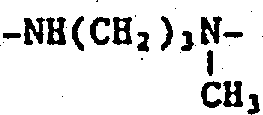具有纤维反应性基团的发色化合物或其盐或其混合物在含羟基或含氮有机基质染色或印花中的应用
本发明涉及含有纤维反应性基团的发色化合物及其制备方法。这些化合物适于在任何传统染色和印花工艺中用作纤维反应性染料。
更具体地说,本发明提供了式I化合物的游离酸或盐,和式I化合物的混合物,
式中Fc是无金属的或金属配合物形式的水溶性偶氮、甲_、酞菁、偶氮甲碱、噁嗪、噻嗪、吩嗪或三苯甲烷染料的基团,这些基团可以含有另外的纤维反应性基团,
a各自独立表示0或1,
b是1或2,
X各自独立表示直接键、-CO-或-SO2-,
R1各自独立表示氢、未取代的C1-4烷基或被羟基、卤素、-SO3H、-OSO3H或-COOH单取代的C1-4烷基,
Alk各自独立表示C2-4亚烷基;
W 各自独立表示
亚烷基
亚烷基
或
式中m是0或1,
B
1是C
2-6亚烷基,间有-O-或-NR
1-的C
2-6亚烷基链,被一个或两个羟基或被一个羧基取代的C
3-6亚烷基,
或
式中n是0、1、2、3或4,
R2是氢,C1-4烷基,C1-4烷氧基,-COOH或-SO3H。
说明书中的任何烷基、链烯基、炔基或亚烷基,如不另外指明,都是直链或支链基团。在任何由羟基取代的、连接到氮原子上的烷基或亚烷基中,该羟基最好连在不直接连在氮原子上的碳原子上。在所有含两个羟基的亚烷基中,这些羟基连在最好彼此不相邻的不同的碳原子上。在间有-O-或-NR1-、并且连在氮原子上的任何亚烷基中,-Q-或-NR1-最好连在不直接与氮原子相连的碳原子上。
任何卤素优先指氟、氯或溴;氯或溴更好,氯最好。
Fc最好是无金属的或含有金属的单偶氮或双偶氮染料的水溶性基团,当染料为金属配合物时,它最好是甲_的1∶1铜配合物或1∶2铬配合物或1∶2钴配合物形式,酞菁或三苯二噁嗪染料的铜或镍配合物。
X最好各自独立表示直接键或-SO2-。
Alk最好各自独立表示C2-3亚烷基。
R1表示的任何烷基最好含1或2个碳原子。
R1表示的任何取代的烷基最好是C1-3烷基,并且其中最好含一个选自羟基、-SO3H、-OSO3H或-COOH(尤其是羟基)的取代基。
所有R1是R1a较好,其中R1a各自独立表示氢、甲基、乙基、2-羟基乙基、-(CH2)r-SO3H、-(CH2)r-OSO3H或-(CH2)q-COOH,其中r是1或2,而q是1、2或3。
所有R1是R1b更好,其中R1b各自独立表示氢、甲基、乙基或2-羟基乙基。最好各个R1都是氢。
所有R2是R2a较好,其中R2a各自独立表示氢、甲基、甲氧基、-COOH或-SO3H。所有R2是R2b更好,其中R2b各自独立表示氢或-SO3H。
所有B
1是B
1a较好,其中B
1a各自独立表示C
2-4亚烷基、C
2-3亚烷基-O-C
2-3亚烷基、C
2-3亚烷基
亚烷基、单羟基取代的C
3-4亚烷基、
或
式中n
1是0、1、2或3。所有B
1是B
1b更好,其中B
1b各自独立表示C
2-3亚烷基、单羟基取代的C
3-4亚烷基、
或
式中n
2是0、1或2。
所有B1是B1C最好,其中B1C各自独立表示C2-3亚烷基或单羟基取代的C3-4亚烷基。
所有W是W
1较好,其中W
1各自独立表示
亚烷基
或
亚烷基
所有W是W
2更好,W
2各自独立表示
或
所有W是W
3最好,其中W
3各自独立表示-NH-B
1C-NH-。
较好的无金属或金属配合物形式的式(I)化合物相应于下面限定的式(1)至(7)所示的化合物:化合物(1)式中DK是苯型或萘型重氮成分的基团,
KK是苯型、萘型或杂环型偶合成分的或能被烯醇化的CH-酸性化合物的基团,Z是
式中W如上所限定,b是1或2,其中一个或两个基团Z通过
连到重氮和/或偶合成分上。最好b是1。化合物(1)中:DK最好是基团(a)至(h)中的一个,这些基团中每个标以星号的键都是与偶氮基相连的,
式中R1和R2的定义同上,
R3是氢、卤素、C1-4烷基、C1-4烷氧基、乙酰氨基、苯甲酰氨基、-SO3H或-COR13,
R4是氢、卤素、C1-4烷基、C1-4烷氧基或-COR13,
R13是-OH、-OC1-4烷基或-NH2,
Alk1是-C2-3亚烷基,
R5是氢、-SO3H、C1-4烷基或C2-4羟烷基,
R50是氢或-SO3H,
R51是
n是0、1、2、3或4,
p是0、1或2,
q是1、2或3,和
r是1或2;
KK最好是(K1)-(K7)基团之一,其中标以星号的碳原子为发生偶合的位置,式中R1定义同上,
R6是氢、C1-4烷基或C1-4烷氧基,和
R
7是氢、卤素、C
1-4烷基、C
1-4烷氧基、-NHCOC
1-4烷基或-NHCONH
2;
式中R
1的定义同上,
R8是氢、卤素、C1-4烷基或C1-4烷氧基,
R9是氢、卤素、C1-4烷基、C1-4烷氧基、-COOH或-SO3H,和
R
10是氢、-SO
3H或-NR
1-(Z);
式中R
11是-OH或-NH
2,
R12是C1-4烷基或-COR13,
R13的定义同上,
R8和R9的定义同上,和
-SO
3H或
其中R
1和a定义同上,B
3是二价桥连基团;
式中Q
2是氢,C
1-4烷基,C
5-6环烷基,苯基或苯基(C
1-4烷基)(其中的各个苯环是未取代的或被1-3个选自C
1-4烷基、C
1-4烷氧基、卤素、-COOH和-SO
3H的取代基取代的),-COR
13或被-SO
3H、-OSO
3H或-COR
13单取代的C
1-4烷基,
Q
3是氢,-CN,-SO
3H,-COR
13,C
1-4烷基,被-OH、卤素、-CN、C
1-4烷氧基、
,-SO
3H,-OSO
3H或-NH
2单取代的C
1-4烷基,-SO
2NH
2 或
式中R
15是氢、C
1-4烷基或C
2-4羟烷基,及An
_是非发色阴离子,Q
4是氢,
C
1-6烷基,C
2-4链烯基,C
2-4炔基,C
5-6环烷基,苯基或苯基(C
1-4烷基)(其中的各苯环是未取代的或由1-3个选自卤素、C
1-4烷基、C
1-4烷氧基、-SO
3H、-COOH和
的取代基取代的),或-C
1-6亚烷基-Y,式中Y是-COOH,-COOC
1-4烷基,-SO
3H,-OSO
3H,-OH,-CN,C
1-4烷氧基,-NH
2或一个可质子化的脂族、环脂族、芳族或杂环的氨基或季铵基,
B
2是C
2-6亚烷基,单羟基取代的C
3-6亚烷基,一个间有-O-或-NR
1-的C
2-6亚烷基链,
-C
1-4亚烷基
或
亚烷基 或者-B
2-NR
1-(Z)是-C
2-4亚烷基
并且R
1,R
9,R
10和R
13定义同上;
式中R
18是C
1-4烷基,苯基或被1-3个选自卤素、C
1-4烷基和C
1-4烷氧基的取代基取代的苯基,
R1、a和m定义同上。化合物(2)式中R6、R7、R1和Z定义同上,
t是2或3,
R6X独立地具有R6含义中的一种,和
R7X独立地具有R7含义中的一种。
较好的化合物(2)是其中R6、R6X和R7X各自为氢的化合物。
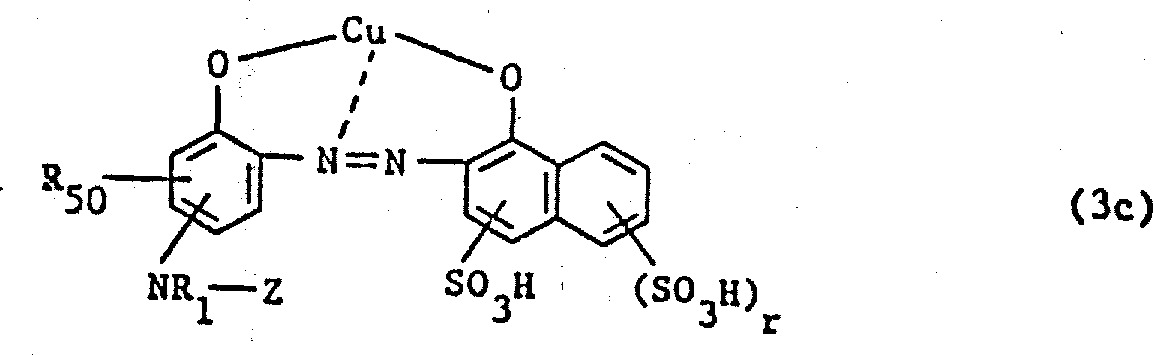
提供的化合物(3)为以下述无金属化合物(3a)或(3b)为基础的金属配合物,较好的是1∶1铜配合物和1∶2铬配合物或1∶2钴配合物,
式中R
1、Z和m定义同上,A是
或
式中m和q定义同上,
R17是氢,卤素,硝基,C1-4烷基,C1-4烷氧基,-COR13,-SO3H或-NHCOCH3,
R22是OH,OCH3或NH2,和
R13定义同上。
在1∶2铬配合物或1∶2钴配合物中,两个偶氮化合物可以相同或不同,即它是对称的或不对称1∶2金属配合物。
特别好的是基于化合物(3a)的1∶1铜配合物,其中A是苯型重氮成分的基团,而萘型偶合成分的基团如下式所示,
此外,还有相应于下述式(3c)-(3e)的1∶1铜配合物:
其中R
50是氢或SO
3H,
R1、Z和r定义同上;式中R52是氢,C1-4烷基或C1-4烷氧基,
m是0或1,r是1或2,
m+r是2或3,
R
1、R
2、R
50和Z定义同上;
式中D
3是
或
其中R
29是氢,COOH或SO
3H,
R30是氢,C1-4烷基,C1-4烷氧基或-O-Alk1-OR5,而t、R5和Alk1定义同上,
R1、R52和Z定义同上。化合物(4)式中D1和D2各自独立表示至少含有一个磺基、磺酰氨基或羧基的氨基苯或氨基萘系列的重氮成分的基团,
各个m是0或1,但要求至少一个m是1,
R1、Z和n定义同上。
D1是D1a较好,D1a是式(ax)或(cx)基团,式中R18是磺基,磺酰氨基或-COR13,
R19是氢、卤素、C1-4烷基、C1-4烷氧基、-NHCOCH3或SO3H,
R13、m和p定义同上,
m+p是1或2。
D2是D2a较好,D2a是式(dx)基团,式中R4和R18定义同上,标星号的键接到偶氮基上,另一个自由键接到活性基上。
更好的情况是,(dx)基团中R
18在2位(偶氮基在1位),而接在活性基上的自由键在4位或5位。化合物(5)
式中X
1和X
2中之一是-O-,而另一个是羧基,
R20和R21各自独立表示氢,卤素,C1-4烷基,C1-4烷氧基,-COR13或-NHCO(C1-4烷基),
R1、R13、Z和m定义同上,
p各自独立表示0、1或2,并且
m+2p至少是2。化合物(6)及其混合物
式中Q是-O-或-S-,
R23各自独立表示氢或-SO2CH2CH2OSO3H,
m各自独立表示0或1,
X3各自独立表示-SO2-,-NR1-,-*CONR1-,或-*SO2NR1-,其中标星号的原子接在环系碳原子上,
Alk2各自独立表示C2-4亚烷基,及
R1和Z各自独立表示上述的定义,
在一个化合物(6)中,两个Z最好相同。化合物(7)及其混合物式中c是1、2或3,
d是0、1或2,但要求c+d不大于3;
Pc是酞菁基,
Me是铜、镍、钴、铁或铝,
R
24和R
25各自独立表示氢或C
1-6烷基,或-NR
24R
25是可再包含一个-O-或
的饱和的5或6员杂环,其中R
31是氢、C
1-4烷基、2-羟基乙基或2-氨基乙基,
R26是氢,卤素,羟基,C1-4烷基,C1-4烷氧基,-COOH或-SO3H,
a是0或1,X
4是可任选地含有
的二价的脂族、芳脂族或杂环桥连基团,
或是-N=N-KK
2,基中KK
2是
或
R
9、R
11、R
12、Q
2、-B
2-、-B
3-和a定义同上,各个标有星号的碳原子指出偶合位置,Q
3a是氢,CN,-SO
3H,-COR
13,C
1-4烷基,被羟基、卤素、-CN,C
1-4烷氧基
,-SO
3H,-OSO
3H或-NH
2;单取代的C
1-4烷基,-SO
2NH
2;
或
An
_,其中R
9、R
13、R
15和An
_定义同上。纤维反应性基团Z为相应于Za结构的基团较好,
Z是Zb更好,其中Zb是W
1等于W
2的Za基团。
Z是Zc最好,其中Zc是W1等于W3的Za基团。
较好的式(1)化合物是相应于式(1a)~(1d)的化合物:式(1a)化合物
式中Za定义同上并连在DK
1或KK
1上,
DK
1是(a
1)~(f
1)基团之一或基团(h
1),其中标有星号的键连在偶氮基上:
式中p是0、1或2,R
3a和R
4a各自独立表示氢,氯,甲基,甲氧基,乙氧基或-COOH;
式中m是0或1,t是2或3,R
5a是氢,甲基,乙基,-SO
3H或2-羟基乙基;
式中m是0或1,r是1或2,m+r是2或3;
式中R
1b、R
4a和p定义同上;
式中R
1b和r定义同上;
式中R
51a是
或
R
50、R
1b、a和r定义同上;KK
1是下面限定的(K
2a)、(K
4a)和(K
5a)所示基团之一或是上面限定的(K
3)基团:
式中R
8a是氢,氯,甲基或甲氧基,R
9a是氢,氯,甲基,甲氧基,-SO
3H或-COOH,R
10a是氢,-SO
3H或
式中R
11、R
8a和R
9a定义同上,R
12a是甲基,-COOH或-CONH
2,R
14a是氢,氯,甲基,乙基,-SO
3H或
式中Q
2a是氢,甲基,乙基,苯基,-COR
13,-CH
2SO
3H或-CH
2OSO
3H,Q
3b是氢,-CN,-SO
3H,-COR
13,甲基,乙基,-CH
2SO
3H或-CH
2NH
2,
Q
4a是氢,
甲基,乙基,环己基,苯基或苯基(C
1-2烷基),在后面两个基团中,苯环是未取代的或被1或2个选自氯、甲基、甲氧基、-SO
3H、-COOH、-NH
2和
的取代基取代的,或是-C
1-4亚
烷基-Y
2,B
2a是-C
2-4亚烷基,单羟基取代的-C
3-4亚烷基-,
-C
1-2亚烷基
或
亚烷基或B
2a和与之相连的
一起形成-C
2-3亚烷基
Y
2是-COOH,-SO
3H,-OSO
3H,-OH,-CN,甲氧基或-NR
27R
28,R
27和R
28各自独立表示氢,未取代的C
1-4烷基,被羟基、C
1-4烷氧基、-COOH、-SO
3H、-NH(C
1-4烷基)或N(C
1-4烷基)
2单取代的C
1-4烷基,未取代的环己基,被1-3个甲基取代的环己基,苯基或苯基(C
1-4烷基),其中各苯环是未取代的或被1或2个选自卤素(最好是氯)、C
1-4烷基、C
1-4烷氧基、-SO
3H和-COOH的取代基取代的,或者-NR
27R
28是一个哌啶、吗啉或哌嗪环,其中的各环是未取代的或含有1~3个甲基的;式(1b)化合物,
式中m+r等于2或3,
R
6a是氢,甲基或甲氧基,R
7a是氢,氯、甲基,甲氧基,-NHCOCH
3或-NHCONH
2;式(1c)化合物,
式中DK
2为上面限定的式(a
1)、(b
1)或(c
1)基团;式(1d)化合物,
式中磺基在萘环的3位或4位,
DK
3是式(a
2)或(c
2)的基团,
式中R
3a和R
4a定义同上,
式中m+p等于1或2。较好的化合物(2)相应于式(2a),
式中R
7a定义同上,m+r等于2或3。
较好的化合物(3)是基于无金属化合物(3a
1)或(3b
1)的1∶1铜配合物,
其中A
1是
或
R
17a是氢,氯,溴,硝基,甲基,甲氧基,-SO
3H或-COOH,R
22a是OH或OCH
3,以及m+r等于2或3;
式中R
22a定义同上。
此外,较好的化合物(3)还有相应于下述通式(3c1)、(3d1)和(3e1)的1∶1铜配合物,式中R50和R2a定义同上,R52a是氢,甲基或甲氧基,D3a是
式中R30a是氢,甲基或甲氧基。较好的化合物(4)为式(4a)化合物,式中D1a、R4和R18定义同上;
更好的是下述式(4a)化合物:其中R4是氢,R18在苯基的2位,而
在苯基的4或5位。较好的式(5)化合物是其中R20和R21各为R20a和R21a的化合物,而R20a和R21a各自独立表示氢、氯、甲基、甲氧基、-COOH或-NHCOCH3。
较好的化合物(6)是下述化合物:其中Q是-O-,X
3各自独立表示
或
R1各自独立表示R1b,
Alk2各自独立表示-C2-3亚烷基-,
Z各自独立表示Za。
更好的化合物(6)是其中两个含有Za的基团相同的化合物。
较好的化合物(7)是其中Me是铜或镍、而R24和R25各为氢的那些化合物。
更好的化合物(7)是下述化合物:其中Me是铜或镍,c是2或3,而d是0,R
1各自独立表示R
1b,Z是Za,R
26是氢,-COOH或-SO
3H,以及a是0,或a是1并且X
4是-N=N-KK
2,其中KK
2是
式中Q
2a、Q
3b和-B
2a-定义同上。
此外,特别好的情况是在上述所有好的无金属化合物和金属配合物中
(1)Za是Zb;
(2)Za是Zc;
(3)Za是Zc,而R1b是氢。
当式I化合物是盐的形式时,与磺基和任何羧基结合的阳离子不严格,可以是纤维反应性染料领域中那些常规的任意非发色阳离子,但要求相应的盐是水溶性的。这些阳离子的实例有碱金属阳离子和未取代的和取代的铵离子,如锂、钠,钾,铵,一、二、三和四甲基铵三乙基铵和一、二和三乙醇铵等离子。
较好的阳离子是碱金属阳离子和铵离子,而以钠离子为最好。
式I化合物中与磺基和任意羧基结合的阳离子可以相同或不同,例如可以将上述阳离子混合使用,这意味着式I化合物可以是混合盐形式的。
本发明进一步提供了式I化合物及其混合物的制备方法,包括使式II化合物或其混合物在Y
3是-W-H时与5-氰基-2,4,6-三氯嘧啶反应,或者在Y
3是氯时与式III化合物(按式II化合物和式III化合物1∶1摩尔比)反应,
式中Y
3是-W-H或氯,Fc、X、R
1、Alk、a、b和W定义同上,
式中W定义同上。
式II化合物中Y3是-W-H较好。式II(Y3是-W-H)化合物与5-氰基-2,4,6-三氯嘧啶的缩合反应适于在0-40℃和pH7-9的条件下进行。通常以水为反应介质,但当5-氰基-2,4,6-三氯嘧啶溶于有机溶剂如丙酮中时,它也可以使用。
另外,式(4)的双偶氮化合物也可以通过使式IV化合物重氮化并与式V化合物碱性偶合来制备,
式中D
2、R
1、Z、m和n定义同上,
式中D
1、R
1、Z、m和n定义同上。
式I化合物可按照已知方法,例如常规的使用碱金属盐进行盐析、过滤并干燥(需要时可用真空和稍微提高温度),分离出来。
根据反应条件和分离条件,得到的式I化合物是游离酸或者比较好的是含有例如一种或多种上述阳离子的盐的形式或甚至是混合盐的形式。可以通过常规方法使其从游离酸形式转变为盐或混合盐的形式,或者按相反方向转变,或者从一种盐转变为另一种盐的形式。
式II、III、IV和V的起始化合物或是已知物,或是可以按照已知方法由已知原料制备的。
式I化合物及其混合物可用作纤维反应性染料将含羟基或含氮有机被染物进行染色或印花。较好的基质是皮革或含有下述材料或由所述材料构成的纤维材料:天然或合成聚酰胺,特别是天然或再生纤维素如棉、粘胶纤维和人造棉纱。最好的基质是含棉或由棉构成的纺织材料。
染色或印花是按照纤维反应性染料领域常用的已知方法进行的。对于式I化合物,在30-80℃温度范围内使用浸染法。
本发明化合物能与其它纤维反应性染料很好地相容;它们可以单独使用,也可以与具有类似染色性能的适当的同类纤维反应性染料结合使用,这些性能包括例如共同的染色牢度、由染缸到纤维的上染能力大小等等。用这种结合物染出的颜色牢度好,并且比得上用单一染料所获得的染色效果。
从它们的显著的集结能力来看,式I化合物具有很好的上染率和固色率。没有固着的那一部分染料可以很容易地从基质上冲脱掉。所得到的染色和印花表明具有好的干态和湿态的耐光牢度,也具有好的耐湿牢度如耐洗、耐水、耐海水和耐汗牢度都很好。它们也能抗氧化影响,例如能抗含氯的水、次氯酸盐漂白液和含有过氧化物或过硼酸盐的洗涤液的氧化作用。
用下述实例进一步说明本发明。实例中所有份数和百分比都是按重量或体积计。温度为摄氏度。实例1
在25°往在500份水中的18.3份(0.025摩尔)式B化合物中一次加入6.1份(20%过量)5-氰基-2,4,6-三氯嘧啶丙酮溶液。持续加入20%碳酸钠溶液,使混合物的自发降低的pH值保持在7-7.5。同时加热混合物达最终温度为35°。3-4小时后,TLC证明该缩合反应已终止。
为了分离反应产物,将所得暗橙红色溶液加热到40°并与过滤用白土搅拌约10分钟。然后过滤,边搅拌边在滤液中加入氯化钠(约10%体积)。滤出细沉淀物并在约50°真空干燥。所得的下式所示的染料将棉制品染成橙色。按通常方法在棉制品上得到的染色和印花表现出好的耐湿牢度性能和很好的耐光牢度,此外,它们也具有抗氧化作用。
起始化合物B的制备
a)将9.6份2-氨基萘-3,6,8-三磺酸溶于已加入4.3份30%氢氧化钠溶液的pH12的60份水中。往此溶液中滴加11份30%盐酸,得到一种悬浮液,将其充分搅拌。加入25份冰以后,在0-5°加入6.5份4N亚硝酸钠溶液进行重氮化反应。将此重氮盐溶液在30分钟内加到由3.9份3-氨基苯基脲在25份冰水中组成的3-7°的悬浮液中。加料过程中,加入22份20%碳酸钠溶液,使pH保持在5.0。形成的红色溶液中含有式A的氨基偶氮化合物。
b)用30分钟将5.1份氰尿酰氯搅拌加入到30份冰水中。然后在5分钟内加入a)步所得染料溶液,加入8份20%碳酸钠溶液,在pH6.0进行缩合反应。往得到的红橙色溶液中加入在50份冰水中含有3.3份1,2-二氨基丙烷的、已加入8份30%盐酸将其pH调至6.0的溶液。1小时内,该反应混合物的温度慢慢升到48-50°。同时,加入20份20%碳酸钠溶液使混合物pH保持在6.0。在分离形成的此橙色染料以前,加入100份氯化钠,使其沉淀。于是便得到式B化合物。实例2-200
按照与实例1所述方法类似的方法,用适当的起始化合物以形成所需的发色部分Fc,可进一步制得下面表1-11所列的式I所示的无金属化合物。在所有这些表的顶部都给出了相应的结构式,式中的符号如表中所限定。
实例2-200化合物可按照常规的浸染法或印花工艺用于含有纤维素纤维或由纤维素纤维构成的基质,特别是用于由棉纱构成的纺织材料,得到指定色调的染色和印花。在棉制品上得到的染色和印花表现出好的耐光牢度和耐湿牢度性能,并对氧化作用有抗性。
表1和其后的各表中的-W-栏限定的二价基团可以是不对称二氨基基团。通常,一边的三嗪碳原子和另一边的嘧啶碳原子之间的联接是按给定顺序进行的。但是当嘧啶化合物与二胺的缩合产物用作起始化合物时,根据起始化合物的制备方法,这一联接顺序也可能反过来,即成为嘧啶/三嗪环方式。
表1/式(T1)化合物
实例号 D
T1 .R
1. R
9 . -W-
(取代位置)2
H -SO
3H(3)
3 do. H do.
