JP5562956B2 - ユニット型陰茎勃起システム及び拡張器 - Google Patents
ユニット型陰茎勃起システム及び拡張器 Download PDFInfo
- Publication number
- JP5562956B2 JP5562956B2 JP2011522060A JP2011522060A JP5562956B2 JP 5562956 B2 JP5562956 B2 JP 5562956B2 JP 2011522060 A JP2011522060 A JP 2011522060A JP 2011522060 A JP2011522060 A JP 2011522060A JP 5562956 B2 JP5562956 B2 JP 5562956B2
- Authority
- JP
- Japan
- Prior art keywords
- penile prosthesis
- implantable penile
- unit
- prosthesis
- implantable
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 230000018052 penile erection Effects 0.000 title 1
- 239000012530 fluid Substances 0.000 claims description 38
- 210000003899 penis Anatomy 0.000 claims description 19
- 238000012546 transfer Methods 0.000 claims description 18
- 210000000711 cavernous sinus Anatomy 0.000 claims description 15
- 239000003638 chemical reducing agent Substances 0.000 claims description 8
- 210000003708 urethra Anatomy 0.000 claims description 3
- 230000006378 damage Effects 0.000 claims description 2
- 238000006073 displacement reaction Methods 0.000 claims description 2
- 230000027939 micturition Effects 0.000 claims 1
- 239000007943 implant Substances 0.000 description 18
- 239000004606 Fillers/Extenders Substances 0.000 description 13
- 230000006835 compression Effects 0.000 description 9
- 238000007906 compression Methods 0.000 description 9
- 210000001519 tissue Anatomy 0.000 description 6
- 210000000056 organ Anatomy 0.000 description 5
- 210000005070 sphincter Anatomy 0.000 description 5
- 238000004140 cleaning Methods 0.000 description 4
- 210000003195 fascia Anatomy 0.000 description 4
- 208000010228 Erectile Dysfunction Diseases 0.000 description 3
- 241000269799 Perca fluviatilis Species 0.000 description 3
- 230000008602 contraction Effects 0.000 description 3
- 238000013461 design Methods 0.000 description 3
- 238000002513 implantation Methods 0.000 description 3
- 201000001881 impotence Diseases 0.000 description 3
- 208000015181 infectious disease Diseases 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- 210000001015 abdomen Anatomy 0.000 description 2
- 238000013270 controlled release Methods 0.000 description 2
- 210000005226 corpus cavernosum Anatomy 0.000 description 2
- 230000004927 fusion Effects 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 210000004706 scrotum Anatomy 0.000 description 2
- 208000032843 Hemorrhage Diseases 0.000 description 1
- 206010020565 Hyperaemia Diseases 0.000 description 1
- 208000008081 Intestinal Fistula Diseases 0.000 description 1
- 206010000196 Urinary abnormalities Diseases 0.000 description 1
- 210000000683 abdominal cavity Anatomy 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 238000004873 anchoring Methods 0.000 description 1
- 208000034158 bleeding Diseases 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 230000009849 deactivation Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000011038 discontinuous diafiltration by volume reduction Methods 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 208000003243 intestinal obstruction Diseases 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 210000001699 lower leg Anatomy 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 238000000034 method Methods 0.000 description 1
- 210000004197 pelvis Anatomy 0.000 description 1
- 210000004303 peritoneum Anatomy 0.000 description 1
- 230000003252 repetitive effect Effects 0.000 description 1
- 239000000837 restrainer Substances 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 239000012945 sealing adhesive Substances 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 229920002379 silicone rubber Polymers 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/26—Penis implants
Landscapes
- Health & Medical Sciences (AREA)
- Reproductive Health (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Prostheses (AREA)
Description
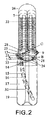
本発明を説明する目的のため、現在の好ましい1つの形態が添付図面に図示されている。本発明は、図示した正確な配置及び実施例に限定することを意図するものではないことが理解される。
Claims (7)
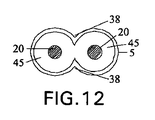
- 液圧作動式の伸長可能な、海綿体洞用の構成要素と、ポンプ及び弁手段を有する手動制御式の流体移送システムと、を含む、ユニット式植え込み型の陰茎補綴具にして、前記伸長可能な構成要素は、伸長可能な内側ユニットを備え、前記伸長可能な内側ユニットは、十分な量の流体が前記伸長可能な内側ユニット内に移送されて該内側ユニットを実質的に満たすとき、弛緩した状態から剛性な状態に変換可能である、前記植込み型の陰茎補綴具において、前記伸長可能な内側ユニットは、該内側ユニットの長さの少なくとも主要な部分に沿って互いに接続された1対の平行な管状通路によって形成されており、該1対の平行な管状通路は、実質的に8の字の形状の断面を有する単一の拡張可能な内部管腔を形成し、前記内側ユニット内に1対の円筒状の形状の容積減少器を更に含む、植込み型陰茎補綴具。
- 請求項1に記載の植込み型陰茎補綴具において、前記1対の管状通路の少なくとも一部分は、アコーディオン状の形態にて伸びる壁により画定されている、植込み型陰茎補綴具。
- 請求項2に記載の植込み型陰茎補綴具において、前記壁は、可撓性の領域を形成する該壁の外周に沿って多数の溝を有している、植込み型陰茎補綴具。
- 請求項1に記載の植込み型陰茎補綴具において、前記伸長可能な構成要素を取り囲む、外側の伸長可能なシースを更に含む、植込み型陰茎補綴具。
- 請求項1に記載の植込み型陰茎補綴具において、前記伸長可能な構成要素は、その外面上に尿道用溝を有し、それによって尿道及び尿道海綿体が邪魔されずに配置されることを許容し、該補綴具が植え込まれた後、排尿又は射精の障害とならないようにした、植込み型陰茎補綴具。
- 請求項1に記載の植込み型陰茎補綴具において、前記手動制御式の流体移送システムは、前記伸長可能な構成要素と接続されたケーシング内に保持されている、植込み型陰茎補綴具。
- 請求項6に記載の植込み型陰茎補綴具において、前記ケーシングは、その外面に形成された尿道用溝を含み、補綴具が植え込まれた後、該尿道用溝の存在により、尿道構造体の偏位、閉塞又は損傷が防止されるようにした、植込み型陰茎補綴具。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/221,962 | 2008-08-08 | ||
| US12/221,962 US7922649B2 (en) | 2008-08-08 | 2008-08-08 | Unitized penile erection system and tissue expander |
| PCT/US2009/004496 WO2010016905A1 (en) | 2008-08-08 | 2009-08-04 | Unitized penile erection system and tissue expander |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2011530325A JP2011530325A (ja) | 2011-12-22 |
| JP5562956B2 true JP5562956B2 (ja) | 2014-07-30 |
Family
ID=41653554
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011522060A Expired - Fee Related JP5562956B2 (ja) | 2008-08-08 | 2009-08-04 | ユニット型陰茎勃起システム及び拡張器 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US7922649B2 (ja) |
| EP (1) | EP2320838A4 (ja) |
| JP (1) | JP5562956B2 (ja) |
| CA (1) | CA2733264C (ja) |
| WO (1) | WO2010016905A1 (ja) |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8241203B2 (en) | 2010-02-12 | 2012-08-14 | Fogarty Terence M | Inflatable penile prosthesis with spool valve |
| US9827102B2 (en) * | 2011-12-21 | 2017-11-28 | Boston Scientific Scimed, Inc. | Penile prosthesis |
| WO2014152968A1 (en) * | 2013-03-14 | 2014-09-25 | The Board Of Regents Of The University Of Texas System | Implantable medical apparatus and systems |
| WO2015093681A1 (ko) * | 2013-12-16 | 2015-06-25 | 이에스에이바이오 (주) | 발기 보조 장치 |
| KR101422100B1 (ko) | 2013-12-16 | 2014-07-23 | (주)이에스에이바이오 | 발기 보조 장치 |
| FR3036611B1 (fr) * | 2015-05-29 | 2017-09-29 | Bernard Andre Noel Costini | Dispositif implantable destine a augmenter la longueur et le diametre de la verge |
| TWI744242B (zh) | 2015-07-31 | 2021-11-01 | 德商安美基研究(慕尼黑)公司 | Egfrviii及cd3抗體構築體 |
| CN106952549B (zh) * | 2017-04-27 | 2023-04-07 | 黄伟 | 仿生男性生殖器 |
| GB2563258B (en) * | 2017-06-08 | 2021-10-13 | Lovehoney Ltd | Self-erecting penis |
| US11324595B2 (en) | 2018-02-06 | 2022-05-10 | Boston Scientific Scimed, Inc. | Inflatable penile prosthesis with a structured cylinder |
| US10413413B1 (en) * | 2018-07-23 | 2019-09-17 | Augmenta, LLC | Penile implants that facilitate tissue expansion |
| US11266563B2 (en) * | 2018-10-15 | 2022-03-08 | Boston Scientific Scimed, Inc. | Extendable penile implant |
| US20230129844A1 (en) | 2020-03-19 | 2023-04-27 | Amgen Inc. | Diagnostic antibodies against mucin 17 and uses thereof |
| CN111956280A (zh) * | 2020-08-25 | 2020-11-20 | 鲍玉梅 | 一种医用外科软组织扩张器 |
| US20230320831A1 (en) * | 2022-03-30 | 2023-10-12 | Boston Scientific Scimed, Inc. | Implantable inflatable device |
Family Cites Families (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3991752A (en) * | 1975-12-04 | 1976-11-16 | Dow Corning Corporation | Penile implant |
| DE2740263A1 (de) * | 1977-09-07 | 1979-03-15 | Max Bernhard Ulrich | Penis-stuetzimplantat |
| US4267829A (en) | 1979-04-11 | 1981-05-19 | American Medical Systems, Inc. | Penile prosthesis |
| US4318396A (en) * | 1980-05-15 | 1982-03-09 | Medical Engineering Corporation | Penile prosthesis |
| US4353360A (en) | 1980-10-31 | 1982-10-12 | Medical Engineering Corporation | Penile erectile system |
| US4369771A (en) | 1981-09-24 | 1983-01-25 | Medical Engineering Corporation | Penile erectile system |
| US4791917A (en) * | 1981-10-22 | 1988-12-20 | Medical Engineering Corporation | Penile prosthesis |
| US4665903A (en) | 1981-12-31 | 1987-05-19 | Whitehead Edgar D | Penile prosthetic device |
| US4549530A (en) * | 1983-08-29 | 1985-10-29 | Medical Engineering Corporation | Male urinary incontinence device and method |
| US4572168A (en) | 1983-12-20 | 1986-02-25 | Fischell Robert | Fully implantable vapor pressure actuated penile erection device and method |
| DE3529613A1 (de) * | 1984-08-20 | 1986-02-27 | Robert Ellentuch Silver Spring Fischell, Md. | Versteifungszylinder fuer ein aufblasbares peniserektionsgeraet |
| US4594997A (en) | 1984-11-13 | 1986-06-17 | Hakky Said I | Self actuated penile implant |
| US4807608A (en) * | 1987-07-22 | 1989-02-28 | American Medical Systems | Mechanical penile prosthesis |
| US4895139A (en) * | 1988-08-29 | 1990-01-23 | American Medical Systems, Inc. | Inflatable penile prosthesis with bend release valve |
| DE9001508U1 (de) | 1990-02-09 | 1990-04-12 | ESKA Kunststofftechnik GmbH & Co. vormals Walter Koss, 6222 Geisenheim | Implantierbare Penisprothese |
| US5067485A (en) | 1990-05-14 | 1991-11-26 | Mentor Corporation | Corpus cavernosum implant device |
| US5167611A (en) * | 1991-07-22 | 1992-12-01 | Mentor Corporation | Penile implant with lengthening cylinder |
| US5250020A (en) | 1991-09-12 | 1993-10-05 | Mentor Corporation | Unitary inflatable penile prosthesis |
| JPH07184940A (ja) * | 1993-11-05 | 1995-07-25 | G Patrick Maxwell | テクスチャー表面を有する人工装具 |
| US5468213A (en) * | 1994-09-22 | 1995-11-21 | American Medical Systems, Inc. | Mechanical penile prosthesis |
| FR2727855B1 (fr) | 1994-12-08 | 1997-10-24 | Subrini Louis | Implants d'extension caverneuse |
| US5669870A (en) | 1996-06-13 | 1997-09-23 | Elist; James J. | Penile implant for improved appearance |
| US5899849A (en) * | 1997-09-22 | 1999-05-04 | Elist; James | Subcutaneous penile implant |
| US7390296B2 (en) * | 2003-06-06 | 2008-06-24 | Ams Research Corporation | Inflatable penile prosthesis with volume displacement materials and devices |
| US8052593B2 (en) * | 2006-10-24 | 2011-11-08 | Ams Research Corporation | Implantable malleable penile prosthetic device |
-
2008
- 2008-08-08 US US12/221,962 patent/US7922649B2/en not_active Expired - Fee Related
-
2009
- 2009-08-04 CA CA2733264A patent/CA2733264C/en not_active Expired - Fee Related
- 2009-08-04 JP JP2011522060A patent/JP5562956B2/ja not_active Expired - Fee Related
- 2009-08-04 WO PCT/US2009/004496 patent/WO2010016905A1/en active Application Filing
- 2009-08-04 EP EP09805271.5A patent/EP2320838A4/en not_active Withdrawn
Also Published As
| Publication number | Publication date |
|---|---|
| US7922649B2 (en) | 2011-04-12 |
| WO2010016905A1 (en) | 2010-02-11 |
| EP2320838A1 (en) | 2011-05-18 |
| US20100036196A1 (en) | 2010-02-11 |
| CA2733264A1 (en) | 2010-02-11 |
| CA2733264C (en) | 2012-03-13 |
| EP2320838A4 (en) | 2018-01-03 |
| JP2011530325A (ja) | 2011-12-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5562956B2 (ja) | ユニット型陰茎勃起システム及び拡張器 | |
| US10426584B2 (en) | Method of treating urinary incontinence by implanting a reservoir around a urethra of the user | |
| US20210393406A1 (en) | Multiple pump system for inflatable penile prosthesis | |
| US4386601A (en) | Artificial sphincter | |
| US4417567A (en) | Artificial sphincter | |
| JPS6160701B2 (ja) | ||
| EP0358380A1 (en) | Inflatable penile prothesis with bend release valve | |
| CN101917928A (zh) | 部分套囊 | |
| JP2019505344A (ja) | 陰茎補綴物 | |
| US10226318B2 (en) | Method of treating urinary incontinence by implanting a tube providing a pressure-regulating storage compartment | |
| US8376930B2 (en) | Implantable pump for erectile dysfunction treatment | |
| DK2566414T3 (en) | BLOWER PROTECTION FOR SUBCUTAN IMPLANTATION | |
| AU2020298335B2 (en) | Penile implant for neophallus | |
| US12208010B2 (en) | Temporary space-filling penile implant for corporal healing and neophallus conditioning | |
| Montague | Prosthetic treatment of male sexual dysfunction |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20120724 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130719 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20131018 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20131025 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140117 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20140513 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20140611 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5562956 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |