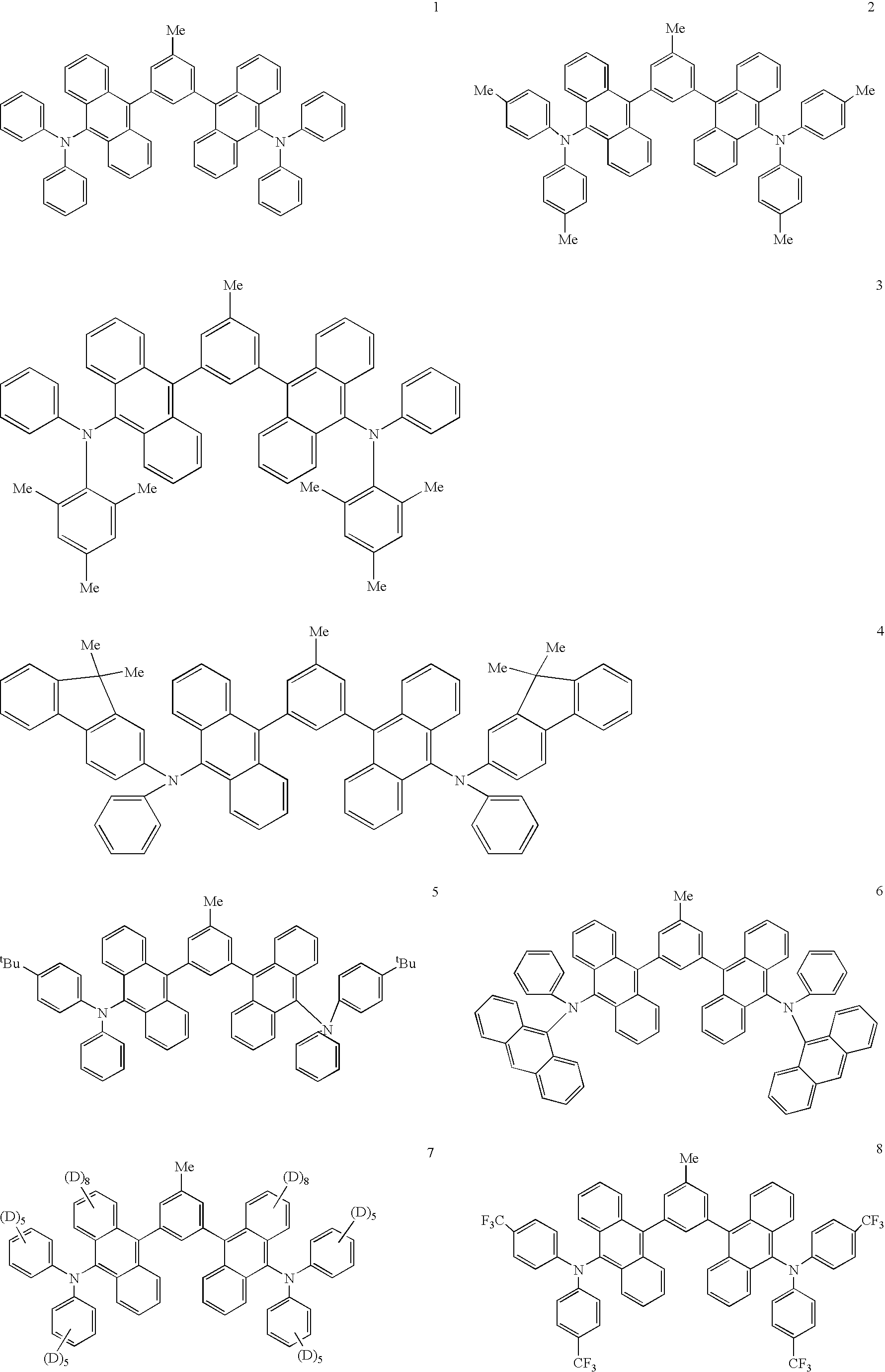
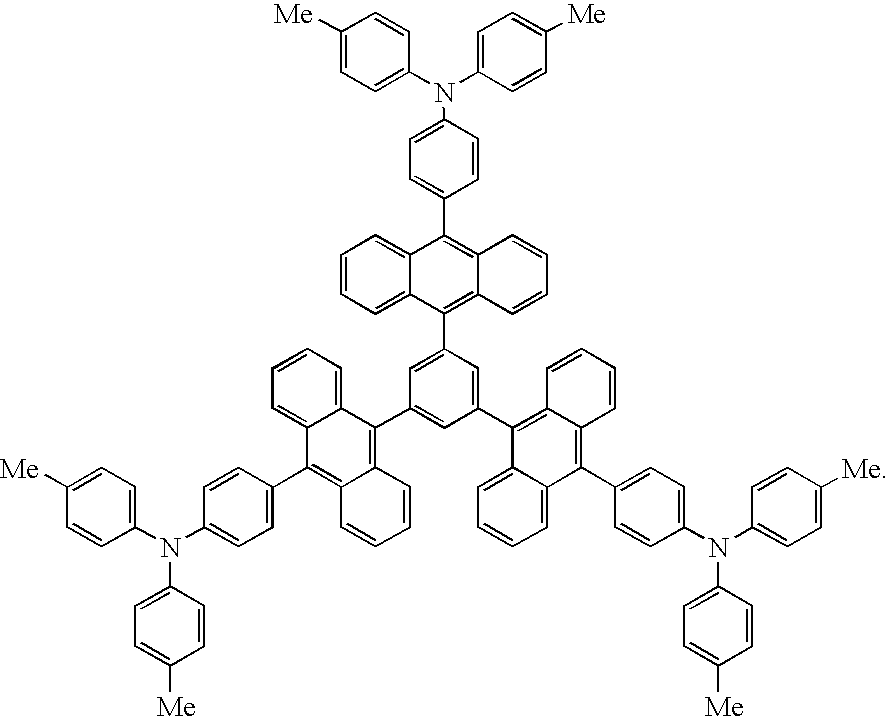
US7173131B2 - Anthryl derivative group substituted compound, and organic luminescent device making use of same - Google Patents
Anthryl derivative group substituted compound, and organic luminescent device making use of same Download PDFInfo
- Publication number
- US7173131B2 US7173131B2 US10/875,241 US87524104A US7173131B2 US 7173131 B2 US7173131 B2 US 7173131B2 US 87524104 A US87524104 A US 87524104A US 7173131 B2 US7173131 B2 US 7173131B2
- Authority
- US
- United States
- Prior art keywords
- group
- substituted
- unsubstituted
- ring
- layer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related, expires
Links
- 150000001875 compounds Chemical class 0.000 title claims abstract description 64
- 125000005428 anthryl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C3C(*)=C([H])C([H])=C([H])C3=C([H])C2=C1[H] 0.000 title claims abstract description 24
- 125000003118 aryl group Chemical group 0.000 claims description 30
- 125000000623 heterocyclic group Chemical group 0.000 claims description 29
- 125000000217 alkyl group Chemical group 0.000 claims description 25
- 229910052805 deuterium Inorganic materials 0.000 claims description 22
- 125000003545 alkoxy group Chemical group 0.000 claims description 16
- 125000003710 aryl alkyl group Chemical group 0.000 claims description 16
- 125000005843 halogen group Chemical group 0.000 claims description 15
- 125000003277 amino group Chemical group 0.000 claims description 14
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 13
- 125000000101 thioether group Chemical group 0.000 claims description 11
- 125000003342 alkenyl group Chemical group 0.000 claims description 7
- 125000000304 alkynyl group Chemical group 0.000 claims description 7
- 125000003808 silyl group Chemical group [H][Si]([H])([H])[*] 0.000 claims description 7
- 125000004431 deuterium atom Chemical group 0.000 claims 2
- 238000004020 luminiscence type Methods 0.000 abstract description 43
- 230000000694 effects Effects 0.000 abstract description 2
- -1 aluminum quinolinol Chemical compound 0.000 description 108
- 239000010410 layer Substances 0.000 description 68
- 239000000463 material Substances 0.000 description 57
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 27
- 239000010408 film Substances 0.000 description 24
- 150000001975 deuterium Chemical group 0.000 description 18
- 239000000243 solution Substances 0.000 description 17
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 16
- 239000000758 substrate Substances 0.000 description 16
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 15
- 230000005525 hole transport Effects 0.000 description 13
- 229920005989 resin Polymers 0.000 description 13
- 239000011347 resin Substances 0.000 description 13
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 12
- 125000000732 arylene group Chemical group 0.000 description 11
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 10
- 229910001148 Al-Li alloy Inorganic materials 0.000 description 9
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 9
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 9
- 238000002347 injection Methods 0.000 description 8
- 239000007924 injection Substances 0.000 description 8
- 229910052757 nitrogen Inorganic materials 0.000 description 8
- 239000012044 organic layer Substances 0.000 description 8
- 239000000047 product Substances 0.000 description 8
- 125000001424 substituent group Chemical group 0.000 description 8
- 238000001771 vacuum deposition Methods 0.000 description 8
- 125000002947 alkylene group Chemical group 0.000 description 7
- 230000015572 biosynthetic process Effects 0.000 description 7
- 150000002894 organic compounds Chemical class 0.000 description 7
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 6
- 0 CC.CC.[1*]C1=C([1*])C([1*])=C2C(CN(C)[Y][Y])=C3C([1*])=C([1*])C([1*])=C([1*])C3=C(CC)C2=C1[1*].[2*]C1=C([2*])C([2*])=C2C(CC)=C3C([2*])=C([2*])C([2*])=C([2*])C3=C(C)C2=C1[2*] Chemical compound CC.CC.[1*]C1=C([1*])C([1*])=C2C(CN(C)[Y][Y])=C3C([1*])=C([1*])C([1*])=C([1*])C3=C(CC)C2=C1[1*].[2*]C1=C([2*])C([2*])=C2C(CC)=C3C([2*])=C([2*])C([2*])=C([2*])C3=C(C)C2=C1[2*] 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 125000004450 alkenylene group Chemical group 0.000 description 6
- 125000004419 alkynylene group Chemical group 0.000 description 6
- 238000006243 chemical reaction Methods 0.000 description 6
- 230000006866 deterioration Effects 0.000 description 6
- 239000002019 doping agent Substances 0.000 description 6
- 125000003983 fluorenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3CC12)* 0.000 description 6
- 229910052751 metal Inorganic materials 0.000 description 6
- 239000002184 metal Substances 0.000 description 6
- NFHFRUOZVGFOOS-UHFFFAOYSA-N palladium;triphenylphosphane Chemical compound [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 6
- 238000003756 stirring Methods 0.000 description 6
- 239000010409 thin film Substances 0.000 description 6
- YJTKZCDBKVTVBY-UHFFFAOYSA-N 1,3-Diphenylbenzene Chemical group C1=CC=CC=C1C1=CC=CC(C=2C=CC=CC=2)=C1 YJTKZCDBKVTVBY-UHFFFAOYSA-N 0.000 description 5
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 5
- 239000011248 coating agent Substances 0.000 description 5
- 238000000576 coating method Methods 0.000 description 5
- 238000010438 heat treatment Methods 0.000 description 5
- 125000002080 perylenyl group Chemical group C1(=CC=C2C=CC=C3C4=CC=CC5=CC=CC(C1=C23)=C45)* 0.000 description 5
- 239000002244 precipitate Substances 0.000 description 5
- 239000002904 solvent Substances 0.000 description 5
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 4
- 125000005577 anthracene group Chemical group 0.000 description 4
- 239000012298 atmosphere Substances 0.000 description 4
- 230000000052 comparative effect Effects 0.000 description 4
- 238000001035 drying Methods 0.000 description 4
- 239000011521 glass Substances 0.000 description 4
- 238000004770 highest occupied molecular orbital Methods 0.000 description 4
- AMGQUBHHOARCQH-UHFFFAOYSA-N indium;oxotin Chemical compound [In].[Sn]=O AMGQUBHHOARCQH-UHFFFAOYSA-N 0.000 description 4
- 238000004768 lowest unoccupied molecular orbital Methods 0.000 description 4
- 238000000034 method Methods 0.000 description 4
- 239000012046 mixed solvent Substances 0.000 description 4
- 125000001624 naphthyl group Chemical group 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 125000000168 pyrrolyl group Chemical group 0.000 description 4
- 238000010898 silica gel chromatography Methods 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- KXSFECAJUBPPFE-UHFFFAOYSA-N 2,2':5',2''-terthiophene Chemical group C1=CSC(C=2SC(=CC=2)C=2SC=CC=2)=C1 KXSFECAJUBPPFE-UHFFFAOYSA-N 0.000 description 3
- JFJNVIPVOCESGZ-UHFFFAOYSA-N 2,3-dipyridin-2-ylpyridine Chemical group N1=CC=CC=C1C1=CC=CN=C1C1=CC=CC=N1 JFJNVIPVOCESGZ-UHFFFAOYSA-N 0.000 description 3
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 3
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical group C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 3
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 3
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical group C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 3
- JFBZPFYRPYOZCQ-UHFFFAOYSA-N [Li].[Al] Chemical compound [Li].[Al] JFBZPFYRPYOZCQ-UHFFFAOYSA-N 0.000 description 3
- 230000000903 blocking effect Effects 0.000 description 3
- 125000000609 carbazolyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3NC12)* 0.000 description 3
- 238000000151 deposition Methods 0.000 description 3
- 230000008021 deposition Effects 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 229910044991 metal oxide Inorganic materials 0.000 description 3
- 150000004706 metal oxides Chemical class 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- YNPNZTXNASCQKK-UHFFFAOYSA-N phenanthrene Chemical group C1=CC=C2C3=CC=CC=C3C=CC2=C1 YNPNZTXNASCQKK-UHFFFAOYSA-N 0.000 description 3
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 3
- 229920000553 poly(phenylenevinylene) Polymers 0.000 description 3
- 125000005581 pyrene group Chemical group 0.000 description 3
- 239000004332 silver Substances 0.000 description 3
- 239000002356 single layer Substances 0.000 description 3
- 125000005579 tetracene group Chemical group 0.000 description 3
- 239000008096 xylene Substances 0.000 description 3
- 125000000094 2-phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 description 2
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 2
- DHDHJYNTEFLIHY-UHFFFAOYSA-N 4,7-diphenyl-1,10-phenanthroline Chemical compound C1=CC=CC=C1C1=CC=NC2=C1C=CC1=C(C=3C=CC=CC=3)C=CN=C21 DHDHJYNTEFLIHY-UHFFFAOYSA-N 0.000 description 2
- 125000004860 4-ethylphenyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)C([H])([H])C([H])([H])[H] 0.000 description 2
- 125000000590 4-methylphenyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)C([H])([H])[H] 0.000 description 2
- 239000004925 Acrylic resin Substances 0.000 description 2
- 229920000178 Acrylic resin Polymers 0.000 description 2
- ROFVEXUMMXZLPA-UHFFFAOYSA-N Bipyridyl Chemical group N1=CC=CC=C1C1=CC=CC=N1 ROFVEXUMMXZLPA-UHFFFAOYSA-N 0.000 description 2
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 2
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical group [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 2
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 2
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- OFBQJSOFQDEBGM-UHFFFAOYSA-N Pentane Chemical group CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 2
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 2
- 229910045601 alloy Inorganic materials 0.000 description 2
- 239000000956 alloy Substances 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- IOJUPLGTWVMSFF-UHFFFAOYSA-N benzothiazole Chemical group C1=CC=C2SC=NC2=C1 IOJUPLGTWVMSFF-UHFFFAOYSA-N 0.000 description 2
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 125000006267 biphenyl group Chemical group 0.000 description 2
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 2
- 229910052794 bromium Inorganic materials 0.000 description 2
- 238000004364 calculation method Methods 0.000 description 2
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 2
- MVPPADPHJFYWMZ-UHFFFAOYSA-N chlorobenzene Chemical compound ClC1=CC=CC=C1 MVPPADPHJFYWMZ-UHFFFAOYSA-N 0.000 description 2
- 238000004140 cleaning Methods 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical group C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 2
- DMBHHRLKUKUOEG-UHFFFAOYSA-N diphenylamine Chemical compound C=1C=CC=CC=1NC1=CC=CC=C1 DMBHHRLKUKUOEG-UHFFFAOYSA-N 0.000 description 2
- 239000007772 electrode material Substances 0.000 description 2
- 125000001033 ether group Chemical group 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- 230000002349 favourable effect Effects 0.000 description 2
- 150000008376 fluorenones Chemical class 0.000 description 2
- 125000002541 furyl group Chemical group 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 2
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 2
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- 229910052744 lithium Inorganic materials 0.000 description 2
- 239000001989 lithium alloy Substances 0.000 description 2
- NEMFQSKAPLGFIP-UHFFFAOYSA-N magnesiosodium Chemical compound [Na].[Mg] NEMFQSKAPLGFIP-UHFFFAOYSA-N 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 125000006384 methylpyridyl group Chemical group 0.000 description 2
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 125000003136 n-heptyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 2
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 239000012299 nitrogen atmosphere Substances 0.000 description 2
- 125000002524 organometallic group Chemical group 0.000 description 2
- 150000004866 oxadiazoles Chemical class 0.000 description 2
- 150000007978 oxazole derivatives Chemical class 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 229910052763 palladium Inorganic materials 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 229920003227 poly(N-vinyl carbazole) Polymers 0.000 description 2
- 229920005990 polystyrene resin Polymers 0.000 description 2
- 229920000123 polythiophene Polymers 0.000 description 2
- 125000004805 propylene group Chemical group [H]C([H])([H])C([H])([*:1])C([H])([H])[*:2] 0.000 description 2
- 239000011241 protective layer Substances 0.000 description 2
- 150000003216 pyrazines Chemical class 0.000 description 2
- 125000004076 pyridyl group Chemical group 0.000 description 2
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 2
- 125000005493 quinolyl group Chemical group 0.000 description 2
- 125000001567 quinoxalinyl group Chemical group N1=C(C=NC2=CC=CC=C12)* 0.000 description 2
- 238000007789 sealing Methods 0.000 description 2
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- 229920002050 silicone resin Polymers 0.000 description 2
- 229910052709 silver Inorganic materials 0.000 description 2
- MFRIHAYPQRLWNB-UHFFFAOYSA-N sodium tert-butoxide Chemical compound [Na+].CC(C)(C)[O-] MFRIHAYPQRLWNB-UHFFFAOYSA-N 0.000 description 2
- 238000004528 spin coating Methods 0.000 description 2
- 238000004544 sputter deposition Methods 0.000 description 2
- PJANXHGTPQOBST-UHFFFAOYSA-N stilbene Chemical class C=1C=CC=CC=1C=CC1=CC=CC=C1 PJANXHGTPQOBST-UHFFFAOYSA-N 0.000 description 2
- 229940042055 systemic antimycotics triazole derivative Drugs 0.000 description 2
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 125000001544 thienyl group Chemical group 0.000 description 2
- BWHDROKFUHTORW-UHFFFAOYSA-N tritert-butylphosphane Chemical compound CC(C)(C)P(C(C)(C)C)C(C)(C)C BWHDROKFUHTORW-UHFFFAOYSA-N 0.000 description 2
- IWZSHWBGHQBIML-ZGGLMWTQSA-N (3S,8S,10R,13S,14S,17S)-17-isoquinolin-7-yl-N,N,10,13-tetramethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-amine Chemical compound CN(C)[C@H]1CC[C@]2(C)C3CC[C@@]4(C)[C@@H](CC[C@@H]4c4ccc5ccncc5c4)[C@@H]3CC=C2C1 IWZSHWBGHQBIML-ZGGLMWTQSA-N 0.000 description 1
- 239000001211 (E)-4-phenylbut-3-en-2-one Substances 0.000 description 1
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical group C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 description 1
- GMVJKSNPLYBFSO-UHFFFAOYSA-N 1,2,3-tribromobenzene Chemical compound BrC1=CC=CC(Br)=C1Br GMVJKSNPLYBFSO-UHFFFAOYSA-N 0.000 description 1
- DXBHBZVCASKNBY-UHFFFAOYSA-N 1,2-Benz(a)anthracene Chemical group C1=CC=C2C3=CC4=CC=CC=C4C=C3C=CC2=C1 DXBHBZVCASKNBY-UHFFFAOYSA-N 0.000 description 1
- BCMCBBGGLRIHSE-UHFFFAOYSA-N 1,3-benzoxazole Chemical class C1=CC=C2OC=NC2=C1 BCMCBBGGLRIHSE-UHFFFAOYSA-N 0.000 description 1
- ONBQEOIKXPHGMB-VBSBHUPXSA-N 1-[2-[(2s,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-4,6-dihydroxyphenyl]-3-(4-hydroxyphenyl)propan-1-one Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1OC1=CC(O)=CC(O)=C1C(=O)CCC1=CC=C(O)C=C1 ONBQEOIKXPHGMB-VBSBHUPXSA-N 0.000 description 1
- 125000004973 1-butenyl group Chemical group C(=CCC)* 0.000 description 1
- NRXWFTYEJYEOGW-UHFFFAOYSA-N 1-methyl-4-(4-methylphenyl)sulfanylbenzene Chemical group C1=CC(C)=CC=C1SC1=CC=C(C)C=C1 NRXWFTYEJYEOGW-UHFFFAOYSA-N 0.000 description 1
- 125000001637 1-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C(*)=C([H])C([H])=C([H])C2=C1[H] 0.000 description 1
- BRSRUYVJULRMRQ-UHFFFAOYSA-N 1-phenylanthracene Chemical class C1=CC=CC=C1C1=CC=CC2=CC3=CC=CC=C3C=C12 BRSRUYVJULRMRQ-UHFFFAOYSA-N 0.000 description 1
- 125000006017 1-propenyl group Chemical group 0.000 description 1
- 125000000530 1-propynyl group Chemical group [H]C([H])([H])C#C* 0.000 description 1
- HYZJCKYKOHLVJF-UHFFFAOYSA-N 1H-benzimidazole Chemical class C1=CC=C2NC=NC2=C1 HYZJCKYKOHLVJF-UHFFFAOYSA-N 0.000 description 1
- 125000000453 2,2,2-trichloroethyl group Chemical group [H]C([H])(*)C(Cl)(Cl)Cl 0.000 description 1
- 125000004206 2,2,2-trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 description 1
- 125000004825 2,2-dimethylpropylene group Chemical group [H]C([H])([H])C(C([H])([H])[H])(C([H])([H])[*:1])C([H])([H])[*:2] 0.000 description 1
- ACYOAZKGYRXBII-UHFFFAOYSA-N 2-(1h-pyrrol-2-yl)-3-pyrrol-2-ylidenepyrrole Chemical group N1=CC=CC1=C1C(C=2NC=CC=2)=NC=C1 ACYOAZKGYRXBII-UHFFFAOYSA-N 0.000 description 1
- 125000003163 2-(2-naphthyl)ethyl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C(C([H])=C([H])C2=C1[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000006280 2-bromobenzyl group Chemical group [H]C1=C([H])C(Br)=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 125000005999 2-bromoethyl group Chemical group 0.000 description 1
- 125000004974 2-butenyl group Chemical group C(C=CC)* 0.000 description 1
- 125000006282 2-chlorobenzyl group Chemical group [H]C1=C([H])C(Cl)=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 125000001340 2-chloroethyl group Chemical group [H]C([H])(Cl)C([H])([H])* 0.000 description 1
- 125000004847 2-fluorobenzyl group Chemical group [H]C1=C([H])C(F)=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 125000004777 2-fluoroethyl group Chemical group [H]C([H])(F)C([H])([H])* 0.000 description 1
- 102100027324 2-hydroxyacyl-CoA lyase 1 Human genes 0.000 description 1
- VQGHOUODWALEFC-UHFFFAOYSA-N 2-phenylpyridine Chemical compound C1=CC=CC=C1C1=CC=CC=N1 VQGHOUODWALEFC-UHFFFAOYSA-N 0.000 description 1
- MGADZUXDNSDTHW-UHFFFAOYSA-N 2H-pyran Chemical class C1OC=CC=C1 MGADZUXDNSDTHW-UHFFFAOYSA-N 0.000 description 1
- 125000006279 3-bromobenzyl group Chemical group [H]C1=C([H])C(=C([H])C(Br)=C1[H])C([H])([H])* 0.000 description 1
- 125000004975 3-butenyl group Chemical group C(CC=C)* 0.000 description 1
- 125000003852 3-chlorobenzyl group Chemical group [H]C1=C([H])C(=C([H])C(Cl)=C1[H])C([H])([H])* 0.000 description 1
- 125000006284 3-fluorobenzyl group Chemical group [H]C1=C([H])C(=C([H])C(F)=C1[H])C([H])([H])* 0.000 description 1
- 125000006281 4-bromobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1Br)C([H])([H])* 0.000 description 1
- 125000006283 4-chlorobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1Cl)C([H])([H])* 0.000 description 1
- 125000004176 4-fluorobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1F)C([H])([H])* 0.000 description 1
- 125000001255 4-fluorophenyl group Chemical group [H]C1=C([H])C(*)=C([H])C([H])=C1F 0.000 description 1
- FCNCGHJSNVOIKE-UHFFFAOYSA-N 9,10-diphenylanthracene Chemical class C1=CC=CC=C1C(C1=CC=CC=C11)=C(C=CC=C2)C2=C1C1=CC=CC=C1 FCNCGHJSNVOIKE-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N Acrylic acid Chemical compound OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 229910001316 Ag alloy Inorganic materials 0.000 description 1
- MHCIFDWZTOJTIF-UHFFFAOYSA-M BrBr.BrBr.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(Br)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(Br)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.ClC(Cl)Cl.I[IH]I.OB(O)C1=CC=C2C=CC=CC2=C1.[V]I Chemical compound BrBr.BrBr.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(Br)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(Br)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.ClC(Cl)Cl.I[IH]I.OB(O)C1=CC=C2C=CC=CC2=C1.[V]I MHCIFDWZTOJTIF-UHFFFAOYSA-M 0.000 description 1
- BIYWYIUJHJPVIF-UHFFFAOYSA-N BrBr.BrC1=C2C=CC=CC2=C(C2=CC(C3=C4C=CC=CC4=C(Br)C4=CC=CC=C43)=CC(C3=C4C=CC=CC4=C(Br)C4=CC=CC=C43)=C2)C2=CC=CC=C21.BrC1=C2C=CC=CC2=C(C2=CC(C3=C4C=CC=CC4=CC4=CC=CC=C43)=CC(C3=C4C=CC=CC4=C(Br)C4=CC=CC=C43)=C2)C2=CC=CC=C21.BrC1=CC(Br)=CC(Br)=C1.C1=CC=C2C(=C1)C=C1C=CC=CC1=C2C1=CC(C2=C3C=CC=CC3=CC3=CC=CC=C32)=CC(C2=C3C=CC=CC3=CC3=CC=CC=C32)=C1.ClCCl.I.II.OB(O)C1=C2C=CC=CC2=CC2=CC=CC=C21 Chemical compound BrBr.BrC1=C2C=CC=CC2=C(C2=CC(C3=C4C=CC=CC4=C(Br)C4=CC=CC=C43)=CC(C3=C4C=CC=CC4=C(Br)C4=CC=CC=C43)=C2)C2=CC=CC=C21.BrC1=C2C=CC=CC2=C(C2=CC(C3=C4C=CC=CC4=CC4=CC=CC=C43)=CC(C3=C4C=CC=CC4=C(Br)C4=CC=CC=C43)=C2)C2=CC=CC=C21.BrC1=CC(Br)=CC(Br)=C1.C1=CC=C2C(=C1)C=C1C=CC=CC1=C2C1=CC(C2=C3C=CC=CC3=CC3=CC=CC=C32)=CC(C2=C3C=CC=CC3=CC3=CC=CC=C32)=C1.ClCCl.I.II.OB(O)C1=C2C=CC=CC2=CC2=CC=CC=C21 BIYWYIUJHJPVIF-UHFFFAOYSA-N 0.000 description 1
- LLBYCEGKQZHBQA-UHFFFAOYSA-N BrC1=C2C=CC=CC2=C(C2=CC(C3=C4C=CC=CC4=C(Br)C4=CC=CC=C43)=CC(C3=C4C=CC=CC4=C(Br)C4=CC=CC=C43)=C2)C2=CC=CC=C21.BrC1=C2C=CC=CC2=C(C2=CC(C3=C4C=CC=CC4=CC4=CC=CC=C43)=CC(C3=C4C=CC=CC4=C(Br)C4=CC=CC=C43)=C2)C2=CC=CC=C21.II Chemical compound BrC1=C2C=CC=CC2=C(C2=CC(C3=C4C=CC=CC4=C(Br)C4=CC=CC=C43)=CC(C3=C4C=CC=CC4=C(Br)C4=CC=CC=C43)=C2)C2=CC=CC=C21.BrC1=C2C=CC=CC2=C(C2=CC(C3=C4C=CC=CC4=CC4=CC=CC=C43)=CC(C3=C4C=CC=CC4=C(Br)C4=CC=CC=C43)=C2)C2=CC=CC=C21.II LLBYCEGKQZHBQA-UHFFFAOYSA-N 0.000 description 1
- KAKZBPTYRLMSJV-UHFFFAOYSA-N Butadiene Chemical group C=CC=C KAKZBPTYRLMSJV-UHFFFAOYSA-N 0.000 description 1
- HXSSCBKZQHUBAN-LIHPPGGZSA-N C/C=C/C1=CC(OC)=C(C)C=C1OCC(CC)CCCC.C/C=C/C1=CC=C(C)C=C1.CC1=CC(C)=C(C)C=C1.CC1=CC(C)=C(C)S1.CC1=CC=C2C(=C1)C(C)(C)/C1=C/C(C)=C\C=C\21.CC=C(C)C1=CC=CC(C)=C1.PP[V].[C-]#[N+]/C(=C\C1=CC(C)=C(C)C=C1C)C1=CC(C)=C(/C(C#N)=C/C)C=C1C Chemical compound C/C=C/C1=CC(OC)=C(C)C=C1OCC(CC)CCCC.C/C=C/C1=CC=C(C)C=C1.CC1=CC(C)=C(C)C=C1.CC1=CC(C)=C(C)S1.CC1=CC=C2C(=C1)C(C)(C)/C1=C/C(C)=C\C=C\21.CC=C(C)C1=CC=CC(C)=C1.PP[V].[C-]#[N+]/C(=C\C1=CC(C)=C(C)C=C1C)C1=CC(C)=C(/C(C#N)=C/C)C=C1C HXSSCBKZQHUBAN-LIHPPGGZSA-N 0.000 description 1
- YSUHSYDZGBZHGF-CIESIZNRSA-N C1=C2=CC=C3=CC=C4=CC=C5=CC=C6=CC=C(=C1)C1=C2C3=C4C5=C16.C1=CC=C(C2=C3C=CC=CC3=C(C3=CC=CC=C3)C3=C(C4=CC=CC=C4)C4=CC=CC=C4C(C4=CC=CC=C4)=C23)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C=CC3=CC=C(C4=CC=C(C=CC5=CC=C(N(C6=CC=CC=C6)C6=CC=CC=C6)C=C5)C=C4)C=C3)C=C2)C=C1.CC1=CC=C(C(=CC2=C3C=CC=CC3=C(C=C(C3=CC=C(C)C=C3)C3=CC=C(C)C=C3)C3=CC=CC=C32)C2=CC=C(C)C=C2)C=C1.CC1=CC=C(C(=CC2=CC=C(C3=CC=C(C=C(C4=CC=C(C)C=C4)C4=CC=C(C)C=C4)C=C3)C=C2)C2=CC=C(C)C=C2)C=C1.CCN(CC)C1=CC2=C(C=C1)C=C(C1=NC3=C(C=CC=C3)S1)C(=O)O2.CCN(CC)C1=CC2=C(C=C1)N=C1C(=CC(=O)C3=C1C=CC=C3)O2.O=C1C2=CC3=C(C=C2NC2=C1C=CC=C2)C(=O)C1=C(C=CC=C1)N3.[C-]#[N+]/C(C#N)=C1\C=C(C)OC(/C=C/C2=CC=C(N(CC)CC)C=C2)=C1 Chemical compound C1=C2=CC=C3=CC=C4=CC=C5=CC=C6=CC=C(=C1)C1=C2C3=C4C5=C16.C1=CC=C(C2=C3C=CC=CC3=C(C3=CC=CC=C3)C3=C(C4=CC=CC=C4)C4=CC=CC=C4C(C4=CC=CC=C4)=C23)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C=CC3=CC=C(C4=CC=C(C=CC5=CC=C(N(C6=CC=CC=C6)C6=CC=CC=C6)C=C5)C=C4)C=C3)C=C2)C=C1.CC1=CC=C(C(=CC2=C3C=CC=CC3=C(C=C(C3=CC=C(C)C=C3)C3=CC=C(C)C=C3)C3=CC=CC=C32)C2=CC=C(C)C=C2)C=C1.CC1=CC=C(C(=CC2=CC=C(C3=CC=C(C=C(C4=CC=C(C)C=C4)C4=CC=C(C)C=C4)C=C3)C=C2)C2=CC=C(C)C=C2)C=C1.CCN(CC)C1=CC2=C(C=C1)C=C(C1=NC3=C(C=CC=C3)S1)C(=O)O2.CCN(CC)C1=CC2=C(C=C1)N=C1C(=CC(=O)C3=C1C=CC=C3)O2.O=C1C2=CC3=C(C=C2NC2=C1C=CC=C2)C(=O)C1=C(C=CC=C1)N3.[C-]#[N+]/C(C#N)=C1\C=C(C)OC(/C=C/C2=CC=C(N(CC)CC)C=C2)=C1 YSUHSYDZGBZHGF-CIESIZNRSA-N 0.000 description 1
- XTKGIQKHKILSIO-UHFFFAOYSA-N C1=CC2=C3N=C4C5C=CC=CC5C5=N4C46N3C(=C2C=C1)/N=C1C2=C(C=CC=C2)C(=N/14)\N=C1\C2=C(C=CC=C2)/C(=N/5)N16.CC.CC1=CC=CC(N(C2=CC=CC(N(C3=CC=CC(C)=C3)C3=CC=CC(C)=C3)=C2)C2=CC(C)=CC=C2)=C1 Chemical compound C1=CC2=C3N=C4C5C=CC=CC5C5=N4C46N3C(=C2C=C1)/N=C1C2=C(C=CC=C2)C(=N/14)\N=C1\C2=C(C=CC=C2)/C(=N/5)N16.CC.CC1=CC=CC(N(C2=CC=CC(N(C3=CC=CC(C)=C3)C3=CC=CC(C)=C3)=C2)C2=CC(C)=CC=C2)=C1 XTKGIQKHKILSIO-UHFFFAOYSA-N 0.000 description 1
- RPMKHARCJXGPPV-UHFFFAOYSA-K C1=CC2=CC=CC3=C2N(=C1)[Al]12(O3)(OC3=C4C(=CC=C3)C=CC=N41)OC1=C3C(=CC=C1)/C=C\C=N/32.C1=CC=C(C2=CC=NC3=C2C=CC2=C3N=CC=C2C2=CC=CC=C2)C=C1 Chemical compound C1=CC2=CC=CC3=C2N(=C1)[Al]12(O3)(OC3=C4C(=CC=C3)C=CC=N41)OC1=C3C(=CC=C1)/C=C\C=N/32.C1=CC=C(C2=CC=NC3=C2C=CC2=C3N=CC=C2C2=CC=CC=C2)C=C1 RPMKHARCJXGPPV-UHFFFAOYSA-K 0.000 description 1
- JOMKZODHAASGBT-SVLHFQGPSA-N C1=CC=C(/C=C/C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(/C=C/C5=CC=CC=C5)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(/C=C/C5=CC=CC=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.C1=CC=C(/C=C/C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(/C=C/C5=CC=CC=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.C1=CC=C2C=C(C3=C4C=CC=CC4=C(C4=CC(C5=C6C=CC=CC6=C(C6=CC=C7C=CC=CC7=C6)C6=CC=CC=C65)=CC(C5=C6C=CC=CC6=C(C6=CC=C7C=CC=CC7=C6)C6=CC=CC=C65)=C4)C4=CC=CC=C43)C=CC2=C1.C1=CC=C2C=C(C3=C4C=CC=CC4=C(C4=CC(C5=C6C=CC=CC6=C(C6=CC=C7C=CC=CC7=C6)C6=CC=CC=C65)=CC(C5=C6C=CC=CC6=CC6=CC=CC=C65)=C4)C4=CC=CC=C43)C=CC2=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CSC1=CC=C(C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(C5=CC=C(SC)C=C5)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(C5=CC=C(SC)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.[2H]C.[2H]C.[2H]C.[2H]C.[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H]C.[2H][2H][2H] Chemical compound C1=CC=C(/C=C/C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(/C=C/C5=CC=CC=C5)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(/C=C/C5=CC=CC=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.C1=CC=C(/C=C/C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(/C=C/C5=CC=CC=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.C1=CC=C2C=C(C3=C4C=CC=CC4=C(C4=CC(C5=C6C=CC=CC6=C(C6=CC=C7C=CC=CC7=C6)C6=CC=CC=C65)=CC(C5=C6C=CC=CC6=C(C6=CC=C7C=CC=CC7=C6)C6=CC=CC=C65)=C4)C4=CC=CC=C43)C=CC2=C1.C1=CC=C2C=C(C3=C4C=CC=CC4=C(C4=CC(C5=C6C=CC=CC6=C(C6=CC=C7C=CC=CC7=C6)C6=CC=CC=C65)=CC(C5=C6C=CC=CC6=CC6=CC=CC=C65)=C4)C4=CC=CC=C43)C=CC2=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CSC1=CC=C(C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(C5=CC=C(SC)C=C5)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(C5=CC=C(SC)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.[2H]C.[2H]C.[2H]C.[2H]C.[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H]C.[2H][2H][2H] JOMKZODHAASGBT-SVLHFQGPSA-N 0.000 description 1
- NFLVMWFUIZKYLI-LYLVRHOXSA-N C1=CC=C(/C=C/C2=CC=C(N(C3=CC=CC=C3)C3=C4C=CC=CC4=C(C4=CC(C5=C6C=CC=CC6=CC6=CC=CC=C65)=CC(C5=C6C=CC=CC6=C(N(C6=CC=CC=C6)C6=CC=C(/C=C/C7=CC=CC=C7)C=C6)C6=CC=CC=C65)=C4)C4=CC=CC=C43)C=C2)C=C1.C1=CC=C(N(C2=CC=CS2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=CS5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.C1=CC=C2C(=C1)C=C1C=CC=CC1=C2C1=CC(C2=C3C=CC=CC3=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C3=CC=CC=C32)=CC(C2=C3C=CC=CC3=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C3=CC=CC=C32)=C1.C1=CC=C2C=C(C3=C4C=CC=CC4=C(C4=CC(C5=C6C=CC=CC6=C(C6=CC=C7C=CC=CC7=C6)C6=CC=CC=C65)=CC(C5=C6C=CC=CC6=CC6=CC=CC=C65)=C4)C4=CC=CC=C43)C=CC2=C1.CC1(C)C2=C(C=CC=C2)C2=C1C=C(N(C1=CC=CC=C1)C1=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC6=C(C=C5)C5=C(C=CC=C5)C6(C)C)C5=CC=CC=C54)=C3)C3=CC=CC=C31)C=C2.CC1=CC=C2C=C3C=C(C)C=CC3=C(C3=CC(C4=C5C=CC(C)=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC=C(C)C=C54)=CC(C4=C5C=C(C)C=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC(C)=CC=C54)=C3)C2=C1.FC(F)(F)C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=C(C(F)(F)F)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1 Chemical compound C1=CC=C(/C=C/C2=CC=C(N(C3=CC=CC=C3)C3=C4C=CC=CC4=C(C4=CC(C5=C6C=CC=CC6=CC6=CC=CC=C65)=CC(C5=C6C=CC=CC6=C(N(C6=CC=CC=C6)C6=CC=C(/C=C/C7=CC=CC=C7)C=C6)C6=CC=CC=C65)=C4)C4=CC=CC=C43)C=C2)C=C1.C1=CC=C(N(C2=CC=CS2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=CS5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.C1=CC=C2C(=C1)C=C1C=CC=CC1=C2C1=CC(C2=C3C=CC=CC3=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C3=CC=CC=C32)=CC(C2=C3C=CC=CC3=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C3=CC=CC=C32)=C1.C1=CC=C2C=C(C3=C4C=CC=CC4=C(C4=CC(C5=C6C=CC=CC6=C(C6=CC=C7C=CC=CC7=C6)C6=CC=CC=C65)=CC(C5=C6C=CC=CC6=CC6=CC=CC=C65)=C4)C4=CC=CC=C43)C=CC2=C1.CC1(C)C2=C(C=CC=C2)C2=C1C=C(N(C1=CC=CC=C1)C1=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC6=C(C=C5)C5=C(C=CC=C5)C6(C)C)C5=CC=CC=C54)=C3)C3=CC=CC=C31)C=C2.CC1=CC=C2C=C3C=C(C)C=CC3=C(C3=CC(C4=C5C=CC(C)=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC=C(C)C=C54)=CC(C4=C5C=C(C)C=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC(C)=CC=C54)=C3)C2=C1.FC(F)(F)C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=C(C(F)(F)F)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1 NFLVMWFUIZKYLI-LYLVRHOXSA-N 0.000 description 1
- PUOBSULBYYCAHX-XLTQAHQASA-N C1=CC=C(N(C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=C5C=CC=CC5=CC=C4)C=C3)C=C2)C2=CC=CC3=C2C=CC=C3)C=C1.CC1=CC=CC(N(C2=CC=CC=C2)C2=C/C3=C(/C=C/2)C2=CC=C(N(C4=CC=CC=C4)C4=CC=CC(C)=C4)C=C2C3(C)C)=C1.CC1=CC=CC(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=CC=CC(C)=C4)C=C3)C=C2)=C1.CC1=CC=CC(N(C2=CC=CC=C2)C2=CC=C(N(C3=CC=C(N(C4=CC=CC=C4)C4=CC(C)=CC=C4)C=C3)C3=CC=C(N(C4=CC=CC=C4)C4=CC(C)=CC=C4)C=C3)C=C2)=C1.[2H]P[3H] Chemical compound C1=CC=C(N(C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=C5C=CC=CC5=CC=C4)C=C3)C=C2)C2=CC=CC3=C2C=CC=C3)C=C1.CC1=CC=CC(N(C2=CC=CC=C2)C2=C/C3=C(/C=C/2)C2=CC=C(N(C4=CC=CC=C4)C4=CC=CC(C)=C4)C=C2C3(C)C)=C1.CC1=CC=CC(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=CC=CC(C)=C4)C=C3)C=C2)=C1.CC1=CC=CC(N(C2=CC=CC=C2)C2=CC=C(N(C3=CC=C(N(C4=CC=CC=C4)C4=CC(C)=CC=C4)C=C3)C3=CC=C(N(C4=CC=CC=C4)C4=CC(C)=CC=C4)C=C3)C=C2)=C1.[2H]P[3H] PUOBSULBYYCAHX-XLTQAHQASA-N 0.000 description 1
- NCQUGRPBQGPFKD-UHFFFAOYSA-N C1=CC=C(N(C2=CC=C3C(=C2)[C@]2(C4=CC(N(C5=CC=CC=C5)C5=C6C=CC=CC6=CC=C5)=CC=C4C4=C2C=C(N(C2=CC=CC=C2)C2=CC=CC5=C2C=CC=C5)C=C4)C2=C3C=CC(N(C3=CC=CC=C3)C3=C4C=CC=CC4=CC=C3)=C2)C2=CC=CC3=CC=CC=C32)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C2(C4=CC(N(C5=CC=CC=C5)C5=CC=CC=C5)=CC=C4C4=C2C=C(N(C2=CC=CC=C2)C2=CC=CC=C2)C=C4)C2=C3C=CC(N(C3=CC=CC=C3)C3=CC=CC=C3)=C2)C=C1.CC1(C)C2=CC(N(C3=CC=C4C(=C3)C(C)(C)C3=C4C=CC=C3)C3=C/C4=C(/C=C/3)C3=CC=C(N(C5=C/C6=C(/C=C/5)C5=CC=CC=C5C6(C)C)C5=C/C6=C(/C=C/5)C5=CC=CC=C5C6(C)C)C=C3C4(C)C)=C=C2C2=C1C=CC=C2.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=CC=C(C3(C4=CC=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C=C4)CCCCC3)C=C2)C=C1 Chemical compound C1=CC=C(N(C2=CC=C3C(=C2)[C@]2(C4=CC(N(C5=CC=CC=C5)C5=C6C=CC=CC6=CC=C5)=CC=C4C4=C2C=C(N(C2=CC=CC=C2)C2=CC=CC5=C2C=CC=C5)C=C4)C2=C3C=CC(N(C3=CC=CC=C3)C3=C4C=CC=CC4=CC=C3)=C2)C2=CC=CC3=CC=CC=C32)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C2(C4=CC(N(C5=CC=CC=C5)C5=CC=CC=C5)=CC=C4C4=C2C=C(N(C2=CC=CC=C2)C2=CC=CC=C2)C=C4)C2=C3C=CC(N(C3=CC=CC=C3)C3=CC=CC=C3)=C2)C=C1.CC1(C)C2=CC(N(C3=CC=C4C(=C3)C(C)(C)C3=C4C=CC=C3)C3=C/C4=C(/C=C/3)C3=CC=C(N(C5=C/C6=C(/C=C/5)C5=CC=CC=C5C6(C)C)C5=C/C6=C(/C=C/5)C5=CC=CC=C5C6(C)C)C=C3C4(C)C)=C=C2C2=C1C=CC=C2.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=CC=C(C3(C4=CC=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C=C4)CCCCC3)C=C2)C=C1 NCQUGRPBQGPFKD-UHFFFAOYSA-N 0.000 description 1
- SNSWRBBKAGMULJ-JHFGWRTGSA-N C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC(C)=CC3=C(C3=CC(C)=CC(C4=C5C=CC(C)=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=C(C)C=C54)=C3)C3=CC=C(C)C=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC(C)=CC3=C(C3=CC(F)=CC(C4=C5C=CC(C)=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=C(C)C=C54)=C3)C3=CC=C(C)C=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C)=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(F)C=C5)C5=CC=C(F)C=C5)C5=CC=CC=C54)=CC(C)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C2C(=C1)C(C1=CC=C3C=CC=CC3=C1)=C1C=CC(C)=CC1=C2C1=CC(C)=CC(C2=C3C=CC(C)=CC3=C(C3=CC4=CC=CC=C4C=C3)C3=CC=C(C)C=C32)=C1.C[Si](C)(C)C1=C2C=CC=CC2=C(C2=CC(C3=C4/C=C\C5=CC=CC6=CC=C(C=C3)C4=C65)=CC(C3=C4C=CC=CC4=C([Si](C)(C)C)C4=CC=CC=C43)=C2)C2=CC=CC=C21.[2H]C.[2H]C.[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H]C.[2H][2H][2H] Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC(C)=CC3=C(C3=CC(C)=CC(C4=C5C=CC(C)=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=C(C)C=C54)=C3)C3=CC=C(C)C=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC(C)=CC3=C(C3=CC(F)=CC(C4=C5C=CC(C)=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=C(C)C=C54)=C3)C3=CC=C(C)C=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C)=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(F)C=C5)C5=CC=C(F)C=C5)C5=CC=CC=C54)=CC(C)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C2C(=C1)C(C1=CC=C3C=CC=CC3=C1)=C1C=CC(C)=CC1=C2C1=CC(C)=CC(C2=C3C=CC(C)=CC3=C(C3=CC4=CC=CC=C4C=C3)C3=CC=C(C)C=C32)=C1.C[Si](C)(C)C1=C2C=CC=CC2=C(C2=CC(C3=C4/C=C\C5=CC=CC6=CC=C(C=C3)C4=C65)=CC(C3=C4C=CC=CC4=C([Si](C)(C)C)C4=CC=CC=C43)=C2)C2=CC=CC=C21.[2H]C.[2H]C.[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H]C.[2H][2H][2H] SNSWRBBKAGMULJ-JHFGWRTGSA-N 0.000 description 1
- KVOQESCVQLUJKW-FGOLCCDCSA-N C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C4=CC(C5=C6C=CC=CC6=C(N(C6=CC=CC=C6)C6=CC=CC=C6)C6=CC=CC=C65)=CC(C5=C6C=CC=CC6=C(N(C6=CC=CC=C6)C6=CC=CC=C6)C6=CC=CC=C65)=C4)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C4=CC(C5=C6C=CC=CC6=CC6=CC=CC=C65)=CC(C5=C6C=CC=CC6=C(N(C6=CC=CC=C6)C6=CC=CC=C6)C6=CC=CC=C65)=C4)=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.C1=CC=C(OC2=C3C=CC=CC3=C(C3=CC(C4=CC(C5=C6C=CC=CC6=C(OC6=CC=CC=C6)C6=CC=CC=C65)=CC(C5=C6C=CC=CC6=C(OC6=CC=CC=C6)C6=CC=CC=C65)=C4)=CC(C4=C5C=CC=CC5=C(OC5=CC=CC=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=CC(C5=C6C=CC=CC6=C(N(C6=CC=C(C)C=C6)C6=CC=C(C)C=C6)C6=CC=CC=C65)=CC(C5=C6C=CC=CC6=CC6=CC=CC=C65)=C4)=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=CC(C5=C6C=CC=CC6=CC6=CC=CC=C65)=CC(C5=C6C=CC=CC6=C(N(C6=CC=C(C)C=C6)C6=CC=C(C)C=C6)C6=CC=CC=C65)=C4)=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC=C4C=CC(C5=C6C=CC=CC6=C(N(C6=CC=C(C)C=C6)C6=CC=C(C)C=C6)C6=CC=CC=C65)=CC4=C3)C3=CC=CC=C32)C=C1.[2H]C.[2H]C.[2H]C.[2H]C.[2H]C.[2H]C.[2H]C.[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H]C.[2H][2H][2H] Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C4=CC(C5=C6C=CC=CC6=C(N(C6=CC=CC=C6)C6=CC=CC=C6)C6=CC=CC=C65)=CC(C5=C6C=CC=CC6=C(N(C6=CC=CC=C6)C6=CC=CC=C6)C6=CC=CC=C65)=C4)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C4=CC(C5=C6C=CC=CC6=CC6=CC=CC=C65)=CC(C5=C6C=CC=CC6=C(N(C6=CC=CC=C6)C6=CC=CC=C6)C6=CC=CC=C65)=C4)=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.C1=CC=C(OC2=C3C=CC=CC3=C(C3=CC(C4=CC(C5=C6C=CC=CC6=C(OC6=CC=CC=C6)C6=CC=CC=C65)=CC(C5=C6C=CC=CC6=C(OC6=CC=CC=C6)C6=CC=CC=C65)=C4)=CC(C4=C5C=CC=CC5=C(OC5=CC=CC=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=CC(C5=C6C=CC=CC6=C(N(C6=CC=C(C)C=C6)C6=CC=C(C)C=C6)C6=CC=CC=C65)=CC(C5=C6C=CC=CC6=CC6=CC=CC=C65)=C4)=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=CC(C5=C6C=CC=CC6=CC6=CC=CC=C65)=CC(C5=C6C=CC=CC6=C(N(C6=CC=C(C)C=C6)C6=CC=C(C)C=C6)C6=CC=CC=C65)=C4)=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC=C4C=CC(C5=C6C=CC=CC6=C(N(C6=CC=C(C)C=C6)C6=CC=C(C)C=C6)C6=CC=CC=C65)=CC4=C3)C3=CC=CC=C32)C=C1.[2H]C.[2H]C.[2H]C.[2H]C.[2H]C.[2H]C.[2H]C.[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H]C.[2H][2H][2H] KVOQESCVQLUJKW-FGOLCCDCSA-N 0.000 description 1
- GCHUDZLAZBXTJD-AMYVVHSRSA-N C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C4=CC=C(C5=CC(C6=C7C=CC=CC7=C(N(C7=CC=CC=C7)C7=CC=CC=C7)C7=CC=CC=C76)=CC(C6=C7C=CC=CC7=C(N(C7=CC=CC=C7)C7=CC=CC=C7)C7=CC=CC=C76)=C5)C=C4)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1(C)C2=C(C=CC(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=C3)=C2)C2=C1C=C(C1=CC(C3=C4C=CC=CC4=CC4=CC=CC=C43)=CC(C3=C4C=CC=CC4=CC4=CC=CC=C43)=C1)C=C2.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(C)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(C)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(C5=CC6=CC=CC=C6C=C5)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.[2H]C.[2H]C.[2H]C.[2H]C.[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H] Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C4=CC=C(C5=CC(C6=C7C=CC=CC7=C(N(C7=CC=CC=C7)C7=CC=CC=C7)C7=CC=CC=C76)=CC(C6=C7C=CC=CC7=C(N(C7=CC=CC=C7)C7=CC=CC=C7)C7=CC=CC=C76)=C5)C=C4)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1(C)C2=C(C=CC(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=C3)=C2)C2=C1C=C(C1=CC(C3=C4C=CC=CC4=CC4=CC=CC=C43)=CC(C3=C4C=CC=CC4=CC4=CC=CC=C43)=C1)C=C2.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(C)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(C)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(C5=CC6=CC=CC=C6C=C5)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.[2H]C.[2H]C.[2H]C.[2H]C.[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H] GCHUDZLAZBXTJD-AMYVVHSRSA-N 0.000 description 1
- IUKIUHPAWMEWQK-UHFFFAOYSA-N C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC=C4C=C(C5=C6C=CC=CC6=C(N(C6=CC=CC=C6)C6=CC=CC=C6)C6=CC=CC=C65)C=CC4=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=NC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=C(C4=C5C=CC=CC5=CC5=CC=CC=C54)N=C3C3=C4C=CC=CC4=C(N(C4=CC=C(C)C=C4)C4=CC=C(C)C=C4)C4=CC=CC=C43)C3=CC=CC=C32)C=C1 Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC=C4C=C(C5=C6C=CC=CC6=C(N(C6=CC=CC=C6)C6=CC=CC=C6)C6=CC=CC=C65)C=CC4=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=NC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=C(C4=C5C=CC=CC5=CC5=CC=CC=C54)N=C3C3=C4C=CC=CC4=C(N(C4=CC=C(C)C=C4)C4=CC=C(C)C=C4)C4=CC=CC=C43)C3=CC=CC=C32)C=C1 IUKIUHPAWMEWQK-UHFFFAOYSA-N 0.000 description 1
- LUGOYHPSACYLRM-UHFFFAOYSA-N C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=CC3=CC=CC=C32)C=C1 Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=CC3=CC=CC=C32)C=C1 LUGOYHPSACYLRM-UHFFFAOYSA-N 0.000 description 1
- RDVXPHSOVKZCBJ-UHFFFAOYSA-N C1=CC=C2C(=C1)C=C1C=CC=CC1=C2C1=CC(C2=C3C=CC=CC3=CC3=CC=CC=C32)=CC(C2=C3C=CC=CC3=CC3=CC=CC=C32)=C1 Chemical compound C1=CC=C2C(=C1)C=C1C=CC=CC1=C2C1=CC(C2=C3C=CC=CC3=CC3=CC=CC=C32)=CC(C2=C3C=CC=CC3=CC3=CC=CC=C32)=C1 RDVXPHSOVKZCBJ-UHFFFAOYSA-N 0.000 description 1
- FXYUGSFUJQBMFL-UHFFFAOYSA-N C1=CC=C2C(=C1)C=CC=C2C1=NN=C(C2=CC(C3=NN=C(C4=CC=CC5=CC=CC=C54)O3)=CC(C3=NN=C(C4=C5C=CC=CC5=CC=C4)O3)=C2)O1.CC(C)(C)C1=CC=C(C2=NN=C(C3=CC=C(C4=CC=CC=C4)C=C3)N2C2=CC=CC=C2)C=C1.CC1=CC=C(C2=NN=C(C3=CC(C4=NN=C(C5=CC=C(C(C)(C)C)C=C5)O4)=CC=C3)O2)C=C1.CCC.O=C1C2=C3C(=CC=C2)/C=C\C=C/3C2=NC3=C(C4=C(C=CC=C4)C4=C3C=CC=C4)N12.O=C1C2=C3C4=C(C=C2)C2=C5C6=C(C=C2)C(=O)N(C2=CC=CC=C2)C(=O)/C6=C/C=C5/C4=C/C=C\3C(=O)N1C1=CC=CC=C1.O=C1C2=C3\C(=CC=C4C5=CC=C6C(=O)N7C8=C(C=CC=C8)/N=C\7C7=C6C5=C(/C=C\7)C(=C43)\C=C/2)C2=NC3=C(C=CC=C3)N12 Chemical compound C1=CC=C2C(=C1)C=CC=C2C1=NN=C(C2=CC(C3=NN=C(C4=CC=CC5=CC=CC=C54)O3)=CC(C3=NN=C(C4=C5C=CC=CC5=CC=C4)O3)=C2)O1.CC(C)(C)C1=CC=C(C2=NN=C(C3=CC=C(C4=CC=CC=C4)C=C3)N2C2=CC=CC=C2)C=C1.CC1=CC=C(C2=NN=C(C3=CC(C4=NN=C(C5=CC=C(C(C)(C)C)C=C5)O4)=CC=C3)O2)C=C1.CCC.O=C1C2=C3C(=CC=C2)/C=C\C=C/3C2=NC3=C(C4=C(C=CC=C4)C4=C3C=CC=C4)N12.O=C1C2=C3C4=C(C=C2)C2=C5C6=C(C=C2)C(=O)N(C2=CC=CC=C2)C(=O)/C6=C/C=C5/C4=C/C=C\3C(=O)N1C1=CC=CC=C1.O=C1C2=C3\C(=CC=C4C5=CC=C6C(=O)N7C8=C(C=CC=C8)/N=C\7C7=C6C5=C(/C=C\7)C(=C43)\C=C/2)C2=NC3=C(C=CC=C3)N12 FXYUGSFUJQBMFL-UHFFFAOYSA-N 0.000 description 1
- OJRUSAPKCPIVBY-KQYNXXCUSA-N C1=NC2=C(N=C(N=C2N1[C@H]3[C@@H]([C@@H]([C@H](O3)COP(=O)(CP(=O)(O)O)O)O)O)I)N Chemical compound C1=NC2=C(N=C(N=C2N1[C@H]3[C@@H]([C@@H]([C@H](O3)COP(=O)(CP(=O)(O)O)O)O)O)I)N OJRUSAPKCPIVBY-KQYNXXCUSA-N 0.000 description 1
- FPHVYYLLKARGGO-UHFFFAOYSA-N CC1(C)C2=C(C=CC(C3=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC=CC=C54)=C3)=C2)C2=C1C=C(C1=CC(C3=C4C=CC=CC4=C(N(C4=CC=CC=C4)C4=CC=CC=C4)C4=CC=CC=C43)=CC(C3=C4C=CC=CC4=C(N(C4=CC=CC=C4)C4=CC=CC=C4)C4=CC=CC=C43)=C1)C=C2.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=C(C4=C5C=CC=CC5=CC5=CC=CC=C54)C=C3C3=C4C=CC=CC4=C(N(C4=CC=C(C)C=C4)C4=CC=C(C)C=C4)C4=CC=CC=C43)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC=C(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)C(C4=C5C=CC=CC5=CC5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC=C(C4=C5C=CC=CC5=CC5=CC=CC=C54)C(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CN(C1=CC=CC=C1)C1=C2C=CC=CC2=C(C2=CC=C(C3=C4C=CC=CC4=C(N(C)C4=CC=CC=C4)C4=CC=CC=C43)C(C3=C4C=CC=CC4=C(N(C)C4=CC=CC=C4)C4=CC=CC=C43)=C2)C2=CC=CC=C21 Chemical compound CC1(C)C2=C(C=CC(C3=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C5=CC=CC=C54)=C3)=C2)C2=C1C=C(C1=CC(C3=C4C=CC=CC4=C(N(C4=CC=CC=C4)C4=CC=CC=C4)C4=CC=CC=C43)=CC(C3=C4C=CC=CC4=C(N(C4=CC=CC=C4)C4=CC=CC=C4)C4=CC=CC=C43)=C1)C=C2.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=C(C4=C5C=CC=CC5=CC5=CC=CC=C54)C=C3C3=C4C=CC=CC4=C(N(C4=CC=C(C)C=C4)C4=CC=C(C)C=C4)C4=CC=CC=C43)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC=C(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)C(C4=C5C=CC=CC5=CC5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC=C(C4=C5C=CC=CC5=CC5=CC=CC=C54)C(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CN(C1=CC=CC=C1)C1=C2C=CC=CC2=C(C2=CC=C(C3=C4C=CC=CC4=C(N(C)C4=CC=CC=C4)C4=CC=CC=C43)C(C3=C4C=CC=CC4=C(N(C)C4=CC=CC=C4)C4=CC=CC=C43)=C2)C2=CC=CC=C21 FPHVYYLLKARGGO-UHFFFAOYSA-N 0.000 description 1
- VZYZZKOUCVXTOJ-UHFFFAOYSA-N CC1(C)C2=CC=CC=C2C2=CC=C(N(C3=CC=CC=C3)C3=CC=C(C4=CC=C(N(C5=CC=CC=C5)C5=CC=C6C7=CC=CC=C7C(C)(C)C6=C5)C=C4)C=C3)C=C21 Chemical compound CC1(C)C2=CC=CC=C2C2=CC=C(N(C3=CC=CC=C3)C3=CC=C(C4=CC=C(N(C5=CC=CC=C5)C5=CC=C6C7=CC=CC=C7C(C)(C)C6=C5)C=C4)C=C3)C=C21 VZYZZKOUCVXTOJ-UHFFFAOYSA-N 0.000 description 1
- LKYYAOMPUWIBJZ-UHFFFAOYSA-N CC1=CC(C)=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=C(C)C=C(C)C=C5C)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C(C)=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=CC=C(C3=C4C=CC=CC4=C(C4=CC(C5=C6C=CC=CC6=CC6=CC=CC=C65)=CC(C5=C6C=CC=CC6=C(C6=CC=C(N(C7=CC=C(C)C=C7)C7=CC=C(C)C=C7)C=C6)C6=CC=CC=C65)=C4)C4=CC=CC=C43)C=C2)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=CC=C(C3=C4C=CC=CC4=C(C4=CC(C5=CC=C(C6=C7C=CC=CC7=CC7=CC=CC=C76)C=C5)=CC(C5=C6C=CC=CC6=C(C6=CC=C(N(C7=CC=C(C)C=C7)C7=CC=C(C)C=C7)C=C6)C6=CC=CC=C65)=C4)C4=CC=CC=C43)C=C2)C=C1.CC1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1 Chemical compound CC1=CC(C)=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=C(C)C=C(C)C=C5C)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C(C)=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=CC=C(C3=C4C=CC=CC4=C(C4=CC(C5=C6C=CC=CC6=CC6=CC=CC=C65)=CC(C5=C6C=CC=CC6=C(C6=CC=C(N(C7=CC=C(C)C=C7)C7=CC=C(C)C=C7)C=C6)C6=CC=CC=C65)=C4)C4=CC=CC=C43)C=C2)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=CC=C(C3=C4C=CC=CC4=C(C4=CC(C5=CC=C(C6=C7C=CC=CC7=CC7=CC=CC=C76)C=C5)=CC(C5=C6C=CC=CC6=C(C6=CC=C(N(C7=CC=C(C)C=C7)C7=CC=C(C)C=C7)C=C6)C6=CC=CC=C65)=C4)C4=CC=CC=C43)C=C2)C=C1.CC1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=CC5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1 LKYYAOMPUWIBJZ-UHFFFAOYSA-N 0.000 description 1
