CN114213952A - Environment-friendly water-based paint and preparation method thereof - Google Patents
Environment-friendly water-based paint and preparation method thereof Download PDFInfo
- Publication number
- CN114213952A CN114213952A CN202111630433.1A CN202111630433A CN114213952A CN 114213952 A CN114213952 A CN 114213952A CN 202111630433 A CN202111630433 A CN 202111630433A CN 114213952 A CN114213952 A CN 114213952A
- Authority
- CN
- China
- Prior art keywords
- parts
- environment
- water
- based paint
- starch
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 title claims abstract description 81
- 239000003973 paint Substances 0.000 title claims abstract description 65
- 238000002360 preparation method Methods 0.000 title claims abstract description 13
- 229920002472 Starch Polymers 0.000 claims abstract description 51
- 239000008107 starch Substances 0.000 claims abstract description 51
- 235000019698 starch Nutrition 0.000 claims abstract description 51
- 239000004814 polyurethane Substances 0.000 claims abstract description 32
- 229920002635 polyurethane Polymers 0.000 claims abstract description 32
- HBMJWWWQQXIZIP-UHFFFAOYSA-N silicon carbide Chemical compound [Si+]#[C-] HBMJWWWQQXIZIP-UHFFFAOYSA-N 0.000 claims abstract description 19
- 229910010271 silicon carbide Inorganic materials 0.000 claims abstract description 19
- 239000004925 Acrylic resin Substances 0.000 claims abstract description 13
- 229920000178 Acrylic resin Polymers 0.000 claims abstract description 13
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims abstract description 13
- 239000008367 deionised water Substances 0.000 claims abstract description 13
- 229910021641 deionized water Inorganic materials 0.000 claims abstract description 13
- 239000012752 auxiliary agent Substances 0.000 claims abstract description 10
- 238000000576 coating method Methods 0.000 claims description 23
- 239000011248 coating agent Substances 0.000 claims description 18
- 238000002156 mixing Methods 0.000 claims description 18
- 238000005303 weighing Methods 0.000 claims description 16
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 claims description 15
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 claims description 12
- 238000003756 stirring Methods 0.000 claims description 12
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 claims description 10
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 claims description 10
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 claims description 10
- 239000002202 Polyethylene glycol Substances 0.000 claims description 10
- 238000001816 cooling Methods 0.000 claims description 10
- 239000011259 mixed solution Substances 0.000 claims description 10
- 229920001223 polyethylene glycol Polymers 0.000 claims description 10
- 239000003795 chemical substances by application Substances 0.000 claims description 8
- 239000005058 Isophorone diisocyanate Substances 0.000 claims description 5
- 235000016735 Manihot esculenta subsp esculenta Nutrition 0.000 claims description 5
- 229960000583 acetic acid Drugs 0.000 claims description 5
- 238000006243 chemical reaction Methods 0.000 claims description 5
- 238000007599 discharging Methods 0.000 claims description 5
- 238000001914 filtration Methods 0.000 claims description 5
- 239000012362 glacial acetic acid Substances 0.000 claims description 5
- 238000010438 heat treatment Methods 0.000 claims description 5
- NIMLQBUJDJZYEJ-UHFFFAOYSA-N isophorone diisocyanate Chemical compound CC1(C)CC(N=C=O)CC(C)(CN=C=O)C1 NIMLQBUJDJZYEJ-UHFFFAOYSA-N 0.000 claims description 5
- 229940098779 methanesulfonic acid Drugs 0.000 claims description 5
- KSBAEPSJVUENNK-UHFFFAOYSA-L tin(ii) 2-ethylhexanoate Chemical compound [Sn+2].CCCCC(CC)C([O-])=O.CCCCC(CC)C([O-])=O KSBAEPSJVUENNK-UHFFFAOYSA-L 0.000 claims description 5
- 239000002518 antifoaming agent Substances 0.000 claims description 4
- 239000002562 thickening agent Substances 0.000 claims description 4
- 239000002245 particle Substances 0.000 claims description 3
- CUVLMZNMSPJDON-UHFFFAOYSA-N 1-(1-butoxypropan-2-yloxy)propan-2-ol Chemical compound CCCCOCC(C)OCC(C)O CUVLMZNMSPJDON-UHFFFAOYSA-N 0.000 claims description 2
- IBLKWZIFZMJLFL-UHFFFAOYSA-N 1-phenoxypropan-2-ol Chemical compound CC(O)COC1=CC=CC=C1 IBLKWZIFZMJLFL-UHFFFAOYSA-N 0.000 claims description 2
- 229920002134 Carboxymethyl cellulose Polymers 0.000 claims description 2
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 claims description 2
- 239000004354 Hydroxyethyl cellulose Substances 0.000 claims description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 claims description 2
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims description 2
- 239000000654 additive Substances 0.000 claims description 2
- 230000000996 additive effect Effects 0.000 claims description 2
- -1 alcohol ester Chemical class 0.000 claims description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 claims description 2
- 235000010948 carboxy methyl cellulose Nutrition 0.000 claims description 2
- 239000008112 carboxymethyl-cellulose Substances 0.000 claims description 2
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 claims description 2
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 claims description 2
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 claims description 2
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 claims description 2
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 claims description 2
- 229920000609 methyl cellulose Polymers 0.000 claims description 2
- 239000001923 methylcellulose Substances 0.000 claims description 2
- 235000010981 methylcellulose Nutrition 0.000 claims description 2
- 239000000203 mixture Substances 0.000 claims description 2
- 229920000058 polyacrylate Polymers 0.000 claims description 2
- 229940113115 polyethylene glycol 200 Drugs 0.000 claims description 2
- 229940068886 polyethylene glycol 300 Drugs 0.000 claims description 2
- 229940068918 polyethylene glycol 400 Drugs 0.000 claims description 2
- 239000011591 potassium Substances 0.000 claims description 2
- 229910052700 potassium Inorganic materials 0.000 claims description 2
- 235000007686 potassium Nutrition 0.000 claims description 2
- 239000010703 silicon Substances 0.000 claims description 2
- 229910052710 silicon Inorganic materials 0.000 claims description 2
- GCLGEJMYGQKIIW-UHFFFAOYSA-H sodium hexametaphosphate Chemical compound [Na]OP1(=O)OP(=O)(O[Na])OP(=O)(O[Na])OP(=O)(O[Na])OP(=O)(O[Na])OP(=O)(O[Na])O1 GCLGEJMYGQKIIW-UHFFFAOYSA-H 0.000 claims description 2
- 235000019982 sodium hexametaphosphate Nutrition 0.000 claims description 2
- 235000019830 sodium polyphosphate Nutrition 0.000 claims description 2
- 235000019832 sodium triphosphate Nutrition 0.000 claims description 2
- RYCLIXPGLDDLTM-UHFFFAOYSA-J tetrapotassium;phosphonato phosphate Chemical compound [K+].[K+].[K+].[K+].[O-]P([O-])(=O)OP([O-])([O-])=O RYCLIXPGLDDLTM-UHFFFAOYSA-J 0.000 claims description 2
- 239000001577 tetrasodium phosphonato phosphate Substances 0.000 claims description 2
- UNXRWKVEANCORM-UHFFFAOYSA-I triphosphate(5-) Chemical compound [O-]P([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O UNXRWKVEANCORM-UHFFFAOYSA-I 0.000 claims description 2
- 241000658379 Manihot esculenta subsp. esculenta Species 0.000 claims 1
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 abstract description 9
- 238000001035 drying Methods 0.000 abstract description 7
- 239000012855 volatile organic compound Substances 0.000 abstract description 7
- 239000000853 adhesive Substances 0.000 abstract description 4
- 230000001070 adhesive effect Effects 0.000 abstract description 4
- 230000000694 effects Effects 0.000 abstract description 3
- 238000003912 environmental pollution Methods 0.000 abstract description 2
- 238000012360 testing method Methods 0.000 description 8
- 230000000052 comparative effect Effects 0.000 description 6
- 238000003860 storage Methods 0.000 description 6
- 230000008901 benefit Effects 0.000 description 5
- 238000000034 method Methods 0.000 description 5
- 240000003183 Manihot esculenta Species 0.000 description 4
- 238000005299 abrasion Methods 0.000 description 4
- 230000007613 environmental effect Effects 0.000 description 4
- 239000000126 substance Substances 0.000 description 3
- 230000032683 aging Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 239000002612 dispersion medium Substances 0.000 description 2
- 239000003960 organic solvent Substances 0.000 description 2
- 238000001556 precipitation Methods 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 239000002966 varnish Substances 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 239000004593 Epoxy Substances 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 238000004887 air purification Methods 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000004568 cement Substances 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000005034 decoration Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000009776 industrial production Methods 0.000 description 1
- 238000013101 initial test Methods 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000011056 performance test Methods 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D175/00—Coating compositions based on polyureas or polyurethanes; Coating compositions based on derivatives of such polymers
- C09D175/04—Polyurethanes
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D7/00—Features of coating compositions, not provided for in group C09D5/00; Processes for incorporating ingredients in coating compositions
- C09D7/20—Diluents or solvents
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D7/00—Features of coating compositions, not provided for in group C09D5/00; Processes for incorporating ingredients in coating compositions
- C09D7/40—Additives
- C09D7/60—Additives non-macromolecular
- C09D7/61—Additives non-macromolecular inorganic
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Paints Or Removers (AREA)
Abstract
The invention provides an environment-friendly water-based paint and a preparation method thereof. The environment-friendly water-based paint comprises the following components in parts by weight: 20-30 parts of acrylic resin, 2-3 parts of diatomite, 25-40 parts of starch modified polyurethane, 3-8 parts of silicon carbide, 5-10 parts of an auxiliary agent and 20-25 parts of deionized water. According to the environment-friendly water-based paint provided by the invention, the acrylic resin and the starch modified polyurethane are matched for use, and the silicon carbide is added into the paint, so that the hardness, the adhesive force, the water resistance and the wear resistance of the environment-friendly water-based paint can be improved, the surface drying time is short, the paint has the effects of removing formaldehyde and peculiar smell by adding the diatomite, the air can be purified, the VOC (volatile organic compound) emission amount of the water-based paint is low, the environmental pollution is small, and the environment-friendly water-based paint is more environment-friendly.
Description
Technical Field
The invention relates to the technical field of coatings, and particularly relates to an environment-friendly water-based coating and a preparation method thereof.
Background
With the improvement of infrastructure, the vigorous development in the fields of building industry and the like and the rapid growth of industrial production, the growth and the use of the coating are greatly improved. The traditional coating mainly uses organic substances as a dispersion medium, but contains more volatile organic solvents (VOC), and can pose certain threats to the surrounding environment and human health.
In recent years, with the enhancement of environmental awareness of people, laws and regulations related to the production and use of chemical products are getting stricter, and the water-based coating is favored by consumers by virtue of the advantages of 'green', 'environmental protection', 'low carbon', 'high performance', 'wide use', and the like, is gradually replacing the leading position of the traditional coating, and is an important direction for the development of the current coating.
The water-based paint refers to a paint using water as a solvent or a dispersion medium, and the waterproof paint mainly has four types: water-based inorganic zinc-rich paint, water-based epoxy paint, water-based acrylic paint and water-based polyurethane paint. Compared with the organic solvent type coating, the water-based coating can reduce the emission of VOC, save resources and energy sources and is convenient to store and transport. However, most of the water-based coatings on the market at present have the problems of long film-forming drying time, low hardness, poor wear resistance and water resistance and the like, are easy to crack and fall off, and cannot well meet the use requirements.
Disclosure of Invention
The invention aims to provide an environment-friendly water-based paint and a preparation method thereof, and the environment-friendly water-based paint can solve the problems of long film-forming drying time, low hardness, and poor wear resistance and water resistance of the existing water-based paint.
According to a first aspect of the invention, an environment-friendly water-based paint is provided, which comprises the following components in parts by weight: 20-30 parts of acrylic resin, 2-3 parts of diatomite, 25-40 parts of starch modified polyurethane, 3-8 parts of silicon carbide, 5-10 parts of an auxiliary agent and 20-25 parts of deionized water;
the starch modified polyurethane is prepared by the following steps:
(1) weighing 2-4 parts of isophorone diisocyanate, 3001-2 parts of polyethylene glycol and 5-10 parts of butanone according to parts by weight, uniformly mixing, heating to 65-75 ℃, and preserving heat for 1-3 hours to obtain a mixed solution;
(2) weighing 5-7 parts of starch, 20003-4 parts of polyethylene glycol and 0.1-0.5 part of stannous octoate according to parts by weight, uniformly mixing, adding into the mixed solution, reacting for 3-5 hours at 65-75 ℃, cooling to 40-50 ℃, adding deionized water into the reaction system, and uniformly stirring to obtain the starch modified polyurethane.
The environment-friendly water-based paint provided by the invention contains acrylic resin, diatomite, starch modified polyurethane, silicon carbide, an auxiliary agent and deionized water, the starch is used for modifying polyurethane to prepare the starch modified polyurethane, the particle size of the starch modified polyurethane is small and uniform step by step, and the starch modified polyurethane is matched with the acrylic resin for use, so that the surface drying time of the paint during film forming is short, the surface of a coated film is smooth, the hardness of the coated film is high, the adhesive force is strong, the water resistance, the wear resistance and other comprehensive properties are improved, and the coating film is easier to degrade than the traditional water-based polyurethane and has less environmental pollution; the addition of the silicon carbide further improves the hardness and the wear resistance of the coating in the film forming process and has the advantage of ageing resistance; the addition of the diatomite enables the water-based paint to have the effects of removing formaldehyde and peculiar smell and further achieving air purification, but the addition of the silicon carbide often causes the storage stability of the paint to be poor, and the polyurethane adopted in the invention is modified by the starch, and the starch base is introduced, so that the phenomenon of precipitation of the paint caused by the addition of the silicon carbide can be avoided, and the storage stability of the water-based paint is greatly improved. Therefore, the acrylic resin, the diatomite, the starch modified polyurethane and the silicon carbide contained in the environment-friendly water-based paint have good synergistic effect, so that the prepared paint has excellent performance, can effectively solve the problems of long film forming and drying time, low hardness, poor wear resistance and water resistance of the existing water-based paint, and has the advantages of environmental protection.
Preferably, in the preparation process of the starch modified polyurethane, the starch used in the step (2) is acetate starch.
Preferably, the acetate starch is prepared by the following steps: weighing 10-15 parts of cassava starch, 15-25 parts of glacial acetic acid, 15-25 parts of acetic anhydride and 0.1-0.5 part of methanesulfonic acid according to parts by weight, uniformly mixing, reacting at 70-80 ℃ for 3-5 h, cooling, adjusting pH, filtering and discharging to obtain the acetate starch.
Preferably, the particle size of the silicon carbide is 1 to 10 μm.
The silicon carbide with moderate grain diameter is selected in the environment-friendly water-based paint provided by the invention, so that the uniformity and the film-forming performance of the paint are better, the phenomenon that the paint is precipitated in the storage process due to too large grain diameter of the silicon carbide is avoided, and the storage stability of the paint is favorably improved.
Preferably, the auxiliary agent in the environment-friendly water-based paint comprises the following components in parts by weight: 1-2 parts of defoaming agent, 1-2 parts of film forming additive, 2-4 parts of thickening agent and 1-2 parts of flatting agent.
Preferably, the antifoaming agent comprises at least one of sodium hexametaphosphate, sodium polyphosphate, potassium tripolyphosphate, and potassium pyrophosphate.
Preferably, the film forming aid comprises at least one of polyethylene glycol 200, polyethylene glycol 300, polyethylene glycol 400, propylene glycol phenyl ether PPH, alcohol ester twelve, dipropylene glycol butyl ether.
Preferably, the thickener comprises at least one of hydroxyethyl cellulose, methyl cellulose, carboxymethyl cellulose, hydroxypropylmethyl cellulose.
Preferably, the leveling agent comprises at least one of a pure polyacrylate leveling agent and an aqueous silicon-containing leveling agent.
According to a second aspect of the present invention, there is provided a method for preparing the above-mentioned environment-friendly water-based paint, comprising the steps of: uniformly mixing acrylic resin, diatomite, starch modified polyurethane and deionized water, adding the auxiliary agent and silicon carbide while stirring, and stirring for 30-45 min to obtain the environment-friendly water-based paint.
The invention has the beneficial effects that:
1. according to the environment-friendly water-based paint provided by the invention, the acrylic resin and the starch modified polyurethane are matched for use, and the silicon carbide is added into the paint, so that the hardness, the adhesive force, the water resistance and the wear resistance of the environment-friendly water-based paint can be improved, the surface drying time is short, and the diatomite is added, so that the paint has the effects of removing formaldehyde and peculiar smell, and can play a role in purifying air.
2. The polyurethane contained in the environment-friendly water-based paint provided by the invention is more easily degraded, has less pollution to the environment and is more environment-friendly than the traditional water-based polyurethane due to the introduction of the starch group.
3. In the preparation process of the environment-friendly water-based paint, the acrylic resin, the diatomite, the starch modified polyurethane and the deionized water are uniformly mixed, and then the auxiliary agent and the silicon carbide are added into the mixture for stirring treatment, so that the hardness and the wear resistance of the water-based paint can be further improved, and the prepared water-based paint has the advantage of ageing resistance.
4. The environment-friendly water-based paint provided by the invention is simple in preparation process, suitable for industrial application and good in economic benefit.
Detailed Description
Technical features in the technical solutions provided by the present invention are further clearly and completely described below with reference to specific embodiments, and it is obvious that the described embodiments are only a part of embodiments of the present invention, and not all embodiments. All other embodiments, which can be derived by a person skilled in the art from the embodiments given herein without making any creative effort, shall fall within the protection scope of the present invention.
Example 1
The preparation method of the starch modified polyurethane comprises the following steps:
(1) weighing 10 parts of cassava starch, 25 parts of glacial acetic acid, 25 parts of acetic anhydride and 0.1 part of methanesulfonic acid according to parts by weight, uniformly mixing, reacting at 75 ℃ for 3 hours, cooling, adjusting pH, filtering and discharging to obtain acetate starch;
(2) weighing 2 parts of isophorone diisocyanate, 3002 parts of polyethylene glycol and 5 parts of butanone according to parts by weight, uniformly mixing, heating to 65 ℃, and keeping the temperature for 2 hours to obtain a mixed solution;
(3) weighing 5 parts of acetate starch, 20003.5 parts of polyethylene glycol and 0.1 part of stannous octoate according to parts by weight, uniformly mixing, adding into the mixed solution, reacting for 4 hours at 75 ℃, cooling to 40 ℃, adding deionized water into the reaction system, and uniformly stirring to obtain the starch modified polyurethane.
Example 2
The preparation method of the starch modified polyurethane comprises the following steps:
(1) weighing 13 parts of cassava starch, 20 parts of glacial acetic acid, 20 parts of acetic anhydride and 0.3 part of methanesulfonic acid according to parts by weight, uniformly mixing, reacting at 70 ℃ for 4 hours, cooling, adjusting pH, filtering and discharging to obtain acetate starch;
(2) weighing 3 parts of isophorone diisocyanate, 3001.5 parts of polyethylene glycol and 8 parts of butanone according to parts by weight, uniformly mixing, heating to 70 ℃, and preserving heat for 3 hours to obtain a mixed solution;
(3) weighing 6 parts of acetate starch, 20003 parts of polyethylene glycol and 0.3 part of stannous octoate according to parts by weight, uniformly mixing, adding into the mixed solution, reacting for 3 hours at 70 ℃, cooling to 45 ℃, adding deionized water into the reaction system, and uniformly stirring to obtain the starch modified polyurethane.
Example 3
The preparation method of the starch modified polyurethane comprises the following steps:
(1) weighing 15 parts of cassava starch, 15 parts of glacial acetic acid, 15 parts of acetic anhydride and 0.5 part of methanesulfonic acid according to parts by weight, uniformly mixing, reacting at 80 ℃ for 5 hours, cooling, adjusting pH, filtering and discharging to obtain acetate starch;
(2) weighing 4 parts of isophorone diisocyanate, 3001 parts of polyethylene glycol and 10 parts of butanone according to parts by weight, uniformly mixing, heating to 75 ℃, and keeping the temperature for 1 hour to obtain a mixed solution;
(3) weighing 7 parts of acetate starch, 20004 parts of polyethylene glycol and 0.5 part of stannous octoate according to parts by weight, uniformly mixing, adding into the mixed solution, reacting for 5 hours at 65 ℃, cooling to 50 ℃, adding deionized water into the reaction system, and uniformly stirring to obtain the starch modified polyurethane.
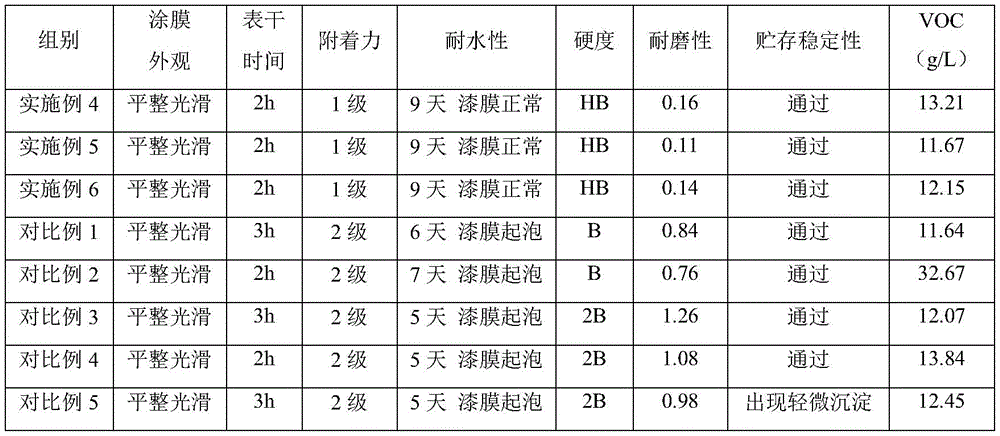
Examples 4 to 6 and comparative examples 1 to 4
The components of the water-based environment-friendly coating are shown in Table 1, and the preparation method comprises the following steps:
weighing 20-30 parts of acrylic resin, 2-3 parts of diatomite, 25-40 parts of starch modified polyurethane in examples 1-3 and 20-25 parts of deionized water according to the weight parts, uniformly mixing, adding 5-10 parts of an auxiliary agent and 3-8 parts of silicon carbide while stirring, and stirring for 30-45 min to obtain the environment-friendly water-based paint.
TABLE 1 Components of Environment-friendly Water-based paint
Test example
1. Experimental construction mode
The reference objects of the test examples are the environment-friendly water-based coatings prepared in the examples 4-6 and the comparative examples 1-5, and various performances of the coatings are tested.