6
H -SO
3H(2)
7
H do. do.8 do. H do.
9
H -SO
3H(3) do.表1(续)实例号 D
T1 .R
1. R
9 . -W-
(取代位置)10 do. H do.
11
H H
12 do. H SO
3H(3)
13 do. H SO
3H(2)
14
H H
15 do. H H -NHCH
2CH
2NH-16 do. H SO
3H(3)
17
H H do.12 do. CH
3 H do.19 do. H SO
3H(2) do.在上表和以后的表中,do.=同上。用实例2-19的染料在棉制品上进行的染色和印花具有绿黄色调。表2/式(T2)化合物
实例号 R
2 . R
3 . R
4 . R
8 . R
9 . R
10 . -W-20 H H -SO
3H H H H -NHCH
2CH
2NH-21 H H do. CH
3 -SO
3H CH
3
22 CH
3 OCH
3 H do. do. OCH
3 do.23 do. CH
3 H do. do. do. do.24 H H -SO
3H do. do. do.
25 CH
3 OCH
3 H -SO
3H H -SO
3H
用实例20-25的染料在棉制品上进行的染色和印花具有绿黄色调。表3/式(T3)化合物
实例号 -W-26
27
28 -NHCH
2CH
2NH-29
用实例26-29的染料在棉制品上进行的染色和印花具有绿黄色调。表4/式(T4)化合物
在最后一栏I中给出了各自将棉制品染成的染色,其中a是绿黄色,b是黄色。
取代位置实例号 D
T4 . R
12 . R
13 . -NH- . -W- . I30
CH
3 H 5
b31 do. do. H 5
b32
do. H 4 do. b33 do. do. H 4 -NHCH
2CH
2NH- b34 do. do. H 5
b35
do. H 4 do. b36
do. H 5 do. b
表4(续)
取代位置实例号 D
T4 . R
12 . R
13 . -NH- . -W- . I37
-COOH -SO
3H 5
a38
do. do. 4
a39
do. do. 5 do. a40
do. do. 4 do. b41 do. do. do. 4 -NHCH
2CH
2NH- b
在最后一栏I中给出了各自将棉制品染成的染色,其中a是绿黄色,b是黄色。
取代位置实例号 -W- -N=N- . R
2 . R
3 . R
11 . R
12 . R
13 . R
14 . R
15 . I42 -NHCH
2CH
2NH- 4 H 5-SO
3H OH CH
3 H 4-SO
3H H b43 do. 4 H do. OH do. Cl do. 5-Cl b44 do. 5 H 4-SO
3H OH COOH CH
3 do. H a45 do. 5 SO
3H do. OH CH
3 Cl 5-SO
3H H a46 do. 4 H 5-SO
3H NH
2 do. H 3-SO
3H H b47
5 H 4-SO
3H do. do. H 4-SO
3H H a48 do. 5 H do. OH do. Cl do. H a
表5(续)
取代位置实例号 -W- -N=N- . R
2 . R
3 . R
11 . R
12 . R
13 . R
14 . R
15 . I49
4 H 5-SO
3H OH CH
3 SO
3H 5-SO
3H H b50 do. 4 H do. OH COOH H 4-SO
3H H b51 do. 5 SO
3H 4-SO
3H OH CH
3 SO
3H do. H a52
4 H 5-SO
3H NH
2 do. H 3-SO
3H H b53 do. 4 H H do. do. SO
3H 5-SO
3H H b54 do. 4 H 5-SO
3H OH do. Cl do. H b55 do. 5 H 4-SO
3H OH COOH CH
3 4-SO
3H H a56 do. 5 SO
3H do. OH do. H do. H a57 do. 5 H do. OH CH
3 Cl do. H a58
4 H 5-SO
3H OH COOH CH
3 do. H b59 do. 5 H 4-SO3H OH CH
3 Cl 5-SO
3H H a60
4 H 5-SO
3H OH do. SO
3H do. H b61
5 H 4-SO
3H OH do. do. do. H a表6/式(T6)化合物
在最后一栏I中给出了各自将棉制品染色后呈现的色调,其中a是绿黄色,b是黄色。
取代位置 取代位置实例号 -W- . -CONH- . -N=N- R
3 R
11 R
12 R
13 R
14 I62 -NHCH
2CH
2NH- 3′ 3 4-SO
3H OH CH
3 -SO
3H 4-SO
3H a63 do. 4′ 4 3-SO
3H OH do. do. 5-SO
3H b64 do. 3′ 4 do. NH
2 do. H 4-SO
3H a65
3′ 4 do. OH do. -SO
3H 5-SO
3H b66 do. 4′ 3 4-SO
3H OH COOH CH
3 4-SO
3H a67 do. 4′ 3 do. OH do. H do. a68
3′ 4 3-SO
3H OH CH
3 Cl do. b69 do. 3′ 3 4-SO
3H NH
2 do. Cl 5-SO
3H a70 do. 4′ 4 3-SO
3H OH do. -SO
3H 4-SO
3H b71 do. 4′ 4 do. OH COOH CH
3 do. b表7/式(T7)化合物
实例号 R
3 R
4 Q
2 Q
3 Q
4 -W-72 -SO
3H H CH
3 -SO
3H CH
3
73 do. H do. do. do.
74 do. -SO
3H do. -CONH
2 do. do.75 do. do. do. do. -CH
2CH
3 do.76 do. H do. -CH
2SO
3H do. -NHCH
2CH
2NH-77 do. -SO
3H do. H do.
78 do. do. -COOH H H do.79 do. H do. H H
80 H H -CH
2SO
3H H CH
3 do.81 -SO
3H H do. -CONH
2 do.
82 do. H CH
3 H -CH
2CH
2SO
3H
83 do. -SO
3H do. H -(CH
2)
3NHCH
3
84 do. do. do. -CONH
2 -CH
2CH
3
表7(续)实例号 R
3 R
4 Q
2 Q
3 Q
4 -W-85 -SO
3H -SO
3H CH
3 -CONH
2 -CH
2CH
3
86 do. H do. -CH
2SO
3H H
用实例72-86的各种染料将棉制品进行染色和印花,呈现绿黄色调。
取代位置实例号 R4 Q2 Q3 Q4 -NH- -W-87 H CH3 -CH2SO3H H 3
88 H do. do. CH3 4 do.89 H do. do. -CH2CH3 3 do.90 H -COOH H H 3 do.91 H CH3 -SO3H H 3 do.92 -SO3H do. -CH2SO3H H 4
93 do. do. -CONH2 -CH2CH3 4 do.94 H do. -CH2SO3H do. 3 do.
表8(续)
取代位置实例号 R
4 Q
2 Q
3 Q
4 -NH- -W-95 H -CH
2SO
3H -CONH
2 CH
3 3
96 -SO
3H CH
3 H -CH
2CH
2SO
3H 4 do.97 H -CH
2SO
3H -CH
2SO
3H CH
3 3
98 -SO
3H CH
3 -CONH
2 -CH
2CH
2SO
3H 4
99 H CH
3 H -CH
2CH
3 4
用实例87-99的各种染料将棉制品进行染色和印花,呈现绿黄色调。表9/式(T9)化合物
在最后一栏I中给出了各自将棉制品染成的色调,其中a是绿黄色,b是黄色。
-B-栏中限定的二价基中所有标以星号的碳原子连接在吡啶酮的氮原子上。
表9中的D
T9可以是下式基团,
根据R
50和R
51的定义,相应的基团表示为DD
1至DD
12。下面给出DD
1至DD
12各个基团的意义。DD
1 : R
51 为
和R
50 为 HDD
2 : R
51 为 do. 和R
50 为 SO
3HDD
3 : R
51 为
和R
50 为 HDD
4 : R
51 为
和R
50 为 HDD
5 : R
51 为
和R
50 为 SO
3HDD
6 : R
51 为
和R
50 为 HDD
7 : R
51 为
和R
50 为 HDD
8 : R
51 为 do. 和R
50 为 SO
3HDD
9 : R
51 为
和R
50 为 HDD
10 : R
51 为
和R
50 为 HDD
11 : R
51 为
和R
50 为 SO
3HDD
12 : R
51 为
和R
50 为 H.此外,D
T9可以是下式基团,
在DD
13-DD
21表示的相应基团中,R
50和R
51限定如下:DD
13 : R
51 为
和R
50 为 HDD
14 : R
51 为
和R
50 为 SO
3HDD
15 : R
51 为
和R
50 为 HDD
16 : R
51 为
和R
50 为 H
DD
19 : R
51 为
和R
50 为 SO
3HDD
20 : R
51 为
和R
50 为 SO
3HDD
21 : R
51 为
和R
50 为 H.
