WO2000015256A2 - Emulsion immunostimulante - Google Patents
Emulsion immunostimulante Download PDFInfo
- Publication number
- WO2000015256A2 WO2000015256A2 PCT/FR1999/002177 FR9902177W WO0015256A2 WO 2000015256 A2 WO2000015256 A2 WO 2000015256A2 FR 9902177 W FR9902177 W FR 9902177W WO 0015256 A2 WO0015256 A2 WO 0015256A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- emulsion
- emulsion according
- molecule
- polynucleotide
- lipid
- Prior art date
Links
- 239000000839 emulsion Substances 0.000 title claims abstract description 59
- 230000003308 immunostimulating effect Effects 0.000 title claims abstract description 22
- 229960001438 immunostimulant agent Drugs 0.000 title abstract description 4
- 239000003022 immunostimulating agent Substances 0.000 title abstract description 4
- 239000000203 mixture Substances 0.000 claims abstract description 18
- 150000002632 lipids Chemical class 0.000 claims abstract description 16
- 229960005486 vaccine Drugs 0.000 claims abstract description 14
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims abstract description 8
- 239000008346 aqueous phase Substances 0.000 claims abstract description 6
- 239000012071 phase Substances 0.000 claims abstract description 6
- 239000000427 antigen Substances 0.000 claims description 34
- 102000036639 antigens Human genes 0.000 claims description 34
- 108091007433 antigens Proteins 0.000 claims description 34
- 108091033319 polynucleotide Proteins 0.000 claims description 26
- 102000040430 polynucleotide Human genes 0.000 claims description 26
- 239000002157 polynucleotide Substances 0.000 claims description 26
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 claims description 17
- 230000004044 response Effects 0.000 claims description 10
- 235000012000 cholesterol Nutrition 0.000 claims description 9
- 210000000987 immune system Anatomy 0.000 claims description 9
- 238000004519 manufacturing process Methods 0.000 claims description 9
- YYGNTYWPHWGJRM-UHFFFAOYSA-N (6E,10E,14E,18E)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene Chemical compound CC(C)=CCCC(C)=CCCC(C)=CCCC=C(C)CCC=C(C)CCC=C(C)C YYGNTYWPHWGJRM-UHFFFAOYSA-N 0.000 claims description 7
- BHEOSNUKNHRBNM-UHFFFAOYSA-N Tetramethylsqualene Natural products CC(=C)C(C)CCC(=C)C(C)CCC(C)=CCCC=C(C)CCC(C)C(=C)CCC(C)C(C)=C BHEOSNUKNHRBNM-UHFFFAOYSA-N 0.000 claims description 7
- PRAKJMSDJKAYCZ-UHFFFAOYSA-N dodecahydrosqualene Natural products CC(C)CCCC(C)CCCC(C)CCCCC(C)CCCC(C)CCCC(C)C PRAKJMSDJKAYCZ-UHFFFAOYSA-N 0.000 claims description 7
- 229940031439 squalene Drugs 0.000 claims description 7
- TUHBEKDERLKLEC-UHFFFAOYSA-N squalene Natural products CC(=CCCC(=CCCC(=CCCC=C(/C)CCC=C(/C)CC=C(C)C)C)C)C TUHBEKDERLKLEC-UHFFFAOYSA-N 0.000 claims description 7
- 239000004094 surface-active agent Substances 0.000 claims description 6
- 108091081548 Palindromic sequence Proteins 0.000 claims description 3
- 241000700605 Viruses Species 0.000 claims description 3
- 206010022000 influenza Diseases 0.000 claims description 3
- -1 phosphodiester phosphorothioate Chemical class 0.000 claims description 3
- 150000004713 phosphodiesters Chemical class 0.000 claims description 3
- 230000000241 respiratory effect Effects 0.000 claims description 3
- 239000003795 chemical substances by application Substances 0.000 claims description 2
- RYYWUUFWQRZTIU-UHFFFAOYSA-K thiophosphate Chemical compound [O-]P([O-])([O-])=S RYYWUUFWQRZTIU-UHFFFAOYSA-K 0.000 claims description 2
- 239000003814 drug Substances 0.000 claims 3
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 claims 1
- 229920000053 polysorbate 80 Polymers 0.000 claims 1
- 230000028327 secretion Effects 0.000 claims 1
- 230000001571 immunoadjuvant effect Effects 0.000 abstract description 5
- 239000000568 immunological adjuvant Substances 0.000 abstract description 4
- 108091034117 Oligonucleotide Proteins 0.000 description 30
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 17
- 239000000243 solution Substances 0.000 description 14
- 241000699670 Mus sp. Species 0.000 description 8
- 239000000872 buffer Substances 0.000 description 7
- 230000000694 effects Effects 0.000 description 7
- 239000003921 oil Substances 0.000 description 7
- 235000019198 oils Nutrition 0.000 description 7
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 6
- 229910052782 aluminium Inorganic materials 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 6
- 230000003053 immunization Effects 0.000 description 6
- 238000002649 immunization Methods 0.000 description 6
- 238000002360 preparation method Methods 0.000 description 6
- 238000003756 stirring Methods 0.000 description 6
- 244000005700 microbiome Species 0.000 description 5
- 238000002156 mixing Methods 0.000 description 5
- 238000003786 synthesis reaction Methods 0.000 description 5
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 4
- 239000003153 chemical reaction reagent Substances 0.000 description 4
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical compound NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 4
- AUZONCFQVSMFAP-UHFFFAOYSA-N disulfiram Chemical compound CCN(CC)C(=S)SSC(=S)N(CC)CC AUZONCFQVSMFAP-UHFFFAOYSA-N 0.000 description 4
- 150000008300 phosphoramidites Chemical class 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- 101100454807 Caenorhabditis elegans lgg-1 gene Proteins 0.000 description 3
- 102000004127 Cytokines Human genes 0.000 description 3
- 108090000695 Cytokines Proteins 0.000 description 3
- 230000003647 oxidation Effects 0.000 description 3
- 238000007254 oxidation reaction Methods 0.000 description 3
- 230000002195 synergetic effect Effects 0.000 description 3
- 235000015112 vegetable and seed oil Nutrition 0.000 description 3
- 239000008158 vegetable oil Substances 0.000 description 3