(1) Time to surface dry
Reference is made to the national standard GB/T1728-;
(2) adhesion force
Refer to national standard GB/T9286-1998;
(3) water resistance
Refer to national standard GB/T1733 + 1993 (A method);
(4) hardness of
Refer to national standard GB/T6739-2006;
(5) wear resistance
The environment-friendly water-based paint prepared in the embodiments 4 to 6 and the comparative examples 1 to 5 is coated on the surface of a fiber reinforced cement plate with the diameter of 25mm, which is specified in GB/T9271-2008 color paint and varnish standard test plate, and a paint film with the thickness of 250 micrometers is obtained after the environment-friendly water-based paint is placed for 24 hours at the temperature of 25 ℃ and the relative humidity of 50%. According to GB/T1768-2006 method for measuring abrasion resistance of colored paint and varnish, the test is carried out under the conditions of temperature of 25 ℃ and relative humidity of 50%, the rotating speed of an abrasion tester is 60r/min, and the test time is 3 min. The abrasion resistance of the coating is expressed in terms of abrasion mass loss (unit: g), and the calculation formula is as follows: wear mass loss is the initial test plate mass-the test plate mass after the test is completed;
(6) storage stability
Placing the environment-friendly water-based paint prepared in the embodiments 4-6 and the comparative examples 1-5 in a constant-temperature oven (the temperature is controlled at 50 +/-5 ℃) for 30 days or 90 days, and observing whether flooding, precipitation, water diversion or the like occurs or not, wherein if the phenomenon does not occur, the environment-friendly water-based paint passes through the environment-friendly water-based paint;
(7) volatile Organic Compound (VOC) content
Refer to the limit of harmful substances in interior decoration materials GB 18582-2008.
2. Results of the experiment
Table 2 results of various property tests of the environmental friendly water based paint
The results of the performance tests of the environmentally friendly water-based coatings prepared in examples 4 to 6 and comparative examples 1 to 5 are shown in Table 2. As shown in Table 2, compared with comparative examples 1 to 5, the environment-friendly water-based paint prepared in examples 4 to 6 simultaneously contains acrylic resin, diatomite, starch modified polyurethane and silicon carbide, has short surface drying time when a paint film is formed, has high hardness, excellent wear resistance and water resistance, high storage stability and strong adhesive force to an adherend, and has low VOC emission, so that the water-based paint is more environment-friendly.
Although the present invention has been described in detail with reference to the above embodiments, it should be understood by those skilled in the art that various changes and modifications may be made therein without departing from the spirit and scope of the invention as defined by the appended claims.
Claims (10)
1. The environment-friendly water-based paint is characterized by comprising the following components in parts by weight: 20-30 parts of acrylic resin, 2-3 parts of diatomite, 25-40 parts of starch modified polyurethane, 3-8 parts of silicon carbide, 5-10 parts of an auxiliary agent and 20-25 parts of deionized water;
the starch modified polyurethane is prepared by the following steps:
(1) weighing 2-4 parts of isophorone diisocyanate, 3001-2 parts of polyethylene glycol and 5-10 parts of butanone according to parts by weight, uniformly mixing, heating to 65-75 ℃, and preserving heat for 1-3 hours to obtain a mixed solution;
(2) weighing 5-7 parts of starch, 20003-4 parts of polyethylene glycol and 0.1-0.5 part of stannous octoate according to parts by weight, uniformly mixing, adding into the mixed solution, reacting at 65-75 ℃ for 3-5 h, cooling to 40-50 ℃, adding deionized water into the reaction system, and uniformly stirring to obtain the starch modified polyurethane.
2. The environmentally friendly aqueous coating of claim 1, wherein: in the step (2), the starch is acetate starch.
3. The environment-friendly water-based paint as claimed in claim 2, wherein the acetate starch is prepared by the following steps: weighing 10-15 parts of cassava starch, 15-25 parts of glacial acetic acid, 15-25 parts of acetic anhydride and 0.1-0.5 part of methanesulfonic acid according to parts by weight, uniformly mixing, reacting at 70-80 ℃ for 3-5 h, cooling, adjusting pH, filtering and discharging to obtain the acetate starch.
4. The environmentally friendly aqueous coating of claim 1, wherein: the particle size of the silicon carbide is 1-10 mu m.
5. The environment-friendly water-based paint as claimed in claim 1, wherein the auxiliary comprises the following components in parts by weight: 1-2 parts of defoaming agent, 1-2 parts of film forming additive, 2-4 parts of thickening agent and 1-2 parts of flatting agent.
6. The environmentally friendly aqueous coating of claim 5, wherein: the defoaming agent comprises at least one of sodium hexametaphosphate, sodium polyphosphate, potassium tripolyphosphate and potassium pyrophosphate.
7. The environmentally friendly aqueous coating of claim 5, wherein: the film forming auxiliary agent comprises at least one of polyethylene glycol 200, polyethylene glycol 300, polyethylene glycol 400, propylene glycol phenyl ether PPH, alcohol ester twelve and dipropylene glycol butyl ether.
8. The environmentally friendly aqueous coating of claim 5, wherein: the thickener comprises at least one of hydroxyethyl cellulose, methyl cellulose, carboxymethyl cellulose and hydroxypropyl methyl cellulose.
9. The environmentally friendly aqueous coating of claim 1, wherein: the leveling agent comprises at least one of a pure polyacrylate leveling agent and a water-based silicon-containing leveling agent.
10. The preparation method of the environment-friendly water-based paint according to any one of claims 1 to 9, characterized by comprising the following steps: and uniformly mixing the acrylic resin, the diatomite, the starch modified polyurethane and the deionized water, adding the auxiliary agent and the silicon carbide into the mixture while stirring, wherein the stirring time is 30-45 min, and thus obtaining the environment-friendly water-based paint.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202111630433.1A CN114213952B (en) | 2021-12-28 | 2021-12-28 | Environment-friendly water-based paint and preparation method thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202111630433.1A CN114213952B (en) | 2021-12-28 | 2021-12-28 | Environment-friendly water-based paint and preparation method thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN114213952A true CN114213952A (en) | 2022-03-22 |
| CN114213952B CN114213952B (en) | 2022-07-12 |
Family
ID=80706552
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202111630433.1A Active CN114213952B (en) | 2021-12-28 | 2021-12-28 | Environment-friendly water-based paint and preparation method thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN114213952B (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN117165174A (en) * | 2023-11-03 | 2023-12-05 | 广州哲铭新材料科技有限公司 | Water-based multifunctional paint and preparation method thereof |
Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5138006A (en) * | 1991-02-11 | 1992-08-11 | Eastman Kodak Company | Radiation polymerizable starch ester-urethanes |
| CN1183791A (en) * | 1995-06-30 | 1998-06-03 | 旭化成工业株式会社 | Highly emulsifiable and stable polyisocyanate composition and water-based coating composition containing the composition |
| US6008276A (en) * | 1995-06-01 | 1999-12-28 | Bayer Aktiengesellschaft | Polymer blends containing starch and polyurethane |
| CN101085891A (en) * | 2006-06-07 | 2007-12-12 | 关西涂料株式会社 | Starch-based coating composition |
| US20100034978A1 (en) * | 2008-08-08 | 2010-02-11 | Formulated Solutions Llc | Compositions for use in construction and methods of applying the same |
| US20120178858A1 (en) * | 2011-01-10 | 2012-07-12 | Andrew Julian Wnuk | Isosorbide-Plasticized Starch And Uses Thereof |
| CN105670438A (en) * | 2016-03-18 | 2016-06-15 | 湖州国信物资有限公司 | Waterborne acrylic wood coating and preparation method thereof |
| CN106243880A (en) * | 2016-08-25 | 2016-12-21 | 阜阳富瑞雪化工科技有限公司 | A kind of environment-friendly type high life true mineral varnish of anti-soil |
| CN107936766A (en) * | 2017-11-30 | 2018-04-20 | 明光市泰丰新材料有限公司 | A kind of interior wall coating for purifying air and preparation method thereof |
| CN108795179A (en) * | 2018-06-28 | 2018-11-13 | 芜湖市棠华建材科技有限公司 | Aqueous woodware paint |
| CN109575697A (en) * | 2018-11-19 | 2019-04-05 | 西华大学 | A kind of starch-based aqueous coating composition and preparation method thereof |
| WO2019216700A1 (en) * | 2018-05-11 | 2019-11-14 | 주식회사 삼양사 | Solid dispersion, preparation method therefor, chain-extended polyurethane using same, and epoxy resin composition comprising same |
| CN110607116A (en) * | 2019-10-10 | 2019-12-24 | 宁波宣威彩色世界涂料有限公司 | Water-based multipurpose coating and preparation process thereof |
| CN111019505A (en) * | 2019-12-25 | 2020-04-17 | 上海五岳木业有限公司 | Woodenware paint and preparation method thereof |
-
2021
- 2021-12-28 CN CN202111630433.1A patent/CN114213952B/en active Active
Patent Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5138006A (en) * | 1991-02-11 | 1992-08-11 | Eastman Kodak Company | Radiation polymerizable starch ester-urethanes |
| US6008276A (en) * | 1995-06-01 | 1999-12-28 | Bayer Aktiengesellschaft | Polymer blends containing starch and polyurethane |
| CN1183791A (en) * | 1995-06-30 | 1998-06-03 | 旭化成工业株式会社 | Highly emulsifiable and stable polyisocyanate composition and water-based coating composition containing the composition |
| CN101085891A (en) * | 2006-06-07 | 2007-12-12 | 关西涂料株式会社 | Starch-based coating composition |
| US20100034978A1 (en) * | 2008-08-08 | 2010-02-11 | Formulated Solutions Llc | Compositions for use in construction and methods of applying the same |
| US20120178858A1 (en) * | 2011-01-10 | 2012-07-12 | Andrew Julian Wnuk | Isosorbide-Plasticized Starch And Uses Thereof |
