X-ray Imaging & X-ray Diffraction Imaging
X-ray imaging research at LAS encompasses technological, methodological, and experimental aspects of 2D, 3D and 4D mapping of materials structure from macroscopic down to sub-microscopic length scales. A particular emphasis of research activities is the development of fast, time-resolved methods which allow dynamic processes such as cell growth or materials processing to be investigated.
The main X-ray imaging research activities at LAS cover technical and methodological research and development in the fields of:
- X-ray optics, X-ray Detectors
- Hard X-ray Microscopy
- 3D imaging
- Biomimetics
- Spectroscopic Imaging, Phase Imaging, Diffraction Imaging
- Algorithms and Computational Approaches
and their application in the fields of life sciences:
- 4D imaging of living organisms, hierarchical and correlated imaging of organisms, tissues, cells and structures, morphological dynamics, mutation etc.
and materials research:
- in-situ & in-operando characterisation of materials for electronics and energy storage, light-weight high-performance materials, microsystems devices, multi-phase fluidics
X-ray imaging research at LAS makes use of facilities available both at the synchrotron radiation source ANKA at KIT, as well as at the ESRF. These facilities encompass beamlines for a wide range of tomographic and diffraction-imaging applications and off-line laboratories for radiography and tomography in absorption contrast (distribution of the attenuation coefficients in 2D and 3D), propagation-based phase contrast imaging for low-Z and weakly absorbing materials, element-specific imaging at absorption edges for mapping the spatial distribution of specific elements. Further available techniques include full-field X-ray microdiffraction (Rocking Curve) imaging with a spatial resolution at or below the 1 µm scale for analysis and quantification of the spatial distribution of crystal lattice misorientations, defect densities and of local lattice quality in crystalline specimens, and tomography and laminography for 3D imaging of objects in absorption and phase contrast.
Life Science Imaging
Evolutionary development in fossilized insect species
(A) Onthophilus intermedius ventrally embedded in a stony matrix. (B) Beetle digitally isolated from the stone, revealing well-preserved morphology hidden by the matrix. (C) Perspective view of the fossil showing parts of exoskeleton, tracheal network, alimentary canal and genitals.
1. Method: Non-invasive, fast white beam X-ray microtomography
2. Data processing: Flat-, dark-field corrections, artefact filtering and tomographic reconstruction
3. Image analysis: Segmentation of stone matrix and beetle, including exoskeletal parts and remains of soft tissue
A.H. Schwermann, T. dos Santos Rolo, M.S. Caterino, G. Bechly, H. Schmied, T. Baumbach, T. van de Kamp, “Preservation of three-dimensional anatomy in phosphatized fossil arthropods enriches evolutionary inference”, elife 5: e12129 (2016) doi: 10.7554/eLife.12129
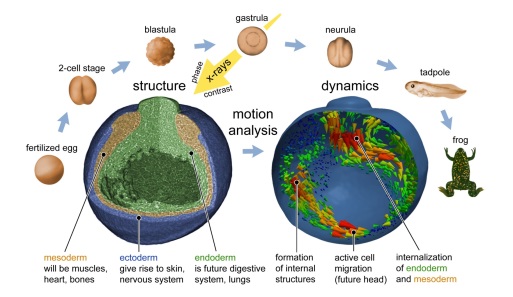
4D Imaging of Xenopus Gastrulation via X-ray Phase Contrast Microtomography
1. Method: Non-invasive in vivo, time-lapsed X-ray microtomography using single-distance, propagation-based phase contrast
2. Data processing: Flat-, dark-field corrections, hot pixel removal, artefact filtering, phase retrieval and tomographic reconstruction
3. Image analysis: Segmentation of cells, tissues and cavity volumes, flow-field calculation to visualize overall dynamics and to track individual cells
J. Moosmann, A. Ershov, V. Altapova, T. Baumbach, M.S., C. LaBonne, X. Xiao, J. Kashef, R. Hofmann, “X-ray phase-contrast in vivo microtomography probes new aspects of Xenopus gastrulation”, Nature 16;497(7449):374-7 (2013) doi: 10.1038/nature12116
Morphometric analysis of adult teleost fish by X-ray CT
Vertebrate models provide indispensable paradigms to study development and disease. Their analysis requires quantitative morphometric study of the body, organs and tissues. This is often impeded by pigmentation and sample size. X-ray micro-computed tomography (micro-CT) allows high-resolution volumetric tissue analysis, largely independent of sample size and transparency to visual light. Importantly, micro-CT data are inherently quantitative.
|
Left: Micro-CT of a juvenile zebrafish. Coronal and sagittal virtual sections of a 38 dpf zebrafish stained with PTA. Boxed regions are shown in higher magnification. As in adult medaka, all tissues and body regions show high and differential contrast: (1) eye, note distinct layers of neural retina (red arrowhead); (2) heart (red arrowhead); (3) brain (red arrowhead). Scale bar: 6 mm. Right: 3D anatomical atlas (a) sagittal, (b) coronal, (c) transverse planes through the 3D interactive model. Each colour represents a unique anatomical organ. |
- Method: X-ray absorption microtomography combined with contrast agent staining of a whole adult fish
- Data processing: Flat-, dark-field corrections, hot pixel removal, artefact filtering, noise removal, tomographic reconstruction and mosaic imaging (six tomograms per sample)
- Image analysis: Automatic segmentation of organs and tissues, calculation and correlation of positions and sizes between different inbred strains
V. Weinhardt, R. Shkarin, T. Wernet, J. Wittbrodt, T. Baumbach & F. Loosli Quantitative morphometric analysis of adult teleost fish by X-ray computed tomography
In vivo cine-tomography of fast-moving beetles
| In vivo X-ray cine-tomography is a 4D imaging technique developed to study real-time dynamics in small living organisms with micrometer spatial resolution and subsecond time resolution. The method enables insights into the physiology of small animals by tracking the 4D morphological dynamics of minute anatomical features as demonstrated here by the analysis of fast-moving screw-and-nut–type weevil hip joints. |
- Method: Ultrafast white beam X-ray cine-tomography
- Data processing: Flat-, dark-field corrections, artefact filtering and tomographic reconstruction
- Image analysis: volume rendering, joint segmentation, motion tracking, optical flow analysis
T. dos Santos Rolo, A. Ershov, T. van de Kamp, T. Baumbach, “In vivo X-ray cine-tomography for tracking morphological dynamics”, PNAS 111(11): 3921-3926 (2014) doi: 10.1073/pnas.1308650111
X-ray Diffraction Imaging
The goal is the development and optimization of correlative full-field diffraction imaging methods with high spatial and temporal resolution. Methodical and instrumental aspects include micro-diffraction imaging, diffraction laminography, and tomography, algorithm development and optimization, simulation, and image analysis.
|
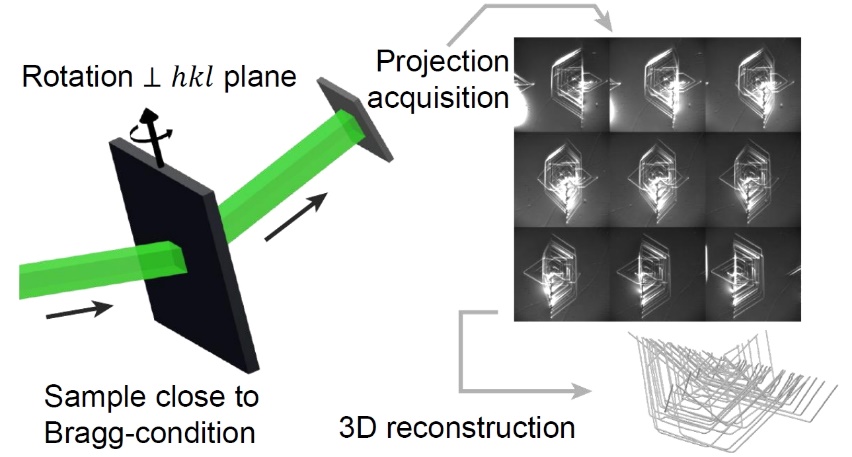
Correlative, time-resolved full-field X-ray diffraction imaging for 2D and 3D reconstruction of materials structures (eg. nanostructures, layers, defects), based on using reciprocal space x-ray diffraction data to reconstruct real space image structures. The experiment consists of the measuring of 2D-series of rocking curves with a suitable detector. The 3D-intensity distribution thus obtained (x,y,θ) is the input information for development of our techniques (solution methods of inverse problems). Applications include:
|
Correlative, time-resolved full-field X-ray diffraction imaging for 2D and 3D reconstruction of materials structures (eg. nanostructures, layers, defects), based on using reciprocal space x-ray diffraction data to reconstruct real space image structures.
The experiment consists of the measuring of 2D-series of rocking curves with a suitable detector. The 3D-intensity distribution thus obtained (x,y,θ) is the input information for development of our techniques (solution methods of inverse problems).
Applications include:
- non-destructive multi-scale 3D characterization of dislocation networks in industrially-relevant materials
- combination with methods such as X-ray white beam topography and optical microscopy to allow correlation of bulk and microscopic physical data
D. Hänschke, A. Danilewsky, L. Helfen, E. Hamann, and T. Baumbach, „Correlated Three-Dimensional Imaging of Dislocations: Insights into the Onset of Thermal Slip in Semiconductor Wafers”, Phys. Rev. Lett. 119, 215504 (2017). (doi: 10.1103/PhysRevLett.119.215504)