CN1541216A - 8-甲氧基-(1,2,4)三唑并(1,5-a)吡啶衍生物及其作为腺苷受体配体的应用 - Google Patents
8-甲氧基-(1,2,4)三唑并(1,5-a)吡啶衍生物及其作为腺苷受体配体的应用 Download PDFInfo
- Publication number
- CN1541216A CN1541216A CNA028145089A CN02814508A CN1541216A CN 1541216 A CN1541216 A CN 1541216A CN A028145089 A CNA028145089 A CN A028145089A CN 02814508 A CN02814508 A CN 02814508A CN 1541216 A CN1541216 A CN 1541216A
- Authority
- CN
- China
- Prior art keywords
- compound
- methoxy
- triazolo
- formula
- phenyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 102000009346 Adenosine receptors Human genes 0.000 title claims abstract description 12
- 108050000203 Adenosine receptors Proteins 0.000 title claims abstract description 12
- 239000003446 ligand Substances 0.000 title description 4
- ARVIDGIGSCDGHQ-UHFFFAOYSA-N 8-methoxy-[1,2,4]triazolo[1,5-a]pyridine Chemical class COC1=CC=CN2N=CN=C12 ARVIDGIGSCDGHQ-UHFFFAOYSA-N 0.000 title description 2
- 150000001875 compounds Chemical class 0.000 claims abstract description 88
- 125000000217 alkyl group Chemical group 0.000 claims abstract description 24
- 150000003839 salts Chemical class 0.000 claims abstract description 21
- 102000005962 receptors Human genes 0.000 claims abstract description 18
- 108020003175 receptors Proteins 0.000 claims abstract description 18
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims abstract description 16
- 229910052736 halogen Inorganic materials 0.000 claims abstract description 14
- 125000001544 thienyl group Chemical group 0.000 claims abstract description 14
- 125000003545 alkoxy group Chemical group 0.000 claims abstract description 11
- 150000002367 halogens Chemical class 0.000 claims abstract description 10
- -1 acetamido, acetyl Chemical group 0.000 claims abstract description 9
- 125000001424 substituent group Chemical group 0.000 claims abstract description 9
- 125000003342 alkenyl group Chemical group 0.000 claims abstract description 6
- 201000010099 disease Diseases 0.000 claims abstract description 6
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 6
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 claims abstract description 3
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 claims description 40
- 239000002126 C01EB10 - Adenosine Substances 0.000 claims description 20
- 229960005305 adenosine Drugs 0.000 claims description 20
- 238000002360 preparation method Methods 0.000 claims description 9
- 239000003814 drug Substances 0.000 claims description 8
- 238000000034 method Methods 0.000 claims description 7
- 239000002253 acid Substances 0.000 claims description 4
- 238000006243 chemical reaction Methods 0.000 claims description 4
- 230000008569 process Effects 0.000 claims description 4
- MXQNJVZJTPNVHQ-UHFFFAOYSA-N 3-bromo-n-(8-methoxy-5-phenyl-[1,2,4]triazolo[1,5-a]pyridin-2-yl)benzamide Chemical compound N12N=C(NC(=O)C=3C=C(Br)C=CC=3)N=C2C(OC)=CC=C1C1=CC=CC=C1 MXQNJVZJTPNVHQ-UHFFFAOYSA-N 0.000 claims description 2
- BFCDYUGWLLNNSS-UHFFFAOYSA-N 3-chloro-n-(8-methoxy-5-thiophen-2-yl-[1,2,4]triazolo[1,5-a]pyridin-2-yl)benzamide Chemical compound N12N=C(NC(=O)C=3C=C(Cl)C=CC=3)N=C2C(OC)=CC=C1C1=CC=CS1 BFCDYUGWLLNNSS-UHFFFAOYSA-N 0.000 claims description 2
- GLUANVBNFOCEEP-UHFFFAOYSA-N 4-bromo-n-(8-methoxy-5-phenyl-[1,2,4]triazolo[1,5-a]pyridin-2-yl)benzamide Chemical compound N12N=C(NC(=O)C=3C=CC(Br)=CC=3)N=C2C(OC)=CC=C1C1=CC=CC=C1 GLUANVBNFOCEEP-UHFFFAOYSA-N 0.000 claims description 2
- BZFPBNHQNOHGDJ-UHFFFAOYSA-N 4-bromo-n-(8-methoxy-5-thiophen-2-yl-[1,2,4]triazolo[1,5-a]pyridin-2-yl)benzamide Chemical compound N12N=C(NC(=O)C=3C=CC(Br)=CC=3)N=C2C(OC)=CC=C1C1=CC=CS1 BZFPBNHQNOHGDJ-UHFFFAOYSA-N 0.000 claims description 2
- FPSAHLITTJPJNO-UHFFFAOYSA-N 4-chloro-n-(8-methoxy-5-thiophen-2-yl-[1,2,4]triazolo[1,5-a]pyridin-2-yl)benzamide Chemical compound N12N=C(NC(=O)C=3C=CC(Cl)=CC=3)N=C2C(OC)=CC=C1C1=CC=CS1 FPSAHLITTJPJNO-UHFFFAOYSA-N 0.000 claims description 2
- FERDNEBQIWWMAX-UHFFFAOYSA-N 4-ethyl-n-(8-methoxy-5-thiophen-2-yl-[1,2,4]triazolo[1,5-a]pyridin-2-yl)benzamide Chemical compound C1=CC(CC)=CC=C1C(=O)NC1=NN2C(C=3SC=CC=3)=CC=C(OC)C2=N1 FERDNEBQIWWMAX-UHFFFAOYSA-N 0.000 claims description 2
- KNHYXMSFKHVMJF-UHFFFAOYSA-N 4-fluoro-n-(8-methoxy-5-phenyl-[1,2,4]triazolo[1,5-a]pyridin-2-yl)benzamide Chemical compound N12N=C(NC(=O)C=3C=CC(F)=CC=3)N=C2C(OC)=CC=C1C1=CC=CC=C1 KNHYXMSFKHVMJF-UHFFFAOYSA-N 0.000 claims description 2
- 238000004519 manufacturing process Methods 0.000 claims description 2
- VNTLTZBHOCXFKF-UHFFFAOYSA-N n-(8-methoxy-5-phenyl-[1,2,4]triazolo[1,5-a]pyridin-2-yl)-5-methylthiophene-2-carboxamide Chemical compound N12N=C(NC(=O)C=3SC(C)=CC=3)N=C2C(OC)=CC=C1C1=CC=CC=C1 VNTLTZBHOCXFKF-UHFFFAOYSA-N 0.000 claims description 2
- PVEYHUQYLPGVNL-UHFFFAOYSA-N n-(8-methoxy-5-thiophen-2-yl-[1,2,4]triazolo[1,5-a]pyridin-2-yl)benzamide Chemical compound N12N=C(NC(=O)C=3C=CC=CC=3)N=C2C(OC)=CC=C1C1=CC=CS1 PVEYHUQYLPGVNL-UHFFFAOYSA-N 0.000 claims description 2
- SRODMGPRIHTQCW-UHFFFAOYSA-N n-(8-methoxy-5-thiophen-3-yl-[1,2,4]triazolo[1,5-a]pyridin-2-yl)-5-methylthiophene-2-carboxamide Chemical compound N12N=C(NC(=O)C=3SC(C)=CC=3)N=C2C(OC)=CC=C1C=1C=CSC=1 SRODMGPRIHTQCW-UHFFFAOYSA-N 0.000 claims description 2
- USFMGERTCUCJTA-UHFFFAOYSA-N n-[5-(2-fluorophenyl)-8-methoxy-[1,2,4]triazolo[1,5-a]pyridin-2-yl]-5-methylthiophene-2-carboxamide Chemical class N12N=C(NC(=O)C=3SC(C)=CC=3)N=C2C(OC)=CC=C1C1=CC=CC=C1F USFMGERTCUCJTA-UHFFFAOYSA-N 0.000 claims description 2
- MWDHTIHNUWROLA-UHFFFAOYSA-N n-[5-(3-ethoxyphenyl)-8-methoxy-[1,2,4]triazolo[1,5-a]pyridin-2-yl]-4-fluorobenzamide Chemical compound CCOC1=CC=CC(C=2N3N=C(NC(=O)C=4C=CC(F)=CC=4)N=C3C(OC)=CC=2)=C1 MWDHTIHNUWROLA-UHFFFAOYSA-N 0.000 claims description 2
- PDJLENTTXUDAHY-UHFFFAOYSA-N n-[5-(3-ethoxyphenyl)-8-methoxy-[1,2,4]triazolo[1,5-a]pyridin-2-yl]-5-methylthiophene-2-carboxamide Chemical compound CCOC1=CC=CC(C=2N3N=C(NC(=O)C=4SC(C)=CC=4)N=C3C(OC)=CC=2)=C1 PDJLENTTXUDAHY-UHFFFAOYSA-N 0.000 claims description 2
- SKAAOGJUJIKDJB-UHFFFAOYSA-N n-[5-(3-fluorophenyl)-8-methoxy-[1,2,4]triazolo[1,5-a]pyridin-2-yl]-5-methylthiophene-2-carboxamide Chemical compound N12N=C(NC(=O)C=3SC(C)=CC=3)N=C2C(OC)=CC=C1C1=CC=CC(F)=C1 SKAAOGJUJIKDJB-UHFFFAOYSA-N 0.000 claims description 2
- UYEZGWUJGIDNNO-UHFFFAOYSA-N n-[8-methoxy-5-(3-methoxyphenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-yl]-5-methylthiophene-2-carboxamide Chemical compound COC1=CC=CC(C=2N3N=C(NC(=O)C=4SC(C)=CC=4)N=C3C(OC)=CC=2)=C1 UYEZGWUJGIDNNO-UHFFFAOYSA-N 0.000 claims description 2
- TUQQULZPOAEGQL-UHFFFAOYSA-N n-[8-methoxy-5-(4-methoxyphenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-yl]-5-methylthiophene-2-carboxamide Chemical compound C1=CC(OC)=CC=C1C1=CC=C(OC)C2=NC(NC(=O)C=3SC(C)=CC=3)=NN12 TUQQULZPOAEGQL-UHFFFAOYSA-N 0.000 claims description 2
- LMBFAGIMSUYTBN-MPZNNTNKSA-N teixobactin Chemical compound C([C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@H]1C(N[C@@H](C)C(=O)N[C@@H](C[C@@H]2NC(=N)NC2)C(=O)N[C@H](C(=O)O[C@H]1C)[C@@H](C)CC)=O)NC)C1=CC=CC=C1 LMBFAGIMSUYTBN-MPZNNTNKSA-N 0.000 claims description 2
- 125000005843 halogen group Chemical group 0.000 claims 4
- KXDXTQZSKLGJIQ-UHFFFAOYSA-N n-[5-(5-chlorothiophen-2-yl)-8-methoxy-[1,2,4]triazolo[1,5-a]pyridin-2-yl]-5-methylthiophene-2-carboxamide Chemical compound N12N=C(NC(=O)C=3SC(C)=CC=3)N=C2C(OC)=CC=C1C1=CC=C(Cl)S1 KXDXTQZSKLGJIQ-UHFFFAOYSA-N 0.000 claims 1
- 239000000546 pharmaceutical excipient Substances 0.000 claims 1
- 239000000203 mixture Substances 0.000 description 15
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 10
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 9
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 8
- 241000282414 Homo sapiens Species 0.000 description 7
- 230000000694 effects Effects 0.000 description 7
- 238000005160 1H NMR spectroscopy Methods 0.000 description 6
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 239000001961 anticonvulsive agent Substances 0.000 description 6
- 239000000969 carrier Substances 0.000 description 6
- IVOMOUWHDPKRLL-KQYNXXCUSA-N Cyclic adenosine monophosphate Chemical compound C([C@H]1O2)OP(O)(=O)O[C@H]1[C@@H](O)[C@@H]2N1C(N=CN=C2N)=C2N=C1 IVOMOUWHDPKRLL-KQYNXXCUSA-N 0.000 description 5
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N DMSO-d6 Substances [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 5
- 206010021143 Hypoxia Diseases 0.000 description 5
- 208000018737 Parkinson disease Diseases 0.000 description 5
- 210000004027 cell Anatomy 0.000 description 5
- 230000007954 hypoxia Effects 0.000 description 5
- 208000028867 ischemia Diseases 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- BMTSZVZQNMNPCT-UHFFFAOYSA-N 2-aminopyridin-3-ol Chemical compound NC1=NC=CC=C1O BMTSZVZQNMNPCT-UHFFFAOYSA-N 0.000 description 4
- 241000282472 Canis lupus familiaris Species 0.000 description 4
- 206010010904 Convulsion Diseases 0.000 description 4
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 4
- 241000700159 Rattus Species 0.000 description 4
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 4
- 239000013543 active substance Substances 0.000 description 4
- 150000001413 amino acids Chemical class 0.000 description 4
- 208000006673 asthma Diseases 0.000 description 4
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 4
- 230000033228 biological regulation Effects 0.000 description 4
- 239000000872 buffer Substances 0.000 description 4
- 125000004432 carbon atom Chemical group C* 0.000 description 4
- 229940045200 cardioprotective agent Drugs 0.000 description 4
- 239000013078 crystal Substances 0.000 description 4
- 230000002401 inhibitory effect Effects 0.000 description 4
- 208000014674 injury Diseases 0.000 description 4
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 4
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 4
- 230000004112 neuroprotection Effects 0.000 description 4
- 229910052760 oxygen Inorganic materials 0.000 description 4
- 239000001301 oxygen Substances 0.000 description 4
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 3
- HJCUIJYQAULEJR-UHFFFAOYSA-N 5-iodo-8-methoxy-[1,2,4]triazolo[1,5-a]pyridin-2-amine Chemical compound COC1=CC=C(I)N2N=C(N)N=C12 HJCUIJYQAULEJR-UHFFFAOYSA-N 0.000 description 3
- JKWVYDCURGHICU-UHFFFAOYSA-N 8-methoxy-[1,2,4]triazolo[1,5-a]pyridin-2-amine Chemical compound COC1=CC=CN2N=C(N)N=C12 JKWVYDCURGHICU-UHFFFAOYSA-N 0.000 description 3
- ZKHQWZAMYRWXGA-KQYNXXCUSA-J ATP(4-) Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O)[C@@H](O)[C@H]1O ZKHQWZAMYRWXGA-KQYNXXCUSA-J 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 208000024827 Alzheimer disease Diseases 0.000 description 3
- 208000019901 Anxiety disease Diseases 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 3
- 206010020751 Hypersensitivity Diseases 0.000 description 3
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 3
- 208000002193 Pain Diseases 0.000 description 3
- 102000030621 adenylate cyclase Human genes 0.000 description 3
- 108060000200 adenylate cyclase Proteins 0.000 description 3
- 230000007815 allergy Effects 0.000 description 3
- 229960003965 antiepileptics Drugs 0.000 description 3
- 239000000164 antipsychotic agent Substances 0.000 description 3
- 229940005529 antipsychotics Drugs 0.000 description 3
- 230000036506 anxiety Effects 0.000 description 3
- 238000003556 assay Methods 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- HVGDTXRMGSMOOG-UHFFFAOYSA-N ethyl n-[(3-methoxypyridin-2-yl)carbamothioyl]carbamate Chemical compound CCOC(=O)NC(=S)NC1=NC=CC=C1OC HVGDTXRMGSMOOG-UHFFFAOYSA-N 0.000 description 3
- 239000007903 gelatin capsule Substances 0.000 description 3
- 239000008187 granular material Substances 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 3
- 229940035363 muscle relaxants Drugs 0.000 description 3
- 239000003158 myorelaxant agent Substances 0.000 description 3
- 239000002858 neurotransmitter agent Substances 0.000 description 3
- 230000036407 pain Effects 0.000 description 3
- 239000000825 pharmaceutical preparation Substances 0.000 description 3
- 229920005862 polyol Polymers 0.000 description 3
- 150000003077 polyols Chemical class 0.000 description 3
- 239000003379 purinergic P1 receptor agonist Substances 0.000 description 3
- 201000000980 schizophrenia Diseases 0.000 description 3
- 239000000932 sedative agent Substances 0.000 description 3
- 229940125723 sedative agent Drugs 0.000 description 3
- 201000009032 substance abuse Diseases 0.000 description 3
- 231100000736 substance abuse Toxicity 0.000 description 3
- 208000011117 substance-related disease Diseases 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 3
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 3
- 230000008733 trauma Effects 0.000 description 3
- HNAYRVKSWGSQTP-UHFFFAOYSA-N 3-methoxypyridin-2-amine Chemical compound COC1=CC=CN=C1N HNAYRVKSWGSQTP-UHFFFAOYSA-N 0.000 description 2
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 2
- XTWYTFMLZFPYCI-KQYNXXCUSA-N 5'-adenylphosphoric acid Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O XTWYTFMLZFPYCI-KQYNXXCUSA-N 0.000 description 2
- LTVVHAZBEXVWHI-UHFFFAOYSA-N 8-methoxy-5-phenyl-[1,2,4]triazolo[1,5-a]pyridin-2-amine Chemical compound N12N=C(N)N=C2C(OC)=CC=C1C1=CC=CC=C1 LTVVHAZBEXVWHI-UHFFFAOYSA-N 0.000 description 2
- XTWYTFMLZFPYCI-UHFFFAOYSA-N Adenosine diphosphate Natural products C1=NC=2C(N)=NC=NC=2N1C1OC(COP(O)(=O)OP(O)(O)=O)C(O)C1O XTWYTFMLZFPYCI-UHFFFAOYSA-N 0.000 description 2
- ZKHQWZAMYRWXGA-UHFFFAOYSA-N Adenosine triphosphate Natural products C1=NC=2C(N)=NC=NC=2N1C1OC(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)C(O)C1O ZKHQWZAMYRWXGA-UHFFFAOYSA-N 0.000 description 2
- 108090000312 Calcium Channels Proteins 0.000 description 2
- 102000003922 Calcium Channels Human genes 0.000 description 2
- 208000020401 Depressive disease Diseases 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- WTDHULULXKLSOZ-UHFFFAOYSA-N Hydroxylamine hydrochloride Chemical compound Cl.ON WTDHULULXKLSOZ-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 102000001708 Protein Isoforms Human genes 0.000 description 2
- 108010029485 Protein Isoforms Proteins 0.000 description 2
- MEFKEPWMEQBLKI-AIRLBKTGSA-N S-adenosyl-L-methioninate Chemical compound O[C@@H]1[C@H](O)[C@@H](C[S+](CC[C@H](N)C([O-])=O)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 MEFKEPWMEQBLKI-AIRLBKTGSA-N 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 2
- OKJPEAGHQZHRQV-UHFFFAOYSA-N Triiodomethane Natural products IC(I)I OKJPEAGHQZHRQV-UHFFFAOYSA-N 0.000 description 2
- 102000014384 Type C Phospholipases Human genes 0.000 description 2
- 108010079194 Type C Phospholipases Proteins 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- UDMBCSSLTHHNCD-KQYNXXCUSA-N adenosine 5'-monophosphate Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O UDMBCSSLTHHNCD-KQYNXXCUSA-N 0.000 description 2
- 239000005557 antagonist Substances 0.000 description 2
- 230000003556 anti-epileptic effect Effects 0.000 description 2
- 229940125681 anticonvulsant agent Drugs 0.000 description 2
- 239000012659 cardioprotective agent Substances 0.000 description 2
- 210000003169 central nervous system Anatomy 0.000 description 2
- 208000015114 central nervous system disease Diseases 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- YMWUJEATGCHHMB-UHFFFAOYSA-N dichloromethane Natural products ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 2
- VYFYYTLLBUKUHU-UHFFFAOYSA-N dopamine Chemical compound NCCC1=CC=C(O)C(O)=C1 VYFYYTLLBUKUHU-UHFFFAOYSA-N 0.000 description 2
- 239000008298 dragée Substances 0.000 description 2
- BDTDECDAHYOJRO-UHFFFAOYSA-N ethyl n-(sulfanylidenemethylidene)carbamate Chemical compound CCOC(=O)N=C=S BDTDECDAHYOJRO-UHFFFAOYSA-N 0.000 description 2
- 239000003925 fat Substances 0.000 description 2
- 235000019253 formic acid Nutrition 0.000 description 2
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 2
- 235000019359 magnesium stearate Nutrition 0.000 description 2
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 2
- 235000019341 magnesium sulphate Nutrition 0.000 description 2
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 150000007522 mineralic acids Chemical class 0.000 description 2
- 230000009456 molecular mechanism Effects 0.000 description 2
- FIQGIOAELHTLHM-UHFFFAOYSA-N n-(2-aminoethyl)-2-[4-(2,6-dioxo-1,3-dipropyl-7h-purin-8-yl)phenoxy]acetamide Chemical compound N1C=2C(=O)N(CCC)C(=O)N(CCC)C=2N=C1C1=CC=C(OCC(=O)NCCN)C=C1 FIQGIOAELHTLHM-UHFFFAOYSA-N 0.000 description 2
- PYEATDFLQHAKQK-UHFFFAOYSA-N n-(8-methoxy-5-phenyl-[1,2,4]triazolo[1,5-a]pyridin-2-yl)benzamide Chemical compound N12N=C(NC(=O)C=3C=CC=CC=3)N=C2C(OC)=CC=C1C1=CC=CC=C1 PYEATDFLQHAKQK-UHFFFAOYSA-N 0.000 description 2
- 239000004090 neuroprotective agent Substances 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 235000005985 organic acids Nutrition 0.000 description 2
- 239000012044 organic layer Substances 0.000 description 2
- HXITXNWTGFUOAU-UHFFFAOYSA-N phenylboronic acid Chemical compound OB(O)C1=CC=CC=C1 HXITXNWTGFUOAU-UHFFFAOYSA-N 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- 238000002953 preparative HPLC Methods 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 239000000296 purinergic P1 receptor antagonist Substances 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 235000017550 sodium carbonate Nutrition 0.000 description 2
- 229910000029 sodium carbonate Inorganic materials 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 239000000829 suppository Substances 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 235000015112 vegetable and seed oil Nutrition 0.000 description 2
- 239000008158 vegetable oil Substances 0.000 description 2
- 239000001993 wax Substances 0.000 description 2
- HGUFODBRKLSHSI-UHFFFAOYSA-N 2,3,7,8-tetrachloro-dibenzo-p-dioxin Chemical compound O1C2=CC(Cl)=C(Cl)C=C2OC2=C1C=C(Cl)C(Cl)=C2 HGUFODBRKLSHSI-UHFFFAOYSA-N 0.000 description 1
- NGNBDVOYPDDBFK-UHFFFAOYSA-N 2-[2,4-di(pentan-2-yl)phenoxy]acetyl chloride Chemical compound CCCC(C)C1=CC=C(OCC(Cl)=O)C(C(C)CCC)=C1 NGNBDVOYPDDBFK-UHFFFAOYSA-N 0.000 description 1
- YLYPIBBGWLKELC-RMKNXTFCSA-N 2-[2-[(e)-2-[4-(dimethylamino)phenyl]ethenyl]-6-methylpyran-4-ylidene]propanedinitrile Chemical compound C1=CC(N(C)C)=CC=C1\C=C\C1=CC(=C(C#N)C#N)C=C(C)O1 YLYPIBBGWLKELC-RMKNXTFCSA-N 0.000 description 1
- 125000004974 2-butenyl group Chemical group C(C=CC)* 0.000 description 1
- CZKLEJHVLCMVQR-UHFFFAOYSA-N 4-fluorobenzoyl chloride Chemical compound FC1=CC=C(C(Cl)=O)C=C1 CZKLEJHVLCMVQR-UHFFFAOYSA-N 0.000 description 1
- 101710169336 5'-deoxyadenosine deaminase Proteins 0.000 description 1
- VCNGNQLPFHVODE-UHFFFAOYSA-N 5-methylthiophene-2-carboxylic acid Chemical compound CC1=CC=C(C(O)=O)S1 VCNGNQLPFHVODE-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical group [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 102000055025 Adenosine deaminases Human genes 0.000 description 1
- WSVLPVUVIUVCRA-KPKNDVKVSA-N Alpha-lactose monohydrate Chemical compound O.O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O WSVLPVUVIUVCRA-KPKNDVKVSA-N 0.000 description 1
- GUBGYTABKSRVRQ-DCSYEGIMSA-N Beta-Lactose Chemical compound OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-DCSYEGIMSA-N 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 208000007204 Brain death Diseases 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 241000700198 Cavia Species 0.000 description 1
- 241000700199 Cavia porcellus Species 0.000 description 1
- 102000000844 Cell Surface Receptors Human genes 0.000 description 1
- 108010001857 Cell Surface Receptors Proteins 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- RGJOEKWQDUBAIZ-IBOSZNHHSA-N CoASH Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCS)O[C@H]1N1C2=NC=NC(N)=C2N=C1 RGJOEKWQDUBAIZ-IBOSZNHHSA-N 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 241000699802 Cricetulus griseus Species 0.000 description 1
- 206010012289 Dementia Diseases 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 208000000059 Dyspnea Diseases 0.000 description 1
- 206010013975 Dyspnoeas Diseases 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- 108091006027 G proteins Proteins 0.000 description 1
- 102000003688 G-Protein-Coupled Receptors Human genes 0.000 description 1
- 108090000045 G-Protein-Coupled Receptors Proteins 0.000 description 1
- 102000030782 GTP binding Human genes 0.000 description 1
- 108091000058 GTP-Binding Proteins 0.000 description 1
- 241000287828 Gallus gallus Species 0.000 description 1
- 239000001828 Gelatine Substances 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- 206010021079 Hypopnoea Diseases 0.000 description 1
- 208000001953 Hypotension Diseases 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- UTLPKQYUXOEJIL-UHFFFAOYSA-N LSM-3822 Chemical compound N1=CC=2C3=NC(C=4OC=CC=4)=NN3C(N)=NC=2N1CCC1=CC=CC=C1 UTLPKQYUXOEJIL-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 102000004257 Potassium Channel Human genes 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 102100028255 Renin Human genes 0.000 description 1
- 108090000783 Renin Proteins 0.000 description 1
- 206010039897 Sedation Diseases 0.000 description 1
- 241000710961 Semliki Forest virus Species 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 241000209149 Zea Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- MMWCIQZXVOZEGG-HOZKJCLWSA-N [(1S,2R,3S,4S,5R,6S)-2,3,5-trihydroxy-4,6-diphosphonooxycyclohexyl] dihydrogen phosphate Chemical compound O[C@H]1[C@@H](O)[C@H](OP(O)(O)=O)[C@@H](OP(O)(O)=O)[C@H](O)[C@H]1OP(O)(O)=O MMWCIQZXVOZEGG-HOZKJCLWSA-N 0.000 description 1
- DACWQSNZECJJGG-UHFFFAOYSA-N [1,2,4]triazolo[1,5-a]pyridine Chemical class C1=CC=CN2N=CN=C21 DACWQSNZECJJGG-UHFFFAOYSA-N 0.000 description 1
- 125000000738 acetamido group Chemical group [H]C([H])([H])C(=O)N([H])[*] 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- 239000002582 adenosine A1 receptor agonist Substances 0.000 description 1
- 239000003449 adenosine A2 receptor antagonist Substances 0.000 description 1
- 229940121359 adenosine receptor antagonist Drugs 0.000 description 1
- 150000001335 aliphatic alkanes Chemical class 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 229940035676 analgesics Drugs 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 230000001773 anti-convulsant effect Effects 0.000 description 1
- 239000000935 antidepressant agent Substances 0.000 description 1
- 229940005513 antidepressants Drugs 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000002249 anxiolytic agent Substances 0.000 description 1
- 230000000949 anxiolytic effect Effects 0.000 description 1
- 229940005530 anxiolytics Drugs 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- PASDCCFISLVPSO-UHFFFAOYSA-N benzoyl chloride Chemical compound ClC(=O)C1=CC=CC=C1 PASDCCFISLVPSO-UHFFFAOYSA-N 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 230000027455 binding Effects 0.000 description 1
- ZADPBFCGQRWHPN-UHFFFAOYSA-N boronic acid Chemical class OBO ZADPBFCGQRWHPN-UHFFFAOYSA-N 0.000 description 1
- 125000005620 boronic acid group Chemical class 0.000 description 1
- 230000006931 brain damage Effects 0.000 description 1
- 231100000874 brain damage Toxicity 0.000 description 1
- 230000003925 brain function Effects 0.000 description 1
- 208000029028 brain injury Diseases 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000007963 capsule composition Substances 0.000 description 1
- 210000000748 cardiovascular system Anatomy 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000019522 cellular metabolic process Effects 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- RGJOEKWQDUBAIZ-UHFFFAOYSA-N coenzime A Natural products OC1C(OP(O)(O)=O)C(COP(O)(=O)OP(O)(=O)OCC(C)(C)C(O)C(=O)NCCC(=O)NCCS)OC1N1C2=NC=NC(N)=C2N=C1 RGJOEKWQDUBAIZ-UHFFFAOYSA-N 0.000 description 1
- 239000005515 coenzyme Substances 0.000 description 1
- 239000005516 coenzyme A Substances 0.000 description 1
- 229940093530 coenzyme a Drugs 0.000 description 1
- 239000002475 cognitive enhancer Substances 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- RVOJTCZRIKWHDX-UHFFFAOYSA-N cyclohexanecarbonyl chloride Chemical compound ClC(=O)C1CCCCC1 RVOJTCZRIKWHDX-UHFFFAOYSA-N 0.000 description 1
- USVZFSNDGFNNJT-UHFFFAOYSA-N cyclopenta-1,4-dien-1-yl(diphenyl)phosphane (2,3-dichlorocyclopenta-1,4-dien-1-yl)-diphenylphosphane iron(2+) Chemical compound [Fe++].c1cc[c-](c1)P(c1ccccc1)c1ccccc1.Clc1c(cc[c-]1Cl)P(c1ccccc1)c1ccccc1 USVZFSNDGFNNJT-UHFFFAOYSA-N 0.000 description 1
- KDTSHFARGAKYJN-UHFFFAOYSA-N dephosphocoenzyme A Natural products OC1C(O)C(COP(O)(=O)OP(O)(=O)OCC(C)(C)C(O)C(=O)NCCC(=O)NCCS)OC1N1C2=NC=NC(N)=C2N=C1 KDTSHFARGAKYJN-UHFFFAOYSA-N 0.000 description 1
- BSHICDXRSZQYBP-UHFFFAOYSA-N dichloromethane;palladium(2+) Chemical compound [Pd+2].ClCCl BSHICDXRSZQYBP-UHFFFAOYSA-N 0.000 description 1
- UXGNZZKBCMGWAZ-UHFFFAOYSA-N dimethylformamide dmf Chemical compound CN(C)C=O.CN(C)C=O UXGNZZKBCMGWAZ-UHFFFAOYSA-N 0.000 description 1
- FPAFDBFIGPHWGO-UHFFFAOYSA-N dioxosilane;oxomagnesium;hydrate Chemical compound O.[Mg]=O.[Mg]=O.[Mg]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O FPAFDBFIGPHWGO-UHFFFAOYSA-N 0.000 description 1
- CETRZFQIITUQQL-UHFFFAOYSA-N dmso dimethylsulfoxide Chemical compound CS(C)=O.CS(C)=O CETRZFQIITUQQL-UHFFFAOYSA-N 0.000 description 1
- 229960003638 dopamine Drugs 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 239000003596 drug target Substances 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 230000002964 excitative effect Effects 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 235000019634 flavors Nutrition 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 230000024924 glomerular filtration Effects 0.000 description 1
- 229930195712 glutamate Natural products 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 210000005003 heart tissue Anatomy 0.000 description 1
- 210000003630 histaminocyte Anatomy 0.000 description 1
- 230000000147 hypnotic effect Effects 0.000 description 1
- 230000036543 hypotension Effects 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- PNDPGZBMCMUPRI-UHFFFAOYSA-N iodine Chemical compound II PNDPGZBMCMUPRI-UHFFFAOYSA-N 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000000555 isopropenyl group Chemical group [H]\C([H])=C(\*)C([H])([H])[H] 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 230000003907 kidney function Effects 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 230000006742 locomotor activity Effects 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 230000000873 masking effect Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 239000012022 methylating agents Substances 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 238000003801 milling Methods 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 239000012452 mother liquor Substances 0.000 description 1
- PEECTLLHENGOKU-UHFFFAOYSA-N n,n-dimethylpyridin-4-amine Chemical compound CN(C)C1=CC=NC=C1.CN(C)C1=CC=NC=C1 PEECTLLHENGOKU-UHFFFAOYSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 230000001607 nephroprotective effect Effects 0.000 description 1
- 230000004770 neurodegeneration Effects 0.000 description 1
- 210000002569 neuron Anatomy 0.000 description 1
- 230000008062 neuronal firing Effects 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 230000009871 nonspecific binding Effects 0.000 description 1
- 239000002773 nucleotide Substances 0.000 description 1
- 125000003729 nucleotide group Chemical group 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 239000008194 pharmaceutical composition Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 229920000729 poly(L-lysine) polymer Polymers 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 108020001213 potassium channel Proteins 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 210000000063 presynaptic terminal Anatomy 0.000 description 1
- 125000004368 propenyl group Chemical group C(=CC)* 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 239000008213 purified water Substances 0.000 description 1
- 229940044551 receptor antagonist Drugs 0.000 description 1
- 239000002464 receptor antagonist Substances 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000008327 renal blood flow Effects 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 239000003169 respiratory stimulant agent Substances 0.000 description 1
- 229940066293 respiratory stimulants Drugs 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 238000007363 ring formation reaction Methods 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 230000036280 sedation Effects 0.000 description 1
- 239000013049 sediment Substances 0.000 description 1
- 208000013220 shortness of breath Diseases 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 239000007916 tablet composition Substances 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- WHRNULOCNSKMGB-UHFFFAOYSA-N tetrahydrofuran thf Chemical compound C1CCOC1.C1CCOC1 WHRNULOCNSKMGB-UHFFFAOYSA-N 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 239000003039 volatile agent Substances 0.000 description 1
- 238000005550 wet granulation Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/08—Antiepileptics; Anticonvulsants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/18—Antipsychotics, i.e. neuroleptics; Drugs for mania or schizophrenia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/22—Anxiolytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/30—Drugs for disorders of the nervous system for treating abuse or dependence
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Neurology (AREA)
- Psychiatry (AREA)
- Pulmonology (AREA)
- Pain & Pain Management (AREA)
- Vascular Medicine (AREA)
- Immunology (AREA)
- Urology & Nephrology (AREA)
- Psychology (AREA)
- Heart & Thoracic Surgery (AREA)
- Addiction (AREA)
- Cardiology (AREA)
- Hospice & Palliative Care (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
Abstract
本发明涉及通式(I)化合物及其可药用盐,其中R1为苯基或噻吩基,其中的环可以是未取代的或被一个或两个取代基取代,所述取代基选自卤素、三氟甲基、低级烷基、低级烷氧基、乙酰氨基、乙酰基、低级链烯基、-C(O)O-低级烷基或硫代-低级烷基;R2为苯基或噻吩基,其中的环可以是未取代的或被一个或两个取代基取代,所述取代基选自卤素、低级烷基、卤素-低级烷基或低级烷氧基。这些化合物对腺苷受体具有良好的亲和力,因此它们可用于治疗与该受体有关的疾病。
Description
本发明涉及通式I化合物及其可药用盐
其中
R1为苯基或噻吩基,其中的环可以是未取代的或被一个或两个取代基取代,所述取代基选自卤素、三氟甲基、低级烷基、低级烷氧基、乙酰氨基、乙酰基、低级链烯基、-C(O)O-低级烷基或硫代-低级烷基;
R2为苯基或噻吩基,其中的环可以是未取代的或被一个或两个取代基取代,所述取代基选自卤素、低级烷基、卤素-低级烷基或低级烷氧基。
现已意外地发现,通式I化合物是腺苷受体配体。
腺苷通过与特定细胞的表面受体相互作用调节广泛的生理功能。腺苷受体作为药物靶点的可能性于1982年首次被评论。腺苷在结构上和代谢上都与生物活性的核苷酸三磷酸腺苷(ATP)、二磷酸腺苷(ADP)、一磷酸腺苷(AMP)和环一磷酸腺苷(cAMP有关);与生物化学甲基化剂S-腺苷-L-甲硫氨酸(SAM)有关;并且在结构上与辅酶NAD、FAD和辅酶A有关;还与RNA有关。这些有关的化合物与腺苷一起在调节细胞代谢的很多方面以及调节不同的中枢神经系统活动中是重要的。
腺苷受体分为A1、A2A、A2B和A3受体,它们属于G蛋白-偶联受体家族。由腺苷激活腺苷受体启动信号转导机制。这些机制依赖于与G蛋白有关的受体。经典地,各腺苷受体亚型的特性由利用cAMP作为第二信使的腺苷酸环化酶效应物系统表示。与Gi蛋白偶联的A1和A3受体抑制腺苷酸环化酶,导致细胞的cAMP水平降低,而A2A和A2B受体与Gs蛋白偶联并激活腺苷酸环化酶,导致细胞的cAMP水平升高。已知A1受体系统包括激活磷脂酶C和调节钾和钙离子通道。除了与腺苷酸环化酶缔合外,A3亚型也刺激磷脂酶C并激活钙离子通道。
A1受体(326-328氨基酸)由各种物种(犬、人、大鼠、狗、鸡、牛、豚鼠)克隆得到,在哺乳动物物种间有90-95%的序列同一性。A2A受体(409-412氨基酸)由犬、大鼠、人、豚鼠和小鼠克隆得到。A2B受体(332氨基酸)由人和小鼠克隆得到,其中人A2B与人A1和A2A受体有45%的同源性。A3受体(317-320氨基酸)由人、大鼠、狗、兔子和羊克隆得到。
有人提出,A1和A2A受体亚型在腺苷调节能量供给中发挥互补作用。ATP代谢产物腺苷从细胞中扩散出来并发挥局部激活腺苷受体的作用以减少氧的需要(A1)或增加氧的供给(A2A),从而使得能量供给与组织内的要求恢复平衡。两种亚型的作用是增加组织可获得的氧的量和保护细胞免受由短期氧不平衡引起的伤害。内源性腺苷的一个重要作用是防止创伤如缺氧、局部缺血、低血压和癫痫发作活动过程中的伤害。
此外,已知腺苷受体激动剂与表达大鼠A3受体的肥大细胞结合导致肌醇三磷酸和细胞内钙浓度的增加,这使得抗原诱导的炎性介质的分泌作用加强。因此,A3受体在介导哮喘发作和其他变态反应中发挥作用。
腺苷还是一种神经调质,它通过介导中枢抑制作用在涉及生理学脑功能的很多方面的分子机理的调节中具有全局的重要性。神经递质释放的增加导致创伤如缺氧、局部缺血和癫痫发作。这些神经递质最终是引起脑损伤或个体死亡的神经变性和神经死亡的原因。因此,模仿腺苷中枢抑制作用的腺苷A1激动剂可用作神经保护剂。已有人提出将腺苷用作内源性抗惊厥药,抑制谷氨酸盐从兴奋性神经元释放和抑制神经元放电。因此,腺苷激动剂可用作抗癫痫药。腺苷拮抗剂刺激CNS的活性并已证明是有效的认知增强剂。选择性A2a-拮抗剂在各种形式的痴呆,例如早老性痴呆的治疗中具有治疗潜力并可用作神经保护剂。腺苷A2-受体拮抗剂抑制多巴胺从中枢突触末端释放并减少运动活性,因此可改善帕金森氏病患者的症状。在与镇静、催眠、精神分裂症、焦虑、疼痛、呼吸、抑郁和物质滥用有关的分子机理中也涉及腺苷的中枢活性。因此,作用于腺苷受体的药物也具有作为镇静剂、肌肉松弛药、抗精神病药、抗焦虑药、镇痛药、呼吸兴奋剂和抗抑郁药的治疗潜力。
腺苷在心血管系统的一个重要作用是作为心脏保护剂。作为对局部缺血和缺氧的反应,内源性腺苷的水平增加并且在创伤期间和创伤后保护心脏组织(预适应)。因此,腺苷激动剂具有作为心脏保护剂的潜力。
腺苷调节肾功能的很多方面,包括肾素释放、肾小球滤过率和肾血流。拮抗腺苷对肾脏的影响的化合物具有作为肾保护剂的潜力。此外,腺苷A3和/或A2B拮抗剂可用于治疗哮喘和其他变态反应。
许多文献中描述了目前关于腺苷受体的知识,例如下列出版物:
Bioorganic & Medicinal Chemistry,6,(1998),619-641,
Bioorganic & Medicinal Chemistry,6,(1998),707-719,
J.Med.Chem.,(1998),41,2835-2845,
J.Med.Chem.,(1998),41,3186-3201,
J.Med.Chem.,(1998),41,2126-2133,
J.Med.Chem.,(1999),42,706-721,
J.Med.Chem.,(1996),39,1164-1171,
Arch.Pharm.Med.Chem.,(1999),332,39-41。
本发明的目的是式I化合物及其可药用盐本身及其作为药物活性物质、其生产、基于本发明化合物的药物及其制备以及式I化合物在控制或预防基于调节腺苷系统的疾病如早老性痴呆、帕金森氏病、神经保护、精神分裂症、焦虑、疼痛、呼吸不足、抑郁、哮喘、变态反应、缺氧、局部缺血、癫痫发作和物质滥用中的用途。此外,本发明的化合物还可用作镇静剂、肌肉松弛药、抗精神病药、抗癫痫药、抗惊厥药和心脏保护剂。本发明最优选的适应症是那些基于A2A受体拮抗剂活性的病症并且包括中枢神经系统病症,例如治疗或预防某些抑郁症、神经保护和帕金森氏病。
本文所使用的术语"低级烷基"表示含有1-6个碳原子的饱和直链或支链烷基,例如,甲基、乙基、丙基、异丙基、正-丁基、异-丁基、2-丁基、叔-丁基等。优选的低级烷基是具有1-4个碳原子的基团。
本文所使用的术语"低级链烯基"表示含有2-6个碳原子和至少一个双键的不饱和直链或支链链烯基,例如,乙烯基、丙烯基、异丙烯基、正-丁烯基、异-丁烯基、2-丁烯基、叔-丁烯基等。优选的低级链烯基是具有2-4个碳原子的基团。
术语"卤素"表示氯、碘、氟和溴。
术语"低级烷氧基"表示其中的烷基残基如上所定义并通过氧原子连接的基团。
"乙酰氨基"是指-NHC(O)CH3并且"乙酰基"为-C(O)CH3。
术语"可药用酸加成盐"包括与无机酸和有机酸形成的盐,所述的酸为例如盐酸、硝酸、硫酸、磷酸、柠檬酸、甲酸、富马酸、马来酸、乙酸、琥珀酸、酒石酸、甲磺酸和对甲苯磺酸等。
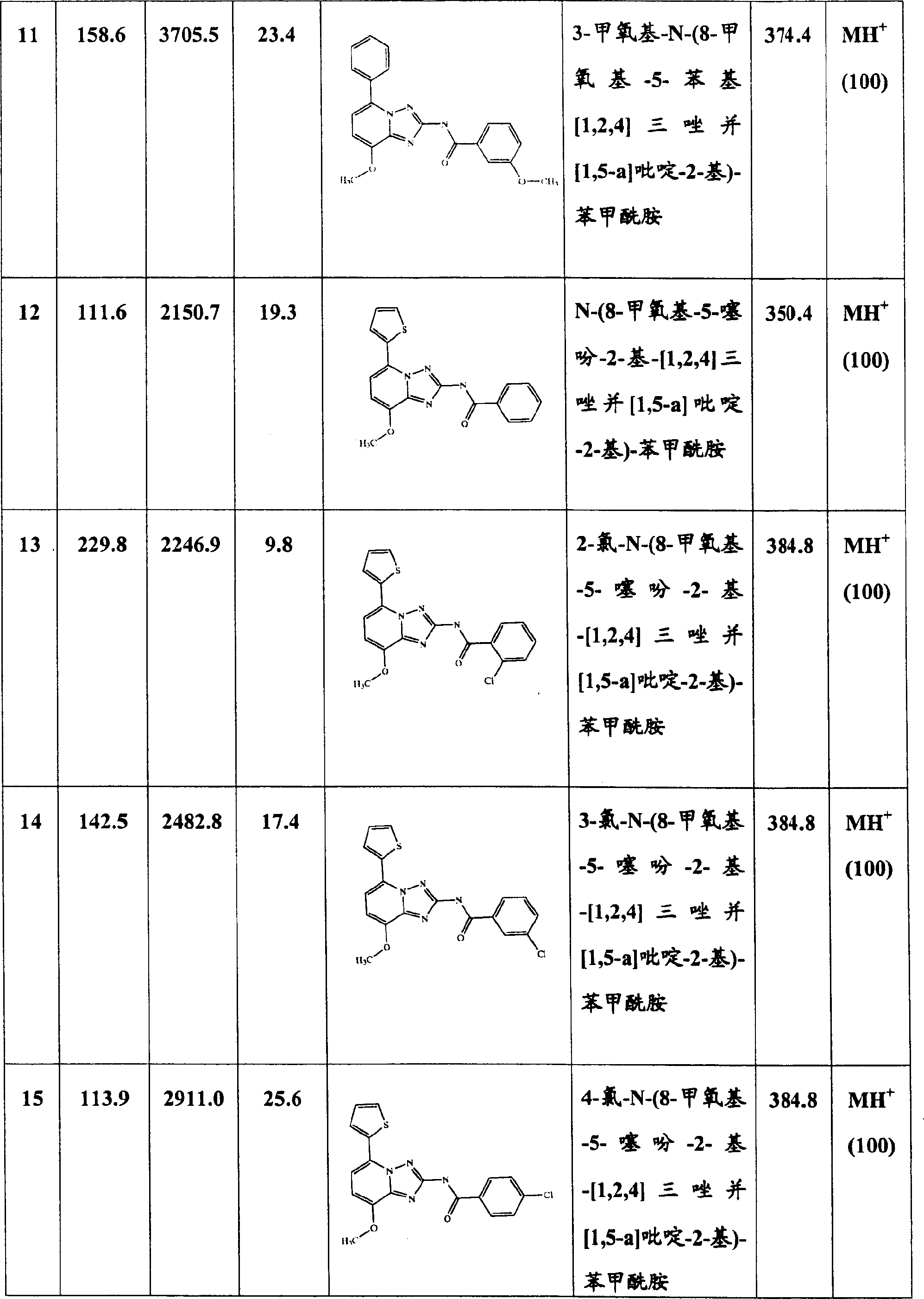
其中R1为噻吩基并且R2为未取代的或被卤素或低级烷基取代的苯基的本发明式I化合物是优选的。例如下列化合物:
4-溴-N-(8-甲氧基-5-噻吩-2-基-[1,2,4]三唑并[1,5-a]吡啶-2-基)苯甲酰胺,
N-(8-甲氧基-5-噻吩-2-基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-苯甲酰胺,
3-氯-N-(8-甲氧基-5-噻吩-2-基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-苯甲酰胺,
4-氯-N-(8-甲氧基-5-噻吩-2-基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-苯甲酰胺或
4-乙基-N-(8-甲氧基-5-噻吩-2-基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-苯甲酰胺。
进一步优选的是其中R1为未取代的或被低级烷氧基取代的苯基并且R2为未取代的或被卤素取代的苯基的化合物。该组的实例为下列化合物:
4-氟-N-(8-甲氧基-5-苯基-[1,2,4]三唑并[1,5-a]吡啶-2-基)苯甲酰胺,
3-溴-N-(8-甲氧基-5-苯基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-苯甲酰胺,
4-溴-N-(8-甲氧基-5-苯基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-苯甲酰胺或
N-[5-(3-乙氧基-苯基)-8-甲氧基-[1,2,4]三唑并[1,5-a]吡啶-2-基]-4-氟-苯甲酰胺。
其中R1为未取代的或被卤素或低级烷氧基取代的苯基并且R2为未取代的或被低级烷基取代的噻吩基的本发明式I化合物也是优选的。例如下列化合物:
5-甲基-噻吩-2-甲酸(8-甲氧基-5-苯基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-酰胺,
5-甲基-噻吩-2-甲酸[5-(2-氟-苯基)-8-甲氧基-[1,2,4]三唑并[1,5-a]吡啶-2-基]-酰胺,
5-甲基-噻吩-2-甲酸[8-甲氧基-5-(3-甲氧基-苯基)-[1,2,4]三唑并[1,5-a]吡啶-2-基]-酰胺,
5-甲基-噻吩-2-甲酸[8-甲氧基-5-(4-甲氧基-苯基)-[1,2,4]三唑并[1,5-a]吡啶-2-基]-酰胺,
5-甲基-噻吩-2-甲酸[5-(3-乙氧基-苯基)-8-甲氧基-[1,2,4]三唑并[1,5-a]吡啶-2-基]-酰胺或
5-甲基-噻吩-2-甲酸[5-(3-氟-苯基)-8-甲氧基-[1,2,4]三唑并[1,5-a]吡啶-2-基]-酰胺。
进一步优选的是其中R1为未取代的或被卤素取代的噻吩基并且R2为未取代的或被低级烷基取代的噻吩基的化合物。该组的实例为下列化合物:
5-甲基-噻吩-2-甲酸(8-甲氧基-5-噻吩-3-基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-酰胺或
5-甲基-噻吩-2-甲酸[5-(5-氯-噻吩-2-基-)-8-甲氧基-[1,2,4]三唑并[1,5-a]吡啶-2-基]-酰胺。
这些式I化合物及其可药用盐可通过本领域已知的方法,例如通过下文描述的方法制备,该方法包括
a)、将式II化合物
与式III化合物反应
R2COCl III
得到式I化合物
其中R1和R2如上所定义。
或者
b)、将式IV化合物
与KIO3反应,得到式V化合物,
然后与式VIa或VIb的化合物反应
R1-B(OH)2或VIa
得到式I化合物
其中R1和R2具有以上给出的含义。
或者
d)、在以上定义的范围内修饰一个或多个取代基R1或R2,并且
需要时,将所得到的化合物转化为可药用酸加成盐。
在实施例1-51和下列方案1中更详细地描述了式I化合物的制备。
使用下列缩写:
DMSO 二甲基亚砜
DMF N,N-二甲基甲酰胺
DMAP 4-二甲基氨基吡啶
DCM 4-(二氰基亚甲基)-2-甲基-6-(4-二甲基氨基苯乙烯基)-4H-吡喃
THF 四氢呋喃
方案1
其中R1和R2如上所定义。
方案1描述了由式VII化合物(通过商业渠道可获得的)开始,制备8-甲氧基-[1,2,4]三唑并[1,5-a]吡啶衍生物的方法。向KOH和DMSO的悬浮液中加入2-氨基-3-羟基吡啶(VII)并搅拌该溶液。然后加入碘甲烷得到式VIII化合物。将在二噁烷中的式VIII化合物和乙氧羰基异硫氰酸酯在室温下搅拌得到式IX化合物。然后向盐酸羟胺和N-乙基二异丙胺在甲醇/乙醇混合物中的溶液中加入N-(3-甲氧基-2-吡啶基)-N′-乙氧羰基-硫脲得到式X化合物。然后将该化合物(8-甲氧基-[1,2,4]三唑并[1,5-a]吡啶-2-基-酰胺)溶解在式R2COCl(III)化合物中得到式IV化合物。式III化合物可以是:苯甲酰氯、4-F-苯甲酰氯、环己烷羰基氯等。
此外,可将式X化合物溶解在硫酸中,然后加热至大约100℃并在1小时内分次加入KIO3。然后由8-甲氧基-5-碘-[1,2,4]三唑并[1,5-a]吡啶-2-基-胺和例如以下所列的各种硼酸或硼酸酯开始,
用取代基R1代替所得到的式X化合物中的碘原子,得到式I化合物。
按照本领域任一技术人员已知和熟悉的方法,在室温下完成盐的制备。不仅考虑到与无机酸的盐,而且考虑到与有机酸的盐。盐酸盐、氢溴酸盐、硫酸盐、硝酸盐、柠檬酸盐、乙酸盐、马来酸盐、琥珀酸盐、甲磺酸盐、对甲苯磺酸盐等是所述盐的实例。
式I化合物及其可药用加成盐具有有价值的药理学特性。具体地说,已发现本发明的化合物是腺苷受体的配体。
按照下文给出的试验研究化合物。
人腺苷A2A受体
利用塞姆利基森林病毒表达系统将人腺苷A2A受体通过重组在中国仓鼠卵巢(CHO)细胞中表达。捕获细胞,通过离心洗涤两次,均化并通过离心再次洗涤。将最终洗涤的膜沉积物悬浮在含有120mM NaCl、5mMKCl、2mM CaCl2和10mM MgCl2(pH 7.4)的Tris(50mM)缓冲液(缓冲液A)中。在96-孔板中,在2.5μg膜蛋白、0.5mg Ysi-聚-l-赖氨酸SPA珠和0.1U腺苷脱氨基酶存在下,在最终体积为200μl的缓冲液A中进行[3H]-SCH-58261(Dionisotti等人,1997,Br.J.Pharmacol.121,353)结合测定。利用黄嘌呤胺同源物(XAC;2μM)来定义非特异性结合。在10μM-0.3nM的10个浓度下测试化合物。所有测定都一式两份并至少重复两次。将测定板在室温下培养1小时后离心,然后用Packard Topcount闪烁计数器测定结合的配体。用非线性曲线拟合程序计算IC50值并用Cheng-Prussoff方程计算Ki值。
根据本发明,已证实式I化合物对A2A受体具有高亲和力。下表描述了所制备的Ki小于1000的化合物的具体值。优选的化合物是其中hA2a Ki小于150nM的化合物。
式I化合物以及式I化合物的可药用盐可以,例如以药物制剂的形式用作药物。药物制剂可以通过口服给药,例如以片剂、包衣片、糖衣丸、硬和软明胶胶囊剂、溶液、乳液或混悬液的形式给药。然而,也可以例如以栓剂的形式通过直肠给药,以例如注射液的形式通过非肠道给药。
可将式I化合物用药物惰性的无机或有机载体处理来制备药物制剂。例如,乳糖、玉米淀粉或其衍生物、滑石粉、硬脂酸或其盐等,可用作片剂、包衣片、糖衣丸和硬明胶胶囊剂的载体。适宜的软明胶胶囊剂的载体为,例如植物油、蜡、脂肪、半固体和液体多元醇等。然而,依赖于活性物质的性质,软明胶胶囊剂通常不需要载体。适宜的制备溶液剂和糖浆剂的载体为,例如水、多元醇、甘油、植物油等。适宜的栓剂载体为例如天然的或硬化油、蜡、脂肪、半液体或液体多元醇等。
此外,药物制剂可包含防腐剂、增溶剂、稳定剂、润湿剂、乳化剂、甜味剂、着色剂、矫味剂、用于改变渗透压的盐、缓冲剂、掩蔽剂或抗氧化剂。它们也可以进一步包含其他具有治疗作用的物质。
包含式I化合物或其可药用盐和治疗惰性载体的药物及其制备方法也是本发明的目的,所述方法包括将一种或多种式I化合物和/或其可药用酸加成盐和一种或多种其他具有治疗作用的物质(如果需要的话)以及一种或多种治疗惰性载体一起制备成盖仑制剂给药形式。
本发明的式I化合物及其可药用盐可用于控制或预防基于腺苷受体拮抗剂活性的疾病如早老性痴呆、帕金森氏病、神经保护、精神分裂症、焦虑、疼痛、呼吸不足、抑郁、哮喘、变态反应、缺氧、局部缺血、癫痫发作和物质滥用。而且本发明化合物可用作镇静剂、肌肉松弛药、抗精神病药、抗癫痫药、抗惊厥药和心脏保护剂以及用于制备相应的药物。
本发明最优选的适应症包括中枢神经系统病症,例如治疗或预防某些抑郁病症、神经保护和帕金森氏病。
剂量可以在宽范围内改变并且当然在各具体病例中应按个体需求调整。在口服给药的情况下,成人的剂量可以为每天大约0.01mg-1000mg的通式I化合物或相当量的其可药用盐。每天的剂量可作为单剂量或以分次剂量形式给予,另外,当需要时,也可以超过上限。
实施例1
N-(8-甲氧基-5-苯基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-苯甲酰胺a)、3-甲氧基-吡啶-2-基-胺
向33.2g(0.592mol)KOH的DMSO(80ml)悬浮液中加入15.9g(0.144mol)2-氨基-3-羟基吡啶并搅拌90分钟。在60分钟内加入9.9ml(0.158mol)碘甲烷并将该混合物在室温下搅拌14小时。将混合物用450ml水终止反应并用8×500ml乙醚提取。将合并的有机层用水洗涤,用MgSO4干燥并蒸发至干。将残余物在乙酸乙酯中重结晶,得到6.99g(56.3mmol,39%)白色结晶状标题化合物。
1-H-NMR(250MHz-DMSO-d6):δ=7.49(dd,J1=5Hz,J2=1.4Hz,1H,H-4),6.98(dd,J1=7.7Hz,J2=1.4Hz,1H,H-6),6.48(dd,J1=7.7Hz,J2=5Hz,1H,H-5),5.60(s,br,2H,NH2),3.75(s,3H,OCH3)。
b)、N-(3-甲氧基-2-吡啶基)-N′-乙氧羰基-硫脲
将8g(64mmol)3-甲氧基-吡啶-2-基胺和7.29ml(64mmol)乙氧羰基异硫氰酸酯(64mmol)在120ml二噁烷中的混合物在室温下搅拌1小时并蒸发至干,得到16g(62.7mmol,97%)黄色结晶状的标题化合物。
1-H-NMR(250MHz-DMSO-d6):δ=11.65(s,br,1H,NH),11.09(s,br,1H,NH),8.01(dd,J1=4.7Hz,J2=1.3Hz,1H,H-4),7.53(dd,J1=7Hz,J2=1.3Hz,1H,H-6),7.33(dd,J1=7Hz,J2=4.7Hz,1H,H-5),4.22(q,J=7.1Hz,2H,CH2),3.33(s,3H,OCH3),1.26(t,J=7.1Hz,3H,CH3)。
c)、8-甲氧基-[1,2,4]三唑并[1,5-a]吡啶-2-基-胺
向21.8g(313.7mmol)盐酸羟胺和32.2ml(188.2mmol)N-乙基二异丙基胺在甲醇/乙醇1∶1混合物(130ml)中的溶液中加入16g(62.7mmol)N-(3-甲氧基-2-吡啶基)-N′-乙氧羰基-硫脲并在室温下搅拌2小时,接下来在60℃下搅拌3小时。减压除去挥发物,残余物用100ml水研制。所收集的沉淀用25ml甲醇/乙醚(4∶1)洗涤,然后用25ml乙醚洗涤。在高真空下干燥后,收集到8g(78%,48.7mmol)灰白色结晶状的标题化合物。
1-H-NMR(250MHz-DMSO-d6):δ=8.13(dd,J1=6.6Hz,J2=1Hz,1H,H-5),6.89(dd,J1=7.1Hz,J2=1Hz,1H,H-7),6.77(dd,J1=7.1Hz,J2=6.6Hz,1H,H-5),5.88(s,br,2H,NH2),3.90(s,3H,OCH3)。
MS(m/e):163.0(M-H,100%)
d)、5-碘-8-甲氧基-[1,2,4]三唑并[1,5-a]吡啶-2-基-胺
将3g(18.3mmol)8-甲氧基-[1,2,4]三唑并[1,5-a]吡啶-2-基-胺在6ml水和6ml硫酸(97%)中的混合物加热至100℃并在1小时内分次加入4.3g(20.1mmol)KIO3。将该混合物加热至120℃ 3小时并补加6ml水和6ml硫酸(97%)。冷却至0℃后,收集沉淀并用2×15ml水洗涤,得到标题化合物的米色结晶。母液用Na2CO3处理并用5×250ml DCM提取。合并的有机层用MgSO4干燥并蒸发至干,得到额外量的标题化合物。将产物在乙醇中重结晶,得到总量为2.59g(49%,8.9mmol)的产物。
1-H-NMR(250MHz-DMSO-d6):δ=7.22(d,J=8.2Hz,1H,H-7),6.76(d,J=8.2Hz,1H,H-6),6.10(s,br,2H,NH2),3.89(s,3H,OCH3)。
e)、8-甲氧基-5-苯基-[1,2,4]三唑并[1,5-a]吡啶-2-基-胺
将50mg(0.17mmol)5-碘-8-甲氧基-[1,2,4]三唑并[1,5-a]吡啶-2-基-胺、46.2mg(0.38mmol)苯基硼酸、6.3mg(0.008mmol)二氯化[1,1′-双(二苯基膦)二茂铁]钯(II)二氯甲烷加合物和0.3ml 2M Na2CO3水溶液在1ml二噁烷中的混合物在80℃下加热90分钟。将该混合物经过短的二氧化硅垫过滤并用30ml乙酸乙酯洗脱。将滤液减压浓缩,将残余物通过反相制备HPLC纯化,用水/乙腈梯度洗脱,得到12mg(29%)标题化合物。
MS m/e(%):241.3(MH+,100)
1-H-NMR(250MHz-DMSO-d6):δ=7.82(dd,J1=8.1Hz,J2=1Hz,2H,苯基),7.48(m,3H,苯基),6.81(dd,J1=8.4Hz,J2=1Hz,2H,H-6和H-7),4.53(s,br,2H,NH2),4.03(s,3H,OCH3)。
f)、N-(8-甲氧基-5-苯基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-苯甲酰胺
将30mg(0.125mmol)8-甲氧基-5-苯基-[1,2,4]三唑并[1,5-a]吡啶-2-基-胺、19.2mg(0.137mmol)苯甲酰氯和27mg NEt3在0.5ml二噁烷中的混合物在室温下振摇4天。加入0.05ml甲酸后,将混合物直接通过反相制备HPLC纯化,用水/乙腈梯度洗脱,得到8.6mg(19%)标题化合物。
MS m/e(%):345(MH+,100)
1-H-NMR(500MHz,DMSO)δ=7.95(m,4H,Ph),7.61(m,6H,Ph),7.30(m,3H,Ph/NH),4.05(s,3H,s,OCH3)。
按照实施例1合成了其它[1,2,4]三唑并[1,5-a]吡啶衍生物。结果列于包含实施例2至实施例51的下表中。
片剂配方(湿法制粒)
项目
成分
mg/片
5mg 25mg 100mg 500mg
1. 式I化合物 5 25 100 500
2. 乳糖无水DTG 125 105 30 150
3. Sta-Rx 1500 6 6 6 30
4. 微晶纤维素 30 30 30 150
5. 硬脂酸镁 1 1 1 1
总量
167
167
167
831
制备方法
1、将项目1、2、3和4混合并用净化水制粒。
2、将颗粒在50℃下干燥。
3、将颗粒通过适宜的制粉装置。
4、加入项目5并混合3分钟;在适宜的压片机上压片。
胶囊剂配方
项目
组成
mg/胶囊
5mg 25mg 100mg 500mg
1. 式I化合物 5 25 100 500
2. 含水乳糖 159 123 148 ---
3. 玉米地方 25 35 40 70
4. 滑石粉 10 15 10 25
5. 硬脂酸镁 1 2 2 5
总量
200
200
300
600
制备方法
1、将项目1、2和3在适宜的混合器中混合30分钟。
2、加入项目4和5并混合3分钟。
3、填充到适宜的胶囊中。
Claims (16)
2、权利要求1的式I化合物,其中R1为噻吩基并且R2为未取代的或被卤素或低级烷基取代的苯基。
3、权利要求2的式I化合物,其中所述化合物为
4-溴-N-(8-甲氧基-5-噻吩-2-基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-苯甲酰胺,
N-(8-甲氧基-5-噻吩-2-基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-苯甲酰胺,
3-氯-N-(8-甲氧基-5-噻吩-2-基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-苯甲酰胺,
4-氯-N-(8-甲氧基-5-噻吩-2-基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-苯甲酰胺或
4-乙基-N-(8-甲氧基-5-噻吩-2-基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-苯甲酰胺。
4、权利要求1的式I化合物,其中R1为未取代的或被低级烷氧基取代的苯基并且R2为未取代的或被卤素取代的苯基。
5、权利要求4的式I化合物,其中所述化合物为
4-氟-N-(8-甲氧基-5-苯基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-苯甲酰胺,
3-溴-N-(8-甲氧基-5-苯基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-苯甲酰胺,
4-溴-N-(8-甲氧基-5-苯基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-苯甲酰胺或
N-[5-(3-乙氧基-苯基)-8-甲氧基-[1,2,4]三唑并[1,5-a]吡啶-2-基]-4-氟-苯甲酰胺。
6、权利要求1的式I化合物,其中R1为未取代的或被卤素或低级烷氧基取代的苯基并且R2为未取代的或被低级烷基取代的噻吩基。
7、权利要求6的式I化合物,其中所述化合物为
5-甲基-噻吩-2-甲酸(8-甲氧基-5-苯基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-酰胺,
5-甲基-噻吩-2-甲酸[5-(2-氟-苯基)-8-甲氧基[1,2,4]三唑并[1,5-a]吡啶-2-基]-酰胺,
5-甲基-噻吩-2-甲酸[8-甲氧基-5-(3-甲氧基-苯基)-[1,2,4]三唑并[1,5-a]吡啶-2-基]-酰胺,
5-甲基-噻吩-2-甲酸[8-甲氧基-5-(4-甲氧基-苯基)-[1,2,4]三唑并[1,5-a]吡啶-2-基]-酰胺,
5-甲基-噻吩-2-甲酸[5-(3-乙氧基-苯基)-8-甲氧基-[1,2,4]三唑并[1,5-a]吡啶-2-基]-酰胺或
5-甲基-噻吩-2-甲酸[5-(3-氟-苯基)-8-甲氧基-[1,2,4]三唑并[1,5-a]吡啶-2-基]-酰胺。
8、权利要求1的式I化合物,其中R1为未取代的或被卤素取代的噻吩基并且R2为未取代的或被低级烷基取代的噻吩基。
9、权利要求8的式I化合物,其中所述化合物为
5-甲基-噻吩-2-甲酸(8-甲氧基-5-噻吩-3-基-[1,2,4]三唑并[1,5-a]吡啶-2-基)-酰胺或
5-甲基-噻吩-2-甲酸[5-(5-氯-噻吩-2-基)-8-甲氧基-[1,2,4]三唑并[1,5-a]吡啶-2-基]-酰胺。
10、包含一种或多种权利要求1-9任一项所述的式I化合物和可药用赋形剂的药物。
11、用于治疗与腺苷受体有关之疾病的权利要求10的药物。
13、通过权利要求12的方法或通过等同的方法制备的权利要求1-9任一项所述的化合物。
14、权利要求1-9任一项所述的化合物在治疗疾病中的应用。
15、权利要求1-9任一项所述的化合物在生产用于治疗与腺苷A2A受体有关之疾病的药物中的应用。
16、以上描述的本发明。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP01117539 | 2001-07-20 | ||
| EP01117539.5 | 2001-07-20 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN1541216A true CN1541216A (zh) | 2004-10-27 |
| CN1264840C CN1264840C (zh) | 2006-07-19 |
Family
ID=8178085
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNB028145089A Expired - Fee Related CN1264840C (zh) | 2001-07-20 | 2002-07-10 | 8-甲氧基-(1,2,4)三唑并(1,5-a)吡啶衍生物及其作为腺苷受体配体的应用 |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US6514989B1 (zh) |
| EP (1) | EP1412352A1 (zh) |
| JP (1) | JP4105089B2 (zh) |
| KR (1) | KR100592939B1 (zh) |
| CN (1) | CN1264840C (zh) |
| AR (1) | AR034870A1 (zh) |
| AU (1) | AU2002325316B2 (zh) |
| BR (1) | BR0211206A (zh) |
| CA (1) | CA2453919A1 (zh) |
| MX (1) | MXPA04000532A (zh) |
| PL (1) | PL373414A1 (zh) |
| RU (1) | RU2297416C2 (zh) |
| WO (1) | WO2003010167A1 (zh) |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1830978B (zh) * | 2005-03-07 | 2010-06-16 | 中国人民解放军军事医学科学院毒物药物研究所 | 吡唑并[4,3-c]喹啉-3-酮化合物、其制备方法及其应用 |
| CN102105471A (zh) * | 2008-07-25 | 2011-06-22 | 加拉帕戈斯股份有限公司 | 用于治疗变性和炎性疾病的新化合物 |
| CN102131389A (zh) * | 2008-06-20 | 2011-07-20 | 健泰科生物技术公司 | 三唑并吡啶jak抑制剂化合物和方法 |
| CN101535307B (zh) * | 2006-08-30 | 2013-10-30 | 塞尔佐姆有限公司 | 作为激酶抑制剂的三唑衍生物 |
| CN103641829A (zh) * | 2006-08-30 | 2014-03-19 | 塞尔佐姆有限公司 | 作为激酶抑制剂的三唑衍生物 |
| WO2020192762A1 (zh) * | 2019-03-28 | 2020-10-01 | 基石药业(苏州)有限公司 | 一种a2a受体拮抗剂的盐型、晶型及其制备方法 |
| CN111892592A (zh) * | 2019-08-06 | 2020-11-06 | 江苏柯菲平医药股份有限公司 | Jak激酶抑制剂及其用途 |
| CN114907182A (zh) * | 2019-09-09 | 2022-08-16 | 武汉诺安药业有限公司 | 一种4-碘代叔戊基苯的清洁制备方法 |
Families Citing this family (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6693116B2 (en) * | 2001-10-08 | 2004-02-17 | Hoffmann-La Roche Inc. | Adenosine receptor ligands |
| AU2002358597C1 (en) * | 2001-12-12 | 2008-09-18 | F. Hoffmann-La Roche Ag | Benzothiophenes as adenosine receptor modulators |
| MY139563A (en) * | 2002-09-04 | 2009-10-30 | Bristol Myers Squibb Co | Heterocyclic aromatic compounds useful as growth hormone secretagogues |
| CA2655310A1 (en) * | 2006-06-22 | 2008-05-29 | Cv Therapeutics, Inc. | Use of a2a adenosine receptor agonists in the treatment of ischemia |
| HUP0700395A2 (en) | 2007-06-07 | 2009-03-02 | Sanofi Aventis | Substituted [1,2,4] triazolo [1,5-a] quinolines, process for their preparation, pharmaceutical compositions thereof, and intermediates |
| GB0719803D0 (en) | 2007-10-10 | 2007-11-21 | Cancer Rec Tech Ltd | Therapeutic compounds and their use |
| CA2706578A1 (en) * | 2007-11-27 | 2009-06-04 | Cellzome Limited | Amino triazoles as pi3k inhibitors |
| AU2009259867A1 (en) | 2008-06-20 | 2009-12-23 | Genentech, Inc. | Triazolopyridine JAK inhibitor compounds and methods |
| WO2010010188A1 (en) * | 2008-07-25 | 2010-01-28 | Galapagos Nv | Novel compounds useful for the treatment of degenerative and inflammatory diseases. |
| TWI453207B (zh) * | 2008-09-08 | 2014-09-21 | Signal Pharm Llc | 胺基三唑并吡啶,其組合物及使用其之治療方法 |
| CA2747022A1 (en) * | 2008-12-19 | 2010-06-24 | Leo Pharma A/S | Triazolopyridines as phosphodiesterase inhibitors for treatment of dermal diseases |
| US8501936B2 (en) | 2009-06-05 | 2013-08-06 | Cephalon, Inc. | Preparation and uses of 1,2,4-triazolo [1,5a] pyridine derivatives |
| JO3030B1 (ar) * | 2009-06-26 | 2016-09-05 | Galapagos Nv | مركب جديد مفيد لمعالجة الامراض التنكسية والالتهابات |
| TWI462920B (zh) | 2009-06-26 | 2014-12-01 | 葛萊伯格有限公司 | 用於治療退化性及發炎疾病之新穎化合物 |
| NZ598242A (en) * | 2009-07-17 | 2013-09-27 | Japan Tobacco Inc | Triazolopyridine compound, and action thereof as prolyl hydroxylase inhibitor and erythropoietin production inducer |
| EP2343297A1 (en) | 2009-11-30 | 2011-07-13 | Bayer Schering Pharma AG | Triazolopyridines |
| US8080568B1 (en) * | 2010-06-29 | 2011-12-20 | Ewha University - Industry Collaboration Foundation | 2-pyridyl substituted imidazoles as therapeutic ALK5 and/or ALK4 inhibitors |
| RU2628616C2 (ru) * | 2011-11-03 | 2017-08-21 | Ф. Хоффманн-Ля Рош Аг | Бициклические соединения пиперазина |
| BR112014014802A2 (pt) * | 2011-12-21 | 2017-06-13 | Leo Pharma As | composto, composição farmacêutica, uso de um composto, métodos para tratamento ou para alívio de uma doença ou de um distúrbio ou de uma condição, e para tratamento ou melhora de doenças ou de condições |
| CA2867061A1 (en) | 2012-03-14 | 2013-09-19 | Bayer Intellectual Property Gmbh | Substituted imidazopyridazines |
| WO2014198647A2 (en) | 2013-06-11 | 2014-12-18 | Bayer Pharma Aktiengesellschaft | Prodrug derivatives of substituted triazolopyridines |
| RU2563254C2 (ru) * | 2013-07-08 | 2015-09-20 | Федеральное государственное бюджетное образовательное учреждение высшего профессионального образования "Московский государственный университет имени М.В. Ломоносова" (МГУ) | Способ получения производных 7-(гетеро)арил-4,5,6,7-тетрагидро[1,2,3]триазоло[1,5-a]пиридина |
| GB201402071D0 (en) | 2014-02-07 | 2014-03-26 | Galapagos Nv | Novel salts and pharmaceutical compositions thereof for the treatment of inflammatory disorders |
| GB201402070D0 (en) | 2014-02-07 | 2014-03-26 | Galapagos Nv | Pharmaceutical compositions for the treatment of inflammatory disorders |
| AR117291A1 (es) | 2018-12-14 | 2021-07-28 | Syngenta Crop Protection Ag | Compuestos heterocíclicos de cianamida con actividad pesticida |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB9226735D0 (en) | 1992-12-22 | 1993-02-17 | Ici Plc | Azole derivatives |
| US5571775A (en) | 1994-07-11 | 1996-11-05 | Dowelanco | N-aryl[1,2,4]triazolo[1,5-a]pyridine-2-sulfonamide herbicides |
| US6355653B1 (en) | 1999-09-06 | 2002-03-12 | Hoffmann-La Roche Inc. | Amino-triazolopyridine derivatives |
-
2002
- 2002-05-29 US US10/157,346 patent/US6514989B1/en not_active Expired - Fee Related
- 2002-07-10 EP EP02758330A patent/EP1412352A1/en not_active Withdrawn
- 2002-07-10 CN CNB028145089A patent/CN1264840C/zh not_active Expired - Fee Related
- 2002-07-10 RU RU2004105260/04A patent/RU2297416C2/ru not_active IP Right Cessation
- 2002-07-10 BR BR0211206-0A patent/BR0211206A/pt not_active IP Right Cessation
- 2002-07-10 JP JP2003515526A patent/JP4105089B2/ja not_active Expired - Fee Related
- 2002-07-10 PL PL02373414A patent/PL373414A1/xx not_active Application Discontinuation
- 2002-07-10 KR KR1020047000914A patent/KR100592939B1/ko not_active IP Right Cessation
- 2002-07-10 AU AU2002325316A patent/AU2002325316B2/en not_active Ceased
- 2002-07-10 WO PCT/EP2002/007661 patent/WO2003010167A1/en active Application Filing
- 2002-07-10 CA CA002453919A patent/CA2453919A1/en not_active Abandoned
- 2002-07-10 MX MXPA04000532A patent/MXPA04000532A/es active IP Right Grant
- 2002-07-18 AR ARP020102688A patent/AR034870A1/es unknown
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1830978B (zh) * | 2005-03-07 | 2010-06-16 | 中国人民解放军军事医学科学院毒物药物研究所 | 吡唑并[4,3-c]喹啉-3-酮化合物、其制备方法及其应用 |
| CN101535307B (zh) * | 2006-08-30 | 2013-10-30 | 塞尔佐姆有限公司 | 作为激酶抑制剂的三唑衍生物 |
| CN103641829A (zh) * | 2006-08-30 | 2014-03-19 | 塞尔佐姆有限公司 | 作为激酶抑制剂的三唑衍生物 |
| CN102131389A (zh) * | 2008-06-20 | 2011-07-20 | 健泰科生物技术公司 | 三唑并吡啶jak抑制剂化合物和方法 |
| CN102105471A (zh) * | 2008-07-25 | 2011-06-22 | 加拉帕戈斯股份有限公司 | 用于治疗变性和炎性疾病的新化合物 |
| CN102105471B (zh) * | 2008-07-25 | 2014-10-15 | 加拉帕戈斯股份有限公司 | 用于治疗变性和炎性疾病的化合物 |
| WO2020192762A1 (zh) * | 2019-03-28 | 2020-10-01 | 基石药业(苏州)有限公司 | 一种a2a受体拮抗剂的盐型、晶型及其制备方法 |
| CN113646313A (zh) * | 2019-03-28 | 2021-11-12 | 基石药业(苏州)有限公司 | 一种a2a受体拮抗剂的盐型、晶型及其制备方法 |
| CN113646313B (zh) * | 2019-03-28 | 2023-05-30 | 基石药业(苏州)有限公司 | 一种a2a受体拮抗剂的盐型、晶型及其制备方法 |
| CN111892592A (zh) * | 2019-08-06 | 2020-11-06 | 江苏柯菲平医药股份有限公司 | Jak激酶抑制剂及其用途 |
| CN111892592B (zh) * | 2019-08-06 | 2023-09-19 | 江苏柯菲平医药股份有限公司 | Jak激酶抑制剂及其用途 |
| CN114907182A (zh) * | 2019-09-09 | 2022-08-16 | 武汉诺安药业有限公司 | 一种4-碘代叔戊基苯的清洁制备方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| US6514989B1 (en) | 2003-02-04 |
| MXPA04000532A (es) | 2004-05-04 |
| CA2453919A1 (en) | 2003-02-06 |
| AU2002325316B2 (en) | 2007-11-01 |
| PL373414A1 (en) | 2005-08-22 |
| KR20040023667A (ko) | 2004-03-18 |
| JP4105089B2 (ja) | 2008-06-18 |
| AU2002325316B8 (en) | 2003-02-17 |
| CN1264840C (zh) | 2006-07-19 |
| BR0211206A (pt) | 2004-07-13 |
| JP2005511492A (ja) | 2005-04-28 |
| AR034870A1 (es) | 2004-03-24 |
| RU2297416C2 (ru) | 2007-04-20 |
| RU2004105260A (ru) | 2005-07-10 |
| EP1412352A1 (en) | 2004-04-28 |
| KR100592939B1 (ko) | 2006-06-26 |
| WO2003010167A1 (en) | 2003-02-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1541216A (zh) | 8-甲氧基-(1,2,4)三唑并(1,5-a)吡啶衍生物及其作为腺苷受体配体的应用 | |
| AU2002325316A1 (en) | Aromatic and heteroaromatic substituted 1,2,4-triazolo pyridine derivatives | |
| CN100346792C (zh) | 用作腺苷受体配体的苯并噻唑衍生物 | |
| CN1956983B (zh) | 4-羟基-4-甲基-哌啶-1-甲酸(4-甲氧基-7-吗啉-4-基-苯并噻唑-2-基)-酰胺 | |
| CN1254475C (zh) | 用作腺苷受体拮抗剂的5-甲氧基-8-芳基-[1,2,4]三唑并[1,5-a]吡啶衍生物 | |
| US6689790B2 (en) | Substituted triazolopyridine compounds | |
| AU2005263500B2 (en) | Benzothiazole derivatives | |
| AU2002338827A1 (en) | Adenosine Receptor Ligands | |
| AU2002347055A1 (en) | Substituted Triazolopyridine Compounds | |
| EP1587799B1 (en) | Benzoxazole derivatives and their use as adenosine receptor ligands | |
| CN1596112A (zh) | 苯并噻唑衍生物 | |
| AU2004203909B8 (en) | Benzoxazol Derivatives |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| C17 | Cessation of patent right | ||
| CF01 | Termination of patent right due to non-payment of annual fee |
Granted publication date: 20060719 Termination date: 20090810 |