CN114560854A - Synthetic method of aromatic amino pyrimidine azole compound - Google Patents
Synthetic method of aromatic amino pyrimidine azole compound Download PDFInfo
- Publication number
- CN114560854A CN114560854A CN202210127744.4A CN202210127744A CN114560854A CN 114560854 A CN114560854 A CN 114560854A CN 202210127744 A CN202210127744 A CN 202210127744A CN 114560854 A CN114560854 A CN 114560854A
- Authority
- CN
- China
- Prior art keywords
- unsubstituted
- substituted
- formula
- hydrogen
- aromatic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- -1 aromatic amino pyrimidine azole compound Chemical class 0.000 title claims abstract description 63
- 238000010189 synthetic method Methods 0.000 title claims description 4
- 230000015572 biosynthetic process Effects 0.000 claims abstract description 27
- 238000003786 synthesis reaction Methods 0.000 claims abstract description 27
- 238000005576 amination reaction Methods 0.000 claims abstract description 24
- 238000000034 method Methods 0.000 claims abstract description 23
- 150000001875 compounds Chemical class 0.000 claims abstract description 20
- 150000004982 aromatic amines Chemical class 0.000 claims abstract description 14
- 239000001257 hydrogen Substances 0.000 claims description 34
- 229910052739 hydrogen Inorganic materials 0.000 claims description 34
- 150000002431 hydrogen Chemical class 0.000 claims description 28
- 125000000217 alkyl group Chemical group 0.000 claims description 27
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 claims description 26
- 125000003118 aryl group Chemical group 0.000 claims description 20
- 229910052736 halogen Inorganic materials 0.000 claims description 18
- 150000002367 halogens Chemical class 0.000 claims description 18
- 125000000623 heterocyclic group Chemical group 0.000 claims description 15
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 claims description 11
- 125000003860 C1-C20 alkoxy group Chemical group 0.000 claims description 8
- 125000003545 alkoxy group Chemical group 0.000 claims description 8
- 125000006615 aromatic heterocyclic group Chemical group 0.000 claims description 8
- 239000002904 solvent Substances 0.000 claims description 8
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 claims description 7
- 239000000460 chlorine Substances 0.000 claims description 7
- 229910052801 chlorine Inorganic materials 0.000 claims description 7
- 125000001931 aliphatic group Chemical group 0.000 claims description 6
- 150000005698 chloropyrimidines Chemical class 0.000 claims description 6
- 230000008569 process Effects 0.000 claims description 6
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical group [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 claims description 5
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical group [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 5
- 238000005660 chlorination reaction Methods 0.000 claims description 5
- 229910052760 oxygen Inorganic materials 0.000 claims description 5
- 239000001301 oxygen Substances 0.000 claims description 5
- 239000011593 sulfur Chemical group 0.000 claims description 5
- 229910052717 sulfur Chemical group 0.000 claims description 5
- 125000000027 (C1-C10) alkoxy group Chemical group 0.000 claims description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 4
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 claims description 4
- YBBRCQOCSYXUOC-UHFFFAOYSA-N sulfuryl dichloride Chemical compound ClS(Cl)(=O)=O YBBRCQOCSYXUOC-UHFFFAOYSA-N 0.000 claims description 4
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 claims description 4
- 125000001624 naphthyl group Chemical group 0.000 claims description 3
- 125000005561 phenanthryl group Chemical group 0.000 claims description 3
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 3
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 claims description 2
- 125000005428 anthryl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C3C(*)=C([H])C([H])=C([H])C3=C([H])C2=C1[H] 0.000 claims 1
- 238000006243 chemical reaction Methods 0.000 abstract description 14
- 230000002194 synthesizing effect Effects 0.000 abstract description 9
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 abstract description 8
- 239000000758 substrate Substances 0.000 abstract description 6
- 239000003054 catalyst Substances 0.000 abstract description 5
- 229910052763 palladium Inorganic materials 0.000 abstract description 4
- 238000007086 side reaction Methods 0.000 abstract description 4
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 12
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 12
- 125000004432 carbon atom Chemical group C* 0.000 description 12
- 125000000753 cycloalkyl group Chemical group 0.000 description 10
- 229960000583 acetic acid Drugs 0.000 description 9
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 8
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 8
- 125000005842 heteroatom Chemical group 0.000 description 6
- HBWKAAMMLXPLGV-UHFFFAOYSA-N CC1=C(C(C(OC)=N2)=CN=C2Cl)ON=C1 Chemical compound CC1=C(C(C(OC)=N2)=CN=C2Cl)ON=C1 HBWKAAMMLXPLGV-UHFFFAOYSA-N 0.000 description 5
- GADHLZGESPBTCN-UHFFFAOYSA-N COC1=NC(Cl)=NC=C1C1=CC=NO1 Chemical compound COC1=NC(Cl)=NC=C1C1=CC=NO1 GADHLZGESPBTCN-UHFFFAOYSA-N 0.000 description 5
- 125000001072 heteroaryl group Chemical group 0.000 description 5
- 125000006736 (C6-C20) aryl group Chemical group 0.000 description 4
- RUFPHBVGCFYCNW-UHFFFAOYSA-N 1-naphthylamine Chemical compound C1=CC=C2C(N)=CC=CC2=C1 RUFPHBVGCFYCNW-UHFFFAOYSA-N 0.000 description 4
- 238000005160 1H NMR spectroscopy Methods 0.000 description 4
- 238000005481 NMR spectroscopy Methods 0.000 description 4
- 229910052786 argon Inorganic materials 0.000 description 4
- 238000004440 column chromatography Methods 0.000 description 4
- 239000012362 glacial acetic acid Substances 0.000 description 4
- 238000004949 mass spectrometry Methods 0.000 description 4
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 4
- 125000002950 monocyclic group Chemical group 0.000 description 4
- 125000003367 polycyclic group Chemical group 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 3
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 3
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 3
- 125000004430 oxygen atom Chemical group O* 0.000 description 3
- 125000001637 1-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C(*)=C([H])C([H])=C([H])C2=C1[H] 0.000 description 2
- 241000711549 Hepacivirus C Species 0.000 description 2
- KAESVJOAVNADME-UHFFFAOYSA-N Pyrrole Chemical compound C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 description 2
- 125000002723 alicyclic group Chemical group 0.000 description 2
- 125000002178 anthracenyl group Chemical group C1(=CC=CC2=CC3=CC=CC=C3C=C12)* 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 239000002532 enzyme inhibitor Substances 0.000 description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 2
- 125000001041 indolyl group Chemical group 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- ZHKNLJLMDFQVHJ-RUZDIDTESA-N (2r)-2-[3-[[1,3-benzoxazol-2-yl-[3-(4-methoxyphenoxy)propyl]amino]methyl]phenoxy]butanoic acid Chemical compound CC[C@H](C(O)=O)OC1=CC=CC(CN(CCCOC=2C=CC(OC)=CC=2)C=2OC3=CC=CC=C3N=2)=C1 ZHKNLJLMDFQVHJ-RUZDIDTESA-N 0.000 description 1
- 125000003837 (C1-C20) alkyl group Chemical group 0.000 description 1
- NGNBDVOYPDDBFK-UHFFFAOYSA-N 2-[2,4-di(pentan-2-yl)phenoxy]acetyl chloride Chemical compound CCCC(C)C1=CC=C(OCC(Cl)=O)C(C(C)CCC)=C1 NGNBDVOYPDDBFK-UHFFFAOYSA-N 0.000 description 1
- 125000001622 2-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C(*)C([H])=C([H])C2=C1[H] 0.000 description 1
- JBIJLHTVPXGSAM-UHFFFAOYSA-N 2-naphthylamine Chemical compound C1=CC=CC2=CC(N)=CC=C21 JBIJLHTVPXGSAM-UHFFFAOYSA-N 0.000 description 1
- GERJIEKMNDGSCS-DQEYMECFSA-N 4-[[(1s,4s)-2-[[4-[4-(1,3-oxazol-2-yl)phenoxy]phenyl]methyl]-2,5-diazabicyclo[2.2.1]heptan-5-yl]methyl]benzoic acid Chemical compound C([C@]1(N(C[C@]2([H])C1)CC=1C=CC(OC=3C=CC(=CC=3)C=3OC=CN=3)=CC=1)[H])N2CC1=CC=C(C(O)=O)C=C1 GERJIEKMNDGSCS-DQEYMECFSA-N 0.000 description 1
- MYQKFUYFJRBFRV-UHFFFAOYSA-N 5-(4-methoxy-2-methylsulfanylpyrimidin-5-yl)-1,2-oxazole Chemical compound COC1=NC(SC)=NC=C1C1=CC=NO1 MYQKFUYFJRBFRV-UHFFFAOYSA-N 0.000 description 1
