CN102202619B - Reduced-pressure, wound-treatment dressings and systems - Google Patents
Reduced-pressure, wound-treatment dressings and systems Download PDFInfo
- Publication number
- CN102202619B CN102202619B CN200980143982.7A CN200980143982A CN102202619B CN 102202619 B CN102202619 B CN 102202619B CN 200980143982 A CN200980143982 A CN 200980143982A CN 102202619 B CN102202619 B CN 102202619B
- Authority
- CN
- China
- Prior art keywords
- wound
- activated
- sealing drape
- drape
- reduced pressure
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/90—Negative pressure wound therapy devices, i.e. devices for applying suction to a wound to promote healing, e.g. including a vacuum dressing
- A61M1/91—Suction aspects of the dressing
- A61M1/915—Constructional details of the pressure distribution manifold
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/08—Wound clamps or clips, i.e. not or only partly penetrating the tissue ; Devices for bringing together the edges of a wound
- A61B17/085—Wound clamps or clips, i.e. not or only partly penetrating the tissue ; Devices for bringing together the edges of a wound with adhesive layer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/00987—Apparatus or processes for manufacturing non-adhesive dressings or bandages
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/05—Bandages or dressings; Absorbent pads specially adapted for use with sub-pressure or over-pressure therapy, wound drainage or wound irrigation, e.g. for use with negative-pressure wound therapy [NPWT]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00526—Methods of manufacturing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00831—Material properties
- A61B2017/00867—Material properties shape memory effect
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00831—Material properties
- A61B2017/00884—Material properties enhancing wound closure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00831—Material properties
- A61B2017/00889—Material properties antimicrobial, disinfectant
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00831—Material properties
- A61B2017/00893—Material properties pharmaceutically effective
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/08—Wound clamps or clips, i.e. not or only partly penetrating the tissue ; Devices for bringing together the edges of a wound
- A61B17/085—Wound clamps or clips, i.e. not or only partly penetrating the tissue ; Devices for bringing together the edges of a wound with adhesive layer
- A61B2017/086—Wound clamps or clips, i.e. not or only partly penetrating the tissue ; Devices for bringing together the edges of a wound with adhesive layer having flexible threads, filaments, laces or wires, e.g. parallel threads, extending laterally from a strip, e.g. for tying to opposing threads extending from a similar strip
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/34—Trocars; Puncturing needles
- A61B17/3417—Details of tips or shafts, e.g. grooves, expandable, bendable; Multiple coaxial sliding cannulas, e.g. for dilating
- A61B17/3421—Cannulas
- A61B2017/345—Cannulas for introduction into a natural body opening
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/90—Negative pressure wound therapy devices, i.e. devices for applying suction to a wound to promote healing, e.g. including a vacuum dressing
- A61M1/91—Suction aspects of the dressing
- A61M1/916—Suction aspects of the dressing specially adapted for deep wounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/90—Negative pressure wound therapy devices, i.e. devices for applying suction to a wound to promote healing, e.g. including a vacuum dressing
- A61M1/92—Negative pressure wound therapy devices, i.e. devices for applying suction to a wound to promote healing, e.g. including a vacuum dressing with liquid supply means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/02—General characteristics of the apparatus characterised by a particular materials
- A61M2205/0266—Shape memory materials
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T29/00—Metal working
- Y10T29/49—Method of mechanical manufacture
- Y10T29/49826—Assembling or joining
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Biomedical Technology (AREA)
- Surgery (AREA)
- Vascular Medicine (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Hematology (AREA)
- Anesthesiology (AREA)
- Manufacturing & Machinery (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- External Artificial Organs (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
- Materials For Medical Uses (AREA)
Abstract
Description
相关申请 related application
本发明根据35U.S.C.§119(e)要求于2008年11月7日提交的题目为“Reduced-Pressure Wound Treatment Dressing and System(减压创伤治疗敷料和系统)”的美国临时专利申请序列号61/112,371的权益,61/112,371为了所有目的通过引用被并入本文。 U.S. Provisional Patent Application Serial No. 61 entitled "Reduced-Pressure Wound Treatment Dressing and System" filed on November 7, 2008 pursuant to 35 U.S.C. §119(e) Interest of /112,371, 61/112,371 is hereby incorporated by reference for all purposes. the
技术领域 technical field
本发明大体上涉及医学治疗系统,并且更特别地,涉及减压创伤治疗敷料、系统和方法。 The present invention relates generally to medical treatment systems, and more particularly, to reduced pressure wound treatment dressings, systems and methods. the
背景技术 Background technique
创伤可以被有意地,例如外科手术切口,或无意地,例如在事故中,被产生。在两种情况下,创伤的闭合对于防止重要体液的损失和微生物的侵入来说是重要的。创伤典型地通过使用缝线或钉而被闭合。 Trauma can be created intentionally, such as a surgical incision, or unintentionally, such as in an accident. In both cases, closure of the wound is important to prevent loss of vital body fluids and intrusion of microorganisms. Wounds are typically closed using sutures or staples. the
然而,缝线或钉的使用可能具有不期望的副作用。例如,缝线或钉的插入一定涉及使患者遭受用于使缝线或钉进入患者的表皮的额外的创伤。这些额外的创伤还经受可能的感染。此外,虽然创伤自身可以导致疤痕,但是来自缝线或钉的额外的创伤也可以导致额外的伤痕,这可能不必要地突出最初的创伤伤痕的已经是美学上不期望的本质。 However, the use of sutures or staples may have undesired side effects. For example, insertion of sutures or staples necessarily involves subjecting the patient to additional trauma for the sutures or staples to enter the patient's epidermis. These additional wounds are also subject to possible infection. Furthermore, while trauma itself can result in scarring, additional trauma from sutures or staples can also result in additional scarring, which may unnecessarily accentuate the already aesthetically undesirable nature of the original traumatic scar. the
发明概述 Summary of the invention
本发明解决了创伤护理的缺点,如在本文的各种例证性的非限制性的实施方案中示出和描述的。根据例证性的非限制性的实施方案,用于治疗患者身上的创伤的减压创伤治疗系统包括创伤闭合敷料、用于布置在密封盖布的面向组织的表面和创伤之间的歧管构件;以及用于向创伤闭合敷料传送减压的减压子系统。创伤闭合敷料包括具有第一表面和面向组织的表面的密封盖布、耦合于密封盖布的收缩元件以及耦合于密封盖布的夹紧构件(gripping member)。密封盖布用于放置在创伤上。收缩元件被配置为在被激活时收缩并且由此产生闭合力。夹紧构件被配置为将闭合力传递至患者的表皮。密封盖布和夹紧构件被配置为在创伤上形成流体密封。 The present invention addresses the shortcomings of wound care, as shown and described herein in various illustrative, non-limiting embodiments. According to an illustrative, non-limiting embodiment, a reduced pressure wound treatment system for treating a wound on a patient includes a wound closure dressing, a manifold member for positioning between a tissue-facing surface of a sealing drape and the wound; and a reduced pressure subsystem for delivering reduced pressure to the wound closure dressing. A wound closure dressing includes a sealing drape having a first surface and a tissue-facing surface, a constricting element coupled to the sealing drape, and a gripping member coupled to the sealing drape. Sealing drape is used to place over the wound. The contraction element is configured to contract and thereby generate a closure force when activated. The clamping member is configured to transmit a closure force to the patient's epidermis. The sealing drape and the gripping members are configured to form a fluid seal over the wound. the
