JP5430803B2 - Peptides and angiotensin converting enzyme inhibitors - Google Patents
Peptides and angiotensin converting enzyme inhibitors Download PDFInfo
- Publication number
- JP5430803B2 JP5430803B2 JP2013536124A JP2013536124A JP5430803B2 JP 5430803 B2 JP5430803 B2 JP 5430803B2 JP 2013536124 A JP2013536124 A JP 2013536124A JP 2013536124 A JP2013536124 A JP 2013536124A JP 5430803 B2 JP5430803 B2 JP 5430803B2
- Authority
- JP
- Japan
- Prior art keywords
- peptide
- converting enzyme
- angiotensin converting
- blood pressure
- present
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 108090000765 processed proteins & peptides Proteins 0.000 title claims description 85
- 239000005541 ACE inhibitor Substances 0.000 title claims description 20
- 229940044094 angiotensin-converting-enzyme inhibitor Drugs 0.000 title claims description 20
- 102000004196 processed proteins & peptides Human genes 0.000 title description 19
- 230000036772 blood pressure Effects 0.000 claims description 29
- 101710129690 Angiotensin-converting enzyme inhibitor Proteins 0.000 claims description 16
- 101710086378 Bradykinin-potentiating and C-type natriuretic peptides Proteins 0.000 claims description 16
- 150000001413 amino acids Chemical class 0.000 claims description 10
- 239000004480 active ingredient Substances 0.000 claims description 9
- 238000000034 method Methods 0.000 claims description 8
- 229940030600 antihypertensive agent Drugs 0.000 claims description 2
- 239000002220 antihypertensive agent Substances 0.000 claims description 2
- UQDJGEHQDNVPGU-UHFFFAOYSA-N serine phosphoethanolamine Chemical compound [NH3+]CCOP([O-])(=O)OCC([NH3+])C([O-])=O UQDJGEHQDNVPGU-UHFFFAOYSA-N 0.000 claims description 2
- 125000003275 alpha amino acid group Chemical group 0.000 claims 1
- UUUHXMGGBIUAPW-UHFFFAOYSA-N 1-[1-[2-[[5-amino-2-[[1-[5-(diaminomethylideneamino)-2-[[1-[3-(1h-indol-3-yl)-2-[(5-oxopyrrolidine-2-carbonyl)amino]propanoyl]pyrrolidine-2-carbonyl]amino]pentanoyl]pyrrolidine-2-carbonyl]amino]-5-oxopentanoyl]amino]-3-methylpentanoyl]pyrrolidine-2-carbon Chemical compound C1CCC(C(=O)N2C(CCC2)C(O)=O)N1C(=O)C(C(C)CC)NC(=O)C(CCC(N)=O)NC(=O)C1CCCN1C(=O)C(CCCN=C(N)N)NC(=O)C1CCCN1C(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C1CCC(=O)N1 UUUHXMGGBIUAPW-UHFFFAOYSA-N 0.000 description 25
- 102000004270 Peptidyl-Dipeptidase A Human genes 0.000 description 25
- 108090000882 Peptidyl-Dipeptidase A Proteins 0.000 description 25
- 239000003814 drug Substances 0.000 description 18
- 235000013305 food Nutrition 0.000 description 18
- 230000002401 inhibitory effect Effects 0.000 description 18
- 239000000203 mixture Substances 0.000 description 14
- 239000000843 powder Substances 0.000 description 10
- 239000000047 product Substances 0.000 description 10
- 102000004190 Enzymes Human genes 0.000 description 9
- 108090000790 Enzymes Proteins 0.000 description 9
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 9
- 239000003795 chemical substances by application Substances 0.000 description 9
- 229940079593 drug Drugs 0.000 description 9
- 235000009508 confectionery Nutrition 0.000 description 8
- 230000000694 effects Effects 0.000 description 8
- 239000003480 eluent Substances 0.000 description 7
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 6
- 229940024606 amino acid Drugs 0.000 description 6
- 235000001014 amino acid Nutrition 0.000 description 6
- 235000013361 beverage Nutrition 0.000 description 6
- 201000010099 disease Diseases 0.000 description 6
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 6
- 238000003786 synthesis reaction Methods 0.000 description 6
- 206010020772 Hypertension Diseases 0.000 description 5
- 241001465754 Metazoa Species 0.000 description 5
- 241000700159 Rattus Species 0.000 description 5
- 238000002835 absorbance Methods 0.000 description 5
- 238000004128 high performance liquid chromatography Methods 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 4
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 4
- 240000007594 Oryza sativa Species 0.000 description 4
- 235000007164 Oryza sativa Nutrition 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 240000008042 Zea mays Species 0.000 description 4
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 4
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 4
- 230000004531 blood pressure lowering effect Effects 0.000 description 4
- 235000005822 corn Nutrition 0.000 description 4
- 235000013611 frozen food Nutrition 0.000 description 4
- 239000008187 granular material Substances 0.000 description 4
- 244000144972 livestock Species 0.000 description 4
- 235000016709 nutrition Nutrition 0.000 description 4
- 235000009566 rice Nutrition 0.000 description 4
- 239000002904 solvent Substances 0.000 description 4
- 239000003826 tablet Substances 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- 241000251468 Actinopterygii Species 0.000 description 3
- 206010002383 Angina Pectoris Diseases 0.000 description 3
- 102400000345 Angiotensin-2 Human genes 0.000 description 3
- 101800000733 Angiotensin-2 Proteins 0.000 description 3
- 239000004278 EU approved seasoning Substances 0.000 description 3
- 208000007530 Essential hypertension Diseases 0.000 description 3
- 108010010803 Gelatin Proteins 0.000 description 3
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 3
- CZGUSIXMZVURDU-JZXHSEFVSA-N Ile(5)-angiotensin II Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC=CC=1)C([O-])=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@@H]([NH3+])CC([O-])=O)C(C)C)C1=CC=C(O)C=C1 CZGUSIXMZVURDU-JZXHSEFVSA-N 0.000 description 3
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 3
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 3
- 229950006323 angiotensin ii Drugs 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 229910000019 calcium carbonate Inorganic materials 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- 229920002678 cellulose Polymers 0.000 description 3
- 239000001913 cellulose Substances 0.000 description 3
- 235000010980 cellulose Nutrition 0.000 description 3
- 235000013339 cereals Nutrition 0.000 description 3
- 235000013399 edible fruits Nutrition 0.000 description 3
- 235000011194 food seasoning agent Nutrition 0.000 description 3
- 235000013350 formula milk Nutrition 0.000 description 3
- 235000015203 fruit juice Nutrition 0.000 description 3
- 239000008273 gelatin Substances 0.000 description 3
- 229920000159 gelatin Polymers 0.000 description 3
- 235000019322 gelatine Nutrition 0.000 description 3
- 235000011852 gelatine desserts Nutrition 0.000 description 3
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 3
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 235000012054 meals Nutrition 0.000 description 3
- 235000012149 noodles Nutrition 0.000 description 3
- 239000008194 pharmaceutical composition Substances 0.000 description 3
- 235000018102 proteins Nutrition 0.000 description 3
- 102000004169 proteins and genes Human genes 0.000 description 3
- 108090000623 proteins and genes Proteins 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- -1 renibase Chemical compound 0.000 description 3
- 235000014347 soups Nutrition 0.000 description 3
- 239000006188 syrup Substances 0.000 description 3
- 235000020357 syrup Nutrition 0.000 description 3
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 2
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 2
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 2
- 101800004538 Bradykinin Proteins 0.000 description 2
- 102400000967 Bradykinin Human genes 0.000 description 2
- 206010007572 Cardiac hypertrophy Diseases 0.000 description 2
- 208000006029 Cardiomegaly Diseases 0.000 description 2
- 239000001856 Ethyl cellulose Substances 0.000 description 2
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- QXZGBUJJYSLZLT-UHFFFAOYSA-N H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH Natural products NC(N)=NCCCC(N)C(=O)N1CCCC1C(=O)N1C(C(=O)NCC(=O)NC(CC=2C=CC=CC=2)C(=O)NC(CO)C(=O)N2C(CCC2)C(=O)NC(CC=2C=CC=CC=2)C(=O)NC(CCCN=C(N)N)C(O)=O)CCC1 QXZGBUJJYSLZLT-UHFFFAOYSA-N 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 2
- 125000000174 L-prolyl group Chemical group [H]N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C(*)=O 0.000 description 2
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 2
- 244000294411 Mirabilis expansa Species 0.000 description 2
- 235000015429 Mirabilis expansa Nutrition 0.000 description 2
- 102000015636 Oligopeptides Human genes 0.000 description 2
- 108010038807 Oligopeptides Proteins 0.000 description 2
- 235000010627 Phaseolus vulgaris Nutrition 0.000 description 2
- 244000046052 Phaseolus vulgaris Species 0.000 description 2
- 108020004511 Recombinant DNA Proteins 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- QXZGBUJJYSLZLT-FDISYFBBSA-N bradykinin Chemical compound NC(=N)NCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(=O)NCC(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CO)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)CCC1 QXZGBUJJYSLZLT-FDISYFBBSA-N 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 235000010216 calcium carbonate Nutrition 0.000 description 2
- 239000001506 calcium phosphate Substances 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 208000026106 cerebrovascular disease Diseases 0.000 description 2
- 239000003086 colorant Substances 0.000 description 2
- 239000002537 cosmetic Substances 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 239000007884 disintegrant Substances 0.000 description 2
- 239000012153 distilled water Substances 0.000 description 2
- 235000019325 ethyl cellulose Nutrition 0.000 description 2
- 229920001249 ethyl cellulose Polymers 0.000 description 2
- 239000003925 fat Substances 0.000 description 2
- 235000019197 fats Nutrition 0.000 description 2
- 239000000796 flavoring agent Substances 0.000 description 2
- 235000013312 flour Nutrition 0.000 description 2
- 235000013355 food flavoring agent Nutrition 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 235000013376 functional food Nutrition 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 239000007791 liquid phase Substances 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 235000013336 milk Nutrition 0.000 description 2
- 239000008267 milk Substances 0.000 description 2
- 210000004080 milk Anatomy 0.000 description 2
- 235000013536 miso Nutrition 0.000 description 2
- 230000035764 nutrition Effects 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 235000019198 oils Nutrition 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 2
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 2
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 2
- 230000003449 preventive effect Effects 0.000 description 2
- 238000004007 reversed phase HPLC Methods 0.000 description 2
- 235000015067 sauces Nutrition 0.000 description 2
- 235000013580 sausages Nutrition 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 235000013322 soy milk Nutrition 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 230000035488 systolic blood pressure Effects 0.000 description 2
- 235000013616 tea Nutrition 0.000 description 2
- 238000010998 test method Methods 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 2
- 235000015112 vegetable and seed oil Nutrition 0.000 description 2
- 239000008158 vegetable oil Substances 0.000 description 2
- 239000008215 water for injection Substances 0.000 description 2
- MJYQFWSXKFLTAY-OVEQLNGDSA-N (2r,3r)-2,3-bis[(4-hydroxy-3-methoxyphenyl)methyl]butane-1,4-diol;(2r,3r,4s,5s,6r)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O.C1=C(O)C(OC)=CC(C[C@@H](CO)[C@H](CO)CC=2C=C(OC)C(O)=CC=2)=C1 MJYQFWSXKFLTAY-OVEQLNGDSA-N 0.000 description 1
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 1
- CBPJQFCAFFNICX-IBGZPJMESA-N (2s)-2-(9h-fluoren-9-ylmethoxycarbonylamino)-4-methylpentanoic acid Chemical compound C1=CC=C2C(COC(=O)N[C@@H](CC(C)C)C(O)=O)C3=CC=CC=C3C2=C1 CBPJQFCAFFNICX-IBGZPJMESA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- MIDXCONKKJTLDX-UHFFFAOYSA-N 3,5-dimethylcyclopentane-1,2-dione Chemical compound CC1CC(C)C(=O)C1=O MIDXCONKKJTLDX-UHFFFAOYSA-N 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- WLDHEUZGFKACJH-ZRUFZDNISA-K Amaranth Chemical compound [Na+].[Na+].[Na+].C12=CC=C(S([O-])(=O)=O)C=C2C=C(S([O-])(=O)=O)C(O)=C1\N=N\C1=CC=C(S([O-])(=O)=O)C2=CC=CC=C12 WLDHEUZGFKACJH-ZRUFZDNISA-K 0.000 description 1
- 102400000344 Angiotensin-1 Human genes 0.000 description 1
- 101800000734 Angiotensin-1 Proteins 0.000 description 1
- 102000004881 Angiotensinogen Human genes 0.000 description 1
- 108090001067 Angiotensinogen Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- SGHZXLIDFTYFHQ-UHFFFAOYSA-L Brilliant Blue Chemical compound [Na+].[Na+].C=1C=C(C(=C2C=CC(C=C2)=[N+](CC)CC=2C=C(C=CC=2)S([O-])(=O)=O)C=2C(=CC=CC=2)S([O-])(=O)=O)C=CC=1N(CC)CC1=CC=CC(S([O-])(=O)=O)=C1 SGHZXLIDFTYFHQ-UHFFFAOYSA-L 0.000 description 1
- 125000001433 C-terminal amino-acid group Chemical group 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 206010008111 Cerebral haemorrhage Diseases 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 1
- 235000019733 Fish meal Nutrition 0.000 description 1
- 241001071795 Gentiana Species 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 108010068370 Glutens Proteins 0.000 description 1
- 240000004670 Glycyrrhiza echinata Species 0.000 description 1
- 235000001453 Glycyrrhiza echinata Nutrition 0.000 description 1
- 235000006200 Glycyrrhiza glabra Nutrition 0.000 description 1
- 235000017382 Glycyrrhiza lepidota Nutrition 0.000 description 1
- 240000005979 Hordeum vulgare Species 0.000 description 1
- 235000007340 Hordeum vulgare Nutrition 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-N L-arginine Chemical compound OC(=O)[C@@H](N)CCCN=C(N)N ODKSFYDXXFIFQN-BYPYZUCNSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- 125000002059 L-arginyl group Chemical group O=C([*])[C@](N([H])[H])([H])C([H])([H])C([H])([H])C([H])([H])N([H])C(=N[H])N([H])[H] 0.000 description 1
- 125000002061 L-isoleucyl group Chemical group [H]N([H])[C@]([H])(C(=O)[*])[C@](C([H])([H])[H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 1
- 125000003440 L-leucyl group Chemical group O=C([*])[C@](N([H])[H])([H])C([H])([H])C(C([H])([H])[H])([H])C([H])([H])[H] 0.000 description 1
- 125000001176 L-lysyl group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C([H])([H])C([H])([H])C([H])([H])C(N([H])[H])([H])[H] 0.000 description 1
- 125000002842 L-seryl group Chemical group O=C([*])[C@](N([H])[H])([H])C([H])([H])O[H] 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 108010007859 Lisinopril Proteins 0.000 description 1
- 235000007688 Lycopersicon esculentum Nutrition 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- IRVONVRHHJXWTK-RWMBFGLXSA-N Met-Lys-Pro Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)O)N IRVONVRHHJXWTK-RWMBFGLXSA-N 0.000 description 1
- 208000001145 Metabolic Syndrome Diseases 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 1
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- ILZZTQYNTRWQSJ-UHFFFAOYSA-N Phe-Phe-Val-Ala-Pro-Phe-Pro-Glu-Val-Phe-Gly-Lys Natural products CC(C)C(NC(=O)C(Cc1ccccc1)NC(=O)C(N)Cc2ccccc2)C(=O)NC(C)C(=O)N3CCCC3C(=O)NC(Cc4ccccc4)C(=O)N5CCCC5C(=O)NC(CCC(=O)O)C(=O)NC(C(C)C)C(=O)NC(Cc6ccccc6)C(=O)NCC(=O)NC(CCCCN)C(=O)O ILZZTQYNTRWQSJ-UHFFFAOYSA-N 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- 235000019484 Rapeseed oil Nutrition 0.000 description 1
- 208000001647 Renal Insufficiency Diseases 0.000 description 1
- 102100028255 Renin Human genes 0.000 description 1
- 108090000783 Renin Proteins 0.000 description 1
- 241000209056 Secale Species 0.000 description 1
- 235000007238 Secale cereale Nutrition 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 229920001800 Shellac Polymers 0.000 description 1
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 1
- 240000003768 Solanum lycopersicum Species 0.000 description 1
- 240000006394 Sorghum bicolor Species 0.000 description 1
- 235000011684 Sorghum saccharatum Nutrition 0.000 description 1
- 244000269722 Thea sinensis Species 0.000 description 1
- 235000009430 Thespesia populnea Nutrition 0.000 description 1
- 241000006364 Torula Species 0.000 description 1
- 235000021307 Triticum Nutrition 0.000 description 1
- 244000098338 Triticum aestivum Species 0.000 description 1
- 239000005862 Whey Substances 0.000 description 1
- 102000007544 Whey Proteins Human genes 0.000 description 1
- 108010046377 Whey Proteins Proteins 0.000 description 1
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- GAMPNQJDUFQVQO-UHFFFAOYSA-N acetic acid;phthalic acid Chemical compound CC(O)=O.OC(=O)C1=CC=CC=C1C(O)=O GAMPNQJDUFQVQO-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 238000005377 adsorption chromatography Methods 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 235000013334 alcoholic beverage Nutrition 0.000 description 1
- 150000003862 amino acid derivatives Chemical class 0.000 description 1
- ORWYRWWVDCYOMK-HBZPZAIKSA-N angiotensin I Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C1=CC=C(O)C=C1 ORWYRWWVDCYOMK-HBZPZAIKSA-N 0.000 description 1
- 230000003276 anti-hypertensive effect Effects 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 235000009697 arginine Nutrition 0.000 description 1
- 229960003121 arginine Drugs 0.000 description 1
- 235000008452 baby food Nutrition 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 235000015895 biscuits Nutrition 0.000 description 1
- 235000008429 bread Nutrition 0.000 description 1
- 235000012813 breadcrumbs Nutrition 0.000 description 1
- 239000006189 buccal tablet Substances 0.000 description 1
- 235000014121 butter Nutrition 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 235000012970 cakes Nutrition 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 235000011132 calcium sulphate Nutrition 0.000 description 1
- 235000013574 canned fruits Nutrition 0.000 description 1
- 235000014613 canned/preserved soup Nutrition 0.000 description 1
- 229960000830 captopril Drugs 0.000 description 1
- FAKRSMQSSFJEIM-RQJHMYQMSA-N captopril Chemical compound SC[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O FAKRSMQSSFJEIM-RQJHMYQMSA-N 0.000 description 1
- 235000013736 caramel Nutrition 0.000 description 1
- 235000014171 carbonated beverage Nutrition 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 229940105329 carboxymethylcellulose Drugs 0.000 description 1
- 229940084030 carboxymethylcellulose calcium Drugs 0.000 description 1
- 239000005018 casein Substances 0.000 description 1
- BECPQYXYKAMYBN-UHFFFAOYSA-N casein, tech. Chemical compound NCCCCC(C(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(CC(C)C)N=C(O)C(CCC(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(C(C)O)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(COP(O)(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(N)CC1=CC=CC=C1 BECPQYXYKAMYBN-UHFFFAOYSA-N 0.000 description 1
- 235000021240 caseins Nutrition 0.000 description 1
- 210000004027 cell Anatomy 0.000 description 1
- 206010008118 cerebral infarction Diseases 0.000 description 1
- 235000013351 cheese Nutrition 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 229940112822 chewing gum Drugs 0.000 description 1
- 235000015218 chewing gum Nutrition 0.000 description 1
- 235000019219 chocolate Nutrition 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 235000019864 coconut oil Nutrition 0.000 description 1
- 239000003240 coconut oil Substances 0.000 description 1
- 235000013353 coffee beverage Nutrition 0.000 description 1
- 235000021557 concentrated beverage Nutrition 0.000 description 1
- 238000010411 cooking Methods 0.000 description 1
- 235000014510 cooky Nutrition 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 235000012495 crackers Nutrition 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 235000021438 curry Nutrition 0.000 description 1
- 235000013365 dairy product Nutrition 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 230000000994 depressogenic effect Effects 0.000 description 1
- 235000021185 dessert Nutrition 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 239000008298 dragée Substances 0.000 description 1
- 235000015071 dressings Nutrition 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 239000002662 enteric coated tablet Substances 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 239000004467 fishmeal Substances 0.000 description 1
- 235000004426 flaxseed Nutrition 0.000 description 1
- 238000001641 gel filtration chromatography Methods 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 235000021312 gluten Nutrition 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 239000004519 grease Substances 0.000 description 1
- 235000013402 health food Nutrition 0.000 description 1
- 208000019622 heart disease Diseases 0.000 description 1
- 239000008172 hydrogenated vegetable oil Substances 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 1
- 229920003132 hydroxypropyl methylcellulose phthalate Polymers 0.000 description 1
- 229940031704 hydroxypropyl methylcellulose phthalate Drugs 0.000 description 1
- 230000001077 hypotensive effect Effects 0.000 description 1
- 235000015243 ice cream Nutrition 0.000 description 1
- 230000000415 inactivating effect Effects 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 235000008446 instant noodles Nutrition 0.000 description 1
- 235000014109 instant soup Nutrition 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 208000017169 kidney disease Diseases 0.000 description 1
- 201000006370 kidney failure Diseases 0.000 description 1
- 229940124280 l-arginine Drugs 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 229960003136 leucine Drugs 0.000 description 1
- 229940010454 licorice Drugs 0.000 description 1
- 235000021388 linseed oil Nutrition 0.000 description 1
- 239000000944 linseed oil Substances 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 235000021056 liquid food Nutrition 0.000 description 1
- 229960002394 lisinopril Drugs 0.000 description 1
- RLAWWYSOJDYHDC-BZSNNMDCSA-N lisinopril Chemical compound C([C@H](N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(O)=O)C(O)=O)CC1=CC=CC=C1 RLAWWYSOJDYHDC-BZSNNMDCSA-N 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 235000018977 lysine Nutrition 0.000 description 1
- 229960003511 macrogol Drugs 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 235000013310 margarine Nutrition 0.000 description 1
- 239000003264 margarine Substances 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 108010022588 methionyl-lysyl-proline Proteins 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 235000020124 milk-based beverage Nutrition 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 235000016046 other dairy product Nutrition 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 238000004810 partition chromatography Methods 0.000 description 1
- 239000006072 paste Substances 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 238000010647 peptide synthesis reaction Methods 0.000 description 1
- 235000021110 pickles Nutrition 0.000 description 1
- 235000015108 pies Nutrition 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 1
- 229920001289 polyvinyl ether Polymers 0.000 description 1
- 229920001592 potato starch Polymers 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 235000013324 preserved food Nutrition 0.000 description 1
- 229960002429 proline Drugs 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 238000004366 reverse phase liquid chromatography Methods 0.000 description 1
- 238000005185 salting out Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 229960001153 serine Drugs 0.000 description 1
- 239000004208 shellac Substances 0.000 description 1
- 229940113147 shellac Drugs 0.000 description 1
- 235000013874 shellac Nutrition 0.000 description 1
- ZLGIYFNHBLSMPS-ATJNOEHPSA-N shellac Chemical compound OCCCCCC(O)C(O)CCCCCCCC(O)=O.C1C23[C@H](C(O)=O)CCC2[C@](C)(CO)[C@@H]1C(C(O)=O)=C[C@@H]3O ZLGIYFNHBLSMPS-ATJNOEHPSA-N 0.000 description 1
- 235000020183 skimmed milk Nutrition 0.000 description 1
- 235000011888 snacks Nutrition 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000007901 soft capsule Substances 0.000 description 1
- 235000014214 soft drink Nutrition 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000010532 solid phase synthesis reaction Methods 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 235000010356 sorbitol Nutrition 0.000 description 1
- 235000013555 soy sauce Nutrition 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 235000013599 spices Nutrition 0.000 description 1
- 235000013547 stew Nutrition 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 239000007940 sugar coated tablet Substances 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 239000003760 tallow Substances 0.000 description 1
- 239000013076 target substance Substances 0.000 description 1
- 229940078499 tricalcium phosphate Drugs 0.000 description 1
- 229910000391 tricalcium phosphate Inorganic materials 0.000 description 1
- 235000019731 tricalcium phosphate Nutrition 0.000 description 1
- 239000013598 vector Substances 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 239000000052 vinegar Substances 0.000 description 1
- 235000021419 vinegar Nutrition 0.000 description 1
- 235000013618 yogurt Nutrition 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
- C07K7/04—Linear peptides containing only normal peptide links
- C07K7/06—Linear peptides containing only normal peptide links having 5 to 11 amino acids
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/17—Amino acids, peptides or proteins
- A23L33/18—Peptides; Protein hydrolysates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Molecular Biology (AREA)
- Pharmacology & Pharmacy (AREA)
- General Chemical & Material Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Public Health (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Mycology (AREA)
- Nutrition Science (AREA)
- Food Science & Technology (AREA)
- Polymers & Plastics (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Coloring Foods And Improving Nutritive Qualities (AREA)
Description
本発明は、新規なペプチド及び同ペプチドを含有するアンジオテンシン変換酵素阻害剤に関する。アンジオテンシン変換酵素阻害剤は、食品、飼料、及び医薬として利用することができる。 The present invention relates to a novel peptide and an angiotensin converting enzyme inhibitor containing the peptide. An angiotensin converting enzyme inhibitor can be used as food, feed, and medicine.
アンジオテンシン変換酵素(ACE)は、レニンによる切断によりアンジオテンシノーゲンから生じるアンジオテンシンIに働き、C末端の2個のアミノ酸を遊離させて、アンジオテンシンIIに変換する酵素である。アンジオテンシン変換酵素は強い昇圧作用を有するアンジオテンシンIIを生成させるとともに、降圧作用を有するブラジキニンを不活性化する作用も有している。このような作用から、アンジオテンシン変換酵素阻害剤は、高血圧の治療薬として使用されており、カプトプリル、レニベース、リシノプリルなどが市販薬として知られている。また、アンジオテンシン変換酵素阻害剤は、心疾患、腎疾患、脳血管障害、動脈硬化性疾患、糖尿病、メタボリックシンドローム、高齢者高血圧など多くの病態に対して第一選択薬として積極的な使用が勧められている(高血圧治療ガイドライン2009)。 Angiotensin converting enzyme (ACE) is an enzyme that acts on angiotensin I generated from angiotensinogen by cleavage with renin, liberates two C-terminal amino acids, and converts them into angiotensin II. Angiotensin converting enzyme generates angiotensin II having a strong pressor action and also has an action of inactivating bradykinin having a hypotensive action. Because of these actions, angiotensin converting enzyme inhibitors are used as therapeutic agents for hypertension, and captopril, renibase, lisinopril and the like are known as commercially available drugs. In addition, angiotensin converting enzyme inhibitors are recommended to be actively used as first-line drugs for many pathologies such as heart disease, kidney disease, cerebrovascular disorder, arteriosclerotic disease, diabetes, metabolic syndrome, and elderly hypertension. (High Blood Pressure Treatment Guidelines 2009).