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- 108020004414 DNA Proteins 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- RYYWUUFWQRZTIU-UHFFFAOYSA-N Thiophosphoric acid Chemical class OP(O)(S)=O RYYWUUFWQRZTIU-UHFFFAOYSA-N 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 230000000890 antigenic effect Effects 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 239000004359 castor oil Substances 0.000 description 2
- 235000019438 castor oil Nutrition 0.000 description 2
- 230000036755 cellular response Effects 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 229940104302 cytosine Drugs 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 2
- 150000002314 glycerols Chemical class 0.000 description 2
- UYTPUPDQBNUYGX-UHFFFAOYSA-N guanine Chemical compound O=C1NC(N)=NC2=C1N=CN2 UYTPUPDQBNUYGX-UHFFFAOYSA-N 0.000 description 2
- 230000000899 immune system response Effects 0.000 description 2
- 230000001965 increasing effect Effects 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 229910052500 inorganic mineral Inorganic materials 0.000 description 2
- 238000000034 method Methods 0.000 description 2
- 235000010755 mineral Nutrition 0.000 description 2
- 239000002773 nucleotide Substances 0.000 description 2
- 239000007764 o/w emulsion Substances 0.000 description 2
- 230000001717 pathogenic effect Effects 0.000 description 2
- 229920000136 polysorbate Polymers 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 2
- 238000002255 vaccination Methods 0.000 description 2
- 239000012646 vaccine adjuvant Substances 0.000 description 2
- 229940124931 vaccine adjuvant Drugs 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- JIAARYAFYJHUJI-UHFFFAOYSA-L zinc dichloride Chemical compound [Cl-].[Cl-].[Zn+2] JIAARYAFYJHUJI-UHFFFAOYSA-L 0.000 description 2
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical class OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 1
- ALSTYHKOOCGGFT-KTKRTIGZSA-N (9Z)-octadecen-1-ol Chemical compound CCCCCCCC\C=C/CCCCCCCCO ALSTYHKOOCGGFT-KTKRTIGZSA-N 0.000 description 1
- FKOKUHFZNIUSLW-UHFFFAOYSA-N 2-Hydroxypropyl stearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(C)O FKOKUHFZNIUSLW-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 238000012286 ELISA Assay Methods 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 101710154606 Hemagglutinin Proteins 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 108091028043 Nucleic acid sequence Proteins 0.000 description 1
- 101710093908 Outer capsid protein VP4 Proteins 0.000 description 1
- 101710135467 Outer capsid protein sigma-1 Proteins 0.000 description 1
- 101710176177 Protein A56 Proteins 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- 229930182558 Sterol Natural products 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 235000019486 Sunflower oil Nutrition 0.000 description 1
- NWGKJDSIEKMTRX-MGMRWDBRSA-N [(2R)-2-[(2R,3R,4R)-3,4-dihydroxyoxolan-2-yl]-2-hydroxyethyl] (Z)-octadec-9-enoate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@@H](O)[C@H]1O NWGKJDSIEKMTRX-MGMRWDBRSA-N 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- ILRRQNADMUWWFW-UHFFFAOYSA-K aluminium phosphate Chemical compound O1[Al]2OP1(=O)O2 ILRRQNADMUWWFW-UHFFFAOYSA-K 0.000 description 1
- 239000010775 animal oil Substances 0.000 description 1
- 230000005875 antibody response Effects 0.000 description 1
- 230000002238 attenuated effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 229940082500 cetostearyl alcohol Drugs 0.000 description 1
- 150000001841 cholesterols Chemical class 0.000 description 1
- ALSTYHKOOCGGFT-UHFFFAOYSA-N cis-oleyl alcohol Natural products CCCCCCCCC=CCCCCCCCCO ALSTYHKOOCGGFT-UHFFFAOYSA-N 0.000 description 1
- 229940071160 cocoate Drugs 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 239000002612 dispersion medium Substances 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 238000012869 ethanol precipitation Methods 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- 230000003631 expected effect Effects 0.000 description 1
- YOCZDGRJDQLKHW-NDHQNRPVSA-N glucose 6-monomycolate (C36) Chemical compound CCCCCCCCCCCCCCCCCCC[C@@H](O)[C@@H](CCCCCCCCCCCCCC)C(=O)OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O YOCZDGRJDQLKHW-NDHQNRPVSA-N 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 239000000185 hemagglutinin Substances 0.000 description 1
- 230000008348 humoral response Effects 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 229940079322 interferon Drugs 0.000 description 1
- 229960003130 interferon gamma Drugs 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- GZQKNULLWNGMCW-PWQABINMSA-N lipid A (E. coli) Chemical compound O1[C@H](CO)[C@@H](OP(O)(O)=O)[C@H](OC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCCCC)[C@@H](NC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCC)[C@@H]1OC[C@@H]1[C@@H](O)[C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](OP(O)(O)=O)O1 GZQKNULLWNGMCW-PWQABINMSA-N 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 229960003511 macrogol Drugs 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 229940035032 monophosphoryl lipid a Drugs 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 125000003729 nucleotide group Chemical group 0.000 description 1
- GLDOVTGHNKAZLK-UHFFFAOYSA-N octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical class CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- 150000002889 oleic acids Chemical class 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 229930182490 saponin Natural products 0.000 description 1
- 150000007949 saponins Chemical class 0.000 description 1
- 235000017709 saponins Nutrition 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 1
- RYYKJJJTJZKILX-UHFFFAOYSA-M sodium octadecanoate Chemical compound [Na+].CCCCCCCCCCCCCCCCCC([O-])=O RYYKJJJTJZKILX-UHFFFAOYSA-M 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 235000003702 sterols Nutrition 0.000 description 1
- 150000003432 sterols Chemical class 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 239000002600 sunflower oil Substances 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- OULAJFUGPPVRBK-UHFFFAOYSA-N tetratriacontyl alcohol Natural products CCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCO OULAJFUGPPVRBK-UHFFFAOYSA-N 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 239000000277 virosome Substances 0.000 description 1
- 239000011592 zinc chloride Substances 0.000 description 1
- 235000005074 zinc chloride Nutrition 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/39—Medicinal preparations containing antigens or antibodies characterised by the immunostimulating additives, e.g. chemical adjuvants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/16—Antivirals for RNA viruses for influenza or rhinoviruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55566—Emulsions, e.g. Freund's adjuvant, MF59
Definitions
- the invention relates to the field of vaccines and more particularly vaccine adjuvants.
- Vaccines whether prophylactic or therapeutic, are intended to stimulate the immune system of the human or animal body to which they are administered, the immune system response can be either a humoral type response (production of antibodies), either a cell type response, or a combination of the two types of responses.
- the immune system response can be either a humoral type response (production of antibodies), either a cell type response, or a combination of the two types of responses.
- vaccination has consisted in administering to an organism a non-pathogenic version of a microorganism so as to prepare the immune system to react effectively in the event that the organism is subsequently caused to encounter the same microorganism, in its pathogenic version.
- the antigen administered during vaccination can.
- oligonucleotides that may immunostimuiatrice activity
- these oligonucleotides can be administered as a vaccine adjuvant.
- This reference also mentions the possibility of associating with these oligonucleotides by ionic, covalent bond or by encapsulation, means for targeting the administration of the oligonucleotide.
- Such means can in particular consist of sterol, of lipid (for example a catiodic lipid, a virosome or a liposome) or of a binding agent specific to the target cell (for example a binder recognized by a receptor specific for the cell). target).
- the invention relates to an immu ⁇ ostimulant emulsion of the oil in water type, comprising at least one aqueous phase and one oily phase, characterized in that it also comprises at least one immunostimulating polynucleotide of which at least a part is covalently coupled to at least one lipid molecule.
- oil-in-water emulsion means a dispersion of oil droplets in an aqueous phase which may consist of a buffer such as the PBS buffer.
- the oily phase consists of a pharmaceutically acceptable oil, which can be a mineral, animal or vegetable oil.
- a metabolizable oil is used such as squalene, esters (in particular ethyl oleate, - j - isopropyl myristate), a vegetable oil (for example castor oil, sunflower oil, olive oil ...) or a modified vegetable oil (eg macrogol glycerides) .
- a satisfactory emulsion by mixing 500 mg of squalene with 10 ml of PBS buffer in a device such as an ULTRA-TURRAX TM, then by microfluidizing the dispersion obtained using a microfluidizer such as Microfluidics TM, which allows to obtain oily particles whose diameter is less than 200 nm.
- a surface-active agent in particular a surface-active agent whose HLB value (Hydrophilic / Lipophilic Balance) is between 6 and 14.
- a surfactant chosen from the list of the following products: sorbitan esters and poiysorbates, ethoxylated castor oil hydrogenated or not, ethoxylated stearic acid, oleic alcohol 10 EO, cetostearyl alcohol 20 EO, glycerol stearate, propylene glycol stearate, lecithins, sodium lauryl sulfate, sodium stearate, ethoxylated glycerol cocoate. 70E, ethoxylated glycerol esters, ethoxylated oleic acids, l 'mannitan oleate. Particularly good results have been obtained using TWEEN TM 80.
- the emulsion obtained is considered to be immunostimulatory if it is capable of causing or increasing the stimulation of the immune system, for example when it is administered together with a vaccine antigen.
- the emulsion is used as an immunoadjuvant.
- This immuno-adjuvant activity can be expressed in different ways:
- - modify the nature of the response of the immune system to the administration of the antigen (for example, inducing a cellular response when the administration of the antigen alone provoked only a humoral response), - induce or increase the production of cytokines, or certain cytokines in particular
- polynucleotide within the meaning of the present invention, is understood a single-stranded oligonucleotide having from 6 to 100 nuciéotides, preferably from 6 to 30 nuciéotides It can be oligonbonucleotide, or o godesoxy ⁇ bo-nucleotide preferably polynucleotides comprising basic sequences with reverse symmetry, as is the case in palindromic sequences (that is to say sequences of the ABCDEE'D'C'B'A 'type where A and A, B and B ', C and C, D and D' E and E 'are complementary bases in the sense of Watson and C ⁇ ck), and more particularly polynucleotides comprising at least one di ⁇ ucleotide sequence Cytosine, Guanme, in which Cytosine and Guanine are not methylated Any other polynucleotide known to be, by its very nature, immunostimulant, may be suitable for
- At least one lipid molecule is covalently coupled to the polynucleotide.
