasive peripheral route of administration such as intravenous intramuscular, subcutaneous, intraperitoneal, or even oral administration. Owing to the BBB transport properties of this new class of molecule, it is not necessary to administer the BDNF directly into the CNS with an invasive delivery procedure requiring penetration of the skull or spinal canal. The reformulated ftision protein of the BDNF variant and the HIR MAb now enables entry of BDNF into the brain from the blood, and the development of BDNF as a neurotherapeutic for human diseases.
[0091] The forms of BDNF used in various embodiments of the invention may include pharmaceutically acceptable salts and prodrugs, and prodrugs of the salts, polymorphs, hydrates, solvates, biologically-active fragments, biologically active variants and stereoisomers of the naturally-occurring BDNF, as well as agonist, mimetic, and antagonist variants of the naturally- occurring BDNF and polypeptide fusions thereof. Variants that include one or more deletions, substitutions, or insertions in the natural sequence of the BDNF, in particular truncated versions of the native BDNF comprising deletion of one or more amino acids at the amino terminus, carboxyl terminus, or both, may also be used in certain embodiments.
[0092] In some embodiments, the invention utilizes a carboxy-truncated variant of the native
BDNF, e.g., a variant in which 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more than 10 amino acids are absent from the carboxy-terminus of the BDNF. BDNF variants include the complete 119 amino acid BDNF, the 117 or 118 amino acid variant with a truncated carboxyl terminus, variants with a truncated amino terminus, or variants with up to about a 20, 30, or 40% change in amino acid composition, as long as the fusion protein variant still binds to the brain neuroprotection receptor with high affinity. When an Ab, e.g., a MAb such as HIRMAb is used, additional fusion protein variants can be produced with the substitution of amino acids within either the framework region (FR) or the complementarity determining region (CDR) of either the light chain or the heavy chain of the Ab, e.g., HIRMAb, as long as the fusion protein binds with high affinity to the endogenous receptor, e.g., HIR to promote transport across the human BBB. Additional fusion protein variants can be produced by changing the composition or length of the linker peptide separating the fusion protein from the HIRMAb.
[0093] In some embodiments, the full-length 119 a.a. sequence of BDNF is utilized (SEQ ID NO:
39). In some embodiments, a one amino-acid carboxy-truncated variant of BDNF is utilized (amino acids 1-118 of SEQ ID NO: 39). In some embodiments, a two amino-acid carboxy-truncated variant of BDNF is utilized (amino acids 1-117 of SEQ ID NO: 39). In some embodiments, a three amino-acid carboxy-truncated variant of BDNF is utilized (amino acids 1-116 of SEQ ID NO: 39).
In some embodiments, a four amino-acid carboxy-truncated variant of BDNF is utilized (amino acids 1-115 of SEQ ID NO: 39). In some embodiments, a five amino-acid carboxy-truncated variant of BDNF is utilized (amino acids 1-114 of SEQ ID NO: 39).
[0094] The sequence of human BDNF is given in SEQ ID NO: 39. In some embodiments, the invention utilizes a BDNF that is about 60, 70, 80, 90, 95, 99, or 100% identical with the sequence of SEQ ID NO: 39, or a truncated version thereof, e.g., the 117 or 118 amino acid variant with a one- or two-amino acid truncated carboxyl terminus, or variants with a truncated amino terminus. In some embodiments, the invention utilizes a two amino-acid carboxy-truncated 117 amino acid variant human BDNF with a sequence that is at least about 60, 70, 80, 90, 95, 99 or 100% identical to the sequence of amino acids 466-582 of SEQ ID NO: 24. In some embodiments, the invention utilizes a two amino-acid carboxy-truncated human 117 amino acid BDNF with a sequence that includes amino acids 466-582 of SEQ ID NO: 24.
[0095] Accordingly, BDNFs useful in the invention include peptides having at least about 60%, at least about 70%, at least about 80%, at least about 90%, at least about 95%, at least about 99%, or greater than 95% or greater than 99% sequence identity, e.g., 100% sequence identity, to the amino acid sequences disclosed herein. C. Glial-Derived Neurotrophic Factor.
[0096] Another particularly useful neurotrophin in embodiments of the invention is glial-derived neurotrophic factor (GDNF). GDNF is a neurotrophic factor that can be used in the treatment of many acute and chronic brain diseases. However, the lack of transport of GDNF across the BBB has prevented the development of this molecule as a neurotherapeutic for the brain and spinal cord.
[0097] Acute brain conditions that can be treated by the GDNF-containing compositions described herein include, but are not limited to, spinal cord injury, brain injury (e.g., traumatic brain injury), focal brain ischemia, and global brain ischemia.
[0098] Chronic brain conditions that can be treated by the GDNF-containing compositions described herein include, but are not limited to neurodegenerative disease such as Parkinson's disease, motor neuron disease (e.g., spinal cord motor neuron disease such as amyotrophic lateral sclerosis); and substance abuse, e.g., addiction, including drug addiction and alchohol addiction.
[0099] The forms of GDNF used in various embodiments of the invention may include acceptable salts and prodrugs, and prodrugs of the salts, polymorphs, hydrates, solvates, biologically-active fragments, biologically active variants and stereoisomers of the naturally-occurring GDNF (e.g., human GDNF and mature human GDNF), as well as agonist, mimetic, and antagonist variants of the naturally-occurring GDNF and polypeptide fusions thereof. Variants that include one or more deletions, substitutions, or insertions in the natural sequence of the GDNF may also be used in certain embodiments, insofar as the variants retain binding and/or activation of GFRαl . The structure-function relationship of GDNF and its ability to bind and activate GFRαl has been studied extensively. See, e.g., Eketjal et al (1999), EMBO J, 18(21):5901-5910; and Baloh et al (2000), J Biol Chem, 275(5):3412-3420. For example mutations known to be particularly sensitive to
mutation include, e.g., the following residues in rat GDNF: D52, E61, E62, Dl 16, 164, Ll 14, Ll 18, Y120, 1122, and ClOl. Functional assays for GDNF are known in the art. See, e.g., Eketjal et al, or Baloh et al supra. Functional assays for GDNF are also described in Example 15 herein.
[00100] When an Ab, e.g., a MAb such as HIRMAb is used, additional fusion protein variants can be produced with the substitution of amino acids within either the framework region (FR) or the complementarity determining region (CDR) of either the light chain or the heavy chain of the Ab, e.g., HIRMAb, as long as the fusion protein binds with high affinity to the endogenous receptor, e.g., HIR to promote transport across the human BBB. Additional fusion protein variants can be produced by changing the composition or length of the linker peptide separating the fusion protein from the HIRMAb.
[00101] In some embodiments, the full-length 211 a.a. sequence of human prepro GDNF is utilized
(GenBank P39905). The sequence of human preproGDNF is given in SEQ ID NO: 44. In other embodiments, the mature form of human GDNF comprising amino acids Ser-78 to He 211 (SEQ ID NO: 52) is utilized:
SPDKQMAVLPRRERNRQAAAANPENSRGKGRRGQRGKNRGCVLTA1HLNVTDLGLGYET KEEL
IFRYCSGSCDAAETTYDKILKNLSRNRRLVSDKVGQACCRPIAFDDDLSFLDDNLVYHILRK HSAKRCGCI (SEQ ID NO:52).
[00102] In some embodiments, the invention utilizes a GDNF that is about 60, 70, 80, 90, 95, 99, or 100% identical with the sequence of SEQ ID NO:52.
[00103] Accordingly, GDNFs useful in the invention include peptides having at least about 60%, at least about 70%, at least about 80%, at least about 90%, at least about 95%, at least about 99%, or greater man 95% or greater than 99% sequence identity, e.g., 100% sequence identity, to the amino acid sequences disclosed herein
[00104] Percent sequence identity is determined by conventional methods. See, for example,
Altschul et al, Bull. Math. Bio. 48:603 (1986), and Henikoff and Henikoff, Proc. Natl Acad. ScL USA 89: 10915 (1992). Briefly, two amino acid sequences are aligned to optimize the alignment scores using a gap opening penalty of 10, a gap extension penalty of 1, and the "BLOSUM62" scoring matrix of Henikoff and Henikoff (ibid.). The percent identity is then calculated as: ([Total number of identical matches]/[length of the longer sequence plus the number of gaps introduced into the longer sequence in order to align the two sequences])(100).
[00105] Those skilled in the art appreciate that there are many established algorithms available to align two amino acid sequences. The "FASTA" similarity search algorithm of Pearson and Lipman is a suitable protein alignment method for examining the level of identity shared by an amino acid sequence disclosed herein and the amino acid sequence of another peptide. The FASTA algorithm is described by Pearson and Lipman, Proc. Nat'lAcad. Set USA 85:2444 (1988), and by Pearson, Meth. En∑ymol. 183:63 (1990). Briefly, FASTA first characterizes sequence similarity by identifying regions shared by the query sequence (e.g., SEQ ID NO:44 or SEO ID NO:521 and a
test sequence that have either the highest density of identities (if the ktup variable is 1) or pairs of identities (if ktup=2), without considering conservative amino acid substitutions, insertions, or deletions. The ten regions with the highest density of identities are then rescored by comparing the similarity of all paired amino acids using an amino acid substitution matrix, and the ends of the regions are "trimmed" to include only those residues that contribute to the highest score. If there are several regions with scores greater than the "cutoff' value (calculated by a predetermined formula based upon the length of the sequence and the ktup value), then the trimmed initial regions are examined to determine whether the regions can be joined to form an approximate alignment with gaps. Finally, the highest scoring regions of the two amino acid sequences are aligned using a modification of the Needleman-Wunsch- Sellers algorithm (Needleman and Wunsch, J. MoL Biol, 48:444 (1970); Sellers, SIAM J. Appl. Math. 26:787 (1974)), which allows for amino acid insertions and deletions. Illustrative parameters for FASTA analysis are: ktup=l, gap opening penalty=10, gap extension penalty=l, and substitution matrix=BLOSUM62. These parameters can be introduced into a FASTA program by modifying the scoring matrix file ("SMATRIX"), as explained in Appendix 2 of Pearson, Meth. Enzymol. 183:63 (1990). The present invention also includes peptides having a conservative amino acid change, compared with an amino acid sequence disclosed herein. Many such changes have been described specifically. More generally, for example, variants can be obtained that contain one or more amino acid substitutions of SEQ ID NO: 52. In some embodiments sequence variants include conservative amino acid substitutions, e.g., an alkyl amino acid is substituted for an alkyl amino acid in a GDNF peptide amino acid sequence, an aromatic amino acid is substituted for an aromatic amino acid in a GDNF peptide amino acid sequence, a sulfur-containing amino acid is substituted for a sulfur- containing amino acid in a GDNF peptide amino acid sequence, a hydroxy-containing amino acid is substituted for a hydroxy-containing amino acid in a GDNF peptide amino acid sequence, an acidic amino acid is substituted for an acidic amino acid in a GDNF peptide amino acid sequence, a basic amino acid is substituted for a basic amino acid in GDNF peptide amino acid sequence, or a dibasic monocarboxylic amino acid is substituted for a dibasic monocarboxylic amino acid in a GDNF peptide amino acid sequence. Among the common amino acids, for example, a "conservative amino acid substitution" is illustrated by a substitution among amino acids within each of the following groups: (1) glycine, alanine, valine, leucine, and isoleucine, (2) phenylalanine, tyrosine, and tryptophan, (3) serine and threonine, (4) aspartate and glutamate, (5) glutamine and asparagine, and (6) lysine, arginine and histidine. The BLOSUM62 table is an amino acid substitution matrix derived from about 2,000 local multiple alignments of protein sequence segments, representing highly conserved regions of more than 500 groups of related proteins (Henikoff and Henikoff, Proc. Nat'lAcad. ScL USA 89:10915 (1992)). Accordingly, the BLOSUM62 substitution frequencies can be used to define conservative amino acid substitutions that may be introduced into the amino acid sequences of the present invention. Although it is possible to design amino acid substitutions based solely upon chemical properties (as discussed above), the language "conservative amino acid
substitution" preferably refers to a substitution represented by a BLOSUM62 value of greater than - 1. For example, an amino acid substitution is conservative if the substitution is characterized by a BLOSUM62 value of 0, 1, 2, or 3. According to this system, preferred conservative amino acid substitutions are characterized by a BLOSUM62 value of at least 1 (e.g., 1, 2 or 3), while more preferred conservative amino acid substitutions are characterized by a BLOSUM62 value of at least 2 (e.g., 2 or 3).
[00107] It also will be understood that amino acid sequences may include additional residues, such as additional N- or C-terminal amino acids, and yet still be essentially as set forth in one of the sequences disclosed herein, so long as the sequence retains sufficient biological protein activity to be functional in the compositions and methods of the invention.
[00108] In some embodiments, where GDNF sequence variants (e.g., variants of SEQ ID NO: 52) are to be utilized, mutation tolerance prediction programs can be used to greatly reduce the number of non-functional sequence variants that would be generated by strictly random mutagenesis. Various programs for predicting the effects of amino acid substitutions in a protein sequence on protein function (e.g., SIFT, PolyPhen, PANTHER PSEC, PMUT, and TopoSNP) are described in, e.g., Henikoff et al., (2006), Anna. Rev. Genomics Hum. Genet., 7:61-80..
V. Compositions
[00109] Compositions of the invention are useful in one or more of: increasing serum half-life of a cationic compound, transporting an agent across the BBB, and/or retaining activity of the agent once transported across the BBB. Accordingly, in some embodiments, the invention provides compositions containing a neurotherapeutic agent covalently linked to a structure that is capable of crossing the blood brain barrier (BBB), where the composition is capable of producing an average elevation of concentration in the brain of the neurotherapeutic agent of at least about 1 , 2, 3, 4, 5, 10, 20, 30, 40, or 50 ng/gram brain following peripheral administration. The invention also provides compositions containing an agent that is covalently linked to a chimeric MAb to the human BBB insulin receptor. The invention further provides a fusion protein containing a structure capable of crossing the BBB, covalently linked to a peptide that is active in the central nervous system (CNS), where the structure capable of crossing the blood brain barrier and the peptide that is active in the central nervous system each retain an average of at least about 10, 20, 30, 40, 50, 60, 70, 80, 90, 95, 99, or 100% of their activities, compared to their activities as separate entities. In certain embodiments, the invention further provides compositions that increase the serum half-life of cationic substances. The invention also provides pharmaceutical compositions that contain one or more compositions of the invention and a pharmaceutically acceptable excipient.
[00110] In some embodiments, the invention provides compositions containing a neurotherapeutic agent covalently linked to a structure that is capable of crossing the blood brain barrier (BBB), where the composition is capable of producing an average elevation of concentration in the brain of
the neurotherapeutic agent of at least about 1, 2, 3, 4, 5, 10, 20, 30, 40, or 50 ng/gram brain following peripheral administration.
[00111] "Elevation" of the agent is an increase in the brain concentration of the agent compared to the concentration of the agent administered alone (i.e., not covalently linked to a structure that is capable of crossing the BBB). In the case of agents for which only a small amount of the agent alone normally crosses the BBB, "elevation" may be an increase in the agent compared to resting brain levels. "Average" refers to the mean of at least three, four, five, or more than five measurements, preferably in different individuals. The individual in which the elevation is measured is a mammal, such as a rat, or, preferably, a primate, e.g., a monkey. An example of measurements of elevation of the level of a neurotherapeutic agent (BDNF) is given in Example 7.
[00112] In some embodiments, the structure that is capable of crossing the BBB utilizes an endogenous BBB receptor mediated transport system, such as a system that utilizes the insulin receptor, transferrin receptor, leptin receptor, LDL receptor, or IGF receptor. In some embodiments, the endogenous BBB receptor mediated transport system is the insulin BBB receptor mediated transport system. In some embodiments, the structure that is capable of crossing the BBB is an antibody, e.g., a monoclonal antibody (MAb) such as a chimeric MAb. The antibody can be a chimeric antibody with sufficient human sequence that it is suitable for administration to a human. The antibody can be glycosylated or nonglycosylated; in some embodiments, the antibody is glycosylated, e.g., in a glycosylation pattern produced by its synthesis in a CHO cell. In embodiments in which the structure is an antibody, the covalent linkage between the antibody and the neurotherapeutic agent may be a linkage between any suitable portion of the antibody and the neurotherapeutic agent, as long as it allows the antibody-agent fusion to cross the blood brain barrier and the neurotherapeutic agent to retain a therapeutically useful portion of its activity within the CNS. In certain embodiments, the covalent link is between one or more light chains of the antibody and the neurotherapeutic agent In the case of a peptide neurotherapeutic agent (e.g., a neurotrophin such as GDNF), the peptide can be covalently linked by its carboxy or amino terminus to the carboxy or amino terminus of the light chain (LC) or heavy chain (HC) of the antibody. Any suitable linkage may be used, e.g., carboxy terminus of light chain to amino terminus of peptide, carboxy terminus of heavy chain to amino terminus of peptide, amino terminus of light chain to amino terminus of peptide, amino terminus of heavy chain to amino terminus of peptide, carboxy terminus of light chain to carboxy terminus of peptide, carboxy terminus of heavy chain to carboxy terminus of peptide, amino terminus of light chain to carboxy terminus of peptide, or amino terminus of heavy chain to carboxy terminus of peptide. In some embodiments, the linkage is from the carboxy terminus of the HC to the amino terminus of the peptide. It will be appreciated that a linkage between terminal amino acids is not required, and any linkage which meets the requirements of the invention may be used; such linkages between non-terminal amino acids of peptides are readily accomplished by those of skill in the art.
[00113] In some embodiments, the invention utilizes BDNF, either the native form or truncated variants. Strikingly, it has been found that fusion proteins of these forms of BDNF retain foil transport and activity. This is surprising because the neurotrophin is translated in vivo in cells as a prepro form and the prepro-BDNF is then converted into mature BDNF following cleavage of the prepro peptide from the amino terminus of the BDNF. In order to preserve the prepro form of the BDNF, and the subsequent cleavability of the prepro peptide, it would seem to be necessary to fuse the prepro BDNF to the amino terminus of either the HC or the LC of the targeting MAb. This could, however, inhibit the binding of the MAb for the target antigen, since the complementarity determining regions (CDR) of the heavy chain or light chain of the MAb molecule, which comprise the antigen binding site of the MAb, are situated near the amino terminus of the heavy chain or light chains of the antibody. Therefore, fusion of the prepro-neurotrophin to the amino terminus of the antibody chains is expected to result in not only impairment of antibody activity, but also an impairment of antibody folding following translation. The present invention shows the unexpected finding that it is possible to fuse the mature form of a neurotrophin, such as a BDNF variant (vBDNF), to the carboxyl terminus of the heavy chain of the HIR MAb. The production of this new genetically engineered fusion protein creates a bi-functional molecule that binds with high affinity to both the HIR and the trkB receptors.
[00114] In other embodiments, the invention utilizes GDNF (e.g., mature human GDNF) or a sequence variant of GDNF as described herein..
[00115] In some embodiments, more than one molecule of the same neurotherapeutic agent is attached to the structure that crosses the BBB. For example, in compositions of the invention where a single neurotrophin is attached to an antibody, one molecule of the neurotrophin is attached to each heavy chain, naturally producing a structure that is ideal for homodimer formation. This is the case for compositions containing BDNF or GDNF. Neurotrophins such as BDNF or GDNF require an obligatory formation of a homo-dimeric structure to be biologically active, and to bind with high affinity to the cognate receptor, e.g. TrkB or GFRαl . A naturally occurring homo-dimeric structure between two BDNF molecules is formed when the neurotrophin is fused to a carboxyl terminus of the CH3 region of an IgG molecule, as illustrated in Figure 18. Without being bound by theory, it is thought that this may account for the unexpected finding of essentially 100% of activity for the BDNF when bound to the IgG (see, e.g., Fig. 24).
[00116] In some embodiments, more than one type of neurotherapeutic agent can be attached to the structure that is capable of crossing the blood brain barrier. In some embodiments, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more than 10 different neurotherapeutic agents may be attached to the structure that is capable of crossing the blood brain barrier. In certain embodiments, 2 different neurotrophins are attached to an antibody to an endogenous BBB receptor-mediated transport system. Any combination of neurotrophins may be used. Particularly useful in some embodiments of the invention are neurotrophins used as precursors for fusion proteins that cross the BBB are those that naturally form dimeric structures, similar to BDNF or GDNF. Certain neurotrophins such as BDNF
or NT-3 may form hetero-dimeric structures, and in some embodiments the invention provides a fusion protein constructed of one neurotrophin monomer fused to one chain (e.g., heavy chain) of an antibody, e.g., of the HIRMAb, and another neurotrophin monomer fused to the second chain of the antibody. Typically, the molecular weight range of recombinant proteins that may be fused to the molecular Trojan horse ranges from 1000 Daltons to 500,000 Daltons.
[00117] In some embodiments, more than one type of structure capable of crossing the BBB, e.g., molecular Trojan horse, may be used. The different structures may be covalently attached to a single neurotherapeutic agent, e.g., a single neurotrophin such as GDNF, or multiple neurotherapeutics, e.g., multiple neurotrophins, or any combination thereof. Thus, for example, in some embodiments either with the same neurotrophin attached to each MTH or a different neurotrophin attached, or combinations of neurotrophins attached. Thus the neuroprotective recombinant protein can be fused to multiple molecular Trojan horses that undergo receptor- mediated transport across the blood-brain barrier, including monoclonal antibodies to the insulin receptor, transferrin receptor, insulin-like growth factor (IGF) receptor, or the low density lipoprotein (LDL) receptor or the endogenous ligand, including insulin, transferrin, the IGFs, or LDL. Ligands that traverse the blood-brain barrier via absorptive-mediated transport may also be used as molecular Trojan horses including cationic proteins, or carbohydrate bearing proteins that bind to membrane lectins. The molecular weight range of molecular Trojan horses is 1000 Daltons to 500,000 Daltons.
[00118] The covalent linkage between the structure capable of crossing the BBB and the neurotherapeutic agent may be direct, e.g., a peptide bond between the terminal amino acid of one peptide and the terminal amino acid of the other peptide to which it is linked, or indirect, via a linker. If a linker is used, it may be any suitable linker, e.g., a peptide linker. If a peptide linker is used, it may be 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more than 10 amino acids in length. In some embodiments, a three amino acid linker is used. In some embodiments, the linker has the sequence ser-ser-met. The covalent linkage may be cleavable, however this is not a requirement for activity of the system in some embodiments; indeed, an advantage of these embodiments of the present invention is that the fusion protein, without cleavage, is partially or fully active both for transport and for activity once across the BBB.
[00119] In some embodiments, a noncovalent attachment may be used. An example of noncovalent attachment of the MTH, e.g., MAb, to the large molecule therapeutic neuroprotective factor is avidin/streptavidin-biotin attachment. Such an approach is further described in U.S. Patent Application No. 10/858,729, entitled "Anti-growth factor receptor avidin fusion proteins as universal vectors for drug delivery," filed April 21, 2005, which is hereby incorporated by reference in its entirety.
[00120] The neurotherapeutic agent may be any suitable neurotherapeutic agent, such as a neurotrophin. In some embodiments, the neurotherapeutic agent is a neurotrophin such as brain derived neurotrophic factor (BDNF), nerve growth factor (NGF), neurotrophin-4/5, fibroblast
growth factor (FGF)-2 and other FGFs, neurotrophin (NT>3, erythropoietin (EPO), hepatocyte growth factor (HGF), epidermal growth factor (EGF), transforming growth factor (TGF)-α, TGF-β, vascular endothelial growth factor (VEGF), interleukin-1 receptor antagonist (IL- Ira), ciliary neurotrophic factor (CNTF), glial-derived neurotrophic factor (GDNF), neurturin, platelet-derived growth factor (PDGF), heregulin, neuregulin, artemin, persephin, interleukins, granulocyte-colony stimulating factor (CSF), granulocyte-macrophage-CSF, netrins, cardiotrophin-1, hedgehogs, leukemia inhibitory factor (LIF), midkine, pleiotrophin, bone morphogenetic proteins (BMPs)3 netrins, saposins, semaphorins, or stem cell factor (SCF). In some embodiments, the neurotrophin is BDNF. The BDNF may be native BDNF or a variant BDNF. Some embodiments utilize a two amino acid carboxyl-truncated variant. The BDNF can be a human BDNF. In some embodiments, the BDNF contains a sequence that is about 60, 70, 80, 90, 95, 99, or 100% identical to the sequence of amino acids 466-582 of SEQ ID NO: 24.
[00121] In some embodiments, the invention provides compositions containing a neurotherapeutic agent covalently linked to a structure that is capable of crossing the BBB where the composition is capable of producing an average elevation of concentration in the brain of the neurotherapeutic agent of at least about 1, 2, 3, 4, 5, 10, 20, 30, 40, or 50 ng/gram brain following peripheral administration, where the neurotherapeutic agent is a neurotrophin and the structure that is capable of crossing the BBB is a MAb to an endogenous BBB receptor mediated transport system. The antibody can be glycosylated or nonglycosylated; in some embodiments, the antibody is glycosylated, e.g., in a glycosylation pattern produced by its synthesis in a CHO cell. In certain embodiments, the neurotrophin is GDNF5 e.g., a mature human GDNF (SEQ ID NO:52) or a sequence variant thereof. The MAb can be an antibody to the insulin BBB receptor mediated transport system, e.g., a chimeric MAb. The antibody can be a chimeric antibody with sufficient human sequence that it is suitable for administration to a human, e.g., at least about 80% human sequence, e.g., 85%, 90%, 95%, or another percent human amino acid sequence from about 80% to about 100% human sequence. In some embodiments, the insulin receptor is a human insulin receptor and the GDNF is a human GDNF. In some embodiments, the GDNF contains a sequence that is about 60, 70, 80, 90, 95, 99, or 100% identical to the sequence of SEQ ID NO: 44. The GDNF can be covalently linked at its amino terminus to the carboxy terminus of the heavy chain of the MAb, optionally with a linker between the termini, such as the three amino-acid linker ser-ser- met, or the two amino acid linker ser-ser. In some embodiments, the heavy chain of the MAb contains a sequence that is about 60, 70, 80, 90, 95, 99, or 100% identical to amino acids 20-462 of SEQ ID NO: 24. In some embodiments, the light chain of the MAb contains a sequence that is about 60, 70, 80, 90, 95, 99, or 100% identical to amino acids 21-234 of SEQ ID NO: 36.
[00122] The invention also provides compositions containing an agent that is covalently linked to a chimeric MAb to the human BBB insulin receptor. In some embodiments, the heavy chain of the MAb is covalently linked to the agent to form a fusion protein. The agent can be any agent described herein, i.e., any agent for which transport across the BBB is desired. In some
embodiments, the agent is a therapeutic agent, such as a neurotherapeutic agent as described herein, e.g., a neurotrophin such as GDNF Strikingly, it has been found that multifunctional fusion proteins of the invention, e.g., difunctional fusion proteins, retain a high proportion of the activity of the separate portions, e.g., the portion that is capable of crossing the BBB and the portion that is active in the CNS. Accordingly, the invention further provides a fusion protein containing a structure capable of crossing the BBB, covalently linked to a peptide that is active in the central nervous system (CNS), where the structure capable of crossing the BBB and the peptide that is active in the central nervous system each retain an average of at least about 10, 20, 30, 40, 50, 60, 70, 80, 90, 95, 99, or 100% of their activities, compared to their activities as separate entities. In some embodiments, the structure capapble of crossing the BBB, and the peptide that is active in the central nervous system each retain about 20% to about 80% of their activities (e.g., about 30% to about 70, or about 40% to about 60%) compared to their activities as separate entities. In some embodiments, the invention provides a fusion protein containing a structure capable of crossing the BBB, covalently linked to a peptide that is active in the central nervous system (CNS), where the structure capable of crossing the blood brain barrier and the peptide that is active in the central nervous system each retain an average of at least about 50% of their activities, compared to their activities as separate entities. In some embodiments, the invention provides a fusion protein containing a structure capable of crossing the BBB, covalently linked to a peptide that is active in the central nervous system (CNS), where the structure capable of crossing the blood brain barrier and the peptide that is active in the central nervous system each retain an average of at least about 60% of their activities, compared to their activities as separate entities. In some embodiments, the invention provides a fusion protein containing a structure capable of crossing the BBB, covalently linked to a peptide that is active in the central nervous system (CNS), where the structure capable of crossing the blood brain barrier and the peptide that is active in the central nervous system each retain an average of at least about 70% of their activities, compared to their activities as separate entities. In some embodiments, the invention provides a fusion protein containing a structure capable of crossing the BBB, covalently linked to a peptide that is active in the central nervous system (CNS), where the structure capable of crossing the blood brain barrier and the peptide that is active in the central nervous system each retain an average of at least about 80% of their activities, compared to their activities as separate entities. In some embodiments, the invention provides a fusion protein containing a structure capable of crossing the BBB, covalently linked to a peptide that is active in the central nervous system (CNS), where the structure capable of crossing the blood brain barrier and the peptide that is active in the central nervous system each retain an average of at least about 90% of their activities, compared to their activities as separate entities. In some embodiments, the structure capable of crossing the blood brain barrier retains at least about 10, 20, 30, 40, 50, 60, 70, 80, 90, 95, 99, or 100% of its activity, compared to its activity as a separate entity, and the peptide that is active in the central nervous
system retains at least about 10, 20, 30, 40, 50, 60, 70, 80, 90, 95, 99, or 100% of its activity, compared to its activity as a separate entity.
[00124] As used herein, "activity" includes physiological activity, e.g., ability to cross the BBB and/or therapeutic activity and also binding affinity of the structures for their respective receptors.
[00125] Transport of the structure capable of crossing the BBB across the BBB may be compared for the structure alone and for the structure as part of a fusion structure of the invention by standard methods. For example, pharmacokinetics and brain uptake of the fusion structure, e.g., fusion protein, by a model animal, e.g., a mammal such as a primate, may be used. Such techniques are illustrated in Example 7, which demonstrates pharmacokinetics and brain uptake of a fusion protein of the invention by the adult Rhesus monkey. Similarly, standard models for the function of an agent, e.g. the therapeutic or protective function of a therapeutic agent, may also be used to compare the function of the agent alone and the function of the agent as part of a fusion structure of the invention. See, e.g., Example 5, which demonstrates the activity of a neurotrophin alone and the same neurotrophin bound to a fusion protein in a model system (hypoxia-reoxygenation in human neural cells). In both Example 5 and Example 7, the fusion protein of the invention retained about 100% of the transport ability and the therapeutic function of its individual components, i.e., a structure capable of crossing the BBB (a MAb to the human insulin receptor) and a therapeutic agent (BDNF).
[00126] Alternatively, binding affinity for receptors may be used as a marker of activity. Binding affinity for the receptor is compared for the structure alone and for the structure when part of the fusion protein. A suitable type of binding affinity assay is the competitive ligand binding assay (CLBA). For example, for fusion proteins containing MAbs to endogenous BBB receptor-mediated transport systems fused to a neurotrophin, a CLBA may be used both to assay the affinity of the MAb for its receptor and the neurotrophin for its receptor, either as part of the fusion protein or as separate entities, and percentage affinity calculated. If, as in some embodiments, the peptide that is active in the CNS is highly ionic, e.g., cationic, causing a high degree of non-specific binding, suitable measures should be taken to eliminate the nonspecific binding. See, e.g., Example 4. "Average" measurements are the average of at least three separate measurements.
[00127] In embodiments of the above fusion proteins, the structure capable of crossing the blood brain barrier crosses the BBB on an endogenous BBB receptor-mediated transporter, such as a transporter selected from the group consisting of the insulin transporter, the transferrin transporter, the leptήi transporter, the LDL transporter, and the IGF receptor. In some embodiments, the endogenous BBB receptor-mediated transporter is selected from the group consisting of the insulin transporter and the transferrin transporter. In some embodiments, the endogenous BBB receptor- mediated transporter is the insulin transporter, e.g., the human insulin transporter. The structure capable of crossing the BBB can be an antibody, e.g., a MAb such as a chimeric MAb. The antibody can be an antibody to an endogenous BBB receptor-mediated transporter, as described herein. The peptide that is active in the CNS can be a neurotherapeutic agent, e.g., a neurotrophin.
In some embodiments, the neurotrophin is selected from the group consisting of brain-derived neurotrophic factor, nerve growth factor (NGF), neurotrophin-4/5, fibroblast growth factor (FGF)-2 and other FGFs, neurotrophin (NT)-3, erythropoietin (EPO), hepatocyte growth factor (HGF), epidermal growth factor (EGF), transforming growth factor (TGF)-α, TGF-β, vascular endothelial growth factor (VEGF), interleukin-1 receptor antagonist (IL- Ira), ciliary neurotrophic factor (CNTF), glial-derived neurotrophic factor (GDNF), neurturin, platelet-derived growth factor (PDGF), heregulin, neuregulin, artemin, persephin, interleukins, granulocyte-colony stimulating factor (CSF), granulocyte-macrophage-CSF, netrins, cardiotrophin- 1, hedgehogs, leukemia inhibitory factor (LIF), midkine, pleiotrophin, bone morphogenetic proteins (BMPs), netrins, saposins, semaphorins, or stem cell factor (SCF). In some embodiments, the neurotrophin is BDNF such as a truncated BDNF, e.g., a carboxyl-tnuicated BDNF. The carboxyl-truncated BDNF is lacking the two carboxyl terminal amino acids in some embodiments. The structure capable of crossing the BBB and the neurotherapeutic agent are covalently linked by a peptide linker in some embodiments. In certain embodiments, the invention provides compositions that increase the serum half- life of cationic substances. One limitation for many current therapeutics, especially cationic therapeutic peptides (e.g., BDNF) is their rapid clearance from the circulation. The positive charge on the cationic substance, such as cationic peptides, rapidly interacts with negative charges on cell membranes, which triggers an absorptive-mediated endocytosis into the cell, particularly liver and spleen. This is true not only for neurotherapeutics (where rapid clearance means only limited contact with the BBB and thus limited ability to cross the BBB) but for other agents as well, such as cationic import peptides such as the tat peptide, or cationic proteins (e.g. protamine, polylysine, polyarginine) that bind nucleic acids, or cationic proteins such as avidin that bind biotinylated drugs. Surprisingly, fusion compositions of the invention that include a cationic therapeutic peptide covalently linked to an immunoglobulin show greatly enhanced serum half-life compared to the same peptide when it was not covalently part of a fusion immunoglobulin. This is an important finding, because it shows that the fusion of a highly cationic protein, e.g., BDNF, to an immunoglobulin, e.g. HIRMAb5 has two important and unexpected effects: 1) it greatly enhances the serum half-life of the cationic protein, and 2) it does not accelerate the blood clearance of the immunoglobulin to which it is attached, e.g., the HIRMAb. Prior work shows that the noncovalent attachment of a cationic therapeutic peptide, e.g., the cationic BDNF to a monoclonal antibody greatly accelerated the blood clearance of the antibody, owing to the cationic nature of the BDNF, which greatly enhances hepatic uptake. The work in Figure 27A and Example 7 shows that when the cationic therapeutic peptide, e.g., BDNF is re-engineered as an IgG fusion protein, the plasma pharmacokinetics is dominated by the IgG moiety, and that the blood level of the BDNF remains high for a prolonged period; indeed, the serum half-life of the BDNF in the fusion protein is at least about 100 times that of the BDNF alone.