一方、アンジオテンシン変換酵素阻害作用を有するペプチドが天然物中から見出されている。これらは天然型のアミノ酸から構成されているため栄養源として経口摂取は勿論のこと、血圧低下作用を示す以外には、重い副作用を示すことはなく安全性が高いため、血圧降下作用を有する特定保健用食品として利用されている。例えば、カゼインを酵素により分解して得たペプチド類(特許文献1〜11、非特許文献1)がその例である。また、その他にもアンジオテンシン変換酵素阻害作用を有する天然型のペプチドが数多く知られている(非特許文献2)。
On the other hand, peptides having an angiotensin converting enzyme inhibitory action have been found in natural products. Since these are composed of natural amino acids, they can be taken orally as nutritional sources, as well as exhibiting blood pressure lowering effects. It is used as a health food. For example, peptides (
上記のように、アンジオテンシン変換酵素阻害作用を有する種々のペプチドが知られているが、これらのペプチド類では、未だ食品中の機能として、アンジオテンシン変換酵素阻害活性は不十分である。よって、天然物型で、一層高いアンジオテンシン変換酵素阻害活性を有するペプチドの取得と、その食品又は医薬等への応用が望まれているところである。
本発明は、アンジオテンシン変換酵素阻害作用を有する新規ペプチド、及びそれを含むアンジオテンシン変換酵素阻害剤を提供することを課題とする。As described above, various peptides having an angiotensin converting enzyme inhibitory action are known, but these peptides still have insufficient angiotensin converting enzyme inhibitory activity as a function in food. Therefore, it is desired to obtain a natural product-type peptide having higher angiotensin converting enzyme inhibitory activity and to apply it to food or medicine.
An object of the present invention is to provide a novel peptide having an angiotensin converting enzyme inhibitory action and an angiotensin converting enzyme inhibitor containing the same.
本発明者らは、前記課題を解決するために鋭意検討を行った。すなわち、リジンをC端側に固定して5種類のアミノ酸(アルギニン、ロイシン、イソロイシン、プロリン、セリン)をランダムにペプチド結合させたヘキサペプチドを合成することにより、その混合物中に高いアンジオテンシン変換酵素阻害活性を有する新規なペプチドが存在し、さらに同ペプチドが特定の配列を有することを見出し、本発明を完成するに至った。 The present inventors have intensively studied to solve the above problems. That is, by synthesizing a hexapeptide in which lysine is fixed on the C-terminal side and 5 types of amino acids (arginine, leucine, isoleucine, proline, serine) are randomly bound to the peptide, high angiotensin converting enzyme inhibition is observed in the mixture. It has been found that there is a novel peptide having activity, and that the peptide has a specific sequence, and the present invention has been completed.
本発明は、下記のアミノ酸配列からなるペプチドを提供する。
Xaa−Arg−Xaa−Pro−Ser−Lys(配列番号1)
(但し、各アミノ酸はL−体であり、Xaaは、独立してIle又はLeuである。)
本発明のペプチドは、以下のアミノ酸配列のいずれかを有することを好ましい形態としている。
a)Ile−Arg−Ile−Pro−Ser−Lys(配列番号2)
b)Ile−Arg−Leu−Pro−Ser−Lys(配列番号3)
c)Leu−Arg−Leu−Pro−Ser−Lys(配列番号4)
d)Leu−Arg−Ile−Pro−Ser−Lys(配列番号5)
本発明はまた、前記ペプチドを有効成分として含有するアンジオテンシン変換酵素阻害剤を提供する。
また本発明は、前記ペプチドを有効成分として含有する血圧降下剤を提供する。
また本発明は、血圧降下を必要とする対象に、前記ペプチドを投与することを含む、前記対象の血圧を降下させる方法を提供する。
また本発明は、血圧降下を必要とする対象の血圧を降下させるための、前記ペプチドの使用を提供する。The present invention provides a peptide consisting of the following amino acid sequence.
Xaa-Arg-Xaa-Pro-Ser-Lys (SEQ ID NO: 1)
(However, each amino acid is L-form, and Xaa is independently Ile or Leu.)
The peptide of the present invention preferably has any of the following amino acid sequences.
a) Ile-Arg-Ile-Pro-Ser-Lys (SEQ ID NO: 2)
b) Ile-Arg-Leu-Pro-Ser-Lys (SEQ ID NO: 3)
c) Leu-Arg-Leu-Pro-Ser-Lys (SEQ ID NO: 4)
d) Leu-Arg-Ile-Pro-Ser-Lys (SEQ ID NO: 5)
The present invention also provides an angiotensin converting enzyme inhibitor containing the peptide as an active ingredient.
The present invention also provides a blood pressure lowering agent containing the peptide as an active ingredient.
The present invention also provides a method for lowering blood pressure in a subject comprising administering the peptide to a subject in need of blood pressure reduction.
The present invention also provides use of the peptide for lowering blood pressure in a subject in need of blood pressure reduction.
次に、本発明の好ましい実施形態について詳細に説明する。ただし、本発明は以下の好ましい実施形態に限定されず、本発明の範囲内で自由に変更することができるものである。尚、本明細書において百分率は特に断りのない限り質量による表示である。
本発明のペプチドは、Xaa−Arg−Xaa−Pro−Ser−Lys(配列番号1)で表される配列を有する。また、本発明のペプチドは、このペプチドの塩類であってもよい。本発明において、LeuはL−ロイシン残基、ArgはL−アルギニン残基、IleはL−イソロイシン残基、ProはL−プロリン残基、SerはL−セリン残基、LysはL−リジン残基を示す。また、Xaaは、独立してIle又はLeuである。Next, a preferred embodiment of the present invention will be described in detail. However, the present invention is not limited to the following preferred embodiments, and can be freely changed within the scope of the present invention. In the present specification, percentages are expressed by mass unless otherwise specified.
The peptide of the present invention has a sequence represented by Xaa-Arg-Xaa-Pro-Ser-Lys (SEQ ID NO: 1). The peptide of the present invention may be a salt of this peptide. In the present invention, Leu is an L-leucine residue, Arg is an L-arginine residue, Ile is an L-isoleucine residue, Pro is an L-proline residue, Ser is an L-serine residue, and Lys is an L-lysine residue. Indicates a group. Xaa is independently Ile or Leu.
すなわち、本発明のペプチドは以下のアミノ酸配列のいずれかを有する。
a)Ile−Arg−Ile−Pro−Ser−Lys(配列番号2)
b)Ile−Arg−Leu−Pro−Ser−Lys(配列番号3)
c)Leu−Arg−Leu−Pro−Ser−Lys(配列番号4)
d)Leu−Arg−Ile−Pro−Ser−Lys(配列番号5)That is, the peptide of the present invention has any of the following amino acid sequences.
a) Ile-Arg-Ile-Pro-Ser-Lys (SEQ ID NO: 2)
b) Ile-Arg-Leu-Pro-Ser-Lys (SEQ ID NO: 3)
c) Leu-Arg-Leu-Pro-Ser-Lys (SEQ ID NO: 4)
d) Leu-Arg-Ile-Pro-Ser-Lys (SEQ ID NO: 5)
本発明のペプチドは、化学合成によって製造することができる。本発明のペプチドの化学合成は、オリゴペプチドの合成に通常用いられている液相法または固相法によって行うことができる。合成されたペプチドは、必要に応じて脱保護され、未反応試薬、副生物等を除去する。このようなペプチドの合成は、市販のペプチド合成装置を用いて行うことができる。上記合成(混合)物から、好ましくは本発明のペプチドを単離、精製する。ペプチドの精製は、通常、オリゴペプチドの精製に用いられているのと同様の手法、例えばイオン交換クロマトグラフィー、吸着クロマトグラフィー、逆相クロマトグラフィー、分配クロマトグラフィー、ゲル濾過クロマトグラフィー等の各種クロマトグラフィー、溶媒沈殿、塩析、2種の液相間での分配等の方法を適宜組み合わせることによって、行うことができる。本発明のペプチドの精製に際しては、目的物質を含む画分は、後述するアンジオテンシン変換酵素阻害作用を指標として決定することができ、それらの画分の活性成分は質量分析法又は/およびプロテインシーケンサーにより同定することができる。 The peptide of the present invention can be produced by chemical synthesis. The chemical synthesis of the peptide of the present invention can be carried out by a liquid phase method or a solid phase method usually used for the synthesis of oligopeptides. The synthesized peptide is deprotected as necessary to remove unreacted reagents, by-products and the like. Such peptide synthesis can be performed using a commercially available peptide synthesizer. The peptide of the present invention is preferably isolated and purified from the above synthesized (mixed) product. Peptide purification is usually carried out in the same manner as that used for oligopeptide purification, such as ion exchange chromatography, adsorption chromatography, reverse phase chromatography, partition chromatography, gel filtration chromatography and other various chromatography methods. , Solvent precipitation, salting out, and partitioning between the two liquid phases, and the like. In the purification of the peptide of the present invention, the fraction containing the target substance can be determined using the angiotensin converting enzyme inhibitory action described below as an index, and the active component of these fractions can be determined by mass spectrometry or / and a protein sequencer. Can be identified.
また、本発明のペプチドは、同ペプチドをコードする組換えDNAを適当な宿主細胞で発現させることによっても、製造することができる。組換えDNAの作成に必要なベクター、及び宿主は、通常タンパク質やペプチドの製造に用いられているものを使用することができる。 The peptide of the present invention can also be produced by expressing a recombinant DNA encoding the peptide in an appropriate host cell. As vectors and hosts necessary for the production of recombinant DNA, those usually used for the production of proteins and peptides can be used.