- This lipid molecule is preferably a cholesterol or cholesterol derivative molecule.
- the coupling can be carried out by covalent bonding at one or at each end of the polynucleotide, or again by inserting at least one lipid molecule next to each base.
- Antigens whose effect it is possible to potentiate thanks to the emulsion according to the present invention, can be of varied nature, it can in particular be proteins, glycoproteins, glycoconjugates, polysaccharides or polynucleotides comprising fractions of DNA likely to lead to the expression of molecules ules of interest, it can also be a mixture of different antigens. Particularly good results have been obtained with a composition comprising influenza antigens as they are present in the commercial vaccine VAXIGRIP TM
- An emulsion according to the invention can be obtained by proceeding in the following manner, first mixing, with stirring, the oil with the aqueous phase optionally consisting of a buffer solution in which a surfactant has been incorporated.
- the mixture obtained is homogenized. by means, for example, of a propeller stirrer, in order to lead to an emulsion of the type oil in water.
- the emulsion obtained is then treated using a microfluidizer in order to reduce the oil droplets to a diameter less than 200 nm
- this emulsion When this emulsion is intended to be used as an immunoadjuvant, it is mixed with stirring, to a composition comprising the antigen whose effect it is desired to potentiate.
- the mixing can advantageously be carried out in a volume ratio of 1. verify the unexpected effect and in particular the synergistic effect obtained on the stimulation of the immune system by the simultaneous use of a polynucleotide coupled to at least one lipid molecule, and its incorporation into an oil-in-water emulsion
- compositions comprising only the antigen or the mixture of antigens vis-à-vis which it is desired to test the immunostimulating effect of the emulsion according to the invention, - or a composition, comprising the antigen or antigens d interest to which has been added a solution comprising only polynucleotides coupled to at least one lipid molecule,
- compositions comprising the antigen or antigens of interest to which has been added an oil-in-water type emulsion, without polynucleotide, or with a polynucleotide lacking immunostimulatory activity with respect to the antigens administered,
- composition comprising the antigen or antigens of interest to which an emulsion according to the invention has been added
- the results obtained showed a significant synergistic effect of the elements constituting the emulsion according to the invention.
- the emulsion obtained according to the invention has increased stability compared to emulsions of the same nature, i.e. those consisting of an identical aqueous phase and an oily phase, but devoid of polynucleotides.
- Oligonucleotides are prepared using a synthesizer automaton provided by Applied Biosystems which implements the standard chemical method with phosphoramidite and which includes an oxidation step at each cycle. This oxidation step is carried out using an iodine / water / tetrahydrofuran / acetonitrile solution to obtain a phosphodiester bond and using a tetraethylthiuram / acetonitrile solution to obtain a phosphorothioate bond.
- a 3 Db (S) oligonucleotide is thus prepared, the sequence of which is reproduced under SEQ ID NO 1 and which comprises phosphorothioate bonds over its entire length.
- An MGC (S) oligonucleotide is also prepared, the sequence of which is reproduced in SEQ ID NO 2, which comprises both phosphodiester bonds and phosphorothioate bonds.
- the phosphorothiate bonds are located at each end; there are 2 phosphorothioate bonds in 3 'and 5 phosphorothioate bonds in 5'.
- This oligonucleotide does not have a palindromic sequence, and in particular no CG sequence.
- Oligonucleotides are prepared which are coupled to the ends of the cholesterol molecules.
- the synthesis of these 3 Db (S) -chol oligonucleotides and MGC (S) -chol is carried out in the same manner as in Example 1, with the exception of the Phosphoramidite reagent which is replaced by a specific reagent, Cholesterol-ON TM Phosphoramidite supplied by the company CLONTECH Lab. Inc, (USA), during the first and last synthesis cycle in order to obtain a cholesterol molecule inserted before each of the end nucleotides.
- the nucleotide sequences obtained are identical to those of the oligonucleotides described in the previous example.
- An immunostimulatory emulsion according to the invention is prepared by mixing 435 ⁇ l of the 2.3 g / l solution of 3Db (S) coupled to the cholesterol obtained in Example 2 (ie 1 mg of oligonucleotide), with 2 ml of the squalene / PBS emulsion obtained in Example 3, kept stirring.
- Another emulsion is prepared by mixing 263 ⁇ l of the 3.81 g / l solution of MGC (S) coupled with cholesterol obtained in Example 2 (ie 1 mg of oligonucleotide) with 2 ml of the squalene emulsion / PBS obtained in Example 3, kept stirring.
- Example 5
- Immunization doses of different natures are prepared by adding, with stirring, 2 ml of splitted vaccine against influenza NIB16 (monovalent A / Singapore H1 N1) containing 100 ⁇ g of hemagglutinin HA in PBS buffer to 2 ml of each of the following preparations •
- mice There are groups of 6 Balb / c female mice aged 6 to 8 weeks, each group corresponding to one of the preparations carried out in Example 6
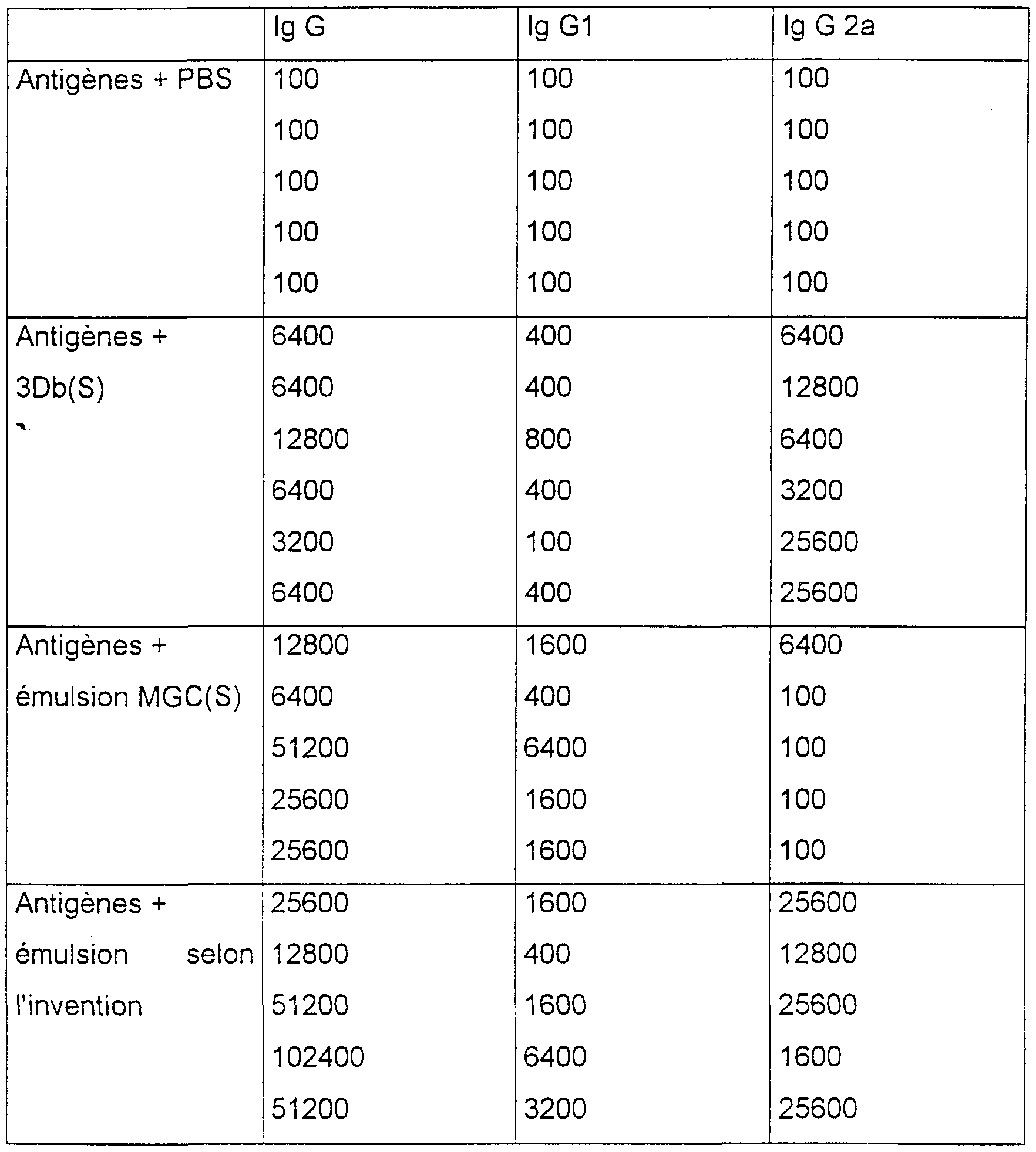
- Each of the mice is immunized with 200 ⁇ l of the preparation corresponding to its group and therefore receives 5 ⁇ g of HA by immunization, each mouse being immunized 2 times 3 weeks apart, with the same preparation 2 weeks after the second injection, the response to specific anti-HA antibodies is measured, using an ELISA test, and the GMT for lgG1 as well as for lgG2a The results obtained are indicated below:
- results obtained confirm that the oligonucleotide 3Db (S) is well endowed with immunostimulatory properties because it is capable of inducing an increase in the antibody response compared to what is obtained during the administration of the antigens alone.
- results obtained with the oligonucleotide MGC (S) do not demonstrate immunostimulatory activity.
- the emulsion containing an immunostimulating polynucleotide such as the polynucleotide 3Db (S) leads to a production of antibodies clearly greater than that obtained with an emulsion containing the polynucleotide MGC (S) -chol; this effect is even more remarkable with regard to the production of IgG2a; which is indicative of an orientation of the immune response towards a TH1 type, orientation sometimes desired in certain vaccine targets.
- Vaccine compositions are prepared comprising the following elements:
- the doses are 50 ⁇ litres and include 50 micrograms of oligonucleotides.
- compositions are administered to mice at OJ and D28; 5 to 6 weeks after the booster injection, the spleens of the mice are removed in order to evaluate the amount of n ⁇ interferer produced.
- Immunization doses are prepared identical to those of Example 7, with the exception of the RSV antigens which are not in the presence of aluminum gel.
- the doses of 50 ⁇ litres are administered intramuscularly to groups of 6 mice.
- mice 4 weeks after immunization, the mice are bled and the anti-F protein antibody levels are determined by ELISA titration. The results obtained are summarized in the following table:
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Immunology (AREA)
- Virology (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Mycology (AREA)
- Oncology (AREA)
- Communicable Diseases (AREA)
- Epidemiology (AREA)
- Microbiology (AREA)
- Molecular Biology (AREA)
- Pulmonology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
Claims
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CA002342313A CA2342313A1 (fr) | 1998-09-11 | 1999-09-13 | Emulsion immunostimulante |
| US09/786,532 US6610308B1 (en) | 1998-09-11 | 1999-09-13 | Immunostimulant emulsion |
| AU56278/99A AU749282B2 (en) | 1998-09-11 | 1999-09-13 | Immunostimulant emulsion |
| EP99942961A EP1121148A2 (fr) | 1998-09-11 | 1999-09-13 | Emulsion immunostimulante |
| NZ510419A NZ510419A (en) | 1998-09-11 | 1999-09-13 | A vaccine adjuvant that is an oil-in-water immunoslimulant emulsion |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR98/11520 | 1998-09-11 | ||
| FR9811520A FR2783170B1 (fr) | 1998-09-11 | 1998-09-11 | Emulsion immunostimulante |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2000015256A2 true WO2000015256A2 (fr) | 2000-03-23 |
| WO2000015256A3 WO2000015256A3 (fr) | 2000-07-13 |
Family
ID=9530468
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/FR1999/002177 WO2000015256A2 (fr) | 1998-09-11 | 1999-09-13 | Emulsion immunostimulante |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US6610308B1 (fr) |
| EP (1) | EP1121148A2 (fr) |
| AU (1) | AU749282B2 (fr) |
| CA (1) | CA2342313A1 (fr) |
| FR (1) | FR2783170B1 (fr) |
| NZ (1) | NZ510419A (fr) |
| WO (1) | WO2000015256A2 (fr) |
Cited By (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2814958A1 (fr) * | 2000-10-06 | 2002-04-12 | Aventis Pasteur | Composition vaccinale |
| US7223741B2 (en) | 1994-07-15 | 2007-05-29 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US7488490B2 (en) | 1997-03-10 | 2009-02-10 | University Of Iowa Research Foundation | Method of inducing an antigen-specific immune response by administering a synergistic combination of adjuvants comprising unmethylated CpG-containing nucleic acids and a non-nucleic acid adjuvant |
| US7569553B2 (en) | 2002-07-03 | 2009-08-04 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US7576066B2 (en) | 2002-07-03 | 2009-08-18 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US7585847B2 (en) | 2000-02-03 | 2009-09-08 | Coley Pharmaceutical Group, Inc. | Immunostimulatory nucleic acids for the treatment of asthma and allergy |
| US7605138B2 (en) | 2002-07-03 | 2009-10-20 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US7615539B2 (en) | 2003-09-25 | 2009-11-10 | Coley Pharmaceutical Group, Inc. | Nucleic acid-lipophilic conjugates |
| US7674777B2 (en) | 1994-07-15 | 2010-03-09 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US7776343B1 (en) | 1999-02-17 | 2010-08-17 | Csl Limited | Immunogenic complexes and methods relating thereto |
| US7807803B2 (en) | 2002-07-03 | 2010-10-05 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US7935675B1 (en) | 1994-07-15 | 2011-05-03 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US7956043B2 (en) | 2002-12-11 | 2011-06-07 | Coley Pharmaceutical Group, Inc. | 5′ CpG nucleic acids and methods of use |
| US7998492B2 (en) | 2002-10-29 | 2011-08-16 | Coley Pharmaceutical Group, Inc. | Methods and products related to treatment and prevention of hepatitis C virus infection |
| US8114419B2 (en) | 2002-07-03 | 2012-02-14 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US8574599B1 (en) | 1998-05-22 | 2013-11-05 | Ottawa Hospital Research Institute | Methods and products for inducing mucosal immunity |
| US8580268B2 (en) | 2006-09-27 | 2013-11-12 | Coley Pharmaceutical Gmbh | CpG oligonucleotide analogs containing hydrophobic T analogs with enhanced immunostimulatory activity |
| EP1434602B1 (fr) * | 2001-10-06 | 2014-12-17 | Merial Limited | CpG plus huile en eau émulsions adjuvants pour herpèsvirus bovin de type 1 glycoprotéine D tronquéeÜ |
| US10837018B2 (en) | 2013-07-25 | 2020-11-17 | Exicure, Inc. | Spherical nucleic acid-based constructs as immunostimulatory agents for prophylactic and therapeutic use |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030026782A1 (en) * | 1995-02-07 | 2003-02-06 | Arthur M. Krieg | Immunomodulatory oligonucleotides |
| US20030022854A1 (en) | 1998-06-25 | 2003-01-30 | Dow Steven W. | Vaccines using nucleic acid-lipid complexes |
| HK1047892B (en) * | 1999-11-19 | 2009-01-09 | Csl Limited | HCV vaccine ingredients |
| IL160157A0 (en) | 2001-08-17 | 2004-07-25 | Coley Pharm Group Inc | Combination motif immune stimulation oligonucleotides with improved activity |
| AU2005326144A1 (en) * | 2004-06-08 | 2006-08-03 | Coley Pharmaceutical Gmbh | Abasic oligonucleotide as carrier platform for antigen and immunostimulatory agonist and antagonist |
| JP6034285B2 (ja) * | 2010-05-28 | 2016-11-30 | ゾエティス・ベルジャム・エス・アー | 抗原および免疫調節ワクチンおよびコレステロール、ならびにその使用 |
| PL3164113T3 (pl) | 2014-06-04 | 2019-09-30 | Exicure, Inc. | Wielowartościowe dostarczanie modulatorów immunologicznych w liposomowych kolistych kwasach nukleinowych do zastosowań profilaktycznych lub terapeutycznych |
| JP2017537619A (ja) | 2014-11-21 | 2017-12-21 | ノースウェスタン ユニバーシティ | 球状核酸ナノ粒子複合体の配列特異的細胞内取込 |
| WO2018039629A2 (fr) | 2016-08-25 | 2018-03-01 | Northwestern University | Acides nucléiques sphériques micellaires obtenus à partir de matrices thermosensibles sans trace |
| WO2018201090A1 (fr) | 2017-04-28 | 2018-11-01 | Exicure, Inc. | Synthèse d'acides nucléiques sphériques à l'aide de fractions lipophiles |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2560114A1 (fr) * | 1994-07-15 | 1996-02-01 | The University Of Iowa Research Foundation | Oligonucleotides immunomodulateurs |
| US5866699A (en) * | 1994-07-18 | 1999-02-02 | Hybridon, Inc. | Oligonucleotides with anti-MDR-1 gene activity |
| US5908777A (en) * | 1995-06-23 | 1999-06-01 | University Of Pittsburgh | Lipidic vector for nucleic acid delivery |
| PT1005368E (pt) * | 1997-03-10 | 2009-11-19 | Coley Pharm Gmbh | Utilização de ácidos nucleicos contendo dinucleótidos cpg não metilados em combinação com alúmen como adjuvante |
-
1998
- 1998-09-11 FR FR9811520A patent/FR2783170B1/fr not_active Expired - Fee Related
-
1999
- 1999-09-13 WO PCT/FR1999/002177 patent/WO2000015256A2/fr not_active Application Discontinuation
- 1999-09-13 NZ NZ510419A patent/NZ510419A/xx unknown
- 1999-09-13 CA CA002342313A patent/CA2342313A1/fr not_active Abandoned
- 1999-09-13 AU AU56278/99A patent/AU749282B2/en not_active Ceased
- 1999-09-13 US US09/786,532 patent/US6610308B1/en not_active Expired - Fee Related
- 1999-09-13 EP EP99942961A patent/EP1121148A2/fr not_active Withdrawn
Cited By (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7674777B2 (en) | 1994-07-15 | 2010-03-09 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US8258106B2 (en) | 1994-07-15 | 2012-09-04 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US7223741B2 (en) | 1994-07-15 | 2007-05-29 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US8129351B2 (en) | 1994-07-15 | 2012-03-06 | The University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US7935675B1 (en) | 1994-07-15 | 2011-05-03 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US7888327B2 (en) | 1994-07-15 | 2011-02-15 | University Of Iowa Research Foundation | Methods of using immunostimulatory nucleic acid molecules to treat allergic conditions |
| US7879810B2 (en) | 1994-07-15 | 2011-02-01 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US7723500B2 (en) | 1994-07-15 | 2010-05-25 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US8202688B2 (en) | 1997-03-10 | 2012-06-19 | University Of Iowa Research Foundation | Use of nucleic acids containing unmethylated CpG dinucleotide as an adjuvant |
| US7488490B2 (en) | 1997-03-10 | 2009-02-10 | University Of Iowa Research Foundation | Method of inducing an antigen-specific immune response by administering a synergistic combination of adjuvants comprising unmethylated CpG-containing nucleic acids and a non-nucleic acid adjuvant |
| US8574599B1 (en) | 1998-05-22 | 2013-11-05 | Ottawa Hospital Research Institute | Methods and products for inducing mucosal immunity |
| US7776343B1 (en) | 1999-02-17 | 2010-08-17 | Csl Limited | Immunogenic complexes and methods relating thereto |
| US8173141B2 (en) | 1999-02-17 | 2012-05-08 | Csl Limited | Immunogenic complexes and methods relating thereto |
| US7585847B2 (en) | 2000-02-03 | 2009-09-08 | Coley Pharmaceutical Group, Inc. | Immunostimulatory nucleic acids for the treatment of asthma and allergy |
| WO2002028428A3 (fr) * | 2000-10-06 | 2003-02-20 | Aventis Pasteur | Composition vaccinale |
| FR2814958A1 (fr) * | 2000-10-06 | 2002-04-12 | Aventis Pasteur | Composition vaccinale |
| EP1434602B1 (fr) * | 2001-10-06 | 2014-12-17 | Merial Limited | CpG plus huile en eau émulsions adjuvants pour herpèsvirus bovin de type 1 glycoprotéine D tronquéeÜ |
| US7576066B2 (en) | 2002-07-03 | 2009-08-18 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US8114419B2 (en) | 2002-07-03 | 2012-02-14 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US7569553B2 (en) | 2002-07-03 | 2009-08-04 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US7807803B2 (en) | 2002-07-03 | 2010-10-05 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US7605138B2 (en) | 2002-07-03 | 2009-10-20 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US7998492B2 (en) | 2002-10-29 | 2011-08-16 | Coley Pharmaceutical Group, Inc. | Methods and products related to treatment and prevention of hepatitis C virus infection |
| US7956043B2 (en) | 2002-12-11 | 2011-06-07 | Coley Pharmaceutical Group, Inc. | 5′ CpG nucleic acids and methods of use |
| US7615539B2 (en) | 2003-09-25 | 2009-11-10 | Coley Pharmaceutical Group, Inc. | Nucleic acid-lipophilic conjugates |
| US8580268B2 (en) | 2006-09-27 | 2013-11-12 | Coley Pharmaceutical Gmbh | CpG oligonucleotide analogs containing hydrophobic T analogs with enhanced immunostimulatory activity |