- IKLDXWZXACEQAR-MOBJMLQXSA-N CC1=CC(C2=C3C=CC=CC3=C(N(C3=CC=C(C(F)(F)F)C=C3)C3=CC=C(C(F)(F)F)C=C3)C3=CC=CC=C32)=CC(C2=C3C=CC=CC3=C(N(C3=CC=C(C(F)(F)F)C=C3)C3=CC=C(C(F)(F)F)C=C3)C3=CC=CC=C32)=C1.CC1=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=C(C)C=C(C)C=C3C)C3=CC=CC=C32)=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=C(C)C=C(C)C=C3C)C3=CC=CC=C32)=C1.CC1=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=C4C=CC=CC4=CC4=CC=CC=C43)C3=CC=CC=C32)=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=C4C=CC=CC4=CC4=CC=CC=C43)C3=CC=CC=C32)=C1.CC1=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=CC4=C(C=C3)C3=C(C=CC=C3)C4(C)C)C3=CC=CC=C32)=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=CC=C4C(=C3)C(C)(C)C3=C4C=CC=C3)C3=CC=CC=C32)=C1.CC1=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=CC=CC=C3)C3=CC=CC=C32)=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=CC=CC=C3)C3=CC=CC=C32)=C1.CC1=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=CC=CC=C3)C3=CC=CC=C32)=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=CC=CC=C3)C3=CC=CC=C32)=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C)=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.[2H]C.[2H]C.[2H]C.[2H]C.[2H]C.[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H]C.[2H][2H][2H] Chemical compound CC1=CC(C2=C3C=CC=CC3=C(N(C3=CC=C(C(F)(F)F)C=C3)C3=CC=C(C(F)(F)F)C=C3)C3=CC=CC=C32)=CC(C2=C3C=CC=CC3=C(N(C3=CC=C(C(F)(F)F)C=C3)C3=CC=C(C(F)(F)F)C=C3)C3=CC=CC=C32)=C1.CC1=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=C(C)C=C(C)C=C3C)C3=CC=CC=C32)=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=C(C)C=C(C)C=C3C)C3=CC=CC=C32)=C1.CC1=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=C4C=CC=CC4=CC4=CC=CC=C43)C3=CC=CC=C32)=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=C4C=CC=CC4=CC4=CC=CC=C43)C3=CC=CC=C32)=C1.CC1=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=CC4=C(C=C3)C3=C(C=CC=C3)C4(C)C)C3=CC=CC=C32)=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=CC=C4C(=C3)C(C)(C)C3=C4C=CC=C3)C3=CC=CC=C32)=C1.CC1=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=CC=CC=C3)C3=CC=CC=C32)=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=CC=CC=C3)C3=CC=CC=C32)=C1.CC1=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=CC=CC=C3)C3=CC=CC=C32)=CC(C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=CC=CC=C3)C3=CC=CC=C32)=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C)=CC(C4=C5C=CC=CC5=C(N(C5=CC=C(C)C=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C(C3=CC(C)=CC(C4=C5C=CC=CC5=C(N(C5=CC=CC=C5)C5=CC=C(C)C=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.[2H]C.[2H]C.[2H]C.[2H]C.[2H]C.[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H].[2H][2H]C.[2H][2H][2H] IKLDXWZXACEQAR-MOBJMLQXSA-N 0.000 description 1
- WBSUVGZIUZCOME-UHFFFAOYSA-N CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(C)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(C)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(C5=CC6=CC=CC=C6C=C5)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(C5=CC=C6C=CC=CC6=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(OC5=CC=CC=C5)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(OC5=CC=CC=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C([Si](C)(C)C)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C([Si](C)(C)C)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=CC=C(C3=C4C=CC=CC4=C(C4=CC(C5=C6C=CC=CC6=C(C6=CC=C(N(C7=CC=C(C)C=C7)C7=CC=C(C)C=C7)C=C6)C6=CC=CC=C65)=CC(C5=C6C=CC=CC6=C(N(C6=CC=C(C)C=C6)C6=CC=C(C)C=C6)C6=CC=CC=C65)=C4)C4=CC=CC=C43)C=C2)C=C1 Chemical compound CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(C)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(C)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(C5=CC6=CC=CC=C6C=C5)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(C5=CC=C6C=CC=CC6=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C(OC5=CC=CC=C5)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C(OC5=CC=CC=C5)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=C3C=CC=CC3=C(C3=CC(C4=C5C=CC=CC5=C([Si](C)(C)C)C5=CC=CC=C54)=CC(C4=C5C=CC=CC5=C([Si](C)(C)C)C5=CC=CC=C54)=C3)C3=CC=CC=C32)C=C1.CC1=CC=C(N(C2=CC=C(C)C=C2)C2=CC=C(C3=C4C=CC=CC4=C(C4=CC(C5=C6C=CC=CC6=C(C6=CC=C(N(C7=CC=C(C)C=C7)C7=CC=C(C)C=C7)C=C6)C6=CC=CC=C65)=CC(C5=C6C=CC=CC6=C(N(C6=CC=C(C)C=C6)C6=CC=C(C)C=C6)C6=CC=CC=C65)=C4)C4=CC=CC=C43)C=C2)C=C1 WBSUVGZIUZCOME-UHFFFAOYSA-N 0.000 description 1
- FWCFXANGZIRZSD-UHFFFAOYSA-N CC1=CC=C(N(C2=CC=C(C)C=C2)C2=CC=C(B(O)O)C=C2)C=C1.II Chemical compound CC1=CC=C(N(C2=CC=C(C)C=C2)C2=CC=C(B(O)O)C=C2)C=C1.II FWCFXANGZIRZSD-UHFFFAOYSA-N 0.000 description 1
- XECKCVGTZNBSRW-UHFFFAOYSA-N CC1=CC=C(N(C2=CC=C(C)C=C2)C2=CC=C(C3=C4C=CC=CC4=C(C4=CC(C5=C6C=CC=CC6=C(C6=CC=C(N(C7=CC=C(C)C=C7)C7=CC=C(C)C=C7)C=C6)C6=CC=CC=C65)=CC(/C5=C6\C=CC=C\C6=C(/C6=CC=C(N(C7=CC=C(C)C=C7)C7=CC=C(C)C=C7)C=C6)C6=CC=CC=C65)=C4)C4=CC=CC=C43)C=C2)C=C1 Chemical compound CC1=CC=C(N(C2=CC=C(C)C=C2)C2=CC=C(C3=C4C=CC=CC4=C(C4=CC(C5=C6C=CC=CC6=C(C6=CC=C(N(C7=CC=C(C)C=C7)C7=CC=C(C)C=C7)C=C6)C6=CC=CC=C65)=CC(/C5=C6\C=CC=C\C6=C(/C6=CC=C(N(C7=CC=C(C)C=C7)C7=CC=C(C)C=C7)C=C6)C6=CC=CC=C65)=C4)C4=CC=CC=C43)C=C2)C=C1 XECKCVGTZNBSRW-UHFFFAOYSA-N 0.000 description 1
- AIBOMTNZBUDFMQ-UHFFFAOYSA-N CC1=CC=C(N(C2=CC=C(C)C=C2)C2=CC=C(C3=C4C=CC=CC4=C(C4=CC(C5=C6C=CC=CC6=C(C6=CC=C(N(C7=CC=C(C)C=C7)C7=CC=C(C)C=C7)C=C6)C6=CC=CC=C65)=CC(/C5=C6\C=CC=C\C6=C\C6=CC=CC=C65)=C4)C4=CC=CC=C43)C=C2)C=C1 Chemical compound CC1=CC=C(N(C2=CC=C(C)C=C2)C2=CC=C(C3=C4C=CC=CC4=C(C4=CC(C5=C6C=CC=CC6=C(C6=CC=C(N(C7=CC=C(C)C=C7)C7=CC=C(C)C=C7)C=C6)C6=CC=CC=C65)=CC(/C5=C6\C=CC=C\C6=C\C6=CC=CC=C65)=C4)C4=CC=CC=C43)C=C2)C=C1 AIBOMTNZBUDFMQ-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- 241000284156 Clerodendrum quadriloculare Species 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 238000004057 DFT-B3LYP calculation Methods 0.000 description 1
- 239000004641 Diallyl-phthalate Substances 0.000 description 1
- VGGSQFUCUMXWEO-LNLMKGTHSA-N Ethene-d4 Chemical group [2H]C([2H])=C([2H])[2H] VGGSQFUCUMXWEO-LNLMKGTHSA-N 0.000 description 1
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 1
- 101001009252 Homo sapiens 2-hydroxyacyl-CoA lyase 1 Proteins 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 229910000861 Mg alloy Inorganic materials 0.000 description 1
- CBENFWSGALASAD-UHFFFAOYSA-N Ozone Chemical compound [O-][O+]=O CBENFWSGALASAD-UHFFFAOYSA-N 0.000 description 1
- 239000005062 Polybutadiene Substances 0.000 description 1
- 239000004695 Polyether sulfone Substances 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 229920000265 Polyparaphenylene Chemical class 0.000 description 1
- 239000004734 Polyphenylene sulfide Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- NRCMAYZCPIVABH-UHFFFAOYSA-N Quinacridone Chemical class N1C2=CC=CC=C2C(=O)C2=C1C=C1C(=O)C3=CC=CC=C3NC1=C2 NRCMAYZCPIVABH-UHFFFAOYSA-N 0.000 description 1
- KJTLSVCANCCWHF-UHFFFAOYSA-N Ruthenium Chemical compound [Ru] KJTLSVCANCCWHF-UHFFFAOYSA-N 0.000 description 1
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical compound [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical group C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 229920001807 Urea-formaldehyde Polymers 0.000 description 1
- DGEZNRSVGBDHLK-UHFFFAOYSA-N [1,10]phenanthroline Chemical compound C1=CN=C2C3=NC=CC=C3C=CC2=C1 DGEZNRSVGBDHLK-UHFFFAOYSA-N 0.000 description 1
- XDARIDUNZZQZBQ-UHFFFAOYSA-N [4-(4-methyl-n-(4-methylphenyl)anilino)phenyl]boronic acid Chemical compound C1=CC(C)=CC=C1N(C=1C=CC(=CC=1)B(O)O)C1=CC=C(C)C=C1 XDARIDUNZZQZBQ-UHFFFAOYSA-N 0.000 description 1
- JHYLKGDXMUDNEO-UHFFFAOYSA-N [Mg].[In] Chemical compound [Mg].[In] JHYLKGDXMUDNEO-UHFFFAOYSA-N 0.000 description 1
- 239000006096 absorbing agent Substances 0.000 description 1
- 239000011354 acetal resin Substances 0.000 description 1
- FZEYVTFCMJSGMP-UHFFFAOYSA-N acridone Chemical class C1=CC=C2C(=O)C3=CC=CC=C3NC2=C1 FZEYVTFCMJSGMP-UHFFFAOYSA-N 0.000 description 1
- 229920000122 acrylonitrile butadiene styrene Polymers 0.000 description 1
- 125000005073 adamantyl group Chemical group C12(CC3CC(CC(C1)C3)C2)* 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 239000012790 adhesive layer Substances 0.000 description 1
- 150000001336 alkenes Chemical group 0.000 description 1
- SNAAJJQQZSMGQD-UHFFFAOYSA-N aluminum magnesium Chemical compound [Mg].[Al] SNAAJJQQZSMGQD-UHFFFAOYSA-N 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 125000002490 anilino group Chemical group [H]N(*)C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- YUENFNPLGJCNRB-UHFFFAOYSA-N anthracen-1-amine Chemical class C1=CC=C2C=C3C(N)=CC=CC3=CC2=C1 YUENFNPLGJCNRB-UHFFFAOYSA-N 0.000 description 1
- 125000002078 anthracen-1-yl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C3C([*])=C([H])C([H])=C([H])C3=C([H])C2=C1[H] 0.000 description 1
- 125000000748 anthracen-2-yl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C3C([H])=C([*])C([H])=C([H])C3=C([H])C2=C1[H] 0.000 description 1
- VHHDLIWHHXBLBK-UHFFFAOYSA-N anthracen-9-ylboronic acid Chemical compound C1=CC=C2C(B(O)O)=C(C=CC=C3)C3=CC2=C1 VHHDLIWHHXBLBK-UHFFFAOYSA-N 0.000 description 1
- MWPLVEDNUUSJAV-UHFFFAOYSA-N anthracene Natural products C1=CC=CC2=CC3=CC=CC=C3C=C21 MWPLVEDNUUSJAV-UHFFFAOYSA-N 0.000 description 1
- NDMVXIYCFFFPLE-UHFFFAOYSA-N anthracene-9,10-diamine Chemical class C1=CC=C2C(N)=C(C=CC=C3)C3=C(N)C2=C1 NDMVXIYCFFFPLE-UHFFFAOYSA-N 0.000 description 1
- 150000001454 anthracenes Chemical class 0.000 description 1
- 125000004653 anthracenylene group Chemical group 0.000 description 1
- 150000008425 anthrones Chemical class 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 229940027991 antiseptic and disinfectant quinoline derivative Drugs 0.000 description 1
- 150000001491 aromatic compounds Chemical class 0.000 description 1
- 125000002102 aryl alkyloxo group Chemical group 0.000 description 1
- 125000004104 aryloxy group Chemical group 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 229930008407 benzylideneacetone Natural products 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- 239000004305 biphenyl Substances 0.000 description 1
- JRXXLCKWQFKACW-UHFFFAOYSA-N biphenylacetylene Chemical group C1=CC=CC=C1C#CC1=CC=CC=C1 JRXXLCKWQFKACW-UHFFFAOYSA-N 0.000 description 1
- 125000002529 biphenylenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3C12)* 0.000 description 1
- QUDWYFHPNIMBFC-UHFFFAOYSA-N bis(prop-2-enyl) benzene-1,2-dicarboxylate Chemical compound C=CCOC(=O)C1=CC=CC=C1C(=O)OCC=C QUDWYFHPNIMBFC-UHFFFAOYSA-N 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 125000005997 bromomethyl group Chemical group 0.000 description 1
- QHIWVLPBUQWDMQ-UHFFFAOYSA-N butyl prop-2-enoate;methyl 2-methylprop-2-enoate;prop-2-enoic acid Chemical compound OC(=O)C=C.COC(=O)C(C)=C.CCCCOC(=O)C=C QHIWVLPBUQWDMQ-UHFFFAOYSA-N 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 125000005566 carbazolylene group Chemical group 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 238000005266 casting Methods 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- YHKCQNIXEBHWPG-UHFFFAOYSA-N chlorobenzene;heptane Chemical compound CCCCCCC.ClC1=CC=CC=C1 YHKCQNIXEBHWPG-UHFFFAOYSA-N 0.000 description 1
- 125000004218 chloromethyl group Chemical group [H]C([H])(Cl)* 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- 125000005578 chrysene group Chemical group 0.000 description 1
- 150000001846 chrysenes Chemical class 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 229940125758 compound 15 Drugs 0.000 description 1
- 229940126142 compound 16 Drugs 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 229920001940 conductive polymer Polymers 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 150000004696 coordination complex Chemical class 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 150000001882 coronenes Chemical class 0.000 description 1
- ZYGHJZDHTFUPRJ-UHFFFAOYSA-N coumarin Chemical class C1=CC=C2OC(=O)C=CC2=C1 ZYGHJZDHTFUPRJ-UHFFFAOYSA-N 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 125000004093 cyano group Chemical group *C#N 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000004210 cyclohexylmethyl group Chemical group [H]C([H])(*)C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000004851 cyclopentylmethyl group Chemical group C1(CCCC1)C* 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 230000009849 deactivation Effects 0.000 description 1
- SWXVUIWOUIDPGS-UHFFFAOYSA-N diacetone alcohol Natural products CC(=O)CC(C)(C)O SWXVUIWOUIDPGS-UHFFFAOYSA-N 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 229910003460 diamond Inorganic materials 0.000 description 1
- LJSQFQKUNVCTIA-UHFFFAOYSA-N diethyl sulfide Chemical group CCSCC LJSQFQKUNVCTIA-UHFFFAOYSA-N 0.000 description 1
- 125000001028 difluoromethyl group Chemical group [H]C(F)(F)* 0.000 description 1
- LTYMSROWYAPPGB-UHFFFAOYSA-N diphenyl sulfide Chemical group C=1C=CC=CC=1SC1=CC=CC=C1 LTYMSROWYAPPGB-UHFFFAOYSA-N 0.000 description 1
- 125000006840 diphenylmethane group Chemical group 0.000 description 1
- 238000007598 dipping method Methods 0.000 description 1
- 230000005684 electric field Effects 0.000 description 1
- 239000003822 epoxy resin Substances 0.000 description 1
- 125000005678 ethenylene group Chemical group [H]C([*:1])=C([H])[*:2] 0.000 description 1
- 125000005677 ethinylene group Chemical group [*:2]C#C[*:1] 0.000 description 1
- 125000000816 ethylene group Chemical group [H]C([H])([*:1])C([H])([H])[*:2] 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- 125000004216 fluoromethyl group Chemical group [H]C([H])(F)* 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 230000005283 ground state Effects 0.000 description 1
- LNEPOXFFQSENCJ-UHFFFAOYSA-N haloperidol Chemical compound C1CC(O)(C=2C=CC(Cl)=CC=2)CCN1CCCC(=O)C1=CC=C(F)C=C1 LNEPOXFFQSENCJ-UHFFFAOYSA-N 0.000 description 1
- 229940083761 high-ceiling diuretics pyrazolone derivative Drugs 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 150000007857 hydrazones Chemical class 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 description 1
- 150000002460 imidazoles Chemical class 0.000 description 1
- 229910052738 indium Inorganic materials 0.000 description 1
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 1
- LHJOPRPDWDXEIY-UHFFFAOYSA-N indium lithium Chemical compound [Li].[In] LHJOPRPDWDXEIY-UHFFFAOYSA-N 0.000 description 1
- 229910003437 indium oxide Inorganic materials 0.000 description 1
- PJXISJQVUVHSOJ-UHFFFAOYSA-N indium(iii) oxide Chemical compound [O-2].[O-2].[O-2].[In+3].[In+3] PJXISJQVUVHSOJ-UHFFFAOYSA-N 0.000 description 1
- 125000001041 indolyl group Chemical group 0.000 description 1
- 238000007641 inkjet printing Methods 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 239000011147 inorganic material Substances 0.000 description 1
- 229940079865 intestinal antiinfectives imidazole derivative Drugs 0.000 description 1
- 229910052741 iridium Inorganic materials 0.000 description 1
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 description 1
- 125000000555 isopropenyl group Chemical group [H]\C([H])=C(\*)C([H])([H])[H] 0.000 description 1
- 230000005445 isotope effect Effects 0.000 description 1
- 239000011133 lead Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- SJCKRGFTWFGHGZ-UHFFFAOYSA-N magnesium silver Chemical compound [Mg].[Ag] SJCKRGFTWFGHGZ-UHFFFAOYSA-N 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 125000005395 methacrylic acid group Chemical group 0.000 description 1
- 125000006217 methyl sulfide group Chemical group [H]C([H])([H])S* 0.000 description 1
- 125000000250 methylamino group Chemical group [H]N(*)C([H])([H])[H] 0.000 description 1
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 1
- KPTRDYONBVUWPD-UHFFFAOYSA-N naphthalen-2-ylboronic acid Chemical compound C1=CC=CC2=CC(B(O)O)=CC=C21 KPTRDYONBVUWPD-UHFFFAOYSA-N 0.000 description 1
- 150000002790 naphthalenes Chemical class 0.000 description 1
- 125000004957 naphthylene group Chemical group 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- VOFUROIFQGPCGE-UHFFFAOYSA-N nile red Chemical compound C1=CC=C2C3=NC4=CC=C(N(CC)CC)C=C4OC3=CC(=O)C2=C1 VOFUROIFQGPCGE-UHFFFAOYSA-N 0.000 description 1
- 150000004767 nitrides Chemical class 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- 125000002868 norbornyl group Chemical group C12(CCC(CC1)C2)* 0.000 description 1
- 125000001715 oxadiazolyl group Chemical group 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 125000005564 oxazolylene group Chemical group 0.000 description 1
- 125000003933 pentacenyl group Chemical group C1(=CC=CC2=CC3=CC4=CC5=CC=CC=C5C=C4C=C3C=C12)* 0.000 description 1
- 125000005003 perfluorobutyl group Chemical group FC(F)(F)C(F)(F)C(F)(F)C(F)(F)* 0.000 description 1
- 125000005004 perfluoroethyl group Chemical group FC(F)(F)C(F)(F)* 0.000 description 1
- 125000005009 perfluoropropyl group Chemical group FC(C(C(F)(F)F)(F)F)(F)* 0.000 description 1
- 125000005563 perylenylene group Chemical group 0.000 description 1
- 150000002987 phenanthrenes Chemical class 0.000 description 1
- 150000005041 phenanthrolines Chemical class 0.000 description 1
- 125000005561 phenanthryl group Chemical group 0.000 description 1
- 125000005562 phenanthrylene group Chemical group 0.000 description 1
- 125000001791 phenazinyl group Chemical group C1(=CC=CC2=NC3=CC=CC=C3N=C12)* 0.000 description 1
- 239000005011 phenolic resin Substances 0.000 description 1
- 125000000843 phenylene group Chemical group C1(=C(C=CC=C1)*)* 0.000 description 1
- 150000004986 phenylenediamines Chemical class 0.000 description 1
- LFSXCDWNBUNEEM-UHFFFAOYSA-N phthalazine Chemical group C1=NN=CC2=CC=CC=C21 LFSXCDWNBUNEEM-UHFFFAOYSA-N 0.000 description 1
- IEQIEDJGQAUEQZ-UHFFFAOYSA-N phthalocyanine Chemical class N1C(N=C2C3=CC=CC=C3C(N=C3C4=CC=CC=C4C(=N4)N3)=N2)=C(C=CC=C2)C2=C1N=C1C2=CC=CC=C2C4=N1 IEQIEDJGQAUEQZ-UHFFFAOYSA-N 0.000 description 1
- 239000002985 plastic film Substances 0.000 description 1
- 239000004014 plasticizer Substances 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229920002492 poly(sulfone) Polymers 0.000 description 1
- 229920002037 poly(vinyl butyral) polymer Polymers 0.000 description 1
- 229920006122 polyamide resin Polymers 0.000 description 1
- 229920000767 polyaniline Polymers 0.000 description 1
- 229920001230 polyarylate Polymers 0.000 description 1
- 229920002857 polybutadiene Polymers 0.000 description 1
- 229920005668 polycarbonate resin Polymers 0.000 description 1
- 239000004431 polycarbonate resin Substances 0.000 description 1
- 125000003367 polycyclic group Chemical group 0.000 description 1
- 229920000647 polyepoxide Polymers 0.000 description 1
- 229920001225 polyester resin Polymers 0.000 description 1
- 239000004645 polyester resin Substances 0.000 description 1
- 229920006393 polyether sulfone Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920013716 polyethylene resin Polymers 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- 239000009719 polyimide resin Substances 0.000 description 1
- 229920006324 polyoxymethylene Polymers 0.000 description 1
- 229920000069 polyphenylene sulfide Polymers 0.000 description 1
- 229920000128 polypyrrole Polymers 0.000 description 1
- 229920005749 polyurethane resin Polymers 0.000 description 1
- 150000004033 porphyrin derivatives Chemical class 0.000 description 1
- BITYAPCSNKJESK-UHFFFAOYSA-N potassiosodium Chemical compound [Na].[K] BITYAPCSNKJESK-UHFFFAOYSA-N 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 125000003373 pyrazinyl group Chemical group 0.000 description 1
- JEXVQSWXXUJEMA-UHFFFAOYSA-N pyrazol-3-one Chemical class O=C1C=CN=N1 JEXVQSWXXUJEMA-UHFFFAOYSA-N 0.000 description 1
- 150000003219 pyrazolines Chemical class 0.000 description 1
- 125000003226 pyrazolyl group Chemical group 0.000 description 1
- 150000003220 pyrenes Chemical class 0.000 description 1
- 125000001725 pyrenyl group Chemical group 0.000 description 1
- 125000005548 pyrenylene group Chemical group 0.000 description 1
- 125000005551 pyridylene group Chemical group 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- YYMBJDOZVAITBP-UHFFFAOYSA-N rubrene Chemical compound C1=CC=CC=C1C(C1=C(C=2C=CC=CC=2)C2=CC=CC=C2C(C=2C=CC=CC=2)=C11)=C(C=CC=C2)C2=C1C1=CC=CC=C1 YYMBJDOZVAITBP-UHFFFAOYSA-N 0.000 description 1
- 229910052707 ruthenium Inorganic materials 0.000 description 1
- 229910052711 selenium Inorganic materials 0.000 description 1
- 239000011669 selenium Substances 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 125000005504 styryl group Chemical group 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 150000003518 tetracenes Chemical class 0.000 description 1
- 125000001935 tetracenyl group Chemical group C1(=CC=CC2=CC3=CC4=CC=CC=C4C=C3C=C12)* 0.000 description 1
- BFKJFAAPBSQJPD-UHFFFAOYSA-N tetrafluoroethene Chemical group FC(F)=C(F)F BFKJFAAPBSQJPD-UHFFFAOYSA-N 0.000 description 1
- 125000000383 tetramethylene group Chemical group [H]C([H])([*:1])C([H])([H])C([H])([H])C([H])([H])[*:2] 0.000 description 1
- ILEXMONMGUVLRM-UHFFFAOYSA-N tetraphenylgermane Chemical compound C1=CC=CC=C1[Ge](C=1C=CC=CC=1)(C=1C=CC=CC=1)C1=CC=CC=C1 ILEXMONMGUVLRM-UHFFFAOYSA-N 0.000 description 1
- PEQHIRFAKIASBK-UHFFFAOYSA-N tetraphenylmethane Chemical compound C1=CC=CC=C1C(C=1C=CC=CC=1)(C=1C=CC=CC=1)C1=CC=CC=C1 PEQHIRFAKIASBK-UHFFFAOYSA-N 0.000 description 1
- JLAVCPKULITDHO-UHFFFAOYSA-N tetraphenylsilane Chemical compound C1=CC=CC=C1[Si](C=1C=CC=CC=1)(C=1C=CC=CC=1)C1=CC=CC=C1 JLAVCPKULITDHO-UHFFFAOYSA-N 0.000 description 1
- 125000001113 thiadiazolyl group Chemical group 0.000 description 1
- 150000007979 thiazole derivatives Chemical class 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 125000005557 thiazolylene group Chemical group 0.000 description 1
- 125000005556 thienylene group Chemical group 0.000 description 1
- 239000011135 tin Substances 0.000 description 1
- XOLBLPGZBRYERU-UHFFFAOYSA-N tin dioxide Chemical compound O=[Sn]=O XOLBLPGZBRYERU-UHFFFAOYSA-N 0.000 description 1
- 229910001887 tin oxide Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- BWHOZHOGCMHOBV-BQYQJAHWSA-N trans-benzylideneacetone Chemical compound CC(=O)\C=C\C1=CC=CC=C1 BWHOZHOGCMHOBV-BQYQJAHWSA-N 0.000 description 1
- TVIVIEFSHFOWTE-UHFFFAOYSA-K tri(quinolin-8-yloxy)alumane Chemical compound [Al+3].C1=CN=C2C([O-])=CC=CC2=C1.C1=CN=C2C([O-])=CC=CC2=C1.C1=CN=C2C([O-])=CC=CC2=C1 TVIVIEFSHFOWTE-UHFFFAOYSA-K 0.000 description 1
- 125000005259 triarylamine group Chemical group 0.000 description 1
- 150000003918 triazines Chemical class 0.000 description 1
- 125000003866 trichloromethyl group Chemical group ClC(Cl)(Cl)* 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- 125000006617 triphenylamine group Chemical group 0.000 description 1
- 125000005580 triphenylene group Chemical group 0.000 description 1
- 125000003960 triphenylenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3C3=CC=CC=C3C12)* 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- 238000004506 ultrasonic cleaning Methods 0.000 description 1
- 238000007738 vacuum evaporation Methods 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
- GPPXJZIENCGNKB-UHFFFAOYSA-N vanadium Chemical compound [V]#[V] GPPXJZIENCGNKB-UHFFFAOYSA-N 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 229910052727 yttrium Inorganic materials 0.000 description 1
- VWQVUPCCIRVNHF-UHFFFAOYSA-N yttrium atom Chemical compound [Y] VWQVUPCCIRVNHF-UHFFFAOYSA-N 0.000 description 1
- YVTHLONGBIQYBO-UHFFFAOYSA-N zinc indium(3+) oxygen(2-) Chemical compound [O--].[Zn++].[In+3] YVTHLONGBIQYBO-UHFFFAOYSA-N 0.000 description 1
- 239000011787 zinc oxide Substances 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/615—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene
- H10K85/626—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene containing more than one polycyclic condensed aromatic rings, e.g. bis-anthracene
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05B—ELECTRIC HEATING; ELECTRIC LIGHT SOURCES NOT OTHERWISE PROVIDED FOR; CIRCUIT ARRANGEMENTS FOR ELECTRIC LIGHT SOURCES, IN GENERAL
- H05B33/00—Electroluminescent light sources
- H05B33/12—Light sources with substantially two-dimensional radiating surfaces
- H05B33/14—Light sources with substantially two-dimensional radiating surfaces characterised by the chemical or physical composition or the arrangement of the electroluminescent material, or by the simultaneous addition of the electroluminescent material in or onto the light source
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
- H10K85/633—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine comprising polycyclic condensed aromatic hydrocarbons as substituents on the nitrogen atom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1007—Non-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1011—Condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1014—Carbocyclic compounds bridged by heteroatoms, e.g. N, P, Si or B
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1029—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom
- C09K2211/1037—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom with sulfur
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1044—Heterocyclic compounds characterised by ligands containing two nitrogen atoms as heteroatoms
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1044—Heterocyclic compounds characterised by ligands containing two nitrogen atoms as heteroatoms
- C09K2211/1048—Heterocyclic compounds characterised by ligands containing two nitrogen atoms as heteroatoms with oxygen
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1088—Heterocyclic compounds characterised by ligands containing oxygen as the only heteroatom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1092—Heterocyclic compounds characterised by ligands containing sulfur as the only heteroatom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/18—Metal complexes
- C09K2211/186—Metal complexes of the light metals other than alkali metals and alkaline earth metals, i.e. Be, Al or Mg
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/111—Organic polymers or oligomers comprising aromatic, heteroaromatic, or aryl chains, e.g. polyaniline, polyphenylene or polyphenylene vinylene
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/111—Organic polymers or oligomers comprising aromatic, heteroaromatic, or aryl chains, e.g. polyaniline, polyphenylene or polyphenylene vinylene
- H10K85/113—Heteroaromatic compounds comprising sulfur or selene, e.g. polythiophene
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/111—Organic polymers or oligomers comprising aromatic, heteroaromatic, or aryl chains, e.g. polyaniline, polyphenylene or polyphenylene vinylene
- H10K85/114—Poly-phenylenevinylene; Derivatives thereof
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/111—Organic polymers or oligomers comprising aromatic, heteroaromatic, or aryl chains, e.g. polyaniline, polyphenylene or polyphenylene vinylene
- H10K85/115—Polyfluorene; Derivatives thereof
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/141—Organic polymers or oligomers comprising aliphatic or olefinic chains, e.g. poly N-vinylcarbazol, PVC or PTFE
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/141—Organic polymers or oligomers comprising aliphatic or olefinic chains, e.g. poly N-vinylcarbazol, PVC or PTFE
- H10K85/146—Organic polymers or oligomers comprising aliphatic or olefinic chains, e.g. poly N-vinylcarbazol, PVC or PTFE poly N-vinylcarbazol; Derivatives thereof
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
- H10K85/311—Phthalocyanine
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
- H10K85/321—Metal complexes comprising a group IIIA element, e.g. Tris (8-hydroxyquinoline) gallium [Gaq3]
- H10K85/324—Metal complexes comprising a group IIIA element, e.g. Tris (8-hydroxyquinoline) gallium [Gaq3] comprising aluminium, e.g. Alq3
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/615—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene
- H10K85/621—Aromatic anhydride or imide compounds, e.g. perylene tetra-carboxylic dianhydride or perylene tetracarboxylic di-imide
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/656—Aromatic compounds comprising a hetero atom comprising two or more different heteroatoms per ring
- H10K85/6565—Oxadiazole compounds
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S428/00—Stock material or miscellaneous articles
- Y10S428/917—Electroluminescent
Definitions
- This invention relates to an organic luminescent device, and more particularly to a device which emits light upon application of an electric field to a thin film formed of an organic compound.
- the organic luminescent device is a device in which a thin film containing a fluorescent organic compound is held between an anode and a cathode, electrons and holes are injected from each electrode to produce excitons of the fluorescent organic compound, and the light is utilized that is emitted when the excitons return to the ground state.
- an organic emission-device making use of a conjugated type high-molecular material is further reported by a group of members of Cambridge University (Nature, 347, 539, 1990).
- polyphenylene vinylene (PPV) is formed into a film by a coating system to ascertain luminescence in a single layer.
- Related patents of such a conjugated type high-molecular material may include U.S. Pat. Nos. 5,247,190, 5,514,878 and 5,672,678, and Japanese Patent Application Laid-Open Nos. H04-145192 and H05-247460.
- a phosphorescent organic luminescent device making use of an iridium complex such as Ir(ppy) 3 as a luminescent material also has lately attracted attention, and a high emission efficiency is reported.
- Japanese Patent Application Laid-Open No. H08-012600 discloses a phenyl anthracene derivative. This reports that a good organic film can be formed because of low crystallizability especially when used as a blue luminescent material or an electron transporting material. However, its luminescence efficiency and durability lifetime have been insufficient in practical use.
- Japanese Patent Application Laid-Open Nos. H09-157643 and H10-072579 disclose an amino anthracene derivative and a diamino anthracene derivative, respectively. They report that green luminescence is obtained when used as a luminescent material. However, the device has a low luminescence efficiency, and also has been insufficient in practical use in regard to durability lifetime.
- Japanese Patent No. 3,008,897 discloses a device making use of a specific bianthryl compound as a luminescent material, which, however, has no disclosure in regard to luminescence efficiency and durability lifetime.
- Japanese Patent Application Laid-Open No. H11-008068 discloses a device making use of, as a luminescent material, a specific anthracene compound containing an olefin moiety, and reports that luminescence of from yellow light to red light is obtained. It, however, has been insufficient in practical use in regard to luminescence efficiency.
- Japanese Patent Application Laid-Open No. 2001-284050 discloses a device having a luminescent medium layer which contains an anthracene derivative with a specific structure, an electron transporting compound and another luminescent compound. This reports that a red luminescent device improved in reliability is obtained. However, the device has an insufficient luminescence efficiency in practical use, and also it has been difficult to obtain blue luminescence because of device structure.
- an object of the present invention is to provide an organic luminescent device which can emit luminescent hues with a very high purity, and effect light emission with high luminance at a high luminescence efficiency and a long lifetime, and also to provide an organic luminescent device which can be easily produced at relatively low cost.
- the present inventors have conducted extensive studies in order to solve the above problems. As a result, they have accomplished the present invention.
- anthryl derivative group substituted compound represented by the following general formula [1]:
- a is an integer of 0 to 4
- b+c+d is an integer of (6 ⁇ a) or less
- A represents a molecular unit including an aromatic ring, a condensed multiple ring or a heterocyclic ring, selected from the group consisting of a terphenyl ring, a naphthalene ring, a phenanthrene ring, a pyrene ring, a tetracene ring, a pentane ring, a perylene ring, a pyrrole ring, a pyridine ring, a terpyridine ring, a thiophene ring and a terthiophene ring which may be substituted or unsubstituted, and a fluorene ring;
- Y 1 and Y 2 are each independently a group selected from the group consisting of an alkyl group, an aralkyl group, an aryl group and a heterocyclic group which are substituted or unsubstituted; Y 1 and Y 2 may be combined to togetherer to form a ring; and Y 1 's and Y 2 's in different anthryl derivative groups may be the same or different;
- Z 1 is a direct bond or a group selected from the group consisting of an arylene group and a divalent heterocyclic group which may be substituted or unsubstituted, and a divalent group having a bridging group, and may be the same or different when a is two or more;
- Z 2 and Z 3 are each independently a direct bond or a group selected from the group consisting of an alkylene group, an alkenylene group, an alkynylene group, an aralkylene group and an arylene group which may be substituted or unsubstituted, and a divalent group having a bridging group, provided that Z 2 and Z 3 are not a direct bond where A is a fluorene ring; and when a is two or more, Z 2 may be the same or different, and when b is two or more, Z 3 may be the same or different;
- X 1 is a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group, an alkenyl group, an alkynyl group, an aralkyl group, an alkoxyl group and a sulfide group which may be substituted or unsubstituted, an aryl group, a heterocyclic group, and a substituted silyl group, and may be the same or different when b is two or more;
- X 2 is a group selected from the group consisting of an aryl group and a heterocyclic group which may be substituted or unsubstituted, and may be the same or different when c is two or more;
- R 1 's and R 2 's are each independently a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group, an aryl group, an alkoxyl group and an amino group which may be substituted or unsubstituted; and R 3 is a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group and an alkoxyl group which may be substituted or unsubstituted.
- the present invention also provides an anthryl derivative group substituted compound represented by the following general formula [2]:
- e is an integer of 0 to 4
- f+g+H is an integer of (6 ⁇ e) or less, provided that e+f is 2 to 5, and h+g is ⁇ 1 when e+f is 2;
- Y 3 and Y 4 are each independently a group selected from the group consisting of an alkyl group, an aralkyl group, an aryl group and a heterocyclic group which may be substituted or unsubstituted, and a divalent group having a bridging group; Y 3 and Y 4 may be combined together to form a ring; and Y 3 's and Y 4 's in different anthryl derivative groups may be the same or different;
- Z 4 is a direct bond, or a group selected from the group consisting of an arylene group and a divalent heterocyclic group which may be substituted or unsubstituted, and a divalent group having a bridging group, and may be the same or different when e is two or more;
- Z 5 and Z 6 are each independently a direct bond or a group selected from the group consisting of an alkylene group, an alkenylene group, an alkynylene group, an aralkylene group and an arylene group which are substituted or unsubstituted, and a divalent group having a bridging group; and when e is two or more, Z 5 may be the same or different, and when f is two or more, Z 6 may be the same or different;
- X 3 is a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group, an alkenyl group, an alkynyl group, an aralkyl group, an alkoxyl group and a sulfide group which may be substituted or unsubstituted, an aryl group, a heterocyclic group, and a substituted silyl group, and may be the same or different when f is two or more;
- X 4 is a group selected from the group consisting of an aryl group and a heterocyclic group which may be substituted or unsubstituted, and may be the same or different when g is two or more;
- R 4 's and R 5 's are each independently a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group, an aryl group, an alkoxyl group and an amino group which may be substituted or unsubstituted; and R 6 is a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group and an alkoxyl group which may be substituted or unsubstituted, and may be the same or different when h is two or more.
- FIG. 1 is a sectional view showing an example of an organic luminescent device in the present invention.
- FIG. 2 is a sectional view showing another example of an organic luminescent device in the present invention.
- FIG. 3 is a sectional view showing still another example of an organic luminescent device in the present invention.
- FIG. 4 is a sectional view showing a further example of an organic luminescent device in the present invention.
- FIG. 5 is a sectional view showing a still further example of an organic luminescent device in the present invention.
- the present invention is an anthryl derivative group substituted compound represented by the following general formula [1] or [2]:
- a is 0 to 4, and b+c+d is an integer of (6 ⁇ a) or less;
- A represents a molecular unit containing an aromatic ring, a condensed multiple ring or a heterocyclic ring, selected from the group consisting of a terphenyl ring, a naphthalene ring, a phenanthrene ring, a pyrene ring, a tetracene ring, a pentane ring, a perylene ring, a pyrrole ring, a pyridine ring, a terpyridine ring, a thiophene ring and a terthiophene ring which are connected to anthracene rings via or not via bridging groups Z 2 and Z 3 and are substituted or unsubstituted, and a fluorene ring which is connected to anthracene rings via bridging groups Z 2 and Z 3 ;
- Y 1 and Y 2 are each a group selected from the group consisting of an alkyl group, an aralkyl group, an aryl group and a heterocyclic group which are substituted or unsubstituted and may be the same or different; Y 1 and Y 2 may be combined together to form a ring; and Y 1 's and Y 2 's in different anthryl derivative groups may be the same or different;
- Z 1 is a direct bond or a group selected from the group consisting of an arylene group and a divalent heterocyclic group which are substituted or unsubstituted, and a divalent group having a bridging group, and Z 1 's in different anthryl derivative groups may be the same or different;
- Z 2 and Z 3 are each a direct bond or a group selected from the group consisting of an alkylene group, an alkenylene group, an alkynylene group, an aralkylene group and an arylene group which are substituted or unsubstituted, and a divalent group having a bridging group; and when a is two or more, Z 2 may be the same or different, and when b is two or more, Z 3 may be the same or different;
- X 1 is a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group, an alkenyl group, an alkynyl group, an aralkyl group, an alkoxyl group and a sulfide group which are substituted or unsubstituted, an aryl group, a heterocyclic group, and a substituted silyl group, and may be the same or different when b is two or more;
- X 2 is a group selected from the group consisting of an aryl group and a heterocyclic group which are substituted or unsubstituted, and may be the same or different when c is two or more;
- R 1 's and R 2 's are each independently a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group, an aryl group, an alkoxyl group and an amino group which are substituted or unsubstituted; and R 3 is a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group and an alkoxyl group which are substituted or unsubstituted, and may be the same or different when d is two or more.
- e is 0 to 4
- f+g+h is an integer of (6 ⁇ e) or less, provided that e+f is 2 to 5, and h+g is ⁇ 1 when e+f is 2;
- Y 3 and Y 4 are each independently a group selected from the group consisting of an alkyl group, an aralkyl group, an aryl group and a heterocyclic group which may be substituted or unsubstituted, and a divalent group having a bridging group; Y 3 and Y 4 may be combined together to form a ring; and Y 3 's and Y 4 's in different anthryl derivative groups may be the same or different;
- Z 4 is a direct bond-or a group selected from the group consisting of an arylene group and a divalent heterocyclic group which are substituted or unsubstituted, and a divalent group having a bridging group, and may be the same or different when e is two or more;
- Z 5 and Z 6 are each independently a direct bond or a group selected from the group consisting of an alkylene group, an alkenylene group, an alkynylene group, an aralkylene group and an arylene group which are substituted or unsubstituted, and a divalent group having a bridging group; and when e is two or more, Z 5 may be the same or different, and when f is two or more, Z 6 may be the same or different;
- X 3 is a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group, an alkenyl group, an alkynyl group, an aralkyl group, an alkoxyl group and a sulfide group which are substituted or unsubstituted, an aryl group, a heterocyclic group, and a substituted silyl group, and may be the same or different when f is two or more;
- X 4 is a group selected from the group consisting of an aryl group and a heterocyclic group which are substituted or unsubstituted, and may be the same or different when g is two or more;
- R 4 's and R 5 's are each independently a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group, an aryl group, an alkoxyl group and an amino group which are substituted or unsubstituted; and R 6 is a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group and an alkoxyl group which are substituted or unsubstituted, and may be the same or different when h is two or more.
- the compounds represented by the general formulas [1] and [2] may chiefly be used as materials for organic luminescent devices.
- they when used as luminescent materials, they may each be used alone in a luminescent layer, and may be used for the purposes of dopant materials and host materials to obtain devices with high color purity, high luminescence efficiency and long lifetime.
- the compounds represented by the general formulas [1] and [2] each have a core unit including an aromatic ring, a condensed multiple ring or a heterocyclic ring, the core unit being substituted with at least two anthryl groups as highly efficient luminescent units and each of the anthryl groups being substituted with an amino group, an amino group having a bridging group, an aryl group or the like.
- HOMO/LUMO levels of the material may be controlled by replacement of substituents, where blue, green and a luminescence color on a longer wavelength side can be obtained with ease (examples of calculation of HOMO/LUMO levels by B3LYP/3-21G: Exemplary Compound 15: ⁇ 5.030/ ⁇ 1.877; Exemplary Compound 19: ⁇ 4.805/ ⁇ 1.696).
- changes in HOMO/LUMO levels by the replacement of substituents may be predicted by calculation, where the material can be designed and synthesized with ease according to the HOMO/LUMO levels of a host material.
- Steric hindrance groups or fluorine atoms may be introduced into the core molecule at the center, the anthryl groups or other aryl groups and the substituents on amino-groups, whereby it can be expected to restrain molecules from cohering to one another and especially improve the device lifetime.
- the materials of the present invention considering that in virtue of an isotope effect, molecular vibration is controlled and thermal deactivation is inhibited, the idea of introducing molecular units substituted with deuterium has been incorporated in molecular designing. In the present invention, molecular designing has been made on the basis of such consideration, thus the present invention has been made.
- the compounds When used as dopant materials, they may each be in a dopant concentration of from 0.01% to 80%, and preferably from 1% to 40%, in respect to the host material.
- the dopant material may be contained uniformly, or with a concentration gradient, in the whole layer formed of the host material, or may partially be contained in some regions to form regions of a host material layer containing no dopant material.
- the bridging group may include, but is not limited to, a substituted or unsubstituted arylene group and a divalent heterocyclic ring group as shown later.
- the divalent group having a bridging group may include, but is not limited to, an alkylene group, an alkenylene group, an alkynylene group, an aralkylene group and an amino group which are substituted or unsubstituted, a substituted silyl group, an ether group, a thioether group and a carbonyl group which have a bridging group.
- the substituted or unsubstituted alkyl group may include, but is not limited to, a methyl group, a methyl-d1group, a methyl-d3 group, an ethyl group, an ethyl-d5 group, an n-propyl group, an n-butyl group, an n-pentyl group, an n-hexyl group, an n-heptyl group, an n-octyl group, an n-decyl group, an iso-propyl group, an iso-propyl-d7 group, an iso-butyl group, a sec-butyl group, a tert-butyl group, a tert-butyl-d9 group, an iso-pentyl group, a neopentyl group, a tert-octyl group, a fluoromethyl group, a difluoromethyl group, a triflu
- the substituted or unsubstituted aralkyl group may include, but is not limited to, a benzyl group, a 2-phenylethyl group, a 2-phenylisopropyl group, a 1-naphthylmethyl group, a 2-naphthylmethyl group, a 2-(1-naphthyl) ethyl group, a 2-(2-naphthyl) ethyl group, a 9-anthrylmethyl group, a 2-(9-anthryl)ethyl group, a 2-fluorobenzyl group, a 3-fluorobenzyl group, a 4-fluorobenzyl group, a 2-chlorobenzyl group, a 3-chlorobenzyl group, a 4-chlorobenzyl group, a 2-bromobenzyl group, a 3-bromobenzyl group and a 4-bromobenzyl group.
- the substituted or unsubstituted alkenyl group may include, but is not limited to, a vinyl group, an allyl group, a 2-propenyl group, a 1-propenyl group, an iso-propenyl group, a 1-butenyl group, a 2-butenyl group, a 3-butenyl group and a styryl group.
- the substituted or unsubstituted alkynyl group may include, but of course is not limited to, an acetylenyl group, a phenylacetylenyl group and a 1-propynyl group.
- the substituted or unsubstituted aryl group may include, but is not limited to, a phenyl group, a phenyl-d5 group, a 4-methylphenyl group, a 4-methoxylphenyl group, a 4-ethylphenyl group, a 4-fluorophenyl group, a 4-trifluorophenyl group, a 3,5-dimethylphenyl group, a 2,6-diethylphenyl group, a mesityl group, a 4-tert-butylphenyl group, a ditolylaminophenyl group, a biphenyl group, a terphenyl group, a 1-naphthyl group, a 1-naphthyl-d7 group, a 2-naphthyl-d7 group, a 1-anthryl group, a 2-anthryl group, a 9-anthryl group, a 9-anthryl-d9 group
- the substituted or unsubstituted heterocyclic group may include, but is not limited to, a pyrrolyl group, a pyridyl group, a pyridyl-d5 group, a bipyridyl group, a methylpyridyl group, a terpyrrolyl group, a thienyl group, a thienyl-d4 group, a terthienyl group, a propylthienyl group, a furyl group, a furyl-d4 group, an indolyl group, a 1,10-phenanthrolyl group, a phenazinyl group, a quinolyl group, a carbazolyl group, an oxazolyl group, an oxadiazolyl group, a thiazolyl group and a thiadiazolyl group.
- the substituted or unsubstituted alkylene group may include, but is not limited to, a methylene group, a methylene-d2 group, a difluoromethylene group, an ethylene group, an ethylene-d4 group, a perfluoroethylene group, a propylene group, an iso-propylene group, a butylene group and a 2,2-dimethylpropylene group.
- the substituted or unsubstituted aralkylene group may include, but is not limited to, a benzylene group, a 2-phenylethylene group, a 2-phenylisopropylene group, a 1-napthylmethylene group, a 2-napthylmethylene group, a 9-anthrylmethylene group, a 2-fluorobenzylene group, a 3-fluorobenzylene group, a 4-fluorobenzylene group, a 4-chlorobenzylene group and a 4-bromobenzylene group.
- the substituted or unsubstituted alkenyl group may include, but of course is not limited to, a vinylene group, an iso-propenylene group, a styrylene group and a 1 , 2 -diphenylvinylene group.
- the substituted or unsubstituted alkynyl group may include, but of course is not limited to, an acetylenylene group and a 1,2-phenylacetylenylene group.
- the substituted or unsubstituted arylene group may include, but is not limited to, a phenylene group, a biphenylene group, a tetrafluorophenylene group, a dimethylphenylene group, a naphthylene group, an anthrylene group, a phenanthrylene group, a pyrenylene group, a tetracenylene group, a pentacenylene group and a perylenylene group.
- the substituted or unsubstituted divalent heterocyclic group may include, but is not limited to, a furylene group, a pyrrolylene group, a pyridylene group, a terpyridylene group, a thienylene group, a terthienylene group, an oxazolylene group, a thiazolylene group and a carbazolylene group.
- the substituted or unsubstituted amino group may be a group in which R′ and R′′ each represent a hydrogen atom, a deuterium atom, any of an alkylene group, an alkenylene group, an alkynylene group, an aralkylene group and an amino group each having a bridging group consisting of the above substituted or unsubstituted alkyl group, aralkyl group, aryl group or heterocyclic group or the substituted or unsubstituted arylene group or divalent heterocyclic group, a substituted silyl group, an ether group, a thioether group, or a carbonyl group, and may include, but is not limited to, e.g., an amino group, an N-methylamino group, an N-ethylamino group, an N,N-dimethylamino group, an N,N-diethylamino group, an N-methyl-N-ethylamin
- the substituted or unsubstituted alkoxyl group may include an alkyloxyl group and an aralkyloxyl group each having the above substituted or unsubstituted alkyl group or aralkyl group, or an aryloxyl group having the above substituted or unsubstituted aryl group or heterocyclic group, and may include, but is not limited to, e.g., a methoxyl group, an ethoxyl group, a propoxyl group, a 2-ethyl-octyloxyl group, a phenoxyl group, a 4-tert-butylphenoxyl group, a benzyloxyl group and a thienyloxyl group.
- the substituted or unsubstituted sulfide group may include an alkyl sulfide group and an aralkyl sulfide group each having the above substituted or unsubstituted alkyl group or aralkyl group, or an aryl sulfide group having the above substituted or unsubstituted aryl group or heterocyclic group, and may include, but is not limited to, e.g., a methyl sulfide group, an ethyl sulfide group, a phenyl sulfide group and a 4-methylphenyl sulfide group.
- the molecular unit A may include, but is not limited to, a benzene ring, a biphenyl ring, a terphenyl ring, a naphthalene ring, a phenanthrene ring, an anthracene ring, a tetracene ring, a benzanthracene ring, a chrysene ring, a pyrene ring, a perylene ring, a choranthlene ring, a triphenylene ring, a thiophene ring, a pyridine ring, a pyrazine ring, a pyrazole ring, a pyrrole ring, a terthiophene ring, a terpyridine ring, a terpyrrole ring, a triazine ring, a carbazole ring, a benzimidazole ring, a benzothiazole
- Substituents the above substituents, bridging groups and molecular unit A may further have may include, but is not limited to, a deuterium atom, an alkyl group such as a methyl group, an ethyl group, an n-propyl group, an n-butyl-group, an n-pentyl group, an n-hexyl group, an n-heptyl group, an n-octyl group, an n-decyl group, an iso-propyl group, an iso-butyl group, a sec-butyl group, a tert-butyl group, an iso-pentyl group, a neopentyl group, a tert-octyl group, a benzyl group or a 2-phenylethyl group; an aralkyl group; an alkoxyl group such as a methoxyl group, an ethoxyl
- At least one of Y 1 , Y 2 , Z 1 to Z 3 , X 1 , X 2 and R 1 to R 3 is a group containing a deuterium atom, or a deuterium atom and at least one of Y 3 , Y 4 , Z 4 to Z 6 , X 3 , X 4 and R 4 to R 6 is a group containing a deuterium atom, or a deuterium atom.
- the organic luminescent device of the present invention is described below in detail.
- the organic luminescent device of the present invention is an organic luminescent device having at least a pair of electrodes at least one of which comprises a transparent or semitransparent anode or cathode, and one or more layers containing an organic compound, held between the electrodes (the anode and the cathode), wherein;
- At least one layer of the layers containing an organic compound contains at least one compound of the anthryl derivative group substituted compounds represented by the general formulas [1] and [2].
- FIGS. 1 to 5 Preferred examples of the organic luminescent device of the present invention are shown in FIGS. 1 to 5 .
- reference numerals shown in the drawings denote the following:
- FIG. 1 is a sectional view showing an example of the organic luminescent device of the present invention.
- FIG. 1 shows a constitution in which an anode 2 , a luminescent layer 3 and a cathode 4 in this order are provided on a substrate 1 .
- the luminescent device used here is useful where the device has by itself hole transportability, electron transportability and luminescence function, or where compounds having the respective properties are used in mixture.
- FIG. 2 is a sectional view showing another example in the organic luminescent device of the present invention.
- FIG. 2 shows a constitution in which an anode 2 , a hole transport layer 5 , an electron transport layer 6 and a cathode 4 in this order are provided on a substrate 1 .
- the device in this case is useful where a material having either hole transport function or electron transport function or both the functions is used as a luminescent material in each layer and is used in combination with a mere hole transporting material or electron transporting material having no luminescent property.
- the luminescent layer 3 is composed of either of the hole transport layer 5 and the electron transport layer 6 .
- FIG. 3 is a sectional view showing still another example in the organic luminescent device of the present invention.
- FIG. 3 shows a constitution in which an anode 2 , a hole transport layer 5 , a luminescent layer 3 , an electron transport layer 6 and a cathode 4 in this order are provided on a substrate 1 .
- This device is one in which the functions of carrier transport and luminescence are separated, and is used in appropriate combination with a compound having respective functions of a hole transport function, an electron transport function and a luminescence function.
- FIG. 4 is a sectional view showing a further example in the organic luminescent device of the present invention.
- FIG. 4 shows a constitution in which in the constitution as shown in FIG. 3 , a hole injection layer 7 is inserted on the anode 2 side. This is effective in improving adherence between the anode 2 and the hole transport layer 5 and improving hole injection, and also is effective in low-voltage luminescence.
- FIG. 5 is a sectional view showing a still further example in the organic luminescent device of the present invention.
- FIG. 5 shows a constitution in which in the constitution as shown in FIG. 3 , a layer (hole/exciton blocking layer 8 ) which prevents holes or excitons from passing through toward the cathode 4 side, is inserted between the luminescent layer 3 and the electron transport layer 6 .
- a compound having a very high ionization potential may be used in the hole/exciton blocking layer 8 to provide a constitution highly effective in improving luminescence efficiency.
- FIGS. 1 to 5 show quite basic constitutions to the end, and the constitutions of the organic luminescent device using the compound of the present invention are by no means limited thereto.
- the device can have various constitutions such that insulating layers are provided at the interfaces between electrodes and organic layers, an adhesive layer or an interference layer is provided, and the hole transport layer is constituted of two layers having different ionization potentials.
- the compounds represented by the general formulas [1] and [2] may be used in any of the forms shown in FIGS. 1 to 5 .
- an organic layer making use of the compound of the present invention is useful as the luminescent layer, the electron transport layer or the hole transport layer.
- a layer formed by vacuum deposition (vacuum evaporation) or solution coating can not easily cause crystallization and is superior in stability over time.
- any of the anthryl derivative group substituted compounds represented by the general formulas [1] and [2] may be used as a constituent of, in particular, the luminescent layer, and may also optionally be used together with a hole transporting compound, a luminescent compound and/or an electron transporting compound which is/are known in the art.
- a hole injecting and transporting material is preferably one which facilitates the injection of holes from the anode and has mobility sufficient to transport the injected holes to the luminescent layer.
- Low-molecular and high-molecular materials having a hole injection and transport function may include, but is not limited to, triarylamine derivatives, phenylenediamine derivatives, triazole derivatives, oxadiazole derivatives, imidazole derivatives, pyrazoline derivatives, pyrazolone derivatives, oxazole derivatives, fluorenone derivatives, hydrazone derivatives, stilbene derivatives, phthalocyanine derivatives, porphyrin derivatives, poly(vinylcarbazole), poly(silylene), poly(thiophene), and other conductive high-molecular materials. Some specific examples of these are shown below.
- Materials concerned chiefly in luminescence function which are usable besides the anthryl derivative group substituted compounds represented by the general formulas [1] and [2] may include, but is not limited to, high-molecular derivatives such as polycyclic condensation aromatic compounds (e.g., naphthalene derivatives, phenanthrene derivatives, fluorene derivatives, pyrene derivatives, tetracene derivatives, coronene derivatives, chrysene derivatives, perylene derivatives, 9,10-diphenylanthracene derivatives, and rubrene), quinacridone derivatives, acridone derivatives, coumarine derivatives, pyrane derivatives, Nile Red, pyrazine derivatives, benzoimidazole derivatives, benzothiazole derivatives, benzoxazole derivatives, stilbene derivatives, organometallic complexes (e.g., organoaluminum complexes such as tris(8-quinolinolato
- An electron injecting and transporting material may be selected from materials which facilitate the injection of electrons from the cathode and have a function to transport electrons to the luminescent layer, and may be selected taking into account a balance with the carrier mobility of the hole transporting material.
- Materials having the electron injection and transport function may include, but is not limited to, oxadiazole derivatives, oxazole derivatives, thiazole derivatives, thidiazole derivatives, pyrazine derivatives, triazole derivatives, triazine derivatives, perylene derivatives, quinoline derivatives, quinoxaline derivatives, fluorenone derivatives, anthrone derivatives, phenanthroline derivatives, and organometallic complexes.
- the layers containing the anthryl derivative group substituted compounds represented by the general formulas [1] and [2] and the layers formed of other organic compounds are commonly formed of thin films produced by vacuum deposition, ionization deposition, sputtering, plasma deposition, or known coating (e.g., spin coating, dipping, casting, the LB (Langmuir Blodgett) process, or ink-jet printing) of a coating material prepared by dissolving materials in a suitable solvent.
- the films may be formed using materials in combination-with a suitable binder resin.
- the binder resin may be selected from extensive binding resins, and may include, but is not limited to, polyvinyl carbazole resins, polycarbonate resins, polyester resins, polyarylate resins, polystyrene resins, ABS resins, polybutadiene resins, polyurethane resins, acrylic resins, methacrylic resins, butyral resins, polyvinyl acetal resins, polyamide resins, polyimide resins, polyethylene resins, polyether sulfone resins, diallyl phthalate resins, phenol resins, epoxy resins, silicone resins, polysulfone resins, and urea resins. These may also be used alone as homopolymers or in a mixture of two or more types as copolymers. Additives such as known plasticizers, antioxidants and ultraviolet absorbers may further optionally be used in a combination of two or more types.
- Materials for the anode having a work function as large as possible are favorable, and usable are, e.g., metals themselves such as gold, platinum, silver, copper, nickel, palladium, cobalt, selenium, vanadium and tungsten, or alloys of any of these; and metal oxides such as tin oxide, zinc oxide, indium oxide, indium-tin oxide (ITO), and indium-zinc oxide.
- metals themselves such as gold, platinum, silver, copper, nickel, palladium, cobalt, selenium, vanadium and tungsten, or alloys of any of these
- metal oxides such as tin oxide, zinc oxide, indium oxide, indium-tin oxide (ITO), and indium-zinc oxide.
- conductive polymers such as polyaniline, polypyrrole, polythiophene, and polyphenylene sulfide. Any of these electrode materials may be used alone or may be used in combination.
- the anode may be constituted of a single layer
- materials for the cathode having a small work function are favorable, and may be used in the form of, e.g., metals themselves such as lithium, sodium, potassium, calcium, magnesium, aluminum, indium, ruthenium, titanium, manganese, yttrium, silver, lead, tin, and chromium; or alloys of two or more of these, such as lithium-indium, sodium-potassium, magnesium-silver, aluminum-lithium, aluminum-magnesium, and magnesium-indium.
- Metal oxides such as indium-tin oxide (ITO) may also be used. Any of these electrode materials may be used alone or may be used in a combination of two or more types.
- the cathode may be constituted of a single layer or may be constituted of a multiple layer.
- At least one of the anode and the cathode is transparent or semitransparent.
- the substrate used in the present invention there are no particular limitations. There may be used opaque substrates such as substrate made of metal and substrates made of ceramic, and transparent substrates such as glass, quartz and plastic sheets. A color filter film, a fluorescent color conversion filter film, a dielectric reflecting film or the like may be applied to the substrate to control luminescence light.
- a protective layer or a sealing layer may be provided in order to prevent contact with oxygen, moisture, etc.
- the protective layer may include diamond thin film; films of inorganic materials such as metal oxides and metal nitrides; films of high-molecular materials such as fluorine resins, polyparaxylene, polyethylene, silicone resins and polystyrene resins; and photocurable resins.
- the device may be covered with glass, a gas-impermeable film, metal or the like and itself may be packed in a suitable sealing resin.
- the resulting solution was stirred for 30 minutes, and thereafter 347 mg (0.3 mmol) of tetrakis(triphenylphosphine)palladium was added, followed by heating and stirring for about 5 hours on an oil bath heated to 80° C.
- the resultant reaction solution was cooled to room temperature, and thereafter 70 ml of water and 70 ml of ethyl acetate were added to separate a water layer and an organic layer.
- the water layer was extracted with toluene and ethyl acetate, and then combined with the previous organic layer, followed by drying with sodium magnesium.
- the resultant reaction solution was cooled to room temperature, and thereafter 70 ml of water and 70 ml of ethyl acetate were added to separate a water layer and an organic layer.
- the water layer was extracted with toluene and ethyl acetate, and then combined with the previous organic layer, followed by drying over sodium magnesium.
- An organic luminescent device having the structure shown in FIG. 3 was produced in the, following way.
- an indium-tin oxide (ITO) film as the anode 2 was formed by sputtering in a layer thickness of 120 nm, and this was used as a transparent conductive support substrate.
- This substrate was subjected to ultrasonic cleaning with acetone and isopropyl alcohol (IPA) in order, and subsequently to boiling cleaning with IPA, followed by drying.
- the substrate was further subjected to UV/ozone cleaning, and was used as the transparent conductive support substrate.
- This solution was dropped on the above ITO electrode, and spin-coating was carried out first at the number of revolutions of 500 rpm for 10 seconds and then at the number of revolutions of 1,000 rpm for 1 minute to form a film (thin film). Thereafter, the film was dried for 10 minutes in a 80° C. vacuum oven to completely remove the solvent remaining in the thin film.
- the hole transport layer 5 thus formed was in a thickness of 50 nm.
- the above Exemplary Compound No. 21 was vacuum-deposited to provide as the luminescent layer 3 a luminescent layer 3 of 20 nm in thickness. At the time of the vacuum deposition, the film was formed under the conditions of a degree of vacuum of 1.0 ⁇ 10 ⁇ 4 Pa and a rate of film formation of 0.2 to 0.3 nm/sec.
- a batho-phenanthroline (BPhen) film was formed by vacuum deposition in a layer thickness of 40 nm. At the time of the vacuum deposition, the film was formed under the conditions of a degree of vacuum of 1.0 ⁇ 10 ⁇ 4 Pa and a rate of film formation of 0.2 to 0.3 nm/sec.
- BPhen batho-phenanthroline
- a vacuum deposition material composed of an aluminum-lithium alloy (lithium concentration: 1 atom %), a metal layer film of 10 nm in thickness was formed by vacuum deposition, and an aluminum film of 150 nm in thickness was further formed by vacuum deposition to produce an organic luminescent device having an aluminum-lithium alloy film as an electron injection electrode (the cathode 4 ).
- the film was formed under the conditions of a degree of vacuum of 1.0 ⁇ 10 ⁇ 4 Pa and a rate of film formation of 1.0 to 1.2 nm/sec.
- the organic luminescent device (organic EL device) thus obtained was covered with a protecting glass plate in a dry air atmosphere and sealed with an acrylic-resin adhesive so as not to cause device deterioration due to adsorption of moisture.
- the voltage was further applied for 100 hours in a nitrogen atmosphere, keeping current density at 3.0 mA/cm 2 .
- the initial luminance 300 cd/m 2 came to be 280 cd/m 2 after 100 hours, showing small luminance deterioration.
- a device was produced in the same manner as in Example 4 except that a comparative compound shown below was used in place of Exemplary Compound No. 21. Evaluation was made in the same way. Green luminescence of 190 cd/m 2 in luminance and 2 lm/W in luminescence efficiency was observed at an applied voltage of 3 V.
- the voltage was further applied for 100 hours in a nitrogen atmosphere, keeping current density at 3.0 mA/cm 2 .
- the initial luminance 180 cd/m 2 came to be 80 cd/m 2 after 100 hours, showing a great luminance deterioration.
- An organic luminescent device having the structure shown in FIG. 3 was produced in the same manner as in Example 4 except that 2,9-bis[2-(9,9-diemethylfluorenyl)]phenanthroline was used in the electron transport layer 6 and Exemplary Compound No. 15 shown previously was vacuum-deposited to form the luminescent layer 3 .
- An organic luminescent device was produced in the same manner as in Example 8 except that Exemplary Compound No. 19 shown previously was vacuum-deposited to form the luminescent layer 3 .
- An organic luminescent device was produced in the same manner as in Example 8 except that Exemplary Compound No. 6 shown previously was vacuum-deposited to form the luminescent layer 3 .
- An organic luminescent device having the structure shown in FIG. 3 was produced in the same manner as in Example 7 except that Exemplary Compound No. 1 and Exemplary Compound No. 36 were vacuum-deposited together (weight ratio: 15:100) to from the luminescent layer 3 .
- Example 8 To the device produced in Example 8, a voltage was applied for 100 hours in an atmosphere of nitrogen, keeping current density at 3.0 mA/cm 2 . As a result, the initial luminance 290 cd/m 2 came to be 270 cd/m 2 after 100 hours, showing small luminance deterioration.
- Example 14 To the device produced in Example 14, a voltage was applied for 100 hours in an atmosphere of nitrogen, keeping current density at 3.0 mA/cm 2 . As a result, the initial luminance 280 cd/m 2 came to be 270 cd/m 2 after 100 hours, showing small luminance deterioration.
- An organic luminescent device was produced in the same manner as in Example 8 except that the following unsubstituted comparative compound was used to form the luminescent layer 3 .
- the anthryl derivative group substituted compounds represented by the general formulas [1] and [2] have been developed on the basis of such designing precept as stated previously.
- the organic luminescent device making use of the material of the present invention has afforded high-efficiency luminescence at a low applied voltage. Also, by the replacement of substituents, various luminescence colors are obtainable with ease and superior durability is also obtainable.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Organic Chemistry (AREA)
- Electroluminescent Light Sources (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
An anthryl derivative group substituted compound having a specific chemical structure is provided. This compound is used for producing an organic luminescent device which can emit luminescent hues with a very high purity and effect light emission with a high luminescence efficiency and a long lifetime.
Description
1. Field of the Invention
This invention relates to an organic luminescent device, and more particularly to a device which emits light upon application of an electric field to a thin film formed of an organic compound.
2. Related Background Art
The organic luminescent device is a device in which a thin film containing a fluorescent organic compound is held between an anode and a cathode, electrons and holes are injected from each electrode to produce excitons of the fluorescent organic compound, and the light is utilized that is emitted when the excitons return to the ground state.
In research made by Eastman Kodak Company in 1987 (Appl. Phys. Lett. 51, 913, 1987), a function-separated double-layer structure device is reported in which ITO (indium-tin-oxide) is used as an anode and an alloy of magnesium and silver is used as a cathode, where an aluminum quinolinol complex is used as an electron transporting material and a luminescent material, and a triphenylamine derivative is used as a hole transporting material, which device enables light of about 1,000 cd/m2 to be emitted under application of a voltage of about 10 V. Related patents may include U.S. Pat. Nos. 4,539,507, 4,720,432 and 4,885,211.
By changing types of the fluorescent organic compound, emission from ultlaviolet up to infrared light is possible, and various compounds are recently energetically researched. For example, they are disclosed in U.S. Pat. Nos. 5,151,629, 5,409,783 and 5,382,477, and Japanese Patent Application Laid-Open Nos. H02-247278, H03-255190, H05-202356, H09-202878 and H09-227576.
Besides the organic luminescent devices making use of low-molecular weight materials as mentioned above, an organic emission-device making use of a conjugated type high-molecular material is further reported by a group of members of Cambridge University (Nature, 347, 539, 1990). In this report, polyphenylene vinylene (PPV) is formed into a film by a coating system to ascertain luminescence in a single layer. Related patents of such a conjugated type high-molecular material may include U.S. Pat. Nos. 5,247,190, 5,514,878 and 5,672,678, and Japanese Patent Application Laid-Open Nos. H04-145192 and H05-247460.
A phosphorescent organic luminescent device making use of an iridium complex such as Ir(ppy)3 as a luminescent material (Appl. Phys. Lett. 75, 4, 1999) also has lately attracted attention, and a high emission efficiency is reported.
Recent advance in organic luminescent devices is noticeable, which are characterized by high luminance at a low applied voltage, variety of luminescence wavelengths, high-speed response, and thin-gage and light-weight, and hence they suggest possibility for extensive uses. However, there are many problems in durability such as changes over time in long-time use and deterioration due to oxygen-containing atmospheric gas or moisture. Where their application to full-color display and the like is taking into account, blue, green and red luminescence with much longer lifetime, higher conversion efficiency and higher color purity is required under the existing conditions.
As an example of materials containing an anthracene ring and organic luminescent devices, Japanese Patent Application Laid-Open No. H08-012600 discloses a phenyl anthracene derivative. This reports that a good organic film can be formed because of low crystallizability especially when used as a blue luminescent material or an electron transporting material. However, its luminescence efficiency and durability lifetime have been insufficient in practical use.
As another example, Japanese Patent Application Laid-Open Nos. H09-157643 and H10-072579 disclose an amino anthracene derivative and a diamino anthracene derivative, respectively. They report that green luminescence is obtained when used as a luminescent material. However, the device has a low luminescence efficiency, and also has been insufficient in practical use in regard to durability lifetime.
As still another example, Japanese Patent No. 3,008,897 discloses a device making use of a specific bianthryl compound as a luminescent material, which, however, has no disclosure in regard to luminescence efficiency and durability lifetime.
As a further example, Japanese Patent Application Laid-Open No. H11-008068 discloses a device making use of, as a luminescent material, a specific anthracene compound containing an olefin moiety, and reports that luminescence of from yellow light to red light is obtained. It, however, has been insufficient in practical use in regard to luminescence efficiency.
As a still further example, Japanese Patent Application Laid-Open No. 2001-284050 discloses a device having a luminescent medium layer which contains an anthracene derivative with a specific structure, an electron transporting compound and another luminescent compound. This reports that a red luminescent device improved in reliability is obtained. However, the device has an insufficient luminescence efficiency in practical use, and also it has been difficult to obtain blue luminescence because of device structure.
The present invention has been made in order to solve such problems the related background art has had. Accordingly, an object of the present invention is to provide an organic luminescent device which can emit luminescent hues with a very high purity, and effect light emission with high luminance at a high luminescence efficiency and a long lifetime, and also to provide an organic luminescent device which can be easily produced at relatively low cost.
The present inventors have conducted extensive studies in order to solve the above problems. As a result, they have accomplished the present invention.
That is, the present invention provides an anthryl derivative group substituted compound represented by the following general formula [1]:
In the general formula [1], a is an integer of 0 to 4, and b+c+d is an integer of (6−a) or less;
A represents a molecular unit including an aromatic ring, a condensed multiple ring or a heterocyclic ring, selected from the group consisting of a terphenyl ring, a naphthalene ring, a phenanthrene ring, a pyrene ring, a tetracene ring, a pentane ring, a perylene ring, a pyrrole ring, a pyridine ring, a terpyridine ring, a thiophene ring and a terthiophene ring which may be substituted or unsubstituted, and a fluorene ring;
Y1 and Y2 are each independently a group selected from the group consisting of an alkyl group, an aralkyl group, an aryl group and a heterocyclic group which are substituted or unsubstituted; Y1 and Y2 may be combined togehter to form a ring; and Y1's and Y2's in different anthryl derivative groups may be the same or different;
Z1 is a direct bond or a group selected from the group consisting of an arylene group and a divalent heterocyclic group which may be substituted or unsubstituted, and a divalent group having a bridging group, and may be the same or different when a is two or more;
Z2 and Z3 are each independently a direct bond or a group selected from the group consisting of an alkylene group, an alkenylene group, an alkynylene group, an aralkylene group and an arylene group which may be substituted or unsubstituted, and a divalent group having a bridging group, provided that Z2 and Z3 are not a direct bond where A is a fluorene ring; and when a is two or more, Z2 may be the same or different, and when b is two or more, Z3 may be the same or different;
X1 is a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group, an alkenyl group, an alkynyl group, an aralkyl group, an alkoxyl group and a sulfide group which may be substituted or unsubstituted, an aryl group, a heterocyclic group, and a substituted silyl group, and may be the same or different when b is two or more;
X2 is a group selected from the group consisting of an aryl group and a heterocyclic group which may be substituted or unsubstituted, and may be the same or different when c is two or more; and
R1's and R2's are each independently a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group, an aryl group, an alkoxyl group and an amino group which may be substituted or unsubstituted; and R3 is a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group and an alkoxyl group which may be substituted or unsubstituted.
The present invention also provides an anthryl derivative group substituted compound represented by the following general formula [2]:
In the general formula [2], e is an integer of 0 to 4, and f+g+H is an integer of (6−e) or less, provided that e+f is 2 to 5, and h+g is ≧1 when e+f is 2;
Y3 and Y4 are each independently a group selected from the group consisting of an alkyl group, an aralkyl group, an aryl group and a heterocyclic group which may be substituted or unsubstituted, and a divalent group having a bridging group; Y3 and Y4 may be combined together to form a ring; and Y3's and Y4's in different anthryl derivative groups may be the same or different;
Z4 is a direct bond, or a group selected from the group consisting of an arylene group and a divalent heterocyclic group which may be substituted or unsubstituted, and a divalent group having a bridging group, and may be the same or different when e is two or more;
Z5 and Z6 are each independently a direct bond or a group selected from the group consisting of an alkylene group, an alkenylene group, an alkynylene group, an aralkylene group and an arylene group which are substituted or unsubstituted, and a divalent group having a bridging group; and when e is two or more, Z5 may be the same or different, and when f is two or more, Z6 may be the same or different;
X3 is a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group, an alkenyl group, an alkynyl group, an aralkyl group, an alkoxyl group and a sulfide group which may be substituted or unsubstituted, an aryl group, a heterocyclic group, and a substituted silyl group, and may be the same or different when f is two or more;
X4 is a group selected from the group consisting of an aryl group and a heterocyclic group which may be substituted or unsubstituted, and may be the same or different when g is two or more; and
R4's and R5's are each independently a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group, an aryl group, an alkoxyl group and an amino group which may be substituted or unsubstituted; and R6 is a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group and an alkoxyl group which may be substituted or unsubstituted, and may be the same or different when h is two or more.
The present invention is an anthryl derivative group substituted compound represented by the following general formula [1] or [2]:
A represents a molecular unit containing an aromatic ring, a condensed multiple ring or a heterocyclic ring, selected from the group consisting of a terphenyl ring, a naphthalene ring, a phenanthrene ring, a pyrene ring, a tetracene ring, a pentane ring, a perylene ring, a pyrrole ring, a pyridine ring, a terpyridine ring, a thiophene ring and a terthiophene ring which are connected to anthracene rings via or not via bridging groups Z2 and Z3 and are substituted or unsubstituted, and a fluorene ring which is connected to anthracene rings via bridging groups Z2 and Z3;
Y1 and Y2 are each a group selected from the group consisting of an alkyl group, an aralkyl group, an aryl group and a heterocyclic group which are substituted or unsubstituted and may be the same or different; Y1 and Y2 may be combined together to form a ring; and Y1's and Y2's in different anthryl derivative groups may be the same or different;
Z1 is a direct bond or a group selected from the group consisting of an arylene group and a divalent heterocyclic group which are substituted or unsubstituted, and a divalent group having a bridging group, and Z1's in different anthryl derivative groups may be the same or different;
Z2 and Z3 are each a direct bond or a group selected from the group consisting of an alkylene group, an alkenylene group, an alkynylene group, an aralkylene group and an arylene group which are substituted or unsubstituted, and a divalent group having a bridging group; and when a is two or more, Z2 may be the same or different, and when b is two or more, Z3 may be the same or different;
X1 is a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group, an alkenyl group, an alkynyl group, an aralkyl group, an alkoxyl group and a sulfide group which are substituted or unsubstituted, an aryl group, a heterocyclic group, and a substituted silyl group, and may be the same or different when b is two or more;
X2 is a group selected from the group consisting of an aryl group and a heterocyclic group which are substituted or unsubstituted, and may be the same or different when c is two or more; and
R1's and R2's are each independently a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group, an aryl group, an alkoxyl group and an amino group which are substituted or unsubstituted; and R3 is a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group and an alkoxyl group which are substituted or unsubstituted, and may be the same or different when d is two or more.
In the general formula [2], e is 0 to 4, and f+g+h is an integer of (6−e) or less, provided that e+f is 2 to 5, and h+g is ≧1 when e+f is 2;
Y3 and Y4 are each independently a group selected from the group consisting of an alkyl group, an aralkyl group, an aryl group and a heterocyclic group which may be substituted or unsubstituted, and a divalent group having a bridging group; Y3 and Y4 may be combined together to form a ring; and Y3's and Y4's in different anthryl derivative groups may be the same or different;
Z4 is a direct bond-or a group selected from the group consisting of an arylene group and a divalent heterocyclic group which are substituted or unsubstituted, and a divalent group having a bridging group, and may be the same or different when e is two or more;
Z5 and Z6 are each independently a direct bond or a group selected from the group consisting of an alkylene group, an alkenylene group, an alkynylene group, an aralkylene group and an arylene group which are substituted or unsubstituted, and a divalent group having a bridging group; and when e is two or more, Z5 may be the same or different, and when f is two or more, Z6 may be the same or different;
X3 is a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group, an alkenyl group, an alkynyl group, an aralkyl group, an alkoxyl group and a sulfide group which are substituted or unsubstituted, an aryl group, a heterocyclic group, and a substituted silyl group, and may be the same or different when f is two or more;
X4 is a group selected from the group consisting of an aryl group and a heterocyclic group which are substituted or unsubstituted, and may be the same or different when g is two or more; and
R4's and R5's are each independently a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group, an aryl group, an alkoxyl group and an amino group which are substituted or unsubstituted; and R6 is a hydrogen atom, a deuterium atom, a halogen atom, or a group selected from the group consisting of an alkyl group and an alkoxyl group which are substituted or unsubstituted, and may be the same or different when h is two or more.
The compounds represented by the general formulas [1] and [2] may chiefly be used as materials for organic luminescent devices. In such use, when used as luminescent materials, they may each be used alone in a luminescent layer, and may be used for the purposes of dopant materials and host materials to obtain devices with high color purity, high luminescence efficiency and long lifetime.
The compounds represented by the general formulas [1] and [2] each have a core unit including an aromatic ring, a condensed multiple ring or a heterocyclic ring, the core unit being substituted with at least two anthryl groups as highly efficient luminescent units and each of the anthryl groups being substituted with an amino group, an amino group having a bridging group, an aryl group or the like. In the introduction of substituents, HOMO/LUMO levels of the material may be controlled by replacement of substituents, where blue, green and a luminescence color on a longer wavelength side can be obtained with ease (examples of calculation of HOMO/LUMO levels by B3LYP/3-21G: Exemplary Compound 15: −5.030/−1.877; Exemplary Compound 19: −4.805/−1.696). When used as dopant materials, changes in HOMO/LUMO levels by the replacement of substituents may be predicted by calculation, where the material can be designed and synthesized with ease according to the HOMO/LUMO levels of a host material. The same applies also when the compounds are used as host materials, and molecular designing is also easy while taking into account a difference in energy level between a hole transport layer and an electron transport layer. In the introduction of substituted amino groups, an improvement in hole transport performance can be expected. Substitution of the anthryl groups with amino groups or the like enables the compounds to have higher Tg (for example, Exemplary Compound 16 has Tg=215° C.) and affords a dendritic molecular shape of a starburst type, whereby a material having good film properties and heat stability can be obtained. Steric hindrance groups or fluorine atoms, the latter having so large electronegativity as to tend to cause electrostatic repulsion against adjacent molecules, may be introduced into the core molecule at the center, the anthryl groups or other aryl groups and the substituents on amino-groups, whereby it can be expected to restrain molecules from cohering to one another and especially improve the device lifetime. In addition to the foregoing consideration, with the materials of the present invention, considering that in virtue of an isotope effect, molecular vibration is controlled and thermal deactivation is inhibited, the idea of introducing molecular units substituted with deuterium has been incorporated in molecular designing. In the present invention, molecular designing has been made on the basis of such consideration, thus the present invention has been made.
When the compounds are used as dopant materials, they may each be in a dopant concentration of from 0.01% to 80%, and preferably from 1% to 40%, in respect to the host material. The dopant material may be contained uniformly, or with a concentration gradient, in the whole layer formed of the host material, or may partially be contained in some regions to form regions of a host material layer containing no dopant material.
The present invention is described below in detail.
Specific examples of the bridging groups, substituents and molecular unit A or core unit in the compounds represented by the general formulas [1] and [2] are shown below.
In the compounds represented by the general formulas [1] and [2], the bridging group may include, but is not limited to, a substituted or unsubstituted arylene group and a divalent heterocyclic ring group as shown later.
In the compounds represented by the general formulas [1] and [2], the divalent group having a bridging group may include, but is not limited to, an alkylene group, an alkenylene group, an alkynylene group, an aralkylene group and an amino group which are substituted or unsubstituted, a substituted silyl group, an ether group, a thioether group and a carbonyl group which have a bridging group.
The substituted or unsubstituted alkyl group may include, but is not limited to, a methyl group, a methyl-d1group, a methyl-d3 group, an ethyl group, an ethyl-d5 group, an n-propyl group, an n-butyl group, an n-pentyl group, an n-hexyl group, an n-heptyl group, an n-octyl group, an n-decyl group, an iso-propyl group, an iso-propyl-d7 group, an iso-butyl group, a sec-butyl group, a tert-butyl group, a tert-butyl-d9 group, an iso-pentyl group, a neopentyl group, a tert-octyl group, a fluoromethyl group, a difluoromethyl group, a trifluoromethyl group, a 2-fluoroethyl group, a 2,2,2-trifluoroethyl group, a perfluoroethyl group, a 3-fluoropropyl group, a perfluoropropyl group, a 4-fluorobutyl group, a perfluorobutyl group, a 5-fluoropentyl group, a 6-fluorohexyl group, a chloromethyl group, a trichloromethyl group, a 2-chloroethyl group, a 2,2,2-trichloroethyl group, a 4-chlorobutyl group, a 5-chloropentyl group, a 6-chlorohexyl group, a bromomethyl group, a 2-bromoethyl group, an iodomethyl group, a 2-iodoethyl group, a hydroxymethyl group, a hydroxyethyl group, a cyclopropyl group, a cyclobutyl group, a cyclopentyl group, a cyclohexyl group, a cyclopentylmethyl group, a cyclohexylmethyl group, a cyclohexylethyl group, a 4-fluorocyclohexyl group, a norbornyl group and an adamantyl group.
The substituted or unsubstituted aralkyl group may include, but is not limited to, a benzyl group, a 2-phenylethyl group, a 2-phenylisopropyl group, a 1-naphthylmethyl group, a 2-naphthylmethyl group, a 2-(1-naphthyl) ethyl group, a 2-(2-naphthyl) ethyl group, a 9-anthrylmethyl group, a 2-(9-anthryl)ethyl group, a 2-fluorobenzyl group, a 3-fluorobenzyl group, a 4-fluorobenzyl group, a 2-chlorobenzyl group, a 3-chlorobenzyl group, a 4-chlorobenzyl group, a 2-bromobenzyl group, a 3-bromobenzyl group and a 4-bromobenzyl group.
The substituted or unsubstituted alkenyl group may include, but is not limited to, a vinyl group, an allyl group, a 2-propenyl group, a 1-propenyl group, an iso-propenyl group, a 1-butenyl group, a 2-butenyl group, a 3-butenyl group and a styryl group.
The substituted or unsubstituted alkynyl group may include, but of course is not limited to, an acetylenyl group, a phenylacetylenyl group and a 1-propynyl group.
The substituted or unsubstituted aryl group may include, but is not limited to, a phenyl group, a phenyl-d5 group, a 4-methylphenyl group, a 4-methoxylphenyl group, a 4-ethylphenyl group, a 4-fluorophenyl group, a 4-trifluorophenyl group, a 3,5-dimethylphenyl group, a 2,6-diethylphenyl group, a mesityl group, a 4-tert-butylphenyl group, a ditolylaminophenyl group, a biphenyl group, a terphenyl group, a 1-naphthyl group, a 1-naphthyl-d7 group, a 2-naphthyl-d7 group, a 1-anthryl group, a 2-anthryl group, a 9-anthryl group, a 9-anthryl-d9 group, a 2-phenanthryl group, a 3-phenanthryl group, a 4-phenanthryl group, a 9-phenanthryl group, a 9-phenanthryl-d9 group, a 1-pyrenyl group, a 1-pyrenyl-d9 group, a 2-pyrenyl group, a 4-pyrenyl group, a tetracenyl group, a pentacenyl group, a fluorenyl group, a triphenylenyl group and a perilenyl group.
The substituted or unsubstituted heterocyclic group may include, but is not limited to, a pyrrolyl group, a pyridyl group, a pyridyl-d5 group, a bipyridyl group, a methylpyridyl group, a terpyrrolyl group, a thienyl group, a thienyl-d4 group, a terthienyl group, a propylthienyl group, a furyl group, a furyl-d4 group, an indolyl group, a 1,10-phenanthrolyl group, a phenazinyl group, a quinolyl group, a carbazolyl group, an oxazolyl group, an oxadiazolyl group, a thiazolyl group and a thiadiazolyl group.
The substituted or unsubstituted alkylene group may include, but is not limited to, a methylene group, a methylene-d2 group, a difluoromethylene group, an ethylene group, an ethylene-d4 group, a perfluoroethylene group, a propylene group, an iso-propylene group, a butylene group and a 2,2-dimethylpropylene group.
The substituted or unsubstituted aralkylene group may include, but is not limited to, a benzylene group, a 2-phenylethylene group, a 2-phenylisopropylene group, a 1-napthylmethylene group, a 2-napthylmethylene group, a 9-anthrylmethylene group, a 2-fluorobenzylene group, a 3-fluorobenzylene group, a 4-fluorobenzylene group, a 4-chlorobenzylene group and a 4-bromobenzylene group.
The substituted or unsubstituted alkenyl group may include, but of course is not limited to, a vinylene group, an iso-propenylene group, a styrylene group and a 1,2-diphenylvinylene group.
The substituted or unsubstituted alkynyl group may include, but of course is not limited to, an acetylenylene group and a 1,2-phenylacetylenylene group.
The substituted or unsubstituted arylene group may include, but is not limited to, a phenylene group, a biphenylene group, a tetrafluorophenylene group, a dimethylphenylene group, a naphthylene group, an anthrylene group, a phenanthrylene group, a pyrenylene group, a tetracenylene group, a pentacenylene group and a perylenylene group.
The substituted or unsubstituted divalent heterocyclic group may include, but is not limited to, a furylene group, a pyrrolylene group, a pyridylene group, a terpyridylene group, a thienylene group, a terthienylene group, an oxazolylene group, a thiazolylene group and a carbazolylene group.
The substituted or unsubstituted amino group (—NR′R″) may be a group in which R′ and R″ each represent a hydrogen atom, a deuterium atom, any of an alkylene group, an alkenylene group, an alkynylene group, an aralkylene group and an amino group each having a bridging group consisting of the above substituted or unsubstituted alkyl group, aralkyl group, aryl group or heterocyclic group or the substituted or unsubstituted arylene group or divalent heterocyclic group, a substituted silyl group, an ether group, a thioether group, or a carbonyl group, and may include, but is not limited to, e.g., an amino group, an N-methylamino group, an N-ethylamino group, an N,N-dimethylamino group, an N,N-diethylamino group, an N-methyl-N-ethylamino group, an N-benzylamino group, an N-methyl-N-benzylamino group, an N,N-dibenzylamino group, an anilino group, an N,N-diphenylamino group, an N-phenyl-N-tolylamino group, an N,N-ditolylamino group, an N-methyl-N-phenylamino group, an N,N-dianisolylamino group, an N-mesityl-N-phenylamino group, an N,N-dimethylamino group, an N-phenyl-N-(4-tert-butylphenyl)amino group and an N-phenyl-N-(4-trifluoromethylphenyl)amino group.
The substituted or unsubstituted alkoxyl group may include an alkyloxyl group and an aralkyloxyl group each having the above substituted or unsubstituted alkyl group or aralkyl group, or an aryloxyl group having the above substituted or unsubstituted aryl group or heterocyclic group, and may include, but is not limited to, e.g., a methoxyl group, an ethoxyl group, a propoxyl group, a 2-ethyl-octyloxyl group, a phenoxyl group, a 4-tert-butylphenoxyl group, a benzyloxyl group and a thienyloxyl group.
The substituted or unsubstituted sulfide group may include an alkyl sulfide group and an aralkyl sulfide group each having the above substituted or unsubstituted alkyl group or aralkyl group, or an aryl sulfide group having the above substituted or unsubstituted aryl group or heterocyclic group, and may include, but is not limited to, e.g., a methyl sulfide group, an ethyl sulfide group, a phenyl sulfide group and a 4-methylphenyl sulfide group.
The molecular unit A may include, but is not limited to, a benzene ring, a biphenyl ring, a terphenyl ring, a naphthalene ring, a phenanthrene ring, an anthracene ring, a tetracene ring, a benzanthracene ring, a chrysene ring, a pyrene ring, a perylene ring, a choranthlene ring, a triphenylene ring, a thiophene ring, a pyridine ring, a pyrazine ring, a pyrazole ring, a pyrrole ring, a terthiophene ring, a terpyridine ring, a terpyrrole ring, a triazine ring, a carbazole ring, a benzimidazole ring, a benzothiazole ring, a quinoline ring, a quinoxaline ring, a quinazoline ring, a phthalazine ring, a diphenylmethane ring, tetraphenylsilane, tetraphenylgermane and tetraphenylmethane which contain deuterium and are substituted or unsubstituted.
Substituents the above substituents, bridging groups and molecular unit A may further have may include, but is not limited to, a deuterium atom, an alkyl group such as a methyl group, an ethyl group, an n-propyl group, an n-butyl-group, an n-pentyl group, an n-hexyl group, an n-heptyl group, an n-octyl group, an n-decyl group, an iso-propyl group, an iso-butyl group, a sec-butyl group, a tert-butyl group, an iso-pentyl group, a neopentyl group, a tert-octyl group, a benzyl group or a 2-phenylethyl group; an aralkyl group; an alkoxyl group such as a methoxyl group, an ethoxyl group, a propoxyl group, a 2-ethyl-octyloxyl group, a phenoxyl group, a 4-tert-butylphenoxyl group or a benzyloxyl group; an aryl group such as a phenyl group, a 4-methylphenyl group, a 4-ethylphenyl group, a 3-chlorphenyl group, a 3,5-dimethylphenyl group, a triphenylamino group, a biphenyl group, a terphenyl group, a naphtyl group, an anthryl group, a phenanthryl group or a pyrenyl group; a heterocyclic group such as a pyridyl group, a bipyridyl group, a methylpyridyl group, a thienyl group, a terthienyl group, a propylthienyl group, a furyl group, a quinolyl group, a carbazolyl group or an N-ethylcarbazolyl group; a halogen atom, a hydroxyl group, a cyano group and a nitro group.
In the compounds represented by the general formulas [1] and [2], it is preferable that at least one of Y1, Y2, Z1 to Z3, X1, X2 and R1 to R3 is a group containing a deuterium atom, or a deuterium atom and at least one of Y3, Y4, Z4 to Z6, X3, X4 and R4 to R6 is a group containing a deuterium atom, or a deuterium atom.
Typical examples of the compounds represented by the general formulas [1] and [2] are shown below. However, it should be noted that examples are by no means limited to these.
Exemplary Compounds
The organic luminescent device of the present invention is described below in detail.
The organic luminescent device of the present invention is an organic luminescent device having at least a pair of electrodes at least one of which comprises a transparent or semitransparent anode or cathode, and one or more layers containing an organic compound, held between the electrodes (the anode and the cathode), wherein;
at least one layer of the layers containing an organic compound contains at least one compound of the anthryl derivative group substituted compounds represented by the general formulas [1] and [2].
Preferred examples of the organic luminescent device of the present invention are shown in FIGS. 1 to 5 . Here, reference numerals shown in the drawings denote the following:
1: a substrate;
2: an anode;
3: a luminescent layer;
4: a cathode;
5: a hole transport layer;
6: an electron transport layer;
7: a hole injection layer; and
8: a hole/exciton blocking layer.
Note that FIGS. 1 to 5 show quite basic constitutions to the end, and the constitutions of the organic luminescent device using the compound of the present invention are by no means limited thereto. For example, the device can have various constitutions such that insulating layers are provided at the interfaces between electrodes and organic layers, an adhesive layer or an interference layer is provided, and the hole transport layer is constituted of two layers having different ionization potentials.
The compounds represented by the general formulas [1] and [2] may be used in any of the forms shown in FIGS. 1 to 5 .
In particular, an organic layer making use of the compound of the present invention is useful as the luminescent layer, the electron transport layer or the hole transport layer. Also, a layer formed by vacuum deposition (vacuum evaporation) or solution coating can not easily cause crystallization and is superior in stability over time.
In the present invention, any of the anthryl derivative group substituted compounds represented by the general formulas [1] and [2] may be used as a constituent of, in particular, the luminescent layer, and may also optionally be used together with a hole transporting compound, a luminescent compound and/or an electron transporting compound which is/are known in the art.
Examples of such compounds are given below.
A hole injecting and transporting material is preferably one which facilitates the injection of holes from the anode and has mobility sufficient to transport the injected holes to the luminescent layer. Low-molecular and high-molecular materials having a hole injection and transport function may include, but is not limited to, triarylamine derivatives, phenylenediamine derivatives, triazole derivatives, oxadiazole derivatives, imidazole derivatives, pyrazoline derivatives, pyrazolone derivatives, oxazole derivatives, fluorenone derivatives, hydrazone derivatives, stilbene derivatives, phthalocyanine derivatives, porphyrin derivatives, poly(vinylcarbazole), poly(silylene), poly(thiophene), and other conductive high-molecular materials. Some specific examples of these are shown below.
Low-molecular Hole Injecting and Transporting Materials:
Materials concerned chiefly in luminescence function which are usable besides the anthryl derivative group substituted compounds represented by the general formulas [1] and [2] may include, but is not limited to, high-molecular derivatives such as polycyclic condensation aromatic compounds (e.g., naphthalene derivatives, phenanthrene derivatives, fluorene derivatives, pyrene derivatives, tetracene derivatives, coronene derivatives, chrysene derivatives, perylene derivatives, 9,10-diphenylanthracene derivatives, and rubrene), quinacridone derivatives, acridone derivatives, coumarine derivatives, pyrane derivatives, Nile Red, pyrazine derivatives, benzoimidazole derivatives, benzothiazole derivatives, benzoxazole derivatives, stilbene derivatives, organometallic complexes (e.g., organoaluminum complexes such as tris(8-quinolinolato)aluminum, and organoberyllium complexes), poly(phenylenevinylene) derivatives, poly(fluorene) derivatives, poly(phenylene) derivatives, poly(thienylenevinylene) derivatives, and poly(acetylene) derivatives. Some specific examples of these are shown below.
Low-molecular Luminescent Materials:
An electron injecting and transporting material may be selected from materials which facilitate the injection of electrons from the cathode and have a function to transport electrons to the luminescent layer, and may be selected taking into account a balance with the carrier mobility of the hole transporting material. Materials having the electron injection and transport function may include, but is not limited to, oxadiazole derivatives, oxazole derivatives, thiazole derivatives, thidiazole derivatives, pyrazine derivatives, triazole derivatives, triazine derivatives, perylene derivatives, quinoline derivatives, quinoxaline derivatives, fluorenone derivatives, anthrone derivatives, phenanthroline derivatives, and organometallic complexes. Some specific examples of these are shown below.
In the organic luminescent device of the present invention, the layers containing the anthryl derivative group substituted compounds represented by the general formulas [1] and [2] and the layers formed of other organic compounds are commonly formed of thin films produced by vacuum deposition, ionization deposition, sputtering, plasma deposition, or known coating (e.g., spin coating, dipping, casting, the LB (Langmuir Blodgett) process, or ink-jet printing) of a coating material prepared by dissolving materials in a suitable solvent. Especially when the films are formed by coating, the films may be formed using materials in combination-with a suitable binder resin.
The binder resin may be selected from extensive binding resins, and may include, but is not limited to, polyvinyl carbazole resins, polycarbonate resins, polyester resins, polyarylate resins, polystyrene resins, ABS resins, polybutadiene resins, polyurethane resins, acrylic resins, methacrylic resins, butyral resins, polyvinyl acetal resins, polyamide resins, polyimide resins, polyethylene resins, polyether sulfone resins, diallyl phthalate resins, phenol resins, epoxy resins, silicone resins, polysulfone resins, and urea resins. These may also be used alone as homopolymers or in a mixture of two or more types as copolymers. Additives such as known plasticizers, antioxidants and ultraviolet absorbers may further optionally be used in a combination of two or more types.
Materials for the anode having a work function as large as possible are favorable, and usable are, e.g., metals themselves such as gold, platinum, silver, copper, nickel, palladium, cobalt, selenium, vanadium and tungsten, or alloys of any of these; and metal oxides such as tin oxide, zinc oxide, indium oxide, indium-tin oxide (ITO), and indium-zinc oxide. Also usable are conductive polymers such as polyaniline, polypyrrole, polythiophene, and polyphenylene sulfide. Any of these electrode materials may be used alone or may be used in combination. Also, the anode may be constituted of a single layer or may be constituted of a multiple layer.
On the other hand, materials for the cathode having a small work function are favorable, and may be used in the form of, e.g., metals themselves such as lithium, sodium, potassium, calcium, magnesium, aluminum, indium, ruthenium, titanium, manganese, yttrium, silver, lead, tin, and chromium; or alloys of two or more of these, such as lithium-indium, sodium-potassium, magnesium-silver, aluminum-lithium, aluminum-magnesium, and magnesium-indium. Metal oxides such as indium-tin oxide (ITO) may also be used. Any of these electrode materials may be used alone or may be used in a combination of two or more types. Also, the cathode may be constituted of a single layer or may be constituted of a multiple layer.
It is also preferable that at least one of the anode and the cathode is transparent or semitransparent.
In the substrate used in the present invention, there are no particular limitations. There may be used opaque substrates such as substrate made of metal and substrates made of ceramic, and transparent substrates such as glass, quartz and plastic sheets. A color filter film, a fluorescent color conversion filter film, a dielectric reflecting film or the like may be applied to the substrate to control luminescence light.
On the device produced, a protective layer or a sealing layer may be provided in order to prevent contact with oxygen, moisture, etc. The protective layer may include diamond thin film; films of inorganic materials such as metal oxides and metal nitrides; films of high-molecular materials such as fluorine resins, polyparaxylene, polyethylene, silicone resins and polystyrene resins; and photocurable resins. Also, the device may be covered with glass, a gas-impermeable film, metal or the like and itself may be packed in a suitable sealing resin.
The present invention will be described below in greater detail by giving Examples. The present invention is by no means limited to these.
(Process of Producing Exemplary Compounds No. 15 and No. 34)
Synthesis of Intermediate (II):
In a stream of nitrogen, 25.8 g (82 mmol) of tribromobenzene and 109.5 g (0.491 moles) of anthracene-9-boronic acid were dissolved in a mixed solvent of 1 L of deaerated toluene and 500 ml of ethanol and were stirred, followed by dropwise adding an aqueous sodium carbonate solution prepared by dissolving 86.9 g of anhydrous sodium carbonate in 800 ml of water. In a stream of nitrogen, the resulting solution was stirred for 1 hour on an oil bath heated to 80° C., and thereafter 14.2 g (12.3 mmol) of tetrakis(triphenylphosphine)palladium was added, followed by heating and stirring for about 4 hours on an oil bath heated to 80° C. The resultant reaction solution was cooled to room temperature and further cooled to 5° C., and crystals precipitated were filtered off. The crystals were dissolved in a chlorobenzene-heptane mixed solvent with heating and purified by silica gel column chromatography (chlorobenzene:heptane=1:3) to produce 25 g of an intermediate (I).
A solution of 16.7 g (27.6 mmol) of the intermediate (I) in 300 ml of chloroform was cooled to 5° C., and 9.69 g (60.6 mmol) of bromine dissolved in 70 ml of chloroform was slowly dropwise added. After the dropwise addition, the resulting solution were stirred at room temperature for 2 hours, and 300 ml of methanol was further added, followed by stirring at 5° C. for 2 hours. The precipitate formed was filtered off and dispersedly washed with acetone. The resultant solution was cooled again to 5° C., and the precipitate was filtered to produce 21 g of an intermediate (II) as a mixture of a monobromo-product, a dibromo-product and a tribromo-product (HPCL analysis, UV; area ratio: monobromo-product:dibromo-product:tribromo-product=1:2.3:3.3).
Synthesis of Exemplary Compounds No. 15 and No. 34:
In an atmosphere of nitrogen, 361 mg (0.627 mmol) of palladium bis(benzylideneacetone) and 0.76 g (3.76 mmol) of tri-tert-butylphosphine were dissolved in 230 ml of xylene and stirred at room temperature for 1 hour. Then, 100 ml of xylene was further added, and thereafter, in a stream of nitrogen, 3 g (3.92 mmol, in terms of the dibromo-product) of the intermediate (II) was added and stirred for 5 minutes on an oil bath heated to 50° C. Then, 1.32 g (7.84 mmol) of diphenylamine dissolved in 50 ml of xylene was dropwise added and subsequently 1.13 g (11.8 mmol) of sodium tert-butoxide was added, followed by heating and stirring for about 6 hours on an oil bath heated to 130° C. The resultant reaction solution was cooled to room temperature, and 100 ml of water was added to separate a water layer and an organic layer. The water layer was extracted with toluene and ethyl acetate, and then combined with the previous organic layer, followed by drying over sodium sulfate. The solvent was distilled away, and the residue was subjected to separation and purification by silica gel column chromatography (toluene:hexane=1:3) to produce 1 g of Exemplary Compound No. 15 and 1.5 g of Exemplary Compound No. 34.
(Process of Producing Exemplary Compounds No. 21 and No. 39)
In a stream of nitrogen, 1.53 g (2 mmol, in terms of the dibromo-product) of the intermediate (II) and 1.90 g (6 mmol) of bis(4-methylphenyl)aminobenzene-4-boronic acid were dissolved in a mixed solvent of 180 ml of deaerated toluene and 90 ml of ethanol and were stirred, followed by dropwise adding an aqueous solution prepared by dissolving 0.98 g of anhydrous sodium carbonate in 30 ml of water. The resulting solution was stirred for 30 minutes, and thereafter 347 mg (0.3 mmol) of tetrakis(triphenylphosphine)palladium was added, followed by heating and stirring for about 5 hours on an oil bath heated to 80° C. The resultant reaction solution was cooled to room temperature, and thereafter 70 ml of water and 70 ml of ethyl acetate were added to separate a water layer and an organic layer. The water layer was extracted with toluene and ethyl acetate, and then combined with the previous organic layer, followed by drying with sodium magnesium. The solvent was distlled away, and the residue was purified by silica gel column chromatography (toluene:hexane=1:3) to produce 0.65 g of Exemplary Compound No. 21 and 0.7 g of Exemplary Compound No. 39.
(Process of Producing Exemplary Compound No. 40)
Synthesis of Intermediate (IV):
A solution of 3 g (3.74 mmol) of the intermediate (III) in 120 ml of chloroform was cooled to 5° C., and 1.2 g (7.48 mmol) of bromine dissolved in 30 ml of chloroform was slowly dropwise added. After the dropwise addition, the resulting solution was stirred at room temperature for 2 hours, and 120 ml of methanol was further added, followed by stirring at 5° C. for 2 hours. The precipitate formed was filtered off, and the precipitate filtered was dispersedly washed with acetone. The resultant solution was cooled again to 5° C., and the precipitate was filtered off to produce 2.9 g of an intermediate (IV).
Synthesis of Exemplary Compound No. 40:
In a stream of nitrogen, 2 g (2.08 mmol) of the intermediate (IV) and 1.10 g (6.42 mmol) of naphthalene-2-boronic acid were dissolved in a mixed solvent of 150 ml of deaerated toluene and 30 ml of ethanol and were stirred, followed by dropwise adding an aqueous solution prepared by dissolving 1.36 g of anhydrous sodium carbonate in 30 ml of water. The resulting solution was stirred for 30 minutes, and thereafter 0.22 g (0.19 mmol) of tetrakis(triphenylphosphine)palladium was added, followed by heating and stirring for about 7 hours on an oil bath heated to 80° C. The resultant reaction solution was cooled to room temperature, and thereafter 70 ml of water and 70 ml of ethyl acetate were added to separate a water layer and an organic layer. The water layer was extracted with toluene and ethyl acetate, and then combined with the previous organic layer, followed by drying over sodium magnesium. The solvent was distilled away, and the residue was purified by silica gel column chromatography (toluene:hexane=1:3) to produce 1.3 g of Exemplary Compound No. 40.
An organic luminescent device having the structure shown in FIG. 3 was produced in the, following way.
On a glass substrate as the substrate 1, an indium-tin oxide (ITO) film as the anode 2 was formed by sputtering in a layer thickness of 120 nm, and this was used as a transparent conductive support substrate. This substrate was subjected to ultrasonic cleaning with acetone and isopropyl alcohol (IPA) in order, and subsequently to boiling cleaning with IPA, followed by drying. The substrate was further subjected to UV/ozone cleaning, and was used as the transparent conductive support substrate.
Using as a hole transporting material a compound represented by the following structural formula, its chloroform solution was so prepared as to be in a concentration of 0.5% by weight.
This solution was dropped on the above ITO electrode, and spin-coating was carried out first at the number of revolutions of 500 rpm for 10 seconds and then at the number of revolutions of 1,000 rpm for 1 minute to form a film (thin film). Thereafter, the film was dried for 10 minutes in a 80° C. vacuum oven to completely remove the solvent remaining in the thin film. The hole transport layer 5 thus formed was in a thickness of 50 nm. Next, on the hole transport layer 5, the above Exemplary Compound No. 21 was vacuum-deposited to provide as the luminescent layer 3 a luminescent layer 3 of 20 nm in thickness. At the time of the vacuum deposition, the film was formed under the conditions of a degree of vacuum of 1.0×10−4 Pa and a rate of film formation of 0.2 to 0.3 nm/sec.
As the electron transport layer 6, a batho-phenanthroline (BPhen) film was formed by vacuum deposition in a layer thickness of 40 nm. At the time of the vacuum deposition, the film was formed under the conditions of a degree of vacuum of 1.0×10−4 Pa and a rate of film formation of 0.2 to 0.3 nm/sec.
Next, using a vacuum deposition material composed of an aluminum-lithium alloy (lithium concentration: 1 atom %), a metal layer film of 10 nm in thickness was formed by vacuum deposition, and an aluminum film of 150 nm in thickness was further formed by vacuum deposition to produce an organic luminescent device having an aluminum-lithium alloy film as an electron injection electrode (the cathode 4). At the time of the vacuum, deposition, the film was formed under the conditions of a degree of vacuum of 1.0×10−4 Pa and a rate of film formation of 1.0 to 1.2 nm/sec.
The organic luminescent device (organic EL device) thus obtained was covered with a protecting glass plate in a dry air atmosphere and sealed with an acrylic-resin adhesive so as not to cause device deterioration due to adsorption of moisture.
To the device thus obtained, a voltage was applied setting the ITO electrode (anode 2) as the positive pole and the Al—Li electrode (cathode 4) as the negative pole, where green luminescence of 320 cd/m2 in luminance and 8 lm/W in luminescence efficiency was observed at an applied voltage of 3 V.
The voltage was further applied for 100 hours in a nitrogen atmosphere, keeping current density at 3.0 mA/cm2. As a result, the initial luminance 300 cd/m2 came to be 280 cd/m2 after 100 hours, showing small luminance deterioration.
A device was produced in the same manner as in Example 4 except that a comparative compound shown below was used in place of Exemplary Compound No. 21. Evaluation was made in the same way. Green luminescence of 190 cd/m2 in luminance and 2 lm/W in luminescence efficiency was observed at an applied voltage of 3 V.
The voltage was further applied for 100 hours in a nitrogen atmosphere, keeping current density at 3.0 mA/cm2. As a result, the initial luminance 180 cd/m2 came to be 80 cd/m2 after 100 hours, showing a great luminance deterioration.
Devices were produced in the same manner as in Example 4 except that compounds shown in Table 1 were used in place of Exemplary-Compound No. 21. Evaluation was made in the same way. Results obtained are shown in Table 1.
| TABLE 1 | ||||
| Exemplary | Applied | Luminescence | ||
| Compound | voltage | Luminance | efficiency | |
| Example: | No. | (V) | (cd/m2) | (lm/W) |
| 5 | 1 | 3 | 245 | 4 |
| 6 | 7 | 3 | 260 | 5 |
| 7 | 29 | 3 | 300 | 7 |
An organic luminescent device having the structure shown in FIG. 3 was produced in the same manner as in Example 4 except that 2,9-bis[2-(9,9-diemethylfluorenyl)]phenanthroline was used in the electron transport layer 6 and Exemplary Compound No. 15 shown previously was vacuum-deposited to form the luminescent layer 3.
To the device thus obtained, a voltage was applied setting the ITO electrode (anode 2) as the positive pole and the Al—Li electrode (cathode 4) as the negative pole, where green luminescence of 300 cd/m2 in luminance and 7 lm/W in luminescence efficiency was observed at an applied voltage of 3 V.
An organic luminescent device was produced in the same manner as in Example 8 except that Exemplary Compound No. 19 shown previously was vacuum-deposited to form the luminescent layer 3.
To the device thus obtained, a voltage was applied setting the ITO electrode (anode 2) as the positive pole and the Al—Li electrode (cathode 4) as the negative pole, where blue-green luminescence of 250 cd/m2 in luminance and 4 lm/W in luminescence efficiency was observed at an applied voltage of 3 V.
An organic luminescent device was produced in the same manner as in Example 8 except that Exemplary Compound No. 6 shown previously was vacuum-deposited to form the luminescent layer 3.
To the device thus obtained, a voltage was applied setting the ITO electrode (anode 2) as the positive pole and the Al—Li electrode (cathode 4) as the negative pole, where orange luminescence of 220 cd/m2 in luminance and 3 lm/W in luminescence efficiency was observed at an applied voltage of 3 V.
Devices were produced in the same manner as in Example 8 except that compounds shown in Table 2 were used to form the luminescent layer 3. Evaluation was made in the same way. Results obtained are shown in Table 2.
| TABLE 2 | ||||
| Exemplary | Applied | Luminescence | ||
| Compound | voltage | Luminance | efficiency | |
| Example: | No. | (V) | (cd/m2) | (lm/W) |
| 11 | 5 | 3 | 280 | 7 |
| 12 | 16 | 3 | 380 | 10 |
| 13 | 17 | 3 | 345 | 8 |
| 14 | 23 | 3 | 285 | 7 |
| 15 | 40 | 3 | 250 | 5 |
| 16 | 51 | 3 | 270 | 6 |
An organic luminescent device having the structure shown in FIG. 3 was produced in the same manner as in Example 7 except that Exemplary Compound No. 1 and Exemplary Compound No. 36 were vacuum-deposited together (weight ratio: 15:100) to from the luminescent layer 3.
To the device thus obtained, a voltage was applied setting the ITO electrode (anode 2) as the positive pole and the Al—Li electrode (cathode 4) as the negative pole, where green luminescence of 225 cd/m2 in luminance and 5 lm/W in luminescence efficiency was observed at an applied voltage of 3 V.
To the device produced in Example 8, a voltage was applied for 100 hours in an atmosphere of nitrogen, keeping current density at 3.0 mA/cm2. As a result, the initial luminance 290 cd/m2 came to be 270 cd/m2 after 100 hours, showing small luminance deterioration.
To the device produced in Example 14, a voltage was applied for 100 hours in an atmosphere of nitrogen, keeping current density at 3.0 mA/cm2. As a result, the initial luminance 280 cd/m2 came to be 270 cd/m2 after 100 hours, showing small luminance deterioration.
An organic luminescent device was produced in the same manner as in Example 8 except that the following unsubstituted comparative compound was used to form the luminescent layer 3.
To the device thus obtained, a voltage was applied setting the ITO electrode 2 as the positive pole and the Al—Li electrode 4 as the negative pole, where blue weak luminescence of 240 cd/m2 in luminance and 0.2 lm/W in luminescence efficiency was observed at an applied voltage of 6 V.
As having been described above by giving embodiments and examples, the anthryl derivative group substituted compounds represented by the general formulas [1] and [2] have been developed on the basis of such designing precept as stated previously. The organic luminescent device making use of the material of the present invention has afforded high-efficiency luminescence at a low applied voltage. Also, by the replacement of substituents, various luminescence colors are obtainable with ease and superior durability is also obtainable.
Claims (3)
1. An anthryl derivative substituted compound represented by the following general formula [1]:
wherein:
Y1 and Y2 are each independently a group selected from the group consisting of a substituted or unsubstituted alkyl group, a substituted or unsubstituted aralkyl group, a substituted or unsubstituted aryl group and a substituted or unsubstituted heterocyclic group, or Y1 and Y2 may be combined together to form a ring, and Y1's and Y2's in different anthryl derivative groups may be the same or different;
X1 is a group selected from the group consisting of a hydrogen atom, a deuterium atom, a halogen atom, a substituted or unsubstituted alkyl group, a substituted or unsubstituted alkenyl group, a substituted or unsubstituted alkynyl group, a substituted or unsubstituted aralkyl group, a substituted or unsubstituted alkoxyl group, a substituted or unsubstituted sulfide group, a substituted or unsubstituted aryl group, a substituted or unsubstituted heterocyclic group, and a substituted silyl group; and
R1's and R2's are each independently a group selected from the group consisting of a hydrogen atom, a deuterium atom, a halogen atom, a substituted or unsubstituted alkyl group, a substituted or unsubstituted aryl group, a substituted or unsubstituted alkoxyl group, and a substituted or unsubstituted amino group.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/500,885 US7309533B2 (en) | 2003-06-27 | 2006-08-09 | Substituted anthryl derivative and electroluminescence device using the same |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2003-184261 | 2003-06-27 | ||
| JP2003184261A JP3848306B2 (en) | 2003-06-27 | 2003-06-27 | Anthryl derivative-substituted compound and organic light emitting device using the same |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/500,885 Division US7309533B2 (en) | 2003-06-27 | 2006-08-09 | Substituted anthryl derivative and electroluminescence device using the same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20040263067A1 US20040263067A1 (en) | 2004-12-30 |
| US7173131B2 true US7173131B2 (en) | 2007-02-06 |
Family
ID=33535370
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/875,241 Expired - Fee Related US7173131B2 (en) | 2003-06-27 | 2004-06-25 | Anthryl derivative group substituted compound, and organic luminescent device making use of same |
| US11/500,885 Expired - Fee Related US7309533B2 (en) | 2003-06-27 | 2006-08-09 | Substituted anthryl derivative and electroluminescence device using the same |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/500,885 Expired - Fee Related US7309533B2 (en) | 2003-06-27 | 2006-08-09 | Substituted anthryl derivative and electroluminescence device using the same |
Country Status (2)
| Country | Link |
|---|---|
| US (2) | US7173131B2 (en) |
| JP (1) | JP3848306B2 (en) |
Cited By (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060125378A1 (en) * | 2003-06-27 | 2006-06-15 | Canon Kabushiki Kaisha | Aminoanthryl derivative substitution compound and organic electroluminescence device using the same |
| US20070049779A1 (en) * | 2005-08-26 | 2007-03-01 | Canon Kabushiki Kaisha | Corannulene compound and organic light-emitting device |
| US20070207346A1 (en) * | 2006-03-02 | 2007-09-06 | Canon Kabushiki Kaisha | Organic light-emitting device |
| US20080124577A1 (en) * | 2006-04-24 | 2008-05-29 | Canon Kabushiki Kaisha | Compound and organic light-emitting element |
| US20080306303A1 (en) * | 2007-06-01 | 2008-12-11 | Vsevolod Rostovtsev | Chrysenes for deep blue luminescent applications |
| US20080303430A1 (en) * | 2007-06-01 | 2008-12-11 | Norman Herron | Blue luminescent materials |
| US20080303428A1 (en) * | 2007-06-01 | 2008-12-11 | Vsevolod Rostovtsev | Chrysenes for green luminescent applications |
| US20090033210A1 (en) * | 2006-04-20 | 2009-02-05 | Canon Kabushiki Kaisha | Compound and organic light emitting device |
| US20090091244A1 (en) * | 2005-12-20 | 2009-04-09 | Canon Kabushiki Kaisha | Amine compound, organic light-emitting device, and organic blue-light-emitting device |
| US20090162693A1 (en) * | 2006-12-28 | 2009-06-25 | Alex Sergey Ionkin | Tetra-substituted chrysenes for luminescent applications |
| US20100187983A1 (en) * | 2008-12-22 | 2010-07-29 | E. I. Du Pont De Nemours And Company | Deuterated compounds for luminescent applications |
| US20100187981A1 (en) * | 2008-12-19 | 2010-07-29 | E. I. Du Pont De Nemours And Company | Chrysene derivative host materials |
| US20100244665A1 (en) * | 2008-01-11 | 2010-09-30 | E. I. Du Pont De Nemours And Company | Substituted pyrenes and associated production methods for luminescent applications |
| WO2010114583A1 (en) * | 2009-04-03 | 2010-10-07 | E. I. Du Pont De Nemours And Company | Electroactive materials |
| WO2010128996A1 (en) * | 2009-05-07 | 2010-11-11 | E. I. Du Pont De Nemours And Company | Deuterated compounds for luminescent applications |
| WO2010099534A3 (en) * | 2009-02-27 | 2011-01-06 | E. I. Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| US20110017980A1 (en) * | 2009-07-27 | 2011-01-27 | E. I. Du Pont De Nemours And Company | Process and materials for making contained layers and devices made with same |
| US20110037381A1 (en) * | 2009-08-13 | 2011-02-17 | E. I. Du Pont De Nemours And Company | Chrysene derivative materials |
| US20110037056A1 (en) * | 2008-12-12 | 2011-02-17 | E. I. Du Pont De Nemours And Company | Photoactive composition and electronic device made with the composition |
| US20110037057A1 (en) * | 2009-02-27 | 2011-02-17 | E.I. Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| US20110095273A1 (en) * | 2009-09-29 | 2011-04-28 | E. I. Du Pont De Nemours And Company | Deuterated compounds for luminescent applications |
| US20110101312A1 (en) * | 2009-10-29 | 2011-05-05 | E. I. Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| US20110121269A1 (en) * | 2009-05-19 | 2011-05-26 | E.I. Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| US20110133632A1 (en) * | 2009-12-09 | 2011-06-09 | E.I. Du Pont De Nemours And Company | Deuterated compound as part of a combination of compounds for electronic applications |
| US20110215715A1 (en) * | 2008-11-19 | 2011-09-08 | E.I. Du Pont De Nemours And Company | Chrysene compounds for blue or green luminescent applications |
| US8257836B2 (en) | 2006-12-29 | 2012-09-04 | E I Du Pont De Nemours And Company | Di-substituted pyrenes for luminescent applications |
| US8263973B2 (en) | 2008-12-19 | 2012-09-11 | E I Du Pont De Nemours And Company | Anthracene compounds for luminescent applications |
| US8273468B2 (en) | 2007-06-01 | 2012-09-25 | E I Du Pont De Nemours And Company | Green luminescent materials |
| US8617720B2 (en) | 2009-12-21 | 2013-12-31 | E I Du Pont De Nemours And Company | Electroactive composition and electronic device made with the composition |
| US8648333B2 (en) | 2009-10-19 | 2014-02-11 | E I Du Pont De Nemours And Company | Triarylamine compounds for use in organic light-emitting diodes |
| US8937300B2 (en) | 2009-10-19 | 2015-01-20 | E I Du Pont De Nemours And Company | Triarylamine compounds for use in organic light-emitting diodes |
| US9133095B2 (en) | 2009-07-01 | 2015-09-15 | E I Du Pont De Nemours And Company | Chrysene compounds for luminescent applications |
| US9260657B2 (en) | 2009-05-19 | 2016-02-16 | E I Du Pont De Nemours And Company | Chrysene compounds for luminescent applications |
| US9293716B2 (en) | 2010-12-20 | 2016-03-22 | Ei Du Pont De Nemours And Company | Compositions for electronic applications |
Families Citing this family (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4585750B2 (en) * | 2002-08-27 | 2010-11-24 | キヤノン株式会社 | Fused polycyclic compound and organic light emitting device using the same |
| JP3848224B2 (en) | 2002-08-27 | 2006-11-22 | キヤノン株式会社 | Spiro compound and organic light emitting device using the same |
| JP4164317B2 (en) * | 2002-08-28 | 2008-10-15 | キヤノン株式会社 | Organic light emitting device |
| JP4311707B2 (en) * | 2002-08-28 | 2009-08-12 | キヤノン株式会社 | Organic light emitting device |
| JP4035482B2 (en) * | 2003-06-27 | 2008-01-23 | キヤノン株式会社 | Substituted anthryl derivative and organic light emitting device using the same |
| KR100560783B1 (en) * | 2003-09-03 | 2006-03-13 | 삼성에스디아이 주식회사 | Organic electroluminescent device having doped light emitting layer |
| JP4065547B2 (en) * | 2004-04-12 | 2008-03-26 | キヤノン株式会社 | Fluorene compound and organic light emitting device using the same |
| JP4599142B2 (en) * | 2004-11-26 | 2010-12-15 | キヤノン株式会社 | Organic light emitting device |
| JP4677221B2 (en) * | 2004-11-26 | 2011-04-27 | キヤノン株式会社 | Organic light emitting device |
| JP4955971B2 (en) * | 2004-11-26 | 2012-06-20 | キヤノン株式会社 | Aminoanthryl derivative-substituted pyrene compound and organic light-emitting device |
| CN101193842A (en) * | 2005-07-14 | 2008-06-04 | 出光兴产株式会社 | Biphenyl derivative, material for organic electroluminescent device, and organic electroluminescent device using same |
| JP5317386B2 (en) * | 2005-08-05 | 2013-10-16 | 出光興産株式会社 | Nitrogen-containing heterocyclic derivative and organic electroluminescence device using the same |
| CN1315764C (en) * | 2005-08-25 | 2007-05-16 | 复旦大学 | Conjugate derivative material of 9-phenyl-9-pyrenyl fluorene substituted pyrene its preparation method and application |
| DE102005058557A1 (en) * | 2005-12-08 | 2007-06-14 | Merck Patent Gmbh | Organic electroluminescent device |
| JP2007265763A (en) * | 2006-03-28 | 2007-10-11 | Canon Inc | Full-color organic electroluminescent panel |
| WO2007116828A1 (en) * | 2006-04-03 | 2007-10-18 | Idemitsu Kosan Co., Ltd. | Bisanthracene derivative and organic electroluminescent device using the same |
| KR100864154B1 (en) * | 2006-04-21 | 2008-10-16 | 주식회사 엘지화학 | Novel anthracene derivatives, preparation method thereof and organic electronic device using same |
| WO2008024378A2 (en) * | 2006-08-24 | 2008-02-28 | E. I. Du Pont De Nemours And Company | Hole transport polymers |
| US8465848B2 (en) * | 2006-12-29 | 2013-06-18 | E I Du Pont De Nemours And Company | Benzofluorenes for luminescent applications |
| CN101689467B (en) * | 2007-06-01 | 2012-10-03 | E.I.内穆尔杜邦公司 | Charge transport materials for luminescent applications |
| JP5153227B2 (en) * | 2007-06-26 | 2013-02-27 | キヤノン株式会社 | Organic light emitting device |
| JP5458516B2 (en) * | 2007-06-28 | 2014-04-02 | 三菱化学株式会社 | Anthracene compound, charge transport material for wet film formation, charge transport material composition for wet film formation, organic electroluminescence device, and organic EL display |
| JP2009076865A (en) * | 2007-08-29 | 2009-04-09 | Fujifilm Corp | Organic electroluminescence device |
| US8063399B2 (en) | 2007-11-19 | 2011-11-22 | E. I. Du Pont De Nemours And Company | Electroactive materials |
| US8343381B1 (en) | 2008-05-16 | 2013-01-01 | E I Du Pont De Nemours And Company | Hole transport composition |
| US8541113B2 (en) * | 2008-08-26 | 2013-09-24 | Sfc Co., Ltd. | Pyrene compounds and organic electroluminescent devices using the same |
| JP2012510540A (en) * | 2008-12-01 | 2012-05-10 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニー | Electroactive materials |
| JP2012510474A (en) * | 2008-12-01 | 2012-05-10 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニー | Electroactive materials |
| WO2010065700A2 (en) * | 2008-12-04 | 2010-06-10 | E. I. Du Pont De Nemours And Company | Electroactive materials |
| KR20120058602A (en) * | 2009-09-03 | 2012-06-07 | 이 아이 듀폰 디 네모아 앤드 캄파니 | Deuterated compounds for electronic applications |
| JP5335103B2 (en) * | 2010-01-26 | 2013-11-06 | 保土谷化学工業株式会社 | Compound having triphenylamine structure and organic electroluminescence device |
| TW201229010A (en) * | 2010-12-13 | 2012-07-16 | Du Pont | Electroactive materials |
| EP3812367B1 (en) | 2018-06-25 | 2024-10-23 | Hodogaya Chemical Co., Ltd. | Compound having triarylamine structure and electroluminescence device |
| CN113851588A (en) * | 2020-06-28 | 2021-12-28 | 冠能光电材料(深圳)有限责任公司 | Electroluminescent material and application thereof in organic light-emitting device |
| TW202222772A (en) * | 2020-10-16 | 2022-06-16 | 日商保土谷化學工業股份有限公司 | Arylamine compound, organic electroluminescent element, and electronic device |
Citations (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4539507A (en) | 1983-03-25 | 1985-09-03 | Eastman Kodak Company | Organic electroluminescent devices having improved power conversion efficiencies |
| US4720432A (en) | 1987-02-11 | 1988-01-19 | Eastman Kodak Company | Electroluminescent device with organic luminescent medium |
| US4885211A (en) | 1987-02-11 | 1989-12-05 | Eastman Kodak Company | Electroluminescent device with improved cathode |
| JPH02247278A (en) | 1989-03-20 | 1990-10-03 | Idemitsu Kosan Co Ltd | Electroluminescence element |
| JPH02282262A (en) | 1989-04-24 | 1990-11-19 | Kao Corp | Electrophotographic sensitive body |
| JPH038897A (en) | 1989-03-25 | 1991-01-16 | Lion Corp | Water-degradable and water-absorptive sheet-like form and production thereof |
| JPH03255190A (en) | 1990-01-22 | 1991-11-14 | Pioneer Electron Corp | Electroluminescent element |
| JPH03284160A (en) | 1990-03-29 | 1991-12-13 | Chodendo Hatsuden Kanren Kiki Zairyo Gijutsu Kenkyu Kumiai | Rotor of superconductive revolving armature |
| JPH04145192A (en) | 1990-10-04 | 1992-05-19 | Sumitomo Chem Co Ltd | organic electroluminescent device |
| US5130603A (en) | 1989-03-20 | 1992-07-14 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device |
| US5151629A (en) | 1991-08-01 | 1992-09-29 | Eastman Kodak Company | Blue emitting internal junction organic electroluminescent device (I) |
| JPH05202356A (en) | 1991-09-11 | 1993-08-10 | Pioneer Electron Corp | Organic electroluminescence element |
| US5247190A (en) | 1989-04-20 | 1993-09-21 | Cambridge Research And Innovation Limited | Electroluminescent devices |
| JPH05247460A (en) | 1991-12-05 | 1993-09-24 | Sumitomo Chem Co Ltd | Organic electroluminescent device |
| US5317169A (en) | 1990-02-23 | 1994-05-31 | Sumitomo Chemical Company, Limited | Organic electroluminescence device |
| US5382477A (en) | 1991-02-27 | 1995-01-17 | Sanyo Electric Co., Ltd. | Organic electroluminescent element and process for producing the same |
| US5409783A (en) | 1994-02-24 | 1995-04-25 | Eastman Kodak Company | Red-emitting organic electroluminescent device |
| JPH0812600A (en) | 1994-04-26 | 1996-01-16 | Tdk Corp | Phenylanthracene derivative and organic el element |
| US5514878A (en) | 1994-03-18 | 1996-05-07 | Holmes; Andrew B. | Polymers for electroluminescent devices |
| US5635308A (en) | 1994-04-26 | 1997-06-03 | Tdk Corporation | Phenylanthracene derivative and organic EL element |
| JPH09157643A (en) | 1995-12-11 | 1997-06-17 | Toyo Ink Mfg Co Ltd | Luminescent material for organic electroluminescent element and organic electroluminescent element using the same |
| JPH09202878A (en) | 1996-01-25 | 1997-08-05 | Nippon Telegr & Teleph Corp <Ntt> | Near ultraviolet or ultraviolet region light-emission element |
| JPH09227576A (en) | 1996-02-23 | 1997-09-02 | Sony Corp | Metallic multinuclear complex, its production and optical element |
| US5672678A (en) | 1990-08-24 | 1997-09-30 | Cambridge Display Technology Limited | Semiconductive copolymers for use in luminescent devices |
| JPH1072579A (en) | 1995-09-25 | 1998-03-17 | Toyo Ink Mfg Co Ltd | Luminescent material for organic electroluminescent element |
| US5759444A (en) | 1995-09-25 | 1998-06-02 | Toyo Ink Manufacturing Co., Ltd. | Light-emitting material for organic electroluminescence device, and organic electroluminescence device for which the light-emitting material is adapted |
| JPH10251633A (en) | 1997-03-17 | 1998-09-22 | Toyo Ink Mfg Co Ltd | Luminescent material for organic electroluminescent element and organic electroluminescent element made by using it |
| JPH118068A (en) | 1997-06-18 | 1999-01-12 | Toyo Ink Mfg Co Ltd | Organic electroluminescent element material and organic electroluminescent element using it |
| JPH11111460A (en) * | 1997-10-06 | 1999-04-23 | Toyo Ink Mfg Co Ltd | Organic electroluminescent element material and organic electroluminescent element using the same |
| JPH11228951A (en) | 1998-02-12 | 1999-08-24 | Nec Corp | Organic electroluminescent element |
| JP2000007604A (en) | 1998-06-18 | 2000-01-11 | Idemitsu Kosan Co Ltd | Distyrylarylene derivatives and organic electroluminescent devices |
| JP2001035668A (en) | 1999-07-19 | 2001-02-09 | Nec Corp | Organic electroluminescence element and its manufacture |
| JP2001240854A (en) | 2000-02-29 | 2001-09-04 | Ricoh Co Ltd | Organic luminescent material and organic luminescent element using the same organic luminescent material |
| JP2001284050A (en) | 2000-03-30 | 2001-10-12 | Idemitsu Kosan Co Ltd | Organic electroluminescence device and organic light emitting medium |
| WO2002047440A1 (en) | 2000-12-07 | 2002-06-13 | Canon Kabushiki Kaisha | Deuterated semiconducting organic compounds used for opto-electronic devices |
| US6660408B1 (en) * | 1998-05-22 | 2003-12-09 | Nec Corporation | Organic electroluminescent device |
| WO2004018588A1 (en) | 2002-07-19 | 2004-03-04 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent devices and organic luminescent medium |
| JP2004530527A (en) | 2001-07-02 | 2004-10-07 | サージクラフト リミテッド | Contractile and expandable device for insertion into the thoracic spine |
| US20040265632A1 (en) * | 2003-06-27 | 2004-12-30 | Canon Kabushiki Kaisha | Organic electroluminescent device |
| JP2005015419A (en) | 2003-06-27 | 2005-01-20 | Canon Inc | Aminoanthryl derivative-substituted compound and organic light-emitting device using the same |
| US7129386B2 (en) * | 2003-06-27 | 2006-10-31 | Canon Kabushiki Kaisha | Substituted anthryl derivative and electroluminescence device using the same |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5317769A (en) * | 1992-11-10 | 1994-06-07 | Hill-Rom Company, Inc. | Hospital bed |
| US6660405B2 (en) * | 2001-05-24 | 2003-12-09 | General Electric Co. | High temperature abradable coating for turbine shrouds without bucket tipping |
| JP4429149B2 (en) * | 2004-11-26 | 2010-03-10 | キヤノン株式会社 | Fluorene compound and organic light emitting device |
-
2003
- 2003-06-27 JP JP2003184261A patent/JP3848306B2/en not_active Expired - Fee Related
-
2004
- 2004-06-25 US US10/875,241 patent/US7173131B2/en not_active Expired - Fee Related
-
2006
- 2006-08-09 US US11/500,885 patent/US7309533B2/en not_active Expired - Fee Related
Patent Citations (47)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4539507A (en) | 1983-03-25 | 1985-09-03 | Eastman Kodak Company | Organic electroluminescent devices having improved power conversion efficiencies |
| US4720432A (en) | 1987-02-11 | 1988-01-19 | Eastman Kodak Company | Electroluminescent device with organic luminescent medium |
| US4885211A (en) | 1987-02-11 | 1989-12-05 | Eastman Kodak Company | Electroluminescent device with improved cathode |
| US5130603A (en) | 1989-03-20 | 1992-07-14 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device |
| JPH02247278A (en) | 1989-03-20 | 1990-10-03 | Idemitsu Kosan Co Ltd | Electroluminescence element |
| US6093864A (en) | 1989-03-20 | 2000-07-25 | Idemitsu Kosan Co., Ltd. | Aromatic dimethylidyne compounds |
| JPH038897A (en) | 1989-03-25 | 1991-01-16 | Lion Corp | Water-degradable and water-absorptive sheet-like form and production thereof |
| US5247190A (en) | 1989-04-20 | 1993-09-21 | Cambridge Research And Innovation Limited | Electroluminescent devices |
| JPH02282262A (en) | 1989-04-24 | 1990-11-19 | Kao Corp | Electrophotographic sensitive body |
| US5227252A (en) | 1990-01-22 | 1993-07-13 | Pioneer Electronic Corporation | Electroluminescent device |
| JPH03255190A (en) | 1990-01-22 | 1991-11-14 | Pioneer Electron Corp | Electroluminescent element |
| US5317169A (en) | 1990-02-23 | 1994-05-31 | Sumitomo Chemical Company, Limited | Organic electroluminescence device |
| US5726457A (en) | 1990-02-23 | 1998-03-10 | Sumitomo Chemical Company, Limited | Organic electroluminescence device |
| JPH03284160A (en) | 1990-03-29 | 1991-12-13 | Chodendo Hatsuden Kanren Kiki Zairyo Gijutsu Kenkyu Kumiai | Rotor of superconductive revolving armature |
| US5672678A (en) | 1990-08-24 | 1997-09-30 | Cambridge Display Technology Limited | Semiconductive copolymers for use in luminescent devices |
| JPH04145192A (en) | 1990-10-04 | 1992-05-19 | Sumitomo Chem Co Ltd | organic electroluminescent device |
| US5382477A (en) | 1991-02-27 | 1995-01-17 | Sanyo Electric Co., Ltd. | Organic electroluminescent element and process for producing the same |
| US5151629A (en) | 1991-08-01 | 1992-09-29 | Eastman Kodak Company | Blue emitting internal junction organic electroluminescent device (I) |
| JPH05202356A (en) | 1991-09-11 | 1993-08-10 | Pioneer Electron Corp | Organic electroluminescence element |
| JPH05247460A (en) | 1991-12-05 | 1993-09-24 | Sumitomo Chem Co Ltd | Organic electroluminescent device |
| US5409783A (en) | 1994-02-24 | 1995-04-25 | Eastman Kodak Company | Red-emitting organic electroluminescent device |
| US5514878A (en) | 1994-03-18 | 1996-05-07 | Holmes; Andrew B. | Polymers for electroluminescent devices |
| JPH0812600A (en) | 1994-04-26 | 1996-01-16 | Tdk Corp | Phenylanthracene derivative and organic el element |
| US5635308A (en) | 1994-04-26 | 1997-06-03 | Tdk Corporation | Phenylanthracene derivative and organic EL element |
| JPH1072579A (en) | 1995-09-25 | 1998-03-17 | Toyo Ink Mfg Co Ltd | Luminescent material for organic electroluminescent element |
| US5759444A (en) | 1995-09-25 | 1998-06-02 | Toyo Ink Manufacturing Co., Ltd. | Light-emitting material for organic electroluminescence device, and organic electroluminescence device for which the light-emitting material is adapted |
| JPH09157643A (en) | 1995-12-11 | 1997-06-17 | Toyo Ink Mfg Co Ltd | Luminescent material for organic electroluminescent element and organic electroluminescent element using the same |
| JPH09202878A (en) | 1996-01-25 | 1997-08-05 | Nippon Telegr & Teleph Corp <Ntt> | Near ultraviolet or ultraviolet region light-emission element |
| JPH09227576A (en) | 1996-02-23 | 1997-09-02 | Sony Corp | Metallic multinuclear complex, its production and optical element |
| JPH10251633A (en) | 1997-03-17 | 1998-09-22 | Toyo Ink Mfg Co Ltd | Luminescent material for organic electroluminescent element and organic electroluminescent element made by using it |
| JPH118068A (en) | 1997-06-18 | 1999-01-12 | Toyo Ink Mfg Co Ltd | Organic electroluminescent element material and organic electroluminescent element using it |
| JPH11111460A (en) * | 1997-10-06 | 1999-04-23 | Toyo Ink Mfg Co Ltd | Organic electroluminescent element material and organic electroluminescent element using the same |
| JPH11228951A (en) | 1998-02-12 | 1999-08-24 | Nec Corp | Organic electroluminescent element |
| US6660408B1 (en) * | 1998-05-22 | 2003-12-09 | Nec Corporation | Organic electroluminescent device |
| JP2000007604A (en) | 1998-06-18 | 2000-01-11 | Idemitsu Kosan Co Ltd | Distyrylarylene derivatives and organic electroluminescent devices |
| JP2001035668A (en) | 1999-07-19 | 2001-02-09 | Nec Corp | Organic electroluminescence element and its manufacture |
| JP2001240854A (en) | 2000-02-29 | 2001-09-04 | Ricoh Co Ltd | Organic luminescent material and organic luminescent element using the same organic luminescent material |
| US6713192B2 (en) | 2000-03-30 | 2004-03-30 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device and organic light emitting medium |
| JP2001284050A (en) | 2000-03-30 | 2001-10-12 | Idemitsu Kosan Co Ltd | Organic electroluminescence device and organic light emitting medium |
| US20020048688A1 (en) | 2000-03-30 | 2002-04-25 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device and organic light emitting medium |
| WO2002047440A1 (en) | 2000-12-07 | 2002-06-13 | Canon Kabushiki Kaisha | Deuterated semiconducting organic compounds used for opto-electronic devices |
| JP2004530527A (en) | 2001-07-02 | 2004-10-07 | サージクラフト リミテッド | Contractile and expandable device for insertion into the thoracic spine |
| WO2004018588A1 (en) | 2002-07-19 | 2004-03-04 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent devices and organic luminescent medium |
| US20040265632A1 (en) * | 2003-06-27 | 2004-12-30 | Canon Kabushiki Kaisha | Organic electroluminescent device |
| JP2005015419A (en) | 2003-06-27 | 2005-01-20 | Canon Inc | Aminoanthryl derivative-substituted compound and organic light-emitting device using the same |
| US20060125378A1 (en) * | 2003-06-27 | 2006-06-15 | Canon Kabushiki Kaisha | Aminoanthryl derivative substitution compound and organic electroluminescence device using the same |
| US7129386B2 (en) * | 2003-06-27 | 2006-10-31 | Canon Kabushiki Kaisha | Substituted anthryl derivative and electroluminescence device using the same |
Non-Patent Citations (4)
| Title |
|---|
| Abstract of Japanese Patent 11-111460, Apr. 23, 1999. * |
| Baldo et al., "Very High-Efficiency Green Organic Light-Emitting Devices Based on Electrophosphorescence", Appl. Phys. Lett., vol. 75, No. 1, Jul. 5, 1999, pp. 4-6. |
| Burroughs et al., "Light-Emitting Diodes Based on Conjugated Polymers", Nature, vol. 347, Oct. 11, 1990, pp. 539-541. |
| Tang et al., "Organic Electroluminescent Diodes", Appl. Phys. Lett., vol. 51, No. 12, Sep. 21, 1987, pp. 913-915. |
Cited By (56)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060125378A1 (en) * | 2003-06-27 | 2006-06-15 | Canon Kabushiki Kaisha | Aminoanthryl derivative substitution compound and organic electroluminescence device using the same |
| US7375250B2 (en) * | 2003-06-27 | 2008-05-20 | Canon Kabushiki Kaisha | Aminoanthryl derivative substitution compound and organic electroluminescence device using the same |
| US20070049779A1 (en) * | 2005-08-26 | 2007-03-01 | Canon Kabushiki Kaisha | Corannulene compound and organic light-emitting device |
| US7794854B2 (en) | 2005-08-26 | 2010-09-14 | Canon Kabushiki Kaisha | Corannulene compound and organic light-emitting device |
| US20090091244A1 (en) * | 2005-12-20 | 2009-04-09 | Canon Kabushiki Kaisha | Amine compound, organic light-emitting device, and organic blue-light-emitting device |
| US20070207346A1 (en) * | 2006-03-02 | 2007-09-06 | Canon Kabushiki Kaisha | Organic light-emitting device |
| US7740958B2 (en) | 2006-03-02 | 2010-06-22 | Canon Kabushiki Kaisha | Organic light-emitting device |
| US8026664B2 (en) | 2006-04-20 | 2011-09-27 | Canon Kabushiki Kaisha | Compound and organic light emitting device |
| US20090033210A1 (en) * | 2006-04-20 | 2009-02-05 | Canon Kabushiki Kaisha | Compound and organic light emitting device |
| US20080124577A1 (en) * | 2006-04-24 | 2008-05-29 | Canon Kabushiki Kaisha | Compound and organic light-emitting element |
| US7932592B2 (en) | 2006-04-24 | 2011-04-26 | Canon Kabushiki Kaisha | Compound and organic light-emitting element |
| US8115378B2 (en) | 2006-12-28 | 2012-02-14 | E. I. Du Pont De Nemours And Company | Tetra-substituted chrysenes for luminescent applications |
| US20090162693A1 (en) * | 2006-12-28 | 2009-06-25 | Alex Sergey Ionkin | Tetra-substituted chrysenes for luminescent applications |
| US8257836B2 (en) | 2006-12-29 | 2012-09-04 | E I Du Pont De Nemours And Company | Di-substituted pyrenes for luminescent applications |
| US8158831B2 (en) | 2007-06-01 | 2012-04-17 | E I Du Pont De Nemours And Company | Chrysene compounds for deep blue luminescent applications |
| US20080303428A1 (en) * | 2007-06-01 | 2008-12-11 | Vsevolod Rostovtsev | Chrysenes for green luminescent applications |
| US20080303430A1 (en) * | 2007-06-01 | 2008-12-11 | Norman Herron | Blue luminescent materials |
| US8604247B2 (en) | 2007-06-01 | 2013-12-10 | E I Du Pont De Nemours And Company | Chrysenes for deep blue luminescent applications |
| US8273468B2 (en) | 2007-06-01 | 2012-09-25 | E I Du Pont De Nemours And Company | Green luminescent materials |
| US20080306303A1 (en) * | 2007-06-01 | 2008-12-11 | Vsevolod Rostovtsev | Chrysenes for deep blue luminescent applications |
| US20100244665A1 (en) * | 2008-01-11 | 2010-09-30 | E. I. Du Pont De Nemours And Company | Substituted pyrenes and associated production methods for luminescent applications |
| US8192848B2 (en) | 2008-01-11 | 2012-06-05 | E I Du Pont De Nemours And Company | Substituted pyrenes and associated production methods for luminescent applications |
| US8383251B2 (en) | 2008-01-11 | 2013-02-26 | E I Du Pont De Nemours And Company | Substituted pyrenes and associated production methods for luminescent applications |
| US20110215715A1 (en) * | 2008-11-19 | 2011-09-08 | E.I. Du Pont De Nemours And Company | Chrysene compounds for blue or green luminescent applications |
| US20110037056A1 (en) * | 2008-12-12 | 2011-02-17 | E. I. Du Pont De Nemours And Company | Photoactive composition and electronic device made with the composition |
| US8932733B2 (en) | 2008-12-19 | 2015-01-13 | E I Du Pont De Nemours And Company | Chrysene derivative host materials |
| US8263973B2 (en) | 2008-12-19 | 2012-09-11 | E I Du Pont De Nemours And Company | Anthracene compounds for luminescent applications |
| US20100187981A1 (en) * | 2008-12-19 | 2010-07-29 | E. I. Du Pont De Nemours And Company | Chrysene derivative host materials |
| US8531100B2 (en) | 2008-12-22 | 2013-09-10 | E I Du Pont De Nemours And Company | Deuterated compounds for luminescent applications |
| US20100187983A1 (en) * | 2008-12-22 | 2010-07-29 | E. I. Du Pont De Nemours And Company | Deuterated compounds for luminescent applications |
| US20110057173A1 (en) * | 2009-02-27 | 2011-03-10 | E. I. Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| US20110037057A1 (en) * | 2009-02-27 | 2011-02-17 | E.I. Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| WO2010099534A3 (en) * | 2009-02-27 | 2011-01-06 | E. I. Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| US8759818B2 (en) | 2009-02-27 | 2014-06-24 | E I Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| US8890131B2 (en) | 2009-02-27 | 2014-11-18 | E I Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| US8497495B2 (en) | 2009-04-03 | 2013-07-30 | E I Du Pont De Nemours And Company | Electroactive materials |
| WO2010114583A1 (en) * | 2009-04-03 | 2010-10-07 | E. I. Du Pont De Nemours And Company | Electroactive materials |
| US20100252819A1 (en) * | 2009-04-03 | 2010-10-07 | E. I. Du Pont De Nemours And Company | Electroactive materials |
| WO2010128996A1 (en) * | 2009-05-07 | 2010-11-11 | E. I. Du Pont De Nemours And Company | Deuterated compounds for luminescent applications |
| US9260657B2 (en) | 2009-05-19 | 2016-02-16 | E I Du Pont De Nemours And Company | Chrysene compounds for luminescent applications |
| US20110121269A1 (en) * | 2009-05-19 | 2011-05-26 | E.I. Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| CN102428158A (en) * | 2009-05-19 | 2012-04-25 | E.I.内穆尔杜邦公司 | Deuterated Compounds For Electronic Applications |
| US9133095B2 (en) | 2009-07-01 | 2015-09-15 | E I Du Pont De Nemours And Company | Chrysene compounds for luminescent applications |
| US8592239B2 (en) | 2009-07-27 | 2013-11-26 | E I Du Pont De Nemours And Company | Process and materials for making contained layers and devices made with same |
| US20110017980A1 (en) * | 2009-07-27 | 2011-01-27 | E. I. Du Pont De Nemours And Company | Process and materials for making contained layers and devices made with same |
| US8968883B2 (en) | 2009-08-13 | 2015-03-03 | E I Du Pont De Nemours And Company | Chrysene derivative materials |
| US20110037381A1 (en) * | 2009-08-13 | 2011-02-17 | E. I. Du Pont De Nemours And Company | Chrysene derivative materials |
| US20110095273A1 (en) * | 2009-09-29 | 2011-04-28 | E. I. Du Pont De Nemours And Company | Deuterated compounds for luminescent applications |
| US8431245B2 (en) | 2009-09-29 | 2013-04-30 | E. I. Du Pont De Nemours And Company | Deuterated compounds for luminescent applications |
| US8648333B2 (en) | 2009-10-19 | 2014-02-11 | E I Du Pont De Nemours And Company | Triarylamine compounds for use in organic light-emitting diodes |
| US8937300B2 (en) | 2009-10-19 | 2015-01-20 | E I Du Pont De Nemours And Company | Triarylamine compounds for use in organic light-emitting diodes |
| US20110101312A1 (en) * | 2009-10-29 | 2011-05-05 | E. I. Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| US9496506B2 (en) | 2009-10-29 | 2016-11-15 | E I Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| US20110133632A1 (en) * | 2009-12-09 | 2011-06-09 | E.I. Du Pont De Nemours And Company | Deuterated compound as part of a combination of compounds for electronic applications |
| US8617720B2 (en) | 2009-12-21 | 2013-12-31 | E I Du Pont De Nemours And Company | Electroactive composition and electronic device made with the composition |
| US9293716B2 (en) | 2010-12-20 | 2016-03-22 | Ei Du Pont De Nemours And Company | Compositions for electronic applications |
Also Published As
| Publication number | Publication date |
|---|---|
| JP3848306B2 (en) | 2006-11-22 |
| US7309533B2 (en) | 2007-12-18 |
| JP2005015418A (en) | 2005-01-20 |
| US20060267488A1 (en) | 2006-11-30 |
| US20040263067A1 (en) | 2004-12-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7173131B2 (en) | Anthryl derivative group substituted compound, and organic luminescent device making use of same | |
| US7129386B2 (en) | Substituted anthryl derivative and electroluminescence device using the same | |
| US7375250B2 (en) | Aminoanthryl derivative substitution compound and organic electroluminescence device using the same | |
| JP4429149B2 (en) | Fluorene compound and organic light emitting device | |
| US7977869B2 (en) | Organic luminescent device and benzo[k]fluoranthene compound | |
| JP4677221B2 (en) | Organic light emitting device | |
| US7491450B2 (en) | Organic electroluminescent device | |
| JP4955971B2 (en) | Aminoanthryl derivative-substituted pyrene compound and organic light-emitting device | |
| US8026664B2 (en) | Compound and organic light emitting device | |
| US7229702B2 (en) | Oligofluorenlylene compounds | |
| US7952269B2 (en) | Organic light-emitting device | |
| US8021766B2 (en) | Benzo(GHI)fluoranthene derivative and organic light emitting device using the same | |
| US7365198B2 (en) | Silyl compound, light emitting material, and organic light emitting device using the same | |
| JP2007186449A (en) | Compound substituted with aminobisanthryl-derived group and organic light-emitting element by using the same | |
| US20070155998A1 (en) | Fluorenylene compound and organic light-emitting device using same | |
| JP2006219392A (en) | Bisanthryl derived group-substituted compound and organic light emitting device | |
| US7740958B2 (en) | Organic light-emitting device |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: CANON KABUSHIKI KAISHA, JAPAN Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:SAITOH, AKIHITO;SUZUKI, KOICHI;SENOO, AKIHIRO;AND OTHERS;REEL/FRAME:015516/0566 Effective date: 20040615 |
|
| CC | Certificate of correction | ||
| FPAY | Fee payment |
Year of fee payment: 4 |
|
| REMI | Maintenance fee reminder mailed | ||
| LAPS | Lapse for failure to pay maintenance fees | ||
| STCH | Information on status: patent discontinuation |
Free format text: PATENT EXPIRED DUE TO NONPAYMENT OF MAINTENANCE FEES UNDER 37 CFR 1.362 |
|
| FP | Lapsed due to failure to pay maintenance fee |
Effective date: 20150206 |