EP2627751B1 - Top-loading laundry vessel method - Google Patents
Top-loading laundry vessel method Download PDFInfo
- Publication number
- EP2627751B1 EP2627751B1 EP11763944.3A EP11763944A EP2627751B1 EP 2627751 B1 EP2627751 B1 EP 2627751B1 EP 11763944 A EP11763944 A EP 11763944A EP 2627751 B1 EP2627751 B1 EP 2627751B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- surfactant
- process according
- particle
- water
- dyes
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D17/00—Detergent materials or soaps characterised by their shape or physical properties
- C11D17/0039—Coated compositions or coated components in the compositions, (micro)capsules
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/02—Inorganic compounds ; Elemental compounds
- C11D3/04—Water-soluble compounds
- C11D3/046—Salts
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/02—Inorganic compounds ; Elemental compounds
- C11D3/04—Water-soluble compounds
- C11D3/08—Silicates
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/02—Inorganic compounds ; Elemental compounds
- C11D3/04—Water-soluble compounds
- C11D3/10—Carbonates ; Bicarbonates
Definitions
- the present invention relates to laundry cleaning methods using shaped laundry detergent particles.
- Liquid detergents do not have these same residue problems, but as they are largely un-built systems, they can suffer cleaning disadvantages especially in hard-water areas. Liquids have other advantages over powders, for example they do not have the caking problems of solids.
- WO1999/32599 describes a method of manufacturing laundry detergent particles, by extrusion of a builder and surfactant to form noodles.
- a composition with a major component of a sulphated or sulphonated anionic surfactant is fed into an extruder, mechanically worked at a temperature of at least 40 °C, preferably at least 60°C, and extruded through an extrusion head having a multiplicity of extrusion apertures.
- the surfactant is fed to the extruder along with builder in a weight ratio of more than 1 part builder to 2 parts surfactant.
- the extruded material apparently required further drying.
- PAS paste was dried and extruded.
- Such PAS noodles are well known in the prior art.
- the noodles are typically cylindrical in shape and their length exceeds their diameter, as described in example 2.
- EP application 09158717.0 ( WO 2010/122051 ) discloses high active detergent particles with a low surface roughness produced by extrusion of an anionic surfactant rich composition, and cutting of the extruded product to form slices which are thinner than they are wide. These slices are then spray-coated with, for example, an aqueous solution of sodium carbonate, to form smooth-surfaced, free-flowing particles which may look like "lentils".
- a builder salt such as sodium carbonate is advantageous because it buffers the pH at a level where the anionic surfactant is particularly effective, and increases the ionic strength.
- the particles are also said to dissolve well in water and are suitable for use in a front loading washing machine (page 6, line 19).
- coated extruded particles with a particular shape and formulation show exceptionally good cleaning and care as perceived by a panel of users.
- the particles do not exhibit a "hot on hands" feeling when used.
- the present invention provides a laundry process which comprises the steps of:
- the surfactant to builder ratio in the detergent particles is in the range 1.5-2.5:1, more preferably 1.8-2.2:1. As the ratio shifts further towards high builder levels the wash liquor becomes too alkaline and as it shifts towards high surfactant levels buffering capacity is lost.
- the builder present as a coating encasing the surfactant, which is soft and sticky, a particulate concentrate with the preferred ratio of surfactant to builder can be provided.
- the absence of other materials reduce the unit dose required for a wash and therefore reduces transportation costs and increases shelf-use efficiency.
- the coated laundry detergent particle has a non-spherical surface. Preferably this surface is in part curved.
- the coated laundry detergent particle may be described as disk-like, lenticular or an oblate spheroid/ellipsoid, where z and y are the equatorial diameters and x is the polar diameter. It is preferred that y and z are similar in value, although with some methods of manufacture they may differ slightly due to pressures exerted during slicing of an extruded product.
- the particles have an axis of rotational symmetry along the polar axis.
- the coated laundry detergent particle does not have any holes; that is to say, the coated laundry detergent particle has a topologic genus of zero.
- y and z are independently in the range 3 to 8 mm. This moves the shape further from a sphere and increases the ratio of surface area to volume, improving solubility.
- the thickness of coating obtainable by use of a particular coating level is significantly greater than would be achieved on typically sized detergent granules (0.5-2mm diameter sphere). This makes for a more robust coating and reduces the tendency of the surfactant to bleed through the coating and make the particles sticky. It is also the case that a significant proportion of the builder will dissolve before the surfactant starts to dissolve so that the wash liquor will be at least partially built before the surfactant is dissolved.
- a further advantage of these relatively large particles in the top-loader is that they sink rapidly and come into contact with the impeller at an early stage in the wash.
- this surface area to volume ratio must be greater than 3 mm -1 .
- the coating thickness is inversely proportional to this coefficient and hence for the coating the ratio "Surface area of coated particle" divided by "Volume of coated particle” should be less than 15 mm -1 .
- the coated laundry detergent particle is such that at least 90 to 100% of the coated laundry detergent particles in the in the average x, y and z dimensions of individual particles are within a 20%, preferably 10%, variable from the largest to the smallest coated laundry detergent particle.
- These aggregations of particles with similar sizes show good flow properties, can be,packaged in bottles and can be dosed like a liquid.
- the detergent particles comprise from 0.0001 to 0.1 wt % dye, preferably 0.001 to 0.01 wt % dye, wherein the dye is selected: from anionic dyes; and, non-ionic dyes.
- Dyes serve several useful functions. They may assist the user in recognising different products, and/or may act as shading agents to mask yellowness of articles being laundered. If is preferable that not all of the particles are of the same colour, and that two or more colours of particles are present, for example white and blue, white and pink or white and orange.
- wt % refer to the total percentage in the particle as dry weights (including water of hydration where present).
- a top loading washing vessel is any vessel which is used by end-users to launder clothes.
- such vessels have a capacity in excess of 4 litres.
- Such vessels include, and preferentially comprise, top loading semi-automatic and automatic washing machines, preferably with means to agitate the wash liquor within them, and, in addition or in the alternative, other vessels where the agitation is manual, preferably a bath, tub, bucket, basin, bowl or sink.
- Machines include machines such as and similar to a Brazilian “Tanquinho".
- the vessel is open at the top during the washing operation and at least part of the agitation is provided by hand.
- the coated laundry detergent particle comprises between 50 to 90 wt% of a surfactant, most preferably 70 to 90 wt%.
- a surfactant most preferably 70 to 90 wt%.
- the nonionic and anionic surfactants of the surfactant system may be chosen from the surfactants described " Surface Active Agents" Vol. 1, by Schwartz & Perry, Interscience 1949 , Vol. 2 by Schwartz, Perry & Berch, Interscience 1958 , in the current edition of "McCutcheon's Emulsifiers and Detergents” published by Manufacturing Confectioners Company or in " Tenside-Taschenbuch", H. Stache, 2nd Edn., Carl Hauser Verlag, 1981 .
- the surfactants used are saturated.
- Suitable anionic detergent compounds which may be used are usually water-soluble alkali metal salts of organic sulphates and sulphonates having alkyl radicals containing from about 8 to about 22 carbon atoms, the term alkyl being used to include the alkyl portion of higher acyl radicals.
- suitable synthetic anionic detergent compounds are sodium and potassium alkyl sulphates, especially those obtained by sulphating higher C 8 to C 18 alcohols, produced for example from tallow or coconut oil, sodium and potassium alkyl C 9 to C 20 benzene sulphonates, particularly sodium linear secondary alkyl C 10 to C 15 benzene sulphonates; and sodium alkyl glyceryl ether sulphates, especially those ethers of the higher alcohols derived from tallow or coconut oil and synthetic alcohols derived from petroleum.
- anionic surfactants are sodium lauryl ether sulfate (SLES), particularly preferred with 1 to 3 ethoxy groups, sodium C 10 to C 15 alkyl benzene sulphonates (LAS) and sodium C 12 to C 18 alkyl sulphates (PAS).
- Alkyl ester suphonates such as Methyl ester sulphonates (MES) may be used in whole or part replacement for the other anionics.
- surfactants such as those described in EP-A-328 177 (Unilever), which show resistance to salting-out, the alkyl polyglycoside surfactants described in EP-A-070 074 , and alkyl monoglycosides.
- the chains of the surfactants may be branched or linear.
- the fatty acid soap used preferably contains from about 16 to about 22 carbon atoms, preferably in a straight chain configuration.
- the anionic contribution from soap is preferably from 0 to 30 wt% of the total anionic.
- At least 50 wt % of the anionic surfactant is selected from: sodium C 11 to C 15 alkyl benzene sulphonates; and, sodium C 12 to C 18 alkyl sulphates. Even more preferably, the anionic surfactant is sodium C 11 to C 15 alkyl benzene sulphonates.
- the anionic surfactant is present in the coated laundry detergent particle at levels between 15 to 85 wt%, more preferably 50 to 80wt% on total surfactant.
- Suitable nonionic detergent compounds which may be used include, in particular, the reaction products of compounds having a hydrophobic group and a reactive hydrogen atom, for example, aliphatic alcohols, acids, amides or alkyl phenols with alkylene oxides, especially ethylene oxide either alone or with propylene oxide.
- Preferred nonionic detergent compounds are C 6 to C 22 alkyl phenol-ethylene oxide condensates, generally 5 to 25 EO, i.e. 5 to 25 units of ethylene oxide per molecule, and the condensation products of aliphatic C 8 to C 18 primary or secondary linear or branched alcohols with ethylene oxide, generally 5 to 50 EO.
- the non-ionic is 10 to 50 EO, more preferably 20 to 35 EO.
- Alkyl ethoxylates are particularly preferred, most particularly those with 25-35 EO, as these give a particularly good foam profile.