表9实例号 D
T9 Q
2 Q
3 -B- -W- I
102
do. -CH
2SO
3H
a
105
do. -CONH
2
a106
-CH
2SO
3H H
do. b107
CH
3 H -
*CH
2CH
2NH- -NHCH
2CH
2NH- b
表9(续)实例号 D
T9 Q
2 Q
3 -B- -W- I108
-CH
2SO
3H -CH
2SO
3H -
*CH
2CH
2NH-
b109
CH
3 -CONH
2
a110
do. H -
*CH
2CH
2NH-
a111
CH
3 -CH
2SO
3H
b112
do. do.
b113
-CH
2SO
3H -CONH
2
do. a114
CH
3 -CH
2SO
3H do. -NHCH
2CH
2NH- a
表9(续)实例号 D
T9 Q
2 Q
3 -B- -W- I115
CH
3 -CH
2SO
3H
a116
do. do.
a117 DD
1 CH
3 H -
*CH
2CH
2NH- -NHCH
2CH
2NH- a118 do. do. -CONH
2
do. a119 do. do. H do.
a120 DD
2 do. -CONH
2 do.
a121 do. do. -CH
2SO
3NH -
*CH
2CH
2NH- do. a122 DD
3 do. SO
3H
a123 DD
4 do. H do.
a124 do. do. -CONH
2 -
*(CH
2)
3NH- do. a125 DD
5 do. -CH
2SO
3H
do. a126 DD
6 do. -CONH
2 -
*CH
2CH
2NH- do. a127 do. do. H do.
a128 DD
7 do. H do.
a129 DD
8 do. -CONH
2
do. a130 do. do. H do. -NHCH
2CH
2NH- a
表9(续)实例号 D
T9 Q
2 Q
3 -B- -W- I131 DD
9 CH
3 -CH
2SO
3H -
*CH
2CH
2NH-
a132 DD
10 do. H do. do. a133 DD
11 -CH
2SO
3H -CH
2SO
3H do. do. a134 DD
12 do. -CONH
2
a135 DD
13 CH
3 do. do. do. a136 do. do. H -
*CH
2CH
2NH- do. a137 DD
14 do. -CH
2SO
3H do.
a138 DD
15 do. H do. do. a139 DD
16 do. -CONH
2
do. a140 do. do. H -
*CH
2CH
2NH- do. a141 DD
17 do. -CH
2SO
3H
a142 DD
18 do. H do.
a143 DD
19 do. -CH
2SO
3H -
*CH
2CH
2NH- do. a144 DD
20 do. -CONH
2 do. do. a145 do. do. H
do. a146 DD
21 do. -CONH
2 do. -NHCH
2CH
2NH- a
表10/式(T10)化合物
在最后一栏I中给出了各自将棉制品染成的色调,其中b是黄色,c是黄橙色,d是橙色。实例 取代位置 m R
6 R
7 -W- I号 -N=N- (取代位置)147 2 1(3) H -NHCONH
2 d148 2 1(4) H do. do. d149 1 1(3) H do. do. d150 2 0 H CH
3 do. b151 2 1(4) H do. do. b152 1 1(3) H do. do. b153 2 1(3) H H do. b154 2 0 H H do. b155 2 1(3) CH
3 CH
3 do. b156 2 1(4) OCH
3 H do. b157 2 1(3) H -NHCOCH
3 do. c158 1 1(3) H do. do. c159 1 1(3) H -NHCONH
2 d160 2 0 H do. do. d161 2 1(4) H do. do. d162 2 1(3) H H do. b
表10(续)实例 取代位置 m R
6 R
7 -W- I号 -N=N- (取代位置)163 2 1(3) H -NHCOCH
3
c164 1 1(3) H do. do. c165 2 0 H do. do. c166 2 1(3) H CH
3 do. b167 2 1(3) OCH
3 H do. b168 2 1(3) H -NHCONH
2 -NHCH
2CH
2NH- d169 2 1(4) H do. do. d170 1 1(3) H do. do. d171 2 1(3) H CH
3 do. b172 2 1(3) H H do. b173 2 1(3) H -NHCONH
2
d174 2 1(4) H CH
3 do. b175 2 1(3) H -NHCONH
2 d
表11/式(T11)化合物
m实例 (取代位置) R
7 -W-号176 1(4) CH
3
177 1(4) -CH
2CH
3 do.178 0 CH
3 do.179 1(3) do. do.180 1(4) do.
181 1(4) -CH
2CH
3 do.182 0 do. do.183 0 CH
3 do.184 1(3) -CH
2CH
3 do.185 1(4) CH
3 -NHCH
2CH
2NH-186 1(3) do. do.187 0 do. do.188 1(4) do.
189 1(4) do.
190 1(3) -CH
2CH
3 do.191 0 CH
3
192 1(3) H
193 1(4) H do.
表11(续)
m实例 (取代位置) R7 -W-号194 1(3) -NHCOCH3 -NH(CH2)3NH-195 1(3) do.
196 1(3) -NHCONH2 do.197 1(4) do. do.198 1(3) H do.199 1(3) -NHCONH2
200 1(3) do. -NH(CH2)3NH-
分别用实例176-200的染料对棉制品染色和印花,呈现橙色。实例201
将15.6份(0.02mol)下式所示的染料溶于300份pH8.5-9的水中。加入少量盐酸调pH至6.0。在10-15°和搅拌条件下,往此溶液中加入3.9份(0.02mol+5%)氰尿酰氯,并且通过加入氢氧化钠溶液保持pH为5.5-6.5。约1小时后反应完成。
往此反应混合物(0.02mol)中加入1.6份(0.02mol+10%)1,2-二氨基丙烷。在40-50°进行缩合,加入氢氧化钠溶液将pH保持在5.5-6.5。大约2小时后反应完成。
往得到的细的染料悬浮液(0.02mol)中加入4.6份(0.02mol+10%)5-氰基-2,4,6-三氯嘧啶。在15-20°和pH8.5-9.0进行反应。通过常规的盐析、过滤和干燥方法分离出相应于下式的染料,
它将棉制品染成蓝色。这些棉制品的颜色具有高的耐光和耐湿性能,并且能抗氧化作用。实例202-291
按照实例201所述的类似方法,用适当的起始化合物制得所需发色部分Fc,进一步制得下述表12-17所列的式I的含金属化合物。在每个表的顶部给出相应的结构式,式中的符号在表中给予限定。
采用常规的浸染法或印花工艺,实例202-291的金属配合物将含有纤维素纤维或由纤维素纤维构成的基质(尤其是棉纺织物)染成所指定的色调。如此形成的棉制品上的染色和印花具有高的耐光或耐湿性能,并且能抗氧化作用。
表12/式(T12)化合物
实例号 D
T12 R
52 -W-202
CH
3 -NHCH
2CH
2NH-203 do. do.
204 do. do.
205
H
206
H do.207
CH
3
208
do. do.209 do. H
表12(续)实例号 D
T12 R
52 -W-210
H
211 do. CH
3 -NHCH
2CH
2NH-212
do.