[00129] Accordingly, in some embodiments, the invention provides composition comprising a cationic therapeutic peptide covaiently linked to an immunoglobulin, wherein the cationic therapeutic peptide in the composition has a serum half-life that is an average of at least about 1.5, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 100, or more than about 100-fold greater than the serum half-life of the cationic therapeutic peptide alone. In some embodiments, the invention provides a composition comprising a cationic therapeutic peptide covaiently linked to an immunoglobulin, wherein the cationic therapeutic peptide in the composition has a mean residence time (MRT) in the serum that is an average of at least about 1.5, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 100, or more than about 100-fold greater than the serum half-life of the cationic therapeutic peptide alone. In some embodiments, the invention provides composition comprising a cationic therapeutic peptide covaiently linked to an immunoglobulin, wherein the cationic therapeutic peptide in the composition has a systemic clearance rate that is an average of at least about 1.5, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 100, or more than about 100-fold slower than the systemic clearance rate of the cationic therapeutic peptide alone. In some embodiments, the invention provides composition comprising a cationic therapeutic peptide covaiently linked to an immunoglobulin, wherein the cationic therapeutic peptide in the composition has average blood level after peripheral administration that is an average of at least about 1.5, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 100, or more than about 100- fold greater than the average blood level after peripheral administration of the cationic therapeutic peptide alone.
[00130] In some embodiments, the cationic therapeutic peptide comprises a neurotherapeutic agent.
Examples of neurotherapeutic agents that are cationic peptides interferons, interleukins, cytokines, or growth factors with an isoelectric point (pi) above 8. In some embodiments, the neurotherapeutic agent is a neurotrophin. Cationic peptide neurotrophins include BDNF, GDNF, NT-3, NT-4/5, NGF, and FGF-2. In some embodiments, the neurotrophin is BDNF or GDNF.
[00131] In some embodiments, the immunoglobulin is an antibody to an endogenous BBB receptor-mediated transport system. In some embodiments, the endogenous BBB receptor- mediated transport system is selected from the group consisting of the insulin BBB transport system, the BBB transferrin receptor, the BBB leptin receptor, the BBB IGF receptor, or the BBB lipoprotein receptor. In some embodiments, the antibody is an antibody to the endogenous insulin BBB receptor-mediated transport system. Antibodies can be any suitable antibody as described herein.
[00132] Pharmaceutical compositions The invention also provides pharmaceutical compositions that contain one or more compositions of the invention and a pharmaceutically acceptable excipient. A thorough discussion of pharmaceutically acceptable carriers/excipients can be found in Remington 's Pharmaceutical Sciences, Gennaro, AR, ed., 20th edition, 2000: Williams and Wilkins PA, USA.. Pharmaceutical compostions of the invention include compositions suitable for administration via any peripheral route, including intravenous, subcutaneous, intrmuscular,
intraperitoneal injection; oral, rectal, transbuccal, pulmonary, transdermal, intranasal, or any other suitable route of peripheral administration.
[00133] The compostions of the invention are particular suited for injection, e.g., as a pharmaceutical composition for intravenous, subcutaneous, intramuscular, or intraperitonaϊ administration. Aqueous compositions of the present invention comprise an effective amount of a composition of the present invention, which may be dissolved or dispersed in a pharmaceutically acceptable carrier or aqueous medium. The phrases "pharmaceutically or pharmacologically acceptable" refer to molecular entities and compositions that do not produce an adverse, allergic or other untoward reaction when administered to an animal, e.g., a human, as appropriate. As used herein, "pharmaceutically acceptable carrier" includes any and all solvents, dispersion media, coatings, antibacterial and antifungal agents, isotonic and absorption delaying agents and the like. The use of such media and agents for pharmaceutically active substances is well known in the art. Except insofar as any conventional media or agent is incompatible with the active ingredient, its use in the therapeutic compositions is contemplated. Supplementary active ingredients can also be incorporated into the compositions.
[00134] Exemplary pharmaceutically acceptable carriers for injectable compositions can include salts, for example, mineral acid salts such as hydrochlorides, hydrobromides, phosphates, sulfates, and the like; and the salts of organic acids such as acetates, propionates, malonates, benzoates, and the like. For example, compositions of the invention may be provided in liquid form, and formulated in saline based aqueous solution of varying pH (5-8), with or without detergents such polysorbate-80 at 0.01-1%, or carbohydrate additives, such mannitol, sorbitol, or trehalose. Commonly used buffers include histidine, acetate, phosphate, or citrate. Under ordinary conditions of storage and use, these preparations can contain a preservative to prevent the growth of microorganisms. The prevention of the action of microorganisms can be brought about by various antibacterial and antifungal agents, for example, parabens, chlorobutanol; phenol, sorbic acid, thimerosal, and the like, In many cases, it will be preferable to include isotonic agents, for example, sugars or sodium chloride. Prolonged absorption of the injectable compositions can be brought about by the use in the compositions of agents delaying absorption, for example, aluminum monostearate, and gelatin.
[00135] For human administration, preparations meet sterility, pyrogenicity, general safety, and purity standards as required by FDA and other regulatory agency standards. The active compounds will generally be formulated for parenteral administration, e.g., formulated for injection via the intravenous, intramuscular, subcutaneous, intralesional, or intraperitoneal routes. The preparation of an aqueous composition that contains an active component or ingredient will be known to those of skill in the art in light of the present disclosure. Typically, such compositions can be prepared as injectables, either as liquid solutions or suspensions; solid forms suitable for use in preparing solutions or suspensions upon the addition of a liquid prior to injection can also be prepared; and the preparations can also be emulsified.
[00136] Sterile injectable solutions are prepared by incorporating the active compounds in the required amount in the appropriate solvent with various of the other ingredients enumerated above, as required, followed by filtered sterilization. Generally, dispersions are prepared by incorporating the various sterilized active ingredients into a sterile vehicle which contains the basic dispersion medium and the required other ingredients from those enumerated above. In the case of sterile powders for the preparation of sterile injectable solutions, methods of preparation include vacuum- drying and freeze-drying techniques which yield a powder of the active ingredient plus any additional desired ingredient from a previously sterile-filtered solution thereof.
[00137] Upon formulation, solutions will be administered in a manner compatible with the dosage formulation and in such amount as is therapeutically effective. The formulations are easily administered in a variety of dosage forms, such as the type of injectable solutions described above, but drug release capsules and the like can also be employed
[00138] The term "unit dose" refers to physically discrete units suitable for use in a subject, each unit containing a predetermined-quantity of the therapeutic composition calculated to produce the desired responses, discussed above, in association with its administration, i.e., the appropriate route and treatment regimen. The quantity to be administered, both according to number of treatments and unit dose, depends on the subject to be treated, the state of the subject and the protection desired. The person responsible for administration will, in any event, determine the appropriate dose for the individual subject.
[00139] The active therapeutic agents may be formulated within a mixture to comprise about
0.0001 to 1.0 milligrams, or about 0.001 to 0.1 milligrams, or about 1.0 to 100 milligrams or even about 0.01 to 1.0 grams per dose or so. Multiple doses can also be administered. In some embodiments, a dosage of about 2.5 to about 25 mg of a fusion protein of the invention is used as a unit dose for administration to a human, e.g., about 2.5 to about 25 mg of a fusion protein of GDNF and a HIR MAb.
[00140] In addition to the compounds formulated for parenteral administration, such as intravenous or intramuscular injection, other alternative methods of administration of the present invention may also be used, including but not limited to intradermal administration (See U.S. Pat. Nos. 5,997,501 ; 5,848,991; and 5,527,288), pulmonary administration (See U.S. Pat. Nos. 6,361,760; 6,060,069; and 6,041,775), buccal administration (See U.S. Pat. Nos. 6,375,975; and 6,284,262), transdermal administration (See U.S. Pat. Nos. 6,348,210; and 6,322,808) and transmucosal administration (See U.S. Pat. No. 5,656,284). All such methods of administration are well known in the art. One may also use intranasal administration of the present invention, such as with nasal solutions or sprays, aerosols or inhalants. Nasal solutions are usually aqueous solutions designed to be administered to the nasal passages in drops or sprays. Nasal solutions are prepared so that they are similar in many respects to nasal secretions. Thus, the aqueous nasal solutions usually are isotonic and slightly buffered to maintain a pH of 5.5 to 6.5. In addition, antimicrobial preservatives, similar to those used in ophthalmic preparations and appropriate drug stabilizers, if required, may be included in the
formulation. Various commercial nasal preparations are known and include, for example, antibiotics and antihistamines and are used for asthma prophylaxis.
[00141] Additional formulations, which are suitable for other modes of administration, include suppositories and pessaries. A rectal pessary or suppository may also be used. Suppositories are solid dosage forms of various weights and shapes, usually medicated, for insertion into the rectum or the urethra. After insertion, suppositories soften, melt or dissolve in the cavity fluids. For suppositories, traditional binders and carriers generally include, for example, polyalkylene glycols or triglycerides; such suppositories may be formed from mixtures containing the active ingredient in any suitable range, e.g., in the range of 0.5% to 10%, preferably l%-2%.
[00142] Oral formulations include such normally employed excipients as, for example, pharmaceutical grades of mannitol, lactose, starch, magnesium stearate, sodium saccharine, cellulose, magnesium carbonate and the like. These compositions take the form of solutions, suspensions, tablets, pills, capsules, sustained release formulations, or powders. In certain defined embodiments, oral pharmaceutical compositions will comprise an inert diluent or assimilable edible carrier, or they may be enclosed in a hard or soft shell gelatin capsule, or they may be compressed into tablets, or they may be incorporated directly with the food of the diet. For oral therapeutic administration, the active compounds may be incorporated with excipients and used in the form of ingestible tablets, buccal tables, troches, capsules, elixirs, suspensions, syrups, wafers, and the like. Such compositions and preparations can contain at least 0.1% of active compound. The percentage of the compositions and preparations may, of course, be varied, and may conveniently be between about 2 to about 75% of the weight of the unit, or between about 25-60%. The amount of active compounds in such therapeutically useful compositions is such that a suitable dosage will be obtained.
[00143] The tablets, troches, pills, capsules and the like may also contain the following: a binder, such as gum tragacanth, acacia, cornstarch, or gelatin; excipients, such as dicalcium phosphate; a disintegrating agent, such as corn starch, potato starch, alginic acid and the like; a lubricant, such as magnesium stearate; and a sweetening agent, such as sucrose, lactose or saccharin may be added or a flavoring agent, such as peppermint, oil of wintergreen, or cherry flavoring. When the dosage unit form is a capsule, it may contain, in addition to materials of the above type, a liquid carrier. Various other materials may be present as coatings or to otherwise modify the physical form of the dosage unit. For instance, tablets, pills, or capsules may be coated with shellac, sugar or both. A syrup of elixir may contain the active compounds sucrose as a sweetening agent, methylene and propyl parabens as preservatives, a dye and flavoring, such as cherry or orange flavor. In some embodiments, an oral pharmaceutical composition may be enterically coated to protect the active ingredients from the environment of the stomach; enteric coating methods and formulations are well-known in the art.
VI. Nucleic acids, vectors, cells, and manufacture.
[00144] The invention also provides nucleic acids, vectors, cells, and methods of production.
A. Nucleic acids
[00145] In some embodiments, the invention provides nucleic acids that code for proteins or peptides of the invention. In certain embodiments, the invention provides a single nucleic acid sequence containing a first sequence coding for a light chain of an immunoglobulin and second sequence coding a heavy chain of the immunoglobulin, where either the first sequence also codes for a peptide that is expressed as a fusion protein of the peptide covalently linked to the light chain, or the second sequence also codes for a peptide that is expressed as a fusion protein of the peptide covalently linked to the heavy chain. In some embodiments, the invention provides nucleic acid sequences, and in some embodiments the invention provides nucleic acid sequences that are at least about 60, 70, 80, 90, 95, 99, or 100% identical to a particular nucleotide sequence. For example, in some embodiments, the invention provides a nucleic acid containing a first sequence that is at least about 60, 70, 80, 90, 95, 99, or 100% identical to SEQ ID NO:45and a second sequence that is at least about 60, 70, 80, 90, 95, 99, or 100% identical to nucleotides 1396-1746 of SEQ ID NO: 33.
[00146] In other embodiments, the invention provides a nucleic acid containing a sequence that is at least about 60, 70, 80, 90, 95, 99, or 100% identical to SEQ ID NO:45.
[00147] For sequence comparison, of two nucleic acids, typically one sequence acts as a reference sequence, to which test sequences are compared. When using a sequence comparison algorithm, test and reference sequences are entered into a computer, subsequence coordinates are designated, if necessary, and sequence algorithm program parameters are designated. Default program parameters can be used, or alternative parameters can be designated. The sequence comparison algorithm then calculates the percent sequence identities for the test sequences relative to the reference sequence, based on the program parameters.
[00148] A "comparison window", as used herein, includes reference to a segment of any one of the number of contiguous positions selected from the group consisting of from 20 to 600, usually about 50 to about 200, more usually about 100 to about 150 in which a sequence may be compared to a reference sequence of the same number of contiguous positions after the two sequences are optimally aligned. Methods of alignment of sequences for comparison are well-known in the art. Optimal alignment of sequences for comparison can be conducted, including but not limited to, by the local homology algorithm of Smith and Waterman (1970) Adv. Appt. Math. 2:482c, by the homology alignment algorithm of Needϊeman and Wunsch (1970) J. MoI. Biol. 48:443, by the search for similarity method of Pearson and Lipman (1988) Proc. Natl Acad Sci. USA 85:2444, by computerized implementations of these algorithms (GAP, BESTFIT, FASTA, and TFASTA in the Wisconsin Genetics Software Package, Genetics Computer Group, 575 Science Dr., Madison, WI), or by manual alignment and visual inspection (see, e.g., Ausubel et al, Current Protocols in Molecular Biology (1995 supplement)).
[00149] One example of an algorithm that is suitable for determining percent sequence identity and sequence similarity are the BLAST and BLAST 2.0 algorithms, which are described in Altschul et al. (1977) Nuc. Acids Res. 25:3389-3402, and Altschul et al (1990) J. MoI Biol. 215:403-410, respectively. Software for performing BLAST analyses is publicly available through the National Center for Biotechnology Information. The BLAST algorithm parameters W, T, and X determine the sensitivity and speed of the alignment. The BLASTN program (for nucleotide sequences) uses as defaults a wordlength (W) of 11 , an expectation (E) or 10, M=5, N=-4 and a comparison of both strands. The BLAST algorithm is typically performed with the "low complexity" filter turned off. The BLAST algorithm also performs a statistical analysis of the similarity between two sequences {see, e.g., Karlin and Altschul (1993) Proc. Natl. Acad. ScL USA 90:5873-5787). One measure of similarity provided by the BLAST algorithm is the smallest sum probability (P(N)), which provides an indication of the probability by which a match between two nucleotide or amino acid sequences would occur by chance. For example, a nucleic acid is considered similar to a reference sequence if the smallest sum probability in a comparison of the test nucleic acid to the reference nucleic acid is less than about 0.2, more preferably less than about 0.01, and most preferably less than about 0.001.
[00150] The invention provides nucleic acids that code for any of the peptides of the invention. In some embodiments, the invention provides a single nucleic acid sequence containing a gene coding for a light chain of an immunoglobulin and a gene coding for a fusion protein, where the fusion protein includes a heavy chain of the immunoglobulin covalently linked to a peptide. In some embodiments, the peptide is a therapeutic peptide. In some embodiments the peptide is a neurotherapeutic peptide, e.g., a neurotrophin such as BDNF or GDNF (e.g., mature human GDNF). In some embodiments, the BDNF is a two amino acid carboxy-truncated BDNF. In some embodiments, the immunoglobulin is an IgG. In some embodiments, the IgG is a MAb, such as a chimeric MAb. The antibody can be an antibody to a transport system, e.g., an endogenous BBB receptor-mediated transport system such as the endogenous BBB receptor-mediated insulin transport system. In some embodiments, the endogenous BBB receptor-mediated insulin transport system is a human endogenous BBB receptor-mediated insulin transport system and wherein the peptide to which the immunoglobulin heavy chain is covalently linked is human BDNF or human GDNF. Any suitable peptide, neurotherapeutic peptide, neurotrophin, BDNF, GDNF (e.g., mature human GDNF), antibody, monoclonal antibody, or chimeric antibody, as described herein, may be coded for by the nucleic acid, combined as a fusion protein and coded for in a single nucleic acid sequence. As is well-known in the art, owing to the degeneracy of the genetic code, any combination of suitable codons may be used to code for the desired fusion protein. In addition, other elements useful in recombinant technology, such as promoters, termination signals, and the like, may also be included in the nucleic acid sequence. Such elements are well-known in the art. In addition, all nucleic acid sequences described and claimed herein include the complement of the sequence.
[00151] In some embodiments the nucleic acid codes for a BDNF, e.g., a variant BDNF, or GDNF
(e.g., mature human GDNF) as a component of the fusion protein, which also comprises an immunoglobulin sequence. In some embodiments, the BDNF contains a sequence that is about 60, 70,80, 90, 95, 99, or 100% identical to the sequence of amino acids 466-582 of SEQ ID NO: 24. In some embodiments, the GDNF contains a sequence that is at least about 60, 70,80, 90, 95, 99, or 100% identical to the sequence of SEQ ID NO:52. In some embodiments, the amino acid sequence of the encoded GDNF consists essentially of SEQ ID NO:52. In some embodiments, the nucleic acid codes for a fusion protein comprising an amino acid sequence that is at least about 60, 70,80, 90, 95, 99, or 100% identical to the sequence of SEQ ID NO:46. In some embodiments, the encoded nucleic acid comprises the amino acid sequence of SEQ ID NO:46. In some embodiments, the BDNF or GDNF is linked at its amino terminus to carboxy terminus of the heavy chain of the immunoglobulin, e.g., MAb. The heavy chain of the MAb can comprise a sequence that is about 60, 70, 80, 90, 95, 99 or 100% identical to amino acids 20-462 of SEQ ID NO: 24. In some embodiments, the light chain of the immunoglobulin, e.g., MAb, comprises a sequence that is about 60, 70, 80, 90, 95, 99 or 100% identical to amino acids 21-234 of SEQ ID NO: 36. The nucleic acid can further contain a nucleic acid sequence that codes for a peptide linker between the heavy chain of the MAb and the BDNF or GDNF. In some embodiments, the linker is S-S-M. The nucleic acid may further contain a nucleic acid sequence coding for a signal peptide, wherein the signal peptide is linked to the heavy chain. Any suitable signal peptide, as known in the art or subsequently developed, may be used. In some embodiments, the signal peptide attached to the heavy chain comprises a sequence that is about 60, 70, 80, 90, 95, 99, or 100% identical to amino acids 1-19 of SEQ ID NO: 24. In some embodiments, the nucleic acid contains a nucleic acid sequence coding for another signal peptide, wherein the other signal peptide is linked to the light chain. The signal peptide linked to the light chain can comprise a sequence that is about 60, 70, 80, 90, 95, 99, or 100% identical to amino acids 1-20 of SEQ ID NO: 36. The nucleic acid can contain a nucleic acid sequence coding for a selectable marker. In some embodiments the selectable marker is DHFR. The sequence of the DHFR can be about 60, 70, 80, 90, 95, 99, or 100% identical to amino acids 1-187 of SEQ ID NO: 38.
[00152] In certain embodiments, the invention provides a nucleic acid comprising a first sequence that codes for a neurotherapeutic peptide, e.g., a neurotrophin such as BDNF, in the same open reading frame as a second sequence that codes for an immunoglobulin component. The immunoglobulin component can be, e.g., a light chain or a heavy chain, e.g., that is at least about 60, 70, 80, 90, 95, 99, or 100% identical to nucleotides 58-1386-of SEQ ID NO: 33 and a second sequence that is at least about 60, 70, 80, 90, 95, 99, or 100% identical to nucleotides 1396-1746 of SEQ ID NO: 33. In some embodiments, the nucleic acid also contains a third sequence that is at least about 60, 70, 80, 90, 95, 99, or 100% identical to nucleotides 61-702 of SEQ ID NO: 35. In some embodiments, the nucleic acid further contains a fourth sequence that codes for a first signal peptide and a fifth sequence that codes for a second signal peptide. In some embodiments, the
fourth sequence is at least about 60, 70, 80, 90, 95, 99, or 100% identical to nucleotides 1-57 of SEQ ID NO: 33 and the fifth sequence is at least about 60, 70, 80, 90, 95, 99, or 100% identical to nucleotides 1-60 of SEQ ID NO: 35. In some embodiments, the nucleic acid further contains a sequence that codes for a selectable marker, such as dihydrofolate reductase (DHFR). In some embodiments, the sequence that codes for the DHFR is at least about 60, 70, 80, 90, 95, 99, or 100% identical to nucleotides 1-561 of SEQ ID NO: 37.
B. Vectors
[00153] The invention also provides vectors. The vector can contain any of the nucleic acid sequences described herein. In some embodiments, the invention provides a single tandem expression vector containing nucleic acid coding for an antibody heavy chain fused to a peptide, e.g., a therapeutic peptide such as a neurotrophin, and nucleic acid coding for a light chain of the antibody, all incorporated into a single piece of nucleic acid, e.g., a single piece of DNA. The singie tandem vector can also include one or more selection and/or amplification genes. A method of making an exemplary vector of the invention is provided in the Examples. However, any suitable techniques, as known in the art, may be used to construct the vector.
[00154] The use of a single tandem vector has several advantages over previous techniques. The transfection of a eukaryotic cell line with immunoglobulin G (IgG) genes generally involves the co- transfection of the cell line with separate plasmids encoding the heavy chain (HC) and the light chain (LC) comprising the IgG. In the case of a IgG fusion protein, the gene encoding the recombinant therapeutic protein may be fused to either the HC or LC gene. However, this co- transfection approach makes it difficult to select a cell line that has equally high integration of both the HC and LC-fusion genes, or the HC-fusion and LC genes. The approach to manufacturing the fusion protein utilized in certain embodiments of the invention is the production of a cell line that is stably transfected with a single plasmid DNA that contains all the required genes on a single strand of DNA, including the HC-fusion protein gene, the LC gene, the selection gene, e.g. neo, and the amplification gene, e.g. the dihydrofolate reductase gene. As shown in the diagram of the fusion protein tandem vector in Figure 12, the HC-fusion gene, the LC gene, the neo gene, and the DHFR gene are all under the control of separate, but tandem promoters and separate but tandem transcription termination sequences. Therefore, all genes are equally integrated into the host cell genome, including the fusion gene of the therapeutic protein and either the HC or LC IgG gene.
C. Cells
[00155] The invention further provides cells that incorporate one or more of the vectors of the invention. The cell may be a prokaryotic cell or a eukaryotic cell. In some embodiments, the cell is a eukaryotic cell. In some embodiments, the cell is a mouse myeloma hybridoma cell. In some embodiments, the cell is a Chinese hamster ovary (CHO) cell. Exemplary methods for incorporation of the vector(s) into the cell are given in the Examples. However, any suitable techniques, as known in the art, may be used to incorporate the vector(s) into the cell. In some embodiments, the invention provides a cell capable of expressing an immunoglobulin fusion
protein, where the cell is a cell into which has been introduced a single tandem expression vector, where both the immunoglobulin light chain gene and the gene for the immunoglobulin heavy chain fused to the therapeutic agent, are incorporated into a single piece of nucleic acid, e.g., DNA. In some embodiments, the invention provides a cell capable of expressing an immunoglobulin fusion protein, where the cell is a cell into which has been stably transfected a single tandem expression vector, where both the immunoglobulin heavy chain gene and the gene for the immunoglobulin light chain fused to the therapeutic agent, are incorporated into a single piece of nucleic acid, e.g., DNA. The introduction of the tandem vector may be by, e.g., permanent integration into the chromsomal nucleic acid, or by, e.g., introduction of an episomal genetic element.
D. Methods of manufacture
[00156] In addition, the invention provides methods of manufacture. In some embodiments, the invention provides a method of manufacturing an immunoglobulin fusion protein, where the fusion protein contains an immunoglobulin heavy chain fused to a therapeutic agent, by introducing into a eukaryotic cell a single tandem expression vector, where both the immunoglobulin light chain gene and the gene for the immunoglobulin heavy chain fused to the therapeutic agent, are incorporated into a single piece of nucleic acid, e.g., DNA. hi some embodiments, the invention provides a method of manufacturing an immunoglobulin fusion protein, where the fusion protein contains an immunoglobulin light chain fused to a therapeutic agent, by Iy introducing into a eukaryotic cell a single tandem expression vector, where both the immunoglobulin heavy chain gene and the gene for the immunoglobulin light chain fused to the therapeutic agent, are incorporated into a single piece of nucleic acid, e.g., DNA. In some embodiments, the introduction of the vector is accomplished by integration into the host cell genome. In some embodiments, the introduction of the vector is accomplished by introduction of an episomal genetic element containing the vector into the host cell. Episomal genetic elements are well-known in the art In some embodiments, the therapeutic agent is a neurotherapeutic agent. In some embodiments, the single piece of nucleic acid further includes one or more genes for selectable markers. In some embodiments, the single piece of nucleic acid further includes one or more amplification genes. In some embodiments, the immunoglobulin is an IgG, e.g., a MAb such as a chimeric MAb. The methods may further include expressing the immunoglobulin fusion protein, and/or purifying the immunoglobulin fusion protein. Exemplary methods for manufacture, including expression and purification, are given in the Examples.
[00157] However, any suitable techniques, as known in the art, may be used to manufacture, optionally express, and purify the proteins. These include non-recombinant techniques of protein synthesis, such as solid phase synthesis, manual or automated, as first developed by Merrifield and described by Stewart et al. in Solid Phase Peptide Synthesis (1984). Chemical synthesis joins the amino acids in the predetermined sequence starting at the C-terminus. Basic solid phase methods require coupling the C-terminal protected α-amino acid to a suitable insoluble resin support. Amino
acids for synthesis require protection on the α-amino group to ensure proper peptide bond formation with the preceding residue (or resin support). Following completion of the condensation reaction at the carboxyl end, the α-amino protecting group is removed to allow the addition of the next residue. Several classes of α-protecting groups have been described, see Stewart et al. in Solid Phase Peptide Synthesis (1984), with the acid labile, urethane-based tertiary-butyloxycarbonyl (Boc) being the historically preferred. Other protecting groups, and the related chemical strategies, may be used, including the base labile 9-fluorenylmethyloxycarbonyl (FMOC). Also, the reactive amino acid sidechain functional groups require blocking until the synthesis is completed. The complex array of functional blocking groups, along with strategies and limitations to their use, have been reviewed by Bodansky in Peptide Synthesis (1976) and, Stewart et al. in Solid Phase Peptide Synthesis (1984).
[00158] Solid phase synthesis is initiated by the coupling of the described C-terminal α-protected amino acid residue. Coupling requires activating agents, such as dicyclohexycarbodiimide (DCC) with or without l-hydroxybenzo-triazole (HOBT), diisopropylcarbodiimide (DIIPC), or ethyldimethylaminopropylcarbodiimide (EDC). After coupling the C-terminal residue, the α-amino protected group is removed by trifluoroacetic acid (25% or greater) in dichloromethane in the case of acid labile tertiary-butyloxycarbonyl (Boc) groups. A neutralizing step with triethylamine (10%) in dichloro-methane recovers the free amine (versus the salt). After the C-terminal residue is added to the resin, the cycle of deprotection, neutralization and coupling, with intermediate wash steps, is repeated in order to extend the protected peptide chain. Each protected amino acid is introduced in excess (three to five fold) with equimolar amounts of coupling reagent in suitable solvent. Finally, after the completely blocked peptide is assembled on the resin support, reagents are applied to cleave the peptide form the resin and to remove the side chain blocking groups. Anhydrous hydrogen fluoride (HF) cleaves the acid labile tertiary-butyloxycarbonyl (Boc) chemistry groups. Several nucleophilic scavengers, such as dimethylsulfϊde and anisole, are included to avoid side reactions especially on side chain functional groups.
VII. Methods
[00159] The invention also provides methods. In some embodiments, the invention provides methods for transport of an agent active in the CNS across the BBB in an effective amount. In some embodiments, the invention provides therapeutic, diagnostic, or research methods. Diagnostic methods include the development of peptide radiopharmaceuticals capable of transport across the BBB, such as the fusion of a peptide ligand, or peptidomimetic MAb for an endogenous receptor in the brain, followed by the radiolabelling of the fusion protein, followed by systemic administration, and external imaging of the localization within the brain of the peptide radiopharmaceutical.
[00160] Prior to the present invention, neurotrophins such as BDNF or GDNF were injected directly into the brain to achieve a therapeutic effect, because the neurotrophin does not cross the BBB. Therefore, it is not expected that neurotrophic factors will have beneficial effects on brain disorders following the peripheral (intravenous, subcutaneous) administration of these molecules,
[00161] However, neurotherapeutics can be developed as drugs for peripheral routes of administration, providing the neurotherapeutic is enabled to cross the BBB. Attachment of the neurotherapeutic, e.g. a neurotrophin such as BDNF or GDNF to a MTH, e.g., the chimeric HIRMAb, offers a new approach to the non-invasive delivery of neurotherapeutics to the CNS in animals, e.g., mammals such as humans for the treatment of acute brain and spinal cord conditions, such as focal brain ischemia, global brain ischemia, and spinal cord injury, and chronic treatment of neurodegenerative disease, including prion diseases, Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), ALS, multiple sclerosis, transverse myelitis, motor neuron disease, Pick's disease, addiction (e.g., drug addiction), tuberous sclerosis, lysosomal storage disorders, Canavan's disease, Rett's syndrome, spinocerebellar ataxias, Friedreich's ataxia, optic atrophy, and retinal degeneration.
[00162] Accordingly, in some embodiments the invention provides methods of transport of an agent active in the CNS from the peripheral circulation across the BBB in an effective amount, where the agent is covalently attached to a structure that crosses the BBB, and where the agent alone is not transported across the BBB in an effective amount. In some embodiments the invention provides methods of transport of neurotherapeutic agent from the peripheral circulation across the BBB in a therapeutically effective amount, where the neurotherapeutic agent is covalently attached to a structure that crosses the BBB, and where the neurotherapeutic agent alone is not transported across the BBB in a therapeutically effective amount
[00163] The invention also provides, in some embodiments, methods of treatment of disorders of the CNS by peripheral administration of an effective amount of a therapeutic agent, e.g., a neurotherapeutic agent covalently linked to a structure that is capable of crossing the BBB, where the agent alone is not capable of crossing the BBB in an effective amount when administered peripherally. In some embodiments, the CNS disorder is an acute disorder, and, in some cases, may require only a single administration of the agent. In some embodiments, the CNS disorder is a chronic disorder and may require more than one administration of the agent.
[00164] In some embodiments, the effective amount, e.g., therapeutically effective amount is such that a concentration in the brain is reached of at least about 0.001, 0.01, 0.1, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 35, 40, 45, 50, 100, or more than 100 ng/gram brain. In some embodiments, a therapeutically effective amount, e.g., of a neurotrophin such as BDNF or GDNF, is such that a brain level is achieved of about 0.1 to 1000, or about 1-100, or about 5-50 ng/g brain. In some embodiments, the neurotherapeutic agent is a neurotrophin, In some embodiments, the neurotrophin is selected from the group consisting of BDNF, nerve growth factor (NGF), neurotrophin-4/5, fibroblast growth factor (FGF)-2 and other FGFs, neurotrophin (NT)-3,
erythropoietin (EPO), hepatocyte growth factor (HGF), epidermal growth factor (EGF), transforming growth factor (TGF)-α, TGF-β, vascular endothelial growth factor (VEGF), interleukin-1 receptor antagonist (IL- Ira), ciliary neurotrophic factor (CNTF), glial-derived neurotrophic factor (GDNF), neurturin, platelet-derived growth factor (PDGF), heregulin, neuregulin, artemin, persephin, interleukins, gramilocyte-colony stimulating factor (CSF), granulocyte-macrophage-CSF, netrins, cardiotrophin-1, hedgehogs, leukemia inhibitory factor (LIF), midkine, pleiotrophin, bone morphogenetic proteins (BMPs), netrins, saposins, semaphorins, or stem cell factor (SCF).. In some embodiments, the neurotrophin is BDNF, e.g. a truncated BDNF, such as the carboxyl-truncated BDNFs described herein.
[00165] In some embodiments, the invention provides methods of treating a disorder of the CNS by peripherally administering to an individual in need of such treatment an effective amount of a neurotrophin, where the neurotrophin is capable of crossing the BBB to produce an average elevation of neurotrophin concentration in the brain of at least about 0.1, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 35, 40, 45, 50, 100, or more than 100 ng/gram brain following said peripheral administration, and where the neurotrophin remains at the elevated level for about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more than 10 days after a single administration. In some embodiments, the neurotrophin remains at a level of greater than about 1 ng/g brain, or about 2 ng/g brain, or about 5 ng/g brain for about 2, 3, 4, 5, 6, 7, 8, 9, 10, or more than 10 days after a single administration. In some embodiments, the neurotrophin is BDNF, including truncated versions thereof.
[00166] In some embodiments, the invention provides methods of treating a disorder of the CNS by peripherally administering to an individual in need of such treatment an effective amount of a composition of the invention. The term "peripheral administration," as used herein, includes any method of administration that is not direct administration into the CNS, i.e., that does not involve physical penetration or disruption of the BBB. "Peripheral administration" includes, but is not limited to, intravenous intramuscular, subcutaneous, intraperitoneal, intranasal, transbuccal, transdermal, rectal, transalveolar (inhalation), or oral administration. Any suitable composition of the invention, as described herein, may be used. In some embodiments, the composition is a neurotrophin covalently linked to a chimeric HIR-MAb. In some embodiments, the neurotrophin is a BDNF. In some embodiments, the BDNF is a variant as described herein, such as a carboxyl- terminal truncated variant. In other embodiments, the neurotrophin is a GDNF (e.g., mature human GDNF).
[00167] A "disorder of the CNS" or "CNS disorder," as those terms are used herein, encompasses any condition that affects the brain and/or spinal cord and that leads to suboptimal function. In some embodiments, the disorder is an acute disorder. Acute disorders of the CNS include focal brain ischemia, global brain ischemia, brain trauma, spinal cord injury, acute infections, status epilepticus, migrane headache, acute psychosis, suicidal depression, and acute anxiety/phobia. In some embodiments, the disorder is a chronic disorder. Chronic disorders of the CNS include chronic neurodegeneration, retinal degeneration, depression, chronic affective disorders, lysosmal
storage disorders, chronic infections of the brain, brain cancer, stroke rehabilitation, inborn errors of metabolism, autism, mental retardation. Chronic neurodegeneration includes neurodegenerative diseases such as prion diseases, Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), transverse myelitis, motor neuron disease, Pick's disease, tuberous sclerosis, lysosomal storage disorders, Canavan's disease, Rett's syndrome, spinocerebellar ataxias, Friedreich's ataxia, optic atrophy, and retinal degeneration, and aging of the CNS.
[00168] In some embodiments, the invention provides methods of treatment of the retina, or for treatment or prevention of blindness. The retina, like the brain, is protected from the blood by the blood-retinal barrier (BRB). The insulin receptor is expressed on both the BBB and the BRB, and the HIRMAb has been shown to deliver therapeutics to the retina via RMT across the BRB. BDNF is neuroprotective in retinal degeneration, but it was necessary to inject the neurotrophin directly into the eyeball, because BDNF does not cross the BRB. In some embodiments, fusion proteins of the invention are used to treat retinal degeneration and blindness with a route of administration no more invasive than an intravenous or subcutaneous injection, because the HIRMAb delivers the BDNF across the BRB, so that the neurotrophin is exposed to retinal neural cells from the blood compartment.
[00169] In some embodiments, the invention provides a method of treatment for depression. A subset of patients with depression may have a brain deficiency of BDNF, and the correlation of single nucleotide polymorphisms (SNPs) with affective disorders has been reported. The direct injection of BDNF into the brain has durable anti-depressant effects in rodent model. The BDNF must be injected directly into the brain, because the neurotrophin does not cross the BBB. In some embodiments, the invention provides a method for treating depression by chronic administration of a fusion protein of the invention, thus elevating the brain levels of BDNF and being therapeutic in those patients with depression and a reduced production of brain BDNF.
