KR101122370B1 - 열수 안정성 알루미나 - Google Patents
열수 안정성 알루미나 Download PDFInfo
- Publication number
- KR101122370B1 KR101122370B1 KR1020087029345A KR20087029345A KR101122370B1 KR 101122370 B1 KR101122370 B1 KR 101122370B1 KR 1020087029345 A KR1020087029345 A KR 1020087029345A KR 20087029345 A KR20087029345 A KR 20087029345A KR 101122370 B1 KR101122370 B1 KR 101122370B1
- Authority
- KR
- South Korea
- Prior art keywords
- alumina
- silica
- adsorbent
- delete delete
- core
- Prior art date
Links
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 title claims abstract description 95
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims abstract description 78
- 239000000377 silicon dioxide Substances 0.000 claims abstract description 33
- 239000003463 adsorbent Substances 0.000 claims abstract description 28
- 239000008119 colloidal silica Substances 0.000 claims abstract description 13
- -1 silicon inorganic compounds Chemical class 0.000 claims abstract description 9
- 239000010419 fine particle Substances 0.000 claims abstract description 7
- 239000000843 powder Substances 0.000 claims abstract description 7
- 238000004519 manufacturing process Methods 0.000 claims abstract description 6
- 238000000034 method Methods 0.000 claims description 14
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 14
- 239000002245 particle Substances 0.000 claims description 10
- 238000005507 spraying Methods 0.000 claims description 9
- 229910052910 alkali metal silicate Inorganic materials 0.000 claims description 3
- 238000002156 mixing Methods 0.000 claims description 3
- 230000003213 activating effect Effects 0.000 claims description 2
- 229910052915 alkaline earth metal silicate Inorganic materials 0.000 claims 1
- 150000001875 compounds Chemical class 0.000 claims 1
- 239000004115 Sodium Silicate Substances 0.000 abstract description 7
- 239000007788 liquid Substances 0.000 abstract description 7
- 229910052710 silicon Inorganic materials 0.000 abstract description 7
- 239000010703 silicon Substances 0.000 abstract description 7
- NTHWMYGWWRZVTN-UHFFFAOYSA-N sodium silicate Chemical compound [Na+].[Na+].[O-][Si]([O-])=O NTHWMYGWWRZVTN-UHFFFAOYSA-N 0.000 abstract description 7
- 229910052911 sodium silicate Inorganic materials 0.000 abstract description 7
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 abstract description 5
- 239000003054 catalyst Substances 0.000 abstract description 5
- 238000010438 heat treatment Methods 0.000 abstract description 4
- 229910010272 inorganic material Inorganic materials 0.000 abstract description 4
- 239000000428 dust Substances 0.000 abstract description 3
- 150000003377 silicon compounds Chemical class 0.000 abstract description 2
- 238000007725 thermal activation Methods 0.000 abstract description 2
- 239000012467 final product Substances 0.000 abstract 1
- 230000015572 biosynthetic process Effects 0.000 description 8
- 239000002274 desiccant Substances 0.000 description 8
- 230000008569 process Effects 0.000 description 8
- 230000006872 improvement Effects 0.000 description 7
- 238000001179 sorption measurement Methods 0.000 description 7
- 238000001035 drying Methods 0.000 description 6
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 6
- 230000008929 regeneration Effects 0.000 description 6
- 238000011069 regeneration method Methods 0.000 description 6
- 239000011324 bead Substances 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 230000007704 transition Effects 0.000 description 5
- 230000032683 aging Effects 0.000 description 4
- 229910052783 alkali metal Inorganic materials 0.000 description 4
- 239000007789 gas Substances 0.000 description 4
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 3
- 229910004298 SiO 2 Inorganic materials 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 238000005054 agglomeration Methods 0.000 description 3
- 230000002776 aggregation Effects 0.000 description 3
- 229910001593 boehmite Inorganic materials 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- FAHBNUUHRFUEAI-UHFFFAOYSA-M hydroxidooxidoaluminium Chemical compound O[Al]=O FAHBNUUHRFUEAI-UHFFFAOYSA-M 0.000 description 3
- 239000003345 natural gas Substances 0.000 description 3
- 239000011574 phosphorus Substances 0.000 description 3
- 229910052698 phosphorus Inorganic materials 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 238000002441 X-ray diffraction Methods 0.000 description 2
- 150000001340 alkali metals Chemical class 0.000 description 2
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 2
- 229910000323 aluminium silicate Inorganic materials 0.000 description 2
- ANBBXQWFNXMHLD-UHFFFAOYSA-N aluminum;sodium;oxygen(2-) Chemical compound [O-2].[O-2].[Na+].[Al+3] ANBBXQWFNXMHLD-UHFFFAOYSA-N 0.000 description 2
- 238000001354 calcination Methods 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 150000002430 hydrocarbons Chemical class 0.000 description 2
- 238000010335 hydrothermal treatment Methods 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 229910021331 inorganic silicon compound Inorganic materials 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 239000002808 molecular sieve Substances 0.000 description 2
- 239000011241 protective layer Substances 0.000 description 2
- 238000005245 sintering Methods 0.000 description 2
- 229910001388 sodium aluminate Inorganic materials 0.000 description 2
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 2
- 238000002411 thermogravimetry Methods 0.000 description 2
- 238000004131 Bayer process Methods 0.000 description 1
- 229910052684 Cerium Inorganic materials 0.000 description 1
- 238000005033 Fourier transform infrared spectroscopy Methods 0.000 description 1
- 206010067482 No adverse event Diseases 0.000 description 1
- 238000001994 activation Methods 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- DIZPMCHEQGEION-UHFFFAOYSA-H aluminium sulfate (anhydrous) Chemical compound [Al+3].[Al+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O DIZPMCHEQGEION-UHFFFAOYSA-H 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 229910001680 bayerite Inorganic materials 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 238000004523 catalytic cracking Methods 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- ZMIGMASIKSOYAM-UHFFFAOYSA-N cerium Chemical compound [Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce] ZMIGMASIKSOYAM-UHFFFAOYSA-N 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000005336 cracking Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 229910001679 gibbsite Inorganic materials 0.000 description 1
- 238000003837 high-temperature calcination Methods 0.000 description 1
- 230000003301 hydrolyzing effect Effects 0.000 description 1
- 238000012844 infrared spectroscopy analysis Methods 0.000 description 1
- 150000002484 inorganic compounds Chemical class 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 description 1
- 239000000347 magnesium hydroxide Substances 0.000 description 1
- 229910001862 magnesium hydroxide Inorganic materials 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000011859 microparticle Substances 0.000 description 1
- DDTIGTPWGISMKL-UHFFFAOYSA-N molybdenum nickel Chemical compound [Ni].[Mo] DDTIGTPWGISMKL-UHFFFAOYSA-N 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 150000003961 organosilicon compounds Chemical class 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 1
- 239000012466 permeate Substances 0.000 description 1
- 230000008092 positive effect Effects 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 229910052761 rare earth metal Inorganic materials 0.000 description 1
- 150000002910 rare earth metals Chemical class 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 238000007670 refining Methods 0.000 description 1
- 238000009738 saturating Methods 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 230000002226 simultaneous effect Effects 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 238000007669 thermal treatment Methods 0.000 description 1
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/02—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols by adsorption, e.g. preparative gas chromatography
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/02—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material
- B01J20/06—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising oxides or hydroxides of metals not provided for in group B01J20/04
- B01J20/08—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising oxides or hydroxides of metals not provided for in group B01J20/04 comprising aluminium oxide or hydroxide; comprising bauxite
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/02—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols by adsorption, e.g. preparative gas chromatography
- B01D53/04—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols by adsorption, e.g. preparative gas chromatography with stationary adsorbents
- B01D53/047—Pressure swing adsorption
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/02—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material
- B01J20/10—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising silica or silicate
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/02—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material
- B01J20/10—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising silica or silicate
- B01J20/103—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising silica or silicate comprising silica
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/28—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/30—Processes for preparing, regenerating, or reactivating
- B01J20/32—Impregnating or coating ; Solid sorbent compositions obtained from processes involving impregnating or coating
- B01J20/3291—Characterised by the shape of the carrier, the coating or the obtained coated product
- B01J20/3293—Coatings on a core, the core being particle or fiber shaped, e.g. encapsulated particles, coated fibers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2253/00—Adsorbents used in seperation treatment of gases and vapours
- B01D2253/10—Inorganic adsorbents
- B01D2253/104—Alumina
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2253/00—Adsorbents used in seperation treatment of gases and vapours
- B01D2253/10—Inorganic adsorbents
- B01D2253/106—Silica or silicates
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/80—Water
Landscapes
- Chemical & Material Sciences (AREA)
- Analytical Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- General Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Solid-Sorbent Or Filter-Aiding Compositions (AREA)
- Catalysts (AREA)
Abstract
Description
Claims (10)
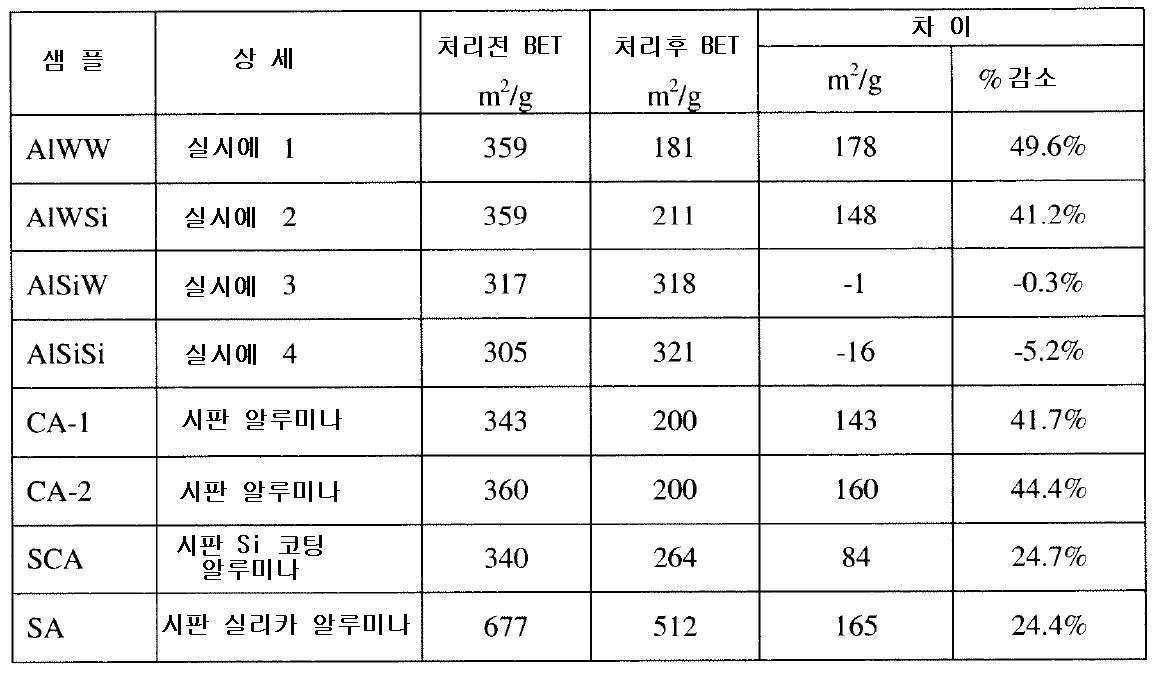
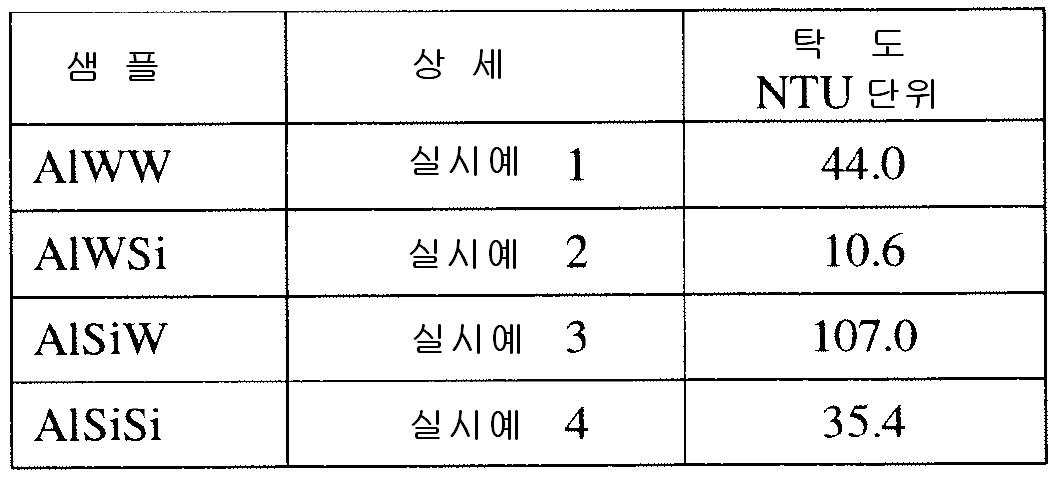
- 실리카 화합물을 함유하는 용액을 다량의 알루미나 분말과 함께 혼합하여 알루미나 미립자를 생성하는 단계;상기 알루미나 미립자를 경화시키는 단계; 및이후 생성된 경화 알루미나 미립자를 활성화하여 열수 안정성 알루미나 흡착제를 생성하는 단계를 포함하는 열수 안정성 알루미나 흡착제를 제조하는 방법으로서,상기 열수 안정성 알루미나 흡착제는 0.4 내지 4 중량%의 실리카를 함유하는 코어, 쉘 및 외표면을 포함하는 실리카 함유 알루미나 입자를 포함하고, 상기 실리카는 상기 코어에 걸쳐 균질하게 분포되고, 상기 쉘은 상기 외표면으로부터 상기 코어를 향해 50 ㎛ 이하로 연장되며, 상기 쉘은 평균적으로 상기 코어보다 2배 이상의 실리카를 함유하는 것인 방법.
- 제1항에 있어서, 상기 알루미나 미립자를 경화시키는 단계 이전 또는 이후에 상기 알루미나 미립자를 물 또는 콜로이드 실리카 용액으로 분무하는 단계를 더 포함하는 방법.
- 제1항에 있어서, 상기 실리카 화합물은 알칼리 금속 실리케이트 및 알칼리 토금속 실리케이트로 이루어진 군으로부터 선택되는 1종 이상의 화합물인 방법.
- 삭제
- 삭제
- 삭제
- 삭제
- 삭제
- 삭제
- 삭제
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/439,547 US7981836B2 (en) | 2006-05-24 | 2006-05-24 | Hydrothermally stable alumina |
| US11/439,547 | 2006-05-24 | ||
| PCT/US2007/068779 WO2007140103A1 (en) | 2006-05-24 | 2007-05-11 | Hydrothermally stable alumina |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020117025924A Division KR101208769B1 (ko) | 2006-05-24 | 2007-05-11 | 열수 안정성 알루미나 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20090007474A KR20090007474A (ko) | 2009-01-16 |
| KR101122370B1 true KR101122370B1 (ko) | 2012-03-23 |
Family
ID=38750197
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020117025924A KR101208769B1 (ko) | 2006-05-24 | 2007-05-11 | 열수 안정성 알루미나 |
| KR1020087029345A KR101122370B1 (ko) | 2006-05-24 | 2007-05-11 | 열수 안정성 알루미나 |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020117025924A KR101208769B1 (ko) | 2006-05-24 | 2007-05-11 | 열수 안정성 알루미나 |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US7981836B2 (ko) |
| EP (1) | EP2019729A4 (ko) |
| KR (2) | KR101208769B1 (ko) |
| CN (1) | CN101448565B (ko) |
| AU (1) | AU2007267719B2 (ko) |
| CA (1) | CA2651503C (ko) |
| WO (1) | WO2007140103A1 (ko) |
Families Citing this family (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8278242B2 (en) * | 2006-05-24 | 2012-10-02 | Uop Llc | Hydrothermally stable alumina |
| US8187997B2 (en) * | 2008-10-06 | 2012-05-29 | Union Carbide Chemicals & Technology LLC | Low metal loaded, catalyst compositions including acidic mixed metal oxide as support |
| BRPI0914045A2 (pt) | 2008-10-06 | 2015-11-03 | Union Carbide Chem Plastic | método para transferir um grupo hidroxialquila de um composto alcoxilado para um composto nucleofílico, método para transalcoxilar um composto alcoxilado, método para transalcoxilar um composto de alcanolamina e método para transoxilar um composto de alcanolamina |
| US8618108B2 (en) * | 2008-10-06 | 2013-12-31 | Union Carbide Chemicals & Plastics Technology Llc | Methods of making cyclic, N-amino functional triamines |
| BRPI0914085A2 (pt) * | 2008-10-06 | 2015-10-27 | Dow Global Technologies Llc | processo para a produção de uma ou mais etanolaminas e uma ou mais etilenoaminas e processo para separar uma ou mais alquiletilenodiaminas de etilenodiamina |
| BRPI0914042A2 (pt) * | 2008-10-06 | 2015-11-03 | Union Carbide Chem Plastic | processo para a produção de uma ou mais etilenoaminas por uma reação de transaminação |
| CN102224129B (zh) * | 2008-10-06 | 2015-02-11 | 联合碳化化学品及塑料技术公司 | 制备乙撑胺的方法 |
| JP5469173B2 (ja) * | 2008-10-06 | 2014-04-09 | ユニオン カーバイド ケミカルズ アンド プラスティックス テクノロジー エルエルシー | 金属低配合、アルミナ担持触媒組成物及びアミノ化方法 |
| US7947250B2 (en) * | 2008-12-11 | 2011-05-24 | Uop Llc | Process for conversion of aluminum oxide hydroxide |
| US8007760B2 (en) * | 2008-12-11 | 2011-08-30 | Uop Llc | Process for producing enhanced alumina |
| WO2010088013A1 (en) * | 2009-01-29 | 2010-08-05 | W. R. Grace & Co.-Conn. | Catalyst on silica clad alumina support |
| CN103464128A (zh) * | 2013-08-23 | 2013-12-25 | 上海华明高纳稀土新材料有限公司 | 高热稳定改性氧化铝及其制备方法 |
| JP2017500386A (ja) | 2013-12-02 | 2017-01-05 | ダウ グローバル テクノロジーズ エルエルシー | 高分子量の分岐鎖非環式ポリアルキレンアミン及びその混合物の調製 |
| KR101549359B1 (ko) | 2014-12-31 | 2015-09-01 | 주식회사 에코프로 | 마이크로파 흡수특성을 가진 흡착제 |
| CN106179289B (zh) * | 2015-04-29 | 2019-08-16 | 中国石油化工股份有限公司 | 一种硅改性氧化铝的制备方法、产品及其应用 |
| CN105126930B (zh) * | 2015-08-28 | 2017-12-12 | 烟台大学 | 一种催化剂载体的制备方法及其在氯化氢催化氧化中的应用 |
| CN105289613B (zh) * | 2015-11-04 | 2017-12-19 | 中国科学院山西煤炭化学研究所 | 氧化铝负载钴费托合成催化剂及制法和应用 |
| CN111099593B (zh) * | 2018-10-26 | 2021-08-03 | 中国石油化工股份有限公司 | 一种固体还原剂及其制备方法和应用 |
| WO2020106836A2 (en) * | 2018-11-21 | 2020-05-28 | Sasol (Usa) Corporation | Silica alumina composition with improved stability and method for making same |
| CN113860344B (zh) * | 2020-06-30 | 2023-04-25 | 中国石油天然气股份有限公司 | 一种具有高水热稳定性的多孔氧化铝基质及其制备方法 |
| CN113019310B (zh) * | 2021-03-30 | 2024-03-29 | 中国建筑设计研究院有限公司 | 一种活性氧化铝包裹建筑垃圾核心骨料的氧化铝球及制备方法 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030134742A1 (en) | 2001-02-28 | 2003-07-17 | Kanazirev Vladislav I. | Low dust adsorbents and catalysts and method for preparation |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3923692A (en) * | 1972-08-03 | 1975-12-02 | Nalco Chemical Co | Hydrotreating catalyst |
| IT1001614B (it) | 1973-10-31 | 1976-04-30 | Snam Progetti | Procedimento per preparare materia li aventi proprieta meccaniche e termiche migliorate e materiali ottenuti |
| CA1276002C (en) | 1985-12-31 | 1990-11-06 | Lawrence L. Murrell | Catalysts comprising silica supported on alumina their preparation and use |
| US5096871A (en) | 1990-07-03 | 1992-03-17 | Alcan International Limited | Alumina-alkali metal aluminum silicate agglomerate acid adsorbents |
| US5147836A (en) | 1991-10-18 | 1992-09-15 | W. R. Grace & Co.-Conn. | Catalytic cracking catalysts |
| US5304526A (en) | 1991-10-18 | 1994-04-19 | W. R. Grace & Co.-Conn. | Silica bayerite/eta alumina |
| CN1089032C (zh) * | 1996-12-10 | 2002-08-14 | 中国石油化工集团公司 | 一种多孔性含硅氧化铝载体小球及其制法 |
| US6159898A (en) * | 1997-05-23 | 2000-12-12 | Uop Llc | Alumina bodies containing alkali or alkaline earth metal compounds |
| US6680271B1 (en) | 1997-12-17 | 2004-01-20 | Grace Gmbh & Co. Kg | Solid zeolite granulates having improved abrasion resistance, and methods of making them |
-
2006
- 2006-05-24 US US11/439,547 patent/US7981836B2/en not_active Expired - Fee Related
-
2007
- 2007-05-11 CA CA2651503A patent/CA2651503C/en not_active Expired - Fee Related
- 2007-05-11 EP EP07762139A patent/EP2019729A4/en not_active Withdrawn
- 2007-05-11 WO PCT/US2007/068779 patent/WO2007140103A1/en active Application Filing
- 2007-05-11 CN CN2007800183903A patent/CN101448565B/zh not_active Expired - Fee Related
- 2007-05-11 AU AU2007267719A patent/AU2007267719B2/en not_active Ceased
- 2007-05-11 KR KR1020117025924A patent/KR101208769B1/ko not_active IP Right Cessation
- 2007-05-11 KR KR1020087029345A patent/KR101122370B1/ko not_active IP Right Cessation
-
2009
- 2009-11-17 US US12/620,225 patent/US20100075846A1/en not_active Abandoned
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030134742A1 (en) | 2001-02-28 | 2003-07-17 | Kanazirev Vladislav I. | Low dust adsorbents and catalysts and method for preparation |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20090007474A (ko) | 2009-01-16 |
| CN101448565B (zh) | 2012-11-21 |
| AU2007267719A1 (en) | 2007-12-06 |
| CA2651503A1 (en) | 2007-12-06 |
| US20100075846A1 (en) | 2010-03-25 |
| AU2007267719B2 (en) | 2010-12-23 |
| US7981836B2 (en) | 2011-07-19 |
| KR101208769B1 (ko) | 2012-12-05 |
| KR20110126190A (ko) | 2011-11-22 |
| WO2007140103A1 (en) | 2007-12-06 |
| CN101448565A (zh) | 2009-06-03 |
| EP2019729A4 (en) | 2011-12-14 |
| CA2651503C (en) | 2011-09-13 |
| EP2019729A1 (en) | 2009-02-04 |
| US20070275846A1 (en) | 2007-11-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101122370B1 (ko) | 열수 안정성 알루미나 | |
| US6736882B2 (en) | Low dust adsorbents and catalysts and method for preparation | |
| EP1749571B1 (en) | Co-formed base-treated aluminas for water and co2 removal | |
| TWI526428B (zh) | 用於自烯烴流移除含氧物之較低反應性吸附收劑及較高含氧物容量 | |
| JP4134031B2 (ja) | メソ細孔性ゼオライトの合成方法 | |
| JP5504446B2 (ja) | 疎水性ゼオライトの製造方法及びその方法で得られた疎水性ゼオライト | |
| CN111683744A (zh) | 用于vsa/vpsa/psa系统的优异的壳包核组分复合吸附剂 | |
| KR20190072322A (ko) | Voc 제거용 복합 성형체 및 이의 제조방법 | |
| US3594982A (en) | Process for drying unsaturated organic gaseous compounds | |
| US8278242B2 (en) | Hydrothermally stable alumina | |
| KR101911151B1 (ko) | 분자체 흡착제 블렌드 및 이의 용도 | |
| US5002742A (en) | Inorganic oxide sorbents for sulfur oxides | |
| JP2000210557A (ja) | X型ゼオライト含有成形体及びその製造方法並びにその用途 | |
| US3623993A (en) | Alumina desiccant for drying unsaturated organic gaseous compounds | |
| CA1226858A (en) | Inorganic oxide sorbents | |
| JP2001347123A (ja) | 二酸化炭素の吸着分離方法 | |
| KR101155980B1 (ko) | 산소제조용 제올라이트 분자체의 제조방법 | |
| CN101643216B (zh) | 一种降低分子筛落粉度的方法 | |
| RU2804129C1 (ru) | Поглотитель хлороводорода и способ очистки газовых смесей | |
| RU2097124C1 (ru) | Способ получения сорбента и сорбент | |
| CN102689913B (zh) | 一种利用分子自组装制备γ-Al2O3的方法 | |
| KR102198162B1 (ko) | 고강도 구형 제올라이트 성형체와 그 제조방법 | |
| JPH06190274A (ja) | 酸性成分吸着剤 | |
| JP2005263529A (ja) | 低アルカリ活性アルミナの製造方法 | |
| KR20230128278A (ko) | 거주 공간의 이산화탄소의 저감 방법, 그리고, 이산화탄소 흡착재 및 그 제조 방법 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| PA0105 | International application |
Patent event date: 20081201 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PA0201 | Request for examination | ||
| PG1501 | Laying open of application | ||
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20101113 Patent event code: PE09021S01D |
|
| AMND | Amendment | ||
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20110830 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20101113 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| J201 | Request for trial against refusal decision | ||
| PJ0201 | Trial against decision of rejection |
Patent event date: 20110930 Comment text: Request for Trial against Decision on Refusal Patent event code: PJ02012R01D Patent event date: 20110830 Comment text: Decision to Refuse Application Patent event code: PJ02011S01I Appeal kind category: Appeal against decision to decline refusal Decision date: 20111130 Appeal identifier: 2011101007183 Request date: 20110930 |
|
| A107 | Divisional application of patent | ||
| AMND | Amendment | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20111031 |
|
| PB0901 | Examination by re-examination before a trial |
Comment text: Amendment to Specification, etc. Patent event date: 20111031 Patent event code: PB09011R02I Comment text: Request for Trial against Decision on Refusal Patent event date: 20110930 Patent event code: PB09011R01I Comment text: Amendment to Specification, etc. Patent event date: 20110211 Patent event code: PB09011R02I |
|
| B701 | Decision to grant | ||
| PB0701 | Decision of registration after re-examination before a trial |
Patent event date: 20111130 Comment text: Decision to Grant Registration Patent event code: PB07012S01D Patent event date: 20111110 Comment text: Transfer of Trial File for Re-examination before a Trial Patent event code: PB07011S01I |
|
| GRNT | Written decision to grant | ||
| PR0701 | Registration of establishment |
Comment text: Registration of Establishment Patent event date: 20120223 Patent event code: PR07011E01D |
|
| PR1002 | Payment of registration fee |
Payment date: 20120223 End annual number: 3 Start annual number: 1 |
|
| PG1601 | Publication of registration | ||
| FPAY | Annual fee payment |
Payment date: 20150217 Year of fee payment: 4 |
|
| PR1001 | Payment of annual fee |
Payment date: 20150217 Start annual number: 4 End annual number: 4 |
|
| FPAY | Annual fee payment |
Payment date: 20151230 Year of fee payment: 5 |
|
| PR1001 | Payment of annual fee |
Payment date: 20151230 Start annual number: 5 End annual number: 5 |
|
| LAPS | Lapse due to unpaid annual fee | ||
| PC1903 | Unpaid annual fee |
Termination category: Default of registration fee Termination date: 20171205 |