实例202-214的各种染料将棉制品染成蓝色。表13/式(T13)化合物
表13
取代位置实例号 R
2 R
29 R
52 -NH- -W- q(取代位置)215 H H H 4 -NHCH
2CH
2NH- 2(3,6)216 2-OCH
3 3-SO
3H CH
3 5
2(3,8)217 H do. H 5 do. 3(3,6,8)218 2-OCH
3 H CH
3 4
do.219 2-CH
3 H H 5 do. 2(4,6)220 H 2-SO
3H H 4 -NH(CH
2)
3NH- 2(3,8)221 H do. H 4
do.222 H do. CH
3 5 do. 2(3,6)223 H do. H 4 do. 3(3,6,8)224 2-OCR
3 3-SO
3H H 5 do. do.225 do. do. H 5
do.226 do. do. H 5 -NHCH
2CH
2NH- do.实例215-226的染料将棉制品染成蓝色。
表14/式(T14)化合物
表14
取代位置实例号 SO
3H R
17 R
1 -W-227 4 H H
228 4 H CH
3 do.229 6 H H do.230 4 6-Cl H do.231 4 do CH
3 do.232 6 4-Cl H do.233 4 6-COOH H do.234 4 6-SO
3H H do.235 4 do. CH
3 do.236 4 H H
237 6 H H do.238 6 4-CH
3 H do.239 4 6-Cl H do.240 4 6-SO
3H H do.241 4 do. H -NHCH
2CH
2NH-242 4 H H do.243 4 6-NO
2 H do.244 4 6-Cl H do.245 6 4-Cl H do.246 4 H H -NH(CH
2)
3NH-247 4 6-Cl H do.248 4 6-SO
3H H do.249 4 do. H
250 4 do. H
实例227-250的染料将棉制品染成宝石红色。
表15/式(T15)化合物
实例号 -W-251 -NHCH
2CH
2NH-252
253 -NH(CH
2)
3NH-254
255
256
实例251-256的染料将棉制品染成深蓝色。表16/式(T16)化合物
表中最后一栏I给出了各自的棉制品染色色调,其中g是宝石红色,h是紫色。
表16
取代位置实例号 R
50 -NH- -W- r(取代位置) I257 5-SO
3H 3 -NHCH
2CH
2NH- 1(6) g258 do. 3
do. g259 3-SO
3H 5 do. do. g260 do. 5
do. g261 5-SO
3H 3 do. do. g262 do. 3
do. g263 H 4 do. 2(6,8) h264 H 4
do. h265 H 5 do. do. h266 H 4 -NHCH
2CH
2NH- do. h267 H 4 -NH(CH
2)
3NH- do. h268 H 4
do. h表17,式(T17)化合物
表17
p 取代位置 m实例号 取代位置 X
1 X
2 -NH- (取代位置) R
20 R
21 -W-269 2(3′,5′) -O- -COOH 4 0 2-SO
3H H -NHCH
2CH
2NH-270 do. do. do. 4 0 do. H
271 do. do. do. 4 0 do. H
272 do. do. do. 4 0 3-SO
3H do.273 do. do. do. 4 0 do. H
274 1(4′) -COOH- -O- 3 1(5) H H -NHCH
2CH
2NH-275 do. do. do. 3 do. H H -NH(CH
2)
3NH-276 do. do. do. 3 do. H H
277 do. do. do. 3 do. H H
278 do. do. do. 3 do. 3-SO
3H H do.279 1(5′) do. do. 3 do. 2-Cl H do.280 do. do. do. 3 do. 2-Cl 5-Cl do.281 do. do. do. 3 do. 2-SO
3H H do.
表17(续)
p 取代位置 m实例号 取代位置 X
1 X
2 -NH- (取代位置) R
20 R
23 -W-282 1(5′) -COOH -O- 3 1(5) 3-SO
3H H
283 do. do. do. 3 do. 4-SO
3H H do.284 do. do. do. 3 do. 4-CH
3 H -NHCH
2CH
2NH-285 do. do. do. 3 do. 4-OCH
3 H do.286 do. do. do. 3 do. 3-CH
3 4-Cl
287 1(4′) -O- -COOH- 3 1(5) 2-CH
3 H
288 1(5′) do. do. 5 1(3) H H do.289 do. do. do. 5 do. 2-CH
3 H
290 do. do. do. 5 do. 4-SO
3H H -NHCH
2CH
2NH-291 do. do. do. 3 1(5) H H do.实例269-291的染料将棉制品染成蓝色。实例292
将45.3份按照已知方法制备的下式所示的单偶氮染料溶于700份水中,
在20-25°下用30分钟往其中加入18.5份固体氰尿酰氯。在pH6.0-6.5搅拌混合物,直至不再能检测到游离氨基。然后,加入11.1份1,2-二氨基丙烷,将反应混合物搅拌2小时,同时加入稀的碳酸钠溶液保持pH7.0。加入氯化钠,使缩合产物盐析出来,过滤。将得到的湿糊状物溶于900份水中。往此溶液中加入24.9份5-氰基-2,4,6-三氯嘧啶,并在0-5°搅拌2小时。反应过程中通过加入稀的碳酸钠溶液使pH保持在8-9。用氯化钠使所得染料沉淀,分离。它相应于下述结构式
干燥后该染料为暗红色粉末,它溶于水时呈现红色,能将棉制品染成猩红色。染出的这些颜色具有好的耐光和耐湿性能,并且能抗氧化作用。实例293-319
按照实例292所述的类似方法,用适当的起始化合物,可以制得另一些式I化合物。它们相应于结构式(T18)其中的符号在下面表18中给出定义。用实例293-319的染料在棉制品上染出的颜色具有好的耐光和耐湿性能,并且能抗氧化作用。
在最后一栏I中给出了各自在棉制品上的染色,其中d是橙色,k是猩红色。表18/式(T18)化合物实例号 R
3 R
4 -W- I293 OC
2H
5 H
k294 OCH
3 SO
3H do. k295 -OC
2H
4OH H do. k296 OCH
3 H
k297 do. SO
3H do. k298 -OC
2H
4OH H do. k299 OCH
3 H -NHCH
2CH
3NH- k
表18(续)实例号 R
3 R
4 -W- I300 OCH
3 SO
3H -NHCH
2CH
2NH- k301 OC
2H
5 H do. k302 -OC
2H
4OH H do. k303 OCH
3 H -NH(CH
2)
3NH- k304 do. SO
3H do. k305 -OC
2H
4OH H do. k306 OC
2H5 H
k307 OCH
3 H
k308 do. SO
3H do. k309 -OC
2H
4OH H do. k310 -OC
2H
4OC
2H
4OH H do. k311 do. H -NHCH
2CH
2NH- k312 do. H -NH(CH
2)
3NH- k313 do. H
k314 do. H
k315 CH
3 SO
3H do. d316 do. do.
d317 do. do. -NHCH
2CH
2NH- d318 do. do.
d319 do. H do. d实例320
在10份水和20份冰的混合物中将5份氰尿酰氯搅拌成均匀悬浮液。往此悬浮液中加入30份下式染料的四钠盐在160份水中的pH7的中性溶液,
在5°和pH5.5-6.0条件下搅拌,直到不再能检测出游离氨基。在pH6.5-7.0将温度慢慢升到15°,然后加入3.25份1,2-二氨基丙烷。加入盐酸使pH保持在6.0。混合物在50°加热3小时,此段时间之后反应完全。再加入70份氯化钠,过滤此红色悬浮液。用500份20%氯化钠溶液洗滤饼。将所得糊状物置于500份水中,加入4.5份5-氰基-2,4,6-三氯嘧啶。在20°搅拌12小时,直至反应完全,然后加入80份氯化钠。将得到的染料进行沉淀并过滤;它相应于下述结构式:
该染料干燥后为暗红色粉末,溶于水时显红色,染出的棉制品呈蓝红色。在棉制品上染出的颜色总的牢度性能很好。实例321-416
用实例320中所述的类似方法,用适当的起始化合物,还制得了下面表19-22中所列的其它式I化合物。在每个表的顶部,给出了相应的结构式,其中的符号在下面表中给出定义。用实例321-416的染料按常规的浸染法可将纤维素纤维、尤其是棉制品染色。染出的所有颜色都是蓝红色,并且牢度强。
表19/式(T19)化合物
实例 m SO
3H基的 -W-号 取代位置321 0 1
322 0 1
323 1 1,5 do.324 1 3,6 do.325 0 1
326 1 1,5 do.327 0 1 -NHCH
2CH
2NH-328 1 1,5 do.329 1 4,8 do.330 0 1 -NH(CH
2)
3NH-331 1 1,5 do.332 0 1
333 1 1,5 do.334 1 3,6 do.335 1 4,8 do.336 0 1
337 1 4,8
表20/式(T20)化合物
实例 m(SO
3H取 R
3 R
4 -W-号 代位置)338 1(2) H H
339 do. 5-SO
3H H do.340 do. do. 4-CH
3 do.341 do. do. 4-OCH
3 do.342 do. H 4-CH
3 do.343 do. H 4-OCH
3 do.344 do. 4-SO
3H H do.345 do. H H
346 do. H 4-OCH
3 do.347 do. 5-SO
3H H do.348 do. H 4-CH
3
349 do. H 4-OCH
3 do.350 do. H H do.351 do. 5-SO
3H H do.352 do. 4-SO
3H H do.353 0 2-COOH H do.354 0 3-COOH H do.355 0 4-COOH H do.356 1(2) 5-SO
3H 4-OCH
3
357 do. H H do.358 do. H 4-CH
3 do.
表20(续)实例 m(SO
3H取 R
3 R
4 -W-号 代位置)359 1(2) H 4-OCH
3
360 do. 4-SO
3H H do.361 0 3-COOH H do.362 1(2) H H
363 do. 5-SO
3H H do.364 do. do. 4-CH
3 do.365 do. 4-SO
3H H do.366 0 2-COOH H do.367 0 3-COOH H do.368 0 4-COOH H do.369 1(2) H H -NHCH
2CH
2NH-370 do. H 4-OCH
3 do.371 0 2-COOH H do.372 1(2) 5-SO
3H H -NH(CH
2)
3NH-表21/式(T21)化合物
实例
-W- R16号373 -NHCH2CH2NH- 甲基374 do. 乙基375 do. 苯基
表21(续)实例号 -W- R
16376
苯基377 do. 甲基378 do. 乙基379
甲基380 do. 苯基381
do.382 do. 乙基383
甲基384 -NH(CH
2)
3NH- 苯基表22/式(T22)化合物
表22
取代位置 取代位置实例号 D
T22 SO
3H -NH- -W-385
3 3 -NHCH
2CH
2NH-386 do. 3 4 do.387 do. 3 3
388 do. 3 3
389 do. 3 3
390 do. 3 3
391 do. 4 3 do.392
3 4 do.393 do. 4 3 do.394 do. 3 3 do.395
3 4 do.396 do. 3 3 do.397 do. 4 4 do.398 do. 3 3
表22(续)
取代位置 取代位置实例号 D
T22 SO
3H -NH- -W-399
3 4
400 do. 3 3 -NHCH
2CH
2NH-401 do. 3 3
402 do. 3 4
403
3 3
404 do. 3 4
405 do. 4 4 do.406
3 4 do.407 do. 3 3 -NHCH
2CH
2NH-408
3 3 do.409 do. 4 3 do.410 do. 3 4
411 do. 3 3
412
3 4 do.表22(续)
取代位置取代位置实例号 D
T22 SO
3H -NH- -W-413
3 4
414
3 3 do.415 do. 4 4 -NH(CH
2)
3NH-416 do. 3 4
实例417
将212份氰尿酰氯在560份冰和200份水中搅拌45分钟。往其中加入由在920份水和112份30%氢氧化钠溶液中的190份2,4-二氨基苯-1-磺酸构成的溶液,将此混合物在冰浴冷却下搅拌6小时。随后将此白色悬浮液间接重氮化。
其间,按已知方法将140份4-氨基苯甲酸重氮化,并在酸性反应条件下与320份1-氨基-8-羟基萘-3,6-二磺酸偶联。往此红色悬浮液中加入上述重氮化合物溶液,进行碱性偶联。
往得到的蓝色溶液中加入120份1,2-二氨基丙烷。缩合反应进行2小时。然后用氯化钠(25%体积)使反应混合物盐析,过滤。将蓝色滤饼重新溶于10倍量的水中。
在两小时内往此水溶液中加入已经在350份水和150份冰中搅拌45分钟的175份5-氰基-2,4,6-三氯嘧啶的悬浮液。然后使该反应混合物的pH在9保持3小时。加入氯化钠(10%体x
积)使所得产物盐析出来,过滤,滤饼在40°真空干燥。该染料具有下述结构式,
该染料将纤维素纤维、特别是棉制品染成藏青色。该色具有好的牢度性能,如耐光和耐湿性能,并能抗氧化作用。实例418-456
按照实例417所述的类似方法,用适当的起始化合物,可制得另外一些式I化合物,它们相应于式(T23)所示化合物,
式中的符号在下面表23中给出定义。采用常规的浸染法,该实例418-456染料将纤维素纤维、特别是棉制品染成藏青色。这些染色具有很好的各种常规牢度性能。
表23/式(T23)化合物实例号 R3 R4 取代位置 R5 -W-
-NH-418 4-SO
3H H 5 SO
3H
419 3-COOH H 4 do. do.420 4-COOH H 4 COOH do.421 do. H 4 SO
2H
422 3-SO
3H H 5 do. do.423 5-COOH 2-Cl 4 do. do.424 do. do. 5 do. do.425 do. do. 4 COOH do.426 3-COOH H 5 SO
3H do.427 4-SO
3H H 4 COOH do.428 2-5O
3H 4-Cl 4 do. do.429 4-SO
3H H 4 SO
2H do.430 2-SO
3H 4-Cl 4 do. do.431 4-SO
3H H 5 do. do.432 2-SO
3H 4-Cl 5 do. do.433 5-COOH 2-Cl 5 do.
434 4-COOH H 5 do. do.435 4-SO
3H H 5 do. do.436 3-COOH H 5 do. do.437 3-COOH H 4 do. do.438 3-SO
3H H 4 do. do.439 do. H 4 COOH do.440 do. H 5 SO
3H -NHCH
2CH
2NH-441 5-COOH 2-Cl 5 do. do.442 5-SO
3H do. 5 do. do.443 3-COOH H 5 do. do.444 2-SO
3H 4-Cl 5 do. -NH(CH
2)
3NH-
表23(续)实例号 R3 R4 取代位置 R5 -W-
-NH-445 5-SO
3H 2-Cl 4 SO
3H -NH(CH
2)
3NH-446 4-COOH H 4 COOH
447 5-SO
3H 2-Cl 5 SO
3H do.448 4-COOH H 5 do. do.449 5-COOH 2-Cl 5 do. do.450 3-COOH H 4 COOH
451 3-SO
3H H 4 do. do.452 do. H 5 SO
3H do.453 5-COOH 2-Cl 4 COOH do.454 4-COOH H 5 SO
3H
455 5-SO
3H 2-Cl 4 COOH do.456 2-SO
3H 4-Cl 4 do. do.实例457
将65.9份按已知方法制备的上式染料溶于1200份水中。在20-25°用30分钟加入37份固体氰尿酰氯。通过往混合物中连续加入稀碳酸钠溶液,保持pH为6.0-6.5,搅拌1小时。加入22.2份1,2-二氨基丙烷,在40-50°搅拌2小时,同时加入稀碳酸钠溶液,保持pH7.0。用氯化钠使缩合产物盐析出来,过滤。将所得糊状物溶于1400份水中。往此溶液中加入49.5份5-氰基-2,4,6-三氯嘧啶,在5-10°搅拌2小时。在反应过程中加入稀碳酸钠溶液使pH保持在8-9。加入氯化钠使所得染料盐析出来,分离。它相应于下述结构式,干燥后为黑色粉末,溶于水后呈深蓝色。该染料将棉制品染成深蓝色。这种染色具有好的耐光和耐湿牢度。
实例458-472
按照实例457所述的类似方法可制得另外一些式1化合物。它们相应于结构式(T24)。
式中的符号在下面表24中给出定义。实例458-472的染料将棉制品染成深蓝色。这些染色具有好的耐光和耐湿性能。表24/式(T24)化合物实例 m R
1 -W-号458 1 H -NHCH
2CH
2NH-459 1 H -NH(CH
2)
3NH-460 1 H
461 1 H
462 1 H
463 0 -SO
2CH
2CH
2OSO
3H do.464 0 do. -NHCH
2CH
2NH-465 0 do. -NH(CH
2)
3NH-466 0 do.
467 0 do.
468 1 do. do.469 1 do.
470 1 do. -NHCH
2CH
2NH-471 1 do. -NH(CH
2)
3NH-472 1 do.