[00170] Formulations and administration. Any suitable formulation, route of administration, and dose of the compositions of the invention may be used. Formulations, doses, and routes of administration are determined by those of ordinary skill in the art with no more than routine experimentation. Compositions of the invention, e.g., GDNF fusion proteins are typically administered in a single dose, e.g., an intravenous dose, of about 0.01-1000 mg, or about 0.05-500 mg, or about 0.1-100 mg, or about 1-100 mg, or about 0.5-50 mg, or about 5-50 mg, or about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 15, 20, 25, 30, 25, 40, 45, 50, 60, 70, 80, 90, or 100 mg. Typically, for the treatment of acute brain disease, such as stroke, cardiac arrest, spinal cord injury, or brain trauma, higher doses may be used, whereas for the treatment of chronic conditions such as Alzheimer's disease, Parkinson's disease, Huntington's disease, MS, ALS, transverse myelitis, motor neuron disease, Pick's disease, tuberous sclerosis, addiction (e.g., drug addiction), lysosomal storage disorders, Canavan's disease, Rett's syndrome, spinocerebellar ataxias, Friedreich's ataxia, optic atrophy, and retinal degeneration, and aging, lower, chronic dosing may be used. Oral
administration can require a higher dosage than intravenous or subcutaneous dosing, depending on the efficiency of absorption and possible metabolism of the protein, as is known in the art, and may be adjusted from the foregoing based on routine experimentation.
[00171] For intravenous or subcutaneous administration, formulations of the invention may be provided in liquid form, and formulated in saline based aqueous solution of varying pH (5-8), with or without detergents such ρolysorbate-80 at 0.01-1%, or carbohydrate additives, such mannitol, sorbitol, or trehalose. Commonly used buffers include histidine, acetate, phosphate, or citrate.
[00172] Dosages for humans can be calculated from appropriate animal data. For example, human dosing of a BDNF-MAB conjugate is based on pre-clinical pharmacokinetic studies, and these measurements have been performed in 2 species, rats, and Rhesus monkeys. Prior work in 3 models of cerebral ischemia in rats demonstrated the range of effective doses of the BDNF-MAb conjugate is 5-50 μg/rat or 20-200 μg/kg of BDNF in the form of the BDNF-MAb conjugate. Since the BDNF component of the fusion protein molecule is 16%, and the HIRMAb component is 84%, the dose of fusion protein is 6-fold greater than the equivalent BDNF dose. Pharmacokinetic studies in rats show these doses produce a concentration of the BDNF in the form of conjugate in plasma of 50-500 ng/mL, and in brain of 5-50 ng/g. Pharmacokinetic studies in adult Rhesus monkeys with the HIRMAb show that the average plasma concentration in the first hour is 0.1% injected dose (ID)/mL, and that the brain concentration is 0.02% ID/g. The brain concentration of the fusion protein is about 0.01% ID/g (Figure 27). Owing to the scaling effect between species, and to the 10-fold larger body size and brain size of humans relative to Rhesus monkeys, the projected plasma and brain concentrations in humans are 0.01% ID/mL and 0.001% ID/g respectively. Since the human brain is 1200 grams, then >1% of the injected dose is delivered to the human brain, which is a level of brain uptake comparable to small molecules. Given an injected dose of fusion protein of 2.5-25 tng in humans, the expected 60 min plasma concentration is 250- 2500 ng/ml of fusion protein, and the expected 60 min brain concentration is 25-250 ng/g of fusion protein, which is equivalent to 4-40 ng/gram brain of BDNF. The 5 mg and 25 mg fusion protein doses in humans will produce a brain concentration of the BDNF that is neuroprotective in either global or regional brain ischemia. Since the BDNF comprises 16% of the fusion protein, the effective doses of BDNF administered to humans is 0.4 or 4.0 mg, respectively, for the 2.5 or 25 mg dose of fusion protein.
[00173] The fusion protein may also be formulated for chronic use for the treatment of a chronic
CNS disorder, e.g., neurodegenerative disease, stroke or brain/spinal cord injury rehabilitation, or depression. Chronic treatment may involve daily, weekly, bi-weekly administration of the composition of the invention, e.g., fusion protein either intravenously, intra-muscularly, or subcutaneous in formulations similar to that used for acute treatment. Alternatively, the composition, e.g., fusion protein may be formulated as part of a bio-degradable polymer, and administered on a monthly schedule.
[00174] Combination therapies. The composition of the invention, e.g., fusion protein may be administered as part of a combination therapy. The combination therapy involves the administration of a composition of the invention in combination with another therapy for the CNS disorder being treated. If the composition of the invention is used in combination with another CNS disorder method or composition, any combination of the composition of the invention and the additional method or composition may be used. Thus, for example, if use of a composition of the invention is in combination with another CNS disorder treatment agent, the two may be administered simultaneously, consecutively, in overlapping durations, in similar, the same, or different frequencies, etc. In some cases a composition will be used that contains a composition of the invention in combination with one or more other CNS disorder treatment agents.
[00175] Other CNS disorder treatment agents that may be used in methods of the invention include, without limitation, thromolytic therapy for stroke, amyloid-directed therapy for Alzheimers disease, dopamine restoration therapy for Parkinsons disease, RNA interference therapy for genetic disorders, cancer, or infections, and anticonvulsant therapy for epilepsy. Dosages, routes of administration, administration regimes, and the like for these agents are well-known in the art.
[00176] In some embodiments, the composition, e.g., fusion protein is co-administered to the patient with another medication, either within the same formulation or as a separate composition. For example, the fusion protein could be formulated with another fusion protein that is also designed to deliver across the human biood-brain barrier a recombinant protein other than BDNF (e.g., GDNF). The fusion protein may be formulated in combination with other large or small molecules.
VIII. Kits
[00177] Compositions of the invention, e.g., fusion proteins, may be provided as a kit that includes the formulation, e.g., fusion protein in a container and in suitable packaging. The composition can be provided in a dry powder form, in solid form (i.e., lyophilized), in solution, or in suspension. If the composition is a protein, to the proteins may have been added emulsifiers, salts, preservatives, other proteins, nucleic acids, protease inhibitors, antibiotics, perfumes, polysaccharides, adhesive agents, polymers, microfibrils, oils, etc. The composition is packaged for transport, storage and/or use by a consumer. Such packaging of therapeutic compositions for transport, storage, and use is well-known in the art. Packaged compositions may include further components for the dispensing and storage of the composition, and may also include separately packaged diluent comprised of, e.g., sterile water or a suitable buffer, for solubilizing the formulation, e.g., fusion protein prior to administration to the patient. Kits of the invention may also include written materials, including instructions for use, results of clinical studies, desired outcome and expected course of treatment, information about precautions and side effects, and the like. The kits may optionally further contain other components, such as gloves, scissors, tape, implements for disposal of used vials and other waste, masks, antiseptic, antibiotics, and the like.
EXAMPLES
Example 1
Construction of the single tandem vector containing complete genes for IgG-neuro therapeutic fusion
[00178] Genetic engineering of a eukaryotic expression vector encoding the heavy chain (HC) of the fusion protein is outlined in Figure 1. The final fusion protein HC expression vector was designated pHIRMAb-BDNF, or clone 416. This vector was designed to produce a fusion protein, comprised of a BDNF variant fused to the HC of the HIRMAb. Either BDNF or a variant of BDNF (vBDNF) can be fused to the HIRMAb. The vBDNF differs from native human BDNF by substitution of certain amino acids, such as a vBDNF where the 2 amino acids at the carboxyl terminus of BDNF are absent in vBDNF. The clone 416 plasmid was derived from clone 400, which produces the HC of the chimeric form of the HIRMAb, and a cDNA encoding mature human vBDNF, which was produced as described in Figure 2. Clone 400 encodes a chimeric human IgGl that is derived from a chromosomal fragment encoding the human IgGl constant region, and is comprised of both intron and exon sequences. The HC gene of the chimeric HIRMAb in clone 400 was subcloned at the BamHI site of the pCR II plasmid to facilitate engineering of the stop codon located at the 3 '-end of the CH3 region by site directed mutagenesis (SDM). The engineering of the stop codon located at the end of the CH3 region was performed by site-directed mutagenesis to produce a Sspl site. The Sspl site allows for insertion of the vBDNF cDNA (Figure 3) by blunt-end ligation into clone 400 to form clone 415. SDM was performed using the QuickChange SDM kit (Stratagene, CA). Sense and complementary mutagenic primers were designed in a way that the CH3 stop codon (aaTGAg) is mutated to Sspl site (aaTATt). In addition, primers contained 15 nucleotides of the stop codon 5'- and 3 '-surrounding region; the sequence of these primers, designated SDM-SspI forward (FWD) and reverse (REV) are given in Table 1.
[00179] DNA sequence analysis of the IgG promoter region revealed the presence of additional
Sspl sites in this region. Therefore, it was first necessary to release the HC promoter region (PRO- VH) by digestion of clone 404 with Xhol and Nhel, and the clone 404 was re-closed with a Xhol- Nhel linker which produced clone 405 (-Pro-VH). The sequence of the forward and reverse ODNs used to produce the Xhol-Nhel linker are given in Table 1. Plasmid clone 405 (-Pro-VH) now carries the single Sspl site introduced by SDM. The human vBDNF cDNA was subcloned at Sspl to form an intermediate plasmid named clone 414 (not shown). The complete fusion protein HC expression cassette was then reconstructed by subcloning of the PRO-VH fragment previously deleted to form clone 415. The fusion protein HC gene was then subcloned in the eukaryotic expression vector, clone 400, at the BamHI site to form clone 416.
[00180] The vBDNF cDNA was produced by PCR via either of 2 equivalent approaches. In one approach, a prokaryotic expression plasmid, pHTBSOl, isolated as an expressed sequence tag (EST), and encoding human BDNF, was digested with BamHI and BpII, and gel purified, and re- ligated with T4 ligase and the 5'-end linker to produce clone 412 (Figure 2). The sequence of the forward and reverse ODNs used to produce the 5'-end linker are given in Table 2.
[00181]
[00182] Clone 412 was then digested with Xhol and BsiWI, and gel purified, and re-ligated with
T4 iigase and the 3'end linker to produce clone 413 (Figure 2). The sequence of the forward and reverse ODNs used to produce the 3 '-end linker are given in Table 2. The vBDNF cDNA, encoding the vBDNF with a reconstructed stop codon, was released from clone 413 by Nrul, and gel purified; the ethidium bromide stain of the agarose gel is shown in Figure 3 A. This gel shows the expected size of the vBDNF cDNA, 0.4 kb, and the vector backbone, 3.5 kb. Alternatively, the BDNF cDNA was produced by PCR from cDNA derived by reverse transcription of polyA+RNA isolated
from human U87 glioma cells, which produce neurotrophins. The primers used to produce the vBDNF by PCR from the U87-derived cDNA are given in Table 2. This PCR produced the expected 0.4 kb vBDNF cDNA (Figure 3B). The 0.4 kb vBDNF fragment was then digested with Nrul, and subcloned into clone 415, as described in Figure 1 , to produce the full fusion protein HC expression cassette, which was released by BamHI and subcloned into the original eukaryotic expression plasmid to produce clone 416 (Figure 1), the final expression plasmid for the fusion protein HC. Clone 416 was analyzed by double digestion with Nhel and BamHI and compared with that of the original clone 400, which lacks the vBDNF. The agarose gel-separated products are shown in Figure 3C, where lanes 1 and 3 show the fragments generated from clone 416 and clone 400, respectively. Both plasmids produce a 6 kb vector backbone (upper of 3 bands in lanes 1 and 3), and a 2.5 kb promoter region (lower of 3 bands in lanes 1 and 3). However, the size of the middle band is 0.4 kb larger for clone 416, as compared to clone 400 (middle band, lanes 1 and 3). A negative clone is shown in lane 2 of Figure 3C.
[00183] The nucleotide and amino acid sequence of the reconstructed carboxyl terminus at the
CH3 region of the HIRMAb HC, a 3-amino acid linker (Ser-Ser-Met), the vBDNF sequence, followed by a stop codon is shown in Figure 4. The entire 2711 nucleotides (nt) comprising the fusion protein HC gene of clone 416 is shown in Figure 5. The ATG initiation codon and the TGA stop codon are underlined. The human IgGl constant region intron and exon sequences are shown in italics and bold font, respectively, in Figure 5. The vBDNF nt sequence hi the clone 416 vector is underlined in Figure 5. These data show that intronic sequence is found between CHl and the hinge region, between the hinge region and CH2, and between CH2 and CH3 regions of the human IgGl constant region. The open reading frame (orf) of the fusion protein HC gene encodes for a 563 amino acid protein, following cleavage of a 19 amino acid signal peptide, and the amino acid sequence of the fusion protein HC is shown in Figure 6. The signal peptide is underlined; the cysteine (C) residues within the constant region that form inter- or intra-chain disulfide bridges are shown in bold font; the serine-serine-methionine (SSM) linker between the CH3 region of the IgG and the vBDNF is underlined; the single N-linked glycosylation site, at the asparagine residue within CH2 in shown by bold underlined font (Figure 6). The amino acid sequences of the individual domains of the fusion protein HC protein are given in Figure 7. The vBDNF domain of the fusion protein is comprised of 117 amino acids.
[00184] Clone 416 plasmid DNA was electroporated into mouse myeloma cells that had previously been transfected with an expression plasmid encoding the light chain (LC) of the chimeric HIRMAb. Since the vBDNF is fused only to the HC, there is no modification of the LC of the chimeric HIRMAb. Following selection of transfected cell lines, media from 96-well plates were screened with an ELISA comprised of 2 anti-human IgG antibodies; one antibody is directed against the heavy chain of human IgGl, and the other antibody is directed against human kappa light chains. Myeloma clones encoding for intact fusion protein were isolated, and propagated in a 10 L bioreactor. However, the production levels of the fusion protein were low. This low
production was attributed to several factors, including (i) transfection of the myeloma line by 3 separate expression plasmids encoding the heavy chain gene, the light chain gene, and the antibiotic resistance gene; and (ii) the use of genomic fragment of the heavy and light chain genes with large intronic sequences. Therefore, the fusion protein expression plasmid was re-engineered with the following features:
(1) the polymerase chain reaction (PCR) was used to convert genomic fragments of the fusion protein HC and LC genes into 'intron-less' cDNA forms of the 2 genes
(2) the cDNA forms the fusion protein HC and LC genes were placed on a single 'tandem vector1 in which the 2 genes were placed in separate and tandem expression cassettes with separate promoters
(3) the promoter driving the expression of the fusion protein HC and LC genes was changed from the human IgG promoters to the cytomegalovirus (CMV) promoter, to enable transfection of non-myeloma cells, such as Chinese hamster ovary (CHO) cells
(4) the tandem vector encoding fusion protein contains a gene encoding for the dihydrofolate reductase (DHFR) gene, under a separate SV40 promoter, to allow for methotrexate (MTX) selection of CHO lines which contain amplification of the genome in the region of the insertion of the expression vector. In order to produce the fusion protein tandem vector, it was first necessary to produce intermediate plasmids, which separately encode cDNA forms of the fusion protein HC and LC genes. Eukaryotic expression plasmids carrying the CMV promoter and the bovine growth hormone (BGH) poly-A (pA) transcription termination sequences, and designated pCD, were digested with Nhel and Xhol and re-ligated with T4 ligase and an Nhel-EcoRV-Kpnl-Scal-BamHI- Xhol linker, as shown in Figure 8. The sequence of the forward and reverse ODNs used to produce this linker are given in Table 3.
[00186] The resulting plasmid, designated pCD-linker (Figure 8) was digested with EcoRV and
BamHI and reclosed with T4 ligase and the fusion protein HC cDNA generated by PCR. For the PCR reaction, the above mentioned myeloma line that had been dual transfected with genomic constructs of the fusion protein HC (clone 416) and LC genes were digested and myeloma derived polyA÷ RNA was produced (part A in Figure 8). Oligodeoxythymidine (ODT) primers were used to produced myeloma cDNA with reverse transcriptase from 0.5 ug of myeloma polyA+RNA, followed by a final RNase digestion. From this cDNA, PCR was used to produce the cDNA form of the fusion protein HC gene, using the forward and reverse primers shown in Table 3, and high fidelity Pfu DNA polymerase. Similarly, the fusion protein LC cDNA was produced by PCR from the myeloma derived cDNA, and the sequences of the forward and reverse PCR primers used to amplify the fusion protein LC cDNA are given in Table 3. Following PCR, the cDNA was applied to an 0.8% agarose gel, and all amplifications yielded a single product, a 1.8 kb fusion protein HC cDNA (lane 1, Figure 3D), and a 0.7 kb fusion protein LC cDNA (lane 2, Figure 3D). The fusion protein HC PCR product was digested with Sspl and BamHI and subckmed into CD-linker to produce the clone 422a (Figure 8), which is an intronless eukaryotic expression plasmid encoding the fusion protein HC cDNA. Clone 422a was analyzed by restriction endonuclease using Nhel; digestion with this enzyme, which has a site in the new multiple cloning region of the pCD vector, produced the expected 0.4 kb fragment corresponding to the fiision protein heavy chain variable region (VH) cDNA (lanes 1-4, Figure 3E). The nucleotide sequence of the fusion protein HC cDNA encoded by clone 422a is shown in Figure 9A, which shows the intron sequences present in clone 416 (Figure 5) have been deleted by the PCR of processed myeloma RNA. The amino acid sequence encoded by the fusion protein HC cDNA is given in Figure 9B, and this ammo acid sequence is identical to that produced by the genomic fragment in clone 416 (Figure 6).
[00187] The fusion protein LC PCR product was digested with EcoRV and Xhol and subcloned into CD-linker to produce the clone 423a (Figure 10), which is an intronless eukaryotic expression plasmid encoding the fusion protein LC cDNA. Clone 423a was analyzed by restriction endonuclease using EcoRV and BamHI; digestion with these enzymes, which have a site in the new multiple cloning region of the pCD vector, produced the expected 0.7 kb fragment corresponding to the fusion protein LC cDNA (lanes 1-5, Figure 3F). The nucleotide sequence of the fusion protein LC cDNA encoded by clone 423a is shown in Figure 1 IA, which shows the intron sequences have
been deleted by the PCR of processed myeloma RNA. The amino acid sequence encoded by the fusion protein LC cDNA is shown in Figure 1 IB.
[00188] Clones 422a and 423a were the precursors to the fusion protein tandem vector, as outlined in Figure 12. In 2 steps, clone 422a was subjected to SDM to introduce an EcoRI site at the 3'-end of the fusion protein HC expression cassette; the sequences of the forward and reverse SDM primers are given in Table 4.
[00189] In step 2, the mutated clone 422a was digested with EcoRI, blunt-ended, and re-ligated with the EcoRI-Hindlll-Notl-Xcal linker to produce clone 422a-I (Figure 12). The sequence of the ODNs used to produce this EcoRI linker are given in Table 4. Clone 422a-I was digested with EcoRI and Hindlll, and closed with T4 ligase in the presence of the fusion protein LC expression cassette to produce clone 422a-II (Figure 12). The fusion protein LC expression cassette was generated by digestion of clone pBS-LC-1 with EcoRI and Hindlll. Clone pB S-LC-I was produced from EcoRV-digested pBS (Bluescript), T4 ligase, and the fusion protein LC expression cassette produced by digestion of clone 423a with Sspl (Figure 12). In parallel, a mouse DHFR expression cassette, containing the SV40 promoter and the hepatitis C virus polyA region, was produced from the pFR400 plasmid (designated pDHFR) by digestion of the plasmid with Smal and Sail (Figure 12). The final fusion protein tandem vector was produced by subcloning the DHFR expression cassette into Xcal digested clone 422a-II followed by closure with T4 ligase (Figure 12). The fusion protein tandem vector was analyzed by restriction endonuclease, and the 11 kb plasmid was linearized by Pvul (lane 1, Figure 3G). The 1.8 kb fusion protein LC and 1.5 kb DHFR expression cassettes, and the 8 kb vector backbone including the fusion protein HC expression
cassette were released by digestion with EcoRI and HindIII (lane 2, Figure 3G). The tandem vector was subjected to DNA sequencing in both directions, and the nucleotide sequence, and the deduced amino acid sequence of the fusion protein HC, the fusion protein LC, and the DHFR genes are shown in Figures 14, 15, and 16, respectively. The calculated MW of the fusion protein HC and LC are 62,220 and 25,760 Da, respectively, not accounting for any carbohydrate content of the fusion protein HC.
Example 2
[00190] Electroporation of CHO cells with fusion protein tandem vector and cultivation in a bioreactor. The fusion protein tandem vector (Figure 12) was linearized with Pvul and electroporated into CHO-KI cells followed by selection with G418 (375 ug/ml) for 3 weeks. Positive clones were detected in 96 well plates with a human IgG ELISA that uses 2 primary antibodies to both the human IgGl HC and the human kappa LC. Cell lines of high copy number of the transgene were selected by graded increases in MTX to 600 nM. The MTX-selected cell line was grown in T 175 flasks and then transferred to a 2OL bioreactor with a 1OL volume of CHO cell serum free medium (SFM). As shown in Figure 17, the CHO cells were maintained at high density in excess of 10 million viable cells/mL for nearly 50 days in perfusion mode in the bioreactor. The secretion by these cells of the fusion protein was detected by ELISA using antibodies to either human IgG or to human BDNF. As shown in Figure 18, the fusion protein is a 1: 1 fusion of the vBDNF to the carboxyl terminus of the HIRMAb heavy chain, which results in formation of the fusion protein heavy chain. This heavy chain complexes with the light chain, as shown in Figure 18. Therefore, the fusion protein should react equally well to 3 antibodies directed against: (i) the human IgGl HC, (ii) the human kappa LC; or (iii) human BDNF. As shown in Figure 19, there is a direct correlation in measurement of the fusion protein in the CHO cell medium depending on whether anti-human IgG or anti-human BDNF antibodies are used in the ELISA. These ELISA results were confirmed with immunocytochemistry (ICC), which showed the CHO cells transfected with TV- 120 were immunoreactive with antibodies to either human IgG or to human BDNF, and that the BDNF immune signal was eliminated by absorption of the anti-BDNF antibody with recombinant BDNF.
Example 3 [00191] Purification and characterization of bioreactor produced fusion protein. The conditioned medium obtained from the bioreactor under perfusion mode was passed through a 1 μm filter, and the medium collected in a 200 L Bioprocess container under sterile conditions, which were maintained at 4°C in a glass door refrigerator contiguous with the bioreactor. Then, 200 L batches of conditioned medium were passed through 1 μm and 0.4 μm pre-filters for the removal of cell debris. The medium was then concentrated with tangential flow filtration (TFF). The TFF system was a Pellicon 2 model from Millipore and was comprised of five 0.5 m2 filtration cassettes
with a 30 kDa molecular weight cutoff and a total surface area of 2.5 m2. A transmembrane gradient of 15 PSI was produced, which results in a reduction in volume of the 200 L to 2 L within 2 hours. The concentrated medium was passed through an 0.22 μ filter prior to elution through 100 mL Prosep A (Millipore) recombinant protein A affinity column. Following application of the sample, the column was washed with buffer A (0.025 M NaCl, 0.025 M Tris, pH=7.4, 3 mM EDTA). The elution of CHO cell host protein (CHOP) was monitored at A280 with a Shimadzu detector. The fusion protein was eluted with 0.1 M citric acid (pH=3) in tubes containing Tris base to cause immediate neutralization to pH 7. The neutralized acid eluate pool was diluted with double distilled water until the conductivity was <7 mS, and the material was applied to a 50 mL Sepharose SP cation exchange column (Amersham) that has been equilibrated with a 0.02 M Tris, pH=7.5. Following washing in the Tris buffer, the residual CHOP was separated from the fusion protein with a linear NaCl gradient from 0 to 1 M NaCl. The fusion protein peak was pooled and buffer exchanged and concentrated with a Millipore diafiltration unit with a 30 kDa molecular weight cutoff. The final concentrated antibody solution was sterile filtered (0.22 μm) and stored at 4°C. The fusion protein was purified to homogeneity on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), as demonstrated in Figure 20. The size of the fusion protein heavy chain was 68 kDa as compared to the size of the HIRMAb heavy chain, which was 54 kDa. The difference between the size of the fusion protein and HIRMAb heavy chains reflects the added vBDNF monomer (14 kDa) fused to each heavy chain of the fusion protein. The fusion protein reacts with both anti-human IgG antibodies and anti-human BDNF antibodies on Western blotting with the expected molecular weight size of the immunoreactive bands (Figure 21). Isoelectric focusing (IEF) shows the isoelectric point (pi) of recombinant BDNF was highly cationic with a pI>10 (Figure 22). The observed pi of the fusion protein was 8.5, and approximates the pi of the HIRMAb (Figure 22). The observed pi of the fusion protein, 8.5, was consistent with the calculated pi, which is 9.04 and 5.27 for the fusion protein HC and LC, respectively (http://scansite.mit.edu/).
Example 4 The fusion protein is bi-functionat and binds with high affinity to both the human insulin receptor and to the human trkB receptor. The affinity of the fusion protein for the HIR extracellular domain (ECD) was determined with a competitive ligand binding assay (CLBA) using the lectin affinity purified HIR ECD. CHO cells transfected with the HIR ECD were grown in serum free media (SFM), and the HIR ECD was purified with a wheat germ agglutinin affinity column. The HIR ECD was plated on Nunc-Maxisorb 96 well dishes and the binding of the murine HIRMAb to the HIR ECD was detected by radioactivity measurements following addition of [125I] murine HIRMAb as the ligand in the binding assay (Figure 23A). The binding of the [125I] murine HIRMAb to the HIR ECD was displaced by the addition of unlabeled fusion protein or HIRMAb as demonstrated in Figure 23B. The CLBA shows comparable binding of the HIRMAb or the fusion protein. A Scatchard analysis using a high affinity and low affinity binding site model and
nonlinear regression analysis was performed to determine the affinity constant of the fusion protein binding to the HIR. Both the fusion protein and the HIRMAb bind equally well to the HIR with a high affinity binding constant, Ki=0.63±0.07 nM (Figure 23B),
[00193] The TrkB CLBA was designed for measurement of the affinity of the fusion protein for recombinant human TrkB ECD. The design of a TrkB CLBA was made difficult by the cationic nature of BDNF, which causes a high degree of nonspecific binding in the assay and this reduces the sensitivity of the assay. The nonspecific binding of BDNF could be eliminated by conjugation of 2000 Da polyethyleneglycol (PEG) to the protein. A bifunctional PEG molecule, biotin-PEG2000- hydrazide (Hz), was commercially obtained, and conjugated to BDNF to produce BDNF-PEG2000- biotin, as outlined in Figure 24A; this molecule was used as the "tracer" in the CLBA. The TrkB ECD was absorbed to ELISA plates and binding of BDNF-PEG2C00-biotin to the TrkB was detected colorimetrically with avidin and biotin peroxidase (Figure 24A). Prior studies showed the ELISA signal (A490) was directly proportional to the amount of TrkB added to the well. In addition, the assay had a very low blank and the A490 was <0.04 when no TrkB is plated. The binding of the BDNF-PEG2000-biotin to the TrkB was competitively displaced by the recombinant BDNF (Figure 24B) or the fusion protein (Figure 24C). The Scatchard analysis of the binding data using nonlinear regression analysis allowed for the computation of the Ki of binding of either BDNF or fusion protein to TrkB, as shown in Figure 24B and 24C, respectively. The affinity of the fusion protein for TrkB was not statistically different from the affinity of the recombinant BDNF (Figure 19 B,C). The nonspecific binding (NSB) of the assay was comparable for either BDNF or the fusion protein. The NSB likely represents nonlinear cooperative binding of the neurotrophin to the TrkB extracellular domain. The TrkB CLBA results shown in Figure 24 indicate the affinity of fusion protein for the TrkB receptor was not changed following fusion of the vBDNF to the carboxyi terminus of the HIRMAb heavy chain.
[00194] Neurotrophins such as BDNF require an obligatory formation of a homo-dimeric structure to be biologically active, and to bind with high affinity to the cognate receptor, e.g. TrkB. A naturally occurring homo-dimeric structure between two BDNF molecules was formed when the neurotrophin was fused to a carboxyi terminus of the CH3 region of an IgG molecule, as illustrated in Figure 18. The surprising observation of the maintenance of the high affinity binding of BDNF for TrkB (Figure 24), despite fusion to the HIRMAb heavy chain (Figure 18), is consistent with the fact that BDNF normally binds to TrkB as a dimer.
Example 5
[00195] Human neural cells subjected to hypoxia are neuroprotcctcd by the fusion protein with equal activity as recombinant BDNF. Human SH-SY5Y neural cells were exposed to 10 uM retinoic acid for 7 days, which induces gene expression of trkB, the BDNF receptor. The cells were then exposed to 16 hours of oxygen deprivation in a sealed chamber, with oxygen sensor. Excitotoxic neural damage was then induced by 4 hours of re-oxygenation (Figure 25A). During
this 4 hour re-oxygenation period, the cells were exposed to either no treatment or equi-molar concentrations of human recombinant BDNF or fusion protein. As shown in Figure 25B5 the fusion protein was equipotent with native human BDNF with respect to inducing neuroprotection in human neural cells exposed to excitoxic ischemia-re-oxygenation.
Example 6
[00196] High affinity binding of fusion protein to human blood-brain barrier insulin receptor in isolated human brain capillaries. Isolated human brain capillaries are used as an in vitro model system of the human BBB (Figure 26A). The fusion protein was radiolabeled with 3 H-N- succinimidyl propionate, and added to the human brain capillaries to establish a radio-receptor assay (RRA) of fusion protein binding to the HIR of the human BBB. [3H]-fusion protein is specifically bound to the BBB, as the binding is self-inhibited by unlabeled fusion protein (Figure 26B). The fusion protein is bound by the insulin receptor of the human BBB, because the murine HIRMAb (mHIRMAb) also inhibits binding of [3H] -fusion protein to the human BBB. The binding data in Figure 26B were fit to a Scatchard plot with a non-linear regression analysis to produce the binding constants: KD= 0.55 ± 0.07 nM, Bmax = 1.35 ± 0.10 pmol/mgp, and NSB = 0.39 ± 0.02 pmol/mgp, where KD is the dissociation constant, Bmax is the maximal binding, and NSB is the non-saturable binding. The KD is <1 nM, which indicate the fusion protein binds the HIR on the human BBB with very high affinity.
Example 7 [00197] Pharmacokinetics and brain uptake of fusion protein by the adult Rhesus monkey.
The fusion protein was tritiated with [3H]-N-succinimidyl propionate to a specific activity of 2.0 μCi/μg. A 5 year old female Rhesus monkey, weighing 5.2 kg, was administered by a single intravenous injection a dose of 746 μCi (373 μg), and serum was collected at multiple time points over a 180 min period. The serum glucose of the anesthetized, overnight-fasted primate was constant throughout the 180 min study period, and averaged 72 ± 2 mg%, which indicates that the administration of the HIRMAb based fusion protein caused no interference of the endogenous insulin receptor, and had no effect on glycemia control. [00198] The serum removed from the anesthetized Rhesus monkey was analyzed for total radioactivity (Figure 27A), and radioactivity that was precipitable by trichloroacetic acid (TCA) (Figure 27B). At 180 minutes after drug injection, the animal was euthanized, and brain radioactivity was analyzed with the capillary depletion method (Figure 27C), similar to prior work on the brain uptake of [125I]-labeled murine HIRMAb in the Rhesus monkey. Based on the specific activity of the [3H]-fusion protein, the brain radioactivity was converted to ng per gram (g) brain, as shown in Figure 27D, and this level was compared to the reported endogenous concentration of BDNF in the adult primate brain.
[00199] The plasma pharmacokinetics analysis (Figure 27A) shows that the fusion protein of the genetically engineered HLRMAb and the BDNF is removed from blood at the same rate as the original murine HIRMAb. This is an important finding, because it shows that the fusion of BDNF, a highly cationic protein, to the HIRMAb does not accelerate the blood clearance of the HIRMAb. Prior work shows that the attachment of the cationic BDNF to a monoclonal antibody greatly accelerates the blood clearance of the antibody, owing to the cationic nature of the BDNF, which greatly enhances hepatic uptake. The work in Figure 27A shows that when the cationic BDNF was re-engineered as an IgG fusion protein, the plasma pharmacokinetics was dominated by the IgG moiety, and that the blood level of the BDNF remains high for a prolonged period.
[00200] The data in Figure 27B show that when BDNF was re-formulated as an IgG fusion protein, the metabolic stability of the neurotrophin in blood was greatly enhanced, as compared to the native BDNF. Owing to its cationic nature, the native BDNF was rapidly removed from blood, and was rapidly degraded into TCA-soluble radioactive metabolites {Figure 27B). However, the TCA- insoluble form of the labeled fusion protein remains high during the 3 hours after an intravenous injection in the primate (Figure 27B). The data in Figures 27A,B show the advantages of re- engineering a neurotrophin pharmaceutical as a fusion protein. The native neurotrophin was rapidly removed from blood and was rapidly degraded. However, the plasma pharmacokinetics profile, and metabolic stability profile, of the neurotrophin resemble those of an IgG molecule, when the IgG- neurotrophm fusion protein was produced.
[00201] Native BDNF is not transported across the BBB. Similarly, a [3H]-mouse IgG2a isotype control antibody was not transported across the BBB in the adult Rhesus monkey, as the brain volume of distribution (VD) of the IgG at 180 minutes after an intravenous injection was equal to the plasma volume, 18 μL/g (Figure 27C, open bars). Conversely, the brain VD of the [3H]-fusion protein exceeds 140 μl/g brain (Figure 27C, closed bars). Capillary depletion analysis separates the brain vasculature from the post-vascular supernatant, and allows detection of the transport of a drug through the BBB and into brain, as opposed to simple sequestration of the drug by the brain vasculature. The brain VD of the post-vascular supernatant of the [3H]-fusion protein was equal to the Vn of the brain homogenate (Figure 27C), which indicates the fusion protein was transported through the BBB and into brain parenchyma.
[00202] The brain VD of the fusion protein was converted into ng fusion protein per gram brain, based on the specific activity of the [3H]-fusion protein, and this allowed for calculation of the total mass of fusion protein in the brain, 24 ± 1 ng/g, as shown in Figure 27D. This value is > 10-fold higher than the endogenous brain concentration of BDNF in the adult primate (45). Therefore, the administration of a dose of 373 μg to a 5.2 kg Rhesus monkey, which is equal to a normalized dose of 72 μg/kg of fusion protein, results in a marked increase in the brain concentration of BDNF. Such an increase in brain BDNF, following intravenous administration, is not possible with native BDNF, because the native BDNF does not cross the BBB. However, when BDNF is re-engineered
in the form of the fusion protein, then pharmacologically active levels of the neurotrophin in brain are achieved (Figure 27D).
[00203] The data shows that: (1) the plasma mean residence time (MRT) of the fusion protein, 312 minutes, was 100-fold greater than the MRT for native BDNF, which was 3.0 minutes, and (2) the systemic clearance of the fusion protein, 0.94 mL/min/kg, was 39-fold slower than the systemic clearance of the BDNF, which was 37 mL/min/kg. In other words, the average blood level of the recombinant protein was up to 100-fold greater when the recombinant protein was re-formulated as an IgG fusion protein. Thus, fusion of the BDNF to the molecular Trojan horse had 2 benefits: (1) the molecular Trojan horse carried the BDNF across the blood-brain barrier (BBB), whereas the BDNF alone cannot cross the BBB, and (2) the molecular Trojan horse prevented the rapid loss from blood of the neurotrophin; BDNF by itself lasts only about 3 minutes in the blood. Both of these properties serve to enhance the pharmacological effect of the BDNF in brain following administration into the blood stream. See, e.g., Table 5.