- MHWJJOSUHJRLSJ-UHFFFAOYSA-N 5-(4-methoxy-2-methylsulfanylpyrimidin-5-yl)-4-methyl-1,2-oxazole Chemical compound CC1=C(C(C(OC)=N2)=CN=C2SC)ON=C1 MHWJJOSUHJRLSJ-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- 125000005915 C6-C14 aryl group Chemical group 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- 208000031226 Hyperlipidaemia Diseases 0.000 description 1
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 1
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 1
- 125000000066 S-methyl group Chemical group [H]C([H])([H])S* 0.000 description 1
- 239000000370 acceptor Substances 0.000 description 1
- 229950006575 acebilustat Drugs 0.000 description 1
- 125000006848 alicyclic heterocyclic group Chemical group 0.000 description 1
- 150000001335 aliphatic alkanes Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- YCSBALJAGZKWFF-UHFFFAOYSA-N anthracen-2-amine Chemical compound C1=CC=CC2=CC3=CC(N)=CC=C3C=C21 YCSBALJAGZKWFF-UHFFFAOYSA-N 0.000 description 1
- 150000003851 azoles Chemical class 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000004619 benzopyranyl group Chemical group O1C(C=CC2=C1C=CC=C2)* 0.000 description 1
- IOJUPLGTWVMSFF-UHFFFAOYSA-N benzothiazole Chemical class C1=CC=C2SC=NC2=C1 IOJUPLGTWVMSFF-UHFFFAOYSA-N 0.000 description 1
- 125000001164 benzothiazolyl group Chemical group S1C(=NC2=C1C=CC=C2)* 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000004541 benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 description 1
- VEZXCJBBBCKRPI-UHFFFAOYSA-N beta-propiolactone Chemical group O=C1CCO1 VEZXCJBBBCKRPI-UHFFFAOYSA-N 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 239000007810 chemical reaction solvent Substances 0.000 description 1
- 150000001805 chlorine compounds Chemical class 0.000 description 1
- 125000004093 cyano group Chemical group *C#N 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- 230000014509 gene expression Effects 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 125000003453 indazolyl group Chemical group N1N=C(C2=C1C=CC=C2)* 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000000122 inhibitory effect on hepatitis Effects 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 125000001977 isobenzofuranyl group Chemical group C=1(OC=C2C=CC=CC12)* 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000000904 isoindolyl group Chemical group C=1(NC=C2C=CC=CC12)* 0.000 description 1
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 125000002183 isoquinolinyl group Chemical group C1(=NC=CC2=CC=CC=C12)* 0.000 description 1
- 125000001786 isothiazolyl group Chemical group 0.000 description 1
- 125000000842 isoxazolyl group Chemical group 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 125000004593 naphthyridinyl group Chemical group N1=C(C=CC2=CC=CN=C12)* 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 125000001715 oxadiazolyl group Chemical group 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 125000000466 oxiranyl group Chemical group 0.000 description 1
- 229950009401 pemafibrate Drugs 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 125000004592 phthalazinyl group Chemical group C1(=NN=CC2=CC=CC=C12)* 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000000561 purinyl group Chemical group N1=C(N=C2N=CNC2=C1)* 0.000 description 1
- 125000004309 pyranyl group Chemical group O1C(C=CC=C1)* 0.000 description 1
- 125000003373 pyrazinyl group Chemical group 0.000 description 1
- 125000003226 pyrazolyl group Chemical group 0.000 description 1
- 125000002098 pyridazinyl group Chemical group 0.000 description 1
- 125000004076 pyridyl group Chemical group 0.000 description 1
- JQYYPSMCISCIRS-UHFFFAOYSA-N pyrimidin-2-ylmethanethiol Chemical class SCC1=NC=CC=N1 JQYYPSMCISCIRS-UHFFFAOYSA-N 0.000 description 1
- 229940083082 pyrimidine derivative acting on arteriolar smooth muscle Drugs 0.000 description 1
- DDWJFSYHYPDQEL-UHFFFAOYSA-N pyrimidine;1h-pyrrole Chemical class C=1C=CNC=1.C1=CN=CN=C1 DDWJFSYHYPDQEL-UHFFFAOYSA-N 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 125000000714 pyrimidinyl group Chemical group 0.000 description 1
- 125000000168 pyrrolyl group Chemical group 0.000 description 1
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 1
- XSCHRSMBECNVNS-UHFFFAOYSA-N quinoxaline Chemical group N1=CC=NC2=CC=CC=C21 XSCHRSMBECNVNS-UHFFFAOYSA-N 0.000 description 1
- 125000001567 quinoxalinyl group Chemical group N1=C(C=NC2=CC=CC=C12)* 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 230000035484 reaction time Effects 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- PXQLVRUNWNTZOS-UHFFFAOYSA-N sulfanyl Chemical class [SH] PXQLVRUNWNTZOS-UHFFFAOYSA-N 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 125000004213 tert-butoxy group Chemical group [H]C([H])([H])C(O*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 125000001113 thiadiazolyl group Chemical group 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- 125000004306 triazinyl group Chemical group 0.000 description 1
- 125000001425 triazolyl group Chemical group 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/04—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Plural Heterocyclic Compounds (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
本申请涉及合成化学技术领域,尤其涉及一种芳香胺基嘧啶唑类化合物的合成方法。该芳香胺基嘧啶唑类化合物的合成方法包括:将式II1或式II2所示的氯嘧啶类化合物与芳香胺R5‑NH2混合,进行胺化反应,得到式I1或式I2所示的芳香胺基嘧啶唑类化合物;该反应过程的底物适用性较好,而且可以不需要钯催化剂,反应条件温和,无副反应,收率较高,因此可以适合规模化合成。The present application relates to the technical field of synthetic chemistry, in particular to a method for synthesizing aromatic aminopyrimidine azole compounds. The method for synthesizing the aromatic aminopyrimidine azole compound includes: mixing the chloropyrimidine compound represented by the formula II1 or the formula II2 with the aromatic amine R 5 -NH 2 , and carrying out an amination reaction to obtain the compound represented by the formula I1 or the formula I2 Aromatic aminopyrimidine azole compounds; the substrate in this reaction process has good applicability, and no palladium catalyst is required, the reaction conditions are mild, there is no side reaction, and the yield is high, so it can be suitable for large-scale synthesis.
Description
技术领域technical field
本申请属于合成化学技术领域,尤其涉及一种芳香胺基嘧啶唑类化合物的合成方法。The application belongs to the technical field of synthetic chemistry, and in particular relates to a method for synthesizing aromatic aminopyrimidine azole compounds.
背景技术Background technique
嘧啶胺类衍生物可以用于各类酶抑制剂研究工作中,例如有报道嘧啶胺类衍生物可以用于光激酶抑制剂,或者用于核内酪氨酸激酶Wee-1抑制剂。嘧啶胺类骨架可作为优异的氢键受体和氢键供体,因此引入嘧啶胺类骨架对提升酶抑制剂的活性有重要帮助。嘧啶衍生物的胺化技术已经多次报道,但现有合成方法相对较为复杂,存在反应温度较高(如高达到150℃)、条件较为苛刻(如需要用到催化剂)、后续处理较为繁琐等诸多不足。Pyrimidine amine derivatives can be used in the research of various enzyme inhibitors. For example, it has been reported that pyrimidine amine derivatives can be used as photokinase inhibitors, or as inhibitors of nuclear tyrosine kinase Wee-1. Pyrimidine amine skeletons can be used as excellent hydrogen bond acceptors and hydrogen bond donors, so the introduction of pyrimidine amine skeletons is of great help to enhance the activity of enzyme inhibitors. The amination technology of pyrimidine derivatives has been reported many times, but the existing synthetic methods are relatively complicated, and there are relatively high reaction temperatures (such as up to 150 ° C), harsh conditions (such as the need to use catalysts), and subsequent processing is relatively trivial, etc. Many deficiencies.
含唑类化合物可以广泛用于医药研究领域,前景广阔。比如用于治疗高血脂症药物Pemafibrate和呼吸系统药物Acebilustat,另外苯并噻唑类药物对丙型肝炎病毒(Hepatitis C Virus,HCV)也具备较好的抑制作用。但是,相关化合物的胺化合成技术对底物适用性不强,且耗时较长,反应条件比较苛刻,当芳香底物含有氰基等基团时极易被破坏掉,不利于规模化合成。The azole-containing compounds can be widely used in the field of medical research and have broad prospects. For example, Pemafibrate, a drug used to treat hyperlipidemia, and Acebilustat, a respiratory drug, and benzothiazoles also have a good inhibitory effect on Hepatitis C Virus (HCV). However, the amination synthesis technology of related compounds is not suitable for substrates, and it takes a long time and the reaction conditions are harsh. When the aromatic substrate contains groups such as cyano groups, it is easily destroyed, which is not conducive to large-scale synthesis. .
发明内容SUMMARY OF THE INVENTION
本申请的目的在于提供一种芳香胺基嘧啶唑类化合物的合成方法,旨在解决如何简单、低成本地制备芳香胺基嘧啶唑类化合物的技术问题。The purpose of this application is to provide a method for synthesizing aromatic aminopyrimidineazole compounds, aiming to solve the technical problem of how to prepare aromatic aminopyrimidineazole compounds simply and at low cost.
为实现上述申请目的,本申请采用的技术方案如下:In order to realize the above-mentioned application purpose, the technical scheme adopted in this application is as follows:
本申请提供一种芳香胺基嘧啶唑类化合物的合成方法,包括如下步骤:The application provides a method for synthesizing an aromatic aminopyrimidine azole compound, comprising the following steps:
将式II1或式II2所示的氯嘧啶类化合物与芳香胺R5-NH2混合,进行胺化反应,得到式I1或式I2所示的芳香胺基嘧啶唑类化合物;Mixing the chloropyrimidine compound represented by the formula II1 or the formula II2 with the aromatic amine R 5 -NH 2 to carry out an amination reaction to obtain the aromatic aminopyrimidine azole compound represented by the formula I1 or the formula I2;
当所述氯嘧啶类化合物的结构通式为式II1时,所述芳香胺基嘧啶唑类化合物的结构通式为式I1;当所述氯嘧啶类化合物的结构通式为式II2时,所述芳香胺基嘧啶唑类化合物的结构通式为式I2;When the general structural formula of the chloropyrimidine compound is formula III1, the general structural formula of the aromatic aminopyrimidineazole compound is formula I1; when the general structural formula of the chloropyrimidine compound is formula II2, the The general structural formula of the aromatic aminopyrimidine azole compounds is formula I2;
其中,in,
R1选自氢、卤素、被取代的或未被取代的C1-C20烷基、被取代的或未被取代的C1-C20烷氧基中的至少一种;R 1 is selected from at least one of hydrogen, halogen, substituted or unsubstituted C 1 -C 20 alkyl, substituted or unsubstituted C 1 -C 20 alkoxy;
R2选自氢、卤素、被取代的或未被取代的C1-C20烷基、被取代的或未被取代的C1-C20烷氧基、被取代的或未被取代的C3-C20环烷基、被取代的或未被取代的多元脂杂环基、被取代的或未被取代的C6-C20芳基中的至少一种;R 2 is selected from hydrogen, halogen, substituted or unsubstituted C 1 -C 20 alkyl, substituted or unsubstituted C 1 -C 20 alkoxy, substituted or unsubstituted C At least one of 3 -C 20 cycloalkyl, substituted or unsubstituted polyaliphatic heterocyclic group, substituted or unsubstituted C 6 -C 20 aryl group;
R3选自氢、被取代的或未被取代的C1-C20烷基中的至少一种;R 3 is selected from at least one of hydrogen, substituted or unsubstituted C 1 -C 20 alkyl;
R4选自氢、被取代的或未被取代的C1-C20烷基中的至少一种;R 4 is selected from at least one of hydrogen, substituted or unsubstituted C 1 -C 20 alkyl;
R5选自被取代的或未被取代的C6-C20芳基、被取代的或未被取代的芳香杂环基中的至少一种;R 5 is selected from at least one of substituted or unsubstituted C 6 -C 20 aryl, substituted or unsubstituted aromatic heterocyclic group;
X为氧或硫。X is oxygen or sulfur.
本申请提供的芳香胺基嘧啶唑类化合物合成方法,将式II1或式II2所示的氯嘧啶类化合物与芳香胺R5-NH2混合进行胺化反应从而可以得到相应的芳香胺基嘧啶唑类化合物,该反应过程的底物适用性较好,而且可以不需要钯催化剂,反应条件温和,无副反应,收率较高,因此可以适合规模化合成。In the method for synthesizing aromatic aminopyrimidine azole compounds provided in this application, the chloropyrimidine compound represented by formula II1 or formula II2 is mixed with aromatic amine R 5 -NH 2 to carry out amination reaction to obtain the corresponding aromatic amino pyrimidine azoles The reaction process has good substrate applicability, and does not need a palladium catalyst, the reaction conditions are mild, there is no side reaction, and the yield is high, so it can be suitable for large-scale synthesis.
具体实施方式Detailed ways
为了使本申请要解决的技术问题、技术方案及有益效果更加清楚明白,以下结合实施例,对本申请进行进一步详细说明。应当理解,此处所描述的具体实施例仅仅用以解释本申请,并不用于限定本申请。In order to make the technical problems, technical solutions and beneficial effects to be solved by the present application more clear, the present application will be further described in detail below with reference to the embodiments. It should be understood that the specific embodiments described herein are only used to explain the present application, but not to limit the present application.
本申请中,术语“和/或”,描述关联对象的关联关系,表示可以存在三种关系,例如,A和/或B,可以表示:单独存在A,同时存在A和B,单独存在B的情况。其中A,B可以是单数或者复数。字符“/”一般表示前后关联对象是一种“或”的关系。In this application, the term "and/or", which describes the relationship between related objects, means that there can be three relationships, for example, A and/or B, which can mean that A exists alone, A and B exist at the same time, and B exists alone Happening. where A and B can be singular or plural. The character "/" generally indicates that the associated objects are an "or" relationship.
本申请中,“至少一种”是指一种或者多种,“多种”是指两种或两种以上。“以下至少一项(个)”或其类似表达,是指的这些项中的任意组合,包括单项(个)或复数项(个)的任意组合。In this application, "at least one" refers to one or more, and "multiple" refers to two or more. "At least one item(s) below" or similar expressions thereof refer to any combination of these items, including any combination of single item(s) or plural items(s).
应理解,在本申请的各种实施例中,上述各过程的序号的大小并不意味着执行顺序的先后,部分或全部步骤可以并行执行或先后执行,各过程的执行顺序应以其功能和内在逻辑确定,而不应对本申请实施例的实施过程构成任何限定。It should be understood that, in various embodiments of the present application, the size of the sequence numbers of the above-mentioned processes does not imply the sequence of execution, some or all of the steps may be executed in parallel or sequentially, and the execution sequence of each process should be based on its functions and It is determined by the internal logic and should not constitute any limitation on the implementation process of the embodiments of the present application.
在本申请实施例中使用的术语是仅仅出于描述特定实施例的目的,而非旨在限制本申请。在本申请实施例和所附权利要求书中所使用的单数形式的“一种”、“所述”和“该”也旨在包括多数形式,除非上下文清楚地表示其他含义。The terms used in the embodiments of the present application are only for the purpose of describing specific embodiments, and are not intended to limit the present application. As used in the embodiments of this application and the appended claims, the singular forms "a," "the," and "the" are intended to include the plural forms as well, unless the context clearly dictates otherwise.
本申请实施例说明书中所提到的相关成分的重量不仅仅可以指代各组分的具体含量,也可以表示各组分间重量的比例关系,因此,只要是按照本申请实施例说明书相关组分的含量按比例放大或缩小均在本申请实施例说明书公开的范围之内。具体地,本申请实施例说明书中所述的质量可以是μg、mg、g、kg等化工领域公知的质量单位。The weight of the relevant components mentioned in the description of the examples of the present application can not only refer to the specific content of each component, but also can represent the proportional relationship between the weights of the components. It is within the scope disclosed in the description of the embodiments of the present application that the content of the ingredients is scaled up or down. Specifically, the mass described in the description of the embodiment of the present application may be a mass unit known in the chemical field, such as μg, mg, g, kg, etc.
本申请实施例中所涉及的化合物及其衍生物均是按照IUPAC(国际纯粹与应用化学联合会)或CAS(化学文摘服务社,位于俄亥俄州哥伦布市)命名系统命名的。因此,本申请实施例中具体涉及到的化合物基团做如下阐述与说明:The compounds and their derivatives involved in the examples of the present application are all named according to the IUPAC (International Union of Pure and Applied Chemistry) or CAS (Chemical Abstracts Service, Columbus, Ohio) nomenclature system. Therefore, the compound groups specifically involved in the examples of this application are described and explained as follows:
“烷基”是指直链或带有支链的、单价的、饱和脂肪链,包括但不限于如甲基、乙基、丙基、异丙基、丁基、异丁基、戊基、异戊基、己基以及其它类似基团。"Alkyl" means a straight or branched, monovalent, saturated aliphatic chain including, but not limited to, methyl, ethyl, propyl, isopropyl, butyl, isobutyl, pentyl, Isopentyl, hexyl and other similar groups.
“烷氧基”是指与一氧原子键合的直链或带有支链的饱和脂肪链,包括但不限于如甲氧基、乙氧基、丙氧基、丁氧基、异丁氧基、叔丁氧基以及其它类似基团。(Ca-Cb)烷氧基指任何含“a”至“b”个碳原子的烷基与一氧原子键合的直链或带有支链的、单价的、饱和脂肪链。"Alkoxy" refers to a straight or branched saturated aliphatic chain bonded to an oxygen atom, including but not limited to, for example, methoxy, ethoxy, propoxy, butoxy, isobutoxy group, tert-butoxy, and other similar groups. (C a -C b )Alkoxy refers to any straight or branched, monovalent, saturated aliphatic chain of any alkyl group containing "a" to "b" carbon atoms bonded to an oxygen atom.
“环烷基”是指饱和的单环或多环烷基。环烷基包括但不限于如环丙基、环丁基、环戊基、环己基、环庚基以及其它类似基团。"Cycloalkyl" refers to a saturated monocyclic or polycyclic alkyl group. Cycloalkyl groups include, but are not limited to, groups such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and other similar groups.
“多元脂杂环基”是指没有芳香特征的杂环基,是指环烷基中的一个或多个碳原子已被如氮、氧或硫等杂原子取代。可以是三元杂环基如环氧乙烷基、四元杂环基如丙内酯基、五元杂环基等。A "polyaliphatic heterocyclyl" refers to a heterocyclyl group without aromatic character, meaning that one or more carbon atoms in the cycloalkyl group have been substituted with a heteroatom such as nitrogen, oxygen, or sulfur. It may be a three-membered heterocyclic group such as an oxiranyl group, a four-membered heterocyclic group such as a propiolactone group, a five-membered heterocyclic group, and the like.
“芳基”是指一种环状的芳香烃,可以是单环或多环或稠环芳香烃,包括但不限于如苯基、萘基、蒽基、菲基以及其它类似基团。"Aryl" refers to a cyclic aromatic hydrocarbon, which may be a monocyclic or polycyclic or fused ring aromatic hydrocarbon, including, but not limited to, groups such as phenyl, naphthyl, anthracenyl, phenanthryl and other similar groups.
“芳香杂环基”又称杂芳基,是指单环或多环或稠环芳香烃中的一个或多个碳原子已被如氮、氧或硫等杂原子取代。如果杂芳基含有不止一个杂原子,则这些杂原子可能是相同,也可能是不同的。杂芳基包括但不限于如苯并呋喃基、苯并噻吩基、苯并咪唑基、苯并恶唑基、苯并噻唑基、苯并吡喃基、呋喃基、咪唑基、吲唑基、吲嗪基、吲哚基、异苯并呋喃基、异吲哚基、异喹啉基、异噻唑基、异恶唑基、萘啶基、噁二唑基、噁嗪基、噁唑基、酞嗪基、蝶啶基、嘌呤基、吡喃基、吡嗪基、吡唑基、哒嗪基、吡啶[3,4-b]吲哚基、吡啶基、嘧啶基、吡咯基、喹嗪基、喹啉基、喹喔啉基、噻二唑基、噻三唑基、噻唑基、噻吩基、三嗪基、三唑基、呫吨基以及其它类似基团。"Aromatic heterocyclyl", also known as heteroaryl, refers to a monocyclic or polycyclic or fused-ring aromatic hydrocarbon in which one or more carbon atoms have been replaced by heteroatoms such as nitrogen, oxygen or sulfur. If the heteroaryl group contains more than one heteroatom, these may or may not be the same. Heteroaryl groups include, but are not limited to, groups such as benzofuranyl, benzothienyl, benzimidazolyl, benzoxazolyl, benzothiazolyl, benzopyranyl, furyl, imidazolyl, indazolyl, Indolyl, indolyl, isobenzofuranyl, isoindolyl, isoquinolinyl, isothiazolyl, isoxazolyl, naphthyridinyl, oxadiazolyl, oxazinyl, oxazolyl, Phthalazinyl, pteridyl, purinyl, pyranyl, pyrazinyl, pyrazolyl, pyridazinyl, pyrido[3,4-b]indolyl, pyridyl, pyrimidinyl, pyrrolyl, quinazine group, quinolinyl, quinoxalinyl, thiadiazolyl, thitriazolyl, thiazolyl, thienyl, triazinyl, triazolyl, xanthyl and other similar groups.
上述杂原子,可以是氧原子、氮原子、硫原子等。卤素可以是包括氟、氯、溴、碘。The above-mentioned hetero atoms may be oxygen atoms, nitrogen atoms, sulfur atoms and the like. Halogen may include fluorine, chlorine, bromine, iodine.
未被取代的,是指上述烷基、烷氧基、环烷基、多元脂杂环基、芳基、芳香杂环基等基团的碳原子上的氢没有被取代后的基团;被取代的,是指上述烷基、烷氧基、环烷基、多元脂杂环基、芳基、芳香杂环基的碳原子上的部分氢有被取代后的基团;取代基可以是卤素、C1-C5烷基、C1-C5烷氧基、羟基、巯基、硝基等。Unsubstituted refers to the groups in which the hydrogens on the carbon atoms of the above-mentioned alkyl groups, alkoxy groups, cycloalkyl groups, polybasic alicyclic groups, aryl groups, aromatic heterocyclic groups and other groups are not substituted; Substituted refers to the group in which some hydrogens on the carbon atoms of the above-mentioned alkyl group, alkoxy group, cycloalkyl group, polybasic alicyclic group, aryl group and aromatic heterocyclic group are substituted; the substituent can be halogen , C 1 -C 5 alkyl, C 1 -C 5 alkoxy, hydroxyl, mercapto, nitro, etc.
本申请实施例提供一种芳香胺基嘧啶唑类化合物的合成方法,该合成方法包括如下步骤:The embodiment of the present application provides a method for synthesizing an aromatic aminopyrimidine azole compound, and the method for synthesizing comprises the following steps:
将式II1所示的氯嘧啶类化合物与芳香胺R5-NH2混合,进行胺化反应,得到式I1所示的芳香胺基嘧啶唑类化合物;或者,The chloropyrimidine compound represented by the formula II1 is mixed with the aromatic amine R 5 -NH 2 to carry out an amination reaction to obtain the aromatic aminopyrimidine azole compound represented by the formula I1; or,
将式II2所示的氯嘧啶类化合物与芳香胺R5-NH2混合,进行胺化反应,得到式I2所示的芳香胺基嘧啶唑类化合物;The chloropyrimidine compound shown in formula II2 is mixed with aromatic amine R 5 -NH 2 to carry out amination reaction to obtain the aromatic aminopyrimidine azole compound shown in formula I2;
其中,in,
R1选自氢、卤素、被取代的或未被取代的C1-C20烷基、被取代的或未被取代的C1-C20烷氧基中的至少一种;R 1 is selected from at least one of hydrogen, halogen, substituted or unsubstituted C 1 -C 20 alkyl, substituted or unsubstituted C 1 -C 20 alkoxy;
R2选自氢、卤素、被取代的或未被取代的C1-C20烷基、被取代的或未被取代的C1-C20烷氧基、被取代的或未被取代的C3-C20环烷基、被取代的或未被取代的多元脂杂环基、被取代的或未被取代的C6-C20芳基中的至少一种;R 2 is selected from hydrogen, halogen, substituted or unsubstituted C 1 -C 20 alkyl, substituted or unsubstituted C 1 -C 20 alkoxy, substituted or unsubstituted C At least one of 3 -C 20 cycloalkyl, substituted or unsubstituted polyaliphatic heterocyclic group, substituted or unsubstituted C 6 -C 20 aryl group;
R3选自氢、被取代的或未被取代的C1-C20烷基中的至少一种;R 3 is selected from at least one of hydrogen, substituted or unsubstituted C 1 -C 20 alkyl;
R4选自氢、被取代的或未被取代的C1-C20烷基中的至少一种;R 4 is selected from at least one of hydrogen, substituted or unsubstituted C 1 -C 20 alkyl;
R5选自被取代的或未被取代的C6-C20芳基、被取代的或未被取代的芳香杂环基中的至少一种;R 5 is selected from at least one of substituted or unsubstituted C 6 -C 20 aryl, substituted or unsubstituted aromatic heterocyclic group;
X为氧或硫。X is oxygen or sulfur.
本申请实施例提供的芳香胺基嘧啶唑类化合物合成方法,将式II1或式II2所示的氯嘧啶类化合物与芳香胺R5-NH2混合进行胺化反应从而可以得到相应的芳香胺基嘧啶唑类化合物,该反应过程的底物适用性较好,而且可以不需要钯催化剂,反应条件温和,无副反应,收率较高,因此可以适合规模化合成。In the synthesis method of aromatic aminopyrimidine azole compounds provided in the examples of this application, the chloropyrimidine compound represented by formula II1 or formula II2 is mixed with aromatic amine R 5 -NH 2 to carry out amination reaction to obtain the corresponding aromatic amino group The pyrimidine azole compounds have good substrate applicability in the reaction process, and do not need a palladium catalyst, the reaction conditions are mild, there is no side reaction, and the yield is high, so it can be suitable for large-scale synthesis.
在一些实施例中,式II1或式II2所示的氯嘧啶类化合物中,R1选自氢、卤素、被取代的或未被取代的C1-C20烷基、被取代的或未被取代的C1-C20烷氧基中的至少一种;其中,被取代的或未被取代的C1-C20烷基可以是含1-20个碳原子的直链或者带支链的烷基,被取代的或未被取代的C1-C20烷氧基可以是含1-20个碳原子的直链或者带支链的烷氧基。具体地,R1选自氢、卤素、被取代的或未被取代的C1-C10烷基、被取代的或未被取代的C1-C10烷氧基中的至少一种。在本申请一些实施例中,R1选自氢、卤素、未被取代的C1-C6烷基或未被取代的C1-C6烷氧基。In some embodiments, in the chloropyrimidine compounds represented by formula III1 or formula II2, R 1 is selected from hydrogen, halogen, substituted or unsubstituted C 1 -C 20 alkyl, substituted or unsubstituted At least one of the substituted C 1 -C 20 alkoxy groups; wherein, the substituted or unsubstituted C 1 -C 20 alkyl group may be a straight chain or branched chain containing 1-20 carbon atoms The alkyl, substituted or unsubstituted C 1 -C 20 alkoxy group may be a straight-chain or branched-chain alkoxy group containing 1 to 20 carbon atoms. Specifically, R 1 is selected from at least one of hydrogen, halogen, substituted or unsubstituted C 1 -C 10 alkyl, and substituted or unsubstituted C 1 -C 10 alkoxy. In some embodiments of the present application, R 1 is selected from hydrogen, halogen, unsubstituted C 1 -C 6 alkyl or unsubstituted C 1 -C 6 alkoxy.
在一些实施例中,式II1或式II2所示的氯嘧啶类化合物中,R2选自氢、卤素、被取代的或未被取代的C1-C20烷基、被取代的或未被取代的C1-C20烷氧基、被取代的或未被取代的C3-C20环烷基、被取代的或未被取代的多元脂杂环基、被取代的或未被取代的C6-C20芳基中的至少一种;其中,被取代的或未被取代的C3-C20环烷基可以是含3-20个碳原子的单环或多环烷基,被取代的或未被取代的多元脂杂环基可以是含杂原子和2-20个碳原子的杂环基,被取代的或未被取代的C6-C20芳基可以是含6-20个碳原子的芳香烃。具体地,R2选自氢、卤素、被取代的或未被取代的C1-C10烷基、被取代的或未被取代的C1-C10烷氧基、被取代的或未被取代的C3-C10环烷基、被取代的或未被取代的多元脂杂环基、被取代的或未被取代的C6-C14芳基中的至少一种。在本申请一些实施例中,R2选自氢、卤素、未被取代的C1-C6烷基、未被取代的C1-C6烷氧基、未被取代的C3-C6环烷基、未被取代的三~七元脂杂环基或未被取代的C6-C14芳基。In some embodiments, in the chloropyrimidine compound represented by formula III1 or formula II2, R 2 is selected from hydrogen, halogen, substituted or unsubstituted C 1 -C 20 alkyl, substituted or unsubstituted Substituted C 1 -C 20 alkoxy, substituted or unsubstituted C 3 -C 20 cycloalkyl, substituted or unsubstituted polyaliphatic heterocyclyl, substituted or unsubstituted At least one of C 6 -C 20 aryl groups; wherein, substituted or unsubstituted C 3 -C 20 cycloalkyl groups may be monocyclic or polycyclic alkyl groups containing 3-20 carbon atoms, which are The substituted or unsubstituted polyaliphatic heterocyclic group may be a heterocyclic group containing heteroatoms and 2-20 carbon atoms, and the substituted or unsubstituted C6 - C20 aryl group may be a heterocyclic group containing 6-20 carbon atoms. Aromatic hydrocarbons with carbon atoms. Specifically, R 2 is selected from hydrogen, halogen, substituted or unsubstituted C 1 -C 10 alkyl, substituted or unsubstituted C 1 -C 10 alkoxy, substituted or unsubstituted C 1 -C 10 alkoxy At least one of substituted C 3 -C 10 cycloalkyl, substituted or unsubstituted polyaliphatic heterocyclic group, and substituted or unsubstituted C 6 -C 14 aryl group. In some embodiments of the present application, R 2 is selected from hydrogen, halogen, unsubstituted C 1 -C 6 alkyl, unsubstituted C 1 -C 6 alkoxy, unsubstituted C 3 -C 6 Cycloalkyl, unsubstituted three- to seven-membered alicyclic heterocyclic group or unsubstituted C 6 -C 14 aryl.
在一些实施例中,式II1或式II2所示的氯嘧啶类化合物中,R3选自氢、被取代的或未被取代的C1-C20烷基中的至少一种;R4选自氢、被取代的或未被取代的C1-C20烷基中的至少一种;R3和R4可以相同或不同。具体地,R3选自氢、被取代的或未被取代的C1-C10烷基中的至少一种;R4选自氢、被取代的或未被取代的C1-C10烷基中的至少一种。在本申请一些实施例中,R3选自氢或未被取代的C1-C6烷基,R4选自氢或未被取代的C1-C6烷基。In some embodiments, in the chloropyrimidine compound represented by formula III1 or formula II2, R 3 is selected from at least one of hydrogen, substituted or unsubstituted C 1 -C 20 alkyl; R 4 is selected from From at least one of hydrogen, substituted or unsubstituted C 1 -C 20 alkyl; R 3 and R 4 may be the same or different. Specifically, R 3 is selected from at least one of hydrogen, substituted or unsubstituted C 1 -C 10 alkyl; R 4 is selected from hydrogen, substituted or unsubstituted C 1 -C 10 alkane at least one of the bases. In some embodiments of the present application, R 3 is selected from hydrogen or unsubstituted C 1 -C 6 alkyl, and R 4 is selected from hydrogen or unsubstituted C 1 -C 6 alkyl.
在一些实施例中,芳香胺R5-NH2中的选自被取代的或未被取代的C6-C20芳基、被取代的或未被取代的芳香杂环基中的至少一种;其中,被取代的或未被取代的芳香杂环基可以是含杂原子和3-20个碳原子的杂芳基,如五元或六元杂芳基、苯并杂环、杂环并杂环。具体地,R5选自被取代的或未被取代的C6-C14芳基、被取代的或未被取代的芳香杂环基中的至少一种。在本申请一些实施例中,R5选自苯基、萘基、蒽基或菲基。In some embodiments, the aromatic amine R 5 -NH 2 is at least one selected from substituted or unsubstituted C 6 -C 20 aryl, substituted or unsubstituted aromatic heterocyclic group ; Wherein, the substituted or unsubstituted aromatic heterocyclic group can be a heteroaryl group containing heteroatoms and 3-20 carbon atoms, such as five- or six-membered heteroaryl, benzoheterocycle, heterocyclic Heterocycle. Specifically, R 5 is selected from at least one of substituted or unsubstituted C 6 -C 14 aryl, substituted or unsubstituted aromatic heterocyclic group. In some embodiments of the present application, R 5 is selected from phenyl, naphthyl, anthracenyl or phenanthryl.
在一些实施例中,式II1或式II2所示的氯嘧啶类化合物与芳香胺R5-NH2以摩尔比1:0.6~0.8混合进行胺化反应。通过氯嘧啶类化合物的过量,可以更好地生成芳香胺基嘧啶唑类化合物。In some embodiments, the chloropyrimidine compound represented by the formula III1 or the formula II2 is mixed with the aromatic amine R 5 -NH 2 in a molar ratio of 1:0.6-0.8 to carry out the amination reaction. Through the excess of chloropyrimidines, aromatic aminopyrimidineazoles can be better generated.
在一些实施例中,将式II1或式II2所示的氯嘧啶类化合物与芳香胺R5-NH2溶于溶剂中,进行胺化反应。其中,溶剂包括1,4-二氧六环。进一步地,溶剂包括1,4-二氧六环和醋酸;以1,4-二氧六环和醋酸混合形成的溶剂,可以更好地促进氯嘧啶类化合物与芳香胺进行胺化反应。具体的,以1,4-二氧六环与醋酸体积比1~10:1混合,形成反应溶剂。更具体的,1,4-二氧六环与醋酸体积比优选1~5:1。In some embodiments, the chloropyrimidine compound represented by the formula III1 or the formula II2 and the aromatic amine R 5 -NH 2 are dissolved in a solvent to carry out an amination reaction. Wherein, the solvent includes 1,4-dioxane. Further, the solvent includes 1,4-dioxane and acetic acid; the solvent formed by mixing 1,4-dioxane and acetic acid can better promote the amination reaction of chloropyrimidine compounds and aromatic amines. Specifically, 1,4-dioxane and acetic acid are mixed in a volume ratio of 1-10:1 to form a reaction solvent. More specifically, the volume ratio of 1,4-dioxane to acetic acid is preferably 1 to 5:1.
在一些实施例中,式II1或式II2所示的氯嘧啶类化合物与芳香胺R5-NH2混合进行胺化反应的温度为50~100℃,时间为1~10h。更进一步地,温度为60~100℃,时间为2~4h。上述温度和时间条件下可以更好地进行胺化反应。In some embodiments, the chloropyrimidine compound represented by the formula II1 or the formula II2 is mixed with the aromatic amine R 5 -NH 2 to carry out the amination reaction at a temperature of 50-100° C. and a time of 1-10 h. Further, the temperature is 60-100°C, and the time is 2-4h. The amination reaction can be better carried out under the above temperature and time conditions.
在一些实施例中,式II1或式II2所示的氯嘧啶类化合物,由对应的硫甲基嘧啶类化合物和含氯化合物进行氯化反应得到。In some embodiments, the chloropyrimidine compound represented by formula III1 or formula II2 is obtained by chlorination of the corresponding thiomethylpyrimidine compound and a chlorine-containing compound.
具体地,上述含氯化合物选自磺酰氯、氯化亚砜和三氯氧磷中的至少一种;上述氯化反应的温度为0~室温(25℃),时间为0.5~24h,具体可以是2~4h。硫甲基嘧啶类化合物和含氯化合物可以在溶剂中进行氯化反应,具体地,硫甲基氯化所用溶剂可以是二氯甲烷(DCM)、醋酸等。Specifically, the above-mentioned chlorine-containing compound is selected from at least one of sulfonyl chloride, thionyl chloride and phosphorus oxychloride; the temperature of the above-mentioned chlorination reaction is 0 to room temperature (25° C.), and the time is 0.5 to 24 hours. is 2 to 4 hours. The thiomethylpyrimidine compound and the chlorine-containing compound can be chlorinated in a solvent, and specifically, the solvent used for the thiomethyl chlorination can be dichloromethane (DCM), acetic acid, and the like.
具体地,本申请实施例的芳香胺基嘧啶唑类化合物的合成可以包括:(1)硫甲基嘧啶类化合物和磺酰氯等含氯化合物反应,得到产物:式II1或式II2所示的氯嘧啶类化合物;(2)上述氯嘧啶类化合物与相应芳香胺R5-NH2进行反应,得到目标化合物:式I1或式I2所示的芳香胺基嘧啶唑类化合物。具体过程如下所示:Specifically, the synthesis of the aromatic aminopyrimidine azole compounds in the embodiments of the present application may include: (1) the reaction of a thiomethylpyrimidine compound with a chlorine-containing compound such as sulfonyl chloride to obtain a product: a chlorine compound represented by formula II1 or formula II2 A pyrimidine compound; (2) the above-mentioned chloropyrimidine compound is reacted with a corresponding aromatic amine R 5 -NH 2 to obtain the target compound: an aromatic aminopyrimidine azole compound represented by formula I1 or formula I2. The specific process is as follows:
本申请实施例的上述式I1或式I2所示的芳香胺基嘧啶唑类化合物的合成方法,可以采用硫甲基嘧啶唑类化合物经反应得到对应的氯嘧啶唑类化合物后,再与芳香胺类化合物经过胺化反应即可获得。该过程可以避免钯催化剂的使用,使用的试剂及原料易得,底物适用性较好,具有反应时间短且温和、无副反应、后处理简单等诸多优点,适合将I1或式I2所示的芳香胺基嘧啶唑类化合物即芳香胺基嘧啶恶(噻)唑类化合物的规模化合成。The method for synthesizing the aromatic aminopyrimidineazole compounds represented by the above-mentioned formula I1 or formula I2 in the embodiments of the present application can use the thiomethylpyrimidineazole compound to obtain the corresponding chloropyrimidineazole compound through the reaction, and then react with the aromatic amine compound to obtain the corresponding chloropyrimidineazole compound. These compounds can be obtained by amination reaction. This process can avoid the use of palladium catalyst, the reagents and raw materials used are easy to obtain, the substrate applicability is good, and it has many advantages such as short and mild reaction time, no side reactions, and simple post-treatment. Large-scale synthesis of aromatic aminopyrimidine azoles, namely aromatic aminopyrimidine ox(thi)azoles.
下面结合具体实施例进行说明。The following description will be given in conjunction with specific embodiments.
实施例中的硫甲基嘧啶类化合物的合成可参考(J.Med.Chem.2009,52,1081–1099),(Chem.Eur.J.2011,17,9385–9394),(Bioorg.Med.Chem.2015,23,5725–5733),(J.Med.Chem.2012,55,2846-2857),US 9133178 B2以及WO 2012055942。For the synthesis of thiomethylpyrimidine compounds in the examples, please refer to (J.Med.Chem.2009,52,1081-1099), (Chem.Eur.J.2011,17,9385-9394), (Bioorg.Med Chem. 2015, 23, 5725-5733), (J. Med. Chem. 2012, 55, 2846-2857), US 9133178 B2 and WO 2012055942.
实施例1Example 1
一种芳香胺基嘧啶唑类化合物的合成:A kind of synthesis of aromatic aminopyrimidine azoles:
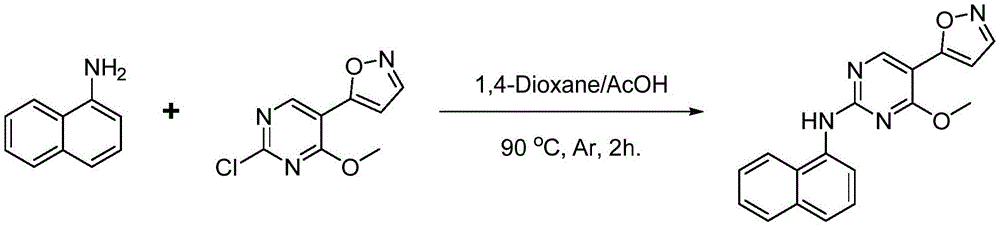
式I1中,X为O,R1为H,R2为甲氧基,R3与R4均为H,R5为1-萘基。具体合成步骤如下:In formula I1, X is O, R 1 is H, R 2 is methoxy, R 3 and R 4 are both H, and R 5 is 1-naphthyl. The specific synthesis steps are as follows:
(1)5-(2-氯-4-甲氧基嘧啶-5-基)异恶唑合成(1) Synthesis of 5-(2-chloro-4-methoxypyrimidin-5-yl)isoxazole
在100mL圆底烧瓶中加入5-(2-甲硫基-4-甲氧基嘧啶-5-基)异恶唑500mg,加入二氯甲烷50mL,于0℃下缓慢滴加磺酰氯0.5mL,加毕后自然升至室温下反应2h反应完毕,旋干即可得到产品5-(2-氯-4-甲氧基嘧啶-5-基)异恶唑450mg,收率95.0%。用于进行下一步胺化反应。500 mg of 5-(2-methylthio-4-methoxypyrimidin-5-yl)isoxazole was added to a 100 mL round-bottomed flask, 50 mL of dichloromethane was added, and 0.5 mL of sulfonyl chloride was slowly added dropwise at 0°C. After the addition, the reaction was naturally raised to room temperature for 2 hours. The reaction was completed, and the product was 450 mg of 5-(2-chloro-4-methoxypyrimidin-5-yl)isoxazole with a yield of 95.0%. for the next step of amination reaction.
(2)胺化反应(2) Amination reaction
于5mL圆底烧瓶中加入1-萘胺(20mg,0.14mmol),5-(2-氯-4-甲氧基嘧啶-5-基)异恶唑(44mg,0.21mmol),1,4-二氧六环2mL,冰醋酸1mL,对体系氩气置换3次,90℃反应2h,降至室温(25℃),乙酸乙酯萃取,柱层析(PE:EA=5:1),得到5-(异恶唑-5-基)-4-甲氧基-N-(萘-1-基)嘧啶-2-胺30mg,收率68.0%,纯度95%。Into a 5mL round-bottomed flask was added 1-naphthylamine (20mg, 0.14mmol), 5-(2-chloro-4-methoxypyrimidin-5-yl)isoxazole (44mg, 0.21mmol), 1,4- 2 mL of dioxane, 1 mL of glacial acetic acid, replaced the system with argon for 3 times, reacted at 90 °C for 2 h, cooled to room temperature (25 °C), extracted with ethyl acetate, and subjected to column chromatography (PE:EA=5:1) to obtain 5-(Isoxazol-5-yl)-4-methoxy-N-(naphthalen-1-yl)pyrimidin-2-amine 30 mg, yield 68.0%, purity 95%.
所得到的产物经质谱和核磁共振确认结构,结果为:1H NMR(300MHz,CDCl3)δ(ppm):8.72(s,1H),8.18(d,J=3Hz,1H),8.12(d,J=3Hz,1H),7.80(d,J=3Hz,1H),7.69(d,J=3Hz,1H),7.54-7.59(m,1H),7.50-7.53(m,1H),7.47-7.48(m,1H),7.45(d,J=3Hz,1H),6.87(d,J=3Hz,1H),4.00(s,3H).MS(ESI):[M+H+]:319.The structure of the obtained product was confirmed by mass spectrometry and nuclear magnetic resonance, and the results were: 1H NMR (300MHz, CDCl3)δ(ppm): 8.72(s, 1H), 8.18(d, J=3Hz, 1H), 8.12(d, J =3Hz,1H),7.80(d,J=3Hz,1H),7.69(d,J=3Hz,1H),7.54-7.59(m,1H),7.50-7.53(m,1H),7.47-7.48( m,1H),7.45(d,J=3Hz,1H),6.87(d,J=3Hz,1H),4.00(s,3H).MS(ESI):[M+H+]:319.
实施例2Example 2
一种芳香胺基嘧啶唑类化合物的合成:A kind of synthesis of aromatic aminopyrimidine azoles:
式I1中,X为O,R1为H,R2为甲氧基,R3与R4均为H,R5为2-蒽基。具体合成步骤如下:In formula I1, X is O, R 1 is H, R 2 is methoxy, R 3 and R 4 are both H, and R 5 is 2-anthracenyl. The specific synthesis steps are as follows:
(1)5-(2-氯-4-甲氧基嘧啶-5-基)异恶唑合成(1) Synthesis of 5-(2-chloro-4-methoxypyrimidin-5-yl)isoxazole
参考实施例1。Refer to Example 1.
(2)胺化反应(2) Amination reaction
于5mL圆底烧瓶中加入2-蒽基胺(20mg,0.10mmol),5-(2-氯-4-甲氧基嘧啶-5-基)异恶唑(33mg,0.16mmol),1,4-二氧六环2mL,冰醋酸1mL,对体系氩气置换3次,90℃反应2h,降至室温,乙酸乙酯萃取,柱层析(PE:EA=5:1),得到5-(异恶唑-5-基)-4-甲氧基-N-(2-蒽基)嘧啶-2-胺30mg,收率78.9%,纯度95%。2-Anthracenylamine (20 mg, 0.10 mmol), 5-(2-chloro-4-methoxypyrimidin-5-yl)isoxazole (33 mg, 0.16 mmol), 1,4 was added to a 5 mL round-bottomed flask - 2 mL of dioxane, 1 mL of glacial acetic acid, replaced the system with argon for 3 times, reacted at 90°C for 2 h, cooled to room temperature, extracted with ethyl acetate, and subjected to column chromatography (PE:EA=5:1) to obtain 5-( Isoxazol-5-yl)-4-methoxy-N-(2-anthryl)pyrimidin-2-amine 30 mg, yield 78.9%, purity 95%.
所得到的产物经质谱和核磁共振确认结构,结果为:1H NMR(500MHz,CDCl3)δ(ppm):8.82(s,1H),8.72(s,1H),8.34(s,1H),8.30(s,1H),8.18(d,J=5Hz,1H),8.01(d,J=5Hz,1H),7.99(d,J=5Hz,1H),7.86(d,J=10Hz,1H),7.56(d,J=5Hz,1H),7.52-7.55(m,1H),7.50-7.52(m,1H),6.87(d,J=5Hz,1H),4.00(s,3H).MS(ESI):[M+H+]:367.The structure of the obtained product was confirmed by mass spectrometry and nuclear magnetic resonance, and the results were: 1H NMR (500MHz, CDCl3)δ(ppm): 8.82(s,1H), 8.72(s,1H), 8.34(s,1H), 8.30( s,1H),8.18(d,J=5Hz,1H),8.01(d,J=5Hz,1H),7.99(d,J=5Hz,1H),7.86(d,J=10Hz,1H),7.56 (d,J=5Hz,1H),7.52-7.55(m,1H),7.50-7.52(m,1H),6.87(d,J=5Hz,1H),4.00(s,3H).MS(ESI) :[M+H+]:367.
实施例3Example 3
一种芳香胺基嘧啶唑类化合物的合成:A kind of synthesis of aromatic aminopyrimidine azoles:
式I1中,X为O,R1为H,R2为甲氧基,R3甲基,R4为H,R5为1-萘基。具体合成步骤如下:In formula I1, X is O, R 1 is H, R 2 is methoxy, R 3 is methyl, R 4 is H, and R 5 is 1-naphthyl. The specific synthesis steps are as follows:
(1)5-(2-氯-4-甲氧基嘧啶-5-基)4-甲基异恶唑合成(1) Synthesis of 5-(2-chloro-4-methoxypyrimidin-5-yl)4-methylisoxazole
在100mL圆底烧瓶中加入5-(2-甲硫基-4-甲氧基嘧啶-5-基)4-甲基异恶唑500mg,加入二氯甲烷50mL,于0℃下缓慢滴加磺酰氯0.4mL,加毕后自然升至室温下反应2h反应完毕,旋干即可得到产品5-(2-氯-4-甲氧基嘧啶-5-基)4-甲基异恶唑460mg,收率97.0%。用于进行下一步胺化反应。500 mg of 5-(2-methylthio-4-methoxypyrimidin-5-yl) 4-methylisoxazole was added to a 100 mL round-bottomed flask, 50 mL of dichloromethane was added, and sulfonic acid was slowly added dropwise at 0°C. Acid chloride 0.4mL, after the addition, the reaction was naturally raised to room temperature for 2h. The reaction was completed, and the product 5-(2-chloro-4-methoxypyrimidin-5-yl)4-methylisoxazole 460mg was obtained by spin drying, Yield 97.0%. for the next step of amination reaction.
(2)胺化反应(2) Amination reaction
于5mL圆底烧瓶中加入1-萘胺(20mg,0.14mmol),5-(2-氯-4-甲氧基嘧啶-5-基)4-甲基异恶唑(47mg,0.21mmol),1,4-二氧六环2mL,冰醋酸1mL,对体系氩气置换3次,90℃反应2h,降至室温,乙酸乙酯萃取,柱层析(PE:EA=5:1),得到5-(4-甲基异恶唑-5-基)-4-甲氧基-N-(萘-1-基)嘧啶-2-胺30mg,收率64.7%,纯度95%。In a 5mL round-bottomed flask, 1-naphthylamine (20mg, 0.14mmol), 5-(2-chloro-4-methoxypyrimidin-5-yl)4-methylisoxazole (47mg, 0.21mmol) were added, 2 mL of 1,4-dioxane, 1 mL of glacial acetic acid, replaced the system with argon three times, reacted at 90°C for 2 h, cooled to room temperature, extracted with ethyl acetate, and subjected to column chromatography (PE:EA=5:1) to obtain 5-(4-Methylisoxazol-5-yl)-4-methoxy-N-(naphthalen-1-yl)pyrimidin-2-amine 30 mg, yield 64.7%, purity 95%.
所得到的产物经质谱和核磁共振确认结构,结果为:1H NMR(400MHz,CDCl3)δ(ppm):8.70(s,1H),8.45(s,1H),8.12(d,J=8Hz,1H),7.79(d,J=4Hz,1H),7.69(d,J=4Hz,1H),7.56-7.58(m,1H),7.50-7.52(m,1H),7.47-7.48(m,1H),7.45(d,J=4Hz,1H),3.99(s,3H),2.32(s,3H).MS(ESI):[M+H+]:333.The structure of the obtained product was confirmed by mass spectrometry and nuclear magnetic resonance, and the results were: 1H NMR (400MHz, CDCl3)δ(ppm): 8.70(s, 1H), 8.45(s, 1H), 8.12(d, J=8Hz, 1H ),7.79(d,J=4Hz,1H),7.69(d,J=4Hz,1H),7.56-7.58(m,1H),7.50-7.52(m,1H),7.47-7.48(m,1H) ,7.45(d,J=4Hz,1H),3.99(s,3H),2.32(s,3H).MS(ESI):[M+H+]:333.
实施例4Example 4
一种芳香胺基嘧啶唑类化合物的合成:A kind of synthesis of aromatic aminopyrimidine azoles:
式I1中,X为O,R1为H,R2为甲氧基,R3甲基,R4为H,R5为2-萘基,具体合成步骤如下:In formula I1, X is O, R 1 is H, R 2 is methoxy, R 3 is methyl, R 4 is H, R 5 is 2-naphthyl, and the specific synthesis steps are as follows:
(1)5-(2-氯-4-甲氧基嘧啶-5-基)4-甲基异恶唑合成(1) Synthesis of 5-(2-chloro-4-methoxypyrimidin-5-yl)4-methylisoxazole
参考实施例3。Refer to Example 3.
(2)胺化反应(2) Amination reaction
于5mL圆底烧瓶中加入2-萘胺(20mg,0.14mmol),5-(2-氯-4-甲氧基嘧啶-5-基)4-甲基异恶唑(47mg,0.21mmol),1,4-二氧六环2mL,冰醋酸1mL,对体系氩气置换3次,90℃反应2h,降至室温,乙酸乙酯萃取,柱层析(PE:EA=5:1),得到5-(4-甲基异恶唑-5-基)-4-甲氧基-N-(萘-2-基)嘧啶-2-胺32mg,收率69.6%,纯度95%。2-naphthylamine (20mg, 0.14mmol), 5-(2-chloro-4-methoxypyrimidin-5-yl)4-methylisoxazole (47mg, 0.21mmol) were added to a 5mL round bottom flask, 2 mL of 1,4-dioxane, 1 mL of glacial acetic acid, replaced the system with argon three times, reacted at 90°C for 2 h, cooled to room temperature, extracted with ethyl acetate, and subjected to column chromatography (PE:EA=5:1) to obtain 5-(4-Methylisoxazol-5-yl)-4-methoxy-N-(naphthalen-2-yl)pyrimidin-2-amine 32 mg, yield 69.6%, purity 95%.
所得到的产物经质谱和核磁共振确认结构,结果为:1H NMR(400MHz,CDCl3)δ(ppm):8.70(s,1H),8.52(s,1H),8.45(s,1H),7.82(d,J=4Hz,1H),7.77(d,J=4Hz,1H),7.74(d,J=4Hz,1H),7.59(d,J=8Hz,1H),7.47-7.49(m,1H),7.44-7.46(m,1H),4.00(s,3H),2.31(s,3H).MS(ESI):[M+H+]:333.The structure of the obtained product was confirmed by mass spectrometry and nuclear magnetic resonance, and the results were: 1H NMR (400MHz, CDCl3)δ(ppm): 8.70(s,1H), 8.52(s,1H), 8.45(s,1H), 7.82( d,J=4Hz,1H),7.77(d,J=4Hz,1H),7.74(d,J=4Hz,1H),7.59(d,J=8Hz,1H),7.47-7.49(m,1H) ,7.44-7.46(m,1H),4.00(s,3H),2.31(s,3H).MS(ESI):[M+H+]:333.
以上所述仅为本申请的较佳实施例而已,并不用以限制本申请,凡在本申请的精神和原则之内所作的任何修改、等同替换和改进等,均应包含在本申请的保护范围之内。The above descriptions are only preferred embodiments of the present application and are not intended to limit the present application. Any modifications, equivalent replacements and improvements made within the spirit and principles of the present application shall be included in the protection of the present application. within the range.
Claims (10)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210127744.4A CN114560854A (en) | 2022-02-11 | 2022-02-11 | Synthetic method of aromatic amino pyrimidine azole compound |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210127744.4A CN114560854A (en) | 2022-02-11 | 2022-02-11 | Synthetic method of aromatic amino pyrimidine azole compound |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN114560854A true CN114560854A (en) | 2022-05-31 |
Family
ID=81713221
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202210127744.4A Pending CN114560854A (en) | 2022-02-11 | 2022-02-11 | Synthetic method of aromatic amino pyrimidine azole compound |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN114560854A (en) |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010022121A1 (en) * | 2008-08-20 | 2010-02-25 | Schering Corporation | Substituted pyridine and pyrimidine derivatives and their use in treating viral infections |
| WO2013066729A1 (en) * | 2011-10-31 | 2013-05-10 | Merck Sharp & Dohme Corp. | Aminopyrimidinones as interleukin receptor-associated kinase inhibitors |
| WO2021115375A1 (en) * | 2019-12-11 | 2021-06-17 | 四川海思科制药有限公司 | Nitrogen-containing heterocyclic autotaxin inhibitor, and composition containing same and use thereof |
| WO2021249892A1 (en) * | 2020-06-08 | 2021-12-16 | F. Hoffmann-La Roche Ag | Substituted amino-pyrimidines |
-
2022
- 2022-02-11 CN CN202210127744.4A patent/CN114560854A/en active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010022121A1 (en) * | 2008-08-20 | 2010-02-25 | Schering Corporation | Substituted pyridine and pyrimidine derivatives and their use in treating viral infections |
| WO2013066729A1 (en) * | 2011-10-31 | 2013-05-10 | Merck Sharp & Dohme Corp. | Aminopyrimidinones as interleukin receptor-associated kinase inhibitors |
| WO2021115375A1 (en) * | 2019-12-11 | 2021-06-17 | 四川海思科制药有限公司 | Nitrogen-containing heterocyclic autotaxin inhibitor, and composition containing same and use thereof |
| WO2021249892A1 (en) * | 2020-06-08 | 2021-12-16 | F. Hoffmann-La Roche Ag | Substituted amino-pyrimidines |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN110719902B (en) | SSAO inhibitor | |
| CN110343088B (en) | A kind of derivative based on PARP inhibitor Niraparib and its preparation method and application | |
| WO2011082098A1 (en) | Lysine and arginine methyltransferase inhibitors for treating cancer | |
| CN103864793B (en) | Substituted purin-9-acetylamino hydroxamic acid histone deacetylases inhibitor and preparation method and application | |
| KR20180038460A (en) | Method for preparing cytotoxic benzodiazepine derivatives | |
| CN107936022A (en) | Xanthine LSD1 inhibitor and its preparation method and application | |
| RS55186B1 (en) | CONDENSED Pyridine Compounds as CB2 Cannabinoid Receptor Ligands | |
| CN107445899A (en) | A kind of benzimidazoles compound and preparation method thereof | |
| CN106146518A (en) | A kind of bruton's tyrosine kinase inhibitor intermediate and preparation method thereof | |
| CN106946868B (en) | Nitric oxide donating type coumarin derivative, its preparation method and medical application | |
| CN113200932B (en) | Synthetic method of oxazolidinone | |
| CN114560854A (en) | Synthetic method of aromatic amino pyrimidine azole compound | |
| FR2891825A1 (en) | 1-AMINO-ISOQUINOLINE DERIVATIVES, THEIR PREPARATION AND THEIR THERAPEUTIC APPLICATION | |
| CN115197261B (en) | Synthesis method of oxadiazine boron derivative | |
| TW202102477A (en) | Production method of quinolinecarboxamide derivative or production intermediate thereof | |
| TWI642658B (en) | Method for preparing indenoisoquinoline derivatives | |
| CN114507180B (en) | Methyl-substituted azaheterocyclic compound C (sp 3 ) Method for self dehydroalkenylation of H bonds | |
| CN114195818B (en) | A kind of 4-arylthiocoumarin compound and preparation method thereof | |
| CN108794375A (en) | A kind of pabishta intermediate and its synthesis and application | |
| CN113943281B (en) | Synthetic method and application of isoxazole pyrimidine derivative | |
| CN107501153A (en) | A kind of preparation method of 2-(2,2,2-trifluoroethyl)-3-aryl-2H-azyridine compound | |
| CN107382769B (en) | A kind of preparation method of 1-aryl-3-azido-4,4,4-trifluoro-1-butene compound | |
| CN115023421B (en) | Fluorine-containing pyrimidine compound and process for producing the same | |
| CN110922402B (en) | A kind of C-3-position iodo indolizine compound and preparation method thereof | |
| CN111560022A (en) | Tetrahydrobenzofuran [3,2-d ] pyrimidine derivative and preparation method and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| RJ01 | Rejection of invention patent application after publication | ||
| RJ01 | Rejection of invention patent application after publication |
Application publication date: 20220531 |