CN116287354A - Method and kit for detecting bacterial fusarium wilt of corn - Google Patents
Method and kit for detecting bacterial fusarium wilt of corn Download PDFInfo
- Publication number
- CN116287354A CN116287354A CN202310525899.8A CN202310525899A CN116287354A CN 116287354 A CN116287354 A CN 116287354A CN 202310525899 A CN202310525899 A CN 202310525899A CN 116287354 A CN116287354 A CN 116287354A
- Authority
- CN
- China
- Prior art keywords
- corn
- stewartii
- detection
- sample
- bacterial
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 230000001580 bacterial effect Effects 0.000 title claims abstract description 33
- 238000000034 method Methods 0.000 title claims abstract description 33
- 240000008042 Zea mays Species 0.000 title claims abstract description 32
- 235000002017 Zea mays subsp mays Nutrition 0.000 title claims abstract description 32
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 title claims abstract description 24
- 235000005822 corn Nutrition 0.000 title claims abstract description 24
- UHPMCKVQTMMPCG-UHFFFAOYSA-N 5,8-dihydroxy-2-methoxy-6-methyl-7-(2-oxopropyl)naphthalene-1,4-dione Chemical compound CC1=C(CC(C)=O)C(O)=C2C(=O)C(OC)=CC(=O)C2=C1O UHPMCKVQTMMPCG-UHFFFAOYSA-N 0.000 title claims description 5
- 241000223218 Fusarium Species 0.000 title claims description 5
- 238000001514 detection method Methods 0.000 claims abstract description 32
- 235000016383 Zea mays subsp huehuetenangensis Nutrition 0.000 claims abstract description 8
- 235000009973 maize Nutrition 0.000 claims abstract description 8
- 239000000523 sample Substances 0.000 claims description 53
- 238000003199 nucleic acid amplification method Methods 0.000 claims description 22
- 230000003321 amplification Effects 0.000 claims description 21
- 239000003153 chemical reaction reagent Substances 0.000 claims description 14
- 108020004707 nucleic acids Proteins 0.000 claims description 14
- 102000039446 nucleic acids Human genes 0.000 claims description 14
- 150000007523 nucleic acids Chemical class 0.000 claims description 14
- 125000006850 spacer group Chemical group 0.000 claims description 11
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 9
- 125000003729 nucleotide group Chemical group 0.000 claims description 8
- 238000005516 engineering process Methods 0.000 claims description 6
- 208000035657 Abasia Diseases 0.000 claims description 4
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 claims description 4
- 239000002981 blocking agent Substances 0.000 claims description 4
- 238000000605 extraction Methods 0.000 claims description 3
- 239000013642 negative control Substances 0.000 claims description 3
- 238000010791 quenching Methods 0.000 claims description 3
- 230000000171 quenching effect Effects 0.000 claims description 3
- 239000002689 soil Substances 0.000 claims description 3
- 239000013641 positive control Substances 0.000 claims description 2
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 claims description 2
- 241000894006 Bacteria Species 0.000 abstract description 14
- 238000006243 chemical reaction Methods 0.000 abstract description 9
- 230000035945 sensitivity Effects 0.000 abstract description 9
- 238000011081 inoculation Methods 0.000 abstract description 4
- 238000004088 simulation Methods 0.000 abstract description 3
- 238000012544 monitoring process Methods 0.000 abstract description 2
- 108020004414 DNA Proteins 0.000 description 17
- 238000012360 testing method Methods 0.000 description 17
- 201000003042 peeling skin syndrome Diseases 0.000 description 16
- 102000018120 Recombinases Human genes 0.000 description 8
- 108010091086 Recombinases Proteins 0.000 description 8
- 238000002474 experimental method Methods 0.000 description 7
- 239000000463 material Substances 0.000 description 7
- 238000012986 modification Methods 0.000 description 7
- 230000004048 modification Effects 0.000 description 7
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 6
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 6
- 239000000203 mixture Substances 0.000 description 6
- 108090000623 proteins and genes Proteins 0.000 description 6
- 239000000243 solution Substances 0.000 description 6
- 241000196324 Embryophyta Species 0.000 description 4
- 201000010099 disease Diseases 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 239000002773 nucleotide Substances 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- 238000000926 separation method Methods 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 239000000725 suspension Substances 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 241000932831 Pantoea stewartii Species 0.000 description 3
- -1 aqua phoor 593 Chemical compound 0.000 description 3
- 244000052616 bacterial pathogen Species 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 230000008929 regeneration Effects 0.000 description 3
- 238000011069 regeneration method Methods 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 238000011282 treatment Methods 0.000 description 3
- SGTNSNPWRIOYBX-UHFFFAOYSA-N 2-(3,4-dimethoxyphenyl)-5-{[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino}-2-(propan-2-yl)pentanenitrile Chemical compound C1=C(OC)C(OC)=CC=C1CCN(C)CCCC(C#N)(C(C)C)C1=CC=C(OC)C(OC)=C1 SGTNSNPWRIOYBX-UHFFFAOYSA-N 0.000 description 2
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- 244000099147 Ananas comosus Species 0.000 description 2
- 235000007119 Ananas comosus Nutrition 0.000 description 2
- LZZYPRNAOMGNLH-UHFFFAOYSA-M Cetrimonium bromide Chemical compound [Br-].CCCCCCCCCCCCCCCC[N+](C)(C)C LZZYPRNAOMGNLH-UHFFFAOYSA-M 0.000 description 2
- 108010017826 DNA Polymerase I Proteins 0.000 description 2
- 102000004594 DNA Polymerase I Human genes 0.000 description 2
- 238000007400 DNA extraction Methods 0.000 description 2
- 238000007399 DNA isolation Methods 0.000 description 2
- 102000052510 DNA-Binding Proteins Human genes 0.000 description 2
- 101710116602 DNA-Binding protein G5P Proteins 0.000 description 2
- 101100440961 Drosophila melanogaster Cpsf160 gene Proteins 0.000 description 2
- JLVVSXFLKOJNIY-UHFFFAOYSA-N Magnesium ion Chemical compound [Mg+2] JLVVSXFLKOJNIY-UHFFFAOYSA-N 0.000 description 2
- DRBBFCLWYRJSJZ-UHFFFAOYSA-N N-phosphocreatine Chemical compound OC(=O)CN(C)C(=N)NP(O)(O)=O DRBBFCLWYRJSJZ-UHFFFAOYSA-N 0.000 description 2
- 108091028043 Nucleic acid sequence Proteins 0.000 description 2
- 108091034117 Oligonucleotide Proteins 0.000 description 2
- 238000011529 RT qPCR Methods 0.000 description 2
- 101710162453 Replication factor A Proteins 0.000 description 2
- 101710176758 Replication protein A 70 kDa DNA-binding subunit Proteins 0.000 description 2
- 101710176276 SSB protein Proteins 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 101710126859 Single-stranded DNA-binding protein Proteins 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 241000191967 Staphylococcus aureus Species 0.000 description 2
- 239000007983 Tris buffer Substances 0.000 description 2
- 238000000246 agarose gel electrophoresis Methods 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 238000006073 displacement reaction Methods 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 235000013305 food Nutrition 0.000 description 2
- 239000001963 growth medium Substances 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- 238000002372 labelling Methods 0.000 description 2
- 229910001425 magnesium ion Inorganic materials 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 230000001717 pathogenic effect Effects 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 238000001556 precipitation Methods 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 238000012163 sequencing technique Methods 0.000 description 2
- 238000002791 soaking Methods 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 238000011144 upstream manufacturing Methods 0.000 description 2
- KHQCCEUMCUSZKM-KVQBGUIXSA-N (2r,3s,5r)-5-(6,8-diaminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-ol Chemical compound NC1=NC2=C(N)N=CN=C2N1[C@H]1C[C@H](O)[C@@H](CO)O1 KHQCCEUMCUSZKM-KVQBGUIXSA-N 0.000 description 1
- RVWUFECBVNXJEN-YGOYTEALSA-N 1-[(2s,4s,5r)-4-hydroxy-5-(hydroxymethyl)-2-prop-1-ynyloxolan-2-yl]pyrimidine-2,4-dione Chemical compound C1=CC(=O)NC(=O)N1[C@@]1(C#CC)C[C@H](O)[C@@H](CO)O1 RVWUFECBVNXJEN-YGOYTEALSA-N 0.000 description 1
- CFBILACNYSPRPM-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;2-[[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]amino]acetic acid Chemical group OCC(N)(CO)CO.OCC(CO)(CO)NCC(O)=O CFBILACNYSPRPM-UHFFFAOYSA-N 0.000 description 1
- MWBWWFOAEOYUST-UHFFFAOYSA-N 2-aminopurine Chemical compound NC1=NC=C2N=CNC2=N1 MWBWWFOAEOYUST-UHFFFAOYSA-N 0.000 description 1
- UDGUGZTYGWUUSG-UHFFFAOYSA-N 4-[4-[[2,5-dimethoxy-4-[(4-nitrophenyl)diazenyl]phenyl]diazenyl]-n-methylanilino]butanoic acid Chemical compound COC=1C=C(N=NC=2C=CC(=CC=2)N(C)CCCC(O)=O)C(OC)=CC=1N=NC1=CC=C([N+]([O-])=O)C=C1 UDGUGZTYGWUUSG-UHFFFAOYSA-N 0.000 description 1
- WCKQPPQRFNHPRJ-UHFFFAOYSA-N 4-[[4-(dimethylamino)phenyl]diazenyl]benzoic acid Chemical compound C1=CC(N(C)C)=CC=C1N=NC1=CC=C(C(O)=O)C=C1 WCKQPPQRFNHPRJ-UHFFFAOYSA-N 0.000 description 1
- XSGZNYKZSOJIAM-XUOJEKSQSA-N 4-amino-1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one;2-(10h-phenoxazin-1-yl)ethanamine Chemical compound O=C1N=C(N)C=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1.O1C2=CC=CC=C2NC2=C1C=CC=C2CCN XSGZNYKZSOJIAM-XUOJEKSQSA-N 0.000 description 1
- MSHADEGKNJRMIU-YGOYTEALSA-N 4-amino-1-[(2s,4s,5r)-4-hydroxy-5-(hydroxymethyl)-2-prop-1-ynyloxolan-2-yl]pyrimidin-2-one Chemical compound C1=CC(N)=NC(=O)N1[C@@]1(C#CC)C[C@H](O)[C@@H](CO)O1 MSHADEGKNJRMIU-YGOYTEALSA-N 0.000 description 1
- OZFPSOBLQZPIAV-UHFFFAOYSA-N 5-nitro-1h-indole Chemical compound [O-][N+](=O)C1=CC=C2NC=CC2=C1 OZFPSOBLQZPIAV-UHFFFAOYSA-N 0.000 description 1
- MSSXOMSJDRHRMC-UHFFFAOYSA-N 9H-purine-2,6-diamine Chemical compound NC1=NC(N)=C2NC=NC2=N1 MSSXOMSJDRHRMC-UHFFFAOYSA-N 0.000 description 1
- WNDDWSAHNYBXKY-UHFFFAOYSA-N ATTO 425-2 Chemical compound CC1CC(C)(C)N(CCCC(O)=O)C2=C1C=C1C=C(C(=O)OCC)C(=O)OC1=C2 WNDDWSAHNYBXKY-UHFFFAOYSA-N 0.000 description 1
- PWZJEXGKUHVUFP-UHFFFAOYSA-N ATTO 590 meta-isomer Chemical compound [O-]Cl(=O)(=O)=O.C1=2C=C3C(C)=CC(C)(C)N(CC)C3=CC=2OC2=CC3=[N+](CC)C(C)(C)C=C(C)C3=CC2=C1C1=CC=C(C(O)=O)C=C1C(O)=O PWZJEXGKUHVUFP-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 239000012103 Alexa Fluor 488 Substances 0.000 description 1
- 244000063299 Bacillus subtilis Species 0.000 description 1
- 235000014469 Bacillus subtilis Nutrition 0.000 description 1
- 101900090047 Bacillus subtilis DNA polymerase I Proteins 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 108091026890 Coding region Proteins 0.000 description 1
- 102000004420 Creatine Kinase Human genes 0.000 description 1
- 108010042126 Creatine kinase Proteins 0.000 description 1
- HMFHBZSHGGEWLO-SOOFDHNKSA-N D-ribofuranose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H]1O HMFHBZSHGGEWLO-SOOFDHNKSA-N 0.000 description 1
- 230000004543 DNA replication Effects 0.000 description 1
- 101100048967 Enterobacteria phage T4 uvsY gene Proteins 0.000 description 1
- 101800001466 Envelope glycoprotein E1 Proteins 0.000 description 1
- 108060002716 Exonuclease Proteins 0.000 description 1
- 102100034343 Integrase Human genes 0.000 description 1
- 240000007594 Oryza sativa Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- 238000012408 PCR amplification Methods 0.000 description 1
- 241000932843 Pantoea stewartii subsp. indologenes Species 0.000 description 1
- 239000001888 Peptone Substances 0.000 description 1
- 108010080698 Peptones Proteins 0.000 description 1
- 108020005120 Plant DNA Proteins 0.000 description 1
- 102000009609 Pyrophosphatases Human genes 0.000 description 1
- 108010009413 Pyrophosphatases Proteins 0.000 description 1
- 108010092799 RNA-directed DNA polymerase Proteins 0.000 description 1
- 102000001218 Rec A Recombinases Human genes 0.000 description 1
- 108010055016 Rec A Recombinases Proteins 0.000 description 1
- PYMYPHUHKUWMLA-LMVFSUKVSA-N Ribose Natural products OC[C@@H](O)[C@@H](O)[C@@H](O)C=O PYMYPHUHKUWMLA-LMVFSUKVSA-N 0.000 description 1
- 244000062793 Sorghum vulgare Species 0.000 description 1
- 244000046127 Sorghum vulgare var. technicum Species 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 108020000005 Sucrose phosphorylase Proteins 0.000 description 1
- 108010006785 Taq Polymerase Proteins 0.000 description 1
- GYDJEQRTZSCIOI-UHFFFAOYSA-N Tranexamic acid Chemical compound NCC1CCC(C(O)=O)CC1 GYDJEQRTZSCIOI-UHFFFAOYSA-N 0.000 description 1
- 101800001690 Transmembrane protein gp41 Proteins 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- JFVJZFMWJVSZNC-KVQBGUIXSA-N [[(2r,3s,5r)-5-(2,6-diaminopurin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] phosphono hydrogen phosphate Chemical compound C12=NC(N)=NC(N)=C2N=CN1[C@H]1C[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 JFVJZFMWJVSZNC-KVQBGUIXSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 125000003275 alpha amino acid group Chemical group 0.000 description 1
- HMFHBZSHGGEWLO-UHFFFAOYSA-N alpha-D-Furanose-Ribose Natural products OCC1OC(O)C(O)C1O HMFHBZSHGGEWLO-UHFFFAOYSA-N 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 238000000429 assembly Methods 0.000 description 1
- 230000000712 assembly Effects 0.000 description 1
- 235000015278 beef Nutrition 0.000 description 1
- 235000013361 beverage Nutrition 0.000 description 1
- 230000027455 binding Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000013068 control sample Substances 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 235000013399 edible fruits Nutrition 0.000 description 1
- 210000002257 embryonic structure Anatomy 0.000 description 1
- 108010052305 exodeoxyribonuclease III Proteins 0.000 description 1
- 102000013165 exonuclease Human genes 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 239000012678 infectious agent Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 239000002054 inoculum Substances 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 238000011901 isothermal amplification Methods 0.000 description 1
- 238000004898 kneading Methods 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 239000008176 lyophilized powder Substances 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 235000019713 millet Nutrition 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 239000003002 pH adjusting agent Substances 0.000 description 1
- VYNDHICBIRRPFP-UHFFFAOYSA-N pacific blue Chemical compound FC1=C(O)C(F)=C2OC(=O)C(C(=O)O)=CC2=C1 VYNDHICBIRRPFP-UHFFFAOYSA-N 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- GJVFBWCTGUSGDD-UHFFFAOYSA-L pentamethonium bromide Chemical compound [Br-].[Br-].C[N+](C)(C)CCCCC[N+](C)(C)C GJVFBWCTGUSGDD-UHFFFAOYSA-L 0.000 description 1
- 235000019319 peptone Nutrition 0.000 description 1
- 238000005191 phase separation Methods 0.000 description 1
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 230000008092 positive effect Effects 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 238000004080 punching Methods 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 239000011535 reaction buffer Substances 0.000 description 1
- 238000003753 real-time PCR Methods 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 238000010845 search algorithm Methods 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 108010068698 spleen exonuclease Proteins 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 230000000153 supplemental effect Effects 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- ABZLKHKQJHEPAX-UHFFFAOYSA-N tetramethylrhodamine Chemical compound C=12C=CC(N(C)C)=CC2=[O+]C2=CC(N(C)C)=CC=C2C=1C1=CC=CC=C1C([O-])=O ABZLKHKQJHEPAX-UHFFFAOYSA-N 0.000 description 1
- MPLHNVLQVRSVEE-UHFFFAOYSA-N texas red Chemical compound [O-]S(=O)(=O)C1=CC(S(Cl)(=O)=O)=CC=C1C(C1=CC=2CCCN3CCCC(C=23)=C1O1)=C2C1=C(CCC1)C3=[N+]1CCCC3=C2 MPLHNVLQVRSVEE-UHFFFAOYSA-N 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 238000011179 visual inspection Methods 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6888—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for detection or identification of organisms
- C12Q1/689—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for detection or identification of organisms for bacteria
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6844—Nucleic acid amplification reactions
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12R—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES C12C - C12Q, RELATING TO MICROORGANISMS
- C12R2001/00—Microorganisms ; Processes using microorganisms
- C12R2001/01—Bacteria or Actinomycetales ; using bacteria or Actinomycetales
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Analytical Chemistry (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Immunology (AREA)
- Molecular Biology (AREA)
- Biotechnology (AREA)
- Biophysics (AREA)
- Physics & Mathematics (AREA)
- Biochemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Abstract
The invention relates to the technical field of molecular biology, in particular to a method and a kit for detecting corn bacterial wilt bacteria. The invention can be used for improving the rapid monitoring and quarantine of the maize bacterial wilt bacteria in customs and basic laboratories. The detection sensitivity of the CLL001F/R primer was determined to be 10 cfu/. Mu.L by a real-time fluorescent RPA reaction. The detection results of the simulation seed tape bacteria and the blade needling inoculation result are ideal. Compared with other detection methods, the RPA detection has high sensitivity, short detection time, easy operation and higher sensitivity and specificity, and is suitable for on-site quick quarantine of corn bacterial wilt bacteria.
Description
Technical Field
The invention relates to the technical field of molecular biology, in particular to a method and a kit for detecting corn bacterial wilt bacteria.
Background
Bacterial fusarium wilt of cornPantoea stewartiisubsp.stewartiiPSS) is one of the infectious agents of the inbound plant, which is transmitted over a long distance mainly through seeds, and which causes bacterial wilt of corn, a disease on corn.
At present, PCR is widely applied to the rapid detection of PSS in imported corn, and a plurality of pairs of specific primers or probes have been reported. Due to PSS andP. stewartiisubsp. indologenesthe gene sequences of (PSI) are very similar, and a plurality of pairs of PSS specific primers can amplify PSI to cause false positive detection results, including primers adopted by PSS diagnosis standards of EPPO and primers adopted in national industry standard SN/T3756-2013 corn bacterial blight quarantine identification method PCR method.
Disclosure of Invention
According to the invention, based on a recombinase polymerase amplification technology (RPA), 12 pairs of PSS specific primers/probes are tested through 26 collected PSS and related strains thereof to evaluate the specificity of the primers/probes, so that a rapid detection method for corn bacterial wilt bacteria based on the RPA is finally established, and the requirements of rapid and accurate detection (on the port) are met.
One aspect of the invention relates to detection of bacterial blight germ of cornPantoea stewartiisubsp. stewartiiSubspecies based on recombinant polymerase amplification technology and used for exclusionP. stewartiisubsp.indologenesDetection of false yang caused by subspeciesThe method comprises the step of detecting the sample to be detected by adopting a primer pair shown in SEQ ID NO. 1 and SEQ ID NO. 2.
A further aspect of the invention relates to a method as described above for differentiating maize bacterial wilt bacteriaPantoea stewartiisubsp. stewartiiSubspecies andP. stewartiisubsp.indologenessubspecies, and the use thereof.
Yet another aspect of the invention relates to a method for detecting bacterial blight of cornPantoea stewartiisubsp. stewartiiA kit of subspecies comprising primers as shown in SEQ ID NO. 1 and SEQ ID NO. 2 and a probe.
The invention can be used for improving the rapid monitoring and quarantine of the maize bacterial wilt bacteria in customs and basic laboratories. The detection sensitivity of the CLL001F/R primer was determined to be 10 cfu/. Mu.L by a real-time fluorescent RPA reaction. The detection results of the simulation seed tape bacteria and the blade needling inoculation result are ideal. Compared with other detection methods, the RPA detection has high sensitivity, short detection time, easy operation and higher sensitivity and specificity, and is suitable for on-site quick quarantine of corn bacterial wilt bacteria.
Drawings
In order to more clearly illustrate the embodiments of the present invention or the technical solutions in the prior art, the drawings that are needed in the description of the embodiments or the prior art will be briefly described, and it is obvious that the drawings in the description below are some embodiments of the present invention, and other drawings can be obtained according to the drawings without inventive effort for a person skilled in the art.
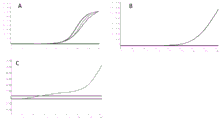
FIG. 1 is a primer-specific RPA assay; 26 pathogenic bacteria on corn are taken for identification, the specificity of the primer and the probe is determined to be very good, and all five positive strains are detected.
FIG. 2 shows the primer sensitivity RPA detection results; wherein the bacterial liquid is diluted in a gradient way, and the minimum detection concentration is 10 cfu/. Mu.L.
FIG. 3 is a simulated seed and leaf carrier RPA test;
A. simulate seed tape experimentsAfter washing the overnight activated bacteria, the OD600 was adjusted to 0.6 (about 10 8 cfu/ml), then soaking seeds for 6 hours by using bacterial liquid after gradient dilution, drying, adding 1ml of water to soak the seeds for 4 hours, then kneading, and taking 2 mu L to carry out RPA experiment;
B. corn leaf bacteria detection, needle spot CT value 19, and CT value 29.9 at a slightly distant place.
Detailed Description
Reference now will be made in detail to embodiments of the invention, one or more examples of which are described below. Each example is provided by way of explanation, not limitation, of the invention. Indeed, it will be apparent to those skilled in the art that various modifications and variations can be made to the present invention without departing from the scope or spirit of the invention. For example, features illustrated or described as part of one embodiment can be used on another embodiment to yield still a further embodiment.
Unless otherwise defined, all terms (including technical and scientific terms) used to describe the invention have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. By way of further guidance, the following definitions are used to better understand the teachings of the present invention. The terminology used herein in the description of the invention is for the purpose of describing particular embodiments only and is not intended to be limiting of the invention.
The term "and/or," "and/or," as used herein, includes any one of two or more of the listed items in relation to each other, as well as any and all combinations of the listed items in relation to each other, including any two of the listed items in relation to each other, any more of the listed items in relation to each other, or all combinations of the listed items in relation to each other. It should be noted that, when at least three items are connected by a combination of at least two conjunctions selected from "and/or", "or/and", "and/or", it should be understood that, in this application, the technical solutions certainly include technical solutions that all use "logical and" connection, and also certainly include technical solutions that all use "logical or" connection. For example, "a and/or B" includes three parallel schemes A, B and a+b. For another example, the technical schemes of "a, and/or B, and/or C, and/or D" include any one of A, B, C, D (i.e., the technical scheme of "logical or" connection), and also include any and all combinations of A, B, C, D, i.e., any two or three of A, B, C, D, and also include four combinations of A, B, C, D (i.e., the technical scheme of "logical and" connection).
The terms "comprising," "including," and "comprising," as used herein, are synonymous, inclusive or open-ended, and do not exclude additional, unrecited members, elements, or method steps.
The recitation of numerical ranges by endpoints of the present invention includes all numbers and fractions subsumed within that range, as well as the recited endpoint.
Concentration values are referred to in this invention, the meaning of which includes fluctuations within a certain range. For example, it may fluctuate within a corresponding accuracy range. For example, 2%, may allow fluctuations within + -0.1%. For values that are larger or do not require finer control, it is also permissible for the meaning to include larger fluctuations. For example, 100mM, fluctuations in the range of.+ -. 1%,.+ -. 2%,.+ -. 5%, etc. can be tolerated. Molecular weight is referred to, allowing its meaning to include fluctuations of + -10%.
In the present invention, the terms "plurality", and the like refer to, unless otherwise specified, 2 or more in number.
In the invention, the technical characteristics described in an open mode comprise a closed technical scheme composed of the listed characteristics and also comprise an open technical scheme comprising the listed characteristics.
In the present invention, "preferred", "better", "preferred" are merely embodiments or examples which are better described, and it should be understood that they do not limit the scope of the present invention. In the present invention, "optional" means optional or not, that is, means any one selected from two parallel schemes of "with" or "without". If multiple "alternatives" occur in a technical solution, if no particular description exists and there is no contradiction or mutual constraint, then each "alternative" is independent.
All documents mentioned in this application are incorporated by reference as if each were individually incorporated by reference. Unless otherwise contradicted by purpose and/or technical solution of the present application, the cited documents related to the present invention are incorporated by reference in their entirety for all purposes. When reference is made to a cited document in the present invention, the definitions of the relevant technical features, terms, nouns, phrases, etc. in the cited document are also incorporated. In the case of the cited documents, examples and preferred modes of the cited relevant technical features are incorporated into the present application by reference, but are not limited to the embodiments that can be implemented. It should be understood that when a reference is made to the description herein, it is intended to control or adapt the present application in light of the description herein.
The first aspect of the invention relates to the detection of bacterial blight germ of cornPantoea stewartiisubsp.stewartiiSubspecies based on recombinant polymerase amplification technology and used for exclusionP. stewartiisubsp.indologenesThe method comprises the step of detecting a sample to be detected by using primer pairs shown in SEQ ID NO. 1 and SEQ ID NO. 2.
In some embodiments, the method further employs a probe as shown below:
CATGGTGTCGTATTTTAGGTAAATAAGTTGTTTAG [ luminophore ] [ abasic nucleotide analog ] GT [ quencher ] TTTTTTCGCCATGCCG [ blocker ];
the blocking agent is used to block polymerase extension of the probe.
In some embodiments, the abasic nucleotide analog is tetrahydrofuran. It can act as a recognition site for exonucleases.
In some embodiments, the blocking agent is selected from a spacer, a phosphate group, biotin-TEG, or an amine (e.g., C6 amine).
In some embodiments, the arms are selected from any of ethylene glycol, C9 arms (Spacer 9), C18 arms (Spacer 18), dideoxy arms [1',2' -Dideoxyribose (dSpacer) ], C3 arms (C3 Spacer).
In some embodiments, the spacer is selected from a C3 spacer.
The Spacer (Spacer) can provide the necessary spacing for oligonucleotide labeling to reduce interactions between the labeling groups and the oligonucleotides, and is mainly used in DNA hairpin structure and double-stranded structure studies. C3 The spacer is used primarily to mimic the three carbon spacing between the 3 'and 5' hydroxyl groups of ribose, or "substitute" for an unknown base in a sequence. 3'-Spacer C3 is used to introduce a 3' Spacer to prevent the 3 'exonuclease and 3' polymerase from acting.
In some embodiments, the luminescent group is selected from any one of AMCA, pacific Blue, atto 425, BODIPY FL, FAM, alexa Fluor 488, TET, JOE, yakima Yellow, VIC, HEX, quasar 570, cy3, NED, TAMRA, ROX, aqua phoor 593, texas Red, atto 590, cy5, quasar 670, cy5.5, and Cy 5.5.
In some embodiments, the quenching group is selected from any of BHQ1, BHQ2, BHQ3, dabcyl, eclipse, and MGB.
In some embodiments, the luminescent group is FAM and the quenching group is BHQ1.
In one aspect, useful primers and probes include nucleotide sequences that are greater than 60%, 65%, 70%, 75%, 80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98% or 99% identical to the upstream primer, downstream primer or probe of the specific sequences provided above. Such primer and probe modifications are also contemplated and can be prepared according to standard techniques.
The term "% identity" in the context of two or more nucleotide sequences or amino acid sequences refers to two or more sequences or subsequences that are the same or have a specified percentage of amino acid residues or nucleotides that are the same, when compared and aligned for maximum correspondence, as measured using one of the following sequence comparison algorithms or by visual inspection. For example,% identity is the entire length of the coding region relative to the sequences to be compared.
For sequence comparison, typically one sequence is used as a reference sequence, and the test sequence is compared to that sequence. When using a sequence comparison algorithm, the test sequence and reference sequence are input into a computer, subsequence coordinates are designated, if necessary, and sequence algorithm program parameters are designated. The sequence comparison algorithm then calculates the percent sequence identity of the test sequence relative to the reference sequence based on the specified program parameters. The percent identity can be determined using search algorithms such as BLAST and PSI-BLAST (Altschul et al, 1990, J Mol Biol 215:3, 403-410; altschul et al, 1997, nucleic Acids Res 25:17, 3389-402).
The primer and probe modification may be performed by a known method. Modified versions of these primer and/or probe sequences can include, by way of non-limiting example, adding one or more nucleotides to the 5 'end, one or more nucleotides to the 3' end, one or more nucleotides to the 5 'and 3' ends, adding tails, shortening the sequence, extending the sequence, shifting the sequence several bases upstream and downstream, or any combination thereof. In addition to the modifications already mentioned above, other modifications such as 3'P, 5'P, 5-nitroindole, 2-aminopurine, 8-amino-2 ' -deoxyadenosine, C-5 propynyl-deoxycytidine, C-5 propynyl-deoxyuridine, 2-amino-2 ' -deoxyadenosine-5 ' -triphosphate, 2, 6-diaminopurine (2-amino-dA), inverted dT, inverted dideoxy-T, hydroxymethyl dC, iso-dC, 5-methyl dC, aminoethyl-phenoxazine-deoxycytidine, and locked nucleic acids (LNA's) may be applied without significantly adversely affecting the function of the primer, and include at least one mismatched base at one of the bases, or at least one of the bases is replaced with an RNA base, to effect, for example, a nucleic acid interaction that increases the 3' end of the mutant specific primer to increase Tm. The addition of double-stranded stable base modifications has a positive effect on PCR, enabling it to be performed at higher temperatures, within which Taq polymerase is known to exhibit maximum activity. The modified probe should retain the ability to distinguish between the mutation site to be detected and the wild-type site.
The sample to be tested for use in the present invention may be any sample suspected of containing corn pathogen. In some embodiments, the sample to be tested comprises at least one of a corn sample, a soil sample, and a water sample (particularly a water source and soil near a corn planting environment).
As used herein, "corn sample" refers to a solid, viscous or liquid substance or formulation, typical examples of which may include cuttings, roots, leaves, stems, flowers, pollen, embryos, anthers, seeds, fruits or other parts of rice.
The corn sample may be subjected to conventional treatments such as grinding, compacting, degrading, drying, wetting, etc., in advance, provided that the treatment does not significantly interfere with the detection of the pathogenic bacteria.
In some embodiments, the method of detecting maize bacterial wilt further comprises isolating DNA (particularly genomic DNA) from a component suspected of containing the pathogenic bacteria. As used herein, the term "isolated" refers to: (1) substantially or essentially free of components that normally accompany or interact with the material in a naturally occurring environment, (2) substantially or essentially free of components of the material in a concomitantly or interacted processed form, such as in a beverage or food product or from substances used in the separation process, or (3) if the material is in its natural environment, the material has been altered and/or placed in a cell at a site different from the site inherent to the material by deliberate human intervention of the composition. When the term "substantially purified" is used, the designation will refer to a composition in which the protein or peptide forms the major component of the composition, e.g., about 50%, about 60%, about 70%, about 80%, about 90%, about 95% or more (i.e., e.g., weight/weight and/or weight/volume) of the composition. As used herein, the term "substantially purified" refers to nucleic acid molecules (particularly DNA) that are removed, isolated or separated from their natural environment or plant, and are at least 60% free, preferably 75% free, more preferably 90% free of other components with which they are naturally associated.
Isolation of the plant DNA fragments from the sample to be tested may involve the use of an isolation solvent such as methanol, ethanol, water, acetone or a combination thereof. In some embodiments, kits of DNA isolation kits may be used, including, for example, DNA isolation protocols using the Dneasy Mericon food kit (Qiagen, germanten, MD, USA) or cetyl trimethylammonium bromide (CTAB). Other separation techniques include lysis, heating, alcohol precipitation, salt precipitation, organic separation, solid phase separation, silica crude membrane separation, CSCL gradient purification, or any combination thereof. In some embodiments, the DNA in the sample to be detected is extracted and purified using the purification reagents described above.
A second aspect of the invention relates to a method as described above for differentiating maize bacterial wilt bacteriaPantoea stewartiisubsp. stewartiiSubspecies andP. stewartiisubsp.indologenessubspecies, and the use thereof.
A third aspect of the present invention relates to a method for detecting bacterial blight of cornPantoea stewartiisubsp. stewartiiA kit of subspecies comprising primers as defined in SEQ ID No. 1 and SEQ ID No. 2 and a probe as defined in the first aspect of the invention.
The term "kit" refers to any article of manufacture (e.g., package or container) comprising at least one device, which may further comprise instructions, supplemental reagents, and/or components or assemblies for use in the methods described herein or steps thereof.
In some embodiments, the kit further comprises one or more of a nucleic acid extraction reagent, a reagent for isothermal nucleic acid amplification, a positive control, and a negative control.
In some embodiments, the reagents used for isothermal nucleic acid amplification include one or more of a recombinase, a single-stranded DNA binding protein, a strand-displacement DNA polymerase, an accessory protein, an exonuclease III, a reverse transcriptase, ATP, reagents for ATP regeneration systems, pH adjusters, dntps, BSA and/or PEG of various molecular weight distributions, DTT, and water, which bind to single-stranded nucleic acids;
wherein the auxiliary protein is used for changing the reversible reaction process of dissociation and recombination of the recombinase-primer complex, so that the reaction is more favorable for isothermal nucleic acid amplification.
The pH adjuster may comprise acids and bases that do not significantly affect the progress of the reaction, as well as buffer components (e.g., tris and acetate, etc.). Further, the Tris buffer is Tris-tricine, which may be present at a working concentration of about 80mM to 120mM.
In some embodiments, the recombinase is selected from uvsX and/or RecA;
in some embodiments, the single-stranded DNA binding protein is gp32;
in some embodiments, the strand displacement DNA polymerase is selected from BSu DNA polymerase and/or Sau DNA polymerase. The DNA polymerase used in the recombinase-mediated isothermal amplification of nucleic acids is bacillus subtilis DNA polymerase I (Bacillus subtilis Pol I, bsu) or staphylococcus aureus (Staphylococcus aureus Pol I, sau), both of which belong to the DNA polymerase I family. The DNA polymerase I family is a polymerase responsible for repair of lesions during DNA replication, and most of the DNA polymerases in this family have low continuous synthesis capacity, i.e., the polymerase in this family has a small number of polymerization reactions that can be catalyzed by a single binding of the polymerase to a template.
In some embodiments, the accessory protein is selected from uvsY;
in the case where a recombinase is used for the strand insertion step, the system may require an energy source. Most of these enzymes utilize ATP as an energy source, but because of the magnesium ion necessary for ATP-finishing (collate) enzyme activity, it is advantageous to provide an additional ATP regeneration system rather than to increase the concentration of ATP. In some embodiments, the reagent used in the ATP regeneration system is selected from one or more of magnesium ion, creatine phosphate and its counter ion, creatine kinase, pyrophosphatase, sucrose, and sucrose phosphorylase.
From the above components, the kits of the present invention may employ and preferably employ methods of Recombinase polymerase amplification (Recombinase Polymerase Amplification, RPA), but may also employ improved methods over this technology, such as Recombinase-dependent amplification (RDA).
In some embodiments, the reagents used for isothermal nucleic acid amplification are lyophilized powder reagents or mixed liquid reagents.
The components are preferably realized in lyophilized form, for example in the form of one or more so-called lyophilized beads. Lyophilization beads are generally understood to mean lyophilisates which are pressed into spheres after manufacture, after which the substance is usually present as a powder. Thus, the components required for the amplification reaction, in particular the various enzymes, nucleic acid components and reaction buffer components, may be provided in lyophilized form. In this way, the amplification process can be started directly in a very user-friendly manner by adding the sample to be quantified and optionally other desired components. In particular, the provision of a lyophilized form is very advantageous for automated applications.
Embodiments of the present invention will be described in detail below with reference to examples. It is to be understood that these examples are illustrative of the present invention and are not intended to limit the scope of the present invention. The experimental methods in the following examples, in which specific conditions are not noted, are preferably referred to in the guidelines given in the present invention, and may be according to the experimental manuals or conventional conditions in the art, and may be referred to other experimental methods known in the art, or according to the conditions suggested by the manufacturer.
In the specific examples described below, the measurement parameters relating to the raw material components, unless otherwise specified, may have fine deviations within the accuracy of weighing. Temperature and time parameters are involved, allowing acceptable deviations from instrument testing accuracy or operational accuracy.
Examples
1 materials and methods
1.1 materials
Test strain: the 26 strains of the strain are collected together, and the strain comprises 5 PSS, 2 PSI and 19 other strains. Wherein 5 PSS, 2 PSI and related species are confirmed by adopting a 16S sequencing method, and strain information is shown in Table 1.
TABLE 1 Strain information
Culture medium: NA medium (peptone 10 g, beef extract 3g, sodium chloride 5 g, agar powder 15 g, distilled water 1L).
Reagent: bacterial genomic DNA extraction kits were purchased from the root biochemistry technologies (beijing) limited company; DL2000 DNA marker, 2X Taq PCR Master Mix, premix Ex Taq (Probe qPCR) was purchased from TaKaRa, japan; the rest reagents are all made or imported analytically pure.
Instrument: t100 PCR instrument, bio-rad, USA; real-time fluorescence PCR instrument of Quan Studio 5, ABI company, USA; geldoc XR gel imaging System, bio-rad, inc., america, anpu future RPA rapid detector.
1.2 primer/Probe
The test primers/probes included 12 pairs of specific primers/probes for detecting PSS reported in the literature (table 2), which were synthesized by hua major genes limited company.
TABLE 2 primer/probe sequences
1.3DNA extraction
Purifying the strain to be tested on NA culture medium, taking single colony for propagation, eluting with sterile water, centrifuging the bacterial suspension, extracting bacterial genome DNA from the precipitate by using a bacterial genome DNA extraction kit, and preserving at-20 ℃ for later use.
1.4 primer test
The tested 26 strain DNA was tested with 12 pairs of primers, respectively, and the reaction procedure was referred to its original literature. The PCR amplified products were analyzed by agarose gel electrophoresis at 1.2%.
1.5 sensitivity test
2 pairs of specific primers CLL001F/R and cpsAB2313F/cpsR were selected by test, genomic DNA of strain 29277 was subjected to 10-fold serial dilutions at DNA concentrations of 50 ng/. Mu.L, 5 ng/. Mu.L, 0.5 ng/. Mu.L, 0.05 ng/. Mu.L, 0.005 ng/. Mu.L, 0.5 pg/. Mu.L, 0.05 pg/. Mu.L, 0.005 pg/. Mu.L and 0.0005 pg/. Mu.L, respectively, PCR amplification was performed with the above 2 pairs of primers, 2. Mu.L of DNA template was added, and the reaction procedure was as in the original document. The PCR amplified products were analyzed by 1% agarose gel electrophoresis.
1.6 simulation seed and leaf area detection
Taking PSS strain29277 preparation 10 8 And (3) carrying out gradient dilution on CFU/mL bacterial suspension, taking 1mL bacterial suspension into 6 corn seeds, uniformly mixing, naturally airing to serve as an artificial bacteria sample, and then extracting DNA after soaking treatment for RPA experiments. Meanwhile, 10≡8 CFU/mL bacterial suspension of the strain 29277 is inoculated with tender leaves by a needle punching method, and after two weeks of water injection as negative control, the disease condition is observed, and RPA experiments are carried out. The primers cpsAB2313F/cpsR and cpsF/cpsR were used to detect the above sample DNA, respectively, and the primer CLL001F/R and probe were also used for RPA detection.
2 results and analysis
2.1 primer specificity test
The genomic DNA of 26 strains was tested by PCR or qPCR using 12 pairs of specific primer/probe pairs, and the test results showed that the primers CLL001F/R and cpsAB2313F/cpsR were both positive for the tested PSS and negative for the other test strains, and the specificity of 2 pairs of primers was superior to that of the other test primers/probes (Table 3). The primer cpsAB2313F/cpsR has weak nonspecific amplification when part of strains are amplified, but the size of a nonspecific band is far different from that of a target band, and the judgment of the result is not influenced.
TABLE 3 primer/probe detection results
Note that: positive for "+"; "-" is negative;
"-" indicates that the result is negative, but that there is non-specific amplification, which does not affect the judgment of the result.
Experimental results show that 10 pairs of test primers have different degrees of false positive or weak false positive amplification, and the strain which is easy to cause the false positive or weak false positive amplification mainly comprises PSI,P. stewartii、P. alliiEtc. (Table 3). Primer cpsF/cpsR can amplify 4 strains of PSI and 3 strainsP. stewartiiAn unexpected band of 193 bp was obtained, whereas the band size upon expansion of PSS was 375 bp. Sequencing results of amplified products are shown (data not shown) due to PSI andP. stewartiithe amplified region lacks 182 bases, resulting in yieldThe sizes of the substances differ by 182bp. In addition, the primer can be used for nonspecific amplificationP. alliiThe amplified band is very close to the target band (only a fraction slightly larger than the target band), which easily affects the outcome determination.
2.2 sample-specific and sensitive RPA detection
The screened primer CLL001F/R and the probe are used for carrying out RPA specificity detection on 26 strains, and experimental results show that the primer and the probe have strong specificity, the corresponding data of A, B, C are shown in the table 4, the table 5 and the table 6 in the figure 1, and PSS and other strains can be successfully distinguished. Sensitivity tests of the primers CLL001F/R and probes were performed using genomic DNA series gradient concentrations of strain 29277 at 50 ng/. Mu.L, 5 ng/. Mu.L, 0.5 ng/. Mu.L, 0.05 ng/. Mu.L, 0.005 ng/. Mu.L, 0.5 pg/. Mu.L, 0.05 pg/. Mu.L, 0.005 pg/. Mu.L, 0.0005 pg/. Mu.L. The results of the RPA experiments showed that the lowest concentration of DNA detected by the RPA method was 0.0005 pg/. Mu.L (FIG. 2, see Table 7 for corresponding data).
TABLE 4 Table 4
TABLE 5
TABLE 6
TABLE 7
2.3 analog seed sample detection with bacteria
The invention prepares 6 parts of corn seeds with different bacterial loads (inoculum size is 1 multiplied by 10) 8 ~1×10 2 CFU/ml), RPA experiments using CLL001F/R and probes showed a detection limit of 1X 10 for seed samples 2 CFU/ml, i.e. in a single reaction system2 cells (see table 8 for corresponding data in fig. 3 a) can be detected. After the young corn leaves are subjected to needling inoculation, sampling is carried out after the occurrence of the disease is confirmed, DNA is extracted, RPA detection is carried out, the CT value of a sample at an inoculation position is found to be 19.3, the same piece is about 27 a little away, the sample is positive, the result of the control sample is negative (B in fig. 3, corresponding data are shown in table 9).
TABLE 8
TABLE 9
Discussion of the invention
At presentP. stewartiiThe subspecies have 2 subspecies, namely PSI and PSS, and the genetic relationship among subspecies is similar, but PSI is not pathogenic to corn, and can cause millet to be treatedSetaria italica) Andpennisetum amer- icanumleaf spot and pineapple of (a)Ananas comosus) Is decomposed. Based on the belief of the learnerP. stewartiiCan be separated into 2 different species. Because of the high sequence similarity of these 2 subspecies, many PSS specific primers can amplify PSI, thereby resulting in false positive amplification, affecting the accuracy of the detection results. Wang, etc [1] 5 pairs of reported PSS specific primers are verified by 39 strains, and 3 pairs of primers can be found to amplify PSI; block et al [2] 9 pairs of reported primers are verified, and the false positive amplification phenomena with different degrees are found; pal et al [3] 8 pairs of reported primers were verified and each of these 8 pairs of primers was found to amplify PSI. Based on previous researches, 26 strains including similar strains separated from corn seed samples are collected, the specificity of 18 pairs of primers reported at present is evaluated, the test results are consistent with the previous researches, most of the tested primers have false positive amplification, and only the DC283galE/DC283galEc and cpsAB2313F/cpsR primers have good specificity.
[1] Wang Ying, zhou Guoliang, yin Liping, et al, maize bacterial wilt bacteria PCR detection, plant pathology journal, 2009, 39 (4): 368-376.
[2] Block C C,Shepherd L M,Munkvold G. Comparison of nine PCR primer sets designed to detect Pantoea stewartii subsp. stewartii in maize[EB/OL].(2021-06-06)[2021-06-07].https://www.ars.usda.gov/research/publications/publication/?seqNo115=266266.
[3] Pal N,Block C C,Gardner C A G. A real-time PCR differentiation Pantoea stewartii subsp. stewartii from P.stewartii subsp. indologenes in corn seed. Plant Disease,2019,103(7):1474-1486.
The above examples illustrate only a few embodiments of the invention, which are described in detail and are not to be construed as limiting the scope of the invention. It should be noted that it will be apparent to those skilled in the art that several variations and modifications can be made without departing from the spirit of the invention, which are all within the scope of the invention. The scope of the invention is therefore intended to be covered by the appended claims, and the description and drawings may be interpreted in accordance with the contents of the claims.
Claims (10)
1. Detection of bacterial fusarium wilt of cornPantoea stewartii subsp. stewartiiSubspecies based on recombinant polymerase amplification technology and used for exclusionP. stewartii subsp. indologenesThe method comprises the step of detecting a sample to be detected by using primer pairs shown in SEQ ID NO. 1 and SEQ ID NO. 2.
2. The method of claim 1, further employing a probe as follows:
CATGGTGTCGTATTTTAGGTAAATAAGTTGTTTAG [ luminophore ] [ abasic nucleotide analog ] GT [ quencher ] TTTTTTCGCCATGCCG [ blocker ];
the blocking agent is used to block polymerase extension of the probe.
3. The method of claim 2, wherein the abasic nucleotide analog is tetrahydrofuran.
4. The method of claim 2, wherein the blocking agent is a spacer.
5. The method of claim 4, wherein the arm is a C3 arm.
6. The method of claim 2, wherein the luminescent group is FAM and the quenching group is BHQ1.
7. The method of any one of claims 1-6, wherein the sample to be tested comprises at least one of a corn sample, a soil sample, and a water sample.
8. The method of any one of claims 1 to 7 for differentiating maize bacterial wilt bacteriaPantoea stewartii subsp. stewartiiSubspecies andP. stewartii subsp. indologenessubspecies, and the use thereof.
9. Is used for detecting bacterial fusarium wilt of cornPantoea stewartii subsp. stewartiiA kit of subspecies comprising primers as defined in any one of claims 2 to 6 as set forth in SEQ ID No. 1 and SEQ ID No. 2.
10. The kit of claim 9, further comprising one or more of a nucleic acid extraction reagent, a reagent for isothermal nucleic acid amplification, a positive control, and a negative control.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202310525899.8A CN116287354A (en) | 2023-05-11 | 2023-05-11 | Method and kit for detecting bacterial fusarium wilt of corn |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202310525899.8A CN116287354A (en) | 2023-05-11 | 2023-05-11 | Method and kit for detecting bacterial fusarium wilt of corn |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN116287354A true CN116287354A (en) | 2023-06-23 |
Family
ID=86799833
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202310525899.8A Pending CN116287354A (en) | 2023-05-11 | 2023-05-11 | Method and kit for detecting bacterial fusarium wilt of corn |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN116287354A (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116926235A (en) * | 2023-09-18 | 2023-10-24 | 三亚中国检科院生物安全中心 | Fusarium RPA-CRISPR/Cas detection kit and method |
| CN116970735A (en) * | 2023-09-22 | 2023-10-31 | 三亚中国检科院生物安全中心 | Rapid detection method and kit for maize rust recombinant polymerase |
| CN118581250A (en) * | 2024-08-07 | 2024-09-03 | 三亚中国检科院生物安全中心 | Primer pair and method for rapid detection of corn wilt pathogen |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101724692A (en) * | 2009-12-31 | 2010-06-09 | 浙江大学 | Primer and diagnosis method used for diagnosing mulberry bacterial wilt disease |
| KR20120107056A (en) * | 2012-08-13 | 2012-09-28 | 강원대학교산학협력단 | Composition for identifying pantoea stewartii subsp. stewartii and use thereof |
| CN105784996A (en) * | 2014-12-21 | 2016-07-20 | 尚杰 | Quarantine identification method of corn bacterial wilt disease |
| CN109504788A (en) * | 2018-10-18 | 2019-03-22 | 中检国研(北京)科技有限公司 | A kind of P.stwartii subsp.stewartii ring mediated isothermal amplification test strips detection method |
| CN112280876A (en) * | 2020-10-20 | 2021-01-29 | 舟山海关综合技术服务中心 | Primers, probes, kits and applications for detection of recombinase-mediated isothermal amplification |
| CN112586509A (en) * | 2020-11-13 | 2021-04-02 | 遵义医科大学 | Compound microorganism effervescent tablet for preventing tobacco bacterial wilt and promoting growth, and preparation method and application thereof |
| CN113144002A (en) * | 2021-03-15 | 2021-07-23 | 北京亿康倍尔生物科技有限公司 | Probiotic composition for maintaining oral health and application thereof |
| CN114958835A (en) * | 2022-05-25 | 2022-08-30 | 三亚中国检科院生物安全中心 | Combination product and kit for detecting bacterial rice blight bacteria |
-
2023
- 2023-05-11 CN CN202310525899.8A patent/CN116287354A/en active Pending
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101724692A (en) * | 2009-12-31 | 2010-06-09 | 浙江大学 | Primer and diagnosis method used for diagnosing mulberry bacterial wilt disease |
| KR20120107056A (en) * | 2012-08-13 | 2012-09-28 | 강원대학교산학협력단 | Composition for identifying pantoea stewartii subsp. stewartii and use thereof |
| CN105784996A (en) * | 2014-12-21 | 2016-07-20 | 尚杰 | Quarantine identification method of corn bacterial wilt disease |
| CN109504788A (en) * | 2018-10-18 | 2019-03-22 | 中检国研(北京)科技有限公司 | A kind of P.stwartii subsp.stewartii ring mediated isothermal amplification test strips detection method |
| CN112280876A (en) * | 2020-10-20 | 2021-01-29 | 舟山海关综合技术服务中心 | Primers, probes, kits and applications for detection of recombinase-mediated isothermal amplification |
| CN112586509A (en) * | 2020-11-13 | 2021-04-02 | 遵义医科大学 | Compound microorganism effervescent tablet for preventing tobacco bacterial wilt and promoting growth, and preparation method and application thereof |
| CN113144002A (en) * | 2021-03-15 | 2021-07-23 | 北京亿康倍尔生物科技有限公司 | Probiotic composition for maintaining oral health and application thereof |
| CN114958835A (en) * | 2022-05-25 | 2022-08-30 | 三亚中国检科院生物安全中心 | Combination product and kit for detecting bacterial rice blight bacteria |
Non-Patent Citations (3)
| Title |
|---|
| I GEHRING 等: "Molecular differentiation of Pantoea stewartii subsp. indologenes from subspecies stewartii and identification of new isolates from maize seeds", 《J APPL MICROBIOL》, vol. 116, no. 6, pages 1553 - 1562 * |
| 单长林 等: "玉米细菌性枯萎病菌荧光重组酶介导等温扩增检测方法的建立与应用", 《广东农业科学》, vol. 48, no. 1, pages 112 * |
| 吴琼,陈枝楠,范怀忠,金显忠: "16S nested-PCR技术检测玉米细菌性枯萎病菌(英文)", 植物病理学报, no. 05 * |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116926235A (en) * | 2023-09-18 | 2023-10-24 | 三亚中国检科院生物安全中心 | Fusarium RPA-CRISPR/Cas detection kit and method |
| CN116926235B (en) * | 2023-09-18 | 2023-12-05 | 三亚中国检科院生物安全中心 | Fusarium RPA-CRISPR/Cas detection kit and method |
| CN116970735A (en) * | 2023-09-22 | 2023-10-31 | 三亚中国检科院生物安全中心 | Rapid detection method and kit for maize rust recombinant polymerase |
| CN116970735B (en) * | 2023-09-22 | 2024-01-19 | 三亚中国检科院生物安全中心 | Rapid detection method and kit for maize rust recombinant polymerase |
| CN118581250A (en) * | 2024-08-07 | 2024-09-03 | 三亚中国检科院生物安全中心 | Primer pair and method for rapid detection of corn wilt pathogen |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN116287354A (en) | Method and kit for detecting bacterial fusarium wilt of corn | |
| JP2005504508A5 (en) | ||
| CN111979303A (en) | Nucleic acid detection kit, method and application thereof | |
| CN114350828B (en) | A specific primer for amplifying Pantoea pineapple and its application | |
| JP2013500008A (en) | Sequences for the detection and characterization of E. coli O157: H7 and their use | |
| CN114075607B (en) | On-site visualization kit for detecting listeria monocytogenes based on SHERLOCK and application | |
| KR20180053743A (en) | Improved detection of short homopolymeric repeat sequences | |
| CN104641000A (en) | Compositions and methods for detection of clostridium difficile | |
| CN114958835B (en) | Combined product and kit for detecting bacterial rhizoctonia cerealis of rice | |
| CN113913498A (en) | Method for detecting target mutation based on CRISPR technology | |
| CN112522375A (en) | Detection kit and detection method for gene mutation of folate metabolism related molecular marker | |
| CN109735645B (en) | Real-time fluorescent PCR (polymerase chain reaction) primer, probe and kit for detecting Sporothrix globosum | |
| CN113862394B (en) | RPA detection method for tomato infertility virus | |
| CN115029470A (en) | Combination product and kit for detection of bacterial blight of rice | |
| US20060088849A1 (en) | Compositions and methods for the diagnosis of group B streptococcus infection | |
| JPH10210980A (en) | Oligonucleotide for detecting lactic acid bacteria and method for detecting the bacteria | |
| CN116200514B (en) | Primer probe combination product for detecting rice pathogenic bacteria and application thereof | |
| CN117947195B (en) | One-step CRISPR/Cas12b detection kit and method for detecting Salmonella | |
| AU2020277502A1 (en) | Methods for detection of rare DNA sequences in fecal samples | |
| CN118048478B (en) | Specific detection target Dv_1135 of blueberry fruit rot and application thereof | |
| CN117947194B (en) | Indiana salmonella molecular detection method and kit | |
| CN115044649B (en) | Improved method for detecting target nucleic acid based on CRISPR technology | |
| CN112301151B (en) | RDA method and kit for rapidly detecting Canine Distemper Virus (CDV) | |
| WO2012003412A2 (en) | Inducible nucleic acid targets for detection of pathogens, methods and compositions thereof | |
| Chen et al. | Rapid detection of multiple phytoplasma with an All-In-One Dual (AIOD) CRISPR assay |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| RJ01 | Rejection of invention patent application after publication |
Application publication date: 20230623 |
|
| RJ01 | Rejection of invention patent application after publication |