根据另一个例证性的非限制性的实施方案,创伤闭合敷料包括具有第一表面和面向组织的表面的密封盖布、耦合于密封盖布的收缩元件以及耦合于密封盖布的夹紧构件。密封盖布用于放置在创伤上。收缩元件被配置为在被激活时收缩并且由此产生闭合力。夹紧构件被配置为将闭合力传递至患者的表皮。密封盖布和夹紧构件被配置为在创伤上形成流体密封。 According to another illustrative, non-limiting embodiment, a wound closure dressing includes an airtight drape having a first surface and a tissue-facing surface, a constriction element coupled to the airtight drape, and a gripping member coupled to the airtight drape. Sealing drape is used to place over the wound. The contraction element is configured to contract and thereby generate a closure force when activated. The clamping member is configured to transmit a closure force to the patient's epidermis. The sealing drape and the gripping members are configured to form a fluid seal over the wound. the
根据另一个例证性的非限制性的实施方案,创伤闭合敷料包括具有第一表面和面向组织的表面的密封盖布、耦合于密封盖布的可溶解体以及在拉伸位置中耦合于可溶解体的弹性构件。密封盖布用于放置在创伤上。弹性构件在可溶解体的至少一部分溶解时收缩至自由位置,由此产生闭合力。创伤闭合敷料还包括耦合于密封盖布和弹性构件中的至少一个的夹紧构件。夹紧构件被配置为将闭合力传递至患者的表皮。 According to another illustrative, non-limiting embodiment, a wound closure dressing includes an airtight drape having a first surface and a tissue-facing surface, a dissolvable body coupled to the airtight drape, and a dissolvable body coupled to the dissolvable body in a stretched position. Elastic components of the body. Sealing drape is used to place over the wound. The elastic member contracts to a free position upon dissolution of at least a portion of the dissolvable body, thereby generating a closure force. The wound closure dressing also includes a grip member coupled to at least one of the seal drape and the elastic member. The clamping member is configured to transmit a closure force to the patient's epidermis. the
根据另一个例证性的非限制性的实施方案,用于治疗创伤的方法包括以下步骤:将收缩元件固定于患者的表皮,使得收缩元件跨越患者的创伤的至少一部分,以及激活收缩元件,使得收缩元件产生闭合力。收缩元件被配置为在被激活时从延伸位置收缩至收缩位置并且从而产生闭合力。 According to another illustrative, non-limiting embodiment, a method for treating a wound comprises the steps of: securing a constricting element to the epidermis of a patient such that the constricting element spans at least a portion of the patient's wound, and activating the constricting element such that the constricting element The element generates the closing force. The retraction element is configured to retract from an extended position to a retracted position when activated and thereby generate a closure force. the
根据另一个例证性的非限制性的实施方案,制造创伤闭合敷料的方法包括以下步骤:形成具有第一表面和面向组织的表面的密封盖布,将收缩元件耦合于密封盖布,以及将夹紧构件耦合于密封盖布。夹紧构件被配置为将闭合力传递至患者的表皮。收缩元件被配置为在被激活时从延伸位置收缩至收缩位置并且从而产生闭合力。 According to another illustrative, non-limiting embodiment, a method of making a wound closure dressing includes the steps of forming an airtight drape having a first surface and a tissue-facing surface, coupling a constriction element to the airtight drape, and attaching the clip to the airtight drape. The tightening member is coupled to the sealing drape. The clamping member is configured to transmit a closure force to the patient's epidermis. The retraction element is configured to retract from an extended position to a retracted position when activated and thereby generate a closure force. the
附图简述 Brief description of the drawings
当结合附图参照以下详细描述时,可以获得对本发明的更完全的理解,在附图中: A more complete understanding of the invention can be gained when reference is made to the following detailed description when taken in conjunction with the accompanying drawings, in which:
图1是用于治疗患者身上的创伤的系统的例证性的非限制性的实施方案的示意性的透视图; Figure 1 is a schematic perspective view of an illustrative, non-limiting embodiment of a system for treating wounds on a patient;
图2是图1的系统的沿图1中的线2-2取的示意性的横截面视图; Figure 2 is a schematic cross-sectional view taken along line 2-2 in Figure 1 of the system of Figure 1;
图3A是图1的系统的示意性的俯视图; Figure 3A is a schematic top view of the system of Figure 1;
图3B是图1的系统的示意性的俯视图,示出了某些被激活由此产生闭合力的收缩元件; FIG. 3B is a schematic top view of the system of FIG. 1 showing certain contraction elements activated thereby generating a closing force;
图4A是被示出为覆盖创伤的创伤闭合敷料的例证性的非限制性的实施方案的示意性的俯视图; Figure 4A is a schematic top view of an illustrative, non-limiting embodiment of a wound closure dressing shown covering a wound;
图4B是图4A的敷料的示意性的俯视图,示出了被激活由此产生闭合力的收缩元件; Figure 4B is a schematic top view of the dressing of Figure 4A, showing the contraction element being activated thereby generating a closure force;
图5是用于治疗创伤的系统的例证性的非限制性的实施方案的示意性的俯视图; Figure 5 is a schematic top view of an illustrative, non-limiting embodiment of a system for treating wounds;
图6是所示的用于治疗创伤的系统的例证性的非限制性的实施方案的示意性的俯视图; Figure 6 is a schematic top view of an illustrative, non-limiting embodiment of the system shown for treating wounds;
图7A是用于治疗创伤的系统的例证性的非限制性的实施方案的一部分的示意性的仰视图; Figure 7A is a schematic bottom view of a portion of an illustrative, non-limiting embodiment of a system for treating wounds;
图7B是图7A的系统的示意性的透视图; Figure 7B is a schematic perspective view of the system of Figure 7A;
图8是用于治疗创伤的系统的例证性的非限制性的实施方案的一部分的示意性的透视图; Figure 8 is a schematic perspective view of a portion of an illustrative, non-limiting embodiment of a system for treating wounds;
图9A是图8的系统部分的示意性的俯视图;以及 Figure 9A is a schematic top view of the system portion of Figure 8; and
图9B是图8的系统部分的示意性的俯视图,示出了某些被溶解从而使相应的弹性构件产生闭合力的珠。 Fig. 9B is a schematic top view of the system portion of Fig. 8, showing certain beads being dissolved to cause the corresponding elastic member to generate a closing force. the
发明详述 Detailed description of the invention
在以下的对优选的实施方案的详细描述中,参考了附图,附图形成例证性的实施方案的一部分,并且其中作为例子示出了本发明可以被实践的具体的实施方案。这些实施方案被足够详细地描述以使本领域的技术人员能够实践本发明,并且应当理解,可以利用其他的实施方案并且可以做出逻辑结构的、机械的、电的以及化学的变化而不偏离本发明的精神或范围。 为了避免对使本领域的技术人员能够实践本发明不必要的细节,描述可能省去了本领域的技术人员已知的某些信息。因此,以下的详细描述不应在限制的意义理解,并且本发明的范围仅由所附的权利要求限定。 In the following detailed description of the preferred embodiments, reference is made to the accompanying drawings, which form a part hereof, and which show by way of example specific embodiments in which the invention may be practiced. These embodiments are described in sufficient detail to enable those skilled in the art to practice the invention, and it is to be understood that other embodiments may be utilized and logical structural, mechanical, electrical and chemical changes may be made without departing from spirit or scope of the invention. To avoid detail not necessary to enable those skilled in the art to practice the invention, the description may omit certain information known to those skilled in the art. Accordingly, the following detailed description should not be taken in a limiting sense, and the scope of the present invention is defined only by the appended claims. the
现在主要参照图1-3B,示出了用于治疗患者身上的创伤102的减压创伤治疗系统100的第一例证性的非限制性的实施方案。减压创伤治疗系统100大体上包括创伤闭合敷料110、歧管构件112和减压子系统114。减压创伤治疗系统100被示出为在围绕创伤102的区域中。在本图示中,创伤102经过表皮104(或皮肤)、真皮106,并且到达下皮或皮下组织108。皮下组织108可以包括许多组织类型,例如脂肪组织或肌肉。虽然创伤102在本例证性的实施方案中被示出为经过表皮104、真皮106并且到达皮下组织108中,但是将意识到,减压创伤治疗系统100可以用于治疗具有任何深度的创伤。
Referring now primarily to FIGS. 1-3B , a first illustrative, non-limiting embodiment of a reduced pressure wound
创伤闭合敷料110包括密封盖布116、一个或多个收缩元件118和夹紧构件120。密封盖布116包括第一表面122和面向组织的第二表面124。密封盖布116可以被控制尺寸,使得密封盖布116以使得盖布延伸部(drape extension)126延伸超过创伤102的外周的方式与创伤102重叠。
The wound closure dressing 110 includes a sealing
密封盖布116可以是弹性体性的材料。“弹性体性的”意指具有弹性体的性质。其通常是指具有像橡胶一样的性质的聚合物材料。更具体地,大多数弹性体具有大于100%的伸长率和相当大的回弹性。材料的回弹性是指材料从弹性形变恢复的能力。弹性体的示例可以包括但不限于:天然橡胶、聚异戊二烯、丁苯橡胶、氯丁橡胶、聚丁二烯、丁腈橡胶、丁基橡胶、乙丙橡胶、三元乙丙单体、氯磺化聚乙烯、聚硫橡胶、聚氨基甲酸酯、EVA膜、共聚酯以及硅树脂。此外,密封构件材料的具体的示例包括硅树脂盖布材料、3M 盖布材料、丙烯酸盖布材料例如可从Avery Dennison获得的丙烯酸盖布材料或切割盖布材料(incise drape material)。然而,将意识到,密封盖布116可以由任何合适的材料形成。在一个替代形式中,密封盖布116可以被穿孔以允许湿气或水蒸气经过以激活收缩元件(下文进一步讨论)。除非另外指明,如本文所使用的,“或”不要求相互排他。
