KR20240089673A - Pigment dispersion compositions, photosensitive coloring compositions and color filters - Google Patents
Pigment dispersion compositions, photosensitive coloring compositions and color filters Download PDFInfo
- Publication number
- KR20240089673A KR20240089673A KR1020247015826A KR20247015826A KR20240089673A KR 20240089673 A KR20240089673 A KR 20240089673A KR 1020247015826 A KR1020247015826 A KR 1020247015826A KR 20247015826 A KR20247015826 A KR 20247015826A KR 20240089673 A KR20240089673 A KR 20240089673A
- Authority
- KR
- South Korea
- Prior art keywords
- group
- functional group
- pigment
- binder resin
- pigment dispersion
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000000049 pigment Substances 0.000 title claims abstract description 181
- 239000000203 mixture Substances 0.000 title claims abstract description 166
- 239000006185 dispersion Substances 0.000 title claims abstract description 99
- 238000004040 coloring Methods 0.000 title claims abstract description 76
- 125000000524 functional group Chemical group 0.000 claims abstract description 162
- 229920000642 polymer Polymers 0.000 claims abstract description 116
- 239000011347 resin Substances 0.000 claims abstract description 116
- 229920005989 resin Polymers 0.000 claims abstract description 116
- 239000011230 binding agent Substances 0.000 claims abstract description 97
- 239000002270 dispersing agent Substances 0.000 claims abstract description 90
- 239000002904 solvent Substances 0.000 claims abstract description 89
- 150000001875 compounds Chemical class 0.000 claims description 102
- 125000003277 amino group Chemical group 0.000 claims description 54
- 150000002366 halogen compounds Chemical class 0.000 claims description 54
- 238000000034 method Methods 0.000 claims description 33
- 125000003700 epoxy group Chemical group 0.000 claims description 24
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 23
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 23
- 125000001302 tertiary amino group Chemical group 0.000 claims description 19
- 125000001261 isocyanato group Chemical group *N=C=O 0.000 claims description 17
- 239000000047 product Substances 0.000 claims description 16
- 239000003085 diluting agent Substances 0.000 claims description 14
- 239000003999 initiator Substances 0.000 claims description 13
- 125000003566 oxetanyl group Chemical group 0.000 claims description 13
- 125000003396 thiol group Chemical group [H]S* 0.000 claims description 13
- 125000005907 alkyl ester group Chemical group 0.000 claims description 12
- IEQIEDJGQAUEQZ-UHFFFAOYSA-N phthalocyanine Chemical group N1C(N=C2C3=CC=CC=C3C(N=C3C4=CC=CC=C4C(=N4)N3)=N2)=C(C=CC=C2)C2=C1N=C1C2=CC=CC=C2C4=N1 IEQIEDJGQAUEQZ-UHFFFAOYSA-N 0.000 claims description 12
- 125000001453 quaternary ammonium group Chemical group 0.000 claims description 12
- 239000007795 chemical reaction product Substances 0.000 claims description 5
- 238000003860 storage Methods 0.000 abstract description 23
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 98
- -1 methacryloyloxy group Chemical group 0.000 description 47
- 239000000178 monomer Substances 0.000 description 41
- 230000015572 biosynthetic process Effects 0.000 description 34
- 238000003786 synthesis reaction Methods 0.000 description 34
- 239000000758 substrate Substances 0.000 description 28
- 150000001412 amines Chemical class 0.000 description 17
- 239000002253 acid Substances 0.000 description 16
- 238000004519 manufacturing process Methods 0.000 description 16
- 239000007787 solid Substances 0.000 description 16
- 238000000576 coating method Methods 0.000 description 15
- 238000006116 polymerization reaction Methods 0.000 description 15
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 14
- 239000011248 coating agent Substances 0.000 description 14
- 230000000052 comparative effect Effects 0.000 description 13
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 12
- 239000002585 base Substances 0.000 description 12
- 238000006243 chemical reaction Methods 0.000 description 12
- 239000000975 dye Substances 0.000 description 12
- LLHKCFNBLRBOGN-UHFFFAOYSA-N propylene glycol methyl ether acetate Chemical compound COCC(C)OC(C)=O LLHKCFNBLRBOGN-UHFFFAOYSA-N 0.000 description 12
- 239000011521 glass Substances 0.000 description 11
- 239000011159 matrix material Substances 0.000 description 11
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 10
- 238000002156 mixing Methods 0.000 description 10
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 9
- 239000003086 colorant Substances 0.000 description 9
- 238000010438 heat treatment Methods 0.000 description 9
- 239000002994 raw material Substances 0.000 description 9
- 239000004925 Acrylic resin Substances 0.000 description 8
- 229920000178 Acrylic resin Polymers 0.000 description 8
- 239000011324 bead Substances 0.000 description 8
- 125000004432 carbon atom Chemical group C* 0.000 description 8
- 238000011161 development Methods 0.000 description 8
- 239000011342 resin composition Substances 0.000 description 8
- 150000003512 tertiary amines Chemical class 0.000 description 8
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 7
- 239000007864 aqueous solution Substances 0.000 description 7
- 238000007334 copolymerization reaction Methods 0.000 description 7
- 238000011156 evaluation Methods 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 6
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 6
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 6
- 125000001309 chloro group Chemical group Cl* 0.000 description 6
- 238000001723 curing Methods 0.000 description 6
- 229910052757 nitrogen Inorganic materials 0.000 description 6
- 229920000768 polyamine Polymers 0.000 description 6
- 239000002243 precursor Substances 0.000 description 6
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 6
- 230000001681 protective effect Effects 0.000 description 6
- 229920001567 vinyl ester resin Polymers 0.000 description 6
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 5
- SJEYSFABYSGQBG-UHFFFAOYSA-M Patent blue Chemical compound [Na+].C1=CC(N(CC)CC)=CC=C1C(C=1C(=CC(=CC=1)S([O-])(=O)=O)S([O-])(=O)=O)=C1C=CC(=[N+](CC)CC)C=C1 SJEYSFABYSGQBG-UHFFFAOYSA-M 0.000 description 5
- 125000000217 alkyl group Chemical group 0.000 description 5
- 125000001246 bromo group Chemical group Br* 0.000 description 5
- 239000004202 carbamide Substances 0.000 description 5
- 239000003054 catalyst Substances 0.000 description 5
- 239000001056 green pigment Substances 0.000 description 5
- 239000004973 liquid crystal related substance Substances 0.000 description 5
- 238000005259 measurement Methods 0.000 description 5
- 239000003505 polymerization initiator Substances 0.000 description 5
- 230000008569 process Effects 0.000 description 5
- WLTSXAIICPDFKI-UHFFFAOYSA-N 3-dodecene Chemical compound CCCCCCCCC=CCC WLTSXAIICPDFKI-UHFFFAOYSA-N 0.000 description 4
- KWOLFJPFCHCOCG-UHFFFAOYSA-N Acetophenone Chemical compound CC(=O)C1=CC=CC=C1 KWOLFJPFCHCOCG-UHFFFAOYSA-N 0.000 description 4
- VVJKKWFAADXIJK-UHFFFAOYSA-N Allylamine Chemical compound NCC=C VVJKKWFAADXIJK-UHFFFAOYSA-N 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical group N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical group [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 4
- 241001561902 Chaetodon citrinellus Species 0.000 description 4
- WOBHKFSMXKNTIM-UHFFFAOYSA-N Hydroxyethyl methacrylate Chemical compound CC(=C)C(=O)OCCO WOBHKFSMXKNTIM-UHFFFAOYSA-N 0.000 description 4
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 4
- 239000000980 acid dye Substances 0.000 description 4
- 125000003118 aryl group Chemical group 0.000 description 4
- ISAOCJYIOMOJEB-UHFFFAOYSA-N benzoin Chemical compound C=1C=CC=CC=1C(O)C(=O)C1=CC=CC=C1 ISAOCJYIOMOJEB-UHFFFAOYSA-N 0.000 description 4
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 description 4
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 4
- NKHAVTQWNUWKEO-UHFFFAOYSA-N fumaric acid monomethyl ester Natural products COC(=O)C=CC(O)=O NKHAVTQWNUWKEO-UHFFFAOYSA-N 0.000 description 4
- 239000000463 material Substances 0.000 description 4
- 125000004433 nitrogen atom Chemical group N* 0.000 description 4
- 239000003973 paint Substances 0.000 description 4
- 238000000206 photolithography Methods 0.000 description 4
- 238000012360 testing method Methods 0.000 description 4
- ARXJGSRGQADJSQ-UHFFFAOYSA-N 1-methoxypropan-2-ol Chemical compound COCC(C)O ARXJGSRGQADJSQ-UHFFFAOYSA-N 0.000 description 3
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 3
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- CERQOIWHTDAKMF-UHFFFAOYSA-M Methacrylate Chemical compound CC(=C)C([O-])=O CERQOIWHTDAKMF-UHFFFAOYSA-M 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- ZJCCRDAZUWHFQH-UHFFFAOYSA-N Trimethylolpropane Chemical compound CCC(CO)(CO)CO ZJCCRDAZUWHFQH-UHFFFAOYSA-N 0.000 description 3
- XTXRWKRVRITETP-UHFFFAOYSA-N Vinyl acetate Chemical compound CC(=O)OC=C XTXRWKRVRITETP-UHFFFAOYSA-N 0.000 description 3
- 150000008065 acid anhydrides Chemical class 0.000 description 3
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 3
- 238000007259 addition reaction Methods 0.000 description 3
- 150000001408 amides Chemical class 0.000 description 3
- 150000004056 anthraquinones Chemical class 0.000 description 3
- AOJOEFVRHOZDFN-UHFFFAOYSA-N benzyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCC1=CC=CC=C1 AOJOEFVRHOZDFN-UHFFFAOYSA-N 0.000 description 3
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 3
- 239000002981 blocking agent Substances 0.000 description 3
- 229910052801 chlorine Inorganic materials 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- GKIPXFAANLTWBM-UHFFFAOYSA-N epibromohydrin Chemical compound BrCC1CO1 GKIPXFAANLTWBM-UHFFFAOYSA-N 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- XPFVYQJUAUNWIW-UHFFFAOYSA-N furfuryl alcohol Chemical compound OCC1=CC=CO1 XPFVYQJUAUNWIW-UHFFFAOYSA-N 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- VOZRXNHHFUQHIL-UHFFFAOYSA-N glycidyl methacrylate Chemical compound CC(=C)C(=O)OCC1CO1 VOZRXNHHFUQHIL-UHFFFAOYSA-N 0.000 description 3
- 125000005843 halogen group Chemical group 0.000 description 3
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- 150000003949 imides Chemical class 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 238000013035 low temperature curing Methods 0.000 description 3
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 3
- 229910052753 mercury Inorganic materials 0.000 description 3
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 3
- WHIVNJATOVLWBW-UHFFFAOYSA-N n-butan-2-ylidenehydroxylamine Chemical compound CCC(C)=NO WHIVNJATOVLWBW-UHFFFAOYSA-N 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 150000007519 polyprotic acids Polymers 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 230000009257 reactivity Effects 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- RMVRSNDYEFQCLF-UHFFFAOYSA-N thiophenol Chemical compound SC1=CC=CC=C1 RMVRSNDYEFQCLF-UHFFFAOYSA-N 0.000 description 3
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 3
- DTGKSKDOIYIVQL-WEDXCCLWSA-N (+)-borneol Chemical group C1C[C@@]2(C)[C@@H](O)C[C@@H]1C2(C)C DTGKSKDOIYIVQL-WEDXCCLWSA-N 0.000 description 2
- FVQMJJQUGGVLEP-UHFFFAOYSA-N (2-methylpropan-2-yl)oxy 2-ethylhexaneperoxoate Chemical compound CCCCC(CC)C(=O)OOOC(C)(C)C FVQMJJQUGGVLEP-UHFFFAOYSA-N 0.000 description 2
- FWUIHQFQLSWYED-ARJAWSKDSA-N (z)-4-oxo-4-propan-2-yloxybut-2-enoic acid Chemical compound CC(C)OC(=O)\C=C/C(O)=O FWUIHQFQLSWYED-ARJAWSKDSA-N 0.000 description 2
- WBYWAXJHAXSJNI-VOTSOKGWSA-M .beta-Phenylacrylic acid Natural products [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 2
- MYRTYDVEIRVNKP-UHFFFAOYSA-N 1,2-Divinylbenzene Chemical compound C=CC1=CC=CC=C1C=C MYRTYDVEIRVNKP-UHFFFAOYSA-N 0.000 description 2
- IKVISLWYYHGTTN-UHFFFAOYSA-N 11-bromoundecane-1-thiol Chemical compound SCCCCCCCCCCCBr IKVISLWYYHGTTN-UHFFFAOYSA-N 0.000 description 2
- LTMRRSWNXVJMBA-UHFFFAOYSA-L 2,2-diethylpropanedioate Chemical compound CCC(CC)(C([O-])=O)C([O-])=O LTMRRSWNXVJMBA-UHFFFAOYSA-L 0.000 description 2
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 2
- SBASXUCJHJRPEV-UHFFFAOYSA-N 2-(2-methoxyethoxy)ethanol Chemical compound COCCOCCO SBASXUCJHJRPEV-UHFFFAOYSA-N 0.000 description 2
- FALRKNHUBBKYCC-UHFFFAOYSA-N 2-(chloromethyl)pyridine-3-carbonitrile Chemical compound ClCC1=NC=CC=C1C#N FALRKNHUBBKYCC-UHFFFAOYSA-N 0.000 description 2
- XNWFRZJHXBZDAG-UHFFFAOYSA-N 2-METHOXYETHANOL Chemical compound COCCO XNWFRZJHXBZDAG-UHFFFAOYSA-N 0.000 description 2
- TXBCBTDQIULDIA-UHFFFAOYSA-N 2-[[3-hydroxy-2,2-bis(hydroxymethyl)propoxy]methyl]-2-(hydroxymethyl)propane-1,3-diol Chemical compound OCC(CO)(CO)COCC(CO)(CO)CO TXBCBTDQIULDIA-UHFFFAOYSA-N 0.000 description 2
- LDLCZOVUSADOIV-UHFFFAOYSA-N 2-bromoethanol Chemical compound OCCBr LDLCZOVUSADOIV-UHFFFAOYSA-N 0.000 description 2
- GJYCVCVHRSWLNY-UHFFFAOYSA-N 2-butylphenol Chemical compound CCCCC1=CC=CC=C1O GJYCVCVHRSWLNY-UHFFFAOYSA-N 0.000 description 2
- CRBJBYGJVIBWIY-UHFFFAOYSA-N 2-isopropylphenol Chemical compound CC(C)C1=CC=CC=C1O CRBJBYGJVIBWIY-UHFFFAOYSA-N 0.000 description 2
- NJWGQARXZDRHCD-UHFFFAOYSA-N 2-methylanthraquinone Chemical compound C1=CC=C2C(=O)C3=CC(C)=CC=C3C(=O)C2=C1 NJWGQARXZDRHCD-UHFFFAOYSA-N 0.000 description 2
- SDXAWLJRERMRKF-UHFFFAOYSA-N 3,5-dimethyl-1h-pyrazole Chemical compound CC=1C=C(C)NN=1 SDXAWLJRERMRKF-UHFFFAOYSA-N 0.000 description 2
- ZPLCXHWYPWVJDL-UHFFFAOYSA-N 4-[(4-hydroxyphenyl)methyl]-1,3-oxazolidin-2-one Chemical compound C1=CC(O)=CC=C1CC1NC(=O)OC1 ZPLCXHWYPWVJDL-UHFFFAOYSA-N 0.000 description 2
- RTTAGBVNSDJDTE-UHFFFAOYSA-N 4-ethoxy-2-methylidene-4-oxobutanoic acid Chemical compound CCOC(=O)CC(=C)C(O)=O RTTAGBVNSDJDTE-UHFFFAOYSA-N 0.000 description 2
- UJOBWOGCFQCDNV-UHFFFAOYSA-N 9H-carbazole Chemical compound C1=CC=C2C3=CC=CC=C3NC2=C1 UJOBWOGCFQCDNV-UHFFFAOYSA-N 0.000 description 2
- GJCOSYZMQJWQCA-UHFFFAOYSA-N 9H-xanthene Chemical compound C1=CC=C2CC3=CC=CC=C3OC2=C1 GJCOSYZMQJWQCA-UHFFFAOYSA-N 0.000 description 2
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 2
- KXDAEFPNCMNJSK-UHFFFAOYSA-N Benzamide Chemical compound NC(=O)C1=CC=CC=C1 KXDAEFPNCMNJSK-UHFFFAOYSA-N 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- WBYWAXJHAXSJNI-SREVYHEPSA-N Cinnamic acid Chemical compound OC(=O)\C=C/C1=CC=CC=C1 WBYWAXJHAXSJNI-SREVYHEPSA-N 0.000 description 2
- 102100026735 Coagulation factor VIII Human genes 0.000 description 2
- XLYMOEINVGRTEX-ARJAWSKDSA-N Ethyl hydrogen fumarate Chemical compound CCOC(=O)\C=C/C(O)=O XLYMOEINVGRTEX-ARJAWSKDSA-N 0.000 description 2
- 101000911390 Homo sapiens Coagulation factor VIII Proteins 0.000 description 2
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- FZERHIULMFGESH-UHFFFAOYSA-N N-phenylacetamide Chemical compound CC(=O)NC1=CC=CC=C1 FZERHIULMFGESH-UHFFFAOYSA-N 0.000 description 2
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 2
- BELBBZDIHDAJOR-UHFFFAOYSA-N Phenolsulfonephthalein Chemical compound C1=CC(O)=CC=C1C1(C=2C=CC(O)=CC=2)C2=CC=CC=C2S(=O)(=O)O1 BELBBZDIHDAJOR-UHFFFAOYSA-N 0.000 description 2
- 239000004793 Polystyrene Substances 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- 244000028419 Styrax benzoin Species 0.000 description 2
- 235000000126 Styrax benzoin Nutrition 0.000 description 2
- 235000008411 Sumatra benzointree Nutrition 0.000 description 2
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 2
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 2
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 2
- YRKCREAYFQTBPV-UHFFFAOYSA-N acetylacetone Chemical compound CC(=O)CC(C)=O YRKCREAYFQTBPV-UHFFFAOYSA-N 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 239000012670 alkaline solution Substances 0.000 description 2
- XYLMUPLGERFSHI-UHFFFAOYSA-N alpha-Methylstyrene Chemical compound CC(=C)C1=CC=CC=C1 XYLMUPLGERFSHI-UHFFFAOYSA-N 0.000 description 2
- MWPLVEDNUUSJAV-UHFFFAOYSA-N anthracene Chemical compound C1=CC=CC2=CC3=CC=CC=C3C=C21 MWPLVEDNUUSJAV-UHFFFAOYSA-N 0.000 description 2
- PYKYMHQGRFAEBM-UHFFFAOYSA-N anthraquinone Natural products CCC(=O)c1c(O)c2C(=O)C3C(C=CC=C3O)C(=O)c2cc1CC(=O)OC PYKYMHQGRFAEBM-UHFFFAOYSA-N 0.000 description 2
- 229960002130 benzoin Drugs 0.000 description 2
- RWCCWEUUXYIKHB-UHFFFAOYSA-N benzophenone Chemical compound C=1C=CC=CC=1C(=O)C1=CC=CC=C1 RWCCWEUUXYIKHB-UHFFFAOYSA-N 0.000 description 2
- 239000012965 benzophenone Substances 0.000 description 2
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 2
- 229910052794 bromium Inorganic materials 0.000 description 2
- WQAQPCDUOCURKW-UHFFFAOYSA-N butanethiol Chemical compound CCCCS WQAQPCDUOCURKW-UHFFFAOYSA-N 0.000 description 2
- 239000006229 carbon black Substances 0.000 description 2
- 125000006297 carbonyl amino group Chemical group [H]N([*:2])C([*:1])=O 0.000 description 2
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 2
- BEQIIZJSZSVJJK-UHFFFAOYSA-M chembl2028372 Chemical compound [Na+].OC1=CC=C(S([O-])(=O)=O)C=C1N=NC1=C(O)C=CC2=CC=CC=C12 BEQIIZJSZSVJJK-UHFFFAOYSA-M 0.000 description 2
- 229930016911 cinnamic acid Natural products 0.000 description 2
- 235000013985 cinnamic acid Nutrition 0.000 description 2
- 238000004132 cross linking Methods 0.000 description 2
- LDHQCZJRKDOVOX-NSCUHMNNSA-N crotonic acid Chemical compound C\C=C\C(O)=O LDHQCZJRKDOVOX-NSCUHMNNSA-N 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- VEZUQRBDRNJBJY-UHFFFAOYSA-N cyclohexanone oxime Chemical compound ON=C1CCCCC1 VEZUQRBDRNJBJY-UHFFFAOYSA-N 0.000 description 2
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 229910001873 dinitrogen Inorganic materials 0.000 description 2
- DMBHHRLKUKUOEG-UHFFFAOYSA-N diphenylamine Chemical compound C=1C=CC=CC=1NC1=CC=CC=C1 DMBHHRLKUKUOEG-UHFFFAOYSA-N 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 239000003822 epoxy resin Substances 0.000 description 2
- JBKVHLHDHHXQEQ-UHFFFAOYSA-N epsilon-caprolactam Chemical compound O=C1CCCCCN1 JBKVHLHDHHXQEQ-UHFFFAOYSA-N 0.000 description 2
- UYMKPFRHYYNDTL-UHFFFAOYSA-N ethenamine Chemical compound NC=C UYMKPFRHYYNDTL-UHFFFAOYSA-N 0.000 description 2
- FJKIXWOMBXYWOQ-UHFFFAOYSA-N ethenoxyethane Chemical compound CCOC=C FJKIXWOMBXYWOQ-UHFFFAOYSA-N 0.000 description 2
- XLYMOEINVGRTEX-UHFFFAOYSA-N fumaric acid monoethyl ester Natural products CCOC(=O)C=CC(O)=O XLYMOEINVGRTEX-UHFFFAOYSA-N 0.000 description 2
- 238000005227 gel permeation chromatography Methods 0.000 description 2
- 238000001879 gelation Methods 0.000 description 2
- 235000019382 gum benzoic Nutrition 0.000 description 2
- NAQMVNRVTILPCV-UHFFFAOYSA-N hexane-1,6-diamine Chemical compound NCCCCCCN NAQMVNRVTILPCV-UHFFFAOYSA-N 0.000 description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- KQNPFQTWMSNSAP-UHFFFAOYSA-N isobutyric acid Chemical compound CC(C)C(O)=O KQNPFQTWMSNSAP-UHFFFAOYSA-N 0.000 description 2
- 239000012948 isocyanate Substances 0.000 description 2
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- QDLAGTHXVHQKRE-UHFFFAOYSA-N lichenxanthone Natural products COC1=CC(O)=C2C(=O)C3=C(C)C=C(OC)C=C3OC2=C1 QDLAGTHXVHQKRE-UHFFFAOYSA-N 0.000 description 2
- NKHAVTQWNUWKEO-IHWYPQMZSA-N methyl hydrogen fumarate Chemical compound COC(=O)\C=C/C(O)=O NKHAVTQWNUWKEO-IHWYPQMZSA-N 0.000 description 2
- WBYWAXJHAXSJNI-UHFFFAOYSA-N methyl p-hydroxycinnamate Natural products OC(=O)C=CC1=CC=CC=C1 WBYWAXJHAXSJNI-UHFFFAOYSA-N 0.000 description 2
- NKHAVTQWNUWKEO-NSCUHMNNSA-N monomethyl fumarate Chemical compound COC(=O)\C=C\C(O)=O NKHAVTQWNUWKEO-NSCUHMNNSA-N 0.000 description 2
- 229940005650 monomethyl fumarate Drugs 0.000 description 2
- QYZFTMMPKCOTAN-UHFFFAOYSA-N n-[2-(2-hydroxyethylamino)ethyl]-2-[[1-[2-(2-hydroxyethylamino)ethylamino]-2-methyl-1-oxopropan-2-yl]diazenyl]-2-methylpropanamide Chemical compound OCCNCCNC(=O)C(C)(C)N=NC(C)(C)C(=O)NCCNCCO QYZFTMMPKCOTAN-UHFFFAOYSA-N 0.000 description 2
- JFNLZVQOOSMTJK-KNVOCYPGSA-N norbornene Chemical compound C1[C@@H]2CC[C@H]1C=C2 JFNLZVQOOSMTJK-KNVOCYPGSA-N 0.000 description 2
- IXQGCWUGDFDQMF-UHFFFAOYSA-N o-Hydroxyethylbenzene Natural products CCC1=CC=CC=C1O IXQGCWUGDFDQMF-UHFFFAOYSA-N 0.000 description 2
- WXZMFSXDPGVJKK-UHFFFAOYSA-N pentaerythritol Chemical compound OCC(CO)(CO)CO WXZMFSXDPGVJKK-UHFFFAOYSA-N 0.000 description 2
- 229960003531 phenolsulfonphthalein Drugs 0.000 description 2
- XUWHAWMETYGRKB-UHFFFAOYSA-N piperidin-2-one Chemical compound O=C1CCCCN1 XUWHAWMETYGRKB-UHFFFAOYSA-N 0.000 description 2
- 229920000647 polyepoxide Polymers 0.000 description 2
- 229920002223 polystyrene Polymers 0.000 description 2
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 2
- 150000003141 primary amines Chemical class 0.000 description 2
- HJWLCRVIBGQPNF-UHFFFAOYSA-N prop-2-enylbenzene Chemical compound C=CCC1=CC=CC=C1 HJWLCRVIBGQPNF-UHFFFAOYSA-N 0.000 description 2
- HNJBEVLQSNELDL-UHFFFAOYSA-N pyrrolidin-2-one Chemical compound O=C1CCCN1 HNJBEVLQSNELDL-UHFFFAOYSA-N 0.000 description 2
- 238000007142 ring opening reaction Methods 0.000 description 2
- 150000003335 secondary amines Chemical class 0.000 description 2
- 125000000467 secondary amino group Chemical group [H]N([*:1])[*:2] 0.000 description 2
- 238000001179 sorption measurement Methods 0.000 description 2
- 150000003440 styrenes Chemical class 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 229940014800 succinic anhydride Drugs 0.000 description 2
- KZNICNPSHKQLFF-UHFFFAOYSA-N succinimide Chemical compound O=C1CCC(=O)N1 KZNICNPSHKQLFF-UHFFFAOYSA-N 0.000 description 2
- 230000002194 synthesizing effect Effects 0.000 description 2
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 2
- UMGDCJDMYOKAJW-UHFFFAOYSA-N thiourea Chemical compound NC(N)=S UMGDCJDMYOKAJW-UHFFFAOYSA-N 0.000 description 2
- MGSRCZKZVOBKFT-UHFFFAOYSA-N thymol Chemical compound CC(C)C1=CC=C(C)C=C1O MGSRCZKZVOBKFT-UHFFFAOYSA-N 0.000 description 2
- 239000010936 titanium Substances 0.000 description 2
- 229910052719 titanium Inorganic materials 0.000 description 2
- LDHQCZJRKDOVOX-UHFFFAOYSA-N trans-crotonic acid Natural products CC=CC(O)=O LDHQCZJRKDOVOX-UHFFFAOYSA-N 0.000 description 2
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 2
- 229920002554 vinyl polymer Polymers 0.000 description 2
- 229910052725 zinc Inorganic materials 0.000 description 2
- 239000011701 zinc Substances 0.000 description 2
- RSHKWPIEJYAPCL-UHFFFAOYSA-N (3-ethyloxetan-3-yl)methyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCC1(CC)COC1 RSHKWPIEJYAPCL-UHFFFAOYSA-N 0.000 description 1
- MUTGBJKUEZFXGO-OLQVQODUSA-N (3as,7ar)-3a,4,5,6,7,7a-hexahydro-2-benzofuran-1,3-dione Chemical compound C1CCC[C@@H]2C(=O)OC(=O)[C@@H]21 MUTGBJKUEZFXGO-OLQVQODUSA-N 0.000 description 1
- FZENGILVLUJGJX-NSCUHMNNSA-N (E)-acetaldehyde oxime Chemical compound C\C=N\O FZENGILVLUJGJX-NSCUHMNNSA-N 0.000 description 1
- 229920002818 (Hydroxyethyl)methacrylate Polymers 0.000 description 1
- CDUQMGQIHYISOP-RMKNXTFCSA-N (e)-2-cyano-3-phenylprop-2-enoic acid Chemical compound OC(=O)C(\C#N)=C\C1=CC=CC=C1 CDUQMGQIHYISOP-RMKNXTFCSA-N 0.000 description 1
- ZKALVNREMFLWAN-VOTSOKGWSA-N (ne)-n-(4-methylpentan-2-ylidene)hydroxylamine Chemical compound CC(C)C\C(C)=N\O ZKALVNREMFLWAN-VOTSOKGWSA-N 0.000 description 1
- XKSUVRWJZCEYQQ-UHFFFAOYSA-N 1,1-dimethoxyethylbenzene Chemical compound COC(C)(OC)C1=CC=CC=C1 XKSUVRWJZCEYQQ-UHFFFAOYSA-N 0.000 description 1
- FMZITCJWMYHQFG-UHFFFAOYSA-N 1,2,3,4,4a,5,8,8a-octahydro-2-methyl-1,4:5,8-dimethanonaphthalene Chemical compound C1C(C23)C=CC1C3C1CC2CC1C FMZITCJWMYHQFG-UHFFFAOYSA-N 0.000 description 1
- KMOUUZVZFBCRAM-UHFFFAOYSA-N 1,2,3,6-tetrahydrophthalic anhydride Chemical compound C1C=CCC2C(=O)OC(=O)C21 KMOUUZVZFBCRAM-UHFFFAOYSA-N 0.000 description 1
- BPXVHIRIPLPOPT-UHFFFAOYSA-N 1,3,5-tris(2-hydroxyethyl)-1,3,5-triazinane-2,4,6-trione Chemical compound OCCN1C(=O)N(CCO)C(=O)N(CCO)C1=O BPXVHIRIPLPOPT-UHFFFAOYSA-N 0.000 description 1
- ILBBNQMSDGAAPF-UHFFFAOYSA-N 1-(6-hydroxy-6-methylcyclohexa-2,4-dien-1-yl)propan-1-one Chemical compound CCC(=O)C1C=CC=CC1(C)O ILBBNQMSDGAAPF-UHFFFAOYSA-N 0.000 description 1
- BOCJQSFSGAZAPQ-UHFFFAOYSA-N 1-chloroanthracene-9,10-dione Chemical compound O=C1C2=CC=CC=C2C(=O)C2=C1C=CC=C2Cl BOCJQSFSGAZAPQ-UHFFFAOYSA-N 0.000 description 1
- OZCMOJQQLBXBKI-UHFFFAOYSA-N 1-ethenoxy-2-methylpropane Chemical compound CC(C)COC=C OZCMOJQQLBXBKI-UHFFFAOYSA-N 0.000 description 1
- UZKWTJUDCOPSNM-UHFFFAOYSA-N 1-ethenoxybutane Chemical compound CCCCOC=C UZKWTJUDCOPSNM-UHFFFAOYSA-N 0.000 description 1
- OVGRCEFMXPHEBL-UHFFFAOYSA-N 1-ethenoxypropane Chemical compound CCCOC=C OVGRCEFMXPHEBL-UHFFFAOYSA-N 0.000 description 1
- XLPJNCYCZORXHG-UHFFFAOYSA-N 1-morpholin-4-ylprop-2-en-1-one Chemical compound C=CC(=O)N1CCOCC1 XLPJNCYCZORXHG-UHFFFAOYSA-N 0.000 description 1
- HECLRDQVFMWTQS-RGOKHQFPSA-N 1755-01-7 Chemical compound C1[C@H]2[C@@H]3CC=C[C@@H]3[C@@H]1C=C2 HECLRDQVFMWTQS-RGOKHQFPSA-N 0.000 description 1
- CERJZAHSUZVMCH-UHFFFAOYSA-N 2,2-dichloro-1-phenylethanone Chemical compound ClC(Cl)C(=O)C1=CC=CC=C1 CERJZAHSUZVMCH-UHFFFAOYSA-N 0.000 description 1
- KWVGIHKZDCUPEU-UHFFFAOYSA-N 2,2-dimethoxy-2-phenylacetophenone Chemical compound C=1C=CC=CC=1C(OC)(OC)C(=O)C1=CC=CC=C1 KWVGIHKZDCUPEU-UHFFFAOYSA-N 0.000 description 1
- YAJYJWXEWKRTPO-UHFFFAOYSA-N 2,3,3,4,4,5-hexamethylhexane-2-thiol Chemical compound CC(C)C(C)(C)C(C)(C)C(C)(C)S YAJYJWXEWKRTPO-UHFFFAOYSA-N 0.000 description 1
- JKTAIYGNOFSMCE-UHFFFAOYSA-N 2,3-di(nonyl)phenol Chemical compound CCCCCCCCCC1=CC=CC(O)=C1CCCCCCCCC JKTAIYGNOFSMCE-UHFFFAOYSA-N 0.000 description 1
- BJELTSYBAHKXRW-UHFFFAOYSA-N 2,4,6-triallyloxy-1,3,5-triazine Chemical compound C=CCOC1=NC(OCC=C)=NC(OCC=C)=N1 BJELTSYBAHKXRW-UHFFFAOYSA-N 0.000 description 1
- BYLSIPUARIZAHZ-UHFFFAOYSA-N 2,4,6-tris(1-phenylethyl)phenol Chemical compound C=1C(C(C)C=2C=CC=CC=2)=C(O)C(C(C)C=2C=CC=CC=2)=CC=1C(C)C1=CC=CC=C1 BYLSIPUARIZAHZ-UHFFFAOYSA-N 0.000 description 1
- BRKORVYTKKLNKX-UHFFFAOYSA-N 2,4-di(propan-2-yl)thioxanthen-9-one Chemical compound C1=CC=C2C(=O)C3=CC(C(C)C)=CC(C(C)C)=C3SC2=C1 BRKORVYTKKLNKX-UHFFFAOYSA-N 0.000 description 1
- LCHAFMWSFCONOO-UHFFFAOYSA-N 2,4-dimethylthioxanthen-9-one Chemical compound C1=CC=C2C(=O)C3=CC(C)=CC(C)=C3SC2=C1 LCHAFMWSFCONOO-UHFFFAOYSA-N 0.000 description 1
- OZAIFHULBGXAKX-UHFFFAOYSA-N 2-(2-cyanopropan-2-yldiazenyl)-2-methylpropanenitrile Chemical compound N#CC(C)(C)N=NC(C)(C)C#N OZAIFHULBGXAKX-UHFFFAOYSA-N 0.000 description 1
- FPZWZCWUIYYYBU-UHFFFAOYSA-N 2-(2-ethoxyethoxy)ethyl acetate Chemical compound CCOCCOCCOC(C)=O FPZWZCWUIYYYBU-UHFFFAOYSA-N 0.000 description 1
- HLIQLHSBZXDKLV-UHFFFAOYSA-N 2-(2-hydroxyethoxy)-1-phenoxyethanol Chemical compound OCCOCC(O)OC1=CC=CC=C1 HLIQLHSBZXDKLV-UHFFFAOYSA-N 0.000 description 1
- DRLRGHZJOQGQEC-UHFFFAOYSA-N 2-(2-methoxypropoxy)propyl acetate Chemical compound COC(C)COC(C)COC(C)=O DRLRGHZJOQGQEC-UHFFFAOYSA-N 0.000 description 1
- JAHNSTQSQJOJLO-UHFFFAOYSA-N 2-(3-fluorophenyl)-1h-imidazole Chemical compound FC1=CC=CC(C=2NC=CN=2)=C1 JAHNSTQSQJOJLO-UHFFFAOYSA-N 0.000 description 1
- QTLHLXYADXCVCF-UHFFFAOYSA-N 2-(4-amino-n-ethyl-3-methylanilino)ethanol Chemical compound OCCN(CC)C1=CC=C(N)C(C)=C1 QTLHLXYADXCVCF-UHFFFAOYSA-N 0.000 description 1
- GOXQRTZXKQZDDN-UHFFFAOYSA-N 2-Ethylhexyl acrylate Chemical compound CCCCC(CC)COC(=O)C=C GOXQRTZXKQZDDN-UHFFFAOYSA-N 0.000 description 1
- IZXIZTKNFFYFOF-UHFFFAOYSA-N 2-Oxazolidone Chemical compound O=C1NCCO1 IZXIZTKNFFYFOF-UHFFFAOYSA-N 0.000 description 1
- CPBHXURKKFMQFI-UHFFFAOYSA-N 2-[(3,5-dimethyl-1h-pyrazole-4-carbonyl)amino]ethyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCNC(=O)C=1C(C)=NNC=1C CPBHXURKKFMQFI-UHFFFAOYSA-N 0.000 description 1
- WAEVWDZKMBQDEJ-UHFFFAOYSA-N 2-[2-(2-methoxypropoxy)propoxy]propan-1-ol Chemical compound COC(C)COC(C)COC(C)CO WAEVWDZKMBQDEJ-UHFFFAOYSA-N 0.000 description 1
- UHFFVFAKEGKNAQ-UHFFFAOYSA-N 2-benzyl-2-(dimethylamino)-1-(4-morpholin-4-ylphenyl)butan-1-one Chemical compound C=1C=C(N2CCOCC2)C=CC=1C(=O)C(CC)(N(C)C)CC1=CC=CC=C1 UHFFVFAKEGKNAQ-UHFFFAOYSA-N 0.000 description 1
- XXSPGBOGLXKMDU-UHFFFAOYSA-N 2-bromo-2-methylpropanoic acid Chemical compound CC(C)(Br)C(O)=O XXSPGBOGLXKMDU-UHFFFAOYSA-N 0.000 description 1
- POAOYUHQDCAZBD-UHFFFAOYSA-N 2-butoxyethanol Chemical compound CCCCOCCO POAOYUHQDCAZBD-UHFFFAOYSA-N 0.000 description 1
- ZCDADJXRUCOCJE-UHFFFAOYSA-N 2-chlorothioxanthen-9-one Chemical compound C1=CC=C2C(=O)C3=CC(Cl)=CC=C3SC2=C1 ZCDADJXRUCOCJE-UHFFFAOYSA-N 0.000 description 1
- BHOJJZTVUGWMQA-UHFFFAOYSA-N 2-ethenyl-2-methyloxetane Chemical compound C=CC1(C)CCO1 BHOJJZTVUGWMQA-UHFFFAOYSA-N 0.000 description 1
- NADUDFSNHZPLIN-UHFFFAOYSA-N 2-ethenylbenzenethiol Chemical compound SC1=CC=CC=C1C=C NADUDFSNHZPLIN-UHFFFAOYSA-N 0.000 description 1
- IXHCBFVEIPFXDC-UHFFFAOYSA-N 2-ethenylhexanedioic acid Chemical compound OC(=O)CCCC(C=C)C(O)=O IXHCBFVEIPFXDC-UHFFFAOYSA-N 0.000 description 1
- KMNCBSZOIQAUFX-UHFFFAOYSA-N 2-ethoxy-1,2-diphenylethanone Chemical compound C=1C=CC=CC=1C(OCC)C(=O)C1=CC=CC=C1 KMNCBSZOIQAUFX-UHFFFAOYSA-N 0.000 description 1
- SVONRAPFKPVNKG-UHFFFAOYSA-N 2-ethoxyethyl acetate Chemical compound CCOCCOC(C)=O SVONRAPFKPVNKG-UHFFFAOYSA-N 0.000 description 1
- PQAMFDRRWURCFQ-UHFFFAOYSA-N 2-ethyl-1h-imidazole Chemical compound CCC1=NC=CN1 PQAMFDRRWURCFQ-UHFFFAOYSA-N 0.000 description 1
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 description 1
- BQZJOQXSCSZQPS-UHFFFAOYSA-N 2-methoxy-1,2-diphenylethanone Chemical compound C=1C=CC=CC=1C(OC)C(=O)C1=CC=CC=C1 BQZJOQXSCSZQPS-UHFFFAOYSA-N 0.000 description 1
- LWRBVKNFOYUCNP-UHFFFAOYSA-N 2-methyl-1-(4-methylsulfanylphenyl)-2-morpholin-4-ylpropan-1-one Chemical compound C1=CC(SC)=CC=C1C(=O)C(C)(C)N1CCOCC1 LWRBVKNFOYUCNP-UHFFFAOYSA-N 0.000 description 1
- LXBGSDVWAMZHDD-UHFFFAOYSA-N 2-methyl-1h-imidazole Chemical compound CC1=NC=CN1 LXBGSDVWAMZHDD-UHFFFAOYSA-N 0.000 description 1
- QTWJRLJHJPIABL-UHFFFAOYSA-N 2-methylphenol;3-methylphenol;4-methylphenol Chemical compound CC1=CC=C(O)C=C1.CC1=CC=CC(O)=C1.CC1=CC=CC=C1O QTWJRLJHJPIABL-UHFFFAOYSA-N 0.000 description 1
- JEHFRMABGJJCPF-UHFFFAOYSA-N 2-methylprop-2-enoyl isocyanate Chemical compound CC(=C)C(=O)N=C=O JEHFRMABGJJCPF-UHFFFAOYSA-N 0.000 description 1
- 150000000376 2-oxazolines Chemical class 0.000 description 1
- UMWZLYTVXQBTTE-UHFFFAOYSA-N 2-pentylanthracene-9,10-dione Chemical compound C1=CC=C2C(=O)C3=CC(CCCCC)=CC=C3C(=O)C2=C1 UMWZLYTVXQBTTE-UHFFFAOYSA-N 0.000 description 1
- QCDWFXQBSFUVSP-UHFFFAOYSA-N 2-phenoxyethanol Chemical compound OCCOC1=CC=CC=C1 QCDWFXQBSFUVSP-UHFFFAOYSA-N 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- YTPSFXZMJKMUJE-UHFFFAOYSA-N 2-tert-butylanthracene-9,10-dione Chemical compound C1=CC=C2C(=O)C3=CC(C(C)(C)C)=CC=C3C(=O)C2=C1 YTPSFXZMJKMUJE-UHFFFAOYSA-N 0.000 description 1
- YUYCFQVUWMCDAF-UHFFFAOYSA-N 3-[3-(4-ethenylphenoxy)propoxymethyl]-3-ethyloxetane Chemical compound C=1C=C(C=C)C=CC=1OCCCOCC1(CC)COC1 YUYCFQVUWMCDAF-UHFFFAOYSA-N 0.000 description 1
- PSXYOONUOUFVFP-UHFFFAOYSA-N 3-[6-(4-ethenylphenoxy)hexoxymethyl]-3-ethyloxetane Chemical compound C=1C=C(C=C)C=CC=1OCCCCCCOCC1(CC)COC1 PSXYOONUOUFVFP-UHFFFAOYSA-N 0.000 description 1
- SKKHNUKNMQLBTJ-UHFFFAOYSA-N 3-bicyclo[2.2.1]heptanyl 2-methylprop-2-enoate Chemical compound C1CC2C(OC(=O)C(=C)C)CC1C2 SKKHNUKNMQLBTJ-UHFFFAOYSA-N 0.000 description 1
- SIBFQOUHOCRXDL-UHFFFAOYSA-N 3-bromopropane-1,2-diol Chemical compound OCC(O)CBr SIBFQOUHOCRXDL-UHFFFAOYSA-N 0.000 description 1
- QQGZSWNELYFQPD-UHFFFAOYSA-N 3-chloroprop-1-en-2-ylbenzene Chemical compound ClCC(=C)C1=CC=CC=C1 QQGZSWNELYFQPD-UHFFFAOYSA-N 0.000 description 1
- YHFGMFYKZBWPRW-UHFFFAOYSA-N 3-methylpentane-1,1-diol Chemical compound CCC(C)CC(O)O YHFGMFYKZBWPRW-UHFFFAOYSA-N 0.000 description 1
- FWTBRYBHCBCJEQ-UHFFFAOYSA-N 4-[(4-phenyldiazenylnaphthalen-1-yl)diazenyl]phenol Chemical compound C1=CC(O)=CC=C1N=NC(C1=CC=CC=C11)=CC=C1N=NC1=CC=CC=C1 FWTBRYBHCBCJEQ-UHFFFAOYSA-N 0.000 description 1
- WXNZTHHGJRFXKQ-UHFFFAOYSA-N 4-chlorophenol Chemical compound OC1=CC=C(Cl)C=C1 WXNZTHHGJRFXKQ-UHFFFAOYSA-N 0.000 description 1
- SXIFAEWFOJETOA-UHFFFAOYSA-N 4-hydroxy-butyl Chemical group [CH2]CCCO SXIFAEWFOJETOA-UHFFFAOYSA-N 0.000 description 1
- NDWUBGAGUCISDV-UHFFFAOYSA-N 4-hydroxybutyl prop-2-enoate Chemical compound OCCCCOC(=O)C=C NDWUBGAGUCISDV-UHFFFAOYSA-N 0.000 description 1
- XBTWVJKPQPQTDW-UHFFFAOYSA-N 4-n,4-n-diethyl-2-methylbenzene-1,4-diamine Chemical compound CCN(CC)C1=CC=C(N)C(C)=C1 XBTWVJKPQPQTDW-UHFFFAOYSA-N 0.000 description 1
- FFAJEKUNEVVYCW-UHFFFAOYSA-N 4-n-ethyl-4-n-(2-methoxyethyl)-2-methylbenzene-1,4-diamine Chemical compound COCCN(CC)C1=CC=C(N)C(C)=C1 FFAJEKUNEVVYCW-UHFFFAOYSA-N 0.000 description 1
- BTJIUGUIPKRLHP-UHFFFAOYSA-N 4-nitrophenol Chemical compound OC1=CC=C([N+]([O-])=O)C=C1 BTJIUGUIPKRLHP-UHFFFAOYSA-N 0.000 description 1
- ISAVYTVYFVQUDY-UHFFFAOYSA-N 4-tert-Octylphenol Chemical compound CC(C)(C)CC(C)(C)C1=CC=C(O)C=C1 ISAVYTVYFVQUDY-UHFFFAOYSA-N 0.000 description 1
- QHPQWRBYOIRBIT-UHFFFAOYSA-N 4-tert-butylphenol Chemical compound CC(C)(C)C1=CC=C(O)C=C1 QHPQWRBYOIRBIT-UHFFFAOYSA-N 0.000 description 1
- QHJIJNGGGLNBNJ-UHFFFAOYSA-N 5-ethylbicyclo[2.2.1]hept-2-ene Chemical compound C1C2C(CC)CC1C=C2 QHJIJNGGGLNBNJ-UHFFFAOYSA-N 0.000 description 1
- FKBMTBAXDISZGN-UHFFFAOYSA-N 5-methyl-3a,4,5,6,7,7a-hexahydro-2-benzofuran-1,3-dione Chemical compound C1C(C)CCC2C(=O)OC(=O)C12 FKBMTBAXDISZGN-UHFFFAOYSA-N 0.000 description 1
- PCBPVYHMZBWMAZ-UHFFFAOYSA-N 5-methylbicyclo[2.2.1]hept-2-ene Chemical compound C1C2C(C)CC1C=C2 PCBPVYHMZBWMAZ-UHFFFAOYSA-N 0.000 description 1
- LQOPXMZSGSTGMF-UHFFFAOYSA-N 6004-79-1 Chemical compound C1CC2C3C(=O)OC(=O)C3C1C2 LQOPXMZSGSTGMF-UHFFFAOYSA-N 0.000 description 1
- YXALYBMHAYZKAP-UHFFFAOYSA-N 7-oxabicyclo[4.1.0]heptan-4-ylmethyl 7-oxabicyclo[4.1.0]heptane-4-carboxylate Chemical compound C1CC2OC2CC1C(=O)OCC1CC2OC2CC1 YXALYBMHAYZKAP-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- DLFVBJFMPXGRIB-UHFFFAOYSA-N Acetamide Chemical compound CC(N)=O DLFVBJFMPXGRIB-UHFFFAOYSA-N 0.000 description 1
- AOMZHDJXSYHPKS-DROYEMJCSA-L Amido Black 10B Chemical compound [Na+].[Na+].[O-]S(=O)(=O)C1=CC2=CC(S([O-])(=O)=O)=C(\N=N\C=3C=CC=CC=3)C(O)=C2C(N)=C1\N=N\C1=CC=C(N(=O)=O)C=C1 AOMZHDJXSYHPKS-DROYEMJCSA-L 0.000 description 1
- NOWKCMXCCJGMRR-UHFFFAOYSA-N Aziridine Chemical compound C1CN1 NOWKCMXCCJGMRR-UHFFFAOYSA-N 0.000 description 1
- 239000004342 Benzoyl peroxide Substances 0.000 description 1
- OMPJBNCRMGITSC-UHFFFAOYSA-N Benzoylperoxide Chemical compound C=1C=CC=CC=1C(=O)OOC(=O)C1=CC=CC=C1 OMPJBNCRMGITSC-UHFFFAOYSA-N 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- DKPFZGUDAPQIHT-UHFFFAOYSA-N Butyl acetate Natural products CCCCOC(C)=O DKPFZGUDAPQIHT-UHFFFAOYSA-N 0.000 description 1
- GAWIXWVDTYZWAW-UHFFFAOYSA-N C[CH]O Chemical group C[CH]O GAWIXWVDTYZWAW-UHFFFAOYSA-N 0.000 description 1
- 101100061273 Caenorhabditis elegans cpr-3 gene Proteins 0.000 description 1
- KZBUYRJDOAKODT-UHFFFAOYSA-N Chlorine Chemical compound ClCl KZBUYRJDOAKODT-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 229910021591 Copper(I) chloride Inorganic materials 0.000 description 1
- 239000004641 Diallyl-phthalate Substances 0.000 description 1
- RWSOTUBLDIXVET-UHFFFAOYSA-N Dihydrogen sulfide Chemical class S RWSOTUBLDIXVET-UHFFFAOYSA-N 0.000 description 1
- LCGLNKUTAGEVQW-UHFFFAOYSA-N Dimethyl ether Chemical compound COC LCGLNKUTAGEVQW-UHFFFAOYSA-N 0.000 description 1
- SNRUBQQJIBEYMU-UHFFFAOYSA-N Dodecane Natural products CCCCCCCCCCCC SNRUBQQJIBEYMU-UHFFFAOYSA-N 0.000 description 1
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- PEEHTFAAVSWFBL-UHFFFAOYSA-N Maleimide Chemical compound O=C1NC(=O)C=C1 PEEHTFAAVSWFBL-UHFFFAOYSA-N 0.000 description 1
- NTIZESTWPVYFNL-UHFFFAOYSA-N Methyl isobutyl ketone Chemical compound CC(C)CC(C)=O NTIZESTWPVYFNL-UHFFFAOYSA-N 0.000 description 1
- UIHCLUNTQKBZGK-UHFFFAOYSA-N Methyl isobutyl ketone Natural products CCC(C)C(C)=O UIHCLUNTQKBZGK-UHFFFAOYSA-N 0.000 description 1
- WRQNANDWMGAFTP-UHFFFAOYSA-N Methylacetoacetic acid Chemical compound COC(=O)CC(C)=O WRQNANDWMGAFTP-UHFFFAOYSA-N 0.000 description 1
- KWYHDKDOAIKMQN-UHFFFAOYSA-N N,N,N',N'-tetramethylethylenediamine Chemical compound CN(C)CCN(C)C KWYHDKDOAIKMQN-UHFFFAOYSA-N 0.000 description 1
- XQVWYOYUZDUNRW-UHFFFAOYSA-N N-Phenyl-1-naphthylamine Chemical compound C=1C=CC2=CC=CC=C2C=1NC1=CC=CC=C1 XQVWYOYUZDUNRW-UHFFFAOYSA-N 0.000 description 1
- IGFHQQFPSIBGKE-UHFFFAOYSA-N Nonylphenol Natural products CCCCCCCCCC1=CC=C(O)C=C1 IGFHQQFPSIBGKE-UHFFFAOYSA-N 0.000 description 1
- 101000900567 Pisum sativum Disease resistance response protein Pi49 Proteins 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 239000004962 Polyamide-imide Substances 0.000 description 1
- 229920002873 Polyethylenimine Polymers 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- 101000621511 Potato virus M (strain German) RNA silencing suppressor Proteins 0.000 description 1
- NRCMAYZCPIVABH-UHFFFAOYSA-N Quinacridone Chemical compound N1C2=CC=CC=C2C(=O)C2=C1C=C1C(=O)C3=CC=CC=C3NC1=C2 NRCMAYZCPIVABH-UHFFFAOYSA-N 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- DWAQJAXMDSEUJJ-UHFFFAOYSA-M Sodium bisulfite Chemical compound [Na+].OS([O-])=O DWAQJAXMDSEUJJ-UHFFFAOYSA-M 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 239000005844 Thymol Substances 0.000 description 1
- 239000007983 Tris buffer Chemical class 0.000 description 1
- QYKIQEUNHZKYBP-UHFFFAOYSA-N Vinyl ether Chemical class C=COC=C QYKIQEUNHZKYBP-UHFFFAOYSA-N 0.000 description 1
- IAXXETNIOYFMLW-COPLHBTASA-N [(1s,3s,4s)-4,7,7-trimethyl-3-bicyclo[2.2.1]heptanyl] 2-methylprop-2-enoate Chemical compound C1C[C@]2(C)[C@@H](OC(=O)C(=C)C)C[C@H]1C2(C)C IAXXETNIOYFMLW-COPLHBTASA-N 0.000 description 1
- MPIAGWXWVAHQBB-UHFFFAOYSA-N [3-prop-2-enoyloxy-2-[[3-prop-2-enoyloxy-2,2-bis(prop-2-enoyloxymethyl)propoxy]methyl]-2-(prop-2-enoyloxymethyl)propyl] prop-2-enoate Chemical compound C=CC(=O)OCC(COC(=O)C=C)(COC(=O)C=C)COCC(COC(=O)C=C)(COC(=O)C=C)COC(=O)C=C MPIAGWXWVAHQBB-UHFFFAOYSA-N 0.000 description 1
- IETYNJBCXVFYIQ-UHFFFAOYSA-N [4-(2-tert-butylperoxypropan-2-yl)phenyl]-phenylmethanone Chemical compound C1=CC(C(C)(C)OOC(C)(C)C)=CC=C1C(=O)C1=CC=CC=C1 IETYNJBCXVFYIQ-UHFFFAOYSA-N 0.000 description 1
- MZVQCMJNVPIDEA-UHFFFAOYSA-N [CH2]CN(CC)CC Chemical group [CH2]CN(CC)CC MZVQCMJNVPIDEA-UHFFFAOYSA-N 0.000 description 1
- YJOLYFYTSAVBML-UHFFFAOYSA-N ac1l4f97 Chemical compound C1C(C23)C=CC1C3C1CC2CC1CC YJOLYFYTSAVBML-UHFFFAOYSA-N 0.000 description 1
- 229960001413 acetanilide Drugs 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-N acetic acid Substances CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 1
- TUVYSBJZBYRDHP-UHFFFAOYSA-N acetic acid;methoxymethane Chemical compound COC.CC(O)=O TUVYSBJZBYRDHP-UHFFFAOYSA-N 0.000 description 1
- PXAJQJMDEXJWFB-UHFFFAOYSA-N acetone oxime Chemical compound CC(C)=NO PXAJQJMDEXJWFB-UHFFFAOYSA-N 0.000 description 1
- 150000008062 acetophenones Chemical class 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 150000003926 acrylamides Chemical class 0.000 description 1
- 125000003647 acryloyl group Chemical group O=C([*])C([H])=C([H])[H] 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 125000005073 adamantyl group Chemical group C12(CC3CC(CC(C1)C3)C2)* 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 239000012615 aggregate Substances 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 125000002723 alicyclic group Chemical group 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 150000005215 alkyl ethers Chemical class 0.000 description 1
- 150000001350 alkyl halides Chemical class 0.000 description 1
- BHELZAPQIKSEDF-UHFFFAOYSA-N allyl bromide Chemical compound BrCC=C BHELZAPQIKSEDF-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- APUPEJJSWDHEBO-UHFFFAOYSA-P ammonium molybdate Chemical compound [NH4+].[NH4+].[O-][Mo]([O-])(=O)=O APUPEJJSWDHEBO-UHFFFAOYSA-P 0.000 description 1
- 239000011609 ammonium molybdate Substances 0.000 description 1
- 229940010552 ammonium molybdate Drugs 0.000 description 1
- 235000018660 ammonium molybdate Nutrition 0.000 description 1
- 235000010208 anthocyanin Nutrition 0.000 description 1
- 239000004410 anthocyanin Substances 0.000 description 1
- 229930002877 anthocyanin Natural products 0.000 description 1
- 150000004636 anthocyanins Chemical class 0.000 description 1
- RJGDLRCDCYRQOQ-UHFFFAOYSA-N anthrone Chemical compound C1=CC=C2C(=O)C3=CC=CC=C3CC2=C1 RJGDLRCDCYRQOQ-UHFFFAOYSA-N 0.000 description 1
- 239000002518 antifoaming agent Substances 0.000 description 1
- 125000003710 aryl alkyl group Chemical group 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- MNFORVFSTILPAW-UHFFFAOYSA-N azetidin-2-one Chemical compound O=C1CCN1 MNFORVFSTILPAW-UHFFFAOYSA-N 0.000 description 1
- 125000000751 azo group Chemical group [*]N=N[*] 0.000 description 1
- WXLFIFHRGFOVCD-UHFFFAOYSA-L azophloxine Chemical compound [Na+].[Na+].OC1=C2C(NC(=O)C)=CC(S([O-])(=O)=O)=CC2=CC(S([O-])(=O)=O)=C1N=NC1=CC=CC=C1 WXLFIFHRGFOVCD-UHFFFAOYSA-L 0.000 description 1
- 235000019400 benzoyl peroxide Nutrition 0.000 description 1
- QUDWYFHPNIMBFC-UHFFFAOYSA-N bis(prop-2-enyl) benzene-1,2-dicarboxylate Chemical compound C=CCOC(=O)C1=CC=CC=C1C(=O)OCC=C QUDWYFHPNIMBFC-UHFFFAOYSA-N 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 239000001055 blue pigment Substances 0.000 description 1
- 150000001649 bromium compounds Chemical class 0.000 description 1
- INLLPKCGLOXCIV-UHFFFAOYSA-N bromoethene Chemical group BrC=C INLLPKCGLOXCIV-UHFFFAOYSA-N 0.000 description 1
- 239000001058 brown pigment Substances 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 150000004657 carbamic acid derivatives Chemical class 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 125000004112 carboxyamino group Chemical group [H]OC(=O)N([H])[*] 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- OIQPTROHQCGFEF-UHFFFAOYSA-L chembl1371409 Chemical compound [Na+].[Na+].OC1=CC=C2C=C(S([O-])(=O)=O)C=CC2=C1N=NC1=CC=C(S([O-])(=O)=O)C=C1 OIQPTROHQCGFEF-UHFFFAOYSA-L 0.000 description 1
- 239000012295 chemical reaction liquid Substances 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000005660 chlorination reaction Methods 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 150000001805 chlorine compounds Chemical class 0.000 description 1
- 239000013065 commercial product Substances 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- OXBLHERUFWYNTN-UHFFFAOYSA-M copper(I) chloride Chemical compound [Cu]Cl OXBLHERUFWYNTN-UHFFFAOYSA-M 0.000 description 1
- XCJYREBRNVKWGJ-UHFFFAOYSA-N copper(II) phthalocyanine Chemical compound [Cu+2].C12=CC=CC=C2C(N=C2[N-]C(C3=CC=CC=C32)=N2)=NC1=NC([C]1C=CC=CC1=1)=NC=1N=C1[C]3C=CC=CC3=C2[N-]1 XCJYREBRNVKWGJ-UHFFFAOYSA-N 0.000 description 1
- 239000007822 coupling agent Substances 0.000 description 1
- 101150046305 cpr-1 gene Proteins 0.000 description 1
- 229930003836 cresol Natural products 0.000 description 1
- 229940045803 cuprous chloride Drugs 0.000 description 1
- 238000007766 curtain coating Methods 0.000 description 1
- 125000000753 cycloalkyl group Chemical group 0.000 description 1
- FFQYMTMKMLRXLJ-UHFFFAOYSA-N cyclohexanol;phenol Chemical compound OC1CCCCC1.OC1=CC=CC=C1 FFQYMTMKMLRXLJ-UHFFFAOYSA-N 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 238000011981 development test Methods 0.000 description 1
- SWXVUIWOUIDPGS-UHFFFAOYSA-N diacetone alcohol Chemical compound CC(=O)CC(C)(C)O SWXVUIWOUIDPGS-UHFFFAOYSA-N 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- ZEFVHSWKYCYFFL-UHFFFAOYSA-N diethyl 2-methylidenebutanedioate Chemical compound CCOC(=O)CC(=C)C(=O)OCC ZEFVHSWKYCYFFL-UHFFFAOYSA-N 0.000 description 1
- IEPRKVQEAMIZSS-AATRIKPKSA-N diethyl fumarate Chemical compound CCOC(=O)\C=C\C(=O)OCC IEPRKVQEAMIZSS-AATRIKPKSA-N 0.000 description 1
- 125000001664 diethylamino group Chemical group [H]C([H])([H])C([H])([H])N(*)C([H])([H])C([H])([H])[H] 0.000 description 1
- FLISWPFVWWWNNP-BQYQJAHWSA-N dihydro-3-(1-octenyl)-2,5-furandione Chemical compound CCCCCC\C=C\C1CC(=O)OC1=O FLISWPFVWWWNNP-BQYQJAHWSA-N 0.000 description 1
- HANKSFAYJLDDKP-UHFFFAOYSA-N dihydrodicyclopentadiene Chemical compound C12CC=CC2C2CCC1C2 HANKSFAYJLDDKP-UHFFFAOYSA-N 0.000 description 1
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 description 1
- BEPAFCGSDWSTEL-UHFFFAOYSA-N dimethyl malonate Chemical compound COC(=O)CC(=O)OC BEPAFCGSDWSTEL-UHFFFAOYSA-N 0.000 description 1
- PPSZHCXTGRHULJ-UHFFFAOYSA-N dioxazine Chemical compound O1ON=CC=C1 PPSZHCXTGRHULJ-UHFFFAOYSA-N 0.000 description 1
- SZXQTJUDPRGNJN-UHFFFAOYSA-N dipropylene glycol Chemical compound OCCCOCCCO SZXQTJUDPRGNJN-UHFFFAOYSA-N 0.000 description 1
- KGGOIDKBHYYNIC-UHFFFAOYSA-N ditert-butyl 4-[3,4-bis(tert-butylperoxycarbonyl)benzoyl]benzene-1,2-dicarboperoxoate Chemical compound C1=C(C(=O)OOC(C)(C)C)C(C(=O)OOC(C)(C)C)=CC=C1C(=O)C1=CC=C(C(=O)OOC(C)(C)C)C(C(=O)OOC(C)(C)C)=C1 KGGOIDKBHYYNIC-UHFFFAOYSA-N 0.000 description 1
- 238000009837 dry grinding Methods 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- 238000010894 electron beam technology Methods 0.000 description 1
- 239000012776 electronic material Substances 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 238000004134 energy conservation Methods 0.000 description 1
- UIWXSTHGICQLQT-UHFFFAOYSA-N ethenyl propanoate Chemical compound CCC(=O)OC=C UIWXSTHGICQLQT-UHFFFAOYSA-N 0.000 description 1
- 239000004210 ether based solvent Substances 0.000 description 1
- IOLQWGVDEFWYNP-UHFFFAOYSA-N ethyl 2-bromo-2-methylpropanoate Chemical compound CCOC(=O)C(C)(C)Br IOLQWGVDEFWYNP-UHFFFAOYSA-N 0.000 description 1
- SUPCQIBBMFXVTL-UHFFFAOYSA-N ethyl 2-methylprop-2-enoate Chemical compound CCOC(=O)C(C)=C SUPCQIBBMFXVTL-UHFFFAOYSA-N 0.000 description 1
- XYIBRDXRRQCHLP-UHFFFAOYSA-N ethyl acetoacetate Chemical compound CCOC(=O)CC(C)=O XYIBRDXRRQCHLP-UHFFFAOYSA-N 0.000 description 1
- WBJINCZRORDGAQ-UHFFFAOYSA-N ethyl formate Chemical compound CCOC=O WBJINCZRORDGAQ-UHFFFAOYSA-N 0.000 description 1
- 125000000031 ethylamino group Chemical group [H]C([H])([H])C([H])([H])N([H])[*] 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 229960002428 fentanyl Drugs 0.000 description 1
- IVLVTNPOHDFFCJ-UHFFFAOYSA-N fentanyl citrate Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O.C=1C=CC=CC=1N(C(=O)CC)C(CC1)CCN1CCC1=CC=CC=C1 IVLVTNPOHDFFCJ-UHFFFAOYSA-N 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 125000003055 glycidyl group Chemical group C(C1CO1)* 0.000 description 1
- 235000021384 green leafy vegetables Nutrition 0.000 description 1
- 230000026030 halogenation Effects 0.000 description 1
- 238000005658 halogenation reaction Methods 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- 125000002768 hydroxyalkyl group Chemical group 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 150000002460 imidazoles Chemical class 0.000 description 1
- YAMHXTCMCPHKLN-UHFFFAOYSA-N imidazolidin-2-one Chemical compound O=C1NCCN1 YAMHXTCMCPHKLN-UHFFFAOYSA-N 0.000 description 1
- 150000002466 imines Chemical class 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000001023 inorganic pigment Substances 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 150000004694 iodide salts Chemical class 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 230000001678 irradiating effect Effects 0.000 description 1
- 229940119545 isobornyl methacrylate Drugs 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- ZFSLODLOARCGLH-UHFFFAOYSA-N isocyanuric acid Chemical compound OC1=NC(O)=NC(O)=N1 ZFSLODLOARCGLH-UHFFFAOYSA-N 0.000 description 1
- PXZQEOJJUGGUIB-UHFFFAOYSA-N isoindolin-1-one Chemical compound C1=CC=C2C(=O)NCC2=C1 PXZQEOJJUGGUIB-UHFFFAOYSA-N 0.000 description 1
- GWVMLCQWXVFZCN-UHFFFAOYSA-N isoindoline Chemical compound C1=CC=C2CNCC2=C1 GWVMLCQWXVFZCN-UHFFFAOYSA-N 0.000 description 1
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- JMMWKPVZQRWMSS-UHFFFAOYSA-N isopropanol acetate Natural products CC(C)OC(C)=O JMMWKPVZQRWMSS-UHFFFAOYSA-N 0.000 description 1
- 229940011051 isopropyl acetate Drugs 0.000 description 1
- GWYFCOCPABKNJV-UHFFFAOYSA-N isovaleric acid Chemical compound CC(C)CC(O)=O GWYFCOCPABKNJV-UHFFFAOYSA-N 0.000 description 1
- 150000003951 lactams Chemical class 0.000 description 1
- 150000002596 lactones Chemical class 0.000 description 1
- FPYJFEHAWHCUMM-UHFFFAOYSA-N maleic anhydride Chemical compound O=C1OC(=O)C=C1 FPYJFEHAWHCUMM-UHFFFAOYSA-N 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229910001507 metal halide Inorganic materials 0.000 description 1
- 150000005309 metal halides Chemical class 0.000 description 1
- LVHBHZANLOWSRM-UHFFFAOYSA-N methylenebutanedioic acid Natural products OC(=O)CC(=C)C(O)=O LVHBHZANLOWSRM-UHFFFAOYSA-N 0.000 description 1
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 description 1
- 238000003801 milling Methods 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- SQDFHQJTAWCFIB-UHFFFAOYSA-N n-methylidenehydroxylamine Chemical compound ON=C SQDFHQJTAWCFIB-UHFFFAOYSA-N 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- JMXROTHPANUTOJ-UHFFFAOYSA-H naphthol green b Chemical compound [Na+].[Na+].[Na+].[Fe+3].C1=C(S([O-])(=O)=O)C=CC2=C(N=O)C([O-])=CC=C21.C1=C(S([O-])(=O)=O)C=CC2=C(N=O)C([O-])=CC=C21.C1=C(S([O-])(=O)=O)C=CC2=C(N=O)C([O-])=CC=C21 JMXROTHPANUTOJ-UHFFFAOYSA-H 0.000 description 1
- CTIQLGJVGNGFEW-UHFFFAOYSA-L naphthol yellow S Chemical compound [Na+].[Na+].C1=C(S([O-])(=O)=O)C=C2C([O-])=C([N+]([O-])=O)C=C([N+]([O-])=O)C2=C1 CTIQLGJVGNGFEW-UHFFFAOYSA-L 0.000 description 1
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 239000012299 nitrogen atmosphere Substances 0.000 description 1
- 229910017464 nitrogen compound Inorganic materials 0.000 description 1
- 150000002830 nitrogen compounds Chemical class 0.000 description 1
- SNQQPOLDUKLAAF-UHFFFAOYSA-N nonylphenol Chemical compound CCCCCCCCCC1=CC=CC=C1O SNQQPOLDUKLAAF-UHFFFAOYSA-N 0.000 description 1
- 238000000655 nuclear magnetic resonance spectrum Methods 0.000 description 1
- 239000001053 orange pigment Substances 0.000 description 1
- 239000012860 organic pigment Substances 0.000 description 1
- 150000002923 oximes Chemical class 0.000 description 1
- 150000002924 oxiranes Chemical class 0.000 description 1
- 125000005702 oxyalkylene group Chemical group 0.000 description 1
- NWVVVBRKAWDGAB-UHFFFAOYSA-N p-methoxyphenol Chemical compound COC1=CC=C(O)C=C1 NWVVVBRKAWDGAB-UHFFFAOYSA-N 0.000 description 1
- VGXOXHRUFVBLBN-UHFFFAOYSA-N pentacyclo[6.5.1.13,6.02,7.09,13]pentadec-4-ene Chemical compound C1C2C3C(C=C4)CC4C3C1C1C2CCC1 VGXOXHRUFVBLBN-UHFFFAOYSA-N 0.000 description 1
- 125000004115 pentoxy group Chemical group [*]OC([H])([H])C([H])([H])C([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- DGBWPZSGHAXYGK-UHFFFAOYSA-N perinone Chemical compound C12=NC3=CC=CC=C3N2C(=O)C2=CC=C3C4=C2C1=CC=C4C(=O)N1C2=CC=CC=C2N=C13 DGBWPZSGHAXYGK-UHFFFAOYSA-N 0.000 description 1
- 125000002080 perylenyl group Chemical group C1(=CC=C2C=CC=C3C4=CC=CC5=CC=CC(C1=C23)=C45)* 0.000 description 1
- CSHWQDPOILHKBI-UHFFFAOYSA-N peryrene Natural products C1=CC(C2=CC=CC=3C2=C2C=CC=3)=C3C2=CC=CC3=C1 CSHWQDPOILHKBI-UHFFFAOYSA-N 0.000 description 1
- 150000002989 phenols Chemical class 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- XVNKRRXASPPECQ-UHFFFAOYSA-N phenyl n-phenylcarbamate Chemical compound C=1C=CC=CC=1OC(=O)NC1=CC=CC=C1 XVNKRRXASPPECQ-UHFFFAOYSA-N 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- 238000000016 photochemical curing Methods 0.000 description 1
- 229920002120 photoresistant polymer Polymers 0.000 description 1
- XQZYPMVTSDWCCE-UHFFFAOYSA-N phthalonitrile Chemical compound N#CC1=CC=CC=C1C#N XQZYPMVTSDWCCE-UHFFFAOYSA-N 0.000 description 1
- 229920006391 phthalonitrile polymer Polymers 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920002312 polyamide-imide Polymers 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 238000006068 polycondensation reaction Methods 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- DJEHXEMURTVAOE-UHFFFAOYSA-M potassium bisulfite Chemical compound [K+].OS([O-])=O DJEHXEMURTVAOE-UHFFFAOYSA-M 0.000 description 1
- 229940099427 potassium bisulfite Drugs 0.000 description 1
- 229910000027 potassium carbonate Inorganic materials 0.000 description 1
- 235000010259 potassium hydrogen sulphite Nutrition 0.000 description 1
