RU99128075A - DERIVATIVES OF 8-AZABICYCLO (3,2,1) OCT-2-ENA AND OCTANE AS CHOLINERGIC LIGANDS ON ACH RECEPTORS - Google Patents
DERIVATIVES OF 8-AZABICYCLO (3,2,1) OCT-2-ENA AND OCTANE AS CHOLINERGIC LIGANDS ON ACH RECEPTORSInfo
- Publication number
- RU99128075A RU99128075A RU99128075/04A RU99128075A RU99128075A RU 99128075 A RU99128075 A RU 99128075A RU 99128075/04 A RU99128075/04 A RU 99128075/04A RU 99128075 A RU99128075 A RU 99128075A RU 99128075 A RU99128075 A RU 99128075A
- Authority
- RU
- Russia
- Prior art keywords
- azabicyclo
- methyl
- oct
- ene
- disease
- Prior art date
Links
Claims (14)
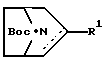
любой из его энантиомеров или любая их смесь, или его фармацевтически приемлемая соль,
где является простой или двойной связью;
R является водородом, алкилом, алкенилом, алкинилом, циклоалкилом, циклоалкилалкилом, арилом или аралкилом; и
R1 представляет собой
где R2 является водородом, алкилом, алкенилом, алкинилом, циклоалкилом, циклоалкилалкилом, амино; или
арил, который может быть замещен один или более чем один раз заместителями, выбранными из группы, состоящей из алкила, циклоалкила, циклоалкилалкила, алкенила, алкинила, алкокси, циклоалкокси, тиоалкокси, тиоциклоалкокси, метилендиокси, арилокси, галогена, CF3, OCF3, CN, амино, аминоацила, нитро, арила и моноциклической 5-6-членной гетероарильной группы; моноциклическую 5-6-членную гетероарильную группу, которая может быть замещена один или более чем один раз заместителями, выбранными из группы, состоящей из алкила, циклоалкила, циклоалкилалкила, алкенила, алкинила, алкокси, циклоалкокси, тиоалкокси, тиоциклоалкокси, метилендиокси, арилокси, галогена, CF3, OCF3, CN, амино, иитро, арила и моноциклической 5-6-членной гетероарильной группы; или
бициклическую гетероарильную группу, состоящую из моноциклической 5-6-членной гетероарильной группы, конденсированной с бензольным кольцом или конденсированной с другим моноциклическим 5-6-членным гетероарилом, причем все они могут быть замещены один или более чем один раз заместителями, выбранными из группы, состоящей из алкила, циклоалкила, циклоалкилалкила, алкенила, алкинила, алкокси, циклоалкокси, тиоалкокси, тиоциклоалкокси, метилендиокси, арилокси, галогена, CF3, OCF3, CN, амино, нитро, арила и моноциклической 5-6-членной гетероарильной группы;
при условии, однако, что соединение не является
моногидратом гидрохлорида 3-(1,2)-бензоизоксазол-3-ил)-8-метил-8-азабицикло[3.2.1]октана,
моногидратом гидрохлорида 3-(1,2)-бензоизоксазол-3-ил)-8-азабицикло[3.2.1]октана,
гидрохлоридом 3-(6-фтор-1,2-бензоизоксазол-3-ил)-8-метил-8-азабицикло[3.2.1]октана,
гидрохлоридом 3-(6-фтор-1,2-бензоизоксазол-3-ил)-8-азабицикло[3.2.1] октана,
3-(1Н-индазол-3-ил)-8-метил-8-азабицикло[3.2.1]октаном,
3-(1Н-индазол-3-ил)8-азабицикло[3.2.1]октаном,
3-(6-фтор-1Н-индазол-3-ил)-8-метил-8-азабицикло[3.2.1]октаном,
3-(6-фтор-1Н-индазол-3-ил)8-азабицикло[3.2.1]октаном,
гидрохлоридом 3-[1,2-бензизотизол-3-ил] -8-метил-8-азабицикло[3.2.1]октана,
3-(1,2-бензизотиазол-3-ил)8-азабицикло[3.2.1]октаном,
8-метил-3-(3,4-дихлорфенил)-8-азабицикло[3.2.1]окт-2-еном,
8-метил-3-(4-хлорфенил)-8-азабицикло[3.2.1]окт-2-еном,
8-метил-3-фенил-8-азабицикло[3.2.1]окт-2-еном,
8-метил-3-(4-метилфенил)-8-азабицикло[3.2.1]окт-2-еном,
8-метил-3-(4-трифторметилфенил)-8-азабицикло[3.2.1]окт-2-еном,
8-метил-3-(4-фторфенил)-8-азабицикло[3.2.1]окт-2-еном,
3-(4-хлорфенил)-8-азабицикло[3,2,1]окт-2-еном,
3-(3,4-дихлорфенил)-8-азабицикло[3.2.1]окт-2-еном,
4-хлор-2,6-диамино-5-[8-(1-нафтил)-8-азабицикло[3,2.1] окт-3-ил] пиримидином,
4-хлор-2,6-диамино-5-[8-(2-нафтил)-8-азабицикло[3.2.1] окт-3-ил] пиримидином,
8-метил-3-фенил-8-азабицикло[3.2.1]октаном,
