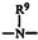TWI770291B - 用於厭氧性可固化組合物之固化加速劑 - Google Patents
用於厭氧性可固化組合物之固化加速劑 Download PDFInfo
- Publication number
- TWI770291B TWI770291B TW107135451A TW107135451A TWI770291B TW I770291 B TWI770291 B TW I770291B TW 107135451 A TW107135451 A TW 107135451A TW 107135451 A TW107135451 A TW 107135451A TW I770291 B TWI770291 B TW I770291B
- Authority
- TW
- Taiwan
- Prior art keywords
- composition
- accelerator
- hydrogen
- anaerobic
- alkyl
- Prior art date
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 95
- 125000000217 alkyl group Chemical group 0.000 claims abstract description 32
- 229910052739 hydrogen Inorganic materials 0.000 claims abstract description 32
- 239000001257 hydrogen Substances 0.000 claims abstract description 32
- 150000002431 hydrogen Chemical class 0.000 claims abstract description 25
- -1 amino, carboxyl Chemical group 0.000 claims abstract description 24
- 125000003342 alkenyl group Chemical group 0.000 claims abstract description 23
- 125000002768 hydroxyalkyl group Chemical group 0.000 claims abstract description 23
- 125000005020 hydroxyalkenyl group Chemical group 0.000 claims abstract description 20
- 125000000304 alkynyl group Chemical group 0.000 claims abstract description 18
- 125000005016 hydroxyalkynyl group Chemical group 0.000 claims abstract description 18
- 125000002877 alkyl aryl group Chemical group 0.000 claims abstract description 16
- 229910052736 halogen Inorganic materials 0.000 claims abstract description 16
- 150000002367 halogens Chemical class 0.000 claims abstract description 16
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims abstract description 11
- 229910052760 oxygen Inorganic materials 0.000 claims abstract description 10
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims abstract description 7
- 229910052717 sulfur Inorganic materials 0.000 claims abstract description 7
- WKJMQLMWPMZUQH-UHFFFAOYSA-N 1,2,3,4-tetrahydrobenzo[h]quinolin-3-ol Chemical group C1=CC2=CC=CC=C2C2=C1CC(O)CN2 WKJMQLMWPMZUQH-UHFFFAOYSA-N 0.000 claims abstract description 5
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 claims description 42
- 230000001939 inductive effect Effects 0.000 claims description 12
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 claims description 6
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 claims description 6
- 229940081974 saccharin Drugs 0.000 claims description 6
- 235000019204 saccharin Nutrition 0.000 claims description 6
- 239000000901 saccharin and its Na,K and Ca salt Substances 0.000 claims description 6
- CIHOLLKRGTVIJN-UHFFFAOYSA-N tert‐butyl hydroperoxide Chemical compound CC(C)(C)OO CIHOLLKRGTVIJN-UHFFFAOYSA-N 0.000 claims description 6
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 claims description 4
- 150000001412 amines Chemical group 0.000 claims description 4
- 229910052751 metal Inorganic materials 0.000 claims description 4
- 239000002184 metal Substances 0.000 claims description 4
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 claims description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 claims description 3
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 claims description 3
- 239000011976 maleic acid Substances 0.000 claims description 3
- 239000003381 stabilizer Substances 0.000 claims description 3
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 claims description 3
- 239000002253 acid Substances 0.000 claims description 2
- 150000007513 acids Chemical class 0.000 claims description 2
- HKJNHYJTVPWVGV-UHFFFAOYSA-N n,n-diethyl-4-methylaniline Chemical compound CCN(CC)C1=CC=C(C)C=C1 HKJNHYJTVPWVGV-UHFFFAOYSA-N 0.000 claims description 2
- 150000003839 salts Chemical class 0.000 claims description 2
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 claims 3
- LAQYHRQFABOIFD-UHFFFAOYSA-N 2-methoxyhydroquinone Chemical compound COC1=CC(O)=CC=C1O LAQYHRQFABOIFD-UHFFFAOYSA-N 0.000 claims 2
- QIGBRXMKCJKVMJ-UHFFFAOYSA-N Hydroquinone Chemical compound OC1=CC=C(O)C=C1 QIGBRXMKCJKVMJ-UHFFFAOYSA-N 0.000 claims 2
- JLTDJTHDQAWBAV-UHFFFAOYSA-N N,N-dimethylaniline Chemical compound CN(C)C1=CC=CC=C1 JLTDJTHDQAWBAV-UHFFFAOYSA-N 0.000 claims 2
- HQABUPZFAYXKJW-UHFFFAOYSA-N butan-1-amine Chemical compound CCCCN HQABUPZFAYXKJW-UHFFFAOYSA-N 0.000 claims 2
- PAFZNILMFXTMIY-UHFFFAOYSA-N cyclohexylamine Chemical compound NC1CCCCC1 PAFZNILMFXTMIY-UHFFFAOYSA-N 0.000 claims 2
- ODLMAHJVESYWTB-UHFFFAOYSA-N propylbenzene Chemical compound CCCC1=CC=CC=C1 ODLMAHJVESYWTB-UHFFFAOYSA-N 0.000 claims 2
- WMNXTXGAMHTQTA-UHFFFAOYSA-N (N-aminoanilino) acetate Chemical compound CC(=O)ON(N)C1=CC=CC=C1 WMNXTXGAMHTQTA-UHFFFAOYSA-N 0.000 claims 1
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical group C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 claims 1
- AZQWKYJCGOJGHM-UHFFFAOYSA-N 1,4-benzoquinone Chemical group O=C1C=CC(=O)C=C1 AZQWKYJCGOJGHM-UHFFFAOYSA-N 0.000 claims 1
- 239000004322 Butylated hydroxytoluene Substances 0.000 claims 1
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 claims 1
- 229930192627 Naphthoquinone Natural products 0.000 claims 1
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 claims 1
- PYKYMHQGRFAEBM-UHFFFAOYSA-N anthraquinone Natural products CCC(=O)c1c(O)c2C(=O)C3C(C=CC=C3O)C(=O)c2cc1CC(=O)OC PYKYMHQGRFAEBM-UHFFFAOYSA-N 0.000 claims 1
- 150000004056 anthraquinones Chemical class 0.000 claims 1
- 229940095259 butylated hydroxytoluene Drugs 0.000 claims 1
- 235000010354 butylated hydroxytoluene Nutrition 0.000 claims 1
- SPTHWAJJMLCAQF-UHFFFAOYSA-M ctk4f8481 Chemical compound [O-]O.CC(C)C1=CC=CC=C1C(C)C SPTHWAJJMLCAQF-UHFFFAOYSA-M 0.000 claims 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 claims 1
- JDEJGVSZUIJWBM-UHFFFAOYSA-N n,n,2-trimethylaniline Chemical compound CN(C)C1=CC=CC=C1C JDEJGVSZUIJWBM-UHFFFAOYSA-N 0.000 claims 1
- 150000002791 naphthoquinones Chemical class 0.000 claims 1
- 229940124530 sulfonamide Drugs 0.000 claims 1
- 150000003456 sulfonamides Chemical class 0.000 claims 1
- 239000013466 adhesive and sealant Substances 0.000 abstract description 4
- 239000000853 adhesive Substances 0.000 description 32
- 230000001070 adhesive effect Effects 0.000 description 32
- LBUJPTNKIBCYBY-UHFFFAOYSA-N 1,2,3,4-tetrahydroquinoline Chemical compound C1=CC=C2CCCNC2=C1 LBUJPTNKIBCYBY-UHFFFAOYSA-N 0.000 description 27
- 125000004432 carbon atom Chemical group C* 0.000 description 16
- 150000001875 compounds Chemical class 0.000 description 14
- 229910001209 Low-carbon steel Inorganic materials 0.000 description 13
- 239000007795 chemical reaction product Substances 0.000 description 10
- 239000000178 monomer Substances 0.000 description 10
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 8
- 238000000926 separation method Methods 0.000 description 8
- 239000000758 substrate Substances 0.000 description 8
- 239000000463 material Substances 0.000 description 7
- 239000000523 sample Substances 0.000 description 7
- 229910001369 Brass Inorganic materials 0.000 description 6
- 125000003118 aryl group Chemical group 0.000 description 6
- 239000010951 brass Substances 0.000 description 6
- 125000000962 organic group Chemical group 0.000 description 6
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Natural products NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 5
- 239000003795 chemical substances by application Substances 0.000 description 5
- 238000010276 construction Methods 0.000 description 5
- 239000010935 stainless steel Substances 0.000 description 5
- 229910001220 stainless steel Inorganic materials 0.000 description 5
- HCLJOFJIQIJXHS-UHFFFAOYSA-N 2-[2-[2-(2-prop-2-enoyloxyethoxy)ethoxy]ethoxy]ethyl prop-2-enoate Chemical compound C=CC(=O)OCCOCCOCCOCCOC(=O)C=C HCLJOFJIQIJXHS-UHFFFAOYSA-N 0.000 description 4
- WOBHKFSMXKNTIM-UHFFFAOYSA-N Hydroxyethyl methacrylate Chemical compound CC(=C)C(=O)OCCO WOBHKFSMXKNTIM-UHFFFAOYSA-N 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 4
- 238000000034 method Methods 0.000 description 4
- 230000000704 physical effect Effects 0.000 description 4
- 239000000376 reactant Substances 0.000 description 4
- KHADWTWCQJVOQO-UHFFFAOYSA-N zinc;oxido-(oxido(dioxo)chromio)oxy-dioxochromium Chemical compound [Zn+2].[O-][Cr](=O)(=O)O[Cr]([O-])(=O)=O KHADWTWCQJVOQO-UHFFFAOYSA-N 0.000 description 4
- OMIGHNLMNHATMP-UHFFFAOYSA-N 2-hydroxyethyl prop-2-enoate Chemical compound OCCOC(=O)C=C OMIGHNLMNHATMP-UHFFFAOYSA-N 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- IISBACLAFKSPIT-UHFFFAOYSA-N bisphenol A Chemical compound C=1C=C(O)C=CC=1C(C)(C)C1=CC=C(O)C=C1 IISBACLAFKSPIT-UHFFFAOYSA-N 0.000 description 3
- PXKLMJQFEQBVLD-UHFFFAOYSA-N bisphenol F Chemical compound C1=CC(O)=CC=C1CC1=CC=C(O)C=C1 PXKLMJQFEQBVLD-UHFFFAOYSA-N 0.000 description 3
- 229910052799 carbon Inorganic materials 0.000 description 3
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 3
- 239000002738 chelating agent Substances 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- 125000001475 halogen functional group Chemical group 0.000 description 3
- 239000003112 inhibitor Substances 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- UICBCXONCUFSOI-UHFFFAOYSA-N n'-phenylacetohydrazide Chemical compound CC(=O)NNC1=CC=CC=C1 UICBCXONCUFSOI-UHFFFAOYSA-N 0.000 description 3
- 239000004014 plasticizer Substances 0.000 description 3
- 229920000058 polyacrylate Polymers 0.000 description 3
- 150000003254 radicals Chemical class 0.000 description 3
- UMGDCJDMYOKAJW-UHFFFAOYSA-N thiourea Chemical compound NC(N)=S UMGDCJDMYOKAJW-UHFFFAOYSA-N 0.000 description 3
- XFCMNSHQOZQILR-UHFFFAOYSA-N 2-[2-(2-methylprop-2-enoyloxy)ethoxy]ethyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCOCCOC(=O)C(C)=C XFCMNSHQOZQILR-UHFFFAOYSA-N 0.000 description 2
- VHSHLMUCYSAUQU-UHFFFAOYSA-N 2-hydroxypropyl methacrylate Chemical compound CC(O)COC(=O)C(C)=C VHSHLMUCYSAUQU-UHFFFAOYSA-N 0.000 description 2
- FRIBMENBGGCKPD-UHFFFAOYSA-N 3-(2,3-dimethoxyphenyl)prop-2-enal Chemical compound COC1=CC=CC(C=CC=O)=C1OC FRIBMENBGGCKPD-UHFFFAOYSA-N 0.000 description 2
- GNSFRPWPOGYVLO-UHFFFAOYSA-N 3-hydroxypropyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCCO GNSFRPWPOGYVLO-UHFFFAOYSA-N 0.000 description 2
- NDWUBGAGUCISDV-UHFFFAOYSA-N 4-hydroxybutyl prop-2-enoate Chemical compound OCCCCOC(=O)C=C NDWUBGAGUCISDV-UHFFFAOYSA-N 0.000 description 2
- 102100026735 Coagulation factor VIII Human genes 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 2
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 2
- 101000911390 Homo sapiens Coagulation factor VIII Proteins 0.000 description 2
- PEEHTFAAVSWFBL-UHFFFAOYSA-N Maleimide Chemical compound O=C1NC(=O)C=C1 PEEHTFAAVSWFBL-UHFFFAOYSA-N 0.000 description 2
- 229910000831 Steel Inorganic materials 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 2
- HVVWZTWDBSEWIH-UHFFFAOYSA-N [2-(hydroxymethyl)-3-prop-2-enoyloxy-2-(prop-2-enoyloxymethyl)propyl] prop-2-enoate Chemical compound C=CC(=O)OCC(CO)(COC(=O)C=C)COC(=O)C=C HVVWZTWDBSEWIH-UHFFFAOYSA-N 0.000 description 2
- 239000000654 additive Substances 0.000 description 2
- 125000003545 alkoxy group Chemical group 0.000 description 2
- 125000002947 alkylene group Chemical group 0.000 description 2
- 150000001408 amides Chemical class 0.000 description 2
- 125000003710 aryl alkyl group Chemical group 0.000 description 2
- 230000000712 assembly Effects 0.000 description 2
