CN100469394C - Implant with FK506 - Google Patents
Implant with FK506 Download PDFInfo
- Publication number
- CN100469394C CN100469394C CNB028050916A CN02805091A CN100469394C CN 100469394 C CN100469394 C CN 100469394C CN B028050916 A CNB028050916 A CN B028050916A CN 02805091 A CN02805091 A CN 02805091A CN 100469394 C CN100469394 C CN 100469394C
- Authority
- CN
- China
- Prior art keywords
- implant
- layer
- polymer
- stent
- metal
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/04—Hollow or tubular parts of organs, e.g. bladders, tracheae, bronchi or bile ducts
- A61F2/06—Blood vessels
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/14—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L31/16—Biologically active materials, e.g. therapeutic substances
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/02—Inorganic materials
- A61L27/04—Metals or alloys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/14—Macromolecular materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/416—Anti-neoplastic or anti-proliferative or anti-restenosis or anti-angiogenic agents, e.g. paclitaxel, sirolimus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/60—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a special physical form
- A61L2300/606—Coatings
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Chemical & Material Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Transplantation (AREA)
- Vascular Medicine (AREA)
- Epidemiology (AREA)
- Medicinal Chemistry (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Molecular Biology (AREA)
- Dermatology (AREA)
- Surgery (AREA)
- Cardiology (AREA)
- Inorganic Chemistry (AREA)
- Gastroenterology & Hepatology (AREA)
- Pulmonology (AREA)
- Materials For Medical Uses (AREA)
- Prostheses (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Glass Compositions (AREA)
Abstract
Description
技术领域 technical field
本发明涉及植入体,特别是腔内或脉管内植入体,优选用于治疗或预防冠脉或外周血管闭塞,或血管缩窄,特别是缩窄和狭窄或再狭窄,优选用于预防再狭窄。它包括化学共价结合或非共价结合或以物理固定形式的FK596,本发明还涉及用于生产和使用它的方法。The present invention relates to implants, in particular intraluminal or intravascular implants, preferably for the treatment or prevention of coronary or peripheral vascular occlusion, or vascular narrowing, in particular narrowing and stenosis or restenosis, preferably for the prophylaxis Restenosis. It includes chemically covalently or non-covalently bound or physically immobilized FK596, and the invention also relates to methods for producing and using it.
背景技术 Background technique
动脉血管中动脉硬化病损伤的形成是大范围临床症状,包括从心绞痛到间歇性跛行到心肌梗死和缺血性中风的潜在性疾病,所有这些都是基于粥样硬化形成(atheromer formation)和/或狭窄病损。术语狭窄病损是指脉管腔局部减小到其正常直径的60-70%以下。这反过来又导致向特定组织提供氧气和营养的显著下降。尽管近十年来药物治疗(statin、ACE抑制剂、gp II a/III b阻断剂和血纤蛋白溶酶原激活物)已经显示具有良好的治疗结果,特别是对于心血管疾病领域,但对于已经发展为完全缺血状态的病人来说仍然需要手术介入(旁路手术等)。这些手术相当复杂并且费用昂贵,还涉及严重的并发症的风险。The formation of atherosclerotic lesions in arterial vessels is a broad spectrum of clinical symptoms, ranging from angina pectoris to intermittent claudication to myocardial infarction and ischemic stroke, all of which are based on atheromer formation and/or or narrow lesions. The term stenotic lesion refers to a local reduction of the lumen of a vessel to less than 60-70% of its normal diameter. This in turn leads to a significant decrease in the supply of oxygen and nutrients to specific tissues. Although drug therapy (statins, ACE inhibitors, gpIIa/IIIb blockers, and plasminogen activators) has shown promising therapeutic outcomes in the last decade, especially in the field of cardiovascular disease, the Surgical intervention (bypass surgery, etc.) is still required for patients who have developed a complete ischemic state. These procedures are quite complex and expensive and involve the risk of serious complications.
为了预防发展成为缺血性心脏病,已经开发了最小的侵入性手术方法。在70年代末期发展的经皮腔内冠状血管成形术(PTCA)是心脏病学领域的一个重大突破。PTCA包括使用可行进到远至冠状动脉狭窄病损处的可充气气囊。然后这些气囊在特定靶位被充气并达到扩张狭窄区域的目的。类似的手术也可用于颈动脉或周围动脉的扩张。To prevent the development of ischemic heart disease, minimally invasive surgical methods have been developed. The development of percutaneous transluminal coronary angioplasty (PTCA) in the late 1970s was a major breakthrough in the field of cardiology. PTCA involves the use of an inflatable balloon that can be advanced as far as the coronary artery stenosis lesion. These balloons are then inflated at specific target locations to dilate the narrowed area. Similar procedures are also used for dilation of the carotid or peripheral arteries.
尽管如此,但发现相对快的时间后,在相当大比例的PTCA患者中在用气囊导管扩张的部位又复发狭窄。在这方面,发现这种被称作再狭窄的原因是由于组织层的血管结构的重构。被称作斯滕特固定模的管形血管金属植入体的引入,在狭窄的腔内(transluminal)治疗中改善了这种情况。临床研究已经显示(Serruys等,N.Engl.J.Med.331(1994)489-495)在气囊扩张位点使用斯滕特固定模能够减少大约45%到30%的再狭窄的发生。尽管这被认为在防止再狭窄后遗症方面具有明显的改善,但对于治疗改善来说仍然是个明显的刺激因素。Nevertheless, relapse of stenosis at sites dilated with balloon catheters was observed in a substantial proportion of PTCA patients after a relatively short time. In this respect, it was found that the cause of this so-called restenosis is due to the remodeling of the vascular structure of the tissue layers. The introduction of tubular vascular metal implants known as stents has ameliorated the situation in transluminal treatment of stenosis. Clinical studies have shown (Serruys et al., N. Engl. J. Med. 331 (1994) 489-495) that the use of a stent at the site of balloon inflation can reduce the incidence of restenosis by approximately 45% to 30%. Although this is considered to be a significant improvement in preventing the sequelae of restenosis, it is still a significant stimulus for improvement in treatment.
在对再狭窄的病理生理学的详细研究中已经发现,在斯滕特固定模中的再狭窄与PTCA诱导的再狭窄不同。炎症反应、过度增生和平滑肌细胞(SMCs)的迁移是导致斯滕特固定模中再狭窄的新内膜形成的重要因素。在再狭窄的动物模型,甚至在人的组织中已经发现平滑肌细胞的过度增生与巨噬细胞和T细胞浸润到斯滕特固定模加强区域周围的组织有关(Grewe等,J.Am.Coll.Cardiol.35(2000)157-63)。类似于涉及炎症反应和细胞的过度增生、并且可以用医学治疗控制的其它临床指征,已经尝试用药物来治疗再狭窄。选定的活性剂已经通过口服或静脉内给予或通过有孔导管送到作用位点。不幸的是,到目前为止,还没有哪种活性剂能够明显减小再狭窄(Gruberg等,Exp.Opin.Inyest.Active agents 9(2000)2555-2578)。In a detailed study of the pathophysiology of restenosis, it has been found that restenosis in stents differs from PTCA-induced restenosis. Inflammation, hyperproliferation, and migration of smooth muscle cells (SMCs) are important factors in neointima formation leading to restenosis in stents. In animal models of restenosis, and even in human tissues, hyperproliferation of smooth muscle cells has been found to be associated with infiltration of macrophages and T cells into the tissue surrounding the area of stent reinforcement (Grewe et al., J. Am. Coll. Cardiol. 35 (2000) 157-63). Similar to other clinical indications involving inflammatory responses and hyperproliferation of cells, and which can be controlled with medical treatment, attempts have been made to treat restenosis with drugs. The selected active agent has been administered orally or intravenously or delivered to the site of action by a perforated catheter. Unfortunately, so far no active agent has been able to significantly reduce restenosis (Gruberg et al., Exp. Opin. Inyest. Active agents 9 (2000) 2555-2578).
药物活性剂从活性剂包被的斯滕特固定模直接传送是这里选择的一种方法。用活性剂包被的斯特特固定模进行的动物试验和临床试验的初步结果得到的印象是免疫抑制剂或抗增生活性剂的延迟释放可减小再狭窄的风险。帕尼特西,一种细胞生长抑制剂,和免疫抑制剂并细胞生长抑制剂雷帕霉素,已经进行了动物试验。两种化合物都能够抑制新内膜的形成(Herdeg等,Semin Intervent Cardiol 3(1998)197-199;Hunter等,Adv.Active agent.Delivery Rev.26(1997)199-207;Burke等,J.Cardiovasc Pharmacol.33(1999)829=835;Gallo等,Circulation 99(1999)2164-2170)。在猪身上植入用帕尼特西包被的斯滕特固定模后6个月观察到作用消失(Heldman,International Local Active agent DeliveryMeeting and Cardiovascular Course on Radiation,Geneva,Jan25-27,2001)。在最初的临床应用中,雷帕霉素显示了再狭窄完全消失的良好的作用(Sousa等,Circulation 103(2001)192-195)。另一方面,这显示与气囊血管成形术和斯滕特固定模植入中血管壁损伤的延迟愈合相一致。Direct delivery of pharmaceutically active agents from active agent-coated stents is the method of choice here. Preliminary results from animal experiments and clinical trials with active agent-coated stents gave the impression that delayed release of immunosuppressive or antiproliferative active agents reduces the risk of restenosis. Panitesi, a cytostatic agent, and the immunosuppressant and cytostatic agent rapamycin, have been tested in animals. Both compounds were able to inhibit neointima formation (Herdeg et al., Semin Intervent Cardiol 3 (1998) 197-199; Hunter et al., Adv. Active agent. Delivery Rev. 26 (1997) 199-207; Burke et al., J. Cardiovasc Pharmacol. 33 (1999) 829=835; Gallo et al., Circulation 99 (1999) 2164-2170). Loss of effect was observed 6 months after implantation of Panitesi-coated stents in pigs (Heldman, International Local Active agent Delivery Meeting and Cardiovascular Course on Radiation, Geneva, Jan 25-27, 2001). In initial clinical use, rapamycin showed a good effect of complete resolution of restenosis (Sousa et al., Circulation 103 (2001) 192-195). On the other hand, this appears to be consistent with delayed healing of vessel wall injuries in balloon angioplasty and stent implantation.
总的来说,在血管成形术和斯滕特固定模放置后的动脉血管壁的愈合和控制新内膜形成之间的平衡非常重要。为了获得这种平衡,应该使用能够选择性地干扰导致新内膜形成的特殊机制的活性剂。Overall, the balance between arterial wall healing and controlled neointima formation after angioplasty and stent placement is very important. To achieve this balance, active agents that selectively interfere with the specific mechanisms leading to neointima formation should be used.
发明内容 Contents of the invention
因此,本发明的一个目的是提供一种植入体,它具有治疗和预防再狭窄的有利特性。It is therefore an object of the present invention to provide an implant having advantageous properties for the treatment and prevention of restenosis.
因此,本发明涉及一种植入体,它包含化学共价结合或非共价结合或物理固定之形式的FK506,和任选的至少一种其它的活性剂。Accordingly, the present invention relates to an implant comprising FK506 in chemically covalently bound or non-covalently bound or physically immobilized form, and optionally at least one other active agent.
在此方面,以下说明适用于上面提到的用于本发明目的的每种活性剂,包括活性剂FK506:术语“活性剂”还包括活性剂的直接的衍生物,并且活性剂还可以以任何类型的盐、对映体、外消旋物、活性剂的碱或游离酸,以及它们的混合物的形式存在。In this regard, the following description applies to each active agent mentioned above for the purposes of the present invention, including the active agent FK506: The term "active agent" also includes direct derivatives of the active agent, and the active agent can also be used in any Types of salts, enantiomers, racemates, bases or free acids of active agents, and mixtures thereof.
优选植入体是腔内植入体,优选是血管内植入体。Preferably the implant is an intraluminal implant, preferably an intravascular implant.
在此,腔内意思是在空腔内,特别是在一个或一些空腔器官,诸如血管、食道、输尿管、胆管等内。Here, intraluminal means within a cavity, especially within a hollow organ or organs such as a blood vessel, esophagus, ureter, bile duct or the like.
血管内,具体地说,意思是应用在血管内。Intravascular, specifically, means application within a blood vessel.
还优选该植入体适用于治疗或预防冠状血管或周围血管的缩窄或闭塞,特别是缩窄或狭窄或再狭窄,优选用于预防再狭窄。It is also preferred that the implant is suitable for the treatment or prevention of narrowing or occlusion of coronary or peripheral vessels, in particular narrowing or stenosis or restenosis, preferably for the prevention of restenosis.
因此,特别优选的是腔内,优选是血管内植入体,用于治疗或预防冠状血管或周围血管缩窄或闭塞、特别是缩窄或狭窄或再狭窄,优选用于预防再狭窄,该植入体包括化学共价结合或非共价结合或物理固定之形式的FK506,和任选的至少一种其它活性剂。Therefore, particularly preferred are intraluminal, preferably intravascular, implants for the treatment or prevention of coronary or peripheral vascular constriction or occlusion, in particular constriction or stenosis or restenosis, preferably for the prevention of restenosis, which The implant comprises FK506 in chemically covalently bound or non-covalently bound or physically immobilized form, and optionally at least one other active agent.
大环内酯抗菌素FK506(他克莫司,[3S-[3R*[E(1S*,3S*,4S*)],4S*,5R*,-8S*,9E,12R*,14R*,15S*,16R*,18S*,19S*,26aR*]]-5,6,8,11,12,13,14,15,16,17,18,19,24,25,25,26,26a-十六氢-5,19-二羟基—3-[2-(4-羟基-3-甲氧基环己基)-1-甲基乙烯基]-14,16-二甲氧基-4,10,12,18-四甲基-8-(2-丙烯基)-15,19-环氧-3H-吡啶并-[2,1-c][1,4]氧杂氮杂-环二十三烷(cyclotricosine)-1,7-20,21(4H,23H)-四酮;Merck索引号9000)是一种研发用作移植药物的活性剂。FK506抑制白介素-2(IL-2)和干扰素-γ(IFN-γ)从T细胞的释放,因此阻断了对植入体(移植体)的排斥反应(Wiederrecht等,Ann.NY Acad.Sci.696(1993)9-19)。还研究了FK506在平滑肌细胞培养中抑制平滑肌细胞增殖的作用(Mohacsi等,J.Heart Lung Transplant.16(1997)484-492;Marx等,Circulation Res.76(1995)412-417)和抑制平滑肌细胞迁移(Poon等,J.Clin.Invest.98(1996)2777-2283)。一般来说,许多研究者评价FK506不合适,因为它预防再狭窄的活性低(Mohasci等,(1997);Poon等(1996);Marx等1995;Dell,Curr Med Chem5(1998)179-94)。Mohacsi等人发现在100nM和1μM之间时对平滑肌增生有半数最大抑制,而Marx等人观察到在浓度高达123nm时完全没有作用。与此相反,雷怕霉素在纳摩尔浓度范围内具有抑制培养的平滑肌细胞增生的活性。Macrolide antibiotic FK506 (tacrolimus, [3S-[3R*[E(1S*, 3S*, 4S*)], 4S*, 5R*, -8S*, 9E, 12R*, 14R*, 15S*, 16R*, 18S*, 19S*, 26aR*]]-5, 6, 8, 11, 12, 13, 14, 15, 16, 17, 18, 19, 24, 25, 25, 26, 26a -Hexadecahydro-5,19-dihydroxy-3-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methylvinyl]-14,16-dimethoxy-4, 10,12,18-Tetramethyl-8-(2-propenyl)-15,19-epoxy-3H-pyrido-[2,1-c][1,4]oxazepine-cyclobis Tridecane (cyclotricosine)-1,7-20,21(4H,23H)-tetraketone; Merck Index No. 9000) is an active agent developed for use as a transplant medicine. FK506 inhibits the release of interleukin-2 (IL-2) and interferon-γ (IFN-γ) from T cells, thus blocking the rejection of implants (grafts) (Wiederrecht et al., Ann.NY Acad. Sci. 696 (1993) 9-19). The effect of FK506 on smooth muscle cell proliferation inhibition in smooth muscle cell culture was also studied (Mohacsi et al., J.Heart Lung Transplant.16(1997)484-492; Marx et al., Circulation Res.76(1995)412-417) and inhibition of smooth muscle Cell migration (Poon et al., J. Clin. Invest. 98 (1996) 2777-2283). In general, many investigators have evaluated FK506 as inappropriate because of its low activity in preventing restenosis (Mohasci et al., (1997); Poon et al. (1996); Marx et al. 1995; Dell, Curr Med Chem 5 (1998) 179-94) . Mohacsi et al. found half-maximal inhibition of smooth muscle proliferation between 100 nM and 1 μM, while Marx et al. observed no effect at all at concentrations as high as 123 nM. In contrast, rapamycin was active in inhibiting the proliferation of cultured smooth muscle cells in the nanomolar concentration range.
根据这些现有技术,一点也不会想到特别地利用FK506来抑制再狭窄(Mohacsi等(1997);Poon等(1996)。然而,与本领域技术人员的观点相反,出人意料地发现特别使用FK506作为斯滕特固定模,当然也可以是其它植入体的一部分可以有效地治疗和预防再狭窄。特别地局部给予FK506有利于预防再狭窄,该作用平衡得非常好,因为它还使得受伤的血管壁内皮重新愈合良好。According to these prior art, it would not at all conceivable to use FK506 specifically to inhibit restenosis (Mohacsi et al. (1997); Poon et al. (1996). Stents, which can of course be part of other implants, are effective in the treatment and prevention of restenosis. In particular, local administration of FK506 is beneficial in the prevention of restenosis, which is very well balanced because it also The wall endothelium re-healed well.
不是一开始就这样假设,这可能能解释为FK506的免疫调节活性,后者可以由约0.1nM浓度时对IL-2释放的半数最大抑制(Kimo等,J.Antibiot.40(1987)1256-1265)和在大约300-500nM时对平滑肌细胞增生的抑制作用来证明。因此,使用FK506是有利的。Rather than assuming this at the outset, this may be explained by the immunomodulatory activity of FK506, which can be inhibited half-maximally by IL-2 release at a concentration of about 0.1 nM (Kimo et al., J.Antibiot.40 (1987) 1256- 1265) and inhibition of smooth muscle cell proliferation at approximately 300-500 nM. Therefore, it is advantageous to use FK506.
在本文中,狭窄意味着血管的闭塞或缩窄,再狭窄意味着狭窄的再次发生。Herein, stenosis means occlusion or narrowing of blood vessels, and restenosis means recurrence of stenosis.
另外,在本文中,“包含”也意味着特别是例如非共价结合的涂层。In addition, in this context, "comprising" also means, in particular, eg non-covalently bound coatings.
另外,在本文中,“周围”意思是指心脏和冠状血管以外的血管或其它空腔器官。In addition, herein, "peripheral" means blood vessels or other hollow organs other than the heart and coronary vessels.
“化学地非共价”结合意思特别是指通过诸如氢键、疏水相互作用、范德华力等的相互作用而发生的连接。Binding by "chemically non-covalent" means inter alia linkage via interactions such as hydrogen bonds, hydrophobic interactions, van der Waals forces, and the like.
物理固定方法是指,例如用膜包封在洞孔中,或通过选定口径进行空间捕获。Physical immobilization means, for example, membrane encapsulation in holes, or spatial trapping through selected apertures.
植入体是指引入体内(甚至有限的时间)的任何类型的人造物体。它们可以是例如腔内,脉管内植入体。实例为斯滕特固定模、移植物、斯特特固定模移植物、移植物连接体、引导线(guidewire)、导管泵或导管。An implant is any type of man-made object that is introduced into the body (even for a limited time). They may be eg intraluminal, intravascular implants. Examples are stents, grafts, stent grafts, graft connectors, guidewires, catheter pumps or catheters.
用于本发明目的的斯滕特固定模(stent)是指延长的植入体,它具有中空的内部和至少两个口,通常具有环形或椭圆形、但也可以是其它任何形状的截面(大多数由金属、但任选也可以是塑料材料或聚合物制成),优选具有穿孔的、格子状结构,它被植入到管腔中,尤其是血管中,以保持其开放和发挥功能。A stent for the purposes of the present invention refers to an elongated implant having a hollow interior and at least two orifices, usually with a circular or oval cross-section, but may be of any other shape ( Mostly made of metal, but optionally also plastic materials or polymers), preferably with a perforated, lattice-like structure, which is implanted in the lumen, especially a blood vessel, to keep it open and functional .
用于本发明目的的移植物是指一种延长的植入体,具有中空的内部和至少两个口,通常有环形或椭圆形的、但也可以是其它任何形状的截面,并具有至少一个封闭的聚合物表面,它是均质的,或者任选由各种细带(strands)编织成的,不能透过血液的颗粒成分(corpuscular constituents)和/或水,这种植入体一般用作血管假体并通常被用于损伤的血管或代替血管。Graft for the purposes of the present invention means an elongated implant having a hollow interior and at least two orifices, usually of circular or oval, but also any other shape, cross-section, and having at least one Closed polymeric surface, which is homogeneous, or optionally woven from various strands, impermeable to corpuscular constituents of blood and/or water, such implants are generally used as Vascular prostheses are often used to replace damaged blood vessels or to replace blood vessels.
用于本发明目的的斯滕特固定模移植物(stent graft)是指斯滕特固定模和移植物之间的连接。因此,斯滕特固定模移植物基本上是一种由斯滕特固定模(见上面所述的移植物)加强的血管假体,其中的聚合物层是均质的或任选由各种细带编制而成,不能透过血液的颗粒成分和/或水。狭义的讲,这是一种斯滕特固定模,其在至少20%的植入体表面具有穿孔(格子状)的、优选由金属构成的、外层和至少一个位于该外层的内侧或外侧、是均质的或任选由各种细带编制而成的、对血液的颗粒成分和/或水不能通透的封闭的聚合物层,和任选的(当穿孔层位于外部的情况下)另外的穿孔的(格子状的)、优选是金属的、位于该聚合物层内部的内层,或位于外部的、位于穿孔层的外部的、为均质的或任选由各种细带编制而成的、并且不能透过血液的颗粒成分和/或水的封闭的聚合物层。A stent graft for the purposes of the present invention refers to the connection between the stent and the graft. Thus, a stent graft is essentially a vascular prosthesis reinforced by a stent (see grafts described above) in which the polymer layer is homogeneous or optionally composed of various The thin bands are woven and impermeable to the particulate components of blood and/or water. In the narrow sense, this is a stent having, on at least 20% of the implant surface, a perforated (lattice-like), preferably metallic, outer layer and at least one inner or outer layer located on the outer layer. An outer, closed polymeric layer that is homogeneous or optionally woven from various thin strips, impermeable to the particulate components of blood and/or water, and optionally (when the perforated layer is located on the outer Bottom) An additional perforated (lattice-like), preferably metallic, inner layer inside the polymer layer, or external, outside the perforated layer, homogeneous or optionally composed of various fine A closed polymer layer that is woven and impermeable to the particulate components of blood and/or water.
用于本发明目的的移植物连接体(graft connector)是指连接至少两个中空脏器、血管或移植物的植入体,它由用于移植物或斯滕特固定模移植物的材料构成和/或具有后者的结构,并相应地有至少两个、优选三个或四个口,特别是呈现为非对称的“T”形。Graft connector for the purposes of the present invention means an implant connecting at least two hollow organs, vessels or grafts, which consists of materials used in grafts or stent grafts And/or have the latter structure, and correspondingly have at least two, preferably three or four openings, especially in an asymmetrical "T" shape.
用于本发明目的的导管是指用于引入中空脏器的管形器械。狭义上讲,优选它们是导引、血管成形术或气囊导管。A catheter for the purposes of the present invention refers to a tubular instrument for introduction into a hollow organ. In the narrow sense, they are preferably guide, angioplasty or balloon catheters.
用于本发明目的的导管泵是指在导管的尖端配置了有助于心肌的泵血的推进器的导管。A catheter pump for the purposes of the present invention refers to a catheter in which a pusher that assists the pumping of the myocardium is disposed at the tip of the catheter.
本发明植入体的另一个优选的实施方案是,该植入体具有至少一个封闭的或穿孔的层或表面,后者由金属或金属合金构成,为均质的或由各种细带编织而成。Another preferred embodiment of the implant according to the invention is that the implant has at least one closed or perforated layer or surface, the latter consisting of metal or metal alloy, homogeneous or braided by various thin strips made.
用于本发明目的的金属或金属合金尤其地是指钢或钢合金或者为镍或镍合金,术语金属从开头还包括金属合金。A metal or a metal alloy for the purposes of the present invention refers in particular to steel or a steel alloy or to nickel or a nickel alloy, the term metal from the beginning also including metal alloys.
穿孔结构是指尤其是格子状或编织的或编成的结构。A perforated structure means especially a lattice-like or woven or braided structure.
本发明植入体的另一个优选的实施方案是该植入体具有至少一个封闭的或穿孔的层或表面,后者由聚合物构成,为均质的或由各种细带编织而成。Another preferred embodiment of the implant according to the invention is that the implant has at least one closed or perforated layer or surface, the latter consisting of a polymer, homogeneous or braided from various thin strips.
在一个优选的实施方案中,植入体具有至少一个聚合物层,它完全或部分地覆盖均质的或由各种细带形成的、由金属或合金构成的封闭的或有孔的层或表面,后者优选为由金属或合金构成的任选格子状的结构。In a preferred embodiment, the implant has at least one polymer layer which completely or partially covers a homogeneous or closed or porous layer formed of metal or alloy or formed of various thin strips or The surface, the latter preferably having an optionally lattice-like structure composed of a metal or alloy.
在一个特别优选的实施方案中,植入体具有至少一个封闭的或穿孔的层或表面,它由金属或金属合金构成,并且是均质的或由各种细带形成,以及至少一个封闭的或穿孔的由聚合物构成、并且是均质的或由各种细带形成的层或表面。In a particularly preferred embodiment, the implant has at least one closed or perforated layer or surface, which consists of metal or a metal alloy and is homogeneous or formed of various thin bands, and at least one closed or perforated layer or surface made of polymer and homogeneous or formed of various thin bands.
对于该植入体来说更特别优选的是,由金属或金属合金构成的层或表面是由金属或金属合金构成的任选格子状的结构,和/或由聚合物构成的层或表面为均质地封闭的或编织的,和/或对水和/或颗粒(corpuscle)不能通透,和/或层和表面的顺序将由外向内为金属-聚合物、聚合物-金属、金属-聚合物-金属或聚合物-金属-聚合物,和/或由聚合物构成的层或表面将非化学地(共价地或非共价地)连接到由金属或金属合金构成的层或表面上,或由聚合物构成的层或表面将通过粘合剂连接到由金属或金属合金构成的层或表面上。It is very particularly preferred for the implant that the layer or surface made of metal or metal alloy is an optionally lattice-like structure made of metal or metal alloy and/or the layer or surface made of polymer is Homogeneously closed or woven, and/or impermeable to water and/or corpuscle, and/or the order of layers and surfaces will be metal-polymer, polymer-metal, metal-polymer from outside to inside - a metal or polymer-metal-polymer, and/or a layer or surface composed of a polymer will be non-chemically (covalently or non-covalently) bonded to a layer or surface composed of a metal or metal alloy, Or a layer or surface composed of a polymer will be attached to a layer or surface composed of a metal or metal alloy by an adhesive.
进一步优选用于植入体的聚合物选自涤纶(Dacron)、聚四氟乙烯(PTFE/Teflon)(可发泡的或不可发泡的)、或聚氨基甲酸酯。优选选自聚四氟乙烯(PTFE)(可发泡的或不可发泡的)或聚氨基甲酸酯,更优选PTFE。It is further preferred that the polymer used for the implant is selected from Dacron, PTFE/Teflon (foamable or non-foamable), or polyurethane. It is preferably selected from polytetrafluoroethylene (PTFE) (expandable or non-expandable) or polyurethane, more preferably PTFE.
在本发明优选的实施方案中植入体是斯滕特固定模、斯滕特固定模移植物、移植物、移植物连接体、引导线、导管或导管泵,优选为斯滕特固定模、斯滕特固定模移植物、移植物或移植物连接体,更优选斯滕特固定模或斯滕特固定模移植物。In a preferred embodiment of the invention the implant is a stent, a stent graft, a graft, a graft connector, a guide wire, a catheter or a catheter pump, preferably a stent, A stent graft, graft or graft connector, more preferably a stent or a stent graft.
特别优选的本发明植入体是用FK506涂敷的植入体。A particularly preferred implant of the invention is an implant coated with FK506.
通过从冠状或周围血管斯滕特固定模的活性剂荷载表面直接传输,可以达到FK506的局部给药。通过使用各种技术方法可以获得斯滕特固定模的活性剂荷载表面。每种方法可通过这样一种方式来执行,即活性剂从表面上短时(数小时)或延长的期限(数天)内释放。通过对表面进行特定的修饰,例如聚合物载体或陶瓷表面的疏水或亲水侧链,可以调节释放动力学。这些表面还可以在表面上被修饰,例如通过氧化铝层上的Si基团来修饰。Local delivery of FK506 can be achieved by direct delivery from the active agent-loaded surface of a coronary or peripheral vascular stent. The active agent loaded surface of the stent can be obtained by using various technical methods. Each method can be performed in such a way that the active agent is released from the surface for a short period (hours) or for an extended period (days). Release kinetics can be tuned by specific modifications to the surface, such as hydrophobic or hydrophilic side chains on polymeric supports or ceramic surfaces. These surfaces can also be modified on the surface, for example by Si groups on the aluminum oxide layer.
通过使用单独或层结构的具体聚合物、嵌段聚合物、聚合物混合物、接枝聚合物可以调节释放动力学。通过利用毫微胶囊(nanocapsules)和/或脂质体和上述聚合物组合,可以特别适当地控制释放动力学。毫微胶囊一般是指胶束体系或胶状固体的涂层,以得到有固体涂层的极细的颗粒。其大小在毫微米范围内的涂覆的颗粒形成胶体溶液。因此,可以使用毫微胶囊包封的具有延长活性的活性剂。脂质体一般通过将磷脂分散于水性介质中而形成,这是本文中感兴趣的,因为亲水性活性剂可以掺入到水性内部体积中和进入水性中间层,而疏水性活性剂可以进入到脂质层。如果使用组成不同的毫微胶囊和/或脂质体,则后者可以荷载不同的活性剂,从而使活性剂的组合可以以定向方式释放。The release kinetics can be adjusted by using specific polymers, block polymers, polymer mixtures, grafted polymers alone or in layered structures. The release kinetics can be particularly suitably controlled by using nanocapsules and/or liposomes in combination with the aforementioned polymers. Nanocapsules generally refer to micellar systems or coatings of colloidal solids to obtain very fine particles coated with solids. Coated particles whose size is in the nanometer range form a colloidal solution. Thus, nanoencapsulated active agents with prolonged activity may be used. Liposomes are generally formed by dispersing phospholipids in an aqueous medium, which is of interest here because hydrophilic active agents can be incorporated into the aqueous interior volume and into the aqueous middle layer, while hydrophobic active agents can enter to the lipid layer. If nanocapsules and/or liposomes of different composition are used, the latter can be loaded with different active agents so that combinations of active agents can be released in a targeted manner.
陶瓷涂层ceramic coating
可以通过浸渍、喷雾或类似技术将10微克到10毫克量的FK506荷载到具有多孔表面的氧化铝涂层上(专利申请DE19855421、DE19910188、WO 00/25841)。活性剂的剂量依赖于目标血管的类型和病人的状况,并可进行选择使得能够充分地抑制增生、迁移和T细胞反应,同时不影响痊愈过程。FK506可以用在水性或有机溶液,例如DMSO、DMF和乙醇中。在喷雾或浸渍后(任选在弱真空条件下),将处理的斯滕特固定模干燥并将该程序重复2-10次。另一个应用的可能性是借助于微量移液器或自动移液器直接将活性剂溶液传输到斯滕特固定模的细丝(strands)上。在最后的干燥步骤后,可以在室温下用水或等渗盐水冲洗斯滕特固定模1分钟,然后再干燥。在已经用适当溶剂将活性剂溶解之后,可通过标准方法(HPLC、LC-MS)来分析活性剂的含量。使用标准释放测量仪可以测量释放动力学。可以将陶瓷方法与聚合物(任选生物可降解的)涂层方法结合。Amounts of 10 micrograms to 10 milligrams of FK506 can be loaded onto alumina coatings with porous surfaces by dipping, spraying or similar techniques (patent applications DE19855421, DE19910188, WO 00/25841). The dosage of the active agent depends on the type of target vessel and the condition of the patient, and can be selected so as to sufficiently inhibit proliferation, migration and T cell responses without affecting the healing process. FK506 can be used in aqueous or organic solutions such as DMSO, DMF and ethanol. After spraying or dipping (optionally under mild vacuum), the treated stent is dried and the procedure is repeated 2-10 times. Another application possibility is the direct transfer of the active agent solution onto the strands of the stent by means of a micropipette or automatic pipette. After the final drying step, the stent can be rinsed with water or isotonic saline for 1 min at room temperature before drying. After the active agent has been dissolved with a suitable solvent, the content of the active agent can be analyzed by standard methods (HPLC, LC-MS). Release kinetics can be measured using standard release measuring apparatus. The ceramic approach can be combined with a polymeric (optionally biodegradable) coating approach.
而且,可能类似地使用各种类型的金属氧化物涂层,例如,如美国专利6245104B1中公开的氧化铱。因此,下面每次提到的氧化铝都应该被理解成也可以使用其它金属氧化物,如也应该理解成包括氧化铱。Also, it is possible to similarly use various types of metal oxide coatings, eg iridium oxide as disclosed in US Patent 6245104B1. Accordingly, each reference below to alumina should be understood to also include other metal oxides, such as iridium oxide.
PTFE膜:斯滕特固定模移植物PTFE Membrane: Stent Graft
在这种情况下使用与上面描述的类似的方法。FK506沉积到多孔PTFE膜的凹陷中。In this case a method similar to that described above is used. FK506 was deposited into the recesses of the porous PTFE membrane.
一般聚合物涂层General Polymer Coating
各种聚合物都适用于荷载活性剂:甲基丙烯酸酯聚合物、聚氨基甲酸酯涂层、PTFE涂层、水凝胶涂层。活性剂可以应用到终表面上(如上所述),也可以直接加到聚合反应溶液中。这种技术方法在其它细节上与上面已经描述的那些方法对应。Various polymers are suitable for loading active agents: methacrylate polymers, polyurethane coatings, PTFE coatings, hydrogel coatings. The active agent can be applied to the final surface (as described above) or it can be added directly to the polymerization solution. This technical method corresponds in other details to those already described above.
可以使用各种形式的聚合物和后者的组合,如聚合物混合物、具有层结构的系统、嵌段共聚物和接枝共聚物。适当的聚合物是丙烯酸酯和甲基丙烯酸酯,聚硅氧烷,如聚二甲基硅氧烷、聚丙二酸亚甲基酯、聚醚、聚酯、可生物吸收的聚合物、来自乙烯单体的聚合物,如聚乙烯吡咯烷酮和乙烯醚、聚-顺式-1,4-丁二烯、聚-顺式-1,4-异戊二烯、聚-反式-1,4-异戊二烯,和硫化产品、聚氨基甲酸酯、聚脲、聚酰胺、聚酰亚胺、聚磺酸酯、和生物聚合物,如纤维素和其衍生物和蛋白质和血纤蛋白溶胶。水凝胶显示了特别感兴趣的特性,因为它显示具有高的水摄取性,作为最外层(顶层涂层)具有非常好的血相容性。在这里有可能使用水凝胶,如聚丙烯酰胺、聚丙烯酸、在主链上有氧作为杂原子的聚合物,如聚环氧乙烷、聚环氧丙烷、聚四氢呋喃。活性剂可以应用到最终表面或包埋到毫微胶囊和/或脂质体中给药。然而,活性剂还可以直接存在于聚合反应溶液中或在聚合物溶液中。对一些聚合物/活性剂系统,活性剂有可能通过溶涨(swelling)来固定。Various forms of polymers and combinations of the latter can be used, such as polymer mixtures, systems with layer structures, block copolymers and graft copolymers. Suitable polymers are acrylates and methacrylates, polysiloxanes such as polydimethylsiloxane, polymethylene malonate, polyethers, polyesters, bioabsorbable polymers, from vinyl Polymers of monomers such as polyvinylpyrrolidone and vinyl ether, poly-cis-1,4-butadiene, poly-cis-1,4-isoprene, poly-trans-1,4- Isoprene, and vulcanized products, polyurethanes, polyureas, polyamides, polyimides, polysulfonates, and biopolymers such as cellulose and its derivatives and proteins and fibrinsols . The hydrogel exhibits particularly interesting properties, since it appears to have a high water uptake and as the outermost layer (top coat) has very good hemocompatibility. Here it is possible to use hydrogels such as polyacrylamide, polyacrylic acid, polymers with oxygen as heteroatoms in the main chain, such as polyethylene oxide, polypropylene oxide, polytetrahydrofuran. Active agents can be applied to the final surface or entrapped in nanocapsules and/or liposomes for administration. However, the active agent can also be present directly in the polymerization solution or in the polymer solution. For some polymer/active agent systems, it is possible for the active agent to be immobilized by swelling.
机械方法mechanical method
机械方法基于通过激光形成在斯滕特固定模支架上的凹陷。然后可以用FK506充填这些凹陷。机械(凹陷)方法可以与聚合物的、任本身荷载有活性剂的任选可生物降解的涂层结合。在从聚合物涂层上最初释放后,活性剂可以从填充活性剂的凹陷中长期释放。这种技术方法在其它细节上与上面已经描述的那些方法相对应。The mechanical method is based on the formation of depressions in the stent holder by laser. These depressions can then be filled with FK506. Mechanical (depression) methods can be combined with an optionally biodegradable coating of the polymer, either itself loaded with an active agent. After the initial release from the polymer coating, the active agent can be released long term from the active agent filled recesses. This technical method corresponds in other details to those already described above.
因此,本发明植入体的另一个优选的实施方案是,该植入体具有结合有FK506的陶瓷涂层,尤其是氧化铝涂层。A further preferred embodiment of the implant according to the invention is therefore that the implant has a ceramic coating, in particular an aluminum oxide coating, in which FK506 is incorporated.
本发明植入体的另一个优选的实施方案是,该植入体具有聚合物涂层,尤其是甲基丙烯酸酯聚合物、聚氨基甲酸酯、PTFE、水凝胶或水凝胶/聚氨基甲酸酯共混物,特别是PTFE,上面结合有FK506或者在形成涂层前FK506已经溶解在其中。Another preferred embodiment of the implant according to the invention is that the implant has a polymer coating, especially a methacrylate polymer, polyurethane, PTFE, hydrogel or hydrogel/poly Urethane blends, especially PTFE, have FK506 bonded thereto or have FK506 dissolved therein prior to forming the coating.
本发明植入体的另一个优选的实施方案是植入体的金属具有通过激光引入的凹陷,其中填充了FK506。在这种情况下特别有利的是配置有充填FK506的凹陷的金属或至少是凹陷用可生物降解聚合物材料涂覆,在这种情况下FK506任选被结合到聚合物涂层上,或FK506已经在涂层的聚合反应之前溶解到聚合物材料中。Another preferred embodiment of the implant according to the invention is that the metal of the implant has recesses introduced by laser, which are filled with FK506. It is particularly advantageous in this case that the metal provided with the recess filled with FK506 or at least that the recess be coated with a biodegradable polymer material, in which case FK506 is optionally bonded to the polymer coating, or FK506 Already dissolved into the polymer material prior to the polymerization of the coating.
在本发明的植入体的另一个有利的实施方案中,可以通过下列方法产生植入体:其中In another advantageous embodiment of the implant according to the invention, the implant can be produced by the following method: wherein
a)使用具有至少一个封闭的或穿孔的层或表面的植入体,该层或表面由金属或金属合金构成,是均质的或由各种细带形成,如权利要求4、6或7至10中任一项所要求的一样,这种植入体用陶瓷涂层,具体地说是氧化铝涂覆;或a) use of implants with at least one closed or perforated layer or surface consisting of metal or metal alloys, homogeneous or formed of various thin bands, as claimed in claim 4, 6 or 7 As required by any one of 10 to 10, the implant is coated with a ceramic coating, in particular alumina; or
b)使用具有至少一个封闭的或穿孔的层或表面的植入体,该层或表面由聚合物构成,为均质的或由各种细带形成,如权利要求5到10中任一项所要求的一样;或b) use of an implant having at least one closed or perforated layer or surface consisting of a polymer, homogeneous or formed by various thin bands, as claimed in any one of
c)使用权利要求1到10中任一项所要求的植入体,其用已经聚合的或在表面上进行聚合的涂层涂敷,尤其是甲基丙烯酸酯聚合物、聚氨酯、PTFE、水凝胶或水凝胶/聚氨酯混合物涂层;或c) use of an implant as claimed in any one of
d)使用如权利要求4、6、或7至10中任一项所要求的植入体,其具有至少一个封闭的或穿孔的层或表面,后者由金属或金属合金构成,为均质的或由各种细带形成,在上面有通过激光形成的凹陷,凹陷中充填了FK506,然后用已经聚合的或在表面上进行聚合的可生物降解涂层涂敷该植入体;d) use of an implant as claimed in any one of claims 4, 6, or 7 to 10, having at least one closed or perforated layer or surface, the latter consisting of a metal or metal alloy, being homogeneous or formed from various thin strips, on which there are depressions formed by laser, the depressions are filled with FK506, and then the implant is coated with a biodegradable coating already polymerized or polymerized on the surface;
e)然后将根据a)、b)、c)或d)的植入体与在水性或有机溶剂中的FK506溶液,例如通过(任选在真空下)喷洒、喷雾或浸渍发生接触;e) then contacting the implant according to a), b), c) or d) with a solution of FK506 in an aqueous or organic solvent, for example by spraying, misting or dipping (optionally under vacuum);
f)然后,任选干燥该植入体,优选直到步骤e)的溶剂被除去为止;f) then, optionally drying the implant, preferably until the solvent of step e) is removed;
g)然后,任选重复步骤e),任选接着是步骤f),优选重复几次,具体地说为1到5次;和g) then, optionally repeating step e), optionally followed by step f), preferably several times, in particular 1 to 5 times; and
h)接着任选用水或等渗盐水冲洗植入体一或多次;和h) followed by optionally rinsing the implant one or more times with water or isotonic saline; and
i)下一步,任选使其干燥。i) Next step, optionally allowing it to dry.
在本文中,优选在生产本发明的植入体期间,可以以这种方式生产本发明的植入体,即,在步骤e)中将FK506溶解在醇,优选溶解在乙醇中,具体地说,FK506以浓度为0.5-5g/L溶解在乙醇中和/或在步骤e)中,通过在真空下浸渍,优选过夜,使植入体与FK506在水性或有机溶剂中的溶液接触,和/或不执行步骤f)和/或g)和/或在步骤h)中用盐水洗涤植入体数次和/或在步骤i)中使植入体干燥过夜。In this context, preferably during the production of the implant according to the invention, the implant according to the invention can be produced in such a way that in step e) FK506 is dissolved in alcohol, preferably ethanol, in particular , FK506 is dissolved in ethanol at a concentration of 0.5-5 g/L and/or in step e), the implant is brought into contact with a solution of FK506 in an aqueous or organic solvent by immersion under vacuum, preferably overnight, and/or Either steps f) and/or g) are not performed and/or the implant is washed several times with saline in step h) and/or the implant is allowed to dry overnight in step i).
在本发明的另一个可供选择的优选的实施方案中,优选在可如上面所述生产的本发明的植入体的生产中,在步骤e)中,植入体被优选无菌地引入到优选无菌的容器中,该容器上带有可以穿孔的并在完成穿孔后可以封闭的封闭物(closure),所述无菌容器例如是注射用小瓶,将FK506优选无菌地引入到该容器中,后者用封闭物封闭,其中所述封闭物可以被穿孔并在完成穿孔后封闭,将细的、优选无菌的透气的通风管(ventilation tube),例如插管(cannula),穿孔通过该封闭物,应用真空,然后优选搅动FK506溶液,最后,优选大约在12小时后,将细的、优选无菌的透气通风管取出和/或在步骤e)中使FK506溶解在醇,优选乙醇中,具体地说是以3.3毫克的FK506于1毫升乙醇中,和/或从步骤e)将植入体保留在优选无菌的、封闭的玻璃容器中待用,和/或步骤f)到i)被省略。In another alternative preferred embodiment of the invention, preferably in the production of the implant of the invention which can be produced as described above, in step e) the implant is introduced preferably aseptically Into a preferably sterile container with a closure (closure) that can be perforated and can be closed after the perforation is completed, such as a vial for injection, FK506 is preferably aseptically introduced into the container. In the container, the latter is closed with a closure, wherein said closure can be perforated and closed after the perforation is completed, a thin, preferably sterile, gas-permeable ventilation tube (ventilation tube), such as a cannula, is perforated Through this closure, a vacuum is applied, then preferably the FK506 solution is agitated, and finally, preferably after about 12 hours, the thin, preferably sterile vent tube is removed and/or the FK506 is dissolved in alcohol, preferably in ethanol, specifically 3.3 mg of FK506 in 1 ml of ethanol, and/or retain the implant in a preferably sterile, closed glass container from step e), and/or step f) to i) are omitted.
在本发明植入体的另一个有利的实施方案中,可以通过下列方法生产植入体,其中在形成至少一个封闭的或有孔的层或表面之前,FK506已经被溶解在聚合反应材料中,其中所述的层或表面是由聚合物或植入体的聚合物涂层构成的。In another advantageous embodiment of the implant of the present invention, the implant can be produced by the following method, wherein before forming at least one closed or porous layer or surface, FK506 has been dissolved in the polymeric reaction material, The layers or surfaces described therein consist of a polymer or a polymer coating of the implant.
特别优选的是,FK506在本发明的植入体植入后释放。更为有利的是延迟释放。在此,本发明的特别优选的实施方案是,在植入后,FK506从植入体上释放24小时、优选48小时、更优选96小时以上。特别有利的是:FK506It is particularly preferred that FK506 is released after implantation of the implant of the invention. Even more advantageous is delayed release. Here, a particularly preferred embodiment of the present invention is that, after implantation, FK506 is released from the implant for 24 hours, preferably 48 hours, more preferably more than 96 hours. Particularly advantageous: FK506
a)在小于48小时的时间内释放,或a) released in less than 48 hours, or
b)在植入后从植入体释放至少48小时、优选至少7天,具体地说至少2天至多达21天;或b) release from the implant for at least 48 hours, preferably at least 7 days, in particular at least 2 days up to 21 days after implantation; or
c)植入体显示a)和b)两种释放模式。c) The implant shows both a) and b) release patterns.
通过使用两种不同类型的涂层、结合或物理固定可具体地获得后一种变型。在一个实施例中,用荷载FK506的可生物降解的膜封闭装有FK506的激光凹陷。从膜中迅速的释放之后是从凹陷中的长期释放。The latter variant can in particular be obtained by using two different types of coatings, bonding or physical fixation. In one embodiment, the FK506-loaded laser pits are sealed with a FK506-loaded biodegradable film. A rapid release from the membrane was followed by a long-term release from the depression.
本发明的再一个优选的实施方案是,在植入体中还存在至少一种其它的活性剂,优选是药物活性剂,特别地,另一种活性剂选自下列活性剂或其衍生物:Another preferred embodiment of the present invention is that at least one other active agent, preferably a pharmaceutical active agent, is also present in the implant, in particular, another active agent is selected from the following active agents or derivatives thereof:
(组1):吗多明、林西多明、硝普钠、硝酸甘油或一般的NO供体、可溶性鸟苷酸环化酶(sGC)的刺激物,例如BAY41-2272(5-(环丙基-2-[1-(2-氟代苯甲基)-1H-吡唑并[3,4-n]吡啶-3-基]-嘧啶-4-基胺);肼屈嗪、维拉帕米、地尔硫、硝苯地平、尼莫地平或其他Ca++通道阻断剂、卡托普利、依那普利、赖诺普利、喹那普利或血管紧张素转化酶的其它抑制剂(血管紧张素转化酶抑制剂)、氯沙坦、candesartan、irbesartan、缬沙坦、或血管紧张素II受体的其它拮抗剂;(Group 1): morphine, lincidomine, sodium nitroprusside, nitroglycerin or general NO donors, stimulators of soluble guanylate cyclase (sGC), such as BAY41-2272 (5-(cyclo Propyl-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-n]pyridin-3-yl]-pyrimidin-4-ylamine); hydralazine, vitamin lapamil, diltiazem , nifedipine, nimodipine or other Ca ++ channel blockers, captopril, enalapril, lisinopril, quinapril or other inhibitors of angiotensin converting enzyme (angiotensin converting enzyme inhibitors), losartan, candesartan, irbesartan, valsartan, or other antagonists of the angiotensin II receptor;
(组2):地塞米松、倍他米松、泼尼松或其他皮质类固醇药、17-β-雌二醇、环孢菌素、麦考酚酸、VEGF、VEGF受体活化剂、曲尼司特、美洛昔康、celebrex、vioxx或其他COX-2拮抗剂、吲哚美辛、双氯芬酸、布洛芬、萘普生或其他COX-1抑制剂、血纤蛋白溶酶原激活物的抑制剂1(血纤蛋白溶酶原激活物抑制剂-1)或丝氨酸蛋白酶抑制剂(serpins);凝血酶抑制剂、例如水蛭素、hirulog、agratroban、PPACK或白介素-10;(Group 2): dexamethasone, betamethasone, prednisone or other corticosteroids, 17-beta-estradiol, cyclosporine, mycophenolic acid, VEGF, VEGF receptor activators, tranis Naproxen, meloxicam, celebrex, vioxx or other COX-2 antagonists, indomethacin, diclofenac, ibuprofen, naproxen or other COX-1 inhibitors, plasminogen activators Inhibitor 1 (plasminogen activator inhibitor-1) or serine protease inhibitors (serpins); thrombin inhibitors such as hirudin, hirulog, agratroban, PPACK or interleukin-10;
(组3):雷怕霉素、SDZ RAD(40-O-(2-羟乙基)雷怕霉素或其他雷怕霉素衍生物、PDGF拮抗剂、紫杉醇(paclitaxel)或7-己酰-紫杉酚(taxol)、顺铂、长春碱、米托蒽醌、combretastatin A4、托泊替堪、甲氨蝶呤、flavopiridol、放线菌素D、Rheopro/阿昔单抗或普罗布考。(Group 3): Rapamycin, SDZ RAD (40-O-(2-hydroxyethyl)rapamycin or other rapamycin derivatives, PDGF antagonists, paclitaxel or 7-hexanoyl - Taxol, cisplatin, vinblastine, mitoxantrone, combretastatin A4, topotecan, methotrexate, flavopiridol, actinomycin D, Rheopro/abciximab, or probucol .
更特别优选的其它活性剂选自组1,并在植入后头24-72小时内从植入体释放,和/或如果其它活性剂选自组2,则在植入后头48小时-21天内从植入体释放,和/或如果其它活性剂选自组3,则在植入后14天到3个月的时间内从植入体释放。More particularly preferred other active agents are selected from
本发明还涉及用于生产本发明的植入体的方法,其中在至少一个由聚合物或植入体的聚合物涂层构成的封闭的或有孔的层或表面形成之前,FK506已经溶于聚合反应材料中。The invention also relates to a method for producing the implant according to the invention, wherein FK506 has been dissolved in Polymerized materials.
本发明进一步涉及用于生产本发明的植入体的方法,包括下列步骤:The invention further relates to a method for producing an implant according to the invention, comprising the following steps:
a)植入体具有至少一个由金属或金属合金构成的、均质的或由各种细带形成的封闭的或穿孔的层或表面,如权利要求4、6、或7至10中任一项所要求的一样,该植入体用陶瓷涂层,特别是用氧化铝涂覆;或a) The implant has at least one closed or perforated layer or surface of metal or metal alloy, homogeneous or formed by various thin bands, as claimed in any one of claims 4, 6, or 7 to 10 As required by
b)具有至少一个由聚合物构成的、均质的或由各种细带形成的封闭的或穿孔的层或表面的植入体,如权利要求5到10中任一项所要求的一样;或b) an implant with at least one closed or perforated layer or surface made of a polymer, homogeneous or formed by various thin bands, as claimed in any one of
c)权利要求1到10中任一项所要求的植入体,其用已经聚合的或在表面上进行聚合的涂层涂敷,具体地说是甲基丙烯酸酯聚合物、聚氨酯、PTFE、水凝胶或水凝胶/聚氨酯混合物涂层;或c) The implant as claimed in any one of
d)使用如权利要求4、6、7至10中任一项所要求的植入体,其具有至少一个封闭的或穿孔的层或表面,后者由金属或金属合金构成,为均质的或由各种细带形成,在上面有通过激光形成的凹陷,凹陷中充填了FK506,然后用已经聚合的或在表面聚合的可生物降解涂层材料涂敷该植入体;d) use of an implant as claimed in any one of claims 4, 6, 7 to 10, which has at least one closed or perforated layer or surface, the latter consisting of a metal or metal alloy, being homogeneous Or formed from various thin strips, on which there are depressions formed by laser, the depressions are filled with FK506, and then the implant is coated with a biodegradable coating material that has been polymerized or polymerized on the surface;
e)然后将根据a)、b)、c)或d)的植入体与在水性或有机溶剂中的FK506溶液,例如通过(任选在真空下)喷洒、喷雾或浸渍发生接触;e) then contacting the implant according to a), b), c) or d) with a solution of FK506 in an aqueous or organic solvent, for example by spraying, misting or dipping (optionally under vacuum);
f)然后,任选干燥该植入体,优选直到步骤e)的溶剂被除去为止;f) then, optionally drying the implant, preferably until the solvent of step e) is removed;
g)然后,任选重复步骤e),任选接着步骤f),优选重复几次,具体地说为1到5次;和g) then, optionally repeating step e), optionally followed by step f), preferably several times, in particular 1 to 5 times; and
h)接着任选用水或等渗盐水冲洗植入体一或多次;和h) followed by optionally rinsing the implant one or more times with water or isotonic saline; and
i)下一步,任选使其干燥。i) Next step, optionally allowing it to dry.
在以下情况下特别优选这种方法:即,在步骤e)中将FK506溶解在醇,优选溶解在乙醇中,特别地,FK506以浓度为0.5-5g/L溶解在乙醇中和/或在步骤e)中,通过在真空下浸渍,优选过夜,使植入体与FK506在水性或有机溶剂中的溶液接触,和/或不执行步骤f)和/或g),和/或在步骤h)中用盐水洗涤植入体数次,和/或在步骤i)中使植入体干燥过夜。This method is particularly preferred if, in step e), FK506 is dissolved in alcohol, preferably ethanol, in particular, FK506 is dissolved in ethanol at a concentration of 0.5-5 g/L and/or in step e) In e), the implant is brought into contact with a solution of FK506 in an aqueous or organic solvent by immersion under vacuum, preferably overnight, and/or steps f) and/or g) are not carried out, and/or steps h) Wash the implant several times with saline and/or allow the implant to dry overnight in step i).
在本发明方法的另一个优选的备选方案中,在步骤e)中,植入体被优选无菌地引入到优选无菌的容器中,该容器上有可以穿孔的并在完成穿孔后封闭的封闭物,所述无菌容器例如是注射用小瓶,将FK506优选无菌地引入到该容器中,后者用封闭物封闭,其中所述封闭物可以被穿孔并在完成穿孔后封闭,将细的、优选无菌的透气的通风管,例如导管,穿孔通过该封闭物,应用真空,然后优选搅动FK506溶液,最后,优选大约在12小时后,将细的、优选无菌的透气通风管取出,和/或在步骤e)中使FK506溶解在醇,优选乙醇中,尤其是以3.3毫克的FK506于1毫升乙醇中,和/或从步骤e)将植入体保留在优选无菌的、封闭的玻璃容器中待用,和/或步骤f)到i)被省略。In another preferred alternative of the method according to the invention, in step e), the implant is introduced, preferably aseptically, into a preferably sterile container with a perforable and closed after perforation. The closure of the sterile container, such as a vial for injection, FK506 is preferably aseptically introduced into the container, the latter is closed with a closure, wherein the closure can be perforated and closed after completion of the perforation, the A thin, preferably sterile, gas-permeable vent tube, such as a catheter, is pierced through the closure, vacuum is applied, the FK506 solution is then preferably agitated, and finally, preferably after about 12 hours, the thin, preferably sterile gas-permeable vent tube is Remove, and/or dissolve FK506 in alcohol, preferably ethanol, in particular 3.3 mg of FK506 in 1 ml of ethanol in step e), and/or leave the implant in a preferably sterile , in a closed glass container for use, and/or steps f) to i) are omitted.
这另一种方法特别有利,这是现有技术完全没有披露的,并且在成本和时间以及生产步骤上都极端有利,特别是植入体可直接获得并已经在无菌包装里。当然,这是一般适用的,不限于FK506而是可以用于许多活性剂。然而,这种有利并简单的方法有不足,所以这是一个问题。This alternative method is particularly advantageous, which is not disclosed at all in the prior art, and is extremely advantageous in terms of costs and time as well as production steps, especially since the implants are available directly and already in sterile packaging. Of course, this is generally applicable and is not limited to FK506 but can be used for many active agents. However, this advantageous and simple approach has drawbacks, so this is a problem.
因此,本申请进一步单独涉及一种生产用活性剂涂敷的植入体的方法,下面将进行一般描述,其具有以下步骤:Accordingly, the present application further relates solely to a method of producing an implant coated with an active agent, generally described below, having the following steps:
a)将该植入体优选无菌地引入优选无菌的容器,例如注射用小瓶中,该无菌容器具有可穿孔的并且在完成穿孔后封闭的封闭物;a) introducing the implant preferably aseptically into a preferably sterile container, such as a vial for injection, which has a closure that is perforable and closed after completion of the perforation;
b)将优选在具有低蒸气压的有机溶剂,尤其是诸如乙醇或甲醇等醇类中的优选无菌的活性剂溶液引入该容器;b) introducing into the container a preferably sterile solution of the active agent, preferably in an organic solvent with low vapor pressure, especially an alcohol such as ethanol or methanol;
c)用封闭物将该容器封闭,其中所述封闭物上可以穿孔并且在完成穿孔后封闭;c) closing the container with a closure which may be perforated and closed after the perforation has been completed;
d)将细的、优选无菌的、透气的通风管,例如插管,穿孔通过该封闭物;d) perforating a thin, preferably sterile, gas-permeable ventilation tube, such as a cannula, through the closure;
e)任选施加真空,并由此优选使活性剂溶液被搅动;e) optionally applying a vacuum and thereby preferably agitating the active agent solution;
f)最后,大约12小时后,优选将细的、优选无菌的、透气的通风管取出,和f) Finally, after about 12 hours, preferably the thin, preferably sterile, gas-permeable ventilation tube is removed, and
g)任选将该植入体保留在步骤a)的优选无菌的、封闭的玻璃容器中直到使用为止。g) optionally keeping the implant in the preferably sterile, closed glass container of step a) until use.
对于这种一般的方法更有利的是,步骤a)的植入体具有至少一个金属的穿孔的或封闭的表面或层、具有陶瓷涂层、具有聚合物涂层和/或具有至少一个聚合物的、有孔的或封闭的表面或层。It is more advantageous for this general method that the implant of step a) has at least one metallic perforated or closed surface or layer, has a ceramic coating, has a polymer coating and/or has at least one polymer coating A porous, porous or closed surface or layer.
同样地,该一般方法优选的植入体为斯滕特固定模、斯滕特固定模移植物、移植物、移植物连接体、聚合物表面的斯滕特固定模或导管。Likewise, preferred implants for this general method are stents, stent grafts, grafts, graft connectors, polymer surfaced stents, or catheters.
如果活性剂选自药物活性剂,诸如免疫抑制剂或抗生素,则该一般方法更为优选。其中所述活性剂优选选自下列活性剂和其衍生物:This general approach is more preferred if the active agent is selected from pharmaceutical active agents, such as immunosuppressants or antibiotics. Wherein said active agent is preferably selected from following active agents and derivatives thereof:
(组1):吗多明、林西多明、硝普钠、硝酸甘油或一般的NO供体、可溶性鸟苷酸环化酶(sGC)的刺激物,例如BAY 41-2272(5-(环丙基-2-[1-(2-氟代苯甲基)-1H-吡唑并[3,4-n]吡啶-3-基]-嘧啶-4-基胺);肼屈嗪、维拉帕米、地尔硫、硝苯地平、尼莫地平或其他Ca++通道阻断剂、卡托普利、依那普利、赖诺普利、喹那普利或血管紧张素转化酶的其它抑制剂(血管紧张素转化酶抑制剂)、氯沙坦、candesartan、irbesartan、缬沙坦、或血管紧张素II受体拮抗剂;(Group 1): morphine, lincidomine, sodium nitroprusside, nitroglycerin or general NO donors, stimulators of soluble guanylate cyclase (sGC), such as BAY 41-2272 (5-( Cyclopropyl-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-n]pyridin-3-yl]-pyrimidin-4-ylamine); Hydralazine, verapamil, diltiazem , nifedipine, nimodipine or other Ca ++ channel blockers, captopril, enalapril, lisinopril, quinapril or other inhibitors of angiotensin converting enzyme (angiotensin converting enzyme inhibitors), losartan, candesartan, irbesartan, valsartan, or angiotensin II receptor antagonists;
(组2):地塞米松、倍他米松、泼尼松或其他皮质类固醇药、17-β-雌二醇、环孢菌素、麦考酚酸、VEGF、VEGF受体活化剂、曲尼司特、美洛昔康、celebrex、vioxx或其他COX-2拮抗剂、吲哚美辛、双氯芬酸、布洛芬、萘普生或其他COX-1抑制剂、血纤蛋白溶酶原激活物的抑制剂1(血纤蛋白溶酶原激活物抑制剂-1)或丝氨酸蛋白酶抑制剂;凝血酶抑制剂、例如水蛭素、hirulog、agratroban、PPACK或白介素-10;(Group 2): dexamethasone, betamethasone, prednisone or other corticosteroids, 17-beta-estradiol, cyclosporine, mycophenolic acid, VEGF, VEGF receptor activators, tranis Naproxen, meloxicam, celebrex, vioxx or other COX-2 antagonists, indomethacin, diclofenac, ibuprofen, naproxen or other COX-1 inhibitors, plasminogen activators Inhibitor 1 (plasminogen activator inhibitor-1) or a serine protease inhibitor; thrombin inhibitors such as hirudin, hirulog, agratroban, PPACK or interleukin-10;
(组3):西罗莫司、雷怕霉素、SDZ RAD(40-O-(2-羟乙基)雷怕霉素或其他雷怕霉素衍生物、PDGF拮抗剂、紫杉醇或7-己酰-紫杉酚、顺铂、长春碱、米托蒽醌、combretastatin A4、托泊替堪、甲氨蝶呤、flavopiridol、放线菌素D、Rheopro/阿昔单抗或普罗布考;(Group 3): sirolimus, rapamycin, SDZ RAD (40-O-(2-hydroxyethyl)rapamycin or other rapamycin derivatives, PDGF antagonists, paclitaxel or 7- Caproyl-paclitaxel, cisplatin, vinblastine, mitoxantrone, combretastatin A4, topotecan, methotrexate, flavopiridol, actinomycin D, Rheopro/abciximab, or probucol;
特别选自:Especially selected from:
(组1):吗多明、林西多明、硝普钠、硝酸甘油或一般的NO供体、可溶性鸟苷酸环化酶(sGC)的刺激物,例如BAY 41-2272(5-(环丙基-2-[1-(2-氟代苯甲基)-1H-吡唑并[3,4-n]吡啶-3-基]-嘧啶-4-基胺);卡托普利、依那普利、赖诺普利、喹那普利或血管紧张素转化酶的其它抑制剂(血管紧张素转化酶抑制剂)、氯沙坦、candesartan、irbesartan、缬沙坦、或血管紧张素II受体的其它拮抗剂。(Group 1): morphine, lincidomine, sodium nitroprusside, nitroglycerin or general NO donors, stimulators of soluble guanylate cyclase (sGC), such as BAY 41-2272 (5-( Cyclopropyl-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-n]pyridin-3-yl]-pyrimidin-4-ylamine); Captopril , enalapril, lisinopril, quinapril, or other inhibitors of angiotensin-converting enzyme (ACE inhibitors), losartan, candesartan, irbesartan, valsartan, or angiotensin Other antagonists of hormone II receptors.
(组2):地塞米松、倍他米松、泼尼松或皮质类固醇药、FK506(他克莫司)、VEGF、VEGF受体活化剂、血纤蛋白溶酶原激活物的抑制剂1(血纤蛋白溶酶原激活物抑制剂-1)或丝氨酸蛋白酶抑制剂;(Group 2): dexamethasone, betamethasone, prednisone or corticosteroids, FK506 (tacrolimus), VEGF, VEGF receptor activators, inhibitors of plasminogen activator 1 ( Plasminogen activator inhibitor-1) or a serine protease inhibitor;
(组3):西罗莫司、雷怕霉素、SDZ RAD(40-O-(2-羟乙基)雷怕霉素或其他雷怕霉素衍生物、PDGF拮抗剂、紫杉醇或7-己酰-紫杉酚、米托蒽醌、combretastatin A4、flavopiridol。(Group 3): sirolimus, rapamycin, SDZ RAD (40-O-(2-hydroxyethyl)rapamycin or other rapamycin derivatives, PDGF antagonists, paclitaxel or 7- Caproyl-paclitaxel, mitoxantrone, combretastatin A4, flavopiridol.
本发明进一步涉及本发明的植入体用于治疗或预防冠状或周围血管缩窄或闭塞,特别是缩窄或狭窄或再狭窄、优选用于预防再狭窄的用途。The present invention further relates to the use of the implant according to the invention for the treatment or prevention of coronary or peripheral vascular constriction or occlusion, in particular constriction or stenosis or restenosis, preferably for the prevention of restenosis.
本申请进一步涉及FK506(以下称为FK506)用于涂覆或用于生产治疗或预防冠状或周围血管缩窄或闭塞,特别是缩窄或狭窄或再狭窄、优选预防再狭窄的植入体的用途。The present application further relates to the use of FK506 (hereinafter referred to as FK506) for coating or for the production of implants for the treatment or prevention of coronary or peripheral vascular constriction or occlusion, in particular constriction or stenosis or restenosis, preferably for the prevention of restenosis use.
如果植入体是斯滕特固定模、斯滕特固定模移植物、移植物、移植物连接体、引导线、导管或导管泵、优选斯滕特固定模、斯滕特固定模移植物、移植物、移植物连接体,特别是斯滕特固定模、斯滕特固定模移植物或聚合物表面的斯滕特固定模,则对于FK506的该用途是优选的。If the implant is a stent, stent graft, graft, graft connector, guide wire, catheter or catheter pump, preferably a stent, stent graft, Grafts, graft connectors, in particular stents, stent grafts or polymer surfaced stents are then preferred for this use of FK506.
对于使用FK506,还优选FK506以这样一种方式结合或连接到植入体上,使其在植入体植入后从植入体上释放,优选以延迟方式释放。For the use of FK506, it is also preferred that the FK506 is bound or attached to the implant in such a way that it is released from the implant after implantation, preferably in a delayed manner.
本发明还单独涉及使用FK506用于治疗或预防冠状或周围血管缩窄或闭塞,特别是缩窄狭窄或再狭窄,优选用于预防再狭窄的用途。如上所述,FK506在此已经被发现在本申请范围内具有特别有利的特性。The present invention also relates solely to the use of FK506 for the treatment or prevention of coronary or peripheral vascular constriction or occlusion, in particular constriction or restenosis, preferably for the prevention of restenosis. As mentioned above, FK506 has been found here to have particularly advantageous properties within the scope of the present application.
在本发明的范围内,具有聚合物层或由聚合物构成的斯滕特固定模,或移植物或斯滕特固定模移植物被证明特别适用于FK506。这种类型的植入体,在本发明范围内被总称为聚合物表面斯滕特固定模,以前未被用活性剂涂覆。然而,出人意料地发现它们特别适用于本发明的目的,如在本申请范围内所进行的研究证明的一样,因为它们很容易荷载活性剂并均匀和有效地传输活性剂。然而,这种特性并不限于FK506,所以本申请还单独涉及聚合物表面斯滕特固定模,它包含化学共价结合或非共价结合或物理固定之形式的至少一种有生理和/或药学活性的活性剂。为了本发明的目的,聚合物表面斯滕特固定模意思是指用于本发明目的的具有聚合物表面的血管内植入体。在广义上讲,因此,聚合物表面斯滕特固定模包括用聚合物包被的或由聚合物构成的移植物和斯滕特固定模移植物、移植物连接体、斯滕特固定模。狭义上讲,是指用聚合物包被的或由聚合物构成的斯滕特固定模,和斯滕特固定模移植物。Within the scope of the present invention, stents, or grafts or stent grafts having or consisting of a polymer layer have proven to be particularly suitable for FK506. Implants of this type, collectively referred to within the scope of the present invention as polymer surface stents, have not previously been coated with an active agent. However, it has surprisingly been found that they are particularly suitable for the purposes of the present invention, as the studies carried out within the scope of the present application demonstrate, since they are easily loaded with active agents and transport them uniformly and effectively. However, this property is not limited to FK506, so the present application also relates solely to polymeric surface stents comprising at least one physiologically and/or Pharmaceutically active active agent. For the purposes of the present invention, a polymer surface stent means an endovascular implant having a polymer surface for the purposes of the present invention. In a broad sense, therefore, polymeric surface stents include polymer-coated or polymer-constructed grafts and stent grafts, graft connectors, stents. In a narrow sense, it refers to stents coated with or composed of polymers, and stent grafts.
对于聚合物表面斯滕特固定模,优选的是活性剂选自以下的药物活性剂,这些活性剂例如是免疫抑制剂或抗生素,优选选自下列活性剂和其衍生物:For polymeric surface stents, it is preferred that the active agent is selected from pharmaceutically active agents such as immunosuppressants or antibiotics, preferably selected from the following active agents and their derivatives:
(组1):吗多明、林西多明、硝普钠、硝酸甘油或一般的NO供体、可溶性鸟苷酸环化酶(sGC)的刺激物,例如BAY41-2272(5-(环丙基-2-[1-(2-氟代苯甲基)-1H-吡唑并[3,4-n]吡啶-3-基]-嘧啶-4-基胺);肼屈嗪、维拉帕米、地尔硫、硝苯地平、尼莫地平或其他Ca++通道阻断剂、卡托普利、依那普利、赖诺普利、喹那普利或血管紧张素转化酶的其它抑制剂(血管紧张素转化酶抑制剂)、氯沙坦、candesartan、irbesartan、缬沙坦、或血管紧张素II受体的其它拮抗剂;(Group 1): morphine, lincidomine, sodium nitroprusside, nitroglycerin or general NO donors, stimulators of soluble guanylate cyclase (sGC), such as BAY41-2272 (5-(cyclo Propyl-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-n]pyridin-3-yl]-pyrimidin-4-ylamine); hydralazine, vitamin lapamil, diltiazem , nifedipine, nimodipine or other Ca ++ channel blockers, captopril, enalapril, lisinopril, quinapril or other inhibitors of angiotensin converting enzyme (angiotensin converting enzyme inhibitors), losartan, candesartan, irbesartan, valsartan, or other antagonists of the angiotensin II receptor;
(组2):地塞米松、倍他米松、泼尼松或其他皮质类固醇药、FK506(他克莫司)、17-β-雌二醇、环孢菌素麦考酚酸、VEGF、VEGF受体活化剂、曲尼司特、美洛昔康、celebrex、vioxx或其他COX-2拮抗剂、吲哚美辛、双氯芬酸、布洛芬、萘普生或其他COX-1抑制剂、血纤蛋白溶酶原激活物的抑制剂1(血纤蛋白溶酶原激活物抑制剂-1)或丝氨酸蛋白酶抑制剂;凝血酶抑制剂、例如水蛭素、hirulog、agratroban、PPACK或白介素-10;(Group 2): dexamethasone, betamethasone, prednisone or other corticosteroids, FK506 (tacrolimus), 17-β-estradiol, cyclosporine mycophenolic acid, VEGF, VEGF Receptor activators, tranilast, meloxicam, celebrex, vioxx or other COX-2 antagonists, indomethacin, diclofenac, ibuprofen, naproxen or other COX-1 inhibitors, fibrin Inhibitor of lysinogen activator 1 (plasminogen activator inhibitor-1) or a serine protease inhibitor; thrombin inhibitors such as hirudin, hirulog, agratroban, PPACK or interleukin-10;
(组3):西罗莫司、雷怕霉素、SDZ RAD(40-O-(2-羟乙基)雷怕霉素或其他雷怕霉素衍生物、PDGF拮抗剂、紫杉醇或7-己酰-紫杉酚、顺铂、长春碱、米托蒽醌、combretastatin A4、托泊替堪、甲氨蝶呤、flavopiridol、放线菌素D、Rheopro/阿昔单抗或普罗布考;(Group 3): sirolimus, rapamycin, SDZ RAD (40-O-(2-hydroxyethyl)rapamycin or other rapamycin derivatives, PDGF antagonists, paclitaxel or 7- Caproyl-paclitaxel, cisplatin, vinblastine, mitoxantrone, combretastatin A4, topotecan, methotrexate, flavopiridol, actinomycin D, Rheopro/abciximab, or probucol;
特别是选自:In particular selected from:
(组1):吗多明、林西多明、硝普钠、硝酸甘油或一般的NO供体、可溶性鸟苷酸环化酶(sGC)的刺激物,例如BAY 41-2272(5-(环丙基-2-[1-(2-氟代苯甲基)-1H-吡唑并[3,4-n]吡啶-3-基]-嘧啶-4-基胺);卡托普利、依那普利、赖诺普利、喹那普利或血管紧张素转化酶的其它抑制剂(血管紧张素转化酶抑制剂)、氯沙坦、candesartan、irbesartan、缬沙坦、或血管紧张素II受体的其它拮抗剂。(Group 1): morphine, lincidomine, sodium nitroprusside, nitroglycerin or general NO donors, stimulators of soluble guanylate cyclase (sGC), such as BAY 41-2272 (5-( Cyclopropyl-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-n]pyridin-3-yl]-pyrimidin-4-ylamine); Captopril , enalapril, lisinopril, quinapril, or other inhibitors of angiotensin-converting enzyme (ACE inhibitors), losartan, candesartan, irbesartan, valsartan, or angiotensin Other antagonists of hormone II receptors.
(组2):地塞米松、倍他米松、泼尼松或其他皮质类固醇药、FK506(他克莫司)、VEGF、VEGF受体活化剂、血纤蛋白溶酶原激活物的抑制剂1(血纤蛋白溶酶原激活物抑制剂-1)或丝氨酸蛋白酶抑制剂;(Group 2): Inhibitors of dexamethasone, betamethasone, prednisone or other corticosteroids, FK506 (tacrolimus), VEGF, VEGF receptor activators, plasminogen activators1 (plasminogen activator inhibitor-1) or a serine protease inhibitor;
(组3):西罗莫司、雷怕霉素、SDZ RAD(40-O-(2-羟乙基)雷怕霉素或其他雷怕霉素衍生物、PDGF拮抗剂、紫杉醇或7-己酰-紫杉酚、米托蒽醌、combretastatin A4、flavopiridol。(Group 3): sirolimus, rapamycin, SDZ RAD (40-O-(2-hydroxyethyl)rapamycin or other rapamycin derivatives, PDGF antagonists, paclitaxel or 7- Caproyl-paclitaxel, mitoxantrone, combretastatin A4, flavopiridol.
和/或聚合物表面斯滕特固定模包括至少两个,优选2或3个选自组1到3中之一的生理和/或药物活性剂,优选一组最多一种活性剂。and/or polymeric surface stents comprising at least two, preferably 2 or 3, physiologically and/or pharmaceutically active agents selected from one of
在这种聚合物表面斯滕特固定模的优选实施方案中,当其它活性剂选自前面所述的组1时,这种活性剂在植入后头24-72小时内从植入体内释放,和/或如果其它活性剂选自上述组2,则它在植入后头48小时-21天内从植入体释放,和/或如果其它活性剂选自上述组3,则它在植入后14天到3个月的时间内从植入体释放。In a preferred embodiment of this polymeric surface stent, when the other active agent is selected from
一般地适用于本发明的聚合物表面斯滕特固定模的是,特别包含FK506的植入体的所有上文所述的实施方式、生产方法和用途对于本发明的聚合物表面斯滕特固定模也是优选的,因此,本发明也涉及这些,只要所述实施方式仍然是聚合物表面斯滕特固定模。Generally applicable to the polymer surface stent according to the invention, all the above-described embodiments, production methods and uses of the implant comprising FK506 in particular are applicable to the polymer surface stent according to the invention Stents are also preferred, and the invention therefore also relates to these, as long as the embodiment is still a polymer surface stent.
本发明还涉及用或通过本发明的植入体或聚合物表面斯滕特固定模治疗需要此治疗的人或动物。The invention also relates to the treatment of a human or animal in need of such treatment with or by the implant or polymer surface stent of the invention.
本发明进一步通过下面部分的实施例进行解释,但不应理解为对本发明的限制。The present invention is further illustrated by the examples in the following section, which should not be construed as limiting the invention.
实施例和附图Examples and drawings
附图:Attached picture:
图1显示了FK506从冠状血管斯滕特固定模移植物上释放,其表面由已经荷载了FK506的PTFE构成。Figure 1 shows the release of FK506 from a coronary stent graft whose surface consisted of PTFE that had been loaded with FK506.
图2显示candesartan和喹那普利从斯滕特固定模移植物释放,其中其表面是由已经荷载了candesartan和喹那普利的PTFE构成。Figure 2 shows the release of candesartan and quinapril from a stent graft whose surface is composed of PTFE that has been loaded with candesartan and quinapril.
图3显示candesartan和喹那普利从聚氨基甲酸酯涂覆的斯滕特固定模上的释放,其中该涂层已经添加有candesartan和喹那普利。Figure 3 shows the release of candesartan and quinapril from a polyurethane coated stent to which the coating has been added candesartan and quinapril.
图4显示candesartan和喹那普利从用聚氨基甲酸酯/水凝胶共混物涂覆的斯滕特固定模上的释放,其中该涂层已经添加了candesartan和喹那普利。Figure 4 shows the release of candesartan and quinapril from a stent coated with a polyurethane/hydrogel blend to which candesartan and quinapril had been added.
图5显示相应涂覆的斯滕特固定模植入兔子中后,血液中FK506的释放。Figure 5 shows the release of FK506 in the blood after corresponding coated stents were implanted in rabbits.
图6显示在有或没有含FK506的相应涂层的植入的斯滕特固定模上的内膜面积。Figure 6 shows the intimal area on implanted stents with and without corresponding coatings containing FK506.
图7显示对有或没有含FK506的相应涂层的斯滕特固定模之植入的炎症反应。Figure 7 shows the inflammatory response to implantation of stents with and without corresponding coatings containing FK506.
实施例Example
实施例1:Example 1:
在斯滕特固定模移植物上进行目标化合物的荷载所有的值都用微克表示。Loading of target compounds on stent grafts All values are expressed in micrograms.
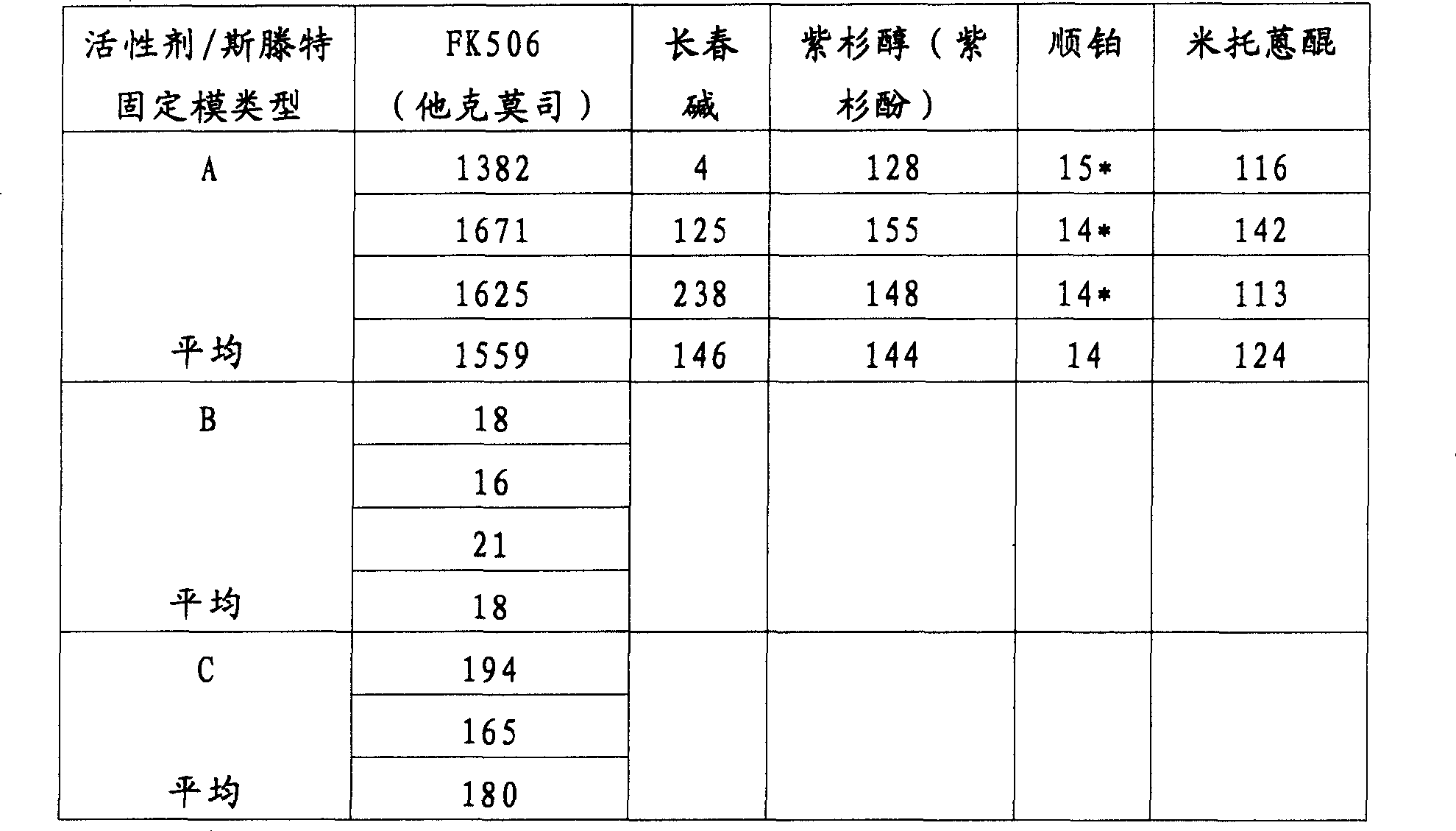
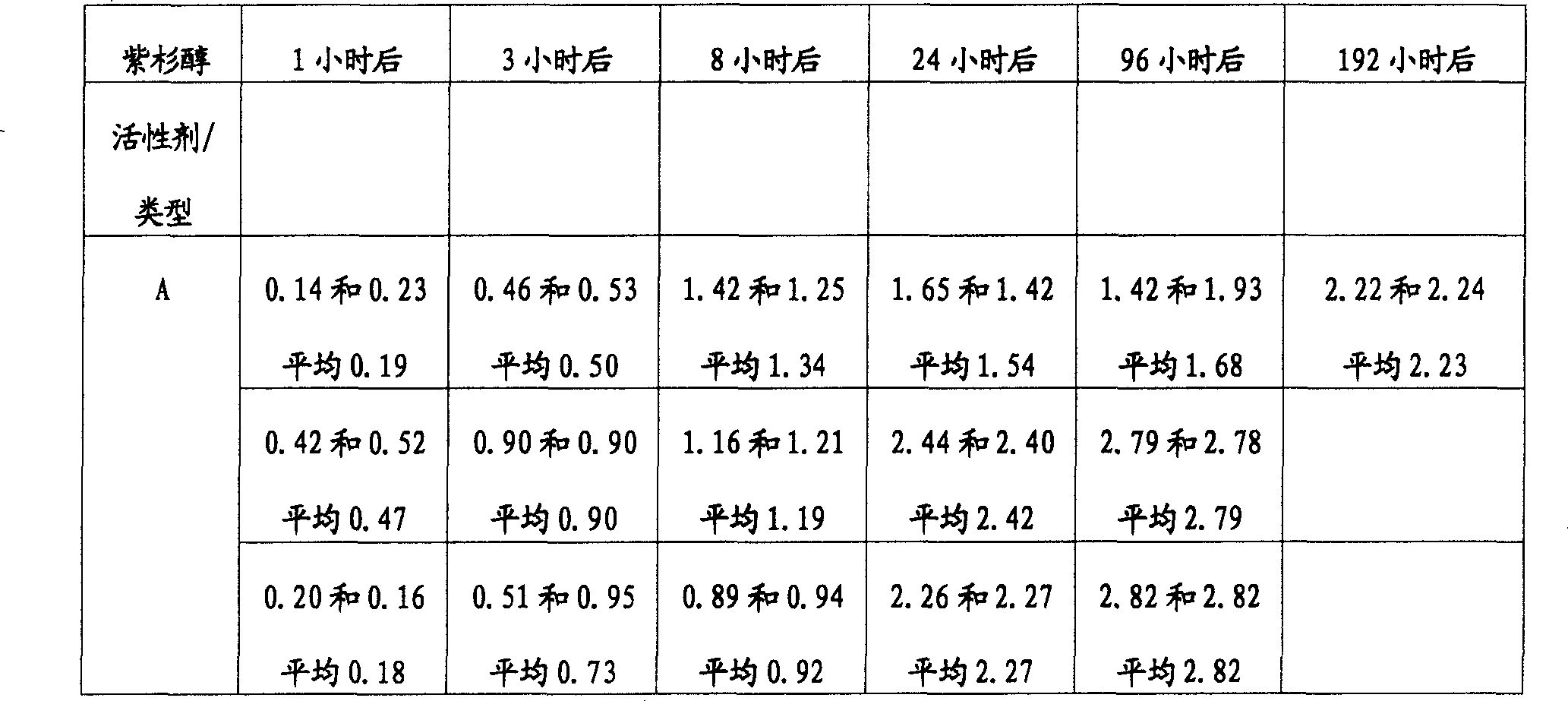
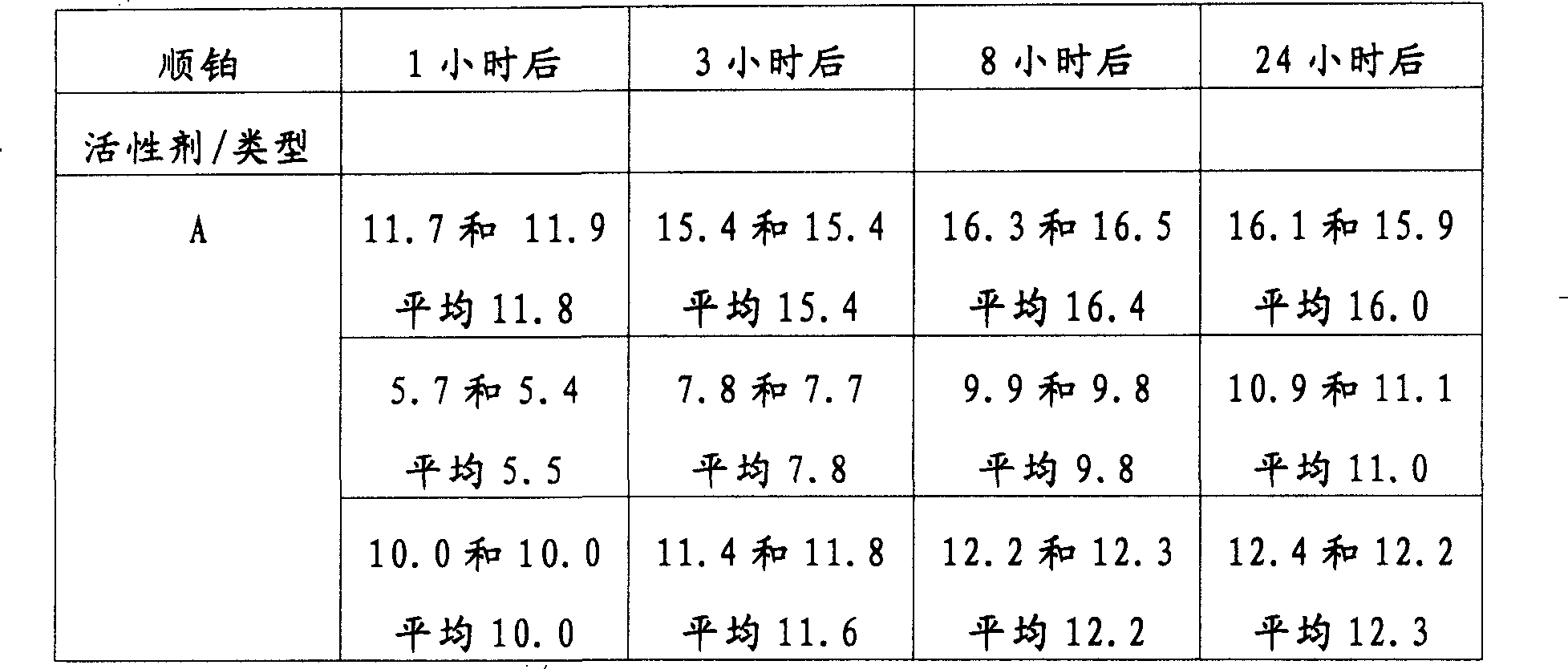
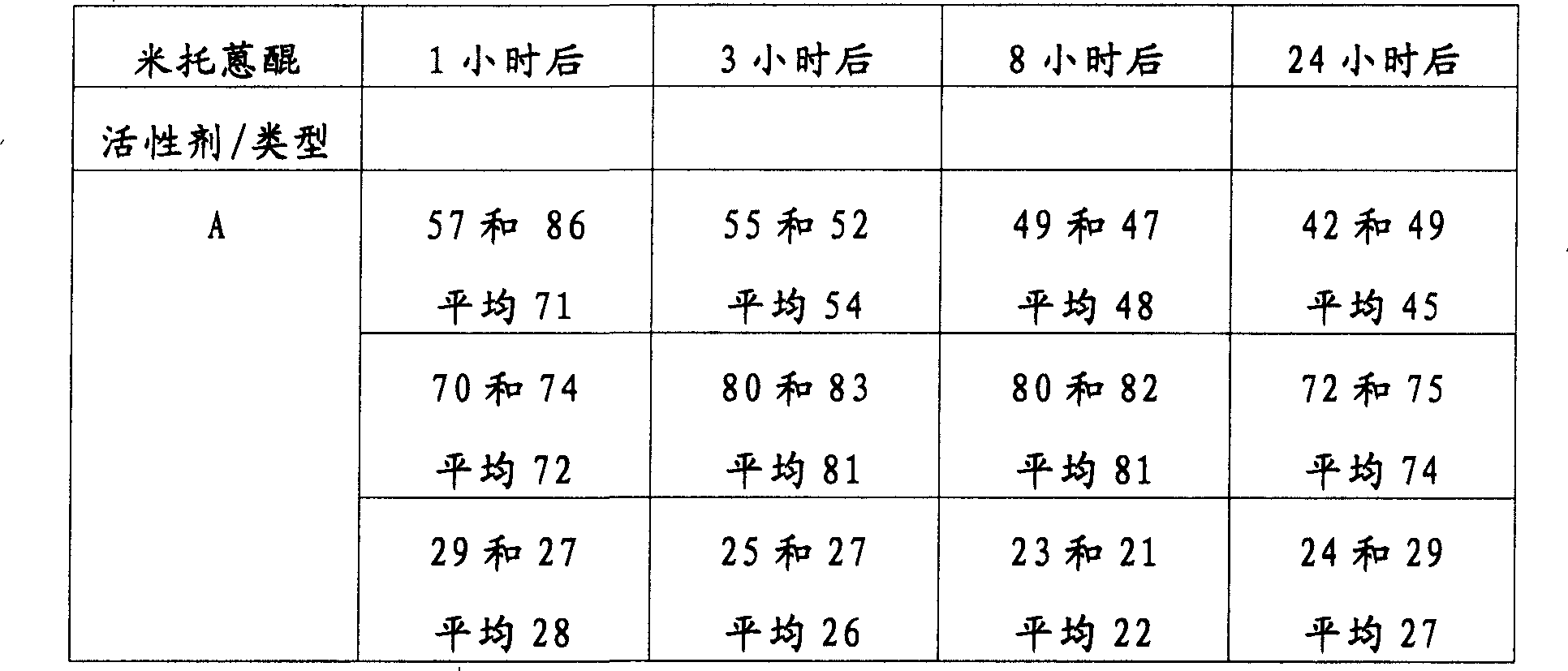
表1Table 1
*由AAS测量*Measured by AAS
A:用溶解的固体进行的试验,其中具有PTFE聚合物层的斯滕特固定模移植物浸渍到该溶液中。A: Test performed with dissolved solids in which a stent graft with a PTFE polymer layer was dipped.
B:用静脉注射(i.v.)溶液进行的试验,其中具有PTFE聚合物层的斯滕特固定模移植物浸渍到该溶液中。B: Test performed with an intravenous (i.v.) solution into which a stent graft with a PTFE polymer layer is dipped.
C:用静脉注射溶液进行的试验,其中将聚氨基甲酸酯涂覆的斯滕特固定模浸渍到该溶液中。C: Test performed with an intravenous solution into which a polyurethane-coated stent was dipped.
实施例2Example 2
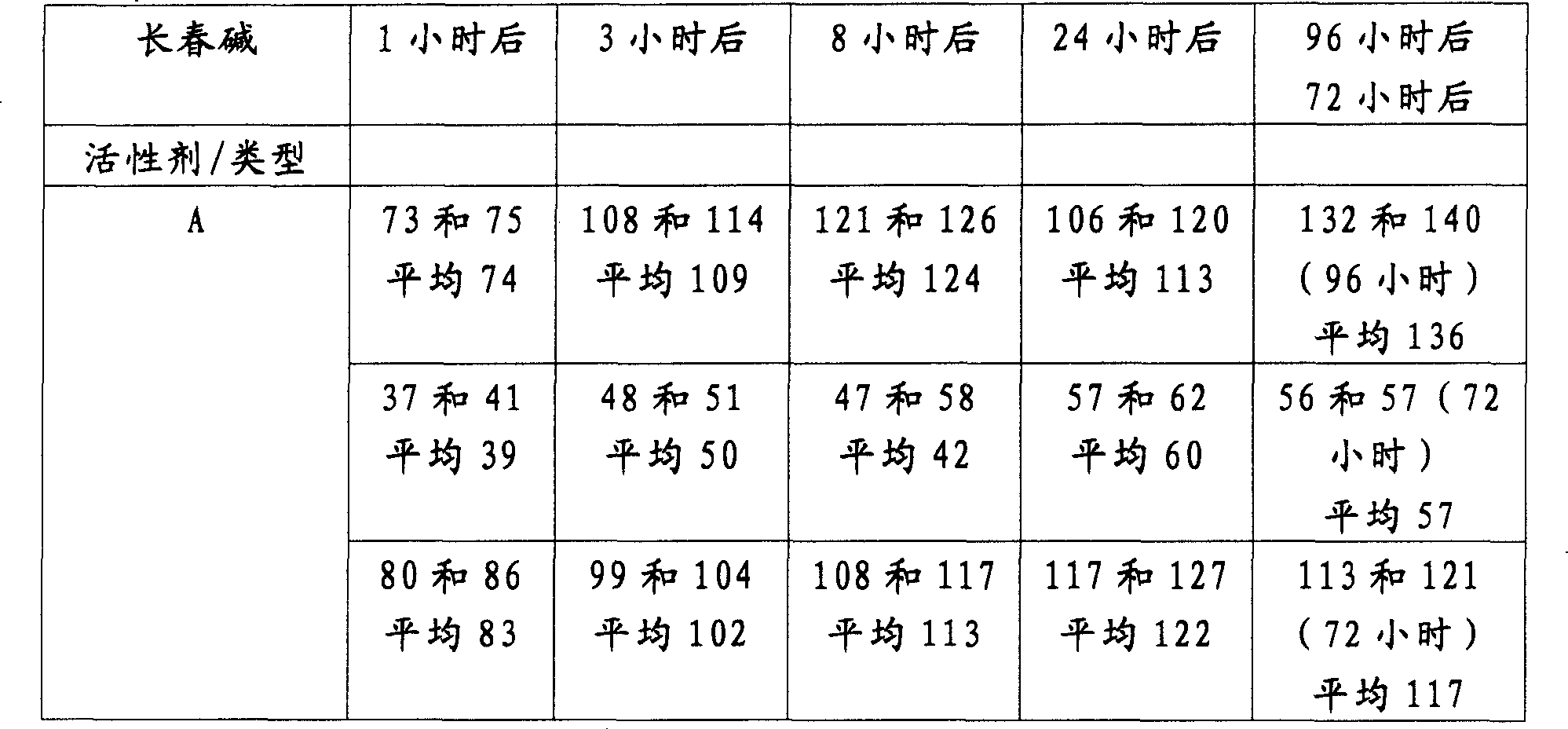
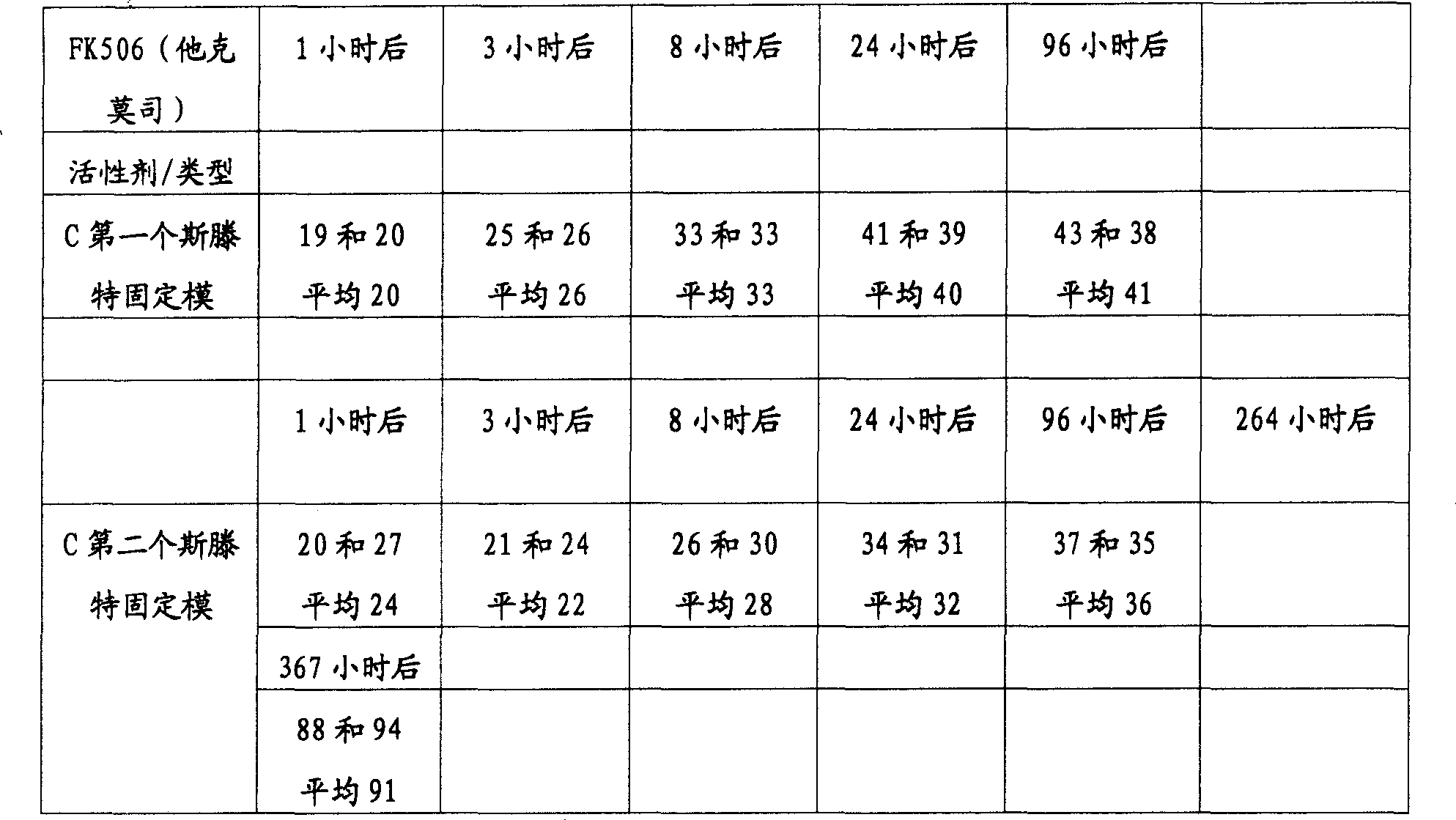
用各种方法生产的本发明的斯滕特固定模移植物以及一般的聚合物表面斯滕特固定模的释放型式:Release profiles of stent grafts of the present invention and polymer surface stents in general produced by various methods:
所有的值都用微克表示。All values are expressed in micrograms.
为了分析活性剂的释放,将斯滕特固定模在370C、10毫升的PBS缓冲液(用叠氮化钠稳定的)中温育。在限定时间过后,取出2×1毫升的溶液并进行分析。用新鲜的PBS缓冲液(用叠氮化钠稳定的)代替这2毫升溶液。To analyze the release of active agent, stents were incubated at 370C in 10 ml of PBS buffer (stabilized with sodium azide). After a defined time, 2 x 1 ml of the solution was withdrawn and analyzed. This 2 ml solution was replaced with fresh PBS buffer (stabilized with sodium azide).
下表表示活性剂在溶液中的总的释放量。这意味着在取出的分析用的缓冲液中的活性剂的量被加入到下一次取出的量上。The table below shows the total release of active agent in solution. This means that the amount of active agent in the withdrawn assay buffer is added to the next withdrawn amount.
表2Table 2
A:用溶解的固体进行的试验,其中具有PTFE聚合物层的斯滕特固定模移植物浸渍到该溶液中。A: Test performed with dissolved solids in which a stent graft with a PTFE polymer layer was dipped.
表3table 3
A:用溶解的固体进行的试验,其中具有PTFE聚合物层的斯滕特固定模移植物浸渍到该溶液中。A: Test performed with dissolved solids in which a stent graft with a PTFE polymer layer was dipped.
表4Table 4
C:用静脉注射溶液进行的试验,其中将聚氨基甲酸酯涂覆的斯滕特固定模浸渍到该溶液中。C: Test performed with an intravenous solution into which a polyurethane-coated stent was dipped.
表5table 5
A:用溶解的固体进行的试验,其中具有PTFE聚合物层的斯滕特固定模移植物浸渍到该溶液中。A: Test performed with dissolved solids in which a stent graft with a PTFE polymer layer was dipped.
表6Table 6
A:用溶解的固体进行的试验,其中具有PTFE聚合物层的斯滕特固定模移植物浸渍到该溶液中。A: Test performed with dissolved solids in which a stent graft with a PTFE polymer layer was dipped.
表7Table 7
A:用溶解的固体进行的试验,其中具有PTFE聚合物层的斯滕特固定模移植物浸渍到该溶液中。A: Test performed with dissolved solids in which a stent graft with a PTFE polymer layer was dipped.
实施例3Example 3
FK506涂覆的植入体的生产方法(1)Production method of FK506 coated implant (1)
●10毫克FK506溶解于3毫升乙醇中;Dissolve 10 mg of FK506 in 3 ml of ethanol;
●在室温、真空下将未涂覆的不锈钢的钢斯滕特固定模浸渍于该溶液中过夜;Immerse an uncoated stainless steel stent in this solution overnight under vacuum at room temperature;
●用盐水洗三次,每次1分钟;●Wash three times with salt water, each time for 1 minute;
●干燥过夜。●Let dry overnight.
实施例4Example 4
FK506涂覆的斯滕特固定模移植物的生产方法(2)Production method of FK506 coated stent graft (2)
●可以根据斯滕特固定模的长度和直径和根据在身体内的用途来改变FK506的用量。在这里,使用每厘米斯滕特固定模长度10-200微克FK506的剂量。●The amount of FK506 can be changed according to the length and diameter of the stent and according to the use in the body. Here, doses of 10-200 micrograms of FK506 per cm of stent length were used.
●将FK506溶解(适于所需剂量)于小玻璃容器内的乙醇中,溶液用视觉检查以察看有没有晶体。• Dissolve FK506 (appropriate for the desired dose) in ethanol in a small glass container and visually inspect the solution for crystals.
●将未进行预处理的斯滕特固定模移植物(”JOSTENT CoronaryStent Graft”)安装在支架上,所述斯滕特固定模移植物由两层不锈钢的斯滕特固定模和PTFE膜的三明治结构构成。A non-pretreated stent graft ("JOSTENT CoronaryStent Graft") consisting of a sandwich of two layers of stainless steel stent and PTFE membrane was mounted on the stent Structural composition.
●使用吸液管将适当量的FK506溶液(5到30微升)吸取到安装的斯滕特固定模移植物上。• Use a pipette to pipette an appropriate amount of FK506 solution (5 to 30 microliters) onto the mounted stent graft.
●可按照需要重复这一步,以便将大量的FK506滴加到植入体上,通常一次到两次。在这里,该程序重复一次。●This step can be repeated as needed to apply large amounts of FK506 to the implant, usually once or twice. Here, the procedure is repeated once.
●在斯滕特固定模移植物全部荷载后,将其小心地从支架上取下。• After the stent graft is fully loaded, it is carefully removed from the stent.
●经过在空气中短暂的干燥后(大约5分钟)(任选输入热),将斯滕特固定模包装。• After a brief drying (approximately 5 minutes) in air (with optional heat input), the stent is packaged.
●在透镜下检查所有斯滕特固定模,如果发现医学絮状物,则丢弃。• Inspect all stents under the lens and discard if medical floe is found.
实施例5Example 5
用FK506涂覆的具有陶瓷涂层的斯滕特固定模的生产方法(3)Production method of stent with ceramic coating coated with FK506 (3)
●将50毫克FK506溶解于玻璃容器内的10毫升乙醇中。根据需要,通过稀释(在1:1和1:20之间稀释)用该储备液制备所有其它浓度的液体;• Dissolve 50 mg of FK506 in 10 ml of ethanol in a glass container. Prepare all other concentrations of fluids from this stock solution by dilution (between 1:1 and 1:20) as required;
●视觉检查该溶液中有无晶体;- Visually inspect the solution for crystals;
●将用氧化铝层涂覆的未荷载的斯滕特固定模(如在PCT申请WO00/25841中公开的一样,也见实施例7陶瓷涂层)安装在支架上;An unloaded stent coated with an aluminum oxide layer (as disclosed in PCT application WO 00/25841, see also Example 7 Ceramic coating) was mounted on the bracket;
●使用皮下注射器将FK506溶液(5到50微升)滴加到斯滕特固定模上。这应该使溶液分布到整个斯滕特固定模上;• Add FK506 solution (5 to 50 microliters) dropwise onto the stent using a hypodermic syringe. This should distribute the solution over the entire stent;
●从支架上小心地取下荷载的斯滕特固定模;● Carefully remove the loaded stent from the bracket;
●经过在空气中短暂的干燥后(大约5分钟)(任选输入热),将斯滕特固定模包装。• After a brief drying (approximately 5 minutes) in air (with optional heat input), the stent is packaged.
●在透镜下检查所有斯滕特固定模是否有医学絮状物,任选丢弃。• Inspect all stents for medical floc under the lens and optionally discard.
实施例6Example 6
新的备选生产方法(4)(尤其适用于FK506,但也适用于其它活性剂),特别是用于生产无菌斯滕特固定模、斯滕特固定模移植物和/或聚合物表面斯滕特固定模:New alternative production method (4) (especially for FK506, but also for other active agents), especially for the production of sterile stents, stent grafts and/or polymeric surfaces Stent stent:
●使用不比所用的斯滕特固定模大多少的小的注射瓶;Use small injection vials not much larger than the stent used;
●将无菌的冠状血管斯滕特固定模(CSGs)无菌地放置在无菌的注射瓶中;Aseptically place sterile coronary stents (CSGs) in sterile injection vials;
●将0.5毫升无菌过滤的FK506溶液(3.3毫克/毫升,在乙醇中)加入到小瓶中;Add 0.5 mL of sterile-filtered FK506 solution (3.3 mg/mL in ethanol) to the vial;
●用橡皮塞封闭小瓶;●Close the vial with a rubber stopper;
●用带有无菌过滤器的无菌注射插管从橡皮塞的中部刺破;pierce the middle of the rubber stopper with a sterile injection cannula with a sterile filter;
●将小瓶水平放置在干燥器中真空下的滚筒装置上;- place the vial horizontally on a tumbler unit under vacuum in a desiccator;
●在真空下将该小瓶滚动过夜;• Roll the vial under vacuum overnight;
●取出注射针;●Remove the injection needle;
●不进行冲洗;●No flushing;
●无菌CSGs待用。●Sterile CSGs are ready for use.
实施例7Example 7
释放活性剂的斯滕特固定模的可能活性剂的选择,该固定模尤其是具有多层,例如聚合物表面斯滕特固定模或斯滕特固定模移植物等Selection of possible active agents for active agent-releasing stents, especially with multiple layers, such as polymer surface stents or stent grafts, etc.
荷载方法描述如下面的技术方法。The loading method is described as the technical method below.
列举的活性剂还包括衍生物和所有类型的盐、对映体、外消旋物、碱或游离酸。The active agents listed also include derivatives and all types of salts, enantiomers, racemates, bases or free acids.
这里最感兴趣的是这样的斯滕特固定模、斯滕特固定模移植物和聚合物表面斯滕特固定模,它们包含并相应地释放至少一种、两种或三种下面所列的活性剂。Of greatest interest here are stents, stent grafts and polymeric surface stents which contain and correspondingly release at least one, two or three of the following listed active agent.
根据它们优选的释放曲线或释放时间,将所列的活性剂划分为组1-3。The active agents listed are divided into groups 1-3 according to their preferred release profile or release time.
而且优选斯滕特固定模、斯滕特固定模移植物和聚合物表面斯滕特固定模包含来自不同组的活性剂。It is also preferred that the stents, stent grafts and polymeric surface stents comprise active agents from different groups.
表8Table 8
表9Table 9
表10Table 10
通过从冠状或周围血管斯滕特固定模的活性剂荷载表面的直接传输可获得活性剂的局部给药。通过使用各种技术方法可获得斯滕特固定模的活性剂荷载表面。这些方法中的每一种都可以这样的方式进行,即,活性剂从表面短暂地(数小时)或长期释放(数天)。通过对表面进行特定的修饰,例如聚合物载体或陶瓷表面的疏水性或亲水性侧链,可以调节释放动力学。Localized administration of the active agent can be achieved by direct delivery from the active agent-loaded surface of a coronary or peripheral vascular stent. The active agent loaded surface of the stent can be obtained by using various technical methods. Each of these methods can be performed in such a way that the active agent is released from the surface either briefly (hours) or long-term (days). Release kinetics can be tuned by specific modifications of the surface, such as hydrophobic or hydrophilic side chains on polymeric supports or ceramic surfaces.
●陶瓷涂层●Ceramic coating
可以将活性剂(例如以10微克到10毫克的FK506),通过浸渍、喷雾或类似技术荷载到具有多孔表面的氧化铝涂层上(专利申请DE19855421、DE19910188、WO 00/25841)。活性剂的剂量依赖于目标血管的类型和病人的状况,选择该剂量使得能够充分地抑制增生、迁移和T细胞反应,同时不影响痊愈过程。活性剂可以用作水性或有机溶液,例如在DMSO、DMF和乙醇中。在喷雾或浸渍后(任选在弱真空条件下),将处理的斯滕特固定模干燥并将该程序重复1-50次。在最后的干燥步骤后,可以在室温下用水或等渗盐水冲洗斯滕特固定模1分钟,然后再干燥。在已经用适当溶剂将活性剂溶解之后,可通过标准方法(HPLC、LC-MS)来分析活性剂的含量。使用标准释放测量仪器可以测量释放动力学。The active agent (eg FK506 at 10 micrograms to 10 milligrams) can be loaded onto alumina coatings with porous surfaces by dipping, spraying or similar techniques (patent applications DE19855421, DE19910188, WO 00/25841). The dosage of the active agent is selected such that proliferation, migration and T cell responses are sufficiently inhibited while not affecting the healing process, depending on the type of target vessel and the condition of the patient. Active agents can be employed as aqueous or organic solutions, for example in DMSO, DMF and ethanol. After spraying or dipping (optionally under mild vacuum), the treated stent is dried and the procedure is repeated 1-50 times. After the final drying step, the stent can be rinsed with water or isotonic saline for 1 min at room temperature before drying. After the active agent has been dissolved with a suitable solvent, the content of the active agent can be analyzed by standard methods (HPLC, LC-MS). Release kinetics can be measured using standard release measurement equipment.
●PTFE膜:斯滕特固定模移植物PTFE membrane: stent graft
这里使用与上面描述的类似的方法。活性剂沉积到多孔PTFE膜的凹陷中。A similar approach to that described above is used here. The active agent is deposited into the depressions of the porous PTFE membrane.
●一般的聚合物涂层●General polymer coating
许多聚合物适用于荷载活性剂:Many polymers are suitable for loading active agents:
甲基丙烯酸酯聚合物、聚氨基甲酸酯涂层、PTFE涂层、水凝胶涂层。活性剂可以应用到终表面上(如上所述),也可以直接加到聚合反应溶液中。这种技术方法在其它细节上与上面已经描述的那些方法对应。Methacrylate polymer, polyurethane coating, PTFE coating, hydrogel coating. The active agent can be applied to the final surface (as described above) or it can be added directly to the polymerization solution. This technical method corresponds in other details to those already described above.
●机械方法●Mechanical method
机械方法基于通过切割激光形成在斯滕特固定模支架上的凹陷。然后可以将活性剂充填这些凹陷。机械(凹陷)方法可以与本身荷载有活性剂的可生物降解的薄的涂层结合。在从生物可降解涂层上最初释放后,活性剂可以从填充活性剂的凹陷中长期释放。这种技术方法在其它细节上与上面已经描述的那些方法相对应。The mechanical method is based on indentations formed in the stent holder by cutting lasers. These depressions can then be filled with active agent. The mechanical (depression) method can be combined with a thin biodegradable coating itself loaded with active agent. After the initial release from the biodegradable coating, the active agent can be released long term from the active agent filled depressions. This technical method corresponds in other details to those already described above.
实施例8Example 8
candesartan和喹那普利从(聚合物)涂覆的植入体上的活性剂释放:Candesartan and Quinapril Release of Active Agents from (Polymer) Coated Implants:
a)给斯滕特固定模上配置多孔的PTFE膜。然后将这种膜荷载上candesartan和喹那普利(各1毫克)的活性剂混合物。这两种活性剂同时释放。测量在PBS(磷酸缓冲的盐水)中的释放。图2显示candesartan和喹那普利从斯滕特固定模上的活性剂的释放。a) Arranging a porous PTFE membrane on the stent. This membrane was then loaded with an active agent mixture of candesartan and quinapril (1 mg each). The two active agents are released simultaneously. Release was measured in PBS (phosphate buffered saline). Figure 2 shows the release of candesartan and quinapril from the active agents on the stent.
b)给开放式(open-cell)不锈钢斯滕特固定模上配置聚氨基甲酸酯涂层。将candesartan和喹那普利(基于聚氨基甲酸酯的含量各为15%)的活性剂混合物引入到5%的聚氨基甲酸酯在二甲基乙酰胺里的溶液中,并通过喷雾方法将其施用到斯滕特固定模上。图3显示相应的活性剂的释放。b) Applying a polyurethane coating to an open-cell stainless steel stent. An active agent mixture of candesartan and quinapril (15% each based on polyurethane content) was introduced into a 5% solution of polyurethane in dimethylacetamide and sprayed Apply it to the stent. Figure 3 shows the release of the corresponding active agent.
c)给开放式不锈钢斯滕特固定模上配置聚氨基甲酸酯/水凝胶涂层。将candesartan和喹那普利(基于聚氨基甲酸酯的含量各为15%)的活性剂混合物引入到5%的聚合物共混物在二甲基乙酰胺里的溶液中,并通过喷雾方法将其施用到斯滕特固定模上。图4显示相应的活性剂的释放。c) A polyurethane/hydrogel coating is applied to the open stainless steel stent. An active agent mixture of candesartan and quinapril (15% each based on polyurethane) was introduced into a 5% solution of the polymer blend in dimethylacetamide and sprayed by spraying. Apply it to the stent. Figure 4 shows the release of the corresponding active agent.
实施例9Example 9
FK506的释放动力学和作用模式的动物研究Animal studies on the release kinetics and mode of action of FK506
用氧化铝陶瓷层涂覆不锈钢冠状血管斯滕特固定模(JOSTENTFlex,16毫米)。这种涂层用作FK506的载体(如在实施例5和7的“陶瓷涂层”中描述的)。在这里,斯滕特固定模荷载总量60微克的FK506。在兔子的动物研究中(n=7),将这些涂覆有FK506的斯滕特固定模和未涂覆的通常的斯滕特固定模植入到新西兰兔的颈动脉中。研究FK506的释放和FK506对内膜的生长和巨噬细胞和淋巴细胞的形成的影响。A stainless steel coronary stent (JOSTENTFlex, 16 mm) was coated with an alumina ceramic layer. This coating was used as a support for FK506 (as described in Examples 5 and 7 under "Ceramic Coating"). Here, the stent was loaded with a total of 60 µg of FK506. In an animal study in rabbits (n=7), these FK506-coated stents and uncoated conventional stents were implanted into the carotid arteries of New Zealand rabbits. The release of FK506 and the effect of FK506 on the growth of intima and the formation of macrophages and lymphocytes were studied.
用HPLC测量1小时、8小时、24小时和48小时后在兔子的血液中FK506的量,以确定FK506的释放。在图5中可以清楚地看出FK506随时间的极好的释放动力学。在28天后处死这些兔子,并检查植入的斯滕特固定模。在光学显微镜下定量在斯滕特固定模内的新生长内膜(新内膜)的面积。从图6中可以清楚地看到,与通常的斯滕特固定模相比,荷载有FK506的陶瓷涂覆的斯滕特固定模内新内膜的形成明显减少。在荷载有60微克的FK506的情况下有53%的下降。而且这种下降伴随着可检测的炎症病灶的减少。从图7中可以明显看出,与通常的斯滕特固定模相比,巨噬细胞和淋巴细胞的形成也明显下降。The release of FK506 was determined by measuring the amount of FK506 in blood of rabbits after 1 hour, 8 hours, 24 hours and 48 hours by HPLC. The excellent release kinetics of FK506 over time can be clearly seen in FIG. 5 . The rabbits were sacrificed after 28 days and the implanted stents were examined. The area of newly grown intima (neointima) within the stents was quantified under a light microscope. From Fig. 6, it can be clearly seen that the formation of neointima in the ceramic-coated stents loaded with FK506 was significantly reduced compared with the usual stents. There was a 53% reduction in the loading with 60 micrograms of FK506. And this decline was accompanied by a reduction in detectable inflammatory foci. As evident from Figure 7, the formation of macrophages and lymphocytes was also significantly reduced compared to the usual stents.
因此,使用带有其上施加了FK506的陶瓷涂层的斯滕特固定模在植入后导致新内膜和炎症病灶的明显减少。Thus, the use of a stent with a ceramic coating on which FK506 was applied resulted in a significant reduction in neointima and inflammatory foci after implantation.
Claims (48)
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE10107339.9 | 2001-02-16 | ||
| DE2001107339 DE10107339A1 (en) | 2001-02-16 | 2001-02-16 | Implant used for treating vascular narrowing or occlusion, especially for controlling restenosis contains FK506 in chemically bound or physically fixed form |
| DE2001127011 DE10127011A1 (en) | 2001-06-05 | 2001-06-05 | Implant used for treating vascular narrowing or occlusion, especially for controlling restenosis contains FK506 in chemically bound or physically fixed form |
| DE10127011.9 | 2001-06-05 | ||
| DE10127330.4 | 2001-06-06 | ||
| DE2001127330 DE10127330A1 (en) | 2001-06-06 | 2001-06-06 | Implant used for treating vascular narrowing or occlusion, especially for controlling restenosis contains FK506 in chemically bound or physically fixed form |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN1547490A CN1547490A (en) | 2004-11-17 |
| CN100469394C true CN100469394C (en) | 2009-03-18 |
Family
ID=27214297
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNB028050916A Expired - Fee Related CN100469394C (en) | 2001-02-16 | 2002-02-18 | Implant with FK506 |
Country Status (13)
| Country | Link |
|---|---|
| US (2) | US8187320B2 (en) |
| EP (2) | EP1372753B1 (en) |
| JP (1) | JP4290985B2 (en) |
| KR (2) | KR20090130879A (en) |
| CN (1) | CN100469394C (en) |
| AT (1) | ATE368482T1 (en) |
| AU (2) | AU2002233342B2 (en) |
| BR (1) | BR0207321A (en) |
| CA (1) | CA2435306C (en) |
| DE (1) | DE50210591D1 (en) |
| IL (2) | IL156887A0 (en) |
| NZ (1) | NZ540000A (en) |
| WO (1) | WO2002065947A2 (en) |
Families Citing this family (135)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7611533B2 (en) * | 1995-06-07 | 2009-11-03 | Cook Incorporated | Coated implantable medical device |
| US6774278B1 (en) * | 1995-06-07 | 2004-08-10 | Cook Incorporated | Coated implantable medical device |
| US7713297B2 (en) | 1998-04-11 | 2010-05-11 | Boston Scientific Scimed, Inc. | Drug-releasing stent with ceramic-containing layer |
| EP1372753B1 (en) | 2001-02-16 | 2007-08-01 | Abbott Laboratories Vascular Enterprises Limited | Implants with fk506 for prophylaxis and treatment of restonoses |
| US6613083B2 (en) † | 2001-05-02 | 2003-09-02 | Eckhard Alt | Stent device and method |
| WO2003002243A2 (en) | 2001-06-27 | 2003-01-09 | Remon Medical Technologies Ltd. | Method and device for electrochemical formation of therapeutic species in vivo |
| WO2003020331A1 (en) * | 2001-09-03 | 2003-03-13 | Oxigene, Inc. | Implants containing combretastatin a-4 |
| GB0121980D0 (en) | 2001-09-11 | 2001-10-31 | Cathnet Science Holding As | Expandable stent |
| US7351255B2 (en) | 2001-12-03 | 2008-04-01 | Xtent, Inc. | Stent delivery apparatus and method |
| US7147656B2 (en) | 2001-12-03 | 2006-12-12 | Xtent, Inc. | Apparatus and methods for delivery of braided prostheses |
| US8080048B2 (en) | 2001-12-03 | 2011-12-20 | Xtent, Inc. | Stent delivery for bifurcated vessels |
| US7294146B2 (en) | 2001-12-03 | 2007-11-13 | Xtent, Inc. | Apparatus and methods for delivery of variable length stents |
| US7137993B2 (en) | 2001-12-03 | 2006-11-21 | Xtent, Inc. | Apparatus and methods for delivery of multiple distributed stents |
| US7309350B2 (en) | 2001-12-03 | 2007-12-18 | Xtent, Inc. | Apparatus and methods for deployment of vascular prostheses |
| US20040186551A1 (en) | 2003-01-17 | 2004-09-23 | Xtent, Inc. | Multiple independent nested stent structures and methods for their preparation and deployment |
| US7892273B2 (en) | 2001-12-03 | 2011-02-22 | Xtent, Inc. | Custom length stent apparatus |
| US7182779B2 (en) | 2001-12-03 | 2007-02-27 | Xtent, Inc. | Apparatus and methods for positioning prostheses for deployment from a catheter |
| US20030135266A1 (en) | 2001-12-03 | 2003-07-17 | Xtent, Inc. | Apparatus and methods for delivery of multiple distributed stents |
| AU2003250913A1 (en) * | 2002-07-08 | 2004-01-23 | Abbott Laboratories Vascular Enterprises Limited | Drug eluting stent and methods of manufacture |
| DE60226236T3 (en) * | 2002-09-20 | 2011-12-15 | Abbott Laboratories Vascular Enterprises Ltd. | A rough-surfaced stent and its manufacturing process |
| DE10244847A1 (en) | 2002-09-20 | 2004-04-01 | Ulrich Prof. Dr. Speck | Medical device for drug delivery |
| PL376169A1 (en) * | 2002-11-15 | 2005-12-27 | Novartis Ag | Drug delivery system |
| US8685428B2 (en) * | 2002-12-10 | 2014-04-01 | Advanced Cardiovascular Systems, Inc. | Therapeutic composition and a method of coating implantable medical devices |
| EP1449546B1 (en) | 2003-02-21 | 2010-10-27 | Sorin Biomedica Cardio S.R.L. | A process for producing stents and corresponding stent |
| EP1603485A4 (en) * | 2003-02-26 | 2011-03-30 | Medivas Llc | Bioactive stents and methods for use thereof |
| US20040236414A1 (en) * | 2003-05-23 | 2004-11-25 | Brar Balbir S. | Devices and methods for treatment of stenotic regions |
| US7241308B2 (en) | 2003-06-09 | 2007-07-10 | Xtent, Inc. | Stent deployment systems and methods |
| US7553324B2 (en) | 2003-10-14 | 2009-06-30 | Xtent, Inc. | Fixed stent delivery devices and methods |
| US7326236B2 (en) | 2003-12-23 | 2008-02-05 | Xtent, Inc. | Devices and methods for controlling and indicating the length of an interventional element |
| ITTO20040056A1 (en) * | 2004-02-05 | 2004-05-05 | Sorin Biomedica Cardio Spa | STENT FOR THE ENDOLIMINAL DELIVERY OF PRINCIPLES OR ACTIVE AGENTS |
| AU2005222085B2 (en) * | 2004-03-05 | 2011-01-06 | Regenerx Biopharmaceuticals, Inc. | Treating or preventing extracellular matrix build-up |
| US7323006B2 (en) | 2004-03-30 | 2008-01-29 | Xtent, Inc. | Rapid exchange interventional devices and methods |
| WO2005097186A2 (en) * | 2004-04-05 | 2005-10-20 | Medivas, Llc | Bioactive stents for type ii diabetics and methods for use thereof |
| US8317859B2 (en) | 2004-06-28 | 2012-11-27 | J.W. Medical Systems Ltd. | Devices and methods for controlling expandable prostheses during deployment |
| US20050288766A1 (en) | 2004-06-28 | 2005-12-29 | Xtent, Inc. | Devices and methods for controlling expandable prostheses during deployment |
| US7919110B2 (en) * | 2005-01-25 | 2011-04-05 | Boston Scientific Scimed, Inc. | Medical device drug release regions containing non-covalently bound polymers |
| US9452001B2 (en) * | 2005-02-22 | 2016-09-27 | Tecres S.P.A. | Disposable device for treatment of infections of human limbs |
| US20060222627A1 (en) * | 2005-03-30 | 2006-10-05 | Andrew Carter | Optimizing pharmacodynamics of therapeutic agents for treating vascular tissue |
| US9034025B2 (en) | 2005-05-23 | 2015-05-19 | Ostial Corporation | Balloon catheters and methods for use |
| US8118852B2 (en) * | 2005-07-13 | 2012-02-21 | Cook Medical Technologies Llc | Introducer for self-expandable medical device |
| WO2007024964A1 (en) * | 2005-08-22 | 2007-03-01 | Incept, Llc | Flared stents and apparatus and methods for making and using them |
| WO2007040249A1 (en) | 2005-10-06 | 2007-04-12 | Kaneka Corporation | Stent to be placed in the living body |
| WO2007046935A2 (en) * | 2005-10-14 | 2007-04-26 | Abbott Laboratories | Compositions, systems, kits, and methods of administering rapamycin analogs with paclitaxel using medical devices |
| US8840660B2 (en) | 2006-01-05 | 2014-09-23 | Boston Scientific Scimed, Inc. | Bioerodible endoprostheses and methods of making the same |
| US8089029B2 (en) | 2006-02-01 | 2012-01-03 | Boston Scientific Scimed, Inc. | Bioabsorbable metal medical device and method of manufacture |
| US8652198B2 (en) | 2006-03-20 | 2014-02-18 | J.W. Medical Systems Ltd. | Apparatus and methods for deployment of linked prosthetic segments |
| US20070224234A1 (en) * | 2006-03-22 | 2007-09-27 | Mark Steckel | Medical devices having biodegradable polymeric regions |
| US20070224235A1 (en) | 2006-03-24 | 2007-09-27 | Barron Tenney | Medical devices having nanoporous coatings for controlled therapeutic agent delivery |
| US8187620B2 (en) | 2006-03-27 | 2012-05-29 | Boston Scientific Scimed, Inc. | Medical devices comprising a porous metal oxide or metal material and a polymer coating for delivering therapeutic agents |
| DE102006016598A1 (en) * | 2006-04-06 | 2007-11-15 | Heraeus Kulzer Gmbh | Coated vascular implants |
| US8048150B2 (en) | 2006-04-12 | 2011-11-01 | Boston Scientific Scimed, Inc. | Endoprosthesis having a fiber meshwork disposed thereon |
| US8815275B2 (en) | 2006-06-28 | 2014-08-26 | Boston Scientific Scimed, Inc. | Coatings for medical devices comprising a therapeutic agent and a metallic material |
| JP2009542359A (en) | 2006-06-29 | 2009-12-03 | ボストン サイエンティフィック リミテッド | Medical device with selective covering |
| WO2008017028A2 (en) | 2006-08-02 | 2008-02-07 | Boston Scientific Scimed, Inc. | Endoprosthesis with three-dimensional disintegration control |
| US8088789B2 (en) | 2006-09-13 | 2012-01-03 | Elixir Medical Corporation | Macrocyclic lactone compounds and methods for their use |
| EP2083834B1 (en) | 2006-09-13 | 2017-06-21 | Elixir Medical Corporation | Macrocyclic lactone compounds and methods for their use |
| US10695327B2 (en) | 2006-09-13 | 2020-06-30 | Elixir Medical Corporation | Macrocyclic lactone compounds and methods for their use |
| WO2008033711A2 (en) | 2006-09-14 | 2008-03-20 | Boston Scientific Limited | Medical devices with drug-eluting coating |
| WO2008034031A2 (en) | 2006-09-15 | 2008-03-20 | Boston Scientific Limited | Bioerodible endoprostheses and methods of making the same |
| JP2010503485A (en) | 2006-09-15 | 2010-02-04 | ボストン サイエンティフィック リミテッド | Medical device and method for manufacturing the same |
| ATE516827T1 (en) * | 2006-09-15 | 2011-08-15 | Boston Scient Scimed | BIOLOGICALLY ERODABLE ENDOPROSTHESIS WITH BIOSTABLE INORGANIC LAYERS |
| ATE517590T1 (en) | 2006-09-15 | 2011-08-15 | Boston Scient Ltd | BIOLOGICALLY ERODABLE ENDOPROTHESES |
| WO2008036548A2 (en) | 2006-09-18 | 2008-03-27 | Boston Scientific Limited | Endoprostheses |
| US20080294236A1 (en) * | 2007-05-23 | 2008-11-27 | Boston Scientific Scimed, Inc. | Endoprosthesis with Select Ceramic and Polymer Coatings |
| US7981150B2 (en) | 2006-11-09 | 2011-07-19 | Boston Scientific Scimed, Inc. | Endoprosthesis with coatings |
| US20080181928A1 (en) * | 2006-12-22 | 2008-07-31 | Miv Therapeutics, Inc. | Coatings for implantable medical devices for liposome delivery |
| EP2125065B1 (en) | 2006-12-28 | 2010-11-17 | Boston Scientific Limited | Bioerodible endoprostheses and methods of making same |
| US20080199510A1 (en) | 2007-02-20 | 2008-08-21 | Xtent, Inc. | Thermo-mechanically controlled implants and methods of use |
| US8431149B2 (en) | 2007-03-01 | 2013-04-30 | Boston Scientific Scimed, Inc. | Coated medical devices for abluminal drug delivery |
| US8070797B2 (en) | 2007-03-01 | 2011-12-06 | Boston Scientific Scimed, Inc. | Medical device with a porous surface for delivery of a therapeutic agent |
| US8486132B2 (en) | 2007-03-22 | 2013-07-16 | J.W. Medical Systems Ltd. | Devices and methods for controlling expandable prostheses during deployment |
| US8067054B2 (en) | 2007-04-05 | 2011-11-29 | Boston Scientific Scimed, Inc. | Stents with ceramic drug reservoir layer and methods of making and using the same |
| US7976915B2 (en) | 2007-05-23 | 2011-07-12 | Boston Scientific Scimed, Inc. | Endoprosthesis with select ceramic morphology |
| US8002823B2 (en) | 2007-07-11 | 2011-08-23 | Boston Scientific Scimed, Inc. | Endoprosthesis coating |
| US7942926B2 (en) | 2007-07-11 | 2011-05-17 | Boston Scientific Scimed, Inc. | Endoprosthesis coating |
| US9284409B2 (en) | 2007-07-19 | 2016-03-15 | Boston Scientific Scimed, Inc. | Endoprosthesis having a non-fouling surface |
| US7931683B2 (en) | 2007-07-27 | 2011-04-26 | Boston Scientific Scimed, Inc. | Articles having ceramic coated surfaces |
| US8815273B2 (en) | 2007-07-27 | 2014-08-26 | Boston Scientific Scimed, Inc. | Drug eluting medical devices having porous layers |
| WO2009018340A2 (en) | 2007-07-31 | 2009-02-05 | Boston Scientific Scimed, Inc. | Medical device coating by laser cladding |
| JP2010535541A (en) | 2007-08-03 | 2010-11-25 | ボストン サイエンティフィック リミテッド | Coating for medical devices with large surface area |
| US8052745B2 (en) | 2007-09-13 | 2011-11-08 | Boston Scientific Scimed, Inc. | Endoprosthesis |
| US20090076591A1 (en) * | 2007-09-19 | 2009-03-19 | Boston Scientific Scimed, Inc. | Stent Design Allowing Extended Release of Drug and/or Enhanced Adhesion of Polymer to OD Surface |
| CA2702183A1 (en) * | 2007-10-10 | 2009-04-16 | Miv Therapeutics Inc. | Lipid coatings for implantable medical devices |
| US7938855B2 (en) | 2007-11-02 | 2011-05-10 | Boston Scientific Scimed, Inc. | Deformable underlayer for stent |
| US8216632B2 (en) | 2007-11-02 | 2012-07-10 | Boston Scientific Scimed, Inc. | Endoprosthesis coating |
| US8029554B2 (en) | 2007-11-02 | 2011-10-04 | Boston Scientific Scimed, Inc. | Stent with embedded material |
| US7833266B2 (en) * | 2007-11-28 | 2010-11-16 | Boston Scientific Scimed, Inc. | Bifurcated stent with drug wells for specific ostial, carina, and side branch treatment |
| US9101503B2 (en) | 2008-03-06 | 2015-08-11 | J.W. Medical Systems Ltd. | Apparatus having variable strut length and methods of use |
| JP5581311B2 (en) | 2008-04-22 | 2014-08-27 | ボストン サイエンティフィック サイムド,インコーポレイテッド | MEDICAL DEVICE HAVING INORGANIC MATERIAL COATING AND MANUFACTURING METHOD THEREOF |
| WO2009132176A2 (en) | 2008-04-24 | 2009-10-29 | Boston Scientific Scimed, Inc. | Medical devices having inorganic particle layers |
| US7998192B2 (en) | 2008-05-09 | 2011-08-16 | Boston Scientific Scimed, Inc. | Endoprostheses |
| US8236046B2 (en) | 2008-06-10 | 2012-08-07 | Boston Scientific Scimed, Inc. | Bioerodible endoprosthesis |
| EP2303350A2 (en) | 2008-06-18 | 2011-04-06 | Boston Scientific Scimed, Inc. | Endoprosthesis coating |
| US7951193B2 (en) * | 2008-07-23 | 2011-05-31 | Boston Scientific Scimed, Inc. | Drug-eluting stent |
| US7985252B2 (en) | 2008-07-30 | 2011-07-26 | Boston Scientific Scimed, Inc. | Bioerodible endoprosthesis |
| US12076258B2 (en) | 2008-09-25 | 2024-09-03 | Advanced Bifurcation Systems Inc. | Selective stent crimping |
| US8821562B2 (en) | 2008-09-25 | 2014-09-02 | Advanced Bifurcation Systems, Inc. | Partially crimped stent |
| CN102215780B (en) | 2008-09-25 | 2015-10-14 | 高级分支系统股份有限公司 | Part crimped stent |
| US12324756B2 (en) | 2008-09-25 | 2025-06-10 | Advanced Bifurcation Systems Inc. | System and methods for treating a bifurcation |
| US8828071B2 (en) | 2008-09-25 | 2014-09-09 | Advanced Bifurcation Systems, Inc. | Methods and systems for ostial stenting of a bifurcation |
| US11298252B2 (en) | 2008-09-25 | 2022-04-12 | Advanced Bifurcation Systems Inc. | Stent alignment during treatment of a bifurcation |
| US8382824B2 (en) | 2008-10-03 | 2013-02-26 | Boston Scientific Scimed, Inc. | Medical implant having NANO-crystal grains with barrier layers of metal nitrides or fluorides |
| US8231980B2 (en) | 2008-12-03 | 2012-07-31 | Boston Scientific Scimed, Inc. | Medical implants including iridium oxide |
| US8267992B2 (en) | 2009-03-02 | 2012-09-18 | Boston Scientific Scimed, Inc. | Self-buffering medical implants |
| US8071156B2 (en) | 2009-03-04 | 2011-12-06 | Boston Scientific Scimed, Inc. | Endoprostheses |
| US8287937B2 (en) | 2009-04-24 | 2012-10-16 | Boston Scientific Scimed, Inc. | Endoprosthese |
| US8888840B2 (en) * | 2009-05-20 | 2014-11-18 | Boston Scientific Scimed, Inc. | Drug eluting medical implant |
| US9265633B2 (en) | 2009-05-20 | 2016-02-23 | 480 Biomedical, Inc. | Drug-eluting medical implants |
| US20110319987A1 (en) * | 2009-05-20 | 2011-12-29 | Arsenal Medical | Medical implant |
| EP3858299B1 (en) * | 2009-05-20 | 2025-10-08 | Lyra Therapeutics, Inc. | Method of loading a self-expanding implant |
| ES2676537T3 (en) * | 2009-06-02 | 2018-07-20 | Concept Medical Inc. | Rejuvenation of the coronary artery by improving blood flow with the help of inserting nano-balls (encapsulated nanoparticles) containing therapeutic agents by means of a non-implantable tissue device and, thus, providing tissue release treat the required cell cycle |
| KR101060607B1 (en) | 2009-07-09 | 2011-08-31 | 전남대학교산학협력단 | Method of manufacturing drug-releasing stent using titanium oxide thin film coating |
| WO2011119573A1 (en) | 2010-03-23 | 2011-09-29 | Boston Scientific Scimed, Inc. | Surface treated bioerodible metal endoprostheses |
| WO2011119882A1 (en) | 2010-03-24 | 2011-09-29 | Advanced Bifurcation Systems, Inc | Methods and systems for treating a bifurcation with provisional side branch stenting |
| WO2011119884A1 (en) | 2010-03-24 | 2011-09-29 | Advanced Bifurcation Systems, Inc | System and methods for treating a bifurcation |
| CN103068345B (en) | 2010-03-24 | 2015-10-14 | 高级分支系统股份有限公司 | Furcation is being carried out to the rack alignment in processing procedure |
| EP4424283A3 (en) | 2011-02-08 | 2024-12-25 | Advanced Bifurcation Systems Inc. | System and methods for treating a bifurcation with a fully crimped stent |
| EP2672925B1 (en) | 2011-02-08 | 2017-05-03 | Advanced Bifurcation Systems, Inc. | Multi-stent and multi-balloon apparatus for treating bifurcations |
| US9005270B2 (en) * | 2012-03-27 | 2015-04-14 | Medtronic Vascular, Inc. | High metal to vessel ratio stent and method |
| KR101649305B1 (en) | 2015-03-03 | 2016-08-18 | 한국전기연구원 | Medical stent and method of manufacturing irregularities are formed on the surface |
| CN110944689B (en) | 2017-06-07 | 2022-12-09 | 施菲姆德控股有限责任公司 | Intravascular fluid movement devices, systems, and methods of use |
| US11529318B2 (en) * | 2017-06-23 | 2022-12-20 | University of Pittsburgh—of the Commonwealth System of Higher Education | Devices and methods for local delivery of tacrolimus |
| US11511103B2 (en) | 2017-11-13 | 2022-11-29 | Shifamed Holdings, Llc | Intravascular fluid movement devices, systems, and methods of use |
| EP4085965A1 (en) | 2018-02-01 | 2022-11-09 | Shifamed Holdings, LLC | Intravascular blood pumps and methods of use and manufacture |
| WO2020028537A1 (en) | 2018-07-31 | 2020-02-06 | Shifamed Holdings, Llc | Intravascaular blood pumps and methods of use |
| JP7470108B2 (en) | 2018-10-05 | 2024-04-17 | シファメド・ホールディングス・エルエルシー | Intravascular blood pump and method of use |
| WO2021011473A1 (en) | 2019-07-12 | 2021-01-21 | Shifamed Holdings, Llc | Intravascular blood pumps and methods of manufacture and use |
| WO2021016372A1 (en) | 2019-07-22 | 2021-01-28 | Shifamed Holdings, Llc | Intravascular blood pumps with struts and methods of use and manufacture |
| US12465748B2 (en) | 2019-08-07 | 2025-11-11 | Supira Medical, Inc. | Catheter blood pumps and collapsible pump housings |
| EP4021369A4 (en) * | 2019-08-26 | 2023-12-27 | Mervyn B. Forman | MEDICAL DEVICES FOR THE CONTINUOUS DELIVERY OF THERAPEUTIC AGENTS |
| EP4034192B1 (en) | 2019-09-25 | 2025-12-24 | Supira Medical, Inc. | Intravascular blood pump systems and methods of use and control thereof |
| US12102815B2 (en) | 2019-09-25 | 2024-10-01 | Shifamed Holdings, Llc | Catheter blood pumps and collapsible pump housings |
| WO2021062260A1 (en) | 2019-09-25 | 2021-04-01 | Shifamed Holdings, Llc | Catheter blood pumps and collapsible blood conduits |
| US12409310B2 (en) | 2019-12-11 | 2025-09-09 | Shifamed Holdings, Llc | Descending aorta and vena cava blood pumps |
| KR102532990B1 (en) | 2021-01-28 | 2023-05-15 | 경북대학교 산학협력단 | A manufacturing method for medical stent using laser |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1162259A (en) * | 1994-10-26 | 1997-10-15 | 诺瓦蒂斯有限公司 | pharmaceutical composition |
| CN1250382A (en) * | 1997-01-27 | 2000-04-12 | 斯蒂斯生物聚合物公司 | Bonding layer for surface coating of medical devices |
| CN1360951A (en) * | 2000-12-28 | 2002-07-31 | 微创医疗器械(上海)有限公司 | Blood vessel support with coating to prevent blood vessel from becoming strictured again |
Family Cites Families (46)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ATE121954T1 (en) | 1988-08-24 | 1995-05-15 | Marvin J Slepian | ENDOLUMINAL SEAL WITH BIDEGRADABLE POLYMERS. |
| US5477864A (en) * | 1989-12-21 | 1995-12-26 | Smith & Nephew Richards, Inc. | Cardiovascular guidewire of enhanced biocompatibility |
| IL94138A (en) | 1990-04-19 | 1997-03-18 | Instent Inc | Device for the treatment of constricted fluid conducting ducts |
| US5411552A (en) | 1990-05-18 | 1995-05-02 | Andersen; Henning R. | Valve prothesis for implantation in the body and a catheter for implanting such valve prothesis |
| US5443498A (en) | 1991-10-01 | 1995-08-22 | Cook Incorporated | Vascular stent and method of making and implanting a vacsular stent |
| US5464450A (en) | 1991-10-04 | 1995-11-07 | Scimed Lifesystems Inc. | Biodegradable drug delivery vascular stent |
| US5500013A (en) | 1991-10-04 | 1996-03-19 | Scimed Life Systems, Inc. | Biodegradable drug delivery vascular stent |
| WO1993006819A1 (en) | 1991-10-10 | 1993-04-15 | Alza Corporation | Osmotic drug delivery devices with hydrophobic wall materials |
| US5449382A (en) | 1992-11-04 | 1995-09-12 | Dayton; Michael P. | Minimally invasive bioactivated endoprosthesis for vessel repair |
| US5443458A (en) | 1992-12-22 | 1995-08-22 | Advanced Cardiovascular Systems, Inc. | Multilayered biodegradable stent and method of manufacture |
| JPH0767895A (en) | 1993-06-25 | 1995-03-14 | Sumitomo Electric Ind Ltd | Antibacterial artificial blood vessel and antibacterial surgical suture |
| US5519042A (en) * | 1994-01-13 | 1996-05-21 | Hoechst Aktiengesellschaft | Method of treating hyperproliferative vascular disease |
| US5891108A (en) | 1994-09-12 | 1999-04-06 | Cordis Corporation | Drug delivery stent |
| AU5025096A (en) | 1995-02-27 | 1996-09-18 | Instent Inc. | Hollow stent |
| US6099562A (en) | 1996-06-13 | 2000-08-08 | Schneider (Usa) Inc. | Drug coating with topcoat |
| CA2178541C (en) * | 1995-06-07 | 2009-11-24 | Neal E. Fearnot | Implantable medical device |
| US6132765A (en) * | 1996-04-12 | 2000-10-17 | Uroteq Inc. | Drug delivery via therapeutic hydrogels |
| KR0176334B1 (en) | 1996-08-19 | 1999-04-01 | 박원훈 | Coating method of endogenous infectious insert metal surface and its treatment technology |
| ZA9710342B (en) * | 1996-11-25 | 1998-06-10 | Alza Corp | Directional drug delivery stent and method of use. |
| US5843172A (en) | 1997-04-15 | 1998-12-01 | Advanced Cardiovascular Systems, Inc. | Porous medicated stent |
| US5836966A (en) | 1997-05-22 | 1998-11-17 | Scimed Life Systems, Inc. | Variable expansion force stent |
| KR100244164B1 (en) * | 1997-07-15 | 2000-03-02 | 김용옥 | Water soluble polymer takurolimus conjugate compounds and its manufacturing process |
| DE19744135C1 (en) * | 1997-09-29 | 1999-03-25 | Schering Ag | Medical implants coated with epothilone |
| US5972027A (en) | 1997-09-30 | 1999-10-26 | Scimed Life Systems, Inc | Porous stent drug delivery system |
| US6153252A (en) * | 1998-06-30 | 2000-11-28 | Ethicon, Inc. | Process for coating stents |
| IL125336A0 (en) * | 1998-07-14 | 1999-03-12 | Yissum Res Dev Co | Compositions for inhibition and treatment of restinosis |
| US20020038146A1 (en) | 1998-07-29 | 2002-03-28 | Ulf Harry | Expandable stent with relief cuts for carrying medicines and other materials |
| AUPP584198A0 (en) * | 1998-09-14 | 1998-10-08 | Fujisawa Pharmaceutical Co., Ltd. | New use |
| US6358276B1 (en) | 1998-09-30 | 2002-03-19 | Impra, Inc. | Fluid containing endoluminal stent |
| US6245104B1 (en) | 1999-02-28 | 2001-06-12 | Inflow Dynamics Inc. | Method of fabricating a biocompatible stent |
| DE19855421C2 (en) * | 1998-11-02 | 2001-09-20 | Alcove Surfaces Gmbh | Implant |
| US6214042B1 (en) | 1998-11-10 | 2001-04-10 | Precision Vascular Systems, Inc. | Micro-machined stent for vessels, body ducts and the like |
| EP1057460A1 (en) | 1999-06-01 | 2000-12-06 | Numed, Inc. | Replacement valve assembly and method of implanting same |
| AU1366701A (en) | 1999-09-23 | 2001-04-24 | Clarence C. Lee | Antimicrobial and anti-inflammatory endovascular (cardiovascular) stent |
| CA2392006C (en) | 1999-11-17 | 2011-03-15 | Microchips, Inc. | Microfabricated devices for the delivery of molecules into a carrier fluid |
| US6776796B2 (en) | 2000-05-12 | 2004-08-17 | Cordis Corportation | Antiinflammatory drug and delivery device |
| US6709451B1 (en) | 2000-07-14 | 2004-03-23 | Norman Noble, Inc. | Channeled vascular stent apparatus and method |
| US6758859B1 (en) | 2000-10-30 | 2004-07-06 | Kenny L. Dang | Increased drug-loading and reduced stress drug delivery device |
| US6471980B2 (en) | 2000-12-22 | 2002-10-29 | Avantec Vascular Corporation | Intravascular delivery of mycophenolic acid |
| US7077859B2 (en) | 2000-12-22 | 2006-07-18 | Avantec Vascular Corporation | Apparatus and methods for variably controlled substance delivery from implanted prostheses |
| US6752829B2 (en) | 2001-01-30 | 2004-06-22 | Scimed Life Systems, Inc. | Stent with channel(s) for containing and delivering a biologically active material and method for manufacturing the same |
| EP1372753B1 (en) | 2001-02-16 | 2007-08-01 | Abbott Laboratories Vascular Enterprises Limited | Implants with fk506 for prophylaxis and treatment of restonoses |
| US6613083B2 (en) | 2001-05-02 | 2003-09-02 | Eckhard Alt | Stent device and method |
| US6685745B2 (en) | 2001-05-15 | 2004-02-03 | Scimed Life Systems, Inc. | Delivering an agent to a patient's body |
| EP1465624A1 (en) * | 2002-01-10 | 2004-10-13 | Novartis AG | Drug delivery systems for the prevention and treatment of vascular diseases comprising rapamycin and derivatives thereof |
| AU2003250913A1 (en) | 2002-07-08 | 2004-01-23 | Abbott Laboratories Vascular Enterprises Limited | Drug eluting stent and methods of manufacture |
-
2002
- 2002-02-18 EP EP02700248A patent/EP1372753B1/en not_active Expired - Lifetime
- 2002-02-18 IL IL15688702A patent/IL156887A0/en unknown
- 2002-02-18 JP JP2002565512A patent/JP4290985B2/en not_active Expired - Fee Related
- 2002-02-18 CN CNB028050916A patent/CN100469394C/en not_active Expired - Fee Related
- 2002-02-18 DE DE50210591T patent/DE50210591D1/en not_active Expired - Lifetime
- 2002-02-18 WO PCT/EP2002/001707 patent/WO2002065947A2/en not_active Ceased
- 2002-02-18 AU AU2002233342A patent/AU2002233342B2/en not_active Ceased
- 2002-02-18 NZ NZ540000A patent/NZ540000A/en not_active IP Right Cessation
- 2002-02-18 BR BR0207321-8A patent/BR0207321A/en not_active IP Right Cessation
- 2002-02-18 CA CA2435306A patent/CA2435306C/en not_active Expired - Fee Related
- 2002-02-18 KR KR1020097025020A patent/KR20090130879A/en not_active Ceased
- 2002-02-18 EP EP07113417A patent/EP1842569A3/en not_active Withdrawn
- 2002-02-18 AT AT02700248T patent/ATE368482T1/en not_active IP Right Cessation
- 2002-02-18 KR KR1020037010759A patent/KR100994543B1/en not_active Expired - Fee Related
-
2003
- 2003-07-10 IL IL156887A patent/IL156887A/en not_active IP Right Cessation
- 2003-08-15 US US10/641,787 patent/US8187320B2/en not_active Expired - Fee Related
-
2008
- 2008-01-22 AU AU2008200300A patent/AU2008200300B2/en not_active Ceased
-
2012
- 2012-05-25 US US13/481,129 patent/US20120239139A1/en not_active Abandoned
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1162259A (en) * | 1994-10-26 | 1997-10-15 | 诺瓦蒂斯有限公司 | pharmaceutical composition |
| CN1250382A (en) * | 1997-01-27 | 2000-04-12 | 斯蒂斯生物聚合物公司 | Bonding layer for surface coating of medical devices |
| CN1360951A (en) * | 2000-12-28 | 2002-07-31 | 微创医疗器械(上海)有限公司 | Blood vessel support with coating to prevent blood vessel from becoming strictured again |
Also Published As
| Publication number | Publication date |
|---|---|
| IL156887A0 (en) | 2004-02-08 |
| EP1372753A2 (en) | 2004-01-02 |
| EP1842569A2 (en) | 2007-10-10 |
| JP4290985B2 (en) | 2009-07-08 |
| KR100994543B1 (en) | 2010-11-16 |
| CA2435306C (en) | 2010-12-21 |
| EP1842569A3 (en) | 2010-03-03 |
| WO2002065947A3 (en) | 2003-09-18 |
| US20040117008A1 (en) | 2004-06-17 |
| JP2004531299A (en) | 2004-10-14 |
| NZ540000A (en) | 2007-05-31 |
| CA2435306A1 (en) | 2002-08-29 |
| AU2008200300A1 (en) | 2008-02-14 |
| WO2002065947A2 (en) | 2002-08-29 |
| KR20090130879A (en) | 2009-12-24 |
| EP1372753B1 (en) | 2007-08-01 |
| BR0207321A (en) | 2004-02-10 |
| AU2008200300B2 (en) | 2011-04-21 |
| ATE368482T1 (en) | 2007-08-15 |
| CN1547490A (en) | 2004-11-17 |
| US20120239139A1 (en) | 2012-09-20 |
| IL156887A (en) | 2009-09-01 |
| US8187320B2 (en) | 2012-05-29 |
| DE50210591D1 (en) | 2007-09-13 |
| KR20040011463A (en) | 2004-02-05 |
| AU2002233342B2 (en) | 2007-10-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN100469394C (en) | Implant with FK506 | |
| CN101808676B (en) | Improved drug-coated medicinal product and its manufacture and use | |
| KR101123528B1 (en) | Medical device for dispensing medicaments | |
| JP5271892B2 (en) | Delivery of highly lipophilic drugs through medical devices | |
| DE10127011A1 (en) | Implant used for treating vascular narrowing or occlusion, especially for controlling restenosis contains FK506 in chemically bound or physically fixed form | |
| CN101474455A (en) | Nano-scale microporous structure medicine elution apparatus for storing and releasing various medicines and preparation method thereof | |
| US20050065595A1 (en) | Implants containing combretastatin a-4 | |
| RU2332959C2 (en) | Implants with fk506 | |
| DE10127330A1 (en) | Implant used for treating vascular narrowing or occlusion, especially for controlling restenosis contains FK506 in chemically bound or physically fixed form | |
| HK1139879B (en) | Improved pharmaceutical-coated medical products and the production thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| C17 | Cessation of patent right | ||
| CF01 | Termination of patent right due to non-payment of annual fee |
Granted publication date: 20090318 Termination date: 20140218 |