8-метил-3-(2-метил-фенил)-8-азабицикло[3.2.1]окт-2-еном,
8-метил-3-(2-метил-фенил)-8-азабицикло[3.2.1]октаном,
3-(4-фторфенил)-8-азабицикло[3.2.1]окт-2-еном,
8-метил-3-(1-метил-индол-2-ил)-8-азабицикло[3.2.1]октаном или
8-метил-3-(1-метил-индол-2-ил)-8-азабицикло[3.2.1]окт-2-еном.1. The compound having the formula
any of its enantiomers or any mixture thereof, or a pharmaceutically acceptable salt thereof,
Where is a single or double bond;
R is hydrogen, alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl, aryl, or aralkyl; and
R 1 is
where R 2 is hydrogen, alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl, amino; or
aryl, which may be substituted one or more than once with substituents selected from the group consisting of alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, alkoxy, cycloalkoxy, thioalkoxy, thiocycloalkoxy, methylenedioxy, aryloxy, halogen, CF 3 , OCF 3 , CN, amino, aminoacyl, nitro, aryl, and a monocyclic 5-6 membered heteroaryl group; a monocyclic 5-6 membered heteroaryl group which may be substituted one or more times with substituents selected from the group consisting of alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, alkoxy, cycloalkoxy, thioalkoxy, thiocycloalkoxy, methylenedioxy, aryloxy, alkyloxy, alkoxy, alkoxy , CF 3 , OCF 3 , CN, amino, and nitro, aryl, and a monocyclic 5-6 membered heteroaryl group; or
a bicyclic heteroaryl group consisting of a monocyclic 5-6-membered heteroaryl group condensed with a benzene ring or condensed with another monocyclic 5-6-membered heteroaryl, all of which can be replaced one or more times with substituents selected from the group consisting from alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, alkoxy, cycloalkoxy, thioalkoxy, thiocycloalkoxy, methylenedioxy, aryloxy, halogen, CF 3 , OCF 3 , CN, amino, nitro, aryl and monocyclic 5-6-membered heteroaryl groups;
provided, however, that the compound is not
3- (1,2) -benzoisoxazol-3-yl) -8-methyl-8-azabicyclo [3.2.1] octane hydrochloride monohydrate,
3- (1,2) -benzoisoxazol-3-yl) -8-azabicyclo [3.2.1] octane hydrochloride monohydrate,
3- (6-fluoro-1,2-benzoisoxazol-3-yl) -8-methyl-8-azabicyclo [3.2.1] octane hydrochloride,
3- (6-fluoro-1,2-benzoisoxazol-3-yl) -8-azabicyclo [3.2.1] octane hydrochloride,
3- (1H-indazol-3-yl) -8-methyl-8-azabicyclo [3.2.1] octane,
3- (1H-indazol-3-yl) 8-azabicyclo [3.2.1] octane,
3- (6-fluoro-1H-indazol-3-yl) -8-methyl-8-azabicyclo [3.2.1] octane,
3- (6-fluoro-1H-indazol-3-yl) 8-azabicyclo [3.2.1] octane,
3- [1,2-benzisotisol-3-yl] -8-methyl-8-azabicyclo [3.2.1] octane hydrochloride,
3- (1,2-benzisothiazol-3-yl) 8-azabicyclo [3.2.1] octane,
8-methyl-3- (3,4-dichlorophenyl) -8-azabicyclo [3.2.1] oct-2-ene,
8-methyl-3- (4-chlorophenyl) -8-azabicyclo [3.2.1] oct-2-ene,
8-methyl-3-phenyl-8-azabicyclo [3.2.1] oct-2-ene,
8-methyl-3- (4-methylphenyl) -8-azabicyclo [3.2.1] oct-2-ene,
8-methyl-3- (4-trifluoromethylphenyl) -8-azabicyclo [3.2.1] oct-2-ene,
8-methyl-3- (4-fluorophenyl) -8-azabicyclo [3.2.1] oct-2-ene,
3- (4-chlorophenyl) -8-azabicyclo [3,2,1] oct-2-ene,
3- (3,4-dichlorophenyl) -8-azabicyclo [3.2.1] oct-2-ene,
4-chloro-2,6-diamino-5- [8- (1-naphthyl) -8-azabicyclo [3.2.1] oct-3-yl] pyrimidine,
4-chloro-2,6-diamino-5- [8- (2-naphthyl) -8-azabicyclo [3.2.1] oct-3-yl] pyrimidine,
8-methyl-3-phenyl-8-azabicyclo [3.2.1] octane,
8-methyl-3- (2-methyl-phenyl) -8-azabicyclo [3.2.1] oct-2-ene,
8-methyl-3- (2-methyl-phenyl) -8-azabicyclo [3.2.1] octane,
3- (4-fluorophenyl) -8-azabicyclo [3.2.1] oct-2-ene,
8-methyl-3- (1-methyl-indole-2-yl) -8-azabicyclo [3.2.1] octane or
8-methyl-3- (1-methyl-indol-2-yl) -8-azabicyclo [3.2.1] oct-2-ene.
где R2 является водородом, алкилом, алкенилом, алкинилом, циклоалкилом, циклоалкилалкилом, амино; или
арил, который замещен один или более чем один раз заместителями, выбранными из группы, состоящей из циклоалкила, циклоалкилалкила, алкенила, алкинила, алкокси, циклоалкокси, тиоалкокси, тиоциклоалкокси, метилендиокси, арилокси, OCF3, CN, амино, аминоацила, нитро, арила и моноциклической 5-6-членной гетероарильной группы;
моноциклическую 5-6-членную гетероарильную группу, которая может быть замещена один или более чем один раз заместителями, выбранными из группы, состоящей из алкила, циклоалкила, циклоалкилалкила, алкенила, алкинила, алкокси, циклоалкокси, тиоалкокси, тиоциклоалкокси, метилендиокси, арилокси, галогена, CF3, OCF3, CN, нитро, арила и моноциклической 5-6-членной гетероарильной группы; или
бициклическую гетероарильную группу, состоящую из моноциклической 5-6-членной гетероарильной группы с одним гетероатомом, конденсированной с бензольным кольцом или конденсированной с другим моноциклическим 5-6-членным гетероарилом, причем все они могут быть замещены один или более чем один раз заместителями, выбранными из группы, состоящей из алкила, циклоалкила, циклоалкилалкила, алкенила, алкинила, алкокси, циклоалкокси, тиоалкокси, тиоциклоалкокси, метилендиокси, арилокси, галогена, CF3, OCF3, CN, амино, нитро, арила и моноциклической 5-6-членной гетероарильной группы.2. The compound of formula 1 according to claim 1, wherein R is hydrogen, alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl, aryl, or aralkyl; and R 1 is
where R 2 is hydrogen, alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl, amino; or
aryl, which is substituted once or more than once with substituents selected from the group consisting of cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, alkoxy, cycloalkoxy, thioalkoxy, thiocycloalkoxy, methylenedioxy, aryloxy, OCF 3 , CN, amino, aminoacyl, nitro, aryl and a monocyclic 5-6 membered heteroaryl group;
a monocyclic 5-6 membered heteroaryl group which may be substituted one or more times with substituents selected from the group consisting of alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, alkoxy, cycloalkoxy, thioalkoxy, thiocycloalkoxy, methylenedioxy, aryloxy, alkyloxy, alkoxy, alkoxy , CF 3 , OCF 3 , CN, nitro, aryl, and monocyclic 5-6 membered heteroaryl group; or
a bicyclic heteroaryl group consisting of a monocyclic 5-6-membered heteroaryl group with one heteroatom condensed with a benzene ring or condensed with another monocyclic 5-6-membered heteroaryl, all of which can be replaced one or more than once with substituents selected from a group consisting of alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, alkoxy, cycloalkoxy, thioalkoxy, thiocycloalkoxy, methylenedioxy, aryloxy, halogen, CF 3 , OCF 3 , CN, amino, nitro, aryl, and monocyclic 5-6-hl heteroaryl group.
(±)-8-бензил-3-(3-пиридил)-8-азабицикло[3.2.1]окт-2-еном,
(±)-8-метил-3-(3-пиридил)-8-азабицикло[3.2.1]окт-2-еном,
(±)-8-метил-3-(3-хинолинил)-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-(3-бензофурил)-8-метил-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-(3-бензотиенил)-8-метил-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-(2-тиазолил)-8-метил-8-азабицикло[3.2.1]окт-2-еном,
(±)-8-метил-3-(2-метоксифенил)-8-азабицикло[3.2.1]окт-2-еном,
(±)-8-метил-3-(3-тиенил)-8-азабицикло[3.2.1]окт-2-еном,
(±)-8-метил-3-(2-нафтил)-8-азабицикло[3.2.1]окт-2-еном,
экзо-8-метил-3-(3-пиридил)-8-азабицикло[3.2.1]октаном,
(±)-8-Н-3-(3-пиридил)-8-азабицикло[3.2.1]окт-2-еном,
(±)-8-метил-3-[3-(6-метокси)-пиридил]-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-ацетил-8-метил-8-азабицикло[3.2.1]окт-2-еном,
(±)-8-метил-3-[3-(6-хлор)-пиридил]-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-(2-бензофурил)-8-метил-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-(2-бензотиенил)-8-метил-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-(3-ацетамидофенил)-8-метил-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-(3-аминофенил)-8-метил-8-азабицикло[3.2,1]окт-2-еном,
(±)-3-(2-тиенил)-8-метил-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-[2-(3-метоксиметилтиенил)]-8-метил-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-(2-бензотиазолил)-8-метил-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-(2-фурил)-8-метил-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-(2-тиено[3.2-b]тиенил)-8-метил-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-(2-тиено[2.3-b]тиенил)-8-метил-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-(2-селенофенил)-8-метил-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-(2-бензофурил)-8-Н-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-[3-(3-фурил)-2-тиенил]-8-Н-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-(2-бензофурил)-8-этил-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-[2-(3-бромтиенил)]-8-метил-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-[2-(3-бромбензофурил)]-8-метил-8-азабицикло[3.2.1]окт-2-еном,
(±)-3-[2-(3-бромбензотиенил)]-8-метил-8-азабицикло[3.2.1]окт-2-еном,
3-[2-(3-хлортиенил)]-8-метил-8-азабицикло[3.2.1 ]окт-2-еном или
(±)-3-[3-(3-фурил)-2-тиенил]-8-метил-8-азабицикло[3.2.1]окт-2-еном,
или их фармацевтически приемлемой солью присоединения.4. The compound according to claim 1, which is
(±) -8-benzyl-3- (3-pyridyl) -8-azabicyclo [3.2.1] oct-2-ene,
(±) -8-methyl-3- (3-pyridyl) -8-azabicyclo [3.2.1] oct-2-ene,
(±) -8-methyl-3- (3-quinolinyl) -8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- (3-benzofuryl) -8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- (3-benzothienyl) -8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- (2-thiazolyl) -8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -8-methyl-3- (2-methoxyphenyl) -8-azabicyclo [3.2.1] oct-2-ene,
(±) -8-methyl-3- (3-thienyl) -8-azabicyclo [3.2.1] oct-2-ene,
(±) -8-methyl-3- (2-naphthyl) -8-azabicyclo [3.2.1] oct-2-ene,
exo-8-methyl-3- (3-pyridyl) -8-azabicyclo [3.2.1] octane,
(±) -8-H-3- (3-pyridyl) -8-azabicyclo [3.2.1] oct-2-ene,
(±) -8-methyl-3- [3- (6-methoxy) -pyridyl] -8-azabicyclo [3.2.1] oct-2-ene,
(±) -3-acetyl-8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -8-methyl-3- [3- (6-chloro) -pyridyl] -8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- (2-benzofuryl) -8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- (2-benzothienyl) -8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- (3-acetamidophenyl) -8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- (3-aminophenyl) -8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- (2-thienyl) -8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- [2- (3-methoxymethylthienyl)] - 8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- (2-benzothiazolyl) -8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- (2-furyl) -8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- (2-thieno [3.2-b] thienyl) -8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- (2-thieno [2.3-b] thienyl) -8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- (2-selenophenyl) -8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- (2-benzofuryl) -8-H-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- [3- (3-furyl) -2-thienyl] -8-H-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- (2-benzofuryl) -8-ethyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- [2- (3-bromothienyl)] - 8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- [2- (3-bromobenzofuryl)] - 8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
(±) -3- [2- (3-bromobenzothienyl)] - 8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
3- [2- (3-chlorothienyl)] - 8-methyl-8-azabicyclo [3.2.1] oct-2-ene or
(±) -3- [3- (3-furyl) -2-thienyl] -8-methyl-8-azabicyclo [3.2.1] oct-2-ene,
or a pharmaceutically acceptable addition salt thereof.
а) стадию взаимодействия соединения, имеющего формулу
где R является таким, как определено выше, с соединением формулы R1-Li, где R1 является таким, как определено выше, с последующей дегидратацией полученного соединения;
б) стадию взаимодействия соединения, имеющего формулу
где R является таким, как определено выше, с соединением формулы R1-X, где R1 является таким, как определено выше, а Х является галогеном, бороновой кислотой или триалкилстаннилом; или
в) стадию восстановления соединения, имеющего формулу
где R1 является таким, как определено выше.10. The method of producing compounds according to claim 1, wherein
a) the stage of interaction of compounds having the formula
where R is the same as defined above, with a compound of the formula R 1 -Li, where R 1 is as defined above, followed by dehydration of the obtained compound;
b) the stage of interaction of the compound having the formula
where R is the same as defined above, with a compound of the formula R 1 -X, where R 1 is the same as defined above, and X is halogen, boronic acid or trialkylstannyl; or
C) the stage of recovery of compounds having the formula
where R 1 is as defined above.
Applications Claiming Priority (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DK0627/97 | 1997-05-30 | ||
| DK62797 | 1997-05-30 | ||
| DK1502/97 | 1997-12-19 | ||
| DK150297 | 1997-12-19 | ||
| DK0408/98 | 1998-03-24 | ||
| DK40898 | 1998-03-24 | ||
| DK53498 | 1998-04-16 | ||
| DK0534/98 | 1998-04-16 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU99128075A true RU99128075A (en) | 2001-09-10 |
| RU2186780C2 RU2186780C2 (en) | 2002-08-10 |
Family
ID=27439314
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU99128075/04A RU2186780C2 (en) | 1997-05-30 | 1998-05-29 | Derivative of 8-azabicyclo[3,2,1]oct-2-ene, methods of its synthesis, pharmaceutical composition |
Country Status (23)
| Country | Link |
|---|---|
| US (2) | US6645977B1 (en) |
| EP (1) | EP0984965B1 (en) |
| JP (1) | JP4447663B2 (en) |
| KR (1) | KR100589872B1 (en) |
| CN (1) | CN1157392C (en) |
| AT (1) | ATE267199T1 (en) |
| AU (1) | AU745964B2 (en) |
| BR (1) | BR9809697A (en) |
| CA (1) | CA2289574C (en) |
| CZ (1) | CZ298824B6 (en) |
| DE (1) | DE69823994T2 (en) |
| DK (1) | DK0984965T3 (en) |
| EE (1) | EE04057B1 (en) |
| HK (1) | HK1027353A1 (en) |
| HU (1) | HUP0002713A3 (en) |
| IL (2) | IL132437A0 (en) |
| IS (1) | IS2092B (en) |
| NO (1) | NO325513B1 (en) |
| NZ (1) | NZ500642A (en) |
| RU (1) | RU2186780C2 (en) |
| SK (1) | SK284994B6 (en) |
| TR (1) | TR199902942T2 (en) |
| WO (1) | WO1998054181A1 (en) |
Families Citing this family (49)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU5790598A (en) * | 1996-12-02 | 1998-06-29 | Georgetown University | Tropane derivatives and method for their synthesis |
| CA2335336A1 (en) | 1998-06-19 | 1999-12-23 | James Edmund Audia | Inhibition of serotonin reuptake |
| US6218383B1 (en) * | 1998-08-07 | 2001-04-17 | Targacept, Inc. | Pharmaceutical compositions for the prevention and treatment of central nervous system disorders |
| DE69914935T2 (en) | 1998-11-27 | 2009-10-01 | Neurosearch A/S | 8-AZABICYCLO [3.2.1] OCT-2-EN- AND -OKTANDERIVATE |
| US6953855B2 (en) | 1998-12-11 | 2005-10-11 | Targacept, Inc. | 3-substituted-2(arylalkyl)-1-azabicycloalkanes and methods of use thereof |
| US6432975B1 (en) * | 1998-12-11 | 2002-08-13 | Targacept, Inc. | Pharmaceutical compositions and methods for use |
| AU2278700A (en) * | 1999-01-28 | 2000-08-18 | Neurosearch A/S | Novel azabicyclo derivatives and their use |
| CZ20012716A3 (en) * | 1999-01-29 | 2001-11-14 | Abbott Laboratories | Diazabicyclic derivatives functioning as ligands of acetylcholine nicotine receptor |
| US7265115B2 (en) | 1999-01-29 | 2007-09-04 | Abbott Laboratories | Diazabicyclic CNS active agents |
| ATE261448T1 (en) | 1999-05-04 | 2004-03-15 | Neurosearch As | HETEROARYL DIAZABICYCLOALKENES, THEIR PREPARATION AND USE |
| US6890935B2 (en) | 1999-11-01 | 2005-05-10 | Targacept, Inc. | Pharmaceutical compositions and methods for use |
| MY137020A (en) * | 2000-04-27 | 2008-12-31 | Abbott Lab | Diazabicyclic central nervous system active agents |
| US7060699B2 (en) | 2000-07-04 | 2006-06-13 | Neurosearch A/S | Aryl and heteroaryl diazabicycloalkanes, their preparation and use |
| NZ524202A (en) * | 2000-10-13 | 2004-08-27 | Neurosearch As | Treatment of affective disorders by the combined action of a nicotinic receptor agonist and a monoaminergic substance |
| WO2003029252A1 (en) | 2001-10-02 | 2003-04-10 | Pharmacia & Upjohn Company | Azabicyclic-substituted fused-heteroaryl compounds for the treatment of disease |
| DE10164139A1 (en) | 2001-12-27 | 2003-07-10 | Bayer Ag | 2-heteroaryl carboxamides |
| WO2003070732A1 (en) | 2002-02-19 | 2003-08-28 | Pharmacia & Upjohn Company | Fused bicyclic-n-bridged-heteroaromatic carboxamides for the treatment of disease |
| WO2003094830A2 (en) * | 2002-05-07 | 2003-11-20 | Neurosearch A/S | Novel azacyclic ethynyl derivatives |
| CN1777596A (en) * | 2003-02-28 | 2006-05-24 | 先灵公司 | Biaryltetrahydroisoquinoline piperidines as selective mch receptor antagonists for the treatment of obesity and related disorders |
| WO2004081007A1 (en) | 2003-03-12 | 2004-09-23 | Pfizer Products Inc. | Pyridyloxymethyl and benzisoxazole azabicyclic derivatives |
| EP1802258A4 (en) | 2004-09-13 | 2015-09-23 | Chrono Therapeutics Inc | Biosynchronous transdermal drug delivery |
| US20060173037A1 (en) * | 2005-01-10 | 2006-08-03 | Nathalie Schlienger | Aminophenyl derivatives as selective androgen receptor modulators |
| ATE495178T1 (en) | 2005-02-16 | 2011-01-15 | Neurosearch As | DIAZABICYCLIC ARYL DERIVATIVES AND THEIR USE AS QUINOLINERGIC LIGANDS ON NICOTINE-ACETYLCHOLINE RECEPTORS |
| AU2006233884A1 (en) * | 2005-04-08 | 2006-10-19 | Neurosearch A/S | (+) - and (-) -8-alkyl-3-(trifluoralkylsulfonyloxy)-8-azabicycl (3.2.1.)oct-2-ene |
| FR2889701B1 (en) * | 2005-08-12 | 2007-10-05 | Sanofi Aventis Sa | 5-PYRIDINYL-1-AZABICYCLO [3.2.1] OCTANE DERIVATIVES, THEIR PREPARATION IN THERAPEUTICS. |
| KR20080079250A (en) | 2005-12-06 | 2008-08-29 | 뉴로서치 에이/에스 | Novel diazabicyclic aryl derivatives and their medical uses |
| US20070134169A1 (en) * | 2005-12-11 | 2007-06-14 | Rabinoff Michael D | Methods for smoking cessation or alcohol cessation or other addiction cessation |
| AU2007213671A1 (en) | 2006-02-10 | 2007-08-16 | Neurosearch A/S | 3-heteroaryl- 3,9-diazabicyclo[3.3.1]nonane derivatives as nicotinic acetylcholine receptor agonists |
| AU2007213670A1 (en) | 2006-02-10 | 2007-08-16 | Neurosearch A/S | 3,9-diazabicyclo[3.3.1]nonane derivatives and their use as monoamine neurotransmitter re-uptake inhibitors |
| CA2641678A1 (en) | 2006-02-10 | 2007-08-16 | Neurosearch A/S | 3,9-diazabicyclo[3.3.1]nonane derivatives and their use as monoamine neurotransmitter re-uptake inhibitors |
| EP1977746B8 (en) | 2007-04-02 | 2014-09-24 | Parkinson's Institute | Methods and compositions for reduction of side effects of therapeutic treatments |
| TWI415850B (en) * | 2007-07-20 | 2013-11-21 | Theravance Inc | Process for preparing an intermediate to mu opioid receptor antagonists |
| SA08290475B1 (en) | 2007-08-02 | 2013-06-22 | Targacept Inc | (2S,3R)-N-(2-((3-pyrdinyl)methyl)-1-aza bicyclo[2,2,2]oct-3-yl)benzofuran-2-carboxamide, its new salt forms and methods of use |
| US8697722B2 (en) * | 2007-11-02 | 2014-04-15 | Sri International | Nicotinic acetylcholine receptor modulators |
| WO2010014257A2 (en) * | 2008-08-01 | 2010-02-04 | Purdue Pharma L.P. | Tetrahydropyridinyl and dihydropyrrolyl compounds and the use thereof |
| EP2367818B1 (en) * | 2008-11-18 | 2018-07-25 | Initiator Pharma A/S | 8-azabicyclo[3.2.1]oct-2-ene derivatives and their use as mono-amine neurotransmitter re-uptake inhibitors |
| NZ613291A (en) | 2008-11-19 | 2014-11-28 | Forum Pharmaceuticals Inc | Treatment of cognitive disorders with (r)-7-chloro-n-(quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide and pharmaceutically acceptable salts thereof |
| TW201031664A (en) | 2009-01-26 | 2010-09-01 | Targacept Inc | Preparation and therapeutic applications of (2S,3R)-N-2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]oct-3-yl)-3,5-difluorobenzamide |
| RU2011150248A (en) * | 2009-05-11 | 2013-06-20 | Энвиво Фармасьютикалз, Инк. | TREATMENT OF COGNITIVE DISORDERS WITH SPECIFIC ALPHA-7 NICOTIN ACID RECEPTORS IN COMBINATION WITH ACETYLCHOLINESTERASE INHIBITORS |
| JP5840143B2 (en) | 2010-01-11 | 2016-01-06 | アストライア セラピューティクス, エルエルシーAstraea Therapeutics, Llc | Nicotinic acetylcholine receptor modulators |
| SG185594A1 (en) | 2010-05-17 | 2012-12-28 | Envivo Pharmaceuticals Inc | A crystalline form of (r)-7-chloro-n-(quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide hydrochloride monohydrate |
| RU2017136693A (en) | 2012-05-08 | 2019-02-08 | Форум Фармасьютикалз, Инк. | METHODS FOR MAINTAINING, TREATING OR IMPROVING COGNITIVE FUNCTION |
| WO2014011768A1 (en) * | 2012-07-10 | 2014-01-16 | Adispell, Inc. | Anti-anxiety treatment |
| WO2016123406A1 (en) | 2015-01-28 | 2016-08-04 | Chrono Therapeutics Inc. | Drug delivery methods and systems |
| US9724340B2 (en) | 2015-07-31 | 2017-08-08 | Attenua, Inc. | Antitussive compositions and methods |
| CA3049529A1 (en) | 2017-01-06 | 2018-07-12 | Chrono Therapeutics Inc. | Transdermal drug delivery devices and methods |
| US11596779B2 (en) | 2018-05-29 | 2023-03-07 | Morningside Venture Investments Limited | Drug delivery methods and systems |
| KR102272452B1 (en) | 2020-12-11 | 2021-07-05 | (주)예광솔라 | Spiral pile constructing method and spiral pile applied by the same |
| KR200497569Y1 (en) | 2021-02-18 | 2023-12-21 | 주식회사 알파인랩 | Screw pile |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3133073A (en) * | 1959-12-10 | 1964-05-12 | Sterling Drug Inc | 3-aryl-1, 5-iminocycloalkanes and preparation thereof |
| US3657257A (en) * | 1970-08-31 | 1972-04-18 | Robins Co Inc A H | 3-aryl-8-carbamoyl nortropanes |
| CA1244028A (en) * | 1983-04-14 | 1988-11-01 | Hans Maag | Pyrimidine derivatives |
| GB9019973D0 (en) | 1990-09-12 | 1990-10-24 | Wyeth John & Brother Ltd | Azabicyclic derivatives |
| US5045546A (en) | 1990-10-26 | 1991-09-03 | Hoechst-Roussel Pharmaceuticals Inc. | 8-azabicyclo[3.2.1]octylalkylthiazolidines |
| FI111367B (en) | 1991-02-04 | 2003-07-15 | Aventis Pharma Inc | Process for the preparation of therapeutically useful N- (aryloxyalkyl) heteroaryl-8-azabicyclo [3.2.1] octane derivatives and intermediates used in the process |
| EP0588917B1 (en) * | 1991-05-29 | 2000-11-08 | Abbott Laboratories | Isoxazole and isothiazole compounds that enhance cognitive function |
| US5731317A (en) * | 1995-03-10 | 1998-03-24 | Merck & Co., Inc. | Bridged piperidines promote release of growth hormone |
| GB9507203D0 (en) * | 1995-04-07 | 1995-05-31 | Smithkline Beecham Plc | Novel compounds |
| AU7083596A (en) * | 1995-09-22 | 1997-04-09 | Novo Nordisk A/S | Novel substituted azacyclic or azabicyclic compounds |
| TR199800628T2 (en) * | 1995-10-13 | 1998-07-21 | Neurosearch A/S | 8-Azabisiklo $ 3.2.1] oct-2-en t�revleri, büllar�n haz�rlanmas� ve kullan�m�. |
| GB9706222D0 (en) * | 1997-03-26 | 1997-05-14 | Zeneca Ltd | Bicyclic amine derivatives |
| EP0955301A3 (en) | 1998-04-27 | 2001-04-18 | Pfizer Products Inc. | 7-aza-bicyclo[2.2.1]-heptane derivatives, their preparation and use according to their affinity for neuronal nicotinic acetylcholine receptors |
| CA2335336A1 (en) * | 1998-06-19 | 1999-12-23 | James Edmund Audia | Inhibition of serotonin reuptake |
-
1998
- 1998-05-29 JP JP50013099A patent/JP4447663B2/en not_active Expired - Fee Related
- 1998-05-29 DK DK98921378T patent/DK0984965T3/en active
- 1998-05-29 EP EP98921378A patent/EP0984965B1/en not_active Expired - Lifetime
- 1998-05-29 WO PCT/DK1998/000225 patent/WO1998054181A1/en active IP Right Grant
- 1998-05-29 DE DE69823994T patent/DE69823994T2/en not_active Expired - Lifetime
- 1998-05-29 SK SK1626-99A patent/SK284994B6/en not_active IP Right Cessation
- 1998-05-29 BR BR9809697-4A patent/BR9809697A/en not_active Application Discontinuation
- 1998-05-29 HU HU0002713A patent/HUP0002713A3/en unknown
- 1998-05-29 RU RU99128075/04A patent/RU2186780C2/en not_active IP Right Cessation
- 1998-05-29 IL IL13243798A patent/IL132437A0/en active IP Right Grant
- 1998-05-29 EE EEP199900529A patent/EE04057B1/en not_active IP Right Cessation
- 1998-05-29 AT AT98921378T patent/ATE267199T1/en not_active IP Right Cessation
- 1998-05-29 NZ NZ500642A patent/NZ500642A/en unknown
- 1998-05-29 TR TR1999/02942T patent/TR199902942T2/en unknown
- 1998-05-29 CZ CZ0414799A patent/CZ298824B6/en not_active IP Right Cessation
- 1998-05-29 CN CNB988054132A patent/CN1157392C/en not_active Expired - Fee Related
- 1998-05-29 KR KR1019997011137A patent/KR100589872B1/en not_active IP Right Cessation
- 1998-05-29 AU AU74261/98A patent/AU745964B2/en not_active Ceased
- 1998-05-29 CA CA002289574A patent/CA2289574C/en not_active Expired - Fee Related
-
1999
- 1999-10-17 IL IL132437A patent/IL132437A/en not_active IP Right Cessation
- 1999-11-10 IS IS5243A patent/IS2092B/en unknown
- 1999-11-29 NO NO19995850A patent/NO325513B1/en not_active IP Right Cessation
- 1999-11-29 US US09/450,637 patent/US6645977B1/en not_active Expired - Fee Related
-
2000
- 2000-10-10 HK HK00106419A patent/HK1027353A1/en not_active IP Right Cessation
-
2003
- 2003-07-17 US US10/620,559 patent/US6964972B2/en not_active Expired - Fee Related
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU99128075A (en) | DERIVATIVES OF 8-AZABICYCLO (3,2,1) OCT-2-ENA AND OCTANE AS CHOLINERGIC LIGANDS ON ACH RECEPTORS | |
| EE04057B1 (en) | 8-Azabicyclo [3,2,1] oct-2-ene and octane derivatives, process for their preparation, use and pharmaceutical composition | |
| KR100614900B1 (en) | Azabicyclic-Substituted Fusion-Heteroaryl Compounds for the Treatment of Disease | |
| ES2242653T3 (en) | NEW USE AND NEW DERIVATIVES OF N-AZABICICLO-AMIDA. | |
| US8586746B2 (en) | Amino-aza-adamantane derivatives and methods of use | |
| MXPA05005666A (en) | Treatment of diseases with combinations of alpha 7 nicotinic acetylcholine receptor agonists and other compounds. | |
| TW200526667A (en) | Substituted diazabicycloalkane derivatives | |
| AU2003248592A1 (en) | Biaryl diazabicycloalkane amides as nicotinic acetylcholine agonists | |
| KR20040018266A (en) | Quinuclidines-Substituted-Multi-Cyclic-Heteroaryls For The Treatment Of Disease | |
| CA2460075A1 (en) | Substituted 7-aza[2.2.1] bicycloheptanes for the treatment of diseases | |
| US20060019984A1 (en) | Treatment of diseases with alpha-7nACh receptor full agonists | |
| BRPI0610028A2 (en) | therapeutic combinations for the treatment or prevention of psychotic disorders | |
| KR20090007753A (en) | Nicotinic Acetylcholine Receptor Ligand 110 | |
| JP2001521523A (en) | Furopyridine, thienopyridine, pyrrolopyridine and related pyrimidine, pyridazine and triazine compounds useful in controlling chemical synaptic transmission | |
| JP2006510663A (en) | Treatment of hyperattention activity | |
| CA2425638A1 (en) | Treatment of affective disorders by the combined action of a nicotinic receptor agonist and a monoaminergic substance | |
| JPH11510171A (en) | Furopyridine, thienopyridine, pyrrolopyridine and related pyrimidine, pyridazine and triazine compounds useful for controlling chemical synaptic transmission | |
| CA2443868A1 (en) | 1,3,8-triazaspiro[4.5]decan-4-one derivatives useful for the treatment of orl-1 receptor mediated disorders | |
| US7041695B2 (en) | N-sulfonylheterocyclopyrrolyl-alkylamine compounds as 5-hydroxytryptamine-6 ligands | |
| CN101646650A (en) | Sub-type selective azabicycloalkane derivatives | |
| CN104418842B (en) | Substituted benzazolyl compounds and its application method and purposes | |
| JP2002518331A (en) | Inhibition of serotonin reuptake | |
| Zhao et al. | Chemical synthesis and pharmacology of 6-and 7-hydroxylated 2-carbomethoxy-3-(p-tolyl) tropanes: antagonism of cocaine's locomotor stimulant effects | |
| DE3929763A1 (en) | PAF ANTAGONISTS FOR THE MANUFACTURE OF A MEDICAMENT SUITABLE FOR TREATING HEART DISEASES CAUSED BY REDUCED SS RECEPTOR STIMULATION | |
| CN101309922A (en) | Amino-aza-adamantane derivatives and methods of use |