实例473将25.7份(0.025mol)染料(按已知方法将铜酞菁氯磺化,然后与1,3-二氨基苯-4-磺酸反应制得,每个分子中平均约含有2.5个磺酸基和1个磺酰氨基)溶于200份pH6.5-7.0的水中。加入150份冰以后,在0-5°加入4.6份氰尿酰氯,在pH6.0-6.5搅拌两小时。然后加入2.8份1,2-二氨基丙烷,在0-5°搅拌1小时后,使温度在两小时内升到30-35°,加入20%碳酸钠溶液将pH调到7.5-8.0。将用70份氯化钠盐析出来并过滤的反应产物再溶于250份水中。在0-5°加入6.3份5-氰基2,4,6-三氯嘧啶,在此温度下搅拌混合物3小时。同时通过加入碳酸钠将pH保持在8-8.5。缩合反应完成后,用45份氯化钠使混合物盐析。抽滤出沉淀的染料并在35°干燥。得到的染料相应于下述结构式,
它将纤维素纤维、尤其是棉制品染成鲜艳的青绿色。这些染色具有好的耐光牢度和极好的耐湿牢度性能。实例474
按照实例473所述的方法,用等当量的镍酞菁染料碳(即25.5份(0.025mol))代替25.7份铜酞菁染料碳。得到相应的〔NiPc〕染料(其结构式类似于实例473所给的式子),它将棉制品染成蓝绿色。该染色具有好的牢度性能。实例475-480
按照类似于实例473和474所述的方法,可制得另外一些酞菁染料。它们相应于下述结构式,式中的符号的定义在下面表25中给出。在最后一栏I中给出了各自将棉制品染成的色调,其中e是鲜青绿色,f是蓝绿色。这些染色具有好的牢度性能。表25/式(T25)化合物
取代位置实例号 Me c d R -NH- -W- I475 Cu 3 0 H 3
e476 Cu 2 1 H 3 do. e477 Cu 1 2 4-SO
3H 3 do. e478 Cu 2 1 2-COOH H do. e479 Ni 2 1 H 3 do. f480 Ni 1 2 4-SO
3H 3
f实例481
将25.5份染料(按已知方法将镍酞菁氯磺化、然后与1,3-二氨基苯-4-磺酸反应制得,每个分子中平均约含2.5个磺酸基和1个磺酰氨基)在150份水中与1.8份亚硝酸钠一起搅拌。将该溶液冷至0-2°,滴加到100份冰水混合物和12份30%盐酸中。然后将所得重氮盐悬浮液加到由5.9份1-(3′-甲胺基丙基)-6-羟基-4-甲基吡啶酮-(2)在300份冰/水中构成的溶液中,同时保持温度在0-5°。偶合反应过程中,通过加入30%氢氧化钠溶液使混合物pH保持在9-9.5。按类似于实例473中所述的方法,使所得绿色溶液先后与氰尿酰氯、1,2-二氨基丙烷和5-氰基-2,4,6-三氯嘧啶反应,得到下式所示的染料,
按实例473所述类似方法将其分离。该染料将纤维素纤维、尤其是棉制品染成鲜绿色。这些染色具有高的耐光牢度和极好的耐湿牢度。实例482-519
按照实例481所述的类似方法可制得另外一些酞菁染料。它们具有下述结构式,
式中的符号在下面表26和27中给出定义。
实例482-519的染料将棉花染成绿色,当使用镍酞菁时,颜色更加鲜艳。这些染色具有很好的耐光和耐湿牢度。
表26/式(T26)化合物实例
Me R Q
3 -B
2-NR
1- -W-号482 Cu SO
3H H
483 Ni H H do. do.484 Ni H -CONH
2 do. do.485 Ni SO
3H H -(CH
2)
3NH- do.486 Cu do. H -CH
2CH
2NH- do.487 Ni do. H do.
488 Ni do. -CONH
2 -(CH
2)
3NH- do.489 Ni do. H do. do.490 Ni do. H
do.491 Ni do. H do.
492 Cu H H do. do.493 Ni SO
3H -CONH
2 do. do.494 Ni do. H -CH
2CH
2NH- do.495 Ni SO
3H H
496 Cu H -CONH
2 do. -NHCH
2CH
2NH-497 Ni SO
3H H do.
498 Ni do. H -(CH
2)
3NH- -NH(CH
2)
3NH-
表27/式(T27)化合物
取代位置实例
Me R R
11 R
12 R
9 -NH- -W-号499 Cu SO
3H OH CH
3 H 5
500 Ni do. OH do. H 5 do.501 Ni do. NH
2 do. H 5 do.502 Ni H OH do. 2-SO
3H 5 do.503 Ni SO
3H OH -COOH H 4 do.504 Cu do. OH CH
3 H 5
505 Ni do. OH do. 2-SO
3H 5 do.506 Ni do. OH do. H 4 do.507 Cu do. OH -COOH H 5 do.508 Ni H OH CH
3 2-SO
3H 5 do.509 Ni H OH do. do. 5
510 Ni SO
3H OH do. H 4 do.511 Cu do. OH do. 2-SO
3H 5 do.512 Ni do. NH
2 do. do. 5 do.513 Ni do. OH do. do. 4
514 Cu do. OH do. H 5
515 Ni H OH -COOH H 5 -NHCH
2CH
2NH-516 Cu H OH CH
3 H 4 do.517 Ni SO
3H OH do. H 5 do.518 Ni do. NH
2 do. H 4 do.519 Ni do. OH do. 2-SO
3H 5 -NH(CH
2)
3NH-实例520
按照实例481所述的类似方法,用适当的起始化合物,可以制得并分离出下式所示的染料,它将棉制品染成鲜艳的绿色。所染出的颜色具有好的耐光和耐湿牢度。
按照所述的方法,可以得到实例1-520染料的钠盐形式。根据不同的反应/分离条件,或按已知方法使该钠盐起反应,也可以得到它们的游离酸形式或其它盐形式,例如那些含有一种或多种上述阳离子的盐。
在下面的实例中说明本发明化合物的应用。应用实例A
将0.3份实例1的染料溶于300份软化水中,加入15份芒硝(烘过)。将染缸加热到40°,然后加入10份漂白过的棉织物。在40°保持30分钟后,往缸中分批加入6份碳酸钠(烘过),每隔10分钟加一次,所加的量依次为0.2、0.6、1.2和最后的4.0份。在加碳酸钠的过程中,温度保持在40°。然后在40°再染1小时。
然后将染过的棉织物用冷的流水漂洗3分钟,之后再用热的流水漂洗3分钟。在含有0.25份市售阴离子活性洗涤剂的500份软化水中将染后的织物煮沸洗涤15分钟。用流动热水漂洗3分钟并离心处理后,染过的织物在约70°的干燥橱中干燥。得到的棉织物的橙色表现出好的耐光和耐湿牢度,并能抗氧化作用。应用实例B
在盛有300份软化水的染缸中加入10份芒硝(烘过)、10份棉织物(漂白过)。在10分钟内将染缸加热到40°,加入0.5份实例1的染料。在40°再保持30分钟后加入3份烘过的碳酸钠,在40°再继续染45分钟。
染过的织物按应用实例A给出的方法先后用冷流水和热流水漂洗,并煮沸洗涤。漂洗并干燥后,得到的棉织物的橙色具有与应用实例1指出的同样好的牢度性能。应用实例C
将应用实例A中所述的方法做某些变动,使用0.3份实例481的染料,只用2份烘过的碳酸钠并且一次加入,代替分批加入的总量6份的碳酸钠。将40°起始温度提高到60°,后面的染色过程在60°进行1小时。在其它方面,类似地采用应用实例A所述的方法。得到的棉织物染色为鲜艳的绿色,染深性能好,并表现出很好的耐光性能。应用实例D
将2.5份实例473的染料溶于2000份水中。加入100份棉织物,在10分钟内将染缸温度提高到80°。加入100份烘过的芒硝,30分钟后加入20份烘过的碳酸钠。在80°继续染色1小时。然后,按照应用实例A给出的方法依次用流动的冷水和流动的热水漂洗和煮沸洗涤染过的织物。漂洗和干燥后,棉织物上的染色为鲜艳的青绿色,具有好的牢度性能。
类似地,其余实例的染料或所举出染料的混合物可以用来按照应用实例A-D所述方法将棉制品染色。应用实例E
用具有下列成分的印花色浆按常规印花方法将棉织物印花:
40份实例1的染料
100份尿素
350份水
50份4%藻酸钠增稠剂
10份碳酸氢钠
1000份。
将印花后的织物干燥并在102-104°的蒸汽中固色4-8分钟,按照应用实例A所述方法将其用冷水、再用热水漂洗和煮沸洗涤,并干燥。得到的橙色印花具有好的各种常规牢度性能。
类似地,实例2-520的染料或所举出的染料的混合物可以按照应用实例E给出的方法将棉制品印花。得到的所有印花都表现出好的牢度性能。