- 238000007639 printing Methods 0.000 description 1
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- UORVCLMRJXCDCP-UHFFFAOYSA-N propynoic acid Chemical compound OC(=O)C#C UORVCLMRJXCDCP-UHFFFAOYSA-N 0.000 description 1
- 238000010926 purge Methods 0.000 description 1
- 239000001057 purple pigment Substances 0.000 description 1
- FYNROBRQIVCIQF-UHFFFAOYSA-N pyrrolo[3,2-b]pyrrole-5,6-dione Chemical compound C1=CN=C2C(=O)C(=O)N=C21 FYNROBRQIVCIQF-UHFFFAOYSA-N 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 235000012739 red 2G Nutrition 0.000 description 1
- 239000001054 red pigment Substances 0.000 description 1
- 238000007670 refining Methods 0.000 description 1
- 238000007151 ring opening polymerisation reaction Methods 0.000 description 1
- 238000007650 screen-printing Methods 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 150000003384 small molecules Chemical group 0.000 description 1
- COEZWFYORILMOM-UHFFFAOYSA-M sodium 4-[(2,4-dihydroxyphenyl)diazenyl]benzenesulfonate Chemical compound [Na+].OC1=CC(O)=CC=C1N=NC1=CC=C(S([O-])(=O)=O)C=C1 COEZWFYORILMOM-UHFFFAOYSA-M 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 235000010267 sodium hydrogen sulphite Nutrition 0.000 description 1
- AXMCIYLNKNGNOT-UHFFFAOYSA-M sodium;3-[[4-[(4-dimethylazaniumylidenecyclohexa-2,5-dien-1-ylidene)-[4-[ethyl-[(3-sulfonatophenyl)methyl]amino]phenyl]methyl]-n-ethylanilino]methyl]benzenesulfonate Chemical compound [Na+].C=1C=C(C(=C2C=CC(C=C2)=[N+](C)C)C=2C=CC(=CC=2)N(CC)CC=2C=C(C=CC=2)S([O-])(=O)=O)C=CC=1N(CC)CC1=CC=CC(S([O-])(=O)=O)=C1 AXMCIYLNKNGNOT-UHFFFAOYSA-M 0.000 description 1
- 229910000679 solder Inorganic materials 0.000 description 1
- 239000011973 solid acid Substances 0.000 description 1
- 125000006850 spacer group Chemical group 0.000 description 1
- 238000004528 spin coating Methods 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 125000004079 stearyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000004575 stone Substances 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 229960002317 succinimide Drugs 0.000 description 1
- 125000000565 sulfonamide group Chemical group 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- XBFJAVXCNXDMBH-UHFFFAOYSA-N tetracyclo[6.2.1.1(3,6).0(2,7)]dodec-4-ene Chemical compound C1C(C23)C=CC1C3C1CC2CC1 XBFJAVXCNXDMBH-UHFFFAOYSA-N 0.000 description 1
- QEMXHQIAXOOASZ-UHFFFAOYSA-N tetramethylammonium Chemical compound C[N+](C)(C)C QEMXHQIAXOOASZ-UHFFFAOYSA-N 0.000 description 1
- YRHRIQCWCFGUEQ-UHFFFAOYSA-N thioxanthen-9-one Chemical compound C1=CC=C2C(=O)C3=CC=CC=C3SC2=C1 YRHRIQCWCFGUEQ-UHFFFAOYSA-N 0.000 description 1
- 229960000790 thymol Drugs 0.000 description 1
- 239000004408 titanium dioxide Substances 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical class CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000002834 transmittance Methods 0.000 description 1
- WHNYVDJJCTVMGO-UHFFFAOYSA-N tricyclo[5.2.1.02,6]dec-8-ene Chemical compound C1=CC2CC1C1C2CCC1 WHNYVDJJCTVMGO-UHFFFAOYSA-N 0.000 description 1
- 125000002221 trityl group Chemical group [H]C1=C([H])C([H])=C([H])C([H])=C1C([*])(C1=C(C(=C(C(=C1[H])[H])[H])[H])[H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 229910052724 xenon Inorganic materials 0.000 description 1
- FHNFHKCVQCLJFQ-UHFFFAOYSA-N xenon atom Chemical compound [Xe] FHNFHKCVQCLJFQ-UHFFFAOYSA-N 0.000 description 1
- 150000003739 xylenols Chemical class 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B67/00—Influencing the physical, e.g. the dyeing or printing properties of dyestuffs without chemical reactions, e.g. by treating with solvents grinding or grinding assistants, coating of pigments or dyes; Process features in the making of dyestuff preparations; Dyestuff preparations of a special physical nature, e.g. tablets, films
- C09B67/006—Preparation of organic pigments
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F20/00—Homopolymers and copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride, ester, amide, imide or nitrile thereof
- C08F20/02—Monocarboxylic acids having less than ten carbon atoms, Derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/10—Esters
- C08F220/12—Esters of monohydric alcohols or phenols
- C08F220/16—Esters of monohydric alcohols or phenols of phenols or of alcohols containing two or more carbon atoms
- C08F220/18—Esters of monohydric alcohols or phenols of phenols or of alcohols containing two or more carbon atoms with acrylic or methacrylic acids
- C08F220/1811—C10or C11-(Meth)acrylate, e.g. isodecyl (meth)acrylate, isobornyl (meth)acrylate or 2-naphthyl (meth)acrylate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/10—Esters
- C08F220/34—Esters containing nitrogen, e.g. N,N-dimethylaminoethyl (meth)acrylate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F8/00—Chemical modification by after-treatment
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D133/00—Coating compositions based on homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides, or nitriles thereof; Coating compositions based on derivatives of such polymers
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D17/00—Pigment pastes, e.g. for mixing in paints
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D7/00—Features of coating compositions, not provided for in group C09D5/00; Processes for incorporating ingredients in coating compositions
- C09D7/40—Additives
- C09D7/41—Organic pigments; Organic dyes
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D7/00—Features of coating compositions, not provided for in group C09D5/00; Processes for incorporating ingredients in coating compositions
- C09D7/40—Additives
- C09D7/45—Anti-settling agents
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/20—Filters
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/004—Photosensitive materials
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/004—Photosensitive materials
- G03F7/027—Non-macromolecular photopolymerisable compounds having carbon-to-carbon double bonds, e.g. ethylenic compounds
- G03F7/032—Non-macromolecular photopolymerisable compounds having carbon-to-carbon double bonds, e.g. ethylenic compounds with binders
- G03F7/033—Non-macromolecular photopolymerisable compounds having carbon-to-carbon double bonds, e.g. ethylenic compounds with binders the binders being polymers obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. vinyl polymers
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/004—Photosensitive materials
- G03F7/038—Macromolecular compounds which are rendered insoluble or differentially wettable
Landscapes
- Chemical & Material Sciences (AREA)
- Physics & Mathematics (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Physics & Mathematics (AREA)
- Polymers & Plastics (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Wood Science & Technology (AREA)
- Optics & Photonics (AREA)
- General Chemical & Material Sciences (AREA)
- Optical Filters (AREA)
- Materials For Photolithography (AREA)
Abstract
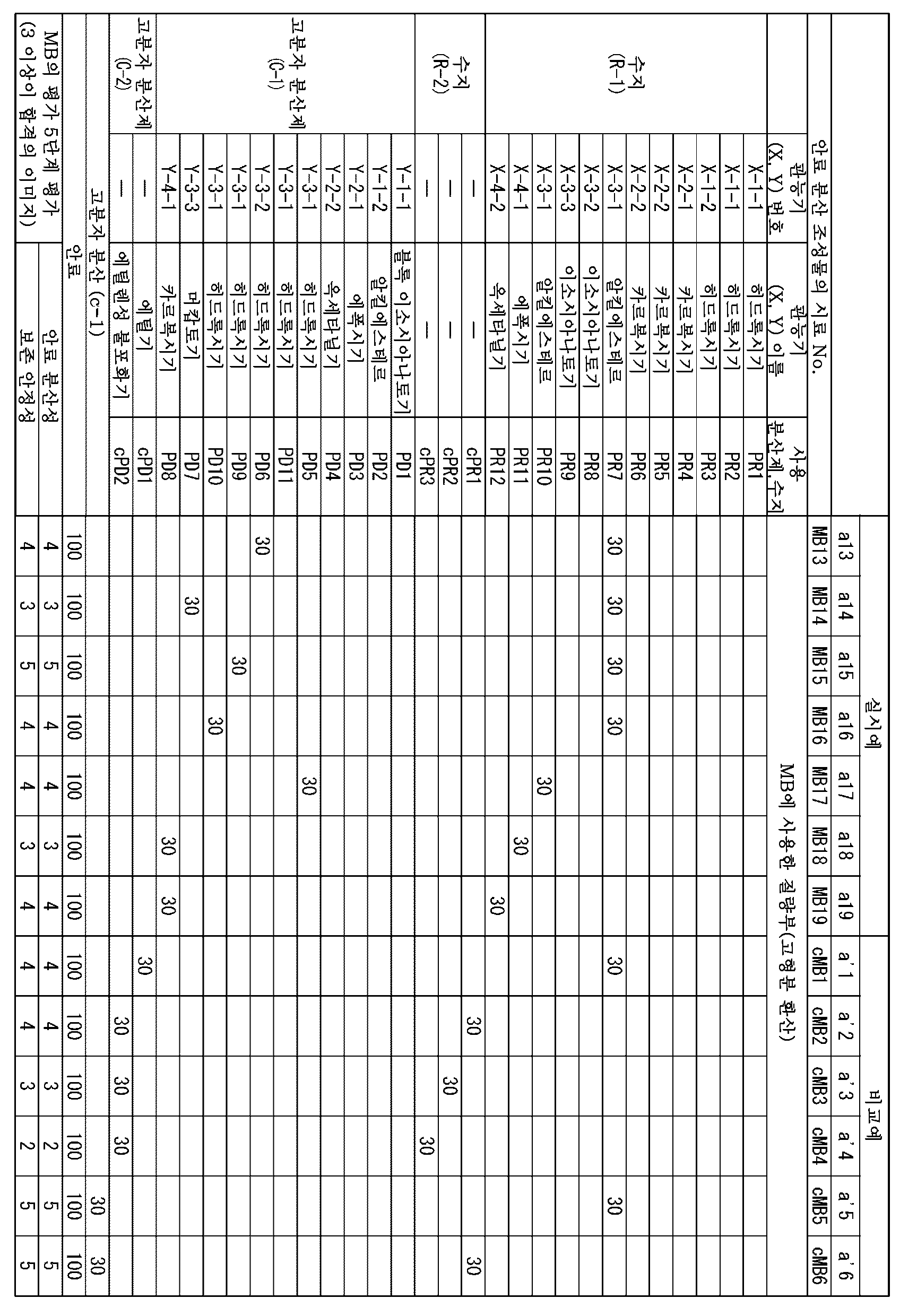
안료 분산성이나 보존 안정성이 양호한 안료 분산 조성물을 제공한다. 또한, 당해 안료 분산 조성물을 감광성 착색 조성물에 사용함으로써, 저온에서 경화가 진행되고, 우수한 내용제성을 갖고, 현상성이 양호한 경화물이 얻어진다. 또한, 당해 감광성 착색 조성물의 경화물을 갖는 컬러 필터, 이것을 구비하는 화상 표시 소자를 제공한다. 본 발명의 안료 분산 조성물은, 결합제 수지 (A-1)과, 안료 (B)와, 고분자 분산제 (C)와, 용제 (D-1)을 함유한다. 상기 결합제 수지 (A-1)이, 관능기 (X)를 갖고, 상기 고분자 분산제 (C)가, 상기 관능기 (X)와 반응성을 갖는 관능기 (Y)를 갖는다.A pigment dispersion composition having good pigment dispersibility and storage stability is provided. Furthermore, by using the pigment dispersion composition in a photosensitive coloring composition, curing proceeds at low temperature, and a cured product having excellent solvent resistance and good developability is obtained. Additionally, a color filter comprising a cured product of the photosensitive coloring composition and an image display element comprising the same are provided. The pigment dispersion composition of the present invention contains a binder resin (A-1), a pigment (B), a polymer dispersant (C), and a solvent (D-1). The binder resin (A-1) has a functional group (X), and the polymer dispersant (C) has a functional group (Y) that is reactive with the functional group (X).
Description
본 발명은 안료 분산 조성물, 감광성 착색 조성물 및 컬러 필터에 관한 것이다.The present invention relates to pigment dispersion compositions, photosensitive coloring compositions and color filters.
본원은, 2021년 12월 23일에, 일본에 출원된 일본 특허 출원 제2021-209989호에 기초하여 우선권을 주장하고, 그 내용을 여기에 원용한다.This application claims priority based on Japanese Patent Application No. 2021-209989, filed in Japan on December 23, 2021, and uses the content here.
현재, 자원 절약 및 에너지 절약의 관점에서, 각종 코팅, 인쇄, 도료, 접착제 등의 분야에 있어서, 자외선, 전자선 등의 활성 에너지선에 의해 경화 가능한 감광성 수지 조성물이 널리 사용되고 있다. 감광성 수지 조성물은, 전자 재료의 분야에 있어서, 프린트 배선 기판 등의 솔더 레지스트, 액정이나 유기 EL 등의 디스플레이의 컬러 필터용 레지스트 등에 사용되고 있다.Currently, from the viewpoint of resource conservation and energy conservation, photosensitive resin compositions that can be cured by active energy rays such as ultraviolet rays and electron beams are widely used in various fields such as coatings, printing, paints, and adhesives. Photosensitive resin compositions are used in the field of electronic materials, such as solder resists for printed wiring boards and other resists for color filters in displays such as liquid crystal and organic EL.
컬러 필터는, 일반적으로, 유리 기판 등의 투명 기판과, 투명 기판 상에 형성된 적색, 녹색 및 청색의 화소와, 화소의 경계에 형성되는 블랙 매트릭스와, 화소 및 블랙 매트릭스 상에 형성되는 보호막으로 구성된다. 이와 같은 구성을 갖는 컬러 필터는, 통상 투명 기판 상에 블랙 매트릭스나 화소와 같은 착색 패턴이나, 보호막과 같은 패턴을 순차 형성함으로써 제조된다. 각종 패턴의 형성 방법으로서는, 다양한 방법이 제안되어 있다. 그 중에서, 안료/염료가 분산된 감광성 수지 조성물을 레지스트로서 사용하고, 도포, 노광, 현상 및 베이킹을 반복하는 포토리소그래피 공법으로 제작된 컬러 필터는, 내구성이 우수하고, 핀 홀 등의 결함이 적은 착색 패턴을 부여한다. 그 때문에, 포토리소그래피 공법이, 현재의 주류로 되어 있다.A color filter generally consists of a transparent substrate such as a glass substrate, red, green, and blue pixels formed on the transparent substrate, a black matrix formed at the boundary of the pixel, and a protective film formed on the pixel and the black matrix. do. A color filter having such a structure is usually manufactured by sequentially forming a colored pattern such as a black matrix or pixel, or a pattern such as a protective film on a transparent substrate. As a method of forming various patterns, various methods have been proposed. Among them, color filters manufactured using a photosensitive resin composition in which pigments/dyes are dispersed as a resist and using a photolithography method that repeats application, exposure, development, and baking have excellent durability and fewer defects such as pinholes. Gives a coloring pattern. For that reason, the photolithography method is currently mainstream.
일반적으로, 포토리소그래피 공법에 사용되는 감광성 수지 조성물은, 알칼리 가용성이 있는 결합제 수지, 반응성 희석제, 광중합 개시제, 안료/염료(착색제라고도 함) 및 용제를 함유한다. 이 감광성 수지 조성물을 사용한 컬러 필터를 제작하는 방법에서는, 블랙 매트릭스, 적색, 녹색, 청색의 패턴을 반복하여 형성하는 점에서, 감광성 수지 조성물의 경화물에는, 제조 공정 중에 노출되는 각종 용제에 대한 내성이 요구된다.Generally, the photosensitive resin composition used in the photolithography method contains an alkali-soluble binder resin, a reactive diluent, a photopolymerization initiator, a pigment/dye (also called a colorant), and a solvent. In the method of producing a color filter using this photosensitive resin composition, a black matrix and a pattern of red, green, and blue are formed repeatedly, and the cured product of the photosensitive resin composition has resistance to various solvents exposed during the manufacturing process. This is required.
게다가, 유기 EL의 발광층은, 열에 약한 부재를 사용하고 있는 경우가 많고, 낮은 경화 온도에서 경화하는 컬러 필터가 요구되도록 되어 있다.In addition, the organic EL light-emitting layer often uses members that are weak to heat, and a color filter that cures at a low curing temperature is required.
근년, 액정이나 유기 EL 등의 디스플레이에는 고화질화, 고정밀화가 요구되고 있고, 컬러 필터에 대해서도 고휘도화 및 색 재현 범위의 확대를 달성하는 설계가 요구되고 있다. 고휘도화나 색 재현 범위 확대를 실현하기 위해서, 착색제로서 염료만을 사용하는 예도 보이지만, 염료는 안료에 비해 내열성이나 용제 내성이 떨어지고, 사용 비율이나 종류가 한정되기 때문에, 많은 경우에는 색재에 안료를 함유하고 있다. 안료를 사용하여 컬러 필터를 형성하는 경우, 안료의 균일한 미세화가 불가결하다. 안료를 미세화함으로써, 컬러 필터를 투과하는 광의 안료 입자에 의한 산란이 저감되어, 투과율에 대한 기여가 높고, 고휘도화가 달성된다.In recent years, displays such as liquid crystal and organic EL have been required to have higher image quality and higher precision, and color filters have also been required to be designed to achieve higher brightness and an expanded color reproduction range. In order to achieve higher brightness or expand the color reproduction range, there are examples of using only dye as a colorant, but dyes have lower heat resistance and solvent resistance than pigments, and the rate and type of use are limited, so in many cases, pigments are added to the colorant. there is. When forming a color filter using a pigment, uniform micronization of the pigment is essential. By refining the pigment, scattering of light passing through the color filter by the pigment particles is reduced, contributing to the transmittance is high, and high brightness is achieved.
그러나, 미세화된 안료 입자는 응집하기 쉽고, 안료의 분산성이나 안료 분산 조성물의 보존 안정성이 저하되기 쉬운 문제가 있었다. 안료 분산 조성물에 있어서 미세화된 안료의 분산성을 향상시키는 방법으로서, 안료와 분산제를 병용하는 것이 유효하다.However, micronized pigment particles are prone to aggregation, and there is a problem that the dispersibility of the pigment and the storage stability of the pigment dispersion composition tend to deteriorate. As a method of improving the dispersibility of micronized pigment in a pigment dispersion composition, it is effective to use a pigment and a dispersant in combination.
또한, 색 재현 범위를 확대하기 위해서, 착색제를 고농도 배합하는 대응도 행해지고 있다. 착색제의 고농도화에 수반하여, 분산제의 농도나 다른 조성물의 농도가 상대적으로 저감하기 때문에, 보다 소량의 분산제라도, 안료 분산성이나 안료 분산 조성물의 보존 안정성, 내열성, 내용제성, 패턴 밀착성 등의 각종 레지스트 특성을 발현할 것이 요구된다.Additionally, in order to expand the color reproduction range, efforts are being made to blend colorants in high concentrations. As the concentration of the colorant increases, the concentration of the dispersant and other compositions relatively decreases, so even with a smaller amount of the dispersant, the pigment dispersibility, storage stability, heat resistance, solvent resistance, and pattern adhesion of the pigment dispersion composition are affected. It is required to exhibit resist characteristics.
일반적으로 분산제는, 안료에 흡착되는 부위와, 용제나 다른 수지 조성물과 친화성이 높은 부위를 가지고 있다. 안료에 흡착되는 부위는, 안료의 표면 상태에 맞춰서 최적 구조가 변화된다. 예를 들어, 산성에 치우친 표면을 갖는 안료에는, 염기성의 흡착 부위를 갖는 분산제가 사용되고, 많은 경우, 특허문헌 1과 같이 염기성의 흡착 부위에는 아미노기가 사용된다.Generally, a dispersant has a site that is adsorbed to a pigment and a site that has high affinity with a solvent or other resin composition. The optimal structure of the site adsorbed to the pigment changes depending on the surface condition of the pigment. For example, a dispersant having a basic adsorption site is used for a pigment having a surface biased toward acidity, and in many cases, an amino group is used for the basic adsorption site as in Patent Document 1.
또한, 근년에는, 특허문헌 1 내지 3과 같이, 분산제에 감광성기인 에틸렌성 불포화기를 도입하고, 경화 기능을 부여하여, 보다 좋게 광경화하는 내용제성이 우수한 분산제도 개발되고 있다.In addition, in recent years, as in Patent Documents 1 to 3, dispersants excellent in solvent resistance have been developed by introducing an ethylenically unsaturated group, which is a photosensitive group, into the dispersant, imparting a curing function, and improving photocuring.
그러나, 특허문헌 1 내지 3에 개시되는 분산제를 사용한 경우, 내용제성이 양호한 경화물이 얻어지지만, 안료 분산성이 불충분하여 단독으로의 사용이 곤란한 경우나, 안료 분산 조성물의 보존 안정성도 개선이 필요하였다. 또한, 도입되고 있는 경화성기가 에틸렌성 불포화기이기 때문에, 높은 경화 온도가 필요해지고, 열에 약한 재료 등과 병용할 수 없어, 개선이 요구되고 있었다.However, when the dispersant disclosed in Patent Documents 1 to 3 is used, a cured product with good solvent resistance is obtained, but the pigment dispersibility is insufficient and use alone is difficult, and the storage stability of the pigment dispersion composition also needs to be improved. did. In addition, since the curable group being introduced is an ethylenically unsaturated group, a high curing temperature is required, and it cannot be used in combination with materials vulnerable to heat, etc., so improvements have been required.
본 발명은, 상기와 같은 과제를 해결하기 위하여 이루어진 것이고, 안료 분산성이나 보존 안정성이 양호한 안료 분산 조성물을 제공하는 것을 목적으로 한다. 또한, 본 발명은, 본 안료 분산 조성물을 사용한 경우, 저온에서 경화하고, 우수한 내용제성을 갖는 현상성이 양호한 경화물이 얻어지는 감광성 착색 조성물을 제공하는 것을 목적으로 하고, 그 경화물을 갖는 컬러 필터, 이것을 구비하는 화상 표시 소자를 제공하는 것을 목적으로 한다.The present invention was made to solve the problems described above, and its purpose is to provide a pigment dispersion composition with good pigment dispersibility and good storage stability. Another object of the present invention is to provide a photosensitive coloring composition that cures at a low temperature when using the present pigment dispersion composition and gives a cured product with excellent solvent resistance and good developability, and a color filter having the cured product. The purpose is to provide an image display element having this.
본 발명은 이하의 양태를 포함한다.The present invention includes the following aspects.
〔1〕 결합제 수지 (A-1)과,[1] Binder resin (A-1),
안료 (B)와,Pigment (B),
고분자 분산제 (C)와,A polymer dispersant (C),
용제 (D-1)Solvent (D-1)
을 함유하는 안료 분산 조성물이며,It is a pigment dispersion composition containing,
상기 결합제 수지 (A-1)이, 아미노기 및 4급 암모늄 양이온기에서 선택되는 1종 이상을 갖고, 관능기 (X)를 갖고,The binder resin (A-1) has at least one selected from amino groups and quaternary ammonium cation groups, and has a functional group (X),
상기 고분자 분산제 (C)가, 상기 관능기 (X)와 반응성을 갖는 관능기 (Y)를 갖는 것을 특징으로 하는 안료 분산 조성물.A pigment dispersion composition characterized in that the polymer dispersant (C) has a functional group (Y) that is reactive with the functional group (X).
〔2〕 상기 고분자 분산제 (C)가, 아미노기 함유 고분자 화합물 (c-1)과, 상기 관능기 (Y)를 갖는 할로겐 화합물 (c-2)의 반응물인 〔1〕에 기재된 안료 분산 조성물.[2] The pigment dispersion composition according to [1], wherein the polymer dispersant (C) is a reaction product of the amino group-containing polymer compound (c-1) and the halogen compound (c-2) having the functional group (Y).
〔3〕 상기 아미노기 함유 고분자 화합물 (c-1)의 아미노기가 3급 아미노기인 〔2〕에 기재된 안료 분산 조성물.[3] The pigment dispersion composition according to [2], wherein the amino group of the amino group-containing polymer compound (c-1) is a tertiary amino group.
〔4〕 상기 결합제 수지 (A-1)이 갖는 관능기 (X)가, 히드록시기 및 머캅토기에서 선택되는 1종 이상이고,[4] The functional group (X) of the binder resin (A-1) is at least one selected from hydroxy groups and mercapto groups,
상기 고분자 분산제 (C)가 갖는 관능기 (Y)가, 블록 이소시아나토기 및 알킬에스테르를 갖는 기에서 선택되는 1종 이상인 〔1〕 내지 〔3〕의 어느 것에 기재된 안료 분산 조성물.The pigment dispersion composition according to any one of [1] to [3], wherein the functional group (Y) of the polymer dispersant (C) is at least one selected from the group consisting of a block isocyanato group and an alkyl ester.
〔5〕 상기 결합제 수지 (A-1)이 갖는 관능기 (X)가, 카르복시기이고, 상기 고분자 분산제 (C)가 갖는 관능기 (Y)가, 에폭시기 및 옥세타닐기에서 선택되는 1종 이상인 〔1〕 내지 〔3〕의 어느 것에 기재된 안료 분산 조성물.[5] [1] wherein the functional group (X) of the binder resin (A-1) is a carboxyl group, and the functional group (Y) of the polymer dispersant (C) is at least one selected from epoxy groups and oxetanyl groups. The pigment dispersion composition according to any one of [3] to [3].
〔6〕 상기 결합제 수지 (A-1)이 갖는 관능기 (X)가, 블록 이소시아나토기 및 알킬에스테르를 갖는 기에서 선택되는 1종 이상이고,[6] The functional group (X) of the binder resin (A-1) is at least one selected from the group consisting of a block isocyanato group and an alkyl ester,
상기 고분자 분산제 (C)가 갖는 관능기 (Y)가, 히드록시기 및 머캅토기에서 선택되는 1종 이상인 〔1〕 내지 〔3〕의 어느 것에 기재된 안료 분산 조성물.The pigment dispersion composition according to any one of [1] to [3], wherein the functional group (Y) of the polymer dispersant (C) is at least one selected from a hydroxy group and a mercapto group.
〔7〕 상기 결합제 수지 (A-1)이 갖는 관능기 (X)가, 에폭시기 및 옥세타닐기에서 선택되는 1종 이상이고,[7] The functional group (X) of the binder resin (A-1) is at least one selected from an epoxy group and an oxetanyl group,
상기 고분자 분산제 (C)가 갖는 관능기 (Y)가, 카르복시기인 〔1〕 내지 〔3〕의 어느 것에 기재된 안료 분산 조성물.The pigment dispersion composition according to any one of [1] to [3], wherein the functional group (Y) of the polymer dispersant (C) is a carboxyl group.
〔8〕 상기 안료 (B)가, 할로겐화 프탈로시아닌 골격을 갖는 안료를 포함하는 〔1〕 내지 〔7〕의 어느 것에 기재된 안료 분산 조성물.[8] The pigment dispersion composition according to any one of [1] to [7], wherein the pigment (B) contains a pigment having a halogenated phthalocyanine skeleton.
〔9〕 상기 안료 (B) 100질량부에 대하여,[9] For 100 parts by mass of the above pigment (B),
상기 결합제 수지 (A-1)을 10 내지 80질량부 함유하고,Containing 10 to 80 parts by mass of the binder resin (A-1),
상기 고분자 분산제 (C)를 10 내지 80질량부 함유하는 〔1〕 내지 〔8〕의 어느 것에 기재된 안료 분산 조성물.The pigment dispersion composition according to any one of [1] to [8], containing 10 to 80 parts by mass of the polymer dispersant (C).
〔10〕 〔1〕 내지 〔9〕의 어느 것에 기재된 안료 분산 조성물과,[10] The pigment dispersion composition according to any one of [1] to [9],
결합제 수지 (A-2)와,Binder resin (A-2),
반응성 희석제 (E)와,a reactive diluent (E),
광중합 개시제 (F)Photopolymerization initiator (F)
를 함유하는 것을 특징으로 하는 감광성 착색 조성물.A photosensitive coloring composition comprising:
〔11〕 상기 안료 (B) 100질량부에 대하여,[11] For 100 parts by mass of the above pigment (B),
상기 결합제 수지 (A-1)을 10 내지 80질량부 함유하고,Containing 10 to 80 parts by mass of the binder resin (A-1),
상기 고분자 분산제 (C)를 10 내지 80질량부 함유하고,Containing 10 to 80 parts by mass of the polymer dispersant (C),
상기 결합제 수지 (A-2)를 50 내지 280질량부 함유하고,Containing 50 to 280 parts by mass of the binder resin (A-2),
상기 반응성 희석제 (E)를 40 내지 200질량부 함유하고,Containing 40 to 200 parts by mass of the reactive diluent (E),
상기 광중합 개시제 (F)를 0.1 내지 10질량부 함유하는Containing 0.1 to 10 parts by mass of the photopolymerization initiator (F)
〔10〕에 기재된 감광성 착색 조성물.The photosensitive coloring composition according to [10].
〔12〕 〔10〕 또는 〔11〕에 기재된 감광성 착색 조성물을 경화시켜 이루어지는 수지 경화막.[12] A resin cured film obtained by curing the photosensitive coloring composition according to [10] or [11].
〔13〕 〔10〕 또는 〔11〕에 기재된 감광성 착색 조성물의 경화물을 갖는 컬러 필터.[13] A color filter having a cured product of the photosensitive coloring composition according to [10] or [11].
〔14〕 청구항 13에 기재된 컬러 필터를 구비하는 화상 표시 소자.[14] An image display element including the color filter according to claim 13.
본 발명에 따르면, 안료 분산성이나 보존 안정성이 양호한 안료 분산 조성물을 제공할 수 있다. 또한, 당해 안료 분산 조성물을 감광성 착색 조성물에 사용함으로써, 저온에서 경화가 진행되고, 우수한 내용제성을 갖고, 현상성이 양호한 경화물이 얻어진다. 또한, 당해 감광성 착색 조성물의 경화물을 갖는 컬러 필터, 이것을 구비하는 화상 표시 소자를 제공할 수 있다.According to the present invention, it is possible to provide a pigment dispersion composition with good pigment dispersibility and good storage stability. Furthermore, by using the pigment dispersion composition in a photosensitive coloring composition, curing proceeds at low temperature, and a cured product having excellent solvent resistance and good developability is obtained. Additionally, a color filter containing a cured product of the photosensitive coloring composition and an image display element containing the same can be provided.
이하, 본 발명의 실시 형태에 대하여 상세하게 설명한다. 단, 본 발명은 이하에 기재하는 실시 형태에 한정되는 것은 아니다.Hereinafter, embodiments of the present invention will be described in detail. However, the present invention is not limited to the embodiments described below.
이하, 본 발명의 실시 형태에 대하여 상세하게 설명한다. 단, 본 발명은 이하에 기재하는 실시 형태에 한정되는 것은 아니다. 또한, 본 명세서에 있어서 「(메트)아크릴로일옥시기」란, 메타크릴로일옥시기 및 아크릴로일옥시기에서 선택되는 1종 이상을 나타낸다. 「(메트)아크릴산」도 마찬가지이다.Hereinafter, embodiments of the present invention will be described in detail. However, the present invention is not limited to the embodiments described below. In addition, in this specification, “(meth)acryloyloxy group” refers to one or more types selected from methacryloyloxy group and acryloyloxy group. The same applies to “(meth)acrylic acid.”
<안료 분산 조성물><Pigment dispersion composition>
본 실시 형태의 안료 분산 조성물은, 결합제 수지 (A-1)과, 안료 (B)와, 고분자 분산제 (C)와, 용제 (D-1)을 함유한다.The pigment dispersion composition of this embodiment contains binder resin (A-1), pigment (B), polymer dispersant (C), and solvent (D-1).
〔결합제 수지 (A-1)〕[Binder Resin (A-1)]
본 실시 형태의 결합제 수지 (A-1)은, 아미노기나 4급 암모늄 구조를 갖지 않고, 관능기 (X)를 갖는다. 결합제 수지 (A-1)을 사용함으로써, 관능기 (X)가, 후술하는 고분자 분산제 (C)가 갖는 상기 관능기 (X)와 반응성을 갖는 관능기 (Y)와 가교하기 때문에, 감광성 착색 조성물로서 사용했을 때에 우수한 저온 경화성을 발현한다. 또한, 저온에서 경화시킨 경우에도 얻어진 수지 경화막은 우수한 내용제성을 발현한다.The binder resin (A-1) of this embodiment does not have an amino group or a quaternary ammonium structure, but has a functional group (X). By using the binder resin (A-1), the functional group (X) is crosslinked with the functional group (Y) that is reactive with the functional group (X) contained in the polymer dispersant (C) described later, so it can be used as a photosensitive coloring composition. It exhibits excellent low-temperature curing properties. In addition, even when cured at low temperature, the obtained resin cured film exhibits excellent solvent resistance.
결합제 수지 (A-1)이 갖는 관능기 (X)로서는, 아미노기나 4급 암모늄 구조를 갖지 않고, 후술하는 고분자 분산제 (C)가 갖는 상기 관능기 (Y)와, 가열에 의해 가교할 수 있는 기라면 특별히 한정되지 않는다. 관능기 (X)로서 예를 들어, 히드록시기, 카르복시기, 블록 이소시아나토기, 에폭시기, 옥세타닐기, 알킬에스테르기(바람직하게는, 탄소수 1 내지 3의 알킬에스테르기), 머캅토기 등을 들 수 있다. 이들 중에서도, 높은 반응성을 갖고, 밀 베이스로 사용했을 때의 안정성의 관점에서, 히드록시기, 블록 이소시아나토기가 바람직하다. 이들의 관능기 (X)는 1종을 단독으로 포함하고 있어도, 2종 이상을 포함하고 있어도 된다.The functional group (X) possessed by the binder resin (A-1) is a group that does not have an amino group or a quaternary ammonium structure and can be crosslinked by heating with the functional group (Y) possessed by the polymer dispersant (C) described later. There is no particular limitation. Examples of the functional group (X) include a hydroxy group, a carboxyl group, a block isocyanato group, an epoxy group, an oxetanyl group, an alkyl ester group (preferably an alkyl ester group having 1 to 3 carbon atoms), and a mercapto group. . Among these, a hydroxy group and a block isocyanato group are preferable because they have high reactivity and from the viewpoint of stability when used as a mill base. These functional groups (X) may be included individually or in combination of two or more.
결합제 수지 (A-1)의 종류로서는, 네가티브형 레지스트에 일반적으로 사용되는 결합제 수지라면 특별히 한정되지 않는다. 예를 들어, (메트)아크릴 수지, 비닐에스테르 수지 등을 들 수 있다. 그 중에서도, 감광성 착색 조성물로서 우수한 현상성을 갖는 점에서, (메트)아크릴 수지가 바람직하다.The type of binder resin (A-1) is not particularly limited as long as it is a binder resin generally used in negative resists. For example, (meth)acrylic resin, vinyl ester resin, etc. can be mentioned. Among them, (meth)acrylic resin is preferable because it has excellent developability as a photosensitive coloring composition.
(메트)아크릴 수지로서는, 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물과, (메트)아크릴레이트 모노머의 공중합체가 바람직하다. 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물은, 관능기 (X)와 에틸렌성 불포화기를 갖는 화합물이다. 에틸렌성 불포화기로서는, 비닐기, 알릴기, (메트)아크릴로일기 등을 들 수 있다.As the (meth)acrylic resin, a copolymer of an ethylenically unsaturated group-containing compound having a functional group (X) and a (meth)acrylate monomer is preferable. An ethylenically unsaturated group-containing compound having a functional group (X) is a compound having a functional group (X) and an ethylenically unsaturated group. Examples of the ethylenically unsaturated group include vinyl group, allyl group, and (meth)acryloyl group.
관능기 (X)가 히드록시기인 경우, 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물로서는, 예를 들어 2-히드록시에틸(메트)아크릴레이트, 4-히드록시부틸(메트)아크릴레이트, 1,6-헥산디올(메트)아크릴레이트, 3-메틸펜탄디올(메트)아크릴레이트 등의 히드록시기를 갖는 에틸렌성 불포화기 함유 화합물을 들 수 있다.When the functional group (X) is a hydroxy group, examples of the ethylenically unsaturated group-containing compound having the functional group (X) include 2-hydroxyethyl (meth)acrylate, 4-hydroxybutyl (meth)acrylate, 1, and ethylenically unsaturated group-containing compounds having a hydroxy group, such as 6-hexanediol (meth)acrylate and 3-methylpentanediol (meth)acrylate.
관능기 (X)가 카르복시기인 경우, 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물로서는, 예를 들어 (메트)아크릴산, 이타콘산, 크로톤산, 신남산, 2-(메트)아크릴로일옥시에틸숙신산, 2-(메트)아크릴로일옥시에틸프탈산, 2-(메트)아크릴로일옥시에틸헥사히드로프탈산, α-브로모(메트)아크릴산, β-푸릴(메트)아크릴산, 크로톤산, 프로피올산, 신남산, α-시아노신남산, 말레산모노메틸, 말레산모노에틸, 말레산모노이소프로필, 푸마르산모노메틸, 이타콘산모노에틸 등의 불포화 일염기산, 말레산모노메틸, 말레산모노에틸, 말레산모노이소프로필, 푸마르산모노메틸, 이타콘산모노에틸 등의 불포화 이염기산모노에스테르, 혹은, 카르복시기를 갖는 에틸렌성 불포화기 함유 화합물을 들 수 있다.When the functional group (X) is a carboxyl group, examples of the ethylenically unsaturated group-containing compound having the functional group (X) include (meth)acrylic acid, itaconic acid, crotonic acid, cinnamic acid, and 2-(meth)acryloyloxyethyl. Succinic acid, 2-(meth)acryloyloxyethylphthalic acid, 2-(meth)acryloyloxyethylhexahydrophthalic acid, α-bromo(meth)acrylic acid, β-furyl(meth)acrylic acid, crotonic acid, propiolic acid , unsaturated monobasic acids such as cinnamic acid, α-cyanocinnamic acid, monomethyl maleate, monoethyl maleate, monoisopropyl maleate, monomethyl fumarate, monoethyl itaconate, monomethyl maleate, monoethyl maleate , unsaturated dibasic acid monoesters such as monoisopropyl maleate, monomethyl fumarate, and monoethyl itaconate, or compounds containing an ethylenically unsaturated group having a carboxyl group.
관능기 (X)가 블록 이소시아나토기인 경우, 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물로서는, 예를 들어 2-이소시아나토에틸(메트)아크릴레이트, 2-이소시아나토프로필(메트)아크릴레이트, 3-이소시아나토프로필(메트)아크릴레이트, 2-이소시아나토-1-메틸에틸(메트)아크릴레이트, 2-이소시아나토-1,1-디메틸에틸(메트)아크릴레이트, 4-이소시아나토시클로헥실(메트)아크릴레이트, 메타크릴로일이소시아네이트 등의 이소시아네이트 화합물에 있어서의 이소시아나토기를 블록제로 블록화한 화합물 등의 블록 이소시아나토기를 갖는 에틸렌성 불포화기 함유 화합물을 들 수 있다.When the functional group (X) is a block isocyanato group, examples of the ethylenically unsaturated group-containing compound having the functional group ( ) Acrylate, 3-isocyanatopropyl (meth)acrylate, 2-isocyanato-1-methylethyl (meth)acrylate, 2-isocyanato-1,1-dimethylethyl (meth)acrylate, Compounds containing an ethylenically unsaturated group having a blocked isocyanato group, such as compounds obtained by blocking the isocyanato group in isocyanate compounds such as 4-isocyanatocyclohexyl (meth)acrylate and methacryloyl isocyanate with a blocking agent. can be mentioned.
관능기 (X)가 에폭시기인 경우, 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물로서는, 예를 들어 글리시딜(메트)아크릴레이트, 3,4-에폭시시클로헥실메틸(메트)아크릴레이트, 지환식 에폭시기를 갖는 (메트)아크릴레이트 및 그의 락톤 부가물, 3,4-에폭시시클로헥실메틸-3',4'-에폭시시클로헥산카르복실레이트, 디시클로펜테닐(메트)아크릴레이트의 에폭시화물, 그리고 디시클로펜테닐옥시에틸(메트)아크릴레이트의 에폭시화물 등의 에폭시기 함유 (메트)아크릴레이트 등의 에폭시기를 갖는 에틸렌성 불포화기 함유 화합물을 들 수 있다.When the functional group (X) is an epoxy group, examples of ethylenically unsaturated group-containing compounds having the functional group (X) include glycidyl (meth)acrylate, 3,4-epoxycyclohexylmethyl (meth)acrylate, and alicyclic (meth)acrylate having an epoxy group and its lactone adduct, 3,4-epoxycyclohexylmethyl-3',4'-epoxycyclohexanecarboxylate, epoxide of dicyclopentenyl (meth)acrylate, And ethylenically unsaturated group-containing compounds having an epoxy group, such as epoxy group-containing (meth)acrylate, such as an epoxidized product of dicyclopentenyloxyethyl (meth)acrylate.
관능기 (X)가 옥세타닐기인 경우, 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물로서는, 예를 들어 (3-에틸옥세탄-3-일)메틸(메트)아크릴레이트, 4-[3-(3-에틸옥세탄-3-일메톡시)프로폭시]스티렌, 4-[6-(3-에틸옥세탄-3-일메톡시)헥실옥시]스티렌, 4-[5-(3-에틸옥세탄-3-일메톡시)펜틸옥시]스티렌, 2-비닐-2-메틸옥세탄 등의 옥세타닐기를 갖는 에틸렌성 불포화기 함유 화합물을 들 수 있다.When the functional group (X) is an oxetanyl group, examples of the ethylenically unsaturated group-containing compound having the functional group (X) include (3-ethyloxetan-3-yl)methyl (meth)acrylate, 4-[3 -(3-ethyloxetan-3-ylmethoxy)propoxy]styrene, 4-[6-(3-ethyloxetan-3-ylmethoxy)hexyloxy]styrene, 4-[5-(3-ethyl and ethylenically unsaturated group-containing compounds having an oxetanyl group, such as oxetan-3-ylmethoxy)pentyloxy]styrene and 2-vinyl-2-methyloxetane.
관능기 (X)가 알킬에스테르기인 경우, 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물로서는, 예를 들어 메틸(메트)아크릴레이트, 에틸(메트)아크릴레이트, 시트라콘산디에틸, 말레산디에틸, 푸마르산디에틸, 이타콘산디에틸 등의 탄소수 1 내지 3의 알킬에스테르기를 갖는 에틸렌성 불포화기 함유 화합물을 들 수 있다.When the functional group ( and ethylenically unsaturated group-containing compounds having an alkyl ester group having 1 to 3 carbon atoms, such as diethyl fumarate and diethyl itaconate.
관능기 (X)가 머캅토기인 경우, 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물로서는, 예를 들어 2-술파닐에틸(메트)아크릴레이트, 3-술파닐프로필(메트)아크릴레이트 또는 4-술파닐부틸(메트)아크릴레이트, 2-비닐벤젠티올, 11-브로모-1-운데칸티올 등의 머캅토기를 갖는 에틸렌성 불포화기 함유 화합물 등을 들 수 있다.When the functional group (X) is a mercapto group, examples of the ethylenically unsaturated group-containing compound having the functional group ( -Ethylenically unsaturated group-containing compounds having a mercapto group such as sulfanylbutyl (meth)acrylate, 2-vinylbenzenethiol, and 11-bromo-1-undecanethiol can be mentioned.
상기 이소시아네이트 화합물을 블록화하는 상기 블록제로서는, 예를 들어 ε-카프로락탐, δ-발레로락탐, γ-부티로락탐, β-프로피오락탐 등의 락탐계; 메탄올, 에탄올, 프로판올, 부탄올, 에틸렌글리콜, 메틸셀로솔브, 부틸셀로솔브, 메틸 카르비톨, 벤질알코올, 페닐셀로솔브, 푸르푸릴알코올, 시클로헥산올 등의 알코올계; 페놀, 크레졸, 크실레놀, 에틸페놀, o-이소프로필페놀, p-tert-부틸페놀 등의 부틸페놀, p-tert-옥틸페놀, 노닐페놀, 디노닐페놀, 스티렌화 페놀, 옥시벤조산에스테르, 티몰, p-나프톨, p-니트로페놀, p-클로로페놀 등의 페놀계; 말론산디메틸, 말론산디에틸, 아세토아세트산메틸, 아세토아세트산에틸, 아세틸아세톤 등의 활성 메틸렌계; 부틸머캅탄, 티오페놀, tert-도데실머캅탄 등의 머캅탄계; 디페닐아민, 페닐나프틸아민, 아닐린, 카르바졸 등의 아민계; 아세트아닐리드, 아세트아니시디드, 아세트산아미드, 벤즈아미드 등의 산 아미드계; 숙신산이미드, 말레산이미드 등의 산 이미드계; 이미다졸, 2-메틸이미다졸, 2-에틸이미다졸 등의 이미다졸계; 요소, 티오요소, 에틸렌요소 등의 요소계; N-페닐카르밤산페닐, 2-옥사졸리돈 등의 카르밤산염계: 에틸렌이민, 폴리에틸렌이민 등의 이민계; 포름알독심, 아세트알독심, 아세톡심, 메틸에틸케톡심, 메틸이소부틸케톡심, 시클로헥사논옥심 등의 옥심계; 중아황산소다, 중아황산칼륨 등의 중 아황산염계 등을 들 수 있다. 이들 중에서도, 감광성 착색 조성물로서의 저온 경화성이 양호한 관점에서, 상기 블록제는 말론산디에틸, 3,5-디메틸피라졸 및 메틸에틸케톡심이 바람직하고, 3,5-디메틸피라졸 및 메틸에틸케톡심이 보다 바람직하다.Examples of the blocking agent that blocks the isocyanate compound include lactams such as ε-caprolactam, δ-valerolactam, γ-butyrolactam, and β-propiolactam; Alcohols such as methanol, ethanol, propanol, butanol, ethylene glycol, methyl cellosolve, butyl cellosolve, methyl carbitol, benzyl alcohol, phenyl cellosolve, furfuryl alcohol, and cyclohexanol; Phenol, cresol, xylenol, ethyl phenol, o-isopropyl phenol, butyl phenol such as p-tert-butyl phenol, p-tert-octyl phenol, nonyl phenol, dinonyl phenol, styrenated phenol, oxybenzoic acid ester, phenols such as thymol, p-naphthol, p-nitrophenol, and p-chlorophenol; Active methylene series such as dimethyl malonate, diethyl malonate, methyl acetoacetate, ethyl acetoacetate, and acetylacetone; mercaptans such as butyl mercaptan, thiophenol, and tert-dodecyl mercaptan; Amines such as diphenylamine, phenylnaphthylamine, aniline, and carbazole; Acid amide series such as acetanilide, acetanisidide, acetic acid amide, and benzamide; Acid imide series such as succinimide and maleimide; Imidazole series such as imidazole, 2-methylimidazole, and 2-ethylimidazole; Urea systems such as urea, thiourea, and ethylene urea; Carbamate series such as phenyl N-phenylcarbamate and 2-oxazolidone; Imine series such as ethyleneimine and polyethyleneimine; oximes such as formaldoxime, acetaldoxime, acetoxime, methyl ethyl ketoxime, methyl isobutyl ketoxime, and cyclohexanone oxime; Bisulfite-based salts such as sodium bisulfite and potassium bisulfite can be mentioned. Among these, from the viewpoint of good low-temperature curing properties as a photosensitive coloring composition, diethyl malonate, 3,5-dimethylpyrazole, and methylethylketoxime are preferable as the blocking agent, and 3,5-dimethylpyrazole and methylethylketoxime are more preferable. desirable.
상기 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물 중에서도, 합성 용이성의 관점에서, 히드록시기를 갖는 에틸렌성 불포화기 함유 화합물, 카르복시기를 갖는 에틸렌성 불포화기 함유 화합물, 블록 이소시아나토기를 갖는 에틸렌성 불포화기 함유 화합물, 에폭시기를 갖는 에틸렌성 불포화기 함유 화합물이 바람직하고; 히드록시알킬(메트)아크릴레이트, (메트)아크릴산, 블록이소시아나토기 함유 (메트)아크릴레이트, 에폭시기 함유 (메트)아크릴레이트가 보다 바람직하고; 블록 이소시아나토기 함유 (메트)아크릴레이트가 더욱 바람직하다. 이들 화합물은 1종을 단독으로 사용해도, 2종 이상을 사용해도 된다.Among the ethylenically unsaturated group-containing compounds having the above-mentioned functional group ( Unsaturated group-containing compounds and ethylenically unsaturated group-containing compounds having an epoxy group are preferred; Hydroxyalkyl (meth)acrylates, (meth)acrylic acid, (meth)acrylates containing a block isocyanato group, and (meth)acrylates containing an epoxy group are more preferable; Block isocyanato group-containing (meth)acrylates are more preferred. These compounds may be used individually or in combination of two or more.
전술 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물과 공중합하여, 결합제 수지 (A-1)을 부여하는 (메트)아크릴레이트 모노머로서는, 상기 관능기 (X)를 갖지 않고, 상술한 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물과 공중합 가능한 (메트)아크릴레이트 모노머라면 특별히 한정되지 않는다. 상기 (메트)아크릴레이트 모노머로서는, 예를 들어 탄소수 4 이상의 알킬(메트)아크릴레이트, 지환식 알킬(메트)아크릴레이트, 방향족 함유 (메트)아크릴레이트 등을 들 수 있다.As a (meth)acrylate monomer that is copolymerized with an ethylenically unsaturated group-containing compound having the above-mentioned functional group ( There is no particular limitation as long as it is a (meth)acrylate monomer that can be copolymerized with an ethylenically unsaturated group-containing compound. Examples of the (meth)acrylate monomer include alkyl (meth)acrylate having 4 or more carbon atoms, alicyclic alkyl (meth)acrylate, and aromatic-containing (meth)acrylate.
상기 탄소수 4 이상의 알킬(메트)아크릴레이트로서는, 예를 들어 tert-부틸(메트)아크릴레이트, 펜틸(메트)아크릴레이트, 네오펜틸(메트)아크릴레이트, 2-에틸헥실(메트)아크릴레이트, 이소아밀(메트)아크릴레이트, 도데실(메트)아크릴레이트 등을 들 수 있다.Examples of the alkyl (meth)acrylate having 4 or more carbon atoms include tert-butyl (meth)acrylate, pentyl (meth)acrylate, neopentyl (meth)acrylate, 2-ethylhexyl (meth)acrylate, iso Amyl (meth)acrylate, dodecyl (meth)acrylate, etc. are mentioned.
상기 지환식 알킬(메트)아크릴레이트로서는, 예를 들어 시클로펜틸(메트)아크릴레이트, 시클로헥실(메트)아크릴레이트, 에틸시클로헥실(메트)아크릴레이트, 노르보르닐(메트)아크릴레이트, 디시클로펜타닐(메트)아크릴레이트, 이소보르닐(메트)아크릴레이트, 아다만틸(메트)아크릴레이트 등을 들 수 있다.Examples of the alicyclic alkyl (meth)acrylate include cyclopentyl (meth)acrylate, cyclohexyl (meth)acrylate, ethylcyclohexyl (meth)acrylate, norbornyl (meth)acrylate, and dicyclo. Fentanyl (meth)acrylate, isobornyl (meth)acrylate, adamantyl (meth)acrylate, etc. can be mentioned.
상기 방향족 함유 (메트)아크릴레이트로서는, 예를 들어 벤질(메트)아크릴레이트, 트리페닐메틸(메트)아크릴레이트, 쿠밀(메트)아크릴레이트, 나프탈렌(메트)아크릴레이트, 안트라센(메트)아크릴레이트 등을 들 수 있다.Examples of the aromatic-containing (meth)acrylate include benzyl (meth)acrylate, triphenylmethyl (meth)acrylate, cumyl (meth)acrylate, naphthalene (meth)acrylate, anthracene (meth)acrylate, etc. can be mentioned.
이들 중에서도, 밀 베이스로 사용했을 때의 분산 안정성의 관점에서, 탄소수 6 이상의 알킬(메트)아크릴레이트(예를 들어, 2-에틸헥실(메트)아크릴레이트), 디시클로펜타닐(메트)아크릴레이트, 벤질(메트)아크릴레이트가 바람직하다. 이들 화합물은 1종을 단독으로 사용해도, 2종 이상을 사용해도 된다.Among these, from the viewpoint of dispersion stability when used as a mill base, alkyl (meth)acrylates having 6 or more carbon atoms (for example, 2-ethylhexyl (meth)acrylate), dicyclofentanyl (meth)acrylate, Benzyl (meth)acrylate is preferred. These compounds may be used individually or in combination of two or more.
결합제 수지 (A-1)이 (메트)아크릴 수지인 경우, 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물 및 (메트)아크릴레이트 모노머에 추가하여, 다른 에틸렌성 불포화기 함유 모노머를 공중합시켜도 된다. 다른 에틸렌성 불포화기 함유 모노머로서는, 스티렌, 스티렌의 α-, o-, m-, p- 알킬 유도체 등의 스티렌류, 노르보르넨(비시클로[2.2.1]헵트-2-엔), 5-메틸비시클로[2.2.1]헵트-2-엔, 5-에틸비시클로[2.2.1]헵트-2-엔, 테트라시클로[4.4.0.12,5.17,10]도데카-3-엔, 8-메틸테트라시클로[4.4.0.12,5.17,10]도데카-3-엔, 8-에틸테트라시클로[4.4.0.12,5.17,10]도데카-3-엔, 디시클로펜타디엔, 트리시클로[5.2.1.02,6]데크-8-엔, 트리시클로[5.2.1.02,6]데크-3-엔, 트리시클로[4.4.0.12,5]운데카-3-엔, 트리시클로[6.2.1.01,8]운데카-9-엔, 트리시클로[6.2.1.01,8]운데카-4-엔, 테트라시클로[4.4.0.12,5.17,10.01,6]도데카-3-엔, 8-메틸테트라시클로[4.4.0.12,5.17,10.01,6]도데카-3-엔, 8-에틸리덴테트라시클로[4.4.0.12,5.17,12]도데카-3-엔, 8-에틸리덴테트라시클로[4.4.0.12,5.17,10.01,6]도데카-3-엔, 펜타시클로[6.5.1.13,6.02,7.09,13]펜타데크-4-엔, 펜타시클로[7.4.0.12,5.19,12.08,13]펜타데크-3-엔, 아세트산비닐, 비닐톨루엔 등의 비닐 화합물을 들 수 있다.When the binder resin (A-1) is a (meth)acrylic resin, in addition to the ethylenically unsaturated group-containing compound having a functional group (X) and the (meth)acrylate monomer, other ethylenically unsaturated group-containing monomers may be copolymerized. . Other ethylenically unsaturated group-containing monomers include styrenes such as styrene and α-, o-, m-, and p-alkyl derivatives of styrene, norbornene (bicyclo[2.2.1]hept-2-ene), 5 -methylbicyclo[2.2.1]hept-2-ene, 5-ethylbicyclo[2.2.1]hept-2-ene, tetracyclo[4.4.0.12,5.17,10]dodeca-3-ene, 8 -Methyltetracyclo[4.4.0.12,5.17,10]dodeca-3-ene, 8-ethyltetracyclo[4.4.0.12,5.17,10]dodeca-3-ene, dicyclopentadiene, tricyclo[5.2 .1.02,6]dec-8-ene, tricyclo[5.2.1.02,6]dec-3-ene, tricyclo[4.4.0.12,5]undeca-3-ene, tricyclo[6.2.1.01,8 ]undeca-9-ene, tricyclo[6.2.1.01,8]undeca-4-ene, tetracyclo[4.4.0.12,5.17,10.01,6]dodeca-3-ene, 8-methyltetracyclo[ 4.4.0.12,5.17,10.01,6]dodeca-3-ene, 8-ethylidenetetracyclo[4.4.0.12,5.17,12]dodeca-3-ene, 8-ethylidenetetracyclo[4.4.0.12, 5.17,10.01,6]dodeca-3-ene, pentacyclo[6.5.1.13,6.02,7.09,13]pentadec-4-ene, pentacyclo[7.4.0.12,5.19,12.08,13]pentadec-3 Vinyl compounds such as -ene, vinyl acetate, and vinyl toluene can be mentioned.
결합제 수지 (A-1)로서 비닐에스테르 수지를 사용하는 경우, 에폭시 수지와 (메트)아크릴산을 반응시켜서 비닐에스테르 수지를 합성하는 과정에서, 에폭시기가 개환하여 히드록시기가 생성됨으로써, 관능기 (X)로서의 히드록시기가 도입된다.When using a vinyl ester resin as the binder resin (A-1), in the process of synthesizing a vinyl ester resin by reacting an epoxy resin with (meth)acrylic acid, the epoxy group is ring-opened to generate a hydroxy group, thereby forming a hydroxy group as the functional group (X) is introduced.
관능기 (X)로서 또한 카르복시기를 도입하는 경우에는, 비닐에스테르 수지가 갖는 히드록시기에, 숙신산 무수물, 말레산 무수물 등의 다염기산 무수물을 부가시킴으로써, 카르복시기를 도입하면 된다.When introducing a carboxyl group as the functional group (X), the carboxyl group can be introduced by adding a polybasic acid anhydride such as succinic anhydride or maleic anhydride to the hydroxy group of the vinyl ester resin.
관능기 (X)로서 에폭시기를 도입하는 경우에는, 비닐에스테르 수지를 합성하는 과정에서, 원료가 되는 에폭시 수지의 에폭시기의 일부를 반응시키지 않고 남기면 된다.When introducing an epoxy group as the functional group (X), a part of the epoxy group of the raw material epoxy resin may be left unreacted in the process of synthesizing the vinyl ester resin.
관능기 (X)로서, 블록 이소시아나토기, 알킬에스테르기, 머캅토기, 옥세타닐기 등을 도입하고 싶은 경우에는, 예를 들어 이들의 관능기와 카르복시기를 갖는 화합물을 비닐에스테르 수지가 갖는 히드록시기에 부가시켜서 도입하는 방법 등을 들 수 있다.When it is desired to introduce a block isocyanato group, an alkyl ester group, a mercapto group, an oxetanyl group, etc. as a functional group (X), for example, a compound having these functional groups and a carboxyl group is added to the hydroxy group of the vinyl ester resin. Methods of introducing it by ordering it are included.
결합제 수지 (A-1)은, 감광성 착색 조성물을 노광, 현상할 때의 우수한 현상성이나, 수지 경화막의 기재에 대한 밀착성 향상의 관점에서, 에틸렌성 불포화기가 도입되어 있어도 된다. 예를 들어, 결합제 수지 (A-1)이 갖는 관능기 (X)의 일부에, 관능기 (X)와 반응성을 갖는 관능기 (Y) 및 에틸렌성 불포화기를 갖는 화합물을 부가함으로써, 결합제 수지 (A-1)에 단독으로의 경화 기능을 부여할 수 있다. 관능기 (Y) 및 에틸렌성 불포화기를 갖는 화합물로서는, 예를 들어 관능기 (X)로서 히드록시기 및 머캅토기에서 선택되는 1종 이상을 갖는 결합제 수지 (A-1)에 대하여, 블록 이소시아나토기 및 알킬에스테르를 갖는 기에서 선택되는 1종 이상과, 에틸렌성 불포화기를 갖는 화합물을 들 수 있다.The binder resin (A-1) may have an ethylenically unsaturated group introduced from the viewpoint of excellent developing properties when exposing and developing the photosensitive coloring composition and improving the adhesion of the resin cured film to the substrate. For example, by adding a compound having a functional group (Y) reactive with the functional group (X) and an ethylenically unsaturated group to a portion of the functional group (X) of the binder resin (A-1), the binder resin (A-1) ) can be given a stand-alone hardening function. Examples of the compound having a functional group (Y) and an ethylenically unsaturated group include, for example, a block isocyanato group and an alkyl binder resin (A-1) having at least one selected from the group consisting of a hydroxy group and a mercapto group as the functional group (X). One or more types selected from groups having an ester and a compound having an ethylenically unsaturated group can be mentioned.
또한, 관능기 (X)로서 카르복시기를 갖는 결합제 수지 (A-1)에 대하여, 에폭시기 및 옥세타닐기에서 선택되는 1종 이상과, 에틸렌성 불포화기를 갖는 화합물을 들 수 있다.Furthermore, with respect to the binder resin (A-1) having a carboxyl group as the functional group (X), a compound having at least one type selected from an epoxy group and an oxetanyl group and an ethylenically unsaturated group can be mentioned.
또한, 관능기 (X)로서 블록 이소시아나토기 및 알킬에스테르를 갖는 기에서 선택되는 1종 이상을 갖는 결합제 수지 (A-1)에 대하여, 히드록시기 및 머캅토기에서 선택되는 1종 이상과, 에틸렌성 불포화기를 갖는 화합물을 들 수 있다.In addition, for the binder resin (A-1) having at least one selected from the group having a block isocyanato group and an alkyl ester as the functional group (X), one or more types selected from the group consisting of a hydroxy group and a mercapto group, and an ethylenic group. Compounds having an unsaturated group can be mentioned.
또한, 관능기 (X)로서 에폭시기 및 옥세타닐기에서 선택되는 1종 이상을 갖는 결합제 수지 (A-1)에 대하여, 카르복시기와, 에틸렌성 불포화기를 갖는 화합물을 들 수 있다.Moreover, with respect to the binder resin (A-1) which has at least one type selected from an epoxy group and an oxetanyl group as a functional group (X), a compound which has a carboxyl group and an ethylenically unsaturated group can be mentioned.
각각 구체적으로는, 전술한 결합제 수지 (A-1)이 (메트)아크릴 수지인 경우의, 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물과 구체예로서 든 것과 마찬가지의 화합물을 적절히 선택할 수 있다.Specifically, in the case where the above-mentioned binder resin (A-1) is a (meth)acrylic resin, compounds containing an ethylenically unsaturated group having a functional group (X) and compounds similar to those listed as specific examples can be appropriately selected. .
결합제 수지 (A-1)의 고형분 산가는, 감광성 착색 조성물을 노광, 현상할 때의 현상성을 향상시키는 관점에서, 10 내지 300mgKOH/g인 것이 바람직하고, 20 내지 200mgKOH/g인 것이 보다 바람직하고, 30 내지 150mgKOH/g인 것이 더욱 바람직하다. 산가가 10 이상이면, 감광성 착색 조성물의 현상성 향상 효과가 충분히 발휘된다. 산가가 300 이하이면, 안료 분산 조성물로서의 안정성이 양호하다.The solid acid value of the binder resin (A-1) is preferably 10 to 300 mgKOH/g, and more preferably 20 to 200 mgKOH/g, from the viewpoint of improving developability when exposing and developing the photosensitive coloring composition. , more preferably 30 to 150 mgKOH/g. When the acid value is 10 or more, the developability improvement effect of the photosensitive coloring composition is sufficiently exhibited. If the acid value is 300 or less, the stability as a pigment dispersion composition is good.
또한, 결합제 수지 (A-1)의 산가는, JIS K6901 5.3을 따라서 브로모티몰 블루와 페놀 레드의 혼합 지시약을 사용하여 측정된 값이다. 결합제 수지 (A-1)의 산가란, 결합제 수지 (A-1) 1g 중에 포함되는 산성 성분을 중화하는데 요하는 수산화칼륨의 mg수를 의미한다.In addition, the acid value of the binder resin (A-1) is a value measured using a mixed indicator of bromothymol blue and phenol red according to JIS K6901 5.3. The acid value of binder resin (A-1) means the number of mg of potassium hydroxide required to neutralize the acidic component contained in 1 g of binder resin (A-1).
결합제 수지 (A-1)의 산가는, 결합제 수지 (A-1)의 공중합 시에, 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물로서 카르복시기를 갖는 에틸렌성 불포화기 함유 화합물을 사용하고, 이 배합량에 의해 조정할 수 있다. 또한, 결합제 수지 (A-1)이 히드록시기를 갖는 경우에는, 여기에 다염기산 무수물을 부가하여 카르복시기를 도입함으로써, 산가를 조정할 수 있다. 다염기산 무수물로서는 1,2,3,6-테트라히드로 무수 프탈산, 헥사히드로 무수 프탈산, 4-메틸헥사히드로 무수 프탈산, 비시클로[2.2.1]헵탄-2,3-디카르복실산 무수물, 메틸비시클로[2.2.1]헵탄-2,3-디카르복실산 무수물, 무수 숙신산, 옥테닐숙신산 무수물 등을 들 수 있다.The acid value of the binder resin (A-1) is determined by using an ethylenically unsaturated group-containing compound having a carboxyl group as an ethylenically unsaturated group-containing compound having a functional group (X) during copolymerization of the binder resin (A-1). It can be adjusted depending on the mixing amount. Additionally, when the binder resin (A-1) has a hydroxy group, the acid value can be adjusted by adding a polybasic acid anhydride to introduce a carboxyl group. Polybasic acid anhydrides include 1,2,3,6-tetrahydrophthalic anhydride, hexahydrophthalic anhydride, 4-methylhexahydrophthalic anhydride, bicyclo[2.2.1]heptane-2,3-dicarboxylic anhydride, and methyl ratio. Cyclo[2.2.1]heptane-2,3-dicarboxylic acid anhydride, succinic anhydride, octenylsuccinic anhydride, etc. are mentioned.
결합제 수지 (A-1)에 히드록시기를 함유시키는 방법으로서는, 이하의 방법 (1) 내지 (3) 등을 들 수 있다.Methods for making the binder resin (A-1) contain a hydroxy group include the following methods (1) to (3).
방법 (1): 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물로서, 히드록시기를 갖는 에틸렌성 불포화기 함유 화합물을 사용하고, 결합제 수지 (A-1)을 공중합하는 방법.Method (1): A method of copolymerizing a binder resin (A-1) using an ethylenically unsaturated group-containing compound having a hydroxy group as an ethylenically unsaturated group-containing compound having a functional group (X).
방법 (2): 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물로서, 카르복시기를 갖는 에틸렌성 불포화기 함유 화합물을 사용하고, 결합제 수지 (A-1)의 전구체를 공중합하고; 그 후, 관능기 (Y) 및 에틸렌성 불포화기를 갖는 화합물로서, 에폭시기를 갖는 에틸렌성 불포화기 함유 화합물을 상기 전구체의 카르복시기에 부가하여 에틸렌성 불포화기를 도입하고; 그것과 함께, 에폭시기의 개환에 의해 히드록시기를 도입하는 방법.Method (2): As the ethylenically unsaturated group-containing compound having a functional group (X), an ethylenically unsaturated group-containing compound having a carboxyl group is used, and the precursor of the binder resin (A-1) is copolymerized; Thereafter, an ethylenically unsaturated group-containing compound having an epoxy group, as a compound having a functional group (Y) and an ethylenically unsaturated group, is added to the carboxyl group of the precursor to introduce an ethylenically unsaturated group; Along with that, a method of introducing a hydroxy group by ring opening of an epoxy group.
방법 (3): 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물로서, 에폭시기를 갖는 에틸렌성 불포화기 함유 화합물을 사용하고, 결합제 수지 (A-1)의 전구체를 공중합하고; 그 후, 관능기 (Y) 및 에틸렌성 불포화기를 갖는 화합물로서, 카르복시기를 갖는 에틸렌성 불포화기 함유 화합물을 상기 전구체의 에폭시기에 부가하여 에틸렌성 불포화기를 도입하고; 그것과 함께, 에폭시기의 개환에 의해 히드록시기를 도입하는 방법.Method (3): As the ethylenically unsaturated group-containing compound having a functional group (X), an ethylenically unsaturated group-containing compound having an epoxy group is used, and the precursor of the binder resin (A-1) is copolymerized; Thereafter, an ethylenically unsaturated group-containing compound having a carboxyl group, as a compound having a functional group (Y) and an ethylenically unsaturated group, is added to the epoxy group of the precursor to introduce an ethylenically unsaturated group; Along with that, a method of introducing a hydroxy group by ring opening of an epoxy group.
결합제 수지 (A-1)의 중량 평균 분자량으로서는, 1000 내지 50000이 바람직하고, 3000 내지 30000이 보다 바람직하고, 5000 내지 20000이 더욱 바람직하다. 중량 평균 분자량이 5000 이상, 20000 이하이면, 밀 베이스로 했을 때에 양호한 분산 안정성이 얻어진다.The weight average molecular weight of the binder resin (A-1) is preferably 1,000 to 50,000, more preferably 3,000 to 30,000, and even more preferably 5,000 to 20,000. If the weight average molecular weight is 5000 or more and 20000 or less, good dispersion stability is obtained when used as a mill base.
결합제 수지 (A-1) 고형 1g 중에 포함되는 관능기 (X)의 양은, 100 내지 3000㎛ol/g인 것이 바람직하고, 300 내지 2500㎛ol/g인 것이 보다 바람직하고, 500 내지 2000㎛ol/g인 것이 더욱 바람직하다. 관능기 (X)의 양이, 100㎛ol/g 이상이면, 도막으로 했을 때에 양호한 내용제성을 갖는 밀 베이스를 제작할 수 있다. 관능기 (X)의 양이, 3000㎛ol/g 이하이면 밀 베이스로 했을 때에 양호한 분산 안정성이 얻어진다.The amount of functional group (X) contained in 1 g of solid binder resin (A-1) is preferably 100 to 3000 μmol/g, more preferably 300 to 2500 μmol/g, and 500 to 2000 μmol/g. It is more preferable that it is g. If the amount of functional group (X) is 100 μmol/g or more, a mill base with good solvent resistance when formed into a coating film can be produced. If the amount of functional group (X) is 3000 μmol/g or less, good dispersion stability can be obtained when used as a mill base.
〔결합제 수지 (A-1)의 구성비〕[Composition of binder resin (A-1)]
결합제 수지 (A-1)이 (메트)아크릴 수지인 경우, 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물의 함유량은, 공중합 모노머를 100몰%로 하여, 10 내지 75몰%가 바람직하고, 20 내지 80몰%가 보다 바람직하고, 30 내지 70몰%가 더욱 바람직하다. 함유량이 10몰% 이상이면, 후술하는 고분자 분산제 (C)가 갖는 관능기 (Y)와의 가교량을 충분히 확보할 수 있고, 저온 경화성이 양호한 감광성 착색 조성물이 얻어진다. 함유량이 75몰% 이하이면, 안료 분산 조성물의 보존 안정성이 양호하다.When the binder resin (A-1) is a (meth)acrylic resin, the content of the ethylenically unsaturated group-containing compound having a functional group (X) is preferably 10 to 75 mol%, with the copolymerization monomer being 100 mol%, 20 to 80 mol% is more preferable, and 30 to 70 mol% is still more preferable. If the content is 10 mol% or more, a sufficient amount of crosslinking with the functional group (Y) of the polymer dispersant (C) described later can be secured, and a photosensitive coloring composition with good low-temperature curability can be obtained. If the content is 75 mol% or less, the pigment dispersion composition has good storage stability.
또한, 관능기 (Y) 및 에틸렌성 불포화기를 갖는 화합물을 관능기 (X)에 부가하고, 결합제 수지 (A-1)에 에틸렌성 불포화기를 도입하는 경우에는, 그 부가율은, 관능기 (X)의 총 몰수에 대하여, 10 내지 80몰%인 것이 바람직하고, 20 내지 70몰%인 것이 보다 바람직하다. 부가율이 10몰% 이상이면 밀 베이스를 도막으로 했을 때에 내용제성이 향상되고, 부가율이 80몰% 이하이면 밀 베이스로 했을 때에 양호한 분산 안정성이 얻어진다.In addition, when a compound having a functional group (Y) and an ethylenically unsaturated group is added to the functional group (X) and an ethylenically unsaturated group is introduced into the binder resin (A-1), the addition rate is the total number of moles of the functional group (X) It is preferably 10 to 80 mol%, and more preferably 20 to 70 mol%. If the addition ratio is 10 mol% or more, solvent resistance is improved when using the mill base as a coating film, and if the addition ratio is 80 mol% or less, good dispersion stability is obtained when using the mill base as a coating film.
(메트)아크릴레이트 모노머의 함유량은, 공중합 모노머를 100몰%로 하여, 25 내지 90몰%가 바람직하고, 30 내지 70몰%가 바람직하고, 35 내지 60몰%가 바람직하다.The content of the (meth)acrylate monomer is preferably 25 to 90 mol%, preferably 30 to 70 mol%, and preferably 35 to 60 mol%, based on 100 mol% of the copolymerized monomer.
〔결합제 수지 (A-1)의 제조 방법〕[Method for producing binder resin (A-1)]
결합제 수지 (A-1)이 (메트)아크릴 수지인 경우, 예를 들어 이하에 나타내는 제조 방법을 사용하여 제조할 수 있다. 즉, 관능기 (X)를 갖는 에틸렌성 불포화기 함유 화합물과, (메트)아크릴레이트 모노머와, 필요에 따라서 사용하는 다른 에틸렌성 불포화기 함유 모노머를 포함하는 원료 모노머와, 중합용 용제와, 중합 개시제를 혼합하고, 질소 분위기 하에서, 통상 70℃ 내지 130℃의 조건 하에서 라디칼 중합하여, 결합제 수지 (A-1)인 (메트)아크릴 수지가 얻어진다. 중합용 용제로서는, 원료 모노머의 공중합 반응에 불활성의 용제이면 되고, 특별히 한정되지 않는다. 후술하는 용제 (D-1)에 포함되는 용제와 일부 또는 전부가 다른 것이어도 된다. 중합용 용제가, 안료 분산 조성물이 함유하고 있는 용제 (D-1)에 포함되는 용제와 일부 또는 전부가 동일한 경우, 공중합 반응 종료 후의 반응액으로부터 중합용 용매를 분리, 제거하지 않고, 용제 (D-1)의 일부로서 사용할 수 있어, 바람직하다.When the binder resin (A-1) is a (meth)acrylic resin, it can be manufactured using, for example, the manufacturing method shown below. That is, a compound containing an ethylenically unsaturated group having a functional group ( are mixed and radically polymerized under nitrogen atmosphere, usually under conditions of 70°C to 130°C, to obtain a (meth)acrylic resin that is the binder resin (A-1). The solvent for polymerization may be any solvent that is inert to the copolymerization reaction of the raw material monomer and is not particularly limited. The solvent may be partially or entirely different from the solvent contained in solvent (D-1) described later. When the solvent for polymerization is partly or entirely the same as the solvent contained in solvent (D-1) contained in the pigment dispersion composition, the solvent for polymerization is not separated or removed from the reaction liquid after completion of the copolymerization reaction, and the solvent (D It is preferable because it can be used as part of -1).
중합용 용제로서는, 후술하는 용제 (D-1)이 함유해도 되는 용제로서 예시하는 것과 마찬가지의 것을 들 수 있다. 이들 중에서도, 취급의 관점에서, (폴리)알킬렌글리콜모노알킬에테르아세테이트류를 사용하는 것이 바람직하고, 특히 프로필렌글리콜모노메틸에테르아세테이트를 사용하는 것이 바람직하다.Examples of the solvent for polymerization include those exemplified as solvents that may be contained in the solvent (D-1) described later. Among these, from the viewpoint of handling, it is preferable to use (poly)alkylene glycol monoalkyl ether acetates, and it is especially preferable to use propylene glycol monomethyl ether acetate.
중합용 용제의 사용량은, 특별히 한정되지 않지만, 원료 모노머 100질량부에 대하여, 바람직하게는 30질량부 내지 1000질량부이고, 보다 바람직하게는 50질량부 내지 800질량부이다. 중합용 용제의 사용량이 30질량부 이상이면, 원료 모노머의 공중합 반응을 안정되게 행할 수 있고, 관능기 함유 수지 전구체 (a)의 착색 및 겔화를 방지할 수 있다. 중합용 용제의 사용량이 1000질량부 이하이면, 연쇄 이동 작용에 의한 관능기 함유 수지 전구체 (a)의 분자량의 저하를 억제할 수 있음과 함께, 반응 용액의 점도를 적절한 범위로 제어할 수 있다.The amount of the solvent for polymerization is not particularly limited, but is preferably 30 parts by mass to 1000 parts by mass, more preferably 50 parts by mass to 800 parts by mass, based on 100 parts by mass of the raw material monomer. If the amount of the solvent for polymerization is 30 parts by mass or more, the copolymerization reaction of the raw material monomer can be performed stably, and coloring and gelation of the functional group-containing resin precursor (a) can be prevented. If the amount of the polymerization solvent used is 1000 parts by mass or less, a decrease in the molecular weight of the functional group-containing resin precursor (a) due to chain transfer action can be suppressed, and the viscosity of the reaction solution can be controlled to an appropriate range.
(중합 개시제)(polymerization initiator)
원료 모노머의 공중합 반응에 사용하는 것이 가능한 중합 개시제로서는, 특별히 한정되지 않지만, 예를 들어 2,2'-아조비스(이소부티로니트릴), 2,2'-아조비스(2,4-디메틸발레로니트릴), 2,2'-아조비스(이소부티르산)디메틸, 과산화벤조일, t-부틸퍼옥시-2-에틸헥사노에이트 등을 들 수 있다. 이들의 중합 개시제는, 1종을 단독으로 사용해도 되고, 2종 이상을 조합하여 사용해도 된다.The polymerization initiator that can be used in the copolymerization reaction of the raw material monomer is not particularly limited, but examples include 2,2'-azobis(isobutyronitrile), 2,2'-azobis(2,4-dimethylvalene) Ronitrile), 2,2'-azobis(isobutyric acid)dimethyl, benzoyl peroxide, t-butylperoxy-2-ethylhexanoate, etc. These polymerization initiators may be used individually by 1 type, or may be used in combination of 2 or more types.
중합 개시제의 사용량은, 특별히 한정되지 않지만, 원료 모노머 100질량부에 대하여, 바람직하게는 0.1질량부 내지 20질량부이고, 보다 바람직하게는 0.5질량부 내지 16질량부이다.The amount of the polymerization initiator used is not particularly limited, but is preferably 0.1 parts by mass to 20 parts by mass, more preferably 0.5 parts by mass to 16 parts by mass, based on 100 parts by mass of the raw material monomer.
결합제 수지 (A-1)에 에틸렌성 불포화기를 도입하는 경우에는, 필요에 따라 중합 금지제나 촉매를 추가하고, 관능기 (Y) 및 에틸렌성 불포화기를 갖는 화합물을 첨가하고, 50 내지 150℃의 조건 하에서, 1 내지 10시간 반응시키면 된다.When introducing an ethylenically unsaturated group into the binder resin (A-1), a polymerization inhibitor or catalyst is added as necessary, a compound having a functional group (Y) and an ethylenically unsaturated group is added, and the mixture is incubated under conditions of 50 to 150°C. , react for 1 to 10 hours.
〔안료 (B)〕[Pigment (B)]
본 실시 형태에 있어서의 안료 (B)는, 다른 조성물과 균일하게 분산하여 컬러 필터의 화소를 형성할 수 있으면, 특별히 한정되지 않는다. 적색, 녹색, 청색과 같은 광의 삼원색 안료를 비롯하여, 보색으로서 이용할 수 있는 황색, 주황색, 자색 등의 안료 및 블랙 매트릭스에서 이용되는 흑색, 갈색 등의 안료 등, 각 색의 안료를 사용할 수 있다. 또한, 안료 (B)의 화학 구조로서는, 이소인돌리논, 이소인돌린, 아조메틴, 안트라퀴논, 안트론, 크산텐, 디케토피롤로피롤, 페릴렌, 페리논, 퀴나크리돈, 인디고이드, 디옥사진, 인디고이드, 프탈로시아닌, 안토시아닌, 아조계 등의 모든 유기 안료나, 카본 블랙이나 티타늄 블랙, 이산화티타늄 등의 무기 안료를 들 수 있다.The pigment (B) in this embodiment is not particularly limited as long as it can be uniformly dispersed with other compositions to form a pixel of a color filter. Pigments of each color can be used, including pigments of the three primary colors of light such as red, green, and blue, pigments such as yellow, orange, and purple that can be used as complementary colors, and pigments such as black and brown used in the black matrix. In addition, the chemical structure of the pigment (B) includes isoindolinone, isoindoline, azomethine, anthraquinone, anthrone, xanthene, diketopyrrolopyrrole, perylene, perinone, quinacridone, indigoid, These include all organic pigments such as dioxazine, indigoid, phthalocyanine, anthocyanin, and azo, and inorganic pigments such as carbon black, titanium black, and titanium dioxide.
그 중에서도, 본 실시 형태에 있어서의 안료 (B)는, 하기 식 (1)로 표시되는 할로겐화 프탈로시아닌 골격을 갖는 녹색 안료를 포함하는 것이 바람직하다.Especially, it is preferable that the pigment (B) in this embodiment contains a green pigment which has a halogenated phthalocyanine skeleton represented by the following formula (1).
식 (1) 중, M은 2가 또는 4가의 금속 원자를 나타낸다. 색 재현성의 관점에서 아연 또는 구리가 바람직하고, 특히 아연이 바람직하다. X는 수소 원자, 염소 원자, 브롬 원자 중 어느 1종을 나타내고, 염소 원자 또는 브롬 원자를 적어도 1 이상 포함한다. 휘도나 색 재현성에 따라서 염소 원자와 브롬 원자의 부가 비율이 바뀌고, 염소 원자가 많고 브롬 원자가 적을수록 고휘도가 되고, 반대로 브롬 원자가 많고 염소 원자가 적을수록 색 재현성이 좋은 경향이 있다. 염소 원자는 1 이상 10 이하가 바람직하고, 1.5 이상 8 이하가 보다 바람직하다. 브롬 원자는 5 이상 15 이하가 바람직하고, 7 이상 14 이하가 보다 바람직하다.In formula (1), M represents a divalent or tetravalent metal atom. From the viewpoint of color reproducibility, zinc or copper is preferable, and zinc is especially preferable. X represents any one of a hydrogen atom, a chlorine atom, and a bromine atom, and contains at least one chlorine atom or a bromine atom. The addition ratio of chlorine atoms and bromine atoms changes depending on brightness or color reproducibility. The more chlorine atoms and fewer bromine atoms, the higher the brightness. Conversely, the more bromine atoms and fewer chlorine atoms, the better the color reproducibility. The number of chlorine atoms is preferably 1 to 10, and more preferably 1.5 to 8. The number of bromine atoms is preferably 5 to 15, and more preferably 7 to 14.
할로겐화 프탈로시아닌 골격을 갖는 녹색 안료와 상기 고분자 분산제 (C)를 사용함으로써, 충분한 안료 분산성이나 안료 분산 조성물의 보존 안정성이 얻어지고, 고휘도 또한 색 재현 범위가 넓은 컬러 필터를 제공할 수 있음과 함께, 착색 패턴의 내열성, 내용제성, 패턴 밀착성을 부여할 수 있다.By using a green pigment having a halogenated phthalocyanine skeleton and the polymer dispersant (C), sufficient pigment dispersibility and storage stability of the pigment dispersion composition are obtained, and a color filter with high brightness and a wide color reproduction range can be provided. It can provide heat resistance, solvent resistance, and pattern adhesion to the colored pattern.
할로겐화 프탈로시아닌 안료로서는, 시판품을 사용해도 되고, 스스로 준비해도 된다. 시판품의 일례로서는, C.I. 피그먼트 그린 7, 36, 58, 59 등을 들 수 있고, 그 중에서 고휘도화나 색 재현성의 우수성으로부터 C.I. 피그먼트 그린 58, 59의 사용이 바람직하다.As the halogenated phthalocyanine pigment, you may use a commercially available product or prepare it yourself. As an example of a commercially available product, C.I. Pigment Green 7, 36, 58, 59, etc. are included, and among them, C.I. is superior in terms of high brightness and color reproducibility. The use of pigment greens 58 and 59 is preferred.
스스로 준비하는 경우에는, 공지된 제조 방법을 이용한다. 예를 들어, 방향환의 수소 원자의 일부 또는 모두가 할로겐 원자로 치환된 프탈로산이나 프탈로니트릴 등을 출발 원료로 하고, 몰리브덴산암모늄 등의 촉매 하 프탈로시아닌 골격을 형성하는 방법이나, 프탈로시아닌을 염소 가스나 브롬 가스로 할로겐화하는 방법을 들 수 있다. 이들의 방법으로 얻어진 조(粗) 안료를 볼 밀, 진동 밀 등의 분쇄기 내에서 건식 마쇄하고, 공지된 솔벤트 솔트 밀링법 등으로 처리함으로써, 원하는 녹색 안료를 얻을 수 있다.When preparing it yourself, use known preparation methods. For example, a method of forming a phthalocyanine skeleton under a catalyst such as ammonium molybdate using phthalocyanine or phthalonitrile in which some or all of the hydrogen atoms of the aromatic ring are replaced with halogen atoms as a starting material, or phthalocyanine is converted into chlorine gas. One example is halogenation using bromine gas. The desired green pigment can be obtained by dry grinding the crude pigment obtained by these methods in a grinder such as a ball mill or vibrating mill and treating it by a known solvent salt milling method or the like.
본 실시 형태에 있어서의 안료 (B)에 대해서, 할로겐화 프탈로시아닌 골격을 갖는 녹색 안료는 단독으로 사용해도 되고, 2종 이상을 조합하여 사용해도 된다. 또한, 할로겐화 프탈로시아닌 골격을 갖는 녹색 안료가 아닌 다른 안료를 병용해도 된다.About the pigment (B) in this embodiment, the green pigment which has a halogenated phthalocyanine skeleton may be used individually, or may be used in combination of 2 or more types. Additionally, pigments other than the green pigment having a halogenated phthalocyanine skeleton may be used together.
다른 안료의 구체예로서는, C.I. 피그먼트 옐로우 1, 3, 12, 13, 14, 15, 16, 17, 20, 24, 31, 53, 83, 86, 93, 94, 109, 110, 117, 125, 128, 137, 138, 139, 147, 148, 150, 153, 154, 166, 173, 194, 214 등의 황색 안료; C.I. 피그먼트 오렌지 13, 31, 36, 38, 40, 42, 43, 51, 55, 59, 61, 64, 65, 71, 73 등의 주황색 안료; C.I. 피그먼트 레드 9, 97, 105, 122, 123, 144, 149, 166, 168, 176, 177, 180, 192, 209, 215, 216, 224, 242, 254, 255, 264, 265 등의 적색 안료; C.I. 피그먼트 블루 15, 15: 3, 15: 4, 15: 6, 60 등의 청색 안료; C.I. 피그먼트 바이올렛 1, 19, 23, 29, 32, 36, 38 등의 자색 안료; C.I. 피그먼트 브라운 23, 25 등의 갈색 안료; C.I. 피그먼트 블랙 1, 7, 카본 블랙, 티타늄 블랙, 산화철 등의 흑색 안료 등을 들 수 있다. 이들의 안료는, 목적으로 하는 화소의 색에 따라, 단독으로 사용해도 되고, 2종 이상을 조합하여 사용해도 된다.Specific examples of other pigments include C.I. Pigment Yellow 1, 3, 12, 13, 14, 15, 16, 17, 20, 24, 31, 53, 83, 86, 93, 94, 109, 110, 117, 125, 128, 137, 138, 139 , 147, 148, 150, 153, 154, 166, 173, 194, 214, etc.; C.I. Orange pigments such as Pigment Orange 13, 31, 36, 38, 40, 42, 43, 51, 55, 59, 61, 64, 65, 71, and 73; C.I. Red pigments such as Pigment Red 9, 97, 105, 122, 123, 144, 149, 166, 168, 176, 177, 180, 192, 209, 215, 216, 224, 242, 254, 255, 264, 265 ; C.I. Blue pigments such as Pigment Blue 15, 15: 3, 15: 4, 15: 6, and 60; C.I. Purple pigments such as Pigment Violet 1, 19, 23, 29, 32, 36, and 38; C.I. Brown pigments such as Pigment Brown 23 and 25; C.I. Black pigments such as pigment black 1 and 7, carbon black, titanium black, and iron oxide can be mentioned. These pigments may be used individually or in combination of two or more types, depending on the color of the target pixel.
또한, 본 실시 형태에 있어서의 안료 (B)에 대해서, 안료뿐만 아니라 염료를 병용해도 된다. 염료를 병용하는 경우, 안료를 사용하는 경우와 비교하여 고휘도화, 색 재현 범위의 확대, 양호한 현상성의 발현을 기대할 수 있다. 한편, 안료를 병용하는 경우, 염료와 비교하여 내열성이 우수하고, 착색 패턴 형성 후의 색 변화가 적다. 요구되는 성능이나 목적으로 하는 화소의 색에 따라, 염료와 안료를 병용해도 된다.Additionally, with respect to the pigment (B) in this embodiment, not only the pigment but also the dye may be used in combination. When a dye is used in combination, higher brightness, an expansion of the color reproduction range, and good developability can be expected compared to when a pigment is used. On the other hand, when a pigment is used together, heat resistance is excellent compared to dye, and color change after forming a colored pattern is small. Depending on the required performance or the target pixel color, dye and pigment may be used together.
염료로서는, 용제 (D)나 알칼리 현상액에 대한 용해성, 수지 조성물 중의 다른 성분과의 상호 작용, 내열성 등의 관점에서, 카르복시기 등의 산성기를 갖는 산성 염료, 산성 염료의 질소 화합물과의 염, 산성 염료의 술폰아미드체 등을 사용하는 것이 바람직하다. 이러한 염료의 구체예로서는, 애시드 알리자린 바이올렛 N; 애시드 블랙 1, 2, 24, 48; 애시드 블루 1, 7, 9, 25, 29, 40, 45, 62, 70, 74, 80, 83, 90, 92, 112, 113, 120, 129, 147; 애시드 크롬 바이올렛 K; 애시드 푹신; 애시드 그린 1, 3, 5, 25, 27, 50; 애시드 오렌지 6, 7, 8, 10, 12, 50, 51, 52, 56, 63, 74, 95; 애시드 레드 1, 4, 8, 14, 17, 18, 26, 27, 29, 31, 34, 35, 37, 42, 44, 50, 51, 52, 57, 69, 73, 80, 87, 88, 91, 92, 94, 97, 103, 111, 114, 129, 133, 134, 138, 143, 145, 150, 151, 158, 176, 183, 198, 211, 215, 216, 217, 249, 252, 257, 260, 266, 274; 애시드 바이올렛 6B, 7, 9, 17, 19; 애시드 옐로우 1, 3, 9, 11, 17, 23, 25, 29, 34, 36, 42, 54, 72, 73, 76, 79, 98, 99, 111, 112, 114, 116; 푸드 옐로우 3 및 이들의 유도체 등을 들 수 있다. 이들 중에서도, 아조계, 크산텐계, 안트라퀴논계 또는 프탈로시아닌계의 산성 염료가 바람직하다. 이들의 염료는, 단독으로 사용해도 되고, 2종 이상을 조합하여 사용해도 된다. 염료를 사용하는 경우, 안료 (B) 100질량부에 대하여 20 내지 80질량부가 바람직하다.As dyes, from the viewpoint of solubility in solvent (D) or alkaline developer, interaction with other components in the resin composition, heat resistance, etc., acid dyes with acidic groups such as carboxyl groups, salts of acid dyes with nitrogen compounds, and acid dyes It is preferable to use a sulfonamide form, etc. Specific examples of such dyes include Acid Alizarin Violet N; Acid Black 1, 2, 24, 48; Acid Blue 1, 7, 9, 25, 29, 40, 45, 62, 70, 74, 80, 83, 90, 92, 112, 113, 120, 129, 147; Acid Chrome Violet K; Acid Fuzz; Acid Green 1, 3, 5, 25, 27, 50; Acid Orange 6, 7, 8, 10, 12, 50, 51, 52, 56, 63, 74, 95; Acid Red 1, 4, 8, 14, 17, 18, 26, 27, 29, 31, 34, 35, 37, 42, 44, 50, 51, 52, 57, 69, 73, 80, 87, 88, 91, 92, 94, 97, 103, 111, 114, 129, 133, 134, 138, 143, 145, 150, 151, 158, 176, 183, 198, 211, 215, 216, 217, 249, 252, 257, 260, 266, 274; Acid Violet 6B, 7, 9, 17, 19; Acid Yellow 1, 3, 9, 11, 17, 23, 25, 29, 34, 36, 42, 54, 72, 73, 76, 79, 98, 99, 111, 112, 114, 116; Food Yellow 3 and derivatives thereof may be included. Among these, azo-based, xanthene-based, anthraquinone-based or phthalocyanine-based acid dyes are preferable. These dyes may be used individually, or may be used in combination of two or more types. When using a dye, the amount is preferably 20 to 80 parts by mass with respect to 100 parts by mass of the pigment (B).
〔고분자 분산제 (C)〕[Polymer dispersant (C)]
본 실시 형태의 고분자 분산제 (C)는, 아미노기 및 4급 암모늄 양이온기에서 선택되는 1종 이상을 갖고, 결합제 수지 (A-1)이 갖는 관능기 (X)와 반응성을 갖는 관능기 (Y)를 갖는 것이면, 특별히 한정되지 않는다. 3급 아미노기 및 4급 암모늄 양이온기로 이루어지는 군에서 선택되는 적어도 1종의 치환기를 갖는 고분자 분산제가 더욱 바람직하다. 고분자 분산제 (C)는 관능기 (Y)를 가짐으로써, 결합제 수지 (A-1)이 갖는 관능기 (X)와 반응하여 가교할 수 있다. 따라서, 감광성 착색 조성물을 저온 경화시킨 경우에도, 내용제성이 우수한 수지 경화막이 얻어진다. 또한, 고분자 분산제 (C)가 아미노기 및 4급 암모늄 양이온기에서 선택되는 1종 이상을 갖는 경우에는, 안료 분산 조성물 중의 안료 분산성이 향상됨과 함께, 감광성 착색 조성물로 했을 때의 현상성이 양호하다.The polymer dispersant (C) of the present embodiment has at least one selected from amino groups and quaternary ammonium cation groups, and has a functional group (Y) that is reactive with the functional group (X) possessed by the binder resin (A-1). There is no particular limitation as long as it is. A polymer dispersant having at least one substituent selected from the group consisting of a tertiary amino group and a quaternary ammonium cation group is more preferable. Since the polymer dispersant (C) has a functional group (Y), it can react with the functional group (X) of the binder resin (A-1) to crosslink it. Therefore, even when the photosensitive coloring composition is cured at low temperature, a cured resin film with excellent solvent resistance is obtained. In addition, when the polymer dispersant (C) has at least one selected from amino groups and quaternary ammonium cation groups, the dispersibility of the pigment in the pigment dispersion composition is improved and the developability when used as a photosensitive coloring composition is good. .
본 명세서에 있어서, 4급 암모늄 양이온기란, 질소 원자에 4개의 탄소 원자가 결합하고 있는 기를 가리키고, 아미드 결합이나 우레아 결합은 포함되지 않는다.In this specification, a quaternary ammonium cation group refers to a group in which four carbon atoms are bonded to a nitrogen atom, and does not include an amide bond or a urea bond.
관능기 (X)와 반응성을 갖는 관능기 (Y)로서는, 결합제 수지 (A)가 함유하는 관능기 (X)와 반응성을 갖는 관능기라면 특별히 한정되지 않는다. 구체적으로는, 관능기 (X)와의 반응성이나 고분자 분산제 (C)의 합성 용이성의 관점에서, 관능기 (Y)로서, 블록 이소시아나토기, 알킬에스테르기, 에폭시기, 옥세타닐기, 히드록시기, 머캅토기, 카르복시기 등을 들 수 있다.The functional group (Y) reactive with the functional group (X) is not particularly limited as long as it is a functional group reactive with the functional group (X) contained in the binder resin (A). Specifically, from the viewpoint of reactivity with the functional group ( A carboxyl group, etc. can be mentioned.
고분자 분산제 (C)가 갖고 있어도 되는 아미노기로서는, 1급, 2급, 3급의 아미노기를 들 수 있다. 각각, 1급 아미노기는 질소 원자에 1개의 탄소 원자가 결합하고 있는 기, 2급 아미노기는 질소 원자에 2개의 탄소 원자가 결합하고 있는 기, 3급 아미노기는 질소 원자에 3개의 탄소 원자가 결합하고 있는 기를 가리킨다.Examples of the amino group that the polymer dispersant (C) may have include primary, secondary, and tertiary amino groups. Respectively, a primary amino group refers to a group in which one carbon atom is bonded to a nitrogen atom, a secondary amino group refers to a group in which two carbon atoms are bonded to a nitrogen atom, and a tertiary amino group refers to a group in which three carbon atoms are bonded to a nitrogen atom. .
아미노기 및 4급 암모늄 양이온기 중, 안료 분산 조성물의 분산성과 보존 안정성의 관점에서, 적어도 3급 아미노기를 포함하는 것이 바람직하다.Among amino groups and quaternary ammonium cation groups, those containing at least a tertiary amino group are preferred from the viewpoint of dispersibility and storage stability of the pigment dispersion composition.
고분자 분산제 (C)로서는, 아미노기 함유 고분자 화합물 (c-1)과, 상기 관능기 (Y)를 갖는 할로겐 화합물 (c-2)의 반응물인 것이 바람직하다. 할로겐 화합물이란, 염소, 브롬, 요오드 등의 할로겐 원자가 결합하고 있는 유기 화학 물질을 의미한다.The polymer dispersant (C) is preferably a reaction product of an amino group-containing polymer compound (c-1) and a halogen compound (c-2) having the functional group (Y). Halogen compounds refer to organic chemical substances in which halogen atoms such as chlorine, bromine, and iodine are bonded.
아미노기 함유 고분자 화합물 (c-1)과, 상기 관능기 (Y)를 갖는 할로겐 화합물 (c-2)가 반응할 때, 할로겐 화합물 (c-2)의 할로겐 원자가 떨어져나간 잔기가 아미노기 함유 고분자 화합물 (c-1)의 아미노기에 배위한다. 그 중에서도, 3급 아미노기를 포함하는 아미노기 함유 고분자 화합물 (c-1)과, 상기 관능기 (Y)를 갖는 할로겐 화합물 (c-2)를 반응시켜서, 상기 3급 아미노기를 4급 암모늄 양이온기로 한 고분자 분산제 (C)가 바람직하다.When the amino group-containing polymer compound (c-1) reacts with the halogen compound (c-2) having the functional group (Y), the residue from which the halogen atom of the halogen compound (c-2) has been removed becomes the amino group-containing polymer compound (c-2). -1) Coordinates to the amino group. Among them, a polymer containing an amino group (c-1) containing a tertiary amino group is reacted with a halogen compound (c-2) having the functional group (Y) to form a polymer in which the tertiary amino group is a quaternary ammonium cation group. Dispersant (C) is preferred.
「아미노기 함유 고분자 화합물 (c-1)」“Amino group-containing polymer compound (c-1)”
아미노기 함유 고분자 화합물 (c-1)로서는, 예를 들어 아미노기와 에틸렌성 불포화기를 갖는 모노머를 단독 중합 또는 다른 에틸렌성 불포화기를 갖는 모노머와 공중합시킨 폴리아민을 들 수 있다. 이러한 고분자 화합물로서는, 예를 들어 비닐아민의 중합체 또는 알릴아민의 중합체인 폴리아민; 또는 비닐아민 혹은 알릴아민과, 다른 에틸렌성 불포화기를 갖는 모노머와 공중합시킨 폴리아민; 1-치환 아지리딘류나 2-옥사졸린 등을 개환 중합시킨 폴리머; 에틸렌디아민이나 헥사메틸렌디아민 등의 다관능 아민 화합물과 할로알칸 등을 중축합시킨 폴리알킬렌이민 등을 들 수 있다. 상기 고분자 화합물 (c-1)은 아미노기 이외에도, 효과를 손상시키지 않는 범위에서 아미드, 이미드, 우레아, 우레탄 등이 포함되어 있어도 되고, 이들에 상기 관능기 (Y)를 갖는 할로겐 화합물 (c-2)가 부가되는 것을 방해하지 않는다. 상술한 바와 같이, 안료 분산성에 크게 기여하는 것은 아민 구조이기 때문에, 아미드, 이미드, 우레아, 우레탄 결합은 실질적으로 포함하지 않는 쪽이 바람직하다. 그 중에서도 아미노기와 에틸렌성 불포화기를 갖는 모노머와 다른 에틸렌성 불포화기를 갖는 모노머를 블록 중합시킨 폴리아민이 바람직하고, 3급 아미노기와 에틸렌성 불포화기를 갖는 모노머와 다른 에틸렌성 불포화기를 갖는 모노머를 블록 중합시킨 폴리아민이 보다 바람직하다. 고분자 분산제 (C)로서 블록 중합시킨 폴리아민을 사용함으로써 편말단 영역에 아미노기가 편재되고, 안료 (B)와의 친화성이 향상된다. 또한, 다른 한쪽의 편말단 영역은, 다른 안료 분산 조성물, 구체적으로는 결합제 수지 (A-1)이나 후술하는 용제 (D-1)과의 친화성이 높아짐으로써, 안료 분산성이나 안료 분산 조성물의 보존 안정성을 비약적으로 향상시킬 수 있다.Examples of the amino group-containing polymer compound (c-1) include polyamine obtained by homopolymerizing a monomer having an amino group and an ethylenically unsaturated group or copolymerizing it with another monomer having an ethylenically unsaturated group. Examples of such high molecular compounds include polyamines, which are polymers of vinylamine or polymers of allylamine; or polyamine copolymerized with vinylamine or allylamine and a monomer having another ethylenically unsaturated group; Polymers obtained by ring-opening polymerization of 1-substituted aziridines, 2-oxazolines, etc.; Examples include polyalkyleneimines obtained by polycondensation of polyfunctional amine compounds such as ethylenediamine and hexamethylenediamine and haloalkanes. In addition to the amino group, the polymer compound (c-1) may contain amide, imide, urea, urethane, etc. as long as the effect is not impaired, and the halogen compound (c-2) having the above functional group (Y) does not prevent addition. As described above, since it is the amine structure that greatly contributes to pigment dispersibility, it is preferable that amide, imide, urea, and urethane bonds are substantially not included. Among them, polyamines obtained by block polymerization of a monomer having an amino group and an ethylenically unsaturated group and a monomer having another ethylenically unsaturated group are preferred, and polyamines obtained by block polymerization of a monomer having a tertiary amino group and an ethylenically unsaturated group and a monomer having another ethylenically unsaturated group are preferred. This is more preferable. By using block-polymerized polyamine as the polymer dispersant (C), amino groups are localized in the partial terminal region, and affinity with the pigment (B) is improved. In addition, the other end region increases the affinity with other pigment dispersion compositions, specifically binder resin (A-1) and the solvent (D-1) described later, thereby improving the pigment dispersibility and the pigment dispersion composition. Storage stability can be dramatically improved.
아미노기와 에틸렌성 불포화기를 갖는 모노머의 구체예로서는, 디메틸아미노에틸(메트)아크릴레이트, 디메틸아미노프로필(메트)아크릴레이트, 디메틸아미노부틸(메트)아크릴레이트, 디에틸아미노에틸(메트)아크릴레이트, 디에틸아미노프로필(메트)아크릴레이트, 디에틸아미노부틸(메트)아크릴레이트, 펜타메틸피페리딜(메트)아크릴레이트, 테트라메틸피페리딜(메트)아크릴레이트, N,N-디메틸(메트)아크릴아미드, N,N-디에틸(메트)아크릴아미드, N-이소프로필(메트)아크릴아미드, 디메틸아미노에틸(메트)아크릴아미드, 디메틸아미노프로필(메트)아크릴아미드, 디아세톤(메트)아크릴아미드, 또는 아크릴로일모르폴린 등의 (메트)아크릴아미드류 등을 들 수 있다. 이들의 모노머는 단독으로 사용해도 되고, 2종 이상을 사용해도 된다. 그 중에서도, 안료 분산 조성물의 분산성 향상이나 보존 안정성 향상에 크게 기여하는 점에서, 3급 아미노기와 에틸렌성 불포화기를 갖는 모노머가 바람직하고, 디메틸아미노에틸(메트)아크릴레이트가 보다 바람직하다. 또한, 3급 아미노기와 에틸렌성 불포화기를 갖는 모노머를 사용하여, 3급 아미노기를 갖는 아미노기 함유 고분자 화합물 (c-1)로 하고, 3급 아미노기의 일부에 상기 관능기 (Y)를 갖는 할로겐 화합물 (c-2)를 반응시켜서 4급 암모늄 양이온기로 하면, 친수성이 향상되고, 감광성 착색 조성물로서의 현상성이 향상된다.Specific examples of monomers having an amino group and an ethylenically unsaturated group include dimethylaminoethyl (meth)acrylate, dimethylaminopropyl (meth)acrylate, dimethylaminobutyl (meth)acrylate, diethylaminoethyl (meth)acrylate, and dimethylaminoethyl (meth)acrylate. Ethylaminopropyl (meth)acrylate, diethylaminobutyl (meth)acrylate, pentamethylpiperidyl (meth)acrylate, tetramethylpiperidyl (meth)acrylate, N, N-dimethyl (meth)acrylate Amide, N,N-diethyl (meth)acrylamide, N-isopropyl (meth)acrylamide, dimethylaminoethyl (meth)acrylamide, dimethylaminopropyl (meth)acrylamide, diacetone (meth)acrylamide, or (meth)acrylamides such as acryloylmorpholine. These monomers may be used individually, or two or more types may be used. Among them, monomers having a tertiary amino group and an ethylenically unsaturated group are preferable, and dimethylaminoethyl (meth)acrylate is more preferable because it greatly contributes to improving the dispersibility and storage stability of the pigment dispersion composition. In addition, a monomer having a tertiary amino group and an ethylenically unsaturated group is used to prepare an amino group-containing polymer compound (c-1) having a tertiary amino group, and a halogen compound (c) having the above functional group (Y) in part of the tertiary amino group. When -2) is reacted to form a quaternary ammonium cation, hydrophilicity is improved and developability as a photosensitive coloring composition is improved.
다른 에틸렌성 불포화기를 갖는 모노머로서는, 일례로서, 알킬기, 아릴기, 아르알킬기, 시클로알킬기, (폴리)옥시알킬렌 골격 등을 갖는 (메트)아크릴레이트를 들 수 있지만, 다른 안료 분산 조성물과 친화성을 손상시키는 것이 아니면 특별히 제한은 없다. 구체예로서는, 메틸(메트)아크릴레이트, 에틸(메트)아크릴레이트, n-프로필(메트)아크릴레이트, 이소프로필(메트)아크릴레이트, n-부틸(메트)아크릴레이트, 이소부틸(메트)아크릴레이트, t-부틸(메트)아크릴레이트, 2-에틸헥실(메트)아크릴레이트, 시클로헥실(메트)아크릴레이트, 스테아릴(메트)아크릴레이트, 라우릴(메트)아크릴레이트, 테트라히드로푸르푸릴(메트)아크릴레이트, 이소보르닐(메트)아크릴레이트, 페닐(메트)아크릴레이트, 벤질(메트)아크릴레이트, 페녹시에틸(메트)아크릴레이트, 페녹시디에틸렌글리콜(메트)아크릴레이트, 메톡시폴리프로필렌글리콜(메트)아크릴레이트, 또는 에톡시폴리에틸렌글리콜(메트)아크릴레이트 등의 (메트)아크릴레이트류, 스티렌 또는 α-메틸스티렌 등의 스티렌류, 에틸비닐에테르, n-프로필비닐에테르, 이소프로필비닐에테르, n-부틸비닐에테르, 또는 이소부틸비닐에테르 등의 비닐에테르류, 아세트산비닐, 또는 프로피온산비닐 등의 지방산 비닐류 등을 들 수 있다. 이들의 모노머는 단독으로 사용해도 되고, 2종 이상을 사용해도 된다.Examples of monomers having other ethylenically unsaturated groups include (meth)acrylates having an alkyl group, aryl group, aralkyl group, cycloalkyl group, (poly)oxyalkylene skeleton, etc., but they have compatibility with other pigment dispersion compositions. There are no particular restrictions as long as it does not cause damage. Specific examples include methyl (meth)acrylate, ethyl (meth)acrylate, n-propyl (meth)acrylate, isopropyl (meth)acrylate, n-butyl (meth)acrylate, and isobutyl (meth)acrylate. , t-butyl (meth)acrylate, 2-ethylhexyl (meth)acrylate, cyclohexyl (meth)acrylate, stearyl (meth)acrylate, lauryl (meth)acrylate, tetrahydrofurfuryl (meth)acrylate ) Acrylate, isobornyl (meth)acrylate, phenyl (meth)acrylate, benzyl (meth)acrylate, phenoxyethyl (meth)acrylate, phenoxydiethylene glycol (meth)acrylate, methoxypolypropylene (meth)acrylates such as glycol (meth)acrylate or ethoxypolyethylene glycol (meth)acrylate, styrenes such as styrene or α-methylstyrene, ethyl vinyl ether, n-propyl vinyl ether, and isopropyl vinyl. Examples include vinyl ethers such as ether, n-butyl vinyl ether, or isobutyl vinyl ether, and fatty acid vinyls such as vinyl acetate or vinyl propionate. These monomers may be used individually, or two or more types may be used.
아미노기 함유 고분자 화합물(c-1)은 시판품을 사용해도 되고, 스스로 준비 해도 된다. 시판품을 이용하는 경우, 적합한 아미노기 함유 고분자 화합물 (c-1)의 일례로서, 빅 케미사제의 DISPERBYK 시리즈, 루브리졸사제의 솔스퍼스 시리즈, BASF사제의 EFKA-PX 시리즈 등을 들 수 있다. 스스로 준비하는 경우에는, 공지된 중합체 제조 방법을 이용한다. 이들의 아미노기 함유 고분자 화합물 (c-1)은 필요에 따라, 단독으로 사용해도 되고, 2종 이상을 조합하여 사용해도 된다.The amino group-containing polymer compound (c-1) may be a commercially available product or may be prepared yourself. When using a commercial product, examples of suitable amino group-containing polymer compounds (c-1) include the DISPERBYK series manufactured by Big Chemistry, the Solsperse series manufactured by Lubrizol, and the EFKA-PX series manufactured by BASF. When preparing yourself, use known polymer preparation methods. These amino group-containing polymer compounds (c-1) may be used individually, or in combination of two or more, as needed.
「할로겐 화합물 (c-2)」“Halogen compound (c-2)”
할로겐 화합물 (c-2)로서는, 상기 관능기 (Y)를 갖는 모노 할로겐 화합물이면 특별히 한정되지 않는다. 상기 관능기 (Y)의 수는 특별히 한정되지 않는다. 예를 들어, 블록 이소시아나토기, 알킬에스테르기, 에폭시기, 옥세타닐기, 히드록시기, 머캅토기 및 카르복시기로 이루어지는 군에서 선택되는 1종 이상을 갖는 화합물의 브롬화물, 염소화물, 요오드화물 등을 들 수 있다. 구체적으로는, 2-브로모에탄올, 에피브로모히드린 등을 들 수 있다. 이들 화합물은 단독으로 사용해도 되고, 2종 이상을 사용해도 된다. 그 중에서도, 반응성의 관점에서, 브롬화물이 바람직하다.The halogen compound (c-2) is not particularly limited as long as it is a monohalogen compound having the above functional group (Y). The number of functional groups (Y) is not particularly limited. For example, bromides, chlorides, iodides, etc. of compounds having one or more types selected from the group consisting of block isocyanato groups, alkyl ester groups, epoxy groups, oxetanyl groups, hydroxy groups, mercapto groups, and carboxyl groups. You can. Specifically, 2-bromoethanol, epibromohydrin, etc. can be mentioned. These compounds may be used individually, or two or more types may be used. Among them, bromide is preferable from the viewpoint of reactivity.
아미노기 함유 고분자 화합물 (c-1)이 갖는 아미노기의 총량에 대한, 할로겐 화합물 (c-2)의 부가율은, 5 내지 80%인 것이 바람직하고, 10 내지 60%인 것이 보다 바람직하고, 15 내지 40%인 것이 더욱 바람직하다. 할로겐 화합물 (c-2)의 부가율이 5% 이상이면, 고분자 분산제 (C)에 충분량의 관능기 (Y)가 도입된다. 그 때문에, 결합제 수지 (A-1)이 갖는 관능기 (X)와의 가교량을 충분히 확보할 수 있다. 그 결과, 감광성 착색 조성물로서의 저온 경화성이 양호하고, 우수한 내용제성을 갖는 수지 경화막이 얻어진다. 할로겐 화합물 (c-2)의 부가율이 80% 이하이면, 고분자 분산제 (C)의 합성 시에 겔화하지 않고 용이하게 제조할 수 있고, 밀 베이스로 했을 때에 양호한 분산 안정성을 유지할 수 있다. 특히 아미노기 함유 고분자 화합물 (c-1)이 갖는 아미노기가 3급 아미노기인 경우에는, 할로겐 화합물 (c-2)의 부가율이 80% 이하이면, 잔존하는 3급 아미노기량을 충분히 확보할 수 있다. 그 때문에, 안료 분산 조성물로서의 분산성이나 보존 안정성이 충분한 것이 된다.The addition ratio of the halogen compound (c-2) relative to the total amount of amino groups in the amino group-containing polymer compound (c-1) is preferably 5 to 80%, more preferably 10 to 60%, and 15 to 40%. It is more preferable that it is %. If the addition ratio of the halogen compound (c-2) is 5% or more, a sufficient amount of functional group (Y) is introduced into the polymer dispersant (C). Therefore, a sufficient amount of crosslinking with the functional group (X) of the binder resin (A-1) can be secured. As a result, a resin cured film having good low-temperature curability as a photosensitive coloring composition and excellent solvent resistance is obtained. If the addition ratio of the halogen compound (c-2) is 80% or less, the polymer dispersant (C) can be easily manufactured without gelation during synthesis, and good dispersion stability can be maintained when used as a mill base. In particular, when the amino group of the amino group-containing polymer compound (c-1) is a tertiary amino group, if the addition ratio of the halogen compound (c-2) is 80% or less, the amount of remaining tertiary amino group can be sufficiently secured. Therefore, the dispersibility and storage stability as a pigment dispersion composition are sufficient.
고분자 분산제 (C)가 아미노기 및 4급 암모늄 양이온기에서 선택되는 1종 이상을 갖는 경우의 아민 함유량(1급 아민, 2급 아민, 3급 아민의 합계량임)은, 아민가(JIS K7237 등에 나타내는 규격을 따라 측정된 값)를 측정함으로써, 그 양을 정량적으로 판단할 수 있다. 본 실시 형태의 고분자 화합물 (c-1)의 아민 함유량은 특별히 한정되지 않지만, 바람직하게는 10mgKOH/g 내지 400mgKOH/g, 보다 바람직하게는 20mgKOH/g 내지 200mgKOH/g, 더욱 바람직하게는 30mgKOH/g 내지 100mgKOH/g이다. 아민가가 10mgKOH/g 이상이면, 안료 분산 조성물이 충분한 분산성이나 보존 안정성이 얻어진다.When the polymer dispersant (C) has one or more types selected from amino groups and quaternary ammonium cation groups, the amine content (the total amount of primary amine, secondary amine, and tertiary amine) is determined by the amine value (standards shown in JIS K7237, etc. By measuring the measured value according to , the amount can be quantitatively determined. The amine content of the polymer compound (c-1) of the present embodiment is not particularly limited, but is preferably 10 mgKOH/g to 400 mgKOH/g, more preferably 20 mgKOH/g to 200 mgKOH/g, and even more preferably 30 mgKOH/g. to 100mgKOH/g. If the amine value is 10 mgKOH/g or more, the pigment dispersion composition will have sufficient dispersibility and storage stability.
고분자 분산제 (C)가 아미노기를 갖는 경우, 안료 분산 조성물의 분산성이나 보존 안정성의 향상에 대하여, 그 중에서도 3급 아미노기의 기여율이 크다. 따라서, 고분자 분산제 (C)의 3급 아민 함유량을 충분히 확보하는 것이 바람직하다. 그러한 관점에서, 고분자 분산제 (C)의 3급 아민가는, 바람직하게는 10mgKOH/g 내지 400mgKOH/g, 보다 바람직하게는 30mgKOH/g 내지 200mgKOH/g, 더욱 바람직하게는 40mgKOH/g 내지 100mgKOH/g이다.When the polymer dispersant (C) has an amino group, the contribution rate of the tertiary amino group is particularly large to the improvement of the dispersibility and storage stability of the pigment dispersion composition. Therefore, it is desirable to ensure a sufficient tertiary amine content of the polymer dispersant (C). From that viewpoint, the tertiary amine value of the polymer dispersant (C) is preferably 10 mgKOH/g to 400 mgKOH/g, more preferably 30 mgKOH/g to 200 mgKOH/g, and still more preferably 40 mgKOH/g to 100 mgKOH/g. .
3급 아민가는, 아민가 V0(JIS K7237 등에 나타내는 규격을 따라 측정된 값)을 베이스로 아미노기 함유 모노머와 할로겐 화합물 (c-2)의 사용량에 기초하여 산출한 값이다.The tertiary amine titer is a value calculated based on the amine titer V 0 (value measured according to the standards shown in JIS K7237, etc.) and the amount of amino group-containing monomer and halogen compound (c-2) used.
고분자 분산제 (C) 고형 1g 중에 포함되는 관능기 (Y)의 양은, 50 내지 5000㎛ol/g인 것이 바람직하고, 100 내지 3000㎛ol/g인 것이 보다 바람직하고, 150 내지 1000㎛ol/g인 것이 더욱 바람직하다. 관능기 (X)의 양이, 50㎛ol/g 이상이면, 감광성 착색 조성물로서의 현상성, 저온 경화성이 우수하고, 내용제성이 양호한 수지 경화막이 얻어진다. 관능기 (X)의 양이, 5000㎛ol/g 이하이면 밀 베이스로 했을 때에 양호한 분산 안정성이 얻어진다.The amount of functional group (Y) contained in 1g of solid polymer dispersant (C) is preferably 50 to 5000 μmol/g, more preferably 100 to 3000 μmol/g, and 150 to 1000 μmol/g. It is more desirable. When the amount of the functional group (X) is 50 μmol/g or more, a resin cured film with excellent developability and low-temperature curing properties as a photosensitive coloring composition and good solvent resistance is obtained. If the amount of functional group (X) is 5000 μmol/g or less, good dispersion stability can be obtained when used as a mill base.
본 실시 형태의 고분자 분산제 (C)의 중량 평균 분자량은, 바람직하게는 1000 내지 40000, 보다 바람직하게는 1000 내지 30000이다. 또한, 고분자 분산제 (C)의 분자량 분포(폴리스티렌 환산의 중량 평균 분자량을 수 평균 분자량으로 제산한 값)는, 바람직하게는 1.0 내지 3.0, 보다 바람직하게는 1.0 내지 2.0의 범위 내이다. 고분자 분산제 (C)의 분자량이나 분자량 분포가 상기 범위 내에 있으면, 안료 분산 조성물의 점도를 적절한 범위로 제어할 수 있다. 그 결과, 충분한 안료 분산성이나 안료 분산 조성물의 보존 안정성이 얻어짐과 함께, 우수한 내열성, 내용제성, 패턴 밀착성, 현상성 등을 확보할 수 있다.The weight average molecular weight of the polymer dispersant (C) of this embodiment is preferably 1,000 to 40,000, more preferably 1,000 to 30,000. In addition, the molecular weight distribution (value obtained by dividing the weight average molecular weight in terms of polystyrene by the number average molecular weight) of the polymer dispersant (C) is preferably in the range of 1.0 to 3.0, more preferably in the range of 1.0 to 2.0. If the molecular weight or molecular weight distribution of the polymer dispersant (C) is within the above range, the viscosity of the pigment dispersion composition can be controlled to an appropriate range. As a result, sufficient pigment dispersibility and storage stability of the pigment dispersion composition are obtained, and excellent heat resistance, solvent resistance, pattern adhesion, developability, etc. can be secured.
〔관능기 (X)와 관능기 (Y)의 바람직한 조합〕[Preferable combination of functional group (X) and functional group (Y)]
결합제 수지 (A-1)이 갖는 관능기 (X)와, 고분자 분산제 (C)가 갖는 관능기 (Y)의 바람직한 조합으로서는, 감광성 착색 조성물로서의 저온 경화성 향상의 관점에서, 이하의 조합 (1) 내지 (4)를 들 수 있다.As a preferable combination of the functional group (X) possessed by the binder resin (A-1) and the functional group (Y) possessed by the polymer dispersant (C), from the viewpoint of improving low-temperature curability as a photosensitive coloring composition, the following combinations (1) to ( 4) can be mentioned.
조합 (1): 관능기 (X)로서 히드록시기 및 머캅토기에서 선택되는 1종 이상, 관능기 (Y)로서 블록 이소시아나토기 및 알킬에스테르를 갖는 기에서 선택되는 1종 이상의 조합.Combination (1): A combination of at least one type selected from a hydroxy group and a mercapto group as the functional group (X), and a group having a block isocyanato group and an alkyl ester as the functional group (Y).
조합 (2): 관능기 (X)로서 카르복시기, 관능기 (Y)로서 에폭시기 및 옥세타닐기에서 선택되는 1종 이상의 조합.Combination (2): A combination of one or more types selected from a carboxyl group as the functional group (X), an epoxy group, and an oxetanyl group as the functional group (Y).
조합 (3): 관능기 (X)로서 블록 이소시아나토기 및 알킬에스테르를 갖는 기에서 선택되는 1종 이상, 관능기 (Y)로서 히드록시기 및 머캅토기에서 선택되는 1종 이상의 조합.Combination (3): A combination of at least one type selected from groups having a block isocyanato group and an alkyl ester as the functional group (X), and at least one type selected from a hydroxy group and a mercapto group as the functional group (Y).
조합 (4): 관능기 (X)로서 에폭시기 및 옥세타닐기에서 선택되는 1종 이상, 관능기 (Y)로서 카르복시기의 조합.Combination (4): A combination of at least one selected from epoxy group and oxetanyl group as the functional group (X) and a carboxyl group as the functional group (Y).
또한 상기 조합 이외의 관능기가 병용되어 있어도 된다. 예를 들어, 결합제 수지 (A-1)이 갖는 관능기 (X)로서는, 감광성 착색 조성물로서의 현상성 향상의 관점에서, 어느 조합에 있어서도 카르복시기가 병용되어 있는 것이 바람직하다.Additionally, functional groups other than the above combination may be used together. For example, as the functional group (X) of the binder resin (A-1), it is preferable that a carboxyl group is used together in any combination from the viewpoint of improving developability as a photosensitive coloring composition.
<고분자 분산제 (C)의 제조 방법><Method for producing polymer dispersant (C)>
고분자 분산제 (C)는, 예를 들어 이하의 방법으로 제조할 수 있다. 즉, 아미노기 함유 고분자 화합물 (c-1)의 아미노기에, 상기 관능기 (Y)를 갖는 할로겐 화합물 (c-2)를 부가함으로써 제조할 수 있다. 상기 고분자 화합물 (c-1)의 아미노기에, 상기 관능기 (Y)를 갖는 할로겐 화합물 (c-2)를 부가하는 방법으로서는, 브로모 염화 반응을 이용하는 것이 바람직하다. 구체적으로는, 고분자 화합물 (c-1), 상기 관능기 (Y)를 갖는 할로겐 화합물 (c-2), 그리고 임의의 비율로 용액을 혼합시켜, 실온 내지 150℃, 바람직하게는 30℃ 내지 130℃의 조건 하에서 반응시킴으로써, 고분자 화합물 (c-1)과 상기 관능기 (Y)를 갖는 할로겐 화합물 (c-2)의 반응물이 되는 고분자 분산제 (C)를 얻는다.The polymer dispersant (C) can be produced, for example, by the following method. That is, it can be produced by adding the halogen compound (c-2) having the above functional group (Y) to the amino group of the amino group-containing polymer compound (c-1). As a method of adding the halogen compound (c-2) having the functional group (Y) to the amino group of the polymer compound (c-1), it is preferable to use a bromo chlorination reaction. Specifically, the polymer compound (c-1), the halogen compound (c-2) having the functional group (Y), and the solution are mixed in an arbitrary ratio, and the temperature is room temperature to 150°C, preferably 30°C to 130°C. By reacting under the conditions, a polymer dispersant (C), which is a reaction product of the polymer compound (c-1) and the halogen compound (c-2) having the functional group (Y), is obtained.
〔용제 (D-1)〕[Solvent (D-1)]
용제 (D-1)은 본 실시 형태의 안료 분산 조성물, 또는 감광성 착색 조성물에 포함되는 각 성분과 반응하지 않고, 또한 이들을 용해 또는 분산 가능한 용제라면 특별히 한정되지 않는다. 용제 (D-1)로서는, 결합제 수지 (A-1) 혹은 고분자 분산제 (C)를 제조할 때에 사용한 용제와 동일한 것을 사용할 수 있고, 반응 후에 포함되어 있는 용제를 그대로 사용할 수도 있고, 더 첨가할 수도 있다. 또한, 그 밖의 성분을 첨가할 때에, 거기에 공존하고 있는 것이어도 된다. 용제 (D-1)의 구체예로서는, 프로필렌글리콜모노메틸에테르, 프로필렌글리콜모노메틸에테르아세테이트, 디프로필렌글리콜모노메틸에테르아세테이트, 아세트산에틸, 아세트산부틸, 아세트산이소프로필, 프로필렌글리콜모노메틸에테르, 디프로필렌글리콜모노메틸에테르, 트리프로필렌글리콜모노메틸에테르, 에틸렌글리콜모노메틸에테르, 디에틸렌글리콜모노메틸에테르, 메틸에틸케톤, 메틸이소부틸케톤, 시클로헥사논, 에틸렌글리콜모노에틸에테르아세테이트, 디에틸렌글리콜에틸에테르아세테이트 등을 들 수 있다. 이들은, 단독으로 사용해도 되고, 2종 이상을 조합하여 사용해도 된다. 또한, 이들 중에서도, 컬러 필터를 제조할 때에 바람직하게 사용되는 프로필렌글리콜모노메틸에테르, 프로필렌글리콜모노메틸에테르아세테이트 등의 글리콜에테르계 용제가 바람직하다.The solvent (D-1) is not particularly limited as long as it is a solvent that does not react with each component contained in the pigment dispersion composition of the present embodiment or the photosensitive coloring composition and is capable of dissolving or dispersing them. As the solvent (D-1), the same solvent as the solvent used when producing the binder resin (A-1) or the polymer dispersant (C) can be used, and the solvent contained after the reaction can be used as is, or more can be added. there is. Additionally, when adding other components, they may coexist there. Specific examples of the solvent (D-1) include propylene glycol monomethyl ether, propylene glycol monomethyl ether acetate, dipropylene glycol monomethyl ether acetate, ethyl acetate, butyl acetate, isopropyl acetate, propylene glycol monomethyl ether, and dipropylene glycol. Monomethyl ether, tripropylene glycol monomethyl ether, ethylene glycol monomethyl ether, diethylene glycol monomethyl ether, methyl ethyl ketone, methyl isobutyl ketone, cyclohexanone, ethylene glycol monoethyl ether acetate, diethylene glycol ethyl ether acetate. etc. can be mentioned. These may be used individually, or may be used in combination of two or more types. Moreover, among these, glycol ether-based solvents such as propylene glycol monomethyl ether and propylene glycol monomethyl ether acetate, which are preferably used when manufacturing color filters, are preferable.
<안료 분산 조성물의 제조 방법><Method for producing pigment dispersion composition>
본 실시 형태의 안료 분산 조성물은, 결합제 수지 (A-1), 고분자 분산제 (C), 안료 (B), 용제 (C-1)을 소정량 칭량하고, 공지된 분산 처리 공정을 사용하여 안료 (B)를 미세화하여 분산한다. 이 분산 처리 공정에 있어서, 페인트 셰이커, 비즈 밀, 볼 밀, 롤밀, 스톤 밀, 제트 밀, 균질기, 플래니터리 믹서, 자전 공전 믹서 등의 장치가 다용된다. 또한, 이 분산 처리 공정에 있어서, 직경 0.01 내지 10mm의 비즈를 사용하면, 안료 (B)의 균일한 미세화를 효율적으로 행할 수 있다. 비즈의 재질에 제한은 없지만, 경도나 안료 분산 조성물에 대한 오염 등을 감안하면, 글래스 비즈나 지르코니아 비즈의 사용이 바람직하다. 분산 처리의 적합한 시간, 온도, 비즈의 직경, 사용량에 대해서는, 안료 분산 조성물의 조성이나 장치의 크기 등에 의해 적정한 조건이 다르기 때문에, 적절히 조정하면 된다.The pigment dispersion composition of the present embodiment is prepared by weighing a predetermined amount of binder resin (A-1), polymer dispersant (C), pigment (B), and solvent (C-1), and dispersing the pigment (C-1) using a known dispersion treatment process. B) is micronized and dispersed. In this dispersion treatment process, various devices such as paint shakers, bead mills, ball mills, roll mills, stone mills, jet mills, homogenizers, planetary mixers, and rotation/revolution mixers are used. Additionally, in this dispersion treatment step, if beads with a diameter of 0.01 to 10 mm are used, the pigment (B) can be uniformly refined efficiently. There are no restrictions on the material of the beads, but considering hardness and contamination of the pigment dispersion composition, it is preferable to use glass beads or zirconia beads. The appropriate time, temperature, bead diameter, and usage amount for the dispersion treatment may be adjusted appropriately because the appropriate conditions vary depending on the composition of the pigment dispersion composition, the size of the device, etc.
마지막으로, 안료 분산 조성물에 미세한 티끌, 그리고 안료 (B)의 조립(粗粒)이나 응집물을 제거하는 목적으로, 안료 분산 조성물을 유리 필터 등으로 여과 처리하는 것이 바람직하다.Finally, for the purpose of removing fine dust and particles or aggregates of the pigment (B) from the pigment dispersion composition, it is preferable to filter the pigment dispersion composition through a glass filter or the like.
〔안료 분산 조성물의 배합비〕[Mixing ratio of pigment dispersion composition]
결합제 수지 (A-1)의 함유량은, 안료 (B) 100질량부에 대하여, 10 내지 80질량부인 것이 바람직하고, 15 내지 50질량부인 것이 보다 바람직하고, 20 내지 40질량부인 것이 더욱 바람직하다. 결합제 수지 (A-1)의 함유량이 10질량부 이상이면, 충분한 현상성이 얻어진다. 결합제 수지 (A-1)의 함유량이 80질량부 이하이면, 밀 베이스로 했을 때에 양호한 분산 안정성을 얻을 수 있다.The content of the binder resin (A-1) is preferably 10 to 80 parts by mass, more preferably 15 to 50 parts by mass, and still more preferably 20 to 40 parts by mass, based on 100 parts by mass of the pigment (B). When the content of binder resin (A-1) is 10 parts by mass or more, sufficient developability is obtained. If the content of the binder resin (A-1) is 80 parts by mass or less, good dispersion stability can be obtained when used as a mill base.
고분자 분산제 (C)의 함유량은, 안료 (B) 100질량부에 대하여, 10 내지 80질량부인 것이 바람직하고, 15 내지 50질량부인 것이 보다 바람직하고, 20 내지 40질량부인 것이 더욱 바람직하다. 고분자 분산제 (C)의 함유량이 10질량부 이상이면, 밀 베이스로 했을 때에 양호한 분산 안정성이 얻어진다. 고분자 분산제 (C)의 함유량이 80질량부 이하이면, 충분한 현상성이 얻어진다.The content of the polymer dispersant (C) is preferably 10 to 80 parts by mass, more preferably 15 to 50 parts by mass, and still more preferably 20 to 40 parts by mass, based on 100 parts by mass of the pigment (B). If the content of the polymer dispersant (C) is 10 parts by mass or more, good dispersion stability is obtained when used as a mill base. If the content of the polymer dispersant (C) is 80 parts by mass or less, sufficient developability is obtained.
용제 (D-1)의 배합량은, 용제 (D-1)을 제외한 안료 분산 조성물의 성분 총합을 100질량부로 했을 때에, 바람직하게는 100 내지 900부, 보다 바람직하게는 120 내지 600부, 더욱 바람직하게는 150 내지 400부이다. 용제 (D-1)의 함유량이 상기 범위 내이면, 적절한 점도를 갖는 안료 분산 조성물이 된다.The compounding amount of solvent (D-1) is preferably 100 to 900 parts, more preferably 120 to 600 parts, and still more preferably 100 parts by mass of the total components of the pigment dispersion composition excluding solvent (D-1). Typically, it is 150 to 400 copies. If the content of the solvent (D-1) is within the above range, a pigment dispersion composition having an appropriate viscosity is obtained.
<감광성 착색 조성물><Photosensitive coloring composition>
고분자 분산제 (C)의 함유량은, 안료 (B) 100질량부에 대하여, 10 내지 80질량부인 것이 바람직하고, 15 내지 50질량부인 것이 보다 바람직하고, 20 내지 40질량부인 것이 더욱 바람직하다. 고분자 분산제 (C)의 함유량이 10질량부 이상이면, 밀 베이스로 했을 때에 양호한 분산 안정성이 얻어진다. 고분자 분산제 (C)의 함유량이 80질량부 이하이면, 충분한 현상성이 얻어진다.The content of the polymer dispersant (C) is preferably 10 to 80 parts by mass, more preferably 15 to 50 parts by mass, and still more preferably 20 to 40 parts by mass, based on 100 parts by mass of the pigment (B). If the content of the polymer dispersant (C) is 10 parts by mass or more, good dispersion stability is obtained when used as a mill base. If the content of the polymer dispersant (C) is 80 parts by mass or less, sufficient developability is obtained.
본 실시 형태의 감광성 착색 조성물은, 상기 안료 분산 조성물과, 결합제 수지 (A-2)와, 반응성 희석제 (E)와, 광중합 개시제 (F)를 함유한다. 본 실시 형태의 감광성 착색 조성물은 용제 (D-2)를 포함해도 된다.The photosensitive coloring composition of this embodiment contains the pigment dispersion composition, binder resin (A-2), reactive diluent (E), and photopolymerization initiator (F). The photosensitive coloring composition of this embodiment may contain a solvent (D-2).
본 실시 형태의 감광성 착색 조성물에 있어서, 안료 (B) 100질량부에 대하여, 상기 결합제 수지 (A-1)과 (A-2)의 합계 결합제 수지 (A)를 50 내지 280질량부, 상기 고분자 분산제 (C)를 10 내지 80질량부, 상기 반응성 희석제 (E)를 40 내지 200질량부, 상기 광중합 개시제 (F)를 0.1 내지 10질량부 함유하는 것이 바람직하다.In the photosensitive coloring composition of the present embodiment, the total binder resin (A) of the binder resin (A-1) and (A-2) is 50 to 280 parts by mass, with respect to 100 parts by mass of the pigment (B), and the polymer. It is preferable to contain 10 to 80 parts by mass of the dispersant (C), 40 to 200 parts by mass of the reactive diluent (E), and 0.1 to 10 parts by mass of the photopolymerization initiator (F).
용제 (D-2)는, 상기 안료 분산 조성물에 포함되어 있는 용제 (D-1)과 공통의 것을 사용해도 되고, 다른 것을 추가 첨부해도 된다.The solvent (D-2) may be the same as the solvent (D-1) contained in the pigment dispersion composition, or another solvent may be added.
〔결합제 수지 (A-2)〕[Binder Resin (A-2)]
결합제 수지 (A-2)는, 감광성 착색 조성물로서 사용할 수 있는 종래 공지된 수지를 특별히 제한 없이 사용할 수 있고, 상기 안료 분산 조성물에 포함되어 있는 결합제 수지 (A-1)과 공통의 것을 사용해도 되고, 다른 것이어도 된다. 또한, 결합제 수지 (A-2)로서 새롭게 추가하지 않고, 결합제 수지 (A-1)을 (A-2)로서 이용해도 된다.As the binder resin (A-2), conventionally known resins that can be used as photosensitive coloring compositions can be used without particular restrictions, and those common to the binder resin (A-1) contained in the pigment dispersion composition may be used. , it can be anything else. In addition, binder resin (A-1) may be used as (A-2) instead of adding it as binder resin (A-2).
상기 결합제 수지 (A-1)과 (A-2)의 합계 결합제 수지 (A)의 함유량은, 안료 (B) 100질량부에 대하여, 50 내지 280질량부인 것이 바람직하고, 75 내지 230질량부인 것이 보다 바람직하고, 100 내지 200질량부인 것이 더욱 바람직하다.The total binder resin (A) content of the binder resin (A-1) and (A-2) is preferably 50 to 280 parts by mass, and 75 to 230 parts by mass, based on 100 parts by mass of the pigment (B). It is more preferable that it is 100 to 200 parts by mass.
〔반응성 희석제 (E)〕[Reactive diluent (E)]
반응성 희석제 (E)는 비닐기, (메트)아크릴로일옥시기 등의 에틸렌성 불포화 이중 결합을 포함하는 저분자 화합물이면 특별히 한정되지 않는다. 반응성 희석제 (C)의 구체예로서는, 스티렌, α-메틸스티렌, α-클로로메틸스티렌, 비닐톨루엔, 디비닐벤젠, 디알릴프탈레이트, 디알릴벤젠포스포네이트 등의 방향족 비닐계 모노머류; 아세트산비닐, 아디프산비닐 등의 폴리카르복실산 모노머류; 메틸(메트)아크릴레이트, 에틸(메트)아크릴레이트, 프로필(메트)아크릴레이트, 부틸(메트)아크릴레이트, β-히드록시에틸(메트)아크릴레이트, 히드록시프로필(메트)아크릴레이트, 에틸렌글리콜디(메트)아크릴레이트, 디에틸렌글리콜디(메트)아크릴레이트, 프로필렌글리콜디(메트)아크릴레이트, 에틸렌글리콜디(메트)아크릴레이트, 트리메틸올프로판디(메트)아크릴레이트, 트리메틸올프로판트리(메트)아크릴레이트, 펜타에리트리톨테트라(메트)아크릴레이트, 디펜타에리트리톨헥사(메트)아크릴레이트, 트리스(히드록시에틸)이소시아누레이트의 트리(메트)아크릴레이트 등의 (메트)아크릴계 모노머; 트리알릴시아누레이트 등을 들 수 있다. 이들은, 단독으로 사용해도 되고, 2종 이상을 조합하여 사용해도 된다. 이들 중에서도, (메트)아크릴로일옥시기를 복수 갖는 화합물이 바람직하고, 트리메틸올프로판트리(메트)아크릴레이트, 펜타에리트리톨테트라(메트)아크릴레이트, 디펜타에리트리톨헥사(메트)아크릴레이트, 트리스(히드록시에틸)이소시아누레이트의 트리(메트)아크릴레이트 등의 (메트)아크릴로일옥시기를 3개 이상 갖는 화합물이 보다 바람직하다.The reactive diluent (E) is not particularly limited as long as it is a low molecular weight compound containing an ethylenically unsaturated double bond such as a vinyl group or (meth)acryloyloxy group. Specific examples of the reactive diluent (C) include aromatic vinyl monomers such as styrene, α-methylstyrene, α-chloromethylstyrene, vinyltoluene, divinylbenzene, diallyl phthalate, and diallylbenzenephosphonate; polycarboxylic acid monomers such as vinyl acetate and vinyl adipic acid; Methyl (meth)acrylate, ethyl (meth)acrylate, propyl (meth)acrylate, butyl (meth)acrylate, β-hydroxyethyl (meth)acrylate, hydroxypropyl (meth)acrylate, ethylene glycol Di(meth)acrylate, diethylene glycol di(meth)acrylate, propylene glycol di(meth)acrylate, ethylene glycol di(meth)acrylate, trimethylolpropane di(meth)acrylate, trimethylolpropane tri( (meth)acrylic monomers such as meth)acrylate, pentaerythritol tetra(meth)acrylate, dipentaerythritol hexa(meth)acrylate, and tri(meth)acrylate of tris(hydroxyethyl)isocyanurate. ; Triallyl cyanurate, etc. can be mentioned. These may be used individually, or may be used in combination of two or more types. Among these, compounds having multiple (meth)acryloyloxy groups are preferable, such as trimethylolpropane tri(meth)acrylate, pentaerythritol tetra(meth)acrylate, dipentaerythritol hexa(meth)acrylate, and tris Compounds having three or more (meth)acryloyloxy groups, such as tri(meth)acrylate of (hydroxyethyl)isocyanurate, are more preferable.
반응성 희석제 (E)의 함유량은, 안료 (B) 100질량부에 대하여, 40 내지 200질량부인 것이 바람직하고, 60 내지 180질량부인 것이 보다 바람직하고, 80 내지 160질량부인 것이 더욱 바람직하다.The content of the reactive diluent (E) is preferably 40 to 200 parts by mass, more preferably 60 to 180 parts by mass, and still more preferably 80 to 160 parts by mass, based on 100 parts by mass of the pigment (B).
〔광중합 개시제 (F)〕[Photopolymerization initiator (F)]
광중합 개시제 (F)는 광 라디칼 발생제가 바람직하고, 그 구체예로서는, 벤조인, 벤조인메틸에테르, 벤조인에틸에테르 등의 벤조인과 그 알킬에테르류; 아세토페논, 2,2-디메톡시-2-페닐아세토페논, 1,1-디클로로아세토페논, 4-(1-t-부틸디옥시-1-메틸에틸)아세토페논 등의 아세토페논류; 2-메틸안트라퀴논, 2-아밀안트라퀴논, 2-t-부틸안트라퀴논, 1-클로로안트라퀴논 등의 안트라퀴논류; 2,4-디메틸티오크산톤, 2,4-디이소프로필티오크산톤, 2-클로로티오크산톤 등의 티오크산톤류; 아세토페논디메틸케탈, 벤질디메틸케탈 등의 케탈류; 벤조페논, 4-(1-t-부틸디옥시-1-메틸에틸)벤조페논, 3,3',4,4'-테트라키스(t-부틸디옥시카르보닐)벤조페논 등의 벤조페논류; 2-메틸-1-[4-(메틸티오)페닐]-2-모르폴리노-프로판-1-온; 2-벤질-2-디메틸아미노-1-(4-모르폴리노페닐)부타논-1; 아실포스핀옥사이드류; 및 크산톤류 등을 들 수 있다. 이들은, 단독으로 사용해도 되고, 2종 이상을 조합하여 사용해도 된다.The photopolymerization initiator (F) is preferably a photo radical generator, and specific examples thereof include benzoin and its alkyl ethers such as benzoin, benzoin methyl ether, and benzoin ethyl ether; Acetophenones such as acetophenone, 2,2-dimethoxy-2-phenylacetophenone, 1,1-dichloroacetophenone, and 4-(1-t-butyldioxy-1-methylethyl)acetophenone; Anthraquinones such as 2-methylanthraquinone, 2-amylanthraquinone, 2-t-butylanthraquinone, and 1-chloroanthraquinone; Thioxanthone such as 2,4-dimethylthioxanthone, 2,4-diisopropylthioxanthone, and 2-chlorothioxanthone; Ketals such as acetophenone dimethyl ketal and benzyl dimethyl ketal; Benzophenone, such as benzophenone, 4-(1-t-butyldioxy-1-methylethyl)benzophenone, and 3,3',4,4'-tetrakis(t-butyldioxycarbonyl)benzophenone. ; 2-methyl-1-[4-(methylthio)phenyl]-2-morpholino-propan-1-one; 2-benzyl-2-dimethylamino-1-(4-morpholinophenyl)butanone-1; Acylphosphine oxides; and xanthons. These may be used individually, or may be used in combination of two or more types.
광중합 개시제 (F)의 함유량은, 안료 (B) 100질량부에 대하여, 0.1 내지 10질량부인 것이 바람직하고, 1 내지 9질량부인 것이 보다 바람직하고, 2 내지 8질량부인 것이 더욱 바람직하다.The content of the photopolymerization initiator (F) is preferably 0.1 to 10 parts by mass, more preferably 1 to 9 parts by mass, and even more preferably 2 to 8 parts by mass, based on 100 parts by mass of the pigment (B).
〔그 밖의 성분〕[Other ingredients]
본 실시 형태의 감광성 착색 조성물은, 상기의 성분에 추가하여, 소정의 특성을 부여하기 위하여 광 산 발생제, 광 염기 발생제, 커플링제, 레벨링제 등의 공지된 첨가제, 필러 등을 배합해도 된다. 이들 성분의 배합량은, 본 발명의 효과를 저해하지 않는 범위라면 특별히 한정되지 않는다.In addition to the above components, the photosensitive coloring composition of the present embodiment may contain known additives such as a photoacid generator, a photobase generator, a coupling agent, and a leveling agent, fillers, etc., in order to impart desired properties. . The mixing amount of these components is not particularly limited as long as it is within a range that does not impair the effect of the present invention.
<감광성 착색 조성물의 제조 방법><Method for producing photosensitive coloring composition>
본 실시 형태의 감광성 착색 조성물은, 공지된 혼합 장치를 사용하여, 상기의 각 성분을 혼합함으로써 제조할 수 있다. 예를 들어, 먼저 안료 분산 조성물을 조정한 후, 결합제 수지 (A-2), 반응성 희석제 (E), 광중합 개시제 (F) 등을 차례로 혼합함으로써 제조할 수 있다. 또한, 필요에 따라, 안료 분산 조성물에 포함되어 있는 용제 (D-1) 이외에, 용제 (D-2)를 첨가해도 된다. 그 경우, 감광성 착색 조성물에 포함되어 있는 용제 (D)가, 용제 (D-1)과 용제 (D-2)를 포함한다. 또한, 용제 (D-2)는 용제 (D-1)과 공통의 것을 사용해도 되고, 다른 것이어도 된다.The photosensitive coloring composition of this embodiment can be manufactured by mixing each of the above components using a known mixing device. For example, it can be prepared by first adjusting the pigment dispersion composition, and then sequentially mixing the binder resin (A-2), the reactive diluent (E), the photopolymerization initiator (F), etc. Additionally, if necessary, solvent (D-2) may be added in addition to solvent (D-1) contained in the pigment dispersion composition. In that case, the solvent (D) contained in the photosensitive coloring composition includes solvent (D-1) and solvent (D-2). In addition, the solvent (D-2) may be the same as the solvent (D-1), or may be different.
상기와 같이 하여 얻어지는 감광성 착색 조성물은, 안료 분산성이나 안료 분산 조성물의 보존 안정성이 충분하고, 고휘도화의 달성이 가능하고, 또한 분산제나 착색제의 블리드 아웃을 억제하여 우수한 내열성, 내용제성, 패턴 밀착성, 현상성 등의 각종 레지스트 특성을 발현할 수 있다. 그 결과, 신뢰성이 우수한 착색 패턴을 형성할 수 있다. 즉, 상기 감광성 착색 조성물을 사용함으로써, 신뢰성이 우수한 컬러 필터를 제공할 수 있다.The photosensitive coloring composition obtained as described above has sufficient pigment dispersibility and storage stability of the pigment dispersion composition, can achieve high brightness, and suppresses bleed-out of the dispersant and colorant, and has excellent heat resistance, solvent resistance, and pattern adhesion. , various resist characteristics such as developability can be expressed. As a result, a highly reliable coloring pattern can be formed. That is, by using the photosensitive coloring composition, a highly reliable color filter can be provided.
<수지 경화막><Resin cured film>
본 실시 형태의 수지 경화막을, 예를 들어 이하의 방법으로 제작할 수 있다.The resin cured film of this embodiment can be produced, for example, by the following method.
본 실시 형태의 감광성 착색 조성물을, 유리 등의 기판 상에, 목적에 따라 최종의 경화 도막의 평균 두께가 소정의 값이 되도록 도막한 후, 예를 들어 50 내지 150℃에서 1 내지 50분간 가열하고 용제를 휘발시킨다. 다음으로 도막의 전체 면을 노광(예를 들어, 램프로서, 우시오 덴키 가부시키가이샤제 USH-250BY를 사용, 노광량 40mJ/㎠)시키고, 또한, 예를 들어 50 내지 200℃에서 10 내지 180분간 베이킹함으로써 경화 도막이 얻어진다. 또한, 포토마스크를 통해 도막을 노광하고, 알칼리 현상액으로 현상하면 소정의 착색 패턴을 갖는 경화 도막이 얻어진다.The photosensitive coloring composition of the present embodiment is coated on a substrate such as glass so that the average thickness of the final cured film is a predetermined value depending on the purpose, and then heated at, for example, 50 to 150°C for 1 to 50 minutes. Volatilize the solvent. Next, the entire surface of the coating film is exposed (for example, using USH-250BY manufactured by Ushio Denki Co., Ltd. as a lamp, exposure amount of 40 mJ/cm2), and then baked at, for example, 50 to 200°C for 10 to 180 minutes. By doing this, a cured coating film is obtained. Additionally, by exposing the coating film through a photomask and developing it with an alkaline developer, a cured coating film with a predetermined coloring pattern is obtained.
<컬러 필터><Color Filter>
이어서, 본 발명의 감광성 착색 조성물이 경화물을 포함하는 착색 패턴을 갖는 컬러 필터에 대하여 설명한다. 본 발명의 컬러 필터는, 상기의 감광성 착색 조성물을 사용하여 형성되는 착색 패턴을 갖는다. 컬러 필터는, 통상 기판과, 그 위에 형성되는 RGB의 화소, 각각의 화소의 경계에 형성되는 블랙 매트릭스 및 화소와 블랙 매트릭스 상에 형성되는 보호막으로 구성된다. 이 구성에 있어서, 화소 및 블랙 매트릭스(착색 패턴)가 상기의 감광성 착색 조성물을 사용하여 형성되는 것을 제외하면, 그 밖의 구성은 공지된 것을 채용할 수 있다.Next, a color filter having a coloring pattern containing a cured product of the photosensitive coloring composition of the present invention will be described. The color filter of the present invention has a coloring pattern formed using the photosensitive coloring composition described above. A color filter usually consists of a substrate, RGB pixels formed thereon, a black matrix formed at the boundary of each pixel, and a protective film formed on the pixels and the black matrix. In this configuration, except that the pixels and the black matrix (coloring pattern) are formed using the above-mentioned photosensitive coloring composition, other known configurations can be adopted.
이어서, 컬러 필터의 제조 방법의 일 실시 형태에 대하여 설명한다. 먼저, 기판 상에 착색 패턴을 형성한다. 구체적으로는, 기판 상에, 블랙 매트릭스 및 RGB의 화소를 순차 형성한다. 기판의 재질은, 특별히 한정되는 것은 아니고, 유리 기판, 실리콘 기판, 폴리카르보네이트 기판, 폴리에스테르 기판, 폴리아미드 기판, 폴리아미드이미드 기판, 폴리이미드 기판, 알루미늄 기판, 프린트 배선 기판, 어레이 기판 등을 적절히 사용할 수 있다.Next, one embodiment of a method for manufacturing a color filter will be described. First, a colored pattern is formed on the substrate. Specifically, a black matrix and RGB pixels are sequentially formed on the substrate. The material of the substrate is not particularly limited and includes glass substrate, silicon substrate, polycarbonate substrate, polyester substrate, polyamide substrate, polyamideimide substrate, polyimide substrate, aluminum substrate, printed wiring substrate, array substrate, etc. can be used appropriately.
착색 패턴은, 포토리소그래피법에 의해 형성할 수 있다. 구체적으로는, 상기의 감광성 착색 조성물을 기판 상에 도포하여 도포막을 형성한 후, 소정의 패턴의 포토마스크를 통해 도포막을 노광하여 노광 부분을 광경화시킨다. 그리고, 미노광 부분을 알칼리 수용액으로 현상한 후, 베이킹함으로써, 소정의 착색 패턴을 형성할 수 있다.The colored pattern can be formed by photolithography. Specifically, the above photosensitive coloring composition is applied on a substrate to form a coating film, and then the coating film is exposed through a photomask with a predetermined pattern to photocure the exposed portion. Then, a predetermined colored pattern can be formed by developing the unexposed portion with an aqueous alkaline solution and then baking it.
감광성 착색 조성물의 도포 방법으로서는, 특별히 한정되지 않지만, 스크린 인쇄법, 롤 코팅법, 커튼 코팅법, 스프레이 코팅법, 스핀 코팅법 등을 사용할 수 있다. 또한, 감광성 착색 조성물의 도포 후, 필요에 따라, 순환식 오븐, 적외선 히터, 핫 플레이트 등의 가열 수단을 사용하여 가열함으로써 용제 (D)를 휘발시켜도 된다. 가열 조건은, 특별히 한정되지 않고, 사용하는 감광성 착색 조성물의 종류에 따라 적절히 설정하면 된다. 일반적으로는, 50℃ 내지 120℃의 온도에서 30초 내지 30분 가열하면 된다.The application method of the photosensitive coloring composition is not particularly limited, and screen printing, roll coating, curtain coating, spray coating, spin coating, etc. can be used. Additionally, after application of the photosensitive coloring composition, if necessary, the solvent (D) may be volatilized by heating using a heating means such as a circulation oven, an infrared heater, or a hot plate. Heating conditions are not particularly limited and may be set appropriately depending on the type of photosensitive coloring composition to be used. In general, heating may be performed at a temperature of 50°C to 120°C for 30 seconds to 30 minutes.
이어서, 형성된 도막에 네가티브형의 마스크를 통해 자외선, 엑시머 레이저 광 등의 활성 에너지선을 조사하여 부분적으로 노광한다. 조사하는 에너지선량은, 감광성 착색 조성물의 조성에 따라서 적절히 선택하면 되고, 예를 들어 30 내지 2000mJ/㎠인 것이 바람직하다. 노광에 사용되는 광원으로서는, 특별히 한정되지 않지만, 저압 수은 램프, 중압 수은 램프, 고압 수은 램프, 크세논 램프, 메탈 할 라이드 램프 등을 사용할 수 있다.Next, the formed coating film is partially exposed by irradiating active energy rays such as ultraviolet rays or excimer laser light through a negative mask. The energy dose to be irradiated may be appropriately selected depending on the composition of the photosensitive coloring composition, and is preferably, for example, 30 to 2000 mJ/cm2. The light source used for exposure is not particularly limited, and a low-pressure mercury lamp, a medium-pressure mercury lamp, a high-pressure mercury lamp, a xenon lamp, a metal halide lamp, etc. can be used.
현상에 사용되는 알칼리 수용액으로서는, 특별히 한정되지 않지만, 탄산나트륨, 탄산칼륨, 탄산칼슘, 수산화나트륨, 수산화칼륨 등의 수용액; 에틸아미노기, 디에틸아미노기, 디메틸에탄올아미노기 등의 아미노기계 화합물의 수용액; 테트라메틸암모늄, 3-메틸-4-아미노-N,N-디에틸아닐린, 3-메틸-4-아미노-N-에틸-N-β-히드록시에틸아닐린, 3-메틸-4-아미노-N-에틸-N-β-메탄술폰아미드에틸아닐린, 3-메틸-4-아미노-N-에틸-N-β-메톡시에틸아닐린 및 이들의 황산염, 염산염 또는 p-톨루엔술폰산염 등의 p-페닐렌디아미노기계 화합물의 수용액 등을 사용할 수 있다. 또한, 이들의 수용액에는, 필요에 따라 소포제나 계면 활성제를 첨가해도 된다. 또한, 상기의 알칼리 수용액에 의한 현상 후, 수세하여 건조시키는 것이 바람직하다.The alkaline aqueous solution used for development is not particularly limited, but includes aqueous solutions such as sodium carbonate, potassium carbonate, calcium carbonate, sodium hydroxide, and potassium hydroxide; Aqueous solutions of amino compounds such as ethyl amino group, diethylamino group, and dimethylethanolamino group; Tetramethylammonium, 3-methyl-4-amino-N,N-diethylaniline, 3-methyl-4-amino-N-ethyl-N-β-hydroxyethylaniline, 3-methyl-4-amino-N -P-phenyl such as ethyl-N-β-methanesulfonamideethylaniline, 3-methyl-4-amino-N-ethyl-N-β-methoxyethylaniline and their sulfate, hydrochloride or p-toluenesulfonate salts. An aqueous solution of a lendiamino compound may be used. Additionally, an antifoaming agent or surfactant may be added to these aqueous solutions as needed. In addition, after developing with the aqueous alkaline solution described above, it is preferable to wash with water and dry.
또한, 베이킹의 조건은, 특별히 한정되지 않고, 사용하는 감광성 착색 조성물의 종류에 따라 가열 처리를 행하면 된다. 일반적으로는, 80 내지 250℃에서 10 내지 60분간 가열하면 된다.In addition, the baking conditions are not particularly limited, and heat treatment may be performed depending on the type of photosensitive coloring composition to be used. In general, heating at 80 to 250°C for 10 to 60 minutes is sufficient.
상기와 같은 도포, 노광, 현상 및 베이킹을, 블랙 매트릭스용의 감광성 착색 조성물 및 적색, 녹색, 청색의 화소용 감광성 착색 조성물을 사용하여 순차 반복함으로써, 원하는 착색 패턴을 형성할 수 있다. 그 후, 착색 패턴(RGB의 각 화소 및 블랙 매트릭스) 상에 보호막을 형성하지만, 보호막으로서는, 특별히 한정되지 않고, 공지된 것을 사용하여 형성하면 된다.By sequentially repeating the above application, exposure, development and baking using the photosensitive coloring composition for the black matrix and the photosensitive coloring composition for red, green, and blue pixels, a desired coloring pattern can be formed. After that, a protective film is formed on the colored pattern (each pixel of RGB and the black matrix). The protective film is not particularly limited and may be formed using a known protective film.
이와 같이 하여 제조되는 컬러 필터는, 안료가 균일한 미세화를 실현함으로써 안료 분산성이 우수하고, 고휘도화를 달성하고, 우수한 내열성, 내용제성, 밀착성을 갖고, 분산제나 착색제의 블리드 아웃 억제를 기대할 수 있다.The color filter manufactured in this way has excellent pigment dispersibility by realizing uniform micronization of the pigment, achieves high brightness, has excellent heat resistance, solvent resistance, and adhesion, and can be expected to suppress bleed out of dispersants and colorants. there is.
<화상 표시 소자><Image display element>
본 실시 형태의 화상 표시 소자는, 상기의 컬러 필터를 구비한 화상 표시 소자이고, 그 구체예로서, 액정 표시 소자, 유기 EL 표시 소자, CCD 소자나 CMOS 소자 등의 고체 촬상 소자 등을 들 수 있다. 본 실시 형태의 화상 표시 소자의 제조는, 상기의 컬러 필터를 사용하는 것 이외, 통상법에 따라서 행하면 된다. 예를 들어, 액정 표시 소자를 제조하는 경우에는, 기판 상에, 상기 컬러 필터를 형성하고, 이어서 전극, 스페이서 등을 순차 형성한다. 그리고, 다른 1매의 기판 상에 전극 등을 형성하고, 양자를 맞대어 붙여서 소정량의 액정을 주입, 밀봉하면 된다.The image display element of this embodiment is an image display element provided with the color filter described above, and specific examples thereof include solid-state imaging elements such as liquid crystal display elements, organic EL display elements, CCD elements and CMOS elements. . Manufacturing of the image display element of this embodiment may be performed according to conventional methods other than using the color filter described above. For example, when manufacturing a liquid crystal display element, the color filter is formed on a substrate, and then electrodes, spacers, etc. are sequentially formed. Then, an electrode or the like is formed on another substrate, the two are placed against each other, and a predetermined amount of liquid crystal is injected and sealed.
실시예Example
이하, 실시예를 참조하여 본 발명을 상세하게 설명하지만, 본 발명은 이들 실시예에 의해 한정되지 않는다. 또한, 이 실시예에 있어서, 부 및 퍼센트라고 하는 것은 특별히 언급하지 않는 한, 모두 질량 기준이다.Hereinafter, the present invention will be described in detail with reference to examples, but the present invention is not limited to these examples. Additionally, in this example, parts and percentages are all based on mass, unless otherwise specified.
<결합제 수지 (A-1)이 갖는 관능기 (X)의 함유량><Content of functional group (X) in binder resin (A-1)>
결합제 수지 (A-1)의 질량당, 관능기 (X)의 몰수이고, 원료 모노머의 사용량에 기초하여 산출한 계산값이다.It is the number of moles of functional group (X) per mass of binder resin (A-1), and is a calculated value calculated based on the amount of raw material monomer used.
<관능기 (X) 함유 화합물량><Amount of compound containing functional group (X)>
관능기 (X) 함유 화합물량은, 결합제 수지 (A-1)의 원료로서의 전체 모노머 100몰%에 대하여, 관능기 (X) 함유 화합물의 사용량(몰%)이다. 실제의 결합제 수지 (A-1)의 원료에 포함되어 있는 전체 모노머의 사용량 및 관능기 (X) 함유 화합물의 사용량에 기초하여 산출한 계산값이다.The amount of the functional group (X)-containing compound is the amount (mol%) of the functional group (X)-containing compound used relative to 100 mol% of all monomers as the raw material for the binder resin (A-1). This is a calculated value calculated based on the amount of total monomers and the amount of the functional group (X)-containing compound contained in the actual raw materials of the binder resin (A-1).
<미반응 관능기 (X)양><Amount of unreacted functional group (X)>
미반응 관능기 (X)양은, 상기 관능기 (X) 함유 화합물량으로부터 부가 반응에서 소비한 관능기 (X)양을 제외한 후의 양이다. 상기 산출한 관능기 (X) 함유 화합물량, 결합제 수지 (A-1)에 대한 부가 반응을 위해 첨가한 모노머의 실제 사용량에 기초하여 산출한 계산값이다.The amount of unreacted functional group (X) is the amount after subtracting the amount of functional group (X) consumed in the addition reaction from the amount of the compound containing the functional group (X). This is a calculated value based on the amount of the functional group (X)-containing compound calculated above and the actual amount of monomer added for the addition reaction to the binder resin (A-1).
<결합제 수지 (A-1)의 관능기 (X)의 총량에 대한, 부가율(C=C 도입률)> <Addition rate (C=C introduction rate) relative to the total amount of functional group (X) of binder resin (A-1)>
관능기 (Y) 및 에틸렌성 불포화기를 갖는 화합물을 관능기 (X)에 부가하고, 결합제 수지 (A-1)에 에틸렌성 불포화기를 도입하는 경우에는, 그 부가율은, 관능기 (X)의 총 몰수 100몰%에 대하여, 상기 화합물의 몰비(몰%)이다. 실제의 관능기 (X) 함유 모노머의 사용량 및 부가 반응용 상기 화합물의 사용량에 기초하여 산출하였다.When a compound having a functional group (Y) and an ethylenically unsaturated group is added to the functional group (X) and an ethylenically unsaturated group is introduced into the binder resin (A-1), the addition rate is 100 moles of the total number of moles of the functional group (X). % is the molar ratio (mol%) of the compound. It was calculated based on the actual amount of functional group (X)-containing monomer used and the amount of the compound used for the addition reaction.
<고형분 농도><Solid content concentration>
결합제 수지 (A-1) 합성 후의 용액 중, 용제를 제외한 성분을 합계한 비율이다.It is the ratio of the total components excluding the solvent in the solution after the binder resin (A-1) synthesis.
<고형분 산가><Solid content acid value>
산가는, JIS K6901 5.3을 따라서 브로모티몰 블루와 페놀 레드의 혼합 지시약을 사용하여 측정하였다.The acid value was measured using a mixed indicator of bromothymol blue and phenol red according to JIS K6901 5.3.
<중량 평균 분자량, 수 평균 분자량의 측정법><Method for measuring weight average molecular weight and number average molecular weight>
중량 평균 분자량, 수 평균 분자량은, 겔 투과 크로마토그래피(GPC)를 사용하여, 하기 조건에서 측정한 표준 폴리스티렌 환산 중량 평균 분자량, 수 평균 분자량을 의미한다.The weight average molecular weight and number average molecular weight mean the standard polystyrene conversion weight average molecular weight and number average molecular weight measured under the following conditions using gel permeation chromatography (GPC).
칼럼: 쇼덱스(등록 상표) LF-804+LF-804(쇼와 덴코 가부시키가이샤제)Column: Showex (registered trademark) LF-804+LF-804 (made by Showa Denko Co., Ltd.)
칼럼 온도: 40℃Column temperature: 40℃
시료: 측정 대상물의 0.2% 테트라히드로푸란 용액Sample: 0.2% tetrahydrofuran solution of the measurement object
전개 용매: 테트라히드로푸란Development solvent: tetrahydrofuran
검출기: 시차 굴절계(쇼덱스 RI-71S)(쇼와 덴코 가부시키가이샤제)Detector: Differential refractometer (Shodex RI-71S) (made by Showa Denko Co., Ltd.)
유속: 1mL/minFlow rate: 1mL/min
<아미노기 함유 고분자 화합물 (c-1)이 갖는 아미노기의 총량에 대한, 할로겐 화합물 (c-2)의 부가율><Addition rate of halogen compound (c-2) relative to the total amount of amino groups possessed by amino group-containing polymer compound (c-1)>
아미노기 함유 고분자 화합물 (c-1)이 갖는 아미노기의 총량에 대한, 할로겐 화합물 (c-2)의 부가율은, 실제의 고분자 화합물 (c-1) 및 상기 관능기 (Y)를 갖는 할로겐 화합물 (c-2)의 사용량, 고분자 분산제 (C)의 관능기 (Y)의 양, 고분자 분산제 (C)의 아민가, 고분자 분산제 (C)의 3급 아민가에 기초하여 산출한 계산값이다.The addition ratio of the halogen compound (c-2) relative to the total amount of amino groups in the amino group-containing polymer compound (c-1) is the difference between the actual polymer compound (c-1) and the halogen compound (c-) having the functional group (Y). It is a calculated value calculated based on the usage amount of 2), the amount of functional group (Y) of the polymer dispersant (C), the amine value of the polymer dispersant (C), and the tertiary amine value of the polymer dispersant (C).
<고분자 분산제 (C)의 아민가><Amine value of polymer dispersant (C)>
고분자 분산제 (C)의 아민가는, 전술한 방법(JIS K7237)에 기초하여 측정하였다. 또한, 본 명세서에 기재된 계산 방법을 사용하여 3급 아민가를 산출하였다. 각각의 결과를, 표 1a 및 표 1b에 나타낸다.The amine titer of the polymer dispersant (C) was measured based on the method described above (JIS K7237). Additionally, the tertiary amine number was calculated using the calculation method described herein. Each result is shown in Table 1a and Table 1b.
<고분자 분산제 (C)의 관능기 (Y)양><Amount of functional group (Y) of polymer dispersant (C)>
고분자 분산제 (C)의 질량당, 관능기 (Y)의 몰수이고, 실제의 고분자 화합물 (c-1) 및 상기 관능기 (Y)를 갖는 할로겐 화합물 (c-2)의 사용량에 기초하여 산출한 계산값이다.It is the number of moles of the functional group (Y) per mass of the polymer dispersant (C), and is a calculated value calculated based on the actual usage amount of the polymer compound (c-1) and the halogen compound (c-2) having the functional group (Y). am.
결합제 수지 (A-1)의 제조예를 이하에 나타낸다.A production example of binder resin (A-1) is shown below.
「결합제 수지의 합성(합성예 a1 내지 a12, 비교 합성예 a'1 내지 a'3)」“Synthesis of binder resin (Synthesis Examples a1 to a12, Comparative Synthesis Examples a’1 to a’3)”
<합성예 a1><Synthesis example a1>
교반 장치, 적하 깔때기, 콘덴서, 온도계 및 가스 도입관을 구비한 플라스크에, 73.7g의 프로필렌글리콜모노메틸에테르아세테이트를 넣은 후, 질소 가스로 치환하면서 교반하고, 120℃로 승온하였다. 이어서, 27.5g의 트리시클로데카닐메타크릴레이트, 66.0g의 벤질메타크릴레이트 및 45.0g의 아크릴산, 16.3g의 에틸렌글리콜메타크릴레이트(HEMA)를 포함하는 모노머 혼합물에, 중합 개시제로서 15.5g의 t-부틸퍼옥시-2-에틸헥사노에이트를 첨가한 것을 적하 깔때기로부터 상기 플라스크 중에 적하하였다. 적하 종료 후, 120℃에서 2시간 교반하여 공중합 반응을 행하였다.73.7 g of propylene glycol monomethyl ether acetate was added to a flask equipped with a stirring device, a dropping funnel, a condenser, a thermometer, and a gas introduction tube, and the mixture was stirred while being replaced with nitrogen gas, and the temperature was raised to 120°C. Next, 15.5 g of as a polymerization initiator was added to the monomer mixture containing 27.5 g of tricyclodecanyl methacrylate, 66.0 g of benzyl methacrylate, 45.0 g of acrylic acid, and 16.3 g of ethylene glycol methacrylate (HEMA). t-Butylperoxy-2-ethylhexanoate was added dropwise into the flask from a dropping funnel. After completion of the dropwise addition, the mixture was stirred at 120°C for 2 hours to perform a copolymerization reaction.
이어서, 상기 플라스크 내를 드라이에어로 치환한 후, 26.6g의 글리시딜메타크릴레이트, 촉매로서 0.5g의 트리페닐포스핀 및 중합 금지제로서 0.4g의 히드로퀴논모노메틸에테르를 첨가하고, 120℃에서 5시간 반응을 행한 후, 용제로서 프로필렌글리콜모노메틸에테르아세테이트 132g을 첨가하여 고형분이 40%가 되도록 조제하고, 관능기 (Y)로서 히드록시기, 알칼리 가용성으로서 카르복실산을 갖는 결합제 수지 (A-1) 또는 (A-2)로서 시료 PR1을 얻었다.Next, after replacing the flask with dry air, 26.6 g of glycidyl methacrylate, 0.5 g of triphenylphosphine as a catalyst, and 0.4 g of hydroquinone monomethyl ether as a polymerization inhibitor were added, and the mixture was heated at 120°C. After conducting the reaction for 5 hours, 132 g of propylene glycol monomethyl ether acetate was added as a solvent to adjust the solid content to 40%, and a binder resin (A-1) having a hydroxyl group as a functional group (Y) and a carboxylic acid as an alkali soluble Alternatively, sample PR1 was obtained as (A-2).
<합성예 a2 내지 a12, 비교 합성예 a'1 내지 a'3><Synthesis Examples a2 to a12, Comparative Synthesis Examples a'1 to a'3>
표 2a 및 표 2b에 기재된 모노머를 사용하고, 중합 온도, 모노머 비율 이외는, 합성예 a1과 마찬가지의 방법에 따라, 결합제 수지 (A-1) 또는 (A-2)로서 시료 PR2 내지 PR12, cPR1 내지 cPR3을 얻었다.Using the monomers shown in Tables 2a and 2b, samples PR2 to PR12 and cPR1 were prepared as binder resins (A-1) or (A-2) in the same manner as in Synthesis Example a1, except for polymerization temperature and monomer ratio. to obtain cPR3.
[표 1a][Table 1a]
[표 1b][Table 1b]
표 1a 및 표 1b 중 표기의 설명은 이하이다.The description of the notation in Table 1a and Table 1b is as follows.
TCDMA: 트리시클로데카닐메타크릴레이트TCDMA: Tricyclodecanyl methacrylate
BZMA: 벤질메타크릴레이트BZMA: Benzyl methacrylate
2EHA: 2-에틸헥실아크릴레이트2EHA: 2-ethylhexyl acrylate
HEMA: 히드록시에틸메타크릴레이트HEMA: Hydroxyethyl methacrylate
4HBA: 4-히드록시부틸아크릴레이트4HBA: 4-hydroxybutylacrylate
MAA: 메타크릴산MAA: methacrylic acid
AA: 아크릴산AA: Acrylic acid
MOI-DEM: 말론산-2-[[[2-메틸-1-옥소-2-프로페닐]옥시]에틸]아미노]카르보닐]-1,3디에틸에스테르MOI-DEM: malonic acid-2-[[[2-methyl-1-oxo-2-propenyl]oxy]ethyl]amino]carbonyl]-1,3 diethyl ester
MOI-BP: 2-[(3,5-디메틸피라졸릴)카르보닐아미노]에틸메타크릴레이트MOI-BP: 2-[(3,5-dimethylpyrazolyl)carbonylamino]ethyl methacrylate
MOI-BM: 2-[0-(1'-메틸프로필리덴아미노)카르복시아미노]에틸메타크릴레이트MOI-BM: 2-[0-(1'-methylpropylidene amino)carboxyamino]ethyl methacrylate
GMA: 글리시딜메타크릴레이트GMA: Glycidyl methacrylate
3EOXMA: (3-에틸옥세탄-3-일)메틸메타크릴레이트3EOXMA: (3-ethyloxetan-3-yl)methyl methacrylate
고분자 분산제 (C)의 제조예를 이하에 나타낸다.A production example of the polymer dispersant (C) is shown below.
「아미노기 함유 고분자 화합물 (c-1)의 합성(합성예 b1 내지 b2)」“Synthesis of amino group-containing polymer compound (c-1) (Synthesis examples b1 to b2)”
<합성예 b1><Synthesis example b1>
교반 장치, 적하 깔때기, 콘덴서, 온도계 및 가스 도입관을 구비한 플라스크에, 133g의 프로필렌글리콜모노메틸에테르아세테이트, 80.5g의 이소보르닐메타크릴레이트 및 촉매로서 13.2g의 테트라메틸에틸렌디아민을 넣고, 플라스크 내를 질소로 치환하면서 50℃에서 1시간 교반하였다. 그 후, 촉매로서 9.3g의 브로모이소부티르산에틸 및 5.6g의 염화제1 구리를 넣고, 플라스크를 110℃로 승온한 후 4시간 중합 반응을 행하였다. 반응 후, 용액을 샘플링하여 불휘발분을 측정하고, 불휘발분으로부터 환산하여 중합 전화율이 98% 이상인 것을 확인한 후, 61g의 프로필렌글리콜모노메틸에테르아세테이트 및 3급 아미노기를 갖는 모노머로서 19.5g의 디메틸아미노에틸메타크릴레이트를 첨가하고, 또한 110℃에서 2시간 반응을 행하였다. 반응 후, 다시 용액을 샘플링하여 불휘발분을 측정하고, 불휘발분으로부터 환산하여 중합 전화율이 98% 이상인 것을 확인한 후, 용액을 냉각하였다. 마지막으로, 용제로서 프로필렌글리콜모노메틸에테르아세테이트를 고형분이 40.0%가 되도록 첨가하고, 3급 아미노기를 함유하는 고분자 화합물 (c-1)로서 bPD1(아민가 70mgKOH/g, 중량 평균 분자량 5500)을 얻었다.In a flask equipped with a stirring device, dropping funnel, condenser, thermometer, and gas introduction tube, 133 g of propylene glycol monomethyl ether acetate, 80.5 g of isobornyl methacrylate, and 13.2 g of tetramethylethylenediamine as a catalyst were added, The flask was stirred at 50°C for 1 hour while purging with nitrogen. After that, 9.3 g of ethyl bromoisobutyrate and 5.6 g of cuprous chloride were added as catalysts, the flask was heated to 110°C, and a polymerization reaction was performed for 4 hours. After the reaction, the solution was sampled to measure the non-volatile content, and after confirming that the polymerization conversion rate was 98% or more in terms of the non-volatile content, 61 g of propylene glycol monomethyl ether acetate and 19.5 g of dimethylaminoethyl as a monomer having a tertiary amino group were added. Methacrylate was added, and reaction was performed at 110°C for 2 hours. After the reaction, the solution was sampled again to measure the non-volatile content, and after confirming that the polymerization conversion ratio was 98% or more in conversion from the non-volatile content, the solution was cooled. Finally, propylene glycol monomethyl ether acetate was added as a solvent so that the solid content was 40.0%, and bPD1 (amine value 70 mgKOH/g, weight average molecular weight 5500) was obtained as a polymer compound (c-1) containing a tertiary amino group.
<합성예 b2><Synthesis example b2>
이소보르닐메타크릴레이트와 디메틸아미노에틸메타크릴레이트의 사용량을 변경하는 것 이외에는 상기 합성 1과 마찬가지로 하여, 3급 아미노기를 함유하는 고분자 화합물 (c-1)로서, bPD2(아민가 160mgKOH/g, 중량 평균 분자량 5500)를 얻었다.As a polymer compound (c-1) containing a tertiary amino group, bPD2 (amine value 160 mgKOH/g, weight Average molecular weight 5500) was obtained.
「고분자 분산제의 합성(합성예 c1 내지 c11, 비교 합성예 c'1 내지 c'2)」“Synthesis of polymer dispersant (Synthesis Examples c1 to c11, Comparative Synthesis Examples c’1 to c’2)”
<합성예 c1><Synthesis Example c1>
고분자 화합물 (c-1)로서 합성예 b1에서 얻어진 bPD1을 35g(용제를 제외한 고형분 환산), 상기 관능기 (Y)를 갖는 할로겐 화합물 (c-2)로서 3-브로모-2-메틸프로판올-2-[(3,5-디메틸피라졸릴)카르보닐아미노]-에틸에스테르를 2.87g(고분자 화합물 (c-1)의 아미노기의 총량에 대하여 20몰%), 용제로서 프로필렌글리콜모노메틸에테르아세테이트를 투입량으로부터 계산한 불휘발분이 30%가 되도록 첨가하고, 플라스크 내를 질소 가스로 치환하면서 교반하고, 실온으로부터 100℃까지 승온하였다. NMR 스펙트럼에 의해 3-브로모-2-메틸프로판올-2-[(3,5-디메틸피라졸릴)카르보닐아미노]-에틸에스테르의 피크 시프트를 확인할 때까지 교반한 후, 실온까지 강온하고, 아미노기 함유 고분자 화합물 (c-1)과 상기 관능기 (Y)를 갖는 할로겐 화합물 (c-2)의 반응물로서 고분자 분산제 (PD1)을 얻었다.35 g (converted to solid content excluding solvent) of bPD1 obtained in Synthesis Example b1 as polymer compound (c-1), and 3-bromo-2-methylpropanol-2 as halogen compound (c-2) having the above functional group (Y). 2.87 g of -[(3,5-dimethylpyrazolyl)carbonylamino]-ethyl ester (20 mol% based on the total amount of amino groups in polymer compound (c-1)) and propylene glycol monomethyl ether acetate as a solvent. It was added so that the non-volatile matter calculated from 30% was stirred while replacing the inside of the flask with nitrogen gas, and the temperature was raised from room temperature to 100°C. After stirring until the peak shift of 3-bromo-2-methylpropanol-2-[(3,5-dimethylpyrazolyl)carbonylamino]-ethyl ester was confirmed in the NMR spectrum, the temperature was lowered to room temperature, and the amino group was added. A polymer dispersant (PD1) was obtained as a reaction product of the containing polymer compound (c-1) and the halogen compound (c-2) having the above functional group (Y).
<합성예 c2 내지 c8, c10><Synthesis examples c2 to c8, c10>
아미노기 함유 고분자 화합물 (c-1)의 종류와 사용량, 상기 관능기 (Y)를 갖는 할로겐 화합물 (c-2)의 종류와 사용량을 변경하는 것 이외에는 합성예 1과 마찬가지로 하여, 고분자 분산제 (C)로서, 시료 PD2 내지 PD8, PD10을 얻었다. 고분자 분산제 (C)의 관능기 (Y)양을 표 2a 및 표 2b에 나타낸다.Except for changing the type and amount of the amino group-containing polymer compound (c-1) and the type and amount of the halogen compound (c-2) having the functional group (Y), the same procedure as in Synthesis Example 1 was used as the polymer dispersant (C). , samples PD2 to PD8 and PD10 were obtained. The amount of functional group (Y) of the polymer dispersant (C) is shown in Tables 2a and 2b.
<합성예 c9><Synthesis example c9>
아미노기 함유 고분자 화합물 (c-1)로서 3급 아민 분산제 EFKA-PX4300(BASF사제, 고형분 30%, 아민가 57mgKOH/g, 중량 평균 분자량 25000)을 사용하는 것 이외에는, 합성예 c5와 마찬가지로 하여 시료 PD9를 얻었다. 관능기 (Y) 함유 고분자 분산제의 관능기 (Y)양을 표 2b에 나타낸다.Sample PD9 was prepared in the same manner as in Synthesis Example c5, except that tertiary amine dispersant EFKA-PX4300 (manufactured by BASF, solid content 30%, amine value 57 mgKOH/g, weight average molecular weight 25000) was used as the amino group-containing polymer compound (c-1). got it The amount of functional group (Y) of the functional group (Y)-containing polymer dispersant is shown in Table 2b.
<합성예 c11><Synthesis example c11>
아미노기 함유 고분자 화합물 (c-1)로서 폴리멘트 NK-380(닛폰 쇼쿠바이사제, 고형분 30%, 아민가 56mgKOH/g, 중량 평균 분자량 100000)을 사용하는 것 이외에는, 합성예 c3과 마찬가지로 하여 시료 PD11을 얻었다. 관능기 (Y) 함유 고분자 분산제의 관능기 (Y)양을 표 2b에 나타낸다.Sample PD11 was prepared in the same manner as in Synthesis Example c3, except that Polyment NK-380 (manufactured by Nippon Shokubai, solid content 30%, amine value 56 mgKOH/g, weight average molecular weight 100000) was used as the amino group-containing polymer compound (c-1). got it The amount of functional group (Y) of the functional group (Y)-containing polymer dispersant is shown in Table 2b.
<비교 합성예 c'1 내지 c'2><Comparative synthesis examples c'1 to c'2>
상기 관능기 (Y)를 갖는 할로겐 화합물 (c-2) 대신에 표 1a 및 표 1b에 기재된 관능기 (Y)를 포함하지 않는 할로겐 화합물을 사용하는 것과, 반응 온도를 40℃로 하는 것 이외에는 분산제 합성예 1과 마찬가지로 하여, 관능기 (Y)를 포함하지 않는 고분자 분산제로서 시료 cPD1 내지 cPD2를 얻었다. 관능기 (Y) 비함유 고분자 분산제 관능기 (Y)양을 표 2b에 나타낸다.Dispersant synthesis example except that a halogen compound not containing the functional group (Y) shown in Tables 1a and 1b is used instead of the halogen compound (c-2) having the above functional group (Y), and the reaction temperature is set to 40°C. In the same manner as 1, samples cPD1 to cPD2 were obtained as a polymer dispersant not containing a functional group (Y). The amount of functional group (Y) in the polymer dispersant without functional group (Y) is shown in Table 2b.
[표 2a][Table 2a]
[표 2b][Table 2b]
표 2a 및 표 2b 중 표기의 설명은 이하이다.The description of the notation in Table 2a and Table 2b is as follows.
EFKA-PX4300: BASF제, 고형분 30%, 3급 아민 분산제, 아민가 57mgKOH/g, 중량 평균 분자량 25000EFKA-PX4300: BASF product, solid content 30%, tertiary amine dispersant, amine value 57mgKOH/g, weight average molecular weight 25000
bPD1: 합성예 b1에서 얻어진 아미노기 함유 고분자 화합물 (c-1) 아민가 70mgKOH/gbPD1: Amino group-containing polymer compound (c-1) obtained in Synthesis Example b1, amine value 70 mgKOH/g
bPD2: 합성예 b2에서 얻어진 아미노기 함유 고분자 화합물 (c-1) 아민가 160mgKOH/gbPD2: Amino group-containing polymer compound (c-1) obtained in Synthesis Example b2, amine value 160 mgKOH/g
폴리멘트 NK-380: 닛폰 쇼쿠바이사제, 고형분 30%, 1, 2급 아민 분산제, 아민가 56mgKOH/g, 중량 평균 분자량 100000)Polyment NK-380: manufactured by Nippon Shokubai, solid content 30%, primary and secondary amine dispersant, amine value 56 mgKOH/g, weight average molecular weight 100000)
브로모화 MOI-BP: 2-브로모-2-메틸프로피온산, 2-[(3,5-디메틸피라졸릴)카르보닐아미노]에틸에스테르Brominated MOI-BP: 2-bromo-2-methylpropionic acid, 2-[(3,5-dimethylpyrazolyl)carbonylamino]ethyl ester
브로모화 MOI-DEM: 말론산-2-[[[2-브로모-2-메틸-1-옥소-2-프로파닐]옥시]에틸]아미노]카르보닐]-1,3디에틸에스테르 에피브로모히드린: 3-브로모프로필렌옥시드Brominated MOI-DEM: malonic acid-2-[[[2-bromo-2-methyl-1-oxo-2-propanyl]oxy]ethyl]amino]carbonyl]-1,3diethyl ester epibro Mohydrin: 3-bromopropylene oxide
브로모화 EVMO: 3-에틸-3-[2-브로모에톡시메틸]-옥세탄Brominated EVMO: 3-ethyl-3-[2-bromoethoxymethyl]-oxetane
EBr: 에틸렌브로모히드린:EBr: Ethylene bromohydrin:
3BrP: 3-브로모-1,2-프로판디올:3BrP: 3-Bromo-1,2-propanediol:
11BrUn: 11-브로모-1-운데칸티올:11BrUn: 11-bromo-1-undecanthiol:
BrAc: 브로모아세트산BrAc: bromoacetic acid
BrE: 브로모에틸렌BrE: Bromoethylene
ABr: 알릴브로마이드ABr: Allyl bromide
안료 분산 조성물의 제조예를 이하에 나타낸다.A production example of the pigment dispersion composition is shown below.
<실시예 a1 내지 a19><Examples a1 to a19>
직경 0.1mm의 지르코니아 비즈(닛카토제 YTZ 볼) 200g을 충전한 SUS 용기(내경 50mm×높이 100mm)에, 안료 (B)로서 C.I. 피그먼트 그린 58(DIC제 Fastogen Green A110)을 7.5g(100질량부), 고분자 분산제 (C)로서 합성예 c1 내지 c11에서 얻어진 시료 PD1 내지 PD11의 어느 것, 결합제 수지 (A)로서 합성예 a1 내지 a11에서 얻어진 시료 PR1 내지 PR11을 표 3a 및 표 3b에 나타내는 조성으로 혼합하고, 용제 (D-1)로서 프로필렌글리콜모노메틸에테르아세테이트를 첨가하고, 지르코니아 비즈를 제외한 고형분이 24%가 되도록 조제하고, 페인트 셰이커(Red Devil Equipment제 Red Devil 5400)로 실온 하에서, 2시간 혼합하여 분산시킨 후, 내용물을 유리 필터로 흡인 여과함으로써, 안료 분산 조성물 MB1 내지 MB19를 얻었다.C.I. was added as a pigment (B) to a SUS container (inner diameter 50 mm 7.5 g (100 parts by mass) of Pigment Green 58 (Fastogen Green A110 manufactured by DIC), any of the samples PD1 to PD11 obtained in Synthesis Examples c1 to c11 as the polymer dispersant (C), and Synthesis Example a1 as the binder resin (A). Samples PR1 to PR11 obtained in to a11 were mixed with the composition shown in Tables 3a and 3b, propylene glycol monomethyl ether acetate was added as a solvent (D-1), and the solid content excluding zirconia beads was adjusted to 24%. After mixing and dispersing for 2 hours at room temperature with a paint shaker (Red Devil 5400 manufactured by Red Devil Equipment), the contents were suction filtered through a glass filter to obtain pigment dispersion compositions MB1 to MB19.
<비교예 a'1 내지 a'6><Comparative examples a'1 to a'6>
직경 0.1mm의 지르코니아 비즈(닛카토제 YTZ 볼) 200g을 충전한 SUS 용기(내경 50mm×높이 100mm)에, 안료 (B)로서 C.I. 피그먼트 그린 58(DIC제 Fastogen Green A110)을 7.5g(100질량부), 고분자 분산제로서 비교 합성예 c'1, c'2에서 얻어진 시료 cPD1 내지 cPD2의 어느 것, 결합제 수지로서 합성예 a1 내지 a11, 비교 합성예 a'1 내지 a'3에서 얻어진 시료를 표 3b에 나타내는 조성으로 혼합하고, 용제 (D-1)로서 프로필렌글리콜모노메틸에테르아세테이트를 첨가하고, 지르코니아 비즈를 제외한 고형분이 24%가 되도록 조제하고, 페인트 셰이커(Red Devil Equipment제 Red Devil 5400)로 실온 하에서, 2시간 혼합하여 분산시킨 후, 내용물을 유리 필터로 흡인 여과함으로써, 안료 분산 조성물 cMB1 내지 cMB6을 얻었다.C.I. was added as a pigment (B) to a SUS container (inner diameter 50 mm 7.5 g (100 parts by mass) of Pigment Green 58 (Fastogen Green A110 manufactured by DIC) as a polymer dispersant, any of the samples cPD1 to cPD2 obtained in Comparative Synthesis Examples c'1 and c'2, and Synthesis Examples a1 to cPD2 as a binder resin. a11, Comparative Synthesis Examples The samples obtained in a'1 to a'3 were mixed with the composition shown in Table 3b, propylene glycol monomethyl ether acetate was added as a solvent (D-1), and the solid content excluding zirconia beads was 24%. After mixing and dispersing for 2 hours at room temperature with a paint shaker (Red Devil 5400 manufactured by Red Devil Equipment), the contents were suction filtered through a glass filter to obtain pigment dispersion compositions cMB1 to cMB6.
[표 3a][Table 3a]
[표 3b][Table 3b]
<안료 분산성의 평가><Evaluation of pigment dispersibility>
조제한 직후의 안료 분산 조성물의 점도를 E형 점도계(도끼 산교 가부시키가이샤제 RE-80L, 콘 사이즈 4.8cm, 회전수 20rpm, 측정 온도 25℃)로 측정함으로써 평가하였다. 또한, 점도의 측정 결과로부터, 안료 분산성을 하기 기준에 의해 1 내지 5의 5단계로 평가하고, 3 이상을 합격으로 하였다. 평가 결과를 상기 표 3a 및 표 3b에 나타내었다.The viscosity of the pigment dispersion composition immediately after preparation was evaluated by measuring it with an E-type viscometer (RE-80L manufactured by Toki Sangyo Co., Ltd., cone size 4.8 cm, rotation speed 20 rpm, measurement temperature 25°C). In addition, from the viscosity measurement results, the pigment dispersibility was evaluated on a 5-level scale from 1 to 5 according to the following criteria, and 3 or more were considered passing. The evaluation results are shown in Tables 3a and 3b above.
「5」: 6.0mPa·s 미만「5」: Less than 6.0mPa·s
「4」: 6.0mPa·s 이상 8.0mPa·s 미만“4”: 6.0 mPa·s or more and less than 8.0 mPa·s
「3」: 8.0mPa·s 이상 10.0mPa·s 미만“3”: 8.0 mPa·s or more but less than 10.0 mPa·s
「2」: 10.0mPa·s 이상「2」: 10.0 mPa·s or more
「1」: 유동성 없음「1」: No liquidity
<안료 분산 조성물의 보존 안정성의 평가><Evaluation of storage stability of pigment dispersion composition>
조제한 안료 분산 조성물을 실온에서 1개월 보관한 후의 안료 분산 조성물의 점도를 E형 점도계(도끼 산교 가부시키가이샤제 RE-80L, 측정 온도 25℃)로 측정함으로써 평가하였다. 실온에서 1개월 보관한 후의 안료 분산 조성물의 점도 상승률 「(보관 후 점도-조정 후 점도)×100/조정 후 점도」가, 조제한 직후와 비교하여, 하기 기준에 의해 1 내지 5의 5단계로 평가하였다. 평가 결과를 상기 표 3a 및 표 3b에 나타내었다.The viscosity of the prepared pigment dispersion composition after being stored at room temperature for 1 month was evaluated by measuring it with an E-type viscometer (RE-80L manufactured by Toki Sangyo Co., Ltd., measurement temperature 25°C). The viscosity increase rate of the pigment dispersion composition after storage at room temperature for 1 month "(viscosity after storage - viscosity after adjustment) did. The evaluation results are shown in Tables 3a and 3b above.
「5」: 5% 미만「5」: Less than 5%
「4」: 5% 이상 10% 미만“4”: 5% or more and less than 10%
「3」: 10% 이상 20% 미만「3」: 10% or more and less than 20%
「2」: 20% 이상 40% 미만「2」: 20% or more but less than 40%
「1」: 14일 이내에 유동성을 상실한다「1」: Liquidity is lost within 14 days
감광성 착색 조성물 (CR)의 제조예를 이하에 나타낸다.A production example of the photosensitive coloring composition (CR) is shown below.
<실시예 b1 내지 b19, 비교예 b'1 내지 b'6><Examples b1 to b19, Comparative Examples b'1 to b'6>
상기 실시예 a1 내지 a19에서 얻어진 안료 분산 조성물 MB1 내지 MB19 및 비교예 a'1 내지 a'6에서 얻어진 안료 분산 조성물 cMB1 내지 cMB4의 고형분(용제 이외의 성분의 총량)을 100질량부로 했을 때에, 결합제 수지 (A-2)로서 수지 합성예 a10에서 얻어진 시료 PR10을 고형분으로서 80질량부, 반응성 희석제 (E)로서 디펜타에리트리톨헥사아크릴레이트를 80질량부, 광중합 개시제 (F)로서 1-[9-에틸-6-(2-메틸벤조일)-9H-카르바졸-3-일-]-, -1-(O-아세틸옥심)을 4질량부 혼합하고, 용제 (D-2)로서 프로필렌글리콜모노메틸에테르아세테이트를 첨가하여 전체의 고형분이 30%가 되도록 혼합함으로써, 감광성 착색 조성물 CR1 내지 19 및 cCR1 내지 cCR6을 조제하였다. 또한, 안료 (B) 100질량부에 대한 각 성분 배합량으로서는, 결합제 수지 (A-2)가 128질량부, 반응성 희석제 (E)가 128질량부, 광중합 개시제 (F)가 6.4질량부이다. 표 4a 및 표 4b에 각종 재료의 사용 부수를 나타낸다.When the solid content (total amount of components other than the solvent) of the pigment dispersion compositions MB1 to MB19 obtained in Examples a1 to a19 and the pigment dispersion compositions cMB1 to cMB4 obtained in Comparative Examples a'1 to a'6 is 100 parts by mass, the binder As the resin (A-2), 80 parts by mass of sample PR10 obtained in Resin Synthesis Example a10 was used as a solid content, 80 parts by mass of dipentaerythritol hexaacrylate as a reactive diluent (E), and 1-[9 as a photopolymerization initiator (F). -Ethyl-6-(2-methylbenzoyl)-9H-carbazol-3-yl-]-, -1-(O-acetyloxime) were mixed and 4 parts by mass were mixed with propylene glycol mono as solvent (D-2). Photosensitive coloring compositions CR1 to 19 and cCR1 to cCR6 were prepared by adding methyl ether acetate and mixing so that the total solid content was 30%. In addition, the mixing amount of each component with respect to 100 parts by mass of the pigment (B) is 128 parts by mass for the binder resin (A-2), 128 parts by mass of the reactive diluent (E), and 6.4 parts by mass of the photopolymerization initiator (F). Table 4a and Table 4b show the number of copies of various materials used.
[표 4a][Table 4a]
[표 4b][Table 4b]
<내용제 시험용 녹색 레지스트 (1)의 제작><Production of green resist (1) for solvent testing>
얻어진 감광성 착색 조성물 CR1 내지 CR19, cCR1 내지 cCR6을, 한 변이 5cm인 정사각형 유리 기판(무알칼리 유리 기판) 상에, 최종의 경화 도막의 평균 두께가 1.5㎛가 되도록 스핀 코팅한 후, 100℃에서 3분간 가열하여 용제를 휘발시켰다. 다음으로 도막의 전체 면을 노광(램프로서, 우시오덴키 가부시키가이샤제 USH-250BY를 사용, 노광량 40mJ/㎠)시킨 후, 또한 100℃에서 30분간 베이킹함으로써 경화 도막인 녹색 레지스트 (1)을 얻었다.The obtained photosensitive coloring compositions CR1 to CR19 and cCR1 to cCR6 were spin-coated on a square glass substrate (alkali-free glass substrate) with a side of 5 cm so that the average thickness of the final cured film was 1.5 ㎛, and then 3 times at 100°C. The solvent was volatilized by heating for a minute. Next, the entire surface of the coating film was exposed (using USH-250BY manufactured by Ushio Denki Co., Ltd. as a lamp, exposure dose 40 mJ/cm2), and then baked at 100°C for 30 minutes to obtain a cured coating film, green resist (1). .
<현상성 시험용 녹색 레지스트 (2)의 제작><Production of green resist (2) for developability test>
얻어진 감광성 착색 조성물 CR1 내지 19, cCR1 내지 cCR6을, 한 변이 5cm인 정사각형 유리 기판(무알칼리 유리 기판) 상에, 최종의 경화 도막의 평균 두께가 1.5㎛가 되도록 스핀 코팅한 후, 100℃에서 3분간 가열하여 용제를 휘발시켰다. 이어서, 기판 상에 라인 앤 스페이스나 도트 패턴의 포토마스크를 설치하여 도막을 노광(램프로서, 우시오덴키 가부시키가이샤제 USH-250BY를 사용, 노광량 40mJ/㎠)하고, 광경화시킨 후, 0.2질량%의 수산화칼륨 수용액으로 현상하고, 또한 100℃에서 30분간 베이킹함으로써 경화 도막인 녹색 컬러 레지스트 (2)를 얻었다.The obtained photosensitive coloring compositions CR1 to 19 and cCR1 to cCR6 were spin-coated on a square glass substrate (alkali-free glass substrate) with a side of 5 cm so that the average thickness of the final cured film was 1.5 μm, and then applied at 100°C for 3 hours. The solvent was volatilized by heating for a minute. Next, a photomask with a line and space or dot pattern was installed on the substrate, the coating film was exposed (as a lamp, USH-250BY manufactured by Ushio Denki Co., Ltd. was used, exposure dose was 40 mJ/cm2), and photocured, then 0.2 mass % potassium hydroxide aqueous solution and baking at 100°C for 30 minutes to obtain a green color resist (2) as a cured film.
<내용제성 시험><Solvent resistance test>
상기 녹색 레지스트 전체 면을 25℃의 프로필렌글리콜모노메틸에테르아세테이트에 15분간 침지시켰다. 그 후, 녹색 레지스트를 취출하고, 공기 건조시켰다. 녹색 레지스트의 잔막률을 확인함으로써, 감광성 착색 조성물의 내용제성을 평가하였다. 측정 결과로부터, 감광성 착색 조성물의 내용제성을 하기 기준에 의해 1 내지 5의 5단계로 평가하고, 3 이상을 합격으로 하였다. 평가 결과를 상기 표 4a 및 표 4b에 나타내었다.The entire surface of the green resist was immersed in propylene glycol monomethyl ether acetate at 25°C for 15 minutes. Afterwards, the green resist was taken out and air dried. The solvent resistance of the photosensitive coloring composition was evaluated by confirming the residual film rate of the green resist. From the measurement results, the solvent resistance of the photosensitive coloring composition was evaluated on a 5-grade scale from 1 to 5 according to the following criteria, and 3 or more were considered passing. The evaluation results are shown in Tables 4a and 4b above.
「5」: 잔막률 95% 이상「5」: Remaining film rate of 95% or more
「4」: 잔막률 90% 이상 95% 미만“4”: Residual film rate 90% or more but less than 95%
「3」: 잔막률 85% 이상 90% 미만“3”: Residual film rate 85% or more but less than 90%
「2」: 잔막률 80% 이상 85% 미만“2”: Residual film rate 80% or more but less than 85%
「1」: 잔막률 80% 미만「1」: Remaining film rate less than 80%
<현상성 시험><Development test>
현상성 시험은, 현상 속도를 평가하였다. 현상 속도에 대해서는, 상기 녹색 컬러 레지스트 (2)의 현상 공정에 있어서, 0.2질량%의 수산화칼륨 수용액에서의 현상 중, 패턴이 다 보일 때까지 걸리는 시간을 측정하고, 하기 기준에 의해 1 내지 5의 5단계로 평가하고, 3 이상을 합격으로 하였다. 평가 결과를 상기 표 4a 및 표 4b에 나타내었다.The developability test evaluated the development speed. Regarding the development speed, in the development process of the green color resist (2), the time taken until the pattern is fully visible during development in a 0.2% by mass potassium hydroxide aqueous solution is measured, and the time taken for the pattern to be fully visible is measured, and the time taken for the pattern to be fully visible is measured, and the time taken for the pattern to be fully visible is measured, and the time taken for the pattern to be fully visible is measured according to the following criteria. Evaluation was made on a 5-level scale, and a score of 3 or higher was considered passing. The evaluation results are shown in Tables 4a and 4b above.
「5」: 40초 미만「5」: Less than 40 seconds
「4」: 40초 이상 50초 미만「4」: More than 40 seconds but less than 50 seconds
「3」: 50초 이상 60초 미만「3」: More than 50 seconds but less than 60 seconds
「2」: 60초 이상 70초 미만「2」: More than 60 seconds but less than 70 seconds
「1」: 70초 이상「1」: 70 seconds or more
표 3a 및 표 3b, 표 4a 및 표 4b의 결과로부터 알 수 있는 바와 같이, 실시예 a1 내지 a19 및 실시예 b1 내지 b19에서는, 안료 분산 조성물의 우수한 안료 분산성 및 보존 안정성이 얻어졌다. 또한 실시예 a1 내지 a19에서 얻어진 안료 분산 조성물을 사용하여 제작한 감광성 착색 조성물은, 관능기 X와 관능기 Y의 반응에 의해, 100℃라고 하는 저온에서 경화하고, 우수한 내용제성, 우수한 현상성이 얻어졌다.As can be seen from the results of Tables 3a and 3b, Tables 4a and 4b, in Examples a1 to a19 and Examples b1 to b19, excellent pigment dispersibility and storage stability of the pigment dispersion composition were obtained. Additionally, the photosensitive coloring composition produced using the pigment dispersion composition obtained in Examples a1 to a19 was cured at a low temperature of 100°C through the reaction of functional group X and functional group Y, and excellent solvent resistance and excellent developability were obtained. .
이에 비해, 비교예 a'4 및 비교예 b'4에서는, 안료 분산 조성물의 안료 분산성이 현저하게 나쁘고, 안료 분산 조성물로서 사용하는 것은 곤란하고, 감광성 착색 조성물로 했을 때의 경화성이 불충분하고, 내용제성이 나쁜 결과가 되어 감광성 착색 조성물로서 사용하는 것도 곤란하였다. 또한, 비교예 a'1, a'5, a'6 및 비교예 b'1, b'5, b'6에서는, 안료 분산 조성물의 안료 분산성 및 보존 안정성, 감광성 착색 조성물의 현상성은 좋았던 한편, 감광성 착색 조성물의 내용제성은 나빴다.In contrast, in Comparative Example a'4 and Comparative Example b'4, the pigment dispersibility of the pigment dispersion composition was significantly poor, it was difficult to use it as a pigment dispersion composition, and the curing property when used as a photosensitive coloring composition was insufficient; The solvent resistance was poor, making it difficult to use it as a photosensitive coloring composition. In addition, in Comparative Examples a'1, a'5, and a'6 and Comparative Examples b'1, b'5, and b'6, the pigment dispersibility and storage stability of the pigment dispersion composition and the developability of the photosensitive coloring composition were good. , the solvent resistance of the photosensitive coloring composition was poor.
이상으로부터, 본 발명에 의해, 안료 분산성이나 보존 안정성이 우수한 안료 분산 조성물, 및 그 안료 분산 조성물을 포함하는 감광성 착색 조성물, 및 그 감광성 착색 조성물을 포함하고, 내용제성, 현상성이 우수하고, 분산제나 안료의 용출이나 블리드 아웃의 억제가 기대되는 컬러 필터를 제공할 수 있다.From the above, according to the present invention, a pigment dispersion composition excellent in pigment dispersibility and storage stability, a photosensitive coloring composition containing the pigment dispersion composition, and the photosensitive coloring composition are excellent in solvent resistance and developability, A color filter expected to suppress elution and bleed-out of dispersants and pigments can be provided.
본 발명에 따르면, 안료 분산성이나 보존 안정성이 양호한 안료 분산 조성물을 제공할 수 있다. 또한, 당해 안료 분산 조성물을 사용함으로써, 우수한 내용제성, 현상성을 갖는 경화물이 얻어지는 감광성 착색 조성물을 제공할 수 있다. 또한, 당해 감광성 착색 조성물의 경화물을 갖는 컬러 필터, 이것을 구비하는 화상 표시 소자를 제공할 수 있다.According to the present invention, it is possible to provide a pigment dispersion composition with good pigment dispersibility and good storage stability. Furthermore, by using the pigment dispersion composition, it is possible to provide a photosensitive coloring composition from which a cured product having excellent solvent resistance and developability can be obtained. Additionally, a color filter containing a cured product of the photosensitive coloring composition and an image display element containing the same can be provided.
Claims (14)
안료 (B)와,
고분자 분산제 (C)와,
용제 (D-1)
을 함유하는 안료 분산 조성물이며,
상기 결합제 수지 (A-1)이, 관능기 (X)를 갖고,
상기 고분자 분산제 (C)가, 아미노기 및 4급 암모늄 양이온기에서 선택되는 1종 이상을 갖고, 상기 관능기 (X)와 반응성을 갖는 관능기 (Y)를 갖는 것을 특징으로 하는 안료 분산 조성물.Binder resin (A-1),
Pigment (B),
A polymer dispersant (C),
Solvent (D-1)
It is a pigment dispersion composition containing,
The binder resin (A-1) has a functional group (X),
A pigment dispersion composition characterized in that the polymer dispersant (C) has at least one selected from amino groups and quaternary ammonium cation groups, and has a functional group (Y) reactive with the functional group (X).
상기 고분자 분산제 (C)가 갖는 관능기 (Y)가, 블록 이소시아나토기 및 알킬에스테르를 갖는 기에서 선택되는 1종 이상인, 안료 분산 조성물.The functional group (X) of the binder resin (A-1) according to any one of claims 1 to 3 is at least one selected from a hydroxy group and a mercapto group,
A pigment dispersion composition, wherein the functional group (Y) of the polymer dispersant (C) is at least one selected from a group having a block isocyanato group and an alkyl ester.
상기 고분자 분산제 (C)가 갖는, 관능기 (Y)가, 에폭시기 및 옥세타닐기에서 선택되는 1종 이상인, 안료 분산 조성물.The functional group (X) of the binder resin (A-1) is a carboxyl group according to any one of claims 1 to 3,
A pigment dispersion composition in which the functional group (Y) of the polymer dispersant (C) is at least one selected from an epoxy group and an oxetanyl group.
상기 고분자 분산제 (C)가 갖는 관능기 (Y)가, 히드록시기 및 머캅토기에서 선택되는 1종 이상인, 안료 분산 조성물.The functional group (X) of the binder resin (A-1) according to any one of claims 1 to 3 is at least one selected from a block isocyanato group and an alkyl ester group,
A pigment dispersion composition, wherein the functional group (Y) of the polymer dispersant (C) is at least one selected from a hydroxy group and a mercapto group.
상기 고분자 분산제 (C)가 갖는 관능기 (Y)가, 카르복시기인, 안료 분산 조성물.The functional group (X) of the binder resin (A-1) according to any one of claims 1 to 3 is at least one selected from an epoxy group and an oxetanyl group,
A pigment dispersion composition wherein the functional group (Y) of the polymer dispersant (C) is a carboxyl group.
상기 결합제 수지 (A-1)을 10 내지 80질량부 함유하고,
상기 고분자 분산제 (C)를 10 내지 80질량부 함유하는, 안료 분산 조성물.The method according to any one of claims 1 to 3, with respect to 100 parts by mass of the pigment (B),
Containing 10 to 80 parts by mass of the binder resin (A-1),
A pigment dispersion composition containing 10 to 80 parts by mass of the polymer dispersant (C).
결합제 수지 (A-2)와,
반응성 희석제 (E)와,
광중합 개시제 (F)
를 함유하는 것을 특징으로 하는 감광성 착색 조성물.The pigment dispersion composition according to any one of claims 1 to 3,
Binder resin (A-2),
a reactive diluent (E),
Photopolymerization initiator (F)
A photosensitive coloring composition comprising:
상기 결합제 수지 (A-1)을 10 내지 80질량부 함유하고,
상기 고분자 분산제 (C)를 10 내지 80질량부 함유하고,
상기 결합제 수지 (A-2)를 50 내지 280질량부 함유하고,
상기 반응성 희석제 (E)를 40 내지 200질량부 함유하고,
상기 광중합 개시제 (F)를 0.1 내지 10질량부 함유하는,
감광성 착색 조성물.The method of claim 10, wherein for 100 parts by mass of the pigment (B),
Containing 10 to 80 parts by mass of the binder resin (A-1),
Containing 10 to 80 parts by mass of the polymer dispersant (C),
Containing 50 to 280 parts by mass of the binder resin (A-2),
Containing 40 to 200 parts by mass of the reactive diluent (E),
Containing 0.1 to 10 parts by mass of the photopolymerization initiator (F),
Photosensitive coloring composition.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2021209989 | 2021-12-23 | ||
| JPJP-P-2021-209989 | 2021-12-23 | ||
| PCT/JP2022/040986 WO2023119898A1 (en) | 2021-12-23 | 2022-11-02 | Pigment dispersion composition, photosensitive colored composition, and color filter |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20240089673A true KR20240089673A (en) | 2024-06-20 |
Family
ID=86902050
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020247015826A Pending KR20240089673A (en) | 2021-12-23 | 2022-11-02 | Pigment dispersion compositions, photosensitive coloring compositions and color filters |
Country Status (5)
| Country | Link |
|---|---|
| JP (1) | JPWO2023119898A1 (en) |
| KR (1) | KR20240089673A (en) |
| CN (1) | CN118265757A (en) |
| TW (1) | TW202338013A (en) |
| WO (1) | WO2023119898A1 (en) |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006075754A1 (en) | 2005-01-17 | 2006-07-20 | Toagosei Co., Ltd. | Compositions curable with actinic energy ray |
| JP2008542523A (en) | 2005-06-07 | 2008-11-27 | ルブリゾル アドバンスド マテリアルズ, インコーポレイテッド | Polyurethane-based pigment dispersants containing reactive double bonds |
| WO2013175978A1 (en) | 2012-05-23 | 2013-11-28 | 東レ株式会社 | Coloring material dispersed liquid and photosensitive coloring resin composition |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016191909A (en) * | 2015-03-30 | 2016-11-10 | 東洋インキScホールディングス株式会社 | Colored composition for color filter, and color filter |
| JP2017058652A (en) * | 2015-09-15 | 2017-03-23 | 東洋インキScホールディングス株式会社 | Colored composition for color filter and color filter |
| JP6705212B2 (en) * | 2016-03-04 | 2020-06-03 | 東洋インキScホールディングス株式会社 | Coloring composition for color filter and color filter |
| JP6733525B2 (en) * | 2016-11-30 | 2020-08-05 | 東洋インキScホールディングス株式会社 | Photosensitive coloring composition for color filter and color filter |
| JP7533037B2 (en) * | 2019-11-20 | 2024-08-14 | artience株式会社 | Pigment composition, photosensitive pigment composition, color filter and display device |
-
2022
- 2022-11-02 KR KR1020247015826A patent/KR20240089673A/en active Pending
- 2022-11-02 JP JP2023569134A patent/JPWO2023119898A1/ja active Pending
- 2022-11-02 WO PCT/JP2022/040986 patent/WO2023119898A1/en active Application Filing
- 2022-11-02 CN CN202280076903.0A patent/CN118265757A/en active Pending
- 2022-11-16 TW TW111143683A patent/TW202338013A/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006075754A1 (en) | 2005-01-17 | 2006-07-20 | Toagosei Co., Ltd. | Compositions curable with actinic energy ray |
| JP2008542523A (en) | 2005-06-07 | 2008-11-27 | ルブリゾル アドバンスド マテリアルズ, インコーポレイテッド | Polyurethane-based pigment dispersants containing reactive double bonds |
| WO2013175978A1 (en) | 2012-05-23 | 2013-11-28 | 東レ株式会社 | Coloring material dispersed liquid and photosensitive coloring resin composition |
Also Published As
| Publication number | Publication date |
|---|---|
| CN118265757A (en) | 2024-06-28 |
| JPWO2023119898A1 (en) | 2023-06-29 |
| WO2023119898A1 (en) | 2023-06-29 |
| TW202338013A (en) | 2023-10-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7563509B2 (en) | Manufacturing method of cured coating film | |
| KR101473511B1 (en) | Photosensitive graft polymer, and photosensitive resin composition comprising the same | |
| JP5665615B2 (en) | Photosensitive resin composition for color filter | |
| JP2018123274A (en) | Alkali-soluble resin, photosensitive resin composition and use therefor | |
| JP7364020B2 (en) | Photosensitive resin composition | |
| WO2008056750A1 (en) | Photosensitive resin composition | |
| KR102270594B1 (en) | Resin composition for color filter, manufacturing method thereof, and color filter | |
| WO2016203905A1 (en) | Coloring composition for color filter, color filter, and image display element | |
| KR102475680B1 (en) | Photosensitive resin composition for color filters, color filter, image display element, and manufacturing method of color filter | |
| TW202235514A (en) | Pigment dispersion composition and photosensitive coloring composition | |
| TW202244072A (en) | Polymeric dispersant, pigment dispersion composition, and photosensitive colored composition | |
| KR20240089673A (en) | Pigment dispersion compositions, photosensitive coloring compositions and color filters | |
| JP2019031627A (en) | Alkali-soluble resin, photosensitive resin composition for color filter containing the same, and color filter | |
| JP2008181016A (en) | Composition for forming protrusion for liquid crystal alignment control | |
| KR20230098322A (en) | Photosensitive resin composition and method for producing the photosensitive resin composition | |
| JP2024061349A (en) | Method for producing pigment dispersant | |
| JP2023167167A (en) | Pigment dispersion composition, photosensitive coloring composition and color filter | |
| TW202436403A (en) | Modified polyol compound and photosensitive resin composition | |
| WO2022102367A1 (en) | Polymerizable polymer dispersant, pigment dispersion composition, and photosensitive coloring composition | |
| JP2023060757A (en) | Resin composition, photosensitive resin composition, resin cured film, color filter, and image display element | |
| JP2024089995A (en) | Pigment dispersion composition and photosensitive colored composition | |
| WO2023119900A1 (en) | Photosensitive resin composition and color filter | |
| JP2024089991A (en) | Pigment dispersion composition and photosensitive colored composition | |
| JP2024089994A (en) | Image display element | |
| KR20250022137A (en) | Photosensitive resin composition, resin cured film and image display element |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20240513 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PA0201 | Request for examination | ||
| PG1501 | Laying open of application |