本発明のペプチドは、アンジオテンシン変換酵素阻害剤の有効成分として使用することができる。本発明のペプチドは、アンジオテンシン変換酵素阻害作用を有している。アンジオテンシン変換酵素が阻害されると、同酵素によるアンジオテンシンIIの生成、及び、ブラジキニンの不活性化が抑制されるため、結果として血圧降下作用を示す。したがって、本発明のペプチド又はアンジオテンシン変換酵素阻害剤は、高血圧に由来する種々の疾患、例えば脳出血、脳梗塞、狭心症、心筋梗塞、腎不全等に対する予防剤又は治療剤、具体的には血圧降下剤等として使用することができる。すなわち、本発明の一形態は、本発明のペプチドを有効成分として含有する血圧降下剤である。また、アンジオテンシン変換酵素阻害剤は、原因不明の本態性高血圧にも効果があることが知られており、本発明のペプチドも本態性高血圧に対する治療又は予防効果を示すことが期待される。その他、アンジオテンシン変換酵素阻害剤が有効であることが知られている心肥大、狭心病等の疾患に対しても、治療、予防薬として使用することができる。
本発明の他の形態は、血圧降下を必要とする対象に本発明のペプチドを投与することを含む、前記対象の血圧を降下させる方法である。
また、本発明の他の形態は、血圧降下を必要とする対象の血圧を降下させるための本発明のペプチドの使用である。
血圧降下を必要とする対象としては、アンジオテンシン変換酵素を阻害することが有効である限り特に制限されないが、上記の高血圧に由来する種々の疾患を有する患者、本態性高血圧の患者、及び、心肥大、狭心病等の疾患を有する患者が挙げられる。また、これらの方法及び使用において、「血圧の降下」は「疾患の治療」であり得る。The peptide of the present invention can be used as an active ingredient of an angiotensin converting enzyme inhibitor. The peptide of the present invention has an angiotensin converting enzyme inhibitory action. When an angiotensin converting enzyme is inhibited, the production of angiotensin II and the inactivation of bradykinin by the enzyme are suppressed, resulting in a blood pressure lowering effect. Therefore, the peptide or angiotensin converting enzyme inhibitor of the present invention is a preventive or therapeutic agent for various diseases derived from hypertension such as cerebral hemorrhage, cerebral infarction, angina pectoris, myocardial infarction, renal failure, etc., specifically blood pressure. It can be used as a depressant. That is, one form of the present invention is a blood pressure lowering agent containing the peptide of the present invention as an active ingredient. Moreover, an angiotensin converting enzyme inhibitor is known to have an effect on essential hypertension of unknown cause, and the peptide of the present invention is expected to show a therapeutic or preventive effect on essential hypertension. In addition, it can also be used as a therapeutic or prophylactic agent for diseases such as cardiac hypertrophy and angina which are known to be effective for angiotensin converting enzyme inhibitors.
Another aspect of the present invention is a method for lowering blood pressure in a subject comprising administering a peptide of the present invention to a subject in need of blood pressure reduction.
Another aspect of the present invention is the use of a peptide of the present invention for lowering blood pressure in a subject in need of blood pressure reduction.
The subject requiring blood pressure lowering is not particularly limited as long as it is effective to inhibit angiotensin converting enzyme, but patients having various diseases derived from the above-mentioned hypertension, patients with essential hypertension, and cardiac hypertrophy And patients having diseases such as angina. Also, in these methods and uses, “decrease in blood pressure” can be “treatment of disease”.
本発明のペプチドは、医薬、飲食品、飼料、化粧品、医薬部外品等に配合することができる。配合されるペプチドは、1種類でもよく、任意の2種以上の混合物であってもよい。
例えば、医薬の製剤化にあたっては、担体、賦形剤、結合剤、崩壊剤、滑沢剤、着色剤安定剤、矯味矯臭剤、希釈剤、注射剤用溶剤等の添加剤を使用できる。具体的製剤として、錠剤(糖衣錠、腸溶性コーティング錠、バッカル錠を含む。)、散剤、カプセル剤(腸溶性カプセル、ソフトカプセルを含む。)、顆粒剤(コーティングしたものを含む。)、丸剤、トローチ剤、封入リポソーム剤、液剤、又はこれらの製剤学的に許容され得る徐放製剤等を例示することができる。The peptide of this invention can be mix | blended with a pharmaceutical, food-drinks, feed, cosmetics, a quasi-drug, etc. One type of peptide may be blended, or a mixture of any two or more types may be used.
For example, additives such as carriers, excipients, binders, disintegrants, lubricants, colorant stabilizers, flavoring agents, diluents, and solvents for injections can be used for pharmaceutical formulation. Specific preparations include tablets (including sugar-coated tablets, enteric-coated tablets, buccal tablets), powders, capsules (including enteric capsules and soft capsules), granules (including those coated), pills, Illustrative examples include troches, encapsulated liposomes, solutions, and pharmaceutically acceptable sustained release formulations.
これらの製剤に用いる担体及び賦形剤としては、乳糖、ブドウ糖、白糖、マンニトール、馬鈴薯澱粉、トウモロコシ澱粉、炭酸カルシウム、リン酸カルシウム、硫酸カルシウム、結晶セルロース、カンゾウ末、ゲンチアナ末等を、結合剤としては澱粉、ゼラチン、シロップ、ポリビニルアルコール、ポリビニルエーテル、ポリビニルピロリドン、ヒドロキシプロピルセルロース、エチルセルロース、メチルセルロース、カルボキシメチルセルロース等を例示することができる。
また、崩壊剤としては、澱粉、寒天、ゼラチン末、カルボキシメチルセルロースナトリウム、カルボキシメチルセルロースカルシウム、結晶セルロース、炭酸カルシウム、炭酸水素ナトリウム、及びアルギン酸ナトリウム等を例示することができる。
更に、滑沢剤としては、ステアリン酸マグネシウム、水素添加植物油、及びマクロゴール等を、着色剤としては医薬品に添加することが許容されている赤色2号、黄色4号、及び青色1号等を、それぞれ例示することができる。
その他、安定剤、矯味矯臭剤、希釈剤、注射剤用溶剤等についても、通常、医薬の製造に用いられる成分を用いることができる。Carriers and excipients used in these formulations include lactose, glucose, sucrose, mannitol, potato starch, corn starch, calcium carbonate, calcium phosphate, calcium sulfate, crystalline cellulose, licorice powder, gentian powder and the like as binders Examples thereof include starch, gelatin, syrup, polyvinyl alcohol, polyvinyl ether, polyvinyl pyrrolidone, hydroxypropyl cellulose, ethyl cellulose, methyl cellulose, carboxymethyl cellulose and the like.
Examples of the disintegrant include starch, agar, gelatin powder, sodium carboxymethyl cellulose, carboxymethyl cellulose calcium, crystalline cellulose, calcium carbonate, sodium bicarbonate, and sodium alginate.
Furthermore, as the lubricant, magnesium stearate, hydrogenated vegetable oil, macrogol, etc., and as the colorant, red No. 2, yellow No. 4, and blue No. 1, etc., which are allowed to be added to pharmaceuticals, etc. , Respectively.
In addition, as for stabilizers, flavoring agents, diluents, solvents for injections, and the like, components usually used in the production of pharmaceuticals can be used.
錠剤及び顆粒剤は、必要に応じ白糖、ヒドロキシプロピルセルロース、精製セラック、ゼラチン、ソルビトール、グリセリン、エチルセルロース、ヒドロキシプロピルセルロース、ヒドロキシプロピルメチルセルロース、ポリビニルピロリドン、フタル酸セルロースアセテート、フタル酸ヒドロキシプロピルメチルセルロース、メチルメタクリレート、及びメタアクリル酸重合体等により被膜することもできる。 Tablets and granules are sucrose, hydroxypropylcellulose, purified shellac, gelatin, sorbitol, glycerin, ethylcellulose, hydroxypropylcellulose, hydroxypropylmethylcellulose, polyvinylpyrrolidone, cellulose phthalate acetate, hydroxypropylmethylcellulose phthalate, methyl methacrylate as necessary And a methacrylic acid polymer.
本発明のアンジオテンシン変換酵素阻害剤又は血圧降下剤は、経口投与、非経口投与のいずれによって投与されてもよいが、経口投与が好ましい。非経口投与としては、静注、直腸投与、吸入等が挙げられる。経口投与の剤型としては、錠剤、カプセル剤、トローチ剤、シロップ剤、顆粒剤、散剤、軟膏等が挙げられる。また、公知の、もしくは将来的に見出されるアンジオテンシン変換酵素阻害作用を有する薬剤、医薬組成物を本発明のペプチドと併用することもできる。併用する薬剤又は医薬組成物は、本発明の薬剤中に有効成分の一つとして含有させてもよいし、本発明の薬剤中には含有させずに、別個の薬剤として本発明の薬剤と組み合わせて商品化してもよい。 The angiotensin converting enzyme inhibitor or blood pressure lowering agent of the present invention may be administered either orally or parenterally, but oral administration is preferred. Parenteral administration includes intravenous injection, rectal administration, inhalation and the like. Examples of the dosage form for oral administration include tablets, capsules, troches, syrups, granules, powders, ointments and the like. Moreover, the medicine and pharmaceutical composition which have the angiotensin converting enzyme inhibitory action known or discovered in the future can also be used together with the peptide of this invention. The drug or pharmaceutical composition to be used in combination may be contained as one of the active ingredients in the drug of the present invention, or it is not contained in the drug of the present invention and is combined with the drug of the present invention as a separate drug. May be commercialized.
また、本発明のペプチドを有効成分として食品中に含有させ、アンジオテンシン変換酵素阻害剤又は血圧降下剤の一態様として、アンジオテンシン変換酵素阻害作用又は血圧降下作用を有する食品として加工することも可能である。
このような食品としては、液状、ペースト状、固体、粉末等の形態を問わず、錠菓、流動食、飼料(ペット用を含む)等のほか、例えば、パン、マカロニ、スパゲッティ、めん類、ケーキミックス、から揚げ粉、パン粉等の小麦粉製品;即席めん、カップめん、レトルト・調理食品、調理缶詰、電子レンジ食品、即席スープ・シチュー、即席みそ汁・吸い物、スープ缶詰、フリーズ・ドライ食品、その他の即席食品等の即席食品類;農産缶詰、果実缶詰、ジャム・マーマレード類、漬物、煮豆類、農産乾物類、シリアル(穀物加工品)等の農産加工品; 水産缶詰、魚肉ハム・ソーセージ、水産練り製品、水産珍味類、つくだ煮類等の水産加工品;畜産缶詰・ペースト類、畜肉ハム・ソーセージ等の畜産加工品;加工乳、乳飲料、ヨーグルト類、乳酸菌飲料類、チーズ、アイスクリーム類、調製粉乳類、クリーム、その他の乳製品等の乳・乳製品;バター、マーガリン類、植物油等の油脂類;しょうゆ、みそ、ソース類、トマト加工調味料、みりん類、食酢類等の基礎調味料;調理ミックス、カレーの素類、たれ類、ドレッシング類、めんつゆ類、スパイス類、その他の複合調味料等の複合調味料・食品類;素材冷凍食品、半調理冷凍食品、調理済冷凍食品等の冷凍食品;キャラメル、キャンディー、チューインガム、チョコレート、クッキー、ビスケット、ケーキ、パイ、スナック、クラッカー、和菓子、米菓子、豆菓子、デザート菓子、その他の菓子などの菓子類;炭酸飲料、天然果汁、果汁飲料、果汁入り清涼飲料、果肉飲料、果粒入り果実飲料、野菜系飲料、豆乳、豆乳飲料、コーヒー飲料、お茶飲料、粉末飲料、濃縮飲料、スポーツ飲料、栄養飲料、アルコール飲料、その他の嗜好飲料等の嗜好飲料類、ベビーフード、ふりかけ、お茶潰けのり等のその他の市販食品等;育児用調製粉乳;経腸栄養食;機能性食品(特定保健用食品、栄養機能食品)等が挙げられる。Further, the peptide of the present invention can be contained in food as an active ingredient, and processed as a food having an angiotensin converting enzyme inhibitory action or blood pressure lowering action as one embodiment of an angiotensin converting enzyme inhibitor or blood pressure lowering agent. .
Such foods may be in the form of liquids, pastes, solids, powders, etc., in addition to tablet confections, liquid foods, feeds (including for pets), etc., for example, bread, macaroni, spaghetti, noodles, cakes Flour products such as mix, fried flour, bread crumbs, instant noodles, cup noodles, retort / cooked food, cooked canned food, microwave food, instant soup / stew, instant miso soup / soup, canned soup, freeze-dried food, other instant foods Instant foods such as foods; Canned agricultural products, canned fruits, jams and marmalades, pickles, boiled beans, dried agricultural products, processed cereals (cereal processed products); canned marine products, fish ham and sausages, fish paste products, Processed marine products such as marine delicacy, tsukudani, etc .; Livestock canned and pasted products, livestock processed products such as livestock ham and sausage; processed milk, milk drinks, yogurts Milk and dairy products such as lactic acid bacteria beverages, cheese, ice cream, formula milk powder, cream and other dairy products; fats and oils such as butter, margarine, vegetable oil; soy sauce, miso, sauces, tomato processed seasonings , Mirins, basic seasonings such as vinegar; cooking mixes, curry ingredients, sauces, dressings, noodle soups, spices, and other complex seasonings and foods; frozen foods, Semi-cooked frozen foods, frozen foods such as cooked frozen foods; caramel, candy, chewing gum, chocolate, cookies, biscuits, cakes, pie, snacks, crackers, Japanese confectionery, rice confectionery, bean confectionery, dessert confectionery, other confectionery Sweets: carbonated drink, natural fruit juice, fruit juice drink, soft drink with fruit juice, fruit drink, fruit drink with fruit granules, vegetable drink, soy milk, soy milk drink Coffee beverages, tea beverages, powdered beverages, concentrated beverages, sports beverages, nutritional beverages, alcoholic beverages, other beverages such as other favorite beverages, baby food, sprinkles, other marketed foods such as tea paste, etc. Examples include formula milk powder; enteral nutrition; functional food (food for specified health use, functional food for nutrition).
さらに、アンジオテンシン変換酵素阻害剤又は血圧降下剤の一態様として、本発明のペプチドを有効成分として飼料中に含有させ、アンジオテンシン変換酵素阻害作用又は血圧降下作用を有する飼料として加工することも可能である。
飼料の形態としては特に制限されず、例えば、本発明のペプチドの粉末やこれらの水溶液(シロップ等)等を、トウモロコシ、小麦、大麦、ライ麦、マイロ等の穀類;大豆油粕、ナタネ油粕、ヤシ油粕、アマニ油粕等の植物性油粕類;フスマ、麦糠、米糠、脱脂米糠等の糠類;コーングルテンミール、コーンジャムミール等の製造粕類;魚粉、脱脂粉乳、ホエイ、イエローグリース、タロー等の動物性飼料類;トルラ酵母、ビール酵母等の酵母類;第三リン酸カルシウム、炭酸カルシウム等の鉱物質飼料;油脂類;単体アミノ酸;糖類等に配合することにより製造できる。飼料の形態としては、例えば、ペットフード、家畜飼料、養魚飼料等が挙げられる。Furthermore, as an embodiment of an angiotensin converting enzyme inhibitor or blood pressure lowering agent, the peptide of the present invention can be contained in the feed as an active ingredient, and processed as a feed having an angiotensin converting enzyme inhibitory action or blood pressure lowering action. .
The form of the feed is not particularly limited. For example, the peptide powder of the present invention or an aqueous solution thereof (syrup or the like) is used for cereals such as corn, wheat, barley, rye, and milo; soybean oil cake, rapeseed oil cake, coconut oil cake Vegetable oils such as flaxseed, flaxseed oil, rice bran, rice bran, defatted rice bran, etc .; production corn such as corn gluten meal, corn jam meal; fish meal, skim milk powder, whey, yellow grease, tallow, etc. Animal feeds; yeasts such as torula yeast and beer yeast; mineral feeds such as tricalcium phosphate and calcium carbonate; fats and oils; simple amino acids; sugars and the like. Examples of the form of the feed include pet food, livestock feed, and fish feed.
本発明のアンジオテンシン変換酵素阻害剤又は血圧降下剤(医薬品、飲食品、飼料、化粧品、医薬部外品等の各態様)において、本発明のペプチドの配合量(ペプチドが複数種の場合は合計量)は、アンジオテンシン変換酵素阻害剤又は血圧降下剤の最終組成物に対して、0.001質量%以上であることが好ましく、0.01質量%以上であることがより好ましく、0.1質量%以上であることが特に好ましい。 In the angiotensin converting enzyme inhibitor or antihypertensive agent of the present invention (each aspect of pharmaceuticals, foods and drinks, feeds, cosmetics, quasi drugs, etc.), the amount of the peptide of the present invention (the total amount in the case of multiple peptides) ) Is preferably 0.001% by mass or more, more preferably 0.01% by mass or more, and 0.1% by mass with respect to the final composition of the angiotensin converting enzyme inhibitor or blood pressure lowering agent. The above is particularly preferable.
本発明のアンジオテンシン変換酵素阻害剤又は血圧降下剤の投与量は、年齢、症状等により異なるが、通常、本発明のペプチドの量として0.001〜3000mg/日、好ましくは0.01〜30mg/日であり、1日1回、又は2回から3回に分けて投与してもよい。また、本発明のアンジオテンシン変換酵素阻害剤又は血圧降下剤を摂取又は服用する場合は、食前、食間、食後のいずれのタイミングであっても、本発明の効果は十分に発揮されるものである。 The dose of the angiotensin converting enzyme inhibitor or blood pressure lowering agent of the present invention varies depending on age, symptoms, etc., but is usually 0.001 to 3000 mg / day, preferably 0.01 to 30 mg / day as the amount of the peptide of the present invention. It may be administered once a day or divided into 2 to 3 times a day. In addition, when the angiotensin converting enzyme inhibitor or blood pressure lowering agent of the present invention is ingested or taken, the effects of the present invention are sufficiently exhibited at any timing before, between, and after a meal.
以下に実施例を用いて本発明をさらに詳しく説明するが、本発明はこれら実施例に限定されるものではない。 Hereinafter, the present invention will be described in more detail using examples, but the present invention is not limited to these examples.
<実施例1>
(1)ペプチドの化学合成
ペプチドシンセサイザー(Model 433A型、アプライドバイオシステムズ社)を使用し、5種類のアミノ酸誘導体混合物〔Fmoc−L−Leu、Fmoc−L−Arg(Mtr)、Fmoc−L−Ile、Fmoc−L−Ser(tBu)、Fmoc−L−Pro〕、Fmoc−L−Lys(Boc)−Wang Resin(いずれも国産化学)を原料に用いて、固相合成法によりAA(6)−AA(5)−AA(4)−AA(3)−AA(2)−Lys〔AA(n)は、5種類のアミノ酸のいずれかであり、合成されるペプチドは55=3125種類〕(配列番号6)の混合物の合成を行った。操作はアプライドバイオシステムズ社のマニュアルに従って行った。合成反応後、脱保護した。得られたペプチドは、下記HPLC条件で精製した。<Example 1>
(1) Chemical synthesis of peptides Using a peptide synthesizer (Model 433A type, Applied Biosystems), a mixture of five amino acid derivatives [Fmoc-L-Leu, Fmoc-L-Arg (Mtr), Fmoc-L-Ile , Fmoc-L-Ser (tBu), Fmoc-L-Pro], Fmoc-L-Lys (Boc) -Wang Resin (both domestic chemistry) as raw materials, AA (6)- AA (5) -AA (4) -AA (3) -AA (2) -Lys [AA (n) is one of five amino acids, and 5 5 = 3125 peptides are synthesized] ( A mixture of SEQ ID NO: 6) was synthesized. The operation was performed according to the manual of Applied Biosystems. After the synthesis reaction, it was deprotected. The obtained peptide was purified under the following HPLC conditions.
(2)HPLCによるペプチドの分離
逆相HPLCで上記合成混合物の分離を行った。このHPLC条件は下記HPLC条件1に示した。(2) Separation of peptides by HPLC The synthesis mixture was separated by reverse phase HPLC. The HPLC conditions are shown in the following
〔HPLC条件1〕
カラム :CadenzaCD−18 10mmI.D.×250mm(インタクト社製)
検出:UV 215nm
流速:3ml/分
溶離液A:0.1% TFAを含む水溶液
溶離液B:0.1% TFAを含むアセトニトリル溶液[HPLC condition 1]
Column: Cadenza CD-18 10 mmI. D. × 250mm (manufactured by Intact)
Detection: UV 215nm
Flow rate: 3 ml / min Eluent A: Aqueous solution containing 0.1% TFA Eluent B: Acetonitrile solution containing 0.1% TFA
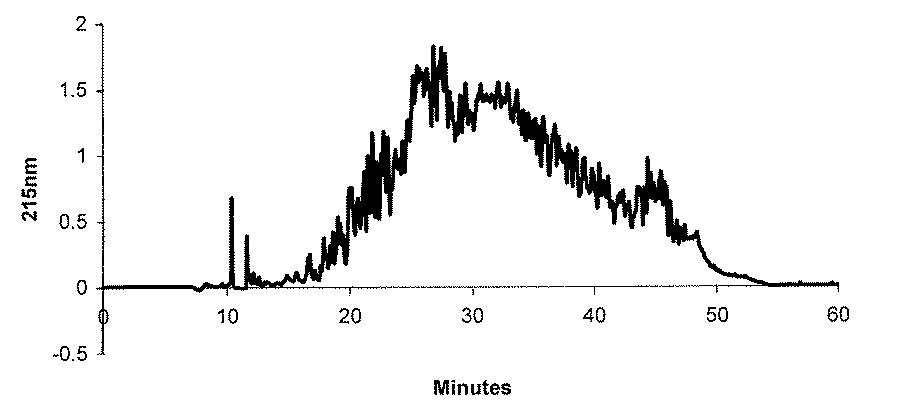
サンプルをカラムに導入後、溶離液(A/B)を98/2で5分間維持し、溶離液(75/25)まで30分間、溶離液(50/50)まで10分間、更に溶離液(20/80)まで3分間でグラジエント溶出を行い、その後5分間溶離液(20/80)を維持するグラジエント条件で、合成ペプチド混合物を分離した(クロマトグラム:図1)。
各ピーク毎に溶出液を分画し、後述する方法でアンジオテンシン変換酵素阻害能を測定したところ、強いアンジオテンシン変換酵素阻害能を持つ画分が、リテンションタイム34.0分、34.4分、34.7分、及び35.1分に溶出された。After the sample is introduced into the column, the eluent (A / B) is maintained at 98/2 for 5 minutes, to the eluent (75/25) for 30 minutes, to the eluent (50/50) for 10 minutes, and further to the eluent ( Gradient elution was performed in 3 minutes until 20/80), and then the synthetic peptide mixture was separated under a gradient condition of maintaining the eluent (20/80) for 5 minutes (chromatogram: FIG. 1).
The eluate was fractionated for each peak, and the angiotensin converting enzyme inhibitory ability was measured by the method described later, and the fractions having strong angiotensin converting enzyme inhibitory ability were retention times of 34.0 minutes, 34.4 minutes, 34.7 minutes, and 35.1. Eluted in minutes.
これらの画分に含まれるペプチドについて、島津製作所製のプロテイン・シーケンサー(PPSQ−23A)で分析を行ったところ、それぞれIle−Arg−Ile−Pro−Ser−Lys(以下、HP1と記載する)、Ile−Arg−Leu−Pro−Ser−Lys(以下、HP2と記載する)、Leu−Arg−Leu−Pro−Ser−Lys(以下、HP3と記載する)、及び、Leu−Arg−Ile−Pro−Ser−Lys(以下、HP4と記載する)であった。 The peptides contained in these fractions were analyzed with a protein sequencer (PPSQ-23A) manufactured by Shimadzu Corporation. As a result, Ile-Arg-Ile-Pro-Ser-Lys (hereinafter referred to as HP1), Ile-Arg-Leu-Pro-Ser-Lys (hereinafter referred to as HP2), Leu-Arg-Leu-Pro-Ser-Lys (hereinafter referred to as HP3), and Leu-Arg-Ile-Pro- Ser-Lys (hereinafter referred to as HP4).
<実施例2>
[ペプチドのアンジオテンシン変換酵素阻害作用]
(1)試験方法
アンジオテンシン変換酵素阻害の測定は、カッシュマンらの方法〔バイオケミカル・ファーマコロジー20巻、1637〜1648頁(1971)〕に準じて行った。
試料として、実施例1で得られた本発明ペプチド(HP1、HP2、HP3、HP4)、Agricultural and Biological Chemistry 49(5), 1405-1409, 1985に記載のペプチド(Phe−Phe−Val−Ala−Pro−Phe−Pro−Glu−Val−Phe−Gly−Lys:配列番号7)、及び特許第3816921号(WO2003/044044))に記載のペプチド(Met−Lys−Pro)を用いた。これらのペプチドは、いずれも実施例1と同様にして化学合成したものである。<Example 2>
[Angiotensin-converting enzyme inhibitory action of peptides]
(1) Test method Angiotensin converting enzyme inhibition was measured according to the method of Kashman et al. [Biochemical pharmacology, Vol. 20, pages 1637 to 1648 (1971)].
As a sample, the peptide of the present invention (HP1, HP2, HP3, HP4) obtained in Example 1, the peptide described in Agricultural and Biological Chemistry 49 (5), 1405-1409, 1985 (Phe-Phe-Val-Ala- Pro-Phe-Pro-Glu-Val-Phe-Gly-Lys: SEQ ID NO: 7) and a peptide (Met-Lys-Pro) described in Japanese Patent No. 38169921 (WO2003 / 044044)) were used. All of these peptides were chemically synthesized in the same manner as in Example 1.
試料を0.1Mホウ酸緩衝液(0.3M NaClを含む、pH8.3)に溶解し、試験管に0.08ml入れた後、0.1Mホウ酸緩衝液(0.3M NaClを含む、pH8.3)で5mMに調製した酵素基質(ヒプリルヒスチジルロイシン、シグマ社製)0.2mlを添加し、37℃で3分間保温した。次いで、蒸留水を添加して0.1U/mlになるように調製したウサギ肺のアンジオテンシン変換酵素(シグマ社製)0.02mlを添加し、37℃で30分間反応させた。 The sample was dissolved in 0.1 M borate buffer (containing 0.3 M NaCl, pH 8.3), 0.08 ml was placed in a test tube, and then 0.1 M borate buffer (containing 0.3 M NaCl). 0.2 ml of an enzyme substrate (Hiprilhistidylleucine, Sigma) adjusted to 5 mM at pH 8.3) was added, and the mixture was incubated at 37 ° C. for 3 minutes. Subsequently, 0.02 ml of rabbit lung angiotensin converting enzyme (manufactured by Sigma) prepared to be 0.1 U / ml by adding distilled water was added and reacted at 37 ° C. for 30 minutes.
その後、1N塩酸0.25mlを添加して反応を終了した後、1.7mlの酢酸エチルを加え、20秒間激しく攪件し、3000rpmで10分間遠心分離して、酢酸エチル層を1.4ml採取した。得られた酢酸エチル層を加熱して溶媒を除去した後、蒸留水を1.0ml添加し、抽出したヒプリル酸の吸収(228nmの吸光度)を測定して、これを酵素活性とした。 Thereafter, 0.25 ml of 1N hydrochloric acid was added to complete the reaction, 1.7 ml of ethyl acetate was added, and the mixture was vigorously stirred for 20 seconds, centrifuged at 3000 rpm for 10 minutes, and 1.4 ml of the ethyl acetate layer was collected. did. The obtained ethyl acetate layer was heated to remove the solvent, and then 1.0 ml of distilled water was added, and the absorption of the extracted hyprilic acid (absorbance at 228 nm) was measured to determine the enzyme activity.
次の式から、阻害活性を求め、IC50[アンジオテンシン変換酵素の活性を50%阻害するために必要な試料濃度(μM)]を決定した。 The inhibitory activity was calculated from the following formula, and IC50 [sample concentration (μM) necessary for inhibiting 50% of the activity of angiotensin converting enzyme] was determined.
阻害率=(A−B)/(A−C)×100%
A:試料(ペプチド)を含まない場合の酵素活性(228nmの吸光度)
B:試料添加の場合の酵素活性(228nmの吸光度)
C:酵素および試料を添加しない場合の酵素活性(228nmの吸光度)Inhibition rate = (A−B) / (A−C) × 100%
A: Enzyme activity when sample (peptide) is not included (absorbance at 228 nm)
B: Enzyme activity when adding sample (absorbance at 228 nm)
C: Enzyme activity when no enzyme and sample are added (absorbance at 228 nm)
(2)試験結果
本試験結果を表1に示す。表1から明らかなとおり、本発明のペプチド(HP1、HP2、HP3、HP4)は、いずれも強力なアンジオテンシン変換酵素阻害活性を有することが判明した。(2) Test results The test results are shown in Table 1. As is clear from Table 1, all of the peptides of the present invention (HP1, HP2, HP3, HP4) were found to have potent angiotensin converting enzyme inhibitory activity.
<実施例3>
[ペプチドの動物における血圧降下作用]
(1)試験方法
12週齢のSHR/Hos雄性ラット14匹(日本エスエルシー株式会社より購入)を、1週間予備飼育し、ラットの血圧を小動物非観血式自動血圧計(MK−2000,室町機械(株)社製)を用いて測定した。<Example 3>
[Antihypertensive action of peptides in animals]
(1) Test method 14 12-week-old SHR / Hos male rats (purchased from Japan SLC Co., Ltd.) were preliminarily raised for 1 week, and the blood pressure of the rats was measured using a small animal non-invasive automatic sphygmomanometer (MK-2000, Muromachi Kikai Co., Ltd.).
収縮期血圧を指標とし、群毎の投与前の平均収縮期血圧がほぼ同じ値になるように、1群あたり7匹となるように2群に分けた後、約14時間絶食し、試験群には実施例1で得られたペプチド(Leu−Arg−Ile−Pro−Ser−Lys:HP4)を注射用水に溶解し、ラットに5mL/体重kg(HP4として3mg/体重kg)の割合で経口投与し、試料投与直前、試料投与の1、3、6及び8時間後に、ラットの血圧を測定した。 Using the systolic blood pressure as an index, the group was divided into two groups so that the average systolic blood pressure before administration for each group was almost the same value, and then the animals were fasted for about 14 hours after being divided into two groups. In Example 1, the peptide obtained in Example 1 (Leu-Arg-Ile-Pro-Ser-Lys: HP4) was dissolved in water for injection and orally administered to rats at a rate of 5 mL / kg body weight (3 mg / kg body weight as HP4). The blood pressure of the rats was measured immediately before sample administration, 1, 3, 6 and 8 hours after sample administration.
対照群には、前記HP4水溶液の代わりに注射用水を同容量経口投与し、試料投与直前、試料投与の1、3、6及び8時間後に、ラットの血圧を測定した。
(2)試験結果In the control group, the same volume of water for injection was orally administered instead of the HP4 aqueous solution, and the blood pressure of the rats was measured immediately before sample administration, 1, 3, 6 and 8 hours after sample administration.
(2) Test results
本試験結果を表2に示す。表2から明らかなとおり、試験群(HP4投与群)において持続的に血圧降下が認められたのに対し、対照群では認められなかった。従って、本発明のペプチド(Leu−Arg−Ile−Pro−Ser−Lys:HP4)に、動物に対する血圧降下作用があることが判明した。
なお、実施例1で得られた他のペプチド(HP1、HP2、HP3)についても同様の試験を行った結果、HP4と同じように血圧降下作用を有することが判明した。The test results are shown in Table 2. As is clear from Table 2, blood pressure was continuously decreased in the test group (HP4 administration group), but not in the control group. Therefore, it was found that the peptide of the present invention (Leu-Arg-Ile-Pro-Ser-Lys: HP4) has a blood pressure lowering effect on animals.
In addition, as a result of conducting the same test also about the other peptides (HP1, HP2, HP3) obtained in Example 1, it was found to have a blood pressure lowering effect like HP4.
本発明によれば、アンジオテンシン変換酵素阻害作用を有する新規ペプチドが提供される。同ペプチドを含有するアンジオテンシン変換酵素阻害剤は、医薬として利用することができる。本発明のペプチドは、すべて天然型(L体)のアミノ酸から構成されるため、安全性が高く食品にも用いることができる。 According to the present invention, a novel peptide having an angiotensin converting enzyme inhibitory action is provided. An angiotensin converting enzyme inhibitor containing the peptide can be used as a medicine. Since the peptides of the present invention are all composed of natural (L) amino acids, they are highly safe and can be used in foods.
Claims (9)
Xaa−Arg−Xaa−Pro−Ser−Lys
(但し、各アミノ酸はL−体であり、Xaaは、独立してIle又はLeuである。) A peptide consisting of the following amino acid sequence.
Xaa-Arg-Xaa-Pro-Ser-Lys
(However, each amino acid is L-form, and Xaa is independently Ile or Leu.)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013536124A JP5430803B2 (en) | 2011-09-28 | 2012-09-05 | Peptides and angiotensin converting enzyme inhibitors |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011213334 | 2011-09-28 | ||
| JP2011213334 | 2011-09-28 | ||
| PCT/JP2012/072609 WO2013047128A1 (en) | 2011-09-28 | 2012-09-05 | Peptide and angiotensin-converting enzyme inhibitor |
| JP2013536124A JP5430803B2 (en) | 2011-09-28 | 2012-09-05 | Peptides and angiotensin converting enzyme inhibitors |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP5430803B2 true JP5430803B2 (en) | 2014-03-05 |
| JPWO2013047128A1 JPWO2013047128A1 (en) | 2015-03-26 |
Family
ID=47995172
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013536124A Active JP5430803B2 (en) | 2011-09-28 | 2012-09-05 | Peptides and angiotensin converting enzyme inhibitors |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP5430803B2 (en) |
| WO (1) | WO2013047128A1 (en) |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001106699A (en) * | 1999-10-05 | 2001-04-17 | Suetsuna Yoko | New hexapeptide and angiotensin-converting enzyme inhibitor |
| EP1907412B1 (en) * | 2005-06-30 | 2012-04-11 | Campina Nederland Holding B.V. | Peptides inhibiting angiotensin-converting enzyme |
| JP2007261999A (en) * | 2006-03-28 | 2007-10-11 | Yamada Bee Farm Corp | Hypotensive peptide derived from royal jelly |
| JP5060741B2 (en) * | 2006-06-06 | 2012-10-31 | 公立大学法人青森県立保健大学 | Apios acetic acid / enzyme processed product production method, Apios-derived peptide and production method thereof |
| JP2008156307A (en) * | 2006-12-26 | 2008-07-10 | Asahi Breweries Ltd | Angiotensin converting enzyme inhibitor, angiotensin converting enzyme inhibitor composition, and food and drink containing them |
| EP1967524A1 (en) * | 2007-03-06 | 2008-09-10 | Friesland Brands B.V. | Methods for producing ACE-inhibitory peptides from whey and peptides obtained thereby |
-
2012
- 2012-09-05 WO PCT/JP2012/072609 patent/WO2013047128A1/en active Application Filing
- 2012-09-05 JP JP2013536124A patent/JP5430803B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2013047128A1 (en) | 2015-03-26 |
| WO2013047128A1 (en) | 2013-04-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6189994B2 (en) | Dipeptidyl peptidase-IV inhibitory food and beverage composition | |
| ES2281567T3 (en) | NEW PEPTIDE THAT HAS INHIBITING EFFECT OF ANGIOTENSIN CONVERTASA. | |
| JP5832049B2 (en) | Dipeptidyl peptidase-IV inhibitor | |
| JP6344796B2 (en) | Alzheimer-type dementia remedy for elderly | |
| JP5875603B2 (en) | Bifidobacterium growth promoter | |
| JP2007261999A (en) | Hypotensive peptide derived from royal jelly | |
| JP3592593B2 (en) | Angiotensin converting enzyme inhibitor | |
| JP5976004B2 (en) | Dipeptidyl peptidase-IV inhibitor | |
| JP2015084694A (en) | Dipeptidyl peptidase-iv inhibitors | |
| JP5877560B2 (en) | Dipeptidyl peptidase-IV inhibitor | |
| WO2022202985A1 (en) | Peptide and composition containing peptide as active ingredient | |
| JPWO2019058609A1 (en) | Composition for promoting energy consumption | |
| JP5879356B2 (en) | peptide | |
| JP5430803B2 (en) | Peptides and angiotensin converting enzyme inhibitors | |
| WO2024038888A1 (en) | Composition | |
| JP2018154640A (en) | Oral compositions for improving cerebral dysfunction | |
| JP6826726B2 (en) | Oral composition for promoting sugar uptake | |
| JP2016222601A (en) | Novel peptide and its use |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20131105 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20131203 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5430803 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |