KR100250830B1 - 자가분해에 의해 폴리하이드록시알킨산으로부터 광학활성을 가진 단량체 하이드록시카르복실산의 제조방법 - Google Patents
자가분해에 의해 폴리하이드록시알킨산으로부터 광학활성을 가진 단량체 하이드록시카르복실산의 제조방법 Download PDFInfo
- Publication number
- KR100250830B1 KR100250830B1 KR1019970066842A KR19970066842A KR100250830B1 KR 100250830 B1 KR100250830 B1 KR 100250830B1 KR 1019970066842 A KR1019970066842 A KR 1019970066842A KR 19970066842 A KR19970066842 A KR 19970066842A KR 100250830 B1 KR100250830 B1 KR 100250830B1
- Authority
- KR
- South Korea
- Prior art keywords
- acid
- hydroxy
- microorganisms
- producing
- autolysis
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000004519 manufacturing process Methods 0.000 title claims abstract description 74
- 239000002253 acid Substances 0.000 title claims abstract description 40
- 230000003287 optical effect Effects 0.000 title claims abstract description 21
- 208000035404 Autolysis Diseases 0.000 title claims description 78
- 206010057248 Cell death Diseases 0.000 title claims description 78
- 230000028043 self proteolysis Effects 0.000 title claims description 78
- BVKZGUZCCUSVTD-UHFFFAOYSA-N carbonic acid Chemical compound OC(O)=O BVKZGUZCCUSVTD-UHFFFAOYSA-N 0.000 title claims description 15
- 239000000178 monomer Substances 0.000 claims abstract description 69
- 239000005014 poly(hydroxyalkanoate) Substances 0.000 claims abstract description 68
- 229920000903 polyhydroxyalkanoate Polymers 0.000 claims abstract description 68
- 239000000243 solution Substances 0.000 claims abstract description 47
- 238000000034 method Methods 0.000 claims abstract description 41
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims abstract description 26
- 239000007853 buffer solution Substances 0.000 claims abstract description 13
- 230000008569 process Effects 0.000 claims abstract description 13
- 241000193033 Azohydromonas lata Species 0.000 claims abstract description 10
- 150000007513 acids Chemical class 0.000 claims abstract description 9
- 239000003960 organic solvent Substances 0.000 claims abstract description 9
- 230000000694 effects Effects 0.000 claims abstract description 5
- 230000003834 intracellular effect Effects 0.000 claims abstract description 5
- 108010081808 poly(3-hydroxyalkanoic acid) depolymerase Proteins 0.000 claims abstract description 3
- 229920000070 poly-3-hydroxybutyrate Polymers 0.000 claims description 74
- 244000005700 microbiome Species 0.000 claims description 54
- 238000000354 decomposition reaction Methods 0.000 claims description 49
- FYSSBMZUBSBFJL-UHFFFAOYSA-N 3-hydroxydecanoic acid Chemical compound CCCCCCCC(O)CC(O)=O FYSSBMZUBSBFJL-UHFFFAOYSA-N 0.000 claims description 26
- NDPLAKGOSZHTPH-UHFFFAOYSA-N 3-hydroxyoctanoic acid Chemical compound CCCCCC(O)CC(O)=O NDPLAKGOSZHTPH-UHFFFAOYSA-N 0.000 claims description 26
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 claims description 21
- 238000004128 high performance liquid chromatography Methods 0.000 claims description 21
- 239000000539 dimer Substances 0.000 claims description 18
- 238000004811 liquid chromatography Methods 0.000 claims description 18
- 239000000203 mixture Substances 0.000 claims description 17
- WHBMMWSBFZVSSR-UHFFFAOYSA-N R3HBA Natural products CC(O)CC(O)=O WHBMMWSBFZVSSR-UHFFFAOYSA-N 0.000 claims description 15
- 229920001577 copolymer Polymers 0.000 claims description 15
- 238000012258 culturing Methods 0.000 claims description 15
- FARPMBPKLYEDIL-UHFFFAOYSA-N 3-hydroxyundecanoic acid Chemical compound CCCCCCCCC(O)CC(O)=O FARPMBPKLYEDIL-UHFFFAOYSA-N 0.000 claims description 14
- 239000012266 salt solution Substances 0.000 claims description 14
- HPMGFDVTYHWBAG-UHFFFAOYSA-N 3-hydroxyhexanoic acid Chemical compound CCCC(O)CC(O)=O HPMGFDVTYHWBAG-UHFFFAOYSA-N 0.000 claims description 13
- 238000000746 purification Methods 0.000 claims description 13
- 125000004432 carbon atom Chemical group C* 0.000 claims description 12
- SJZRECIVHVDYJC-UHFFFAOYSA-N 4-hydroxybutyric acid Chemical compound OCCCC(O)=O SJZRECIVHVDYJC-UHFFFAOYSA-N 0.000 claims description 11
- 241000252867 Cupriavidus metallidurans Species 0.000 claims description 11
- -1 3-hydroxy-7-octenic acid octenoic acid Chemical compound 0.000 claims description 9
- OXSSIXNFGTZQMZ-UHFFFAOYSA-N 3-hydroxyheptanoic acid Chemical compound CCCCC(O)CC(O)=O OXSSIXNFGTZQMZ-UHFFFAOYSA-N 0.000 claims description 9
- 238000001035 drying Methods 0.000 claims description 9
- 239000000843 powder Substances 0.000 claims description 9
- 238000000926 separation method Methods 0.000 claims description 9
- MUCMKTPAZLSKTL-UHFFFAOYSA-N 3-hydroxylauric acid Chemical compound CCCCCCCCCC(O)CC(O)=O MUCMKTPAZLSKTL-UHFFFAOYSA-N 0.000 claims description 8
- 238000009472 formulation Methods 0.000 claims description 8
- 239000012535 impurity Substances 0.000 claims description 8
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 claims description 8
- REKYPYSUBKSCAT-UHFFFAOYSA-N 3-hydroxypentanoic acid Chemical compound CCC(O)CC(O)=O REKYPYSUBKSCAT-UHFFFAOYSA-N 0.000 claims description 6
- ALRHLSYJTWAHJZ-UHFFFAOYSA-N 3-hydroxypropionic acid Chemical group OCCC(O)=O ALRHLSYJTWAHJZ-UHFFFAOYSA-N 0.000 claims description 6
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 claims description 6
- 241000589516 Pseudomonas Species 0.000 claims description 6
- 239000007864 aqueous solution Substances 0.000 claims description 6
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 6
- 150000007524 organic acids Chemical class 0.000 claims description 6
- 241000589517 Pseudomonas aeruginosa Species 0.000 claims description 5
- 108700005078 Synthetic Genes Proteins 0.000 claims description 5
- VEXDRERIMPLZLU-UHFFFAOYSA-N 3-hydroxy-2-methylbutanoic acid Chemical compound CC(O)C(C)C(O)=O VEXDRERIMPLZLU-UHFFFAOYSA-N 0.000 claims description 4
- FMHKPLXYWVCLME-UHFFFAOYSA-N 4-hydroxy-valeric acid Chemical compound CC(O)CCC(O)=O FMHKPLXYWVCLME-UHFFFAOYSA-N 0.000 claims description 4
- SJZRECIVHVDYJC-UHFFFAOYSA-M 4-hydroxybutyrate Chemical compound OCCCC([O-])=O SJZRECIVHVDYJC-UHFFFAOYSA-M 0.000 claims description 4
- JSSMLGKWQYPKBS-UHFFFAOYSA-N 6-hydroxydodecanoic acid Chemical compound CCCCCCC(O)CCCCC(O)=O JSSMLGKWQYPKBS-UHFFFAOYSA-N 0.000 claims description 4
- 241000186249 Corynebacterium sp. Species 0.000 claims description 4
- 241000589781 Pseudomonas oleovorans Species 0.000 claims description 4
- 230000000243 photosynthetic effect Effects 0.000 claims description 4
- 238000002360 preparation method Methods 0.000 claims description 4
- 238000000638 solvent extraction Methods 0.000 claims description 4
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N valeric acid Chemical compound CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 claims description 4
- PHOJOSOUIAQEDH-UHFFFAOYSA-N 5-hydroxypentanoic acid Chemical compound OCCCCC(O)=O PHOJOSOUIAQEDH-UHFFFAOYSA-N 0.000 claims description 3
- 241000193830 Bacillus <bacterium> Species 0.000 claims description 3
- 241000194110 Bacillus sp. (in: Bacteria) Species 0.000 claims description 3
- 241000186216 Corynebacterium Species 0.000 claims description 3
- 241000589351 Methylosinus trichosporium Species 0.000 claims description 3
- 241000190984 Rhodospirillum rubrum Species 0.000 claims description 3
- 239000008346 aqueous phase Substances 0.000 claims description 3
- 239000006227 byproduct Substances 0.000 claims description 3
- 239000012074 organic phase Substances 0.000 claims description 3
- GUXUIBFKSWOPEY-YUMQZZPRSA-N (3S,4S)-3-hydroxy-4-methyloctanoic acid Chemical compound CCCC[C@H](C)[C@@H](O)CC(O)=O GUXUIBFKSWOPEY-YUMQZZPRSA-N 0.000 claims description 2
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 claims description 2
- NVPCGXUWUBHZBD-IHWYPQMZSA-N (z)-3-hydroxybut-2-enoic acid Chemical compound C\C(O)=C\C(O)=O NVPCGXUWUBHZBD-IHWYPQMZSA-N 0.000 claims description 2
- GGYAGRTZMIRKTE-UHFFFAOYSA-N 2-(3-hydroxycyclohexyl)butanoic acid Chemical compound CCC(C(O)=O)C1CCCC(O)C1 GGYAGRTZMIRKTE-UHFFFAOYSA-N 0.000 claims description 2
- HKJLYGUVYQCXAP-UHFFFAOYSA-N 2-hydroxydecanoic acid 4-hydroxydecanoic acid Chemical compound OC(C(=O)O)CCCCCCCC.OC(CCC(=O)O)CCCCCC HKJLYGUVYQCXAP-UHFFFAOYSA-N 0.000 claims description 2
- JGEFOCOPBYAGDE-UHFFFAOYSA-N 3,12-dihydroxylauric acid Chemical compound OCCCCCCCCCC(O)CC(O)=O JGEFOCOPBYAGDE-UHFFFAOYSA-N 0.000 claims description 2
- SCAWECGFPWPHAR-UHFFFAOYSA-N 3-Hydroxyisoheptanoic acid Chemical compound CC(C)CC(O)CC(O)=O SCAWECGFPWPHAR-UHFFFAOYSA-N 0.000 claims description 2
- WMFPBJRTSLJPTJ-UHFFFAOYSA-N 3-hydroxy-2-phenoxypentanoic acid Chemical compound CCC(O)C(C(O)=O)OC1=CC=CC=C1 WMFPBJRTSLJPTJ-UHFFFAOYSA-N 0.000 claims description 2
- GFSKSFDEQIWSQH-UHFFFAOYSA-N 3-hydroxy-3-(3-pentyloxiran-2-yl)propanoic acid Chemical compound CCCCCC1OC1C(O)CC(O)=O GFSKSFDEQIWSQH-UHFFFAOYSA-N 0.000 claims description 2
- GXQJBKQQEGWPLR-UHFFFAOYSA-N 3-hydroxy-6-methylnonanoic acid Chemical compound CCCC(C)CCC(O)CC(O)=O GXQJBKQQEGWPLR-UHFFFAOYSA-N 0.000 claims description 2
- CKOHYUUVZVLYIP-UHFFFAOYSA-N 3-hydroxy-6-methyloctanoic acid Chemical compound CCC(C)CCC(O)CC(O)=O CKOHYUUVZVLYIP-UHFFFAOYSA-N 0.000 claims description 2
- NWOBUQRAAUPEEC-UHFFFAOYSA-N 3-hydroxy-7-methylnonanoic acid Chemical compound CCC(C)CCCC(O)CC(O)=O NWOBUQRAAUPEEC-UHFFFAOYSA-N 0.000 claims description 2
- FQYYTNDVLHRMGY-UHFFFAOYSA-N 3-hydroxy-7-methyloct-6-enoic acid Chemical compound CC(C)=CCCC(O)CC(O)=O FQYYTNDVLHRMGY-UHFFFAOYSA-N 0.000 claims description 2
- UXJJZNZXKAPKLQ-UHFFFAOYSA-N 3-hydroxy-8-methylnonanoic acid Chemical compound CC(C)CCCCC(O)CC(O)=O UXJJZNZXKAPKLQ-UHFFFAOYSA-N 0.000 claims description 2
- ODCCWURQWWXXKS-UHFFFAOYSA-N 3-hydroxy-9-methyldecanoic acid Chemical compound CC(C)CCCCCC(O)CC(O)=O ODCCWURQWWXXKS-UHFFFAOYSA-N 0.000 claims description 2
- BVAXVQDOCBTYID-UHFFFAOYSA-N 3-hydroxy-isoheptanoic acid Chemical compound CC(C)CCC(O)CC(O)=O BVAXVQDOCBTYID-UHFFFAOYSA-N 0.000 claims description 2
- ANJVCBASFCUSLC-UHFFFAOYSA-N 3-hydroxydec-9-enoic acid Chemical compound OC(=O)CC(O)CCCCCC=C ANJVCBASFCUSLC-UHFFFAOYSA-N 0.000 claims description 2
- XGWGWKJFMLNRSQ-UHFFFAOYSA-N 3-hydroxyhex-5-enoic acid Chemical compound C=CCC(O)CC(O)=O XGWGWKJFMLNRSQ-UHFFFAOYSA-N 0.000 claims description 2
- YOYJVCHIWPIPFH-UHFFFAOYSA-N 3-hydroxynon-8-enoic acid Chemical compound OC(=O)CC(O)CCCCC=C YOYJVCHIWPIPFH-UHFFFAOYSA-N 0.000 claims description 2
- UZGRZSHGRZYCQV-UHFFFAOYSA-N 4,6-dichloro-1,3-benzothiazol-2-amine Chemical compound C1=C(Cl)C=C2SC(N)=NC2=C1Cl UZGRZSHGRZYCQV-UHFFFAOYSA-N 0.000 claims description 2
- MYCCAWPBMVOJQF-UHFFFAOYSA-N 4-Hydroxyenanthoic acid Chemical compound CCCC(O)CCC(O)=O MYCCAWPBMVOJQF-UHFFFAOYSA-N 0.000 claims description 2
- SEBFOLWMHMRBOG-UHFFFAOYSA-N 4-hydroxy-6-methoxy-6-oxohexanoic acid Chemical compound COC(=O)CC(O)CCC(O)=O SEBFOLWMHMRBOG-UHFFFAOYSA-N 0.000 claims description 2
- ZRNOVONGMRDZEL-UHFFFAOYSA-N 4-hydroxy-octanoic acid Chemical compound CCCCC(O)CCC(O)=O ZRNOVONGMRDZEL-UHFFFAOYSA-N 0.000 claims description 2
- SMHOCEUFSUDPPJ-UHFFFAOYSA-N 5-hydroxy-7-oxo-7-propoxyheptanoic acid Chemical compound CCCOC(=O)CC(O)CCCC(O)=O SMHOCEUFSUDPPJ-UHFFFAOYSA-N 0.000 claims description 2
- YDCRNMJQROAWFT-UHFFFAOYSA-N 5-hydroxyhexanoic acid Chemical compound CC(O)CCCC(O)=O YDCRNMJQROAWFT-UHFFFAOYSA-N 0.000 claims description 2
- JJIXPLIFDQBBMD-UHFFFAOYSA-N 6-chloro-3-hydroxyhexanoic acid Chemical compound OC(=O)CC(O)CCCCl JJIXPLIFDQBBMD-UHFFFAOYSA-N 0.000 claims description 2
- SBIYXEINNITUSB-UHFFFAOYSA-N 6-hydroxy-8-methoxy-8-oxooctanoic acid Chemical compound COC(=O)CC(O)CCCCC(O)=O SBIYXEINNITUSB-UHFFFAOYSA-N 0.000 claims description 2
- OCYCYTKZMANKAA-UHFFFAOYSA-N 6-hydroxydodec-3-enoic acid Chemical compound CCCCCCC(O)CC=CCC(O)=O OCYCYTKZMANKAA-UHFFFAOYSA-N 0.000 claims description 2
- QQEAQDPGHTVQII-UHFFFAOYSA-N 7-fluoro-3-hydroxyheptanoic acid Chemical compound OC(=O)CC(O)CCCCF QQEAQDPGHTVQII-UHFFFAOYSA-N 0.000 claims description 2
- XHBMGVZHTRNEOW-UHFFFAOYSA-N 8-acetyloxy-3-hydroxyoctanoic acid Chemical compound CC(=O)OCCCCCC(O)CC(O)=O XHBMGVZHTRNEOW-UHFFFAOYSA-N 0.000 claims description 2
- SPBBSBZTCXPXRG-UHFFFAOYSA-N 8-bromo-3-hydroxyoctanoic acid Chemical compound OC(=O)CC(O)CCCCCBr SPBBSBZTCXPXRG-UHFFFAOYSA-N 0.000 claims description 2
- WCURZZCYTAPZPN-UHFFFAOYSA-N 8-chloro-3-hydroxyoctanoic acid Chemical compound OC(=O)CC(O)CCCCCCl WCURZZCYTAPZPN-UHFFFAOYSA-N 0.000 claims description 2
- RQJZNJSIDNBENW-UHFFFAOYSA-N 8-ethoxy-6-hydroxy-8-oxooctanoic acid Chemical compound CCOC(=O)CC(O)CCCCC(O)=O RQJZNJSIDNBENW-UHFFFAOYSA-N 0.000 claims description 2
- YDJKYCWLWDTNAY-UHFFFAOYSA-N 8-hydroxy-10-oxo-10-phenylmethoxydecanoic acid Chemical compound OC(=O)CCCCCCC(O)CC(=O)OCC1=CC=CC=C1 YDJKYCWLWDTNAY-UHFFFAOYSA-N 0.000 claims description 2
- JOLPHFUTBQKLCD-UHFFFAOYSA-N 9-acetyloxy-3-hydroxynonanoic acid Chemical compound CC(=O)OCCCCCCC(O)CC(O)=O JOLPHFUTBQKLCD-UHFFFAOYSA-N 0.000 claims description 2
- QGJMHMYCARESKY-UHFFFAOYSA-N 9-fluoro-3-hydroxynonanoic acid Chemical compound OC(=O)CC(O)CCCCCCF QGJMHMYCARESKY-UHFFFAOYSA-N 0.000 claims description 2
- ATRNZOYKSNPPBF-UHFFFAOYSA-N D-beta-hydroxymyristic acid Natural products CCCCCCCCCCCC(O)CC(O)=O ATRNZOYKSNPPBF-UHFFFAOYSA-N 0.000 claims description 2
- ABIKNKURIGPIRJ-UHFFFAOYSA-N DL-4-hydroxy caproic acid Chemical compound CCC(O)CCC(O)=O ABIKNKURIGPIRJ-UHFFFAOYSA-N 0.000 claims description 2
- GZZPOFFXKUVNSW-UHFFFAOYSA-N Dodecenoic acid Natural products OC(=O)CCCCCCCCCC=C GZZPOFFXKUVNSW-UHFFFAOYSA-N 0.000 claims description 2
- 241000589651 Zoogloea Species 0.000 claims description 2
- 230000002378 acidificating effect Effects 0.000 claims description 2
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 claims description 2
- 230000029087 digestion Effects 0.000 claims description 2
- 239000001630 malic acid Substances 0.000 claims description 2
- 235000011090 malic acid Nutrition 0.000 claims description 2
- 229940005605 valeric acid Drugs 0.000 claims description 2
- CBWALJHXHCJYTE-UHFFFAOYSA-N 3-hydroxypalmitic acid Chemical compound CCCCCCCCCCCCCC(O)CC(O)=O CBWALJHXHCJYTE-UHFFFAOYSA-N 0.000 claims 2
- 241000190932 Rhodopseudomonas Species 0.000 claims 2
- 241000191001 Thiocapsa Species 0.000 claims 2
- UHZWUNLTEZCDMA-UHFFFAOYSA-N (10r)-10-hydroxyundecanoic acid Chemical compound CC(O)CCCCCCCCC(O)=O UHZWUNLTEZCDMA-UHFFFAOYSA-N 0.000 claims 1
- IWXFIKFCUPXJLW-UHFFFAOYSA-N 2,3-dihydroxy-4-methylpentanoic acid Chemical compound CC(C)C(O)C(O)C(O)=O IWXFIKFCUPXJLW-UHFFFAOYSA-N 0.000 claims 1
- MCJAZCYLVCHELT-UHFFFAOYSA-N 2-cyano-3-hydroxy-2-phenoxyhexanoic acid Chemical compound CCCC(O)C(C(O)=O)(C#N)OC1=CC=CC=C1 MCJAZCYLVCHELT-UHFFFAOYSA-N 0.000 claims 1
- YBTWUESFQWFDMR-UHFFFAOYSA-N 3-Hydroxyhexadecanoic acid Natural products CCCCCCCCCCCCCC(O)CC(=O)OC YBTWUESFQWFDMR-UHFFFAOYSA-N 0.000 claims 1
- COJMCLUAEMVCKL-UHFFFAOYSA-N 3-hydroxy-2-phenoxybutanoic acid Chemical compound CC(O)C(C(O)=O)OC1=CC=CC=C1 COJMCLUAEMVCKL-UHFFFAOYSA-N 0.000 claims 1
- BFBQYHBRGKIUEG-UHFFFAOYSA-N 3-hydroxy-4-methylhexanoic acid Chemical compound CCC(C)C(O)CC(O)=O BFBQYHBRGKIUEG-UHFFFAOYSA-N 0.000 claims 1
- PYHGGQCTNHKLGY-UHFFFAOYSA-N 3-hydroxy-5-(3-pentyloxiran-2-yl)pentanoic acid Chemical compound CCCCCC1OC1CCC(O)CC(O)=O PYHGGQCTNHKLGY-UHFFFAOYSA-N 0.000 claims 1
- URSDOFWLDXJRKM-UHFFFAOYSA-N 3-hydroxy-7-methyloctanoic acid Chemical compound CC(C)CCCC(O)CC(O)=O URSDOFWLDXJRKM-UHFFFAOYSA-N 0.000 claims 1
- WHBMMWSBFZVSSR-UHFFFAOYSA-M 3-hydroxybutyrate Chemical compound CC(O)CC([O-])=O WHBMMWSBFZVSSR-UHFFFAOYSA-M 0.000 claims 1
- AINRQBNLOBQURT-UHFFFAOYSA-N 3-hydroxypent-4-enoic acid Chemical compound C=CC(O)CC(O)=O AINRQBNLOBQURT-UHFFFAOYSA-N 0.000 claims 1
- BYHDDXPKOZIZRV-UHFFFAOYSA-N 5-phenylpentanoic acid Chemical compound OC(=O)CCCCC1=CC=CC=C1 BYHDDXPKOZIZRV-UHFFFAOYSA-N 0.000 claims 1
- VQNWTTQUKRRSOQ-UHFFFAOYSA-N 7-cyano-3-hydroxyheptanoic acid Chemical compound OC(=O)CC(O)CCCCC#N VQNWTTQUKRRSOQ-UHFFFAOYSA-N 0.000 claims 1
- OUHJCYSMDKXCSR-UHFFFAOYSA-N 7-hydroxy-9-methoxy-9-oxononanoic acid Chemical compound COC(=O)CC(O)CCCCCC(O)=O OUHJCYSMDKXCSR-UHFFFAOYSA-N 0.000 claims 1
- OTPYHQICHMCIHD-UHFFFAOYSA-N 9-hydroxy-10-methoxy-10-oxodecanoic acid Chemical compound COC(C(CCCCCCCC(=O)O)O)=O OTPYHQICHMCIHD-UHFFFAOYSA-N 0.000 claims 1
- 241000590020 Achromobacter Species 0.000 claims 1
- 241000218592 Acidovorax delafieldii Species 0.000 claims 1
- 241000726118 Acidovorax facilis Species 0.000 claims 1
- 241000589291 Acinetobacter Species 0.000 claims 1
- 241001147825 Actinomyces sp. Species 0.000 claims 1
- 241000588986 Alcaligenes Species 0.000 claims 1
- 241001135315 Alteromonas macleodii Species 0.000 claims 1
- 241000178563 Aphanothece sp. Species 0.000 claims 1
- 235000016425 Arthrospira platensis Nutrition 0.000 claims 1
- 240000002900 Arthrospira platensis Species 0.000 claims 1
- 241000894009 Azorhizobium caulinodans Species 0.000 claims 1
- 241000589941 Azospirillum Species 0.000 claims 1
- 241000589151 Azotobacter Species 0.000 claims 1
- 241000190909 Beggiatoa Species 0.000 claims 1
- 241000588882 Beijerinckia Species 0.000 claims 1
- BVKRZYCFTNFTAI-UHFFFAOYSA-N C(C)(=O)OC(C(=O)O)CCCCCCC Chemical compound C(C)(=O)OC(C(=O)O)CCCCCCC BVKRZYCFTNFTAI-UHFFFAOYSA-N 0.000 claims 1
- 241001353638 Chlorogloea Species 0.000 claims 1
- 241000190831 Chromatium Species 0.000 claims 1
- 241000588881 Chromobacterium Species 0.000 claims 1
- 241000193403 Clostridium Species 0.000 claims 1
- 241000589519 Comamonas Species 0.000 claims 1
- 241001180360 Derxia Species 0.000 claims 1
- 241000605827 Desulfococcus multivorans Species 0.000 claims 1
- 241000193104 Desulfonema Species 0.000 claims 1
- 241000190986 Ectothiorhodospira Species 0.000 claims 1
- 241000589564 Flavobacterium sp. Species 0.000 claims 1
- 241000606768 Haemophilus influenzae Species 0.000 claims 1
- 241000205062 Halobacterium Species 0.000 claims 1
- 241000204988 Haloferax mediterranei Species 0.000 claims 1
- 241001051274 Herbaspirillum autotrophicum Species 0.000 claims 1
- 241000216643 Hydrogenophaga Species 0.000 claims 1
- 241000922030 Hydrogenophaga flava Species 0.000 claims 1
- 241000862974 Hyphomicrobium Species 0.000 claims 1
- 241000411968 Ilyobacter Species 0.000 claims 1
- 241000862991 Leptothrix <Bacteria> Species 0.000 claims 1
- 241000589323 Methylobacterium Species 0.000 claims 1
- 241000520880 Methylococcus thermophilus Species 0.000 claims 1
- 241000589964 Methylocystis parvus Species 0.000 claims 1
- 241000589348 Methylomonas methanica Species 0.000 claims 1
- 241000589354 Methylosinus Species 0.000 claims 1
- 241000192041 Micrococcus Species 0.000 claims 1
- 241000186359 Mycobacterium Species 0.000 claims 1
- 241000605159 Nitrobacter Species 0.000 claims 1
- RQXYPNMJOJLPLB-UHFFFAOYSA-N OC(CC(=O)O)CC(CCC)C.CC(C(=O)O)CCCCCC Chemical compound OC(CC(=O)O)CC(CCC)C.CC(C(=O)O)CCCCCC RQXYPNMJOJLPLB-UHFFFAOYSA-N 0.000 claims 1
- 241001663787 Oscillatoria limosa Species 0.000 claims 1
- 241001507683 Penicillium aurantiogriseum Species 0.000 claims 1
- 241000607568 Photobacterium Species 0.000 claims 1
- 241000224486 Physarum polycephalum Species 0.000 claims 1
- 241000232299 Ralstonia Species 0.000 claims 1
- 241000589180 Rhizobium Species 0.000 claims 1
- 241000191025 Rhodobacter Species 0.000 claims 1
- 241000316848 Rhodococcus <scale insect> Species 0.000 claims 1
- 241000191042 Rhodocyclus Species 0.000 claims 1
- 241000190967 Rhodospirillum Species 0.000 claims 1
- 241000736110 Sphingomonas paucimobilis Species 0.000 claims 1
- 241000605008 Spirillum Species 0.000 claims 1
- 241000191940 Staphylococcus Species 0.000 claims 1
- 241000862969 Stella Species 0.000 claims 1
- 241000122973 Stenotrophomonas maltophilia Species 0.000 claims 1
- 241000187747 Streptomyces Species 0.000 claims 1
- 241000606014 Syntrophomonas wolfei Species 0.000 claims 1
- 241000736888 Thiocystis violacea Species 0.000 claims 1
- 241000607598 Vibrio Species 0.000 claims 1
- 241000607272 Vibrio parahaemolyticus Species 0.000 claims 1
- 241000589494 Xanthobacter autotrophicus Species 0.000 claims 1
- 229940011158 alteromonas macleodii Drugs 0.000 claims 1
- 229940047650 haemophilus influenzae Drugs 0.000 claims 1
- 238000011002 quantification Methods 0.000 claims 1
- 238000007670 refining Methods 0.000 claims 1
- 229940082787 spirulina Drugs 0.000 claims 1
- 229910052799 carbon Inorganic materials 0.000 abstract description 38
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 abstract 1
- 229910052801 chlorine Inorganic materials 0.000 abstract 1
- 239000000460 chlorine Substances 0.000 abstract 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 37
- 238000006243 chemical reaction Methods 0.000 description 29
- 230000035484 reaction time Effects 0.000 description 16
- 229920000642 polymer Polymers 0.000 description 13
- FBUKVWPVBMHYJY-UHFFFAOYSA-N nonanoic acid Chemical compound CCCCCCCCC(O)=O FBUKVWPVBMHYJY-UHFFFAOYSA-N 0.000 description 12
- 108090000623 proteins and genes Proteins 0.000 description 12
- 239000003513 alkali Substances 0.000 description 11
- XBUXARJOYUQNTC-UHFFFAOYSA-N ()-3-Hydroxynonanoic acid Chemical compound CCCCCCC(O)CC(O)=O XBUXARJOYUQNTC-UHFFFAOYSA-N 0.000 description 10
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 8
- 108090000790 Enzymes Proteins 0.000 description 8
- 102000004190 Enzymes Human genes 0.000 description 8
- 238000005119 centrifugation Methods 0.000 description 7
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 6
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 6
- WWZKQHOCKIZLMA-UHFFFAOYSA-N Caprylic acid Natural products CCCCCCCC(O)=O WWZKQHOCKIZLMA-UHFFFAOYSA-N 0.000 description 6
- RGHNJXZEOKUKBD-SQOUGZDYSA-N D-gluconic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 description 6
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 6
- 239000002585 base Substances 0.000 description 6
- 239000013612 plasmid Substances 0.000 description 5
- 238000009825 accumulation Methods 0.000 description 4
- 230000001851 biosynthetic effect Effects 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 4
- MNWFXJYAOYHMED-UHFFFAOYSA-N heptanoic acid Chemical compound CCCCCCC(O)=O MNWFXJYAOYHMED-UHFFFAOYSA-N 0.000 description 4
- 239000002243 precursor Substances 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- BYKRNSHANADUFY-UHFFFAOYSA-M sodium octanoate Chemical compound [Na+].CCCCCCCC([O-])=O BYKRNSHANADUFY-UHFFFAOYSA-M 0.000 description 4
- GYSCBCSGKXNZRH-UHFFFAOYSA-N 1-benzothiophene-2-carboxamide Chemical compound C1=CC=C2SC(C(=O)N)=CC2=C1 GYSCBCSGKXNZRH-UHFFFAOYSA-N 0.000 description 3
- 241000894006 Bacteria Species 0.000 description 3
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Natural products OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 description 3
- GHVNFZFCNZKVNT-UHFFFAOYSA-N Decanoic acid Natural products CCCCCCCCCC(O)=O GHVNFZFCNZKVNT-UHFFFAOYSA-N 0.000 description 3
- 241000588724 Escherichia coli Species 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 229930091371 Fructose Natural products 0.000 description 3
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 3
- 239000005715 Fructose Substances 0.000 description 3
- 241001240958 Pseudomonas aeruginosa PAO1 Species 0.000 description 3
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 3
- 238000013019 agitation Methods 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- 238000007796 conventional method Methods 0.000 description 3
- 230000000593 degrading effect Effects 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 239000000174 gluconic acid Substances 0.000 description 3
- 235000012208 gluconic acid Nutrition 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- PKAUICCNAWQPAU-UHFFFAOYSA-N 2-(4-chloro-2-methylphenoxy)acetic acid;n-methylmethanamine Chemical compound CNC.CC1=CC(Cl)=CC=C1OCC(O)=O PKAUICCNAWQPAU-UHFFFAOYSA-N 0.000 description 2
- CVKMFSAVYPAZTQ-UHFFFAOYSA-N 2-methylhexanoic acid Chemical compound CCCCC(C)C(O)=O CVKMFSAVYPAZTQ-UHFFFAOYSA-N 0.000 description 2
- SRBFZHDQGSBBOR-IOVATXLUSA-N D-xylopyranose Chemical compound O[C@@H]1COC(O)[C@H](O)[C@H]1O SRBFZHDQGSBBOR-IOVATXLUSA-N 0.000 description 2
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 150000001298 alcohols Chemical class 0.000 description 2
- OBETXYAYXDNJHR-UHFFFAOYSA-N alpha-ethylcaproic acid Natural products CCCCC(CC)C(O)=O OBETXYAYXDNJHR-UHFFFAOYSA-N 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 229940088710 antibiotic agent Drugs 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- GONOPSZTUGRENK-UHFFFAOYSA-N benzyl(trichloro)silane Chemical compound Cl[Si](Cl)(Cl)CC1=CC=CC=C1 GONOPSZTUGRENK-UHFFFAOYSA-N 0.000 description 2
- BOAHYOWLXYZJSN-UHFFFAOYSA-N beta-Oxy-gamma-methyl-butan-alpha-carbonsaeure Natural products CC(C)C(O)CC(O)=O BOAHYOWLXYZJSN-UHFFFAOYSA-N 0.000 description 2
- 238000006065 biodegradation reaction Methods 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 230000007613 environmental effect Effects 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 239000003102 growth factor Substances 0.000 description 2
- FUZZWVXGSFPDMH-UHFFFAOYSA-N n-hexanoic acid Natural products CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 2
- 235000005985 organic acids Nutrition 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 235000019260 propionic acid Nutrition 0.000 description 2
- 238000000425 proton nuclear magnetic resonance spectrum Methods 0.000 description 2
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 2
- 239000011232 storage material Substances 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 230000002194 synthesizing effect Effects 0.000 description 2
- 230000003407 synthetizing effect Effects 0.000 description 2
- IMMRMPAXYUIDLR-UHFFFAOYSA-N (R)-3-Hydroxy-5-phenylpentanoic acid Chemical compound OC(=O)CC(O)CCC1=CC=CC=C1 IMMRMPAXYUIDLR-UHFFFAOYSA-N 0.000 description 1
- BSIMZHVOQZIAOY-SCSAIBSYSA-N 1-carbapenem-3-carboxylic acid Chemical compound OC(=O)C1=CC[C@@H]2CC(=O)N12 BSIMZHVOQZIAOY-SCSAIBSYSA-N 0.000 description 1
- HWXNNTPBPKZXDE-UHFFFAOYSA-N 10-ethoxy-8-hydroxy-10-oxodecanoic acid Chemical compound CCOC(=O)CC(O)CCCCCCC(O)=O HWXNNTPBPKZXDE-UHFFFAOYSA-N 0.000 description 1
- KTHRQDFQEQQGDF-UHFFFAOYSA-N 2,3-dihydroxyhexadecanoic acid Chemical compound CCCCCCCCCCCCCC(O)C(O)C(O)=O KTHRQDFQEQQGDF-UHFFFAOYSA-N 0.000 description 1
- NVIHALDXJWGLFD-UHFFFAOYSA-N 2-Methyl-3-hydroxyvaleric acid Chemical compound CCC(O)C(C)C(O)=O NVIHALDXJWGLFD-UHFFFAOYSA-N 0.000 description 1
- IXMRKZOZPMKYMT-UHFFFAOYSA-N 2-cyano-3-hydroxyheptanoic acid Chemical compound C(#N)C(C(=O)O)C(CCCC)O IXMRKZOZPMKYMT-UHFFFAOYSA-N 0.000 description 1
- SZKQQZKIGFBZHS-UHFFFAOYSA-N 2-fluoroheptanoic acid Chemical compound CCCCCC(F)C(O)=O SZKQQZKIGFBZHS-UHFFFAOYSA-N 0.000 description 1
- ZJDXMXMEZZVUKO-UHFFFAOYSA-N 2-hydroxy-4-methoxy-4-oxobutanoic acid Chemical compound COC(=O)CC(O)C(O)=O ZJDXMXMEZZVUKO-UHFFFAOYSA-N 0.000 description 1
- NYHNVHGFPZAZGA-UHFFFAOYSA-N 2-hydroxyhexanoic acid Chemical compound CCCCC(O)C(O)=O NYHNVHGFPZAZGA-UHFFFAOYSA-N 0.000 description 1
- YLZOPXRUQYQQID-UHFFFAOYSA-N 3-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)-1-[4-[2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidin-5-yl]piperazin-1-yl]propan-1-one Chemical compound N1N=NC=2CN(CCC=21)CCC(=O)N1CCN(CC1)C=1C=NC(=NC=1)NCC1=CC(=CC=C1)OC(F)(F)F YLZOPXRUQYQQID-UHFFFAOYSA-N 0.000 description 1
- FYSMRQJFXUUGIA-UHFFFAOYSA-N 3-hydroxy-2,6-dimethylhept-5-enoic acid Chemical compound OC(=O)C(C)C(O)CC=C(C)C FYSMRQJFXUUGIA-UHFFFAOYSA-N 0.000 description 1
- AWXNFKICMHSAOP-UHFFFAOYSA-N 3-hydroxy-2-phenoxyheptanoic acid Chemical compound CCCCC(O)C(C(O)=O)OC1=CC=CC=C1 AWXNFKICMHSAOP-UHFFFAOYSA-N 0.000 description 1
- KHKSLWMQZMLZKO-UHFFFAOYSA-N 3-hydroxy-5-methyloctanoic acid Chemical compound CCCC(C)CC(O)CC(O)=O KHKSLWMQZMLZKO-UHFFFAOYSA-N 0.000 description 1
- QYQFJLBAOZQKQU-UHFFFAOYSA-N 3-hydroxyoct-7-enoic acid Chemical compound OC(=O)CC(O)CCCC=C QYQFJLBAOZQKQU-UHFFFAOYSA-N 0.000 description 1
- NZPGYIBESMMUFU-UHFFFAOYSA-N 4-methylhexan-3-ol Chemical compound CCC(C)C(O)CC NZPGYIBESMMUFU-UHFFFAOYSA-N 0.000 description 1
- AWQSAIIDOMEEOD-UHFFFAOYSA-N 5,5-Dimethyl-4-(3-oxobutyl)dihydro-2(3H)-furanone Chemical compound CC(=O)CCC1CC(=O)OC1(C)C AWQSAIIDOMEEOD-UHFFFAOYSA-N 0.000 description 1
- PRMNZQRHYSTJDR-UHFFFAOYSA-N 6-bromo-3-hydroxyhexanoic acid Chemical compound OC(=O)CC(O)CCCBr PRMNZQRHYSTJDR-UHFFFAOYSA-N 0.000 description 1
- HZMLRXHYPOCPEV-UHFFFAOYSA-N 8-hydroxy-10-methoxy-10-oxodecanoic acid Chemical compound COC(=O)CC(O)CCCCCCC(O)=O HZMLRXHYPOCPEV-UHFFFAOYSA-N 0.000 description 1
- AQNPYPFXFCNZPO-UHFFFAOYSA-N 8-hydroxy-9-methoxy-9-oxononanoic acid Chemical compound COC(C(CCCCCCC(=O)O)O)=O AQNPYPFXFCNZPO-UHFFFAOYSA-N 0.000 description 1
- GQUHZVSHQWCDPD-UHFFFAOYSA-N 9-cyano-3-hydroxynonanoic acid Chemical compound OC(=O)CC(O)CCCCCCC#N GQUHZVSHQWCDPD-UHFFFAOYSA-N 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 1
- 241001522878 Escherichia coli B Species 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 239000007993 MOPS buffer Substances 0.000 description 1
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical class [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 229920000331 Polyhydroxybutyrate Polymers 0.000 description 1
- 241000589776 Pseudomonas putida Species 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- WKDDRNSBRWANNC-ATRFCDNQSA-N Thienamycin Chemical compound C1C(SCCN)=C(C(O)=O)N2C(=O)[C@H]([C@H](O)C)[C@H]21 WKDDRNSBRWANNC-ATRFCDNQSA-N 0.000 description 1
- WKDDRNSBRWANNC-UHFFFAOYSA-N Thienamycin Natural products C1C(SCCN)=C(C(O)=O)N2C(=O)C(C(O)C)C21 WKDDRNSBRWANNC-UHFFFAOYSA-N 0.000 description 1
- 239000008351 acetate buffer Substances 0.000 description 1
- 150000001335 aliphatic alkanes Chemical class 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- YIYBQIKDCADOSF-UHFFFAOYSA-N alpha-Butylen-alpha-carbonsaeure Natural products CCC=CC(O)=O YIYBQIKDCADOSF-UHFFFAOYSA-N 0.000 description 1
- 235000011114 ammonium hydroxide Nutrition 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- PYMYPHUHKUWMLA-UHFFFAOYSA-N arabinose Natural products OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 description 1
- 239000003782 beta lactam antibiotic agent Substances 0.000 description 1
- SRBFZHDQGSBBOR-UHFFFAOYSA-N beta-D-Pyranose-Lyxose Natural products OC1COC(O)C(O)C1O SRBFZHDQGSBBOR-UHFFFAOYSA-N 0.000 description 1
- 239000001273 butane Substances 0.000 description 1
- 229940041011 carbapenems Drugs 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 238000003889 chemical engineering Methods 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 230000006837 decompression Effects 0.000 description 1
- 125000005594 diketone group Chemical group 0.000 description 1
- 238000011038 discontinuous diafiltration by volume reduction Methods 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 238000009510 drug design Methods 0.000 description 1
- 238000004146 energy storage Methods 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 238000000855 fermentation Methods 0.000 description 1
- 230000004151 fermentation Effects 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- 239000003205 fragrance Substances 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 229940050410 gluconate Drugs 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 238000005984 hydrogenation reaction Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 238000005805 hydroxylation reaction Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 239000000644 isotonic solution Substances 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 230000005415 magnetization Effects 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 1
- OFBQJSOFQDEBGM-UHFFFAOYSA-N n-pentane Natural products CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 239000002547 new drug Substances 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 229940021222 peritoneal dialysis isotonic solution Drugs 0.000 description 1
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 description 1
- 239000003016 pheromone Substances 0.000 description 1
- 239000008055 phosphate buffer solution Substances 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 229920001013 poly(3-hydroxybutyrate-co-4-hydroxybutyrate) Polymers 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 239000002689 soil Substances 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 238000005979 thermal decomposition reaction Methods 0.000 description 1
- YIYBQIKDCADOSF-ONEGZZNKSA-N trans-pent-2-enoic acid Chemical compound CC\C=C\C(O)=O YIYBQIKDCADOSF-ONEGZZNKSA-N 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 230000001131 transforming effect Effects 0.000 description 1
- 238000005292 vacuum distillation Methods 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P7/00—Preparation of oxygen-containing organic compounds
- C12P7/40—Preparation of oxygen-containing organic compounds containing a carboxyl group including Peroxycarboxylic acids
- C12P7/42—Hydroxy-carboxylic acids
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S435/00—Chemistry: molecular biology and microbiology
- Y10S435/8215—Microorganisms
- Y10S435/822—Microorganisms using bacteria or actinomycetales
Landscapes
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Zoology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Wood Science & Technology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Microbiology (AREA)
- General Chemical & Material Sciences (AREA)
- Biotechnology (AREA)
- Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Abstract
Description
Claims (24)
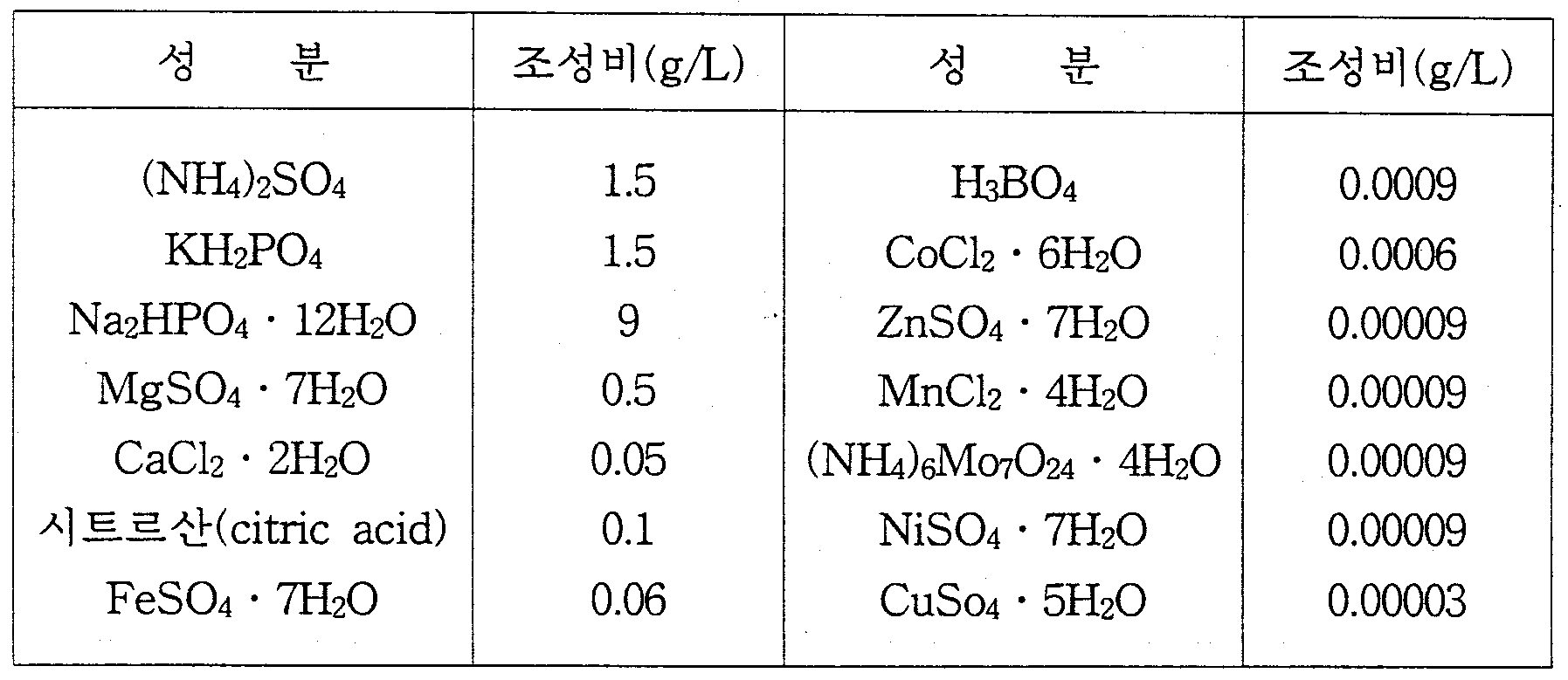
- 세포내 폴리하이드록시알칸산 분해효소(intracellular polyhydroxyalkanoate depolymerase)의 활성도가 있는 미생물의 비양에 의해 폴리하이드록시알칸산(polyhydroxyalkanoates)을 생산ㆍ축적한 다음, 그 균체를 물, 염 수용액 또는 완충액의 분해용액에 방치하여 폴리하이드록시알칸산을 자가분해시킴으로써, 광학 활성을 갖는 그의 단량체 (D)-하이드록시카르복실산(hydroxycarboxylic acids)의 제조방법.
- 제1항에서 광학활성을 갖는 단량체 (D)-하이드록시카르복실산(hydroxycarboxylic acids)을 제조한 다음, 액체크로마토그래피(LC)나 고속액체크로마토그래피(HPLC)를 이용한 전기 단량체의 분리공정을 추가로 포함하는, (D)-하이드록시카르복실산의 제조방법.
- 제1항 또는 제2항에서 순수분리된 광학활성을 갖는 단량체 (D)-하이드록시카르복실산을 용매추출에 의한 불순물 제거 및 건조시키는 정제 및 분말 제재화 공정을 추가로 포함하는, (D)-하이드록시카르복실산의 제조방법.
- 제2항에 있어서, LC나 HPLC는 유기산 분리 컬럼을 채용하고, pH 범위가 1~3인 산성 수용액을 유동상으로 사용하는 것을 특징으로 하는 (D)-하이드록시카르복실산의 제조방법.
- 제3항에 있어서, 정제 및 분말 제재화 공정은 순수분리된 (D)-하이드록시카르복실산 함유 용액에 강염기를 첨가하여 pH 9 이상의 염기영역으로 조정한 다음, 유기용매를 사용한 추출에 의해 불순물을 제거하고 건조시키는 공정만으로 구성되는 것을 특징으로 하는 (D)-하이드록시카르복실산의 제조방법.
- 제5항에 있어서, 강염기로 수산화나트륨을 사용하여 pH 11로 조정한 다음, 클로로포름을 첨가하여 유기상으로 추출된 불순물을 제거한 후, 수용상을 건조기(drying oven)에서 건조시키는 것을 특징으로 하는 (D)-하이드록시카르복실산의 제조방법.
- 제1항에 있어서, 고농도의 폴리하이드록시알칸산을 자가분해하여 그의 단량체를 고농도로 생산하는 것을 특징으로 하는 (D)-하이드록시카르복실산의 제조방법.
- 제1항에 있어서, 폴리하이드록시알칸산의 자가분해시 부산물로 생성되는 이량체를 염기성 조건에서 가열 분해함으로써 단량체 생산 수율을 향상시키는 것을 특징으로 하는 (D)-하이드록시카르복실산의 제조방법.
- 제1항에 있어서, 미생물은 Achromobacter 속 미생물들, Acidovorax delafieldii, Acidovax facilis, Acinetobacter 속 미생물들, Actinomyces sp., Alcaligenes 속 미생물들, Alteromonas macleodii, Amoebobacter 속 미생물들, Aphanocapa sp., Aphanothece sp., Aquaspirillum autotrophicum, Azorhizobium caulinodans, Azospirillum 속 미생물들, Azotobacter 속 미생물들, Bacillus 속 미생물들, Beggiatoa 속 미생물들, Beijerinckia 속 미생물들, Beneckea 속 미생물들, Bordetella pertussis, Bradyrhizobium japonicum, Caryophamon latum, Caulobacter 속 미생물들, Chloroflexus aurantiacus, Chlorogloea 속 미생물들, Chromatium 속 미생물들, Chromobacterium 속 미생물들, Clostridium 속 미생물들, Comamonas 속 미생물들, Corynebacterium 속 미생물들, Derxia 속 미생물들, Desulfococcus multivorans, Desulfonema 속 미생물들, Desulfosacina variabilis, Desulfovibrio sapovorans, Ectothiorhodospira 속 미생물들, Ferrobacillus ferroxidans, Flavobacterium sp., Haemophilus influenzae, Halobacterium 속 미생물들, Haloferax mediterranei, Hydroclathratus clathratus, Hydrogenomonas facilis, Hydrogenophaga flava, Hydrogenophaga 속 미생물들, Hyphomicrobium 속 미생물들, Ilyobacter delafieldii, Labrys monachus, Lamprocystis reseopersicina, Lampropedia hyalina, Legionella sp., Leptothrix discophorus, Methylobacterium 속 미생물들, Methylococcus thermophilus, methylocystis parvus, Methylomonas methanica, Methylosinus 속 미생물들, Methylovibrio soehngenii, Micrococcus 속 미생물들, Mycobacterium 속 미생물들, Nitrobacter 속 미생물들, Nocardia 속 미생물들, Paracoccus dentrificans, Oscillatoria limosa, Penicillium cyclopium, Photobacterium 속 미생물들, Physarum polycephalum, Pseudomonas 속 미생물들, Ralstonia 속 미생물들, Rhizobium 속 미생물들, Rhodobacillus 속 미생물들, Rhodobacter속 미생물들, Rhodococcus 속 미생물들, Rhodocyclus 속 미생물들, Rhodomicrobium vannielii, Rhodopseudomonas 속 미생물들, Rhodospirillum 속 미생물들, Sphingomonas paucimobilis, Spirillum 속 미생물들, Spirulina 속 미생물들, Staphylococcus 속 미생물들, Stella 속 미생물들, Streptomyces 속 미생물들, Syntrophomonas wolfei, Thiobacillus 속 미생물들, Thiocapsa 속 미생물들, Thiocystis violacea, Vibrio parahaemolyticus, Xanthobacter autotrophicus, Xanthomonas maltophilia 및 Zoogloea 속 미생물들로 구성된 그룹으로부터 선택되는 1종인 것을 특징으로 하는 (D)-하이드록시카르복실산의 제조방법.
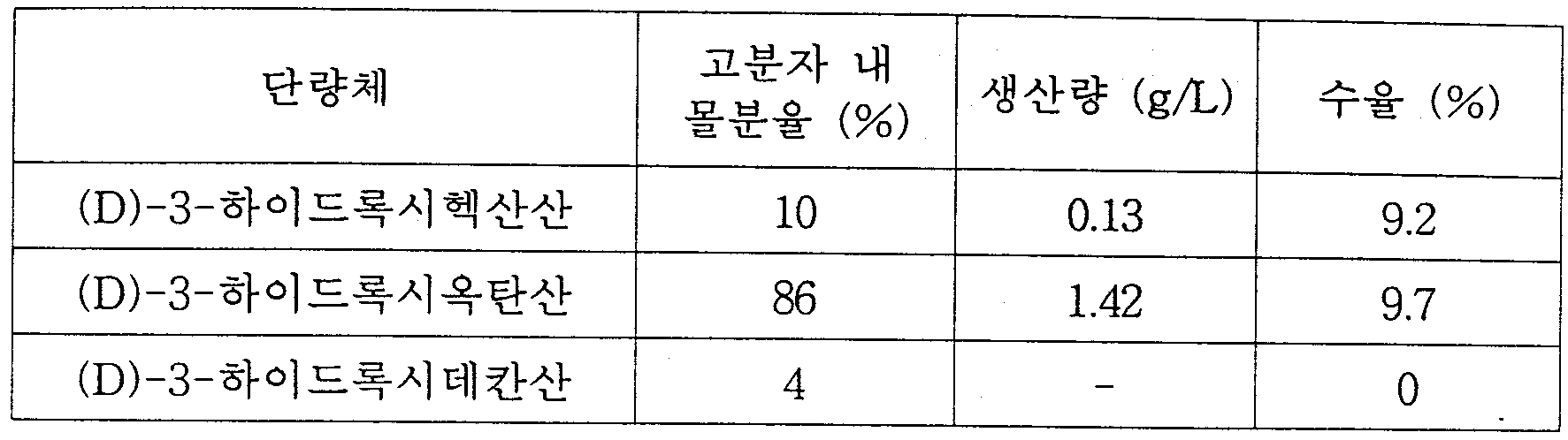
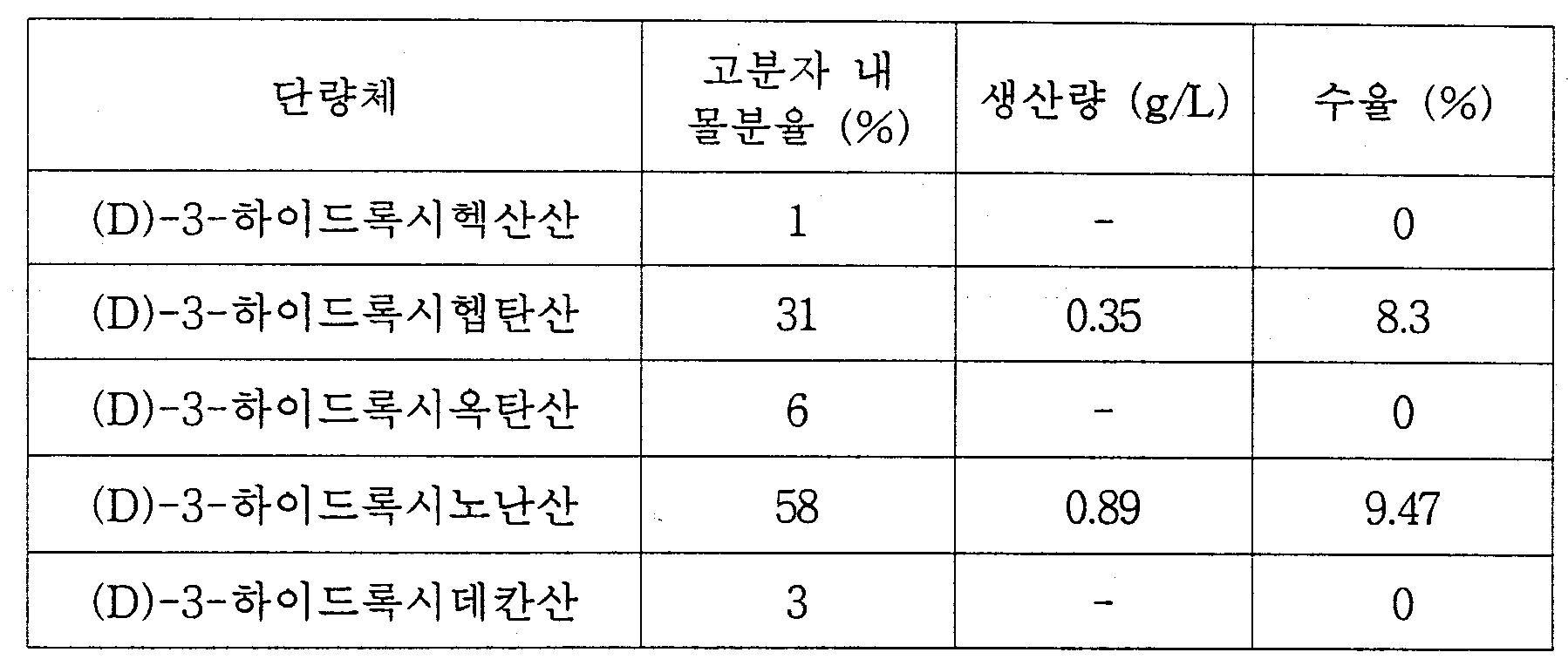
- 제1항에 있어서, 단량체는 3-하이드록시프로피온산(hydroxypropionic acid), 3-하이드록시부탄산(hydroxybutyrate), 3-하이드록시발레르산(hydroxvalerate), 3-하이드록시헥산산(hydroxyhexanoic acid), 3-하이드록시헵탄산(hydroxyheptanoic acid), 3-하이드록시옥탄산(hydroxyoctanoic acid), 3-하이드록시노난산(hydroxynonoic acid), 3-하이드록시데칸산(hydroxydecanoic acid), 3-하이드록시운데칸산(hydroxyundecanoic acid), 3-하이드록시도데칸산(hydroxydodecanoic acid), 3-하이드록시테트라데칸산(hydroxytetradecanoic acid), 3-하이드록시헥사데칸산(hydroxyhexadecanoic acid), 4-하이드록시부탄산(hydroxybutyrate), 4-하이드록시발레르산(hydroxvaleric acid),4-하이드록시헥산산(hydroxyhexanoic acid), 4-하이드록시헵탄산(hydroxyheptanoic acid), 4-하이드록시옥탄산(hydroxyoctanoic acid), 4-하이드록시데칸산(hydroxydecanoic acid), 5-하이드록시발레르산(hydroxvaleric acid), 5-하이드록시헥산산(hydroxyhexanoic acid), 6-하이드록시도데칸산(hydroxydodecanoic acid), 3-하이드록시(hydroxy)-4-펜탄산(pentenoic acid), 3-하이드록시(hydroxy)-4-trans-헥센산(hexenoic acid), 3-하이드록시(hydroxy)-4-cis-헥센산(hexenoic acid), 3-하이드록시(hydroxy)-5-헥센산(hexenoic acid), 3-하이드록시(hydroxy)-6-trans-옥텐산(octenoic acid), 3-하이드록시(hydroxy)-6-cis-옥텐산(octenoic acid), 3-하이드록시(hydroxy)-7-옥텐산(octenoic acid), 3-하이드록시(hydroxy)-8-노넨산(nonenoic acid), 3-하이드록시(hydroxy)-9-데센산(decenoic acid), 3-하이드록시(hydroxy)-5-cis-도데센산(dodecenoic acid), 3-하이드록시(hydroxy)-6-cis-도데센산(dodecenoic acid), 3-하이드록시(hydroxy)-5-cis-테트라데센산(tetradecenoic acid), 3-하이드록시(hydroxy)-7-cis-테트라데센산(tetradecenoic acid), 3-하이드록시(hydroxy)-5,8-cis-cis-테트라데센산(tetradecenoic acid), 3-하이드록시(hydroxy)-4-메틸발레르산(methylvaleric acid), 3-하이드록시(hydroxy)-4-메틸헥산산(methylhexanoic acid), 3-하이드록시(hydroxy)-5-메틸헥산산(methylhexanoic acid), 3-하이드록시(hydroxy)-6-메틸헵산산(methylheptanoic acid), 3-하이드록시(hydroxy)-4-메틸옥탄산(methyloctanoic acid), 3-하이드록시(hydroxy)-5-메틸옥탄산(methyloctanoic acid), 3-하이드록시(hydroxy)-6-메틸옥탄산(methyloctanoic acid), 3-하이드록시(hydroxy)-7-메틸옥탄산(methyloctanoic acid), 3-하이드록시(hydroxy)-6-메틸노난산(methylnonanoic acid), 3-하이드록시(hydroxy)-7-메틸노난산(methylnonanoic acid), 3-하이드록시(hydroxy)-8-메틸노난산(methylnonanoic acid), 3-하이드록시(hydroxy)-7-메틸데칸산(methyldecanoic acid), 3-하이드록시(hydroxy)-9-메틸데칸산(methyldecanoic acid), 3-하이드록시(hydroxy)-7-메틸-6-옥텐산(octenoic acid), 말산(malic acid), 3-하이드록시숙신산(hydroxysuccinic acid)-메틸 에스테르, 3-하이드록시아디핀산(hydroxyadipinic acid)-메틸 에스테르, 3-하이드록시스베린산(hydroxysuberic acid)-메틸 에스테르, 3-하이드록시아젤라인산(hydroxyazelaic acid)-메틸 에스테르, 3-하이드록시세바신산(hydroxysebacic acid)-메틸 에스테르, 3-하이드록시스베린산(hydroxysuberic acid)-에틸 에스테르, -하이드록시세바신산(hydroxysebacic acid)-에틸 에스테르, 3-하이드록시피메린산(hydroxypimelic acid)-프로필 에스테르, 3-하이드록시세바신산(hydroxysebacic acid)-벤질 에스테르, 3-하이드록시(hydroxy)-8-아세톡시옥탄산(acetoxyoctanoic acid), 3-하이드록시(hydroxy)-9-아세톡시노난산(acetoxynonanoic acid), 페녹시(phenoxy)-3-하이드록시부탄산(hydroxybutyric acid), 페녹시(phenoxy)-3-하이드록시발레르산(hydroxyvaleric acid), 페녹시(phenoxy)-3-하이드록시헵탄산(hydroxyheptanoic acid), 페녹시(phenoxy)-3-하이드록시옥탄산(hydroxyoctanoic acid), para-시아노페녹시(cyanophenoxy)-3-하이드록시부탄산(hydroxybutyric acid), para-시아노페녹시(cyanophenoxy)-3-하이드록시발레르산(hydroxyvaleric acid), para-시아노페녹시(cyanophenoxy)-3-하이드록시헥산산(hydroxyhexanoic acid), para-니트로페녹시(nitrophenoxy)-3-하이드록시헥산산(hydroxyhexanoic acid), 3-하이드록시(hydroxy)-5-페닐발레르산(phenylvaleric acid), 3-하이드록시(hydroxy)-5-시클로헥실부탄산(cyclohexylbutyric acid), 3,12-디하이드록시도데칸산(dihydroxydodecanoic acid), 3,8-디하이드록시(dihydroxy)5-cis-테트라데센산(tetradecenoic acid), 3-하이드록시(hydroxy)-4,5-에폭시데칸산(epoxydecanoic acid), 3-하이드록시(hydroxy)-6,7-에폭시도데칸산(epoxydodecanoic acid), 3-하이드록시(hydroxy)-8,9-에폭시(epoxy)-5,6-cis-테트라데칸산(tetradecanoic acid), 7-시아노(cyano)-3-하이드록시헵탄산(hydroxyheptanoic acid), 9-시아노(cyano)-3-하이드록시노난산(hydroxynonanoic acid), 3-하이드록시(hydroxy)-7-플루오로헵탄산(fluoroheptanoic acid), 3-하이드록시(hydroxy)-9-플루오로노난산(fluorononanoic acid), 3-하이드록시(hydroxy)-6-클로로헥산산(chlorohexanoic acid), 3-하이드록시(hydroxy)-8-클로로옥탄산(chlorooctanoic acid), 3-하이드록시(hydroxy)-6-브로모헥산산(bromohexanoic acid), 3-하이드록시(hydroxy)-8-브로모옥탄산(bromooctanoic acid), 3-하이드록시(hydroxy)-11-브로모운데칸산(bromodecanoic acid), 3-하이드록시(hydroxy)-2-부텐산(butenoic acid), 6-하이드록시(hydroxy)-3-도데센산(dodecenoic acid), 3-하이드록시(hydroxy)-2-메틸부탄산(methylbutyric acid), 3-하이드록시(hydroxy)-2-메틸발레르산(methylvaleric acid) 및 3-하이드록시(hydroxy)-2,6-디메틸-5-헵탄산(heptenoic acid)으로 구성된 그룹으로부터 선택되는 1종 이상인 것을 특징으로 하는 (D)-하이드록시카르복실산의 제조방법.
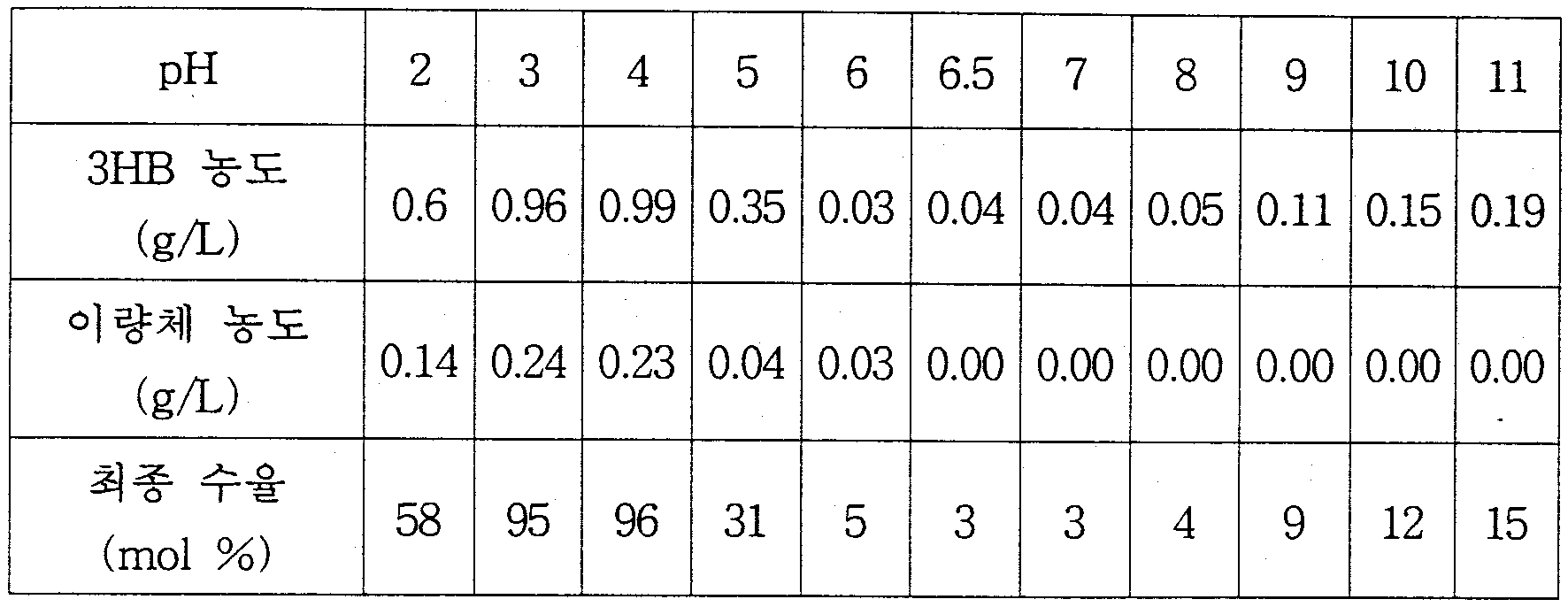
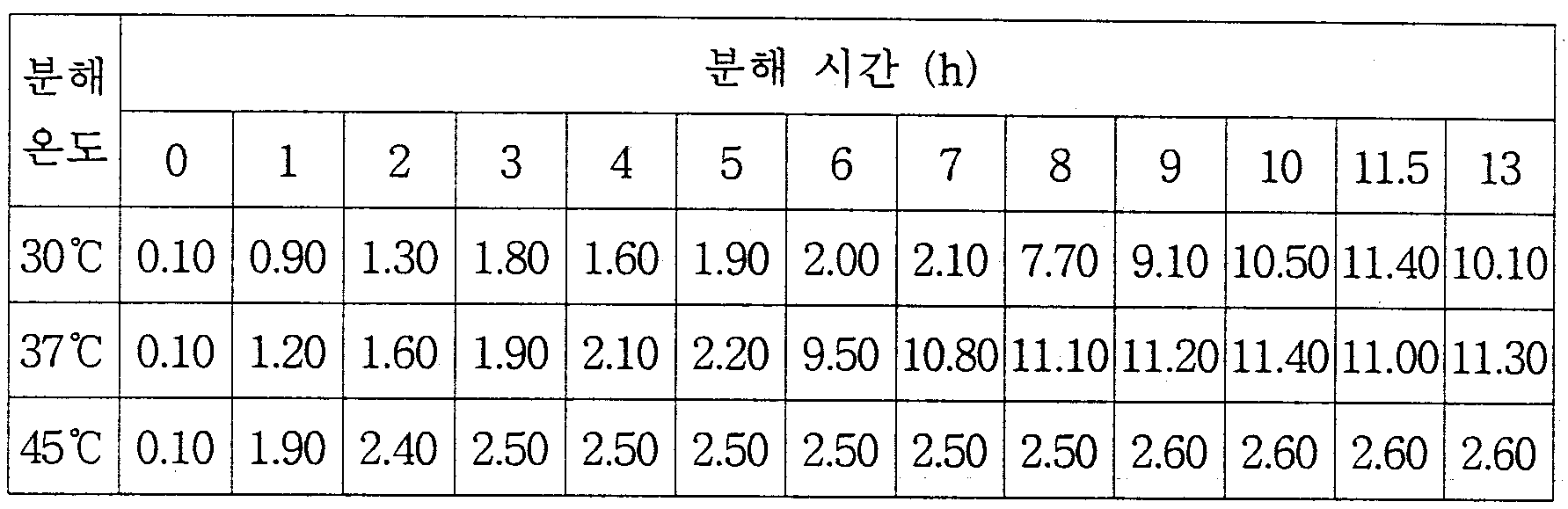
- 미생물 Alcaligenes latus의 배양에 의해 PHB(Poly-3-hydroxybutyrate)를 생산ㆍ축적한 다음, 그 균체를 물, 염 수용액 또는 완충용액의 분해용액(pH 2~11)에서 4~55℃ 온도로 30분~10시간 방치하는 자가분해에 의한 단량체 (D)-하이드록시카르복실산의 제조방법.
- 제11항에 있어서, 미생물 Alcaligenes latus를 pH 2~5의 분해용액에서 37℃ 온도로 반응시키는 것을 특징으로 하는 (D)-하이드록시카르복실산의 제조방법.
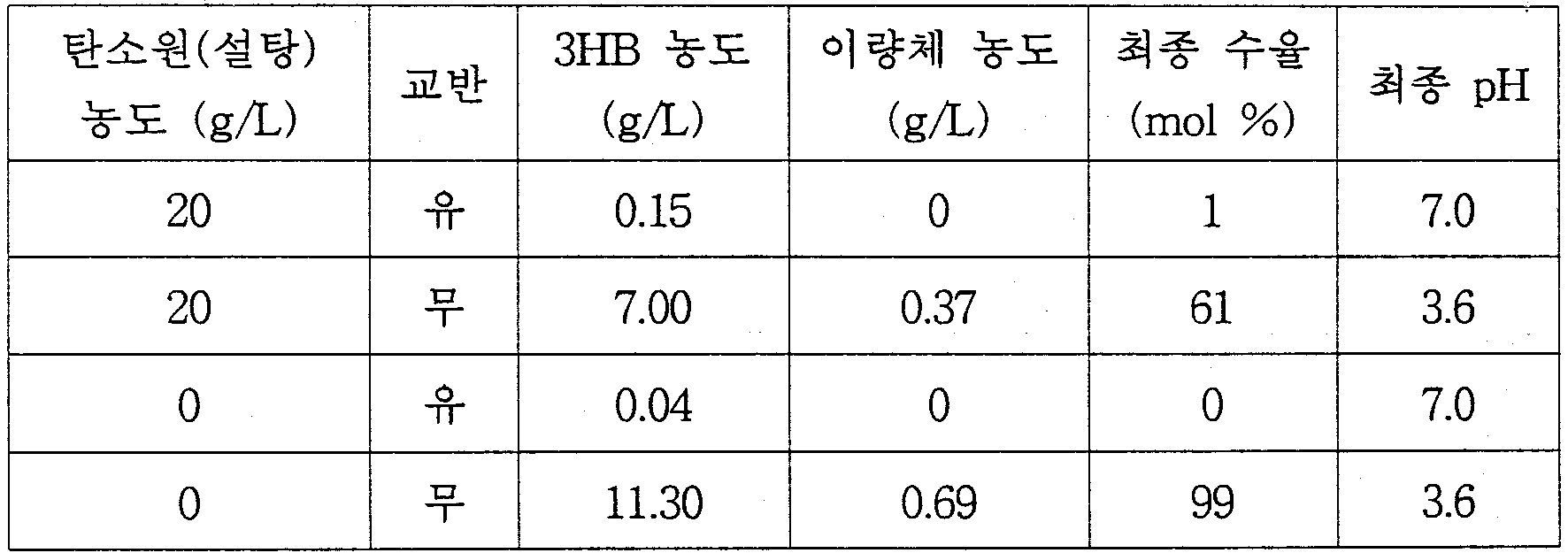
- 미생물 Alcaligenes latus의 배양에 의해 공중합체 폴리-3-하이드록시부탄산-co-4-하이드록시부탄산(poly-3-hydroxybutyrate-co-4-hydroxybutyrate)을 생산ㆍ축적한 다음, 그 균체를 물, 염 수용액 또는 완충용액의 분해용액(pH 7이하)에서 30~40℃ 온도로 30분~10시간 방치하는 자가분해에 의한 (D)-3-하이드록시부탄산 및 4-하이드록시부탄산의 제조방법.
- 제13항에 있어서, 미생물 Alcaligenes latus를 pH 2~5의 분해용액에서 37℃ 온도로 반응시키는 것을 특징으로 하는 (D)-3-하이드록시부탄산 및 4-하이드록시부탄산의 제조방법.
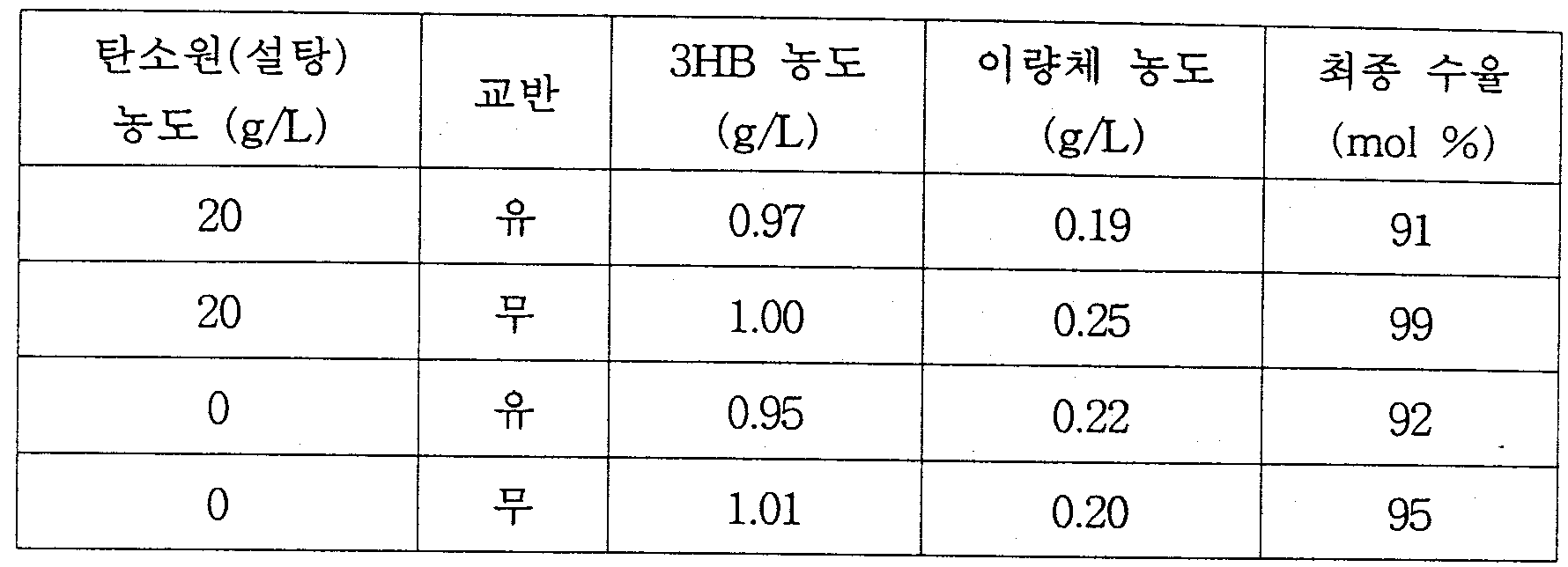
- 미생물 Alcaligenes eutrophus의 배양에 의해 PHB(poly-3-hydroxybutyrate)를 생산ㆍ축적한 다음, 그 균체를 물, 염 수용액 또는 완충용액의 분해용액(pH 2~12)에서 30~40℃ 온도로 20시간 이상 방치하는 자가분해에 의한 (D)-3-하이드록시부탄산의 제조방법.
- 미생물 Alcaligenes eutrophus의 배양에 의해 공중합체 폴리-3-하이드록시부탄산-co-3-하이드록시발레르산(poly-3-hydroxybutyrate-co-4-hydroxyvalerate)을 생산ㆍ축적한 다음, 그 균체를 물, 염 수용액 또는 완충용액의 분해용액(pH 2~12)에서 30~40℃ 온도로 20시간 이상 방치하는 자가분해에 의한 (D)-3-하이드록시부탄산 및 (D)-3-하이드록시발레르산의 제조방법.
- 미생물 Corynebacterium sp.의 배양에 의해 PHB(poly-3-hydroxybutyrate)를 생산ㆍ축적한 다음, 그 균체를 물, 염 수용액 또는 완충용액의 분해용액(pH 5~9)에서 30~40℃ 온도로 2~30시간 이상 방치하는 자가분해에 의한 (D)-3-하이드록시부탄산의 제조방법.
- 미생물 Bacillus sp.의 배양에 의해 PHB(poly-3-hydroxybutyrate)를 생산ㆍ축적한 다음, 그 균체를 물, 염 수용액 또는 완충용액의 분해용액(pH 2~12)에서 30~40℃ 온도로 10시간 이상 방치하는 자가분해에 의한 (D)-3-하이드록시부탄산의 제조방법.
- 혐기성 메탄 자화균 Methylosinus trichosporium의 배양에 의해 PHB(poly-3-hydroxybutyrate)를 생산ㆍ축적한 다음, 그 균체를 물, 염 수용액 또는 완충용액의 분해용액(pH 2~12)에서 30~40℃ 온도로 10시간 이상 방치하는 자가분해에 의한 (D)-3-하이드록시부탄산의 제조방법.
- 혐기성 광합성 세균 Rhodospirillum rubrum의 배양에 의해 PHB(poly-3-hydroxybutyrate)를 생산ㆍ축적한 다음, 그 균체를 물, 염 수용액 또는 완충용액의 분해용액(pH 2~12)에서 30~40℃ 온도로 10시간 이상 방치하는 자가분해에 의한 (D)-3-하이드록시부탄산의 제조방법.
- 과당 이용 균주 Alcaligenes eutrophus H16의 배양에 의해 PHB(poly-3-hydroxybutyrate)를 생산ㆍ축적한 다음, 그 균체를 물, 염 수용액 또는 완충용액의 분해용액(pH 2~12)에서 30~40℃ 온도로 20시간 이상 방치하는 자가분해에 의한 (D)-3-하이드록시부탄산의 제조방법.
- 미생물 Pseudomonas oleovorans 또는 Pseudomonas aeruginosa의 배양에 의해 PHA(polyhydroxyalkanoates)를 생산ㆍ축적한 다음, 그 균체를 물, 염 수용액 또는 완충용액의 분해용액(pH 2~12)에서 30~40℃ 온도로 10시간 이상 방치하는 자가분해에 의한 탄소수 6~14의 (D)-하이드록시카르복실산의 제조방법.
- PHA(polyhydroxyalkanoates) 합성 유전자를 가진 재조합 균주의 배양에 의해 PHA를 생산ㆍ축적한 다음, 그 균체를 물, 염 수용액 또는 완충용액의 분해용액(pH 2~12)에서 30~40℃ 온도로 20시간 이상 방치하는 자가분해에 의한 (D)-하이드록시카르복실산의 제조방법.
- 제23항에 있어서, 재조합 균주는 Alcaligenes eutrophus의 PHA 합성 유전자를 가진 재조합 Alcaligenes eutrophus인 것을 특징으로 하는 (D)-3-하이드록시부탄산의 제조방법.
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1019970066842A KR100250830B1 (ko) | 1997-12-09 | 1997-12-09 | 자가분해에 의해 폴리하이드록시알킨산으로부터 광학활성을 가진 단량체 하이드록시카르복실산의 제조방법 |
| PCT/KR1998/000395 WO1999029889A1 (en) | 1997-12-09 | 1998-12-02 | A method for producing hydroxycarboxylic acids by auto-degradation of polyhydroxyalkanoates |
| EP98959248A EP1036190B1 (en) | 1997-12-09 | 1998-12-02 | A method for producing hydroxycarboxylic acids by auto-degradation of polyhydroxyalkanoates |
| JP2000524460A JP3579352B2 (ja) | 1997-12-09 | 1998-12-02 | 自家分解によるポリヒドロキシアルカノエートからの光学活性をもつ単量体のヒドロキシカルボン酸の製法 |
| CNB988117495A CN1194098C (zh) | 1997-12-09 | 1998-12-02 | 一种通过聚羟基链烷酸酯的自降解生产羟基羧酸的方法 |
| DE69830193T DE69830193T2 (de) | 1997-12-09 | 1998-12-02 | Eine methode zur herstellung von hydroxycarboxylsäuren durch selbstdegradation von polyhydroxyalkanoaten |
| US09/554,948 US6472188B1 (en) | 1997-12-09 | 1998-12-02 | Method for producing hydroxycarboxylic acids by auto-degradation of polyhydroxyalkanoates |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1019970066842A KR100250830B1 (ko) | 1997-12-09 | 1997-12-09 | 자가분해에 의해 폴리하이드록시알킨산으로부터 광학활성을 가진 단량체 하이드록시카르복실산의 제조방법 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR19990048214A KR19990048214A (ko) | 1999-07-05 |
| KR100250830B1 true KR100250830B1 (ko) | 2000-04-01 |
Family
ID=19526756
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1019970066842A Expired - Fee Related KR100250830B1 (ko) | 1997-12-09 | 1997-12-09 | 자가분해에 의해 폴리하이드록시알킨산으로부터 광학활성을 가진 단량체 하이드록시카르복실산의 제조방법 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US6472188B1 (ko) |
| EP (1) | EP1036190B1 (ko) |
| JP (1) | JP3579352B2 (ko) |
| KR (1) | KR100250830B1 (ko) |
| CN (1) | CN1194098C (ko) |
| DE (1) | DE69830193T2 (ko) |
| WO (1) | WO1999029889A1 (ko) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008062996A1 (en) | 2006-11-21 | 2008-05-29 | Lg Chem, Ltd. | Copolymer containing 3-hydroxyalkanoate unit and lactate unit, and its manufacturing method |
| WO2008062995A1 (en) | 2006-11-21 | 2008-05-29 | Lg Chem, Ltd. | Copolymer comprising 4-hydroxybutyrate unit and lactate unit and its manufacturing method |
| US9410167B2 (en) | 2005-05-24 | 2016-08-09 | Lg Chem, Ltd. | Cells or plants producing polylactate or its copolymers and uses thereof |
| WO2021049910A1 (ko) | 2019-09-11 | 2021-03-18 | 주식회사 엘지화학 | 블록 공중합체 제조 방법 |
Families Citing this family (44)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100359171B1 (ko) * | 2000-05-16 | 2002-10-31 | 한국과학기술원 | 폴리하이드록시알칸산 생합성 효소와 세포내폴리하이드록시알칸산 분해효소를 발현시키는 재조합미생물 및 그를 이용한 (r)-3-하이드록시카르복실산의제조방법 |
| US6562603B2 (en) | 2000-08-04 | 2003-05-13 | E. I. Du Pont De Nemours And Company | 3-hydroxycarboxylic acid production and use in branched polymers |
| KR100462543B1 (ko) * | 2000-09-14 | 2004-12-17 | 캐논 가부시끼가이샤 | 폴리하이드록시알카노에이트 및 그 제조방법 |
| KR100447532B1 (ko) * | 2001-11-28 | 2004-09-08 | 한국과학기술원 | (알)-하이드록시카르복실산 생산 재조합 미생물 및 그를이용한 (알)-하이드록시카르복실산의 제조방법 |
| KR100482776B1 (ko) * | 2003-04-23 | 2005-04-14 | 한국과학기술원 | MaoC 단백질을 이용한 폴리히드록시알칸산의 제조방법 |
| US7579176B2 (en) | 2003-10-15 | 2009-08-25 | Newlight Technologies, Llc | Method for the production of polyhydroxyalkanoic acid |
| US8735113B2 (en) | 2003-10-15 | 2014-05-27 | Newlight Technologies, Llc | Methods and systems for production of polyhydroxyalkanoate |
| JP4875494B2 (ja) * | 2003-11-14 | 2012-02-15 | ユーシーエル バイオメディカ ピーエルシー | 免疫調節物質 |
| US7148051B2 (en) | 2004-08-16 | 2006-12-12 | E. I. Du Pont De Nemours And Company | Production of 3-hydroxycarboxylic acid using nitrilase |
| CN101056986B (zh) * | 2004-09-17 | 2012-06-13 | 住友化学株式会社 | 含硫羟基羧酸的制造法 |
| KR100720667B1 (ko) * | 2006-01-04 | 2007-05-21 | 경북대학교 산학협력단 | 슈도모나스 에어루지노사에 의한 트리올레인으로부터하이드록시 지방산의 제조방법 |
| AU2008206403A1 (en) * | 2007-01-12 | 2008-07-24 | The Regents Of The University Of Colorado, A Body Corporate | Compositions and methods for enhancing tolerance for the production of organic chemicals produced by microorganisms |
| WO2008103480A2 (en) * | 2007-02-23 | 2008-08-28 | Massachusetts Institute Of Technology | Conversion of natural products including cellulose to hydrocarbons, hydrogen and/or other related compounds |
| US20100204435A1 (en) * | 2007-09-04 | 2010-08-12 | Keio University | 12-hydroxstearic acid copolymer and method for producing the same |
| US8048624B1 (en) | 2007-12-04 | 2011-11-01 | Opx Biotechnologies, Inc. | Compositions and methods for 3-hydroxypropionate bio-production from biomass |
| JP5396639B2 (ja) | 2008-06-05 | 2014-01-22 | 国立大学法人東京工業大学 | ポリヒドロキシアルカン酸共重合体及びその製造法 |
| DE102008002715A1 (de) * | 2008-06-27 | 2009-12-31 | Evonik Röhm Gmbh | 2-Hydroxyisobuttersäure produzierende rekombinante Zelle |
| US20120165500A1 (en) * | 2009-08-27 | 2012-06-28 | Newlight Technologies, Llc | Process for the production of polyhydroxyalkanoates |
| US8809027B1 (en) | 2009-09-27 | 2014-08-19 | Opx Biotechnologies, Inc. | Genetically modified organisms for increased microbial production of 3-hydroxypropionic acid involving an oxaloacetate alpha-decarboxylase |
| MX2012003604A (es) | 2009-09-27 | 2012-09-12 | Opx Biotechnologies Inc | Metodo para producir acido 3-hidroxipropionico y otros productos. |
| US9040267B2 (en) | 2011-03-08 | 2015-05-26 | Newlight Technologies, Llc | Polyhydroxyalkanoate production method |
| US12060597B2 (en) | 2011-12-02 | 2024-08-13 | Newlight Technologies, Inc. | Polyhydroxyalkanoate production methods and systems for same |
| US20200347417A1 (en) | 2012-03-29 | 2020-11-05 | Newlight Technologies, Inc | Polyhydroxyalkanoate production methods and materials and microorganisms used in same |
| US9085784B1 (en) | 2012-03-29 | 2015-07-21 | Newlight Technologies, Llc | Polyhydroxyalkanoate production methods and materials and microorganisms used in same |
| EP2892870A1 (en) | 2012-05-31 | 2015-07-15 | Micromidas, Inc. | Polyhydroxyalkanoate derivatives, preparation and uses thereof |
| KR20150040359A (ko) | 2012-08-10 | 2015-04-14 | 오피엑스 바이오테크놀로지스, 인크. | 지방산 및 지방산 유도된 산물의 생산을 위한 미생물 및 방법 |
| JP6883384B2 (ja) | 2012-11-05 | 2021-06-09 | ザ ユナイテッド ステイツ オブ アメリカ, アズ リプレゼンテッド バイ ザ セクレタリー, デパートメント オブ ヘルス アンド ヒューマン サービシーズ | 電離放射線による傷害から組織を防護するためのケトン体 |
| US20150057465A1 (en) | 2013-03-15 | 2015-02-26 | Opx Biotechnologies, Inc. | Control of growth-induction-production phases |
| US9512057B2 (en) | 2013-03-15 | 2016-12-06 | Cargill, Incorporated | 3-hydroxypropionic acid compositions |
| US10337038B2 (en) | 2013-07-19 | 2019-07-02 | Cargill, Incorporated | Microorganisms and methods for the production of fatty acids and fatty acid derived products |
| US11408013B2 (en) | 2013-07-19 | 2022-08-09 | Cargill, Incorporated | Microorganisms and methods for the production of fatty acids and fatty acid derived products |
| JP6183817B2 (ja) | 2013-11-12 | 2017-08-23 | 国立研究開発法人産業技術総合研究所 | ハロモナス菌を用いた3−ヒドロキシ酪酸の製造方法 |
| CN103756936B (zh) * | 2014-01-09 | 2015-11-18 | 杭州宝晶生物股份有限公司 | 一种双头菌及其生产l(+)-酒石酸或其盐的方法 |
| CN105199977A (zh) * | 2014-06-25 | 2015-12-30 | 陆祖军 | 一种耐高温产聚羟基丁酸酯细菌的培养基 |
| EP2993228B1 (en) | 2014-09-02 | 2019-10-09 | Cargill, Incorporated | Production of fatty acid esters |
| CN104357496B (zh) * | 2014-10-29 | 2017-06-27 | 中国科学院成都生物研究所 | 一种通过微生物催化乳酸合成己酸的方法 |
| CN105985939B (zh) * | 2015-02-03 | 2019-06-04 | 中国科学院微生物研究所 | 一种聚羟基烷酸颗粒降解酶其用途及(r)-3hb生产方法 |
| JP6478697B2 (ja) * | 2015-02-24 | 2019-03-06 | 国立大学法人東京工業大学 | 高結晶化速度を有する微生物ポリエステル共重合体及びその製造法 |
| US20180237353A1 (en) * | 2015-02-27 | 2018-08-23 | Agrinos AS | Microbial consortia |
| US11345938B2 (en) | 2017-02-02 | 2022-05-31 | Cargill, Incorporated | Genetically modified cells that produce C6-C10 fatty acid derivatives |
| CN110904161A (zh) * | 2019-12-27 | 2020-03-24 | 浙江英玛特生物科技有限公司 | 一种采用酶法生产高纯度(r)-(-)-3-羟基丁酸的方法 |
| EP4363599A1 (en) | 2021-06-28 | 2024-05-08 | PHB Industrial S.A. | Methods to produce therapeutic formulations comprising hydroxybutirate and hydroxyvalerate, therapeutic formulations and uses thereof |
| CN113832070B (zh) * | 2021-10-18 | 2023-06-16 | 北京化工大学 | 一种能利用植物油或淀粉等多种碳源生产聚羟基脂肪酸酯的发光细菌及其应用 |
| CN113736717B (zh) * | 2021-11-03 | 2022-02-11 | 广东省科学院生态环境与土壤研究所 | 一株具有脱氮功能和缺氧抗逆性的甲烷氧化菌及其应用 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2641532B1 (fr) * | 1989-01-06 | 1991-03-29 | Solvay | Procede pour la preparation d'esters de l'acide (beta)-hydroxybutyrique |
| JP2696127B2 (ja) * | 1990-03-22 | 1998-01-14 | 旭化成工業株式会社 | 光学活性な2―ヒドロキシカルボン酸の製造法 |
| JPH0815989A (ja) * | 1994-06-28 | 1996-01-19 | Mita Ind Co Ltd | 画像形成装置における現像ローラ |
| JPH09234091A (ja) * | 1995-12-28 | 1997-09-09 | Akira Shimizu | 発酵法によるR−β−ヒドロキシ酪酸の製造法 |
-
1997
- 1997-12-09 KR KR1019970066842A patent/KR100250830B1/ko not_active Expired - Fee Related
-
1998
- 1998-12-02 JP JP2000524460A patent/JP3579352B2/ja not_active Expired - Fee Related
- 1998-12-02 CN CNB988117495A patent/CN1194098C/zh not_active Expired - Fee Related
- 1998-12-02 EP EP98959248A patent/EP1036190B1/en not_active Expired - Lifetime
- 1998-12-02 WO PCT/KR1998/000395 patent/WO1999029889A1/en active IP Right Grant
- 1998-12-02 US US09/554,948 patent/US6472188B1/en not_active Expired - Fee Related
- 1998-12-02 DE DE69830193T patent/DE69830193T2/de not_active Expired - Fee Related
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9410167B2 (en) | 2005-05-24 | 2016-08-09 | Lg Chem, Ltd. | Cells or plants producing polylactate or its copolymers and uses thereof |
| WO2008062996A1 (en) | 2006-11-21 | 2008-05-29 | Lg Chem, Ltd. | Copolymer containing 3-hydroxyalkanoate unit and lactate unit, and its manufacturing method |
| WO2008062995A1 (en) | 2006-11-21 | 2008-05-29 | Lg Chem, Ltd. | Copolymer comprising 4-hydroxybutyrate unit and lactate unit and its manufacturing method |
| EP4047033A1 (en) | 2006-11-21 | 2022-08-24 | LG Chem, Ltd. | Copolymer comprising 4-hydroxybutyrate unit and lactate unit and its manufacturing method |
| WO2021049910A1 (ko) | 2019-09-11 | 2021-03-18 | 주식회사 엘지화학 | 블록 공중합체 제조 방법 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1036190A1 (en) | 2000-09-20 |
| JP2002518992A (ja) | 2002-07-02 |
| WO1999029889A1 (en) | 1999-06-17 |
| CN1194098C (zh) | 2005-03-23 |
| CN1301310A (zh) | 2001-06-27 |
| KR19990048214A (ko) | 1999-07-05 |
| EP1036190B1 (en) | 2005-05-11 |
| DE69830193D1 (de) | 2005-06-16 |
| US6472188B1 (en) | 2002-10-29 |
| JP3579352B2 (ja) | 2004-10-20 |
| DE69830193T2 (de) | 2006-01-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR100250830B1 (ko) | 자가분해에 의해 폴리하이드록시알킨산으로부터 광학활성을 가진 단량체 하이드록시카르복실산의 제조방법 | |
| Lee et al. | Recent advances in polyhydroxyalkanoate production by bacterial fermentation: mini-review | |
| JP2642937B2 (ja) | 酵素によるポリエステルの製造方法,光学活性カルボン酸およびエステルの製造方法,およびポリエステルを含む製品 | |
| US6770464B2 (en) | Methods for producing poly(hydroxy) fatty acids in bacteria | |
| Lee et al. | Chiral compounds from bacterial polyesters: sugars to plastics to fine chemicals | |
| KR100979694B1 (ko) | 폴리락테이트 또는 그 공중합체 생성능을 가지는 세포 또는식물 및 이를 이용한 폴리락테이트 또는 그 공중합체의제조방법 | |
| Marangoni et al. | Production of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by Ralstonia eutropha in whey and inverted sugar with propionic acid feeding | |
| Ueda et al. | Synthesis of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) from methanol and n-amyl alcohol by the methylotrophic bacteria Paracoccus denitrificans and Methylobacterium extorquens | |
| Sudesh et al. | Effect of increased PHA synthase activity on polyhydroxyalkanoates biosynthesis in Synechocystis sp. PCC6803 | |
| Durner et al. | Accumulation of poly [(R)‐3‐hydroxyalkanoates] in Pseudomonas oleovorans during growth in batch and chemostat culture with different carbon sources | |
| Diniz et al. | High-cell-density cultivation of Pseudomonas putida IPT 046 and medium-chain-length polyhydroxyalkanoate production from sugarcane carbohydrates | |
| US6562603B2 (en) | 3-hydroxycarboxylic acid production and use in branched polymers | |
| Hori et al. | Simultaneous production of polyhydroxyalkanoates and rhamnolipids by Pseudomonas aeruginosa | |
| CN101300354A (zh) | 具有改良的聚羟基链烷酸产生能力的去调节的细菌 | |
| JPH06172501A (ja) | ポリβ−ヒドロキシ酪酸エステル共重合体 | |
| Cui et al. | Improved productivity of poly (3-hydroxybutyrate)(PHB) in thermophilic Chelatococcus daeguensis TAD1 using glycerol as the growth substrate in a fed-batch culture | |
| Martin et al. | High-titer production of monomeric hydroxyvalerates from levulinic acid in Pseudomonas putida | |
| KR100359171B1 (ko) | 폴리하이드록시알칸산 생합성 효소와 세포내폴리하이드록시알칸산 분해효소를 발현시키는 재조합미생물 및 그를 이용한 (r)-3-하이드록시카르복실산의제조방법 | |
| Kato et al. | Production of 3-hydroxybutyric acid trimer by Bacillus megaterium B-124 | |
| Park et al. | Biosynthesis of (R)-3-hydroxyalkanoic acids by metabolically engineered Escherichia coli | |
| KR101076043B1 (ko) | 폴리하이드록시알카노에이트 생성능을 가지는 미생물을 이용한 하이드록시알카노에이트 알킬에스테르의 제조방법 | |
| Chen et al. | A process for simultaneously achieving phenol biodegradation and polyhydroxybutyrate accumulation using Cupriavidus taiwanesis 187 | |
| Li et al. | Efficient production of (R)-3-hydroxybutyric acid by Pseudomonas sp. DS1001a and its extracellular poly (3-hydroxybutyrate) depolymerase | |
| JP2003052368A (ja) | ポリヒドロキシアルカン酸とラムノリピッドの同時生産法 | |
| WO2008113190A1 (en) | Method for the production of r-hydroxycarboxylic acids |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| PA0109 | Patent application |
Patent event code: PA01091R01D Comment text: Patent Application Patent event date: 19971209 |
|
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 19971209 Comment text: Request for Examination of Application |
|
| N231 | Notification of change of applicant | ||
| PN2301 | Change of applicant |
Patent event date: 19981102 Comment text: Notification of Change of Applicant Patent event code: PN23011R01D |
|
| PG1501 | Laying open of application | ||
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
Patent event code: PE07011S01D Comment text: Decision to Grant Registration Patent event date: 19991023 |
|
| GRNT | Written decision to grant | ||
| PR0701 | Registration of establishment |
Comment text: Registration of Establishment Patent event date: 20000107 Patent event code: PR07011E01D |
|
| PR1002 | Payment of registration fee |
Payment date: 20000107 End annual number: 3 Start annual number: 1 |
|
| PG1601 | Publication of registration | ||
| PR1001 | Payment of annual fee |
Payment date: 20030107 Start annual number: 4 End annual number: 4 |
|
| PR1001 | Payment of annual fee |
Payment date: 20040108 Start annual number: 5 End annual number: 5 |
|
| PR1001 | Payment of annual fee |
Payment date: 20050707 Start annual number: 6 End annual number: 6 |
|
| PR1001 | Payment of annual fee |
Payment date: 20051230 Start annual number: 7 End annual number: 7 |
|
| PR1001 | Payment of annual fee |
Payment date: 20070105 Start annual number: 8 End annual number: 8 |
|
| FPAY | Annual fee payment |
Payment date: 20080104 Year of fee payment: 9 |
|
| PR1001 | Payment of annual fee |
Payment date: 20080104 Start annual number: 9 End annual number: 9 |
|
| LAPS | Lapse due to unpaid annual fee | ||
| PC1903 | Unpaid annual fee |
Termination category: Default of registration fee Termination date: 20091210 |