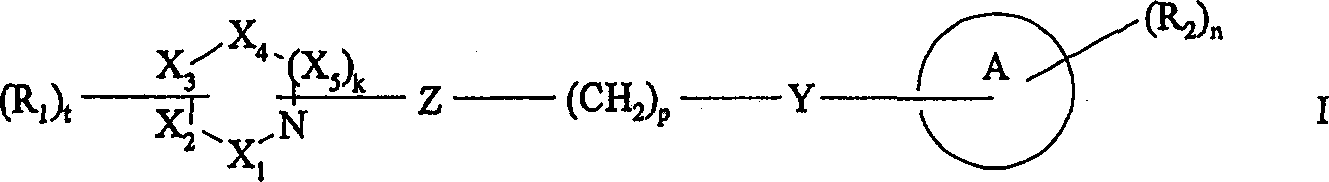CN1303376A - Compound with activity on muscarinic receptors - Google Patents
Compound with activity on muscarinic receptors Download PDFInfo
- Publication number
- CN1303376A CN1303376A CN99806618A CN99806618A CN1303376A CN 1303376 A CN1303376 A CN 1303376A CN 99806618 A CN99806618 A CN 99806618A CN 99806618 A CN99806618 A CN 99806618A CN 1303376 A CN1303376 A CN 1303376A
- Authority
- CN
- China
- Prior art keywords
- alkyl
- compound
- alkoxyl group
- group
- butyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/04—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D207/08—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon radicals, substituted by hetero atoms, attached to ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D295/00—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms
- C07D295/04—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms
- C07D295/10—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by doubly bound oxygen or sulphur atoms
- C07D295/104—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by doubly bound oxygen or sulphur atoms with the ring nitrogen atoms and the doubly bound oxygen or sulfur atoms attached to the same carbon chain, which is not interrupted by carbocyclic rings
- C07D295/108—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by doubly bound oxygen or sulphur atoms with the ring nitrogen atoms and the doubly bound oxygen or sulfur atoms attached to the same carbon chain, which is not interrupted by carbocyclic rings to an acyclic saturated chain
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/06—Antiglaucoma agents or miotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D211/00—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings
- C07D211/04—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D211/06—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D211/08—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms
- C07D211/18—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D211/20—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms with substituted hydrocarbon radicals attached to ring carbon atoms with hydrocarbon radicals, substituted by singly bound oxygen or sulphur atoms
- C07D211/22—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms with substituted hydrocarbon radicals attached to ring carbon atoms with hydrocarbon radicals, substituted by singly bound oxygen or sulphur atoms by oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/24—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D213/28—Radicals substituted by singly-bound oxygen or sulphur atoms
- C07D213/30—Oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/61—Halogen atoms or nitro radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/62—Oxygen or sulfur atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/06—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
Landscapes
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Ophthalmology & Optometry (AREA)
- Hospice & Palliative Care (AREA)
- Psychiatry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Hydrogenated Pyridines (AREA)
- Plural Heterocyclic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
Abstract
Compounds and methods are provided for the alleviation or treatment of diseases or conditions in which modification of muscarinic m1 receptor activity has a beneficial effect. In the method, a therapeutically effective amount of a selective muscarinic m1 agonist compound is administered to a patient in need of such treatment.
Description
Invention field
The present invention relates to that the muscarinic acetylcholine receptor hypotype is had optionally new compound, and activate the method and the treatment of muscarinic receptor or alleviate and wherein improve the method that the muscarinic receptor activity has the disease of beneficial effect.
Background of invention
Muscarinic acetylcholine receptor plays an important role on the senior cognitive function of central nervous system and in the periphery parasympathetic nervous system.Exist (being called m1-m5) of the definite five kinds of different muscarinic receptor hypotypes of cloning (consults T.I.Bonner etc., Science237,1987,527-532 page or leaf; T.I.Bonner etc., Neuron 1,1988, the 403-410 page or leaf).Found that m1 is the main hypotype in the pallium, and think that it relates to the control of cognitive function, m2 preponderates in heart, and think that its control with heart rate is relevant, it is relevant with polysialia with stomach and intestine and urethral stimulant effect and perspiration that m3 is considered to, m4 is present in the brain, and m5 is present in the brain and may relates to some function of the central nervous system relevant with dopaminergic system.
The zooscopy of various muscarinic parts (S.Iversen, Life Sciences 60 (Nos.13/14), 1997,1145-1152 page or leaf) has shown that the muscarinic compound has tangible effect, for example learning and memory to cognitive function.This prompting can be in the disease of feature with the cognitive impairment, comprise relevant with the age (as AlzheimerShi disease or other dementia) and with the age irrelevant (as scatterbrained hyperactivity disorder) in, effectively utilize muscarinic agonists and improve cognitive function.
Be present in various tissues based on the muscarinic receptor hypotype, but as if in pallium, basal ganglion and hippocampus, the m1 receptor subtype is abundanter hypotype, the 35-60% that it accounts for all muscarinic receptor combining sites (consults A.Levey, Proc.Natl.Acad.Sci., USA93,1996, the 13541-13546 page or leaf).Inferred that m1 (and possible m4) hypotype plays a major role as postsynaptic muscarinic receptor (being arranged on the cholinocepter neurone of neocortex and hippocampus) in various cognitions and motor function, and it is likely the main contributor of the m1 response of being measured in these zones of brain.
Finding already that selectivity loses vagusstoff in symptom (as AlzheimerShi disease) relevant with cognitive impairment and the brain takes place simultaneously.Assert that it is the result that cholinergic neuron is degenerated in the forebrain substrate, associated cortex that relates in its innervation level process and hippocampus (consulting above S.Iversen).This discovery shows with the medicine that can increase cholinergic function in the brain involved area can treat or alleviate these symptoms at least.
With acetylcholinesterase (AChE) inhibitor, as 9-amino-1,2,3,4-tetrahydro acridine (tacrine) treatment can make vagusstoff increase in the brain, and it causes the hormesis of muscarinic receptor indirectly.Tacrine treatment can appropriateness and temporarily be improved AlzheimerShi disease patients cognitive (consult above Kasa etc.).On the other hand, found since tacrine to the caused cholinergic side effect of the hormesis of periphery vagusstoff.These side effects comprise angina abdominis, nausea,vomiting,diarrhea, apocleisis, lose weight, myopathy and depression.About 1/3rd the patient who finds to be treated has gastrointestinal side effect.Find that also tacrine can cause tangible liver toxicity, discovery liver transaminase raises and (consults P.Taylor in about 30% patient, " Anticholinergic Agents ", the 8th chapter, Goodman and Gilman:ThePharmacological Basis of Therapeutics, the 9th edition, 1996, the 161-176 page or leaf).The side effect of tacrine has seriously limited its clinical use.Nearest another AChE inhibitor of approved, (R, S)-1-benzyl-4-[5,6-dimethoxy-1-(2, the 3-indone)-2-yl] methyl piperidine hydrochloride (donepezil), be used for the treatment of mild to moderate AlzheimerShi disease (consult above P.Kasa etc.).Do not find the liver injury effect of this compound, but it has and the similar gastrointestinal side effect of tacrine, its reason may be the hormesis of the caused m3 acceptor because the parasympathetic effect raises.
Find already, because the muscarinic m1 acceptor in prefrontal cortex and the hippocampus appears as integral body, so by donation is the medicine of those muscarinic receptor agonists, losing of vagusstoff be may cure or alleviate at least among the AlzheimerShi disease patient and (J.H.Brown and P.Taylor consulted, " Muscarinic Receptor Agonists and Antagonists ", the 7th chapter, Goodman and Gilman:The Pharmacological Basis of Therapeutics, the 9th edition, 1996,147 pages).
Think so far and as methylarecaidin, in clinical experiment, do not demonstrate the effect bigger (consulting above S.V.P.Jones etc.) as yet by the muscarinic agonists (being considered to the m1 selective agonist) that is used for the treatment of AlzheimerShi disease than AChE inhibitor.(consult T.Sunderland etc. a research, Brain Res.Rev.13,1988, the 371-389 page or leaf) find in methylarecaidin very not big as cognitive enhancement to the influence of the frequent observed behavior variation of institute in AlzheimerShi disease patient, as locomotor activity obviously strengthen, mood obviously improves and unable symptom obviously reduces.But, found afterwards that the m1 agonist of being supposed had weak partial agonist selectivity (H.Br uner-Osborne etc., J.Med.Chem.38,1995,2188-2195 page or leaf) to m2 and/or m3 receptor subtype.Show as above, infer that the m2 subtype-selective determines the viewed cardiovascular effect of these agonists, overrun and bradyrhythmia, think that the m3 activity is the major cause of this agonist gastrointestinal side effect as aroused in interest.
Therefore, m2 and/or m3 activity are the main drawbacks for the treatment of this muscarinic agonists that AlzheimerShi disease mentioned so far, and it has seriously limited the dosage of this medicine, and what therefore give the patient may be to be lower than dose,optimum always.Therefore, the low effectiveness that lacks subtype-selective and the general cholinergic compound of testing increases the periphery side effect, and owing to weak in brain and/or opposite effect have limited cognitive effect.So exploitation has higher selectivity to the m1 hypotype but is very significant to the compound that m2 and m3 hypotype have very little or a non-activity.
Summary of the invention
The invention provides and have the active logical formula I compound of muscarinic agonists or its pharmacy acceptable salt, ester or prodrug:
Wherein
X
1, X
2, X
3, X
4And X
5Be selected from C, N and O;
K is 0 or 1;
T is 0,1 or 2;
R
1Be the C of straight or side chain
1-8Alkyl, C
2-8Alkenyl, C
2-8Alkynyl group, C
1-8Alkylidene group, C
1-8Alkoxyl group, C
1-8Assorted alkyl, C
1-8Aminoalkyl group, C
1-8Haloalkyl, C
1-8Alkoxy carbonyl, C
1-8Hydroxy alkoxy base, C
1-8Hydroxyalkyl ,-SH, C
1-8Alkylthio ,-O-CH
2-C
5-6Aryl, by C
1-3Alkyl or halogen replace-C (O)-C
5-6Aryl; Optional contain 1 or the heteroatomic C of a plurality of N of being selected from, S and O
5-6Aryl or C
5-6Cycloalkyl;-C (O) NR
3R
4,-NR
3R
4,-NR
3C (O) NR
4R
5,-CR
3R
4,-OC (O) R
3(the CH of ,-(O)
2)
sNR
3R
4Or-(CH
2)
sNR
3R
4R wherein
3, R
4And R
5Identical or different, independently be selected from H, C separately
1-6Alkyl; Optional contain 1 or the heteroatoms of a plurality of N of being selected from, O and S and optional by halogen or C
1-6The C that alkyl replaces
5-6Aryl; C
3-6Cycloalkyl; Perhaps R
3And R
4Contain 5-6 ring structure that is selected from the atom of C, N, S and O with N atom (when existing) formation; S is the integer of 0-8;
A is C
5-12Aryl or C
5-7Cycloalkyl optionally separately contains 1 or the heteroatoms of a plurality of N of being selected from, S and O;
R
2Be the C of H, amino, hydroxyl, halogen or straight or side chain
1-6Alkyl, C
2-6Alkenyl, C
2-6Alkynyl group, C
1-6Alkoxyl group, C
1-6Assorted alkyl, C
1-6Aminoalkyl group, C
1-6Haloalkyl, C
1-6Alkylthio, C
1-6Alkoxy carbonyl ,-CN ,-CF
3,-OR
3,-COR
3,-NO
2,-NHR
3,-NHC (O) R
3,-C (O) NR
3R
4,-NR
3R
4,-NR
3C (O) NR
4R
5,-OC (O) R
3,-C (O) R
3R
4,-O (CH
2)
qNR
3,-CNR
3R
4Or-(CH
2)
qNR
3R
4Wherein q is the integer of 1-6;
N is 0,1,2,3 or 4, when n>1, and R
2Can be identical or different;
P is 0 or the integer of 1-5;
Y be O, S, CHOH ,-NHC (O)-,-C (O) NH-,-C (O)-,-OC (O)-, NR
7Or-CH=N-, R
7Be H or C
1-4Alkyl; Or do not exist; With
Z is CR
8R
9, R wherein
8And R
9Independently be selected from the C of H and straight or side chain
1-8Alkyl.
The present invention also provides the medicinal compositions that contains significant quantity formula I compound.
The method that also provides treatment and levels of acetylcholine to lower diseases associated or symptom, this method comprise treat significant quantity contain the formula I compound compositions.
In another embodiment, the present invention also provides the method for treatment with intraocular pressure rising diseases associated or symptom (as glaucoma), this method comprise treat significant quantity contain the formula I compound compositions.
The accompanying drawing summary
Fig. 1 is for being presented in the described test of embodiment X VI, to the figure of the raw data of a 96-hole microtiter plate of 35,000 little organic molecules screenings.
Fig. 2 is for showing with reference to the figure of the atropinic distribution of antagonist with the data of comparing with the m1 muscarinic receptor of Samoryl (open triangle) or compd A (embodiment I) (closed triangle) stimulated cells institute transfection.
Detailed Description Of The Invention
The invention provides with respect to other muscarinic hypotype, the m1 receptor subtype is preferably demonstrated high relatively optionally compound, it is at the treatment cognitive impairment, as having useful effect on the relevant symptom of AlzheimerShi disease or other cognitive decline relevant, but avoided the side effect of such medicine on this purposes so far with the age.By screening m1-m5 receptor subtype, isolate the compound that presents this character in surprise.
One embodiment of this invention provides formula I compound or its pharmacy acceptable salt, ester or prodrug, wherein:
X
1, X
2, X
3, X
4And X
5Be C; Perhaps X
1, X
2, X
3, X
4Or X
5One of them is O or N, and remaining is C;
K is 0 or 1;
T is 1;
R
1Be the C of straight or side chain
1-8Alkyl, C
2-8Alkenyl, C
2-8Alkynyl group, C
1-8Alkylidene group, C
1-8Alkoxyl group, C
1-8Aminoalkyl group, C
1-8Haloalkyl, C
1-8Alkoxy carbonyl ,-C (O) NR
3R
4,-NR
3R
4,-NR
3C (O) NR
4R
5,-OC (O) R
3Or-(CH
2)
sNR
3R
4R wherein
3, R
4And R
5Identical or different, independently be selected from H and C separately
1-6Alkyl; S is the integer of 1-8;
N is 1,2 or 3; And
A is a phenyl or naphthyl;
R wherein
2Be the C of straight or side chain
1-6Alkyl, C
2-6Alkenyl, C
2-6Alkynyl group, C
1-6Alkoxyl group, C
1-6Aminoalkyl group, C
1-6Haloalkyl, C
1-6Alkoxy carbonyl ,-CN ,-CF
3,-OH ,-COR
3,-NHR
3,-NHC (O) R
3,-C (O) NR
3R
4,-NR
3R
4,-NR
3C (O) NR
4R
5,-OC (O) R
3Or-(CH
2)
qNR
3R
4Wherein q is the integer of 1-6; Perhaps
A contains 1 or the heteroatomic aryl of a plurality of N of being selected from, S and O;
R
2Be the C of H, halogen, straight or side chain
1-6Alkyl, C
2-6Alkenyl, C
2-6Alkynyl group, C
1-6Alkoxyl group, C
1-6Assorted alkyl, C
1-6Aminoalkyl group, C
1-6Haloalkyl, C
1-6Alkoxy carbonyl ,-CN ,-CF
3,-OH ,-COR
3,-NHR
3,-NHC (O) R
3,-C (O) NR
3R
4,-NE
3R
4,-NR
3C (O) NR
4R
5,-OC (O) R
3Or-(CH
2)
qNR
3R
4
The preferred inferior embodiment of formula II compound comprise formula (II a) and (II b) compound:
According to the embodiment of a preferred series of formula I, II, II a and II b compound, t is 1, Y is-C (O)-,-NHC (O)-, S, O or-OC (O)-.In another embodiment, X
3Be C.Preferred R
1Be alkyl, and R
2Preferably alkyl, aminoalkyl group, alkoxyl group or hydroxyl.In one embodiment, p is 3.In another embodiment, R
1Be C
2-8Alkyl and R
2Be methyl, hydroxyl or alkoxyl group.
In one embodiment, n is 1 or 2; Y is-C (O)-or O, t is 1.Preferred R
2It is halogen.Other embodiment of the present invention, t is 0; Or R
1Be alkoxyl group, benzyl or phenyl.
According to one embodiment of the invention, X
3Can also be N, R
1Be alkyl or alkoxyl group; Perhaps R
1Be benzyl or phenyl; And R
2Be alkyl or alkoxyl group.
According to another embodiment of the invention, X
3Be O, wherein t can be, for example 0.Preferably, R
2Be alkyl or alkoxyl group; Perhaps R
2It is halogen.
Specific embodiments of the present invention comprises: 4-methoxyl group-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-oxyethyl group-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-propoxy--1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-butoxy-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-methoxymethyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-ethoxyl methyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-propoxy-methyl isophthalic acid-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-(2-methoxy ethyl)-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-(2-ethoxyethyl group)-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-methoxyl group-4-methyl isophthalic acid-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-methoxyl group-4-ethyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-methoxyl group-4-propyl group-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-methoxyl group-4-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-oxyethyl group-4-methyl isophthalic acid-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-oxyethyl group-4-ethyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-oxyethyl group-4-propyl group-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-oxyethyl group-4-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-propoxy--4-methyl isophthalic acid-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-propoxy--4-ethyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-propoxy--4-propyl group-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-propoxy--4-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-n-butoxy-4-methyl isophthalic acid-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-n-butoxy-4-ethyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-n-butoxy-4-propyl group-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-n-butoxy-4-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 2-[3-(4-normal-butyl piperidines) propoxy-] toluene; 2-[3-(4-normal-butyl piperidines) rosickyite alkyl (sulfanyl)] toluene; 2-[3-(4-normal-butyl piperidines) third sulfinyl] toluene; 3-(4-normal-butyl piperidines)-o-tolyl-butane-1-thioketones; 3-(4-normal-butyl piperidino-(1-position only) propyl group)-Ortho Toluidine; N-(4-(4-normal-butyl piperidines)-1-o-tolyl-butyl)-azanol; 4-normal-butyl-1-[4-(2-chloro-phenyl-)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-bromophenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-fluorophenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-sulfydryl phenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-sulfane ylmethyl phenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-sulfane base ethylphenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-aminophenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-methylamino-phenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-l-[4-(2-ethylamino phenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-dimethylamino phenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-diethylin phenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(1-H-imidazoles-2-yl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(1-imidazoles-1-yl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(1-thiazol-2-yl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-([1,2,3] triazol-1-yl)-4-oxo-1-butyl] piperidines; 2-[4-normal-butyl-piperidines-1-ethyl]-8-methyl-3,4-hydrogen-2H-naphthalene-1-ketone; 2-[4-normal-butyl-piperidines-1-ethyl]-7-methyl-2, the 3-bihydrogen-1-indenone; 3-[4-normal-butyl-piperidines-1-ethyl]-chromanone; 2-[4-normal-butyl-piperidines-1-ethyl]-the 1H-benzoglyoxaline; 4-normal-butyl-1-[4-(4-fluoro-2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-hydroxy phenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-p-methoxy-phenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(1-thiophene-2-yl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-ethylphenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-ethoxyl phenenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2, the 4-3,5-dimethylphenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2, the 3-3,5-dimethylphenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(3-p-methoxy-phenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-benzyloxy phenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(4-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-N-phenyl-butyramide; 4-methyl isophthalic acid-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(naphthalene-1-yl)-4-oxo-1-butyl] piperidines; 4-benzyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] tetramethyleneimine; 4-benzyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperazine; 2-propyl group-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 2-ethyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-n-propyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperazine; 3,5-dimethyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-methyl isophthalic acid-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperazine; 4-n-hexyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperazine; 4-hydroxyethyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperazine; 4-ethyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperazine; 4-benzyl-1-[4-(4-fluorophenyl)-4-oxo-1-butyl] piperidines; 4-benzyl-1-[4-(4-bromophenyl)-4-oxo-1-butyl] piperidines; 4-phenyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperazine; 3-methylol-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-methyl isophthalic acid-[4-(4-bromophenyl)-4-oxo-1-butyl] piperidines; 1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 2-methylol-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 4-benzyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-amyl group] piperazine; 4-n-hexyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-amyl group] piperazine; 4-(piperidines-1-yl)-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl]-2,3-dihydro-1H-indoles; 4-benzyl-1-[5-(2-aminomethyl phenyl)-5-Oxy-1-amyl group] piperidines; 4-normal-butyl-1-[5-(2-aminomethyl phenyl)-5-Oxy-1-amyl group] piperidines; 4-normal-butyl-1-[4-(2, the 6-3,5-dimethylphenyl)-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-methoxymethyl phenyl)-4-oxo-1-butyl] piperidines; 1-(2-aminomethyl phenyl)-2-(4-benzyl diethylenediamine-1-yl)-ethyl ketone; 3,5-dimethyl-1-[5-(2-aminomethyl phenyl)-5-Oxy-1-amyl group] piperidines; 3,5-dimethyl-1-[4-(4-fluorophenyl)-4-oxo-1-butyl] piperidines; 1-[4-(4-fluorophenyl)-4-oxo-1-butyl] tetramethyleneimine; 4-benzyl-1-[6-(2-aminomethyl phenyl)-6-Oxy-1-hexyl] piperazine; 3,5-dimethyl-1-[6-(2-aminomethyl phenyl)-6-Oxy-1-butyl] piperidines; 4-benzyl-1-[5-(2-p-methoxy-phenyl)-5-Oxy-1-amyl group] piperazine; 4-benzyl-1-[3-phenyl-3-Oxy-1-propyl group] piperazine; 4-normal-butyl-1-[5-(2-p-methoxy-phenyl)-5-Oxy-1-amyl group] piperidines; 3,5-dimethyl-1-[4-(4-fluoro-2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines; 3-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] azetidine; 4-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-2-methyl-1-butene base] piperidines; 4-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-2,2-dimethyl-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-2-ethyl-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-2-propyl group-1-butyl] piperidines; With 4-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-2,2-diethyl-1-butyl] piperidines.
The special compound of getting rid of itself is 4-normal-butyl-1-[4-phenyl-4-oxo-1-butyl in the formula I scope] piperidines; 4-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperazine; 2-[3-(3-normal-butyl piperidines) rosickyite alkyl] toluene; With 4-propoxy--1-[4-(4-fluorophenyl)-4-oxo-1-butyl] and piperidines (that is, wherein-(CH
2)
p-Y-is-(CH
2)
3-C (O)-or-(CH
2)
3-S-; X
1-X
5Be C; So-A-(R
2)
nAnd R
1Be respectively o-methyl-phenyl-and normal-butyl not together; Phenyl and normal-butyl; Or to fluorophenyl and-O-(CH
2)
2CH
3).
The present invention also provides the method for exciting muscarinic receptor, and this method comprises makes this receptor contact with the formula I compound of significant quantity, comprise all compounds in the formula I scope (promptly comprise 4-normal-butyl-1-[4-phenyl-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperazine; 2-[3-(3-normal-butyl piperidines) rosickyite alkyl] toluene; With 4-propoxy--1-[4-(4-fluorophenyl)-4-oxo-1-butyl] piperidines).
The present invention also provides the medicinal compositions that comprises significant quantity formula I compound, it comprise all compounds in the formula I scope (promptly comprise 4-normal-butyl-1-[4-phenyl-4-oxo-1-butyl] piperidines; 4-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperazine; 2-[3-(3-normal-butyl piperidines) rosickyite alkyl] toluene; With 4-propoxy--1-[4-(4-fluorophenyl)-4-oxo-1-butyl] piperidines).
The present invention also provides treatment and levels of acetylcholine to lower the method for diseases associated or symptom, and this method comprises the composition described herein for the treatment of significant quantity.The example of disease or symptom comprises neurodegeneration (neurogenerative) disease, cognitive impairment, cognitive decline or the dementia relevant with the age.
The compounds of this invention also demonstrates the ability that reduces intraocular pressure, therefore can be used for treatment as glaucomatous disease.Glaucoma is the disease that notes abnormalities in anterior chamber's's (being the space that forms between cornea and the lens) that aqueous humor is filled cycle control mechanism.It can cause the increase of aqueous humor volume and the increase of intraocular pressure, thereby causes defect of visual field, because the compulsion of optic disk and contraction even can cause blind.
The compounds of this invention preferably demonstrates optionally agonist activity to the m1 acceptor.It is when contacting with acceptor that this agonist is defined as, and can increase the active compound of m1 muscarinic receptor.It is the character of muscarinic m1 agonist that selectivity is defined as, thereby the amount that effectively strengthens the agonist of m1 receptor active causes the very little activity that maybe can not strengthen m3 and m5 hypotype and preferred m2 and m4 hypotype.
Term used herein " alkyl " is meant the straight or branched alkane alkyl that has 1-6 carbon atom in the chain, for example, and methyl, ethyl, propyl group, sec.-propyl, normal-butyl, sec-butyl, the tertiary butyl etc.Term " assorted alkyl " is meant and contains 1 or 2 heteroatomic alkyl that is selected from O, S or N.
Term used herein " alkenyl " is meant the straight or branched-chain alkene base that has 2-6 carbon atom in the chain; Term " alkynyl group " is meant a straight or alkyne base that has 2-6 carbon atom in the chain.
Term used herein " aryl " and " cycloalkyl " preferably are meant the list that contains 5-12 carbon atom or the ring structure of dicyclo, are more preferably the monocycle that contains 5-6 carbon atom.When these rings contain one or more heteroatoms that is selected from N, S and O (heterocycle), these rings contain 5-12 atom, more preferably 5-6 atom altogether.Heterocycle includes, but are not limited to furyl, pyrryl, pyrazolyl, thienyl, imidazolyl, isoxazolyl, oxazolyl, thiazolyl, isothiazolyl, pyridyl, piperidyl, piperazinyl, pyridazinyl, pyrimidyl, pyrazinyl, morpholinyl, oxadiazole base, thiadiazolyl group, imidazolinyl, imidazolidyl etc.This ring can be by one or more above R
2Group included in the definition replaces.If should understand substituting group C
1-6Alkyl, C
1-6Alkenyl, C
1-6Alkynyl group, C
1-6Alkoxyl group, C
1-6Assorted alkyl, C
1-6Aminoalkyl group, C
1-6Haloalkyl or C
1-6Alkoxy carbonyl exists, and they can be by one or more hydroxyl, C
1-4Alkoxyl group, halogen, cyano group, amino or nitro replace.
Term used herein " halogen " or " halo " comprise chlorine, fluorine, iodine and bromine.
The ring that should understand following structure representative can be saturated and undersaturated:
The compounds of this invention can by with GB 1,142,143 and US 3,816,433 in disclosed similar approach prepare.To those skilled in the art, the approach that changes those methods comprise other reagent etc. is conspicuous.Therefore, for example, formula I compound can prepare shown in following reaction scheme.
Initial compounds with formula (X) can be by the logical method preparation of organic synthesis.The logical method of preparation formula (X) compound can be with reference to Fuller, R.W. etc., J.Med.Chem.14:322-325 (1971); Foye, W.O. etc., J.Pharm.Sci.68:591-595 (1979); Bossier, J.R. etc., Chem.Abstr.66:46195h and 67:21527a (1967); Aldous, F.A.B., J.Med.Chem.17:1100-1111 (1974); Fuller, R.W. etc., J.Pharm.Pharmacol.25:828-829 (1973); Fuller, R.W. etc., Neuropharmacology14:739-746 (1975); Conde, S. etc., J.Med.Chem.21:978-981 (1978); Lukovits, I. etc., Int.J.Ouantum.Chem.20:429-438 (1981); And Law, B. etc., J.Cromatog.407:1-18 (1987), all its disclosed contents are attached among the present invention as a reference.Radiolabeled derivative with formula (XX) can pass through, and for example, forms reduction amination or utilizes the starting raw material of 14C-mark to prepare with the tritiate reductive agent.
In addition, when initial compounds contains carbonyl, available as AlH
3, diborane: methyl-sulfide or other standard carbonyl reduction agent are with formula (X XII) compound reduction production (XXX) part:
Formula (XX XII) receptors ligand can be by preparing with aminoderivative (XX XI) nucleophilic displacement electrophilic reagent (E).The example that is used for the electrophilic reagent of this purpose comprises halogenide, as I, Cl, Br, tosylate or methanesulfonates.
When the Y in the formula (XX XII) be-C (O)-time, this compound can be by secondary alcohol with as pyridinium chlorochromate or N-chlorosuccinimide or CrO
3-H
2SO
4Perhaps nickel peroxide or metal (Al, K) or DCC-DMSO oxidation prepare.
When the Y in the formula (XX XII) be-during O-, this compound can be by under as Cu catalysis, the alkylation of alcohol and aryl halide prepares.
When the Y in the formula (XXX XII) be-during S-, this compound can be by under as Cu catalysis, the alkylation of mercaptan and aryl halide prepares.
When the Y in the formula (XX XII) be-during CHOH-, this compound can be by catalytic hydrogenation or by using NaBH
4Perhaps use LiAlH
4The corresponding ketone of reduction prepares.
The suitable pharmacy acceptable salt of The compounds of this invention comprises acid salt, they are for example by being equipped with this acid example hydrochloric acid, sulfuric acid, fumaric acid, toxilic acid, succsinic acid, acetate, phenylformic acid, oxalic acid, citric acid, tartrate, carbonic acid or phosphoric acid with the solution of The compounds of this invention and the solution mixing system of pharmaceutically acceptable acid.In addition, when The compounds of this invention had acidic moiety, its suitable pharmacy acceptable salt comprised an alkali metal salt, as sodium or sylvite; Alkaline earth salt is as calcium or magnesium salts; And the salt that forms with suitable organic ligand, as quaternary ammonium salt.The example of pharmacy acceptable salt comprises acetate, benzene sulfonate, benzoate, supercarbonate, hydrosulfate, bitartrate, borate, bromide, calcium salt, carbonate, muriate, clavulanate, Citrate trianion, dihydrochloride, fumarate, gluconate, glutaminate, hydrobromate, hydrochloride, Hydroxynaphthoate, iodide, isothionate, lactic acid salt, Lactobionate, lauroleate, maleate, mandelate, mesylate, methyl bromide, methyl nitrate, Methylsulfate, nitrate, N-methylglucosamine ammonium salt, oleate, oxalate, phosphoric acid salt/diphosphate, salicylate, stearate, vitriol, succinate, tannate, tartrate, tosylate, triethiodide and valerate.
The present invention comprises the prodrug of The compounds of this invention in its scope.In general, these prodrugs are nonactive derivatives of The compounds of this invention, and it can be easy to change into needed compound in vivo.The general method of selecting and preparing suitable prodrug derivant sees that edit H.Bundgaard as " prodrug design ", Elsevier is described in 1985.The metabolite of these compounds comprises that The compounds of this invention enters the activity form that physiological environment produces.
When The compounds of this invention had at least one chiral centre, they can exist with racemic modification or enantiomeric form.It should be noted that all these isomer and composition thereof all comprise within the scope of the present invention.In addition, may there be polymorphic in some crystallized form of The compounds of this invention, and all these polymorphics all comprise within the scope of the present invention.Moreover some The compounds of this invention can form solvate with water (being hydrate) or common organic solvents.These solvates are also included within the scope of the present invention.
When the preparation process of The compounds of this invention produces the mixture of steric isomer, can separate these isomer by common technology (as preparation chirality chromatography).It is maybe can be by stereoselectivity synthetic or by splitting the single enantiomorph of preparation that The compounds of this invention can be made into the racemic modification form.For example; can be by standard method; for example by with optically active acid; as (-)-two-toluoyl base-d-tartrate and/or (+)-two-toluoyl base-1-tartrate; it is right that formation salt forms diastereomer; the method of then fractional crystallization, and then formation free alkali splits into its component enantiomorph with compound.Also can follow chromatographic separation by forming the ester or the acid amides of diastereomer, the method for removing chiral adjuvant then splits compound.
In the preparation process of any The compounds of this invention, may and/or the sensitivity or the active group of claimed any related molecule.It can be finished by blocking group commonly used, and blocking group is seen Protective Groups in Organic Chemistry, editor J.F.W.McOmie, Plenum Press, 1973; With T.W.Greene ﹠amp; P.G.M.Wuts, ProtectiveGroups in Organic Synthesis, John Wiley ﹠amp; Sons is described in 1991.Available method known in the art is being removed this protecting group in the later step easily.
No matter when need the active special pharmacology of muscarinic receptor to change, can be with any aforementioned compositions form, and give compound of the present invention according to the determined dosage in this area.
The present invention also provides medicinal compositions, and it comprises one or more The compounds of this invention and pharmaceutically acceptable thinner or vehicle.These compositions are preferably unit dosage form, as tablet, pill, capsule (comprising the preparation that slowly-releasing or time-delay discharge), powder agent, granule, elixir, tincture, syrup and emulsion, aseptic non-enteron aisle solution or suspension, aerosol or liquid spray, drops, ampoule, automatic injector assembly or suppository; In oral, the non-enteron aisle (as intravenously, intramuscular or subcutaneous), nose, the form of hypogloeeis or rectal administration, or by sucking or be blown into administration and can making preparation by suitable mode and according to the working method of being accepted, see Remington ' s Pharmaceutical Sciences as these working method, Gennaro edits, Mack Publishing Co., Easton PA is described in 1990.In addition, said composition can be the slowly-releasing form that is suitable for weekly or was administered once in every month; For example, can adopt the insoluble salt of this active compound,, provide the storage preparation of intramuscularly as caprate.The confession that the present invention also plans to provide suitable such as the topical formulations of eye or skin or mucosa delivery.
For example, for carrying out oral administration with tablet or Capsule form, can be with this active medicine component and oral, avirulent pharmaceutically acceptable inert support, as mixing such as ethanol, glycerine, water.In addition, when needs or in case of necessity, also can in this mixture, add suitable adhesive, lubricant, disintegrating agent, correctives and tinting material.Suitable adhesive includes, but are not limited to: starch, gelatin, natural sugar such as glucose or beta lactose, natural and synthetic is gummy as gum arabic, tragacanth gum or sodiun alginate, carboxymethyl cellulose, polyoxyethylene glycol, beeswax etc.Employed lubricant includes, but are not limited in these formulations: sodium oleate, sodium stearate, Magnesium Stearate, Sodium Benzoate, sodium acetate, sodium-chlor etc.Disintegrating agent includes, but are not limited to: starch, methylcellulose gum, agar, bentonite, xanthan gum etc.
For preparation solids composition such as tablet, with this active ingredient and suitable pharmaceutical excipient, kind as previously discussed, and other medicinal diluent, as water, mix the solid pre-formed composition that forms the uniform mixture that contains The compounds of this invention or its pharmacy acceptable salt.Term " evenly " is meant active ingredient is evenly dispersed in the described composition, so that easily said composition is divided into again the unit dosage form of equivalence, as tablet, pill and capsule.Then, this solid pre-formed composition is divided into the unit dosage form of the above type, it contains the of the present invention active ingredient of 0.1mg to about 50mg again.Can be with the tablet of the present composition or coating of pill or compound in addition and form formulation with prolongation effect characteristics.For example, this tablet or pill can comprise that an inner core that contains this active compound and one are wrapped in the skin around this inner core.This skin dressing can be an enteric layer, its not disintegration and make this inner core intactly enter duodenum or postpone to discharge in stomach.This casing or dressing can use various materials, and these materials comprise many polymeric acid and polymeric acid and common used material, as the mixture of lac, cetyl alcohol and cellulose ethanoate.
The confession liquid form oral or drug administration by injection of the adding present composition comprises syrup, water-based or the oil-based suspension of the aqueous solution, suitable flavoring and contains flavoring emulsion and the elixir and the similar pharmaceutical carrier of edible oil (as Oleum Gossypii semen, sesame oil, Oleum Cocois or peanut oil).The suitable dispersion agent of aqueous suspension or suspension agent comprise synthetic and natural natural gum, as tragacanth gum, gum arabic, alginate, dextran, Xylo-Mucine, gelatin, methylcellulose gum or polyvinylpyrrolidone.Spendable other dispersion agent comprises glycerine etc.For parenterai administration, require sterile suspension and solution.When requiring intravenous administration, use the grade that generally contains suitable sanitas to ooze preparation.Also said composition can be made ophthalmic solution or suspension form, as eye drop for dosing eyes.
Therefore, the invention still further relates to the The compounds of this invention of going up significant quantity by the object treatment that needs this kind treatment, alleviation or treatment wherein improve muscarinic receptor activity (particularly m1 receptor active) and have the disease of beneficial effect or the method for symptom.For example, these diseases or symptom may cause by stimulating inadequately or activating muscarinic receptor.Expectation is by using concrete muscarinic receptor hypotype, particularly m1 compound selectively, can avoid the deleterious side-effect problem found in known muscarinic medicine substantially, overruns or bradyrhythmia or gastrointestinal reaction as aroused in interest.
Term used herein " object " is meant animal, preferred mammal, optimum is chosen, be the object treating, observe or test.
Term used herein " significant quantity in the treatment " is meant the tissue that can cause the person of being studied, animal doctor, doctor or other clinicist and observe, system, animal or human's biology or the active compound of medical response or the amount of medicine, and it comprises the amount of the symptom of the disease that alleviation is treated.
Generalformula preferably demonstrates subtype-selective to muscarinic m1 receptor subtype.Equally, with the acceptor of the human body G-protein coupling of other test, comprise that serotonin, histamine, Dopamine HCL or adrenergic receptor compare, this compound demonstrates selectivity to muscarinic ml receptor subtype.This optionally an important deduction be exactly disease and the disorder that many central nervous systems could be treated or alleviate to these compounds effectively, but the bad side effect of in the past in non-selective compound, not finding.
The compounds of this invention show to muscarinic m1 receptor subtype optionally ability can to make them be used for the treatment of many very effectively be the disease and the disorder of feature with the cognitive impairment, for example, absent minded or neurodegenerative disease such as AlzheimerShi disease, other cognitive decline disease relevant with the age, as senile dementia, perhaps relevant symptom with dementia, as the motion vigor go down, emotional change, anergy, apathy, destabilization and Aggression.It is generally acknowledged that muscarinic m1 acceptor is also relevant with control of intraocular pressure, so muscarinic m1 agonist can be used for treating or alleviation illness in eye, as glaucoma.
The compounds of this invention is favourable with the odd-numbered day dosed administration, perhaps can be with total per daily dose with two, three or four times administered in divided doses every day.In addition, can use that formulation gives The compounds of this invention in the nose that installs in the suitable nose by the part, the perhaps transdermal of being familiar with the those skilled in the art form of pasting is by giving The compounds of this invention through the skin approach.Will be with the form administration of transdermal delivery system, the administration of this formulation must be successive rather than interruption in this dosage so.
Dosage with The compounds of this invention can be selected according to many factors, comprises patient's type, ethnic group, age, body weight, sex and medical conditions; The severity of the symptom of being treated; Route of administration; Patient's kidney and liver function; And used particular compound.Doctor in charge that general technology is skilled or animal doctor can determine at an easy rate and leave the medicine that is used to the required significant quantity of the disease that suppresses, resist or interrupt being treated or disorderly development.
The per daily dose of each adult's this medicine every day can change in the scope of 0.01-100mg.For oral administration, preferably with contain 0.01,0.05,0.1,0.5,1.0,2.5,5.0,10.0,15.0,25.0 or the tablet form of this active ingredient of 50.0mg described composition is provided so that regulate the dosage that gives the patient that treats according to symptom.Unitary dose generally contains the active ingredient of 0.001-50mg approximately, preferably contains the active ingredient of the 1-10mg that has an appointment.This effective amount of drug is generally the dosage level of the about 0.0001-25mg of every kg body weight every day.This scope is preferably the about 0.001-10mg of every kg body weight every day, is preferably the about 0.001-1mg of every kg body weight every day especially.The dosage regimen of this compound is every day 1-4 time.
Can use The compounds of this invention separately so that acquisition to the suitableeest pharmacological action of muscarinic receptor (particularly muscarinic m1 receptor subtype), makes any potential toxicity or deleterious effects ease down to minimum simultaneously with the determined suitable dosage of routine test.In addition, in some cases, need take simultaneously or take in turn with other medicine that can improve this compound effects.
The compounds of this invention can adopt the recombinant receptor hypotype to the pharmacological properties of specific muscarinic receptor hypotype by a series of different test methods with selectivity, and preferred people's acceptor (if any) confirms, as second messenger commonly used or in conjunction with test.Function test system is at US 5 especially easily, 707, disclosed this receptor selectivity and amplification test in 798, this patent description in the presence of this receptor part, the method that the ability that utilization is increased by the receptor dna cells transfected is screened bioactive compounds, as the muscarinic hypotype of encoding different.The increase level of marker that can also be by this cell expressing detects the amplification of cell.
With following embodiment more openly the present invention, this embodiment does not limit the scope of the present invention that proposes claim.
EXAMPLE Example I 4-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines (5)
The inferior normal-butyl piperidines of 1-benzyl-4-(2) in 500mL is equipped with the three-necked bottle of agitator, add sodium hydride (1.61g, 67mmol) and DMSO (40mL).Under 90 ℃, this suspension that obtains was heated 30 minutes, until stopping releasing hydrogen gas.This suspension was cooled off in ice bath 20 minutes, add bromination Ding base San Ben Phosphonium (26.6g, DMSO 67mmol) (70mL) pulpous state liquid then.Under the room temperature, should stir 15 minutes by the redness mixed solution.(14.0g 74mmol), under the room temperature spends the night this mixed solution stirring slowly to add 1-benzyl-4-piperidone 1 with 30 minutes.In this reaction mixture, add entry (200mL), then with heptane (4 * 100mL) and ethyl acetate (2 * 100mL) extractions.With the organic layer drying that merges, be evaporated to driedly, obtain the 38.1g yellow oil.Distill this oily matter and obtain 2 of 14.9g (88%), bp 101-105 ℃ (0.1mmHg).
1H?NMR(CDCl
3)0.90-0.95(t,3H),1.25-1.41(m,2H),1.90-2.20(m,2H),2.18-2.30(m,4H),2.40-2.45(m,4H),2.50(s,2H),5.17(t,1H),7.20-7.42(m,5H)。
4-normal-butyl piperidines (3) adds 2 in 500mL is equipped with the flask of agitator (13.2g 58mmol) and ethanol (70mL) the pulpous state liquid of 10% palladium on carbon (1.2g), then adds concentrated hydrochloric acid (1.5mL).This reaction flask is found time, in this reaction flask, add hydrogen.Consume 2.5dm altogether
3Hydrogen.This reaction mixture is filtered, evaporation, with residue water-soluble (40mL) and NaOH (20mL, 2M) in, then with ethyl acetate (3 * 100mL) extractions.With salt solution (30mL) washing, be evaporated to driedly the organic phase that merges, obtain 7.1g crude product 3.This crude product is through CC[elutriant: heptane: EtOAc (4: 1)] obtain pure product 3 (2.7g, 33%).
1H?NMR(CDCl
3)0.85(t,3H),1.0-1.38(m,9H),1.65(dd,2H),2.38(s,1H),2.55(dt,2H),3.04(dt,2H)。
4-(4-normal-butyl piperidines-1-yl) butyronitrile (4) in 100mL is equipped with the flask of magnetic stirring apparatus, put into 3 (2.3g, 16.4mmol), 4-bromine butyronitrile (2.4g, 16.4mmol), potassium carbonate powder (2.5g, acetonitrile 18mmol) (20mL) liquid.Under the room temperature this reaction mixture was stirred 5 hours, then add entry (15mL).(3 * 30mL) extract, and the organic phase that merges is evaporated to the dried 3.9g of obtaining crude product 4 with ethyl acetate with this mixed solution.This crude product is through CC[elutriant: heptane: EtOAc (1: 1)] obtain pure product 4 (2.3g, 87%).
1H?NMR(CDCl
3)0.82(t,3H),1.19-1.37(m,9H),1.64-1.75(d,2H),1.84-2.01(m,4H),2.39-2.54(m,4H),2.89-2.97(d,2H)。
4-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines (5) in the flask of 25mL oven dry, put into heat gun activatory Mg bits (125mg, 5.2mmol).Under rare gas element, add 2-iodanisol (1.13g, Et 5.2mmol)
2O (4mL) suspension is placed this reaction mixture 1 hour under the room temperature.Add compound 4 (720mg, Et 3.4mmol)
3O (4mL) liquid spends the night this mixed-liquor return.Add THF (15mL) and sulfuric acid (4mL, 2M), with this reaction mixture stirring 4 hours, then add NaOH (6mL, 2M).(3 * 50mL) extract, and the organic phase that merges is evaporated to the dried 1.2g of obtaining crude product 5 with ethyl acetate with this reaction mixture.This crude product is through CC[elutriant: CH
2Cl
2: CH
3OH (99: 1)] obtain pure product 5 (0.42g, 26%).
1H?NMR(CDCl
3)0.83(t,3H),1.20-1.42(m,9H),1.65-1.73(d,2H),1.96-2.20(m,4H),2.53(t,2H),3.02-3.17(m,4H),3.89(s,3H),6.95-7.01(m,2H),7.44(t,1H),7.65(d,1H)。Embodiment II 3-methylol-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines (7)
4-(3-methylol-piperidines-1-yl)-butyronitrile (6) in the flask of 25mL oven dry, put into piperidines-3-base-methyl alcohol (1.12g, acetonitrile 10mmol) (10mL) liquid, then add salt of wormwood (1.38g, 10mmol) and 4-bromine butyronitrile (0.90mL, 9mmol).Under the room temperature, this reaction mixture was stirred 12 hours.This mixed solution is filtered, be evaporated to dried.Add entry (20mL), then (3 * 20mL) extract, with the organic phase drying (MgSO that merges with ethyl acetate
4), being evaporated to the dried 1.50g of obtaining crude product 6, this crude product can be directly used in synthetic compound 7 without being further purified.
(780mg 32mmol), then adds anhydrous THF (7mL) to 3-methylol-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines (7) with heat gun activatory Mg bits under the adding vacuum in the flask of 50mL oven dry.Under rare gas element, (5.3g, THF 24mmol) (10mL) suspension reflux this reaction mixture 4 hours to add the 2-toluene iodide.((23mg, 0.16mmol 2mol%), under the room temperature spend the night this reaction mixture stirring to add CuBr then for 1.50g, THF 8mmol) (5mL) suspension to add compound 6 by syringe.Add H
2SO
4(20mL, 2M) quencher should reaction, stirs under room temperature 2 hours, then add NaOH (8mL, 2M).Add THF (15mL), use CH then
2Cl
2(3 * 20mL) extract, with organic phase drying (MgSO
4), be evaporated to the dried 0.41g of obtaining crude product 7.This crude product is through preparing HPLC CC[elutriant: buffer A: 0.1%TFA; Buffer B: 80%CH
3CN+0.1%TFA] obtain analytically pure sample compound 7.LC-MS[M+H]
+275 (calculated values 275.2).Embodiment III 2-propyl group-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines (9)
Under 4-(2-propyl group-piperidines-1-yl)-butyronitrile (8) room temperature, with 2-propyl group piperidines (550mg, 4.3mmol), 4-bromine butyronitrile (430mg, 3.0mmol) and salt of wormwood (550mg, 4.0mmol) acetonitrile (5mL) mixed solution stirred 12 hours, then add saturated brine (25mL).(3 * 25mL) extract, with the organic phase drying (MgSO that merges with ethyl acetate with this reaction mixture
4), be evaporated to the dried crude product 8 that obtains.This crude product is through CC[elutriant: CH
2Cl
2: MeOH (99: 1)] obtain pure product 8 (0.48g, 83%).LC-MS[M+
H]
+194 (calculated values 194.2).
2-propyl group-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines (9) in the flask of 10mL oven dry, be added under the vacuum with heat gun activatory Mg bits (97mg, 4.1mmol).Under rare gas element, add 2-toluene iodide (380mL, Et 2.8mmol)
2The suspension of O (3mL) refluxes this reaction mixture 1 hour.Add compound 8 (0.43g, CH 2.2mmol) by syringe
2Cl
2Under (3mL) suspension, room temperature this reaction mixture stirring is spent the night.Add H
2SO
4(20mL, 2M) quencher should reaction, stirs under the room temperature 12 hours, then add NaOH (10mL, 2M).Add THF (15mL), (3 * 50mL) extract, with the organic phase that merges salt solution (10mL) and NaOH (10mL, 2M) washing, dry (MgSO to use ethyl acetate then
4), be evaporated to the dried 0.43g of obtaining crude product 9.This crude product is through preparing the HPLC[elutriant: buffer A: 0.1%TFA; Buffer B: 80%CH
3CN+0.1%TFA] obtain analytically pure sample compound 9.LC-MS[M+
H]
+287 (calculated values 287.2).Embodiment IV 1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperidines (11)
In the flask of 10mL oven dry, add under the vacuum with heat gun activatory Mg bits (97mg, 4.1mmol).Under rare gas element, add 2-toluene iodide (380mL, Et 3.0mmol)
2O (3mL) suspension was with this mixed-liquor return 1 hour.Add 4-piperidines-1-base-butyronitrile 10 (Dahlbom etc., Acta.Chem.Scand.1951,5,690-697) (0.305g, CH 2.0mmol) by syringe
2Cl
2(3mL) suspension spends the night this reaction mixture stirring under room temperature then.Add H
2SO
4(10mL, 2M) quencher should the reaction, restir is 12 hours under room temperature, then add NaOH (12mL, 2M).Add THF (15mL), (3 * 50mL) extract, with the organic phase that merges salt solution (10mL) and NaOH (10mL, 2M) washing, dry (MgSO to use ethyl acetate then
4), be evaporated to the dried 0.21g of obtaining crude product 11.This crude product is through preparing the HPLC[elutriant: buffer A: 0.1%TFA; Buffer B: 80%CH
3CN+0.1%TFA] obtain analytically pure sample compound 11.LC-MS[M+H]
+245 (calculated values 245.2).Embodiment V 4-methyl isophthalic acid-[4-(4-bromophenyl)-4-oxo-1-butyl] piperidines (12)
In 10mL exsiccant flask, add the 4-methyl piperidine (719mL, 6mmol), dioxane (5mL), then add salt of wormwood (0.30g, 2.18mmol), potassiumiodide (10mg) and 4-bromo-4-chloropropyl phenyl ketone (785mg, 2.76mmol).With this reaction mixture place 110 ℃ following 12 hours, then use H
2O (10mL) dilution.With this reaction mixture Et
2(3 * 15mL) extract O, with the organic phase drying (MgSO that merges
4), be evaporated to the dried 0.50g of obtaining crude product 12.This crude product is through preparing the HPLC[elutriant: buffer A: 0.1%TFA; Buffer B: 80%CH
3CN+0.1%TFA] obtain analytically pure sample compound 12.LC-MS[M+H]
+322 (calculated values 323.1).Embodiment VI 1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] tetramethyleneimine (13)
In the flask of 10mL oven dry, add under the vacuum with heat gun activatory Mg bits (30mg, 1.2mmol).Under rare gas element, add 2-toluene iodide (0.22g, Et 1.0mmol)
2O (2mL) solution was with this mixed-liquor return 1 hour.Add 4-tetramethyleneimine-1-base-butyronitrile (Burckhalter etc., J.Org.Chem.1961,26,4070-4076) (0.14g, CH 1.0mmol) by syringe
2Cl
2(2mL) suspension spends the night this reaction mixture stirring under room temperature then.Add H
2SO
4(restir is 2 hours under room temperature for 10mL, 2M) this reaction mixture of quencher, then add NaOH (10mL, 2M).Add THF (15mL), (3 * 20mL) extract, with organic phase drying (MgSO to use ethyl acetate then
4), be evaporated to the dried 0.12g of obtaining crude product 13.This crude product is through preparing the HPLC[elutriant: buffer A: 0.1%TFA; Buffer B: 80%CH
3CN+0.1%TFA] obtain analytically pure sample compound 13.LC-MS[M+H]
+231 (calculated values 231.3).Embodiment VII 4-methyl isophthalic acid-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperazine (15)
4-(4-methyl-piperazine-1-yl)-butyronitrile (14) in the 25mL flask, add the 1-methylpiperazine (0.52g, 5.1mmol), 4-bromine butyronitrile (0.78g, 5.3mmol) and salt of wormwood (0.71g, acetonitrile 5.3mmol) (5mL) suspension.Under the room temperature this reaction mixture was stirred 4 hours, then add entry (20mL), (3 * 25mL) extract with ethyl acetate.The organic phase that merges is washed dry (MgSO with salt solution (25mL)
4), being evaporated to the dried 0.72g of obtaining crude product 14, this crude product is directly used in synthetic compound 15 without being further purified.
4-methyl isophthalic acid-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperazine (15) in the flask of 10mL oven dry, add under the vacuum with heat gun activatory Mg bits (116mg, 4.0mmol).Under rare gas element, add 2-toluene iodide (0.65g, Et 3.0mmol)
2O (3mL) mixed solution was with this mixed-liquor return 1 hour.Add compound 14 (0.33g, CH 2.0mmol) by syringe
2Cl
2(3mL) solution under the room temperature spends the night this reaction mixture stirring then.Add H
2SO
4(6mL, 2M) quencher should reaction, stirs under the room temperature 2 hours, then add NaOH (8mL, 2M).Add THF (15mL), use CH then
2Cl
2(3 * 20mL) extract.With organic phase drying (MgSO
4), be evaporated to the dried 0.26g of obtaining crude product 15.This crude product is through preparing the HPLC[elutriant: buffer A: 0.1%TFA; Buffer B: 80%CH
3CN+0.1%TFA] obtain analytically pure sample compound 15.LC-MS[M+H]
+260 (calculated values 260.4).Embodiment VIII 4-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperazine (17)
4-(4-butyl-piperazine-1-yl)-butyronitrile (16) in the 25mL flask, add 1-butyl piperazine (712mg, 5.0mmol), 4-bromine butyronitrile (779mg, 5.3mmol) and salt of wormwood (687mg, acetonitrile 5.0mmol) (5mL) suspension.Under the room temperature this reaction mixture was stirred 12 hours, then add entry (20mL), (3 * 25mL) extract with ethyl acetate.The organic phase that merges is washed dry (MgSO with salt solution (25mL)
4), being evaporated to the dried 0.89g of obtaining crude product 16, this crude product is directly used in synthetic compound 17 without being further purified.
4-normal-butyl-1-[4-(2-aminomethyl phenyl)-4-oxo-1-butyl] piperazine (17) in the flask of 10mL oven dry, add under the vacuum with heat gun activatory Mg bits (100mg, 4.0mmol).Under rare gas element, add 2-toluene iodide (0.66g, Et 3.0mmol)
2O (3mL) suspension was with this mixed-liquor return 1 hour.Add compound 16 (0.43g, CH 2.0mmol) by syringe
2Cl
2(3mL) suspension under the room temperature spends the night this reaction mixture stirring then.Add H
2SO
4(6mL, 2M) quencher should reaction, stirs under room temperature 2 hours again, then add NaOH (8mL, 2M).Add THF (15mL), use CH then
2Cl
2(3 * 20mL) extract, with organic phase drying (MgSO
4), be evaporated to the dried 0.50g of obtaining crude product 17.This crude product is through preparing the HPLC[elutriant: buffer A: 0.1%TFA; Buffer B: 80%CH
3CN+0.1%TFA] obtain analytically pure sample compound 17.LC-MS[M+H]
+302 (calculated values 302.5).Embodiment IX 4-normal-butyl-1-[4-(2-ethoxyl phenenyl)-4-oxo-1-butyl] piperidines (18)
In the flask of 10mL oven dry, add under the vacuum with heat gun activatory Mg bits (94mg, 3.8mmol).Under rare gas element, add 1-oxyethyl group-2-iodobenzene (0.71g, Et 2.9mmol)
2O (3mL) suspension was with this mixed-liquor return 3 hours.Add compound 4 (0.40g, CH 1.9mmol)
2Cl
2(3mL) solution, then under 40 ℃ with this reaction mixture restir 3 hours.Add H
2SO
4(10mL, 2M) quencher should be reacted, and stirred under the room temperature and spent the night, and (20mL is 2M) until alkaline condition then to add NaOH.(3 * 50mL) extract, with the organic phase that merges salt solution (10mL) and NaOH (10mL, 2M) washing, dry (MgSO with ethyl acetate with this reaction mixture
4) organic phase that merges, be evaporated to the dried 0.60g of obtaining crude product 18.This crude product is through the CC[elutriant: toluene: ethyl acetate (1: 1)] obtain pure product 18 (0.32g, 34%); LC-MS[M+H]
+331 (calculated values 331.5).Embodiment X 4-normal-butyl-1-[4-(2, the 3-3,5-dimethylphenyl)-4-oxo-1-butyl] piperidines (19)
In the flask of 10mL oven dry, add under the vacuum with heat gun activatory Mg bits (94mg, 3.8mmol).Under rare gas element, under refluxing automatically, add 1-iodo-2,3-dimethyl benzene (0.69g, Et 3.0mmol)
2O (5mL) suspension was with this mixed-liquor return 4 hours.In this reaction mixture, add compound 4 (0.41g, CH 2.0mmol)
2Cl
2(2mL) suspension spends the night under the room temperature then.Add H
2SO
4(7mL, 2M) quencher should be reacted, and stirred 3 hours under the room temperature, and (20mL is 2M) until alkaline condition then to add NaOH.(3 * 50mL) extract, with the organic phase that merges salt solution (10mL) and NaOH (10mL, 2M) washing, dry (MgSO with ethyl acetate with this reaction mixture
4) organic phase, be evaporated to the dried 0.69g of obtaining crude product 19.This crude product is through CC[elutriant: CH
2Cl
2: MeOH (99: 1)] obtain pure product 19 (0.40g, 64%); LC-MS[M+H]
+315 (calculated values 315.5).Embodiment XI 4-normal-butyl-1-[4-(2, the 4-3,5-dimethylphenyl)-4-oxo-1-butyl] piperidines (20)
In the flask of 10mL oven dry, add under the vacuum with heat gun activatory Mg bits (95mg, 3.9mmol).Under rare gas element, under refluxing automatically, add 1-iodo-2,4-methylbenzene (0.69g, Et 2.9mmol)
2O (4.5mL) suspension was with this mixed-liquor return 3 hours.Under rare gas element, in this reaction mixture, add compound 4 (0.41g, CH 2.0mmol)
2Cl
2(2mL) solution stirs under the room temperature then and spends the night.Add H
2SO
4(8mL, 2M) quencher should be reacted, and stirred 4 hours under the room temperature, and (20mL 2M) makes this reaction mixture be alkalescence then to add NaOH.Add THF (20mL), (3 * 50mL) extract, with the organic phase that merges salt solution (10mL) and NaOH (10mL, 2M) washing, dry (MgSO with ethyl acetate
4) organic phase, be evaporated to the dried 0.61g of obtaining crude product 20.This crude product is through CC[elutriant: CH
2Cl
2: MeOH (99: 1)] obtain pure product 20 (0.21g, 35%); LC-MS[M+H]
+315 (calculated values 315.5).Embodiment XII 4-normal-butyl-1-[4-(2-p-methoxy-phenyl)-4-oxo-1-butyl] piperidines (21)
In the flask of 10mL oven dry, add under the vacuum with heat gun activatory Mg bits (0.12g, 4.9mmol).Under rare gas element, add 1-bromo-2-ethylbenzene (0.66g, Et 3.6mmol)
2O (2mL) suspension refluxes this reaction mixture 2 hours.Add compound 4 (0.50g, CH 2.4mmol) by syringe
2Cl
2(2mL) suspension stirs this mixture overnight then under the room temperature.Add H
2SO
4(14mL, 2M) quencher should reaction, stirs under room temperature 2 hours, then add NaOH (20mL, 2M).Adding THF (20mL), then (3 * 50mL) extract, with the organic phase that merges salt solution (10mL) and NaOH (10mL, 2M) washing, dry (MgSO with ethyl acetate
4) organic phase, be evaporated to the dried 0.75g of obtaining crude product 21.This crude product is through CC[elutriant: CH
2Cl
2: MeOH (99: 1)] obtain pure product 21 (0.68g, 90%); LC-MS[M+H]
+315 (calculated values 315.5).Embodiment X III 4-normal-butyl-1-[4-(2, the 4-3,5-dimethylphenyl)-4-oxo-1-butyl] piperidines (22)
In the flask of 10mL oven dry, add under the vacuum with heat gun activatory Mg bits (88mg, 3.6mmol).Under rare gas element, add 1-iodo-2-methoxymethyl benzene (0.67g, Et 2.7mmol)
2O (4mL) suspension refluxes this reaction mixture 1 hour.Add compound 8 (0.38g, CH 1.8mmol) by syringe
2Cl
2(4mL) suspension under the room temperature spends the night this reaction mixture stirring then.Add H
2SO
4(10mL, 2M) quencher should reaction, stirs under the room temperature 2 hours, then add NaOH (10mL, 2M).Add THF (15mL), (3 * 50mL) extract, with the organic phase that merges salt solution (10mL) and NaOH (10mL, 2M) washing, dry (MgSO with ethyl acetate
4) organic phase, be evaporated to the dried 0.51g of obtaining crude product 22.This crude product is through CC[elutriant: CH
2Cl
2: MeOH (99: 1)] obtain pure product 22 (0.14g, 23%); LC-MS[M+H]
+331 (calculated values 331.5).Embodiment X IV 4-normal-butyl-1-[4-(2-pyridyl)-4-oxo-1-butyl] piperidines (24)
4-(4-butyl-piperidines-1-yl) methyl-butyrate (23) in the 25mL reaction flask, add 4-bromo-butyric acid methyl esters (2.04g, 11.2mmol), compound 3 (1.51g, 10.8mmol) and salt of wormwood (1.63g, CH 11.8mmol)
3CN (10mL) suspension.Under the room temperature this reaction mixture stirring is spent the night, then filter, be evaporated to dried.Add H
2O (50mL), then (3 * 100mL) extract with ethyl acetate.With the organic phase drying (MgSO that merges
4), be evaporated to the dried 2.84g of obtaining crude product 23.This crude product is through CC[elutriant: CH
2Cl
2: MeOH (99: 1)] obtain pure product 23 (1.93g, 75%); LC-MS[M+H]
+241 (calculated values 241.2).
4-normal-butyl-1-[4-(2-pyridyl)-4-oxo-1-butyl-] piperidines (24) adds in exsiccant 25mL reaction flask and is dissolved in CH
2Cl
22-bromopyridine (3mL) (200mg, 1.3mmol), with extremely-78 ℃ of this temperature regulation.Stir after 20 minutes, under rare gas element, add n-Butyl Lithium (0.84mL, 1.4mmol).Add after 30 minutes, adding is dissolved in CH
2Cl
2The solution of 23 (2mL).This reaction mixture temperature to ambient temperature overnight, is used H then
2SO
4(5mL, 1M) quencher.(6 * 25mL) extract, with the organic phase drying (MgSO that merges with ethyl acetate with this reaction mixture
4), be evaporated to the dried 0.31g of obtaining crude product 24.This crude product is through CC[elutriant: CH
2Cl
2: MeOH (10: 1)] obtain pure product 24 (75mg, 12%); LC-MS[M+H]
+288 (calculated values 288.2).Embodiment X V 4-normal-butyl-1-[4-(2-hydroxy phenyl)-4-oxo-1-butyl] piperidines (27)
1-benzyloxy-2-iodobenzene (25) in the flask of 25mL oven dry, with the 2-iodophenol (1.03g, 4.7mmol) and salt of wormwood (0.71g 5.2mmol) is dissolved in the exsiccant acetone (10mL).This mixed solution was stirred 15 minutes, and (0.61mL, 5.2mmol), room temperature is placed and is spent the night then to add bromotoluene.Add H
2O (50mL), then (3 * 50mL) extract, with the organic phase drying (MgSO that merges with ethyl acetate
4), be evaporated to the dried 1.7g of obtaining crude product 25.This crude product is through CC[elutriant: heptane: EtOAc (9: 1)] obtain pure product 25 (1.2g, 81%).LC-MS[M+H]
+310 (calculated values 310.0).
4-normal-butyl-1-[4-(2-benzyloxy phenyl)-4-oxo-1-butyl] piperidines (26) in the flask of 25mL oven dry, add under the vacuum with heat gun activatory Mg bits (123mg, 5.1mmol).Under rare gas element, add 1-benzyloxy-2-iodobenzene (25) (1.18g, Et 3.8mmol)
2O (10mL) solution refluxes this reaction mixture 3.5 hours.Add 4-(4-normal-butyl piperidines-1-yl)-butyronitrile 4 (0.53g, CH 2.5mmol)
2Cl
2(3mL) solution stirs this reaction mixture down at 40 ℃ then and spends the night.Add H
2SO
4(10mL, 2M) quencher should be reacted, restir 1 hour, (20mL is 2M) to alkaline condition then to add NaOH.(3 * 50mL) extract, with the organic phase that merges salt solution (10mL) and NaOH (10mL, 2M) washing, dry (MgSO with ethyl acetate with this reaction mixture
4) organic phase that merges, be evaporated to the dried 1.28g of obtaining crude product 26.This crude product is through the CC[elutriant: toluene: ethyl acetate (1: 1)] obtain pure product 26 (0.51g, 51%); LC-MS[M+H]
+393 (calculated values 393.7).
4-normal-butyl-1-[4-(2-hydroxy phenyl)-4-oxo-1-butyl] piperidines (27) adds 4-normal-butyl-1-[4-(2-benzyloxy the phenyl)-4-oxo-1-butyl that is dissolved in dry EtOH (10mL) and the concentrated hydrochloric acid (0.1mL) in the 25mL reaction flask] piperidines (26) (49mg, 1.2mmol) solution, then add palladium on carbon (40mg).In this reaction flask, add H with ventilating technology then
2, at H
2Under the environment, in stirred overnight at room temperature.(2mL, 2.0M) this reaction mixture that alkalizes passes through diatomite filtration to add NaOH.(3 * 50mL) extract, with the organic phase that merges salt solution (10mL) and NaOH (10mL, 2M) washing, dry (MgSO with ethyl acetate with water
4), be evaporated to the dried 0.42g of obtaining crude product 27.This crude product is through CC[elutriant: CH
2Cl
2: MeOH (99: 1)] obtain pure product 27 (0.21g, 58%); LC-MS[M+H]
+303 (calculated values 303.2).Embodiment X VI is the shaker test compound in the test of muscarinic receptor hypotype m1, m2, m3, m4 and m5
With muscarinic receptor DNAs transfectional cell (logical method) under 37 ℃, at humid gas (5%CO
2) in the incubator, replenishing 4.5g/l glucose, 4mM glutamine, 50 units/ml penicillin, 50 units/ml Streptomycin sulphate (Advanced Biotechnologies, Inc., Gaithersburg, MD provides) and 10% calf serum (Sigma, St.Louis, MI provides) DulbeccoShi improvement EagleShi substratum (DMEM) in NIH 3T3 cell (providing with ATCC CRL 1658 by American type culture collection) growth is provided.This cell is handled with trypsinase-EDTA, centrifugal, with every 15cm ware 2 * 10
6Be inoculated among the DMEM that contains 10% calf serum of 20ml.
Substantially according to Bonner etc., Science 237,1987,527 pages and Bonner etc., and Neuron 1,1988, and 403 pages of described methods are cloned m1-m5 muscarinic receptor hypotype.For m2 and m4 acceptor, this cell is used in the chimeric DNA cotransfection of coding between five carboxyl terminal amino acid of Gq protein and Gi protein (this Gq-i5 construction is seen Conklin etc., and Nature 363,1993, and 274 pages described).
According to the specification sheets of manufacturers, every day is with Superfect transfection reagent (by Qiagen, Valencia, CA provides) this cell of transfection.Add receptor dna, beta-galactosidase enzymes DNA in each flat board (by Promega, Madison, WI provides the pSI-beta-galactosidase enzymes), be used for the mosaic Gq-i5 DNA of m2 and m4 receptor subtype test and as the salmon sperm DNA of weighting material (by Sigma, St.Louis, MI provides) to total amount be 20 μ g DNA.Before in this flat board, adding, in this DNA, add the Superfect of 60 μ l, come thorough mixing by pick up and emit several with suction pipe.Under the room temperature, this mixed solution was hatched 10-15 minute.Extract substratum out, in this flat board, add the fresh DMEM that 12ml contains 10% calf serum and 50 units/ml penicillin/streptomycin.With suction pipe this DNA-Superfect solution is mixed once, add in the flat board, rotation makes this DNA mixed solution uniform distribution in its surface.At 37 ℃ and 5%CO
2Down, with this cell overnight incubation.
After hatching, extract substratum out, with the HankShi buffer saline rinsing of 15ml volume once with this flat board.Rotating this flat board makes rinsing complete.In flat board, add 20ml and replenished the fresh DMEM of 10% calf serum and 50 units/ml penicillin/streptomycin.This cell hatched be paved with until this flat board 100% in 24-48 hour.
Under aseptic condition, heating contains the DMEM of 2%Cyto-SF3 in 37 ℃ of water-baths with the NIH 3T3 cell tests of muscarinic receptor hypotype transfection (logical method).The aseptic active redundancy solution of test compound to be tested is by preparing diluted chemical compound to the octuple of testing ultimate density in DMEM.Included compound (Samoryl) also is diluted to the octuple of ultimate density in will the test as positive control in DMEM.Under aseptic condition, in every hole of 96-hole microtiter plate, add the DMEM that 50 μ l contain 2%Cyto-SF3.Then, in the topped hole of this titer plate, add the compound solution of 16 μ l,, then it is drawn the dilution of carrying out this solution to next round by from topped hole, taking out this compound solution of 16 μ l.Following every round repeats this step, but only adds the substratum of 50 μ l in baseline control hole (contain substratum and cell, but do not contain the hole of test compound) and titer plate control wells (contain substratum, but do not contain test compound and cell).Then this flat board is placed 37 ℃ of incubator equilibrium temperatures and pH.
When this cell culture medium reaches 100% when being paved with, extract this substratum out, with every dull and stereotyped HankShi buffer saline (HBS) rinsing with 15ml.This cell placed became little Huang until HBS in the about 10-15 of incubator minute.Extract HBS then out, in every flat board, add 1ml trypsinase, rotate to and cover this flat board fully.Rap loosening for several times this cell of this plate edge.After treating that cell has moved from the surface, the DMEM that adding 8ml contains 10% calf serum and 50 units/ml penicillin and 50 units/ml Streptomycin sulphate suppresses this trypsinase.Flat board with this substratum rinsing, is drawn this cell to pipe again.With IEC Centra CL2 whizzer (Sorvall manufacturing), under 1000rpm, with this cell centrifugation 5-10 minute.Then, carefully extract this substratum out and this cell is moved.This cell precipitation is suspended in 1600 μ l contains among the DMEM of 10% calf serum and 50 units/ml penicillin and 50 units/ml Streptomycin sulphate, add the DMEM that 20ml has replenished 2%Cyto-SF3 again.This cell suspension that in each hole of the 96-hole of above preparation microtiter plate (except the dull and stereotyped control wells), adds 50 μ l equal portions.Then at 37 ℃ and 5%CO
2Under hatched this plate 4 days.
After hatching, be inverted this microtiter plate and remove substratum, light shaking blots it on thieving paper then.In every hole, add 100 μ l chromogenic substrates (phosphate buffered saline buffer of 3.5mM ortho-nitrophenyl base-β-D-galactopyranoside, 0.5%Nonidet NP-40), under 30 ℃, hatch this flat board until obtain the suitableeest optical density at the 405nm place.From all absorption values, deduct the absorption value of baseline control hole and dull and stereotyped control wells.
The result is with above-described logical method, with the DNAs cotransfection of MH 3T3 cell with coding m1, m3 and m5 receptor subtype.According to above-described method, the compound library (1 in every hole) that contains about 35,000 little organic compound is carried out the acceptor screening.Fig. 1 illustrates the data of a 96-orifice plate in this screening.In this plate, there are two compounds that the acceptor of one or more transfection is had activity.In all screenings, confirm four and demonstrate active related compound.For determining which acceptor is activated in screening, transfection to each acceptor in the separate cell substratum is carried out compound test by the above method.Compd A only has activity to the m1 receptor subtype, and it is effective partial agonist on this receptor, and its inductive peak response value is lower than reference compound Samoryl.
In further testing, find four compounds to the selective activity of m1 acceptor, and m2, m3, m4 or m5 muscarinic receptor are not had remarkable activity.The most activated compound, compd A is not the antagonists of these five kinds of muscarinic receptor hypotypes to the inductive response of Samoryl institute.
Compd A is further carried out to several other acceptors at alpha-adrenergic receptor hypotype 1D, 1B, 1A, 2A, 2B and 2C the agonist activity test on histamine H 1 and serotonin 5-HT1A and the 5-HT2A hypotype.This compound does not show significantly active in these tests.In the antagonist test, compd A can not suppress the response of alpha-adrenergic receptor hypotype 2A, 2B or 2C or 5-hydroxytryptamine receptor hypotype 5-HT1A or 5-HT2A.As Fig. 2 explanation, the response that compd A brought out can be blocked by the muscarinic antagonists coromegine, and its usefulness is identical with the response that the muscarinic agonists Samoryl brings out.
Embodiment X VII-R-SAT test
With useful m1, m3 or m5 acceptor cells transfected be exposed in 7 kinds of compounds of 1.5 μ M concentration, carry out R-SAT test (see United States Patent (USP) 5,707,798, be attached to this as a reference).Cellular response is represented with the per-cent of cell peak response (being defined as the response to 10 μ M Samoryls).The results are shown in following table.
| Compound | Acceptor and concentration | ||||||||
| ??????????m1 ?????????1.5M | ???????????m3 ??????????1.5M | ?????????m5 ????????1.5M | |||||||
| A (embodiment I) | 107 | +/- | 9 | ?7 | +/- | 8 | ?3 | +/- | 8 |
| B (embodiment IX) | 76 | +/- | 11 | ?7 | +/- | 9 | ?-6 | +/- | 10 |
| C (embodiment X V) | 91 | +/- | 9 | ?4 | +/- | 9 | ?0 | +/- | 12 |
| D (embodiment X) | 72 | +/- | 9 | ?13 | +/- | 7 | ?2 | +/- | 15 |
| E (embodiment XI) | 42 | +/- | 13 | ?9 | +/- | 3 | ?-3 | +/- | 2 |
| F (embodiment XII) | 65 | +/- | 9 | ?9 | +/- | 7 | ?5 | +/- | 11 |
| ????G * | 66 | +/- | 19 | ?16 | +/- | 12 | ?7 | +/- | 11 |
*4-normal-butyl-1-[4-phenyl-4-oxo-1-butyl] piperidines
Show that as above this compound is the selective agonist of m1 acceptor.
Described herein and the present invention of proposing claim is not by specific embodiments limited range disclosed herein, because these embodiments are to be used to illustrate several aspect of the present invention.Any suitable embodiment all is included within the scope of the invention.Certainly, according to above explanation, except that change shown in the present and described, obviously those skilled in the art can do various modifications to the present invention.These modifications are also included within the scope of incidental claims.
The present invention quotes various reference, and these disclosed contents all are attached among the present invention as a reference.
Claims (99)
Wherein
X
1, X
2, X
3, X
4And X
5Be selected from C, N and O;
K is 0 or 1;
T is 0,1 or 2;
R
1Be the C of straight or side chain
1-8Alkyl, C
2-8Alkenyl, C
1-8Alkynyl group, C
1-8Alkylidene group, C
1-8Alkoxyl group, C
1-8Assorted alkyl, C
1-8Aminoalkyl group, C
1-8Haloalkyl, C
1-8Alkoxy carbonyl, C
1-8Hydroxy alkoxy base, C
1-8Hydroxyalkyl ,-SH, C
1-8Alkylthio ,-O-CH
2-C
5-6Aryl, by C
1-3Alkyl or halogen replace-C (O)-C
5-6Aryl; Optional contain 1 or the heteroatomic C of a plurality of N of being selected from, S and O
5-6Aryl or C
5-6Cycloalkyl;-C (O) NR
3R
4,-NR
3R
4,-NR
3C (O) NR
4R
5,-CR
3R
4,-OC (O) R
3(the CH of ,-(O)
2)
sNR
3R
4Or-(CH
2)
sNR
3R
4R wherein
3, R
4And R
5Identical or different, independently be selected from H, C separately
1-6Alkyl; Optional contain 1 or the heteroatoms of a plurality of N of being selected from, O and S and optional by halogen or C
1-6The C that alkyl replaces
5-6Aryl; C
3-6Cycloalkyl; Perhaps R
3And R
4Contain 5-6 ring structure that is selected from the atom of C, N, S and O with N atom (when existing) formation; S is the integer of 0-8;
A is C
5-12Aryl or C
5-7Cycloalkyl optionally separately contains 1 or the heteroatoms of a plurality of N of being selected from, S and O;
R
2Be the C of H, amino, hydroxyl, halogen or straight or side chain
1-6Alkyl, C
2-6Alkenyl, C
2-6Alkynyl group, C
1-6Alkoxyl group, C
1-6Assorted alkyl, C
1-6Aminoalkyl group, C
1-6Haloalkyl, C
1-6Alkoxy carbonyl, C
1-6Alkylthio ,-CN ,-CF
3,-OR
3,-COR
3,-NO
2,-NHR
3,-NHC (O) R
3,-C (O) NR
3R
4,-NR
3R
4,-NR
3C (O) NR
4R
5,-OC (O) R
3,-C (O) R
3R
4,-O (CH
2)
qNR
3,-CNR
3R
4Or-(CH
2)
qNR
3R
4Wherein q is the integer of 1-6;
N is 0,1,2,3 or 4, when n>1, and R
2Can be identical or different;
P is 0 or the integer of 1-5;
Y be O, S, CHOH ,-NHC (O)-,-C (O) NH-,-C (O)-,-OC (O)-, NR
7Or-CH=N-, R
7Be H or C
1-4Alkyl; Or do not exist;
Z is CR
8R
9, R wherein
8And R
9Independently be selected from the C of H and straight or side chain
1-8Alkyl;
Condition is: when-(CH
2) p-Y-is-(CH
2)
3-C (O)-or-(CH
2)
3-S-; And X
1-X
5When all being C ,-A-(R
2)
nAnd R
1Be respectively o-methyl-phenyl-and normal-butyl not together; Phenyl and normal-butyl; Or to fluorophenyl and-O-(CH
2)
2CH
3
2. the compound of claim 1 or its pharmacy acceptable salt, ester or prodrug, wherein:
X
1, X
2, X
3, X
4And X
5Be C; Perhaps X
1, X
2, X
3, X
4Or X
5One of them is O or N, and remaining is C;
K is 0 or 1;
T is 1;
R
1Be the C of straight or side chain
1-8Alkyl, C
2-8Alkenyl, C
2-8Alkynyl group, C
1-8Alkylidene group, C
1-8Alkoxyl group, C
1-8Aminoalkyl group, C
1-8Haloalkyl, C
1-8Alkoxy carbonyl ,-C (O) NR
3R
4,-NR
3R
4,-NR
3C (O) NR
4R
5,-OC (O) R
3Or-(CH
2)
sNR
3R
4R wherein
3, R
4And R
5Identical or different, independently be selected from H and C separately
1-6Alkyl; S is the integer of 1-8;
N is 1,2 or 3; And
A is a phenyl or naphthyl;
R wherein
2Be the C of straight or side chain
1-6Alkyl, C
2-6Alkenyl, C
2-6Alkynyl group, C
1-6Alkoxyl group, C
1-6Aminoalkyl group, C
1-6Haloalkyl, C
1-6Alkoxy carbonyl ,-CN ,-CF
3,-OH ,-COR
3,-NHR
3,-NHC (O) R
3,-C (O) NR
3R
4,-NR
3R
4,-NR
3C (O) NR
4R
5,-OC (O) R
3Or-(CH
2)
qNR
3R
4Wherein q is the integer of 1-6; Perhaps
A contains 1 or the heteroatomic aryl of a plurality of N of being selected from, S and O;
R
2Be the C of H, halogen, straight or side chain
1-6Alkyl, C
2-6Alkenyl, C
2-6Alkynyl group, C
1-6Alkoxyl group, C
1-6Assorted alkyl, C
1-6Aminoalkyl group, C
1-6Haloalkyl, C
1-6Alkoxy carbonyl ,-CN ,-CF
3,-OH ,-COR
3,-NHR
3,-NHC (O) R
3,-C (O) NR
3R
4,-NR
3R
4,-NR
3C (O) NR
4R
5,-OC (O) R
3Or-(CH
2)
qNR
3R
4
3. claim 1 or 2 compound, wherein p is 3.
4. claim 1 or 2 compound, wherein k is 0.
7. the compound of claim 6, wherein t be 1 and Y be-C (O)-,-NHC (O)-, S, O or-OC (O)-.
8. the compound of claim 7, wherein X
3Be C.
9. the compound of claim 8, wherein R
1It is alkyl.
10. the compound of claim 9, wherein R
2Be alkyl, aminoalkyl group, alkoxyl group or hydroxyl.
11. the compound of claim 10, wherein p is 3.
12. the compound of claim 11, wherein R
1Be C
2-8Alkyl, R
2Be methyl, hydroxyl or alkoxyl group.
13. the compound of claim 12, wherein Y is-C (O)-or O.
14. the compound of claim 9, wherein R
2It is halogen.
15. the compound of claim 6, wherein t is 0.
16. the compound of claim 8, wherein R
1It is alkoxyl group.
17. the compound of claim 8, wherein R
1Be benzyl or phenyl.
18. the compound of claim 7, wherein X
3Be N.
19. the compound of claim 18, wherein R
1Be alkyl or alkoxyl group.
20. the compound of claim 18, wherein R
1Be benzyl or phenyl.
21. the compound of claim 19, wherein R
2Be alkyl or alkoxyl group.
22. the compound of claim 20, wherein R
2Be alkyl or alkoxyl group.
23. the compound of claim 7, wherein X
3Be O.
24. the compound of claim 23, wherein R
1It is alkyl.
25. the compound of claim 24, wherein R
2Be alkyl or alkoxyl group.
26. the compound of claim 24, wherein R
2It is halogen.
28. the compound of claim 27, wherein Y be-C (O)-,-NHC (O)-, S, O or-OC (O)-.
29. the compound of claim 28, wherein X
3Be C.
30. the compound of claim 29, wherein R
1It is alkyl.
31. the compound of claim 30, wherein R
2Be alkyl, aminoalkyl group, alkoxyl group or hydroxyl.
32. the compound of claim 31, wherein p is 3.
33. the compound of claim 32, wherein R
1Be C
2-8Alkyl, R
2Be methyl, hydroxyl or alkoxyl group.
34. the compound of claim 33, wherein Y is-C (O)-or O.
35. the compound of claim 30, wherein R
2It is halogen.
36. the compound of claim 27, wherein t is 0.
37. the compound of claim 29, wherein R
1It is alkoxyl group.
38. the compound of claim 29, wherein R
1Be benzyl or phenyl.
39. the compound of claim 28, wherein X
3Be N.
40. the compound of claim 39, wherein R
1Be alkyl or alkoxyl group.
41. the compound of claim 39, wherein R
1Be benzyl or phenyl.
42. the compound of claim 40, wherein R
2Be alkyl or alkoxyl group.
43. the compound of claim 41, wherein R
2Be alkyl or alkoxyl group.
44. the compound of claim 28, wherein X
3Be O.
45. the compound of claim 44, wherein R
1It is alkyl.
46. the compound of claim 45, wherein R
2Be alkyl or alkoxyl group.
47. the compound of claim 45, wherein R
2It is halogen.
48. the method for exciting muscarinic receptor, it comprises makes this receptor contact with formula I compound or its pharmacy acceptable salt, ester or the prodrug of significant quantity:
Wherein
X
1, X
2, X
3, X
4And X
5Be selected from C, N and O;
K is 0 or 1;
T is 0 or 1;
R
1Be the C of straight or side chain
1-8Alkyl, C
2-8Alkenyl, C
2-8Alkynyl group, C
1-8Alkylidene group, C
1-8Alkoxyl group, C
1-8Assorted alkyl, C
1-8Aminoalkyl group, C
1-8Haloalkyl, C
1-8Alkoxy carbonyl, C
1-8Hydroxy alkoxy base, C
1-8Hydroxyalkyl ,-SH, C
1-8Alkylthio ,-O-CH
2-C
5-6Aryl, by C
1-3Alkyl or halogen replace-C (O)-C
5-6Aryl; Optional contain 1 or the heteroatomic C of a plurality of N of being selected from, S and O
5-6Aryl or C
5-6Cycloalkyl (89620);-C (O) NR
3R
4,-NR
3R
4,-NR
3C (O) NR
4R
5,-CR
3R
4,-OC (O) R
3,-C (O) (CH
2)
sNR
3R
4Or-(CH
2)
sN-R
3R
4R wherein
3, R
4And R
5Identical or different, independently be selected from H, C separately
1-6Alkyl; Optional contain 1 or the heteroatoms of a plurality of N of being selected from, O and S and optional by halogen or C
1-6The C that alkyl replaces
5-6Aryl; C
3-6Cycloalkyl; Perhaps R
3And R
4Contain 5-6 ring structure that is selected from the atom of C, N, S and O with N atom (when existing) formation; S is the integer of 0-8;
A is C
5-12Aryl or C
5-7Cycloalkyl optionally separately contains 1 or the heteroatoms of a plurality of N of being selected from, S and O;
R
2Be the C of H, amino, hydroxyl, halogen or straight or side chain
1-6Alkyl, C
2-6Alkenyl, C
2-6Alkynyl group, C
1-6Alkoxyl group, C
1-6Assorted alkyl, C
1-6Aminoalkyl group, C
1-6Haloalkyl, C
1-6Alkoxy carbonyl ,-CN ,-CF
3,-OR
3,-COR
3,-NO
2,-NH-R
3,-NHC (O) R
3,-C (O) NR
3R
4,-NR
3R
4,-NR
3C (O) NR
4R
5,-OC (O) R
3,-C (O) R
3R
4,-O (CH
2)
qNR
3,-CNR
3R
4Or-(CH
2)
qNR
3R
4Wherein q is the integer of 1-6;
N is 0,1,2,3 or 4, when n>1, and R
2Can be identical or different;
P is 0 or the integer of 1-5;
Y be O, S, CHOH ,-NHC (O)-,-C (O) NH-,-C (O)-,-OC (O)-, NR
7Or-CH=N-, R
7Be H or C
1-4Alkyl; Or do not exist;
Z is CR
8R
9, R wherein
8And R
9Independently be selected from the C of H and straight or side chain
1-8Alkyl.
50. the method for claim 49, wherein t be 1 and Y be-C (O)-,-NHC (O)-, S, O or-OC (O)-.
51. the method for claim 50, wherein X
3Be C.
52. the method for claim 51, wherein R
1It is alkyl.
53. wherein R washes in the side of claim 52
2Be alkyl, aminoalkyl group, alkoxyl group or hydroxyl.
54. the method for claim 53, wherein p is 3.
55. the method for claim 54, wherein R
1Be C
2-8Alkyl, R
2Be methyl, hydroxyl or alkoxyl group.
56. the method for claim 55, wherein Y is-C (O)-or O.
57. the method for claim 52, wherein R
2It is halogen.
58. the method for claim 49, wherein t is 0.
59. the method for claim 51, wherein R
1It is alkoxyl group.
60. the method for claim 51, wherein R
1Be benzyl or phenyl.
61. the method for claim 52, wherein X
3Be N.
62. the method for claim 61, wherein R
1Be alkyl or alkoxyl group.
63. the method for claim 61, wherein R
1Be benzyl or phenyl.
64. the method for claim 62, wherein R
2Be alkyl or alkoxyl group.
65. the method for claim 63, wherein R
2Be alkyl or alkoxyl group.
66. the method for claim 50, wherein X
3Be O.
67. the method for claim 66, wherein R
1It is alkyl.
68. the method for claim 67, wherein R
2Be alkyl or alkoxyl group.
69. the method for claim 67, wherein R
2It is halogen.
70. medicinal compositions, it comprises formula I compound or its pharmacy acceptable salt, ester or prodrug and the pharmaceutically acceptable carrier of significant quantity:
Wherein
X
1, X
2, X
3, X
4And X
5Be selected from C, N and O;
K is 0 or 1;
T is 0 or 1;
R
1Be the C of straight or side chain
1-8Alkyl, C
2-8Alkenyl, C
2-8Alkynyl group, C
1-8Alkylidene group, C
1-8Alkoxyl group, C
1-8Assorted alkyl, C
1-8Aminoalkyl group, C
1-8Haloalkyl, C
1-8Alkoxy carbonyl, C
1-8Hydroxy alkoxy base, C
1-8Hydroxyalkyl ,-SH, C
1-8Alkylthio ,-O-CH
2-C
5-6Aryl, by C
1-3Alkyl or halogen replace-C (O)-C
5-6Aryl; Optional contain 1 or the heteroatomic C of a plurality of N of being selected from, S and O
5-6Aryl or C
5-6Cycloalkyl;-C (O) NR
3R
4,-NR
3R
4,-NR
3C (O) NR
4R
5,-CR
3R
4,-OC (O) R
3,-C (O) (CH
2)
sNR
3R
4Or-(CH
2)
sNR
3R
4R wherein
3, R
4And R
5Identical or different, independently be selected from H, C separately
1-6Alkyl; Optional contain 1 or the heteroatoms of a plurality of N of being selected from, O and S and optional by halogen or C
1-6The C that alkyl replaces
5-6Aryl; C
3-6Cycloalkyl; Perhaps R
3And R
4Contain 5-6 ring structure that is selected from the atom of C, N, S and O with N atom (when existing) formation; S is the integer of 0-8;
A is C
5-12Aryl or C
5-7Cycloalkyl optionally separately contains 1 or the heteroatoms of a plurality of N of being selected from, S and O;
R
2Be the C of H, amino, hydroxyl, halogen or straight or side chain
1-6Alkyl, C
2-6Alkenyl, C
2-6Alkynyl group, C
1-6Alkoxyl group, C
1-6Assorted alkyl, C
1-6Aminoalkyl group, C
1-6Haloalkyl, C
1-6Alkoxy carbonyl ,-CN ,-CF
3,-OR
3,-COR
3,-NO
2,-NHR
3,-NHC (O) R
3,-C (O) NR
3R
4,-NR
3R
4,-NR
3C (O) NR
4R
5,-OC (O) R
3,-C (O) R
3R
4,-O (CH
2)
qNR
3,-CNR
3R
4Or-(CH
2)
qNR
3R
4Wherein q is the integer of 1-6;
N is 0,1,2,3 or 4, when n>1, and R
2Can be identical or different;
P is 0 or the integer of 1-5;
Y be O, S, CHOH ,-NHC (O)-,-C (O) NH-,-C (O)-,-OC (O)-, NR
7Or-CH=N-, R
7Be H or C
1-4Alkyl; Or do not exist;
Z is CR
8R
9, R wherein
8And R
9Independently be selected from the C of H and straight or side chain
1-8Alkyl.
72. the composition of claim 71, wherein t be 1 and Y be-C (O)-,-NHC (O)-, S, O or-OC (O)-.
73. the composition of claim 72, wherein X
3Be C.
74. the composition of claim 73, wherein R
1It is alkyl.
75. the composition of claim 74, wherein R
2Be alkyl, aminoalkyl group, alkoxyl group or hydroxyl.
76. the composition of claim 75, wherein p is 3.
77. the composition of claim 75, wherein R
1Be C
2-8Alkyl, R
2Be methyl, hydroxyl or alkoxyl group.
78. the composition of claim 77, wherein Y is-C (O)-or O.
79. the composition of claim 74, wherein R
2It is halogen.
80. the composition of claim 71, wherein t is 0.
81. the composition of claim 73, wherein R
1It is alkoxyl group.
82. the composition of claim 73, wherein R
1Be benzyl or phenyl.
83. the composition of claim 74, wherein X
3Be N.
84. the composition of claim 82, wherein R
1Be alkyl or alkoxyl group.
85. the composition of claim 82, wherein R
1Be benzyl or phenyl.
86. the composition of claim 83, wherein R
2Be alkyl or alkoxyl group.
87. the composition of claim 84, wherein R
2Be alkyl or alkoxyl group.
88. the composition of claim 72, wherein X
3Be O.
89. the composition of claim 88, wherein R
1It is alkyl.
90. the composition of claim 89, wherein R
2Be alkyl or alkoxyl group.
91. the composition of claim 87, wherein R
2It is halogen.
92. treatment and levels of acetylcholine lower the method for diseases associated or symptom, this method comprises the compound of one or more claim 1 for the treatment of significant quantity.
93. the method for claim 92, wherein said disease or symptom are neurodegeneration (neurogenerative) disease, cognitive impairment, cognitive decline or the dementia relevant with the age.
94. the method for treatment and intraocular pressure rising diseases associated or symptom, this method comprises the compound of one or more claim 1 for the treatment of significant quantity.
95. the method for claim 94, wherein this disease is a glaucoma.
96. treatment and levels of acetylcholine lower the method for diseases associated or symptom, this method comprises the composition of the claim 70 for the treatment of significant quantity.
97. the method for claim 96, wherein this disease or symptom are neurodegenerative disease, cognitive impairment, cognitive decline or the dementia relevant with the age.
98. the method for treatment and intraocular pressure rising diseases associated or symptom, this method comprises the composition of the claim 70 for the treatment of significant quantity.
99. the method for claim 98, wherein this disease is a glaucoma.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US8013398P | 1998-03-31 | 1998-03-31 | |
| US60/080,133 | 1998-03-31 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN1303376A true CN1303376A (en) | 2001-07-11 |
Family
ID=22155464
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN99806618A Pending CN1303376A (en) | 1998-03-31 | 1999-03-31 | Compound with activity on muscarinic receptors |
Country Status (14)
| Country | Link |
|---|---|
| EP (1) | EP1068185A1 (en) |
| JP (1) | JP2002509918A (en) |
| KR (2) | KR20060120715A (en) |
| CN (1) | CN1303376A (en) |
| AR (1) | AR014974A1 (en) |
| AU (1) | AU762726B2 (en) |
| BR (1) | BR9909277A (en) |
| CA (1) | CA2326804C (en) |
| MX (1) | MXPA00009569A (en) |
| NO (1) | NO319835B1 (en) |
| NZ (2) | NZ525108A (en) |
| RU (2) | RU2230740C2 (en) |
| WO (1) | WO1999050247A1 (en) |
| ZA (1) | ZA200005149B (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN100402032C (en) * | 2001-12-28 | 2008-07-16 | 阿卡蒂亚药品公司 | Tetrahydroquinoline analogues as muscarinic agonists |
| CN102558026A (en) * | 2011-12-27 | 2012-07-11 | 盛世泰科生物医药技术(苏州)有限公司 | Synthesis and optimized process of 4-N-butyl-1-[4-(2-methylphenyl)-4-one-1-butyl]piperidine. N-benzyl-4-piperidone (1) |
Families Citing this family (48)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6528529B1 (en) | 1998-03-31 | 2003-03-04 | Acadia Pharmaceuticals Inc. | Compounds with activity on muscarinic receptors |
| US6624162B2 (en) | 2001-10-22 | 2003-09-23 | Pfizer Inc. | Imidazopyridine compounds as 5-HT4 receptor modulators |
| US7550459B2 (en) | 2001-12-28 | 2009-06-23 | Acadia Pharmaceuticals, Inc. | Tetrahydroquinoline analogues as muscarinic agonists |
| MXPA03000145A (en) | 2002-01-07 | 2003-07-15 | Pfizer | Oxo or oxy-pyridine compounds as 5-ht4 receptor modulators. |
| GB0211230D0 (en) | 2002-05-16 | 2002-06-26 | Medinnova Sf | Treatment of heart failure |
| DOP2003000703A (en) | 2002-09-20 | 2004-03-31 | Pfizer | IMIDAZOPIRADINE COMPOUNDS AS 5-HT4 RECEIVER AGONISTS |
| CA2499494A1 (en) | 2002-09-20 | 2004-04-01 | Pfizer Inc. | N-substituted piperidinyl-imidazopyridine compounds as 5-ht4 receptor modulators |
| HU227534B1 (en) | 2003-08-04 | 2011-08-29 | Richter Gedeon Nyrt | (thio)carbamoyl-cyclohexane derivatives, process for producing them and pharmaceutical compositions containing them |
| CN1863536A (en) * | 2003-08-05 | 2006-11-15 | 萨马里坦药品公司 | Sigma-1 receptor ligand with acetylcholinesterase |
| JP4795022B2 (en) | 2003-09-30 | 2011-10-19 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | Novel antifungal agent containing a heterocyclic compound |
| EP2088147A1 (en) | 2003-12-22 | 2009-08-12 | Arcadia Pharmaceuticals Inc. | Dibenzo-condensed azepine, diazepine, oxazepine and thiazepine derivatives as muscarinic agonists and methods of treatment of neuropsychiatric disorders |
| MXPA06012505A (en) | 2004-04-30 | 2006-12-15 | Warner Lambert Co | Substituted morpholine compounds for the treatment of central nervous system disorders. |
| US7737163B2 (en) | 2004-06-15 | 2010-06-15 | Pfizer Inc. | Benzimidazolone carboxylic acid derivatives |
| EA200801608A1 (en) | 2004-06-15 | 2008-10-30 | Пфайзер Инк. | DERIVATIVES OF BENZIMIDAZOLONIC CARBONIC ACID |
| WO2006074025A1 (en) | 2004-12-30 | 2006-07-13 | Janssen Pharmaceutica N.V. | Piperazinyl and piperidinyl ureas as modulators of fatty acid amide hydrolase |
| JP5507049B2 (en) | 2004-12-30 | 2014-05-28 | アステックス、セラピューティックス、リミテッド | Medicine |
| WO2006106711A1 (en) | 2005-03-30 | 2006-10-12 | Eisai R & D Management Co., Ltd. | Antifungal agent containing pyridine derivative |
| EP1928437A2 (en) | 2005-08-26 | 2008-06-11 | Braincells, Inc. | Neurogenesis by muscarinic receptor modulation |
| EP2258357A3 (en) | 2005-08-26 | 2011-04-06 | Braincells, Inc. | Neurogenesis with acetylcholinesterase inhibitor |
| US8097610B2 (en) | 2005-08-26 | 2012-01-17 | Shionogi & Co., Ltd. | Derivative having PPAR agonistic activity |
| TWI385169B (en) | 2005-10-31 | 2013-02-11 | Eisai R&D Man Co Ltd | Heterocyclic substituted pyridine derivatives and antifungal agent containing same |
| US8399442B2 (en) | 2005-12-30 | 2013-03-19 | Astex Therapeutics Limited | Pharmaceutical compounds |
| US8435970B2 (en) | 2006-06-29 | 2013-05-07 | Astex Therapeutics Limited | Pharmaceutical combinations of 1-cyclopropyl-3-[3-(5-morpholin-4-ylmethyl-1H-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-urea |
| CL2008000119A1 (en) | 2007-01-16 | 2008-05-16 | Wyeth Corp | COMPOUNDS DERIVED FROM PIRAZOL, ANTAGONISTS OF THE NICOTINIC ACETILCOLINE RECEIVER; PHARMACEUTICAL COMPOSITION; AND USE IN THE TREATMENT OF DISEASES SUCH AS SENILE DEMENTIA, ALZHEIMER AND SCHIZOPHRENIA. |
| HUP0700353A2 (en) | 2007-05-18 | 2008-12-29 | Richter Gedeon Nyrt | Metabolites of (thio)carbamoyl-cyclohexane derivatives |
| US7875610B2 (en) | 2007-12-03 | 2011-01-25 | Richter Gedeon Nyrt. | Pyrimidinyl-piperazines useful as D3/D2 receptor ligands |
| US8513287B2 (en) | 2007-12-27 | 2013-08-20 | Eisai R&D Management Co., Ltd. | Heterocyclic ring and phosphonoxymethyl group substituted pyridine derivatives and antifungal agent containing same |
| CA2730287C (en) | 2008-07-16 | 2017-02-07 | Forest Laboratories Holdings Limited | Pharmaceutical formulations containing dopamine receptor ligands |
| HU230067B1 (en) | 2008-12-17 | 2015-06-29 | Richter Gedeon Nyrt | Novel piperazine salt and preparation method thereof |
| HUP0800766A2 (en) | 2008-12-18 | 2010-11-29 | Richter Gedeon Vegyeszet | Process for the preparation of piperazine derivatives |
| HUP0800765A2 (en) | 2008-12-18 | 2010-11-29 | Richter Gedeon Nyrt | A new process for the preparation of piperazine derivatives and their hydrochloric salts |
| UA108233C2 (en) | 2010-05-03 | 2015-04-10 | Fatty acid amide hydrolysis activity modulators | |
| GB201106817D0 (en) | 2011-04-21 | 2011-06-01 | Astex Therapeutics Ltd | New compound |
| US8912197B2 (en) | 2012-08-20 | 2014-12-16 | Forest Laboratories Holdings Ltd. | Crystalline form of carbamoyl-cyclohexane derivatives |
| CN102997438A (en) * | 2012-10-16 | 2013-03-27 | 李誊 | Control method of electric water heater for improving heat utilization ratio |
| GB201218862D0 (en) | 2012-10-19 | 2012-12-05 | Astex Therapeutics Ltd | Bicyclic heterocycle compounds and their uses in therapy |
| US9980973B2 (en) | 2012-10-19 | 2018-05-29 | Astex Therapeutics Limited | Bicyclic heterocycle compounds and their uses in therapy |
| GB201218850D0 (en) | 2012-10-19 | 2012-12-05 | Astex Therapeutics Ltd | Bicyclic heterocycle compounds and their uses in therapy |
| GB201218864D0 (en) | 2012-10-19 | 2012-12-05 | Astex Therapeutics Ltd | Bicyclic heterocycle compounds and their uses in therapy |
| CN105073728A (en) | 2013-03-15 | 2015-11-18 | 全球血液疗法股份有限公司 | Compounds and uses thereof for the modulation of hemoglobin |
| EA201992707A1 (en) | 2013-11-18 | 2020-06-30 | Глобал Блад Терапьютикс, Инк. | COMPOUNDS AND THEIR APPLICATIONS FOR HEMOGLOBIN MODULATION |
| ES2883289T3 (en) | 2013-12-20 | 2021-12-07 | Astex Therapeutics Ltd | Bicyclic heterocyclic compounds and their uses in therapy |
| US11274087B2 (en) | 2016-07-08 | 2022-03-15 | Richter Gedeon Nyrt. | Industrial process for the preparation of cariprazine |
| IL308744A (en) | 2017-06-20 | 2024-01-01 | Imbria Pharmaceuticals Inc | Preparations for use to increase the efficiency of heart metabolism |
| US11547707B2 (en) | 2019-04-10 | 2023-01-10 | Richter Gedeon Nyrt. | Carbamoyl cyclohexane derivatives for treating autism spectrum disorder |
| US11530184B2 (en) | 2020-06-30 | 2022-12-20 | Imbria Pharmaceuticals, Inc. | Crystal forms of 2-[4-[(2,3,4-trimethoxyphenyl)methyl]piperazin-1-yl]ethyl pyridine-3-carboxylate |
| US11780811B2 (en) | 2020-06-30 | 2023-10-10 | Imbria Pharmaceuticals, Inc. | Methods of synthesizing 2-[4-[(2,3,4-trimethoxyphenyl)methyl]piperazin-1-yl]ethyl pyridine-3-carboxylate |
| US11883396B2 (en) | 2021-05-03 | 2024-01-30 | Imbria Pharmaceuticals, Inc. | Methods of treating kidney conditions using modified forms of trimetazidine |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2695295A (en) * | 1952-12-19 | 1954-11-23 | Mcneilab Inc | Unsymmetrical n, n'-substituted ethylenediamine and piperazine compounds |
| GB874206A (en) * | 1956-09-05 | 1961-08-02 | Knoll Ag | Basic derivatives of salicylamide |
| NL271658A (en) * | 1960-12-01 | |||
| FR1382425A (en) * | 1963-01-14 | 1964-12-18 | Ciba Geigy | Process for preparing diaza-cyclo-alkanes, inter alia 3-methyl-4-phenyl-1- (2-phenylmercapto-ethyl) -piperazine |
| GB1053301A (en) * | 1963-01-14 | |||
| US3488352A (en) * | 1966-10-11 | 1970-01-06 | Shulton Inc | Basically substituted alkoxy anthranilamides,their corresponding 2-nitro compounds and derivatives thereof |
| FR1543944A (en) * | 1967-03-10 | 1968-10-31 | Bruneau & Cie Lab | Salicylic acid amide derivatives and their preparation |
| DE2335432A1 (en) * | 1973-07-12 | 1975-01-30 | Boehringer Mannheim Gmbh | 3,4-DIHYDRO-2H-NAPHTHALINONE- (1) 5-OXY-PROPYL-PIPERAZINE DERIVATIVES AND THE METHOD FOR THEIR PRODUCTION |
| BE792187A (en) * | 1971-12-03 | 1973-03-30 | Sumitomo Chemical Co | NEW ALKYLAMINE DERIVATIVES |
| GB1459506A (en) * | 1974-02-18 | 1976-12-22 | Wyeth John & Brother Ltd | Piperidine derivatives |
| GB1498884A (en) * | 1975-04-15 | 1978-01-25 | Wyeth John & Brother Ltd | Aminoacetamide-pyridyl-tetrahydropyridyl and-piperidyl derivatives |
| IL117149A0 (en) * | 1995-02-23 | 1996-06-18 | Schering Corp | Muscarinic antagonists |
| US5889006A (en) * | 1995-02-23 | 1999-03-30 | Schering Corporation | Muscarinic antagonists |
| EP0922029B1 (en) * | 1996-08-15 | 2005-06-08 | Schering Corporation | Ether muscarinic antagonists |
-
1999
- 1999-03-31 NZ NZ525108A patent/NZ525108A/en unknown
- 1999-03-31 KR KR1020067023572A patent/KR20060120715A/en not_active Application Discontinuation
- 1999-03-31 CA CA002326804A patent/CA2326804C/en not_active Expired - Fee Related
- 1999-03-31 WO PCT/US1999/007057 patent/WO1999050247A1/en active IP Right Grant
- 1999-03-31 AU AU32187/99A patent/AU762726B2/en not_active Ceased
- 1999-03-31 RU RU2000127105/04A patent/RU2230740C2/en active
- 1999-03-31 CN CN99806618A patent/CN1303376A/en active Pending
- 1999-03-31 KR KR1020007010780A patent/KR100672186B1/en not_active IP Right Cessation
- 1999-03-31 NZ NZ507204A patent/NZ507204A/en unknown
- 1999-03-31 BR BR9909277-8A patent/BR9909277A/en not_active Application Discontinuation
- 1999-03-31 MX MXPA00009569A patent/MXPA00009569A/en active IP Right Grant
- 1999-03-31 JP JP2000541152A patent/JP2002509918A/en not_active Withdrawn
- 1999-03-31 EP EP99914306A patent/EP1068185A1/en not_active Withdrawn
- 1999-04-05 AR ARP990101516A patent/AR014974A1/en not_active Application Discontinuation
-
2000
- 2000-09-26 ZA ZA200005149A patent/ZA200005149B/en unknown
- 2000-09-29 NO NO20004912A patent/NO319835B1/en unknown
-
2004
- 2004-03-11 RU RU2004107218/04A patent/RU2278111C2/en active
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN100402032C (en) * | 2001-12-28 | 2008-07-16 | 阿卡蒂亚药品公司 | Tetrahydroquinoline analogues as muscarinic agonists |
| CN102558026A (en) * | 2011-12-27 | 2012-07-11 | 盛世泰科生物医药技术(苏州)有限公司 | Synthesis and optimized process of 4-N-butyl-1-[4-(2-methylphenyl)-4-one-1-butyl]piperidine. N-benzyl-4-piperidone (1) |
Also Published As
| Publication number | Publication date |
|---|---|
| NZ525108A (en) | 2005-02-25 |
| KR100672186B1 (en) | 2007-01-19 |
| RU2278111C2 (en) | 2006-06-20 |
| AU3218799A (en) | 1999-10-18 |
| CA2326804A1 (en) | 1999-10-07 |
| AR014974A1 (en) | 2001-04-11 |
| MXPA00009569A (en) | 2002-08-06 |
| NZ507204A (en) | 2003-12-19 |
| NO20004912L (en) | 2000-11-23 |
| KR20010042248A (en) | 2001-05-25 |
| CA2326804C (en) | 2006-05-02 |
| WO1999050247A1 (en) | 1999-10-07 |
| RU2230740C2 (en) | 2004-06-20 |
| ZA200005149B (en) | 2002-01-08 |
| NO319835B1 (en) | 2005-09-19 |
| BR9909277A (en) | 2001-10-16 |
| NO20004912D0 (en) | 2000-09-29 |
| KR20060120715A (en) | 2006-11-27 |
| RU2004107218A (en) | 2005-08-20 |
| EP1068185A1 (en) | 2001-01-17 |
| JP2002509918A (en) | 2002-04-02 |
| AU762726B2 (en) | 2003-07-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1303376A (en) | Compound with activity on muscarinic receptors | |
| US6528529B1 (en) | Compounds with activity on muscarinic receptors | |
| CN1131854C (en) | 1-(1,2-disubstituted piperidinyl)-4-substituted piperidine derivatives as tachykinin receptor antagonists | |
| JP2969359B2 (en) | Cyclic amine compounds | |
| DE60315265T2 (en) | NITROGENIC HETEROCYCLIC COMPOUND AND MEDICINES THEREOF | |
| US6479480B1 (en) | Phenylindole derivatives as 5-ht2a receptor ligands | |
| NO326106B1 (en) | Mustarin agonists, pharmaceutical compositions containing them, and their use in the treatment of a disease state | |
| CN103068800B (en) | Piperidinyl compound as a modulator of chemokine receptor activity | |
| JP5663657B2 (en) | 1-[(4-Hydroxypiperidin-4-yl) methyl] pyridin-2 (1H) -one derivative, process for its preparation and use thereof | |
| CN1372541A (en) | Sulfonylcarboxamide derivatives, process for their preparation and their use as medicaments | |
| JP2007508359A (en) | Derivatives of N- [heteroallyl (piperidin-2-yl) methyl] benzamide, process for its preparation and use in this therapy | |
| CN1368960A (en) | Indole derivatives, process for their preparation, pharmaceutical compositions containing them and their medicinal application | |
| JP2010501566A (en) | Multitransmitter transporter inhibitors for use in the treatment of central nervous system disorders | |
| JP4076234B2 (en) | Tetrahydrobenzindole compounds | |
| CN102076663A (en) | Piperidinyl derivative as modulator of chemokine receptor activity | |
| CN101061123A (en) | Thienopyridinone compounds and methods of treatment | |
| DE3620643A1 (en) | Thiazoles, their preparation and use | |
| JP2003516967A (en) | 1,3,4-Substituted Piperidine Analogs and Their Use in the Treatment of Addiction | |
| US6916822B2 (en) | Phenoxyalkylamine derivatives useful as opioid δ receptor agonists | |
| CN1717390A (en) | Piperazine and piperidine derivatives for treatment of neurological diseases | |
| CN1304929A (en) | 4-hydroxy-4-phenyl piperidine derivative and medicine containing said derivative | |
| HUT65472A (en) | Process for producing 3,4-dihydro-2(1h)-quinolone derivatives and pharmaceutical preparations containing them | |
| JPH08208603A (en) | 3-alkoxybenzylpiperidine derivative as melatonin agent | |
| CN1529599A (en) | Acylic piperazine and piperidine derivatives which are used for treating neuronal damage | |
| CN1629156A (en) | Pyrudyl and pyrimidylpiperazine derivatives, preparation method thereof and pharmaceutical composition containing the derivatives |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C02 | Deemed withdrawal of patent application after publication (patent law 2001) | ||
| WD01 | Invention patent application deemed withdrawn after publication |