- the nonionic surfactant is present in the coated laundry detergent particle at levels between 5 to 75 wt% on total surfactant, more preferably 10 to 40 wt% on total surfactant.
- Cationic surfactant may be present as minor ingredients at levels preferably between 0 to 5 wt% on total surfactant.
- surfactants are mixed together before being dried. Conventional mixing equipment may be used.
- the surfactant core of the laundry detergent particle may be formed by extrusion or roller compaction and subsequently coated with an inorganic salt.
- Foam boosters such as betaines, amine oxides and/or other zwitterionic and aphphoteric surfactants are optional as the compositions of the invention generally show adequate foaming.
- the surfactant system used is calcium tolerant and this is a preferred aspect because this reduces the need for builder.
- Surfactant blends that do not require builders to be present for effective detergency in hard water are preferred. Such blends are called calcium tolerant surfactant blends if they pass the test set out hereinafter. However, the invention may also be of use for washing with soft water, either naturally occurring or made using a water softener. In this case, calcium tolerance is no longer important and blends other than calcium tolerant ones may be used.
- the surfactant blend in question is prepared at a concentration of 0.7 g surfactant solids per litre of water containing sufficient calcium ions to give a French hardness of 40 (4 x 10 -3 Molar Ca 2+ ).
- Other hardness ion free electrolytes such as sodium chloride, sodium sulphate, and sodium hydroxide are added to the solution to adjust the ionic strength to 0.05M and the pH to 10.
- the adsorption of light of wavelength 540 nm through 4 mm of sample is measured 15 minutes after sample preparation. Ten measurements are made and an average value is calculated. Samples that give an absorption value of less than 0.08 are deemed to be calcium tolerant.
- Suitable calcium tolerant co-surfactants include SLES 1-7EO, and alkyl-ethoxylate nonionic surfactants, particularly those with melting points less than 40°C.
- a LAS/SLES surfactant blend has a superior foam profile to a LAS nonionic surfactant blend and is therefore preferred for hand washing formulations requiring high levels of foam.
- SLES may be used at levels of up to 30wt% of the surfactant blend.
- the water-soluble inorganic salts are preferably selected from water soluble salts of carbonate, chloride, silicate and sulphate, or mixtures thereof, most preferably, 70 to 100 wt% sodium carbonate on total water-soluble inorganic salts.
- the water-soluble inorganic salt is present as a coating on the particle.
- the amount of coating should lay in the range 1 to 40 wt% of the particle, preferably 20 to 40 wt%, more preferably 25 to 35 wt% for the best results in terms of anti-caking properties of the detergent particles.
- the coating is preferably applied to the surface of the surfactant core, by deposition from an aqueous solution of the water soluble inorganic salt.
- an aqueous solution of the water soluble inorganic salt can be performed using a slurry.
- the aqueous solution preferably contains greater than 50g/L, more preferably 200 g/L of the salt.
- An aqueous spray-on of the coating solution in a fluidised bed has been found to give good results and may also generate a slight rounding of the detergent particles during the fluidisation process. Drying and/or cooling may be needed to finish the process.
- a preferred calcium tolerant coated laundry detergent particle comprises 15 to 100 wt% on surfactant of anionic surfactant of which 20 to 30 wt% on surfactant is sodium lauryl ether sulphate.
- a highly preferred water soluble inorganic salt for inclusion in the coating is sodium carbonate monohydrate. Where, during the wash process, the coated detergent particles contact both water and the skin of the user, this confers the distinct benefit that upon exposure to water the particles do not generate as much heat as they would if the carbonate was anhydrous. This is an important preferred feature as users often mix detergent products with water by hand, and describe products which employ anhydrous sodium carbonate as having a "hot on hands" feeling.
- the inorganic salts in the coating is 100% by weight sodium carbonate monohydrate. This gives the best protection against moisture.
- the level of sodium carbonate monohydrate can be as low as 50% by weight of the inorganic salts in the coating.
- Dyes are a highly preferred component of the present invention. Suitable dyes are described in Industrial Dyes edited by K. Hunger 2003 Wiley-VCH ISBN 3-527-30426-6 .
- Dyes for use in the current invention are selected from anionic and non-ionic dyes
- Anionic dyes are negatively charged in an aqueous medium at pH 7.
- anionic dyes are found in the classes of acid and direct dyes in the Color Index (Society of Dyers and Colourists and American Association of Textile Chemists and Colorists).
- Anionic dyes preferably contain at least one sulphonate or carboxylate groups.
- Non-ionic dyes are uncharged in an aqueous medium at pH 7, examples are found in the class of disperse dyes in the Color Index.
- the dyes may be alkoxylated.
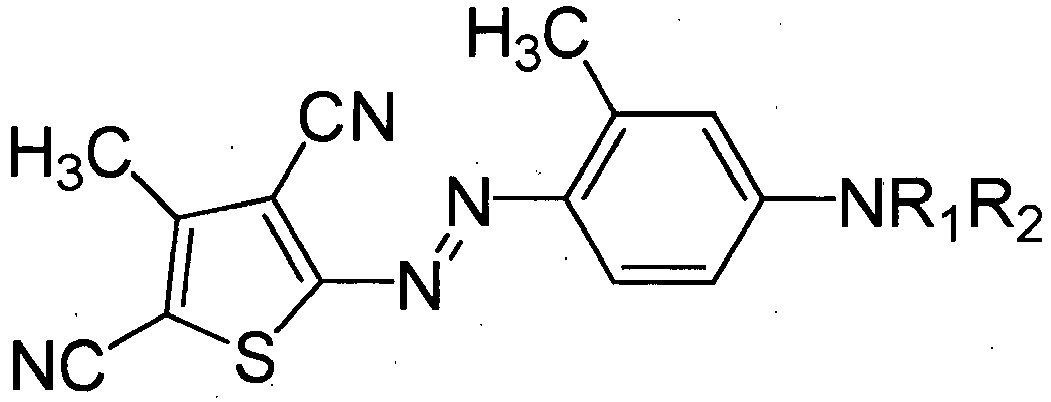
- Alkoxylated dyes are preferably of the following generic form: Dye-NR 1 R 2 .
- the NR 1 R 2 group is attached to an aromatic ring of the dye.
- R 1 and R 2 are independently selected from polyoxyalkylene chains having 2 or more repeating units and preferably having 2 to 20 repeating units. Examples of polyoxyalkylene chains include ethylene oxide, propylene oxide, glycidol oxide, butylene oxide and mixtures thereof.
- a preferred alkoxylated dye for use in the invention is:
- the dye is selected from acid dyes; disperse dyes and alkoxylated dyes.
- the dye is a non-ionic dye.
- the dye is selected from those having: anthraquinone; mono-azo; bis-azo; xanthene; phthalocyanine; and, phenazine chromophores. More preferably the dye is selected from those having: anthraquinone and, mono-azo chromophores.
- the dye is added to the coating solution or slurry and agitated before applying to the core of the particle.
- Application may be by any suitable method, preferably spraying on to the core particle as detailed above.
- the dye may be any colour, preferable the dye is blue, violet, green or red. Most preferably the dye is blue or violet.
- the dye is selected from: acid blue 80, acid blue 62, acid violet 43, acid green 25, direct blue 86, acid blue 59, acid blue 98, direct violet 9, direct violet 99, direct violet 35, direct violet 51, acid violet 50, acid yellow 3, acid red 94, acid red 51, acid red 95, acid red 92, acid red 98, acid red 87, acid yellow 73, acid red 50, acid violet 9, acid red 52, food black 1, food black 2, acid red 163, acid black 1, acid orange 24, acid yellow 23, acid yellow 40, acid yellow 11, acid red 180, acid red 155, acid red 1, acid red 33, acid red 41, acid red 19, acid orange 10, acid red 27, acid red 26, acid orange 20, acid orange 6, sulphonated Al and Zn phthalocyanines, solvent violet 13, disperse violet 26, disperse violet 28, solvent green 3, solvent blue 63, disperse blue 56, disperse violet 27, solvent yellow 33, disperse blue 79:1.
- the dye is preferably a shading dye for imparting a perception of whiteness to a laundry textile.
- the dye may be covalently bound to polymeric species. A combination of dyes may be used.
- the particle preferably comprises from 0 to 15 wt% water, more preferably 0 to 10 wt%, most preferably from 1 to 5 wt% water, at 293K and 50% relative humidity. This facilitates the storage stability of the particle and its mechanical properties.
- adjuncts as described below may be present in the coating or the core of the particle.
- the coated laundry detergent particle preferably comprises a fluorescent agent (optical brightener).
- fluorescent agents are well known and many such fluorescent agents are available commercially. Usually, these fluorescent agents are supplied and used in the form of their alkali metal salts, for example, the sodium salts.
- the total amount of the fluorescent agent or agents used in the composition is generally from 0.005 to 2 wt %, more preferably 0.01 to 0.1 wt %. Suitable fluorescer for use in the invention are described in chapter 7 of Industrial Dyes edited by K. Hunger 2003 Wiley-VCH ISBN 3-527-30426-6 .
- Preferred fluorescers are selected from the classes of distyrylbiphenyls, triazinylaminostilbenes, bis(1,2,3-triazol-2-yl)stilbenes, bis(benzo[b]furan-2-yl)biphenyls, 1,3-diphenyl-2-pyrazolines and courmarins.
- the fluorescer is preferably sulfonated.
- Preferred classes of fluorescer are: Di-styryl biphenyl compounds, e.g. Tinopal (Trade Mark) CBS-X, Di-amine stilbene di-sulphonic acid compounds, e.g. Tinopal DMS pure Xtra and Blankophor (Trade Mark) HRH, and Pyrazoline compounds, e.g. Blankophor SN.
- Di-styryl biphenyl compounds e.g. Tinopal (Trade Mark) CBS-X
- Di-amine stilbene di-sulphonic acid compounds e.g. Tinopal DMS pure Xtra and Blankophor (Trade Mark) HRH
- Pyrazoline compounds e.g. Blankophor SN.
- Preferred fluorescers are: sodium 2 (4-styryl-3-sulfophenyl)-2H-napthol[1,2-d]triazole, disodium 4,4'-bis ⁇ [(4-anilino-6-(N methyl-N-2 hydroxyethyl) amino 1,3,5-triazin-2-yl)]amino ⁇ stilbene-2-2' disulfonate, disodium 4,4'-bis ⁇ [(4-anilino-6-morpholino-1,3,5-triazin-2-yl)]amino ⁇ stilbene-2-2' disulfonate, and disodium 4,4'-bis(2-sulfostyryl)biphenyl.
- Tinopal® DMS is the disodium salt of disodium 4,4'-bis ⁇ [(4-anilino-6-morpholino-1,3,5-triazin-2-yl)]amino ⁇ stilbene-2-2' disulfonate.
- Tinopal® CBS is the disodium salt of disodium 4,4'-bis(2-sulfostyryl)biphenyl.
- the composition comprises a perfume.
- the perfume is preferably in the range from 0.001 to 3 wt%, most preferably 0.1 to 1 wt%.
- CTFA Cosmetic, Toiletry and Fragrance Association
- Many suitable examples of perfumes are provided in the CTFA (Cosmetic, Toiletry and Fragrance Association) 1992 International Buyers Guide, published by CFTA Publications and OPD 1993 Chemicals Buyers Directory 80th Annual Edition, published by Schnell Publishing Co .
- compositions of the present invention it is envisaged that there will be four or more, preferably five or more, more preferably six or more or even seven or more different perfume components.
- top notes are defined by Poucher (Journal of the Society of Cosmetic Chemists 6(2):80 [1955 ]).
- Preferred top-notes are selected from citrus oils, linalool, linalyl acetate, lavender, dihydromyrcenol, rose oxide and cis-3-hexanol.
- the composition may comprise one or more polymers.
- polymers are carboxymethylcellulose, poly (ethylene glycol), poly(vinyl alcohol), polyethylene imines, ethoxylated polyethylene imines, water soluble polyester polymers polycarboxylates such as polyacrylates, maleic/acrylic acid copolymers and lauryl methacrylate/acrylic acid copolymers.
- One or more enzymes are preferred present in a composition of the invention.
- the level of each enzyme is from 0.0001 wt% to 0.5 wt% protein on product.
- enzymes include proteases, alpha-amylases, cellulases, lipases, peroxidases/oxidases, pectate lyases, and mannanases, or mixtures thereof.
- Suitable lipases include those of bacterial or fungal origin. Chemically modified or protein engineered mutants are included. Examples of useful lipases include lipases from Humicola (synonym Thermomyces), e.g. from H. lanuginosa (T. lanuginosus) as described in EP 258 068 and EP 305 216 or from H. insolens as described in WO 96/13580 , a Pseudomonas lipase, e.g. from P. alcaligenes or P. pseudoalcaligenes ( EP 218 272 ), P. cepacia ( EP 331 376 ), P. stutzeri ( GB 1,372,034 ), P .
- lipase variants such as those described in WO 92/05249 , WO 94/01541 , EP 407 225 , EP 260 105 , WO 95/35381 , WO 96/00292 , WO 95/30744 , WO 94/25578 , WO 95/14783 , WO 95/22615 , WO 97/04079 and WO 97/07202 , WO 00/60063 , WO 09/107091 and WO09/111258 .
- LipolaseTM and Lipolase UltraTM LipexTM (Novozymes A/S) and LipocleanTM.
- phospholipase classified as EC 3.1.1.4 and/or EC 3.1.1.32.
- phospholipase is an enzyme which has activity towards phospholipids.
- Phospholipids such as lecithin or phosphatidylcholine, consist of glycerol esterified with two fatty acids in an outer (sn-1) and the middle (sn-2) positions and esterified with phosphoric acid in the third position; the phosphoric acid, in turn, may be esterified to an amino-alcohol.
- Phospholipases are enzymes which participate in the hydrolysis of phospholipids.
- phospholipases A 1 and A 2 which hydrolyze one fatty acyl group (in the sn-1 and sn-2 position, respectively) to form lysophospholipid
- lysophospholipase or phospholipase B
- Phospholipase C and phospholipase D release diacyl glycerol or phosphatidic acid respectively.
- proteases include those of animal, vegetable or microbial origin. Microbial origin is preferred. Chemically modified or protein engineered mutants are included.
- the protease may be a serine protease or a metallo protease, preferably an alkaline microbial protease or a trypsin-like protease.
- Preferred commercially available protease enzymes include AlcalaseTM, SavinaseTM, PrimaseTM, DuralaseTM, DyrazymTM, EsperaseTM, EverlaseTM, PolarzymeTM, and KannaseTM, (Novozymes A/S), MaxataseTM, MaxacalTM, MaxapemTM, ProperaseTM, PurafectTM, Purafect OxPTM, FN2TM, and FN3TM (Genencor International Inc.).
- the method of the invention may be carried out in the presence of cutinase. classified in EC 3.1.1.74.
- the cutinase used according to the invention may be of any origin.
- Preferably cutinases are of microbial origin, in particular of bacterial, of fungal or of yeast origin.
- Suitable amylases include those of bacterial or fungal origin. Chemically modified or protein engineered mutants are included. Amylases include, for example, alpha-amylases obtained from Bacillus, e.g. a special strain of B. licheniformis, described in more detail in GB 1,296,839 , or the Bacillus sp. strains disclosed in WO 95/026397 or WO 00/060060 .
- amylases are DuramylTM, TermamylTM, Termamyl UltraTM, NatalaseTM, StainzymeTM, FungamylTM and BANTM (Novozymes A/S), RapidaseTM and PurastarTM (from Genencor International Inc.).
- Suitable cellulases include those of bacterial or fungal origin. Chemically modified or protein engineered mutants are included. Suitable cellulases include cellulases from the genera Bacillus, Pseudomonas, Humicola, Fusarium, Thielavia, Acremonium, e.g. the fungal cellulases produced from Humicola insolens, Thielavia terrestris, Myceliophthora thermophila, and Fusarium oxysporum disclosed in US 4,435,307 , US 5,648,263 , US 5,691,178 , US 5,776,757 , WO 89/09259 , WO 96/029397 , and WO 98/012307 .
- cellulases include CelluzymeTM, CarezymeTM, EndolaseTM, RenozymeTM (Novozymes A/S), ClazinaseTM and Puradax HATM (Genencor International Inc.), and KAC-500(B)TM (Kao Corporation).
- Suitable peroxidases/oxidases include those of plant, bacterial or fungal origin. Chemically modified or protein engineered mutants are included. Examples of useful peroxidases include peroxidases from Coprinus, e.g. from C. cinereus, and variants thereof as those described in WO 93/24618 , WO 95/10602 , and WO 98/15257 . Commercially available peroxidases include GuardzymeTM and NovozymTM 51004 (Novozymes A/S).
- Combinations of enzymes may be used. It is particularly preferred to use a combination of lipase, protease and one or both of amylase and mannanase.
- Any enzyme present in the composition may be stabilized using conventional stabilizing agents, e.g., a polyol such as propylene glycol or glycerol, a sugar or sugar alcohol, lactic acid, boric acid, or a boric acid derivative, e.g., an aromatic borate ester, or a phenyl boronic acid derivative such as 4-formylphenyl boronic acid, and the composition may be formulated as described in e.g. WO 92/19709 and WO 92/19708 .
- a polyol such as propylene glycol or glycerol
- a sugar or sugar alcohol lactic acid, boric acid, or a boric acid derivative, e.g., an aromatic borate ester, or a phenyl boronic acid derivative such as 4-formylphenyl boronic acid
- Sequesterants for ions other than calcium and magnesium may be present in the coated laundry detergent particles.
- Citrate is preferably present at a level of 5-15%wt on total particle. Citrate can be present either as the salt, or as the acid. When present as the acid evolved gas is generated on exposure of the particles to water. This can generate local turbulence which promotes dissolution and acts as a cue for cleaning.
- Phosphonates, for example such as DequestTM 2010 can also be used as sequestering agents.
- the coated laundry detergent particle does not contain a peroxygen bleach, e.g., percarbonate, perborate, and other peracids.
- a peroxygen bleach e.g., percarbonate, perborate, and other peracids.
- Preferred "chassis” formulations comprise:
- Either of the LAS/NI based or LAS/PAS/SLES based options can be combined with citrate/citric acid at a level of 5-10 wt%.
- Either formulation may comprise a soil release polymer, preferably a polyester material, for example Texcare SRN170 at a level of 1-5 wt%.
- the extruded product was cut after the die-plate using a high speed cutter set up to produce particle with a thickness of ⁇ 1.1 mm.
- the cutter deforms the discs cut from the extruded product to give a slight difference between dimensions y and z.
- the coating solution was fed to the spray nozzle of the Strea 1 via a peristaltic pump (Watson-Marlow model 101 U/R) at an initial rate of 3.3g/min, rising to 9.1 g/min during the course of the coating trial.
- a peristaltic pump Wood-Marlow model 101 U/R
- the Fluid bed coater was operated with an initial air inlet air temperature of 55°C increasing to 90°C during the course of the coating trial whilst maintaining the outlet temperature in the range 45-50°C throughout the coating process.
- compositions of the present invention show significant improvement over the standard and while they are pourable composition they are also significantly better performing than conventional liquids.
- SkipTM powder (ex Argentina) and the composition of the present invention was sewn into a black cotton cloth sachet. Washes were performed using the cycles mentioned in the table below. Before preparing the sachets the black cotton was prewashed 3 times at 60°C to prevent dye transfer during the black sachet test (Miele professional 350 g Robijn, 8 kg black cotton). Sachets were filled with 10 gram of the different detergent products. Results are shown below.
- the temperature rise on dissolution of a composition of the present invention was compared with the temperature rise of commercial laundry compositions in both a tub formed from a plastics material and a glass jar. In both cases 75g of product was added to 70g of water. The temperature rise was monitored at intervals for 300 seconds. In all cases the temperature rise using the particles of the present invention was significantly less than that using the commercial products.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Detergent Compositions (AREA)
Description
- The present invention relates to laundry cleaning methods using shaped laundry detergent particles.
- Many different wash-habits are known around the world. While "front loading", "automatic", horizontal-axis washing machines with sophisticated control systems are commonplace in Western Europe, many people, especially in developing and emerging markets, still use "top loading" washing vessels. These range from bowls and buckets through devices which provide some mechanical agitation (for example the Brazilian "Tanquinho") to rather more sophisticated top-loading machines.
- With the exception of relatively small quantities of flakes, pastes and tablets, and, in some parts of the world, detergent bars, the vast majority of laundry compositions sold and used are provided as liquids and powders. Powders are a traditional format in many places and users value the effective cleaning that they provide. However, some negatives can be perceived due to the build-up of residues on articles being washed. In part, these residues are due to ash, other un-dissolved solids (for example zeolite builders) and/or due to precipitating builders. The eventual consequence of this build-up can be colour changes of articles which have been laundered.
- Liquid detergents do not have these same residue problems, but as they are largely un-built systems, they can suffer cleaning disadvantages especially in hard-water areas. Liquids have other advantages over powders, for example they do not have the caking problems of solids.
- However, many users remain unconvinced that liquids have the cleaning efficacy of powders. The residue problem with powders is especially marked with top-loading wash habits, where rinsing out of precipitated and/or deposited solids presents a problem, especially where water is scarce or relatively expensive.
-
WO1999/32599 -
EP application 09158717.0 WO 2010/122051 ) discloses high active detergent particles with a low surface roughness produced by extrusion of an anionic surfactant rich composition, and cutting of the extruded product to form slices which are thinner than they are wide. These slices are then spray-coated with, for example, an aqueous solution of sodium carbonate, to form smooth-surfaced, free-flowing particles which may look like "lentils". It is stated in that specification that use of a builder salt such as sodium carbonate is advantageous because it buffers the pH at a level where the anionic surfactant is particularly effective, and increases the ionic strength. The particles are also said to dissolve well in water and are suitable for use in a front loading washing machine (page 6, line 19). - We have determined that in a process involving the use of a top-loading washing vessel, coated extruded particles with a particular shape and formulation show exceptionally good cleaning and care as perceived by a panel of users.
- In an especially preferred embodiment, where the coating is formulated with sodium carbonate monohydrate the particles do not exhibit a "hot on hands" feeling when used.
- Accordingly the present invention provides a laundry process which comprises the steps of:
- a) providing a coated detergent particle having perpendicular dimensions x, y and z, wherein x is from 1 mm to less than 2 mm, y is from more than 2mm to 8mm and z is from more than 2mm to 8 mm, wherein the particle comprises:
- i) from 50 to 90 wt% surfactant selected from anionic surfactant and non-ionic surfactant;
- ii) from 1 to 40 wt% water-soluble inorganic salts;
- iii) from 0 to 20 wt% other ingredients, including less than 15wt% water insoluble materials including fragrance, and,
wherein the inorganic salts are present on the laundry detergent particle as a coating and the surfactant is present as a core.
- b) dissolving said coated detergent particle in water to form a wash liquor
- c) treating articles with said wash liquor,
- d) separating said articles from said wash liquor, and e) rinsing and drying said articles
- Panel studies show that the above method produces softer clothes, with brighter colours, less residues, yet parity of cleaning with conventional powders.
- It is very preferred that the surfactant to builder ratio in the detergent particles is in the range 1.5-2.5:1, more preferably 1.8-2.2:1. As the ratio shifts further towards high builder levels the wash liquor becomes too alkaline and as it shifts towards high surfactant levels buffering capacity is lost. By having the builder present as a coating encasing the surfactant, which is soft and sticky, a particulate concentrate with the preferred ratio of surfactant to builder can be provided. The absence of other materials (carriers for the surfactant, fillers etc) reduce the unit dose required for a wash and therefore reduces transportation costs and increases shelf-use efficiency.
- Environmental impact of chemicals is also reduced. Dosage will typically be significantly smaller than with conventional products, around 0.5-1 g/L of fully formulated product for top loading machines and 1-3 g/L for hand-wash vessels.
- As will be noted from the dimensions, the coated laundry detergent particle has a non-spherical surface. Preferably this surface is in part curved. The coated laundry detergent particle may be described as disk-like, lenticular or an oblate spheroid/ellipsoid, where z and y are the equatorial diameters and x is the polar diameter. It is preferred that y and z are similar in value, although with some methods of manufacture they may differ slightly due to pressures exerted during slicing of an extruded product. In general, the particles have an axis of rotational symmetry along the polar axis.
- Preferably, the coated laundry detergent particle does not have any holes; that is to say, the coated laundry detergent particle has a topologic genus of zero. Preferably y and z are independently in the range 3 to 8 mm. This moves the shape further from a sphere and increases the ratio of surface area to volume, improving solubility. The use of the particles which are "short and fat" rather than elongate and thin, improves the product flow and dose control properties. By coating the relatively large detergent particles of the current invention the thickness of coating obtainable by use of a particular coating level is significantly greater than would be achieved on typically sized detergent granules (0.5-2mm diameter sphere). This makes for a more robust coating and reduces the tendency of the surfactant to bleed through the coating and make the particles sticky. It is also the case that a significant proportion of the builder will dissolve before the surfactant starts to dissolve so that the wash liquor will be at least partially built before the surfactant is dissolved.
- A further advantage of these relatively large particles in the top-loader is that they sink rapidly and come into contact with the impeller at an early stage in the wash.
- For optimum dissolution properties, this surface area to volume ratio must be greater than 3 mm-1. However, the coating thickness is inversely proportional to this coefficient and hence for the coating the ratio "Surface area of coated particle" divided by "Volume of coated particle" should be less than 15 mm-1.
- Preferably, the coated laundry detergent particle is such that at least 90 to 100% of the coated laundry detergent particles in the in the average x, y and z dimensions of individual particles are within a 20%, preferably 10%, variable from the largest to the smallest coated laundry detergent particle. These aggregations of particles with similar sizes show good flow properties, can be,packaged in bottles and can be dosed like a liquid.
- Conveniently the detergent particles comprise from 0.0001 to 0.1 wt % dye, preferably 0.001 to 0.01 wt % dye, wherein the dye is selected: from anionic dyes; and, non-ionic dyes. Dyes serve several useful functions. They may assist the user in recognising different products, and/or may act as shading agents to mask yellowness of articles being laundered. If is preferable that not all of the particles are of the same colour, and that two or more colours of particles are present, for example white and blue, white and pink or white and orange.
- Unless otherwise stated all wt % refer to the total percentage in the particle as dry weights (including water of hydration where present).
- In order that the invention may be further and better understood it will be further explained with reference to various preferred and/or optional features.
- For the purposes of the present specification and in the context of the present invention a top loading washing vessel is any vessel which is used by end-users to launder clothes. Typically, such vessels have a capacity in excess of 4 litres.
- Such vessels include, and preferentially comprise, top loading semi-automatic and automatic washing machines, preferably with means to agitate the wash liquor within them, and, in addition or in the alternative, other vessels where the agitation is manual, preferably a bath, tub, bucket, basin, bowl or sink. Machines include machines such as and similar to a Brazilian "Tanquinho".
- In a preferred embodiment of the invention the vessel is open at the top during the washing operation and at least part of the agitation is provided by hand.
- The coated laundry detergent particle comprises between 50 to 90 wt% of a surfactant, most preferably 70 to 90 wt%. In general, the nonionic and anionic surfactants of the surfactant system may be chosen from the surfactants described "Surface Active Agents" Vol. 1, by Schwartz & Perry, Interscience 1949, Vol. 2 by Schwartz, Perry & Berch, Interscience 1958, in the current edition of "McCutcheon's Emulsifiers and Detergents" published by Manufacturing Confectioners Company or in "Tenside-Taschenbuch", H. Stache, 2nd Edn., Carl Hauser Verlag, 1981. Preferably the surfactants used are saturated.
- Suitable anionic detergent compounds which may be used are usually water-soluble alkali metal salts of organic sulphates and sulphonates having alkyl radicals containing from about 8 to about 22 carbon atoms, the term alkyl being used to include the alkyl portion of higher acyl radicals. Examples of suitable synthetic anionic detergent compounds are sodium and potassium alkyl sulphates, especially those obtained by sulphating higher C8 to C18 alcohols, produced for example from tallow or coconut oil, sodium and potassium alkyl C9 to C20 benzene sulphonates, particularly sodium linear secondary alkyl C10 to C15 benzene sulphonates; and sodium alkyl glyceryl ether sulphates, especially those ethers of the higher alcohols derived from tallow or coconut oil and synthetic alcohols derived from petroleum.
- Most preferred anionic surfactants are sodium lauryl ether sulfate (SLES), particularly preferred with 1 to 3 ethoxy groups, sodium C10 to C15 alkyl benzene sulphonates (LAS) and sodium C12 to C18 alkyl sulphates (PAS). Alkyl ester suphonates such as Methyl ester sulphonates (MES) may be used in whole or part replacement for the other anionics.
- Also applicable are surfactants such as those described in
EP-A-328 177 EP-A-070 074 - Soaps may also be present. The fatty acid soap used preferably contains from about 16 to about 22 carbon atoms, preferably in a straight chain configuration. The anionic contribution from soap is preferably from 0 to 30 wt% of the total anionic.
- Preferably, at least 50 wt % of the anionic surfactant is selected from: sodium C11 to C15 alkyl benzene sulphonates; and, sodium C12 to C18 alkyl sulphates. Even more preferably, the anionic surfactant is sodium C11 to C15 alkyl benzene sulphonates.
- Preferably the anionic surfactant is present in the coated laundry detergent particle at levels between 15 to 85 wt%, more preferably 50 to 80wt% on total surfactant.
- Suitable nonionic detergent compounds which may be used include, in particular, the reaction products of compounds having a hydrophobic group and a reactive hydrogen atom, for example, aliphatic alcohols, acids, amides or alkyl phenols with alkylene oxides, especially ethylene oxide either alone or with propylene oxide. Preferred nonionic detergent compounds are C6 to C22 alkyl phenol-ethylene oxide condensates, generally 5 to 25 EO, i.e. 5 to 25 units of ethylene oxide per molecule, and the condensation products of aliphatic C8 to C18 primary or secondary linear or branched alcohols with ethylene oxide, generally 5 to 50 EO. Preferably, the non-ionic is 10 to 50 EO, more preferably 20 to 35 EO. Alkyl ethoxylates are particularly preferred, most particularly those with 25-35 EO, as these give a particularly good foam profile.
- Preferably the nonionic surfactant is present in the coated laundry detergent particle at levels between 5 to 75 wt% on total surfactant, more preferably 10 to 40 wt% on total surfactant.
- Cationic surfactant may be present as minor ingredients at levels preferably between 0 to 5 wt% on total surfactant.
- Preferably all the surfactants are mixed together before being dried. Conventional mixing equipment may be used. The surfactant core of the laundry detergent particle may be formed by extrusion or roller compaction and subsequently coated with an inorganic salt.
- Foam boosters such as betaines, amine oxides and/or other zwitterionic and aphphoteric surfactants are optional as the compositions of the invention generally show adequate foaming.
- In another aspect the surfactant system used is calcium tolerant and this is a preferred aspect because this reduces the need for builder.
- Surfactant blends that do not require builders to be present for effective detergency in hard water are preferred. Such blends are called calcium tolerant surfactant blends if they pass the test set out hereinafter. However, the invention may also be of use for washing with soft water, either naturally occurring or made using a water softener. In this case, calcium tolerance is no longer important and blends other than calcium tolerant ones may be used.
- The surfactant blend in question is prepared at a concentration of 0.7 g surfactant solids per litre of water containing sufficient calcium ions to give a French hardness of 40 (4 x 10-3 Molar Ca2+). Other hardness ion free electrolytes such as sodium chloride, sodium sulphate, and sodium hydroxide are added to the solution to adjust the ionic strength to 0.05M and the pH to 10. The adsorption of light of wavelength 540 nm through 4 mm of sample is measured 15 minutes after sample preparation. Ten measurements are made and an average value is calculated. Samples that give an absorption value of less than 0.08 are deemed to be calcium tolerant.
- Examples of surfactant blends that satisfy the above test for calcium tolerance include those having a major part of LAS surfactant (which is not of itself calcium tolerant) blended with one or more other surfactants (co-surfactants) that are calcium tolerant to give a blend that is sufficiently calcium tolerant to be usable with little or no builder and to pass the given test. Suitable calcium tolerant co-surfactants include SLES 1-7EO, and alkyl-ethoxylate nonionic surfactants, particularly those with melting points less than 40°C.
- A LAS/SLES surfactant blend has a superior foam profile to a LAS nonionic surfactant blend and is therefore preferred for hand washing formulations requiring high levels of foam. SLES may be used at levels of up to 30wt% of the surfactant blend.
- The water-soluble inorganic salts are preferably selected from water soluble salts of carbonate, chloride, silicate and sulphate, or mixtures thereof, most preferably, 70 to 100 wt% sodium carbonate on total water-soluble inorganic salts. The water-soluble inorganic salt is present as a coating on the particle.
- It will be appreciated by those skilled in the art that while multiple layered coatings, of the same or different coating materials, could be applied, a single coating layer is preferred, for simplicity of operation, and to maximise the thickness of the coating. The amount of coating should lay in the range 1 to 40 wt% of the particle, preferably 20 to 40 wt%, more preferably 25 to 35 wt% for the best results in terms of anti-caking properties of the detergent particles.
- The coating is preferably applied to the surface of the surfactant core, by deposition from an aqueous solution of the water soluble inorganic salt. In the alternative coating can be performed using a slurry. The aqueous solution preferably contains greater than 50g/L, more preferably 200 g/L of the salt. An aqueous spray-on of the coating solution in a fluidised bed has been found to give good results and may also generate a slight rounding of the detergent particles during the fluidisation process. Drying and/or cooling may be needed to finish the process.
- A preferred calcium tolerant coated laundry detergent particle comprises 15 to 100 wt% on surfactant of anionic surfactant of which 20 to 30 wt% on surfactant is sodium lauryl ether sulphate.
- A highly preferred water soluble inorganic salt for inclusion in the coating is sodium carbonate monohydrate. Where, during the wash process, the coated detergent particles contact both water and the skin of the user, this confers the distinct benefit that upon exposure to water the particles do not generate as much heat as they would if the carbonate was anhydrous. This is an important preferred feature as users often mix detergent products with water by hand, and describe products which employ anhydrous sodium carbonate as having a "hot on hands" feeling.
- It is preferred that of the inorganic salts in the coating is 100% by weight sodium carbonate monohydrate. This gives the best protection against moisture. The level of sodium carbonate monohydrate can be as low as 50% by weight of the inorganic salts in the coating.
- Dyes are a highly preferred component of the present invention. Suitable dyes are described in Industrial Dyes edited by K. Hunger 2003 Wiley-VCH ISBN 3-527-30426-6.
- Dyes for use in the current invention are selected from anionic and non-ionic dyes Anionic dyes are negatively charged in an aqueous medium at pH 7. Examples of anionic dyes are found in the classes of acid and direct dyes in the Color Index (Society of Dyers and Colourists and American Association of Textile Chemists and Colorists). Anionic dyes preferably contain at least one sulphonate or carboxylate groups. Non-ionic dyes are uncharged in an aqueous medium at pH 7, examples are found in the class of disperse dyes in the Color Index.
- The dyes may be alkoxylated. Alkoxylated dyes are preferably of the following generic form: Dye-NR1R2. The NR1R2 group is attached to an aromatic ring of the dye. R1 and R2 are independently selected from polyoxyalkylene chains having 2 or more repeating units and preferably having 2 to 20 repeating units. Examples of polyoxyalkylene chains include ethylene oxide, propylene oxide, glycidol oxide, butylene oxide and mixtures thereof.
- A preferred polyoxyalkylene chain is [(CH2CR3HO)x(CH2CR4HO)yR5) in which x+y ≤ 5 wherein y ≥ 1 and z = 0 to 5, R3 is selected from: H; CH3; CH2O(CH2CH2O)zH and mixtures thereof; R4 is selected from: H; CH2O(CH2CH2O)zH and mixtures thereof; and, R5 is selected from: H; and, CH3
-
- Preferably the dye is selected from acid dyes; disperse dyes and alkoxylated dyes.
- Most preferably the dye is a non-ionic dye.
- Preferably the dye is selected from those having: anthraquinone; mono-azo; bis-azo; xanthene; phthalocyanine; and, phenazine chromophores. More preferably the dye is selected from those having: anthraquinone and, mono-azo chromophores.
- To obtain coloured particles, the dye is added to the coating solution or slurry and agitated before applying to the core of the particle. Application may be by any suitable method, preferably spraying on to the core particle as detailed above.
- The dye may be any colour, preferable the dye is blue, violet, green or red. Most preferably the dye is blue or violet.
- Preferably the dye is selected from: acid blue 80, acid blue 62, acid violet 43, acid green 25, direct blue 86, acid blue 59, acid blue 98, direct violet 9, direct violet 99, direct violet 35, direct violet 51, acid violet 50, acid yellow 3, acid red 94, acid red 51, acid red 95, acid red 92, acid red 98, acid red 87, acid yellow 73, acid red 50, acid violet 9, acid red 52, food black 1, food black 2, acid red 163, acid black 1, acid orange 24, acid yellow 23, acid yellow 40, acid yellow 11, acid red 180, acid red 155, acid red 1, acid red 33, acid red 41, acid red 19, acid orange 10, acid red 27, acid red 26, acid orange 20, acid orange 6, sulphonated Al and Zn phthalocyanines, solvent violet 13, disperse violet 26, disperse violet 28, solvent green 3, solvent blue 63, disperse blue 56, disperse violet 27, solvent yellow 33, disperse blue 79:1.
- The dye is preferably a shading dye for imparting a perception of whiteness to a laundry textile. The dye may be covalently bound to polymeric species. A combination of dyes may be used.
- The particle preferably comprises from 0 to 15 wt% water, more preferably 0 to 10 wt%, most preferably from 1 to 5 wt% water, at 293K and 50% relative humidity. This facilitates the storage stability of the particle and its mechanical properties.
- The adjuncts as described below may be present in the coating or the core of the particle.
- The coated laundry detergent particle preferably comprises a fluorescent agent (optical brightener). Fluorescent agents are well known and many such fluorescent agents are available commercially. Usually, these fluorescent agents are supplied and used in the form of their alkali metal salts, for example, the sodium salts. The total amount of the fluorescent agent or agents used in the composition is generally from 0.005 to 2 wt %, more preferably 0.01 to 0.1 wt %. Suitable fluorescer for use in the invention are described in chapter 7 of Industrial Dyes edited by K. Hunger 2003 Wiley-VCH ISBN 3-527-30426-6.
