US10835524B2 - Compositions for the treatment of pancreatic cancer and uses thereof - Google Patents
Compositions for the treatment of pancreatic cancer and uses thereof Download PDFInfo
- Publication number
- US10835524B2 US10835524B2 US15/737,545 US201615737545A US10835524B2 US 10835524 B2 US10835524 B2 US 10835524B2 US 201615737545 A US201615737545 A US 201615737545A US 10835524 B2 US10835524 B2 US 10835524B2
- Authority
- US
- United States
- Prior art keywords
- trifluoromethyl
- quinolin
- bis
- inhibitors
- ethanol
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active, expires
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 34
- 206010061902 Pancreatic neoplasm Diseases 0.000 title claims abstract description 14
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 title claims abstract description 14
- 201000002528 pancreatic cancer Diseases 0.000 title claims abstract description 14
- 208000008443 pancreatic carcinoma Diseases 0.000 title claims abstract description 14
- 238000011282 treatment Methods 0.000 title description 6
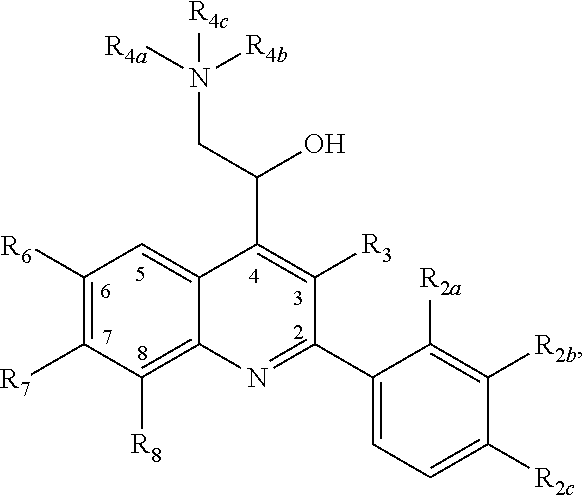
- KXYNLLGPBDUAHW-UHFFFAOYSA-N quinolin-4-ylmethanol Chemical class C1=CC=C2C(CO)=CC=NC2=C1 KXYNLLGPBDUAHW-UHFFFAOYSA-N 0.000 claims abstract description 52
- 238000000034 method Methods 0.000 claims abstract description 41
- 108091007960 PI3Ks Proteins 0.000 claims abstract description 32
- 108010065917 TOR Serine-Threonine Kinases Proteins 0.000 claims abstract description 32
- 102000013530 TOR Serine-Threonine Kinases Human genes 0.000 claims abstract description 32
- 239000003112 inhibitor Substances 0.000 claims abstract description 27
- 239000004480 active ingredient Substances 0.000 claims abstract description 6
- 108091008611 Protein Kinase B Proteins 0.000 claims abstract 4
- 102100033810 RAC-alpha serine/threonine-protein kinase Human genes 0.000 claims abstract 4
- 150000001875 compounds Chemical class 0.000 claims description 41
- 108090000430 Phosphatidylinositol 3-kinases Proteins 0.000 claims description 31
- 239000003197 protein kinase B inhibitor Substances 0.000 claims description 28
- 229940124302 mTOR inhibitor Drugs 0.000 claims description 27
- 239000003628 mammalian target of rapamycin inhibitor Substances 0.000 claims description 27
- 239000012828 PI3K inhibitor Substances 0.000 claims description 19
- 229940043441 phosphoinositide 3-kinase inhibitor Drugs 0.000 claims description 19
- XEEQGYMUWCZPDN-DOMZBBRYSA-N (-)-(11S,2'R)-erythro-mefloquine Chemical compound C([C@@H]1[C@@H](O)C=2C3=CC=CC(=C3N=C(C=2)C(F)(F)F)C(F)(F)F)CCCN1 XEEQGYMUWCZPDN-DOMZBBRYSA-N 0.000 claims description 18
- HKVAMNSJSFKALM-GKUWKFKPSA-N Everolimus Chemical compound C1C[C@@H](OCCO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 HKVAMNSJSFKALM-GKUWKFKPSA-N 0.000 claims description 18
- 229960005167 everolimus Drugs 0.000 claims description 18
- JOGKUKXHTYWRGZ-UHFFFAOYSA-N dactolisib Chemical compound O=C1N(C)C2=CN=C3C=CC(C=4C=C5C=CC=CC5=NC=4)=CC3=C2N1C1=CC=C(C(C)(C)C#N)C=C1 JOGKUKXHTYWRGZ-UHFFFAOYSA-N 0.000 claims description 17
- 229950006418 dactolisib Drugs 0.000 claims description 16
- 229960001962 mefloquine Drugs 0.000 claims description 16
- 229940124640 MK-2206 Drugs 0.000 claims description 15
- ULDXWLCXEDXJGE-UHFFFAOYSA-N MK-2206 Chemical compound C=1C=C(C=2C(=CC=3C=4N(C(NN=4)=O)C=CC=3N=2)C=2C=CC=CC=2)C=CC=1C1(N)CCC1 ULDXWLCXEDXJGE-UHFFFAOYSA-N 0.000 claims description 15
- LOUPRKONTZGTKE-WZBLMQSHSA-N Quinine Chemical compound C([C@H]([C@H](C1)C=C)C2)C[N@@]1[C@@H]2[C@H](O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-WZBLMQSHSA-N 0.000 claims description 14
- 102000009516 Protein Serine-Threonine Kinases Human genes 0.000 claims description 9
- 108010009341 Protein Serine-Threonine Kinases Proteins 0.000 claims description 9
- 229940126638 Akt inhibitor Drugs 0.000 claims description 8
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 claims description 7
- SXHYASJCSPRVCT-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(methylamino)ethanol Chemical compound C1=CC=C2C(C(O)CNC)=CC(C(F)(F)F)=NC2=C1C(F)(F)F SXHYASJCSPRVCT-UHFFFAOYSA-N 0.000 claims description 6
- 235000001258 Cinchona calisaya Nutrition 0.000 claims description 6
- LOUPRKONTZGTKE-UHFFFAOYSA-N cinchonine Natural products C1C(C(C2)C=C)CCN2C1C(O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-UHFFFAOYSA-N 0.000 claims description 6
- 229960003445 idelalisib Drugs 0.000 claims description 6
- PQLXHQMOHUQAKB-UHFFFAOYSA-N miltefosine Chemical compound CCCCCCCCCCCCCCCCOP([O-])(=O)OCC[N+](C)(C)C PQLXHQMOHUQAKB-UHFFFAOYSA-N 0.000 claims description 6
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 6
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 6
- 229960000948 quinine Drugs 0.000 claims description 6
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 claims description 6
- 229960002930 sirolimus Drugs 0.000 claims description 6
- GYLDXIAOMVERTK-UHFFFAOYSA-N 5-(4-amino-1-propan-2-yl-3-pyrazolo[3,4-d]pyrimidinyl)-1,3-benzoxazol-2-amine Chemical compound C12=C(N)N=CN=C2N(C(C)C)N=C1C1=CC=C(OC(N)=N2)C2=C1 GYLDXIAOMVERTK-UHFFFAOYSA-N 0.000 claims description 5
- 238000011260 co-administration Methods 0.000 claims description 5
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 claims description 5
- GRZXWCHAXNAUHY-NSISKUIASA-N (2S)-2-(4-chlorophenyl)-1-[4-[(5R,7R)-7-hydroxy-5-methyl-6,7-dihydro-5H-cyclopenta[d]pyrimidin-4-yl]-1-piperazinyl]-3-(propan-2-ylamino)-1-propanone Chemical compound C1([C@H](C(=O)N2CCN(CC2)C=2C=3[C@H](C)C[C@@H](O)C=3N=CN=2)CNC(C)C)=CC=C(Cl)C=C1 GRZXWCHAXNAUHY-NSISKUIASA-N 0.000 claims description 4
- STUWGJZDJHPWGZ-LBPRGKRZSA-N (2S)-N1-[4-methyl-5-[2-(1,1,1-trifluoro-2-methylpropan-2-yl)-4-pyridinyl]-2-thiazolyl]pyrrolidine-1,2-dicarboxamide Chemical compound S1C(C=2C=C(N=CC=2)C(C)(C)C(F)(F)F)=C(C)N=C1NC(=O)N1CCC[C@H]1C(N)=O STUWGJZDJHPWGZ-LBPRGKRZSA-N 0.000 claims description 4
- HBPXWEPKNBHKAX-NSHDSACASA-N (2S)-N1-[5-(2-tert-butyl-4-thiazolyl)-4-methyl-2-thiazolyl]pyrrolidine-1,2-dicarboxamide Chemical compound S1C(C=2N=C(SC=2)C(C)(C)C)=C(C)N=C1NC(=O)N1CCC[C@H]1C(N)=O HBPXWEPKNBHKAX-NSHDSACASA-N 0.000 claims description 4
- BKVZXESTLGCNPZ-NXCSSKFKSA-N (s)-[6,8-dichloro-2-(trifluoromethyl)quinolin-4-yl]-[(2s)-piperidin-2-yl]methanol;methanesulfonic acid Chemical compound CS(O)(=O)=O.C([C@H]1[C@@H](O)C=2C3=CC(Cl)=CC(Cl)=C3N=C(C=2)C(F)(F)F)CCCN1 BKVZXESTLGCNPZ-NXCSSKFKSA-N 0.000 claims description 4
- IUSVBXZZIYDCHO-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2,2,2-trifluoroethylamino)ethanol Chemical compound C1=CC=C2C(C(CNCC(F)(F)F)O)=CC(C(F)(F)F)=NC2=C1C(F)(F)F IUSVBXZZIYDCHO-UHFFFAOYSA-N 0.000 claims description 4
- VPFPNHVXCIERAP-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2-ethyl-4-methylimidazol-1-yl)ethanol Chemical compound CCC1=NC(C)=CN1CC(O)C1=CC(C(F)(F)F)=NC2=C(C(F)(F)F)C=CC=C12 VPFPNHVXCIERAP-UHFFFAOYSA-N 0.000 claims description 4
- XTYWUWAFQAZOHU-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2-fluoroethylamino)ethanol Chemical compound C1=CC=C2C(C(CNCCF)O)=CC(C(F)(F)F)=NC2=C1C(F)(F)F XTYWUWAFQAZOHU-UHFFFAOYSA-N 0.000 claims description 4
- VXHJCXNRDBFYPT-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2-hydroxyethylamino)ethanol Chemical compound C1=CC=C2C(C(O)CNCCO)=CC(C(F)(F)F)=NC2=C1C(F)(F)F VXHJCXNRDBFYPT-UHFFFAOYSA-N 0.000 claims description 4
- NJNILOUJUMZROM-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2-methoxyethylamino)ethanol Chemical compound C1=CC=C2C(C(O)CNCCOC)=CC(C(F)(F)F)=NC2=C1C(F)(F)F NJNILOUJUMZROM-UHFFFAOYSA-N 0.000 claims description 4
- CKQNYOZESJFGOP-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2-methylbenzimidazol-1-yl)ethanol Chemical compound C1=CC=C2C(C(O)CN3C4=CC=CC=C4N=C3C)=CC(C(F)(F)F)=NC2=C1C(F)(F)F CKQNYOZESJFGOP-UHFFFAOYSA-N 0.000 claims description 4
- RHNLVGHNUAPSRA-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2-methylpropylamino)ethanol Chemical compound C1=CC=C2C(C(O)CNCC(C)C)=CC(C(F)(F)F)=NC2=C1C(F)(F)F RHNLVGHNUAPSRA-UHFFFAOYSA-N 0.000 claims description 4
- HVQUFOSYNSVFOW-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2-phenylethylamino)ethanol Chemical compound C=1C(C(F)(F)F)=NC2=C(C(F)(F)F)C=CC=C2C=1C(O)CNCCC1=CC=CC=C1 HVQUFOSYNSVFOW-UHFFFAOYSA-N 0.000 claims description 4
- NGOKTPCVOHAXIR-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2-propan-2-ylimidazol-1-yl)ethanol Chemical compound CC(C)C1=NC=CN1CC(O)C1=CC(C(F)(F)F)=NC2=C(C(F)(F)F)C=CC=C12 NGOKTPCVOHAXIR-UHFFFAOYSA-N 0.000 claims description 4
- AQTJRCXTFQZPAD-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2-propylimidazol-1-yl)ethanol Chemical compound CCCC1=NC=CN1CC(O)C1=CC(C(F)(F)F)=NC2=C(C(F)(F)F)C=CC=C12 AQTJRCXTFQZPAD-UHFFFAOYSA-N 0.000 claims description 4
- WXMUOGFRYHAMLJ-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(3-methoxypropylamino)ethanol Chemical compound C1=CC=C2C(C(O)CNCCCOC)=CC(C(F)(F)F)=NC2=C1C(F)(F)F WXMUOGFRYHAMLJ-UHFFFAOYSA-N 0.000 claims description 4
- CXWFUEQMTADWKS-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(3-methylsulfanylpropylamino)ethanol Chemical compound C1=CC=C2C(C(O)CNCCCSC)=CC(C(F)(F)F)=NC2=C1C(F)(F)F CXWFUEQMTADWKS-UHFFFAOYSA-N 0.000 claims description 4
- ZFEDHTPDUDQCFL-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(4-methylpentan-2-ylamino)ethanol Chemical compound C1=CC=C2C(C(O)CNC(C)CC(C)C)=CC(C(F)(F)F)=NC2=C1C(F)(F)F ZFEDHTPDUDQCFL-UHFFFAOYSA-N 0.000 claims description 4
- FNAWEZUMHMJQKN-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(butylamino)ethanol Chemical compound C1=CC=C2C(C(O)CNCCCC)=CC(C(F)(F)F)=NC2=C1C(F)(F)F FNAWEZUMHMJQKN-UHFFFAOYSA-N 0.000 claims description 4
- AHDMCPDJZBURTG-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(dibutylamino)ethanol Chemical compound C1=CC=C2C(C(O)CN(CCCC)CCCC)=CC(C(F)(F)F)=NC2=C1C(F)(F)F AHDMCPDJZBURTG-UHFFFAOYSA-N 0.000 claims description 4
- UBNCYTVRBKDHDU-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(diethylamino)ethanol Chemical compound C1=CC=C2C(C(O)CN(CC)CC)=CC(C(F)(F)F)=NC2=C1C(F)(F)F UBNCYTVRBKDHDU-UHFFFAOYSA-N 0.000 claims description 4
- KHHBNVFVJFKYIK-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(dimethylamino)ethanol Chemical compound C1=CC=C2C(C(O)CN(C)C)=CC(C(F)(F)F)=NC2=C1C(F)(F)F KHHBNVFVJFKYIK-UHFFFAOYSA-N 0.000 claims description 4
- GQVYZGLHUGDJLC-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(dipropylamino)ethanol Chemical compound C1=CC=C2C(C(O)CN(CCC)CCC)=CC(C(F)(F)F)=NC2=C1C(F)(F)F GQVYZGLHUGDJLC-UHFFFAOYSA-N 0.000 claims description 4
- JWGHJIKJZGXRNQ-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(hexylamino)ethanol Chemical compound C1=CC=C2C(C(O)CNCCCCCC)=CC(C(F)(F)F)=NC2=C1C(F)(F)F JWGHJIKJZGXRNQ-UHFFFAOYSA-N 0.000 claims description 4
- GHJPGPNNFIPPNV-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(propan-2-ylamino)ethanol Chemical compound C1=CC=C2C(C(O)CNC(C)C)=CC(C(F)(F)F)=NC2=C1C(F)(F)F GHJPGPNNFIPPNV-UHFFFAOYSA-N 0.000 claims description 4
- JVIVAICSLHRRNC-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-[(4,6,6-trimethyl-3-bicyclo[3.1.1]heptanyl)amino]ethanol Chemical compound C1=CC=C2C(C(O)CNC3CC4CC(C4(C)C)C3C)=CC(C(F)(F)F)=NC2=C1C(F)(F)F JVIVAICSLHRRNC-UHFFFAOYSA-N 0.000 claims description 4
- ZBVPAPVVMYMMBS-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-butoxyethanol Chemical compound C1=CC=C2C(C(O)COCCCC)=CC(C(F)(F)F)=NC2=C1C(F)(F)F ZBVPAPVVMYMMBS-UHFFFAOYSA-N 0.000 claims description 4
- XXYBIONWXZDBOY-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-butylsulfanylethanol Chemical compound C1=CC=C2C(C(O)CSCCCC)=CC(C(F)(F)F)=NC2=C1C(F)(F)F XXYBIONWXZDBOY-UHFFFAOYSA-N 0.000 claims description 4
- NEZJFNMPGLCANK-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-3-(tert-butylamino)propan-1-ol Chemical compound C1=CC=C2C(C(O)CCNC(C)(C)C)=CC(C(F)(F)F)=NC2=C1C(F)(F)F NEZJFNMPGLCANK-UHFFFAOYSA-N 0.000 claims description 4
- UMGPTBBPJBOCRQ-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]heptan-1-ol Chemical compound C1=CC=C2C(C(O)CCCCCC)=CC(C(F)(F)F)=NC2=C1C(F)(F)F UMGPTBBPJBOCRQ-UHFFFAOYSA-N 0.000 claims description 4
- IKBSEBRGSVFUHM-UHFFFAOYSA-N 1-[3-[4-(3-bromo-2h-pyrazolo[3,4-d]pyrimidin-4-yl)piperazin-1-yl]-4-methyl-5-(2-pyrrolidin-1-ylethylamino)phenyl]-4,4,4-trifluorobutan-1-one Chemical compound C1=C(C(=O)CCC(F)(F)F)C=C(N2CCN(CC2)C=2C=3C(Br)=NNC=3N=CN=2)C(C)=C1NCCN1CCCC1 IKBSEBRGSVFUHM-UHFFFAOYSA-N 0.000 claims description 4
- RQBRFKKFZPOBFG-UHFFFAOYSA-N 1-ethyl-3-[3-(4-methylanilino)pyrido[2,3-b]pyrazin-6-yl]urea Chemical compound N=1C2=NC(NC(=O)NCC)=CC=C2N=CC=1NC1=CC=C(C)C=C1 RQBRFKKFZPOBFG-UHFFFAOYSA-N 0.000 claims description 4
- QMNUDYFKZYBWQX-UHFFFAOYSA-N 1H-quinazolin-4-one Chemical compound C1=CC=C2C(=O)N=CNC2=C1 QMNUDYFKZYBWQX-UHFFFAOYSA-N 0.000 claims description 4
- VNOVQLJFNCUXLN-UHFFFAOYSA-N 2-(benzimidazol-1-yl)-1-[2,8-bis(trifluoromethyl)quinolin-4-yl]ethanol Chemical compound C1=CC=C2C(C(CN3C4=CC=CC=C4N=C3)O)=CC(C(F)(F)F)=NC2=C1C(F)(F)F VNOVQLJFNCUXLN-UHFFFAOYSA-N 0.000 claims description 4
- NSQSXKXTVAETFT-UHFFFAOYSA-N 2-(benzylamino)-1-[2,8-bis(trifluoromethyl)quinolin-4-yl]ethanol Chemical compound C=1C(C(F)(F)F)=NC2=C(C(F)(F)F)C=CC=C2C=1C(O)CNCC1=CC=CC=C1 NSQSXKXTVAETFT-UHFFFAOYSA-N 0.000 claims description 4
- PGLJCSZNFGDTFZ-UHFFFAOYSA-N 2-(dibutylamino)-1-[6,8-dichloro-2-(3,4-dichlorophenyl)quinolin-4-yl]ethanol Chemical compound N=1C2=C(Cl)C=C(Cl)C=C2C(C(O)CN(CCCC)CCCC)=CC=1C1=CC=C(Cl)C(Cl)=C1 PGLJCSZNFGDTFZ-UHFFFAOYSA-N 0.000 claims description 4
- IUVCFHHAEHNCFT-INIZCTEOSA-N 2-[(1s)-1-[4-amino-3-(3-fluoro-4-propan-2-yloxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]ethyl]-6-fluoro-3-(3-fluorophenyl)chromen-4-one Chemical compound C1=C(F)C(OC(C)C)=CC=C1C(C1=C(N)N=CN=C11)=NN1[C@@H](C)C1=C(C=2C=C(F)C=CC=2)C(=O)C2=CC(F)=CC=C2O1 IUVCFHHAEHNCFT-INIZCTEOSA-N 0.000 claims description 4
- PDXDKZUJFFIYQL-UHFFFAOYSA-N 2-[2-(benzylamino)ethylamino]-1-[2,8-bis(trifluoromethyl)quinolin-4-yl]ethanol Chemical compound C=1C(C(F)(F)F)=NC2=C(C(F)(F)F)C=CC=C2C=1C(O)CNCCNCC1=CC=CC=C1 PDXDKZUJFFIYQL-UHFFFAOYSA-N 0.000 claims description 4
- UAXHPOBBKRWJGA-ZDUSSCGKSA-N 2-[2-[(2s)-2-methyl-2,3-dihydroindol-1-yl]-2-oxoethyl]-6-morpholin-4-yl-1h-pyrimidin-4-one Chemical compound C([C@@H]1C)C2=CC=CC=C2N1C(=O)CC(NC(=O)C=1)=NC=1N1CCOCC1 UAXHPOBBKRWJGA-ZDUSSCGKSA-N 0.000 claims description 4
- GDWQUBHISBTRGK-JYXGGTNWSA-N 2-[[(1r,4s)-2-azabicyclo[2.2.1]heptan-4-yl]methylamino]-1-[2,8-bis(trifluoromethyl)quinolin-4-yl]ethanol Chemical compound C1=CC=C2C(C(CNC[C@@]34C[C@@H](CC3)NC4)O)=CC(C(F)(F)F)=NC2=C1C(F)(F)F GDWQUBHISBTRGK-JYXGGTNWSA-N 0.000 claims description 4
- SESUZCWKFIVUFP-UHFFFAOYSA-N 2-amino-1-[2,8-bis(trifluoromethyl)quinolin-4-yl]ethanol Chemical compound C1=CC=C2C(C(O)CN)=CC(C(F)(F)F)=NC2=C1C(F)(F)F SESUZCWKFIVUFP-UHFFFAOYSA-N 0.000 claims description 4
- MWYDSXOGIBMAET-UHFFFAOYSA-N 2-amino-N-[7-methoxy-8-(3-morpholin-4-ylpropoxy)-2,3-dihydro-1H-imidazo[1,2-c]quinazolin-5-ylidene]pyrimidine-5-carboxamide Chemical compound NC1=NC=C(C=N1)C(=O)N=C1N=C2C(=C(C=CC2=C2N1CCN2)OCCCN1CCOCC1)OC MWYDSXOGIBMAET-UHFFFAOYSA-N 0.000 claims description 4
- AQESSCKRZOFAEF-UHFFFAOYSA-N 2-anilino-1-[2,8-bis(trifluoromethyl)quinolin-4-yl]ethanol Chemical compound C=1C(C(F)(F)F)=NC2=C(C(F)(F)F)C=CC=C2C=1C(O)CNC1=CC=CC=C1 AQESSCKRZOFAEF-UHFFFAOYSA-N 0.000 claims description 4
- XTKLTGBKIDQGQL-UHFFFAOYSA-N 2-methyl-1-[[2-methyl-3-(trifluoromethyl)phenyl]methyl]-6-morpholin-4-ylbenzimidazole-4-carboxylic acid Chemical compound CC1=NC2=C(C(O)=O)C=C(N3CCOCC3)C=C2N1CC1=CC=CC(C(F)(F)F)=C1C XTKLTGBKIDQGQL-UHFFFAOYSA-N 0.000 claims description 4
- BEUQXVWXFDOSAQ-UHFFFAOYSA-N 2-methyl-2-[4-[2-(5-methyl-2-propan-2-yl-1,2,4-triazol-3-yl)-5,6-dihydroimidazo[1,2-d][1,4]benzoxazepin-9-yl]pyrazol-1-yl]propanamide Chemical compound CC(C)N1N=C(C)N=C1C1=CN(CCOC=2C3=CC=C(C=2)C2=CN(N=C2)C(C)(C)C(N)=O)C3=N1 BEUQXVWXFDOSAQ-UHFFFAOYSA-N 0.000 claims description 4
- JUSFANSTBFGBAF-IRXDYDNUSA-N 3-[2,4-bis[(3s)-3-methylmorpholin-4-yl]pyrido[2,3-d]pyrimidin-7-yl]-n-methylbenzamide Chemical compound CNC(=O)C1=CC=CC(C=2N=C3N=C(N=C(C3=CC=2)N2[C@H](COCC2)C)N2[C@H](COCC2)C)=C1 JUSFANSTBFGBAF-IRXDYDNUSA-N 0.000 claims description 4
- UJIAQDJKSXQLIT-UHFFFAOYSA-N 3-[2,4-diamino-7-(3-hydroxyphenyl)-6-pteridinyl]phenol Chemical compound C=1C=CC(O)=CC=1C1=NC2=NC(N)=NC(N)=C2N=C1C1=CC=CC(O)=C1 UJIAQDJKSXQLIT-UHFFFAOYSA-N 0.000 claims description 4
- JDUBGYFRJFOXQC-KRWDZBQOSA-N 4-amino-n-[(1s)-1-(4-chlorophenyl)-3-hydroxypropyl]-1-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide Chemical compound C1([C@H](CCO)NC(=O)C2(CCN(CC2)C=2C=3C=CNC=3N=CN=2)N)=CC=C(Cl)C=C1 JDUBGYFRJFOXQC-KRWDZBQOSA-N 0.000 claims description 4
- QXEUTSONLCRQHV-UHFFFAOYSA-N 4-tert-butyl-2-[(tert-butylamino)methyl]-6-(4-chlorophenyl)phenol Chemical compound CC(C)(C)NCC1=CC(C(C)(C)C)=CC(C=2C=CC(Cl)=CC=2)=C1O QXEUTSONLCRQHV-UHFFFAOYSA-N 0.000 claims description 4
- SJVQHLPISAIATJ-ZDUSSCGKSA-N 8-chloro-2-phenyl-3-[(1S)-1-(7H-purin-6-ylamino)ethyl]-1-isoquinolinone Chemical compound C1([C@@H](NC=2C=3N=CNC=3N=CN=2)C)=CC2=CC=CC(Cl)=C2C(=O)N1C1=CC=CC=C1 SJVQHLPISAIATJ-ZDUSSCGKSA-N 0.000 claims description 4
- CPRAGQJXBLMUEL-UHFFFAOYSA-N 9-(1-anilinoethyl)-7-methyl-2-(4-morpholinyl)-4-pyrido[1,2-a]pyrimidinone Chemical compound C=1C(C)=CN(C(C=C(N=2)N3CCOCC3)=O)C=2C=1C(C)NC1=CC=CC=C1 CPRAGQJXBLMUEL-UHFFFAOYSA-N 0.000 claims description 4
- KGPGFQWBCSZGEL-ZDUSSCGKSA-N GSK690693 Chemical compound C=12N(CC)C(C=3C(=NON=3)N)=NC2=C(C#CC(C)(C)O)N=CC=1OC[C@H]1CCCNC1 KGPGFQWBCSZGEL-ZDUSSCGKSA-N 0.000 claims description 4
- GNWHRHGTIBRNSM-UHFFFAOYSA-N IC-87114 Chemical compound CC1=CC=CC=C1N1C(=O)C2=C(C)C=CC=C2N=C1CN1C2=NC=NC(N)=C2N=C1 GNWHRHGTIBRNSM-UHFFFAOYSA-N 0.000 claims description 4
- CZQHHVNHHHRRDU-UHFFFAOYSA-N LY294002 Chemical compound C1=CC=C2C(=O)C=C(N3CCOCC3)OC2=C1C1=CC=CC=C1 CZQHHVNHHHRRDU-UHFFFAOYSA-N 0.000 claims description 4
- FCKJZIRDZMVDEM-UHFFFAOYSA-N N-(7,8-dimethoxy-2,3-dihydro-1H-imidazo[1,2-c]quinazolin-5-ylidene)pyridine-3-carboxamide Chemical compound COC1=C(C2=NC(=NC(=O)C3=CN=CC=C3)N4CCNC4=C2C=C1)OC FCKJZIRDZMVDEM-UHFFFAOYSA-N 0.000 claims description 4
- QIUASFSNWYMDFS-NILGECQDSA-N PX-866 Chemical compound CC(=O)O[C@@H]1C[C@]2(C)C(=O)CC[C@H]2C2=C1[C@@]1(C)[C@@H](COC)OC(=O)\C(=C\N(CC=C)CC=C)C1=C(O)C2=O QIUASFSNWYMDFS-NILGECQDSA-N 0.000 claims description 4
- CBPNZQVSJQDFBE-FUXHJELOSA-N Temsirolimus Chemical compound C1C[C@@H](OC(=O)C(C)(CO)CO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 CBPNZQVSJQDFBE-FUXHJELOSA-N 0.000 claims description 4
- KCRSJPCXPQESIU-SEYXRHQNSA-N [(z)-docos-13-enyl] 2-(trimethylazaniumyl)ethyl phosphate Chemical compound CCCCCCCC\C=C/CCCCCCCCCCCCOP([O-])(=O)OCC[N+](C)(C)C KCRSJPCXPQESIU-SEYXRHQNSA-N 0.000 claims description 4
- HXIIVEXNDYQSKF-WXMRUQJCSA-N [2,8-bis(trifluoromethyl)quinolin-4-yl]-[[(2r)-pyrrolidin-2-yl]methylamino]methanol Chemical compound C=1C(C(F)(F)F)=NC2=C(C(F)(F)F)C=CC=C2C=1C(O)NC[C@H]1CCCN1 HXIIVEXNDYQSKF-WXMRUQJCSA-N 0.000 claims description 4
- GMHFKOCCMACCAP-HTYZCBBDSA-N [[(1s,4r)-2-azabicyclo[2.2.1]heptan-4-yl]methylamino]-[2,8-bis(trifluoromethyl)quinolin-4-yl]methanol Chemical compound C1=CC=C2C(C(NC[C@@]34C[C@H](CC3)NC4)O)=CC(C(F)(F)F)=NC2=C1C(F)(F)F GMHFKOCCMACCAP-HTYZCBBDSA-N 0.000 claims description 4
- CBFCDTFDPHXCNY-UHFFFAOYSA-N icosane Chemical compound CCCCCCCCCCCCCCCCCCCC CBFCDTFDPHXCNY-UHFFFAOYSA-N 0.000 claims description 4
- 229960003775 miltefosine Drugs 0.000 claims description 4
- KWRYMZHCQIOOEB-LBPRGKRZSA-N n-[(1s)-1-(7-fluoro-2-pyridin-2-ylquinolin-3-yl)ethyl]-7h-purin-6-amine Chemical compound C1([C@@H](NC=2C=3N=CNC=3N=CN=2)C)=CC2=CC=C(F)C=C2N=C1C1=CC=CC=N1 KWRYMZHCQIOOEB-LBPRGKRZSA-N 0.000 claims description 4
- JOWXJLIFIIOYMS-UHFFFAOYSA-N n-hydroxy-2-[[2-(6-methoxypyridin-3-yl)-4-morpholin-4-ylthieno[3,2-d]pyrimidin-6-yl]methyl-methylamino]pyrimidine-5-carboxamide Chemical compound C1=NC(OC)=CC=C1C1=NC(N2CCOCC2)=C(SC(CN(C)C=2N=CC(=CN=2)C(=O)NO)=C2)C2=N1 JOWXJLIFIIOYMS-UHFFFAOYSA-N 0.000 claims description 4
- JLPDBLFIVFSOCC-XYXFTTADSA-N oleandrin Chemical compound O1[C@@H](C)[C@H](O)[C@@H](OC)C[C@@H]1O[C@@H]1C[C@@H](CC[C@H]2[C@]3(C[C@@H]([C@@H]([C@@]3(C)CC[C@H]32)C=2COC(=O)C=2)OC(C)=O)O)[C@]3(C)CC1 JLPDBLFIVFSOCC-XYXFTTADSA-N 0.000 claims description 4
- 201000008129 pancreatic ductal adenocarcinoma Diseases 0.000 claims description 4
- SZFPYBIJACMNJV-UHFFFAOYSA-N perifosine Chemical compound CCCCCCCCCCCCCCCCCCOP([O-])(=O)OC1CC[N+](C)(C)CC1 SZFPYBIJACMNJV-UHFFFAOYSA-N 0.000 claims description 4
- LHNIIDJUOCFXAP-UHFFFAOYSA-N pictrelisib Chemical compound C1CN(S(=O)(=O)C)CCN1CC1=CC2=NC(C=3C=4C=NNC=4C=CC=3)=NC(N3CCOCC3)=C2S1 LHNIIDJUOCFXAP-UHFFFAOYSA-N 0.000 claims description 4
- 229960001302 ridaforolimus Drugs 0.000 claims description 4
- 229950009216 sapanisertib Drugs 0.000 claims description 4
- URLYINUFLXOMHP-HTVVRFAVSA-N tcn-p Chemical compound C=12C3=NC=NC=1N(C)N=C(N)C2=CN3[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O URLYINUFLXOMHP-HTVVRFAVSA-N 0.000 claims description 4
- 229960000235 temsirolimus Drugs 0.000 claims description 4
- QFJCIRLUMZQUOT-UHFFFAOYSA-N temsirolimus Natural products C1CC(O)C(OC)CC1CC(C)C1OC(=O)C2CCCCN2C(=O)C(=O)C(O)(O2)C(C)CCC2CC(OC)C(C)=CC=CC=CC(C)CC(C)C(=O)C(OC)C(O)C(C)=CC(C)C(=O)C1 QFJCIRLUMZQUOT-UHFFFAOYSA-N 0.000 claims description 4
- QDLHCMPXEPAAMD-QAIWCSMKSA-N wortmannin Chemical compound C1([C@]2(C)C3=C(C4=O)OC=C3C(=O)O[C@@H]2COC)=C4[C@@H]2CCC(=O)[C@@]2(C)C[C@H]1OC(C)=O QDLHCMPXEPAAMD-QAIWCSMKSA-N 0.000 claims description 4
- QINPEPAQOBZPOF-UHFFFAOYSA-N 2-amino-n-[3-[[3-(2-chloro-5-methoxyanilino)quinoxalin-2-yl]sulfamoyl]phenyl]-2-methylpropanamide Chemical compound COC1=CC=C(Cl)C(NC=2C(=NC3=CC=CC=C3N=2)NS(=O)(=O)C=2C=C(NC(=O)C(C)(C)N)C=CC=2)=C1 QINPEPAQOBZPOF-UHFFFAOYSA-N 0.000 claims description 3
- HNFMVVHMKGFCMB-UHFFFAOYSA-N 3-[3-[4-(1-aminocyclobutyl)phenyl]-5-phenylimidazo[4,5-b]pyridin-2-yl]pyridin-2-amine Chemical compound NC1=NC=CC=C1C1=NC2=CC=C(C=3C=CC=CC=3)N=C2N1C1=CC=C(C2(N)CCC2)C=C1 HNFMVVHMKGFCMB-UHFFFAOYSA-N 0.000 claims description 3
- BUROJSBIWGDYCN-GAUTUEMISA-N AP 23573 Chemical compound C1C[C@@H](OP(C)(C)=O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 BUROJSBIWGDYCN-GAUTUEMISA-N 0.000 claims description 3
- HGVNLRPZOWWDKD-UHFFFAOYSA-N ZSTK-474 Chemical compound FC(F)C1=NC2=CC=CC=C2N1C(N=1)=NC(N2CCOCC2)=NC=1N1CCOCC1 HGVNLRPZOWWDKD-UHFFFAOYSA-N 0.000 claims description 3
- AXTAPYRUEKNRBA-JTQLQIEISA-N n-[(2s)-1-amino-3-(3,4-difluorophenyl)propan-2-yl]-5-chloro-4-(4-chloro-2-methylpyrazol-3-yl)furan-2-carboxamide Chemical compound CN1N=CC(Cl)=C1C1=C(Cl)OC(C(=O)N[C@H](CN)CC=2C=C(F)C(F)=CC=2)=C1 AXTAPYRUEKNRBA-JTQLQIEISA-N 0.000 claims description 3
- QTHCAAFKVUWAFI-DJKKODMXSA-N n-[(e)-(6-bromoimidazo[1,2-a]pyridin-3-yl)methylideneamino]-n,2-dimethyl-5-nitrobenzenesulfonamide Chemical compound C=1N=C2C=CC(Br)=CN2C=1/C=N/N(C)S(=O)(=O)C1=CC([N+]([O-])=O)=CC=C1C QTHCAAFKVUWAFI-DJKKODMXSA-N 0.000 claims description 3
- FVSKHRXBFJPNKK-UHFFFAOYSA-N propionitrile Chemical compound CCC#N FVSKHRXBFJPNKK-UHFFFAOYSA-N 0.000 claims description 3
- OYYVWNDMOQPMGE-SDQBBNPISA-N (5z)-5-[[5-(4-fluoro-2-hydroxyphenyl)furan-2-yl]methylidene]-1,3-thiazolidine-2,4-dione Chemical compound OC1=CC(F)=CC=C1C(O1)=CC=C1\C=C/1C(=O)NC(=O)S\1 OYYVWNDMOQPMGE-SDQBBNPISA-N 0.000 claims description 2
- ALUZCFMOHFZLKV-UHFFFAOYSA-N (6,8-dichloro-2-phenylquinolin-4-yl)-piperidin-2-ylmethanol Chemical compound C=1C(C=2C=CC=CC=2)=NC2=C(Cl)C=C(Cl)C=C2C=1C(O)C1CCCCN1 ALUZCFMOHFZLKV-UHFFFAOYSA-N 0.000 claims description 2
- ALUZCFMOHFZLKV-NQIIRXRSSA-N (S)-(6,8-dichloro-2-phenylquinolin-4-yl)-[(2R)-piperidin-2-yl]methanol Chemical compound O[C@H]([C@H]1CCCCN1)c1cc(nc2c(Cl)cc(Cl)cc12)-c1ccccc1 ALUZCFMOHFZLKV-NQIIRXRSSA-N 0.000 claims description 2
- YGTWRJXPPATPFF-UHFFFAOYSA-N 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(propylamino)ethanol Chemical compound C1=CC=C2C(C(O)CNCCC)=CC(C(F)(F)F)=NC2=C1C(F)(F)F YGTWRJXPPATPFF-UHFFFAOYSA-N 0.000 claims description 2
- HJQAYDUYDZYGDS-UHFFFAOYSA-N 13-oxapentacyclo[10.6.1.02,10.05,9.015,19]nonadeca-1(19),2(10),3,5(9),7-pentaene-6,11,16-trione Chemical compound C1CC(C2C=3C(OC2)C(C=2C4=C(C=CC=2C1=3)C(C=C4)=O)=O)=O HJQAYDUYDZYGDS-UHFFFAOYSA-N 0.000 claims description 2
- YWOWJQMFMXHLQD-UHFFFAOYSA-N 3-(trifluoromethyl)pyridin-2-amine Chemical compound NC1=NC=CC=C1C(F)(F)F YWOWJQMFMXHLQD-UHFFFAOYSA-N 0.000 claims description 2
- PTBDIHRZYDMNKB-UHFFFAOYSA-M 3-hydroxy-2-(hydroxymethyl)-2-methylpropanoate Chemical compound OCC(C)(CO)C([O-])=O PTBDIHRZYDMNKB-UHFFFAOYSA-M 0.000 claims description 2
- HWUHTJIKQZZBRA-UHFFFAOYSA-N 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-2h-[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3-one;dihydrochloride Chemical compound Cl.Cl.C=1C=C(C=2C(=CC=3C=4N(C(NN=4)=O)C=CC=3N=2)C=2C=CC=CC=2)C=CC=1C1(N)CCC1 HWUHTJIKQZZBRA-UHFFFAOYSA-N 0.000 claims description 2
- 229960005531 AMG 319 Drugs 0.000 claims description 2
- 241000605445 Anemarrhena asphodeloides Species 0.000 claims description 2
- AFJRDFWMXUECEW-LBPRGKRZSA-N N-[(2S)-1-amino-3-(3-fluorophenyl)propan-2-yl]-5-chloro-4-(4-chloro-2-methyl-3-pyrazolyl)-2-thiophenecarboxamide Chemical compound CN1N=CC(Cl)=C1C1=C(Cl)SC(C(=O)N[C@H](CN)CC=2C=C(F)C=CC=2)=C1 AFJRDFWMXUECEW-LBPRGKRZSA-N 0.000 claims description 2
- 241000612118 Samolus valerandi Species 0.000 claims description 2
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 claims description 2
- MMTWXUQMLQGAPC-YXOKLLKRSA-N Timosaponin A-III Chemical compound O([C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1C[C@H]2CC[C@H]3[C@@H]4C[C@H]5[C@@H]([C@]4(CC[C@@H]3[C@@]2(C)CC1)C)[C@@H]([C@]1(OC[C@@H](C)CC1)O5)C)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O MMTWXUQMLQGAPC-YXOKLLKRSA-N 0.000 claims description 2
- 229950010482 alpelisib Drugs 0.000 claims description 2
- 229950003628 buparlisib Drugs 0.000 claims description 2
- GOJNABIZVJCYFL-UHFFFAOYSA-M dimethylphosphinate Chemical compound CP(C)([O-])=O GOJNABIZVJCYFL-UHFFFAOYSA-M 0.000 claims description 2
- 125000000555 isopropenyl group Chemical group [H]\C([H])=C(\*)C([H])([H])[H] 0.000 claims description 2
- 201000002120 neuroendocrine carcinoma Diseases 0.000 claims description 2
- 201000002530 pancreatic endocrine carcinoma Diseases 0.000 claims description 2
- LOUPRKONTZGTKE-LHHVKLHASA-N quinidine Chemical compound C([C@H]([C@H](C1)C=C)C2)C[N@@]1[C@H]2[C@@H](O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-LHHVKLHASA-N 0.000 claims description 2
- 125000004549 quinolin-4-yl group Chemical group N1=CC=C(C2=CC=CC=C12)* 0.000 claims description 2
- BLGWHBSBBJNKJO-UHFFFAOYSA-N serabelisib Chemical compound C=1C=C2OC(N)=NC2=CC=1C(=CN12)C=CC1=NC=C2C(=O)N1CCOCC1 BLGWHBSBBJNKJO-UHFFFAOYSA-N 0.000 claims description 2
- 206010041823 squamous cell carcinoma Diseases 0.000 claims description 2
- 229960001967 tacrolimus Drugs 0.000 claims description 2
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 claims description 2
- 229950001269 taselisib Drugs 0.000 claims description 2
- 229930193981 timosaponin Natural products 0.000 claims description 2
- MMTWXUQMLQGAPC-XWIAVXRASA-N timosaponin A-III Natural products C[C@@H]1CC[C@@]2(OC1)O[C@@H]3C[C@H]4[C@H]5CC[C@@H]6C[C@H](CC[C@]6(C)[C@H]5CC[C@]4(C)[C@@H]3[C@@H]2C)O[C@@H]7O[C@H](CO)[C@H](O)[C@H](O)[C@H]7O[C@@H]8O[C@H](CO)[C@@H](O)[C@H](O)[C@H]8O MMTWXUQMLQGAPC-XWIAVXRASA-N 0.000 claims description 2
- 229950003873 triciribine Drugs 0.000 claims description 2
- 108010086097 viridin Proteins 0.000 claims description 2
- YEIGUXGHHKAURB-UHFFFAOYSA-N viridine Natural products O=C1C2=C3CCC(=O)C3=CC=C2C2(C)C(O)C(OC)C(=O)C3=COC1=C23 YEIGUXGHHKAURB-UHFFFAOYSA-N 0.000 claims description 2
- QDLHCMPXEPAAMD-UHFFFAOYSA-N wortmannin Natural products COCC1OC(=O)C2=COC(C3=O)=C2C1(C)C1=C3C2CCC(=O)C2(C)CC1OC(C)=O QDLHCMPXEPAAMD-UHFFFAOYSA-N 0.000 claims description 2
- 102000038030 PI3Ks Human genes 0.000 claims 6
- 239000000126 substance Substances 0.000 claims 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims 3
- 239000001257 hydrogen Substances 0.000 claims 3
- 229910052739 hydrogen Inorganic materials 0.000 claims 3
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 claims 2
- YKLIKGKUANLGSB-HNNXBMFYSA-N idelalisib Chemical compound C1([C@@H](NC=2[C]3N=CN=C3N=CN=2)CC)=NC2=CC=CC(F)=C2C(=O)N1C1=CC=CC=C1 YKLIKGKUANLGSB-HNNXBMFYSA-N 0.000 claims 2
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 claims 2
- JPBLHOJFMBOCAF-UHFFFAOYSA-N 1,3-benzoxazol-2-amine Chemical compound C1=CC=C2OC(N)=NC2=C1 JPBLHOJFMBOCAF-UHFFFAOYSA-N 0.000 claims 1
- 125000004173 1-benzimidazolyl group Chemical group [H]C1=NC2=C([H])C([H])=C([H])C([H])=C2N1* 0.000 claims 1
- RCALMNBWTVIOSW-UHFFFAOYSA-N 3-chloro-4-(4-chloro-2-methylpyrazol-3-yl)thiophene-2-carboxamide Chemical compound CN1N=CC(Cl)=C1C1=CSC(C(N)=O)=C1Cl RCALMNBWTVIOSW-UHFFFAOYSA-N 0.000 claims 1
- 125000003349 3-pyridyl group Chemical group N1=C([H])C([*])=C([H])C([H])=C1[H] 0.000 claims 1
- GSENCEDSCQYPIL-UHFFFAOYSA-N morpholin-4-ylmethanone Chemical compound O=[C]N1CCOCC1 GSENCEDSCQYPIL-UHFFFAOYSA-N 0.000 claims 1
- VOUDEIAYNKZQKM-MYHMWQFYSA-N n-[(e)-(6-bromoimidazo[1,2-a]pyridin-3-yl)methylideneamino]-n,2-dimethyl-5-nitrobenzenesulfonamide;hydrochloride Chemical compound Cl.C=1N=C2C=CC(Br)=CN2C=1/C=N/N(C)S(=O)(=O)C1=CC([N+]([O-])=O)=CC=C1C VOUDEIAYNKZQKM-MYHMWQFYSA-N 0.000 claims 1
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 claims 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 claims 1
- 206010028980 Neoplasm Diseases 0.000 abstract description 24
- 201000011510 cancer Diseases 0.000 abstract description 11
- 201000009030 Carcinoma Diseases 0.000 abstract description 6
- 206010009944 Colon cancer Diseases 0.000 abstract description 6
- 206010006187 Breast cancer Diseases 0.000 abstract description 5
- 208000026310 Breast neoplasm Diseases 0.000 abstract description 5
- 206010060862 Prostate cancer Diseases 0.000 abstract description 5
- 208000000236 Prostatic Neoplasms Diseases 0.000 abstract description 5
- 201000001441 melanoma Diseases 0.000 abstract description 5
- 208000002154 non-small cell lung carcinoma Diseases 0.000 abstract description 5
- 208000003174 Brain Neoplasms Diseases 0.000 abstract description 4
- 208000001333 Colorectal Neoplasms Diseases 0.000 abstract description 4
- 206010058467 Lung neoplasm malignant Diseases 0.000 abstract description 4
- 208000034578 Multiple myelomas Diseases 0.000 abstract description 4
- 206010035226 Plasma cell myeloma Diseases 0.000 abstract description 4
- 201000005202 lung cancer Diseases 0.000 abstract description 4
- 208000020816 lung neoplasm Diseases 0.000 abstract description 4
- 210000003494 hepatocyte Anatomy 0.000 abstract description 3
- 239000000546 pharmaceutical excipient Substances 0.000 abstract description 3
- 102000010400 1-phosphatidylinositol-3-kinase activity proteins Human genes 0.000 abstract 2
- 239000003795 chemical substances by application Substances 0.000 description 48
- 230000000694 effects Effects 0.000 description 35
- 102000003993 Phosphatidylinositol 3-kinases Human genes 0.000 description 25
- 210000004027 cell Anatomy 0.000 description 23
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 12
- 150000003839 salts Chemical class 0.000 description 10
- 230000001225 therapeutic effect Effects 0.000 description 9
- 201000010099 disease Diseases 0.000 description 8
- 239000003814 drug Substances 0.000 description 7
- 229920000371 poly(diallyldimethylammonium chloride) polymer Polymers 0.000 description 7
- 229940079593 drug Drugs 0.000 description 6
- 230000009977 dual effect Effects 0.000 description 6
- 239000012636 effector Substances 0.000 description 6
- 238000009472 formulation Methods 0.000 description 6
- 238000005303 weighing Methods 0.000 description 6
- 239000012823 PI3K/mTOR inhibitor Substances 0.000 description 5
- 206010039491 Sarcoma Diseases 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- IFSDAJWBUCMOAH-HNNXBMFYSA-N idelalisib Chemical compound C1([C@@H](NC=2C=3N=CNC=3N=CN=2)CC)=NC2=CC=CC(F)=C2C(=O)N1C1=CC=CC=C1 IFSDAJWBUCMOAH-HNNXBMFYSA-N 0.000 description 5
- 230000008685 targeting Effects 0.000 description 5
- 230000035899 viability Effects 0.000 description 5
- 206010018338 Glioma Diseases 0.000 description 4
- 101000653005 Homo sapiens Thromboxane-A synthase Proteins 0.000 description 4
- 101000809797 Homo sapiens Thymidylate synthase Proteins 0.000 description 4
- 241000699666 Mus <mouse, genus> Species 0.000 description 4
- 231100000673 dose–response relationship Toxicity 0.000 description 4
- 230000037361 pathway Effects 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 206010003571 Astrocytoma Diseases 0.000 description 3
- 208000032612 Glial tumor Diseases 0.000 description 3
- 101001059454 Homo sapiens Serine/threonine-protein kinase MARK2 Proteins 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 3
- 229940124647 MEK inhibitor Drugs 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 102000014160 PTEN Phosphohydrolase Human genes 0.000 description 3
- 108010011536 PTEN Phosphohydrolase Proteins 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 3
- 102100028904 Serine/threonine-protein kinase MARK2 Human genes 0.000 description 3
- 208000005718 Stomach Neoplasms Diseases 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 239000005557 antagonist Substances 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000002648 combination therapy Methods 0.000 description 3
- 231100000433 cytotoxic Toxicity 0.000 description 3
- 230000001472 cytotoxic effect Effects 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 206010017758 gastric cancer Diseases 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 208000032839 leukemia Diseases 0.000 description 3
- 230000001394 metastastic effect Effects 0.000 description 3
- 206010061289 metastatic neoplasm Diseases 0.000 description 3
- 239000002829 mitogen activated protein kinase inhibitor Substances 0.000 description 3
- 201000005962 mycosis fungoides Diseases 0.000 description 3
- 230000036961 partial effect Effects 0.000 description 3
- 208000029340 primitive neuroectodermal tumor Diseases 0.000 description 3
- 230000035755 proliferation Effects 0.000 description 3
- CYOHGALHFOKKQC-UHFFFAOYSA-N selumetinib Chemical compound OCCONC(=O)C=1C=C2N(C)C=NC2=C(F)C=1NC1=CC=C(Br)C=C1Cl CYOHGALHFOKKQC-UHFFFAOYSA-N 0.000 description 3
- 229950010746 selumetinib Drugs 0.000 description 3
- 201000011549 stomach cancer Diseases 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- 238000011287 therapeutic dose Methods 0.000 description 3
- 238000002560 therapeutic procedure Methods 0.000 description 3
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 3
- SVNJBEMPMKWDCO-KCHLEUMXSA-N (2s)-2-[[(2s)-3-carboxy-2-[[2-[[(2s)-5-(diaminomethylideneamino)-2-[[4-oxo-4-[[4-(4-oxo-8-phenylchromen-2-yl)morpholin-4-ium-4-yl]methoxy]butanoyl]amino]pentanoyl]amino]acetyl]amino]propanoyl]amino]-3-hydroxypropanoate Chemical compound C=1C(=O)C2=CC=CC(C=3C=CC=CC=3)=C2OC=1[N+]1(COC(=O)CCC(=O)N[C@@H](CCCNC(=N)N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C([O-])=O)CCOCC1 SVNJBEMPMKWDCO-KCHLEUMXSA-N 0.000 description 2
- QDITZBLZQQZVEE-YBEGLDIGSA-N (5z)-5-[(4-pyridin-4-ylquinolin-6-yl)methylidene]-1,3-thiazolidine-2,4-dione Chemical compound S1C(=O)NC(=O)\C1=C\C1=CC=C(N=CC=C2C=3C=CN=CC=3)C2=C1 QDITZBLZQQZVEE-YBEGLDIGSA-N 0.000 description 2
- DWZAEMINVBZMHQ-UHFFFAOYSA-N 1-[4-[4-(dimethylamino)piperidine-1-carbonyl]phenyl]-3-[4-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)phenyl]urea Chemical compound C1CC(N(C)C)CCN1C(=O)C(C=C1)=CC=C1NC(=O)NC1=CC=C(C=2N=C(N=C(N=2)N2CCOCC2)N2CCOCC2)C=C1 DWZAEMINVBZMHQ-UHFFFAOYSA-N 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- AKKCGLXULFRAET-UHFFFAOYSA-N 5-[7-methyl-6-[(4-methylsulfonylpiperazin-1-yl)methyl]-4-morpholin-4-ylthieno[3,2-d]pyrimidin-2-yl]pyrimidin-2-amine Chemical compound S1C2=C(N3CCOCC3)N=C(C=3C=NC(N)=NC=3)N=C2C(C)=C1CN1CCN(S(C)(=O)=O)CC1 AKKCGLXULFRAET-UHFFFAOYSA-N 0.000 description 2
- YEAHTLOYHVWAKW-UHFFFAOYSA-N 8-(1-hydroxyethyl)-2-methoxy-3-[(4-methoxyphenyl)methoxy]benzo[c]chromen-6-one Chemical compound C1=CC(OC)=CC=C1COC(C(=C1)OC)=CC2=C1C1=CC=C(C(C)O)C=C1C(=O)O2 YEAHTLOYHVWAKW-UHFFFAOYSA-N 0.000 description 2
- -1 AKT Proteins 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 2
- 201000003076 Angiosarcoma Diseases 0.000 description 2
- 206010005003 Bladder cancer Diseases 0.000 description 2
- 208000018084 Bone neoplasm Diseases 0.000 description 2
- UFKLYTOEMRFKAD-SHTZXODSSA-N C1C[C@@H](OC)CC[C@@H]1N1C2=NC(C=3C=NC(=CC=3)C(C)(C)O)=CN=C2NCC1=O Chemical compound C1C[C@@H](OC)CC[C@@H]1N1C2=NC(C=3C=NC(=CC=3)C(C)(C)O)=CN=C2NCC1=O UFKLYTOEMRFKAD-SHTZXODSSA-N 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- 206010014733 Endometrial cancer Diseases 0.000 description 2
- 206010014759 Endometrial neoplasm Diseases 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- 208000022072 Gallbladder Neoplasms Diseases 0.000 description 2
- 206010051066 Gastrointestinal stromal tumour Diseases 0.000 description 2
- 208000021309 Germ cell tumor Diseases 0.000 description 2
- 201000010915 Glioblastoma multiforme Diseases 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- 208000001258 Hemangiosarcoma Diseases 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- 208000006404 Large Granular Lymphocytic Leukemia Diseases 0.000 description 2
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 2
- 206010025323 Lymphomas Diseases 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- 206010027476 Metastases Diseases 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- 208000003445 Mouth Neoplasms Diseases 0.000 description 2
- 241001529936 Murinae Species 0.000 description 2
- 241000699670 Mus sp. Species 0.000 description 2
- 201000003793 Myelodysplastic syndrome Diseases 0.000 description 2
- 206010061306 Nasopharyngeal cancer Diseases 0.000 description 2
- 208000034176 Neoplasms, Germ Cell and Embryonal Diseases 0.000 description 2
- 206010029260 Neuroblastoma Diseases 0.000 description 2
- 206010033128 Ovarian cancer Diseases 0.000 description 2
- 206010061535 Ovarian neoplasm Diseases 0.000 description 2
- TUVCWJQQGGETHL-UHFFFAOYSA-N PI-103 Chemical compound OC1=CC=CC(C=2N=C3C4=CC=CN=C4OC3=C(N3CCOCC3)N=2)=C1 TUVCWJQQGGETHL-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 108091000080 Phosphotransferase Proteins 0.000 description 2
- 208000007913 Pituitary Neoplasms Diseases 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- LCTONWCANYUPML-UHFFFAOYSA-N Pyruvic acid Chemical compound CC(=O)C(O)=O LCTONWCANYUPML-UHFFFAOYSA-N 0.000 description 2
- 208000006265 Renal cell carcinoma Diseases 0.000 description 2
- 206010041067 Small cell lung cancer Diseases 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 2
- 201000005969 Uveal melanoma Diseases 0.000 description 2
- 208000033559 Waldenström macroglobulinemia Diseases 0.000 description 2
- HJSSPYJVWLTYHG-UHFFFAOYSA-N XL765 Chemical compound COC1=CC(OC)=CC(NC=2C(=NC3=CC=CC=C3N=2)NS(=O)(=O)C=2C=CC(NC(=O)C=3C=C(OC)C(C)=CC=3)=CC=2)=C1 HJSSPYJVWLTYHG-UHFFFAOYSA-N 0.000 description 2
- 208000009956 adenocarcinoma Diseases 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 208000029742 colonic neoplasm Diseases 0.000 description 2
- 239000000824 cytostatic agent Substances 0.000 description 2
- 230000001085 cytostatic effect Effects 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 206010016629 fibroma Diseases 0.000 description 2
- 201000010175 gallbladder cancer Diseases 0.000 description 2
- 201000011243 gastrointestinal stromal tumor Diseases 0.000 description 2
- 208000005017 glioblastoma Diseases 0.000 description 2
- 230000009036 growth inhibition Effects 0.000 description 2
- 201000009277 hairy cell leukemia Diseases 0.000 description 2
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 150000007529 inorganic bases Chemical class 0.000 description 2
- 208000012987 lip and oral cavity carcinoma Diseases 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- 230000003211 malignant effect Effects 0.000 description 2
- 150000007522 mineralic acids Chemical class 0.000 description 2
- 208000025113 myeloid leukemia Diseases 0.000 description 2
- GDCJHDUWWAKBIW-UHFFFAOYSA-N n-[4-[4-[2-(difluoromethyl)-4-methoxybenzimidazol-1-yl]-6-morpholin-4-yl-1,3,5-triazin-2-yl]phenyl]-2-(dimethylamino)ethanesulfonamide Chemical compound FC(F)C1=NC=2C(OC)=CC=CC=2N1C(N=1)=NC(N2CCOCC2)=NC=1C1=CC=C(NS(=O)(=O)CCN(C)C)C=C1 GDCJHDUWWAKBIW-UHFFFAOYSA-N 0.000 description 2
- CGBJSGAELGCMKE-UHFFFAOYSA-N omipalisib Chemical compound COC1=NC=C(C=2C=C3C(C=4C=NN=CC=4)=CC=NC3=CC=2)C=C1NS(=O)(=O)C1=CC=C(F)C=C1F CGBJSGAELGCMKE-UHFFFAOYSA-N 0.000 description 2
- 231100000590 oncogenic Toxicity 0.000 description 2
- 230000002246 oncogenic effect Effects 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 235000005985 organic acids Nutrition 0.000 description 2
- 150000007530 organic bases Chemical class 0.000 description 2
- 201000008968 osteosarcoma Diseases 0.000 description 2
- 102000020233 phosphotransferase Human genes 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 208000000587 small cell lung carcinoma Diseases 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 230000002195 synergetic effect Effects 0.000 description 2
- 208000008732 thymoma Diseases 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- AKCRNFFTGXBONI-UHFFFAOYSA-N torin 1 Chemical compound C1CN(C(=O)CC)CCN1C1=CC=C(N2C(C=CC3=C2C2=CC(=CC=C2N=C3)C=2C=C3C=CC=CC3=NC=2)=O)C=C1C(F)(F)F AKCRNFFTGXBONI-UHFFFAOYSA-N 0.000 description 2
- 206010044412 transitional cell carcinoma Diseases 0.000 description 2
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 2
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 2
- 210000004881 tumor cell Anatomy 0.000 description 2
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 description 2
- 201000005112 urinary bladder cancer Diseases 0.000 description 2
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- KITJXMWIPMZSEV-GLTSFNQTSA-N (z)-but-2-enedioic acid Chemical compound OC(=O)\C=C/C(O)=O.OC(=O)\C=C/C(O)=O.OC(=O)\C=C/C(O)=O KITJXMWIPMZSEV-GLTSFNQTSA-N 0.000 description 1
- WBYWAXJHAXSJNI-VOTSOKGWSA-M .beta-Phenylacrylic acid Natural products [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- XDLYKKIQACFMJG-UHFFFAOYSA-N 2-amino-8-[4-(2-hydroxyethoxy)cyclohexyl]-6-(6-methoxypyridin-3-yl)-4-methylpyrido[2,3-d]pyrimidin-7-one Chemical compound C1=NC(OC)=CC=C1C(C1=O)=CC2=C(C)N=C(N)N=C2N1C1CCC(OCCO)CC1 XDLYKKIQACFMJG-UHFFFAOYSA-N 0.000 description 1
- ISHNPDWTQUXPSY-UHFFFAOYSA-N 2-methyl-2-[4-[3-methyl-2-oxo-8-(2-pyridin-3-ylethynyl)imidazo[4,5-c]quinolin-1-yl]phenyl]propanenitrile Chemical compound O=C1N(C)C2=CN=C3C=CC(C#CC=4C=NC=CC=4)=CC3=C2N1C1=CC=C(C(C)(C)C#N)C=C1 ISHNPDWTQUXPSY-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- 208000030507 AIDS Diseases 0.000 description 1
- 208000002008 AIDS-Related Lymphoma Diseases 0.000 description 1
- 208000007876 Acrospiroma Diseases 0.000 description 1
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 1
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 description 1
- 206010000871 Acute monocytic leukaemia Diseases 0.000 description 1
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 1
- 208000036762 Acute promyelocytic leukaemia Diseases 0.000 description 1
- 208000001783 Adamantinoma Diseases 0.000 description 1
- 208000003200 Adenoma Diseases 0.000 description 1
- 206010001233 Adenoma benign Diseases 0.000 description 1
- 208000009746 Adult T-Cell Leukemia-Lymphoma Diseases 0.000 description 1
- 208000016683 Adult T-cell leukemia/lymphoma Diseases 0.000 description 1
- 208000037540 Alveolar soft tissue sarcoma Diseases 0.000 description 1
- 206010061424 Anal cancer Diseases 0.000 description 1
- 208000001446 Anaplastic Thyroid Carcinoma Diseases 0.000 description 1
- 206010073478 Anaplastic large-cell lymphoma Diseases 0.000 description 1
- 206010002240 Anaplastic thyroid cancer Diseases 0.000 description 1
- 206010051810 Angiomyolipoma Diseases 0.000 description 1
- 208000007860 Anus Neoplasms Diseases 0.000 description 1
- 206010073360 Appendix cancer Diseases 0.000 description 1
- 206010060971 Astrocytoma malignant Diseases 0.000 description 1
- 201000008271 Atypical teratoid rhabdoid tumor Diseases 0.000 description 1
- 208000004736 B-Cell Leukemia Diseases 0.000 description 1
- 208000036170 B-Cell Marginal Zone Lymphoma Diseases 0.000 description 1
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 1
- 208000003950 B-cell lymphoma Diseases 0.000 description 1
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 description 1
- YUXMAKUNSXIEKN-BTJKTKAUSA-N BGT226 Chemical compound OC(=O)\C=C/C(O)=O.C1=NC(OC)=CC=C1C1=CC=C(N=CC2=C3N(C=4C=C(C(N5CCNCC5)=CC=4)C(F)(F)F)C(=O)N2C)C3=C1 YUXMAKUNSXIEKN-BTJKTKAUSA-N 0.000 description 1
- 206010004146 Basal cell carcinoma Diseases 0.000 description 1
- 206010004453 Benign salivary gland neoplasm Diseases 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- 206010004593 Bile duct cancer Diseases 0.000 description 1
- 206010005949 Bone cancer Diseases 0.000 description 1
- 208000007690 Brenner tumor Diseases 0.000 description 1
- 206010073258 Brenner tumour Diseases 0.000 description 1
- 208000003170 Bronchiolo-Alveolar Adenocarcinoma Diseases 0.000 description 1
- 206010058354 Bronchioloalveolar carcinoma Diseases 0.000 description 1
- 206010070487 Brown tumour Diseases 0.000 description 1
- 208000011691 Burkitt lymphomas Diseases 0.000 description 1
- CACUWZLBIJSTIC-ZDGMYTEDSA-N CC[C@H](NC1=C2N=CNC2=NC=N1)C1NC2=C(C(=O)N1C1=CC=CC=C1)C(F)=CC=C2 Chemical compound CC[C@H](NC1=C2N=CNC2=NC=N1)C1NC2=C(C(=O)N1C1=CC=CC=C1)C(F)=CC=C2 CACUWZLBIJSTIC-ZDGMYTEDSA-N 0.000 description 1
- 206010007275 Carcinoid tumour Diseases 0.000 description 1
- 206010007279 Carcinoid tumour of the gastrointestinal tract Diseases 0.000 description 1
- 208000009458 Carcinoma in Situ Diseases 0.000 description 1
- 201000000274 Carcinosarcoma Diseases 0.000 description 1
- 208000005024 Castleman disease Diseases 0.000 description 1
- 208000037138 Central nervous system embryonal tumor Diseases 0.000 description 1
- 206010007953 Central nervous system lymphoma Diseases 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- 206010008583 Chloroma Diseases 0.000 description 1
- 201000005262 Chondroma Diseases 0.000 description 1
- 208000005243 Chondrosarcoma Diseases 0.000 description 1
- 201000009047 Chordoma Diseases 0.000 description 1
- 208000006332 Choriocarcinoma Diseases 0.000 description 1
- 208000004378 Choroid plexus papilloma Diseases 0.000 description 1
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 description 1
- WBYWAXJHAXSJNI-SREVYHEPSA-N Cinnamic acid Chemical compound OC(=O)\C=C/C1=CC=CC=C1 WBYWAXJHAXSJNI-SREVYHEPSA-N 0.000 description 1
- 206010052012 Congenital teratoma Diseases 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 208000009798 Craniopharyngioma Diseases 0.000 description 1
- 208000008334 Dermatofibrosarcoma Diseases 0.000 description 1
- 206010057070 Dermatofibrosarcoma protuberans Diseases 0.000 description 1
- 208000001154 Dermoid Cyst Diseases 0.000 description 1
- 208000008743 Desmoplastic Small Round Cell Tumor Diseases 0.000 description 1
- 206010064581 Desmoplastic small round cell tumour Diseases 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 201000009051 Embryonal Carcinoma Diseases 0.000 description 1
- 208000002460 Enteropathy-Associated T-Cell Lymphoma Diseases 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 208000033832 Eosinophilic Acute Leukemia Diseases 0.000 description 1
- 201000008228 Ependymoblastoma Diseases 0.000 description 1
- 206010014967 Ependymoma Diseases 0.000 description 1
- 206010014968 Ependymoma malignant Diseases 0.000 description 1
- 201000005231 Epithelioid sarcoma Diseases 0.000 description 1
- 208000031637 Erythroblastic Acute Leukemia Diseases 0.000 description 1
- 208000036566 Erythroleukaemia Diseases 0.000 description 1
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 1
- 208000006168 Ewing Sarcoma Diseases 0.000 description 1
- 208000012468 Ewing sarcoma/peripheral primitive neuroectodermal tumor Diseases 0.000 description 1
- 208000017259 Extragonadal germ cell tumor Diseases 0.000 description 1
- 208000010368 Extramammary Paget Disease Diseases 0.000 description 1
- 206010061850 Extranodal marginal zone B-cell lymphoma (MALT type) Diseases 0.000 description 1
- 201000001342 Fallopian tube cancer Diseases 0.000 description 1
- 208000013452 Fallopian tube neoplasm Diseases 0.000 description 1
- 201000008808 Fibrosarcoma Diseases 0.000 description 1
- 206010016935 Follicular thyroid cancer Diseases 0.000 description 1
- 201000004066 Ganglioglioma Diseases 0.000 description 1
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 description 1
- 206010061183 Genitourinary tract neoplasm Diseases 0.000 description 1
- 208000000527 Germinoma Diseases 0.000 description 1
- 208000002966 Giant Cell Tumor of Bone Diseases 0.000 description 1
- 201000005409 Gliomatosis cerebri Diseases 0.000 description 1
- 206010068601 Glioneuronal tumour Diseases 0.000 description 1
- 206010018381 Glomus tumour Diseases 0.000 description 1
- 206010018404 Glucagonoma Diseases 0.000 description 1
- 208000005234 Granulosa Cell Tumor Diseases 0.000 description 1
- 206010066476 Haematological malignancy Diseases 0.000 description 1
- 208000006050 Hemangiopericytoma Diseases 0.000 description 1
- 208000002250 Hematologic Neoplasms Diseases 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 206010021042 Hypopharyngeal cancer Diseases 0.000 description 1
- 206010056305 Hypopharyngeal neoplasm Diseases 0.000 description 1
- 208000005726 Inflammatory Breast Neoplasms Diseases 0.000 description 1
- 206010021980 Inflammatory carcinoma of the breast Diseases 0.000 description 1
- 206010061252 Intraocular melanoma Diseases 0.000 description 1
- 208000009164 Islet Cell Adenoma Diseases 0.000 description 1
- 208000007766 Kaposi sarcoma Diseases 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- 208000007666 Klatskin Tumor Diseases 0.000 description 1
- 101150105104 Kras gene Proteins 0.000 description 1
- 208000000675 Krukenberg Tumor Diseases 0.000 description 1
- RFSMUFRPPYDYRD-CALCHBBNSA-N Ku-0063794 Chemical compound C1=C(CO)C(OC)=CC=C1C1=CC=C(C(=NC(=N2)N3C[C@@H](C)O[C@@H](C)C3)N3CCOCC3)C2=N1 RFSMUFRPPYDYRD-CALCHBBNSA-N 0.000 description 1
- 208000031671 Large B-Cell Diffuse Lymphoma Diseases 0.000 description 1
- 208000032004 Large-Cell Anaplastic Lymphoma Diseases 0.000 description 1
- 206010023825 Laryngeal cancer Diseases 0.000 description 1
- 206010024218 Lentigo maligna Diseases 0.000 description 1
- 206010024305 Leukaemia monocytic Diseases 0.000 description 1
- 206010061523 Lip and/or oral cavity cancer Diseases 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 201000002171 Luteoma Diseases 0.000 description 1
- 206010025219 Lymphangioma Diseases 0.000 description 1
- 208000028018 Lymphocytic leukaemia Diseases 0.000 description 1
- 206010025312 Lymphoma AIDS related Diseases 0.000 description 1
- 201000003791 MALT lymphoma Diseases 0.000 description 1
- 231100000002 MTT assay Toxicity 0.000 description 1
- 238000000134 MTT assay Methods 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 208000006644 Malignant Fibrous Histiocytoma Diseases 0.000 description 1
- 206010064281 Malignant atrophic papulosis Diseases 0.000 description 1
- 208000030070 Malignant epithelial tumor of ovary Diseases 0.000 description 1
- 206010025557 Malignant fibrous histiocytoma of bone Diseases 0.000 description 1
- 206010073059 Malignant neoplasm of unknown primary site Diseases 0.000 description 1
- 208000032271 Malignant tumor of penis Diseases 0.000 description 1
- 208000025205 Mantle-Cell Lymphoma Diseases 0.000 description 1
- 208000009018 Medullary thyroid cancer Diseases 0.000 description 1
- 208000000172 Medulloblastoma Diseases 0.000 description 1
- 208000035490 Megakaryoblastic Acute Leukemia Diseases 0.000 description 1
- 208000002030 Merkel cell carcinoma Diseases 0.000 description 1
- 206010027406 Mesothelioma Diseases 0.000 description 1
- 206010027462 Metastases to ovary Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 208000035489 Monocytic Acute Leukemia Diseases 0.000 description 1
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 description 1
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 description 1
- 208000037538 Myelomonocytic Juvenile Leukemia Diseases 0.000 description 1
- 208000014767 Myeloproliferative disease Diseases 0.000 description 1
- 201000007224 Myeloproliferative neoplasm Diseases 0.000 description 1
- 206010028729 Nasal cavity cancer Diseases 0.000 description 1
- 206010028767 Nasal sinus cancer Diseases 0.000 description 1
- 208000002454 Nasopharyngeal Carcinoma Diseases 0.000 description 1
- 208000001894 Nasopharyngeal Neoplasms Diseases 0.000 description 1
- 206010029266 Neuroendocrine carcinoma of the skin Diseases 0.000 description 1
- 201000004404 Neurofibroma Diseases 0.000 description 1
- 208000005890 Neuroma Diseases 0.000 description 1
- 208000033755 Neutrophilic Chronic Leukemia Diseases 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 206010029488 Nodular melanoma Diseases 0.000 description 1
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 1
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 1
- 208000000160 Olfactory Esthesioneuroblastoma Diseases 0.000 description 1
- 201000010133 Oligodendroglioma Diseases 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 206010048757 Oncocytoma Diseases 0.000 description 1
- 206010031096 Oropharyngeal cancer Diseases 0.000 description 1
- 206010057444 Oropharyngeal neoplasm Diseases 0.000 description 1
- 208000007571 Ovarian Epithelial Carcinoma Diseases 0.000 description 1
- 206010061328 Ovarian epithelial cancer Diseases 0.000 description 1
- 206010033268 Ovarian low malignant potential tumour Diseases 0.000 description 1
- 206010073261 Ovarian theca cell tumour Diseases 0.000 description 1
- 208000002063 Oxyphilic Adenoma Diseases 0.000 description 1
- 208000025618 Paget disease of nipple Diseases 0.000 description 1
- 201000010630 Pancoast tumor Diseases 0.000 description 1
- 208000015330 Pancoast tumour Diseases 0.000 description 1
- 206010033701 Papillary thyroid cancer Diseases 0.000 description 1
- 208000037064 Papilloma of choroid plexus Diseases 0.000 description 1
- 206010061332 Paraganglion neoplasm Diseases 0.000 description 1
- 208000003937 Paranasal Sinus Neoplasms Diseases 0.000 description 1
- 208000000821 Parathyroid Neoplasms Diseases 0.000 description 1
- 208000002471 Penile Neoplasms Diseases 0.000 description 1
- 206010034299 Penile cancer Diseases 0.000 description 1
- 208000031839 Peripheral nerve sheath tumour malignant Diseases 0.000 description 1
- 208000000360 Perivascular Epithelioid Cell Neoplasms Diseases 0.000 description 1
- 208000009565 Pharyngeal Neoplasms Diseases 0.000 description 1
- 206010034811 Pharyngeal cancer Diseases 0.000 description 1
- 206010050487 Pinealoblastoma Diseases 0.000 description 1
- 208000007641 Pinealoma Diseases 0.000 description 1
- 208000021308 Pituicytoma Diseases 0.000 description 1
- 201000005746 Pituitary adenoma Diseases 0.000 description 1
- 206010061538 Pituitary tumour benign Diseases 0.000 description 1
- 201000008199 Pleuropulmonary blastoma Diseases 0.000 description 1
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 1
- 206010065857 Primary Effusion Lymphoma Diseases 0.000 description 1
- 208000026149 Primary peritoneal carcinoma Diseases 0.000 description 1
- 206010057846 Primitive neuroectodermal tumour Diseases 0.000 description 1
- 208000033759 Prolymphocytic T-Cell Leukemia Diseases 0.000 description 1
- 208000033826 Promyelocytic Acute Leukemia Diseases 0.000 description 1
- 208000006930 Pseudomyxoma Peritonei Diseases 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 208000034541 Rare lymphatic malformation Diseases 0.000 description 1
- 208000015634 Rectal Neoplasms Diseases 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 201000000582 Retinoblastoma Diseases 0.000 description 1
- 208000008938 Rhabdoid tumor Diseases 0.000 description 1
- 208000005678 Rhabdomyoma Diseases 0.000 description 1
- 208000025316 Richter syndrome Diseases 0.000 description 1
- 208000025280 Sacrococcygeal teratoma Diseases 0.000 description 1
- 208000004337 Salivary Gland Neoplasms Diseases 0.000 description 1
- 206010061934 Salivary gland cancer Diseases 0.000 description 1
- 208000006938 Schwannomatosis Diseases 0.000 description 1
- 201000010208 Seminoma Diseases 0.000 description 1
- 208000000097 Sertoli-Leydig cell tumor Diseases 0.000 description 1
- 208000002669 Sex Cord-Gonadal Stromal Tumors Diseases 0.000 description 1
- 208000009359 Sezary Syndrome Diseases 0.000 description 1
- 208000021388 Sezary disease Diseases 0.000 description 1
- 208000003252 Signet Ring Cell Carcinoma Diseases 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- 208000021712 Soft tissue sarcoma Diseases 0.000 description 1
- 206010041329 Somatostatinoma Diseases 0.000 description 1
- 208000000102 Squamous Cell Carcinoma of Head and Neck Diseases 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- 206010042553 Superficial spreading melanoma stage unspecified Diseases 0.000 description 1
- 208000031673 T-Cell Cutaneous Lymphoma Diseases 0.000 description 1
- 208000029052 T-cell acute lymphoblastic leukemia Diseases 0.000 description 1
- 201000008717 T-cell large granular lymphocyte leukemia Diseases 0.000 description 1
- 208000000389 T-cell leukemia Diseases 0.000 description 1
- 208000028530 T-cell lymphoblastic leukemia/lymphoma Diseases 0.000 description 1
- 206010042971 T-cell lymphoma Diseases 0.000 description 1
- 208000027585 T-cell non-Hodgkin lymphoma Diseases 0.000 description 1
- 208000026651 T-cell prolymphocytic leukemia Diseases 0.000 description 1
- 208000020982 T-lymphoblastic lymphoma Diseases 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 206010043276 Teratoma Diseases 0.000 description 1
- 208000024313 Testicular Neoplasms Diseases 0.000 description 1
- 201000000331 Testicular germ cell cancer Diseases 0.000 description 1
- 206010057644 Testis cancer Diseases 0.000 description 1
- 108010022394 Threonine synthase Proteins 0.000 description 1
- 206010043515 Throat cancer Diseases 0.000 description 1
- 201000009365 Thymic carcinoma Diseases 0.000 description 1
- 102000005497 Thymidylate Synthase Human genes 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- 208000015778 Undifferentiated pleomorphic sarcoma Diseases 0.000 description 1
- 206010046431 Urethral cancer Diseases 0.000 description 1
- 206010046458 Urethral neoplasms Diseases 0.000 description 1
- 208000008385 Urogenital Neoplasms Diseases 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- 208000002495 Uterine Neoplasms Diseases 0.000 description 1
- 208000009311 VIPoma Diseases 0.000 description 1
- 208000014070 Vestibular schwannoma Diseases 0.000 description 1
- 206010047741 Vulval cancer Diseases 0.000 description 1
- 208000004354 Vulvar Neoplasms Diseases 0.000 description 1
- 208000021146 Warthin tumor Diseases 0.000 description 1
- 208000000260 Warts Diseases 0.000 description 1
- 208000008383 Wilms tumor Diseases 0.000 description 1
- 208000012018 Yolk sac tumor Diseases 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- RFSMUFRPPYDYRD-UHFFFAOYSA-N [5-[2-(2,6-dimethylmorpholin-4-yl)-4-morpholin-4-ylpyrido[2,3-d]pyrimidin-7-yl]-2-methoxyphenyl]methanol Chemical compound C1=C(CO)C(OC)=CC=C1C1=CC=C(C(=NC(=N2)N3CC(C)OC(C)C3)N3CCOCC3)C2=N1 RFSMUFRPPYDYRD-UHFFFAOYSA-N 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 239000003070 absorption delaying agent Substances 0.000 description 1
- 206010059394 acanthoma Diseases 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 208000006336 acinar cell carcinoma Diseases 0.000 description 1
- 208000004064 acoustic neuroma Diseases 0.000 description 1
- 206010000583 acral lentiginous melanoma Diseases 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 208000021841 acute erythroid leukemia Diseases 0.000 description 1
- 208000013593 acute megakaryoblastic leukemia Diseases 0.000 description 1
- 208000020700 acute megakaryocytic leukemia Diseases 0.000 description 1
- 208000026784 acute myeloblastic leukemia with maturation Diseases 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 208000002517 adenoid cystic carcinoma Diseases 0.000 description 1
- 208000026562 adenomatoid odontogenic tumor Diseases 0.000 description 1
- 208000020990 adrenal cortex carcinoma Diseases 0.000 description 1
- 208000007128 adrenocortical carcinoma Diseases 0.000 description 1
- 201000006966 adult T-cell leukemia Diseases 0.000 description 1
- 208000015230 aggressive NK-cell leukemia Diseases 0.000 description 1
- 229940125528 allosteric inhibitor Drugs 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 208000008524 alveolar soft part sarcoma Diseases 0.000 description 1
- 230000002707 ameloblastic effect Effects 0.000 description 1
- 229940024606 amino acid Drugs 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 125000004397 aminosulfonyl group Chemical group NS(=O)(=O)* 0.000 description 1
- 206010002449 angioimmunoblastic T-cell lymphoma Diseases 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000003096 antiparasitic agent Substances 0.000 description 1
- 229940125687 antiparasitic agent Drugs 0.000 description 1
- 239000000074 antisense oligonucleotide Substances 0.000 description 1
- 238000012230 antisense oligonucleotides Methods 0.000 description 1
- 201000011165 anus cancer Diseases 0.000 description 1
- 208000021780 appendiceal neoplasm Diseases 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 125000005605 benzo group Chemical group 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 201000009036 biliary tract cancer Diseases 0.000 description 1
- 208000020790 biliary tract neoplasm Diseases 0.000 description 1
- 230000008827 biological function Effects 0.000 description 1
- 201000009076 bladder urachal carcinoma Diseases 0.000 description 1
- 201000000053 blastoma Diseases 0.000 description 1
- 201000011143 bone giant cell tumor Diseases 0.000 description 1
- 208000012172 borderline epithelial tumor of ovary Diseases 0.000 description 1
- 210000000133 brain stem Anatomy 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 230000005907 cancer growth Effects 0.000 description 1
- 208000035269 cancer or benign tumor Diseases 0.000 description 1
- 150000003857 carboxamides Chemical class 0.000 description 1
- 208000002458 carcinoid tumor Diseases 0.000 description 1
- 230000003833 cell viability Effects 0.000 description 1
- 201000007335 cerebellar astrocytoma Diseases 0.000 description 1
- 208000030239 cerebral astrocytoma Diseases 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 208000006990 cholangiocarcinoma Diseases 0.000 description 1
- 210000000349 chromosome Anatomy 0.000 description 1
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 1
- 201000006778 chronic monocytic leukemia Diseases 0.000 description 1
- 201000010902 chronic myelomonocytic leukemia Diseases 0.000 description 1
- 201000010903 chronic neutrophilic leukemia Diseases 0.000 description 1
- 235000013985 cinnamic acid Nutrition 0.000 description 1
- 229930016911 cinnamic acid Natural products 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 201000010276 collecting duct carcinoma Diseases 0.000 description 1
- 238000011284 combination treatment Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 208000017563 cutaneous Paget disease Diseases 0.000 description 1
- 201000007241 cutaneous T cell lymphoma Diseases 0.000 description 1
- 208000017763 cutaneous neuroendocrine carcinoma Diseases 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 210000004443 dendritic cell Anatomy 0.000 description 1
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 1
- 206010012818 diffuse large B-cell lymphoma Diseases 0.000 description 1
- 239000002612 dispersion medium Substances 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 201000004428 dysembryoplastic neuroepithelial tumor Diseases 0.000 description 1
- 201000008184 embryoma Diseases 0.000 description 1
- 208000001991 endodermal sinus tumor Diseases 0.000 description 1
- 230000002357 endometrial effect Effects 0.000 description 1
- 208000027858 endometrioid tumor Diseases 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 230000008029 eradication Effects 0.000 description 1
- 201000004101 esophageal cancer Diseases 0.000 description 1
- 208000032099 esthesioneuroblastoma Diseases 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- 201000008819 extrahepatic bile duct carcinoma Diseases 0.000 description 1
- 201000010972 female reproductive endometrioid cancer Diseases 0.000 description 1
- 210000003754 fetus Anatomy 0.000 description 1
- 201000003444 follicular lymphoma Diseases 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- 201000008361 ganglioneuroma Diseases 0.000 description 1
- 201000011587 gastric lymphoma Diseases 0.000 description 1
- 230000014509 gene expression Effects 0.000 description 1
- 201000003115 germ cell cancer Diseases 0.000 description 1
- 201000008822 gestational choriocarcinoma Diseases 0.000 description 1
- 201000007116 gestational trophoblastic neoplasm Diseases 0.000 description 1
- 208000003064 gonadoblastoma Diseases 0.000 description 1
- 201000010536 head and neck cancer Diseases 0.000 description 1
- 208000014829 head and neck neoplasm Diseases 0.000 description 1
- 201000000459 head and neck squamous cell carcinoma Diseases 0.000 description 1
- 201000010235 heart cancer Diseases 0.000 description 1
- 208000024348 heart neoplasm Diseases 0.000 description 1
- 201000002222 hemangioblastoma Diseases 0.000 description 1
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 1
- 206010066957 hepatosplenic T-cell lymphoma Diseases 0.000 description 1
- 201000011045 hereditary breast ovarian cancer syndrome Diseases 0.000 description 1
- 208000029824 high grade glioma Diseases 0.000 description 1
- 208000018060 hilar cholangiocarcinoma Diseases 0.000 description 1
- 201000006866 hypopharynx cancer Diseases 0.000 description 1
- 230000002267 hypothalamic effect Effects 0.000 description 1
- NSHFLKZNBPJNPA-UHFFFAOYSA-N imidazo[4,5-c]quinolin-2-one Chemical compound C1=CC=C2C3=NC(=O)N=C3C=NC2=C1 NSHFLKZNBPJNPA-UHFFFAOYSA-N 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 201000004933 in situ carcinoma Diseases 0.000 description 1
- 201000004653 inflammatory breast carcinoma Diseases 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 201000002529 islet cell tumor Diseases 0.000 description 1
- JJWLVOIRVHMVIS-UHFFFAOYSA-N isopropylamine Chemical compound CC(C)N JJWLVOIRVHMVIS-UHFFFAOYSA-N 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 201000005992 juvenile myelomonocytic leukemia Diseases 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 206010023841 laryngeal neoplasm Diseases 0.000 description 1
- 208000011080 lentigo maligna melanoma Diseases 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 206010024627 liposarcoma Diseases 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 208000016992 lung adenocarcinoma in situ Diseases 0.000 description 1
- 208000024169 luteoma of pregnancy Diseases 0.000 description 1
- 208000012804 lymphangiosarcoma Diseases 0.000 description 1
- 230000001926 lymphatic effect Effects 0.000 description 1
- 208000003747 lymphoid leukemia Diseases 0.000 description 1
- 201000000564 macroglobulinemia Diseases 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 159000000003 magnesium salts Chemical class 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 208000030883 malignant astrocytoma Diseases 0.000 description 1
- 201000011614 malignant glioma Diseases 0.000 description 1
- 208000006178 malignant mesothelioma Diseases 0.000 description 1
- 201000009020 malignant peripheral nerve sheath tumor Diseases 0.000 description 1
- 208000015179 malignant superior sulcus neoplasm Diseases 0.000 description 1
- 201000001117 malignant triton tumor Diseases 0.000 description 1
- 208000026045 malignant tumor of parathyroid gland Diseases 0.000 description 1
- 208000027202 mammary Paget disease Diseases 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 208000000516 mast-cell leukemia Diseases 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 201000000349 mediastinal cancer Diseases 0.000 description 1
- 208000029586 mediastinal germ cell tumor Diseases 0.000 description 1
- 208000023356 medullary thyroid gland carcinoma Diseases 0.000 description 1
- 201000008203 medulloepithelioma Diseases 0.000 description 1
- 206010027191 meningioma Diseases 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 208000037970 metastatic squamous neck cancer Diseases 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- WBYWAXJHAXSJNI-UHFFFAOYSA-N methyl p-hydroxycinnamate Natural products OC(=O)C=CC1=CC=CC=C1 WBYWAXJHAXSJNI-UHFFFAOYSA-N 0.000 description 1
- 208000024191 minimally invasive lung adenocarcinoma Diseases 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 201000006894 monocytic leukemia Diseases 0.000 description 1
- 230000000877 morphologic effect Effects 0.000 description 1
- 208000022669 mucinous neoplasm Diseases 0.000 description 1
- 206010051747 multiple endocrine neoplasia Diseases 0.000 description 1
- 201000005987 myeloid sarcoma Diseases 0.000 description 1
- 208000009091 myxoma Diseases 0.000 description 1
- AHDFWNJLFALBJP-JTQLQIEISA-N n-[(2s)-1-amino-3-(3,4-difluorophenyl)propan-2-yl]-5-chloro-4-(4-chloro-2-methylpyrazol-3-yl)thiophene-2-carboxamide Chemical compound CN1N=CC(Cl)=C1C1=C(Cl)SC(C(=O)N[C@H](CN)CC=2C=C(F)C(F)=CC=2)=C1 AHDFWNJLFALBJP-JTQLQIEISA-N 0.000 description 1
- 208000014761 nasopharyngeal type undifferentiated carcinoma Diseases 0.000 description 1
- 201000011216 nasopharynx carcinoma Diseases 0.000 description 1
- 208000018280 neoplasm of mediastinum Diseases 0.000 description 1
- 208000028732 neoplasm with perivascular epithelioid cell differentiation Diseases 0.000 description 1
- 208000007538 neurilemmoma Diseases 0.000 description 1
- 201000009494 neurilemmomatosis Diseases 0.000 description 1
- 208000027831 neuroepithelial neoplasm Diseases 0.000 description 1
- 208000029974 neurofibrosarcoma Diseases 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 201000000032 nodular malignant melanoma Diseases 0.000 description 1
- 150000007523 nucleic acids Chemical group 0.000 description 1
- 201000002575 ocular melanoma Diseases 0.000 description 1
- 206010073131 oligoastrocytoma Diseases 0.000 description 1
- 230000004650 oncogenic pathway Effects 0.000 description 1
- 230000005959 oncogenic signaling Effects 0.000 description 1
- 201000011130 optic nerve sheath meningioma Diseases 0.000 description 1
- 208000022982 optic pathway glioma Diseases 0.000 description 1
- 201000006958 oropharynx cancer Diseases 0.000 description 1
- 208000021284 ovarian germ cell tumor Diseases 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- QXAMTEJJAZOINB-UHFFFAOYSA-N oxane-3,4,5-triol Chemical compound OC1COCC(O)C1O QXAMTEJJAZOINB-UHFFFAOYSA-N 0.000 description 1
- 201000011116 pancreatic cholera Diseases 0.000 description 1
- 208000022102 pancreatic neuroendocrine neoplasm Diseases 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 208000003154 papilloma Diseases 0.000 description 1
- 208000029211 papillomatosis Diseases 0.000 description 1
- 208000007312 paraganglioma Diseases 0.000 description 1
- 201000007052 paranasal sinus cancer Diseases 0.000 description 1
- 208000030940 penile carcinoma Diseases 0.000 description 1
- 201000005207 perivascular epithelioid cell tumor Diseases 0.000 description 1
- 208000028591 pheochromocytoma Diseases 0.000 description 1
- 201000004119 pineal parenchymal tumor of intermediate differentiation Diseases 0.000 description 1
- 201000003113 pineoblastoma Diseases 0.000 description 1
- 208000021310 pituitary gland adenoma Diseases 0.000 description 1
- 208000010916 pituitary tumor Diseases 0.000 description 1
- 208000010626 plasma cell neoplasm Diseases 0.000 description 1
- 208000024246 polyembryoma Diseases 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- 208000016800 primary central nervous system lymphoma Diseases 0.000 description 1
- 208000025638 primary cutaneous T-cell non-Hodgkin lymphoma Diseases 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 229940107700 pyruvic acid Drugs 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 206010038038 rectal cancer Diseases 0.000 description 1
- 201000001275 rectum cancer Diseases 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 208000030859 renal pelvis/ureter urothelial carcinoma Diseases 0.000 description 1
- 210000002345 respiratory system Anatomy 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 201000009410 rhabdomyosarcoma Diseases 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 201000007416 salivary gland adenoid cystic carcinoma Diseases 0.000 description 1
- 206010039667 schwannoma Diseases 0.000 description 1
- 201000008407 sebaceous adenocarcinoma Diseases 0.000 description 1
- 150000003335 secondary amines Chemical class 0.000 description 1
- 208000011581 secondary neoplasm Diseases 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- 208000028467 sex cord-stromal tumor Diseases 0.000 description 1
- 201000008123 signet ring cell adenocarcinoma Diseases 0.000 description 1
- 201000000849 skin cancer Diseases 0.000 description 1
- 201000008261 skin carcinoma Diseases 0.000 description 1
- 201000010153 skin papilloma Diseases 0.000 description 1
- 208000000649 small cell carcinoma Diseases 0.000 description 1
- 201000002314 small intestine cancer Diseases 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 239000011877 solvent mixture Substances 0.000 description 1
- 239000004071 soot Substances 0.000 description 1
- 206010062261 spinal cord neoplasm Diseases 0.000 description 1
- 208000037959 spinal tumor Diseases 0.000 description 1
- 206010062113 splenic marginal zone lymphoma Diseases 0.000 description 1
- 208000030457 superficial spreading melanoma Diseases 0.000 description 1
- 201000008205 supratentorial primitive neuroectodermal tumor Diseases 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 206010042863 synovial sarcoma Diseases 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- 201000003120 testicular cancer Diseases 0.000 description 1
- 208000001644 thecoma Diseases 0.000 description 1
- 201000002510 thyroid cancer Diseases 0.000 description 1
- 208000030901 thyroid gland follicular carcinoma Diseases 0.000 description 1
- 208000030045 thyroid gland papillary carcinoma Diseases 0.000 description 1
- 208000019179 thyroid gland undifferentiated (anaplastic) carcinoma Diseases 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- GUXXEUUYCAYESJ-UHFFFAOYSA-N torin 2 Chemical compound C1=NC(N)=CC=C1C1=CC=C(N=CC2=C3N(C=4C=C(C=CC=4)C(F)(F)F)C(=O)C=C2)C3=C1 GUXXEUUYCAYESJ-UHFFFAOYSA-N 0.000 description 1
- 201000007363 trachea carcinoma Diseases 0.000 description 1
- YFTHZRPMJXBUME-UHFFFAOYSA-N tripropylamine Chemical compound CCCN(CCC)CCC YFTHZRPMJXBUME-UHFFFAOYSA-N 0.000 description 1
- 208000018417 undifferentiated high grade pleomorphic sarcoma of bone Diseases 0.000 description 1
- 208000023747 urothelial carcinoma Diseases 0.000 description 1
- 206010046766 uterine cancer Diseases 0.000 description 1
- 208000037965 uterine sarcoma Diseases 0.000 description 1
- 206010046885 vaginal cancer Diseases 0.000 description 1
- 208000013139 vaginal neoplasm Diseases 0.000 description 1
- 208000008662 verrucous carcinoma Diseases 0.000 description 1
- 201000005102 vulva cancer Diseases 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4709—Non-condensed quinolines and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
- A61K31/4184—1,3-Diazoles condensed with carbocyclic rings, e.g. benzimidazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/4353—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems
- A61K31/436—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems the heterocyclic ring system containing a six-membered ring having oxygen as a ring hetero atom, e.g. rapamycin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/4353—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems
- A61K31/4375—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems the heterocyclic ring system containing a six-membered ring having nitrogen as a ring heteroatom, e.g. quinolizines, naphthyridines, berberine, vincamine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4738—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems
- A61K31/4745—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems condensed with ring systems having nitrogen as a ring hetero atom, e.g. phenantrolines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
Definitions
- Cancer is the second most prevalent cause of death in the United States and is a complex disease arising after a selection process for cells with acquired functional capabilities, such as limitless proliferative potential and metastatic capabilities.
- the subject application provides a novel combination therapy that targets cancer at the PI3K/AKT/mTOR pathway, which is constitutively activated in many types of cancers.
- the disclosed combination therapy has, surprisingly, been found to be effective in the treatment of cancer, particularly breast cancer, prostate cancer, multiple myeloma, hepatocyte carcinoma, lung cancer, non-small cell lung carcinoma, colorectal cancer, melanoma and/or pancreatic cancer.
- compositions comprising inhibitors of PI3K, AKT and/or mTOR, one or more 4-quinolinemethanols and a pharmaceutically acceptable excipient. These compositions can contain subtherapeutic amounts of each active ingredient (PI3K, AKT, mTOR, one or more 4-quinolinemethanols and various combinations thereof).
- the compositions provided by the subject application can be used for the treatment of various forms of cancer, such as breast cancer, prostate cancer, multiple myeloma, hepatocyte carcinoma, brain cancer, lung cancer, non-small cell lung carcinoma, colorectal cancer, melanoma and/or pancreatic cancer.
- FIG. 1 Outline of Compound P+Ras Effector Inhibitor Experiments.
- FIG. 2 Compound P+Everolimus MIA PaCa-2.
- FIG. 3 Compound P+Everolimus MIA PaCa-2.
- FIG. 4 Compound P+Everolimus MIA PaCa-2.
- FIG. 5 Compound P+Everolimus Panc-1.
- FIG. 6 Compound P+Everolimus Panc-1.
- FIG. 7 Compound P+Everolimus Panc-1.
- FIG. 8 Compound P+Everolimus (mTOR inhibitor) in Pancreatic Ductal Adenocarcinoma Cell Lines Derived from KRAS/PTEN Mutant Mouse Tumors.
- FIG. 9 Compound P+MK-2206 MIA PaCa-2.
- FIG. 10 Compound P+MK-2206 MIA PaCa-2.
- FIG. 11 Compound P+MK-2206 Panc-1.
- FIG. 12 Compound P+MK-2206 Panc-1.
- FIG. 13 Compound P+MK-2206 (AKT inhibitor) in Pancreatic Ductal Adenocarcinoma Cell Lines Derived from KRAS/PTEN Mutant Mouse Tumors.
- FIG. 14 Compound P+BEZ-235 MIA PaCa-2.
- FIG. 15 Compound P+BEZ-235 MIA PaCa-2.
- FIG. 16 Compound P+BEZ-235 MIA PaCa-2.
- FIG. 17 Compound P+BEZ-235 Panc-1.
- FIG. 18 Compound P+BEZ-235 Panc-1.
- FIG. 19 Compound P+BEZ-235 Panc-1.
- FIG. 20 Compound P+BEZ235 (Dual PI-3K/mTOR Inhibitor) in Pancreatic Ductal Adenocarcinoma Cell Lines Derived from KRAS/PTEN Mutant Mouse Tumors.
- FIG. 21 Summary Of Ratio Ranges for PDAC Cell Lines.
- FIG. 22 TS Accelerates KRAS mutant PDAC.
- Table 1 provides a list of exemplary 4-quinolinemethanols that can be used in the formulation of compositions disclosed herein.
- Compound P as used throughout this application, is mefloquine or a pharmaceutically acceptable salt thereof.
- Table 2 provides a list of exemplary PI-3K inhibitors that can be used in the formulation of compositions disclosed herein.
- Table 3 provides a list of exemplary AKT inhibitors that can be used in the formulation of compositions disclosed herein.
- Table 4 provides a list of exemplary mTOR inhibitors that can be used in the formulation of compositions disclosed herein.
- PI3K Phosphoinositide-3-kinase
- AKT serine-threonine protein kinase B
- mTOR Mammalian target of rapamycin).
- the term “about” or “approximately” means within an acceptable error range for the particular value as determined by one of ordinary skill in the art, which will depend in part on how the value is measured or determined, i.e., the limitations of the measurement system. For example, “about” can mean within 1 or more than 1 standard deviation, per the practice in the art. Alternatively, “about” can mean a range of up to 0-20%, 0 to 10%, 0 to 5%, or up to 1% of a given value. Alternatively, particularly with respect to biological systems or processes, the term can mean within an order of magnitude, preferably within 5-fold, and more preferably within 2-fold, of a value.
- compositions containing amounts of ingredients where the terms “about” or “approximately” are used contain the stated amount of the ingredient with a variation (error range) of 0-10% around the value (X ⁇ 10%).
- Treatment “Treatment”, “treating”, “palliating” and “ameliorating” (and grammatical variants of these terms), as used herein, are used interchangeably. These terms refer to an approach for obtaining beneficial or desired results including but not limited to therapeutic benefit. A therapeutic benefit is achieved with the eradication or amelioration of one or more of the physiological symptoms associated with the underlying disorder such that an improvement is observed in the patient, notwithstanding that the patient may still be afflicted with the underlying disorder.
- cancer refers to the presence of cells possessing abnormal growth characteristics, such as uncontrolled proliferation, immortality, metastatic potential, rapid growth and proliferation rate, perturbed oncogenic signaling, and certain characteristic morphological features. This includes but is not limited to the growth of: (1) benign or malignant cells (e.g., tumor cells) that correlates with overexpression of a serine/threonine kinase; or (2) benign or malignant cells (e.g., tumor cells) that correlates with abnormally high levels of serine/threonine kinase activity or lipid kinase activity.
- benign or malignant cells e.g., tumor cells
- benign or malignant cells e.g., tumor cells
- benign or malignant cells e.g., tumor cells
- lipid kinases include but are not limited to PI3 kinases such as PBK ⁇ , PBK ⁇ , PBK ⁇ , and PBK ⁇ .
- the term “effective amount” or “therapeutically effective amount” refers to that amount of an inhibitor described herein that is sufficient to effect the intended application including but not limited to disease treatment.
- the therapeutically effective amount may vary depending upon the intended application (in vitro or in vivo), or the subject and disease condition being treated, e.g., the weight and age of the subject, the severity of the disease condition, the manner of administration and the like, which can readily be determined by one of ordinary skill in the art.
- the term also applies to a dose that will induce a particular response in target cells, e.g., reduction of proliferation or downregulation of activity of a target protein.
- the specific dose will vary depending on the particular compounds chosen, the dosing regimen to be followed, whether it is administered in combination with other compounds, timing of administration, the tissue to which it is administered, and the physical delivery system in which it is carried.
- a “sub-therapeutic amount” of an agent is an amount less than the effective amount for that agent, but which when combined with an effective or sub-therapeutic amount of another agent or therapy can produce a desired result, due to, for example, synergy in the resulting efficacious effects (e.g., therapeutic benefit) for the patient, or reduced side effects associated with the compounds administered to the patient.
- Typical therapeutic amounts for an agent, as disclosed herein can be ascertained from various publicly available sources (e.g., drugs.com, The Physician's Desk Reference, or scientific literature).
- Subtherapeutic amounts of an agent, as provided herein are amounts less than those reported in the publicly available sources.
- the therapeutic dose is 1000 mg per day.
- subtherapeutic doses of mefloquine (per day) are less than 1000 mg and can range from 5 mg to 950 mg, about 5 mg to about 500 mg, about 100 mg to about 500 mg, or about 200 to about 700 mg.
- the therapeutic dose is 648 mg per day.
- subtherapeutic doses of quinine (per day) are less than range from 5 mg to 625 mg, about 5 mg to about 500 mg, about 100 mg to about 575 mg, or about 200 to about 500 mg.
- subtherapeutic doses (per day) are less than 300 mg.
- subtherapeutic doses can range from 5 mg to 250 mg, about 5 mg to about 150 mg, about 100 mg to about 125 mg, or about 50 to about 150 mg.
- subtherapeutic doses (per day) are less than 150 mg per day for subjects of 45 kg or more or less than 100 mg per day for subjects between 30 and 44 kg.
- subtherapeutic doses can range from 5 mg to 125 mg, about 5 mg to about 100 mg, about 100 mg to about 125 mg, or about 25 to about 125 mg.
- subtherapeutic doses (per day) are less than 2 mg per day for subjects of 40 kg or more or less than 1 mg/m 2 (body surface area) per day for subjects less than 40 kg.
- subtherapeutic doses can range from 0.1 mg to 1.95 mg, about 0.5 mg to about 1.75 mg, about 1 mg to about 1.50 mg, or about 0.25 to about 1.50 mg.
- subtherapeutic doses (per day) can range from 0.1 mg to 0.95 mg, about 0.5 mg to about 0.75 mg, about 0.01 mg to about 0.75 mg, or about 0.25 to about 0.75 mg.
- subtherapeutic doses (per day) are less than 10 mg per day.
- subtherapeutic doses (per day) can range from 0.1 mg to 9.50 mg, about 0.5 mg to about 9.00 mg, about 1 mg to about 7.5 mg, or about 2.50 to about 5.0 mg.
- subtherapeutic doses are about 25 mg/week.
- subtherapeutic doses (per week) can range from 1 mg to 24.5 mg, about 0.5 mg to about 22 mg, about 1 mg to about 15 mg, or about 2.50 to about 20 mg.
- a “synergistically effective” therapeutic amount or “synergistically effective” amount of an agent is an amount which, when combined with an effective or subtherapeutic amount of another agent or therapy, produces a greater effect than when either of the two agents are used alone.
- a synergistically effective therapeutic amount of an agent produces a greater effect when used in combination than the additive effects of each of the two agents or therapies when used alone.
- the term “greater effect” encompasses not only a reduction in symptoms of the disorder to be treated, but also an improved side effect profile, improved tolerability, improved patient compliance, improved efficacy, or any other improved clinical outcome.
- agent refers to an inhibitor of PI3K, AKT and/or mTOR and one or more 4-quinolinemethanols. Exemplary agents are provided in Tables 1-4 of this application.
- antagonists may be used interchangeably, and they refer to a compound having the ability to inhibit a biological function of a target protein, whether by inhibiting the activity or expression of the target protein. Accordingly, the terms “antagonist” and “inhibitor” are defined in the context of the biological role of the target protein. Exemplary “antagonists” and “inhibitors” are provided in Tables 1-4 of this application.
- co-administration encompass administration of two or more agents to a subject so that both agents and/or their metabolites are present in the subject at the same time.
- Co-administration includes simultaneous administration in separate compositions, administration at different times in separate compositions, or administration in a composition in which both agents are present.
- Co-administered agents may be in the same formulation.
- Coadministered agents may also be in different formulations.
- a “therapeutic effect,” as used herein, encompasses a therapeutic benefit as described above. This includes delaying the appearance of a disease or condition, delaying the onset of symptoms of a disease or condition, slowing, halting, or reversing the progression of a disease or condition, or any combination thereof.
- pharmaceutically acceptable salt refers to salts derived from a variety of organic and inorganic counter ions well known in the art.
- Pharmaceutically acceptable acid addition salts can be formed with inorganic acids and organic acids.
- Inorganic acids from which salts can be derived include, for example, hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric acid, and the like.
- Organic acids from which salts can be derived include, for example, acetic acid, propionic acid, glycolic acid, pyruvic acid, oxalic acid, maleic acid, malonic acid, succinic acid, fumaric acid, tartaric acid, citric acid, benzoic acid, cinnamic acid, mandelic acid, methanesulfonic acid, ethanesulfonic acid, p-toluenesulfonic acid, salicylic acid, and the like.
- Pharmaceutically acceptable base addition salts can be formed with inorganic and organic bases.
- Inorganic bases from which salts can be derived include, for example, sodium, potassium, lithium, ammonium, calcium, magnesium, iron, zinc, copper, manganese, aluminum, and the like.
- Organic bases from which salts can be derived include, for example, primary, secondary, and tertiary amines, substituted amines including naturally occurring substituted amines, cyclic amines, basic ion exchange resins, and the like, specifically such as isopropylamine, trimethylamine, diethylamine, triethylamine, tripropylamine, and ethanolamine.
- the pharmaceutically acceptable base addition salt is chosen from ammonium, potassium, sodium, calcium, and magnesium salts.
- “Pharmaceutically acceptable carrier” or “pharmaceutically acceptable excipient” includes any and all solvents, dispersion media, coatings, antibacterial and antifungal agents, isotonic and absorption delaying agents and the like. The use of such media and agents for pharmaceutically active substances is well known in the art. Except insofar as any conventional media or agent is incompatible with the active ingredient, its use in the therapeutic compositions of the invention is contemplated. Supplementary active ingredients can also be incorporated into the compositions.
- Subject refers to an animal, such as a mammal, for example a human.
- the methods described herein can be useful in both pre-clinical human therapeutics and veterinary applications.
- the subject is a mammal (such as an animal model of disease), and in some embodiments, the subject is human.
- the terms “subject” and “patient” can be used interchangably.
- the terms “simultaneous” or “simultaneously” as applied to administering agents to a subject refer to administering one or more agents at the same time, or at two different time points that are separated by no more than 1 hour.
- the term “sequentially” refers to administering more than one agent at two different time points that are separated by more than 1 hour, e.g., about 2 hours, about 5 hours, 8 hours, 1 day, 2 days, 3 days, 4 days, 5 days, 6 days, 7 days or even longer.
- the subject application provides methods of treating cancer comprising the administration or co-administration of a composition comprising one or more 4-quinolinemethanols and: a) one or more agents that inhibit the activity of Phosphoinositide-3-kinase (PI-3K); b) one or more agents that inhibit the activity of serine-threonine protein kinase B (AKT); c) one or more agents that inhibit the activity of mammalian target of rapamycin (mTOR); or d) any combination of one or more agents that inhibit the activity of PI-3K, AKT and mTOR.
- PI-3K Phosphoinositide-3-kinase
- AKT serine-threonine protein kinase B
- mTOR mammalian target of rapamycin
- any combination of one or more agents that inhibit the activity of PI-3K, AKT and mTOR any combination of one or more agents that inhibit the activity of PI-3K, AKT and mTOR.
- the 4-quinolinemethanol is selected from Table 1, the PI-3K inhibitor is selected from Table 2, the AKT inhibitor is selected from Table 3 and the mTOR inhibitor is selected from Table 4.
- one or more 4-quinolinemethanols and one or more PI-3K inhibitors are administered simultaneously or sequentially.
- Other embodiments provide for the one or more 4-quinolinemethanols and one or more PI-3K inhibitors to be co-administered as a single composition.
- Yet other embodiments provide for one or more 4-quinolinemethanols and one or more AKT inhibitors to co-administered or administered simultaneously or sequentially.
- Yet another embodiment provides for one or more 4-quinolinemethanols and one or more AKT inhibitors to be co-administered as a single composition.
- the disclosed method can comprise the administration or co-administration of more 4-quinolinemethanols and one or more mTOR inhibitors.
- one or more 4-quinolinemethanols and one or more AKT inhibitors can be administered simultaneously or sequentially or as a single composition.
- the subtherapeutic amounts of these agents are preferably provided in synergistically effective amounts.
- the agents can be administered in subtherapeutic amounts in the following ratios:
- 4QM 4-quinolinemethanols
- mTORI mTOR inhibitors
- Cancers suitable for treatment according to the disclosed methods include, but are not limited to: Acanthoma, Acinic cell carcinoma, Acoustic neuroma, Acral lentiginous melanoma, Acrospiroma, Acute eosinophilic leukemia, Acute lymphoblastic leukemia, Acute megakaryoblastic leukemia, Acute monocytic leukemia, Acute myeloblastic leukemia with maturation, Acute myeloid dendritic cell leukemia, Acute myeloid leukemia, Acute promyelocytic leukemia, Adamantinoma, Adenocarcinoma, Adenoid cystic carcinoma, Adenoma, Adenomatoid odontogenic tumor, Adrenocortical carcinoma, Adult T-cell leukemia, Aggressive NK-cell leukemia, AIDS-related cancers, AIDS-related lymphoma, Alveolar soft part sarcoma, Ameloblastic fibroma, Anal cancer, Anaplastic
- the cancer selected from the group consisting of non-small cell lung cancer, small cell lung cancer, head and neck squamous cell carcinoma, pancreatic cancer, breast cancer, ovarian cancer, renal cell carcinoma, prostate cancer, neuroendocrine cancer, gastric cancer, bladder cancer, a brain cancer (glioma, astrocytoma, glioblastoma multiforme, etc.), colon cancer and endometrial cancer.
- compositions comprising one or more 4-quinolinemethanols (4QM) and: a) one or more agents that inhibit the activity of Phosphoinositide-3-kinase (PI-3K); b) one or more agents that inhibit the activity of serine-threonine protein kinase B (AKT); c) one or more agents that inhibit the activity of mammalian target of rapamycin (mTOR); or d) any combination of one or more agents that inhibit the activity of PI-3K, AKT and mTOR.
- 4Qm and the other agents are provided within the composition.
- Some embodiments provide compositons containing subtherapeutic amounts of the agents disclosed herein in the following ratios:
- compositions comprising 4QM and various agents in subtherapeutic amounts.
- subtherapeutic doses of mefloquine are less than 1000 mg and can range from 5 mg to 950 mg, about 5 mg to about 500 mg, about 100 mg to about 500 mg, or about 200 to about 700 mg.
- the therapeutic dose is 648 mg per day.
- subtherapeutic doses of quinine (per day) can range from 5 mg to 625 mg, about 5 mg to about 500 mg, about 100 mg to about 575 mg, or about 200 to about 500 mg.
- subtherapeutic doses (per day) are less than 300 mg.
- subtherapeutic doses can range from 5 mg to 250 mg, about 5 mg to about 150 mg, about 100 mg to about 125 mg, or about 50 to about 150 mg.
- subtherapeutic doses (per day) are less than 150 mg per day for subjects of 45 kg or more or less than 100 mg per day for subjects between 30 and 44 kg.
- subtherapeutic doses can range from 5 mg to 125 mg, about 5 mg to about 100 mg, about 100 mg to about 125 mg, or about 25 to about 125 mg.
- subtherapeutic doses (per day) are less than 2 mg per day for subjects of 40 kg or more or less than 1 mg/m 2 (body surface area) per day for subjects less than 40 kg.
- subtherapeutic doses can range from 0.1 mg to 1.95 mg, about 0.5 mg to about 1.75 mg, about 1 mg to about 1.50 mg, or about 0.25 to about 1.50 mg.
- subtherapeutic doses (per day) can range from 0.1 mg to 0.95 mg, about 0.5 mg to about 0.75 mg, about 0.01 mg to about 0.75 mg, or about 0.25 to about 0.75 mg.
- subtherapeutic doses (per day) are less than 10 mg per day.
- subtherapeutic doses (per day) can range from 0.1 mg to 9.50 mg, about 0.5 mg to about 9.00 mg, about 1 mg to about 7.5 mg, or about 2.50 to about 5.0 mg.
- subtherapeutic doses are about 25 mg/week.
- subtherapeutic doses per week can range from 1 mg to 24.5 mg, about 0.5 mg to about 22 mg, about 1 mg to about 15 mg, or about 2.50 to about 20 mg.
- compositons containing subtherapeutic amounts of the other agents disclosed in Tables 1-4 can be readily asceratained from the literature and correspond to amounts that are between 5% and 95%, between 5% and 90%, between 5% and 80% or between 5% and 75% of the amounts reported to be therapeutically effective for any compound identified in Tables 1-4.
- compositions comprising one or more 4-quinolinemethanols (4QM) and: a) one or more agents that inhibit the activity of Phosphoinositide-3-kinase (PI-3K); b) one or more agents that inhibit the activity of serine-threonine protein kinase B (AKT); c) one or more agents that inhibit the activity of mammalian target of rapamycin (mTOR); or d) any combination of one or more agents that inhibit the activity of PI-3K, AKT and mTOR.
- 4QM 4-quinolinemethanols
- Subtherapeutic amounts of these components of the composition correspond to amounts that are between 5% and 95%, between 5% and 90%, between 5% and 80% or between 5% and 75% of the amounts reported to be therapeutically effective for a given 4-quinolinemethanol (4QM) agent that inhibits the activity of Phosphoinositide-3-kinase (PI-3K), an agent that inhibits the activity of serine-threonine protein kinase B (AKT), or an agent that inhibit the activity of mammalian target of rapamycin (mTOR).
- 4QM 4-quinolinemethanol
- hTS human TS significantly accelerates PDAC progression and metastases and decreases overall survival of Pdx1-Cre/LSL-Kras G12D/+ mice (Kras G12D/+ mice) and that dual targeting of TS and the Ras/PI-3K/AKT/mTOR pathway results in synergistic growth inhibition of PDAC in vitro and in vivo.
- a widely-used anti-parasitic agent (referred to as Compound P or mefloquine), which inhibits the enzymatic activity of human TS in a partial mixed-type manner, synergizes with inhibitors of the KRAS/PI-3K/AKT/mTOR pathway to inhibit PDAC in vitro and in vivo.
- Compound P a widely-used anti-parasitic agent
- the oncogenes KRAS and TS cooperate to accelerate the progression of PDAC, and propose that combination therapies targeting these two oncogenic pathways should be evaluated in PDAC patients (see FIG. 22).
- GDC-0941 4-[2-(1H-indazol-4-yl)-6-[(4-methylsulfonylpiperazin-1- yl)methyl]thieno[3,2-d]pyrimidin-4-yl]morpholine NVP- 5-(2,6-dimorpholin-4-ylpyrimidin-4-yl)-4- BKM120 (trifluoromethyl)pyridin-2-amine PX-866 [(3aR,6E,9S,9aR,10R,11aS)-6-[[bis(prop-2- enyl)amino]methylidene]-5-hydroxy-9-(methoxymethyl)- 9a,11a-dimethyl-1,4,7-trioxo-2,3,3a,9,10,11- hexahydroindeno[4,5-h]isochromen-10-yl]acetate GDC-0032 2-methyl-2-[4-[2-(5-methyl-2-
- AKT inhibitors MK-2206 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-2H- [1,2,4]triazolo[3,4-f][1,6]naphthyridin-3- one; dihydrochloride Triciribine (2R,3R,4S,5R)-2-(3-amino-5-methyl-1,4,5,6,8- pentaazaacenaphthylen-1(5H)-yl)-5- (hydroxymethyl)tetrahydrofuran-3,4-diol.
- Triciribine-Phosphate 1-(5-O-Phosphono- ⁇ -D-ribofuranosyl)-5-methyl-1,5- dihydro-1,4,5,6,8-pentaazaacenaphthylene-3- amine; 1,4,5,6,8-Pentaazaacenaphthylene-3-amino-1,5- dihydro-5-methyl-1-beta-D-ribofuranosyl 5′-monophosphate Miltefosine 2-(hexadecoxy-oxido-phosphoryl)oxyethyl-trimethyl- azanium Perifosine (KRX-0401) (1,1-dimethylpiperidin-1-ium-4-yl) octadecyl phosphate RX-0201 This is an antisense oligonucleotide having the following sequence: 5′ gctgcatgatctccttggcg 3′ Erucylphosphocholine [(Z)-doc
- Sirolimus (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S, 32S,35R)-1,18-dihydroxy-12-[(2S)-1-[(1S,3R,4R)-4- hydroxy-3-methoxycyclohexyl]propan-2-yl]-19,30- dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4- azatricyclo[30.3.1.0 4 , 9 ]hexatriaconta-16,24,26,28- tetraene-2,3,10,14,20-pentone Everolimus (1S,9R,15R,16E,18R,19R,21S,23R,24E,26E,28E,30S,32R, 35S)-1,18-dihydroxy-12-[(2S)
- GDC-0890 PF-05212384 (PKI-587) 1-[4-[4-(dimethylamino)piperidine-1-carbonyl]phenyl]-3- [4-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)phenyl]urea XL-765 N-[4-[[3-(3,5-dimethoxyanilino)quinoxalin-2- yl]sulfamoyl]phenyl]-3-methoxy-4-methylbenzamide
- GSK2126458 2,4-difluoro-N-[2-methoxy-5-(4-pyridazin-4-ylquinolin-6- yl)pyridin-3-yl]benzenesulfonamide
- PWT33597 (VCD-597) PI-103 3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin-2- y
Landscapes
- Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
| TABLE 1 |
| List of Exemplary 4-Quinolinemethanols |
| WR-142490 | [2,8-bis(trifluoromethyl)quinolin-4-yl]-piperidin-2- |
| (Compound P; | ylmethanol |
| mefloquine) | |
| Quinine | (R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2- |
| yl]-(6-methoxyquinolin-4-yl)methanol | |
| Qunidine | (S)-[(2R,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2- |
| yl]-(6-methoxyquinolin-4-yl)methanol | |
| SN-10275 | (6,8-dichloro-2-phenylquinolin-4-yl)-piperidin-2- |
| ylmethanol | |
| WR-30090 | 2-(dibutylamino)-1-[6,8-dichloro-2-(3,4- |
| dichlorophenyl)quinolin-4-yl]ethanol | |
| WR-176990 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2- |
| (dibutylamino)ethanol | |
| WR-177000 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2- |
| (butylamino)ethanol | |
| WR-177973 | Identified in Table 1 of US 2011/0092488. |
| WR-183544 | 1-[2,8-Bis(trifluoromethyl)-4-quinolinyl]-2- |
| (propylamino)ethanol | |
| WR-183545 | 1-[2,8-Bis(trifluoromethyl)-4-quinolinyl]-2- |
| (methylamino)ethanol | |
| WR-184806 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-3-(tert- |
| butylamino)propan-1-ol | |
| WR-194965 | 4-tert-butyl-2-[(tert-butylamino)methyl]-6-(4- |
| chlorophenyl)phenol | |
| WR-211679 | Identified in Table 1 of US 2011/0092488. |
| WR-211925 | Identified in Table 1 of US 2011/0092488. |
| WR-226253 | (S)-[6,8-dichloro-2-(trifluoromethyl)quinolin-4-yl]-[(2S)- |
| piperidin-2-yl]methanol; methanesulfonic acid | |
| WR-308245 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2- |
| (methylamino)ethanol | |
| WR-308246 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2- |
| (dimethylamino)ethanol | |
| WR-308247 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2- |
| fluoroethylamino)ethanol | |
| WR-308251 | 2-anilino-1-[2,8-bis(trifluoromethyl)quinolin-4-yl]ethanol |
| WR-308252 | 2-(benzylamino)-1-[2,8-bis(trifluoromethyl)quinolin-4- |
| yl]ethanol | |
| WR-308253 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2- |
| phenylethylamino)ethanol | |
| WR-308254 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2- |
| (diethylamino)ethanol | |
| WR-308257 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(propan-2- |
| ylamino)ethanol | |
| WR-308258 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2- |
| hydroxyethylamino)ethanol | |
| WR-308277 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2- |
| (dipropylamino)ethanol | |
| WR-308278 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(3- |
| methylsulfanylpropylamino)ethanol | |
| WR-308314 | 2-amino-1-[2,8-bis(trifluoromethyl)quinolin-4-yl]ethanol |
| WR-308396 | 2-[2-(benzylamino)ethylamino]-1-[2,8- |
| bis(trifluoromethyl)quinolin-4-yl]ethanol | |
| WR-308411 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2,2,2- |
| trifluoroethylamino)ethanol | |
| WR-308412 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2- |
| methoxyethylamino)ethanol | |
| WR-308413 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(4- |
| methylpentan-2-ylamino)ethanol | |
| WR-308437 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2-ethyl-4- |
| methylimidazol-1-yl)ethanol | |
| WR-308442 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2- |
| (hexylamino)ethanol | |
| WR-308446 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-[(4,6,6- |
| trimethyl-3-bicyclo[3.1.1]heptanyl)amino]ethanol | |
| WR-308607 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2- |
| methylpropylamino)ethanol | |
| WR-308621 | [2,8-bis(trifluoromethyl)quinolin-4-yl]-[[(2R)-pyrrolidin- |
| 2-yl]methylamino]methanol | |
| WR-308622 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(3- |
| methoxypropylamino)ethanol | |
| WR-308623 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2- |
| propylimidazol-1-yl)ethanol | |
| WR-308626 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2-propan-2- |
| ylimidazol-1-yl)ethanol | |
| WR-308632 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2- |
| butylsulfanylethanol | |
| WR-308633 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-butoxyethanol |
| WR-308653 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]heptan-1-ol |
| WR-308763 | 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]-2-(2- |
| methylbenzimidazol-1-yl)ethanol | |
| WR-308764 | 2-(benzimidazol-1-yl)-1-[2,8- |
| bis(trifluoromethyl)quinolin-4-yl]ethanol | |
| WR-319535 | [[(1S,4R)-2-azabicyclo[2.2.1]heptan-4-yl]methylamino]- |
| [2,8-bis(trifluoromethyl)quinolin-4-yl]methanol | |
| WR-319581 | 2-[[(1R,4S)-2-azabicyclo[2.2.1]heptan-4- |
| yl]methylamino]-1-[2,8-bis(trifluoromethyl)quinolin-4- | |
| yl]ethanol | |
| TABLE 2 |
| Exemplary PI-3K Inhibitors |
| GDC-0941 | 4-[2-(1H-indazol-4-yl)-6-[(4-methylsulfonylpiperazin-1- |
| yl)methyl]thieno[3,2-d]pyrimidin-4-yl]morpholine | |
| NVP- | 5-(2,6-dimorpholin-4-ylpyrimidin-4-yl)-4- |
| BKM120 | (trifluoromethyl)pyridin-2-amine |
| PX-866 | [(3aR,6E,9S,9aR,10R,11aS)-6-[[bis(prop-2- |
| enyl)amino]methylidene]-5-hydroxy-9-(methoxymethyl)- | |
| 9a,11a-dimethyl-1,4,7-trioxo-2,3,3a,9,10,11- | |
| hexahydroindeno[4,5-h]isochromen-10-yl]acetate | |
| GDC-0032 | 2-methyl-2-[4-[2-(5-methyl-2-propan-2-yl-1,2,4-triazol-3- |
| yl)-5,6-dihydroimidazo[1,2-d][1,4]benzoxazepin-9- | |
| yl]pyrazol-1-yl]propanamide | |
| GSK2636771 | 2-methyl-1-[[2-methyl-3-(trifluoromethyl)phenyl]methyl]-6- |
| morpholin-4-ylbenzimidazole-4-carboxylic acid | |
| IPI-145 | 8-chloro-2-phenyl-3-[(1S)-1-(7H-purin-6- |
| ylamino)ethyl]isoquinolin-1-one | |
| CAL-101 | 5-fluoro-3-phenyl-2-[(1S)-1-(7H-purin-6- |
| (GS-1101) | ylamino)propyl]quinazolin-4-one |
| LY294002 | 2-morpholin-4-yl-8-phenylchromen-4-one |
| Wortmannin | (1S,6bR,9aS,11R,11bR)-9a,11b-dimethyl-1- |
| [(methyloxy)methyl]-3,6,9-trioxo-1,6,6b,7,8,9,9a,10,11,11b- | |
| decahydro-3H-furo[4,3,2-de]indeno[4,5-h]isochromen-11-yl | |
| acetate | |
| Demethoxy- | (1R,11bR)-1-Hydroxy-11b-methyl-1,7,8,11b- |
| viridin | tetrahydrocyclopenta[7,8]phenanthro[10,1-bc]furan- |
| 3,6,9(2H)-trione | |
| XL-147 | 2-amino-N-[3-[[3-(2-chloro-5-methoxyanilino)quinoxalin-2- |
| yl]sulfamoyl]phenyl]-2-methylpropanamide | |
| BAY80-6946 | 2-amino-N-[7-methoxy-8-(3-morpholin-4-ylpropoxy)-2,3- |
| dihydroimidazo[1,2-c]quinazolin-5-yl]pyrimidine-5- | |
| carboxamide | |
| ZSTK474 | 4-[4-[2-(difluoromethyl)benzimidazol-1-yl]-6-morpholin-4- |
| yl-1,3,5-triazin-2-yl]morpholine | |
| BYL719 | (2S)-1-N-[4-methyl-5-[2-(1,1,1-trifluoro-2-methylpropan-2- |
| yl)pyridin-4-yl]-1,3-thiazol-2-yl]pyrrolidine-1,2- | |
| dicarboxamide | |
| MLN01117 | |
| (INK-1117) | |
| SAR260301 | (S)-2-(2-(2-methylindolin-1-yl)-2-oxoethyl)-6- |
| morpholinopyrimidin-4(3H)-one | |
| AMG319 | (S)-N-(1-(7-Fluoro-2-(pyridin-2-yl)quinolin-3-yl)ethyl)-9H- |
| purin-6-amine | |
| TGR-1202 (RP6530) |
|
| IC87114 | 2-[(6-aminopurin-9-yl)methyl]-5-methyl-3-(2- |
| methylphenyl)quinazolin-4-one | |
| TG-100-115 | 3-[2,4-diamino-7-(3-hydroxyphenyl)pteridin-6-yl]phenol |
| CUDC-907 | N-hydroxy-2-[[2-(6-methoxypyridin-3-yl)-4-morpholin-4- |
| ylthieno[3,2-d]pyrimidin-6-yl]methyl- | |
| methylamino]pyrimidine-5-carboxamide | |
| AEZS-136 | |
| NVP- | 2-methyl-2-[4-[2-methyl-8-(2-pyridin-3- |
| BAG956 | ylethynyl)imidazo[4,5-c]quinolin-1-yl]phenyl]propanenitrile |
| PIK-75 | N-[(E)-(6-bromoimidazo[1,2-a]pyridin- |
| 3-yl)methylideneamino]-N,2-dimethyl-5- | |
| nitrobenzenesulfonamide; hydrochloride | |
| PIK-90 | N-(7,8-dimethoxy-2,3-dihydroimidazo[1,2-c]quinazolin-5- |
| yl)pyridine-3-carboxamide | |
| TGX-221 | 9-(1-anilinoethyl)-7-methyl-2-morpholin-4-ylpyrido[1,2- |
| a]pyrimidin-4-one | |
| A5-252424 | 5-[5-(4-fluoro-2-hydroxy-phenyl)-furan-2-ylmethylene]- |
| thiazolidine-2,4-dione | |
| D-106669 | 1-ethyl-3-[3-(4-methylanilino)pyrido[2,3-b]pyrazin-6-yl]urea |
| A-66 | (2S)-N1-[2-(1,1-Dimethylethyl)-4′-methyl[4,5′-bithiazol]-2′- |
| yl]-1,2-pyrrolidinedicarboxamide | |
| BN108 | The solution containing 0.5 mg/mL of dried extract of |
| Anemarrhena asphodeloides Bunge is also referred to herein | |
| as BN108. Active ingredient in BN108 is Timosaponin A3 | |
| Timosaponin | (2S,3R,4S,5S,6R)-2-{[(2R,3R,4S,5R,6R)-4,5-dihydroxy-6- |
| A3 | (hydroxymethyl)-2- |
| [(1′R,2′S,3R,4′S,7′S,8′R,9′S,12′S,13′S,16′S,18′R)-6,7′,9′,13′- | |
| tetramethyl-5′-oxaspiro[oxane-3,6′- | |
| pentacyclo[10.8.0.02,9.04,8.013,18]icosane]oxy]oxan-3- | |
| yl]oxyl-6-(hydroxymethyl)oxane-3,4,5-triol | |
| TABLE 3 |
| Exemplary AKT inhibitors |
| MK-2206 | 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-2H- |
| [1,2,4]triazolo[3,4-f][1,6]naphthyridin-3- | |
| one; dihydrochloride | |
| Triciribine | (2R,3R,4S,5R)-2-(3-amino-5-methyl-1,4,5,6,8- |
| pentaazaacenaphthylen-1(5H)-yl)-5- | |
| (hydroxymethyl)tetrahydrofuran-3,4-diol. | |
| Triciribine-Phosphate | 1-(5-O-Phosphono-β-D-ribofuranosyl)-5-methyl-1,5- |
| dihydro-1,4,5,6,8-pentaazaacenaphthylene-3- | |
| amine; 1,4,5,6,8-Pentaazaacenaphthylene-3-amino-1,5- | |
| dihydro-5-methyl-1-beta-D-ribofuranosyl 5′-monophosphate | |
| Miltefosine | 2-(hexadecoxy-oxido-phosphoryl)oxyethyl-trimethyl- |
| azanium | |
| Perifosine (KRX-0401) | (1,1-dimethylpiperidin-1-ium-4-yl) octadecyl phosphate |
| RX-0201 | This is an antisense oligonucleotide having the following |
| sequence: | |
| 5′ gctgcatgatctccttggcg 3′ | |
| Erucylphosphocholine | [(Z)-docos-13-enyl] 2-(trimethylazaniumyl)ethyl phosphate |
| PBI-05204 | [(3S,5R,8R,9S,10S,13R,14S,16S,17R)-14-hydroxy-3- |
| [(2R,4S,5S,6S)-5-hydroxy-4-methoxy-6-methyloxan-2- | |
| yl]oxy-10,13-dimethyl-17-(5-oxo-2H-furan-3-yl)- | |
| 1,2,3,4,5,6,7,8,9,11,12,15,16,17- | |
| tetradecahydrocyclopenta[a]phenanthren-16-yl] acetate | |
| GSK690693 | 4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[[(3S)- |
| piperidin-3-yl]methoxy]imidazo[4,5-c]pyridin-4-yl]-2- | |
| methylbut-3-yn-2-ol | |
| XL-418 | 1-[3-[4-(3-bromo-2H-pyrazolo[3,4-d]pyrimidin-4- |
| yl)piperazin-1-yl]-4-methyl-5-(2-pyrrolidin-1- | |
| ylethylamino)phenyl]-4,4,4-trifluorobutan-1-one | |
| GDC-0068 | (2S)-2-(4-chlorophenyl)-1-[4-[(5R,7R)-7-hydroxy-5-methyl- |
| 6,7-dihydro-5H-cyclopenta[d]pyrimidin-4-yl]piperazin-1- | |
| yl]-3-(propan-2-ylamino)propan-1-one | |
| GSK2110183 | N-[(2S)-1-amino-3-(3,4-difluorophenyl)propan-2-yl]-5- |
| chloro-4-(4-chloro-2-methylpyrazol-3-yl)thiophene-2- | |
| carboxamide | |
| GSK2141795 | N-[(2S)-1-amino-3-(3,4-difluorophenyl)propan-2-yl]-5- |
| chloro-4-(4-chloro-2-methylpyrazol-3-yl)furan-2- | |
| carboxamide | |
| ARQ-092 | |
| AZD5363 | (S)-4-amino-N-(1-(4-chlorophenyl)-3-hydroxypropyl)-1- |
| (7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide | |
| TABLE 4 |
| mTOR Inhibitors |
| Sirolimus (Rapamycin) | (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S, |
| 32S,35R)-1,18-dihydroxy-12-[(2S)-1-[(1S,3R,4R)-4- | |
| hydroxy-3-methoxycyclohexyl]propan-2-yl]-19,30- | |
| dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4- | |
| azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28- | |
| tetraene-2,3,10,14,20-pentone | |
| Everolimus | (1S,9R,15R,16E,18R,19R,21S,23R,24E,26E,28E,30S,32R, |
| 35S)-1,18-dihydroxy-12-[(2S)-1-[(1S,3R,4R)-4-(2- | |
| hydroxyethoxy)-3-methoxycyclohexyl]propan-2-yl]- | |
| 19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36- | |
| dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta- | |
| 16,24,26,28-tetraene-2,3,10,14,20-pentone | |
| Tacrolimus | (1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)- |
| 1,14-dihydroxy-12-[(1E)-1-[(1R,3R,4R)-4-hydroxy-3- | |
| methoxycyclohexyl]prop-1-en-2-yl]-23,25-dimethoxy- | |
| 13,19,21,27-tetramethyl-17-(prop-2-en-1-yl)-11,28-dioxa- | |
| 4-azatricyclo[22.3.1.04,9]octacos-18-ene-2,3,10,16-tetrone | |
| Temsirolimus | (1R,2R,4S)-4-[(2R)-2- |
| [(1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S, | |
| 32S,35R)-1,18-dihydroxy-19,30-dimethoxy- | |
| 15,17,21,23,29,35-hexamethyl-2,3,10,14,20-pentaoxo- | |
| 11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta- | |
| 16,24,26,28-tetraen-12-yl]propyl]-2-methoxycyclohexyl | |
| 3-hydroxy-2-(hydroxymethyl)-2-methylpropanoate | |
| Deforolimus or Ridaforolimus | (1R,2R,4S)-4-[(2R)-2- |
| [(1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28Z,30S, | |
| 32S,35R)-1,18-dihydroxy-19,30-dimethoxy- | |
| 15,17,21,23,29,35-hexamethyl-2,3,10,14,20-pentaoxo- | |
| 11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta- | |
| 16,24,26,28-tetraen-12-yl]propyl]-2-methoxycyclohexyl | |
| dimethylphosphinate | |
| AZD2014 | 3-[2,4-bis[(3S)-3-methylmorpholin-4-yl]pyrido[2,3- |
| d]pyrimidin-7-yl]-N-methylbenzamide | |
| MLN-0128 (INK-128) | 5-(4-amino-1-propan-2-ylpyrazolo[3,4-d]pyrimidin-3-yl)- |
| 1,3-benzoxazol-2-amine | |
| CC-223 |
|
| Palomid 529 | 8-(1-hydroxyethyl)-2-methoxy-3-((4- |
| methoxybenzyl)oxy)-6H-benzo[c]chromen-6-one | |
| KU-0063794 | [5-[2-[(2R,6S)-2,6-dimethylmorpholin-4-yl]-4-morpholin- |
| 4-ylpyrido[2,3-d]pyrimidin-7-yl]-2- | |
| methoxyphenyl]methanol | |
| Torin-1 | 1-[4-(4-propanoylpiperazin-1-yl)-3- |
| (trifluoromethyl)phenyl]-9-quinolin-3- | |
| ylbenzo[h][1,6]naphthyridin-2-one | |
| Torin-2 | 9-(6-aminopyridin-3-yl)-1-[3- |
| (trifluoromethyl)phenyl[benzo[h][1,6]naphthyridin-2-one | |
| DUAL PI-3K/mTOR Inhibitors |
| NVP-BEZ235 | 2-methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3- |
| ylimidazo[4,5-c]quinolin-1-yl)phenyl]propanenitrile | |
| NVP-BBD130 | 2-methyl-2-(4-(3-methyl-2-oxo-8-(2-(pyridin-3- |
| yl)ethynyl)-2,3-dihydroimidazo[4,5-c]quinolin-1- | |
| yl)phenyl)propanenitrile | |
| NVP-BGT226 | (Z)-but-2-enedioic acid;8-(6-methoxypyridin-3-yl)-3- |
| methyl-1-[4-piperazin-1-yl-3- | |
| (trifluoromethyl)phenyl]imidazo[4,5-c]quinolin-2-one | |
| LY3023414 | Identified in US20140377258 without providing structure |
| or IUPAC name. | |
| GDC-0890 | |
| PF-05212384 (PKI-587) | 1-[4-[4-(dimethylamino)piperidine-1-carbonyl]phenyl]-3- |
| [4-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)phenyl]urea | |
| XL-765 | N-[4-[[3-(3,5-dimethoxyanilino)quinoxalin-2- |
| yl]sulfamoyl]phenyl]-3-methoxy-4-methylbenzamide | |
| GSK2126458 | 2,4-difluoro-N-[2-methoxy-5-(4-pyridazin-4-ylquinolin-6- |
| yl)pyridin-3-yl]benzenesulfonamide | |
| PWT33597 (VCD-597) | |
| PI-103 | 3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin-2- |
| yl)phenol | |
| GNE-477 | 5-[7-methyl-6-[(4-methylsulfonylpiperazin-1-yl)methyl]- |
| 4-morpholin-4-ylthieno[3,2-d]pyrimidin-2-yl]pyrimidin- | |
| 2-amine | |
| SF-1126 | L-Serine, N2-(1,4-dioxo-4-((4-(4-oxo-8-phenyl-4H-1- |
| benzopyran-2-yl)morpholinium-4-yl)methoxy)butyl)-L- | |
| arginylglycyl-L-alpha-aspartyl-, inner salt | |
| GSK1059615 | (5Z)-5-[(4-pyridin-4-ylquinolin-6-yl)methylidene]-1,3- |
| thiazolidine-2,4-dione | |
| PF-04691502 | 2-amino-8-[4-(2-hydroxyethoxy)cyclohexyl]-6-(6- |
| methoxypyridin-3-yl)-4-methylpyrido[2,3-d]pyrimidin-7- | |
| one | |
Claims (28)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/737,545 US10835524B2 (en) | 2015-06-24 | 2016-06-16 | Compositions for the treatment of pancreatic cancer and uses thereof |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562183831P | 2015-06-24 | 2015-06-24 | |
| US15/737,545 US10835524B2 (en) | 2015-06-24 | 2016-06-16 | Compositions for the treatment of pancreatic cancer and uses thereof |
| PCT/US2016/037731 WO2016209688A1 (en) | 2015-06-24 | 2016-06-16 | Compositions for the treatment of cancer and uses thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20180177776A1 US20180177776A1 (en) | 2018-06-28 |
| US10835524B2 true US10835524B2 (en) | 2020-11-17 |
Family
ID=57585320
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US15/737,545 Active 2036-12-31 US10835524B2 (en) | 2015-06-24 | 2016-06-16 | Compositions for the treatment of pancreatic cancer and uses thereof |
Country Status (2)
| Country | Link |
|---|---|
| US (1) | US10835524B2 (en) |
| WO (1) | WO2016209688A1 (en) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TWI795381B (en) | 2016-12-21 | 2023-03-11 | 比利時商健生藥品公司 | Pyrazole derivatives as malt1 inhibitors |
| CN110755426B (en) * | 2018-07-26 | 2022-09-30 | 中国农业大学 | Application of rapamycin and structural analogs thereof in preparing medicines for treating diseases caused by ectopic overexpression of Msi1 gene |
| US12269813B2 (en) * | 2019-02-22 | 2025-04-08 | Janssen Pharmaceutica Nv | Crystalline form of 1-(1-oxo-1,2-dihydroisoquinolin-5-yl)-5-(trifluoromethyl)-N-(2-(trifluoromethyl)pyridin-4-yl)-1H-pyrazole-4-carboxamide monohydrate |
| EP3953345B1 (en) | 2019-04-11 | 2023-04-05 | Janssen Pharmaceutica NV | Pyridine rings containing derivatives as malt1 inhibitors |
| CN111533721B (en) * | 2020-05-15 | 2022-04-26 | 浙江大学 | Benzopyrone or quinolinone compounds and application thereof |
Citations (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5781460A (en) | 1980-11-10 | 1982-05-21 | Mochida Pharmaceut Co Ltd | Novel indolinecarboxylic acid derivative |
| US4992444A (en) | 1986-12-02 | 1991-02-12 | Stevens Malcolm F G | Antifolate agents |
| US5635515A (en) | 1991-02-15 | 1997-06-03 | Debiopharm S.A. | Therapeutic agents for the treatment of multiple drug resistance of cancers |
| WO1998030550A1 (en) | 1997-01-14 | 1998-07-16 | Btg International Limited | 2,4-diaminopyrimidine compounds as anti-cancer agents |
| US6525091B2 (en) | 2001-03-07 | 2003-02-25 | Telik, Inc. | Substituted diarylureas as stimulators for Fas-mediated apoptosis |
| US20030216426A1 (en) | 2002-05-17 | 2003-11-20 | Regents Of The University Of California | Treatment of cancer with mefloquine, its purified enantiomers, and mefloquine analogs |
| US20050038031A1 (en) | 2003-02-28 | 2005-02-17 | Jacques Dumas | Novel bicyclic urea derivatives useful in the treatment of cancer and other disorders |
| US20050143588A1 (en) | 1999-02-15 | 2005-06-30 | Novogen Research Pty Ltd. | Production of isoflavone derivatives |
| US6984647B2 (en) | 2002-05-17 | 2006-01-10 | Janssen Pharmaceutica N.V. | Aminotetralin-derived urea modulators of vanilloid VR1 receptor |
| WO2006049941A2 (en) | 2004-10-27 | 2006-05-11 | Neurogen Corporation | Diaryl ureas as cb1 antagonists |
| US20060166925A1 (en) | 2002-12-30 | 2006-07-27 | Karel Dolezal | Substitution derivatives of n6-benzyladenosine, methods of their preparation, their use for preparation of drugs, cosmetic preparations and growth regulators, pharmaceutical preparations, cosmetic preparations and growth regulators containing these compounds |
| WO2006125540A1 (en) | 2005-05-27 | 2006-11-30 | Bayer Healthcare Ag | Combination therapy comprising a diaryl urea compound and a pi3, akt kinase or mtor inhibitors (rapamycins) for cancer treatment |
| US20070099976A1 (en) | 2004-02-13 | 2007-05-03 | President And Fellows Of Harvard College | 3-3-di-substituted-oxindoles as inhibitors of translation initiation |
| US20070161546A1 (en) | 2005-12-15 | 2007-07-12 | Colm King | Methods and compositions for treatment of cancer |
| US20080045589A1 (en) | 2006-05-26 | 2008-02-21 | Susan Kelley | Drug Combinations with Substituted Diaryl Ureas for the Treatment of Cancer |
| US20090203636A1 (en) | 2006-03-14 | 2009-08-13 | Bondarev Igor E | Prevention and Treatment of Cancer and Other Diseases |
| US20100009934A1 (en) | 2008-06-09 | 2010-01-14 | Combinatorx, Incorporated | Beta adrenergic receptor agonists for the treatment of b-cell proliferative disorders |
| US20100260772A1 (en) | 2007-09-28 | 2010-10-14 | The Trustees Of Columbia University In The City Of New York | Methods for treating or preventing diseases associated with low bone mass |
| WO2010138820A2 (en) | 2009-05-28 | 2010-12-02 | President And Fellows Of Harvard College | N,n-diarylurea compounds and n,n'-diarylthiourea compounds as inhibitors of translation initiation |
| CN102125579A (en) | 2010-12-22 | 2011-07-20 | 中国科学院广州生物医药与健康研究院 | Novel application of 5-bit modified 2'deoxycytidine derivative or phosphate thereof in the preparation of medicaments |
| US20110176996A1 (en) | 2009-12-30 | 2011-07-21 | Brigham Young University | Compositions and methods for cancer management using antibodies binding to nucleotide salvage pathway enzymes and complexes thereof |
| US20110224141A1 (en) | 2008-06-05 | 2011-09-15 | Stc.Unm | Methods and related compositions for the treatment of cancer |
| WO2011151423A1 (en) | 2010-06-04 | 2011-12-08 | Exonhit S.A. | Substituted isoquinolines and their use as tubulin polymerization inhibitors |
| US20120082659A1 (en) | 2007-10-02 | 2012-04-05 | Hartmut Land | Methods And Compositions Related To Synergistic Responses To Oncogenic Mutations |
| US20120260772A1 (en) | 2011-04-12 | 2012-10-18 | Valerio Thomas A | Method and System for Processing an Iron Ore Tailings Byproduct |
| US20130183289A1 (en) | 2007-09-14 | 2013-07-18 | Biogen Idec Ma Inc | Compositions and methods for the treatment of progressive multifocal leukoencephalopathy (pml) |
| US20160067240A1 (en) | 2013-03-15 | 2016-03-10 | University Of Florida Research Foundation, Inc. | Novel allosteric inhibitors of thymidylate synthase |
-
2016
- 2016-06-16 US US15/737,545 patent/US10835524B2/en active Active
- 2016-06-16 WO PCT/US2016/037731 patent/WO2016209688A1/en not_active Ceased
Patent Citations (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5781460A (en) | 1980-11-10 | 1982-05-21 | Mochida Pharmaceut Co Ltd | Novel indolinecarboxylic acid derivative |
| US4992444A (en) | 1986-12-02 | 1991-02-12 | Stevens Malcolm F G | Antifolate agents |
| US5635515A (en) | 1991-02-15 | 1997-06-03 | Debiopharm S.A. | Therapeutic agents for the treatment of multiple drug resistance of cancers |
| WO1998030550A1 (en) | 1997-01-14 | 1998-07-16 | Btg International Limited | 2,4-diaminopyrimidine compounds as anti-cancer agents |
| US20050143588A1 (en) | 1999-02-15 | 2005-06-30 | Novogen Research Pty Ltd. | Production of isoflavone derivatives |
| US6525091B2 (en) | 2001-03-07 | 2003-02-25 | Telik, Inc. | Substituted diarylureas as stimulators for Fas-mediated apoptosis |
| US20030216426A1 (en) | 2002-05-17 | 2003-11-20 | Regents Of The University Of California | Treatment of cancer with mefloquine, its purified enantiomers, and mefloquine analogs |
| WO2003096992A2 (en) | 2002-05-17 | 2003-11-27 | The Regents Of The University Of California | Treatment of cancer with mefloquire |
| US20050154010A1 (en) | 2002-05-17 | 2005-07-14 | The Regents Of University Of California | Treatment of cancer with mefloquine, its purified enantiomers, and mefloquine analogs |
| US6984647B2 (en) | 2002-05-17 | 2006-01-10 | Janssen Pharmaceutica N.V. | Aminotetralin-derived urea modulators of vanilloid VR1 receptor |
| US20060166925A1 (en) | 2002-12-30 | 2006-07-27 | Karel Dolezal | Substitution derivatives of n6-benzyladenosine, methods of their preparation, their use for preparation of drugs, cosmetic preparations and growth regulators, pharmaceutical preparations, cosmetic preparations and growth regulators containing these compounds |
| US20050038031A1 (en) | 2003-02-28 | 2005-02-17 | Jacques Dumas | Novel bicyclic urea derivatives useful in the treatment of cancer and other disorders |
| US20070099976A1 (en) | 2004-02-13 | 2007-05-03 | President And Fellows Of Harvard College | 3-3-di-substituted-oxindoles as inhibitors of translation initiation |
| WO2006049941A2 (en) | 2004-10-27 | 2006-05-11 | Neurogen Corporation | Diaryl ureas as cb1 antagonists |
| WO2006125540A1 (en) | 2005-05-27 | 2006-11-30 | Bayer Healthcare Ag | Combination therapy comprising a diaryl urea compound and a pi3, akt kinase or mtor inhibitors (rapamycins) for cancer treatment |
| US20070161546A1 (en) | 2005-12-15 | 2007-07-12 | Colm King | Methods and compositions for treatment of cancer |
| US20090203636A1 (en) | 2006-03-14 | 2009-08-13 | Bondarev Igor E | Prevention and Treatment of Cancer and Other Diseases |
| US20080045589A1 (en) | 2006-05-26 | 2008-02-21 | Susan Kelley | Drug Combinations with Substituted Diaryl Ureas for the Treatment of Cancer |
| US20130183289A1 (en) | 2007-09-14 | 2013-07-18 | Biogen Idec Ma Inc | Compositions and methods for the treatment of progressive multifocal leukoencephalopathy (pml) |
| US20100260772A1 (en) | 2007-09-28 | 2010-10-14 | The Trustees Of Columbia University In The City Of New York | Methods for treating or preventing diseases associated with low bone mass |
| US20120082659A1 (en) | 2007-10-02 | 2012-04-05 | Hartmut Land | Methods And Compositions Related To Synergistic Responses To Oncogenic Mutations |
| US20110224141A1 (en) | 2008-06-05 | 2011-09-15 | Stc.Unm | Methods and related compositions for the treatment of cancer |
| US20100009934A1 (en) | 2008-06-09 | 2010-01-14 | Combinatorx, Incorporated | Beta adrenergic receptor agonists for the treatment of b-cell proliferative disorders |
| WO2010138820A2 (en) | 2009-05-28 | 2010-12-02 | President And Fellows Of Harvard College | N,n-diarylurea compounds and n,n'-diarylthiourea compounds as inhibitors of translation initiation |
| US20120115915A1 (en) | 2009-05-28 | 2012-05-10 | President And Fellows Of Harvard College | N,n'-diarylurea compounds and n,n'-diarylthiourea compounds as inhibitors of translation initiation |
| US20110176996A1 (en) | 2009-12-30 | 2011-07-21 | Brigham Young University | Compositions and methods for cancer management using antibodies binding to nucleotide salvage pathway enzymes and complexes thereof |
| WO2011151423A1 (en) | 2010-06-04 | 2011-12-08 | Exonhit S.A. | Substituted isoquinolines and their use as tubulin polymerization inhibitors |
| CN102125579A (en) | 2010-12-22 | 2011-07-20 | 中国科学院广州生物医药与健康研究院 | Novel application of 5-bit modified 2'deoxycytidine derivative or phosphate thereof in the preparation of medicaments |
| US20120260772A1 (en) | 2011-04-12 | 2012-10-18 | Valerio Thomas A | Method and System for Processing an Iron Ore Tailings Byproduct |
| US20160067240A1 (en) | 2013-03-15 | 2016-03-10 | University Of Florida Research Foundation, Inc. | Novel allosteric inhibitors of thymidylate synthase |
Non-Patent Citations (68)
| Title |
|---|
| [No Author Listed], CID 280274. Compound Summary. Mar. 26, 2006. https://pubchem.ncbi.nlm.nih.gov/compound/280274#section=Top. [last accessed Jul. 17, 2017]. 14 pages. |
| [No Author Listed], CID 42501056. Compound Summary. May 30, 2009. |
| [No Author Listed], CID 5911417. Compound Summary. Sep. 10, 2005. https://pubchem.ncbi.nlm.nih.gov/compound/5911417#section=Top. [last accessed Jul. 17, 2017]. 15 pages. |
| Aarhus et al., Microarray analysis reveals down-regulation of the tumour suppressor gene WWOX and up-regulation of the oncogene TYMS in intracranial sporadic meningiomas, J Neuro-Oncology, 2008, vol. 88, No. 3, pp. 251-259. |
| Barlesi et al., Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01 ),Annals of Oncology, 2011, vol. 22, pp. 2466-2470. |
| Barrows et al., A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection. Cell Host Microbe. Aug. 10, 2016;20(2):259-70. doi: 10.1016/j.chom.2016.07.004. Epub Jul. 28, 2016. |
| Bermudez et al., Mefloquine and Its Enantiomers Are Active against Mycobacterium tuberculosis In Vitro and in Macrophages. Tuberc Res Treat. 2014;2014:530815. doi: 10.1155/2014/530815. Epub Dec. 11, 2014. |
| Brickelmaier et al., Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother. May 2009;53(5):1840-9. doi: 10.1128/AAC.01614-08. Epub Mar. 2, 2009. |
| Brozell et al., Evaluation of DOCK 6 as a pose generation and database enrichment tool, Journal of Computer-Aided Molecular Design, 2012, vol. 26, pp. 749-773. |
| Cardinale et al., Protein-protein interface-binding peptides inhibit the cancer therapy target human thymidylate synthase, Proceedings of the National Academy of Sciences of the United States of America, Aug. 23, 2011, vol. 108, No. 34, pp. E542-549. |
| Carreras et al., The catalytic mechanism and structure of thymidylate synthase, Annual Review of Biochemistry, 1995, vol. 64, pp. 721-762. |
| Ceppi et al., Squamous Cell Carcinoma of the Lung Compared With Other Histotypes Shows Higher Messenger RNA and Protein Levels for Thymidylate Synthase, Cancer, 2006, 107, No. 7, pp. 1589-1596. |
| Ceppi et al., Thymidylate Synthase Expression in Gastroenteropancreatic and Pulmonary Neuroendocrine Tumors, Clinical Cancer Research, Feb. 15, 2008, vol. 14, No. 4, pp. 1059-1064. |
| Chang et al., PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. Oct. 2, 2014;5:e1437. doi:10.1038/cddis.2014.415. |
| Chavchich et al., Role of pfmdr1 amplification and expression in induction of resistance to artemisinin derivatives in Plasmodium falciparum. Antimicrob Agents Chemother. Jun. 2010;54(6):2455-64. doi: 10.1128/AAC.00947-09. Epub Mar. 29, 2010. |
| Chu et al., Induction of Thymidylate Synthase Associated with Multidrug Resistance in Human Breast and Colon Cancer Cell Lines, Molecular Pharmacology, 1991, vol. 39, No. 2, pp. 136-143. |
| Copur et al., Thymidylate Synthase Gene Amplification in Human Colon Cancer Cell Lines Resistant to 5-Fluorouracil, Biochemical Pharmacology, 1995, vol. 49, No. 10, pp. 1419-1426. |
| Crawford et al., Sigma-2 Receptor Agonists Activate a Novel Apoptotic Pathway and Potentiate Antineoplastic Drugs in Breast Tumor Cell Lines, Cancer Research, 2002, vol. 62, pp. 313-322. |
| Cruikshank et al., Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc Natl Acad Sci U S A. Aug. 17, 2004;101(33):12364-9. Epub Aug. 5, 2004. |
| Danenberg et al., Thymidylate Synthetase-A Target Enzyme in Cancer Chemotherapy, Biochimica et Biophysica Acta, 1977, vol. 473, pp. 73-92. |
| Danenberg et al., Thymidylate Synthetase—A Target Enzyme in Cancer Chemotherapy, Biochimica et Biophysica Acta, 1977, vol. 473, pp. 73-92. |
| Edler et al., Thymidylate Synthase Expression: An Independent Prognostic Factor for Local Recurrence, Distant Metastasis, Disease-free and Overall Survival in Rectal Cancer, Clinical Cancer Research, Apr. 2000, vol. 6, No. 4, pp. 1378-1384. |
| Galvani et al. Thymidylate synthase inhibitors for non-small cell lung cancer. Expert Opinion on Investigational Drugs, 2011, vol. 20, No. 10, pp. 1343-1356. |
| Geng et al., Chloroquine-induced autophagic vacuole accumulation and cell death in glioma cells is p53 independent. Neuro Oncol. May 2010;12(5):473-81. doi: 10.1093/neuonc/nop048. Epub Jan. 27, 2010. |
| Gonçalves et al., Mefloquine-oxazolidine derivatives, derived from mefloquine and arenecarbaldehydes: In vitro activity including against the multidrug-resistant tuberculosis strain T113. Bioorg Med Chem. Jan. 1, 2012;20(1):243-8. doi: 10.1016/j.bmc.2011.11.006. Epub Nov. 12, 2011. |
| Griffin et al., Structural studies on bioactive compounds. 8. Synthesis, crystal structure, and biological properties of a new series of 2,4-diamino-5-aryl-6-ethylpyrimidine dihydrofolate reductase inhibitors with in vivo activity against a methotrexate-resistant tumor cell line. J Med Chem. Nov. 1989;32(11):2468-74. |
| Grimaudo et al., Selective induction of apoptosis in multidrug resistant HL60R cells by the thiazolobenzoimidazole derivative 1-(2,6-difluoropheny1)-1H,3H-thiazolo [3,4-a] benzimidazole (TBZ). Eur J Cancer. Oct. 1998;34(11):1756-63. |
| Grunda et al., Increased expression of thymidylate synthetase (TS), ubiquitin specific protease 10 (USP10) and survivin is associated with poor survival in glioblastoma multiforme (GBM), Journal of Neuro-Oncology, 2006, vol. 80, pp. 261-274. |
| Houssay et al., The Influence Exerted by Desoxycorticosterone Acetate upon the Production of Adrenal Tumors in Gonadectomized Mice, Cancer Research, 1951, vol. 11, No. 5, pp. 297-300. |
| International Preliminary Report on Patentability, dated Sep. 24, 2015, in connection with PCT/U52014/030143. |
| International Search Report and Written Opinion, dated Oct. 19, 2016, in connection with PCT/US2016/037731. |
| International Search Report and Written Opinion, dated Oct. 27, 2014, in connection with PCT/U52014/030143. |
| Irwin et al., ZINC: A Free Tool to Discover Chemistry for Biology, Journal of Chemical Information and Modeling, 2012, vol. 52, No. 7, pp. 1757-1768. |
| Iwama et al., Epidural Administration of Droperidol Suppresses Cisplatin-induced Emesis: Preliminary Findings, Surgery Today, 1998, vol. 28, pp. 231-234. |
| Kim et al., Co-treatment with the anti-malarial drugs mefloquine and primaquine highly sensitizes drug-resistant cancer cells by increasing P-gp inhibition. Biochem Biophys Res Commun. Nov. 22, 2013;441(3):655-60. doi: 10.1016/j.bbrc.2013.10.095. Epub Oct. 26, 2013. |
| Klebe, Virtual ligand screening: strategies, perspectives and limitations. Drug Discovery Today,. Jul. 2006, vol. 11, Nos. 13-14, pp. 580-594. |
| Krieger et al., Mefloquine as a potential drug against multidrug-resistant tuberculosis. Eur Respir J. Nov. 2015;46(5):1503-5. doi: 10.1183/13993003.00321-2015. Epub Jul. 23, 2015. |
| Labianca et al., The role of adjuvant chemotherapy in colon cancer, Surgical Oncology, 2007, vol. 16, Suppl. 1, pp. 893-896. |
| Li et al., Simultaneous determination of vanadium, niobium and tantalum by highperformance liquid chromatography equipped with on-line enrichment using 2-[2-(5-bromoquinolinylazo)]-5-diethylaminophenol as pre-column derivatization agent, Microchimica Acta, 2007, vol. 158, pp. 95-102. |
| Liu et al., Mefloquine effectively targets gastric cancer cells through phosphatase-dependent inhibition of PI3K/Akt/mTOR signaling pathway. Biochem Biophys Res Commun. Feb. 5, 2016;470(2):350-5. doi:10.1016/j.bbrc.2016.01.046. Epub Jan. 11, 2016. |
| Longley et al., 5-fluorouracil: mechanisms of action and clinical strategies. Nature Reviews Cancer, May 2003, vol. 3, No. 5, pp. 330-338. |
| Ma et al., A phase II trial of a combination of pemetrexed and gemcitabine in patients with metastatic breast cancer: an NCCTG study, Annals of Oncology, 2006, vol. 17, No. 2, pp. 226-231. |
| Morgan et al., Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. Mar. 2009;9(2):237-49. |
| Mukherjee et al., Docking Validation Resources: Protein Family and Ligand Flexibility Experiments, Journal of Chemical Information and Modeling, 2010, vol. 50, No. 11, pp. 1986-2000. |
| Pai et al., AKT inhibitors in clinical development for the treatment of cancer, 2010, Expert Opin Investig Drugs, 19(11), pp. 1355-1366. (Year: 2010). * |
| PCT/US2014/030143, Oct. 27, 2014, International Search Report and Written Opinion. |
| PCT/US2014/030143, Sep. 24, 2015 International Preliminary Report on Patentability. |
| PCT/US2016/037731, Oct. 19, 2016, International Search Report and Written Opinion. |
| Pereira et al., Spectrophotometric determination of arsenic in soil samples using 2-(5-bromo-2-pyridylazo)-5-di-ethylaminophenol (Br-PADAP), Ecletica Quimica, 2008, vol. 33, No. 3, pp. 23-28. |
| Phan et al., Human Thymidylate Synthase Is in the Closed Conformation When Complexed with dUMP and Raltitrexed, an Antifolate Drug, Biochemistry, 2001, vol. 40, pp. 1897-1902. |
| Rahman et al., Thymidylate synthase as an oncogene: A novel role for an essential DNA synthesis enzyme, Cancer Cell, Apr. 2004, vol. 5, No. 4, pp. 341-351. |
| Ramaswamyet al., Multiclass cancer diagnosis using tumor gene expression signatures, PNAS. Dec. 18, 2001, vol. 98, No. 26, pp. 15149-15154. |
| Rodrigues et al., Mefloquine-oxazolidine derivatives: a new class of anticancer agents. Chem Biol Drug Des. Jan. 2014;83(1):126-31. doi:10.1111/cbdd.12210. Epub Oct. 5, 2013. |
| Sachlos et al., Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. Jun. 8, 2012;149(6):1284-97. doi: 10.1016/j.cell.2012.03.049. Epub May 24, 2012. |
| Sharma et al., Inhibition of autophagy and induction of breast cancer cell death by mefloquine, an antimalarial agent. Cancer Lett. Dec. 30, 2012;326(2):143-54. doi:10.1016/j.canlet.2012.07.029. Epub Aug. 1, 2012. |
| Sharma et al., Screening of potential chemopreventive agents using biochemical markers of carcinogenesis. Cancer Res. Nov. 15, 1994;54(22):5848-55. |
| Sigmond et al., Induction of resistance to the multitargeted antifolate Pemetrexed (ALIMTA) in WiDr human colon cancer cells is associated with thymidylate synthase overexpression, Biochemical Pharmacology, 2003, vol. 66, No. 3, pp. 431-438. |
| Skibola et al., Polymorphisms and haplotypes in folate-metabolizing genes and risk of non-Hodgkin lymphoma, Blood, Oct. 1, 2004, vol. 104, pp. 2155-2162. |
| Spielmann et al., Activity of Pemetrexed (ALIMTA®, Multitargeted Antifolate, L Y231514) in Metastatic Breast Cancer Patients Previously Treated with an Anthracycline and a Taxane: An Interim Analysis. Clinical Breast Cancer, Apr. 2001, vol. 2, No. 1 pp. 47-51. |
| Sukhai et al., Lysosomal disruption preferentially targets acute myeloid leukemia cells and progenitors. J Clin Invest. Jan. 2013;123(1):315-28. doi:10.1172/JCI64180. Epub Dec. 3, 2012. |
| Van Tri Est et al., Downstream molecular determinants of response to 5-fluorouracil and antifolate thymidylate synthase inhibitors, Annals of Oncology, 2000, vol. 11, No. 4, pp. 385-391. |
| Voeller et al., Elevated Levels of Thymidylate Synthase Linked to Neoplastic Transformation of Mammalian Cells, Cell Cycle, Aug. 2004, vol. 3, No. 8, pp. 1005-1007. |
| Wahba et al., Direct Spectrophotometric Evidence for the Oxidation of Tetrahydrofolate during the Enzymatic Synthesis of Thymidylate. J Biological Chemistry, vol. Mar. 1961, vol. 236, pp. PC11-PC12. |
| Wahba et al., The Enzymatic Synthesis of Thymidylate. I. Early steps in the purification of thymidylate synthetase of Escherichia coli. J Biological Chemistry, Dec. 1962, vol. 237, No. 12, pp. 3794-3801. |
| Yan et al., Mefloquine exerts anticancer activity in prostate cancer cells via ROSmediated modulation of Akt, ERK, JNK and AMPK signaling, Oncology Letters, 2013, vol. 5, pp. 1541-1545. |
| Yan et al., Mefloquine induces cell death in prostate cancer cells and provides a potential novel treatment strategy in vivo . . . Oncol Lett. May 2013;5(5):1567-1571. Epub Mar. 15, 2013. |
| Yang et al., "Pancreatic cancer require autophagy for tumor growth", Genes and Development, vol. 25, pp. 1-13 (Mar. 2011). * |
| Yao et al., "Everolinnus for Advanced Neuroendocrine Tumors", The New England Journal of Medicine, vol. 364, No. 6, pp. 514-523 (2011). * |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2016209688A1 (en) | 2016-12-29 |
| US20180177776A1 (en) | 2018-06-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10835524B2 (en) | Compositions for the treatment of pancreatic cancer and uses thereof | |
| JP7667132B2 (en) | Treatment of breast cancer using a combination therapy comprising an ATP-competitive AKT inhibitor, a CDK4/6 inhibitor, and fulvestrant | |
| JP2025072429A (en) | Methods for Treating Myeloproliferative Neoplasms | |
| US20210213037A1 (en) | Methods for treating fibrosis | |
| US8748428B2 (en) | Use of a PKC inhibitor | |
| WO2016193955A1 (en) | Combinations of kinase inhibitors for treating colorectal cancer | |
| RU2019132893A (en) | COMBINATION THERAPY FOR BREAST CANCER | |
| US20250041300A1 (en) | Pharmaceutical combination comprising abemaciclib and a pi3k and/or a mtor inhibitor for the treatment of mantle cell lymphoma | |
| TW201815395A (en) | Use of dianhydrogalactitol or derivatives or analogs thereof for treatment of pediatric central nervous system malignancies | |
| WO2018153984A1 (en) | Mtor inhibitor-corticoid combination therapy for multiple sclerosis | |
| US20210161897A1 (en) | Epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of cancer | |
| US20250205233A1 (en) | Epidermal growth factor receptor (egfr) tyrosine kinase inhibitors in combination with an akt inhibitor for the treatment of cancer | |
| US20250387383A1 (en) | Methods of treating myeloproliferative neoplasms | |
| Costa et al. | A Compendium of tyrosine-kinase Inhibitors: Powerful and efficient drugs against cancer | |
| EP4551226A1 (en) | Epidermal growth factor receptor tyrosine kinase inhibitors in combination with hgf-receptor inhibitors for the treatment of cancer | |
| WO2025179032A1 (en) | Methods of treating myelofibrosis | |
| TW201139417A (en) | Use of a PKC inhibitor |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| FEPP | Fee payment procedure |
Free format text: ENTITY STATUS SET TO UNDISCOUNTED (ORIGINAL EVENT CODE: BIG.); ENTITY STATUS OF PATENT OWNER: SMALL ENTITY |
|
| FEPP | Fee payment procedure |
Free format text: ENTITY STATUS SET TO SMALL (ORIGINAL EVENT CODE: SMAL); ENTITY STATUS OF PATENT OWNER: SMALL ENTITY |
|
| AS | Assignment |
Owner name: UNIVERSITY OF FLORIDA RESEARCH FOUNDATION, INCORPORATED, FLORIDA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:ZAJAC-KAYE, MARIA;FRANCOIS, RONY A.;REEL/FRAME:046639/0664 Effective date: 20180627 Owner name: UNIVERSITY OF FLORIDA RESEARCH FOUNDATION, INCORPO Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:ZAJAC-KAYE, MARIA;FRANCOIS, RONY A.;REEL/FRAME:046639/0664 Effective date: 20180627 |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: RESPONSE TO NON-FINAL OFFICE ACTION ENTERED AND FORWARDED TO EXAMINER |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: NON FINAL ACTION MAILED |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: RESPONSE TO NON-FINAL OFFICE ACTION ENTERED AND FORWARDED TO EXAMINER |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: NOTICE OF ALLOWANCE MAILED -- APPLICATION RECEIVED IN OFFICE OF PUBLICATIONS |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: PUBLICATIONS -- ISSUE FEE PAYMENT RECEIVED |
|
| STCF | Information on status: patent grant |
Free format text: PATENTED CASE |
|
| CC | Certificate of correction | ||
| MAFP | Maintenance fee payment |
Free format text: PAYMENT OF MAINTENANCE FEE, 4TH YR, SMALL ENTITY (ORIGINAL EVENT CODE: M2551); ENTITY STATUS OF PATENT OWNER: SMALL ENTITY Year of fee payment: 4 |