Example 8 [00204] Neuroprotection in regional brain ischemia by conjugates of BDNF and a BBB molecular Trojan horse. Numerous attempts have been made to develop neuroprotective agents for the treatment of acute stroke. There have been no successes to date because the neuroprotective drugs are either too toxic, in the case of certain small molecules, or ineffective, because the drag does not cross the BBB. BDNF is neuroprotective when injected directly in the brain in parallel with experimental stroke in rodents and regional brain ischemia. The BDNF must be injected across the skull bone into the brain, because this large molecule drug does not cross the BBB. Since the BBB is intact in the early hours after regional brain ischemia, and since BDNF does not cross the BBB, then there is no neuroprotective effect in the ischemic brain following the intravenous administration of BDNF alone. To deliver BDNF across the BBB, the neurotrophin was attached to a mouse MAb to the rat transferrin receptor (TfR). This peptidomimetic MAb carries BDNF across the BBB, and the combined BDNF-MAb conjugate is highly neuroprotective following delayed intravenous administration in experimental stroke, because the BDNF is able to cross the BBB and enter the brain from blood. Once inside the brain, and behind the BBB, the BDNF activates its cognate receptor, trkB, which then induces neuroprotection in ischemic neurons, and stops the apoptotic death cycle. The neuroprotective effect of the BDNF-MAb conjugate demonstrates a dose response effect, a time response effect, and is long-lasting, as the neuroprotection at 7 days is identical to the neuroprotection at 1 day after a single intravenous administration of the BDNF-MAb conjugate. See, e.g., Zhang and Pardridge (2001) Brain Res. 889:49-56, and Zhang and Pardridge (2001) Stroke 32:1378-1374, which are incorporated by reference herein in their entirety. The fusion protein would also be neuroprotective in human stroke, since the BDNF is fused to an MAb to the HIR, which rapidly binds to both the human BBB in vitro, and is rapidly transported across the primate BBB in vivo.
Example 9 [00205] Neuroprotection in global brain ischemia of conjugates of BDNF and a BBB molecular Trojan horse. The direct injection of BDNF into the brain is also neuroprotective in transient forebrain ischemia (TFI), such as might occur after a cardiac arrest. However, intravenous BDNF is not neuroprotective in TFI, because the BDNF does not cross the BBB, and because the BBB is intact in the early hours after TFI, when neuroprotection is still possible. Conversely, intravenous BDNF was neuroprotective in TFI if the BDNF was attached to a mouse MAb against the rat transferrin receptor (TfR), which acts as a molecular Trojan horse to ferry the BDNF across the BBB and into brain. Adult rats were subjected to TFI, which resulted in a flat-line electroencephalogram (EEG) for approximately a 10-minute period. The animals were resuscitated
and then administered 1 of 4 different therapeutics intravenously: (a) buffer, (b) unconjugated BDNF, (c) the receptor specific MAb without the BDNF attached, and (d) the BDNF-MAb conjugate. In the case of the animals treated with saline, unconjugated BDNF, or MAb alone, there was no neuroprotection of pyramidal neurons in the CAl sector of hippocampus. However, in the case of the BDNF-MAb conjugate, there is complete normalization of CAl pyramidal neuron density following delayed intravenous administration. See, e.g., Wu and Pardridge (199), PNAS (USA) 96:254-259, which is incorporated by reference herein in its entirety. This shows that BDNF is strongly neuroprotective in global brain ischemia following delayed intravenous administration, providing the BDNF is attached to a BBB molecular Trojan horse. The recombinant fusion protein of BDNF and a receptor specific MAb could be given following cardiac arrest to prevent permanent brain damage.
Example 10 [00206] BDNF is neuroprotective in brain and spinal cord injury if the neurotrophin can access brain cells. BDNF is neuroprotective in brain injury, providing the neurotrophin is injected directly through the skull bone, because BDNF does not cross the BBB. BDNF is also neuroprotective in brain subjected to excitotoxic injury by neurotoxins, and is neuroprotective in brain infected with the human immune deficiency virus (HIV)-I. BDNF is also neuroprotective in acute spinal cord injury; however, the BDNF must be administered by direct infusion into the spinal canal, because the BDNF does not cross the blood-spinal cord barrier, which is the same as the BBB in the forebrain. In all these cases, the intravenous administration of BDNF would not be neuroprotective, because the BDNF does not cross the BBB, and the BBB is intact in brain injury in the early hours after the injury, when neuroprotection is still possible. Conversely, the BDNF fusion protein would be neuroprotective in these conditions following intravenous administration, because the BDNF is fused to the BBB molecular Trojan horse, and is able to penetrate the brain and spinal cord from the blood following peripheral administration.
Example 11 [00207] BDNF is neuroprotective in chronic neurodegenerative conditions of brain if the neurotrophin can access brain cells. Neurotrophins, such as BDNF can be developed as drugs for peripheral routes of administration, providing the neurotrophin is enabled to cross the BBB. Fusion of BDNF to the chimeric HIRMAb offers a new approach to the non-invasive delivery of BDNF to the brain in humans for the chronic treatment of neurodegenerative disease, including prion diseases, Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), ALS, transverse myelitis, motor neuron disease, Pick's disease, tuberous sclerosis, lysosomal storage disorders, Canavan's disease, Rett's syndrome, spinocerebellar ataxias, Friedreich's ataxia, optic atrophy, and retinal degeneration, and brain aging.
Example 12
[00208] BDNF as a therapeutic in retinal degeneration and blindness. The retina, like the brain, is protected from the blood by the blood-retinal barrier (BRB). The insulin receptor is expressed on both the BBB and the BRB, and the HIRMAb has been shown to deliver therapeutics to the retina via RMT across the BRB (Zhang et al, (2003) MoL Ther. 7: 11-18). BDNF is neuroprotective in retinal degeneration, but it was necessary to inject the neurotrophin directly into the eyeball, because BDNF does not cross the BRB. The fusion protein could be used to treat retinal degeneration and blindness with a route of administration no more invasive than an intravenous or subcutaneous injection, because the HIRMAb would deliver the BDNF across the BRB, so that the neurotrophin would be exposed to retinal neural cells from the blood compartment.
Example 13
[00209] BDNF as a therapeutic for depression. A subset of patients with depression may have a brain deficiency of BDNF, and the correlation of single nucleotide polymorphisms (SNPs) with affective disorders has been reported. The direct injection of BDNF into the brain has durable antidepressant effects in rodent model. The BDNF must be injected directly into the brain, because the neurotrophin does not cross the BBB. The chronic administration of the fusion protein would provide a means for elevating the brain levels of BDNF, and may be therapeutic in those patients with depression and a reduced production of brain BDNF.
Example 14 [00210] Method of manufacturing IgG fusion proteins. The trans fection of a eukaryotic cell line with immunoglobulin G (IgG) genes generally involves the co-transfection of the cell line with separate plasmids encoding the heavy chain (HC) and the light chain (LC) comprising the IgG. In the case of a IgG fusion protein, the gene encoding the recombinant therapeutic protein may be fused to either the HC or LC gene. However, this co-transfection approach makes it difficult to select a cell line that has equally high integration of both the HC and LC-fusion genes, or the HC- fusion and LC genes. The preferred approach to manufacturing the fusion protein is the production of a cell line that is transfected with a single plasmid DNA that contains all the required genes on a single strand of DNA, including the HC-fusion protein gene, the LC gene, the selection gene, e.g. neo, and the amplification gene, e.g. the dihydrofolate reductase gene. As shown in the diagram of the fusion protein tandem vector in Figure 12, the HC-fusion gene, the LC gene, the neo gene, and the DHFR gene are all under the control of separate, but tandem promoters and separate but tandem transcription termination sequences. Therefore, all genes are equally integrated into the host cell genome, including the fusion gene of the therapeutic protein and either the HC or LC IgG gene.
Example 15
[00211] Construction and Use of GDNF-immnnoglobulin fusion proteins. Glial-derived neurotrophic factor (GDNF) is a potent neuroprotective neurotrophin (Lin et al, Science 260:1130- 1132 (1993)). GDNF could be developed as a neuroprotective drug for acute conditions, such as acute ischemic stroke, or chronic conditions, such as Parkinson's disease, or motor neuron disease, or post-stroke neural repair (Lapchak et al, Neuroscience 78:61-72 (1997); Kitagawa et al, Stroke 29:1417-1422 (1998); Bonn, Exp. Neurol. 190:263-275 (2004); Kobayashi, et al. Stroke 37:2361- 2367 (2006)). However, GDNF, like other large molecule drugs, does not cross the blood-brain barrier (BBB) (Kastin et al, Neurosci Lett. 340:239-241(2003)). Owing to the BBB problem, prior human clinical trials with recombinant GDNF delivered the protein to brain via invasive transcranial routes, such as intra-cerebroventricular injection (Lang et al, Ann Neurol. 59:459-466 (2006)) or convention enhanced diffusion (Patel et al, Ann. Neurol. 57:298-302 (2005)). However, both approaches were limited by poor GDNF penetration into the brain, as well as adverse events related to the invasive drug delivery system.
[00212] Large molecule drugs, such as GDNF, could be delivered to brain non-invasively, via the trans-vascular route across the BBB with the use of molecular Trojan horse technology (Pardridge, Pharm. Res. 27:1733-1744 (2007)). A BBB molecular Trojan horse is an endogenous peptide, or receptor-specific peptidomimetic monoclonal antibody (MAb) that undergoes receptor-mediated transport across the BBB via endogenous peptide receptors, such as the transferrin receptor or the insulin receptor. Prior work has developed genetically engineered chimeric or humanized MAb's to the human insulin receptor (HIR), as molecular Trojan horses for the human brain (Boado et al, Biotechnol. Bioeng. 96:381-391 (2007)). The non-transportable protein therapeutic is fused to the heavy chain (HC) of the HIRMAb, It is essential that the genetically engineered fusion protein retain bi-functionality, and both bind with high affinity to the HIR, to cause BBB transport, and bind to the cognate receptor on brain cells, to induce the desired pharmacologic effect, once the fusion protein penetrates the brain.
[00213] The present studies described the genetic engineering of a fusion protein of the chimeric
HIRMAb and human GDNF. The amino terminus of mature human GDNF is fused to the carboxyl terminus of the HC of the HIRMAb (Figure 28). Following expression in COS cells, the bi- functionality of the HIRMAb-GDNF fusion protein is evaluated with receptor binding assays for both the HIR and the human GDNF receptor (GFR)-αl . The GDNF biologic activity of the fusion protein is also evaluated with bio-assays using 2 human neural cell lines, the SH-SY5 Y line, and the SK-N-MC line. Finally, the in vivo neuroprotective effects of the HIRMAb-GDNF fusion protein is demonstrated in rat brain using the middle cerebral artery occlusion model (MCAO) of acute ischemic stroke.
MATERIALS AND METHODS Cloning of GDNF cDNA
[00214] The human prepro GDNF cDNA (GenBank P39905) corresponding to amino acids
Met-"1-Ile211 was cloned by the polymerase chain reaction (PCR) using the oligodexoynucleotides (ODNs) described in Table 6 and cDNA derived from reverse transcription of polyA+RNA isolated from human U87 glial cells. DNA sequence analysis was performed and the nucleic acid and amino acid sequences of the cloned human prepro GDNF are given in SEQ ID NOs :43 and 44, respectively.
[00215] The GDNF cDNA was cloned by PCR using 25 ng polyA+RNA-derived cDNA, 0.2 μM forward and reverse ODN primers (Table 6), 0.2 mM deoxynucleosidetriphosphates, and 2.5 U PMJltra DNA polymerase (Stratagene, San Diego, CA) in a 50 μl Pfu buffer (Stratagene). The amplification was performed in a Mastercycler temperature cycler (Eppendorf, Hamburg, Germany) with an initial denaturing step of 95°C for 2 min followed by 30 cycles of denaturing at 95°C for 30 sec, annealing at 55°C for 30 sec and amplification at 72°C for 1 min; followed by a final incubation at 72°C for 10 min. PCR products were resolved in 0.8% agarose gel electrophoresis, and the expected major single band of -0.6 kb corresponding to the human GDNF cDNA was produced (Figure 29A). The human prepro GDNF cDNA was subcloned into the pcDNA3.1 eukaryotic expression plasmid, and was designated pCD-GDNF. The engineering of this plasmid was validated by DNA sequencing, and by expression of immunoreactive GDNF in COS cells transfected with pCD-GDNF. Engineering of HIRMAb-GDNF expression vector
[00216] For the engineering of the pHIRMAb-GDNF heavy chain (HC) expression plasmid, the mature human GDNF cDNA (SEQ ID NO:51) corresponding to amino acids Ser-78-Ile211 (SEQ ID NO:52) of full length human GDNF was cloned by PCR using the pCD-GDNF as template.
TCACCAGATAAACAAATGGCAGTGCTTCCTAGAAGAGAGCGGAATCGGCAGGCTGCA GCTGCCAACCCAGAGAATTCCAGAGGAAAAGGTCGGAGAGGCCAGAGGGGCAAAAAC CGGGGTTGTGTCTTAACTGCAATACATTTAAATGTCACTGACTTGGGTCTGGGCTATGA Λ Λ rr AAGG AGGAACTGATTTTTAGGTACTGCAGCG^r τrττr;r π Λ TΠΓ Λ r,r TΠ Δ a A Γ A
ACGTACGACAAAATATTGAAAAACTTATCCAGAAATAGAAGGCTGGTGAGTGACAAA GTAGGGCAGGCATGTTGCAGACCCATCGCCTTTGATGATGACCTGTCGTTTTTAGATGA TAACCTGGTTTACCATATTCTAAGAAAGCATTCCGCTAAAAGGTGTGGATGTATCTGA (SEQ ID NO:51)
SPDKQMAVLPRRERNRQAAAANPENSRGKGRRGQRGKNRGCVLTA1HLNVTDLGLGYET KEELIFRYCSGSCDAAETTYDKILKNLSRNRRLVSDKVGQACCRPIAFDDDLSFLDDNLVY HILRKHSAKRCGCI (SEQ ID NO:52) The PCR cloning reaction was performed as described above. The ODNs used for PCR are
5'-phosphorylated for direct insertion into the Hpal site of the pHIRMAb-HC expression plasmid (Figure 29B). The pHIRMAb-HC plasmid encodes the HC of the chimeric HIRMAb, and dual transfection of COS cells with this plasmid and a light chain (LC) expression plasmid, pHIRMAb- LC, allows for transient expression of either the chimeric HIRMAb, or a fusion protein, in COS cells. The mature human GDNF forward (FWD) PCR primer (Table 6) introduces "CA" nucleotides to maintain the open reading frame and to introduce a Ser-Ser linker between the carboxyl terminus of the CH3 region of the HIRMAb HC and the amino terminus of the GDNF. The fusion of the GDNF monomer to the carboxyl terminus of each HC is depicted in Figure 28. This design sterically restricts the GDNF to a dimeric configuration, which replicates the native dimeric conformation of GDNF that binds to the GFRαl (Xu et al, 1998; Eketjall et aL 1999). The GDNF reverse (REV) PCR primer introduces a stop codon, "TGA," immediately after the terminal isoleucine of the mature GDNF protein, and it is identical to the human prepro GDNF REV ODN primer (Table 6) used in the cloning of the human prepro GDNF cDNA. The engineered pHIRMAb-GDNF expression vector was validated by DNA sequencing. The nucleic acid and amino acid sequences for fusion heavy chain (SEQ ID NOs 45 and 46) are as follow:
ATGGACTGGACCTGGAGGGTGTTCTGCCTGCTTGCAGTGGCCCCCGGAGCCCACAGCCAGGTTCAG CTGCAGCAGTCTGGACCTGAGCTGGTGAAGCCTGGGGCTTTAGTGAAGATATCCTGCAAGGCTTCT GGTTACACCTTCACAAACTACGATATACACTGGGTGAAGCAGAGGCCTGGACAGGGACTTGAGTGG ATTGGATGGATTTATCCTGGAGATGGTAGTACTAAGTACAATGAGAAATTCAAGGGCAAGGCCACA CTGACTGCAGACAAATCCTCCAGCACAGCCTACATGCACCTCAGCAGCCTGACTTCTGAGAAATCT GCAGTCTATTTCTGTGCAAGAGAGTGGGCTTACTGGGGCCAAGGGACTCTGGTCACTGTCTCTGCA GCTAGCACCAAGGGCCCATCGGTCTTCCCCCTGGCACCCTCCTCCAAGAGCACCTCTGGGGGCACA GCGGCCCTGGGCTGCCTGGTCAAGGACTACTTCCCCGAACCGGTGACGGTGTCGTGGAACTCAGGC GCCCTGACCAGCGGCGTGCACACCTTCCCGGCTGTCCTACAGTCCTCAGGACTCTACTCCCTCAGC AGCGTGGTGACCGTGCCCTCCAGCAGCTTGGGCACCCAGACCTACATCTGCAACGTGAATCACAAG CCCAGCAACACCAAGGTGGACAAGAAAGTTGAGCCCAAATCTTGTGACAAAACTCACACATGCCCA CCGTGCCCAGCACCTGAACTCCTGGGGGGACCGTCAGTCTTCCTCTTCCCCCCAAAACCCAAGGAC ACCCTCATGATCTCCCGGACCCCTGAGGTCACATGCGTGGTGGTGGACGTGAGCCACGAAGACCCT
GAGGTCAAGTTCAACTGGTACGTGGACGGCGTGGAGGTGCATAATGCCAAGACAAAGCCGCGGGAG GAGCAGTACAACAGCACGTACCGTGTGGTCAGCGTCCTCACCGTCCTGCACCAGGACTGGCTGAAT GGCAAGGAGTACAAGTGCAAGGTCTCCAACAAAGCCCTCCCAGCCCCCATCGAGAAAACCATCTCC AAAGCCAAAGGGCAGCCCCGAGAACCACAGGTGTACACCCTGCCCCCATCCCGGGATGAGCTGACC AAGAACCAGGTCAGCCTGACCTGCCTGGTCAAAGGCTTCTATCCCAGCGACATCGCCGTGGAGTGG GAGAGCAATGGGCAGCCGGAGAACAACTACAAGACCACGCCTCCCGTGCTGGACTCCGACGGCTCC TTCTTCCTCTACAGCAAGCTCACCGTGGACAAGAGCAGGTGGCAGCAGGGGAACGTCTTCTCATGC TCCGTGATGCATGAGGCTCTGCACAACCACTACACGCAGAAGAGCCTCTCCCTGTCTCCGGGTAAA AGTTCATCACCAGATAAACAAATGGCAGTGCTTCCTAGAAGAGAGCGGAATCGGCAGGCTGCAGCT GCCAACCCAGAGAATTCCAGAGGAAAAGGTCGGAGAGGCCAGAGGGGCAAAAACCGGGGTTGTGTC TTAACTGCAATACATTTAAATGTCACTGACTTGGGTCTGGGCTATGAAACCAAGGAGGAACTGATT TTTAGGTACTGCAGCGGCTCTTGCGATGCAGCTGAGACAACGTACGACAAAATATTGAAAAACTTA TCCAGAAATAGAAGGCTGGTGAGTGACAAAGTAGGGCAGGCATGTTGCAGACCCATCGCCTTTGAT GATGACCTGTCGTTTTTAGATGATAACCTGGTTTACCATATTCTAAGAAAGCATTCCGCTAAAAGG TGTGGATGTATCTGA (SEQ ID NO:45)
MDWTWRVFCLLAVAPGAHSQVQLQQSGPELVKPGAL VKISCKASGYTFTNYDIHWVKQRPGQGLEW IGWIYPGDGSTKYNE KFKGKATLTAD KSSSTAYMHLSSLTSEKSAVYFCAREWAYWGQGTLVTVSA ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLS SVVTVPSSSLGTQTYICNVNHKPSNTKVDK-CVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKD TI^ISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRWSVLTVLHQDWLN GKEYKCKVSNKALPAPIΞKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEW ESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK SSSPDKQMAVLPRRERNRQAAAANPENSRGKGRRGQRGKNRGCVLTAIHLNVTDLGLGYETKEELI FRYCSGSCDAAETTYDKILKNLSRNRRLVSDKVGQACCRPIAFDDDLSFLDDNLVYHILRKHSAKR CGCI (SEQ ID NO:46). Also shown in Figure 36.
[00218] The HIRMAb HC and LC cDNA expression cassettes are driven by the cytomegalovirus
(CMV) promoter and contain the bovine growth hormone (BGH) polyadenylation (pA) sequence (Figure 29B). The engineering of the universal pHIRMAb-HC vector was performed by insertion of a single Hpal site at the end of the HIRMAb HC CH3 open reading frame (orf) by site directed mutagenesis (SDM). Transient expression of HIRMAb-GDNF fusion protein in COS cells
[00219] COS cells were dual transfected with pHIRMAb-LC and pHIRM Ab-HC-GDNF using
Lipofectamine 2000, with a ratio of 1:2.5, ug DNA:uL Lipofectamine. Following transfection, the cells were cultured in serum-free VP-SFM (Invitrogen, Carlsbad, CA). The conditioned serum free medium was collected at 3 and 7 days. The fusion protein was purified by protein A affinity chromatography.
Human IgG and GDNF ELISAs
[00220] Human IgG ELISA was performed in Immulon 2 high binding plates (Dynex Tech.,
Chantilly, VA) with COS cell conditioned medium. A goat anti-human IgG primary antibody (Zymed-Invitrogen, Carlsbad, CA) was plated in 0.1 M NaHCO3 (100 μl, 2 μg/ml) and incubated for overnight at 4C. Plates were washed 0.01 M Na2HPO4/0.15 M NaCl/ρH=7.4/0.05% Tween-20 (PBST), and blocked with 1% gelatin in PBST for 30 min at 22°C. Plates were incubated with 100 μL/well of either human IgGl standard or the fusion protein for 60 minutes at room temperature (RT). After washing with PBST, a goat anti-human kappa LC antibody conjugated to alkaline phosphatase (Sigma Chemical Co., St. Louis, MO) was plated for 60 min at 37°C. Color development was performed with p-nitrophenyl phosphate (Sigma) at pH=10.4 in the dark. The reaction was stopped with NaOH, and absorbance at 405 nm was measured in a BioRad ELISA plate reader. Human GDNF immunoreactivity in COS cell conditioned medium was measured with the double antibody sandwich GDNF Emax Immunoassay System kit from Promega (Madison, WI). SDS-PAGE and Western blotting
[00221] The homogeneity of protein A purified fusion protein produced by COS cells was evaluated with a reducing 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS- PAGE), followed by Coomasie Blue staining. For Western blotting, immunoreactivity was tested with a primary rabbit antibody to human GDNF (Santa Cruz Biotechnology, Santa Cruz, CA) or a primary goat antiserum against human IgG heavy and light chains (Vector Labs, Burlingame, CA). HIR receptor assay
[00222] The affinity of the fusion protein for the HIR extracellular domain (ECD) was determined with an ELISA using the lectin affinity purified HIR ECD. CHO cells transfected with the HIR ECD were grown in serum free media (SFM), and the HIR ECD was purified with a wheat germ agglutinin affinity column. The HIR ECD (0.2 μg/well) was plated on Immulon 2 high binding 96- well plates, and the binding of the chimeric HfRMAb, the HIRMAb-GDNF fusion protein, or human IgGl to the HIR ECD was detected with a biotinylated goat anti-human IgG (H+L) antibody (0.3 μg/well), and the ABC Elite detection system (Vector Labs). The concentration that caused 50% binding to the HIR ECD, the ED50, was determined by non-linear regression analysis using the WinNonlin software. GFRαl receptor assay
[00223] Binding of recombinant GDNF (Peproαtech, Rocky Hill, NJ), the chimeric HIRMAb, or the HIRMAb-GDNF fusion protein to the ECD of recombinant human GFRαl was measured with an ELISA. A mouse anti-human (MAH) IgG (Zymed-Invitrogen) was plated in 96-well plates overnight at 2 μg/well. Following aspiration, washing, and blocking with 1% bovine serum albumin (BSA), the Fc fusion of the human GFRαl ECD (R&D Systems, Minneapolis, MN) was plated at 0.4 μg/well. Following an incubation at room temperature (RT) for 60 min, the wells were washed with PBST, and the GDNF, antibody, or fusion protein was plated for 2 hours at RT.
Following washing in PBST, a goat anti-GDNF antibody (R&D Systems) was plated at 0.4 ug/well for 30 min at RT. Following washing in PBST, a conjugate of alkaline phosphatase (AP) and a rabbit anti-goat (RAG) IgG(H+L) (Vector Labs) was plated and detection at 405 nm was performed with an ELISA plate reader after color development with para-nitrophenylphosphate (Sigma Chemical Co.). SK-N-MC bio-assay
[00224] Human neural SK-N-MC cells were dual transfected with the c-ret kinase and a luciferase reporter plasmid under the influence of the 5'-flanking sequence (FS) of the rat tyrosine hydroxylase (TH) gene. The cells were grown in collagen coated 24-well dishes to 70% confluency in Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) and 400 μg/mL G418. To begin the assay, the medium was aspirated, and 400 μL/well of fresh DMEM/ 10% FBS was added along with either recombinant GDNF or fusion protein. After a 24 hour incubation at 37°C, the wells were aspirated, and the cells extracted with 200 μL/well of Luciferase Reporter Lysis buffer (Promega, Madison, WI). Following centrifugation, luciferase enzyme activity was measured in the Iysate with a luminometer, and luciferase enzyme activity, reported as picogram (pg) of luciferase, was normalized per mg sample protein using the bicinchoninic acid (BCA) protein assay (Pierce Chemical Co., Rockford, IL). SH-SY5Y bio-assay
[00225] Human neural SH-SY5Y cells were plated in 96 well plates in DMEM/Ham F12 (1 : 1) in
10% FBS and grown until the cells reached 70% confluency. The medium was supplemented with 10 μM retinoic acid to induce differentiation of the cells over a 10 day period. After cell differentiation, the medium was changed, and supplemented with either GDNF or HIRMAb-GDNF fusion protein at zero time, and cell proliferation was measured over the 5 days with the CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (Promega), which uses the tetrazolium compound, (3-(4,5-dimethylthiazol-2-yI)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H- tetrazolium, inner salt; MTS), and the electron coupling reagent, phenazine methosulfate (PMS). Absorbance at 490 nm is a measure of formazan production, which is proportional to the number of viable cells. Middle cerebral artery occlusion model
[00226] The permanent middle cerebral artery occlusion (MCAO) model was performed as described previously in adult Sprague-Dawley rats (Zhang and Pardridge, Brain Res. 889:49-56 (2001)). The right middle cerebral artery of the anesthetized rat was occluded by insertion of an intra-luminal 4-0 nylon suture; the suture was coated in 0.1% poly-L-lysine and dried at 60°C for 1 hour. Within 15 minutes of the occlusion, the rat was treated with an intra-cerebral injection of either HIRMAb-GDNF fusion protein (130 μg in l0 μL) or an equal volume of saline. Since the fusion protein is 17% GDNF, the dose of fusion protein is equivalent to a dose of GDNF of 22 μg, which is a dose that causes pharmacological effects in the brain (Sullivan et al, 1998). The drug was injected into the brain under stereotaxic guidance with the following coordinates: 0.2 mm posterior
to bregma, 4.0 mm lateral to the midline, and 5.0 mm deep from the dural surface. The arterial nylon suture was left in place for permanent occlusion of the middle cerebral artery. A total of 20 rats were used for the study; 9 rats (5 treated with saline and 4 treated with HIRMAb-GDNF) expired prematurely and were excluded from the study. The rats were allowed to recover, and were euthanized 24 hours later, and coronal sections of brain were prepared with a rat brain matrix (ASI Instruments, Warren, MI), followed by staining with 2% 2,3,5-triphenyltetrazolium chloride (TTC), as described previously (Zhang and Pardridge, Brain Res. 889:49-56 (2001)).TTC stains healthy brain red, and infarcted brain is colorless. The stained brain sections were scanned on a UMAX PowerLook II flatbed scanner, and the area of the hemispheric infarct volumes was determined with the N1H Image software. The volume of the infarct was computed from the area of the infarcted zone and length (2 mm) of the coronal slices of brain hemisphere (Zhang and Pardridge, Brain Res. 889:49-56 (2001)). The scanned color image was converted to an inverted grayscale image in Photoshop, so that infarcted brain appears black and healthy brain appears white.
[00227] The neurologic deficit was determined 24 hours after the occlusion, as described previously (Zhang and Pardridge, Stroke 32:1378-1384 (200I)), and scored as follows: 0, no deficit; 1, failure to extend contralateral forepaw fully; 2, decreased grip of contralateral forelimb while tail is pulled; 3, spontaneous circling to left; 4, walks only when stimulated with decreased level of consciousness. RESULTS
[00228] DNA sequencing of the expression cassette of the pCD-GDNF encompassed 1,752 nucleotides (nt), including a 715 nt CMV promoter, a 636 nt prepro GDNF open reading frame, and a 401 nt BGH sequence, which predicted a 211 amino acid human preproGDNF protein, including a 19 amino acid signal peptide with 100% identity with the known sequence for human GDNF (P39905). Transfection of COS cells with pCD-GDNF resulted in high levels of immunoreactive GDNF the medium at 3 and 7 days following transfection.
[00229] The cDNA corresponding to the 134 amino acid mature GDNF was amplified by PCR using the ODNs in Table 6 and the pCD-GDNF as template, and this cDNA was subcloned into the Hpal site of the pHIRM Ab-HC plasmid, as outlined in Figure 29B. DNA sequencing of the expression cassette of the pHIRMAb-GDNF plasmid encompassed 2,890 nt, including a 714 nt CMV promoter, a 9 nt full Kozak site (GCCGCCACC), a 1,797 nt HIRMAb HC-GDNF fusion protein open reading frame, and a 370 nt BGH sequence. The plasmid encoded for a 598 amino acid protein, comprised of a 19 amino acid IgG signal peptide, the 443 amino acid HIRMAb HC, a 2 amino acid linker (Ser-Ser), and the 134 amino acid human GDNF minus the signal peptide or propeptide. The predicted molecular weight of the heavy chain fusion protein, minus glycosylation, is 63,860 Da, with a predicted isoelectric point (pi) of 9.03.
[00230] Dual transfection of COS cells with the pHIRMAb-GDNF and the pHIRM Ab-LC resulted in medium human IgG levels of 2.0 μg/mL, as determined with a human Fc specific ELISA. The level of immunoreactive GDNF in the medium was measured with a GDNF-specific ELISA. The
data from both the human IgG and human GDNF ELISAs are given in Table 7, and the medium levels are expressed as nM concentrations to allow for comparison of the 2 assays.
[00231] As shown in Table 7, the concentration of the HIRMAb-GDNF fusion protein the medium was the same irrespective of whether the human IgG or GDNF ELISA is used.
[00232] The HIRMAb-GDNF fusion protein was purified by protein A affinity chromatography.
Following SDS-PAGE and Coomasie blue staining, the size of the light chain (LC) was the same for both the HIRMAb and the HIRMAb-GDNF fusion protein (Figure 30). The size of the heavy chain (HC) of the fusion protein was about 15 kDa larger than the HC of the HIRMAb (Figure 30). On Western blotting, the LC of either the HIRMAb or the HIRMAb-GDNF fusion protein reacted equally on the Western with a primary antibody directed against the human IgG (H+L), as shown in Figure 31. The size of the HC of the fusion protein was about 15 kDa larger than the size of the HC of the HIRMAb on both Western blots using either the anti-human IgG primary antibody (Figure 31) or the anti-human GDNF primary antibody (Figure 31). The anti-GDNF primary antibody reacts with the HC of the fusion protein, and with recombinant GDNF, but does not react with the HIRMAb (Figure 31).
[00233] The affinity of the fusion protein for the HIR extracellular domain (ECD) was determined with a Iigand binding assay using lectin affinity purified HIR ECD (Methods). There is comparable binding of either the chimeric HlRMAb or the HIRMAb-GDNF fusion protein for the HIR ECD with ED50 of 0.50±0.11 nM and 0.87±0.14 nM, respectively (Figure 32).
[00234] The affinity of either GDNF or the HIRMAb-GDNF fusion protein for the ECD of the human GFRαl was measured with an ELISA, which is outlined in Figure 33A. Human GDNF bound to the GFRαl with an ED50 of 1.03±0.18 nM (Figure 33B, top panel). The affinity of the HIRMAb-GDNF fusion protein for the GFRαl was also high, with an ED50 of 1 ,68±0.17 nM (Figure 33B, bottom panel). The biologic activity of GDNF or the HIRMAb-GDNF fusion protein was also evaluated with bio-assays in human neural cell lines. The human neural SK-N-MC cell line was dual transfected with the c-ret kinase, and with a luciferase expression plasmid under the
influence of the 5 '-flanking sequence (FS) of the rat TH promoter. Addition of GDNF to the medium activates the TH promoter via activation of the GFRαl/c-ret kinase system, and this leads to increased luciferase expression (Figure 34A). GDNF increased luciferase expression with an ED50 of 1.03±0.31 nM, and the HIRMAb-GDNF fusion protein also increased luciferase expression with an ED50 of 1.68±0.45 nM (Figure 34B). Both GDNF and the HIRMAb-GDNF fusion protein activated cell division of human neural SH-S Y5 Y cells following retinoic acid differentiation, and both proteins increased cell division about 50% over a 5 day incubation incubation period (Table 8).
Mean ± SE (n=6); a: p<0.005. Cell proliferation measured with the MTS assay as described herein.
[00235] The intra-cerebral injection of the HIRMAb-GDNF fusion protein caused a 77% reduction in hemispheric stroke volume, from 331 ± 33 mm3 to 77 ± 22 mm3 (mean ± SE, n=5-6 rats per group), which was significant at the pO.OOl level (Student's t-test), as shown in Figure 35A. Similarly, the cortical stroke volume was reduced 86% from 241 ± 26 mm3 to 34 ± 9 mm3 (ρ<0.001), and the sub-cortical stroke volume was reduced 52% from 90 ± 10 mm3 to 43 ± 16 mm3 (p<0.025), as shown in Figure 35 A. A representative TTC stain of the brain 24 hours after permanent MCAO and intra-cerebral injection of either saline or the HIRMAb-GDNF fusion protein is shown in Figures 35B and 35C, respectively. The neurologic deficit score was 3.0 ± 0.7 and 1.3 ± 0.6 (mean ± SE, n=5-6 rats per group) in the saline treated and HTRMAb-GDNF treated rats, respectively, and this improvement in neurologic deficit was significant at the p<0.05 level (Student's t-test). DISCUSSION
[00236] The results of this study were consistent with the following conclusions. First, a bi- functional IgG-GDNF fusion protein was genetically engineered, wherein mature human GDNF was fused to the carboxyl terminus of the heavy chain (HC) of a chimeric HIRMAb (Figure 28), and expressed and secreted in COS cells (Table 8, Figures 30 and 31). Second, the HIRMAb-GDNF fusion protein was bi-functional and bound the HIR and human GFRαl with high affinity (Figures 32 and 33). Third, the HIRMAb-GDNF fusion protein had activity in bio-assays of human neural cells comparable to recombinant human GDNF (Figure 34, Table 8). Fourth, the HIRMAb-GDNF
was neuroprotective in rat brain in vivo in the permanent MCAO stroke model, and caused a marked reduction in stroke volume (Figure 35).
[00237] GDNF is a member of the transforming growth factor (TGF)-α gene family, along with other neurotrophins, such as neuturin, persephin, or artemin. GDNF reacts with its cognate receptor, GFRαI, as a homo-dimer (Eketjall et al, 1999). Therefore, the GDNF fusion construct described in this work (Figure 28) places GDNF in a dimeric configuration that mimicked the conformation of the neurotrophin at the receptor. The prepro GDNF could be fused to the amino terminus of the HIRMAb HC. However, this would interfere with HIRMAb binding to the HIR, which would impair transport of the fusion protein across the BBB via the insulin receptor. Moreover, the mature GDNF protein folded into a biologically active conformation, despite the absence of the prepro GDNF peptide in the fusion protein described in this work. The goal of fusion protein engineering was to retain the bi-functional characteristics of the fusion construct, and this was accomplished in the case of the HIRMAb-GDNF fusion protein.
[00238] The fusion protein was secreted in high amounts to the medium by COS cells co- transfected with the HC expression plasmid, pHIRMAb-GDNF (Figure 29B), and the LC expression plasmid, pHIRMAb-LC (Table 7). The HC of the fusion protein is about 15 kDa larger than the HC of the chimeric HIRMAb, based on either SDS-PAGE gels with Coomassie blue staining (Figure 30), or on Western blot analysis using primary antibodies to either human IgG or human GDNF (Figure 31). The anti-human IgG antibody reacts equally with the LC of the fusion protein or the chimeric HIRMAb, since both proteins use the same LC. Both the HIRMAb-GDNF fusion protein and the HIRMAb bind with high affinity to the HIR, and with comparable affinity (Figure 32).
[00239] The HIRMAb-GDNF fusion protein retains high affinity binding to the human GFRα 1 receptor, and the affinity constant of binding is comparable to that of recombinant GDNF (Figure 33B). The high affinity binding of the HIRMAb-GDNF fusion protein to the GFRαl was translated into biological activity in two different human neural cell lines. The SH-SH5Y cell line expresses the GFRαl 1, but does not express the c-ret kinase in the undifferentiated state (Xiao et al, J. Neurochem. 82:701-808 (2002)). Both the GFRαl and the c-ret kinase must be expressed to enable GDNF activation of a neural cell (Cik et al, 2000). However, differentiation of SH-SH5Y cells by retinoic acid causes an induction of the expression of the c-ret kinase (Xiao et al, J. Neurochem. 82:701-808 (2002)). Following differentiation by retinoic acid, the SH-S Y5 Y cells demonstrate enhanced proliferation hi response to either recombinant human GDNF or the HIRMAb-GDNF fusion protein (Table 8). The SK-N-MC human neural cell line also expresses the GFRαl receptor, but not the c-ret kinase (Hirata and Kiuchi, Brain Res. 983:1-12 (2003)). However, following co-transfection of these neural cells with a c-ret kinase cDNA and a plasmid encoding luciferase under the influence of 2 kb of the 5'-FS of the rat TH promoter, these cells respond to GDNF (Tanaka et al, Brain res. Brain Res. Protoc. 11 : 119-122 (2003)). As outlined in Figure 34A, the extracellular GDNF binds the GFRαl, which activates the c-ret kinase, which
induces a signal transduction cascade leading to activation of the TH promoter. Accordingly, both recombinant GDNF and the HIRMAb-GDNF fusion protein caused an approximate 500% increase in cellular luciferase enzyme activity. The GDNF ED50 in the luciferase assay is 1.0 ± 0.1 nM, and the HIRMAb-GDNF ED50 in this assay is 1.7 ± 0.5 nM (Figure 34B). These ED50 values are identical to the ED50 of saturable binding of either GDNF or the HIRMAb-GDNF fusion protein in the GFRαl binding assay (Figure 33B). This correlation indicates that the rate-limiting step in activation of neural pathways by GDNF is binding to the cell membrane GFRαl receptor.
[00240] The intracerebral injection of the HIRMAb-GDNF fusion protein in rat brain following permanent middle cerebral artery occlusion (MCAO) resulted in a 77% reduction in hemispheric stroke volume from 331 ± 33 mm3 to 77 ± 22 mm3 (p<0.001), as shown in Figure 35A. The neuroprotection was both cortical and sub-cortical, as the cortical stroke volume was reduced 86% from 241 ± 26 mm3 to 34 ± 9 mm3 (p<0.001), and the sub-cortical stroke volume is reduced 52% from 90 ± 10 mm3 to 43 ± 16 mm3 (ρ<0.025) (Figure 35A). A representative TTC stain of the rat brain at 24 hours after the permanent MCAO is shown in Figure 35B, for the saline treated rat, and in Figure 35C for the HIRMAb-GDNF fusion protein treated rat. The reduction in stroke volume was correlated with a functional improvement as the neurologic deficit was reduced from 3.0 ± 0,3 to 1.3 ± 0.6 (p<0.05) in the rats treated with the HIRMAb-GDNF fusion protein as compared to the saline treated rats. These findings of in vivo neuroprotection of the HIRMAb-GDNF fusion protein correlated with the tissue culture bio-assays (Table 8, Figure 34B), and confirmed the neuroprotective effects of the HIRMAb-GDNF fusion protein on neural cells in brain. The MCAO findings confirmed prior work showing the beneficial effects of intra-cerebral GDNF in acute stroke (Kitagawa et al, 1998). Recent work showed that chronic treatment of the brain with intra- cerebral GDNF may promote striatal neurogenesis following stroke (Kobayashi et al, Stroke 37:2361-2367 (2007)). Irrespective of whether the brain is treated acutely or chronically with GDNF, the neurotrophin had to be re-engineered to cross the BBB before clinical trials in human stroke can be initiated.
[00241] The HIRMAb-GDNF fusion protein could not be delivered to rat brain in the MCAO model following intravenous administration, because the HIRMAb part of the fusion protein is not reactive with the rodent insulin receptor. However, the HIRMAb is active in Old World primates, such as the Rhesus monkey (Pardridge et al, Pharm. Res. 12:807-816 (1995)). Recent work has shown that fusion proteins of the HIRMAb and brain-derived neurotrophic factor (Boado et al, Biotechnol. Bioeng. 97:1376-1386 (2007)), a single chain Fv antibody (Boado et al, Bioconjug Chem. 18:447-455 (2007)), or iduronidase, a lysosomal enzyme (Boado et al, Biotechnol Bioeng.99: 475-484 (2008)), all are rapidly transported across the Rhesus monkey BBB in vivo. In the primate brain, the uptake of the fusion protein is approximately 1% of the injected dose (ID) (Boado et al, Biotechnol Bioeng. 99: 474-484 (2008)),. Since the weight of the human brain is about 10-fold greater than the weight of the Rhesus monkey brain, it is expected that the brain uptake of the HIRMAb-GDNF fusion protein by the human brain will be about 0.1% of the ID.
Given an injection dose of 25 mg, the expected brain concentration of the fusion protein is about 25 ng/gram human brain, which is equivalent to 5 ng/gram of GDNF, since the fusion protein is about 20% GDNF and 80% HIRMAb. The concentration of GDNF in the human brain is 0.2-1 ng/gram (Wiesenhofer et al, Acta Neuropathol (Berl) 99:131-137 (2000)). Therefore, the dosing of 25 mg of the HIRMAb-GDNF fusion protein to a 60 kg human could result in pharmacologically significant increase in GDNF in the brain. In experimental Parkinson's disease, pharmacological effects are achieved with just a 3-fold increase in brain GDNF concentration (Eslamboli et al, J. Neurosci. 25:769-777 (2005)). Aberrant neuronal sprouting is induced when the brain GDNF concentration is increased > 100-fold (Eslamboli et al, J. Neurosci. 25:769-777 (2005)). ). However, such large increases in brain GDNF will not be produced by therapeutic dosing of the HIRMAb-GDNF fusion protein in humans.
[00242] In conclusion, these studies described the genetic engineering, transient expression in COS cells, and validation of a HIRMAb-GDNF fusion protein, which represents a re-engineering of this neurotrophin to enable transport across the human BBB in vivo. The fusion protein can be administered by systemic injection to humans for treatment of multiple neurologic disorders, including stroke, neural repair, Parkinson's disease, or motor neuron disease. Example 16 Expression of HIRMAb-GDNF fusion protein following stable transfection of CHO cells
[00243] A tandem vector (TV) encoding the HIRMAb-GDNF fusion protein was engineered as shown in Figure 37, and is designated HIRMAb-GDNF TV, The TV contains on a single piece of DNA the fusion heavy chain (HC), the light chain (LC), and the gene for DHFR. DNA sequencing of the entire 6,300+ nucleotides (nt) of the HIRMAb-GDNF TV using custom oligodeoxynucleotides (ODNs) showed the sequence was comprised of 6,342 nt (SEQ ID NO. 47), which included the following domains:
• 731 nt cytomegalovirus (CMV) promoter
• 9 nt Kozak sequence (GCCGCCACC)
• 705 nt open reading frame (orf) encoding the HIRMAb LC
• 291 nt bovine growth hormone (BGH) polyA (pA) sequence
• 23 nt linker
• 714 nt CMV promoter
• 9 nt Kozak sequence
• 1 ,797 ORF encoding the fusion gene of the HIRMAb HC and GDNF
• 296 nt BGH pA
• 254 SV40 promoter
• 9 nt Kozak sequence
• 564 murine DHFR orf
• 940 hepatitis B virus (HBV) pA
[00244] The HIRMAb-GDNF TV also included the expression cassette encoding neo, the neomycin resistance gene, to enable selection with G418 (Figure 37). It was necessary to include the HC fusion gene, the LC gene, and the DHFR gene on a single piece of DNA, or tandem vector (Figure 37) to allow for equally high expression of all 3 genes in the transfected CHO cell.
[00245] The HIRMAb-GDNF TV sequence encoded for a 579 amino acid (AA) HC fusion protein
(SEQ ID NO 48), which was comprised of a 19 AA IgG signal peptide, the 443 AA HIRMAb HC, a 2 AA linker, and the 134 AA human GDNF. The predicted molecular weight (MW) of the non- glycosylated HC was 63,860 Daltons (Da) and the predicted isolectric point (pi) of the fusion HC protein was 9.03. The HIRMAb-GDNF TV sequence encoded for a 234 AA LC protein (SEQ ID NO 49), which was comprised of a 20 AA IgG signal peptide, and the 214 AA HIRMAb LC. The predicted MW of the LC was 23,398 Da and the predicted pi of the LC protein was 5.45. The HIRMAb-GDNF TV sequence encoded for a DHFR protein (SEQ ID NO 50), that had an AA sequence 100% identical with the known AA sequence of murine DHFR. The domain structure of the HC is shown in Figure 36 and the domain structure of the LC is shown in Figure 38. Electroporation of CHO cells.
[00246] DG44 CHO cells were grown in serum-free medium (SFM), containing IX HT supplement (hypoxanthine and thymidine). DG44 CHO cells (5 x 106 viable cells) were electroporated with 5 μg Pvul-linearized TV plasmid DNA. The cell-DNA suspension is then incubated for 10 min on ice. Cells are electroporated with pre-set protocol for CHO cells, i.e., square wave with pulse of 15 msec and 160 volts. After electroporation, cells are incubated for 10 min on ice. The cell suspension is transferred to 50 ml culture medium and plated at 125 μl per well in 40 x 96-well plates (10,000 cells per well), and 4,000 wells per study. Selection and amplification with methotrexate and screening IgG ELISA.
[00247] Following electroporation (EP), the CHO cells were placed in the incubator at 37 °C and
8% CO2. Owing to the presence of the neo gen in the TV, transfected cell lines are initially selected with G418. The TV also contains the gene for DHFR, so the transfected cells were also selected with 20 nM methotrexate (MTX) and HT-deficient medium. Once visible colonies are detected at about 21 days after EP, the conditioned medium was sampled for human IgG by ELISA, Plates were removed from the incubator and transferred to the sterile hood where 100 μL samples were taken from each well using a Precision Pipettor system and transferred into a sterile 96-well tissue culture plate, which was then used for the human IgG ELISA. The media taken from the EP plates for the ELISA was replaced with 100 μL of SFM and cells were returned to the incubator at 37 °C and 8% CO2. Wells with high human IgG signals in the ELISA were transferred from the 96-well plate to a 24-well plate with 1 mL of SFM. The 24-well plates were returned to the incubator at 37 °C and 8% CO2. The following week IgG ELISA was performed on the clones in the 24-well plates. This was repeated through the 6- well plates to T75 flasks and finally to 60 mL and 125 mL square plastic bottles on an orbital shaker. After the cells adapted to the 60 mL bottle on the orbital shaker at 120RPM they were transferred into a 125 mL plastic square bottle with 12 mL of SFM.
At this stage, the final MTX concentration was 80 nM, and the medium IgG concentration, which is a measure of HIRMAb-GDNF fusion protein in the medium was >10 mg/L at a cell density of 106/mL. Dilutional cloning of CHO cells.
[00248] Clones selected for dilutional cloning (DC) were removed from the orbital shaker in the incubator and transferred to the sterile hood. The cells were diluted to 500 mL in F-12K medium with 5% dialyzed fetal bovine serum (d-FBS) and penicillin/streptomycin, and the final dilution was 8 cells per mL, so that 4,000 wells in 40 x 96-well plates were plated at a cell density of 1 cell per well (CPW). After the cell suspension was prepared, within the sterile hood, a 125μL aliquot was dispensed into each well of a 96-well plate using an 8-channel pipettor or a Precision Pipettor system. The plates were then returned to the incubator at 37 °C and 8% CO2. The cells diluted to 1 cell/well cannot survive without serum. On day 6 or 7, DC plates were removed from the incubator and transferred to the sterile hood where 125 μL of F-12K medium with 5% dialyzed fetal bovine serum (d-FBS) was added to each well. After this step, the selection media contained 5% d-FBS, 30 nM MTX and 0.25 mg/mL Geneticin. On day 21 after the initial 1 CPW plating, aliquots from each of the 4,000 wells were removed for human IgG ELISA, using robotics equipment. DC plates were removed from the incubator and transferred to the sterile hood, where 100 μL of media was removed per well of the 96-well plate and transferred into a new, sterile sample 96-well plate using an 8-channel pipettor or a Precision Pipettor system. ELISA screening of 4,000 wells.
[00249] On day 20 after the initial 1 CPW plating, 44 96-well Immunoassay plates were plated with 100 μL of 1 μg/mL solution of Primary antibody, a mouse anti-human IgG in 0. IM NaHCO3. Plates were incubated overnight in the 4 °C Revco refrigerator. The following day, the plates were washed with Ix TBST 5 times, and 100 μL of 1 μg/mL solution of secondary antibody and blocking buffer were added. Immulon plates were washed with Ix TBST 5 times. 100 μL of lmg/mL of 4-nitrophenyl phosphate di(2-amino-2-ethyl-1,3-propanediol) salt in 0.1M glycine buffer were added to the Immulon 96-well Immunoassay plates using the BioTek uFill. Plates were read on a microplate reader. The assay produced IgG output data for 4,000 wells/experiment. The highest producing 24-48 wells were selected for further propagation. Adaptation of cloned cells to serum-free medium (SFM).
[00250] The highest producing 24-well plates from the 1 CPW DC transferred to the sterile hood were gradually subcloned through 6-well dishes, T75 flasks, and 125 mL square plastic bottles on an orbital shaker. During this process the serum was reduced to zero, at the final stage of centrifugation of the cells and resuspension in SFM. Second round dilutional cloning.
[00251] The above procedures were repeated with a second round of dilutional cloning, again at
0,5 cells/well (CPW). At this stage, approximately 40% of the wells showed any cell growth, and all wells showing growth also secreted human IgG. These results confirmed that on average only 1
cell is plated per well with these procedures, and that the CHO cell line originated from a single cell.
Downstream processing and 3-column purification of the HIRMAb-GDNF fusion protein. [00252] Following the second round of dilutional cloning, the highest producing cell line secreting the HIRMAb-GDNF fusion protein was propagated in serum- free medium to a total volume of 2,000 mL in several 1 L square plastic bottles on an orbital shaker. The HIRMAb-GDNF fusion protein was purified from the CHO cell conditioned medium using the following down-stream processing:
• Depth filtration with a 0.2 m2 0.65 urn GF filter in series with an 0.05 m20.2 μm ultrafilter
• Volume reduction to 400 mL using a Pellicon-2 tangential flow filtration (TFF) system
• Ultra-filtration with a 0.2 mm ultra-filter and application to a 7 mL column of protein A . Following application to the column, the column was eluted with 1 M NaCl, which elutes DNA non-specifically absorbed to the column, and the product is eluted as a single peak with 0.1 M sodium acetate/ρH=3.7. The acid eluate was neutralized with 1 M Tris base and concentrated to 5 mL.
• Cation exchange (CATEX) chromatography in bind-elute mode was performed with a 5 mL column of SP Sepharose FF equilibrated with 0.02 M MES and 0.05 M NaCl. The conductivity of the sample was reduced to <5 mS/cm prior to application to the column. The column was successively eluted with step gradients of 0.02 M MES/ρH=5.5 containing 0.25 M NaCl, 0.35 M NaCl, 0.5 M NaCl, and IM NaCI. The HIRMAb-GDNF fusion protein eluted in 0.5 M NaCl.
• Anion exchange (ANEX) chromatography in flow-through mode was performed with a 5 mL column of Q Sepharose FF equilibrated with 0.025 M MES/ρH=5.5 and 0.05 M NaCl. The conductivity of the sample was reduced to <7 mS/cm. The HIRMAb-GDNF fusion protein eluted in the flow-through.
Biochemical characterization of 3-column purified CHO cell derived HIRMAb-GDNF fusion protein.
[00253] The purity and potency of the CHO derived HIRMAb-GDNF fusion protein was assessed with the following procedures:
(a) SDS-PAGE. The HIRMAb-GDNF fusion protein was purified to homogeneity based on reducing and non-reducing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS- PAGE). A characteristic "fingerprint" of the HIRMAb-GDNF fusion protein is the detection of 3 heavy chain glycoforms, which arise from differential glycosylation of the GDNF part of the fusion protein. This pattern is reproducible from run to run.
(b) GDNF and human IgG Western blot. The CHO derived HIRMAb-GDNF fusion protein was electrophoresed on a 12% SDS-PAGE gel and blotted to nitrocellulose for Western blotting with primary antibodies to either human IgG (left panel, Figure 39), or to human GDNF (right panel, Figure 39). Both the anti-human IgG antibody and the anti-human GDNF antibody reacted specifically with the heavy chain of the HIRMAb-GDNF fusion protein.
CH2 region and 2 sites within the GDNF region of the fusion protein heavy chain. The neutral monosaccharide content (moles monosaccharide/mole fusion protein) was 7.7 for N-acetyl glucosamine, 5.3 for mannose, 2.3 for galactose, 1.6 for fucose, and 0.19 for N-acetyl galactosamine. These 4 sugars are organized as 1 of 4 different terminal structures, designated GO, Gl(1,6), Gl(1,3), or G2, depending on whether there is 0, 1, or 2 terminal galactose moieties, respectively. The profile for AGT-190 is GO>G1>G2, which is typical of CHO-derived recombinant proteins, and indicative of relative reduced terminal galactosylation. The glycosylation of HIRMAb-GDNF fusion protein on at least 3 different asparagine residues explains the heterogeneity of the heavy chain on Western blotting (Figure 39).
(h) Binding to human blood-brain barrier. Capillaries, which form the BBB in vivo, were isolated from human autopsy brain in a purified preparation as shown by light microscopy (Figure 43), The HIRMAb-GDNF fusion protein was radio-labeled by tritiation, and a radioreceptor assay was performed to demonstrate binding of the labeled HTRMAb-GDNF fusion protein to the HIR at the human BBB, using the isolated human brain capillaries. There was a high level of binding of the HIRMAb-GDNF fusion protein to the human brain capillary and the binding was displaced by unlabeled murine HIRMAb (Figure 43B).
Example 17
Treatment of Parkinson's disease with the HIRMAb-GDNF fusion protein Parkinson's disease (PD) is a neurodegenerative condition that affects the dopaminergic neurons of the nigral-striatal tract. GDNF is a potent trophic factor for these dopaminergic neurons. The intra-cerebral injection of the GDNF protein into the brain of rats with experimental PD can protect these neurons, and blocks further axotomy of the fibers projecting from the substantia nigra to the striatum. Accordingly, recombinant human GDNF has been developed as a new therapeutic for PD. However, GDNF does not cross the BBB. Therefore, GDNF was administered via a transcranial route using intra-cerebroventricular (ICV) injection. However, GDNF delivered by ICV injection was found to not penetrate into the brain parenchyma and was ineffective in patients with PD, and this delivery approach was abandoned. Subsequently, GDNF has been administered by convection enhanced diffusion (CED). In this approach, a reservoir holding the GDNF is implanted in the abdomen, and a catheter is run through the skin from the reservoir to the brain via a transcranial passage. The reservoir has a pump that continuously pumps the GDNF fluid into the brain. However, this highly invasive procedure was found to lead to toxic effects in the brain, was not effective owing to lack of penetration of the GDNF to the nigra-striatal tract, and was abandoned. These failed trans-cranial approaches to the treatment of PD with GDNF highlighted the importance of re-engineering this neurotrophin so that the molecule can cross the BBB. The trans-BBB delivery route has two unique advantages. First, the GDNF can be given by a non-invasive systemic injection, such as an intravenous, subcutaneous, or infra-muscular injection, and no neurosurgical procedure is required. Second, the GDNF enters the brain via the trans-vascular route. Since every
neuron in the brain is perfused by its own blood vessel, the GDNF is delivered to every cell comprising the nigra-striatal tract of brain. The GDNF could be given on a weekly or bi-weekly basis to patients with PD at doses ranging from 1, 3, 10, 30, or 100 mg. The considerations in Example 7 suggest that 25-30 mg may be a preferred dose.
Example 18
Acute and chronic treatment of stroke with the HIRMAb-GDNF fusion protein [00255] GDNF is highly neuroprotective in experimental stroke (Kitagawa et al, Stroke 29:1417-
1422 (1998)). Activation of the GDNF receptor, GFRαl, inhibits apoptosis in the neuron that is induced by an ischemic event. The demonstration of GDNF's neuroprotective effects in experimental stroke have uniformly involved intra-cerebral injection of the neurotrophin, because the GDNF does not cross the BBB, and because the BBB is intact in the first 5 hours after stroke, when the rescue of dying neurons is still possible. The HIRMAb-GDNF is a re-engineered form of GDNF that is both equi-potent with GDNF as a neuroprotective agent, and is able to cross the human BBB following systemic administration. Thus, the patient suffering from an acute stroke could be rapidly treated with intravenous HIRMAb-GDNF in the emergency room. The HIRMAb- GDNF fusion protein will rapidly enter the brain and activate GFRαl receptors on neurons to inhibit the apoptosis cycle induced by the stroke. In addition, chronic treatment with systemic HIRMAb-GDNF fusion protein could be therapeutic in the rehabilitation phase of stroke, since the intra-cerebral infusion of GDNF into the post-stroke brain induces neurogenesis, and neural repair in the post-stroke period (Kobayashi et al, 2006).
Example 19
Treatment of motor neuron disease with the HIRMAb-GDNF fusion protein [00256] Motor neuron diseases such as amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA) cause progressive paralysis leading to premature death. GDNF is highly neuroprotective of spinal cord motor neurons (Bonn supra). However, it is unlikely that GDNF will be developed as a drug for ALS or SMA, given the failed clinical trials of other neurotrophic factors for these conditions. Both BDNF and ciliary neurotrophic factor (CNTF) were administered to ALS patients in the 1990s by subcutaneous administration. The subcutaneous administration phase III clinical trials failed, because BDNF and CNTF, like GDNF, do not cross the BBB. However, with the re-engineering of GDNF as the HIRMAb-GDNF fusion protein demonstrated herein, conditions such as ALS or SMA couid now be amenable to treatment by systemic administration. The HlRMAb fusion proteins cross the blood-spinal cord barrier, which as been demonstrated by brain scanning of adult Rhesus monkeys following intravenous administration of the HIRMAb fusion protein (Boado et al, Biotechnoi Bioeng, 97(6): 1376-1386 (2007); Boado et al, Bioconjug. Chem. 18:447-455 (2007)).
Example 20
Treatment of brain or spinal cord injury with the HIRMAb-GDNF fusion protein
[00257] GDNF promotes neural repair in the period following acute experimental brain injury, such as with a lateral fluid percussion injury (Bakshi et al, Ever. J. Neurosci. 23:2119-2134 (2006)). GDNF does not cross the BBB, and the BBB is intact in the days/weeks after head injury, when the brain attempts to heal from the acute insult. Therefore, the beneficial effects of GDNF on acute head injury could only be demonstrated by the intra-cerebral injection of genetically modified stem cells that secrete GDNF. Although the trans-cranial injection approach may be feasible in the rat brain, the human brain is 1000-fold larger than a rat brain. Diffusion of the GDNF from the depot injection site becomes limiting, and the GDNF cannot penetrate to the wound area, beyond the local injection site. Alternatively, if the GDNF is delivered to brain via the trans-vascular route, then every injured neuron in the brain is exposed to the GDNF. The latter is feasible with the weekly, biweekly, or daily systemic administration of the HIRMAb-GDNF fusion protein during the post- injury repair and rehabilitation period.
[00258] Similarly, motor neurons of the spinal cord are responsive to GDNF, and GDNF can promote neural repair following experimental spinal cord injury (Lu et al, J. Neurotrauma 19: 1081- 1090 (2002)). However, because the opening of the BBB following spinal cord injury is only transient, it is not possible to treat spinal cord injury with systemic GDNF administration. Instead, the GDNF expressing plasmid DNA was injected directly into the rat spinal cord following the acute spinal cord injury. However, the size of the spinal cord in humans is 1000-fold larger than that of the rat Therefore, it is not possible to distribute GDNF to the entire lesioned area following direct spinal injection of the GDNF therapeutic in humans. Alternatively, GDNF is delivered to all injured neurons Ln the spinal cord following a trans-vascular delivery, and this is possible with the systemic administration of the HIRMAb-GDNF fusion protein.
Example 21
Treatment of drug and alchohol addiction with the HIRMAb-GDNF fusion protein [00259] The intra-cerebral injection of GDNF results in treatment of animals addicted to either opioid, or non-opioid drugs. With respect to non-opioid drugs, the intra-cerebral injection of GDNF decreases cocaine self-aministration in rats (Green-Sadan et al, Eur. J. Neurosci., 18: 2093-2098, 2003). Similarly, the intra-cerebral injection of GDNF decreases ethanol self-administration (He et al, J. Neurosci., 25: 619-628, 2005). The GDNF has to be administered via a trans-cranial route, because GDNF does not cross the BBB. The drug, ibogaine, decreases withdrawl symptoms to chronic opioid drugs, cocaine, or alchohol, and works by increasing GDNF in regions of the brain such as the ventral tegmental area (VTA) (He and Ron, FASEB J, 20: E1820-D1827, 2006). Although ibogaine is a small molecule that crosses the BBB, its application in the treatment of addiction is limited by severe side effects. GDNF is implicated in another study related to cocaine addiction. Cocaine increases tyrosine hydroxylase in the VTA, and this effect of cocaine is blocked
by the intra-cerebral injection of GDNF (Messer et al, Neuron, 26: 247-257, 2000). With respect to methamphetamine addiction, a decrease in brain concentration of GDNF, such as occurs in GDNF knock-out heterozygote mice, results in increased methamphetamine self-administration (Yan et al, FASEB J, 21 : 1994-2004, 2007). These forms of addiction could be treated by systemic administration of GDNF if this neurotrophin was re-formulated to cross the BBB, which is the case for the HIRMAb-GDNF fusion protein.
While preferred embodiments of the present invention have been shown and described herein, it will be obvious to those skilled in the art that such embodiments are provided by way of example only. Numerous variations, changes, and substitutions will now occur to those skilled in the art without departing from the invention. It should be understood that various alternatives to the embodiments of the invention described herein may be employed in practicing the invention. It is intended that the following claims define the scope of the invention and that methods and structures within the scope of these claims and their equivalents be covered thereby.
SEQUENCES
<210> 1
<211> 36
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400> 1 cctgtctccg ggtaaatatt tgcgacggcc ggcaag
<2lθ> 2 <211> 36
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400> 2 cttgccggcc gtcgcaaata tttacccgga gacagg
<210> 3
<211> 42 <212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400> 3 atgctcgagg aattcccatg gatgatggct agcaagctta tg
<210> 4
<211> 42
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400> 4 cataagcttg ctagccatca tccatgggaa ttcctcgagc at
<210> 5
<211> 56 <212 > DNA
<213 > Artificial Sequence
<220>
<223 > Description of Artificial Sequence : Synthetic Oligonucleotide
<400> 5 tccggatcct cgcgagtatg cactctgacc ctgcccgtcg aggtgagctg agcgtg
<210> 6
<211> 56
<212 > DNA
<213 > Artificial Sequence
<220>
<223 > Description of Artif icial Sequence : Synthetic Oligonucleotide
<400> 6 cacgctcagc tcacctcgac gggcagggtc agagtgcata ctcgcgagga tccgga
<210 > 7
<211> 119
<212> DNA
<213 > Artif icial Sequence
<220>
<223 > Description of Artificial Sequence : Synthetic Oligonucleotide
<400> 7 agtcgtacgt gcgggccctt accatggata gcaaaaagag aattggctgg cgattcataa ggatagacac ttcttgtgta tgtacattga ccattaaaag gtgatcgcga ctcgagatg
<210 > 8
<211> 119
<212> DNA
<213 > Artificial Sequence
<220>
<223 > Description of Artificial Sequence : Synthetic Oligonucleotide
<400 > 8 catctcgagt cgcgatcacc ttttaatggt caatgtacat acacaagaag tgtctatcct tatgaatcgc cagccaattc tctttttgct atccatggta agggcccgca cgtacgact
<210> 9 <211> 29 <212 > DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400> 9 atctcgcgag tatgcactct gaccctgcc
<210> 10
<211> 27
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400> 10 atctcgcgat caccttttaa tggtcaa
<210> 11 <211> 42
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400> 11 atggctagcg atatcggtac cgtatacgga tccctcgaga tg
<210> 12
<211> 42
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400> 12 catctcgagg gatccgtata cggtaccgat atcgctagcc at
<210> 13
<211> 25
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400> 13 gtgacaaaca cagacatagg atatc
<210> 14
<211> 24
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400> 14 atgctcgagc taacactctc ccct
<210> 15
<211> 26
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400> 15 atgaatattc caccatggaa tgcagc
<2io> 16
<211> 27
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400> 16 ataggatcct caccttttaa tggtcaa
<210> 17
<211> 42
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400> 17 aaaaggccag gaaccgaatt cagatctcgt tgctggcgtt tt
<210> 18
<211> 42
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400> 18 aaaacgccag caacgagatc tgaattcggt tcctggcctt tt
<210> 19
<211> 38
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400> 19 atcgaattca agcttgcggc cgcgtataca gatctatc
<210> 20
<211> 38
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400> 20 gatagatctg tatacgcggc cgcaagcttg aattcgat
<210> 21
<211> 378
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic DNA
<220>
<221> CDS
<222> (1) .. (375)
<400> 21 ctg tct ccg ggt aaa teg agt atg cac tct gac ect gcc cgt cga ggt Leu Ser Pro Gly Lys Ser Ser Met His Ser Asp Pro Ala Arg Arg Gly gag ctg age gtg tgt gac agt att agt gag tgg gta acg gcg gca gac GIu Leu Ser VaI Cy s Asp Ser lie Ser GIu Trp VaI Thr Ala Ala Asp aaa aag act gca gtg gac atg teg ggc ggg acg gtc aca gtc ctt gaa Lys Lys Thr Ala VaI Asp Met Ser Gly Gly Thr VaI Thr VaI Leu GIu aaσ gtc cct gta tea aaa ggc caa ctg aag caa tac ttc tac ga g acc
Lys VaI Pro VaI Ser Lys G l y GIn Leu Lys GIn Tyr Phe Tyr GIu Thr aag tgc aat ccc atg ggt tac aca aaa gaa ggc tgc agg ggc ata gac Lys Cys Asn Pro Met G l y Tyr Thr Lys G l u G l y Cys Arg G l y He Asp aaa agg cat tgg aac tec cag tgc cga act ace cag teg tac gtg egg Lys Arg His Trp Asn Ser Gin Cys Arg Thr Thr Gin Ser Tyr VaI Arg gee ctt ace atg gat age aaa aag aga att ggc tgg cga ttc ata agg Ala Leu Thr Met Asp Ser Lys Lys Arg lie G l y Trp Arg Phe lie Arg ata gac act tct tgt gta tgt aca ttg ace att aaa agg tga lie Asp Thr Ser Cys VaI Cys Thr Leu Thr lie Lys Arg
<210> 22
<211> 125
<212 > PRT
<213 > Artificial Sequence
<220>
<223 > Description of Artificial Sequence : Synthetic Protein
<400 > 22
Leu Ser Pro G l y Lys Ser Ser Met His Ser Asp Pro Ala Arg Arg G l y GIu Leu Ser VaI Cys Asp Ser lie Ser GIu Trp VaI Thr Ala Ala Asp Lys Lys Thr Ala VaI Asp Met Ser G l y G l y Thr VaI Thr VaI Leu GIu Lys VaI Pro VaI Ser Lys G l y Gin Leu Lys Gin Tyr Phe Tyr GIu Thr Lys Cys Asn Pro Met G l y Tyr Thr Lys GIu G l y Cys Arg G l y lie Asp Lys Arg His Trp Asn Ser Gin Cys Arg Thr Thr Gin Ser Tyr VaI Arg Ala Leu Thr Met Asp Ser Lys Lys Arg He G l y Trp Arg Phe He Arg He Asp Thr Ser Cys VaI Cys Thr Leu Thr He Lys Arg
<210> 23
<211> 2711
<212> DNA
<213 > Artificial Sequence
<220>
<223 > Description of Artificial Sequence : Synthetic DNA
<400 > 23 tagtctttct cttcagtgac aaacacagac ataggatatt ccacσatgga atgcagctgg gtcatgctct tcctcctgtc aggaactgca ggtgtccatt gccaggttca gctgcagcag tctggacctg agctggtgaa gcctggggct ttagtgaaga tatcctgcaa ggcttctggt tacaccttca caaactacga tatacactgg gtgaagcaga ggcctggaca gggacttgag tggattggat ggatttatcc tggagatggt agtactaagt acaatgagaa attcaagggc aaggccacac tgactgcaga caaatcctcc agcacagcct acatgcacct cagcagcctg acttctgaga aatctgcagt ctatttctgt gcaagagagt gggcttactg gggccaaggg actctggtca ctgtctctgc agctagcacc aagggcccat cggtcttccc cctggcaccc tcctccaaga gcacctctgg gggcacagcg gccctgggct gcctggtcaa ggactacttc cccgaaccgg tgacggtgtc gtggaactca ggcgccctga ccagcggcgt gcacaccttc ccggctgtcc tacagtcctc aggactctac tccctcagca gcgtggtgac cgtgccctcc agcagcttgg gcacccagac ctacatctgc aacgtgaatc acaagcccag caacaccaag gtggacaaga aagttggtga gaggccagca cagggaggga gggtgtctgc tggaagccag gctcagcgct cctgcctgga cgcatcccgg ctatgcagcc ccagtccagg gcagcaaggc aggccccgtc tgcctcttca cccggaggcc tctgcccgcc ccactcatgc tcagggagag ggtcttctgg ctttttcccc aggctctggg caggcacagg ctaggtgccc ctaacccagg ccctgcacac aaaggggcag gtgctgggct cagacctgcc aagagccata tccgggagga ccctgcccct gacctaagcc caccccaaag gccaaactct ccactccctc aqctcααaca
ccttctctcc tcccagattc cagtaactcc caatcttctc tctgcagagc ccaaatcttg tgacaaaact cacacatgcc caccgtgccc aggtaagcca gcccaggcct cgccctccag ctcaaggcgg gacaggtgcc ctagagtagc ctgcatccag ggacaggccc cagccgggtg ctgacacgtc cacctccatc tcttcctcag cacctgaact cctgggggga ccgtcagtct tcctcttccc cccaaaaccc aaggacaccc tcatgatctc ccggacccct gaggtcacat gcgtggtggt ggacgtgagc cacgaagacc ctgaggtcaa gttcaactgg tacgtggacg gcgtggaggt gcataatgcc aagacaaagc cgcgggagga gcagtacaac agcacgtacc gtgtggtcag ggtcctcacc gtcctgcacc aggactggct gaatggcaag gagtacaagt gcaaggtctc caacaaagcc ctcccagccc ccatcgagaa aaccatctcc aaagccaaag gtgggacccg tggggtgcga gggccacatg gacagaggcc ggctcggccc accctctgcc ctgagagtga ccgctgtacc aacctctgtc cctacagggc agccccgaga accacaggtg tacaccctgc ccccatcccg ggatgagctg accaagaacc aggtcagcct gacctgcctg gtcaaaggct tctatcccag cgacatcgcc gtggagtggg agagcaatgg gcagccggag aacaactaca agaccacgcc tcccgtgctg gactccgacg gctccttctt cctctacagc aagctcaccg tggacaagag caggtggcag caggggaacg tcttctcatg ctccgtgatg catgaggctc tgcacaacca ctacacgcag aagagcctct ccctgtctcc gggtaaatcg agtatgcact ctgaccctgc ccgtcgaggt gagctgagcg tgtgtgacag tattagtgag tgggtaacgg cggcagacaa aaagactgca gtggacatgt cgggcgggac ggtcacagtc cttgaaaagg tccctgtatc aaaaggccaa ctgaagcaat acttctacga gaccaagtgc aatcccatgg gttacacaaa agaaggctgc aggggcatag acaaaaggca ttggaactcc cagtgccgaa ctacccagtc gtacgtgcgg gcccttacca tggatagcaa aaagagaatt ggctggcgat tcataaggat agacacttct tgtgtatgta cattgaccat taaaaggtga tcgattttgc gacggccggc aagcccccgc tccccgggct ctcgcggtcg cacgaggatg cttggcacgt accccctgta catacttccc gggcgcccag catggaaata aagcacccag cgctgccctg ggcccctgcg agactgtgat ggttctttcc acgggtcagg ccgagtctga ggcctgagtg gcatgaggga ggcagagcgg gtcccactgt ccccacactg gcccaggctg tgcaggtgtg cctgggccgc ctagggtggg gctcagccag gggctgccct cggcagggtg ggggatttgc c
<210> 24
<211> 582
<212 > PRT
<213 > Artificial Sequence
<220 >
<223 > Description of Artificial Sequence : Synthetic Protein
<400 > 24
Met GIu Cys Ser Trp VaI Met Leu Phe Leu Leu Ser G l y Thr Ala G l y VaI His Cys GIn VaI GIn Leu GIn Gin Ser G l y Pro GIu Leu VaI Lys Pro G l y Ala Leu VaI Lys lie Ser Cys Lys Ala Ser G l y Tyr Thr Phe Thr Asn Tyr Asp lie His Trp VaI Lys Gin Arg Pro G l y G l n G l y Leu GIu Trp He G l y Trp He Tyr Pro G l y Asp G l y Ser Thr Lys Tyr Asn GIu Lys Phe Lys G l y Lys Ala Thr Leu Thr Ala Asp Lys Ser Ser Ser Thr Ala Tyr Met His Leu Ser Ser Leu Thr Ser GIu Lys Ser Ala VaI Tyr Phe Cys Ala Arg GIu Trp Ala Tyr Trp G l y GIn G l y Thr Leu VaI Thr VaI Ser Ala Ala Ser Thr Lys G l y Pro Ser VaI Phe Pro Leu Ala Pro Ser Ser Lys Ser Thr Ser G l y G l y Thr Ala Ala Leu G l y Cys Leu VaI Lys Asp Tyr Phe Pro GIu Pro VaI Thr VaI Ser Trp Asn Ser G l y Ala Leu Thr Ser G l y VaI His Thr Phe Pro Ala VaI Leu GIn Ser Ser G l y Leu Tyr Ser Leu Ser Ser VaI VaI Thr VaI Pro Ser Ser Ser Leu G l y Thr Gin Thr Tyr He Cys Asn VaI Asn His Lys Pro Ser Asn Thr Lys VaI Asp Lys Lys VaI GIu Pro Lys Ser Cys Asp Lys Thr His Thr Cys Pro Pro Cys Pro Ala Pro GIu Leu Leu G l y G l y Pro Ser VaI Phe Leu Phe Pro Pro Lys Pro Lys Asp Thr Leu Met He Ser Arg Thr Pro G l u VaI Thr Cys VaI VaI VaI Asp VaI Ser His GIu Asp Pro GIu VaI Lys Phe Asn Trp Tyr VaI Asp G l y VaI G l u VaI His Asn Ala Lys Thr
Lys Pro Arg Glu GIu Gln Tyr Asn Ser Thr Tyr Arg VaI VaI Arg VaI
Leu Thr VaI Leu His Gin Asp Trp Leu Asn Gly Lys GIu Tyr Lys Cys
Lys VaI Ser Asn Lys Ala Leu Pro Ala Pro He GIu Lys Thr He Ser
Lys Ala Lys Gly Gin Pro Arg Glu Pro GIn VaI Tyr Thr Leu Pro Pro
Ser Arg Asp Glu Leu Thr Lys Asn Gln VaI Ser Leu Thr Cys Leu VaI
Lys Gly Phe Tyr Pro Ser Asp He Ala VaI Glu Trp GIu Ser Asn Gly
GIn Pro Glu Asn Asn Tyr Lys Thr Thr Pro Pro VaI Leu Asp Ser Asp
Gly Ser Phe Phe Leu Tyr Ser Lys Leu Thr VaI Asp Lys Ser Arg Trp
Gln Gln Gly Asn VaI Phe Ser Cys Ser VaI Met His Glu Ala Leu His
Asn His Tyr Thr Gin Lys Ser Leu Ser Leu Ser Pro Gly Lys Ser Ser
Met His Ser Asp Pro Ala Arg Arg Gly Glu Leu Ser VaI Cys Asp Ser
He Ser Glu Trp VaI Thr Ala Ala Asp Lys Lys Thr Ala VaI Asp Met
Ser Gly Gly Thr VaI Thr VaI Leu Glu Lys VaI Pro VaI Ser Lys Gly
GIn Leu Lys Gln Tyr Phe Tyr GIu Thr Lys Cys Asn Pro Met Gly Tyr
Thr Lys GIu Gly Cys Arg Gly He Asp Lys Arg His Trp Asn Ser GIn
Cys Arg Thr Thr GIn Ser Tyr VaI Arg Ala Leu Thr Met Asp Ser Lys
Lys Arg lie Gly Trp Arg Phe He Arg He Asp Thr Ser Cys VaI Cys
Thr Leu Thr He Lys Arg
<210> 25
<211> 582
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Protein
<400> 25
Met GIu Cys Ser Trp VaI Met Leu Phe Leu Leu Ser Gly Thr Ala Gly VaI His Cys Gin VaI GIn Leu GIn GIn Ser Gly Pro GIu Leu VaI Lys Pro Gly Ala Leu VaI Lys He Ser Cys Lys Ala Ser Gly Tyr Thr Phe Thr Asn Tyr Asp He His Trp VaI Lys GIn Arg Pro Gly Gin Gly Leu Glu Trp He Gly Trp He Tyr Pro Gly Asp Gly Ser Thr Lys Tyr Asn GIu Lys Phe Lys Gly Lys Ala Thr Leu Thr Ala Asp Lys Ser Ser Ser Thr Ala Tyr Met His Leu Ser Ser Leu Thr Ser GIu Lys Ser Ala VaI Tyr Phe Cys Ala Arg GIu Trp Ala Tyr Trp Gly GIn Gly Thr Leu VaI Thr VaI Ser Ala Ala Ser Thr Lys Gly Pro Ser VaI Phe Pro Leu Ala Pro Ser Ser Lys Ser Thr Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu VaI Lys Asp Tyr Phe Pro GIu Pro VaI Thr VaI Ser Trp Asn Ser Gly Ala Leu Thr Ser Gly VaI His Thr Phe Pro Ala VaI Leu Gin Ser Ser Gly Leu Tyr Ser Leu Ser Ser VaI VaI Thr VaI Pro Ser Ser Ser Leu Gly Thr Gin Thr Tyr He Cys Asn VaI Asn His Lys Pro Ser Asn Thr Lys VaI Asp Lys Lys VaI GIu Pro Lys Ser Cys Asp Lys Thr His Thr Cys Pro Pro Cys Pro Ala Pro GIu Leu Leu Gly Gly Pro Ser VaI Phe Leu Phe Pro Pro Lys Pro Lys Asp Thr Leu Met He Ser Arg Thr Pro GIu VaI Thr Cys VaI VaI VaI Asp VaI Ser His GIu Asp Pro GIu VaI Lys Phe Asn Trp Tyr VaI Asp Gly VaI Glu VaI His Asn Ala Lys Thr Lys Pro Arg GIu GIu GIn Tyr Asn Ser Thr Tyr Arg VaI VaI Ser VaI Leu Thr VaI Leu His Gin Asp Trp Leu Asn Gly Lys GIu Tyr Lys Cys Lys VaI Ser Asn Lys Ala Leu Pro Ala Pro He GIu Lys Thr He Ser Lys Ala Lys Gly GIn Pro Arg GIu Pro GIn VaI Tyr Thr Leu Pro Pro Ser Arg Asp GIu Leu Thr Lys Asn Gln VaI Ser Leu Thr Cys Leu VaI Lys Gly Phe Tyr Pro Ser Asp He Ala VaI Glu Trp GIu Ser Asn Gly GIn Pro GIu Asn Asn Tyr Lys Thr Thr Pro Pro VaI Leu Asp Ser Asp Gly Ser Phe Phe Leu Tyr Ser Lys Leu Thr VaI Asp Lys Ser Arg Trp GIn GIn Gly Asn VaI Phe Ser Cys Ser VaI Met His Glu Ala Leu His Asn His Tyr Thr GIn Lys Ser Leu Ser Leu Ser Pro GIy Lys Ser Ser
Met His Ser Asp Pro Ala Arg Arg G l y G l u Leu Ser VaI Cys Asp Ser lie Ser GIu Trp VaI Thr Ala Ala Asp Lys Lys Thr Ala VaI Asp Met Ser G l y G l y Thr VaI Thr VaI Leu GIu Lys VaI Pro VaI Ser Lys G l y GIn Leu Lys GIn Tyr Phe Tyr GIu Thr Lys Cys Asn Pro Met G l y Tyr Thr Lys GIu G l y Cys Arg G l y lie Asp Lys Arg His Trp Asn Ser GIn Cys Arg Thr Thr G l n Ser Tyr VaI Arg Ala Leu Thr Met Asp Ser Lys Lys Arg lie G l y Trp Arg Phe lie Arg lie Asp Thr Ser Cys VaI Cys Thr Leu Thr lie Lys Arg
<210> 26
<211> 1757
<212 > DNA
<213 > Artificial Sequence
<220 >
<223 > Description of Artificial Sequence : Synthetic DNA
<400> 26 attccaccat ggaatgcagc tgggtcatgc tcttcctcct gtcaggaact gcaggtgtcc attgccaggt tcagctgcag cagtctggac ctgagctggt gaagcctggg gctttagtga agatatcctg caaggcttct ggttacacct tcacaaacta cgatatacac tgggtgaagc agaggcctgg acagggactt gagtggattg gatggattta tcctggagat ggtagtacta agtacaatga gaaattcaag ggcaaggcca cactgactgc agacaaatcc tccagcacag cctacatgca cctcagcagc ctgacttctg agaaatctgc agtctatttc tgtgcaagag agtgggctta ctggggccaa gggactctgg tcactgtctc tgcagctagc accaagggcc catcggtctt ccccctggca ccctcctcca agagcacctc tgggggcaca gcggccctgg gctgcctggt caaggactac ttccccgaac cggtgacggt gtcgtggaac tcaggcgccc tgaccagcgg cgtgcacacc ttcccggctg tcctacagtc ctcaggactc tactccctca gcagcgtggt gaccgtgccc tccagcagct tgggcaccca gacctacatc tgcaacgtga atcacaagcc cagcaacacc aaggtggaca agaaagttga gcccaaatct tgtgacaaaa ctcacacatg cccaccgtgc ccagcacctg aactcctggg gggaccgtca gtcttcctct tccccccaaa acccaaggac accctcatga tctcccggac ccctgaggtc acatgcgtgg tggtggacgt gagccacgaa gaccctgagg tcaagttcaa ctggtacgtg gacggcgtgg aggtgcataa tgccaagaca aagccgcggg aggagcagta caacagcacg taccgtgtgg tcagcgtcct caccgtcctg caccaggact ggctgaatgg caaggagtac aagtgcaagg tctccaacaa agccctccca gcccccatcg agaaaaccat ctccaaagcc aaagggcagc cccgagaacc acaggtgtac accctgcccc catcccggga tgagctgacc aagaaccagg tcagcctgac ctgcctggtc aaaggcttct atcccagcga catcgccgtg gagtgggaga gcaatgggca gccggagaac aactacaaga ccacgcctcc cgtgctggac tccgacggct ccttcttcct ctacagcaag ctcaccgtgg acaagagcag gtggcagcag gggaacgtct tctcatgctc cgtgatgcat gaggctctgc acaaccacta cacgcagaag agcctctccc tgtctccggg taaatcgagt atgcactctg accctgcccg tcgaggtgag ctgagcgtgt gtgacagtat tagtgagtgg gtaacggcgg cagacaaaaa gactgcagtg gacatgtcgg gcgggacggt cacagtcctt gaaaaggtcc ctgtatcaaa aggccaactg aagcaatact tctacgagac caagtgcaat cccatgggtt acacaaaaga aggctgcagg ggcatagaca aaaggcattg gaactcccag tgccgaacta cccagtcgta cgtgcgggcc cttaccatgg atagcaaaaa gagaattggc tggcgattca taaggataga cacttcttgt gtatgtacat tgaccattaa aaggtga
<210 > 27 <211> 1749
<212> DNA
<213 > Artificial Sequence
<220>
<223 > Description of Artificial Sequence : Synthetic DNA
<220 >
<221> CDS
<222> ( 1 ) . . ( 1746 )
<400> 27 atg gaa tgc age tgg gtc atg etc ttc etc ctg tea gga act gca ggt Met G l u Cys Ser Trp VaI Met Leu Phe Leu Leu Ser G l y Thr Ala G l y gtc cat tgc cag gtt cag ctg cag cag tct gga cct gag ctg gtg aag VaI His Cys Gin VaI GIn Leu GIn Gin Ser G l y Pro GIu Leu VaI Lys cct ggg get tta gtg aag ata tec tgc aag get tct ggt tac ace ttc Pro G l y Ala Leu VaI Lys lie Ser Cys Lys Ala Ser G l y Tyr Thr Phe aca aac tac gat ata cac tgg gtg aag cag agg cct gga cag gga ctt Thr Asn Tyr Asp lie His Trp VaI Lys GIn Arg Pro G l y Gin G l y Leu gag tgg att gga tgg att tat cct gga gat ggt agt act aag tac aat GIu Trp lie G l y Trp He Tyr Pro G l y Asp G l y Ser Thr Lys Tyr Asn gag aaa ttc aag ggc aag gee aca ctg act gca gac aaa tec tec age GIu Lys Phe Lys G l y Lys Ala Thr Leu Thr Ala Asp Lys Ser Ser Ser aca gcc tac atg cac etc age age ctg act tct gag aaa tct gca gtc Thr Ala Tyr Met His Leu Ser Ser Leu Thr Ser Glu Lys Ser Ala VaI tat ttc tgt gca aga gag tgg get tac tgg ggc caa ggg act ctg gtc Tyr Phe Cys Ala Arg Glu Trp Ala Tyr Trp G l y GIn G l y Thr Leu VaI act gtc tct gca get age ace aag ggc cca teg gtc ttc ccc ctg gca Thr VaI Ser Ala Ala Ser Thr Lys G l y Pro Ser VaI Phe Pro Leu Ala ccc tec tec aag age ace tct ggg ggc aca gcg gcc ctg ggc tgc ctg Pro Ser Ser Lys Ser Thr Ser G l y G l y Thr Ala Ala Leu G l y Cys Leu gtc aag gac tac ttc ccc gaa ccg gtg acg gtg teg tgg aac tea ggc VaI Lys Asp Tyr Phe Pro Glu Pro VaI Thr VaI Ser Trp Asn Ser G l y gcc ctg ace age ggc gtg cac ace ttc ccg get gtc eta cag tec tea Ala Leu Thr Ser G l y VaI His Thr Phe Pro Ala VaI Leu Gin Ser Ser gga etc tac tec etc age age gtg gtg ace gtg ccc tec age age ttg G l y Leu Tyr Ser Leu Ser Ser VaI VaI Thr VaI Pro Ser Ser Ser Leu ggc ace cag ace tac ate tgc aac gtg aat cac aag ccc age aac ace G l y Thr Gin Thr Tyr He Cys Asn VaI Asn His Lys Pro Ser Asn Thr aag gtg gac aag aaa gtt gag ccc aaa tct tgt gac aaa act cac aca Lys VaI Asp Lys Lys VaI Glu Pro Lys Ser Cys Asp Lys Thr His Thr tgc cca ccg tgc cca gca cct gaa etc ctg ggg gga ccg tea gtc ttc Cys Pro Pro Cys Pro Ala Pro Glu Leu Leu G l y Gly Pro Ser VaI Phe etc ttc ccc cca aaa ccc aag gac ace etc atg ate tec egg ace cct Leu Phe Pro Pro Lys Pro Lys Asp Thr Leu Met He Ser Arg Thr Pro gag gtc aca tgc gtg gtg gtg gac gtg age cac gaa gac cct gag gtc Glu VaI Thr Cys VaI VaI VaI Asp VaI Ser His Glu Asp Pro Glu VaI aag ttc aac tgg tac gtg gac ggc gtg gag gtg cat aat gcc aag aca Lys Phe Asn Trp Tyr VaI Asp Gly VaI Glu VaI His Asn Ala Lys Thr aag ccg egg gag gag cag tac aac age acg tac cgt gtg gtc age gtc Lys Pro Arg Glu Glu Gin Tyr Asn Ser Thr Tyr Arg VaI VaI Ser VaI etc ace gtc ctg cac cag gac tgg ctg aat ggc aag gag tac aag tgc Leu Thr VaI Leu His GIn Asp Trp Leu Asn Gly Lys Glu Tyr Lys Cys aag gtc tec aac aaa gcc etc cca gcc ccc ate gag aaa ace ate tec Lys VaI Ser Asn Lys Ala Leu Pro Ala Pro He Glu Lys Thr He Ser aaa gcc aaa ggg cag ccc cga gaa cca cag gtg tac ace ctg ccc cca Lys Ala Lys Gly GIn Pro Arg Glu Pro GIn VaI Tyr Thr Leu Pro Pro tec egg gat gag ctg ace aag aac cag gtc age ctg ace tqc ctcr crtc
Ser Arg Asp GIu Leu Thr Lys Asn GIn VaI Ser Leu Thr Cys Leu VaI aaa ggc ttc tat ccc age gac ate gcc gtg gag tgg gag age aat ggg Lys G l y Phe Tyr Pro Ser Asp lie Ala VaI G l u Trp G l u Ser Asn G l y cag ccg gag aac aac tac aag ace acg cct ccc gtg ctg gac tec gac G l n Pro GIu Asn Asn Tyr Lys Thr Thr Pro Pro VaI Leu Asp Ser Asp ggc tec ttc ttc etc tac age aag etc ace gtg gac aag age agg tgg G l y Ser Phe Phe Leu Tyr Ser Lys Leu Thr VaI Asp Lys Ser Arg Trp cag cag ggg aac gtc ttc tea tgc tec gtg atg cat gag get ctg cac Gin Gin G l y Asn VaI Phe Ser Cys Ser VaI Met His G l u Ala Leu His aac cac tac acg cag aag age etc tec ctg tct ccg ggt aaa teg agt Asn His Tyr Thr GIn Lys Ser Leu Ser Leu Ser Pro G l y Lys Ser Ser atg cac tct gac cct gcc cgt cga ggt gag ctg age gtg tgt gac agt Met His Ser Asp Pro Ala Arg Arg G l y G l u Leu Ser VaI Cys Asp Ser att agt gag tgg gta acg gcg gca gac aaa aag act gca gtg gac atg He Ser GIu Trp VaI Thr Ala Ala Asp Lys Lys Thr Ala VaI Asp Met teg ggc ggg acg gtc aca gtc ctt gaa aag gtc cct gta tea aaa ggc Ser G l y G l y Thr VaI Thr VaI Leu GIu Lys VaI Pro VaI Ser Lys G l y caa ctg aag caa tac ttc tac gag ace aag tgc aat ccc atg ggt tac Gin Leu Lys GIn Tyr Phe Tyr GIu Thr Lys Cys Asn Pro Met G l y Tyr aca aaa gaa ggc tgc agg ggc ata gac aaa agg cat tgg aac tec cag Thr Lys GIu G l y Cys Arg Gly lie Asp Lys Arg His Trp Asn Ser Gin tgc cga act ace cag teg tac gtg egg gcc ctt ace atg gat age aaa Cys Arg Thr Thr GIn Ser Tyr VaI Arg Ala Leu Thr Met Asp Ser Lys aag aga att ggc tgg cga ttc ata agg ata gac act tct tgt gta tgt Lys Arg lie G l y Trp Arg Phe lie Arg He Asp Thr Ser Cys VaI Cys aca ttg ace att aaa agg tga Thr Leu Thr He Lys Arg
<210> 28
<211> 582
<212 > PRT
<213 > Artificial Sequence
<220 >
<223 > Description of Artificial Sequence : Synthetic Protein
<400 > 28
Met GIu Cys Ser Trp VaI Met Leu Phe Leu Leu Ser G l y Thr Ala Gly VaI His Cys Gin VaI GIn Leu G l n G l n Ser Gly Pro GIu Leu VaI Lys Pro Gly Ala Leu VaI Lys He Ser Cys Lys Ala Ser Gly Tyr Thr Phe Thr Asn Tyr Asp He His Trp VaI Lys GIn Arg Pro Gly GIn Gly Leu GIu Trp He Gly Trp He Tyr Pro Gly Asp Gly Ser Thr Lys Tyr Asn GIu Lys Phe Lys Gly Lys Ala Thr Leu Thr Ala Asp Lys Ser Ser Ser Thr Ala Tyr Met His Leu Ser Ser Leu Thr Ser GIu Lys Ser Ala VaI Tyr Phe Cys Ala Arg GIu Trp Ala Tyr Trp Gly GIn Gly Thr Leu VaI Thr VaI Ser Ala Ala Ser Thr Lys Gly Pro Ser VaI Phe Pro Leu Ala Pro Ser Ser Lys Ser Thr Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu VaI Lys Asp Tyr Phe Pro Glu Pro VaI Thr VaI Ser Trp Asn Ser Gly Ala Leu Thr Ser Gly VaI His Thr Phe Pro Ala VaI Leu Gin Ser Ser Gly Leu Tyr Ser Leu Ser Ser VaI VaI Thr VaI Pro Ser Ser Ser Leu Gly Thr G l n Thr Tyr He Cys Asn VaI Asn His Lys Pro Ser Asn Thr Lys VaI Asp Lys Lys VaI Glu Pro Lys Ser Cys Asp Lys Thr His Thr Cys Pro Pro Cys Pro Ala Pro Glu Leu Leu Gly Gly Pro Ser VaI Phe Leu Phe Pro Pro Lys Pro Lys Asp Thr Leu Met He Ser Arg Thr Pro
Glu VaI Thr Cys VaI VaI VaI Asp VaI Ser His Glu Asp Pro Glu VaI Lvs Phe Asn Trp Tyr VaI Asp Gly VaI Glu VaI His Asn Ala Lvs Thr
Lys Pro Arg GIu GIu Gin Tyr Asn Ser Thr Tyr Arg VaI VaI Ser VaI Leu Thr VaI Leu His Gin Asp Trp Leu Asn Gly Lys GIu Tyr Lys Cys Lys VaI Ser Asn Lys Ala Leu Pro Ala Pro lie GIu Lys Thr lie Ser Lys Ala Lys Gly Gin Pro Arg GIu Pro GIn VaI Tyr Thr Leu Pro Pro Ser Arg Asp GIu Leu Thr Lys Asn GIn VaI Ser Leu Thr Cys Leu VaI Lys Gly Phe Tyr Pro Ser Asp lie Ala VaI Glu Trp GIu Ser Asn Gly GIn Pro GIu Asn Asn Tyr Lys Thr Thr Pro Pro VaI Leu Asp Ser Asp Gly Ser Phe Phe Leu Tyr Ser Lys Leu Thr VaI Asp Lys Ser Arg Trp GIn GIn GIy Asn VaI Phe Ser Cys Ser VaI Met His GIu Ala Leu His Asn His Tyr Thr Gln Lys Ser Leu Ser Leu Ser Pro Gly Lys Ser Ser Met His Ser Asp Pro Ala Arg Arg Gly GIu Leu Ser VaI Cys Asp Ser He Ser GIu Trp VaI Thr Ala Ala Asp Lys Lys Thr Ala VaI Asp Met Ser Gly Gly Thr VaI Thr VaI Leu GIu Lys VaI Pro VaI Ser Lys Gly Gin Leu Lys Gin Tyr Phe Tyr Glu Thr Lys Cys Asn Pro Met Gly Tyr Thr Lys GIu Gly Cys Arg Gly He Asp Lys Arg His Trp Asn Ser GIn Cys Arg Thr Thr GIn Ser Tyr VaI Arg Ala Leu Thr Met Asp Ser Lys Lys Arg He Gly Trp Arg Phe He Arg He Asp Thr Ser Cys VaI Cys Thr Leu Thr He Lys Arg
<210> 29
<211> 714
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence; Synthetic DNA
<400> 29 gatatcacca tggagacaga cacactcctg ctatggctct tgttgctcat gtttccaggt accagatgtg acatccagat gacccagtct ccatcctcct tatctgcctc tctgggagaa agagtcagtc tcacttgtcg ggcaagtcag gacattggtg gtaacttata ctggcttcag cagggaccag atggaactat taaacgcctg atctacgcca catccagttt agattctggt gtccccaaaa ggttcagtgg cagtaggtct gggtcagatt attctctcac catcagcagc cttgagtctg aagattttgt agactattac tgtctacagt attctagttc tccgtggacg ttcggtggag cgacaaagat ggaaataaaa cgaactgtgg ctgcaccatc tgtcttcatc ttcccgccat ctgatgagca gttgaaatct ggaactgcct ctgttgtgtg cctgctgaat aacttctatc ccagagaggc caaagtacag tggaaggtgg ataacgccct ccaatcgggt aactcccagg agagtgtcac agagcaggac agcaaggaca gcacctacag cctcagcagc accctgacgc tgagcaaagc agactacgag aaacacaaag tctacgcctg cgaagtcacc catcagggcc tgagctcgcc cgtcacaaag agcttcaaca ggggagagtg ttag
<210> 30
<211> 705
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic DNA
<220>
<221> CDS
<222> (1) .. (702)
<400> 30 atg gag aca gac aca etc ctg eta tgg etc ttg ttg etc atg ttt cca
Met GIu Thr Asp Thr Leu Leu Leu Trp Leu Leu Leu Leu Met Phe Pro
ggt ace aga tgt gac ate cag atg ace cag tct cca tec tec tta tct Gly Thr Arg Cys Asp lie GIn Met Thr GIn Ser Pro Ser Ser Leu Ser gcc tct ctg gga gaa aga gtc agt etc act tgt egg gca agt cag gac Ala Ser Leu Gly GIu Arg VaI Ser Leu Thr Cys Arg Ala Ser Gin Asp att ggt ggt aac tta tac tgg ctt cag cag gga cca gat gga act att lie Gly Gly Asn Leu Tyr Trp Leu Gin GIn Gly Pro Asp Gly Thr lie aaa cgc ctg ate tac gcc aca tec agt tta gat tct ggt gtc ccc aaa Lys Arg Leu lie Tyr Ala Thr Ser Ser Leu Asp Ser Gly VaI Pro Lys agg ttc agt ggc agt agg tct ggg tea gat tat tct etc ace ate age Arg Phe Ser Gly Ser Arg Ser Gly Ser Asp Tyr Ser Leu Thr lie Ser age ctt gag tct gaa gat ttt gta gac tat tac tgt eta cag tat tct Ser Leu GIu Ser GIu Asp Phe VaI Asp Tyr Tyr Cys Leu GIn Tyr Ser agt tct ccg tgg acg ttc ggt gga gcg aca aag atg gaa ata aaa cga Ser Ser Pro Trp Thr Phe Gly Gly Ala Thr Lys Met Glu lie Lys Arg act gtg get gca cca tct gtc ttc ate ttc ccg cca tct gat gag cag Thr VaI Ala Ala Pro Ser VaI Phe lie Phe Pro Pro Ser Asp GIu GIn ttg aaa tct gga act gcc tct gtt gtg tgc ctg ctg aat aac ttc tat Leu Lys Ser Gly Thr Ala Ser VaI VaI Cys Leu Leu Asn Asn Phe Tyr ccc aga gag gcc aaa gta cag tgg aag gtg gat aac gcc etc caa teg Pro Arg GIu Ala Lys VaI Gin Trp Lys VaI Asp Asn Ala Leu Gin Ser ggt aac tec cag gag agt gtc aca gag cag gac age aag gac age ace Gly Asn Ser Gin Glu Ser VaI Thr GIu Gin Asp Ser Lys Asp Ser Thr tac age etc age age ace ctg acg ctg age aaa gca gac tac gag aaa Tyr Ser Leu Ser Ser Thr Leu Thr Leu Ser Lys Ala Asp Tyr Glu Lys cac aaa gtc tac gcc tgc gaa gtc ace cat cag ggc ctg age teg ccc His Lys VaI Tyr Ala Cys GIu VaI Thr His Gin Gly Leu Ser Ser Pro gtc aca aag age ttc aac agg gga gag tgt tag VaI Thr Lys Ser Phe Asn Arg Gly GIu Cys
<210> 31
<211> 234
<212> PRT
<213> Artificial Sequence
<220?
<223> Description of Artificial Sequence: Synthetic Protein
<400> 31
Met GIu Thr Asp Thr Leu Leu Leu Trp Leu Leu Leu Leu Met Phe Pro Gly Thr Arg Cys Asp lie Gin Met Thr GIn Ser Pro Ser Ser Leu Ser Ala Ser Leu Gly GIu Arg VaI Ser Leu Thr Cys Arg Ala Ser GIn Asp lie Gly Gly Asn Leu Tyr Trp Leu Gln Gln Gly Pro Asp Gly Thr He Lys Arg Leu He Tyr Ala Thr Ser Ser Leu Asp Ser Gly VaI Pro Lys Arg Phe Ser Gly Ser Arg Ser Gly Ser Asp Tyr Ser Leu Thr He Ser Ser Leu GIu Ser GIu Asp Phe VaI Asp Tyr Tyr Cys Leu Gin Tyr Ser Ser Ser Pro Trp Thr Phe Gly Gly Ala Thr Lys Met GIu He Lys Arg Thr VaI Ala Ala Pro Ser VaI Phe He Phe Pro Pro Ser Asp GIu Gin Leu Lys Ser Gly Thr Ala Ser VaI VaI Cys Leu Leu Asn Asn Phe Tyr Pro Arg GIu Ala Lys VaI GIn Trp Lys VaI Asp Asn Ala Leu GIn Ser Gly Asn Ser Gin GIu Ser VaI Thr GIu Gln Asp Ser Lys Asp Ser Thr Tyr Ser Leu Ser Ser Thr Leu Thr Leu Ser Lys Ala Asp Tyr GIu Lys His Lys VaI Tyr Ala Cys GIu VaI Thr His GIn Gly Leu Ser Ser Pro VaI Thr Lys Ser Phe Asn Arg Gly Glu Cys
<210> 32 <211> 6505
<212> DNA
<213 > Artificial Sequence
<220>
<223 > Description of Artificial Sequence : Synthetic DNA
<400> 32 cgatgtacgg gccagatata cgcgttgaca ttgattattg actagttatt aatagtaatc aattacgggg tcattagttc atagcccata tatggagttc cgcgttacat aacttacggt aaatggcccg cctggctgac cgcccaacga cccccgccca ttgacgtcaa taatgacgta tgttcccata gtaacgccaa tagggacttt ccattgacgt caatgggtgg agtatttacg gtaaactgcc cacttggcag tacatcaagt gtatcatatg ccaagtacgc cccctattga cgtcaatgac ggtaaatggc ccgcctggca ttatgcccag tacatgacct tatgggactt tcctacttgg cagtacatct acgtattagt catcgctatt accatggtga tgcggttttg gcagtacatc aatgggcgtg gatagcggtt tgactcacgg ggatttccaa gtctccaccc cattgacgtc aatgggagtt tgttttggca ccaaaatcaa cgggactttc caaaatgtcg taacaactcc gccccattga cgcaaatggg cggtaggcgt gtacggtggg aggtctatat aagcagagct ctctggctaa ctagagaacc cactgcttac tggcttatcg aaattaatac gactcactat agggagaccc ttgctagcga tattccacca tggaatgcag ctgggtcatg ctcttcctcc tgtcaggaac tgcaggtgtc cattgccagg ttcagctgca gcagtctgga cctgagctgg tgaagcctgg ggctttagtg aagatatcct gcaaggcttσ tggttacacc ttcacaaact acgatataca ctgggtgaag cagaggcctg gacagggact tgagtggatt ggatggattt atcctggaga tggtagtact aagtacaatg agaaattcaa gggcaaggcc acactgactg cagacaaatc ctccagcaca gcctacatgc acctcagcag cctgacttct gagaaatctg cagtctattt ctgtgcaaga gagtgggctt actggggcca agggactctg gtcactgtct ctgcagctag caccaagggc ccatcggtct tccccctggc accctcctcc aagagcacct ctgggggcac agcggccctg ggctgcctgg tcaaggacta cttccccgaa ccggtgacgg tgtcgtggaa ctcaggcgcc ctgaccagcg gcgtgcacac cttcccggct gtcctacagt cctcaggact ctactccctc agcagcgtgg tgaccgtgcc ctccagcagc ttgggcaccc agacctacat ctgcaacgtg aatcacaagc ccagcaacac caaggtggac aagaaagttg agcccaaatc ttgtgacaaa actcacacat gcccaccgtg cccagcacct gaactcctgg ggggaccgtc agtcttcctc ttccccccaa aacccaagga caccctcatg atctcccgga cccctgaggt cacatgcgtg gtggtggacg tgagccacga agaccctgag gtcaagttca actggtacgt ggacggcgtg gaggtgcata atgccaagac aaagccgcgg gaggagcagt acaacagcac gtaccgtgtg gtcagcgtcc tcaccgtcct gcaccaggac tggctgaatg gcaaggagta caagtgcaag gtctccaaca aagccctccc agcccccatc gagaaaacca tctccaaagc caaagggcag ccccgagaac cacaggtgta caccctgccc ccatcccggg atgagctgac caagaaccag gtcagcctga cctgcctggt caaaggcttc tatcccagcg acatcgccgt ggagtgggag agcaatgggc agccggagaa caactacaag accacgcctc ccgtgctgga ctccgacggc tccttcttcc tctacagcaa gctcaccgtg gacaagagca ggtggcagca ggggaacgtc ttctcatgct ccgtgatgca tgaggctctg cacaaccact acacgcagaa gagcctctcc ctgtctccgg gtaaatcgag tatgcactct gaccctgccc gtcgaggtga gctgagcgtg tgtgacagta ttagtgagtg ggtaacggcg gcagacaaaa agactgcagt ggacatgtcg ggcgggacgg tcacagtcct tgaaaaggtc cctgtatcaa aaggccaact gaagcaatac ttctacgaga ccaagtgcaa tcccatgggt tacacaaaag aaggctgcag gggcatagac aaaaggcatt ggaactccca gtgccgaact acccagtcgt acgtgcgggc ccttaccatg gatagcaaaa agagaattgg ctggcgattc ataaggatag acacttcttg tgtatgtaca ttgaccatta aaaggtgagg atccctcgag catgcatcta gagggcccta ttctatagtg tcacctaaat gctagagctc gctgatcagc ctcgactgtg ccttctagtt gccagccatc tgttgtttgc ccctcccccg tgccttcctt gaccctggaa ggtgccactc ccactgtcct ttcctaataa aatgaggaaa ttgcatcgca ttgtctgagt aggtgtcatt ctattctggg gggtggggtg gggcaggaca gcaaggggga ggattgggaa gacaatagca ggcatgctgg ggatgcggtg ggctctatgg cttctgaggc ggaaagaacc agtggcggta atacggttat ccacagaatc aggggataac gcaggaaaga acatgtgagc aaaaggccag caaaaggcca ggaaccgaat tcgatattcc atacacatac ttctgtgttc ctttgaaagc tggacttttg caggctccac cagacctctc tagatcaatt cctttgccta atttcgctta caatttacgc gcgcgttgac attgattatt gactagttat taatagtaat caattacggg gtcattagtt catagcccat atatggagtt ccgcgttaca
taacttacgg taaatggccc gcctggctga ccgcccaacg acccccgccc attgacgtca ataatgacgt atgttcccat agtaacgcca atagggactt tccattgacg tcaatgggtg gagtatttac ggtaaactgc ccacttggca gtacatcaag tgtatcatat gccaagtacg ccccctattg acgtcaatga cggtaaatgg cccgcctggc attatgccca gtacatgacc ttatgggact ttcctacttg gcagtacatc tacgtattag tcatcgctat taccatggtg atgcggtttt ggcagtacat caatgggcgt ggatagcggt ttgactcacg gggatttcca agtctccacc ccattgacgt caatgggagt ttgttttggc accaaaatca acgggacttt ccaaaatgtc gtaacaactc cgccccattg acgcaaatgg gcggtaggcg tgtacggtgg gaggtctata taagcagagc tctctggcta actagagaac ccactgctta ctggcttatc gaaattaata cgactcacta tagggagacc caagctggct agcgatatca ccatggagac agacacactc ctgctatggc tcttgttgct catgtttcca ggtaccagat gtgacatcca gatgacccag tctccatcct ccttatctgc ctctctggga gaaagagtca gtctcacttg tcgggcaagt caggacattg gtggtaactt atactggctt cagcagggac cagatggaac tattaaacgc ctgatctacg ccacatccag tttagattct ggtgtcccca aaaggttcag tggcagtagg tctgggtcag attattctct caccatcagc agccttgagt ctgaagattt tgtagactat tactgtctac agtattctag ttctccgtgg acgttcggtg gagcgacaaa gatggaaata aaacgaactg tggctgcacc atctgtcttc atcttcccgc catctgatga gcagttgaaa tctggaactg cctctgttgt gtgcctgctg aataacttct atcccagaga ggccaaagta cagtggaagg tggataacgc cctccaatcg ggtaactccc aggagagtgt cacagagcag gacagcaagg acagcaccta cagcctcagc agcaccctga cgctgagcaa agcagactac gagaaacaca aagtctacgc ctgcgaagtc acccatcagg gcctgagctc gcccgtcaca aagagcttca acaggggaga gtgttagctc gagtctagag ggcccgttta aacccgctga tcagcctcga ctgtgccttc tagttgccag ccatctgttg tttgcccctc ccccgtgcct tccttgaccc tggaaggtgc cactcccact gtcctttcct aataaaatga ggaaattgca tcgcattgtc tgagtaggtg tcattctatt ctggggggtg gggtggggca ggacagcaag ggggaggatt gggaagacaa tagcaggcat gctggggatg cggtgggctc tatggcttct gaggcggaaa gaaccagtgg cggtaatacg gttatccaca gaatcagggg ataacgaaat gaggacttaa cctgtggaaa tatcaagctt gcggccgcgt atcgacgctc tcccttatgc gactcctgca ttaggaagca gcccagtagt aggttgaggc cgttgagcac cgccgccgca aggaatggtg catgcaagga gatggcgccc aacagtcccc cggccacggg gcctgccacc atacccacgc cgaaacaagc gctcatgagc ccgaagtggc gagcccgatc ttccccatcg gtgatgtcgg cgatataggc gccagcaacc gcacctgtgg cgccggtgat gccggccacg atgcgtccgg cgtagaggat ctctgacgga aggaaagaag tcagaaggca aaaacgagag taactccaca gtagctccaa attctttata agggtcaatg tccatgcccc aaagccaccc aaggcacagc ttggaggctt gaacagtggg acatgtacaa gagatgatta ggcagaggtg aaaaagttgc atggtgctgg tgcgcagacc aatttatgcc tacagcctcc taatacaaag acctttaacc taatctcctc ccccagctcc tcccagtcct taaacacaca gtctttgaag taggcctcaa ggtcggtcgt tgacattgct gggagtccaa gagtcctctt atgtaagacc ttgggcagga tctgatgggc gttcacggtg gtctccatgc aacgtgcaga ggtgaagcga agtgcacacg gaccggcaga tgagaaggca cagacgggga gaccgcgtaa agagaggtgc gccccgtggt cggctggaac ggcagacgga gaaggggacg agagagtccc aagcggcccc gcgaggggtc gtccgcggga ttcagcgccg acgggacgta aacaaaggac gtcccgcgaa ggatctaaag ccagcaaaag tcccatggtc ttataaaaat gcatagcttt aggaggggag cagagaactt gaaagcatct tcctgttagt ctttcttctc gtagacttca aacttatact tgatgccttt ttcctcctgg acctcagaga ggacgcctgg gtattctggg agaagtttat atttccccaa atcaatttct gggaaaaacg tgtcactttc aaattcctgc atgatccttg tcacaaagag tctgaggtgg cctggttgat tcatggcttc ctggtaaaca gaactgcctc cgactatcca aaccatgtct actttacttg ccaattccgg ttgttcaata agtcttaagg catcatccaa acttttggca agaaaatgag ctcctcgtgg tggttctttg agttctctac tgagaactat attaattctg tcctttaaag gtcgattctt ctcaggaatg gagaaccagg ttttcctacc cataatcacc agattctgtt taccttccac tgaagaggtt gtggtcattc tttggaagta cttgaactcg ttcctgagcg gaggccaggg tcggtctccg ttcttgccaa tccccatatt ttgggacacg gcgacgatgc agttcaatgg tcgaaccatg atggcaaatt ctagaatcga taagcttttt gcaaaagcct aggcctccaa aaaagcctcc tcactacttc tggaatagct cagaggccga ggcggcctcg gcctctgcat aaataaaaaa aattagtcag ccatggggcg gagaatgggc ggaactgggc ggagttaggg gcgggatggg cggagttagg ggcgggacta tggttgctga ctaattgaga tgcagatctc gagctagcac gcgtaagagc tcggtacctc cctac
<210> 33
<211> 1749
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic DNA
<220>
<221> CDS
<222> (1) .. (1746)
<400> 33 atg gaa tgc age tgg gtc atg etc ttc etc ctg tea gga act gca ggt
Met GIu Cys Ser Trp VaI Met Leu Phe Leu Leu Ser Gly Thr Ala Gly gtc cat tgc cag gtt cag ctg cag cag tet gga ect gag ctg gtg aag
VaI His Cys GIn VaI Gin Leu GIn Gin Ser Gly Pro Glu Leu VaI Lys cct ggg get tta gtg aag ata tec tgc aag get tct ggt tac ace ttc
Pro Gly Ala Leu VaI Lys lie Ser Cys Lys Ala Ser Gly Tyr Thr Phe aca aac tac gat ata cac tgg gtg aag cag agg cct gga cag gga ctt Thr Asn Tyr Asp lie His Trp VaI Lys GIn Arg Pro Gly GIn Gly Leu gag tgg att gga tgg att tat cct gga gat ggt agt act aag tac aat GIu Trp lie Gly Trp lie Tyr Pro Gly Asp Gly Ser Thr Lys Tyr Asn gag aaa ttc aag ggc aag gee aca ctg act gca gac aaa tec tec age GIu Lys Phe Lys Gly Lys Ala Thr Leu Thr Ala Asp Lys Ser Ser Ser aca gcc tac atg cac etc age age ctg act tct gag aaa tct gca gtc Thr Ala Tyr Met His Leu Ser Ser Leu Thr Ser GIu Lys Ser Ala VaI tat ttc tgt gca aga gag tgg get tac tgg ggc caa ggg act ctg gtc Tyr Phe Cys Ala Arg GIu Trp Ala Tyr Trp Gly GIn Gly Thr Leu VaI act gtc tct gca get age ace aag ggc cca teg gtc ttc ccc ctg gca Thr VaI Ser Ala Ala Ser Thr Lys Gly Pro Ser VaI Phe Pro Leu Ala ccc tec tec aag age ace tct ggg ggc aca gcg gcc ctg ggc tgc ctg Pro Ser Ser Lys Ser Thr Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu gtc aag gac tac ttc ccc gaa ccg gtg acg gtg teg tgg aac tea ggc VaI Lys Asp Tyr Phe Pro GIu Pro VaI Thr VaI Ser Trp Asn Ser Gly gcc ctg ace age ggc gtg cac ace ttc ccg get gtc eta cag tec tea Ala Leu Thr Ser Gly VaI His Thr Phe Pro Ala VaI Leu GIn Ser Ser gga etc tac tec etc age age gtg gtg ace gtg ccc tec age age ttg Gly Leu Tyr Ser Leu Ser Ser VaI VaI Thr VaI Pro Ser Ser Ser Leu ggc ace cag ace tac ate tgc aac gtg aat cac aag ccc age aac ace Gly Thr GIn Thr Tyr lie Cys Asn VaI Asn His Lys Pro Ser Asn Thr aag gtg gac aag aaa gtt gag ccc aaa tct tgt gac aaa act cac aca Lys VaI Asp Lys Lys VaI GIu Pro Lys Ser Cys Asp Lys Thr His Thr tgc cca ccg tgc cca gca cct gaa etc ctg ggg gga ccg tea gtc ttc Cys Pro Pro Cys Pro Ala Pro GIu Leu Leu Gly Gly Pro Ser VaI Phe etc ttc ccc cca aaa ccc aag gac ace etc atg ate tec egg ace cct Leu Phe Pro Pro Lys Pro Lys Asp Thr Leu Met lie Ser Arg Thr Pro gag gtc aca tgc gtg gtg gtg gac gtg age cac gaa gac cct gag gtc GIu VaI Thr Cys VaI VaI VaI Asp VaI Ser His Glu Asp Pro GIu VaI aag ttc aac tgg tac gtg gac ggc gtg gag gtg cat aat gcc aag aca Lys Phe Asn Trp Tyr VaI Asp Gly VaI GIu VaI His Asn Ala Lys Thr aag ccg egg gag gag cag tac aac age acg tac cgt gtg gtc age gtc Lys Pro Arg GIu GIu GIn Tyr Asn Ser Thr Tyr Arg VaI VaI Ser VaI etc ace gtc ctg cac cag gac tgg ctg aat ggc aag gag tac aag tgc Leu Thr VaI Leu His Gin Asp Trp Leu Asn Gly Lys GIu Tvr Lvs Cvs
aag gtc tec aac aaa gcc etc cca gcc ccc ate gag aaa ace ate tec Lys VaI Ser Asn Lys Ala Leu Pro Ala Pro lie GIu Lys Thr lie Ser aaa gcc aaa ggg cag ccc cga gaa cca cag gtg tac ace ctg ccc cca Lys Ala Lys Gly Gin Pro Arg GIu Pro GIn VaI Tyr Thr Leu Pro Pro tec egg gat gag ctg ace aag aac cag gtc age ctg ace tgc ctg gtc Ser Arg Asp Glu Leu Thr Lys Asn GIn VaI Ser Leu Thr Cys Leu VaI aaa ggc ttc tat ccc age gac ate gcc gtg gag tgg gag age aat ggg Lys Gly Phe Tyr Pro Ser Asp He Ala VaI GIu Trp GIu Ser Asn Gly cag ccg gag aac aac tac aag ace acg cct ccc gtg ctg gac tec gac GIn Pro GIu Asn Asn Tyr Lys Thr Thr Pro Pro VaI Leu Asp Ser Asp ggc tec ttc ttc etc tac age aag etc ace gtg gac aag age agg tgg Gly Ser Phe Phe Leu Tyr Ser Lys Leu Thr VaI Asp Lys Ser Arg Trp cag cag ggg aae gtc ttc tea tgc tec gtg atg cat gag get ctg cac GIn GIn Gly Asn VaI Phe Ser Cys Ser VaI Met His GIu Ala Leu His aac cac tac acg cag aag age etc tec ctg tct ccg ggt aaa teg agt Asn His Tyr Thr Gln Lys Ser Leu Ser Leu Ser Pro Gly Lys Ser Ser atg cac tct gac cct gcc cgt cga ggt gag ctg age gtg tgt gac agt Met His Ser Asp Pro Ala Arg Arg Gly GIu Leu Ser VaI Cys Asp Ser att agt gag tgg gta acg gcg gca gac aaa aag act gca gtg gac atg He Ser Glu Trp VaI Thr Ala Ala Asp Lys Lys Thr Ala VaI Asp Met teg ggc ggg acg gtc aca gtc ctt gaa aag gtc cct gta tea aaa ggc Ser Gly Gly Thr VaI Thr VaI Leu GIu Lys VaI Pro VaI Ser Lys Gly caa ctg aag caa tac ttc tac gag ace aag tgc aat ccc atg ggt tac GIn Leu Lys Gin Tyr Phe Tyr GIu Thr Lys Cys Asn Pro Met Gly Tyr aca aaa gaa ggc tgc agg ggc ata gac aaa agg cat tgg aac tec cag Thr Lys GIu Gly Cys Arg Gly He Asp Lys Arg His Trp Asn Ser GIn tgc cga act ace cag teg tac gtg egg gcc ctt ace atg gat age aaa Cys Arg Thr Thr Gin Ser Tyr VaI Arg Ala Leu Thr Met Asp Ser Lys aag aga att ggc tgg cga ttc ata agg ata gac act tct tgt gta tgt Lys Arg He Gly Trp Arg Phe He Arg He Asp Thr Ser Cys VaI Cys aca ttg ace att aaa agg tga Thr Leu Thr He Lys Arg
<210> 34
<211> 582
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic Protein
<400> 34
Met Glu Cys Ser Trp VaI Met Leu Phe Leu Leu Ser Gly Thr Ala Gly VaI His Cys GIn VaI GIn Leu GIn GIn Ser Gly Pro GIu Leu VaI Lys Pro Gly Ala Leu VaI Lys He Ser Cys Lys Ala Ser Gly Tyr Thr Phe Thr Asn Tyr Asp He His Trp VaI Lys GIn Arg Pro Gly Gin Gly Leu GIu Trp He Gly Trp He Tyr Pro Gly Asp Gly Ser Thr Lys Tyr Asn GIu Lys Phe Lys Gly Lys Ala Thr Leu Thr Ala Asp Lys Ser Ser Ser Thr Ala Tyr Met His Leu Ser Ser Leu Thr Ser GIu Lys Ser Ala VaI Tyr Phe Cys Ala Arg GIu Trp Ala Tyr Trp Gly GIn Gly Thr Leu VaI Thr VaI Ser Ala Ala Ser Thr Lys Gly Pro Ser VaI Phe Pro Leu Ala Pro Ser Ser Lys Ser Thr Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu VaI Lys Asp Tyr Phe Pro GIu Pro VaI Thr VaI Ser Trp Asn Ser Gly Ala Leu Thr Ser Gly VaI His Thr Phe Pro Ala VaI Leu Gin Ser Ser Gly Leu Tyr Ser Leu Ser Ser VaI VaI Thr VaI Pro Ser Ser Ser Leu Gly Thr GIn Thr Tyr He Cys Asn VaI Asn His Lys Pro Ser Asn Thr Lys VaI Asp Lys Lys VaI GIu Pro Lys Ser Cys Asp Lys Thr His Thr
Cys Pro Pro Cys Pro Ala Pro Glu Leu Leu Gly Gly Pro Ser VaI Phe Leu Phe Pro Pro Lys Pro Lys Asp Thr Leu Met He Ser Arg Thr Pro GIu VaI Thr Cys VaI VaI VaI Asp VaI Ser His Glu Asp Pro Glu VaI Lys Phe Asn Trp Tyr VaI Asp Gly VaI Glu VaI His Asn Ala Lys Thr Lys Pro Arg Glu Glu GIn Tyr Asn Ser Thr Tyr Arg VaI VaI Ser VaI Leu Thr VaI Leu His GIn Asp Trp Leu Asn Gly Lys Glu Tyr Lys Cys Lys VaI Ser Asn Lys Ala Leu Pro Ala Pro He Glu Lys Thr He Ser Lys Ala Lys Gly GIn Pro Arg Glu Pro GIn VaI Tyr Thr Leu Pro Pro Ser Arg Asp Glu Leu Thr Lys Asn GIn VaI Ser Leu Thr Cys Leu VaI Lys Gly Phe Tyr Pro Ser Asp He Ala VaI Glu Trp Glu Ser Asn Gly GIn Pro GIu Asn Asn Tyr Lys Thr Thr Pro Pro VaI Leu Asp Ser Asp Gly Ser Phe Phe Leu Tyr Ser Lys Leu Thr VaI Asp Lys Ser Arg Trp GIn GIn Gly Asn VaI Phe Ser Cys Ser VaI Met His Glu Ala Leu His Asn His Tyr Thr GIn Lys Ser Leu Ser Leu Ser Pro Gly Lys Ser Ser Met His Ser Asp Pro Ala Arg Arg Gly Glu Leu Ser VaI Cys Asp Ser He Ser Glu Trp VaI Thr Ala Ala Asp Lys Lys Thr Ala VaI Asp Met Ser Gly Gly Thr VaI Thr VaI Leu Glu Lys VaI Pro VaI Ser Lys Gly GIn Leu Lys Gin Tyr Phe Tyr Glu Thr Lys Cys Asn Pro Met Gly Tyr Thr Lys Glu Gly Cys Arg Gly He Asp Lys Arg His Trp Asn Ser Gln Cys Arg Thr Thr Gin Ser Tyr VaI Arg Ala Leu Thr Met Asp Ser Lys Lys Arg He Gly Trp Arg Phe He Arg He Asp Thr Ser Cys VaI Cys Thr Leu Thr He Lys Arg
<210> 35
<211> 705
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic DNA
<220>
<221> CDS
<222> (1) .. (702)
<400> 35 atg gag aca gac aca etc ctg eta tgg etc ttg ttg etc atg ttt cca Met Glu Thr Asp Thr Leu Leu Leu Trp Leu Leu Leu Leu Met Phe Pro ggt ace aga tgt gac ate cag atg ace cag tct cca tec tec tta tct Gly Thr Arg Cys Asp He GIn Met Thr GIn Ser Pro Ser Ser Leu Ser gee tct ctg gga gaa aga gtc agt etc act tgt egg gca agt cag gac Ala Ser Leu Gly Glu Arg VaI Ser Leu Thr Cys Arg Ala Ser GIn Asp att ggt ggt aac tta tac tgg ctt cag cag gga cca gat gga act att He Gly Gly Asn Leu Tyr Trp Leu GIn Gin Gly Pro Asp Gly Thr He aaa cgc ctg ate tac gcc aca tec agt tta gat tct ggt gtc ccc aaa Lys Arg Leu He Tyr Ala Thr Ser Ser Leu Asp Ser Gly VaI Pro Lys agg ttc agt ggc agt agg tct ggg tea gat tat tct etc ace ate age Arg Phe Ser Gly Ser Arg Ser Gly Ser Asp Tyr Ser Leu Thr He Ser age ctt gag tct gaa gat ttt gta gac tat tac tgt eta cag tat tct Ser Leu Glu Ser Glu Asp Phe VaI Asp Tyr Tyr Cys Leu GIn Tyr Ser agt tct ccg tgg acg ttc ggt gga gcg aca aag atg gaa ata aaa cga Ser Ser Pro Trp Thr Phe Gly Gly Ala Thr Lys Met Glu He Lys Arg act gtg get gca cca tct gtc ttc ate ttc ccg cca tct gat gag cag Thr VaI Ala Ala Pro Ser VaI Phe He Phe Pro Pro Ser Asp Glu Gin ttg aaa tct gga act gcc tct gtt gtg tgc ctg ctg aat aac ttc tat Leu Lys Ser Gly Thr Ala Ser VaI VaI Cys Leu Leu Asn Asn Phe Tyr ccc aga gag gcc aaa gta cag tgg aag gtg gat aac qcc etc caa tccr
Pro Arg GIu Ala Lys VaI Gin Trp Lys VaI Asp Asn Ala Leu GIn Ser ggt aac tec cag gag agt gtc aca gag cag gac age aag gac age ace G l y Asn Ser GIn GIu Ser VaI Thr GIu GIn Asp Ser Lys Asp Ser Thr tac age etc age age ace ctg acg ctg age aaa gca gac tac gag aaa Tyr Ser Leu Ser Ser Thr Leu Thr Leu Ser Lys Ala Asp Tyr GIu Lys cac aaa gtc tac gee tgc gaa gtc ace cat cag ggc ctg age teg ccc His Lys VaI Tyr Ala Cys Glu VaI Thr His GIn G l y Leu Ser Ser Pro gtc aca aag age ttc aac agg gga gag tgt tag VaI Thr Lys Ser Phe Asn Arg G l y Glu Cys
<210 > 36
<211> 234
<212> PRT
<213 > Artificial Sequence
<220 >
<223 > Description of Artificial Sequence : Synthetic Protein
<400 > 36 Met Glu Thr Asp Thr Leu Leu Leu Trp Leu Leu Leu Leu Met Phe Pro G l y Thr Arg Cys Asp He Gin Met Thr G l n Ser Pro Ser Ser Leu Ser Ala Ser Leu G l y Glu Arg VaI Ser Leu Thr Cys Arg Ala Ser GIn Asp He G l y G l y Asn Leu Tyr Trp Leu GIn Gin Gly Pro Asp Gly Thr He Lys Arg Leu He Tyr Ala Thr Ser Ser Leu Asp Ser Gly VaI Pro Lys Arg Phe Ser G l y Ser Arg Ser G l y Ser Asp Tyr Ser Leu Thr He Ser Ser Leu Glu Ser Glu Asp Phe VaI Asp Tyr Tyr Cys Leu GIn Tyr Ser Ser Ser Pro Trp Thr Phe Gly G l y Ala Thr Lys Met Glu He Lys Arg Thr VaI Ala Ala Pro Ser VaI Phe He Phe Pro Pro Ser Asp Glu GIn Leu Lys Ser G l y Thr Ala Ser VaI VaI Cys Leu Leu Asn Asn Phe Tyr Pro Arg Glu Ala Lys VaI Gin Trp Lys VaI Asp Asn Ala Leu GIn Ser G l y Asn Ser Gin Glu Ser VaI Thr Glu GIn Asp Ser Lys Asp Ser Thr Tyr Ser Leu Ser Ser Thr Leu Thr Leu Ser Lys Ala Asp Tyr Glu Lys His Lys VaI Tyr Ala Cys Glu VaI Thr His GIn Gly Leu Ser Ser Pro VaI Thr Lys Ser Phe Asn Arg Gly Glu Cys
<210 > 37
<211> 564
<212 > DNA
<213 > Mus musculus
<220>
<221> CDS
<222 > ( 1 ) . . ( 561 )
<400> 37 atg gtt cga cca ttg aac tgc ate gtc gee gtg tec caa aat atg ggg Met VaI Arg Pro Leu Asn Cys He VaI Ala VaI Ser Gin Asn Met Gly att ggc aag aac gga gac cga ccc tgg cct ccg etc agg aac gag ttc He Gly Lys Asn Gly Asp Arg Pro Trp Pro Pro Leu Arg Asn Glu Phe aag tac ttc caa aga atg ace aca ace tct tea gtg gaa ggt aaa cag Lys Tyr Phe Gin Arg Met Thr Thr Thr Ser Ser VaI Glu Gly Lys Gin aat ctg gtg att atg ggt agg aaa ace tgg ttc tec att cct gag aag Asn Leu VaI He Met Gly Arg Lys Thr Trp Phe Ser He Pro Glu Lys aat cga cct tta aag gac aga att aat ata gtt etc agt aga gaa etc Asn Arg Pro Leu Lys Asp Arg He Asn He VaI Leu Ser Arg Glu Leu aaa gaa cca cca cga gga get cat ttt ctt gee aaa agt ttg gat gat
Lys GIu Pro Pro Arg Gly Ala His Phe Leu Ala Lys Ser Leu Asp Asp gcc tta aga ctt att gaa caa ccg gaa ttg gca agt aaa gta gac atg
Ala Leu Arg Leu lie Glu GIn Pro Glu Leu Ala Ser Lys VaI Asp Met gtt tgg ata gtc gga ggc agt tct gtt tac cag gaa gcc atg aat caa
VaI Trp He VaI Gly Gly Ser Ser VaI Tyr Gin GIu Ala Met Asn GIn cca ggc cac etc aga etc ttt gtg aca agg ate atg cag gaa ttt gaa
Pro Gly His Leu Arg Leu Phe VaI Thr Arg lie Met GIn GIu Phe GIu agt gac acg ttt ttc cca gaa att gat ttg ggg aaa tat aaa ctt etc
Ser Asp Thr Phe Phe Pro GIu lie Asp Leu Gly Lys Tyr Lys Leu Leu cca gaa tac cca ggc gtc etc tct gag gtc cag gag gaa aaa ggc ate
Pro GIu Tyr Pro Gly VaI Leu Ser GIu VaI Gln GIu GIu Lys Gly He aag tat aag ttt gaa gtc tac gag aag aaa gac taa Lys Tyr Lys Phe GIu VaI Tyr Glu Lys Lys Asp
<210> 38
<211> 187
<212> PRT
<213> Mus musculus
<400> 38
Met VaI Arg Pro Leu Asn Cys He VaI Ala VaI Ser GIn Asn Met Gly He Gly Lys Asn Gly Asp Arg Pro Trp Pro Pro Leu Arg Asn GIu Phe Lys Tyr Phe Gin Arg Met Thr Thr Thr Ser Ser VaI GIu Gly Lys Gin Asn Leu VaI He Met Gly Arg Lys Thr Trp Phe Ser He Pro GIu Lys Asn Arg Pro Leu Lys Asp Arg He Asn He VaI Leu Ser Arg GIu Leu Lys GIu Pro Pro Arg Gly Ala His Phe Leu Ala Lys Ser Leu Asp Asp Ala Leu Arg Leu He GIu GIn Pro GIu Leu Ala Ser Lys VaI Asp Met VaI Trp He VaI Gly Gly Ser Ser VaI Tyr GIn GIu Ala Met Asn Gin Pro Gly His Leu Arg Leu Phe VaI Thr Arg He Met GIn GIu Phe GIu Ser Asp Thr Phe Phe Pro GIu He Asp Leu Gly Lys Tyr Lys Leu Leu Pro GIu Tyr Pro Gly VaI Leu Ser GIu VaI GIn GIu Glu Lys Gly He Lys Tyr Lys Phe Glu VaI Tyr GIu Lys Lys Asp
<210> 39
<211> 119
<212> PRT
<213> Homo sapiens
<400> 39
His Ser Asp Pro Ala Arg Arg Gly GIu Leu Ser VaI Cys Asp Ser He Ser Glu Trp VaI Thr Ala Ala Asp Lys Lys Thr Ala VaI Asp Met Ser Gly Gly Thr VaI Thr VaI Leu GIu Lys VaI Pro VaI Ser Lys Gly GIn Leu Lys Gin Tyr Phe Tyr Glu Thr Lys Cys Asn Pro Met Gly Tyr Thr Lys Glu Gly Cys Arg Gly He Asp Lys Arg His Trp Asn Ser Gin Cys Arg Thr Thr GIn Ser Tyr VaI Arg Ala Leu Thr Met Asp Ser Lys Lys Arg He Gly Trp Arg Phe He Arg He Asp Thr Ser Cys VaI Cys Thr Leu Thr He Lys Arg Gly Arg
<210> 40
<211> 25
<212> DNA
<213> artificial sequence
<220>
<223> Description of Artificial Sequence: Synthetic Oligonucleotide
<400 > 40
ATGAAGTTATGGGATGTCGTGGCTG
<210> 41
<211> 25
<212 > DNA
<213 > artif icial sequence
<220 >
<223 > Description of Artificial Sequence : Synthetic
Oligonucleotide
<400 > 41
TCAGATACATC CACACCTTTTAGCG
<210> 42
<211> 26
<212 > DNA
<213 > artificial sequence
<220>
<223 > Description of Artificial Sequence : Synthetic
Oligonucleotide
<400 > 42 CATCACCAGATAAACAAATGGCAGTG
<210> 43
<211> 636
<212 > DNA
<213 > artificial sequence
<220>
<223 > Descriptioi i of Arti
<400> 43
ATGAAGTTATGGGATGTCGTGGCTGTCTGCCTGGTGCTGCTCCACACCGCGTCCGCCTTCCCGCTGCCCGCC
GGTAAGAGGCCTCCCGAGGCGCCCGCCGAAGACCGCTCCCTCGGCCGCCGCCGCGCGCCCTTCGCGCTGAGC
AGTGACTCAAATATGCCAGAGGATTATCCTGATCAGTTCGATGATGTCATGGATTTTATTCAAGCCACCATT
AAAAGACTGAAAAGGTCACCAGATAAACAAATGGCAGTGCTTCCTAGAAGAGAGCGGAATCGGCAGGCTGCA
GCTGCCAACCCAGAGAATTCCAGAGGAAAAGGTCGGAGAGGCCAGAGGGGCAAAAACCGGGGTTGTGTCTTA
ACTGCAATACATTTAAATGTCACTGACTTGGGTCTGGGCTATGAAACCAAGGAGGAACTGATTTTTAGGTAC
TGCAGCGGCTCTTGCGATGCAGCTGAGACAACGTACGACAAAATATTGAAAAACTTATCCAGAAATAGAAGG
GATAACCTGGTTTACCATATTCTAAGAAAGCATTCCGCTAAAAGGTGTGGATGTATCTGA
<210> 44
<211> 211
<212 > PRT
<213 > artificial sequence
<220 >
<223 > DescriDtioi i of Arti
<400> 44
MKLWDWAVCLVLLHTASAFPLPAGKRPPEAPAEDRSLGRRRAPFALSSDSNMPEDYPDQFDDVrøFIQATI KRLKRS PDKQMAVLPRRΞRNRQAAAANPENSRGKGRRGQRGKNKGCVLTA1HLNVTDLGLGYETKEELIFRY CSGSCDAAETTYDKILKNLSRNRRLVSDKVGQACCRPIAFDDDLSFLDDNL VYHI LRKHS AKRCGC I
<210> 45
<211> 1797
<212 > DNA
<213 > artif icial sequence
<220>
<223> Description of Arti
<400> 45
ATGGACTGGACCTGGAGGGTGTTCTGCCTGCTTGCAGTGGCCCCCGGAGCCCACAGCCAGGTTCAG
CTGCAGCAGTCTGGACCTGAGCTGGTGAAGCCTGGGGCTTTAGTGAAGATATCCTGCAAGGCTTCT
GGTTACACCTTCACAAACTACGATATACACTGGGTGAAGCAGAGGCCTGGACAGGGACTTGAGTGG
ATTGGATGGATTTAT C CTGGAGATGGTAGTACTAAGTACAATGAGAAATTCAAGGGCAAGGCCACA
CTGACTGCAGACAAATC CT CCAGCACAGCCTACATGCACCTCAGCAGC CTGACTTCTGAGAAAT CT
GCAGTCTATTTCTGTGCAAGAGAGTGGGCTTACTGGGGCCAAGGGACTCTGGTCACTGTCTCTGCA
GCTAGCACCAAGGGCCCATCGGTCTTCCCCCTGGCACCCTCCTCCAAGAGCACCTCTGGGGGCACA
GCGGCCCTGGGCTGCCTGGTCAAGGACTACTTCCCCGAACCGGTGACGGTGTCGTGGAACTCAGGC
GCCCTGACCAGCGGCGTGCACACCTTCCCGGCTGTCCTACAGTCCTCAGGACTCTACTCCCTCAGC
AGCGTGGTGACCGTGCCCTCCAGCAGCTTGGGCACCCAGACCTACATCTGCAACGTGAATCACAAG
CCCAGC^AC^CCTΛGGTGGACAAGAAAGTTGAGCCCAAATCTTGTGACAAAACTCACACATGCCCA
CCGTGCCCAGCACCTGAACTCCTGGGGGGACCGTCAGTCTTCCTCTTCCCCCCAAAACCCAAGGAC
ACCCTCATGATCTCCCGGACCCCTGAGGTCACATGCGTGGTGGTGGACGTGAGCCACGAAGACCCT
GAGGTCAAGTTCAACTGGTACGTGGACGGCGTGGAGGTGCATAATGCCAAGACAAAGCCGCGGGAG
GAGCAGTACAACAGCACGTACCGTGTGGTCAGCGTCCTCACCGTCCTGCACCAGGACTGGCTGAAT
GGCAAGGAGTACAAGTGCAAGGTCTCCAACAAAGCCCTCCCAGCCCCCATCGAGAAAACCATCTCC
AAAGCCAAAGGGCAGCCCCGAGAACCACAGGTGTACACCCTGCCCCCATCCCGGGATGAGCTGACC
AAGAACCAGGTCAGCCTGACCTGCCTGGTCAAAGGCTTCTATCCCAGCGACATCGCCGTGGAGTGG
GAGAGCAATGGGCAGCCGGAGAACAACTACAAGACCACGCCTCCCGTGCTGGACTCCGACGGCTCC
TTCTTCCTCTACAGCAAGCTCACCGTGGACAAGAGCAGGTGGCAGCAGGGGAACGTCTTCTCATGC
TCCGTGATGCATGAGGCTCTGCACAACCACTACACGCAGAAGAGCCTCTCCCTGTCTCCGGGTAAA
AGTTCATCACCAGATAAACAAATGGCAGTGCTTCCTAGAAGAGAGCGGAATCGGCAGGCTGCAGCT
GCCAACCCAGAGAATTCCAGAGGAAAAGGTCGGAGAGGCCAGAGGGGCAAAAACCGGGGTTGTGTC
TTAACTGCAATACATTTAAATGTCACTGACTTGGGTCTGGGCTATGAAACCAAGGAGGAACTGATT
TTTAGGTACTGCAGCGGCTCTTGCGATGCAGCTGAGACAACGTACGACAAAATATTGAAAAACTTA
TCCAGAAATAGAAGGCTGGTGAGTGACAAAGTAGGGCAGGCATGTTGCAGACCCATCGCCTTTGAT
GATGACCTGTCGTTTTTAGATGATAACCTGGTTTACCATATTCTAAGAAAGCATTCCGCTAAAAGG
TGTGGATGTATCTGA
<210> 46
<211> 598
<212 > PRT
<213 > artificial sequence
<220>
<223 > Descriptioi i of Arti
<400> 46