The sealing
一个或多个收缩元件118耦合于密封盖布116。每个收缩元件118被配置为在被激活时收缩以产生可以辅助闭合创伤102的闭合力(例如在由图3B中的矢量或箭头128图示的方向的)。在本例证性的实施方案中,收缩元件118在创伤闭合敷料110被施用于患者时处在拉伸位置中。收缩元件118可以在拉伸位置中被耦合于密封盖布116,或在创伤闭合敷料110正在被施用于患者时,可以被运动至拉伸位置。在两种情况下,当收缩元件118被激活时,它们试图返回至非拉伸的或者自由位置,并且从而收缩以产生闭合力。下文进一步描述收缩的其他例证性的方式。
One or
创伤闭合敷料110可以包括被以许多配置布置的各种收缩元件118。例如,如图1-3B所示,创伤闭合敷料110可以包括多个收缩元件118,每个收缩元件118作为条被形成。可选择地,创伤闭合敷料110可以包括单一的收缩元件118。在又一个实施方案中,一个或多个收缩元件118可以被编织入密封盖布116中或编织入耦合于密封盖布116的额外的构件,例如纱布、另一个盖布状的部分等等中。另外,每个收缩元件118可以通过任何合适的装置或技术,包括但不限于焊接(例如超声焊接或射频焊接)、捆绑、机械紧固件、粘合剂、胶接剂等等,被耦合于密封盖布116。可选择地,每个收缩元件118可以被模塑入密封盖布116中。收缩元件118耦合于密封盖布116的面向组织的第二表面124或耦合于密封盖布116的第一表面122或耦合于密封盖布116的内部部分。
Wound closure dressing 110 may include various constricting
每个收缩元件118可以由任何合适的响应于被激活而收缩的材料形成。收缩元件118被配置为在被完全激活时从延伸位置运动至收缩位置(或自由位置)。例如,每个收缩元件118可以由纤维素形成,由此湿气使收缩元件118收缩。湿气可以从患者自身的渗出液被引入。可选择地或另外地,湿气可以在创伤闭合敷料110被施用于患者之前以流体例如水、抑菌水、盐水等等的方式被引入创伤102或创伤区域。在又一个替代形式或附加形式中,密封盖布116可以设置有用于在创伤闭合敷料110已经被施用于患者之后将流体引入创伤区域以激活收缩元件118的口(未示出)。
Each
在另一个替代形式或附加形式中,收缩元件118可以由响应于被激活而收缩的形状记忆金属形成。合适的形状记忆金属的一个示例是来自加利 福尼亚州的Nitinol Devices & Components of Fremont的 材料。由形状记忆金属形成的收缩元件118可以被热,例如患者的体热、加热板、热灯等等激活。可选择地或另外地,收缩元件118可以通过电磁感应的引入而被激活。然而,将意识到,收缩元件118可以由任何合适的材料形成,包括但不限于形状记忆合金、磁性形状记忆合金、形状记忆聚合物、压电材料、电活性聚合物、磁流变流体和弹性体以及电流变流体。取决于具体的材料,激活可以采取电场、温度变化、磁场、机械负荷或压力、光、紫外光、环境pH的变化、超声、湿气等等的形式。
In another alternative or additional form, the
夹紧构件120帮助由收缩元件118的收缩产生的闭合力(由箭头128示出)向患者的皮肤传输。被传递的力在图2中作为力矢量130被图示。密封盖布116和夹紧构件120可以共同起作用以在患者的创伤102上方形成流体密封。考虑到所涉及的具体的减压源或子系统,“流体密封”或“密封”意指足以在期望的部位处保持减压的密封。
Gripping
夹紧构件120可以是任何适合于将闭合力从收缩元件118传递至患者的表皮104(患者的表皮104可以被视为包括衬垫或其他材料的层)的材料或辅助在创伤102上形成流体密封。例如,夹紧构件120可以是压敏粘合剂、热活化粘合剂、密封带、双面密封带、胶水、水解胶体、水凝胶、钩、缝线等等。在本例证性的实施方案中,夹紧构件120是粘合剂层并且跨越密封盖布116的面向组织的第二表面124的宽度并且与收缩元件118重叠。然而,将意识到,夹紧构件120可以仅耦合于盖布延伸部126的面向组织的表面。夹紧构件120可以作为分布在密封盖布116上的薄片层或型式(pattern)被形成。可选择地,对于密封带的情况来说,夹紧构件120可以被施用在密封盖布116的整个第一表面122上或施用在盖布延伸部126的第一表面上。
Gripping
歧管构件112或歧管可被定位在密封盖布116的面向组织的第二表面124和创伤102的至少一部分之间。歧管构件112可以被控制尺寸以实质上覆盖创伤102的估计的面积,但是在不同的应用中可以使用较大的或较小的尺寸。歧管构件112由歧管材料制造。
如本文所使用的术语“歧管”通常是指被提供以辅助向创伤102施加 减压、向创伤102传送流体或从创伤102除去流体的物质或结构。歧管构件112典型地包括多个流动通道或路径,该多个流动通道或路径分配被提供至歧管构件112周围的创伤102以及被从歧管构件112周围的创伤102除去的流体。在一个例证性的实施方案中,流动通道或路径互相连接以改进被提供或被从创伤102除去的流体的分配。歧管构件112可以是能够被放置为与创伤102接触并且向创伤102分配减压的生物相容材料。歧管构件112的示例可以包括但不限于例如具有被排列以形成流动通道的结构要素的装置,例如多孔状泡沫、开孔泡沫、多孔组织聚集体以及包括或固化以包括流动通道的液体、凝胶和泡沫。歧管构件112可以是多孔的并且可以由泡沫、纱布、毡化垫或任何其他适合于具体的生物学应用的材料制造。
As used herein, the term "manifold" generally refers to a substance or structure provided to assist in applying reduced pressure to the
在一个实施方案中,歧管构件112是多孔泡沫并且包括作为流动通道的多个互相连接的小室或孔。多孔泡沫可以是聚氨基甲酸酯开孔网状泡沫体,例如由德克萨斯州的圣安东尼奥的Kinetic Concepts,Incorporated生产的 材料。其他实施方案可以包括“闭孔”。在某些条件下,歧管构件112还可以用于向创伤102分配流体,例如药物、抗菌剂、生长因子和各种溶液。在歧管构件112中或歧管构件112上可以包括其他层,例如吸收性材料、毛细管材料(wicking material)、疏水材料和亲水材料。在某些情况下,可以是期望的是,在微焊工艺中将离子银加入泡沫中或向歧管构件112中加入其他物质,例如抗微生物剂。歧管构件112可以是各向同性的或各向异性的,取决于在减压期间所期望的压缩力的精确的取向。此外,歧管材料可以是生物可吸收材料。
In one embodiment, the
歧管构件112可以耦合于密封盖布116。耦合可以以许多方式发生。密封盖布116和歧管构件112可以使用粘合剂,例如丙烯酸粘合剂、有机硅粘合剂、水凝胶、水解胶体等等被耦合。可选择地,密封盖布116和歧管构件112可以通过热结合、超声波结合和射频结合等等被结合。耦合可以图案形式或更完全地发生。结构可以被加入结合部以使密封盖布116在期望的方向表现出各向异性,即制造各向异性的盖布材料。各向异性的盖布材料可以与收缩元件118共同起作用,以主要在给定的方向运动,即仅围绕某一个或多个轴线。例如,各向异性的密封盖布可以与收缩元件共同起作用以产生闭合力以辅助闭合创伤。
The
减压子系统114包括减压源132,减压源132可以采取许多不同的形式。减压源132作为减压创伤治疗系统100的一部分提供减压。减压源132提供减压。减压源132可以是任何用于供应减压的装置,例如真空泵、壁吸入器或其他源。虽然被施加于组织部位或创伤的减压的量和性质将典型地根据应用变化,但是减压将典型地在-5mmHg至-500mmHg之间并且更典型地在-100mmHg至-300mmHg之间,并且更典型地在-100mmHg至-200mmHg的范围内。
Reduced-
如本文所使用的,“减压”通常是指小于在正在经受治疗的组织部位或创伤处的环境压力的压力。在大多数情况下,这种减压将小于患者所处之处的大气压力。可选择地,减压可以小于组织部位处的流体静压。除非另外指明,本文声明的压力的值是表压。被传送的减压可以是恒定的或变化的(有规律的或随机的)并且可以被连续地或间歇地传送。虽然可以使用术语“真空”和“负压”描述被施加于组织部位的压力,但是被施加于组织部位的实际压力可以大于通常与绝对真空相关联的压力。与本文中的使用一致,减压或真空压力的升高典型地是指绝对压力的相对降低。 As used herein, "reduced pressure" generally refers to a pressure that is less than the ambient pressure at the tissue site or wound being treated. In most cases, this reduced pressure will be less than the atmospheric pressure where the patient is located. Alternatively, the reduced pressure may be less than the hydrostatic pressure at the tissue site. Unless otherwise indicated, pressure values stated herein are gauge pressures. The reduced pressure delivered may be constant or variable (regular or random) and may be delivered continuously or intermittently. Although the terms "vacuum" and "negative pressure" may be used to describe pressure applied to a tissue site, the actual pressure applied to the tissue site may be greater than the pressure typically associated with an absolute vacuum. Consistent with the use herein, an increase in reduced or vacuum pressure typically refers to a relative decrease in absolute pressure. the
在本例证性的实施方案中,减压源132被示出为具有电池隔间134和具有窗138的罐区域136,窗138提供罐136内的流体的水平的可见指示。插入的膜过滤器,例如疏水过滤器或疏油过滤器,可以被散置在减压传送导管或管路140和减压源132之间。
In this illustrative embodiment, reduced
由减压源132生成的减压被经过减压传送导管140传送至减压接驳体142,减压接驳体142可以是肘形口144。在一个例证性的实施方案中,肘形口144是可从德克萨斯州的圣安东尼奥的KCI获得的 技术口(technology port)。减压接驳体142允许减压被传送至创伤闭合敷料110并且在创伤闭合敷料110的内部部分内以及歧管构件112内被实现。在本例证性的实施方案中,肘形口144经过密封盖布116延伸到歧管构件112。
Reduced pressure generated by reduced
一个或多个装置141可以被加入减压传送导管140中。例如,装置141可以是用于容纳渗出液和其他被除去的流体的流体储存器或收集构件。可以被包括在减压传送导管140上或以其他方式流体地耦合于减压传送导管140的装置141的其他示例包括以下非限制性的示例:压力反馈装置、体 积检测系统、血液检测系统、感染检测系统、流动监测系统、温度监测系统等等。这些装置中的某些可以被与减压源132一体地形成。
One or
在操作中,减压创伤治疗系统100可以被施用于患者的创伤102。歧管构件112首先被放置在创伤102上。歧管构件112可以被放置在创伤102内或可以覆盖创伤102的一部分。如果创伤闭合敷料110尚未耦合于歧管构件112,那么然后创伤闭合敷料110可以被放置在歧管构件112的顶部上,使得密封盖布116的盖布延伸部126延伸超出创伤102的外周。盖布延伸部126被夹紧构件120固定于患者的表皮104,以在创伤102上形成流体密封。然后,一个或多个收缩元件118可以被激活,使得收缩元件118产生收缩力(由箭头128示出),该收缩力通过夹紧构件120被传输至患者的表皮104,使得创伤边缘101被拉动得更靠近。
In operation, the reduced pressure wound
如果减压接驳体142尚未被安装,那么减压接驳体142被施用,并且减压传送导管140在一端被流体地耦合。减压传送导管140的另一端被流体地耦合于减压源132。然后减压源132可以被激活,使得减压被向创伤闭合敷料110传送。有利地,如果收缩元件118被来自流体的湿气激活,如上文讨论的,那么减压的施加可以部分地起作用以将额外的流体从创伤闭合敷料110的内部拉出来并且拉至收缩元件118上。
If the reduced-
当压力被降低时,歧管构件112横向地压缩和收缩以形成半刚性的基底。减压被进一步地仍然经过歧管构件112传递,使得在患者的表皮104处和在创伤102处经受减压。被传送至歧管构件112的减压可以生成可以提供稳定性和疗法的压缩力146。压缩力146可以不仅在表皮104的顶部;压缩力146可以向下更深地延伸并且可以在皮下组织108的水平处被感受到。
When the pressure is reduced, the
当密封盖布116和歧管构件112在减压的影响下横向地收缩时,以及当向下的力经过表皮104的泊松比作用时,可以生成向内的力148,该向内的力148可以帮助在创伤102上保持额外的闭合力并且可以大体上向创伤102提供额外的稳定性。因此,来自减压的向内的力148和来自收缩元件118的力130可以共同作用以辅助闭合创伤102。同时,被向歧管构件112传送并且被传送经过歧管构件112的减压帮助从创伤102除去任何渗 出液和其他流体并且向创伤102提供减压疗法。所有的这些作用可以改进创伤102的愈合。
When the
参照图3A,创伤闭合敷料110被示出为在收缩元件118的激活之前被布置在创伤102上。图3B示出了在至少三个最内侧的收缩元件118已经至少部分地收缩以提供由箭头128表示的闭合力之后的创伤闭合敷料110。
Referring to FIG. 3A , wound closure dressing 110 is shown deployed on
可以是期望的是,在手术室中施用减压创伤治疗系统100,并且允许减压创伤治疗系统100保留在患者身上,直到已经发生足够的愈合。在这方面,可以是期望的是,由透明材料形成密封盖布116、歧管构件112和任何其他层,以允许护理提供者获得关于创伤102的视觉线索,而不需要必须除去创伤闭合敷料110。此外,应意识到,减压创伤治疗系统100可以用作主要的创伤闭合治疗或作为创伤闭合治疗的中间步骤。此外,将意识到,创伤闭合敷料110可以在没有歧管构件112或减压子系统114的情况下使用。创伤闭合敷料110作为能够向创伤102传送闭合力而不需要减压的独立的绷带可以是有益的。
It may be desirable to administer reduced pressure wound
现在主要参照图4A和4B,示出了创伤闭合敷料210的例证性的实施方案。创伤闭合敷料210在大多数方面与图1-3B的创伤闭合敷料110和有关的部件是类似的,并且各部分的相关性在本实施方案中通常通过将数字调整100来表示。例如,密封盖布216类似于密封盖布116。虽然创伤闭合敷料210作为分离的创伤闭合敷料存在,但是创伤闭合敷料210可以用作减压系统,例如减压创伤治疗系统100的一部分。创伤闭合敷料210可以被成形以接近创伤202的形状或延伸超出创伤202。虽然创伤闭合敷料210的平面图被示出为是实质上圆形的,但是将意识到,创伤闭合敷料210可以具有任何合适的平面图,包括但不限于正方形的、矩形的、三角形的、椭圆形的、六边形的、八边形的、不规则的等等。收缩元件218可以以“草盖(thatched)”的型式被编织在一起,使得在被激活时,实质上相等的闭合力(由图4B中的箭头228代表的)可以被分布于创伤202的整个外周203周围。图4A示出了在收缩元件218的激活之前的创伤闭合敷料210和创伤202,并且图4B示出了在收缩元件218已经被激活之后的创伤闭合敷料210和创伤202。
Referring now primarily to FIGS. 4A and 4B , an illustrative embodiment of a wound closure dressing 210 is shown. Wound closure dressing 210 is similar in most respects to wound closure dressing 110 and related components of FIGS.
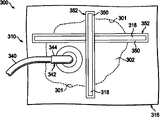
现在主要参照图5,示出了用于治疗患者身上的创伤302的另一个例证性的减压创伤闭合系统300。系统在大多数方面与图1-3B的减压创伤治疗系统100的系统是大体上类似的。类似的部分通过将图1-3B的参考数字调整200来表示。在本例证性的实施方案中,可以利用多个收缩元件318和背垫条350。每个收缩元件318和相应的背垫条350形成收缩条352。因此,可以利用多个收缩条352。每个收缩元件318可脱离地耦合于相应的背垫条350。如前所述,收缩元件318被配置为在被激活时收缩,以产生可以辅助闭合创伤302的闭合力。收缩元件318可以在拉伸位置中被可脱离地耦合于背垫条350,或在收缩条352被施用于患者时,可以被运动至拉伸位置。在两种情况下,当收缩元件318被激活时,收缩元件318试图返回至非拉伸的或者自由位置,并且从而收缩以产生闭合力。
Referring now primarily to FIG. 5 , another illustrative reduced pressure
虽然例证性的实施方案示出每个收缩条352具有单一的收缩元件318,但是将意识到,可以采用收缩元件318的任何合适的数目。此外,在收缩条352包括多个收缩元件318的情况下,多个收缩元件318可以相对于彼此以任何合适的型式被布置,例如平行的、垂直的、成角度的等等。虽然提到了多个收缩元件318、背垫条350和收缩条352,但是也可以使用每个的单一的构件。如同其他实施方案一样,收缩元件318可以由任何合适的响应于被激活而收缩的材料形成并且可以以许多方式被激活。
While the illustrative embodiment shows each
背垫条350可以由任何合适的材料形成,包括但不限于纱布、弹性体、粘合剂等等。任何合适的数目的收缩条352都可以被放置在创伤302和歧管构件,例如图2中的歧管构件112上。每个收缩条352都可以被相应的夹紧构件以任何合适的型式固定于表皮,以辅助闭合创伤302。具有减压接驳体342的密封盖布316可以被放置在收缩条352上,使得减压可以被传送至创伤302;可选择地或另外地,收缩条352可以被放置在密封盖布316的顶部。在可选择的实施方案中,每个所采用的收缩条352的背垫条350可以由盖布材料形成,由此每个背垫条350与一个或多个毗邻的背垫条350共同起作用,从而形成一体的盖布,由此消除对额外的密封盖布的需要;在本实施方案中,可以是期望的是,使用具有高水蒸气传输速率(MVTR)的夹紧构件(例如粘合剂)。在又一个可选择的实施方案中,收缩条352可以用作独立的部件(例如没有歧管和减压子系统)以辅助闭合创伤。
现在主要参照图6,示出了用于治疗创伤402的减压创伤闭合系统400的另一个例证性的非限制性的实施方案。减压创伤闭合系统400在大多数方面与图1-3B的减压创伤治疗系统100是大体上类似的,并且类似的部分通过将图1-3B的参考数字调整300来表示。收缩元件418被以“星形的”型式布置,以向创伤402的外周401传送实质上相等的闭合力。收缩元件418包括中央孔454,中央孔454用于接纳穿过中央孔454的减压接驳体442。虽然例证性的收缩元件418被示出为具有八个“支腿”,但是将意识到,收缩元件418可以包括任何合适的数目的支腿并且可以被作为如所示的一体的单元制造或由多个部件制造。
Referring now primarily to FIG. 6 , another illustrative, non-limiting embodiment of a reduced pressure
现在主要参照图7A和图7B,图7A是用于治疗创伤的系统的例证性的实施方案的一部分的示意性的仰视图。这些图包括用于闭合创伤的创伤闭合敷料500的可选择的实施方案。创伤闭合敷料500包括密封盖布502、可溶解体504、弹性构件506和夹紧构件508。创伤闭合敷料500可以用作辅助创伤闭合的独立部件,或可以用作辅助创伤闭合和治疗的减压系统的一部分。密封盖布502包括第一表面510和面向组织的第二表面512。密封盖布502大体上可以由与图1-3B的密封盖布116相同的或相似的材料形成并且可以以相似的方式操作。可选择地,密封盖布502可以被穿孔以允许湿气从第一表面510通向可溶解体504。
Referring now primarily to FIGS. 7A and 7B , FIG. 7A is a schematic bottom view of a portion of an illustrative embodiment of a system for treating wounds. These figures include an alternative embodiment of a wound closure dressing 500 for closing a wound. Wound closure dressing 500 includes sealing
可溶解体504耦合于密封盖布502的面向组织的第二表面512。可溶解体504可以由任何合适的可溶解材料形成,包括但不限于生物可降解材料或生物可吸收材料,例如聚交酯(PLA)、乳酸-乙醇酸共聚物(poly(lactic-co-glycolic acid))(PLGA)、聚乙醇酸(PGA)、聚已酸内酯(PCL)、氯化钠或类似的。此外,可溶解体504可以包括用于减少感染的氧化过的颗粒(oxygenated particle)或抗微生物颗粒。可溶解体504将弹性构件506保持在拉伸位置中,可溶解体504可以在任何合适的因素,包括但不限于湿气、热、超声等等的影响下溶解。当可溶解体504溶解时,弹性构件506被至少部分地释放,由此产生闭合力,因为弹性构件506试图返回未拉伸位置。虽然例证性的实施方案示出了单一的可溶解体504,但是将意识到,可以采 用可溶解体的任何合适的数目(见例如图8-9B)。
The
弹性构件506在拉伸位置中耦合于可溶解体504,使得当可溶解体504或其一部分溶解时,弹性构件506收缩以产生闭合力。弹性构件506可以通过任何合适的装置或技术,包括但不限于粘合剂、机械紧固件、捆绑、声焊接等等,被耦合于可溶解体504。可选择地或另外地,弹性构件506可以通过将弹性构件506嵌入可溶解体504中被耦合。弹性构件506可以是任何合适的能够在拉伸位置中耦合于可溶解体504并且能够在可溶解体504的至少一部分溶解时收缩的材料。例如但不限于,弹性构件506可以由弹性体形成。如本文所使用的,术语“耦合”包括通过分离的物体的耦合并且包括直接耦合。术语“耦合”还包括两个或更多个部件借助于部件中的每个由同一片材料形成而彼此连续。此外,术语“耦合”可以包括化学的(例如通过化学键)、机械的、热的或电的耦合。耦合可以还包括将一个构件嵌入另一个中。
The
如图7B中清楚地示出的,弹性构件506在本例证性的实施方案中具有实质上圆形的横截面。然而,应意识到,弹性构件506可以具有任何合适的横截面。此外,将意识到,弹性构件506可以具有任何合适的配置。例如,弹性构件506可以被布置为“草垫”型式、十字型式、平行型式等等。此外,虽然例证性的实施方案示出了单一的弹性构件506,但是将意识到,任何合适的数目的弹性构件506都可以耦合于一个或多个可溶解体504。此外,弹性构件506的端可以耦合于密封盖布502,使得由弹性构件506产生的收缩力可以被直接地传递至夹紧构件508(如下文进一步讨论的)。
As best shown in Figure 7B,
夹紧构件508与图1-3B的减压创伤治疗系统100的夹紧构件120是相同的或相似的。夹紧构件508可以被耦合于密封盖布502、可溶解体504或弹性构件506中的至少一个。夹紧构件508被配置为将由弹性构件506的收缩产生的力传递至患者的表皮以辅助闭合创伤。可选择地,如在图7B中最好地示出的,创伤闭合敷料500也可以包括穿孔片材514,穿孔片材514被布置在可溶解体504和创伤之间,以调节来自患者的被允许传至可溶解体504的渗出液的湿气的量。这对于在其中可溶解体504在湿气被引 入可溶解体504时溶解的情况下对可溶解体504溶解的量或速率的控制来说可以是有用的。
Gripping
现在主要参照图8-9B,示出了另一个用于治疗创伤602的创伤闭合敷料610。创伤闭合敷料610在大多数方面与图7A和7B的创伤闭合敷料500是大体上类似的。类似的部分通过将图7A和7B的参考数字调整100来表示。在本实施方案中,可溶解体604包括多个可溶解珠618或其他可溶解的构件。多个可溶解珠618保持由拉伸的弹性构件606产生的力,并且如果多个可溶解珠618被溶解,那么附接于弹性构件606的夹紧构件(未示出)经受升高的力。珠618典型地被来自创伤602的渗出液溶解。当珠618溶解时,由相应的弹性构件606产生的收缩力增加。所产生的闭合力可以与被溶解的珠618的数目具有直接的关系。例如,当被溶解的珠618的数目增加时,所产生的闭合力可以以确定的关系,例如线性地、指数地等等增加。
Referring now primarily to FIGS. 8-9B , another wound closure dressing 610 for treating a
因此,如图9A和9B中清楚地示出的,创伤闭合敷料610可以被“调节”以在创伤602的更宽的部分处产生更大的闭合力。此外,这可以以自调节的方式发生。换句话说,创伤602的具有渗出液的升高的水平的区域,即典型地是创伤602的较宽的部分,经受更大的闭合力,因为渗出液的升高的水平导致更多的珠618溶解,这增加了由弹性构件606产生的闭合力。创伤闭合敷料610可以与相似于图7A和7B的创伤闭合敷料510的密封盖布和夹紧构件的并且作为减压治疗系统的一部分的密封盖布和夹紧构件共同使用。可选择地,每个弹性构件606的每个端可以被固定于患者的表皮以及被用作用于辅助创伤闭合的独立敷料的创伤闭合敷料610。
Thus, as best shown in FIGS. 9A and 9B , wound closure dressing 610 can be "tuned" to produce greater closure force at wider portions of
虽然已经在某些例证性的非限制性实施方案的上下文中公开了本发明以及其优点,但是应当理解,可以作出各种改变、取代、置换和更改而不偏离由所附的权利要求限定的本发明的范围。应认识到,结合任何一个实施方案描述的特征也可以适用于任何其他实施方案。 Although the present invention and its advantages have been disclosed in the context of certain illustrative and non-limiting embodiments, it should be understood that various changes, substitutions, substitutions and alterations can be made without departing from the scope of the invention as defined by the appended claims. scope of the invention. It will be appreciated that features described in connection with any one embodiment may also be applicable to any other embodiment. the
Claims (18)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11237108P | 2008-11-07 | 2008-11-07 | |
| US61/112,371 | 2008-11-07 | ||
| PCT/US2009/062981 WO2010053870A1 (en) | 2008-11-07 | 2009-11-02 | Reduced-pressure, wound-treatment dressings and systems |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN102202619A CN102202619A (en) | 2011-09-28 |
| CN102202619B true CN102202619B (en) | 2014-03-12 |
Family
ID=42153198
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN200980143982.7A Expired - Fee Related CN102202619B (en) | 2008-11-07 | 2009-11-02 | Reduced-pressure, wound-treatment dressings and systems |
Country Status (12)
| Country | Link |
|---|---|
| US (3) | US8460257B2 (en) |
| EP (2) | EP3421020B1 (en) |
| JP (2) | JP2012508037A (en) |
| KR (1) | KR101644206B1 (en) |
| CN (1) | CN102202619B (en) |
| AU (1) | AU2009311352B2 (en) |
| BR (1) | BRPI0916062A2 (en) |
| CA (1) | CA2742962C (en) |
| MX (1) | MX2011004820A (en) |
| RU (1) | RU2011114000A (en) |
| TW (1) | TW201021862A (en) |
| WO (1) | WO2010053870A1 (en) |
Families Citing this family (130)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB0224986D0 (en) | 2002-10-28 | 2002-12-04 | Smith & Nephew | Apparatus |
| GB0325129D0 (en) | 2003-10-28 | 2003-12-03 | Smith & Nephew | Apparatus in situ |
| US7708724B2 (en) * | 2004-04-05 | 2010-05-04 | Blue Sky Medical Group Incorporated | Reduced pressure wound cupping treatment system |
| US7909805B2 (en) | 2004-04-05 | 2011-03-22 | Bluesky Medical Group Incorporated | Flexible reduced pressure treatment appliance |
| US7776028B2 (en) | 2004-04-05 | 2010-08-17 | Bluesky Medical Group Incorporated | Adjustable overlay reduced pressure wound treatment system |
| US10058642B2 (en) | 2004-04-05 | 2018-08-28 | Bluesky Medical Group Incorporated | Reduced pressure treatment system |
| US8062272B2 (en) | 2004-05-21 | 2011-11-22 | Bluesky Medical Group Incorporated | Flexible reduced pressure treatment appliance |
| CA2619929A1 (en) | 2005-09-06 | 2007-03-15 | Tyco Healthcare Group Lp | Self contained wound dressing with micropump |
| US7779625B2 (en) | 2006-05-11 | 2010-08-24 | Kalypto Medical, Inc. | Device and method for wound therapy |
| US9820888B2 (en) | 2006-09-26 | 2017-11-21 | Smith & Nephew, Inc. | Wound dressing |
| EP3000448B2 (en) | 2007-11-21 | 2022-03-09 | Smith & Nephew PLC | Wound dressing |
| GB0722820D0 (en) | 2007-11-21 | 2008-01-02 | Smith & Nephew | Vacuum assisted wound dressing |
| WO2009066105A1 (en) | 2007-11-21 | 2009-05-28 | Smith & Nephew Plc | Wound dressing |
| GB0804654D0 (en) * | 2008-03-13 | 2008-04-16 | Smith & Nephew | Vacuum closure device |
| GB0808376D0 (en) | 2008-05-08 | 2008-06-18 | Bristol Myers Squibb Co | Wound dressing |
| GB0817796D0 (en) | 2008-09-29 | 2008-11-05 | Convatec Inc | wound dressing |
| GB0902368D0 (en) | 2009-02-13 | 2009-04-01 | Smith & Nephew | Wound packing |
| US8444614B2 (en) * | 2009-04-10 | 2013-05-21 | Spiracur, Inc. | Methods and devices for applying closed incision negative pressure wound therapy |
| JP5650199B2 (en) | 2009-04-10 | 2015-01-07 | スピレイカー・インコーポレイテッドSpiracur, Inc. | Method and apparatus for attaching a negative pressure closure therapy system for a closed incision |
| WO2010121186A1 (en) | 2009-04-17 | 2010-10-21 | Kalypto Medical, Inc. | Negative pressure wound therapy device |
| US10159825B2 (en) | 2009-09-17 | 2018-12-25 | Zipline Medical, Inc. | Rapid closing surgical closure device |
| JP2013505057A (en) | 2009-09-17 | 2013-02-14 | ジップライン メディカル, インコーポレイテッド | Rapid closure surgical closure device |
| US8721606B2 (en) | 2010-03-11 | 2014-05-13 | Kci Licensing, Inc. | Dressings, systems, and methods for treating a tissue site |
| US8702665B2 (en) | 2010-04-16 | 2014-04-22 | Kci Licensing, Inc. | Reduced-pressure sources, systems, and methods employing a polymeric, porous, hydrophobic material |
| US20190381222A9 (en) * | 2010-04-16 | 2019-12-19 | Kci Licensing, Inc. | Reduced-Pressure Sources, Systems, And Methods Employing A Polymeric, Porous, Hydrophobic Material |
| US9061095B2 (en) | 2010-04-27 | 2015-06-23 | Smith & Nephew Plc | Wound dressing and method of use |
| US8439945B2 (en) | 2010-05-03 | 2013-05-14 | Zipline Medical, Inc. | Methods for biopsying tissue |
| EP2579828B1 (en) | 2010-06-14 | 2018-06-13 | Zipline Medical Inc. | Apparatus for inhibiting scar formation |
| US9265665B2 (en) * | 2010-07-19 | 2016-02-23 | Kci Licensing, Inc. | Inflatable off-loading wound dressing assemblies, systems, and methods |
| CA140189S (en) | 2010-10-15 | 2011-11-07 | Smith & Nephew | Medical dressing |
| CA140188S (en) | 2010-10-15 | 2011-11-07 | Smith & Nephew | Medical dressing |
| GB201020236D0 (en) | 2010-11-30 | 2011-01-12 | Convatec Technologies Inc | A composition for detecting biofilms on viable tissues |
| US9526816B2 (en) | 2010-12-08 | 2016-12-27 | Convatec Technologies Inc. | Wound exudate monitor accessory |
| WO2012078723A1 (en) | 2010-12-08 | 2012-06-14 | Convatec Technologies Inc. | Method and system for removing exudates from a wound site |
| CA2819475C (en) | 2010-12-08 | 2019-02-12 | Convatec Technologies Inc. | Integrated system for assessing wound exudates |
| US9421132B2 (en) | 2011-02-04 | 2016-08-23 | University Of Massachusetts | Negative pressure wound closure device |
| JP6158096B2 (en) | 2011-02-04 | 2017-07-05 | ユニバーシティー オブ マサチューセッツUniversity of Massachusetts | Negative pressure wound closure device |
| MX2013013739A (en) | 2011-05-24 | 2014-07-28 | Kalypto Medical Inc | Device with controller and pump modules for providing negative pressure for wound therapy. |
| US9058634B2 (en) | 2011-05-24 | 2015-06-16 | Kalypto Medical, Inc. | Method for providing a negative pressure wound therapy pump device |
| US9067003B2 (en) | 2011-05-26 | 2015-06-30 | Kalypto Medical, Inc. | Method for providing negative pressure to a negative pressure wound therapy bandage |
| JP6224581B2 (en) * | 2011-06-24 | 2017-11-01 | ケーシーアイ ライセンシング インコーポレイテッド | Reduced pressure dressing with tissue fixation elements |
| AU2012282287B2 (en) | 2011-07-14 | 2017-06-01 | Smith & Nephew Plc | Wound dressing and method of treatment |
| GB201115182D0 (en) | 2011-09-02 | 2011-10-19 | Trio Healthcare Ltd | Skin contact material |
| US9408941B2 (en) | 2011-09-20 | 2016-08-09 | Kci Licensing, Inc. | Tissue treatment systems and methods having a non-tactile-stimulus-activated, macroscopically-deforming material |
| US9198803B1 (en) | 2011-09-26 | 2015-12-01 | David S. London | Dressing device for offloading and treating an ulcer |
| JP2014530075A (en) * | 2011-10-17 | 2014-11-17 | ケーシーアイライセンシング インコーポレイテッド | System and apparatus with an in-line canister for treating a tissue site |
| US8323313B1 (en) | 2011-11-01 | 2012-12-04 | Zipline Medical, Inc. | Surgical incision and closure apparatus with integrated force distribution |
| US9561034B2 (en) | 2011-11-01 | 2017-02-07 | Zipline Medical, Inc. | Surgical incision and closure apparatus |
| US10123800B2 (en) | 2011-11-01 | 2018-11-13 | Zipline Medical, Inc. | Surgical incision and closure apparatus with integrated force distribution |
| US10123801B2 (en) | 2011-11-01 | 2018-11-13 | Zipline Medical, Inc. | Means to prevent wound dressings from adhering to closure device |
| US9050086B2 (en) | 2011-11-01 | 2015-06-09 | Zipline Medical, Inc. | Surgical incision and closure apparatus |
| US12171432B2 (en) | 2011-11-01 | 2024-12-24 | Zipline Medical, Inc. | Closure apparatuses and methods for ulcers and irregular skin defects |
| DK2773383T3 (en) * | 2011-11-02 | 2018-06-18 | Smith & Nephew | Device for pressure ulcer treatment |
| GB2497406A (en) | 2011-11-29 | 2013-06-12 | Webtec Converting Llc | Dressing with a perforated binder layer |
| GB201120693D0 (en) | 2011-12-01 | 2012-01-11 | Convatec Technologies Inc | Wound dressing for use in vacuum therapy |
| USD733896S1 (en) | 2012-05-04 | 2015-07-07 | Genadyne Biotechnologies, Inc. | Abdominal dressing |
| AU346291S (en) | 2012-05-15 | 2013-01-09 | Smith & Nephew | Medical dressing |
| AU2013291693B2 (en) | 2012-05-22 | 2018-03-01 | Smith & Nephew Plc | Wound closure device |
| JP6382185B2 (en) | 2012-05-22 | 2018-08-29 | スミス アンド ネフュー ピーエルシーSmith & Nephew Public Limited Company | Apparatus and method for wound treatment |
| JP6400570B2 (en) | 2012-05-23 | 2018-10-10 | スミス アンド ネフュー ピーエルシーSmith & Nephew Public Limited Company | Apparatus and method for local negative pressure closure therapy |
| WO2013175309A1 (en) | 2012-05-24 | 2013-11-28 | Smith & Nephew Plc | Devices and methods for treating and closing wounds with negative pressure |
| EP2872085A1 (en) | 2012-07-16 | 2015-05-20 | Smith&Nephew, Inc. | Negative pressure wound closure device |
| CA2895896A1 (en) | 2012-12-20 | 2014-06-26 | Convatec Technologies Inc. | Processing of chemically modified cellulosic fibres |
| JP6407954B2 (en) | 2013-03-13 | 2018-10-17 | スミス アンド ネフュー インコーポレイテッド | Negative pressure wound closure device and system and method of use in wound treatment with negative pressure |
| WO2014140578A1 (en) | 2013-03-14 | 2014-09-18 | Smith & Nephew Plc | Compressible wound fillers and systems and methods of use in treating wounds with negative pressure |
| CN105492035B (en) | 2013-03-14 | 2019-06-14 | 史密夫和内修有限公司 | System and method for applying reduced pressure therapy |
| US9737649B2 (en) | 2013-03-14 | 2017-08-22 | Smith & Nephew, Inc. | Systems and methods for applying reduced pressure therapy |
| JP6670233B2 (en) * | 2013-03-29 | 2020-03-18 | コーニンクレッカ フィリップス エヌ ヴェKoninklijke Philips N.V. | Force feedback grip device with actuator based on magnetic viscosity |
| JP2016528964A (en) | 2013-07-16 | 2016-09-23 | スミス アンド ネフュー ピーエルシーSmith & Nephew Public Limited Company | Apparatus and method for wound treatment |
| US9675504B2 (en) * | 2013-08-28 | 2017-06-13 | Lisa Ann Myers | Disposable water resistant protective cover cast and wound sites |
| EP3060181B1 (en) | 2013-10-21 | 2021-11-03 | Smith & Nephew, Inc. | Negative pressure wound closure device |
| CN105873525A (en) | 2014-01-05 | 2016-08-17 | 奇普林医药公司 | Instrumented wound closure device |
| AU2015208299B2 (en) | 2014-01-21 | 2019-11-21 | Smith & Nephew Plc | Collapsible dressing for negative pressure wound treatment |
| JP6704346B2 (en) | 2014-01-21 | 2020-06-03 | スミス アンド ネフュー ピーエルシーSmith & Nephew Public Limited Company | Wound treatment device |
| US10226566B2 (en) | 2014-04-23 | 2019-03-12 | Genadyne Biotechnologies, Inc. | System and process for removing bodily fluids from a body opening |
| US10441474B1 (en) | 2014-05-27 | 2019-10-15 | Barakat Alhammadin | Bandage for applying arterial pressure |
| US9770369B2 (en) | 2014-08-08 | 2017-09-26 | Neogenix, Llc | Wound care devices, apparatus, and treatment methods |
| CN104188705B (en) * | 2014-09-11 | 2016-10-05 | 黄成� | Moulding of a kind of hemostasis |
| WO2016108585A1 (en) * | 2015-01-02 | 2016-07-07 | 가톨릭관동대학교산학협력단 | Medical magnet band |
| KR101706916B1 (en) * | 2015-01-02 | 2017-02-16 | 가톨릭관동대학교산학협력단 | Medical magnet bandage |
| EP3288509B1 (en) * | 2015-04-29 | 2022-06-29 | Smith & Nephew, Inc | Negative pressure wound closure device |
| EP3326593A4 (en) * | 2015-07-24 | 2019-03-13 | Nichiban Co. Ltd. | Skin-suturing tape or reinforcing tape used after suturing skin |
| GB2543544A (en) | 2015-10-21 | 2017-04-26 | Brightwake Ltd | Wound dressing |
| US10814049B2 (en) | 2015-12-15 | 2020-10-27 | University Of Massachusetts | Negative pressure wound closure devices and methods |
| US10575991B2 (en) | 2015-12-15 | 2020-03-03 | University Of Massachusetts | Negative pressure wound closure devices and methods |
| US11357906B2 (en) * | 2016-02-12 | 2022-06-14 | Smith & Nephew, Inc. | Systems and methods for detecting operational conditions of reduced pressure therapy |
| EP3435941B1 (en) | 2016-03-30 | 2021-09-01 | ConvaTec Technologies Inc. | Detecting microbial infections in wounds |
| KR20190015210A (en) | 2016-03-30 | 2019-02-13 | 퀄리자임 다이아그노스틱스 게엠베하 엔드 코 카게 | Detection of microbial infection in wound |
| GB201608099D0 (en) | 2016-05-09 | 2016-06-22 | Convatec Technologies Inc | Negative pressure wound dressing |
| CN109640904A (en) | 2016-07-08 | 2019-04-16 | 康沃特克科技公司 | fluid collection equipment |
| PL3481360T3 (en) | 2016-07-08 | 2022-05-02 | Convatec Technologies Inc. | Fluid flow sensing |
| CN109688991B (en) | 2016-07-08 | 2021-10-29 | 康沃特克科技公司 | Flexible negative pressure system |
| CN109640903A (en) | 2016-08-30 | 2019-04-16 | 史密夫及内修公开有限公司 | For applying the system of reduced pressure therapy |
| JP6361844B1 (en) * | 2016-09-23 | 2018-07-25 | 株式会社村田製作所 | Negative pressure closure therapy device |
| WO2018060144A1 (en) | 2016-09-27 | 2018-04-05 | Smith & Nephew Plc | Wound closure devices with dissolvable portions |
| US11096817B2 (en) * | 2016-10-19 | 2021-08-24 | 6D Tape Inc | Therapy tape to aid patient recovery |
| GB2555584B (en) | 2016-10-28 | 2020-05-27 | Smith & Nephew | Multi-layered wound dressing and method of manufacture |
| WO2018081795A1 (en) | 2016-10-31 | 2018-05-03 | Zipline Medical, Inc. | Systems and methods for monitoring physical therapy of the knee and other joints |
| US11617684B2 (en) | 2016-11-02 | 2023-04-04 | Smith & Nephew, Inc. | Wound closure devices |
| CN106726146A (en) * | 2017-01-18 | 2017-05-31 | 柴家科 | For the negative pressure wound surface therapeutic system of Wound treating |
| CN110997028B (en) | 2017-02-22 | 2022-12-30 | 康奈尔大学 | Mechanical vacuum dressing for mechanical management, protection and aspiration of small incision wounds |
| WO2018229010A1 (en) | 2017-06-13 | 2018-12-20 | Smith & Nephew Plc | Collapsible structure and method of use |
| CA3063813A1 (en) | 2017-06-13 | 2018-12-20 | Smith & Nephew Plc | Wound closure device and method of use |
| US11395873B2 (en) | 2017-06-14 | 2022-07-26 | Smith & Nephew, Inc. | Control of wound closure and fluid removal management in wound therapy |
| US11583623B2 (en) | 2017-06-14 | 2023-02-21 | Smith & Nephew Plc | Collapsible structure for wound closure and method of use |
| JP2020523052A (en) | 2017-06-14 | 2020-08-06 | スミス アンド ネフュー インコーポレイテッド | Fluid removal management and control of wound closure in wound care |
| JP7419072B2 (en) | 2017-06-14 | 2024-01-22 | スミス アンド ネフュー ピーエルシー | Foldable sheet for wound closure and method of use |
| EP3658090B1 (en) | 2017-07-27 | 2021-11-10 | Smith & Nephew PLC | Customizable wound closure device |
| US11590030B2 (en) | 2017-08-07 | 2023-02-28 | Smith & Nephew Plc | Wound closure device with protective layer and method of use |
| EP3675925B1 (en) | 2017-08-29 | 2025-12-03 | Smith & Nephew PLC | Systems for monitoring wound closure |
| US12161792B2 (en) | 2017-11-16 | 2024-12-10 | Convatec Limited | Fluid collection apparatus |
| JP7424638B2 (en) | 2017-12-06 | 2024-01-30 | コーネル ユニヴァーシティー | Manually operated negative pressure wound therapy (NPWT) bandage with improved pump efficiency, automatic pressure indicator and automatic pressure limiter |
| WO2019199849A1 (en) | 2018-04-13 | 2019-10-17 | Kci Licensing, Inc. | Dressing bolster with area pressure indicator |
| US11896464B2 (en) | 2018-04-13 | 2024-02-13 | Kci Licensing, Inc. | Method to dynamically measure apposition and patient limb movement in a negative pressure closed incision dressing |
| WO2019199798A1 (en) * | 2018-04-13 | 2019-10-17 | Kci Licensing, Inc. | Compression strain and negative pressure delivery indicator for a wound dressing |
| GB2574074B (en) | 2018-07-27 | 2020-05-20 | Mclaren Applied Tech Ltd | Time synchronisation |
| CN109124712B (en) * | 2018-09-20 | 2021-05-07 | 上海锦辰医药科技有限公司 | Wound closure device |
| EP3893825A1 (en) | 2018-12-13 | 2021-10-20 | University of Massachusetts | Negative pressure wound closure devices and methods |
| WO2020166744A1 (en) * | 2019-02-15 | 2020-08-20 | 주식회사 하이로닉 | Skin lifting method using microneedle having deformed structure and skin dressing pad |
| CN109938920B (en) * | 2019-04-29 | 2024-02-20 | 振德医疗用品股份有限公司 | Micro negative pressure foam dressing and manufacturing method thereof |
| WO2020245656A1 (en) | 2019-06-03 | 2020-12-10 | Convatec Limited | Methods and devices to disrupt and contain pathogens |
| US20220305192A1 (en) * | 2019-06-17 | 2022-09-29 | Kci Licensing, Inc. | Abdominal Negative-Pressure Therapy Dressing With Closed-Loop Force Management Control |
| GB2588236B (en) | 2019-10-18 | 2024-03-20 | Mclaren Applied Ltd | Gyroscope bias estimation |
| BR112022012120A2 (en) | 2019-12-23 | 2022-08-30 | Convatec Ltd | POINT OF CARE DEVICES TO DETECT INFECTION STATUS OF A WOUND |
| US11331221B2 (en) | 2019-12-27 | 2022-05-17 | Convatec Limited | Negative pressure wound dressing |
| US11771819B2 (en) | 2019-12-27 | 2023-10-03 | Convatec Limited | Low profile filter devices suitable for use in negative pressure wound therapy systems |
| CN113786282A (en) * | 2021-09-15 | 2021-12-14 | 电子科技大学 | A thermally activated electromechanical synergistic dressing for accelerating wound healing and preparation method thereof |
| CN114432044B (en) * | 2022-01-22 | 2023-06-09 | 佛山市耐思得卫生用品有限公司 | Sanitary towel |
| WO2024062325A1 (en) * | 2022-09-20 | 2024-03-28 | Solventum Intellectual Properties Company | Dressing having an integral closure device |
| CN116439767B (en) * | 2023-03-06 | 2024-11-29 | 上海哈易拿医疗科技有限公司 | Unidirectional air suction type negative pressure tension reducing scar removing patch |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050131327A1 (en) * | 2000-11-29 | 2005-06-16 | Lockwood Jeffrey S. | Vacuum therapy and cleansing dressing for wounds |
| WO2005105174A1 (en) * | 2004-04-27 | 2005-11-10 | Smith & Nephew, Plc | Wound cleansing apparatus with stress |
| US20060079852A1 (en) * | 2002-12-31 | 2006-04-13 | Bubb Stephen K | Externally-applied patient interface system and method |
| CN100349551C (en) * | 2003-03-03 | 2007-11-21 | Kci特许公司 | Tissue processing system |
Family Cites Families (137)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1355846A (en) | 1920-02-06 | 1920-10-19 | David A Rannells | Medical appliance |
| US2012755A (en) * | 1934-07-12 | 1935-08-27 | Muth Otto De | Surgical dressing |
| US2547758A (en) | 1949-01-05 | 1951-04-03 | Wilmer B Keeling | Instrument for treating the male urethra |
| US2632443A (en) | 1949-04-18 | 1953-03-24 | Eleanor P Lesher | Surgical dressing |
| GB692578A (en) | 1949-09-13 | 1953-06-10 | Minnesota Mining & Mfg | Improvements in or relating to drape sheets for surgical use |
| US2682873A (en) | 1952-07-30 | 1954-07-06 | Johnson & Johnson | General purpose protective dressing |
| NL189176B (en) | 1956-07-13 | 1900-01-01 | Hisamitsu Pharmaceutical Co | PLASTER BASED ON A SYNTHETIC RUBBER. |
| US2969057A (en) | 1957-11-04 | 1961-01-24 | Brady Co W H | Nematodic swab |
| US3066672A (en) | 1960-09-27 | 1962-12-04 | Jr William H Crosby | Method and apparatus for serial sampling of intestinal juice |
| US3367332A (en) | 1965-08-27 | 1968-02-06 | Gen Electric | Product and process for establishing a sterile area of skin |
| US3520300A (en) | 1967-03-15 | 1970-07-14 | Amp Inc | Surgical sponge and suction device |
| US3568675A (en) | 1968-08-30 | 1971-03-09 | Clyde B Harvey | Fistula and penetrating wound dressing |
| US3682180A (en) | 1970-06-08 | 1972-08-08 | Coilform Co Inc | Drain clip for surgical drain |
| BE789293Q (en) | 1970-12-07 | 1973-01-15 | Parke Davis & Co | MEDICO-SURGICAL DRESSING FOR BURNS AND SIMILAR LESIONS |
| US3826254A (en) | 1973-02-26 | 1974-07-30 | Verco Ind | Needle or catheter retaining appliance |
| DE2527706A1 (en) | 1975-06-21 | 1976-12-30 | Hanfried Dr Med Weigand | DEVICE FOR THE INTRODUCTION OF CONTRAST AGENTS INTO AN ARTIFICIAL INTESTINAL OUTLET |
| DE2640413C3 (en) | 1976-09-08 | 1980-03-27 | Richard Wolf Gmbh, 7134 Knittlingen | Catheter monitor |
| NL7710909A (en) | 1976-10-08 | 1978-04-11 | Smith & Nephew | COMPOSITE STRAPS. |
| GB1562244A (en) | 1976-11-11 | 1980-03-05 | Lock P M | Wound dressing materials |
| US4080970A (en) | 1976-11-17 | 1978-03-28 | Miller Thomas J | Post-operative combination dressing and internal drain tube with external shield and tube connector |
| US4139004A (en) | 1977-02-17 | 1979-02-13 | Gonzalez Jr Harry | Bandage apparatus for treating burns |
| US4184510A (en) | 1977-03-15 | 1980-01-22 | Fibra-Sonics, Inc. | Valued device for controlling vacuum in surgery |
| US4165748A (en) | 1977-11-07 | 1979-08-28 | Johnson Melissa C | Catheter tube holder |
| US4256109A (en) | 1978-07-10 | 1981-03-17 | Nichols Robert L | Shut off valve for medical suction apparatus |
| SE414994B (en) | 1978-11-28 | 1980-09-01 | Landstingens Inkopscentral | VENKATETERFORBAND |
| DE2953373A1 (en) | 1978-12-06 | 1981-01-08 | P Svedman | Device for treating tissues,for example skin |
| US4266545A (en) | 1979-04-06 | 1981-05-12 | Moss James P | Portable suction device for collecting fluids from a closed wound |
| US4284079A (en) | 1979-06-28 | 1981-08-18 | Adair Edwin Lloyd | Method for applying a male incontinence device |
| US4261363A (en) | 1979-11-09 | 1981-04-14 | C. R. Bard, Inc. | Retention clips for body fluid drains |
| US4569348A (en) | 1980-02-22 | 1986-02-11 | Velcro Usa Inc. | Catheter tube holder strap |
| WO1981002516A1 (en) | 1980-03-11 | 1981-09-17 | E Schmid | Cushion for holding an element of grafted skin |
| US4297995A (en) | 1980-06-03 | 1981-11-03 | Key Pharmaceuticals, Inc. | Bandage containing attachment post |
| US4333468A (en) | 1980-08-18 | 1982-06-08 | Geist Robert W | Mesentery tube holder apparatus |
| US4465485A (en) | 1981-03-06 | 1984-08-14 | Becton, Dickinson And Company | Suction canister with unitary shut-off valve and filter features |
| US4392853A (en) | 1981-03-16 | 1983-07-12 | Rudolph Muto | Sterile assembly for protecting and fastening an indwelling device |
| US4373519A (en) | 1981-06-26 | 1983-02-15 | Minnesota Mining And Manufacturing Company | Composite wound dressing |
| US4392858A (en) | 1981-07-16 | 1983-07-12 | Sherwood Medical Company | Wound drainage device |
| US4419097A (en) | 1981-07-31 | 1983-12-06 | Rexar Industries, Inc. | Attachment for catheter tube |
| AU550575B2 (en) | 1981-08-07 | 1986-03-27 | Richard Christian Wright | Wound drainage device |
| SE429197B (en) | 1981-10-14 | 1983-08-22 | Frese Nielsen | SAR TREATMENT DEVICE |
| DE3146266A1 (en) | 1981-11-21 | 1983-06-01 | B. Braun Melsungen Ag, 3508 Melsungen | COMBINED DEVICE FOR A MEDICAL SUCTION DRAINAGE |
| US4551139A (en) | 1982-02-08 | 1985-11-05 | Marion Laboratories, Inc. | Method and apparatus for burn wound treatment |
| US4475909A (en) | 1982-05-06 | 1984-10-09 | Eisenberg Melvin I | Male urinary device and method for applying the device |
| EP0100148B1 (en) | 1982-07-06 | 1986-01-08 | Dow Corning Limited | Medical-surgical dressing and a process for the production thereof |
| NZ206837A (en) | 1983-01-27 | 1986-08-08 | Johnson & Johnson Prod Inc | Thin film adhesive dressing:backing material in three sections |
| US4548202A (en) | 1983-06-20 | 1985-10-22 | Ethicon, Inc. | Mesh tissue fasteners |
| US4540412A (en) | 1983-07-14 | 1985-09-10 | The Kendall Company | Device for moist heat therapy |
| US4543100A (en) | 1983-11-01 | 1985-09-24 | Brodsky Stuart A | Catheter and drain tube retainer |
| US4525374A (en) | 1984-02-27 | 1985-06-25 | Manresa, Inc. | Treating hydrophobic filters to render them hydrophilic |
| CA1286177C (en) | 1984-05-03 | 1991-07-16 | Smith And Nephew Associated Companies Plc | Adhesive wound dressing |
| US4897081A (en) | 1984-05-25 | 1990-01-30 | Thermedics Inc. | Percutaneous access device |
| US5215522A (en) | 1984-07-23 | 1993-06-01 | Ballard Medical Products | Single use medical aspirating device and method |
| GB8419745D0 (en) | 1984-08-02 | 1984-09-05 | Smith & Nephew Ass | Wound dressing |
| US4872450A (en) | 1984-08-17 | 1989-10-10 | Austad Eric D | Wound dressing and method of forming same |
| US4826494A (en) | 1984-11-09 | 1989-05-02 | Stryker Corporation | Vacuum wound drainage system |
| US4655754A (en) | 1984-11-09 | 1987-04-07 | Stryker Corporation | Vacuum wound drainage system and lipids baffle therefor |
| US4605399A (en) | 1984-12-04 | 1986-08-12 | Complex, Inc. | Transdermal infusion device |
| US5037397A (en) | 1985-05-03 | 1991-08-06 | Medical Distributors, Inc. | Universal clamp |
| US4640688A (en) | 1985-08-23 | 1987-02-03 | Mentor Corporation | Urine collection catheter |
| US4710165A (en) | 1985-09-16 | 1987-12-01 | Mcneil Charles B | Wearable, variable rate suction/collection device |
| US4758220A (en) | 1985-09-26 | 1988-07-19 | Alcon Laboratories, Inc. | Surgical cassette proximity sensing and latching apparatus |
| US4733659A (en) | 1986-01-17 | 1988-03-29 | Seton Company | Foam bandage |
| WO1987004626A1 (en) | 1986-01-31 | 1987-08-13 | Osmond, Roger, L., W. | Suction system for wound and gastro-intestinal drainage |
| US4838883A (en) | 1986-03-07 | 1989-06-13 | Nissho Corporation | Urine-collecting device |
| JPS62281965A (en) | 1986-05-29 | 1987-12-07 | テルモ株式会社 | Catheter and catheter fixing member |
| GB8621884D0 (en) | 1986-09-11 | 1986-10-15 | Bard Ltd | Catheter applicator |
| GB2195255B (en) | 1986-09-30 | 1991-05-01 | Vacutec Uk Limited | Apparatus for vacuum treatment of an epidermal surface |
| US4743232A (en) | 1986-10-06 | 1988-05-10 | The Clinipad Corporation | Package assembly for plastic film bandage |
| DE3634569A1 (en) | 1986-10-10 | 1988-04-21 | Sachse Hans E | CONDOM CATHETER, A URINE TUBE CATHETER FOR PREVENTING RISING INFECTIONS |
| JPS63135179A (en) | 1986-11-26 | 1988-06-07 | 立花 俊郎 | Subcataneous drug administration set |
| GB8628564D0 (en) | 1986-11-28 | 1987-01-07 | Smiths Industries Plc | Anti-foaming agent suction apparatus |
| GB8706116D0 (en) | 1987-03-14 | 1987-04-15 | Smith & Nephew Ass | Adhesive dressings |
| US4787888A (en) | 1987-06-01 | 1988-11-29 | University Of Connecticut | Disposable piezoelectric polymer bandage for percutaneous delivery of drugs and method for such percutaneous delivery (a) |
| US4863449A (en) | 1987-07-06 | 1989-09-05 | Hollister Incorporated | Adhesive-lined elastic condom cathether |
| US5176663A (en) | 1987-12-02 | 1993-01-05 | Pal Svedman | Dressing having pad with compressibility limiting elements |
| US4906240A (en) | 1988-02-01 | 1990-03-06 | Matrix Medica, Inc. | Adhesive-faced porous absorbent sheet and method of making same |
| US4985019A (en) | 1988-03-11 | 1991-01-15 | Michelson Gary K | X-ray marker |
| GB8812803D0 (en) | 1988-05-28 | 1988-06-29 | Smiths Industries Plc | Medico-surgical containers |
| US4919654A (en) | 1988-08-03 | 1990-04-24 | Kalt Medical Corporation | IV clamp with membrane |
| US5000741A (en) | 1988-08-22 | 1991-03-19 | Kalt Medical Corporation | Transparent tracheostomy tube dressing |
| JPH02270874A (en) | 1989-01-16 | 1990-11-05 | Roussel Uclaf | Azabicyclo compounds and their salts, their production, pharmaceutical compound containing them and their use as remedy |
| DK0460040T3 (en) * | 1989-02-25 | 1995-01-23 | Smith & Nephew | Woven or knitted elastic bandage |
| GB8906100D0 (en) | 1989-03-16 | 1989-04-26 | Smith & Nephew | Laminates |
| US5261893A (en) | 1989-04-03 | 1993-11-16 | Zamierowski David S | Fastening system and method |
| US5100396A (en) | 1989-04-03 | 1992-03-31 | Zamierowski David S | Fluidic connection system and method |
| US5527293A (en) | 1989-04-03 | 1996-06-18 | Kinetic Concepts, Inc. | Fastening system and method |
| US4969880A (en) | 1989-04-03 | 1990-11-13 | Zamierowski David S | Wound dressing and treatment method |
| US5358494A (en) | 1989-07-11 | 1994-10-25 | Svedman Paul | Irrigation dressing |
| JP2719671B2 (en) | 1989-07-11 | 1998-02-25 | 日本ゼオン株式会社 | Wound dressing |
| US5232453A (en) | 1989-07-14 | 1993-08-03 | E. R. Squibb & Sons, Inc. | Catheter holder |
| GB2235877A (en) | 1989-09-18 | 1991-03-20 | Antonio Talluri | Closed wound suction apparatus |
| US5134994A (en) | 1990-02-12 | 1992-08-04 | Say Sam L | Field aspirator in a soft pack with externally mounted container |
| US5092858A (en) | 1990-03-20 | 1992-03-03 | Becton, Dickinson And Company | Liquid gelling agent distributor device |
| US5149331A (en) | 1991-05-03 | 1992-09-22 | Ariel Ferdman | Method and device for wound closure |
| US5278100A (en) | 1991-11-08 | 1994-01-11 | Micron Technology, Inc. | Chemical vapor deposition technique for depositing titanium silicide on semiconductor wafers |
| US5645081A (en) | 1991-11-14 | 1997-07-08 | Wake Forest University | Method of treating tissue damage and apparatus for same |
| US5636643A (en) | 1991-11-14 | 1997-06-10 | Wake Forest University | Wound treatment employing reduced pressure |
| US5279550A (en) | 1991-12-19 | 1994-01-18 | Gish Biomedical, Inc. | Orthopedic autotransfusion system |
| US5167613A (en) | 1992-03-23 | 1992-12-01 | The Kendall Company | Composite vented wound dressing |
| FR2690617B1 (en) | 1992-04-29 | 1994-06-24 | Cbh Textile | TRANSPARENT ADHESIVE DRESSING. |
| US5377715A (en) * | 1992-11-09 | 1995-01-03 | Andenmatten; Roy W. | Method for eliminating hazardous materials from cargo tank wet lines |
| DE4306478A1 (en) | 1993-03-02 | 1994-09-08 | Wolfgang Dr Wagner | Drainage device, in particular pleural drainage device, and drainage method |
| US6241747B1 (en) | 1993-05-03 | 2001-06-05 | Quill Medical, Inc. | Barbed Bodily tissue connector |
| US5342376A (en) | 1993-05-03 | 1994-08-30 | Dermagraphics, Inc. | Inserting device for a barbed tissue connector |
| US5344415A (en) | 1993-06-15 | 1994-09-06 | Deroyal Industries, Inc. | Sterile system for dressing vascular access site |
| US5437651A (en) | 1993-09-01 | 1995-08-01 | Research Medical, Inc. | Medical suction apparatus |
| US5549584A (en) | 1994-02-14 | 1996-08-27 | The Kendall Company | Apparatus for removing fluid from a wound |
| US5556375A (en) | 1994-06-16 | 1996-09-17 | Hercules Incorporated | Wound dressing having a fenestrated base layer |
| US5607388A (en) | 1994-06-16 | 1997-03-04 | Hercules Incorporated | Multi-purpose wound dressing |
| US5664270A (en) | 1994-07-19 | 1997-09-09 | Kinetic Concepts, Inc. | Patient interface system |
| DK0853950T3 (en) | 1994-08-22 | 2002-11-25 | Kinetic Concepts Inc | Wound drainage canisters |
| DE29504378U1 (en) | 1995-03-15 | 1995-09-14 | MTG Medizinisch, technische Gerätebau GmbH, 66299 Friedrichsthal | Electronically controlled low-vacuum pump for chest and wound drainage |
| GB9523253D0 (en) | 1995-11-14 | 1996-01-17 | Mediscus Prod Ltd | Portable wound treatment apparatus |
| US6135116A (en) | 1997-07-28 | 2000-10-24 | Kci Licensing, Inc. | Therapeutic method for treating ulcers |
| AU755496B2 (en) | 1997-09-12 | 2002-12-12 | Kci Licensing, Inc. | Surgical drape and suction head for wound treatment |
| GB9719520D0 (en) | 1997-09-12 | 1997-11-19 | Kci Medical Ltd | Surgical drape and suction heads for wound treatment |
| US6071267A (en) | 1998-02-06 | 2000-06-06 | Kinetic Concepts, Inc. | Medical patient fluid management interface system and method |
| US6488643B1 (en) | 1998-10-08 | 2002-12-03 | Kci Licensing, Inc. | Wound healing foot wrap |
| US6287316B1 (en) | 1999-03-26 | 2001-09-11 | Ethicon, Inc. | Knitted surgical mesh |
| US7799004B2 (en) | 2001-03-05 | 2010-09-21 | Kci Licensing, Inc. | Negative pressure wound treatment apparatus and infection identification system and method |
| US6856821B2 (en) | 2000-05-26 | 2005-02-15 | Kci Licensing, Inc. | System for combined transcutaneous blood gas monitoring and vacuum assisted wound closure |
| US6991643B2 (en) | 2000-12-20 | 2006-01-31 | Usgi Medical Inc. | Multi-barbed device for retaining tissue in apposition and methods of use |
| ATE266443T1 (en) | 2000-02-24 | 2004-05-15 | Venetec Int Inc | UNIVERSAL CATHETER FASTENING SYSTEM |
| US7700819B2 (en) | 2001-02-16 | 2010-04-20 | Kci Licensing, Inc. | Biocompatible wound dressing |
| US6540705B2 (en) | 2001-02-22 | 2003-04-01 | Core Products International, Inc. | Ankle brace providing upper and lower ankle adjustment |
| US20030023196A1 (en) | 2001-07-24 | 2003-01-30 | Jim Liguori | Shrink wrap bandage |
| US7390499B2 (en) * | 2002-04-26 | 2008-06-24 | Lohmann & Rauscher Gmbh | Microbial cellulose wound dressing for treating chronic wounds |
| US6838589B2 (en) * | 2003-02-19 | 2005-01-04 | 3M Innovative Properties Company | Conformable wound dressing |
| GB0518826D0 (en) * | 2005-09-15 | 2005-10-26 | Smith & Nephew | Apparatus with actives from tissue - exudialysis |
| JP5263882B2 (en) * | 2006-02-07 | 2013-08-14 | コビディエン・リミテッド・パートナーシップ | Surgical wound dressing |
| AU2007282013B2 (en) * | 2006-08-03 | 2013-07-11 | The Board Of Trustees Of The Leland Stanford Junior University | Devices and bandages for the treatment or prevention of scars and/or keloids and methods and kits therefor |
| US8680360B2 (en) * | 2006-09-26 | 2014-03-25 | Smith & Nephew Inc. | Lattice dressing |
| WO2008064502A1 (en) * | 2006-11-30 | 2008-06-05 | Medela Holding Ag | Device for treating wounds |
| ATE519462T1 (en) * | 2006-11-30 | 2011-08-15 | Medela Holding Ag | DEVICE FOR WOUND TREATMENT |
| EP2109427B1 (en) * | 2007-02-09 | 2014-11-05 | KCI Licensing, Inc. | A breathable interface system for topical reduced pressure |
| WO2008103406A1 (en) * | 2007-02-20 | 2008-08-28 | Kci Licensing Inc. | System and method for distingushing leaks from a disengaged canister condition in a redued pressure treatment system |
| EP2203137B1 (en) | 2007-10-11 | 2016-02-24 | Spiracur, Inc. | Closed incision negative pressure wound therapy device |
-
2009
- 2009-11-02 RU RU2011114000/14A patent/RU2011114000A/en unknown
- 2009-11-02 AU AU2009311352A patent/AU2009311352B2/en not_active Ceased
- 2009-11-02 MX MX2011004820A patent/MX2011004820A/en not_active Application Discontinuation
- 2009-11-02 US US12/610,910 patent/US8460257B2/en active Active
- 2009-11-02 CN CN200980143982.7A patent/CN102202619B/en not_active Expired - Fee Related
- 2009-11-02 JP JP2011534856A patent/JP2012508037A/en active Pending
- 2009-11-02 EP EP18188896.7A patent/EP3421020B1/en active Active
- 2009-11-02 EP EP09825282.8A patent/EP2361069B1/en active Active
- 2009-11-02 BR BRPI0916062A patent/BRPI0916062A2/en not_active IP Right Cessation
- 2009-11-02 WO PCT/US2009/062981 patent/WO2010053870A1/en not_active Ceased
- 2009-11-02 CA CA2742962A patent/CA2742962C/en not_active Expired - Fee Related
- 2009-11-02 KR KR1020117012813A patent/KR101644206B1/en not_active Expired - Fee Related
- 2009-11-06 TW TW098137769A patent/TW201021862A/en unknown
-
2013
- 2013-05-14 US US13/894,217 patent/US10398603B2/en active Active
-
2014
- 2014-12-04 JP JP2014245863A patent/JP5864711B2/en not_active Expired - Fee Related
-
2019
- 2019-06-19 US US16/445,921 patent/US20190298577A1/en not_active Abandoned
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050131327A1 (en) * | 2000-11-29 | 2005-06-16 | Lockwood Jeffrey S. | Vacuum therapy and cleansing dressing for wounds |
| US20060079852A1 (en) * | 2002-12-31 | 2006-04-13 | Bubb Stephen K | Externally-applied patient interface system and method |
| CN100349551C (en) * | 2003-03-03 | 2007-11-21 | Kci特许公司 | Tissue processing system |
| WO2005105174A1 (en) * | 2004-04-27 | 2005-11-10 | Smith & Nephew, Plc | Wound cleansing apparatus with stress |
Also Published As
| Publication number | Publication date |
|---|---|
| MX2011004820A (en) | 2011-05-30 |
| JP2015097792A (en) | 2015-05-28 |
| AU2009311352A1 (en) | 2010-05-14 |
| EP2361069A1 (en) | 2011-08-31 |
| US20190298577A1 (en) | 2019-10-03 |
| EP3421020B1 (en) | 2022-05-11 |
| EP2361069A4 (en) | 2017-06-21 |
| KR20110083709A (en) | 2011-07-20 |
| TW201021862A (en) | 2010-06-16 |
| AU2009311352B2 (en) | 2014-06-26 |
| WO2010053870A1 (en) | 2010-05-14 |
| EP2361069B1 (en) | 2018-12-26 |
| CA2742962A1 (en) | 2010-05-14 |
| US8460257B2 (en) | 2013-06-11 |
| JP5864711B2 (en) | 2016-02-17 |
| RU2011114000A (en) | 2012-12-20 |
| US20130253401A1 (en) | 2013-09-26 |
| US10398603B2 (en) | 2019-09-03 |
| KR101644206B1 (en) | 2016-08-10 |
| CN102202619A (en) | 2011-09-28 |
| BRPI0916062A2 (en) | 2019-09-24 |
| JP2012508037A (en) | 2012-04-05 |
| US20100121286A1 (en) | 2010-05-13 |
| CA2742962C (en) | 2018-09-25 |
| EP3421020A1 (en) | 2019-01-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN102202619B (en) | Reduced-pressure, wound-treatment dressings and systems | |
| CN102026675B (en) | Reduced-pressure surgical wound treatment systems and methods | |
| JP2012523915A (en) | Reduced pressure treatment system and method using variable cover | |
| AU2014233596B2 (en) | Reduced-pressure, wound-treatment dressings and systems | |
| HK1158487A (en) | Reduced-pressure, wound-treatment dressings and systems | |
| HK1153957A (en) | Reduced-pressure, linear-wound treatment systems |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 1158487 Country of ref document: HK |
|
| GR01 | Patent grant | ||
| GR01 | Patent grant | ||
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: WD Ref document number: 1158487 Country of ref document: HK |
|
| TR01 | Transfer of patent right |
Effective date of registration: 20210521 Address after: American Minnesota Patentee after: 3M innovation intellectual property Co. Address before: Texas Patentee before: Kathy Chartered Ltd. |
|
| TR01 | Transfer of patent right | ||
| CF01 | Termination of patent right due to non-payment of annual fee |
Granted publication date: 20140312 Termination date: 20201102 |
|
| CF01 | Termination of patent right due to non-payment of annual fee |