- 238000000429 assembly Methods 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- KQNZLOUWXSAZGD-UHFFFAOYSA-N benzylperoxymethylbenzene Chemical compound C=1C=CC=CC=1COOCC1=CC=CC=C1 KQNZLOUWXSAZGD-UHFFFAOYSA-N 0.000 description 2
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 2
- 239000003054 catalyst Substances 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 125000000392 cycloalkenyl group Chemical group 0.000 description 2
- 125000000753 cycloalkyl group Chemical group 0.000 description 2
- ZQMIGQNCOMNODD-UHFFFAOYSA-N diacetyl peroxide Chemical compound CC(=O)OOC(C)=O ZQMIGQNCOMNODD-UHFFFAOYSA-N 0.000 description 2
- GPLRAVKSCUXZTP-UHFFFAOYSA-N diglycerol Chemical compound OCC(O)COCC(O)CO GPLRAVKSCUXZTP-UHFFFAOYSA-N 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 229920001971 elastomer Polymers 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 125000000623 heterocyclic group Chemical group 0.000 description 2
- 239000003999 initiator Substances 0.000 description 2
- RNVCVTLRINQCPJ-UHFFFAOYSA-N o-toluidine Chemical compound CC1=CC=CC=C1N RNVCVTLRINQCPJ-UHFFFAOYSA-N 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 150000002978 peroxides Chemical class 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 229920001228 polyisocyanate Polymers 0.000 description 2
- 239000005056 polyisocyanate Substances 0.000 description 2
- 229920001296 polysiloxane Polymers 0.000 description 2
- 238000010526 radical polymerization reaction Methods 0.000 description 2
- 239000002994 raw material Substances 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 239000010959 steel Substances 0.000 description 2
- GJBRNHKUVLOCEB-UHFFFAOYSA-N tert-butyl benzenecarboperoxoate Chemical compound CC(C)(C)OOC(=O)C1=CC=CC=C1 GJBRNHKUVLOCEB-UHFFFAOYSA-N 0.000 description 2
- 150000004992 toluidines Chemical class 0.000 description 2
- 239000011701 zinc Substances 0.000 description 2
- 229910052725 zinc Inorganic materials 0.000 description 2
- 125000003837 (C1-C20) alkyl group Chemical group 0.000 description 1
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 1
- 229920002818 (Hydroxyethyl)methacrylate Polymers 0.000 description 1
- JUDOLRSMWHVKGX-UHFFFAOYSA-N 1,1-dioxo-1$l^{6},2-benzodithiol-3-one Chemical compound C1=CC=C2C(=O)SS(=O)(=O)C2=C1 JUDOLRSMWHVKGX-UHFFFAOYSA-N 0.000 description 1
- NCYNKWQXFADUOZ-UHFFFAOYSA-N 1,1-dioxo-2,1$l^{6}-benzoxathiol-3-one Chemical compound C1=CC=C2C(=O)OS(=O)(=O)C2=C1 NCYNKWQXFADUOZ-UHFFFAOYSA-N 0.000 description 1
- UBRWPVTUQDJKCC-UHFFFAOYSA-N 1,3-bis(2-tert-butylperoxypropan-2-yl)benzene Chemical compound CC(C)(C)OOC(C)(C)C1=CC=CC(C(C)(C)OOC(C)(C)C)=C1 UBRWPVTUQDJKCC-UHFFFAOYSA-N 0.000 description 1
- KAJICSGLHKRDLN-UHFFFAOYSA-N 1,3-dicyclohexylthiourea Chemical compound C1CCCCC1NC(=S)NC1CCCCC1 KAJICSGLHKRDLN-UHFFFAOYSA-N 0.000 description 1
- XSZYESUNPWGWFQ-UHFFFAOYSA-N 1-(2-hydroperoxypropan-2-yl)-4-methylcyclohexane Chemical compound CC1CCC(C(C)(C)OO)CC1 XSZYESUNPWGWFQ-UHFFFAOYSA-N 0.000 description 1
- PQXKLSYJDSMALY-UHFFFAOYSA-N 1-chloro-4-[(4-chlorophenyl)methylperoxymethyl]benzene Chemical compound C1=CC(Cl)=CC=C1COOCC1=CC=C(Cl)C=C1 PQXKLSYJDSMALY-UHFFFAOYSA-N 0.000 description 1
- FSWXFPXMYYOZLD-UHFFFAOYSA-N 2-(2-chloroprop-2-enoyloxy)ethyl 2-chloroprop-2-enoate Chemical compound ClC(C(=O)OCCOC(C(=C)Cl)=O)=C FSWXFPXMYYOZLD-UHFFFAOYSA-N 0.000 description 1
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 1
- RTMKOJVCQFWHFA-UHFFFAOYSA-N 2-(anilinocarbamoyl)prop-2-enoic acid Chemical compound OC(=O)C(=C)C(=O)NNC1=CC=CC=C1 RTMKOJVCQFWHFA-UHFFFAOYSA-N 0.000 description 1
- HGIPIEYZJPULIQ-UHFFFAOYSA-N 2-(methylazaniumyl)-2-phenylacetate Chemical compound CNC(C(O)=O)C1=CC=CC=C1 HGIPIEYZJPULIQ-UHFFFAOYSA-N 0.000 description 1
- BEWCNXNIQCLWHP-UHFFFAOYSA-N 2-(tert-butylamino)ethyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCNC(C)(C)C BEWCNXNIQCLWHP-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- HWSSEYVMGDIFMH-UHFFFAOYSA-N 2-[2-[2-(2-methylprop-2-enoyloxy)ethoxy]ethoxy]ethyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCOCCOCCOC(=O)C(C)=C HWSSEYVMGDIFMH-UHFFFAOYSA-N 0.000 description 1
- LTHJXDSHSVNJKG-UHFFFAOYSA-N 2-[2-[2-[2-(2-methylprop-2-enoyloxy)ethoxy]ethoxy]ethoxy]ethyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCOCCOCCOCCOC(=O)C(C)=C LTHJXDSHSVNJKG-UHFFFAOYSA-N 0.000 description 1
- GPOGMJLHWQHEGF-UHFFFAOYSA-N 2-chloroethyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCCl GPOGMJLHWQHEGF-UHFFFAOYSA-N 0.000 description 1
- AEPWOCLBLLCOGZ-UHFFFAOYSA-N 2-cyanoethyl prop-2-enoate Chemical compound C=CC(=O)OCCC#N AEPWOCLBLLCOGZ-UHFFFAOYSA-N 0.000 description 1
- IEVADDDOVGMCSI-UHFFFAOYSA-N 2-hydroxybutyl 2-methylprop-2-enoate Chemical compound CCC(O)COC(=O)C(C)=C IEVADDDOVGMCSI-UHFFFAOYSA-N 0.000 description 1
- GWZMWHWAWHPNHN-UHFFFAOYSA-N 2-hydroxypropyl prop-2-enoate Chemical compound CC(O)COC(=O)C=C GWZMWHWAWHPNHN-UHFFFAOYSA-N 0.000 description 1
- FZSHSWCZYDDOCK-UHFFFAOYSA-N 2-methylprop-2-enoic acid;oxolane Chemical compound C1CCOC1.CC(=C)C(O)=O FZSHSWCZYDDOCK-UHFFFAOYSA-N 0.000 description 1
- RIVJYLGQARVCBI-UHFFFAOYSA-N 2-tert-butylperoxy-2-methylpropane;cumene Chemical compound CC(C)C1=CC=CC=C1.CC(C)(C)OOC(C)(C)C RIVJYLGQARVCBI-UHFFFAOYSA-N 0.000 description 1
- BIISIZOQPWZPPS-UHFFFAOYSA-N 2-tert-butylperoxypropan-2-ylbenzene Chemical compound CC(C)(C)OOC(C)(C)C1=CC=CC=C1 BIISIZOQPWZPPS-UHFFFAOYSA-N 0.000 description 1
- QZPSOSOOLFHYRR-UHFFFAOYSA-N 3-hydroxypropyl prop-2-enoate Chemical compound OCCCOC(=O)C=C QZPSOSOOLFHYRR-UHFFFAOYSA-N 0.000 description 1
- XOJWAAUYNWGQAU-UHFFFAOYSA-N 4-(2-methylprop-2-enoyloxy)butyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCCCOC(=O)C(C)=C XOJWAAUYNWGQAU-UHFFFAOYSA-N 0.000 description 1
- PCOLKDCINSTMQM-UHFFFAOYSA-N 4-(n-aminoanilino)-4-oxobut-2-enoic acid Chemical compound OC(=O)C=CC(=O)N(N)C1=CC=CC=C1 PCOLKDCINSTMQM-UHFFFAOYSA-N 0.000 description 1
- PSMOPJGGDKTSDN-UHFFFAOYSA-N 4-(n-aminoanilino)butanoic acid Chemical compound OC(=O)CCCN(N)C1=CC=CC=C1 PSMOPJGGDKTSDN-UHFFFAOYSA-N 0.000 description 1
- GDZIMHQTKFQCFV-UHFFFAOYSA-N 4-[n-(methylamino)anilino]-4-oxobut-2-enoic acid Chemical compound OC(=O)C=CC(=O)N(NC)C1=CC=CC=C1 GDZIMHQTKFQCFV-UHFFFAOYSA-N 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N 4-hydroxybenzoic acid Chemical compound OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- OKJADYKTJJGKDX-UHFFFAOYSA-N Butyl pentanoate Chemical compound CCCCOC(=O)CCCC OKJADYKTJJGKDX-UHFFFAOYSA-N 0.000 description 1
- OFXOBLFLXCXZSY-UHFFFAOYSA-N C(C(=C)C)(=O)O.C(C(=C)C)(=O)O.C(CCCC)(O)O.C(CCCC)(O)O Chemical compound C(C(=C)C)(=O)O.C(C(=C)C)(=O)O.C(CCCC)(O)O.C(CCCC)(O)O OFXOBLFLXCXZSY-UHFFFAOYSA-N 0.000 description 1
- 125000003358 C2-C20 alkenyl group Chemical group 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- PDQAZBWRQCGBEV-UHFFFAOYSA-N Ethylenethiourea Chemical compound S=C1NCCN1 PDQAZBWRQCGBEV-UHFFFAOYSA-N 0.000 description 1
- ZGUNAGUHMKGQNY-ZETCQYMHSA-N L-alpha-phenylglycine zwitterion Chemical compound OC(=O)[C@@H](N)C1=CC=CC=C1 ZGUNAGUHMKGQNY-ZETCQYMHSA-N 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- WUGQZFFCHPXWKQ-UHFFFAOYSA-N Propanolamine Chemical compound NCCCO WUGQZFFCHPXWKQ-UHFFFAOYSA-N 0.000 description 1
- BLRPTPMANUNPDV-UHFFFAOYSA-N Silane Chemical compound [SiH4] BLRPTPMANUNPDV-UHFFFAOYSA-N 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- MNOILHPDHOHILI-UHFFFAOYSA-N Tetramethylthiourea Chemical compound CN(C)C(=S)N(C)C MNOILHPDHOHILI-UHFFFAOYSA-N 0.000 description 1
- ZJCCRDAZUWHFQH-UHFFFAOYSA-N Trimethylolpropane Chemical compound CCC(CO)(CO)CO ZJCCRDAZUWHFQH-UHFFFAOYSA-N 0.000 description 1
- DAKWPKUUDNSNPN-UHFFFAOYSA-N Trimethylolpropane triacrylate Chemical compound C=CC(=O)OCC(CC)(COC(=O)C=C)COC(=O)C=C DAKWPKUUDNSNPN-UHFFFAOYSA-N 0.000 description 1
- OKKRPWIIYQTPQF-UHFFFAOYSA-N Trimethylolpropane trimethacrylate Chemical compound CC(=C)C(=O)OCC(CC)(COC(=O)C(C)=C)COC(=O)C(C)=C OKKRPWIIYQTPQF-UHFFFAOYSA-N 0.000 description 1
- 125000003158 alcohol group Chemical group 0.000 description 1
- 125000005907 alkyl ester group Chemical group 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 125000004103 aminoalkyl group Chemical group 0.000 description 1
- APLHDUWNMGJBFD-UHFFFAOYSA-N azepane-2-thione Chemical compound S=C1CCCCCN1 APLHDUWNMGJBFD-UHFFFAOYSA-N 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- CREMABGTGYGIQB-UHFFFAOYSA-N carbon carbon Chemical compound C.C CREMABGTGYGIQB-UHFFFAOYSA-N 0.000 description 1
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 239000013068 control sample Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 125000004093 cyano group Chemical group *C#N 0.000 description 1
- OIWOHHBRDFKZNC-UHFFFAOYSA-N cyclohexyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OC1CCCCC1 OIWOHHBRDFKZNC-UHFFFAOYSA-N 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- LSXWFXONGKSEMY-UHFFFAOYSA-N di-tert-butyl peroxide Chemical compound CC(C)(C)OOC(C)(C)C LSXWFXONGKSEMY-UHFFFAOYSA-N 0.000 description 1
- 125000004386 diacrylate group Chemical group 0.000 description 1
- SZXQTJUDPRGNJN-UHFFFAOYSA-N dipropylene glycol Chemical compound OCCCOCCCO SZXQTJUDPRGNJN-UHFFFAOYSA-N 0.000 description 1
- 239000000806 elastomer Substances 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- ACCCMOQWYVYDOT-UHFFFAOYSA-N hexane-1,1-diol Chemical compound CCCCCC(O)O ACCCMOQWYVYDOT-UHFFFAOYSA-N 0.000 description 1
- 150000002429 hydrazines Chemical class 0.000 description 1
- 150000002430 hydrocarbons Chemical group 0.000 description 1
- 125000001183 hydrocarbyl group Chemical group 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 150000002432 hydroperoxides Chemical class 0.000 description 1
- 125000000687 hydroquinonyl group Chemical class C1(O)=C(C=C(O)C=C1)* 0.000 description 1
- 150000002466 imines Chemical class 0.000 description 1
- 239000012948 isocyanate Substances 0.000 description 1
- IQPQWNKOIGAROB-UHFFFAOYSA-N isocyanate group Chemical group [N-]=C=O IQPQWNKOIGAROB-UHFFFAOYSA-N 0.000 description 1
- 150000002513 isocyanates Chemical class 0.000 description 1
- 150000002689 maleic acids Chemical class 0.000 description 1
- 230000013011 mating Effects 0.000 description 1
- 150000002734 metacrylic acid derivatives Chemical class 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- YDKNBNOOCSNPNS-UHFFFAOYSA-N methyl 1,3-benzoxazole-2-carboxylate Chemical compound C1=CC=C2OC(C(=O)OC)=NC2=C1 YDKNBNOOCSNPNS-UHFFFAOYSA-N 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 125000004957 naphthylene group Chemical group 0.000 description 1
- SLCVBVWXLSEKPL-UHFFFAOYSA-N neopentyl glycol Chemical compound OCC(C)(C)CO SLCVBVWXLSEKPL-UHFFFAOYSA-N 0.000 description 1
- 150000007524 organic acids Chemical group 0.000 description 1
- UWJJYHHHVWZFEP-UHFFFAOYSA-N pentane-1,1-diol Chemical compound CCCCC(O)O UWJJYHHHVWZFEP-UHFFFAOYSA-N 0.000 description 1
- 150000002989 phenols Chemical class 0.000 description 1
- 125000000843 phenylene group Chemical group C1(=C(C=CC=C1)*)* 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 150000004053 quinones Chemical class 0.000 description 1
- 239000007870 radical polymerization initiator Substances 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 239000005060 rubber Substances 0.000 description 1
- 239000000565 sealant Substances 0.000 description 1
- 229910000077 silane Inorganic materials 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 125000000547 substituted alkyl group Chemical group 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-L sulfite Chemical class [O-]S([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-L 0.000 description 1
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical group [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 1
- 150000003871 sulfonates Chemical class 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- UWHCKJMYHZGTIT-UHFFFAOYSA-N tetraethylene glycol Chemical compound OCCOCCOCCOCCO UWHCKJMYHZGTIT-UHFFFAOYSA-N 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 125000000383 tetramethylene group Chemical group [H]C([H])([*:1])C([H])([H])C([H])([H])C([H])([H])[*:2] 0.000 description 1
- 229920001187 thermosetting polymer Polymers 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 125000004001 thioalkyl group Chemical group 0.000 description 1
- 239000012745 toughening agent Substances 0.000 description 1
- 229910021654 trace metal Inorganic materials 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/34—Heterocyclic compounds having nitrogen in the ring
- C08K5/3412—Heterocyclic compounds having nitrogen in the ring having one nitrogen atom in the ring
- C08K5/3432—Six-membered rings
- C08K5/3437—Six-membered rings condensed with carbocyclic rings
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F20/00—Homopolymers and copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride, ester, amide, imide or nitrile thereof
- C08F20/02—Monocarboxylic acids having less than ten carbon atoms, Derivatives thereof
- C08F20/10—Esters
- C08F20/20—Esters of polyhydric alcohols or polyhydric phenols, e.g. 2-hydroxyethyl (meth)acrylate or glycerol mono-(meth)acrylate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F20/00—Homopolymers and copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride, ester, amide, imide or nitrile thereof
- C08F20/02—Monocarboxylic acids having less than ten carbon atoms, Derivatives thereof
- C08F20/04—Acids, Metal salts or ammonium salts thereof
- C08F20/06—Acrylic acid; Methacrylic acid; Metal salts or ammonium salts thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F20/00—Homopolymers and copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride, ester, amide, imide or nitrile thereof
- C08F20/02—Monocarboxylic acids having less than ten carbon atoms, Derivatives thereof
- C08F20/10—Esters
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/0008—Organic ingredients according to more than one of the "one dot" groups of C08K5/01 - C08K5/59
- C08K5/0025—Crosslinking or vulcanising agents; including accelerators
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/0008—Organic ingredients according to more than one of the "one dot" groups of C08K5/01 - C08K5/59
- C08K5/005—Stabilisers against oxidation, heat, light, ozone
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/04—Oxygen-containing compounds
- C08K5/07—Aldehydes; Ketones
- C08K5/08—Quinones
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/04—Oxygen-containing compounds
- C08K5/14—Peroxides
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L33/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- C08L33/04—Homopolymers or copolymers of esters
- C08L33/06—Homopolymers or copolymers of esters of esters containing only carbon, hydrogen and oxygen, which oxygen atoms are present only as part of the carboxyl radical
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J133/00—Adhesives based on homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides, or nitriles thereof; Adhesives based on derivatives of such polymers
- C09J133/04—Homopolymers or copolymers of esters
- C09J133/06—Homopolymers or copolymers of esters of esters containing only carbon, hydrogen and oxygen, the oxygen atom being present only as part of the carboxyl radical
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J133/00—Adhesives based on homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides, or nitriles thereof; Adhesives based on derivatives of such polymers
- C09J133/04—Homopolymers or copolymers of esters
- C09J133/14—Homopolymers or copolymers of esters of esters containing halogen, nitrogen, sulfur or oxygen atoms in addition to the carboxy oxygen
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J4/00—Adhesives based on organic non-macromolecular compounds having at least one polymerisable carbon-to-carbon unsaturated bond ; adhesives, based on monomers of macromolecular compounds of groups C09J183/00 - C09J183/16
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K3/00—Materials not provided for elsewhere
- C09K3/10—Materials in mouldable or extrudable form for sealing or packing joints or covers
- C09K2003/1034—Materials or components characterised by specific properties
- C09K2003/1065—Anaerobically hardenable materials
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Adhesives Or Adhesive Processes (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Polymerization Catalysts (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Epoxy Resins (AREA)
Abstract
Description
厭氧性黏著劑組合物一般為眾所周知的。參見例如
R.D. Rich,Handbook of Adhesive Technology
中之「Anaerobic Adhesives」, 29, 467-79, A. Pizzi及K.L. Mittal編, Marcel Dekker, Inc., New York (1994)及其中引用之參考文獻。其用途眾多且繼續開發新的應用。
習知的厭氧性黏著劑通常包括可自由基聚合之丙烯酸酯單體,以及過氧化氫引發劑及抑制劑組分。通常,此類厭氧性黏著劑組合物亦含有加速劑組分以提高組合物固化之速度。
誘導及加速固化之理想的厭氧性固化誘導組合物通常可包括糖精;甲苯胺,諸如N,N-二乙基-對甲苯胺(「DE-p-T」)及N,N-二甲基-鄰甲苯胺(「DM-o-T」);乙醯基苯肼(「APH」);馬來酸中之一或多者。
糖精及APH在厭氧性黏著劑固化系統中用作標準固化加速劑組分。然而,此等組分已在世界某些地區受到監管審查,因此已嘗試將候選物確定為替代物。
用於厭氧性黏著劑之其他固化劑的實例包括硫代己內醯胺(例如
美國專利第5,411,988號)及硫脲[例如
美國專利第3,970,505號(Hauser) (四甲基硫脲),德國專利文件第DE 1 817 989號(烷基硫脲及N,N'-二環己基硫脲)及第2 806 701號(伸乙基硫脲),及日本專利文件第JP 07-308,757號(醯基、烷基、亞烷基、伸烷基及烷基硫脲)],後者中之某些已商業化使用直至約二十年前。
Loctite (R&D) Ltd.發現一種新型材料-三硫雜二氮雜並環戊二烯-有效作為厭氧性黏著劑組合物之固化劑。將此等材料添加至厭氧性黏著劑中作為習知固化劑(諸如APH)之替代物出人意料地為由其形成之反應產物提供至少相當的固化速度及物理特性。參見
美國專利第6,583,289號(McArdle)。
美國專利第6,835,762號(Klemarczyk)提供一種基於(甲基)丙烯酸酯組分之厭氧性可固化組合物,其具有基本上不含乙醯基苯肼及馬來酸之厭氧性固化誘導組合物及在同一分子上具有鍵-C(=O)-NH-NH-及有機酸基團的厭氧性固化加速劑化合物,限制條件為厭氧性固化加速劑化合物不包括1-(2-羧基丙烯醯基)-2-苯肼。厭氧性固化加速劑由以下結構所涵蓋:
其中R1
-R7
各自獨立地選自氫及C1 - 4
;Z為碳-碳單鍵或碳-碳雙鍵;q為0或1;且p為1至5之整數,其實例為3-羧基丙烯醯基苯肼、甲基-3-羧基丙烯醯基苯肼、3-羧基丙醯基苯肼及亞甲基-3-羧基丙醯基苯肼。
美國專利第6,897,277號(Klemarczyk)提供一種基於(甲基)丙烯酸酯組分之厭氧性可固化組合物,其具有基本上不含糖精之厭氧係固化誘導組合物及在以下結構內之厭氧性固化加速劑化合物,
其中R係選自氫、鹵素、烷基、烯基、羥基烷基、羥基烯基、羧基及磺酸酯基,且R1
係選自氫、烷基、烯基、羥基烷基、羥基烯基及烷芳基,其實例為苯基甘胺酸及N-甲基苯基甘胺酸。
美國專利第6,958,368號(Messana)提供一種厭氧性可固化組合物。此組合物係基於(甲基)丙烯酸酯組分,其具有基本上不含糖精之厭氧性固化誘導組合物且在以下結構內,
其中Y為芳環,視情況在至多五個位置處經C1 - 6
烷基或烷氧基或鹵基取代;A為C=O、S=O或O=S=O;X為NH、O或S,且Z為芳環,視情況經在至多五個位置處經C1 - 6
烷基或烷氧基或鹵基取代,或Y及Z一起可連接至相同的芳環或芳環系統,限制條件為當X為NH時,該結構不包括鄰苯甲醯磺醯亞胺。由上述結構所涵蓋之厭氧性固化加速劑化合物的實例包括2-磺基苯甲酸環酐及3H-1,2-苯并二硫醇-3-酮-1,1-二氧化物。
Three Bond Co. Ltd., Tokyo, Japan在過去曾將一種稱為四氫喹啉(「THQ」)之組分描述為厭氧性黏著劑及密封劑組合物中之組分。
且最近的美國專利第8,362,112號描述由包含以下之反應物製備的反應產物:(a)涵蓋在以下結構內之化合物,
其中X為C1 - 20
烷基、C2 - 20
烯基或C7 - 20
烷芳基,其中任一者可間雜有一或多個雜原子,且其經至少一個選自-OH、-NH2
或-SH之基團官能化且z為1-3;及(b)至少一種異氰酸酯官能材料。
美國專利第8,481,659號描述一種厭氧性可固化組合物,其包含(a) (甲基)丙烯酸酯組分;(b)厭氧性固化系統;及(c)由包含以下之反應物製備的反應產物:(i)至少一種選自由以下結構表示之化合物之群的化合物,
其中z為1-3;及(b)以下兩者中之任一者:(i)至少一種選自由以下結構表示之化合物之群的化合物,
其中Z''係選自-O-、-S-及-NH-;q為1至4;R6
獨立地選自由羥基烷基、胺基烷基及硫代烷基組成之群;且n為至少1,其中反應產物包含至少兩個獨立地選自-OH、-NH2
及-SH之側鏈官能基;或(ii)烷基化劑、烯基化劑或烷芳基化劑。
儘管有目前先進技術,但仍期望發現厭氧性固化加速劑之替代技術以區別於現有產品,且在原料供應短缺或停止之情況下提供供應保證。此外,由於習知厭氧性固化誘導組合物中使用的某些原料一定程度上受到監管審查且可能受供應鏈中斷影響,因此需要用於厭氧性固化誘導組合物的替代組分。因此,需要鑑定在厭氧性可固化組合物之固化中充當固化組分的新材料。
本發明係關於可用於厭氧性可固化組合物之固化加速劑,諸如黏著劑及密封劑。
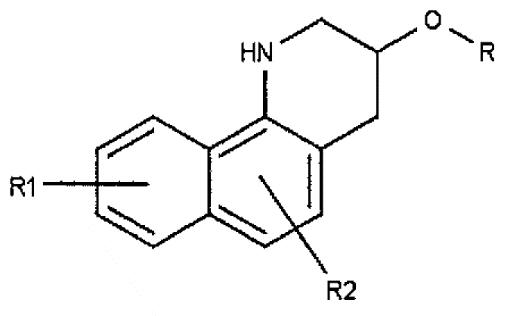
固化加速劑涵蓋於以下結構內,
其中X為CH2
、O、S、NR4
、CR5
R6
或C=O;R為氫、烷基、烯基、炔基、羥基烷基、羥基烯基或羥基炔基中之一或多者;R1
-R6
各自獨立地選自氫、鹵素、胺基、羧基、硝基、烷基、烯基、炔基、羥基烷基、羥基烯基、羥基炔基或烷芳基;R7
為氫或CHR8
R9
,其中R8
及R9
各自獨立地選自氫、鹵素、胺基、羧基、硝基、烷基、烯基、炔基、羥基烷基、羥基烯基、羥基炔基或烷芳基;且n為0或1。羧基、烷基、烯基、炔基、羥基烷基、羥基烯基、羥基炔基或烷芳基中之每一者應視需要含有一至十二個碳原子。特別理想的實例為1,2,3,4-四氫苯并-h-喹啉-3-醇。
此等固化加速劑可用於厭氧性可固化組合物中,該等組合物包含(甲基)丙烯酸酯組分及厭氧性固化誘導組分。
本發明係關於將固化加速劑添加至厭氧性黏著劑中作為一定量或全部量之習知厭氧性固化加速劑[諸如甲苯胺、THQ及/或乙醯基苯肼(「APH」)之替代品,出人意料地為由其形成之反應產物提供與由習知厭氧性可固化組合物觀察到的固化速度及物理特性相比至少相當的固化速度及物理特性。
舉例而言,經由使用本發明固化加速劑可達成減少THQ及/或APH含量(諸如習知厭氧性可固化組合物用量之約50重量%或更少),且理想地,厭氧性可固化組合物基本上不含THQ及/或APH (小於約10重量%、小於約5重量%或小於約1重量%)或不含THQ及/或APH。本發明之固化加速劑可代替一些或全部THQ及/或APH。
適用作本發明中之(甲基)丙烯酸酯組分的(甲基)丙烯酸酯單體可選自多種材料,諸如由H2
C=CGCO2
R8
表示之材料,其中G可為氫、鹵素或具有1至約4個碳原子之烷基,且R8
可選自具有1至約16個碳原子之烷基、環烷基、烯基、環烯基、烷芳基、芳烷基或芳基,其中任一者可視情況經以下各者取代或視具體情況穿插以下各者:矽烷、矽、氧、鹵素、羰基、羥基、酯、羧酸、脲、胺基甲酸酯、碳酸酯、胺、醯胺、硫、磺酸酯、碸及其類似物。
本文中適用作本發明中之(甲基)丙烯酸酯組分或適用作製備反應產物之組分的其他(甲基)丙烯酸酯單體包括多官能(甲基)丙烯酸酯單體,例如二官能或三官能(甲基)丙烯酸酯,諸如聚乙二醇二(甲基)丙烯酸酯、四氫呋喃(甲基)丙烯酸酯及二(甲基)丙烯酸酯、(甲基)丙烯酸羥丙酯(「HPMA」)、己二醇二(甲基)丙烯酸酯、三羥甲基丙烷三(甲基)丙烯酸酯(「TMPTMA」)、二乙二醇二甲基丙烯酸酯、三乙二醇二甲基丙烯酸酯(「TRIEGMA」)、四乙二醇二(甲基)丙烯酸酯、二丙二醇二(甲基)丙烯酸酯、二(戊二醇)二(甲基)丙烯酸酯、四伸乙基二甘醇二(甲基)丙烯酸酯、二甘油四(甲基)丙烯酸酯、二(甲基)丙烯酸丁二酯、二(甲基)丙烯酸乙二酯、新戊二醇二(甲基)丙烯酸酯,及雙酚A單-與二-(甲基)丙烯酸酯,諸如乙氧基化雙酚A (甲基)丙烯酸酯(「EBIPMA」),及雙酚F單-與二-(甲基)丙烯酸酯,諸如乙氧基化雙酚F (甲基)丙烯酸酯。
可用於本文中之其他(甲基)丙烯酸酯單體包括聚矽氧(甲基)丙烯酸酯部分(「SiMA」),諸如美國專利第5,605,999號(Chu)中所教示及主張之聚矽氧(甲基)丙烯酸酯部分,該專利以引用的方式併入本文中。
其他適合之單體包括由下式表示之聚丙烯酸酯,其中R4
為選自氫、鹵素或1至約4個碳原子之烷基的基團;q為至少等於1且較佳等於1至約4之整數;且X為含有至少兩個碳原子且總鍵結容量為q加1之有機基團。關於X中碳原子數之上限,可加工單體基本上以任何值存在。然而,實際上,通常的上限為約50個碳原子,較佳30個且最佳約20個。
其他類別之有用單體為二烷醇胺或三烷醇胺(例如乙醇胺或丙醇胺)與丙烯酸之反應產物,諸如法國專利第1,581,361號中所揭示。
有用的丙烯酸酯寡聚物之實例包括具有以下通式之丙烯酸酯寡聚物:其中R5
表示選自氫、1至約4個碳原子之低碳烷基、1至約4個碳原子之羥基烷基的基團,及其中R4
為選自氫、鹵素或1至約4個碳原子之低碳烷基的基團;R6
為選自氫、羥基之基團,或m為至少等於1之整數,例如
1至約15或更高且較佳1至約8;n為至少等於1之整數,例如
1至約40或更多且較佳約2至約10;且p為0或1。
對應於上述通式之丙烯酸酯寡聚物的典型實例包括二、三及四乙二醇二甲基丙烯酸酯;二(戊二醇)二甲基丙烯酸酯;四乙二醇二丙烯酸酯;四乙二醇二(氯丙烯酸酯);二甘油二丙烯酸酯;二甘油四甲基丙烯酸酯;丁二醇二甲基丙烯酸酯;新戊二醇二丙烯酸酯;及三羥甲基丙烷三丙烯酸酯。
雖然可能需要二丙烯酸酯及其他聚丙烯酸酯,且尤其前述段落中所描述之聚丙烯酸酯,但亦可使用單官能丙烯酸酯(含有一個丙烯酸酯基之酯)。當處理單官能丙烯酸酯時,使用具有相對極性醇部分之酯為極佳的。此類材料之揮發性低於低分子量烷基酯,且更重要的是,極性基團往往會在固化期間及之後提供分子間吸引力,由此產生更理想的固化特性以及更耐用的密封劑或黏著劑。理想地,極性基團係選自不穩定的氫、雜環、羥基、胺基、氰基及鹵基極性基團。此類化合物之典型實例為甲基丙烯酸環己酯、甲基丙烯酸四氫呋喃酯、丙烯酸羥乙酯、甲基丙烯酸羥丙酯、甲基丙烯酸第三丁基胺基乙酯、丙烯酸氰基乙酯及甲基丙烯酸氯乙酯。
另一類有用的單體係藉由在官能取代基上含有活性氫原子之單官能取代之丙烯酸烷基或芳基酯的反應來製備。此單官能丙烯酸酯封端之材料與有機聚異氰酸酯以適合之比例反應,以便將所有異氰酸酯基轉化成胺基甲酸酯基或脲基。單官能丙烯酸烷基及芳基酯較佳為在其非丙烯酸酯部分上含有羥基或胺基官能基的丙烯酸酯及甲基丙烯酸酯。適合使用之丙烯酸酯具有下式,其中X係選自--O--或,且R9
係選自氫或1至7個碳原子之低碳烷基;R7
係選自氫、氯或甲基及乙基;且R8
為選自1至8個碳原子之低碳伸烷基、伸苯基或伸萘基之二價有機基團。此等基團在與聚異氰酸酯適當反應時,產生以下通式之單體:其中n為2至約6之整數;B為多價有機基團,其選自經取代及未經取代之烷基、烯基、環烷基、環烯基、芳基、芳烷基、烷芳基或雜環基;且R7
、R8
及X具有上文給出之含義。
適合之羥基官能性(甲基)丙烯酸酯的實例包括丙烯酸羥乙酯、丙烯酸羥丙酯、丙烯酸羥丁酯、甲基丙烯酸羥乙酯(「HEMA」)、甲基丙烯酸羥丙酯(「HPMA」)、甲基丙烯酸羥丁酯及其混合物。適合之羥基官能性(甲基)丙烯酸酯的其他實例包括丙烯酸2-羥乙酯、丙烯酸2-羥丙酯、甲基丙烯酸2-羥乙酯(「HEMA」)、季戊四醇三丙烯酸酯(「PETA」)及丙烯酸4-羥丁酯。
羥基官能性(甲基)丙烯酸酯之數目平均分子量可為約80至約1,000 g/mol,或約100至約800 g/mol,或約110至約600 g/mol。
當然,亦可使用此等(甲基)丙烯酸酯單體之組合。
以組合物之總重量計,(甲基)丙烯酸酯組分可佔組合物之約10至約90重量%,諸如約60至約90重量%。
厭氧性固化誘導組合物包含選自第三丁基氫過氧化物、對甲烷氫過氧化物、氫過氧化異丙苯、氫過氧化二異丙基苯及其混合物之氫過氧化物。
如上文所指出,提供之固化加速劑涵蓋於以下結構內,
其中X為CH2
、O、S、NR4
、CR5
R6
或C=O;R為氫、烷基、烯基、炔基、羥基烷基、羥基烯基或羥基炔基中之一或多者;R1
-R6
各自獨立地選自氫、鹵素、胺基、羧基、硝基、烷基、烯基、炔基、羥基烷基、羥基烯基、羥基炔基或烷芳基;R7
為氫或CHR8
R9
,其中R8
及R9
各自獨立地選自氫、鹵素、胺基、羧基、硝基、烷基、烯基、炔基、羥基烷基、羥基烯基、羥基炔基或烷芳基;且n為0或1。羧基、烷基、烯基、炔基、羥基烷基、羥基烯基、羥基炔基或烷芳基中之每一者應視需要含有一至十二個碳原子。
在此三個替代實施例中之每一者中,R如上文所定義。
以組合物之總重量計,固化加速劑可以約0.005至約5重量%,諸如約0.01至約2重量%,理想地約0.01至約1.5重量%之量存在。固化加速劑可與習知加速劑組合使用(此處稱為共加速劑,但含量低於此類習知加速劑)。
可向(甲基)丙烯酸酯組分、厭氧性固化誘導組合物及固化加速劑中添加已包含於傳統厭氧性黏著劑中之組分,以改變調配物或其反應產物之物理特性。舉例而言,可包括馬來醯亞胺組分、賦予耐熱性之共反應物、在高溫條件下反應之稀釋劑組分、單羥基烷或聚羥基烷、聚合塑化劑及螯合劑中之一或多者(參見
美國專利第6,391,993號,其以引用的方式併入本文中)以調節調配物之物理特性及/或固化概況及/或固化之黏著劑的濃度或耐熱性。
使用時,馬來醯亞胺、共反應物、反應性稀釋劑、增塑劑及/或單羥基烷或聚羥基烷可以組合物之總重量計約1重量%至約30重量%之量存在。
本發明組合物亦可包括其他習知組分,諸如自由基引發劑、自由基共加速劑及自由基生成抑制劑,以及金屬催化劑。
許多眾所周知的自由基聚合引發劑通常併入本發明組合物中,包括但不限於過氧化物化合物,諸如氫過氧化物,如氫過氧化異丙苯(「CHP」)、對薄荷烷氫過氧化物、第三丁基氫過氧化物(「TBH」)及過苯甲酸第三丁酯。其他過氧化物包括過氧化苯甲醯、過氧化二苯甲醯、1,3-雙(第三丁基過氧基異丙基)苯、過氧化二乙醯、4,4-雙(第三丁基過氧基)戊酸丁酯、過氧化對氯苯甲醯、過氧化第三丁基異丙苯、過苯甲酸第三丁酯、二第三丁基過氧化物、過氧化二異丙苯、2,5-二甲基-2,5-二第三丁基過氧己烷、2,5-二甲基-2,5-二第三丁基過氧己-3-炔、4-甲基-2,2-二第三丁基過氧戊烷及其組合。
以組合物之總重量計,此類過氧化物化合物通常以約0.1至約10重量%用於本發明中,其中約1至約5重量%為理想的。
如所指出,習知自由基聚合加速劑亦可與本發明中所用之固化加速劑結合使用,但其用量少於過去使用之量。此類加速劑(在本文中稱為共加速劑)通常為肼類(例如
APH),如美國專利第4,287,350號(Rich)及第4,321,349號(Rich)所揭示。當選擇APH作為本文使用之共加速劑時,通常亦添加馬來酸。本發明之一個益處為本發明之厭氧性固化加速劑使得在製備厭氧性黏著劑組合物時不必使用此類酸。
其他共加速劑亦可用於本發明之組合物中,包括但不限於有機醯胺及醯亞胺,諸如苯甲醯磺醯亞胺(亦稱為糖精) (參見
美國專利第4,324,349號)。當然,THQ亦可用作共加速劑。
亦可採用穩定劑及抑制劑(諸如苯酚,包括氫醌及醌)以控制及防止本發明組合物過早的過氧化物分解及聚合,以及採用螯合劑[諸如乙二胺四乙酸(「EDTA」)之四鈉鹽]以自其中截獲痕量金屬污染物。使用時,螯合劑通常可以組合物之總重量計約0.001重量%至約0.1重量%之量存在於組合物中。
金屬催化劑溶液或其預混物以約0.03至約0.1重量%之量使用。
其他添加劑,諸如增稠劑、非反應性塑化劑、填料、韌化劑(諸如彈性體及橡膠)及其他眾所周知的添加劑可併入其中,熟習此項技術者認為此舉為合乎需要的。
本發明亦提供製備及使用本發明之厭氧性黏著劑組合物的方法,以及該等組合物之反應產物。
本發明之組合物可使用熟習此項技術者所熟知的習知方法製備。舉例而言,本發明組合物之組分可根據組分在組合物中發揮之作用及功能,依任何合宜的順序混合在一起。可採用使用已知設備的習知混合技術。
本發明之組合物可施用於各種基板,以表現本文所述之所需益處及優點。舉例而言,適當基板可由鋼、黃銅、銅、鋁、鋅及其他金屬及合金、陶瓷及熱固性材料建構。本發明之組合物在鋼、黃銅、銅及鋅上展現特別好的黏合強度。可於所選基板之表面上施用適合厭氧性可固化組合物之底漆,以提高固化速率。或者,本發明之厭氧性固化加速劑可作為底漆施用於基板表面。參見例如
美國專利第5,811,473號(Ramos)。
另外,本發明提供一種製備厭氧性可固化組合物之方法,其步驟包括將(甲基)丙烯酸酯組分、厭氧性固化誘導組合物及厭氧性固化加速劑反應產物混合在一起。
且本發明提供一種使用厭氧性固化加速劑化合物之方法,其包括(I)將厭氧性固化加速劑化合物混合在厭氧性可固化組合物中或(II)將厭氧性固化加速劑化合物施用於基板表面上,且在其上施用厭氧性可固化組合物。當然,本發明亦提供在配合之基板與本發明組合物之間形成的黏合。
鑒於以上對本發明之描述,顯然提供廣泛的實踐機會。以下實例僅用於說明目的,且不應解釋為以任何方式限制本文之教示。實例
在製備樣品時,使用不鏽鋼螺旋槳型混合器混合組分。
評估此等樣品在各種基板上之各種強度量測值,包括分離扭矩、預置扭矩及剪切強度。
分離扭矩為減小或消除非固定總成中之軸向載荷所需的初始扭矩。在螺帽360°旋轉期間之任何點量測黏合初始斷裂後之預置扭矩。預置扭矩通常在螺帽旋轉180°時測定。
將黑色氧化物螺栓及軟鋼脫脂,將黏著劑施用於螺釘,並將螺帽旋擰於螺釘上。針對每種測試之黏著劑調配物組裝五個螺帽及螺釘樣本。對於分離/預置評估,樣本在裝配後在環境溫度下維持1小時及24小時。隨後分別在環境溫度(25℃)及45-50%相對濕度下1小時後及24小時後記錄各黏著劑調配物之五個樣本的分離扭矩強度及預置扭矩強度(N-m)。使用經校準自動扭矩分析儀來量測扭矩強度。關於分離扭矩評估之資料列於下表2A及2B中。表 2A 表 2B
表2A及2B中捕獲之此資料表明,當施用及固化於基板上時,樣品B及D在室溫下顯示出與對照(樣品A及C)相比通常類似的松脫特性。
在大多數情況下,樣品B (具有THBQol)至少表現得與對照樣品A一樣。關於此等資料之圖形描繪參見
圖11-13。
應記住,樣品A中THQ之使用量為1重量%,自表4及5中記錄之資料顯而易見,在許多情況下,以低至0.1重量%之含量替代地使用THBQol可產生至少可接受之效能。關於此資料之圖形描繪亦參見圖1及圖2。
如同24小時分離及預置資料,自表6及7中記錄之1小時分離及預置資料顯而易見,在許多情況下,以低至0.1重量%之含量替代地使用THBQol可生產至少可接受之效能。關於此資料之圖形描繪亦參見圖3及圖4。
具有0.1至1重量%之量的THBQol作為加速劑之厭氧性可固化組合物在24小時固化後在軟鋼銷及套環上顯示出優異的效能。參見
圖5。
具有0.1至1重量%之量的THBQol作為加速劑之厭氧性可固化組合物在1小時固化後在軟鋼銷及套環上顯示出優異的效能。參見
圖6。
具有0.1至1重量%之量的THBQol作為加速劑之厭氧性可固化組合物在24小時固化後在0.15 mm間隙之軟鋼銷及套環上顯示出優異的間隙固化效能。參見
圖7。
亦將THBQol與APH作為固化加速劑進行比較。因此,代替使用樣品A作為對照,樣品C在下面的評估中用作對照,因為其使用APH作為固化加速劑。
此資料表明,THBQol作為加速劑在黏合之軟鋼部件上的剪切強度效能至少與APH相當。關於資料之圖形描繪參見
圖8。
此資料表明,THBQol作為加速劑在黏合之黑色氧化物螺帽及螺栓上的分離扭矩效能至少與APH相當。關於資料之圖形描繪參見
圖9。
此資料表明,THBQol作為加速劑在黏合之黑色氧化物螺帽及螺栓上的分離扭矩效能至少與APH相當。關於資料之圖形描繪參見
圖10。
圖1描繪作為對照之厭氧性黏著劑組合物(其含有0.9重量% THQ作為加速劑)及無THQ但具有各種濃度之THBQol作為本發明固化加速劑之可比厭氧性黏著劑組合物在由黃銅、重鉻酸鋅、不鏽鋼及軟鋼/黑色氧化物建構之M10螺帽及螺栓上之24小時分離扭矩的繪圖。
圖2描繪作為對照之厭氧性黏著劑組合物(其含有0.9重量% THQ作為加速劑)及無THQ但具有各種濃度之THBQol作為本發明固化加速劑之可比厭氧性黏著劑組合物在由黃銅、重鉻酸鋅、不鏽鋼及軟鋼/黑色氧化物建構之M10螺帽及螺栓上之24小時預置扭矩的繪圖。
圖3描繪作為對照之厭氧性黏著劑組合物(其含有0.9重量% THQ作為加速劑)及無THQ但具有各種濃度之THBQol作為本發明固化加速劑之可比厭氧性黏著劑組合物在由黃銅、重鉻酸鋅、不鏽鋼及軟鋼/黑色氧化物建構之M10螺帽及螺栓上之1小時分離扭矩的繪圖。
圖4描繪作為對照之厭氧性黏著劑組合物(其含有0.9重量% THQ作為加速劑)及無THQ但具有各種濃度之THBQol作為本發明固化加速劑之可比厭氧性黏著劑組合物在由黃銅、重鉻酸鋅、不鏽鋼及軟鋼/黑色氧化物建構之M10螺帽及螺栓上之1小時預置扭矩的繪圖。
圖5描繪作為對照之厭氧性黏著劑組合物(其含有0.9重量% THQ作為加速劑)及無THQ但具有各種濃度之THBQol作為本發明固化加速劑之可比厭氧性黏著劑組合物在由軟鋼建構之銷及套環上之24小時剪切強度的繪圖。
圖6描繪作為對照之厭氧性黏著劑組合物(其含有0.9重量% THQ作為加速劑)及無THQ但具有各種濃度之THBQol作為本發明固化加速劑之可比厭氧性黏著劑組合物在由軟鋼建構之銷及套環上之1小時剪切強度的繪圖。
圖7描繪作為對照之厭氧性黏著劑組合物(其含有0.9重量% THQ作為加速劑)及無THQ但具有各種濃度之THBQol作為本發明固化加速劑之可比厭氧性黏著劑組合物在由軟鋼建構之銷及套環上之24小時剪切強度的繪圖,其中銷與套環之間的間隙設置為0.15 mmm。
圖8描繪在1及24小時固化後樣品C及D之剪切強度值的條形圖。
圖9描繪在0.5及24小時固化後樣品C及D之分離扭矩值的條形圖。
圖10描繪在1及24小時固化後樣品C及D之預置扭矩值的條形圖。
圖11描繪在1及24小時固化後樣品A及B在銷及套環總成上之剪切強度值的條形圖。
圖12描繪在1及24小時固化後樣品A及B之分離扭矩值的條形圖。
圖13描繪在1及24小時固化後樣品A及B之預置扭矩值的條形圖。
Claims (10)
- 如請求項1之組合物,其中該厭氧性固化誘導組合物包含選自由以下組成之群的氫過氧化物:第三丁基氫過氧化物、對甲烷氫過氧化物、氫過氧化異丙苯、氫過氧化二異丙基苯及其混合物。
- 如請求項1之組合物,其進一步包含至少一種共加速劑。
- 如請求項3之組合物,其中該共加速劑係選自由以下組成之群:胺、氧化胺、磺醯胺、金屬源、酸及其混合物。
- 如請求項3之組合物,其中該共加速劑係選自由以下組成之群:三嗪、乙醇胺、二乙醇胺、三乙醇胺、N,N二甲基苯胺、苯磺醯亞胺、環己胺、三乙胺、丁胺、糖精、N,N-二乙基-對甲苯胺、N,N-二甲基-鄰甲苯胺、乙醯基苯肼、馬來酸及其混合物。
- 如請求項1之組合物,其進一步包含至少一種穩定劑。
- 如請求項6之組合物,其中該穩定劑係選自由以下組成之群:苯醌、萘醌、蒽醌、氫醌、甲氧基氫醌、丁基化羥基甲苯、乙二胺四乙酸或其鹽,及其混合物。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB1716517.6A GB2567242B (en) | 2017-10-09 | 2017-10-09 | Anaerobically curable compositions comprising 1, 2, 3, 4-tetrahydro benzo(h)quinolin-3-ol or derivatives thereof |
| GB1716517.6 | 2017-10-09 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| TW201922906A TW201922906A (zh) | 2019-06-16 |
| TWI770291B true TWI770291B (zh) | 2022-07-11 |
Family
ID=60326844
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| TW107135451A TWI770291B (zh) | 2017-10-09 | 2018-10-08 | 用於厭氧性可固化組合物之固化加速劑 |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US11274190B2 (zh) |
| EP (1) | EP3694942B1 (zh) |
| JP (1) | JP7312181B2 (zh) |
| KR (1) | KR102601879B1 (zh) |
| CN (1) | CN111417693B (zh) |
| BR (1) | BR112020006459A2 (zh) |
| CA (1) | CA3077392A1 (zh) |
| GB (1) | GB2567242B (zh) |
| MX (1) | MX2020004122A (zh) |
| TW (1) | TWI770291B (zh) |
| WO (1) | WO2019072686A1 (zh) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116323723A (zh) | 2020-08-18 | 2023-06-23 | 汉高股份有限及两合公司 | 用于可厌氧固化组合物的固化促进剂 |
| JP2025507018A (ja) * | 2022-03-04 | 2025-03-13 | ヘンケル・アクチェンゲゼルシャフト・ウント・コムパニー・コマンディットゲゼルシャフト・アウフ・アクチェン | 嫌気性硬化性組成物のための硬化促進剤 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20120157641A1 (en) * | 2009-05-01 | 2012-06-21 | Loctite (R&D) Limited | Cure accelerators for anaerobic curable compositions |
Family Cites Families (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3218305A (en) | 1963-12-26 | 1965-11-16 | Loctite Corp | Accelerated anaerobic compositions and method of using same |
| DE1719144C3 (de) | 1967-12-01 | 1974-08-22 | Henkel & Cie Gmbh, 4000 Duesseldorf | Unter Sauerstoffausschluß beschleunigt erhärtende Klebstoffe oder Dichtungsmittel |
| DE1817989C3 (de) | 1967-12-28 | 1981-11-05 | Loctite Corp., 06111 Newington, Conn. | Verfahren zum Verbinden von nicht porösen Oberflächen mittels polymerisierbaren Klebstoffen |
| DK126006B (da) * | 1969-12-05 | 1973-05-28 | Tokyo Three Bond Co Ltd | Anaerobt hærdende forseglingsmateriale. |
| IL36289A (en) * | 1970-03-18 | 1974-03-14 | Loctite Corp | Highly stable anaerobic compositions comprising acrylate ester monomer |
| US3970505A (en) | 1973-01-15 | 1976-07-20 | Loctite Corporation | Anaerobic compositions and surface activator therefor |
| JPS5247766B2 (zh) | 1973-08-21 | 1977-12-05 | ||
| JPS51132234A (en) * | 1975-04-21 | 1976-11-17 | Suriibondo:Kk | An anaerobic adhesive composition |
| US4321349A (en) | 1975-05-23 | 1982-03-23 | Loctite Corporation | Accelerator for curable compositions |
| JPS53102989A (en) | 1977-02-22 | 1978-09-07 | Denki Kagaku Kogyo Kk | Curable composition |
| JPS5469198A (en) * | 1977-11-14 | 1979-06-02 | Toagosei Chem Ind Co Ltd | Anaerobic curable composition |
| DE2911661A1 (de) | 1979-03-24 | 1980-09-25 | Bayer Ag | Verfahren zur herstellung von thiazolidin-2-thionen |
| JPS59207977A (ja) * | 1983-05-12 | 1984-11-26 | Okura Ind Co Ltd | 嫌気硬化性接着剤 |
| IE882227L (en) * | 1988-07-21 | 1990-01-21 | Loctite Ireland Ltd | Sealant composition |
| US5411988A (en) | 1993-10-27 | 1995-05-02 | Bockow; Barry I. | Compositions and methods for inhibiting inflammation and adhesion formation |
| JPH07308757A (ja) | 1994-05-18 | 1995-11-28 | Tokyo Yogyo Co Ltd | スライディングノズルプレート |
| US5605999A (en) | 1995-06-05 | 1997-02-25 | Loctite Corporation | Anaerobically curable silicones |
| US5811473A (en) | 1996-04-02 | 1998-09-22 | Loctite Corporation | Primer activator composition for anaerobic adhesives |
| US6001517A (en) * | 1996-10-31 | 1999-12-14 | Kabushiki Kaisha Toshiba | Positive photosensitive polymer composition, method of forming a pattern and electronic parts |
| US6043327A (en) | 1997-07-03 | 2000-03-28 | Loctite Corporation | Anaerobic adhesive compositions of acrylates coreactants and maleimides curable under ambient conditions |
| US6583289B1 (en) * | 1999-01-08 | 2003-06-24 | Loctite (R&D) Limited | Curative for anaerobic adhesive compositions |
| CN1181152C (zh) | 1999-06-11 | 2004-12-22 | 株式会社三键 | 厌氧可固化的组合物 |
| DE10046024A1 (de) * | 1999-09-16 | 2001-04-19 | Yokohama Rubber Co Ltd | Thermoreversibel vernetzbares Elastomer und seine Zusammensetzung |
| US6835762B1 (en) * | 2002-05-31 | 2004-12-28 | Henkel Corporation | Cure accelerators for anaerobic curable compositions |
| US6958368B1 (en) * | 2002-05-31 | 2005-10-25 | Henkel Corporation | Cure accelerators for anaerobic curable compositions |
| US6897277B1 (en) * | 2003-12-22 | 2005-05-24 | Henkel Corporation | Cure accelerators for anaerobic curable compositions |
| CN100543097C (zh) * | 2007-05-28 | 2009-09-23 | 黑龙江省石油化学研究院 | 一种具有低拆卸力矩耐高温厌氧胶及其制备方法 |
| US20090012202A1 (en) * | 2007-07-03 | 2009-01-08 | Henkel Corporation | Acrylated Urethanes, Processes for Making the Same and Curable Compositions Including the Same |
| US20110190412A1 (en) * | 2008-01-28 | 2011-08-04 | Basf Se | Photolatent amidine bases for redox curing of radically curable formulations |
| CN101497769B (zh) * | 2009-02-23 | 2010-11-24 | 黑龙江省科学院石油化学研究院 | 无机/有机杂化耐高温厌氧型螺纹锁固密封胶粘剂及其制备方法 |
| EP2424931B1 (en) | 2009-05-01 | 2015-03-04 | Henkel US IP LLC | Cure accelerators for anaerobic curable compositions |
| CN102549059B (zh) * | 2009-10-15 | 2016-05-18 | 汉高知识产权控股有限责任公司 | 厌氧固化组合物 |
| JP5469198B2 (ja) | 2012-05-31 | 2014-04-09 | ニチアス株式会社 | 断熱体 |
| US8945338B2 (en) | 2013-03-15 | 2015-02-03 | Henkel US IP LLC | Anaerobic curable compositions |
-
2017
- 2017-10-09 GB GB1716517.6A patent/GB2567242B/en active Active
-
2018
- 2018-10-04 MX MX2020004122A patent/MX2020004122A/es unknown
- 2018-10-04 BR BR112020006459-9A patent/BR112020006459A2/pt not_active Application Discontinuation
- 2018-10-04 WO PCT/EP2018/077044 patent/WO2019072686A1/en unknown
- 2018-10-04 JP JP2020540676A patent/JP7312181B2/ja active Active
- 2018-10-04 KR KR1020207010797A patent/KR102601879B1/ko active Active
- 2018-10-04 CA CA3077392A patent/CA3077392A1/en active Pending
- 2018-10-04 CN CN201880076133.3A patent/CN111417693B/zh active Active
- 2018-10-04 EP EP18782443.8A patent/EP3694942B1/en active Active
- 2018-10-08 TW TW107135451A patent/TWI770291B/zh not_active IP Right Cessation
-
2020
- 2020-04-09 US US16/844,279 patent/US11274190B2/en active Active
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20120157641A1 (en) * | 2009-05-01 | 2012-06-21 | Loctite (R&D) Limited | Cure accelerators for anaerobic curable compositions |
Also Published As
| Publication number | Publication date |
|---|---|
| CN111417693A (zh) | 2020-07-14 |
| GB2567242A (en) | 2019-04-10 |
| KR102601879B1 (ko) | 2023-11-14 |
| MX2020004122A (es) | 2020-08-13 |
| KR20200067151A (ko) | 2020-06-11 |
| EP3694942B1 (en) | 2021-09-29 |
| TW201922906A (zh) | 2019-06-16 |
| GB201716517D0 (en) | 2017-11-22 |
| JP7312181B2 (ja) | 2023-07-20 |
| GB2567242B (en) | 2021-08-11 |
| EP3694942A1 (en) | 2020-08-19 |
| CN111417693B (zh) | 2022-05-27 |
| BR112020006459A2 (pt) | 2020-10-06 |
| WO2019072686A1 (en) | 2019-04-18 |
| CA3077392A1 (en) | 2019-04-18 |
| JP2020537029A (ja) | 2020-12-17 |
| US11274190B2 (en) | 2022-03-15 |
| US20200299485A1 (en) | 2020-09-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101899270B1 (ko) | 노볼락 비닐 에스테르를 갖는 혐기 경화성 조성물 | |
| US9580530B2 (en) | Anaerobically curable compositions | |
| KR101520513B1 (ko) | 내열성 혐기 경화성 조성물 | |
| TWI770291B (zh) | 用於厭氧性可固化組合物之固化加速劑 | |
| US8519023B2 (en) | Fast,curing two part anaerobic adhesive composition | |
| EP3201266B1 (en) | Cure accelerators for anaerobic curable compositions | |
| US20210139623A1 (en) | Anaerobically Curable Compositions Containing Alpha-Methylene-Lactones | |
| EP3204461B1 (en) | Cure accelerators for anaerobic curable compositions | |
| CN107148411B (zh) | 苯肼/酸酐加合物和使用苯肼/酸酐加合物的厌氧可固化组合物 | |
| EP4200344A1 (en) | Cure accelerators for anaerobic curable compositions | |
| US20240425729A1 (en) | Cure accelerator for anaerobic curable composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | Annulment or lapse of patent due to non-payment of fees |