mWTWRVFCLLAVAPGAHSQVQLQQSGPELVKPGALVKISCKASGYTFTNYD1HWVKQRPGQGLEWIGWIYP GDGSTKYNEKFKGKATLTADKSSSTAYMHLSSLTSEKSAVYFCAREWAYWGQGTLVTVSAASTKGPSVFPLA PSSKSTSGGTAALGCL VKDYFPEPVTVSWNSGALTSGVHTFPAVLQS SGLYSLSSWTVPSSSLGTQTYICN VNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCWVDVSHEDPEV KFNWYVDGVEVHNAKTKPREEQYNSTYRWSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPRΞ PQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPΞNNYKTTPPVLDSDGSFFLYSKLTVDKSRW QQGNVFSCSVMHEALHNHYTQKSLSLSPGKSSSPDKQMAVLPRRERNRQAAAANPENSRGKGRRGQRGKNRG CVLTA1HLNVTDLGLGYETKEELIFRYCSGSCDAAETTYDKILKNLSRNRRLVSDKVGQACCRPIAFDDDLS FLDDNLVYHILRKHSAKRCGCI
<210> 47
<211> 6342
<212> DNA
<213 > artificial sequence
<220>
<223 > DescriDtion of Arti
<400> 47
GTTGACATTGATTATTGACTAGTTATTAATAGTAATCAATTACGGGGTCATTAGTTCATAGCCC
ATATATGGAGTTCCGCGTTACATAACTTACGGTAAATGGCCCGCCTGGCTGACCGCCCAACGAC
C CCCGCCCATTGACGTCAATAATGACGTATGTTCCCATAGTAACGCCAATAGGGACTTTC CATT
GACGTCAATGGGTGGAGTATTTACGGTAAACTGCCCACTTGGCAGTACATCAAGTGTATCATAT
GCCAAGTACGCCCCCTATTGACGTCAATGACGGTAAATGGCCCGCCTGGCATTATGCCCAGTAC
ATGACCTTATGGGACTTTCCTACTTGGCAGTACATCTACGTATTAGTCATCGCTATTACCATGG
TGATGCGGTTTTGGCAGTACATCAATGGGCGTGGATAGCGGTTTGACTCACGGGGATTTCCAAG
TCTCCACCCCATTGACGTCAATGGGAGTTTGTTTTGGCACCAAAATCAACGGGACTTTCCAAAA
TGTCGTAACAACTCCGCCCCATTGACGCAAATGGGCGGTAGGCGTGTACGGTGGGAGGTCTATA
TAAGCAGAGCTCTCTGGCTAACTAGAGAACCCACTGCTTACTGGCTTATCGAAATTAATACGAC
TCACTATAGGGAGACCCAAGCTGGCTAGCGTTTAAACTTAAGCTTGGTACCGAGCTCGGATCCA
CTAGTCCAGTGTGGTGGAATTCTGCAGGCCGCCACCATGGAGACCCCCGCCCAGCTGCTGTTCC
TGTTGCTGCTTTGGCTTCCAGATACTACCGGCGACATCCAGATGACCCAGTCTCCATCCTCCTT
ATCTGCCTCTCTGGGAGAAAGAGTCAGTCTCACTTGTCGGGCAAGTCAGGACATTGGTGGTAAC
TTATACTGGCTTCAGCAGGGACCAGATGGAACTATTAAACGCCTGATCTACGCCACATCCAGTT
TAGATTCTGGTGTCCCCAAAAGGTTCAGTGGCAGTAGGTCTGGGTCAGATTATTCTCTCACCAT
CAGCAGCCTTGAGTCTGAAGATTTTGTAGACTATTACTGTCTACAGTATTCTAGTTCTCCGTGG
ACGTTCGGTGGAGGCACAAAGATGGAAATAAAACGAACTGTGGCTGCACCATCTGTCTTCATCT
TCCCGCCATCTGATGAGCAGTTGAAATCTGGAACTGCCTCTGTTGTGTGCCTGCTGAATAACTT
CTATCCCAGAGAGGCCAAAGTACAGTGGAAGGTGGATAACGCC CTCCAATCGGGTAACTC CCAG
GAGAGTGTCACAGAGCAGGACAGCAAGGACAGCACCTACAGCCTCAGCAGCACCCTGACGCTGA
GCAAAGCAGACTACGAGAAACACAAAGTCTACGCCTGCGAAGTCACCCATCAGGGCCTGAGCTC
GCCCGTCACAAAGAGCTTCAACAGGGGAGAGTGTTAGCTCGAGTCTAGAGGGCCCGTTTAAACC
CGCTGATCAGCCTCGACTGTGCCTTCTAGTTGCCAGCCATCTGTTGTTTGCCCCTCCCCCGTGC
CTTCCTTGACCCTGGAAGGTGCCACTCCCACTGTCCTTTCCTAATAAAATGAGGAAATTGCATC
GCATTGTCTGAGTAGGTGTCATTCTATTCTGGGGGGTGGGGTGGGGCAGGACAGCAAGGGGGAG
GATTGGGAAGACAATAGCAGGCATGCTGGGGATGCGGTGGGCTCTATGGCTTCTGAGGCGGAAA
GAACCAGCCGATGTACGGGCCAGATATACGCGTTGACATTGATTATTGACTAGTTATTAATAGT
AATCAATTACGGGGTCATTAGTTCATAGCCCATATATGGAGTTCCGCGTTACATAACTTACGGT
AAATGGCCCGCCTGGCTGACCGCCCAACGACCCCCGCCCATTGACGTCAATAATGACGTATGTT
CCCATAGTAACGCCAATAGGGACTTTCCATTGACGTCAATGGGTGGAGTATTTACGGTAAACTG
CCCACTTGGCAGTACATCAAGTGTATCATATGCCAAGTACGCCCCCTATTGACGTCAATGACGG
TAAATGGCCCGCCTGGCATTATGCCCAGTACATGACCTTATGGGACTTTCCTACTTGGCAGTAC
ATCTACGTATTAGTCATCGCTATTACCATGGTGATGCGGTTTTGGCAGTACATCAATGGGCGTG
GATAGCGGTTTGACTCACGGGGATTTCCAAGTCTCCACCCCATTGACGTCAATGGGAGTTTGTT
TTGGCACCAAAATCAACGGGACTTTCCAAAATGTCGTAACAACTCCGCCCCATTGACGCAAATG
GGCGGTAGGCGTGTACGGTGGGAGGTCTATATAAGCAGAGCTCTCTGGCTAACTAGAGAACCCA
CTGCTTACTGGCTTATCGAAATTAATACGACTCACTATAGGGAGACCCAAGCTGGCTAGCGTTT
AAACGGGCCCTCTAGACTCGAGCGGCCGCCACTGTGCTGGAGCCGCCACCATGGACTGGACCTG GAGGGTGTTCTGCCTGCTTGCAGTGGCCCCCGGAGCCCACAGCCAGGTTCAGCTGCAGCAGTCT GGACCTGAGCTGGTGAAGCCTGGGGCTTTAGTGAAGATATCCTGCAAGGCTTCTGGTTACACCT TCACAAACTACGATATACACTGGGTGAAGCAGAGGCCTGGACAGGGACTTGAGTGGATTGGATG GATTTATCCTGGAGATGGTAGTACTAAGTACAATGAGAAATTCAAGGGCAAGGCCACACTGACT GCAGACAAATCCTCCAGCACAGCCTACATGCACCTCAGCAGCCTGACTTCTGAGAAATCTGCAG TCTATTTCTGTGCAAGAGAGTGGGCTTACTGGGGCCAAGGGACTCTGGTCACTGTCTCTGCAGC TAGCACCAAGGGCCCATCGGTCTTCCCCCTGGCACCCTCCTCCAAGAGCACCTCTGGGGGCACA GCGGCCCTGGGCTGCCTGGTCAAGGACTACTTCCCCGAACCGGTGACGGTGTCGTGGAACTCAG GCGCCCTGACCAGCGGCGTGCACACCTTCCCGGCTGTCCTACAGTCCTCAGGACTCTACTCCCT CAGCAGCGTGGTGACCGTGCCCTCCAGCAGCTTGGGCACCCAGACCTACATCTGCAACGTGAAT CACAAGCCCAGCAACACCAAGGTGGACAAGAAAGTTGAGCCCAAATCTTGTGACAAAACTCACA CATGCCCACCGTGCCCAGCACCTGAACTCCTGGGGGGACCGTCAGTCTTCCTCTTCCCCCCAAA ACCCAAGGACACCCTCATGATCTCCCGGACCCCTGAGGTCACATGCGTGGTGGTGGACGTGAGC CACGAAGACCCTGAGGTCAAGTTCAACTGGTACGTGGACGGCGTGGAGGTGCATAATGCCAAGA CAAAGCCGCGGGAGGAGCAGTACAACAGCACGTACCGTGTGGTCAGCGTCCTCACCGTCCTGCA CCAGGACTGGCTGAATGGCAAGGAGTACAAGTGCAAGGTCTCCAACAAAGCCCTCCCAGCCCCC ATCGAGAAAACCATCTCCAAAGCCAAAGGGCAGCCCCGAGAACCACAGGTGTACAC CCTGCCC C CATCCCGGGATGAGCTGACCAAGAACCAGGTCAGCCTGACCTGCCTGGTCAAAGGCTTCTATCC CAGCGACATCGCCGTGGAGTGGGAGAGCAATGGGCAGCCGGAGAACAACTACAAGACCACGCCT CCCGTGCTGGACTCCGACGGCTCCTTCTTCCTCTACAGCAAGCTCACCGTGGACAAGAGCAGGT GGCAGCAGGGGAACGTCTTCTCATGCTCCGTGATGCATGAGGCTCTGCACAACCACTACACGCA GAAGAGCCTCTCCCTGTCTCCGGGTAAAAGTTCATCACCAGATAAACAAATGGCAGTGCTTCCT AGAAGAGAGCGGAATCGGCAGGCTGCAGCTGCCAACCCAGAGAATTCCAGAGGAAAAGGTCGGA GAGGCCAGAGGGGCAAAAACCGGGGTTGTGTCTTAACTGCAATACATTTAAATGTCACTGACTT GGGTCTGGGCTATGAAACCAAGGAGGAACTGATTTTTAGGTACTGCAGCGGCTCTTGCGATGCA GCTGAGACAACGTACGACAAAATATTGAAAAACTTATCCAGAAATAGAAGGCTGGTGAGTGACA AAGTAGGGCAGGCATGTTGCAGACCCATCGCCTTTGATGATGACCTGTCGTTTTTAGATGATAA CCTGGTTTACCATATTCTAAGAAAGCATTCCGCTAAAAGGTGTGGATGTATCTGAAACCCGAGC TCGGTACCAAGCTTAAGTTTAAACCGCTGATCAGCCTCGACTGTGCCTTCTAGTTGCCAGCCAT CTGTTGTTTGCCCCTCCCCCGTGCCTTCCTTGACCCTGGAAGGTGCCACTCCCACTGTCCTTTC CTAATAAAATGAGGAAATTGCATCGCATTGTCTGAGTAGGTGTCATTCTATTCTGGGGGGTGGG GTGGGGCAGGACAGCAAGGGGGAGGATTGGGAAGACAATAGCAGGCATGCTGGGGATGCGGTGG GCTCTATGGCTTCTGAGGCGGAAAGAACCAGGGGAGGTACCGAGCTCTTACGCGTGCTAGCTCG AGATCTGCATCTCAATTAGTCAGCAACCATAGTCCCGCCCCTAACTCCGCCCATCCCGCCCCTA ACTCCGCCCAGTTCCGCCCATTCTCCGCCCCATGGCTGACTAATTTTTTTTATTTATGCAGAGG CCGAGGCCGCCTCGGCCTCTGAGCTATTCCAGAAGTAGTGAGGAGGCTTTTTTGGAGGCCTAGG CTTTTGCAAAAAGCTTATCGATTCTAGAAGCCGCCACCATGGTTCGACCATTGAACTGCATCGT CGCCGTGTCCCAAAATATGGGGATTGGCAAGAACGGAGACCTACCCTGGCCTCCGCTCAGGAAC GAGTTCAAGTACTTCCAAAGAATGACCACAACCTCTTCAGTGGAAGGTAAACAGAATCTGGTGA TTATGGGTAGGAAAACCTGGTTCTCCATTCCTGAGAAGAATCGACCTTTAAAGGACAGAATTAA TATAGTTCTCAGTAGAGAACTCAAAGAACCACCACGAGGAGCTCATTTTCTTGCCAAAAGTTTG GATGATGCCTTAAGACTTATTGAACAACCGGAATTGGCAAGTAAAGTAGACATGGTTTGGATAG TCGGAGGCAGTTCTGTTTACCAGGAAGCCATGAATCAACCAGGCCACCTCAGACTCTTTGTGAC AAGGATCATGCAGGAATTTGAAAGTGACACGTTTTTCCCAGAAATTGATTTGGGGAAATATAAA CTTCTCCCAGAATACCCAGGCGTCCTCTCTGAGGTCCAGGAGGAAAAAGGCATCAAGTATAAGT TTGAAGTCTACGAGAAGAAAGACTAACAGGAAGATGCTTTCAAGTTCTCTGCTCCCCTCCTAAA GCTATGCATTTTTATAAGACCATGGGACTTTTGCTGGCTTTAGATCCTTCGCGGGACGTCCTTT GTTTACGTCCCGTCGGCGCTGAATCCCGCGGACGACCCCTCGCGGGGCCGCTTGGGACTCTCTC GTCCCCTTCTCCGTCTGCCGTTCCAGCCGACCACGGGGCGCACCTCTCTTTACGCGGTCTCCCC GTCTGTGCCTTCTCATCTGCCGGTCCGTGTGCACTTCGCTTCACCTCTGCACGTTGCATGGAGA CCACCGTGAACGCCCATCAGATCCTGCCCAAGGTCTTACATAAGAGGACTCTTGGACTCCCAGC AATGTCAACGACCGACCTTGAGGCCTACTTCAAAGACTGTGTGTTTAAGGACTGGGAGGAGCTG GGGGAGGAGATTAGGTTAAAGGTCTTTGTATTAGGAGGCTGTAGGCATAAATTGGTCTGCGCAC CAGCACCATGCAACTTTTTCACCTCTGCCTAATCATCTCTTGTACATGTCCCACTGTTCAAGCC TCCAAGCTGTGCCTTGGGTGGCTTTGGGGCATGGACATTGACCCTTATAAAGAATTTGGAGCTA CTGTGGAGTTACTCTCGTTTTTGCCTTCTGACTTCTTTCCTTCCGTCAGAGATCCTCTACGCCG GACGCATCGTGGCCGGCATCACCGGCGCCACAGGTGCGGTTGCTGGCGCCTATATCGCCGACAT
CACCGATGGGGAAGATCGGGCTCGCCACTTCGGGCTCATGAGCGCTTGTTTCGGCGTGGGTATG GTGGCAGGCCCCGTGGCCGGGGGACTGTTGGGCGCCATCTCCTTGCATGCACCATTCCTTGCGG CGGCGGTGCTCAACGGCCTCAACCTACTACTGGGCTGCTTCCTAATGCAGGAGTCGCATAAGGG AGAGCG
<210> 48
<211> 234
<212 > PRT
<213 > artificial sequence
<220 >
<223 > DescriDtion of Arti
<400 > 48
METPAQLLFLLLLWL PDTTGD IQMTQSPS S LSASLGERVSLTCRAS QDIGGNLYWLQQGPDGT I
KRLIYATSSLDSGVPKRFSGSRSGSDYSLTISSLESΞDFVDYYCLQYSSSPWTFGGGTKMEIKR
TVAAPSVFIFPPSDEQLKSGTASWCLLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDST
YSLSSTLTLSKADYEKHKVYACΞVTHQGLSSPVTKSFNRGEC
<210> 49
<211> 598
<212 > PRT
<213 > artificial sequence
<220>
<223 > Descriptior i of Arti
<400> 49
MDWTWRVFCLLAVAPGAHSQVQLQQSGPELVKPGALVKISCKASGYTFTNYDIHWVKQRPGQGLEWIGWIYP
GDGS TKYNEKFKGKATLTAD KSS S TAYMHLS S LTS EKS AVYFCARE WAYWGQGTL VTVS AASTKGPS VF PLA
PSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSWTVPSSSLGTQTYICN
VNHKPSNTKVDKKVE PKS CD KTHTCP PCPAPELLGGPSVFLFPPKPKDTLMI SRT PEVTCWVDVSHED PEV
KFNWYVDGVEVHNAKTKPREΞQYNSTYRWSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPRE
PQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRW
QQGNVFSCSVMHEALHNHYTQKSLSLSPGKSSSPDKQMAVLPRRΞRNRQAAAANPENSRGKGRRGQRGKNRG
CVLTAIHLKVTDLGLGYETKEELIFRYCSGΞCDAAETTYDKILKNLSRNRRLVSDKVGQACCRPIAFDDDLS
FLDDNLVYHILRKHSAKRCGCI
<210 > 50
<211> 187
<212 > PRT
<213 > artif icial sequence
<220>
<223 > Descriptioi i of Arti
<400> 50
MVRPLNCIVAVSQNMGIGKNGDLPWPPLRNEFKYFQRMTTTSSVEGKQNLVIMGRKTWFSIPEKNRPLKDRI NIVLSRELKEPPRGAHFLAKSLDDALRLIEQPELASKVDMVWIVGGSSVYQEAMNQPGHLRLFVTRIMQEFE SDTFFPEIDLGKYKLLPEYPGVLSEVQEEKGIKYKFSVYEKKD
<210> 51
<211> 405
<212 > DNA
<213 > artificial sequence
<220 >
<223 > Description of Artif icial Sequence : Synthetic DNA
<400 > 51
TCACCAGATAAACAAATGGCAGTGCTTCCTAGAAGAGAGCGGAATCGGCAGGCTGCAGCTGCCAACCCAGAG
AATTC CAGAGGAAAAGGTCGGAGAGGCCAGAGGGGCAAAAAC CGGGGTTGTGTCTTAACTGCAATACATTTA
AATGTCACTGACTTGGGTCTGGGCTATGAAACCAAGGAGGAACTGATTTTTAGGTACTGCAGCGGCTCTTGC
GATGCAGCTGAGACAACGTACGACAAAATATTGAAAAACTTATCCAGAAATAGAAGGCTGGTGAGTGACAAA
GTAGGGCAGGCATGTTGCAGACCCATCGCCTTTGATGATGACCTGTCGTTTTTAGATGATAACCTGGTTTAC
CATATTCTAAGAAAGCATTCCGCTAAAAGGTGTGGATGTATCTGA
<210 > 52
<211> 134
<212 > PRT
<213 > artificial sequence
<220 >
<223 > Description of Artif icial Sequence : Synthetic Protein
<400> 52
SPDKQMAVLPRRΞRNRQAAAANPENSRGKGRRGQRGKNRGCVLTAIHLNVTDLGLGYETKEELIFRYCSGSC DAAETT YDKILKNLSRNRRLVSDKVGQACCRPIAPDDDLSPLDDNLVYHILRKHSAKRCGCI
Claims
1. A composition comprising a neurotberapeutic peptide comprising an amino acid sequence which is at least 80% identical to the amino acid sequence of mature human GDNF (SEQ ID NO:52) covalently linked to a structure that is capable of crossing the blood brain barrier (BBB).
2. The composition of claim 1 wherein the structure that is capable of crossing the BBB crosses the BBB on an endogenous BBB receptor.
3. The composition of claim 2 wherein the endogenous BBB receptor is selected from the group consisting of the insulin receptor, transferrin receptor, leptin receptor, lipoprotein receptor, and the IGF receptor.
4. The composition of claim 3 wherein the endogenous BBB receptor mediated transport system is the insulin BBB receptor mediated transport system.
5. The composition of claim 1 wherein the structure that is capable of crossing the BBB is an antibody.
6. The composition of claim 5 wherein the antibody i« a monoclonal antibody (MAb).
7. The composition of claim 6 wherein the MAb is. a chimeric MAb.
8. The composition of claim 2 wherein the structure that crosses the BBB on an endogenous BBB receptor mediated transport system is an antibody.
9. The composition of claim 8 wherein the antibody is a monoclonal antibody.
10. The composition of claim 9 wherein the MAb is a chimeric MAb.
1 1. The composition of claim lO.wherein the chimeric antibody contains at least about 80% human sequence.
12. The composition of claim I , wherein the neurotherapeutic peptide comprises an amino acid sequence that is at least 95% identical to the amino acid sequence of mature human GDMF (SEQ ID NO:52).
13. The composition of claim 12, wherein the neurotberapeutic peptide comprises the amino acid sequence of mature human GDNF (SEQ ID NO:52).
14. The composition of claim 6. wherein the neurotherapeutic peptide is covalently linked at its amino terminus to the carboxy terminus of the heavy chain of the MAb.
15. A composition for treating a neurological disorder comprising a neurotherapeutic peptide comprising an amino acid sequence which is at least 80% identical to the amino acid sequence of mature human GDN" F (SEQ ID NO:52) covalently linked to an immunoglobulin that is capable of crossing the blood brain barrier, wherein the composition is capable of crossing the BBB in an amount that is effective in treating the neurological disorder.
16. A recombinant mammalian cell comprising the composition of claim 15.
17. A method for treating a CNS disorder in an individual comprising peripherally administering to the individual an effective amount of a composition comprising a neurotherapeutic peptide comprising an amino acid sequence which is at least 80% identical to the amino acid sequence of mature human ODNF (SEQ ID NO:52) covaieiitly attached to a structure capable of crossing the BBB.
18. The method of claim 17, wherein the neurotherapeutic peptide comprises an amino acid sequence at least 95% identical to the amino acid sequence of mature human GDNF (SEQ FD NO:52),
19. The method of claim 18, wherein the neurotherapeutic peptide comprises the amino acid sequence of mature human GDNF (SEQ ID NO:52).
20. The method of claim 17 wherein the administering is selected from the group consisting of oral, intravenous, intramuscular, subcutaneous, intraperitoneal, rectal, transbuccal, intranasal, transdermal, and inhalation administration.
21. The method of claim 20 wherein the administering is intravenous, intramuscular, or subcutaneous.
22. The method of claim 17 wherein the CNS disorder is an acute CNS disorder.
23. The method of claim 22 wherein She acute CNS disorder is selected from the group consisting of spinal cord injury, brain injury, focal brain ischeπiia and giobal brain ischemia.
24. The method of claim 17 wherein Uie CNS disorder is a chronic disorder.
25. The method of claim 24 wherein the chronic disorder is selected from the group consisting of chronic neurodegenerative disease, drug addiction, or alcohol addiction.
26. The method of claim 25 wherein the chronic neurodegenerative disease is selected from the group consisting of Parkinson's disease or a motor neuron disease.
27. The method of claim 17 wherein the individual is administered a dose of the composition that is about 1 lo about 100 mg.
28. A method for treating substance abuse in an individual, comprising administering to the individual an effective amount of a composition comprising a neurotherapeutic peptide comprising an amino acid sequence which is at least 80% identical to the amino acid sequence of mature human GDNF (SEQ 3D NO:52) covaieiitly attached to a structure capable of crossing the BBB.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US99029007P | 2007-11-26 | 2007-11-26 | |
| US60/990,290 | 2007-11-26 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2009070597A2 true WO2009070597A2 (en) | 2009-06-04 |
Family
ID=40679205
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2008/084718 Ceased WO2009070597A2 (en) | 2007-11-26 | 2008-11-25 | Fusion proteins for delivery of gdnf to the cns |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2009070597A2 (en) |
Cited By (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010142035A1 (en) * | 2009-06-11 | 2010-12-16 | Angiochem Inc. | Fusion proteins for delivery of gdnf and bdnf to the central nervous system |
| WO2011000095A1 (en) * | 2009-07-02 | 2011-01-06 | Angiochem Inc. | Multimeric peptide conjugates and uses thereof |
| US7902156B2 (en) | 2005-02-18 | 2011-03-08 | Angiochem Inc. | Aprotinin polypeptides for transporting a compound across the blood-brain barrier |
| US8486399B2 (en) | 2011-12-02 | 2013-07-16 | Armagen Technologies, Inc. | Methods and compositions for increasing arylsulfatase A activity in the CNS |
| US8497246B2 (en) | 2006-08-18 | 2013-07-30 | Armagen Technologies, Inc. | Methods for diagnosing and treating CNS disorders by trans-blood-brain barrier delivery of protein compositions |
| US8741260B2 (en) | 2005-10-07 | 2014-06-03 | Armagen Technologies, Inc. | Fusion proteins for delivery of GDNF to the CNS |
| US8834874B2 (en) | 2009-10-09 | 2014-09-16 | Armagen Technologies, Inc. | Methods and compositions for increasing iduronate 2-sulfatase activity in the CNS |
| US8853353B2 (en) | 2008-12-17 | 2014-10-07 | Angiochem, Inc. | Membrane type-1 matrix metalloprotein inhibitors and uses thereof |
| US8921314B2 (en) | 2008-10-15 | 2014-12-30 | Angiochem, Inc. | Conjugates of GLP-1 agonists and uses thereof |
| US8969310B2 (en) | 2005-07-15 | 2015-03-03 | Angiochem Inc. | Potentiation of anticancer agents |
| WO2015031673A2 (en) | 2013-08-28 | 2015-03-05 | Bioasis Technologies Inc. | Cns-targeted conjugates having modified fc regions and methods of use thereof |
| US8974791B2 (en) | 2007-07-27 | 2015-03-10 | Armagen Technologies, Inc. | Methods and compositions for increasing α-L-iduronidase activity in the CNS |
| US9173891B2 (en) | 2009-04-20 | 2015-11-03 | Angiochem, Inc. | Treatment of ovarian cancer using an anticancer agent conjugated to an angiopep-2 analog |
| US9221867B2 (en) | 2003-01-06 | 2015-12-29 | Angiochem Inc. | Method for transporting a compound across the blood-brain barrier |
| US9365634B2 (en) | 2007-05-29 | 2016-06-14 | Angiochem Inc. | Aprotinin-like polypeptides for delivering agents conjugated thereto to tissues |
| US9533055B2 (en) | 2009-03-18 | 2017-01-03 | Armagen Technologies, Inc. | Compositions and methods for blood-brain barrier delivery of IgG-decoy receptor fusion proteins |
| US9914754B2 (en) | 2008-12-05 | 2018-03-13 | Angiochem Inc. | Conjugates of neurotensin or neurotensin analogs and uses thereof |
| CN110461880A (en) * | 2016-11-10 | 2019-11-15 | 科乐斯疗法公司 | GDNF fusion polypeptides and methods of use thereof |
| US10538589B2 (en) | 2015-01-14 | 2020-01-21 | Armagen Inc. | Methods and compositions for increasing N-acetylglucosaminidase (NAGLU) activity in the CNS using a fusion antibody comprising an anti-human insulin receptor antibody and NAGLU |
| US10980892B2 (en) | 2015-06-15 | 2021-04-20 | Angiochem Inc. | Methods for the treatment of leptomeningeal carcinomatosis |
| EP3954393A1 (en) | 2020-08-13 | 2022-02-16 | Bioasis Technologies Inc. | Combination therapies for delivery across the blood brain barrier |
-
2008
- 2008-11-25 WO PCT/US2008/084718 patent/WO2009070597A2/en not_active Ceased
Cited By (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9221867B2 (en) | 2003-01-06 | 2015-12-29 | Angiochem Inc. | Method for transporting a compound across the blood-brain barrier |
| US7902156B2 (en) | 2005-02-18 | 2011-03-08 | Angiochem Inc. | Aprotinin polypeptides for transporting a compound across the blood-brain barrier |
| US9713646B2 (en) | 2005-07-15 | 2017-07-25 | Angiochem Inc. | Potentiation of anticancer agents |
| US8969310B2 (en) | 2005-07-15 | 2015-03-03 | Angiochem Inc. | Potentiation of anticancer agents |
| US8741260B2 (en) | 2005-10-07 | 2014-06-03 | Armagen Technologies, Inc. | Fusion proteins for delivery of GDNF to the CNS |
| US8759297B2 (en) | 2006-08-18 | 2014-06-24 | Armagen Technologies, Inc. | Genetically encoded multifunctional compositions bidirectionally transported between peripheral blood and the cns |
| US11155631B2 (en) | 2006-08-18 | 2021-10-26 | Armagen, Inc. | Macromolecular compositions that cross the blood-brain barrier and methods of use thereof |
| US10144783B2 (en) | 2006-08-18 | 2018-12-04 | Armagen, Inc. | Macromolecular compositions that cross the blood-brain barrier and methods of use thereof |
| US8497246B2 (en) | 2006-08-18 | 2013-07-30 | Armagen Technologies, Inc. | Methods for diagnosing and treating CNS disorders by trans-blood-brain barrier delivery of protein compositions |
| US8753610B2 (en) | 2006-08-18 | 2014-06-17 | Armagen Technologies, Inc. | Methods for diagnosing CNS disorders with fusion antibodies that cross the blood-brain barrier in both directions |
| US9365634B2 (en) | 2007-05-29 | 2016-06-14 | Angiochem Inc. | Aprotinin-like polypeptides for delivering agents conjugated thereto to tissues |
| US10202467B2 (en) | 2007-07-27 | 2019-02-12 | Armagen, Inc. | Methods and compositions for increasing α-L-iduronidase activity in the CNS |
| US11512145B2 (en) | 2007-07-27 | 2022-11-29 | Armagen, Inc. | Methods and compositions for increasing alpha-L-iduronidase activity in the CNS |
| US8974791B2 (en) | 2007-07-27 | 2015-03-10 | Armagen Technologies, Inc. | Methods and compositions for increasing α-L-iduronidase activity in the CNS |
| US9567400B2 (en) | 2007-07-27 | 2017-02-14 | Armagen Technologies, Inc. | Methods and compositions for increasing α-L-iduronidase activity in the CNS |
| US8921314B2 (en) | 2008-10-15 | 2014-12-30 | Angiochem, Inc. | Conjugates of GLP-1 agonists and uses thereof |
| US9914754B2 (en) | 2008-12-05 | 2018-03-13 | Angiochem Inc. | Conjugates of neurotensin or neurotensin analogs and uses thereof |
| US8853353B2 (en) | 2008-12-17 | 2014-10-07 | Angiochem, Inc. | Membrane type-1 matrix metalloprotein inhibitors and uses thereof |
| US9533055B2 (en) | 2009-03-18 | 2017-01-03 | Armagen Technologies, Inc. | Compositions and methods for blood-brain barrier delivery of IgG-decoy receptor fusion proteins |
| US9173891B2 (en) | 2009-04-20 | 2015-11-03 | Angiochem, Inc. | Treatment of ovarian cancer using an anticancer agent conjugated to an angiopep-2 analog |
| CN103819564A (en) * | 2009-06-11 | 2014-05-28 | 安吉奥开米公司 | Fusion proteins for delivery of GDNF and BDNF to the central nervous system |
| WO2010142035A1 (en) * | 2009-06-11 | 2010-12-16 | Angiochem Inc. | Fusion proteins for delivery of gdnf and bdnf to the central nervous system |
| CN102459348A (en) * | 2009-06-11 | 2012-05-16 | 安吉奥开米公司 | Fusion proteins for delivery of gdnf and bdnf to the central nervous system |
| US9161988B2 (en) | 2009-07-02 | 2015-10-20 | Angiochem Inc. | Multimeric peptide conjugates and uses thereof |
| WO2011000095A1 (en) * | 2009-07-02 | 2011-01-06 | Angiochem Inc. | Multimeric peptide conjugates and uses thereof |
| JP2012531467A (en) * | 2009-07-02 | 2012-12-10 | アンジオケム インコーポレーテッド | Multimeric peptide conjugates and uses thereof |
| US10011651B2 (en) | 2009-10-09 | 2018-07-03 | Armagen, Inc. | Methods and compositions for increasing iduronate 2-sulfatase activity in the CNS |
| US8834874B2 (en) | 2009-10-09 | 2014-09-16 | Armagen Technologies, Inc. | Methods and compositions for increasing iduronate 2-sulfatase activity in the CNS |
| US12043661B2 (en) | 2009-10-09 | 2024-07-23 | Armagen, Inc. | Methods and compositions for increasing iduronate 2-sulfatase activity in the CNS |
| US11028156B2 (en) | 2009-10-09 | 2021-06-08 | Armagen, Inc. | Methods and compositions for increasing iduronate 2-sulfatase activity in the CNS |
| US8715661B2 (en) | 2011-12-02 | 2014-05-06 | Armagen Technologies, Inc. | Methods and compositions for increasing arylsulfatase A activity in the CNS |
| US9975955B2 (en) | 2011-12-02 | 2018-05-22 | Armagen, Inc. | Methods and compositions for increasing arylsulfatase A activity in the CNS |
| US8486399B2 (en) | 2011-12-02 | 2013-07-16 | Armagen Technologies, Inc. | Methods and compositions for increasing arylsulfatase A activity in the CNS |
| US8920801B2 (en) | 2011-12-02 | 2014-12-30 | Armagen Technologies, Inc. | Methods and compositions for increasing arylsulfatase A activity in the CNS |
| WO2015031673A2 (en) | 2013-08-28 | 2015-03-05 | Bioasis Technologies Inc. | Cns-targeted conjugates having modified fc regions and methods of use thereof |
| US10538589B2 (en) | 2015-01-14 | 2020-01-21 | Armagen Inc. | Methods and compositions for increasing N-acetylglucosaminidase (NAGLU) activity in the CNS using a fusion antibody comprising an anti-human insulin receptor antibody and NAGLU |
| US10980892B2 (en) | 2015-06-15 | 2021-04-20 | Angiochem Inc. | Methods for the treatment of leptomeningeal carcinomatosis |
| US11912750B2 (en) * | 2016-11-10 | 2024-02-27 | Keros Therapeutics, Inc. | GDNF fusion polypeptides and methods of use thereof |
| CN110461880A (en) * | 2016-11-10 | 2019-11-15 | 科乐斯疗法公司 | GDNF fusion polypeptides and methods of use thereof |
| US12404313B2 (en) | 2016-11-10 | 2025-09-02 | Keros Therapeutics, Inc. | GDNF fusion polypeptides and methods of use thereof |
| EP3954393A1 (en) | 2020-08-13 | 2022-02-16 | Bioasis Technologies Inc. | Combination therapies for delivery across the blood brain barrier |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20220378932A1 (en) | Fusion proteins for delivery of gdnf to the cns | |
| WO2009070597A2 (en) | Fusion proteins for delivery of gdnf to the cns | |
| US8124095B2 (en) | Fusion proteins for delivery of erythropoietin to the CNS | |
| US8053569B2 (en) | Nucleic acids encoding and methods of producing fusion proteins | |
| AU2017202437B2 (en) | Novel immunoconjugates | |
| CN109705218B (en) | ASGPR antibodies and uses thereof | |
| US9533055B2 (en) | Compositions and methods for blood-brain barrier delivery of IgG-decoy receptor fusion proteins | |
| US20100077498A1 (en) | Compositions and methods for blood-brain barrier delivery in the mouse | |
| CN110234662A (en) | Tissue specificity WNT signal enhancing molecule and its purposes | |
| AU2020200250A1 (en) | Methods and compositions for increasing enzyme activity in the CNS | |
| AU2011205186B2 (en) | Fusion proteins for blood-brain barrier delivery | |
| HK40007646A (en) | Asgpr antibodies and uses thereof | |
| HK40007646B (en) | Asgpr antibodies and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 08853640 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase in: |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 08853640 Country of ref document: EP Kind code of ref document: A2 |