| CN105670438A (en) * | 2016-03-18 | 2016-06-15 | 湖州国信物资有限公司 | Waterborne acrylic wood coating and preparation method thereof |
| CN106243880A (en) * | 2016-08-25 | 2016-12-21 | 阜阳富瑞雪化工科技有限公司 | A kind of environment-friendly type high life true mineral varnish of anti-soil |
| CN107936766A (en) * | 2017-11-30 | 2018-04-20 | 明光市泰丰新材料有限公司 | A kind of interior wall coating for purifying air and preparation method thereof |
| WO2019216700A1 (en) * | 2018-05-11 | 2019-11-14 | 주식회사 삼양사 | Solid dispersion, preparation method therefor, chain-extended polyurethane using same, and epoxy resin composition comprising same |
| CN108795179A (en) * | 2018-06-28 | 2018-11-13 | 芜湖市棠华建材科技有限公司 | Aqueous woodware paint |
| CN109575697A (en) * | 2018-11-19 | 2019-04-05 | 西华大学 | A kind of starch-based aqueous coating composition and preparation method thereof |
| CN110607116A (en) * | 2019-10-10 | 2019-12-24 | 宁波宣威彩色世界涂料有限公司 | Water-based multipurpose coating and preparation process thereof |
| CN111019505A (en) * | 2019-12-25 | 2020-04-17 | 上海五岳木业有限公司 | Woodenware paint and preparation method thereof |
Non-Patent Citations (2)
| Title |
|---|
| 孙家干等: "水性聚氨酯的复合改性研究及应用新进展", 《中国皮革》, vol. 38, no. 23, 3 December 2009 (2009-12-03), pages 42 - 46 * |
| 赵青山等: "醋酸淀粉合成的工艺研究", 《山西食品工业》, no. 3, 30 September 2004 (2004-09-30), pages 31 - 33 * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN117165174A (en) * | 2023-11-03 | 2023-12-05 | 广州哲铭新材料科技有限公司 | Water-based multifunctional paint and preparation method thereof |
| CN117165174B (en) * | 2023-11-03 | 2024-02-02 | 广州哲铭新材料科技有限公司 | Water-based multifunctional paint and preparation method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN114213952B (en) | 2022-07-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN110885630A (en) | Organic silicon water-based high-temperature-resistant special coating and preparation method thereof | |
| CN102268221A (en) | Double-component aqueous inorganic non-expandable fireproof coating and preparation method thereof | |
| CN117050627B (en) | Polyaspartic acid ester polyurea coating for SMC (sheet molding compound) substrate and preparation method thereof | |
| CN112358780A (en) | Coating and preparation method and application thereof | |
| CN110229602A (en) | Fluorocarbon coating and preparation method thereof and coating process | |
| CN114716852A (en) | Lithium silicate paint for internal and external walls and preparation method thereof | |
| CN114213952B (en) | Environment-friendly water-based paint and preparation method thereof | |
| CN111019453A (en) | Environment-friendly waterproof coating and preparation method thereof | |
| WO2022088114A1 (en) | Water-based paint special for glass substrates and preparation method therefor | |
| CN112048238A (en) | Two-component isocyanate coating | |
| CN115011190B (en) | Water-based low-temperature quick-drying acrylate coating and preparation method thereof | |
| CN113621290A (en) | Low-VOC high-gloss water-based steel drum outer wall coating and preparation method thereof | |
| CN105238181A (en) | Aqueous flow-coating high-temperature-resistant woodenware paint | |
| CN111253825B (en) | Water-based acrylic acid graft modified polysiloxane coating, preparation method and application | |
| CN107641402B (en) | Nano flame-retardant stone paint and preparation method thereof | |
| CN115975461B (en) | Heat-insulating anti-condensation coating, and preparation method and application thereof | |
| CN110819182A (en) | High-environment-friendly alcohol-resistant wear-resistant water-based plastic paint and preparation method thereof | |
| WO2020056628A1 (en) | Aqueous nano porcelain imitating coating and preparation method therefor | |
| CN114031726A (en) | Hydroxyl acrylic acid dispersion and preparation method and application thereof | |
| CN112063299A (en) | Ceramic tile antifouling agent and preparation method thereof | |
| CN118638462A (en) | A water-based oil-resistant, heat-resistant, insulating and anti-rust primer and its application | |
| CN114149710B (en) | Environment-friendly paint remover and preparation method thereof | |
| CN117844328A (en) | A highly weather-resistant water-based epoxy-modified polyacrylate coating and a preparation method thereof | |
| CN118421190B (en) | Bio-based anticorrosive paint and preparation method thereof | |
| CN111019517B (en) | Water-based ceramic coating and preparation method thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |