CN101899011B - Dithiocarbamates compound, preparation method and application thereof - Google Patents
Dithiocarbamates compound, preparation method and application thereof Download PDFInfo
- Publication number
- CN101899011B CN101899011B CN 200910143757 CN200910143757A CN101899011B CN 101899011 B CN101899011 B CN 101899011B CN 200910143757 CN200910143757 CN 200910143757 CN 200910143757 A CN200910143757 A CN 200910143757A CN 101899011 B CN101899011 B CN 101899011B
- Authority
- CN
- China
- Prior art keywords
- chloro
- acid
- fluorobenzyloxy
- compound
- anilino
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000002360 preparation method Methods 0.000 title claims abstract description 89
- -1 Dithiocarbamates compound Chemical class 0.000 title claims abstract description 52
- 239000012990 dithiocarbamate Substances 0.000 title abstract 2
- 150000001875 compounds Chemical class 0.000 claims abstract description 83
- 150000003839 salts Chemical class 0.000 claims abstract description 17
- 239000002246 antineoplastic agent Substances 0.000 claims abstract description 11
- 229940041181 antineoplastic drug Drugs 0.000 claims abstract description 11
- 229940079593 drug Drugs 0.000 claims abstract description 8
- 239000003814 drug Substances 0.000 claims abstract description 8
- 206010006187 Breast cancer Diseases 0.000 claims abstract description 7
- 208000026310 Breast neoplasm Diseases 0.000 claims abstract description 6
- 208000005718 Stomach Neoplasms Diseases 0.000 claims abstract description 6
- 206010017758 gastric cancer Diseases 0.000 claims abstract description 6
- 201000011549 stomach cancer Diseases 0.000 claims abstract description 6
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 claims abstract description 6
- 239000005483 tyrosine kinase inhibitor Substances 0.000 claims abstract description 6
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims abstract description 5
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims abstract description 5
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims abstract description 5
- 201000002528 pancreatic cancer Diseases 0.000 claims abstract description 5
- 208000008443 pancreatic carcinoma Diseases 0.000 claims abstract description 5
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 claims abstract description 5
- 125000002490 anilino group Chemical group [H]N(*)C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 claims description 36
- QOSMNYMQXIVWKY-UHFFFAOYSA-N Propyl levulinate Chemical compound CCCOC(=O)CCC(C)=O QOSMNYMQXIVWKY-UHFFFAOYSA-N 0.000 claims description 28
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 claims description 27
- 125000004494 ethyl ester group Chemical group 0.000 claims description 24
- 239000000203 mixture Substances 0.000 claims description 15
- 150000002367 halogens Chemical class 0.000 claims description 13
- 229910052736 halogen Inorganic materials 0.000 claims description 12
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 12
- 229910052760 oxygen Inorganic materials 0.000 claims description 11
- 239000001301 oxygen Substances 0.000 claims description 11
- 125000000217 alkyl group Chemical group 0.000 claims description 9
- 229910052757 nitrogen Inorganic materials 0.000 claims description 9
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 9
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 9
- 125000000623 heterocyclic group Chemical group 0.000 claims description 8
- 239000008194 pharmaceutical composition Substances 0.000 claims description 8
- 239000003937 drug carrier Substances 0.000 claims description 7
- 229920006395 saturated elastomer Polymers 0.000 claims description 7
- 229910052717 sulfur Inorganic materials 0.000 claims description 7
- 125000004434 sulfur atom Chemical group 0.000 claims description 7
- 125000004432 carbon atom Chemical group C* 0.000 claims description 6
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 claims description 6
- 125000001424 substituent group Chemical group 0.000 claims description 6
- 239000004480 active ingredient Substances 0.000 claims description 5
- 125000003545 alkoxy group Chemical group 0.000 claims description 5
- BNSRVFGXRITOQK-UHFFFAOYSA-N 2-(1,2-dichloroethyl)-4-methyl-1,3-dioxolane Chemical compound CC1COC(C(Cl)CCl)O1 BNSRVFGXRITOQK-UHFFFAOYSA-N 0.000 claims description 4
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 claims description 4
- 229910052799 carbon Inorganic materials 0.000 claims description 4
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N dimethylmethane Natural products CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 claims description 4
- 239000002552 dosage form Substances 0.000 claims description 4
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 4
- 229910052739 hydrogen Inorganic materials 0.000 claims description 4
- 125000004430 oxygen atom Chemical group O* 0.000 claims description 4
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 4
- 239000001294 propane Substances 0.000 claims description 4
- 125000000051 benzyloxy group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])O* 0.000 claims description 3
- 238000007911 parenteral administration Methods 0.000 claims description 3
- 239000003826 tablet Substances 0.000 claims description 3
- 150000004917 tyrosine kinase inhibitor derivatives Chemical class 0.000 claims description 3
- 239000002775 capsule Substances 0.000 claims description 2
- 125000006297 carbonyl amino group Chemical group [H]N([*:2])C([*:1])=O 0.000 claims description 2
- 239000008187 granular material Substances 0.000 claims description 2
- 239000001257 hydrogen Substances 0.000 claims description 2
- 125000004435 hydrogen atom Chemical class [H]* 0.000 claims description 2
- 238000002347 injection Methods 0.000 claims description 2
- 239000007924 injection Substances 0.000 claims description 2
- 239000007788 liquid Substances 0.000 claims description 2
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 2
- 238000013268 sustained release Methods 0.000 claims description 2
- 239000012730 sustained-release form Substances 0.000 claims description 2
- 238000011200 topical administration Methods 0.000 claims description 2
- 239000011248 coating agent Substances 0.000 claims 1
- 238000000576 coating method Methods 0.000 claims 1
- 239000002702 enteric coating Substances 0.000 claims 1
- 238000009505 enteric coating Methods 0.000 claims 1
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 104
- 238000006243 chemical reaction Methods 0.000 description 67
- 238000000034 method Methods 0.000 description 50
- 239000007787 solid Substances 0.000 description 48
- 239000000243 solution Substances 0.000 description 43
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 38
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 30
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 27
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 27
- 239000002994 raw material Substances 0.000 description 25
- 239000000047 product Substances 0.000 description 23
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 21
- 238000010992 reflux Methods 0.000 description 20
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 18
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 18
- 239000003480 eluent Substances 0.000 description 17
- 238000003756 stirring Methods 0.000 description 17
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 16
- 239000003208 petroleum Substances 0.000 description 16
- QGJOPFRUJISHPQ-UHFFFAOYSA-N Carbon disulfide Chemical compound S=C=S QGJOPFRUJISHPQ-UHFFFAOYSA-N 0.000 description 15
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 15
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 14
- 238000000921 elemental analysis Methods 0.000 description 14
- 239000000741 silica gel Substances 0.000 description 14
- 229910002027 silica gel Inorganic materials 0.000 description 14
- 238000012360 testing method Methods 0.000 description 14
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 12
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 12
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 10
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 10
- 239000000706 filtrate Substances 0.000 description 10
- 239000012044 organic layer Substances 0.000 description 10
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 9
- 230000008859 change Effects 0.000 description 9
- DHYHYLGCQVVLOQ-UHFFFAOYSA-N 3-bromoaniline Chemical compound NC1=CC=CC(Br)=C1 DHYHYLGCQVVLOQ-UHFFFAOYSA-N 0.000 description 8
- AYPFEYDGZDPAPE-UHFFFAOYSA-N 3-chloro-4-[(3-fluorophenyl)methoxy]aniline Chemical compound ClC1=CC(N)=CC=C1OCC1=CC=CC(F)=C1 AYPFEYDGZDPAPE-UHFFFAOYSA-N 0.000 description 8
- 238000004252 FT/ICR mass spectrometry Methods 0.000 description 8
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 8
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 8
- 238000000926 separation method Methods 0.000 description 8
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 8
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 7
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 7
- 238000000746 purification Methods 0.000 description 7
- 239000007858 starting material Substances 0.000 description 7
- PVOAHINGSUIXLS-UHFFFAOYSA-N 1-Methylpiperazine Chemical compound CN1CCNCC1 PVOAHINGSUIXLS-UHFFFAOYSA-N 0.000 description 6
- 206010028980 Neoplasm Diseases 0.000 description 6
- 230000003197 catalytic effect Effects 0.000 description 6
- 239000012141 concentrate Substances 0.000 description 6
- 230000000694 effects Effects 0.000 description 6
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 description 6
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 description 6
- 239000012065 filter cake Substances 0.000 description 6
- 230000002401 inhibitory effect Effects 0.000 description 6
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 description 6
- 238000012216 screening Methods 0.000 description 6
- GGZQLTVZPOGLCC-UHFFFAOYSA-N 2-(2-bromoethyl)-1,3-dioxolane Chemical compound BrCCC1OCCO1 GGZQLTVZPOGLCC-UHFFFAOYSA-N 0.000 description 5
- YSEMCVGMNUUNRK-UHFFFAOYSA-N 3-chloro-4-fluoroaniline Chemical compound NC1=CC=C(F)C(Cl)=C1 YSEMCVGMNUUNRK-UHFFFAOYSA-N 0.000 description 5
- 239000005457 ice water Substances 0.000 description 5
- 239000010410 layer Substances 0.000 description 5
- 239000012265 solid product Substances 0.000 description 5
- 239000002904 solvent Substances 0.000 description 5
- UVBYXMSAEFOVCD-UHFFFAOYSA-N 4-(3-bromoanilino)quinazolin-6-ol Chemical compound C12=CC(O)=CC=C2N=CN=C1NC1=CC=CC(Br)=C1 UVBYXMSAEFOVCD-UHFFFAOYSA-N 0.000 description 4
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 4
- 108060006698 EGF receptor Proteins 0.000 description 4
- 102000001301 EGF receptor Human genes 0.000 description 4
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 4
- 239000002253 acid Substances 0.000 description 4
- 238000007796 conventional method Methods 0.000 description 4
- 238000011156 evaluation Methods 0.000 description 4
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 4
- QPJVMBTYPHYUOC-UHFFFAOYSA-N methyl benzoate Chemical compound COC(=O)C1=CC=CC=C1 QPJVMBTYPHYUOC-UHFFFAOYSA-N 0.000 description 4
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 4
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 4
- 229910000160 potassium phosphate Inorganic materials 0.000 description 4
- 235000011009 potassium phosphates Nutrition 0.000 description 4
- 239000002244 precipitate Substances 0.000 description 4
- JWVCLYRUEFBMGU-UHFFFAOYSA-N quinazoline Chemical compound N1=CN=CC2=CC=CC=C21 JWVCLYRUEFBMGU-UHFFFAOYSA-N 0.000 description 4
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical class O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 4
- RIPXNPZTBKTRRC-UHFFFAOYSA-N 3-bromo-n-[4-(3-chloro-4-fluoroanilino)quinazolin-6-yl]propanamide Chemical compound C1=C(Cl)C(F)=CC=C1NC1=NC=NC2=CC=C(NC(=O)CCBr)C=C12 RIPXNPZTBKTRRC-UHFFFAOYSA-N 0.000 description 3
- HJAGRTPTIMMQPY-UHFFFAOYSA-N 4-n-(3-chloro-4-fluorophenyl)quinazoline-4,6-diamine Chemical compound C12=CC(N)=CC=C2N=CN=C1NC1=CC=C(F)C(Cl)=C1 HJAGRTPTIMMQPY-UHFFFAOYSA-N 0.000 description 3
- MOBNCKURXDGQCB-UHFFFAOYSA-N 6-nitro-1h-quinazolin-4-one Chemical compound N1C=NC(=O)C2=CC([N+](=O)[O-])=CC=C21 MOBNCKURXDGQCB-UHFFFAOYSA-N 0.000 description 3
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 3
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 3
- 239000002136 L01XE07 - Lapatinib Substances 0.000 description 3
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical group C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 3
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 125000003277 amino group Chemical group 0.000 description 3
- 230000000259 anti-tumor effect Effects 0.000 description 3
- 125000003118 aryl group Chemical group 0.000 description 3
- KGNDCEVUMONOKF-UGPLYTSKSA-N benzyl n-[(2r)-1-[(2s,4r)-2-[[(2s)-6-amino-1-(1,3-benzoxazol-2-yl)-1,1-dihydroxyhexan-2-yl]carbamoyl]-4-[(4-methylphenyl)methoxy]pyrrolidin-1-yl]-1-oxo-4-phenylbutan-2-yl]carbamate Chemical compound C1=CC(C)=CC=C1CO[C@H]1CN(C(=O)[C@@H](CCC=2C=CC=CC=2)NC(=O)OCC=2C=CC=CC=2)[C@H](C(=O)N[C@@H](CCCCN)C(O)(O)C=2OC3=CC=CC=C3N=2)C1 KGNDCEVUMONOKF-UGPLYTSKSA-N 0.000 description 3
- 230000037396 body weight Effects 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 229940125833 compound 23 Drugs 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 229940121647 egfr inhibitor Drugs 0.000 description 3
- 239000003112 inhibitor Substances 0.000 description 3
- BCFGMOOMADDAQU-UHFFFAOYSA-N lapatinib Chemical compound O1C(CNCCS(=O)(=O)C)=CC=C1C1=CC=C(N=CN=C2NC=3C=C(Cl)C(OCC=4C=C(F)C=CC=4)=CC=3)C2=C1 BCFGMOOMADDAQU-UHFFFAOYSA-N 0.000 description 3
- 229960004891 lapatinib Drugs 0.000 description 3
- OXLCCXLHESAETR-UHFFFAOYSA-N n-(3-chloro-4-fluorophenyl)-6-nitroquinazolin-4-amine Chemical compound C12=CC([N+](=O)[O-])=CC=C2N=CN=C1NC1=CC=C(F)C(Cl)=C1 OXLCCXLHESAETR-UHFFFAOYSA-N 0.000 description 3
- 230000007935 neutral effect Effects 0.000 description 3
- NLKNQRATVPKPDG-UHFFFAOYSA-M potassium iodide Chemical compound [K+].[I-] NLKNQRATVPKPDG-UHFFFAOYSA-M 0.000 description 3
- DRYRBWIFRVMRPV-UHFFFAOYSA-N quinazolin-4-amine Chemical class C1=CC=C2C(N)=NC=NC2=C1 DRYRBWIFRVMRPV-UHFFFAOYSA-N 0.000 description 3
- 150000003246 quinazolines Chemical class 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 238000010898 silica gel chromatography Methods 0.000 description 3
- 239000011734 sodium Substances 0.000 description 3
- 229910052708 sodium Inorganic materials 0.000 description 3
- 230000002194 synthesizing effect Effects 0.000 description 3
- 238000010189 synthetic method Methods 0.000 description 3
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 2
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 description 2
- JASUZIAETLGSCB-UHFFFAOYSA-N 1-bromo-2-(2-bromoethylsulfonyl)ethane Chemical compound BrCCS(=O)(=O)CCBr JASUZIAETLGSCB-UHFFFAOYSA-N 0.000 description 2
- HYNQTSZBTIOFKH-UHFFFAOYSA-N 2-Amino-5-hydroxybenzoic acid Chemical compound NC1=CC=C(O)C=C1C(O)=O HYNQTSZBTIOFKH-UHFFFAOYSA-N 0.000 description 2
- LBKFGYZQBSGRHY-UHFFFAOYSA-N 3-hydroxy-4-methoxybenzoic acid Chemical compound COC1=CC=C(C(O)=O)C=C1O LBKFGYZQBSGRHY-UHFFFAOYSA-N 0.000 description 2
- JPZHUWGRVIXKIE-UHFFFAOYSA-N 4-(3-chloro-4-fluoroanilino)quinazolin-6-ol Chemical compound C12=CC(O)=CC=C2N=CN=C1NC1=CC=C(F)C(Cl)=C1 JPZHUWGRVIXKIE-UHFFFAOYSA-N 0.000 description 2
- QBADLRDMMIGVMZ-UHFFFAOYSA-N 4-[3-chloro-4-[(3-fluorophenyl)methoxy]anilino]quinazolin-6-ol Chemical compound C12=CC(O)=CC=C2N=CN=C1NC(C=C1Cl)=CC=C1OCC1=CC=CC(F)=C1 QBADLRDMMIGVMZ-UHFFFAOYSA-N 0.000 description 2
- VBMNWPOIPHHUSC-UHFFFAOYSA-N 4-aminoquinoline-3-carbonitrile Chemical class C1=CC=C2C(N)=C(C#N)C=NC2=C1 VBMNWPOIPHHUSC-UHFFFAOYSA-N 0.000 description 2
- XNILKVADCMYCQT-UHFFFAOYSA-N 4-chloro-6-methylquinazoline Chemical compound N1=CN=C(Cl)C2=CC(C)=CC=C21 XNILKVADCMYCQT-UHFFFAOYSA-N 0.000 description 2
- QRZFUQFQXCRJRR-UHFFFAOYSA-N 4-n-(3-chloro-4-fluorophenyl)-7-methoxyquinazoline-4,6-diamine Chemical compound C=12C=C(N)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 QRZFUQFQXCRJRR-UHFFFAOYSA-N 0.000 description 2
- UNMYHYODJHKLOC-UHFFFAOYSA-N 5-Nitroisatin Chemical compound [O-][N+](=O)C1=CC=C2NC(=O)C(=O)C2=C1 UNMYHYODJHKLOC-UHFFFAOYSA-N 0.000 description 2
- RUCHWTKMOWXHLU-UHFFFAOYSA-N 5-nitroanthranilic acid Chemical compound NC1=CC=C([N+]([O-])=O)C=C1C(O)=O RUCHWTKMOWXHLU-UHFFFAOYSA-N 0.000 description 2
- FUWFVMHHNPEPHD-UHFFFAOYSA-N 6-(bromomethyl)-4-chloroquinazoline Chemical compound C1=C(CBr)C=C2C(Cl)=NC=NC2=C1 FUWFVMHHNPEPHD-UHFFFAOYSA-N 0.000 description 2
- JUCDXPIFJIVICL-UHFFFAOYSA-N 6-methyl-1h-quinazolin-4-one Chemical compound N1C=NC(=O)C2=CC(C)=CC=C21 JUCDXPIFJIVICL-UHFFFAOYSA-N 0.000 description 2
- PMLONMIODRHERC-UHFFFAOYSA-N 7-chloro-1h-quinazolin-4-one Chemical compound N1C=NC(=O)C=2C1=CC(Cl)=CC=2 PMLONMIODRHERC-UHFFFAOYSA-N 0.000 description 2
- AJRGEVFKZSWGFX-UHFFFAOYSA-N 7-methoxy-6-nitro-1h-quinazolin-4-one Chemical compound N1=CNC(=O)C2=C1C=C(OC)C([N+]([O-])=O)=C2 AJRGEVFKZSWGFX-UHFFFAOYSA-N 0.000 description 2
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 2
- RGHNJXZEOKUKBD-SQOUGZDYSA-N D-gluconic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 description 2
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- QUSNBJAOOMFDIB-UHFFFAOYSA-N Ethylamine Chemical compound CCN QUSNBJAOOMFDIB-UHFFFAOYSA-N 0.000 description 2
- KRHYYFGTRYWZRS-UHFFFAOYSA-N Fluorane Chemical compound F KRHYYFGTRYWZRS-UHFFFAOYSA-N 0.000 description 2
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- MFESCIUQSIBMSM-UHFFFAOYSA-N I-BCP Chemical compound ClCCCBr MFESCIUQSIBMSM-UHFFFAOYSA-N 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- BAPJBEWLBFYGME-UHFFFAOYSA-N Methyl acrylate Chemical compound COC(=O)C=C BAPJBEWLBFYGME-UHFFFAOYSA-N 0.000 description 2
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 2
- PCLIMKBDDGJMGD-UHFFFAOYSA-N N-bromosuccinimide Chemical compound BrN1C(=O)CCC1=O PCLIMKBDDGJMGD-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- 230000001093 anti-cancer Effects 0.000 description 2
- 239000008346 aqueous phase Substances 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- 229960003328 benzoyl peroxide Drugs 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- XTEOJPUYZWEXFI-UHFFFAOYSA-N butyl n-[3-[4-(imidazol-1-ylmethyl)phenyl]-5-(2-methylpropyl)thiophen-2-yl]sulfonylcarbamate Chemical compound S1C(CC(C)C)=CC(C=2C=CC(CN3C=NC=C3)=CC=2)=C1S(=O)(=O)NC(=O)OCCCC XTEOJPUYZWEXFI-UHFFFAOYSA-N 0.000 description 2
- 150000004657 carbamic acid derivatives Chemical class 0.000 description 2
- 239000000460 chlorine Substances 0.000 description 2
- 229910052801 chlorine Inorganic materials 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- 239000012043 crude product Substances 0.000 description 2
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical compound C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 2
- USIUVYZYUHIAEV-UHFFFAOYSA-N diphenyl ether Chemical compound C=1C=CC=CC=1OC1=CC=CC=C1 USIUVYZYUHIAEV-UHFFFAOYSA-N 0.000 description 2
- POULHZVOKOAJMA-UHFFFAOYSA-N dodecanoic acid Chemical compound CCCCCCCCCCCC(O)=O POULHZVOKOAJMA-UHFFFAOYSA-N 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- 229910052731 fluorine Inorganic materials 0.000 description 2
- 239000011737 fluorine Substances 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- IPCSVZSSVZVIGE-UHFFFAOYSA-N hexadecanoic acid Chemical compound CCCCCCCCCCCCCCCC(O)=O IPCSVZSSVZVIGE-UHFFFAOYSA-N 0.000 description 2
- MSQACBWWAIBWIC-UHFFFAOYSA-N hydron;piperazine;chloride Chemical compound Cl.C1CNCCN1 MSQACBWWAIBWIC-UHFFFAOYSA-N 0.000 description 2
- 230000005918 in vitro anti-tumor Effects 0.000 description 2
- JXDYKVIHCLTXOP-UHFFFAOYSA-N isatin Chemical compound C1=CC=C2C(=O)C(=O)NC2=C1 JXDYKVIHCLTXOP-UHFFFAOYSA-N 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- QXOXUEFXRSIYSW-UHFFFAOYSA-N methyl 3-hydroxy-4-methoxybenzoate Chemical compound COC(=O)C1=CC=C(OC)C(O)=C1 QXOXUEFXRSIYSW-UHFFFAOYSA-N 0.000 description 2
- 229940095102 methyl benzoate Drugs 0.000 description 2
- 150000007522 mineralic acids Chemical class 0.000 description 2
- UHOVQNZJYSORNB-UHFFFAOYSA-N monobenzene Natural products C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 2
- LYHIGZLXZKJTPS-UHFFFAOYSA-N n-(3-chloro-4-fluorophenyl)-6-(3-chloropropoxy)-7-methoxyquinazolin-4-amine Chemical compound C=12C=C(OCCCCl)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 LYHIGZLXZKJTPS-UHFFFAOYSA-N 0.000 description 2
- VKEKKVCWODTGAP-UHFFFAOYSA-N n-(3-chloro-4-fluorophenyl)-7-methoxy-6-nitroquinazolin-4-amine Chemical compound C=12C=C([N+]([O-])=O)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 VKEKKVCWODTGAP-UHFFFAOYSA-N 0.000 description 2
- 125000001624 naphthyl group Chemical group 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 239000012074 organic phase Substances 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- XKJCHHZQLQNZHY-UHFFFAOYSA-N phthalimide Chemical compound C1=CC=C2C(=O)NC(=O)C2=C1 XKJCHHZQLQNZHY-UHFFFAOYSA-N 0.000 description 2
- 239000013641 positive control Substances 0.000 description 2
- XAEFZNCEHLXOMS-UHFFFAOYSA-M potassium benzoate Chemical compound [K+].[O-]C(=O)C1=CC=CC=C1 XAEFZNCEHLXOMS-UHFFFAOYSA-M 0.000 description 2
- 229910000027 potassium carbonate Inorganic materials 0.000 description 2
- 150000003248 quinolines Chemical class 0.000 description 2
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 2
- 235000017557 sodium bicarbonate Nutrition 0.000 description 2
- 238000000967 suction filtration Methods 0.000 description 2
- 238000010998 test method Methods 0.000 description 2
- JRMUNVKIHCOMHV-UHFFFAOYSA-M tetrabutylammonium bromide Chemical compound [Br-].CCCC[N+](CCCC)(CCCC)CCCC JRMUNVKIHCOMHV-UHFFFAOYSA-M 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N valeric acid Chemical compound CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 2
- AOSZTAHDEDLTLQ-AZKQZHLXSA-N (1S,2S,4R,8S,9S,11S,12R,13S,19S)-6-[(3-chlorophenyl)methyl]-12,19-difluoro-11-hydroxy-8-(2-hydroxyacetyl)-9,13-dimethyl-6-azapentacyclo[10.8.0.02,9.04,8.013,18]icosa-14,17-dien-16-one Chemical compound C([C@@H]1C[C@H]2[C@H]3[C@]([C@]4(C=CC(=O)C=C4[C@@H](F)C3)C)(F)[C@@H](O)C[C@@]2([C@@]1(C1)C(=O)CO)C)N1CC1=CC=CC(Cl)=C1 AOSZTAHDEDLTLQ-AZKQZHLXSA-N 0.000 description 1
- SZUVGFMDDVSKSI-WIFOCOSTSA-N (1s,2s,3s,5r)-1-(carboxymethyl)-3,5-bis[(4-phenoxyphenyl)methyl-propylcarbamoyl]cyclopentane-1,2-dicarboxylic acid Chemical compound O=C([C@@H]1[C@@H]([C@](CC(O)=O)([C@H](C(=O)N(CCC)CC=2C=CC(OC=3C=CC=CC=3)=CC=2)C1)C(O)=O)C(O)=O)N(CCC)CC(C=C1)=CC=C1OC1=CC=CC=C1 SZUVGFMDDVSKSI-WIFOCOSTSA-N 0.000 description 1
- GHYOCDFICYLMRF-UTIIJYGPSA-N (2S,3R)-N-[(2S)-3-(cyclopenten-1-yl)-1-[(2R)-2-methyloxiran-2-yl]-1-oxopropan-2-yl]-3-hydroxy-3-(4-methoxyphenyl)-2-[[(2S)-2-[(2-morpholin-4-ylacetyl)amino]propanoyl]amino]propanamide Chemical compound C1(=CCCC1)C[C@@H](C(=O)[C@@]1(OC1)C)NC([C@H]([C@@H](C1=CC=C(C=C1)OC)O)NC([C@H](C)NC(CN1CCOCC1)=O)=O)=O GHYOCDFICYLMRF-UTIIJYGPSA-N 0.000 description 1
- ITOFPJRDSCGOSA-KZLRUDJFSA-N (2s)-2-[[(4r)-4-[(3r,5r,8r,9s,10s,13r,14s,17r)-3-hydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]pentanoyl]amino]-3-(1h-indol-3-yl)propanoic acid Chemical compound C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H](CC[C@]13C)[C@@H]2[C@@H]3CC[C@@H]1[C@H](C)CCC(=O)N[C@H](C(O)=O)CC1=CNC2=CC=CC=C12 ITOFPJRDSCGOSA-KZLRUDJFSA-N 0.000 description 1
- WWTBZEKOSBFBEM-SPWPXUSOSA-N (2s)-2-[[2-benzyl-3-[hydroxy-[(1r)-2-phenyl-1-(phenylmethoxycarbonylamino)ethyl]phosphoryl]propanoyl]amino]-3-(1h-indol-3-yl)propanoic acid Chemical compound N([C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)O)C(=O)C(CP(O)(=O)[C@H](CC=1C=CC=CC=1)NC(=O)OCC=1C=CC=CC=1)CC1=CC=CC=C1 WWTBZEKOSBFBEM-SPWPXUSOSA-N 0.000 description 1
- QFLWZFQWSBQYPS-AWRAUJHKSA-N (3S)-3-[[(2S)-2-[[(2S)-2-[5-[(3aS,6aR)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-[1-bis(4-chlorophenoxy)phosphorylbutylamino]-4-oxobutanoic acid Chemical compound CCCC(NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)C(C)C)P(=O)(Oc1ccc(Cl)cc1)Oc1ccc(Cl)cc1 QFLWZFQWSBQYPS-AWRAUJHKSA-N 0.000 description 1
- IWZSHWBGHQBIML-ZGGLMWTQSA-N (3S,8S,10R,13S,14S,17S)-17-isoquinolin-7-yl-N,N,10,13-tetramethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-amine Chemical compound CN(C)[C@H]1CC[C@]2(C)C3CC[C@@]4(C)[C@@H](CC[C@@H]4c4ccc5ccncc5c4)[C@@H]3CC=C2C1 IWZSHWBGHQBIML-ZGGLMWTQSA-N 0.000 description 1
- LSBGXLOBLBWVEN-UHFFFAOYSA-N (4-chloroquinazolin-6-yl) acetate Chemical compound N1=CN=C(Cl)C2=CC(OC(=O)C)=CC=C21 LSBGXLOBLBWVEN-UHFFFAOYSA-N 0.000 description 1
- QHSILJZBWLFQGR-UHFFFAOYSA-N (4-oxo-1h-quinazolin-6-yl) acetate Chemical compound N1=CNC(=O)C2=CC(OC(=O)C)=CC=C21 QHSILJZBWLFQGR-UHFFFAOYSA-N 0.000 description 1
- 125000006273 (C1-C3) alkyl group Chemical group 0.000 description 1
- 125000006559 (C1-C3) alkylamino group Chemical group 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- OREHUWJLRDJJGY-UHFFFAOYSA-N 1,4-diazepane;hydrochloride Chemical compound Cl.C1CNCCNC1 OREHUWJLRDJJGY-UHFFFAOYSA-N 0.000 description 1
- ONBQEOIKXPHGMB-VBSBHUPXSA-N 1-[2-[(2s,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-4,6-dihydroxyphenyl]-3-(4-hydroxyphenyl)propan-1-one Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1OC1=CC(O)=CC(O)=C1C(=O)CCC1=CC=C(O)C=C1 ONBQEOIKXPHGMB-VBSBHUPXSA-N 0.000 description 1
- UNILWMWFPHPYOR-KXEYIPSPSA-M 1-[6-[2-[3-[3-[3-[2-[2-[3-[[2-[2-[[(2r)-1-[[2-[[(2r)-1-[3-[2-[2-[3-[[2-(2-amino-2-oxoethoxy)acetyl]amino]propoxy]ethoxy]ethoxy]propylamino]-3-hydroxy-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-3-[(2r)-2,3-di(hexadecanoyloxy)propyl]sulfanyl-1-oxopropan-2-yl Chemical compound O=C1C(SCCC(=O)NCCCOCCOCCOCCCNC(=O)COCC(=O)N[C@@H](CSC[C@@H](COC(=O)CCCCCCCCCCCCCCC)OC(=O)CCCCCCCCCCCCCCC)C(=O)NCC(=O)N[C@H](CO)C(=O)NCCCOCCOCCOCCCNC(=O)COCC(N)=O)CC(=O)N1CCNC(=O)CCCCCN\1C2=CC=C(S([O-])(=O)=O)C=C2CC/1=C/C=C/C=C/C1=[N+](CC)C2=CC=C(S([O-])(=O)=O)C=C2C1 UNILWMWFPHPYOR-KXEYIPSPSA-M 0.000 description 1
- TTXIFFYPVGWLSE-UHFFFAOYSA-N 1-[bis(4-fluorophenyl)methyl]piperazine Chemical compound C1=CC(F)=CC=C1C(C=1C=CC(F)=CC=1)N1CCNCC1 TTXIFFYPVGWLSE-UHFFFAOYSA-N 0.000 description 1
- LDMOEFOXLIZJOW-UHFFFAOYSA-N 1-dodecanesulfonic acid Chemical compound CCCCCCCCCCCCS(O)(=O)=O LDMOEFOXLIZJOW-UHFFFAOYSA-N 0.000 description 1
- LBLYYCQCTBFVLH-UHFFFAOYSA-N 2-Methylbenzenesulfonic acid Chemical compound CC1=CC=CC=C1S(O)(=O)=O LBLYYCQCTBFVLH-UHFFFAOYSA-N 0.000 description 1
- IMSODMZESSGVBE-UHFFFAOYSA-N 2-Oxazoline Chemical compound C1CN=CO1 IMSODMZESSGVBE-UHFFFAOYSA-N 0.000 description 1
- JYYLQSCZISREGY-UHFFFAOYSA-N 2-amino-4-chlorobenzoic acid Chemical compound NC1=CC(Cl)=CC=C1C(O)=O JYYLQSCZISREGY-UHFFFAOYSA-N 0.000 description 1
- NBUUUJWWOARGNW-UHFFFAOYSA-N 2-amino-5-methylbenzoic acid Chemical compound CC1=CC=C(N)C(C(O)=O)=C1 NBUUUJWWOARGNW-UHFFFAOYSA-N 0.000 description 1
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- MBXSHBIQMDKTEW-UHFFFAOYSA-N 3-bromobutanenitrile Chemical compound CC(Br)CC#N MBXSHBIQMDKTEW-UHFFFAOYSA-N 0.000 description 1
- UOQHWNPVNXSDDO-UHFFFAOYSA-N 3-bromoimidazo[1,2-a]pyridine-6-carbonitrile Chemical compound C1=CC(C#N)=CN2C(Br)=CN=C21 UOQHWNPVNXSDDO-UHFFFAOYSA-N 0.000 description 1
- IHBVNSPHKMCPST-UHFFFAOYSA-N 3-bromopropanoyl chloride Chemical compound ClC(=O)CCBr IHBVNSPHKMCPST-UHFFFAOYSA-N 0.000 description 1
- ONADZNBSLRAJFW-UHFFFAOYSA-N 3-methoxy-4-methylaniline Chemical compound COC1=CC(N)=CC=C1C ONADZNBSLRAJFW-UHFFFAOYSA-N 0.000 description 1
- RLTQWOJPSCVGSC-UHFFFAOYSA-N 4-chloro-6-(3-chloropropoxy)-7-methoxyquinazoline Chemical compound C1=NC(Cl)=C2C=C(OCCCCl)C(OC)=CC2=N1 RLTQWOJPSCVGSC-UHFFFAOYSA-N 0.000 description 1
- LZOSFEDULGODDH-UHFFFAOYSA-N 4-chloro-6-nitroquinazoline Chemical compound N1=CN=C(Cl)C2=CC([N+](=O)[O-])=CC=C21 LZOSFEDULGODDH-UHFFFAOYSA-N 0.000 description 1
- IRGQTUXDGYVGPA-UHFFFAOYSA-N 4-chloro-7-methoxy-6-methylquinoline-3-carbonitrile Chemical compound C1=C(C#N)C(Cl)=C2C=C(C)C(OC)=CC2=N1 IRGQTUXDGYVGPA-UHFFFAOYSA-N 0.000 description 1
- BAARZNXBWRRHPB-UHFFFAOYSA-N 6-(3-chloropropoxy)-7-methoxy-1h-quinazolin-4-one Chemical compound C1=NC(O)=C2C=C(OCCCCl)C(OC)=CC2=N1 BAARZNXBWRRHPB-UHFFFAOYSA-N 0.000 description 1
- QJRNXXLTDWMENM-UHFFFAOYSA-N 6-hydroxy-1h-quinazolin-4-one Chemical compound N1C=NC(=O)C2=CC(O)=CC=C21 QJRNXXLTDWMENM-UHFFFAOYSA-N 0.000 description 1
- URDYTQYZXZKBQT-UHFFFAOYSA-N 7-chloro-6-nitro-1h-quinazolin-4-one Chemical compound N1C=NC(=O)C2=C1C=C(Cl)C([N+](=O)[O-])=C2 URDYTQYZXZKBQT-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical group [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- 239000004342 Benzoyl peroxide Substances 0.000 description 1
- OMPJBNCRMGITSC-UHFFFAOYSA-N Benzoylperoxide Chemical compound C=1C=CC=CC=1C(=O)OOC(=O)C1=CC=CC=C1 OMPJBNCRMGITSC-UHFFFAOYSA-N 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- OJRUSAPKCPIVBY-KQYNXXCUSA-N C1=NC2=C(N=C(N=C2N1[C@H]3[C@@H]([C@@H]([C@H](O3)COP(=O)(CP(=O)(O)O)O)O)O)I)N Chemical compound C1=NC2=C(N=C(N=C2N1[C@H]3[C@@H]([C@@H]([C@H](O3)COP(=O)(CP(=O)(O)O)O)O)O)I)N OJRUSAPKCPIVBY-KQYNXXCUSA-N 0.000 description 1
- USLCCURGBSJKHS-UHFFFAOYSA-N CN(CC1)CCN1C(SCCC(Nc(cc1)cc2c1ncnc2Nc(cc1Cl)ccc1F)=O)=S Chemical compound CN(CC1)CCN1C(SCCC(Nc(cc1)cc2c1ncnc2Nc(cc1Cl)ccc1F)=O)=S USLCCURGBSJKHS-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 229940126657 Compound 17 Drugs 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Natural products OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 101150029707 ERBB2 gene Proteins 0.000 description 1
- 101150039808 Egfr gene Proteins 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- JIGUQPWFLRLWPJ-UHFFFAOYSA-N Ethyl acrylate Chemical compound CCOC(=O)C=C JIGUQPWFLRLWPJ-UHFFFAOYSA-N 0.000 description 1
- 229940125497 HER2 kinase inhibitor Drugs 0.000 description 1
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 1
- 239000005411 L01XE02 - Gefitinib Substances 0.000 description 1
- 239000005639 Lauric acid Substances 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- OPFJDXRVMFKJJO-ZHHKINOHSA-N N-{[3-(2-benzamido-4-methyl-1,3-thiazol-5-yl)-pyrazol-5-yl]carbonyl}-G-dR-G-dD-dD-dD-NH2 Chemical compound S1C(C=2NN=C(C=2)C(=O)NCC(=O)N[C@H](CCCN=C(N)N)C(=O)NCC(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC(O)=O)C(N)=O)=C(C)N=C1NC(=O)C1=CC=CC=C1 OPFJDXRVMFKJJO-ZHHKINOHSA-N 0.000 description 1
- 208000002454 Nasopharyngeal Carcinoma Diseases 0.000 description 1
- 206010061306 Nasopharyngeal cancer Diseases 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 1
- 235000021314 Palmitic acid Nutrition 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 101710100969 Receptor tyrosine-protein kinase erbB-3 Proteins 0.000 description 1
- 101710100963 Receptor tyrosine-protein kinase erbB-4 Proteins 0.000 description 1
- SKZKKFZAGNVIMN-UHFFFAOYSA-N Salicilamide Chemical class NC(=O)C1=CC=CC=C1O SKZKKFZAGNVIMN-UHFFFAOYSA-N 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- LNUFLCYMSVYYNW-ZPJMAFJPSA-N [(2r,3r,4s,5r,6r)-2-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[[(3s,5s,8r,9s,10s,13r,14s,17r)-10,13-dimethyl-17-[(2r)-6-methylheptan-2-yl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-yl]oxy]-4,5-disulfo Chemical compound O([C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1C[C@@H]2CC[C@H]3[C@@H]4CC[C@@H]([C@]4(CC[C@@H]3[C@@]2(C)CC1)C)[C@H](C)CCCC(C)C)[C@H]1O[C@H](COS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]1OS(O)(=O)=O LNUFLCYMSVYYNW-ZPJMAFJPSA-N 0.000 description 1
- OXFUSTPZKUKRHD-UHFFFAOYSA-N [4-(3-chloro-4-fluoroanilino)quinazolin-6-yl] acetate Chemical compound C12=CC(OC(=O)C)=CC=C2N=CN=C1NC1=CC=C(F)C(Cl)=C1 OXFUSTPZKUKRHD-UHFFFAOYSA-N 0.000 description 1
- PASIGYXSLURDEH-UHFFFAOYSA-N [4-[3-chloro-4-[(3-fluorophenyl)methoxy]anilino]quinazolin-6-yl] acetate Chemical compound C12=CC(OC(=O)C)=CC=C2N=CN=C1NC(C=C1Cl)=CC=C1OCC1=CC=CC(F)=C1 PASIGYXSLURDEH-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 125000003282 alkyl amino group Chemical group 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 125000004202 aminomethyl group Chemical group [H]N([H])C([H])([H])* 0.000 description 1
- 238000005902 aminomethylation reaction Methods 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 235000011114 ammonium hydroxide Nutrition 0.000 description 1
- 229940027991 antiseptic and disinfectant quinoline derivative Drugs 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- CHIHQLCVLOXUJW-UHFFFAOYSA-N benzoic anhydride Chemical compound C=1C=CC=CC=1C(=O)OC(=O)C1=CC=CC=C1 CHIHQLCVLOXUJW-UHFFFAOYSA-N 0.000 description 1
- 235000019400 benzoyl peroxide Nutrition 0.000 description 1
- 239000004305 biphenyl Substances 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 1
- 239000004327 boric acid Substances 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 125000001246 bromo group Chemical group Br* 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 210000004027 cell Anatomy 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 229940125904 compound 1 Drugs 0.000 description 1
- 229940125773 compound 10 Drugs 0.000 description 1
- 229940125797 compound 12 Drugs 0.000 description 1
- 229940126543 compound 14 Drugs 0.000 description 1
- 229940125758 compound 15 Drugs 0.000 description 1
- 229940126142 compound 16 Drugs 0.000 description 1
- 229940125782 compound 2 Drugs 0.000 description 1
- 229940125810 compound 20 Drugs 0.000 description 1
- 229940126086 compound 21 Drugs 0.000 description 1
- 229940126208 compound 22 Drugs 0.000 description 1
- 229940126214 compound 3 Drugs 0.000 description 1
- 229940125898 compound 5 Drugs 0.000 description 1
- GLUUGHFHXGJENI-UHFFFAOYSA-N diethylenediamine Natural products C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 238000002451 electron ionisation mass spectrometry Methods 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- KTMGNAIGXYODKQ-VOTSOKGWSA-N ethyl (e)-2-cyano-3-ethoxyprop-2-enoate Chemical compound CCO\C=C(/C#N)C(=O)OCC KTMGNAIGXYODKQ-VOTSOKGWSA-N 0.000 description 1
- MHYCRLGKOZWVEF-UHFFFAOYSA-N ethyl acetate;hydrate Chemical compound O.CCOC(C)=O MHYCRLGKOZWVEF-UHFFFAOYSA-N 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- XGALLCVXEZPNRQ-UHFFFAOYSA-N gefitinib Chemical compound C=12C=C(OCCCN3CCOCC3)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 XGALLCVXEZPNRQ-UHFFFAOYSA-N 0.000 description 1
- 229960002584 gefitinib Drugs 0.000 description 1
- 239000000174 gluconic acid Substances 0.000 description 1
- 235000012208 gluconic acid Nutrition 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 231100000086 high toxicity Toxicity 0.000 description 1
- 150000002430 hydrocarbons Chemical group 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- ZLVXBBHTMQJRSX-VMGNSXQWSA-N jdtic Chemical compound C1([C@]2(C)CCN(C[C@@H]2C)C[C@H](C(C)C)NC(=O)[C@@H]2NCC3=CC(O)=CC=C3C2)=CC=CC(O)=C1 ZLVXBBHTMQJRSX-VMGNSXQWSA-N 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 229940099563 lactobionic acid Drugs 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 231100000053 low toxicity Toxicity 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- GBMDVOWEEQVZKZ-UHFFFAOYSA-N methanol;hydrate Chemical compound O.OC GBMDVOWEEQVZKZ-UHFFFAOYSA-N 0.000 description 1
- ATTKOTWDBRBING-UHFFFAOYSA-N methyl 3-(3-chloropropoxy)-4-methoxybenzoate Chemical compound COC(=O)C1=CC=C(OC)C(OCCCCl)=C1 ATTKOTWDBRBING-UHFFFAOYSA-N 0.000 description 1
- FHZRIROWYFCPID-RZFZLAGVSA-N molport-002-523-172 Chemical compound O=C([C@@H](N1C([C@]2(C)C3=C(C4=CC=CC=C4N3)CCN2C1=O)=O)C)NCC1=C(F)C=CC=C1Cl FHZRIROWYFCPID-RZFZLAGVSA-N 0.000 description 1
- WQEPLUUGTLDZJY-UHFFFAOYSA-N n-Pentadecanoic acid Natural products CCCCCCCCCCCCCCC(O)=O WQEPLUUGTLDZJY-UHFFFAOYSA-N 0.000 description 1
- NWDJQSVYLFYKEY-UHFFFAOYSA-N n-[3-(4-benzylpiperazin-1-yl)propyl]-5-[(2-chloro-6-fluorophenyl)methyl]-1,3,4-oxadiazole-2-carboxamide Chemical compound FC1=CC=CC(Cl)=C1CC1=NN=C(C(=O)NCCCN2CCN(CC=3C=CC=CC=3)CC2)O1 NWDJQSVYLFYKEY-UHFFFAOYSA-N 0.000 description 1
- 201000011216 nasopharynx carcinoma Diseases 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- 235000021313 oleic acid Nutrition 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- PNJWIWWMYCMZRO-UHFFFAOYSA-N pent‐4‐en‐2‐one Natural products CC(=O)CC=C PNJWIWWMYCMZRO-UHFFFAOYSA-N 0.000 description 1
- 125000000612 phthaloyl group Chemical group C(C=1C(C(=O)*)=CC=CC1)(=O)* 0.000 description 1
- 150000004885 piperazines Chemical class 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000006239 protecting group Chemical group 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 125000004076 pyridyl group Chemical group 0.000 description 1
- 150000003921 pyrrolotriazines Chemical class 0.000 description 1
- 108091008598 receptor tyrosine kinases Proteins 0.000 description 1
- 102000027426 receptor tyrosine kinases Human genes 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 229930195734 saturated hydrocarbon Natural products 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 238000002626 targeted therapy Methods 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- CBXCPBUEXACCNR-UHFFFAOYSA-N tetraethylammonium Chemical compound CC[N+](CC)(CC)CC CBXCPBUEXACCNR-UHFFFAOYSA-N 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- BRNULMACUQOKMR-UHFFFAOYSA-N thiomorpholine Chemical compound C1CSCCN1 BRNULMACUQOKMR-UHFFFAOYSA-N 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 229940005605 valeric acid Drugs 0.000 description 1
- 239000011345 viscous material Substances 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Landscapes
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
技术领域 technical field
本发明涉及一种氨基二硫代甲酸酯类化合物,其制备方法和应用,本发明化合物是一类新的酪氨酸激酶抑制剂,可作为抗肿瘤药物。The invention relates to a carbamate compound, its preparation method and application. The compound of the invention is a novel tyrosine kinase inhibitor and can be used as an antitumor drug.
背景技术 Background technique
表皮生长因子受体(ErbB)家族属于I型受体酪氨酸激酶家族,该家族有四个成员:ErbB-1(EGFR),ErbB-2(HER-2),ErbB-3和ErbB-4。研究表明,在膀胱癌、乳腺癌、结肠癌、肺癌等肿瘤细胞中,EGFR和HER-2均有高表达,实体瘤与它们的高表达之间有着很强的相关性。因此,近年来以EGFR和HER-2作为靶点进行靶向抗肿瘤药物研究受到高度重视。The epidermal growth factor receptor (ErbB) family belongs to the type I receptor tyrosine kinase family, which has four members: ErbB-1 (EGFR), ErbB-2 (HER-2), ErbB-3 and ErbB-4 . Studies have shown that EGFR and HER-2 are highly expressed in bladder cancer, breast cancer, colon cancer, lung cancer and other tumor cells, and there is a strong correlation between solid tumors and their high expression. Therefore, in recent years, research on targeted anti-tumor drugs using EGFR and HER-2 as targets has been highly valued.
目前,已发现的EGFR和HER-2抑制剂的主要结构类型有以下5种,即4-氨基喹唑啉类(4-Aminoquinazolines,A),4-氨基-3-氰基喹啉(4-Aminoquinoline-3-carbonitriles,B),苯亚甲基丙二腈类(Benzylidenemalononitriles,C),水杨酰胺类(Salicylanilides,D)和吡咯并三嗪类(Pyrrolotrazines,E)。这5种结构中研究最多的是4-氨基喹唑啉类(A)和4-氨基-3-氰基喹啉(B)类,例如中国专利申请CN200610023526.7及国际申请公开WO2008/0033748和WO2008/0033749所公开的化合物。并且已发现了一些抗肿瘤活性很好的化合物,有些已进入临床。At present, the main structural types of EGFR and HER-2 inhibitors that have been discovered include the following five types, namely 4-aminoquinazolines (4-Aminoquinazolines, A), 4-amino-3-cyanoquinolines (4- Aminoquinoline-3-carbonitriles, B), benzylidenemalononitriles (C), salicylamides (Salicylanilides, D) and pyrrolotriazines (Pyrrolotrazines, E). Among these five structures, 4-aminoquinazolines (A) and 4-amino-3-cyanoquinolines (B) are the most studied, such as Chinese patent application CN200610023526.7 and international application publications WO2008/0033748 and Compounds disclosed in WO2008/0033749. And some compounds with good anti-tumor activity have been found, some of which have entered clinical practice.
氨基二硫代甲酸酯类化合物是另外一类具有抗肿瘤活性的化合物,李润涛等人在中国发明专利申请CN01118399.3和CN200410054686.9中公开了此类化合物,其中所公开的化合物990208(Hyd)和990227是活性较好的两种化合物。Carbamate compounds are another class of compounds with anti-tumor activity. Li Runtao and others disclosed such compounds in Chinese invention patent applications CN01118399.3 and CN200410054686.9, wherein the disclosed compound 990208 (Hyd) and 990227 are two compounds with better activity.
基于现有技术对氨基二硫代甲酸酯类化合物的研究结果和表皮生长因子受体抑制剂(EGFR inhibitor)的研究进展,本发明人经过大量实验,发现了一类结构全新、活性强、选择性高、毒性低的表皮生长因子受体抑制剂。Based on the research results of the prior art on carbamate compounds and the research progress of epidermal growth factor receptor inhibitors (EGFR inhibitor), the inventors have discovered a class of new structure, strong activity, and selectivity through a large number of experiments. An epidermal growth factor receptor inhibitor with high toxicity and low toxicity.
发明内容Contents of the invention
本发明的目的是提供一类有下述通式(I)结构的化合物或其药用盐:The object of the present invention is to provide a class of compounds with following general formula (I) structure or pharmaceutically acceptable salts thereof:
其中:in:
A是氮原子或-CCN;A is a nitrogen atom or -CCN;
D是-(CH2)m-、-(CH2)m-O-、-(CH2)m-NH-或-CH2CH2CONH-,其中m各自独立的是1-3的整数;D is -(CH 2 ) m -, -(CH 2 ) m -O-, -(CH 2 ) m -NH- or -CH 2 CH 2 CONH-, wherein m is independently an integer of 1-3;
E是硫原子或NR3,其中R3是H、甲基、乙基或苯基;E is a sulfur atom or NR 3 , wherein R 3 is H, methyl, ethyl or phenyl;
R是R4R5N-或R6S-,其中R4和R5各自独立地是H、烷基或芳基,或R4和R5与所连接的氮原子一起表示取代或未取代的、另外还含有1个氮原子、氧原子或硫原子的饱和杂环,其中所述的取代基是C1-4的烷基,所述的C1-4的烷基可被苯基或卤代苯基一取代或多取代;或其中的R6表示下述基团:R is R 4 R 5 N- or R 6 S-, wherein R 4 and R 5 are each independently H, alkyl or aryl, or R 4 and R 5 together with the attached nitrogen atom represent substituted or unsubstituted In addition, a saturated heterocyclic ring containing 1 nitrogen atom, oxygen atom or sulfur atom, wherein the substituent is a C 1-4 alkyl group, and the C 1-4 alkyl group can be replaced by phenyl or Halogenated phenyl monosubstituted or multi-substituted; or wherein R 6 represents the following groups:
其中R7和R8各自独立地是氢、甲基、乙基、苯基或苯甲基;q是1-3的整数;Wherein R 7 and R 8 are each independently hydrogen, methyl, ethyl, phenyl or benzyl; q is an integer of 1-3;
R1选自下述基团:卤素、苯基、苯氧基、苄基、苄氧基、吡啶基甲基或吡啶基甲氧基,其中在所述的苯基、苄基或吡啶基上可具有1-3个选自下述基团的取代基:卤素、羟基、氨基、C1-4烷基或C1-4烷氧基; R is selected from the following groups: halogen, phenyl, phenoxy, benzyl, benzyloxy, pyridylmethyl or pyridylmethoxy, wherein on the phenyl, benzyl or pyridyl Can have 1-3 substituents selected from the following groups: halogen, hydroxyl, amino, C 1-4 alkyl or C 1-4 alkoxy;
n是1-3的整数;和n is an integer from 1 to 3; and
R2是H、C1-4烷基、卤素取代的C1-4烷基、C1-4烷氧基或卤素取代的C1-4烷氧基。R 2 is H, C 1-4 alkyl, halogen substituted C 1-4 alkyl, C 1-4 alkoxy or halogen substituted C 1-4 alkoxy.
本发明所述通式(I)化合物的药用盐是指本发明化合物的酸加成盐,包括无机酸加成盐或有机酸加成盐,所述的无机酸例如是硫酸、盐酸、亚硫酸、硼酸、磷酸、磺酸、氢溴酸或氢氟酸等;所述的有机酸例如是乙酸、戊酸、乙酸、马来酸、富马酸、草酸、油酸、乳酸、棕榈酸、月桂酸、硬脂酸、柠檬酸、琥珀酸、酒石酸、苯甲酸、甲磺酸、甲苯磺酸、葡萄糖酸、乳糖酸或月桂基磺酸等;在所述的盐之中还可以还有碱金属或碱土金属阳离子,例如锂、钠、钾、钙、镁等的阳离子,以及各种有机铵阳离子,例如四甲基胺、四乙基胺、甲胺、二甲胺、乙胺等形成的铵盐阳离子。The pharmaceutically acceptable salt of the compound of general formula (I) of the present invention refers to the acid addition salt of the compound of the present invention, including inorganic acid addition salt or organic acid addition salt, and described inorganic acid is sulfuric acid, hydrochloric acid, Sulfuric acid, boric acid, phosphoric acid, sulfonic acid, hydrobromic acid or hydrofluoric acid etc.; Described organic acid is for example acetic acid, valeric acid, acetic acid, maleic acid, fumaric acid, oxalic acid, oleic acid, lactic acid, palmitic acid, Lauric acid, stearic acid, citric acid, succinic acid, tartaric acid, benzoic acid, methanesulfonic acid, toluenesulfonic acid, gluconic acid, lactobionic acid or laurylsulfonic acid, etc; Metal or alkaline earth metal cations, such as lithium, sodium, potassium, calcium, magnesium, etc., and various organic ammonium cations, such as tetramethylamine, tetraethylamine, methylamine, dimethylamine, ethylamine, etc. Ammonium salt cation.
本发明的上述化合物是表皮生长因子受体抑制剂(EGFR inhibitor),可用于治疗肿瘤,特别适用于治疗由蛋白酪氨酸激酶介导的疾病,如乳腺癌、非小细胞肺癌、胃癌、胰腺癌等。The above-mentioned compound of the present invention is an epidermal growth factor receptor inhibitor (EGFR inhibitor), which can be used to treat tumors, and is especially suitable for treating diseases mediated by protein tyrosine kinases, such as breast cancer, non-small cell lung cancer, gastric cancer, pancreatic cancer, etc. cancer etc.
优选的,本发明通式(I)化合物中的基团D是-CH2-、-(CH2)3-、-(CH2)3-O-、-(CH2)3-NH-或-CH2CH2CONH-;其中的基团E是硫原子或-NH-;更优选其中所述的-E-D-基团是:-NH-CH2-、-NH-(CH2)3-、-S-(CH2)3-O-或-S-CH2CH2CONH-。Preferably, the group D in the compound of general formula (I) of the present invention is -CH 2 -, -(CH 2 ) 3 -, -(CH 2 ) 3 -O-, -(CH 2 ) 3 -NH- or -CH 2 CH 2 CONH-; wherein the group E is a sulfur atom or -NH-; more preferably, the -ED- group is: -NH-CH 2 -, -NH-(CH 2 ) 3 - , -S-(CH 2 ) 3 -O- or -S-CH 2 CH 2 CONH-.
当通式中的R基团为R4R5N-,且其中的R4和R5与所连接的氮原子一起表示取代或未取代的、另外还含有1个氮原子、氧原子或硫原子的饱和杂环时,其中所述的饱和杂环为饱和六元或七元杂环。优选的,其中所述的饱和六元或七元杂环为:When the R group in the general formula is R 4 R 5 N-, and R 4 and R 5 represent substituted or unsubstituted together with the connected nitrogen atom, and also contain 1 nitrogen atom, oxygen atom or sulfur atom, wherein the saturated heterocycle is a saturated six-membered or seven-membered heterocycle. Preferably, the saturated six-membered or seven-membered heterocycle is:
更优选的饱和杂环为取代或未取代的哌嗪或吗啉环。当所述的饱和六元或七元杂环具有取代基时,优选所述的取代基为甲基、卤代苯基甲基或二(氟代苯基)甲基。最优选4-甲基哌嗪基。More preferred saturated heterocycles are substituted or unsubstituted piperazine or morpholine rings. When the saturated six-membered or seven-membered heterocycle has a substituent, preferably the substituent is methyl, halophenylmethyl or bis(fluorophenyl)methyl. Most preferred is 4-methylpiperazinyl.
当通式中所述的R是R6S-时,其中的R6优选表示下述基团:When R described in the general formula is R 6 S-, R 6 therein preferably represents the following groups:
优选的R1基团选自:卤素、苄氧基或具有1-3个卤素取代基的苄氧基,更优选连接于喹啉环或喹唑啉环4-位氨基上的基团是:Preferred R group is selected from: halogen, benzyloxy or benzyloxy with 1-3 halogen substituents, more preferably the group connected to quinoline ring or quinazoline ring 4-position amino is:
优选的R2基团是H、C1-4烷氧基或卤素取代的C1-4烷氧基;更优选H或甲氧基。Preferred R2 groups are H, C1-4 alkoxy or halogen substituted C1-4 alkoxy; more preferably H or methoxy.
在本发明化合物的上述定义中,其中所用的术语“烷基”是指饱和的烃基,优选具有1-6个碳原子的直链或支链的烷基,更优选具有1-3个碳原子的直链或支链的烷基,例如甲基、乙基、丙基、异丙基等。In the above definition of the compounds of the present invention, the term "alkyl" used therein refers to a saturated hydrocarbon group, preferably a linear or branched chain alkyl group having 1-6 carbon atoms, more preferably having 1-3 carbon atoms straight-chain or branched-chain alkyl groups, such as methyl, ethyl, propyl, isopropyl, etc.
本发明所用的术语“芳基”是指苯基、萘基等具有6-10个碳原子的芳香族基团,优选苯基或萘基。The term "aryl" used in the present invention refers to phenyl, naphthyl and other aromatic groups with 6-10 carbon atoms, preferably phenyl or naphthyl.
本发明所使用的术语“卤素”是指氟、氯、溴和碘原子,优选为氟、氯或溴原子。The term "halogen" used in the present invention refers to fluorine, chlorine, bromine and iodine atoms, preferably fluorine, chlorine or bromine atoms.
本发明化合物定义所用的术语“取代或未取代的”中,其中所述的“取代”是指在可取代的任意位置上被所述取代基单取代或多取代的基团。In the term "substituted or unsubstituted" used in the definition of the compounds of the present invention, the "substituted" refers to a group that is mono-substituted or multi-substituted by the substituent at any substitutable position.
本发明优选的化合物是:Preferred compounds of the present invention are:
4-甲基哌嗪-1-二硫代甲酸-[3-[4-(3-溴苯氨基)喹唑啉-6-氧]]丙酯(化合物1);4-methylpiperazine-1-dithiocarboxylic acid-[3-[4-(3-bromoanilino)quinazoline-6-oxygen]]propyl ester (compound 1);
4-甲基哌嗪-1-二硫代甲酸-[3-[4-(3-氯-4-氟苯氨基)喹唑啉-6-氧]]丙酯(化合物2);4-methylpiperazine-1-dithiocarboxylic acid-[3-[4-(3-chloro-4-fluoroanilino)quinazoline-6-oxygen]]propyl ester (compound 2);
4-甲基哌嗪-1-二硫代甲酸-[3-[4-[3-氯-4-(3-氟苄氧基)苯氨基]喹唑啉-6-氧]丙酯(化合物3);4-Methylpiperazine-1-dithiocarboxylic acid-[3-[4-[3-chloro-4-(3-fluorobenzyloxy)anilino]quinazoline-6-oxo]propyl ester (compound 3);
4-甲基哌嗪-1-二硫代甲酸-{3-[4-(3-溴苯氨基)-7-甲氧基喹唑啉-6-氧]}丙酯(化合物4);4-methylpiperazine-1-dithiocarboxylic acid-{3-[4-(3-bromoanilino)-7-methoxyquinazoline-6-oxygen]}propyl ester (compound 4);
4-甲基哌嗪-1-二硫代甲酸-{3-[4-(3-氯-4-氟苯氨基)-7-甲氧基喹唑啉-6-氧]}丙酯(化合物5);4-methylpiperazine-1-dithiocarboxylic acid-{3-[4-(3-chloro-4-fluoroanilino)-7-methoxyquinazoline-6-oxygen]}propyl ester (compound 5);
4-甲基哌嗪-1-二硫代甲酸-[3-[4-[3-氯-4-(3-氟苄氧基)苯氨基]-7-甲氧基喹唑啉-6-氧]]丙酯(化合物6);4-methylpiperazine-1-dithiocarboxylic acid-[3-[4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-7-methoxyquinazoline-6- Oxy]]propyl ester (compound 6);
哌嗪-4-二硫代甲酸-[3-[4-[3-氯-4-(3-氟苄氧基)苯氨基]-7-甲氧基喹唑啉-6-氧]]丙酯(化合物7);Piperazine-4-dithiocarboxylic acid-[3-[4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-7-methoxyquinazoline-6-oxygen]]propane Esters (Compound 7);
高哌嗪-4-二硫代甲酸-[3-[4-[3-氯-4-(3-氟苄氧基)苯氨基]-7-甲氧基喹唑啉-6-氧]]丙酯(化合物8);Homopiperazine-4-dithiocarboxylic acid-[3-[4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-7-methoxyquinazoline-6-oxo]] Propyl ester (compound 8);
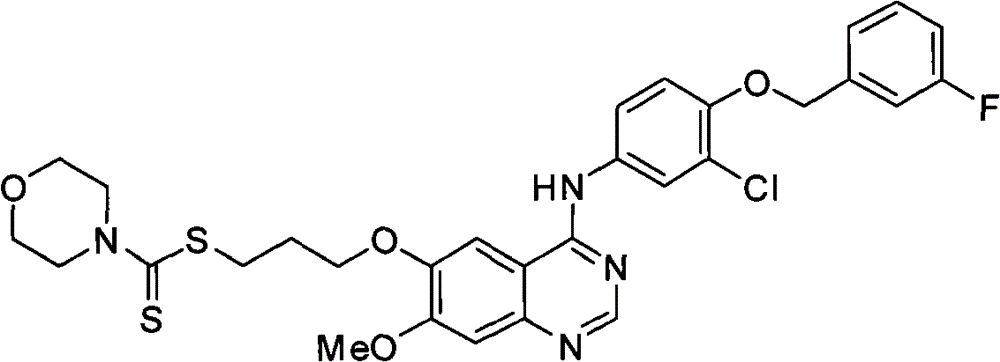
吗啉-4-二硫代甲酸-[3-[4-[3-氯-4-(3-氟苄氧基)苯氨基]-7-甲氧基喹唑啉-6-氧]]丙酯(化合物9);Morpholine-4-dithiocarboxylic acid-[3-[4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-7-methoxyquinazoline-6-oxygen]]propane Esters (Compound 9);
硫代吗啉-4-二硫代甲酸-[3-[4-[3-氯-4-(3-氟苄氧基)苯氨基]-7-甲氧基喹唑啉-6-氧]]丙酯(化合物10);Thiomorpholine-4-dithioformic acid-[3-[4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-7-methoxyquinazoline-6-oxo] ] Propyl ester (compound 10);
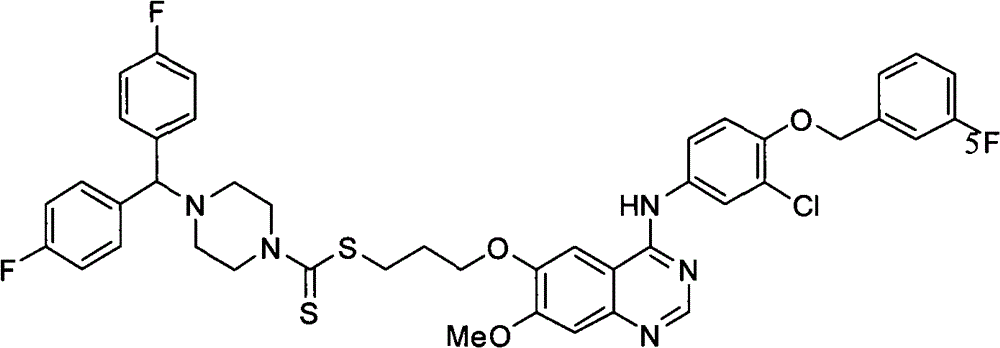
4-[二(4-氟苯基)甲基]哌嗪-1-二硫代甲酸-[3-[4-[3-氯-4-(3-氟苄氧基)苯氨基]-7-甲氧基喹唑啉-6-氧]]丙酯(化合物11);4-[bis(4-fluorophenyl)methyl]piperazine-1-dithiocarboxylic acid-[3-[4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-7 -Methoxyquinazoline-6-oxo]]propyl ester (compound 11);
4-甲基哌嗪-1-二硫代甲酸-[2-[4-(3-氯-4-氟苯氨基)喹唑啉-6-氨甲酰基]]乙酯(化合物12);4-methylpiperazine-1-dithiocarboxylic acid-[2-[4-(3-chloro-4-fluoroanilino)quinazoline-6-carbamoyl]]ethyl ester (compound 12);
4-甲基哌嗪-1-二硫代甲酸-[2-[4-(3-氯-4-氟苯氨基)-7-甲氧基喹唑啉-6-氨甲酰]]乙酯(化合物13);4-Methylpiperazine-1-dithioformic acid-[2-[4-(3-chloro-4-fluoroanilino)-7-methoxyquinazoline-6-carbamoyl]]ethyl ester (compound 13);
{4-[3-氯-4-(3-氟苄氧基)苯氨基]喹唑啉基}-6-甲氨基二硫代甲酸-2-[2-(1,3-二氧环戊基)]乙酯(化合物14);{4-[3-Chloro-4-(3-fluorobenzyloxy)anilino]quinazolinyl}-6-methylaminodithiocarbamate-2-[2-(1,3-dioxolane base)] ethyl ester (compound 14);
[4-(3-氯-4-氟苯氨基)喹唑啉基]-6-甲氨基二硫代甲酸-2-[2-(1,3-二氧环戊基)]乙酯(化合物15);[4-(3-Chloro-4-fluoroanilino)quinazolinyl]-6-methylaminodithiocarbamate-2-[2-(1,3-dioxolane)]ethyl ester (compound 15);
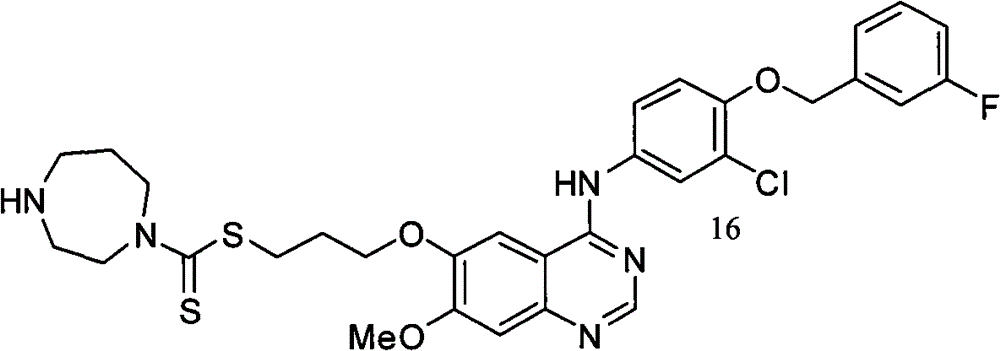
[4-(3-溴苯氨基)喹唑啉基]-6-甲氨基二硫代甲酸-2-[2-(1,3-二氧环戊基)]乙酯(化合物16);[4-(3-bromoanilino)quinazolinyl]-2-[2-(1,3-dioxolopentyl)]ethyl-6-methylaminodithiocarboxylate (compound 16);
{4-[3-氯-4-(3-氟苄氧基)苯氨基]喹唑啉基}-6-甲氨基二硫代甲酸-2-氰基乙酯(化合物17);{4-[3-Chloro-4-(3-fluorobenzyloxy)anilino]quinazolinyl}-6-methylaminodithiocarbamate-2-cyanoethyl ester (compound 17);
{4-[3-氯-4-(3-氟苄氧基)苯氨基]喹唑啉基}-6-甲氨基二硫代甲酸-2-甲氧甲酰基乙酯(化合物18);{4-[3-Chloro-4-(3-fluorobenzyloxy)anilino]quinazolinyl}-6-methylaminodithiocarbamate-2-methoxyformyl ethyl ester (compound 18);
{4-[3-氯-4-(3-氟苄氧基)苯氨基]喹唑啉基}-6-甲氨基二硫代甲酸-2-苄砜基乙酯(化合物19);{4-[3-Chloro-4-(3-fluorobenzyloxy)anilino]quinazolinyl}-2-benzylsulfone ethyl 6-methylaminodithiocarbamate (compound 19);
{4-[3-氯-4-(3-氟苄氧基)苯氨基]-3-氰基-7-甲氧基喹啉基}-6-甲氨基二硫代甲酸-2-[2-(1,3-二氧环戊基)]乙酯(化合物20){4-[3-Chloro-4-(3-fluorobenzyloxy)anilino]-3-cyano-7-methoxyquinolinyl}-6-methylaminodithioformic acid-2-[2 -(1,3-Dioxolane)] ethyl ester (compound 20)
[4-(3-氯-4-氟苯氨基)-3-氰基-7-甲氧基喹啉基]-6-甲氨基二硫代甲酸-2-[2-(1,3-二氧环戊基)]乙酯(化合物21);[4-(3-Chloro-4-fluoroanilino)-3-cyano-7-methoxyquinolinyl]-6-methylaminodithioformic acid-2-[2-(1,3-di Oxycyclopentyl)] ethyl ester (compound 21);
[4-(3-溴苯氨基)-3-氰基-7-甲氧基喹啉基]-6-甲氨基二硫代甲酸-2-[2-(1,3-二氧环戊基)]乙酯(化合物22);和[4-(3-Bromoanilino)-3-cyano-7-methoxyquinolinyl]-6-methylaminodithioformic acid-2-[2-(1,3-dioxolyl )] ethyl ester (compound 22); and
[4-(3-溴苯氨基)喹唑啉基]-6-甲氨基二硫代甲酸-2-苄砜基乙酯(化合物23)。[4-(3-Bromoanilino)quinazolinyl]-2-benzylsulfonylethyl ester of 6-methylaminodithiocarbamate (Compound 23).
本发明的另一目的是提供了上述通式(I)化合物或其药用盐的制备方法,该方法包括首先按照常规的合成方法合成喹啉环或喹唑啉环,然后按照常规方法以任意的顺序先后在其4-位上引入所需的氨基取代基和在其6-位上引入所需基团。Another object of the present invention is to provide the preparation method of above-mentioned general formula (I) compound or pharmaceutically acceptable salt thereof, this method comprises at first synthesizing quinoline ring or quinazoline ring according to conventional synthetic method, then according to conventional method with arbitrary The sequence of introducing the desired amino substituent at its 4-position followed by the desired group at its 6-position.
例如,当通式(I)化合物为喹唑啉衍生物、连接于6-位的是氧原子时,合成喹唑啉环和在其4-位上引入所需氨基取代基的方法可参照WO2008/0033749所公开的反应路线1、2、6或15进行;按照以上各种合成方法可得到喹唑啉环6-位是羟基或被保护羟基的中间体化合物:For example, when the compound of general formula (I) is a quinazoline derivative and is connected to an oxygen atom at the 6-position, the method for synthesizing the quinazoline ring and introducing the desired amino substituent at its 4-position can refer to WO2008 The reaction scheme 1, 2, 6 or 15 disclosed in /0033749 is carried out; according to the above various synthetic methods, the intermediate compound in which the 6-position of the quinazoline ring is a hydroxyl or a protected hydroxyl can be obtained:
然后,使其脱保护,将喹唑啉环6-位是羟基的中间体化合物与通式(III)化合物:X(CH2)mBr进行反应,得到通式(IV)化合物:Then, it is deprotected, and the 6-position of the quinazoline ring is a hydroxyl intermediate compound and the compound of general formula (III): X(CH 2 ) m Br is reacted to obtain the compound of general formula (IV):
其中所述的m如上文定义,X为卤素;wherein m is as defined above, and X is a halogen;
将上述化合物(IV)与二硫化碳和R4R5NH在常规的反应条件下进行反应,即可得到R基团为R4R5N-的目的化合物。The target compound whose R group is R 4 R 5 N- can be obtained by reacting the above compound (IV) with carbon disulfide and R 4 R 5 NH under conventional reaction conditions.
当通式(I)化合物为喹唑啉衍生物、连接于6-位的是氮原子时,合成喹唑啉环和在其4-位上引入所需氨基取代基的方法可参照WO2008/0033749所公开的反应路线3进行;按照以上各种合成方法可得到喹唑啉环的6-位是氨基的中间体化合物,然后,使喹唑啉环6-位是氨基的中间体化合物与R6X和CS2在常规的反应条件下进行反应,即可得到R基团是R6S-的目的化合物。When the compound of general formula (I) is a quinazoline derivative, when the nitrogen atom is connected to the 6-position, the method for synthesizing the quinazoline ring and introducing the desired amino substituent at its 4-position can refer to WO2008/0033749 The disclosed reaction scheme 3 is carried out; According to the above various synthetic methods, the 6-position of the quinazoline ring is the intermediate compound of the amino group, and then the 6-position of the quinazoline ring is the intermediate compound of the amino group and R 6 X and CS 2 are reacted under conventional reaction conditions to obtain the target compound whose R group is R 6 S-.
当通式(I)化合物为喹唑啉衍生物,且连接于喹唑啉环6-位的是C1-3烷基氨基氨基二硫代甲酸酯时,所述目的化合物的制备方法包括首先制备喹唑啉环6-位的烷基氨基被保护的中间体,其中所述的保护基团以邻苯二甲酰基为例:When the compound of general formula (I) is a quinazoline derivative, and what is connected to the 6-position of the quinazoline ring is C 1-3 alkylaminodithiocarbamate, the preparation method of the object compound comprises First prepare the protected intermediate of the alkylamino group at the 6-position of the quinazoline ring, wherein the protecting group is phthaloyl as an example:
然后使所得到的中间体化合物脱保护,并将该中间体化合物与R6X和CS2在常规的反应条件下进行反应,即可得到喹唑啉-6-C1-3烷基氨基二硫代甲酸酯类的目的化合物。Then the obtained intermediate compound is deprotected, and the intermediate compound is reacted with R 6 X and CS 2 under conventional reaction conditions to obtain quinazoline-6-C 1-3 alkylamino di Thioformic esters of interest.
当所述的通式(I)化合物为喹啉衍生物,即连接于喹啉环6-位的是C1-3烷基氨基的氨基二硫代甲酸酯化合物时,可以相应的喹啉衍生物为原料,按照与上述反应流程类似的方法制备相应的目的化合物。When the compound of general formula (I) is a quinoline derivative, that is, when the 6-position of the quinoline ring is connected to a carbamate compound of C 1-3 alkylamino, the corresponding quinoline Derivatives are used as raw materials, and the corresponding target compounds are prepared in a manner similar to the above reaction scheme.
当其中喹啉环6-位氨基被保护的中间体是氨甲基中间体时,另一种得到喹啉环6-位是氨基被保护的氨甲基化中间体的方法例如可以是:When the intermediate in which the 6-position amino of the quinoline ring is protected is an aminomethyl intermediate, another method for obtaining the aminomethylation intermediate in which the amino group is protected at the 6-position of the quinoline ring can be, for example:
然后,使上述中间体化合物脱保护,与R6X和CS2在常规的反应条件下进行反应,即可得到喹啉-6-甲氨基二硫代甲酸酯类目的化合物。Then, the above-mentioned intermediate compound is deprotected and reacted with R 6 X and CS 2 under conventional reaction conditions to obtain quinoline-6-methylaminodithiocarbamate compounds.
在该反应流程中,起始原料化合物原料中相应于上述中间体化合物6-位的位置是甲基,可以理解的是,根据目的化合物所要求的碳原子数目,所述的甲基可以是C1-3的烷基基团。In this reaction process, the position corresponding to the 6-position of the above-mentioned intermediate compound in the starting material compound raw material is a methyl group, it can be understood that, according to the number of carbon atoms required by the target compound, the methyl group can be C 1-3 alkyl groups.
本发明通式(I)化合物的药用盐的制备可在制备该化合物的过程中完成,也可以在制备得到该化合物后,按照制备加成盐的常规方法,使该化合物与相应的酸进行反应获得。The preparation of the pharmaceutically acceptable salt of the compound of general formula (I) of the present invention can be completed in the process of preparing the compound, or after the compound is prepared, the compound is reacted with the corresponding acid according to the conventional method for preparing an addition salt. The response is obtained.
上述方法中所用的各种原料均可在市场上买到,或采用已知方法制备。The various raw materials used in the above methods are available in the market or prepared by known methods.
本发明的另一目的是提供了一种药物组合物,该药物组合物是一种酪氨酸激酶抑制剂,可用于治疗由蛋白酪氨酸激酶介导的疾病,例如可用于治疗肿瘤,特别适用于治疗乳腺癌、非小细胞肺癌、胃癌、胰腺癌等。该组合物以本发明的通式(I)化合物或其药用盐为活性成分,其中还可以任意地含有药用载体。Another object of the present invention is to provide a pharmaceutical composition, which is a tyrosine kinase inhibitor, which can be used to treat diseases mediated by protein tyrosine kinases, for example, to treat tumors, especially It is suitable for the treatment of breast cancer, non-small cell lung cancer, gastric cancer, pancreatic cancer, etc. The composition uses the compound of the general formula (I) of the present invention or a pharmaceutically acceptable salt thereof as an active ingredient, and may optionally contain a pharmaceutically acceptable carrier.
具体的,该组合物含有治疗有效量的本发明通式(I)化合物或其药用盐,以及一种或多种药学上可接受的载体。优选的,本发明组合物中活性成分的含量是0.5%-99%,药用载体的含量是1%-99.5%。Specifically, the composition contains a therapeutically effective amount of the compound of general formula (I) of the present invention or a pharmaceutically acceptable salt thereof, and one or more pharmaceutically acceptable carriers. Preferably, the content of the active ingredient in the composition of the present invention is 0.5%-99%, and the content of the pharmaceutical carrier is 1%-99.5%.
本发明的组合物可以制成各种常规的药用剂型,例如制成口服、肠胃外给药的形式,所述肠胃外给药的形式例如是各种注射给药、局部给药、吸入给药、直肠给药或植入给药的形式。The composition of the present invention can be made into various conventional pharmaceutical dosage forms, such as oral and parenteral administration forms, such as various injection administration, topical administration, inhalation administration, etc. drug, rectal or implanted form.
适于药用的口服给药制剂例如是片剂、胶囊、颗粒剂或其它适于药用的液体形式的制剂如溶液、乳液、悬浮剂等。优选的口服制剂是片剂,并且所述片剂可以制成包衣、肠溶、缓释或定量释放的形式。Pharmaceutically suitable oral administration preparations are, for example, tablets, capsules, granules, or other pharmaceutically suitable liquid forms such as solutions, emulsions, suspensions and the like. The preferred oral formulation is a tablet, and the tablet can be made into a coated, enteric-coated, sustained-release or quantitative-release form.
为了制备适用的剂型,可根据需要在活性成分中添加一种或多种药用载体,所述的药用载体包括各种常规的药用辅剂,例如赋形剂、填充剂、稀释剂、崩解剂、表面活性剂、湿润剂、防腐剂、甜味剂、色素等。In order to prepare a suitable dosage form, one or more pharmaceutical carriers can be added to the active ingredient as required, and the pharmaceutical carrier includes various conventional pharmaceutical adjuvants, such as excipients, fillers, diluents, Disintegrants, surfactants, wetting agents, preservatives, sweeteners, coloring agents, etc.
根据疾病的类型、严重的程度以及患者的状况,例如性别、年龄、体重等选用合适的剂型和施用剂量,通常成人施用剂量在1-200mg/kg体重/天,优选为1-50mg/kg体重/天之间。According to the type of disease, the degree of severity and the condition of the patient, such as gender, age, body weight, etc., the appropriate dosage form and dosage are selected. Usually, the dosage for adults is 1-200 mg/kg body weight/day, preferably 1-50 mg/kg body weight / between days.
本发明的药用组合物和该组合物的各种制剂可按照制药领域已知的常规方法制备。The pharmaceutical composition of the present invention and various formulations of the composition can be prepared according to conventional methods known in the field of pharmacy.
本发明的另一目的是提供了通式(I)化合物或其可接受盐的药用用途,本发明公开了上述通式(I)化合物或其可接受的盐,以及含有上述化合物的药物组合物在制备酪氨酸激酶抑制剂中的应用,特别是在制备抗肿瘤药物中的应用。所述的抗肿瘤药物特别适用于治疗乳腺癌、非小细胞肺癌、胃癌、胰腺癌等肿瘤。Another object of the present invention is to provide the pharmaceutical use of the compound of general formula (I) or its acceptable salt, and the present invention discloses the compound of general formula (I) or its acceptable salt, and the pharmaceutical combination containing the above compound The application of the compound in the preparation of tyrosine kinase inhibitors, especially the application in the preparation of antitumor drugs. The antitumor drug is especially suitable for treating breast cancer, non-small cell lung cancer, gastric cancer, pancreatic cancer and other tumors.
通过对本发明化合物的活性进行筛选,包括进行蛋白酪氨酸激酶抑制活性筛选试验和体外抗肿瘤活性测试的结果可以看出,本发明的通式(I)的化合物或其药用盐具有优异的蛋白酪酸激酶抑制活性和抗癌活性,有望开发成为一类新结构类型的抗肿瘤新药。By screening the activity of the compounds of the present invention, including the results of protein tyrosine kinase inhibitory activity screening tests and in vitro antitumor activity tests, it can be seen that the compounds of the general formula (I) of the present invention or their pharmaceutically acceptable salts have excellent The protein tyrosine kinase inhibitory activity and anticancer activity are expected to be developed into a new type of antitumor drug with a new structure.
本发明的另一目的是提供了一种治疗肿瘤的方法,该方法包括将治疗有效量的通式(I)化合物或其药用盐施用于需要这种治疗的患者。Another object of the present invention is to provide a method for treating tumors, which comprises administering a therapeutically effective amount of a compound of general formula (I) or a pharmaceutically acceptable salt thereof to a patient in need of such treatment.
具体实施方式 Detailed ways
下面通过具体的实施方式对本发明的技术方案作进一步的说明,其中例举的实施例是对本发明的说明,而不以任何方式限制其保护范围。The technical solutions of the present invention will be further described through specific implementation methods below, wherein the illustrated embodiments are descriptions of the present invention, and do not limit its protection scope in any way.
制备实施例1 6-羟基-3H-喹唑啉-4-酮Preparation Example 1 6-hydroxyl-3H-quinazolin-4-ketone
将2-氨基-5-羟基苯甲酸(1.53g,10mmol)加入10mL甲酰胺中,150℃下反应0.5h,反应液冷却至室温,向反应液中加入30mL水,析出大量固体,抽滤,烘干,得1.44g棕色固体产物,收率为88%,m.p.>300℃。1H NMR(300MHz,DMSO-d6):δ=7.25(s,1H),7.40(s,1H),7.52(s,1H),7.90(s,1H),10.07(s,1H),12.03(s,1H)。Add 2-amino-5-hydroxybenzoic acid (1.53g, 10mmol) into 10mL of formamide, react at 150°C for 0.5h, cool the reaction solution to room temperature, add 30mL of water to the reaction solution, a large amount of solid is precipitated, and suction filtered. After drying, 1.44 g of brown solid product was obtained, the yield was 88%, and mp>300°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=7.25(s, 1H), 7.40(s, 1H), 7.52(s, 1H), 7.90(s, 1H), 10.07(s, 1H), 12.03 (s, 1H).
制备实施例2 6-乙酰氧基-3H-喹唑啉-4-酮Preparation Example 2 6-Acetoxyl group-3H-quinazolin-4-ketone
将6-羟基-3H-喹唑啉-4-酮(0.81g,5mmol)加入8mL乙酸酐中,加入1mL吡啶,回流2h,反应液冷却至室温,向反应液中加入30mL冰水,析出大量固体,抽滤,烘干,得0.66g灰白色固体产物,收率64%,m.p.>300℃。1H NMR(300MHz,DMSO-d6):δ=2.32(s,3H),7.60(dd,J=2.7,8.7Hz,1H),7.73(d,J=2.7Hz,1H),7.83(d,J=2.4Hz,1H),8.11(s,1H),12.35(s,1H)。Add 6-hydroxy-3H-quinazolin-4-one (0.81g, 5mmol) to 8mL of acetic anhydride, add 1mL of pyridine, reflux for 2h, cool the reaction solution to room temperature, add 30mL of ice water to the reaction solution, and a large amount of The solid was suction filtered and dried to obtain 0.66 g of off-white solid product, the yield was 64%, and mp>300°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.32(s, 3H), 7.60(dd, J=2.7, 8.7Hz, 1H), 7.73(d, J=2.7Hz, 1H), 7.83(d , J=2.4Hz, 1H), 8.11(s, 1H), 12.35(s, 1H).
制备实施例3 4-(3-溴苯氨基)-6-乙酰氧基喹唑啉Preparation Example 3 4-(3-bromoanilino)-6-acetoxyquinazoline
将6-乙酰氧基-3H-喹唑啉-4-酮(1.03g,5mmol)加入到10mL二氯亚砜中,滴入N,N-二甲基甲酰胺(DMF)(0.5mL),回流反应6h,减压蒸除过量的二氯亚砜,得中间体4-氯-6-乙酰氧基喹唑啉,不经纯化,直接溶于15mL异丙醇中,加入间溴苯胺(0.86g,5mmol),1mL三乙胺,回流反应4h,反应液冷却至室温,抽滤,滤饼依次用异丙醇、水、乙醚洗涤,烘干,得1.24g淡黄色固体产物,收率69%,m.p.238-239℃。1H NMR(300MHz,DMSO-d6):δ=2.40(s,3H),7.44-7.54(m,2H),7.78(d,J=7.5Hz,1H),7.93-8.09(m,3H),8.67(s,1H),8.99(s,1H),11.39(s,1H)。Add 6-acetoxy-3H-quinazolin-4-one (1.03g, 5mmol) into 10mL of thionyl chloride, drop into N,N-dimethylformamide (DMF) (0.5mL), Refluxing for 6 hours, distilling off excess thionyl chloride under reduced pressure to obtain the intermediate 4-chloro-6-acetoxyquinazoline, which was directly dissolved in 15 mL of isopropanol without purification, and m-bromoaniline (0.86 g, 5mmol), 1mL triethylamine, reflux reaction for 4h, the reaction solution was cooled to room temperature, suction filtered, the filter cake was washed with isopropanol, water, ether, and dried to obtain 1.24g light yellow solid product, yield 69 %, mp238-239°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.40(s, 3H), 7.44-7.54(m, 2H), 7.78(d, J=7.5Hz, 1H), 7.93-8.09(m, 3H) , 8.67(s, 1H), 8.99(s, 1H), 11.39(s, 1H).
制备实施例4 4-(3-氯-4-氟苯氨基)-6-乙酰氧基喹唑啉Preparation Example 4 4-(3-chloro-4-fluoroanilino)-6-acetoxyquinazoline
按照制备实施例3所述的方法,只是将间溴苯胺改为3-氯-4-氟苯胺,收率74%,m.p.>300℃。According to the method described in Preparation Example 3, except that m-bromoaniline was changed to 3-chloro-4-fluoroaniline, the yield was 74%, m.p.>300°C.
制备实施例5 4-[3-氯-4-(3-氟苄氧基)苯氨基]-6-乙酰氧基喹唑啉Preparation Example 5 4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-6-acetoxyquinazoline
按制备实施例3所述的方法,只是将间溴苯胺改为3-氯-4-(3-氟苄氧基)苯胺,收率73%,m.p.290-292℃。According to the method described in Preparation Example 3, except that m-bromoaniline was changed to 3-chloro-4-(3-fluorobenzyloxy)aniline, the yield was 73%, m.p.290-292°C.
制备实施例6 4-(3-溴苯氨基)-6-羟基喹唑啉Preparation Example 6 4-(3-bromoanilino)-6-hydroxyquinazoline
将4-(3-溴苯氨基)-6-乙酰氧基喹唑啉(0.72g,2mmol)溶于15mL甲醇中,加入4mL氨水,室温下反应3h,抽滤,烘干,得0.31g灰白色固体,滤液浓缩后经硅胶柱分离纯化(洗脱剂,石油醚∶乙酸乙酯=1∶2,v∶v),得0.28g灰白色固体0.59g,收率93%,m.p.>300℃。1H NMR(300MHz,DMSO-d6):δ=7.28-7.37(m,1H),7.46(d,J=9.0Hz,1H),7.71(d,J=9.0Hz,1H),7.80(s,1H),8.27(s,1H),8.52(s,1H),9.60(s,1H),10.11(s,1H)。Dissolve 4-(3-bromoanilino)-6-acetoxyquinazoline (0.72g, 2mmol) in 15mL of methanol, add 4mL of ammonia water, react at room temperature for 3h, filter with suction, and dry to obtain 0.31g off-white The solid was concentrated and the filtrate was separated and purified by silica gel column (eluent, petroleum ether: ethyl acetate = 1:2, v:v) to obtain 0.28 g of off-white solid 0.59 g, yield 93%, mp > 300°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=7.28-7.37(m, 1H), 7.46(d, J=9.0Hz, 1H), 7.71(d, J=9.0Hz, 1H), 7.80(s , 1H), 8.27(s, 1H), 8.52(s, 1H), 9.60(s, 1H), 10.11(s, 1H).
制备实施例7 4-(3-氯-4-氟苯氨基)-6-羟基喹唑啉Preparation Example 7 4-(3-chloro-4-fluoroanilino)-6-hydroxyquinazoline
按照制备实施例6所述的方法,只是将4-(3-溴苯氨基)-6-乙酰氧基喹唑啉改为4-(3-氯-4-氟苯氨基)-6-乙酰氧基喹唑啉,收率91%,m.p.>300℃。1H NMR(300MHz,DMSO-d6):δ=7.56(t,J=9.0Hz,1H),7.72-7.74(m,2H),7.92(d,J=8.7Hz,1H),8.04-8.07(m,2H),8.87(s,1H),10.98(s,1H),11.38(s,1H)。According to the method described in Preparation Example 6, except that 4-(3-bromoanilino)-6-acetoxyquinazoline was changed to 4-(3-chloro-4-fluoroanilino)-6-acetoxyquinazoline Quinazoline, yield 91%, mp>300°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=7.56(t, J=9.0Hz, 1H), 7.72-7.74(m, 2H), 7.92(d, J=8.7Hz, 1H), 8.04-8.07 (m, 2H), 8.87 (s, 1H), 10.98 (s, 1H), 11.38 (s, 1H).
制备实施例8 4-[3-氯-4-(3-氟苄氧基)苯氨基]-6-羟基喹唑啉Preparation Example 8 4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-6-hydroxyquinazoline
按照制备实施例6所述的方法,只是将4-(3-溴苯氨基)-6-乙酰氧基喹唑啉改为4-[3-氯-4-(3-氟苄氧基)苯氨基]-6-乙酰氧基喹唑啉,收率94%,m.p.>300℃。1H NMR(300MHz,DMSO-d6):δ=5.25(s,1H),7.16-7.34(m,4H),7.40-7.51(m,2H),7.67(d,J=8.7Hz,1H),7.74-7.78(m,2H),8.07(d,J=2.7Hz,1H),8.44(s,1H),9.50(s,1H),10.09(s,1H)。According to the method described in Preparation Example 6, except that 4-(3-bromoanilino)-6-acetoxyquinazoline was changed to 4-[3-chloro-4-(3-fluorobenzyloxy)benzene Amino]-6-acetoxyquinazoline, yield 94%, mp>300°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=5.25(s, 1H), 7.16-7.34(m, 4H), 7.40-7.51(m, 2H), 7.67(d, J=8.7Hz, 1H) , 7.74-7.78 (m, 2H), 8.07 (d, J=2.7Hz, 1H), 8.44 (s, 1H), 9.50 (s, 1H), 10.09 (s, 1H).
制备实施例9 4-(3-溴苯氨基)-6-(3-氯丙氧基)喹唑啉Preparation Example 9 4-(3-bromoanilino)-6-(3-chloropropoxy)quinazoline
将4-(3-溴苯氨基)-6-羟基喹唑啉(0.32g,1mmol)溶于20mL乙腈中,加入碳酸钾(0.56g,4mmol)和催化量的四丁基溴化铵,加热回流20min,加入1,3-溴氯丙烷(0.32g,2mmol),回流反应2h,反应液冷却至室温,过滤,滤饼用乙腈洗涤,将滤液浓缩,硅胶柱分离纯化(洗脱剂,石油醚∶乙酸乙酯=2∶1,v∶v),得0.27g灰白色固体,收率68%,m.p.175-176℃。1H NMR(300MHz,DMSO-d6):δ=2.29(t,J=6.0Hz,2H),3.88(t,J=6.0Hz,2H),4.30(t,J=6.0Hz,2H),7.30-7.41(m,2H),7.54-7.57(m,2H),7.77(d,J=9.0Hz,1H),7.90-7.96(m,2H),8.19(s,1H),8.57(s,1H),9.70(s,1H)。4-(3-bromoanilino)-6-hydroxyquinazoline (0.32g, 1mmol) was dissolved in 20mL of acetonitrile, potassium carbonate (0.56g, 4mmol) and catalytic amount of tetrabutylammonium bromide were added, heated Reflux for 20min, add 1,3-bromochloropropane (0.32g, 2mmol), reflux for 2h, cool the reaction solution to room temperature, filter, wash the filter cake with acetonitrile, concentrate the filtrate, and separate and purify on a silica gel column (eluent, petroleum Ether:ethyl acetate=2:1, v:v), 0.27g off-white solid was obtained, yield 68%, mp 175-176°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.29(t, J=6.0Hz, 2H), 3.88(t, J=6.0Hz, 2H), 4.30(t, J=6.0Hz, 2H), 7.30-7.41(m, 2H), 7.54-7.57(m, 2H), 7.77(d, J=9.0Hz, 1H), 7.90-7.96(m, 2H), 8.19(s, 1H), 8.57(s, 1H), 9.70 (s, 1H).
制备实施例10 4-(3-氯-4-氟苯氨基)-6-(3-氯丙氧基)喹唑啉Preparation Example 10 4-(3-chloro-4-fluoroanilino)-6-(3-chloropropoxy)quinazoline
按照制备实施例9所述的方法,只是将4-(3-溴苯氨基)-6-羟基喹唑啉改为4-(3-氯-4-氟苯氨基)-6-羟基喹唑啉,收率60%,m.p.140-141℃。1H NMR(300MHz,DMSO-d6):δ=2.29(t,J=6.3Hz,2H),3.88(t,J=5.7Hz,2H),4.30(t,J=6.0Hz,2H),7.44-7.57(m,2H),7.75-7.82(m,2H),7.93(s,1H),8.16-8.18(m,1H),8.55(s,1H),9.74(s,1H)。According to the method described in Preparation Example 9, just change 4-(3-bromoanilino)-6-hydroxyquinazoline to 4-(3-chloro-4-fluoroanilino)-6-hydroxyquinazoline , yield 60%, mp 140-141°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.29(t, J=6.3Hz, 2H), 3.88(t, J=5.7Hz, 2H), 4.30(t, J=6.0Hz, 2H), 7.44-7.57 (m, 2H), 7.75-7.82 (m, 2H), 7.93 (s, 1H), 8.16-8.18 (m, 1H), 8.55 (s, 1H), 9.74 (s, 1H).
制备实施例11 4-[3-氯-4-(3-氟苄氧基)苯氨基]-6-(3-氯丙氧基)喹唑啉Preparation Example 11 4-[3-Chloro-4-(3-fluorobenzyloxy)anilino]-6-(3-chloropropoxy)quinazoline
按照制备实施例9所述的方法,只是将4-(3-溴苯氨基)-6-羟基喹唑啉改为4-[3-氯-4-(3-氟苄氧基)苯氨基]-6-羟基喹唑啉,收率67%,m.p.144-146℃。1H NMR(300MHz,DMSO-d6):δ=2.28(t,J=6.6Hz,2H),3.87(t,J=6.0Hz,2H),4.28(t,J=5.7Hz,2H),5.26(s,2H),7.19(m,1H),7.27-7.35(m,3H),7.47-7.54(m,2H),7.72-7.75(m,2H),7.91(m,1H),7.99(d,J=2.4Hz,1H),8.49(s,1H),9.61(s,1H)。Follow the method described in Preparative Example 9, except that 4-(3-bromoanilino)-6-hydroxyquinazoline is changed to 4-[3-chloro-4-(3-fluorobenzyloxy)anilino] -6-Hydroxyquinazoline, yield 67%, mp144-146°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.28(t, J=6.6Hz, 2H), 3.87(t, J=6.0Hz, 2H), 4.28(t, J=5.7Hz, 2H), 5.26(s, 2H), 7.19(m, 1H), 7.27-7.35(m, 3H), 7.47-7.54(m, 2H), 7.72-7.75(m, 2H), 7.91(m, 1H), 7.99( d, J=2.4Hz, 1H), 8.49(s, 1H), 9.61(s, 1H).
实施例1 4-甲基哌嗪-1-二硫代甲酸-[3-[4-(3-溴苯氨基)喹唑啉-6-氧]]丙酯Example 1 4-methylpiperazine-1-dithioformic acid-[3-[4-(3-bromoanilino)quinazoline-6-oxygen]]propyl ester
将N-甲基哌嗪(0.10g,1mmol)溶于15mL DMF中,加入无水磷酸钾(0.43g,2mmol),室温搅拌下滴入二硫化碳(0.19g,2.5mmol),反应30min后,加入4-(3-溴苯氨基)-6-(3-氯丙氧基)喹唑啉(0.40g,1mmol),继续室温搅拌,TLC检测,直至反应结束。向反应体系中加入15mL水,用乙酸乙酯萃取(15mL×3),合并有机层,水洗(15mL×2),无水硫酸钠干燥,浓缩,剩余物用乙腈重结晶,得到0.38g固体,收率71%,m.p.152-153℃。1H NMR(300MHz,DMSO-d6):δ=2.20-2.24(m,5H),2.38(t,J=5.1H z,4H),3.47(t,J=7.2H z,2H),3.91(m,2H),4.24(t,J=5.4H z,4H),7.30-7.40(m,2H),7.54(dd,J=2.7,9.3Hz,1H),7.76(d,J=9.0Hz,1H),7.91-7.93(m,2H),8.19-8.20(m,1H),8.57(s,1H),9.67(s,1H)。元素分析C23H26BrN5OS2:理论值:C,51.88;H,4.92;N,13.15.测定值:C,51.81;H,4.92;N,13.03。Dissolve N-methylpiperazine (0.10g, 1mmol) in 15mL DMF, add anhydrous potassium phosphate (0.43g, 2mmol), add carbon disulfide (0.19g, 2.5mmol) dropwise under stirring at room temperature, react for 30min, add 4-(3-Bromoanilino)-6-(3-chloropropoxy)quinazoline (0.40 g, 1 mmol) was stirred at room temperature and detected by TLC until the reaction was completed. Add 15 mL of water to the reaction system, extract with ethyl acetate (15 mL×3), combine the organic layers, wash with water (15 mL×2), dry over anhydrous sodium sulfate, concentrate, and recrystallize the residue with acetonitrile to obtain 0.38 g of solid, Yield 71%, mp 152-153°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.20-2.24(m, 5H), 2.38(t, J=5.1Hz, 4H), 3.47(t, J=7.2Hz, 2H), 3.91 (m, 2H), 4.24(t, J=5.4Hz, 4H), 7.30-7.40(m, 2H), 7.54(dd, J=2.7, 9.3Hz, 1H), 7.76(d, J=9.0Hz , 1H), 7.91-7.93 (m, 2H), 8.19-8.20 (m, 1H), 8.57 (s, 1H), 9.67 (s, 1H). Elemental analysis C 23 H 26 BrN 5 OS 2 : Theoretical: C, 51.88; H, 4.92; N, 13.15. Found: C, 51.81; H, 4.92; N, 13.03.
实施例2 4-甲基哌嗪-1-二硫代甲酸-[3-[4-(3-氯-4-氟苯氨基)喹唑啉-6-氧]]丙酯Example 2 4-methylpiperazine-1-dithioformic acid-[3-[4-(3-chloro-4-fluoroanilino)quinazoline-6-oxygen]]propyl ester
按照实施例1所述的方法,只是将原料4-(3-溴苯氨基)-6-(3-氯丙氧基)喹唑啉改为4-(3-氯-4-氟苯氨基)-6-(3-氯丙氧基)喹唑啉,收率63%,m.p.154-155℃。1H NMR(300MHz,DMSO-d6):δ=2.20-2.24(m,5H),2.39(t,J=5.1H z,4H),3.46(t,J=7.2H z,2H),3.92(m,2H),4.25(t,J=5.7H z,4H),7.47(t,J=9.0H z,1H),7.54(dd,J=2.4,9.0Hz,1H),7.76(d,J=9.0Hz,1H),7.81-7.87(m,1H),7.91(d,J=2.1Hz,1H),8.17(dd,J=2.7,6.9Hz,1H),8.55(s,1H),9.70(s,1H)。元素分析C23H25ClFN5OS2:理论值:C,54.59;H,4.98;N,13.84.测定值:C,54.65;H,5.00;N,13.75。According to the method described in Example 1, just change the raw material 4-(3-bromoanilino)-6-(3-chloropropoxy)quinazoline into 4-(3-chloro-4-fluoroanilino) -6-(3-chloropropoxy)quinazoline, yield 63%, mp 154-155°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.20-2.24(m, 5H), 2.39(t, J=5.1Hz, 4H), 3.46(t, J=7.2Hz, 2H), 3.92 (m, 2H), 4.25(t, J=5.7Hz, 4H), 7.47(t, J=9.0Hz, 1H), 7.54(dd, J=2.4, 9.0Hz, 1H), 7.76(d, J=9.0Hz, 1H), 7.81-7.87(m, 1H), 7.91(d, J=2.1Hz, 1H), 8.17(dd, J=2.7, 6.9Hz, 1H), 8.55(s, 1H), 9.70 (s, 1H). Elemental analysis C 23 H 25 ClFN 5 OS 2 : Theoretical: C, 54.59; H, 4.98; N, 13.84. Found: C, 54.65; H, 5.00; N, 13.75.
实施例3 4-甲基哌嗪-1-二硫代甲酸-[3-[4-[3-氯-4-(3-氟苄氧基)苯氨基]喹唑啉-6-氧]丙酯Example 3 4-methylpiperazine-1-dithioformic acid-[3-[4-[3-chloro-4-(3-fluorobenzyloxy)anilino]quinazoline-6-oxygen]propane ester
按照实施例1所述的方法,只是将原料4-(3-溴苯氨基)-6-(3-氯丙氧基)喹唑啉改为4-[3-氯-4-(3-氟苄氧基)苯氨基]-6-(3-氯丙氧基)喹唑啉,收率57%,m.p.128-130℃。1H NMR(300MHz,DMSO-d6):δ=2.19(m,5H),2.39(t,J=4.8Hz,4H),3.46(t,J=6.6H z,2H),3.92(m,2H),4.24(s,4H),5.26(s,2H),7.19(t,J=8.7H z,1H),7.27-7.35(m,3H),7.44-7.53(m,2H),7.71-7.74(m,2H),7.90(s,1H),7.99(d,J=2.4Hz,1H),8.49(s,1H),9.59(s,1H)。HRMS:C30H31ClFN5O2S2+H,理论值612.16700;测定值(ESI-FTMS,(M+H)+1),612.16556。According to the method described in Example 1, just change the raw material 4-(3-bromoanilino)-6-(3-chloropropoxy)quinazoline into 4-[3-chloro-4-(3-fluoro Benzyloxy)anilino]-6-(3-chloropropoxy)quinazoline, yield 57%, mp128-130°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.19(m, 5H), 2.39(t, J=4.8Hz, 4H), 3.46(t, J=6.6Hz, 2H), 3.92(m, 2H), 4.24(s, 4H), 5.26(s, 2H), 7.19(t, J=8.7Hz, 1H), 7.27-7.35(m, 3H), 7.44-7.53(m, 2H), 7.71- 7.74 (m, 2H), 7.90 (s, 1H), 7.99 (d, J=2.4Hz, 1H), 8.49 (s, 1H), 9.59 (s, 1H). HRMS: C 30 H 31 ClFN 5 O 2 S 2 +H, calc. 612.16700; found (ESI-FTMS, (M+H) +1 ), 612.16556.
制备实施例12 3-羟基-4-甲氧基苯甲酸甲酯Preparation Example 12 3-Hydroxy-4-methoxybenzoic acid methyl ester
将3-羟基-4-甲氧基苯甲酸(1.68g,10mmol)溶于15mL甲醇中,冰浴下缓慢滴加二氯亚砜(1.1mL),滴毕,室温下反应24h,蒸除过量的甲醇,加入15mL水,用乙酸乙酯萃取(15mL×3),合并有机层,无水硫酸钠干燥,浓缩,硅胶柱分离纯化,洗脱剂(石油醚∶乙酸乙酯=5∶1,v∶v),得1.56g白色固体产物,收率85%,m.p.61-63℃。1H NMR(300MHz,CDCl3):δ=3.88(s,1H),3.95(s,1H),5,67(s,1H),6.87(d,J=8.1Hz,1H),7.60(s,1H),7.63(d,J=1.5Hz,1H)。Dissolve 3-hydroxy-4-methoxybenzoic acid (1.68g, 10mmol) in 15mL of methanol, slowly add thionyl chloride (1.1mL) dropwise under ice-cooling, after the dropwise completion, react at room temperature for 24h, evaporate the excess Add 15mL of water, extract with ethyl acetate (15mL×3), combine the organic layers, dry over anhydrous sodium sulfate, concentrate, separate and purify on a silica gel column, eluent (petroleum ether:ethyl acetate=5:1, v:v) to obtain 1.56 g of white solid product, yield 85%, mp61-63°C. 1 H NMR (300MHz, CDCl 3 ): δ=3.88(s, 1H), 3.95(s, 1H), 5, 67(s, 1H), 6.87(d, J=8.1Hz, 1H), 7.60(s , 1H), 7.63 (d, J=1.5Hz, 1H).
制备实施例13 3-(3-氯丙氧基)-4-甲氧基苯甲酸甲酯Preparation Example 13 3-(3-chloropropoxy)-4-methoxybenzoic acid methyl ester
将制备实施例12产物(1.82g,10mmol)溶于20mL乙腈,加入碳酸钾(2.76g,20mmol)和催化量的四丁基溴化铵,加热回流20min,加入1,3-溴氯丙烷(0.32g,2mmol),回流反应4h,反应液冷却至室温,蒸除乙腈,向反应体系中加入15mL水,用乙酸乙酯萃取(15mL×3),合并有机层,无水硫酸钠干燥,浓缩,硅胶柱分离纯化(洗脱剂,石油醚∶乙酸乙酯=5∶1,v∶v),得2.41g白色固体,收率93%,m.p.51-52℃。1H NMR(300MHz,DMSO-d6):δ=2.14-2.23(m,2H),3.77-3.85(m,8H),4.12(t,J=6.0Hz,2H),7.09(d,J=8.7Hz,1H),7.47(d,J=1.8Hz,1H),7.60(dd,J=1.8,8.4Hz,1H)。Dissolve the product of Preparation Example 12 (1.82g, 10mmol) in 20mL of acetonitrile, add potassium carbonate (2.76g, 20mmol) and a catalytic amount of tetrabutylammonium bromide, heat to reflux for 20min, add 1,3-bromochloropropane ( 0.32g, 2mmol), reflux reaction for 4h, the reaction solution was cooled to room temperature, acetonitrile was distilled off, 15mL water was added to the reaction system, extracted with ethyl acetate (15mL×3), the organic layers were combined, dried over anhydrous sodium sulfate, concentrated , silica gel column separation and purification (eluent, petroleum ether: ethyl acetate = 5: 1, v: v), to obtain 2.41 g of white solid, yield 93%, mp51-52 ° C. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.14-2.23(m, 2H), 3.77-3.85(m, 8H), 4.12(t, J=6.0Hz, 2H), 7.09(d, J= 8.7Hz, 1H), 7.47 (d, J=1.8Hz, 1H), 7.60 (dd, J=1.8, 8.4Hz, 1H).
制备实施例14 2-硝基-4-甲氧基-5-(3-氯丙氧基)苯甲酸甲酯Preparation Example 14 2-nitro-4-methoxy-5-(3-chloropropoxy) methyl benzoate
将制备实施例13产物(6.46g,25mmol)溶于50mL氯仿中,10℃下滴加由浓硝酸(4.5mL)和浓硫酸(5mL)配成的混酸,滴毕,20℃下反应4h,加入50mL冰水稀释反应液,分去水层,氯仿层用20mL饱和碳酸氢钠水溶液洗涤,水洗至中性,无水硫酸钠干燥,蒸除溶剂,硅胶柱分离纯化(洗脱剂,石油醚∶乙酸乙酯=5∶1,v∶v),得6.84g黄色固体,收率90%,m.p.68-70℃。1H NMR(300MHz,DMSO-d6):δ=2.21(t,J=6.0Hz,2H),3.78(t,J=6.3Hz,2H),3.82(s,3H),3.92(s,3H),4.25(t,J=6.0Hz,2H),7.37(s,1H),7.65(s,1H)。The product of Preparation Example 13 (6.46g, 25mmol) was dissolved in 50mL of chloroform, and a mixed acid composed of concentrated nitric acid (4.5mL) and concentrated sulfuric acid (5mL) was added dropwise at 10°C, and the reaction was carried out at 20°C for 4h. Add 50mL ice water to dilute the reaction solution, separate the water layer, wash the chloroform layer with 20mL saturated aqueous sodium bicarbonate solution, wash to neutral, dry over anhydrous sodium sulfate, evaporate the solvent, separate and purify on a silica gel column (eluent, petroleum ether : ethyl acetate=5:1, v:v), 6.84g of yellow solid was obtained, yield 90%, mp68-70°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.21(t, J=6.0Hz, 2H), 3.78(t, J=6.3Hz, 2H), 3.82(s, 3H), 3.92(s, 3H ), 4.25(t, J=6.0Hz, 2H), 7.37(s, 1H), 7.65(s, 1H).
制备实施例15 2-氨基-4-甲氧基-5-(3-氯丙氧基)苯甲酸甲酯Preparation Example 15 2-Amino-4-methoxy-5-(3-chloropropoxy)methyl benzoate
将制备实施例14产物(1.83g,6mmol)、氯化铵(1.61g,30mmol)、还原铁粉(1.11g,19.8mmol)加入30mL甲醇和15mL水中,回流反应2h,将反应液冷却至室温,用饱和碳酸氢钠水溶液将反应液调至中性,抽滤,滤去不溶物,滤液用二氯甲烷(15mL×3)萃取,合并有机层,无水硫酸钠干燥,蒸除溶剂,硅胶柱分离纯化(洗脱剂,石油醚∶乙酸乙酯=4∶1,v∶v),得6.84g白色固体,收率65%,m.p.97-99℃。1H NMR(400MHz,CDCl3):δ=2.20-2.26(m,2H),3.76(t,J=4.8Hz,2H),3.84(s,3H),3.84(s,3H),4.08(t,J=4.5Hz,2H),5.51(s,2H),6.15(s,1H),7.36(s,1H)。Add the product of Preparation Example 14 (1.83g, 6mmol), ammonium chloride (1.61g, 30mmol), and reduced iron powder (1.11g, 19.8mmol) into 30mL of methanol and 15mL of water, reflux for 2h, and cool the reaction solution to room temperature , the reaction solution was adjusted to neutral with saturated aqueous sodium bicarbonate solution, suction filtered, and the insoluble matter was filtered off, the filtrate was extracted with dichloromethane (15mL×3), the organic layers were combined, dried over anhydrous sodium sulfate, the solvent was evaporated, and the silica gel Column separation and purification (eluent, petroleum ether: ethyl acetate = 4: 1, v: v) gave 6.84 g of white solid, yield 65%, mp 97-99°C. 1 H NMR (400MHz, CDCl 3 ): δ=2.20-2.26(m, 2H), 3.76(t, J=4.8Hz, 2H), 3.84(s, 3H), 3.84(s, 3H), 4.08(t , J=4.5Hz, 2H), 5.51(s, 2H), 6.15(s, 1H), 7.36(s, 1H).
制备实施例16 4-羟基-6-(3-氯丙氧基)-7-甲氧基喹唑啉Preparation Example 16 4-hydroxyl-6-(3-chloropropoxy)-7-methoxyquinazoline
将制备实施例15产物(0.55g,2mmol)加入5mL甲酰胺中,油浴加热,搅拌使固体全溶,缓慢滴加1mL三氯氧磷,控制温度在85-95℃之间,滴加完毕,在该温度下反应2h,待反应完全后,向所得的红褐色粘稠物中加入适量冰水,室温下搅拌,用1N的氢氧化钠水溶液调节pH 6~7,得橘红色混浊液,抽滤,得紫褐色固体0.35g,收率65%,m.p.213-215℃。1H NMR(300MHz,DMSO-d6):δ=2.23(t,J=6.3Hz,2H),3.81(t,J=6.3Hz,2H),3.92(s,3H),4.19(t,J=6.3Hz,2H),7.15(s,1H),7.47(s,1H),7.99(s,1H),12.09(s,1H)。Add the product of Preparation Example 15 (0.55 g, 2 mmol) into 5 mL of formamide, heat in an oil bath, stir to completely dissolve the solid, slowly add 1 mL of phosphorus oxychloride dropwise, control the temperature between 85-95 °C, and complete the dropwise addition , react at this temperature for 2 hours, after the reaction is complete, add an appropriate amount of ice water to the obtained reddish-brown viscous substance, stir at room temperature, adjust the pH to 6-7 with 1N aqueous sodium hydroxide solution, and obtain an orange-red turbid solution. After suction filtration, 0.35 g of purple-brown solid was obtained, yield 65%, mp 213-215°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.23(t, J=6.3Hz, 2H), 3.81(t, J=6.3Hz, 2H), 3.92(s, 3H), 4.19(t, J =6.3Hz, 2H), 7.15(s, 1H), 7.47(s, 1H), 7.99(s, 1H), 12.09(s, 1H).
制备实施例17 4-(3-溴苯氨基)-6-(3-氯丙氧基)-7-甲氧基喹唑啉Preparation Example 17 4-(3-bromoanilino)-6-(3-chloropropoxy)-7-methoxyquinazoline
将制备实施例16产物(1.08g,4mmol)加入10mL二氯亚砜中,滴入0.5mL DMF,回流反应3h,减压蒸除过量二氯亚砜,得中间体4-氯-6-(3-氯丙氧基)-7-甲氧基喹唑啉,不作分离,将中间体溶于15mL异丙醇中,加入间溴苯胺(0.69g,4mmol),1mL三乙胺,回流反应3h,将反应液冷却至室温,抽滤,冷异丙醇洗涤,干燥,得淡黄色固体1.52g,收率89%,m.p.198-200℃。1H NMR(400MHz,DMSO-d6):δ=2.27-2.34(m,2H),3.86(t,J=4.8Hz,2H),3.96(s,3H),4.29(t,J=4.5Hz,2H),7.23(s,1H),7.27-7.30(m,1H),7.36(t,J=6.0Hz,1H),7.84-7.89(m,1H),7.96(s,1H),8.15(t,J=1.5Hz,1H),8.53(s,1H),9.53(s,1H)。The product of Preparation Example 16 (1.08 g, 4 mmol) was added to 10 mL of thionyl chloride, 0.5 mL of DMF was added dropwise, refluxed for 3 hours, and excess thionyl chloride was evaporated under reduced pressure to obtain the intermediate 4-chloro-6-( 3-chloropropoxy)-7-methoxyquinazoline, without separation, the intermediate was dissolved in 15mL of isopropanol, m-bromoaniline (0.69g, 4mmol), 1mL of triethylamine were added, and the reaction was refluxed for 3h , the reaction solution was cooled to room temperature, filtered with suction, washed with cold isopropanol, and dried to obtain 1.52 g of light yellow solid, yield 89%, mp 198-200°C. 1 H NMR (400MHz, DMSO-d 6 ): δ=2.27-2.34(m, 2H), 3.86(t, J=4.8Hz, 2H), 3.96(s, 3H), 4.29(t, J=4.5Hz , 2H), 7.23(s, 1H), 7.27-7.30(m, 1H), 7.36(t, J=6.0Hz, 1H), 7.84-7.89(m, 1H), 7.96(s, 1H), 8.15( t, J=1.5Hz, 1H), 8.53(s, 1H), 9.53(s, 1H).
制备实施例18 4-(3-氯-4-氟苯氨基)-6-(3-氯丙氧基)-7-甲氧基喹唑啉Preparation Example 18 4-(3-chloro-4-fluoroanilino)-6-(3-chloropropoxy)-7-methoxyquinazoline
按照制备实施例17所述的方法,只是将原料间溴苯胺改为3-氯-4-氟苯胺,收率82%,m.p.224-226℃。1H NMR(300MHz,DMSO-d6):δ=2.29(t,J=6.0Hz,2H),3.85(t,J=5.4Hz,2H),4.02(s,3H),4.34(t,J=5.7Hz,2H),7.31(s,1H),7.57(t,J=8.7Hz,1H),7.70(s,1H),8.01(d,J=7.2Hz,1H),8.23(s,1H),8.87(s,1H),11.21(s,1H)。According to the method described in Preparation Example 17, except that the raw material m-bromoaniline was changed to 3-chloro-4-fluoroaniline, the yield was 82%, mp 224-226°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.29(t, J=6.0Hz, 2H), 3.85(t, J=5.4Hz, 2H), 4.02(s, 3H), 4.34(t, J =5.7Hz, 2H), 7.31(s, 1H), 7.57(t, J=8.7Hz, 1H), 7.70(s, 1H), 8.01(d, J=7.2Hz, 1H), 8.23(s, 1H ), 8.87(s, 1H), 11.21(s, 1H).
制备实施例19 4-[3-氯-4-(3-氟苄氧基)苯氨基]-6-(3-氯丙氧基)-7-甲氧基喹唑啉Preparation Example 19 4-[3-Chloro-4-(3-fluorobenzyloxy)anilino]-6-(3-chloropropoxy)-7-methoxyquinazoline
按照制备实施例17所述的方法,只是将原料间溴苯胺改为3-氯-4-(3-氟苄氧基)苯胺,收率72%,m.p.194-196℃。1H NMR(300MHz,DMSO-d6):δ=2.31(t,J=4.8Hz,2H),3.85(t,J=4.8Hz,2H),4.01(s,3H),4.34(t,J=4.5Hz,2H),5.31(s,2H),7.17-7.22(m,1H),7.32-7.36(m,4H),7.46-7.51(m,1H),7.61(dd,J=1.8,6.6Hz,1H),7.85(d,J=1.8Hz,1H),8.26(s,1H),8.84(s,1H),11.25(s,1H)。According to the method described in Preparation Example 17, except that the raw material m-bromoaniline was changed to 3-chloro-4-(3-fluorobenzyloxy)aniline, the yield was 72%, mp 194-196°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.31(t, J=4.8Hz, 2H), 3.85(t, J=4.8Hz, 2H), 4.01(s, 3H), 4.34(t, J =4.5Hz, 2H), 5.31(s, 2H), 7.17-7.22(m, 1H), 7.32-7.36(m, 4H), 7.46-7.51(m, 1H), 7.61(dd, J=1.8, 6.6 Hz, 1H), 7.85 (d, J = 1.8 Hz, 1H), 8.26 (s, 1H), 8.84 (s, 1H), 11.25 (s, 1H).
实施例4 4-甲基哌嗪-1-二硫代甲酸-{3-[4-(3-溴苯氨基)-7-甲氧基喹唑啉-6-氧]}丙酯Example 4 4-methylpiperazine-1-dithioformic acid-{3-[4-(3-bromoanilino)-7-methoxyquinazoline-6-oxygen]}propyl ester
将N-甲基哌嗪(0.10g,1mmol)溶于15mL DMF,加入无水磷酸钾(0.43g,2mmol),室温搅拌下滴入二硫化碳(0.19g,2.5mmol),反应30min后,加入4-(3-溴苯氨基)-6-(3-氯丙氧基)-7-甲氧基喹唑啉(0.43g,1mmol),继续室温搅拌,由TLC检测,直至反应结束。向反应体系中加入15mL水,用乙酸乙酯萃取(15mL×3),合并有机层,水洗(15mL×2),无水硫酸钠干燥,浓缩,剩余物用乙腈重结晶,得到0.42g固体,收率74%,m.p.166-168℃。1H NMR(300MHz,DMSO-d6):δ=2.20-2.25(m,5H),2.39(t,J=4.5Hz,4H),3.44(t,J=6.6Hz,2H),3.95(s,5H),4.25(t,J=5.7Hz,4H),7.23(s,1H),7.27-7.39(m,2H),7.86-7.90(m,2H),8.15(s,1H),8.53(s,1H),9.52(s,1H)。元素分析C24H28BrN5O2S2:理论值:C,51.24;H,5.02;N,12.45.测定值:C,51.29;H,5.06;N,12.61。Dissolve N-methylpiperazine (0.10g, 1mmol) in 15mL DMF, add anhydrous potassium phosphate (0.43g, 2mmol), add carbon disulfide (0.19g, 2.5mmol) dropwise under stirring at room temperature, react for 30min, add 4 -(3-Bromoanilino)-6-(3-chloropropoxy)-7-methoxyquinazoline (0.43g, 1mmol), continue to stir at room temperature, and detect by TLC until the reaction is complete. Add 15 mL of water to the reaction system, extract with ethyl acetate (15 mL×3), combine the organic layers, wash with water (15 mL×2), dry over anhydrous sodium sulfate, concentrate, and recrystallize the residue with acetonitrile to obtain 0.42 g of solid, Yield 74%, mp 166-168°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.20-2.25(m, 5H), 2.39(t, J=4.5Hz, 4H), 3.44(t, J=6.6Hz, 2H), 3.95(s , 5H), 4.25(t, J=5.7Hz, 4H), 7.23(s, 1H), 7.27-7.39(m, 2H), 7.86-7.90(m, 2H), 8.15(s, 1H), 8.53( s, 1H), 9.52 (s, 1H). Elemental analysis C 24 H 28 BrN 5 O 2 S 2 : Theoretical: C, 51.24; H, 5.02; N, 12.45. Found: C, 51.29; H, 5.06; N, 12.61.
实施例5 4-甲基哌嗪-1-二硫代甲酸-{3-[4-(3-氯-4-氟苯氨基)-7-甲氧基喹唑啉-6-氧]}丙酯Example 5 4-methylpiperazine-1-dithioformic acid-{3-[4-(3-chloro-4-fluoroanilino)-7-methoxyquinazoline-6-oxygen]}propane ester
按照实施例4所述的方法,只是将原料4-(3-溴苯氨基)-6-(3-氯丙氧基)-7-甲氧基喹唑啉改为4-(3-氯-4-氟苯氨基)-6-(3-氯丙氧基)-7-甲氧基喹唑啉,收率69%,m.p.86-87℃。1H NMR(300MHz,DMSO-d6):δ=2.20-2.25(m,5H),2.38(t,J=5.1Hz,4H),3.44(t,J=7.2Hz,2H),3.95(s,5H),4.22(t,J=5.7Hz,4H),7.22(s,1H),7.46(t,J=9.0Hz,4H),7.79-7.84(m,2H),8.12(dd,J=2.4,6.9Hz,1H),8.51(s,1H),9.55(s,1H)。元素分析C24H27ClFN5O2S2:理论值:C,53.77;H,5.08;N,13.06.测定:C,53.69;H,5.06;N,12.97。According to the method described in Example 4, just change the raw material 4-(3-bromoanilino)-6-(3-chloropropoxy)-7-methoxyquinazoline into 4-(3-chloro- 4-fluoroanilino)-6-(3-chloropropoxy)-7-methoxyquinazoline, yield 69%, mp86-87℃. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.20-2.25(m, 5H), 2.38(t, J=5.1Hz, 4H), 3.44(t, J=7.2Hz, 2H), 3.95(s , 5H), 4.22(t, J=5.7Hz, 4H), 7.22(s, 1H), 7.46(t, J=9.0Hz, 4H), 7.79-7.84(m, 2H), 8.12(dd, J= 2.4, 6.9Hz, 1H), 8.51(s, 1H), 9.55(s, 1H). Elemental analysis C 24 H 27 ClFN 5 O 2 S 2 : Theoretical: C, 53.77; H, 5.08; N, 13.06. Determined: C, 53.69; H, 5.06; N, 12.97.
实施例6 4-甲基哌嗪-1-二硫代甲酸-[3-[4-[3-氯-4-(3-氟苄氧基)苯氨基]-7-甲氧基喹唑啉-6-氧]]丙酯Example 6 4-methylpiperazine-1-dithioformic acid-[3-[4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-7-methoxyquinazoline -6-Oxo]]propyl ester
按照实施例4所述的方法,只是将原料4-(3-溴苯氨基)-6-(3-氯丙氧基)-7-甲氧基喹唑啉改为4-[3-氯-4-(3-氟苄氧基)苯氨基]-6-(3-氯丙氧基)-7-甲氧基喹唑啉,收率62%,m.p.84-85℃。1H NMR(300MHz,DMSO-d6):δ=2.19-2.23(m,5H),2.37(t,J=4.5Hz,4H),3.42(t,J=7.2Hz,2H),3.92(s,5H),4.21(s,4H),5.24(s,2H),7.15-7.33(m,6H),7.42-7.50(m,1H),7.69(dd,J=2.4,8.7Hz,1H),7.81(s,1H),7.94(d,J=2.4Hz,1H),8.44(s,1H)。元素分析C31H33ClFN5O3S2.CH3CN:理论值:C,58.01;H,5.31;N,12.30.测定值:C,57.90;H,5.42;N,12.23。According to the method described in Example 4, just change the raw material 4-(3-bromoanilino)-6-(3-chloropropoxy)-7-methoxyquinazoline into 4-[3-chloro- 4-(3-fluorobenzyloxy)anilino]-6-(3-chloropropoxy)-7-methoxyquinazoline, yield 62%, mp84-85℃. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.19-2.23(m, 5H), 2.37(t, J=4.5Hz, 4H), 3.42(t, J=7.2Hz, 2H), 3.92(s , 5H), 4.21(s, 4H), 5.24(s, 2H), 7.15-7.33(m, 6H), 7.42-7.50(m, 1H), 7.69(dd, J=2.4, 8.7Hz, 1H), 7.81 (s, 1H), 7.94 (d, J=2.4Hz, 1H), 8.44 (s, 1H). Elemental analysis C 31 H 33 ClFN 5 O 3 S 2 .CH 3 CN: Theoretical: C, 58.01; H, 5.31; N, 12.30. Found: C, 57.90; H, 5.42; N, 12.23.
实施例7 哌嗪-4-二硫代甲酸-[3-[4-[3-氯-4-(3-氟苄氧基)苯氨基]-7-甲氧基喹唑啉-6-氧]]丙酯Example 7 Piperazine-4-dithioformic acid-[3-[4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-7-methoxyquinazoline-6-oxo ]] Propyl ester
将哌嗪单盐酸盐(0.1mmol)溶于10mL DMF中,缓慢滴入二硫化碳(0.1mmol),室温搅拌0.5h后,加入无水磷酸钾(0.2mmol)以及4-[3-氯-4-(3-氟苄氧基)苯氨基]-6-(3-氯丙氧基)-7-甲氧基喹唑啉(0.1mmol),继续室温搅拌反应,TLC监测反应,至反应完全。向反应体系中加入15mL水,用乙酸乙酯萃取(15mL×3),合并有机层,水洗(15mL×2),无水硫酸钠干燥,浓缩,剩余物用乙腈重结晶,得到35mg固体,收率55%,m.p.124-126℃。HRMS:C30H31ClFN5O3S2+H,理论值628.16191;测定值(ESI-FTMS,(M+H)+1),628.16196。Dissolve piperazine monohydrochloride (0.1mmol) in 10mL DMF, slowly drop into carbon disulfide (0.1mmol), stir at room temperature for 0.5h, add anhydrous potassium phosphate (0.2mmol) and 4-[3-chloro-4 -(3-fluorobenzyloxy)anilino]-6-(3-chloropropoxy)-7-methoxyquinazoline (0.1 mmol), continue to stir the reaction at room temperature, and monitor the reaction by TLC until the reaction is complete. Add 15 mL of water to the reaction system, extract with ethyl acetate (15 mL×3), combine the organic layers, wash with water (15 mL×2), dry over anhydrous sodium sulfate, concentrate, and recrystallize the residue with acetonitrile to obtain 35 mg of solid, Rate 55%, mp124-126°C. HRMS: C 30 H 31 ClFN 5 O 3 S 2 +H, calc. 628.16191; found (ESI-FTMS, (M+H) +1 ), 628.16196.
实施例8 高哌嗪-4-二硫代甲酸-[3-[4-[3-氯-4-(3-氟苄氧基)苯氨基]-7-甲氧基喹唑啉-6-氧]]丙酯Example 8 Homopiperazine-4-dithioformic acid-[3-[4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-7-methoxyquinazoline-6- Oxy]]propyl ester
按照实施例7所述的方法,只是将原料哌嗪单盐酸盐改为高哌嗪单盐酸盐,收率47%,m.p.179-180℃。1H NMR(400MHz,DMSO-d6):δ=1.21(s,1H),2.03-2.20(m,5H),3.92-4.55(m,14H),5.24(s,2H),7.15-7.20(m,2H),7.24-7.34(m,3H),7.44-7.49(m,1H),7.69(dd,J=1.8,6.9Hz,1H),7.81(s,1H),7.94(d,J=1.5Hz,1H),8.45(s,1H),9.42(s,1H)。According to the method described in Example 7, except that the raw material piperazine monohydrochloride was changed to homopiperazine monohydrochloride, the yield was 47%, mp 179-180°C. 1 H NMR (400 MHz, DMSO-d 6 ): δ=1.21 (s, 1H), 2.03-2.20 (m, 5H), 3.92-4.55 (m, 14H), 5.24 (s, 2H), 7.15-7.20 ( m, 2H), 7.24-7.34(m, 3H), 7.44-7.49(m, 1H), 7.69(dd, J=1.8, 6.9Hz, 1H), 7.81(s, 1H), 7.94(d, J= 1.5Hz, 1H), 8.45(s, 1H), 9.42(s, 1H).
实施例9 吗啉-4-二硫代甲酸-[3-[4-[3-氯-4-(3-氟苄氧基)苯氨基]-7-甲氧基喹唑啉-6-氧]]丙酯Example 9 Morpholine-4-dithioformic acid-[3-[4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-7-methoxyquinazoline-6-oxo ]] Propyl ester
按照实施例6所述的方法,只是将原料N-甲基哌嗪改为吗啉,收率66%,m.p.80-82℃。1H NMR(400MHz,DMSO-d6):δ=2.20-2.27(m,2H),3.46(t,J=5.4Hz,2H),3.66((t,J=3.3Hz,4H),3.94(s,5H),4.23(t,J=4.5Hz,4H),5.25(s,2H),7.16-2.21(m,2H),7.26-7.35(m,3H),7.45-7.50(m,1H),7.69(dd,J=1.8,6.6Hz,1H),7.83(s,1H),7.95(d,J=1.8Hz,1H),8.46(s,1H),9.42(s,1H)。元素分析C30H30ClFN4O4S2:理论值:C,57.27;H,4.81;N,8.90.测定值:C,57.69;H,4.91;N,8.70。According to the method described in Example 6, except that the raw material N-methylpiperazine was changed to morpholine, the yield was 66%, mp80-82°C. 1 H NMR (400MHz, DMSO-d 6 ): δ=2.20-2.27(m, 2H), 3.46(t, J=5.4Hz, 2H), 3.66((t, J=3.3Hz, 4H), 3.94( s, 5H), 4.23(t, J=4.5Hz, 4H), 5.25(s, 2H), 7.16-2.21(m, 2H), 7.26-7.35(m, 3H), 7.45-7.50(m, 1H) , 7.69 (dd, J = 1.8, 6.6Hz, 1H), 7.83 (s, 1H), 7.95 (d, J = 1.8Hz, 1H), 8.46 (s, 1H), 9.42 (s, 1H). Elemental analysis C 30 H 30 ClFN 4 O 4 S 2 : Cal.: C, 57.27; H, 4.81; N, 8.90. Found: C, 57.69; H, 4.91; N, 8.70.
实施例10 硫代吗啉-4-二硫代甲酸-[3-[4-[3-氯-4-(3-氟苄氧基)苯氨基]-7-甲氧基喹唑啉-6-氧]]丙酯Example 10 Thiomorpholine-4-dithioformic acid-[3-[4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-7-methoxyquinazoline-6 -oxy]]propyl ester
按照实施例6所述的方法,只是将原料N-甲基哌嗪改为硫代吗啉,收率80%,m.p.176-177℃。1HNMR(400MHz,DMSO-d6):δ=2.20-2.27(m,2H),2.71-2.74(m,4H),3.46(t,J=5.4Hz,2H),3.94(s,3H),4.22-4.52(m,6H),5.25(s,2H),7.16-7.21(m,2H),7.26-7.35(m,3H),7.45-7.50(m,1H),7.69(dd,J=2.1,6.9Hz,1H),7.82(s,1H),7.95(d,J=1.8Hz,1H),8.46(s,1H),9.42(s,1H)。元素分析C30H30ClFN4O3S3:理论值:C,55.84;H,4.69;N,8.68.测定值:C,55.86;H,4.91;N,8.67。According to the method described in Example 6, except that the raw material N-methylpiperazine was changed to thiomorpholine, the yield was 80%, and the mp was 176-177°C. 1 HNMR (400MHz, DMSO-d 6 ): δ=2.20-2.27(m, 2H), 2.71-2.74(m, 4H), 3.46(t, J=5.4Hz, 2H), 3.94(s, 3H), 4.22-4.52(m, 6H), 5.25(s, 2H), 7.16-7.21(m, 2H), 7.26-7.35(m, 3H), 7.45-7.50(m, 1H), 7.69(dd, J=2.1 , 6.9Hz, 1H), 7.82(s, 1H), 7.95(d, J=1.8Hz, 1H), 8.46(s, 1H), 9.42(s, 1H). Elemental analysis C 30 H 30 ClFN 4 O 3 S 3 : Theoretical: C, 55.84; H, 4.69; N, 8.68. Found: C, 55.86; H, 4.91; N, 8.67.
实施例11 4-[二(4-氟苯基)甲基]哌嗪-1-二硫代甲酸-[3-[4-[3-氯-4-(3-氟苄氧基)苯氨基]-7-甲氧基喹唑啉-6-氧]]丙酯Example 11 4-[bis(4-fluorophenyl)methyl]piperazine-1-dithioformic acid-[3-[4-[3-chloro-4-(3-fluorobenzyloxy)phenylamino ]-7-methoxyquinazoline-6-oxy]]propyl ester
按照实施例6所述的方法,只是将原料N-甲基哌嗪改为1-[二(4-氟苯基)甲基]哌嗪,收率45%,m.p.151-153℃。1H NMR(400MHz,DMSO-d6):δ=2.09(s,1H),2.21(t,J=5.1Hz,2H),2.36(t,J=3.3Hz,4H),3.42(t,J=5.4Hz,2H),3.93(s,4H),4.21(t,J=4.5Hz,4H),4.46(s,1H),5.26(s,2H),7.12-7.21(m,6H),7.26-7.35(m,3H),7.42-7.50(m,5H),7.70(dd,J=1.8,6.6Hz,1H),7.81(s,1H),7.94(d,J=1.8Hz,1H),8.46(s,1H),9.41(s,1H)。元素分析C43H39ClF3N5O3S2:理论值:C,62.20;H,4.73;N,8.43.测定值:C,62.41;H,4.97;N,8.29。According to the method described in Example 6, except that the raw material N-methylpiperazine was changed to 1-[bis(4-fluorophenyl)methyl]piperazine, the yield was 45%, and the mp was 151-153°C. 1 H NMR (400MHz, DMSO-d 6 ): δ=2.09(s, 1H), 2.21(t, J=5.1Hz, 2H), 2.36(t, J=3.3Hz, 4H), 3.42(t, J =5.4Hz, 2H), 3.93(s, 4H), 4.21(t, J=4.5Hz, 4H), 4.46(s, 1H), 5.26(s, 2H), 7.12-7.21(m, 6H), 7.26 -7.35(m, 3H), 7.42-7.50(m, 5H), 7.70(dd, J=1.8, 6.6Hz, 1H), 7.81(s, 1H), 7.94(d, J=1.8Hz, 1H), 8.46(s, 1H), 9.41(s, 1H). Elemental analysis C 43 H 39 ClF 3 N 5 O 3 S 2 : Theoretical: C, 62.20; H, 4.73; N, 8.43. Found: C, 62.41; H, 4.97; N, 8.29.
制备实施例20 5-硝基靛红Preparation Example 20 5-Nitroisatin
将靛红(5.88g,40mmol)溶于25.2mL浓硫酸中,冰浴下搅拌,缓慢滴加1.88mL发烟硝酸,0℃下反应45min,反应完毕,将反应液倒入碎冰中,析出桔红色固体,抽滤,用水洗至中性,烘干,得6.3g桔红色固体,收率81%,m.p.253-254℃。Dissolve isatin (5.88g, 40mmol) in 25.2mL concentrated sulfuric acid, stir under ice bath, slowly add 1.88mL fuming nitric acid dropwise, and react at 0°C for 45min. After the reaction is complete, pour the reaction solution into crushed ice to precipitate The orange-red solid was suction-filtered, washed with water until neutral, and dried to obtain 6.3 g of orange-red solid, yield 81%, m.p.253-254°C.
制备实施例21 2-氨基-5-硝基苯甲酸Preparation Example 21 2-amino-5-nitrobenzoic acid
将5-硝基靛红(1.92g,10mmol)溶于50mL 1N氢氧化钠水溶液中,滴加3.2mL双氧水,滴毕,10~15℃下反应30min,反应完毕,将反应液抽滤,滤去不溶物,滤液用3N盐酸溶液调至pH=5~6,析出大量黄色固体,抽滤,烘干,得1.24g黄色固体,收率68%,m.p.275-276℃。1H NMR(300MHz,DMSO-d6):δ=6.87(d,J=9.3Hz,1H),7.88(s,2H),8.08(dd,J=3.0,9.3Hz,1H),8.59(d,J=2.7Hz,1H),13.29(br s,1H)。Dissolve 5-nitroisatin (1.92g, 10mmol) in 50mL of 1N aqueous sodium hydroxide solution, add 3.2mL of hydrogen peroxide dropwise, and react at 10-15°C for 30min. Insoluble matter was removed, and the filtrate was adjusted to pH=5-6 with 3N hydrochloric acid solution, a large amount of yellow solid was precipitated, filtered by suction, and dried to obtain 1.24 g of yellow solid, yield 68%, mp275-276°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=6.87(d, J=9.3Hz, 1H), 7.88(s, 2H), 8.08(dd, J=3.0, 9.3Hz, 1H), 8.59(d , J=2.7Hz, 1H), 13.29 (br s, 1H).
制备实施例22 4-羟基-6-硝基喹唑啉Preparation Example 22 4-hydroxyl-6-nitroquinazoline
将2-氨基-5-硝基苯甲酸(0.91g,5mmol)加入5mL甲酰胺中,150℃下反应6h,将反应液冷至室温,往反应瓶中加入30mL乙酸乙酯,抽滤,除去不溶物,滤液用乙酸乙酯-水混合体系萃取,合并有机层,无水硫酸钠干燥,浓缩,剩余物用甲醇-水重结晶,得0.60g黄色固体,收率62%,m.p.284-286℃。1H NMR(300MHz,DMSO-d6):δ=7.87(d,J=9.0Hz,1H),8.32(s,1H),8.56(dd,J=2.7,9.0Hz,1H),8.81(d,J=2.7Hz,1H),12.77(s,1H)。Add 2-amino-5-nitrobenzoic acid (0.91g, 5mmol) into 5mL formamide, react at 150°C for 6h, cool the reaction solution to room temperature, add 30mL ethyl acetate to the reaction flask, suction filter, remove Insoluble matter, the filtrate was extracted with ethyl acetate-water mixed system, the organic layers were combined, dried over anhydrous sodium sulfate, concentrated, and the residue was recrystallized with methanol-water to obtain 0.60g of yellow solid, yield 62%, mp284-286℃ . 1 H NMR (300MHz, DMSO-d 6 ): δ=7.87(d, J=9.0Hz, 1H), 8.32(s, 1H), 8.56(dd, J=2.7, 9.0Hz, 1H), 8.81(d , J=2.7Hz, 1H), 12.77(s, 1H).
制备实施例23 4-(3-氯-4-氟苯氨基)-6-硝基喹唑啉Preparation Example 23 4-(3-chloro-4-fluoroanilino)-6-nitroquinazoline
将4-羟基-6-硝基喹唑啉(0.96g,5mmol)加入10mL二氯亚砜中,滴入0.5mL DMF,回流反应6h,减压蒸除过量二氯亚砜,得中间体4-氯-6-硝基-喹唑啉,不作分离,将中间体溶于15mL异丙醇中,加入3-氯-4-氟苯胺(0.73g,5mmol),1mL三乙胺,回流反应4h,反应液冷却至室温,抽滤,滤饼依次用异丙醇、水、乙醚洗涤,烘干,得1.24g浅黄色固体,两步总收率87%,m.p.244-246℃。4-Hydroxy-6-nitroquinazoline (0.96g, 5mmol) was added in 10mL of thionyl chloride, dripped into 0.5mL of DMF, refluxed for 6h, and the excess thionyl chloride was evaporated under reduced pressure to obtain intermediate 4 -Chloro-6-nitro-quinazoline, without separation, the intermediate was dissolved in 15 mL of isopropanol, added 3-chloro-4-fluoroaniline (0.73 g, 5 mmol), 1 mL of triethylamine, and refluxed for 4 h , the reaction solution was cooled to room temperature, filtered with suction, and the filter cake was washed with isopropanol, water, and ether in sequence, and dried to obtain 1.24 g of light yellow solid, the total yield of two steps was 87%, m.p.244-246°C.
制备实施例24 4-(3-氯-4-氟苯氨基)-6-氨基喹唑啉Preparation Example 24 4-(3-chloro-4-fluoroanilino)-6-aminoquinazoline
将4-(3-氯-4-氟苯氨基)-6-硝基喹唑啉(0.64g,2mmol)溶于30mL无水乙醇中,加入锡粉(1.19g,10mmol),室温下搅拌,加入10mL浓盐酸,继续室温下反应12h,往反应液中加入50ml乙酸乙酯和50mL水,剧烈搅拌,用1N氢氧化钠水溶液将混合液的pH调至8~9,抽滤,除去不溶物,分液,水层用乙酸乙酯(15mL×2)萃取,合并有机层,无水硫酸钠干燥,浓缩,硅胶柱层析分离(洗脱剂,石油醚∶乙酸乙酯=1∶2,v∶v),得0.38g黄色固体,收率65%,m.p.260-262℃。Dissolve 4-(3-chloro-4-fluoroanilino)-6-nitroquinazoline (0.64g, 2mmol) in 30mL of absolute ethanol, add tin powder (1.19g, 10mmol), stir at room temperature, Add 10mL of concentrated hydrochloric acid, continue to react at room temperature for 12h, add 50ml of ethyl acetate and 50mL of water to the reaction solution, stir vigorously, adjust the pH of the mixture to 8-9 with 1N aqueous sodium hydroxide solution, and filter with suction to remove insoluble matter , separated, the aqueous layer was extracted with ethyl acetate (15mL×2), the organic layers were combined, dried over anhydrous sodium sulfate, concentrated, and separated by silica gel column chromatography (eluent, petroleum ether: ethyl acetate=1:2, v:v), 0.38 g of yellow solid was obtained, the yield was 65%, m.p.260-262°C.
制备实施例25 4-(3-氯-4-氟苯氨基)-6-(3-溴丙酰胺基)喹唑啉Preparation Example 25 4-(3-chloro-4-fluoroanilino)-6-(3-bromopropionamido)quinazoline
将4-(3-氯-4-氟苯氨基)-6-氨基喹唑啉(0.58g,2mmol)溶于20mL四氢呋喃中,加入1mL三乙胺,冰浴下滴加3-溴丙酰氯(0.69g,4mmol),滴毕,室温反应2h,向反应体系中加入15mL水,用乙酸乙酯萃取(15mL×3),合并有机层,无水硫酸钠干燥,浓缩,硅胶柱层析分离(洗脱剂,石油醚∶乙酸乙酯=1∶1,v∶v),得0.45g黄色固体,收率53%。4-(3-Chloro-4-fluoroanilino)-6-aminoquinazoline (0.58g, 2mmol) was dissolved in 20mL of tetrahydrofuran, 1mL of triethylamine was added, and 3-bromopropionyl chloride ( 0.69g, 4mmol), dropwise, reacted at room temperature for 2h, added 15mL water to the reaction system, extracted with ethyl acetate (15mL×3), combined organic layers, dried over anhydrous sodium sulfate, concentrated, and separated by silica gel column chromatography ( Eluent, petroleum ether: ethyl acetate = 1:1, v:v), to obtain 0.45 g of yellow solid, yield 53%.
实施例12 4-甲基哌嗪-1-二硫代甲酸-[2-[4-(3-氯-4-氟苯氨基)喹唑啉-6-氨基甲酰基]]乙酯Example 12 4-methylpiperazine-1-dithioformic acid-[2-[4-(3-chloro-4-fluoroanilino)quinazoline-6-carbamoyl]]ethyl ester
将N-甲基哌嗪(0.10g,1mmol)溶于15mL DMF中,加入无水磷酸钾(0.43g,2mmol),室温搅拌下滴入二硫化碳(0.19g,2.5mmol),反应30min后,加入4-(3-氯-4-氟苯氨基)-6-(3-溴丙酰胺基)喹唑啉(0.43g,1mmol),继续室温搅拌,TLC检测直至反应结束。向反应体系中加入15mL水,用乙酸乙酯萃取(15mL×3),合并有机层,水洗(15mL×2),无水硫酸钠干燥,浓缩,硅胶柱层析分离(洗脱剂,乙酸乙酯∶甲醇=15∶1,v∶v),得到0.25g固体,收率48%,m.p.236-237℃。1H NMR(300MHz,DMSO-d6):δ=2.19(s,3H),2.37(s,4H),2.87(t,J=6.0Hz,2H),3.57(t,J=5.4Hz,2H),3.88(s,2H),4.23(s,2H),7.39-7.46(m,1H),7.78-7.79(m,3H),8.12(d,J=6.9Hz,1H),8.54(d,J=1.8Hz,1H),8.74(s,1H),9.93(s,1H),10.36(s,1H)。元素分析C23H24ClFN6OS2:理论值:C,53.22;H,4.66;N,16.19.测定值:C,53.20;H,4.68;N,16.16。Dissolve N-methylpiperazine (0.10g, 1mmol) in 15mL DMF, add anhydrous potassium phosphate (0.43g, 2mmol), add carbon disulfide (0.19g, 2.5mmol) dropwise under stirring at room temperature, react for 30min, add 4-(3-Chloro-4-fluoroanilino)-6-(3-bromopropionamido)quinazoline (0.43 g, 1 mmol) was stirred at room temperature, and detected by TLC until the reaction was completed. Add 15 mL of water to the reaction system, extract with ethyl acetate (15 mL × 3), combine the organic layers, wash with water (15 mL × 2), dry over anhydrous sodium sulfate, concentrate, and separate by silica gel column chromatography (eluent, ethyl acetate Ester:methanol=15:1, v:v), 0.25 g of solid was obtained, yield 48%, mp 236-237°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.19(s, 3H), 2.37(s, 4H), 2.87(t, J=6.0Hz, 2H), 3.57(t, J=5.4Hz, 2H ), 3.88(s, 2H), 4.23(s, 2H), 7.39-7.46(m, 1H), 7.78-7.79(m, 3H), 8.12(d, J=6.9Hz, 1H), 8.54(d, J=1.8Hz, 1H), 8.74(s, 1H), 9.93(s, 1H), 10.36(s, 1H). Elemental analysis C 23 H 24 ClFN 6 OS 2 : Theoretical: C, 53.22; H, 4.66; N, 16.19. Found: C, 53.20; H, 4.68; N, 16.16.
制备实施例26 4-羟基-7-氯喹唑啉Preparation Example 26 4-hydroxyl-7-chloroquinazoline
将4-氯-2-氨基苯甲酸(1.72g,10mmol)加入10mL甲酰胺中,150℃下反应5h,反应液冷却至室温,析出大量固体,往反应液中加入10mL水,抽滤,烘干,得1.65g灰白色固体,收率91%,m.p.248-250℃。1H NMR(300MHz,DMSO-d6):δ=7.56(d,J=7.8Hz,1H),7.73(s,1H),8.10-8.15(m,2H),12.40(s,1H)。Add 4-chloro-2-aminobenzoic acid (1.72g, 10mmol) into 10mL formamide, react at 150°C for 5h, cool the reaction solution to room temperature, and a large amount of solid precipitates, add 10mL water to the reaction solution, suction filter, and dry After drying, 1.65 g of off-white solid was obtained, yield 91%, mp 248-250°C. 1 H NMR (300 MHz, DMSO-d 6 ): δ=7.56 (d, J=7.8 Hz, 1H), 7.73 (s, 1H), 8.10-8.15 (m, 2H), 12.40 (s, 1H).
制备实施例27 4-羟基-6-硝基-7-氯喹唑啉Preparation Example 27 4-hydroxyl-6-nitro-7-chloroquinazoline
将4-羟基-7-氯喹唑啉(1.60g,8.86mmol)在冰浴下缓慢加入到浓硫酸(4mL)和发烟硝酸(4mL)组成混酸中,加毕,升温至90℃反应3h,反应完毕,冷却至室温,将反应液倒入100mL冰水中,析出大量淡黄色固体,抽滤,烘干,得1.80g淡黄色固体,收率90%,m.p.>300℃。1H NMR(300MHz,DMSO-d6):δ=8.05(s,1H),8.33(s,1H),8.69(s,1H),12.80(s,1H)。4-Hydroxy-7-chloroquinazoline (1.60g, 8.86mmol) was slowly added to the mixed acid composed of concentrated sulfuric acid (4mL) and fuming nitric acid (4mL) in an ice bath. After the reaction was completed, cool to room temperature, pour the reaction solution into 100 mL of ice water, a large amount of light yellow solid was precipitated, filtered by suction, and dried to obtain 1.80 g of light yellow solid, yield 90%, mp>300°C. 1 H NMR (300 MHz, DMSO-d 6 ): δ=8.05 (s, 1H), 8.33 (s, 1H), 8.69 (s, 1H), 12.80 (s, 1H).
制备实施例28 4-羟基-6-硝基-7-甲氧基喹唑啉Preparation Example 28 4-Hydroxy-6-nitro-7-methoxyquinazoline
在冰浴条件下,将金属钠(0.41g,17.64mmol)加入到20mL无水甲醇中,搅拌直至钠完全溶解,将4-(3-氯-4-氟苯氨基)-6-硝基喹唑啉(1.26g,5,58ml)的DMF(20ml)溶液加入到反应体系中,再加入0.98g(5.58mmol)碘化钾,反应液在90℃下反应24h,反应完毕,用醋酸将反应液pH调至7左右,将20mL水加入到反应液中,搅拌,出现大量黄色沉淀,抽滤,烘干,得0.91g黄色固体,收率73%,m.p.240-242℃。Under ice-bath conditions, metal sodium (0.41g, 17.64mmol) was added to 20mL of anhydrous methanol, stirred until the sodium was completely dissolved, and 4-(3-chloro-4-fluorophenylamino)-6-nitroquine The DMF (20ml) solution of oxazoline (1.26g, 5,58ml) was added to the reaction system, and then 0.98g (5.58mmol) potassium iodide was added, and the reaction solution was reacted at 90°C for 24h. After the reaction was completed, the pH of the reaction solution was adjusted with acetic acid Adjust to about 7, add 20mL of water into the reaction solution, stir, a large amount of yellow precipitate appears, filter with suction, and dry to obtain 0.91g of yellow solid, yield 73%, m.p.240-242°C.
制备实施例29 4-(3-氯-4-氟苯氨基)-6-硝基-7-甲氧基喹唑啉Preparation Example 29 4-(3-chloro-4-fluoroanilino)-6-nitro-7-methoxyquinazoline
按照制备实施例23所述的方法,将原料4-羟基-6-硝基喹唑啉改为4-羟基-6-硝基-7-甲氧基喹唑啉,收率45%,m.p.234-236℃。1H NMR(300MHz,DMSO-d6):δ=4.11(s,1H),7.53-7.59(m,1H),7.62-7.64(m,1H),7.75-7.76(m,1H),8.06-8.08(m,1H),8.95(s,1H),9.58-9.63(m,1H),11.71(s,1H)。According to the method described in Preparation Example 23, the raw material 4-hydroxyl-6-nitroquinazoline was changed to 4-hydroxyl-6-nitro-7-methoxyquinazoline, the yield was 45%, mp234- 236°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=4.11(s, 1H), 7.53-7.59(m, 1H), 7.62-7.64(m, 1H), 7.75-7.76(m, 1H), 8.06- 8.08 (m, 1H), 8.95 (s, 1H), 9.58-9.63 (m, 1H), 11.71 (s, 1H).
制备实施例30 4-(3-氯-4-氟苯氨基)-6-氨基-7-甲氧基喹唑啉Preparation Example 30 4-(3-chloro-4-fluoroanilino)-6-amino-7-methoxyquinazoline
按照制备实施例24所述的方法,只是将原料4-(3-氯-4-氟苯氨基)-6-硝基喹唑啉改为4-(3-氯-4-氟苯氨基)-6-硝基-7-甲氧基喹唑啉,收率61%,m.p.260-262℃。According to the method described in Preparation Example 24, except that the starting material 4-(3-chloro-4-fluorophenylamino)-6-nitroquinazoline was changed to 4-(3-chloro-4-fluorophenylamino)- 6-nitro-7-methoxyquinazoline, yield 61%, m.p.260-262°C.
制备实施例31 4-(3-氯-4-氟苯氨基)-6-(3-溴丙酰胺基)-7-甲氧基喹唑啉Preparation Example 31 4-(3-chloro-4-fluoroanilino)-6-(3-bromopropionamido)-7-methoxyquinazoline
按照制备实施例25所述的方法,只是将原料4-(3-氯-4-氟苯氨基)-6-氨基喹唑啉改为4-(3-氯-4-氟苯氨基)-6-氨基-7-甲氧基喹唑啉,收率47%。According to the method described in Preparation Example 25, except that the starting material 4-(3-chloro-4-fluoroanilino)-6-aminoquinazoline was changed to 4-(3-chloro-4-fluorophenylamino)-6 -Amino-7-methoxyquinazoline, yield 47%.
实施例13 4-甲基哌嗪-1-二硫代甲酸-[2-[4-(3-氯-4-氟苯氨基)-7-甲氧基喹唑啉-6-氨甲酰]]乙酯Example 13 4-methylpiperazine-1-dithioformic acid-[2-[4-(3-chloro-4-fluoroanilino)-7-methoxyquinazoline-6-carbamoyl] ] ethyl ester
按照实施例12所述的方法,只是将原料4-(3-氯-4-氟苯氨基)-6-(3-溴丙酰胺基)喹唑啉改为4-(3-氯-4-氟苯氨基)-6-(3-溴丙酰胺基)-7-甲氧基喹唑啉,收率43%,m.p.242-244℃。1H NMR(300MHz,DMSO-d6):δ=2.21(s,3H),2.40(s,4H),2.93(s,2H),3.56(t,J=7.2Hz,2H),3.90-4.00(m,5H),4.24(s,2H),7.28(s 1H),7.42(t,J=8.7Hz,1H),7.78-7.79(m,1H),8.12(d,J=5.1Hz,1H),8.53(s,1H),8.53(s,1H),9.63(s,1H),9.82(s,1H)。HRMS:C24H26ClFN6O2S2+H,理论值549.13095;测定值(ESI-FTMS,(M+H)+1),549.12920。According to the method described in Example 12, just change the raw material 4-(3-chloro-4-fluoroanilino)-6-(3-bromopropionamido)quinazoline into 4-(3-chloro-4- Fluoroanilino)-6-(3-bromopropionamido)-7-methoxyquinazoline, yield 43%, mp242-244°C. 1 H NMR (300MHz, DMSO-d 6 ): δ=2.21(s, 3H), 2.40(s, 4H), 2.93(s, 2H), 3.56(t, J=7.2Hz, 2H), 3.90-4.00 (m, 5H), 4.24(s, 2H), 7.28(s 1H), 7.42(t, J=8.7Hz, 1H), 7.78-7.79(m, 1H), 8.12(d, J=5.1Hz, 1H ), 8.53(s, 1H), 8.53(s, 1H), 9.63(s, 1H), 9.82(s, 1H). HRMS: C 24 H 26 ClFN 6 O 2 S 2 +H, calc. 549.13095; found (ESI-FTMS, (M+H) +1 ), 549.12920.
制备实施例32 4-羟基-6-甲基喹唑啉Preparation Example 32 4-hydroxyl-6-methylquinazoline
将2-氨基-5-甲基苯甲酸(7.20g,48mmol)和甲酰胺(7.6mL,192mmol)混合,加热至130-135℃,回流反应4h。反应结束后滴入1mL水破坏反应,温度降至60℃时再加入20mL水,冷却至室温,继续搅拌30min。抽滤,水洗,得灰白色固体。甲醇重结晶,得到白色絮状固体5.50g,收率77%,m.p.266-267℃。1H NMR(300MHz,DMSO-d6):δ2.44(s,3H),7.57(d,J=8.4Hz,1H),7.64(dd,J=8.25Hz,1.2Hz,1H),7.92(d,J=0.9Hz,1H),8.03(s,1H),12.17(br,1H)。Mix 2-amino-5-methylbenzoic acid (7.20g, 48mmol) and formamide (7.6mL, 192mmol), heat to 130-135°C, and reflux for 4h. After the reaction was completed, 1 mL of water was added dropwise to destroy the reaction, and when the temperature dropped to 60°C, 20 mL of water was added, cooled to room temperature, and continued to stir for 30 min. Suction filtration and washing with water gave off-white solid. Methanol was recrystallized to obtain 5.50 g of white flocculent solid, yield 77%, mp 266-267°C. 1 H NMR (300MHz, DMSO-d 6 ): δ2.44(s, 3H), 7.57(d, J=8.4Hz, 1H), 7.64(dd, J=8.25Hz, 1.2Hz, 1H), 7.92( d, J = 0.9 Hz, 1H), 8.03 (s, 1H), 12.17 (br, 1H).
制备实施例33 4-氯-6-甲基喹唑啉Preparation Example 33 4-chloro-6-methylquinazoline
将4-羟基-6-甲基喹唑啉(3.12g,21mmol)加入二氯亚砜(52mL)中,滴入催化量的DMF(1mL),加热回流反应3h。反应结束后,减压蒸除二氯亚砜,剩余物利用硅胶柱分离纯化(洗脱剂,石油醚∶乙酸乙酯=3∶1,v∶v),得黄色固体化合物1.36g,收率36%,m.p.107-108℃。1H NMR(300MHz,CDCl3):δ2.62(s,3H),7.81(dd,J=8.5Hz,1.5Hz,1H),7.98(d,J=8.7Hz,1H),8.04(s,1H),8.99(s,1H)。4-Hydroxy-6-methylquinazoline (3.12g, 21mmol) was added into thionyl chloride (52mL), a catalytic amount of DMF (1mL) was added dropwise, and heated under reflux for 3h. After the reaction, thionyl chloride was distilled off under reduced pressure, and the residue was separated and purified by silica gel column (eluent, petroleum ether: ethyl acetate = 3:1, v:v) to obtain 1.36 g of yellow solid compound, the yield 36%, mp 107-108°C. 1 H NMR (300MHz, CDCl 3 ): δ2.62(s, 3H), 7.81(dd, J=8.5Hz, 1.5Hz, 1H), 7.98(d, J=8.7Hz, 1H), 8.04(s, 1H), 8.99 (s, 1H).
制备实施例34 6-溴甲基-4-氯-喹唑啉Preparation Example 34 6-bromomethyl-4-chloro-quinazoline
将4-氯-6-甲基喹唑啉(0.89g,5mmol)和N-溴代琥珀酰亚胺(NBS)(0.89g,5mmol)溶于CCl4(10mL)中,加入催化量的过氧化苯甲酰,加热回流0.5h。TLC检测反应结束后,冷却至室温,过滤,减压蒸除溶剂,粗产物经硅胶柱分离纯化(洗脱剂,石油醚∶乙酸乙酯=15∶1,v∶v),得白色固体0.60g,收率45%,m.p.118-120℃。1H NMR(300MHz,CDCl3):δ4.69(s,2H),8.01(d,J=8.7Hz,2.1Hz,1H),8.09(d,J=8.4Hz,1H),8.26(d,J=1.5Hz,1H),9.07(s,1H)。4-Chloro-6-methylquinazoline (0.89 g, 5 mmol) and N-bromosuccinimide (NBS) (0.89 g, 5 mmol) were dissolved in CCl 4 (10 mL), and a catalytic amount of Benzoyl oxide was heated to reflux for 0.5h. After the reaction was detected by TLC, it was cooled to room temperature, filtered, and the solvent was evaporated under reduced pressure. The crude product was separated and purified by silica gel column (eluent, petroleum ether:ethyl acetate=15:1, v:v) to obtain a white solid 0.60 g, yield 45%, mp118-120°C. 1 H NMR (300MHz, CDCl 3 ): δ4.69(s, 2H), 8.01(d, J=8.7Hz, 2.1Hz, 1H), 8.09(d, J=8.4Hz, 1H), 8.26(d, J=1.5Hz, 1H), 9.07(s, 1H).
制备实施例35 4-氯-6-(邻苯二甲酰亚胺甲基)喹唑啉Preparation Example 35 4-Chloro-6-(phthalimide methyl) quinazoline
将邻苯二甲酰亚胺的钾盐(0.74g,4mmol)溶于丙酮(10mL)中,加热至回流,滴入6-溴甲基-4-氯-喹唑啉(1.03g,4mmol)的丙酮溶液,继续回流1h。TLC检测原料消失,停止反应,冷却至室温,过滤,滤饼为目标化合物,滤液浓缩后利用硅胶柱分离纯化(洗脱剂,石油醚∶乙酸乙酯=1∶1,v∶v),得白色固体1.20g,收率92%,m.p.212-214℃。1H NMR(300MHz,CDCl3):δ5.09(s,2H),7.74-7.77(m,2H),7.88-7.91(m,2H),8.06(s,2H),8.34(s,1H),9.04(s,1H)。EI-MS:[M+]=323.1.(分子式:C17H10ClN3O2,分子量:323.733)The potassium salt of phthalimide (0.74g, 4mmol) was dissolved in acetone (10mL), heated to reflux, and 6-bromomethyl-4-chloro-quinazoline (1.03g, 4mmol) was added dropwise acetone solution, continue to reflux for 1h. TLC detects that the raw material disappears, stop the reaction, cool to room temperature, filter, the filter cake is the target compound, and the filtrate is concentrated and purified by silica gel column separation and purification (eluent, petroleum ether:ethyl acetate=1:1, v:v), to obtain White solid 1.20g, yield 92%, mp212-214°C. 1 H NMR (300MHz, CDCl 3 ): δ5.09(s, 2H), 7.74-7.77(m, 2H), 7.88-7.91(m, 2H), 8.06(s, 2H), 8.34(s, 1H) , 9.04(s, 1H). EI-MS: [M+]=323.1. (Molecular formula: C 17 H 10 ClN 3 O 2 , molecular weight: 323.733)
制备实施例36 4-[3-氯-4-(3-氟苄氧基)苯氨基]-6-(邻苯二甲酰亚胺甲基)喹唑啉Preparation Example 36 4-[3-Chloro-4-(3-fluorobenzyloxy)anilino]-6-(phthalimidemethyl)quinazoline
将制备实施例35产物(1mmol)和3-氯-4-(3-氟苄氧基)苯胺(1mmol)混合加入异丙醇(2mL)中,加热至回流,有固体析出,停止反应,冷却,过滤,干燥,得黄色固体产物,收率77%,m.p.259-262℃。1H NMR(300MHz,DMSO-d6):δ5.01(s,2H),5.30(s,2H),7.17-7.22(m,1H),7.30-7.35(m,3H),7.44-7.57(m,2H),7.82(d,J=5.7Hz,1H),7.87-7.97(m,5H),8.07-8.10(d,J=8.7Hz,1H),8.64(s,1H),8.90(s,1H),11.49(br,1H)。Mix the product of Preparation Example 35 (1mmol) and 3-chloro-4-(3-fluorobenzyloxy)aniline (1mmol) into isopropanol (2mL), heat to reflux, a solid precipitates out, stop the reaction, and cool , filtered and dried to obtain a yellow solid product with a yield of 77%, mp259-262°C. 1 H NMR (300MHz, DMSO-d 6 ): δ5.01(s, 2H), 5.30(s, 2H), 7.17-7.22(m, 1H), 7.30-7.35(m, 3H), 7.44-7.57( m, 2H), 7.82(d, J=5.7Hz, 1H), 7.87-7.97(m, 5H), 8.07-8.10(d, J=8.7Hz, 1H), 8.64(s, 1H), 8.90(s , 1H), 11.49 (br, 1H).
制备实施例37 2-[4-(3-氯-4-氟苯氨基)-6-(邻苯二甲酰亚胺甲基)喹唑啉Preparation Example 37 2-[4-(3-Chloro-4-fluoroanilino)-6-(phthalimidemethyl)quinazoline
按照制备实施例36所述的方法,只是将原料3-氯-4-(3-氟苄氧基)苯胺改为3-氯-4-氟苯胺,收率81%,m.p.267-268℃。1H NMR(300MHz,DMSO-d6):δ4.98(s,2H),7.45(t,J=9.0Hz,1H),7.74-7.95(m,7H),8.13(d,J=6.0Hz,1H),8.44(s,1H),8.61(s,1H),9.98(br,1H)。Following the method described in Preparation Example 36, except that the starting material 3-chloro-4-(3-fluorobenzyloxy)aniline was changed to 3-chloro-4-fluoroaniline, the yield was 81%, mp 267-268°C. 1 H NMR (300MHz, DMSO-d 6 ): δ4.98(s, 2H), 7.45(t, J=9.0Hz, 1H), 7.74-7.95(m, 7H), 8.13(d, J=6.0Hz , 1H), 8.44 (s, 1H), 8.61 (s, 1H), 9.98 (br, 1H).
制备实施例38 2-[4-(3-溴苯氨基)6-(邻苯二甲酰亚胺甲基)喹唑啉Preparation Example 38 2-[4-(3-bromoanilino) 6-(phthalimide methyl) quinazoline
按照制备实施例36所述的方法,只是将原料3-氯-4-(3-氟苄氧基)苯胺改为间溴苯胺,收率92%,m.p.236-237℃。1H NMR(300MHz,DMSO-d6):δ5.02(s,2H),7.43-7.56(m,2H),7.68(d,J=8.1Hz,1H),7.87-7.98(m,6H),8.10(d,J=8.7Hz,1H),8.70(s,1H),8.96(s,1H),11.57(br,1H)。Following the method described in Preparation Example 36, except that the starting material 3-chloro-4-(3-fluorobenzyloxy)aniline was changed to m-bromoaniline, the yield was 92%, mp 236-237°C. 1 H NMR (300MHz, DMSO-d 6 ): δ5.02(s, 2H), 7.43-7.56(m, 2H), 7.68(d, J=8.1Hz, 1H), 7.87-7.98(m, 6H) , 8.10 (d, J=8.7Hz, 1H), 8.70 (s, 1H), 8.96 (s, 1H), 11.57 (br, 1H).
实施例14 {4-[3-氯-4-(3-氟苄氧基)苯氨基]喹唑啉基}-6-甲氨基二硫代甲酸-2-[2-(1,3-二氧环戊基)]乙酯Example 14 {4-[3-chloro-4-(3-fluorobenzyloxy)anilino]quinazolinyl}-6-methylaminodithioformic acid-2-[2-(1,3-di Oxycyclopentyl)] ethyl ester
将制备实施例36的产物(0.20g)溶于甲醇(2mL)中,搅拌均匀后滴入甲胺醇溶液(10mL),搅拌10min,加甲醇至反应液澄清,室温搅拌过夜。反应结束后,减压蒸除溶剂,剩余物用3N盐酸(5mL)溶液酸化,有固体析出,加水至固体全溶,乙酸乙酯萃取(20mL×2),水相用1N氢氧化钠溶液碱化至pH 7-8,乙酸乙酯萃取(20mL×3),合并碱化后乙酸乙酯层,饱和食盐水洗(30mL),无水硫酸钠干燥,过滤,减压蒸干得固体。固体(1mmol)溶于DMF(2mL)中,滴入Et3N(2mmol),搅拌10min后,滴入CS2(4mmol),搅拌15min后,滴入2-(2-溴乙基)-1,3-二氧戊环(1.2mmol),室温搅拌过夜。反应结束后,加入过量水(50mL),用乙酸乙酯萃取(30mL×3),合并有机相,饱和食盐水洗(30mL),无水硫酸钠干燥,过滤,滤液浓缩后利用硅胶柱分离纯化(洗脱剂,石油醚∶乙酸乙酯=1∶1.5,v∶v),得标题产物,收率35%,m.p.158-160℃。1H NMR(300MHz,DMSO-d6):δ1.95-2.04(m,2H),3.31(t,J=7.5Hz,2H),3.80-3.97(m,4H),4.92(t,J=7.5Hz,1H),5.05(d,J=5.1Hz,2H),5.31(s,2H),7.20-7.39(m,4H),7.48-7.56(m,1H),7.77-7.82(m,3H),8.07(d,J=2.1Hz,1H),8.50(s,1H),8.63(s,1H),9.88(br,1H),10.57(br,1H)。元素分析C28H26ClFN4O3S2:理论值:C,57.48;H,4.48;N,9.58.测定值:C,57.32;H,4.75;N,9.43。The product of Preparation Example 36 (0.20 g) was dissolved in methanol (2 mL), stirred evenly, and added dropwise into methylamino alcohol solution (10 mL), stirred for 10 min, methanol was added until the reaction solution was clear, and stirred overnight at room temperature. After the reaction, the solvent was evaporated under reduced pressure, and the residue was acidified with 3N hydrochloric acid (5mL) solution, and a solid precipitated, and water was added until the solid was completely dissolved, extracted with ethyl acetate (20mL×2), and the aqueous phase was alkalinized with 1N sodium hydroxide solution. The mixture was adjusted to pH 7-8, extracted with ethyl acetate (20 mL×3), combined and basified ethyl acetate layers were washed with saturated brine (30 mL), dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure to obtain a solid. The solid (1mmol) was dissolved in DMF (2mL), Et 3 N (2mmol) was added dropwise, after stirring for 10min, CS 2 (4mmol) was added dropwise, after stirring for 15min, 2-(2-bromoethyl)-1 , 3-dioxolane (1.2 mmol), stirred overnight at room temperature. After the reaction was completed, excess water (50 mL) was added, extracted with ethyl acetate (30 mL×3), the organic phases were combined, washed with saturated brine (30 mL), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated and purified by silica gel column separation and purification ( Eluent, petroleum ether: ethyl acetate = 1:1.5, v:v), the title product was obtained, yield 35%, mp 158-160°C. 1 H NMR (300MHz, DMSO-d 6 ): δ1.95-2.04(m, 2H), 3.31(t, J=7.5Hz, 2H), 3.80-3.97(m, 4H), 4.92(t, J= 7.5Hz, 1H), 5.05(d, J=5.1Hz, 2H), 5.31(s, 2H), 7.20-7.39(m, 4H), 7.48-7.56(m, 1H), 7.77-7.82(m, 3H ), 8.07 (d, J=2.1 Hz, 1H), 8.50 (s, 1H), 8.63 (s, 1H), 9.88 (br, 1H), 10.57 (br, 1H). Elemental analysis C 28 H 26 ClFN 4 O 3 S 2 : Theoretical: C, 57.48; H, 4.48; N, 9.58. Found: C, 57.32; H, 4.75; N, 9.43.
实施例15 [4-(3-氯-4-氟苯氨基)喹唑啉基]-6-甲氨基二硫代甲酸-2-[2-(1,3-二氧环戊基)]乙酯Example 15 [4-(3-chloro-4-fluorophenylamino)quinazolinyl]-6-methylaminodithioformic acid-2-[2-(1,3-dioxolyl)]B ester
按照实施例14所述的方法,只是将原料制备实施例36的产物改为制备实施例37的产物,收率40%,m.p.162-163℃。1H NMR(300MHz,DMSO-d6):δ1.90-1.97(m,2H),3.26(t,J=7.5Hz,2H),3.75-3.92(m,4H),4.88(t,J=7.5Hz,1H),5.01(d,J=5.4Hz,2H),7.47(t,J=9.0Hz,1H),7.80(s,3H),8.19-8.21(m,1H),8.47(s,1H),8.64(s,1H),9.96(br,1H),10.54(br,1H)。元素分析C21H20ClFN4O2S2:理论值:C,52.66;H,7.21;N,11.70.测定值:C,52.51;H,4.233;N,11.36。According to the method described in Example 14, only the product of Example 36 was changed from the raw material to the product of Example 37, the yield was 40%, mp 162-163°C. 1 H NMR (300MHz, DMSO-d 6 ): δ1.90-1.97(m, 2H), 3.26(t, J=7.5Hz, 2H), 3.75-3.92(m, 4H), 4.88(t, J= 7.5Hz, 1H), 5.01(d, J=5.4Hz, 2H), 7.47(t, J=9.0Hz, 1H), 7.80(s, 3H), 8.19-8.21(m, 1H), 8.47(s, 1H), 8.64 (s, 1H), 9.96 (br, 1H), 10.54 (br, 1H). Elemental analysis C 21 H 20 ClFN 4 O 2 S 2 : Theoretical: C, 52.66; H, 7.21; N, 11.70. Found: C, 52.51; H, 4.233; N, 11.36.
实施例16 [4-(3-溴苯氨基)喹唑啉基]-6-甲氨基二硫代甲酸-2-[2-(1,3-二氧环戊基)]乙酯Example 16 [4-(3-bromoanilino)quinazolinyl]-6-methylaminodithioformic acid-2-[2-(1,3-dioxolyl)]ethyl ester
按照实施例14所述的方法,只是将原料制备实施例36的产物改为制备实施例38的产物,收率36%,m.p.90-92℃。1H NMR(300MHz,DMSO-d6):δ1.90-1.99(m,2H),3.26(t,J=7.5Hz,2H),3.75-3.92(m,4H),4.88(t,J=7.5Hz,1H),5.02(d,J=5.4Hz,2H),7.30-7.40(m,2H),7.80(s,2H),7.93(t,J=6.9Hz,1H),8.23(s,1H),8.51(s,1H),8.67(s,1H),9.92(br,1H),10.54(br,1H)。HRMS:C21H21BrN4O2S2+H,理论值505.03621;测定值(ESI-FTMS,(M+H)+1),505.03601。According to the method described in Example 14, except that the raw material was changed from the product of Example 36 to the product of Example 38, the yield was 36%, and mp90-92°C. 1 H NMR (300MHz, DMSO-d 6 ): δ1.90-1.99(m, 2H), 3.26(t, J=7.5Hz, 2H), 3.75-3.92(m, 4H), 4.88(t, J= 7.5Hz, 1H), 5.02(d, J=5.4Hz, 2H), 7.30-7.40(m, 2H), 7.80(s, 2H), 7.93(t, J=6.9Hz, 1H), 8.23(s, 1H), 8.51 (s, 1H), 8.67 (s, 1H), 9.92 (br, 1H), 10.54 (br, 1H). HRMS: C 21 H 21 BrN 4 O 2 S 2 +H, calc. 505.03621; found (ESI-FTMS, (M+H) +1 ), 505.03601.
实施例17 {4-[3-氯-4-(3-氟苄氧基)苯氨基]喹唑啉基}-6-甲氨基二硫代甲酸-2-氰基乙酯Example 17 {4-[3-Chloro-4-(3-fluorobenzyloxy)anilino]quinazolinyl}-6-methylaminodithiocarbamate-2-cyanoethyl ester
按照实施例14所述的方法,只是将原料2-(2-溴乙基)-1,3-二氧戊环改为β-溴丙氰,收率45%,m.p.173-177℃。1H NMR(300MHz,DMSO-d6):δ2.93(t,J=6.3Hz,2H),3.50(t,J=6.9Hz,2H),5.02(s,2H),5.27(s,2H),7.17-7.49(m,5H),7.72-7.79(m,3H),8.03(s,1H),8.47(s,1H),8.59(s,1H),9.84(br,1H),10.75(br,1H)。HRMS:C26H21ClFN5OS2+H,理论值538.09328;测定值(ESI-FTMS,(M+H)+1),538.09230。According to the method described in Example 14, except that the raw material 2-(2-bromoethyl)-1,3-dioxolane was changed to β-bromopropylcyanide, the yield was 45%, and the mp was 173-177°C. 1 H NMR (300MHz, DMSO-d 6 ): δ2.93(t, J=6.3Hz, 2H), 3.50(t, J=6.9Hz, 2H), 5.02(s, 2H), 5.27(s, 2H ), 7.17-7.49(m, 5H), 7.72-7.79(m, 3H), 8.03(s, 1H), 8.47(s, 1H), 8.59(s, 1H), 9.84(br, 1H), 10.75( br, 1H). HRMS: C 26 H 21 ClFN 5 OS 2 +H, calc. 538.09328; found (ESI-FTMS, (M+H) +1 ), 538.09230.
实施例18 {4-[3-氯-4-(3-氟苄氧基)苯氨基]喹唑啉基}-6-甲氨基二硫代甲酸-2-甲氧甲酰基乙酯Example 18 {4-[3-Chloro-4-(3-fluorobenzyloxy)anilino]quinazolinyl}-6-methylcarbamodithioate-2-methoxyformyl ethyl ester
按照实施例14所述的方法,只是将原料2-(2-溴乙基)-1,3-二氧戊环改为丙烯酸甲酯,收率38%,m.p.169-171℃。1H NMR(300MHz,DMSO-d6):δ2.74(t,J=6.3Hz,2H),3.50(t,J=6.9Hz,2H),3.61(s,3H),5.00(d,J=4.5Hz,2H),5.26(s,2H),7.16-7.52(m,5H),7.12-7.78(m,3H),8.03(s,1H),8.45(s,1H),8.58(s,1H),9.84(br,1H),10.59(br,1H)。元素分析C27H24ClFN4O3S2:理论值:C,56.78;H,4.24;N,9.81.测定值:C,56.85;H,4.255;N,9.757。According to the method described in Example 14, except that the raw material 2-(2-bromoethyl)-1,3-dioxolane was changed to methyl acrylate, the yield was 38%, mp 169-171°C. 1 H NMR (300MHz, DMSO-d 6 ): δ2.74(t, J=6.3Hz, 2H), 3.50(t, J=6.9Hz, 2H), 3.61(s, 3H), 5.00(d, J =4.5Hz, 2H), 5.26(s, 2H), 7.16-7.52(m, 5H), 7.12-7.78(m, 3H), 8.03(s, 1H), 8.45(s, 1H), 8.58(s, 1H), 9.84 (br, 1H), 10.59 (br, 1H). Elemental analysis C 27 H 24 ClFN 4 O 3 S 2 : Theoretical: C, 56.78; H, 4.24; N, 9.81. Found: C, 56.85; H, 4.255; N, 9.757.
实施例19 {4-[3-氯-4-(3-氟苄氧基)苯氨基]喹唑啉基}-6-甲氨基二硫代甲酸-2-苄砜基乙酯Example 19 {4-[3-Chloro-4-(3-fluorobenzyloxy)anilino]quinazolinyl}-6-methylcarbamodithioate-2-benzylsulfone ethyl ester
按照实施例14所述的方法,只是将原料2-(2-溴乙基)-1,3-二氧戊环改为(2-溴乙基砜基)甲基苯,收率24%,m.p.168-170℃。1H NMR(400MHz,DMSO-d6):δ3.40-3.44(m,2H),3.57-3.61(t,J=8.4,2.4Hz,2H),4.57(s,2H),5.00-5.01(d,J=5.2Hz,2H),5.26(s,2H),7.16-7.21(m,1H),7.29-7.35(m,3H),7.38-7.50(m,6H),7.73(dd,J=8.8,2.8Hz,1H),7.78(s,1H),8.02(d,J=2.8Hz,1H),8.46(s,1H),8.59(s,1H),9.85(br,1H),10.70(br,1H)。元素分析C32H28ClFN4O3S3:理论值:C,57.60;H,4.23;N,8.40.测定值:C,57.15;H,4.23;N,8.38。According to the method described in Example 14, just change the raw material 2-(2-bromoethyl)-1,3-dioxolane into (2-bromoethylsulfone)methylbenzene, the yield is 24%, mp168-170°C. 1 H NMR (400MHz, DMSO-d 6 ): δ3.40-3.44(m, 2H), 3.57-3.61(t, J=8.4, 2.4Hz, 2H), 4.57(s, 2H), 5.00-5.01( d, J=5.2Hz, 2H), 5.26(s, 2H), 7.16-7.21(m, 1H), 7.29-7.35(m, 3H), 7.38-7.50(m, 6H), 7.73(dd, J= 8.8, 2.8Hz, 1H), 7.78(s, 1H), 8.02(d, J=2.8Hz, 1H), 8.46(s, 1H), 8.59(s, 1H), 9.85(br, 1H), 10.70( br, 1H). Elemental analysis C 32 H 28 ClFN 4 O 3 S 3 : Theoretical: C, 57.60; H, 4.23; N, 8.40. Found: C, 57.15; H, 4.23; N, 8.38.
制备实施例39 2-氰基-3-(3-甲氧基-4-甲基苯氨基)丙烯酸乙酯Preparation Example 39 2-cyano-3-(3-methoxy-4-methylanilino) ethyl acrylate
将3-甲氧基-4-甲基苯胺(10.96g,80mmol)和2-氰基-3-乙氧基丙烯酸乙酯(13.54g,80mmol)溶于甲苯(32mL)中,加热回流5h,静置冷却,析出大量黄色固体,过滤,乙醚洗涤,干燥,得黄色固体18.6g,收率94%,m.p.152-156℃。1H NMR(300MHz,CDCl3):δ1.37(t,J=7.2Hz,3H),2.19(s,3H),3.85(s,3H),4.26-4.33(m,1H),6.51(d,J=1.8Hz,1H),6.60(dd,J=7.8,2.1Hz,1H),7.12(d,J=8.1Hz,1H),7.83(d,J=13.5Hz,1H),10.72(br,1H)。Dissolve 3-methoxy-4-methylaniline (10.96g, 80mmol) and ethyl 2-cyano-3-ethoxyacrylate (13.54g, 80mmol) in toluene (32mL), heat to reflux for 5h, After standing and cooling, a large amount of yellow solid precipitated, filtered, washed with ether, and dried to obtain 18.6 g of yellow solid, yield 94%, mp 152-156°C. 1 H NMR (300MHz, CDCl 3 ): δ1.37(t, J=7.2Hz, 3H), 2.19(s, 3H), 3.85(s, 3H), 4.26-4.33(m, 1H), 6.51(d , J=1.8Hz, 1H), 6.60(dd, J=7.8, 2.1Hz, 1H), 7.12(d, J=8.1Hz, 1H), 7.83(d, J=13.5Hz, 1H), 10.72(br , 1H).
制备实施例40 3-氰基-4-羟基-6-甲基-7-甲氧基喹啉Preparation Example 40 3-cyano-4-hydroxyl-6-methyl-7-methoxyquinoline
将制备实施例39产物(5.00g,19mmol)溶于二苯醚和联苯混合液(200mL,v∶v,3∶1)中,加热至258℃回流24h,冷却,倒入100mL环己烷中,过滤,得黑色固体化合物2.14g,收率53%,m.p.>300℃。1H NMR(300MHz,DMSO-d6):δ2.25(s,3H),3.91(s,3H),6.99(s,1H),7.88(s,1H),8.63(s,1H),12.58(br,1H)。The product of Preparation Example 39 (5.00g, 19mmol) was dissolved in diphenyl ether and biphenyl mixture (200mL, v:v, 3:1), heated to 258°C and refluxed for 24h, cooled, and poured into 100mL cyclohexane , and filtered to obtain 2.14 g of black solid compound, yield 53%, mp>300°C. 1 H NMR (300MHz, DMSO-d 6 ): δ2.25(s, 3H), 3.91(s, 3H), 6.99(s, 1H), 7.88(s, 1H), 8.63(s, 1H), 12.58 (br, 1H).
制备实施例41 3-氰基-4-氯-6-甲基-7-甲氧基喹啉Preparation Example 41 3-cyano-4-chloro-6-methyl-7-methoxyquinoline
将制备实施例40产物(0.39g,2mmol)加入三氯氧磷(2mL)中,滴入催化量DMF(3滴),110℃加热回流2h。冷却,将反应液滴入冰水中,加10N氢氧化钠至溶液pH=7,过滤,滤饼经硅胶柱分离纯化(洗脱剂,石油醚∶乙酸乙酯=1∶1,v∶v),得淡黄色固体化合物0.37g,收率84%,m.p.196-197℃。1H NMR(300MHz,DMSO-d6):δ2.42(s,3H),4.04(s,3H),7.53(s,1H),8.08(s,1H),9.06(s,1H)。The product of Preparation Example 40 (0.39 g, 2 mmol) was added to phosphorus oxychloride (2 mL), and a catalytic amount of DMF (3 drops) was added dropwise, and heated to reflux at 110° C. for 2 h. Cool, drop the reaction solution into ice water, add 10N sodium hydroxide until the solution pH = 7, filter, and separate and purify the filter cake through a silica gel column (eluent, petroleum ether: ethyl acetate = 1:1, v:v) , to obtain 0.37 g of light yellow solid compound, yield 84%, mp 196-197°C. 1 H NMR (300 MHz, DMSO-d 6 ): δ 2.42 (s, 3H), 4.04 (s, 3H), 7.53 (s, 1H), 8.08 (s, 1H), 9.06 (s, 1H).
制备实施例42 3-氰基-4-氯-6-溴甲基-7-甲氧基喹啉Preparation Example 42 3-cyano-4-chloro-6-bromomethyl-7-methoxyquinoline
将制备实施例41产物(0.37g,1.7mmol)和N-溴代琥珀酰亚胺(NBS)(0.36g,2mmol)溶于CCl4(5mL)中,加入催化量的过氧化苯甲酰,加热回流0.5h。TLC检测反应结束后,停止加热,冷至室温,过滤,滤液浓缩,粗产物经硅胶柱分离纯化(洗脱剂,石油醚∶乙酸乙酯=4∶1,v∶v),得白色固体0.43g,收率85%,m.p.178-182℃。1H NMR(300MHz,DMSO-d6):δ4.10(s,3H),4.14(s,3H),4.90(s,2H),7.58(s,1H),7.63(s,1H),7.69(s,1H),8.45(s,1H),8.60(s,1H),9.13(s,1H),9.17(s,1H)。The product of Preparation Example 41 (0.37 g, 1.7 mmol) and N-bromosuccinimide (NBS) (0.36 g, 2 mmol) were dissolved in CCl 4 (5 mL), and a catalytic amount of benzoyl peroxide was added, Heat to reflux for 0.5h. After the reaction was detected by TLC, the heating was stopped, cooled to room temperature, filtered, the filtrate was concentrated, and the crude product was separated and purified on a silica gel column (eluent, petroleum ether:ethyl acetate=4:1, v:v) to obtain a white solid 0.43 g, yield 85%, mp 178-182°C. 1 H NMR (300MHz, DMSO-d 6 ): δ4.10(s, 3H), 4.14(s, 3H), 4.90(s, 2H), 7.58(s, 1H), 7.63(s, 1H), 7.69 (s, 1H), 8.45 (s, 1H), 8.60 (s, 1H), 9.13 (s, 1H), 9.17 (s, 1H).
制备实施例43 3-氰-4-氯-6-邻苯二甲酰亚胺甲基-7-甲氧基喹啉Preparation Example 43 3-cyano-4-chloro-6-phthalimidemethyl-7-methoxyquinoline
将邻苯二甲酰亚胺的钾盐(0.55g,3mmol)溶于丙酮(20mL)中,加热至回流,滴入制备实施例42产物(0.43g,1.5mmol)的丙酮溶液,继续回流1h。TLC检测原料消失,停止反应,冷却至室温,过滤,滤饼为化合物c-7,滤液浓缩后利用硅胶柱分离纯化(洗脱剂,石油醚∶乙酸乙酯=2∶1,v∶v),得白色固体化合物0.23g,收率45%,m.p.240-244℃。1H NMR(300MHz,DMSO-d6):δ4.06(s,3H),4.96(s,2H),7.63(s,1H),7.88-7.97(m,5H),9.11(s,1H)。Dissolve the potassium salt of phthalimide (0.55g, 3mmol) in acetone (20mL), heat to reflux, drop into the acetone solution of the product of Preparation Example 42 (0.43g, 1.5mmol), and continue to reflux for 1h . TLC detects that the raw material disappears, stop the reaction, cool to room temperature, filter, the filter cake is compound c-7, and the filtrate is concentrated and purified by silica gel column separation and purification (eluent, petroleum ether: ethyl acetate = 2: 1, v: v) , to obtain 0.23 g of white solid compound, yield 45%, mp 240-244°C. 1 H NMR (300MHz, DMSO-d 6 ): δ4.06(s, 3H), 4.96(s, 2H), 7.63(s, 1H), 7.88-7.97(m, 5H), 9.11(s, 1H) .
制备实施例44 3-氰基-4-[3-氯-4-(3-氟苄氧基)苯氨基]-6-邻苯二甲酰亚胺甲基-7-甲氧基喹啉Preparation Example 44 3-cyano-4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-6-phthalimidemethyl-7-methoxyquinoline
将制备实施例43化合物(1mmol)和3-氯-4-(3-氟苄氧基)苯胺(1mmol)混合加入异丙醇(2mL)中,加热至回流,有固体析出,停止反应,冷却,过滤,干燥,收率80%,m.p.278-282℃。1H NMR(300MHz,DMSO-d6):δ4.01(s,3H),4.84(s,3H),5.24(s,2H),7.15-7.46(m,8H),7.83-7.94(m,4H),8.05(s,1H),8.50(s,1H),9.61(br,1H)。Mix the compound of Preparation Example 43 (1mmol) and 3-chloro-4-(3-fluorobenzyloxy)aniline (1mmol) into isopropanol (2mL), heat to reflux, a solid precipitates out, stop the reaction, and cool , filtered, dried, yield 80%, mp278-282°C. 1 H NMR (300MHz, DMSO-d 6 ): δ4.01(s, 3H), 4.84(s, 3H), 5.24(s, 2H), 7.15-7.46(m, 8H), 7.83-7.94(m, 4H), 8.05 (s, 1H), 8.50 (s, 1H), 9.61 (br, 1H).
制备实施例45 3-氰基-4-(3-氯-4-氟苯氨基)-6-邻苯二甲酰亚胺甲基-7-甲氧基喹啉Preparation Example 45 3-cyano-4-(3-chloro-4-fluoroanilino)-6-phthalimidemethyl-7-methoxyquinoline
按照制备实施例44所述的方法,只是将原料3-氯-4-(3-氟苄氧基)苯胺改为3-氯-4-氟苯胺,收率83%,m.p.310-313℃。1H NMR (300MHz,DMSO-d6):δ4.05(s,3H),4.85(s,2H),7.37-7.47(m,3H),7.64(d,J=1.8Hz,1H),7.90(d,J=8.4Hz,4H),8.15(s,1H),8.83(s,1H),10.44(br,1H)。Following the method described in Preparation Example 44, except that the starting material 3-chloro-4-(3-fluorobenzyloxy)aniline was changed to 3-chloro-4-fluoroaniline, the yield was 83%, mp 310-313°C. 1 H NMR (300MHz, DMSO-d 6 ): δ4.05(s, 3H), 4.85(s, 2H), 7.37-7.47(m, 3H), 7.64(d, J=1.8Hz, 1H), 7.90 (d, J=8.4Hz, 4H), 8.15(s, 1H), 8.83(s, 1H), 10.44(br, 1H).
制备实施例46 3-氰基-4-(3-溴苯氨基)-6-邻苯二甲酰亚胺甲基-7-甲氧基喹啉Preparation Example 46 3-cyano-4-(3-bromoanilino)-6-phthalimidemethyl-7-methoxyquinoline
按照制备实施例44所述的方法,只是将原料3-氯-4-(3-氟苄氧基)苯胺改为3-溴苯胺,收率99%,m.p.310-312℃。1H NMR(300MHz,DMSO-d6):δ4.05(s,3H),4.85(s,2H),7.31-7.60(m,5H),7.86-7.94(m,5H),8.25(s,1H),8.95(s,1H),10.91(br,1H)。Following the method described in Preparation Example 44, except that the starting material 3-chloro-4-(3-fluorobenzyloxy)aniline was changed to 3-bromoaniline, the yield was 99%, mp 310-312°C. 1 H NMR (300MHz, DMSO-d 6 ): δ4.05(s, 3H), 4.85(s, 2H), 7.31-7.60(m, 5H), 7.86-7.94(m, 5H), 8.25(s, 1H), 8.95 (s, 1H), 10.91 (br, 1H).
实施例20 {4-[3-氯-4-(3-氟苄氧基)苯氨基]-3-氰基-7-甲氧基喹啉基}-6-甲氨基二硫代甲酸-2-[2-(1,3-二氧环戊基)]乙酯Example 20 {4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-3-cyano-7-methoxyquinolinyl}-6-methylaminodithioformic acid-2 -[2-(1,3-dioxolopentyl)]ethyl ester
将制备实施例44的产物(0.20g)溶于甲醇(2mL)中,搅拌均匀后滴入甲胺醇溶液(10mL),搅拌10min,加甲醇至反应液澄清,室温搅拌过夜。反应结束后,减压蒸除溶剂,剩余物用3N盐酸溶液(5mL)酸化,有固体析出,加水至固体全溶,乙酸乙酯萃取(20mL×2),水相用1N氢氧化钠溶液碱化至pH 7-8,乙酸乙酯萃取(20mL×3),合并碱化后乙酸乙酯层,饱和食盐水洗(30mL),无水硫酸钠干燥,过滤,减压蒸干得固体化合物。将上述化合物(1mmol)溶于DMF(2mL)中,滴入Et3N(2mmol),搅拌10min后,滴入CS2(4mmol),搅拌15min后,滴入2-(2-溴乙基)-1,3-二氧戊环,室温搅拌过夜。反应结束后,加入过量水(50mL),用乙酸乙酯萃取(30mL×3),合并有机相,饱和食盐水洗(30mL),无水硫酸钠干燥,过滤,滤液浓缩后利用硅胶柱分离纯化(洗脱剂,石油醚∶乙酸乙酯=1∶1.5,v∶v),得目标化合物,收率24%,m.p.174-177℃。1H NMR(300MHz,DMSO-d6):δ1.86-1.93(m,2H),3.20(t,J=7.5Hz,2H),3.72-3.89(m,4H),3.97(s,3H),4.83(t,J=4.5Hz,3H),5.25(s,2H),7.17-7.47(m,8H),8.27(s,1H),8.51(s,1H)。HRMS:C31H28ClFN4O4S2+H,理论值639.12973;测定值(ESI-FTMS,(M+H)+1),639.12921。The product of Preparation Example 44 (0.20 g) was dissolved in methanol (2 mL), stirred evenly, and added dropwise into methylamino alcohol solution (10 mL), stirred for 10 min, methanol was added until the reaction solution was clear, and stirred overnight at room temperature. After the reaction, the solvent was distilled off under reduced pressure, and the residue was acidified with 3N hydrochloric acid solution (5mL), and a solid precipitated, and water was added until the solid was completely dissolved, extracted with ethyl acetate (20mL×2), and the aqueous phase was alkalized with 1N sodium hydroxide solution. The mixture was adjusted to pH 7-8, extracted with ethyl acetate (20 mL×3), combined and basified ethyl acetate layers were washed with saturated brine (30 mL), dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure to obtain a solid compound. The above compound (1mmol) was dissolved in DMF (2mL), Et 3 N (2mmol) was added dropwise, after stirring for 10min, CS 2 (4mmol) was added dropwise, after stirring for 15min, 2-(2-bromoethyl) was added dropwise -1,3-dioxolane, stirred overnight at room temperature. After the reaction was completed, excess water (50 mL) was added, extracted with ethyl acetate (30 mL×3), the organic phases were combined, washed with saturated brine (30 mL), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated and purified by silica gel column separation and purification ( Eluent, petroleum ether: ethyl acetate=1:1.5, v:v), the target compound was obtained with a yield of 24%, mp174-177°C. 1 H NMR (300MHz, DMSO-d 6 ): δ1.86-1.93(m, 2H), 3.20(t, J=7.5Hz, 2H), 3.72-3.89(m, 4H), 3.97(s, 3H) , 4.83 (t, J=4.5Hz, 3H), 5.25 (s, 2H), 7.17-7.47 (m, 8H), 8.27 (s, 1H), 8.51 (s, 1H). HRMS: C 31 H 28 ClFN 4 O 4 S 2 +H, calc. 639.12973; found (ESI-FTMS, (M+H) +1 ), 639.12921.
实施例21 [4-(3-氯-4-氟苯氨基)-3-氰基-7-甲氧基喹啉基]-6-甲氨基二硫代甲酸-2-[2-(1,3-二氧环戊基)]乙酯Example 21 [4-(3-chloro-4-fluoroanilino)-3-cyano-7-methoxyquinolinyl]-6-methylaminodithioformic acid-2-[2-(1, 3-Dioxocyclopentyl)] ethyl ester
按照实施例20所述的方法,只是将原料制备实施例44产物的改为制备实施例45的产物,收率53%,m.p.190-192℃。1H NMR(300MHz,DMSO-d6):δ1.88-1.90(m,2H),3.21(t,J=7.5Hz,2H),3.77-3.99(m,9H),4.86(s,3H),7.25-7.47(m,4H),8.21(s,1H),8.61(s,1H),9.78(br,1H),10.19(br,1H)。HRMS:C24H22ClFN4O3S2+H,理论值533.08786;测定值(ESI-FTMS,(M+H)+1),533.08724。According to the method described in Example 20, except that the raw materials used to prepare the product of Example 44 were changed to the product of Example 45, the yield was 53%, and mp 190-192°C. 1 H NMR (300MHz, DMSO-d 6 ): δ1.88-1.90(m, 2H), 3.21(t, J=7.5Hz, 2H), 3.77-3.99(m, 9H), 4.86(s, 3H) , 7.25-7.47 (m, 4H), 8.21 (s, 1H), 8.61 (s, 1H), 9.78 (br, 1H), 10.19 (br, 1H). HRMS: C 24 H 22 ClFN 4 O 3 S 2 +H, calc. 533.08786; found (ESI-FTMS, (M+H) +1 ), 533.08724.
实施例22 [4-(3-溴苯氨基)-3-氰基-7-甲氧基喹啉基]-6-甲氨基二硫代甲酸-2-[2-(1,3-二氧环戊基)]乙酯Example 22 [4-(3-bromoanilino)-3-cyano-7-methoxyquinolinyl]-6-methylaminodithioformic acid-2-[2-(1,3-dioxo Cyclopentyl)] ethyl ester
按照实施例20所述的方法,只是将制备实施例44的产物改为制备实施例46的产物,收率41%,m.p.155-157℃。1H NMR(300MHz,DMSO-d6):δ1.88-1.99(m,2H),3.21(t,J=7.5Hz,2H),3.74-4.05(m,7H),4.85(t,J=4.5Hz,3H),7.16-7.41(m,5H),8.22(s,1H),8.66(s,1H),9.80(br,1H),10.19(br,1H)。元素分析C24H23BrN4O3S2:理论值:C,51.52;H,4.14;N,10.01.测定值:C,51.26;H,4.15;N,9.94。According to the method described in Example 20, except that the product of Preparation Example 44 was changed to the product of Preparation Example 46, the yield was 41%, mp 155-157°C. 1 H NMR (300MHz, DMSO-d 6 ): δ1.88-1.99(m, 2H), 3.21(t, J=7.5Hz, 2H), 3.74-4.05(m, 7H), 4.85(t, J= 4.5Hz, 3H), 7.16-7.41(m, 5H), 8.22(s, 1H), 8.66(s, 1H), 9.80(br, 1H), 10.19(br, 1H). Elemental analysis C 24 H 23 BrN 4 O 3 S 2 : Theoretical: C, 51.52; H, 4.14; N, 10.01. Found: C, 51.26; H, 4.15; N, 9.94.
实施例23 [4-(3-溴苯氨基)喹唑啉基]-6-甲氨基二硫代甲酸-2-苄砜基乙酯Example 23 [4-(3-bromoanilino)quinazolinyl]-2-benzylsulfonyl ethyl ester of 6-methylaminodithiocarbamate
按照实施例14所述的方法,只是将原料制备实施例36的产物改为制备实施例38的产物,将原料2-(2-溴乙基)-1,3-二氧戊环改为(2-溴乙基砜基)甲基苯,收率39%,m.p.180-182℃。1H NMR(300MHz,DMSO-d6):δ3.38-3.43(m,2H),3.55-3.60(m,2H),4.57(s,2H),4.55(s,2H),4.50(s,2H),7.29-7.39(m,8H),7.80(s,2H),7.91(d,J=8.1Hz,1H),8.22(s,1H),8.50(s,1H),8.65(s,1H).HRMS:C25H23BrN4O2S3+H,理论值587.02393;测定值(ESI-FTMS,(M+H)+1),587.02408。According to the method described in embodiment 14, just change the product of raw material preparation embodiment 36 into the product of preparation embodiment 38, change raw material 2-(2-bromoethyl)-1,3-dioxolane into ( 2-Bromoethylsulfone) methylbenzene, yield 39%, mp180-182°C. 1 H NMR (300MHz, DMSO-d 6 ): δ3.38-3.43(m, 2H), 3.55-3.60(m, 2H), 4.57(s, 2H), 4.55(s, 2H), 4.50(s, 2H), 7.29-7.39(m, 8H), 7.80(s, 2H), 7.91(d, J=8.1Hz, 1H), 8.22(s, 1H), 8.50(s, 1H), 8.65(s, 1H ). HRMS: C 25 H 23 BrN 4 O 2 S 3 +H, calc. 587.02393; found (ESI-FTMS, (M+H) +1 ), 587.02408.
试验实施例1化合物的生物活性评价-蛋白酪氨酸激酶抑制活性筛选Biological Activity Evaluation of Compounds of Test Example 1 - Protein Tyrosine Kinase Inhibitory Activity Screening
筛选方法:酶联免疫吸附测定(ELISA)Screening method: enzyme-linked immunosorbent assay (ELISA)
本实施例采用药学领域蛋白酪氨酸激酶抑制活性筛选试验的常规方法,例如中国专利公开CN1944398A所描述的方法。试验结果见下表1。This example adopts the conventional method of screening test for protein tyrosine kinase inhibitory activity in the field of pharmacy, such as the method described in Chinese patent publication CN1944398A. The test results are shown in Table 1 below.
表1酪氨酸激酶活性的抑制率(%)The inhibitory rate (%) of table 1 tyrosine kinase activity
其中的拉帕替尼(Lapatinib)是已知的抗肿瘤药物,是一种新的EGFR靶向治疗药物乳腺癌晚期的新药,在上述试验中作为阳性对照药。Among them, lapatinib (Lapatinib) is a known anti-tumor drug and a new EGFR-targeted therapy drug for advanced breast cancer. It was used as a positive control drug in the above-mentioned test.
在上述试验中,酪氨酸激酶(EGFR)浓度:10μM;作用时间:1h;结果评定:无效:10μM<50%;有效:10μM≥50%。In the above test, EGFR concentration: 10 μM; action time: 1 h; result evaluation: invalid: 10 μM<50%; effective: 10 μM≥50%.
试验实施例2化合物的生物活性评价-体外抗肿瘤活性测试Biological Activity Evaluation of Test Example 2 Compound - In Vitro Antitumor Activity Test
本实施例所采用的试验方法是药学领域常规的抗肿瘤活性试验方法,例如可参见下述文献:(J.Immunol Method,1983,65,55)。试验以化合物23作为本发明化合物的代表性实例,以已知药物吉非替尼为阳性对照药物,试验结果见下表2。The test method used in this example is a conventional anti-tumor activity test method in the pharmaceutical field, for example, refer to the following literature: (J. Immunol Method, 1983, 65, 55). In the test, compound 23 was used as a representative example of the compound of the present invention, and the known drug gefitinib was used as a positive control drug. The test results are shown in Table 2 below.
表2实施例化合物23的细胞活性试验结果The cell activity test result of table 2 embodiment compound 23
*筛选方法及测试模型:A:MTT法(HL-60人白血病);B:SRB法(BGC-823人胃癌);C:SRB法(Bel-7402人肝癌);D:SRB法(KB人鼻咽癌)。*Screening method and test model: A: MTT method (HL-60 human leukemia); B: SRB method (BGC-823 human gastric cancer); C: SRB method (Bel-7402 human liver cancer); D: SRB method (KB human nasopharyngeal carcinoma).
从上述试验结果可以清楚地看出,本发明要求获得专利保护的通式(I)的化合物具有优异的蛋白酪酸激酶抑制活性和抗癌活性。有望开发成为一类新结构类型的抗肿瘤新药。From the above test results, it can be clearly seen that the compound of general formula (I) for which patent protection is claimed in the present invention has excellent protein tyrosine kinase inhibitory activity and anticancer activity. It is expected to be developed into a new anti-tumor drug with a new structure type.
现在已经详细描述了本发明的实施方案,对本领域技术人员来说很明显可以做很多改进和变化而不会背离本发明的基本精神,所有这些变化和改进都在是本发明的范围之内,其特征由上述说明书确定。Now having described the embodiment of the present invention in detail, it is obvious to those skilled in the art that many improvements and changes can be made without departing from the basic spirit of the present invention, and all these changes and improvements are within the scope of the present invention. Its characteristics are determined by the above description.
Claims (17)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN 200910143757 CN101899011B (en) | 2009-05-26 | 2009-05-26 | Dithiocarbamates compound, preparation method and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN 200910143757 CN101899011B (en) | 2009-05-26 | 2009-05-26 | Dithiocarbamates compound, preparation method and application thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN101899011A CN101899011A (en) | 2010-12-01 |
| CN101899011B true CN101899011B (en) | 2013-01-16 |
Family
ID=43224974
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN 200910143757 Expired - Fee Related CN101899011B (en) | 2009-05-26 | 2009-05-26 | Dithiocarbamates compound, preparation method and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN101899011B (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106928249A (en) * | 2017-03-14 | 2017-07-07 | 宁夏大学 | A kind of preparation method of easy, efficient sweet wormwood sulfone-dithiocarbamates compound |
Families Citing this family (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103304572A (en) * | 2012-03-09 | 2013-09-18 | 上海医药集团股份有限公司 | 3-cyanoquinoline compound as well as pharmaceutical composition and application thereof |
| CN102657647A (en) * | 2012-04-06 | 2012-09-12 | 澳门大学 | Application of IC-4 in antiangiogenesis |
| UY35084A (en) | 2012-10-16 | 2014-04-30 | Janssen Pharmaceutica Nv | MODULATORS OF RORYT OF QUINOLINILO UNITED BY METHYLENE |
| BR112015008515A2 (en) | 2012-10-16 | 2017-07-04 | Janssen Pharmaceutica Nv | heteroaryl-linked quinolinyl ror t modulators |
| WO2014062667A1 (en) | 2012-10-16 | 2014-04-24 | Janssen Pharmaceutica Nv | Phenyl linked quinolinyl modulators of ror-gamma-t |
| WO2014086284A1 (en) * | 2012-12-04 | 2014-06-12 | 上海医药集团股份有限公司 | Deuterated 3-cyano quinoline compound, pharmaceutical composition, preparation method and use thereof |
| EP3057422B1 (en) | 2013-10-15 | 2019-05-15 | Janssen Pharmaceutica NV | Quinolinyl modulators of ror(gamma)t |
| US9328095B2 (en) | 2013-10-15 | 2016-05-03 | Janssen Pharmaceutica Nv | Heteroaryl linked quinolinyl modulators of RORgammat |
| US9346782B2 (en) | 2013-10-15 | 2016-05-24 | Janssen Pharmaceutica Nv | Alkyl linked quinolinyl modulators of RORγt |
| US10555941B2 (en) | 2013-10-15 | 2020-02-11 | Janssen Pharmaceutica Nv | Alkyl linked quinolinyl modulators of RORγt |
| US9284308B2 (en) | 2013-10-15 | 2016-03-15 | Janssen Pharmaceutica Nv | Methylene linked quinolinyl modulators of RORγt |
| US9403816B2 (en) | 2013-10-15 | 2016-08-02 | Janssen Pharmaceutica Nv | Phenyl linked quinolinyl modulators of RORγt |
| US9221804B2 (en) | 2013-10-15 | 2015-12-29 | Janssen Pharmaceutica Nv | Secondary alcohol quinolinyl modulators of RORγt |
| CN106146410B (en) * | 2015-04-03 | 2018-10-12 | 中南大学 | 6- amino -4 (3H)-Quinazol derivative and its preparation method and use |
| CN106146487B (en) * | 2015-04-27 | 2019-05-10 | 北京大学 | Heteroarylcycloalkyl picolinate amino dithiocarboxylate compound and preparation method and use thereof |
| CN106432208B (en) * | 2015-08-10 | 2019-12-13 | 北京大学 | Aminodithiocarboxylate (sulfamoyl) ethyl ester compound and preparation method and use thereof |
| CN108721304B (en) * | 2017-04-17 | 2020-10-16 | 北京大学 | Pharmaceutical composition for treating tumor and application thereof |
| CN111072571B (en) * | 2018-10-18 | 2021-05-14 | 北京西博医药研究有限公司 | Dithiocarbamates as FAK inhibitors |
| CN112341416A (en) * | 2019-08-09 | 2021-02-09 | 天津津艺医药科技发展有限责任公司 | Method for preparing benzo oxygen-containing aliphatic heterocyclic derivative |
| CN113024514B (en) * | 2021-03-26 | 2022-02-15 | 北京大学 | Amino dithiocarbamate compound and its preparation method and pharmaceutical composition and application |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1328999A (en) * | 2001-05-29 | 2002-01-02 | 北京大学 | Piperazine dithioformates compounds, their preparation method and application in cancer-resisting medicine |
| CN1727332A (en) * | 2004-07-27 | 2006-02-01 | 北京大学 | Arylmethylcarbamate compound and its preparation method and application |
| CN101003514A (en) * | 2006-01-20 | 2007-07-25 | 上海艾力斯医药科技有限公司 | Derivative of quinazoline, preparation method and usage |
| WO2008033749A2 (en) * | 2006-09-11 | 2008-03-20 | Curis, Inc. | Quinazoline based egfr inhibitors containing a zinc binding moiety |
-
2009
- 2009-05-26 CN CN 200910143757 patent/CN101899011B/en not_active Expired - Fee Related
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1328999A (en) * | 2001-05-29 | 2002-01-02 | 北京大学 | Piperazine dithioformates compounds, their preparation method and application in cancer-resisting medicine |
| CN1727332A (en) * | 2004-07-27 | 2006-02-01 | 北京大学 | Arylmethylcarbamate compound and its preparation method and application |
| CN101003514A (en) * | 2006-01-20 | 2007-07-25 | 上海艾力斯医药科技有限公司 | Derivative of quinazoline, preparation method and usage |
| WO2008033749A2 (en) * | 2006-09-11 | 2008-03-20 | Curis, Inc. | Quinazoline based egfr inhibitors containing a zinc binding moiety |
Non-Patent Citations (2)
| Title |
|---|
| 1-[二-(4-氟苯)甲基]-4-取代哌嗪类化合物的合成及抗肿瘤活性;郭保国等;《北京大学学报(医学版)》;20010630;第33卷(第3期);第213-216页 * |
| 郭保国等.1-[二-(4-氟苯)甲基]-4-取代哌嗪类化合物的合成及抗肿瘤活性.《北京大学学报(医学版)》.2001,第33卷(第3期),第213-216页. |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106928249A (en) * | 2017-03-14 | 2017-07-07 | 宁夏大学 | A kind of preparation method of easy, efficient sweet wormwood sulfone-dithiocarbamates compound |
Also Published As
| Publication number | Publication date |
|---|---|
| CN101899011A (en) | 2010-12-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN101899011B (en) | Dithiocarbamates compound, preparation method and application thereof | |
| US11008306B2 (en) | Quinazolines as potassium ion channel inhibitors | |
| JP3088018B2 (en) | Quinazoline derivatives | |
| US7312330B2 (en) | Bicycloheteroarylamine compounds as ion channel ligands and uses thereof | |
| CN106831730B (en) | A kind of substituted diaminopyrimidines and its preparing the purposes in anti-malignant tumor medicine | |
| EP3687540A1 (en) | Heteroaryl compounds as cxcr4 inhibitors, composition and method using the same | |
| TWI699360B (en) | Substituted pyrimidine compounds as inhibitors of phosphoinositide 3-kinase delta and applications thereof | |
| AU2018226922B2 (en) | Urea-substituted aromatic ring-linked dioxane-quinazoline and -linked dioxane-quinoline compounds, preparation method therefor and use thereof | |
| WO2013013614A1 (en) | 4-(3-heteroarylarylamino)quinazoline and 1-(3-heteroarylarylamino)isoquinoline as hedgehog pathway inhibitor and use thereof | |
| PT3013814T (en) | Substituted tetrahydrocarbazole and carbazole carboxamide compounds useful as kinase inhibitors | |
| AU2006330072A1 (en) | Selective benzylamine derivatives and their utility as cholesterol ester-transfer protein inhibitors | |
| EP0799826A1 (en) | Heteroaryl substituted phenylene derivatives, their preparation and their use as leukotriene receptor antagonists | |
| WO2016023217A1 (en) | Quinazoline derivative, preparation method therefor, and pharmaceutical composition and application thereof | |
| WO2017198196A1 (en) | Quinoline derivative having anti-tumor activity | |
| WO2023005280A1 (en) | Preparation and application of aminopyrimidine derivative selectively targeting cdk9 | |
| JP2013526590A (en) | Fused ring quinazoline derivatives and uses thereof | |
| EA007066B1 (en) | Triazolo-quinolin derivatives useful as adenosine receptor ligands | |
| KR102030016B1 (en) | Novel compound, method for preparing the same, and pharmaceutical composition for use in preventing or treating poly(ADP-ribose)polymerase-1 related diseases containing the same as an active ingredient | |
| CN103864680B (en) | There is the 7-chloro-4-oxo-quinoline derivative of anti-tumor activity | |
| WO2021164793A1 (en) | Compound used as kinase inhibitor and use thereof | |
| KR102017115B1 (en) | Pharmaceutical composition for use in preventing or treating poly(ADP-ribose)polymerase-1 related diseases containing the same as an active ingredient | |
| CN109666022B (en) | Triazole derivatives and their preparation methods and uses | |
| CN110372666A (en) | Quinazoline compounds are as tri- inhibition from mutation agent of EGFR and its application | |
| JP7110335B2 (en) | Pyridoquinazoline derivatives useful as protein kinase inhibitors | |
| CN113788835B (en) | Triazole tetrazine compound containing morpholine and quinoline ring and preparation method and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| CF01 | Termination of patent right due to non-payment of annual fee |
Granted publication date: 20130116 Termination date: 20150526 |
|
| EXPY | Termination of patent right or utility model |