- Preferred fluorescers are selected from the classes of distyrylbiphenyls, triazinylaminostilbenes, bis(1,2,3-triazol-2-yl)stilbenes, bis(benzo[b]furan-2-yl)biphenyls, 1,3-diphenyl-2-pyrazolines and courmarins. The fluorescer is preferably sulfonated.
- Preferred classes of fluorescer are: Di-styryl biphenyl compounds, e.g. Tinopal (Trade Mark) CBS-X, Di-amine stilbene di-sulphonic acid compounds, e.g. Tinopal DMS pure Xtra and Blankophor (Trade Mark) HRH, and Pyrazoline compounds, e.g. Blankophor SN. Preferred fluorescers are: sodium 2 (4-styryl-3-sulfophenyl)-2H-napthol[1,2-d]triazole, disodium 4,4'-bis{[(4-anilino-6-(N methyl-N-2 hydroxyethyl) amino 1,3,5-triazin-2-yl)]amino}stilbene-2-2' disulfonate, disodium 4,4'-bis{[(4-anilino-6-morpholino-1,3,5-triazin-2-yl)]amino} stilbene-2-2' disulfonate, and disodium 4,4'-bis(2-sulfostyryl)biphenyl.
- Tinopal® DMS is the disodium salt of disodium 4,4'-bis{[(4-anilino-6-morpholino-1,3,5-triazin-2-yl)]amino} stilbene-2-2' disulfonate. Tinopal® CBS is the disodium salt of disodium 4,4'-bis(2-sulfostyryl)biphenyl.
- Preferably the composition comprises a perfume. The perfume is preferably in the range from 0.001 to 3 wt%, most preferably 0.1 to 1 wt%. Many suitable examples of perfumes are provided in the CTFA (Cosmetic, Toiletry and Fragrance Association) 1992 International Buyers Guide, published by CFTA Publications and OPD 1993 Chemicals Buyers Directory 80th Annual Edition, published by Schnell Publishing Co.
- It is commonplace for a plurality of perfume components to be present in a formulation. In the compositions of the present invention it is envisaged that there will be four or more, preferably five or more, more preferably six or more or even seven or more different perfume components.
- In perfume mixtures preferably 15 to 25 wt% are top notes. Top notes are defined by Poucher (Journal of the Society of Cosmetic Chemists 6(2):80 [1955]). Preferred top-notes are selected from citrus oils, linalool, linalyl acetate, lavender, dihydromyrcenol, rose oxide and cis-3-hexanol.
- The composition may comprise one or more polymers. Examples are carboxymethylcellulose, poly (ethylene glycol), poly(vinyl alcohol), polyethylene imines, ethoxylated polyethylene imines, water soluble polyester polymers polycarboxylates such as polyacrylates, maleic/acrylic acid copolymers and lauryl methacrylate/acrylic acid copolymers.
- One or more enzymes are preferred present in a composition of the invention.
- Preferably the level of each enzyme is from 0.0001 wt% to 0.5 wt% protein on product.
- Especially contemplated enzymes include proteases, alpha-amylases, cellulases, lipases, peroxidases/oxidases, pectate lyases, and mannanases, or mixtures thereof.
- Suitable lipases include those of bacterial or fungal origin. Chemically modified or protein engineered mutants are included. Examples of useful lipases include lipases from Humicola (synonym Thermomyces), e.g. from H. lanuginosa (T. lanuginosus) as described in
EP 258 068 EP 305 216 WO 96/13580 EP 218 272 EP 331 376 GB 1,372,034 WO 95/06720 WO 96/27002 WO 96/12012 JP 64/744992 WO 91/16422 - Other examples are lipase variants such as those described in
WO 92/05249 WO 94/01541 EP 407 225 EP 260 105 WO 95/35381 WO 96/00292 WO 95/30744 WO 94/25578 WO 95/14783 WO 95/22615 WO 97/04079 WO 97/07202 WO 00/60063 WO 09/107091 WO09/111258 - Preferred commercially available lipase enzymes include Lipolase™ and Lipolase Ultra™, Lipex™ (Novozymes A/S) and Lipoclean™.
- The method of the invention may be carried out in the presence of phospholipase classified as EC 3.1.1.4 and/or EC 3.1.1.32. As used herein, the term phospholipase is an enzyme which has activity towards phospholipids. Phospholipids, such as lecithin or phosphatidylcholine, consist of glycerol esterified with two fatty acids in an outer (sn-1) and the middle (sn-2) positions and esterified with phosphoric acid in the third position; the phosphoric acid, in turn, may be esterified to an amino-alcohol. Phospholipases are enzymes which participate in the hydrolysis of phospholipids. Several types of phospholipase activity can be distinguished, including phospholipases A1 and A2 which hydrolyze one fatty acyl group (in the sn-1 and sn-2 position, respectively) to form lysophospholipid; and lysophospholipase (or phospholipase B) which can hydrolyze the remaining fatty acyl group in lysophospholipid. Phospholipase C and phospholipase D (phosphodiesterases) release diacyl glycerol or phosphatidic acid respectively.
- Suitable proteases include those of animal, vegetable or microbial origin. Microbial origin is preferred. Chemically modified or protein engineered mutants are included. The protease may be a serine protease or a metallo protease, preferably an alkaline microbial protease or a trypsin-like protease. Preferred commercially available protease enzymes include Alcalase™, Savinase™, Primase™, Duralase™, Dyrazym™, Esperase™, Everlase™, Polarzyme™, and Kannase™, (Novozymes A/S), Maxatase™, Maxacal™, Maxapem™, Properase™, Purafect™, Purafect OxP™, FN2™, and FN3™ (Genencor International Inc.).
- The method of the invention may be carried out in the presence of cutinase. classified in EC 3.1.1.74. The cutinase used according to the invention may be of any origin. Preferably cutinases are of microbial origin, in particular of bacterial, of fungal or of yeast origin.
- Suitable amylases (alpha and/or beta) include those of bacterial or fungal origin. Chemically modified or protein engineered mutants are included. Amylases include, for example, alpha-amylases obtained from Bacillus, e.g. a special strain of B. licheniformis, described in more detail in
GB 1,296,839 WO 95/026397 WO 00/060060 - Suitable cellulases include those of bacterial or fungal origin. Chemically modified or protein engineered mutants are included. Suitable cellulases include cellulases from the genera Bacillus, Pseudomonas, Humicola, Fusarium, Thielavia, Acremonium, e.g. the fungal cellulases produced from Humicola insolens, Thielavia terrestris, Myceliophthora thermophila, and Fusarium oxysporum disclosed in
US 4,435,307 ,US 5,648,263 ,US 5,691,178 ,US 5,776,757 ,WO 89/09259 WO 96/029397 WO 98/012307 - Suitable peroxidases/oxidases include those of plant, bacterial or fungal origin. Chemically modified or protein engineered mutants are included. Examples of useful peroxidases include peroxidases from Coprinus, e.g. from C. cinereus, and variants thereof as those described in
WO 93/24618 WO 95/10602 WO 98/15257 - Further enzymes suitable for use are disclosed in
WO2009/087524 ,WO2009/090576 ,WO2009/148983 andWO2008/007318 . - Combinations of enzymes may be used. It is particularly preferred to use a combination of lipase, protease and one or both of amylase and mannanase.
- Any enzyme present in the composition may be stabilized using conventional stabilizing agents, e.g., a polyol such as propylene glycol or glycerol, a sugar or sugar alcohol, lactic acid, boric acid, or a boric acid derivative, e.g., an aromatic borate ester, or a phenyl boronic acid derivative such as 4-formylphenyl boronic acid, and the composition may be formulated as described in e.g.
WO 92/19709 WO 92/19708 - Sequesterants for ions other than calcium and magnesium may be present in the coated laundry detergent particles. One suitable sequesterant which also functions as a builder as removing iron, is citrate. Citrate is preferably present at a level of 5-15%wt on total particle. Citrate can be present either as the salt, or as the acid. When present as the acid evolved gas is generated on exposure of the particles to water. This can generate local turbulence which promotes dissolution and acts as a cue for cleaning. Phosphonates, for example such as Dequest™ 2010 can also be used as sequestering agents.
- It is preferred that the coated laundry detergent particle does not contain a peroxygen bleach, e.g., percarbonate, perborate, and other peracids.
- Preferred "chassis" formulations comprise:
- LAS/NI based (lower foam option):
- (i) from 50 to 70 wt% sodium LAS and 7-35EO nonionic;
- (ii) from 20 to 30 wt% sodium carbonate;
- (iii) from 1-3 wt% carboxy methyl cellulose;
- (iv) from 1-3 wt% fragrance; and,
- (v) 0-2wt% moisture.
- In the above formulation some of the LAS can be replaced with PAS.
- LAS/PAS/SLES based (high foam option):
- (i) from 50 to 70 wt% sodium LAS, PAS and SLES;
- (ii) from 20 to 30 wt% sodium carbonate;
- (iii) from 1-3 wt% carboxy methyl cellulose;
- (iv) from 1-3 wt% fragrance; and,
- (v) 0-2wt% moisture.
- Either of the LAS/NI based or LAS/PAS/SLES based options can be combined with citrate/citric acid at a level of 5-10 wt%.
- Either formulation may comprise a soil release polymer, preferably a polyester material, for example Texcare SRN170 at a level of 1-5 wt%.
- In order that the invention may be further understood and carried forth in practice it will be further described by way of example.
- 1962.5g of dried, milled surfactant blend (LAS/PAS/NI 68/17/15 by weight) was thoroughly mixed with 37.38g of perfume oil. The mixture was then extruded using a ThermoFisher 24HC twin screw extruder, operated at a rate of 8kg/hr. Inlet temperature of the extruder was set at 20°C, rising to 40°C just prior to the die-plate. The die-plate used was drilled with 6 circular orifices of 5mm diameter.
- The extruded product was cut after the die-plate using a high speed cutter set up to produce particle with a thickness of ∼1.1 mm. The cutter deforms the discs cut from the extruded product to give a slight difference between dimensions y and z.
- 764g of the extrudates above were charged to the fluidising chamber of a Strea 1 laboratory fluid bed drier (Aeromatic-Fielder AG) and spray coated using 1069g of a solution containing 320.7g of sodium carbonate in 748.3g of water, using a top-spray configuration.
- The coating solution was fed to the spray nozzle of the Strea 1 via a peristaltic pump (Watson-Marlow model 101 U/R) at an initial rate of 3.3g/min, rising to 9.1 g/min during the course of the coating trial.
- The Fluid bed coater was operated with an initial air inlet air temperature of 55°C increasing to 90°C during the course of the coating trial whilst maintaining the outlet temperature in the range 45-50°C throughout the coating process.
- The resulting particles were oblate elipisoids which had the following average dimensions: x= 1.1 mm y= 4.0 mm z= 5.0 mm. The particles weighed ∼0.013g each.
- Wash studies were performed in Brazilian Whirlpool top loading washing machine set on cold wash medium fill (45 L) with 12FH water. Load comprised 3kg with 31% knitted cotton, 38% poly-cotton and 31% knitted polyester. Six repeat washes were performed, using a product dosage of 20-25g. Detergency was measured using an extensive monitor set with particulate, fatty and oily, enzyme-sensitive and bleachable stains. The results are given in the table below.
- Higher numerical results show a better score against the standard which has been set at the "Skip"™ powder.
Stain Class Skip (powder ex. Argentina) 50-60g/wash OMO (liquid ex. China) 75ml/wash Present invention 20-25g/wash Particulate 1 -3.5 4.2 Fatty & Oily 1 -2.9 1.0 Enzymatic 1 -3.9 2.0 Bleachable 1 -2.3 -1.0 Redep 1 -0.1 -0.1 - From these results it can be seen that the compositions of the present invention show significant improvement over the standard and while they are pourable composition they are also significantly better performing than conventional liquids.
- Skip™ powder (ex Argentina) and the composition of the present invention was sewn into a black cotton cloth sachet. Washes were performed using the cycles mentioned in the table below. Before preparing the sachets the black cotton was prewashed 3 times at 60°C to prevent dye transfer during the black sachet test (Miele professional 350 g Robijn, 8 kg black cotton). Sachets were filled with 10 gram of the different detergent products. Results are shown below.
Skip (comparative) Invention 40 degrees cotton cycle some residue no residue 30 degrees woollens cycle much residue slight residues - The results show that the embodiments of the invention, while starting with a larger particle size than the powder, still gave fewer residues.
- A panel of 80 consumers from Thailand and South Africa were asked to compare the product according to the invention with their current powder products under confidentiality.
- Statistically significant findings were that the product according to the invention "dissolves completely", "cleans effectively", "leaves the clothes bright", "leaves the clothes soft" and "left clothes easier to iron".
- In this technical observation study the consumers noted that the product according to the invention was not, in comparison with their current powder product, "hot on hands".
- The temperature rise on dissolution of a composition of the present invention was compared with the temperature rise of commercial laundry compositions in both a tub formed from a plastics material and a glass jar. In both cases 75g of product was added to 70g of water. The temperature rise was monitored at intervals for 300 seconds. In all cases the temperature rise using the particles of the present invention was significantly less than that using the commercial products.
- The results given below are temperatures for products as listed on the left, used with the tub.
Recorded Temperature (Celsius) Time (seconds) 0 20 40 60 120 300 Persil ™ (ex UK) 23.2 32.0 34.1 33.7 34.1 32.5 Brilhante™ (ex Brazil) 23.5 28.6 30.8 31.0 30.9 29.8 Embodiment 23.5 22.9 23.2 25.2 24.8 24.3 - It can be seen that the initial temperature rise for the embodiment of the invention was far lower than that for the compared commercial products.
Claims (15)
- A laundry process which comprises the steps of:a) providing a coated detergent particle having perpendicular dimensions x, y and z, wherein x is from 1 mm to less than 2 mm, y is from more than 2mm to 8mm and z is from more than 2mm to 8 mm, wherein the particle comprises:i) from 50 to 90 wt% surfactant selected from anionic surfactant, nonionic surfactant and mixtures thereof;ii) from 1 to 40 wt% water-soluble inorganic salts;iii) from 0 to 20 wt% other ingredients, including less than 15wt% water insoluble materials including fragrance, and,
wherein, the inorganic salts are present on the laundry detergent particle as a coating and the surfactant is present as a core;b) dissolving said coated detergent particle in water to form a wash liquor;c) treating articles with said wash liquor;d) separating said articles from said wash liquor; and,e) rinsing and drying said articles;
wherein, at least step (c) is performed in a top-loading washing vessel. - A process according to claim 1 wherein the coated detergent particle further comprises one or more dyes, preferably selected from acid dyes; disperse dyes and alkoxylated dyes, more preferably selected from those having: anthraquinone; mono-azo; bis-azo; xanthene; phthalocyanine; and, phenazine chromophores, most preferably selected from those having: anthraquinone and mono-azo; chromophores.
- A process according to claim 1 or 2, wherein the inorganic salts present on the laundry detergent particle as a coating comprise sodium carbonate.
- A process according to any of the preceding claims, wherein the anionic surfactant comprises alkyl benzene sulphonates, preferably sodium C10 to C15 alkyl benzene sulphonates; alkyl ether sulphates, preferably sodium lauryl ether sulfate with 1 to 3 ethoxy groups; and/or alkyl sulphates, preferably sodium C12 to C18 alkyl sulphates.
- A process according to any of claims 1 to 4, wherein the surfactant comprises 15 to 100 wt% anionic surfactant of which 20 to 30 wt% is sodium lauryl ether sulphate, based on total surfactant.
- A process according to any of claims 1 to 4, wherein the surfactant comprises from 15 to 85 wt% anionic surfactant and from 5 to 75 wt% non-ionic surfactant, based on total surfactant.
- A process according to any of claims 1 to 4 and 6, wherein the nonionic surfactant is 10 to 50 EO alcohol ethoxylate.
- A process according to claim 7, wherein the non-ionic surfactant is the condensation products of aliphatic C8 to C18 primary or secondary linear or branched alcohols with 20 to 35 ethylene oxide groups.
- A process according to any of the preceding claims, wherein the coated detergent particle comprises 20 to 40 wt%, preferably 25 to 35 wt% of the inorganic builder salts as a coating.
- A process according to any of the preceding claims, wherein the particle comprises from 0 to 15 wt%, preferably 1 to 5 wt% water.
- A process according to any of the preceding claims, wherein at least 90 to 100 number% of the coated detergent particles in the in the x, y and z dimensions are within a 20, preferably a 10 number% variable from the largest to the smallest coated detergent particle.
- A process according to any of the preceding claims wherein the top-loading washing vessel is selected from top loading semi-automatic or automatic washing machines.
- A process according to claim 12 wherein the machine is provided with means to agitate the wash liquor.
- A process according to any of claims 1-11 wherein the top-loading washing vessel comprise a bath, tub, bucket, basin, bowl or sink.
- A process according to any of the preceding claims wherein during the wash process the coated detergent particles contact both water and the skin of the user.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP11763944.3A EP2627751B1 (en) | 2010-10-14 | 2011-09-30 | Top-loading laundry vessel method |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP10187500 | 2010-10-14 | ||
| EP11763944.3A EP2627751B1 (en) | 2010-10-14 | 2011-09-30 | Top-loading laundry vessel method |
| PCT/EP2011/067159 WO2012049033A1 (en) | 2010-10-14 | 2011-09-30 | Top-loading laundry vessel method |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2627751A1 EP2627751A1 (en) | 2013-08-21 |
| EP2627751B1 true EP2627751B1 (en) | 2015-06-03 |
Family
ID=43759466
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP11763944.3A Active EP2627751B1 (en) | 2010-10-14 | 2011-09-30 | Top-loading laundry vessel method |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP2627751B1 (en) |
| CN (1) | CN103282477B (en) |
| BR (1) | BR112013009127B1 (en) |
| WO (1) | WO2012049033A1 (en) |
| ZA (1) | ZA201302655B (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2020109227A1 (en) | 2018-11-28 | 2020-06-04 | Unilever N.V. | Large particles |
Family Cites Families (52)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1296839A (en) | 1969-05-29 | 1972-11-22 | ||
| GB1372034A (en) | 1970-12-31 | 1974-10-30 | Unilever Ltd | Detergent compositions |
| DK187280A (en) | 1980-04-30 | 1981-10-31 | Novo Industri As | RUIT REDUCING AGENT FOR A COMPLETE LAUNDRY |
| GR76050B (en) * | 1981-02-04 | 1984-08-03 | Unilever Nv | |
| AU556758B2 (en) | 1981-07-13 | 1986-11-20 | Procter & Gamble Company, The | Foaming compositions based on alkylpolysaccharide |
| JPH0697997B2 (en) | 1985-08-09 | 1994-12-07 | ギスト ブロカデス ナ−ムロ−ゼ フエンノ−トチヤツプ | New enzymatic detergent additive |
| ES2058119T3 (en) | 1986-08-29 | 1994-11-01 | Novo Nordisk As | ENZYMATIC DETERGENT ADDITIVE. |
| NZ221627A (en) | 1986-09-09 | 1993-04-28 | Genencor Inc | Preparation of enzymes, modifications, catalytic triads to alter ratios or transesterification/hydrolysis ratios |
| DE3854249T2 (en) | 1987-08-28 | 1996-02-29 | Novonordisk As | Recombinant Humicola Lipase and Process for the Production of Recombinant Humicola Lipases. |
| GB8803036D0 (en) | 1988-02-10 | 1988-03-09 | Unilever Plc | Liquid detergents |
| JP3079276B2 (en) | 1988-02-28 | 2000-08-21 | 天野製薬株式会社 | Recombinant DNA, Pseudomonas sp. Containing the same, and method for producing lipase using the same |
| US5776757A (en) | 1988-03-24 | 1998-07-07 | Novo Nordisk A/S | Fungal cellulase composition containing alkaline CMC-endoglucanase and essentially no cellobiohydrolase and method of making thereof |
| EP0406314B1 (en) | 1988-03-24 | 1993-12-01 | Novo Nordisk A/S | A cellulase preparation |
| GB8915658D0 (en) | 1989-07-07 | 1989-08-23 | Unilever Plc | Enzymes,their production and use |
| KR100236540B1 (en) | 1990-04-14 | 2000-01-15 | 레클로우크스 라우에르 | Alkaline bacillus lipases, coding dna sequences thereof and bacilli which produce these lipases |
| DK0548228T3 (en) | 1990-09-13 | 1999-05-10 | Novo Nordisk As | lipase variants |
| EP0511456A1 (en) | 1991-04-30 | 1992-11-04 | The Procter & Gamble Company | Liquid detergents with aromatic borate ester to inhibit proteolytic enzyme |
| ES2085024T3 (en) | 1991-04-30 | 1996-05-16 | Procter & Gamble | LIQUID DETERGENTS REINFORCED WITH BORICO-POLYOL ACID COMPLEX TO INHIBIT THE PROTEOLYTIC ENZYME. |
| US5332518A (en) * | 1992-04-23 | 1994-07-26 | Kao Corporation | Stable slurry-coated sodium percarbonate, process for producing the same and bleach detergent composition containing the same |
| DK72992D0 (en) | 1992-06-01 | 1992-06-01 | Novo Nordisk As | ENZYME |
| DK88892D0 (en) | 1992-07-06 | 1992-07-06 | Novo Nordisk As | CONNECTION |
| AU673078B2 (en) | 1993-04-27 | 1996-10-24 | Genencor International, Inc. | New lipase variants for use in detergent applications |
| JP2859520B2 (en) | 1993-08-30 | 1999-02-17 | ノボ ノルディスク アクティーゼルスカブ | Lipase, microorganism producing the same, method for producing lipase, and detergent composition containing lipase |
| US5817495A (en) | 1993-10-13 | 1998-10-06 | Novo Nordisk A/S | H2 O2 -stable peroxidase variants |
| JPH07143883A (en) | 1993-11-24 | 1995-06-06 | Showa Denko Kk | Lipase gene and mutant lipase |
| ATE222604T1 (en) | 1994-02-22 | 2002-09-15 | Novozymes As | METHOD FOR PRODUCING A VARIANT OF A LIPOLYTIC ENZYME |
| DE69534464T2 (en) | 1994-03-29 | 2006-09-28 | Novozymes A/S | ALKALIC AMYLASE FROM BACELLUS |
| WO1995030744A2 (en) | 1994-05-04 | 1995-11-16 | Genencor International Inc. | Lipases with improved surfactant resistance |
| WO1995035381A1 (en) | 1994-06-20 | 1995-12-28 | Unilever N.V. | Modified pseudomonas lipases and their use |
| WO1996000292A1 (en) | 1994-06-23 | 1996-01-04 | Unilever N.V. | Modified pseudomonas lipases and their use |
| BE1008998A3 (en) | 1994-10-14 | 1996-10-01 | Solvay | Lipase, microorganism producing the preparation process for the lipase and uses thereof. |
| AU3697995A (en) | 1994-10-26 | 1996-05-23 | Novo Nordisk A/S | An enzyme with lipolytic activity |
| JPH08228778A (en) | 1995-02-27 | 1996-09-10 | Showa Denko Kk | Novel lipase gene and method for producing lipase using the same |
| CN1122361A (en) * | 1995-03-15 | 1996-05-15 | 梁健 | Hyperconcentrated detergent powder |
| CN1182451A (en) | 1995-03-17 | 1998-05-20 | 诺沃挪第克公司 | Novel endoglucanases |
| WO1997004078A1 (en) | 1995-07-14 | 1997-02-06 | Novo Nordisk A/S | A modified enzyme with lipolytic activity |
| WO1997007202A1 (en) | 1995-08-11 | 1997-02-27 | Novo Nordisk A/S | Novel lipolytic enzymes |
| DE69735767T2 (en) | 1996-09-17 | 2007-04-05 | Novozymes A/S | cellulase |
| WO1998015257A1 (en) | 1996-10-08 | 1998-04-16 | Novo Nordisk A/S | Diaminobenzoic acid derivatives as dye precursors |
| CA2307377A1 (en) * | 1997-10-22 | 1999-04-29 | Unilever Plc | Detergent compositions in tablet form |
| GB9726824D0 (en) | 1997-12-19 | 1998-02-18 | Manro Performance Chemicals Lt | Method of manufacturing particles |
| ES2532606T3 (en) | 1999-03-31 | 2015-03-30 | Novozymes A/S | Polypeptides with alkaline alpha-amylase activity and nucleic acids encoding them |
| KR20010108379A (en) | 1999-03-31 | 2001-12-07 | 피아 스타르 | Lipase variant |
| DE19941934A1 (en) * | 1999-09-03 | 2001-03-15 | Cognis Deutschland Gmbh | Solid detergents |
| EP1876226B1 (en) | 2006-07-07 | 2011-03-23 | The Procter & Gamble Company | Detergent compositions |
| CN1916148A (en) * | 2006-09-01 | 2007-02-21 | 王涛 | Encapsulated washing monomer, and preparation method |
| EP2242830B2 (en) | 2008-01-04 | 2020-03-11 | The Procter & Gamble Company | Enzyme and fabric hueing agent containing compositions |
| EP2085070A1 (en) | 2008-01-11 | 2009-08-05 | Procter & Gamble International Operations SA. | Cleaning and/or treatment compositions |
| AR070497A1 (en) | 2008-02-29 | 2010-04-07 | Procter & Gamble | DETERGENT COMPOSITION THAT LIPASA INCLUDES |
| MX2010009457A (en) | 2008-02-29 | 2010-09-24 | Procter & Gamble | Detergent composition comprising lipase. |
| EP2300588B1 (en) | 2008-06-06 | 2019-02-06 | The Procter and Gamble Company | Detergent composition comprising a variant of a family 44 xyloglucanase |
| WO2010122051A1 (en) | 2009-04-24 | 2010-10-28 | Unilever Plc | High active detergent particles |
-
2011
- 2011-09-30 EP EP11763944.3A patent/EP2627751B1/en active Active
- 2011-09-30 BR BR112013009127-4A patent/BR112013009127B1/en not_active IP Right Cessation
- 2011-09-30 WO PCT/EP2011/067159 patent/WO2012049033A1/en not_active Ceased
- 2011-09-30 CN CN201180049085.7A patent/CN103282477B/en not_active Expired - Fee Related
-
2013
- 2013-04-12 ZA ZA2013/02655A patent/ZA201302655B/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| CN103282477B (en) | 2015-04-01 |
| BR112013009127B1 (en) | 2020-05-26 |
| ZA201302655B (en) | 2014-06-25 |
| EP2627751A1 (en) | 2013-08-21 |
| WO2012049033A1 (en) | 2012-04-19 |
| CN103282477A (en) | 2013-09-04 |
| BR112013009127A2 (en) | 2016-07-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2627757B1 (en) | Laundry detergent particles | |
| CA2813791C (en) | Laundry detergent particles | |
| EP2627754B1 (en) | Laundry detergent particles | |
| EP2834336B1 (en) | Laundry detergent particles | |
| EP2627758B1 (en) | Laundry detergent particles | |
| WO2013149754A1 (en) | Laundry detergent particle | |
| WO2013149752A1 (en) | Laundry detergent particles | |
| EP2627749B1 (en) | Laundry detergent particles | |
| EP2627753B1 (en) | Laundry detergent particle | |
| EP2627751B1 (en) | Top-loading laundry vessel method | |
| EP2441822A1 (en) | Laundry detergent particles |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20130408 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| DAX | Request for extension of the european patent (deleted) | ||
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| INTG | Intention to grant announced |
Effective date: 20150318 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: KENINGLEY, STEPHEN, THOMAS Inventor name: RAJAPANDIAN, BENJAMIN, JESUKUMAR |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 729930 Country of ref document: AT Kind code of ref document: T Effective date: 20150715 Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602011016950 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: RO Ref legal event code: EPE |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 729930 Country of ref document: AT Kind code of ref document: T Effective date: 20150603 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150903 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20150603 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150903 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150904 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20151006 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20151003 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602011016950 Country of ref document: DE |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150930 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| 26N | No opposition filed |
Effective date: 20160304 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20150930 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20150930 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20150930 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20160921 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: RO Payment date: 20160824 Year of fee payment: 6 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20110930 Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 7 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 602011016950 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RO Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180404 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 8 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150603 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E Free format text: REGISTERED BETWEEN 20220127 AND 20220202 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: TR Payment date: 20230929 Year of fee payment: 13 Ref country code: IT Payment date: 20230921 Year of fee payment: 13 Ref country code: GB Payment date: 20230920 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20230928 Year of fee payment: 13 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20240930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20240930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20240930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20240930 |