KR100818202B1 - 미생물의 성장에 필수적인 대사산물의 스크리닝 방법 - Google Patents
미생물의 성장에 필수적인 대사산물의 스크리닝 방법 Download PDFInfo
- Publication number
- KR100818202B1 KR100818202B1 KR1020060133119A KR20060133119A KR100818202B1 KR 100818202 B1 KR100818202 B1 KR 100818202B1 KR 1020060133119 A KR1020060133119 A KR 1020060133119A KR 20060133119 A KR20060133119 A KR 20060133119A KR 100818202 B1 KR100818202 B1 KR 100818202B1
- Authority
- KR
- South Korea
- Prior art keywords
- phosphate
- metabolite
- growth
- essential
- metabolic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000002207 metabolite Substances 0.000 title claims abstract description 159
- 230000012010 growth Effects 0.000 title claims abstract description 67
- 238000000034 method Methods 0.000 title claims abstract description 49
- 244000005700 microbiome Species 0.000 title claims abstract description 45
- 238000012216 screening Methods 0.000 title claims abstract description 18
- 230000002503 metabolic effect Effects 0.000 claims abstract description 67
- 108090000623 proteins and genes Proteins 0.000 claims abstract description 53
- 230000004907 flux Effects 0.000 claims abstract description 47
- 244000000010 microbial pathogen Species 0.000 claims abstract description 24
- 230000000813 microbial effect Effects 0.000 claims abstract description 11
- 239000000126 substance Substances 0.000 claims abstract description 11
- 230000010261 cell growth Effects 0.000 claims description 51
- 241000588724 Escherichia coli Species 0.000 claims description 26
- 238000006241 metabolic reaction Methods 0.000 claims description 25
- 230000007423 decrease Effects 0.000 claims description 23
- -1 CO 2 Chemical compound 0.000 claims description 17
- 238000005206 flow analysis Methods 0.000 claims description 16
- 230000002829 reductive effect Effects 0.000 claims description 14
- KYQCXUMVJGMDNG-SHUUEZRQSA-N keto-3-deoxy-D-manno-octulosonic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CC(=O)C(O)=O KYQCXUMVJGMDNG-SHUUEZRQSA-N 0.000 claims description 10
- 229910019142 PO4 Inorganic materials 0.000 claims description 9
- 230000000415 inactivating effect Effects 0.000 claims description 9
- 239000010452 phosphate Substances 0.000 claims description 9
- 230000004060 metabolic process Effects 0.000 claims description 8
- 238000004519 manufacturing process Methods 0.000 claims description 7
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 claims description 6
- BGWQRWREUZVRGI-NNPWBXLPSA-N (3s,4s,5s,6r)-6-[(1r)-1,2-dihydroxyethyl]oxane-2,3,4,5-tetrol Chemical compound OC[C@@H](O)[C@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1O BGWQRWREUZVRGI-NNPWBXLPSA-N 0.000 claims description 6
- XAOLJGCZESYRFT-VHSKNIDJSA-N (KDO)2-lipid IVA Chemical compound O[C@H]1[C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](OP(O)(O)=O)O[C@@H]1CO[C@H]1[C@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@H](OP(O)(O)=O)[C@@H](CO[C@]2(O[C@@H]([C@H](O)[C@H](O[C@]3(O[C@@H]([C@H](O)[C@H](O)C3)[C@H](O)CO)C(O)=O)C2)[C@H](O)CO)C(O)=O)O1 XAOLJGCZESYRFT-VHSKNIDJSA-N 0.000 claims description 6
- 101710146995 Acyl carrier protein Proteins 0.000 claims description 6
- BGWQRWREUZVRGI-WABJIWILSA-N D-glycero-D-manno-Heptose Natural products OC[C@H](O)[C@@H]1O[C@@H](O)[C@H](O)[C@H](O)[C@@H]1O BGWQRWREUZVRGI-WABJIWILSA-N 0.000 claims description 6
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 claims description 6
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 claims description 6
- UKAUYVFTDYCKQA-VKHMYHEASA-N L-homoserine Chemical compound OC(=O)[C@@H](N)CCO UKAUYVFTDYCKQA-VKHMYHEASA-N 0.000 claims description 6
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 claims description 6
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 claims description 6
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 claims description 6
- 102000002933 Thioredoxin Human genes 0.000 claims description 6
- UCMIRNVEIXFBKS-UHFFFAOYSA-N beta-alanine Chemical compound NCCC(O)=O UCMIRNVEIXFBKS-UHFFFAOYSA-N 0.000 claims description 6
- GNGACRATGGDKBX-UHFFFAOYSA-N dihydroxyacetone phosphate Chemical compound OCC(=O)COP(O)(O)=O GNGACRATGGDKBX-UHFFFAOYSA-N 0.000 claims description 6
- FPWMCUPFBRFMLH-UHFFFAOYSA-N prephenic acid Chemical compound OC1C=CC(CC(=O)C(O)=O)(C(O)=O)C=C1 FPWMCUPFBRFMLH-UHFFFAOYSA-N 0.000 claims description 6
- KIDHWZJUCRJVML-UHFFFAOYSA-N putrescine Chemical compound NCCCCN KIDHWZJUCRJVML-UHFFFAOYSA-N 0.000 claims description 6
- ATHGHQPFGPMSJY-UHFFFAOYSA-N spermidine Chemical compound NCCCCNCCCN ATHGHQPFGPMSJY-UHFFFAOYSA-N 0.000 claims description 6
- 108060008226 thioredoxin Proteins 0.000 claims description 6
- 229940094937 thioredoxin Drugs 0.000 claims description 6
- MSTNYGQPCMXVAQ-RYUDHWBXSA-N (6S)-5,6,7,8-tetrahydrofolic acid Chemical compound C([C@H]1CNC=2N=C(NC(=O)C=2N1)N)NC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 MSTNYGQPCMXVAQ-RYUDHWBXSA-N 0.000 claims description 5
- QNAYBMKLOCPYGJ-UHFFFAOYSA-N D-alpha-Ala Natural products CC([NH3+])C([O-])=O QNAYBMKLOCPYGJ-UHFFFAOYSA-N 0.000 claims description 5
- QNAYBMKLOCPYGJ-UWTATZPHSA-N L-Alanine Natural products C[C@@H](N)C(O)=O QNAYBMKLOCPYGJ-UWTATZPHSA-N 0.000 claims description 5
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 claims description 5
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 claims description 5
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 claims description 5
- LCTONWCANYUPML-UHFFFAOYSA-M Pyruvate Chemical compound CC(=O)C([O-])=O LCTONWCANYUPML-UHFFFAOYSA-M 0.000 claims description 5
- NJMGRJLQRLFQQX-HYXAFXHYSA-N 2-isopropylmaleic acid Chemical compound CC(C)C(\C(O)=O)=C\C(O)=O NJMGRJLQRLFQQX-HYXAFXHYSA-N 0.000 claims description 4
- NGHMDNPXVRFFGS-IUYQGCFVSA-N D-erythrose 4-phosphate Chemical compound O=C[C@H](O)[C@H](O)COP(O)(O)=O NGHMDNPXVRFFGS-IUYQGCFVSA-N 0.000 claims description 4
- ATBOMIWRCZXYSZ-XZBBILGWSA-N [1-[2,3-dihydroxypropoxy(hydroxy)phosphoryl]oxy-3-hexadecanoyloxypropan-2-yl] (9e,12e)-octadeca-9,12-dienoate Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCC\C=C\C\C=C\CCCCC ATBOMIWRCZXYSZ-XZBBILGWSA-N 0.000 claims description 4
- ZSLZBFCDCINBPY-ZSJPKINUSA-N acetyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 ZSLZBFCDCINBPY-ZSJPKINUSA-N 0.000 claims description 4
- AWUCVROLDVIAJX-UHFFFAOYSA-N alpha-glycerophosphate Natural products OCC(O)COP(O)(O)=O AWUCVROLDVIAJX-UHFFFAOYSA-N 0.000 claims description 4
- 238000012258 culturing Methods 0.000 claims description 4
- 230000003247 decreasing effect Effects 0.000 claims description 4
- 230000002401 inhibitory effect Effects 0.000 claims description 4
- 230000009467 reduction Effects 0.000 claims description 4
- WTFXTQVDAKGDEY-UHFFFAOYSA-N (-)-chorismic acid Natural products OC1C=CC(C(O)=O)=CC1OC(=C)C(O)=O WTFXTQVDAKGDEY-UHFFFAOYSA-N 0.000 claims description 3
- NMDWGEGFJUBKLB-YFKPBYRVSA-N (2S)-2-hydroxy-2-methyl-3-oxobutanoic acid Chemical compound CC(=O)[C@](C)(O)C(O)=O NMDWGEGFJUBKLB-YFKPBYRVSA-N 0.000 claims description 3
- OKYHYXLCTGGOLM-BYPYZUCNSA-N (2S)-2-hydroxy-3-oxobutyl phosphate Chemical compound CC(=O)[C@@H](O)COP(O)(O)=O OKYHYXLCTGGOLM-BYPYZUCNSA-N 0.000 claims description 3
- HIIZAGQWABAMRR-BYPYZUCNSA-N (2S)-2-isopropyl-3-oxosuccinic acid Chemical compound CC(C)[C@H](C(O)=O)C(=O)C(O)=O HIIZAGQWABAMRR-BYPYZUCNSA-N 0.000 claims description 3
- BITYXLXUCSKTJS-ZETCQYMHSA-N (2S)-2-isopropylmalic acid Chemical compound CC(C)[C@](O)(C(O)=O)CC(O)=O BITYXLXUCSKTJS-ZETCQYMHSA-N 0.000 claims description 3
- QYNUQALWYRSVHF-OLZOCXBDSA-N (6R)-5,10-methylenetetrahydrofolic acid Chemical compound C([C@H]1CNC=2N=C(NC(=O)C=2N1C1)N)N1C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 QYNUQALWYRSVHF-OLZOCXBDSA-N 0.000 claims description 3
- GPNCBCJEDRRCDW-ACUQGRCXSA-N (KDO)-lipid IVA Chemical compound O[C@H]1[C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](OP(O)(O)=O)O[C@@H]1CO[C@H]1[C@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@H](OP(O)(O)=O)[C@@H](CO[C@]2(O[C@@H]([C@H](O)[C@H](O)C2)[C@H](O)CO)C(O)=O)O1 GPNCBCJEDRRCDW-ACUQGRCXSA-N 0.000 claims description 3
- JTEYKUFKXGDTEU-VKHMYHEASA-N (R)-2,3-dihydroxy-3-methylbutanoic acid Chemical compound CC(C)(O)[C@@H](O)C(O)=O JTEYKUFKXGDTEU-VKHMYHEASA-N 0.000 claims description 3
- LXJXRIRHZLFYRP-VKHMYHEASA-L (R)-2-Hydroxy-3-(phosphonooxy)-propanal Natural products O=C[C@H](O)COP([O-])([O-])=O LXJXRIRHZLFYRP-VKHMYHEASA-L 0.000 claims description 3
- XHFVGHPGDLDEQO-ZETCQYMHSA-N (R)-4'-phosphopantothenic acid Chemical compound OP(=O)(O)OCC(C)(C)[C@@H](O)C(=O)NCCC(O)=O XHFVGHPGDLDEQO-ZETCQYMHSA-N 0.000 claims description 3
- OTOIIPJYVQJATP-BYPYZUCNSA-N (R)-pantoic acid Chemical compound OCC(C)(C)[C@@H](O)C(O)=O OTOIIPJYVQJATP-BYPYZUCNSA-N 0.000 claims description 3
- GHOKWGTUZJEAQD-ZETCQYMHSA-M (R)-pantothenate Chemical compound OCC(C)(C)[C@@H](O)C(=O)NCCC([O-])=O GHOKWGTUZJEAQD-ZETCQYMHSA-M 0.000 claims description 3
- DWAKNKKXGALPNW-BYPYZUCNSA-N (S)-1-pyrroline-5-carboxylic acid Chemical compound OC(=O)[C@@H]1CCC=N1 DWAKNKKXGALPNW-BYPYZUCNSA-N 0.000 claims description 3
- CXMBCXQHOXUCEO-BYPYZUCNSA-N (S)-2,3,4,5-tetrahydrodipicolinic acid Chemical compound OC(=O)[C@@H]1CCCC(C(O)=O)=N1 CXMBCXQHOXUCEO-BYPYZUCNSA-N 0.000 claims description 3
- UWOCFOFVIBZJGH-YFKPBYRVSA-N (S)-2,3-dihydrodipicolinic acid Chemical compound OC(=O)[C@@H]1CC=CC(C(O)=O)=N1 UWOCFOFVIBZJGH-YFKPBYRVSA-N 0.000 claims description 3
- VUQLHQFKACOHNZ-LURJTMIESA-N (S)-2-acetyl-2-hydroxybutanoic acid Chemical compound CC[C@](O)(C(C)=O)C(O)=O VUQLHQFKACOHNZ-LURJTMIESA-N 0.000 claims description 3
- JVQYSWDUAOAHFM-BYPYZUCNSA-N (S)-3-methyl-2-oxovaleric acid Chemical compound CC[C@H](C)C(=O)C(O)=O JVQYSWDUAOAHFM-BYPYZUCNSA-N 0.000 claims description 3
- UFIVEPVSAGBUSI-REOHCLBHSA-N (S)-dihydroorotic acid Chemical compound OC(=O)[C@@H]1CC(=O)NC(=O)N1 UFIVEPVSAGBUSI-REOHCLBHSA-N 0.000 claims description 3
- UKAUYVFTDYCKQA-UHFFFAOYSA-N -2-Amino-4-hydroxybutanoic acid Natural products OC(=O)C(N)CCO UKAUYVFTDYCKQA-UHFFFAOYSA-N 0.000 claims description 3
- TZCPCKNHXULUIY-RGULYWFUSA-N 1,2-distearoyl-sn-glycero-3-phosphoserine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCC TZCPCKNHXULUIY-RGULYWFUSA-N 0.000 claims description 3
- QOUSHGMTBIIAHR-KEOHHSTQSA-N 1-(5-phospho-beta-D-ribosyl)-5-[(5-phospho-beta-D-ribosylamino)methylideneamino]imidazole-4-carboxamide Chemical compound N([C@H]1[C@@H]([C@H](O)[C@@H](COP(O)(O)=O)O1)O)/C=N/C1=C(C(=O)N)N=CN1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O QOUSHGMTBIIAHR-KEOHHSTQSA-N 0.000 claims description 3
- RKNHJBVBFHDXGR-KEOHHSTQSA-N 1-(5-phospho-beta-D-ribosyl)-ATP Chemical compound O[C@@H]1[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@H]1N1C(N=CN([C@H]2[C@@H]([C@H](O)[C@@H](COP(O)(O)=O)O2)O)C2=N)=C2N=C1 RKNHJBVBFHDXGR-KEOHHSTQSA-N 0.000 claims description 3
- AUFGTPPARQZWDO-YPMHNXCESA-N 10-formyltetrahydrofolic acid Chemical compound C([C@H]1CNC=2N=C(NC(=O)C=2N1)N)N(C=O)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 AUFGTPPARQZWDO-YPMHNXCESA-N 0.000 claims description 3
- RQFCJASXJCIDSX-UHFFFAOYSA-N 14C-Guanosin-5'-monophosphat Natural products C1=2NC(N)=NC(=O)C=2N=CN1C1OC(COP(O)(O)=O)C(O)C1O RQFCJASXJCIDSX-UHFFFAOYSA-N 0.000 claims description 3
- HLDJGHAAKRKPAV-QDORLFPLSA-N 2,3,2',3'-tetrakis(3-hydroxytetradecanoyl)-alpha-D-glucosaminyl-1,6-beta-D-glucosamine 1-phosphate Chemical compound O1[C@H](CO)[C@@H](O)[C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H]1OC[C@@H]1[C@@H](O)[C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](OP(O)(O)=O)O1 HLDJGHAAKRKPAV-QDORLFPLSA-N 0.000 claims description 3
- FZLJPEPAYPUMMR-RTRLPJTCSA-N 2-acetamido-2-deoxy-D-glucopyranose 1-phosphate Chemical compound CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)OC1OP(O)(O)=O FZLJPEPAYPUMMR-RTRLPJTCSA-N 0.000 claims description 3
- PWKSKIMOESPYIA-UHFFFAOYSA-N 2-acetamido-3-sulfanylpropanoic acid Chemical compound CC(=O)NC(CS)C(O)=O PWKSKIMOESPYIA-UHFFFAOYSA-N 0.000 claims description 3
- XHMJOUIAFHJHBW-IVMDWMLBSA-N 2-amino-2-deoxy-D-glucopyranose 6-phosphate Chemical compound N[C@H]1C(O)O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O XHMJOUIAFHJHBW-IVMDWMLBSA-N 0.000 claims description 3
- QDGAVODICPCDMU-UHFFFAOYSA-N 2-amino-3-[3-[bis(2-chloroethyl)amino]phenyl]propanoic acid Chemical compound OC(=O)C(N)CC1=CC=CC(N(CCCl)CCCl)=C1 QDGAVODICPCDMU-UHFFFAOYSA-N 0.000 claims description 3
- DPTKHUJHZQQZJX-UHFFFAOYSA-N 2-amino-6-(hydroxymethyl)-7,8-dihydro-1h-pteridin-4-one;phosphono dihydrogen phosphate Chemical compound OP(O)(=O)OP(O)(O)=O.N1=C(CO)CNC2=NC(N)=NC(O)=C21 DPTKHUJHZQQZJX-UHFFFAOYSA-N 0.000 claims description 3
- CQQNNQTXUGLUEV-UHFFFAOYSA-N 2-amino-6-(hydroxymethyl)-7,8-dihydropteridin-4-ol Chemical compound N1CC(CO)=NC2=C1N=C(N)NC2=O CQQNNQTXUGLUEV-UHFFFAOYSA-N 0.000 claims description 3
- PKVVTUWHANFMQC-UHFFFAOYSA-N 2-dehydropantoic acid Chemical compound OCC(C)(C)C(=O)C(O)=O PKVVTUWHANFMQC-UHFFFAOYSA-N 0.000 claims description 3
- TYEYBOSBBBHJIV-UHFFFAOYSA-N 2-oxobutanoic acid Chemical compound CCC(=O)C(O)=O TYEYBOSBBBHJIV-UHFFFAOYSA-N 0.000 claims description 3
- KPGXRSRHYNQIFN-UHFFFAOYSA-N 2-oxoglutaric acid Chemical compound OC(=O)CCC(=O)C(O)=O KPGXRSRHYNQIFN-UHFFFAOYSA-N 0.000 claims description 3
- KDTSHFARGAKYJN-IBOSZNHHSA-N 3'-dephospho-CoA Chemical compound O[C@@H]1[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCS)O[C@H]1N1C2=NC=NC(N)=C2N=C1 KDTSHFARGAKYJN-IBOSZNHHSA-N 0.000 claims description 3
- GACDQMDRPRGCTN-KQYNXXCUSA-N 3'-phospho-5'-adenylyl sulfate Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP(O)(=O)OS(O)(=O)=O)[C@@H](OP(O)(O)=O)[C@H]1O GACDQMDRPRGCTN-KQYNXXCUSA-N 0.000 claims description 3
- YCFFMSOLUMRAMD-UHFFFAOYSA-N 3-(imidazol-4-yl)-2-oxopropyl dihydrogen phosphate Chemical compound OP(O)(=O)OCC(=O)CC1=CNC=N1 YCFFMSOLUMRAMD-UHFFFAOYSA-N 0.000 claims description 3
- WVMWZWGZRAXUBK-SYTVJDICSA-N 3-dehydroquinic acid Chemical compound O[C@@H]1C[C@](O)(C(O)=O)CC(=O)[C@H]1O WVMWZWGZRAXUBK-SYTVJDICSA-N 0.000 claims description 3
- WVMWZWGZRAXUBK-UHFFFAOYSA-N 3-dehydroquinic acid Natural products OC1CC(O)(C(O)=O)CC(=O)C1O WVMWZWGZRAXUBK-UHFFFAOYSA-N 0.000 claims description 3
- YVYKOQWMJZXRRM-PUFIMZNGSA-N 3-dehydroshikimate Chemical compound O[C@@H]1C[C@H](C(O)=O)C=C(O)[C@@H]1O YVYKOQWMJZXRRM-PUFIMZNGSA-N 0.000 claims description 3
- QHKABHOOEWYVLI-UHFFFAOYSA-N 3-methyl-2-oxobutanoic acid Chemical compound CC(C)C(=O)C(O)=O QHKABHOOEWYVLI-UHFFFAOYSA-N 0.000 claims description 3
- OSJPPGNTCRNQQC-UWTATZPHSA-N 3-phospho-D-glyceric acid Chemical compound OC(=O)[C@H](O)COP(O)(O)=O OSJPPGNTCRNQQC-UWTATZPHSA-N 0.000 claims description 3
- LFLUCDOSQPJJBE-UHFFFAOYSA-N 3-phosphonooxypyruvic acid Chemical compound OC(=O)C(=O)COP(O)(O)=O LFLUCDOSQPJJBE-UHFFFAOYSA-N 0.000 claims description 3
- QYOJSKGCWNAKGW-PBXRRBTRSA-N 3-phosphoshikimic acid Chemical compound O[C@@H]1CC(C(O)=O)=C[C@@H](OP(O)(O)=O)[C@H]1O QYOJSKGCWNAKGW-PBXRRBTRSA-N 0.000 claims description 3
- 229940086681 4-aminobenzoate Drugs 0.000 claims description 3
- ALYNCZNDIQEVRV-UHFFFAOYSA-N 4-aminobenzoic acid Chemical compound NC1=CC=C(C(O)=O)C=C1 ALYNCZNDIQEVRV-UHFFFAOYSA-N 0.000 claims description 3
- BKAJNAXTPSGJCU-UHFFFAOYSA-N 4-methyl-2-oxopentanoic acid Chemical compound CC(C)CC(=O)C(O)=O BKAJNAXTPSGJCU-UHFFFAOYSA-N 0.000 claims description 3
- IXZNKTPIYKDIGG-REOHCLBHSA-N 4-phospho-L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(=O)OP(O)(O)=O IXZNKTPIYKDIGG-REOHCLBHSA-N 0.000 claims description 3
- XTWYTFMLZFPYCI-KQYNXXCUSA-N 5'-adenylphosphoric acid Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O XTWYTFMLZFPYCI-KQYNXXCUSA-N 0.000 claims description 3
- DCTLYFZHFGENCW-UUOKFMHZSA-N 5'-xanthylic acid Chemical compound O[C@@H]1[C@H](O)[C@@H](COP(O)(O)=O)O[C@H]1N1C(NC(=O)NC2=O)=C2N=C1 DCTLYFZHFGENCW-UUOKFMHZSA-N 0.000 claims description 3
- QUTYKIXIUDQOLK-PRJMDXOYSA-N 5-O-(1-carboxyvinyl)-3-phosphoshikimic acid Chemical compound O[C@H]1[C@H](OC(=C)C(O)=O)CC(C(O)=O)=C[C@H]1OP(O)(O)=O QUTYKIXIUDQOLK-PRJMDXOYSA-N 0.000 claims description 3
- PQGCEDQWHSBAJP-TXICZTDVSA-N 5-O-phosphono-alpha-D-ribofuranosyl diphosphate Chemical compound O[C@H]1[C@@H](O)[C@@H](O[P@](O)(=O)OP(O)(O)=O)O[C@@H]1COP(O)(O)=O PQGCEDQWHSBAJP-TXICZTDVSA-N 0.000 claims description 3
- BLKFNHOCHNCLII-GHVQHMAVSA-N 5-[(5-phospho-1-deoxy-D-ribulos-1-ylimino)methylamino]-1-(5-phospho-beta-D-ribosyl)imidazole-4-carboxamide Chemical compound OP(=O)(O)OC[C@@H](O)[C@@H](O)C(=O)CN=CNC1=C(C(=O)N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](COP(O)(O)=O)O1 BLKFNHOCHNCLII-GHVQHMAVSA-N 0.000 claims description 3
- XFVULMDJZXYMSG-ZIYNGMLESA-N 5-amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxylic acid Chemical compound NC1=C(C(O)=O)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](COP(O)(O)=O)O1 XFVULMDJZXYMSG-ZIYNGMLESA-N 0.000 claims description 3
- PDACUKOKVHBVHJ-XVFCMESISA-N 5-amino-1-(5-phospho-beta-D-ribosyl)imidazole Chemical compound NC1=CN=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](COP(O)(O)=O)O1 PDACUKOKVHBVHJ-XVFCMESISA-N 0.000 claims description 3
- XKQZIXVJVUPORE-RPDRRWSUSA-N 5-amino-6-(D-ribitylamino)uracil Chemical compound NC1=C(NC[C@H](O)[C@H](O)[C@H](O)CO)NC(=O)NC1=O XKQZIXVJVUPORE-RPDRRWSUSA-N 0.000 claims description 3
- ABCOOORLYAOBOZ-KQYNXXCUSA-N 5-formamido-1-(5-phospho-D-ribosyl)imidazole-4-carboxamide Chemical compound O=CNC1=C(C(=O)N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](COP(O)(O)=O)O1 ABCOOORLYAOBOZ-KQYNXXCUSA-N 0.000 claims description 3
- SKCBPEVYGOQGJN-TXICZTDVSA-N 5-phospho-beta-D-ribosylamine Chemical compound N[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O SKCBPEVYGOQGJN-TXICZTDVSA-N 0.000 claims description 3
- YQIFAMYNGGOTFB-XINAWCOVSA-N 7,8-dihydroneopterin Chemical compound N1CC([C@H](O)[C@H](O)CO)=NC2=C1N=C(N)NC2=O YQIFAMYNGGOTFB-XINAWCOVSA-N 0.000 claims description 3
- DGGUVLXVLHAAGT-XINAWCOVSA-N 7,8-dihydroneopterin 3'-triphosphate Chemical compound N1CC([C@H](O)[C@H](O)COP(O)(=O)OP(O)(=O)OP(O)(O)=O)=NC2=C1N=C(N)NC2=O DGGUVLXVLHAAGT-XINAWCOVSA-N 0.000 claims description 3
- WBFYVDCHGVNRBH-UHFFFAOYSA-N 7,8-dihydropteroic acid Chemical compound N=1C=2C(=O)NC(N)=NC=2NCC=1CNC1=CC=C(C(O)=O)C=C1 WBFYVDCHGVNRBH-UHFFFAOYSA-N 0.000 claims description 3
- PJWIPEXIFFQAQZ-PUFIMZNGSA-N 7-phospho-2-dehydro-3-deoxy-D-arabino-heptonic acid Chemical compound OP(=O)(O)OC[C@@H](O)[C@@H](O)[C@H](O)CC(=O)C(O)=O PJWIPEXIFFQAQZ-PUFIMZNGSA-N 0.000 claims description 3
- KMSFWBYFWSKGGR-RQWOTHMISA-N ADP-D-glycero-D-manno-heptose Chemical compound C([C@H]1O[C@H]([C@@H]([C@@H]1O)O)N1C=2N=CN=C(C=2N=C1)N)OP(O)(=O)OP(O)(=O)OC1O[C@H]([C@H](O)CO)[C@@H](O)[C@H](O)[C@@H]1O KMSFWBYFWSKGGR-RQWOTHMISA-N 0.000 claims description 3
- KMSFWBYFWSKGGR-XRLZOAFQSA-N ADP-L-glycero-D-manno-heptose Chemical compound C([C@H]1O[C@H]([C@@H]([C@@H]1O)O)N1C=2N=CN=C(C=2N=C1)N)OP(O)(=O)OP(O)(=O)OC1O[C@H]([C@@H](O)CO)[C@@H](O)[C@H](O)[C@@H]1O KMSFWBYFWSKGGR-XRLZOAFQSA-N 0.000 claims description 3
- NOTGFIUVDGNKRI-UUOKFMHZSA-N AICA ribonucleotide Chemical compound NC1=C(C(=O)N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](COP(O)(O)=O)O1 NOTGFIUVDGNKRI-UUOKFMHZSA-N 0.000 claims description 3
- 108700027273 Acyl Carrier Proteins 0.000 claims description 3
- XTWYTFMLZFPYCI-UHFFFAOYSA-N Adenosine diphosphate Natural products C1=NC=2C(N)=NC=NC=2N1C1OC(COP(O)(=O)OP(O)(O)=O)C(O)C1O XTWYTFMLZFPYCI-UHFFFAOYSA-N 0.000 claims description 3
- ZKHQWZAMYRWXGA-KQYNXXCUSA-N Adenosine triphosphate Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O ZKHQWZAMYRWXGA-KQYNXXCUSA-N 0.000 claims description 3
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 claims description 3
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 claims description 3
- WVIMUEUQJFPNDK-PEBGCTIMSA-N CDP-ethanolamine Chemical compound O[C@@H]1[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OCCN)O[C@H]1N1C(=O)N=C(N)C=C1 WVIMUEUQJFPNDK-PEBGCTIMSA-N 0.000 claims description 3
- IERHLVCPSMICTF-XVFCMESISA-N CMP group Chemical group P(=O)(O)(O)OC[C@@H]1[C@H]([C@H]([C@@H](O1)N1C(=O)N=C(N)C=C1)O)O IERHLVCPSMICTF-XVFCMESISA-N 0.000 claims description 3
- YWWJKULNWGRYAS-UOVSKDHASA-N CMP-3-deoxy-beta-D-manno-octulosonic acid Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](COP(O)(=O)O[C@@]2(O[C@@H]([C@H](O)[C@H](O)C2)[C@H](O)CO)C(O)=O)O1 YWWJKULNWGRYAS-UOVSKDHASA-N 0.000 claims description 3
- PCDQPRRSZKQHHS-XVFCMESISA-N CTP Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 PCDQPRRSZKQHHS-XVFCMESISA-N 0.000 claims description 3
- RGJOEKWQDUBAIZ-IBOSZNHHSA-N CoASH Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCS)O[C@H]1N1C2=NC=NC(N)=C2N=C1 RGJOEKWQDUBAIZ-IBOSZNHHSA-N 0.000 claims description 3
- GSXOAOHZAIYLCY-UHFFFAOYSA-N D-F6P Natural products OCC(=O)C(O)C(O)C(O)COP(O)(O)=O GSXOAOHZAIYLCY-UHFFFAOYSA-N 0.000 claims description 3
- NBSCHQHZLSJFNQ-GASJEMHNSA-N D-Glucose 6-phosphate Chemical compound OC1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H](O)[C@H]1O NBSCHQHZLSJFNQ-GASJEMHNSA-N 0.000 claims description 3
- DEFJQIDDEAULHB-QWWZWVQMSA-N D-alanyl-D-alanine Chemical compound C[C@@H]([NH3+])C(=O)N[C@H](C)C([O-])=O DEFJQIDDEAULHB-QWWZWVQMSA-N 0.000 claims description 3
- ILRYLPWNYFXEMH-UHFFFAOYSA-N D-cystathionine Natural products OC(=O)C(N)CCSCC(N)C(O)=O ILRYLPWNYFXEMH-UHFFFAOYSA-N 0.000 claims description 3
- HFYBTHCYPKEDQQ-RITPCOANSA-N D-erythro-1-(imidazol-4-yl)glycerol 3-phosphate Chemical compound OP(=O)(O)OC[C@@H](O)[C@@H](O)C1=CNC=N1 HFYBTHCYPKEDQQ-RITPCOANSA-N 0.000 claims description 3
- 229930195713 D-glutamate Natural products 0.000 claims description 3
- WHUUTDBJXJRKMK-GSVOUGTGSA-N D-glutamic acid Chemical compound OC(=O)[C@H](N)CCC(O)=O WHUUTDBJXJRKMK-GSVOUGTGSA-N 0.000 claims description 3
- LXJXRIRHZLFYRP-VKHMYHEASA-N D-glyceraldehyde 3-phosphate Chemical compound O=C[C@H](O)COP(O)(O)=O LXJXRIRHZLFYRP-VKHMYHEASA-N 0.000 claims description 3
- JDMUPRLRUUMCTL-VIFPVBQESA-N D-pantetheine 4'-phosphate Chemical compound OP(=O)(O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCS JDMUPRLRUUMCTL-VIFPVBQESA-N 0.000 claims description 3
- FNZLKVNUWIIPSJ-UHNVWZDZSA-N D-ribulose 5-phosphate Chemical compound OCC(=O)[C@H](O)[C@H](O)COP(O)(O)=O FNZLKVNUWIIPSJ-UHNVWZDZSA-N 0.000 claims description 3
- SLWWJZMPHJJOPH-UHFFFAOYSA-N DHS Natural products OC1CC(C(O)=O)=CC(=O)C1O SLWWJZMPHJJOPH-UHFFFAOYSA-N 0.000 claims description 3
- RWSOTUBLDIXVET-UHFFFAOYSA-N Dihydrogen sulfide Chemical compound S RWSOTUBLDIXVET-UHFFFAOYSA-N 0.000 claims description 3
- QGWNDRXFNXRZMB-UUOKFMHZSA-K GDP(3-) Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@@H]1O[C@H](COP([O-])(=O)OP([O-])([O-])=O)[C@@H](O)[C@H]1O QGWNDRXFNXRZMB-UUOKFMHZSA-K 0.000 claims description 3
- VFRROHXSMXFLSN-UHFFFAOYSA-N Glc6P Natural products OP(=O)(O)OCC(O)C(O)C(O)C(O)C=O VFRROHXSMXFLSN-UHFFFAOYSA-N 0.000 claims description 3
- JZNWSCPGTDBMEW-UHFFFAOYSA-N Glycerophosphorylethanolamin Natural products NCCOP(O)(=O)OCC(O)CO JZNWSCPGTDBMEW-UHFFFAOYSA-N 0.000 claims description 3
- ZWZWYGMENQVNFU-UHFFFAOYSA-N Glycerophosphorylserin Natural products OC(=O)C(N)COP(O)(=O)OCC(O)CO ZWZWYGMENQVNFU-UHFFFAOYSA-N 0.000 claims description 3
- 239000004471 Glycine Substances 0.000 claims description 3
- 229920002527 Glycogen Polymers 0.000 claims description 3
- XKMLYUALXHKNFT-UUOKFMHZSA-N Guanosine-5'-triphosphate Chemical compound C1=2NC(N)=NC(=O)C=2N=CN1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O XKMLYUALXHKNFT-UUOKFMHZSA-N 0.000 claims description 3
- GRSZFWQUAKGDAV-UHFFFAOYSA-N Inosinic acid Natural products OC1C(O)C(COP(O)(O)=O)OC1N1C(NC=NC2=O)=C2N=C1 GRSZFWQUAKGDAV-UHFFFAOYSA-N 0.000 claims description 3
- SDVXSCSNVVZWDD-LURJTMIESA-N L-2-succinylamino-6-oxoheptanedioic acid Chemical compound OC(=O)CCC(=O)N[C@H](C(O)=O)CCCC(=O)C(O)=O SDVXSCSNVVZWDD-LURJTMIESA-N 0.000 claims description 3
- ZQISRDCJNBUVMM-UHFFFAOYSA-N L-Histidinol Natural products OCC(N)CC1=CN=CN1 ZQISRDCJNBUVMM-UHFFFAOYSA-N 0.000 claims description 3
- FFFHZYDWPBMWHY-UHFFFAOYSA-N L-Homocysteine Natural products OC(=O)C(N)CCS FFFHZYDWPBMWHY-UHFFFAOYSA-N 0.000 claims description 3
- FFEARJCKVFRZRR-UHFFFAOYSA-N L-Methionine Natural products CSCCC(N)C(O)=O FFEARJCKVFRZRR-UHFFFAOYSA-N 0.000 claims description 3
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 claims description 3
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 claims description 3
- ODKSFYDXXFIFQN-BYPYZUCNSA-N L-arginine Chemical compound OC(=O)[C@@H](N)CCCN=C(N)N ODKSFYDXXFIFQN-BYPYZUCNSA-N 0.000 claims description 3
- HOSWPDPVFBCLSY-VKHMYHEASA-N L-aspartic 4-semialdehyde Chemical compound [O-]C(=O)[C@@H]([NH3+])CC=O HOSWPDPVFBCLSY-VKHMYHEASA-N 0.000 claims description 3
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 claims description 3
- ILRYLPWNYFXEMH-WHFBIAKZSA-N L-cystathionine Chemical compound [O-]C(=O)[C@@H]([NH3+])CCSC[C@H]([NH3+])C([O-])=O ILRYLPWNYFXEMH-WHFBIAKZSA-N 0.000 claims description 3
- 229930195714 L-glutamate Natural products 0.000 claims description 3
- KABXUUFDPUOJMW-BYPYZUCNSA-N L-glutamic 5-semialdehyde Chemical compound OC(=O)[C@@H](N)CCC=O KABXUUFDPUOJMW-BYPYZUCNSA-N 0.000 claims description 3
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 claims description 3
- 229930182816 L-glutamine Natural products 0.000 claims description 3
- ZQISRDCJNBUVMM-YFKPBYRVSA-N L-histidinol Chemical compound OC[C@@H](N)CC1=CNC=N1 ZQISRDCJNBUVMM-YFKPBYRVSA-N 0.000 claims description 3
- CWNDERHTHMWBSI-YFKPBYRVSA-N L-histidinol phosphate Chemical compound OP(=O)(O)OC[C@@H](N)CC1=CNC=N1 CWNDERHTHMWBSI-YFKPBYRVSA-N 0.000 claims description 3
- FFFHZYDWPBMWHY-VKHMYHEASA-N L-homocysteine Chemical compound OC(=O)[C@@H](N)CCS FFFHZYDWPBMWHY-VKHMYHEASA-N 0.000 claims description 3
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 claims description 3
- 229930182844 L-isoleucine Natural products 0.000 claims description 3
- 235000019454 L-leucine Nutrition 0.000 claims description 3
- 239000004395 L-leucine Substances 0.000 claims description 3
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 claims description 3
- 229930195722 L-methionine Natural products 0.000 claims description 3
- 229930182821 L-proline Natural products 0.000 claims description 3
- GMKMEZVLHJARHF-WHFBIAKZSA-N LL-2,6-diaminopimelic acid Chemical compound OC(=O)[C@@H](N)CCC[C@H](N)C(O)=O GMKMEZVLHJARHF-WHFBIAKZSA-N 0.000 claims description 3
- LTYOQGRJFJAKNA-KKIMTKSISA-N Malonyl CoA Natural products S(C(=O)CC(=O)O)CCNC(=O)CCNC(=O)[C@@H](O)C(CO[P@](=O)(O[P@](=O)(OC[C@H]1[C@@H](OP(=O)(O)O)[C@@H](O)[C@@H](n2c3ncnc(N)c3nc2)O1)O)O)(C)C LTYOQGRJFJAKNA-KKIMTKSISA-N 0.000 claims description 3
- MSFSPUZXLOGKHJ-UHFFFAOYSA-N Muraminsaeure Natural products OC(=O)C(C)OC1C(N)C(O)OC(CO)C1O MSFSPUZXLOGKHJ-UHFFFAOYSA-N 0.000 claims description 3
- VDXLUNDMVKSKHO-ZRTZXPPTSA-N N(2)-formyl-N(1)-(5-phospho-D-ribosyl)glycinamide Chemical compound O[C@H]1[C@@H](O)C(NC(=O)CNC=O)O[C@@H]1COP(O)(O)=O VDXLUNDMVKSKHO-ZRTZXPPTSA-N 0.000 claims description 3
- DEFJQIDDEAULHB-UHFFFAOYSA-N N-D-alanyl-D-alanine Natural products CC(N)C(=O)NC(C)C(O)=O DEFJQIDDEAULHB-UHFFFAOYSA-N 0.000 claims description 3
- OVRNDRQMDRJTHS-UHFFFAOYSA-N N-acelyl-D-glucosamine Natural products CC(=O)NC1C(O)OC(CO)C(O)C1O OVRNDRQMDRJTHS-UHFFFAOYSA-N 0.000 claims description 3
- OVRNDRQMDRJTHS-FMDGEEDCSA-N N-acetyl-beta-D-glucosamine Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O OVRNDRQMDRJTHS-FMDGEEDCSA-N 0.000 claims description 3
- MBLBDJOUHNCFQT-LXGUWJNJSA-N N-acetylglucosamine Natural products CC(=O)N[C@@H](C=O)[C@@H](O)[C@H](O)[C@H](O)CO MBLBDJOUHNCFQT-LXGUWJNJSA-N 0.000 claims description 3
- HLKXYZVTANABHZ-REOHCLBHSA-N N-carbamoyl-L-aspartic acid Chemical compound NC(=O)N[C@H](C(O)=O)CC(O)=O HLKXYZVTANABHZ-REOHCLBHSA-N 0.000 claims description 3
- GLXUWZBUPATPBR-BQBZGAKWSA-N N-succinyl-LL-2,6-diaminopimelic acid Chemical compound OC(=O)[C@@H](N)CCC[C@@H](C(O)=O)NC(=O)CCC(O)=O GLXUWZBUPATPBR-BQBZGAKWSA-N 0.000 claims description 3
- BAWFJGJZGIEFAR-NNYOXOHSSA-N NAD zwitterion Chemical compound NC(=O)C1=CC=C[N+]([C@H]2[C@@H]([C@H](O)[C@@H](COP([O-])(=O)OP(O)(=O)OC[C@@H]3[C@H]([C@@H](O)[C@@H](O3)N3C4=NC=NC(N)=C4N=C3)O)O2)O)=C1 BAWFJGJZGIEFAR-NNYOXOHSSA-N 0.000 claims description 3
- BOPGDPNILDQYTO-NNYOXOHSSA-L NADH(2-) Chemical compound C1=CCC(C(=O)N)=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](COP([O-])(=O)OP([O-])(=O)OC[C@@H]2[C@H]([C@@H](O)[C@@H](O2)N2C3=NC=NC(N)=C3N=C2)O)O1 BOPGDPNILDQYTO-NNYOXOHSSA-L 0.000 claims description 3
- XJLXINKUBYWONI-NNYOXOHSSA-N NADP zwitterion Chemical compound NC(=O)C1=CC=C[N+]([C@H]2[C@@H]([C@H](O)[C@@H](COP([O-])(=O)OP(O)(=O)OC[C@@H]3[C@H]([C@@H](OP(O)(O)=O)[C@@H](O3)N3C4=NC=NC(N)=C4N=C3)O)O2)O)=C1 XJLXINKUBYWONI-NNYOXOHSSA-N 0.000 claims description 3
- ACFIXJIJDZMPPO-NNYOXOHSSA-N NADPH Chemical compound C1=CCC(C(=O)N)=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OC[C@@H]2[C@H]([C@@H](OP(O)(O)=O)[C@@H](O2)N2C3=NC=NC(N)=C3N=C2)O)O1 ACFIXJIJDZMPPO-NNYOXOHSSA-N 0.000 claims description 3
- VZXPDPZARILFQX-BYPYZUCNSA-N O-acetyl-L-serine Chemical compound CC(=O)OC[C@H]([NH3+])C([O-])=O VZXPDPZARILFQX-BYPYZUCNSA-N 0.000 claims description 3
- GNISQJGXJIDKDJ-YFKPBYRVSA-N O-succinyl-L-homoserine Chemical compound OC(=O)[C@@H](N)CCOC(=O)CCC(O)=O GNISQJGXJIDKDJ-YFKPBYRVSA-N 0.000 claims description 3
- 108010013639 Peptidoglycan Proteins 0.000 claims description 3
- PMCOGCVKOAOZQM-XVFCMESISA-N Phosphoribosylformylglycineamidine Chemical compound O=CNCC(/N)=N/[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O PMCOGCVKOAOZQM-XVFCMESISA-N 0.000 claims description 3
- 239000005700 Putrescine Substances 0.000 claims description 3
- FNZLKVNUWIIPSJ-UHFFFAOYSA-N Rbl5P Natural products OCC(=O)C(O)C(O)COP(O)(O)=O FNZLKVNUWIIPSJ-UHFFFAOYSA-N 0.000 claims description 3
- MEFKEPWMEQBLKI-AIRLBKTGSA-N S-adenosyl-L-methioninate Chemical compound O[C@@H]1[C@H](O)[C@@H](C[S+](CC[C@H](N)C([O-])=O)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 MEFKEPWMEQBLKI-AIRLBKTGSA-N 0.000 claims description 3
- ZUNBITIXDCPNSD-LSRJEVITSA-N S-adenosylmethioninamine Chemical compound O[C@@H]1[C@H](O)[C@@H](C[S+](CCCN)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 ZUNBITIXDCPNSD-LSRJEVITSA-N 0.000 claims description 3
- NAQGHJTUZRHGAC-ZZZDFHIKSA-N SAICAR Chemical compound NC1=C(C(=O)N[C@@H](CC(O)=O)C(O)=O)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](COP(O)(O)=O)O1 NAQGHJTUZRHGAC-ZZZDFHIKSA-N 0.000 claims description 3
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 claims description 3
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 claims description 3
- KOJCFMYSTWNMQW-RUAJDYCTSA-N UDP-2,3-bis[(3R)-3-hydroxytetradecanoyl]-alpha-D-glucosamine Chemical compound O1[C@H](CO)[C@@H](O)[C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@H]1OP(O)(=O)OP(O)(=O)OC[C@@H]1[C@@H](O)[C@@H](O)[C@H](N2C(NC(=O)C=C2)=O)O1 KOJCFMYSTWNMQW-RUAJDYCTSA-N 0.000 claims description 3
- TZSJGZGYQDNRRX-VCEDRBFZSA-N UDP-3-O-(3-hydroxytetradecanoyl)-N-acetyl-beta-glucosamine Chemical compound CC(=O)N[C@@H]1[C@@H](OC(=O)CC(O)CCCCCCCCCCC)[C@H](O)[C@@H](CO)O[C@H]1OP(O)(=O)OP(O)(=O)OC[C@@H]1[C@@H](O)[C@@H](O)[C@H](N2C(NC(=O)C=C2)=O)O1 TZSJGZGYQDNRRX-VCEDRBFZSA-N 0.000 claims description 3
- ZFPNNOXCEDQJQS-SSVOXRMNSA-N UDP-3-O-[(3R)-3-hydroxytetradecanoyl]-alpha-D-glucosamine Chemical compound O1[C@H](CO)[C@@H](O)[C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](N)[C@H]1OP(O)(=O)OP(O)(=O)OC[C@@H]1[C@@H](O)[C@@H](O)[C@H](N2C(NC(=O)C=C2)=O)O1 ZFPNNOXCEDQJQS-SSVOXRMNSA-N 0.000 claims description 3
- BEGZZYPUNCJHKP-DBYWSUQTSA-N UDP-N-acetyl-3-O-(1-carboxyvinyl)-alpha-D-glucosamine Chemical compound O1[C@H](CO)[C@@H](O)[C@H](OC(=C)C(O)=O)[C@@H](NC(=O)C)[C@H]1OP(O)(=O)OP(O)(=O)OC[C@@H]1[C@@H](O)[C@@H](O)[C@H](N2C(NC(=O)C=C2)=O)O1 BEGZZYPUNCJHKP-DBYWSUQTSA-N 0.000 claims description 3
- LFTYTUAZOPRMMI-CFRASDGPSA-N UDP-N-acetyl-alpha-D-glucosamine Chemical compound O1[C@H](CO)[C@@H](O)[C@H](O)[C@@H](NC(=O)C)[C@H]1OP(O)(=O)OP(O)(=O)OC[C@@H]1[C@@H](O)[C@@H](O)[C@H](N2C(NC(=O)C=C2)=O)O1 LFTYTUAZOPRMMI-CFRASDGPSA-N 0.000 claims description 3
- NQBRVZNDBBMBLJ-MQTLHLSBSA-N UDP-N-acetyl-alpha-D-muramic acid Chemical compound CC(=O)N[C@@H]1[C@@H](O[C@H](C)C(O)=O)[C@H](O)[C@@H](CO)O[C@@H]1OP(O)(=O)OP(O)(=O)OC[C@@H]1[C@@H](O)[C@@H](O)[C@H](N2C(NC(=O)C=C2)=O)O1 NQBRVZNDBBMBLJ-MQTLHLSBSA-N 0.000 claims description 3
- NTMMCWJNQNKACG-KBKUWGQMSA-N UDP-N-acetyl-alpha-D-muramoyl-L-alanine Chemical compound CC(=O)N[C@@H]1[C@@H](O[C@H](C)C(=O)N[C@@H](C)C(O)=O)[C@H](O)[C@@H](CO)O[C@@H]1OP(O)(=O)OP(O)(=O)OC[C@@H]1[C@@H](O)[C@@H](O)[C@H](N2C(NC(=O)C=C2)=O)O1 NTMMCWJNQNKACG-KBKUWGQMSA-N 0.000 claims description 3
- QUHLBZKCGUXHGP-BHBBPGSKSA-N UDP-N-acetyl-alpha-D-muramoyl-L-alanyl-D-gamma-glutamyl-meso-2,6-diaminoheptanedioic acid Chemical compound CC(=O)N[C@@H]1[C@@H](O[C@H](C)C(=O)N[C@@H](C)C(=O)N[C@H](CCC(=O)N[C@@H](CCC[C@@H](N)C(O)=O)C(O)=O)C(O)=O)[C@H](O)[C@@H](CO)O[C@@H]1OP(O)(=O)OP(O)(=O)OC[C@@H]1[C@@H](O)[C@@H](O)[C@H](N2C(NC(=O)C=C2)=O)O1 QUHLBZKCGUXHGP-BHBBPGSKSA-N 0.000 claims description 3
- OJZCATPXPWFLHF-DNMPHPEFSA-N UDP-N-acetylmuramoyl-L-alanyl-D-glutamic acid Chemical compound CC(=O)N[C@@H]1[C@@H](O[C@H](C)C(=O)N[C@@H](C)C(=O)N[C@H](CCC(O)=O)C(O)=O)[C@H](O)[C@@H](CO)OC1OP(O)(=O)OP(O)(=O)OC[C@@H]1[C@@H](O)[C@@H](O)[C@H](N2C(NC(=O)C=C2)=O)O1 OJZCATPXPWFLHF-DNMPHPEFSA-N 0.000 claims description 3
- IMWOXEZVYQDRDF-MCZXNMLPSA-N UDP-N-acetylmuramoyl-L-alanyl-gamma-D-glutamyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine Chemical compound CC(=O)N[C@@H]1[C@@H](O[C@H](C)C(=O)N[C@@H](C)C(=O)N[C@H](CCC(=O)N[C@@H](CCC[C@@H](N)C(O)=O)C(=O)N[C@H](C)C(=O)N[C@H](C)C(O)=O)C(O)=O)[C@H](O)[C@@H](CO)O[C@@H]1OP(O)(=O)OP(O)(=O)OC[C@@H]1[C@@H](O)[C@@H](O)[C@H](N2C(NC(=O)C=C2)=O)O1 IMWOXEZVYQDRDF-MCZXNMLPSA-N 0.000 claims description 3
- HSCJRCZFDFQWRP-JZMIEXBBSA-N UDP-alpha-D-glucose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OP(O)(=O)OP(O)(=O)OC[C@@H]1[C@@H](O)[C@@H](O)[C@H](N2C(NC(=O)C=C2)=O)O1 HSCJRCZFDFQWRP-JZMIEXBBSA-N 0.000 claims description 3
- LFTYTUAZOPRMMI-UHFFFAOYSA-N UNPD164450 Natural products O1C(CO)C(O)C(O)C(NC(=O)C)C1OP(O)(=O)OP(O)(=O)OCC1C(O)C(O)C(N2C(NC(=O)C=C2)=O)O1 LFTYTUAZOPRMMI-UHFFFAOYSA-N 0.000 claims description 3
- PGAVKCOVUIYSFO-XVFCMESISA-N UTP Chemical compound O[C@@H]1[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@H]1N1C(=O)NC(=O)C=C1 PGAVKCOVUIYSFO-XVFCMESISA-N 0.000 claims description 3
- XCCTYIAWTASOJW-XVFCMESISA-N Uridine-5'-Diphosphate Chemical compound O[C@@H]1[C@H](O)[C@@H](COP(O)(=O)OP(O)(O)=O)O[C@H]1N1C(=O)NC(=O)C=C1 XCCTYIAWTASOJW-XVFCMESISA-N 0.000 claims description 3
- PLROMJHFTOKDCY-GKHCUFPYSA-N [(2r,3r,4r,5s,6s)-2,3,4,5,6-pentahydroxy-7-oxoheptyl] dihydrogen phosphate Chemical compound OP(=O)(O)OC[C@@H](O)[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)C=O PLROMJHFTOKDCY-GKHCUFPYSA-N 0.000 claims description 3
- UDMBCSSLTHHNCD-KQYNXXCUSA-N adenosine 5'-monophosphate Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O UDMBCSSLTHHNCD-KQYNXXCUSA-N 0.000 claims description 3
- IRLPACMLTUPBCL-FCIPNVEPSA-N adenosine-5'-phosphosulfate Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@@H](CO[P@](O)(=O)OS(O)(=O)=O)[C@H](O)[C@H]1O IRLPACMLTUPBCL-FCIPNVEPSA-N 0.000 claims description 3
- OFBHPPMPBOJXRT-VWJPMABRSA-N adenylosuccinic acid Chemical compound O[C@@H]1[C@H](O)[C@@H](COP(O)(O)=O)O[C@H]1N1C2=NC=NC(N[C@@H](CC(O)=O)C(O)=O)=C2N=C1 OFBHPPMPBOJXRT-VWJPMABRSA-N 0.000 claims description 3
- 229960003767 alanine Drugs 0.000 claims description 3
- 108010056243 alanylalanine Proteins 0.000 claims description 3
- PPQRONHOSHZGFQ-WDCZJNDASA-N aldehydo-D-arabinose 5-phosphate Chemical compound OP(=O)(O)OC[C@@H](O)[C@@H](O)[C@H](O)C=O PPQRONHOSHZGFQ-WDCZJNDASA-N 0.000 claims description 3
- NTXGVHCCXVHYCL-RDQGWRCRSA-N all-trans-undecaprenyl diphosphate Chemical compound CC(C)=CCC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\COP(O)(=O)OP(O)(O)=O NTXGVHCCXVHYCL-RDQGWRCRSA-N 0.000 claims description 3
- YMJBYRVFGYXULK-QZABAPFNSA-N alpha-D-glucosamine 1-phosphate Chemical compound N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OP(O)(O)=O YMJBYRVFGYXULK-QZABAPFNSA-N 0.000 claims description 3
- HXXFSFRBOHSIMQ-VFUOTHLCSA-N alpha-D-glucose 1-phosphate Chemical compound OC[C@H]1O[C@H](OP(O)(O)=O)[C@H](O)[C@@H](O)[C@@H]1O HXXFSFRBOHSIMQ-VFUOTHLCSA-N 0.000 claims description 3
- KTVPXOYAKDPRHY-AIHAYLRMSA-N alpha-D-ribofuranose 5-phosphate Chemical compound O[C@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O KTVPXOYAKDPRHY-AIHAYLRMSA-N 0.000 claims description 3
- 229940000635 beta-alanine Drugs 0.000 claims description 3
- FFQKYPRQEYGKAF-UHFFFAOYSA-N carbamoyl phosphate Chemical compound NC(=O)OP(O)(O)=O FFQKYPRQEYGKAF-UHFFFAOYSA-N 0.000 claims description 3
- WTFXTQVDAKGDEY-HTQZYQBOSA-N chorismic acid Chemical compound O[C@@H]1C=CC(C(O)=O)=C[C@H]1OC(=C)C(O)=O WTFXTQVDAKGDEY-HTQZYQBOSA-N 0.000 claims description 3
- RGJOEKWQDUBAIZ-UHFFFAOYSA-N coenzime A Natural products OC1C(OP(O)(O)=O)C(COP(O)(=O)OP(O)(=O)OCC(C)(C)C(O)C(=O)NCCC(=O)NCCS)OC1N1C2=NC=NC(N)=C2N=C1 RGJOEKWQDUBAIZ-UHFFFAOYSA-N 0.000 claims description 3
- 239000005516 coenzyme A Substances 0.000 claims description 3
- 229940093530 coenzyme a Drugs 0.000 claims description 3
- 239000013317 conjugated microporous polymer Substances 0.000 claims description 3
- SUYVUBYJARFZHO-RRKCRQDMSA-N dATP Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@H]1C[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 SUYVUBYJARFZHO-RRKCRQDMSA-N 0.000 claims description 3
- SUYVUBYJARFZHO-UHFFFAOYSA-N dATP Natural products C1=NC=2C(N)=NC=NC=2N1C1CC(O)C(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 SUYVUBYJARFZHO-UHFFFAOYSA-N 0.000 claims description 3
- RGWHQCVHVJXOKC-SHYZEUOFSA-J dCTP(4-) Chemical compound O=C1N=C(N)C=CN1[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O)[C@@H](O)C1 RGWHQCVHVJXOKC-SHYZEUOFSA-J 0.000 claims description 3
- HAAZLUGHYHWQIW-KVQBGUIXSA-N dGTP Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@H]1C[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 HAAZLUGHYHWQIW-KVQBGUIXSA-N 0.000 claims description 3
- UJLXYODCHAELLY-XLPZGREQSA-N dTDP Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](COP(O)(=O)OP(O)(O)=O)[C@@H](O)C1 UJLXYODCHAELLY-XLPZGREQSA-N 0.000 claims description 3
- GYOZYWVXFNDGLU-XLPZGREQSA-N dTMP Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)C1 GYOZYWVXFNDGLU-XLPZGREQSA-N 0.000 claims description 3
- NHVNXKFIZYSCEB-XLPZGREQSA-N dTTP Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)C1 NHVNXKFIZYSCEB-XLPZGREQSA-N 0.000 claims description 3
- SENPVEZBRZQVST-HISDBWNOSA-O deamido-NAD(+) Chemical compound [N+]1([C@@H]2O[C@@H]([C@H]([C@H]2O)O)COP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@@H]([C@@H]2O)O)N2C=3N=CN=C(C=3N=C2)N)=CC=CC(C(O)=O)=C1 SENPVEZBRZQVST-HISDBWNOSA-O 0.000 claims description 3
- KDTSHFARGAKYJN-UHFFFAOYSA-N dephosphocoenzyme A Natural products OC1C(O)C(COP(O)(=O)OP(O)(=O)OCC(C)(C)C(O)C(=O)NCCC(=O)NCCS)OC1N1C2=NC=NC(N)=C2N=C1 KDTSHFARGAKYJN-UHFFFAOYSA-N 0.000 claims description 3
- OZRNSSUDZOLUSN-LBPRGKRZSA-N dihydrofolic acid Chemical compound N=1C=2C(=O)NC(N)=NC=2NCC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OZRNSSUDZOLUSN-LBPRGKRZSA-N 0.000 claims description 3
- ZGSPNIOCEDOHGS-UHFFFAOYSA-L disodium [3-[2,3-di(octadeca-9,12-dienoyloxy)propoxy-oxidophosphoryl]oxy-2-hydroxypropyl] 2,3-di(octadeca-9,12-dienoyloxy)propyl phosphate Chemical compound [Na+].[Na+].CCCCCC=CCC=CCCCCCCCC(=O)OCC(OC(=O)CCCCCCCC=CCC=CCCCCC)COP([O-])(=O)OCC(O)COP([O-])(=O)OCC(OC(=O)CCCCCCCC=CCC=CCCCCC)COC(=O)CCCCCCCC=CCC=CCCCCC ZGSPNIOCEDOHGS-UHFFFAOYSA-L 0.000 claims description 3
- UFPHFKCTOZIAFY-NTDVEAECSA-N ditrans,polycis-undecaprenyl phosphate Chemical compound CC(C)=CCC\C(C)=C\CC\C(C)=C\CC\C(C)=C/CC\C(C)=C/CC\C(C)=C/CC\C(C)=C/CC\C(C)=C/CC\C(C)=C/CC\C(C)=C/CC\C(C)=C/COP(O)(O)=O UFPHFKCTOZIAFY-NTDVEAECSA-N 0.000 claims description 3
- POULHZVOKOAJMA-UHFFFAOYSA-M dodecanoate Chemical compound CCCCCCCCCCCC([O-])=O POULHZVOKOAJMA-UHFFFAOYSA-M 0.000 claims description 3
- 239000002158 endotoxin Substances 0.000 claims description 3
- 229940096919 glycogen Drugs 0.000 claims description 3
- RQFCJASXJCIDSX-UUOKFMHZSA-N guanosine 5'-monophosphate Chemical compound C1=2NC(N)=NC(=O)C=2N=CN1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O RQFCJASXJCIDSX-UUOKFMHZSA-N 0.000 claims description 3
- 229960002885 histidine Drugs 0.000 claims description 3
- 229910000037 hydrogen sulfide Inorganic materials 0.000 claims description 3
- NMUOATVLLQEYHI-UHFFFAOYSA-N iminoaspartic acid Chemical compound OC(=O)CC(=N)C(O)=O NMUOATVLLQEYHI-UHFFFAOYSA-N 0.000 claims description 3
- XKVWLLRDBHAWBL-UHFFFAOYSA-N imperatorin Natural products CC(=CCOc1c2OCCc2cc3C=CC(=O)Oc13)C XKVWLLRDBHAWBL-UHFFFAOYSA-N 0.000 claims description 3
- 229960000310 isoleucine Drugs 0.000 claims description 3
- GSXOAOHZAIYLCY-HSUXUTPPSA-N keto-D-fructose 6-phosphate Chemical compound OCC(=O)[C@@H](O)[C@H](O)[C@H](O)COP(O)(O)=O GSXOAOHZAIYLCY-HSUXUTPPSA-N 0.000 claims description 3
- BTNMPGBKDVTSJY-UHFFFAOYSA-M keto-phenylpyruvate Chemical compound [O-]C(=O)C(=O)CC1=CC=CC=C1 BTNMPGBKDVTSJY-UHFFFAOYSA-M 0.000 claims description 3
- BTNMPGBKDVTSJY-UHFFFAOYSA-N keto-phenylpyruvic acid Chemical compound OC(=O)C(=O)CC1=CC=CC=C1 BTNMPGBKDVTSJY-UHFFFAOYSA-N 0.000 claims description 3
- 229940070765 laurate Drugs 0.000 claims description 3
- 229960003136 leucine Drugs 0.000 claims description 3
- ZNOVTXRBGFNYRX-ABLWVSNPSA-N levomefolic acid Chemical compound C1NC=2NC(N)=NC(=O)C=2N(C)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 ZNOVTXRBGFNYRX-ABLWVSNPSA-N 0.000 claims description 3
- 235000007635 levomefolic acid Nutrition 0.000 claims description 3
- 239000011578 levomefolic acid Substances 0.000 claims description 3
- KVJWZTLXIROHIL-QDORLFPLSA-N lipid IVA Chemical compound O[C@H]1[C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](OP(O)(O)=O)O[C@@H]1CO[C@H]1[C@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@H](OP(O)(O)=O)[C@@H](CO)O1 KVJWZTLXIROHIL-QDORLFPLSA-N 0.000 claims description 3
- 229920006008 lipopolysaccharide Polymers 0.000 claims description 3
- LTYOQGRJFJAKNA-DVVLENMVSA-N malonyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CC(O)=O)O[C@H]1N1C2=NC=NC(N)=C2N=C1 LTYOQGRJFJAKNA-DVVLENMVSA-N 0.000 claims description 3
- GMKMEZVLHJARHF-SYDPRGILSA-N meso-2,6-diaminopimelic acid Chemical compound [O-]C(=O)[C@@H]([NH3+])CCC[C@@H]([NH3+])C([O-])=O GMKMEZVLHJARHF-SYDPRGILSA-N 0.000 claims description 3
- 229960004452 methionine Drugs 0.000 claims description 3
- 229950006780 n-acetylglucosamine Drugs 0.000 claims description 3
- 229950006238 nadide Drugs 0.000 claims description 3
- 229930027945 nicotinamide-adenine dinucleotide Natural products 0.000 claims description 3
- JOUIQRNQJGXQDC-ZYUZMQFOSA-L nicotinate D-ribonucleotide(2-) Chemical compound O1[C@H](COP([O-])([O-])=O)[C@@H](O)[C@@H](O)[C@@H]1[N+]1=CC=CC(C([O-])=O)=C1 JOUIQRNQJGXQDC-ZYUZMQFOSA-L 0.000 claims description 3
- PXQPEWDEAKTCGB-UHFFFAOYSA-N orotic acid Chemical compound OC(=O)C1=CC(=O)NC(=O)N1 PXQPEWDEAKTCGB-UHFFFAOYSA-N 0.000 claims description 3
- KYOBSHFOBAOFBF-XVFCMESISA-N orotidine 5'-phosphate Chemical compound O[C@@H]1[C@H](O)[C@@H](COP(O)(O)=O)O[C@H]1N1C(=O)NC(=O)C=C1C(O)=O KYOBSHFOBAOFBF-XVFCMESISA-N 0.000 claims description 3
- KHPXUQMNIQBQEV-UHFFFAOYSA-N oxaloacetic acid Chemical compound OC(=O)CC(=O)C(O)=O KHPXUQMNIQBQEV-UHFFFAOYSA-N 0.000 claims description 3
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 claims description 3
- 150000008103 phosphatidic acids Chemical class 0.000 claims description 3
- 150000008104 phosphatidylethanolamines Chemical class 0.000 claims description 3
- 229930029653 phosphoenolpyruvate Natural products 0.000 claims description 3
- DTBNBXWJWCWCIK-UHFFFAOYSA-N phosphoenolpyruvic acid Chemical compound OC(=O)C(=C)OP(O)(O)=O DTBNBXWJWCWCIK-UHFFFAOYSA-N 0.000 claims description 3
- BZQFBWGGLXLEPQ-REOHCLBHSA-N phosphoserine Chemical compound OC(=O)[C@@H](N)COP(O)(O)=O BZQFBWGGLXLEPQ-REOHCLBHSA-N 0.000 claims description 3
- 229960002429 proline Drugs 0.000 claims description 3
- GJAWHXHKYYXBSV-UHFFFAOYSA-N quinolinic acid Chemical compound OC(=O)C1=CC=CN=C1C(O)=O GJAWHXHKYYXBSV-UHFFFAOYSA-N 0.000 claims description 3
- JDTUMPKOJBQPKX-GBNDHIKLSA-N sedoheptulose 7-phosphate Chemical compound OCC(=O)[C@@H](O)[C@H](O)[C@H](O)[C@H](O)COP(O)(O)=O JDTUMPKOJBQPKX-GBNDHIKLSA-N 0.000 claims description 3
- 229960001153 serine Drugs 0.000 claims description 3
- JXOHGGNKMLTUBP-HSUXUTPPSA-N shikimic acid Chemical compound O[C@@H]1CC(C(O)=O)=C[C@@H](O)[C@H]1O JXOHGGNKMLTUBP-HSUXUTPPSA-N 0.000 claims description 3
- JXOHGGNKMLTUBP-JKUQZMGJSA-N shikimic acid Natural products O[C@@H]1CC(C(O)=O)=C[C@H](O)[C@@H]1O JXOHGGNKMLTUBP-JKUQZMGJSA-N 0.000 claims description 3
- AWUCVROLDVIAJX-GSVOUGTGSA-N sn-glycerol 3-phosphate Chemical compound OC[C@@H](O)COP(O)(O)=O AWUCVROLDVIAJX-GSVOUGTGSA-N 0.000 claims description 3
- IFGCUJZIWBUILZ-UHFFFAOYSA-N sodium 2-[[2-[[hydroxy-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxyphosphoryl]amino]-4-methylpentanoyl]amino]-3-(1H-indol-3-yl)propanoic acid Chemical compound [Na+].C=1NC2=CC=CC=C2C=1CC(C(O)=O)NC(=O)C(CC(C)C)NP(O)(=O)OC1OC(C)C(O)C(O)C1O IFGCUJZIWBUILZ-UHFFFAOYSA-N 0.000 claims description 3
- 229940063673 spermidine Drugs 0.000 claims description 3
- WPLOVIFNBMNBPD-ATHMIXSHSA-N subtilin Chemical compound CC1SCC(NC2=O)C(=O)NC(CC(N)=O)C(=O)NC(C(=O)NC(CCCCN)C(=O)NC(C(C)CC)C(=O)NC(=C)C(=O)NC(CCCCN)C(O)=O)CSC(C)C2NC(=O)C(CC(C)C)NC(=O)C1NC(=O)C(CCC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C1NC(=O)C(=C/C)/NC(=O)C(CCC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)CNC(=O)C(NC(=O)C(NC(=O)C2NC(=O)CNC(=O)C3CCCN3C(=O)C(NC(=O)C3NC(=O)C(CC(C)C)NC(=O)C(=C)NC(=O)C(CCC(O)=O)NC(=O)C(NC(=O)C(CCCCN)NC(=O)C(N)CC=4C5=CC=CC=C5NC=4)CSC3)C(C)SC2)C(C)C)C(C)SC1)CC1=CC=CC=C1 WPLOVIFNBMNBPD-ATHMIXSHSA-N 0.000 claims description 3
- VNOYUJKHFWYWIR-ITIYDSSPSA-N succinyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CCC(O)=O)O[C@H]1N1C2=NC=NC(N)=C2N=C1 VNOYUJKHFWYWIR-ITIYDSSPSA-N 0.000 claims description 3
- 229960004441 tyrosine Drugs 0.000 claims description 3
- DJJCXFVJDGTHFX-XVFCMESISA-N uridine 5'-monophosphate Chemical compound O[C@@H]1[C@H](O)[C@@H](COP(O)(O)=O)O[C@H]1N1C(=O)NC(=O)C=C1 DJJCXFVJDGTHFX-XVFCMESISA-N 0.000 claims description 3
- 229960004295 valine Drugs 0.000 claims description 3
- RNQHMTFBUSSBJQ-CRCLSJGQSA-N (2R,3S)-3-isopropylmalic acid Chemical compound CC(C)[C@H](C(O)=O)[C@@H](O)C(O)=O RNQHMTFBUSSBJQ-CRCLSJGQSA-N 0.000 claims description 2
- RTQMRTSPTLIIHM-KEOHHSTQSA-N 1-(5-phospho-beta-D-ribosyl)-5'-AMP Chemical compound O[C@@H]1[C@H](O)[C@@H](COP(O)(O)=O)O[C@H]1N1C(N=CN([C@H]2[C@@H]([C@H](O)[C@@H](COP(O)(O)=O)O2)O)C2=N)=C2N=C1 RTQMRTSPTLIIHM-KEOHHSTQSA-N 0.000 claims description 2
- OIUJHGOLFKDBSU-HTQZYQBOSA-N 4-amino-4-deoxychorismic acid Chemical compound N[C@@H]1C=CC(C(O)=O)=C[C@H]1OC(=C)C(O)=O OIUJHGOLFKDBSU-HTQZYQBOSA-N 0.000 claims description 2
- RTNBXJBOAIDPME-SHUUEZRQSA-N 8-phospho-3-deoxy-D-manno-oct-2-ulosonic acid Chemical compound OP(=O)(O)OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CC(=O)C(O)=O RTNBXJBOAIDPME-SHUUEZRQSA-N 0.000 claims description 2
- WFPZSXYXPSUOPY-ROYWQJLOSA-N ADP alpha-D-glucoside Chemical compound C([C@H]1O[C@H]([C@@H]([C@@H]1O)O)N1C=2N=CN=C(C=2N=C1)N)OP(O)(=O)OP(O)(=O)O[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O WFPZSXYXPSUOPY-ROYWQJLOSA-N 0.000 claims description 2
- XQYALQVLCNHCFT-CBAPKCEASA-N N-[(R)-4-phosphopantothenoyl]-L-cysteine Chemical compound OP(=O)(O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)N[C@@H](CS)C(O)=O XQYALQVLCNHCFT-CBAPKCEASA-N 0.000 claims description 2
- 239000004473 Threonine Substances 0.000 claims description 2
- MSCRMMUDYYKUIO-JCJJBUFRSA-N [(2S,3R,4R,5R,6R)-3-amino-4,5-dihydroxy-6-(hydroxymethyl)-3,4-bis(3-hydroxytetradecanoyl)oxan-2-yl] dihydrogen phosphate Chemical compound CCCCCCCCCCCC(CC(=O)[C@@]1([C@@H]([C@H](O[C@H]([C@]1(C(=O)CC(CCCCCCCCCCC)O)N)OP(=O)(O)O)CO)O)O)O MSCRMMUDYYKUIO-JCJJBUFRSA-N 0.000 claims description 2
- 229960001230 asparagine Drugs 0.000 claims description 2
- 229960002898 threonine Drugs 0.000 claims description 2
- 230000001131 transforming effect Effects 0.000 claims description 2
- PNWZQTONLRRPST-KLDRQJOASA-N undecaprenyldiphospho-N-acetylmuramoyl-L-alanyl-D-gamma-glutamyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine Chemical compound OC(=O)[C@@H](C)NC(=O)[C@@H](C)NC(=O)[C@H](CCC[C@@H](N)C(O)=O)NC(=O)CC[C@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](C)O[C@H]1[C@H](O)[C@@H](CO)O[C@H](OP(O)(=O)OP(O)(=O)OC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(/C)CC\C=C(/C)CCC=C(C)C)[C@@H]1NC(C)=O PNWZQTONLRRPST-KLDRQJOASA-N 0.000 claims description 2
- BEBCJVAWIBVWNZ-UHFFFAOYSA-N glycinamide Chemical compound NCC(N)=O BEBCJVAWIBVWNZ-UHFFFAOYSA-N 0.000 claims 1
- 230000009036 growth inhibition Effects 0.000 claims 1
- 238000006243 chemical reaction Methods 0.000 abstract description 9
- 239000003596 drug target Substances 0.000 abstract description 4
- 238000012269 metabolic engineering Methods 0.000 abstract 1
- 210000004027 cell Anatomy 0.000 description 39
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 26
- 239000001301 oxygen Substances 0.000 description 26
- 229910052760 oxygen Inorganic materials 0.000 description 26
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 22
- 239000008103 glucose Substances 0.000 description 22
- 230000000694 effects Effects 0.000 description 11
- 239000013598 vector Substances 0.000 description 8
- 230000008859 change Effects 0.000 description 7
- 238000012217 deletion Methods 0.000 description 7
- 230000037430 deletion Effects 0.000 description 7
- 229940079593 drug Drugs 0.000 description 6
- 239000003814 drug Substances 0.000 description 6
- 108020004414 DNA Proteins 0.000 description 5
- 230000037353 metabolic pathway Effects 0.000 description 5
- 241000196324 Embryophyta Species 0.000 description 4
- 229930091371 Fructose Natural products 0.000 description 4
- 239000005715 Fructose Substances 0.000 description 4
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 4
- 239000013600 plasmid vector Substances 0.000 description 4
- 241000894006 Bacteria Species 0.000 description 3
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 238000012224 gene deletion Methods 0.000 description 3
- 230000002779 inactivation Effects 0.000 description 3
- 231100000225 lethality Toxicity 0.000 description 3
- 239000013612 plasmid Substances 0.000 description 3
- PDGXJDXVGMHUIR-UJURSFKZSA-N (2R,3R)-2,3-dihydroxy-3-methylpentanoic acid Chemical compound CC[C@@](C)(O)[C@@H](O)C(O)=O PDGXJDXVGMHUIR-UJURSFKZSA-N 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 2
- 241000589158 Agrobacterium Species 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- 239000004475 Arginine Substances 0.000 description 2
- 241001646716 Escherichia coli K-12 Species 0.000 description 2
- 229930064664 L-arginine Natural products 0.000 description 2
- 235000014852 L-arginine Nutrition 0.000 description 2
- 241000589516 Pseudomonas Species 0.000 description 2
- XRHZRKQNEFPRSM-HJZCUYRDSA-N [2-[(3r,4s,5r)-3,4-dihydroxy-5-(phosphonooxymethyl)oxolan-2-yl]-1h-imidazol-5-yl]carbamic acid Chemical compound O[C@@H]1[C@H](O)[C@@H](COP(O)(O)=O)OC1C1=NC=C(NC(O)=O)N1 XRHZRKQNEFPRSM-HJZCUYRDSA-N 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 2
- 235000009697 arginine Nutrition 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 238000005842 biochemical reaction Methods 0.000 description 2
- 230000003115 biocidal effect Effects 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 210000003850 cellular structure Anatomy 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 230000007812 deficiency Effects 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 230000006866 deterioration Effects 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 229930195712 glutamate Natural products 0.000 description 2
- OBQMLSFOUZUIOB-SHUUEZRQSA-N glycineamide ribonucleotide Chemical compound NCC(=O)N[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O OBQMLSFOUZUIOB-SHUUEZRQSA-N 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 101150003321 lpdA gene Proteins 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 238000005457 optimization Methods 0.000 description 2
- 101150112726 purN gene Proteins 0.000 description 2
- 230000010076 replication Effects 0.000 description 2
- 108091008146 restriction endonucleases Proteins 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- QDSWNDMHSBZXKX-JTQLQIEISA-N (r)-3-bromo-2-hydroxy-2-methyl-n-[4-nitro-3-(trifluoromethyl)phenyl]propanamide Chemical compound BrC[C@@](O)(C)C(=O)NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 QDSWNDMHSBZXKX-JTQLQIEISA-N 0.000 description 1
- KYQCXUMVJGMDNG-UHFFFAOYSA-N 4,5,6,7,8-pentahydroxy-2-oxooctanoic acid Chemical compound OCC(O)C(O)C(O)C(O)CC(=O)C(O)=O KYQCXUMVJGMDNG-UHFFFAOYSA-N 0.000 description 1
- 244000063299 Bacillus subtilis Species 0.000 description 1
- 235000014469 Bacillus subtilis Nutrition 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 241001522878 Escherichia coli B Species 0.000 description 1
- 241000620209 Escherichia coli DH5[alpha] Species 0.000 description 1
- 241001302584 Escherichia coli str. K-12 substr. W3110 Species 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 108091028043 Nucleic acid sequence Proteins 0.000 description 1
- HEDRRTIJEFSOHK-RFKZQXLXSA-N O=C[C@H](O)[C@H](O)CO.P(O)(O)(O)=O Chemical compound O=C[C@H](O)[C@H](O)CO.P(O)(O)(O)=O HEDRRTIJEFSOHK-RFKZQXLXSA-N 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 241000293869 Salmonella enterica subsp. enterica serovar Typhimurium Species 0.000 description 1
- 241000607720 Serratia Species 0.000 description 1
- 101100157012 Thermoanaerobacterium saccharolyticum (strain DSM 8691 / JW/SL-YS485) xynB gene Proteins 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 125000000218 acetic acid group Chemical group C(C)(=O)* 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000001775 anti-pathogenic effect Effects 0.000 description 1
- 244000052616 bacterial pathogen Species 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 238000009395 breeding Methods 0.000 description 1
- 230000001488 breeding effect Effects 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000008614 cellular interaction Effects 0.000 description 1
- 230000007248 cellular mechanism Effects 0.000 description 1
- 229960005091 chloramphenicol Drugs 0.000 description 1
- WIIZWVCIJKGZOK-RKDXNWHRSA-N chloramphenicol Chemical compound ClC(Cl)C(=O)N[C@H](CO)[C@H](O)C1=CC=C([N+]([O-])=O)C=C1 WIIZWVCIJKGZOK-RKDXNWHRSA-N 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000002900 effect on cell Effects 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 101150097303 glyA gene Proteins 0.000 description 1
- 101150079604 glyA1 gene Proteins 0.000 description 1
- 230000006801 homologous recombination Effects 0.000 description 1
- 238000002744 homologous recombination Methods 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 238000000126 in silico method Methods 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 229930027917 kanamycin Natural products 0.000 description 1
- 229960000318 kanamycin Drugs 0.000 description 1
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 1
- 229930182823 kanamycin A Natural products 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 238000013178 mathematical model Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 239000002773 nucleotide Substances 0.000 description 1
- 125000003729 nucleotide group Chemical group 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 230000007918 pathogenicity Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 210000001236 prokaryotic cell Anatomy 0.000 description 1
- 235000018102 proteins Nutrition 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 238000010187 selection method Methods 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 239000005460 tetrahydrofolate Substances 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 101150110790 xylB gene Proteins 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/02—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving viable microorganisms
- C12Q1/025—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving viable microorganisms for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/02—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving viable microorganisms
- C12Q1/18—Testing for antimicrobial activity of a material
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
- G01N33/5008—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics
- G01N33/502—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics for testing non-proliferative effects
- G01N33/5038—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics for testing non-proliferative effects involving detection of metabolites per se
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Analytical Chemistry (AREA)
- Microbiology (AREA)
- Physics & Mathematics (AREA)
- Toxicology (AREA)
- Biotechnology (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Biophysics (AREA)
- General Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Urology & Nephrology (AREA)
- Medicinal Chemistry (AREA)
- Cell Biology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Food Science & Technology (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Abstract
Description
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 배지조건 | glucose 최소량, 유산소 | glucose 최소량, 무산소 | fructose 최소량, 유산소 | fructose 최소량, 무산소 | acetate 최소량, 유산소 | succinate 최소량, 유산소 | pyruvate 최소량, 유산소 | arginine 최소량, 유산소 | triptophan 최소량, 유산소 | glutamate 최소량 유산소 |
| 배지조성 | glucose:10 산소: 20 | glucose: 10 산소: 0 | fructose: 10 산소: 20 | fructose: 10 산소: 0 | acetate: 10 산소: 20 | succinate: 10 산소: 20 | pyruvate: 10 산소:20 | arginine: 10 산소:20 | triptophan: 10 산소:20 | glutamate: 10 산소:20 |
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |
| 배지조건 | glucose 최소량, C 제한 | glucose 최소량, 무산소, C 제한 | glucose 최소량, P 제한 | glucose 최소량, 무산소, P 제한 | glucose 최소량, N 제한 | glucose 최소량, 무산소, N 제한 | glucose 최소량, S 제한 | glucose 최소량, 무산소, S 제한 | glucose 최소량, 산소제한 |
| 배지조성 | glucose: 6 산소: 20 | glucose: 6 산소: 0 | glucose: 10 산소: 20 PI: 0.5 | glucose: 10 산소: 0 PI: 0.12 | glucose: 10 산소: 20 NH4: 5.91 | glucose: 10 산소: 0 NH4: 1.38 | glucose: 10 산소: 20 SO4: 0.13 | glucose: 10 산소: 0 SO4: 0.03 | glucose: 10 산소: 0.6 |
| 필수 대사산물 리스트 (essential metabolite) : 총 231개 |
| H+, H2O, ATP, Phosphate, ADP, Nicotinamide adenine dinucleotide, Nicotinamide adenine dinucleotide-reduced, CO2, Nicotinamide adenine dinucleotide phosphate, Pyruvate, Nicotinamide adenine dinucleotide phosphate - reduced, L-Glutamate, Coenzyme A, ammonium, AMP, Acetyl-CoA, 2-Oxoglutarate, acyl carrier protein, Phosphoenolpyruvate, L-Aspartate, L-Glutamine, Glyceraldehyde 3-phosphate, CMP, Glycerol 3-phosphate, GTP, 5-Phospho-alpha-D-ribose 1-diphosphate, Dihydroxyacetone phosphate, L-Alanine, L-Serine, D-Fructose 6-phosphate, Malonyl-[acyl-carrier protein], D-Glucose 1-phosphate, GDP, Oxaloacetate, Reduced thioredoxin, FAD, D-Glucose 6-phosphate, Oxidized thioredoxin, UMP, CTP, S-Adenosyl-L-methionine, L-Cysteine, alpha-D-Ribose 5-phosphate, UDPglucose, 5,6,7,8-Tetrahydrofolate, Acetoacetyl-ACP, UDP, UTP, Succinyl-CoA, L-Threonine, Putrescine, Glycine, GMP, Spermidine, IMP, Phosphatidylglycerol, L-Arginine, L-Lysine, 5,10-Methylenetetrahydrofolate, Chorismate, D-Alanine, L-Proline, L-Asparagine, UDP-N-acetyl-D-glucosamine, D-Glucosamine 6-phosphate, Nicotinate D-ribonucleotide, dGTP, Iminoaspartate, D-Ribulose 5-phosphate, Myristoyl-ACP (n-C14:0ACP), 3-Methyl-2-oxobutanoate, L-Methionine, L-Tryptophan, dTMP, phosphatidate , L-Valine, Bicarbonate, dCTP, dUMP, L-Glutamate 5-semialdehyde, meso-2,6-Diaminoheptanedioate, CMP-3-deoxy-D-manno-octulosonate, Undecaprenyl diphosphate, L-Isoleucine, Phosphatidylethanolamine, L-Leucine, L-Histidine, Hexadecenoyl-ACP (n-C16:1ACP), 1-Pyrroline-5-carboxylate, Sulfite, Carbamoyl phosphate, D-Erythrose 4-phosphate, 3-Phospho-D-glycerate, CDPdiacylglycerol, Xanthosine 5'-phosphate, dTTP, L-Tyrosine, L-Homocysteine, dATP, 10-Formyltetrahydrofolate, R-3-hydroxy-myristoyl-ACP, Octadecenoyl-ACP (n-C18:1ACP), Palmitoyl-ACP (n-C16:0ACP), (S)-Dihydroorotate, Tetradecenoyl-ACP (n-C14:1ACP), 2-Oxobutanoate, 5-Amino-1-(5-Phospho-D-ribosyl)imidazole-4-carboxamide, L-Phenylalanine, phosphatidylserine, UDP-2,3-bis(3-hydroxytetradecanoyl)glucosamine, L-Homoserine, Undecaprenyl phosphate, (R)-Pantothenate, Orotate, L-Aspartate 4-semialdehyde, Sedoheptulose 7-phosphate, N2-Formyl-N1-(5-phospho-D-ribosyl)glycinamide, 5-Methyltetrahydrofolate, Hydrogen sulfide, 7,8-Dihydrofolate, dTDP, glycogen, Dodecanoyl-ACP (n-C12:0ACP), 4-(1-D-Ribitylamino)-5-aminouracil, Prephenate, 5-amino-1-(5-phospho-D-ribosyl)imidazole, KDO(2)-lipid IV(A), N1-(5-Phospho-D-ribosyl)glycinamide, 5-[(5-phospho-1-deoxyribulos-1-ylamino)methylideneamino]-1-(5-phosphoribosyl)imidazole-4-carboxamide, Phosphatidylglycerophosphate, UDP-N-acetylmuramoyl-L-alanine, N-Succinyl-LL-2,6-diaminoheptanedioate, 2-Dehydro-3-deoxy-D-arabino-heptonate 7-phosphate, O-Phospho-L-serine, UDP-N-acetylmuramate, (S)-2-[5-Amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamido]succinate, UDP-N-acetylmuramoyl-L-alanyl-D-gamma-glutamyl-meso-2,6-diaminopimelate, 3-Carboxy-3-hydroxy-4-methylpentanoate, 2,3-Bis(3-hydroxytetradecanoyl)-beta-D-glucosaminyl 1-phosphate, 3-Deoxy-D-manno-octulosonate 8-phosphate, N-((R)-4-Phosphopantothenoyl)-L-cysteine, Peptidoglycan subunit of Escherichia coli, Adenosine 5'-phosphosulfate, Dihydropteroate, 3-Dehydroshikimate, 1-(5-Phosphoribosyl)-AMP, 4-Methyl-2-oxopentanoate, D-Glycero-D-manno-heptose 7-phosphate, 2,3,4,5-Tetrahydrodipicolinate, 3-Phosphohydroxypyruvate, D-Glycero-D-manno-heptose 1-phosphate, D-erythro-1-(Imidazol-4-yl)glycerol 3-phosphate, Sulfate, UDP-3-O-(3-hydroxytetradecanoyl)-N-acetylglucosamine, 2-Amino-4-hydroxy-6-(D-erythro-1,2,3-trihydroxypropyl)-7,8-dihydropteridine, UDP-3-O-(3-hydroxytetradecanoyl)-D-glucosamine, Orotidine 5'-phosphate, UDP-N-acetyl-3-O-(1-carboxyvinyl)-D-glucosamine, 6-hydroxymethyl dihydropterin, O-Acetyl-L-serine, |
| UDP-N-acetylmuramoyl-L-alanyl-D-glutamyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine, UDP-N-acetylmuramoyl-L-alanyl-D-glutamate, Cardiolipin, (R)-2,3-Dihydroxy-3-methylbutanoate, (S)-2-Acetolactate, 3'-Phosphoadenylyl sulfate, 1-(5-Phosphoribosyl)-ATP, 2,3,2'3'-Tetrakis(beta-hydroxymyristoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate, Shikimate 5-phosphate, (R)-Pantoate, 2-Dehydropantoate, CDPethanolamine, 5-amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxylate, 5-Phospho-beta-D-ribosylamine, N-Succinyl-2-L-amino-6-oxoheptanedioate, D-4'-Phosphopantothenate, 3-Deoxy-D-manno-2-octulosonate, ADP-L-glycero-D-manno-heptose, beta-Alanine, D-Alanyl-D-alanine, O-Succinyl-L-homoserine, Quinolinate, 2-(Formamido)-N1-(5-phospho-D-ribosyl)acetamidine, D-Arabinose 5-phosphate, 1-(5-Phosphoribosyl)-5-[(5-phosphoribosylamino)methylideneamino]imidazole-4-carboxamide, lipopolysaccharide, 6-hydroxymethyl-dihydropterin pyrophosphate, Shikimate, Undecaprenyl-diphospho-N-acetylmuramoyl-L-alanyl-D-glutamyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine, Undecaprenyl-diphospho-N-acetylmuramoyl-(N-acetylglucosamine)-L-ala-D-glu-meso-2,6-diaminopimeloyl-D-ala-D-ala, 5-phosphoribosyl-5-carboxyaminoimidazole, 3-Carboxy-2-hydroxy-4-methylpentanoate, N-Acetyl-D-glucosamine 1-phosphate, L-Cystathionine, (S)-3-Methyl-2-oxopentanoate, 5-O-(1-Carboxyvinyl)-3-phosphoshikimate, 2-Amino-4-hydroxy-6-(erythro-1,2,3-trihydroxypropyl)dihydropteridine triphosphate, D-Glycero-D-manno-heptose 1,7-bisphosphate, 3-(Imidazol-4-yl)-2-oxopropyl phosphate, 3,4-dihydroxy-2-butanone 4-phosphate, KDO(2)-lipid IV(A) with laurate, 2-Isopropylmaleate, KDO-lipid IV(A), ADP-D-glycero-D-manno-heptose, KDO(2)-lipid (A), N6-(1,2-Dicarboxyethyl)-AMP, 5-Formamido-1-(5-phospho-D-ribosyl)imidazole-4-carboxamide, Lipid A Disaccharide, 2,3-Dihydrodipicolinate, 3-Dehydroquinate, 4-Phospho-L-aspartate, S-Adenosylmethioninamine, 3-Carboxy-4-methyl-2-oxopentanoate, Phenylpyruvate, D-Glucosamine 1-phosphate, Dephospho-CoA, ADPglucose, 4-Aminobenzoate, L-Histidinol phosphate, LL-2,6-Diaminoheptanedioate, Dihydroneopterin monophosphate, Pantetheine 4'-phosphate, N-Carbamoyl-L-aspartate, (R)-2,3-Dihydroxy-3-methylpentanoate, Malonyl-CoA, 4-amino-4-deoxychorismate, D-Glutamate, 3-(4-Hydroxyphenyl)pyruvate, L-Histidinol, Deamino-NAD+, (S)-2-Aceto-2-hydroxybutanoate |
| Mutant | ΔpurN | ΔlpdA | ΔglyA | ΔpurNΔlpdA | ΔpurNΔlpdAΔglyA |
| growth rate(μ) | 0.292h-1 | 0.228h-1 | 0.188h-1 | 0.102h-1 | no growth |
Claims (16)
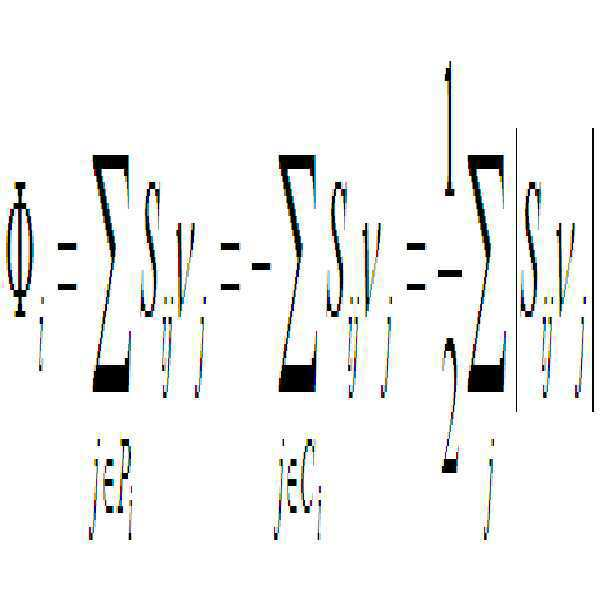
- 다음 단계를 포함하는 대사흐름분석을 이용한 미생물 성장에 필수적인 대사산물의 스크리닝 방법:(a) 목적하는 대상 미생물을 선정하고, 선정된 미생물의 대사회로 모델을 구축하는 단계;(b) 상기 구축된 대사회로 모델에서, 각 대사산물의 소비 반응식의 대사흐름값을 0으로 고정하고, 계산함으로써, 상기 대사산물의 소비 반응식을 불활성화 시켜 대사흐름을 분석하고, 다음 수학식 2를 이용하여, 원래의 세포성장속도에 비해 50%이하로 감소하는 경우의 대사산물을 미생물 성장에 필수적인 대사산물로 선정하는 단계;[수학식 2]여기서, rwild : 원래의 세포 성장속도, rloss : 해당 소비 반응식을 불활성화시켰을 때에 감소하는 세포 성장속도임(c) 상기 (b) 단계에서 선정된 대사산물의 활용도를 수학식 3으로 표현되는 플럭스 섬(flux sum: Φ)으로 정의하고 각 대사산물의 Φ 값을 계산하는 단계; 및[수학식 3]여기서, Φi: i번째 대사물질의 플럭스 섬(Φ), Sij : i번째 대사물질과 j번째 대사반응의 화학양론 계수, νj : j번째 대사반응의 대사흐름, Pi : i번째 대사물질을 생산하는 대사반응들의 집합, Ci : i번째 대사물질을 소비하는 대사반응들의 집합임(d) 각 대사산물의 Φ 값을 감소시킬 경우, 세포 성장이 감소 또는 정지하는 경우의 대사산물을 미생물 성장에 필수적인 대사산물로 확인하는 단계.
- 삭제
- 삭제
- 제1항에 있어서, 상기 미생물의 성장에 필요한 필수 대사산물은 H+, H2O, ATP, Phosphate, ADP, Nicotinamide adenine dinucleotide, Nicotinamide adenine dinucleotide-reduced, CO2, Nicotinamide adenine dinucleotide phosphate, Pyruvate, Nicotinamide adenine dinucleotide phosphate-reduced, L-Glutamate, Coenzyme A, ammonium, AMP, Acetyl-CoA, 2-Oxoglutarate, acyl carrier protein, Phosphoenolpyruvate, L-Aspartate, L-Glutamine, Glyceraldehyde 3-phosphate, CMP, Glycerol 3-phosphate, GTP, 5-Phospho-alpha-D-ribose 1-diphosphate, Dihydroxyacetone phosphate, L-Alanine, L-Serine, D-Fructose 6-phosphate, Malonyl-[acyl-carrier protein], D-Glucose 1-phosphate, GDP, Oxaloacetate, Reduced thioredoxin, FAD, D-Glucose 6-phosphate, Oxidized thioredoxin, UMP, CTP, S-Adenosyl-L-methionine, L-Cysteine, alpha-D-Ribose 5-phosphate, UDPglucose, 5,6,7,8-Tetrahydrofolate, Acetoacetyl-ACP, UDP, UTP, Succinyl-CoA, L-Threonine, Putrescine, Glycine, GMP, Spermidine, IMP, Phosphatidylglycerol, L-Arginine, L-Lysine, 5,10-Methylenetetrahydrofolate, Chorismate, D-Alanine, L-Proline, L-Asparagine, UDP-N-acetyl-D-glucosamine, D-Glucosamine 6-phosphate, Nicotinate D-ribonucleotide, dGTP, Iminoaspartate, D-Ribulose 5-phosphate, Myristoyl-ACP (n-C14:0ACP), 3-Methyl-2-oxobutanoate, L-Methionine, L-Tryptophan, dTMP, phosphatidate, L-Valine, Bicarbonate, dCTP, dUMP, L-Glutamate 5-semialdehyde, meso-2,6-Diaminoheptanedioate, CMP-3-deoxy-D-manno-octulosonate, Undecaprenyl diphosphate, L-Isoleucine, Phosphatidylethanolamine, L-Leucine, L-Histidine, Hexadecenoyl-ACP(n-C16:1ACP), 1-Pyrroline-5-carboxylate, Sulfite, Carbamoyl phosphate, D-Erythrose 4-phosphate, 3-Phospho-D-glycerate, CDPdiacylglycerol, Xanthosine 5'-phosphate, dTTP, L-Tyrosine, L-Homocysteine, dATP, 10-Formyltetrahydrofolate, R-3-hydroxy-myristoyl-ACP, Octadecenoyl-ACP (n-C18:1ACP), Palmitoyl-ACP (n-C16:0ACP), (S)-Dihydroorotate, Tetradecenoyl-ACP (n-C14:1ACP), 2-Oxobutanoate, 5-Amino-1-(5-Phospho-D-ribosyl)imidazole-4-carboxamide, L-Phenylalanine, phosphatidylserine, UDP-2,3-bis(3-hydroxytetradecanoyl)glucosamine, L-Homoserine, Undecaprenyl phosphate, (R)-Pantothenate, Orotate, L-Aspartate 4-semialdehyde, Sedoheptulose 7-phosphate, N2-Formyl-N1-(5-phospho-D-ribosyl)glycinamide, 5-Methyltetrahydrofolate, Hydrogen sulfide, 7,8-Dihydrofolate, dTDP, glycogen, Dodecanoyl-ACP(n-C12:0ACP), 4-(1-D-Ribitylamino)-5-aminouracil, Prephenate, 5-amino-1-(5-phospho-D-ribosyl)imidazole, KDO(2)-lipid IV(A), N1-(5-Phospho-D-ribosyl)glycinamide, 5-[(5-phospho-1-deoxyribulos-1-ylamino)methylideneamino]-1-(5-phosphoribosyl)imidazole-4-carboxamide, Phosphatidylglycerophosphate, UDP-N-acetylmuramoyl-L-alanine, N-Succinyl-LL-2,6-diaminoheptanedioate, 2-Dehydro-3-deoxy-D-arabino-heptonate 7-phosphate, O-Phospho-L-serine, UDP-N-acetylmuramate, (S)-2-[5-Amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamido]succinate, UDP-N-acetylmuramoyl-L-alanyl-D-gamma-glutamyl-meso-2,6-diaminopimelate, 3-Carboxy-3-hydroxy-4-methylpentanoate, 2,3-Bis(3-hydroxytetradecanoyl)-beta-D-glucosaminyl 1-phosphate, 3-Deoxy-D-manno-octulosonate 8-phosphate, N-((R)-4-Phosphopantothenoyl)-L-cysteine, Peptidoglycan subunit of Escherichia coli, Adenosine 5'-phosphosulfate, Dihydropteroate, 3-Dehydroshikimate, 1-(5-Phosphoribosyl)-AMP, 4-Methyl-2-oxopentanoate, D-Glycero-D-manno-heptose 7-phosphate, 2,3,4,5-Tetrahydrodipicolinate, 3-Phosphohydroxypyruvate, D-Glycero-D-manno-heptose 1-phosphate, D-erythro-1-(Imidazol-4-yl)glycerol 3-phosphate, Sulfate, UDP-3-O-(3-hydroxytetradecanoyl)-N-acetylglucosamine, 2-Amino-4-hydroxy-6-(D-erythro-1,2,3-trihydroxypropyl)-7,8-dihydropteridine, UDP-3-O-(3-hydroxytetradecanoyl)-D-glucosamine, Orotidine 5'-phosphate, UDP-N-acetyl-3-O-(1-carboxyvinyl)-D-glucosamine, 6-hydroxymethyl dihydropterin, O-Acetyl-L-serine, UDP-N-acetylmuramoyl-L-alanyl-D-glutamyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine, UDP-N-acetylmuramoyl-L-alanyl-D-glutamate, Cardiolipin, (R)-2,3-Dihydroxy-3-methylbutanoate, (S)-2-Acetolactate, 3'-Phosphoadenylyl sulfate, 1-(5-Phosphoribosyl)-ATP, 2,3,2'3'-Tetrakis(beta-hydroxymyristoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate, Shikimate 5-phosphate, (R)-Pantoate, 2-Dehydropantoate, CDPethanolamine, 5-amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxylate, 5-Phospho-beta-D-ribosylamine, N-Succinyl-2-L-amino-6-oxoheptanedioate, D-4'-Phosphopantothenate, 3-Deoxy-D-manno-2-octulosonate, ADP-L-glycero-D-manno-heptose, beta-Alanine, D-Alanyl-D-alanine, O-Succinyl-L-homoserine, Quinolinate, 2-(Formamido)-N1-(5-phospho-D-ribosyl)acetamidine, D-Arabinose 5-phosphate, 1-(5-Phosphoribosyl)-5-[(5-phosphoribosylamino)methylideneamino]imidazole-4-carboxamide, lipopolysaccharide, 6-hydroxymethyl-dihydropterin pyrophosphate, Shikimate, Undecaprenyl-diphospho-N-acetylmuramoyl-L-alanyl-D-glutamyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine, Undecaprenyl-diphospho-N-acetylmuramoyl-(N-acetylglucosamine)-L-ala-D-glu-meso-2,6-diaminopimeloyl-D-ala-D-ala, 5-phosphoribosyl-5-carboxyaminoimidazole, 3-Carboxy-2-hydroxy-4-methylpentanoate, N-Acetyl-D-glucosamine 1-phosphate, L-Cystathionine, (S)-3-Methyl-2-oxopentanoate, 5-O-(1-Carboxyvinyl)-3-phosphoshikimate, 2-Amino-4-hydroxy-6-(erythro-1,2,3-trihydroxypropyl)dihydropteridine triphosphate, D-Glycero-D-manno-heptose 1,7-bisphosphate, 3-(Imidazol-4-yl)-2-oxopropyl phosphate, 3,4-dihydroxy-2-butanone 4-phosphate, KDO(2)-lipid IV(A) with laurate, 2-Isopropylmaleate, KDO-lipid IV(A), ADP-D-glycero-D-manno-heptose, KDO(2)-lipid (A), N6-(1,2-Dicarboxyethyl)-AMP, 5-Formamido-1-(5-phospho-D-ribosyl)imidazole-4-carboxamide, Lipid A Disaccharide, 2,3-Dihydrodipicolinate, 3-Dehydroquinate, 4-Phospho-L-aspartate, S-Adenosylmethioninamine, 3-Carboxy-4-methyl-2-oxopentanoate, Phenylpyruvate, D-Glucosamine 1-phosphate, Dephospho-CoA, ADPglucose, 4-Aminobenzoate, L-Histidinol phosphate, LL-2,6-Diaminoheptanedioate, Dihydroneopterin monophosphate, Pantetheine 4'-phosphate, N-Carbamoyl-L-aspartate, (R)-2,3-Dihydroxy-3-methylpentanoate, Malonyl-CoA, 4-amino-4-deoxychorismate, D-Glutamate, 3-(4-Hydroxyphenyl)pyruvate, L-Histidinol, Deamino-NAD+ 및 (S)-2-Aceto-2-hydroxybutanoate 로 구성된 군에서 선택되는 것을 특징으로 하는 방법.
- 제1항에 있어서, 플럭스 섬(Φ)의 감소에 따른 세포 성장의 감소 유형은 선형적으로 감소하는 유형, 임계치 이하에서 감소하는 유형 및 비선형적으로 감소해 플럭스 섬(Φ)이 0에 도달하기 전에 세포 성장이 정지하는 유형으로 구성된 군에서 선택되는 것을 특징으로 하는 방법.
- 제5항에 있어서, 상기 세포 성장의 감소 유형에 따라 플럭스 섬(Φ)의 섭동량을 다르게 하는 것을 특징으로 하는 방법.
- 제1항에 있어서, 상기 대상 미생물은 대장균인 것을 특징으로 하는 방법.
- 제1항에 있어서 상기 대상미생물은 병원성 미생물인 것을 특징으로 하는 방법.
- 제1항의 방법에 의해 스크리닝된 미생물 성장에 필수적인 대사산물에 관여하는 유전자를 선별하는 것을 특징으로 하는 미생물의 성장에 필수적인 유전자의 스크리닝 방법.
- 제9항에 있어서, 상기 대상미생물은 대장균인 것을 특징으로 하는 방법.
- 제9항에 있어서, 상기 대상미생물은 병원성 미생물인 것을 특징으로 하는 방법.
- 제10항의 방법에 의해 스크리닝된 대장균 성장에 필수적인 유전자로 모델 미생물을 형질전환하는 것을 특징으로 하는, 형질전환된 미생물의 제조방법.
- 제11항의 방법에 의해 스크리닝된 병원성 미생물 성장에 필수적인 유전자로 모델 미생물을 형질전환하는 것을 특징으로 하는, 형질전환된 미생물의 제조방법.
- 제12항의 방법에 의해 제조되고, 대장균의 성장에 필수적인 유전자로 형질전환된 미생물.
- 제13항의 방법에 의해 제조되고, 병원성 미생물의 성장에 필수적인 유전자로 형질전환된 미생물.
- 다음 단계를 포함하는 병원성 미생물의 성장을 억제하는 물질의 스크리닝 방법:(a) 병원성 미생물의 성장 억제 후보물질의 존재하에 제15항의 형질전환 미생물을 배양하는 단계; 및(b) 상기 성장 억제 후보물질 없이 배양하는 경우와 비교하여, 상기 형질전환 미생물의 성장이 억제되는 경우의 후보물질을 병원성 미생물의 성장을 억제하는 물질로 선정하는 단계.
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020060133119A KR100818202B1 (ko) | 2006-12-22 | 2006-12-22 | 미생물의 성장에 필수적인 대사산물의 스크리닝 방법 |
| EP07851674A EP2097743A1 (en) | 2006-12-22 | 2007-12-21 | Method for screening essential metabolites in growth of microorganisms |
| PCT/KR2007/006708 WO2008078911A1 (en) | 2006-12-22 | 2007-12-21 | Method for screening essential metabolites in growth of microorganisms |
| US12/519,791 US8494779B2 (en) | 2006-12-22 | 2007-12-21 | Method for screening essential metabolites in growth of microorganisms |
| CNA2007800476444A CN101611312A (zh) | 2006-12-22 | 2007-12-21 | 筛选微生物生长中必需代谢物的方法 |
| JP2009542652A JP2010512783A (ja) | 2006-12-22 | 2007-12-21 | 微生物の成長に必須の代謝産物をスクリーニングする方法 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020060133119A KR100818202B1 (ko) | 2006-12-22 | 2006-12-22 | 미생물의 성장에 필수적인 대사산물의 스크리닝 방법 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR100818202B1 true KR100818202B1 (ko) | 2008-03-31 |
Family
ID=39412173
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020060133119A Expired - Fee Related KR100818202B1 (ko) | 2006-12-22 | 2006-12-22 | 미생물의 성장에 필수적인 대사산물의 스크리닝 방법 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US8494779B2 (ko) |
| EP (1) | EP2097743A1 (ko) |
| JP (1) | JP2010512783A (ko) |
| KR (1) | KR100818202B1 (ko) |
| CN (1) | CN101611312A (ko) |
| WO (1) | WO2008078911A1 (ko) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101243378B1 (ko) | 2009-09-18 | 2013-03-25 | 한국과학기술원 | 필수 대사산물을 이용한 병원성 미생물의 약물 표적 예측 및 약물 탐색 방법 |
| WO2019168300A1 (ko) * | 2018-02-28 | 2019-09-06 | 고려대학교 산학협력단 | 합성가스 발효 균의 합성가스 발효 시의 대사체 분석을 위한 대사체 샘플링 및 분석 방법 |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR3029936B1 (fr) | 2014-12-15 | 2020-01-24 | Biomerieux | Procede et dispositif de caracterisation du pouvoir inhibiteur d'une molecule sur un microorganisme |
| CN107102090B (zh) * | 2017-06-09 | 2019-08-09 | 云南中烟工业有限责任公司 | 一种判别烟用香料变质的方法 |
| US11508459B2 (en) * | 2018-01-31 | 2022-11-22 | X Development Llc | Modified FBA in a production network |
| CN108624638B (zh) * | 2018-08-24 | 2021-03-30 | 湖南汇升生物科技有限公司 | 一种发酵生产氨基葡萄糖的方法 |
| CN111979140B (zh) * | 2020-07-10 | 2021-12-21 | 山东瑞泽检测评价技术服务有限公司 | 用于水体污染物监测的明亮发光杆菌生长促进剂及其应用 |
| CN116969799A (zh) * | 2023-07-12 | 2023-10-31 | 宁夏农林科学院园艺研究所(宁夏设施农业工程技术研究中心) | 一种西瓜、甜瓜专用蚯蚓粪肥及其制备方法 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020168654A1 (en) * | 2001-01-10 | 2002-11-14 | Maranas Costas D. | Method and system for modeling cellular metabolism |
| JP2005058226A (ja) | 2003-07-29 | 2005-03-10 | Ajinomoto Co Inc | 物質生産に影響する代謝フラックスの決定方法 |
| KR100655495B1 (ko) | 2005-07-11 | 2006-12-08 | 한국과학기술원 | 대사산물들의 플럭스 섬을 이용한 인-실리코 생물 개량방법 |
| KR100727053B1 (ko) | 2006-05-04 | 2007-06-12 | 한국과학기술원 | 대사산물들의 플럭스 섬 프로파일링을 이용한 생물개량방법 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7127379B2 (en) * | 2001-01-31 | 2006-10-24 | The Regents Of The University Of California | Method for the evolutionary design of biochemical reaction networks |
| KR100630836B1 (ko) * | 2005-04-08 | 2006-10-02 | 한국과학기술원 | 인-실리코 분석을 통한 균주 개량방법 |
-
2006
- 2006-12-22 KR KR1020060133119A patent/KR100818202B1/ko not_active Expired - Fee Related
-
2007
- 2007-12-21 JP JP2009542652A patent/JP2010512783A/ja active Pending
- 2007-12-21 WO PCT/KR2007/006708 patent/WO2008078911A1/en active Application Filing
- 2007-12-21 CN CNA2007800476444A patent/CN101611312A/zh active Pending
- 2007-12-21 EP EP07851674A patent/EP2097743A1/en not_active Withdrawn
- 2007-12-21 US US12/519,791 patent/US8494779B2/en active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020168654A1 (en) * | 2001-01-10 | 2002-11-14 | Maranas Costas D. | Method and system for modeling cellular metabolism |
| JP2005058226A (ja) | 2003-07-29 | 2005-03-10 | Ajinomoto Co Inc | 物質生産に影響する代謝フラックスの決定方法 |
| KR100655495B1 (ko) | 2005-07-11 | 2006-12-08 | 한국과학기술원 | 대사산물들의 플럭스 섬을 이용한 인-실리코 생물 개량방법 |
| KR100727053B1 (ko) | 2006-05-04 | 2007-06-12 | 한국과학기술원 | 대사산물들의 플럭스 섬 프로파일링을 이용한 생물개량방법 |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101243378B1 (ko) | 2009-09-18 | 2013-03-25 | 한국과학기술원 | 필수 대사산물을 이용한 병원성 미생물의 약물 표적 예측 및 약물 탐색 방법 |
| WO2019168300A1 (ko) * | 2018-02-28 | 2019-09-06 | 고려대학교 산학협력단 | 합성가스 발효 균의 합성가스 발효 시의 대사체 분석을 위한 대사체 샘플링 및 분석 방법 |
| US11988651B2 (en) | 2018-02-28 | 2024-05-21 | Korea University Research And Business Foundation | Metabolome sampling and analysis method for analyzing metabolome during synthetic gas fermentation of synthetic gas fermentation microorganisms |
Also Published As
| Publication number | Publication date |
|---|---|
| US8494779B2 (en) | 2013-07-23 |
| JP2010512783A (ja) | 2010-04-30 |
| EP2097743A1 (en) | 2009-09-09 |
| US20100143911A1 (en) | 2010-06-10 |
| WO2008078911A1 (en) | 2008-07-03 |
| CN101611312A (zh) | 2009-12-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR100818202B1 (ko) | 미생물의 성장에 필수적인 대사산물의 스크리닝 방법 | |
| Bochner et al. | Phenotype microarrays for high-throughput phenotypic testing and assay of gene function | |
| Traxler et al. | The global, ppGpp‐mediated stringent response to amino acid starvation in Escherichia coli | |
| Bertels et al. | Design and characterization of auxotrophy-based amino acid biosensors | |
| Record Jr et al. | Responses of E. coli to osmotic stress: large changes in amounts of cytoplasmic solutes and water | |
| Wolfe et al. | Evidence that acetyl phosphate functions as a global signal during biofilm development | |
| Tipples et al. | The obligate intracellular bacterium Chlamydia trachomatis is auxotrophic for three of the four ribonucleoside triphosphates | |
| Fondi et al. | Genome‐scale metabolic reconstruction and constraint‐based modelling of the Antarctic bacterium P seudoalteromonas haloplanktis TAC 125 | |
| Metris et al. | Metabolic shift of Escherichia coli under salt stress in the presence of glycine betaine | |
| Piper | Using artificial diets to understand the nutritional physiology of Drosophila melanogaster | |
| CA2495021A1 (en) | System and methods for measuring at least one metabolic rate of a plurality of cells | |
| Waschina et al. | Metabolic network architecture and carbon source determine metabolite production costs | |
| Macvanin et al. | Fusidic acid‐resistant EF‐G perturbs the accumulation of ppGpp | |
| Bazurto et al. | An unexpected route to an essential cofactor: Escherichia coli relies on threonine for thiamine biosynthesis | |
| Bouvet et al. | Diversity of the auxotrophic requirements in natural isolates of Escherichia coli | |
| Magnus et al. | The identification of enzyme targets for the optimization of a valine producing Corynebacterium glutamicum strain using a kinetic model | |
| Uygun et al. | Investigation of metabolic objectives in cultured hepatocytes | |
| Mader et al. | Role of N-terminal protein formylation in central metabolic processes in Staphylococcus aureus | |
| King et al. | An approach to learn regulation to maximize growth and entropy production rates in metabolism | |
| Dens et al. | Cell division theory and individual-based modeling of microbial lag: Part II. Modeling lag phenomena induced by temperature shifts | |
| Stuer-Lauridsen et al. | Purine salvage in two halophilic archaea: characterization of salvage pathways and isolation of mutants resistant to purine analogs | |
| Hanawalt | A balanced perspective on unbalanced growth and thymineless death | |
| Liang et al. | An analysis of the mechanism underlying photocatalytic disinfection based on integrated metabolic networks and transcriptional data | |
| Weiss | YjjG, a dUMP phosphatase, is critical for thymine utilization by Escherichia coli K-12 | |
| Taylor et al. | A metabolic sum rule dictates bacterial response to short-chain fatty acid stress |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| PA0109 | Patent application |
Patent event code: PA01091R01D Comment text: Patent Application Patent event date: 20061222 |
|
| PA0201 | Request for examination | ||
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20071130 Patent event code: PE09021S01D |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
Patent event code: PE07011S01D Comment text: Decision to Grant Registration Patent event date: 20080321 |
|
| GRNT | Written decision to grant | ||
| PR0701 | Registration of establishment |
Comment text: Registration of Establishment Patent event date: 20080325 Patent event code: PR07011E01D |
|
| PR1002 | Payment of registration fee |
Payment date: 20080326 End annual number: 3 Start annual number: 1 |
|
| PG1601 | Publication of registration | ||
| PR1001 | Payment of annual fee |
Payment date: 20110302 Start annual number: 4 End annual number: 4 |
|
| PR1001 | Payment of annual fee |
Payment date: 20120228 Start annual number: 5 End annual number: 5 |
|
| FPAY | Annual fee payment |
Payment date: 20130304 Year of fee payment: 6 |
|
| PR1001 | Payment of annual fee |
Payment date: 20130304 Start annual number: 6 End annual number: 6 |
|
| FPAY | Annual fee payment |
Payment date: 20131105 Year of fee payment: 8 |
|
| PR1001 | Payment of annual fee |
Payment date: 20131105 Start annual number: 7 End annual number: 8 |
|
| FPAY | Annual fee payment |
Payment date: 20160225 Year of fee payment: 9 |
|
| PR1001 | Payment of annual fee |
Payment date: 20160225 Start annual number: 9 End annual number: 9 |
|
| FPAY | Annual fee payment |
Payment date: 20170224 Year of fee payment: 10 |
|
| PR1001 | Payment of annual fee |
Payment date: 20170224 Start annual number: 10 End annual number: 10 |
|
| FPAY | Annual fee payment |
Payment date: 20180226 Year of fee payment: 11 |
|
| PR1001 | Payment of annual fee |
Payment date: 20180226 Start annual number: 11 End annual number: 11 |
|
| PC1903 | Unpaid annual fee |
Termination category: Default of registration fee Termination date: 20210105 |