| US9382545B2 (en) | 2006-09-27 | 2016-07-05 | Coley Pharmaceutical Gmbh | CpG oligonucleotide analogs containing hydrophobic T analogs with enhanced immunostimulatory activity |
| US10260071B2 (en) | 2006-09-27 | 2019-04-16 | Coley Pharmaceutical Gmbh | CpG oligonucleotide analogs containing hydrophobic T analogs with enhanced immunostimulatory activity |
| US10837018B2 (en) | 2013-07-25 | 2020-11-17 | Exicure, Inc. | Spherical nucleic acid-based constructs as immunostimulatory agents for prophylactic and therapeutic use |
Also Published As
| Publication number | Publication date |
|---|---|
| US6610308B1 (en) | 2003-08-26 |
| CA2342313A1 (fr) | 2000-03-23 |
| NZ510419A (en) | 2003-01-31 |
| EP1121148A2 (fr) | 2001-08-08 |
| AU5627899A (en) | 2000-04-03 |
| FR2783170B1 (fr) | 2004-07-16 |
| AU749282B2 (en) | 2002-06-20 |
| WO2000015256A3 (fr) | 2000-07-13 |
| FR2783170A1 (fr) | 2000-03-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2000015256A2 (fr) | Emulsion immunostimulante | |
| EP1326636B1 (fr) | Composition vaccinale | |
| US5994318A (en) | Cochleate delivery vehicles | |
| JPH11500443A (ja) | コクリエート送達ビヒクル | |
| Rajananthanan et al. | Evaluation of novel aggregate structures as adjuvants: composition, toxicity studies and humoral responses | |
| WO1996014831A1 (fr) | Adjuvant pour composition vaccinale | |
| FR2598622A1 (fr) | Adjuvants immunologiques pour vaccins polysaccharidiques | |
| EP0918541B9 (fr) | Formulation de lipides immunostimulants | |
| WO2005060966A1 (fr) | Composition immunostimulante comprenant au moins un agoniste des recepteurs toll-like 7 ou du recepteur toll-like 8 et un agoniste du recepteur toll-like 4 | |
| WO1999051269A1 (fr) | Vaccins adn adjuves | |
| CN117897487A (zh) | 人工合成的含CpG单链脱氧寡核苷酸在疫苗中的应用 | |
| CA2246754C (fr) | Vecteurs d'apport de structures cochleaires | |
| EP1696954B1 (fr) | Composition vaccinale adjuvantée par une alkylphosphatidylcholine | |
| CA2406949A1 (fr) | Utilisation de vecteurs particulaires dans l'immunomodulation | |
| EP1024830A1 (fr) | Vecteurs d'antigenes sous forme de vesicules multilamellaires | |
| EP0748214B1 (fr) | Liposomes, et leur utilisation notamment pour l'obtention de compositions immunomodulatrices | |
| FR2692148A1 (fr) | Composition adjuvante de l'immunité humorale et à médiation cellulaire n'induisant pas de réponse vis-à-vis de déterminants auto-antigéniques. | |
| WO1993025236A1 (fr) | Composition adjuvante de l'immunite humorale et a mediation cellulaire n'induisant pas de reponse vis-a-vis de determinants auto-antigeniques | |
| FR2805163A1 (fr) | Utilisation d'un detergent de type zwittergent pour la preparation d'une composition pharmaceutique destinee a etre administree par voie nasale | |
| EP1628683A2 (fr) | Composition vaccinale comprenant du phosphate de fer a titre d' adjuvant vaccinal | |
| FR2808194A1 (fr) | Utilisation de particules hydrophiles portant ou non des ligands ioniques pour ameliorer les proprietes immunomodulatrices d'une substance autre qu'un antigene, et les compositions pharmaceutiques ainsi obtenues | |
| WO1986007365A1 (fr) | Produits resultant de la conjugaison d'un support macromoleculaire, d'un haptene et d'un muramyl-peptide. | |
| AU2006236007A1 (en) | Cochleate Delivery Vehicles | |
| WO2004073596A2 (fr) | Composition vaccinale comprenant un antigene proteique du vih incorpore dans des vesicules lipidiques multilamellaires. |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A2 Designated state(s): AE AL AM AT AU AZ BA BB BG BR BY CA CH CN CR CU CZ DE DK DM EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MD MG MK MN MW MX NO NZ PL PT RO RU SD SE SG SI SK SL TJ TM TR TT UA UG US UZ VN YU ZA ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A2 Designated state(s): GH GM KE LS MW SD SL SZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| AK | Designated states |
Kind code of ref document: A3 Designated state(s): AE AL AM AT AU AZ BA BB BG BR BY CA CH CN CR CU CZ DE DK DM EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MD MG MK MN MW MX NO NZ PL PT RO RU SD SE SG SI SK SL TJ TM TR TT UA UG US UZ VN YU ZA ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A3 Designated state(s): GH GM KE LS MW SD SL SZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1999942961 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 56278/99 Country of ref document: AU |
|
| ENP | Entry into the national phase |
Ref document number: 2342313 Country of ref document: CA Ref country code: CA Ref document number: 2342313 Kind code of ref document: A Format of ref document f/p: F |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 510419 Country of ref document: NZ |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 09786532 Country of ref document: US |
|
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |
|
| WWP | Wipo information: published in national office |
Ref document number: 1999942961 Country of ref document: EP |
|
| WWG | Wipo information: grant in national office |
Ref document number: 56278/99 Country of ref document: AU |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: 1999942961 Country of ref document: EP |